Ercole LiPuma

Modelling of Rhizospheric Escherichiacoli and Pseudomonas simiae using COMETS
Ercole LiPuma
Boston University Department of Bioinformatics
Daniel Segrè Lab
Dr. Ilija Dukovski and Dr. Daniel Segrè
Abstract
Studies have observed that the nutritional value of common crops will decrease due to the increased strain that anthropologic and industrial activities will have on the environment. Reduced natural resources in addition to a reduction in fertile farmland caused by climate change have greatly affected crop yield. As thus, an agricultural revolution is required in order to continue producing crops on an increased scale. The rhizosphere defines the region of the root-soil interface affected by the chemical processes of its host, and is a space inhabited by microorganisms. One avenue towards increasing agricultural productivity is to increase rhizosphere metabolic activity through inoculation or colonization of key plant growth promoting rhizobacteria, such as Pseudomonas simiae, which can release vital phytohormones and other compounds which can increase the growth rate of their host plants, such as Brachypodium distachyon. The Microbial Community Analysis and Function Evaluation in Soils scientific advisory board has invested in in vitro methods towards analyzing the productivity of rhizospheric bacterial metabolic processes. The Daniel Segrè Lab used Computational of Microbial Ecosystems in Time and Space to evaluate the growth of bacterial biomasses in soil by way of Voronoi models to simulate ectorhizospheric soil grains. The growth front of the bacterial biomass front was evaluated between dishes of varying porosity, altering both grain density and pore neck width. A basic metabolic model of Escherichia coli was used as a stand in for bacteria with more complex metabolic inputs and outputs. No evidence was found with current models that porosity was a significant determinant of the bacterial growth front. Additionally, the first step of bacterial colonization was investigated: chemotaxis a process by which bacteria can move towards the necessary metabolites required using P. simiae as a model. Successful ring biomass formation was made in COMETS using P. simiae. These
are important steps towards improving in vitro models and towards investigating how the rhizosphere can be a way to improve nutrient uptake in a world where soil fertility is constantly declining.
Introduction
Agriculture is the crutch of modern society, and yet it is an incredibly finicky crutch. 1 Grown plant biomass is dictated by the environment it propagates in: water with varying pH levels, concentrations of CO2 in the atmosphere, or metabolite and exudate levels within the growth medium can all affect total biomass production. 2 Unfavorable environments caused by increased anthropogenic activity can lead to a decrease in total global plant biomass. Improving one environmental variable the growth medium is theorized to lead to an increase in produced biomass, despite the decreasing quality of other environmental factors. 3-6 Thus, improving the growth medium will improve societies crutch.
The Microbial Community Analysis & Functional Evaluation in Soils scientific advisory board (m-CAFEs), a coalition of 6 separate university laboratories including Boston University’s Daniel Segrè laboratory is run out of the Lawrence Berkley national lab, and is interested in investigating propagation mediums for microbial communities residing within the rhizosphere. 7 The rhizosphere defines the three separate layers of space which are influenced by the chemical exudates of the host plant directly adjacent to the root structures of plants. 3 This space hosts a variety of microorganisms which are both beneficial and detrimental to the host plant. 3,8 Microbial communities are integral to natural ecosystems, and inhabit a variety of roles within them, whether they be a symbiotic or non-intrusive dependence on their host plant. 3 An example of a symbiotic relationship would be Pseudomonas simiae, a rhizobacteria, and Brachypodium distachyon, a common grass species. 9 Brachypodium distachyon
has become a model grass species used for research, and is well studied.9 Its relationship with Pseudomonas simiae is of particular relevance because it is well studied so its influences from other environmental factors can be removed, and so the effect solely deriving from rhizospheric bacteria can be isolated. P. simiae aids B. distachyon ’s growth, and B. distachyon provides protection from abiotic factors to P. simiae. The plant biomass growth derived from specifically the symbiotic relationship with P. simiae is significant, and it is not a novel example. Other microorganisms within the rhizosphere can have beneficial effects on biomass production and nutritional makeup of edible components of cash crops. 3,9-14 The proliferation of agricultural methods involving the usage of microorganisms will have a positive impact on the yield and quality of harvested products. 3 This impact is theorized to be a possible method through which to combat the degradation of fertile land and the leaching of nutrients from vascular plants caused by climate change. 3-6,8-14
This study investigates, the relationships by which bacterial biomass propagated by using computational computer models, including Computation of Microbial Ecosystems in Time and Space (COMETS) and Metabolic Constraint-based Reconstruction and Analysis in Python (COBRA-PY). Using these in vitro models along with Voronoi models formed to simulate soil pore systems allow for an investigation of these microbial relationships. Specific traits such as chemotaxis, colonial growth front as hindered through soil, as well as co-dependant and single dependent colonies within soil systems were investigated. The work performed and investigated as part of this study is an important step towards understanding how microbial communities interact with their environments in enclosed soil systems. Greater knowledge regarding these interactions can aid more efficient methods for increasing crop yield, increasing productivity, immune defense, and nutritional value. In an age where climate change
threatens the future of agricultural practices, an increased study of the fundamentals of plant growth can help society overcome selfmade struggles.
The Rhizosphere
Understanding the rhizosphere allows for a more accurate application of systems designed to improve it. The rhizosphere is occupied by thousands of bacteria and fungi, and provides natural protection against biotic and abiotic factors to microorganisms which inhabit it. 3 Biotic factors include pathogenic bacteria, viruses, and predators, and example abiotics include toxic matter, and weather extremes. Those which make their home in a plant’s rhizosphere feed on the produced and secreted nutrients, commonly referred to as exudates or plant rhizodeposition products. 15 These rhizodeposition products range from simple sugars and amino acids to tannins, phenolic acids, and steroids. The surrounding micro-organisms process the rhizodeposition products and release their own nutrients, nutrients which are then uptaken by the host plant for use in future reactions.15-16 These reactions are mainly for continued plant growth including those dealing with cell replication or DNA transcription. Plant rhizospheres can have their own native rhizobacterial species, species evolved for the unique environment provided. Bacteria chose the rhizosphere as it can provide protection against abiotic and biotic stresses, and provides an ideal environment for metabolic processes.3
The rhizosphere defines the endorhizosphere, the rhizoplane, and the ectorhizosphere (Figure 1.2), however, this study focuses solely on the ectorhizosphere, the area of dirt which surrounds the root and is influenced by the rhizodeposition products produced and secreted. 3 The endorhizosphere defines the root cells, mainly the cortex and the endodermis of the root, and
defines the apoplastic space which microbes and cations occupy. The rhizoplane is the transition from the endorhizosphere to the ectorhizosphere and directly influences ease of colonization for pathogenic bacteria, such as Escherichia coli. An example of such a colonization would be the uptake of E. coli from contaminated soil to the vascular system of a plant.17,18,19 The ectorhizosphere of rhizospheric soil is noticeably different from bulk soil due to the rhizosphere effect: soil surrounding a plant system has greater bacterial diversity, concentration, and activity than bulk soil. 3 More than 30,000 bacterial species call rhizospheric soil their home, and 10 11
bacterial cells can be discovered per gram of root. The root of the plant becomes completely covered in bacterial biofilms, which occupy up to 40% of the total root surface. 3
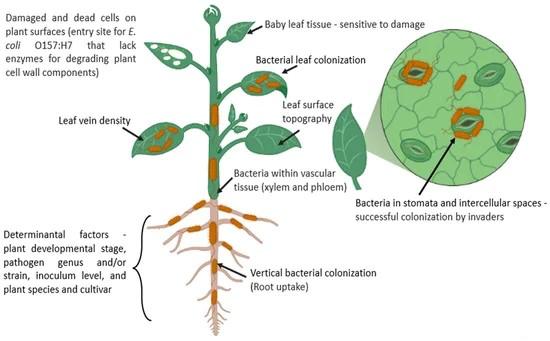
Figure 1.1: Possible locations for bacterial infection. 3

Figure 1.2: A closeup artistic replication of the rhizosphere sections on a root.3
Rhizodeposition Products
The rhizosphere microcosm is unique to the host, and many bacterial species colonize specific species of roots. 3,20 Not all rhizospheric organisms, or bacteria, exhibit this behavior as mycorrhizal fungal relationships are non-specific. The reason for specific bacterial colonization is due to the makeup of the rhizodeposition products; specific bacteria can only metabolize certain compounds metabolites and their host plants produce them. Such compounds can include a variety of LMW or HMW compounds including, phenolic acid and tannins. 15 Many of the nutrients are carbon containing, as plants release between 10 and 40 percent of their total fixed carbon in these rhizodeposition products. 15 The rhizodeposition products are then metabolites for bacteria, which they can process and later release their metabolites for the host plant.21-24 While many of the nutrients may seem like
secondary metabolites, many released compounds can be direct aids to the plant or root system. An example would be the mucilage, a thick oily lubricant, released by the root tip, which aids in the diffusion of the 100 atmospheres of pressure released by the root stem as it pushes through soil. 3
These products allow communication between the host plant and its dependent bacteria. The bacteria then communicate with each other’s biofilms through quorum sensing (QS). 3 QS is a system of communication between bacterial colonies utilizing deposited metabolites.3 One of the studied habits of bacteria within the rhizosphere was how a model bacteria, such as E. coli which metabolized off of exudated glucose, and exudated acetate would interact with a model E coli which metabolized off of the exudated acetate. In this model, a rough view of bacteria-bacteria quorum sensing can then be studied, and form the basis of more advanced examples of QS within COMETS.
Of the bacteria which inhabit the rhizosphere planes, the most relevant to current researchers include the broad class of plant growth-promoting rhizobacteria (PGPRs), which were defined in 1978 and have grown to include two dozen genera of non-pathic rhizobacteria.3 These PGPRs have the ability to increase the growth of their hosts through a variety of methods including the release of phytohormones (auxines or cytokinesis), the fixing or solubilizing of key minerals, induced systemic restoration, the release of antimicrobial compounds, or biocontrol.22 PGPRs are currently being used industrially. 3 This industrial utilization is influenced by tradition; for centuries, crop turning with legumes has been observed to increase the growth of desired crops. Due to the presence of rhizobacteria within the rhizosphere of various legumes belonging to a category of PGPRs known as nitrogen fixing bacteria, which can transform N2 in the atmosphere into bioavailable nitrogen, the soil the legumes grew in was naturally fertilized.3
Another class of PGPRs is Phosphate-Solubilizing Bacteria, or PSB, which are a category of bacteria able to make the soil-insoluble phosphates bioavailable. 3, 25-26 Soil-insoluble phosphates account for the majority of naturally occurring phosphates only 1-2.5% is plant usable. Phosphorus is necessary for plant growth and is the second most important macronutrient behind nitrogen. It is used in vital cell processes including nucleic acid synthesis, cell division, and tissue growth. Its bio-necessity leads it to account for 0.2% of the total dry weight of plants. Because of this, phosphorus fertilizers are often used to distribute vital phosphorus across fields. 3,25-26 However, phosphorus fertilizers contain highly reactive phosphorus which then complexes with heavy metal cations in the soil rendering them biologically inaccessible. Recent concerning estimates regarding natural phosphorus reserves are leading scientists to search for reusable methods of securing phosphorus. PSBs are a hopeful mechanism to supply the world with its necessary phosphorus. PSB makes insoluble phosphorus bioavailable through the secretion of enzymes, the use of organic acids, and chelation.
Escherichia coli
Escherichia coli is the single most studied bacteria in human history, and research on it has dated back more than 100 years. 27 ,38 Much of this research has focused on its role as a pathogen; strains of Verocytotoxigenic E. Coli can cause urinary tract infections, severe cramps, diarrhea, and vomiting. 8, 29 It is for this reason that many studies aim to mitigate and prevent the spread of E. coli, focusing on E. coli O157:H7. 8,17-19,30-31 E. coli ’s enteric nature leads it to inhabit primarily the gastrointestinal tract of ruminants, where improper sanitation techniques (e.g. not fully cooking) before consumption may lead to human infection. 31 However, recent studies have found it able to exist epiphytically on
the surface of plants where it attempts endophytic colonization. 31 E. coli is able to exist within a variety of systems; it can persist in non-rhizospheric soil for upwards of 25 weeks under ideal conditions. It has also been discovered to be able to colonize and inhabit the rhizospheric system of plants, of which Suaeda Salsa was studied. ,6 Studies have observed phosphorus solubilizing capabilities from E.coli-10527, a strain of E. Coli found to survive in extreme saline conditions within the rhizosphere of Suaeda salsa. 6 Saline conditions can occur naturally due to molecular leaching caused by increased acidity, which leads to difficulties in plant survivability. 3 Increasing survivability in non-favorable environments is the essence of current studies using rhizobacteria on plants.
Because of E. coli’s ability to improve the growth of the host plant, there is evidence of practical application of rhizospheric bacteria. However, due to E. coli ’s pathogenic nature, it cannot be used as an effective method for growing any cash crops, such as tomatoes, lettuce, or leeks. Prior outbreaks of E. coli have occurred because E. coli O157:h7 rooted itself into the rhizosphere of lettuce plants and then internalized itself through the vascular system. 30-32 For this reason, non-pathogenic rhizospheric bacteria are required to grow cash crops on an industrial scale. The usage of bacteria in farming also gave rise to another idea: the use of rhizospheric bacteria to prevent outbreaks of E. coli. 8 It was found that certain bacteria possess antibacterial properties, secreting certain chemicals such as pyrrolo(1,2-a)pyrazine-1,4-dione,hexahydro (PPDH) and pyrrolo(1,2-a)pyrazine-1,4-dione,hexahydro-3(2methylpropyl) (PPDHMP), which are detrimental to well known drug-resistant bacteria, such as Staphylococcus aureus, a common cause of cellulitis. 8 ,33 However, all the positive benefits that E. coli can have for its host plant cannot occur if it cannot successfully colonize onto its host. E. coli is unable to chemotax, which requires scientists to inoculate it onto the seed directly. Common study techniques cannot work in vivo as colony sampling is destructive to
the communities, and inoculation techniques cannot be implemented easily in vivo, as they require laboratory equipment which ultimately changes the environment. However, studying E. coli is still practical, as it is so well studied that it provides a basis for further research on more applicable bacteria.
COMETS
The current hypothesis held by m-CAFEs is that maximizing the amount of bacteria which release these secondary compounds can in turn maximize the growth of the host plant. 7 The first step necessary for successful natural bacterial colonization of a root system is chemotaxis, movement towards a secreted chemoattractant by which bacteria inhabit the specific space which secrets the necessary metabolic compound. 34-37
COMETS is being used to accurately detail the relationship between rhizosphere bacteria and plant hosts in chemotaxis a process by which bacteria can move towards the necessary metabolites required. Bacteria movement is dependent on the metabolized metabolite. In areas of high metabolite concentration diffusion is limited and the converse is true in areas of low metabolite concentration. Prior research by the Segrè lab formed a basic model of P. simiae chemotaxis, which is used in this study. 9
The Segrè lab investigated the relationships by which bacterial biomass propagated by using computational computer models, namely: Computation of Microbial Ecosystems in Time and Space (COMETS) and Metabolic Constraint-based Reconstruction and Analysis in Python (COBRA-PY). COMETS is an open-source software launched in 2021 and developed by the Segrè lab, 38 COMETS allows for the analysis of microorganisms within digitally reconstructed environments which simulate natural environments including controlling metabolite concentrations and positions. While the simulation currently operates in two
dimensions, work is being carried out to expand it into three dimensions, simulating a larger volume of rhizospheric soil. Twodimensional models simulate the petri dishes which were resin printed by the Lawrence Livermore laboratory to model real bacterial growth in a faux-soil environment. Thus, twodimensional models are used in this study. COMETS operates off of a form of dynamic flux balance analysis (dFBA), an expansion of the established method of flub balance analysis (FBA). 39 ,40 FBA utilizes genome-scale reconstruction of the studied organisms metabolic network under a steady constant input of metabolites. While it is incredibly strong, it's limited by the fact that it can only function under constant conditions. dFBA is able to incorporate dynamic environments and more complex input streams. dFBA constantly updates its environment to account for the additional variables. For this reason however, it is a more resource consuming method, and experiments can take days to complete.
COMETS uses the open source python library of COBRAPY, a library which contains models of bacteria and their corresponding metabolic reactions.38 ,41 It provides a simple interface for metabolic constraint based analysis, and allows freedom regarding gene activity, nutrient uptake, and more. Using these in vitro models along with Voronoi models formed to simulate soil pore systems allows for an investigation of these microbial relationships. Specific traits such as chemotaxis, colonial growth front as hindered through soil, as well as co-dependant and single dependent colonies within soil systems were investigated. The work performed and investigated as part of this study is an important step towards understanding how microbial communities interact with their environments in enclosed soil systems. Understanding of microbial community interactions will allow the development of systems which improve plant growth Vornonoi Models
In addition to bacterial models, COMETS also requires an environment in which to operate. Voronoi models are models which take a set area, and populate it with a non-random random distribution of points (seeds).42,43 The used environment uses a nonrandom random distribution of seeds. Non-random random distribution ensures a random distribution which is natural, as natural environments don’t have true random points.2 The techniques used to form the non-random random distributions vary, prior attempts were based on Lloyd relaxation techniques, which square and average seed values until natural uniform distribution is achieved. 9, 42 The models used were based on Delaunay triangulation, which used Lloyd relaxation as a basis and then extended the lines formed by the bisectors to form the segments. Each non-seed point within the grid is then systematically calculated to find which seed point its closest to, and the grid is then segmented into polygons based on proximity to seed values. Formation of channels intersecting the values where segments interact forms patterns common in nature, including insect wings, corn growth on husks, leaf vascular systems, and soil.
Methods:
Voronoi Formation:
Open source Voronoi production programs were used to form Voronoi structures for petri dishes of soil object densities of 10, 20, 30, 40, and 50 objects per dish, each density having pore widths of 3, 4, 5, 6, 7, 8 9, and 10 pixels, for a total of 40 environments. These structures were defined first by their density, and second by their pore neck width. The porosity of the environments was varied from a highest porosity of roughly 7% for the dishes with an object density of 10 and neck width of 3, to 30%
for the dishes with an object density of 50 and neck width of 10. 44-

Figure 2.1: An example of Voronoi maker–an open source program which can formulate voronoi structures with a wide range of density, width, and other user-friendly variables.



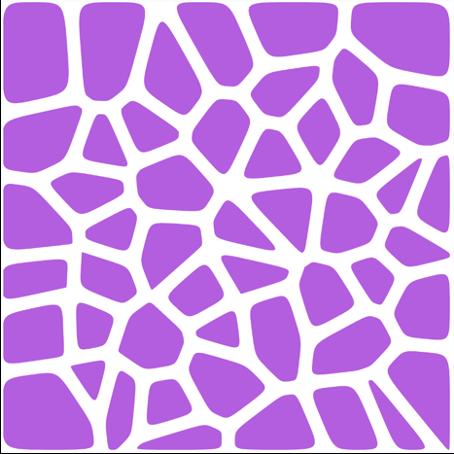
Figure 2.2: Four different Voronoi structures generated, from top left clockwise, 10D 3w, 10D 10W, 50D 10W, 10D 3W.m
Each structure was then copied from the program, and edited to form uniform color spaces. The structure was then imported into Python where colors were matched to define pore space and non-pore space and saved to a CSV file containing all the barrier points. These barrier points were then imported and defined as a barrier within the petri dish. Initial models formed square dishes, continuing with the work previously produced. However, the models were later refitted to form circular dishes to
better match petri dishes which had been sent by the Berkeley Lab as part of the MCAFE’s project. The shapes were resized to a 300 pixel by 300 pixel grid, corresponding to a circular petri radius of 150 pixels.
Populating the Environments
The environment was populated with unlimited oxygen, ammonia, protons, inorganic phosphate, and acetate. The amount of glucose was chosen based on the organic concentration of a natural E. coli colony, but the amount had to be altered to account for the fact that it would be dispersed and well mixed through the dish as opposed to directly concentrated atop the colony. A wellmixed medium within simulations would allow for simpler comparison to in vitro studies of live bacteria. Thus, the Petri dish was additionally loaded with 3e -6 mg of glucose. A textbook E. coli model was loaded into the model, and made to metabolize glucose and acetate, starting as a line across the bottom two layers for the square models. Textbook models of bacteria are models which contain only basic metabolic capabilities. For the circular Voronoi models, the initial population of bacteria was generated as a circle with a radius of 20 pixels, and was made as close to the center as possible without being hindered by an immediate border.
Data Collection
The models were run for five hundred cycles with time steps of 0.2 hours, corresponding to 100 hours of growth in realtime. Images of the diffusion and growth of the biomass were recorded at t=0, 100, 200, 300, 400, 500 cycles. Python code calculated the Euclidean distance from the initial center to the furthest bacterial point, and graphed them per cycle. The distribution of the bacteria was calculated and graphed. The growth
of the biomass front was measured as the highest point that the biomass had grown to. For the circular models, it was measured radially using the center that the biomass had grown around. This results in all the values for growth starting at 20 pixels, as the initial biomass started radially 20 away from the growing center.
Chemotaxis
For modeling chemotaxis a separate bacterial model was used. E. coli doesn’t chemotax, resulting in the usage of a P. simiae model, based on prior studies.9, 47-51 No barriers were added to the Petri dish, although the same code as formulated for the growth front model would still function. The chemotaxic model ran for another 500 cycles, matching a real-time of 25 hours, and images were taken at t=0, 100, 300, 500 cycles.
Results and Discussion:
The result of comparing the different growth rates of E coli colonies within different soil pore widths and densities did not match prior expectations. A linear relationship between pore density and bacterial front was expected, however, there is no simulated experimental evidence that pore density in any way greatly affects the bacterial front of textbook E. coli. The bacterial front is the furthest extent a bacterial colony has grown from its initial site of innoculation, shown (Figure 3.1). Figure 3.2 depicts the front of initial square Voronoi models, using densities of 0, 10, 20, 30, 40, 50 soil particles per area, and for each density was additionally run with pore widths of 4 and 10. Figure 3.3 shows
examples of these initial structures and the bacteria grown within them.

Figure 3.1: The distribution of an E. coli initially inoculated at the bottom two rows of a square model as it propagates upwards through time. Each line depicts the distribution of bacterial biomass per cycle for 100 cycles.
The front data for 500 cycles of 10 separately run circular simulations for densities between 0, 10, 20, 30, 40, 50 soil particles per area, and for each pore density they are being run with a pore width of 3 and 10 pixels is shown in Figure 3.4. Additionally, a control with no barriers was run as well.

Figure 3.2: The bacterial growth front of 10 E. coli simulations, ran for 300 cycles. No significant difference exists between the front of all simulations.

Figure 3.3: An initial square Voronoi structure with a density of 10 and a pixel width of 7 ran for 50 cycles with a Textbook E. coli bacteria originating on the bottom two rows.

Figure 3.4: A revised version of Figure 3.2 using a control and circular petri dishes. It similarly depicts no significant difference between models and growth front.
The data shows that each simulation had a near-exact front, matching with an unhampered wall simulation. Images modeling the bacterial growth through cycles show the similarities in the growth patterns between the different colonies in how the different colonies grow, but additionally show that the front was itself unhampered.
This data is unhelpful for searching for a way to increase the growth rate of bacteria within soil ecosystems. Without any significantly superior pore density, there isn’t any avenue of implementing a way of increasing growth rate in controlled environments.
The data also brings up questions about the accuracy of the COMETS model, as studies indicate that bacteria grown along some form of construct in their ecosystem present the behavior of “sticking” to the construct. 3 However, as indicated in Figure 3.4, the COMETS model currently does not have models which can exhibit this type of behavior. Under the assumption that bacteria
universally stick to constructs, a higher density environment is expected to be proportional to a slower growth rate, as bacteria is more inclined to grow along the construct than move further out. Further refining of the COMETS model to allow for a “sticking” behavior may see this pattern emerge within future simulations, or it may show a new pattern.



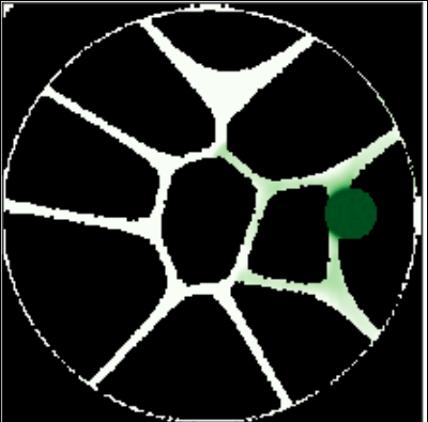
Figure 3.5: Circular petri dishes of varying pore densities and widths. Each has been onnculated with an initial population of bacteria and has been run for varying amounts of time. From top left, top right, bottom left, to bottom right: a control ran for 500 cycles, 50 density 10 pixel pore width ran for 500 cycles, 30 density 3 pixel pore width ran for 300 cycles, 10 density 3 pixel pore width ran for 800 cycles.
Bacteria are also rarely viewed in rhizosphere systems individually, and even bacteria of the same species can have small genetic differences between colonies. Bacteria-bacteria interactions are well studied, and show many differences in behaviors. However, bacteria-bacteria interactions within soil environments have not been extensively investigated. Figure 3.6 shows prior COMETS runs with two strains of E-coli grown together under varying parameters including higher diffusion constants, and the prominence of certain parameters allowed one strain of E. coli to dominate the culture as opposed to the other. Trials were run comparing multiple strains of bacterium within root-soil systems, with different metabolizing compounds. However, no conclusive results were discovered.
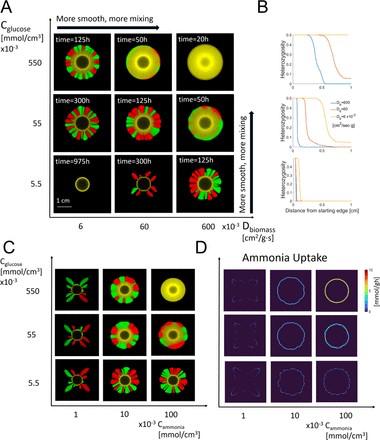
Figure 3.6: Prior research performed by the Segrè lab which shows how diffusivity and glucose concentration can have an effect on multi colonial systems. 52
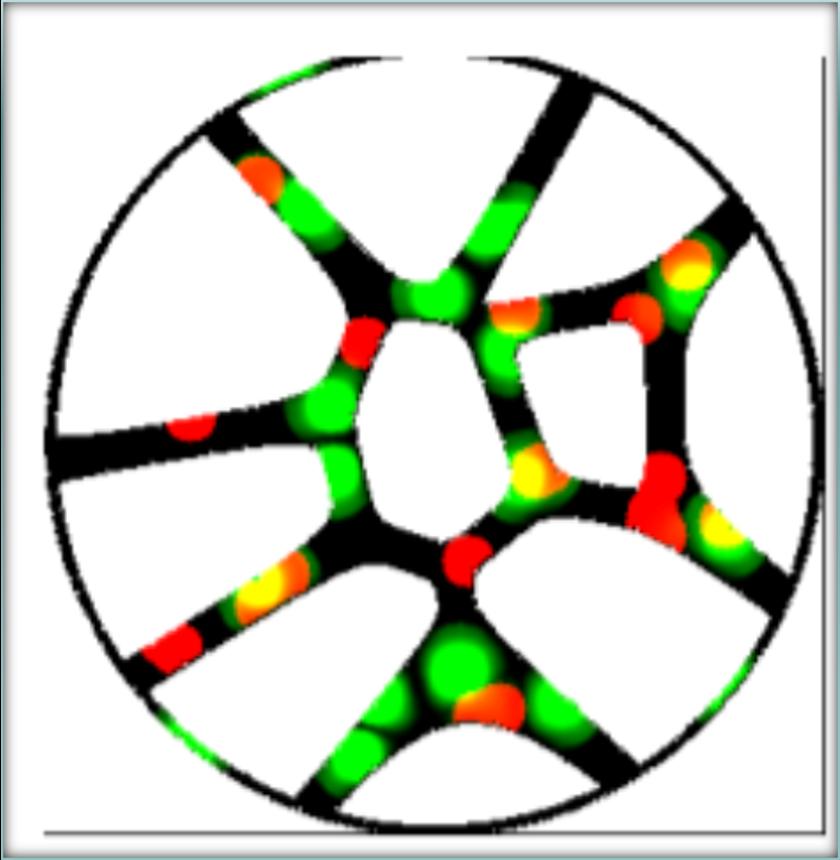
Figure 3.7: A multi colonial petri dish with Voronoi structures. The green bacteria are metabolizing off of glucose and exudating acetate, while the red bacteria can solely consume acetate. Yellow areas mark where the colonies overlap.
A P. simiae chemotactic model was additionally run for 500 cycles, however current research is limited.9 Further research based on COMETS chemotaxis models is being performed. 51



Figure 3.7: Images for chemotaxic P. simiae for cycles 0, 100, 300, and 500 from top left, top right, bottom left, then bottom right.
Conclusion:
While no significant evidence of pore width hindering front growth was found with this current COMETS model, hypotheses exist to explain a lack of association. The work performed by the Segrè lab as part of m-CAFEs has been an important step towards understanding effective ways to model rhizospheric metabolic processes with complex environments. Effective modeling techniques will allow for a basis to form industrial environments which can allow for efficient and effective inoculation of rhizobacteria. Industrial usage of microbial inoculation will allow for improved quality and quantity of
consumable cash crop biomass. The increased amount of produced food can be used to feed the increasing global population.
One next step for model improvement would be to include a method by which biofilms could be generated. Biofilm generation would allow for more advanced modeling of quorum sensing. Additionally, another step would be to implement hindered diffusing when adjacent to barriers. Current models have no discrimination regarding diffusion based on position which is not particularly accurate. Additionally, the COMETS environments utilized a well-mixed medium which is not accurate to true soil environments; a more accurate scattering of soil nutrients would form more accurate biomass distributions.
Other bacterial models should also be tested, as while E. coli can exhibit PGRP properties, it is not considered a concrete rhizobacteria because it cannot chemotax and must be inoculated directly onto the plant. Additionally, it is also harmful to many organisms that would consume the plants it would develop atop. While E. coli ’s metabolic pathways are similar to a rhizobacteria such as P. simiae, P. simiae ought to be more conclusively studied, as well as other PGPRs. While its chemotaxic properties were studied, more testing regarding the curve function of a chemotaxis growth function should performed to form more accurate chemotaxic models.
More models will be created to include the full range of rhizo-organisms, allowing for more complex modeling interactions between rhizo-organisms. Rhizobacteria have complex reactions, including the secretion of antimicrobial compounds, and allowing the modeling of such antimicrobial secretion and its detrimental effect on surroundings will be important for implementation for agricultural processes.
Furthermore, implementation of the COMETS program into three dimensions would allow for more accurate mapping of colonization patterns of rhizospheric bacteria and more accurate visuals of the dynamics of rhizospheric organisms. Currently, the
models work bi-dimensionally, giving an approximation of their spread, but failing to fully capture the entiretyof the bacteria-rootsoil interface. Work is already being performed to implement COMETS into three dimensions, but it is currently non-functional. Implementing these changes would allow for a greater modeling capabilities of microorganisms within the rhizosphere. Effective microorganism modeling would allow for implementation of real-world changes within agricultural practices, thus allowing for inoculation of beneficial bacteria to cash-crops and a return on the investment.
Bibliography:
(1)
World Bank. Agriculture and Food. World Bank. https://www.worldbank.org/en/topic/agriculture/overview. (2)
Vijayalaxmi Kinhal. What Factors Affect Biomass Accumulation in Plants? CID Bio-Science. https://cid-inc.com/blog/whatfactors-affect-biomass-accumulation-in-plants/. (3)
McNear, D. The rhizosphere - Roots, soil and everything in between. Nature.com.
https://www.nature.com/scitable/knowledge/library/therhizosphere-roots-soil-and-67500617/. (4)
Khan, N.; Ali, S.; Shahid, M. A.; Mustafa, A.; Sayyed, R. Z.; Curá, J. A. Insights into the Interactions among Roots, Rhizosphere, and Rhizobacteria for Improving Plant Growth and Tolerance to Abiotic Stresses: A Review. Cells 2021, 10 (6), 1551. https://doi.org/10.3390/cells10061551. (5)
He, T.; Xu, Z.-J.; Wang, J.-F.; Wang, F.-P.; Zhou, X.-F.; Wang, L.L.; Li, Q.-S. Improving Cadmium Accumulation by Solanum Nigrum L. Via Regulating Rhizobacterial Community and Metabolic Function with Phosphate-Solubilizing Bacteria Colonization. Chemosphere 2021, 287, 132209. https://doi.org/10.1016/j.chemosphere.2021.132209. (6)
He, T.; Xu, Z.-M.; Wang, J.-F.; Zhang, K.; Wang, F.-P.; Li, W.-L.; Tian, P.; Li, Q.-S. Inoculation of Escherichia Coli Enriched the Key Functional Bacteria That Intensified Cadmium Accumulation by Halophyte Suaeda Salsa in Saline Soils. Journal of Hazardous Materials 2023, 458 , 131922.
https://doi.org/10.1016/j.jhazmat.2023.131922.
(7) m-CAFEs. m-CAFEs – . Lbl.gov. https://mcafes.lbl.gov/ (accessed 2025-02-26).
(8)
Prasad, J. K.; Dey, R.; Raghuwanshi, R. Antimicrobial Potential of Rhizospheric Bacteria Streptobacillus Sp. Journal of Pure and Applied Microbiology 2021, 15 (4), 1846–1854. https://doi.org/10.22207/jpam.15.4.01.
(9)
Ternoey, S. Modeling Rhizobacterial Colonization of Roots in COMETS. Senior Thesis, Boston University Academy, 2024, pp. 1–38. https://issuu.com/buacademy/docs/ternoey_thesis. (10)
Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11 (12), 2904. https://doi.org/10.3390/microorganisms11122904. (11)
Collavino, M. M.; Sansberro, P. A.; Mroginski, L. A.; Aguilar, O. M. Comparison of in Vitro
Solubilization Activity of Diverse Phosphate-Solubilizing Bacteria
Native to Acid Soil and Their Ability to Promote Phaseolus Vulgaris Growth. Biology and Fertility of Soils 2010, 46 (7), 727–738. https://doi.org/10.1007/s00374-010-0480-x. (12)
Wang, W.; Jia, T.; Qi, T.; Li, S.; A. Allan Degen; Han, J.; Bai, Y.; Zhang, T.; Qi, S.; Huang, M.; Li, Z.; Jiao, J.; Shang, Z. Root Exudates Enhanced Rhizobacteria Complexity and Microbial Carbon Metabolism of Toxic Plants. iScience 2022, 25 (10), 105243–105243. https://doi.org/10.1016/j.isci.2022.105243. (13)
Hu, L.; Robert, C. A. M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M. G. A.;
Schlaeppi, K.; Erb, M. Root Exudate Metabolites Drive Plant-Soil Feedbacks on Growth and Defense by Shaping the Rhizosphere Microbiota. Nature Communications 2018, 9 (1), 2738.
https://doi.org/10.1038/s41467-018-05122-7. (14)
NAL Agricultural Thesaurus: NALT: cash crops. Usda.gov. https://lod.nal.usda.gov/nalt/en/page/22797 (accessed 2025-02-26). (15)
Plant exudates: The root of the matter. Impello® Biosciences. https://impellobio.com/blogs/inoculants/rootexudates?srsltid=Afm BOopzofw1Vw0XZCxRPblA vqXo_cVNfx3qsmC90KqVrVdZrGIgOJcH (accessed 2025-0226).
(16)
Villarino, S. H.; Pinto, P.; Jackson, R. B.; Piñeiro, G. Plant Rhizodeposition: A Key Factor for Soil Organic Matter Formation in Stable Fractions. Science Advances 2021, 7 (16). https://doi.org/10.1126/sciadv.abd3176.
(17) van Overbeek, L.; Duhamel, M.; Aanstoot, S.; van der Plas, C. L.; Nijhuis, E.; Poleij, L.; Russ, L.; van der Zouwen, P.; Andreo-Jimenez, B. Transmission of Escherichia Coli from Manure to Root Zones of Field-Grown Lettuce and Leek Plants. Microorganisms 2021, 9 (11), 2289. https://doi.org/10.3390/microorganisms9112289. (18)
Habteselassie, M. Y.; Bischoff, M.; Applegate, B.; Reuhs, B.; Turco, R. F. Understanding the Role of Agricultural Practices in the Potential Colonization and Contamination by Escherichia Coli in the Rhizospheres of Fresh Produce. Journal of Food Protection 2010, 73 (11), 2001–2009. https://doi.org/10.4315/0362-028x73.11.2001.
(19)
Esmael, A.; Al-Hindi, R. R.; Albiheyri, R. S.; Alharbi, M. G.; Filimban, A. A. R.; Alseghayer, M. S.; Almaneea, A. M.; Alhadlaq,
M. A.; Ayubu, J.; Teklemariam, A. D. Fresh Produce as a Potential Vector and Reservoir for Human Bacterial Pathogens: Revealing the Ambiguity of Interaction and Transmission. Microorganisms 2023, 11 (3), 753. https://doi.org/10.3390/microorganisms11030753. (20)
McLaughlin, S.; Zhalnina, K.; Kosina, S.; Northen, T. R.; Sasse, J. The Core Metabolome and Root Exudation Dynamics of Three Phylogenetically Distinct Plant Species. Nature Communications 2023, 14 (1). https://doi.org/10.1038/s41467-023-37164-x. (21)
Mendes, R.; Garbeva, P.; Raaijmakers, J. M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiology Reviews 2013, 37 (5), 634–663. https://doi.org/10.1111/15746976.12028. (22)
Saeed, Q.; Xiukang, W.; Haider, F. U.; Kučerik, J.; Mumtaz, M. Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; Brtnicky, M.; Mustafa, A. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. International Journal of Molecular Sciences 2021, 22 (19), 10529. https://doi.org/10.3390/ijms221910529. (23)
Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Frontiers in Plant Science 2019, 10. https://doi.org/10.3389/fpls.2019.00157. (24)
Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13 (2), 95–95. https://doi.org/10.3390/biology13020095.
(25)
Ahemad, M. Phosphate-Solubilizing Bacteria-Assisted Phytoremediation of Metalliferous Soils: A Review. 3 Biotech 2014, 5 (2), 111–121. https://doi.org/10.1007/s13205-014-0206-0. (26)
Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11 (16), 2119. https://doi.org/10.3390/plants11162119. (27)
E.coli. whatsinaname.hmnh.harvard.edu.
https://whatsinaname.hmnh.harvard.edu/ecoli. (28)
Hufnagel, D. A.; Depas, W. H.; Chapman, M. R. The Biology of the Escherichia Coli Extracellular Matrix. Microbiology Spectrum 2015, 3 (3). https://doi.org/10.1128/microbiolspec.mb-0014-2014. (29)
Mayo Clinic. E. coli. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/e-coli/symptomscauses/syc-20372058. (30)
A. Mark Ibekwe; Grieve, C. M.; Papiernik, S. K.; Yang, C.-C. Persistence of Escherichia Coli O157:H7 on the Rhizosphere and Phyllosphere of Lettuce. Letters in Applied Microbiology 2009, 49 (6), 784–790. https://doi.org/10.1111/j.1472765x.2009.02745.x. (31)
Quilliam, R. S.; Williams, A. P.; Jones, D. L. Lettuce Cultivar Mediates Both Phyllosphere and Rhizosphere Activity of Escherichia Coli O157:H7. PLoS ONE 2012, 7 (3), e33842. https://doi.org/10.1371/journal.pone.0033842.
(32)
Centers for Disease Control and Prevention. About escherichia coli infection. E. coli Infection (Escherichia coli). https://www.cdc.gov/ecoli/about/index.html. (33)
Barnhart, M. M.; Chapman, M. R. Curli Biogenesis and Function. Annual Review of Microbiology 2006, 60 (1), 131–147. https://doi.org/10.1146/annurev.micro.60.080805.142106. (34)
Adler, J. Chemotaxis in Bacteria. Science (New York, N.Y.) 1966, 153 (3737), 708–716. https://doi.org/10.1126/science.153.3737.708. (35)
Cremer, J.; Honda, T.; Tang, Y.; Wong-Ng, J.; Vergassola, M.; Hwa, T. Chemotaxis as a Navigation Strategy to Boost Range Expansion. Nature 2019, 575 (7784), 658–663. https://doi.org/10.1038/s41586-019-1733-y. (36)
Hadjidemetriou, K.; Kaur, S.; Cassidy, C. K.; Zhang, P. Mechanisms of E. Coli Chemotaxis Signaling Pathways Visualized Using CryoET and Computational Approaches. Biochemical Society Transactions 2022, 50 (6), 1595–1605. https://doi.org/10.1042/bst20220191. (37)
Brunet, M.; Amin, S. A.; Iurii Bodachivskyi; Unnikrishnan Kuzhiumparambil; Seymour, J. R.; Raina, J.-B. An Atlas of Metabolites Driving Chemotaxis in Prokaryotes. Nature Communications 2025, 16 (1). https://doi.org/10.1038/s41467-02556410-y. (38)
Dukovski, I.; Bajić, D.; Chacón, J. M.; Quintin, M.; Vila, J. C. C.; Sulheim, S.; Pacheco, A. R.; Bernstein, D. B.; Riehl, W. J.; Korolev, K. S.; Sanchez, A.; Harcombe, W. R.; Segrè, D. A Metabolic Modeling Platform for the Computation of Microbial
Ecosystems in Time and Space (COMETS). Nature Protocols 2021, 16 (11), 5030–5082. https://doi.org/10.1038/s41596-02100593-3.
(39)
Mahadevan, R.; Edwards, J. S.; Doyle, F. J. Dynamic Flux Balance Analysis of Diauxic Growth in Escherichia Coli. Biophysical Journal 2002, 83 (3), 1331–1340. https://doi.org/10.1016/s00063495(02)73903-9.
(40)
Orth, J. D.; Thiele, I.; Palsson, B. Ø. What Is Flux Balance
Analysis? Nature Biotechnology 2010, 28 (3), 245–248. https://doi.org/10.1038/nbt.1614. (41)
cobrapy - constraint-based metabolic modeling in Python. opencobra.github.io. https://opencobra.github.io/cobrapy/. (42)
Bellelli, F. The fascinating world of Voronoi diagrams | Towards Data Science. Towards Data Science. https://towardsdatascience.com/the-fascinating-world-of-voronoidiagrams-da8fc700fa1b/. (43)
Voronoi Editor. voronoi-editor.web.app. https://voronoieditor.web.app/. (44)
Nimmo, J. Porosity and Pore Size Distribution; 2004. https://wwwrcamnl.wr.usgs.gov/uzf/abs_pubs/papers/nimmo.04.en cyc.por.ese.pdf. (45)
Jaber Taheri-Shakib; Adil Al-Mayah. A Review of Microstructure Characterization of Asphalt Mixtures Using Computed Tomography Imaging: Prospects for Properties and Phase Determination. Construction and Building Materials 2023, 385, 131419–131419.
https://doi.org/10.1016/j.conbuildmat.2023.131419.
(46)
Lopez, J. Pedology: The Dirt on Soil - Long Acres Ranch. Long Acres Ranch. https://longacresranch.org/pedology-the-dirt-on-soil/ (accessed 2025-03-06). (47)
Bhattacharjee, T.; Amchin, D. B.; Ott, J. A.; Kratz, F.; Datta, S. S. Chemotactic Migration of Bacteria in Porous Media. Biophysical Journal 2021, 120 (16), 3483–3497. https://doi.org/10.1016/j.bpj.2021.05.012. (48)
Zhang, J. PREDICTION and DESIGN of SYNTHETIC MICROBIAL CONSORTIA through INTEGRATION of COMPUTATIONAL and EXPERIMENTAL APPROACHES.
Dissertation, BOSTON UNIVERSITY FACULTY OF COMPUTING & DATA SCIENCES, 2023. (49)
AldénL.; Demoling, F.; BååthE. Rapid Method of Determining Factors Limiting Bacterial Growth in Soil. Applied and Environmental Microbiology 2001, 67 (4), 1830–1838. https://doi.org/10.1128/aem.67.4.1830-1838.2001. (50)
Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K. A.; Tomita, M.; Wanner, B. L.; Mori, H. Construction of Escherichia Coli K-12 In-Frame, Single-Gene Knockout Mutants: The Keio Collection. Molecular Systems Biology 2006, 2 (1). https://doi.org/10.1038/msb4100050. (51)
Shi, H. DEVELOPING and TESTING a COMPUTATIONAL MODEL of CHEMOTAXIS in a COLLABORATIVE LATFORM for the SYSTEMS BIOLOGY of MICROBIAL COMMUNITIES.
Dissertation, BOSTON UNIVERSITY FACULTY OF COMPUTING & DATA SCIENCES, 2023.
Dukovski, I.; Golden, L.; Zhang, J.; Osborne, M.; Segrè, D.; S. Korolev, K. Biophysical Metabolic Modeling of Complex Bacterial Colony Morphology. Biophysics. https://doi.org/10.1101/2024.03.13.584915 . (Forthcoming)

