Mira Chu-Shore
Senior Thesis | 2025

Long
short-term memory neural network to identify surgical targets in human epilepsy
Mira Chu-Shore
Introduction:
Epilepsy is the most common neurological disorder, affecting approximately 50 million people worldwide and accounting for 1% of the global disease burden.1 Epilepsy impacts individuals of all ages, backgrounds, and socioeconomic statuses, regardless of race, ethnicity, or intelligence, making it a truly universal condition. Characterized by recurrent, unprovoked seizures, epilepsy can significantly affect a person’s psychological well-being, independence, emotional adjustment, and ability to maintain employment.
Epilepsy negatively impacts patients’ mental health and sleep and is associated with higher indirect costs and lower productivity.2 In a series of studies with sample sizes up to 1611 individuals, patients recently diagnosed with epilepsy were compared to patients who had been diagnosed for decades. Epilepsy symptoms significantly affect patients' daily lives, often worsened by poor sleep or declining mental health in both patients and caregivers. Epilepsy negatively affects patient’s mental health, specifically depression and anxiety, with the proportion of patients with depression ranging from 16% to 68% across studies. Characteristics associated with poor seizure control, like high seizure frequency, recurrent seizures, and drug-resistance were found to especially correlate to increased mental health impairment. In terms of sleep, some studies found no difference in outcome between epileptic and normal controls. However, factors including excessive daytime sleepiness, insomnia, and sleep quality were all shown to worsen with epilepsy.2
Beyond physical effects, epilepsy influences patients’ personality and self-esteem.2 When evaluated with the Buss-Perry Aggression Questionnaire and the Rosenberg Self-Esteem Scale, irritability levels increased with poor seizure control in patients with epilepsy. Self-esteem and work motivation were lower in patients with epilepsy and aggression was higher in epileptic
patients with depression than in controls. Family and social support is also negatively affected by epilepsy. Using the Family Adaptability, Partnership, Growth, Affection, and Resolve Scale (APGAR), at least mild familial dysfunction was shown in at least half of patients with epilepsy and their caregivers. Familial support and satisfaction was further impacted by mental health illness such as depression and perceived stigma. Perceived stigma specifically is experienced by 35.5% of people with epilepsy and 23.8% of patients report having experienced enacted stigma.
Seizures are caused by abnormal neuronal discharges or hyperexcitability of neurons. Epilepsy is diagnosed by the presence of either at least two unprovoked seizures or having one unprovoked seizure and an electroencephalogram (EEG) with abnormal epileptic activity. An EEG is a test that measures electrical activity of the brain using electrodes that either attach to the scalp or are inserted surgically into the brain.3 Epileptic activity consists of brief bursts of abnormal neuronal activity that do not cause clinical symptoms, but indicate that neurons are firing abnormally and have the potential to generate a seizure.
Seizures are classified into two broad types based on patterns of onset in the brain: focal or generalized.4 Focal seizures are seizures that initially affect one hemisphere of the brain, whereas seizures with generalized onset arise from widespread, interconnected neural networks in both hemispheres. Depending on the part of the brain affected, seizures can present with a wide variety of symptoms. If they occur in the part of the brain responsible for movement, seizures can cause muscles to stiffen or jerk. If seizures involve the part of the brain responsible for language, seizures can cause confusion and inability to communicate. During tonic-clonic seizures the whole brain is involved, causing loss of consciousness and whole body jerking and stiffness. If seizures involve a brain area without a specific function, they do not cause any clinical signs (known as subclinical).
The clinical evaluation after epilepsy diagnosis often involves studies to localize seizure onset include computed tomography scan, magnetic resonance imaging (MRI), positron emission tomography, single photon emission computed tomography, and genetic testing. MRI is an imaging technique to produce 3-dimensional images used in disease detection, diagnosis, and monitoring.5 A computed tomography scan, or CT scan, is a type of imaging that uses X-ray techniques and computer technology to create detailed, cross-sectional images of the body.6 A positron emission tomography scan, or PET scan, is another imaging technique that uses a radioactive drug, or tracer, to show metabolic activity. PET scans can often detect abnormal metabolism of the tracer drug before other signs of disease appear in other imaging tests.7 A single photon emission computed tomography scan, or SPECT scan, is a third type of imaging test that uses radioactive substances and a camera to generate a 3dimensional image. SPECT scans can reveal how well organs are working, often used in epilepsy diagnosis to create a detailed map of brain blood flow activity to pinpoint the affected area of the brain.8 Genetic testing in epilepsy diagnosis consists of analysis of the entire genome to identify variants in genes associated with epilepsy.9
The causes of epilepsy are generally categorized into three subgroups: acquired, idiopathic, and of genetic or developmental origin.1 Acquired epilepsy is due to structural injuries of the brain after birth, such as those caused by head trauma, tumor, infection, perinatal or neonatal injury, or various other disorders causing physical damage. These causes are typically identified through MRI of the brain. Idiopathic epilepsy lacks a known cause but is presumed to be genetic or epigenetic in origin and is related to abnormalities in protein channels that regulate neuronal excitability. Epilepsy categorized as of genetic origin is distinct from idiopathic in that its genetic origin is confirmed through genetic testing.
To diagnose or learn more about a patient’s epilepsy through EEG, clinicians look for specific biomarkers. The term “biomarker” refers to an objectively defined characteristic that is a measurable indicator of normal or pathological biological processes.10 The identification of reliable biomarkers is essential to many aspects of patient diagnosis and care. In the case of epilepsy, a reliable biomarker can confirm an epileptic diagnosis, allowing treatment to begin before the patient experiences subsequent confirming seizures. Epileptic biomarkers can also be used to assess the efficacy of treatment instead of requiring the patient to experience seizures and thus be subject to their risks. Similarly, biomarkers can be used to more efficiently test potential new treatments. Finally, using biomarkers drastically lowers the cost while increasing the safety and accuracy of locating the area of the brain responsible for generating seizures. The most common epileptic biomarkers are signals in the EEG, which include spikes, defined as high amplitude, sharp, voltage fluctuations, ripples, defined as brief, low amplitude bursts between 80 and 250 Hz, and spike ripples, defined as the co-occurrence of a spike and a ripple.11
As epilepsy is so prevalent and can be detrimental both physically and psychologically, effective treatment is essential. While non-invasive treatments such as medications are preferred, more than 30% of patients with epilepsy do not experience remission despite antiepileptic drug therapy. Persistent seizures were found to be higher in patients with epilepsy that also have developmental or structural comorbidities (40%) than in those with idiopathic epilepsy (26%), and also higher in patients who had more than 20 seizures before starting treatment (51%) than in those with fewer than 20 seizures before starting treatment (29%). 47% of patients are seizure free with the first medication that is tried. If a patient continues to have seizures after the initial medication, then 14% of remaining patients become seizure free with a second medication. If a patient does not respond to the first two treatments, then less than 3% of remaining patients will respond to further
medication trials. Therefore, the definition of drug-refractory epilepsy is failure to have seizure freedom after treatment with at least two medications.12
Epilepsy surgery is the treatment of choice for patients with drug refractory epilepsy. While a surgical referral results in actual resection only 5% of the time,13 an evaluation is key as it can indicate other treatment paths such as laser interstitial thermal therapy and neuromodulation. Traditional surgeries for epilepsy treatment include focal resections (removal of small, specific brain tissue), multilobar resections (removal of multiple lobes of the brain), and hemispherotomies (removal or disconnection of one hemisphere of the brain) depending on the location of the epileptogenic zone. The epileptogenic zone is defined as the location of the brain responsible for generating seizures, and thus resection of the epileptogenic zone is necessary for surgical cure. For example, a randomized clinical trial demonstrated that in children with drug refractory epilepsy, surgical intervention led to a significantly higher proportion of epilepsy cure compared to continued treatment with medication. Predictors of epilepsy cure include a greater area of the brain resected, the cause of the brain abnormality, and whether or not the complete epileptiform foci is removed. In general, between half and two thirds of patients are seizure free after at least 10 years of follow up after resective epilepsy surgery, a drastic improvement compared to drug outcomes. These 10-year outcomes only differ significantly from one and two year follow-ups, but not five year follow-up results, demonstrating that the proportion of patients that experience seizure freedom for two years generally indicate cure.14 In summary, resective surgery is a potentially curative treatment method for many patients. Despite the benefits of timely epilepsy surgery referral, epilepsy surgery is still considered by many a last resort, with delays in treatment especially prevalent among pediatric patients. Delays are especially harmful as earlier surgery results in optimal cognitive and developmental outcomes can
prevent severe cognitive delay over time, so timely evaluation and intervention operation is extremely beneficial. This delay in epilepsy surgery referral is due to many factors such as overestimation of the risks of neurosurgery, underestimation of the risks of persistent seizures, and lack of access to the necessary healthcare.13
For patients that undergo an epilepsy surgery evaluation, resection of the epileptogenic zone is not always an option. There are many scenarios that can make curative surgery not achievable, including if the patient has multifocal epileptogenic zones across many regions of the brain, the seizures are generalized at onset, the epileptogenic zone overlaps with regions of the brain that would cause loss of function if removed, or if surgical resection was attempted but unsuccessful, or the epileptogenic zone could not be properly localized. For these patients, neuromodulation is emerging as another prominent treatment method.15 Neuromodulation is the electrical stimulation of a nervous system structure to decrease epileptic activity through a variety of targets, stimulation parameters, and mechanisms of action. It has recently been proven to be more effective to stimulate the epileptogenic zone than the brain as a whole, and thus also relies on accurate identification of the epileptogenic zone. The three most understood methods of neuromodulation are open-loop vagus nerve stimulation (VNS), open-loop deep brain stimulation (DBS), and closed-loop responsive neuromodulation (RNS). All three methods are proven to be clinically relevant, as they all lead to a decrease in seizure frequency.15
As both successful resective epilepsy surgery and neuromodulation rely on accurate identification of the epileptogenic zone, identifying reliable biomarkers to locate it is essential for patient care. Two widely used EEG biomarkers for the epileptogenic zone are interictal (between seizures) spikes and high frequency oscillations (HFOs) or ripples, but both have demonstrated suboptimal specificity in actual patient care.16 Spikes
are specific for pathology, meaning they only occur in an epileptic brain, but are not spatially specific. Ripples are spatially focal but are not specific for pathology as they also occur in healthy brains. However, spike ripples have recently emerged as the most reliable biomarker for identifying the epileptogenic zone.16 A recent study used automated detection on intracranial EEG recordings from 109 subjects with drug refractory epilepsy and curative surgical outcomes, meaning for these patients, the epileptogenic zone was successfully localized and resected. Spike ripples were found to identify the epileptogenic zone more accurately among these patients than spikes, spike-gamma, wideband HFOs, ripples, and fast ripples. As spike-ripples are the co-occurrence of spikes and ripples, they are both pathologically and focally specific, making spike ripples a highly effective biomarker for the epileptogenic zone.
The current validated method to detect spike ripples in intracranial EEG data combines a feature-based algorithm (an algorithm trained to identify specific features in time series data) and a convolutional neural network detector (CNN).16 A neural network is a computer algorithm modeled on the human brain where the algorithm learns to perform a task by analyzing training examples. Much like the brain, it is organized into layers of nodes, or neurons, through which the data is moved through and processed.17 A convolutional neural network learns to detect and recognize patterns, such as objects or features, specifically within input data like images or videos. Although this current spike ripple detector performs well to detect spike ripples, it is computationally inefficient and therefore impractical to use on the multi day and multichannel intracranial EEG recordings that are collected in epilepsy patients during their surgical evaluation. For example, the average patient recording lasts seven days and includes up to 256 channels of data and analyzing this becomes computationally intractable. The bulk of the previous detector’s inefficiency was due to the CNN detector, as it relies on the creation, saving, and
reading of images. The time required to analyze 10 minutes of data from one channel is: 1.8 minutes for feature-based analysis; 5 minutes for CNN analysis. The long time required to analyze a small amount of data from one channel makes analyzing large quantities of patient data computationally intractable.
The goals of this research are to contribute to efforts to localize the epileptogenic zone using EEG biomarkers in patients with drug refractory epilepsy who are undergoing surgical evaluation. This project aimed to develop and test a detector that would have similar performance statistics but improved speed to detect spike ripples compared to the current gold standard. If successful, this tool could then be more practically applied in a clinical setting to help identify the epileptogenic zone and improve surgical outcomes for patients with drug refractory epilepsy.
Materials and Methods:
Subject data
The LSTM network was trained on data from a multiinstitutional intracranial EEG dataset from 18 epilepsy subjects with available intracranial EEG recordings prior to surgery, known resection volumes (e.g., the epileptogenic zone), and epilepsy cure after resection. Epilepsy cure after resection is key as it ensures that for each of these patients, the epileptogenic zone was correctly identified. In each case, a feature-based spike ripple detector was applied to 10 minutes of data from 10 intracranial EEG channels (5 within and 5 outside of the epileptogenic zone). All candidate events within the epileptogenic zone were hand-classified by experts as spike ripples or false positives. A false positive represents an event from within the epileptogenic zone that is not a spike ripple. All candidate events outside of the epileptogenic zone were classified as false positives. In total, the training dataset
included 1700 true spike ripples, 950 false positives, and 2550 true negatives.
Long Short-Term Memory network architecture
The detector was based on a long short-term memory (LSTM) neural network architecture. LSTM networks are a type of recurrent neural network (RNN). RNNs are specifically designed to process and predict data sequences over time, as they can capture long term dependencies in data.18 A dependency means the RNN network can look at information in the past and can use it to inform its decision in the future (hence both the “long” and “short-term memory” component of the name LSTM). The key features of RNN neural networks are the presence of a hidden state and selfconnections or loops (Figure 1). The RNN hidden state h stores depends on past information (h at time t-1) and operates on input sequences X. A RNN contains two sets of weights with one for the hidden state vector and one for the inputs. The RNN learns weights, or modifiers, for both the input and the hidden state during training. This is so the output Y is influenced by both the current input and the hidden state, which is itself based on previous inputs.

Figure 1. Data flow at time step t for a basic RNN.
Long short-term memory networks are a specific type of RNN created to solve a recurring issue in training RNN networks. RNNs are typically trained through a method called backpropagation, in which there are often vanishing or exploding gradient problems, causing the network weights to either become too small or too large. LSTM layers contain additional gates that control what information in the hidden state is carried to the next hidden state. LSTM blocks typically contain a memory cell, input gate, output gate, and forget gate, all of which let the LSTM network learn long term dependencies more effectively (Figure 2). The memory cell keeps the cell state to remember information from the past, and is a combination of many past steps. The forget gate determines how much of the previous state to forget, or not use to influence the next cell state. The input gate determines how much of the input the network should use to determine the next cell state. The output gate depends on what the LSTM neuron did one timestate before. LSTM networks also have lowered sensitivity to the
time gap compared to RNNs, making them better for understanding and analyzing sequential data than basic RNN networks.
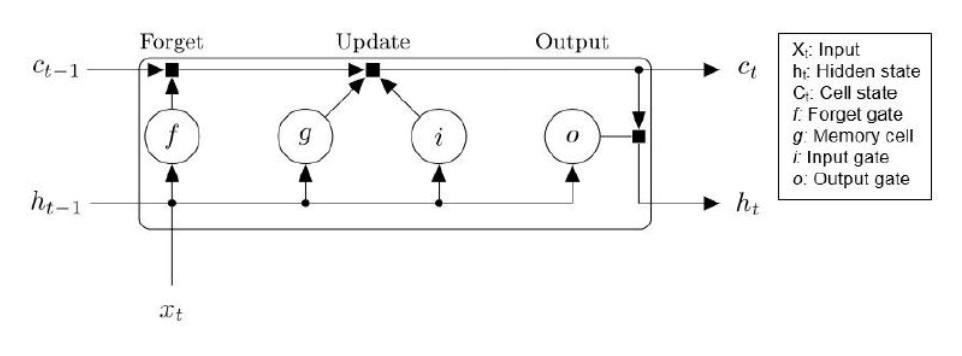
Figure 2. Data flow at time step t for one LSTM unit.
This more complicated architecture stabilizes the derivatives computed in the process of backpropagation and thus protects from gradients vanishing. LSTM networks are thus wellsuited to time series data including EEG19 as an EEG is a time series voltage recording. LSTM networks have previously been used to successfully detect epileptic spikes and spike ripples in extracranial EEG,20 suggesting that that the long short-term memory neural network would have similar accuracy but improved computational efficiency to detect spike ripples in human intracranial EEG compared to the current approach.
LSTM
implementation
The neural network consisted of 5 layers: (i) a sequence input layer with two inputs (the real and imaginary parts of the Fourier transform, or the sine and cosine representations of an event at frequencies 1 to 300 Hz), (ii) a bidirectional LSTM layer with 256 hidden units (individual units can be thought of as neurons, and here these neurons will compare the real and imaginary parts of the Fourier transform), (iii) a fully connected
layer with two outputs (representing the two possible outcomes of a spike ripple or not), (iv) a softmax layer (which transforms information into sets of probabilities), and (v) a classification layer that computes the cross-entropy loss for classification (decides if the event is a spike ripple or not the LSTM spike ripple detector does not distinguish between false positive events and true negative events).

Figure 3. The LSTM network consists of five layers.
a) A sequence input layer with two inputs (the real and imaginary parts of the Fourier transform of an event at frequencies 1-300 Hz.)
b) A bidirectional LSTM layer with 256 hidden units.
c) A fully connected layer with two outputs.
d) A softmax layer.
e) A classification layer that computes the cross-entropy loss for classification.
Detector validation
To evaluate the performance of the long short-term memory neural network to classify spike ripples, leave-one-out crossvalidation was performed. To do so, the neural network was trained on the data of all subjects but one and the network’s performance was tested on the left-out subject’s data. To minimize the impact on the results of subjects with few spike ripples detected, the long short-term memory neural network’s performance was tested on subjects with greater than or equal to 50 true spike ripples (8 subjects qualified). This process was repeated for all subjects, and ensured no overlap in training and testing data per iteration. For each cycle, the F1 score, specificity, and positive predictive value (PPV) were computed. The F1 score is the harmonic mean of precision and recall, balancing the trade-off between false positives and false negatives. It reflects the LSTM detector’s ability to correctly identify spike ripples while minimizing missed detections and false positives. Specificity is defined as the proportion of true negatives correctly identified. A high specificity ensures the detector avoids excessive false positives. Positive predictive value (PPV) is the proportion of true positive detections out of all positive detections, and measures the likelihood that detected spike ripple events are true spike ripple events identified by human experts. The measured performance statistics and speed were then compared to the previous spike ripple detector’s performance statistics and speed.
Results:
Subject characteristics
Subjects with drug refractory epilepsy and successful surgical outcomes after one year of follow-up were included. The training and testing data came from subjects with diverse demographic and clinical characteristics. Subject characteristics are provided in Table 1.
Center Age Sex Epilepsy Etiology
Univ Freiburg 16 F Focal Cortical Dysplasia
Univ Freiburg 12 M Focal Cortical Dysplasia
Univ Freiburg 54 M Focal Cortical Dysplasia
Univ Freiburg 29 F Tumor
Univ Freiburg 8 F Focal Cortical Dysplasia
Univ Freiburg 62 F Encephalocele
Univ Freiburg 46 M Focal Cortical Dysplasia
Univ Freiburg 22 M Mesial Temporal Sclerosis
Mayo Clinic 39 M Non-Lesional
Mayo Clinic 46 M Cavitation
Univ
Univ
Table 1. Epilepsy subject characteristics. Intracranial data from 18 subjects from four centers was used to train and test the detector.
LSTM achieves similar performance to current gold standard
Performance statistics were recorded across probability thresholds ranging from 0 to 1 to compensate for any overidentification or under-identification by the LSTM network. A probability threshold is the probability that an event is a spike ripple and therefore each probability threshold has corresponding performance statistics. For example, a low probability threshold would correspond to a high sensitivity of detecting events but the detected events will have a low positive predictive value. The optimal mean F1 score achieved by the LSTM detector was 0.76,
which was achieved using a probability threshold of 0.13. At that threshold, the mean PPV was 0.73 and the mean sensitivity was 0.84 (Figure 4). This performance was similar to or improved compared to the currently available detector (F1 = 0.68, specificity = 0.42, positive predictive value (PPV) = 0.79).
LSTM performs with improved efficiency compared to current gold standard
In addition, the LSTM detector required ~150 seconds to analyze 10 minutes of data from 10 channels on a single processor with 2.3 GHz CPU (32 GB RAM). This was ~320% faster than the currently available CNN detector which required ~480 seconds to analyze 10 minutes of data from 10 channels on a faster processor.
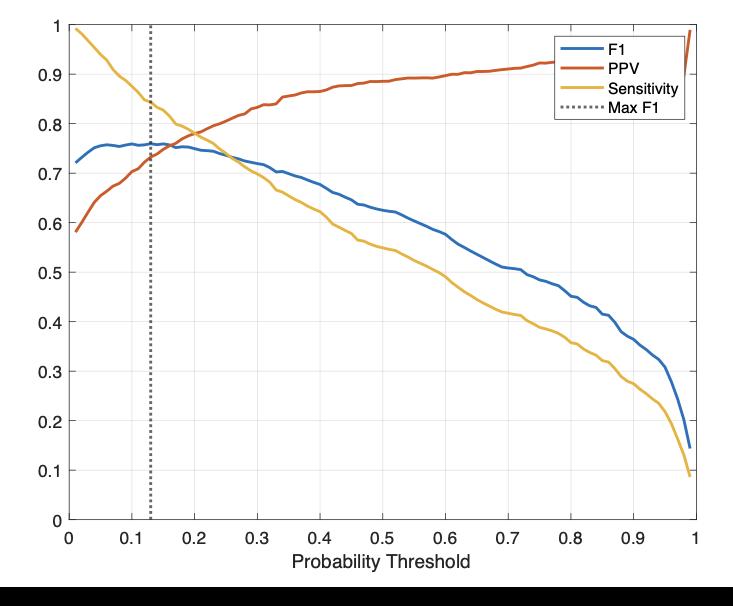
Figure 4. Performance statistics of the LSTM detector. The optimal F1 score was at a probability threshold of 0.13.
Discussion:
The LSTM-based spike ripple detector matched the performance of the current gold standard detector while significantly improving computational efficiency. The LSTM detector was 320% faster than the CNN-based approach, making it more practical for analyzing large-scale intracranial EEG data in a clinical setting. These results suggest that this detector can allow for more accessible identification of the epileptogenic zone, improving surgical planning and patient outcomes.
The LSTM detector results are promising, but to know its true efficacy would require a prospective clinical trial. This involves randomizing patients to either use the detector or not in identifying the epileptogenic zone and assessing whether its use leads to improved clinical outcomes. A well-designed randomized controlled trial would help establish causality by minimizing bias and ensuring that differences in outcomes are attributable to the detector itself.
While satisfactory, the LSTM detector’s performance statistics are far from perfect. To improve these statistics, the LSTM network would need to be trained and tested on a larger dataset. However, intracranial EEG recordings are limited due to their highly invasive nature, especially from patients with ILAE1 (successful cure of epilepsy after surgery) surgical outcomes. Additionally, a limitation of this study is that the dataset only included patients with one-year seizure freedom, whereas some proportion of patients experience seizure recurrence beyond this period. Longer-term follow-up data would provide a more comprehensive evaluation of the detector’s clinical impact. Nonetheless, with a larger dataset, the next steps are to explore the limit of the long short-term memory neural network in accurate, fast spike ripple detection for an even more widely accessible detector for surgical targets in epilepsy.
Artificial intelligence is advancing at a massive rate, and LSTM networks are just one approach to localize the epileptogenic zone. Other members in the Chu Kramer lab are currently evaluating reservoir networks, with the goal of providing even more accurate and efficient spike ripple detection. Development of a CNN network that can save and create images faster would be another area to explore with recent AI advancements, as the current CNN detector’s main limitation was speed.
Recent research suggests that fast ripples, a type of highfrequency oscillation, specifically greater than 250 Hz, are an even more accurate biomarker.21 So to improve the detector in the future, one step could be to increase the minimum frequency threshold or identifying the necessary ripples, detecting only what is termed “fast ripples,” which should exclude the ripples present in normal physiological function. For some patients, this threshold may need to be raised to 500 to 1000 Hz. However, a detector using this threshold was tested, and led to decreased sensitivity and decreased detections per patient, thus reducing successful epileptogenic localization.
Applying computational tools to improve analysis of complex brain data and build tools to localize the epileptogenic zone will provide better surgical outcomes, increase access to effective treatment, and enhance the quality of life for patients with epilepsy.
Conclusions:
Accurately identifying the epileptogenic zone is critical for successful epilepsy surgery, but existing spike ripple detectors are computationally inefficient, making large-scale intracranial EEG analysis impractical in clinical settings. The long short-term memory network performed with similar or superior accuracy to the currently available detector and had improved efficiency, making it more suitable to identify the epileptogenic zone in patients undergoing epilepsy surgery evaluation. For patients, this advancement offers the potential for a better quality of life, greater independence, and relief from the emotional and cognitive burdens of uncontrolled epilepsy.
Acknowledgements:
This project is supported by NINDS R01NS119483
References:
1. Anwar H, Khan QU, Nadeem N, Pervaiz I, Ali M, Cheema FF. Epileptic seizures. Discoveries. 2020;8(2):e110.
doi:https://doi.org/10.15190/d.2020.7
2. Boccaletti S, Lucas E, Nixon A, Boskovic N, Di Dato G. Systematic literature review of the humanistic and economic burden of focal epilepsy and primary generalized tonic–clonic seizures in adults. Epilepsia Open. 2024;9(6):2055-2086. doi:10.1002/epi4.13011
3. Mayo Clinic . EEG (electroencephalogram) - Mayo Clinic. Mayoclinic.org. Published May 29, 2024. https://www.mayoclinic.org/testsprocedures/eeg/about/pac-20393875
4. Mayo Clinic. Seizures - Symptoms and Causes. Mayo Clinic. Published September 2, 2023. https://www.mayoclinic.org/diseasesconditions/seizure/symptoms-causes/syc-20365711
5. National Institute of Biomedical Imaging and Bioengineering. Magnetic Resonance Imaging (MRI). National Institute of Biomedical Imaging and Bioengineering. Published July 17, 2019. https://www.nibib.nih.gov/scienceeducation/science-topics/magnetic-resonanceimaging-mri
6. Mayo Clinic. CT Scan . mayo clinic. Published May 7, 2024. https://www.mayoclinic.org/testsprocedures/ct-scan/about/pac-20393675
7. Mayo Clinic. Positron Emission Tomography Scan. Mayoclinic.org. Published September 10, 2024. https://www.mayoclinic.org/tests-procedures/petscan/about/pac-20385078
8. Mayo Clinic. SPECT scan - Mayo Clinic. Mayoclinic.org. Published 2016. https://www.mayoclinic.org/tests-procedures/spectscan/about/pac-20384925
9. Genetic Testing for Epilepsy. Epilepsy Foundation. https://www.epilepsy.com/causes/genetic/testing
10. Califf RM. Biomarker Definitions and Their Applications. Experimental Biology and Medicine. 2018;243(3):213-221.
doi:https://doi.org/10.1177/1535370217750088
11. Engel Jr J. Biomarkers in epilepsy: Introduction. Biomarkers in Medicine. 2011;5(5):537-544. doi:10.2217/bmm.11.62
12. Kwan P, Brodie MJ. Early Identification of Refractory Epilepsy. New England Journal of Medicine. 2000;342(5):314-319.
doi:https://doi.org/10.1056/nejm200002033420503
13. Jehi L, Jette N, Kwon C, et al. Timing of referral to evaluate for epilepsy surgery: Expert consensus recommendations from the Surgical Therapies Commission of the International League Against Epilepsy. Epilepsia. 2022;63(10):2491-2506. doi:10.1111/epi.17350
14. Harris WB, Brunette-Clement T, Wang A, et al. Longterm outcomes of pediatric epilepsy surgery: Individual Participant Data and study level metaanalyses. Seizure: European Journal of Epilepsy.
2022;101:227-236.
doi:10.1016/j.seizure.2022.08.010
15. Touma L, Dansereau B, Chan AY, et al. Neurostimulation in people with drug‐resistant epilepsy: Systematic review and meta‐analysis from the Ilae Surgical Therapies Commission. Epilepsia. 2022;63(6):1314-1329. doi:10.1111/epi.17243
16. Shi W, Shaw D, Walsh KG, et al. Spike ripples localize the epileptogenic zone best: An international intracranial study. Brain. 2024;147(7):2496-2506. doi:10.1093/brain/awae037
17. Hardesty L. Explained: Neural networks. MIT News. Published April 14, 2017. https://news.mit.edu/2017/explained-neuralnetworks-deep-learning-0414
18. Long Short-Term Memory (LSTM) Networks. www.mathworks.com. https://www.mathworks.com/discovery/lstm.html
19. Schlafly ED, Carbonero D, Chu CJ, Kramer MA, 2024. A data augmentation procedure to improve detection of spike ripples in Brain Voltage Recordings. Neuroscience Research. doi:10.1016/j.neures.2024.07.005
20. Xu Z, Wang T, Cao J, Bao Z, Jiang T, Gao F, 2021. BECT spike detection based on novel EEG sequence features and LSTM algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 29, 1734–1743. doi:10.1109/TNSRE.2021.3107142.
21. Quraishi IH. Spike ripples: Hidden clues to the mystery of the epileptogenic zone. Epilepsy Currents. Published online January 6, 2025. doi:10.1177/15357597241306610

