The Effects of the Overexpression of CKAP5 on Facial Morphology and the Development of Craniofacial Disorders
Priya Roy
Senior Thesis | 2025



Priya Roy, Jasming Senel, Chiedza Sibanda, Burcu Erdogan, Laura Lowery
Department of Medicine, Boston University Chobanian and Avedisian School of Medicine and Boston Medical Center, Boston, MA, USA
Craniofacial disorders impact roughly 3% of the population worldwide, making it a major global health concern14 . They appear in patients at birth and can either be inherited from a parent or occur spontaneously in newborns with no known history of facial deformities in their family. Craniofacial disorders can appear in a variety of ways, affecting all parts of the face, but the majority of cases occur through a patient’s mouth forming incorrectly, causing complications such as cleft lip and cleft palate. Both oral clefts are among the most common birth defects, just after Down Syndrome, which is the number one outcome of genetic anomalies.10 Cleft lip and cleft palate are recognized by gaps in either the upper lip or roof of the mouth, and both clefts can occur either individually or simultaneously11 .
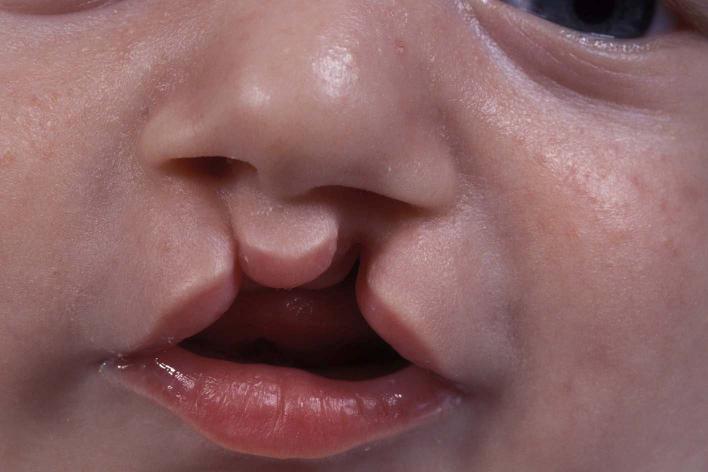
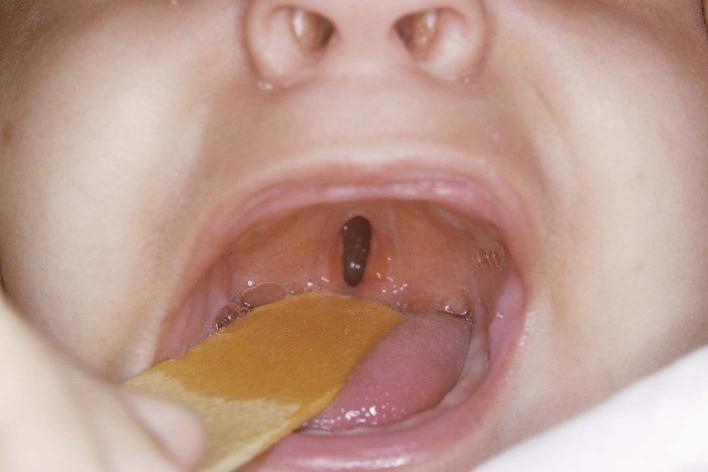
Figure 1: An infant with cleft lip. Cleft lip can take place as one gap or, as shown, two gaps in the lip that affect the left and right sides of the mouth. These clefts can be diverse in width and length, and can form as a small slit as shown on the left side, or a broad open space that reaches the nostril, as shown on the right side9.
Figure 2: An infant with cleft palate. Similar to clefted lips, clefted palates range in severity, with some cases only being a small opening either at the back of the mouth or roof of the mouth (the latter shown above). A more severe version takes place through a gap that completely splits the palate in half, from the back of the mouth to the upper front teeth9.
The global incidence of cleft lip with or without cleft palate is approximately 0.01%, which is 1 in 1,000 live births11 . There are many factors that are suspected to cause infants to develop these disorders, encompassing both genetic and environmental factors10. These disorders severely hinder children from developing at a normal rate, and not only affect patients physically but also neurologically and emotionally. Early intervention, most importantly surgically repairing facial deformities, is crucial for patients to continue to thrive and minimize the detrimental effects of these conditions3 .
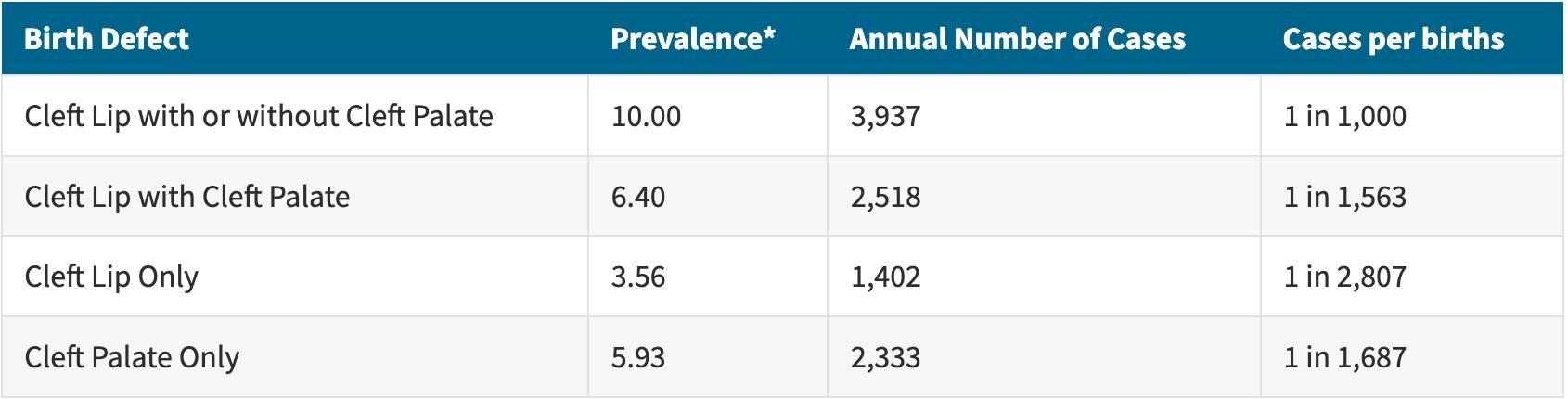
Figure 3: The average annual prevalence of cleft lip and cleft palate, along with the number of patients affected in the United States from 2010 to 2014. The birth defects quantified were cleft lip either with or without cleft palate, cleft lip strictly with cleft palate, cleft lip only, and cleft palate only. According to the data collected from 13 U.S. states and one territory, cleft lip occurring by itself was the rarest out of all these combinations. A child being born with cleft lip in general (with or without cleft palate) was the most common outcome, and cleft lip was more likely to develop along with cleft palate than without11.
There are a variety of craniofacial disorders that stem from genetic defects, which then cause errors during the embryonic development stage. Some problems that can occur are cranial cartilage merging prematurely or undeveloped tissue in the face. These disorders, if untreated, can severely decrease the quality of patients lives and continue to cause problems throughout childhood. Patients often have trouble to performing daily tasks such as eating, comprehensibly speaking, and breathing1,3. Due to the gaps in their mouth, babies with cleft lip and/or palate struggle with nursing and bottle feeding since they cannot effectively latch on to feed. Craniofacial disorders can also cause fluid to accumulate in the ears and head of the patient, which makes them prone to ear infections and puts their hearing at risk. Untreated mouth clefts, if they reach the gums, commonly cause teeth to come in at an incorrect angle or in the wrong position. Children with mouth clefts are also more likely to experience tooth decay as the incorrect alignment of teeth promotes the accumulation of plaque9,16. The patient can even be at risk for death in severe cases where the intracranial pressure is severely high or the airway path is obstructed3 .
Families of patients also face a financial burden due to the cost of treatment, which often includes surgery, speech therapy, specialized feeding support, dental treatment, and continuous monitoring of the ear canal. The cost of healthcare for children ages one through ten with cleft lip is three times higher than the cost for an average child. On the other hand, the cost is nearly six times higher for a child with cleft palate, with or without cleft lip. A 2007 study conducted that surgery to treat cleft palate with or without cleft lip was around $19,227 per procedure. A study in 1992 discovered that the lifetime costs for these patients are much higher, and accounting for special learning accommodations, impacted productivity, medical treatment, and developmental aid overall, the cost was around $100,00011 .
The majority of patients who suffer from clefts that receive treatment early on continue to lead normal lives, and no longer need any specialized support. The ideal treatment plan for clefts includes surgery early in the infant’s development stage, with a cleft lip needing to be repaired at 3 to 6 months old, and a cleft palate closed at 6 to 12 months old. A cleft lip surgery takes one to two hours, and the cleft is closed with stitches which are removed after a couple of days. A cleft palate surgery takes around the same time, and the surgery includes closing the gap with dissolvable stitches as well as reforming the palate and muscles in the roof of the mouth. In some cases, additional surgeries may be necessary to correct facial appearance or repair other parts of the face impacted9 .
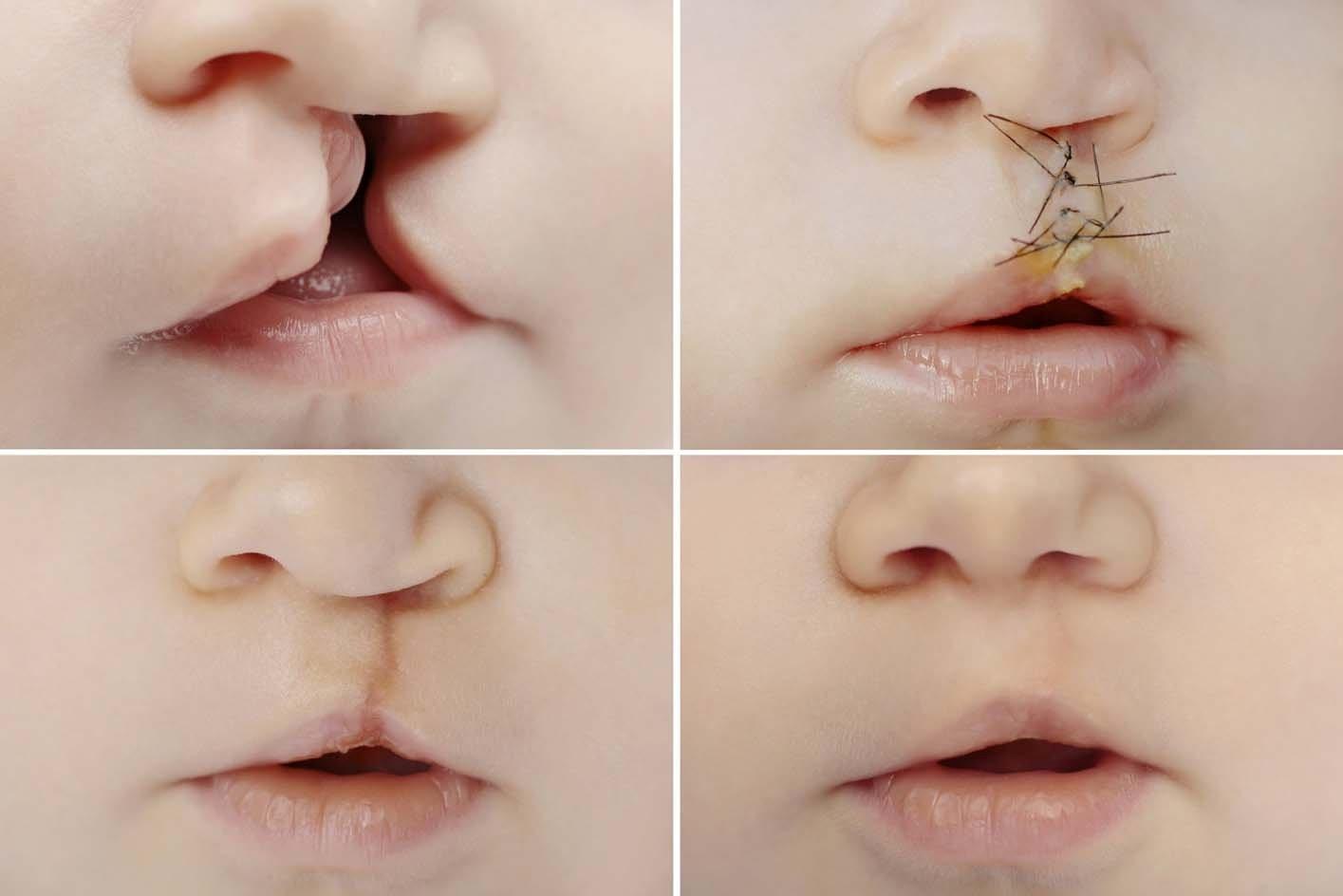
Figure 4: The cleft lip surgery process to repair a single cleft in the upper lip. There is a noticeable scar after the procedure, and the surgeon attempts to stitch in a way that will cause the scar to form along the natural curvature of the upper lip, so it will be as faint as possible after the healing process. Normally, the scar fades significantly over time9.
Up until the teenage years, patients continue receiving speech therapy and orthodontic treatment to monitor oral development, and assistance tapers off around the time adulthood is reached9. But since the ideal treatment plan is lengthy and costly, in countries such as India the majority of these disorders go untreated due to inaccessible healthcare and lack of affordability for surgeries1 .
Medical and surgical care for craniofacial disorders has improved drastically in the last few decades in terms of restoring functionality post-surgery and correcting the appearance of facial features. But despite these advancements, the surgical techniques for repairing the tissue are still inconsistent, and often unsatisfactory. Treatment for craniofacial disorders can be improved if scientists can further understand the craniofacial development process15. An important step toward understanding craniofacial disorders is determining how these genetic defects are caused on a molecular level.
There are many genes that are known effectors of the craniofacial development process and are being studied to determine exactly what roles they play in causing cartilage deformities. Scientists are investigating such genes that influence early cell migration, and therefore cytoskeletal structure which eventually forms into facial cartilage. One specific gene that is being studied is cytoskeleton-associated protein 5 (CKAP5). CKAP5 aids in the formation of the cytoskeleton, and has many functions during microtubule assembly such as helping tubulin molecules bind and form microtubules, binding to the microtubules which increases their stability, and helping microtubules separate chromosomes during mitosis. CKAP5’s regulation of microtubules is crucial as microtubule structures determine the composition and behavior of the cell2,12.
Scientists were led to study CKAP5 by other similar genes that were studied prior, one of these being transforming acidic coiled-coil protein 3 (TACC3). TACC3 works in similar areas as CKAP5, and shares the same role of regulating microtubules and promoting mitosis during neural development. TACC3 resides in the pharyngeal arches, which are structures in the embryo that later turn into cartilage and tissue, the face, and the frontal brain of the Xenopus laevis species of frog, or the specific spices used for embryonic injections when researching craniofacial disorders. Scientists discovered that knocking down TACC3 in Xenopus embryos, or decreasing the amount of the gene expressed, caused observable deformities in the facial cartilage and craniofacial structure. These deformities included lesser facial width, smaller faces, and elongated facial features compared to the control embryos6. Due to its involvement in the early developmental stage and the findings of the TACC3 knockdown experiment, CKAP5, a similar gene, is being studied.
When a gene is expressed abnormally, it can impact the neural crest cells during the developmental stage, potentially causing an insufficient amount of neural crest cells to be produced, or causing them to migrate incorrectly. Neural crest cells follow specific and precise pathways to reach their destinations and form into pharyngeal arches, so complications
during this process can cause the pharyngeal arches to migrate to the incorrect places and result in the facial tissue forming incompletely5,15 .
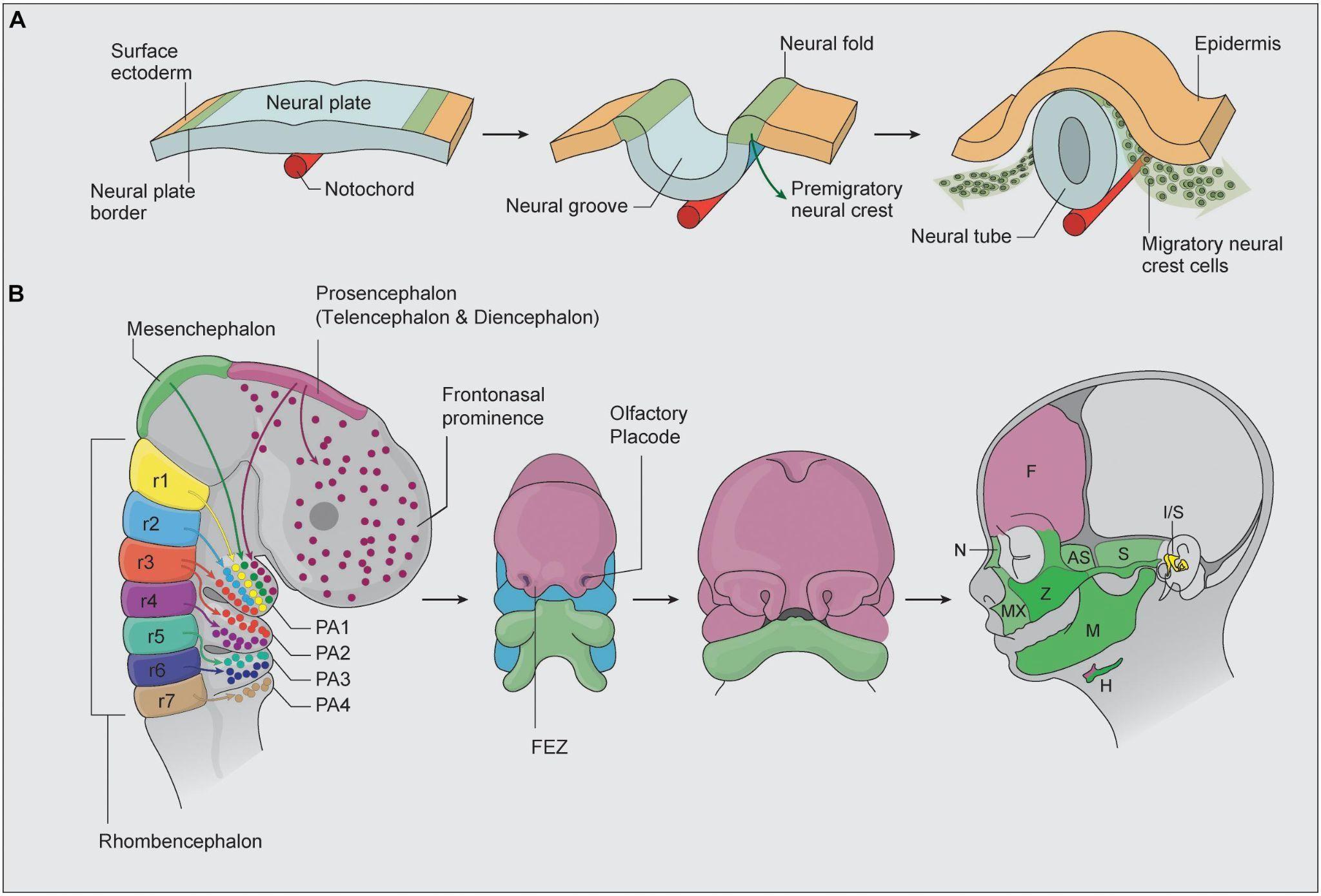
Figure 5: During the development stage, neural crest cells all migrate to different regions to eventually form the cranial structure4
As seen in Figure 5, the development of the cranial neural crest determines both facial and brain morphology. Neural crest cells that incorrectly migrate to the prosencephalon region or an insufficient amount of cells can ultimately change the structure of the frontal portion of the brain4. Certain genes such as CKAP5 share a role of expressing themselves around the area of the neural tube, pharyngeal arches, and facial structure. CKAP5 being expressed incorrectly is suspected to contribute to craniofacial disorders, and therefore impact neural crest cell migration. The precise migration of these cells are crucial to the correct facial and brain structure forming, and complications during this process can cause neurodevelopmental and craniofacial disorders. As a result, deformed facial structures and delayed intellectual ability are often associated8 .
Since it is known that craniofacial disorders are caused by complications in early neural crest cell migration, which is influenced by CKAP5, this study hypothesized that increasing the levels of CKAP5 in Xenopus laevis species will lead to craniofacial deformities in the embryos. Since Xenopus is genetically similar to humans, studies done on craniofacial disorders in this species are applicable to deformities developed in humans. The equivalent of CKAP5 that is translatable to Xenopus is named “Xenopus Microtubule-associated protein 215” (XMAP215). This experiment utilized this clone of CKAP5 so they could be expressed during the development stage of the Xenopus embryos.
Female frogs are first injected with human chorionic gonadotropin at 400 units per frog, and after 12-18 hours, their eggs are collected in 1X Marc’s Modified Ringer solution (MMR) (0.1 M NaCl, 2.0 mM KCl, 1.0 mM MgSO4, 2.0 mM CaCl2, 5.0 mM HEPES, pH 7.4). The testicles are extracted from the male frog, minced up to release the sperm, and used to fertilize the eggs. Then, after 20 minutes, the embyros washed with a 2% cysteine 1X MMR solution (brought to pH 7.8 using NaOH) to dissolve the jelly coating around them, which makes them easier to inject. Afterward, the embryos are washed 3-5 times with 0.1X MMR to avoid damage from the cysteine solution. Before injections, the embryos are stored in a fridge at 14-18 °C to slow down the development. Storing the embryos at a lower heat than room temperature elongates the time it takes to reach cell stage two by approximately 30 minutes, where room temperature allows the embryos to progress to the next stage in an hour and a half, while the experimental embryos take around 2 hours before they are ready for injections.
Once the embryos have divided and reached cell stage two, they are placed in a plastic mesh and injected on both sides with fluorescent XMAP215 mRNA, which is XMAP215 mRNA fused with a green fluorescent protein (GFP), to overexpress the levels of this gene.. XMAP215 mRNA at 750 pg/mL is prepared. The XMAP215 mRNA was in vitro transcribed, meaning it was synthesized to be used in a laboratory and to imitate naturally occurring mRNA. A hollow injection needle with a tip diameter of approximately 0.2 μm is prepared and filled with XMAP215 mRNA with a pipette that is inserted inside the needle. The needle
tip is then slightly broken with forceps at an angle, so that it releases a drop of mRNA that is 9 μm in diameter.
Once the injected embryos reach cell stages 15-17 a few days later, they are examined under a fluorescent microscope to verify whether the injections worked, which is signified by the mRNA glowing green. As a control, another set of embryos with in vitro transcribed GFP mRNA are injected at only 100 pg/mL. The following day, all embryos are checked under a fluorescent microscope for GFP expression, and embryos that displayed GFP expression are left to grow to stage 42. Then, the embryos are fixed with 4% paraformaldehyde (PFA) for one hour at room temperature. The embryos are then rinsed in 1X phosphatebuffered saline (PBS) and stored in a fridge at 4˚C until imaging.
For the imaging, embryos are first cut in half, and only the anterior half of the embryo was used to simplify the imaging process. The anterior half of the embryos are then placed in a clay dish filled with 1X PBS. Wells in the clay are made so that the embryos can placed in them and kept still while they are imaged. The embryos were viewed using a Zeiss SteREO Discovery.V8 light microscope at 5X magnification at 5X concentration. The images were taken with a Zeiss AxioCam MRc camera and analyzed using ImageJ to determine whether the increased levels of CKAP5 impacted facial structure. All of the experiments and protocols conducted by the Lowery Lab were approved by the Boston College Institutional Animal Care and Use Committee, and pertain to national regulatory standards.7
The embryos’ left and right eye areas, face height, face width, midface angle, midface area, mouth height, mouth width, mouth roundness, and left and right snout length were measured. A disparity was seen between the control and CKAP5 overexpressed embryos.
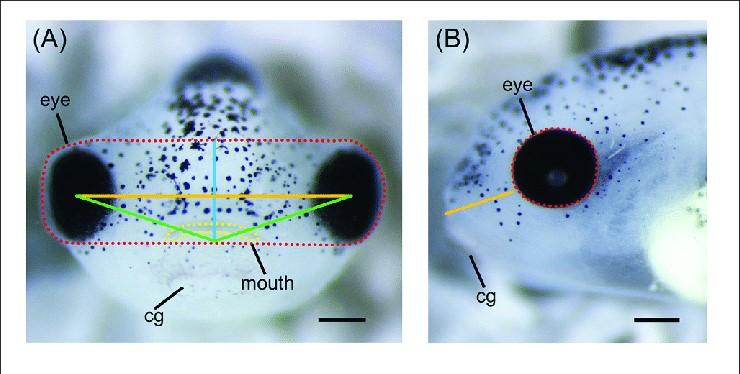
Figure 6: This image showcases how all the different measurements were taken exactly in reference to the embryo’s feautures. In panel A, the area surrounded by the red dotted line is the midface area, and the yellow dotted line represents the mouth area. The solid orange line represents the face width, and the upwards-facing angle formed by the solid green lines was measured as the midface angle. In panel B, the area enclosed by the red dotted line represents the eye area, and the solid orange line measures the length of the snout. The cg, or cement gland, was much more visible in the experimental embryos, but was not considered while taking measurements because it is a feature that humans do not possess during the developmental stage8,13.
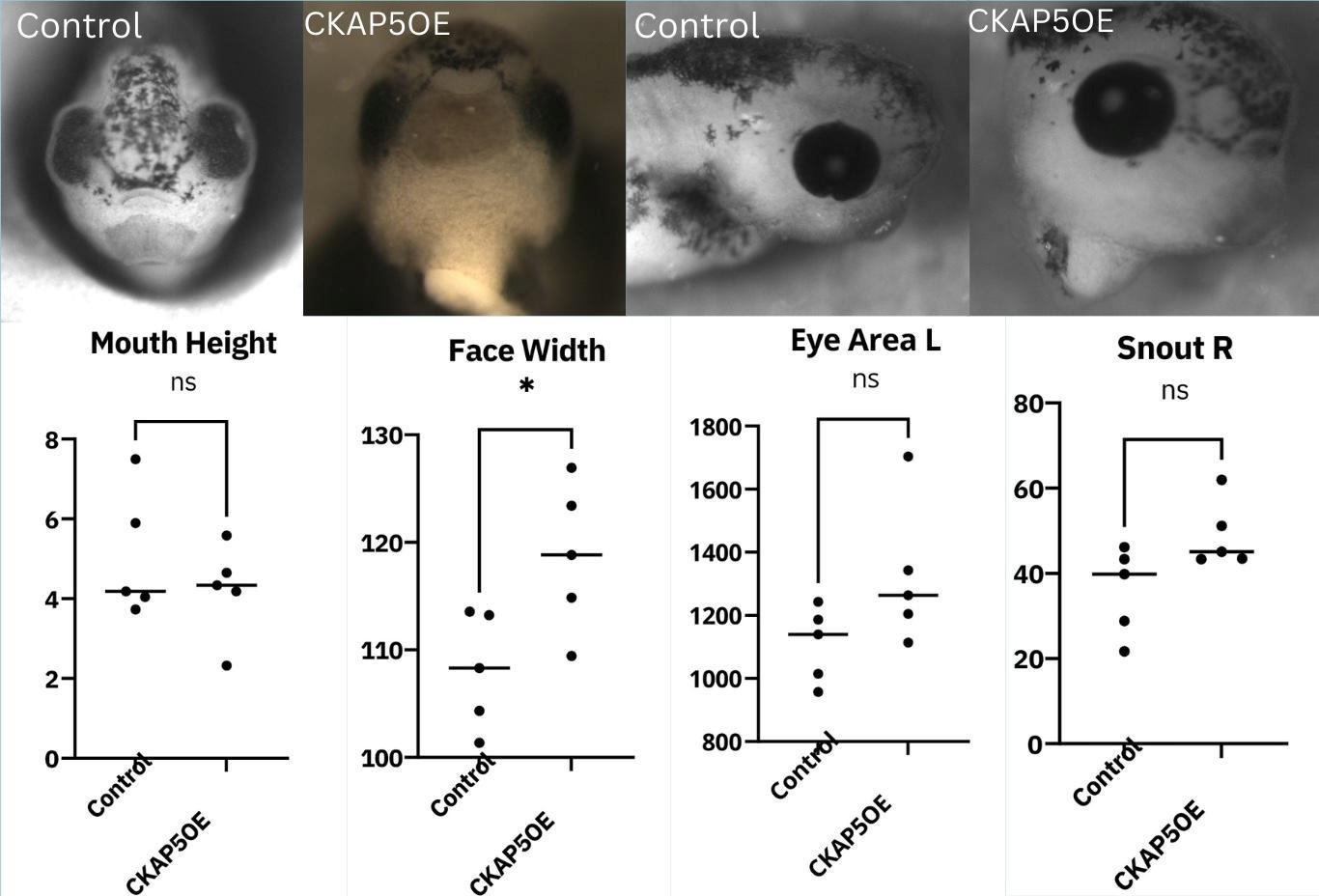
Figure 7: The effect of CKAP5 overexpression on facial morphology (p < 0.05, n=5).
For all measurements, there was a trend where the CKAP5 overexpressed embryos seemed to produce larger measurements overall, except for mouth height, where the control embryos had a higher mouth height on average. However, out of all the results collected, only the face width was significant, where the width of the XMAP215 OE embryos’ faces had a significantly larger width (Figure 7). Since we only measured 5 embryos from each of the two categories, this could be due to the small sample size. Another limitation was the inconsistent lighting provided by the microscope, and images that came out darker could have produced errors during measurement. But according to the findings, CKAP5 overexpression has the potential to significantly impact at least one area of facial development.
The control embryos had a higher mouth height on average, but a significantly smaller facial width. The embryos subjected to overexpression had a larger eye area, most strongly demonstrated by the left eye, and a longer snout, mostly strongly demonstrated on the right side. Four images display a comparison between the two groups, including a frontal and lateral view of the embryos. The control embryo from the front view has a much taller head with symmetrical facial features, a midface angle of around 90 degrees, and a thick mouth. The embryo injected with CKAP5 mRNA displayed clustered facial features, and a rather wide midface angle. The mouth appears longer and thinner and the head overall rounder. The face also appears asymmetrical, as the eyes are different shapes from one another. The cement gland is bigger as well, which could suggest that the embryo needs more support due to its abnormal cranial features13. The lateral view of the control embryo displays a short snout, while the CKAP5 OE embryo has a much longer snout and larger eyes (Figure 7).
There are a host of genes with diverse functions within the cell, yet they are all speculated to play a role in cranial crest cell migration, and therefore the formation of the brain and facial structure. After performing our experiments, the embryos with overexpressed CKAP5 showed trends of larger eye areas, larger face width, larger midface area, and mouths that are shorter, wider, and have larger areas. The snout lengths showed inconsistent trends, with the left side of the snout being shorter than the control, and the right side of the snout being longer. However, more experiments involving CKAP5’s impact on facial morphology must be completed to determine if it is a factor that causes craniofacial disorders. There have also been studies conducted involving the knockdown of CKAP5, in which morphilio was injected into Xenopus embryos in order to block the expression of this gene, which also showed promising results and trends. But in addition to CKAP5, other genes that express themselves along the neural tube during embryonic development must be investigated to determine if they cause craniofacial disorders. The process is complex in that both knockdown and overexpression must be looked into.
Once it can be proven that CKAP5 is involved in the formation of the facial structure, in situ hybridization can be conducted, which is a process that examines how the pharyngeal arches develop when the genetic information is altered. There is twist RNA in the embryo which makes proteins, which allows the pharyngeal arches to migrate and develop into facial cartilage.
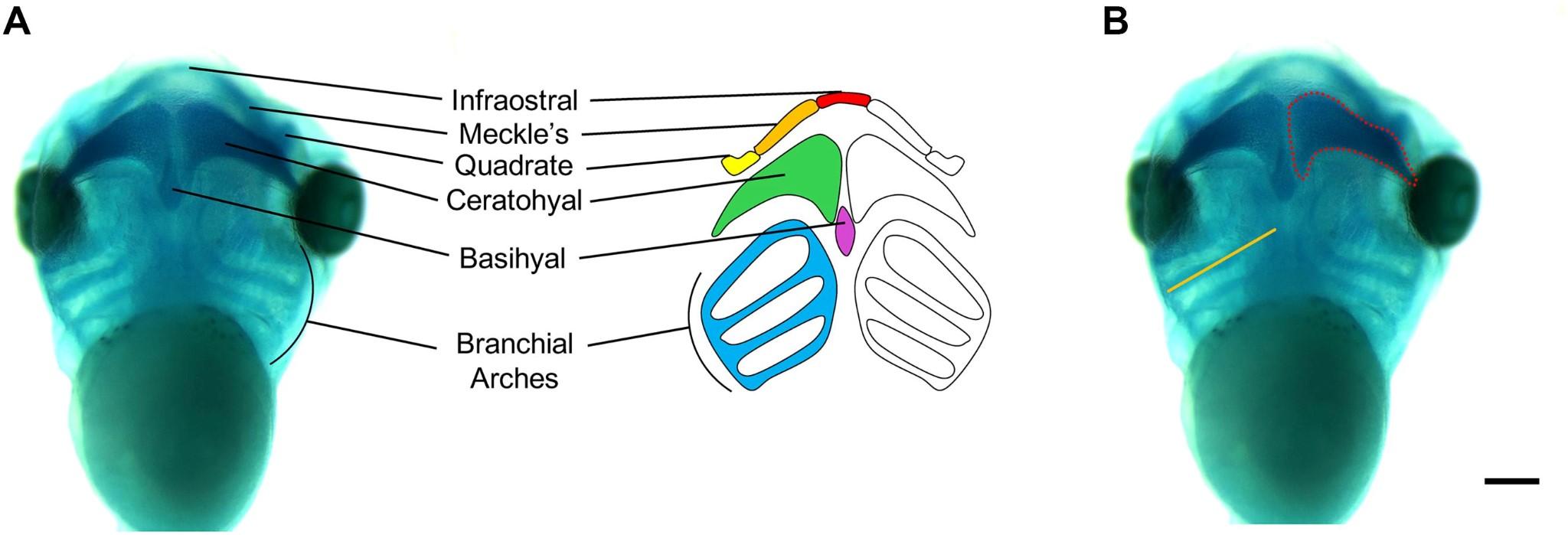
Figure 8: The cartilage morphology of the Xenopus embryo is split into different regions. The pharyngeal or branchial arches are in the lateral sides of the head and mouth of the embryo8
This experiment will involve staining the pharyngeal arches and looking in vivo at the embryo, or creating set conditions for it to thrive so its developmental process can be observed. By imaging, we can examine the size and length of the arches to see if changing the level of a certain gene will cause deformities. Imaging the craniofacial features of the tadpoles could potentially prove that there is a change caused, but through in situ hybridization, it can be determined exactly when and how changes occur throughout the process.
In order to directly test the role of CKAP5 in cell migration, in vitro cell migration assays should be performed. This process requires the harvestation of neural crest cells from Xenopus by dissecting and isolating the neural tube. These cells will then be cultured so they can continue to develop while maintaining their natural properties. We will begin conducting the migration essay by placing the neural crest cells on a substrate which would imitate the extracellular matrix that they would exist within in vivo.
Time-lapse imaging can then be performed to capture cell movements, which will allow the migration of the cells over an
extended period of time to be monitored. We will record information about the cell migration such as overall displacement and speed of cell movement, and compare the results with the control group. In vitro cell migration assays will allow us to pinpoint CKAP5’s impact on cell migration, which therefore influences the formation of the neural tube during the development process.
Interestingly, a study in India found that only 15% of patients with a cleft had facial deformities in their family history, while 85% did not. There have been some leads involving genetic causes for craniofacial disorders, and studies have concluded that having a positive family history can increase the risk of an affected child being born. An affected parent’s chances of having an affected child is approximately at a small percentage of 4%. It has also been found that inbreeding deems a child at risk for being affected, but there are additional environmental factors that appear to be the root cause, rather than just genetics. The majority of individuals with clefts were found to be in families of lower socioeconomic status and living in rural areas. These conditions could cause lack of proper nutrition, and impact the infant during pregnancy. Smoking and alcohol consumption during pregnancy were also found to be risk factors1. In the future, studies can be conducted to examine family history of families of lower socioeconomic status, take certain risk factors into account, and investigate which genes exist in the family lineage. Sociological studies can bring us closer to finding the root cause of craniofacial disorders and provide recommendations to minimize environmental factors.
1. AN Mahamad Irfanulla Khan, CS Prashanth, N Srinath. Genetic etiology of cleft lip and cleft palate[J]. AIMS Molecular Science, 2020, 7(4): 328-348. doi: 10.3934/molsci.2020016
2. Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. Microtubules. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9932/
3. Forrest, Christopher R. M.D., M.Sc.; Hopper, Richard A. M.D., M.Sc.. Craniofacial Syndromes and Surgery. Plastic and Reconstructive Surgery 131(1):p 86e-109e, January 2013. doi:10.1097/PRS.0b013e318272c12b.
4. Fitriasari S, Trainor PA. Diabetes, Oxidative Stress, and DNA Damage Modulate Cranial Neural Crest Cell Development and the Phenotype Variability of Craniofacial Disorders. Front Cell Dev Biol. 2021;9:644410. Published 2021 May 20. doi:10.3389/fcell.2021.644410.
5. Lasser M, Bolduc J, Murphy L, O’Brien C, Lee S, Girirajan S, Lowery LA. (2022) 16p12.1 deletion orthologs are expressed in motile neural crest cells and are important for regulating craniofacial development in Xenopus laevis, Frontiers in Genetics - Genetics of Common and Rare Diseases, www.frontiersin.org/articles/10.3389/fgene.2022.833083/f ull.
6. Lasser M/Pratt B, Monahan C, Kim SW, Lowery LA. (2019) The many faces of Xenopus: Xenopus laevis as a model system to study Wolf-Hirschhorn Syndrome. Front. Physiol. Jun 26;10:817.
7. Lowery LA, Faris AE, Stout A, Van Vactor D. Neural Explant Cultures from Xenopus laevis. J Vis Exp. 2012;(68):e4232. Published 2012 Oct 15. doi:10.3791/4232
8. Mills A/Bearce E, Cella R, Kim SW, Selig M, Lee S, Lowery LA. (2019)
Wolf-Hirschhorn Syndrome-Associated Genes Are Enriched in Motile Neural Crest Cells and Affect Craniofacial Development in Xenopus laevis. Front Physiol. Apr 12;10:431.
9. National Health Service (NHS). Cleft lip and palate. NHS. Updated March 23, 2023. (Accessed March 6, 2025).
https://www.nhs.uk/conditions/cleft-lip-and-palate/.
10. National Institute of Dental and Craniofacial Research. Craniofacial Birth Defects: Oral Clefts. National Institute of Dental and Craniofacial Research. Updated August, 2022.
(Accessed March 6, 2025).
https://www.nidcr.nih.gov/research/datastatistics/craniofacial-birth-defects.
11. National Institute of Dental and Craniofacial Research. Prevalence of Cleft Lip & Cleft Palate. National Institute of Dental and Craniofacial Research. Updated November, 2022. (Accessed March 6, 2025).
https://www.nidcr.nih.gov/research/datastatistics/craniofacial-birth-defects/prevalence.
12. Rossi F, Beltran M, Damizia M, et al. Circular RNA
ZNF609/CKAP5 mRNA interaction regulates microtubule dynamics and tumorigenicity. Molecular Cell. 2022;82(1):75-89.
https://doi.org/10.1016/j.molcel.2021.11.032.
13. Sive H, Bradley L. A sticky problem: the Xenopus cement gland as a paradigm for anteroposterior patterning. Dev Dyn. 1996;205(3):265-280. doi:10.1002/(SICI)10970177(199603)205:3<265::AID-AJA7>3.0.CO;2-G
14. Slah-Ud-Din S, Ali K, Mahd SM, Nisar S, Nisar O. Factors Associated With an Increased Risk of Facial Malformations. Cureus. 2023;15(7):e41641. Published 2023 Jul 10. doi:10.7759/cureus.41641
15. Trainor PA. Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher
Collins syndrome and the potential for prevention. Am J Med
Genet A. 2010;152A(12):2984-2994. doi:10.1002/ajmg.a.33454
16. Wu Q, Li Z, Zhang Y, Peng X, Zhou X. Dental caries and periodontitis risk factors in cleft lip and palate patients. Front Pediatr. 2023;10:1092809. Published 2023 Jan 4. doi:10.3389/fped.2022.1092809
