SPEAKER BIOGRAPHIES
APARNA BHADURI, PH.D.
SHUIBING CHEN, PH.D.
GEORGE DALEY, M.D., PH.D.
MARK KRASNOW, M.D., PH.D.
TIPPI MACKENZIE, M.D.









SPEAKER BIOGRAPHIES
APARNA BHADURI, PH.D.
SHUIBING CHEN, PH.D.
GEORGE DALEY, M.D., PH.D.
MARK KRASNOW, M.D., PH.D.
TIPPI MACKENZIE, M.D.




Dear Colleagues and Friends:
On behalf of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, it is my privilege to welcome you to the 21st Annual Stem Cell Symposium. This year’s gathering carries particular significance as it coincides with the 20th anniversary of our center’s founding — two decades of groundbreaking research, transformative innovation, and a shared commitment to improving human health.
We come together in the aftermath of the recent wildfires, which have had a profound impact on our Los Angeles community. Many have faced devastating losses — of homes, cherished possessions, and stability — and we extend our heartfelt support to all who are rebuilding in the wake of these events. In such trying times, the power of community becomes a vital force, reminding us of the resilience and compassion that define our shared humanity.
This symposium has always been more than an annual event. Over the past 21 years, it has been a touchpoint — a place for collaboration, conversation, and connection. It has brought together a cadre of researchers who understand that stem cell science is not just about discovery but about addressing the most urgent health challenges of our time.
This year, we are thrilled to showcase an exceptional lineup of speakers who will share cutting-edge advancements in stem cell research, along with nearly 60 early-career scientists presenting their work in our poster session. These scholars, trained through our center’s interdisciplinary mentoring programs, represent the future of regenerative medicine and the hope it brings to patients worldwide.
Looking back, our 20-year journey has been nothing short of extraordinary. But what excites me most is what lies ahead. From paradigm-shifting technologies to life-changing therapies, the field of stem cell science continues to transform possibilities into reality. At the Broad Stem Cell Research Center, we remain committed to driving this progress, guided by the power of collaboration and the shared vision of what is possible.
Of course, none of this progress happens in isolation. It is your collective efforts as researchers, clinicians, educators, and philanthropists that propel our field forward. As we celebrate this milestone, we do so with immense gratitude for your dedication and partnership. You are an integral part of our journey, and together we will continue to forge new paths in stem cell research and regenerative medicine.
Thank you for being here and for being part of this vibrant and enduring community.
Sincerely,
Dr. Thomas A. Rando
Director, UCLA Broad Stem Cell Research Center

21STANNUAL
8:00-8:30am
8:30-8:45am
8:45-9:25am
9:25-10:05am
10:05-10:30am
10:30-11:10am
Check-in Begins
Welcome Remarks by Thomas Rando, M.D., Ph.D.
FRIDAY, FEBRUARY 7, 2025 COVEL COMMONS 8:00AM-4:35PM
Director, Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research
Mark Krasnow, M.D., Ph.D. (Stanford University)
Lung Stem Cells, their Niches, and the Initiation of Lung Cancer
Moderator: Brigitte Gomperts, M D
Aparna Bhaduri, Ph.D. (UCLA)
Understanding Cell Types in the Developing Human Brain and Glioblastoma
Moderator: Lili Yang, Ph D
Coffee Break
Samira Musah, Ph.D. (Duke University)
Advancing Stem Cell Differentiation and Human Disease Modeling with Organ Chips
Moderator: Melody Li, Ph D
David V. Schaffer, Ph.D. (University of California, Berkeley)
11:10-11:50am
11:50am-12:30pm
12:30-1:30pm
1:30-2:10pm
2:10-2:50pm
2:50-3:15pm
3:15-3:55pm
3:55-4:35pm
4:35-6:00pm
Engineering Biomaterial Microenvironments to Scale Stem Cell Expansion and Differentiation for Clinical Translation
Moderator: April Pyle, Ph D
Lunch
Charles Murry, M.D., Ph.D. (University of Southern California)
Heart Regeneration: Peeling an Onion?
Moderator: Arjun Deb, M D
Shuibing Chen, Ph.D. (Cornell University)
Human Pluripotent Stem Cells, Organoids and Drug Screening
Moderator: Song Li, Ph D
Coffee Break
Tippi MacKenzie, M.D. (University of California, San Francisco)
Prenatal Therapies for Single Gene Disorders
Moderator: Amander Clark, Ph.D.
George Daley, M.D., Ph.D. (Harvard University)
Hematopoietic Lineages from Pluripotent Stem Cells
Moderator: Thomas Rando, M D , Ph D
Poster Session Reception
In 2005, UCLA launched the Institute for Stem Cell Biology and Medicine, now the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, to bring together scientific, legal, and ethical experts from across the university to focus on fulfilling the great promise of stem cell research
Since then, our scientists have advanced the field in meaningful ways—initiating cell and gene therapy clinical trials for patients with deadly immune diseases, late-stage cancers, blinding eye diseases and more.
Our center supports innovation, excellence, and the highest ethical standards focused on taking groundbreaking stem cell research from the laboratory to the patient. We're committed to a multidisciplinary and integrated collaboration of scientific, academic, and medical disciplines for the purpose of revolutionizing the treatment of disease through personalized cellular therapies and regenerative medicine.
Our research is made possible by granting agencies such as the California Institute for Regenerative Medicine (CIRM) and the National Institutes of Health (NIH). Many of our hallmark programs are generously supported by philanthropic organizations including The Eli and Edythe Broad Foundation and The Rose Hills Foundation, as well as by individual contributors.
Thomas Rando, M.D., Ph.D. Director, UCLA Broad Stem Cell Research Center Professor, Neurology and Molecular, Cell and Developmental Biology
Melody Man Hing Li, Ph.D.
Assistant Professor, Microbiology, Immunology and Molecular Genetics
Song Li, Ph.D. Chancellor’s Professor, Bioengineering
April Pyle, Ph.D.
Professor and Vice Chair, Microbiology, Immunology and Molecular Genetics
Lili Yang, Ph.D.
Professor, Microbiology, Immunology and Molecular Genetics

University of California, Los Angeles
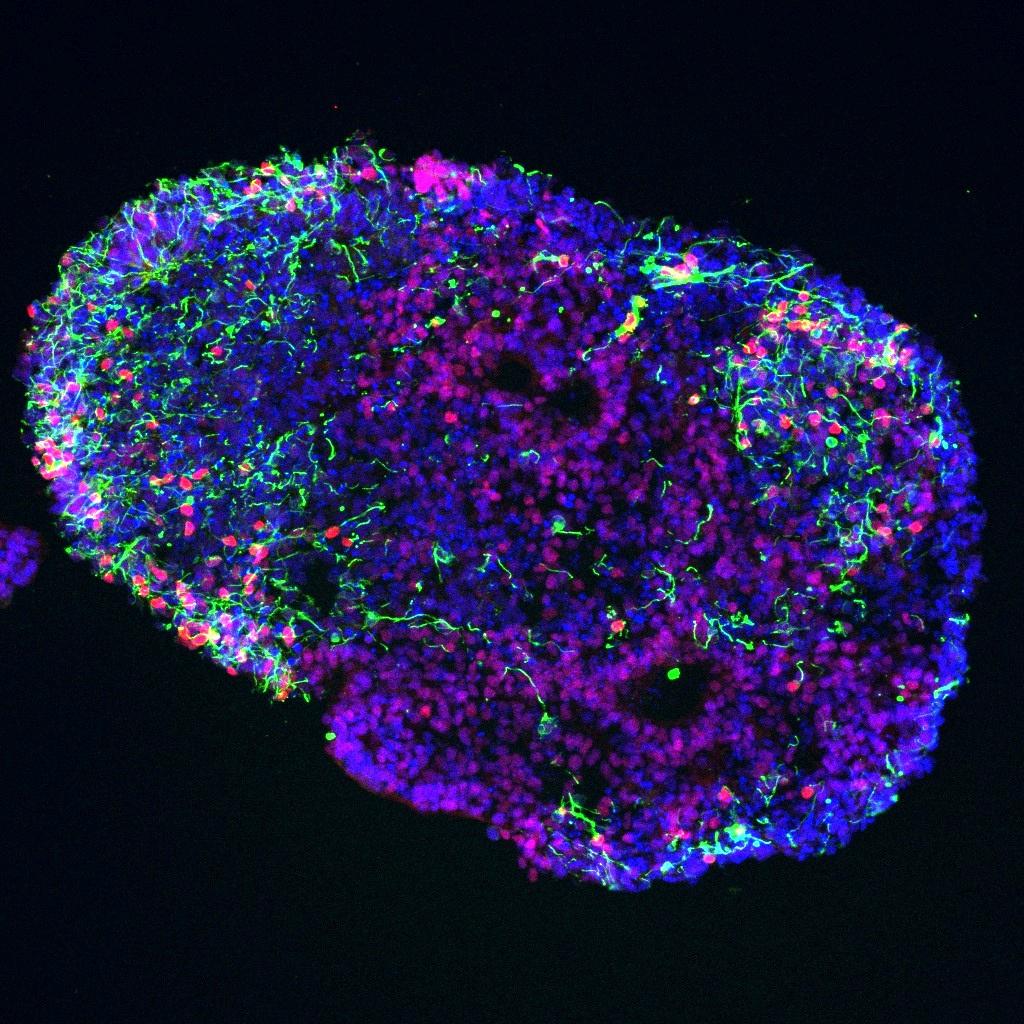
Dr. Aparna Bhaduri earned a B.S. in Biochemistry and Cell Biology and a B.A. in Political Science from Rice University in 2010. She completed her doctoral studies in Cancer Biology at Stanford University in 2016, where she focused on epithelial tissue differentiation and neoplasms. She completed her postdoctoral training in Dr. Arnold Kriegstein's lab at the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research at the University of California, San Francisco. She has used single-cell RNA sequencing to characterize cell types in the developing cortex across cortical areas in human and non-human primates, and in glioblastoma. Because experimental manipulations of the developing human cortex will require in vitro models, she has been using similar approaches to compare cells types in organoid models and primary tissues. Her long-term interest is in understanding how stem cells give rise to the human brain during cortical development, and how aspects of these developmental programs can be hijacked in cancers such as glioblastoma. In order to explore these questions, the Bhaduri Lab uses single-cell genomics, informatic analysis, and organoid models.

Kilts Family Professor, Surgery and Biochemistry
Cornell University
Dr. Shuibing Chen is the Kilts Family Professor and Vice Chair of Innovation in the Department of Surgeryand the Director of the Center for Genomic Health (CGH) at Weill Cornell Medicine. Dr. Chen was trained as a biochemist in Dr. Peter Schultz's lab at Scripps. Following her postdoctoral training in Dr. Doug Melton's lab at Harvard, she transitioned to the stem cell field and began her independent career at WCM in 2011. Early in her career, Dr. Chen conducted chemical screens to identify compounds that control stem cell differentiation. In recent years, she has focused on using human stem cell-derived organoids for disease modeling and drug screening.
Her laboratory has published in top peer-reviewed journals, including Nature, Nature Medicine, Nature Cell Biology, Cell Stem Cell, Cell Metabolism, etc. Dr. Chen serves on the Board of Directors for the International Society for Stem Cell Research and the International Chemical Biology Society. She is the President of the Chinese American Diabetes Association. She also serves on the editorial boards of leading stem cell journals, including Cell Stem Cell and Stem Cell Reports. Her exemplary contributions have been recognized with numerous prestigious awards, including the New York Stem Cell Foundation Robertson Investigator Award, the American Diabetes Association (ADA) Innovative Award, the NIH Director’s New Innovator Award, and the ISSCR Dr. Susan Lim Award for Outstanding Young Investigator, etc.

Dean,
Caroline Shields Walker Professor, Medicine
Professor, Biological Chemistry and Molecular Pharmacology
Harvard University
Dr. George Daley is Dean, Caroline Shields Walker Professor of Medicine, and Professor of Biological Chemistry and Molecular Pharmacology at Harvard Medical School. His research has focused on stem cell and cancer biology, with an emphasis on hematopoietic development and diseases of the bone marrow, blood and immune system. Daley earned his A.B. and M.D. degrees from Harvard and a Ph.D. in biology from MIT. Prior to becoming Dean at HMS, he was an investigator of the Howard Hughes Medical Institute and Director of the Pediatric Stem Cell Transplantation Program of the Dana-Farber Cancer Institute and Boston Children’s Hospital.

Executive Director, Wall Center for Pulmonary Vascular Disease
Paul and Mildred Berg Professor, Biochemistry
Stanford University
Dr. Mark Krasnow is the Paul and Mildred Berg Professor, Executive Director of the Wall Center for Pulmonary Vascular Disease, and past Chair of the Department of Biochemistry at Stanford University, and an Investigator of the Howard Hughes Medical Institute. He and his lab pioneered single-cell genetic and genomic approaches including single-cell RNA sequencing to elucidate organ development and stem cell programs, focusing on the respiratory system. They started with Drosophila where they defined the genes and molecular programs controlling each of the fundamental steps in organogenesis.
They developed similar approaches for mouse lung and used them to define at single-cell resolution the progenitors, stem cells and their niches for many lung cell types. They created a molecular cell atlas of the human lung, identifying 58 molecular cell types, 14 of which are new, including specialized cells and stem cells on each side (air and blood) of the alveolar gas exchange surface. They are using this comprehensive atlas to map the targeted cell types and how they are transformed in major lung diseases including lung cancer, COPD/emphysema, pulmonary fibrosis, pulmonary hypertension, and respiratory infections such as COVID-19. They have also led establishment of a new genetic model organism, the mouse lemur, to study primate physiology, behavior, disease, and ecology, He is a member of the National Academy of Sciences and the National Academy of Medicine, and a fellow of the American Academy of Arts and Sciences.

University of California, San Francisco
Dr. Tippi MacKenzie is a Professor of Surgery and Director of the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research at the University of California, San Francisco. She is a pediatric and fetal surgeon who is focused on developing better ways to diagnose and treat genetic diseases before birth. She runs a translational research lab examining fetal immunology and maternalfetal tolerance, with the ultimate goal of inventing new fetal therapies for patients with genetic diseases or pregnancy complications. She has moved two fetal molecular therapies from the lab to the clinic as phase 1 clinical trials after obtaining FDA approval: in utero hematopoietic stem cell transplantation to treat fetuses with alpha thalassemia and in utero enzyme replacement therapy in fetuses with lysosomal storage disorders. Her research has been supported by the National Institutes of Health, the March of Dimes, the California Institute for Regenerative Medicine, and the Burroughs Wellcome Fund. Dr. MacKenzie has been elected to the American Society for Clinical Investigation and the National Academy of Medicine for her innovative work.
Dr. MacKenzie trained in classical piano at Juilliard before obtaining her undergraduate degree from Harvard College and her medical degree from Stanford University. She completed her surgical residency at Brigham and Women’s Hospital in Boston and obtained additional fellowships in Fetal Surgery and Pediatric Surgery at the Children’s Hospital of Philadelphia. She joined the faculty at the University of California, San Francisco in 2007 and is now a Professor of Surgery. She recently co-founded the Center for Maternal-Fetal Precision Medicine, with the aim of accelerating the processes that link basic research to clinical trials to improve maternal, fetal, and neonatal health. This Center is testing methods to improve prenatal diagnosis of birth defects and developing new cellular and molecular therapies for definitive fetal treatment.

Ph.D.
Dr. Charles (Chuck) Murry received his bachelor’s degree in chemistry from the University of North Dakota, followed by M.D.-Ph.D. training at Duke University, where he studied myocardial ischemiareperfusion injury (heart attacks). He did residency training in Anatomic Pathology at the University of Washington, followed by fellowship training in vascular biology and diagnostic cardiovascular pathology. At the UW, Murry was the founding director of the Center for Cardiovascular Biology, and he cofounded and for many years directed the Institute for Stem Cell and Regenerative Medicine. Murry recently was recruited to the University of Southern California Keck School of Medicine, where he chairs the department of Stem Cell Biology and Regenerative Medicine and directs the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research.
Dr. Murry’s research focuses on stem cell biology, with an emphasis on understanding differentiation of the human cardiovascular system and using these cells to study diseases and to regenerate damaged tissues. His group is a world leader in heart regeneration and is working toward a clinical trial using cardiomyocyte therapy. He has served on many local, national, and international committees, spoken widely about stem cells and cardiovascular medicine, and he has received numerous awards for teaching and scientific achievement. Dr. Murry is a past member of the International Society for Stem Cell Research Board of Directors and currently serves on its Manufacturing, Clinical Translation, and Industry Committee. In addition to his academic work, Murry has worked to promote commercialization of novel cardiovascular therapies. He cofounded BEAT Biotherapeutics, Sana Biotechnology, and most recently a Los Angeles-based startup called StemCardia Inc.

Ph.D. Assistant Professor, Biomedical Engineering and Medicine, Nephrology
Dr. Samira Musah is an Assistant Professor at Duke University, jointly appointed in the Department of Biomedical Engineering and the Department of Medicine, Division of Nephrology. Research in her laboratory aims to understand the roles of molecular and biophysical cues in organ development and how these processes can be harnessed to understand disease mechanisms and develop new therapeutic strategies.
She is known for developing novel methods for stem cell differentiation and engineering functional models of human tissues and organs. Dr. Musah is a recipient of many prestigious research awards, including the NIH Director's New Innovator Award, the Whitehead Scholarship in Biomedical Research, the Burroughs Wellcome Fund Career Transition Award, the Baxter’s Young Investigator Award, the Genentech Research Award, the MEDx Biomechanics of Injury Repair Award, the Innovation & Entrepreneurship Initiative Award, and the Howard Hughes Medical Institute Gilliam Advisor Award. She was featured in Cell Stem Cell Early-Career Researchers and Nature Biotechnology Outstanding & Trailblazing Researchers. She was named among the inaugural "100 Inspiring Black Scientists in America" by Cell Press and named a Rising Star in Biomedical Engineering at MIT.

Dr. David Schaffer is the Hubbard Howe Professor, Chemical and Biomolecular Engineering, Bioengineering, and Molecular and Cell Biology at the University of California, Berkeley, and he also serves as the Executive Director of QB3 and the Director, Bakar BioEnginuity Hub, Bakar Labs, Bakar Fellows, and Bakar ClimatEnginuity Hub. He completed his B.S. in chemical engineering at Stanford University in 1993, his Ph.D. in chemical engineering at MIT in 1998, and a postdoctoral fellowship at the Salk Institute for Biological Studies in 1999 before joining Berkeley in 1999. There, he applies engineering principles to optimize gene and stem cell therapies, work that includes developing the concept of applying directed evolution to engineer targeted and efficient viral gene therapy vectors as well as new technologies to investigate and control stem cell function. He has published >250 papers, has advised >90 graduate students and postdoctoral fellows, is an inventor on >50 issued patents, and developed technologies that are being used in nine human clinical trials. In addition, he has co-founded eight companies, including 4D Molecular Therapeutics (NASDAQ: FDMT), Ignite Immunotherapies (acquired by Pfizer), and Rewrite (acquired by Intellia). He has received numerous recognitions, including election to the National Academy of Inventors and awards such as the Andreas Acrivos Award for Professional Progress, the American Institute of Chemical Engineers Pharmaceutical and Bioengineering Award, the American Chemical Society Marvin Johnson Award, and the Biomedical Engineering Society Rita Schaffer Young Investigator Award.
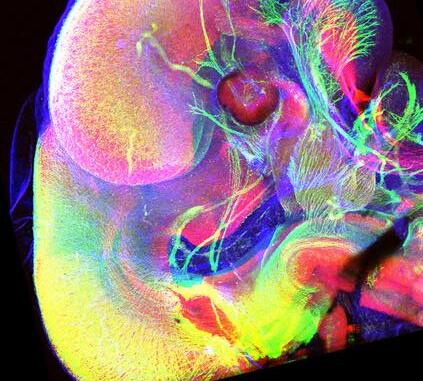
Developingarapidprotocoltoderivehumanlumbar/sacralspinalsensoryinterneuronsforspinalcord regeneration
TalinDemirjie
UCLA–CSUNCIRMBridgesTrainee
LabPI:SamanthaJ.Butler,Ph.D.
Thespinalcordcontainsdifferentpopulationsofsensoryinterneurons,knownasdorsalinterneurons(dIs),crucialforprocessingtouch, proprioception,pain,anditch Inspinalcordinjuries(SCIs)ordisease,dIdysfunctionleadstosensoryloss ToadvanceSCItherapies,weare developinghumanstemcellprotocolstogeneratedIsthroughneuromesodermalprogenitors(NMPs),thekeyprogenitorsthatendogenously giverisetothespinalcord.However,noefficientprotocolscurrentlyexisttorapidlyinducethemostposteriordIs,whichrelaygutandbladder sensationsinvivo WealsowanttoensurethattheNMPprotocolshavewidepatientapplicability Thus,weareusinganAfrican-American human-inducedpluripotentstemcell(hiPSC)donorline(F3.5.2)toidentifytheconditionsthatinducehumandIswithregionalidentitiesfrom theneck(brachial),trunk(thoracic),ortail(lumbar/sacral)spinalcordregions,thusfacilitatingregion-specifictransplantation Specifically,we aretestingwhetherNMPscanbeposteriorizedwithin1-4daysbyanalyzingtheeffectsoftwogrowthfactors:GDF11,whichishighly expressedintheposteriorspinalcord,orNotch,whichalsopatternsthisregion Spinalpatterningwasinducedusingretinoicacid(RA)and bonemorphogeneticprotein4(BMP4)in3Dembryoid-body(EB)cultures EBswerethenanalyzedwithimmunohistochemistry(IHC)toassess regionalspecificitythroughHOXexpressionanddIdiversityforaxial-specificidentities.Preliminaryresultssuggesthighconcentrationsof GDF11andNotchactivationhavelimitedeffectsonNMPposteriorization However,wehaveunexpectedlyfoundthattreatingtheF352line withBMP4,resultsinawiderrangeofcellfatedecisionsonday20,thanobservedpreviously.Thesefindingsprovideinsightintocriticaltime pointsindifferentiationforgeneratingspecificdIsubtypeswithdesiredregionalidentitiesforregenerativetherapies
ModulatingretinoicacidandWNTsignalingtodiversifyhumanstemcell-derivedsensoryinterneurons
YahirVerdin
UCLA–CSUNCIRMBridgesTrainee
LabPI:SamanthaJ Butler,PhD
Sensorymodalitiesarerelayedfromthebodytothebrainbydorsalinterneurons(dIs)inthespinalcord Lossoftheseneuronsafterinjuryor diseasecanresultindysfunctionalcontrolovertouch,pain,andmotoroutput Decipheringthemechanismsthatdirectembryonicstemcells (ESC)towarddIfatesisessentialtodevelopingcellularregenerativetherapiestoreplaceimpairedorinjuredsensorycircuits Previousstudies intheButlerlabhaveshownthatdirectingmouseESC-derivedneuromesodermalprogenitors(NMP)towardsdIscangenerateinvitro-derived dIsthatphenocopytheirinvivocounterparts TheseNMPprotocolsuseretinoicacid(RA)±bonemorphogenicprotein4(BMP4)toderivealldI populations,dI1-dI6.Moreover,thesedIslargelyresembleneck,andtrunkregions,whiletheyieldofmostposteriordIs,suchastailregionsis minimal Indevelopingembryos,RAandactiveWntsignalingplaycriticalrolesindeterminingboththeneuronalfatesinthespinalcordand theirlumbar/sacralidentitiesbyinducingposteriorHOXgeneexpression Thus,wetestedifdifferentconcentrationsofRAandWNTscan generatedIswithalteredregionalidentities TodevelopahumanNMPprotocolthatcanbeappliedtoawidepatientbase,wearederiving NMPsfromancestrallydiversehumaninducedpluripotentstemcell(iPSC)lines SpinalcordpatterningwasinducedinNMPembryoidbody (EB)cultureswithlowandhighRAconcentrationsacrossaCHIR(WNTagonist)gradient.Immunohistochemicalapproacheswereusedto analyzeifdIswithalteredaxialidentitiesweregenerated WhilewearestillinvestigatingtheeffectsoftheseconditionsondIposteriorization, weunexpectedlydiscoveredthatlowRAwithhighCHIRconcentrationsinduceddI1,dI4-dI6.Thisisthefirstinstancewherewehaveidentified thedevelopmentofdorsal-mostcelltypeswithoutBMP4treatmentandsuggeststhatWNTsignalinghasacriticalroleinhumanspinalcord patterninginvitro
Cyto-Tag:ATooltoRapidlyIsolateRareCellPopulationsinVivo
SaraFrigui
BSCRC–CIRMPredoctoralTrainee
LabPI:HeatherChristofk,PhD
Thisproject’sobjectivewastodevelopatechnologytostudythemetabolismofstemcellsintheinvivonichetounderstandhowmetabolism drivescellfatedecisions Isolatingrarecelltypesinvivofordownstreammetabolomicanalysishasposedanunsolvedchallenge Current methodscharacterizinginvivometabolismarelengthyandexertmechanicalstress,resultinginalterationofthecellularmetabolomeand incompatibilitywithstableisotope-labelednutrienttracing WehavedevelopedtheCYTO-Tag,atooltoisolaterarecellpopulationsthatis compatiblewithinvivonutrienttracing.TheCYTO-TagisanaffinitytagcontrolledbyCre-recombinasedrivenbyacell-typespecificpromoter. Additionally,itcanbeusedinconjunctionwithanewtissuedigestiontechniqueusingproteasesactiveat4°C Rapidcellisolationatcold temperaturesshouldallowgreaterpreservationofinvivocellularmetabolism
WehavevalidatedCYTO-TagmRNAexpressioninLgr5+intestinalstemcells(ISCs) WewillfurtheruseCYTO-Tagandinvivonutrienttracing toassesshowfasting,diet,andpregnancyimpactthemetabolismofISCs Additionally,wehavegeneratedmiceexpressingtheCYTO-Tagin Pax7+Musclestemcells(MuSCs)andwillassesstheimpactsofinjuryandchronicexerciseonMuSCmetabolism
InvestigatingtheDevelopmentalProgressionofMousePrimordialGermCell-LikeCellsinaReconstitutedOvary AzraCruz
UCLA–CIRMCOMPASSScholar
LabPI:AmanderT Clark,PhD
Infertilityaffectsaround15%oftheglobalpopulation,andexistingclinicaltreatmentsfailtotreatinfertilityatagermcelllevel,leadingtothe importanceofproducingoocytesasaformofregenerativemedicine Thoughthereconstitutedovary(rOvary)isamodelcapableofcreating oocytes,theprocessremainsinefficientduetothehighpercentageofembryoloss TobeginimprovingtherOvary,itisessentialtounderstand thedevelopmentofgermcellsintheinvitrosystem.FollowingtherOvaryprotocol,wegeneratedrOvariesandcollectedthemalongtheir developmentaltrajectory Utilizingimmunostaining,westudiedprimordialgermcell-likecells(PGCLCs)astheydevelopedintooocyte-likecells usinggreen-fluorescentprotein(GFP)torepresentthetransgenicreporterused,DAZLtomarkPGCLCscompetentofmeiosis,andSCP3to markPGCLCsinmeiosis TheexpressionofSCP3ledustohypothesizethattheinefficiencyoftherOvaryisduetoanabnormalprogressionof meiosisinPGCLCsandapotentialdevelopmentalmisalignment ExpandingourknowledgeoftherOvaryiscriticaltogaininginsightintothe developmentofPGCLCs,improvingtheefficiencyofIVO,creatinghigher-qualityoocytes,andaddressinginfertilityatagermcelllevel
AtemporallyresolvedBMP4responseintheperi-implantationhumanembryonicdisciscriticalfortheinductionof multipotentTFAP2A+cellsandEMT
AurianaArabpour
BSCRC–RoseHillsFoundationPredoctoralTrainee
LabPI:AmanderT Clark,PhD
Theunilaminarembryonicdisc,asheetofepithelizedSOX2+epiblastcells,undergoescomplexmorphogenesistocreateamulti-dimensional humangastrula Thisprocessendowsearlylineagespecificationandspatialidentitiesthatbeginattheposteriorendofthedisctocreatea coordinatesystemforembryogenesis Here,wefindthatsimplyaddingbasementmembraneextracts(BMEs)tothestemcellmediais sufficienttomodeltheearlyperi-gastrulatingembryonicdisc Wedemonstratethattheemergenceofamnion,primordialgermcells(PGCs), andmesodermarisefromBMP4-responsivedisccellsthatinducetransientTFAP2AexpressionandSOX2repression.Wetracktheorderof embryoniceventsthattakeplaceduringdiscmorphogenesisandshowthatamnion-likecells(AMLCs)andPGC-likecells(PGCLCs)are specifiedfirstfromepithelizedTFAP2A+progenitors Shortlyafter,gastrulatingmesoderm-likecells(MeLCs)arisefromtransientlyexpressed TFAP2A+disccellsthatthenundergoEMT WefindthatBMP4-responsivenessiscriticaltoSOX2repression,TFAP2Aexpression,lineage inductionandEMT,whiletheprocessofEMTitselfisnot Thus,weshowthattheextracellularmatrixisnecessarytopromotedisc morphogenesisandEMT
StrategiesforAnalyzingGermCellNestBreakdown,FollicleFormation,andFollicleActivationintheReconstituted Ovary
AutumnJackson
LabPI:AmanderT Clark,PhD
Mousereconstitutedovaries(rOvaries)arebiomedicalresearchtoolsusedtomodelovariandevelopmentinvitro Whilemanyoftheovary’s cellularprocessesarereproducibleintherOvary,keydifferencesbetweentheinvitroandtheinvivosystemstillexist Theprocessoffollicle formation,whichendowsreproductivecapacityinthefemale,isonesuchkeyevent Follicleformationhappenswhenaneggcell(oocyte)is encapsulatedbyhelpercellsknownasgranulosacells,whichsupporttheoocyte’sgrowthintomaturity Twoclassesoffolliclesexist:onethat activatesgrowthimmediatelyafterassembly,andanotherwhichremainsdormantintheovaryuntilpuberty TherOvaryisthoughttoonly producethefirstclassofimmediatelygrowingfollicles.However,thisideawasonlyshownfromtheperspectiveoftheoocyte.Togathera moreholisticviewoffollicleassemblyintherOvary,weturnedtoinvivoovaries WeperformedimmunofluorescencewithaFOXL2antibodyto targetgranulosacells,STELLAantibodyforoocytes,SCP3antibodyformeiosis,andFOXO3antibodytoidentifyimmediatelygrowingor dormantoocytesinfollicles Wecombinedourimmunofluorescencewithgranulosacellnumberquantification,whichmaybeareliablemethod toclassifyfolliclesasdormantorgrowing WefoundthatnuclearFOXO3expressionandanaveragegranulosacellcountof3-4arereliable indicatorsfordormantfollicles However,FOXO3wasexpressedinthreeclassesoffolliclesoverthecourseofgrowth Therefore,follicle classificationrequiresanintegratedapproachofproteinmarkersandmorphologicalindicatorslikegranulosacellcountandshape Withthe informationgleanedfromtheinvivoovary,wewillbeabletobetterunderstandinvitrofollicleformationintherOvary,andhowtoimprovethe processtomakeitmorereflectiveoftheinvivosystem,increasingitsrelevanceasaresearchandtherapeutictool
A2Dculturesystemfortheefficientinductionandstudyofearlyhumangermcells
MarkLarsen
LabPI:AmanderT Clark,PhD
Duringimplantation,earlyhumanembryocellsdiversifyintoseveralcelllineagesincludingprimordialgermcells,cellscrucialforreproduction Ourlabstudiesthesecellstounderstandgermcellspecification,whichoccurs2-3weekspost-conception,makingthemchallengingtostudy invivo Valuableinvitromodelsofgermcellspecificationhavebeendeveloped,butexistingconditionsgeneratecellline-dependentvariability ingermcellinduction Thisvariabilitymakesitdifficulttostudymolecularmechanismsofgermcellspecificationthatarerepresentativeof theirinvivocounterparts.Toreducevariability,ourlabhasdevelopedahighlydefinedembryomodelthatrecapitulatesdifferentiationof multiplehumanembryoniccellscatalyzedbyanoverlayofbasementmembraneextracttocellsplatedinaxeno-freemedia Herewehave optimizedthisembryomodeltoinduceaconsistentpercentageofgermcellsforthestudyofgermcellspecificationintwoseparatecelllines. Additionofatime-specificdoseofBMP4directsmoreculturedstemcellsintospecifiedgermcells,markedbySOX17andTFAP2Cexpression Toshowthepotentialofthemodelforthemechanisticstudyofgermcellbiology,wefocusedonthetranscriptionfactorTFAP2A,aknown downstreameffectorofBMP4involvedingermcellspecification CulturingstemcellsharboringmutatedTFAP2Aledtosignificantreduction ingermcellpercentages Thisnovelinvitromodelprovidesthefieldwithareliableandreproduciblemeanstostudygermcelldevelopment
RaymondAlvarado
UCLA–CIRMCOMPASSScholar
LabPI:HilaryColler,Ph.D.
Autophagy,acellulardegradationprocess,isessentialformaintainingcellularhomeostasisandrespondingtostress Incancerbiology, autophagyplaysacomplexroleasitcansuppresstumorinitiationandsupporttumorgrowthinestablishedcancers Furthermore,anessential elementincancerinitiationandprogressionisthetumormicroenvironment(TME),whichincludesimmunecells,extracellularmatrixproteins, andchemokines.Previousresearchfoundthatawhole-bodyknockout(KO)ofautophagyleadstosmallertumors.Theroleofautophagyin specificcellpopulationsontumordevelopmentremainspoorlyunderstood Myresearchaddressesthisgapbystudyingtheeffectsofan autophagyKOintheimmunecellswithintheTME
MacrophagesandDendriticCells(DCs)playintricateroleswithintheTME,capableofeitherpromotingorinhibitingtumorprogression As importantcellsinprimingandactivatingT-cellresponses,bothcelltypesaretargetsforinvestigation Ourstudyinvestigatesthetumor responseofautophagy-deficientmacrophageandDC ThemacrophagesandDCswereculturedinvitrofrombonemarrowthatwasisolated fromeitherautophagy-proficientordeficientmice.TheculturedmacrophagesandDCswerethentreatedwithB16-F10,mousemelanoma, conditionedmediatosimulateaTIME Then,RTqPCRwasusedtoanalyzetheirexpressionofproinflammatorycytokines Ourresults demonstratethatautophagy-deficientmacrophagesandDCsexpresshigherlevelsofproinflammatorycytokinesthantheirautophagyproficientcounterparts Thesefindingssuggestthatautophagy-deficientmacrophagesandDCsexhibitaheightenedactivity,whichmayplaya roleinslowingcancerprogression Thisstudyhighlightstheimportanceofstudyingimmunecellresponsesincancerbiology Ourfindings maypavethepathfornovelimmunotherapytechniquesthatusemacrophageandDCactivitytobattletumorprogression
GrasielaTorres
LabPI:AnthonyJ.Covarrubias,Ph.D.
Agingisthenumberoneriskfactorformultiplediseasesincludingcancer,diabetes,andneurodegeneration,creatingurgencyforsolutions regardinghealth-spanmaintenanceaslifespanincreases Cellularsenescenceisemergingasamaindriverofage-relateddiseasesduetothe chroniclow-gradeinflammationthatresultsfromaccumulationofsenescentcellsinvarioustissuesthroughoutthebody Cellsthatundergo senescencearecharacterizedbypermanentcellcyclearrest,alteredmetabolismandsecretionofinflammatorymolecules,knownasthe senescent-associatedsecretoryphenotype(SASP) Ourlabhasshownthatsenescentmacrophagesaccumulateinagingtissuesandcan becomesenescentusingDNAdamagingstimulisuchasirradiationandchemotherapeuticdrugs.Ourdatasuggestthatmacrophages representakeysourceofsenescentcellsinagingtissues,howeverthemolecularmechanismsdrivingsenescenceinmacrophagesandtheir linktoage-relateddiseasesremainunclear Ourstudyaimstounderstandroleofsenescentmacrophagesasadriverofinflammagingand aging-relateddiseases Todoso,wewillidentifygenesandpathwaysthatcontributetocellularsenescenceofmacrophages Ourapproach utilizesinvivostudies,useofanovelinvitrosenescentmacrophagesystemusingprimarybonemarrowmacrophagesfromWTandknockout mice,andCRISPR-Cas9genomiceditingtoinvestigatehowgenecandidatesregulatevariousfeaturesofsenescence.Understandingthe molecularmechanismsregulatingmacrophagesenescencemayprovideinsightintotreatingage-relateddiseases
Macrophagesrepresentasourceofp21+senescentcellsinagedandsteatoticliver
IvanSalladay
LabPI:AnthonyJ Covarrubias,PhD
Senescentcellsplaycausalrolesinsterileinflammationinagingtissuesthroughthesenescence-associatedsecretoryphenotype(SASP) Targetingsenescentcellspresentpromisingstrategiesfortreatingage-relateddiseaseslinkedtometabolicdysfunction;however,identifying specificsenescentcelltypes,especiallymyeloidcellslikemacrophages,poseschallengesininflammatoryconditions Toaddressthisissue, aninvitromodelofDNAdamage-inducedmacrophagesenescencewasdevelopedandfacilitatedtheidentificationofuniquebiomarkersthat distinguishsenescencefromotherpolarizedstates Interestingly,excessivecholesterolstorageisadistinctivefeatureandmajordriverof p21+macrophagesenescence,particularlyinmicefedahighcholesteroldiet Treatmentwithsenolyticsreducedthelevelsofsenescent macrophagesandimprovedthefattyliverpathology Furthermore,humansenescentmacrophagesarealsop21+andareenrichedinhuman livercirrhosissamples Thesefindingsprovideevidencethatsenescentmacrophagesplaypivotalrolesindrivinghomeostaticdysfunctionin agingtissues,potentiallycontributingtothedevelopmentofliverfailure.Thisunderscoresthepotentialoftargetingmacrophagesenescence asatherapeuticapproachtoaddressthesourcesofage-relatedchronicinflammation
TylerMcCaw,M.D.,Ph.D.
BSCRC–CIRMClinicalFellow
LabPI:JosephG Crompton,MD,PhD
Immunotherapyisapowerfulcancertreatmentstrategy,occasionallyresultingincures;however,Tcellexhaustionseverelycurtailsitscurrent clinicalefficacy.Exhaustionisanepigenetically-mediatedterminaldifferentiationwhereinTcellslosestemnessandtumor-killingability.Thus, theproblemisthis:forimmunotherapytobeeffective,stem-likeTcellsareabsolutelyrequiredbutbythetimeofclinicalpresentationthevast majorityofapatient’savailabletumor-specificTcellsareexhausted Wethereforehypothesizedthattranscriptionfactorreprogrammingcan overcometheepigeneticmechanismsenforcingTcellexhaustiontoregeneratestem-liketumor-specificTcells Ourapproachistwo-part First,exhaustedtumor-specificCD8Tcellsarededifferentiatedintoinducedpluripotentstemcells(iPSCs)viatransductionwithOct3/4,Sox2, Klf4,andc-Myctranscriptionfactors(OSKM;DE-differentiation).Second,CD8-derivediPSCsarere-differentiatedtomature,stem-likeCD8T cellsusinganartificialthymicorganoid(ATO;RE-differentiation) De-differentiatingCD8Tcellsprocuredfrompatientswithsofttissue sarcomas(STS),wedemonstratedthataTcell’sstartingepigenomedictatesitsOSKMreprogrammingpotential.Notably,exhausted, polyclonalCD8TcellsisolatedfromsurgicallyresectedSTScangeneratestableiPSCcolonies Re-differentiationoftheseCD8-derivediPSC intomatureCD8abTcellswasthenaccomplishedusinganATO,reachingsimilarefficaciesasiPSCderivedfromnon-exhausted,monoclonal CD8Tcells Ourfindingsdemonstratethattumor-specificTcellexhaustioncanbeovercomeandstemnessrestoredusingOSKM reprogramming Thesestem-likeCD8Tcellsarethenspecifictothepatient’sowntumorfromwhichtheywereisolatedandcanthenbe reinfusedintothepatientasapotentimmunotherapeuticplatform.
Investigatetheroleofancestry-associatedgermlinevariantsinprostatecancerinitiation
ShileZhang
BSCRC–RoseHillsFoundationPredoctoralTrainee
LabPI:AndrewS Goldstein,PhD
ProstateCancer(PCa)isoneofthemostcommonanddeadlycancersinAmericanmen ThePCaincidenceanddeathratesaredispersed amongstminorethnicgroups,withAfrican-AmericanmenhavingbothhigherincidenceandmortalityratesinPCathanCaucasianmen In additiontoknownfactorsassociatedwithhealthdisparities,includingsocioeconomicstatus,accessibilitytohealthcare,andracialbias,littleis knownaboutthegeneticdriversofancestry-associateddisparitiesinPCa RecentstudiesfoundthattheheritableHOXB13variants,orgenetic mutations,increasePCarisksandprogressionindifferentancestrygroups.HOXB13isakeygenefornormalprostatedevelopment.Its variantsofinterestincludeG84EinCaucasians,G132EinJapanese,G135EinChinese,andX285KinindividualsofWestAfricanancestry However,itremainsunclearhowtheancestry-specificHOXB13variantsinfluencethedevelopmentofaprostatethateventuallyincreasesthe riskofPCa Weemployacombinationof3Dhumanprostatecellmodelandmouseembryonicprostatecellmodeltodefinetheearlyrolethat thesevariantsplayinprostatetissue OurdatashowsthatsomeHOXB13variantsacquirestrongerregenerativecapacityandrelevant functionalchangesincellculture EvaluatingtheunderlyingcauseofthevaryingPCariskindifferentancestrygroupsiscrucialtoachievinga moreequitablehealthcaresysteminAmerica NostudieshaveattemptedtodefinethecommonmechanismbehindmultipleHOXB13germline variantsinpre-cancerousprostatetissues,largelyduetothechallengesinmodelingearlyhumanprostatedevelopment.Ourlabhasdeveloped bothhumanandmousecell-basedmodelsystemstostrategicallyaddresstheroleoftheheritableHOXB13variants
InvestigatingPerilipinFunctioninProstateCancerCellLinestoExploreTheirRoleinLipidMetabolismand AndrogenReceptorSignaling
JavierMartinez
UCLA–CIRMCOMPASSScholar
LabPI:AndrewS Goldstein,PhD
Prostatecanceristhesecondmostcommoncancerinmenandaleadingcauseofcancer-relateddeaths Akeytherapeuticapproachtargets theandrogenreceptor(AR)duetoitsroleintumordevelopment TheAR,onceactivatedbybindingtoandrogens,translocatestothenucleus toactasatranscriptionfactorforgenesthatdrivecellsurvivalandtumorgrowth Androgendeprivationtherapy(ADT)reducesandrogen levelsorblocksandrogenbindingtotheAR However,mosttumorseventuallybecomeresistanttoADT,leadingtocastration-resistant prostatecancer(CRPC).Lipidmetabolismiscriticalfortumorigenesis,ascellsrequireenhancedlipidsynthesisanduptakeoflipidstofuel rapidcancercellgrowth UnderstandinglipidomicchangesinCRPCcanidentifyvulnerabilitiestodeveloptherapeutictargets Usingwestern blots,weaimtocharacterizeendogenousperilipin(Plin2,Plin3,andPlin5P)expressioninvariousprostatecancer(PCa)celllinesdisplayinga widerangeofARexpression Furthermore,toexploreeffectsonlipidmetabolismandARsignaling,wewilloverexpressPlin2,Plin3,andPlin5 inPCacelllinesandperformlipidomicsandmetabolomics Currently,ourpreliminaryresultsshowthatAR-low-expressingPCacelllines displayhighlevelsofPlin2expression AR-lowPCacelllinesarealsomarkedbylowlevelsofFASNandACAC-A,twocriticalproteinsimportant forlipogenesis TheseexperimentswillhelpusunderstandhowoverexpressionofeachPlinaffectslipidmetabolisminARhigh/lowPCacells ThisresearchwillprovideinsightintolipidregulationandhowitisinterconnectedwithARactivity,whichiscrucialfordevelopingstrategiesto targetaggressivecancercells
MingxiaGu,MD,PhD
AssociateProfessor,AnesthesiologyandPerioperativeMedicine
Thevasculatureandmesenchymeexhibitdistinctorgan-specificcharacteristicsadaptedtomeetlocalphysiologicaldemands The microenvironmentandcell-cellinteractionsarecrucialindrivingtheadoptionoforganotypicfeaturessincetheearliestdevelopmentalstages Torecapitulatethisentireprocess,weco-differentiatedmesodermandendodermlineageswithinthesamespheroidtovascularizelungand intestinalorganoidsfromiPSCs Theratioofendodermandmesodermlineageswasfine-tunedbyBMPsignalingduringtheinitialstageof differentiation,acriticalstepingeneratingtheappropriateproportionsofendothelialandepithelialprogenitorswithtissuespecificityfor subsequentorganoidpatterningandvascularization Single-cellRNA-seqanalysisfurtherdemonstratedtheorgan-specificgenesignaturesof endotheliumandmesenchyme,andidentifiedkeyligandsdrivingendothelialspecificationinbothlungandintestine.Theorganotypic endotheliumexhibitedtissue-specificbarrierfunction,enhancedorganoidmaturation,promotedcellulardiversity,andsupportedalveolar structureformationonbioengineeredscaffolds Upontransplantationintomice,thevasculatureretaineditsorganspecificityandintegrated withthehostcirculation,furtherenhancingthematurationandpatterningoftheorganoids Additionally,ourmodelrevealedabnormal endothelial-epithelialcrosstalkinpatientswithFOXF1deletionormutations Multilineageorganoidsprovideauniqueplatformtostudy developmentalcuesguidingendothelialandmesenchymalcellfatedetermination,andinvestigateintricatecell-cellcommunicationsinhuman organogenesisanddisease
DistinctrolesforNFκBsignalinginthebonemarrownicheandinhematopoieticstemcellsuponhematopoietic aging
JenniferChia,M.D.,Ph.D.
BSCRC–CIRMClinicalFellow
LabPI:AlexanderHoffmann,PhD
Hematopoieticagingischaracterizedbychronicinflammationassociatedwithmyeloidbias,HSCaccumulation,andfunctionalHSC impairment.Yetitremainsunclearhowinflammationpromotesagingphenotypes.NFκBbothrespondstoanddirectsinflammation,andwe presentanexperimentalmodelofelevatedNFκBactivity(“IκB–”)todissectitsroleinhematopoieticagingphenotypes Wefoundthatwhile elevatedNFκBactivityisnotsufficientforHSCaccumulation,HSC-autonomousNFκBactivityimpairstheirfunctionality,leadingtoreduced bonemarrowreconstitution Incontrast,myeloidbiasisdrivenbytheIκB–proinflammatorybonemarrowmilieuasobservedfunctionally, epigenomically,andtranscriptomically AnewscRNA-seqHSPClabelingframeworkenabledfurthercomparisonswithagedmurineandhuman HSCdatasets,documentinganassociationbetweenHSC-intrinsicNFκBactivityandquiescence,butnotmyeloidbias.Thesefindingsdelineate separateregulatorymechanismsthatunderliethethreehallmarksofhematopoieticaging,suggestingthattheyarespecificallyand independentlytherapeuticallytargetable.
ViraldeliveryofanRNA-guidedgenomeeditorfortransgene-freegermlineeditinginArabidopsis
DiegoSahagun
UCLA–CIRMCOMPASSScholar
LabPI:SteveJacobsen,PhD
GenomeeditingistransformingplantbiologybyenablingpreciseDNAmodifications However,deliveryofeditingsystemsintoplantsremains challenging,oftenrequiringslow,genotype-specificmethodssuchastissuecultureortransformation Plantviruses,whichnaturallyinfectand spreadtomosttissues,presentapromisingdeliverysystemforeditingreagents.Butmostviruseshavelimitedcargocapacities,restricting theirabilitytocarrylargeCRISPR-Cassystems Here,weengineeredtobaccorattlevirus(TRV)tocarrythecompactRNA-guidedTnpBenzyme ISYmu1anditsguideRNA Thisinnovationallowedtransgene-freeeditingofArabidopsisthalianainasinglestep,witheditsinheritedinthe subsequentgeneration Byovercomingtraditionalreagentdeliverybarriers,thisapproachoffersanovelplatformforgenomeediting,which cangreatlyaccelerateplantbiotechnologyandbasicresearch
ElucidatingtheRegulationofaCriticalComponentoftheSWI/SNFChromatinRemodelingComplex KalenBunch
UCLA–CIRMCOMPASSScholar
LabPI:TracyL.Johnson,Ph.D.
Stemcellsrequiretightregulationofgeneexpressionprogramstomaintainpotencyanddifferentiateattherighttime TheSWI/SNFchromatin remodelingcomplexplaysacriticalroleinthisprocessbyregulatinggenesrequiredforgrowthanddifferentiation Thiscomplex’sabilityto integratesignalsandcontrolgeneexpressionisconservedfrommammalstoyeast WhilethemechanismsofSWI/SNF-dependentgene regulationarebeingstudied,littleisknownabouthowtheSWI/SNFcomplexisitselfregulated.Here,weelucidatethemechanismsbywhich Snf2,thecatalyticcomponentoftheSWI/SNFcomplex,isregulatedinthebiochemicallytractableorganismSaccharomycescerevisiae ThroughrandomamplificationofcDNAends(RACE)wehavefoundtwodistinctSNF2RNAisoformswiththelongisoformcontaining upstreamopenreadingframes(uORFs) Thetranscriptionoftheseisoformsisinfluencedbyglucoseavailability ToassesstheeffectsofSnf2 RNAisoformsonproteinexpressionwecreatedaSNF25’UTR-Ub-GFPreportersystemthatallowsforinductionoflongisoform-transcription ExperimentswiththissystemcorroborateamodelwherebySNF2’stwoRNAisoformshavedivergingeffectsonSnf2proteinexpression
AProposedMurineModeltoStudytheTumorMicroenvironmentInteractionofGBMandMicroglia AmirFilabs
UCLA–CSUNCIRMBridgesTrainee
LabPI:HarleyKornblum,MD,PhD
Glioblastoma(GBM)isahighlyaggressivebraincancerthatischaracterizedbyacomplextumormicroenvironment(TME)thatsuppressesthe immunesystemandreprogramsimmunecellsintoanimmunosuppressivephenotype,allowingforunregulatedcellularproliferation Previous in-vitrostudiessuchasconditionedmediaexperimentshaveshownthatmicroglia,theresidentmacrophagesinthebrain,arepolarizedtoan anti-inflammatory,immunosuppressivephenotypeintheGBMTMEtherebypermittingtumorgrowth Wehypothesizethatthiseffectispartly duetocytokinesecretionfromnearbytumorendothelialcells’(TECs)withintheTME.Ourstudyaimstoinvestigatethemechanismsunderlying thisTEC-microgliainteractionusingahumanizedmicroglia-mousemodel Wewillco-implanthumanmicroglia,tumorendothelialcellsand patient-derivedGBMcellsintomousebrains,creatingaphysiologicallyrelevantmodeloftheGBMTME QuantitativePCRdatafromin-vitro conditionedmediaexperimentsconfirmedtheexpressionofanti-inflammatorymarkersandidentifiedkeysignalingpathwaysinvolvedinthis process,includingJAK/STATandTGF-βsignaling Therefore,wehypothesizethattherewillbesimilarresultsfromourinvivoexperiments UsingsinglecellRNAseq,wewillinvestigatethetumor-microgliaandtumor-ECsinteractionusingUMAPplots,geneexpression,andCellChat. OurfindingsthusfarhighlightthepotentialfortargetingTEC-derivedsignalstorestoremicroglialanti-tumoractivity,offeringanovel therapeuticapproachforimprovingGBMtreatmentoutcomes.ByunderstandingthemechanismsofTEC-microgliainteractions,ourwork contributestothebroaderunderstandingoftheTMEanditsimpactontumorprogressionandtherapyresistance
OvercomingGlioblastomaTherapyResistancebyInterferingwithintercellularvascular-to-cancercrosstalk
ShaneYu
LabPI:HarleyKornblum,MD,PhD
Glioblastoma(GBM),anaggressiveformofbraincancer,ischaracterizedbyextensiveneovascularization Inadditiontosupplyingbloodand nutrients,vascularendothelial(VE)cellsprovidetrophicsupporttoGBMcellsviaparacrinesignaling,theprecisemechanismsofwhichare beingunraveled.OurlabhasrecentlyshownthatoneofthestandardtreatmentsforGBM,radiation,inducedaphenotypicconversionofGSCs tovascular-likecells,whichinturn,providetrophicsupporttotheremainingtumor Inourcurrentstudy,usingpatient-derivedGBMandVEcells aswellasorthotopicGBMmousemodels,wedemonstratedthatEndocan(ESM1),anendothelial-secretedproteoglycan,promotesmalignancy andradioresistanceinGBM UsingMassspectrometryanalysis,weidentifiedPDGFRA,acommonlydysregulatedReceptorTyrosineKinase (RTK)inGBM,asapotentialreceptorforEndocan BiacoreexperimentconfirmedthatEndocanbindstoPDGFRAwithnanomolaraffinity Consistentwiththisresult,wedemonstratedthatEndocanactivatesthePDGFRApathway Subsequentdownstreamsignalingincreases chromatinaccessibilityoftheMycpromoterandupregulatesMycexpressioninducinghighlystablephenotypicchangesinGBMcells InhibitionofEndocan-PDGFRAsignalingwithponatinib,aPDGFRAinhibitorincreasessurvivalintheEsm1wild-typebutnotintheEsm1knockoutmouseGBMmodel IdentificationoftheEndocan/PDGFRA/MycaxisdemonstratesanimportantroleofVEcellsinGBMmalignancywhile targetingthispathwaymightsubduetherecurrenceofGBMandfurtherhighlighttheimportanceofvasculartotumorcellsignalingforGBM biology
GabrielleAnneGo
LabPI:HarleyKornblum,M.D,Ph.D.
Histonedeacetylases(HDACs)arealargefamilyofenzymesthatdiminishacetylationofhistonesandotherproteins HDACinhibitionhas beeninvestigatedasapotentialtreatmenttoenhanceneuralrepair,however,broadspectrumHDACinhibitorshavesignificanttoxicities.In priorstudiesofrodentmodels,HDAC6inhibitionincreasedneuriteoutgrowthandsurvival However,thepotentialrolefortargetingHDAC6in humanneuralrepairisstillunknown TreatmentofNGN2-inducedhumanneuronswitheitherthebroadspectrumHDACinhibitorbelinostator therelativelyselectiveHDAC6inhibitortubastatinAresultedinenhancedneuriteoutgrowth However,whiletubastatinAtreatmentresultedin elevatedlevelsofacetylatedα-tubulin,adirecttargetofHDAC6,belinostattreatmenthadnoeffectontubulinacetylation,suggestingthatthe effectofbelinostatonneuriteoutgrowthmaybeindependentofHDAC6 TodirectlyassesstheroleofHDAC6inneuritegrowth,weknocked outHDAC6inhumanESCsusingCRISPR/Cas9 NGN2-inducedneuronswerederivedfromtheseESCs LossofHDAC6enhancedneurite outgrowthandacetylatedα-tubulinintheinducedneurons,confirmingtheroleofHDAC6inneuritegrowth.Thesestudiessupportaroleforthe studyofselectiveHDAC6inhibitorsinneuralrepair
AutismandaCompletePTENKnockout
SofiaIrvin
LabPI:HarleyKornblum,MD,PhD
HeterozygousgermlinemutationsinPTENareassociatedwithautism(ASD)andmacrocephaly WhilehomozygousdeletionofPteninmurine neuralstemcellsandtheirprogenyisalsoassociatedwithenlargedbrains,therearefewphenotypicchangesinmicewithheterozygousPten deletions Thisraisesthepossibilitythathumandiseasecausingmutationsplayadominantnegativefunction,effectivelyeliminatingPTEN functionality Totestthishypothesis,weutilizediPSCsderivedfromapatientcarryingaheterozygouspH93YmutationinPTENandwho exhibitedmacrocephalyandASDwithisogenicCRISPR-correctedcontrollinesandthesamelinesinwhichPTENwasfullydeletedusing CRISPR-Cas9 WemeasuredactivationoftheAKT-mTORpathway,thegrowthofcerebralorganoidsandneuriteoutgrowthinorganoid-derived andNGN2-inducedneurons Whilefulldeletionresultedinenhancedorganoidgrowthandneuriteoutgrowthininducedandorganoid-derived neurons,therewasverylittleeffectoftheheterozygouspatientmutationonorganoidgrowthcomparedtotheCRISPRcorrectedline,andno cleareffectonneuriteoutgrowth.TreatmentwiththeselectivemTORinhibitor,rapamycin,inhibitedneuriteoutgrowthinallconditions, suggestingthatsomeleveloftonicmTORactivationispresentinallconditionsandregulateshumanneuriteoutgrowth Surprisingly,no conditionresultedinelevatedlevelsofpS6,whileonlythecompletePTENknockoutsresultedinelevatedpAKT,suggestingthatchronic inhibitionofPTENfunctionresultsinactivationofmTORC2 Thesefindingssuggestthatthemutationstudieddoesnotresultinafull disruptionofPTENfunction,indicatingthatenvironmentalorotherfactorsmayplayaroleininactivatingtheWTalleleinthispatient Future experimentswilldeterminewhetherthesameholdsforothermutationsassociatedwithASDandmacrocephaly
Quinn Mortensen
UCLA – CSUN CIRM Bridges Trainee
Lab PI: Siavash K Kurdistani, M D
Small peptides, or microproteins, are being increasingly identified, but their roles in cellular biology remain mostly unknown Our laboratory has discovered a novel 29-amino acid peptide, provisionally named CBP-29, that has not been previously characterized This peptide is encoded in the ribosomal DNA (rDNA) locus of yeast within the non-transcribed spacer 2 (NTS2) region While NTS2 was initially believed not to be transcribed, it is now known that NTS2 generates several mRNAs with unknown functions. Two proteins, the Sir2 lysine deacetylase, and the Set1 lysine methyltransferase, prevent high levels of transcription of NTS2 in rich media. Still, our preliminary data indicate that they do not affect the expression levels of CBP-29 My project focuses on characterizing the cellular impact of the loss of CBP-29 to delineate its function I have found that loss of CBP-29 may increase cellular resistance to starvation and may be involved in Zn homeostasis
BinaryColloidalCrystalsInfluencetheDirectConversionofFibroblastsintoNeurons
JohnathanHorn
UCLA–CIRMCOMPASSScholar
LabPI:SongLi,PhD
Directreprogrammingistheprocessofconvertingfromonecelltypeintoaverydistantlyrelatedcelltypewithouttheuseofanintermediate proliferativestem-cell-likestage Studieshaveshownthatsomaticcellscanbedirectlyreprogrammedintoneuronalsubtypesusingdifferent combinationsoftranscriptionfactorsandmicroRNAs.Biochemicalfactorshavealsobeenshowntohelpreprogramcellsintoinduced neuronal(iN)cells Unlikebiochemicalfactors,theroleofbiophysicalfactorsduringdirectreprogrammingislimited Somebiophysicalfactors havebeenshowntoregulatevaryingcellularbehaviors,andtheuseofbinarycolloidalcrystals(BCCs)havebeenusedascellsubstratesfor celldifferentiation Althoughtherolesoftranscriptionfactorsandsolublebiochemicalfactorsinreprogramminghavebeenwidelystudied, howbiophysicalcuesprovidedbyBCCsregulateiNreprogrammingisnotwellunderstood HereweshowthataspecificBCCsubstrate,ie BCC6(2µmSi/70nmPS),canenhanceiNreprogrammingafterscreeningsevencombinationsofBCCs Wefoundthatincreasingthesizeof thepolystyreneparticlesusedintheBCCsurfacesfrom100nmto400nmimprovediNreprogrammingefficiencyandpurity Ontheother hand,decreasingthesizeofsilicaparticles(5µmto2µm)increasediNcellyieldandpurity.Withregardstosurfacechemistry,wefoundthat carboxylatedpolystyreneparticlesdecreasedboththereprogrammingefficiencyandiNpurityby14-fold Findingsfromthisstudyaimto broadentheunderstandingofcell-matrixinteractions'influenceonthefibroblast-to-neuronreprogrammingprocess
YifanWu
LabPI:SongLi,PhD
Extracellularmatricesoflivingtissuesexhibitviscoelasticproperties Althoughseveralstudieshaveinvestigatedthecytoskeletal reorganizationonviscoelasticsubstratestoconnectextracellularmechanicalchangeswithintracellularsignaling,therolesofthecellnucleus asanimportantmechanosensoryunitinresponsetomatrixviscoelasticityislargelyunknown.Decipheringwhetherandhowmatrix viscoelasticityaffectsthecellnucleustomodulatecellfate,especiallychromatinandtheepigenome,willshedlightsonthedevelopmentof smartmaterialstocontrolcellphenotypeconversion.Toachievethis,weengineeredthestiffnessandviscoelasticityofcell-adhesive substrates,andinvestigatedthegenome-widebiophysicalandbiochemicalchangesgloballyandatspecificsites Wefoundthatviscoelastic substratesinducenuclearstructuralreorganizationandepigeneticchanges,withmorepronouncedeffectsonsoftersurfaces Fibroblastson viscoelasticsubstratesdisplayedlargernuclei,looselyorganizedchromatin,anddifferentialexpressionofdistinctsetsofgenesrelatedtothe cytoskeletonandnuclearfunctioncomparedtothoseonpurelyelasticsurfaces Slow-relaxingviscoelasticsubstratesreducedlaminA/C expressionandenhancednuclearremodeling.Thesestructuralchangeswereaccompaniedbyaglobalincreaseineuchromatinmarksand localincreaseinchromatinaccessibilityatcis-regulatoryelementsassociatedwithneuronalandpluripotentgenes Consequently,viscoelastic substratesimprovedthereprogrammingefficiencyfromfibroblastsintoneuronsandinducedpluripotentstemcells.Collectively,ourfindings unraveltherolesofmatrixviscoelasticityinepigeneticregulationandcellreprogramming,potentiallyleadingtoanewtechnologyplatformto engineercellfatefortissueregeneration,diseasemodeling,anddrugscreening
YouchengYang
LabPI:SongLi,PhD
RecentadvancementsinTCR-TtherapyhaveemphasizedtheimportanceofeffectiveTcellactivationfortherapeuticapplications However, currentmethodsforTcellactivationandexpansion,suchasantibody-coatedparamagneticpolystyrenebeads(eg,Dynabeads),oftenresultin suboptimalcellactivation.OnereasonisthatDynabeadsarestiffandhaveaverydifferentmechanicalpropertyincomparisontoantigenpresentingcells(APCs)whicharesofterandhavetheviscoelasticpropertythatiscriticaltomediateligand-receptorengagementand activation.Toaddressthis,weexploredtheuseoftunableviscoelasticsyntheticcellsasanovelplatformforactivatingantigen-specificT cells Thesesyntheticcells,designedtomimicthemechanicalpropertiesofantigen-presentingcells,provideacustomizable microenvironmentthatenhancesTcellreceptor(TCR)engagementandsubsequentactivation Weemployedaclick-chemistrybasedmethod toconjugateanti-CD28andMHC(HLA-A2-NY-ESO1)onalginatemicrobeadstofabricatethesesyntheticcells Theycanhavethesame stiffnessbutvaryingdegreesofviscoelasticity,whicharefast-relaxingbeads(FRB),medium-relaxingbeads(MRB),andslow-relaxingbeads (SRB).OurexperimentsdemonstratedthatTcellsculturedwiththesebeadsexhibitincreasedproliferationandcytokineproductioncompared tocell-basedactivationmethods Moreover,thedifferenceintheviscoelasticityofthebeadsallowsforthechangeofTcellactivationproximal signalingandmetabolism,whichmaycontributetothebetterinvivotherapeuticeffect.Thisinnovativeapproachholdspromiseforimproving thedesignofTCR-Tbasedtherapies,offeringaversatiletoolforimmunologicalresearchandclinicalapplications
ExploringDNADamage-InducedSenescencePathwayswithinCDKL5DeficiencyDisorder,DownSyndrome, Bohring-OpitzSyndrome,andArboleda-ThamSyndromeneuronalcellmodels
LeslieRodriguez
UCLA–CSUNCIRMBridgesTrainee
LabPI:WilliamLowry,PhD
DownSyndrome,Arboleda-ThamSyndrome,Bohring-OpitzSyndrome,andCDKL5DeficiencyDisorderareallneurologicaldiseasescausedbya singlegeneticmutation ThisresearchaimedtodeterminewhetherthesamesenescencepathwayscontributedbyelevatedDNAdamage observedinRettSyndromearealsopresentinotherintellectualdiseasesincludingDownSyndrome,Arboleda-ThamSyndrome,Bohring-Opitz Syndrome,andCDKL5DeficiencyDisorder UsingsimilarmolecularprofilingtechniquesasintheRettSyndromemodel,theresearchshown heretakesadvantageofthediseaseinadishmodeltogrowpatient-derivedinducedpluripotentstemcells(iPSCs)intoneuronstomimicthese threeneurodevelopmentaldisorders.Althoughthenumberofaffectedgroupsishigh,molecularramificationsofthesediseaseshaveyettobe investigated Throughoutthisprocess,weconductedaBeta-GalactosidaseAssay,CometAssay,andaseriesofImmunofluorescentstainingto deducewhethertheneuralprogenitorcellsorneuronalcellsweresenescentandwhethertheyhadahigherincidenceofDNAdamage/breaks.
ThepreliminaryresultssuggestelevatedsenescencepatternsandDNAdamagemarkersexpressioninsomelinesbutnotothers TheDown SyndromecellslineshadanincreaseincellularsenescenceandDNAdamage.Arboleda-ThanSyndromeandBohring-OpitzSyndromelines showcompetingresultsthatdonotfullysupportsenescenceandDNAdamageasacomponentforthedisease CDKL5deficiencydisorder linesalsoshowedcrossingresultsbetweeneachDNAdamagemarkerstainsbutpreliminarysenescencestainingrevealedhigherincidenceof senescenceinmutantlines Thishelpeduscharacterizekeycellularstressphenotypesforeachdisease Ifaconnectionbetweenthese molecularmechanismsismade,wecanidentifypossibletreatmentsforthecellstofurthertheresearchquestionandpossiblyprovidean avenuefortreatmentoptionsforpatients
MatthewHeffel
LabPI:ChongyuanLuo,Ph.D.
Thehumanhippocampusandprefrontalcortexplaycriticalrolesinlearningandcognition,yetthedynamicmolecularcharacteristicsoftheir developmentremainenigmatic Hereweinvestigatedtheepigenomicandthree-dimensionalchromatinconformationalreorganizationduring thedevelopmentofthehippocampusandprefrontalcortex,usingmorethan53,000jointsingle-nucleusprofilesofchromatinconformation andDNAmethylationgeneratedbysingle-nucleusmethyl-3Csequencing(snm3C-seq3).TheremodellingofDNAmethylationistemporally separatedfromchromatinconformationdynamics Usingsingle-cellprofilingandmultimodalsingle-moleculeimagingapproaches,wehave foundthatshort-rangechromatininteractionsareenrichedinneurons,whereaslong-rangeinteractionsareenrichedinglialcellsandnon-brain tissues Wereconstructedtheregulatoryprogramsofcell-typedevelopmentanddifferentiationfromearlyradialgliastem-likeprogenitorsto fullydevelopedadultneuronsandglialsupportcells,findingputativelycausalcommonvariantsforschizophreniastronglyoverlappingwith chromatinloop-connected,cell-type-specificregulatoryregions Ourdataprovidemultimodalresourcesforstudyinggeneregulatorydynamics inbraindevelopmentanddemonstratethatsingle-cellthree-dimensionalmulti-omicsisapowerfulapproachfordissectingneuropsychiatric riskloci.
DecipheringthefunctionsofMLLT3isoformsinhematopoieticandleukemiastemcellfatedecisions
YuanyuanWang,PhD
LabPI:HannaK.A.Mikkola,M.D.,Ph.D.
Leukemiastemcells(LSCs)andnormalhematopoieticstemcells(HSCs)sharetheuniqueabilitytoself-renew,makingitessentialto distinguishtheircommonmechanismsfromLSC-specificpathwaystodeveloptargetedleukemiatherapies.OurlabidentifiedtwoMLLT3 isoforms MLLT3-L(long)andMLLT3-S(short) askey,yetopposing,regulatorsofhumanHSCstemness MLLT3-Lpromotesstemness,while MLLT3-SservesasapotentialinhibitorbysequesteringkeyproteinpartnersofMLLT3-L,suchasDOT1Landthesuperelongationcomplex (SEC) MLLT3isalsoinvolvedinvariousleukemiasandfrequentlyservesasafusionpartnerwithMLL However,littleisknownaboutthe functionofMLLT3isoformsininitiatingormaintainingLSCs
Ourbulk,long-read,andsingle-cellRNA-SeqdatarevealedanimbalanceofMLLT3isoformsinaggressiveleukemias,includingMLL-MLLT3and MECOM-rearrangedleukemias,characterizedbyreducedorabsentexpressionofMLLT3-S InHSCs,MLLT3-LandMLLT3-SmodulateMECOM expressionviaDOT1L-dependentH3K79me2modification,whereasinMECOM-rearrangedLSCs,full-lengthMLLT3fuelsMECOM overexpression,whileMLLT3-Sisabsent ChIP-seqdatashowthatwhileMLLT3bindsthetranscriptionstartsite(TSS)ofbothlongandshort MECOMisoformsinHSCs,itselectivelybindstheleukemogenicshortMECOM(EVI1)isoforminLSCs Furthermore,ChIP-seqidentifiedafeedforwardregulatoryloopinwhichEVI1bindstheTSSofMLLT3-L,reinforcingthestemnessprograminMECOMLSCs
WearenowdefiningthefunctionalroleofMLLT3isoformsinLSCfateusinglentiviralknockdownandoverexpression,andwilltestwhether MLLT3-ScandisruptMLLT3-LandMLL-MLLT3complexesinLSCs ThesefindingswillprovidemechanisticinsightsintoLSCregulationand suggestnoveltherapeuticstrategies
Meng-WeiKo,PhD
LabPI:HannaKA Mikkola,MD,PhD
Hematopoieticstemcells(HSC)transplantationisacurativetreatmentforvariousblooddisorders However,manypatientswhocouldbenefit fromthistreatmenthaveasignificantchallengeoffindingamatchingdonor,highlightingtheneedforalternativesthatcouldmakethislifesavingtherapyavailabletoallpatients Tomitigatethehurdle,ourlong-termgoalhasbeentogenerateHSCsinthelaboratoryfrompluripotent stemcells(PSC).ThemostadvancedprotocolstodategeneratePSC-HSCthatcanbetransplanted,butwithlowefficiency,whichstemsfrom theirdevelopmentalimmaturity ThisposesasignificantchallengetotheclinicalapplicabilityofPSC-HSCs,astheircapacitytoengraftand producebloodcellsovertimeislimited.Toaddressthis,ourlaboratorycreatedadetailedmapofhumanHSCdevelopmentbyemploying single-cellRNAsequencinganalysis ThismapprovidesacomprehensiveframeworkforunderstandingthevariousstagesofhumanHSC developmentatthemolecularlevel Whenreferencingtothisdevelopmentalmap,wefoundthatPSC-HSCcloselyresembletheimmatureHSC generatedearlyindevelopment AlthoughthesePSC-HSCrepresentanimportantstepforward,theylackcriticalfeaturesofdevelopmentally matureHSC,whichlikelycontributestotheirimpairedfunctionintransplantationsettings TopromotethematurationofPSC-HSCs,we optimizedatwo-stepculturesystemcombiningPSC-HSCgenerationwithourestablishedHSCexpansionprotocols.Interestingly,a subpopulationofPSC-HSCsgraduallyacquiredhallmarksofmorematureHSCinculture WewillnowevaluateifthesemorematurePSC-HSCs showimprovedengraftmentandbloodcellproductioncomparedtoearly-stagePSC-HSCs.IfwecanenhancePSCHSCtransplantation efficacy,thisapproachcouldprovideamoreaccessibletreatmentforpatientswithbloodcancersandhematologicaldisorders
StephanieFrenz-Wiessner,PhD
LabPI:HannaKA
Mikkola,MD,PhD
InfantswithDownsyndrome(DS)havea150-foldincreasedriskofdevelopingmyeloidleukemia(ML-DS)withinthefirstyearsoflife,attributed toaberranthematopoieticstem/progenitorcelldevelopment ML-DShasafavorableprognosisatdiagnosisbutisassociatedwithsignificant treatment-relatedtoxicitiesandpooroutcomesafterrelapse,emphasizingtheneedfortargetedtherapiesandmolecularriskstratification MLDSisprecededbytransientabnormalmyelopoiesis(TAM),apre-leukemicconditionarisinginuteroduetoGATA1mutationsthatresultinthe truncatedproteinGATA1s Thisdisruptserythroidandmegakaryocyticdifferentiation,leadingtoanaccumulationofimmatureprogenitors AlthoughTAMresolvesinmostinfantsby3months,itislinkedtohighmortality,and20%ofcasesprogresstoML-DSwithouteffective preventionstrategies
However,thedevelopmentaloriginandleukemicevolutionofTAMandML-DSatthemolecularlevelisnotwellunderstood Wecreatedacomprehensivesingle-cellmapofdiseaseorigin,progression,treatmentresponse,andrelapse,byperforming5′single-cellRNA sequencingoflongitudinalsamplesfrom5TAMand14ML-DSpatients,including4relapses,aswellas16and20-weekfetalliverandbone marrowsamplesfromTrisomy21(T21)andeuploidtissues
TotraceML-DSevolutionanddevelopmentaloriginwedefinedstage-specificmolecularsignaturesandidentifiedaTAMprecursorcell populationexpandedinT21fetalliver WeassessedGATA1transcriptsatthesingle-celllevelanddetectedGATA1swithexon2skippingthat enablesclonaltrackingandleukemiastemcell(LSC)characterizationacrossdiseasestages.Flowcytometryvalidatednovelblastsurface markersassociatedwithstemnessprograms BytracingleukemiaevolutionfromafetalcelloforiginthroughTAM,ML-DS,andrelapse,our studybridgesbasicresearchandclinicalapplication,offeringinsightsintootherinfantleukemiasoriginatinginutero.
Isiah Estrada
UCLA – CSUN CIRM Bridges Trainee
Lab PI: Bennett Novitch, Ph D
Cerebral organoids created from human induced pluripotent stem cells (hiPSC) have emerged as one of the best experimental platforms for recapitulating features of brain development in vivo and pathologies associated with neurological disease In our study, cerebral organoids are used to model FOXP1 syndrome, a neurodevelopmental disorder associated with developmental delays in language milestones, epilepsy, and high incidence of autism spectrum disorder. A crucial aspect to cerebral brain organoid development is the manipulation of small molecule inhibitors, as they direct stem cell differentiation by manipulating developmental signaling pathways Different concentrations of TGF- β, Wnt, and Ras/Raf/MAP Kinase pathway inhibitors were tested to establish which combinations could best yield the formation of well-structured organoids suitable for modeling the impact of FOXP1 gene loss on cortical development Our preliminary findings show that the combined inhibition of TGF- β and Wnt pathways resulted in a large number of undifferentiated cells and excessive cell death By contrast, Ras/Raf/MAP Kinase pathway inhibition alone increased the overall formation of neuroepithelial progenitors, though lower than expected numbers of neural rosette structures as visualized through immunohistochemistry. In our ongoing and future experiments, we aim to further optimize the organoid differentiation conditions by modifying the concentration and combinations of these and other signaling pathway modulators. In the future, we will seek to create WT and FOXP1-deficient hippocampal and ganglionic eminence organoids and use these to form assembloids in which functional neural circuits can be constructed to enable us to examine the impact of FOXP1 deficiency on neural network activities These findings will provide a foundation to learn more about the underlying causes of FOXP1 syndrome and potentially therapeutic interventions to improve the well-being of patients with this disorder
ModelingRettSyndromewithPatientDerivedBrainOrganoids
NatellaBaliaouri
BSCRC–RoseHillsFoundationPredoctoralTrainee
LabPI:BennettNovitch,PhD
Rettsyndrome(RTT)isanX-linkedneurodevelopmentaldisorderprimarilycausedbyheterozygousmutationsintheMECP2gene RTTis characterizedbysymptomssuchasdevelopmentaldelays,stereotypedmovements,andseizures DysfunctionalandfunctionalMECP2 expressionismosaicduetotherandomizedpatternofX-chromosomeinactivationinindividualcells,meaningcellsexpresseitherfunctional ordysfunctionalMECP2 Boththeproportionofdysfunctionalcellsandthespecificmutationimpactsymptomseverity Priororganoidmodels usedRTT-patientderivedinducedpluripotentstemcellstocreateorganoidswithhomogenouscellpopulations Wehavecreatedandvalidated amosaicorganoidmodelforRTTtobettermodelthedisorderandexaminethecellautonomousandnon-cellautonomouseffectsofMECP2 dysfunction Ourmosaicmodelsdemonstratedistinctelectrophysiologicalphenotypesrelativetothefullymutantandcontrolmodels MECP2 dysfunctionappearstoimpactneuralnetworkdevelopmentbyaffectingtheearlyneurodevelopmentaltimelineofmutantcellsrelativeto controlcellsinmosaicorganoids,whichlikelycontributestotheelectrophysiologicaldifferencesinmorematureorganoids Weplantofurther analyzethedifferencesincellswithfunctionalMECP2infullycontrolorganoidsascomparedtomosaicorganoidstomodelthenon-cell autonomouseffectsofcellswithdysfunctionalMECP2 Incorporatingcellularmosaicismintheorganoidmodelrecapitulatesthecellular compositionofthebrainoffemaleRTTpatientsmoreaccuratelythananypreviousinvitromodel Additionally,mosaicorganoidsmayhave broaderapplicabilitytootherdisordersinwhichmosaicismisanotablefeature Mosaicorganoidstudieswillhelpprovidenewinsightsinto someofthekeydownstreameffectorsofMECP2bothintercellularlyandwithinthecell
Maria Caballero
UCLA – CIRM COMPASS Scholar
Lab PI: Bennett Novitch, Ph D
Human induced pluripotent stem cell (hiPSC)-derived brain organoids are increasingly becoming a reliable model to investigate neurodevelopmental disorders such as Rett syndrome, which is caused by heterozygous mutations in the MECP2 gene located in the X chromosome In females, variability in phenotypes and symptom severity can result from random patterns of X-chromosome inactivation that lead to mosaic expression of wild-type and mutant MECP2 alleles in patient brains. To recapitulate this feature of Rett syndrome, we have successfully produced mosaic organoids by intermixing isogenic wild-type control and MECP2 mutant cells, which result in structures that exhibit abnormal neural network activities To further refine our investigation, we assessed alterations in neurogenesis across wild-type, mutant, and a range of mosaic organoids by quantifying the expression of key markers reflecting distinct populations and developmental states of cells in the developing cortex Our analysis reveals some significant shifts in the cellular composition across our mosaic models, providing insights into how MECP2 mosaicism might influence early cortical development in patients’ brains This approach provides a relevant model for the study of the developmental basis of Rett syndrome, deepening our physiological understanding of the disorder
GeorgeRadionov
UCLA–CSUNCIRMBridgesTrainee
LabPI:AprilPyle,Ph.D.
Volumetricmuscleloss(VML)ischaracterizedbyasignificantlossofskeletalmuscle,resultinginpermanentimpairmentduetothe overwhelmingdemandforrepair Withnostandardtreatmentavailable,breakthroughsinstemcelltherapieshavesparkedinterestinusingin vitro-generatedmusclestemcells,calledsatellitecells,fromhumanpluripotentstemcells(hPSCs)tocombatmusclelossandenhancerepair However,currentinvitroprotocolsforgeneratingskeletalmuscleprogenitorcells(SMPCs)arelimitedtomodelingembryonicstagesof development Thesecellsexhibitlownicheoccupancyandlimitedregenerativecapacitywhenengraftedinvivo Totacklethesechallenges, thePyleLabhascreatedaprotocolallowingfortheco-differentiationofhiPSCsintovascularizedmusclefibersinnervatedbymotorneurons TheintegrationofendothelialcellsincreasesSMPCproliferationandfusioninadditiontoenhancingPAX7+cellmaturity Althoughthisnew protocolproducesfetalmuscleMYH8fibersandSMPCs,resultshaveshownlimitationsinreachingfulladultmaturity Wehypothesizethat integratingthismodelina3Denvironmentwillprovideamoreaccuratesettingforthecapillarizationofskeletalmuscleandtheformationof adultmuscleMYH2fibersandsatellitecellniches Toachievethis,wecreateda3Dfibrinogen-thrombin-matrigelhydrogeltoobtainlong-term structuralintegrityandcentralizedsuspensionofthecellsduringdifferentiation,thencomparedtoits2Dcounterpart.Geneexpression analysiswasthenoptimizedtoextractRNAfromthecellscultivatedwithintheextracellularmatrix(ECM)hydrogelatdays5and14 Our preliminaryresultsshowedhighexpressionofmyogenicandendothelialmarkersafter14days Byintegratingstage-specificECMas discoveredbythePyleLabinvivo,intothe3Dmodel,weaimtogeneratemorematureSMPCswithenhancedregenerativecapabilitiesfor cellulartherapeuticstotreatconditionslikeVML
Enhancingskeletalmuscleprogenitorcellmaturityusingendothelialcellstotreatvolumetricmuscleloss
KilianMazaleyrat,PhD
BSCRC–CIRMPostdoctoralTrainee
LabPI:AprilPyle,PhD
Volumetricmuscleloss(VML)injuriesoccurduetoorthopedictraumaorthesurgicalremovalofskeletalmuscle(SkM)andresultsin irreversibledamageinmusclestructureandfunctionduetothelossofasignificantportionofSkM CurrentclinicaloptionstotreatVMLare inefficient,asadvancesintissueengineeredSkMofferapotentialsolutiontoaddresslostordamagedmuscletissuebutarelimitedbydonor availabilityandissuesassociatedwithimmuneincompatibility Theuseofhumaninducedpluripotentstemcells(hiPSCs)derivedSkMis extremelypromisingforthetreatmentofVMLduetotheirabilitytogeneratepersonalizedmusclecellsinadish However,thePylelabhas shownthatcurrentlyavailableprotocolsforgeneratinghiPSCmuscleprogenitors(SMPCs)arelimitedtomodelingtheembryonicstageof development Wehypothesizedthatintroducingendothelialcellsinourestablishedprotocol,allowingtheco-differentiationofhPSCsinto innervatedmusclefibers,wouldenhancetheproliferationandthematurityoftheSMPCs Tooptimizeourmodel,weinducedthecodifferentiationofthelateralmesodermalongtheparaxialmesodermandtheneuroectodermandtheninducedthedifferentiationand proliferationofendothelialcells After60daysofdifferentiation,asshownbyimmunofluorescence,weobtainedvascularizedmillimeterlong fetalmusclebundlesinnervatedbymotorneuronscapableofrepeatedcontractionsandsurroundedbyahighnumberofSMPCs Our snRNAseqanalysis,aswellasthefusionindexofthesortedSMPCs,suggeststhatthepresenceofendothelialcellsincreasestheproliferation andenhancethematurityofourSMPCsaligningwithourdataoffetalinvivoSMPCs.ThePylelabhasshownthatfetalSMPCscanbetter regenerateskeletalmusclecomparedtoembryonicSMPCsandourongoingstudiesareevaluatingSMPCsandendothelialcellsinmouse modelsofVMLforfutureuseinacell-basedtherapyfortreatmentofVMLinjuries
PhenotypicvariationsintheretinalpigmentepithelialcellsofpatientswithdiverseABCA4mutations ArpitaDave,PhD LabPI:RoxanaA.Radu,M.D.
RecessiveStargardtdisease(STGD1)isthemostprevalentinheritedretinaldegenerativedisordercharacterizedbyprogressivecentraland colorvisionlossandiscurrentlyuntreatable MutationsintheABCA4genearetheleadingcauseofSTGD1 Withover2,000knownABCA4 variantsassociatedwithSTGD1,patientsexhibitdiversediseaseseverityandprogressionrates,creatingchallengesinunderstandingthe precisepathophysiologicalmechanisms.WhileABCA4knockoutmousemodelshaveprovidedvaluableinsightsintoneuralretinaandretinal pigmentepithelium(RPE)dysfunction,thetranslationalrelevanceofthesefindingstohumandiseaseprogressionremainsunclear Tobridge thisgap,wedevelopedinducedpluripotentstemcell-derivedRPE(iPSC-RPE)cellsfromskinbiopsiesofthreeSTGD1patientswithdistinct ABCA4mutations Ourpatient-derivediPSC-RPEcellsdemonstratedkeypathologicalfeaturespreviouslyobservedinmousemodels,including elevatedlysosomalpH,reducedCathepsinDactivity,andimpairedphagocytosis Notably,weobservedvaryingdegreesofα-synuclein accumulation,lipidaggregation,andmitochondrialmetabolicdysfunctionacrossdifferentpatientsamples,correlatingwiththeirunique ABCA4mutations ThesefindingsvalidateandextendourunderstandingofSTGD1pathophysiologyinhumancells,establishingarobust ""diseaseinadish""modelthatrecapitulatespatient-specificmolecularsignaturesandprovidesaplatformtoevaluatenewtherapeutic approachesforABCA4-mediatedretinopathies
CorticalVersusHippocampalNetworkDysfunctioninaHumanBrainAssembloidModelofEpilepsyandIntellectual Disability
ColinMcCrimmon,MD,PhD
LabPI:RanmalA Samarasinghe,MD,PhD
Neurodevelopmentaldisordersoftenimpairmultiplecognitivedomains Forinstance,ageneticepilepsysyndromemightcauseseizuresdueto corticalhyperexcitabilityandpresentwithmemoryimpairmentsarisingfromhippocampaldysfunction Thisstudyexamineshowasingle disorderdifferentiallyaffectsdistinctbrainregionsbyusinghumanpatientiPSC-derivedcortical-andhippocampal-ganglioniceminence assembloidstomodelDevelopmentalandEpilepticEncephalopathy13(DEE-13),aconditionarisingfromgain-of-functionmutationsinthe SCN8Agene Whilecorticalassembloidsshowednetworkhyperexcitabilityakintoepileptogenictissue,hippocampalassembloidsdidnot,and insteaddisplayednetworkdysregulationpatternssimilartoinvivohippocampalrecordingsfromepilepsypatients.Predictivecomputational modeling,immunohistochemistry,andsingle-nucleusRNAsequencingrevealedchangesinexcitatoryandinhibitoryneuronorganizationthat werespecifictohippocampalassembloids Thesefindingshighlighttheuniqueimpactsofasinglepathogenicvariantacrossbrainregions andestablishhippocampalassembloidsasaplatformforstudyingneurodevelopmentaldisorders
Distinctnucleotidemetabolismfluctuationsdefinepluripotentstemcellfatetransitions
MaryDoan
BSCRC–CIRMPredoctoralTrainee
LabPI:MichaelTeitell,MD,PhD
Changesinmetabolitelevelsandfluxesguidehumanpluripotentstemcell(hPSC)self-renewalanddifferentiation Differentiationbeginswith thelossofpluripotencyandactivationoflineage-specifictranscriptionfactorsforectoderm,endoderm,ormesoderm.Ourrecentfindings revealeachlineagedependsdifferentlyonglutamine(Gln)forfatetransitions Uniquely,inectoderm,adequateacquiredorgeneratedGln levelsprovideanessentialfate-specifyingsignalthatactivatesmTORC1duringtheinitial14hoursofdifferentiation Conversely,Glnlevelsare insufficienttoactivatemTORC1inendodermormesoderm,andforcedactivationintheseconditionsreducesviability Endodermand mesodermprioritizeexogenousGlnforTCAcycleanaplerosisviaGLS1,whileectodermdependslessonthisprocess Beyondenergy,Gln supportsanabolicpathwayslikeproteinanddenovonucleotidebiosynthesis(DNS),withmTORC1-regulatedDNSpromotingcancergrowth. However,theroleofDNSinhPSCfatedecisionsremainsunclear,andwhetherDNSpathwayactivationdownstreamofGln-mTORC1signaling isnecessaryforectodermfatespecificationwarrantsinvestigation.Usingnutrient-balancedmedia,weguidedhPSCsintogermlineagesand profilednucleotidemetabolism WeobservedreducednucleotidelevelsduringthehPSCtransitiontoendoderm,elevatedlevelsinectoderm, andnosignificantchangeinmesoderm Supportingthis,treatmentwithDNSinhibitorsduringtrilineagedifferentiationselectivelyimpacted hPSCsdevelopingintoectoderm Finally,weexaminedDNSinhibitoreffectsduringthefirst14hoursofectodermdifferentiation acritical windowforlineagespecification followedbywashoutuntilcommitmentcompletion EarlyinterventioninDNSpathwaysduringthiswindow impairedidentityswitchinginectodermdifferentiation.Basedontheseresults,weproposearefinedmodelwhereGln-drivennucleotide productioninhPSCsiscrucialforectodermlineagespecification
VahanMartirosian,PhD
BSCRC–CIRMPostdoctoralTrainee
LabPI:MichaelTeitell,MD,PhD
Pluripotentstemcelldifferentiationisacomplexprocesstraditionallyattributedtotranscriptionalchangesspecifictoeachgermlineage However,emergingevidenceunderscoresthepivotalroleofmetabolitesandmetabolicenzymesinguidingdifferentiationtrajectories The subcellularlocalizationofthesemediatorsiscriticaltotheirfunction,withrecentstudieshighlightingtheinvolvementoftricarboxylicacid (TCA)cycleenzymesinnuclearprocesses Whilenuclearproteinshavebeencharacterizedtosomeextent,thespecificcompositionof pluripotentstemcellnuclei,thedynamicchangesinnuclearcomponentsduringdifferentiation,andthequantificationofnuclearmetabolites remainlargelyunexplored Toaddressthesechallenges,wedevelopedanovelmodelsystembasedonpreviousmethodologiestoimprove nuclearisolationfor-omicsanalyses.Humanpluripotentstemcellsweretransfectedwithacustomvectorencodingchimericproteinsthat labelmitochondriaandnucleiwithdistinctepitopetags Immunoprecipitationusingmagneticallylabeledbeadsspecifictoeachtag demonstratedsuccessfulenrichmentofmitochondrialandnuclearproteinsinseparatefractions,validatingthereliabilityofourconstructsfor isolatingtheseorganelles Ournextstepsinvolveleveragingthissystemtomeasurenuclearmetabolitesandquantifynuclearconstituents throughoutdifferentiation Thisinnovativeapproachpromisestorevealnewinsightsintothedynamicsubcellularlocalizationofproteinsand metabolites,advancingourunderstandingofnuclearmetabolicregulationinpluripotentstemcelldifferentiation
PGC-1αdrivessmallcellneuroendocrinecancerprogressiontowardanASCL1-expressingsubtypewithincreased mitochondrialcapacity
GrigorVaruzhanyan,PhD
LabPI:OwenN Witte,MD
Adenocarcinomasfrommultipletissuescanconvergetotreatment-resistantsmallcellneuroendocrine(SCN)cancerscomposedofASCL1, POU2F3,NEUROD1,andYAP1subtypes WeinvestigatedhowmitochondrialmetabolisminfluencesSCNcancer(SCNC)progression Extensive bioinformaticsanalysesencompassingthousandsofpatienttumorsandhumancancercelllinesuncoveredenhancedexpressionof proliferator-activatedreceptorgammacoactivator1-alpha(PGC-1α),apotentregulatorofmitochondrialoxidativephosphorylation(OXPHOS), acrossseveralSCNCs.PGC-1αcorrelatedtightlywithincreasedexpressionofthelineagemarkerAchaete-scutehomolog1,(ASCL1)througha positivefeedbackmechanism Analysesusingahumanprostatetissue-basedSCNtransformationsystemshowedthattheASCL1subtypehas heightenedPGC-1αexpressionandOXPHOSactivity PGC-1αinhibitiondiminishedOXPHOS,reducedSCNCcellproliferation,andblockedSCN prostatetumorformation Conversely,PGC-1αoverexpressionenhancedOXPHOS,validatedbysmall-animalPositronEmissionTomography mitochondrialimaging,tripledtheSCNprostatetumorformationrate,andpromotedcommitmenttotheASCL1lineage Theseresults establishPGC-1αasadriverofSCNCprogressionandsubtypedetermination,highlightingmetabolicvulnerabilitiesinSCNCsacrossdifferent tissues
FunctionallyvalidatingTcellreceptorstargetingprostaticacidphosphataseforprostatecancer
CaitlinGee
LabPI:OwenN
Witte,MD
Prostatecancerremainsoneoftheleadingcausesofcancerdeathsinmales,withrecurrentandmetastaticformsofthediseaseposing significanttherapeuticchallenges Currenttherapeuticsofferlimitedefficacyandareprimarilypalliative,highlightingtheurgentneedfornovel approacheswithcurativeintentandlong-termimmunesurveillance Toaddressthisgap,ourprojectexploresaTcell-basedtherapeutic approach Specifically,weaimtouseadefinedTcellreceptor(TCR)thatactivatesafterbindingtocancer-associatedantigenfragments presentedbymajorhistocompatibilitycomplexes(MHCs)onthecancercellsurface Thisinteractioncaninducetumorcelldeathandpromote immuneresponseagainstprostatecancercells PrioreffortsofTCR-Tcell-mediatedtherapeuticsmainlyfocusonsynovialsarcomaand metastaticmelanoma,leadingtothefirstFDA-approvedTCR-Ttherapy,afamicel,in2024.However,onlylimitedsuccesshasbeenachieved againstepithelialcancerssuchasprostate Ourlabhasbeendedicatedtoisolating,defining,andimprovingTCRswiththepotentialto effectivelytargetprostaticacidphosphatase(PAP),awell-definedprostatecancerantigen HighserumPAPleveloftencorrelateswithworse prognosis,makingitanidealtargetforearlyintervention OurpreviousworkhasthoroughlydefinedthepeptidesofPAPonvariousHLAalleles, socalledimmunopeptidome Inthisproject,weuseisolatedandfinelytunedTCRsspecifictoPAPtofunctionallyvalidateandscreen candidatesviacytokinereleasemeasurementsandcytotoxicityevaluation OurfindingsindicateTCRscaneffectivelyinducethekillingofPAPpositiveprostatecancercells TheseresultshighlightthepotentialofTCR-basedimmunotherapyintreatingprostatecancer
culturemethod
Yan-RuideLi,PhD
BSCRC–CIRMPostdoctoralTrainee
LabPI:LiliYang,PhD
Cancerimmunotherapywithautologouschimericantigenreceptor(CAR)Tcellsfaceschallengesinmanufacturingandpatientselectionthat couldbeavoidedbyusing‘off-the-shelf’products,suchasallogeneicCARnaturalkillerT(AlloCAR-NKT)cells Previously,wereportedasystem fordifferentiatinghumanhematopoieticstemandprogenitorcellsintoAlloCAR-NKTcells,buttheuseofthree-dimensionalcultureand xenogeneicfeedersprecludeditsclinicalapplication HerewedescribeaclinicallyguidedmethodtodifferentiateandexpandIL-15-enhanced AlloCAR-NKTcellswithhighyieldandpurity.WegeneratedAlloCAR-NKTcellstargetingsevencancersand,inamultiplemyelomamodel, demonstratedtheirantitumorefficacy,expansionandpersistence Thecellsalsoselectivelydepletedimmunosuppressivecellsinthetumor microenviromentandantagonizedtumorimmuneevasionviatripletargetingofCAR,TCRandNKreceptors Theyexhibitedastable hypoimmunogenicphenotypeassociatedwithepigeneticandsignalingregulationanddidnotinducedetectablegraftversushostdiseaseor cytokinereleasesyndrome ThesepropertiesofAlloCAR-NKTcellssupporttheirpotentialforclinicaltranslation
KuangyiZhou
BSCRC–RoseHillsFoundationPredoctoralTrainee
LabPI:LiliYang,Ph.D.
Allogeneicchimericantigenreceptor(CAR)-Tcellsholdpotentialformoreaccessibleandaffordablecancertreatments,butchallengessuchas limiteddonorcells,manufacturingvariability,andmultiplexgeneengineeringpersist iPSCsprovideapromisingsolutionduetotheirunlimited self-renewalpropertiesandtheabilityforsingle-instancegeneticmodifications However,efficientlydifferentiatingiPSCsintomatureCAR-T cellsremainsahurdle,particularlyasCARengineeringmayarrestTcelldevelopmentfromstemcells,andiPSC-derivedCAR-Tcellsoftenlack invivopersistenceandclonalexpansionneededforlong-termtumorsuppression
Mucosal-associatedinvariantT(MAIT)cells,asubsetofαβTcellsthatrecognizeMR1anddonotcausegraft-versus-hostdiseasein allogeneicsettings,haveshownuniquepropertiesintissuehomingandtumorinfiltration Yet,theirlimitednumbersinhumanshindertheirfull therapeuticpotential.
Wehavedevelopedafeeder-freeandserum-freeexvivoculturemethodcapableofgenerating1013CARMAITcellsfrom106iPSCs Our culturemethodallowsiPSCstodifferentiateintomatureCARMAITcellswithouttheneedofTRACknockin,TCRknockout,orengineering iPSCswithCARthatcontainscompromisedsignalingdomain TheseiPSC-derivedCARMAITcells,canbeeithersinglepositive(SP)-CD4or SP-CD8,exhibitpotentantitumorcapabilitiesandrobustclonalexpansioninvivo.
Asaproof-of-conceptforthisinnovativeplatform,weemployedaBCMA-targetingCAR(BCAR)totreatBCMA+multiplemyeloma,anda mesothelin-targetingCAR(MCAR)totreatmesothelin+mesothelioma.
Engineeringallorejection-resistantCAR-NKTcellsfromhematopoieticstemcellsforoff-the-shelfcancer immunotherapy
YichenZhu
LabPI:LiliYang,PhD
TheclinicalpotentialofcurrentFDA-approvedchimericanti-genreceptor(CAR)-engineeredT(CAR-T)celltherapyisencumberedbyits autologousnature,whichpresentsnotablechallengesrelatedtomanufacturingcomplexities,heightenedcosts,andlimitationsinpatient selection Therefore,thereisagrowingdemandforoff-the-shelfuniversalcelltherapies Inthisstudy,wehavegenerateduniversalCARengineeredNKT(UCAR-NKT)cellsbyintegratingiNKTTCRengineeringandHLAgeneeditingonhematopoieticstemcells(HSCs),alongwith anexvivo,feeder-freeHSCdifferentiationculture TheUCAR-NKTcellsareproducedwithhighyield,purity,androbustness,andtheydisplaya stableHLA-ablatedphenotypethatenablesresistancetohostcell-mediatedallorejection.TheseUCAR-NKTcellsexhibitpotentantitumor efficacytobloodcancersandsolidtumors,bothinvitroandinvivo,employingamultifacetedarrayoftumor-targetingmechanisms These cellsarefurthercapableofalteringthetumormicroenvironmentbyselectivelydepletingimmunosuppressivetumor-associatedmacrophages andmyeloid-derivedsuppressorcells Inaddition,UCAR-NKTcellsdemonstrateafavorablesafetyprofilewithlowrisksofgraft-versus-host diseaseandcytokinereleasesyndrome Collectively,thesepreclinicalstudiesunder-scorethefeasibilityandsignificanttherapeuticpotential ofUCAR-NKTcellproductsandlayafoundationfortheirtranslationalandclinicaldevelopment IdentifyingAberrantCellularPhenotypesinNeuralCulturesDerivedfromArboleda-ThamSyndromeiPSCsthrough AI-AssistedImmunofluorescenceAnalysis
YanethPerez
UCLA–CIRMCOMPASSScholar
LabPI:ValerieA Arboleda,MD,PhD
Arboleda-ThamSyndrome(ARTHS)isarareMendelianneurodevelopmentaldisordercausedbydominantmutationsintheKAT6Agene–with patientsexhibitingvariabledegreesofdevelopmentaldelay,intellectualdisability,speech/languagedeficits,andcraniofacialabnormalities Sinceitsdiscoveryin2015,therehavebeenover200identifiedcases.However,themechanismsbywhichKAT6Amutationscauseabnormal neurologicalphenotypesinARTHSpatientsremainunknown Tobetterunderstandthedevelopmentalstagesatwhichthesephenotypic abnormalitiesarise,wefirstplatedARTHS/controlinducedpluripotentstemcells(iPSCs),neuralprogenitorcells(NPCs),andneuronsonglass slides Wethenperformedimmunofluorescence(IF)forcellfateandproliferationmarkersontheseslides–followedbyconfocalimaging The imagesfromourIFexperimentswerethenanalyzedusinganartificialintelligence(AI)-assistedquantificationplatformknownasBiodock Statisticalanalyses,includingt-tests,wereperformedusingRStudiotocreateboxplotsthatcomparetheaveragefluorescentintensities betweenpatientandcontrolsamples PreliminarydatashowssignificantdifferencesintheexpressionofpluripotencybetweenARTHSand controlsamples.UnderstandingtheseearlydevelopmentalvariationsmayhelpdevelopfuturetherapeuticstrategiesforArboleda-Tham Syndrome AMeta-AtlasoftheDevelopingHumanCortexIdentifiesModulesDrivingCellSubtypeSpecification
PatriciaNano,PhD
BSCRC–CIRMPostdoctoralTrainee
LabPI:AparnaBhaduri,PhD
Humanbraindevelopmentrequiresthegenerationofhundredsofdiversecelltypes,aprocesstargetedbyrecentsingle-celltranscriptomic profilingefforts Throughparallelmeta-analysesofthehumancortexindevelopment(7datasets)andadulthood(16datasets),wehave generatedover500geneco-expressionnetworksthatcandescribemechanismsofcorticaldevelopment These“meta-modules”show dynamiccellsubtype-specificitiesthroughoutcorticaldevelopment,withseveraldevelopmentalmeta-modulesdisplayingspatiotemporal expressionpatternsthatalludetopotentialrolesincellfatespecification Wevalidatedtheexpressionofthesemodulesinprimaryhuman corticaltissues Theseincludemeta-module20,amoduleelevatedinFEZF2+deeplayerneuronsthatincludesTSHZ3,atranscriptionfactor associatedwithneurodevelopmentaldisorders Humancorticalchimeroidexperimentsvalidatedthatbothfactorsarerequiredtodrivemodule 20activityanddeeplayerneuronspecification,butthroughdistinctmodalities.Thesestudiesdemonstratehowourmeta-atlasescanengender furthermechanisticanalysesofcorticalfatespecification
Understandingtheroleofglucosemetabolismincellfatespecificationeventsduringcorticaldevelopment JessenyaMil
BSCRC–CIRMPredoctoralTrainee
LabPI:AparnaBhaduri,PhD
Corticalorganoidsarerevolutionaryasamodelforhumancorticaldevelopment The3Dinvitromodelprovidesaframeworkforthe developmentalarchitectureofthecerebralcortex.However,workfromourlabandothershaveshownthatcorticalorganoidsdisplayimpaired cellsubtypediversityandgeneticmaturationsignaturesincomparisontohumanprimarycorticaltissue Moreover,theseimpairmentshave alsobeenfoundtocorrelatewithanupregulatedglycolyticgeneexpressionincorticalorganoids Consideringthatneuralstemcells(NSCs) relyonaerobicglycolysistoretaintheirstemnessandtransitiontoutilizingmitochondrialoxidativephosphorylation(OXPHOS)during activationanddifferentiationintoaneuronallineage,Ipredicttheupregulatedglycolyticstressincorticalorganoidsmaybeadrivinglimitation ofsubtypespecificationandimpairedcorrelationtogenematurationnetworks.Thus,themetabolicdifferencesseenbetweencortical organoidsandhumanprimarycorticaltissueprovideuswithamodeltounderstandhowthemanipulationofglucosemetabolisminfluences cellfatespecificationandneuronalmaturation.Iaimtoestablishthefunctionalmetabolicprofileofbothcorticalorganoidsandhuman primarycorticaltissueduringkeyneurodifferentiationevents,allowingforthecomparisonofactiveandinactivemetabolicpathways throughoutcorticaldevelopmentinbothmodels Additionally,Iwillintroducemetabolicperturbationsduringkeydevelopmentaltimepointsto characterizeitsimpactoncellsubtypespecificationandgeneticmaturationsignatures Byidentifyingtheinfluentialmetabolicpathways duringcorticaldevelopment,wecanprovidenewinsightsastohowdysregulationofcellfateandmaturationmayimpactbraindevelopment andidentifytheetiologyofneurodevelopmentaldisordersdrivenbymetabolicmutations.
XianglongTan,Ph.D.
LabPI:MichaelF Carey,PhD
Theorganizationandexpressionofgeneticinformationwithineukaryoticgenomesrelyonahighlycompacted,three-dimensionalchromatin architecture This3Dgenomicstructure,hypothesizedtoorchestrategenetranscriptionpredominantlythroughpromoter-enhancer interactions,iscarefullyconstructedandmaintainedbycrucialmolecularmachineries,includingthePreinitiationComplex(PIC),thecohesin complex,andothers OurlabhaspreviouslydepletedthePIC,observingadrasticreductionintranscriptionbutasurprisinglymodesteffecton the3Dgenomestructure Despiteaplethoraofexistingdata,theexactrolesthesecomplexesplayinmediatingpromoter-enhancer interactionscontrollinggenetranscriptionremainsomewhatelusive Toaddressthisproblem,weemployedarobustexperimentalsetup leveragingthe2ndgenerationauxin-induceddegradationsystemtosystematicallydepletetheRad21subunitofthecohesincomplexandthe Taf12subunitoftheTFIIDcomplexinmouseembryonicstemcellsindividuallyandincombination.ChIP-Seqanalysisofepigenomichallmarks revealedasignificantdepletionofthePICandMediatorcomponentsfollowingTaf12degradation,alongwithadepletionofcohesin componentsfollowingRad21degradation Wefurtheremployedpromoter-captureHi-C,whichenablesustoexaminechromatin conformationalchangeswith15-kbresolution OurfindingsrevealthatboththePICandcohesincomplexescontributetopromoterlooping, thoughtheyimpactdifferentpromoterloopgroups Additionally,byintegratingwithChromHMMstates,weuncoveredasubtleyetimportant correlationbetweentranscriptionandpromoterlooping,whichdisappearsuponcohesindepletion,indicatingonlyacertaingroupofpromoter loopingisnecessaryforgenetranscription OurresearchshedsnewlightonthedualrolesofthePICandcohesincomplexesinmediating functionalpromoter-enhancerinteractions.
PreranaKasanagottu
LabPI:HilaryColler,PhD
Quiescence,areversibleexitfromthecellcycleinG1,isrequiredforproperformationoforgansandtissuesandtheabilitytohealafterinjury Inresponsetonutrientdeprivation,autophagy,apathwayinwhichcellssequesteranddegradecellularmaterialatlysosomes,canprovide metabolitesandenergy However,notallquiescentcellsarenutrientdeprived Wesoughttounderstandhowtheautophagypathwayis regulatedwhencellsbecomequiescentinresponsetosignalsbesidesnutrientdeprivation Imagingandimmunoblottingrevealedthatserumstarvedandcontact-inhibited(CI)primaryhumandermalfibroblastsaccumulateautophagosomesandautolysosomes.ForCIfibroblasts, ratherthandegradingtheengulfedmaterial,itaccumulatesexpandedlysosomes WhenCIcellsarerestimulated,autophagosomelevelsare stronglyreduced,lysosomesshrink,andthedegradationrateofautophagyproteinsbecomesevenmorerapidthaninproliferatingfibroblasts. TheincreaseinLC3+punctaandlipidatedLC3formedduringCIlikelyreflectsnoncanonicalpathwayasLC3-IIlevelswerelowerinCImouse embryonicfibroblastsunabletoperformLC3-associatedendocytosis ThispathwayisimportantforthesurvivalofCIfibroblastsasCI fibroblastsunabletoperformitexhibitreducedviability Thefindingssuggestamodelinwhichquiescent,CIfibroblastsactivatenoncanonical autophagytoengulfextracellularmaterialthatisstoredinlysosomesuntilitisrequiredforthecells’bioenergeticneeds
ClonalstudiesidentifytheeffectsofhematopoieticstemcellagingonTcelldevelopment
JuliaGensheimer
LabPI:GayM Crooks,MD
Tcellproductiondeclineswithageandcontributestodecreasedimmunefunctioninolderadults ThisdeclineinTcelloutputmaybedueto changesinagedprogenitorcellsand/orchangesinthethymicmicroenvironment Duringaging,theHSCpooliscomprisedofincreasing proportionsofmyeloid-biasedHSCs(My-HSCs)comparedtolymphoid-biasedHSCs(Ly-HSCs).Thus,whetherreducedTcellpotentialisdueto changesintheproportionsofLy-andMy-HSCsorareducedintrinsiccapacityofeachsubtypeisunclear Tobetterunderstandtheeffectsof HSCagingonTcelloutput,westudiedphenotypically-definedagedandyoungHSCsusingtheinvitroArtificialThymicOrganoid(ATO)system. TheATOrecapitulatesallstagesofTcelldevelopmentfromasingleHSC,allowingfordetaileddissectionandquantificationofthymopoiesis ataclonallevelwithouttheinfluenceofanagedmicroenvironment OurpreliminarydatasuggestthatthelineagebiasofanHSCimpactsitsT cellpotentialregardlessofage,withbothyoungandagedLy-HSCsgeneratingmoreTcommittedclonesthanaged-matchedMy-HSCs Further, ATOsderivedfromyoungandagedLy-HSCsproducecomparablecellnumbers,whichsuggeststhatremovingagedHSCsfromtheir microenvironmentrestorestheirTpotential.
Developmentandcharacterizationofnon-alloreactiveTandCARTcellsfromiPSCs
LindsayLathrop
LabPI:GayM.Crooks,M.D.
AprimarybarriertoChimericAntigenReceptor(CAR)TcelltherapiesisthecurrentrelianceonautologousTcellsourcesandtheresulting unpredictableresponseratesduetoproductionartifacts.Apotentialsolutionistouseinduced-pluripotentstemcells(iPSCs)astheinitial sourcefordevelopingthesetherapies Comparedtootherallogeneicsources,iPSCsallowforcomplexmulti-geneeditingthatcanbeusedto changethenatureoftheTcellstocreateauniversalrenewableCARTcellproductthatcanbereadilyandwidelyavailabletoallpatients.As iPSCsareanallogenicsourcetheendogenousTCRandMHCmustberemovedtopreventGvHDand/orrejectionrespectively However,since thesegeneticalterationsareintroducedatthestemcellstagetheywillaffectdevelopmentoftheTcells Removingtheseelementscreatesa blockindevelopmentasTCRstimulationbyMHC-peptideantigenpresentationmediatespositiveselectionofdoublepositive(DP)Tcellsinto singlepositiveCD4+(SP4s)andCD8+(SP8s) Hereourgroupdemonstrateshowstage-specifictransgenicTCRandCARexpression overcomesthisblockinpositiveselectionandallowsfordevelopmentofTCR-lessCARTcells.Infuturestudies,weaimtoelucidatehow singleCAR-mediatedpositiveselectionaffectsTcellintracellularsignaling,phenotype,transcriptionandfunction
AnthonyAzzun
LabPI:GayM Crooks,MD
RegulatoryTcells(Tregs)areessentialformaintainingimmunebalanceandpreventingautoimmunediseaseslikelupusandtype1diabetes. Despitetheirtherapeuticpotential,currentstrategiesforgeneratingTregsrelyonconvertingmatureTcellsintoinducedTregs,whichoften exhibitinstabilityandlossoffunctionovertime Ourresearchseekstoaddressthislimitationbydevelopinganovelmethodtogeneratestable Tregsfrominducedpluripotentstemcells(iPSCs),providinganunlimited,renewablesourceofthesecrucialimmunecells
WefocusonFOXP3,themasterregulatorofTregdevelopment,tounderstandhowitsexpressioninfluencesTcellfate.Usinganartificial thymicorganoid(ATO)system,theonlyinvitromodelcapableofproducingmatureTcellsfromstemcells,weinvestigatehowintroducing FOXP3intoiPSCsaffectstheirdifferentiation Weemploymultiplestrategies,includingconstitutiveFOXP3overexpressionvialentiviral transductionandtargetedinsertionusingCRISPR-Cas9tomimicphysiologicalexpression Additionally,weexploresmall-moleculemodulation toenhanceendogenousFOXP3expression
OurpreliminaryfindingsrevealthatthymiclevelsofFOXP3expressionblocknormalTcelldevelopmentatearlydifferentiationstagesiniPSCs whilerestrictingFOXP3tolaterstagespreservesmatureTcellformation TheseinsightspavethewayforoptimizedTreggenerationand functionaltestinginsuppressorassays ByuncoveringmechanismsthatcontrolFOXP3expressioniniPSC-derivedTcells,ourworkadvances thefieldofstemcell-basedimmunotherapy ThisresearchholdspromisefordevelopingmoreeffectiveTreg-basedtreatmentsforautoimmune diseases,potentiallyrevolutionizingpersonalizedcelltherapiesandimprovingoutcomesformillionsofpatients
EffectsonNeurogenesisandCellSurvivalFollowingtheKnockdownofCARHSP1inPrimaryHumanNeural ProgenitorCells
NolanFernandez
UCLA–CIRMCOMPASSScholar
LabPI:LuisdelaTorre-Ubieta,PhD
Thedevelopmentofthehumanneocortex,responsibleforourmostdistinguishingcognitiveandsocialcapabilities,ispreciselyregulatedby geneticprogramsdrivingcelltype-specificmolecularmechanisms However,manygene-regulatorymechanismsgoverningcortical neurogenesisremaintobecharacterized Previously,weidentifiedtheRNA-bindingproteinandputativetranscriptionalregulatorCARHSP1as anovelcell-specificmarkerandpotentialregulatorofradialglialprogenitoridentity LeveragingCRISPRinterference(CRISPRi)weknocked downCARHSP1indifferentiatingprimaryhumanneuralprogenitorcells(phNPCs)andassessedneurogenesisusingimmunocytochemistryfor knownmarkersofneuronsandprogenitors WedidnotobservesignificantchangesinneurogenesiswithlossofCARHSP1,butobserveda trendtowardsreducedinfectionrateandnumberofknockdowncellssuggestingchangesinprogenitorproliferationand/orsurvival.Inongoing work,weaimtodefinewhetherCARHSP1affectsneuronalprogenitorproliferationorsurvival Altogether,ourfindingssuggestCARHSP1is essentialforprogenitorsurvivalorproliferationdynamics
Definingmolecularandgene-regulatorydysregulationinDownSyndromeneocortex
AlexisWeber
LabPI:LuisdelaTorre-Ubieta,PhD
Downsyndrome(DS)isthemostcommonformofgeneticintellectualdisability,occurringin1/700newbornsandpresentingwithcognitive, learning,memory,andlanguagedeficits DSresultsfromtrisomyofchromosome21(hsa21),causingimpairedcorticaldevelopmentandbrain architecture.However,thecellularandmolecularmechanismsdrivingthesepathologiesremainunclear.Wehypothesizethatincreased dosageofhsa21-encodedtranscriptionfactors(TFs)andepigeneticregulatorsaltersglobalgeneexpressionleadingtochangesin neurogenesisandneuralspecification.Toaddressthisgapinknowledge,weprofiled~110Kcellsfrom26age/sex-matchedneurotypical(NT) andDSmid-gestationneocorticesviasnMultiometechnology InDStissue,weobservedasignificantreductioninneuralprogenitorsanddeep layercorticothalamic(CT)neuronsandconcomitantincreaseofinter-telencephalic(IT)neurons,accompaniedbyacceleratedtimetoneural specificationasdemonstratedbylineagetrajectoryanalysis Inadditiontothe25%ofhsa21-encodedgenesdetectedasexclusively upregulated,wefoundwidespreadchangesinnon-hsa21geneexpressionandchromatinaccessibility Usinggeneco-expression(hdWGCNA), weidentifiedcelltype-specificchangesinmodulesinvolvedinneurogenesisandaxondevelopment.Tounderstandthemolecularmechanisms drivingthesechanges,weperformedgeneregulatorynetworkanalysis(SCENIC+),findingsignificantchangesintheactivityofregulons controllingCTvsITneuronspecificationthataredownstreamofhsa21-encodedTFs,includingBACH1.Together,ourdatarevealsacascade ofmoleculardysregulationoriginatingfromhsa21-encodedTFsleadingtoalterationsinneurogenesisandmaturation,providingafoundation fordevelopingfuturetherapeutictargets
Machinelearning-assistedquantificationofcellcompositionindevelopingDownSyndromeneocortex Moonkyu(Patrick)Seong
LabPI:LuisdelaTorre-Ubieta,PhD
Downsyndrome(DS),resultingfromtrisomyofchromosome21,isthemostcommongeneticcauseofintellectualdisability,affecting approximately1in700livebirths DSisassociatedwithdisruptionsinthedevelopmentofthecerebralcortex,leadingtocognitive,learning, andmemorydeficits HistologicalandbioinformaticstudiesinpostmortemDSbraintissuehavefoundconflictingresultswithregardsto changesincellularcompositionandidentity Whilepathologicalimagingstudiesareoftenthoughtasthegoldstandard,theyareoftenlimited bysamplesizeandlaboriousmanualquantification Workinourlaboratoryusingsingle-nuclearmultiomicsofmid-gestationDSneocortex identifiedchangesinneuronalcomposition,inparticulardeep-layercorticothalamic(CT)andinter-telencephalic(IT)neuronspecification.To validatethesefindings,weperformedimmunohistochemistryindevelopingDSandneurotypical(NT)neocorticaltissueusingmarkerslabelling CT(BCL11B/CTIP2)andIT(SATB2)neurons Weimplementedamachinelearning-assistedcellsegmentation(CellPose)andautomated thresholdingpipelinetoquantifythousandsofcellsacross6age-matchedNTandDSsamplesWevalidatedourpipelinebycomparingtheir resultstothoseofmanualquantificationsonsmallerregionsofneocorticaltissuestains Confirmingtheaccuracyofthesemethods,wethen scaleduptheautomaticapproachtolargerregionsofcorticaltissue,efficientlyclassifyingmanynucleitodeterminetheneuronalcomposition ofoursamples Ourworkprovidesapipelinetoquantifycellcompositionwithnuclearmarkersinbraintissuesectionsatscale Together, theseeffortscontributetoadeeperunderstandingofthecellularalterationsunderlyingDSandultimatelythesearchfortargetedtherapeutic interventions
SummerParr
UCLA–CIRMCOMPASSScholar
LabPI:BrigitteGomperts,MD
Virusesaredependentonhostcellularproteinsforeachstepoftheirlifecycle,andtheyhijackmanyofthehostcellproteinsfortheirfunction ManyRNAvirusescaninduceasignificantDNAdamageresponse(DDR)whichcancontributetothepathogenesisofRNAvirusesthroughthe triggeringofapoptosisandstimulationofinflammatoryimmuneresponses Cigarettesmoke(CS)canalsoinduceDDRanditisknownthat patientswhosmokeareatincreasedriskforsevereCOVID-19lungdiseaseanddeath WeidentifiedberzosertibasaninhibitorofSARS-CoV-2 infection.Berzosertibexhibitedanti-SARS-CoV-2activityandwaseffectiveatreducingSARS-CoV-2infectioninthehumanair-liquidinterface (ALI)culturemodel WeexposedhumanalveolartypeII(ATII)cellsgrowninorganoidsandALIculturestoCSfollowedbyliveSARS-Cov-2viral infectionandfoundthatCSincreasedthenumberofinfectedcells2-3-fold ATIIorganoidsexposedtocigarettesmokeextract(CSE)also showedincreasedproliferationandafive-foldincreaseinSARS-CoV-2infection AsbothcigarettesmokingandviralinfectioninducetheDDR, wehypothesizedthatberzosertib,aDDRinhibitor,wouldbeanespeciallyeffectivetherapytoblockSARS-CoV-2replication Treatmentwith BerzosertibduringCSexposureandSARS-CoV-2infectionreducedviralreplicationinhumanALIandATIIcellcultures Inaddition,K18-ACE2 miceexposedtoCSandinfectedwithSARS-CoV-2showedlesslungconsolidationwhentreatedwithBerzosertib Berzosertibiseffectiveat reducingSARS-CoV-2infectioninprimaryhumanairwayandalveolarlungculturesandtheK18-ACE2mousemodel.Wearecurrently elucidatingthetoxicityandefficacyofberzosertibduringsmokinginthesethreemodels Thisdatawillhelptoestablishtheefficacyof berzosertibinthecontextofSARS-CoV2,especiallyinvulnerablepatientpopulationssuchassmokers
AndrewLund LabPI:BrigitteGomperts,MD
Airwaysubmucosalglands(SMGs)houseatleasttwodistinctstempopulations,namelytheductbasalandmyoepithelialcells(MECs) Based onstudiesutilizingsevereairwayinjurymodalitiesinmurinemodels,bothpopulationsareseeminglycompetenttoregenerateSMGand surfaceairwayepithelium(SAE) However,equivalentregenerativecapacityhasnotbeenestablishedforthehumancounterpartstothese stempopulations
SortedSMGductbasal,surfacebasal,MECs,andunsortedSMGisolateswereseededtoinitiateorganoidcultures Organoid-forming efficienciesandorganoidareaweremeasured.Single-cellgeneexpressionoforganoidsfromeachpopulationwasinterrogated.
SortedMECsexhibiteddiminishedorganoid-formingefficienciescomparedtounsortedSMGisolates,sortedductbasal,andsortedsurface basalcells Single-cellfixedRNAprofilingrevealedthatMECspreferentiallydifferentiateintosecretorylineagesattheexpenseofstem maintenance,insteadgeneratingcyclingsecretoryintermediatesthatarefate-committed Conversely,surfacebasalcellsrapidlymobilize underexpansionconditionsinculture,accountingforthegreatestorganoid-formingefficiencyandtotalbiomassofthesortedpopulations, whereasductbasalcellsdemonstrategrowthdynamicsintermediatebetweenMECsandsurfacebasalcells.Moreover,surfaceandductbasal cellsaremoreproficientinmaintenanceof“stemness”comparedtoMECsandhaveamorebalanceddistributionacrossfate-committed clusters,includingprogenythataredirectedtowardssurface/duct-likeluminalcelltypes.
Identificationofalong-livedairwaystempopulationwithbroadmultipotencywouldproduceanidealcandidatefortherapeuticconcerns SMG ductbasalcellsisolatedfromhumanairwaysdemonstratedexcellentself-renewalcapacityandmultipotencyinorganoidcultures,markingthis populationasanattractiveprospectforadvancingregenerativemedicineintherespiratorysystem
CarolineCherry
LabPI:BrigitteGomperts,M.D.
Woodfiresmoke(WFS)permeatesourenvironment,butthehealthrisksfromtheseexposuresarenotknown Althoughtherelationship betweenlungdiseaseandciliarydysfunctionhasbeenestablished,littleworkhasbeendonetoidentifytheeffectsofenvironmentalexposures onairwaycilia Ofnote,theMAPKinase(MAPK)signalingpathwayhasbeenshowntobeassociatedwithciliarymotorbehaviorand cytoskeletalrearrangement.Therefore,thisproposalaimedtounderstandthemechanismsofciliaryinjuryfollowingWFSexposureandidentify potentialtherapeuticagentstomitigatethisinjury Utilizinghealthyhumanbronchialsamples,weexposedcellculturestoWFS Airwaybasal cellinjuryfollowingWFSexposurewasassessedthroughbulk-omicsanalysis,ciliarybeatanalysis,andtransmissionelectronmicroscopy (TEM) WeidentifiedglobaldownregulationoftranscriptionofciliarelatedpathwaysandanupregulationofMAPK Proteomicanalysis revealedlittlechangeinproteinabundance,coupledwithaglobaldownregulationofphosphorylationofciliarelatedproteins WFSexposure for5daysabolishedciliarymovementinadosedependentmanner Interestingly,treatmentwithvariousMEK1/2inhibitorsincreasedciliary movementbyapproximately70% Furthermore,weinvestigatedtheultrastructureofciliatedcellsfollowingWFSexposuresandMEKinhibitors TEManalysisrevealednormalciliaryaxonemesacrosscontrolsamples,and~90%lossofthecentralpairfollowingWFS,whichwasrescued byMEKinhibition Together,wehavecreatedanexposurechambertomimicthehumanairwayexposuretoWFS WeshowthatWFSis responsibleforreducingciliarymovementintheairwaybydamagingthecentralpairofproteinsinciliaryaxonemesandthatMAPKsignaling regulatestheseciliaryproteins InhibitionofMEKpreservesciliaryultrastructureandciliarymovement,therefore,posingpotentialtobeused asamitigatorofairwaydiseaseinWFSexposedindividuals
DesigningTargetingLipidNanoparticlesforinvivoCARTCellGeneration
ItzelHuerta
UCLA–CSUNCIRMBridgesTrainee
LabPI:StevenJ Jonas,MD,PhD
CellbasedimmunotherapiesintheformofTcellsgeneticallymodifiedtoexpresschimericantigenreceptors(CAR)areprovidingadditional treatmentoptionsforrelapsedand/orrefractorypediatriccancers;however,currentCARTcelltherapiesrequirecostlyandtimelyviralexvivo modification Lipidnanoparticle(LNP)technologieshaveshownsignificantpromiseinthenon-viralengineeringofCARTcells,yettheir clinical,invivotranslationislimitedbytheirnon-specificdelivery
Herewereportthedesignofamodular,antibodyconjugatedLNPplatformanditsoptimizationfortargetedengineeringofprimary,humanT Cells OptimizationofourLNPplatformwasachievedthroughmicrofluidicsynthesisofseveralformulationsintegratingdifferentionizable lipidsformRNAdelivery,withthemostefficientformulasdisplayingnanoparticlediameters80%byRibogreenfluorescence-basedassay.The surfaceoftheLNPswerethendecoratedwithαCD3monoclonalantibodiesutilizingabioorthogonalclickchemistryconjugationstrategy,and sizeexclusionchromatography(SEC)enabledremovalofunboundantibodiesconfirmedviafluorescentproteinlabelling.αCD3-modifiedLNPs showedcomparabletransfectionofactivated,primaryhumanTcellstothatofoptimized,non-targetingLNPswithatleast70%oftreatedcells expressinggreenfluorescenceprotein(GFP)24hourspostLNPtreatment OuroptimizedαCD3-LNPsenabledthestimulationandtransfection ofrestingTcells,with10%oftreatedcellsexpressingGFPafter24hours,increasingto20%after48hours Thesenanotechnologiesserveasa modular,foundationalplatformforTcellengineering,enablingtargetspecificitytoempowernextgenerationcellularimmunotherapeutic approaches.
Toolsformaintaininghematopoieticstemcellsin3Dmatricesforstemcellgenetherapies:collagen-based hydrogelsforHSCculturingandrecovery
LuisReyes
UCLA–CSUNCIRMBridgesTrainee
LabPI:StevenJ Jonas,MD,PhD
Hematopoieticstemcells(HSCs)canbeusedtotreatmalignantandnon-malignantdiseases Whilethedifferentiationandself-renewalof hematopoieticstemcells(HSCs)inbonemarrowhasbeenextensivelystudied,theimpactofex-vivoculturingandtheabilitytomaintainthe stem-likenatureofthesecellsmustbeimprovedtoadvanceHSCgeneandcelltherapies AchallengewithHSCsistheabsenceofinvitro modelsthatcaneffectivelymimicthehumanbonemarrowmicroenvironment Herewepresentthedevelopmentoftoolstoenablethe culturingofHSCsina3Dhydrogelmatrix,andtheirimpactonHSCself-renewal.Ourpreliminarystudiesindicatethatthesuccessful encapsulation,maintenanceandrecoveryofHSCsfromthese3DculturesenablestheholdingofHSCmarkerswhencomparedtostandard2D culturepractices ThisresearchprojectprimarilyaimstoinvestigatewhetherHSCscanbemaintainedina3Dcultureenvironmentthat preventsdifferentiationandpreservestheirstem-likeproperties Forourexperiments,weutilizedtheKG-1acelllineasamodeltostudyHSC behaviorbeforeexperimentingwiththeHSCsinhydrogels Thehydrogelswerefine-tunedbyevaluatingdifferentcollagenandfibrinogen formulationsthatmosteffectivelykeptviabilityabove90%,regulatedproliferationofKG-1acells,andallowedforthemostamountofcellsto berecoveredaftermelting TheviabilityofHSCswasassessedthroughflowcytometrybothatthetimeofencapsulationand24hoursafter OurfindingsindicatedthatacombinationofcollagenandfibrinogeninthehydrogelsledtoadecreaseintheproliferationofKG-1acellsand HSCpopulationswhilekeepingviabilitylevelsabove90% ThisworkwillenablefurtherinvestigationintothemechanismsofHSCself-renewal regulationandfatedecisionofcommittedprogenitorstoimprovecurrentinvitromaintenanceofHSCsforgeneandcellulartherapy manufacturing
AmaduTadesse
LabPI:StevenJ Jonas,MD,PhD
Alveolarrhabdomyosarcoma(aRMS)isarareandaggressivesofttissuecancerarisingfromskeletalmusclestemcellsinchildrenandyoung adults Thisprojectleveragesnanotechnology-basedstrategiestodevelopalipidnanoparticle(LNP)platformdesignedtodeliver CRISPR/Cas9reagentsconfiguredtodisruptthePAX3-FOXO1translocationthatdrivesthemostaggressivephenotypesofaRMSandmitigate itsoncogeniceffects Herewepresentatwo-prongedapproach:first,designingandvalidatingsingleguideRNA(sgRNA)sequences configuredforthetargetedknockoutofPAX3-FOXO1translocations;second,engineeringLNPsfortheefficientdeliveryofthesegene-editing reagentsfordirecttherapeuticuseonaRMStumors.PAX3-FOXO1sgRNAsweredesignedusingconservedfusionregionsidentifiedfrom ChimerDB40andtheUCSCGenomedatabase GuideswereoptimizedusingBenchlingandIntegratedDNATechnologytoolstomaximizeontargetefficiencywhileminimizingoff-targetrisks GuidecandidateswerethentestedinarepresentativePAX3-FOXO1positiveaRMScellline (RH30)byelectroporatingCas9ribonucleoproteincomplexesassembledwithPAX3-FOXO1sgRNAconstructsandvalidatedviaqPCRanalysis Greenfluorescentprotein(GFP)-encodingmRNAwasinitiallyencapsulatedinLNPstooptimizeformulationdeliveryefficiency Nanoparticles werepreparedviamicrofluidicmixingwithmeandiameters80%determinedbyRibogreenfluorescence-basedassay Ourbestperforming formulationsachieved>90%GFPexpression24hposttreatment PreliminarystudiesapplyingLNPscarryingPAX3-FOXO1CRISPR/Cas9 cargoes,observeda>50%reductioninPAX3-FOXO1expressionintargetedaRMScells,asconfirmedbyqPCR.Thesefindingsdemonstratethe potentialofLNP-basedCRISPR/Cas9strategiestotargetoncogenicmutationstherapeuticallyinhigh-riskchildhoodmalignancieslikeaRMS
AugmentingNaturalKillerCellFunctionThroughChimericAntigenReceptorandEndogenousSignalingPathways
JedricGonzales
UCLA–CIRMCOMPASSScholar
LabPI:ScottG.Kitchen,Ph.D.
Currentresearchhasestablishedtheeffectwhichthetransductionofchimericantigenreceptor(CAR)machineryintonaturalkiller(NK)cells andT-cells(TC)hasonthecytotoxiccapabilitiesofsuchcells Simultaneously,researchshowsthattheendogenouspathwayscombinedwith CARmachineryhelpstoincreasethecytotoxicefficiencyofbothTandNKcells Whilethesestudieshaveshownthepotentialforadjoined engineeringofCARtherapywithendogenouselements,researchonadjacent,NK-specificformsofendogenoussignalinginthecontextofCAR therapyisnotasthorough,despitesuchactivationplayingamajorroleintheactivationofNKcells Thus,thisstudyfocusesonhowthe functionalityofNKcellscanbeimprovedbycombiningtheelementsofCARmachineryalongsideimportantNK-specificsignalingpathways. Toconductthisstudy,wewillusefourdifferentgeneticconstructsthatwillbeincorporatedintofourNK92celllinesvialentiviraltransduction EachconstructcontainsCD4,CAR,CD3(��)machinery,andthefluorocombMScarlet3,whichaidsincellstainingproceduresandverificationof transduction ThreeconstructscontaincombinationsofNKsignaling:onecontainingDAP12,anothercontainingDAP12,CD40,andCD3(ε) insteadofCD3(��),andthelastcontainingDAP12andNKG2C Currentanalysisofthecontrolcelllineconfirmsthestrongefficacyofcurrent transductionmethodsandestablishesthebaselineofunmodifiedCAR-NKfunction.Futureprocedureswillfocusonthetransductionofthe threeexperimentalconstructsintorespectiveNK92celllinesbeforeconductingcellularstaining,cytokineassay,andcytotoxicityprocedures
OptimizationofBTKDonorforCRISPR/Cas9GeneTherapyofX-LinkedAgammaglobulinemia(XLA) ElyceVelazquez
UCLA–CSUNCIRMBridgesTrainee
LabPI:DonaldB.Kohn,M.D.
TheBruton’sTyrosineKinase(BTK)geneencodesforaproteinthatplaysacrucialroleinBcellsignalingandisessentialforBcelldevelopment, proliferation,anddifferentiation MutationsintheBTKgenecausesX-LinkedAgammaglobulinemia(XLA),aprimaryimmunodeficiencydisorder leadingtotheinabilitytoproduceBcellsorimmunoglobulins,conferringthesusceptibilitytoinfections XLAistreatedthroughregularand frequentintravenousimmunoglobulinreplacementtherapy,howeverthistreatmentiscostlyanddoesnotprovideapermanentcureforpatients. PreviousresearchhasshownitispossibletoedittheBTKlocustorestorefunctiontotheBTKgene,howeverfurtherresearchisnecessarytofind theoptimalexpressionandintegrationoftheBTKdonorDNA ItisespeciallyimportanttohaveapreciselevelofBTKexpressionasoverexpression iscorrelatedwithBlymphoidleukemias UsingintronicregulatorsinthegenetherapyofXLAmayrestoretheproperexpressionpatterns,thuswe willoptimizetherepairtemplatetoreachthiscorrectlevelofexpressionoftheBTKgene Severalcandidatesequenceshavebeenclonedintothe BTKdonortemplateinpreviousstudiesandintron4hasshownanimprovementoftheexpressionlevel Intron4and5oftheendogenousBTK genehavebeenclonedandtestedforexpressionlevelinduced AtthesametimeremovingoftheWPREsequencewastestedtoseeiftheintronic sequencewouldmaintaintheexpressionintheabsenceofthisviralregulator.Intron4’ssequenceistoolargetobepackagedintoanAAVvector, sowewilldesignandtest3differentconstructsgeneratedwithsubsequentdeletionsandcontainingthenecessaryportionsofIntron4tofitinto anAAVvector These3DNAconstructswillthenbetestedfortheinducedexpressionoftheBTKtransgene
IDLVvsAAV:OptimizingVectorDeliveryforBTKGeneEditing
PatienceAkok
UCLA–CIRMCOMPASSScholar
LabPI:DonaldB.Kohn,M.D.
X-linkedagammaglobulinemia(XLA)isadiseaseaffecting1in200,000malescausedbyamutationoftheBrutontyrosinekinase(BTK)gene, responsibleforthedevelopmentofBcells.SinceBcellsproduceantibodies,patientsaffectedbyXLAhaveanincreasedsusceptibilityto infections Allogeneictransplantofbonemarrowfromahealthydonortothepatientwouldresultinacure However,itposesthethreatofgraftvs hostdisease.Alternatively,geneeditingthepatient’shematopoieticstemcells(HSCs)wouldallowforcorrectionofthemutationandrestorethe HSCsabilitytodifferentiateintofunctionalBcells Beforeclinicalapplications,thismustbetestedandoptimizedinvitro Wefocusedontheuseof viralvectorswhendeliveringahealthyBTKdonorsequence Adeno-associatedviralvectors(AAV)havepreviouslyledtohighintegrationofthe BTKdonor,howeveritwastoxictocellsin-vitro Ontheotherhand,integration-defectivelentiviralvectors(IDLV)hadlessintegrationpotential,but preservedcellviability Inordertotestoptimalconditionsforgeneediting,weelectroporatedHSCswithaCRISPR/Cas9complexandtransduced themwithaDNAdonorusingeitherAAVorIDLV.Then,wecomparedBTKintegrationbyddPCRandBTKexpressionusingflowcytometry. PreliminaryfindingsconcludedthatAAVintegratedBTKmoreefficientlyandhadhigherBTKexpression,butmoreoptimizationsofbothvectors mustbedonetoconfirm Oncedonesuccessfully,itwillbepossibletoselectthebestvectorfordeliveryinpatientcells
iPSC-derived blood-brain barrier cellular model reveals distinct mechanisms of Old World alphavirus neuroinvasion
Pablo Alvarez
Lab PI: Melody Man Hing Li, Ph D
Several alphaviruses bypass the blood-brain barrier (BBB), causing debilitating or fatal encephalitis Sindbis virus (SINV) has been extensively studied in vivo to understand alphavirus neuropathogenesis; yet the molecular details of neuroinvasion at the BBB remain poorly understood We investigated alphavirus-BBB interactions by pairing a physiologically relevant, human pluripotent stem cell derived model of brain microvascular endothelial cells (BMECs) with SINV strains of opposite neuroinvasiveness Our system demonstrates that SINV neuroinvasion correlates with robust infection of the BBB Specifically, SINV genetic determinants of neuroinvasion enhance viral entry into BMECs We also identify solute carrier family 2 member 3 (SLC2A3, also named GLUT3) as a potential BMEC-specific entry factor exploited for neuroinvasion. Strikingly, efficient BBB infection is a conserved phenotype that correlates with the neuroinvasive capacity of several Old World alphaviruses, including chikungunya virus. Here, we reveal BBB infection as a shared pathway for alphavirus neuroinvasion that can be targeted for preventing alphavirus-induced encephalitis.
UnravelingtheSLC6A8Mutation:InsightsintoNeurodevelopmentthroughOrganoidResearch
MayaMoreno
UCLA–CIRMCOMPASSScholar
LabPI:GeraldS.Lipshutz,M.D.
CreatineisafundamentalnitrogenousmetaboliteessentialforATPproduction,osmoregulation,andneurotransmission Disruptionsincreatine metabolism,arisingfrommutationsaffectingitssynthesis(AGAT,GAMT)ortransport(SLC6A8),leadtosevereintellectualandphysical disabilities,collectivelyknownasCerebralCreatineDeficiencySyndromes(CCDS) DespitetheprofoundneurologicalimpactofSLC6A8 deficiencies,theunderlyingdiseasemechanismsremainpoorlyunderstood Thisstudyaimstoelucidatethecellularandmolecularpathways disruptedbySLC6A8mutationsandexplorepotentialtherapeuticstrategies Wewillgenerateandanalyzepatient-derivedorganoids,isogenic corrected-celllines,andcontrolcelllinestomodelCCDSinahumanneurodevelopmentalcontext Additionally,wewillassesstheefficacyof currentbutlimitedtherapeuticinterventionsoncellularfunctiontodeterminetheircorrectivepotential Bybridgingcriticalknowledgegapsin SLC6A8-relatedneurodevelopmentaldeficits,thisresearchwilladvanceourunderstandingofCCDSpathologyandcontributetothedevelopment oftargetedtherapies.Ultimately,theseinsightshavethepotentialtodriveinnovativetreatmentsthatimprovethequalityoflifeforaffected individualsandtheirfamilies
MechanotransductionintheGut:InvestigatingPiezo1/2FunctioninPDGFRα⁺Cells
AllisonFlores
LabPI:MartínG.Martín,M.D.,M.P.P.
Efficientgastrointestinal(GI)motilityreliesoncoordinatedinteractionsbetweensmoothmusclecells(SMCs),interstitialcellsofCajal(ICCs), andPDGFRα+cellswithinthesubintestinalplexus(SIP)syncytium.PDGFRα+cellsfunctionasfibroblast-likeinterstitialcells,modulatingSMC excitability,mechanosensation,andcontractions However,themolecularmechanismsthroughwhichPiezo1/2mechanosensitivecalcium channelscontributetothesefunctionsremainunclear.ToinvestigatePiezo1/2function,weutilizedaPiezo1/2;mTmG;PDGFRα-ERT2-Cre/LoxP modeltogeneratePiezo1/2control(Piezo1/2WT)andknockout(Piezo1/2ΔPDGFRα)mice WeconfirmedmembraneGFP(mG+)expression andmappedPDGFRα+celllocalizationwithintheintestinalmuscularis OurfindingsrevealedPiezo1/2WTmiceshoweddenseclusteringof mG+PDGFRα+cellswithinthehorizontalandcircularmuscularis,formingaweb-likestructureintheinnerlongitudinalmuscle Incontrast, Piezo1/2ΔPDGFRαmiceexhibitedacontinuousPDGFRα+celldistributionfromtheserosatothesubmucosa,withuniqueserosalpatterns BothgroupsdisplayedelongatedPDGFRα+cellsnearthecircularmuscle,likelyshapedbytissueorientation,withnoobservedsexdifferences. ImmunostainingconfirmedPiezo1/2expressioninPDGFRα+GFP+cells,suggestingaregulatoryroleinmechanosensitiveionchannel signaling.FuturestudieswilluseaSALSAreporterlinetoassessPiezo1/2depletioneffectsoncellmorphology,Ca²⁺flux,andcontractility.By elucidatingPiezo1/2functioninPDGFRα+cells,thisstudyadvancesourunderstandingofSIPgenicmodulationinGImotilityandmayreveal newmechanisticpathwaysunderlyingintestinalmotilityregulation
InvestigatingtheImpactofPiezo1DepletioninSmoothMuscleCellsonIntestinalFunction MireyaCruz
UCLA–CSUNCIRMBridgesTrainee
LabPI:MartínG Martín,MD,MPP
Piezo1/2aremechanosensitivechannelsthatareresponsivetostretchandleadtothereleaseofdi-cationicelementssuchasCa++ To investigatethespecificfunctionsofthesechannelsinGImotilityandhomeostasis,wegeneratedfourgeneralmousestrainsthatallowfor targeteddeletionofPiezo1/2inparticularcelltypesinthesmallintestines.Thisincludesepithelialcells,smoothmusclecells(SMCs), InterstitialcellsofCajalandPDGFRαcells EntericSMCcontractionresultsfromtheinfluxofCa++inthecytoplasm IntheSMCs,Piezo1,not Piezo2,isexpressedathighlevels Here,wedeterminedtheroleofPiezo1inSMCsinmediatingintestinalmuscularisfunction We hypothesizedthatlossofPiezo1intheSMCswillleadtointestinaldysmotility UsingthePiezo1/mTmG/Myh11-ERT2/Cre-LoxPsystem,we generatedamodelwithtamoxifen(Tam)-Piezo1control(Piezo1WT)andKO(Piezo1ΔSMC)inSMCs Tamwasgivento6-wk-oldmiceand assessed21dayslater WehaveperformedacomprehensiveassessmentoftheroleofPiezo1inentericSMCs,andhavefoundthatselective depletionofPiezo1inSMCsofthesmallbowelleadstosignificantchangesinintestinalmotilityandfunction,asevidencedby1)delayedgut transitandweightlosswithoutanincreaseinmortality;2)thinningofthemuscularisandalossofSMCs;3)diminishedcontractilepatternand strength;4)alterationsintissuepropertiesincludingtonicityandviscoelasticproperties;5)dysregulatedexpressionofionchannelsinSMCs; and6)predominantlocalizationofPiezo1intheSRofSMCswithreducedIP3Rexpression Thisstudywaslimitedto21dayspost-tamoxifen Despitemuscularisthinning,nochangesinSMCapoptosisorproliferationwerefound Toinvestigateearlycellularchanges,weareperforming atime-courseanalysisusingimmunofluorescenceonwhole-mountdistalboweltoassessSMCabundance,apoptosis(CC3),andproliferation (Ki67)withinthefirst21dayspost-tamoxifen
InvestigatingtheRoleofPiezo1/2inc-Kit+InterstitialCellsofCajalandTheirContributiontoGastrointestinal Motility
NicoleKushnir
LabPI:MartínG.Martín,M.D.,M.P.P.
Thegastrointestinal(GI)tractreliesoncoordinatedmechanicalpropertiestofacilitatenutrientassimilation,regulatedbysmoothmusclecells (SMCs),interstitialcellsofCajal(ICCs),andPdgfrα+cellswithintheSIPsyncytium Aspacemakercells,ICCsgenerateslowwaves,rhythmic contractions,andmediateneurotransmissiontoregulatemotility AllcellularcomponentsoftheSIPsyncytiumrichlyexpressthe mechanosensitivecalciumchannels,Piezo1&2,yettheirroleinthesecelltypesremainsunclear.Piezo1&2arestrongcandidatesfor modulatingintracellularCa2+dynamics WehypothesizedthatthelossofPiezo1/2inICCswouldimpairintestinalmotility Tofurtherassess Piezo1/2functioninICCcells,wehaveestablishedac-Kit-CreERT2;Piezo1/2LoxP;mTmGorSalsasystemtodevelopPiezo1WTand Piezo1/2ΔICCknockoutmodelinICCs ThemTmGreportermarksCrebychangingmembranetdTomato(mT)toGFP(mG),whiletheSalsa reporteristdTomatolinkedtoGCaMP6f Tamoxifenwasadministeredto6-week-oldmiceandanalyzedafter21days MembraneGFP expressionconfirmedc-Kit+cellswerelocalizedwithintheupperlongitudinal,innerlongitudinal,circularmuscularislayers,andepithelialcells alongthecryptsandvilli Co-localizationwithac-Kitantibodyvalidatedtheiridentity Maleandfemaleassessmentsshowednosignificant differencesinICCdistribution.ImmunostainingconfirmedPiezo1/2inICCs,supportingtheirpotentialroleincalciummodulationwithinICCs. AnalysisofPiezo1/2ΔICCknockoutmicehasjustbegun OngoingstudieswillexaminehowPiezo1/2deletionaffectsICCCa2+fluxand contractilityusingaSALSAreporterlinethatwillallowustomeasurethatpatternofCa+fluxinlivecellsisolatedfrompupsandadultmice ThisstudyadvancesourunderstandingofICCfunctioninSIPgenicGImotilityandprovidesinsightsintomechanisticpathwaysthatcoulddrive researchontherapeutictargetsformotilitydisorders
CharacterizationofPlatinum-ResistantHigh-GradeSerousOvarianCancer(HGSOC)Patient-DerivedXenograft Models(PDX):InvestigatingCancerStemCellPopulationsandTheirRoleinTumorRecurrenceand Chemoresistance
JessicaGebreel
UCLA–CIRMCOMPASSScholar
LabPI:SanazMemarzadeh,M.D.,Ph.D.
High-gradeserousovariancancer(HGSOC)isthemostcommontypeofovariancancerandaleadingcauseofdeathfromgynecological tumors.Afterinitialtreatment,upto85%ofpatientsexperiencearecurrent,andtypicallymoreaggressiveformofthedisease.Recurrent tumorsareoftenresistanttostandardplatinumchemotherapy,resultinginandaggressivediseaseprogressionandpoorpatientoutcomes Consequently,improvedtreatmentsandabetterunderstandingofHGSOCareneeded Patient-derivedxenograft(PDX)modelsareaviable modelforcancerresearch,duetotheirabilitytoretainsimilarcharacteristicstotheirparenttumorandcanbeusedaspretherapymodelsfor noveltherapeutictesting WeaimedtoestablishandcharacterizeplatinumresistantHGSOCPDXmodelsandfurtherourunderstandingof cancerstemcell(CSC)populationsandtheirroleindrivingtumorrecurrenceandresistance HGSOCcellswereisolatedfromplatinumresistantpatientsamplesandinjectedintoNSGmiceintraperitoneally(n=9) Tumorcellsandmajororganswerecollectedfrommiceandused fordownstreamanalyses.Immunohistochemistry(IHC)demonstratedtheexpressionofHGSOCmarkersincludingPAX8andp53,inparent HGSOCcells,tumorcells,andorganscollectedfrommice InvasivemetastaticspreadwasobservedinallPDXmice Toadvancethisproject, anemphasisonCSCmarkerslikeALDH(aldehydedehydrogenase),CD44,CD133,andCD117willbestudiedviaflowcytometry Weplanto sortALDH+andALDH-populationsandsubcutaneouslyinjecttheseintomicetoassesstumorigenesis Tumorgrowthwillbemonitored, followedbyanalysisusingIHCandflowcytometrytoassessphenotypicandfunctionaldifferencesbetweenthesepopulations Utilizingthese CSCmarkerswillenableidentificationofcancerstemcellpopulationswiththehopetotargetthesecellswithnovelimmunotherapiesand consequentlyimprovepatientoutcomesinovariancancer
ProfilingofgynecologiccarcinosarcomasrevealsuniquevulnerabilitiestoNKcell-basedimmunotherapies ChristopherOchoa BSCRC–RoseHillsFoundationPredoctoralTrainee LabPI:SanazMemarzadeh,MD,PhD
Gynecologiccarcinosarcomas(GyCS)arerare,aggressivetumorsoriginatingintheuterusorovaries,characterizedbybothcarcinomaand sarcomaelements Thesetumorsexhibithighrecurrencerates,resistancetoconventionaltreatments,andlimitedtherapeuticoptions We investigatedwhetherastem-likecellpopulationandanimmunoevasivephenotypedriveGyCSprogression.
Single-cellRNAsequencingofeightprimaryGyCSpatientsamplesidentifiedapotentialprogenitorpopulationwithahighstemnesssignature, supportedbyCytoTRACEandpseudotimeanalyses.Thisstem-likephenotypewasfurthervalidatedbyflowcytometry,whichrevealed heterogeneousexpressionofcancerstemcell(CSC)markers Functionalstudiesinauterinecarcinosarcomapatient-derivedxenograftmodel showedthatALDH,anenzymelinkedtomaintainingstemness,marksahighlytumorigenicpopulation.ALDH+cellsoutgrewALDH-cellsin vitroandinvivo,confirmingastem-likeroleinGyCSprogression
WealsofoundevidenceofanimmunoevasivephenotypeinGyCStumors,markedbyreducednaturalkiller(NK)cellinfiltration.Despitethis, tumorcellsexpressedNK-activatingligands(CD112,CD155)andthetumor-associatedantigenHER2,suggestingtheirpotentialsusceptibility toNK-basedtherapies.InvitroandinvivostudiesdemonstratedthatcombiningNKcelltherapieswithHER2monoclonalantibodytreatment significantlyoutperformedHER2antibody-drugconjugates,resultinginimprovedtumorclearanceandsurvival
OurfindingsconfirmthatGyCStumorspossessastem-like,immunoevasivephenotypeandhighlightNKcell-basedimmunotherapiesasa potentialstrategytoimprovepatientoutcomes
ImpairedCardiomyocyteMaturationandMitochondrialDysfunctionintheembryonicheartofdiabeticpregnancy
HarukoNakano
LabPI:Atsushi(Austin)Nakano,MD,PhD
Glucoseisacrucialenergysourceforadevelopingbaby'sheart However,whenamotherhashighbloodsugar(aconditioncalledmaternal hyperglycemia),itcanleadtoheartdefectsinthebaby.Ourpreviousresearchfoundthattoomuchglucoseinterfereswiththeheartcells' abilitytomatureproperly,partlybecauseitincreasestheproductionofcertaincellularbuildingblockscallednucleotides Tobetterunderstand howhighmaternalbloodsugaraffectsorgandevelopment,weanalyzedfetaltissuesamplesfromnormalanddiabeticpregnancies Using advancedtechniquestotrackhowglucoseisusedinthebody,wefoundthatfetalorgansfromdiabeticpregnanciesreliedmoreonaprocess calledglycolysis(whichbreaksdownglucoseforenergy)butdidnotefficientlyconnecttoanotherimportantenergypathway,theTCAcycle Wealsosawconsistentchangesinanothermetabolicpathwaycalledthepentosephosphatepathway
Furtherinvestigationofindividualheartcellsconfirmedthatfetalheartcellsfromdiabeticpregnanciesshowedreducedsignsofmaturityand anincreasedtendencytowardmitochondrialfragmentation(whichisassociatedwithstressanddysfunction) However,whenweactivated AMPKusingachemicalcalledAICAR,weobservedimprovedmitochondrialcontentandincreasedexpressionofaheartmaturitymarker,even inhighglucoseconditions.
Insummary,ourstudysuggeststhatanexcessofnucleotidesandthesuppressionofAMPKsignalingarekeyreasonswhyfetalheartcellsfail tomatureproperlyindiabeticpregnancies Thisinsightcouldleadtonewstrategiesforpreventingheartdefectsinbabiesofmotherswithhigh bloodsugar
Thechiralnematicpatternunderliescardiactissueorganizationandfunction
KawahiraNaofumi,PhD
LabPI:Atsushi(Austin)Nakano,M.D.,Ph.D.
Thehelicalmotionduringthemacroscopiccontractionoftheheartisdrivenbythetwistedarrangementofcardiomyocytes Thecardiacfibers exhibitadistinctchiralitycharacterizedbyacounterclockwiserotationfromtheepicardialtotheendocardialsurface Thisuniquestructureof thecardiacfibersformsthenematicpatternakintothatobservedinliquidcrystal Althoughthenematicpatternofcardiacfibershasbeen studiedin2D,theabsenceof3Dcharacterizationhaslimitedourunderstandingofhowthemicroscopiccellchiralitytranslatesinto macroscopiccontractilemotionoftheheart
Analysesoftheleftventricularfreewalloflight-sheetandconfocalimagingofclearedmouseheartsanddiffusiontensorMRIdatafrom humanheartsrevealedacounterclockwiserotationofcardiomyocytes ThischiralpatternisestablishedduringstagesE125-145concomitant withthedevelopmentofcompactlayerinmouseembryo.TheformationofthelocaltissuechiralityisindependentofsystemicLeft-Right signalasheterotaxyheartsdisplaythesamecounterclockwiserotationinmice Aprominentfeatureofliquidcrystalsistheformationof topologicaldefects,discontinuitiesoforientationfieldwithnematicorder Our3Danalysesrevealeddistincttopologicaldefectsintheleftapex andintheanteriorinterventricularseptum Mechanicalsimulationbasedonthesedatashowedthatthe135-degreecounterclockwisefiber rotationisadvantageousforthemacroscopiccontractilefunctionandthattheworkproductionisreducedinthetopologicaldefectregions Together,thisfirst3Danalysisofthemusclefiberorientationinmammalianheartprovidesasolidbasisforhowchiralnematicbehaviorofthe cardiomyocytesshapesthearchitectureandfunctionoftheheart Thedemonstrationofthetheoreticalfoundationfortheorganfunctionby cellulartopologyisofgreatsignificancetoourunderstandingofthephysiologyandpathologyoftheheart.
PreventingerosionoftheinactiveXchromosomewithFrepeatinfemalehumanpluripotentstemcells
Liang-WeiHuang
BSCRC–CIRMPredoctoralTrainee
LabPI:KathrinPlath,PhD
X-chromosomeinactivation(XCI)isessentialinfemalemammalstobalancethedosageofgenesontheXchromosomewithmales.XCIis achievedthroughtheexpressionofXIST,alongnon-codingRNAthatcoatsandsilencesoneXchromosome However,inhumanfemale inducedpluripotentstemcells(iPSCs)andembryonicstemcells(ESCs),prolongedcultureleadstothesilencingofXISTanderosionofgene silencingoftheinactiveXchromosome(Xi),resultinginreactivationofasubsetofgenesandfurtheraffectingdifferentiationefficiency Hence,itiscriticaltounderstandthemechanismofthesilenceofXISTinhumanasitistheleadingcauseofXierosion However,itisunclear howtheXISTissilencedinfemalehumaniPSCs Wefoundthatsimilarepigeneticpatternsbetweenasubregion:FrepeatofXISTandFIRRE locusduringXierosionfromthepublisheddata Inaddition,wefoundsimilartranscriptionfactorsbindtoXISTandFIRRElocusonlyinXi Consequently,weareinterestedinhowtheseregionregulatetheprocessofXISTsilencing.Importantly,thesetworegionsformlong-range interaction,termedsuperloop,exclusivelyintheXi Therefore,weareinterestedinwhetherthesuperloopisdisruptedduringXierosion,and whetherthestructureofthesuperloopfacilitatesthemaintenanceofXISTexpression.FromtheHi-Cresultgeneratedfromtheearlyandlate passageshumaniPSCs,wefoundthatFIRRElocusformssuperloopwithDXZ4,anotherlocusknowntoformsuper-loopsontheXi,onlyinthe earlypassageiPSCs Hence,thesuper-loopwasdisruptedduringXierosion,indicatingthecontributionofXimaintenancefromhigher-order structureoftheXichromosome Wewillfurtherdeciphertheroleofthe"common"transcriptionfactorsbetweenXISTandFIRREregardingto theXISTexpressionandsuperloopformationonXi OurfindingswillclarifythemechanismsofXISTsilencingandmayhelppreventXierosion inhumaniPSCs.
Metabolicregulationofstemcellfatethroughthemodulationoftheextracellularmatrix
ZeenatRashida,PhD
BSCRC–CIRMPostdoctoralTrainee
LabPI:KathrinPlath,PhD
Thevariousfunctionsofstemcellsduringdevelopment,regenerationandinhomeostasisaredeterminedbytheirtrajectorytowards differentiation,self-renewal,orquiescence,anddisruptingthesepathscanleadtoavarietyofdiseases Whilegeneticfactorsregulate pluripotencyandcellfate,increasingevidenceshowsthatextrinsicfactorslikethenutrientenvironmentandtheextracellularmatrix(ECM)can regulatestemcellfunctions TheECMisacomplexnetworkofproteinsthatsurroundsthecells,providesstructuralsupportandtransduces mechanicalandbiochemicalsignalstoinfluencecellbehavior,includingproliferation,migration,anddifferentiation Despiteitsimportance,the roleofECMinregulatingstemcellfunction,particularlyduringinducedpluripotentstemcell(iPSC)reprogramming,remainsinadequately explored Ourworkaimstoaddressthisgapbyinvestigatinghownutrient-drivenchangesinmetabolisminfluenceECMcompositionandstem cellreprogramming Specifically,wefocusonVitaminC(VitC),anessentialnutrientthatenhancespluripotencyinductionandiscommonly usediniPSCculturemedia WeproposethatVitCmodulatesECMdepositionthroughitseffectoncellularmetabolism,particularlyby enhancingglycosylationpathwaysnecessaryforglycosaminoglycanandcollagenproduction.This,inturn,altersECMcompositionand promotesiPSCgeneration ThesefindingsprovidenovelinsightsintohowmetabolismandECMinteracttoregulatestemcellfatetransitions OurworkalsoadvancestheunderstandingofECM’sroleinstemcellfunction,withbroadimplicationsforimprovingstemcelltherapies,tissue engineering,andregenerativemedicine,aswellasaddressingdiseaseslinkedtoECMdysregulation
InvestigatingErosionoftheInactiveXChromosomeinHumanPluripotentStemCells
SophiaPeavy
UCLA–CIRMCOMPASSScholar
LabPI:KathrinPlath,PhD
Reprogrammingfemalehumanfibroblaststoinducedpluripotentstemcells(hiPSCs)hasenablednewdiseasestudiesandpossibilitiesin regenerativemedicine However,allfemalehiPSCsdisplayaculture-inducederosionofX-chromosomeinactivation(XCI)whichimpairs differentiationmechanismsthereforelimitingtheutilizationoffemalehiPSCs Inthisprocess,XISTRNA,whichisresponsibleforsilencingthe genesontheinactiveXchromosome(Xi),islost,whichleadstotheXire-exhibitinggeneexpression.ThecauseofXierosionisultimatelyXIST loss,butthemechanismsleadingtoitsdownregulationarestillunknown ThisprojectaimstofindapracticalsolutiontopreventXierosion andtoestablishabetterunderstandingofthemechanismscontributingtotheprocess.ThePolycombRepressiveComplex2(PRC2)is responsiblefortrimethylatinghistoneH3atlysine27(H3K27me3)andishighlyenrichedontheXiinhiPSCsbutnotinfibroblasts,albeit H3K27me3accumulatesontheXiinboth AtimecourseofreprogrammingintermediatesrevealsstrongXilocalizedEzh2enrichmentoccurs lateinthereprogrammingprocessaftermesenchymaltoepithelialtransition SincePRC2andH3K27me3facilitategenesilencingontheXi downstreamtoXISTonthenormalXi,wehypothesizethatthehighPRC2enrichmentontheXi,whichisspecifictohiPSCs,contributesto theXCI-erosionprocess.WeculturedhiPSCswithPRC2inhibitorstoaddressthisquestionandfollowedXierosionovertime.Therewasno evidenceofasignificanteffectofPRC2inhibitiononerosion,however,wedemonstratedtheabilitytodramaticallydepleteH3K27me3globally andlocallyontheXiinfemalehiPSCs.Additionally,wehavebeguninvestigatingbothcisandtranselementsinvolvedinXCItobetter understandtheirrelationshiptoXierosion Xierosionisadeeplycomplexprocessthatappearstobeestablishedbymanydifferentfactorsand conditions
AlexanderLee,PhD
LabPI:KathrinPlath,PhD
Adipocyte-derivedstemcells(ADSCs)areapromisingsourceofstemcellsforregenerativemedicineduetotheirrelativeeaseofisolationand multipotentdifferentiationcapacity However,theirlimitedpluripotencyrestrictstheirbroaderclinicalapplications Inducedpluripotentstem cells(iPSCs)offeramoreversatilealternativebuttypicallyrequireoverexpressionofoncogeneslikec-Mycviaviralvectorstoachieve pluripotency,reducingtheirclinicalutility Inthisstudy,weexploreasmall-molecule-basedapproachtodirectlyreprogramADSCsintoiPSCs, followingthemethodologyoutlinedbyLiuyangetal.(2023).Ourgoalwastoovercomethechallengesofcurrentreprogrammingtechniquesby utilizingsmallmoleculesalone,thusenhancingclinicalsafety Byapplyingacocktailofepigeneticmodulatorsandsignalingmolecules,we successfullyreprogrammedADSCsintoiPSCsthatexhibitcharacteristicpluripotentmarkersandself-renewal.Ourresultsdemonstratethat small-molecule-basedreprogrammingcanpotentiallyserveasanalternativetoconventionalviralreprogrammingmethods,whichcouldbe moresuitableforclinicalapplications Thesefindingscontributetoadvancingcell-basedtherapiesbyenablingthegenerationofpluripotent stemcellsfromareadilyaccessibleandclinicallyrelevantcellsource Thebroaderimplicationsofthisworkmayincludeimprovedstrategies forpersonalizedmedicineandregenerativetherapies,facilitatingtheuseofpatient-specificiPSCsfordiseasemodelingandtherapeutic interventions.
AnaHernandez
UCLA–CIRMCOMPASSScholar
PearlQuijada,PhD
Slit2isaglycoproteinknownforitsroleinaxonguidanceandangiogenesisinthecentralnervoussystem.However,itsfunctioninadult cardiacfibroblastshasnotbeenwelldefined WehypothesizethatSlit2regulatestheexpressionofcriticalgenesinvolvedinextracellular matrix(ECM)productionandangiogenesis,regulatingfibroticremodelingintheheart.Weisolatedadultcardiacfibroblaststoinvestigatethe roleofSlit2inthecardiacfibroblasts Wetreatedthemwitheithervehicleortransforminggrowthfactorb-1(Tgfb1)andAngiotensinII(AngII) toinducefibroblastactivationandwithorwithoutrecombinantSLIT2proteinfor48hours Totestourhypothesis,RNAwasisolatedfromadult cardiacfibroblasttreatmentgroupsandsubjectedtoquantitativeqPCRtomeasuretheexpressionlevelsofangiogenicandfibrosis-related genes OurresultsshowthatSlit2enhancestheupregulationofPostn,Acta2,andAngpt1,indicatingitssignificantregulatoryroleinECM productionandangiogenesisduringfibroblastactivation.Conversely,expressionlevelsofgeneslikeVegfcandPdfgaweredownregulatedor unchanged OurdatasuggeststhatSlit2maysignificantlyregulateECMproductionandangiogenesisduringischemia-inducedcardiac remodelinginvivo.
Invivopartialcellularreprogrammingofmyofibersleadstosystemicrejuvenationacrossothertissues
MinoriOhashi,MD,PhD
BSCRC–CIRMClinicalFellow
LabPI:ThomasA.Rando,M.D.,Ph.D.
Skeletalmusclehealthisacriticalpredictoroflongevityandhasaprofoundimpactonorganismalaging Theprogressivedeclineinskeletal musclefunctionwithagingcontributestofrailtyandage-relateddisorders Importantly,musclespecificinterventionshavedemonstrated significantpotentialinslowingdownorevenreversingtheagingprocess Partialcellularreprogrammingisaninnovativeanti-agingtechnique thatresetsthebiologicalclockandrestoresyouthfulcellularfeaturesbyoverexpressingkeyreprogrammingfactors.Skeletalmusclespecific reprogramminghasshownpromiseinpromotingskeletalmusclerejuvenationandrestoringregenerativefunction Inthisstudy,wetestedthe hypothesisthatskeletalmuscleplaysacentralroleinregulatingaging Weproposedthatrejuvenatingskeletalmuscleusingpartialcellular reprogrammingcouldleadtosystemicanti-agingeffectsacrossthebody Usingskeletalmuscle-specificpartialcellularreprogramming techniquesinagedmice,weobservedrejuvenationnotonlyinskeletalmusclebutalsoinotherorgans,includingthespleen,kidney,andskin Thesesystemiceffectsarelikelymediatedbymuscle-derivedsecretoryfactorsthatenterthebloodstreamandinfluenceothertissues Our findingsunderscorethepotentialoftargetingskeletalmuscletoachievesystemicrejuvenation Furthermore,identifyingandcharacterizingthe muscle-secretedfactorsinducedbypartialcellularreprogrammingcouldpavethewayforthedevelopmentofnoveltherapeuticcandidatesfor systemicanti-agingtreatments
YutzilHerrera
UCLA–CIRMCOMPASSScholar
LabPI:ThomasA Rando,MD,PhD
LipidDroplets(LDs)areorganellespresentinthecytoplasmacrossvariouscelltypes Theirprimaryroleinvolvesstoringfreefattyacids (FFAs)forsubsequentuseinmanymetabolicpathways,asasourceofenergy,andformembranesynthesis.Thegoalofthestudyisto investigatewhetherchangesinLDdynamicsimpactSCdifferentiationandtheabilitytofuse WetargetedenzymesinvolvedinLDformation andbreakdownbyusingspecificinhibitors:Atglistatin(ATGLin)toincreasethenumberofLDs,andA922500andPF-06424439(DGATin)to inhibitLDformation WefoundthatATGLinsignificantlydecreasedmyoblastfusionby79%(p=00003)at3hoursand38%(p=00061)at5 hours,andtheirabilitytogrowwhencomparedtocontrol(36%MHC+areavs27%,p<00001) IncubationofSCswithDGATinresultedinan increaseofmyosinheavychain(MHC)positiveareaby18%whencomparedtothecontrol(p=0410) DGATin,LinoleicAcid(LA),and Ferrostatin-1partiallyreversedthenegativeeffectsofATGLin ThosedataindicatethatLDdynamicsmayplayanimportantroleinmyogenic progression.TheobservedresultslikelyarisefromanaugmentedcellulardemandforFFAsduringperiodsofheightenedgrowthandthe capabilityofFerrostatin-1topreventferroptosis,celldeathduetolipidperoxidation Theexactmechanismunderlyingthisrelationshipandits potentialimplicationsforthemyogenicprogression Thisdatamighthelpdevelopnewtherapiestargetingconditionsresultinginreduced muscleregenerationcapacity
DevelopingahumaniPSC-derivedmodelofwhiteadipocytestostudythecontributionofgeneticriskfactorsto metabolicdiseases
HannaLiliom,PhD
LabPI:DéboraSobreira,PhD
Metabolicdisorderssuchasobesityandtype2diabetespresentmajorhealthproblemsworldwideandtheriskoftheirdevelopmentistoa certainextentheritable Genome-wideassociationstudies(GWAS)haveidentifiednumerousgeneticvariantslinkedtotheseconditions,but comprehendingtheirmolecularmechanismsremainschallenging,largelyduetomanydisease-associatedvariantsbeingnon-coding Tissue type,celltypeanddevelopmentalstageareimportantdeterminantsofhowrisklociexerttheireffects,emphasizingtheimportanceof developingaccuratehumancellularmodelsforinvitroexperimentstoidentifycausalriskvariants,aswellastoelucidatetheirmechanismof action Adipocytesofwhiteadiposetissuearecrucialplayersinmaintainingwhole-bodyenergyhomeostasisandregulatingthemetabolic responsethroughvariouspathways,andtheirfunctionisimpairedinvariousmetabolicdisorders Directstudyofhumanwhiteadipocytesis challengingsincethematurecellsarepost-mitotic,aswellasfragileandbuoyantduetotheirlargeintracellularlipiddroplet.
Toovercomethesechallenges,wearedevelopinganinvitromodelofwhiteadipocytesderivedfrominducedpluripotentstemcells(iPSCs) Utilizingtheknownfunctional-phenotypicplasticityofbeigeadipocytes,weadaptedapublisheddirecteddifferentiationprotocoltogenerate maturebeigeadipocytesfromhumaniPSCsthroughamesenchymalstemcellphase,andtestedwhetherwecouldshiftthematurebeige adipocytestowardsawhiteadipocyte-likeidentitythroughdifferentmediaandreagentcompositions.Wecomparedcellmorphologyandthe molecularphenotypeoftheiPSC-derivedbeigeandwhite-likeadipocytes Ourresultsdemonstratethat‘whitening’induceddrasticincreasesof theintracellularlipiddropletsizesanddownregulationofkeymarkersassociatedwithbeigeadipocyteidentityinthe‘whitened’adipocytes OurgoalistorefineourcellularmodelandestablishtheframeworkofitsuseasatoolforstudyingGWAScandidatesimplicatedinmetabolic disorders,enablingustounderstandhowriskvariantsexerttheireffectwithinthewidergeneticcontextofadiversesetofindividuals.
HarryChen
LabPI:MelissaJ
Spencer,PhD
DuchenneMuscularDystrophy(DMD)isararegeneticdiseasecausedbyframeshiftingmutationsinDMD,resultinginalackofdystrophin proteinproduction WhileAAV-microdystrophingenereplacementtherapywasrecentlygivenacceleratedFDAapprovalforDMD,the therapeuticefficacyappearstoreducewithtime,likelyduetoturnoverofskeletalmuscleandreplacementbymusclestemcells Redosing AAVisnotanoption;however,astrategytoretaindurabledystrophinistotargetMuscleStemCells(MuSCs)withCRISPR/Cas9tocorrect genomicDNAintheproliferatingcellpopulation Wehavepreviouslyidentified5MuSCtargetingand5SkeletalMuscle(SkMu)targetingAAVs throughdirectedevolutionofa7aminoacidsequenceinvivo,insertedbetweenaminoacids589Aand590QinthesurfaceregionVIII(VR-VIII) ofAAV9 Individualvalidationofthesevariantsinhumanmusclecellshasrevealedthattheyperformnearly40xbetterthanwtAAV9and15x betterthanMyoAAV1AinMuSCtargetingwhileretainingtheirmyotubetropism.Inaddition,invivostudiesinmicehavedemonstrateda similarenhancedtropismcomparedtowtAAV9 Thesedatahavebeenconfirmedthroughflowcytometry,luciferase,andqPCRofviral genomes Whilefurthercharacterizationmustbedone,thesenewvariantsprovideapotentialnewplatformforachievinglong-termdystrophin restorationanddurabilitywhenAAVisusedtodeliverCRISPRcomponentsinDMDandothermyogenicdiseasesthatinvolvemuscle degenerationandregeneration
Stimulus-dependentneurodevelopmentalmechanismsinchromosome16p11.2microdeletionsyndrome
RachelFox
BSCRC–CIRMPredoctoralTrainee
LabPI:MichaelF Wells,PhD
Copynumbervariation(CNV)atthechromosome16p11.2locusisassociatedwithneuropsychiatricandneurodevelopmentaldisorders (NDDs) Theheterozygousproximaldeletionofchromosome16p112(16pdel)accountsfor~05%ofautismspectrumdisorder(ASD)cases andoftenco-occurswithmacrocephaly,developmentaldelay,languagedisorders,andobesity.Themechanismsthatlink16pdeltoASDand driveassociatedphenotypesremainunknown,andnocleartherapeutictargetshavebeenidentified Neuralprogenitorcells(NPCs)respondto spatialandtemporalcuesastheyproliferateanddifferentiate,establishingregionalidentityanddeterminingtheproportionofneuronsandglia inthedevelopingbrain Interconnectedandhighly-coordinatedsignalingpathways,suchassonichedgehog(SHH),regulatethisprocessin NPCs,andgeneticmutationsintheseandrelatedsignalingpathwaysarealsoassociatedwithNDDs In16pdeliPSCs,researchershave observeddifferentialexpressionofdevelopmentalsignaltransductionrelatedgenes,buttheextenttowhichaberrantresponsetopathways themselvesdrivesdownstream16pdeldysfunctionhasnotbeeninterrogated Inacellvillageof16pdelandcontroliPSC-derivedNPCstreated withSHH,wehaveobservedthat16pdelNPCsappearhyperresponsivetoSHHstimulation.ExposuretoSHHduringneurodevelopment thereforeprovidesacontextinwhich16pdelcellulardysfunctionmaybemostdysregulatedandmayunderliephenotypeslikemacrocephaly
VAMP2/3-dependentexocytosisinoligodendrocytesisrequiredformaintainingoligodendrocyteplasma membraneintegrityandthehomeostaticstateofmicrogliaandastrocytes
CaitlinHooper
UCLA–CIRMCOMPASSScholar
LabPI:YeZhang,PhD
Oligodendroccytesproduceextensivemyelinmembranestoinsulateaxons Asaxonsandmyelinexpandduringdevelopment,the oligodendrocytes’plasmamembranesundergophysicalstretching Inmultiplesclerosis,thecomplementmembraneattackcomplexforms poresinthemyelinmembrane.Giventhechallengestoplasmamembraneintegrity,maintainingthisintegrityisvitalforoligodendrocyte functionalityandsurvival,althoughtheunderlyingmechanismsareunknown WehypothesizedthatVAMP2/3-dependentexocytosisactively repairsplasmamembranedamageinmatureoligodendrocytes Totestthis,weemployedgenetictoolstoinhibitVAMP2/3-dependent exocytosisinthesecells OurfindingsindicatethatinhibitingVAMP2/3-dependentexocytosiscompromisesoligodendrocytemembranerepair followinglaser-inducedmembraneinjuryinvitro Intriguingly,blockingoligodendrocyteexocytosisinvivoresultsintheprolongedreactivityof microgliaandastrocytes Ongoingstudiesaimtodeterminewhetheroligodendrocytemembraneintegrityiscompromisedinvivoandhow oligodendrocyteexocytosiscontributestomaintainingthehomeostaticstateofmicrogliaandastrocytes Thisresearchshedslightonthe underexploreddomainofmyelinplasmamembranerepair,revealingpotentialtherapeutictargetsfordemyelinatingdisorders.Additionally,it unveilsnewinsightsintotheinteractionbetweenoligodendrocytes,microglia,andastrocytes