




Partnering to produce high quality, life-changing biotechnological treatments for patients
State-of-the-art mammalian cell culture manufacturing facilities in Europe and Asia for clinical and commercial material, ranging from 150 to 13,000 liters.
Customized solutions and efficient handling for your microbial-based products, with upstream and downstream capabilities ranging from 150 to 40,000 liters.
High-quality plasmid DNA and RNA manufacturing for various types of RNA drugs (including siRNA), mRNA vaccines and innovative gene and cell therapies, up to a scale of 3,000 liters.
Rely on a leading Cell & Gene therapy manufacturer with an excellent team and high-tech facilities.
Producing viral vectors for vaccines or cell and gene therapy in a state-of-the-art facility.

A fully integrated set of services and solutions for aseptic fill and finish of medicinal products. Available packaging systems and volumes include:
• Vials (liquid, lyophilized)
• Pre-filled syringes
• Low-level siliconized syringes
• Droptainers
• Ampules
• Tubes
Manufacturing master cell and working cell banks to enable high-quality clinical and commercial production.
We follow a comprehensive Novartis quality assurance system, with all our facilities operating in compliance with cGMP regulations.
To find out how we can help, get in touch at biotech.cooperations@novartis.com www.novartis.com/globalbiotechcooperations
In a world where biotechnology has been enabling products and processes for decades, it’s time to step out from behind the technology and claim the resulting products and their value in our everyday life. It needs higher visibility and recognition of impact, moving beyond its ‘enabling’ brand which forever makes it sound like the benefits are just around the corner, rather than the existing significant economic and industrial impact.
CLAIRE SKENTELBERY, PH.D., a biochemist by training, has worked in the development of scientific associations for 20 years. She started her career within the UK Cambridge biotech cluster and was a co-founder of the Council of European BioRegions. In Brussels from 2009, she was also Secretary General for the European Biotechnology Network, working across sectors, organisations and countries as part of the mission to facilitate partnerships. Claire became Director General of the globally-focused Nanotechnology Industries Association until November 2020 when she joined EuropaBio as Director General.

Step up ‘Biomanufacturing’. It has relevance across sectors and the language is accessible and tangible. When we speak of biomanufacturing, we speak of employment, skills, products, trade and economic growth. It perfectly fits the evolving narrative landscape for biotech. Within EuropaBio, we see biomanufacturing delivering advanced healthcare, resilient food supply chains, sustainable manufacturing across sectors…the list is a long one and it’s time to speak with a single voice. This comes at a time of critical industrial transition. Industrial processes are responding to climate change, stretched and fractured supply chains, technologies able to deliver ever more complex products and biotechnology is right in the centre.
Europe is one player amongst many in this critical industrial transition. The White House Executive Order on Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy kind of says it all really. Published in September 2022, the US aims to wire up its own complex landscape so that biomanufacturing can flourish. And the US is far from alone. China also has an innovative bioeconomy strategy and can move at a scale and speed beyond both Europe and the US.
What Europe needs, is its own joint plan for biomanufacturing. In the melee of General Pharma Legislation, Net Zero Industry Act, Novel Genomic Techniques, Taxonomy Regulation, Recovery and Resilience plans plus the 27 countries through whom these are delivered, biomanufacturing needs a focus and a strategy across sectors. We know from experience that policy can pull advanced technologies apart rather than give them the critical mass needed to bring investment for impactful outcomes. To that end, EuropaBio has launched a Bio manufacturing Platform, as an expansion of the 26-year-old association, that reflects the changing position and recognition of biotechnology through the lens of biomanufacture. It projects the activities and priorities of our members to deliver the investment and critical mass so crucial to the delivery of biomanufacturing. By the time of publication, we will have hosted our first Biomanufacturing Policy Summit, which will build visibility for biomanufacturing within European industrial and sector strategies. It marks the recognition of an important journey for Europe, one that it has already begun but needs to go faster and further, with a smooth road, clear signposts and the right destination.
Pre-clinical through commercial
Protein biologics | Advanced therapies





























































Choose a CDMO with experience at all stages of the pharmaceutical journey. AGC Biologics provides development, analytical services, and manufacturing capabilities customized to fit your project.







100+
150+
cell culture-based products

8 EFSA: Insect protein is only the beginning
12 Statement: BioNTech leaves; what does Germany do?
14 Interview: José Maria Fernández Sousa-Faro, Chairman of the Board at PharmaMar SA
18 EU stakeholder platform set to speed up clinical trials; Petroleum giants and the greenhouse-gas lie ECONOMY
Nearly thirty years ago, Bill Gates commented that “DNA is like a computer program, but far more advanced than any software ever created.”

That recognition, however, was only the first step on a long road. Reconfiguring cellular or viral DNA to create something entirely new remained stubbornly expensive for decades. Now programmable biology is maturing, and finally looks set to drive revolutions in medicine, chemistry and consumer markets.
80
64 Northern Europe: Sweden, Denmark, Norway and Finland
66 Western Europe: France, Belgium, The Netherlands and the UK
68 Central Europe: Germany, Switzerland and Austria
70 S outhern Europe: Italy, Spain, Slovenia and Portugal
72 Eastern Europe: Estonia, Hungary and t he Czech Rebublic
74 SOS from adipose cells; Modelling fatty liver disease; Gut microbiome affects onset of multiple sclerosis
76 The price of eating ultra-processed food
PICK & MIX
81 News from Biotech Austria; the European Circular Bioeconomy Fund, Young European Biotech Network, Swiss Biotech Association, German Association of Synthetic Biology and the European Biotechnology Network
88 Company index/New product 89 Events
IMPRINT European Biotechnology (ISSN 2364-2351) is published quarterly by: BIOCOM AG, Jacobsenweg 61, D-13509 Berlin, Germany, Tel.: +49-30-264921-0, Fax: +49-30-264921-11, Email: service@ european-biotechnology.com, Internet: www.european-biotechnology.com; Publisher: Andreas Mietzsch; Editorial Team: Thomas Gabrielczyk (Editor-in-chief), Derrick Williams (Co-editor), Dr Georg Kääb, Uta Mommert, Gwendolyn Dorow, Margarita Milidakis, Maren Kühr, Arno Fricke; Advertising: Oliver Schnell, +49-30-264921-45, Christian Böhm, +49-30-264921-49, Andreas Macht, +49-30264921-54; Distribution: Lukas Bannert, +49-30-264921-72; Graphic Design: Michaela Reblin; Production: Martina Willnow; Printed at: Königsdruck, Berlin; European Biotechnology Life Sciences & Industry Magazine is only regularly available through subscription with a BIOCOM CARD. Annual subscription BIOCOM CARD Europe: €80 for private individuals (students €40) incl. VAT, €120 plus VAT for corporates. Prices includes postage & packaging. Ordered subscriptions can be cancelled within two weeks directly at BIOCOM AG. The subscription is initially valid for one calendar year and is automatically renewed every year after. The subscription can be cancelled at any time and is valid until the end of that calendar month. Failures of delivery, which BIOCOM AG is not responsible for, do not entitle the subscriber to delivery or reimbursement of pre-paid fees. Seat of court is Berlin, Germany. As regards contents: individually named articles are published within the sole responsibility of their respective authors. All material published is protected by copyright. No article or part thereof may be reproduced in any way or processed, copied, and proliferated by electronic means without the prior written consent of the publisher. Cover Photo: © Alexander Limbach - stock.adobe.com; ® BIOCOM is a registered trademark of BIOCOM AG, Berlin, Germany.
BIOSIMILARS
Europe has been very proactive in the early adoption of biosimilars, with over 70 authorised in the bloc. But distribution on the continent is uneven. Some countries use them far more than others. Market opportunities abroad are also beckoning, but only a few companies have organised strategies to expand into the Middle East or Africa. If they miss the boat, India and South Korea could slip ahead.
Pressure to focus on sustainability issues are rising for companies in every industry, and pharmaceuticals is no exception. Some firms are trying to meet requirements for environmentally-friendly packaging alternatives. They were in the limelight at this year’s Pharmapack Europe.


As recently as the 1950s, petroleum was seen as a futuristic source of progress and prosperity: Cars became a symbol of freedom, plastics an earmark of progress. Just 70 years later, fossil resources stand for environmental pollution and global warming. The EU Commission is set to ban non-reusable and non-recyclable plastic packaging by 2030, and is aiming to prohibit combustion engines by 2035. In a range of sectors, the potential consequences of the EU Plastics Strategy are already evident. At this year’s Pharmapack event in Paris, sustainability was in the front row (see p. 46). The situation is similar for CDMOs that rely on single-use bags. As Big Pharma demands ESGcompliant services, working on your green footprint has become a matter of survival for companies in the sector (see p. 41). The cosmetics, food and chemical industries have to follow suit. Many biotech firms stand to profit from the Green Deal. Driven by the belief that GDP as a parameter for prosperity can be completely decoupled from resource consumption through efficiency increases and a circular economy, the focus is now shifting to bioengineering and synbio (see p. 20), protein substitutes from the fermenter, along with biomaterials and enzymes that enable degradable plastic substitutes. But regardless of whether the green dream of growth without sacrifice comes true, a new era in biosynthesis has already begun.
Thomas Gabrielczyk Editor-in-Chief

FOOD While the EU market for organic products for the first time stagnated in 2022 by 5%, the market for sustainable imitation meat and fish is booming. By 2030, global revenues for plant, insect, microbial and cell culture-based food is expected to grow from US $4.2bn to US $118bn. In January, the EU authority EFSA approved insect protein as a Novel Food.
At the end of January, the European Food Safety Authority (EFSA) classified consumption of house crickets ( Acheta domesticus) and larvae of the grain mould beetle ( Alphitobius diaperinus) as safe, adding two further insects to the list of insect protein-based foodstuffs. The new alternative sources of protein to meat or fish may be added to bread, pasta, crisps or meat preparations and are expected to have a better eco-footprint than animal meat production that occupies 71% of the global agricultural area for feedstuff production, while food production just occupies 18%.
The European Circular Economy Fund (ECBF) welcomed the decision, pointing to the high market potential of sustainably produced alternative proteins: “The world faces the dual challenge of increasing protein production to feed an ever-growing population while reducing carbon emissions,” stressed Stephané Roussel, partner at the ECBF, that last year co-invested €50m into the Dutch Black Soldier Fly producer Protix BV, along with BNP Paribas, the Prince Albert II Foundation, The Good Investors and existing investors. “While great efforts are being made to reduce losses in food production, there is still a need to develop additional sources of protein,” he added. “Insect breeding is fast becoming one of the most promising ways to meet this demand in the most environmentally friendly way,” according to Roussel.
Insects that can be fed with low-value materials or waste and its mass cultivation require only limited space. This might reduce greenhouse gas emissions according to model comparisons to animal-based protein production, making insects, including the four approved EU products, a potentially sustainable, circular source of protein: Mealybugs and migratory locusts have already been approved in 2021 under the EU Novel Food Regulation (EC 2015/2283). Contract producers for the flour beetle are SAS EAP Group (France), for the migratory locust and the house cricket Fair Insects BV, for the larvae of the grain mould beetle Ynsect NL BV (both: The Netherlands), and for the partially defatted powder of the house Cricket One Co Ltd (UK). However, applications of insects are not limited to the food sector. According to Spanish Tebrio Srl, which will finish construction
of Europe’s biggest mealworm farm, applications of Tebrio molitor go far beyond protein production for the food and feed sectors: insect ingredients such as chitosan for example can be used in water purification, as an additive in the food industry, as a fungicide and fertilizer in agriculture, as a compound to reduce cholesterol in the medical field, as well as an immobilising agent for enzymes. Others can be used to manufacture biodegradable plastics that dissolve in water, production of creams or cosmetics etc.

More than a dozen of insect breeding companies in Europe are recently scaling up production. However, insects are not the only field of bio-based protein production that competes with the already established €5.2bn world market (2021) of plant-based protein alternatives, which is led by the EU with €2.3bn and the US (€1.9bn). According to the brandnew survey “New Food Frontiers” conducted by Danish Lindberg International commissioned by plant manufacturer GEA Group AG among 1,000 chefs, at least 25% of all foods by 2040 will be manufactured using new proteins such as vegan eggs, meat, fish, and dairy products. About 90% of the chefs already perceive growing customer interest for alternative proteins. In the next decade, they see particular high consumer demand for plant based (95%) products, and microbially or cell-culture-produced proteins (45%), but only minor interest (36%) for insect-based protein alternatives. Con -
Sino Biological provides the most cost-effective solution for the rapid production of a large number of antibodies. In response to the rapidly increasing therapeutic needs, Sino Biological combines its expertise in gene synthesis, vector design and transient antibody expression technology to produce highly efficient antibodies in HEK293 and CHO cells in as fast as 2 weeks.
•

sequently, the market is booming, with €2.2bn in capital raised by European protein makers.
Several biotech protein producers such as Finnish Solar Foods (vegan meat), which already gained Novel Food approval in Asia, or German cheese maker Formo Bio GmbH (milk protein), who
raised €50m in a Series A financing, have just begun to scale up production, a field in which GEA also sees growth potential. But this is just the tip of an iceberg that is driven by consumer demand in animalfree protein for ecological (79%), ethical (50%) and health reasons, according to the survey. More than two thirds of the chefs surveyed think that quality improvements – particularly in taste or texture – will be essential for further mar-
A new study named “Politics of protein” published by the Belgian think tank Ipes-Food recommends shift the focus from a protein transition to a sustainable food system transition and sustainable food policies. The expert panel stresses that impacts on biodiversity, resource efficiency, circularity, resilience, sustainable livelihoods, local nutrient availability and food security, territorial cohesion, and food cultures must be taken
into account, alongside GHG emissions, in order to comprehensively assess the sustainability of livestock and fishery systems. To prevent consumer resistance – such as in the debate on genetic engineered crops – due to false claims, they recommend to define a clear set of parameters to assess technologies and realign innovation pathways with the public good. www.ipes-food.org/pages/ politicsofprotein L
ket success. Companies such as Belgian Paleo BV, which produce species-specific myoglobins that mediate meat taste and colour, are building a B2B business. In January, German start-up Project Eaden expanded its seed financing to €10.1m. The company produces edible protein fibres that mimic the texture and appearance of animal flesh ultra-realistically – the machine of the Berlin-based company spins 0.2 millimetre-threads from a stock of vegetable proteins, bundles 250 fibres, like muscle strands, and combines them with vegetable fats. The red and white marbled imitation meat that is almost indistinguishable in appearance and texture from the animal original.

EU discounters such as ALDI or LIDL have now entered the debate about use of insect proteins in their products because in contrast to Asian consumers, EU consumers reject insect-based food admixtures as strange. This shows that European protein producers and policy makers are well-advised not to disappoint EU consumer expectations concerning sustainability and health impact of products because most consumers are highly sceptical concerning processed vs. naturally appearing foods.
Sustainable foods made by means of biotech and fermentation will be in the focus of INDUSTRIA BIOTEC (21-22 September 2023) in Berlin. At the second edition of the European meeting of biotech innovators, politicians and NGOs, stakeholders will discuss how (bio)technology can contribute to mitigate global warming within a broader plan to make food and production systems inherently sustainable and compatible with nature’s circles. On the one hand, companies will stress what kind of regulatory framework might help to sustainably improve Europe’s ranking in protein alternatives, decarbonisation of industrial production, resource conservation and circularisation in different sectors. On the other hand, policy makers discuss how to achieve a sustainable system. L
t.gabrielczyk@biocom.de

STATEMENT In soccer, such a transfer would raise tempers: A superstar leaves his team because he no longer trusts it to succeed. A warning sign, a headline event, fodder for weeks of debate in the media. Something similar has just happened to the biotech industry, but things remain strangely quiet in the fan block of Germany’s top research – whispers at best, just whispers, but no outcry anywhere.
BioNTech, which has become the superstar of mRNA research with its corona vaccines, wants to cause a sensation in the UK and develop personalised mRNA cancer immunotherapies and new vaccines there. In addition to 70 highly qualified scientists moving to the island, a regional headquarters will be established in London – with such central departments as Regulatory Affairs, Medical Affairs, Intellectual Property and Legal.
It all sounds like systematic planning for the next big thing. Except, of course, that it will be British. And what happens here? A bit of whistling in the woods that BioNTech is by no means turning its back on Germany. According to experts, the Mainz-based company is moving to Great Britain because of the prohibitive and increasing bureaucracy, lack of skilled workers and slow processes in Germany (see p. 18).
Otherwise: isolated complaints about dwindling competitiveness that usual appeals to politicians. This is nothing new, and much has already been done. Is not Rhineland-Palatinate a prime example of good biotech support? For an innovation climate in which science and industry, university research, startups and pharmaceutical companies cooperate for the benefit of all? And yet, Mainz’s flagship company chose the British. Someone must have put even
Dr Ute Kilger and Elmar Jehn

better cards on the table or made the appropriate framework conditions possible. As Bio NTech co-founder Prof. Dr Özlem Türeci said diplomatically, “BioNTech relies on the strengths of the respective countries for research and development.”
Touchez! But what exactly are the German and European non-strengths or weaknesses? Clinical research and development for cell and gene therapies are now almost exclusively in the USA and Asia. Are other areas now migrating? The lament about more difficult data situations, regulatory freedoms, and risk averse investors in Germany is as
understandable as it is well known. Of course, all of this needs to be analysed, addressed, and directed into the proper channels with the necessary pressure.
And preferably at Germany’s pace, i.e. subito, as Chancellor Olaf Scholz just demanded during a BioNTech visit. What is possible for LNG terminals must also apply to research, he said. “We must make faster approval procedures possible for factories, new medicines, research projects and the use of research data,” he said. He added that the German government “now wants to help the medical industry and healthcare industry make progress in a very short time with many very concrete legislative projects.”
So far, so hopeful. But all this threatens to come to nothing if something fundamental is lacking: The industry’s self-confidence, the broad chest that can only be shown by those who fundamentally believe in themselves and the opportunities for Germany as a business location.
Ugur Sahin and Özlem Türeci have been elevated to exceptional researchers, celebrated, admired and highly respected for their success in the historic situation of the COVID -19 pandemic. Self-starters, so to speak. But are there not many Sahins and Türecis in laboratories in this country? World-class scientists, passionate doers, highly idealistic physicians? We hear too little about them; instead, it seems the industry is
labouring under a strange kind of despondency. Pharmaceuticals do not have a good reputation; distrust rustles through every package insert.
So it is better to keep a low profile and not appear too wide-eyed? The crux of the matter is that anyone who behaves this way finds it challenging to generate the economic and political support they so urgently need. Politicians, in particular, are prone to opportunistic reflexes. Why go out on a limb for a cause that cannot win a pot of gold in the public eye?
The ball is already in the court of the industry itself. It must find itself, become louder, more offensive. It must make clear to the public what an immense contribution it can make to the well-being of people – if only it is allowed to. If the industry doesn’t like political developments that lead to anti-innovative conditions, it must engage in public discussion and amend public perception.
This is another lesson from the COVID-19 pandemic: research and science must face the challenges the media poses. They must explain, they must advertise, and they must speak the language of the people. And they must not shy away from addressing their doubts, uncertainties and conflicts. This is the only way they can prove their relevance and credibility. On the other hand, what is not possible is to keep one’s feet still. No, when one superstar leaves, the others must run all the more.
CLINICAL STUDIES At the beginning of January, BioNTech SE announced a strategic partnership with the UK Government to provide up to 10,000 patients with personalised mRNA cancer immunotherapies by 2030, either in clinical trials or as authorised treatments. To achieve this, the parties plan to utilise the UK’s clinical trial network, genomics and health data assets. The next steps of the collaboration will be the selection of candidates, trial sites and the set-up of a development plan with the aim of being ready to enrol the first cancer patient in the second half of 2023. “The fact that BioNTech is now going to the UK is a signal that German politicians cannot approve of,”. commented Martin Krauss, the Chairman of the Board of BVMA e.V., which represents the interests of Contract Research Organizations (CROs) operating in Germany. BioNTech told EuropEan BiotEchnology that “the UK is an excellent partner for collaboration for a number of reasons: the country has succeeded in rapidly approving COVID-19 vaccines and rolling out the vaccination campaign; the country is an international leader in genomic analysis of cancer patients and is very experienced in conducting clinical trials in both oncology and infectious diseases.”
SigmaPlot® is an easy-to-use scientific graphing & statistical data analysis software package for researchers, scientists and engineers who need to create precise, publication-quality graphs that best communicates their research results for presentations, technical publications, and the web. Along with advanced curve fitting, a vector-based programming language, macro capability and over 50 frequently used statistical tests, SigmaPlot also provides more than 100 different 2D & 3D graph types from which one can choose a full range of graphing options such as technical axis scales, multiple axes, multiple intersecting 3-D graphs and much more. SigmaPlot now has SigmaStat included with it which is a perfect tool to visualize and understand basic and advanced statistics.
SigmaPlot offers:
•Complete Advisory Statistical Analysis
•Award-Winning Technical Graphing Capability
•Powerful and Easy-to-use Data Analysis
•Ability to Customize every Element of Graph
•Precise Publication-Quality Graphs
•Ability to Publish your Work Anywhere
Latest updates:
•Includes a New Heat Map Macro
•Removes all dependencies on old redistributable by removing Lead Tools and uses Windows Graphics Device Interface + (GDI+) for graph export
•Uses the latest Sentinel License Manager which is compatible with the latest Microsoft Server 2022
•Uses a hosted licensing service for smooth license activation and validation
•Has a new and refreshed ribbon manager that enhances the already commendable user experience in SigmaPlot
North America & South America
Inpixon USA
2479 E. Bayshore Rd, Suite 195. Palo Alto, CA 94303, United States saves.sales@inpixon.com
Germany, Austria, & Eastern Europe
Inpixon GmBH
Königsallee 92a, 40212 Düsseldorf, Germany saveskontakt@inpixon.com
UK & Western Europe
Inpixon UK Ltd
268 Bath Road, Slough, SLI 4DX, UK saves.sales@inpixon.com
©2022 by SYSTAT Software, Inc. SYSTAT, SigmaPlot, SigmaStat, TableCurve2D, TableCurve3D and PeakFit are trademakes of SYSTAT Software, Inc. All other product or brand names are trademarks or registered trademarks of their respective holders.
PHARMAMAR VS. EMA In 2016, PharmaMar filed a European marketing authorisation application for Aplidin as a treatment for patients with multiple myeloma. After the European Medicines Agency rejected the MAA twice, which was accepted in Australia with the same data, PharmaMar sued the EC. EuropE an BiotEchnology spoke with PharmaMar’s Chairman José María Fernández Sousa-Faro.
EuroBiotech _The cancer drug Aplidin (plitidepsin) is approved in Australia as a 4th-line treatment for patients with relapsed and refractory multiple myeloma, but not in Europe. How did the regulatory authorities justify this on the basis of the clinical data submitted?
Sousa-Faro_The data submitted to both agencies were the same. EMA’s rapporteur and co-rapporteur recommended the approval of plitidepsin with no outstanding issues. Then inexplicably, the Committee for Medicinal Products for Human Uses (CHMP) summoned an oral explanation in a short period of time making use of a deliberately predated document.
The Chairman conducted an incredible oral hearing in which the Swedish and Danish CHMP´s members attacked the recommendation of rapporteur and corapporteur leading to a negative vote of the majority with no need to justify it. Besides, it was contrary to the recommendation of rapporteurs. As far as we know, there is no precedent. In Australia, the authorities with the same Phase III study that we presented to the EMA concluded that the drug was safe and there was a positive benefit for the patients, where some stages of the disease had not many treatment options.
EuroBiotech_How did development continue in Europe after orphan drug status was granted in 2003?
Sousa-Faro_The development continued because we had the basis to believe that it could be a drug for multiple myeloma, so our objective was the registration for marketing authorisation. We conducted a Phase I and II and a Phase III study. The results were positive and that was precisely what encouraged us to register Aplidin in
SOUSA-FARO holds the position of Chairman of the Board of Directors of Pharma Mar SA and Chairman of Fundación Bankinter para la Innovación. He has been a member of the Board of the following companies: Antibióticos SA, ICI-Farma SA, Transportes Ferroviarios Especiales SA; Pescanova SA; Cooper Zeltia SA; Biolys SA; ICIZeltia SA; Penibérica SA; Banco Guipuzcoano SA and Zeltia SA. SousaFaro has a degree in Chemistry (1967) and a PhD in Biochemistry (1971) from Universidad Complutense, Madrid. Between 1971 and 1979, he was tenured professor and subsequently held the Chair of Biochemistry at the University of Santiago de Compostela. MBA from IESE (Universidad de Navarra), Madrid (1986-1987). He has over 100 academic publications and patents in the fields of biochemistry, molecular biology, and anti-infective and anti-tumour drugs.

the EMA and to other regulatory agencies. At that time, due to the good tolerance of plitidepsin, that allowed its addition to other three drugs, we invested in a quadruple combination with bortezomib, lenalidomide, dexamethasone and plitidepsin for multiple myeloma. This study was abandoned after the EMA’s refusal.
EuroBiotech _What reasons did the EMA give for refusing marketing authorisation in 2017 and in the appeal procedure in 2018?
Sousa-Faro_There were no real differences between the examination and the reexamination, the speech at the CHMP was always the same, despite the fact that a total of three of four rapporteurs and co-rapporteurs were positively recommending the approval. As usual, they stated that the benefit/risk ratio was negative. Other previously approved drugs in multiple myeloma had a similar profile.
EuroBiotech_How did PharmaMar assess this and why did PharmaMar initiate proceedings before the ECJ?
Sousa-Faro_During the evaluation procedure, we witnessed things that we thought were out of the usual procedure. As I said, they recommended us a method that later they did not accept, additionally there were errors of form. However, the most surprising was that the CHMP bypassed the rapporteur and co-rapporteur’s positive opinion, which is unusual and unique. We asked for a reexamination. Here, it is worth recalling the Swedish nationalities and Karolinska Institute´s training of the four members, who were key to the EMA’s negative opinion on plitidepsin. Tomas Salmonson, chair of the EMA’s CHMP, the rapporteur of the appeal, Filip Josephson,


the chair of the Scientific Advisory Group (SAG) assessment, Jonas Bergh, and SAG’s multiple myeloma expert, Hareth Nahi, who between 2015 and 2021 served as Senior Medical Advisor to the Swedish company XNK Therapeutics AB; a very high endogamy. I would like to highlight that the EMA does not have a compliance department as the FDA does, that verifies the declarations of the members regarding their independence.

Sousa-Faro_In 2020, the Court ruled in favour of PharmaMar, annulling the decision and ordering the European Commission (EC), the EMA depends on EC, to pay costs. The Court concluded that there was no guarantee that members of the EMA were free from a conflict of interest with the pharmaceutical industry. The EC did not appeal, so they accepted the sentence but Estonia and Germany did it in 2021, joined later by the Netherlands and the EMA itself as well. The question still unresolved here is: Why did Estonia and Germany appeal in opposition to the criterion of the only European institution concerned, the EC? Even more so when the Estonian CHMP abstained from voting in the Aplidin process. Last January, the Advocate General of the EU published his opinion about the case. His opinion was based on the fact that there was no conflict of interest because the Karolinska Institute is not a company; we all know that and this is not the point. The point is the SAG member that was working at that time for a company; XNK Therapeutics was linked to the Karolinska Institute. The Advocate General argues also, that there are multiple drugs for the treatment of multiple myeloma, which in his view should have led the judgment under appeal to declare the “rival products” rules on conflicts of interest inapplicable. However, he ignores that with respect to a drug there are first, second, third, fourth and fifth lines of treatment, so that competition per line is very limited, normally one or two, contrary to what the Advocate General says. Therefore, he inexplicably argues that since there are many treatments for multiple myeloma there is no competition from any product.
EuroBiotech _What is PharmaMar’s view on this and why and what did the EMA say in the proceedings?
Sousa-Faro_For us it is very clear. Our claim to the Court of Luxembourg had five allegations. The first one was conflict of interest and for which the Court ruled saying “the procedure that led to the adoption of the contested decision did not provide sufficient guarantees to exclude any legitimate doubt as to possible bias”.
It was also very clear for the Court because they did not examine the rest of the claim that referred to: breach of the principle of good administration, the principle of equal treatment, infringement of the requirement to provide a statement of reasons and infringement of the right of defence.
EuroBiotech _What is the explanation for the differences in the approval of Aplidin in Australia versus Europe?
Sousa-Faro_The Australian authorities can grant the independence of the members and therefore of the process versus EMA who has a sentence of the General Court of the EU for conflict of interest, an institution with the lack of a compliance department. This could be resolved with clear, transparent rules and if the decisions made by each member state belonging to the EMA were public and of course the motivation of the vote as happens in the oral hearings of the FDA that are of free public access.
EuroBiotech _What is the next step from PharmaMar’s point of view?
Sousa-Faro_We will fight until the end. Meanwhile, we are waiting for the Court of Justice of the EU to rule, from there we will take the decisions we have to take. If the Court again gives us the reason, the EMA has to re-evaluate the drug as it was left in 2017. In the event that the Court follows the opinion of the Advocate General, he recommended to cancel the previous sentence and to send the case back to the General Court, which will have to examine the other four points of the lawsuit that we filed against the EMA. L
t.gabrielczyk@biocom.eu

GLOBAL WARMING About a year and a half ago, a US Congressional Subcommittee questioned executives from ExxonMobil, BP, Chevron, Shell and US American Petroleum Institute about industry efforts to downplay the role of fossil fuels in climate change. Darren Wood, a member of Exxon’s board of directors, testified before MPs that the company’s public statements “have always been and continue to be truthful” and that the corporation “does not spread misinformation regarding climate change”. Internal company documents from the past 60 years indicate otherwise. A new study adds weight to ongoing legal investigations against ExxonMobil and partners for deliberate climate malfeasance.
For decades, the members of the oil industry tried to convince the public that a causative link between fossil fuel use and climate warming could not be made because the models used to project warming were too uncertain. As early as 1977, climate scenarios of ExxonMobil predicted global warming as a result of burning fossil fuels with an accuracy of 63% to 83%. This is confirmed by a German-US-American team’s analysis of the company's internal forecasts, that became public in 2015 (SciEncE, doi: 10.1126/science.abk0063).
According to study co-author Stefan Rahmstorf of the Potsdam Institute for Climate Impact Research, Exxon’s projections predicted global warming of 0.20 ± 0.04 degrees Celsius per decade as early as 1977. According to the study, the internal analyses accurately predicted when human-induced global warming would first be detected in measured data, and even realistically estimated the carbon budget for limiting warming to below 2°C.” Currently, Big Oil is greening its image with biotech partnerships. Exxon’s internal research from 1979 found that if the industry abandoned fossil fuel use and focused instead on renewable energy, this could reduce fossil fuel pollution in the 1990s and avoid a major climate crisis.
CTIS A new platform is set to facilitate the exchange of information on the optimisation of the electronic reporting system on clinical trials CTIS next year.
Following strong criticism ahead of the mandatory launch of the CTIS electronic clinical trial reporting portal by the European Medicines Agency (EMA), the EU SME Office has now launched a public consultation on the development of a multi-stakeholder platform to foster collaboration on improving clinical trials in the EU, as envisaged in Priority Action 3 of Accelerating Clinical Trials in the EU.
In particular, an alliance of German biopharma, physician and CRO associations as well as the ethics committees had complained before Christmas 2022 about technical errors making the portal “partially unusable” during the one-year transition phase. If these were not eliminated by the start of the portal on 1 February 2023, the goal of shortening the time to approval of a clinical trial would be missed and Europe would fall further behind the USA, Asia and the UK as a trial location, the Working Group of Medical Ethics Committees in the Federal Republic of Germany (AKEK), German University Medicine, the Network of Coordination Centres for Clinical Trials as well as
the German pharmaceutical associations BAH, BPI and vfa, the German CRO Association BVMA, the Association of Medical Faculties, the Association of University Clinics and the German Medical Association had complained. After the launch of CTIS, the EU pharma federation EFPIA, which was fierce behind the scenes, had thanked the EMA for its efforts in fixing the technical bugs and system errors.
The new platform will now host the previously public dialogue among stakeholders on clinical trials and facilitate the development of the clinical trial environment, according to a statement. It will help communicate advances in clinical trial methods, technology and science and serve as a neutral space to discuss “challenges” and “develop practical solutions”. There will be several development phases leading up to the final design of the platform, which have been summarised in a concept paper. The aim of the consultation, which runs until March 3rd, is to gauge interest in the platform, get feedback on priority discussion topics and gather comments on the proposal. The kick-off meeting of the multi-stakeholder platform will take place in Q2/2023.
In mid-January, the Good Clinical Trials Collaborative (GCTC) called for less bureaucracy and modernisation of clinical trials. The World Heart Federation, the two US-American heart associations AHA and ACG as well as the European and three German Cardiovacular Societies (DGTHG, DGK, DGPK) call for less bureaucracy in the planning and conduct of clinical trials as well as the possibility of using national electronic health records for the selection of subjects and validation of randomised clinical trials (RCTs). The ICH regulations have made these too complex, costly and lengthy, leading to frequent trial cancellations.


Innovative technology is the key to shifting from a fossil-based to a bio-based circular economy, crucial for achieving the European climate targets.

As a venture capital fund, our primary goal is to invest in scale-up companies with high potential for excellence on a pan-European or global scaledelivering both impact and financial returns at the highest level.

Our entire experience in venture capital and our investment scope spanning the circular economy and bioeconomy is focused on this purpose.

Supported by:

Investors:


BIOTECH Way back in 1995, Bill Gates commented that “DNA is like a computer program, but far more advanced than any software ever created.” That recognition was just the first step on a long road. For many years, reconfiguring cellular or viral DNA to create something entirely new was simply too expensive. Finally – two decades after its inception – programmable biology is set to drive revolutions in medicine, chemistry and consumption.
Although many people view genetic engineering and its advanced cousin – synthetic biology – as unnatural, a bio(tech) wave is just beginning to crest. Synbio products are rapidly permeating societies and by 2030, it’s very likely that you’ll have eaten, worn, used or been treated with at least one. Two decades after the human genome was comprehensively deciphered, DNA engineering looks set to become the dominant 21st-century technology. Products that involve reading, writing and editing DNA to redesign existing systems found in nature are already well advanced, or about to enter the market. They include synthetically produced viruses (among them polio, flu or SARSCoV-2), AI sequence-optimised mRNA vaccines, genetically engineered CAR-T cells, living therapeutics released to restore the gut microbiome after disease biomarkers have been detected, gene-scissored crops, programmed production strains for chemical manufacture of chemicals, microplastics-free fibres and biofuels. The field is already changing medicine, agriculture and – to some extent – petroleumbased chemistry.
“Since the publication of the first papers in natur E in 2000, synthetic biology has diversified so much,” says Martin Fussenegger from the ETH Zurich/University of Basel. Five years ago, he healed

mice of both gout and diabetes by means of encapsulated gene circuits. By 2021, scientists had created the first self-replicating xenobot – a programmable syn-
!We have some catching up to do, especially in the transfer from academic research – where Europe plays a serious role in many areas –to commercial application. Incentives are needed for researchers, venture capitalists and critical infrastructure managers to exchange more. Policymakers also seem to be still divided, despite success stories like Impossible Foods or BioNTech.
thetic organism derived from frog cells and designed by artificial intelligence (doi: 10.1073/pnas.2112672118). According to Fussenegger, “synbio has evolved to become the engineering science of biology, similar to chemical engineering for chemistry.” Depending on how and where they are applied, human-designed DNA technologies have the potential to stop the destruction of the environment and the climate catastrophe through new production methods, to offer healthy alternatives to factory farming, and to restore natural cycles that have been thrown out of balance by toxic fossil chemistry and the endless quest for profit. For instance, continuing to produce polyethylene, a material that persists for hundreds of years in the environment before breaking down into toxic microplastics, is not a good idea, even if it is made from waste or industrial emissions. It’s now time to move away from the old ‘jobs and growth’ mentality of the petroleum age, because permanent growth is neither natural nor sustainable. Economists like Maja Goepel, who has advised the German government, believe that the current economic paradigm must change in ways that allow companies to make profits without destroying frameworks.
According to Galya Laskar’s Equity Research team at Barclay’s Investment Bank, “consumer goods are the next big thing”
in synthetic biology, with animal-free meat, fish and dairy products at the forefront (see EuropE an BiotEchnology 2/22). Analysts at Vantage Market Research valued the synthetic biology market at more than US$10bn in revenue in 2021. As the commercialisation rate of academic synbio inventions is significantly higher than in any other product segment, they predict the market will triple to US$32.7bn by 2028. Among the most rapidly developing fields of innovation: medicines and diagnostics, sustainable plastics and petrochemical substitutes, and microbial or cell-culture-based foods. The US, China and the UK are leading the pack, followed by the Netherlands, Denmark, Switzerland, Germany and France. A recent McKinsey report predicts synbio applications will hit US$4tn annually by 2040, providing treatments for 45% of the world’s current disease burden and 60% of the world’s physical input.
Progress using synbio tools in combination with AI happened first in drug discovery and rational drug design. During the COVID-19 pandemic, mRNA vaccines were developed by BioNTech and Moderna that were genetically optimised
to improve intracellular stability and antigen expression, while reducing mRNA toxicity.
“The time has come for synthetic biologists to develop more real-world applications. The field has had its hype phase. Now it needs to deliver.”
Following the complete synthesis of polio and flu viruses by bioengineers, an attenuated codon-deoptimised SARS-CoV-2 virus (carrying all virus antigens, and thus mutation-insensitive) is currently being tested within the WHO’s Phase III Solidarity trial by Merck, Sharp & Dohme and Codagenix Inc. One drawback of codon deoptimisation compared to genetically optimised DNA or mRNA (i.e. cancer) vaccines may be that there is a trade-off in production between the degree of attenuation and viral recovery. The optimal mutational load may also need to be empirically determined for each virus. Another downside of new technology: it could also be used against humanity. Although researchers headed by David Evans were criticised for publishing a work-
flow in 2018 on how to resurrect the eradicated horsepox virus from synthesised DNA, their work demonstrated that viral bioweapons could theoretically be reconstructed via reverse genetics.
On the diagnostics side, paper-based toehold-switch RNA-sensor diagnostics have been able to detect femto molar amounts of viral nucleic acid, i.e. gut micro biome bacteria (including C. difficile) at a cost of just 0.1-1US$ per test. Switch RNA sensors in their off state have a stem-loop structure that prevents reporter gene translation. Target RNA binding to this toehold allows downstream translation. These gene circuits are freeze-dried on paper, stable for 12 months at room temperature, and can be administered anywhere – even sites with no hospital infrastructure.
Two FDA-approved CRISPR-based diagnostics for detecting SARS-CoV-2 RNA sequences through cleavage at specific sites and subsequent colour reaction with an attomolar sensitivity were marketed by Sherlock Biosciences and Mammoth Bio sciences during the pandemic. And yet another success using synbio has been engineering CAR-T cell therapies, which made US$2bn in revenues as 2nd and 3rd -line blood cancer treatments in 2022. They are expected to grow to
High-profile seed investors in synthetic biology include Bill Gates (Microsoft), Eric Schmidt (Google), Vinod Khosla (Sun Microsystems), Jerry Yang (Yahoo), Bryan Johnson (Venmo/Kernel), Peter Thiel and Max Levchin (PayPal).
strain engineering for industrial production, medicine, fashion / machine-learning genome mining for chemical-producing GMOs
Number of publications on synthetic biology (blue: 2000-2017, red: 2017-2022)
produce annual turnover of US$10bn by 2030. Traditionally, CAR-Ts link a singlechain variable fragment (scFv) to a CD8 transmembrane domain, an intracellular CD3 domain from the T cell receptor, and a co-stimulatory domain. When a target antigen is bound by the scFv, activation of both stimulatory and co-stimulatory domains is required to promote T cell proliferation and target-cell killing. As cytokine storms are common, biologically engineered control systems such as logic gates and split safety switches – like those developed by Bellicum Pharmaceuticals or Cogent Bio – are under development to balance safety and efficacy.
Another field of medical innovation through synbio is gut dysbiosis and its associated pathologies, which include inflammatory bowel disease, multiple sclerosis, ADHD, hypertension and other conditions. Living therapeutics such as rationally designed microbes and synthetic microbial communities also offer a platform for treating inherited disorders such as phenylketonuria and cancer, as well as communicable diseases such as chronic C. difficile infection (Seres Therapeutics).
Many companies have begun to combine synthetic biology and AI. “Whether in protein design, drug discovery or the optimisation of production strains that produce chemicals or animal-free protein from residues or CO2, AI and synthetic biology play together everywhere,” says Hendrick Cooper from the German Association for Synthetic Biology (GSAB). Together with the European Synthetic Biology Society (EUSynBioS), the GSAB is fighting for more acceptance and bet-
ter conditions for the research in Europe. “Scientists from Europe have been playing an important role in progressing the field, as their contributions rival the amount of literature produced in the US and other parts of the world, with the UK and Germany having the highest publishing output,“ stresses EUSynBioS head Stefano Donati from the The Novo Nordisk Foundation Center for Biosustainability. In contrast, the US has been the dominant driver in establishing industrial solutions, filing more than 50% of global patents and investing ten times more than Europe. But Europe could still catch up, provided policy makers support SMEs to scale up production of sustainable plastics, chemicals and protein alternatives – the next big market opportunities – and provide fast-track market approval like Singapore does. Many European food and feed innovators intend to target Asian markets first with their sustainable products.
According to Donati, the European Investment Bank (EIB) has launched ESCALAR, a €300m pilot programme focusing on providing finance for the scale-up companies. It was complemented in 2022 by an EIB committment of €500m for the European Tech Champions Initiative (ETCI), a multi-investor fund structure aimed at fostering growth in innovative European tech companies. Additionally, the Circular Bioeconomy Joint Undertaking (CBE JU) contributed €215.5m last year to finance projects. The eureKARE – a €60m fund – has invested in multi-

High Quality Grade Plasmid & Minicircle DNA




Customized High Quality Grade DNA for GMP production of viral vectors, RNA and CAR-T cells QC including CGE service





















pDG/pDP plasmids for AAV production

2 plasmid system
Serotypes including AAV8 & AAV9 GFP transfer plasmids

ITRRESCUETM In Stock service
The better way to DNA! Coming soon

› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2023
› Guide to German Biotech Companies 2023
› conveniently accessible ePapers
› Discount for BIOCOM events for 1 person
› additional partner offers
Price: 80 Euro p.a. (incl. VAT)
Students 50% discount (subject to proof of enrolment)
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2023
› Guide to German Biotech Companies 2023
› conveniently accessible ePapers
› Discount for BIOCOM events for 2 persons
› additional partner offers
Price: 120 Euro p.a. (plus VAT)
ple AI/synbio/microbiome-driven companies since 2021, among them biomanufacturing SPAC eureKing, French DNA synthesis company DNA Script, gene therapy delivery specialist Coave Therapeutics, Fecal Micro biota Transplant expert MaaT Pharma, live biotherapeutic product developer NovoBiome, food specialist Gynov and DNA data-storage expert Biomemory. Additionally, it supports cell-free manufacturing specialist EonBio and German Dirac Bio sciences at its Belgian biopharma suite. The latter develops gene circuits that release cancer drugs upon detection of specific cancer biomarkers.
Big players like Novozymes, Cargill, BASF, L’Oréal (see p. 46) and others are currently greening their product portfolio with partners like BRAIN Biotech, which use genetic engineering to mine metagenomes, finding and optimising new enzymes or production strains. There’s a wide range of such cell factories.
With help from engineered Clostridia chassis, companies like Lanzatech can already commercially produce ethylene and ethanol from industrial emissions or gasified organic waste. Using engineered bacterial enzymes, French Carbios SAS can recycle polyethylene terephthalate (PET) bottles and textiles down to the original mono mers at commercial scale. And German microbial nanocellulose maker Bioweg and US synbio contract reseach organisation Ginkgo Bioworks partnered in February to reduce microplastic waste. Their approach is to optimise bacteria that produce cellu lose to replace naphtha-based polyethylene, acrylates and polystyrol, which remain not accessible to enzymatic recycling (see p. 42). Most recently AMsilk, which produces natural high-performance biomaterials from recombinant silk protein, entered into a protein optimisation partnership with BRAIN Biotech, which has a wide range of such projects over different industries, including microbial lithium recycling.
The Next Big Thing is vegan protein alternatives, such as casein produced in optimised bacteria by vegan cheesemakers Formo Bio or Standing Ovation SA, the winner of last year’s INDUSTRIA BIOTEC start-up pitch, the sector’s annual meeting in Berlin (see EuropE an BiotEchnology 4/22, p. 59). Finnish Solar Foods has already received a Novel Food authorisation in Singapore for its protein alternative Solein, which is made from CO2 and regenerative energy. US Company Impossible Foods and Belgian Paleo BV have engineered microorganisms to produce leghemoglobin or species-specific myoglobins, respectively, which when added to vegan meat improve meaty flavours, colour and aromas. The B2C approach sold to vegan protein-makers is set to revolutionise the market for CO2-neutral, animal-free meat alternatives made with food additives copied from nature rather than unhealthy chemical supplements that may cause negative health effects in ultra-processed foods (see p. 76). Business approaches with ingredients or materials designed by nature, but produced sustainably and circularly with the help of synthetic biology, offer a way to unite the hitherto opposing interests of eco- and biotechnology stakeholders. If vegan products can be established worldwide in a CO2-neutral fashion, following nature’s example, part of the +70% of agricultural land currently used for concentrated feed production can be freed up for organic cultivation.
Mandatory designation of the seeds used could protect organic farming from unwanted contamination by GM and geneedited plants obtained through targeted biological mutagenesis. Only that kind of regulation will allow coexistence for the two types of farming on equal terms.
The technology has delivered. Now it’s time for politicians to step up and create a sensible framework that does not serve capital interests alone. In funding science, Europe can continue to focus just on the interests of chemical giants or seed- and crop-protection companies, or it can finally deliver a real Green Deal that reconciles business and nature. L
t.gabrielczyk@biocom.de
New product launch: The Bioprocess Autosampler
Delegate bioprocess sampling
Regular measurement of bioprocess parameters is the basis for process optimization.





The Bioprocess Autosampler from Eppendorf facilitates sampling 24/7 in short and regular intervals and like this gaining complete datasets.
> E ciency: Enables regular sampling 24/7

> Space-saving: No sterile hood is necessary for operation
> Flexibility: Compatible with di erently sized glass and single-use bioreactors operated with a DASbox ® Mini Bioreactor System or a DASGIP ® Parallel Bioreactor System


DR MARCUS WIEPRECHT, STIFEL EUROPE BANK AG From its all-time high in August 2021 the largest and most important index for the biotech industry also in Europe (NBI) lost around 25%. After a period of excessive capital inflows, money is much harder to come by these days. The answer can only be more creativity in structuring deals.

There are many reasons behind this quite hard and persistant landing. For sure, too many early-stage companies went public and find themselves today with limited cash runways combined with limited options to access fresh capital, resulting in a near record high number of c.200 listed biotech companies globally (out of a total of c.850) trading below their cash levels (i.e. a negative enterprise value).
However, Biotech stocks trade on longterm expectations for future cash flows and therefore trade inversely to interest rates, which have seen a record-breaking rise in the last months. As it turns out, inflation may not disappear quickly and higher interest rates may stay for longer. Have the fundamentals of the biotech industry changed after all? Probably not as there is a stronger than ever innovation power and leading-edge technology out there. But markets probably overshot on the upside, and now we are living the consequences.
Money is certainly available. However, going forward, greater scrutiny from public, private and venture capital investors can be expected on how capital is deployed. Successful biotechs will ensure a clear connection between capital allocation and value creation in R&D. Against this backdrop, transaction activity and average deal values continue to decline from 2021 highs through the beginning of 2023. As inflation remains elevated, Central Banks likely maintain their hawkish stance and current market conditions, if they persist much longer, will force issuers in need of capital to make tough decisions.
In summary, a prolonged buyers’ market means the opportunities to support issuers will require increasingly investor-friendly terms. Strong companies with recurring revenues, profitability, and compelling uses of proceeds can find success in accessing the record amounts of investors’ dry powder even in this challenging market.
Hookipa Pharma Inc. Vienna-based company (NASDAQ: HOOK) has received a US$10m milestone under its 2022 agreement with Roche to develop an arenaviral immunotherapy for KRAS-mutated cancers. The non-dilutive milestone payment was due for
the start of the manufacturing process to support a Phase I clinical trial of HB700, a novel arenaviral immunotherapy for KRAS-mutated cancers. Hookipa plans to submit an IND to the US Food and Drug Administration in the first half of 2024. In October 2022, Hookipa and
Also, large pharma companies collectively are sitting on historically high cash piles to fund M&A and strategic partnerships, while facing significant product exclusivity losses in the coming years themselves. However, large pharma M&A may or may not pick up any time soon and in case it does, it will for sure not rescue all biotech companies running out of cash.
Despite this tough funding environment, a few follow-ons were successfully placed in the market recently, including in Europe. Some biotechs have secured funding from existing investors, often through private investments in public equity, but these options are unattractive or unobtainable for many smaller companies given current valuations.
How can hundreds of public biotechs gain financing through the downturn? The answer can only be more creativity in structuring deals including alternative funding options such as royalty deals, structured financing for clinical trials, an expanded role for private equity, convertible bonds and even non-traditional venture debt financing. L
Roche announced this strategic collaboration agreement under which the company from Austria is eligible for research, development and commercialisation milestone-based payments up to approximately US$930m in two programs, plus royalties. L
The unique and most complete list of share price developments of biotech companies listed in Europe – exclusively in European Biotechnology Magazine.
How
do I find a new job or a new employee? Now, there is an easy solution: eurobiotechjobs.net, the Europe-wide job market for biotechnology and the life sciences. Presented by the European Biotechnology Network.
VENTURE CAPITAL Jeito Capital is a young global investment company, founded 2018 in France. Initially unveiled with €200m in January 2020, backed by Sanofi and others, Jeito I has raised €534m in September 2021 with the aim of financing between 12 and 15 European biopharmas. Europ E an Biot Echnology spoke to the US-Partner of Jeito Capital, Rachel Mears.
EuroBiotech _Before we talk about some special characteristics with the people at Jeito Capital, can you describe the investment focus in your strategy?
Rachel Maers_Jeito is therapeutics focused, modality agnostic from biologics to gene therapy or small molecules, anything, with a focus on immuno-oncology in our portfolio lately, we believe in datadriven innovation where the application of new tools like AI is a new opportunity, defining the right patient at the right time for example.
EuroBiotech _Our last issue of Europ E anB iot E chnology n E ws was titled ‘Crisiswhat crisis?’ Do you see any negative trends in your area of investment in these challenging times?
Rachel Maers_The fundamentals of investing in the health care sector are not affected by any crises. Human health is a major driver and enduring unmet medical need is economically non-cyclical. On the other side there is an enduring need for innovation by pharma, since patents are running out. These enduring needs from both sides are perhaps not crisis-proof, but in the personal meetings at partnering conferences you can sense the excitement to act in our times.
EuroBiotech _With your experience from many years in the US and your perspective from the other side of the Atlantic: What – if at all – makes investing in Europe attractive?
Rachel Maers_We see more traditional investors coming to European rounds, also from the US. We at Jeito Capital think, that key for innovation is to have strong syndicates, the European ecosystem holds a multitude of opportunities.
RACHEL MAERS Partner with Jeito Capital, residing in New York City. She adds the US-perspective to European investments but is also looking for investment opportunities in the USA.

Strong science is the basis, but strong talent is key too. The serial drug developer is essential, with talent, strong science and capital you have it right in the making.
EuroBiotech _Can you describe the process of validating an investment target at Jeito? Is there a ‘Jeito way’ of decision making?
Rachel Maers_First we come in on validated science, we focus on the path to the patient, what is the need? Which data set will deliver on the unmet medical need? Who will treat this patient, what is the path to the patient for the therapy? With this picture in mind, we look at companies who address this issue.
EuroBiotech _Why is this? These opportunities have been there in Europe for a long time. What has changed in the awareness in the US?
Rachel Maers_The biotech innovation profits from the ecosystem around. Such ecosystems have to become robust to challenges from outside the sector and Europe is on a good way.
EuroBiotech _So to say, there is more experience in starting and running a company available in Europe?
Rachel Maers_Yes, that´s right. Jeito believes that there are tremendous opportunities in EU, and saying this means that there are also the people to leverage.
EuroBiotech _Jeito once started as an “all women” company, founded by Rafaèle Tordjman, who was tasked by the French government with boosting the biotech ecosystem in the country. Meanwhile, at least some male investment experts are working at Jeito. Is gender an issue in venture capital?
Rachel Maers_Yes. It is difficult to describe, and gender is not the bottom line, there are more and divers factors.
EuroBiotech _Can you elaborate on this point a little more?
Rachel Maers_We focus on an approach called ‘collective intelligence’, so not the gender, the function or the level of a person is important as we collect everything that delivers on the whole picture for an intelligence driven investment that fits into our strategy for patient driven benefit.
EuroBiotech _If gender is not key on the investors side, what about the patient
side, where Jeito is claiming to ‘go faster’ to the bedside?
Rachel Maers_We see that the patient population is very important to look at, to understand disease and differences in treatment and outcomes.
EuroBiotech _But is it about gender?
Rachel Maers _It is about not leaving groups outside the research. Not only gender, but from a holistic perspective having representative diversity in place.
EuroBiotech _Let´s come back to the dependence of innovation on the fitting ecosystem. You said, Europe has kind of evolved with more experienced people. Is this possible everywhere?
Rachel Maers_Years ago I would have said, innovation is location driven. Today, I and we at Jeito as a team see, that the way investors are acting in the (local) ecosystem is important. They are at best on a mission, focused to find the best approach on a global scale.
EuroBiotech _So the investor is shaping the local ecosystem?
Rachel Maers_The investor’s behaviour can transform an ecosystem. The growing talent pool of experienced managers and investors in Europe is an asset today.
EuroBiotech _How is Jeito Capital transforming the European ecosystem in health innovation?
Rachel Maers_.Jeito’s strategy is focused on supporting and empowering ambitious entrepreneurs to bring groundbreaking treatments to market quickly, benefiting patients worldwide. CDR Life, SparingVision, and CatalYm [see box] are excellent examples of companies that fit this strategy due to their focus on developing treatments for areas of high unmet medical need. These companies’ dedication to innovation aligns with Jeito’s approach, and we’re committed to supporting them as they work to bring new treatments to patients in need. By working together, we believe that we can make a meaningful difference in the lives of patients and their families.
g.kaeaeb@biocom.eu
JEITO CAPITAL Jeito selects companies according to its investment strategy to support the development of the most promising European Biopharma with growth and acceleration potential. In November 2022 Jeito co-led a €50m Series C financing round in CatalYm GmbH, a clinical-stage biopharmaceutical company developing novel immunotherapies to fight cancer. CatalYm was founded in 2016 as a spin-off from Würzburg University and is based in Munich (Germany). The lead candidate, visugromab is a humanized monoclonal antibody engineered to neutralise the tumour-produced Growth Differentiation Factor-15 (GDF-15). GDF-15 acts as a key regulator of immune cell activation and as an inhibitor of immune cell infiltration into the tumour tissue and is currently in Phase II clinical studies in patients with solid tumours that are relapsed/refractory to prior anti-PD1/-PD-L1 treatment. Dr. Andreas Wallnoefer, Partner at Jeito Capital, has joined CatalYm’s Board of Directors.
S paringVision In September 2022 Jeito co-led a €75m Series B financing round in SparingVision, a privately held French biotech company. As a spinoff of the Paris Vision Institute and the result of more than 20 years of scientific research, SparingVision develops genetic innovative therapies in ophthalmology for the treatment of blinding inherited retinal diseases. Proceeds from the financing will be primarily used to advance the development of SparingVision’s breakthrough lead treatments, SPVN06 and SPVN20, for a unique mutation-agnostic approach leading to new treatments in retinitis pigmentosa, one of the leading inherited causes of blindness. Most notably, the funding will support SparingVision’s first-in-human studies, bringing lead assets to clinical validation. Proceeds will also accelerate the development of other innovative genomic medicines products of the pipeline. SparingVision was Jeito’s first French investment following Jeito’s Fund I launch in January 2020.
CDR Life Already in April 2022 Jeito had co-led a $76m Series A financing round in CDR-Life, a Swiss privately-held biotech company developing a new generation of immuno-oncology therapies. Dr Rafaèle Tordjman, founder and CEO of Jeito Capital joined the company’s board of directors. Founded in 2017, CDR-Life leverages the exceptional expertise and track record of its founders, Christian Leisner, Dominik Escher, Konstantin von Schulthess, Leonardo Borras and Rouven Bingel-Erlenmeyer, who together have developed new antibody drugs, notably FDA approved Beovu ®, and closed major biotech deals, such as ESBATech, in the field of ophthalmology. CDR-Life is currently advancing its lead program, CDR404, the first dual MAGE-A4 T-cell engager that targets solid tumours across multiple indications, based on the company’s unique M-gager ® technology. The Series A funding will advance CDR404 through potential clinical proof-of-concept readout as well as expansion of the pipeline leveraging the Company’s M-gager ® technology for targeting intracellular antigens positioned to deliver unparalleled specificity and affinity in solid tumours.
Noema Pharma WIth Noema Pharma as the latest addition to the portfolio on March 7th this year, Jeito Capital co-lead a €104m financing of the Swiss clinical-stage company targeting debilitating central nervous system disorders. L
Pharming Group
NV enrolled the first of 15 children aged 4 to 11 with heritable activated phosphoinositide 3-kinase delta syndrome in a global Phase III clinical trial (NCT05438407) evaluating leniolisib in mid-February. According to Phase II/III results, the oral, selective phosphoinositide 3-kinase delta (PI3Kd) blocker reached the primary efficacy endpoints of reduction in index lymph node size and an increased proportion of naïve B cells out of total B cells from baseline at 12 weeks. Secondary endpoints of the pivotal Phase III trial additionally include a patient-reported assessment of the ability of leniolisib to modify health-related quality of life. FDA will decide on an accelerated US approval on 29 March, the EMA’s decision is expected in Q2/2023. PI3Kd is expressed predominately in heamatopoietic cells and is essential to normal immune system function through conversion of phosphatidylinositol-4-5-trisphosphate (PIP2) to phosphatidylinositol-3-4-5-trisphosphate (PIP3).
In February, Neogap Therapeutics AB has got the green light to start a monocentric Phase I/IIa trial of pTTL, its personalised cell therapy against colorectal cancer in H1/2023. Using its machine learning software PIOR, Neogap identifies a patient’s most prominent tumour neoantigens and uses its EpiTCer platform to proliferate T cells attacking these targets. The ultimate goal of the trial is to treat solid tumours by training the immune system to recognise neoantigens and thereby attack the cancer.
Swiss Hoffmann-La Roche AG’s faricimab has met the primary endpoints of noninferior visual acuity gains at 24 weeks compared to treatment with Bayer AG/ Regeneron Pharmaceuticals’ vacular endothelian growth factor (VEGF) blocker aflibercept in two global Phase III studies (BALATON and COMINO) in patients with macular oedema due to branch and central retinal vein occlusion. Roche’s bispecific antibody, which will be evaluated for further 48 weeks, targets and inhibits angiopoietin-2 (Ang-2) and VEGFA. For the first 20 weeks, patients were randomised 1:1 to receive six monthly injections of either faricimab (6 mg) or aflibercept (2 mg). From weeks 24-72, all patients (will) receive faricimab (6 mg) up to every four months – according to a personalised treatment interval dosing regimen. While In BALATON, one third of patients (34%) treated with faricimab had an absence of leakage compared to one fifth (21%) of aflibercept patients. In COMINO, the rates were 44% for faricimab patients vs. 30% for aflibercept patients. However, the average vision gains from baseline were comparable between the two treatments (faricimab: +16.9 letter vs. aflibercept: +17.5/17.3 letters) in both studies.
However, as the patent of Bayer/Regeneron’s aflibercept will expire in 2026, biosimilars are already on the horizon. In February, German Formycon AG published positive preliminary efficacy and safety data from MAGELLAN-AMD Phase III clinical trial for FYB203, its biosimilar candidate to the €9bn blockbuster aflibercept. According to an FDA-specific interim analysis of the randomised, doubleblind, multi-centre Phase III study, FYB203 met the primary efficacy endpoint, demonstrating comparable efficacy between FYB203 and aflibercept in patients with neovascular age-related macular degeneration (nAMD). Most recently, Klinge Biopharma GmbH, licensee and exclusive holder of the worldwide commercialisation rights of FYB203, had entered into
a binding term sheet for its exclusive commercialisation in the US with Coherus BioSciences Inc
In February, Abionyx Pharma SA has announced positive data from Phase IIa testing of iCER-001, a recombinant apoA-I, as a treatment for septic patients at high risk of developing Acute Kidney Injury (AKI). According to a Phase IIa pilot study enrolling 20 patients with bacterially induced sepsis at high risk for developing AKI, Abionyx Pharma SA’s recombinant apoA-I iCER-001 was able to scavenge endotoxins, to modulate the cytokine storm, and to prevent endothelial disruption. So far, the latter was only achieved up by adrecizumab, an antibody drug developed by German Adrenomed AG, that has entered a pivotal Phase III clinical trial in December 2022.
Tübingen-based CureVac NV and its partner GlaxoSmithKline plc reported positive data from two ongoing Phase I trials at the beginning of January. After evaluation of five dose levels of the mRNACOVID-19 vaccine candidate CV0501 between 12.5µg and 100µg, an eightfold increase in the titre of the antibody directed against the BA-1 region of the Omicron variant was observed in young patients. A 3.3-fold increase in antibody titre against seasonal influenza virus haemagglutinin antigens was observed following administration of three dose levels of FluSV mRNA in young patients. After evaluating data from the dose levels given to older patients, the companies plan to start two Phase II trials later this year. Both vaccines have a backbone of modified mRNA, unlike previous vaccine candidates. With the British partner, CureVac, which has relied exclusively on almost unmodified mRNA vaccines since 2000, is thus making a major turnaround. CureVac is the first company publicly comparing whether modified mRNA (CV0501) actually shows advantages in safety and efficacy over unmodified
mRNA (CV2CoV). Although other developers have been able to achieve great successes with modified mRNA vaccines, full data transparency does not yet exist. The scientific journal naturE speaks of a historic head-to-head study.
The Swiss subsidiary of Italian immuno-oncology specialist Nouscom Srl is collaborating with global pharma player Merck Sharp & Dohme (Merck & Co., Inc.). Under the terms of the collaboration, announced in early January, the companies are conducting a Phase II combination trial comparing the safety and efficacy of Nouscom’s lead candidate NOUS-209 together with MSD’s anti-PD-1 cancer immunotherapy pembrolizumab versus pembrolizumab monotherapy. Patients with metastatic colorectal cancer (CRC) with the biomarker Mismatch Repair/Microsatellite Instable High (dMMR/MSI-H) will be recruited. NOUS-209 is an immunotherapy that targets 209 cancer neoantigens found in unresectable or metastatic dMMR/MSI-H tumours of the stomach, colorectum and gastroesophageal tract. The Phase II study (NCT04041310) includes two cohorts: a randomised patient group eligible for firstline treatment randomised in a 2:1 ratio to NOUS-209 plus pembrolizumab versus monotherapy, and a single-arm cohort without control group, enrolling pa-
tients who have failed to respond to prior anti-PD1 treatment.
In mid-February, Scancell Holdings plc announced the completion of an openlabel single-arm dose finding study of its Phase I/II ModiFY basket trial of its cancer vaccine Modi-1. Modi-1 combines three citrullinated peptides: two vimentins, which are commonly found after epithelial mesenchymal transition of endometrial, renal, sarcomas, lymphomas and lung cancers, and an alpha-enolase involved in the tumours metabolic switch to glycolytic lactic acid production. Modi-1 peptides have each been conjugated to a tolllike receptor (TLR) 1/2 agonist, which acts as an adjuvant and was licenced from Dutch ISA Pharmaceuticals. Data from patients with advanced head and neck, ovarian, triple-negative breast and renal carcinomas receiving the Modi-1 cancer vaccine as a monotherapy demonstrated that it was safe and well tolerated. Furthermore, Scancell reported hints to early efficacy in a head and neck cancer patient and in high grade serous ovarian carcinoma (HGSOC) and triple negative breast cancer (TNBC). Modi-1 is the first candidate from Scancell’s Moditope ® platform. Up to 138 cancer patients will be recruited into either the monotherapy groups of the trial, or treated in combination with Merck Sharp & Dohme’s checkpoint inhibitor pembrolizumab.
According to CGT Catapult ’s annual UK ATMP Clinical Trials Report, the UK remained a global leader in clinical research, with the ongoing trials increasing from 168 in 2021 to 178 in 2022. This is in contrast to the global landscape, where there has been a 13% overall decline in the number of such trials. The UK was involved in 14% of global ongoing ATMP commercial Phase I-III trials. Of the UK ATMP clinical trials observed in 2022, 37% were in Phase I/II and the number of Phase II/III trials increased from three trials in 2021 to seven trials in 2022. Oncology again was the dominant therapeutic area under investigation in ATMP clinical trials (38%), followed by haematological indications (11%), metabolic disorders (10%), and ophthalmology (9%). Cell types investigated in ATMP clinical trials remain largely unchanged from 2021; with the dominance of T cells as the most commonly investigated cell type continuing to increase, accounting for 58% of UK ATMP clinical trials. The proportion of commercially sponsored ATMP clinical trials in the UK has also continued to rise, with now over 80% (131 trials in 2021, 145 in 2022), highlighting that the UK remains recognised as a good location for clinical research by industry. In January, BioNTech SE expanded its footprint in the UK, signing a MOU with the British Government to enrol 10,000 cancer patients in mRNA vaccine trials.

DEPENDANCE FROM:
Chair of Physics of Synthetic Biosystems, TU Munich
NUMBER OF EMPLOYEES
AT THE IZB:
6
BUSINESS SEGMENT: Product developer Drug Discovery Services
Dr Patrick Großmann, PhD, Dr Kilian Vogele, PhD, MBA; CEO & Co-Founder CTO & Co-Founder
Dr Kilian Vogele, PhD, MBA; CEO & Co-Founder CTO & Co-Founder

At Invitris, we are driven by the passion for making a lasting impact in the pharmaceutical industry. Our goal is to revolutionise the way protein-based drugs are developed and bring innovative solutions to the market.
One of our primary focuses is addressing the growing threat of multidrug resistant infections. Our universal technology platform enables us to synthesise different protein classes in vitro, providing a highly scalable and costeffective solution for both drug discovery and production.
“The IZB is a lighthouse for innovation. We’re grateful to be part of this exceptional community. We look forward to utilizing the resources and network offered by the IZB and contributing to its thriving ecosystem.
Invitris is transforming the way life science companies develop protein-based drugs, to accomplish this, we developed a pioneering technology platform capable of universally synthesizing novel therapeutic modalities completely in vitro. This breakthrough enables us to highly scale both drug discovery and production. Our achievements have earned us recognition with multiple awards and international accolades.
panies to bring innovative products into their drug development pipeline. Our initial focus is on antimicrobial proteins, such as bacteriophages or endolysins, to combat the biggest health-economic threat of the coming decades: antimicrobial resistance and antibiotic resistance in particular (AMR).
Our shared goal is to make a lasting impact in the pharmaceutical industry and thereby contribute to a healthier, more sustainable future.

The team of dedicated entrepreneurs and scientists share this vision.
Our business strategy involves collaborating with biopharmaceutical com-
Am Klopferspitz 19 82152 Planegg-Martinsried www.invitris.com
Fördergesellschaft IZB mbH Am Klopferspitz 19 82152 Planegg/Martinsried

Tel. + 49 (0)89.55 279 48-0




Fax + 49 (0)89.55 279 48-29
info@izb-online.de www.izb-online.de

Site: 26,000 m2, S1 laboratories










Real estate and facility management on site







Faculty Club and conference rooms for up to 100 people


Kindergarden (Bio Kids), Chemistry School Elhardt
Hotel CAMPUS AT HOME
Restaurant SEVEN AND MORE, The Bowl
On the Martinsried Campus: over 50 start-ups in the IZB, two Max Planck Institutes, ten faculties of the LMU, Clinic of the University Munich




LIFE SCIENCES HUBS The Berlin-Brandenburg capital region is one of the leading life science regions in Europe and a lot is being done to keep it that way: with a wide variety of space offerings and support programs for founders and start-ups.

Berlin is the hotspot for German startups: According to the Start-up Barometer of the management consulting firm E&Y, the capital was once again in first place in Germany in every respect in the first half of 2022. Berlin is also ahead in the health sector. According to E&Y, the most investment capital in this sector flowed into Berlin start-ups last year. These figures are certainly not only the result of the attractiveness of the region, but also of the good framework conditions that start-ups find here in the form of a large number of funding offers for founders, an effective infrastructure and corresponding space offers in the capital region.
Among other things, many scientific institutions offer extensive support to founders from their ranks. For example, the Berlin University Alliance – an alliance of the Free University of Berlin, Humboldt University of Berlin, the Technical University of Berlin and CharitéUniversitätsmedizin Berlin – has bundled its start-up activities in the “Science & Startups” initiative. It is the virtual umbrella brand for the joint activities of the start-up centres of four institutions and supports university spin-offs by raising funds, offering workshops and coaching, establishing contacts with academic mentors and investors, and creating incubator space.
Early support in the further development of medical innovations is also pro -
vided by the SPARK mentoring program at the Berlin Institute of Health at Charité. The program was founded in 2015 and is based on the successful SPARK program, which was developed at Stanford University and is now established at more than 50 institutions worldwide.
In addition to academic offerings aimed primarily at spin-offs from its own institutions, there is also a wide range of support offers in the region for start-ups in the early phase. In total, more than 80 accelerators and incubators support start-ups in the early phase. Some of these have focused on health issues –such as the Vision Health Pioneers Incubator.
Another example of comprehensive support for founders in Berlin’s healthcare industry is the Creative Destruction Lab (CDL). The global non-profit organ -
isation opened its first location in Germany in Berlin at the European School of Management and Technology Berlin (ESMT), mid-year 2022.
In addition to support through programs, an effective and efficient infrastructure with fast routes, a high density of research and development as well as close networking are also of great value to founders. Besides the successful healthcare industries cluster Berlin-Brandenburg, these include eight technology parks in the region, offering companies optimal space as well as modern laboratories.
Since the metropolitan region is also supposed to remain attractive in the future for start-ups in the life sciences, several expansions of the centres and new space offers for founders are already being implemented or planned in the next years. L


CDMOS Despite full books due to the booming market for biologics, CDMOs are facing major challenges in the face of the climate and Ukraine crises. In addition to the diversification of drug formats, the industry, which is built on plastic, has to convert to sustainability.
The green challenge comes in addition to the global competition for qualified specialists, who have become scarce due to capacity expansions through progress with new drug formats such as CAR-Ts, gene therapies, mRNAs, synthetic vaccines, peptides, conjugates during the last decade. Experts are rightly asking how this is to be achieved.
"Those who don't convert by 2030 will be out of the market. Then, major pharma companies will no longer place orders with CDMOs or customers who do not produce in a demonstrably sustainable way," one of the medium-sized contract manufacturers for biologics and cell therapies told E urop E an Biot Echnology
Therefore, the numerous specialists in the production of antibodies, recom -


binant proteins, virus-based cell and gene therapies, mRNAs as well as conjugates are feverishly looking for solutions to solve the three main problems of the deeply unsustainable industry: to reduce the high consumption of energy, water and single-use plastic consumables.
It is precisely the replacement of the multi-layer bags, fittings, tubing, filter cartridges, which emit a minimum of leachables, that have approval for the demanding cGMP production, that causes problems. This is because their essential components, polyethylene, polyamide and polyvinyl chloride are still based on fossil raw materials and are neither biodegradable nor readily recyclable. Also, for reasons of patent protection of the drug candidates adhering to them even after use, they are incinerated to this day – not exactly favourable for the decarbonisation or eco-balance of the processes.
Under the dictates of ESG criteria and newly pending EU regulations on sustainable production, CDMO customers are now looking for solutions to make these hitherto anything but climateneutral or waste-avoiding processes more sustainable.
Sustainability will affect biopharma developers and contractors in many ways in the near future as sustainability parameters are going to be mandatory audited within the EU. According to the recent CPHI annual survey among (bio-)pharma professionals, 83% believe that specific sustainability metrics will be implemented within the next five years in all CDMO contracts. Nearly 60% believe CDMOs will be required to provide their partners with both sustainability metrics for projects and specific corporate ESG goals. Greenhouse
Profound understanding of customer requirements based on 15+ years of experience

UNLOCK PICHIA® toolbox – broad set of expression tools and strategies
Process intensification with methanol-free Pichia allowing shorter batch times Industry proven and regulatory compliant expression platform
Tailor made glycoproteins – UNLOCK PICHIA® boosting Pichia GlycoSwitch®
gas (GHG) emission reduction, energy efficiency, abatement of water or resource adoption, waste management as well as driving health equity and improving diversity in clinical trials are some concrete examples of what matters for producers and its contractors when it comes to sustainability.
According to Heiko Schmidt, Strategy & Consulting Life Sciences lead, Accenture AG, establishing sustainability metrics is an investment for the future as investment decisions will be affected by the sustainability footprint of Big Pharma companies in the medium-term. These pass this on to their service providers and are expected to demand documentation of a CDMO’s sustainability performance that is aligned with the corporate strategy.
Since there is no common reporting standard for sustainability, currently companies are applying various principles. Rating agencies build their own metrics and indices. For the moment, GHG emission-related sustainability ambitions and corresponding plans can already be certified by the Science Based Targets initiative, a coalition of UN agencies, business and industry leaders. “Since 2020, our German sites are climate-neutral and no longer have a CO 2 footprint. Our production sites and sales offices in Austria, the US and Asia have followed this CO2 neutrality in 2021,” says Henryk Badack, Senior VP Technical Service and Internal Project Management at Vetter.
Improvement of pharma packaging, reuse loops, manufacturing efficiencies including yield optimisation and improvements in monitoring waste of materials and takeback programs are among the low-hanging fruits to achieve an apparently greener footprint. Using regenerative energy to fuel the energycostly automated air circulation systems in cleanroom facilities and advanced water management strategies are also a topic for CDMOs such as Vetter. However, the sustainable recycling or incineration of bags and other cGMP-compatible single-use plastics, which form the basis of flexible, fast and cost-effective biomanufacturing, will remain an unsolved problem for at least the next decade
The single-use plastics topic is a dilemma for many CDMOs who want to prepare for a
greener future: On the one hand, contract manufacturers, must, continue to achieve the high regulatory requirements. On the other hand, they must strive to enable environmentally-friendly usage of resources to contribute to meeting the Green Deal climate goals.
However, replacing high-performance plastics such as polyethylene, polyamides and polyvinyl chloride in approved processes would require costly new process certifications by the regulatory authorities. This is something that will take some time as the sector is conservative and patient safety is a priority over sustanainability, yet.
Currently, there are neither biodegradable alternatives to PE, PA or polyethers nor enzymes that are able to degrade these persistant oil-based polymers. Thus, a sustainable solution is not in sight in the mid-term. Some companies, however, are developing nano-cellulose-based polyethylene, polypropylene and polystryrol alternative "bioplastics" that are fully biodegradable. In the long-term metagenome mining, structural motif mining or even synthetic biology might identify plastic-degrading enzymes or alternatives. However, it's a long way to market adoption so that the sector will remain only semi-sustainable.
t.gabrielczyk@biocom.eu
Currently, only 9% of the 391 million tonnes of plastic produced annually worldwide and 14 % of the almost 142 million tonnes of plastic packaging are recycled. Incidentally, the large amounts of plastic waste that the EU and North America export to Asia are also considered recycled. In fact, 40% of non-degradable plastics containing toxic additives end up in landfills, 14%
are incinerated and about 32% end up in the environment. Since packaging is supposed to be lightweight, functional and durable, the chemical industry mainly produces plastics that take at least 500 years to be ground into microplastics; plastics that are biodegradable at 37° C in industrial recycling plants currently account for only 2% of global production. L






PRODUCTION The switch from lab-scale to industrial-scale AAV production requires deep hands-on expertise in various technological solutions, their limitations, and their suitability for GMP large-scale manufacturing. Direct application of lab-scale process leads to expensive AAV-based products and unnecessarily laborious industrial processes. The complexity and growing demands for controllable critical quality attributes (CQAs), create a challenging blend.
› Artur Padzik, Ph.D., AAV Technology Manager, BiovianBroadly, AAV manufacturing consists of the upstream process, where producer cells, upon transfection, start generating proteinous and genomic components that self-assemble to form active AAV particles. Breaking cells to release AAVs marks the end of the upstream production and transition to downstream purification.
Large-scale clinical AAV production poses numerous hurdles not visible in a preclinical scale. The early discovery phase typically utilises adherent cells, which at a scale translate to fixed-bed reactors. The expansion phase in the adherent cells process introduces variability due to difficulty in delivering consistent parameters such as pH, oxygen concentration, or cell distribution across multiple vessels. While fixedbed reactors allow effective viral particle collection from the supernatant recovering AAV from biomass is more challenging. Depending on the serotype and harvesting time, about 20-40% of AAV particles remain in cells.
Biovian believes in its AAV platform process approach, where true scala -
bility is possible with serum-free suspension culture in stirred tank bioreactors and a well-characterised in-house cell line. In suspension culture, volume dimension increases faster than surface area. Based on our experience, huge differences exist between HEK293 cell clones available on the market in their aggregation susceptibility, growth speed, and toxicity response to commercial transfection reagents leading to up to 10-fold titer differences. Above mentioned differences still do not consider the substantial effect on titer imposed by transfection conditions, plasmid ratios, the genetic background of plasmids, and plasmid quality. Our in-house plasmid production allows a comprehensive understanding and control of each quality attribute that converts into consistency in AAV manufacturing. Simultaneously, we generated a well-defined upstream process design space using the Design-of-Ex-
periments methodology to control production with titers >1014 vg/l or >10 5 vg/ cell. The suspension process has lower cell densities per volume than adherent cultures and can be further intensified by perfusion, with transfections possible even at 9x10 6 cells/ml and titers improved by 5-10-fold.
The small-scale downstream process utilises problematic procedures such as cell collection by centrifugation, freezethawing to release AAV from cells, or centrifugation to separate empty AAVs. Through our AAV platform process philosophy, we focused on scalable chromatography. We achieved a uniform affinity chromatography process that produces stable > 80% recovery with multiple serotypes. When looking at empty-full AAV separation, a typical preclinical scale cesium chloride and ultracentrifugation-based separation generates challenges in upscaling. We tackled scalable anion exchange chromatography and developed a robust protocol allowing stable baseline separation of empty and full AAV particles regardless of therapeutic gene size and serotype. The compatibility with largescale should happen early to deliver low-cost, high-quality products. L
D e s i g n , m a n u f a c t u r e a n d m a i n t a i n b i o p r o c e s s e q u i p m e n t a n d s y s t e m s
A u t o m a t e d s t e r i l i z a b l e s t a i n l e s s - s t e e l a n d a u t o c l a v a b l e g l a s s b i o r e a c t o r s

F r o m l a b - s c a l e e x p e r i m e n t a l r e a c t o r s t o i n d u s t r i a l p r o d u c t i o n s i z e
T a i l o r e d t o i n d i v i d u a l u s e r r e q u i r e m e n t s
w w w b e l a c h s e
i n f o @ b e l a c h s e
D u m p e r v ä g e n 6
1 4 2 5 0 S k o g å s , S w e d e n


PHARMAPACK Pressures to handle sustainability risks are increasing globally, and the pharmaceutical industry is no exception. There is a growing feeling of urgency to replace traditional cutting-edge processes to meet the requirements for environmentally-friendly packaging alternatives. These were extensively discussed at this year's Pharmapack Europe.
Whether it be glass vials, cardboard applications, auto-injectors, or biodegradable containers, at the first post-pandemic in-person Pharmapack Europe 2023 (1-2 February, Paris) the shift in priorities of the industry was clearly visible. Nearly 5,000 on site visitors, more than 4,000 online attendees, and over 350 exhibitors discussed the transformation the pharmaceutical packaging industry is experiencing, and showcased innovations emphasising the priority of environmentally friendly packaging. Despite some still existing travel restrictions and the strike rendering the commute within France a challenge, 78 countries were represented, and 42 content sessions held.

The displayed products, the eager exhibitors and alluring goodies were not the only attraction of this year’s Pharmapack. The “learning labs”, a space for research and product presentations, were well attended, as well as the innovation gallery, which allowed the close-up inspection of high-end products. All of this was sprinkled with the luxurious feeling of Paris by the provided crêpes, coffee, and croissants for everyone, as well as live music. The latest innovations from packaging companies within the sector were celebrated at the annual awards, which recognised improved efficacy, user safety and environmental impact reduction of the products. The entire event reflected the new confidence, positivity, and relief coming out of a pandemic instilled in the public.
Challenges posed by the COVID-19 pandemic lay in the numbers of nov -
el vaccines, medical equipment, and medications needing to be rapidly manufactured, parcelled, stored, and distributed all over the world while preserving the day-to-day business as usual. The supply chain was heavily affected by the pandemic, especially early on. This crisis demanded creativity, action, and collaboration which generated a wide spectrum of opportunities in new possible methods across the industry. The rapid development of digital applications and connected gadgets is one such beneficiary. For instance, many countries have introduced track and trace schemes, vaccine cards, virtual appointments, digital doctor’s notices, digital prescriptions, and have quickly increased the use of self-administration of therapies, with apps possibly becoming the new model for mental health treatments.
Another post-pandemic trend is the shift toward self-administration. One only needs to consider the impact of overburdened institutions during the epidemic to see the long- and shortterm advantages. With a growing number of patients now using injectable de-
vices that can also be monitored at home, it is perhaps predictable that wearable injectors capable of providing high-volume biologics, another field of recent innovation, is growing financially in the industry. Unsurprisingly, gadget usability is a critical component of future design of new medications, and there has been a change in how companies perform R&D, with patient experience teams and usability studies now being a necessity rather than an option. Patient centricity as well as painless delivery are essential elements in device choice, especially in terms of the need to optimise safety and efficacy while minimising possible user errors.
While the COVID-19 pandemic has obviously captured the attention of the world, including the pharmaceutical industry, there is also another looming issue on the horizon: the climate change. Pharma has seen a drive towards sustainability in the last years, with an increasing number of companies looking to minimise the impact they have on the environment by measuring their carbon footprint, reducing waste, lessening emissions, and removing plastics wherever possible. Since most medical packaging is derived from polymers, with the majority of waste being disposed of in landfills, the packaging sector has come into focus in this discussion. Waste and energy management, as well as the supply chain are the three biggest contributors to the industry’s carbon footprint, thus those are the things that need to be tackled. “What we are seeing, particularly at this year’s








Pharmapack, is how pharma is now taking a very holistic view to sustainability and looking more deeply at how we can reduce ‘Lifecycle impact’ of products and therapies”, affirms the event’s brand manager, Laura Murina-Indriksone.
The technological surge towards digitalisation and the apparent advantages that would bring in the medical care sector are pushing towards building digital into single-use devices. This of course raises the issue of electronic waste and how it should be dealt with. Governments are creating wider sustainability strategies, with a recent drive towards green chemistry like solvent use reduction initiatives in API manufacturing. Investment in eco-packaging is expected to radically increase within the next years, and this will be largely driven by companies looking to lower their carbon footprint in line with the global goals in the 2030 agenda, as set out by the United Nations.
At the same time, one must remember the delicacy of handling medical equipment and medication in a way that complies with a whole array of regulatory requirements. For example, aseptic manufacturing and cold chain are both energy intensive but crucial to supplying injectables safely. It would be potentially detrimental to cut corners around regulatory requirements. Additionally, the search for reusable and
plastic-free alternatives is in stark contrast to the expectations the industry had a few decades ago and the trajectory it has had to date. If one asked what the trend is going to be in the pharma and the packaging industry 15 years ago, they would have explained that we were heading into a future where everything was going to be single use and thus easy and safe to use.
Indeed today, the use of fast hybrid processes that allow to quickly get medications to patients are widespread. So, it requires a break-free of the traditional ways of manufacturing and regulatory regime, which raises many concerns about the feasibility, necessity and most importantly the quality of the final product. That in turn takes a lot of time, data, and confidence, since it is linked to the switch from one process to another for an already established and successful product. This is one of the biggest hindrances. To respond to this element of risk, new technologies, and support for the developers from a financial and political point of view are needed.
It is understandable, thus, that even though sustainability has been at the forefront of global discussions for years now, the pharmaceutical industry has been slow to react. This cannot be used as an excuse any more sustainability should be heavily embedded in each company’s strategy and balancing eco-
nomic performance with social and ecological responsibility.
This year’s Pharmapack Awards mirrored the sentiment and showed how a focus on improving sustainability and reducing waste by replacing singleuse plastic devices with reusable ones through modular systems one can still create novel drug delivery solutions, reusable connected devices, and recyclable packaging. In the category “sustainability initiative” for example, the winner, Körber Pharma Packaging Materials AG – who won the sustainability initiative award for their pharmaceutical grass paper packaging in 2022 as well – got awarded for their sustainable COVID Rapid Tester out of recyclable cardboard mono material. The award of “Eco-Design” went to the 100% PET, PVC- and aluminium-free, and thus recyclable blister by Rotor Print SL and even the “Drug delivery Innovation” award, which didn’t need to be a sustainable product was awarded to Owen Mumford GmbH for UniSafe®, the reusable connected auto-injector. “Different parts of the industry are responding to this challenge differently. We have seen companies looking to make plastic only devices so that they can be recycled [anything with multiple components parts is often unsuitable for recycling], and others that are reducing or replacing plastic”, explains Murina-Indriksone. “Then there are many device manufacturers exploring how to make single use devices reusable or to have parts of these devices made reusable. We also see companies looking at how automation and even AI can make the manufacturing of devices more efficient.”

For such solutions to be feasible and widely used to make a difference, collaboration between the industry, investors, and politicians is crucial. There is no way for a single company to tackle sustainability in the entire production circle, while preserving standards and regulations that are not designed for such a huge undertaking. Real change can be enacted with a collaborative strategy that acknowledges the obstacles and establishes standards. This change is what we are striving towards.
m.milidakis@biocom.de





BIOPROCESS EQUIPMENT Sweden’s Belach Bioteknik has been partnering mostly with researchers and pharma customers to design suited bioreactor systems for biomanufacturing. Most recently, the company has entered the growing cultured meat sector, in which many companies are currently scaling up their production and thus need expertise to get optimal results.
EuroBiotech _Can you tell us about Belach Bioteknik's background and how it became the biotechnology company that it is today?
György Rajkai Belach was established in 1985 by Swedish engineers –our name is the acronym of the founders –and aimed to design and build labscale bioreactors for universities, research institutes and R&D departments of production companies.

The first customers contacted Belach from the Swedish universities (KTH, Lund, Uppsala University), but we also had industrial partners, mostly pharmaceutical companies. The first products were developed jointly with the academic researchers, in line with their needs. I can mention two of our unique products, a high-throughput multibioreactor system that was developed in a cooperation with academic researchers of Karolinska Institute and the biogas research reactor system that was developed together with SLU, Uppsala. In the 90`s, the company started to offer digital control systems for reactors and bioprocess systems, which was a cutting-edge and pioneer solution at that time. Besides, the Swedish market, Belach entered the Scandinavian market as well. The business philosophy remained the same, we started to serve Norwegian and Finnish universities and research institutes.
The business policy has been unchanged since the beginning: we are at our potential customers/users’ dispos-
György Rajkai started his career as an Automation Engineer at Belach Bioteknik in the 90s. Using its expertise, the company developed fully automated bioprocess systems. In 2013, he was appointed CEO. Since 2019, he has been working as CTO, managing the technical design and execution of projects, product developments and unique solutions.
al from the very beginning of their projects, supporting them to create and describe the exact requirements that bioreactors and bioprocess systems shall fulfil. We are their partners from the design of the simplest equipment to manufacturing of the most complex automated system.
EuroBiotech_What have been some of your major milestones?
Rajkai In the 2000s, the company was entrusted by a Swedish vaccine producer to design and build an automated production plant. That was a big challenge for Belach, but this plant had been in operation for 20 years. It meant a real milestone in progression for Belach, by gaining robust experience from the design and execution of stainless steel to in-situ sterilisable bioreactors and productionscale automated bioprocess systems. We also enlarged our portfolio, developing a parallel multibioreactor system, that is suitable for research purposes, but for small-scale production as well. The last was originally designed for a customer in Germany, a couple of years ago, who produces animal vaccines especially cultivated for farms individually. So low volume for production purposes is needed. These multibioreactos can be run by only one or two persons. At the same time, they can produce a diversity of products, for which different parameters define the process. These reactors can also run several functions such as cultivation, CIP or sterilisation, in parallel in its different vessels. Belach enlarged the portfolio with other equipment relating to bioprocesses, like decontamination equipment. These can be good solutions in terms of environmental requirements for laboratories, hospitals, as well as industrial user.
24-26 October 2023 | Barcelona, Spain
Pharma’s largest event is making an exciting return to Barcelona on 24-26 October 2023.

45,000+ Attendees
160+ Countries 2000+ Exhibitors
Join us at CPHI Barcelona to position your company at the heart of pharma and to connect with international pharma professionals. Grow your business and expand your network in one of Europe’s leading countries in pharma. cphi.com/europe
EuroBiotech _Can you discuss your current biotechnology projects and their potential impact on the market?
Rajkai In recent years, we have been contacted by several companies that are dedicated to make research with cultured meat. We were also contacted by a start-up company from the USA, who intended to start their production in this field. The process has been developed by the researchers, but the bioprocess system has been designed by Belach. Since the bioprocess and the equipment is strictly confidential, I just can mention that as a very interesting and challenging task for our engineers. Should the experiments deem successful in the current lab scale and pilot scale systems, we could step ahead to design the production scale reactors and systems.
The significance of this sort of research and experiments to the market can be revolutionary, of course, if they prove realistic and the scale up be -
comes applicable. We are glad to be among the first “bioneers”.
EuroBiotech _How do you see your company's expertise in biotechnology translating to success in the cultured protein industry?
Rajkai The cultured protein industry is expected to continue growing in the coming years, driven by concerns over environmental sustainability, animal welfare, and food security. Biotechnology companies need to stay up to date with emerging technologies and market trends to remain competitive in this rapidly changing field. Additionally, biotechnology companies may need to focus on developing unique technologies or products that differentiate them from competitors. Our philosophy follows the same approach that we have been believing for decades: the bioprocess technology shall be developed by the researchers and users, but we can provide design and build the hard -
ware and the software solutions for the bioprocess so that the researchers can achieve their goals. Belach Bioteknik has senior engineers, like chemical engineers, automation engineers, and mechanical and electric engineers, who have designed and built many systems, with unique solutions. Besides the internal team, Belach also works with constant external engineers, consultants and subcontractors who have robust industrial experience in their professional area and our cooperation has lasted for years or decades.
EuroBiotech _What are the key challenges your company is facing now and how are you overcoming them?
Rajkai One of the key challenges is the uncertainty of the customers, who have become more cautious in the last three years, due to the turbulent circumstances. Based on our experience, the investments are limited, suspended, postponed, or eventually cancelled. Therefore, the pricing strategy is a crucial factor. Our goal is that the customers can continue their research work, and their investments in production plants and find us for mutual professional cooperation, for an affordable price. An additional challenge for the company is to find additional specialists for various key roles. This is crucial to ensure the company’s continuity and expansion.
EuroBiotech _Can you discuss which geographic markets your company is currently targeting for expansion?
Rajkai Belach Bioteknik – being a small enterprise – entered the European biotech market five to six years ago. We are now present in eleven European countries with benchtop and stainless steel bioreactors that are applicable for both microbial and cell cultivation. In addition to sales, we can reliably provide parts supply and service from our office in Stockholm. We already have –mostly completely unique – equipment in the USA, too, and this expansion – in addition to further expansion in Europe – is also part of our long-term vision. L
t.gabrielczyk@biocom.de


DSP Tosoh Bioscience recently launched Octave© BIO, the first in a series of MCC instruments targeting all stages of biomolecule manufacturing. Here, Tosoh discusses the potential of MCC to help alleviate downstream bottlenecks and a process intensification collaboration they recently performed with Catalent Biologics. Emily Schirmer, Interim General Manager at Catalent Biologics, provides selected insights on Catalent’s findings from the collaboration.
With broad expertise in development sciences, delivery technologies, and manufacturing, Catalent is a reliable drug development and delivery partner for the industry. In an ongoing approach to increase the efficiency of manufacturing processes, scientists at Catalent evaluated a newly developed multicolumn chromatography system, the Octave BIO, that was released in September 2022 by Tosoh Bioscience.

Emily Schirmer explained the background of this project and how bioprocessing needs are changing the industry: “From large pharma companies to contract manufacturers, several trends are changing the industry landscape. We are seeing higher productivity cell lines and increased use of process intensification and continuous processing methods to further drive higher productivity. These technologies and product development approaches have enabled a significant increase in the amount of material that can be generated upstream. The increase in upstream material means there is a need for efficient downstream purification methods – to avoid bottlenecks and reduce manufacturing footprints.”
Purification platforms for monoclonal antibodies (mAbs) typically contain an affinity capture step based on protein A followed by polishing steps
for further impurities reduction. Today these steps are typically performed in batch chromatography with process columns. Innovations in downstream mAb processing, including continuous chromatography approaches such as multi-column chromatography (MCC), have been shown to increase purification productivity and reduce operating costs significantly. Although batch processes are inherently less complex, MCC takes significant advantage of spreading the sample load across
multiple columns in a more efficient cyclical process. As a result, a several-fold increase in productivity typically is achieved.
Multi-column chromatography relies on a series of small columns instead of one large column, reducing the total resin volume required by as much as 90 percent. The various operations of the process protocol (loading, washing,
elution, and cleaning) are carried out simultaneously in different columns under the control of individual pumps. Periodic switching of the inlet and outlet streams to downstream column positions via a valve system enables the progression of process steps in a continuous cycle. MCC also allows maximum productivity, as mass transfer to the Protein A chromatography resin allows the total capacity of columns to be reached as fast as possible while maintaining high purity and recovery.
Some biopharma manufacturers no longer want or need to deal with the large stainless steel equipment, as well as buffer and resin volumes associated with batch processes. MCC addresses the bottlenecks that companies may experience and can provide significant economic advantages compared to traditional batch methods for mAb purification, including 3–10-fold increased productivity, 85–95 percent resin capacity utilisation, 30–50 percent reduced buffer consumption, decreased column volume, and smaller versatile process skids.

From batch to continuous Emily stated: “Catalent has been and is continuing to consider alternatives to traditional batch chromatography to address process intensification upstream. Protein A media can be costly – often requiring a large upfront investment to establish the downstream process. The investment required for highcost chromatography matrices can be limiting for biotechnology firms and can be mitigated by using MCC since smaller columns are used, which reduces resin usage. And that is how our partnership with Tosoh came about. We worked with Tosoh’s MCC systems and completed several pilot and manufacturing scale demonstration runs.”
Octave BIO is a comprehensive and versatile multi-column chromatography system with a modular design and added functionalities that support a range of process scales, implementations,

and applications, including continuous purification. Octave BIO is the first in a series of MCC instruments targeting all stages of biologic manufacturing, from pre-clinical to clinical and commercial GMP manufacturing. SkillPakTM BIO pre-packed columns are designed with standard shorter bed heights optimised for the fast flow rates and short residence times of MCC. The Octave solution consisting of the MCC instrument combined with bespoke columns enables customers to quickly and efficiently develop pre-clinical processes.
MCC affinity capturing of mAbs
TOYOPEARL® Protein A and Protein L resins provide the ideal combination of capacity, flow properties, and resulting product purity to achieve the highest productivity when used in MCC methods for the capture of antibody-based therapeutics. Octave systems paired with TOYOPEARL AF-rProtein A HC650F resin, for example, allow flow rates of > 600 cm/h and loading res -
idence time as short as 0.25 min for Protein A adsorption of the mAb versus 4 min or more in a single column batch process. As all non-loading steps are carried out simultaneously in the other columns, there is no delay in completing each step. Protein A capture is achieved with greater speed and efficiency than a single-column process. Flexibility is afforded by adjusting the column number, size, and configuration to suit feed and adsorbent properties, accommodate all process steps, and satisfy run time requirements.
Tosoh has been investing in the support infrastructure to help educate current and future MCC users with virtual and in-person offerings. At MCC Centers of Excellence in the U.S. headquarters in King of Prussia, PA, and in the European headquarters in Griesheim, Germany, Tosoh's renowned chromatography experts are ready to train and support the industry from the lab to the field.
Emily Schirmer’s conclusion after the demonstration runs: “The results of the in-depth MCC evaluation clearly illustrated the potential for significant time and cost savings for Catalent’s partners. The automated system also provides benefits to operating costs and time. As the industry looks to overcome the bottlenecks that can occur during intensified processes, MCC can offer a pertinent solution.” Based on the positive feedback from the industry, we at Tosoh Bioscience are convinced that MCC fits the growing industry trend of switching from batch to continuous processes and meets the four design principles for biologic facilities of the future: fast, flexible, small, and sustainable.
Contact: Regina Roemling, Senior Marketing Communication Manager at Tosoh Bioscience GmbH, regina.roemling@tosoh.comBIOMANUFACTURING Implementing high-quality process development that considers GMP manufacturing from the start is critical to reducing costs and timeline disruptions associated with raw material supply chain insecurity, adhering to regulatory compliance, and avoiding process re-design at a later stage.

In overly ambitious biopharmaceutical development timelines, overlooking a GMP-suited process is common. The key milestone is producing during a pre-booked GMP slot to achieve firstto-clinical Phase I status, but manufacturing capacity is typically booked far in advance, making rescheduling difficult and costly.
Amidst the rush to meet timelines, bio pharma companies and their partners often overlook crucial factors such as raw material availability and equipment compatibility. For instance, limited availability of resins may impede timely scaling up of a process, while a process designed for Phase I may lack scalability for commercial production. As an example, only a portion of USP material is utilised in Phase I's downstream process since minimal material is needed, but a
scalability issue arises when moving to commercial scale.
In some instances, biopharma companies collaborate with CROs who have different objectives, solely focused on developing the drug substance process for early-phase development, which permits sponsors to evaluate the protein's/ antibody's mode of action in a research environment. Sponsors may be at fault for overlooking this, prioritising meeting pre-set milestones in Phases I, II, and III, while compromising on complete process development, often leading to issues like yield or purity shortcomings, or a non-scalable/non-robust process. Only after that they consider scale-up, commercialisation, and ways to increase efficiencies and reduce process costs. Inadequate process development has severe consequences. If significant process
redesign is necessary, it may require repeating toxicology and Phase I/Phase II studies, resulting in a loss of both time and money. This further shortens the commercialisation period until patent expiration, exacerbating the situation. Consequently, finding a licensing partner for commercialisation becomes challenging, even with a persuasive scientific mode of action. The cost of goods sold (COGS) for that partner, potentially during a shorter commercialisation phase due to the impending patent expiration, does not warrant the investment needed to redesign a suboptimal GMP production process. Ultimately, high-quality process development aims to maintain consistency from toxicology through GMP production runs. Process adaptations are usually necessary for tech transfer or scale-up to achieve higher yield, greater purity, or a more stable supply chain (e.g., changing to a resin with a shorter lead time). However, significant changes invite additional regulatory scrutiny.
To ensure thorough, well-considered process development, it is crucial to secure a CDMO partner as early as possible in a project. Biopharma companies often understand that their proposed process needs optimisation and seek external expertise to achieve this. The CDMO-client
After being held digitally the last three years, BIO-Europe Spring is back in-person March 20–22, in Basel, Switzerland. The enthusiasm of being back face-to-face in tangible with a record-breaking 3,200+ attendees expected to be in Basel and 18,000 meetings requested weekly. Register now to meet one-to-one with your next strategic partner. .

conversation covers various areas, such as yield and purity improvement, investment in the process, and desired outcomes. An experienced CDMO will prioritise optimising process steps that need attention. However, some companies demand running their heavily invested process exactly as designed or resist changes due to regulatory compliance concerns. Supply chain issues may arise, leading to missed GMP runs. Even if the GMP run occurs on time, issues may expose the process as unsuitable for the scale, equipment, or facility, affecting the entire process. Therefore, regardless of when the CDMO joins a project, discussing process optimisation is crucial to ensure yield and purity improvement.
A biopharma company typically knows what kind of CDMO it wants to work with and seeks out expertise and experience with a certain process or protein. However, it is unlikely that any CDMO will have experience with a client’s exact process or protein, so adaptability and capability in handling diverse molecules are essential. Northway Biotech stands out in this regard, with experience not only in monoclonal antibodies but also bispecific and trispecific antibodies, as well as microbially produced recombinant proteins, which have different purification requirements. This expertise allows for the development of optimised processes, overcoming specific problems, and is facilitated by in-house knowledge of equipment limitations, supply chain, and facility, ensuring a suitable process for GMP-scale production.
Do not underestimate the importance of process development. A process that works in the early stages may not respond well to GMP manufacturing and scale-up, leading to additional costs and time loss. Process development is crucial not only for on-time GMP production but also for presenting a complete package to potential partners or acquirers. For more information, contact the authors and visit www.northwaybiotech. com
BAYER The Supervisory Board of Bayer AG has appointed Roche Pharmaceuticals CEO Bill Anderson (56) as new Chairman of the Management Board of Bayer AG, with effect from June 2023. Anderson will join the life sciences company based in Leverkusen, Germany, as a member of the Management board on April. Bayer's current CEO, Werner Baumann (60), will work closely with him to ensure a smooth transition before he retires at the end of May after 35 years of service at Bayer. The chemical engineer
Bill Anderson has held various leadership positions in the life sciences industry over the past 25 years. In 2019, he became CEO of Roche Pharmaceuticals, based in Switzerland. Prior to that, Anderson was CEO of biotech pioneer Genentech, and he held several senior positions in general management, product development and finance at Biogen and at technology and electronics company Raychem. L
BIOSENIC Dr Carole Nicco took up her position as CSO of Belgium-based BioSenic SA in mid-January. Nicco will oversee the development of BioSenic's cell therapy and autoimmune disease platform pipeline and be responsible for R&D programmes. She has more than two decades of experience in cancer biology and immunology, inflammation, immunity, novel target identification and drug dis -
covery. From 2005 to 2023, Nicco was one of the principal investigators and laboratory manager of the research team


“Pathogenesis and innovative treatments for chronic fibro-inflammatory diseases” at the Institute Cochin, a biomedical research centre affiliated to INSERM (Unit 1016), CNRS (UMR 8104) and Paris Cité University. In 2023, Dr Nicco will become President of the international nonprofit organisation Redox Medicine Society (formerly the International Society of Antioxidants in Nutrition and Health). She holds a PhD in Human Physiology and Physiopathology from Denis Diderot University in Paris, France. L
OXFORD BIOMEDICA Former Rentschler-CEO Dr Frank Mathias officially takes up his new position as CEO and board member of Oxford Biomedica plc at the end of March. He has led Rentschler Biopharma SE since 2016. In addition, Oxford Biomedica announced two changes to its Board, both of which will also take effect on the 27 th March 2023. Stuart Henderson will become Vice Chair, a newly created position which replaces the previously combined role of Deputy Chair and Senior Independent Director, the position Stuart has filled since June 2020. Professor Dame Kay Davies will assume the role of Senior Independent Director, following her appointment as a Non-Executive Director in March 2021.L


› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2023
› Guide to German Biotech Companies 2023
› conveniently accessible ePapers
› Discount for BIOCOM events for 1 person
› additional partner offers
Price: 80 Euro p.a. (incl. VAT)
Students 50% discount (subject to proof of enrolment)
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2023
› Guide to German Biotech Companies 2023
› conveniently accessible ePapers
› Discount for BIOCOM events for 2 persons
› additional partner offers
Price: 120 Euro p.a. (plus VAT)
BIOSIMILARS The region regulated by the European Medicines Agency (EMA) has been the most proactive globally in the early adoption of biosimilars. By February 2023, it had approved around 75 products containing them. The distribution of such compounds within Europe, however, has been uneven, with some countries offering more market opportunities than others. Beyond Europe, few producers have a strategy for the Middle East and/or Africa, and India and South Korea could soon jump in to fill the gap.
The European Union core (EU5) and the Nordic countries in particular have seen very high market penetration of biosimilars – medicines that mimic specific blockbuster biologics. In general, biosimilars tend to follow the global distribution path taken by their originator biologics, with a key difference: in the area, the European healthcare market is ahead of the US. The popularity of a biosimilars business model has grown steadily and constantly. In 2021, for example, the global biologics market was estimated to be worth almost US$366bn, with biosimilars making up only around 4% of the total (US$16bn). However, according to a report by IQVIA, they saw particularly strong growth of about 80% CAGR in the period 2015-2019. That translates into a near doubling in value annually.
The general trend towards high market penetration for biosimilars is expected to continue, as several blockbuster reference biologics are soon going off patent. Two of the latest prominent additions to the list are Avastin/bevacizumab and Lucentis/ranibizumab. It’s interesting to note though that across the major world regions, biosimilar penetration varies widely, and appears to run somewhat counter to conventional economic rules.
One surprising example of this is that the US appears to be lagging behind Europe in the use of biosimilars. Since 2007, only 30 biosimilars have launched in the United States, although at least 10 more are in the approval process and are expected to hit the market by the end of 2023. In addition to the 12 specific active ingredients already being copied (some active ingredients have several biosimilar substitutes), a total of 20 different molecules are then expected to be either approved or in advanced stages of clinical development in the US. In terms of numbers of active ingredients with biosimilar imitation products, the US and Europe are therefore comparable. But the variety of products issued by different manufacturers is much greater in Europe.
The experience has shown, however, that market penetration of biosimilars in the US can happen rapidly. An American study of Community Oncology Alliance practices found a dramatic increase in biosimilar uptake for trastuzumab (Herceptin) – which first hit the market in July 2019 – and bevacizumab (Avastin). In the fourth quarter of that year, participating practices reported 8,000 bevacizumab biosimilar administrations compared to 21,000 for the reference product. In other words, around 28% of all administrations were for biosimilar bevacizumab. By the fourth quarter of 2020, just
15 months later, bevacizumab biosimilars had already reached a share of nearly 60% of all administrations. In addition, participating practices reported a 27% increase in the number of patients treated after the biosimilars entered the market, almost certainly because the therapy had grown more affordable.
Estimates from the Association for Accessible Medicines, the trade association for generic and biosimilar manufacturers, say that savings from biosimilars in the United States will total US$85-US$133bn by 2025. And as Joe Biden’s Inflation Reduction Act kicks in, the landscape should grow even more dynamic in the future.
But even when the EMA gives central approval for biosimilars, Europe is not a uniform market. This is because the agency says nothing about availability, nor how a newly approved biosimilar will rank in comparison to the original preparation in the 27 different reimbursement systems of the bloc’s respective healthcare systems.
The latest (2021) biosimilar report from the European industry association Medicines for Europe examines the availability of the 15 biosimilar active ingredients across Europe at that time. All the bio -
similars listed were only available without gaps in Germany, France, Hungary and Italy. In Switzerland one biosimilar active ingredient was missing, in Austria two, similar to Belgium and other countries. In the UK, only 10 of the 15 biosimilars were available at the time of the study, which corresponds to a 30% gap in overall supply possible through a marketing authorisation. In some Eastern European countries (with the exception of Hungary), the biosimilar supply gaps were even more pronounced. One reason for this could be the issue of logistics and prioritisation by pharmaceutical manufacturers. But another is reimbursement and models for how reimbursement processes are designed. Free pricing is only used as a model for biosimilars in five European countries.
All the other countries use ‘regulated pricing’ based on different reference prices and reference pricing systems. One can’t really speak of a uniform EU area, especially when it comes to reimbursement, cost and thus the actual business model. How much cheaper a biosimilar is than the original can vary greatly in Europe. While the Scandinavian countries regularly demand discounts of over 40% for certain biosimilars, savings in France or Italy might only be in the low single-digit range. Market penetration can also generally be viewed as very rapid and significant in Europe, but there are country-specific features there as well. For example, after introduction, the trastuzumab biosimilar in Switzerland was able to gain a market share of just 13% from the previous reference preparation between Q1/2020 and Q4/ 2021 (Source: Biosimilars.ch).
The high approval figures in Europe reveal on the one hand that the EU – and in particular the EMA – created a practicable framework at an early stage to make the process of comparing reference products with biosimilar candidates transparent and watertight. A basic prerequisite is precise knowledge of the reference product in combination
with powerful analytical tools that largely predict clinical comparability, subject to confirmation by a comparative pharmacokinetic study.
On the other hand, it is astonishing how differently market penetration happens in individual European nations after a marketing authorisation. In fact, it doesn’t make much sense that equally good but cheaper products don’t spread much more dynamically in financially weaker EU countries, where they would have the greatest impact.
One possible reason why could be marketing strategy among biosimilar developers. Put simply, this strategy is generally to wrest as much market share as possible as quickly as possible from a blockbuster in its blockbuster market. Various delaying tactics – such as patent extensions, patent infringement suits, formulation adjustments and strong lobbying with the medical profession –can often give the original manufacturer more time to profit from the highest prices. Bio similar developers are heavily involved in all these defensive battles, but also have to keep an eye on copycat competition and exactly time their own approvals so as not to compete with the original too early or too late.
But even if all this works out in a biosimilar developer’s favour, it still has to negotiate prices amidst competition for reimbursement by respective healthcare systems. In Germany, for example, there is currently a lot of criticism of what’s called ‘automatic substitution’. This rule dictates that a patient’s health insurance company determines via price negotiation which biosimilar is to be administered in place of an original preparation. Until now, this decision was made by the treating physician. Biosimilar companies now fear that exclusive general agreements might be made for individual active substances, and that if there are numerous suppliers of a suitable biosimilar, it could lead to a destructive price-war spiral. That could result in the end in a ‘last man left standing’ supplier for each
active ingredient. But ‘winner takes it all’ isn’t a desirable outcome.
A look at the production locations shows why the scenario would be bad for Europe. Currently, almost 60% of the biosimilar doses administered in Germany for instance are produced within Europe. If a massive discount battle were to kick off, Asian suppliers would ultimately be able to exploit cost advantages to the full, and Europe could once again grow dependent on them. InnerEuropean markets for biosimilars (especially in Eastern Europe) are insufficiently developed, and still offer big development potential for European pharmaceutical companies.
But the European biosimilar community could also try to adopt a completely different perspective for once. Instead of primarily trying to conquer the blockbuster market, it might make more sense to look to other regions in the world where the ‘Made in Europe’ badge is still considered a seal of quality, and where large numbers of patients still don’t have access to advanced but affordable treatments. The Middle East and Africa both fit this description.
Unlike Europe, the MEA market is still considered underdeveloped when it comes to biosimilars. Biologics as a whole, however, have certainly laid the groundwork for a market there. According to a white paper from IQVIA, they made up about 15% of the roughly US$27bn overall spent on therapeutics in 2019. According to the paper’s figures, Saudi Arabia leads the pack in the use and administration of biologics by a wide margin, spending about US$2bn. Biosimilars still have a very small footprint though. They made up only around 2% of the total biologics market in Saudi Arabia, and even less in the United Arab Emirates. At a much lower level in terms of overall numbers of treatment courses, they were around 5% of all prescriptions in Lebanon and 3% in Tunisia.
Observers point out that the commercial environment in the MEA region is
growing more conducive to the strong uptake of biosimilars, in light of an increased government focus on expanding patient access to medicines, budget constraints and availability of a regulatory framework. Countries there have discovered the topic of biosimilars for themselves, and usually closely follow approval rules issued by the EMA or the FDA.
The 54 African nations, on the other hand, have a patchwork of regulations and rules that can affect comparison procedures between an original biologic and any biosimilars, but also pricing. But if you can manage the diverse international European landscape, why not in Africa too? Individual European countries even offer training programmes on how countries in the MEA region, and Africa in particular, should position themselves in regulatory terms with regard to biosimilars. In 2022, for example, the German Ministry of Health’s Global Health Protection Programme (GHPP) sponsored a workshop on the ‘Assessment of Biosimilars’. It lasted several days in the Reg-Train/Pharm-Train project, and was attended by health authority representatives from Zimbabwe, Ghana, South Africa and Tanzania. South Africa is one of the few African countries to have regulated biosimilar approval, but the first was only greenlighted in 2019.

The cost-effective drug development of biosimilars is particularly attractive for resource-constraint settings, and helps enable the availability of biologics on these markets. However, the larger molecular size and complexity of therapeutically active proteins demand specific requirements in assessing the corresponding marketing authorisation applications. Before anything can happen, assessors first have to acquire the skills needed to compare biosimilar and original biologic, as well as experience with pharmaceutical quality, efficacy and safety in the biosimilar landscape.
Some pharmaceutical companies are playing more active roles in the MEA and
African regions, and investment by local and regional companies in the still largely untapped biosimilar space is flowering. Examples include Spimaco in Saudi Arabia in partnership with Roche, Julphar in the UAE in partnership with Biocad, Sedico and Pharco in Egypt, Arwan and Benta in Lebanon, El Kendi in partnership with mAbxience in Algeria, Medis in Tunisia and Sothema in Morocco in partnership with Biocad. Algerian firm Hikma, in partnership with Cell trion, launched an infliximab biosimilar in the main MEA markets, while Cipla from India has not only entered the market but also has its own production sites.
Interestingly, Iceland-based Alvotech is one of the rare pure biosimilar players from Europe to have a business strategy in Africa. In partnership with local or other strong pharma companies, every biosimilar the firm has in development also has a specific roll-out plan for the continent. Alvotech has also signed an exclusive licensing agreement with Bioventure (Dubai), Minapharm Pharmaceuticals (Cairo) and MiGenTra GmbH
(daughter company of ProBioGen AG in Berlin) for the commercialisation of multiple biosimilar candidates in Egypt and 18 additional countries in Africa and the Middle East. The partnership will now see the latter companies share the launch and marketing responsibilities of Alvotech-manufactured biosimilars in these regions. This kind of network approach might provide a template for overcoming the fragmented African health space, and pave the way for more European players with experience in both drug development and production. That will be key for African market penetration, as infrastructure for the logistics of biologics drugs and their application to patients is still often missing.
And there’s a final reason for biosimilar makers to focus on emerging markets like those in the Middle East and Africa. In places where an original biologic had little impact because it was never widely available, biosimilars don’t have to fight unfounded beliefs they’re just cheaper or ‘bad copies’. The doors and markets are open there for European biosimilar developers and manufacturers. But the clock is ticking.
PROTEOMICS In January, Novo Holdings invested US$40m in the eight-yearold clinical proteomics specialist Evosep Aps to benefit from the advancement of the company’s LC-MS platform that allows highly reproducible diagnostic protein panel analyses and is set to enable sensitive and high-throughput proteomebased drug discovery and biomarker/target identification in the future. Evosep said it will expand its global presence, particularly in the rapidly growing US market. According to Novo Holdings, the investment arm of Nordisk A/S, the global market for clinical diagnostics alone is estimated to yield revenues of more than US$50bn per year.
“Evosep’s technology can profoundly impact the diagnosis and treatment of diseases,” said Stephen Van Helden, the Principal of Novo Holdings, who will join Evosep’s Board of Directors. “Its platform addresses some of the most common technical pain points that have historically held back the widespread application of proteomics.”
GENOMICS FinnGen, one of the world’s leading biobank-based genomic research projects, which aims to integrate genomic information from half a million Finns, has published its first results: The flagship study, published in naturE (doi: 10.1038/s41586-022-05473-8), describes results from the data analysis based on 224,737 Finnish biobank participants. After performing comprehensive genetic analyses for more than 1,900 diseases, the researchers identified almost 2,500 genomic regions that are linked with at least one of these diseases. Among these variants, the researchers highlight 29 that are located in genes not previously linked to any disease, for example a variant in a gene called TNRC18 that predisposes to inflammatory bowel disease and other inflammatory conditions. “Even with less than half of the recruited 500,000 participants analysed

ONCOLOGY Karolinska University Hospital and Bristol-Myers Squibb have agreed to start a research collaboration in oncology and haematology. The focus will be on research and generating data with the goal of delivering innovative therapies. A committee with experts from both organisations will discuss the scope of work, including clinical trials, translational and preclinical research, and other collaborative research. The initiative aims to optimise the use of innovative therapies to inform precision medicine in cancer, potentially ranging from early-phase studies to biomarker research and real-world data.
at this stage, this snapshot of results describes a wealth of important genetic discoveries emerging from FinnGen, including novel risk and protective variants for both common and rare diseases”, says FinnGen Scientific Director Aarno Palotie.
In Finland, a strong founding bottleneck about 120 generations ago was followed by rapid population expansion. This resulted in numerous strongly deleterious alleles occurring more frequently in Finland. “The Finnish health registers containing health and medication data throughout an individual’s lifetime, allowed us to rapidly and accurately identify disease cases. This accurate phenotyping, coupled with Finnish population history is a very powerful combination for novel genetic discoveries in a wide variety of diseases”, comments first author Mitja Kurki.
FINANCING Cytovation A/S has raised NOK180m (€16.4m) in a Series A financing. The Bergen-based immune oncology company will use the funds to advance the clinical development of its first-in-class tumour membrane targeted immunotherapy CyPep-1. CyPep-1 eliminates cancer cells by forming pores in the plasma membrane, releasing cancer specific antigens to the immune system, promoting an inflammatory microenvironment and inducing a tumour-specific immune response by in situ vaccination The round was led by the Norwegian venture capital firm Sandwater and Canica, an investment company operating out of Norway and Switzerland.
ApS is not one to set small goals: The biotech company, which develops therapeutics for serious, underserved bleeding and thrombosis disorders, has stated it aims to develop five clinical assets by 2025. Now, the company has taken a big step towards that goal by raising the impressive sum of US$135m (~€128m) in a Series B financing. With the funds, Hemab will complete its ongoing Phase I/ II clinical study of lead candidate HMB001 in Glanzmann Thrombasthenia, in which the first patient has been dosed in January, and start and complete a Phase I/II clinical evaluation for HMB-VWF in von Willebrand disease.
Hemab also plans to initiate pivotal studies and advance its pipeline further.
“Hemab is fundamentally reimagining the treatment paradigm for underserved bleeding and thrombotic disorders. This financing will allow us to progress our clinical programs for the first prophylactic treatments for Glanzmann Thrombasthenia and von Willebrand Disease, delivering functional cures for patients in need,” said Benny Sorensen, CEO of Hemab.

The oversubscribed Series B financing was led by Access Biotechnology with participation from new investors Deep Track Capital, Avoro Ventures, Invus,
Rock Springs Capital, and Maj Invest Equity. Current investors including HealthCap, Novo Holdings, and RA Capital Management also invested. “We’re grateful for this robust syndicate of investors who support our approach of leveraging validated advanced technologies and deep insights into the biology of clotting to overcome decades of scientific stagnation,” said Benny Sorensen, CEO and president of Hemab. The company had already raised US$55m in a Series A led by RA Capital Management, Novo Holdings and HealthCap in July 2021.
Evaxion Biotech A/S has received FDA fast-track designation for its peptide-based cancer immunotherapy EVX-01 in combination with Merck’s PD-1 inhibitor Keytruda. The company can now proceed with its Phase IIb clinical trial, where the personalised cancer vaccine is given to patients with metastatic melanoma.
Swedish medtech company Capitainer AB launched a research collaboration with AstraZeneca plc. The aim is to use Capitainer’s novel self-sampling product delivering cell-free blood to develop protocols for biomarkers relevant to AstraZeneca’s clinical drug programmes.
ONCOLOGY Finnish pharma company Orion Corporation AB and Alligator Bioscience, a clinical-stage biotechnology company developing tumour-directed immuno-oncology antibody drugs, have expanded their existing immuno-oncology research collaboration: They have initiated a second program targeting a bispecific antibody with potential applications in solid tumours. Using Alligator’s proprietary bispecific platform, each company will provide validated monospecific binders for one tar-
get. Under the initial agreement signed in 2021, Alligator employs its proprietary phage display libraries and RUBY™ bispecific platform to develop immunooncology product candidates based on design criteria identified by Orion. Alligator remains eligible for development, approval and sales milestone payments of up to €469m across the three potential programmes, in addition to royalties if Orion exercises its options to continue development and commercialisation of the resulting product candidates.
Akiram Therapeutics AB has raised SEK68m (€6m) to start clinical studies of its targeted radioimmunotherapy for anaplastic thyroid cancer. The share issue was led by Sciety and the network Sciety Venture Partners, and was joined by Linc AB. The proceeds will go towards the GMP production of the drug candidate and initiating a Phase I clinical study.
Contract research organisation TFS HealthScience AB expanded its expertise in ophthalmology by acquiring Swiss Appletree CI Group. The takeover will bring the CRO closer to becoming a market leader in the ophthalmology field, TFS hopes.
BIOMATERIALS British researchers have created a new polymer material that may help to prevent urinary tract infections (UTIs) caused by catheters
The acrylate co-polymer reported by Jean-Frédéric Dubern and colleagues in S ciEncE a dvancES (doi: 10.1126/sciadv. add7474) have the potential to prevent 80% of hospital-acquired UTIs worldwide mostly caused by silicone or latex catheders. Preventing such cathederassociated infections could account for healthcare savings of up to US$1.7bn in the US alone.
In contrast to common catheder polymers, which can’t prevent infections through biofilm formation, encrustation, and migration via swarming, the novel acrylate co-polymer was better than antimicrobial coatings that have proven ineffective at preventing infections, mostly due to their inability to prevent swarming. A microarray screen of around 400 polymer materials, found that poly(tert-butyl cyclohexyl acrylate, tBCHA) possessed anti-biofilm properties and poly(2-hydroxy-3-phenoxypropyl acrylate) (HPhOPA) inhibited swarming. A 2.4:1 ratio of these polymers significantly reduced biofilm formation, swarming speed, and encrustation compared to silicone materials.
The biocompatible copolymer “offers a potential solution for the prevention of such infections but will require further in vivo evaluation to confirm our in vitro and ex vivo clinical findings,” Dubern et al. write.
BIOPRODUTION A €150m loan from the EIB is financing the construction of the first modular J.POD biologics production facility in Europe. According to German Evotec SE the plant will be constructed in Toulouse. Evotec’s 12,000 m2 biologics manufacturing plant is expected to facilitate scaling up from fedbatch facilities, create 200 jobs and enable new partnerships and corporate bridge financing. The loan, repayable over sev-
en years each, is low-interest and will be disbursed over three annual tranches. Additional €50m will be provided by the French Government, Occitanie Region, Bpifrance, Haute-Garonne Prefecture and Toulouse Métropole. The current financial agreement, as well as a €75m loan from the EIB to Evotec in 2017, was developed together with the kENUP Foundation, a non-governmental organisation promoting innovation in Europe.
PVH Corp has joined the fibre-to-fibre consortium established in January 2023 to support circular reuse of synthetic fossil-based PET fibres through French Carbios SAS patented cutinase technology. The consortium members include On, Patagonia, PUMA and Salomon. Carbios’ esterase-based technology can recycle blended PET feedstocks, therefore reducing extensive sorting required by current thermomechanical recycling methods. For mixed fiber textile materials, Carbios’ patented cutinase acts solely on the PET polyester found within. This process creates recycled PET (r-PET), equivalent in quality to virgin PET, that will take advantage of German T.EN Zimmer GmbH’s monomer repolymerisation technology to create 100% recycled PET. Deloitte will carry out an environmental performance analysis of the process for plastic and textile waste to produce new textile fibers. Globally, only 13% of textile waste is currently recycled.
During the two-year collaboration, the consortium will collaborate to deliver thorough sorting and dismantling technologies for complex textile waste from apparel, underwear, footwear and sportswear. The ultimate aim is to prove fiber-to-fiber closed circularity using Carbios’ process at an industrial scale to support the partners’ sustainability goals.
Next year, Carbios will start the very first 8,000 ton PET waste facility in Europe which is supported with €3.3m of EU money from the „LIFE Cycle of PET“ project. This comes in anticipation of future regulations, such as the separate collection of textile waste to be made mandatory in Europe from 1 January 2025.
The plan is to reduce the waste from the 14.7 Mt of PET (60% in textiles, 40% in bottles) consumed annually in the EU by at least 30% by 2030 and licence the technology to eight major PET producers worldwide.
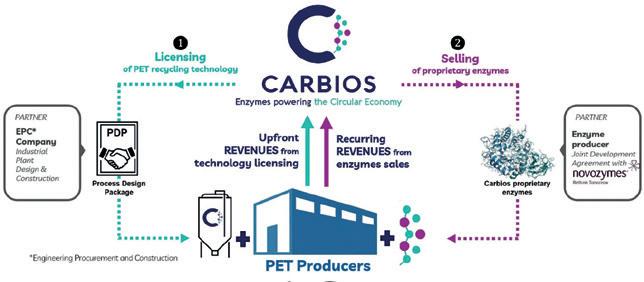
CONJUGATES In mid-February, Amsterdam-based Synaffix BV has licenced its ADC platform to South Korean Chong Kun Dang Pharm. Though the companies did not give any financial details, former licencing deals of Synaffix to global pharma companies such as Amgen ranged between €400m and €500m per programme including upfront and milestone payments. Under the deal with the South-Korean Chong Kun Dang Pharm, Synaffix will provide access to its proprietary Antibody-Drug Conjugate (ADC) technologies GlycoConnect™ and Hy-
draSpace™, in combination with an undisclosed toxSYN™ linker-payload, to develop an ADC portfolio against a single cancer target.
Under the terms of the agreement, Chong Kun Dang Pharm will be responsible for the research, development, manufacturing and commercialisation of appropriate ADCs produced by Synaffix.
Synaffix has deals with Amgen, ADC Therapeutics, Mersana Therapeutics, Shanghai Miracogen, Innovent Biologics, ProfoundBio, Kyowa Kirin, Genmab, Macrogenics and Emergence Therapeutics. L
GREEN CHEMISTRY Dutch bioplastics specialist Avantium NV has inked a five-year agreement to supply German Henkel KGaA with plant sugar-based furandicarboxylic acid (FDCA), a building block of polyethylen furanoate (PEF) bioplastics, in Janurary. According to Avantium, PEF provides a fully degradable alternative to oil-based polyethylene terephtalate (PET). Starting from 2024, Henkel will buy FDCA from Avantiums’s new production plant (see photo) to bring environmentally friendly high-performance polyurethane adhesives for electronic applications to market. Henkel and Avantium have been partners in the PEFerence

consortium since 2019, which is building a supply chain for both FDCA and polyethylene furanoate (PEF), a renewable bioplastic for the beverage and textile industries. Like many chemical giants, Henkel wants to polish its sustainability image. “Avantium’s 100% plant-based FDCA supports our strategy to incorporate renewable carbon into our technology roadmap while improving the performance of our products,” said Adrian Brandt, head of the biorenewable materials team at Henkel Adhesive Technologies. “We see great potential for FDCA in application areas beyond electronics, such as packaging, wood construction or textile lamination,” he added. L
Belgian remyelination specialist Rewind Therapeutics BV has increased its 2018 Series A funding led by new investor Sunstone Life Siences A/S. The company, founded by CD3 (Leuven, Belgium) and Axxam S.p.A. (Milan, Italy), did not reveal the exact amount added to the €15.2m in its 2018 Series A financing and subsequent 2019 €2.9m funding to screen small molecule GPCR blockers and remyelination inducers, it now wants to advance to clinical testing. About a dozen companies are active in remyelination to cure multiple sclerosis and similar neurodegenerative disorders.
Toulouse White Biotech (TWB) has signed a strategic partnership agreement with Canadian multinational Premier Tech. Under the contract, a dozen team members from Premier Tech’s Life Sciences group will move to TWB’s premises full time to accelerate the development of new natural alternatives to antibiotics in animal nutrition and health treatments, and as functional solutions in the human nutrition and health sector.
Phage therapy specialist
Vésale Bioscience BV has bagged €1.8m from European Innovation Council (EIC) to fight antimicrobial resistance (AMR). Under the grant provided by the EIC Accelerator Fund for the PhageDiag project, the Belgian company will develop an AI-based phage therapy diagnostic platform to fight multidrug-resistant infections.
LABWARE Cologne-based BioEcho Life Sciences GmbH, a biotechnology company specialising in nucleic acid extraction products and services, has been granted two new patents (No. US 11,578,319 B2 and No. JP 7122373 B2, respectively) for its EchoLUTION technology for nucleic acid extraction by both the U.S. Patent and Trademark Office and the Japan Patent Office.
“The existing intellectual property/patent protection landscape is very dense these days, making this news a significant and exciting recognition for our company. Our patent approvals in Japan and the US prove that BioEcho’s technology is truly innovative,” comments Dr Markus Müller, CEO of BioEcho.
The patents strengthen BioEcho’s intellectual property and represent the company’s idea that even traditional routine molecular biology protocols can be improved. For decades, silica-based methods have been used for DNA/RNA extraction, involving multiple washing steps before the purified nucleic acids are eluted. The technology enables DNA and RNA extraction in a single centrifugation step. “EchoLUTION is unique in that it reverses the principle of purification: not binding, washing and eluting first, but eluting directly,” Markus Müller explains the simplicity of the technology. Due to the smaller number of steps, fewer consumables are also needed, which leads to an enormous reduction in the amount of plastic, but also in the chemicals used in purification, leading to a more sustainable laboratory supply.
ACQUISITION BioNTech SE will pay £562m in cash to acquire its former partner InstaDeep Ltd. to expand its capabilities in AI-based drug discovery.

According to Bio NTech, the acquisition is part of its strategy to “build worldleading capabilities in AI-driven drug discovery and the development of nextgeneration immunotherapies and vaccines”. In addition to the approximately 240 highly skilled professionals with expertise in AI, ML, bioengineering, data science and software development, the Mainz-based company will also gain ac-
cess to a network of global research collaborations in this field and presences of the combined company in locations in the US, Europe, Africa and the Middle East. This is of enormous importance for recruiting employees in these high-demand fields, the company says.
The acquisition builds on the successful collaboration between the two companies that has been in place since 2019. Only shortly afterwards, in autumn 2020, they announced a multi-year strategic collaboration as well as the establishment of a joint AI Innovation Lab. L
CRISPR AND BEYOND As part of a strategic cooperation, BRAIN Biotech AG in Zwingenberg and A M silk GmbH (Munich) are combining their expertise in the fields of high-performance biomaterials, such as recombinantly produced spider silk, and rational protein design. The aim of the cooperation is to establish sustainable protein fibres instead of petroleum-based fibres and to offer them to the textile market. Also in the novel food industry, Brain has a footprint in developing fermentative enzymes and for example collaborates with Berlin/ Frankfurt based Formo Bio, who produces lab grown milk and cheese products from casein. But BRAIN was even more busy in the first few weeks and months of 2023 to spin-out the proprietary genome editing development platform which overcomes the heavily patent protected operational CRISPR-Cas9 space. The experts in microbiological data mining have found a multitude of highly divers classes of Cas enzymes and will bundle this know-how and its own universe of patent rights in the spin-off company Akribion Genomics. Just before regular foundation, US-company TransCode Therapeutics (Nasdaq: RNAZ) and Brain/Akribion Genomics published a joint development agreement to use a cell killing Cas-enzyme variant in oncology. L
AGROTECH At the beginning of February, the Swiss Federal Council approved the report of the expert commission on the regulation of new genetic engineering methods.

The report of the Federal Council states that new genetic engineering methods and their plant breeding products, for example via CRISPR-Cas, should be specifically regulated by the law, but not generally treated as genetically modified organisms (GMOs). One of the greatest challenges with the new techniques still is traceability, since the current detection methods do not allow
clear conclusions to be drawn for the mutations caused by the new genetic engineering techniques.
The Advisory Committee on Agriculture (BEKO) appointed by the Swiss Federal Council welcomes the broad interpretation and concludes (by a majority) that special treatment of new genetic engineering techniques with a risk-based authorisation regime is justified. The BEKO writes that it would be preferable to adopt a case-specific approach, which has yet to be defined, and which incorporates elements of process and product authorisation.
SYNTHETIC BIOTECH The Austrian company Ribbon Biolabs GmbH, residing north of Vienna at the IST-Cube, is providing long DNA – in lengths beyond 10KB. The underlying technology is to combine paired DNA-strands from a pool of DNA fragments via sticky ends to build such very long sequences.
These DNA synthesis technologies (also provided by others like Twist Bioscience) are elementary to close the gene writing gap in the many approaches where synthetic biotechnology is
used for creating new DNA-software. The DNA synthesis industry is rapidly growing. New industry partnerships have been formed to introduce innovative technologies in the enzymatic DNA synthesis space. Examples are the joint ventures between Codexis and Molecular Assemblies and between Integrated DNA Technologies Inc and Danaher Inc. Ribbon Biolab´s technology was now featured in an article by Hoose et al. in n atur E c h E mi S try r E vi E w, https://doi. org/10.1038/s41570-022-00456-9
The Austrian Life Sciences research funding programme continues to promote entrepreneurial, applied research in Austria with a focus on life sciences. In order to provide targeted support for research into medicines and medical products, the “Austrian Life Sciences” package was initiated by the Ministry of Economics last year with a total volume of €50m. Of this amount, €11.5m have been approved for project funding so far. This means that around €40m are still available for funding further projects in the current year. This also applies for companies and research being just relocated to Austria.
LegoChem Biosciences Inc. from South Korea announced a licence agreement with Elthera AG, a biotechnology company in Switzerland, to develop and commercialize a novel antibody-drug conjugate (ADC) therapy using a monoclonal antibody developed by Elthera. The licensed asset is a monoclonal antibody with a target expressed in a variety of solid tumours including pancreatic, ovarian, breast, lung, and colorectal cancer. “We are very pleased to enter this licence agreement with LegoChemBio,” said Anne Schmidt, CEO of Elthera.
A larger selection of news and views from Germany, Austria, Switzerland can be obtained in the sister magazine |transkript or online: www.transkript.de
DRUG REPURPOSING Anticancer drugs were shown to limit inflammation and are as such potential treatments for sepsis. In a study published in late 2022, the Portuguese Instituto Gulbenkian de Ciência in collaboration with the Instituto de Medicina Molecular, the Institute of Structural Biology and the European Molecular Biology Laboratory in Germany, and the Leiden University Medical Center in the Netherlands found anthracyclines to control relevant inflammatory genes in the immune system cells and might thus be an effective treatment for exaggerated inflammation, such as sepsis or rheumatoid arthritis.
FUNDING Portugal’s renewable energy supplier Greenvolt – Energias Renováveis SA harnesses the wind, the sun and biomass by using and developing multiple technologies. In January, they announced their agreement with US global investment fund manager Kohlberg Kravis Roberts LP (KKR) to issue bonds worth €200m, maturing in seven years. The planned issue of convertible bonds is subject to approval by shareholders at the next general meeting, to be held on May 31st. This fund will go towards the continuation of Greenvolt’s projects in the transition to green resources, renewable energy and a circular sustainable economy.
JOINT VENTURE From athletic footwear and the beauty industry all the way over to the aerospace industry, plastic is an unavoidable material. Countless companies are currently working on the development of plastic alternatives. The Israeli company Smart Resilin is genetically engineering Resilin, an elastomeric protein found in insects, that is considered to be the most elastic material in nature. With that, the company created a raw substance which is cheaper and easier to produce than other silk-based recombinant structural proteins used in the industry.
As announced in January, Smart Resilin now partnered with Acies Bio Ldt, a biotech company from Slovenia, to further develop and commercialise resilin. The joint
DRUG DEVELOPMENT AptaTargets SL developed a neuroprotective drug to use alongside the standard surgical removal of clots that cause strokes, to reduce the volume of damaged brain tissue. Their preliminary study shows the drug reducing mortality from 18% to 5% in a Phase Ib/ IIa trial in acute ischemic stroke patients, as well as brain damage and disability. According to the researchers, the new medication, called ApTOLL, shields brain tissue from ongoing damage by cooling down inflammation. For this achievement, AptaTargets was awarded €2.5m from the Spanish government for a Phase IIa clinical trial to evaluate and further develop the cardioprotective effect. Confirmatory studies, in larger populations, are to start in the last quarter of 2023.
venture will combine Smart Resilin’s process technology and Acies Bio’s expertise in genetic, microbiological, and scale-up development. The aim is to provide a nontoxic, biodegradable protein for various potential products.
CLINICAL TRIAL In January, Newron Pharmaceuticals SpA announced their six month interim findings from its exploratory clinical Phase II study assessing evenamide as add-on therapy for patients with treatment-resistant schizophrenia, who show no adequate response to the commonly used clozapine medication.
The Milan-based biopharmaceutical company, which focuses on the development of novel therapies for patients with central and peripheral nervous system diseases, presented promising results from the first 85 enrolled patients in its evenamide study after 30 weeks of therapy. Evenamide is an orally administered blocker of voltage-gated sodium channels, thereby normalising the release of glutamate and modulating repetitive neuronal firing. The findings show continued improvement of the treatment-resistant schizophrenia symptoms after six months.
This year Newron intends to begin a key trial in treatment-resistant schizophrenia joining the US-based biotech Karuna Therapeutics Inc., who is planning to submit a xanomeline-trospium drug application to the FDA by mid-2023.
BIOSTIMULANTS The excessive use of fertilizers of mineral origin leads to the acidification and eutrophication of seas and oceans, where the non assimilated nitrogen and phosphorus ends up. This ecological hazard is fought by the “Nutrialgae” project launched in 2020 in Spain, which achieves its goal of reducing the use of chemical fertilizers by 30% while increasing crop productivity by up to 20% with the help of biostimulants. This project is part of the Ocean Innovation Challenge Initiative (OIC) and the United Nations Development Program (UNDP), and was created by the marine biotech company Ficosterra SL in collaboration with the Center for Scientific Research and Higher Education of Ensenada, Mexico, GN Productores Agrícolas, Mexico, and the Hassan II University of Casablanca, Morocco. Part of the reward was the development of
a support program for the company to do more research. Ficosterra’s aim has been to complement the conventional fertilization program with biostimulants made out of seaweed and microorganism-extracts. These prevent contamination of aquifers, caused by phosphorous, nitrogen, and potassium residues that are not fully assimilated by crops.

With their trials on broccoli-cultures, both in the field and in the laboratory, they show that the use of their biostimulants, cystium-k® and ficosagro®, which are certified by organic agriculture, allows to significantly reduce mineral fertilizers without reducing crop productivity. The biostimulants increased agricultural yields by 15% on average, while decreasing ocean pollution. Ficosterra is currently planning technology transfer services to assist nations in meeting their sustainability goals
Methinks Software SL, a digital health company specialising in early detection and management of acute stroke through the use of artificial intelligence, received the CE mark in February for its medical imaging software Methinks Stroke Suite, which assists in detecting large vessel occlusions, allowing commercialization in Europe and assisting in emergency settings for the benefit of patients.
Progressive Supranuclear Palsy (PSP) is a rare neurological condition that causes problems with walking, balance, speech, swallowing and vision. The Swiss clinical stage biotechnology company Asceneuron SA developed a chemical entity at clinical stage for the treatment of PSP, ASN90. In February 2023 they announced the signing of a licensing agreement with Spanish pharmaceutical company Grupo Ferrer Internacional, SA by which Ferrer obtains the exclusive worldwide rights to develop and commercialize ASN90.
BREAST CANCER The Italian pharmaceutical and diagnostics company A. Menarini Industrie Farmaceutiche
Riunite Srl announced today that the US Food and Drug Administration has approved their elacestrant ORSERDU for the treatment of postmenopausal women or adult men with ER+, HER2- or ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine
hormonal therapy. These mutations are present in up to 40% of such cases. It works by blocking the effects of estrogen on hormone receptor-positive breast cancer cells. The elacestrant will be commercialised in the United States by Stemline Therapeutics Inc, a whollyowned affiliate of the Menarini Group based in New York and focused on de
livering innovative oncology therapies to cancer patients.
Chiesi Global Rare Diseases Inc, a division of Chiesi Farmaceutici SpA, announced at the beginning of February that the FDA approved Lamzede ® (velmanase alfa-tycv) for the treatment of non-central nervous system manifestations of alphamannosidosis (AM) in adults and children. AM is an uncommon, progressive lysosomal storage disorder induced by a-mannosidase enzyme deficiency.
ENERGY Finnish plant manufacturer Valmet is converting the coal-fired power plant of Hungary’s CHP-Invest Kft in Oroszlány to “to-be-sustainable”. Through boiler conversions and emission reduction solutions that will be finished by Q2/2024, renewable biomass-based fuel will be burned CO2-neutrally in the power plant instead of coal in the future. The €25m order includes the conversion of two coal-fired boilers to bubbling fluidised bed combustion that can be run mainly on biomass fuel.
“Our company intends to include environmentally conscious solutions in all its investments, targeting a long-term solution for the sustainability of the industry. We are gradually converting our existing coal-fired power plants to run on more environmentally friendly and sustainable fuels. After the recommissioning, the Oroszlány power plant will produce more than 600 GWh of renewable electricity, making up about 1.5% of today’s electricity consumption in Hungary,” says György Palkó, CEO of Veolia Energia Magyarország Zrt.
Bioethanol industry representatives and the German government are currently in a conflict about the energetic use of biomass as the green party ministers favour material cascade use over energetic use of biomass. They argue that the CO2 fixed in materials is released back into the atmosphere more slowly than that bound in energy crops. The bioethanol industry points to a 99% reduction in CO2 compared to the burning of fossil resources.
The European Investment Bank (EIB) has granted a €18m loan to Estonian Icosagen AS (Tartu) that will support the financing of Icosagen’s new cGMP-compliant production facility for biologics and to strengthen its R&D technology platforms. The total investment in the project exceeds €40m. Icosagen expects to kick-start the biological drug industry in Estonia by providing lots for clinical testing.
The construction of the new 1,600m2 production plant expands on Icosagen’s existing laboratories in Tartu, and will be completed in September 2023. The plant is expected to become operational in 2024. This new facility will help Icosagen become a one-stop shop for its biotechnology and pharmaceutical clients, and a full contract research, development and manufacturing organisation (CRDMO), offering seamless discovery, development and manufacturing capabilities of protein drug candidates in mammalian cells.
“We have steadily been working towards this important milestone for a long time,” said CEO and founder of Icosagen Professor Mart Ustav, Ph.D. “Developing
novel technologies and workflows will help to increase our competitiveness in providing contract research and development service to our clients in Europe and other regions of the world. The cGMP facility will provide further state-of-theart training and employment opportunities for local and regional scientists and, at the same time, expand the capabilities and impact of the European biopharmaceutical drug production industry. We are very grateful to the EIB for their crucial support in this endeavour.”
Senior Director Business Development of Icosagen Cell Factory Dr Oliver Schub added: “In 2022, Icosagen Cell Factory worked with over 100 protein drug/vaccine developing companies, in well over 500 projects, producing more than 3,000 different recombinant proteins. Our partner clients can now seamlessly proceed from antibody discovery, over transient preclinical protein production, to stable CHO cell line development, and clinical GMP manufacturing, all from one team on a single site. Our unique CRDMO concept results in minimised development timelines, costs and project management efforts.”
DRUG DEVELOPMENT Czech PPF
Group-owned immuno-oncology specialist SOTIO Biotech SL dosed the first of 52 colorectal cancer (CRC) patients with its IL-15 superagonist nanrilkefusp alfa (SOT101)plus cetuximab in a non-blinded Phase II multi-centre trial without control arm, in mid-January. “In addition to our previous and ongoing studies to determine the efficacy of nanrilkefusp alfa in combination with checkpoint blockers, we now aim to determine its ability to enhance the antibody dependent cellular cytotoxicity (ADCC) exerted by cetuximab,” commented Richard Sachse, CMO of SOTIO. In animal models the biologic increased anti-tumour activity and prolif-
eration of cytotoxic T cells. Patients enrolled have been stratified to show the RAS wild-type, had a prior chemotherapy with irinotecan- and oxaliplatin. Currently, patients with advanced refractory colorectal cancer have poor long-term survival rates. Nanrilkefusp alfa (SOT101) is a subcutaneously administered IL-15 superagonist that is fused to the sushi+ domain of the IL-15 receptor alpha chain. In disease models it has demonstrated increased long-term survival and tumour regression, as well as a favourable toxicology profile. Preclinically, it synergised with checkpoint blockers and ADCC enhancing antibodies. IL-15 is a T killer cell and NK cell booster
16/17 MAY
THE LARGEST NETWORKING EVENT FOR LIFE SCIENCES AND HEALTHCARE INDUSTRIES IN BERLIN



























Keynote and Sessions | Matchmaking | Industry Exhibition | Cooperation/Venture Track | Speed Lecture Award | Networking

New technologies changing the way we diagnose and cure diseases







Register now for free: bionnale2023.b2match.io
PLATIN SPONSORS
GOLD SPONSORS
SILVER SPONSORS
MEDIA PARTNERS
In February, British Owlstone Medical plc reported the identification of seven volatile organic compounds (VOCs) that reliably differentiated 46 patients with advanced liver disease such as cirrhosis from 22 healthy controls (doi: 10.14218/JCTH.2022.00309). The study is part of the company’s strategy to receive US Food and Drug Administration (FDA) approval for exhaled biomarkers for diagnosis and monitoring of chronic liver diseases such as NASH, NAFLD and cirrhosis. A subset of 11 VOCs was correlated with blood metrics of liver function (bilirubin, albumin, prothrombin time) and separated patients by cirrhosis severity using principal component analysis.
Disease-modifying therapies for multiple sclerosis (MS) may positively influence the composition of the intestinal flora. This is indicated by a pilot study carried out by scientists from the University of Basel and the University Hospital Basel under Prof. Dr. Adrian Egli with 20 MS patients who received dimethyl fumarate therapy (gut microBES. doi: 10.1080/19490976.2022.2147055).
Significantly fewer MS-associated pro-inflammatory gut microbes such as A. muciniphilia and C. eutactus appeared after the first three months of DMF treatment within the longitudinal study. Furthermore, the presence of the bacterial strain Akkermansia muciniphila and the absence of Prevotella copri proved to be a risk factor for lymphopenias. The doctors now want to evaluate this diagnostic potential in larger studies.
DIABETES So far, not much is known about the exosome-based signalling system in the body, only that blood cells and cancer cells lace signalling molecules into the 50-90µm large vesicles, triggering cell programming in target organs. Now German scientists have discovered that fat cells and the pancreas can counteract insulin resistance. In February, they delineated the role of adipocyte-derived extracellular vesicles (AdEVs) as messengers that regulate the blood glucose level. Pairing fluorescence AdEV-tracing and SILAC-labeling with (phospho)proteomics, the group of Kerstin Stemmer at German Center for Diabetes Research in Munich revealed that AdEVs transfer functional insulinotropic protein cargo into pancreatic beta-cells. Upon transfer, AdEV proteins were subject to phosphorylation, augmented insulinotropic GPCR/cAMP/PKA signaling by increasing total protein abun-
dances, phosphosite dynamics, and ultimately boosted glucose-stimulated insulin secretion in murine islets. Notably, insulinotropic effects were restricted to AdEVs isolated from obese and insulin resistant, but not lean mice. Likewise, in vivo pretreatment with AdEVs from obese but not lean mice amplified insulin secretion and glucose tolerance in mice.
“This data suggests that secreted AdEVs can inform pancreatic beta-cells about insulin resistance in adipose tissue in order to amplify glucose-stimulated insulin secretion in times of increased insulin demand. “Overall, extracellular vesicles have very wide application potential for the diagnosis and therapy of a wide variety of diseases,” said Stemmer. “Our ongoing studies are aimed at loading the vesicles in a targeted manner in order to be able to use them for therapeutic purposes.” L
FATTY LIVER DISEASE Dutch researchers from Hubrecht Institute headed by Roche’s new pRED chief Hans Clevers have found a novel drug candidate using human organoid models that capture different triggers of fatty liver disease development (naturE BiotEchn., doi: 10.1038/ s41587-023-01680-4). Firstly, they “fed” the organoids with a mixture of fatty acids to mimic a Western diet and witnessed the rapid development of fatty liv-
er organoids. Using CRISPR prime editing, the team introduced the top risk mutation for fatty liver disease into their organoid system that increased severe fat accumulation in organoids. Finally, the researchers also modelled genetic lipid disorders using CRISPR-Cas9 to investigate how these disorders influence the development of fatty liver disease. These mutant organoids spontaneously developed severe fatty livers as a result of a build-up of sugar-derived fats.
Spontaneously forming fatty liver organoids. Lipids are depth-colored.
Screening a drug library, the researchers identified a drug subset that had in common that the generation of lipids from sugars was blocked. Organoids having the top risk mutation for fatty liver disease did not react to all drugs in the same way. Using a CRISPR-based loss-offunction screening platform named FatTracer, they found that overexpression of fatty acid desaturase-2 reduced the fat content of the organoids, suggesting it is a potential novel therapeutic target.

NUTRITION A UK Biobank analysis of data from 200,000 people over more than ten years, carried out by British researchers, have linked consumption of ultra-processed foods to an increased risk of developing and dying from cancer. However, according to the authors from Imperial College London it doesn’t provide a causal relationship.
Long-term prospective data from the UK Biobank support the assumption that convenience foods and other ultra-highprocessed (UPF) foods are hazardous to health. While higher UPF consumption has been linked to increased risk for obesity and cardiometabolic diseases, prospective evidence on cancer outcomes was limited so far. In February, a FrenchBrazil-Portuguese research team headed by Dr Eszter Vamos from Imperial College London demonstrated that UPFs such as breakfast cereals, protein concentrates, mass-produced packaged bread, cheese or ready meals are significantly increasing the risk of developing almost three dozens of different cancers. The most comprehensive assessment to date of the association between ultra-processed foods and cancer risk is not yet a proof that processed foods cause cancer, however, is an important risk factor.
It’s not yet clear if the study is bad news for organic foodstores selling ready meals or vegan protein makers as first author Kiara Chang told Europ E an B iot E chnology magazine: “We assigned plant-based meat and dairy substitutes to the ultra-processed food category if they used artificial ingredients or additives [such as food additives to adjust colour, flavour, consistency, texture], but it is not within scope of this study to explore the difference between protein sources [such as insect, plant, microbial or cell-culture-based protein] so I am afraid we are not able to provide a comment at this stage.” However, consumers are well advised to pay attention to the degree of processing and the list

of ingredients when buying supposedly healthy or sustainable food.
The study used UK Biobank records to collect information on the diets of 200,000 middle-aged adult participants. Researchers monitored participants’ health over a ten-year period, looking at the risk of dying from 34 types of cancer overall as well as the specific risk of developing each.
According to the study results, higher consumption of UPF was associated with a greater risk of developing cancer overall, specifically with ovarian and brain cancers. It was also associated with an increased risk of dying from cancer, most notably with ovarian and breast cancers.
For every 10% increase in UPF in a person’s diet, there was an increased incidence of 2% for cancer overall, with a 19% increase for ovarian cancer specifically. Each 10% increase in UPF con -
sumption was also associated with increased mortality for cancer overall by at least 6%, with a 16% and a 30% increase for breast cancer and ovarian cancer, respectively. These links remained after adjusting for a range of socio-economic, behavioural and dietary factors, such as smoking status, physical activity and body mass index (BMI).
The team also confirmed earlier studies that found out that higher consumption of UPF was associated with a greater risk of developing obesity and type 2 diabetes, and a greater weight gain in UK children extending from childhood to young adulthood. “This study adds to the growing evidence that UPFs are likely to negatively impact our health,” said Vamos. “Given the high levels of consumption in UK adults and children, this has important implications for future health outcomes.” L
t.gabrielczyk@biocom.eu
April 24-25, 2023
As one of the leading biotechnology conferences, the Swiss Biotech Day has developed into a truly global networking day, attracting many international delegations.
What you can expect:
› Meet 1,000+ senior experts from the life science industry
› 70+ exhibitors and global village with international delegations from all over the world
› Swiss Biotech Success Stories Awards
› Innovative biotech start-ups and medium-sized biotech companies
the opmodern bioor indusapplying these SBA takes working biotechnology.
Consortium is an includes support white growth. The OECD (see pages programmes and useimportance so that education. the right profits
› Thematically focused panel discussions
› Swiss Biotech Report 2023
sector is internationally visible. The project-specific participating companies (most of them young and internationally less savvy) find a comprehensive partner which is helping to put them in the public window.
SWISS BIOTECH...
› Pre-scheduled one-to-one partnering meetings
The participating Life Science Regions are important internal carriers of the dynamics in the Biotech sector, thus enhancing the common understanding of the industry. This and more knowledge is brought into Europa Bio, the European Biotech Association, where the SBA is an active member.
› General Assembly of the Swiss Biotech Association

...is an alliance of four leading Biotech regions of Switzerland (Bio Alps, BioPolo Ticino, Basel Area and Greater Zurich Area). They have combined efforts to streamline interests of the national biotech sector. The SWX Swiss Exchange holds a leading position in terms of lifescience listings and offers companies from that industry – be they located in Switzerland or abroad – access to an internationally recognised financial marketplace. The initiative was co-founded by the SBA which also manages the executive office of Swiss Biotech.
Sign in on our website at www.swissbiotechday.ch and stay updated about any news.

Media partners:
Organized by:
© Swiss Biotech AssociationNETWORKING With a highly specialised profile, Chemspec Europe is a key event for the fine and speciality chemicals industry, taking place from May 24 th -25th at the Messe Basel exhibition centre in Switzerland.
Opening times: from 9 am to 5 pm on both days of the show
Visitor registration for a free ticket for the two days of the show is now open on the show's official website: www. chemspeceurope.com. Free registration is available until May 9th, before the ticket price increases to CHF 50 from May 10th. On-site ticket purchases will incur a further rise to CHF 70.
For more information on exhibitors, products & services showcase, conference agenda and travel, please visit www.chemspeceurope.com L
Chemspec Europe brings together suppliers and purchasers looking for bespoke products or specific substances to enhance a product/drug or develop new chemical solutions. The exhibition is a powerful gateway to global business and industry knowledge exchange.
Chemspec Europe is an international event with a solid representation of Swiss, German, Austrian, French, Belgian, American and Indian firms. The exhibitor list includes Arxada AG, CABB AG, ESIM Chemicals GmbH, ORGANICA Feinchemie GmbH Wolfen, PI INDUSTRIES LTD, Robinson Brothers Limited, Saltigo GmbH (Lanxess), SEQENS SA, Society of Chemical Manufacturers & Affiliates (SOCMA), Sumitomo Chemical Europe NV, Weylchem International GmbH and many more.

Discussions, exchange of expertise and R&D are an integral part of Chemspec Europe. Look forward to a first-rate conference programme and benefit from exciting insight into ongoing R&D projects, interesting discussions and valuable networking opportunities. The conference programme takes place in five theatres:
Agrochemicals, Pharma, the Royal Society of Chemistry Symposium, Regulatory Services and Innovative Start-ups. Details on the conference programme will be released in due course.
Chemspec Europe offers a free-ofcharge Matchmaking Programme to enhance visitors' networking opportunities on-site. The matchmaking platform is an important time-saving asset for visitors of the show, aiming to connect visitors and exhibitors with complementary buyer and seller interests. A compatibility function will help attendees find the perfect match and organise private meetings with industry suppliers ahead of the event. Visitors who register via the Chemspec Europe website will be invited to use the networking service at a later stage.
Chemspec Europe 2023
36th International Exhibition for Fine and Speciality Chemicals
May 24th –25th 2023 | Hall 1, Messe Basel, Switzerland
Christiane Beck -
Chemspec Europe Event Manager, RX
“The chemicals industry is one of Europe's largest manufacturing sectors and plays a pivotal role in providing innovative materials and technological solutions. It operates in a rapidly changing market and faces many challenges. The roadmap for the European Green Deal sets the expectations that, by 2050, the EU chemicals sector will need to become climate neutral, digitalise its processes and transition to safe and sustainable chemicals, whilst adapting to changing legislative measures and a circular economy. Choosing the right suppliers and exchanging knowledge within international industry networks is more important than ever. Chemspec Europe 2023 is the ideal platform for enabling new learning opportunities, networking with industry peers and showcasing this dynamic industry.”
Looking for new standards in mixing, temperature control, automation, safety and design? Here comes the perfect HABITAT
HABITAT research is the new smart laboratory bioreactor from IKA. As the first bioreactor with a lid stand, it ensures ergonomic working and a tidy laboratory. In combination with a circulator, it is also your fermenter. And if you connect light panels, your new photobioreactor.

www.ika.com/habitat

ANNEX 1 Modern vaccines, patient-specific therapies, robotic isolators - regulatory as well as scientific and technical changes and innovations require pharmaceutical and biopharmaceutical manufacturers to keep up with the latest developments in manufacturing and quality assurance.

With becoming effective of several regulatory directives – like the European Directive EC 1394/2007 for Advanced Therapy Medicinal Products (ATMPs) – ATMPs have been classified as medicinal products and have thus to comply with EU requirements for medicinal products. However, many of these products are currently developed and manufactured at universities or in small and medium-sized enterprises and differ from classical pharmaceuticals in terms of manufacturing conditions (e.g., open manipulation during extraction at the surgical level, short
shelf lives). This leads to challenges for manufacturers in terms of quality, safety, GMP aspects and regulatory approval.
The EU tried to address this with its own “Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products”. However, after four years, this still leads to new questions. For instance, even though the EU GMP Guide Annex 1 for aseptic manufacturing has been comprehensively revised with the involvement of the WHO, the PIC/s and the FDA and thus represents the current state of science and
technology, it is still not clear whether it can simply be ignored for sterile products falling under the ATMP guidelines. This is a question that will probably have to be clarified by the authorities in the near future.
Among other topics, e.g. Process-, QCand Release Strategies for ATMPs, this question will also be discussed during the ATMP Track of the PharmaCongress 2023 on 28/29 March in Wiesbaden. You will find details at www.pharmacongress.com.
Axel H SchroederGMP for ATMPs vs Annex 1
Join the conference track ATMP – Manufacturing, Quality & Safety as part of the PharmaCongress on 28/29 March at the RheinMain CongressCenter in Wiesbaden and learn from peers what the challenges are in manufacturing – as the GMP regulations – but also about quality control issues, appropriate ways to maintain, assure and control the expected quality.

European Biotechnology Network
Europe: european-biotechnology.net
Europe: yebn.eu

Europe: ecbf.vc
Austria: biotechaustria.org

Austria: lifescienceaustria.at
Switzerland: swissbiotech.org

BIOPLASTICS At the 17th EUPB Conference, European Bioplastics (EUBP) presented a positive outlook for the global bioplastics industry, based on market data compiled in cooperation with novaInstitute. “After a stagnation of the overall plastics production in 2020, mainly due to challenges posed by the COVID -19 pandemic, there is now a new momentum for global bioplastics production,” the European bioplastics industry association said. Capacity of production is set to increase significantly from around 2.23 million tonnes in 2022 to approximately 6.3 million tonnes in 2027. Packaging remains the largest field of application with 48 percent of the total bioplastics market in 2022. “The positive development of bioplastics production capacity needs to be seen within the wider global context of a climate crisis, rising energy costs, and disrupted value chains. Yet, despite these challenges, bioplastics production capacity is growing. Once again, this shows the resilience and importance of our industry”, states Hasso von Pogrell, Managing Director of European Bioplastics.
Germany: gasb.de

Europe: medicinesforeurope.com
hollandbio.nl
Germany: biodeutschland.org Portugal: www.p-bio.org UK: biopartner.co.uk France: france-biotech.org
Italy: assobiotec.it

swedenbio.com Hungary: hungarianbiotech.org
EuropE an BiotEchnology covers the biotechnology sector of the current 27 EU member states, Norway, Switzerland, and UK. If you would like to subscribe, please refer to european-biotechnology.com
WOMEN IN SCIENCE On the occasion of the International Day of Women and Girls in Science in February, the Spanish Bioindustry Association AseBio launched its first programme to pair professional women from the Spanish biotech sector with young students. This new initiative, “Day with a #BiotechWoman”, had talented young women meet and closely follow the professional work of the biotech industry for a whole day. “With this initiative, we want to expose young people to different professional careers within a biotechnology company and also offer women in our sector more direct contact with universities, facilitating the exchange of knowledge and life experiences,” explained AseBio Chairwoman Ana Polanco.
BASEL Switzerland’s most important biotech conference will once again attract more than 1,000 life science experts to Basel on April 24 and 25, 2023. The networking event is all about new partnerships, innovation, research and international collaboration.
The Swiss Biotech Day in Basel is a permanent fixture in the biotechnology community’s calendar. With close to 1,200 participants from 36 nations, it again demonstrated in 2022 the impressive growth of the Swiss biotech industry and offered an exciting program with ideal networking opportunities across borders. One of Europe’s most important biotechnology conferences is equally attractive for biotech companies, their suppliers, investors and pharma partners.
During one and a half days, the Swiss Biotech Day will again stage high-level
experts from the life science industry, offer space for networking, discussions and to discover trends in R&D, manufacturing, data management, artificial intelligence and innovative financing. Partnering allows to take advantage of opportunities and synergies arising from the close collaboration between biotech and pharma, or to find interesting partners for future projects and jointly drive the development of innovative products and platform technologies. In addition, international biotech delegations use the “Global Village” to strengthen their ties with Switzerland as a biotech location
› 1,000+ high-ranking experts from the life science industry
› Thematically focused panel discussions
› Pre-arranged one-to-one partnering meetings
› Presentations from innovative biotech start-ups and mid-sized biotech companies

› 70+ exhibitors from all over Europe and Global Village with international delegations

› Presentation of the Swiss Biotech Success Stories Awards

› Swiss Biotech Report 2023 with the most important facts, trends, innovation factors and sources of innovation in the Swiss biotech industry
› General Assembly of the Swiss Biotech Association

and to promote cross-border investments, public-private partnerships, research and development collaborations, and the exchange of talent.
The program will be presented in English with thematically focused panel discussions. About 70 exhibitors, company presentations, and partnering complement the cornerstones of the event. L
Find more info and register at swissbiotechday.ch
Over 1,000 participants are also expected at the Swiss Biotech Day 2023The Global Village offers international biotech delegations a versatile and effective platform Parallel sessions feature numerous thematically focused panel discussions to captivate the audience

SYNTHETIC BIOLOGY The interplay of advances in life sciences triggers a new wave of innovation that scratches from healthcare and agriculture to consumer goods while having a significant socio-economic impact. Purely analytical biology is becoming Synthetic Biology.
For Synthetic Biology to develop its full potential, it is essential to connect all the stakeholders in this discipline. We, the German Association for Synthetic Biology (GASB), have dedicated ourselves to this task for six years now. With more than 200 members, we are the hub for everyone interested in this field: From students to experts, from academic research labs to industry, from professional scientists to Synthetic Biology enthusiasts, not just from Germany but from all around the world.
Academic research is core to Synthetic Biology. Therefore, GASB focuses on promoting its importance and results within the community and beyond. By organizing conferences and other events, we bring together key players in the field to foster collaboration and spark new ideas. For this year’s GASB7 conference, we partnered with the Designer Biology Meeting to explore possibilities at the intersection of Synthetic Biology and material sciences.
Despite broad-based academic research, the industrial sector of Synthetic Biology in Germany is still in its infancy. With our flagship project - the “SynBio World Café” - GASB provides a platform to connect bright minds with their innovative ideas and accelerates the integration of Synthetic Biology products into our daily lives.
To create the necessary framework for these advancements in Germany, we have the mission to raise political aware -
With a variety of projects and events, GASB connects all areas and stakeholders within Synthetic Biology.
ness and inform elected representatives about the opportunities of the key technology of Synthetic Biology. Therefore, we take on our role to represent and communicate the needs of the Synthetic Biology community on a political level to create a supportive framework for future growth.
A dynamic and disruptive new field like Synthetic Biology often encounters mistrust and prejudices. At GASB, we believe open discourse and education are the best ways to overcome outdated conventions. We pave the way for a comprehensive understanding and access to Synthetic Biology technologies through our projects aimed at the general public and young students. In particular, we want to highlight one of our education projects, where we provide students with access to cell-free gene expression experiments, allowing them
› June 27-28, Darmstadt SynBio World Café
› September 18-20, Freiburg GASB 7 x Designer Biology Conference
to participate in Synthetic Biology at an early age.
Given the far-reaching possibilities of Synthetic Biology, GASB stimulates discussions on biosafety. To ensure a safe and thriving environment for Synthetic Biology in the future, GASB acts as a facilitator for discourse by organizing events and panel discussions and representing the voice of Synthetic Biology at key professional meetings (e.g., the G7 Global Partnership Conference on Biosafety).
Since our Association’s founding, we have strived to build a broad network to improve the position of Synthetic Biology in Germany. Therefore, we collaborate with industry, institutes, and NGOs, notably our long-standing support for iGEM teams in Germany, partnerships with other Synthetic Biology associations in Europe and around the globe, as well as national science communication and interest groups like Öko-Progressives Netzwerk e.V. Now, we are particularly pleased to have the opportunity to report on our work in the European Biotechnology Magazine. We look forward to strengthening our ties with all players within the rapidly growing field of Synthetic Biology.
by Lasse Middendorf (Active Member)ANGIOS GMBH What is the recipe for building a biotech cluster? Paramount for success is to bring together talented companies to work on promising projects and to add well-equipped facilities and experienced leadership.

› 27 April 2023
2. BIOTECH CIRCLE AUSTRIA, Vienna
In theory it is a straightforward task. One would assume that in Tyrol – home to eight recognized higher-education institutions with over 40 000 students and an established background of highgrade research in biology, chemistry, and physics – this would be an easy goal to achieve. However, the reality looks different. With most graduates opting to go to established life science hubs, such as Vienna, Munich, or Basel there is little workforce left to fuel startup biotech companies in Tyrol. Even the very few that do choose to stay in the Alps would join the large well- established “big players” in the area – Novartis, Sandoz and MED-EL.
Angios GmbH – a young biotech startup – managed to break this trend and establish itself in Tyrol and is a new breed of companies. It all starts, as outlined at the very beginning, with the basic ingredients. The idea with which it all began-


the promising project- originates from the work from Professor Josef Penninger and his team, who developed a unique protocol to grow small blood vessels in a dish. It was immediately evident that besides a groundbreaking discovery in science, one could also use this technology in a clinical setting. The small blood vessels could be transplanted into a host organism, where they successfully connect to and expanded the existing vasculature. It is well established that a functional vasculature is a major bottleneck for engrafting of virtually all cellular transplants and therefore being able to supply this missing piece will hopefully increase the realization of most cell-based therapies. The success of this project will depend on the coming together of the rest of the major ingredients outlined before. Angios employs top-notch scientists from all over the world, it is guided by CEO Dr Gregor Wick and Head of R&D Dr
Teo dor Yordanov, who can draw upon the help of an internationally recognized advisory board, and has set up its laboratories in the beautiful Tyrolean capitalInnsbruck. With numerous collaboration partners and the support of the province Tyrol, Angios has already founded a Spin Off, secured a globally renowned North American Biotech company, as a development partner and first equity investor. Building a Biotech cluster also means connecting people. Gregor represents western Austria in BIOTECH AUSTRIA to get more recognition among Tyrol’s Biotech scene. It’s a long way ahead to establish a Biotech cluster that is sustainable and dynamic to attract additional international venture capital, but the foundation has been laid for a bright future of a Biotech industry in Tyrol and BIOTECH AUSTRIA continues its intensive efforts to further establish Tyrolean biotech companies in Austria and the world.
ACTIVE INGREDIENTS Biotech innovator Biosyntia ApS, the latest addition to the ECBF, is applying proprietary biological processes to produce sustainable nutritional ingredients – a mission that has captured the imagination of seasoned biotech investors and major ingredient players.
Active ingredients that can be produced at scale in a sustainable and natural way are in short supply and high demand.
For many applications, manufacturers of nutraceuticals, dietary supplements, pharmaceuticals and personal care products have little choice but to use ingredients that are produced via highly polluting chemical synthesis. Vitamins are a case in point. Most brands on the market today rely on vitamins that are produced synthetically from non-renewable petrochemicals, most often in Asia. However, as we transition towards a greener economy, it is becoming increasingly apparent that such ingredients fall short of both consumer and manufacturer expectations in terms of their transparency, sustainability and natural credentials.
Danish biotech firm Biosyntia has pioneered a unique biotechnology that has the potential to transform the way that active nutritional ingredients are produced. The company has developed a first-of-itskind proprietary precision fermentation platform that offers a sustainable alternative to inefficient botanical extraction and petrochemical production methods. The highly efficient fermentation process uses carbohydrate from sugar beets, significantly reducing land use and solvent use.
Biosyntia ApS has already used the platform to build a portfolio of bio-based active ingredients that includes B-vitamins, terpenes and flavonoids, for use in personal care, human and animal nutrition products.
BIO-B7 – a sustainably produced B-vitamin, is the most advanced ingredient in the company’s pipeline. Raising €17.5m

in its latest funding round has enabled Biosyntia to realise its ambition of commercialising the world’s first natural and sustainably produced biotin (vitamin B7); pilot production has now commenced and samples are available.

Biosyntia’s partnership with Munichbased WACKER Group will contribute towards successful scale-up of biotin production. The two companies are jointly developing a large-scale biotin production process based on Biosyntia’s fermentation technology. Biosyntia has also signed an in-licensing deal with French biotech firm Lantana, with the aim of introducing the first sustainably produced fermented flavonoids to the nutraceutical ingredients market. Indeed, value-sharing collaborations are a cornerstone of Biosyntia’s business model. Since the company’s inception, it has joined forces with more than 20 academic partners and 20 industry partners.
Strategic partnerships with in-market players facilitate market access and ensure the relevance of Biosyntia’s active ingredients to target audiences. For example, in 2021, the company entered into a long term partnership with flavours and fragrances giant Givaudan, with the joint objective of exploring the application of fermentation technology for producing flavour ingredients that are not currently available from natural sources.
Building the company with In-licensing and acquisition of new technologies is also a focus of Biosyntia. Martin Plambech elaborates: “Our in-house team has decades of industry expertise in microbial engineering, process optimisation, process scale-up and production, analytics as well as sales and marketing. This means we can commercialise new technology relatively fast. And the more ingredients we have in our pipeline, the more synergies we can harvest across operations and sales, which is why we are actively looking for opportunities to acquire, in-license or partner organisations with complementary capabilities.“
YEBN The YEBN is an association of students, young researchers, and professionals working to build a bridge between academia and industry and foster European collaborations to create a sustainable impact on our society.

› AYB – Association of Young Biotechnologists (Poland)
› Biotecnologi Italiani - National Association of Italian Biotechnologists (Italy)
› NBV – The Dutch Biotechnology Association (Netherlands)
We are working on expanding our Institutional Members, and establishing connections with Hungary and the Baltics, as well as developing joint projects with fellow associations. These include the btS (Biotechnological Student Initiative e.V.), in Germany, or Health Valley Netherlands, the biggest Life Sciences & Health innovation Companies Cluster in the Netherlands. We also want to establish deeper collaborations with companies and connect young professionals and students.
In YEBN, we create tools to help young professionals and last year’s students develop themselves at a professional and personal level. We want to empower and give voice to young bioleaders concerned with positively impacting society. We are looking for new members to join our Executive Board. If you want to be part of an international association fostering collaboration and synergies and have the opportunity to expand your experiences and network, keep reading!
Our initials spell our DNA, the characteristics of our association:
› Young: YEBN is powered by young developing bioleaders from students and young professionals in academia and industry.

› European: we have a European target. We work hand in hand with regional
and national associations, aiming for the benefit of the whole society and having an impact at international level.
› Biotech: we focus on Biotechnology and the Life Sciences sector.
› Network: A network full of inspiration, information, connection, best practices exchange, training, and innovation.
YEBN is currently collaborating with many partners throughout Europe. Our primary stakeholders are our Institutional Members or fellow associations that believe in a common objective for our associations. Our current institutional members are presented here:
› NGB – New Generation of Biotechnologists (France)
YEBN acts as a catalyst and provides a platform for accessing and exchanging information and communication. We provide a common place for people to meet and exchange ideas in events organized throughout Europe, with multiple events organized in the last few years. The YEBN, together with our Institutional Members and fellow associations, works on developing events and partnerships that create impact. At the centre of all actions, we aim to strengthen YEBN, our institutions, and our partners to foster innovation and keep working on our common goals.
Contact us if you are an individual, an association, or a company aligned with our goals. We will be thrilled to discuss any possible collaboration and shared endeavour.
Drop us an e-mail at contact@yebn.eu Get inspired, get involved!


DRUG DISCOVERY Seasonal flu is a common infectious disease of the respiratory tract caused by influenza virus. Vaccination is the most effective way to prevent flu infection. Each year, several different flu strains are selected as vaccine candidates based on surveillance data of the recent isolates and the performance of the vaccines from the previous season.
On February 24 th , 2023, the World Health Organization (WHO) announced the recommendations for influenza vaccine composition for the 2023/2024 northern hemisphere influenza season. To support vaccine research, Sino Biological has released the recombinant antigens for 2023/2024 influenza vaccine strains.

With the world’s largest viral antigen collection, ProVir ®, Sino Biological provides a range of recombinant influenza antigen products, including hemagglutinin (HA), neuraminidase (NA), and nucleoprotein (NP) proteins from all WHO-recommended vaccine strains in recent years. A large collection of monoclonal antibodies against the flu antigens are also available to support the development of relevant assays and diagnostic kits.
Learn more about our reagents

AG
13.–15.6.2023 LILLE PBS is the leading international event where biobased chemistry players and end-users imagine, build and develop the next sustainable market with: 30 high level conferences, 100+ international key speakers,50 exhibitors, business meetings, 700+ participants, a third day dedicated to sites industrial visits. www.plantbasedsummit.com
15.-16.5.23
BioEquity Europe 2023, Dublin (IRL) Info: EBD Group/Biocentury https://conferences.biocentury.com
18.–20.5.2023
EuSARC 2023, Vienna (AT) Info: St. Anna Kinderkrebsforschung GmbH Children’s Cancer Research Institute / CCRI https://ccri.at/eusarc/
24.-25.5.23
Chemspec Europe , Basel (CH) Info: Mac Brooks Exhibitions www.chemspeceurope.com
5.-8.6.23
BIO International Convention, Boston (USA) Info: BIO – Biotechnology Innovation Organization www.bio.org/events/bio-international-convention
14.-15.6.23
11th International Bioeconomy Conference, Halle/Saale (DE) Info: BioEconomy Cluster/ Science Campus Halle/S. www.bioeconomy-conference.eu
14.-16.6.23
26.-28.9.23
BIOSPAIN 2023, Barcelona (ES)
Info: ASEBIO https://biospain2021.org
26.9.-2.10.23
European Biotech Week 2023
Info: EuopaBio https://biotechweek.org/
21./22.9. 2023 BERLIN Industry representatives will discuss the latest opportunities and developments. On the second day, the conference will open up to identify obstacles to growth together with politicians and organisations and look for ways to speed up market access.
https://industria-biotec.com
20.-22.3.23
BIO-Europe Spring 2023, Basel (CH)
Info: Olivia Guiliana, EBD Group https://informaconnect.com/bioeurope-spring/
19.4.2023
Takeda R&D Science Day, Vienna (AT)

Info: LISAvienna/Takeda www.lisavienna.at
24.-25.4.23
Swiss Biotech Day 2023, Basel (CH)
Info: Swiss Biotech Association, https://swissbiotechday.ch
24.-25.4.23
BioVaria 2023, Munich (DE)
Info: Esther Lange, Ascenion GmbH, www.biovaria.org/
3.-4.5.23
OTC – Outsourcing in Clinical Trials Europe 2023, Barcelona (ES)
Info: Katie Giorgadze, Arena International Event Group www.arena-international.com/octeurope/
3.-4.5.23
7 th Euro BioMAT 2023 – European Symposium on Biomaterials and Related Areas, Weimar (DE), +++ online +++
Info: German Materials Society, https://dgm.de/biomat
9.-11.5.23
Labvolution 2023, Hannover (DE)
Info: Deutsche Messe AG, www.labvolution.de
10.-11.5.23
World Bio Markets – Driving the commercialisation of the bioeconomy, Le Hague (NL)
Info: TNP Media Ltd., https://www.worldbiomarkets.com
Greentech Festival 2023, Berlin (DE) Info: Greentech Show GmbH, https://greentechfestival.com/
6.9.23
New Updates in Drug Formulation & Bioavailability, Copenhagen (DK) Info: Maria Eriksson, Life Science Sweden www.formulationsbioavailability.com
26.–30.6.2023 BERLIN The MPS World Summit will bring together the academic research community, medical centres, the pharmaceutical, cosmetics, chemical and food industries, regulatory agencies, health foundations, charities, patients associations and policymakers.

https://mpsworldsummit.com/
19.-20.10.23
European Conference of the Global Biotech, Pharma & Healthtech Supply Chain Community –BSMA, Brussels (B)
Info: BSMA – Bio Supply Management Alliance https://www.bsmaeurope.com/
24.-26.10.23
CPhI Worldwide 2023, Barcelona (ES)
Info: CPhI Global Office https://www.cphi.com
24.-25.10.23
European Forum for Industrial Biotechnology & the Bioeconomy – EFIB, Rotterdam (NL)

Info: EuropaBio https://efibforum.com
6.-8.11.23
BIO-Europe 2023, Munich (DE)
Info: EBD Group https://informaconnect.com/bioeurope/
29.-30.11.23
Nordic Life Science Days 2023, Copenhagen (DK)
Info: Olivier Duchamp, SwedenBio www.nlsdays.com
BILL ANDERSON, most recently CEO of the pharmaceuticals division of the Roche Pharmaceuticals, was announced as new CEO of Bayer AG at the beginning of February. The American is already the bearer of hope for a comprehensive transformation program and increased share prices.

WERNER BAUMANN had to retire early after public criticism from US investor Jeff Ubben, who accused him of incompetence in driving the share price after the devastating consequences of Bayer’s Monsanto take-over. Share prices soared up 7% after the announcement in early February. Bayer’s supervisory board had already been looking for a successor.
“...I got so bored working as a basic research scientist in discovery mode. Biotechnologists engineer biological systems to advance technology and shape solutions of the future. We make things happen.”
SPAC As sustainability becomes a global consensus in the form of new laws, more and more companies are investing to become carbon-neutral. As a result, the LanzaTech Inc. has a large influx of partners from the cosmetic and chemical sector: with the companies Lululemon and L’Oréal the first “sustainable” polyethylene packaging was developed. The ethylene needed is no longer produced from fossil oil, but fermented from gasified biomass or gaseous emissions to ethanol and then dehydrated. Lanzatech went public on February 10th with funding from Chinese VC firm Qiming Venture Partners Ltd. via the SPAC route. The new company, LanzaTech Global, was issued at US$10 per share, giving it a market value of US$2 billion. However, the share price fell 21% at launch, making it the fourth company from Qiming’s portfolio to go public this year.
A broad transition to alternative proteins – whether those be plant-, algae-, or fungi-based – has the potential to help solve global issues ranging from basic food supply to climate change. According to the data of the GEA Chef Survey 2022, for which 1000 chefs were questioned, at least a quarter of all food could be produced from alternative proteins by 2040. Around 90% of chefs have already observed a growing interest in alternatives to conventional products.
Mushrooms-based Packaging? That’s #IKEA’s Concept of Sustainable Supply Chain. @tracktheirmoney
Bacteria to the Future: Microbes could make perfumes more sustainable https:// bit.ly/41I1VNJ @ IBioIC
Unlike the pills and gels previously tested as male contraceptives, which needed regular and long-term application to be effective, the new possible
male birth control is fast-acting and temporary @TheEconomist
us
The Bioeconomy 500 for 2023 – outstanding leaders of the bioeconomy’s development and deployment https://bit. ly/41I1VNJ
SUMMER EDITION Personalised cardiology and virus driven inflammation as well as Biotech in the UK will be in the focus of our next issue. Players in these fields are invited to present their offering in our special in EuropE an BiotEchnology Want to participate? Please contact Andreas Macht (+49-30-264921-54), Oliver Schnell (-45), or drop us an email: marketing@biocom.de. Publishing date is 17th May, 2023; deadline for ads is 5th May, 2023.
For biotechnologists, Europe is a collaborative research area. For the biotech industry it is a huge common market with almost 500 million potential customers. The 13th edition of the European Biotechnology Guide presents an attractive cross-section of companies and institutions from 16 nations.

188 pages, available at local bookstores or online.
ISBN 978-3-928383-87-5
19.80 Euro

END-TO-END CDMO
Live and inactivated bacterial and viral vaccines up to BSL-2
Aseptic processing systems
_ Aseptic filling into vials
Lyophilization for clinical trial material and large-scale production
Process development and optimization
Quality control testing: microbiological, sterile/nonsterile, chemical-physical, and biological
Storage: (2º - 8ºC), (-20ºC), (-30ºC), (-80ºC)
WE ARE A FULL-SERVICE PROVIDER WITH 30 YEARS OF EXPERIENCE IN VACCINE DEVELOPMENT AND MANUFACTURING.
LARGE-SCALE BIOTECH MANUFACTURING

CZ VACCINES has several GMP certified production plants in Spain and Portugal.


HUMAN AREA

O Porriño (SPAIN)
Paredes de Coura (PORTUGAL)
VETERINARY AREA
León (SPAIN)
cGMP PRODUCTION CAPABILITIES
cGMP preparation and control of Master and Working Seed Banks of cells, virus and bacteria
Roller bottles, cell factory and wave bioreactor
Single-use bioreactors: up to 2 x 2,000L
Stainless steel bioreactors: up to 2 x 2,000L
Single-use blending mixers: 2 × 3,000L
Stainless steel vessels: 4 × 6,000L
State-of the art purification processes: Chromatography (ÄKTA ready), centrifugation, ultrafiltration, diafiltration and tangential flow filtration
cGMP FILL & FINISH CAPABILITIES
Liquid and lyophilized products up to BSL-2
4 filling machines: 6,000 vials/hour, 2 x 12,000 vials/hour and 24,000 vials/hour
2 freeze dryers: up to 24,000 vials per run, and up to 180,000 vials per run with an automatic loading & unloading system

Component size: 2R-10R-20R
Manual & automatic visual inspection Labeling & packaging
We cover the entire value chain, from contract development through clinical phases I to III, to commercial production of vaccines and other biological products.