modeling
Sophie Baden
Senior Thesis | 2025

Human in vitro modeling assesses the immune response elicited by vaccine adjuvant formulations in children
Sophie Baden, Senior Thesis 2025
Abstract
Improving vaccine induced immune responses is important and is age dependent. The potential of adjuvants to improve vaccine responses is poorly studied. The Precision Vaccines Program studied the immune responses in 12 children to Influenza, RSV, and SARS-CoV-2 and the impact of certain adjuvants on these immune responses in an in vitro system. Using flow cytometry, we characterized the CD4, CD8, Th1 and Th2 responses.1 In 12 children: blood samples were analyzed. Antigenspecific (influenza, SARS-CoV2 and RSV) CD4 and CD8 T cell memory responses were detected.2 This in vitro platform detects immune responses. Impact of adjuvants on type of immune response is underway.
Introduction
Improving vaccine responses in children is important to prevent the consequences of infection and associated illness. This project is a study of children, ages 2-6 years old, and testing them for immunity to different viruses and the role of adjuvants in shaping this immune response.
The immune system of children (ages 2-6) works differently than other age groups, as the immune system is growing (like the rest of the child) and it is exposed to numerous pathogens and vaccines for the first time (Figure 1; the vaccines recommended for all children by the CDC). The vaccines children receive protect them from the serious illness that these infections cause like measles (currently surging in Texas with at least 1 child dying from infection), however, the immune response can be variable in this age group. This variability is across the different kinds of immune responses, such as antibodies (from B-cells) and cell-mediated immunity (from T cells), and across different pathogens like influenza (FLU), respiratory syncytial virus (RSV), and SARS-CoV-2 (SCV2 or Covid-19). This assessment of immune responses is further complicated by whether the child had prior infection with any of these pathogens and the number of vaccines (and which ones) they have received. So, understanding immune responses is complicated but important to improve vaccine efficacy.

Figure 1: Which vaccines and approximate time intervals for children 0-18 years old, as recommended by the Centers for Disease Control (CDC)
The immune responses of children are fundamentally different from those of adults. For example, children produce a stronger Th2 (T helper cell type 2) immune response, which supports B cell maturation and proliferation (B cells are white blood cells that create antibodies). Antibodies are proteins produced by the immune system which attach to antigens (foreign substances) on pathogens, such as bacteria, fungi, and viruses, and bind toxins (e.g., diphtheria), and neutralize them from causing infection/illness and facilitating their removal from the body.17 The studies now described explore important types of immune responses brought out by different vaccines and adjuvants.
Key Immunologic Principles
In order to set the stage for this work several key immunologic parameters need to be defined and this sets the stage for the analyses are presented. An important immunological parameter is T helper cells (Th). These cells have an important surface marker called CD4 (Cluster of Differentiation 4) which is how they are identified. These T-cells provide critical functions like helping B-cells make antibodies (Th2 type) and helping other T cells fight bugs (typically have a CD8 surface marker and are called Th1 type response). These types of cell markers allow the research to identify the type of immune response generated after an infection or a vaccination. A third subset of T helper cells are regulatory helper T cells which are called Th17.
Cytokines are small molecules that exist in cells throughout the body, and are messenger proteins responsible for many of the biological effects in the immune system. Cells typically ‘talk’ to each other by secreting specific types of cytokines depending on what the message is. Lymphocytes, which contain many cytokines, are a type of white blood cell that help the body fight foreign viruses and bacteria. There are two main types of lymphocytes; T lymphocytes (known as T cells) control the body’s immune system response and directly attack and kill infected cells, and B lymphocytes (B cells) which make antibodies, proteins that target viruses, bacteria, and other infections. All T lymphocytes use cytokines to communicate. Th1 polarized T cells (CD8 type), which is the immune response more dominant in adults, recognizes viruses in the body, actually in individual cells, and instantly knows if it is a threat. These cells then directly kill and remove the virus, a process called cell mediated immunity (CMI).
During childhood, most immune responses are predominantly Th2 (T helper 2) polarized responses, as the body at this time is like a blank canvas. This immune response tries to figure out what viruses and bacteria are important to neutralize (kill) as they are dangerous. The
immune system of children in this age range have little to no exposure to most infections, thus the immune system has to be educated about how to fight them for the first time, for each bug. This is vital to childhood development that the right antibodies are made against bugs but not against self - as would cause autoimmune illnesses (like many rheumatologic conditions, type 1 diabetes, bad rash like eczema, and more). Though Th2 responses are trying to help B-cells make good antibodies to fight infections if not done just right, like the Goldielocks story, too strong an immune response can cause very extreme reactions, which can be dangerous and possibly lead to death. This is where allergies and rheumatologic diseases stem from. As antibodies are like bullets and T cells are like infantry, it is important for children to develop all the necessary antibodies against all the different common childhood infections as they provide protection for years and are much easier for the body to make than T cells. Adjuvants are compounds which help direct the immune system to make particular types of immune responses such as more of a Th1 or a Th2 type response.
Hypothesis
Identification of adjuvants with greatest Th1/CD8 vs Th2/B cell potential in children ages 2-6, which will allow us to guide immune responses to what is the best type of immunity for a given pathogen.
Methods
For this study we focused on the Th1, and Th2 responses to Covid, Flu, and RSV in children. Blood was collected from children and the lymphocytes (peripheral blood mononuclear cells or PBMCs) were harvested. These immune cells were then exposed to different vaccine adjuvants and different vaccine antigens (Flu, RSV, and SCV2) to define the types of immune responses elicited.
We used the Memphis - in vitro assay platform that evaluates the potency and specificity of immunomodulators. In vitro is referring to the studies conducted outside the organism, in a controlled environment (ex: a laboratory) usually in a test tube or a petri dish. On the contrary, In vivo means experiments studied within a living organism. All assays are performed using the cells from a single blood draw in each study participant. We plated the sample from each child on a 96 well plate in triplicate plus a control (Figure 2, Panels A-D). For each sample we averaged the 3 values measured and subtracted the background values to determine a value.


Figure 2: Plate 1 layout of all samples. Panel A is the plate and Panel B depicts the plate layout. The darker shading (A1 – D1), and (E5 – H12) depicts empty wells, without any cells/ adjuvants present. Plate 2 layout of all samples Panel C is the plate and Panel D depicts the plate layout. The darker shading (where there are empty wells) - in E4-B4 and (E6 – H12)
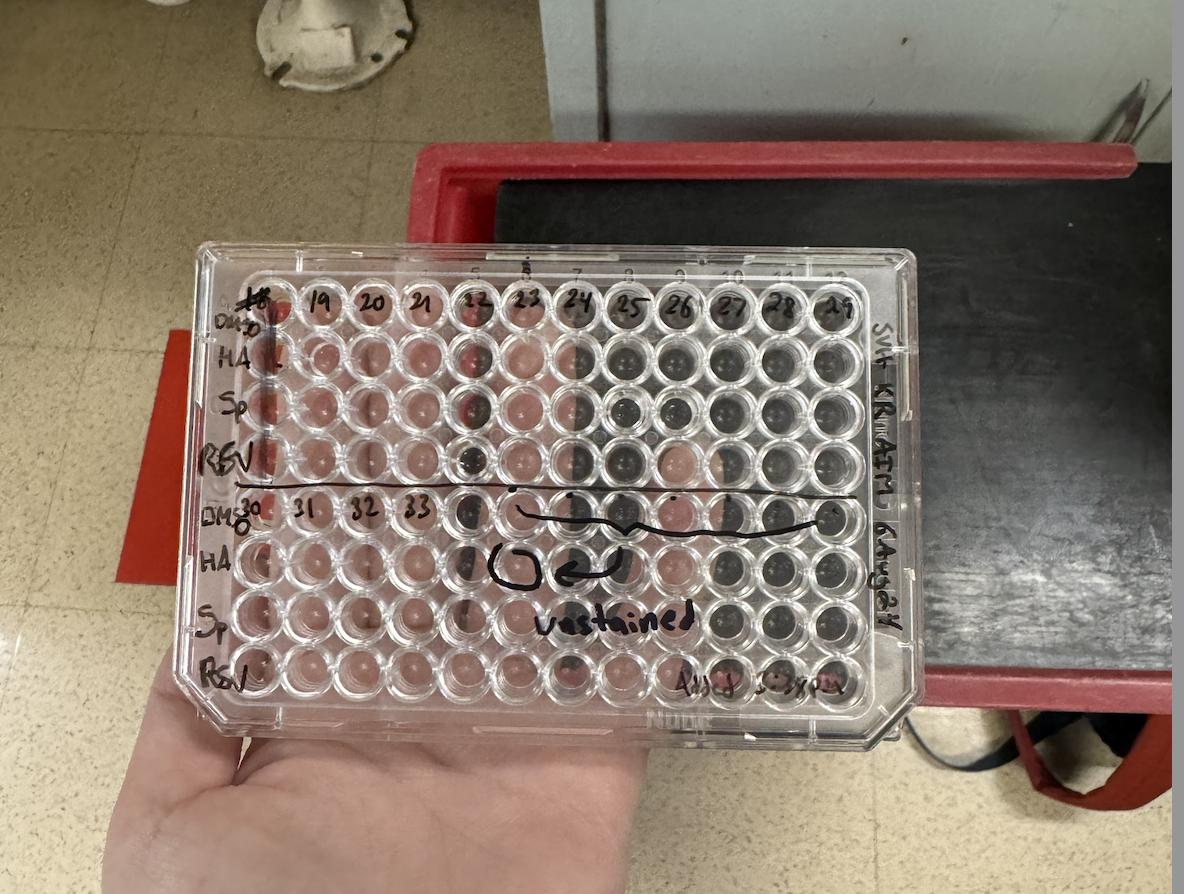

Figure 2: Plate 1 layout of all samples. Panel A is the plate and Panel B depicts the plate layout. The darker shading (A1 – D1), and (E5 – H12) depicts empty wells, without any cells/ adjuvants present. Plate 2 layout of all samples Panel C is the plate and Panel D depicts the plate layout. The darker shading (where there are empty wells) - in E4-B4 and (E6 – H12)
PBMCs
Human peripheral blood mononuclear cells (PBMCs) are lymphocytes. These cells are easily isolated from the blood of healthy donors, which makes them favorable when trying to single out the cells from the blood we want to analyze. PBMCs include lymphocytes (T cells, B cells, and NK cells), monocytes, and dendritic cells, which only make up a small portion of the original sample of blood, so it is important that PBMCs can be easily separated. A gradient medium with a density of 1.077 g/ml separates whole blood into two fractions; PBMCs make up the population of cells that have a lower density, whilst red blood cells and PMNs (polymorphonuclear neutrophils) have a higher density. CD4+, CD8+, and Th17+ Cells
CD4+ is a glycoprotein found on the surface of selected immune cells. It is found on the T helper cells. Its main role in the immune system is to help regulate immune responses. CD4 helps T cells recognize antigens; a marker which tells your immune system whether something in your body is harmful or not. 2 They are found on viruses, bacteria, tumors, and normal cells on the human body. It binds to dendritic cells, an immune cell found in tissues, and boosts immune responses by displaying antigens on the surface of other cells in the immune system. This interaction helps activate the T cell, which then activates an immune response to fight infections. CD8+ is also a glycoprotein found on the surface of primarily cytotoxic T cells (also known as CD8+ T cells) but can also be present on other immune cells like Natural Killer (NK) cells and specific dendritic cells. Glycoproteins are molecules that comprise protein and carbohydrate chains that are involved in many physiological functions; including immunity. Many viruses have glycoproteins that help them enter bodily cells, but they are also immune targets.18 CD8 functions as a co-receptor that enhances the ability for these cells to recognize and respond to infected or abnormal cells in the body. CD8+ T cells are a critical part of the immune system, as they specialize in identifying and
killing cancerous or infected cells. They develop long-term protection against re-infection, as it remembers previous pathogens.
Th17 is known as the mediator helper cell, as itself and its effector cytokines mediate host defensive mechanisms to various infections. 15 Th17 cells have been recognized as a distinct T helper cell population that plays a crucial role in CD4+ T cellmediated adaptive immunity.^15 There are three immediate categories of cell-mediated effector immunity, the first being; Tbet(+) IFN-γ-producing group 1 ILCs (ILC1 and natural killer cells), CD8(+) cytotoxic T cells (TC1), and CD4(+) Th1 cells, which protect against intracellular microbes. The second type of immunity includes GATA-3(+) ILC2s, TC2 cells, and Th2 cells producing IL-4, IL-5, and IL-13 which induce mast cell, basophil, and eosinophil activation. These cells help regulate the immune system. The T cell regulatory phenotype - also known as Treg (Figure 3).

Figure 3: In this schematic, it is distinguishing Treg to self (e) and pathogen/bug (p)
Sample Preparation
A 1X perm wash (50 mL) was made, after which cells were spun for 5 minutes at 500G in a centrifuge at room temperature. Then the plate was flicked empty, then 100 microliters were added in each well with 1X perm wash, left to sit for 15 minutes at room temperature, flicked empty, (process repeats). Add again → 100 microliters were added in each well with 1X perm wash and left to sit for 15 minutes at room temperature. While that spun, an antibody mix was made (20 microliters per antibody: 7 antibodies). The cells were spun for 5 minutes at 500 G at room temperature, and the well plates were flicked empty. After this process, there were 20 microliters left, which were spun briefly, and then 1 microliter per antibody was added in each well (so 7 microliters of mix per well). This mixture was left for 30 minutes at room temperature in a drawer (a dark space), then washed with 100 microliters of perm wash, spun for 5 minutes at 500 G at room temperature, flicked empty, washed with PBS (to get rid of the perm wash), spun for 5 minutes at 500 G at room temperature, flicked empty, then the final volume was resuspended in 30 microliters of PBS, and ultimately stored in fridge at 4C until assays run.
The following cellular markers were used to identify which T cells were stimulated: 1. characterizing the T cells (CD4 and CD8); 2. characterizing if Th1 (T-bet+); Th2 (GATA3+); or Treg (CD25+) polarized; and 3. type of T cell population stimulated TCM (T cell central memory); TEM (T cell effector memory); TEMRA (T cell effector memory CD45RA+) that are more differentiated than TEM cells.The IMPACC triple AIM panel was utilized to assess different adjuvants including: CAF08 (which induces TLR7/8 and CLR Mincle), R848 (which cannot be used in
humans but induces TLR7/8), MPLA+QS21 (which induces TLR7/8/9), CpG+Alum, and AS01 (which is a liposomal coformulation of MPLA+QS21 and the same as MPLA+QS2, but patented by GSK; this induces TLR7/8/9).
Single Color Controls
Single Color controls are vital to make sure the experiment is functioning correctly. The single color control plate (example in Figure 4) was placed into the machine before the adjuvant and antigen mix was tested; this ensures that the activations of each color (that are supposed to happen) are the ones that are in effect. We put each of these colors in their respective wells, on the third plate to pass through the analyzer first, to make sure we had valid single color control samples before starting the actual analysis. We then put the 2 plates for each participant through the AIM analyzer: called the Aurora, testing the amount/percentages of cellular stimulation in each well of the two plates. This was done once the control plate confirmed the machine was running properly. In order to assess the adjuvant responses, we put the same amount of donor sample in each well, but varied the amount of each adjuvant (these data are not being shared now as still undergoing analyses).

4: Depicts the final plate layout for the single color control plate before we ran the cells through the AIM analyzer.
Activation-Induced Marker (AIM) assay
Activation-induced marker (AIM) assays are a method of antigen-specific T-cell detection. Recognition of peptide-MHC complexes by T-cell receptors then induces upregulation of activation markers on the T cells that can then be detected by flow cytometry. By activating the T cells with specific viral antigens, the AIM assay enables these specific T cells to be analyzed using flow cytometry.25
Flow Cytometry
Flow cytometers utilize lasers as light sources to produce both scattered and fluorescent light signals that are read by detectors. These signals are converted into electronic signals that are analyzed by a computer and written to a data file. Cell populations can be analyzed and/or
purified based on their fluorescent or light scattering characteristics. A variety of fluorescent reagents are utilized in flow cytometry. The activated T cells from the AIM assay were then detected by flow cytometry (Figure 5).

5: Showing the process of flow cytometry25
Results
At this time we present the responses to the 3 viruses –influenza, RSV and SARS-CoV-2 as the data for the adjuvant responses are still being analyzed. In Figure 6 the data from the control assay (Group A), Influenza HA peptides (Group B), SARSCoV-2 spike peptides (Group C), and RSV N peptides (Group D) are presented. Twelve different participants are used to measure each set of responses (the average of the triplicate measurements for each volunteer are presented). This is done as the immune responses can differ between people, so we need to measure many people to understand the average immune responses in general.
Group A is the control group for how the assay background works. The focus is on the details for the CD4 and CD8 responses as this is an important part of how the immune system behaves. So we looked at the types of CD4 and CD8 cells that are stimulated by the different conditions – 3 important viruses.
Background Cellular Stimulation
In Figure 6, the types of immune responses brought out by the assay control are shown. This tells us about the background immune stimulation in the assay, so when we look at the assays for the antiviral responses, we can see the difference. The DMSO control causes stimulation of different types of CD4 and CD8 cells with the highest types of immune cells being stimulated being: CD4: CD25+CD69+, TEMRA and TN, and for CD8: TEMRA, CD25+CD69+, TCM and TN. Of note, in the CD4 cells there is slightly more GATA3+ (Th2) compared to T-bet+ (Th1) skewing. This represents assay issues and not the specific immune response to each of the 3 viruses. It is also interesting to see how variable these responses are across all 12 participants.
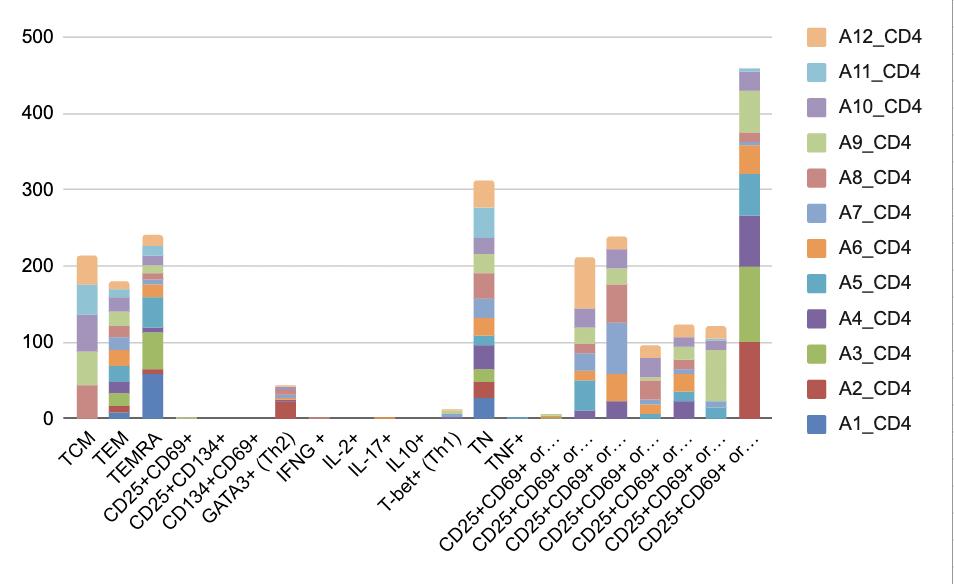
Figure 6, Panel A: CD4 Controls, A1-12 represent the 12 participants

Figure 6, Panel B: CD8 Controls, A1-12 represent the 12 participants
Antigen Specific Cellular Stimulation
The immune responses to the 3 viruses are different. In Figure 7 the immune responses to influenza HA are shown. This is the interesting part of the data – as our lab group had many discussions over why certain viruses would elicit different T cell responses. Of note the CD4 response to influenza HA is high in TCM compared to background without evidence for Th1 or Th2 skewing. The CD8 responses are high for TEMRA suggesting significant prior influenza exposure thus increased T cell memory populations.

Figure 7, Panel A: CD4 responses to influenza HA. B1-12 represents the 12 participants

Figure 7, Panel B: CD8 responses to influenza HA. B1-12 represents the 12 participants

Figure 8, Panel A: CD4 responses to SARS-CoV2. C1-12 represents the 12 participants

Figure 8, Panel B: CD8 responses to SARS-CoV2. C1-12 represents the 12 participants
In Group D the immune responses to RSV are measured as seen in Figure 9. The CD4 and CD8+ TEMRA responses are highest with little Th1 or Th2 skewing.

Figure 9, Panel A: CD4 responses to RSV. D1-12 represents the 12 participants

Figure 9, Panel B: CD8 responses to RSV. D1-12 represents the 12 participants
Taken together, this data shows evidence for some T cell memory, likely from prior exposure or vaccination to these 3 viruses. However, there is little evidence for Th1 or Th2 skewing to the 3 different viruses when exposed to antigen without any in vitro adjuvant stimulation.
We are in the process of analyzing the impact of adding different adjuvants to see how that affects the kinds of CD4 and CD8 responses to the 3 viruses. We hypothesize that different adjuvants will cause different in vitro Th1 or Th2 skewing of the immune response. These additional groups are currently being analyzed.
Discussion
This data shows the immune responses of children to 3 important viruses – influenza, SARS-CoV-2 and RSV. All children have evidence for T cell memory responses. The variability of the immune responses between children is notable and shows the importance of studying larger numbers to better understand the immune responses across people. There is little evidence for Th1 or Th2 skewing when only viral antigens are used without any adjuvant. The in vitro data on the adjuvants is in progress.
There is experience with SARS-CoV2 vaccines and immune responses in children. One study, is the ‘Comparative immunogenicity and safety of SpikoGen®, a recombinant SARSCoV-2 spike protein vaccine in children and young adults: An immuno-bridging clinical trial.’ SpikoGen is a spike protein ectodomain vaccine, manufactured in insect cells.21 The study aimed to compare the safety and immunogenicity of the SpikoGen vaccine in children, adolescents, and young adults. Two doses were given to each participant, except for children 5-12 years old, who received a half dose. These participants returned 14 days after the second dose, where the vaccine immunogenicity was tested via assessment of serum anti-spike and neutralizing antibodies - as neutralizing antibodies measure the antibody function, and then
neutralize the virus. Serum anti spike addresses antibodies in the blood directed against the spike protein. Spike proteins are a protein on the surface of the virus that binds to the host cell, and come as trimmers (3 spike proteins bonded together) in COVID19. Children were followed up until 6 months after the second dosage was given.
Two weeks after the second dose, the seroconversion rates, which is the immune response to the vaccine; neutralizing antibody levels were not significantly different for children (59.50 %), adolescents (52.06 %) and adults (56.01 %).21 This study is comparable to my research, as it is studying the immunogenicity of different age ranges concerning this vaccine, and my lab was considering the effects of covid, pertaining to the amount of exposure children had faced.
Another study is “Human in vitro modeling of adjuvant formulations demonstrates enhancement of immune responses to SARS-CoV-2 antigen.’ Adjuvants are known to boost vaccine immunogenicity, but their mechanisms are often not fully understood, which limits their immediate use in pandemic vaccine development. In this study, they explored the cellular and molecular activities of various adjuvant formulations that are available for pre-clinical testing, including some designed for global public access. These scientists utilized four human in vitro platforms to evaluate both individual and combined adjuvants in different delivery systems: unformulated, oil-in-water, and liposomal. The liposomal co-formulation of MPLA and QS-21 proved to be the most effective in promoting dendritic cell maturation, selectively driving Th1-polarizing cytokine production, and activating SARS-CoV-2 Spike-specific CD4+ and CD8+ T cells in a co-culture assay. Some formulations also significantly enhanced Spike antigen-specific humoral immunity in vivo. This study demonstrates the value of using a combination of human in vitro models to predict the adjuvanticity of different formulations. As such, human in vitro modeling could help
accelerate the development of affordable and scalable adjuvants for vaccines aimed at vulnerable populations, ultimately benefiting public health as a whole.28
This article emphasizes the general study of the SARSCoV-2 antigen in terms of making the adjuvants for vaccines accessible, but also pertains to T cell isolation (which includes CD4+ and CD8+ isolation), and dendritic cell formulation. This is relevant to our study, as this article dives into the formation of a vaccine (for Covid) utilizing CD4+ and CD8+ spike specific T cells. This testing showed the relevance of these T-cells in activating the immune system and clearing a virus.
Another study is ‘Human In vitro Modeling Identifies Adjuvant Combinations that Unlock Antigen Cross-presentation and Promote T-helper 1 Development in Newborns, Adults and Elders.’ Adjuvants are vaccine components that boost type, magnitude, breadth, and durability of an immune response. Certain adjuvant combinations can act synergistically to enhance and shape immunogenicity, promoting Th1 and cytotoxic T cell development. The combination also promotes protective immunity – to vulnerable populations such as newborns. The study’s aim was to identify adjuvant combinations that could synergistically promote the expansion of vaccine-specific CD4+ cells, induce crosspresentation on MHC class I, resulting in antigen-specific activation of CD8+ cells, and direct the balance of immune response to favor the production of Th1-promoting cytokines. A combination of TLR9 and STING agonists (CpG + 2,3-cGAMP) promoted influenzaspecific CD4+ and CD8+ T cell activation and selectively favored production of Th1-polarizing cytokines TNF and IL-12p70 over co-regulated cytokines IL-6 and IL-12p40, respectively.
The identification of the adjuvant combination CpG + 2,3cGAMP may therefore be essential to the future development of vaccines against respiratory viral infections tailored for the
functionally distinct immune systems of vulnerable populations such as older adults and newborns.29
This study is in tandem with the research presented, as the immunodeficiency of children due to limited exposure is critical to how the body will respond to serious viral infections (such as RSV, FLU, and SARS-CoV-2).
Animal studies with the same antigens and adjuvants are being conducted to validate the in vitro profiles determined in this study. These in vivo mouse and monkey studies will provide correlation between in vitro and in vivo models to further allow development and refinement of potential vaccine adjuvants for human use.
References
1. Ruterbusch, Mikel, et al. “In Vivo CD4+ T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm.” PubMed, 26 April 2020, https://pubmed.ncbi.nlm.nih.gov/32340571/. Accessed 7 January 2025.
2. Cleveland Clinic. “Antigen: What It Is, Function, Types, & Testing.” Cleveland Clinic, Cleveland Clinic, https://my.clevelandclinic.org/health/diseases/24067antigen. Accessed 7 January 2025.
3. Doss-Gollin, Simon et al. “Human in vitro modeling of adjuvant formulations demonstrates enhancement of immune responses to SARS-CoV-2 antigen.” NPJ vaccines vol. 8,1 163. 26 Oct. 2023, doi:10.1038/s41541-023-00759y
4. Diray-Arce, Joann et al. “Multi-omic longitudinal study reveals immune correlates of clinical course among hospitalized COVID-19 patients.” Cell reports. Medicine vol. 4,6 (2023): 101079. doi:10.1016/j.xcrm.2023.101079
5. Ozonoff, Al et al. “Features of acute COVID-19 associated with post-acute sequelae of SARS-CoV-2 phenotypes: results from the IMPACC study.” Nature communications vol. 15,1 216. 3 Jan. 2024, doi:10.1038/s41467-023-440905
6. Morrocchi, Elena et al. “Modeling human immune responses to vaccination in vitro.” Trends in immunology vol. 45,1 (2024): 32-47. doi:10.1016/j.it.2023.11.002
7. Zimmermann, Julie et al. “Co-adjuvanting DDA/TDB liposomes with a TLR7 agonist allows for IgG2a/c classswitching in the absence of Th1 cells.” NPJ vaccines vol. 8,1 189. 22 Dec. 2023, doi:10.1038/s41541-023-00781-0
8. Berger, Abi. “Th1 and Th2 Responses: What Are They?” The BMJ, British Medical Journal Publishing Group, 12 Aug. 2000, www.bmj.com/content/321/7258/424.1.
9. professional, Cleveland Clinic medical. “Lymphocytes: Function, Definition, Levels & Ranges.” Cleveland Clinic, 1 May 2024, my.clevelandclinic.org/health/body/23342lymphocytes.
10. Manoylov, MK. “What Are Cytokines?” LiveScience, Purch, 6 Nov. 2020, www.livescience.com/what-arecytokines.html.
11. professional, Cleveland Clinic medical. “Helper T Cells: Overview & Function.” Cleveland Clinic, 10 Sept. 2024, my.clevelandclinic.org/health/body/23193-helper-t-cells.
12. Yang, Mingyue & Meng, Fanzheng & Gao, Man & Cheng, Genhong & Wang, Xiaosong. (2019). Cytokine signatures associate with disease severity in children with Mycoplasma pneumoniae pneumonia. Scientific Reports. 9. 10.1038/s41598-019-54313-9.
13. Wu X;Wu P;Shen Y;Jiang X;Xu F; “Cd8+ Resident Memory T Cells and Viral Infection.” Frontiers in Immunology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/30283442/. Accessed 7 Jan. 2025.
14. Ong, Guang Han, et al. “Exploration of Pattern Recognition Receptor Agonists as Candidate Adjuvants.” Frontiers in Cellular and Infection Microbiology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/34692565/. Accessed 7 Jan. 2025.
15. Ouyang, Wenjun, et al. “The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation.” Immunity, U.S. National Library of Medicine, Apr. 2008, pmc.ncbi.nlm.nih.gov/articles/PMC3424508/.
16. Campe, Julia, and Evelyn Ullrich. “Frontiers | T Helper Cell Lineage-Defining Transcription Factors: Potent Targets for Specific GVHD Therapy?” Frontiers in Immunology , 4 Jan. 2022,
www.frontiersin.org/journals/immunology/articles/10.3389 /fimmu.2021.806529/full.
17. Professional, Cleveland Clinic medical. “Antibodies: Definition, Types & Function.” Cleveland Clinic, 13 Sept. 2024, my.clevelandclinic.org/health/body/22971antibodies.
18. Shabir, Osman. “What Is a Glycoprotein?” News, 24 Feb. 2021, www.news-medical.net/health/What-is-aGlycoprotein.aspx.
19. Markov, Peter V, and Oliver G Pybus. “Evolution and Diversity of the Human Leukocyte Antigen(HLA).” Evolution, Medicine, and Public Health, U.S. National Library of Medicine, 10 Jan. 2015, pmc.ncbi.nlm.nih.gov/articles/PMC4315060/.
20. Strzepa, Anna, et al. “Myeloperoxidase: A New Player in Autoimmunity.” Cellular Immunology, U.S. National Library of Medicine, July 2017, pmc.ncbi.nlm.nih.gov/articles/PMC5665680/.
21. Tabarsi, Payam, et al. “Comparative Immunogenicity and Safety of SpikoGen®, a Recombinant SARS-COV-2 Spike Protein Vaccine in Children and Young Adults: An Immuno-Bridging Clinical Trial.” International Immunopharmacology, U.S. National Library of Medicine, 25 Dec. 2023, pubmed.ncbi.nlm.nih.gov/38147778/.
22. Li, Fang. “Structure, Function, and Evolution of Coronavirus Spike Proteins.” Annual review of virology vol. 3,1 (2016): 237-261. doi:10.1146/annurev-virology110615-042301
23. Corti, C et al. “Seroconversion rate after vaccination against COVID-19 in patients with cancer-a systematic review.” Annals of oncology : official journal of the European Society for Medical Oncology vol. 33,2 (2022): 158-168. doi:10.1016/j.annonc.2021.10.014
24. Lazarevic, Vanja et al. “T-bet: a bridge between innate and adaptive immunity.” Nature reviews. Immunology vol. 13,11 (2013): 777-89. doi:10.1038/nri3536
25. Poloni, Chad et al. “T-cell activation-induced marker assays in health and disease.” Immunology and cell biology vol. 101,6 (2023): 491-503. doi:10.1111/imcb.12636
26. McKinnon, Katherine M. “Flow Cytometry: An Overview.” Current protocols in immunology vol. 120 5.1.1-5.1.11. 21 Feb. 2018, doi:10.1002/cpim.40
27. Kleiveland CR. Peripheral Blood Mononuclear Cells. In: Verhoeckx K, Cotter P, López-Expósito I, et al., editors. The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet]. Cham (CH): Springer; 2015. Chapter 15. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500157/ doi: 10.1007/978-3-319-16104-4_15
28. Doss-Gollin, Simon et al. “Human in vitro modeling of adjuvant formulations demonstrates enhancement of immune responses to SARS-CoV-2 antigen.” NPJ vaccines vol. 8,1 163. 26 Oct. 2023, doi:10.1038/s41541-023-00759y
29. Thomas, Sanya et al. “Human In vitro Modeling Identifies Adjuvant Combinations that Unlock Antigen Crosspresentation and Promote T-helper 1 Development in Newborns, Adults and Elders.” Journal of molecular biology vol. 436,4 (2024): 168446. doi:10.1016/j.jmb.2024.168446

