Sahithi Lingareddy
Senior Thesis | 2025

Identifying the Optimal Parameters of Neuromodulation in Epilepsy
S.R. Lingareddy1, K. Isaac 2-3, W. Shi 2-3, M.A. Kramer 4-5, C.J. Chu 2-3
1Boston University Academy, Boston, Massachusetts, US; 2Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts, US; 3Harvard Medical School, Boston, Massachusetts, US; 4Department of Mathematics and Statistics, Boston University, Boston, Massachusetts, US; 5Center for Systems Neuroscience, Boston University, Boston, Massachusetts, US.
Abstract
Epilepsy is the most common neurological disease in the world. As of 2021, according to the National Institute of Health, around 3 million people in the US have been diagnosed with epilepsy. Of these people, only ⅔ respond to medications and many are not candidates for surgical cure through resection. Neuromodulation is an innovative approach for those with drug refractory epilepsy. This treatment involves the surgical placement of a device, or devices, that directly send electrical pulses intracranially to disrupt epileptiform activity, consequently preventing seizures and/or lessening their severity. Devices include Deep Brain Stimulation (DBS) and Responsive Neurostimulation (RNS). DBS constantly sends stimulation following a duty cycle, with patient-specific parameter settings, whereas RNS reads the brain activity and sends simulations based on detected brain signals. While innovative, neuromodulation poses limitations as well. Efficacy studies have shown that attaining the patient-specific parameters for either the RNS or DBS is a time-consuming process as it’s done through an ad hoc approach. Although, even after the optimal parameters are attained, neuromodulation does not immediately act at its peak efficacy. Its efficacy improves over time, taking several years for patients to achieve optimal reduction in the severity and abundance of their seizures. In addition to the general time limitation, the parameters of stimulation, including frequency, amplitude, and duration of stimulation, are not well known, meaning all patients
must start from the same baseline when finding their specific parameters. Strategic changes cannot be made based on the behaviors of both the parameters and the patient's epileptiform activity. This, too, prevents patients from getting closer to a better quality of life quickly. This study, conducted in the Department of Neurology at Massachusetts General Hospital, analyzes the effects of different frequencies of stimulation on epileptic brain activity in patients with drug refractory epilepsy. Subjects with intracranial depth electrodes were stimulated with 5 different frequencies (1 Hz, 20 Hz, 40 Hz, 140 Hz, and 200 Hz) in a randomized paradigm in the brain region responsible for generating seizures (the epileptogenic zone, EZ). Both the stimulation amplitude and duration remained constant for all stimulation bursts. Rates of spikes, the canonical biomarker for the EZ, and spike ripples, the most accurate biomarker for the EZ, were measured after each individual stimulation burst for analysis, using trained, validated detectors. Comparisons between the rates from different frequencies were conducted using a linear mixed effects model. Results show that stimulation reduced both spike rate and spike ripple rate compared to baseline. In addition, higher frequencies (>20Hz) are significantly more disruptive to spikes and spike ripples than lower frequencies (<20Hz). This suggests that higher frequencies are more disruptive to epileptiform activity in general. With more knowledge and understanding of one of the parameters of stimulation, the time consumption in the process of determining the optimal patient-specific parameters for neuromodulation will decrease, allowing patients to achieve a better quality of life faster with lessened seizure severity and/or abundance.
Background
Epilepsy
Epilepsy is the most common neurological disease in the world. It can be caused by genetic diseases, abnormalities in brain development, injury such as head trauma, or from unknown
reasons. Those with epilepsy experience recurrent, unpredictable seizures. Semiologically seizures can present differently in different people; they can include convulsions, contractions, loss of consciousness, auras, confusion, jerking movements, weak or limp muscles, tense or rigid muscles, twitching, spasms, automatisms, behavioral arrest, changes in sensations, emotions, thinking, etc. Triggers for seizures also vary from person to person; they include but are not limited to stress, light, flashing lights, temperature differences, caffeine, or nothing at all. In terms of brain activity, seizures are uncontrolled bursts of electrical activity that disrupt healthy neural messages. There are several biomarkers for epilepsy, but two leading biomarkers are spikes and spike ripples. These biomarkers are typically measured in the epileptogenic zone (EZ) of the brain-- the part of the brain where seizures usually begin.1
In addition to the physical toll of seizures that patients experience, their overall well-being can be significantly altered. Patients can experience cognitive impairments from seizures, regarding memory, attention and executive functioning, depending on the location of the EZ, as well as other neurological deficits and behavioral issues, such as depression and anxiety.2-3 As well as physical effects, there are effects impacting their quality of life. Patients will face job and driving restrictions due to their disease; in Massachusetts, patients are required to be at least 6 months seizure free before being able to drive.4 Hospitalization may occur if seizures are frequent or severe, or do not end on their own, and is a toll in and of itself. Patients with epilepsy have higher rates of premature death, but, no matter the length of their lives, they must deal with a low quality of life.1
Treatment Options
There are a couple of effective treatment options to decrease seizure frequency and severity. Antiepileptic drugs (AED) can be prescribed. AEDs either inhibit excitation of neurons or enhance
inhibition of neurons.5 Communication between neurons is essential for functioning. Neural communication is conducted through action potentials, regulated by ions: sodium and potassium. AEDs regulate the channels for sodium and potassium that conduct action potentials or neurotransmitter receptors that induce action potentials, controlling the electrical activity of the neurons. For example, Benzodiazepines, which are also used as sedatives, tranquilizers and muscle relaxants, inhibit electrical activity, by enhancing the activity of GABAA, the main inhibitory neurotransmitter, receptors. Phenytoin, Carbamazepine, and Valproate decrease the firing of action potentials by increasing the inactivation of sodium channels, crucial to beginning and sustaining an action potential.6
Oftentimes, monotherapy, or the prescription of only one drug, is not enough to control the symptomatic seizures. Epileptologists have to create a combination of drugs, or polytherapy, in order to achieve the desired outcome.7 Older AED combinations include Phenytoin, carbamazepine and valproate.8 Newer medications, such as Levetiracetam, or Keppra, are commonly prescribed in seizure-specific clinics as they have fewer side effects compared to Phenytoin. Phenytoin, as well as other AEDs, can result in unsteadiness, cognitive deficits and long term cosmetic and bone issues. Additionally, various cognitive issues can occur due to the lowered excitability of the neurons caused by the drug.5
However, medications are only effective in ⅔ of patients who suffer from epilepsy,1 and can have a number of side effects including, but not limited to, dizziness, blurred vision, rashes, unsteadiness, and worsening of cognitive functions. An alternative option for refractory epilepsy is resection surgery, which can be curative. In order to be considered for a resective surgery an intracranial electroencephalogram (EEG) recording may be performed. Intracranial electrodes record directly from the brain to map the EZ. With about 8-16 contacts on each electrode, they measure the electrical activity of that point in the brain. The epileptiform activity that’s measured, such as spikes and spike
ripples, will determine the area of the brain that needs to be resected: the EZ. If this area is concise, the effects of resection of this particular area of the brain is not drastic, and surgery is feasible for the family, then this is a possible option for drug refractory epilepsy. However, resective surgery may not be a feasible option as the seizure focus may either be in multiple areas or too risky of an area.1
For those with refractory epilepsy and are not candidates for resective surgery, neuromodulation is an innovative technique that is proven to be effective. Neuromodulation is the method of sending small electrical pulses to areas of the brain in hopes of disrupting epileptic activity and therefore decreasing seizure abundance and severity. As neural messages and brain function is based on electrical signals, electrical pulses have the ability to manipulate those signals. If the epileptiform activity is disrupted with these electrical manipulations, it’ll allow for normal brain function and activity as well as prevent the seizures associated with the specific epileptiform activity present. If a patient's EZ is multifocal or is too dangerous to remove, this technique can safely modify the area. There are three currently used approaches for neuromodulation: Deep Brain Stimulation (DBS), an open looped stimulation procedure in multiple points of the brain, Responsive Neurostimulation (RNS), a closed looped stimulation that sends stimulation in response to detecting specific epileptiform activity, and Vagal Nerve Stimulation (VNS), open loop stimulation of the vagus nerve, a key nerve that transmits information between the brain and other vital organs and modulates activity in specific areas of the brain. VNS and DBS, as they’re both open looped systems, constantly send stimulation despite the response, whereas RNS responds to the brain activity and sends stimulation accordingly, making it a closed loop system. DBS is often used when the EZ is near the thalamus as that is where it’s most effective9 or when the EZ cannot be localized to one specific area; the thalamus is a part of a greater circuit, directly connecting it to many areas of the brain. VNS is used when the EZ is near the brainstem, specifically
the nucleus of the solitary tract, as that is the nerve’s most direct contact. RNS is used when the detection of seizure onsets is crucial as the device can read the activity accurately and efficiently and send stimulations accordingly.10
Neuromodulation is a generally limited technique even though it is very innovative. Epilepsy is specific to each individual, as their EZs, epileptiform activities, and semiologies, or the physical symptoms that are presented when seizing, such as jerking or staring spells, all vary.1 Therefore, parameters for each individual vary as well. Finding the correct parameters is done through an ad hoc approach, meaning trial and error. This approach is incredibly time consuming; there can only be small changes over long periods of time to prevent any dangerous outcomes. Once these parameters are found, studies have shown that neurostimulation does not reach its peak efficacy until months later, adding to the amount of time it takes for patients to attain a better quality of life. In addition to the time-consuming nature of the technique, the different parameters themselves and the ways that they can be changed also add time.12
Efficacy and longevity of these devices have all been measured through studies where each subject implanted with the device, set to patient specific electric pulses, note the amount of seizures they experience ranging from 3 months to 2 years post implantation. A general trend of increase in improvement in seizure abundance and severity is seen even after the immediate improvement after the implantation, meaning reaching a better quality of life is a time consuming process with neuromodulation.10-11 However, as each of the parameters were specific to each patient, the effects and behaviors of the parameters are unknown. All that can be concluded is that some type of neuromodulation is effective for those with drug refractory epilepsy.9, 11-12
Previous Studies on Neuromodulation
A study in 2020 examines the efficacy and safety with RNS for focal epilepsy. Patients who participated in the previous 2-year feasibility study, assessing whether the results are worth testing with a larger subject size or not, were used in this study, meaning they were required to have three or more disabling partial-onset, that originate from one or two locations over a 12-week baseline period, refractory epilepsy, and ineligible for other epilepsy treatments based on the previous study’s conditions. For this study specifically, subjects must also have intractable focal onset seizures, seizures originating only from one hemisphere, in addition to the previous requirements. For the feasibility study, after implantation, subjects were randomly split into “Treatment” and “Sham” groups; the Treatment group received stimulation whereas the Sham group received no stimulation. This was recorded for 3 weeks. Then, both groups would receive stimulation until the 2 year mark since stimulation. For the pivotal study, the larger subject group, the same system occurred, but observed for an additional 7 years instead. Results from both studies are considered. During the study, medication changes were permitted, though they were deemed unrelated to the improvements caused by the RNS stimulations. Results have shown that there was up to a 75% seizure reduction at the 9 year mark and 18.4% of 256 subjects experienced at least 1 year of seizure freedom; for these patients, the average seizure free period was 3.2 years after observation.There were correlations between those with only one seizure focus rather than multiple and younger subjects with a better outcome. Overall, the hypothesis was supported in that RNS can be considered for a safe and beneficial additional treatment for those with medically intractable focal onset seizures.11-12
In 2016, a study was done to determine the efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. The thalamus is considered a hub of the brain as it’s connected to many parts of the cortex, where seizures start. including the frontal and temporal lobe structures. The reasoning behind simulating this area
is so that the positive effects of the stimulation, the regulation of epileptiform activity, can travel along with typical thalamic communication to the other areas of the brain. After one month post-implantation, subjects were randomly separated into two different groups: Treatment and Sham, where treatment received stimulation. This blinded treatment occurred for 3 months. After 3 months, everyone received stimulation. Safety and efficacy was measured for 5 years after implantation. Through their research, results showed that 68% of subjects demonstrated a decrease in seizure frequency. Although parameters were not directly tested in this study, there were observations of better responses to higher amplitudes and rapid cycling. There was a focus on a big risk that comes with neurostimulation: kindling, the increased likelihood of a recurring seizure after being induced by stimulation. Though poor results did occur in this regard, they were rare. Overall, results indicate that DBS in the thalamus is a tolerated treatment that is associated with significant and sustained reduction in frequency and severity of seizures.9
Factors of Neurostimulation
Parameters for stimulation include: frequency, amplitude and pulse width. These parameters and their behaviors themselves are relatively unknown, meaning everyone starts at zero for all parameters. Small changes in only one of these parameters are very spaced out, time-wise; making drastic changes quickly would result in difficulty interpreting the impact of the changes on the brain activity and potentially dangerous effects. If the parameter behaviors that are most effective in reducing pathological brain activity can be identified, specialists can start with settings that only require minimal adjustments thereafter, taking less time. With this, patients will be able to attain seizure reduction, or at least seizure severity reduction, and, therefore, a better quality of life faster.15
The goal of changing these parameters is to disrupt epileptiform activity and therefore prevent seizures. The epileptiform activity
exclusively examined in this study are spike ripples and spikes. Spikes, shown in Figure 1, are interictal sharp synchronous neuronal discharges, relative to the normal brain activity. The frequency for spikes range from 80 to 600 Hz.13 They are specific to epilepsy and easily identifiable, making them the canonical biomarker. Spike ripples are a combination of spikes and high frequency oscillations, as seen in Figure 2.14 They are the most accurate biomarker for the EZ as they are specific to epilepsy, due to the spikes, and spatially accurate, due to the ripples.14
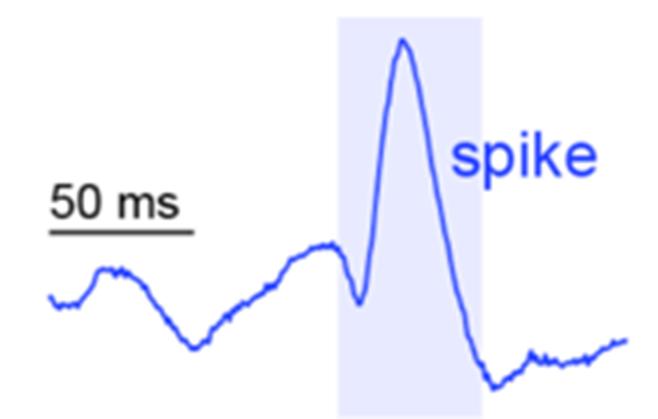
1: Example spike in brain activity.

Figure 2: Example spike ripple in brain activity. The red activity highlights the ripple component, and the blue activity highlights the spike component. When these two features occur at the same time, that is considered a spike ripple. The overlap, referring to the highlighted section of the figure, illustrates this biomarker.
Both epileptiform activities were studied as the spikes would provide helpful information for physicians, as they generally work with spikes due to their conspicuity, and spike ripples provide the most accurate information.
The purpose of this study is to determine whether different neurostimulation frequencies in the EZ optimally reduce pathologic spike and spike ripple activity, in order to improve patients’ quality of life, despite the varying semiological and electrical activities. A better understanding of the impact of these parameters will decrease the amount of time consumed in the treatment process using neuromodulation to get there.
Subjects
Subjects in this study were undergoing their surgical evaluation at Massachusetts General Hospital. The mean subject age is 27.5 years (5-66 years of age range); subjects must be a minimum of 2 years old, with no maximum age cap, as infants' skulls are too young for current neuromodulation devices. Of the 11 subjects, 6 are female. As a part of their surgical evaluation, patients have depth electrodes implanted into their brain in order to collect adequate data for their evaluation. Depth electrodes are small metal points implanted into the brain that sense and record electrical activity in that specific area. The electrodes go straight into the brain. They measure a more accurate reading of brain activity as opposed to scalp or subdural electrodes that only record from the surface of either the scalp or the brain. Taking advantage of this invasive procedure, data for the study was collected through those depth electrodes. There were no other restrictions for subjects in this study as the goal is to generate a better understanding of the disease as a whole; patients with comorbidities, common and associated with epilepsy, are accepted.
Methods
To identify the epileptogenic zone, we first used an automated, validated spike ripple detector applied to all intracranial channels to identify the channel with the highest spike ripple rate. This channel was then selected for stimulation. We designed an automated code to deliver electrical stimulations across varying stimulation parameters in a random order over the course of 3 hours. Here, we focused on the impact of changing stimulation frequency, where charge density and stimulation duration were kept constant across the different trials. The experimental design included 10 second long trials consisting of 100 ms of stimulation occurring every second, leaving 900 ms of time in between each stimulation. Each 10 second trial was separated by 5 seconds. Every stimulation had the constant amplitude of 1 milliamp and pulse width of 100ms. Across each 10 second trial the frequency varied randomly between 1 Hz, 20 Hz, 40 Hz, 140 Hz and 200 Hz. The frequency was changed randomly across trials in order to minimize any bias that could affect the results.
With the collected data, an automated and validated spike detector, Persyst, was applied to each dataset. The detector was first calibrated for each subject using the subject’s pre-stimulation baseline data. The detector defines a spike based on the presence of high frequency activity in comparison to the normal range of frequencies in that channel. Using baseline data to set the detection threshold was an important step so that the detector wasn’t skewed by the high frequency artifact introduced by the stimulation experiment. Once spike detection was complete, the spike rate in the 900 ms post stimulation was compared to the spike rate in the last second of the 5 second intervals between trials, which was used as a baseline rate, using a linear mixed effects model. Linear mixed effects models are essentially a series of linear equations that define a relationship between the frequency, the independent variable, and the spike or spike ripple rate, the dependent variable, and allow multiple observations for the subject. These relationships account for both fixed and random effects, or the
general trends of the experiment and variations amongst the individuals themselves. This method of analysis was specially chosen as it accounts for the individual variability as epilepsy is a particularly unique disease. This same process was used to analyze spike ripple rate as well, using an automated, validated spike ripple detector.
Results

Figure 3: (A) Spike rates under each stimulation frequency for each subject and (B) respective relative spike rates relative to the within stim baselines, defined by the last 1 sec recording during the 5-sec pause after each stimulation trial throughout the experiment. Using a previously validated spike detector, we find that spike rates are reduced by higher frequency stimulation compared to baseline (p<0.01, frequency ≥ 40Hz, linear mixed effects models).
** p<0.01
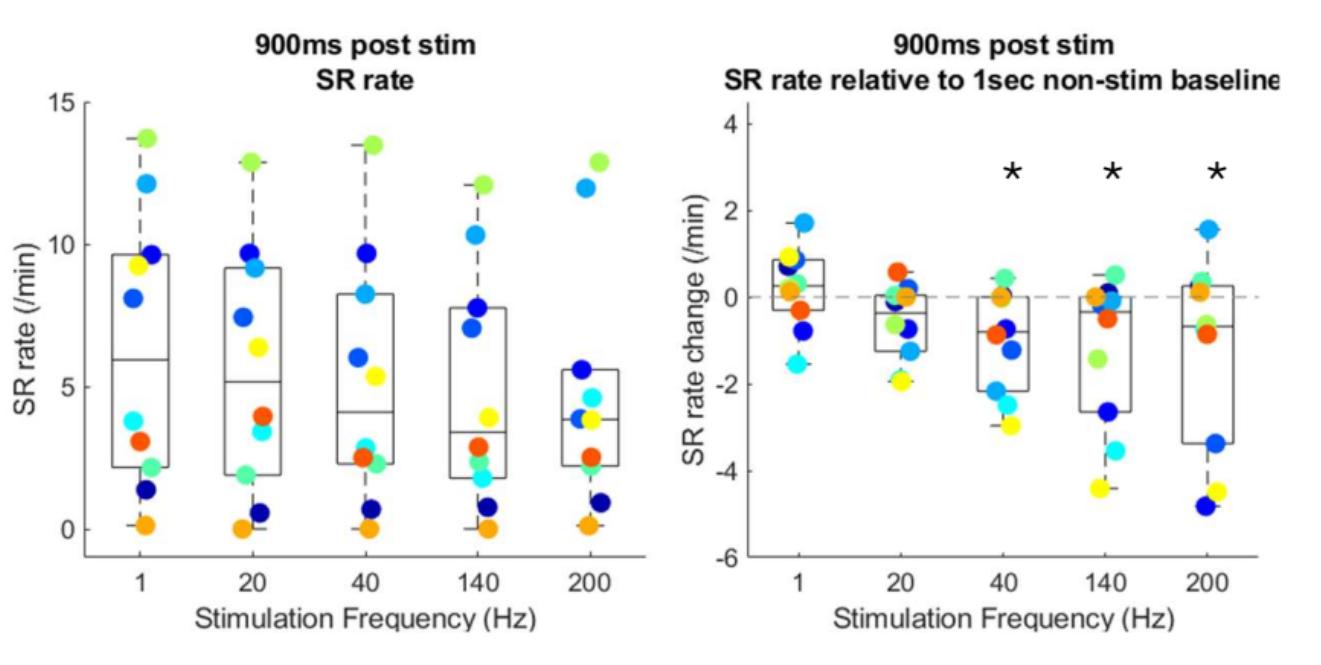
Figure 4: (A) Spike ripple (SR) rates under each stimulation frequency for each subject and (B) respective relative SR rates relative to the within stim baselines, defined by the last 1 sec recording during the 5-sec pause after each stimulation trial throughout the experiment. Using a previously validated spike ripple detector, we find that spike ripple rates are reduced by higher frequency stimulation compared to baseline (p<0.04, frequency ≥ 40Hz, linear mixed effects models).
* p<0.04

Figure 5: For each subject, spike rates were normalized to the baseline spike rate from the same subject, which are all last 1-sec recordings within the 5-sec inter-trial pauses throughout the experiment. (2-sided t-test)
+ p<0.1; * p<0.05, ** p<0.01, *** p<0.001

Figure 6: For each subject, SR rates were normalized to the baseline SR rate from the same subject, which are all last 1-sec recordings within the 5-sec inter-trial pauses throughout the experiment. (2-sided t-test)
+ p<0.1; * p<0.05, ** p<0.01, *** p<0.001
Group Results
Spike rates are reduced at higher stimulation frequencies compared to baseline (40Hz, 140Hz, 200Hz, p=0.01), with increased effect sizes also observed at higher frequencies (Figure 3). Spike ripple rates are reduced at higher stimulation frequencies compared to baseline (40 Hz, 140 Hz, 200 Hz (p<0.04, Figure 4).
Individual Subject Analysis
When analyzing the spike rates within individuals, SRN001, SRN002, and SRN008 all had significant decreases for 1 Hz(p<0.05 only for SRN001; p<0.001), 40 Hz(p<0.001), 140 Hz (p<0.001) and 200 Hz (p<0.001). SRN003, SRN006, SRN007, and SRN0015 all have significant decreases in spike rates for all five frequencies (p<0.001). Generally, the individual significant results are to the higher frequencies but most are individual frequencies that are most effective for an unknown reason (Figure 5).
When analyzing the spike ripple rates, one subject, SRN008, has increasingly significant results, starting from 20 Hz: p<0.05 for 20 Hz, p<0.01 for 40 Hz, and p<0.001 for both 140 Hz and 200 Hz. SRN004 only had a significant decrease in spike ripple rate for 40 Hz (p<0.05) as well as SRN005 (p<0.01). SRN002 and SRN003 only had significant decreases for 200 Hz (p<0.01). Thus, although group results suggest higher frequencies disrupt pathologic activity better, at the individual level, different frequencies had different effects (Figure 6).
There were no adverse events, an undesired clinical outcome, of note during this study.
Discussion
The objective of this study is to identify which frequency of neurostimulation optimally disrupts pathologic epileptiform activity in patients with drug refractory epilepsy, specifically spikes and spike ripples. Based on the results, the hypothesis is supported: higher frequencies are associated with a lower spike and spike ripple rate (Figure 3 and Figure 4). This information contributes greatly to our knowledge on neuromodulation which allows physicians and neuromodulation specialists to make thoughtful adjustments to parameters, reducing the amount of time it takes to acquire a better quality of life.
As this study only analyzed and measured the effects of one of the many parameters and two of the many epileptiform activities, there is still a lot more to uncover. Future research can focus on amplitude, pulse width, and other stimulation settings like how many pulses are in a burst and the timings between each pulse and/or burst. In addition to these foci, other epileptiform activities that patients have, like sharp waves, ripples, etc. can be analyzed. Another factor to consider is the location of the EZ. The brain is interconnected in many ways; there is a possibility that there is a zone that communicates with the EZ that has a more significant impact than directly stimulating the EZ. While, with this study the behavior of neuromodulation is more well-known, it highlights how much more is left to uncover.
This study specifically does not include many subjects. With the limited number of subjects, the results are not as reliable. Although, the results are still indicative of the behaviors of different frequencies. With more subjects, it will strengthen the analysis and provide better evidence for physicians to rely on.
The individuality of epilepsy creates room for more research to be conducted regarding why these differences are there. EZ differences, epileptiform activity differences, as well as age differences could all be significant to an unknown extent. Specific factors could be indicative to the specific neuromodulation settings
they need in order to gain control over seizures and attain a better quality of life.
While epilepsy remains relatively unknown, these findings contribute to the treatment of this common disease, helping improve patients’ quality of life faster and more efficiently.
References
1. Mayo Clinic. Epilepsy - Symptoms and Causes. Mayo Clinic. Published October 7, 2021. https://www.mayoclinic.org/diseasesconditions/epilepsy/symptoms-causes/syc-20350093
2. Novak A, Vizjak K, Rakusa M. Cognitive Impairment in People with Epilepsy. J Clin Med. 2022;11(1):267. Published 2022 Jan 5
3. Moods and Behavior. Epilepsy Foundation. https://www.epilepsy.com/complications-risks/moods-behavior
4. Massachusetts. Epilepsy Foundation. Published 2020. Accessed March 6, 2025. https://www.epilepsy.com/lifestyle/driving-andtransportation/laws/massachusetts
5. Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36 Suppl 2:S2-12.
6. Epilepsy Foundation. Summary of Anti-Seizure Medications. Epilepsy Foundation. https://www.epilepsy.com/stories/summaryanti-seizure-medications
7. Lee JW, Dworetzky B. Rational Polytherapy with Antiepileptic Drugs. Pharmaceuticals (Basel). 2010;3(8):2362-2379. Published 2010 Jul 26.
8. Pellock JM. Standard approach to antiepileptic drug treatment in the United States. Epilepsia. 1994;35 Suppl 4:S11-8.
9. Fisher R, et al. 2010. Epilepsia. 51(5):899-908.
10 .The RNS System. NeuroPace, Inc. https://www.neuropace.com/patients/neuropace-rns-system/#howit-works
11. Heck CN, et al. 2014. Epilepsia. 55(3):432-441.
12. Nair DR, et al. 2020. Neurology. 95(9):e1244-e1256.
13. Jing Xiang, Ellen Maue, Han Tong, Francesco T Mangano, Hansel Greiner, Jeffrey Tenney, Neuromagnetic high frequency
spikes are a new and noninvasive biomarker for localization of epileptogenic zones, Seizure, Volume 89, 2021, Pages 30-37, ISSN 1059-1311
14. Shi W, et al. 2024. Brain.147(7):2496-2506
15. Denison T, Morrell MJ. Neuromodulation in 2035: The Neurology Future Forecasting Series. Neurology. 2022;98(2):6572.

