Corinna LaPlume
Senior Thesis | 2025

Tuberous Sclerosis Complex
Corinna LaPlume
Abstract
The Sahin Lab at Boston Children's Hospital is a translational lab, meaning animal models are used to predict outcomes in humans. The lab’s focus is on Tuberous Sclerosis Complex (TSC), a genetic disease that can cause autism, behavioral problems, non-cancerous tumors, learning disabilities, and various neuropsychological issues (2). But the most glaring issue of this disease is epilepsy, which causes life threatening seizures. While most patients with TSC are able to lead a normal life, many still die from epileptic seizures. About 1/6000 children are diagnosed with TSC every year (National Institute of Neurological Disorders and Stroke, 2024). The Sahin Lab's goal is to continue to find solutions to TSC, specifically the epilepsy aspect of the disease since it is the most dangerous aspect. Some things the Sahin Lab does is test new drugs, breed the genetically correct mice for testing, dissections in search of neurons, and more. Currently, there are very few medicines for TSC, and the ones available right now are still unable to treat all manifestations of TSC. They can also be incredibly expensive, some costing upwards of 10,000 dollars for long term treatment (Drugs.com, 2024). Unfortunately, while the best current drug on the market for TSC, named Everolimus, does work, it does not eliminate all symptoms of the disease. Everolimus targets a specific part of the cell called ‘Rapamycin’, a topic of interest for a lot of current and older papers on TSC. Many papers from the Sahin lab have been written about TSC. Currently, more papers are focused on targeting the PTEN protein to try to diminish the effects of TSC because it would affect both the TSC1 and the TSC2 complexes. These papers are saying that though targeting Rapamycin works, there may be even better medicines made by targeting different parts of the cell. Because of this, the Sahin Lab is trying to alter different parts of the pathway that causes TSC to see if there are more effective ways to reverse the effects. Understanding and hopefully curing the side effects of TSC is done through alteration of the TSC pathway.
Introduction
Tuberous Sclerosis Complex (TSC) is a genetic disorder that can cause a variety of side effects, epilepsy, which can lead to death, as well as skin lesions and autism (Winden, K. D. et al., 2018). The Sahin Lab is one of many trying to improve medicines for TSC. Understanding the TSC pathway is vital to understanding how TSC works and how it can be fixed. First of all, the TSC pathway is located in the brain. In the pathway, there are two regions of focus, TSC1 and TSC2. These two parts of a neuron work together to inhibit mTOR. The inhibition of mTOR, which is responsible for cell growth, makes sure that cells grow at the right time and the right amount (Karalis, V. et al., 2024). When TSC1 or 2 is damaged or missing, mTOR is constantly active and cells can grow unregulated (Dhamne, S. C. et al., 2023). This causes the noncancerous tumors and other symptoms. Other parts of the pathway include PTEN, which is a tumor suppressor and a regulator of the AKT pathway, further ‘upstream’ of TSC1/2. PTEN dephosphorylates PIP3 into PIP2. This means there is less PIP3. PIP3 activates PDK1 which activates AKT. AKT inhibits TSC1 and 2, which both inhibit mTORc1. mTORc1 is a protein that is crucial for cell growth. In summary, PTEN inhibits AKT, which in turn leads to more activity from TSC2, which eventually inhibits mTORc1. Since AKT encourages cell and tumor growth, PTEN regulates uncontrolled cell growth (Han, J. M., & Sahin, M., 2011). This also means that loss or damage of PTEN can lead to uncontrolled cell growth, like tumors, which can contribute to oncogenesis, which is the process of normal cells turning into cancer cells(Park, K. K. et al., 2008). But this also means that more PTEN could fix some of the issues with the hyperactivation of mTORc1.
Rapamycin directly affects mTORc1, making it an ideal target for resolving issues within mTORc1. Rapamycin inhibits mTORc1, so if mTOR is overactivating, extra Rapamycin would, in theory, reverse these effects. However Figure 1 shows that Rapamycin only connects to mTORc1, and usually there are issues with mTORc2 as well, so even though targeting Rapamycin would potentially fix some issues, it would not fix any issues occurring in the mTORc2 complex. More complexly, rapamycin binds to FKBP12 that forms a complex that inhibits the activity of mTORc1. It binds with mTORc1, preventing any other factors from binding with it. Because it inhibits the cell growth abilities of
mTORc1, it can be used to stop the benign tumors that often appear as a side effect of TSC. Because it does not affect both mTOR complexes, it is not seen as the ideal medicine, and is not as much of a focus for labs anymore. Instead, the ideal target could affect both mTORc1 and mTORc2 in a single action. For example, the PTEN and AKT proteins are both so high upstream that they affect both mTORs. This makes them ideal targets for combating TSC because they should be the most efficient and effective option. Currently, PTEN and other upstream ).

The line at the top represents the cell membrane, where the glucose, amino acids, and growth factors are coming from outside the cell to the edge of the cell. mTORc1 and 2 are outlined in a red dotted line, and the TSC complex is outlined in a dark gray dotted line. The light gray shaded area is the inside of the neuron. In this map it is clear how each of the mTOR pathways interact with each other. It is also clear to see where the problem arises in the TSC bubble. Figure 1 shows how every complex being tested, rapamycin, AMPK, PTEN, PI3K are all connected directly to this pathway.
Methods
One of the more common experiments at the Sahin Lab was something called a Polymerase Chain Reaction (PCR) test. In a PCR test, specific primers are used to identify a gene of interest. If, for example, the primers are set to target a sequence named “x”, if “x” is present in the sample it will be multiplied over and over again. If “x” is not there, nothing will get duplicated. In this case there were two genes of interest, the cre positive and the TSC2 presence. Therefore, two separate PCR tests are run, one for the cre and one for the TSC2 presence. The cre is what activates the mutation in the TSC2 gene. The cre is indicated by a + or a -, and positive for TSC2 is CC while negative for TSC2 is KC on paper. In an ideal mouse, it is necessary for TSC2 to be present and the mutation to be active(CC+). If TSC2 is not present, it is likely the mouse’s offspring won't survive. So for breeding purposes, KC- is preferred. For testing the effects of the mutations, CC+ is preferred.
In a PCR reaction, the mutations of the genes were seen visually. First, a DNA sample was prepared. For mice, a toe or a piece of the ear was used and placed in sealable tubes. These are best taken at about 1 week after birth. Each tube contains one piece of one mouse's DNA, and was mixed with 10µl of lysis buffer. The lysis buffer breaks open the cells by disrupting the outer area of the cells, beginning the DNA extraction process. Then the mixture was heated to 65 and eventually 96 degrees C, and then it was put in the 4 degree celsius fridge overnight. After 24 hours, the DNA samples were removed from the fridge and the next step can begin. The DNA sample was then mixed into liquid to extract as much of the DNA as possible. Usually the lab tested for two different pieces of the genome, so there were two primer solutions named
T2 and PV. T2 was for KC/CC, and PV is for +/-. The recipes for PV and T2 are different because different primers were needed to attach to different parts of the DNA.Then primers were added into each tube, and samples were prepared for the PCR machine. In the PV recipe, 1µl of primer was used per tube being tested. So for the first primer if there were 5 samples plus 2 controls, 7µl of primer would be used in our PV solution. In the T2 solution it was the same thing except it is 1/2µl. After each of the primer solutions were mixed, 12µl were put in each test tube as well as 3.5µl of the DNA lysis solution. In total, there were 15.5µl of solution in each tube containing the primers and the DNA. Since two different segments of DNA were getting duplicated, it was necessary to separate the two experiments and run them at different temperatures in the PCR machine for different amounts of time. After putting them in the PCR machine, which takes about an hour and a half, the gel mixture was prepared. It was made by mixing a buffer, 2 grams per 100ml of agar powder, and 5µl per 100ml of dye. This gel mixture was how the results were displayed. Meanwhile, the PCR machine was cycling through 3 different temperatures. At one temperature, the DNA was peeled apart and separated. At the next temperature, the primers went in and found the target sequence and ‘marked it’. Then at the final temperature, the two separated strands of DNA will each have been completed by the DNA polymerase, so what was once two strands of data had become four. This repeated about 32 times depending on how long the samples are left in the machine. After the samples had been sitting in the PCR machine, they were transferred to the gel. On the top end of the gel, a negative charge was placed and at the bottom a positive charge was placed. This is because DNA is negatively charged because of the phosphate groups in the nucleotides, so it will move away from the negative charge on top and towards the positive charge at the bottom. The positive charge will draw the duplicated target sequence down and out of the little well where you placed it. Based on the pattern formed by the sample as it leaves the well, one can tell if it was positive or negative for the target sequence. Typically two lines are positive for the target sequence and one line is negative. These results tell us which mice are preferable for breeding, which mice are preferable for testing, and which mice should not be bred or tested.
Unfortunately, the image is never perfect. Maybe some of the sample was lost to evaporation so there was a weaker response, or maybe your controls just didn’t work. Lines are also not always a perfect 2 or 1 line response. And maybe some information is lost after taking the photo. The photo of your results can be useful for later testing and other experiments. Unfortunately the consequence of an unclear image/results is large. Because you cannot retrieve a second sample from the mice, the mice may die since their alleles are unknown and therefore they cannot be used. If you saved the DNA, though, then you may have a second shot of retrieving the data so long as you found your mistake or your contamination.
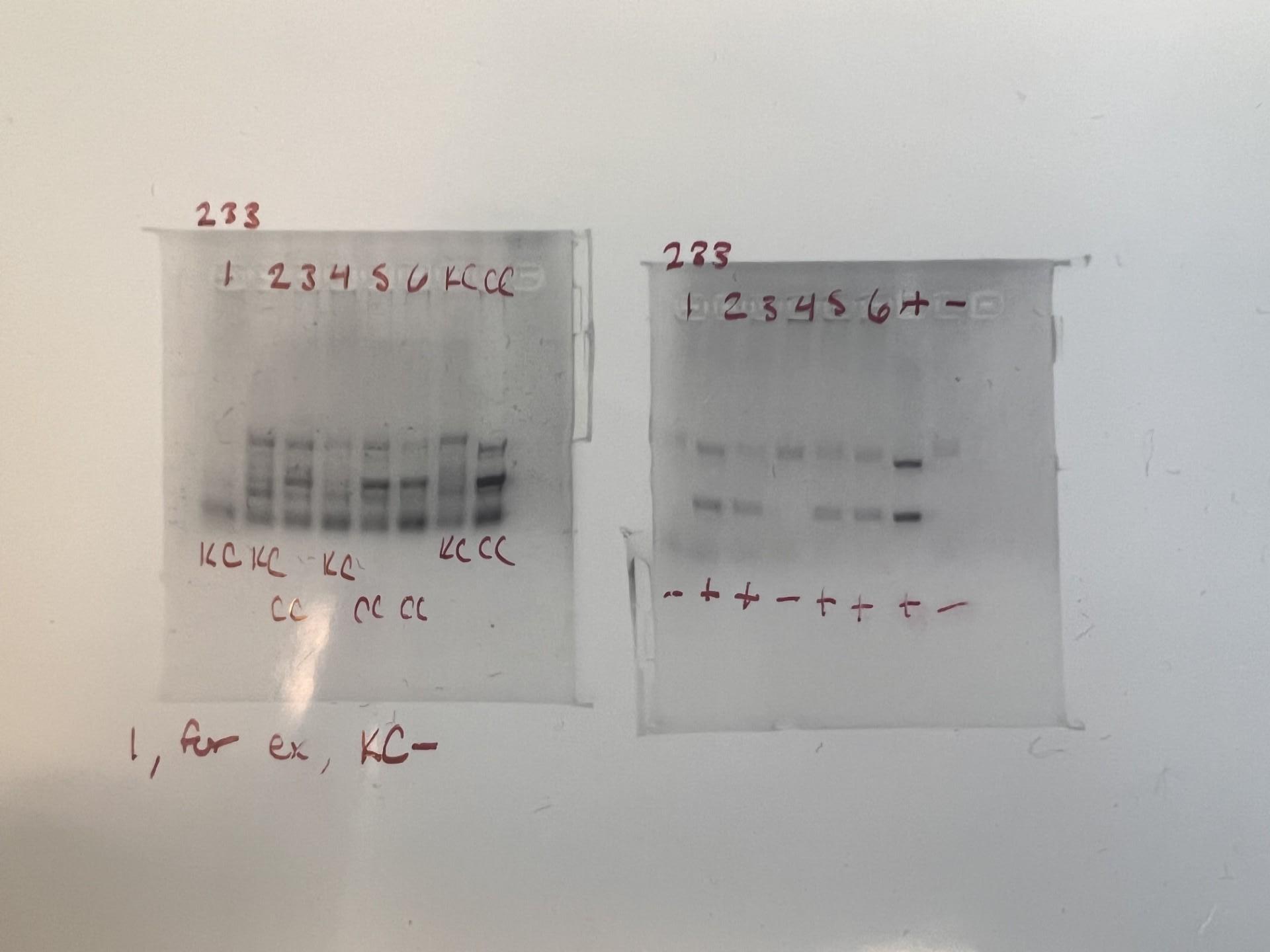
In Figure 2, it is shown that from one litter there were pups, marked 1-6 on the image. At the end of each row are the controls. The box to the left shows whether the mice are homozygous or heterozygous. If they are homozygous, it means that they have the TSC mutation and can potentially be used for testing, represented by a CC. If they are heterozygous it means they are suitable for breeding, and do not have the
TSC mutation, represented by KC. On the right side, the box represents whether the mutation is active. The plus means that if the mouse is CC, they are experiencing symptoms of TSC, and if it is minus then the mutation is not activated, no matter if the mouse is CC or KC. The controls at the end of each box tell us what the results should look like for KC, CC, +, and -. It becomes very tricky to determine the difference between KC and CC because they never look exactly like the controls. The very bold band in the middle is the marker for CC. So if the result looked like it may be CC but it was missing the bold stripe, it was probably KC. The +/- is a little easier to tell because it's only two bands. Two bands means positive, one band means negative. The results for mouse one in both boxes are combined. Since mouse one is KC in the left box and - in the right box, its genotype is KC-, making it ideal for breeding. But mouse three’s results from the right box shows that it is a CC mouse, and the left box shows that it is positive and the mutation is active. Overall, this PCR result from a mouse three of litter 233 shows that the genotype CC+, making it ideal for testing because the mutation is there and activated.
Brain dissection of a mouse is one of the most important experiments for studying TSC. In the pups, taken from the stomach of a pregnant mouse, the entire brain is removed from the pup’s head (must be at least 17 days pregnant for cre to be active), then the white matter is removed, the olfactory bulbs are removed, and the hippocampus is removed so that only the cortex remains, and the rest is discarded. In an adult mouse the process is similar, but everything is saved. Typically these dissections are to retrieve neurons, or sometimes even to observe cilia. Then, if the sample is good enough, the neurons will be saved and cultured. Maintaining the culture takes patience and focus. The culture tends to stick to the bottom of the petri dish, so when it's time to change the media, which is basically a mixture of chemicals the culture floats around in, it is important to gently remove and replace the media without displacing the culture. After the culture has aged a little bit, typically a few days or weeks, then the culture can be tested with drugs. Typically, the culture will be split into multiple sections so that old drugs can be tested right next to new drugs, and the results can be compared. In the Sahin Lab, Everolimus, the most common drug for TSC, is almost
always tested against new variations in drugs to see what changes a new drug offers in combating TSC.
Another experiment is called perfusion. This experiment is less common, and entails replacing the blood of a mouse with a clear liquid to remove the blood from the brain so that imaging of the mouse’s brain is clear. In this experiment, an adult mouse is sedated and placed on a sturdy pedestal of sorts. The mouse’s heart area is cut open, and the diaphragm is cut. Once the diaphragm is cut, the mouse is considered dead. Then, a needle that slowly releases clear liquid is put into the left atrium so that the clear liquid can be expelled throughout the body via the right ventricle. The blood slowly leaves the mouse’s body, being replaced by the clear liquid, a process that only takes a few minutes. At this point, all the blood has been flushed out of the brain, making the brain ideal for a variety of models such as tissue slicing and in vivo imaging.
To make all this possible, to be able to induce TSC in certain mice and to test it on human cells, you need to do something called gene editing. This is done through the crips-cas9 technology. Crisprcas9 has 3 steps: recognition, cleavage, and repair. During recognition, a CAS9 enzyme cuts off a gene with a guide RNA to help locate the target gene sequence. In cleavage, a part of that target sequence is cut out and the new, edited, replacement piece of DNA is inserted. Then, during repair, all the loose ends are tied up, meaning the new piece of DNA is connected to the old strands, and the edit in the sequence has been successfully planted into the genome.
Crispr RNAs contain the data to match to a specific part of the genome. CAS9 is an enzyme capable of cutting the genome and placing the RNA inside. The RNA is built to be a perfect match to the target sequence. Together, both parts of the system work to replace issues in the genome or to do something else. And this process doesn't just happen in one cell. It goes to all the target cells that express the segment of the DNA. Gene editing is a new technique, and is vital to the work done at the Sahin Lab.
Because this lab is testing medicines, both human cells and animals are needed to ensure effective and safe results. Because animals are involved, there are a lot of ethical guidelines that the lab follows. For example, mice are put to sleep before any dissection or experiment
occurs. Because mice are genetically different from humans, their spine connects to their brain and you can actually shift their spine down to cause immediate and painless death for the mouse. While it sounds gruesome, this is the most humane way to put the mouse down before a dissection. The mice are given food and water often, as well as toys and companions to keep them entertained. Of course the lab is always trying to use the least amount of mice as possible. When large DNA samples are needed from the mice, small pieces of skin or flesh are removed from the toes and tip of the tail from the mice at a really young age. And, as a general rule for all labs, there is a very strict guideline that must be followed, enforced by the government.
Important findings/papers
More papers are focused on targeting the PTEN protein to try to diminish the effects of TSC because it would affect both the TSC1 and the TSC2 complexes. Previous papers have focused on Rapamycin, but Rapamycin doesn’t work as well because it only affects TSC1. The research article Seizure reduction in TSC2-mutant mouse model by an mTOR catalytic inhibitor states “rapamycin[ ] inhibits mTORC1 but does not acutely affect the mTORC2 complex”. This is the problem with the most common TSC medicine, too, called Everolimus. It targets mTORc1 inhibitors that are too far downstream to affect mTORc2. In the end, the medicine works, but not to its full potential. Since mTORc1 affects cell growth, Everolimus would fix the benign tumors and the epileptic symptoms. But the metabolism and cell survival issues controlled by mTORc2 would not be fixed. Some examples of this would be the presence of ADHD or ASD, skin or lung issues, and even persistent epilepsy issues. While it is the most common medicine for TSC, it is not ideal and can be fixed. An example of a different kind of target is mGluR5, where inhibiting mGluR5, similar to other medicines, mainly prevents seizures (Kelly, E et al., 2018).
The review article by Karalis V. describes the effect the upregulation/downregulation of the TSC pathway can have on neurons. Increased activity of TSC1/2 complexes means that the axons of the neuron never form, and dendrites remain small. If the pathway is downregulated, and TSC1/2 are less active, neurons grow abnormally, forming multiple axons. This will greatly affect the nervous systems
development and more. It will also affect the myelination of the neurons in both the PNS and the CNS. In both, the myelin sheath will be noticeably thinner, which means that the signals will not be insulated as well and will not travel as fast. Sometimes in the PNS there can even be over myelination after remyelination, which can destroy the neuron. Both myelination and remyelination issues can cause greater issues in neurons and the nervous system.
The research article Autistic-like behavior and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice by Dalal, J. discusses the role the cerebellum plays in ASD when it comes from TSC. One common theme found was that in TSC patients who also had ASD, there was considerable loss of cells in the cerebellum. They also found that in the autistic mice, there were issues with their olfactory systems that had effects on social interactions with other mice. To test the behavior of the mice, they placed them in a water T test. This test involves placing the mice in water and providing an elevated platform that sometimes switches locations. Mice with ASD often took more time to find the platform after its location had been moved, demonstrating behavioral inflexibility.
Another common research paper topic is about 16p deletion, which means the partial deletion of the 16th chromosome. The article 16p11.2 is associated with hyperactivation of human iPSC-derived dopaminergic neuron networks and is rescued by RHOA inhibition in vitro by Sundberg, M et al. discussed how the negative effects of 16p deletion may be rescued by the inhibition of RHOA via Rhosin. They found that by using Rhosin, the hyperactivation of RHOA is rescued. The findings from this article show that Rhosin and other RHOA inhibitors may open doors to other solutions to 19pdel in the future. Though it may not seem like it, there are small connections between 16p deletion and TSC. If a deletion on 16p includes the TSC2 gene, it could lead to TSC.
Results/Discussion
Each finding in the lab can have greater effects and uses outside of what was done in the lab. For example, all the work on trying to treat TSC can be used to treat other forms of epilepsy or other disorders that occur in a similar pathway. And all the work with elongated cilia can be used on different types of cells in different diseases and disorders. Almost every experiment in this lab has results that can be used for an incredibly wide variety of subjects beyond what the original experiment was for.
So as discussed before, the lab is currently testing the PTEN pathway, in hopes that PTEN is high up enough in the pathways that the effects will transfer all the way down the pathway, affecting both mTORc1 and mTORc2. If this works, which looks more likely, then in a few years, Everolimus may not be the best available medicine for TSC anymore. The new medicine that would target PTEN would successfully eliminate a wider range of side effects, and be the most effective drug for TSC yet. However, if it doesn’t work, a few things will be learned. It could mean that PTEN is unable to affect the mTORs and will not work for TSC or in any other disease that affects this pathway, and a new protein will have to be targeted, like AKT (which has already been done) or growth factors. It could mean that this pathway isn’t behaving as expected, and no ‘trickle down’ theory is going to work, so switching to a new protein doesn’t fix any issues. In that case, the next step may be to target the mTORs directly. But since the results for the PTEN pathway are already looking good, hopefully in a few years that new medicine will become a reality.
Conclusion
Tuberous Sclerosis Complex’s cause affects many children across the globe every year, even causing death in some cases. Everolimus, the current most common medicine for TSC, lessens the risk of death, but doesn’t fix every issue TSC causes. The Sahin Lab works to try and find new cures for TSC that fixes more of the side effects, such as tumours and skin lesions. Though the specific part of the TSC pathway that causes TSC is still unknown, testing new pathways and targeting different proteins could result in a more effective medicine against TSC. As new varieties in the medicine for TSC are created and tested, not only does it help the children affected, but it could also help future experiments find the cause of diseases more efficiently.
References
1) Dalal, J et al. (2021) Loss of Tsc1 in cerebellar Purkinje cells induces transcriptional and translation changes in FMRP target transcripts. eLife. https://doi.org/10.7554/eLife.67399
2) Winden, K. D. et al. (2018) Abnormal mTOR Activation in Autism. Annual Review of Neuroscience
3) Kelly, E et al. (2018). mGluR5 Modulation of Behavioral and Epileptic Phenotypes in a Mouse Model of Tuberous Sclerosis Complex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 43(6), 1457–1465. https://doi.org/10.1038/npp.2017.295
4) Dhamne, S. C. et al. (2023). Seizure reduction in TSC2-mutant mouse model by an mTOR catalytic inhibitor. Annals of clinical and translational neurology, 10(10), 1790–1801. https://doi.org/10.1002/acn3.51868
5) Han, J. M., & Sahin, M. (2011). TSC1/TSC2 signaling in the CNS. FEBS letters, 585(7), 973–980. https://doi.org/10.1016/j.febslet.2011.02.001
6) Karalis, V. et al. (2024). The role of TSC1 and TSC2 proteins in neuronal axons. Molecular psychiatry, 29(4), 1165–1178. https://doi.org/10.1038/s41380-023-02402-7
7) Nie, D., & Sahin, M. (2012). A genetic model to dissect the role of Tsc-mTORC1 in neuronal cultures. Methods in molecular biology (Clifton, N.J.), 821, 393–405. https://doi.org/10.1007/978-1-61779-430-8_25
8) Park, K. K. et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science (New York, N.Y.), 322(5903), 963–966. https://doi.org/10.1126/science.1161566
9) DiMario, F. J., Jr, Sahin, M., & Ebrahimi-Fakhari, D. (2015). Tuberous sclerosis complex. Pediatric clinics of North America, 62(3), 633–648. https://doi.org/10.1016/j.pcl.2015.03.005
10) National Institute of Neurological Disorders and Stroke. (n.d). Tuberous Sclerosis Complex. National Institutes of Health. Retrieved July 29, 2024 from https://www.ninds.nih.gov/healthinformation/disorders/tuberous-sclerosis-complex
11) Drugs.com. (n.d). Everolimus prices, coupons, copay cards & assistance. Retrieved July 29, 2024 from https://www.drugs.com/price-guide/everolimus
12) Sundberg, M., Pinson, H., Smith, R. S., Winden, K. D., Venugopal, P., Tai, D. J. C., Gusella, J. F., Talkowski, M. E., Walsh, C. A., Tegmark, M., & Sahin, M. (2021). 16p11.2 deletion is associated with hyperactivation of human iPSCderived dopaminergic neuron networks and is rescued by RHOA inhibition in vitro. Nature communications, 12(1), 2897. https://doi.org/10.1038/s41467-021-23113-z

