
3 minute read
GROB-WERKE GmbH & Co. KG
Name ›
Address/P.O. Box › Postal Code/City › State › Contact Person › Telephone › Fax › Email › Website ›
Social Media › Number of Employees › Founded (year) ›
Areas of Activity ›
Annual Turnover ›
FRIWO Gerätebau GmbH
Von-Liebig-Str. 11 48346 Ostbevern North-Rhine Westphalia Gerrit Menzel +49-2532-81-0 +49-2532-81-112 hello@friwo.com www.friwo.com www.friwo-shop.com
F LI Q
2,600 1971
| Power Supplies | Chargers | Battery Packs | LED Drivers | Motor Control Units | Drive Systems
€100m
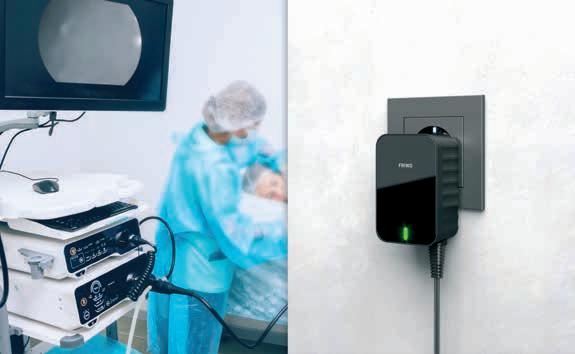
Medical power supplies: the heart of your application
Heart failure – one of the most dreaded incidents in medicine. If the heart fails, so does everything else. The same applies to your application’s power supply: if its service diminishes, the entire system will be affected – which can be disastrous in medical technology.
It is therefore of utmost importance to have a reliable partner for power supplies. We want to meet this need: ever since the invention of the world’s first plug-in power supply in 1971, our customers have been relying on our expertise, since we have already manufactured more than one billion power solutions. 50 years of know-how and our German engineering skills safeguard the delivery reliability of your application – and thus the maximum safety of the patient.
One-stop-shop for power supply and drive system solutions
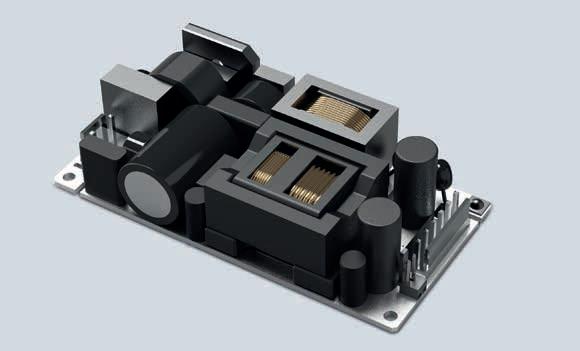
Today FRIWO is an international manufacturer of stateof-the-art chargers, battery packs, power supply units, and LED drivers. In addition, the company acts as a system provider and supplies digitally controllable drive solutions from a single source. The product range includes all components required for a modern electric drive train: from the display and engine control to the battery, charger, and control software.
Innovative solutions for the toughest requirements
FRIWO’s medical power supply solutions are designed for the most demanding conditions. Whether it is about surviving falls during hectic emergency treatment with a patented encapsulation technique, protecting the patient with minimal leakage currents from ≤ 10 μA, or securing the power supply with redundant systems and battery-operated backup solutions: FRIWO develops and manufactures reliable power supplies.

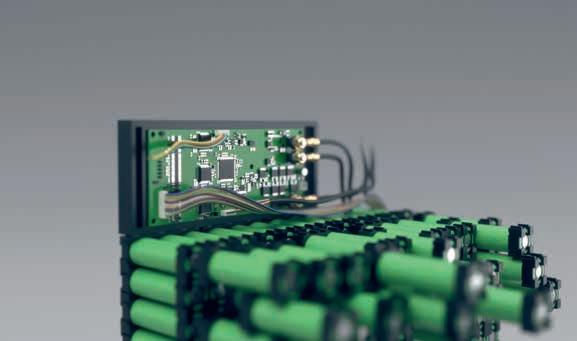
We always think from the user’s point of view and develop innovative concepts to make everyday medical life easier. In the field of inductive charging technology, for example, we already offer contactless energy solutions with up to 150 W transmission power and parallel data communication. The use of inductive charging technology enables the development of medical devices with completely closed housings – an invaluable advantage for sterile workplaces!
FRIWO always develops and produces taking into account possible future changes in standards and increasing efficiency requirements in order to ensure smooth and long-term marketing of your product. FRIWO is also at your side as a reliable partner for new legal regulations such as the Medical Device Regulation (MDR) or the upcoming UKCA.
Global network for maximum flexibility
Thanks to our competent global network, customer requirements and wishes can be flexibly implemented. With modern engineering and production centres in Germany and Vietnam, as well as sales offices in Europe, Asia, and North America, we are present in all important markets of the world.
In particular, certification according to ISO13485 is an additional quality promise for medical technology, as it defines regulatory requirements for a comprehensive management system of medical device manufacturers. As an internationally recognised standard, this regulation contains guidelines for design and development, production, installation, maintenance, and sales. This certification places high demands on the exact adherence to all process steps. Particular attention is paid to risk management and consistent and complete documentation; not only with regard to minimising risks, but also with regard to optimum traceability of products and components.










