




Experience newfound clarity with the Nexera XS inert UHPLC. Offering reliable, robust performance, the Nexera XS inert represents a new peak in the analysis of biopolymers. It features a metal-free sample flow path prepared from corrosion-resistant materials, so that results will be clear and unaffected by sample adsorption or surface corrosion. Together with a new range of consumables, Shimadzu now offers the complete solution for bioanalysis.
Unconstrained recovery and sensitivity
Bioinert flow path prevents sample loss due to adsorption.
Clear resolution without restrictions UHPLC performance for high efficiency bioanalysis.
Assured reliability and reproducibility
Corrosion-resistant material ensures long-term stability and reliable data acquisition.

Ultra High Performance
Liquid Chromatograph
Nexera XS inert

Learn more!

ADRIEN SAMSON is Healthcare Policy Senior Manager at EuropaBio, the European Biotech Industry Association. He leads the association’s policy work on health biotech issues. He has expertise on EU pharmaceutical and industrial policies and works with a diverse range of stakeholders, including policymakers, regulators, industry representatives, patient groups, and research organisations, to promote the benefits and potential of biotech for health and society.
The 2023 State of the Union speech saw biotechnology rise as a priority for the European Union. It has been designated as a critical technology for Europe’s economic security. In March 2024, the European Commission presented its biotechnology and biomanufacturing strategy with a clear leadership ambition for Europe’s biotech industries. The Letta Report on the future of the Single Market, published April 2024, proposed a fifth freedom on research and innovation which would directly benefit biotech.
In a sign of how quickly policy priorities change in Brussels, six months before the European Commission published its much-anticipated revision of the EU General Pharmaceutical Legislation (GPL). The proposals make no reference to biotech and the impact on the biotech ecosystem was not specifically assessed. The revision proposed several welcomed improvements, including reducing the assessment periods and the creation of sandboxes for cutting edge products, but also proposed a lower baseline incentives and unpredictable modulation for novel medicines, including for rare diseases that will negatively impact the biotech ecosystem.
Within healthcare, biotech is increasingly the primary source of new therapies, bringing previously untreatable diseases within reach and transitioning from ‘manage’ to ‘cure’ with improvements to quality of life, freeing patients, families, and healthcare systems. Higher risk and long development timelines characterise the translation of biotech into therapies, with smaller companies being the primary vehicle for translation of Europe’s research into development pipelines. The pathway to patients is a highly collaborative ecosystem between companies of all sizes. Innovators, especially emerging and small companies, are highly reliant on a strong and predictable incentives framework to secure early investment for long term programmes.
The GPL is a force for growth in EU biotech innovation and patient benefit. The Commission’s proposals will negatively impact Europe’s biotech ecosystem, with small innovators are at greatest risk and with them the EU’s engine for novel medicines. Biotech companies are strongly inter-dependent for successful development of medicines. Proposed changes negatively impact partnerships and Europe’s healthcare autonomy. Reducing incentives and certainty for early programmes is a barrier to the delivery of innovative medicines through biotech.
Despite the European Parliament having adopted its position on the proposals in record time, it must not be forgotten that GPL is legislation for future innovation and will only become law at the end of the decade. There is still time and opportunity for the Member States in the Council to ensure the GPL is aligned with Europe’s priorities on competitiveness and open strategic autonomy, and delivers a more ambitious vision for the future of biotech innovation.

The Three R (Replacement, Reduction and Refinement) principles developed over 60 years ago provide a framework for more humane lab animal testing and, more importantly, less of it. A range of in-vitro model systems that might one day replace animal-based programmes is now out there, but only recently has the use of multi-organ chips gained serious momentum. High-tech developments are increasingly allowing the replication of human tissues and organs for testing purposes.
6 European Commission raises hopes for biotech boost in next term
12 Parliament approves highly criticised EU pharma package with minor improvements; European Court of Auditors presents flawed mobility report; EuropaBio criticises rules for joint clinical assessments
13 Clinical trials: deregulation to bring Germany back to the forefront of the European Union
14 ECCP 2024 – doomed to innovation
16 Interview: Montse Daban, President, Council of European Bioregions (CEBR)
24 Interview: Alexander Schueller, CEO cellvie AG, Zurich
27 Analyst commentary
28 European Biotech Stocks
31 Swiss Biotech Day(s): vibrant ecosystem showcase
34 Carbios starts biorecycling of PET waste
36 Update on clinical trials
40 Interview: Prof. Dr. Salah-Eddin Al-Batran Frankfurt Institute for Clinical Cancer Research IKF GmbH
60 Northern Europe: Sweden, Denmark, Norway and Finland
62 Western Europe: France, Belgium, The Netherlands and the UK
64 Central Europe: Germany, Switzerland and Austria
66 Southern Europe: Italy, Spain, Greece, Slovenia and Portugal
68 Eastern Europe: Poland, Hungary, Lithuania, and the Czech Republic
SCIENCE & TECHNOLOGY
69 Next-gen dry AMD drug
70 Cure for Hurler sydrome
71 Fish flesh goes production
72 RNA replacing pesticides
73 Microbial plastic-eaters
74 Smart paths to cultured coffee
75 mRNA versus metabolic disease
44 Biopeople
76 News from Biotech Associations
80 Company index/ New product
81 Events
82 Encore
IMPRINT European Biotechnology (ISSN 2364-2351) is published quarterly by: BIOCOM Interrelations GmbH, Jacobsenweg 61, D-13509 Berlin, Germany, Tel.: +49-30-264921-0, Fax: +49-30-264921-11, Email: service@european-biotechnology.com, Internet: www.european-biotechnology.com; Publisher: Andreas Mietzsch; Editorial Team: Thomas Gabrielczyk (Editor in Chief), Derrick Williams (Co-editor), Dr. Georg Kääb, Uta Mommert, Maren Kühr; Advertising: Oliver Schnell, +49-30-264921-45, Christian Böhm, +49-30-264921-49, Andreas Macht, +49-30-264921-54; Distribution: Ianuaria Cipolletta, +49-30-264921-72; Graphic Design: Michaela Reblin; Production: Martina Willnow; Printed at: Königsdruck, Berlin; European Biotechnology Life Sciences & Industry Magazine is only regularly available through subscription with a BIOCOM CARD. Annual subscription BIOCOM CARD Europe: €80 for private individuals (students €40) incl. VAT, €120 plus VAT for corporates. Prices includes postage & packaging. Ordered subscriptions can be cancelled within two weeks directly at BIOCOM AG. The subscription is initially valid for one calendar year and is automatically renewed every year after. The subscription can be cancelled at any time and is valid until the end of that calendar month. Failures of delivery, which BIOCOM AG is not responsible for, do not entitle the subscriber to delivery or reimbursement of pre-paid fees. Seat of court is Berlin, Germany. As regards contents: individually named articles are published within the sole responsibility of their respective authors. All material published is protected by copyright. No article or part thereof may be reproduced in any way or processed, copied, and proliferated by electronic means without the prior written consent of the publisher. Bound-in inserts: Life Science Factory, Göttingen; Cover Photo: © William - stock.adobe.com; ® BIOCOM is a registered trademark of BIOCOM AG, Berlin, Germany.
With the presentation of a biotech and biomanufacturing initiative shortly before its putative re-election, the EU Commission has raised hopes in the biotech sector for good framework conditions in Europe. However, in addition to the vague promise to draft by an EU Biotech Act, the non-binding paper contains many ifs and buts.


Within a single year, new treatments for obesity – incretin agonists – have propelled Novo Nordisk and Eli Lilly to first and second place in the global ranks of top-selling pharma companies. In a global race, developers are now targeting hunger control with combination therapies that boost the effect.
47 New event season
48 CPHI Milan 2024, Milan, Italy
50 Global Bioeconomy Summit, Nairobi, Kenya
52 Bio-Europe 2024, Stockholm, Sweden
With a grand gesture, the European Court of Auditors (ECA) issued its first statement on the future of the internal combustion engine in the EU with a ‘report’ – and cast a clear vote against synthetic and biofuels and in favour of electric mobility in passenger transport. That would be all well and good, if only the statement of opinion disguised as an analysis were not riddled with outdated facts and errors that also politically obstruct the future of a sustainable solution to the fuel problem in heavy goods transport. The auditors are apparently unaware that, in addition to bio-based fuels, there are also fuels that can be produced directly from sunlight and a few minerals without any sourcing problems. Several European and US companies are also working on diesel from nonGM algae that are 70% oils that can be easily converted into certified diesel fuel.
The auditors also failed to realise that these and other biofuels are not overpriced, but can be produced at a price of €1.50/litre even before upscaling. There are just two problems. First, it will cost money to build the ponds for algae cultivation, and second, these would have to be located in places that get a lot of sunlight, such as Africa. Hopefully postpandemic Europe has learnt from its mistakes in the past, as well as a thing or two about cooperation.
 Thomas Gabrielczyk Editor-in-Chief
Thomas Gabrielczyk Editor-in-Chief

EU Europe is good when it comes to visions. The EU pioneered a bioeconomy strategy, it was first globally to authorise a biosimilar and establish rules for ATMPs. The only thing the Eurocrats are not so good at is implementation. However, in the power vacuum before the elections of the new EU Commission, the old one has raised hopes for an Biotech Act that might unveil the potential of biotech in Europe.
At the end of March, Danish EU VicePresident Margrethe Vestager took to the lectern and announced – unusually clearly for the EU Commission, which is never at a loss for words – eight measures to realise the potential of biotechnology in Europe through a EU Biotech and Biomanufacturing Initiative.
“Everywhere across Europe, we are faced with the same challenges: climate change affects us all. Resource scarcity affects us all,” said Vestager. “Biotechnology can contribute to solving these challenges. Biotech also largely supports Europe’s economy and contributes to our competitiveness, with high growth potential and labour productivity. And by reducing Europe’s dependency on fos -
sil-based input and other sources of raw materials biotech also increases circularity and strengthens our path towards independence of fossil fuels,” the Commissioner for the Digital Age stressed. “With today’s proposal we want to create the right environment for this sector to grow and deliver global solutions to societal and environmental problems,” Vestager closed applauded by EU industry federations Cefic, EuropaBio, Bio-based Industries and others.
Nobody in the life sciences sector had seriously believed yet in the non-legislative EU Biotech and Biomanufacturing

Initiative, which Commission President Ursula von der Leyen had promised in her State of the Union address in September 2023. This was because von der Leyen failed to make some promises of her legendary speech come true. The most important was to prioritise the underfunded EU biotech sector with an investment boost of up to €160bn through a new vehicle termed STEP (the Strategic Technologies for Europe Platform). In the end, the budget earmarked for STEP’s priority areas, namely biotech, cleantech and IT/deeptech, failed to be approved due to the resistance of EU member states, whose representatives did not want to give any extra cent even as innovation support, and particularly not the €10bn of seed capital for setting up STEP. With the financial cut, STEP, which was the EU’s central driver to catch up internationally now appears like a toothless tiger. The instrument that was formally adopted by the EU Council and Parliament at the end of February, does have some money available through the redistribution of existing EU money from 11 EU financial instruments. However, the starting capital for STEP has been shrinked to €1.5bn. Zero extra investment is now expected to finally unleash the huge potential of bio technologies for setting up a fossilfree industrial production in Europe. As no extra money is available for the EU’s new biotech priority, the Commission is focussed on making the EU regulatory framework and its implementation smoother. The sector‘s industry inter-




Profit from


















est group EuropaBio stressed the importance of rapidly addressing regulatory challenges in a statement. “If Europe is to succeed, this initiative has to rapidly transform ‘rhetoric’ into policy and legislative action for competitiveness, enabling innovators to thrive and grow, and creating long-term investment into infrastructures, employment and skills in Europe,” the association stressed.
“What we hear from industry is that there are indeed huge differences, in what time it takes to have a product that is finalised and tested from the side of the business to being able to market it,” Vestager explained presenting the proposal. “The claim is that in the US you can do that in two to three years, but within the European Union, it will take you six to eight years,” she added.
So what exactly can we expect from the eight actions (see below) published along with the legally non-binding communication about the biotechnology and biomanufacturing initiative? Biotech entrepreneurs can be pleased that EU policy is increasingly recognising the importance of biotechnology, albeit the initiative includes many ifs and buts
and still relies on safeguarding through lengthy studies instead of immediate implementation. In it, the EU Biotech Act is also described vaguely as an option instead of taking bold and swift action following the prioritisation of biotechnology. Measured against the claim to establish Europe as a leading biotech region, the Commission is publicly denying important facts.
According to EuropaBio Director Claire Skentelbery “we actually have everything we need for a good location: excellently trained staff, good research and expertise. We have also seen during the pandemic that much is possible if the political will is there.” European biotech, medtech and CDMO CEOs, however, recently complained at a panel moderated by the Brit that they always face exactly the same problem: a lack of harmonised rules and extremely slow processing times due to downstream EU bureaucracy.
Vestager’s initiative picks up on this and promises improvement but ignores that actually, Europe is lagging behind in biotechnology. The US market share of biotech sales is around 60%, that of the EU 12%, followed by China with 11%, which is growing much faster due to massive strategic state subsidies. The US

administration has already recognised the geopolitical importance of its leading role in biotech and protect it against the emerging Middle Kingdom, which has already left it behind in terms of the number of clinical gene therapy studies, by classifying biotech as important for the national security. Europe does not protect its sector. Figures from the Commission’s scientific service, the JRC, show that the USA has six times as many jobs in biotechnology in relation to the population as Europe. The EU is also increasingly losing ground in terms of biotech patent applications (as of Patstat 2023) compared to emerging China, and even more so compared to the USA (see graph). The fact that the EU’s current biotech strategy is 22 years old, while the USA has only recently set itself ambitious goals in pharmaceutical, industrial and plant biotechnology, shows the real pressure on Europe and a certain verbal whitewashing of EU policy. Instead of concentrating on financial support, it is focussing on improving the political framework without, however, clearly naming and eliminating past mistakes. EuropE an Biot Echnology has learnt from Commission circles that it is important to publicly name future priorities even before the new EU Commission is elected and that the new bioeconomy strategy planned for the end of 2025 will have ‘surprising’ things in store.
Although the German Christian Democrats support the re-nomination of current EU Commission President von der Leyen, who only received around 52% of votes in favour in the last EU Parliament confirmatory vote, the parent party is calling for the Green Deal, which many Christian Democrats see as a bureaucratic monster that is stifling any growth in Europe, to be de-prioritised. Instead, more emphasis should be placed on competitiveness and growth. How this fits in with the half-hearted commitment of the biotech and biomanufacturing initiative remains to be seen. What is clear, however, is that von der Leyen’s EPP can



The US and China are the top performers in more investment performance metrics than Europe, exhibiting stronger growth trends
count on a clear majority in the European elections and that a second term in office is therefore likely.
But now to the actions associated with biotech/biomanufacturing initiative, presented by Vestager, and the associated EU thinking. According to the EU paper, the EU biotechnology and biomanufacturing sector is facing several challenges: research and technology transfer to the market, regulatory complexity, access to finance, skills, value chain obstacles, intellectual property, public acceptance and economic security. The EU biotech and biomanufacturing initiative aims to map current challenges and barriers for biotechnologies and biomanufacturing and proposes eight actions “to address these challenges in a timely manner”. Most of them will require lengthy studies instead implementing action:
› Leveraging research and boosting innovation: To help to identify drivers and bottlenecks of innovation and of technology adoption, the Commis -
sion has commissioned another study to investigate again the EU’s position compared to other global leaders in emerging biotechnology generation and transfer to the biomanufacturing industry. On top, the Commission seeks to explore ways to accelerate the development and use of the Industrial Biotechnology Innovation and Synthetic Biology Accelerator (EU IBISBA) as a digital repository and service network for the sector.
› Stimulating market demand: To succeed on the market, bio-based products need to prove their lower environmental impact when compared, for instance, to petrochemical products. The Commission will review the assessment of fossil-based and bio-based products to ensure equivalence of treatment and incorporate methods for carbon storage in construction materials. To accelerate the substitution of fossil feedstock and to stimulate the demand and market uptake of bio-manufactured products, the Commission will conduct an in-depth impact assessment of the feasibility of bio-based content requirements in specific product categories and in public
procurement instead eliminating subsidies making the fossil-based industry more cost-effective. Furthermore, the Commission said it will explore how bio-manufactured non-food products could profile themselves better through labelling.
› Streamlining regulatory pathways : The old Commission promises to assess how EU legislation and its implementation could be streamlined and shorten the time to market for biotech innovations. Another study is set to lay the foundations for a ”possible EU Biotech Act“. The Commission plans also to establishing an EU Biotech Hub that helps biotechs to navigate through the regulatory framework and identify support to scale up, by the end of this year. In addition it is planned to establish so-called regulatory sandboxes that allow to test novel solutions in a controlled environment for a limited amount of time under the supervision of regulators, as a way of bringing more of them quickly to the market.
› Fostering public and private investments: The EU names a lot of exist-
ing financing instruments to support biotechnology and biomanufacturing such as Horizon Europe; the Innovation Fund; and STEP. To develop and scale up innovations with the potential to create new markets, a policy goal is to support specific challenges on biotech and biomanufacturing in the European Innovation Council (EIC) accelerator Work Programme 2025. Furthermore, the Commission will launch a study by the end of 2024 to identify barriers and ways to support the consolidation of investment funds, stock exchanges and post-trading infrastructure in order to enable the development of the necessary scale, enhance the knowledge base, create deeper pools of liquidity and help lower the cost of financing for highgrowth companies.
› Strengthening biotech-related skills: According to the paper, large-scale and regional skills partnerships might play
an important role in providing upskilling and reskilling opportunities on biotech and biomanufacturing. A specific large-scale partnership for biotech and biomanufacturing players will be explored, which can be co-financed through the Blueprint Alliances activity of the Erasmus+ programme.
› Updating standards: The Commission said it will encourage the elaboration and updating of European standards for biotech and biomanufacturing to facilitate market access.
› Supporting collaboration: The Commission will encourage the deployment of technologies related to biotech processes across EU regions through so called Regional Innovation Valleys.
› Fostering international cooperation. The Commission will explore the possibility of launching international biotech and biomanufacturing partnerships with international partners to collaborate on research and tech-transfer, in strategic
cooperations on regulatory and market access-related topics.
› Using AI and generative AI: the Commission wants to accelerate the uptake of AI, and generative AI in biotech and biomanufacturing in the context of GenAI4EU.
› Reviewing the bioeconomy strategy: Finally, the Commission will review its EU Bioeconomy Strategy by the end of 2025.
According to Skentelbery, “a global technology and market race is underway within biotechnology and biomanufacturing. Successful regions will lead global trade and supply chains for sectors that Europe considers vital and grow next generation economies based on biotech innovation. It is vital that the proposed Initiative recognises the need for tangible industrial impact during the next Commission mandate and is bold in its ambition”. L
t.gabrielczyk@biocom.eu

BioSpring is your trusted partner for manufacturing and analysis of therapeutic siRNA, ASOs, guide RNA and many more. With more than 27 years of experience, we know what’s needed to help you succeed with your therapeutic program.

EU PARLIAMENT In mid-April, just one year after the European Commission proposed a pharmaceutical package to revise the EU’s pharmaceutical legislation, MEPs adopted their proposals to revamp EU pharmaceutical legislation (495 votes in favour, 57 against and 45 abstentions) and regulation (488 votes in favour, 67 against and 34 abstentions). MEPs want to introduce a minimum regulatory data protection period of seven and a half years, in addition to two years of market protection during which generic, hybrid or biosimilar products cannot be sold, following a marketing authorisation. One year will be added if the product addresses an unmet medical need, if the product adds benefit in another indication plus six months, if comparative clinical trials are being conducted on the product. Orphan drugs would benefit from up to 11 years of market exclusivity if they address a high unmet medical need.
To incentivise R&D of novel antimicrobials, MEPs want to introduce market entry rewards and milestone payment reward schemes. These would be complemented by a subscription model scheme through voluntary joint procurement agreements, to encourage investment in antimicrobials.
The MEPs also support the introduction of a transferable data exclusivity voucher for priority antimicrobials, providing for a maximum of 12 additional months of data protection for an authorised product. The voucher could not be used for a product that has already benefited from maximum regulatory data protection and would be transferable only once to another marketing authorisation holder. “The revision of the EU pharmaceutical legislation is vital for patients, industry and society,” said Pernille Weiss, the Danish Rapporteur for the Directive. “We hope Council takes note of our ambition and commitment to create a robust legislative framework, setting the scene for effective negotiations.”

Tiemo Wölken, Rapporteur for the regulation added: “This revision paves the way to addressing challenges such as medicines shortages and AMR. Measures improving access to medicines, whilst incentivising areas of unmet medical needs, are crucial parts of this reform.” The file will be followed up by the new Parliament after the 6 - 9 June European elections.
The EU pharma industry federation EFPIA and national pharma associations criticised the Parliament. “An opportunity has been missed for the pharmaceutical industry,” stressed German pharma lobbyist Han Steutel, President of the vfa. “Europe‘s profile as a centre of innovation has not been raised, nor has patient care been improved. In particular, the planned weakening of document protection is hostile to innovation,” said Steutel. “Although the reports by MEPs in Parliament are moving in the right direction compared to the EU Commission‘s previous proposals, they still fall short in many respects,” added Dr Kai Joachimsen, Managing Director of German BPI. “It is now up to the EU member states to sharpen up and set the course for futureproof legislation.”
ECA In an analysis of the ban on vehicles with combustion engines registered after 2035, the European Court of Auditors (ECA) described the future of HVO100, e- or synfuels and biofuels as “uncertain”. Vehicle emissions were still as high as they were eleven years ago, said Court of Auditors member Nikolaos Milionis. As 1st generation biofuels – i.e. biogenic fuels not produced from residual materials or by photosynthetic microorganism – were “more expensive than fossil fuels”, it would be “cheaper to purchase emission certificates than to reduce CO2 emissions with the help of biofuels, which are not always favoured by the taxation policies of EU countries”, the auditors stated. Both the competition for biogenic raw materials and their limited quantity make combustion engines appear to auditors as no alternative to battery-powered electric vehicles, which the ECA members – similar to many EU decision-makers – presented as the only viable alternative. The report does not consider carbon negative fuels produced from photosynthetic micro- and macroalgae that cost €1.50/l. Contrary to the auditors’ statement, these primary producers growing in brackish, fresh and seawater do not require a sugary nutrient medium, but use CO2 as a source of carbon and sunlight as a source of energy.
HTA JCA EU Biotech federation EuropaBio has warned that the draft Joint Clinical Assessment Implementing Act (HTA JCA) could limit patient access to innovative biotech meds due to double work, delays, and resource waste On top, lack of early involvement of developers could hinder biotech innovator abilities to deliver on the aims of the Regulation. According to Claire Skentelbery, Director of EuropaBio “it is essential to give health technology innovators the predictability and time required to successfully complete the JCA.” L
CLINICAL TRIALS With a new law, the German government wants to increase the number of clinical trials conducted in Germany and make the country number one in the EU again. The draft Medical Research Act presented by German Health Minister Karl Lauterbach in Berlin before Easter is the centrepiece of Germany’s pharma strategy, which aims at making Germany an attractive location for biopharma production and R&D. The Medical Research Act is designed to help remove bureaucratic and legal obstacles that currently prevent the rapid start of industry-sponsored and investigator-initiatied clinical trials in Germany.
As Germany had fallen from 2nd to 6th place worldwide in terms of the number of clinical trials between 2016 and 2021, policy makers saw a need for action following strong pressure from the pharmaceutical industry, CRO and medical associations. To this end, the authorisation procedures for clinical trials at the German regulatory authority BFArM, which is subordinate to the Federal Ministry of Health, will be shortened to 26 days and a central ethics committee subordinate to the ministry will take over the role of the previous state ethics committees. In addition, the Ministry of Health recommends using model contract clauses drawn up by industry and medical associations in a legally non-binding manner in order to speed up negotiations between study clinics and study sponsors, which are particularly lengthy in Germany.
Pharmaceutical, biotech, CRO and medical associations had recommended that the model contract clauses they developed and publicly presented at the end of 2023 should be made legally binding by ordinance, as a quick conclusion of contracts between study sponsors and clinics is considered essential for a quick start to studies. While it usually takes 4-6 months to conclude such a contract in Germany, it takes much less time in neighbouring countries such as France (76 days), Spain (111 days) and the
UK (134 days), as binding standard contractual clauses have been in place there for years.
When presenting the draft bill, German Health Minister Karl Lauterbach, who is known to be resistant to consultation, was highly optimistic that the yearslong backlog would be made up: “We will see significantly more pharmaceutical and academic studies in Germany in the coming years,” he said. However, as essential wishes of the industry – such as legally binding model contract clauses or the authority of the well-established state ethics commissions to issue directives –were ignored and sacrificed to the ministry’s claim to leadership, the draft law was met with disappointment in the in dustry.
“It is regrettable that the current ver sion of the Medical Research Act misses the opportunity to achieve a significant breakthrough that could automatical ly bring us closer to the international forefront of clinical trials,“ said Martin Krauss, head of the VDMA, which repre sents the interests of Germany’s contract research organisations (CROs) that carry out 60% of clinical trials. The planned establishment of a central ethics commit tee calls into question the independence of the ethical evaluation of study pro jects, criticised the German Medical As sociation BÄK. The state ethics commit tees organised in AKEK also do not find the draft law expedient: “The Medical Research Act does not solve any prob lems regarding the regulation of clinical studies. Instead, it creates new problems by creating a parallel bureaucracy,” said Georg Schmidt, Chairman of the AKEK.
From the point of view of lawyers who were significantly involved in the drafting of the model contract clauses, the draft law does not advance Germany one centimetre as a study location. “The regulations related to standard contractual clauses for the conduct of clinical trials leaves much room for improvement,” stressed Dr Ute Kilger, Partner at Boehmert & Boehmert in Berlin. L









The better way to DNA!




Customized High Quality Grade DNA for GMP production of viral vectors, RNA and CAR-T cells QC including CGE service pDG/pDP plasmids for AAV production
High Quality Grade Plasmid & Minicircle DNA ask for



2 plasmid system
Serotypes including AAV8 & AAV9 GFP transfer plasmids
ITRRESCUE® In Stock service







now!




EU Compared to the US and China, EU biotech clusters appear to be dwarfed by the lack of industry-specific support in terms of access to funding, resources and scaling up (bio-)production. At the beginning of May, more than 900 experts from clusters and the EU Commission discussed at the sold-out 9 th European Cluster Conference what cluster policy is needed to make Europe a world leading place for the Green and Digital Transition.
Although Europe is highly innovative in biotech research it does not translate this into marketable solutions as effectively as its global competitors (see p.8), albeit modellings of the EU bio-industry (https://datam.jrc.ec.europa.eu/datam/mashup/BIOECONOMICS/) paint another picture. From the perspective of the European Federation of Pharmaceutical Industries and Associations (EFPIA), the solution lies in concentrating resources in a few large clusters in order to keep pace with global biopharma hubs such as Boston, San Francisco or Maryland. In view of the high number of innovative SMEs in Europe, however, the networking of expertise flanked by appropriate funding and sector-specific political and regulatory support could also help Europe to achieve sustainable growth. At the beginning of May, more than 900 experts from clusters and the EU Commission discussed at the sold-out 9th European Cluster Conference (ECCP.2024, Brussels) what cluster policy is needed to make Europe a world leader in the technology fields that have already been prioritised by the EU Commission, namely the green and digital transition and biotechnology. The major topic: how to attract investors.
In 2022, experts from Charles River Associates made seven recommendations on behalf of EFPIA to improve the factors that attract pharmaceutical investments in the EU. Greenfield projects outside of clusters favoured by EU Horizon funding were sharply criticised. Instead, the pharmaceutical experts want to see the EU concentrating scientific, clinical and transla-

Full house: 900 cluster professionals and policy makers in Brussels at the 9th European Cluster Conference
tional expertise in a few hubs, along the lines of the global pharma cluster Basel, the hubs around Oxford and Cambridge or the world‘s third largest gene therapy centre Catapult in the UK, where biotech value creation can be measured by deal volumes. Cluster experts from various sectors expressed a completely different view at ECCP.2024. Montse Daban, President of the Council of European Bioregions, CEBR, spoke out in favour of more networking and mapping of existing expertise in topic-specific metaclusters.
At the opening of the conference, Thierry Breton, European Commissioner for the Internal Market, addressed the topic of cluster networking with reference to the Euroclusters project. “We must overcome cross-border barriers to the internal market”, he said in a video message. That this is not always easy was not difficult to understand from conversations on the fringes
of the conference. „There are two worlds in EU cluster policy,” according to stakesholders from a Slovenian cluster, „a wellfunded one and one with fewer resources that is concerned with survival.”
How the claim “it is important to grow small clusters to innovation networks” formulated by Antonio Novo, President of the European Clusters Alliance, could look in reality, however, was not specifically addressed. In discussions, the cluster managers conveyed that although the EU Commission is endeavouring to create the conditions for growth and an appropriate regulatory environment, the clusters, with their very different and specific expertise, feel that their sector-specific needs are not being heard or understood. Whether the USA and emerging Asian countries are better able at providing the relevant expertise in the responsible bodies – keyword: mindset – was also not thematised
at the conference. Hard facts on innovation scores and the corresponding global ranking in key technologies were presented at the conference by Jan-Philippe Kramer, Team Leader, Data and Policy, European Cluster Collaboration Platform, at Prognos.
According to Prognos analyses, China has already caught up with the EU in terms of innovation through massive investment and sector-specific policies and is on its way – with a steep growth curve – to catching up with the vice-innovation champion USA (and – not shown –world champion Switzerland). According to Prognos, 17% of the mapped EU cluster organisations are in the healthcare and agrifood sectors, respectively, and 18% in the energy sector. Kramer emphasised the great importance of clusters in the innovation process, citing that the densely clustered Spanish province of Catalonia alone accounts for around a third of Spanish patents. Kramer noted that the most innovative clusters are those that are well-positioned in the green and digital transition and highlighted that the achievement of innovation policy goals depends crucially on cooperation with clusters, but that the success of clusters is also highly dependent on long-term, coherent and practical political support through stable political framework conditions. To date, 14 EU member states are active in cluster policy.
Cleantech and biotech solutions play a strategically important role when it comes to the competitive reorganisation of existing value chains and the creation of new ones that are climate-friendly and low in fossil fuels. Montse Daban, President of CEBR, which represents 50 European Bioregions, emphasised: “We have to map the technological competencies that are available in clusters.” Due to the increasing fusion and simultaneous use of technology fields such as AI/data sciences, biotech and med-tech/diagnostics, it is crucial for success to develop solutions cross-sectorally and to network competences, such as in the EU Fab Initiative from the pandemic year 2022. It is about networking many
smaller contract manufacturers in order to be able to produce a wide variety of vaccines in sufficient quantities if needed. In Europe, with its numerous SMEs, this is a prime example of how expertise can be bundled thematically without geographical clustering. Like many visitors to the conference, Daban called for more stability and predictability in political priorities: “Investors need only two things: stability and critical mass, that is how we can attract money”, she said (see p. 16). This probably meant a long-term commitment and rapid implementation, as recently demonstrated by the UK government with its 10-year biomanufacturing plan and the US with its modern bioeconomy strategy. According to current plans, the new EU Commission will not take up this important topic until mid-2015 and adapt it to the current global state of development.
The discussion then turned to the priorities of the eagerly awaited new (old) Commission. It is clear that the desired green and digital transformation will require a lot of investment while fresh money is not available due to the mid-term financial plannings of the EU. However, how the money is to be distributed across the fields of SME funding, scaling and product roll-out will not be decided until mid-2025.
During an interactive brainstorming session initiated by the Commission on
cluster needs in various fields of technology, it became clear that not much can be created in the clusters without funding. However, Christian Ketels from Innovation Fund Denmark previously had summarised well what the many cluster managers gathered in Brussels are actually concerned about. Much has changed as a result of the current geopolitical crises, he said: “The current challenges must be tackled in co-operation. No single company can do that any more. That‘s why we need new forms of co-operation and that is expressed in the clusters and in its cooperation. It is a real challenge, because only a few recognise the problem-solving expertise of clusters.” Their actual task is not to offer services for companies, but to offer industry the strategic solutions it needs. “We need to talk to politicians with one voice, because we hold the means of implementation in our hands. It is important that EU policy does not see clusters as recipients of funding, but as partners in solving problems.” With regard to the maximum funding period of ten years, Marc Andres from Flanders innovation & entrepreneurship added “making clusters self-sufficient is not a good idea, because it drives them into dependency from industry.”
Verónique Willems, Secretary-General of SMEunited, made it clear in Brussels that the pandemic had driven a lot of SMEs into bankruptcy, but that state subsidies did not necessarily lead to a loss of

competitiveness. “You can‘t buy them,” she explained. Companies would tend to avoid the funding offer simply because of the time-consuming application process, but would love clusters with a thematic focus, as these help take away investors fear of risk and offer infrastructure and network value.
In addition to the biotech incubators‘ permanent hunger for funds, the 70 exhibitors in Brussels – despite major support from EU funding – are also struggling with a completely different problem: regulatory issues. While the US has a single authority responsible for the approval of therapeutics and their accompanying diagnostics, particularly in personalised medicine, there is a lack of notified bodies for diagnostics subject to EU medical device regulation, it was said on the fringes of the conference. Coordinated authorisation of early and companion diagnostics for novel medicinal products is hardly possible, according to various stakeholders. It is therefore no wonder that companies such as Adrenomed AG are seeking FDA accelerated approval procedure for the first effective sepsis medicine in a patient population previously stratified with two biomarkers. Furthermore, the early cancer diagnostics company Harbinger Health Inc., founded by German researchers in the USA and seeded with US$80m in capital, is validating its approval-relevant methylation pattern liquid biopsy tests, which cover the Big Five cancer indications in Stage I and II, on 10,000 US citizens.
It remains to be seen whether, as suggested in the Green Transition session moderated by Kirstin Dunlop, CEO of the EIT Climate KIC, the clusters can only be helped by a “green dictatorship” that implements industry-friendly rules as quickly as Singapore, China and the USA. The newly elected European Commission and the decision criteria extracted from the dialogue with the experts will be in the focus of attention and discussion. L
t.gabrielczyk@biocom.eu
CEBR Biotech clusters and ecosystems are seen as the cradle of innovation. At the European Cluster Conference 2024, Europ E an Biot Echnology spoke with Montse Daban, President of the Council of European Bioregions, about Europe’s position and outlooks.
EuroBiotech_Europe’s biotech sector and clusters lag far behind the USA and now also behind Asian countries such as China and South Korea in terms of value creation and financing. What are the reasons and possible solutions?
Daban_If we look at Europe as a whole, according to a recent EuropaBio report,

DR MONTSE DABAN holds master’s degrees in science communication with a focus on medicine, biology, and diplomacy and foreign relations. She also holds a doctorate in molecular biology. She has over 35 years of experience as a life sciences and scientific communication researcher, having worked in Cleveland, Barcelona, Lille, France, and Ohio. In addition to her work as a scientific publisher at Editorial Rubes, she has advised the Department of Research and Universities of the Generalitat of Catalonia on R&I policies and international relations. Dr Daban has worked at Biocat, the BioRegion of Catalonia since 2006, currently as Director fo Strategic Foresight and International Relations. She serves as President of the Council of European BioRegions (CEBR) and as member of the Board of Directors of the European Clusters Alliance (ECA).
the industry has a production turnover of €425bn and there are around 9,51 million people employed in healthcare in the EU, and another 840,000 in the life sciences industry. Europe is a biotech hub. It’s true that biotech companies in the US receive more financing than the companies in Europe but along with the United States and China, the EU is a major hub for biotechnology. Compared to these regions, Europe is a more complex market. Thousands of EU companies are developing new therapies, new diagnostic equipment, vaccines, and advanced medicinal products, but Europe’s rules for innovation and financing are complex and there are differences among Member States that complicate the landscape while offering an attractive diversity and huge possibilities. At the same time, there’s a huge effort in life sciences funding in the EU from public EU funds and investment instruments and Member States to leverage private funding. The pharma package is seen as an opportunity to increase access and affordability, improve resilience and foster innovation, even if some players have expressed fears that it could attract or slow investment and reduce or not close the gap with US and China.
EuroBiotech _How can clusters help to improve the situation?
Daban_We are at the European Clusters Conference. The main take-home message is: it is unlikely that companies attracting investment are not in a cluster, connected synergistically with other key players and profiles in the ecosystem. Clusters, understood as ecosystems and not just industrial associations, are more than connectors. Provide stability and critical mass, the two main drivers to attract investment. Talent, funding, policy making, connection, dem-
onstration spaces, critical mass to drive change – this happens in a cluster. Cluster organisations are multipliers of value.
EuroBiotech _Why don’t we simply copy the approach of large clusters from the US? What makes sense in Europe and promises success?
Daban_It does not work the same way in Europe as it works in the US. Even if there are large hubs in Europe, every country has its policies, regulations, legislation. The European model for innovation is about not leaving anyone behind. We cannot concentrate and leave others behind. The policies in Europe tend to reduce the gaps not increase them. The model is different and my belief is that Europe can only be competitive if all the territories are competitive. And clusters, with metaclusters like the Council of European BioRegions and the European Clusters Alliance, contribute to this connection across Europe
EuroBiotech _Which political flanking measures do biotech SMEs in the EU clusters need in order to bring the high level of innovation in research to the market?
Daban_There are several measures but I would like to highlight three enablers: skilled talent across the value chains, reduction of regulatory burdens and increased innovation procurement.

Global biotech ranking map. Since 2009, Thinkbiotech gives updates on parameters determining the global competitivity of biotech in a nation regarding productivity, intensity, enterprise support, education & workforce, foundations, as well as policy and stability. In contrast to scores that focus on pharma biotech, the map ranges from advanced medicines to and improvements in agriculture to feedstocks and biofuels, and further to exotic industrial materials and processes and is a a distillation of the Scientific American WorldView Scorecard which was developed by Dr. Yali Friedman in 2009.
EuroBiotech_How can the networking of small clusters and open innovation contribute to creating success stories similar to the large clusters in Boston or San Francisco?
Daban_European Clusters do have success stories. Existing metaclusters connecting these not-so-small clusters across member states, collaborating to reduce the gap and increasing impact
on European population, connecting sectors, growing business that put the patient not at the center but at the origin is a success story. These clusters reduced disruptions in value chains during the pandemics through daily collaboration (calls, exchanges, connections) for months. That is a clusters’ success story too. L
t.gabrielczyk@biocom.eu


MODELLING The Three R (Replacement, Reduction and Refinement) principles developed over 60 years ago provide a framework for more humane animal testing and, more importantly, less of it. A number of in-vitro model systems are now out there, but only recently has the use of multi-organ chips gained serious momentum. 3D printing technologies loaded with stem cells are increasingly allowing the replication of human tissues and organs for testing purposes.
The Three Rs were first defined in 1957 by Russell and Burch in their book The Principles of Humane Experimental Technique. Since then, the guidelines have been incorporated into national and international laws and regulations governing the use of animals in scientific procedures, as well as into the policies of organisations that fund or conduct animal experiments. But despite some headway, animal testing in the life sciences continues to decline slowly, if at all.
For many years, the EU’s statistical database ALURES (AnimaL Use REporting System) on the use of animals for scientific purposes in accordance with Directive 2010/63/EU has attempted to provide a numerical basis for animal testing in research and product approvals. However, the data framework changed in 2019 due to Brexit and new inclusion for Norway, leaving room for misinterpretation. In spite of those complicating factors, indications imply that the number of animal experiments in the EU has fallen. There were around 8.1 million lab animals in the bloc in 2019, and just 7.3 million in 2020. That’s the first time the number of animals has dropped below 8 million since the introduction of Di -
rective 2010/63/EU. There’s an ongoing debate as to whether this fall might only be a result of pandemic-driven lockdowns. Viewed positively, however, it’s hard to see why the number of animals involved in clinical testing might have actually dropped despite massive efforts to get vaccines and therapeutics against COVID -19 over the approval line.
One way or the other, there is major interest in finding alternatives to animal testing, as animal data are not just costly, time-consuming and ethically questionable, but also often fail to predict results in human trials. A lack of humanrelevant preclinical models – and the resulting high failure rates for therapeutics in the clinic – have led to unsustainable rises in healthcare costs and fewer effective medicines reaching patients. Pressure from societies and governments to find alternatives to animal testing is also growing harder to ignore, as evidenced by the US Food and Drug Administration (FDA) Modernisation Act of 2021 and the Humane Research and Testing Act (HR 1744) passed by the US Congress. This new law disperses with the requirement that drugs in development have to be tested on animals before being given to participants in human trials. Signed by
US President Joe Biden in December as part of a larger spending package, however, the new legislation doesn’t ban testing new drugs on animals completely. Instead, it simply removes the former hard requirement that pharma companies use animals to test new drugs before testing them on humans. Companies are still allowed to test drugs on animals if they wish. Even so, the act is a sign of how strongly the FDA is promoting the development of alternative testing methods.
The stage is thus set for other methods pharma companies can use to evaluate new treatments, such as computer modelling and “organs-on-a-chip” (OOCs) –microfluidic devices that can mimic how organ function is affected by drugs. And companies that provide technology or equipment for organoids and OOC models have indeed seen increased demand since the new FDA law was passed, according to Reyk Horland of Berlin-based TissUse GmbH.
Currently, however, genetically modified mice and other animal models continue to dominate both basic research and drug development. Aware of this fact, the FDA has not mandated an immediate end to the most widely used research methods. Avoiding animal testing is hence still

There are many different options when it comes to organ-on-a-chip systems
a recommendation rather than a requirement. Most industry experts say the transition will take time, because almost every benchmark animal model will have to be replaced by an alternative system on a case-by-case basis, and each replacement will have to prove its efficacy. So these are uncertain times for companies that choose to use alternative test methods to replace animal testing. And as always, uncertain times harbour both risks and opportunities. New technologies could very well be able detect potential toxicities and safely capture all the conceivable types of toxicity that have been observed in the past. But it’s also important to be aware of their limitations.
The pharmaceutical and biotechnology industries spend billions every year to take compounds from discovery in the lab to approval by healthcare authorities. In many parts of the world today, regulatory approval continues to require animal testing for safety and efficacy – even though many studies have shown that results from animal testing often don’t accurately predict results in humans. Over and over again, that approach has led to drugs and vaccines that successfully pass through the preclinical development pipeline – including studies in small animals or even non-human primates –but then either fail to show efficacy (e.g. tuberculosis MV85a, HIV-1 DNA/rAd5,
hepatitis C vaccines) or cause potentially life-threatening toxicities. That was the case for instance with monoclonal antibody Hu5c8 (CD40L), which showed unexpected thrombotic complications. Like many other discontinued candidates, it led to human trials that resulted in serious side effects (e.g. TGN1412/ Tegenero). Some have even caused deaths. Equally worrisome is that there are probably drug candidates that would have proven safe and effective in humans, but never reached clinical trials in our species because they were dropped from development pipelines due to erroneous results in animals.
The poor predictive power of preclinical models is particularly troublesome given their heavy reliance on animals – especially genetically modified mice – in basic research, which is where potential drug targets are often first identified. When researchers have directly compared the predictive value of mouse models for a number of human diseases (among them Alzheimer’s, sepsis and acute respiratory distress syndrome) the results have proven dismal. Even when animal models of disease are phenotypically similar to humans, underlying molecular and cellular mechanisms are often different, and potential therapeutic targets identified in these models may therefore lack clinical relevance.
The greatest attrition in development pipelines still happens when making the jump to the clinic, with failure rates of 45% in Phase I and another 50% in Phase II. And those numbers are also often related to poor comparability of animal and human data. One attempt to circumvent this uncomfortable fact has been to implant more and more humanlike tissues and cell structures into experimental animals. But although there are now ‘human’ immune systems in GM mice or ‘human’ organs in large animals like pigs, comparability remains limited.
The call for relevant models
The development of therapeutics has been additionally transformed in recent decades by the creation of biological therapies like monoclonal antibodies, adeno-associated viral gene vectors, small interfering RNAs and CRISPR-RNA therapies. What all these approaches have in common is that some of these compounds may be so specific to human target sequences or conformations that they should either have no effects in non-human models or initiate completely different activities. With biological therapies approaching personalised medicine levels in almost half of all drugs in development, preclinical models that are relevant to individual humans have become a critical need.
Other challenges also require human modelling. For example, one recent paradigm shift in medicine has been the recognition of the central role that the microbiome plays in human health and disease. However, the complex communities of microorganisms that make up the microbiome differ in humans between different individuals, and even in the same person at different times or places. This challenge is compounded by the fact that it still isn’t possible to culture complex microbial communities in direct contact with human cells, as the process often leads to contamination and rapid cell-death.
The need for personalised preclinical models has grown even more important as the healthcare industry shifts focus to precision medicine. For example,
findings of a high variability in response to therapies between different genetic or ethnic populations, which is seen in some clinical trials, raises the question of whether it still makes sense to model human genetic diseases using inbred animal lines. Iconic geneticist Craig Venter has often said in plenary discussions that the mouse and human genomes are simply too different to be compared at all. There are ways to address these new challenges. Among them are preclinical human in-vitro models that use organspecific cells isolated from surgical specimens, as well as organoids containing adult stem cells or induced pluripotent stem cells (iPS cells) from individual patients.
Organ-on-a-chip (OOC) micro fluidic culture devices represent one recent success in the quest for in-vitro microphysiological systems (MPS) that can recapitulate organ-level realities and even organ-level functions. To avoid linguistic confusion between spheroids, organoids, organs-on-chips and many other terms, the lowest common denominator of all these approaches is termed the “microphysiological system”. On a chip, the microfluidic form of a microphysiological system can be of different sizes and shapes, but all contain hollow channels lined with living cells and tissues cultured under dynamic fluid flow. Some devices replicate organ-level structures (e.g., tissue-tissue interfaces) and provide the relevant mechanical cues (such as respiration and peristaltic-like motions) needed to faithfully model organ physiology and disease states. By fluidically coupling two or more organ chips, human “body-on-chips” can be created. These multi-organ systems mimic whole-body physiology, along with drug distribution and disposition. Advances in stem-cell technology, like those involving iPS cells and organoids, have enabled the cultivation of personalised stem cells that can now be integrated and differentiated into OOCs to create patient-specific preclinical models.
To date, developers of microfluidic devices have focused on design and construction, along with an experimental demonstration of their ability to replicate relevant tissue and organ functions. Over the years, a range of different companies have built up high levels of expertise in chip design, the use of suitable materials, miniature pumps and the application of microphysical forces to move fluids, as well as in the detailed analysis of the components in these systems. They involve tiny volumes, but also extremely fast interactions. And it all takes place in a small space, a situation that has to be resolved using various spectroscopic and microscopic methods.
The challenge now is to take things to the next level by demonstrating equivalence or even superiority to animal models. If human OOCs prove to be better, they can be used not only to reduce animal testing, but also truly used to develop or select therapeutics that have been personalised for individual patients, specific genetic subpopulations or even subgroups with specific disease comorbidities. The field has the potential to revolutionise clinical trial design.
The first microfluidic culture device recapitulated structures and functions at the organ level (i.e. multiple tissues) of a major functional unit of a human organ – the pulmonary alveolus. It was created using a soft lithography-based manufacturing approach borrowed from the microchip industry.
The Lung Alveolar Chip is a device around the size of a flash drive made from an optically clear, elastomeric polydimethylsiloxane (PDMS) material that contains two parallel hollow channels separated by a porous membrane. It’s coated with extracellular cell matrices (ECM) lined with human lung alveolar epithelial cells on one side and human vascular endothelium on the other, mimicking the alveolar-capillary interface. A culture medium is perfused through the endothelium-lined channel to mimic vascular perfusion, and air is introduced into the epithelial channel to mimic the airliquid interface of the lung. This is critical for lung differentiation and function, and instigates cyclic aspiration on the hollow side-chambers. That in turn allows cyclic tissue deformation to occur at the flexible tissue-tissue interface, mimicking respiratory movements.
This dual-channel chip design has since been modified to permit open access to one channel, allowing the formation of a thicker tissue construct while still applying cyclic mechanical stresses (e.g. for a skin chip). It has been used by several groups to create microfluidic models of various organ types with fluids flowing through both channels. Other single device and multiplexed versions have been fabricated using a range of methods (among them soft lithography, physical removal of sacrificial cylindrical mandrels, injection moulding) or by replacing the porous membrane with an ECM gel to support tissue ECM formation, tissue-tissue interface formation,

or cellular ingrowth to study dynamic 3D morpho genetic processes (e.g. tumour angiogenesis and lymphangiogenesis).
“An extremely large number of new methods have emerged,” says Reyk Horland from TissUse. “In a chip, you try to reproduce not only the cell architecture but also the physiological conditions in a way that is as close as possible to the human situation. This is commonly not the case with cells or tissue cultured in a standard cell culture plate,” he remarks, clarifying the differences between OOC developers and the organoid community. The presence of dynamic fluid flow and organ-specific mechanical cues has been repeatedly shown to promote higher levels of tissue-specific differentiation and improve organ-level functions – even beyond those seen in static 3D organoid cultures, when organoid-derived cells are used to line the chips. As these chips are actively perfused, immune cells can be channeled through the vascular endothelium-lined channel, like in circulating blood. Resident immune cells can also be placed on-chip in 3D ECM gels
to mimic lymphatic and haematopoietic microenvironments, thereby reconstituting specialised lymphoid follicular and bone marrow structures and functions in vitro.
As OOCs are typically fabricated using microengineering approaches, it is also possible to incorporate various types of in-line sensors to monitor tissue viability and function, including real-time monitoring of oxygen levels, changes in tissue barrier integrity (e.g. use of integrated electrodes to measure transepithelial and/or transendothelial electrical resistance or impedance spectro scopy) and cellular electrical activity (e.g. use of multi-electrode arrays).
As interest in the field skyrockets, all of these approaches are entering the limelight at once, and it isn’t easy to select the best system to answer a particular question. For example, growing numbers of special-tissue organoids modelling brain or hearts (like those from

ahead.bio or Heartbeat.Bio in Vienna) are growing in importance in early drug testing. They also have a lot to teach us about the minimal necessary interplay of cell types and tissue architecture when it comes to transferring such systems to chip platforms and a high-throughput testing environment.
But observing how tissue develops in an organ culture can also bring new insights. Sasha Mendjan from the Institute of Molecular Biotechnology (IMBA) and co-founder of Heartbeat.Bio recently published a study in the journal cEll (autumn 2023) on the first heart organoid to have multi-chamber architecture. These cardioids are licensed to Heartbeat.Bio and allow researchers to analyse chamberspecific defects. As a proof-of-principle, the Mendjan team has established a defect screening platform in which they are investigating how known teratogens and mutations affect hundreds of cardiac organoids simultaneously.
Thalidomide is known to cause severe heart defects in foetuses. The same is true of retinoid derivatives used to treat leukaemia, psoriasis and acne. Both teratogens induced similar severe ventricular defects in cardiac organoids. Similarly, mutations in three cardiac transcription factor genes led to ventricle-specific defects like those observed sometimes in human development. “Our tests show that multi-chamber cardiac organoids can recapitulate embryonic heart development and reveal perturbing effects on the whole heart with high specificity. We do this with a holistic approach by looking at multiple measurements simultaneously,” Mendjan explains.
OOCs developed in the last decade now model a wide range of human disorders in virtually all organ systems, providing new insights into the molecular and cellular basis of various physiological and pathophysiological processes. They’ve also been used to simulate different types of drug delivery approaches, and to recapitulate clinical responses to therapeutics in human patients.

Human OOCs have been produced using primary cells from human donors or established cell lines and patient-derived organoids or iPS cells. Hamburg-based Evotec SE is very confident in its iPS stem-cell platform, which makes it possible to generate different cell types and use them in a high-throughput process for screening in the early analysis of active substances. Its multi-billion research collaboration with Bristol Myers Squibb shows how much interest this approach to organ-like cellular test systems is attracting.
Based on recent advances with iPS cells in the field, it is now possible to envisage a future where single OOCs – or multiorgan chip systems made with organotypic cells from the same patient – could be used as personalised living avatars (organ-twins) to repurpose or select more effective and less toxic therapeutics, optimise routes of administration and develop dosing regimens for a specific patient, opening up new avenues in precision medicine. Such an approach will be costly, so one early step might be to produce chips loaded with primary or stem cells from patients who represent unique genetic subpopulations, and use these chips to develop or select new drugs for specific groups, and only then conducting clinical trials with these patients. That could reduce both time and cost factors and increase the likelihood of success in the drug development process.
The community is taking it step by step, according to Horland: “Many experts believe that eleven organs are probably enough to represent a mini-organism of the human. Preferably with a female and a male version to meet the growing demand for gender-specific analysis methods. But we are not there yet.”
Although OOC technology has a lot of potential, there are still technical, social and economic hurdles left to overcome before significant numbers and types of animals can be replaced. For some areas of testing, like behaviour or cognition studies, or ones looking emotions or anxiety, chip formats won’t work. But in places like ADME (toxicity testing of Absorption, Distribution, Metabolism and Excretion) studies could be adequately performed on a device.
Sourcing high-quality human cells has also always presented major challenges, but with iPS cells and organoids growing more available, this is less of an issue today. Most OOCs are also low-throughput but high-content. So they generate a lot of data. And that can be valuable in later stages of the drug development pipeline (e.g. when lead compounds need to be selected for further drug development), although not in the earlier discovery phase. Also, artifical intelligence is being integrated in simulation procedures.
Higher-throughput systems are necessary to enable statistically significant studies with a lot of replication, which is essential for pharmaceutical validation. The recent development of higher-throughput OOCs (i.e. single devices with many parallel culture chambers) is addressing this issue. If they can retain many of the key features required for drugs testing, this route should also be pursued. Another aspect that needs improving is automation. There new players from very different industry sectors have been entering the OOC space, among them assembly line experts from the Festo Group, who collaborated on an automated chip environ ment with robot-assisted chip handling process in the incubator.
Perhaps the biggest challenge facing organoid/organ-on-a-chip technologies, however, is a conceptual one. Many pharmaceutical, regulatory and academic researchers have invested heavily in the way they currently conduct research, and may be reluctant to change their methods. They will demand convincing data demonstrating the advantages of human OOCs over animal models before accepting the new technology in their labs. However, the new rules from the FDA should help accelerate acceptance of devices as a replacement for animal models. It feels as though we have reached a turning point in the field, with a real reduction in the use of animals on the horizon, along with applications that will help make more effective approaches to drug development and personalised medicine a reality.
The European Commission, on the other hand, has been slow to move in this direction, stating last year that it “does not share the view that a legislative proposal is necessary to achieve the objective of phasing out the use of animals in research, education and training.” However, it continued uninspired, the EU would “begin to explore the possibility of coordinating the activities of member states and national authorities in this area.” L g.kaeaeb@biocom.eu
There is a growing body of evidence showing that mitochondria are not only the powerhouses of a cell, but that their transfer may also function in rescue of cells in distress. Still early times, start-ups are already working to bring mitochondria therapy to the patient. EBM spoke to Alexander Schueller, CEO of Switzerland-based cellvie, about a long and winding road.
EuroBiotech _Mitochondria are not part of the standard repertoire of research, let alone clinical practice. Are the systems for dealing with these cell organelles sufficiently developed?
Alexander Schueller_Mitochondria remain somewhat elusive, given that most laboratory technologies available today were designed for research on cells –which are often more than 1000-fold larger than your average mitochondrion. This presents a challenge to the field as it limits our ability to characterise and trace mitochondria for example.
EuroBiotech _Would you say that regulation is more refined at this point, where does mitochondrial transplantation stand?
Schueller_The regulation for mitochondria transplantation is still evolving, but we expect that the therapy will be regulated as a biologic in the USA. Now, while there is little explicit precedent to go on, we can take cues from the regulation on cell and gene therapies or exosomes. So, I would say we roughly know what to expect, but the details remain to be determined. On the upside, as the ones pioneering the field, we will have the chance to work closely with the FDA or EMA to help shape the regulation.
EuroBiotech _What exactly is cellvie’s therapy and how to use it?
Schueller_We take mitochondria from cells and purify them. Most importantly, we developed processes and excipients to be able to freeze these mitochondria

ALEXANDER SCHUELLER CEO of cellvie AG, located in Zurich, Switzerland and Houston, Texas (USA). The company with founders from Harvard Medical School was seedfinanced by Kizoo of Michael Greve.
without compromising their therapeutic potential. This is essential to enable centralised manufacturing, allowing for the mandated process controls and documentation, as well as the production at scale and thus reasonable costs. So, the product are frozen mitochondria in a vial, provided with a delivery buffer, ready to use within minutes, allowing seamless integration into the clinical workflow. In terms of applications, we view mitochondria as a platform technology. We are pursuing kidney transplantation first, as a steppingstone to the large
cardiac markets of ischemia-reperfusion injury. By rescuing cells that are damaged in conjunction with organ extraction, transport and transplantation, we expect to improve organ viability, longevity as well as availability. Importantly, this is not a day dream. The efficacy of our stored human mitochondria has been shown in corresponding large animal experiments. In addition to our lead-asset for ischemia-reperfusion injury, we have been developing a promising pipeline in cell and gene therapy, for example the use of mitochondria as non-viral vectors for gene therapy delivery to solid organs other than the liver.
EuroBiotech _What is known and understood to date about the therapeutic potential of mitochondria to ameliorate the ischemia-reperfusion injury, for example during an organ transplantation? Schueller_There are still unknowns, given the relatively early stage of the field. But researchers in academia and industry have been converging on a key mechanism by which mitochondria transplantation likely rescues cells or improves their performance. Specifically, we and others have observed mitochondria transplantation to trigger mitophagy and mitogenesis and thereby reinvigorate the cell energy metabolism, which has been disturbed due to ischemia – i.e., the lack of blood flow. A finding, which was confirmed very recently in a Nature publication, one of the most rigorous and hence impactful scientific journals. Whilst a consensus seems to be building in that mitophagy and mitogenesis are key mechanisms of
action, they may not be the only ones. The exact function and effect of the transplanted mitochondria are hence still being debated, particularly across different applications. But their complex nature and multi-dimensional effects are precisely why, in my opinion, they may offer a completely new starting point for thus far intractable human conditions such as ischemia-reperfusion injury.
EuroBiotech _What therapeutic applications do you foresee for mitochondria transplantation?
Schueller_We believe in mitochondria as a platform technology. However, one has to be careful to not overstate the expected use-cases. Mitochondria are not pixiedust. But there are already a range of medical conditions wherein mitochondria transplantation has shown promise. Most notably and repeatedly, in ischemia-reperfusion injury, which was the application wherein my co-founder, Dr.
McCully, pioneered the field. Ischemiareperfusion injury arises whenever the flow of blood is interrupted and subsequently re-introduced. Medical conditions include heart attacks, organ transplantations, strokes, or long surgical procedures. There are hence millions of patients that stand to benefit each year. Recently, a group in the US, in collaboration with Dr. McCully, also showed that the performance of the aging muscle may be improved by mitochondria transplantation. Another interesting area, which we are also working in, is the amplification of cell therapies by ex-vivo transplantation of mitochondria. There may be more, and, with the zeroing in on mitophagy and mitogenesis as a key mechanism of action, I believe a roadmap may be emerging to find them.
EuroBiotech _But do investors react favourably to such early, high-risk projects in the current climate?
Schueller_I have prior experience in founding a company, but in medical devices. We developed a surgical adhesive. There the story was more simple. You could see the glue, you could put it on your skin to showcase its characteristics or perform acute animal workshops for that same purpose. There was also a clear understanding of the regulations and the path to follow for approval. Now with mitochondria it’s completely different. Something this new does not commonly trigger enthusiasm among investors, but uncertainty. I frankly underestimated the extent to which ambiguity may overshadow upside potential.So, no, I would not say that investors generally view such early stage projects favorably, and even less so in the current climate.
EuroBiotech_And your experience didn’t play so much of a role in convincing investors?



Schueller_It helped, but it was insufficient. Even with our Harvard origin, a strong founding team, and first clinical proof of concept data, mitochondria transplantation was seen as too risky, or fringy by most people we spoke to. We were fundraising for nearly two years until Michael Greve with his venture capital firm Kizoo gave us the first major capital to start addressing the uncertainties that kept more traditional VC funds at bay. We are very grateful for that. Without his impact-driven investment philosophy, we would not have been able to bring Taiho Ventures on board, who led our funding round last year. Generally speaking, the average investor, especially in Europe, does not have the fund size or mandate to fund the emergence of a new field of medicine.
EuroBiotech _Buzzwords such as longevity are not enough to open the check books?
Schueller_While the attraction of longevity is real, buzzwords won’t do with sophisticated investors. I would also say that while interest in longevity has risen exponentially in the general population and with certain groups of highly affluent individuals, it has not yet come close to hype-status amongst institutional investors. In addition to the insufficiently understood biology of many aging related degenerations, time frames to results of longevity approaches commonly do not align well with traditional funds’ investment horizons.
EuroBiotech _So the reluctance to invest in mitochondria transplantation is not due to insufficient marketing within the longevity community?
Schueller_The potential application in aging-related degeneration has gotten us off the ground. It has been the shared vision of Michael Greve and cellvie. But as we are eying a substantial Series A, we must look towards more timely and tangible areas of development. Such as in organ transplantation.
EuroBiotech _Would you characterise Europe’s VC landscape as more risk
averse and hence consider the US as more likely to back a concept such as mitochondria transplantation?
Schueller_Absolutely. Particularly, as we are now aiming for a Series A financing of US$30-40m. As a lead investor, a fund would need to commit US$10-15m in such a round. For one, there are not that many funds in Europe large enough to write checks of this size. And within those funds that can, there will be only a limited number of investment allocations reserved for truly novel concepts. So, it’s not just a question of risk-philosophy, but also a numbers game. Although, I would assume that there are relatively more tickets reserved for high-risk-high-rewards investments in US funds. If we were to raise a smaller round, which we might have to, we would have the benefit of a considerably larger number of fitting European VCs.
EuroBiotech _How do you sell such a novel concept? A lot of basics to teach, I guess. Do you focus your pitch on the basics of mitochondria transplantation, or on the vision of its potential?
Schueller_It’s always a bit of both. Ideally, you capture the interest and imagination of the investor with your pitch for impact, and turn him or her into a believer and advocate by presenting a wellfounded rationale for why such impact is possible and likely. In particular in the early days, when we got started, there was a lot of skepticism, fueled by the lack of understanding and consensus on the mitochondria’s mechanisms of action. With the reproduction of my cofounder’s results across various labs, the field has been experiencing a discernable shift from skepticism to curiosity and hope. I believe, several investors may even be close to action. The recent Nature article will hopefully further substantiate the confidence in the technology. It also helps that we are not alone. Luca Science, in Japan, for example raised a US$30m Series B and Minovia from Israel just started a clinical trial with mitochondria-enriched stem cells. I hope we will be able to benefit from this building momentum.
EuroBiotech _What is important to you now for the next steps?
Schueller_Given the round size we are after, we need to further substantiate our products’ efficacy, safety and manufacturability. We expect to be ready to fundraise in late Q3, early Q4 this year. If we succeed, the next inflection point will be the clinical trial of our allogeneic off-theshelf mitochondria in kidney transplantation. Nothing short of a clinical proof of concept will truly elevate the field.
EuroBiotech _How do you view clinicianled initiatives to start experimenting with mitochondria transplantation?
Schueller_Very critical. The field is already suffering somewhat from the history of stem cell therapies, where some doctors took it upon themselves to determine the appropriateness of the approach along with the therapy’s manufacturing and administration. Of course, we need clinicians to become early adopters and work with us in investigational clinical trials. But we don’t need these unregulated individual clinical treatments. We need to ensure patient safety and create reliable evidence of efficacy.
EuroBiotech _In other words, you need the regulation that is commonly criticised so much?
Schueller_Of course we need it. While we may sometimes rightfully grumble about its bureaucracy and rigidity, it serves to ensure patient safety and provides a framework for companies like us to test our therapy with confidence in patients. It also serves to build trust in new therapies amongst clinicians and patients. It is hence indispensable for a healthcare system to work.
EuroBiotech _ Your enthusiasm and vision in honour but he whole thing sounds like a long and winding road ahead, right?
Schueller_If it was easy, anyone could do it. On the bright side, the road ahead may feel and actually be shorter than the one already traveled. L g.kaeaeb@biocom.eu
SOO ROMANOFF, ANALYST EDISON GROUP 2023 was another particularly tumultuous year for life sciences and, although the lingering effects of the last year are unlikely to change dramatically, the stabilisation of interest rates (off 16-year highs) and emerging direction of travel (market data points) tilt our bias to positive for the new year.
Out with the old… As the smallest life science companies often champion innovation, they have the heaviest burden of proving safety and efficacy before commercialisation. The required lengthy development horizon is particularly challenging in times of elevated interest rates as clinical activities are capital intensive. Although this was not a concern before 2021, elevated interest rates have adversely tainted investor sentiment, especially in 2023, reflected in the 80% decline of microcap stocks included in the SPDR S&P Biotech ETF, XBI (since the peak on 8 February 2021 versus the 25% overall ETF performance decline and a slight increase for large caps within the ETF), which

has resulted in most of the smaller companies trading below cash.
Positive bias towards the new: Robust science will prevail (as in previous cycles). An improving macro environment is expected to rally investor sentiment. With the stabilisation in rates, we have seen more market activity, including the announcement of large acquisitions to enter or expand into specialised areas, including AbbVie’s foray into CNS (Cerevel for US$8.7bn) and entry into immunotherapy (ImmunoGen for US$10.1bn); Eli Lilly, Novo Nordisk, Bristol Myers Squibb with billions in other acquisitions. Prior to these, we had observed tepid risk tolerance de-
Idorsia , has troubled times in adressing short-term liquidity needs. Therefore the company of Actelion founder couple Clozel is trying to persuade holders of its outstanding convertible bond to be refunded with shares.
Novartis, is in radiopharmacy again. The swiss-based company announced that it has entered into an agreement to acquire Mariana Oncology Inc, a preclinical-stage biotechnology company based in Watertown, Massachusetts focused on developing novel radioligand thera-
pies (RLTs) to treat cancers with high unmet patient need. The transaction bolsters the Novartis RLT pipeline supporting Novartis strategic priorities in oncology and RLT platform innovation. Novartis will make an upfront payment of US$1bn and additional US$750m in payments upon completion of pre-specified milestones.
Bayer, and the also German-based life science company, Evotec SE, announced that they have updated the focus of their strategic collaboration to developing innovative precision treatments for cardio -
spite the approaching patent expirations for big pharma. Big pharma companies cannot rely on their current portfolios, resonating with Pfizer’s announced plans to eliminate US$3.5bn in overhead with the displacement of COVID revenues. Increased activity from alternative investors (with longer investment horizons) as they take advantage of the unique arbitrage opportunity (higher biotech cash levels versus market caps). Innovative therapies and devices are expected to stem from smaller, more agile/ entrepreneurial biotechs as development requires specialised resources. Although disease-altering or new mechanisms of action remain the holy grail, generalist and more conservative investors have buoyed solutions with niche expertise and/or shorter development horizons. L
vascular diseases (CVDs). The collaboration aims to identify and validate novel targets, with the goal of building a portfolio of precision cardiology therapeutics by leveraging Evotec’s disease modelling capabilities using human induced pluripotent stem cells (iPSCs). The iPSC-platform technology and highthroughput analytics of Evotec provide unique opportunities to identify new disease mechanisms and pathways for novel treatments simulating human conditions (see also article p. 18). L
2cureX AB 0.03 595k
4SC AG 8.20 82,930k
AB Science SA 2.04 106,000k
Abionyx Pharma SA 1.02 33,110k
Abivax SA 13.16 828,140k
Ablivia AB 0.02 18,590k
AC Immune SA 2.87 84,200k
Acticor Biotech SA 0.80 12,570k
Active Biotech AB 0.03 9,030k
Adaptimmune Therapeutics plc 1.05 265,000k
ADC Therapeutics SA 3.92 324,477k
Addex Therapeutics Ltd 0.07 6,820k
ADL Bionatur Solutions SA 0.35 20,100k
Adocia SAS 9.13 131,460k
Advicenne SA 1.85 22,700k
Aelis Farma SAS 12.70 168,370k
Affimed NV 4.81 73,240k
Akari Therapeutics plc 1.49 11,130k
ALK-Abelló A/S 20.22 4,100,000k
Alkermes plc 22.40 3,790,000k
Allarity Therapeutics A/S 0.74 1,759,212k
Alligator Bioscience AB 0.06 758,000k
Altamira Therapeutics Ltd 1.50 3,360k
Alvotech SAS 11.75 3,140,000k
Alzinova AB 0.09 4,060k
Amniotics AB 0.00 542k
Annexin Pharmaceuticals AB 0.02 5,960k
Aprea Therapeutics AB 5.39 29,270k
Aqua Bio Technology ASA 0.37 21,560k
Arctic Zymes Technologies ASA 1.91 96,960k
Arecor Therapeutics plc 1.46 44,720k
Argenx BV 342.20 59,430k
Arocell AB 0.04 8,110k
Arterra Bioscience SpA 2.00 13,320k
Asarina Pharma AB 0.07 1,610k
Ascelia Pharma AB 0.85 28,830k
Ascendis Pharma A/S 124.00 7,030,000k
Autolus Therapeutics plc 3.58 982,276k
Avacta Group plc 0.51 138,110k
Avadel Pharmaceuticals plc 16.00 1,540,000k
Axichem AB 0.07 1,530k
Basilea Pharmaceutica AG 43.04 519,560k
Bavarian Nordic A/S 22.30 1,740,000k
BB Biotech 41.40 2,270,000k
Bergenbio ASA 0.01 510k
BICO Group 3.61 249,640k
Bicycle Therapeutics plc 21.40 905,360k
Bioarctic AB 17.03 1,050,000k
Biocartis NV 0.29 27,240k
Bioextrax AB 0.16 4,800k
Biofrontera AG 0.33 1,000k
Biogaia AB 10.84 1,050,000k
Bioinvent International AB 2.20 144,440k
Biomed-Lublin SA 1.17 82,230k
Biomérieux SA 97.00 11,480,000k
BioNTech SE 85.90 20,420,000k
Biophytis SA 0.75 2,920k
Bioporto Diagnostics A/S 0.26
BioSenic SA 0.01 3,810k
Biosergen AB 0.02 3,270k
Biotage Sweden AB 14.58 1,170,000k
Bioventix plc 50.00 260,980k
Biovica International AB 0.13 10,450k
Bioxxmed AG 0.41 2,020k
Bivictrix Therapeutics plc 0.12 82,530k
Brain AG 2.72 59,430k
C4X Discovery Holdings plc 0.12 29,260k
Calliditas Therapeutics AB 9.79 582,990k
Camurus AB
Cantargia AB
Carbios SA 23.10 389,130k
The unique and most complete list of share price developments of biotech companies listed in Europe – exclusively in European Biotechnology Magazine.
Celyad Oncology SA
Centessa Pharmaceuticals plc
Centogene NV
Circio Holding 0.19 1,610k
Cline Scientific AB
Co.don AG
Coegin Pharma AB
CombiGene AB
Cosmo Pharmaceuticals NV 73.00 1,170,000k
CRISPR Therapeutics AG 40.00 3,400,000k
CSL Ltd 172.30 83,260,000k
Curevac NV 2.78 622,840k
Cyxone AB 0.02 1,930k
DBV Technologies SA 1.18 113,790k
Destiny Pharma plc 0.19 17,730k
Diagonal Bio AB 0.01 408k
Diamyd Medical AB 0.96 92,760k
Diasorin SpA 101.95 5,440,000k
DMS Imaging 0.02 26,810k
e-Therapeutics plc 0.10 60,770k
Elicera Therapeutics AB 0.05 950k
Ellen AB 0.11 1,247k
Enzymatica AB 0.25 43,520k
Epigenomics AG 1.64 56k
Erytech Pharma SA 2.90 17,620k
Eurobio Scientific SA 14.68 150,450k
Eurocine Vaccines AB 0.01 1,040k
Eurofins Scientific SE 57.36 11,010,000k
Evaxion Biotech A/S 3.64 17,741k
Evgen Pharma plc 0.00 214k
Evolva N 1.02 7,352k
Evotec SE 9.97 1,772,900k
Expres2ion Biotech Holding AB 0.15 7,720k
Faron Pharmaceuticals Oy 1.88 135,370k
Fermentalg SA 0.50 27,080k
Fluicell 0.00 299k
Formycon AG 42.75 754,830k
Fusion Antibodies plc 3.95 3,762k
Gabather AB 0.15 2,890k
Galapagos NV 26.50 1,750,000k
Genedrive PLC 0.02 3,360k
Geneuro SA 1.49 44,340k
Genfit SA 3.42 170,190k
Genflow Biosciences plc 0.02 6,990k
GENinCode plc 0.09 16,630k
Genmab A/S 270.51 17,889,000k
Genovis AB 2.54 166,280k
Genoway SA 4.14 38,390k
Gensight Biologics SA 0.41 41,770k
Gentian Diagnostics AS 3.18 49,040k
Genus plc 21.60 1,420,000k
Global Bioenergies SA 1.85 33,590k
Glycorex Transplantation AB 0.08 25,846k
Guard Therapeutics International AB 3.14 31,590k
Hansa Biopharma AB 3.03 191,925k
HBM Healthcare Investments AG 164.80 1,150,000k
Heidelberg Pharma AG 3.00 139,800k
Hemogenyx Pharmaceuticals plc 0.02 20,800k
Herantis Pharma Oyj 1.43 28,730k
Hofseth Biocare ASA 0.18 72,286k
Hookipa Biotech AG 0.71 68,870k
Hybrigenics SA 0.01 3,840k
Idorsia Ltd 2.26 427,601k
Immatics NV 10.08 1,040,000k
Immunic AG 1.16 104,850k
Immunovia AB 0.13 6,845k
Immupharma plc 0.02 8,330k
Index Pharm. Holding AB 0.03 18,176k
Infant Bacterial Therapeutics AB 8.14 105,772k
InflaRx NV 1.18 69,250k
Innate Pharma SA 2.31 186,790k
Integragen SA 0.67 4,510k
Intervacc AB 0.33 25,030k
Inventiva SA 3.46 181,310k
IO Biotech Inc. 1.31 86,462k
IRLAB Therapeutics AB 1.39 71,730k
Isofol Medical AB 0.04 7,110k
ISR Holding AB 0.19 13,350k
Kancera AB 0.15 17,770k
Kuros Biosciences AG 7.23 266,777k
Lipigon Pharmaceuticals AB 0.02 1,450k
Lipum AB 0.54 5,045k
Lytix Biopharma AS 0.49 19,750k
MaaT Pharma
Medesis
Medivir AB 0.22 24,990k
Mendus AB 0.04 35,650k
BV
Metabolic Explorer SA 0.12 6,180k
Mithra Pharmaceuticals SA 0.22 14,930k
Modus Therapeutics Holding AB 0.08 2,790k Molecular Partners AG 3.28 108,892k Morphosys AG
Newron Pharmaceuticals SpA
Nextcell Pharma AB 0.06 2,220k NFL Biosciences SA 2.07 19,850k
Nicox SA 0.37 18,840k Niox Group plc 0.82 347,720k
NLS Pharmaceutics AG 0.13 5,175k
Novacyt SA 0.75 53,180k
Novozymes Biopharma DK A/S 56.01 23,014,500k
NuCana ADR 3.42 7,240k
Nykode Therapeutics ASA 1.20 390,880k
ObsEva SA 0.00 102k
Okyo Pharma Ltd 1.21 40,220k
Oncimmune Holdings plc 0.21 15,270k
Oncoarendi Therapeutics SA 3.57 60,100k
Oncopeptides AB 0.27 24,333k
OncoZenge AB 0.32 3,790k
Open Orphan plc 0.33 223,160k
Optibiotix Health plc 0.20 19,980k
Orphazyme A/S 139.00 4,910k
Oryzon Genomics SA 1.95 122,920k
OSE Immuno SA 6.59 143,450k
Ovoca Bio plc 0.00 204k
Oxford Biodynamics plc 0.09 28,850k
Oxford Biomedica plc 3.72 372,000k
Oxurion NV 0.00 101,000k
Paion AG 0.04 250k
Pangaea Oncology SA 1.84 56,100k
PCI Biotech Holding ASA 0.14 5,102k
Pharma Mar SA 33.32 611,590k
Pharming Group NV 0.87 588,590k
Pharnext SA 0.07 39k
Photocure ASA 4.95 134,250k
Physiomics plc 0.01 1,490k
Pieris Pharmaceuticals Inc. 11.09 13,710k
Plant Advanced Technologies SA 14.70 16,210k
PMD Device Solutions AB 0.71 14,510k
PolyPeptide Group SF 32.76 1,085,334k
Poolbeg Pharma plc 0.15 75,000k
Poxel SA 0.67 31,310k
Predilife SA 7.00 26,040k
Probi AB 17.80 202,820k
ProQR Therapeutics BV 1.83 147,810k
Prostatype Genomics AB 0.00 2,530k
Proteome Sciences plc 0.04 11,810k
Prothena Corporation plc 19.90 1,070,000k
Pure Biologics SA 1.64 5,440k
Q-Linea AB 0.20 23,290k
Qiagen NV 40.71 9,010,000k
Quantum Genomics SAS 0.13 9,020k
Redx Pharma plc 0.17 66,130k
Relief Therapeutics Holding AG 1.29 14,212k
Reneuron Group plc 0.03 1,940k
Ryvu Therapeutics SA 11.48 265,420k
Saniona AB 0.13 14,710k
Santhera Pharmaceuticals AG 9.30 105,220k
Sareum Holdings plc 0.42 45,300k
Sartorius AG 218.50 7,480,000k
Scancell Holdings plc 0.09 86,300k
Scandion Oncology A/S 0.11 4,500k
Selvita SA 15.58 285,980k
Sensorion SA 0.70 210,180k
SenzaGen AB 0.79 19,110k
Shield Therapeutics plc 2.15 13,851k
Silence Therapeutics plc 18.70 899,000k
Simris Group AB 0.01 3,360k
Skinbiotherapeutics plc 9.98 20,352k
Softox Solutions AS 0.01 5,030k
Sophia
Spexis AG 0.07 4,853k
Sprint Bioscience AB 0.11 7,400k
Stayble Therapeutics AB 0.02 606k
SynAct
Synairgen Research Ltd
Theranexus
Tissue Regenix Group plc 0.69 48,340k
Tiziana Life Sciences plc 0.59 59,830k
TME Pharma NV 0.25 4,370k
Transgene SA 1.32 133,130k
Trinity Biotech plc 7.21 15,190k
uniQure NV 4.58 248,000k
Valbiotis SAS 4.46 70,440k
Valerio Therapeutics 0.11 17,040k
Valirx plc 0.02 2,780k
Valneva SE 3.40 473,400k
Verici Dx plc 0.08 19,400k
Verona Pharma plc 13.70 1,040,000k
Vicore Pharma Holding AB 1.73 193,060k
Virax Biolabs Group Ltd 0.76 1,806k
Vivoryon Therapeutics AG 0.77 20,010k
WntResearch AB 0.00 650k
X4 Pharmaceuticals Inc. 0.96 161,810k
Xbrane Biopharma AB 0.02 33,500k
Xintela AB 0.02 12,020k
Xlife Sciences AG 38.29 218,398k
Xspray Pharma AB 3.55 88,710k
Zealand Pharmaceuticals A/S 84.35 5,280,000k


For biotechnologists, Europe is a collaborative research area. For the biotech industry it is a huge common market with almost 500 million potential customers. The 14th edition of the European Biotechnology Guide presents an attractive cross-section of companies and institutions from 16 nations.
164 pages, available in all bookshops and at biospheria.shop/en
ISBN 978-3-928383-91-2 19.80 Euro


PARTNERING The Swiss Biotech Day 2024 set a new record in participants with almost 2.500 attendees. More than 1.000 delegates from outside Switzerland made the event an international melting pot with all the actors of the whole added value chain of (bio)pharmaceuticals.
The record revenues of CHF7.3bn (US$8.1bn) and a capital investments increase by more than 50% to CHF2bn made the headlines of the summary of 2023 in Swiss Biotech Report by EY and Swiss Biotech Association.
In terms of investments, CHF1.4bn were acquired by public companies, CHF0.6bn by private companies. Also there was significant licensing and M&A activity seen in 2023 whereas R&D investments of publicly traded biotech companies have decreased in line with global markets. On the contrary, privately financed companies were able to increase both R&D investments and liquidity reserves.
During 2023, Switzerland’s biotech sector again demonstrated considerable agility particularly in commercialisation and financing activities to continue to play a
 Michael Altorfer, CEO Swiss Biotech Association
Michael Altorfer, CEO Swiss Biotech Association
key role in driving global healthcare innovation. In addition to record revenues of CHF7.3bn, the industry raised more than CHF2bn – a remarkable 50% increase over 2022. This comprised around CHF1.4bn collected by public companies, and the remaining CHF0.6bn by private companies. Although R&D investments dipped slightly to CHF2.4bn, they are still at very high levels. The latest edition of the Swiss Biotech Report launched at the first day of the Swiss Biotech convention by the Swiss Biotech Association, in conjunction with EY and eight other partner organisations, provides an analysis of the 2023 biotech funding as well as other 2023 key ratios and statistics. The 2024 Swiss Biotech Report’s theme of ‘Reliable Partners Beyond Borders’ focuses on the positive impact of Swiss collaborations at all levels of the global life sciences ecosystem – from research & development to manufacturing and regulatory harmonisation.
Michael Altorfer, CEO, Swiss Biotech Association, commented: «The World Intellectual Property Organization, WIPO, has placed Switzerland top of the Global Innovation Index for the past 13 consecutive years. The Swiss Biotech Report shows that Swiss biotech companies continue to harness this innovation power to develop effective new products and solutions that address global needs. Around CHF1.5bnw were dedicated to collaborations all over the world.»
Frederik Schmachtenberg, EY Partner and Global Life Sciences Lead for Financial Accounting Advisory Services, added «Swiss biotech companies attracted more than CHF2bn, a more than 50% increase compared to 2022. While public biotech companies seemed to have been at least partially impacted by a dire global market sentiment, privately financed companies have raised a solid CHF0.6bn, which even allowed them to increase their overall liquidity reserves, despite record investments in R&D projects.”
Once again, the Swiss biotech industry generated record revenues of CHF7.3bn compared to CHF6.8bn in 2022. This was driven by significant collaboration and licensing deals, where Swiss biotech companies often successfully partner with large pharma companies, and prod-

uct sales boosted by a record number of approvals from Swissmedic, EMA, FDA and other global regulatory authorities, including breakthrough advanced therapies from CRISPR Therapeutics, Santhera Pharmaceuticals, Idorsia, Relief Therapeutics and Basilea.
In a challenging global public market, the main fundraises were achieved by Oculis SA, with a SPAC transaction on NASDAQ and follow-on financing collecting US$144m and MoonLake Immunotherapeutics, with CHF415m through a follow-on transaction from 2022’s IPO. While the public market sentiment remained dire ever since the end of the Covid pandemic, the private funding environment held up well in 2023. Noema Pharma with CHF103m raised, Al -

entis Therapeutics (CHF94m), Rejuveron (CHF67m), Nouscom (CHF65m), and NewBiologix with CHF45m led the way.
... and lead to merger eventually
2023 also saw significant M&A activity at all levels from early stage to established companies demonstrating the maturity of the Swiss biotech sector. Particularly worth noting are Pierre Fabre Laboratories purchase of Vertical Bio, Ironwood Pharma’s takeover of VectivBio for US$1bn and Boehringer Ingelheim’s acquisition of T3 Pharma for an amount of up to CHF450m.
The number of FTEs working in Swiss R&D biotech companies remained with over 19,000 people almost unchanged in 2023 compared to 2022. Despite an overall slight decrease in R&D expenses, the private companies’ share continued to grow.
Finally, following the creation of an innovation office in 2022, Swissmedic looked to foster international collaboration and harmonisation by establishing ‘The Access Consortium’ with medium-sized regulatory authorities, including Australia's Therapeutic Goods Administration (TGA), Health Canada (HC), Singapore's Health Sciences Authority (HSA), and the UK’s Medicines and Healthcare Products

Regulatory Agency (MHRA). By this, the small swiss market in terms of patients and volume of products in a population of about 9 million people can be easily multiplied by nearly the factor of 20. A record number of product approvals by Swissmedic, EMA and FDA – including world’s first CRISPR gene editing therapy – is a testament for acceleration in regulation and the path of innovation from bench to bedside.
The life sciences sector has been a solid pillar of the Swiss economy and foreign trade for decades. In 2013 it took the lead as the largest export industry of the country. It has shown remarkable resilience, continuing to grow even during the peak year of the COVID pandemic in 2020, when exports almost universally slumped. While exports from all other sectors combined grew by 81% in the twenty-five years from 1999 to 2023, the contribution of the life sciences sector grew by 400% over the same period. Today, the life sciences sector is Switzerland’s largest export industry. In 2023, it accounted for 38.5% of total Swiss exports and contributed CHF 105.5bn to the foreign export trade.
The Swiss Biotech Report 2024 was launched at the Swiss Biotech Day which


is attracting increasing numbers of international delegates. One contributing factor is the Global Village, a platform hosted by the Swiss Biotech Association in partnership with Switzerland Global Enterprise to stimulate sustainable worldwide networking and international collaboration in biotech and life sciences. In just its second year, the Global Village has already grown by 70% and featured 17 international delegations.
To recognise outstanding achievements, the Swiss Biotech Association was also presenting the Swiss Biotech Success Stories Awards at this year’s Swiss Biotech Day to the winners Fondation Sui-
sse de Recherche sur les Maladies Musculaires (FSRMM) and Dr. Hans-Peter Strebel.
FSRMM was founded in 1985 by Jacques and Monique Rognon together with two existing patient organisations to promote research on neuromuscular diseases. Since its inception, FSRMM has raised in excess of CHF30m and funded more than 200 research projects at Swiss universities and hospitals.
Dr. Hans-Peter Strebel founded Fumapharm AG with three other scientists in 1983. This was the start of more than 40 years of successful research leading to the development of Tecfidera in relapsing multiple sclerosis which has so far benefitted many patients worldwide. L g.kaeaeb@biocom.eu

BIOECONOMY French Carbios celebrated the groundbreaking ceremony for the world’s first PET biorecycling plant in Longlaville in the Grand-Est-Region. Carbios’ recycling technology aims to depolymerise PET waste based on an enzymatic process resulting in monomers that can be used for the production of new, virgin-like PET.
Carbios SA (Euronext Growth Paris: ALCRB) celebrated the groundbreaking ceremony for the world’s first PET biorecycling plant in the presence of representatives of local authorities, partner brands and industrial partners in April. Located in Longlaville, in the Grand-Est region of France, Carbios’ first commercial plant shall play a key role in the fight against plastic pollution, offering an industrial-scale solution for the enzymatic depolymerisation of PET waste in order to accelerate a circular economy for plastic and textiles. Work is progressing on schedule, as the company stated with “significant quantities delivered to customers in 2026”.
The world's first PET biorecycling plant with Carbios’ technology enables PET circularity and provides an alternative raw
material to fossil-based monomers, offering PET producers, waste management companies, public bodies and brands an effective solution to meet regulatory requirements and their own commitments to sustainable development. The plant will have the capacity to process 50,000 tons of PET waste per year (equivalent to 2 billion coloured bottles, 2.5 billion food trays or 300 million T-shirts).
The plant will create 150 direct and indirect jobs in the region. In October 2023, after only 10 months (the average duration in France is 17 months), Carbios obtained the building and operating permits for the site. The factory is currently still under construction on land officially acquired from Indorama Ventures on 14 February 2024. In February 2024, Carbios and De Smet Engineers & Contractors (DSEC) announced their collaboration to manage construction. Several feedstock supply agreements, notably

with CITEO and Landbell Group, will secure the vast majority of the raw materials required. Close to the borders with Belgium, Germany and Luxembourg, the plant’s location is strategic for access to nearby waste supplies.
French president Emanuel Macron honoured the ceremony with a special post and commented: “At a time when governments are negotiating an international treaty against plastic pollution in Ottowa, the groundbreaking of Carbios’ biorecycling plant is particularly significant. It illustrates France’s commitment to the ecological transition, and demonstrates our ability to turn challenges into opportunities for a more sustainable future. This plant, a world first, embodies French innovation in responding to complex environmental challenges, offering a sustainable solution for a future where plastic and textile waste will be transformed into valuable resources for a truly circular economy.”
As part of France 2030, Carbios will receive grants totaling €54m from French State and Grand-Est Region for the proceedings.
Carbios was recently awarded 1st prize in the “So French So Innovative” Award organised by Business France, the Hong Kong Committee of French Foreign Trade Advisors (CCEF), La French Tech and its partners at InnoEX 2024 taking place in
AMELIE REIGL
STORYTELLING FOR SUCCESS
INVESTMENT
FINANCING IN ECONOMICALLY CHALLENGING TIMES

Science
Magazine No. 2/2024
Perfect conditions for successful start-ups
P Start-up incubator for life sciences and medicine of the future
P State-of-the-art laboratory infrastructure, co-working and community spaces
P Tailored incubation process & training programs
P Coaching and mentoring from experienced entrepreneurs and experts
P Partnerships with industry key players and strategic partners


Dear readers, Dear startup enthusiasts,
We are delighted to share the second issue of our Catalyser magazine with you – the navigator through the world of life science startups and innovations with all their challenges and triumphs that make entrepreneurship so fascinating.
Our inspiring hero story by Barbara Stegmann is a lesson in perseverance and demonstrates how important it is to build a strong personal brand to succeed in today's business world. A particularly significant hurdle for academic startups in current times of economic uncertainty is to receive funding. Grit Zahn and Marco Janezic shed light on fundraising strategies and provide insights on how startups can secure investments even in a recession. With a twinkle, we demystify "investor jargon" in this context and explain the terms that every founder should know.
In the startup industry, communication as well as flexibility are important components on the path to success. Amelie Reigl, expert in science communication, shares her insights and tips on how startups can effectively convey their key messages. With Siro Gottardi, we discuss when and how a strategic realignment makes sense, and why a pivot can often be intimidating but also opens new opportunities.
In this issue, we also dare to think big: Patrick Rose discusses moonshot projects that might have the potential to change our future. Additionally, we get an exclusive insight into the prototyping area of the Life Science Factory, which reveals the creativity and innovation flourishing in the startup hub on-site.
Join us on this journey full of insights for life science startups focused on the German market and inspiration for your own projects. We hope you enjoy this issue of CATALYSER and, as always, we look forward to your feedback.
Yours sincerely,
Irina Reimer and Svenja Hodel
3 EDITORIAL 4 JUST SOME CRAZY THOUGHTS
Patrick Rose reveals three moonshot projects worth investing

6 SHARE WHAT YOU DO
Barbara Stegmann: Successful founder and personal brand
9 STORYTELLING FOR SUCCESS
Why communication is an important key to success for startups
11 CHANGING DIRECTION
When and how to pivot your business successfully

13 BUILD IT YOURSELF
Advantages of prototyping for life science startups
15 GIMME SHELTER
Financing in economically challenging times
17 INVESTOR JARGON FOR FOUNDERS
Understanding frequently used terms of investors
Adress: Life Science Factory Management GmbH, Annastr. 27, 37075 Göttingen; E-Mail: info@lifescience-factory.de; Website: catalyser.de; Publisher: Svenja Hodel, Irina Reimer; Editors: Frau Wenk GmbH, BIOCOM Interrelations GmbH; Idea and concept: Life Science Factory Management GmbH, Frau Wenk GmbH; Design: Michaela Reblin; Printed by: Königsdruck, Berlin; Cover picture: © dieWissenschaftlerin
INNOVATION SPRIND stands for the Federal Agency for Disruptive Innovation. But what exactly is disruptive innovation? And why do we need it?
How about a 300-metre-high wind turbine that harvests wind at high altitudes and converts it into electrical energy? Does that sound crazy? It’s not at all, says Patrick Rose, Innovation Manager at SPRIND. The Federal Agency is on the lookout for exactly those types of absurd moonshot ideas.
Rose and his team offer people, who would like to realise their ideas, a home and a space to try things out and show that even the supposedly impossible is possible. The aim is to create products, services, and systems that noticeably and sustainably improve everyone’s lives.
“There are no stupid ideas,” says Patrick Rose. “It’s quite the opposite. The more unusual a technology or vision of an application seems, the more likely we are to give it a go! There are no limits.” The aim is to advance technologies that nobody knows about yet but nobody will be able to live without.
The organisation thinks holistically and creates an entrepreneurial environment where it is possible to take risks and be courageous. Patrick Rose, who describes

himself as a fan of Star Trek and the Marvel films, sees courage as a factor that is in short supply in Germany. “Germans often lack the grit and the passion to think big about something big and crazy.
BACKROUND
Since November 2022, Patrick Rose has been Innovation Manager at SPRIND. He celebrates unique perspectives and distinct approaches that challenge conventional thinking, he says about himself. Before taking his position at SPRIND, Rose was Chief Scientist at ONR Global and gained many years of experience as an Adjunct Assistant Professor in the Department of Epidemiology and Public Health at the University of Maryland School of Medicine.
The sentiment is very different in other countries, such as the USA. Here, people are working on really crazy ideas.” He adds, “If you ask postdocs in the Baltic countries, for example, which one of them has ever thought about founding their own company, almost everyone raises their arm.” In Germany, on the other hand, hardly anyone would come forward. Rose finds this very regrettable because there is more than enough potential. Germans are too afraid to make mistakes. Most academics in the life sciences would rather stay at university than try out new things.
Crazy and big ideas
However, by putting its approach out there, the Federal Agency wants to foster a new way of thinking - crazy and big. SPRIND

“It may sound like utopia, but it’s not. What the USB stick and the hard drive can do, our DNA can do at least just as well: store a huge amount of data and information externally. As the storage medium of the future, DNA offers a huge field that we believe should be explored as a matter of urgency. After decoding DNA more than 20 years ago, an algorithm can translate information into the nucleic bases that make up our DNA. These short DNA strands can then be put together in the laboratory to easily retrieve the data they contain. It is also plausible that we will use DNA to build smaller organic chips for computers.”

“Migratory birds use the Earth’s magnetic field to navigate. They have a magnetoreception sensor in their eyes that works independently of light. When flying, birds use this sensor to determine the inclination angle of the lines of the Earth’s magnetic field and thus always know exactly where they need to fly. The sensor functions like a magnetic compass. The entire process takes place at a quantum level. It would be possible to build a biological quantum computer using this mechanism as a model. It can be demonstrated mathematically that quantum computers work much faster than a classic computer for all problems that can be solved by trial and error.”

“Who hasn’t seen those scenes from Star Trek when Captain Kirk goes to one of the replicator terminals on the Starship Enterprise and orders a cup of tea. In the science fiction series, the replicator is designed so that food and other things can be replicated simply by command. This is a project that we would like to realise and in which we are willing to invest money. So we really want to build the 1st generation replicator. The 3D printers are the first preliminary stage of the replicators. But it will be even more exciting if the replicator works assembles and produces objects at a molecular level.”
offers financial support and a network for optimising and finalising prototypes. If the SPRIND experts deem a project worthy of funding, it receives between four and fifteen million euros per year for further development.
“In the case of funding, we often set up project subsidiaries. We also finance projects via our SPRIND challenges with between €500,000 and €3 million. The financing is provided here as a pre-commercial contract for research and development services,” explains Patrick Rose.
Inspired by the latest Marvel film, Patrick Rose is thinking about how it might be possible to build a space elevator. But are there any other examples of projects that would immediately inspire him and the other experts at SPRIND?

The first challenge: Develop an end-to-end prototype that processes various carbonaceous waste streams into new products as a continuous bioproduction process
HERO STORY Barbara Stegmann is CEO and co-founder of living brain. During an interview with Catalyser, she talks about her first steps in marketing, the challenges of the German healthcare market and the time when she almost lost her passion for her work.
One simple formula applies to every newly founded company: If you want to be successful, you need to be recognised and heard. There are various ways to achieve this, whether it's press and public relations work, networking and participation in relevant events or cooperations with established companies, which can help startups increase their reach, which is still limited at the beginning.
In April 2019, Barbara Stegmann and Julian Specht founded living brain, a company that specialises in the rehabilitation of patients following an accident or illness. Based on innovative technologies, the company has developed a new treatment method that can be used anytime and anywhere. To achieve this, living brain combines psychological learning strategies, therapeutic science and virtual reality.
Personal branding was an important piece of the puzzle for living brain when it came to raising the company's profile and increasing its reach. In this interview, Barbara Stegmann talks about her experiences on the way to creating herself as a brand and marketing herself as a successful founder.
CATALYSER. Do you remember the moment you had the idea to establish living brain?
Stegmann. Actually, there was no specific moment. I was studying psychology and met my future co-founder Julian during my time at the university. Julian suffered from severe epilepsy, he had two or three epileptic seizures every day and was taking everything you could get on the interna-

tional market to stop them. None of it really helped. In the end, his only option was to have the part of his brain, that caused the seizures, surgically removed. He asked his doctors: "What are the risks?" His doctors replied that he could develop problems with his memory and that this could lead to massive cognitive impairment, such as problems with his attention, memory and concentration.
Then Julian asked: "If that happens, what do I do? What therapies are available?" And his doctors replied: "We have a few exercises with pen and paper, we have some things on the computer, but we don't really trust the methods."
Julian had the operation because it was the only way for him to be free of seizures.
And he was lucky: he never experienced any of these impairments.
But I found it astonishing that his doctors said that although there were treatment methods, they didn't believe in them.
CATALYSER. What happened next?
Stegmann . At that time, Julian and I thought: "What is the problem?" Since cognition is a branch of psychology, we thought we could turn the topic into a student research project to impress our professors. So, we took a closer look. The therapy methods date back to the 1950s. They were never adapted, just digitised. What was striking was the huge problem with knowledge transfer: the exercises are so abstract that our brains are unable to apply them to everyday life. It's like trying to teach a child how to swim: At best, you allow them to go into the pool with floaties, but you don't give them a book and say, "Read this, then you'll know how to swim". That's when we started to come up with a concept. And ultimately, we decided to go for a VR solution.
CATALYSER. After founding your company, you probably asked yourself the question "How do I make myself and my company visible?". Do you still remember your initial ideas?
Stegmann For me, awareness wasn't as important at the beginning as meeting the right people. Initially, we focused on going to startup events, healthcare and life science events. We specifically approached people who support healthcare startups.
First of all, it was important for us to build up a network with the right people. Making the product or the idea more widely known was not the main focus at the time. At the time, I thought that we had to establish something solid before we could even start talking about our product. I always had the principle "Don't overpromise and underdeliver" in mind. After all, how disappointed will someone be if I promise them something and then can't deliver on it because my startup and my product aren't quite ready yet? That's why it was all about building a network with the right people at first.
CATALYSER The life science market is considered complex. As a prospective CEO, what were the challenges for you in gaining a foothold in this market?
Stegmann The healthcare market has completely different rules compared to other markets. It is heavily regulated - by many laws, by standards that you have to comply with, by requirements, regulations, etc. It is also very complex due to the different reimbursement methods that exist in Germany. After all, these are things that you can still read up on. But there are also things that you have to learn through experience along the way, for example the topic of reimbursement values: Why is it important to fit into a certain reimbursement code? What are the unwritten rules of the market? Alternatively, consider the topic of clinical trials, which are expensive, time-consuming and risky. One thing is clear: in the healthcare sector, startups first have to survive a very long "valley of death". Both the number of risks and the capital requirements of healthcare companies are disproportionately higher compared to many other markets. Moreover, the healthcare market is relatively conservative. Sometimes, this makes it harder for young people to gain a foothold. As a young CEO, you have to learn quickly, understand quickly and implement quickly. It's not enough if you can do it in theory; you have to be able to apply it directly to your company.
CATALYSER. You mention that the healthcare market is conservative. You have now
introduced a new product and a technology-supported therapy method to this market. That was probably another special challenge, wasn't it?
Stegmann The advantage is that if you are one of the first, you can still surprise people and establish standards because no one has done it before. The disadvantage is that many people are very skeptical, especially in Germany. There was also scepticism about virtual reality. Therefore, we conducted an acceptance study with living brain at the beginning, because we had often heard: "Older people? Stroke patients? As if they can use VR!" And we were told this by doctors as well as neuropsychologists or occupational therapists and almost even more frequently by the relatives of those affected. Whereas those affected were often totally motivated and said: "I can do it!”
For the acceptance study, we asked: Is acceptance among older people with a stroke worse or better than among people of the same age who have not had a stroke? And is acceptance generally positive or rather negative?
Our study showed that acceptance is positive for both groups. Usability and user experience were positive for both stroke patients and healthy people of the same age.
We have learned for ourselves that many reservations can be anticipated if you have evidence. Emotional selling, i.e. convincing people on an emotional level, works for patients and relatives, but when it comes to decision-makers, for example in hospitals
or insurance companies, you need much evidence in clear terms.
CATALYSER. As an entrepreneur, you have certainly also had to put up with setbacks. What moments of failure do you recall?
Stegmann There were certainly a few moments when things didn't go so well, or at least not as we had imagined. Nevertheless, I wouldn't say that there were moments during which we ultimately failed. There were moments when we went the extra mile to learn something new and develop our product further.
CATALYSER. Can you provide one or two specific examples?
Stegmann. For example, I remember a situation when we set up our quality management system. We did this completely in-house. You have to know that: As a medical device company, you need to have a certified quality management system in accordance with a specific standard. There are companies that employ entire departments for this and have external quality managers who do it for them. We decided to set this up completely in-house. And we did it without any of us ever having done it before, let alone knowing how it works. It took us 18 months. That was a time when I realised that we had reached the limits of what we enjoy doing. This time really brought us closer together. But it was also a time when we asked ourselves:

"What are we actually doing this for right now?". It was an enormous amount of work and complicated and non-transparent in many areas. That was a time when we said: "We'll be working extra hours and will learn something." You can't call it a failure, because we received the certificate the first time around, but it was definitely a very stressful process.
CATALYSER Have situations like this had an impact on your personal branding strategy?
Stegmann. When it came to the quality management system and certification, I tried to talk about it publicly on social media. I tried to share my learnings, also because I realised that it is unusual to set up such a system without external help. I was then able to include this in my personal branding: a competence that I had acquired. As a result, I was able to talk more about it.
I have the impression that what I do and who I am has changed me as a person and how I communicate to the outside world over the years. Through my work in the company, I have acquired skills that I can share and that I can use to support other people, for example with tips, tricks and best practices.
CATALYSER. So you definitely see a difference between the strategies you used for personal branding in the beginning and the ones you are pursuing now, right?
Stegmann. Definitely. When I started out, personal branding was hardly an issue for me - perhaps also because I didn't think I knew enough back then to be able to share anything. I am convinced that I am not alone in this. I think there are a lot of people who say: "I don't share anything because I'm not sure if what I'm saying is important enough." Something has changed for me with regard to this in recent years.
However, I also have to say: I used to have reservations about marketing and personal branding because I always had the "American" version in my head, this overselling: claiming things that aren't true.
CATALYSER And what does it look like today?
Stegmann. This initial feeling of aversion only subsided when we founded a GmbH in 2019 and asked ourselves: "How do we do marketing now?". I started to "label" both marketing and personal branding differently in my head. It's not about selling something to others, but about talking about the good we do: We can help people with our product. We are not selling hope, but a therapy that outperforms the current gold standard.
"When it comes to decision-makers, you need evidence, you always need it in cold print"
We are better than the therapy available on the market. So it is actually our obligation to talk about it because we are doing something good with it and because we need to reach the people who can benefit from us.
I am still developing my personal brand. But even in that case, I said to myself: maybe I can help other people by sharing my expertise and best practices or maybe by talking about my insecurities. I think we all have a lot of thoughts, perspectives or even fears that we don't talk about because we're afraid that someone else will judge them. But we are certainly not alone in this.
CATALYSER What impact does your personal branding have on potential investors?
Stegmann. Personal branding is not just about social media; it's also about being present at events, giving keynotes or talking on panels. If you have a good network, you will be invited to audiences like this. If you talk about it, you get invited more often, etc. I have had the opportunity to meet some
of our investors at many events. Many only became aware of me when I was on a panel for the first time or because they had seen an interview or watched something and then came into contact with us.
One of our investors wrote to me on Linkedin: "Hey, I just happened to see your profile, I looked at a few things, I think it's pretty cool. I saw you're doing a fundraising round. What does it look like? Are you still looking for investors?"
Ultimately, personal branding is "just" an extension of your own network. I have to make sure that people know what I do because it might be relevant and interesting for them, and I can help them, or they can help me. Personal branding is therefore a form of "socialising" and networking, although you don't necessarily talk to anyone directly, but rather present yourself and show what you can do and who you are.
CATALYSER. Is there anything else you can share with aspiring founders and perhaps especially female CEOs from your wealth of experience?
Stegmann. Look for a network of other female founders and CEOs! There are some very cool ones in Germany, for example Encourage Ventures or Mission Female. There are female founder networks in many cities. Have a look around, check it out! I've experienced for myself that you're treated differently when you're a woman in this position. In the beginning, I thought it was because of my skills. It didn't even occur to me that it was because of my gender. When I started talking to other female founders and other female managers and investors, I realised that the things that happened to me also happened to them. We still have to break down prejudices in our society.
What else I can recommend to founders: Find something you really love about what you do. There are bad times and there are always times that are challenging. It's at times like these that you need to find something to hold on to. Something that you love about it so much that you keep going. Whether it's the money or the team or the mission, the product, the problem or whatever: you have to love something about it, otherwise you won't finish it.
COMMUNICATION "If you let data speak for itself, that's science. If you collect data specifically to prove what you've already decided to prove, that's not science, that's marketing," inventor Tom DeMarco once said.

DeMarco sums up the dilemma as to why many scientists shy away from marketing and PR very well. Yet, for startups in particular, both are absolutely essential tools. Amelie Reigl demonstrates how it can be done. Using the name @DieWissenschaftlerin, her videos on Instagram and TikTok reach almost half a million followers. She talks about the difference between soccer and science and raises the question of whether ‘Dry January’ really makes a difference. "I would like to make science more visible. To do so, I talk about everything that affects or concerns me in any way. I want to show my life as a scientist and how diverse it is," says Amelie Reigl about the content she creates. " My videos don't require any prior knowledge and I aim for each video to stand on its own. This also includes not using technical terms. As a researcher, you are trained to speak as precisely as pos-
sible when using terminology and I really enjoy that. However, it is a real challenge to speak in simple terms without losing in-
formation. But that's exactly what I try to do on Instagram and TikTok."
Making the whole team visible Presenting scientific contexts as comprehensively and entertainingly as possible on social media - is this also the formula for success for startups in the science sector? Amelie Reigl certainly thinks so - however, she advises against an overly hasty approach. First of all, companies need a plan, a vision and a goal as to what they want to achieve with their communication. It is essential that the entire team is involved in the creative process: "It's not just me as the founder who represents the company, but the company as a whole. And that's why everyone should have a say in how they would like to present themselves on social media or not," explains Reigl. " Particularly when it comes to science, there's room for creativity. Many startups in
BACKROUND
Amelie Reigl is a biologist and is doing her PhD at the University of Würzburg. For her doctoral thesis, she is developing a human skin model that is intended to be used for testing purposes and should thus reduce animal testing. She is also one of the leading German-speaking content creators in the science sector using the name @dieWissenschaftlerin. Launched during the 2020 coronavirus pandemic, she now has a community of almost half a million followers on TikTok and Instagram. Reigl aims to make the everyday life of a scientist visible, offering up-close insights into research and simple explanatory videos to help people understand science. Her own startup TigerShark Science is already waiting to be launched after her doctoral thesis is completed and will be dedicated to the aforementioned skin model.
this field have great visual images based on their work that are ideal for social media. However, I also often see companies who quite intentionally don't show their product, thus creating a certain level of curiosity."
Reigl also points out that no company should necessarily use all channels from Instagram to TikTok. Instead, it is important to choose according to the objectives and then appoint a person who is in charge of social media, who is allocated the necessary time and who enjoys being in front of the camera from time to time.
Use PR early on
However, LinkedIn is a channel that definitely pays off in terms of business goals and being seen by potential partners, customers or investors. The business network is the common denominator on which startups, business and science are equally represented. Not only Amelie Reigl shares this opinion, but also Alexander Hüsing, founder and editor-in-chief of the specialised business publication deutsche-startups.de: "In addition to your own website and traditional PR work, LinkedIn is an extremely important vehicle for being noticed in the first place. Initially, it's not about posting daily, but simply about being visible on LinkedIn as a company and founding team by having your own page and providing the most important information."
According to Hüsing, startups cannot start too early with visibility on LinkedIn, just as they cannot start too early with traditional PR work. On the contrary: "I find it hard to understand when companies shy away from PR work even though they have already founded a GmbH and set up


a website. To anyone who does this, I can say: there are a hell of a lot of journalists out there who would like to learn as early as possible that there is a team of people working on a specific topic."
Don’t call it „Press Release“
Especially when sending press releases or pitches, however, you should integrate as much individuality as possible in order to stand out from the crowd. Otherwise, the emails could easily get lost in journalists' inboxes. "I often have emails in my inbox with the subject 'Press release', 'Press news' or 'New startup'. None of that works for me at all," explains Hüsing, adding: "Founders should ask themselves before each mailing: 'How does this email look when it ends up in the journalist's inbox?' It should then be tweaked if necessary. For me, the company
name definitely belongs in the subject line as well as a direct salutation in the message. In other words, it shouldn't say 'Hello editorial team at deutsche-startups', but rather 'Hello Alexander'."
Even in the founder's story, there are often hidden gems that can add individual value to your pitch. Be it the company's location, which is particularly relevant for the local press, or the fact that the founders got to know each other at university, or the team has known each other since childhood. Hüsing's tip: "Founders need to learn to take a look at their startup and their history from the outside. Otherwise, a certain degree of 'operational blindness' automatically sets in, so that they no longer notice the truly interesting aspects of their story. To prevent this, it also helps to talk to people outside the company about your own business and its history."
The bottom line: Become visible
Long story short: science startups should always invest time in social media and PR work to attract the attention of potential customers, partners, investors and journalists. The sooner they become visible, the better - but this should not lead to hasty decisions. The following applies to both communication channels: there must be a clear plan of what the company wants to achieve in specific terms and how it wants to do this. It is important to identify the rough diamonds in your own company story in order to stand out from the crowd and attract attention in both social networks and press relations.
BACKROUND
Alexander Hüsing has been working as a journalist for more than 25 years and specialises in new companies and founders. With deutsche-startups.de, he has been reporting daily on news from the German Internet startup scene since 2007. Interviews, portraits of individual startups and founders as well as market overviews of interesting segments supplement the daily news. Background information on investors and business angels as well as guest contributions from well-known founders and other Internet personalities complete the comprehensive range of information on German startups.
STRATEGY The road to business success is not always a straight path. Especially startups often need to make adjustments or even serious changes to their business model. This process is called a pivot. But what about the life sciences segment? Are the rules different in this area?

Siro Gottardi advises startups in the life sciences to always assess the market’s needs and adjust their business models accordingly
More and more scientists are stepping away from typical careers in research to found a startup. However, not all of these entrepreneurs are successful with their young companies at the first attempt. Sometimes, they have to pivot, adapt, or even make extensive changes to their business model. Which factors need to be considered?
Classification makes all the difference “In highly regulated areas of the life sciences, it may often take longer than ten years before a company can launch its product on the market,” says Siro Gottardi from the management consultancy Peak Spirit in an interview with Catalyser. Peak Spirit sup-
ports early-stage life science companies in market entry and remuneration, and works with more than 100 startups every year. Regarding market entry, the life science sector differs greatly from other industries. It usually requires significantly more financing than startups in other sectors. This is because much more research is necessary in life sciences. “It is fundamentally important,” says Siro Gottardi, “that founders consider the market before and also during the ongoing operation of the company and obtain feedback from experts.” They should also set goals that can be quantified. If this does not work, it can be one of the reasons why a startup has to pivot in order not to fail
entirely. In fact, various studies show that around 80 % of startups have to give up their business operations within the first three years.
Possible reasons for a pivot
Besides insufficient observation of the market situation, there are other reasons for a pivot. These are:
• a product expansion,
• a completely new product,
• the customisation of a product,
• changes in the business model.
Changing direction - a game-changer
Many of today’s successful companies have carried out a pivot in the past. By changing direction well prepared, they were able to give their business model a lasting boost in all cases. Examples include Apple and Nokia. The latter was once founded as a paper factory. Apple started as a small computer company under the leadership of Steve Jobs. At the time, the focus was
“Entrepreneurs should always inform themselves about the market’s needs and constantly think outside the box.”
on selling basic computer accessories. The founders quickly realised that the business model could not be scaled up. The pivot plan: Apple was to build its own brand. It now has cult status.
Not insisting on a single vision
These examples show that success does not depend on one particular vision. Founders should be open to other opportunities and develop the original idea further. “Entrepreneurs should always inform themselves about the market’s needs and constantly think outside the box,” advises Gottardi. If a pivot does have to take place, good preparation is essential. “This includes analysing the market in detail and talking to experts,” says Gottardi. It is the only way to ensure that the pivot is successful in the long term.
Get the whole team on board
It is essential to involve the team in the process. The team members must be given the opportunity to contribute their opinions and help shape the pivot transparently. The product should also be confirmed by the market in a way that fundamentally ensures usability by a significant target group. If this is not the case, according to the expert, it often becomes apparent that the market is too small, and the product cannot be successful.
Better to act sooner
It is also essential that companies consider the possibility of a pivot as early as possible. Market validation should occur nine to twelve months before a new financing round. “Entrepreneurs are often not able to let go of their vision. It is much better to be honest with yourself and regularly track
Measure your success
Perfect timing: plan and prepare all departments
Learn and adapt 2 3 4 5 6 7 8
Know your target market and its pain points
8 IMPORTANT STEPS TO PIVOT YOUR BUSINESS SUCCESSFULLY 1
your KPIs so that you have enough time to change direction.”
Look for established partners
Once a pivot has been carried out and the company’s realignment has been finalised, young companies should join forces with established partners from the relevant life sciences sectors. This offers advantages for all parties involved. “Successful, estab-
BACKROUND
Establish new goals
Siro Gottardi is Chief Executive Officer at Peak Spirit. He provides advisory and business coaching for entrepreneurs, executives and investors in health technology and medtech companies. His core topics are: Go-to-market strategies, revenue models, sales growth and organisational development/scaling.
Choose how to pivot
Analyse competitors and differentiate
Identify the need and reason for pivoting
lished companies gain new impetus through collaboration with startups or learn about forward-looking innovations that benefit their KPIs. On the other hand, startups gain important insights through cooperation and often valuable tips or mentors who can coach the young company’s managers at various levels. Ultimately, the partnership may also offer financial benefits for a startup. This is because established partners often suggest further developing a product together or offer access to additional funding.”
However, it is essential not to miss the right time for a pivot. The market situation should be monitored and evaluated over the entire period of research and development. This applies to the life sciences sector just as much as to all other sectors. Good preparation is just as necessary before a pivot as before founding a company. This is the only way to prevent a company that started promisingly suddenly ceasing to exist.
CNC milling machines, 3D printers, 3D scanners in use: Maker's Factory at the Life Science Factory in Göttingen

PROTOTYPING Venturing into the uncharted realms of research often necessitates tools that don't yet exist. This very conundrum is what the Maker's Factory at the Life Science Factory in Göttingen resolves.
Besides fully equipped lab space, a unique prototype workshop can be found at the Life Science Factory. It offers startups and entrepreneurs the swift and cost-effective production of customised aids essential for their research. Whether it's innovative tools, tactile prototypes, or even specific projects like a device for measuring water quality in developing countries, the possibilities are nearly limitless.
Does this sound unconventional? Indeed, the concept of this prototype workshop is quite singular, catering to a variety of applications such as:
• Rapid Prototyping
• Creation of tactile models
• Ad hoc development of individualised tools
To facilitate these diverse applications, the Maker's Factory boasts a high-tech
machine park focused on additive manufacturing, equipped with the latest CNC milling machines, 3D printers, 3D scanners, and even lasers for cutting.
Startups have the opportunity to rapidly develop and produce a prototype based on their idea. This allows for quick testing with subjects and gathering feedback from stakeholders. Iteration cycles are short, enabling prompt enhancements, while rapid prototyping also helps save costs and resources. Creating tactile models is crucial not only for medtech startups but also for those in pharmaceutical development. In this way, processes can be made visible that are otherwise rather invisible because they happen ‘inside the body’. These models can be used by startups during pitches to
investors, with examples including a cell printed on a larger scale or a heart model with arterial and venous inflows, created using a 3D printer.
Sometimes startups reach a point where processes stall due to the lack of the right tool. In the Maker's Factory, tools of all shapes and sizes can be created ad hoc, with the 3D printer often being the method of choice.
Should startup staff lack experience with machinery and tools, they can undertake an introductory course with Maximilian Bieker, the manager of the Maker's Factory. He is the point of contact for all young companies utilising the 300 square metre space and he also advises entrepreneurs on developmental issues.

"For some users, this is their first-ever experience in a workshop. I encourage everyone new to us to try out the machines and discover their technical affinity," the manager emphasises. "There's an opportunity for everyone to learn how to handle the tools in no time, whether it's a laser cutter or a 3D printer."
startups
The Maker's Factory primarily offers entrepreneurs guidance on implementing things themselves in the open workshop. It's about 'learning by doing' and at the same time about the community of diverse researchers who assist and exchange with each other, rather than just a service. "This often leads to new ideas," notes Bieker. Startups also have access to countless forums and open-source resources for file research and template design.
Shadowing and advising
If development stalls, Maximilian Bieker offers his ‘shadowing’. This means he accompanies entrepreneurs for a day in their lab routine and afterwards provides constructive feedback, helping them optimise processes or rapidly develop and build essential tools using a 3D printer. This approach allows for quick and precise resolution of everyday work problems.
Startups can control the quality and speed of implementation themselves. In the case of prototypes, depending on the materials and technology used in development, the prototype can be of high quality
and close to the final product or just a preliminary model not yet fully refined.
"Every life science startup can benefit from the workshop," Maximilian Bieker highlights. The main pain points for young entrepreneurs are time and money. And this is precisely where the Maker's Factory steps in with its unique concept.
Maker's Factory success stories
Digity builds Artus exoskeleton
The startup Digity, based at the Life Science Factory, focuses on automated processes and developments concerning the human hand. The startup has developed an exoskeleton named Artus, consisting of tactile elements to support the wearer's hand during work and protect against injuries – particularly to the finger joints
– without feeling intrusive. For this, Digity developed a custom fitting methodology – the so-called Digity Size Wizard. The exoskeleton is designed to feel like a second skin while providing a secure grip.
Disinfectant dispenser from MAGNUS MAGNUS, a medtech startup, has developed an intuitive hardware solution for everyday medical use. Three years ago, at the peak of the COVID pandemic, the company launched the MAGNUS dispenser. Maximilian Bieker was involved as a consultant on this project. It is a disinfectant dispenser that replaces the conventional door handle, combining door opening with hand disinfection. The innovative product is aimed especially at frequently used rooms with high hygiene standards.
WaterScope develops rapid water testing
The British company WaterScope also finds its base at the Life Science Factory. The startup addresses a particular issue in developing countries – the fact that one in ten people in the world lacks access to clean water. To combat this existential inequality, WaterScope has developed a specialised device for rapid testing of water quality in terms of bacterial density. The device is intuitive and can be easily used by anyone. In developing the measuring device, the startup relies on robust materials and machine learning, enabling automatic detection within eight hours of whether the drinking water is contaminated, for example, with E. coli bacteria.

FINANCING 2023 was not an easy year for the life sciences sector. Investors held back, VC companies prioritised maintaining the existing portfolio and tried to extend the lifetime and reach of financing by focussing.
CATALYSER How does such an arduous year affect a startup ecosystem?
Marco Janezic. It's true, investors are travelling with the handbrake on. If anything, low-risk, advanced projects were sought after and invested in. Even the big pharmaceutical companies are experiencing uncertainty, fear for jobs and a wave of restructuring became a common topic.
CATALYSER. No good times for startups?
Marco Janezic. Not really. Takeover candidates are being sought which are in later stage or with products on the market. Money is available, but not for the early startup phases. Investors are still sitting on a lot of money that they have been able to collect, but there has been a lack of impetus to spread this across the entire spectrum of opportunities. On the contrary, the herd has tended to jump on the diet wave, and everyone is trying to get a slice of the cake. If you´re not in there or some other hot areas, you´ve been held at distance with a firm restraint.
CATALYSER. How can the Life Science Factory help with this?
Grit Zahn The Life Science Factory is a very agile startup centre. You could already see that during the early days, which coincided with the coronavirus pandemic. Life Science Factory had to throw all the plans overboard and build a community through a lot of online work. The fact that this was successful shows that the Life Science Factory team has struck a chord with startups.


MarcoJanezic, Managing Director Blue Ribbon Partners
Grit Zahn Yes, this is true. But it´s less frightening in a group. A Life Science Factory like this is also a shelter, providing support and very specific help, because the combination of the open premises and the lively dialogue with the rapidly growing network of experienced coaches has created a vibrant ecosystem with very short communication channels.
Marco Janezic It helps that the Life Science Factory, as a startup centre of the companies Sartorius and others, is independent thanks to their support. This creates this protected space which, with its proximity to industry, also creates channels for the products but also reflects the special challenges of a marketable product in a very hands-on way.
"All the money from investors has to be spent. We will see strong financing again."
CATALYSER. Now the pandemic is over, and the next challenges are just around the corner. Isn´t it always hard, stormy, and risky in the startup universe, in particular for the beginners?
CATALYSER Many people are now hoping that everything will improve this year, that investors will turn their attention back to the startup scene and that the difficult phase is over. Do you see any signs of this?
Marco Janezic. Occasionally and in special cases. But I am cautiously optimistic, not yet euphoric. Some changes to the framework conditions are positive: the Federal Agency for Disruptive Innovation (SPRIND) has been released from some of its shackles and can now function better as an investor for risky project ideas, which was previously lacking. The Growth Opportunities Act contains a lot of good news for founders, now it just needs to be passed. All the money from investors still has to be spent, so I think there will be a strong acceleration in this area.
Grit Zahn It is important for me to emphasise that a lot has also improved on the startup side. The whole area of startups has become much more professionalised. In Göttingen, it has been possible to successfully complete the development phase of an ecosystem. The Life Science Factory is an anchor for the scene; this critical mass can now develop and stabilise here, which is necessary to ensure that investors are also tied to the location in the long term.
CATALYSER Startups find open doors at the Life Science Factory, but also have to face critical judgement. How do you balance these contrasts?
Grit Zahn I think the network of coaches is very important. We started out with three coaches, now there are 15 of us, who regularly exchange a lot of information about the startup teams. We learn a lot from each other with these different perspectives. And we can pass this on to the startups. It's true that they are welcomed with open arms, but then they have to make a lot of effort. But we work together. We walk the path together and we learn together. When we give homework, we also help them to solve it.
Marco Janezic There has to be a selection process for the startups. It is important to us that the projects have already undergone due diligence, that a filter takes place, which is why we also have very standardised Memberships in the Factory. The added value is the complete offer, the team and its network. We now also help startups with grants so that they can overcome the entry hurdle to the Life Science Factory more easily.
CATALYSER. How are the startups doing? How do you interact?
Grit Zahn . The startups come from Göttingen, but not only from Göttingen. That has already expanded a lot, they come from very different places. And they bring very different experiences with them. How does the technology transfer work there, what gaps are there in the business plan, what is the situation with patents? Is founding a company the right way to go? We have a lot of question marks at the beginning and then try to develop solutions.

Grit Zahn,
"Everyone must be willing to learn. It is a shared journey, we grow together."
Marco Janezic. We have the ELSA programme jointly running with Fraunhofer AHEAD and Helmholtz Munich which helps
life science startups to shape their business idea in a sustainable way and take it to the next level in the shortest possible time. We have investors days, we have the Startup Day Lower Saxony. So, we try to provide a mix of internal training and coaching and the open stage for presentation. And we see and feel the progress every project takes from the start in our multi expert nursery.
CATALYSER. If you could make a wish, what would it be?
Marco Janezic . As I said, the framework has improved but there is always space for more. Politicians who might not have been the first to spring to mind if it comes to the startup environment and conditions for a vibrant ecosystem are now very supportiv. I very much wish that the public will follow and embrace the founders that want to make the future a better place.
Grit Zahn. If have seen a lot of trouble with technology transfer in Germany, and still do with some institutions. There is improvement but a clear priority to help the founders is missing. There is no incentive for startups as it is for high impact publication. Second wish is for simple financing tools for the quick start, without judgement or bureaucracy, that would be nice. More experienced interim managers, entrepreneurs in residence who pass on their experience, that would be a good addition too.
The interview was conducted with Georg Kääb, |transkript
Grit is a biochemist and has been active in drug development for more than 20 years. She has held a number of positions at many institutions and companies, working as a researcher at the Charité university medical center in Berlin; in drug development at the Jerini AG biotech company where she helped develop a product to market maturity; in technology transfer at ipal GmbH; and today at Eternygen GmbH, where she is head of research and also advises other biotech companies.
INVESTMENT Investors speak their own language, which young founders in particular must first learn to understand. They need to know these terms.
From ‘cap table’ to ‘vesting’: Startups are characterised by innovative business ideas. But young companies often need financial support from investors to get off to a flying start and develop their full growth potential. However, investors often do not speak the same language as academic founding teams. We will briefly explain key investor terms below.
A cap table, short for capitalisation table, is listing all company shareholders based on their percentage share in the company. This is particularly important if there are several founders or additional shareholders. The cap table makes managing the startup’s financial interests and ownership structure easier. But beware: The more investors join a startup, the more diluted the cap table becomes. This makes it difficult for founders to secure future financing or negotiate favourable conditions.
Due diligence describes the careful analysis and examination of a startup regarding its various aspects, such as the economic, legal, and financial dimensions. The analysis is conducted before crucial decisions are made, especially in the case of investments in the company. Due diligence is carried out by experts who do not work in the respective startup or by external consultants. The aim of due diligence is to gain a comprehensive understanding of the facts, opportunities, and risks that arise for investors. Thus, the analysis helps to minimise business

risks and to increase the potential for successful cooperation.
Vesting refers to the distribution of shares to the founders or employees of a company to motivate them in the long term. In the case of startups, this usually initially involves gradually releasing shares. It is a common practice to opt for a period of four years, during which founders are credited with 1/48 of the shares as “vested” every month. At the end of this period, the founders can leave the company
without incurring any losses. Important: Investors do not usually receive vesting shares or units.
How quickly does a startup “burn” or use up its capital? This information is given by the cash burn rate and is specified as an amount (“How much money is used each month?”) or as a period (“How long will the available money last?”). The value is calculated for startups not yet earning money and companies in crisis. The cash burn rate indicates how much money flows
from the business. It does not equal the loss incurred by the company and can differ significantly from the profit and loss account prepared. Startups should always compare the actual cash burn rate with the planned cash burn rate, as an increased value forces them to act more quickly. The cash burn rate is an essential indicator for avoiding insolvency and securing the company’s existence.
The pre-money valuation describes the company’s valuation before a funding round, i.e., before investors inject equity into the company. Among other factors, the startup is evaluated based on the turnover, profits, and experience of the founding team. The pre-money valuation is a decisive factor for investors in determining the value of the startup and the amount of the investment, as well as the future company shares. In contrast, the
post-money valuation defines the company value after a financing round plus the capital invested by the investors. The post-money valuation indicates the startup’s success: A higher rating shows that the company is growing and thriving. It also makes the company more attractive to potential investors.
Bootstrapping is a way of starting a business without external funding. The aim is to avoid expenses while maximising income. Bootstrapping requires founders to work with a limited budget and to follow a strict timetable in order to become operational and cash flow positive as quickly as possible. The founders retain full control of their startup as they do not have to work with investors and learn to operate economically and effectively. However, the startup's development options are limited with this form of financing. The main instruments
for bootstrapping are equity, money from friends and family, public grants and small bank loans. In the case of rapid growth and the subsequent decision to seek outside capital, investors are often impressed if a startup has managed to finance itself and grow on its own.
Smart money refers to investors who provide startups not only with money, but also with their knowledge, strategic support, network and experience. Smart money investors, such as venture capitalists, business angels and accelerators, provide startups with valuable insights into the industry and the market, enabling them to grow more quickly and more effectively. They can also help find business partners and build a network of key contacts. To ensure a successful collaboration, startups should always consider which investors are the best fit for their business.

April 29 - 30
Life Science Factory @ BioVaria, Munich
June 27 - 30
Life Science Factory @ Festival der Zukunft, Munich
July 2 - 3
Life Science Factory @ BayOConnect, Munich
September - December ELSA Program, Goettingen
November 4-6
Life Science Factory @ Bio Europe, Stockholm


Don‘t miss the most exciting gathering of tech thought leaders from startups, VCs, corporates, science, politics, arts and media! Munich, 2024 June 27 to 30


lifescience-factory.com/de/catalyser/

Hong Kong mid of April. The award recognises French innovation to promote and support French Tech in the Asia-Pacific region. The final awards ceremony was held on the French pavilion in the presence of members of the Hong Kong Government and Christile Drulhe, Consul General of France in Hong Kong.
This is very much related to the company’s strategy to go global from the very beginning of delivering biorecycled PET into the markets where use of fossil PET is still growing and an increasingly serious issue in pollution of the environment. “In a dynamic global PET market, where the share of recycled PET will expand, Carbios’ ambition is to become a leading r-PET player by 2035”, representatives of Carbios state. CARBIOS has extended its international reach to boost its commercial deployment worldwide. According to Carbios, teams are already in place in key markets to identifying business opportunities and establishing commercial partnerships for PET biorecycling technology.
A first kind of such a business opportunity and contract with waste management companies was released in May. Carbios is now cooperating with Ger-
man Hündgen Entsorgungs GmbH & Co. KG (Hündgen), a waste management expert in logistics, sorting services and the recycling of recyclable materials from waste mixtures. A Memorandum of Understanding was signed relating to the sourcing, preparation and recycling of 15kt/year of post-consumer PET waste using Carbios’ biorecycling technology at Longlaville, from end 2026.
The partnership will leverage Hündgen’s expertise and network in the sourcing and preparation of light packaging waste collected from German households. This PET waste will be prepared into flakes ready for biorecycling using Carbios’ enzymatic depolymerization technology, which produces food-grade PTA and MEG, further re-polymerized into PET.
The strategic location of the Longlaville plant close to nearby waste supplies in Belgium, Germany and Luxembourg seems to pay off early. “Establishing new standards in the field of plastic packaging recycling requires the development and implementation of innovative technologies and the promotion of cross-border cooperations. In this context, we are delighted to be part of one of the leading projects in Europe in the field of PET nonbottle recycling”, said Christian Hündgen, CEO of Hündgen Entsorgungs GmbH & Co.KG
To date, Carbios is represented in three key regions: Europe, North America (including Canada) and Asia (China, Japan, Korea, Singapore, Taiwan, and soon India).
PET, the second most widely used plastic in the world, is mainly manufactured from oil. Thanks to Carbios’ enzymatic biorecycling technology, PET now can be made from its own waste rather than oil. This technological breakthrough enables a pure circular economy and opens up new recycling streams for multilayered, colored and opaque trays made from packaging waste and polyester textile waste. Various brand consortia from the food and textile industries founded by Carbios and its partners, such as L'ORÉAL, Puma, Patagonia, Pepsico among others, are validating Carbios recycling technology for use in their own packaging material and products. Plastic waste, the scourge of our civilization, is becoming an important and, above all, valuable raw material for the future. This innovative technology paves the way to a pure circular economy and offers a viable alternative to the traditional production of plastic from fossil oil.
In addition, this breakthrough positions France as a pioneer in green innovation, contributing to its reindustrialisation and leadership in the transition to a sustainable economy.
Essential Pharma Group has acquired 4-month-old Renaissance Pharma Ltd to commercialise its GD2antibody Hu14.18 that achieved a three-year survival rate of 86% in paedriatric high-risk neuroblastoma.
In April, Vienna-based company G.ST Antivirals GmbH has started a randomised, double-blind, placebo-controlled Phase II trial for its anti-rhino viral lead programme, a broad-spectrum nasal spray containing 3.5% 2-deoxy-D-glucose (2DG) with the enrolment of the first subject out of a total of 128 volunteers in Leiden, The Netherlands. 2-DG is an glucose analogue blocking sugar metabolism of rhinoviruses’ host cells locally, thus blocking viral access to nutrients. This results in virus starvation within the host cell and therefore leeds to complete elimination of viral reproduction.
Euronext-listed TME Pharma NV announced that the US FDA has granted Fast Track designation for NOX-A12 (olaptesed pegol), TME Pharma’s CXCL12 inhibitor, in combination with radiotherapy and bevacizumab for use in the treatment of aggressive glioblastoma. CXCL12 is an intravenously administered, PEGylated L-stereoisomer RNA aptamer that targets the C-X-C Chemokine Ligand 12. A Phase II study is scheduled for the end of the year. In a completed Phase I/II study NOX-A12 demonstrated an unprecedented median Overall Survival of 19.9 months.
Danish NDM Pharma A/S has reported proof-of-priciple for its oral CIC-1 blocker NMD670 at the end of March. Together with a team of researchers from Aarhus University, the company demonstrated that NMD670 restores crosstalk between neurons and muscles in Myasthenia gravis. In S ci Enc E tranS lational M EdicinE the research team headed by Thomas Holm Pedersen from NDM Pharma A/S reported results from animal studies and a Phase I trial to prove that blocking the chloride ion channel CIC-1 can reverse muscle decline in people with the orphan disease. Myasthenia gravis weakens muscles by interrupting the electrical signals that permit neurons to communicate with muscle fibers, leading to severe muscle weakness and fatigue. Patients usually take combinations of drugs that boost muscle signal transmission and target the immune system, but there is no cure that can completely restore muscle function in day-to-day life. In rat models of myasthenia gravis, the compound restored neuromuscular communication, leading to improved grip strength and movement. Moving to humans, the researchers then administered the drug to 12 patients with mild myasthenia gravis, where the compound showed a good safety profile. It led to improvements in a clinical score of myasthenia gravis, including by augmenting grip strength. Researchers have noted similar deficits in signal transmission in other neuromuscular diseases.
GVHD ActiTrexx GmbH (Mainz, Germany) has treated the first patient with the cell therapy actileucel in mid-March as part of a Phase Ib dose-finding study. Actileucel is a novel cell therapy for the prevention and treatment of graft-versus-host disease (GvHD) in patients receiving an allogeneic haematopoietic stem cell transplant for the treatment of leukaemia. For Actileucel, autologous regulatory T cells are activated in vitro so that the cells can be given to the recipient within 24 hours. Ac-
tileucel suppresses the unwanted activation of CD4-positive T cells in the transplant. GvHD occurs in around 50% of patients when the T cells of the stem cell donor attack the patient’s tissue. Currently, non-specific immunosuppressive drugs are used to treat GvHD, which are only effective to a limited extent and cause severe lifelong side effects. The donor T cells do not have to match the patient’s tissue characteristics in order to prevent or contain GvHD. The aim of the prospective, open-label, single-arm, non-randomised, multi-centre Phase Ib/II trial is to assess the safety and feasibility of singledose treatment with Actileucel in initially ten patients shortly after stem cell transplantation. The patients will be treated in three cohorts with increasing doses of Actileucel early after transplantation and followed up for six months. The primary endpoint is the safety and tolerability secondary endpoints include the frequency and severity of GvHD in the treated patients and the feasibility of therapy with actileucel.
ALS
Lyon-based Amyotrophic Lateral Sclerosis (ALS) specialist Axoltis Pharma SA got the green light to conduct a Phase II clinical trial of its drug candidate NX210c, the first drug to target the integrity of the Blood Brain Barrier (BBB). The study’s primary objective is to assess the effect of NX210c via two markers: Neurofilament Light chain (NfL) concentration in the blood, as a diagnostic and prognostic of axonal damage relevant to ALS, as well as the ratio of albumin concentration between cerebrospinal fluid (CSF) and blood, which has long been a reliable biomarker of BBB integrity. The study will also evaluate the effect of NX210c on functional outcomes and select secondary biomarkers. Axoltis aims to enrol a total of 80 ALS patients in France. Patients will receive a 10-minute infusion of NX210c at doses of 5 mg/kg or 10 mg/kg NX210c, or placebo, three times a week over four weeks. First results are expected by early 2026.

COVID-19, climate apocalypse, billions of people in turmoil. New strategies are desperately needed. Biotechnology creates confidence and solutions. The European Biotechnology Network is a non-profit organisation that aims to facilitate cooperation between all professionals in biotechnology and life sciences on the European continent. Find out about (free) membership on our website www.european-biotechnology.net
Biotechnology
AISBL
Louise 65, Box 11 | 1050 Brussels
| Tel: +32 (0)2 588 70 71 www.european-biotechnology.net info@european-biotechnology.net
UCB SA has published data from a post-hoc analysis of its Phase III RAISE study and its ongoing Phase III RAISE-XT open-label extension (OLE) study in the J ournal of n Eurology. In the RAISE study, Zilucoplan demonstrated statistically significant improvements in fatigue scores and severity vs. placebo at week 12 and improvements in fatigue have been sustained to week 60, according to RAISE-XT data.
In May Zerion Pharma A/S and its CRO Insud Pharma SA reported positive outcomes from a comparative oral bioavailability pilot study of their Dispersome ® formulation of Ivacaftor, a drug approved to treat cystic fibrosis. According to the companies, Dispersome ® formulations showed improved bioavailability compared to the worse soluble reference product.
Neurocrine Biosciences Inc (Cambridge, UK) has initiated a firstin-human Phase I study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of NBI-1117567 in healthy adults. NBI-1117567, an oral, M1/M4 selective muscarinic agonist, was licenced back in November 2021 from Tokyo-based Nxera Pharma Co Ltd (former Sosei Heptares) to treat symptoms of cognition in patients with neurological and neuropsychiatric conditions. M1 is validated as a potential drug target in cognition and M4 in psychosis.
German AdrenoMed AG has got FDA Fast Track designation for its first-in-class septic shock antibody enibarcimab (adrecizumab) that restores endothelial integrity. The good news came in mid-April, after Adrenomed AG demonstrated that its septic shock treatment led to a 60% reduction in relative 28-day mortality vs. placebo in a stratified patient population. In the AdrenOSS-2 Phase IIa study (n=301 patients), shock patients treated with enibarcimab showed improvement in organ function and a significant reduction in 28-day mortality from 24% to 8% was achieved in adrenomedullin- and DPP3positive patients. The response to the antibody was highest in patients with elevated blood levels of the stable adrenomedullin fragment ADM, which indicates an increased amount of intravascular adrenomedullin. Additionally, elevated levels of the proprietary biomarker DPP3 indicate a reduced pumping capacity of the heart and impaired renal clearance associated with septic and cardiogenic shock. In the upcoming pivotal Phase IIb trial, the company, founded by serial entrepreneur Dr Andreas Bergmann, wants to confirm this findings and the mechanism of action in a larger patient cohort.
Enibarcimab acts like a hoover for the vasoactive 6kDA peptide adrenomedullin. Excess enibarcimab in the bloodstream binds most of the vasoactive 6kDA peptide adrenomedullin, which freely diffuses between the extra- and intravascular space, fixing it to the intravascular compartment and thereby restoring the barrier function of the endothelium. When located in the intravascular space, the peptide hormone causes vasodilation, which contributes to the rapid drop in blood pressure that precedes septic shock and multiple organ failure. Last year, shock expert Prof. Peter Pickkers (Nijmegen) demonstrated that high levels of circulating DPP3 (cDPP3) indicate a high risk of organ dysfunction and mortality. This pathway is mechanistically independent from the loss of vascular integrity, which is known to be the main driver of mortality in septic shock and is indicated by elevated plasma levels of ADM (>70 pg/mL). If approved,
enibarcimab would have great market potential due to the lack of causative sepsis therapies. With a mortality rate of 20-30% for sepsis and 30-50% for septic shock in industrialised countries, sepsis is the cause of almost 20% of all deaths worldwide
Heidelberg Pharma AG, which specialises in Amanitin-linked antibody drug conjugates, received the green light from the US Food & Drug Administration (FDA) in March for a protocol amendment to its Phase I/IIa dose-escalation study. Its ADC candidate ATAC-HDP-101 for the treatment of multiple myeloma can now be investigated more comprehensively in an expanded cohort for the biological efficacy of the antibody loaded with the toxin of the green tuber leaf fungus alpha-amanitin. At the end of March, Heidelberg Pharma received orphan drug designation for HDP-101 from the FDA.
Swiss MoonLake Immunotherapeutics
AG’s trivalent psoriatic arthritis nanobody sonelokimab has shown statistically significant improvements after 24 weeks of treatment compared to patients treated with placebo in the Phase II ARCO trial. After 24 weeks of treatment, the nanobody that targets IL-17A, IL-17F and human serum albumin achieved a better therapy response with an ACR 50 and ACR 70 in 60%/40% of those treated than after 12 weeks. In addition, around 80% and 60% of patients treated with sonelokimab achieved a Psoriasis Area Severity Index (PASI) of 90 and 100, respectively, at week 24, regardless of whether 60mg or 120mg was administered. The results exceeded those of the standard of care adalimumab and were also higher in the indirect study comparison with competitors vs. adalimumab. In combination of PASI and ACR, there were score differences of 16% to 29% in favour of sonelokimabs. According to the manufacturer, the drop-out rate in the second part of the study remained at 5%. In addition, no signs of suicidal thoughts or elevated liver enzyme levels were found in connection with treatment with sonelokimab.

With more than 38 years of experiences in the life sciences, BIOCOM ensures skilled management and targeted communication for projects such as the EU Framework Programme for Research and Innovation.
Communication & dissemination: strategy development, digital & print communication, public relations, website development, film & animation, social media
Memorable experiences: exhibitions, innovative workshops, hands-on labs
Stakeholder & public engagement: networking hubs, living labs, public dialogue formats
Capacity development: communication trainings, educational toolkits
Analysis & consulting: country and market studies, evidence-based policy advice
For more information, just head to www.biocom.eu/comdis or contact Christin Boldt at c.boldt@biocom.eu
CLINICAL TRIALS
Over the past few years, the Frankfurt Institute of Clinical Cancer Research (IKF) has successfully conducted over 90 clinical studies. Each year, it enrolls approximately 1,500 patients in these trials. Europ E an Biot Echnology spoke with the founder and director Prof. Dr med. Salah-Eddin Al-Batran.
EuroBiotech _Germany is falling behind its European neighbors in terms of the total number of clinical trials conducted. What is different about the IKF?
Salah-Eddin Al-Batran_The IKF is a clinical cancer research institute deeply integrated within the academic community, with close collaborations with leading university hospitals in Europe and worldwide. Simultaneously, it functions as an autonomous entity within the free market economy, facilitating smoother partnerships with the industry. What distinguishes the IKF is its distinctive position bridging the academic and the pharmaceutical sectors. It strives to bring together these two worlds that think and act very differently, in order to create fruitful synergies and carry out innovative research projects that serve the goals of both sides. Symbolically, the IKF could be seen as an adapter that serves to connect these two worlds. The primary product of the IKF is the knowledge derived from these unique intersections.
EuroBiotech _Can you please explain the adapter function in more detail?
Al-Batran_The IKF is based on three pillars. The first one is the role of the sponsor according to the German Medicinal Products Act (Deutsches Arzneimittel Gesetz, AMG) or the European Clinical Trials Regulation (EU CTR). The IKF acts as a sponsor in around 50% of its clinical trials. This function is of crucial importance for our own studies. In addition, the IKF often takes on the role as sponsor in so-called collaborative studies with the pharmaceutical industry or in coopera-
 PROF.
PROF.
DR SALAH-EDDIN AL-BATRAN
Founder,Medical Director, Frankfurt Institute for Clinical Cancer Research IKF GmbH at the Northwest Hospital.
tions with smaller biotech companies that do not have sufficient capacity to meet the now very complex requirements placed on a sponsor. The second pillar is the role as a Full-Service CRO. As FullService CRO, the IKF not only carries out the procedural implementation of its own studies, but also acts as a contract research institute for studies commissioned by academic sponsors or science-driven biotech companies. Hereby, the IKF covers all necessary processes of study planning and execution according to FDA and EMA standards. The third pillar of the IKF is the highly specialised outpatient study
clinic and Phase 1 unit, which were created through a cooperation with the Northwest Hospital of the University Cancer Center (UCT) Frankfurt. This unit functions independently of the sponsor and CRO function and serves the IKF as a highly qualified study center with strong recruitment capabilities.
Partners who work with us benefit considerably from this 3-pillar model. They practically receive an efficient allin-one solution that offers them advantages in many respects.
EuroBiotech_What is the approximate ratio of academic and pharma-sponsored clinical trials you conduct at the IKF? Al-Batran_At the IKF, we conduct clinical trials in a ratio of approximately 70% academically initiated (investigator-initiated) to 30% sponsored by the pharmaceutical industry. However, it is important to note that we are also conducting a significant third group of studies that are part of our so-called Collaborative Academic Drug Development (cADD) program. These studies are a kind of cooperation between academic research and the pharmaceutical industry, which involves the development and approval of drugs for the industry. They are industrial-academic hybrids where industry representatives are integrated into the steering committee of the studies and at the same time, the IKF assumes the role of sponsor conducting the studies in its academic networks. Through this kind of study, we enable the industry to conduct parallel development of their compounds alongside their own study program,
which can save significant resources and development costs. The IKF plans to further expand this flexible model, as we are convinced that it will optimise and accelerate the development of innovative cancer drugs. We are particularly motivated by the prospect that our contribution will enable our partners to obtain marketing authorisation for their drugs in indications that would otherwise not be the focus of pharmaceutical partners and that we can therefore help to meet socalled unmet needs.
EuroBiotech _Can a centre like the IKF “keep up” with the many innovative therapeutic approaches in oncology?
Al-Batran _Not only can the IKF keep pace with the many innovative therapeutic approaches in oncology, such as CAR-T, mRNA, gene and cell therapy and various combinations thereof, but these new therapeutic approaches are also a focus of our work. We are currently conducting numerous immunotherapeutic studies, including immune checkpoint inhibitors, mRNA and immune cell therapies. For example, the IKF is sponsoring the so-called INSIGHT Phase I platform study, in which a novel immunotherapeutic agent is being combined with various other therapeutic agents, such as chemotherapies and checkpoint inhibitors. Each tested combination forms a new arm of the study. Currently INSIGHT has five arms, i.e., five different innovative immunotherapy combinations are being investigated within one study. In addition, partially different types of tumors are treated in the arms. It is therefore a highly complex platform study that allows the rapid identification of promising therapeutic approaches, which can then be further evaluated in larger studies. We would of course like to expand the study further in the near future. In the field of ATMPs (Advanced Therapy Medicinal Products), the IKF is supporting a partner that develops immune cellbased therapies in the validation of its manufacturing process and the planned first-in-men testing of its cell preparations. Here, immune cells (so-called tumour-infiltrating lymphocytes) are isolat-
ed from tumour tissue taken from cancer patients as part of surgical procedures or biopsies, enriched in a special cultivation process and administered back to the patient via infusion. The aim is for these enriched, endogenous immune cells to migrate into the patient’s tumour and stop the growth of the tumour or, in the best case, shrink the tumour. In addition, the IKF has established a unique network called the PLATON Network, in which around 150 clinics are now involved. This network serves to identify patients with rare biomarkers and recruit them for clinical trials. This enables us to advance innovative therapeutic approaches and to further promote research in the field of oncology. Here, collaboration with other centers in this highly specialised field is always useful and a matter of course in order to share knowledge and resources and make progress together.
EuroBiotech _Can you give examples where your collaborative research approach came to fruition and led to improvements for patients?
Al-Batran _We are extremely proud to have significantly influenced the standard of care for esophageal and gastric cancer worldwide through our own trials, as documented, for example, by publications in the renowned medical journals Lancet in 2019 and J O ur N al O f c li N ical
O N cO lO gy in 2023. Currently, the IKF is conducting a total of 15 Phase III trials, underlining our ongoing commitment to advancing cancer treatment and promoting innovation in medical research.
EuroBiotech _What are your wishes for Germany as a place to study and for the IKF in particular?
Al-Batran_I have many wishes for Germany as a place to study, which would also be of great benefit to the IKF. First and foremost, I would like to see the approval processes for clinical trials simplified, ideally at European level. Both the state and private actors such as foundations should invest considerably more money in academic studies and provide more support for young biotech companies. The pharmaceutical industry should focus more on investigator-initiated studies. Currently, the industry tests its substances within only a very narrow context for resource reasons. As a result, the full potential of innovative drugs often remains untapped. This is exactly where the IKF is trying to make a difference. More support and collaboration between different players would accelerate research and development of new therapeutic approaches and significantly increase the chances of progress in cancer treatment. L
g.kaeaeb@biocom.eu


mk2 Biotechnologies redefines the purification of recombinant peptide & proteins. By developing fully scalable, chromatography-free purification processes that can be coupled with various expression systems, we provide the missing puzzle piece for a large variety of products required at large scales and low cost. Using simple and high-throughput unit operations and water-based buffer systems without the necessity of resins, we seek for synergies and collaborations to create a fully integrated low-cost production process at large scales. That way we enable the production of proteins
“We are excited to be part of the fruitful IZB ecosystem - a great foundation to achieve our big goals.”
with uncompromised quality for nonniche applications like bio-preservation, cultured meat production, and antigen vaccines novel biologics for both animal and human use – and many more across different industries. For this reason, we are strongly convinced that our technologies have the potential to tackle major social challenges as well as to provide solutions for a known and yet unknown demand for new classes of active substances.
Future challenges cannot be overcome simply by an incremental improvement of today’s technology. Facing the yet

FOUNDED : 2020
BUSINESS SEGMENT: Industrial Biotechnology
unknown threats of the next 100 years urgently demands open-minded thinking, radical new ideas and revolutionary technologies. We are strongly convinced, that we do not only have the right technology, but also the right team to biologically contribute to coping with future challenges – by developing the new standard in peptide & protein purification.
CONTACT
mk2 Biotechnologies GmbH Am Klopferspitz 19 82152 Planegg-Martinsried info@mk2.bio www.mk2.bio


AMARNA THERAPEUTICS Leiden-based Amarna Therapeutics Ltd welcomed Aurelia Caparrós as its new Chief Business Officer (CBO) in April. Aurelia Caparrós has many years of experience in the healthcare industry. She brings expertise in business development, strategy and global marketing to her new position. Caparrós has held global leadership positions at Novartis AG, among others. She led business transformation programmes and implemented strategic growth initiatives. As the new CBO, she will be responsible for driving Amarna’s future growth and expansion in the Netherlands and Spain. L Aurelia Capparós
FARON PHARMACEUTICALS Faron Pharmaceutical Oy’s Chief Operation Officer, Dr Juho Jalkanen has been appointed as new CEO of Faron Pharmaceuticals (Turku/Boston).

Yrjö Wichmann has taken on the role of Interim Chief Financial Officer. Juho Jalkanen MD, PhD, MSc (Economics), succeeds Dr Markku Jalkanen as CEO. He will turn 70 in 2024 and wishes to retire. But he will continue to serve as a member of the Board of Directors and support the transition of the CEO role until 2024. Juho Jalkanen has been with Faron for more

than six years. He started in 2018 as Chief Development Officer, three years later he took over the position of Chief Operating Officer. Yrjö Wichmann started as Chief Financial Officer in mid-April. His predecessor James O’Brien was leaving the company to take up another position outside the company. Wichmann previously served as CFO of the company between 2014 and 2019. Most recently, he held the position of Senior Vice President, Financing & IR at Faron. Prior to joining Faron, Wichmann held a number of senior positions in life sciences and biotechnology at IP Finland Oy, Biohit Oy, CapMan Oyj, FibroGen Europe Oyj and D. Carnegies & Co AB. L

Margarethe Sørgaard
CALLUNA PHARMA Margrethe Sørgaard strengthens Calluna Pharma Inc with over 25 years’ experience from different leading positions in clinical development and operation, medical affairs, and drug safety/pharmacovigilance. She took over her new position as Senior Vice President of Clinical Operations and Pharmacovigilance in April. Sørgaard joins Calluna from BioInvent International AB where she was the Senior Director of Safety and PV, supporting product safety and assuring safety standards were compliant with global drug safety regulations. She holds an M.Sc. in Biology (Physiology) from the University of Oslo. Calluna Pharma was formed in 2023 following the merger of Oxitope Pharma and Arxx Therapeutics and is based in Oslo, Norway. L
 Matthew G. Hall
Matthew G. Hall
BIOLIZARD NV The bioinformatics service provider BioLizard NV with offices in Lausanne (CH) and Ghent (Belgium), appointed Dr Matthew G. Hall as Director of Business Development in mid-April. He is to expand the presence of the service provider for bioinformatics, data analysis and data management in the life sciences at the new location in Lausanne. In addition to a large network, Hall brings around ten years of experience in the Swiss biotech industry and AI expertise to his position. Hall, who has a doctorate in molecular and cellular neurobiology, has held various management positions in recent years, at Lagosta and at Distalmotion. He was also CEO, co-founder and board member of SimplicityBio SA. L
PROTHYA BIOSOLUTIONS Manja Boerman, MD, has been named as Chief Executive Officer of Amsterdam-based Prothya Biosolutions BV. She is an experienced senior executive who previously held leadership positions with Catalent, DSM, Patheon, Consort Medical and Kiadis Pharma. In her last role as President of Catalent’s Cell, Gene and Protein Therapy Division she led, built and help grow a world class organisation offering development and GMP manufacturing services for new modalities as cell and gene therapies. L
2nd half-year 2024

May 5-6, 2025
Congress Center Basel
As one of the leading biotechnology conferences, the Swiss Biotech Day has developed into a truly global networking day, attracting many international delegations.
What you can expect:
› Meet 2,500+ senior experts from the life science industry
› 100+ exhibitors and Global Village with international delegations from all over the world
› Swiss Biotech Success Stories Awards
› Innovative biotech start-ups and medium-sized biotech companies
“WHITE BIOTECHNOLOGY” WORKING GROUP
› Thematic company pitching sessions
› Thematically focused panel discussions
› Swiss Biotech Report 2025
› Pre-scheduled one-to-one partnering meetings
› General Assembly of the Swiss Biotech Association
Media partners:
Leading chemical companies are exploring the opportunities that have been opened up by modern biotechnology, especially in the field of “white” or industrial biotechnology. And they are also applying these technologies, wherever it makes sense. The SBA takes such initiatives seriously and has formed a working group specifically dedicated to white biotechnology. The Swiss Industrial Biocatalysis Consortium is an important partner in this effort. The group includes leading multinational companies that support white biotechnology as a pillar of economic growth. The planned activities are in agreement with OECD strategies.
In partnership with the Swiss Biotechnet (see pages 14/15) the SBA develops training programmes and useful support tools for the industry. It is of importance that the industry specifies its training needs so that the academic side can create tailor-made education. This strategy ensures that the industry gets the right workforce with the right education. The SBA profits from the marketing alliance “Swiss Biotech” (see box) in a multiplied form. Thanks to Swiss Biotech, the
sector is internationally visible. The project-specific participating companies (most of them young and internationally less savvy) find a comprehensive partner which is helping to put them in the public window. The participating Life Science Regions are important internal carriers of the dynamics in the Biotech sector, thus enhancing the common understanding of the industry. This and more knowledge is brought into Europa Bio, the European Biotech Association, where the SBA is an active member.
Sign in on our website at www.swissbiotechday.ch and stay updated about any news.
...is an alliance of four leading Biotech regions Switzerland (Bio Alps, BioPolo Ticino, Basel and Greater Zurich Area). They have combined forts to streamline interests of the national sector. The SWX Swiss Exchange holds a position in terms of lifescience listings and companies from that industry – be they located Switzerland or abroad – access to an internationally recognised financial marketplace. The initiative was co-founded by the SBA which also manages the executive office of Swiss Biotech.
Host:

EUROBIOFAIRS COMPASS Each manager, scientist, and business developer has their own specific network – so, their must-attend events vary. Where to meet potential new partners can be quite difficult to determine. Euro Biofair’s Compass will help you navigate the European meeting jungle in H2/2024.

With 60,000 attendents and 2,500 exhibitors expected, this year the CPHI Milan 2024 (8 – 10 Oktober 2024) is set to become the largest ever pharma meeting in the history of the event. At CPHI Milan, decision makers along the whole (bio)pharma value chain will meet, including pharma and biopharma developers, API providers, contract manufactureres and many other specialists. To be able to make the most of the event, there is an online partnering
tool along a full programme reflecting all relevant topics in pharmaceutical development and commercialisation (page 48).
Shortly before the global climate tipping points are reached, the venue for the Global Bioeconomy Summit 2024 (p. 50) could not have been better chosen: from 23 to 24 October, the event will take place in Nairobi, East Africa. Around 400 delegates from politics, science, civil society and the business sector will be on
site, thousands will be connected via live stream, to present and discuss solutions in plenary sessions and interactive workshops for the preservation of everyone's livelihood, which is increasingly jeopardised by resource-consuming growth. The African continent has become an important partner in solving the global energy problem, especially with biotechnological solutions such as oil-producing algae. It will be interesting to see whether the biotechnology-supported high-value-output bioeconomy will make the leap from talk to implementation after the USA's entry. The summit organised by the IACGB and BioInnovate Africa can provide an impetus here.
Another partnering must-attend, particularly suited to the needs of bio-pharma and pharma biotech executives, will come to the Nobel prize city of Stockholm: From 4 – 6 November, BIO Europe 2024 (see p. 52) will celebrate its 30 th anniversary in Sweden. Three days of high-level networking through partnering meetings enabled by EBD Group's partneringOne software will be embedded in a programme including strategic panel discussions, and world-class workshops in the centre of the vibrant Nordic biotech network. L
t.gabrielczyk@biocom.eu



CPHI Step into the future of pharma at CPHI Milan 2024 – the largest event for global pharma professionals to meet and grow their business at Fiera Milano 8 th -10 th October 2024.
Join us at CPHI Milan 2024, the ultimate platform for pharma professionals to connect, innovate, and thrive. Celebrating 35 years of excellence, this year's event promises unparalleled opportunities for growth and networking. Don't miss out on being part of the global pharma community at the heart of advancing human health!
At CPHI Milan 2024, we're excited to bring together the world's largest pharma community for three days of inspiration and collaboration. With over 62,000 attendees expected, this year's event is

GREETING Embark on a journey with the pharma industry's manufacturers, innovators, ingredient/ product providers, and outsourcing partners as we delve into pharmaceutical excellence and forge connections for a brighter future to-
set to be our biggest yet. From insightful seminars to cutting-edge exhibitions, CPHI Milan offers a comprehensive experience for industry professionals looking to stay ahead of the curve.
Our programme sessions will provide insights into the industry's current state and future trajectory and showcasing opportunities for start-ups and innovators. The content will engage in discussions on emerging trends, collective solutions to address challenges, and highlight best practices in sustainability, all with the aim of uniting the global pharma com-
gether! Milan, the hub of Italy's pharma industry, anticipates a record-breaking event this October, with over 62,000 attendees. With a world-class conferenceboasting200+sessions,weeagerly await welcoming the entire pharmaceutical supply chain, driving growth and innovation in the industry.

8 – 10 October, 2024
Sherma Ellis Daal Brand Director Pharma Informa Marketsmunity to advance human health safely and effectively. Attendees can also leverage our online platform to connect with industry leaders year-round, maximising their experience at CPHI Milan. The awards night will be back with two new categories introduced this year – ‘Woman of the year’ and ‘Future leader’.
Join us in-person at Fiera Milano, Italy, and immerse yourself in a dynamic environment where ideas flourish and partnerships thrive. Together, let's shape the future of pharma. Visit https://www. cphi.com/europe/en/home.html to learn more and register today! L
Over 2,400 exhibitors expected at Milan with the largest event yet.
Programme Highlights
CPHI offers unparalleled opportunities for business growth and meaningful connections at the 'heart of pharma'. This year, we celebrate 35 years of CPHI with a plethora of special programmes honouring our success in bringing the world's largest pharma community together. Join our global network of pharma professionals through our online platform and elevate your experience by attending CPHI Milan in person. Register here: www.cphi. com/europe/en/home.html




GBS More than 600 attendees already pre-registered for the Global Bioeconomy Summit. The Summit will take place in person (with online streaming) from October 23 to 24 in Nairobi, Kenya.
The GBS took place in Berlin in 2015 and 2018; it was held virtually in 2020. This year, this recogniszed Summit will take place outside of Germany for the first time, in Kenya, East Africa. The Summit brings together experts and representatives from politics, science, civil society and the business sector from all hemispheres and from diverse backgrounds to represent the interdisciplinary and diverse features of the bioeconomy.
The GBS is convened by the International Advisory Council on Global Bio -
Contact www.gbs2024.org
Content
› More than 500 attendees
› Online streaming
› Five plenaries
› 15 interactive workshops
› Exhibition of products and projects
economy (IACGB) in collaboration with East African partners. IACGB is a think tank with the mission to promote the Global Bioeconomy as a sustainable and future looking economic development model that benefits people and the planet (https://www.iacgb.net).
With five plenary sessions and up to 15 interactive workshops, the Summit covers a broad spectrum of discussions within the bioeconomy. Exhibition booths will showcase academic and industry projects as well as start-up initiatives. Special focus will be Bioeconomy Youth networks and indigenous community projects. Proposals for workshops are invited via GBS workshops, with June 17 as the deadline for receiving proposals.
The overall theme of GBS2024 will be “One Planet – Sustainable Bioeconomy Solutions for Global Challenges”. Top-class speakers and panelists will discuss a sustainable bioeconomy as the key to decarbonisation and sustainable transition to less fossil fuel dependent, rural and urban green economies; while building resilient and sustainable food systems, revers-

23-24 October 2024
Nairobi, Kenya
ing biodiversity loss, meeting health challenges, and using innovation as a driver for new economic opportunities, especially jobs for the youth. L
GREETING The Global Bioeconomy Summit will be hosted for the first time outside of Germany.

East Africa is proud to be the host for this prestigious Summit. We invite bioeconomy thought leaders from allhemispherestothe vibrant bioeconomy hub of Nairobi, Kenya to meet and discuss with more than 500 delegates. Together with IACGB, the International Centre of Insect Physiology and Ecology (icipe)/BioInnovate Africa and its co-hosting partners

East African Science and Technology Commission of the East AfricaCommunityand Stockholm Environment Institute are welcoming you to share your experiences and ideas in our forward looking plenaries and workshops.
Julius Ecuru
icipe/BioInnovate Africa
Christine Lang
IACGB

One Planet – Sustainable Bioeconomy Solutions for Global Challenges
Secure your spot at the Global Bioeconomy Summit, hosted for the first time in Africa.
Inclusive discussions on global bioeconomy issues, while also profiling and recognising the importance of bioeconomy for Africa and other regions in advancing sustainable development.
} Over 500 participants from across the globe
} Top-class speakers and panelists
} Interactive workshops
} Exhibition
} Online streaming

23 – 24 October 2024, Nairobi, Kenya Contact: gbs2024@icipe.org www.gbs2024.org




BIO-EUROPE BIO-Europe celebrates 30 years of facilitating partnerships this Fall. In 1994, BIO-Europe launched with the mission to drive biotech innovation and dealmaking forward through one-to-one partnering. Today, it proudly holds the mantle as Europe’s flagship partnering event.
Through the everchanging landscape of life science partnering, BIO-Europe remains committed to its overriding mission of convening the biopharma community to catalyse breakthroughs through meaningful partnerships. In true form, BIO-Europe will mark its 30 th anniversary in Stockholm, Sweden, from November 4-6, 2024. The Nordic region prides itself on developing new

GREETING Whether youareabiotechinnovator seeking the opportunity to showcase your breakthroughs or an investment or pharma representative in the relentless search for tomorrow's cutting-edge therapeutics, BIOEurope will convene delegates spanning the global life science ecosystem. Overthelast30years,we,EBDGroup, haveexplorednewandinnovativewaysto
therapies, technologies, medicines and treatments through strong collaborations, making it the perfect setting for the discovery and dealmaking that takes place at the event.
Evolving from a manual process at its inception to today’s sophisticated partnering process, BIO-Europe will host 30,000 one-to-one meetings between over 5,500 international delegates to
catertotheneedsofthebiopharmacommunityandthisyearwillbenoexception. Through improvements to our partnering platform, highlighting diverse voices intheprogramme,buildingcommunities based on shared values and interests, and wellness activities that keep attendees in top form for partnering, BIO-Europeoffersauniqueexperiencewithyour needs in mind.
Networking opportunities aren’t limited to chance encounters along the busy hallways of the convention centre. BIOEurope features renowned networking receptions held in some of the most memorable venues in Stockholm.

4–6 November, 2024
Stockholm, Sweden
further global drug development. Oneto-one meetings at BIO-Europe are powered by partneringONE™, the gold standard solution for partnering meetings. Its advanced search capabilities allow delegates to pinpoint their ideal partners with ease and efficiency.
Additionaly, through three days of programme sessions, attendees will have the chance to learn about important trends and opportunities in life science from some of the brightest minds in the sector. The agenda has been built around the theme of Collaborative Innovation delivered in three tracks: the Business of Biotech, Therapeutic Insights, and Ecosystem Innovation.
2024 marks a momentous milestone for BIO-Europe, but just as importantly, it marks the beginning of another 30 years. Let’s celebrate what brings us together: innovation and partnerships. L
Start your planning to join us in Stockholm, Sweden, where you can meet more potential partners over three days than you can all year.
Claire Macht EBD Group
BIO-Europe® is set to mark its 30th anniversary in Stockholm, Sweden, from November 4–6, 2024. Bringing together over 5,500 delegates to engage in 30,000+ one-to-one meetings, BIO-Europe is the premier platform connecting the global biopharma community. Additionally, the event offers numerous networking opportunities, a carefully curated program and wellness activities to keep attendees energized during the event.


Without the Gila monster (Heloderma suspectum) there would probably be no GLP-1 receptor agonists today. In 1992, Dr. John Eng isolated and patented the enzyme exendin-4 – which has a sequence >50% homologous and significantly extended half-life compared to GLP-1 – from the venom of the big desert lizards. This laid the foundation for the first blood-sugar-level-lowering GLP-1RA treatment for diabetes from Amylin Pharmaceuticals, which received FDA approval in 2005. The observation that diabetes patients lost weight during therapy has led to the development of optimised GLP-1RA therapies for obesity.
SLIMMING DOWN Within a single year, new treatments for obesity – incretin agonists – have propelled Novo Nordisk and Eli Lilly to first and second place in the global ranks of top-selling pharma companies. Demand for Novo’s GLP-1 receptor agonist (semaglutide) and Lilly’s GLP-1/GIP booster (tirzepatide) currently can’t be met. The drugs reduced weight by 12.7% and 18% over 68 and 72 weeks respectively in patients with BMI>30 and BMI>27 in those with obesity-triggered diseases. In a global race, developers are now targeting hunger control with combinations that boost the effect.
Light is sometimes hard to differentiate from shadow, especially in high-risk sectors like biopharmaceuticals. One tried and tested PR rule is that there are always two pieces of news, and you have to put the good news out front and hide the bad. In the young growth market of obesity treatments, Amgen Inc did exactly the opposite. A day after announcing it had deprioritised its Phase I programme with the oral Delta-5 desaturase (D5D) blocker AMG 786, the multinational rolled out the golden news: not a single patient enrolled in the proof-of-concept Phase II study of its GLP-1RA/GIP blocker maridebart cafraglutide had discontinued treatment due to side effects, and all 11 study arms of the placebo-controlled trial will remain active. Within 24 hours of the conference call, Amgen’s value on the market had ballooned by US$20bn. The company said details of the study will be presented at the end of this year. Some analysts have guessed the combination could reduce body weight by up to 24%.
There has been no stopping obesity therapies since FDA approval of Novo Nordisk A/S’s GLP-1 (glucagonelike peptide 1) receptor agonist Wegovy in April 2021 and Eli Lilly Inc’s GLP-1/GIP (Gastric inhibitory polypeptide) receptor agonist tirzepatide
(Zepbound) in November 2023. Goldrush fever has seized the rapidly growing field. Analysts are predicting sales of at least US$90bn by 2030, and are already calling the two approved obesity drugs “blockbusters of the decade”. As of May 9 th , the pen-injected compounds have made Lilly the top pharma dog in terms of market capitalisation (US$756.56bn) and Novo number two (US$429.99bn). “We are in a new era for
obesity pharmacotherapy where combinations of entero-pancreatic hormones approach the weight loss achieved with bariatric surgery,” stresses diabetes and obesity expert Prof. Dr Melanie Davies from the Leicester Diabetes Centre. In addition, there are several agents with different mechanisms that can complement the effect of the incretin hormones, which lower blood glucose levels, the speed of digestion in the stomach and
In the field of obesity drugs – which has seen a string of failures since the 1980s – the hormone GLP-1 has now taken center stage. It’s released by L-cells in the small intestine after meals and has a variety of effects on the brain-gut axis (see p. 56). The rapidly degraded natural hormone not only lowers blood glucose levels, but also intervenes in lipid metabolism, hunger control and gastric acid production, which are all centrally controlled by the hypothalamus. With GLP-1 receptor agonists, it has for the first time been possible to potentiate the amount of GLP-1 attaching to receptors in the
brain in such a way that the chronic feeling of hunger many obese people experience disappears. Chyme remains in the stomach for longer and – in combination with a change in diet and exercise – significant weight loss occurs. Although the molecular mechanisms are not fully understood, and the long-term effects of GLP-1 receptor agonists are unknown, what is clear is that weight is regained when the therapy is discontinued. Novo Nordisk, Eli Lilly and competitors are currently all working feverishly on new combination therapies that promise to reduce weight even more effectively. L

Figure 1: Biological effects of glucagon-like peptide (GLP-1).
modify lipid metabolism. But despite the enthusiasm surrounding the medical potential of the weight-loss drugs, it remains unclear whether payers will assume costs for Wegovy (over $1,300 monthly) or Zepbound (close to $1,000). If they refuse, the treatments would only remain an option for the wealthy.
After the failure of drugs in the 1990s that targeted appetite-suppressing hormones leptin and ghrelin, drugs that potentiate the GLP-1 concentration in the infundibular nucleus, the appetite centre of our body, and many other brain regions (see Fig. 1) in still unknown ways are very timely. The number of adults worldwide with a body mass index (BMI) over 30 has more than doubled since 1990, and quadrupled in those under 19. According to figures from the World Health Organization published two years ago, a billion people across the globe are now overweight (BMI>25) or obese (BMI>30). The researchers involved in a lancEt paper on the topic called it “a global obesity epidemic”.
The lifestyle disease myth
For decades, obesity has been interpreted by scientists, payors and the pub -
lic alike as a lifestyle disease. At fault were the obese people themselves, due to a supposed lack of willpower. But the new treatment options are now helping to destigmatise a systemic chronic endocrine disease that can trigger severe co-morbidities – among them diabetes, dyslipidemia, congestive heart failure, gallstones, chronic kidney disease, high blood pressure/atherosclerosis, higher cancer risk and psychiatric disorders.
“If there wasn’t the obesity pandemic, there even wouldn’t be a diabetes pandemic.”
However, the new drugs don’t just have a rosy side. Since their introduction, Weight Watchers has lost 70% of its customers worldwide, while possible long-term effects such as an elevated thyroid cancer risk are still under investigation. The therapies also only help with some symptoms of the complex, often deadly disorder. However, the EMA has not found enough evidence that the newly approved incretins trigger suicidal thoughts. Instead, incretin-
based medications have been shown to have potent modulatory effects on glucose and lipid metabolism. Since most diabetic patients have some degree of dyslipidemia, the compounds’ lipid-modulatory effects provide further pharmacological benefits for diabetic patients. They modulate lipid metabolism via at least five cellular pathways: lipogenesis and lipolysis, lipid peroxidation, lipid absorption, cholesterol bio-synthesis, and fatty-acid beta-oxidation
Billions are now being channeled into R&D, licensing, and new manufacturing sites to satisfy demand for the new drug class. According to GlobalData analysts, 13 drug launches can be expected in the US by 2028. Since GLP-1RAs have already been approved as diabetes meds, companies seeking to enter the area can rely on R&D work already done in the diabetes field to jump-start obesity candidates. Even before the FDA approved Zepbound as an obesity medicine for people with BMI>30 and patients with BMI>27 who have at least one obesity-related comorbidity, Novo Nordisk’s Wegovy and Eli Lilly’s diabetes med Mounjaro – in which tirzepatide is also the active ingredient – generated $9.7bn in 2023. That’s a 239% increase on total revenue generated from all obesity drugs in 2022. “Starting in 2024, significant increases in the number of launches are expected per year, culminating in a peak of four launches in both 2027 and 2028,” says Jasper Morley, Pharma Analyst at GlobalData. “This surge in commercial launches over the next five years represents a 333% increase in comparison to the three launches over the preceding fiveyear period (2019–23).” According to Morley, Novo Nordisk will probably cement its position as an obesity frontrunner, as it has six of the 13 estimated launches in its pipeline (see p. 57). Its lead pipeline drug Cagrisema, set to be launched next year, is forecast to generate US$7.4bn by 2029 alone. However, large and small drugmakers alike are now vying for a piece of what analysts view as one of the biggest market pies in the history of the pharma industry. The number of deals is staggering,
Table: © BIOCOM AG. Source: clinicaltrials.gov
but they all have the same goal – finding new targets that boost the effect of GLP-1 receptor agonists. As our understanding of the complex interplay of endocrine signalling remains limited, this is therefore often a game of trial and error.
Novo Nordisk’s biggest competitor Eli Lilly, whose semaglutide rival Zepbound combined its GLP-1RA with a GIP (glucose-dependent insulinotropic peptide) receptor agonist that boosts insulin sensitivity and production, resulted in 6% more weight loss in pivotal trials. It clear-
ly shows how much chance is involved in the search for the ultimate combination therapy. Competitor Amgen’s Phase II candidate AMG-133, with which the company reportedly hopes to shed an additional 8% more weight, does exactly the opposite. Exactly how the GIP receptor blocker works has so far only been the subject of very rough hypotheses, but with no reliable data to back them up. However, as numerous deals prove, the companies involved are relatively indifferent to the reasons why a product works. In a gold rush, that’s secondary. For example Roche AG ponied up US$2.7bn for Australia’s Carmot Therapeutics Ltd, which
should enable the Swiss company to reenter the obesity business with the synthetic GLP-1/GIP receptor agonist CT-388. Meanwhile, Eli Lilly is also keen to buy as it seeks to beef up its pipeline, acquiring Versanis and its lead asset bimagrumab for a total of US$1.92bn. According to ex-Novo Nordisk expert Nicholas Finer, the monoclonal antibody, which reduced fat mass by 21.9% in a Phase IIa trial without any major change in caloric intake, is particularly interesting because of its thermogenic properties – i.e. its ability to convert white, energy-absorbing fat cells into brown, energy-consuming fat cells. Bimagrumab, which comes from the Novartis
Future obesity medications selected from among 120 clinical studies. T2D (type 2 diabetes), HFpEF (heart failure with preserved ejection fraction), MASH (metabolic dysfunction-associated steatohepatitis), CKD (chronic kidney disease), RA (receptor agonist), RAnt (receptor antagonist), GLP-1 (glucagon-like peptide-1), GIP (glucose-dependent insulinotropic polypeptide), GCG (glucagon)
› Semaglutide Novo Nordisk III, completed 50 oral, 1/day
› Orforglipron Eli Lilly III, 09/2027 nP oral, 1/day
› Semaglutide Novo Nordisk III, 12/2024 7.2 oral, 1/day
› Tirzepatide Eli Lilly III, 07/2024 5-15 pen, 1/week GLP-1/GIP RA
› Cagrisema Novo Nordisk III, 10/2026 2.4 pen, 1/week GLP-1/Amylin RA
› Survodutide Boehringer In. III, completed 3.6–6 pen, 1/week GLP-1/GCG RA
› Mazdutide Innovent Biol. III, completed 4–6 pen, 1/week GLP-1/GCG RA
› Mazdutide Innovent Biol. III, completed 4–6 pen, 1/week GLP-1/GCG RA
› Retatrutide Eli Lilly III, 05/2026 4–12 pen, 1/week
GLP-1/GIP/GCG RA
› Danuglipron Pfizer II, completed 40-200 oral, 2/day GLP-1 RA
› Cagrilintide Novo Nordisk II, completed 0.3–4.5 pen, 1/week Amylin RA
› PYY 1875 Novo Nordisk II, completed 0.03-2.4 pen/nP PYY RA
› Efinopegdutide Hamna Pharma II, completed 5-10 pen/nP
› Pemvidutide Altimmune II, completed 1.2–2.4 pen, 1/week
NCT05035095 T2D, Phase III
NCT05869903 T2D, Ph III
NCT05649137 T2D, Ph III
NCT04184622 T2D, Ph III/MASH, CKD, Ph II
NCT05567796 T2D, Ph III
NCT04667377 T2D, Ph III, MASH
NCT05607680 T2D, Ph III, CKD Ph I
NCT06164873 not public (nP)
NCT05929066 T2D, Ph III, CKD Ph II
NCT04707313 nP
NCT03856047 MASH Ph I
NCT03707990 nP
GLP-1/GCG RA NCT03486392 T2D, MASH/MASLD Ph II
GLP-1/GCG RA NCT05295875 MASH/MASLD PhII, T2D PH I
› AMG-133 Amgen II, completed nP pen, 1/week GLP-1/GIP RAnt NCT05669599 nP
› NNC0165-1875 + Semaglutide Novo Nordisk II, completed 1–2 + 2.4 pen, 1-2/month
GLP-1/ PYY RA NCT04969939 nP
› Dapiglutide Zealand Pharma II, 08/2024 4–6 pen, 1/week GLP-1/GLP-2RA
NCT05788601 nP
› Bimagrumab + Semaglutide Versanis Bio II, 09/2025 30 + 1–2.4 pen, 1/week, IV 1/month Activin IIRAnt/ GLP-1RA NCT05616013 nP
› S-309309 Shionogi II, 05/2024 nP pen, 1/week MGAT 2 NCT05925114 nP
› NNC0247-0829 Novo Nordisk I, completed nP pen, 1/week GDF15 analogue NCT05925114 nP
› CT-388 Roche AG I, completed 0.5-12 pen, 1/week GLP-1/GLP-2RA NCT04838405 nP
› CIN109/JNJ9090 J&J/CinRx I nP nP GDF15 analogue none nP
› SCO-267 Scohia Pharma I, completed nP nP
portfolio, was initially discarded after failing in a Phase II/III trial for the treatment of rare muscle wasting. However, Lilly also has in-house proprietary developments on offer, among them its candidate retatrutide. The GLP1/GIP and glucagon (GCG) receptor agonist achieved a weight loss of 24% in Phase II. The hormone amylin, which is secreted by the pancreas together with insulin after food intake, is also considered a promising combination candidate. Like GIP RAs, it slows gastric emptying, triggers a satiety signal and inhibits glucagon responses to meals. The neuropeptide Y inhibitors developed by Janssen and Novo –so-called NPY-Y2 receptor agonists – also enhance the effect of GLP-1RAs. However, there are issues involving the unspecificity of the study endpoint BMI, which does not allow any statement to be made about the type of weight loss and metabolic change, according to analyses by Global Data. Critics are calling for modified study designs that will help us to better understand the mechanisms the new blockbusters tap into.
New research funded by the Novo Nordisk Foundation is also generating hope for a future generation of obesity treatments: drugs that can convert white fat cells into brown fat cells. This ‘thermogenesis’ process also occurs naturally. In May, a German-Danish team reported on a newly discovered adenlyate cyclase 3 fragment that could herald a revolution. If the prin-
ciple they discovered in male mice also applies to humans, it might be used to transform fat-storing adipocytes into their fat-burning brown counterparts. Therapeutics that regulate the activity of this enzyme could therefore open new avenues to obesity resistance (naturE MEtaBoliSM, doi 10.1038/s42255-024-01033-8). – an opportunity to fight the condition at its roots with long-term effect.
Most mammals harbour two morphologically and functionally distinct types of fat cells. Consisting mostly of a single lipid droplet, white adipocytes are largely for energy storage, whereas brown adipo cytes (BAs) are multilocular and can convert foods into heat in a molecular process termed ‘non-shivering thermogenesis’ (NST). Interest in brown adipocytes arose after the discovery that these cells exist not only in small animals and human infants, but also in adults. Exposure to cold ambient temperatures or stimulating beta-adrenergic G-protein-coupled receptors (GPCRs) activates brown adipocytes, ultimately reducing BMI. The cold temperatures activate the sympathetic nervous system and induce release of the neurotransmitter nordrenaline. It in turn activates beta-adrenergic GPCRs, which then stimulate synthesis of the second messenger cAMP by transmembrane adenylyl cyclases. In turn, this activates protein kinase A (PKA), finally inducing lipolysis in brown fat cells
The research team headed by Prof. Jan-Wilhelm Kornfeld (Denmark) and

Dagmar Wachten (Germany) have now discovered that brown fat has a builtin mechanism that switches it off shortly after activation. They also identified a cold-inducible promoter that generates a 5’ truncated AC3 mRNA isoform called Adcy3-at that retains adenylate cyclase 3 in the endoplasmatic reticulum, thus reducing the pool of adenylyl cyclases available for G-protein-mediated cAMP synthesis, which drives lipolysis in brown fat cells.
“Looking ahead, we think that finding ways to block AC3-AT could be a promising strategy for safely activating brown fat and tackling obesity and related health problems,” said first author Hande Topel. “When we investigated mice that genetically didn’t have AC3-AT, we found that they were protected from becoming obese, partly because their bodies were simply better at burning off calories and were able to increase their metabolic rates through activating brown fat”.
In the study, two groups of mice were fed a high-fat diet for 15 weeks that rendered them obese. The group that had the AC3-AT removed gained less weight than the control group, and were metabolically healthier. “As AC3-AT is found not only in mice but also in humans and other species, there are direct therapeutic implications for humans,” said Topel.
› Brown fat burns calories from the food that we eat into heat.
› Brown fat is activated when we are exposed to cold temperatures.
› Scientists are looking for ways to activate brown fat as a strategy to tackle obesity.
Intriguingly, the study didn’t just identify AC3-AT, which is a shorter, previously unknown form of the AC3 protein. The researchers also identified other similar but unknown protein/gene versions that respond to cold exposure. “Understanding these kinds of molecular mechanisms not only sheds light on the regulation of brown fat, but also holds promise for unravelling similar mechanisms in other cellular pathways,” said Kornfeld.
Comprehending the complex mechanisms behind obesity is a huge challenge. However, the ongoing rush to identify treatments should also lead to a deeper understanding of the causes behind this multifaceted disease. L
t.gabrielczyk@biocom.eu
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2024
› Guide to German Biotech Companies 2024
› conveniently accessible ePapers
› Discount for BIOCOM events for 1 person
› additional partner offers
Price: 80 Euro p.a. (incl. VAT)
Students 50% discount (subject to proof of enrolment)
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2024
› Guide to German Biotech Companies 2024
› conveniently accessible ePapers
› Discount for BIOCOM events for 2 persons
› additional partner offers
Price: 120 Euro p.a. (plus VAT)

CULTURED MEAT Plant biotechnology company ORF Genetics, together with its Australian food innovation partner Vow, held a cultured meat tasting in Europe, featuring dishes created from quail cells grown with the help of ORF’s animal-free growth factors produced in barley plants.
ORF’s production technology is based on bioengineering the barley plant (Hordeum vulgare) to produce recombinant proteins in the endosperm compartment of the seed, using the seed as a bioreactor. According to ORF, the barley-grown growth factors allow cultured meat companies not only to grow but to rapidly scale-up its production and meet the needs of the rapidly growing cultured meat market.
Among those attending the tasting was Katrín Jakobsdóttir, Prime Minister and acting Minister of Food, Fisheries and Agriculture of Iceland. “Cultivated meat is one of the solutions to the climate challenge,” commented Jakobsdóttir. “The Icelandic authorities are determined to pave the way for the adoption of new solutions in Iceland and we are eager to see the development of an EU regulatory framework for cultivated meat.”

FINANCING KinSea Lead Discovery A/S, a biopharmaceutical start-up specialising in marine bioactives for treating cancer and other diseases, has closed a seed financing round of undisclosed amount. This round includes an equity investment from German KHAN Technology Transfer Fund I GmbH & Co KG and new investor Berners AS, a Norwegian investment company. Notably, a convertible loan previously extended to KinSea by KHAN-I a year ago has now been converted into shares. The funds go towards advancing KinSea’s lead programme, a highly differentiated FLT3 kinase inhibitor for the treatment of acute myeloid leukemia.
OBESITY Gubra A/S has unveiled the newest addition to its obesity treatment pipeline. Urocortin-2 (UCN2), a potent CRHR2 agonist, is aimed at promoting weight loss with a favourable cardiometabolic profile. In rat models, UCN2 prevented the lean mass loss observed in animals treated with GLP-1 or Amylin agonists while improving fat mass loss. “We see great potential for UCN2 within the area of healthy weight loss,” said CSO Niels Vrang. “UCN2 is well suited as a stand-alone treatment, but also as a future combination partner with other anti-obesity drugs incl. our amylin analogue (GUBamy) currently in Phase I clinical trials.”

ONCOLOGY Bexmarilimab, a myeloid cell-targeting immunotherapy candidate developed by Turku-based Faron Pharmaceuticals Ltd, binds to Clever-1, an immunosuppressive receptor found on macrophages leading to tumour growth and metastases. Scientists at the University of Turku have now uncovered how the immunotherapy changes macrophage behaviour so that they can infiltrate the tumour even in patients that do no respond to other treatments. The study utilised a novel spatial transcriptomics method that allowed the researchers to investigate changes in immune cell gene expression in a spatial context of patient tissue samples. The image shows cancer cells (shown in white-grey) and macrophage immune cells (shown in red). The research was published in the journals c E ll r E ports M E dicin E (.1016/j.xcrm.2023.101307) and c anc E r i MM unology r E s E arch (10.1158/23266066.CIR-23-0350) L
ONCOLOGY Genmab A/S made headlines with its announcement of the acquisition of ProfoundBio, a privately held biotech company, for US$1.8bn in cash. This strategic move is aimed at bolstering Genmab’s cancer pipeline with cutting-edge antibody-drug conjugate (ADC) therapies, leveraging ProfoundBio’s expertise and portfolio.
The acquisition will give Genmab access to ProfoundBio’s portfolio of ADCs, with three candidates already in clinical trials. Notably, the most advanced product in ProfoundBio’s pipeline is rinatabart sesutecan (Rina-S), a potentially first-in-class ADC that works by targeting the folate receptor alpha (FRa). Rina-S is currently in Phase II of a Phase I/II clinical trial designed to assess its therapeutic potential against ovarian and other FRaexpressing cancers. In January 2024, ProfoundBio announced that Rina-S had received Fast Track designation from the FDA for FRa-expressing high-grade serous or endometrioid platinum-resistant ovarian cancer.
Genmab is not new to the burgeoning ADC space, with partnership already established with Seagen, Synaffix and ADC Therapeutics. The ADC arena is becoming increasingly competitive following Pfizer’s US$43bn acquisition of Seagen and other multibillion-dollar deals in -

volving major players like Merck, Daiichi Sankyo, Bristol Myers Squibb, Eli Lilly, ADC Therapeutics, GlycoEra, Heidelberg Pharma, Tubulis, BioNTech, and emerging Chinese firms like MediLink and Duality Biologics. Genmap will also profit from the dual bases of ProfoundBio. The Chinese-American company has its headquarters in Seattle, Washington, and a research facility in Suzhou, near Shanghai. (see EuropE an BiotEchnology, 4/2023)
Genmab’s CEO, Jan van de Winkel, commented: “We believe that ProfoundBio’s ADC candidates, proprietary technology platforms and talented team will be a great addition to Genmab and that, together, we will be able to accelerate the development of innovative, differentiated antibody therapies for cancer patients.”
RESEARCH Blood and tissue samples stored at prestigious Karolinska Institute (KI) were destroyed over the Christmas holidays last year when a freezer malfunctioned. Now, an internal investigation came to the conclusion that “a chain of [technical as well as organisational] shortcomings caused the breakdown that led to the destruction of research material and the loss of years of research work”. KI will now appoint an external review group to further analyse how KI should organise its biobanks and similar infrastructure.
LOAN The European Investment Bank (EIB) has provided a €150m loan to Helen Ltd, a Finnish energy company owned by the City of Helsinki, to finance two new renewable-energy projects The total investment of €209m will support the installation of a new heat pump plant and the conversion of a heating plant from coal to biomass pellets in Helsinki. Helen, one of Finland’s largest energy companies, aims to achieve 100% carbon neutrality in its energy production by 2030 and is making significant investments to this end.
Novo Nordisk A/S has agreed to acquire Cardior Pharmaceuticals, a company active in the discovery and development of therapies that target RNA as a means to treat diseases of the heart, for up to €1.025bn. The agreement includes Cardior’s lead compound CDR132L, currently in Phase II clinical development for the treatment of heart failure. The takeover includes an upfront payment and additional payments if certain development and commercial milestones are achieved.
Bavarian Nordic A/S has secured a €65m contract to supply its MVA-BN smallpox vaccine to the European Union’s strategic reserve in 2025. This third, larger order follows two previous ones for the rescEU stockpiles, enhancing the EU’s readiness for biological threats by ensuring rapid vaccine deployment across member states. The MVA-BN vaccine, is approved by multiple international agencies and can also be used for mpox.
Cyxone AB, a biotech active in autoimmune diseases, has entered into a research collaboration with a group at the School of Infection and Immunity, University of Glasgow, with the objective of performing an exploratory Phase II study with rabeximod in patients with rheumatoid arthritis. The collaboration will be performed together with members of the Glasgow Rheumatic and Musculoskeletal Disease Group.
PLANT-BASED MEAT France’s highest administrative court, the Conseil d’État, has passed a judgement with a European signal effect. In mid-April, the Conseil overruled a government decree that provided for a ban on meat labelling for plant-based protein foods such as veggie steak, veggie sausage or burgers that are not protected under EU law. Six manufacturers of such products took legal action against this and were upheld.
The judges based their decision on the fact that a national ban would put French manufacturers at a disadvantage compared to manufacturers in other EU member states and would therefore constitute an attempt to create illegal trade barriers in the EU. According to the Conseil, the terms are well-established for years and would result in a drop in sales and high costs for companies to re-label their products.
The European Commission had already labelled Italy’s national marketing ban on cell-based foods as unlawful (see European Biotechnology, Winter 2023). A judgement by the European Court of Justice is pending. A proposal by eight EU member states at the EU Council of Agriculture Ministers in February (see EuropE an Biot E chnology, Spring 2024) to ban the marketing of plant- and cell-based foods aimed at creating a preference for traditional agricultural was rejected with a view to a free internal market. Pending a decision by the European Court of Justice, the judgement represents another victory for producers of plant-based meat. L
PIPELINE Ipsen SA and RNA splicing modifier specialist Skyhawk Therapeutics Inc have entered a US$1.8bn licencing option deal in rare neurological disorders. Under the agreement, Skyhawk will provide its discovery platform for RNA-targeting small molecules across several therapeutic areas, including rare neurological diseases. Ipsen, which provided no financial details about the worldwide exclusive agreement, has the option to licence successful candidates for clinical devel-
opment. Overall, the Belgian pharma company is committing up to US$1.8bn biobucks, which include all milestone payments. Skyhawk will also be entitled to tiered royalties on marketed products resulting from the partnership. With the deal, Ipsen is aiming to expand its drug pipeline to treat movement disorders. At the core of Skyhawk’s platform is RNA splicing modification – a way to coax dysfunctional genes into producing functional proteins without altering them permanently. L

BIOECONOMY In mid-April, wastebased biosurfactants producer AmphiStar BV has closed a €6m funding round led by the European Circular Bioeconomy Fund (ECBF), Qbic III and Flanders Future Tech Fund (FFTF) 1). The infusion of new capital will empower the Belgian company to kickstart commercial biosurfactant production in collaboration with external partners. The investment will also be used to support R&D efforts to optimise AmphiStar’s microbially produced sophorolipid and glycolipid biosurfactant platform, which is fully sourced from biobased waste- and side streams. AmphiStars’ surfactants are part of a synbio platform and are not linked to fossil feedstocks, palm oil, direct land use or deforestation to produce shampoos, toothpastes, cosmetics, inks, paints, agrochemicals, food and feed and even construction materials. The new assets will also allow the 2020 spin-
out from University Ghent and BioBase Europe Pilot Plant to complete the regulatory and certification dossiers required for the construction of a 1.000 tonnes per annum plant.
AmphiStar announced it will raise more funding in 2025. To manage the accelerated transition, Pierre-Franck Valentin joined the team as CEO.
AmphiStar’s current biosurfactant technology platform is the result of more than ten years integrated strain and process development. It is mainly based on the highly productive yeast organism Starmerella bombicola, and enables the company to produce over 25 specific variants of well-characterised glycolipid biosurfactants. The technology has been demonstrated at 15m³ scale, generating high purity products for application tests. A first product based on AmphiStar’s biosurfactants was launched by Ecover last May. L
CANCER British pharma giant AstraZeneca has announced to acquire next-generation radio-conjugate specialist Fusion Therapeutics Inc for US$2bn upfront through acquisition of all outstanding shares for US$30 per share. The British-Swedish pharma giant is interested in Fusion’s alpha emitter actinium-225-based pipeline of radiopharmaceutical conjugates that deliver a greater
radiation dose over a shorter distance. They include the Phase II programme FPI-2265, that is being tested in metastatic castration-resistant prostate cancer. FPI-2265, which was licenced from Heidelberg University and Euratom in February 2024, targets prostate-specific membrane antigen (PSMA), a protein that is highly expressed in castration-resistant prostate cancer. L
CELL-BASED MEAT This year, Europe has seen its first official cultivated meat meetings. In mid-February, Australian cell-based meat specialist Vow and its Icelandic partner ORF Genetics, who provides a plant-based growth factor, served gourmet dishes crafted with Vow’s cultivated quail to Iceland’s Agriculture and Prime Minister Katrín Jakobsdóttir and other pre-selected volunteers. Vow’s product, which has been approved in Singapore in late-April, is grown from cells of Coturnix japonica without antibiotic supplementation or animal-derived additives, the company said.
In mid-April, Meatable NV reported a pre-approval cultivated sausage tasting with VIPs Ira von Eelen, Constantijn van Oranje, Prince of the Netherlands, and Michelin-starred chef Ron Blaauwat at its new Leiden headquarters. Meatable’s hybrid sausage, which contains 28% pork fat cells differentiated from stem cells in only four days time and plant-based proteins has been also granted market approval in Singapore. As the first EU country, The Netherlands greenlighted cultivated meat and seafood tastings after rigorous food safety tests back in July 2023. L

Digital health start-up Klineo SAS (Paris) has raised €2m to improve equity of access to clinical cancer trials. The funds came from BPI France and business angels. The company’s AI-based platform, which was piloted in 2022, connects doctors and patients matching the inclusion criteria directly with relevant trials in less than a minute. The proceeds will enable Klineo to extend its platform to all types of cancer in France by the end of 2024. Thereafter, the company plans to expand its platform to other countries in Europe and the US and other pathologies such as rare and neurological diseases.
EQT Life Sciences’, Andera Partners, and Sofinnova Partners portfolio company Amolyt Pharma has agreed to be acquired by Alexion, AstraZeneca Rare Diseases in a transaction worth US$1.05bn, Amolyt Pharma’s Phase III candidate eneboparatide is a peptidic PTH receptor 1 agonist with a novel mode of action to treat hypoparathyroidism caused by a deficiency of parathyroid hormone (PTH) that results in decreased calcium and elevated phosphorus levels in the blood. Clincial data for eneboparatide showed that it normalised serum calcium levels and might end daily vitamin D supplementation. As eneboparatide also normalised calcium in urine in adults with chronic hypoparathyroidism and hypercalciuria, the therapy preserved bone mineral density. Thus, the candidate could complement Alexion’s bone metabolism franchise.
TRAVEL INFECTION Austrian-
French Valneva SE is slowly gaining traction with its three travel vaccinations. Valneva’s total revenues were €32.8m in the first quarter of 2024 and comparable to 2023.
While the two vaccination products against Japanese encephalitis and Cholera where relativley stable in sales, the just approved world first vaccine against chikungunya virus is now starting after getting several further recommendations.
IXCHIQ ® sales just started in the first quarter of 2024. IXCHIQ ® is the world’s first and only licensed chikungunya vaccine available to address this significant unmet medical need. Following adoption of the U.S. Advisory Committee on Immunization Practices (ACIP)’s recommendations by the U.S. Centers for Disease Control and Prevention (CDC) at the beginning of March 2024, Valneva has focused on launching its single-dose chikungunya vaccine in the U.S. and recorded initial sales of €0.2 million in the first quarter.
Other products in the making are vaccines against ZIKA virus and Lyme disease, the latter in collaboration with Pfizer in an ongoing phase 3 trial. The ZIKA clinical development started recently with a randomised, placebo-controlled, phase 1 trial, called VLA1601-102. The trial is planned to enroll approximately 150 participants aged 18 to 49 years in the United States. Participants will receive a low, medium or high dose of VLA1601. Data shall be available mid of 2025.
INNOVATION Johnson & Johnson has opened its first J&J Innovation Hub in mainland Europe at the Switzerland Innovation Park Basel Area. This is an extension of J&J Innovation in London and will serve as a bridge to external innovation.
J&J is the third largest pharmaceutical company in the world in terms of market capitalisation, and the largest in terms of annual sales in 2023 at around US$85bn. Due to the current boom and hype surrounding diet injections from Eli Lilly and Novo Nordisk, these pharma -
ceutical companies are currently ahead of Johnson and Johnson in terms of market capitalisation.
In Switzerland, Johnson & Johnson employs more than 5,700 people in the fields of biotechnology and medical technology, and the opening of the Innovation Hub also emphasised “the important role that Basel and Switzerland as a whole play in biotech and medtech innovation and Johnson & Johnson’s ongoing commitment to the region”, the company commented its decission for Basel.

FACILITIES German Chancellor Olaf Scholz visited the laying of the foundation stone for Merck’s Innovation Centre. Merck KGaA is investing more than €300m in the new research centre at its headquarters in Darmstadt, Hesse. At the symbolic event, Scholz emphasised the importance of such investments for Germany as a research location. The newly designed centre is intended to accelerate biopharmaceutical product development and is part of Merck’s €1.5bn investment programme in Darmstadt until 2025. The Advanced Research Centre will provide space for around 550 employees, starting 2027. Just a few days earlier, Scholz had attended the laying of the foundation stone for Eli Lilly in Alzey near Mainz, which is investing around €2.3bn in a production centre for obesity drugs. His gift, the draft law of Germany´s new Medical Research Act, is now in the “time capsule” that was embedded in the foundation stone of the new building. L
ADDICTION The Swiss company Stalicla SA from Geneva reports progress in clinical development. The first patient visit (First Patient First Visit, FPFV) for the DDI (Drug-Drug Interaction) study of STP7 (mavoglurant) has taken place, the company announced in May. The compound is a negative allosteric modulator of glutamate receptor 5 (mGluR5) licensed to Stalicla by Novartis. The DDI study is the final regulatory requirement in a comprehensive Phase II programme. Its completion may initiate the start of a Phase III trial in the US in 2025.
Mavoglurant is the most clinically advanced negative allosteric modulator of glutamate receptor 5, which not only
plays a role in the pathophysiology of addiction, but is also implicated in mood disorders and neurodevelopmental disorders such as Fragile X and autism spectrum disorders.
Novartis has conducted extensive clinical trials with it, involving more than 1,800 adults over a period of up to two years. They showed good safety and tolerability and a significant reduction in cocaine use, positioning STP7 as a strong therapeutic candidate. The in-licensing of Mavoglurant from Novartis in 2023, was accompanied by a financial dowry totalling up to US$250m upfront plus milestone and possible royalty payments.
SPATIAL BIOLOGY Out of bankruptcy proceedings and against the (significantly lower) bid of the investment firm Patient Square of around US$220m, Bruker acquires the US company Nanostring for around US$390m. Bruker, which was founded in Karlsruhe, Germany, in 1960, is thus seeking to secure further expertise in the field of “spatial biology”, an area of high-resolution anal-
ysis of cell tissue. The US company Nanostring, which has been active for two decades, had previously been unable to assert its right to use a technology licensed from 10x Genomics in a very intense and public patent dispute that had been going on for years. Nanostring, based in Seattle, initiated the sales process by filing for protection under bankruptcy at the beginning of February.

Austria invests more in research in 2024 than ever before. Statistics Austria published its estimate of the research ratio for 2024, which is expected to be 3.34% this year. This means that around €16.6bn will be invested in research and development (R&D) this year. Last year, the figure was €15.6bn. Figures from 2022 are available for the European comparison: Austria ranks third in the EU behind Belgium and Sweden, and remarkably on position eight worldwide.
First field trial with CRISPR barley in Switzerland. Agroscope, the Swiss government’s centre of excellence for agriculture, has launched the first field trial with barley genetically modified using CRISPR/ Cas. The modification affects a single gene and is intended to lead to higher yields. Critics are missing the relevance of the trial for nutrition or climate impact, while Agroscope is more concerned with practical issues in its test setting.
Bavarian Axolabs establishes its RNA GMP production in Berlin. Axolabs GmbH and Asahi Kasei Bioprocess have started a partnership in the field of oligonucleotide therapeutics (RNA). The partners will work together to build a state-ofthe-art cGMP production facility for oligonucleotides on a 5,500 square metre site in Berlin. Axolabs, founded in Kulmbach, is an expert in RNA production issues difficult to treat.
BIOSIMILARS Until now, the Slovenian arm of the Swiss Sandoz AG in Ljubljana has only produced small molecule generics. Last summer, the Swiss biosimilar experts announced a €84m expansion that will make the site one of Sandoz’ biggest biosimilar centres in Europe. In addition, €372m will be invested in the construction of a new biologics manufacturing centre in Lendava that started last December and will create 300 new jobs. In late April, Slovenia’s Lek broke ground for the €84m Lek factory in Ljubljana. The 9,500 sqm facility will employ 200 experts, Lek said in a statement on Friday. The investment is part of Sandoz’ broader US$500m commitment to biosimilar development and production in Slovenia, ensuring access to critical medicines. Last August, Swiss Novartis AG transferred its ownership of the equity capital of Lek to the manufacturing specialist Sandoz.
INVESTMENT The Panhellenic Pharmaceutical Industry Association will invest €1.5bn by 2026 to ensure self-sufficiency of Greece with pharma raw materials and antibodies by expanding R&D and production capabilities, president Theodoros Tryfon confirmed in April. The plan includes breaking ground for 10 production sites, and 14 new R&D centres including biotech incubators.
DEAL Spanish Ferrer Internacional SA has acquired the ex-US rights to VRG50635 from AI drug discovery specialist Verge Genomics Inc to commercialise the oral Phase I experimental ALS treatment in Central and South America, Southeast Asia and Japan.
The €122.5m invested include upfront and milestone payments plus royalties. Verge’s clinical-stage amyotrophic lateral sclerosis (ALS) drug VRG50635 was developed using the AI platform Converge that integrates human omics data, in vitro testing data with clinical data from ALS patients and controls to screen the best drug candidates VRG50635, which is currently being tested in a Phase Ib trial, is believed to improve the survival of neurons in patients affected by ALS by blocking PIKfyve (1 phosphatidylinositol
3 phosphate 5 kinase). In January, Ferrer’s oral ALS formulation of edaravone, a compound from Mitsubishi Tanabe Pharma which got approval in another indication with another formulation, failed in a Phase III trial. Currently, in Europe there is a single approved treatment option in the form of riluzole, marketed by Sanofi as Rilutek, and as Teglutik that is marketed by Italfarmaco SA (France, Germany, Italy, Spain) and by Martindale Pharmaceuticals (UK). According to data from the ADORE trial, edaravone (100mg, once daily) did not demonstrate a significant benefit over placebo in slowing ALS progression, measured by change from baseline in the revised ALS Functional Rating Scale (ALSFRS-R) score after 48 weeks of daily oral edaravone dosing.
ALGAE BIOTECH Faro-based private non-profit organisation GreenCoLab SL has debuted microalgae- and seaweedbased prototype of caviar, beer, and burgers at VitaFoods Europe in mid-May. The food innovations include a microalgaebased alternative to sturgeon roe, microalgae-based beer, and seaweed-derived burgers. GreenCoLab combines different expertises at the academic research centers CCMAR and CIIMAR, the LNEG lab and the University of Algarve with the market know-how of six com -
panies. The academic and industry partners at Allmicroalgae, Necton, Algaplus, Sparos, Iberagar and Gopsis have a common goal: advancing the R&D on the cultivation of algae and algal biomass downstream processing, with the ultimate goal of generating a portfolio of new commercial products and services for partners in the industry.“As the paradigm shifts away from animal-based markets, there is a growing demand for vegan alternatives,” says business and communication head Daniel Mendonça Silva.


BIOECONOMY Following the successful cultivation of muscle and fat cells from chicken feathers in the laboratory, the Feather to meat project launched last year at the University of Trento is now entering the next phase of process development. This was announced at the end of April by research leaders Luciano Conti and Stefano Biressi from the University of Trento, who spun out Italy’s first cellbased meat specialist Bruno Cell Srl. In April, feather to meat project, which has been co-funded by the animal welfare organisation Save the Chickens Foundation announced it will now focus on the selection of production cell lines and their licensing to food companies. The media in
which the stem cells extracted from the feathers are used for propagation and differentiation have already been optimised. Despite Italy’s legal ban on the commercialisation of cell-based foods, the foundation and scientists see a great need for alternatives that make slaughter and factory farming superfluous.
It is not the first trial to use feathers as a protein source. Back in 2000, Thai inventor Sorawut Kittibanthorne presented a 13-step process to bring the protein keratin, that makes up 88% of chicken feathers, into a digestable form. According to Kittibanthorn more than 2 million tonnes of feathers are wasted annually in Europe alone.
OUTSOURCING Verona-based drug discovery specialist Sibylla Biotech SpA has entered a strategic drug discovery collaboration with Osaka-headquartered pharma giant Ono Pharmaceutical Co Ltd in the CNS disease area. Under the agreement inked in mid-March, Sibylla will provide access to its cutting-edge Pharmacological Protein Inactivation by Folding Intermediates Targeting (PPI-FIT) technology to identify and develop candidates for multiple therapeutic targets in the field of Central Nervous System (CNS) disorders. The spin-out of the National Institute of Nuclear Physics and the Trento and Perugia Universities is entitled to receive threedigit millions of US biobucks, including an upfront payment, R&D and sales mile-
stones, as well as royalties on sales. Further financial details on the collaboration have not been disclosed.
Sibylla Biotech utilises its PPI-FIT technology to design Folding Interfering Degraders (FIDs). FIDs are small molecules that induce the degradation of a target protein by interfering with its folding pathway. The technology targets specific proteins, particularly those considered “undruggable” due to the inaccessibility of suitable binding pockets in their native state. Ono Pharmaceutical plans to harness the technology to address some of the most challenging aspects of CNS drug development by targeting proteins involved in complex neurological path
ways.
After 20 years of GMO blockade, Italian Institute for Environmental Protection and Research ISPRA has greenlighted the first field tests of a genome-edited rice that is resistant to the filamentous fungus Magnaporthe oryzae (Pyricularia oryzae), which causes rice blast disease. A research team headed by Vittoria Brambilla from the University of Milan has used CRISPR/Cas9 to make deletions in three genes that determine the risotto rice’s susceptibility to the pathogen, Pi21, HMA1 and HMA2. The NGT-1 plants will be sewed on a 28sqm plot, set within a 400sqm field, that will act as a buffer zone to prevent cross-pollination. Italian researchers have about a dozen NGT-1 breeds in their pipeline.
Spanish energy giant Repsol SA has entered the biomethane market by taking a 40% stake in Genia Bioenergy, which owns 19 biomethane facilities, 11 thereof under early stage construction. Within the EU’s RePower programme, biomethane production is going to be expanded by 2030 to the eightfold amount recorded in 2022. The Spanish Gas Association Sedigas estimates that biomethane could account for almost half of Spain’s natural gas consumption. Gas is used to make 15% of the countries energy.
Igor Mišic and Dušan Glušcevic and their Bio Scanner won the Dubrovnik Hackaton in April with an app detecting diseases in plants.
BIOMANUFACTURING At the beginning of April, Memel Biotech, a Lithuania-based contract development and manufacturing organisation (CDMO) has launched a fully integrated advanced therapy development and manufacturing service at its facility in Klaipeda, which will serve markets in the European Union (EU). With this new service, the company aims to target partnerships with emerging and established biotech companies seeking to enter the ATMP space. The company will operate a new good manufacturing practice (GMP) facility, where it will offer discovery-to-delivery service for all three classes of ATMPs: cell therapy, gene therapy, and tissue-engineered therapy. The company’s manufacturing range encompasses mesenchymal stem cells, induced pluripotent stem cells, extracellular vesicles, immune cells (including natural killer cells and dendritic cells), and gene modified cells, including chimeric antigen receptor T cells (Car-Ts).
ORPHAN DISEASES The Czech patient organisation Rare Disease Czech Republic has published an EFPIA-sponsored article that calls for better access to early diagnosis and treatment of orphan diseases. The patient group’s VP René Brectan called for incentives that assure early, affordable access to the expensive medicines.
CELL THERAPY Gdansk-based public cell therapy developer PolTREG SA has received cGMP certification from Poland’s Chief Pharmaceutical Inspectorate in mid-March. The certification enables the company to produce Tregs at its production facility and seek permission from Poland’s Office for Registration of Medicinal Products, Medical Devices and Biocidal Products (URPL) to perform clinical trials in the facility, for diseases such as Type-1 Diabetes (T1D) and Multiple Sclerosis (MS). PolTREG was the first company in the world to administer Treg therapies to patients, and the first to start receiving revenues from its lead product under a hospital exemption. Its manufacturing facility is one of Europe’s largest, boasting
over 2,100 sqm of laboratory space, including 15 production lines.
PolTREG has the option to substantially expand the facility to accommodate manufacturing of next-generation engineered therapies and cell therapies from future partners. The company has treated more than 100 patients with Treg cells.
The company’s pipeline includes the lead candidate, PTG-007, which is ready for licencing as a Phase II/III candidate to treat early-onset type-1 diabetes. Phase II studies with PTG-007 as a treatment for remitting/relapsing and progressive MS are expected to begin in H2/2024. For CARTregs, the company expects to launch a first-in-human trial in MS and ALS in early 2025.
BIOBASED ECONOMY Even after the latest bioeconomy strategies of the USA and the UK rely heavily on the potential of synthetic biotechnology to drive forward the defossilisation of production, the signs in Europe are still pointing to biobased. At the beginning of July, shortly after taking over the EU Council Presidency, Hungary plans to propose a €500m Horizon Europe co-funded bioeconomy alliance of 11 Central and Eastern European partners. The Bioeast initiative, a policy steering group operating in 11 central and eastern European countries recommends to take the PRIMA alliance of 19 Mediterranean countries, which is funded along the same lines, as a model for the eastern alliance.
The members of the alliance have a strong raw and residual materials base that hasn’t been exploited in a coordinated way in the past to produce biogenic materials. In terms of Horizon funding, the 11 member states cashed in only 7% of the €10bn budget available for research in bioeconomy. Now, they ask for more support to build biorefineries and catch up with the western EU member
states. Though producers from different industries have underlined that there are not enough bio-based resources available to fully replace fossil-resource-based processes, the European Commission resisted in its belief that bio-based products and chemicals will be necessary to reduce fossil fuel use in industry and transport to meet the Paris climate goals. For example, HVO100 biodiesel world market leader Neste (Finland) estimates that globally 40 million tons of residual plant oil and animal fat is available to produce HVO100. That is one tenth of the consumption of the US transport sector alone.
According to Barna Kovacs, Secretary General of the Bioeast initiative, eastern Europe’s biomass is exported but not processed locally, what would be more sustainable and would strengthen Europe’s self sufficiency, a policy maker buzzword that came along with the Ukraine war and its implications for the EU energy sector. It’s remains to be seen, if the new Commission that will be elected after the European election in midJune will continue the Green Deal.
OPHTALMOLOGY Following the FDA approval of the complement C5 inhibitors pegcetacoplan and avacincaptad pegol as the first specific agents against dry AMD, a French company is now starting to block the underlying necroptosis pathways. In late April, five year old SeaBeLife SAS has bagged a €1.5m fund within the i-Nov 2024 innovation competition, which is part of the French government’s France 2030 investment plan. Under the SeaBeEYE project, the preclinical-stage drugmaker will use the fresh proceeds to advance a topical formulation of a small molecule drug to treat geographic atrophy, the progressive, irreversible, advanced form of dry AMD.
SeaBeLife’s approach centers around SBL03, a derivative of a naturally occurring flavanone, isolated from Populus nigra buds. In vitro, SBL03 has been shown to directly and simultaneously block two forms of regulated necrosis that lead to retinal cells’ death and vision loss: cellular necroptosis and ferroptosis. Additionally, SBL03 also blocked oxytosis that plays an important role in CNS diseases.
After successful preclinical in vivo proofof-principle, the SeaBeEYE project focuses on the preclinical and regulatory development of a topical formulation (gel) of the drug candidate as an alternative to sustained-release intravitreal injections. SeaBeLife SAS aims to have a treatment option ready for clinical trials in humans by Q1/2026.Recent FDA-approved drugs
for dry AMD such as pegcetacoplan and avacincaptad pegol block complement activation, which trigger the inflammation in the eye that may support macular degeneration. SeaBeLife SAS joined another patented approach: when a cell is ready to die, there are several modes in which it can do so. Necroptosis, a form of regulated necrosis, results in inflammation, which damages surrounding tissue. SeaBeLife’s dual-acting inhibitor SBL03 has the unique property to fight both, necroptosis and ferroptosis and the complex crosstalk between these pathways.
Founded in 2019 and based in Roscoff in Brittany, France, SeaBeLife is led by CEO and co-founder Morgane Rousselot, who holds a PhD in biochemistry from Sorbonne University/the French National Center for Scientific Research (CNRS)/Roscoff Marine Station. The company’s activities are based on the research work of Stéphane Bach, PhD, CNRS research engineer and scientific lead at the Roscoff screening platform, Marie-Thérèse Dimanche-Boitrel, research director at IRSET, and Claire Delehouzé, PhD, a biotechnology engineer, co-founder and CTO at SeaBeLife. SeaBeLife currently employs eight people and since its creation has raised €5.5M in private equity and grants. The company benefits from the support of partners, including SATT Ouest Valorisation, Biotech Santé Bretagne, Bpifrance and the regional council of Brittany. L
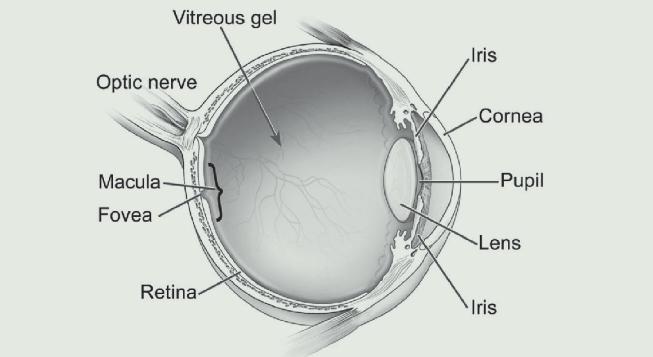
French reseachers have discovered that incomplete silencing of the second X chromosome during female embryonic development protects lupus models from developing autoimmune diseases. Christophe Huret and colleagues from Université Paris Cité deregulated a non-coding RNA called Xist in male and female mice. Doing so caused TLR7, an autoimmunity-mediating gene, to turn on in females.
A bacterium of the gut microbiome seems to spark an autoimmune disease in the kidneys by leaking antigens that trigger the immune system reported by an Irish-French research team at the end of March. Although a direct causal link is yet missing, the group found that the gut microbiomes of 33 patients with the disorder were unusually skewed toward bacterial species that degrade mucin, including Akkermansia muciniphila, which converted IgA1 into a deglycosylated form associated with IgA nephropathy. The newly created antigens crossed the lining of the intestines and entered the blood. The team also found out that antimicrobial alpha-defensins suppressed the growth of A. muciniphila in culture, but that these protective effects seemed to be lost in stool samples from patients.
Flagging of CAR-T cells with the B7H6 protein weakened the response to human leukaemia in models as it marks them for NK cell attack, German researchers reported in April.
ORPHAN DISEASES A Swiss-Italian group of gene therapy specialists report early success in treating the congential lysosomal storage disorder Hurler syndrome after 4-year follow-up of a Phase I/II study conducted with patients who received genetically modified autologous stem cell transplants. Skeletal deformities in children improved more than in those treated with non GM transplants.
In autumn 2021, a team of Italian researchers headed by Alessandro Aiuti at San Raffaele Telethon Institute for Gene Therapy reported that an experimental approach that seemed to be correcting the metabolic issues at the core of a rare genetic disease that left children unable to break down sugar molecules. However the allogeneic hematopoietic stemcell transplantation did not correct skeletal deformities that regularly occur in infants with Hurler syndrome.
A new treatment protocol reported in May, however, combines the stem cell transplants with gene therapy shows signs of correcting skeletal deformities in eight children with Hurler syndrome. Results from the ongoing Phase I/II study (NCT03488394) suggest that similar strategies may counteract one of the most debilitating complications from this rare disorder, which currently has no cure.
Mucopolysaccharidosis type I Hurler variant (PMSIH) is caused by mutations in the gene encoding alpha-1-iduronidase (IDUA). It leads to accumulation of glycosaminoglycans in multiple tissues and is associated with progressive skeletal dysplasia that is not halted by allogeneic hematopoietic stem cell transplantation (HSCT)
Patients with Hurler syndrome face severe complications including heart disease and a drastic slowdown of growth in the skeleton. This lack of growth produces a constellation of deformities in the spine, hips, and other areas, necessitating a lifetime of surgeries and substantially affecting quality of life.

Now, first author Giulia Consiglieri and colleagues report on results from a 3.7 years follow-up of a clinical trial of a stem cell-based gene therapy for skeletal deformities in Hurler syndrome. The objectives of the trial were to evaluate the safety, tolerability, and efficacy (measured as biochemical correction) of autologous CD34 + cells transduced with an lentiviral vector encoding the IDUA gene in paediatric patients following a myeloablative and lymphoablative conditioning regimen. The primary efficacy endpoint of the trial was IDUA activity in DBS at one year after treatment, and secondary efficacy endpoints included IDUA activity in DBS, engraftment of transduced cells at levels above 30%, and growth velocity at one, three, and five years after treatment. Motor function and spine MRI score at one, three, and five years after treatment were evaluated. Overall, patients who received autolo -
gous stem cells containing the corrected alpha-L-iduronidase (IDUA) gene in a lentiviral vector displayed close-to-normal skeletal growth patterns restoring the cells’ ability to dispose waste and reducing the buildup of glycan molecules. The patients also grew taller and showed more complete joint mobility compared with a control cohort of patients with Hurler syndrome who received allogeneic HSCT. Data suggest that the corrected cells stabilised spine defects and had beneficial effects on various skeletal features. Though autologous hematopoietic stem progenitor cell/gene therapy (HSPC-GT) provided superior metabolic correction in patients who received MPSIH instead of HSCT, the gene therapy’s ability to affect skeletal manifestations needs to be confirmed in the long term and in a prospective comparison with HSCT. L
t.gabrielczyk@biocom.eu
AMR Antibiotics have been overused worldwide to treat hospitalised patients with COVID-19, a new report published by the World Health Organization WHO suggests. The authors concluded at the ESCMID Global Congress in Barcelona that the COVID-19 pandemic may have exacerbated the silent spread of antibiotic resistance. While only 8% of hospitalised patients with COVID-19 had a bacterial superinfection around 75% were treated with antibiotics what did not help but in some cases was harmful.
COMA Most recently, glial fibrillary acidic protein (GFAP) has been identified as a biomarker of intracranial hemorrhage. A test conducted at the RKH Klinikum Ludwigsburg, Germany, whether prehospital GFAP measurements on a point-of-care device could help to rapidly differentiate intracranial hemorrhage from other causes of acute coma led to a positive result. Patients with Glasgow Coma Scale scores between 3 and 8 were enrolled prospectively. Blood samples from 143 patients were collected in the prehospital phase. Plasma GFAP measurements were performed on the i-STAT Alinity (Abbott) device shortly after hospital admission. GFAP plasma concentrations were strongly elevated in patients with intracranial hemorrhage (n = 51, 3352 pg/mL) compared to all other coma etiologies (43 pg/mL When using an optimal cut-off value of 101 pg/ mL, sensitivity to identifying intracranial hemorrhage was 94.1% while specificity stood at 78.9%, the positive predictive value was 71.6% while the negative predictive value was 95.9%. In-hospital mortality risk was associated with prehospital GFAP values. Thus, increased GFAP plasma concentrations in patients with acute coma identified intracranial hemorrhage with high diagnostic accuracy.
NOVEL FOOD German BLUU Seafood GmbH, which specialises in the cellbased production of salmon and trout fillets, has opened a production facility in Hamburg. At the opening in mid-April, the company announced it is aiming for market approval in 2025.
Almost four years after it was founded in Lübeck, BLUU Seafood GmbH opened Europe’s first pilot plant for cultivated fish in its new headquarters. The company will initially operate a 65-litre bioreactor on an area of around 2,000 square metres to multiply and differentiate fish muscle cells from stem cells. Following market approval in the novel food mecca of Singapore, which is planned for 2025, the company will scale up to industrial production of up to 2,000 litres. The company, which has raised €23m to date, aims to achieve price parity in three years if the market environment is favourable.
BLUU Seafood is able to cultivate muscle, fat and connective tissue cells from Atlantic salmon and rainbow trout in significantly larger quantities than before. At the optimum temperature and with an appropriate supply of oxygen and nu-
trients, the animal cells grow and divide just as they do in live fish. Unlike many wild-caught fish, the fish products created from the cell mass, such as fish fingers or fish balls, are free from heavy metals and microplastics. They are similar to conventional products in terms of flavour and nutritional content as well as cooking behaviour. “Although cultivated fish is no different from wild-caught or farmed fish at the cellular level, it is considered a novel food in regulatory terms and is thoroughly tested in all markets before being authorised. This is quickest in Singapore and the USA. Here it takes less than a year. The UK and Israel are currently adapting their laws. In the EU, it currently takes up to four years for market authorisation. Nevertheless, BLUU Seafood is aiming for marketing authorisations in the USA and the European Union. If the scaling options and framework conditions are right, we will be able to offer cultivated fish at wholesale fish prices in just three years,” says Managing Director Sebastian Rakers. The new site is an important building block in this development. L

PLANT PROTECTION A German-Italian research team has patented a technology that fights viral plant pathogens with its own weapons. The team headed by Sven-Erik Behrens; CSO of Verovaccines Gmbh at Halle University, used antisense oligodeoxynucleotides (ASOs) introduced into plant cells to trigger enzymatic degradation of the mRNAs produced by viral pathogens to reprogramme the plant cell.
So far, the practicability of antisense technologies was limited because it was difficult to reliably predict the sites accessible to ASOs in complex folded RNAs. Now, the researchers applied a method that reproduces RNA-induced RNA silencing in vitro to reliably identify sites in target RNAs that are accessible to small interfering RNA (siRNA)-guided Argonaute endonucleases. They also demonstrated that this method is suitable for identifying ASOs that are effective in DNA-induced RNA silencing by RNase H.
ASOs identified that way are comparably effective in protecting plants from infection as siRNAs with the corresponding sequence. As the team reports in the intErnational Journal of MolEcular SciEncES, the new active substance was able to ward off the virus in up to 90% of cases. The anti-
viral activity of the ASOs could be further increased by chemical modification. In addition to better target specificity, ASOs are easier and cheaper to synthesise than siRNAs or dsRNAs. What’s more, in the human system, ASOs have been shown to have low immunoreactivity, which is also of significant importance for potential applications in edible plants. However, the application of ASOs in plants is only slowly gaining interest. Mostly used to study gene function, the recent development of methods to introduce ASOs into plants, by infiltration, has opened up further applications.
The patented technology could be useful not only for effective RNA silencing in plants but also in other organisms. The team proved for the first time that their newly developed antisense process can be used to produce ASOs that offer a high level of plant protection. “Antisense oligonucleotides are not only very accurate, they are also relatively easy and cheap to produce. Similar active ingredients have also been used in humans for several years and show few side effects. This is another important point in favour of their potential use in food,” said Behrens, who wants to commercialise the technology.

SYNBIO German biotech start-up Insempra GmbH (Martinsried), which specialises in the biotechnological production of natural ingredients for the food, cosmetics and textile industries, has raised US$20m in May. Bayern Kapital, Henkel dx Ventures and Alante Capital were acquired as new investors for the Series A financing round. Existing investors EQT Ventures (lead), BlueYard Capital, Possible Ventures, Taavet Sten and Acequia Capital also participated.
The company will use the proceeds to scale its screening platform for cell-free precision fermentation of base materials and ingredients for various industries. The company has already launched the flavouring agent alpha-ionon last year. Insempra is currently developning processes for the microbial production of polyester and polyamides from residual and waste materials.
Insempra builds microbial strains that can convert renewable feedstocks to natural ingredients. To make the most efficient microbes for production, the company screens through thousands of metabolic designs. Building each of these designed microbial strains is one of the most time-consuming steps in Insempra’s development process. to reduce development time, the company has built a cell-free platform to test metabolic designs quickly and speed-up time to market.
Together with its scientific advisor
Prof. Michael Jewett from Northwestern University the company is using CellFree Protein Synthesis (CFPS) to synthesise sustainable bioproducts, like styrene, butanol, limonene, acetone, and hexanol. In CFPS, the transcription and translation machinery are reconstructed in cell-free reactions. By introducing DNA bearing one or multiple genes of interest, we are able to synthesize target proteins within a few hours and characterize their functions immediately. This eliminates the time-consuming steps in conventional strain construction before assaying the protein function.
PLASTIC DEGRADERS While French microbial plastics degrading specialist Carbios SA announced a partnership in PET recycling, US-based synbio start-up Breaking Inc announced its discovery of X-32, a microbe able to degrade plastics such as polyesters, polyamides and even polyethylene within only 22 months.
At the beginning of May, Carbios SA and German Hündgen Entsorgungs GmbH & Co. KG, a waste management expert in logistics, sorting services and the recycling of recyclable materials from waste mixtures, announced that they have agreed to sign a non-binding contract relating to the sourcing, preparation and recycling of 15,000t/year of post-consumer polyethylene therephtalate (PET) waste using Carbios’ enzymatic depolymerisation technology at its first commercial plant in Longlaville, from the end 2026 to produce food-grade PTA and MEG, that can be re-polymerised into PET (see p. 34).
At the same time, the US-start up Breaking Inc claimed it has developed a microbe named X-32 that is able to degrade ester bonds such as in PET, polyamide bonds such as in nylon and C-C-bonds like in PE. The company announced X-32 was able to degrade up to 90% of polyesters and polyolefins in less than 22 months but did not give the chemical details of the reaction. Breaking starts with US$10.5m in seed financing to hijack a market of approximately 5,000 million tons of plastic sitting in landfills, oceans, and ecosystems.
Breaking reported it will further opimise X-32 to address the global plastics crisis. In its natural state X-32 can degrade polyolefins and polyesters leaving behind carbon dioxide, water and biomass in just 22 months under optimal conditions. With future synthetic genetic edits, the team co-founded by Sukanya Punthambaker (CEO) and Vaskar Gnyawali (CSO) is focused on making X-32 faster, more efficient and more effective. “I’ve spent my career in synthetic biology and protein engineering with the

Breaking CSO Vaskar Gnyawali in his lab at the Wyss Institute
hope of developing something this transformational,” said Punthambaker. “In the future, our solution will be able to work across terrestrial and marine environments to break down today’s greatest threat to humankind/our existence: the plastic that is choking our world.”
The world’s plastic problem is growing increasingly more severe: On top of the 5,000 million tons of plastic that are sitting in landfills, oceans, and the ecosystems, 390 million tons more of plastic are produced each year; up 22,400% since 1950. Plastics have been found in Antarctic sea ice, the blood, and in marine animals in deep ocean trenches. Even bottled water contains almost a quarter of a million nanoplastic fragments. In its current state, X-32’s performance is al -
ready good: it degrades up to 90% of polyesters and polyolefins from mixed plastics in short time.
The microbe starts to work immediately. In lab tests, X-32 started to break down paint brush bristles, fishing wire and dental floss in less than five days. If left untreated, paint bristles brushes can take 450 to 1000 years to decompose, fishing wire can take 600 years, and dental floss would take 80 years. X-32, which was developed at the Wyss Institute utilises plastics as a primary carbon source and needs no pre-treatment, sorting, cleaning or decontamination and it emits carbon dioxide, water and biomass during the degradation process. Engineers at Breaking will next focus on identifying and isolating the enzyme used by X-32, then editing and optimising it by machine learning. L t.gabrielczyk@biocom.eu
FOOD BIOTECH As coffee harvest is predicted to shrink by more than 50% until 2050 due to climate change, an international research team supported by Swiss food giant Nestlé has developed a database to better identify and breed climate-resistant coffee plants. Meanwhile, biotechnologists are seeking to produce coffee in the lab.
With climate change threatening coffee cultivation, an international research team supported by Nestlé experts have explored how advanced data science and artificial intelligence can be leveraged to help select and breed more climate-resilient plants.
While more than 120 species of coffee exist, around 70% of the world’s coffee production is arabica. However, arabica has a lower tolerance to rising temperatures and is more susceptible to disease than other coffee plants, such as robusta. Additionally, climate change is reducing the amount of arable land it is possible to cultivate coffee on, and water shortages are significantly reducing yields. At the same time, demand for coffee is growing.
To identify new, higher yielding arabica varieties with greater resistance to disease and drought, an international research team from nine countries has developed a high quality arabica reference genome that makes it easier to analyse different traits of coffee varieties to identify specific traits such as better yield, coffee cherry size and greater resilience to disease or drought, as well as flavour characteristics.
According to Jeroen Dijkman, Head of Nestlé’s Institute of Agricultural Sciences, the new reference is “like a high-quality map of a big city. It will help us identify key genetic markers in the arabica genome that are responsible for specific traits in adult plants […] to better identify, select and breed new and improved arabica coffee varieties.”
Biotechnologists such as Henry Kunz, founder of Paris-based Stem SA, believe

that they can solve many of these problems by growing coffee cells in a laboratory instead of on a tree.
They start with undifferentiated coffee cell materials from selected varieties, propagate them in a liquid suspension using a plant growth media before proceeding to upscale the culture in a bioreactor through a fermentation process. To bring added complexity to the cup, Stem make use of natural flavour engineering techniques sourced from coffee by-products. With significantly less energy and water input, they harvest green coffee powder which, just like beans, is then dried and roasted. In contrast to culture media for cell-based meat, the cost of culture media for coffee plant cells is only €10 per litre, or €5 per litre after scaling up, according to Stem. Start-ups, such as Minus, Atomo, Northern Wonder, Prefer, or researchers
at the Finnish research centre VTT believe that their “beanless coffee,” has a future as extreme weather in Brazil sent commodity coffee prices to an 11-year high of $2.58 per pound in 2022.
Last December, VTT researchers led by Heiko Rischer published the recipe for cell-based coffee (see European Biotechnology, Winter 2023). Rischer and his team see their biotechnological process, in which the cells are roasted instead of the green coffee beans, as an alternative to traditional coffee cultivation. This yields up to two harvests per year, while a batch of cell-based coffee can be cultivated in reproducible quality under controlled conditions within a month. L
t.gabrielczyk@biocom.eu
ARTIFICIAL INTELLIGENCE The clinical knowledge and reasoning ability of the AI language model GPT-4 is approaching the level of ophthalmology specialists, according to a study published by UK data experts in early May (10.1371/ journal.pdig.0000341). GPT-4 was tested against doctors at different career stages: residents, physicians in residency training, and ophthalmology specialists. In the head-to-head comparison with practising doctors, the AI was asked to make a diagnosis and select a treatment from the options provided.
The test included questions on a wide range of eye problems, including extreme sensitivity to light, visual impairment, lesions and itchy and painful eyes taken from a textbook that is not freely available on the Internet and is used to test doctors training to become ophthalmologists.
Overall, the performance of GPT-4 (69%) was superior to GPT-3.5 (48%), LLaMA (32%), and PaLM 2 (56%). GPT4 compared favourably with expert ophthalmologists (median 76%, range 64–90%), ophthalmology trainees (median 59%, range 57–63%), and unspecialised junior doctors (median 43%, range 41–44%). In view of the comparable or even better performance of assistant doctors and doctors in specialist training, the researchers assume that modern language models such as GPT-4 could be used in a variety of ways. For example, ophthalmological consultation and diagnosis are conceivable if patients need to be assessed or access to specialists is limited.
“It is realistic that we could use AI to triage patients with eye problems, to decide which cases are emergencies that need to be seen immediately by a specialist, which patients can be treated by a general practitioner and which do not need treatment,” said first author Arun James Thirunavukarasu, who conducted the study at the University of Cambridge’s School of Clinical Medicine.
ORPHAN DISEASES The mRNA technology made famous by the coronavirus vaccine might also be used to treat hereditary metabolic disorders such as Propionic acidaemia. Results of a Phase I/II study were reported by a research group headed by Stephanie Grunewald from Great Ormond Street Hospital for Children in London and the mRNA vaccine specialist Moderna Inc (10.1038/ s41586-024-07266-7). The rare disease is caused by mutations in the PCCA or PCCB genes, which encode the alpha and beta subunit of propionyl-CoA carboxylase. Dysfunction of the amino acid-, fatty acid- and cholesterol-degrading enzyme leads to accumulation of toxic metabolites, which, if left un -
treated, can result in encephalopathy, lethargy, seizures and coma. So far, 16 children were enrolled in the single-arm dose-finding study and injected with mRNA-3927 packaged in lipid nanoparticles, which compensate for the enzyme defect by expressing an intact version of the protein in liver cells. No dose-limiting toxicities occurred. However, 15 of 16 patients showed treatment-emergent adverse events.
Additionally, eight patients experienced a 70% to 80% reduction in the number of recurrent metabolic decompensation events. As there was no placebo group, efficacy studies are pending in the event of a positive outcome. L

MEAT ANALOGUES Researchers at Lund University have found a way to improve the texture of vegan meat. Current vegan food lacks the fibre structure required to provide the chewiness that people appreciate. “With the help of technology, we want to introduce chewiness into vegetable-based foods by imitating muscle fibres,” said team co-leader Karolina Östbring. At the core of the invention is a programmable extruder with myriads of parameters. Additionally, Östbring and her colleague Jeanette Purhagen reduced the energy consumption of the process by 75%. “It was not possible to patent the discovery, as the whole patent system is based on adding a step, rather than removing and simplifying,” said Purhagen L





HOLLANDBIO The Netherlands laid out its ambitious goals in the field of biotechnology in the recently published National Technology Strategy (NTS), which aims for the country to become a global leader in biomolecular and cell technologies by 2035. Hollandbio welcomed the recognition of biotech as a key technology in the NTS. In a statement published in early 2024, the association urged for a comprehensive, long-term government vision on biotechnology, and emphasised the importance of adequate financing, accessible research facilities, skilled personnel, and effective knowledge transfer for maximising impact. Hollandbio points to the crucial role biotechnology plays in accelerating and realising significant transitions. The associations aims to see these policy intentions translated into concrete actions that support biotech leaders in their journey from lab to society, advocating for immediate action rather than delay.
BIOSIMILARS Medicines for Europe is calling for a future-looking Biosimilar Strategy for Europe to prepare for the upcoming decade, emphasizing the need for more comprehensive development and accessibility of biosimilar medicines in Europe. At the 20 th annual biosimilar medicines conference, the association highlighted the impressive track record of biosimilars in providing significant health benefits and substantial savings to healthcare systems. As the demand for biological medicines grows – currently accounting for 40% of all medicines used in Europe – the proposed strategy aims to connect health actors, streamline regulatory processes, overcome barriers, and expand biomanufacturing. Isabell Remus, Chair of the Biosimilar medicines sector group, stressed the urgency of expanding access to these critical therapies to avoid missing future opportunities.
EXPANDING NETWORKS Comprised of passionate individuals committed to driving positive change, YEBN board brings a wealth of diverse experiences and expertise to guide the association towards new heights of success.
As spring, Young European Biotech Network bloomed this April. YEBN is brimming with an abundance of news and updates. Our annual delegate assembly was held in the Eternal City, Rome, making history by ourselves, we had the pleasure to incorporate three new associations to our community: Biocatalyst Foundation (Baltic states), Magyar Biotechnológus-hallgatók Egyesülete (Hungary), and BiotechClub (Finland). These recent incorporations mark just the initial step in what promises to be a substantial expansion of the association. We took the advantage to find common needs and possible solutions. Exciting developments are on the horizon due to this expansion and exchange of ideas. We are thrilled to embark on new projects in collaboration with all affiliated associations and companies.
But that’s not all that has been brewing in Rome—we also have a new board!
Comprising a full team of seven dedicated individuals, this board marks a pivotal moment in YEBN’s evolution. Their collective vision is set to propel the association to greater heights of success, as they work tirelessly to enhance collaboration and foster advancements within the biotech community.
Moreover, the winds of change are sweeping through YEBN as the association announces a relocation to Italy. YEBN returns to where it was founded more than twenty years ago. This strategic move positions YEBN at the heart of Europe’s biotech landscape, offering unparalleled opportunities for networking, collaboration, and growth. Lastly, but certainly not least, the association has taken a significant stride forward by approving new statutes and internal regula-

Join YEBN and make the difference.

tions. These revisions mark a pivotal moment in the evolution of YEBN, poised to elevate its agility and fortify the organisation’s comprehensive framework. With these enhancements, YEBN is primed to navigate the complexities of the everevolving landscape of biotechnology and ensure that it remains at the forefront of innovation and excellence in its field. So stay tuned to know more about our activities!
As always, in our dedication to fostering collaboration and meeting the demands of the biotech community, YEBN is proactively identifying opportunities to create significant change. That is why is important to continue expanding, association, companies and individual members alike. We invite you to join us in this journey of exploration and discovery. Whether you’re a seasoned professional, a budding entrepreneur, a young scientist, or a curious student, there’s a place for you in the YEBN community.
Your journey begins now!
The stage is set, the momentum is building – YEBN is ready to lead the way in driving positive change and making a lasting impact on the biotech industry. Join us as we embark on this thrilling new chapter together. Your journey with YEBN begins now. If you’re interested in learning more about the association and individuals involved, feel free to visit our LinkedIn page or website!

BIOTECH IN AUSTRIA Against the backdrop of Vienna’s historic charm, BIOTECH AUSTRIA, the association for the Austrian biotech industry, recently invited leading industry representatives to the Vienna Stock Exchange to mark the third anniversary of the association.
The Biotech Circle, which was fully booked, kicked off with a warm welcome from Peter Llewellyn-Davies, president of BIOTECH AUSTRIA. He reflected on the association’s journey and achievements of the past year, including highlights such as the inaugural Biotech Summit Austria in Graz in October 2023. Amidst the positive aspects, the audience was reminded that whilst much has been achieved since the inception of BIOTECH AUSTRIA, there is a wealth of untapped potential within Austria’s biotech landscape that needs to be nurtured. With the impending national elections on the horizon, it is vital that the Austrian biotech scene is put firmly on the agenda.
Diving into the main topic of the event, the motto “Seed and Succeed” was summarized in an inspiring presentation by Dr. Norbert W. Bischofberger, the former
CSO of Gilead Science, Inc., and current President and CEO of Kronos Bio, Inc., who shared valuable insights gathered during his long and impressive career. A focus of the talk was the challenges biotech companies encounter when moving beyond the seed stage, with the notion that “seed is easier than succeed” being particularly applicable to European biotechs. Drawing comparisons between the US and Europe, US biotechs enjoy several advantages such as access to venture capital, a strong financial market, and a better founder mentality. Crucially, US-based funds have access to sources of capital such as pension funds or university endowments. Dr Bischofberger also urged a shift in mindset among European biotech entrepreneurs to be more confident during fundraising and emphasized that success isn’t guaranteed by large teams or organizations, but requires creativity, innovation and a patient-centric focus in drug development.

› June 18, 2024, Vienna (hybrid) Taskforce Finance Meeting
› October 10-11, 2024, Innsbruck BIOTECH SUMMIT AUSTRIA
The challenges, but also the opportunities for European, and in particular Austrian biotech companies when moving beyond the seed stage were taken up in a separate panel discussion. Aside from Dr. Bischofberger and Peter Llewellyn-Davies, Dr. Anna Orlova, Co-Founder and CEO of RIANA Therapeutics GmbH and DI Christopher Trummer, Co-Founder and CEO of Celeris Therapeutics GmbH discussed common issues faced by Austrian biotech companies such as fundraising hurdles, a risk-averse mindset, regulatory complexities, and low company valuations. While seed financing in Austria is accessible due to ample public funding, scaling beyond this stage remains difficult. Panellists stressed the need for legislative changes to ease these challenges and make Austria a more attractive destination for biotech investment. There is also a strong need for Austrian biotech companies to increase their visibility globally.
Despite the obstacles, the entrepreneurial spirit within the Austrian biotech community remains strong. The combination of a supportive local ecosystem, a strong talent pool and abundant scientific achievements fuels optimism for overcoming existing barriers and driving future growth. The 3rd Biotech Circle Austria served as a reminder of the industry’s potential for innovation and collaboration and underscored the commitment to drive the biotech scene forward.
AUTOMATION SLAS empowers the global life sciences community to transform research by bringing interdisciplinary researchers and technology providers together to learn, solve challenges and advance life sciences innovation.
SLAS is the leading global professional society of academic, industry and government researchers coupled with the developers and providers of laboratory automation technology and tools. We bring these stakeholders together to advance all areas of pre-clinical drug discovery, life sciences research, and beyond.
We offer tangible educational resources such as our premier international conference in the U.S. with skill-building pre-conference short courses, the annual European conference with local incentives, our four intensive topical symposia (two in the U.S. and two in Europe), two peer-reviewed journals, and opportunities for professional collaboration, networking and career advancement, as well as support for start-up biotech and lab automation companies.
Our annual Graduate Education Fellowship Grant Program provides grants of up
to $50,000 per year, for a maximum of two years, to outstanding students pursuing graduate degrees related to life sciences R&D. At our conferences, we provide generous travel grants making it possible for students and those in their first five years of their professional careers to present their work as either a podium or poster presentation. We bestow Student Poster Awards to up to three students, and at our international conference we present the $10,000 Innovation Award recognizing the work behind one cutting-edge podium presentation. For our technology providers, we honor the best New Products, and celebrate the most promising startup companies with the Ignite Award.
SLAS Ignite underscores the Society’s foundational commitment to collaboration, en-


› September 11-12, 2024, Cambridge SLAS 2024 Microscale Innovation in Life Sciences Symposium
› October 16-17, 2024, Toulouse SLAS 2024 Sample Management Symposium
abling scientists, academic researchers and business development professionals to build relationships as potential strategic partners. The program includes:
› academic collaboration presentations showcasing the latest research partnerships between academia and industry,
› panel discussions and online discussions focused on entrepreneurship,
› Innovation AveNEW -- a designated area of our exhibition floor for start-up companies fully funded by SLAS, and
› the SLAS Ignite Award given to the most promising start-up company featured on Innovation AveNEW.
We never stop innovating
We seek to support our members to lead the industry, not follow or trail. In 2024 we are working to expand on our Lab of the Future initiatives, creating a functional and interactive experience at SLAS2025 for laboratory technology users at every stage in their lab’s automation integration cycle. Watch for more on that initiative soon!
FIND OUT MORE! slas.org
Sign up for Pointto-point, our weekly e-newsletter, to stay in touch.


CYTOKINES Sino Biological offers a comprehensive array of recombinant cytokines tailored for cell culture to advance research in tumor immunotherapy, stem cell therapy, drug screening, and regenerative medicine. With stringent quality control and extensive validation, we guarantee that our cytokines have high purity, validated biological activity, high stability, and low endotoxin levels. Our recombinant cytokines are available for various species, including human, rat, mouse and others, and support diverse cell types such as stem cells, neural cells, immune cells, and organoids, facilitating functional studies and accelerating scientific discoveries.
Sino Biological is committed to developing high-quality reagents for drug development and clinical research. In addition to RUO-grade cytokines, a series of GMP-grade cytokines with greater stability and higher quality have been developed based on the GMP quality management system, comprehensively assisting the process of cell therapy and drug development.

Sino Biological Europe GmbH
Düsseldorfer Str. 40 65760 Eschborn, Germany
+49(0)6196 9678656
order_eu@sinobiologicaleu.com www.sinobiological.com
2cureX A/S (DK)
AbbVie Inc. (USA)
Acequia Capital (USA)
ActiTrexx GmbH (DE)
Threapeutics (CH)
(F)
Inc. (USA)
Amarna Therapeutics (NL)
Amolyt Pharma (F/USA)
AmphiStar BV (B)
Andera Partners (F)
Asahi Kasei Pharma (JP)
AstraZeneca AB (SE/UK)
Axolabs GmbH (DE)
Axoltis Pharma SA (F)
Basilea Pharmaceutica (CH)
Nordic A/S (DK)
AG (DE)
Kapital GmbH (DE)
Berner A/S (NO) 60
BIOCOM Interrelations GmbH (DE) 39, 43, 59
Biolizard NV (CH/B) 44
BioNTech SE (DE) 61
BioSpring Biotechnologie GmbH (DE) 11
BlueYard Capital (DE) 72
Bluu Seafood GmbH (DE) 71
Boehmert & Boehmert (DE) 9
Boehringer Ingelheim (DE) 32, 57
Bristol Myers Squibb (USA) 27, 61
Bruker SA (USA) 65
Bruno Cell Srl. (IT) 66
Calluna Pharma AB (NO) 44
Carbiolice (F) 82
Carbios SA (F) 34, 82
Cardior Pharmaceuticals GmbH (DE) 61
Carmot Therapeutics Ltd. (AUS) 57
Celeris Therapeutics GmbH (AT) 78
cellVie AG (CH) 24, 25
Chemspec Europe 2024 (UK) 46
CinRx Pharma (USA) 57
Conventus – HUPO 2024 (DE) 47
CRISPR Therapeutics (CH/USA) 32
Cyxone AB (SE) 61
Daiichi Sankyo (JP) 61
DASGIP GmbH an Eppendorf Company (DE) 7
Duality Biologics (CN) 61
EBD Group (CH) 50, 51
Eli Lilly & Co Ltd (USA) 61
EQT Partners AB (SE) 63, 72 Essential Pharma Group (UK) 36
European Biotechnology Network (B) 37
Evotec SE (DE) 27
EY (DE) 31
Faron Pharmaceuticals Ltd. (FI) 44, 60
Ferrer Group Int. SA (ES) 66
Festo Group (DE) 23
FGK Clinical Research GmbH (DE) 17
Fördergesellschaft IZB (DE)
Fumapharm AG (CH)
(AT)
Genmab A/S (DK)
Gilead Science Inc.
10.–11.10.2024 INNSBRUCK Biotech Austria, Human.technology Styria and the Tyrol location agency bring together the key players from the Austrian and international biotech industry and illuminate cutting-edge technology from different perspectives. At the agenda: key notes, panels, workshops, start-up pitches, networking. www.biotech-summit-austria.com

4.6.24
Austrian Life Science Day, Graz (AT) Info: LISAvienna, www.lisavienna.at
10.-14.6.24
ACHEMA 2024, Frankfurt am Main (DE)
Info: DECHEMA, www.achema.de
11.-13.6.24
Renewable Materials Conference 2024, Siegburg/Cologne (DE) Info: nova Institute www.renewable-materials.eu
12.-15.6.24
EuroScience Open Forum 2024, Katowice (PL) Info: EuroScience/ University of Silesia in Katowice/ City of Katowice, www.esof.eu/
17.-19.6.24
RESI Europe 2024, Barcelona (ES)/ +++ online +++ Info: Biocat, https://resiconference.com
19.-20.6.24
Chemspec Europe , Düsseldorf (DE)
Info: Mac Brooks Exhibitions, www.chemspeceurope.com
24.-27.6.24
EUBCE 2024 – 31st European Biomass Conference & Exhibition, Marseille (F) Info: ETA Florence Renewable Energies, www.eubce.com
26.-27.6.24
World Bio Markets – Driving the commercialisation of the bioeconomy, Le Hague (NL)
Info: Paul McDonald, TNP Media Ltd., www.worldbiomarkets.com
30.6.-3.7.24
European Congress on Biotechnology – Grand Challenges for Biotechnology: Health, Food Security and Global Warming , Rotterdam (NL) Info: European Federation of Biotechnology, www.ecb2024.com/
11.9.24
The Future of Swedish & Danish Life Science, Lund (SE) Info: Maria Eriksson, Life Science Sweden, www.swedishdanishlifescience.se
11.-12.9.24
SLAS 2024 – Microscale Innovation in Life Sciences Symposium, Cambridge (UK)
Info: Society for Laboratory Automation and Screening www.slas.com/
24.9.24
Prague.bio Conference 2024, Prague (CZ) Info: Prague.bio, https://conference.prague.bio/
25.-26.9.24
ILMAC 2024, Lausanne (CH) Info: Sandy Mauch, MCH Group, www.ilmac.ch
25.-26.9.24
24 th Annual Biotech in Europe Forum, Basel (CH)/+++ online +++ Info: Sachs Associates, www.sachsforum.com
20.–24.10.2024 DRESDEN HUPO will provide a unique opportunity to present and discuss progress in the interdisciplinary field of proteome research from application of new technologies to advancement of biological knowledge, global health and wellness in a variety of workshops, sessions, training courses and poster sessions. https://2024.hupo.org

8.-10.10.24
CPhI Milan 2024, Milan (IT)
Info: CPhI Global Office, www.cphi.com
4.-5.11.24
European Forum for Industrial Biotechnology & the Bioeconomy – EFIB, Marseille (F)
Info: EuropaBio, https://efibforum.com
14.–15.11.2024 ONLINE The Virtual Theranostic Summit will present experts from academia and industry in the field of theranostics in precision oncology – „Alpha emitters such as Actinium and more, as well as radiopharmaceuticals such as FAP and more“. www.icpo.foundation

11.-14.11.24
esib – European Summit of Industrial Biotechnology, Graz (AT)
Info: acib – austrian center of industrial biotechnology, www.esib.at
14.-15.11.24
ICPO Virtual Theranostics Summit 2024, +++ online +++
Info: International Centers for Precision Oncology Foundation – ICPO Foundation, www.icpo.foundation
27.-29.11.24
CellMAT 2024 – International Conference on Cellular Materials, Magdeburg (DE)/ +++ online +++
Info: Deutsche Gesellschaft für Materialkunde, www.dgm.de/cellmat
3.-4.12.24
BioFIT 2024, Lille (F)
Info: Margaux Satola, Eurosanté, www.biofit-event.com
12.12.24
New Horizons in Biologics & Bioprocessing, Stockholm (SE)
Info: Maria Eriksson, Life Science Sweden, www.bioprocessing.se
CARBIOS SA Groundbraking for of the worldwide first recycling plant in France for PET waste with biotechnology measures and enzymatic breakdown of polymers is a major step in providing circularity ethylene monomers for new products.
CHINA The USA ban Chinese companies. Drug manufacturers would have until 2032 to sever their ties with WuXi AppTec and other Chinese bioscience companies. This is according to an draft of the much publicised Biosecure Act which prohibits companies from working closely with certain Chinese biotech companies, but it now includes a clause to allow existing contracts to be grandfathered until 2032.
I’m in biotechnology because
 DR JULIA SCHAFT, Managing Director,
DR JULIA SCHAFT, Managing Director,
BioRN Clustermanagement, Heidelberg, Germany
“... its a fascinating technology that combines efforts from science and industry to contribute to so many areas of innovation including medicine, energy, sustainability and food supply.”
Chinese companies lead the ADC development pipeline. Antibody drug conjugates (ADCs) are a rapidly growing therapeutic class, and Chinese companies are leading, accounting for half of the top 10 developers by active pipeline count* as they seek to capitalise on potential returns. The significant level of Chinese innovation and presence within the new cancer therapy space underlies the country’s emergence as a key player within the ADC market, according to GlobalData

A little industrial history to round off this issue? In 2016, the company Carbiolice was founded in Clermont-Ferrand, France, on the basis of an industrial line of biodegradable compounds developed by Limagrain Ingredients. Five years later, Carbiolice became a wholly owned subsidiary of Carbios SA. And here an enzymatic solution for the biodegradation of PLA (polylactic acid) was created, called Carbios Active. Integrated directly
into plastic conversion processes, it enables the creation of a new generation of PLA that is 100% compostable, even at ambient temperature, without leaving toxic residues or microplastics. Carbios Active was recently certified by the US Biodegradable Products Institute. With this Food Contact Notification and BPI certification – could it be that the yoghurt pot that only lasts a few weeks longer than its contents is finally within reach? A dream would come true.
BerNardO glavO
FOUNDATION MINGLE Early in May, three of the largest foundations in the world announced that they are joining forces for the first time to support science that addresses global challenges such as climate change, infectious diseases and nutrition and wellbeing.
The three-year initiative was announced at the Novo Nordisk Foundation’s Global Science Summit in Denmark, where each organization committed US$100m, for a total of US$300m. Initial funding will support solutions to address the health impacts of climate change; infectious disease and antimicrobial resistance (AMR); and greater understanding of the interplay between nutrition, immunity, disease, and developmental outcomes. The new funding will also include direct support for researchers and institutions to advance locally relevant research agendas.
AUTUMN EDITION In addition to current reports and background information, the next issue of the EuropEan BiotEchnology MagazinE will cover CDMOs and CROs. We will also be focussing on the events CPhI and BIO-Europe. Want to participate with an advertisement? Just contact Christian Böhm (+49-30-264921-49), Oliver Schnell (-45), Andreas Macht (-54) or please send a mail: marketing@biocom.eu. Publishing date is 26 September 24; deadline for ads is 13 September 24.
source: GlobalData, Drugs Database (Accessed April 2, 2024; *active count refers to pre-registration, phase 0-3, IND, preclinical, discovery);

•Pharmaceuticals
•Fine Chemicals
•Chemical Intermediates
•Agrochemicals
•Custom Synthesis
•Adhesives & Sealants
•Paints & Coatings

•Colourants & Dyestuffs
•Flavours & Fragrances
•Green Chemicals
•Household & Industrial Cleaning
•Biocatalysts
•Bio-based Chemicals
•Additives
•Cosmetics
•Polymers
•Surfactants
•Petrochemicals
•Electronic Chemicals
•and much more

•Agrochemical
•EFCG
•Pharma
•RSC
•Regulatory
Lecture Theatre
•Innovative

For the 11th consecutive year, Worldwide was rated as a high-performing CRO based on primary market research from the Industry Standard Research (ISR) Reports. Not only are we the highest-rated service provider for Project Manager Quality among Phase 2/3 CROs, but we are also the highestrated Phase 2/3 CRO service provider for biostatistics.

