Daphne Yesilaltay
Senior Thesis | 2025

Abstract
The Mexican axolotl (A. mexicanum) possesses a unique capability to regenerate limbs, a process critically dependent on nerve supply. Without proper innervation, limb regeneration fails, resulting in tissue regression and histolysis. This study aims to map nerve locations within a regenerating blastema and to characterize cell death in denervated limbs. Using immunohistochemistry (IHC) to stain for nerve markers, the experiment utilized beta-tubulin to successfully visualize locations of regenerated innervation. Once nerve regeneration was established, it was followed by attempts to determine specific types of nerves; staining for sympathetic and sensory nerves using Tyrosine Hydroxylase and HNK1 antibodies, respectively. Additionally, staining for Tyrosine Hydroxylase and HNK1 aims to indicate methods of tissue regression due to denervation. These findings contribute to the scientific understanding of the axolotl’s nerve-dependent regeneration and encourage further investigation.
Introduction
Background on Axolotl
The Mexican axolotl (Ambystoma mexicanum), a type of North American salamander, is critically endangered in the wild and native to the Lake Xochimilco area in the Valley of Mexico. Between 1863 and 1866, seven axolotls were transported to the Paris Natural History Museum, and these specimens are thought to be the direct ancestors of nearly all domestic axolotls alive today. Currently, most axolotls live in captivity, either in aquaria or laboratories. Axolotls typically can grow to be around 15 to 45 cm in length (6 to 18 inches), 56 to 226 grams in weight (2 to 8 ounces), and have a lifespan of about 10 to 15 years (Farkas & Monaghan, 2015).
Axolotls are of the order Urodela (salamanders and newts), but unlike other urodeles, they are paedomorphic, meaning that they do not naturally undergo metamorphosis and reach sexual maturity while retaining their juvenile features (De Groef et al., 2018). However, metamorphosis can be induced in axolotls by injecting them with thyroid hormones. The axolotl is notable for its remarkable ability to regenerate complex body structures, such as limbs, gills, and portions of their hearts and brain, with incredible accuracy (Farkas & Monaghan, 2015). While axolotls are not the only organisms capable of such regeneration, several features make them especially advantageous for research in regenerative biology. These include their large egg size, consistent breeding in captivity, acceptance of embryonic and adult tissue grafts, large clutch sizes, and long-term laboratory viability (Farkas & Monaghan, 2015). These characteristics make axolotls an ideal model organism for investigating regenerative mechanisms. A
central question in axolotl research is understanding the mechanisms behind their exceptional regenerative capabilities.
In limb regeneration, axolotls can reconstruct the entire structure of lost limbs post-amputation through the formation of a cellular stump called a blastema. Blastema formation is not exclusive to axolotls but is essential to their regenerative process. The process of blastema formation is as follows (Figure 1): Within hours postamputation, a wound epithelium closes the open wound. An epithelium is a thin layer of tissue that closes the wound, made up of epithelial cells. Within the following days, the wound epithelium becomes innervated. The innervated wound epithelium becomes a specialized signaling center known as the apical epithelial cap (AEC). Beneath the wound epithelium, cells begin to gather to create the blastema and dedifferentiate because of signaling from the AEC (Wells-Enright et al., 2021; McCusker et al., 2015). When a cell dedifferentiates it loses its original identity and becomes a stem cell before acquiring the specific identity needed for regeneration. The blastema serves as a progenitor cell mass from which an organ or body part develops by supporting cellular proliferation and differentiation, eventually leading to complete limb regrowth (McCusker et al., 2015).
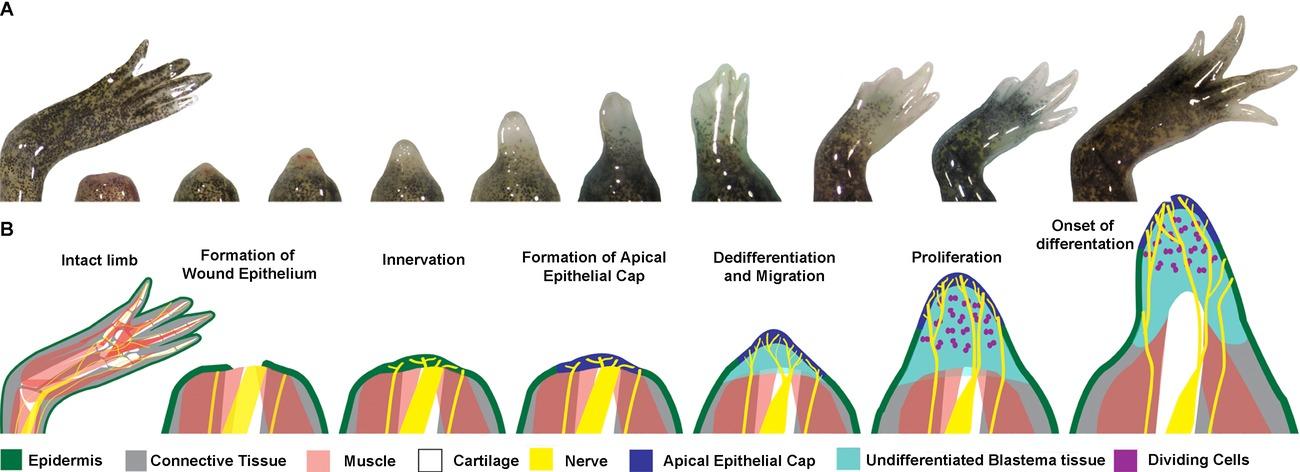
Figure 1: From McCusker et al., 2015. The course of limb regeneration post-amputation. (A) Live images of blastema generation and limb regeneration, recorded at 1 day, 7, 9, 11, 13, 15, 17, 21, 25, and 31 days post amputation. (B) Diagram of key steps of blastema generation during the regeneration process. The tissue components are labeled by the colored key below. Note the prevalence and necessity of innervation in the regeneration process.
A juvenile axolotl (8.5-10 cm in length – note that because axolotls never undergo metamorphosis there are no biological markers for age besides reaching sexual maturity, so age is commonly synonymous with size) reaches the differentiation state approximately 32 days post amputation and after approximately 100 days will fully regenerate the amputated limb. In comparison, a three-month-old axolotl will reach the differentiation stage approximately 22 days post-amputation and around 66 days postamputation the amputated limb will achieve full regeneration (Monaghan et al., 2014). This inverse relationship between age and regenerative/wound healing ability holds true for most other animals, including humans (Gerstein et al., 1993).
Nerve Dependence in Limb Regeneration
One major factor that has emerged in studies of axolotl limb regeneration is the importance of innervation. Innervation means the presence of nerves. Nerves are fibers in the body that transmit electrical signals of sensation to the brain or spinal cord and are part of the central nervous system. Axolotl limb regeneration is highly nerve-dependent, meaning that the limb will not regenerate in the absence of innervation (Farkas & Monaghan, 2017). This dependence remains true for many other vertebrates capable of regenerating tissue. When nerves are removed or damaged the limp halts development and undergoes histolysis; a regression of cellular and tissue structure. The theory of limb regression due to denervation was discovered in 1953 when scientist Charles Thornton conducted a study where he injured an innervated and denervated amphibian limb. He discovered that while the innervated limb could completely regenerate, the denervated limb underwent histolysis, inflammation, and tissue degradation. The histolysis due to denervation has been observed to be so severe that a limb could fall off entirely (Farkas & Monaghan, 2017). This was the first time a connection had been established between denervation to histolysis and inflammation, but has remained relatively understudied. This nerve dependency indicates that innervation provides crucial signals for cell proliferation, patterning, and growth within the blastema. Limb regeneration halts in the absence of nerves, proving the vital role of nerve signals in maintaining regenerative capacity (Farkas & Monaghan 2017). The crucial role of nerves in regeneration remains a mystery, making it essential to explore their functions in the process. Gaining this understanding will ultimately help address the larger question: How do axolotls regenerate their limbs?
Types of Cell Death
Eukaryotic cell death is commonly characterized in two ways: apoptosis and necrosis. Apoptosis is defined as controlled, autonomous, and intentional cell death. Its name comes from the Greek word for “falling off,” in reference to leaves from a tree connoting a controlled physiological process involving the loss of individual components of an organism without destruction or damage to the organism. Necrosis on the other hand is defined as random, uncontrolled, and accidental cell death usually due to environmental factors (Fink & Cookson, 2005). An example of apoptosis is the death of unnecessary cells between developing fetal fingers. Apoptosis is necessary and preprogrammed because if these cells didn’t die the fetus would be born with webbing between their fingers, unable to separate them. Necrosis can occur due to a myriad of reasons such as traumatic injury, autoimmune disease, infection, or toxins (Cell Death, n.d.). Although these two methods of cell death are often presented in dichotomy, the reality is more complex and requires further research to achieve a more nuanced understanding. But for the purpose of this study, apoptosis and necrosis can be thought of as thus: apoptosis is indicated by organized cell death without inflammation, and necrosis is indicated by unorganized cell death.
Importance of Understanding Nerve Mapping in Regeneration
Understanding the dependence that regenerating axolotl limbs have on innervation could be the key to unlocking a deeper understanding of the mechanisms of regenerative biology. Mapping the innervation within the blastema and exploring the cellular mechanisms that underlie nerve dependence is crucial for understanding the full extent of axolotl limb regeneration. This is
why one of the main goals of this study was to determine the location of regenerated innervation in a blastema.
Another goal of this study was to determine if cell death in denervated limbs results from apoptosis or necrosis. In a study published in 2001, scientists Anthony L. Mescher et al. investigated this very question. Their paper, “Apoptosis in regenerating and denervated, non-regenerating urodele forelimbs,” found that the apoptotic index of denervated forelimbs in axolotl larvae within 11 days was 4%. The apoptotic index is a quantitative measure used to assess the extent of apoptosis in a cell population. In contrast, innervated regenerating limbs had an apoptotic index of approximately 1% over that same period of 11 days. While this study established the presence of apoptotic cell death in denervated larval axolotls, little research has been conducted to follow up or build on these findings. Further investigations into this phenomenon may help reveal why nerve signals are so vital for regeneration in axolotls.
Study Objectives
This study aimed to map nerve locations within the regenerating blastema and to investigate the types of cell death occurring in denervated limb tissue. Specifically, the study sought to:
1. Visualize innervation in a regenerating blastema using beta-tubulin as a general nerve marker.
2. Assess the presence of sympathetic and sensory nerves using Tyrosine Hydroxylase and HNK1 antibodies.
3. Characterize the type of cell death (apoptosis or necrosis) in histolysis of denervated limb tissue.
Methods
Animal Model and Sample Collection
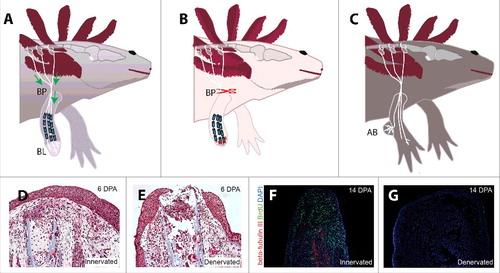
The model organism used for this study was A. mexicanum from the Monaghan Lab at Northeastern University. Following ethical protocols for vertebrate research, the forelimbs of four axolotls were denervated and then amputated. Denervation was achieved by severing the nerves at the brachial plexus, a network of nerves located in the shoulder region that supplies the forelimb. This procedure ensured complete disconnection of nerve signals to the limb (Figure 2). After amputation, blastemas were collected during regeneration for analysis. Each axolotl contributed two forelimbs, providing a total of eight samples for the study.
Immunohistochemistry (IHC) was used to map innervation within the regenerating blastema. Immunohistochemistry is a staining
technique where antibodies stain certain antigens in a tissue sample, allowing visualization beneath a microscope. It uses the antigen-binding properties of antibodies to detect and bind to antigens in a tissue sample. Fluorescently labeled antibodies emit light when excited by a specific wavelength, creating a visual contrast that highlights the distribution of the target antigens otherwise invisible to the naked eye (Im et al., 2019).

Figure 3: From Verma, 2022. Visual representation of antigenantibody specificity that enables detection and localization of a single target. Here, the target antigen is “Protein 5,” and “Antibodies Against Protein 5” are the antibodies that attach to the target antigen, showing the main premise of IHC.
Sample Preparation: Once the denervated regenerating blastemas were collected, the samples were fixed in 4% paraformaldehyde (PFA), a chemical fixative that cross-links proteins to retain the tissue’s chemical composition and harden it. The samples were incubated in the PFA overnight. Fixed samples were then subjected to cryoprotection to prevent damage during freezing. To begin, this process first requires a phosphate buffered saline (PBS) wash to
wash off residual PFA. To conduct a PBS wash, PBS is pipetted onto sample slides where it sits for 5 minutes before the excess liquid is removed. This process is done 3 times, often called a 3 x 5 PBS wash. After the PBS wash, the tissue samples were immersed in a 30% sucrose mixture until the samples sank to the bottom, indicating equilibrium.
Next, the samples were embedded in O.C.T. (Optimal Cutting
Temperature) Compound: a gel-like substance composed of a water-soluble blend of glycols and resins that preserve and stabilize the tissue during freezing, allowing the sample to be worked on at -10 degrees C and below. Each sample was embedded in O.C.T in a small mold, using a microscope to ensure proper orientation with the blastema in the top right corner. This step ensures that the samples are easily identifiable during sectioning, as the blastemas are hard to see with the naked eye due to their small size, and the fact that O.C.T. is opaque when frozen. Finally, the samples in their molds were wrapped in aluminum foil and frozen at -80 degrees C overnight.
Sectioning: The samples were sectioned using a cryostat, a specialized instrument designed to cut ultra-thin histological slides at low temperatures. The instrument’s function is in its name: cryo meaning cold, and stat meaning stable. It cuts cross sections in, essentially, a micro deli-slicer fashion. The samples, frozen in their O.C.T. molds were placed in the cold cryostat and sliced in a process called sectioning. More O.C.T. was used to stick the frozen block containing the sample to a “sample stub” as the cold cryostat froze the O.C.T. in a matter of minutes, ensuring the sample was securely attached. The blastema samples were cut into 12 µm thick sections – for scale, one micron is one-millionth of a meter – and mounted onto slides, with about four sections per slide.

Figure 4: From White, 2011. Diagram with parts of cryostat labeled . The sample is attached to the sample stub using O.C.T., the stub is attached to the chuck, section thickness is adjusted, and a lever outside the machine is cranked to slice the sample against the blade.
Staining: The sections were then washed in PBS to get the remaining O.C.T. off in preparation for staining. The samples were then permeabilized with 0.1% Triton X-100, which grants the antibody easier access to the inside of the cell to detect the target antigen. The sample was then incubated in a “blocking” buffer made of 15 µL goat serum and 1 mL PBS for 30 minutes. Blocking prevents non-specific binding, ensuring that the antibodies only bind to the one desired site. After a PBS wash to remove the blocking buffer, the primary antibody was applied and incubated overnight at 4 degrees C, leaving one slide without any antibody as a negative control. The next day the slides were washed in PBS
and the secondary antibody was applied; its role was to amplify the fluorescent signal of the primary. Once it incubated in complete darkness for about an hour at room temperature – although the IHC protocol calls for 30-minute incubation, the longer the secondary antibody stays on the sample the stronger the signal usually is, so it was left on for an hour – then slides were washed in PBS. Finally, coverslips along with ProLong Gold were applied to the slides. A coverslip is a small, thin (less than one mm thick) piece of glass that sits on top of a slide to hold the sample in place and to protect it from contamination from the environment, and ProLong Gold is an antifade reagent for fluorescence that preserves light signal without dimming it.
Antibodies specific to the central nervous system (CNS) were used to stain for innervation: beta-tubulin, Tyrosine Hydroxylase, and HNK1. The IHC procedure involved preparing tissue sections, applying primary antibodies, and visualizing results through fluorescence microscopy. Beta-tubulin was utilized as a control to visualize general innervation. Tyrosine Hydroxylase and HNK1 were chosen for their specificity in marking sympathetic and sensory nerves, respectively.
Specific Antibodies and Their Roles
● Beta-tubulin: This antibody was used to stain for general nerve innervation. Beta-tubulin is expressed by neurons, which is why staining for it reveals innervation. In the IHC process beta-tubulin is the primary antibody and the corresponding secondary antibody used is anti-rabbit 647.
● Tyrosine Hydroxylase: This antibody was selected to stain for sympathetic nerves. Sympathetic nerves play a role in autonomic functions, and their presence could indicate a regulatory role in regeneration.
● HNK1: This antibody was chosen to stain for sensory nerves. Sensory nerves are responsible for transmitting sensory information, and their mapping could provide insight into feedback mechanisms during regeneration.
Imaging and Analysis
Fluorescence microscopy was employed to visualize stained sections. This method works by illuminating a specimen with light of a specific wavelength, which is absorbed by fluorophores in the sample. The fluorophores then emit light at a longer wavelength, producing a different color than the absorbed light. In the case of immunofluorescence, the technique used in this experiment, antibodies are conjugated to fluorophores, allowing specific proteins or structures to be visualized under the microscope.
A fluorescence microscope typically consists of a light source, which emits excitation light that passes through an excitation filter to select the desired wavelength. This light is then directed toward the sample using a dichroic mirror. If the target antigen is present, the fluorophore-conjugated antibody will bind to it and emit fluorescence when excited. The emitted light is then filtered through an emission filter before being captured by the microscope's detector or camera, allowing for clear visualization of the labeled structures.
One limitation of fluorescence microscopy is autofluorescence, which is the natural emission of light by certain cellular components when excited by ultraviolet or visible light. This can create background noise and make it difficult to distinguish specific staining from non-specific fluorescence. To account for this, it is essential to include a negative control sample, which undergoes the same experimental process but without the addition of fluorophore-conjugated antibodies. This control helps
differentiate true fluorescence from autofluorescence – any signal observed in the experimental sample that is absent in the negative control is attributed to specific staining rather than background fluorescence (Im et al., 2019).
Results
Initial Imaging with Beta-tubulin
Beta-tubulin staining successfully marked nerve locations along the epithelium of the regenerating blastema. Fluorescent imaging indicated clear nerve presence, allowing for a detailed mapping of general innervation in the blastema. The sample stained with the beta-tubulin antibody was also compared to a negative control (Figure 5), which did not have any antibodies on it to account for autofluorescence. Anywhere that the beta-tubulin stained sample showed fluorescence that was absent in the negative control, were regenerated nerves. The most significant nerve regeneration found from the sample stained with beta-tubulin was at the blastema’s epithelium. (Figure 6). This result supports existing studies and understandings of axolotl blastema generation (McCusker et al., 2015).
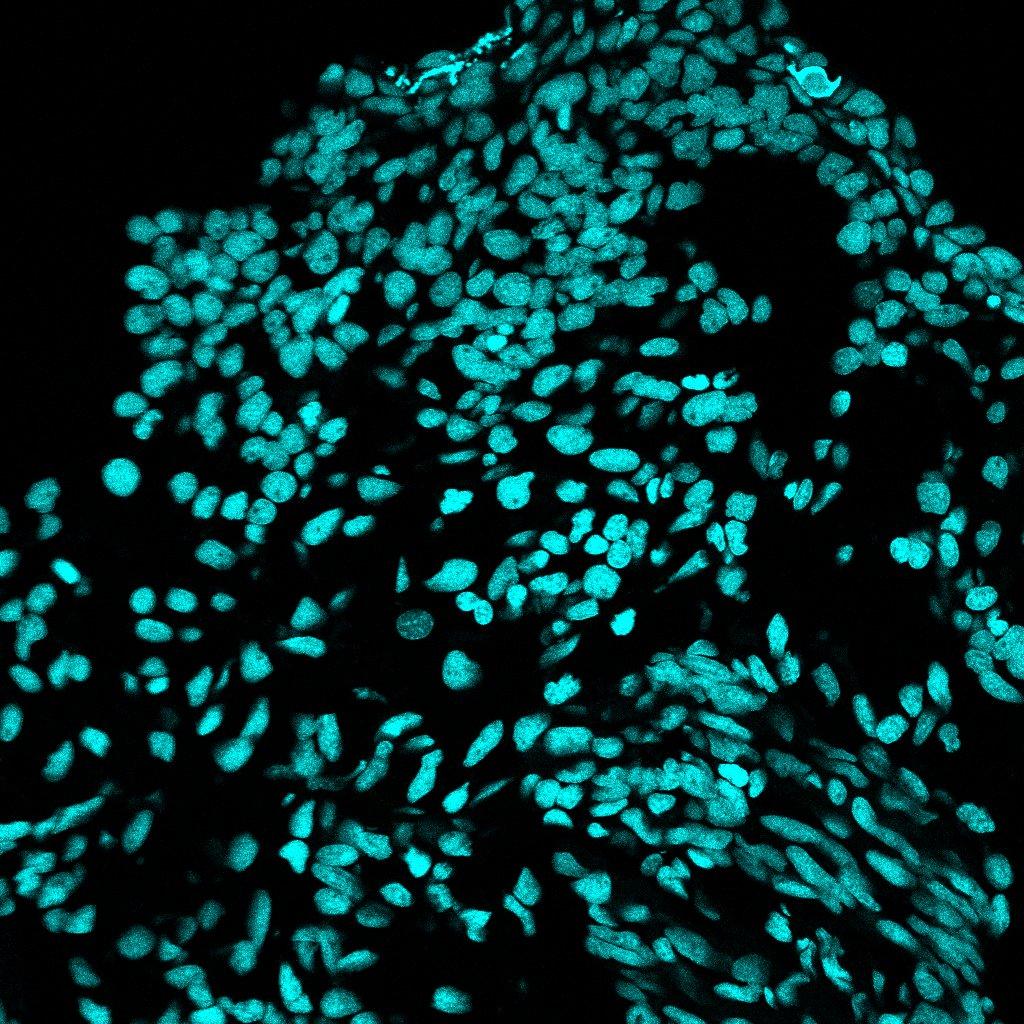
Figure 5: Negative Control Sample, no antibodies. Imaged using Fiji/ImageJ

Tubulin. Imaged using
Second Round with Tyrosine Hydroxylase and HNK1
Despite initial success with beta-tubulin, staining with Tyrosine Hydroxylase and HNK1 did not produce the expected fluorescence. No images are included due to lack of fluorescence. This finding (or lack thereof) calls for further trials in order to get results that can characterize the type of cell death responsible for tissue histolysis of a denervated blastema.
Limitations
The second attempt at an IHC using Tyrosine Hydroxylase and HNK1 did not yield visible fluorescence either, limiting the conclusions on sympathetic and sensory nerve presence. This lack of fluorescence could have resulted from many reasons such as antibody degradation (either primary or secondary), human error, or failure to permeabilize.
Conclusion
This study provides insights into the nerve dependency of axolotl limb regeneration, confirming innervation at a regeneration blastema’s epithelium through beta-tubulin staining. Although challenges with Tyrosine Hydroxylase and HNK1 prevented conclusive evidence of specific nerve types, the findings support further investigation into nerve roles in regeneration.
Discussion
Further research with improved antibody quality or alternative methods is needed to determine the method of cell death in the blastema. This study highlights the importance of nerve signaling in limb regeneration and the potential of axolotls to inform regenerative medicine and nerve-associated tissue repair. Further studies investigating the methods of cell death should also take into consideration apoptotic or necrotic indices later in the regenerative process as this study and the study conducted by Mescher et al. only investigated early in regeneration. Future investigations of methods of cell death and the nerves implicated in axolotls’
regenerative systems should prove useful in shedding light on the nature of growth regulation by nerves during regeneration. An understanding of the mechanisms involved in histolysis due to denervation is important for furthering understanding of mechanisms of regeneration.
Additionally, future research should focus on different aspects of axolotl, such as axolotls that have had metamorphosis induced. Axolotls that have undergone metamorphosis display differences in regenerative ability compared to those that have not undergone metamorphosis. Adult axolotls regenerate more slowly after undergoing metamorphosis, and they show an inability to correctly execute patterning and growth during regeneration (Monaghan et al., 2014). The way metamorphosis affects regeneration requires further investigation, and an investigation of nerve dependency in metamorphs could prove useful in elucidating mechanisms of regeneration and limb regrowth.
While regeneration’s dependence on innervation is well established, the largest looming question that could be the key to understanding tissue regeneration is why; why are nerves necessary for regeneration? An understanding of the nerves involved and the methods of tissue regression would bring scientific understanding one step closer to more fully understanding why nerves are necessary for tissue regeneration.
Gaining a deeper understanding of nerves’ roles in regeneration could be the key to understanding the mystery of axolotl regeneration, and this understanding could ultimately be applied to other species, maybe eventually humans. Axolotl limbs are anatomically similar to humans and studies have found that, to a certain extent, humans share similar nerve dependence in tissue regeneration. For example, paraplegic (partial or complete
paralysis of the lower half of the body) or quadriplegic (paralysis of all four limbs) individuals experience greater difficulty recovering from wounds compared to people with an intact nervous system (Basson & Burney, 1982). Denervated wounds in humans show significant complications in healing these wounds, the same way that axolotls are unable to regenerate tissue in the absence of nerves. Many people around the world have experienced loss of limbs or loss of innervation, and understanding axolotl limb regeneration could be a life changing discovery for millions of people.
References
Axolotl | San Diego Zoo Animals & Plants. (n.d.). Retrieved January 10, 2025, from https://animals.sandiegozoo.org/animals/axolotl
Basson, M. D., & Burney, R. E. (1982). Defective wound healing in patients with paraplegia and quadriplegia. Surgery, Gynecology & Obstetrics, 155(1), 9–12.
Cell Death: Types, Causes & Necrosis. (n.d.). Cleveland Clinic. Retrieved January 16, 2025, from https://my.clevelandclinic.org/health/articles/cell-death
De Groef, B., Grommen, S. V. H., & Darras, V. M. (2018). Forever young: Endocrinology of paedomorphosis in the Mexican axolotl (Ambystoma mexicanum). General and Comparative Endocrinology, 266, 194–201. https://doi.org/10.1016/j.ygcen.2018.05.016
Farkas, J. E., & Monaghan, J. R. (2015). Housing and Maintenance of Ambystoma mexicanum, the Mexican Axolotl. In A. Kumar & A. Simon (Eds.), Salamanders in Regeneration Research: Methods and Protocols (pp. 27–46). Springer. https://doi.org/10.1007/978-1-4939-24950_3
Farkas, J. E., & Monaghan, J. R. (2017). A brief history of the study of nerve dependent regeneration. Neurogenesis, 4(1), e1302216. https://doi.org/10.1080/23262133.2017.1302216
Fink, S. L., & Cookson, B. T. (2005, April 1). Apoptosis, Pyroptosis, and Necrosis: Mechanistic Description of Dead and Dying Eukaryotic Cells | Infection and Immunity. American Society for Microbiology Journals. https://journals.asm.org/doi/10.1128/iai.73.4.19071916.2005
Gerstein, A. D., Phillips, T. J., Rogers, G. S., & Gilchrest, B. A. (1993). Wound healing and aging. Dermatologic Clinics, 11(4), 749–757.
Im, K., Mareninov, S., Diaz, M. F. P., & Yong, W. H. (2019). An introduction to Performing Immunofluorescence Staining. Methods in Molecular Biology (Clifton, N.J.), 1897, 299–311. https://doi.org/10.1007/978-1-4939-89355_26
McCusker, C., Bryant, S. V., & Gardiner, D. M. (2015). The axolotl limb blastema: Cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration, 2(2), 54–71.
https://doi.org/10.1002/reg2.32
Mescher, A. L., White, G. W., & Brokaw, J. J. (2000). Apoptosis in regenerating and denervated, nonregenerating urodele forelimbs. Wound Repair and Regeneration, 8(2), 110–116. https://doi.org/10.1046/j.1524-475x.2000.00110.x
Monaghan, J. R., Stier, A. C., Michonneau, F., Smith, M. D., Pasch, B., Maden, M., & Seifert, A. W. (2014). Experimentally induced metamorphosis in axolotls reduces regenerative rate and fidelity. Regeneration, 1(1), 2–14. https://doi.org/10.1002/reg2.8
Stocum, D. L. (2017). Mechanisms of urodele limb regeneration. Regeneration, 4(4), 159–200. https://doi.org/10.1002/reg2.92
Thornton, C. S. (1968). Amphibian Limb Regeneration. In M. Abercrombie, J. Brachet, & T. J. King (Eds.), Advances in Morphogenesis (Vol. 7, pp. 205–249). Elsevier. https://doi.org/10.1016/B978-1-4831-9954-2.50010-0
Verma, A. (2022, July 5). Immunohistochemistry Techniques, Strengths, Limitations and Applications. Analysis & Separations from Technology Networks.
http://www.technologynetworks.com/analysis/articles/im munohistochemistry-techniques-strengths-limitationsand-applications-363107
Wells-Enright, K. M., Kelley, K., Baumel, M., Vieira, W. A., & McCusker, C. D. (2021). Neurotrophic control of size regulation during axolotl limb regeneration (p. 2021.04.27.441633). bioRxiv. https://doi.org/10.1101/2021.04.27.441633
White, R. (2011, January 22). Cryostat sectioning of frozen tissues. PROMETHEUS.
https://prometheusprotocols.net/structure/anatomy-andmicroscopy/anatomical-sectioning/cryostat-sectioning-offrozen-tissues/

