




British Dermatological Nursing Group
Volume: 23 Issue: 4
Skin of colour Understanding sun safety and risk
Phototherapy
The knowledge gaps facing dermatology nurses
Educating children Promoting skin health awareness in schools
The BDNG Members Journal delivered to you by
1,2
ILUMETRI ® is indicated for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy. 2
ILUMETRI® offers early and long-term control and proven effectiveness in skin treatment including sensitive areas*2-4
ILUMETRI® restores patients’ wellbeing from week 16 up to week 52 in line with general population averages7,9
ILUMETRI® is the only anti-IL-23 giving the flexibility to individualise therapy whilst maintaining an acceptable safety profile1,5,6
Could any of your patients benefit from ILUMETRI® and our enhanced value support services?

*Sensitive areas such as scalp, nails, genitals and palmo-plantar2-4
1. ILUMETRI® SmPC. Almirall. 2. Thaçi D, Piaserico S, Warren RB, et al. Br J Dermatol. 2021;185(2):323–334. 3. Thaçi D, Gerdes S, Jardin K, et al. Dermatol Ther.2022;12(10):2325-2341. 4. Magnolo N, et al. Presented at DDG Congress, Mar 1-3 Wiesbaden, Germany, 2024. P030. 5. Tremfya® Summary of Product Characteristics. Janssen. 6. Skyrizi® Summary of Product Characteristics. AbbVie. 7. Mrowietz U, Augustin M, Sommer R. Abstract presented at 25th World Congress of Dermatology, Singapore, 3–8 July, 2023. 8. Dauden E, Mrowietz U, Sommer R, et al. Presented at 51st National Congress AEDV, 22-25 May; Madrid, Spain. 2024. Current SPA version: 166/2374/199. 9. EuroFound “European Quality of Life Survey - Data visualisation. Eurofound https://www.eurofound.europa.eu/data/european-quality-of-life-survey. Last accessed August 2024.
ILUMETRI® (tildrakizumab) PRESCRIBING INFORMATION
Please consult the Summary of Product Characteristics (SmPC) before prescribing ILUMETRI® is available as 100 mg and 200 mg solution for injection in pre-filled syringes. Active Ingredient: Each pre-filled syringe contains 100 mg or 200 mg of tildrakizumab in 1 mL or 2 mL. Tildrakizumab is a humanised IgG1/k monoclonal antibody produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology. Also contains 0.5 mg/mL polysorbate 80 (E 433). Indication: ILUMETRI is indicated for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy.Dosage and Administration: The recommended dose of ILUMETRI is 100 mg by subcutaneous injection at weeks 0, 4 and every 12 weeks thereafter. In patients with certain characteristics (e.g. high disease burden, body weight ≥ 90 kg) 200 mg may provide greater efficacy. Consideration should be given to discontinuing treatment in patients who have shown no response after 28 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 28 weeks. Injection sites should be alternated. Elderly: No dose adjustment is required. Renal or hepatic impairment: No dosage recommendations can be made. Paediatric population: No data available. Contraindications, Precautions and Warnings: Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in SmPC section 6.1. Clinically important active infection, e.g. active tuberculosis. Precautions: To improve traceability always record the batch number of the administered product. ILUMETRI has the potential to increase the risk of infections. If a patient develops a serious infection, the patient should be closely monitored and treatment with ILUMETRI should not be administered until the infection resolves. Exercise caution in patients with a chronic infection or a history of recurrent or recent serious infection. Instruct patients to seek medical advice if signs or symptoms of an infection occur. Patients should be evaluated for tuberculosis (TB) prior to initiation of treatment and monitored for signs and symptoms of active TB during and after treatment. In patients with a history of latent or active TB, consideration for anti-TB therapy should be given. Discontinue
use if a serious hypersensitivity occurs. All appropriate immunisations should be completed prior to start of treatment with ILUMETRI. If a patient has received live viral or bacterial vaccination it is recommended to wait at least 4 weeks prior to starting treatment with ILUMETRI. Patients treated with ILUMETRI should not receive live vaccine during treatment and for at least 17 weeks after treatment. This medicine contains polysorbate. Polysorbates may cause allergic reactions. Fertility, pregnancy and lactation: Women of childbearing potential should use effective methods of contraception during treatment and for 17 weeks after treatment. As a precautionary measure, it is preferable to avoid the use of ILUMETRI during pregnancy. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from ILUMETRI therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. The effect of ILUMETRI on human fertility has not been evaluated. Adverse Reactions: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): Headache, gastroenteritis, nausea, diarrhoea, injection site pain, back pain. Immunogenicity: In pooled Phase 2b and Phase 3 analyses, 7.3% of tildrakizumab-treated patients developed antibodies to tildrakizumab up to week 64. Of the subjects who developed antibodies to tildrakizumab, 38% (22/57 patients) had neutralizing antibodies. This represents 2.8% of all subjects receiving tildrakizumab.
Please consult Summary of Product Characteristics for further information. Legal Category: Ireland: Subject to prescription which may not be renewed (A). United Kingdom & UK/NI: POM Price & Pack: United Kingdom 100 mg pre-filled syringe - £3,241; 200 mg pre-filled syringe - £3,241 Ireland 100 mg pre-filled syringe Price to wholesaler 200 mg pre-filled syringe Price to wholesaler
Marketing Authorisation Number(s): IE & UK(NI) - EU/1/18/1323/001 ; EU/1/18/1323/003 GB - PLGB 16973/0038 ; PLGB 16973/0045
Further information available from: Almirall Limited, Harman House, 1 George Street, Uxbridge, Middlesex, UB8 1QQ, UK.
Date of Revision: 08/2024 Item code: UK&IE-ILU-2400045
UK-Adverse events should be reported. Reporting forms and information can be found at MHRA https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should be also reported to Almirall Ltd. Tel. 0800 0087 399
IE-Adverse events should be reported. Reporting forms and information can be found at HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse events should be also reported to Almirall Ltd. Tel: +353 1800849322
Celebrating a busy year for PEM
Ingrid Thompson
Skin at School: Educational materials on skin and skin diseases for primary schools
Jolien van der Geugten, Karin Veldman
Professional development
The importance of education to enhance phototherapy nursing practice: Feedback from the BDNG Manchester Meeting 2024
Stephanie Greenleaf
What additional educational materials do nurses need to help them care for patients with cutaneous T-cell lymphoma? A report of a BDNG survey
Rebecca Penzer-Hick
A week in the life of a primary care
Lucy Evans-Hill
cancer and sun protection for people with skin of colour
Conducting a literature review: Part four – critical appraisal
Laura
Management of a patient living with PsA exhibiting symptoms of dactylitis and enthesitis: A case study
Jing Husaini BDNG meets
to understand the psychological impact of skin disease
Ella Guest, Rob Mair
The advantages of taking a Master’s degree in advanced clinical practice
Rod Tucker, Emma Button
classes of oral antibiotics are associated with severe adverse cutaneous reactions? Rod

new in the world of research? Rod Tucker
for the workforce
Dungeons and Dragons: Where stress lessens and adventure beckons Molly Connolly
news
An update from the BDNG’s Member and International Liaison Lead
overseas perspective on the BDNG’s annual conference

Editorial
Jackie Tomlinson
Chair of the Editorial Board
Dermatology Clinical Nurse Specialist, Addenbrooke’s Hospital, Cambridge University Hospitals Foundation Trust
Polly Buchanan
Clinical Editor
Community Dermatology Nurse Specialist, NHS Fife pauline.buchanan2@nhs.scot
Rob Mair
Managing Editor
Rob.Mair@pavpub.com
Lauren Nicolle
Deputy Editor
Lauren.Nicolle@pavpub.com
Tony Pitt
Art Director
Tony.Pitt@pavpub.com
Commercial
Mark Freeman
Journal, Conference & Corporate Sponsorship Sales dnadvertising@bdng.org.uk 07957 404 831
Editorial board
Mandy Aldwin/Sarah Griffiths-Little Ichthyosis Support Group
Tanya O. Bleiker
Consultant Dermatologist, University Hospitals of Derby and Burton NHS Foundation Trust
Julie Brackenbury
Aesthetic Nurse Practitioner, JB Cosmetic, Bath
Ivan Bristow
Podiatrist and Associate Professor, Southampton
Sara Burr
Senior Lecturer, Centre of Postgraduate Medicine and Public Health, University of Hertfordshire. Community Dermatology Specialist Nurse/Clinical Lead Community Skin Integrity Team, Norfolk Community Health and Care
NHS Trust
Fiona Cowdell
Professor of Nursing and Health Research, Faculty of Health, Education and Life Sciences, Birmingham City University
Elfie Deprez
PhD student and Psoriasis Nurse Specialist, University of Ghent, Belgium
Steven Ersser
Professor of Nursing and Dermatology Care and Head of Department of Nursing Science, Bournemouth University
Mark Goodfield
Consultant Dermatologist, Leeds Teaching Hospitals NHS Trust
Karina Jackson
Nurse Consultant, St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, London
Teena Mackenzie
Education and Development Lead, British Dermatological Nursing Group
Dermatological Nursing is the official journal of the BDNG. To contact the BDNG, email: admin@bdng.org.uk or tel: 02892 793 981
Printed in Great Britain by Micropress, Fountain Way, Reydon Business Park, Reydon, Suffolk, IP18 6SZ
The BDNG logo and name are Registered Trademarks. All registered rights apply.
Chris McCabe
UK Medical Strategy Lead for Immunology, UCB Pharma
Jodie Newman
Education Nurse, British Dermatological Nursing Group
Michelle Ogundibo
Paediatric Dermatology Nurse Specialist, Chapel Allerton Hospital, Leeds Teaching Hospitals NHS Trust
Rebecca Penzer-Hick
Senior Lecturer, Postgraduate Medicine, University of Hertfordshire and Dermatology Specialist Nurse, Dermatology Clinic Community Services, Cambridgeshire
Kathy Radley
Senior Lecturer, School of Life and Medical Sciences, University of Hertfordshire and Dermatology Nurse Specialist, Dermatology Community Clinic Services, Cambridgeshire
Alison Schofield
Head of Education, Tissue Viability Nurse Consultant at NHS Pioneer Wound Healing and Lymphoedema centres.
Karen Stephen
Lead Dermatology Nurse, NHS Tayside
Delia Sworm
Trainee Advanced Clinical Practitioner – Skin Cancers (Oncology), St Luke’s Cancer Centre, Royal Surrey NHS Foundation Trust
Rod Tucker
Community Pharmacist/Researcher with a Special Interest in Dermatology, East Yorkshire
Andrew Thompson
Clinical Health Psychologist and Reader in Clinical Psychology, University of Sheffield
Carrie Wingfield
Dermatology Nurse Consultant, Norfolk and Norwich University Hospital
Dermatological Nursing (ISSN 1477-3368) is published by the BDNG, 82 Antrim Street, Lisburn BT28 1AU Tel: 02892 793 981. www.bdng.org.uk
All rights reserved. No part of this publication may be reproduced, stored or transmitted in any form or by any means without the prior written permission of the BDNG. Opinions expressed in articles are those of the authors and do not necessarily reflect those of the BDNG or the editorial/advisory board. Advertisements have no influence on editorial content or presentation.
The advertisements in this journal are for a UK audience only.



























Need 24 hours’ hydration? Prescribe ‘Doublebase Once’ – the yellow pack.
Presentation: White opaque gel.
Uses: An advanced, highly moisturising and protective emollient gel for regular once daily use in the management of dry skin conditions such as eczema, psoriasis or ichthyosis. May also be used as an adjunct to any other emollients or treatments.
Directions: All age groups. Apply direct to dry skin once daily or as often as necessary. Can be used as a soap substitute for washing. If applying another treatment to the same areas of skin, apply treatments alternately leaving sufficient time to allow the previous application to soak in.
Contraindications, warnings, side effects etc: Do not use if sensitive to any of the ingredients. Doublebase Once has been specially designed





Proven to provide at least 24 hours’ hydration1,2,3 from just 1 application.
• For the management of dry skin conditions such as eczema, psoriasis and ichthyosis.
• The Doublebase Once advanced gel formulation provides significantly greater and longer-lasting skin hydration than ointments1,2 following a single application over 24 hours.








for use on dry, problem, or sensitive skin. Rarely skin irritation (mild rashes) or allergic skin reactions can occur on extremely sensitive skin, these tend to occur during or soon after the first few uses and if this occurs stop treatment.
Instruct patients not to smoke or go near naked flames. Fabric (clothing, bedding, dressings etc) that has been in contact with this product burns more easily and is a potential fire hazard. Washing clothing and bedding may reduce product build-up but not totally remove it.
Ingredients: Isopropyl myristate, liquid paraffin, glycerol, steareth-21, macrogol stearyl ether, ethylhexylglycerin, 1,2-hexanediol, caprylyl glycol, povidone, trolamine, carbomer, purified water.




Pack sizes and NHS prices: 100g tube £2.69, 500g pump pack
Legal category: Class I medical device.
Further information is available from: Dermal Laboratories Ltd, Tatmore Place, Gosmore, Hitchin, Herts, SG4 7QR, UK.
Date of preparation: August 2023.
‘Doublebase’ is a trademark.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk. Adverse events should also be reported to Dermal.
10mm
References: 1. Comparison of the skin hydration of Doublebase Once emollient with Epaderm Ointment in a 24-hour, single application study, in subjects with dry skin. Extract report summarising skin hydration results for wiped off sites. Data on file. Dermal Laboratories Ltd, Hitchin, UK. Epaderm® Ointment is a registered trademark of Mölnlycke Health Care. 2. Antonijevic´ MD & Karajcˇic´ J. Ointments or a gel emollient? Randomised and blinded comparison of the hydration effect on ex-vivo human skin. Data presented at the 17th European Academy of Dermatology and Venereology (EADV) Symposium, May 2022, Ljubljana, Slovenia. 3. Antonijevic´ MD & Karajcˇic´ J. Randomised, open, comparative study of the hydration effect on ex-vivo human skin of two emollient formulations with different recommended application regimes. Data presented at the 17th European Academy of Dermatology and Venereology (EADV) Symposium, May 2022, Ljubljana, Slovenia.

Carrie Wingfield
An invitation to write a reflective guest editorial has resulted in a slightly indulgent laying down of words reflecting on my 30 years in this speciality. I hope you will forgive me if I use the opportunity to present you with a frank cathartic offloading of a rollercoaster career that has been my life, my passion, and my nemesis at times.
My career in dermatology started as a healthcare assistant, working in a busy outpatient department for gynaecology, urology, ENT and dermatology. At that time, the dermatology presence was small, consisting of one dermatologist, a registrar and a GP practitioner. My interest in dermatology began in outpatients with a role-model who was not a nurse, but a young consultant dermatologist whose teaching was forthcoming, kind and nurturing. This convinced me to train as an RGN. On qualifying, after a fleeting stint on a surgical ward, I migrated back to dermatology as a staff nurse. In those early days, phototherapy was administered by physiotherapists

Carrie Wingfield is a Nurse Consultant in Dermatology and ICS Advanced Clinical Practice Lead for NHS Norfolk and Waveney Integrated Care System (ICS), Norfolk & Norwich University Foundation Hospital.
and nurse-led clinics were mostly limited to wart and dressing clinics. I questioned my choice of speciality as I started to deskill in acute nursing skills learnt on the wards. Was this a good career move?
As I learnt my craft, the true worth of this speciality emerged. Its diversity, the layered knowledge required to deliver care for chronic lifelong skin disease, debilitating conditions that impacted on every aspect of life, the stigma, and psychological consequences. This was no ‘Dermaholiday’, a misconception and sometimes a put down I have witnessed within our profession, a perception that sometimes views dermatology as a low adrenaline, sedate and ‘Cinderella’ speciality, a cop out in the nursing world. Boy, did we prove them wrong. The rise of dermatology and evolution of service delivery has put nurses at the forefront of innovative nurse-led services, highly qualified specialists and educational leaders. This is a speciality and career path where nurses can make a difference and where education and drive are needed across the board to improve patient experience and access to specialist care. Changing those misconceptions and pushing the scope of service improvements became an obsession throughout my career.
After a couple of years, I found myself in a senior role and joined the BDNG. On reflection, this was the exposure and opportunity to gain experience from my peers that led to personal accomplishment and exposed me to so many innovative role models. I started a challenging process of instigating departmental change, to encourage a growing consultant and nursing team
to see the true worth of a diverse dermatology workforce. We had to evolve, and this meant unpopular decisions, such as losing the ward where increasingly our beds were lost to non-dermatology patients. This meant moving staff out of their comfort zones. It meant taking risks to move on and establish a team that could meet the challenges and changing face of both dermatology and the NHS. There was resistance and anxiety along the way, but as the workforce started to develop it formed the foundation for a benchmark department that is now unrecognisable from those early days.
A supportive and forward-thinking dermatologist team who work in unison with dermatology nurses is imperative for moving services forward. As a service leader you need to be a voice in your department, put your head above the parapet, do not be frightened about taking risks. Be in no doubt that we can be a dysfunctional family at times, especially as we face the challenges of targets, waiting lists, lack of funding, lack of capacity, staff shortages and insourcing services. All presenting, at times, a sense of loss of control.
My message to the future nursing leaders and dermatology teams is simple: nurture your team, value them, support them, retain, and develop their skills and recognise their talent, do not let them slip through your fingers. Do not ignore those who are struggling, do not shy away from the difficult conversations and confrontations, be part of that change process on a personal level and strive against adversity to keep your dermatology service at the top of its game.



















… Adex Gel emollient with an Added Extra anti-inflammatory action.








































Adex Gel emollient offers an effective, simple and different approach to the treatment and management of mild to moderate eczema.


Specially formulated with a high level of oils (30%) and an ancillary anti-inflammatory, nicotinamide (4%) to help reduce inflammation.


Can be used continuously, for as long as necessary, all over the body including on the face, hands and flexures.


Adex Gel may help reduce the need for topical corticosteroids.1,2 Does not contain corticosteroids.


Suitable for patients aged 1 year +.
Presentation: White opaque gel.
Uses: Highly moisturising and protective emollient with an ancillary anti-infl ammatory medicinal substance for the treatment and routine management of dry and infl amed skin conditions such as mild to moderate atopic dermatitis, various forms of eczema, contact dermatitis and psoriasis.


Adex Gel - bridges the gap between plain emollients and topical corticosteroids.

Directions: Adults, the elderly and children from 1 year of age. For generalised all-over application to the skin. Apply three times daily or as often as needed. Adex Gel can be used for as long as necessary either occasionally, such as during flares, or continuously. Seek medical advice if there is no improvement within 2-4 weeks. Contra-indications, warnings, side effects etc: Do not use if sensitive to any of the ingredients. Keep away from the eyes, inside the nostrils and mouth. Temporary tingling, itching or stinging may occur with emollients when applied to damaged skin. Such symptoms usually subside after a few days of treatment, however, if they are troublesome or persist, stop using and seek medical advice. Rarely skin irritation (mild rashes) or allergic skin reactions can occur on extremely sensitive skin, these

tend to occur during or soon after the fi rst few uses and if this occurs stop treatment. Vitamin B derivative requirements are increased during pregnancy and infancy. However, with prolonged use over signifi cant areas, it may be possible to exceed the minimum recommended levels of nicotinamide in pregnancy. Safety trials have not been conducted in pregnancy and breast feeding therefore, as with other treatments, caution should be exercised, particularly in the fi rst three months of pregnancy.
Instruct patients not to smoke or go near naked fl ames. Fabric (clothing, bedding, dressings etc) that has been in contact with this product burns more easily and is a potential fi re hazard. Washing clothing and bedding may reduce product build-up but not totally remove it.
Ingredients: Carbomer, glycerol, isopropyl myristate 15%, liquid paraffi n 15%, nicotinamide 4%, phenoxyethanol, sorbitan laurate, trolamine, purifi ed water.
Pack sizes and NHS prices: 100g tube £2.69, 500g pump pack £5.99. Legal category: Class III medical device with an ancillary medicinal substance.
Further information is available from: Dermal Laboratories, Tatmore Place, Gosmore, Hitchin, Herts, SG4 7QR, UK.
Date of preparation: December 2022.
‘Adex’ is a trademark.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk. Adverse events should also be reported to Dermal.
1. Djokic-Gallagher J., Rosher P., Hart V. & Walker J. Steroid sparing effects and acceptability of a new skin gel containing the anti-infl ammatory medicinal substance nicotinamide. Clinical, Cosmetic and Investigational Dermatology 2019;12:545-552.
2. Gallagher J., Gianfrancesco S., Hart V. and Walker J. Performance of Adex Gel: A retrospective survey of healthcare professionals with an interest in dermatology. Data presented at the Austrian Society of Dermatology and Venereology (OGDV) Science Days, September 2022, Bad Gastein, Austria.

Ingrid Thompson
PEM Friends is a small UK-based charity that supports pemphigus and pemphigoid patients and their carers. In the last year, the PEM Friends support group has made some substantial changes, including becoming a charity in February 2024.
Pemphigus and pemphigoid are rare autoimmune blistering diseases that cause blistering on the skin and mucous membranes. They are often confused with each other because they have similar symptoms, but they affect different layers of the skin and cause different types of blisters. Pemphigus and pemphigoid occur when the body’s immune system attacks healthy skin cells, mistaking them for foreign invaders. The exact cause is unknown, but genetics and environmental factors may play a role.
An accurate diagnosis is important in order to find the right treatment and prevent complications like infection, scarring, and vision problems, and in some cases they can be fatal.
On behalf of the PEM Council, here is our update for the year.
We always seem to say the same, but 2024 has been an exceptionally busy year. A huge amount of change has happened.

Most significantly, we became a charity in February, or a charitable incorporated organisation (CIO) to be exact. Becoming a charity has several benefits, not least of which is that we can claim gift aid on any donations, and we have access to more resources than we had before. However, it does bring some pressures, and our administration and management needs to be even tighter than before. Some of the time spent has been in managing the transition, such as developing more policies, setting up ‘Give as you Live’ donations and creating a new bank account to manage the finances. Changing our bank account involves a lot of work to comply with the bank’s terms and conditions. Most of the council have also become trustees, which is a big personal responsibility.
Our long-standing chair, Isobel, stepped down this year and her last day was 31 October. As a leaving present to us, Isobel organised a PEM Zoom meeting with Molly Connolly, Clinical Nurse Specialist from Scotland and a prominent member of the BDNG. We will be posting this talk to the new website soon. With Isobel leaving we now have a new chair, Trina. She has taken on a huge task and we are very grateful to her. We have also re-modelled the PEM Council, with three new people joining including a new treasurer, John, who has the experience and commitment for the role.
We have launched a new website that is packed with information and has regular updates. Development of this is still ongoing, so please do take a look. You can find the website at www.pemfriends. org.uk or use this QR code.

Additional activities this year:
l We had our 3rd AGM, many of us meeting face-to-face for the first time
l We attended a few conferences but, most notably, the British Association of Dermatologists Annual Meeting in July
l We had a PEM Advisory Group meeting (the advisory group are a team of notable clinicians who help us)
l We have participated in several studies and research activities, for example: The Centre of Evidence Based Dermatology in Nottingham have produced a paper just published in the British Journal of Dermatology on ‘Bullous pemphigoid and drugs that may trigger this.’ Also, there is a trial about to start on a new treatment for scarring in ocular mucous membrane pemphigoid (OcMMP) at Birmingham
l Our leaflet campaign has been slow but still progressing
l We have set up a few regional groups.
At our AGM in July, we discussed how we would manage the group in the absence of Isobel, who has done so much for PEM Friends in the last 10 or so years.
Running PEM Friends is a team effort. With 11 people (at its maximum) keeping things going, we manage to maintain our role as a patient group that is respected and well-known to those in the field of pemphigus and pemphigoid across the UK and beyond. We are all volunteers and patients, though we are looking to employ volunteers from outside to help us.
Our plan constantly evolves.

PREVIOUSLY CALLED HAELAN TAPE


Recommended by The British Association of Aesthetic Plastic Surgeons as a simple steroid treatment for scars & keloids1
Fludroxycortide 4 micrograms per square centimetre Tape effectively treats inflammatory dermatoses and it can be cut to any size or shape.
Including hand eczema and finger tip fissures2
Scan the QR code to view the application video. Alternatively visit typharm.com or call 01603 722480 for more information
Typharm Limited, 14D Wendover Road, Rackheath Industrial Estate, Norwich, NR13 6LH
Can be cut to size for scar site
Reference: 1. Scars & Keloids. BAAPS. Available at https://baaps.org.uk/patients/procedures/16/scars_and_keloids [Last Accessed January 2023]. 2. Layton A. Reviewing th e use of Fludroxycortide tape (Haelan Tape) in Dermatology Practice. Typharm Dermatology.
FLUDROXYCORTIDE TAPE PRESCRIBING INFORMATION: Fludroxycortide 4 micrograms per square centimetre Tape See full Summary of Product Characteristics (SmPC) before prescribing. Presentation: Transparent, plastic surgical tape impregnated with 4 micrograms fludroxycortide per square centimetre. Indications: Adjunctive therapy for chronic, localised, recalcitrant dermatoses that may respond to topical corticosteroids and particularly dry, scaling lesions. Posology and Method of Administration: Adults and the Elderly: For application to the skin, which should be clean, dry, and shorn of hair. In most instances the tape need only remain in place for 12 out of 24 hours. The tape is cut so as to cover the lesion and a quarter inch margin of normal skin. Corners should be rounded off. After removing the lining paper, the tape is applied to the centre of the lesion with gentle pressure and worked to the edges, avoiding excessive tension of the skin. If longer strips of tape are to be applied, the lining paper should be removed progressively. Paediatric population: Courses should be limited to five days and tight coverings should not be used. If irritation or infection develops, remove tape, and consult a physician. Fludroxycortide Tape is waterproof. Cosmetics may be applied over the tape. Contraindications: Chicken pox; vaccinia; tuberculosis of the skin; hypersensitivity to any of the components; facial rosacea, acne vulgaris, perioral dermatitis, perianal and genital pruritus; dermatoses in infancy including eczema, dermatitic napkin eruption, bacterial (impetigo), viral (herpes simplex) and fungal (candida or dermatophyte) infections. Warnings and Precautions: Not advocated for acute and weeping dermatoses. Local and systemic toxicity of medium and high potency topical corticosteroids is common, especially following long-term continuous use, continued use on large areas of damaged skin, flexures and with polythene occlusion. Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression. Long-term continuous therapy should be avoided in all patients irrespective of age. Application under occlusion should be restricted to dermatoses in very limited areas. If used on the face, courses should be limited to five days and occlusion should not be used. In the presence of skin infections, the use of an appropriate antifungal or antibacterial agent should be instituted. Long term continuous or inappropriate use of topical steroids can result in the development of rebound flares after stopping treatment (topical steroid withdrawal syndrome). A severe form of rebound flare can develop which takes the form of a dermatitis with intense redness, stinging and burning that can spread beyond the initial treatment area. It is more likely to occur when delicate skin sites such as the face and flexures are treated. Should there be a reoccurrence of the condition within days to weeks after successful
Waterproof protection once in place
For both children and adults
treatment a withdrawal reaction should be suspected. Reapplication should be with caution and specialist advise is recommended in these cases or other treatment options should be considered. For children, administration of topical corticosteroids should be limited to the least amount compatible with an effective therapeutic regimen. Children may absorb proportionally larger amounts of topical corticosteroids and thus may be more susceptible to systemic toxicity. Pregnancy and Lactation: Use in pregnancy only when there is no safer alternative and when the disease itself carries risks for mother and child. Caution should be exercised when topical corticosteroids are administered to nursing mothers. Undesirable Effects: The following local adverse reactions may occur with the use of occlusive dressings: burning, itching, irritation, dryness, folliculitis, hypertrichosis, acne form eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, miliaria, striae and thinning and dilatations of superficial blood vessels producing telangiectasia. Transient HPA axis suppression. Cushing’s syndrome. Hyperglycaemia. Glycosuria. Adrenal suppression in children may occur. Infected skin lesions, viral, bacterial, or fungal may be substantially exacerbated by topical steroid therapy. Wound healing is significantly retarded. Local hypersensitivity reactions. Stop treatment immediately if hypersensitivity occurs. Withdrawal reactions - redness of the skin which may extend to areas beyond the initial affected area, burning or stinging sensation, itch, skin peeling, oozing pustules. Precautions for Storage: Store in a dry place, below 25oC. Pack Size and Price: Polypropylene dispenser and silica gel desiccant sachet in a polypropylene container, with a polyethylene lid, packed in a cardboard box, containing 20cm or 50cm of translucent, polythene adhesive film, 7.5cm wide, protected by a removable paper liner. 7.5cm x 20cm £19.49. 7.5cm x 50cm £28.95. Legal Category: POM Marketing Authorisation Number: PL 00551/0014 Marketing Authorisation Holder: Typharm Ltd., Unit 1, 39 Mahoney Green, Rackheath, Norwich NR13 6JY. Tel: 01603 722480, Fax:
van der Geugten, Karin Veldman
The implementation of the ‘Skin at School’ programme in the Netherlands was motivated by various factors. Skin complaints are often underestimated, impacting the quality of life for individuals with chronic conditions and contributing to significant bullying effects on children.1-3 In every class, there are children who suffer from skin problems such as warts, athlete’s foot or eczema. Patient representatives reported that children with a chronic skin condition and their parents have questions about how to talk about this at school as not
Summary:
every child is comfortable giving a presentation about his or her condition.
Skin cancer is the most common form of cancer in the Netherlands, with 83,300 reported cases in 2022. Skin cancer is mainly caused by excessive exposure to UV radiation from the sun and tanning beds.4 Children can learn through education about the risk of excessive exposure to UV radiation from the sun and the importance of using sunscreen to prevent skin cancer later in life.
Skin at School aimed to address the underestimation of skin complaints, recognising the impact on children’s quality of life, particularly those who experience bullying as a result of their skin’s appearance. Parents of affected children face challenges discussing these issues at school. The project therefore aimed to empower children aged four to 12 by developing educational materials, fostering knowledge, skills and attitudes about skin. Healthcare professionals, educators, and patient representatives collaborated on a curriculum, resulting in tailored lessons for pupils. Skin at School has been successfully implemented and positively assessed, prompting a call for further research on its broader effects.
Abstract:
Background: The impact skin conditions can have on young people’s quality of life is often underestimated, with many children bullied as a result of their skin’s appearance. Parents of children with skin conditions report facing challenges discussing these issues at school, despite the critical importance of skincare and skin cancer prevention. Moreover, inaccurate information shared on social media is being followed and believed by young people. Education is therefore vital in preventing health risks. Aim: To develop educational materials for children aged four to 12, fostering knowledge, understanding, skills and attitudes about their own and other’s skin. To empower children to prevent health risks and enhance their wellbeing and resilience. Methods: Healthcare professionals, educational experts, and patient representatives collaborated to determine learning objectives, curriculum topics, and assignment designs. The curriculum was carefully crafted to be substantively correct, fitting into the educational context and being appropriate for patient representatives. A children’s council provided advice. Results: The curriculum – comprising five lessons tailored to specific age groups – covers skin basics, skincare, skin colour, temporary and long-lasting skin problems, sun protection, assessing reliable information, acceptance and wellbeing. Conclusion: Skin at school has been successfully developed, implemented and positively assessed. Further research on the effects of this programme is needed.
Author info:
Jolien van der Geugten is Project Manager for Skin at School at the Dutch Skin Coalition (Huid Nederland); Owner and scientific researcher at WYS Research & Education. Karin Veldman is President at the Dutch Skin Coalition (Huid Nederland); President Association for Ichthyosis Networks, The Netherlands; Vice-President European Network Ichthyosis.
Keywords: Skin, Skin disease, Skin cancer, Educational materials, Skin care
Citation:
Van der Geugten J, Veldman K. Skin at School: Educational materials on skin and skin diseases for primary schools. Dermatological Nursing 2024. 23(4):10-16
“Skin cancer is the most common form of cancer in the Netherlands, with 83,300 reported cases in 2022”
Yet dermatologists involved in this project observed inadequate skincare knowledge among participants, including a lack of awareness of the importance of suncream and protecting our skin from sunburn.
Nurses provide care and guidance to children with skin conditions and their parents. Nurses are also involved in the patient journey, and can therefore determine what information children and their parents need at particular times. Educational materials can help nurses inform children and their parents, and these materials can also be used in schools to increase understanding of skin conditions.
We – Jolien Van der Geugten and Karin Veldman – are the initiators of the Skin at School programme, and we found that more knowledge about having a chronic skin condition at school is necessary. In 2009, Veldman was diagnosed with a severe and ultrarare skin condition called Netherton syndrome. Her years in primary school have led to trauma, as she was bullied both mentally and physically. Van der Geugten has three children, one of whom has eczema and one of whom has ichthyosis. Her children are frequently questioned about their flakes or red spots by other pupils. From these experiences, the authors are determined to address the acceptance of children with skin problems, and skin care in general.
In 2020, we carried out a survey among 41 teachers. Around three-quarters of the respondents said students do not know about common and chronic skin problems. Fortunately, a large majority of the teachers believe it is important to have educational materials to improve knowledge about skin and to increase acceptance of students with skin conditions.
Finally, there are many inaccurate messages circulating on social media channels about skincare, which are often followed and believed by young people. For example, the use of blister plasters on acne and that using sunscreen actually causes cancer.5,6 A lack of basic knowledge about the skin is a major contributing factor to the spread of misinformation.
In the Netherlands, there are specific goals and building blocks for primary education including that pupils ‘learn to take care of the physical and psychological health of themselves and others’.7 In addition, there are crosscurricular themes of ‘Health’ and ‘Digital literacy’.8 The textbooks for elementary schools related to biology that were looked at for this project showed that there is limited focus on skin. Elementary schools in the Netherlands do have project work and themes such as health and body, where ‘skin’ could be included.
Optional teaching material on the skin can be found online, however, it is not clear who developed these materials

and for what purpose. Teaching materials developed by patient organisations were mostly focused on informing the child, their parents or the teacher about a specific skin condition. There are children’s books, short animations and educational television programmes about common skin problems and chronic skin conditions, but these have been developed independently of formal education.
At the congress of the European Academy of Dermatology and Venereology in Berlin (2023), various patient associations and medical professionals were interested in an educational programme on skin such as Skin at School. They agreed that this programme should be known and used by many children.
The aim of Skin at School is to develop and implement educational materials about skin conditions and skin care for students at primary schools, enabling them to develop knowledge and understanding, and embrace a more positive attitude to their own skin and that of other students. We intend to contribute to the empowerment of children to prevent health risks and enhance their wellbeing and resilience.
The Dutch Skin Coalition initiated Skin at School on behalf of its members,

“Across five lessons, children developed their knowledge, insight, skills and attitude towards their own skin and that of other students”
including patient associations, patient representatives and their loved ones, dermatologists and skin therapists. As an umbrella organisation, we do not possess independent financial resources. Therefore, in 2020, we submitted a grant application for patient organisations to the Dutch government to develop educational materials on skin care and skin conditions tailored for children aged eight to 12. In 2023, we submitted another grant application for the implementation of (1) the educational materials tailored for children aged eight to 12 years, (2) a specific lesson about acne and (3) the development of educational materials for children aged four to eight years. Both grant applications were approved and assigned to us. The Dutch Association for Dermatology and Venereology, along with a Dutch health insurance company (Under Your Skin Foundation and Foundation CW De Boer) provided co-funding.
To have sufficient support for the outcome of this project, it was important to involve all stakeholders. Therefore, in this project we collaborated with patient organisations, patient representatives, dermatologists, nurse practitioners, skin therapists, teachers, educational experts and children themselves. In addition,
we have partnered with the Dutch association for dermatologists, the Dutch association for skin therapists and the Under Your Skin Foundation.
We assembled a project group with three dermatologists, three teachers/ educational experts, two nursing specialists, a skin therapist and six patient representatives. We formed a junior council with six children aged eight to 12, including some who had skin conditions and some who did not.
As a result of the Covid-19 pandemic and project group members living and working far apart, the project group had online meetings.
The first phase of the project involved formulating goals for teaching materials and selecting topics. For this, we used the knowledge and experience of all project group members together with available guidelines and educational policies.
Based on the meetings, one didactical expert and the project leader worked this into a draft. This was discussed with the project group and presented to the junior council. Practical consideration was given to its feasibility at school and in the classroom. We tested our

materials on the junior council, who provided us with feedback. We also asked some teachers to test the materials at their schools, after which we made some more adjustments.
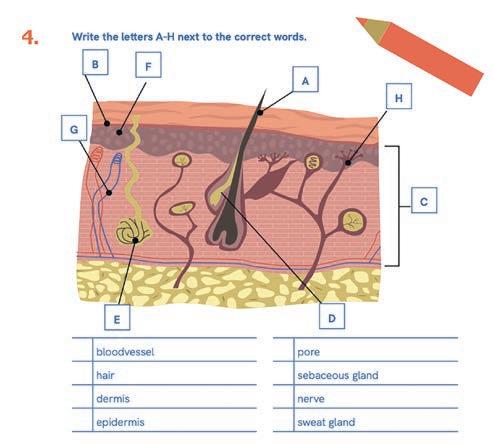
Across five lessons, children developed their knowledge, insight, skills and attitude towards their own skin and that of other students. They learn how to prevent health risks and improve their wellbeing and resilience through the activities within the curriculum. They learn about the structure and function of the skin, the similarities and differences between people’s skin, and the importance of skin care. They are educated on sun protection and preventing skin cancer, and about temporary and permanent skin conditions and their consequences (Table 1). They learn that children’s skin should not be the subject of bullying or stigmatisation.
The Skin at School programme is designed to educate children that everybody can be affected by a skin problem, ranging from temporary to lifelong. With the rise of misinformation on social media, the children also learn how to critique information they read about skin on the internet.
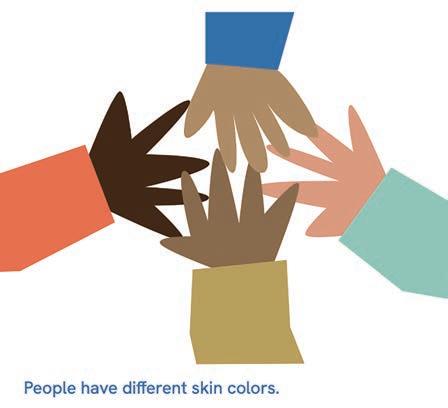
The lesson series consists of five lessons, with each lesson tailored to children of different ages (4-6 years, 6-8 years, 8-10 years and 10-12 years). Each lesson can be covered in 30 to 45 minutes (Table 1). The first lesson covers basic skin knowledge, including skin colour and how the skin works. The second lesson is about taking
care of the skin, such as using creams, showering, good hygiene and hand washing. In the third lesson, we focus on common skin problems such as warts, foot fungus and acne. The fourth lesson is about the risk of sunburn and how children can protect themselves from UV radiation. The fifth lesson is about chronic skin conditions (such as eczema, psoriasis and vitiligo) and what it is like to have one of these conditions.
There are four additional lesson sheets for children aged eight to 12 years. Three are on rare skin disorders such as ichthyosis, congenital naevi and vascular malformations. These sheets can be used when a child or a child’s sibling has one of these skin conditions. There is also one lesson sheet about acne for children aged 10-12, because teachers and skin therapists emphasised this as an important topic for children of this age.

The teaching materials are flexibly designed, so that teachers can decide which materials to cover with their students. We always recommend starting with Lesson 1, as this lesson contains basic concepts. After that, the other lessons can be followed in any order. To provide a safe learning environment, we
Lessons Subject
1 Basics of the skin: how the skin works
Learning objectives
Characteristics about their own skin and that of others.
The structure and function of the skin.a,b The protective function of the skin.b Possible advantages and disadvantages of washing hands with soap.a,b,c
2 Care of the skin Characteristics of skin types.a,b Hygiene and the importance of skin care. The origin and care of an abrasion.
3 Temporary skin problems like warts and acne
4 The protection of the skin against the sun
5 Long-lasting skin problems like eczema, psoriasis, vitiligo and alopecia
a For children aged 8-10 years
b For children aged 10-12 years
c For children aged 6-8 years
Common skin conditions that anyone can get.
The infectiousness of skin conditions.a,b
Skin colour types. Sunburn and protection against it. The relationship between skin and vitamin D.a,b
Chronic skin conditions.a,b,c
The effects of a chronic skin condition.a,b How to treat others with chronic skin conditions.a,b
advise holding a consultation in advance with students and parents. In the materials, students observe their own skin and the skin of their classmates to learn about similarities and differences.
The lessons are light-hearted so that students can process the material easily. There is a manual for teachers, which provides more detailed explanations. The children who can already read can work on the assignments independently or in small groups. Children also watch episodes from educational television programmes and explanations and animations via YouTube to complete assignments. In addition, we ask the older children to reflect on what they read and see, such as the impact of a permanent skin condition. Several assignments lend themselves well to classroom discussion. Each lesson begins with an assignment in which students record personal information about their own skin in their ‘skin passport’.
The teaching materials come in a cardboard case with a manual, skin passport, poster, flyer and skincoloured pencils. There is dish soap and a jar of pepper for doing an experiment. Worksheets and manuals are downloadable for free from the website, and are also available from an online Dutch primary education platform. There are 600 teaching kits distributed to schools in all provinces of the Netherlands. Teaching kits were sent upon teacher request to ensure purposeful use, avoiding random distribution to prevent potential waste.
Teachers applied for the teaching materials for various reasons: some aimed to educate about the skin, others had personal connections through their own skin conditions or a partner’s melanoma, some organised a health week, and others incorporated sun protection into their lessons. Not all schools were interested in our teaching materials and indicated that they were too busy with regular classes. It was mostly individual teachers who ordered the material because they saw possibilities for the subject they had to teach anyway or wanted to pay special attention to.
Healthcare professionals sought the kits to offer to patients or deliver guest lessons at their children’s schools. The Netherlands Association of Skin Therapists (NVH) embraced our project, collaborating on a webinar and ordering an additional 200 cases. They also decided to reward members with accreditation points for conducting guest lessons at schools, emphasising the priority of providing skin care within their professional responsibilities.
During the June 2022 Skin at School launch, Veldman spent two days at a primary school delivering guest lessons on skin to all classes and sharing her personal experiences. Collaborating with authors of children’s books about different-looking skin enriched the lessons. Veldman received compliments from teachers on the quality of our materials.
It is modern, accessible, widely applicable, didactically well put together, and aligns well with the core values of primary school education in the Netherlands. The launch of Skin at School gained additional coverage with reports from local and regional radio. A teacher with almost 2,000 followers on Instagram created a great video about our teaching materials and as a result, over 100 requests for the teaching materials came from other teachers.
We actively promoted Skin at School within patient associations, featuring a booth at the 2022 Dutch World Eczema Day organised by the

eczema patient association. During the event, positive feedback came from children and patients who expressed appreciation for the initiative. Emotional responses were noted, with some individuals saying they wished such lessons existed in their youth. The profound impact of childhood experiences where differences led

to social exclusion were shared. Our teaching materials extended their reach to family meetings of various patient associations, including vitiligo, psoriasis, congenital naevi, vascular malformations, and ichthyosis. Further visibility was achieved through mentions on websites, newsletters, and patient magazines.

“We actively promoted Skin at School within patient associations, featuring a booth at the 2022 Dutch World Eczema Day”
To engage dermatologists, we reached out to the Association for Dermatologists in the Netherlands, published articles in magazines, and posted on LinkedIn. Our presence at the annual symposium for paediatricians and dermatologists allowed us to deliver a presentation on our educational materials, ensuring awareness among these medical

professionals. Many dermatologists and paediatricians are informed about Skin at School and how it offers a valuable resource for children and parents facing skin-related bullying and for those seeking guidance on discussing skin conditions. Flyers in various hospitals and GP practices further disseminated information to children and their parents.
Teacher: ‘I myself have skin problems/a chronic skin condition and one of the students had a chronic skin condition, which allowed us to explain together what it does to your skin and your life.’
Teacher: ‘I think it is very important that children learn about their skin and the different skin types. In addition, it is important that they learn, especially in primary school, about protecting the skin.’
Teacher: ‘Children think it is boring to talk about skin, but you have included social media. This makes it more interesting’.
Parent: ‘Thank you for this interesting material! My child was anxious to talk about his eczema, but during the lessons it turned out he was not the only one in his class!’
Skin therapist: ‘The children really enjoyed the lesson and actively started applying sunscreen.’
Skin therapist: ‘Nice way to offer a lesson in schools, the teaching materials are well put together.’
During implementation, our team utilised social media, disseminating messages on LinkedIn, raising awareness among patient representatives, healthcare professionals, teachers, and educational experts. Our Instagram account successfully reached numerous teachers and skin therapists, providing regular informative posts about the teaching material’s content. Relevant hashtags facilitated discovery by teachers, patients, and patient organisations, fostering engagement and visibility.
In total, 700 teaching kits have been distributed to schools in 170 different cities in the Netherlands. We could not record on the website how many times the materials were downloaded. Our evaluation form was completed by 24 skin therapists who gave guest lectures at schools (70%), teachers (26%) and a parent of a child with a skin condition (4%). In Figure 1 you can see which lessons were taught.
All the respondents mentioned positive aspects about the learning material. For example, that it is interactive, developed clearly and
at the right level, nicely designed, and that assignments were easy to use. Some of them provided advice for improving lessons, such as simplifying the skin passport (less cutting and folding), creating standard presentations for guest teachers by topic, and adding sunscreen to the teaching kit.
Discussion
We have succeeded in implementing educational materials about the skin, skin care and skin problems throughout the Netherlands. The collaboration between various healthcare professionals, educational professionals, patient representatives and a junior council allowed the materials developed to be well placed to achieve the aims of Skin at School.
The strength of the Skin at School material lies in its focus not only on children with skin problems but on all children. Everyone has skin that requires proper care. Through knowledge and understanding of your own skin, you discover that everyone can develop common skin problems, and some individuals have a permanent skin condition. The teaching material is also inclusive regarding skin colour.
Our project team’s analysis showed that teaching material about the skin was scarce and the quality was unclear. Most of the material available focused on children with a specific skin condition. Our materials align with educational policy and current affairs related to information literacy and assessing of health information.7,8 Children can be exposed to fake news or misinformation through social media or prominent individuals who make claims, such as the use of sunscreen.5,6 Education can play a crucial role in preventing health risks.
Our teaching materials can be utilised by teachers themselves. Schools have the option to use a guest teacher, or they can choose to teach the material independently. The teaching materials are available for free by digital download or print. Some teachers may find it challenging to broach the
subject of having a skin condition, and Skin at School is designed to make these conversations easier. We are now mainly contacting teachers who are interested in the subject or looking for teaching materials, as some schools feel they are too busy with regular classes. Therein lies a challenge for us to bring the programme to the attention of all schools and implement it.
The Netherlands is a multicultural society with diversity in skin tone. Of the 17.5 million inhabitants, 14% came to the Netherlands as migrants and 11% were Dutch-born children of migrants. 8 There was no data available on how many people have skin of colour. With our programme, we aim to show children both the similarities and differences in skin tones. We provide them with a set of pencils representing various skin tones from around the world, not limiting it to the commonly used salmon pink, which is often considered a default skin colour by those with white skin. Our lessons are inclusive, covering all skin tones, and we aim to educate children about the consequences of stigmatisation.
In addition, we see it as our responsibility to teach children at a young age to protect themselves from sunburn. Children are at a high risk of developing skin cancer later in life, but they may not be aware of it. Many people do not realise that sunburn during childhood can lead to skin cancer in adulthood. By setting a good example early on in life, we can hopefully encourage healthy behaviours that will last long into adulthood. By doing so, we can influence how many children develop skin cancer. 9
One limitation of our project is that due to limited funding, we have not been able to investigate the effect of our teaching materials on knowledge, attitudes and behaviours. We need to acquire additional funding to investigate the programme’s impact, and to translate our teaching materials to other languages so it can be used in countries other than the Netherlands.
Skin at School has been successfully developed and implemented and the feedback we have received has been largely positive. Further research on the impact of this programme is needed.
1. Ablett K, Thompson AR Parental, child, and adolescent experience of chronic skin conditions: a meta-ethnography and re- view of the qualitative literature. Body Image 2016.19:175–185
2. Golics CJ, Basra MK, Finlay AY, Salek MS. Adolescents with skin disease have specific quality of life issues. Dermatology. 2009;218(4):357-66.
3. Holm EA, Wulf HC, Stegmann H, Jemec GB. Life quality assessment among patients with atopic eczema. Br J Dermatol. 2006 Apr;154(4):719-25.
4. De Groot, J, Kramer-Noels, E. Nationaal actieplan huidkanker. Stuurgroep Huidkankerzorg Nederland; 2021 April.
5. Joshi, M, Korrapati, NH, Reji F, Hasan, A, Kurudamannil RA. The Impact of Social Media on Skin Care: A Narrative Review. Lviv Clinical Bulletin. 2022, 1(37)-2(38):85-96.
6. Borba, AJ, Young, P.M, Read, C. Armstrong, A. Engaging but inaccurate: A cross-sectional analysis of acne videos on social media from non–health care sources. JAAD. 2019 Aug;83(2):610-612.
7. Greven J, Letschert J. Kerndoelenboekje basisonderwijs. Den Haag: Ministerie van Onderwijs, Cultuur en Wetenschap; 2006 April.
8. Curriculum.nu. Samen bouwen aan het primair en voortgezet onderwijs van morgen. Curriculum.nu; 2019 Oktober.
9. CBS. Hoeveel inwoners van Nederland zijn in het buitenland geboren? Retrieved from https://www.cbs.nl/nl-nl/visualisaties/ dashboard-bevolking/herkomst [Accessed May 2024]
10. Baig IT, Petronzio A, Maphet B, Chon S. A Review of the Impact of Sun Safety Interventions in Children. Dermatol Pract Concept. 2023 Jan 1;13(1)
The authors would like to thank the following people for their contribution to the project: Dermatologists Shiarra Stewart, Kirsten Vogelaar-Burghout and Suzanne Pasmans, and all of the patient representatives and educational experts.
KLISYRI® (tirbanibulin) is indicated for the field treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis (AK, Olsen grade 1) of the face or scalp in adults.1

WELL-TOLERATED FIELD THERAPY2 NOVEL MoA1,2
KLISYRI® (TIRBANIBULIN) PRESCRIBING INFORMATION
Please consult the Summary of Product Characteristics (SmPC) before prescribing. Klisyri 10 mg/g ointment Active Ingredient: Each gram of ointment contains 10 mg of tirbanibulin. Each sachet contains 2.5 mg of tirbanibulin in 250 mg ointment. Excipients with known effects: Propylene glycol 890 mg/g ointment Indication: Klisyri is indicated for the field treatment of non-hyperkeratotic, non hypertrophic actinic keratosis (Olsen grade 1) of the face or scalp in adults. Dosage and Administration: Tirbanibulin ointment should be applied to the affected field on the face or scalp once daily for one treatment cycle of 5 consecutive days. A thin layer of ointment should be applied to cover the treatment field of up to 25cm2 Consult SmPC and package leaflet for full method of administration. Contraindications, Precautions and Warnings: Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of SmPC. Precautions: Contact with the eyes should be avoided. Tirbanibulin ointment may cause eye irritation. In the event of accidental contact with the eyes, the eyes should be rinsed immediately with large amounts of water, and the patient should seek medical care as soon as possible. Tirbanibulin ointment must not be ingested. If accidental ingestion occurs, the patient should drink plenty of water and seek medical care. Tirbanibulin ointment should not be used on the inside of the nostrils, on the inside of the ears, or on the lips. Application of tirbanibulin ointment is not recommended until the skin is healed from treatment with any previous medicinal product, procedure or surgical treatment and should not be applied to open wounds or broken skin where the skin barrier is compromised. Local skin reactions in the treated area, may occur after topical application. Treatment effect may not be adequately assessed until resolution of local skin reactions. Due to the nature of the disease, excessive exposure to sunlight (including sunlamps and tanning beds) should be avoided or minimised. Tirbanibulin ointment should be used with caution in immunocompromised patients. Changes in the appearance of actinic keratosis could suggest progression to invasive squamous cell carcinoma. Propylene glycol may cause skin irritation. Consult SmPC and package leaflet for more information. Fertility, pregnancy and lactation: No human data on the effect of tirbanibulin ointment on fertility are available. Tirbanibulin
Reference: 1. KLISYRI® SmPC. Almirall. 2. Blauvelt A, et al. N Engl J Med. 2021;384(6):512-520. 3. Schlesinger T et al. Skin, 2023: 7(3); 771-787. PROVEN EFFICACY2 CONVENIENT SHORT TREATMENT COURSE1
ointment is not recommended during pregnancy and in women of childbearing potential not using contraception. It is unknown whether tirbanibulin/metabolites are excreted in human milk. A risk to the newborns/infants cannot be excluded. Consult SmPC and package leaflet for more information. Adverse Reactions: Very common (≥1/10): Application site - erythema; exfoliation; scab; swelling; erosion. Common (≥1/100 to <1/10): Application site - pain, pruritus and vesicles. Consult SmPC and package leaflet for further information. Legal Category: Ireland: POM Subject to prescription which may not be renewed (A). United Kingdom & Northern Ireland: POM Price: Ireland: Price to wholesaler United Kingdom & Northern Ireland: UK NHS Cost: £59.00 (excluding VAT). Marketing Authorisation Numbers: Ireland and Northern Ireland: EU/1/21/1558/001 Great Britain: PLGB 16973/0043 Marketing Authorisation Holder: Almirall, S.A., Ronda General Mitre, 151 08022 Barcelona, Spain Further information available from: Almirall Limited, Harman House, 1 George Street, Uxbridge, Middlesex, UB8 1QQ, UK. Date of First Issue: August 2021 Item code: IETIRBA-2100011 Complete information on where to find reportingforms and information for adverse events search MHRA Yellow Card in the Google Play or Apple App Store.
UK and UK(NI)-Adverse events should be reported. Reporting forms and information can be found at MHRA https:// yellowcard.mhra.gov.uk Adverse events should be also reported to Almirall Ltd. Tel. 0800 0087 399
IE-Adverse events should be reported. Reporting forms and information can be found at HPRA Website: www.hpra.ie. Adverse events should be also reported to Almirall Ltd. Tel. +353 (0) 1431 9836




Soothe, hydrate and comfort dry skin around the delicate eye area
sensitive, irritated eyelids or for those with eyelid eczema
Helps to relieve eyelids that are:

to find out more
EPIMAX® Eyelid Ointment is available for patients to buy OTC and online
EPIMAX® Eyelid Ointment Product Information
Presentation: Ointment in 4g tube Main ingredients: Yellow soft paraffin 80%, liquid paraffin 10% and wool fat (lanolin) 10%. Indications: Soothing moisturiser for dry, itchy, red, and flaky eyelids. Method of use: Apply to the eyelid skin as frequently as required. Warnings: For external use only. Avoid contact with eyes. If product does accidentally come in to contact with eyes, rinse well with water and seek medical advice. Do not use if allergic to any of the ingredients. This product contains lanolin, which may cause local skin reactions or allergic reactions. Seek medical advice before use if the skin is broken, infected, or bleeding; or after use if any undesirable effects are experienced, or if accidentally swallowed. No special precautions during pregnancy or breast-feeding. Contraindications: Patients with known hypersensitivity to any ingredient. Product classification: Class I Medical Devices. Cost: RRP: £9.95 NHS List Price: £2.35 Legal Manufacturer: Aspire Pharma Ltd, Unit 4, Rotherbrook Court, Bedford Road, Petersfield, Hampshire, GU32 3QG, UK. Date reviewed: March 2023 Version number: 10104511504 v 2.0
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Aspire Pharma Ltd on 01730 231148.
Please advise your patients to always read the label
Stephanie Greenleaf
Phototherapy is a vital treatment for various dermatological conditions including psoriasis, eczema and vitiligo.1 Over the past seven years, I have had the privilege of setting up and leading a phototherapy unit in an acute hospital in Southwest London. This role has not only fuelled my passion for dermatological nursing but also allowed me to engage deeply with both the operational and educational aspects of service provision.
My journey started in a small unit with one nurse and has expanded into a comprehensive service offering extensive treatment options to our local population and surrounding area of patients. This expansion was guided by strict adherence to the British Association of Dermatologists’ (BAD) phototherapy service guidelines and the St John’s Phototherapy Guidelines.2,3 The guidance supports implementation of high standards for patient care and safety. There was an agreed narrative of ensuring excellent leadership, incorporating trust values by addressing staff development through training and access to learning with an overarching aim to build a team, capable of delivering exceptional care to our dermatological patients.4
My role as phototherapy lead has naturally developed. Alongside the managerial aspect, I provide education to junior doctors, student nurses and nursing staff. The opportunity to teach and mentor others has been profoundly rewarding and continuously reinforces my commitment to nurturing the next generation of phototherapy and dermatology professionals.
This aspect of my work led to an invitation from my clinical lead Emma Button to present at the prestigious BDNG Manchester 2024 Meeting, which I proudly accepted.
Preparing for the two-day BDNG meeting involved extensive research to ensure relevant, current and correct information was shared. The meeting covered a wide range of topics within phototherapy designed to engage a diverse audience of professionals. I utilised the BAD Service Guidance and Standards for Phototherapy Units to ensure current and relevant information was used throughout
This article provides an overview of implementing phototherapy guidelines and practices within a clinical setting, considering the importance of education to enhance patient outcomes and streamline services. Additionally, it details how the development of a comprehensive training programme can also improve staff proficiency in line with the NMC Code (2018). The insights shared are drawn from my leadership experience in a phototherapy unit, firsthand observations, and feedback from the attendees at the British Dermatological Nursing Group (BDNG) Manchester 2024 Meeting.
The discussion covers the integration of best practices, the evolution of patient experience and the impact of continuous professional development on treatment efficacy. Reference is also made to the St John’s Phototherapy Guidelines, (2009), which inform best practice by providing detailed protocols and safety measures for the effective and safe use of phototherapy in treating dermatological conditions. Although there are numerous other UK phototherapy guidelines, time constraints limited their use within the meeting.
Keywords:
Phototherapy, Education, Professional Development, BDNG Manchester Meeting
Author info:
Stephanie Greenleaf, Clinical Nurse Specialist Lead for Phototherapy, West London NHS Trust.
Citation: Greenleaf, S. Expert consensus on the systemic treatment of atopic dermatitis. Dermatological Nursing 2024. 23(4):19-23.
the meeting as well as the Nursing and Midwifery Council (NMC) Code (2018) to guide best practices in nursing. In doing so, I was able to deliver a comprehensive timetable of subjects offering relevance and interest to all those working within the phototherapy field.
This process allowed me to creatively convey complex information in an accessible format.5 However, integrating technology such as digital tools into ‘classroom’ tasks would have significantly enhanced learning by promoting oral presentations and collaborative group activities.6
I encourage phototherapy nurses to engage and embrace educational activities, as through personal reflection I am able to advise on personal growth and discovery, shaping my professional practice and reinforcing my commitment to advancing phototherapy nursing through continuous learning and teaching.7
The BDNG meeting provided an update and refresh in education for both me and the nurses in attendance. Reflection is deeply integrated into our practice through revalidation and appraisals, which mandate dedicated time for self-development.8
The feedback from the BDNG meeting was collected through a set of questions answered by participants. This feedback offers a comprehensive understanding of the meeting’s impact and areas for improvement.
The data presented below reflects the overall satisfaction levels, the effectiveness of the presentations and the value of the meeting, highlighting both strengths and opportunities for further enhancement.9
Q1. How did you hear about this event?
The data from 62 respondents shows that 40.32% heard about the meeting through colleagues, underscoring the power of word-of-mouth in professional networks. The BDNG website was the next most common source at 37.10% followed by email notifications at 19.35%.
“Reflection is deeply integrated into our practice through revalidation and appraisals, which mandate dedicated time for self-development.”
A small fraction (3.23%) received information via other means, such as personal anticipation and department managers and no respondents used postal mail.
These insights emphasise the importance of interpersonal communication and official online platforms for effective information dissemination and could help in planning future communication methods to ensure maximum reach and effectiveness.
Q2. Who paid your registration fee?
The data indicates that 66.13% of respondents had their registration fees paid by employers, highlighting strong organisational support for professional development. This suggests employers value their employees’ participation and are keen to invest in their growth, enhancing job performance and satisfaction.
Meanwhile, 30.65% of respondents paid their fees themselves, showing high personal commitment to professional development but also suggesting some employers do not provide financial support for these activities. A small number (3.23%) had their fees covered by third parties, such as sponsors or grants, indicating alternative funding options exist.
Q.3 Who paid your travel costs?
Just under two thirds (64.52%) of respondents had their travel costs paid by their employer, while 32.26% paid their own costs and 3.23% were covered by third parties. This indicates strong employer support for professional development. However, the significant number of self-payers suggests substantial personal commitment and
highlights a gap in employer support, potentially due to budget constraints or policies. The presence of thirdparty payments, though minor, points to alternative funding sources such as sponsorships and grants.
Q4. Did you receive study leave to attend this meeting?
Nearly all (96.77%) respondents received study leave to attend the meeting, showcasing strong organisational support for professional development. Only 3.23% did not receive study leave which may be due to company policies or staffing issues.
This high level of support suggests a positive organisational culture which prioritises continuous educational and professional growth, leading to increased employee satisfaction and retention. However, the small percentage not receiving leave highlights potential areas for improvement, such as exploring flexible solutions for inclusivity in professional development. Overall, the data reflects positively on organisational practices and underscores a commitment to employee growth.
Q5. Please evaluate each presentation
The evaluation data for the presentations, categorised as ‘Excellent’, ‘Good’, ‘Average’, or ‘Poor’, show that high ratings were generally received, with most responses falling under ‘Excellent’ or ‘Good.’
What is phototherapy?
An overwhelmingly positive evaluation with 64.41% of respondents rating this talk as excellent and 30.51% rating it as good. Only 5.08% rated the presentation as average and none rated it as poor. This suggests that the explanation of phototherapy was both
clear and comprehensive, effectively meeting the audience’s expectations and educational needs.
This presentation was well-received with 57.63% of respondents rating it as excellent, 37.29% as good and 5.08% as average. No poor ratings were received. This suggests that the presentation effectively conveyed a strong understanding of conditions responsive to phototherapy, meeting the audience’s expectations and educational needs.
Patient assessment/the patient journey
Results show positive feedback with 55.93% of respondents rating the talk as excellent and 38.98% as good. Only 5.08% rated it as average, and none rated it as poor. This reflects effective communication of the patient assessment processes and the journey through treatment.
Treatment protocols
Nearly half (49.15%) of respondents rated this talk as excellent and 45.76% as good, with only 5.08% rating it as average and none rating it as poor. This suggests that the presentation provided clear and useful treatment guidelines.
Patient safety
This talk also received very positive feedback with 59.32% of respondents rating it as excellent and 37.29% as good. Only 3.39% rated it as average and none rated it as poor. These high ratings indicate that the presentation effectively conveyed vital information on maintaining patient safety.
Photosensitising drugs
This talk was well-regarded with 49.15% of respondents rating it as excellent and 44.07% as good. In total, 6.78% rated it as average and none rated it as poor. This indicates that the information was thorough and effectively communicated, successfully meeting the audience’s needs and expectations.
MED/MPD testing
Just over half (50.85%) of respondents rated this talk as excellent, 38.98% as good, 10.17% as average and none as
Evaluation data for the presentations (Q5)
Photo-responsive dermatoses
Patient assessment/the patient journey
Treatment protocols
Patient safety
Photosensitising drugs
MED/MPD testing
Protective measures
When to seek advice
Discharge of patients
Service standards
Phototherapy in children
Safe calculations
Competences
Topical treatments
% of respondents
poor. These ratings suggest that the presentation clearly communicated the procedures and importance of these testing methods for these critical components in phototherapy.
High ratings were received for this talk, with 57.63% of respondents rating it as excellent and 33.90% as good. A smaller proportion (8.47%) rated it as average and none rated it as poor indicating that the audience found the information valuable and well-communicated, with the talk effectively addressing the importance of protective measures in ensuring safe and effective phototherapy treatments.
A well-received presentation, with 55.17% of respondents rating it as excellent and 37.93% as good. A small percentage (6.90%) rated it as average
and none rated it as poor. This indicates that the information on when to seek additional advice, support and guidance was clearly conveyed to the audience.
Just over half (50.85%) of respondents rated this presentation as excellent and 42.37% as good, with a small percentage (6.78%) rating it as average, and none rating it as poor. This indicates that the presentation provided clear and effective guidelines on patient discharge procedures post-phototherapy.
Service standards
This presentation received strong positive feedback with 50.85% of respondents rating it as excellent and 42.37% as good with only a small portion (6.78%) rating it as average and none rating it as poor. The presentation effectively communicated well-defined service standards in phototherapy
Attendees’ agreement/disagreement with several statements (Q6)
Speakers
offering improved understanding and implementation of high-quality service standards in practice.
This talk received mixed feedback with 40.35% of respondents rating it as excellent and an equal percentage rating it as good. However, 15.79% rated it as average and 3.51% rated it as poor. While the presentation was generally well-received, this suggests there is room for improvement and that it did not fully meet the audience’s expectations or needs. This highlights the need for clearer and more comprehensive explanations of phototherapy specifically tailored for paediatric patients.
Just under half (45.76%) of respondents rated this presentation as excellent and 42.37% as good, with 11.86% giving it an average rating and none rating it as poor. This data suggests the presentation was well-received overall, but some participants may have found the topic more challenging.
Positive feedback was largely received for this talk with 44.83% of respondents rating it as excellent and 46.55% as good. However, 6.90% rated it as
average and 1.72% rated it as poor. This indicates a need for better clarity on competence standards with improved delivery and explanation in order to enhance engagement and satisfaction among attendees.
Generally a positive reception, with 42.86% of respondents rating this talk as excellent and 42.86% rating it as good. However, 10.71% rated it as average and 3.57% rated it as poor. These ratings suggest that while the presentation was valuable and well-received by the majority, further clarity and detail could enhance understanding of topical treatments among participants.
The data indicates that most presentations were well-received, with excellent and good ratings dominating across topics. Top presentations, such as ‘What is phototherapy?’ and ‘Patient safety’, showcased effective communication and clear information.
Conversely, ‘Phototherapy in children’ and ‘Topical treatments’ received lower ratings, indicating areas for improvement. Overall, the feedback reflects strong educational content and a generally high standard of presentation quality.
“The data indicates that most presentations were well-received, with excellent and good ratings dominating across topics”
Overall, it seems the meeting was successful in delivering valuable content and supporting professional development with a consistent emphasis on practical and safety aspects while also offering insight into future enhancements, which will focus on ensuring all topics maintain the same level of clarity and engagement.
Q6. How strongly do you agree or disagree with the following statements?
This data evaluates attendees’ agreement/disagreement with several statements regarding specific aspects of the BDNG Manchester 2024 Meeting. The answers were categorised into ‘Strongly Agree’, ‘Agree’, ‘Neutral’, ‘Disagree’ or ‘Strongly Disagree’.
Generally, speakers covered the topic
The data reveals that 52.54% (31) of respondents strongly agreed, 42.37% (25) agreed, 3.39% (2) were neutral, 1.69% (1) disagreed that speakers covered their topics, with none strongly disagreeing. The vast majority (94.91%) of respondents either strongly agreed or agreed that speakers effectively covered the topics, indicating comprehensive presentations that met attendees’ expectations.
My learning objectives were met
In total, 38.98% (23) of respondents strongly agreed, 47.46% (28) agreed, 6.78% (4) were neutral and 6.78% (4) disagreed that their learning objectives were met, with none strongly disagreeing. The fact that most respondents (86.44%) felt their learning objectives were met is a positive message overall, suggesting some room for improvement in meeting all attendees’ educational goals.
This meeting was good value for money
Over half (55.93%) (33) of respondents strongly agreed, 28.81% (17) agreed, 11.86% (7) were neutral and 3.39% (2) disagreed that the meeting was good value for money, with none strongly disagreeing. A large majority (84.74%) therefore believed the meeting provided good value for money, with
minimal disagreement, reflecting positive perceived cost-effectiveness and satisfaction with the meeting’s return on investment.
I would recommend this to my colleagues
When asked whether they would recommend the event to a colleague, 59.32% (35) of respondents strongly agreed, 33.90% (20) agreed, 5.08% (3) were neutral, 1.69% (1) disagreed and none strongly disagreed. This means an overwhelming 93.22% would recommend the meeting to colleagues, indicating high overall satisfaction and perceived value, with only a small fraction (1.69%) expressing disagreement.
This data shows a strong positive reception for the meeting. The high number of strongly agree and agree responses across all questions suggest that the attendees found the meeting valuable, informative and worth recommending. The minimal negative responses highlight minor areas for potential improvement to ensure that all attendees’ learning objectives are fully met.
The feedback from the BDNG meeting indicates high attendee satisfaction overall, with participants valuing the coverage of phototherapy topics and the expertise of the speakers. Attendees highlighted the importance of ongoing education in developing a knowledgeable nursing workforce capable of addressing the complex needs of phototherapy patients.
Key suggestions for improvement include more detailed BAD phototherapy guidelines and treatment protocols for rare diseases, practical insights on conditions such as psoriasis, eczema and nodular prurigo, along with more interactive sessions and case studies incorporated into the two-day programme.
Attendees also requested insights on advanced treatment protocols and more case studies, particularly on drug
calculations, practical demonstrations and interactive sessions such as group discussions and Q&A sessions at the end of presentations. Additional topics of interest include paediatric phototherapy, patch testing, contact dermatitis, systemic treatments, biologics, JAK inhibitors and managing patients with learning disabilities.
The expertise and approachability of the speakers were noted positively, with sessions praised for being informative and engaging. The meeting was considered good value for money, enhancing both new and experienced attendees’ understanding of phototherapy.
Taking this feedback on board provides me with insight into the specific needs and preferences of the attendees, allowing for optimum planning for next year’s meeting. This constructive feedback not only informs but also inspires me to refine and expand the content of my presentations, ensuring the next meeting is even more informative, engaging and tailored to the professional development needs of our participants.
If you are interested in joining the phototherapy subgroup, please feel free to request my contact details through the BDNG’s education team or by contacting emma@bdng.org.uk.
References
1. Rathod DG, Muneer H, Masood S. Phototherapy. [Updated 2023 Feb 16]. In: StatPearls [online]. Treasure Island (FL): StatPearls Publishing; 2024. Available at: https:// www.ncbi.nlm.nih.gov/books/NBK563140/ [Accessed October 2024]
2. British Association of Dermatologists. Service Guidance and Standards for the use of Pototherapy. 2022. Available at: https:// cdn.bad.org.uk/uploads/2022/05/20125546/ Phototherapy-Guidance-and-ServicesStandards-Public-Consultation-Draft-2022. pdf [Accessed October 2024]
3. St John’s Phototherapy Guidelines. 2009. Available at: https://www. phototherapysupport.net/wp-content/ uploads/2020/01/Phototherapy-Guidelines. pdf [Accessed October 2024]
4. Byrne-Davis L, Cohen SN, Turner RR. Evaluating dermatology education
and training’, Clinical and Experimental Dermatology. 2022. 47(12). Available at: https://doi.org/10.1111/ced.15398 [Accessed October 2024]
5. Naegle, K.M. Ten simple rules for effective presentation slides. PLOS Computational Biology. 2021. 17(12). Available at: https://doi. org/10.1371/journal.pcbi.1009554 [Accessed October 2024]
6. Haleem A, Javaid M, Qadri MA, Suman R. Understanding the role of digital technologies in education: A review. Sustainable Operations and Computers 2022. 3(3): 275-285 Available at: https://doi. org/10.1016/j.susoc.2022.05.004 [Accessed October 2024]
7. Mlambo M, Silén C, McGrath, C. Lifelong learning and nurses’ continuing professional development, a metasynthesis of the literature. BMC Nursing 2021. 20(62):1-13 Available at: https://doi.org/10.1186/s12912021-00579-2 [Accessed October 2024]
8. Nursing & Midwifery Council. The Code: Professional standards of practice and behaviour for nurses, midwives and nursing associates. 2018. Available at: https://www. nmc.org.uk/globalassets/sitedocuments/ nmc-publications/nmc-code.pdf [Accessed October 2024]
9. Gnepp J, Klayman J, Williamson IO, Barlas S. The Future of Feedback: Motivating Performance Improvement Through Futurefocused Feedback. Plos One 2020. 15(6) Available at: https://doi.org/10.1371/journal. pone.0234444 [Accessed October 2024]
More details about the author:
I am the Clinical Nurse Specialist lead for phototherapy in a West London NHS Trust hospital. I have a profound passion for dermatology including the emotional and psychological impact of chronic skin conditions, rooted in my counselling training. This is an area I am dedicated to exploring further in the future. I completed the Clinical Reasoning in Health Assessment Level 7 course, following the Chronic Skin Conditions course, which has now led me to studying MSc in Clinical Dermatology. I thoroughly enjoy this level of education as it allows me to directly apply learning to practice, enhancing my level of understanding of many more dermatological conditions and improving the care and service I provide to my patients.
SOTYKTU® (deucravacitinib) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1 (deucravacitinib)
SOTYKTU® (deucravacitinib) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1
SOTYKTU® (deucravacitinib) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1 (deucravacitinib)
SOTYKTU® (deucravacitinib) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1
SOTYKTU® (deucravacitinib) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1
SOTYKTU® (deucravacitinib) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1

SOTYKTU: A first-in-class, once-daily oral, efficacious and generally well tolerated treatment1–3
SOTYKTU: A first-in-class, once-daily oral, efficacious and generally well tolerated treatment1–3
SOTYKTU: A first-in-class, once-daily oral, efficacious and generally well tolerated treatment1–3
SOTYKTU: A first-in-class, once-daily oral, efficacious and generally well tolerated treatment1–3
SOTYKTU: A first-in-class, once-daily oral, efficacious and generally well tolerated treatment1–3
SOTYKTU: A first-in-class, once-daily oral, efficacious and generally well tolerated treatment1–3
Patients with psoriasis can find injectables and IV treatment burdensome, which may lead to non-adherence4,5 of patients found their injectable or IV treatment burdensome*†4 of patients do not adhere to their prescribed biologic therapy5
Patients with psoriasis can find injectables and IV treatment burdensome, which may lead to non-adherence4,5 of patients found their injectable or IV treatment burdensome*†4 of patients do not adhere to their prescribed biologic therapy5
Patients with psoriasis can find injectables and IV treatment burdensome, which may lead to non-adherence4,5 of patients found their injectable or IV treatment burdensome*†4 of patients do not adhere to their prescribed biologic therapy5
Patients with psoriasis can find injectables and IV treatment burdensome, which may lead to non-adherence4,5 of patients found their injectable or treatment burdensome*†4 of patients do not adhere to their prescribed biologic therapy5
Patients with psoriasis can find injectables and IV treatment burdensome, which may lead to non-adherence4,5 of patients found their injectable or IV treatment burdensome*†4 of patients do not adhere to their prescribed biologic therapy5
Patients with psoriasis can find injectables and IV treatment burdensome, which may lead to non-adherence4,5 of patients found their injectable or IV treatment burdensome*†4 of patients do not adhere to their prescribed biologic therapy5
The time taken to administer injectables in a specialist setting or to train patients to self-inject adds time, cost and healthcare resource utilisation6
The time taken to administer injectables in a specialist setting or to train patients to self-inject adds time, cost and healthcare resource utilisation6
The time taken to administer injectables in a specialist setting or to train patients to self-inject adds time, cost and healthcare resource utilisation6
The time taken to administer injectables in a specialist setting or to train patients to self-inject adds time, cost and healthcare resource utilisation6
The time taken to administer injectables in a specialist setting or to train patients to self-inject adds time, cost and healthcare resource utilisation6
The time taken to administer injectables in a specialist setting or to train patients to self-inject adds time, cost and healthcare resource utilisation6
SOTYKTU offers patients with moderate-to-severe plaque psoriasis:
SOTYKTU offers patients with moderate-to-severe plaque psoriasis:
SOTYKTU offers patients with moderate-to-severe plaque psoriasis:
SOTYKTU offers patients with moderate-to-severe plaque psoriasis:
offers patients with moderate-to-severe plaque psoriasis:
moderate-to-severe plaque psoriasis:
Superior PASI 75 and sPGA 0/1 response rates vs placebo at Week 16 (co-primary endpoints)‡2,3
Superior PASI 75 and sPGA 0/1 response rates vs placebo at Week 16 (co-primary endpoints)‡2,3
Superior PASI 75 and sPGA 0/1 response rates vs placebo at Week 16 (co-primary endpoints)‡2,3
Superior PASI 75 and sPGA 0/1 response rates vs placebo at Week 16 (co-primary endpoints)‡2,3
Superior PASI 75 and sPGA 0/1 response rates vs placebo at Week 16 (co-primary endpoints)‡2,3
Superior PASI 75 and sPGA 0/1 response rates vs placebo at Week 16 (co-primary endpoints)‡2,3
Sustained PASI 75 and PASI 90 responses across 3 years in the LTE study§7
Sustained PASI 75 and PASI 90 responses across 3 years in the LTE study§7
Sustained PASI 75 and PASI 90 responses across 3 years in the LTE study§7
Sustained PASI 75 and PASI 90 responses across 3 years in the LTE study§7
Sustained PASI 75 and PASI 90 responses across 3 years in the LTE study§7
Sustained PASI 75 and PASI 90 responses across 3 years in the LTE study§7
A generally well tolerated treatment with rates of AEs with SOTYKTU remaining consistent across 3 years in the LTE study1–3,7,8
A generally well tolerated treatment with rates of AEs with SOTYKTU remaining consistent across 3 years in the LTE study1–3,7,8
An oral treatment without the need for routine blood monitoring1
An oral treatment without the need for routine blood monitoring1
A generally well tolerated treatment with rates of AEs with SOTYKTU remaining consistent across 3 years in the LTE study1–3,7,8
A generally well tolerated treatment with rates of AEs with SOTYKTU remaining consistent across 3 years in the LTE study1–3,7,8
An oral treatment without the need for routine blood monitoring1
An oral treatment without the need for routine blood monitoring1
An oral treatment without the need for routine blood monitoring1
An oral treatment without the need for routine blood monitoring1
A first-in-class allosteric, selective TYK2 inhibitor1
A generally well tolerated treatment with rates of AEs with SOTYKTU remaining consistent across 3 years in the LTE study1–3,7,8 A first-in-class allosteric, selective TYK2 inhibitor1
A generally well tolerated treatment with rates of AEs with SOTYKTU remaining consistent across 3 years in the LTE study1–3,7,8 A first-in-class allosteric, selective TYK2 inhibitor1
A first-in-class allosteric, selective TYK2 inhibitor1
A first-in-class allosteric, selective TYK2 inhibitor1
A first-in-class allosteric, selective TYK2 inhibitor1
*Based on 3,806 adult patients who self-reported an HCP diagnosis of moderate-to-severe PsO and/or PsA surveyed in the UPLIFT study. The survey was conducted in Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States between March 2, 2020, and June 3, 2020.5
*Based on 3,806 adult patients who self-reported an HCP diagnosis of moderate-to-severe PsO and/or PsA surveyed in the UPLIFT study. The survey was conducted in Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States between March 2, 2020, and June 3, 2020.5
†The therapies surveyed in the UPLIFT study did not include therapies that were not approved as of June 2020, such as SOTYKTU.4
*Based on 3,806 adult patients who self-reported an HCP diagnosis of moderate-to-severe PsO and/or PsA surveyed in the UPLIFT study. The survey was conducted in Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States between March 2, 2020, and June 3, 2020.5 The therapies surveyed in the UPLIFT study did not include therapies that were not approved as of June 2020, such as SOTYKTU.4 SOTYKTU was studied in two global, Phase 3, randomised, multi-arm clinical studies: POETYK PSO-1 and PSO-2. PASI 75 and sPGA 0/1 vs placebo at Week 16 were co-primary endpoints. PASI 75 was defined as Γ75% reduction from baseline in the Psoriasis Area and Severity Index. sPGA was defined as sPGA score of 0 or 1 with Γ2-point improvement from baseline. N numbers: PSO-1: SOTYKTU (n=332), apremilast (n=168), placebo (n=166); PSO-2: SOTYKTU (n=511), apremilast (n=254), placebo (n=255). SOTYKTU delivered superior PASI 75 response rates vs placebo (PSO-1: 58.4% vs 12.7%, p<0.0001; PSO-2: 53.0% vs 9.4%, p<0.0001) at Week 16, and superior results achieving clear or almost clear skin (sPGA 0/1) vs placebo (PSO-1: 53.6% vs 7.2%, p<0.0001; PSO-2: 49.5% vs 8.6%, p<0.0001) at Week 16 (co-primary endpoints).2,3 POETYK PSO-LTE is an in an open-label trial assessing the safety profile and efficacy of SOTYKTU in 1,221 adult patients with moderate-to-severe plaque psoriasis. Patients who completed treatment through Week 52 in either POETYK PSO-1 or PSO-2 were eligible to enrol in an optional LTE PSO-LTE) and be blindly switched to open-label SOTYKTU 6 mg once daily.7
*Based on 3,806 adult patients who self-reported an HCP diagnosis of moderate-to-severe PsO and/or PsA surveyed in the UPLIFT study. The survey was conducted in Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States between March 2, 2020, and June 3, 2020.5 †The therapies surveyed in the UPLIFT study did not include therapies that were not approved as of June 2020, such as SOTYKTU.4
*Based on 3,806 adult patients who self-reported an HCP diagnosis of moderate-to-severe PsO and/or PsA surveyed in the UPLIFT study. The survey was conducted in Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States between March 2, 2020, and June 3, 2020.5
†The therapies surveyed in the UPLIFT study did not include therapies that were not approved as of June 2020, such as SOTYKTU.4
*Based on 3,806 adult patients who self-reported an HCP diagnosis of moderate-to-severe PsO and/or PsA surveyed in the UPLIFT study. The survey was conducted in Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States between March 2, 2020, and June 3, 2020.5 †The therapies surveyed in the UPLIFT study did not include therapies that were not approved as of June 2020, such as SOTYKTU.4
†The therapies surveyed in the UPLIFT study did not include therapies that were not approved as of June 2020, such as SOTYKTU.4
‡SOTYKTU was studied in two global, Phase 3, randomised, multi-arm clinical studies: POETYK PSO-1 and PSO-2. PASI 75 and sPGA 0/1 vs placebo at Week 16 were co-primary endpoints. PASI 75 was defined as Γ75% reduction from baseline in the Psoriasis Area and Severity Index. sPGA was defined as sPGA score of 0 or 1 with Γ2-point improvement from baseline. N numbers: PSO-1: SOTYKTU (n=332), apremilast (n=168), placebo (n=166); PSO-2: SOTYKTU (n=511), apremilast (n=254), placebo (n=255). SOTYKTU delivered superior PASI 75 response rates vs placebo (PSO-1: 58.4% vs 12.7%, p<0.0001; PSO-2: 53.0% vs 9.4%, p<0.0001) at Week 16, and superior results achieving clear or almost clear skin (sPGA 0/1) vs placebo (PSO-1: 53.6% vs 7.2%, p<0.0001; PSO-2: 49.5% vs 8.6%, p<0.0001) at Week 16 (co-primary endpoints).2,3 §POETYK PSO-LTE is an in an open-label trial assessing the safety profile and efficacy of SOTYKTU in 1,221 adult patients with moderate-to-severe plaque psoriasis. Patients who completed treatment through Week 52 in either POETYK PSO-1 or PSO-2 were eligible to enrol in an optional LTE (POETYK PSO-LTE) and be blindly switched to open-label SOTYKTU 6 mg once daily.7
‡SOTYKTU was studied in two global, Phase 3, randomised, multi-arm clinical studies: POETYK PSO-1 and PSO-2. PASI 75 and sPGA 0/1 vs placebo at Week 16 were co-primary endpoints. PASI 75 was defined as Γ75% reduction from baseline in the Psoriasis Area and Severity Index. sPGA was defined as sPGA score of 0 or 1 with Γ2-point improvement from baseline. N numbers: PSO-1: SOTYKTU (n=332), apremilast (n=168), placebo (n=166); PSO-2: SOTYKTU (n=511), apremilast (n=254), placebo (n=255). SOTYKTU delivered superior PASI 75 response rates vs placebo (PSO-1: 58.4% vs 12.7%, p<0.0001; PSO-2: 53.0% vs 9.4%, p<0.0001) at Week 16, and superior results achieving clear or almost clear skin (sPGA 0/1) vs placebo (PSO-1: 53.6% vs 7.2%, p<0.0001; PSO-2: 49.5% vs 8.6%, p<0.0001) at Week 16 (co-primary endpoints).2,3 §POETYK PSO-LTE is an in an open-label trial assessing the safety profile and efficacy of SOTYKTU in 1,221 adult patients with moderate-to-severe plaque psoriasis. Patients who completed treatment through Week 52 in either POETYK PSO-1 or PSO-2 were eligible to enrol in an optional LTE (POETYK PSO-LTE) and be blindly switched to open-label SOTYKTU 6 mg once daily.7
‡SOTYKTU was studied in two global, Phase 3, randomised, multi-arm clinical POETYK PSO-1 and PSO-2. PASI 75 and sPGA 0/1 vs placebo at Week 16 were co-primary endpoints. PASI 75 was defined as Γ75% reduction from baseline in the Psoriasis Area and Severity Index. sPGA was defined as sPGA score of 0 or 1 with Γ2-point improvement from baseline. N numbers: PSO-1: SOTYKTU (n=332), apremilast (n=168), placebo (n=166); PSO-2: SOTYKTU (n=511), apremilast (n=254), placebo (n=255). SOTYKTU delivered superior PASI 75 response rates vs placebo (PSO-1: 58.4% vs 12.7%, p<0.0001; PSO-2: 53.0% vs 9.4%, p<0.0001) at Week 16, and superior results achieving clear or almost clear skin (sPGA 0/1) vs placebo (PSO-1: 53.6% vs 7.2%, p<0.0001; PSO-2: 49.5% vs 8.6%, p<0.0001) at Week 16 (co-primary endpoints).2,3 §POETYK PSO-LTE is an in an open-label trial assessing the safety profile and efficacy of SOTYKTU in 1,221 adult patients with moderate-to-severe plaque psoriasis. Patients who completed treatment through Week 52 in either POETYK PSO-1 or PSO-2 were eligible to enrol in an optional LTE (POETYK PSO-LTE) and be blindly switched to open-label SOTYKTU 6 mg once daily.7
‡SOTYKTU was studied in two global, Phase 3, randomised, multi-arm clinical studies: POETYK PSO-1 and PSO-2. PASI 75 and sPGA 0/1 vs placebo at Week 16 were co-primary endpoints. PASI 75 was defined as Γ75% reduction from baseline in the Psoriasis Area and Severity Index. sPGA was defined as sPGA score of 0 or 1 with Γ2-point improvement from baseline. N numbers: PSO-1: SOTYKTU (n=332), apremilast (n=168), placebo (n=166); PSO-2: SOTYKTU (n=511), apremilast (n=254), placebo (n=255). SOTYKTU delivered superior PASI 75 response rates vs placebo (PSO-1: 58.4% vs 12.7%, p<0.0001; PSO-2: 53.0% vs 9.4%, p<0.0001) at Week 16, and superior results achieving clear or almost clear skin (sPGA 0/1) vs placebo (PSO-1: 53.6% vs 7.2%, p<0.0001; PSO-2: 49.5% vs 8.6%, p<0.0001) at Week 16 (co-primary endpoints).2,3 §POETYK PSO-LTE is an in an open-label trial assessing the safety profile and efficacy of SOTYKTU in 1,221 adult patients with moderate-to-severe plaque psoriasis. Patients who completed treatment through Week 52 in either POETYK PSO-1 or PSO-2 were eligible to enrol in an optional LTE (POETYK PSO-LTE) and be blindly switched to open-label SOTYKTU 6 mg once daily.7
‡SOTYKTU was studied in two global, Phase 3, randomised, multi-arm clinical studies: POETYK PSO-1 and PSO-2. PASI 75 and sPGA 0/1 vs placebo at Week 16 were co-primary endpoints. PASI 75 was defined as Γ75% reduction from baseline in the Psoriasis Area and Severity Index. sPGA was defined as sPGA score of 0 or 1 with Γ2-point improvement from baseline. N numbers: PSO-1: SOTYKTU (n=332), apremilast (n=168), placebo (n=166); PSO-2: SOTYKTU (n=511), apremilast (n=254), placebo (n=255). SOTYKTU delivered superior PASI 75 response rates vs placebo (PSO-1: 58.4% vs 12.7%, p<0.0001; PSO-2: 53.0% vs 9.4%, p<0.0001) at Week 16, and superior results achieving clear or almost clear skin (sPGA 0/1) vs placebo (PSO-1: 53.6% vs 7.2%, p<0.0001; PSO-2: 49.5% vs 8.6%, p<0.0001) at Week 16 (co-primary endpoints).2,3 §POETYK PSO-LTE is in an open-label trial assessing the safety profile and efficacy of SOTYKTU in 1,221 adult patients with moderate-to-severe plaque psoriasis. Patients who completed treatment through Week 52 in either POETYK PSO-1 or PSO-2 were eligible to enrol in an optional LTE (POETYK PSO-LTE) and be blindly switched to open-label SOTYKTU 6 mg once daily.7

References:
1 SOTYKTU (deucravacitinib) Summary of Product Characteristics; 2 Armstrong A et al. J Am Acad Dermatol 2023;88(1):29–39; 3. Strober B et al. J Am Acad Dermatol 2023;88(1):40–51; 4. Lebwohl MG et al. Dermatol Ther (Heidelb) 2022;12(1): 61–78; 5. Piragine E et al. J Clin Med 2022;11(6):1506; 6. Green W et al. Dermatology and Therapy 2021;11:1635–1642; 7. Armstrong AW et al. Oral presentation at the EADV Annual Meeting; 11–14 October 2023; Berlin, Germany. Presentation FC.02.7; 8. SOTYKTU. European Product Assessment Report (EPAR). 26 January 2023. Available at: https://www.ema.europa.eu/en/documents/assessment-report/sotyktu-epar-public-assessment-report en.pdf. Date accessed: May 2024.
Great Britain
Consult Summary of Product Characteristics (SmPC) before prescribing. This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information.
PRESENTATION: Film-coated tablet containing 6 mg of deucravacitinib.
INDICATION: Treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
DOSAGE AND ADMINISTRATION: Treatment should be initiated under the guidance and supervision of a physician experienced in the diagnosis and treatment of psoriasis. Posology: 6 mg orally once daily. If a patient shows no evidence of therapeutic benefit after 24 weeks, treatment discontinuation should be considered. The patient’s response to treatment should be evaluated on a regular basis. Special populations: Elderly: No dose adjustment is required in elderly patients aged 65 years and older. Clinical experience in patients 75 years is very limited and deucravacitinib should be used with caution in this group of patients. Renal Impairment: No dose adjustment is required in patients with renal impairment, including end stage renal disease (ESRD) patients on dialysis. Hepatic impairment: No dose adjustment is required in patients with mild or moderate hepatic impairment. Deucravacitinib is not recommended to be used in patients with severe hepatic impairment. Paediatric population: The safety and efficacy of deucravacitinib in children and adolescents below the age of 18 years have not yet been established. No data are available. Method of administration: For oral use. Tablets can be taken with or without food. Tablets should be swallowed whole and should not be crushed, cut, or chewed.
CONTRAINDICATIONS: Hypersensitivity to the active substance or to any of the excipients (see SmPC). Clinically important active infections (e.g. active tuberculosis).
WARNINGS AND PRECAUTIONS: Infections: Treatment should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated. Caution should be exercised when considering the use in patients with a chronic infection or a history of recurrent infection. Patients treated with deucravacitinib should be instructed to seek medical advice if signs or symptoms suggestive of an infection occur. If a patient develops a clinically important infection or is not responding to standard therapy, monitor carefully and deucravacitinib should not be given until the infection resolves. Pre-treatment evaluation for tuberculosis (TB): Prior to initiating treatment with deucravacitinib, patients should be evaluated
SOTYKTU®
for TB infection. Deucravacitinib should not be given to patients with active TB. Treatment of latent TB should be initiated prior to administering deucravacitinib. Anti-TB therapy should be considered prior to initiation of deucravacitinib in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Patients receiving deucravacitinib should be monitored for signs and symptoms of active TB. Malignancies*: Malignancies, including lymphomas and nonmelanoma skin cancer (NMSC), were observed in clinical studies with deucravacitinib. Limited clinical data are available to assess the potential relationship of exposure to deucravacitinib and the development of malignancies. Long-term safety evaluations are ongoing. The risks and benefits of deucravacitinib treatment should be considered prior to initiating patients**. Major adverse cardiovascular events (MACE), deep venous thrombosis (DVT) and pulmonary embolism (PE)*: An increased risk was not observed in clinical trials with deucravacitinib. Long-term safety evaluations are ongoing. The risks and benefits of deucravacitinib treatment should be considered prior to initiating patients**. Immunisations: Consider completion of all age-appropriate immunisations according to current immunisation guidelines prior to initiating therapy. Use of live vaccines in patients being treated with deucravacitinib should be avoided. Excipients: Contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose galactose malabsorption should not take this medicinal product. Contains less than 1 mmol of sodium (23 mg) per tablet, essentially ‘sodium-free’. *serious. **It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the adverse reactions of Janus Kinase (JAK) inhibition. In a large randomised active-controlled study of a JAK inhibitor in rheumatoid arthritis (RA) patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of malignancies (particularly lung cancer, lymphoma and NMSC), a higher rate of MACE (defined as cardiovascular death, non-fatal myocardial infarction and non-fatal stroke), and a dose dependent higher rate of venous thromboembolism (including DVT and PE) were observed with a JAK inhibitor compared to TNF inhibitors. INTERACTIONS: Deucravacitinib does not have any known clinically relevant drug interactions. Refer to SmPC for full details. PREGNANCY AND LACTATION: Pregnancy: There is a limited amount of data on the use of deucravacitinib in pregnant women. As a precautionary measure, it is preferable to avoid the use of deucravacitinib during pregnancy. Breast-feeding: It is unknown whether deucravacitinib/metabolites are excreted in human milk. A risk to the newborns/infants by breast-feeding cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from deucravacitinib
Northern Ireland / Ireland
Consult Summary of Product Characteristics (SmPC) before prescribing. This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information.
PRESENTATION: Film-coated tablet containing 6 mg of deucravacitinib.
INDICATION: Treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
DOSAGE AND ADMINISTRATION: Treatment should be initiated under the guidance and supervision of a physician experienced in the diagnosis and treatment of psoriasis. Posology: 6 mg orally once daily. If a patient shows no evidence of therapeutic benefit after 24 weeks, treatment discontinuation should be considered. The patient’s response to treatment should be evaluated on a regular basis. Special populations: Elderly: No dose adjustment is required in elderly patients aged 65 years and older. Clinical experience in patients 75 years is very limited and deucravacitinib should be used with caution in this group of patients. Renal Impairment: No dose adjustment is required in patients with renal impairment, including end stage renal disease (ESRD) patients on dialysis. Hepatic impairment: No dose adjustment is required in patients with mild or moderate hepatic impairment. Deucravacitinib is not recommended to be used in patients with severe hepatic impairment. Paediatric population: The safety and efficacy of deucravacitinib in children and adolescents below the age of 18 years have not yet been established. No data are available. Method of administration: For oral use. Tablets can be taken with or without food. Tablets should be swallowed whole and should not be crushed, cut, or chewed.
CONTRAINDICATIONS: Hypersensitivity to the active substance or to any of the excipients (see SmPC). Clinically important active infections (e.g. active tuberculosis).
WARNINGS AND PRECAUTIONS: Infections: Treatment should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated. Caution should be exercised when considering the use in patients with a chronic infection or a history of recurrent infection. Patients treated with deucravacitinib should be instructed to seek medical advice if signs or symptoms suggestive of an infection occur. If a patient develops a clinically important infection or is not responding to standard therapy, monitor carefully and deucravacitinib should not be given until the infection resolves. Pre-treatment evaluation for tuberculosis (TB): Prior to initiating treatment with deucravacitinib, patients should be evaluated for TB infection. Deucravacitinib should not be given to patients
with active TB. Treatment of latent TB should be initiated prior to administering deucravacitinib. Anti-TB therapy should be considered prior to initiation of deucravacitinib in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Patients receiving deucravacitinib should be monitored for signs and symptoms of active TB. Malignancies*: Malignancies, including lymphomas and nonmelanoma skin cancer (NMSC), were observed in clinical studies with deucravacitinib. Limited clinical data are available to assess the potential relationship of exposure to deucravacitinib and the development of malignancies. Long-term safety evaluations are ongoing. The risks and benefits of deucravacitinib treatment should be considered prior to initiating patients**. Major adverse cardiovascular events (MACE), deep venous thrombosis (DVT) and pulmonary embolism (PE)*: An increased risk was not observed in clinical trials with deucravacitinib. Long-term safety evaluations are ongoing. The risks and benefits of deucravacitinib treatment should be considered prior to initiating patients**. Immunisations: Consider completion of all age-appropriate immunisations according to current immunisation guidelines prior to initiating therapy. Use of live vaccines in patients being treated with deucravacitinib should be avoided. Excipients: Contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose galactose malabsorption should not take this medicinal product. Contains less than 1 mmol of sodium (23 mg) per tablet, essentially ‘sodium-free’. *serious. **It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the adverse reactions of Janus Kinase (JAK) inhibition. In a large randomised active-controlled study of a JAK inhibitor in rheumatoid arthritis (RA) patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of malignancies (particularly lung cancer, lymphoma and NMSC), a higher rate of MACE (defined as cardiovascular death, non-fatal myocardial infarction and non-fatal stroke), and a dose dependent higher rate of venous thromboembolism (including DVT and PE) were observed with a JAK inhibitor compared to TNF inhibitors. INTERACTIONS: Deucravacitinib does not have any known clinically relevant drug interactions. Refer to SmPC for full details. PREGNANCY AND LACTATION: Pregnancy: There is a limited amount of data on the use of deucravacitinib in pregnant women. As a precautionary measure, it is preferable to avoid the use of deucravacitinib during pregnancy. Breast-feeding: It is unknown whether deucravacitinib/metabolites are excreted in human milk. A risk to the newborns/infants by breast-feeding cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from deucravacitinib therapy taking into account the benefit of breast feeding for the
therapy taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman. Fertility: The effect of deucravacitinib on human fertility has not been evaluated.
UNDESIRABLE EFFECTS: The most commonly reported adverse reaction is upper respiratory infections (18.9%), most frequently nasopharyngitis. The longer-term safety profile of deucravacitinib was similar and consistent with previous experience. Very common ( 1/10): Upper respiratory infections*** (including nasopharyngitis, upper respiratory tract infection, viral upper respiratory tract infection, pharyngitis, sinusitis, acute sinusitis, rhinitis, tonsillitis, peritonsillar abscess, laryngitis, tracheitis, and rhinotracheitis). Common ( 1/100 to < 1/10): Herpes simplex infections*** (including oral herpes, herpes simplex, genital herpes, and herpes viral infection), Oral ulcers (including aphthous ulcer, mouth ulceration, tongue ulceration, and stomatitis), Acneiform rash (including acne, dermatitis acneiform, rash, rosacea, pustule, rash pustular, and papule), Folliculitis and Blood creatine phosphokinase increased. Uncommon ( 1/1,000 to < 1/100): Herpes zoster***. Refer to SmPC for full details on adverse reactions.
***serious adverse drug reaction
LEGAL CATEGORY: POM
MARKETING AUTHORISATION NUMBER and BASIC NHS PRICE: PLGB 15105/0179: Carton of 28 film-coated tablets 6 mg NHS price: £690.00; Carton of 84 film-coated tablets 6 mg NHS price: £2070.00.
MARKETING AUTHORISATION HOLDER: Bristol-Myers Squibb Pharma EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland.
FOR FURTHER INFORMATION CONTACT: medical.information@bms.com or 0800 731 1736 (Great Britain). DATE OF PREPARATION: May 2023
ADDITIONAL INFORMATION AVAILABLE ON REQUEST Approval code: 1787-GB-2300080
Adverse events should be reported. Reporting forms and information can be found at: Great Britain –www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store; Adverse events should also be reported to Bristol-Myers Squibb via medical.information@bms.com or 0800 731 1736 (Great Britain).
child and the benefit of therapy for the woman. Fertility: The effect of deucravacitinib on human fertility has not been evaluated. UNDESIRABLE EFFECTS: The most commonly reported adverse reaction is upper respiratory infections (18.9%), most frequently nasopharyngitis. The longer-term safety profile of deucravacitinib was similar and consistent with previous experience. Very common ( 1/10): Upper respiratory infections*** (including nasopharyngitis, upper respiratory tract infection, viral upper respiratory tract infection, pharyngitis, sinusitis, acute sinusitis, rhinitis, tonsillitis, peritonsillar abscess, laryngitis, tracheitis, and rhinotracheitis). Common ( 1/100 to < 1/10): Herpes simplex infections*** (including oral herpes, herpes simplex, genital herpes, and herpes viral infection), Oral ulcers (including aphthous ulcer, mouth ulceration, tongue ulceration, and stomatitis), Acneiform rash (including acne, dermatitis acneiform, rash, rosacea, pustule, rash pustular, and papule), Folliculitis and Blood creatine phosphokinase increased. Uncommon ( 1/1,000 to < 1/100): Herpes zoster***. Refer to SmPC for full details on adverse reactions.
***serious adverse drug reaction
LEGAL CATEGORY: POM
MARKETING AUTHORISATION NUMBER and BASIC NHS PRICE: EU/1/23/1718/006: Carton of 28 film-coated tablets 6 mg NHS price: £690.00.
MARKETING AUTHORISATION HOLDER: Bristol-Myers Squibb Pharma EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland.
FOR FURTHER INFORMATION CONTACT: medical.information@bms.com or 0800 731 1736 (Northern Ireland) / 1 800 749 749 (Ireland).
DATE OF PREPARATION: June 2023
ADDITIONAL INFORMATION AVAILABLE ON REQUEST Approval code: 1787-IE-2300001
Adverse events should be reported. Reporting forms and information can be found at: Northern Ireland –www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store; Ireland – via HPRA Pharmacovigilance at www.hpra.ie Adverse events should also be reported to Bristol-Myers Squibb via medical.information@bms.com or 0800 731 1736 (Northern Ireland); 1 800 749 749 (Ireland).
Rebecca Penzer-Hick
Introduction
Cutaneous T-cell lymphoma (CTCL) is a rare malignancy in which there is an accumulation of neoplastic lymphocytes in the skin. A systematic review of epidemiological data indicated that the incidence of CTCL was estimated at between 0.29-0.39 per 100,000 people per year.1 CTCL is represented by several different subtypes, the most common of which is mycosis fungoides (MF), which is thought to account for around 60% of CTCL cases.2 Clinical presentation will vary depending on the specific subtype and the stage of the disease.3 Due to the relative rarity of the condition and the multiple presentations, diagnosis is often not made in a timely manner which can lead to a delay in commencing effective treatment.4 It has been noted in the UK that the care pathway for patients with CTCL is often not clear as CTCL services may be based within oncology, haematology or dermatology depending on local provision. This adds to the challenges of providing effective care.
Patients with CTCL require effective nursing input at all stages of their condition. As a potentially life limiting disease, effective psychological support along with effective use of a range of therapeutic options are required to deliver effective care. Patients require
Summary:
This article presents findings from a recent survey of British Dermatological Nursing Group members, which sought clarity on the educational needs of nurses who care for patients with cutaneous T-cell lymphoma. In total, 52 people took part in the survey.
Keywords:
Cutaneous T-cell lymphoma, Care, Training and education needs, Survey
different medical input depending on the stage of the disease, however nursing support and knowledge is critical at each stage. Although CTCL presents in the skin, as has already been highlighted, patients are not always cared for in a dermatology department. This not only complicates the patient pathway but also increases the challenge of providing effective education and training to nurses who look after patients with CTCL. Consequently, patients with CTCL will encounter nurses with differing levels of experience of the disease (and expertise in managing it), at the different stages of their pathway.
To understand the training and education needs of nurses who care for patients with CTCL, a project was established through the BDNG which was funded by Kyowa Kirin (KK). KK had no influence over the outcomes of the project. This article briefly outlines the methods used and the results obtained and makes some recommendations for how training and education might be provided to optimise care for this group of patients.
Method
The project consisted of two approaches to data collection. Firstly, some qualitative information was obtained through two focus groups with nurses from
Author info:
Rebecca Penzer-Hick is a Senior Lecturer, Postgraduate Medicine, University of Hertfordshire and Dermatology Specialist Nurse, Dermatology Clinic Community Services, Cambridgeshire, and a member of the Dermatological Nursing Editorial Board.
Citation: Penzer-Hick R. What additional educational materials do nurses need to help them care for patients with cutaneous T-cell lymphoma?
A report of a BDNG survey. Dermatological Nursing 2024. 23(4):26-28.
Table 1: Which topics would be most useful?
Topical treatment
Managing common symptoms such as pruritus
Diagnosis
Psychological support
Prognosis
Wound care for advanced disease
Phototherapy
General skin care
Chemotherapy
Radiotherapy
different areas of practice and levels of experience. The transcripts from these focus groups were analysed and key themes were identified. Using these themes, a questionnaire was developed that was sent to 2,402 members of the BDNG, using SurveyMonkey, to access the questionnaire. Numerical data was then extracted from the returned questionnaires.
Results
Fifty-two people (2%) responded to the questionnaire, which is a low response rate. Consequently, the following results should be viewed in that light. However, there were some clear trends in the data which made it possible to generate a set of recommendations.
Table 2: Which method of education delivery would you prefer?
Method of delivery – most to least popular
E-learning modules
Combination of online webinars and face-to-face study days
Live online webinars
Face-to-face study days
Reading articles and guidelines
Learning from others at work
Conferences
The results indicated that most people worked in dermatology, however one respondent worked in oncology, four in a combination of oncology and dermatology, and three in other clinical environments. Most of the respondents claimed to have a basic knowledge of CTCL, with seven saying they had no knowledge and six saying they had a good knowledge. There were too few respondents to be able to make any certain link between speciality background and knowledge level. The only person who worked full time with patients with CTCL worked in oncology. The other respondents were split mainly between working occasionally or regularly with patients with CTCL (n=20 45%) and quite frequently with
“Due to the relative rarity of the condition and the multiple presentations, diagnosis is often not made in a timely manner which can lead to a delay in commencing effective treatment”
patients with CTCL (n=19 43%). Two people stated that they had no contact with CTCL patients.
To get a baseline understanding of educational provision, respondents were asked to identify what education they were currently accessing with regards to CTCL. Eighty percent said they were not accessing education, but they would like to. Fourteen percent said they were accessing education, including reading articles, speaking to others in the workplace, online webinars, conferences and face-to-face study days.
The remainder of the questionnaire aimed to understand what sort of educational provision respondents would find useful.
Although the response rate to this survey was low, some clear patterns emerged which will be helpful when planning education in the future. Most of the nurses who responded to this survey worked in dermatology. Most people described their level of knowledge as basic, with a proportion of nurses from all backgrounds putting themselves in this category. Nobody considered themselves as having advanced knowledge, despite one person declaring that they care for CTCL patients as a main component of their job.
The rarity of the condition was reflected in the fact that 45% (n=20) of people said they had “very little” contact with CTCL patients, where very little contact
was described as less than five patients a year. Forty-three percent (n=19) of respondents described their contact as occasional (6-12 patients a year). Despite the relative infrequency of contact with CTCL patients, there remained a strong appetite for education. This was emphasised by the fact that even though 80% (n=39) were not accessing education currently, they said they would like to in the future. It was interesting that of the small minority (three people) who said that education was not relevant for them, two were seeing patients with CTCL (albeit infrequently). The other person did not see CTCL patients. For some people, education will not be a priority, but they appear to be a small minority.
Only seven respondents stated that they were currently accessing education, with most of the learning coming from others in the workplace and reading articles. This contrasted with the preferred routes for education which were resoundingly in favour of online learning with some face-to-face contact (68% of the votes). As learning from others and reading secured only 15% of the votes, people want approaches to learning which are not available (or being accessed) currently.
“Patients with CTCL require effective nursing input at all stages of their condition”
All the suggested educational topics received some votes, but the top six all secured more than double the number of votes compared to the bottom four. Most popular were topical treatments and managing common symptoms, but it was notable that diagnosis and psychological support were in joint third place. The lower votes were for topics which might be considered more specialised (for example, phototherapy and chemotherapy) and therefore less relevant to most respondents. It was surprising to see general skin care low down the list of priorities; this may be in part because using topical therapies and managing symptoms could be seen as covering the topic of general skin care.
A clear majority of respondents wanted nurses or medics to provide education with these two groups forming the first or second choice for the majority with a slight preference for nurses. The pharmaceutical industry had the highest number of fourth place rankings and it would appear that more people would prefer to hear from patients than from industry.
One of the clearest results of the survey came from the final question, asking which of four resources would be of use. Nursing guidelines with regards to CTCL are clearly a resource which would be valued. ‘How to’ posters and talking head videos saw significant favour with posters receiving marginally more support. Of least interest was the patient support group/website, which was perhaps unsurprising considering the target audience of this survey.
“The rarity of the condition was reflected in the fact that 45% (n=20) of people said they had ‘very little’ contact with CTCL patients”
This cross-sectional survey provides a snapshot view of nurses from a special interest group. Whilst most respondents had infrequent contact with CTCL patients, there was a clear appetite for clinically relevant education. It would appear that easy-to-access education (with an online focus) around topics that are relevant to those who may not be specialists is in demand. It was difficult to make any significant link between level of experience and educational needs because so few nurses described themselves as having good or advanced knowledge of CTCL.
This survey would suggest that providing online education (with some face-to-face components) centred around key topics such as topical therapy and managing key symptoms, including psychological concerns, should be priorities moving forward. Of particular interest to respondents were the development of nursing guidelines, but other resources such as ‘how to’ posters and talking head videos also found favour.
1. Dobos G, Pohrt A, Ram-Wolff C, Lebbe C, Bouazis J, Battistella M et al. Epidemiology of Cutaneous T-Cell Lymphomas: A Systematic Review and Meta-Analysis of 16,953 Patients. Cancers 2020. 12(10):2921
2. Willemze R. Primary cutaneous lymphoma: the 2018 update of the WHO-EORTC classification La Presse Medicale 2022. 51(1)
3. Morgenroth S, Roggo A, Pawlik L, Dummer R, Ramelyte E. What is new in cutaneous T cell lymphoma? Current Oncology Reports 2023. 25:1397-1408
4. Scarisbrick JJ et al. The PROCLIPI international registry of early stage mycosis fungoides identifies substantial diagnostic delay in most patients British Journal of Dermatology 2019. 181(2):350-357.


Formula with Triple Oat Complex benefits the skin in multiple ways


Strengthens the skin moisture barrier




Soothes very dry, itchy skin
Helps support the skin microbiome
Order free samples



Enstilar® is indicated for the topical treatment of psoriasis vulgaris in adults.1
once-daily treatment for 4 weeks during the active flare… 1-3
twice-weekly maintenance treatment for up to 52 weeks in responders1,4*

Enstilar® should be
twice-weekly on two nonconsecutive days to previously affected areas.1 Between applications there should be 2-3 days without Enstilar® treatment.1 The most frequently reported adverse reactions during treatment are application site reactions.1 Local reactions can occur after topical use, especially during prolonged application, including skin atrophy, telangiectasia, striae, folliculitis, hypertrichosis, perioral dermatitis, allergic contact dermatitis, depigmentation, and colloid milia.1 Long-term use of corticosteroids may increase the risk of local and systemic adverse reactions.1 Treatment should be discontinued in case of adverse reactions related to long-term use of corticosteroid.1 For a full list of adverse reactions please refer to the Enstilar® SPC.1 The total dose of all calcipotriol containing products should not exceed 15 g per day.1 The total body surface area treated should not exceed 30%.1
Presc ribing I nformatio n for E ns tilar ® (c alcip ot riol / bet amet haso ne)
5 0 mic rog r ams/g + 0 5 mg /g c ut a ne ous foam Please refer to the full S ummar y of Product Characteristic s (S mP C) (ww w me dicines org uk /emc) before prescribing I n dic atio n: Top c al treatment of psoriasis vulgaris in adults Ac tive ing re die nt s: 5 0 µ g /g c alcipotriol (as monohydrate) and 0 5 mg /g betamethasone (as dipropionate) Dosage a n d ad minis tr atio n: Flare treatme nt: A pply by spraying onto af fe cte d area once daily Re c ommende d treatment period is 4 we eks If it is ne c essar y to continue or restar t treatment af ter this period treatment should be c ontinue d af ter me dic al review and under re gular super vision Long - te r m mainte nanc e treatme nt: Patients who have responde d at 4 we eks treatment using Enstilar onc e daily are suitab e for ong - ter m maintenance treatment Enstilar should be applie d t wic e we ek ly on t wo non - conse cu tive days to areas previously af fe cte d by psor asis vulgaris B et we en applic ations there should be 2- 3 days withou t Enstilar should be re - initiated M ax mu m dose T he daily ma ximum dose of Enstilar should not exce e d 15 g i e one 6 0 g c an should last for at least 4 days of treatment 15 g corresponds to the amount administere d from the c an f the actuator is fully depresse d for approximately one minu te A t wo - se cond applic ation delivers approximately 0 5 g A s a guide 0 5 g of foam should cover an area of sk in roughly c orresponding to the sur face area of an adult hand If using other c alcipotriol - c ontaining me dic al products in add tion to Enstilar the total dose of all c alcipotriol - containing products should not exc e e d 15 g per day Total body sur face area treate d should not exce e d 30 %
be ow 18 years have not be en established S hake the c an for a few se conds before use A pply by spraying hold ng the c an at least 3 cm from the sk in in any orientation except horizontally S pray dire ctly onto each af fe cte d sk in area and r ub in gently If use d on the sc alp, spray into the palm of the hand use d to treat the hands) to avo d accidentally spreading to other par ts of the body as well as un ntende d dr ug absor ption on the hands Avoid applic ation under o cclusive dressings sinc e system c absor pt on of cor t c osteroids increases It is re commende d not to take a shower or bath imme diately af ter applic at on Let the foam remain on the sc alp and /or sk in during the night or during the day C o ntr ain dic atio ns: H y pers ensitivit y to the active substanc es or any of the excipients Er y throder mic and pustular psoriasis Patients with k nown disorders of c alcium metabolism V iral (e g her pes or varicella) sk in lesions , fungal or bacteria sk in infe ctions parasitic infe ct ons sk in
manifestations in relation to tuberculosis perioral der matitis atrophic sk n striae atrophic ae fragilit y of sk in veins ichthyosis acne vulgaris acne rosacea , rosacea , ulcers and wounds Pre c au tio ns an d warnings: Adverse reactions found in conne ction with systemic cor ticostero d treatment e g adreno cor tic al suppression or impaire d glyc aemic control of diabetes mellitus , may o ccur also during topic al cor ticosteroid treatment due to systemic absor ption A pplic ation under o cclusive dressings should be avoide d sinc e t increases the systemic absor pt on of c or tic osteroids A pplic ation on large areas of damage d sk in , or on muc ous membranes or n sk in folds should be avoide d sinc e it increases the system c absor ption of cor tic osteroids V isual distur bance may be repor te d with system c and topic al cor tic osteroid use If a patient presents with sy mptoms such as blurre d vis on or other visua distur bances the patient should be considere d for a referra to an ophthal mologist for evaluation of possible c auses which may include c ataract , glaucoma or rare d seases such as c entral serous chorioretinopathy (C S C R) which have be en repor te d af ter use of systemic and topic al cor ticostero ds D ue to the c ontent of c alcipotriol , hy perc alc aemia may o ccur S er um c alcium is nor malise d when treatment is discontinued T he risk of hy perc alc aemia is min mal when the ma ximum daily dose of Enstil ar (15 g) is not exc e e ded Enstilar contains a potent group III - stero d and concurrent treatment with other stero ds on the same treatment area must be avoided T he sk in on the fac e and genitals is ver y sensitive to cor ticosteroids Ensti ar shou d not be use d in these areas Instr uct the pat ent in the corre ct use of the product to avoid applic ation and accidental transfer to the fac e mou th and eyes Wash han ds af ter each app c ation to avoid accidental transfer to these areas as well as unintende d dr ug absor ption on the hands If lesions be c ome se condarily infe cte d , they should be treate d with antimicrobiologic al therapy However if infe ction worsens treatment w th cor tic osteroids should be d sc ontinued W hen treating psoriasis with topic al cor tic osteroids there may be a risk of rebound ef fe cts when discontin uing treatment Me dic al super vis on should therefore continue in the posttreatment period Long - ter m use of cor ticosteroids may ncrease the risk of lo c al and systemic adverse reactions Treatment should be discont nue d in c ase of adverse reactions relate d to ong - ter m use of c or ticosteroid T here is no experienc e w th the use of Enstilar in gu t tate psorias s Enstilar c ontains bu t ylhydrox y toluene (E3 21), wh ich may c ause lo c al sk in reactions (e g contact der matit s) or irritation to the eyes and mucous membranes Pre g na ncy an d lac t atio n: T here are no ade quate data from the use of Enstilar in pre gnant women Enstilar should on y be use d during pre gnancy when the prescribing Enst ar to women who breast-fe ed T he patient should be instr ucte d not to use Enstilar on the breast when breast-fe e ding S ide ef fe c t s:
T here are no common adverse reactions base d on the clinic al studies T he most fre quently repor te d adverse reactions are applic ation site reactions U ncommon (≥1/1, 00 0 to <1/10 0): Folliculitis , hy persensitivit y, hy perc a c ae mia , sk n hy popigmentation rebound ef fe ct , app lic ation site pr uritus , applic ation site irritation , applic ation site pa n (including applic ation site burning) N ot k nown (c annot be estimate d from availab e data): Blurre d vision , applic ation site er y thema hair colour changes Calcip otrio : Adverse react ons include applic ation site reactions pr uritus sk in irritation burning and stinging sensation , dr y sk in er y thema , rash , der matitis , psoriasis aggrava te d , photosensitivit y and hy persensitiv t y reactions including ver y rare c ases of angio e dema and facial o e dema S ystemic ef fe cts af ter top c al use may appear ver y rarely c ausing hy perc alc aemia or hy perc alciuria B etamethasone: Lo c a reactions c an o ccur af ter topic al use espe cia y during prolonge d applic ation , inc uding sk in atrophy, telangie ctasia striae, folliculitis hy per trichosis perioral der mat t s , a lergic c ontact der matitis , depigmentation and colloid milia W hen treating psoriasis with topic al cor ticosteroids there may be a r sk of generalise d pustular psoriasis S ystemic reactions due to topic a use of cor ticosteroids are rare in adults; however they c an be severe Adreno cor tic al suppression c ataract nfe ctions impa re d glyc aemic c ontrol of diabetes mellitus and ncrease of intra - o cu ar pressu re c an o ccur, espe cia y af ter long - ter m treatment S ystemic reactions o ccur more fre quently when applie d under o cclusion ( plastic , sk in folds), when applie d onto large sk in areas and during long - ter m treatment Pre c au tio ns for s tor age: container May burst if heated Prote ct from sunlight D o not expose to temperatures exc e e ding 5 0 C D o not pierc e or burn even af ter use D o not Le gal
References: 1. Enstilar® (calcipotriol/betamethasone) SPC 2022. 2. Leonardi C et al. J Drugs Dermatol 2015;14(12):1468–1477. 3. King C and Lowson D. Poster 237 presented at the 5th Psoriasis International Network Annual Meeting, Paris, France, 5–7 July 2016. 4. Lebwohl M et al. J Am Acad Dermatol 2021;84:1269-77.
Lucy Evans-Hill
When I qualified as a nurse in 2005, I took the unusual step of going straight into primary care. It wasn’t my plan – I had always wanted to be an ITU nurse – but a student placement in a GP practice in North London changed my mind. After some heavygoing, busy student placements on general wards and a spinal injuries unit, I relished having the time to talk to my patients in a calmer environment. I felt like I could make a difference in those 15-minute appointments. There’s something about a beginning, middle and an end to a patient encounter, and it’s a privilege to help people in this pocket of time. Whilst it can be a challenge at times – there is no other job as rewarding (in my opinion) –and so that was it, I made the decision to focus on being a practice nurse.
I was drawn to wound care from day one – and soon enrolled on the leg ulcer diploma at University of Hertfordshire. I recall weekly clinics in the leg ulcer clinic in Finchley, where I’d go home with fingers stained pink from potassium permanganate. Wound care was my thing – I loved this part of my job. But as the years went by and I qualified in minor illness, injury and as a prescriber, I found myself doing a Postgraduate Diploma in Advanced Nursing and took on the role of Nurse Practitioner, seeing urgent ‘on the day’ patients. Having moved from London to Solihull to Hertfordshire, I eventually settled in Suffolk in 2016, in a lovely semi-rural practice near the sea.
I was lucky enough to work alongside a GP with a special interest in dermatology and minor surgery. Once again, I was hooked! When he announced his retirement in 2019, I decided to embark on the MSc in Skin Lesion Management at the University of Hertfordshire in 2020 (at the beginning of Covid and with a one-year-old – I’m not sure what I was thinking!). Here I am, four years later,
Summary:
having qualified with a distinction in my MSc, juggling various dermatology balls – and with a five-year-old that has got used to me talking about skin. A lot.
When I was asked to write this article, I jumped at the chance. But then I stumbled. How can I write about one day, when every day is so different? So bear with me, I’m going to write about ‘three days in the life of a primary care dermatology nurse’.
These are my days in my GP practice in Saxmundham. I work as a Nurse Practitioner and have been there for more than eight years. I started by seeing patients with minor illness and injuries, and my job has evolved over time. I am very fortunate that the GP partners have supported me throughout my dermatology training, and I think there is now an appreciation and acceptance that I am ‘the skin nurse’. But it’s not all I do. My day is a mix of acute ‘on the day’ patients, patients with inflammatory skin conditions and those with skin lesions. I opted for two modules in my MSc related to inflammatory or chronic skin conditions. I am just as happy to see a patient with poorly controlled acne or undiagnosed hidradenitis suppurativa as I am to see a patient worried about a changing mole. I am very mindful though that I am employed to do a bit of everything – I also run a hypertension clinic, where I manage patients that need medication changes – so I have to keep it all in perspective and try not to just focus on skin.
I am brought rapidly down to earth with a succession of sore throats, headaches and questions about menopause or contraception – so I have to keep up to date with all that’s involved in urgent care. I can’t wave the ‘Oh I’m just a dermatology nurse’ card just yet!
In this article, the author discusses their role in primary care, and explains the work being undertaken locally and regionally to provide first class dermatology care with the setting. They also discuss how some of these initiatives may take some of the burden and demand away from secondary care, ensuring only those who need the service are referred into it.
Citation: Evans-Hill L. A week in the life. Dermatological Nursing 2024. 23(4):31-32.
Keywords:
Primary care, Clinic, Dermoscopy, Practice
Author info:
Lucy Evans-Hill is a Nurse Practitioner at Saxmundham Health and an Honorary Nurse Specialist in Dermatology at Ipswich Hospital.
With the knowledge from the MSc, combined with local and BAD guidelines, I work my patients up to the maximum primary care treatment before referring to secondary care. It is surprising, when talking to secondary care colleagues, to learn just how many primary care clinicians will refer to secondary care before trying all the very effective treatments available at our fingertips. I specialise in dermoscopy, so I put this to good use with any patients with skin lesions that are booked in, and will always take both clinical and dermoscopy photos and load these onto the patient’s record for future reference, or to aid a referral.
I think the patients appreciate my being there – we can never underestimate the psychological impact of a chronic skin condition. And, with the prospect of a six-eight month wait to be seen in secondary care, I will endeavour to keep up to date, research the evidence, listen to the patient, talk to colleagues and provide the best treatment I can in that 15-minute pocket of time.
Wednesday is a fun day. This is when I get to work in a community dermoscopy clinic that I was very fortunate to help establish two years ago. It is facilitated by the Suffolk GP Federation and is available to nine local GP practices to refer into. We have a clinic twice a week on a Wednesday afternoon and a Saturday afternoon. We are basically an extension of the GP service and are able to offer patients an appointment within a twoweek timeframe and will assess any skin lesions that are of concern. Some of these patients will have seen their GP already, others are directed to us by the Care Navigation team at their GP practice. There are only three of us that are able to offer this service, two GPs and myself, so we juggle the rota between us, and the patients love it! We are not able to offer any fast-track service into secondary care, and the option to refer directly for biopsy is not there yet, but we offer a little bit of expertise that the patients and the GPs really appreciate.
Primarily, we are there to screen, and a lot of the time we are seeing patients with benign lesions such as seborrheic keratoses, where we can provide reassurance and discharge them. But with our dermoscopy knowledge we are also there to pick up on the harder to diagnose lesions, and be confident in referring appropriately. Since starting the service, the inappropriate two week wait referrals have reduced, and we are making a small dent in the burden on dermatology in secondary care. We are also able to initiate treatment for pre-cancerous lesions such as actinic keratoses and Bowen’s disease, again often preventing another referral to the hospital.
“We need more knowledge in primary care, and it is now my mission to try and help achieve this”
Thursdays are different again. I am lucky enough to be involved in a pilot that currently has four clinicians (three GPs and myself) working one day a week in secondary care in dermatology, under the watchful eye of a very accommodating consultant. This is happening in both Ipswich and Colchester hospitals, and we all feel exceptionally lucky to have this opportunity.
The premise behind this pilot is to provide a primary care professional development programme that enhances our primary care knowledge that we can then share with our primary care colleagues and improve care for the patient. It has been developed in collaboration with Suffolk and North East Essex (SNEE) Training Hub and East Suffolk and North Essex Foundation Trust (ESNEFT). We are the first cohort and are four months into the programme, with a new cohort champing at the bit behind us. The goal once we have
a stream of clinicians coming out the other side is to provide a dermatology service in the community, with a group of doctors and nurses with varying skills and a knowledge of both primary and secondary care. To be involved, the clinician needs to have done a diploma in dermatology or already have extended dermatology knowledge.
Within the clinics we are seeing a mix of skin lesions, rashes and responding to advice and guidance requests, but all with the oversight of the consultants, who have been so accommodating and helpful.
It has not been without its challenges, however. Making the transition from working in primary care to secondary care (even if it is just for a day a week under supervision) is no mean feat! There are hours upon hours of mandatory training and compliance hoops to jump through, although it is worth it in the end, as the teaching and learning thus far has been invaluable. The consultants are doing this in their own time, and are often supervising our clinics whilst seeing complex cases themselves, so we really appreciate having this opportunity.
In summary, my week is very varied. I’m often asked if I will go and work in secondary care now that I have my qualification, and the answer is no. We need more knowledge in primary care, and it is now my mission to try and help achieve this. Primary care nursing can be quite lonely at times, particularly if you choose to specialise in an area where there are no local nurses working in a similar role. This is where networking and organisations such as the BDNG and PCDS (Primary Care Dermatology Society) are vital. I am now a member of the BDNG primary care subgroup and look forward to getting involved in some of their events, and doing a bit of teaching too. Attending conferences has been invaluable in meeting other nurses doing a similar role, or that have an interest in dermatology.
So, what’s next? Who knows… but I still have Mondays free!


Sebco, managing scaly scalp conditions such as: Psoriasis*, Eczema, Dandruff and Seborrheic Dermatitis... ...by softening and removing the scale as well as having mild antipruritic and antiseptic properties.
*NICE recommend a topical treatment containing salicylic acid and oils as a second line treatment for patients with scalp psoriasis1

go near naked flames - risk of severe burns. Fabric (clothing, bedding, dressings etc) that has been in contact with this product burns more easily and is a serious fire hazard. Washing clothing and bedding may reduce product build-up but not totally remove it. Avoid contact with eyes, and any areas of broken skin. Coal tar may stain clothes and jewellery. Remove or protect these items during treatment. Side Effects: May cause skin irritation, folliculitis and rarely photosensitivity. In the event of such a reaction, discontinue use. Prescribers should consult the Summary of Product Characteristics in relation to other side effects.
Rod Tucker
Sun protection measures in Caucasians help to reduce the risk of skin cancer and photoaging. However, much less emphasis has been placed on the use of these measures for people with skin of colour (SOC), i.e., those of African, Asian, Hispanic or Middle Eastern descent. The term SOC can also be defined using the Fitzpatrick skin type (FST) scale. The FST categorises skin types based on a self-reported likelihood of sunburn and tanning following sun exposure. Based on the FST scale, people with SOC have type IV to VI skin.
For many years, it was believed that coloured skin offered protection against the harmful effects of excessive UV exposure, thereby reducing the risk of skin cancer. Nevertheless, while this risk is reduced, it is not eliminated. Indeed, the most common serious form of skin cancer, melanoma, can still occur in those with SOC, but it often presents on sun-protected regions of the body. Unfortunately, this leads to diagnostic delays and the subsequent skin cancer presenting at a more advanced stage.
This article discusses how sunlight affects the skin, skin cancer in SOC and sun protection advice for those with SOC.
Sunlight represents the portion of the electromagnetic spectrum emitted by the sun that reaches the earth’s surface. It is composed of visible light (roughly 50%) and infra-red radiation (40%), with the remaining 10% being ultra-violet (UV) radiation. This latter UV radiation is further divided into UVA (95%), UVB (5%) and UVC (0%). UVB is responsible for sunburn and the development of skin cancer, whereas UVA penetrates the dermis where it damages collagen and elastin, leading to tanning and premature ageing of the skin. Fortunately, UVC is filtered out by the ozone layer.
Summary:
Over many years, cumulative damage leads to the classic signs of photoaging, i.e, wrinkles, pigmentation changes and broken capillaries. Although sun protection measures have focused largely on the UV region of the solar spectrum, it has also become apparent in recent years that visible light is far from benign. Exposure to visible light causes erythema, pigmentation, thermal damage, as well as the generation of reactive oxygen species which can be especially problematic for those with SOC.2
The skin pigment melanin is a chromophore and able to absorb UV radiation, subsequently dissipating this energy as heat. The higher levels of melanin (particularly eumelanin) and its distribution within the epidermis in people with SOC affords a greater level of protection against UV radiation. This has been known for many years. In fact, a 1979 study showed that around five times as much UV radiation reached the upper dermis in Caucasian compared to Black skin, providing more effective sun protection.3 Based on this work, the authors estimated that Caucasian skin provides a sun protection factor (SPF) of around 3.3, whereas Black skin has an SPF of 13.4. This difference is likely to account for the low levels of cutaneous melanoma in people with Black skin, with one study estimating an incidence of 1 per 100,00.4 However, exposure to UV radiation may not be an important factor in the development of melanoma in those with SOC as noted in a recent systematic review.5
Cutaneous melanoma, which accounts for the majority of skin cancer cases in Caucasians, are much less common in people with SOC. Such individuals have a considerably higher incidence of an acral lentiginous melanoma (ALM) as shown in Figure 1. This frequently occurs on acral surfaces of the skin such as the palms, soles of the feet
This article provides an overview of how skin cancers present in people with skin of colour (SOC) and discusses why photoprotective measures are still required.
Author info:
Rod Tucker is a pharmacist with a special interest in dermatology, and a member of the Dermatological Nursing Editorial Board.
Citation: Tucker R. Skin cancer and sun protection for people with skin of colour Dermatological Nursing 2024. 23(4):34-37.
Keywords:
Skin of colour, Skin cancer, Photoaging, Melanoma, Sun protection
and the nails. For instance, one analysis found that whereas truncal melanomas occurred in 34.5% of Caucasian patients, ALMs were present in 56.1% of African Americans.6 ALM is also more prevent in people of Asian, Chinese and Middle Eastern descent. Furthermore, a recent study of 48 Black patients with melanoma also found that 75% occurred on acral sites.7 However, why this form of melanoma is more common is unclear because while acral sites have less protective melanin, they are also less exposed to sunlight.
Recognising melanoma in skin of colour
Melanomas are more likely to be diagnosed at an advanced stage in people with SOC.8 The reasons for this are uncertain, but probably reflect a mistaken belief that coloured skin is protected against cancer. A further reason may simply reflect a lack of awareness of the development of melanoma in those with SOC. A highprofile example of how melanoma can affect those with SOC is illustrated by the Jamaican musician Bob Marley, who died in 1981 aged 36 from an AML.9 When Marley first noticed a dark spot under his toenail he failed to seek treatment, thinking that it was related to a football injury, which ultimately led to his early death.
In recent years, there has been a growth in the development of artificial
intelligence (AI) systems that have been trained to detect and classify skin cancers through the use of algorithms and deep neural networks. In practice such systems are used to help with the triage of skin malignancies. In fact, a recent systematic review and metaanalysis, found that when compared to clinicians, AI systems had a comparable diagnostic accuracy, based on both sensitivity and specificity.10 Nevertheless, the diagnostic accuracy of an AI system is highly dependent upon the dataset used to train the model and it seems that the current systems are less effective at identifying malignancies in people with SOC.
In one such study, AI systems performed worse on lesions in patients with SOC compared to lighter skin, even when there was a biopsy-proven malignancy.11 A more recent analysis, focusing specifically on the ability of AI systems to detect skin cancers in people with SOC, found that while the models had a good level of accuracy, the systems had only been trained on East Asian populations, largely ignoring those with Fitzpatrick skin types IV to VI.12
As well as AI systems, dermoscopy can be used to aid the diagnosis of skin cancers, and a recent development has been the incorporation of dermoscopy imaging features into AI systems to identify malignant lesions.13 Despite this advance, some neoplasms and skin

(Reproduced with permission from ©DermNet www.dermnetnz.org 2024.)
www.bdng.org.uk
photo-types are underrepresented in studies exploring the value of dermoscopy for the identification of malignancies in darker skin.14
The two main non-melanoma skin cancers, squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), are less commonly seen in people of colour, accounting for around 1-2% of all neoplasms. However, an SCC is the most common form of non-melanoma skin cancer and accounts for around 30% to 65% of all skin cancers in Black and Asian Indian people.15
Unlike with Caucasians, UV radiation is not a primary factor in the development of SCCs in people with SOC, and one of the main factors linked to the development of an SCC is scarring due to either burns or chronic leg ulcers.16 Clinically, an SCC can present as scaling, indurated and well-circumscribed papules or plaques (see Figure 2).
The site of presentation for a BCC in a patient with SOC is largely similar to those seen in Caucasians, occurring on the head, scalp and nose. However, more than half of all lesions are pigmented and the typical, translucent rolled borders and telangiectasis (Figure 3) become much more difficult to see, which can delay the diagnosis.
(Reproduced with permission from ©DermNet www.dermnetnz.org 2024.)
Until recently, little attention has been paid to the effects visible light can have on the skin. It is now recognised that visible light, specifically in people with SOC, leads to increased pigmentation. A 2010 study showed that irradiation with visible light in people with SOC led to the induction of darker skin pigmentation in comparison to those with Fitzpatrick skin type II.17 Visible light has also been shown to trigger the pigmentation disorder melasma (Figure 4) in Filipino women.18 In fact, within the literature, it is widely accepted that those with SOC often use sun protection to guard against further skin darkening and prevent ageing rather than to reduce the risk of skin cancer.19
Sun protection practices among those with skin of colour
Research suggests that awareness of skin cancer risk among people with SOC is low even for those with ALMs.20
For instance, in a recent qualitative study of Black African Americans there was a perceived low risk of malignant melanoma and even surprise that melanoma could develop at acral sites.21
Despite the presence of a darker skin tone, people with SOC still
experience sunburn, which could be due to the perception that sunscreen is unnecessary. This was seen in a recent study of 222 UK individuals of African/ Caribbean descent. Researchers found that 52.2% of participants reported a history of sunburn with 69% stating that they had not used sunscreen at the time.22 Another surprising finding was that non-Hispanic Black people who reported severe sunburn were seven times less likely to use sunscreen than non-Hispanic whites.23 But failure to apply a sunscreen is not exclusive to Black patients. Other work has shown how American Asians are significantly less likely to use sunscreens compared to Caucasians.24
Advice from the British Association of Dermatologists (BAD) states that sun protection to prevent sunburn in the UK is unnecessary for people with SOC, especially for those with very dark skin, due to the low UV index.25 Nevertheless, sun protection is recommended when visiting sunny climates with a higher UV index and particularly when individuals intend to spend large amounts of time outdoors. The BAD still advises that sunscreens are used for those with known photosensitivity, vitiligo and lupus, as well as those who have been prescribed

Basal cell carcinoma
(Reproduced with permission from ©DermNet www.dermnetnz.org 2024.)
immunosuppressive treatments and those with known risk factors for the development of skin cancer.
The general advice from the BAD for all other people with SOC is:
l To seek shade between 11am and and 3pm
l To protect the skin with appropriate clothing including a wide brimmed hat and sunglasses
l Use sunscreens with a minimum SPF (sun protection factor) of 30 and high UVA protection, e.g. a four- or five-star rating if using a UK brand (UVA is circulated in international brands)
l If susceptible to melasma or hyperpigmentation, use physical sunscreens with inorganic filters such as titanium dioxide, zinc or iron oxide.
Physical or mineral sunscreens are advocated for people with SOC because these contain inorganic filters such as titanium dioxide which reflect visible light. Unfortunately, such products leave a white film on the skin which reduces the cosmetic acceptability. Sunscreens that use small particles of titanium improve the appearance on the skin, but such micronized formulations are less protective against visible light. A suggested alternative is

Melasma
Journal of the American Academy of Dermatology Volume 86 Issue 4 Pages 729-739 (April 2022)
DOI: 10.1016/j.jaad.2021.07.080. Copyright © 2022 American Academy of Dermatology, Inc)
the use of a tinted sunscreen, containing pigmentary titanium dioxide with iron oxide. These formulations physically block both visible light and UV radiation and have been found to prevent melasma and hyperpigmentation.26 Nevertheless, insufficient application, cosmetic elegance and cost remain big challenges to the effective use of tinted sunscreens.27
To help increase the recognition of AML, the American Academy of Dermatology has produced a guide on the signs of melanoma on the feet.28 The guide recommends that people with SOC should look out for:
l Any brown or black vertical lines under their toenails
l Any new spot or growth at the site of a prior injury
l Any rapidly growing mass on their foot or a non-healing sore.
Conclusion
All skin tones can experience sun damage and visible light increases the risk of pigmentation disorders for those with SOC. Sun protection is still required in people with SOC because while their risk of cancer is reduced, it is not eliminated. Suitable protection is needed against both UV and visible light, although finding a suitable product has become a matter of trial and error. However, an erroneous belief that darker skin fully protects against cancer and insufficient awareness of acral site melanomas serves only to highlight the importance of educational interventions on skin cancer for people with SOC. Hopefully, such interventions will reduce delays in seeking help, delays in diagnosis and ultimately skin cancer mortality.
References
1. Beani JC. Ultraviolet A-induced DNA damage: role in skin cancer. Bull Acad Natl Med. 2014 Feb;198(2):273-95.
2. Mahmoud BH, Hexsel CL, Hamzavi IH et al. Effects of visible light on the skin. Photochem Photobiol. 2008 MarApr;84(2):450-62.
3. Kaidbey KH, Agin PP, Sayre RM et al. Photoprotection by melanin--a comparison of black and Caucasian skin. J Am Acad Dermatol. 1979 Sep;1(3):249-60.
4. Culp MB, Lunsford NB. Melanoma Among Non-Hispanic Black Americans. Prev Chronic Dis. 2019 Jun 20;16:E79.
5. Lopes FCPS, Sleiman MG, Sebastian K et al. UV Exposure and the Risk of Cutaneous Melanoma in Skin of Color: A Systematic Review. JAMA Dermatol. 2021 Feb 1;157(2):213-219.
6. Mahendraraj K, Sidhu K, Lau CSM et al. Malignant Melanoma in African-Americans: A Population-Based Clinical Outcomes Study Involving 1106 African-American Patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1988-2011). Medicine (Baltimore). 2017 Apr;96(15):e6258.
7. Wix SN, Brown AB, Heberton M et al. Clinical Features and Outcomes of Black Patients with Melanoma. JAMA Dermatol. 2024 Mar 1;160(3):328-333.
8. Hu S, Soza-Vento RM, Parker DF et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006 Jun;142(6):704-8.
9. Skin Cancer Foundation. Bob Marley should not have died from melanoma. Available online at: https://www.skincancer. org/blog/bob-marley-should-not-have-diedfrom-melanoma/ [Accessed May 2024].
10. Salinas MP, Sepúlveda J, Hidalgo L, et al. A systematic review and meta-analysis of artificial intelligence versus clinicians for skin cancer diagnosis. NPJ Digit Med. 2024 May 14;7(1):125. doi: 10.1038/s41746-02401103-x
11. Daneshjou R, Vodrahalli K, Novoa RA et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022 Aug 12;8(32): eabq6147.
12. Liu Y, Primiero CA, Kulkarni V et al. Artificial Intelligence for the Classification of Pigmented Skin Lesions in Populations with Skin of Color: A Systematic Review. Dermatology. 2023;239(4):499-513.
13. Brutti F, La Rosa F, Lazzeri L, et al. Artificial Intelligence Algorithms for Benign vs. Malignant Dermoscopic Skin Lesion Image Classification. Bioengineering (Basel). 2023 Nov 16;10(11):1322. doi: 10.3390/ bioengineering10111322.
14. Enechukwu NA, Behera B, Ding DD, et al. Dermoscopy of Cutaneous Neoplasms in Skin of Color - A Systematic review by the International Dermoscopy Society “Imaging in Skin of Color” Task Force. Dermatol Pract Concept. 2023 Oct 1;13(4 S1): e2023308S. doi: 10.5826/dpc.1304S1a308S
15. . Gupta AK, Bharadwaj M, Mehrotra R. Skin Cancer Concerns in People of Color: Risk Factors and Prevention. Asian Pac J
Cancer Prev. 2016 Dec 1;17(12):5257-5264.
16. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. A review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981 Nov;5(5):535-43.
17. Mahmoud BH, Ruvolo E, Hexsel CL. Et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010 Aug;130(8):2092-7.
18. Verallo-Rowell VM, Pua JM, Bautista D. Visible light photo-patch testing of common photo-contactants in female Filipino adults with and without melasma: a cross-sectional study. J Drugs Dermatol. 2008 Feb;7(2):149-56.
19. Buchanan Lunsford N, Berktold J, Holman DM, Stein K, Prempeh A, Yerkes A. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep 2018 Oct 6; 12:203-209.
20. Dlova, N., Gathers, R., Tsoka-Gwegweni, J., et al. Skin cancer awareness and sunscreen use among outpatients of a South African hospital: need for vigorous public education. South African Family Practice, 2018; 60(4), 132–136.
21. de Vere Hunt I, Owen S, Amuzie A et al. Qualitative exploration of melanoma awareness in black people in the USA. BMJ Open. 2023 Jan 11;13(1): e066967.
22. Bello O, Sudhoff H, Goon P. Sunburn Prevalence is Underestimated in UK-Based People of African Ancestry. Clin Cosmet Investig Dermatol. 2021 Nov 24; 14:17911797.
23. Summers P, Bena J, Arrigain S et al. Sunscreen use: Non-Hispanic Blacks compared with other racial and/or ethnic groups. Arch Dermatol. 2011 Jul;147(7):863-4.
24. Martin A, Liu J, Thatiparthi A, et al. Asian Americans are less likely to wear sunscreen compared with non-Hispanic whites. J Am Acad Dermatol. 2022 Jan;86(1):167-169.
25. British Association of Dermatologists. Sun advice for skin of colour. Available at: https://www.skinhealthinfo.org.uk/sunawareness/sun-advice-for-skin-of-colour [Accessed May 2024]
26. Zhou C, Lee C, Salas J, Luke J. Guide to tinted sunscreens in skin of color. Int J Dermatol. 2024 63(3):272-276.
27. Xue, G.R., Hamzavi, I.H., Kohli, I. and Mohammad, T.F. Challenges of tinted sunscreen in skin of color. Int J Dermatol 2024. https://doi.org/10.1111/ijd.17347
28. American Academy of Dermatology Association. Signs that could be melanoma on your feet. Available at: https://www.aad.org/ public/diseases/skin-cancer/types/common/ melanoma/signs- foot [Accessed May 2024]






Aklief is a topical retinoid indicated for the cutaneous treatment of moderate acne vulgaris of the face and/or trunk in patients from 12 years of age and older when many comedones, papules and pustules are present 5
Aklief is contraindicated during pregnancy or for women planning a pregnancy
Aklief 50 microgram/g cream - Prescribing Information (United Kingdom) Please refer to SmPC before prescribing. Presentation: Aklief is presented in a multidose container with airless pump containing 75g of cream. One gram of cream contains 50 micrograms of trifarotene. Indications: Aklief is indicated for the cutaneous treatment of Acne Vulgaris of the face and/or the trunk in patients from 12 years of age and older, when many comedones, papules and pustules are present. Dosage and Method of administration: Dosage: Apply a thin layer of Aklief cream to the affected areas of the face and/or trunk once a day, in the evening, on clean and dry skin. It is recommended that the physician assesses the continued improvement of the patient after three months of treatment. Special populations: Paediatric population and Elderly patients. The safety and efficacy of Aklief in children below 12 years old and in geriatric patients aged 65 years and above have not been established. Renal and hepatic impairment. Aklief has not been studied in patients with renal and hepatic impairment. Method of administration: For cutaneous use only. Before using the pump for the first time, prime it by pressing down several times until a small amount of medicine is dispensed (up to 10 times maximum). The pump is now ready to use. Apply a thin layer of Aklief cream once a day, to the following affected areas, in the evening, on clean and dry skin: • forehead, nose, chin and right and left cheeks – one pump actuation should be enough. • upper trunk (i.e. reachable upper back, shoulders and chest) – two pump actuations should be enough • one additional pump actuation may be used for middle and lower back if acne is present. Patients should be instructed to avoid contact with the eyes, eyelids, lips and mucous membranes and to wash their hands after applying the medicinal product. Contraindications: • Pregnancy • Women planning a pregnancy • Hypersensitivity to the active substance or to any of the excipients.
Precautions and Warnings: To mitigate the risk of erythema, scaling, dryness, and stinging/burning, patients should be instructed to use a moisturizer from the initiation of treatment (while allowing sufficient time before and after the application of Aklief cream to allow the skin to dry), and, if appropriate, reduce the frequency of application of Aklief cream, or suspend use temporarily. If severe reactions persist the treatment may be discontinued. The product should not be applied to cuts, abrasions, eczematous or sunburned skin. As with other retinoids, use of “waxing” as a depilatory method should be avoided on skin treated with Aklief. If a reaction suggesting sensitivity to any component of the formula occurs, use should be discontinued. Caution should be exercised if cosmetics or acne medications with desquamative, irritant or drying effects are concomitantly used with the medicinal product, as they may produce additive irritant effects. Aklief should not come into contact with the eyes, eyelids, lips, or mucous membranes. If the product enters the eye, wash immediately and abundantly with lukewarm water. Excessive exposure to sunlight, including sunlamps or phototherapy should be avoided during the treatment. Use of a broad-spectrum, waterresistant sunscreen with a Sun Protection Factor (SPF) of 30 or higher and protective clothing over treated areas is recommended when exposure cannot be avoided. This product contains propylene glycol (E1520) that may cause skin irritation. Aklief also contains 50 mg alcohol (ethanol) in each gram which is equivalent to 5% w/w. It may cause burning sensation on damaged skin. Pregnancy, Breast-feeding and Fertility: Orally administered retinoids have been associated with congenital abnormalities. When used in accordance with the prescribing information,
topically administered retinoids are generally assumed to result into low systemic exposure due to minimal dermal absorption. However, there could be individual factors (e.g. damaged skin barrier, excessive use) that contribute to an increased systemic exposure. Pregnancy: Aklief is contraindicated during pregnancy or in women planning a pregnancy. If the product is used during pregnancy, or if the patient becomes pregnant while taking this drug, treatment should be discontinued. Breast-feeding: A risk to the suckling child cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from Aklief therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. To avoid the risk of ingestion by, and/or contact exposure of, an infant, nursing women should not apply trifarotene cream to the chest or breast area. Fertility: No human fertility studies were conducted with Aklief. After oral administration of trifarotene to dogs, findings of Germ cell degeneration were observed. Effects on ability to drive and use machines: Aklief has no or negligible influence on the ability to drive and use machines. Undesirable Effects: The most “commonly” reported adverse reactions (occurring in ≥1/100 to <1/10 patients) of application site irritation, application site pruritus and sunburn (see table below) occurred in 1.2% to 6.5% of patients treated with Aklief cream in clinical studies. Table of “commonly” reported adverse reactions of patients treated with Aklief cream in clinical studies
System Organ Class Adverse reactions
General disorders and administration site conditions Application site irritation, Application site pruritus
Injury, poisoning and procedural complications Sunburn
Prescribers should consult the Summary of Product Characteristics (SmPC) in relation to the uncommon (≥1/1,000 to <1/100) and rarely occurring (≥1/10,000 to <1/1,000) adverse reactions as reported in these studies. Packaging Quantities and Cost: 1 x 75g multidose container with airless pump; £ 27.75 excluding VAT. Marketing
Authorisation Number: PL 10590/0071. Legal Category: POM. Marketing Authorisation Holder: Galderma (UK) Limited, Evergreen House North, Grafton Place, London, NW1 2DX, United Kingdom. Further information is available from: Galderma (UK) Ltd, Evergreen House North, Grafton Place, London, NW1 2DX, United Kingdom. Telephone: +44 (0)300 3035674. Date of Revision: July 2023.
Adverse events should be reported.
Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Galderma (UK) Ltd: E-mail: medinfo.uk@galderma.com Tel: +44 (0)300 3035674
References: 1. Kasser M et al. J Cosmet Dermatol. 2020;00:1–6. 2. Stein Gold L et al. Dermatol Ther (Heidelb) 2021;11:661–664
3. Blume-Peytavi U et al. JEADV 2020, 34, 166–173. 4. Bell K et al. Annals of Pharmacotherapy 2021;55(1) 111–116
5. Aklief Summary of Product Charactistics. Available at: https://www.medicines.org.uk/emc/product/13881/smpc#gref. Accessed: Oct 2024
Laura Crosby
The previous articles in this series looked at the inclusion and exclusion criteria, types of evidence, search terms, PICO (Population, Intervention, Control and Outcome) search strategies and narrowing down the results to focus on the most relevant research to answer the clinical question. In our example we used the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow diagram.
As in the previous article, we will be using the following research question: Is laser treatment effective in the treatment of acne scarring in young adults?
In the last article we identified one systematic review that would be the best source of evidence to answer this question: Interventions for acne scars 1
As healthcare professionals we are required to be evidence-based in our approach to care.2 As such, it is important that we cannot only seek out research that provides answers to clinical questions, but also that we are able to evaluate the quality, validity and relevance of the evidence we find.3
To apply a thorough and systematic approach to critical appraisal, it is often helpful to use a checklist such as the CASP (Critical Appraisal Skills Programme) systematic review checklist.4 There are many different tools available for this, and CASP provide a selection of checklists for appraising different types of research. This particular appraisal tool was used as it is designed for use with health research, it is considered user-friendly and it is endorsed by Cochrane.5-7
Summary:
This is the fourth in a series of articles exploring the process of literature searching. This article focuses on critical appraisal and application to practice.
Keywords:
Literature review, Evidence, Evidencebased practice, Professional development
Citation:
This checklist allows us to methodically work through the review to establish any issues with the quality, validity and relevance. The article chosen for this critical appraisal is a Cochrane systematic review, and this gives us our first insight into its quality. Whilst many reviews are published, Cochrane systematic reviews are considered the gold standard, and as such, give an indication of the rigor with which the research will be scrutinised.8
Below is a worked example of a systematic review checklist followed by a summary of the findings:
Paper for appraisal and reference:
Abdel Hay, R., Shalaby, K., Zaher, H., Hafez, V., Chi, C. C., Dimitri, S., Nabhan, A. F., & Layton, A. M. (2016). Interventions for acne scars. The Cochrane database of systematic reviews, 4(4), CD011946. https://doi. org/10.1002/14651858.CD011946.pub2
“The results of Hay et al.1 are clear. They answer their initial question by stating that the review does not provide support for the use of any intervention in the treatment of acne scars as a gold standard”
Author info:
Laura Crosby is a Clinical Nurse Specialist, Medical Dermatology, at University Hospitals Bristol and Weston NHS Foundation Trust.
Crosby L. Conducting a literature review: Part four – critical appraisal. Dermatological Nursing 2024. 23(4):39-43.
Section A: Are the results of the review valid?
1. Did the review address a clearly focused question?
Yes HINT: An issue can be ‘focused’ In terms of
Can’t Tell No
Comments:
l the population studied
l the intervention given
l the outcome considered
This review addressed the question: Which treatments are effective for acne scars?
Clear list of primary and secondary outcomes –participant reported scar improvement: measured by a scar improvement, grading, or severity scale. Participants with serious adverse events (resulting in withdrawal from the trial).
Identifies acne scarring as population and specifies that participants must have been identified as having atrophic or hypertrophic acne scarring by a dermatologist or experienced investigator. No age range given but age range in included papers was 18 and over.
2. Did the authors look for the right type of papers?
Yes HINT: ‘The best sort of studies’ would
l address the review’s question
3. Do you think all the important, relevant studies were included?
Yes
Can’t Tell No
Comments:
HINT: Look for l which bibliographic databases were used l follow up from reference lists l personal contact with experts l unpublished as well as published studies l non-English language studies
Robust choice of databases in line with Cochrane systematic review guidance in order to identify all relevant research and to minimise risk of bias created by relying on one database – Cochrane, Medline, Embase and LILACS (Latin American and Caribbean Literature on Health Sciences).
Bibliographies of included trials were checked as well as manufacturers’ websites.
Trial registries checked – ISRCTN, US National Institutes of Health Ongoing Trials Register, Australian New Zealand Clinical Trials Registry, World Health Organization International Clinical Trials Registry Platform and EU Clinical Trials Register.
Authors of trials with missing data or information were contacted if required along with authors of ongoing trials. Non-English language studies were eligible for inclusion.
Can’t Tell No
Comments:
l have an appropriate study design (usually randomised controlled trials (RCTs) for papers evaluating interventions)
Appropriate study design searched for as RCTs are best for studying interventions and these were the papers included.
Search terms in appendix show appropriate terms to capture the information in the research question. This paper identifies that their initial objective focused on facial acne, but this did not fit with their overall title. As a consequence, they revised their objectives to reflect their research question and to include research about other body areas as well as the face.
4. Did the review’s authors do enough to assess the quality of the included studies?
Yes
Can’t Tell No
Comments:
HINT: The authors need to consider the rigour of the studies they have identified. Lack of rigour may affect the studies’ results (“All that glitters is not gold” Merchant of Venice – Act II Scene 7)
Bias, study size, study duration, study methodology and design all considered.
Authors have allocated a risk of bias (low, high or unclear) for sequence generation (selection bias), allocation concealment (selection bias), blinding (performance and detection bias), incomplete outcome data (attrition bias), selective reporting bias, other sources of bias (such as baseline imbalance or blocked randomisation in unblinded trials) and overall risk of bias (against criteria in Cochrane Handbook for Systemic Reviews). This demonstrates significant consideration of rigour.
5. If the results of the review have been combined, was it reasonable to do so?
Yes
HINT: Consider whether l results were similar from study to study l results of all the included studies are clearly displayed l results of different studies are similar l reasons for any variations in results are discussed Can’t Tell No
Comments:
The study results have not been combined in a metaanalysis, but this is reasonable as the results cannot be compared due to different interventions.
Results of all the included studies are clearly displayed and similarities are identified where possible. However, this systematic review was not able to identify enough studies of similar interventions to carry out any study comparisons.
Section B: What are the results?
6. What are the overall results of the review?
HINT: Consider
l if you are clear about the review’s ‘bottom line’ results l what these are (numerically if appropriate) l how were the results expressed (NNT, odds ratio etc.)
Comments:
The review does not find the evidence necessary to support the use of any interventions for the treatment of acne scarring. This gives a very clear ‘bottom line’. Lots of numerics for individual trials but not generally for ‘laser’ as a whole. There were multiple types of laser included across the studies, but no overall consensus on laser as a whole.
CI and RR included in discussion of individual trials. Of relevance - improvement in skin texture from the use of CO2 laser compared to placebo with p = 0.032 and p= 0.0001. However, 95% CI ranged from 1.25-12.84 so not a reliable outcome. Put simply, the research around CO2 lasers showed a significant result, however, there is a wide range in the CI which makes it unclear how transferrable these results would be if the trial was repeated in a bigger cohort.
7. How precise are the results?
HINT: Look at the confidence intervals, if given Comments:
Results of the systematic review are likely to be precise, but given the small number of participants the data around confidence intervals (CI) makes it unclear if these results could be repeated in a bigger cohort. Therefore, efficacy of laser cannot be accurately assessed.
Fractional laser versus fractional radiofrequency CI demonstrated no statistical significance. Fractional CO2 laser versus placebo investigator assessment of improvement in texture was shown with statistical significance, but patient assessed improvement was not demonstrated with significance. Fractional laser versus combined chemical peeling showed no difference in efficacy between interventions.
Results reported using the GRADE approach to measure quality.9
Section C: Will the results help locally?
8. Can the results be applied to the local population?
Yes
Can’t Tell
No
Comments:
HINT: Consider whether l the patients covered by the review could be sufficiently different to your population to cause concern l your local setting is likely to differ much from that of the review
All but four studies that consider laser were based in the Middle East or Asia, with only 23 participants in European studies.
Nearly half of the participants in laser studies were from Asia.
Ethnic makeup of England and Wales in 2011 census is 86% white, so we cannot assume that results from Asian trials are transferrable.
Question looks at young adults, but data from the European studies includes people aged 18-60.
9. Were all important outcomes considered?
Yes HINT: Consider whether: l there is other information you would like to have seen
Can’t Tell No
Comments:
The systematic review included all appropriate outcomes (see question 6), but further placebo trials would be helpful in assessing efficacy.
10. Are the benefits worth the harms and costs?
Selection criteria are clear: inclusion of randomised controlled trials (RCTs) for treating acne scars. This review also states exclusion of studies dealing mostly or exclusively with keloid scars. As RCTs are considered the most appropriate study design for evaluating interventions, this choice of study design is appropriate for the question being answered by Hay et al.1 The clear inclusion and exclusion criteria also indicate that the authors have considered the quality and relevance of the included papers. Whilst interventions other than laser were included in this review, the outcomes of this review will be relevant in forming an evidence base for or against the use of lasers for the treatment of acne scarring.
Yes HINT: Consider l even if this is not addressed by the review, what do you think?
Can’t Tell No
Comments:
There is no good quality evidence to support the use of laser treatment.
“As healthcare professionals we are required to be evidence-based in our approach to care”
The Critical Appraisal Skills Programme (CASP) Systematic Review Checklist4 was used to critically appraise Hay et al.1 This Cochrane Review clearly states a focus of interventions for treating acne scars and an assessment of the effectiveness. The review includes several different interventions, not all of which are relevant, but it does consider several different types of laser treatments, which are all relevant to the initial question.
The inclusion criteria did not specify age range, but covers the 19-24 year category identified as a MeSH term relevant to ‘young adult’. There was no data about the impact age or skin type may have in relation to the effectiveness of interventions, however, they were identified as information that should be recorded in future studies. The review states that participant-reported scar improvement and serious adverse events would be used as primary outcomes. This outline of the research focus makes it possible to determine the relevance of this review in assessing the effectiveness of laser treatment in acne scarring.
Hay et al.1 gives a clear list of search databases, trial registers and states that bibliographies and treatment manufacturers were contacted to thoroughly check for any other relevant trials. The paper also states that the authors of papers with missing data or ongoing trials were contacted. A list of these authors is included and the outcome of the attempts to make contact is recorded. The selection of databases and trial registers searched suggests that this review was conducted thoroughly and systematically. This thorough, systematic approach should ensure that the authors have found all relevant literature on the topic and have gone on to consider this in their evaluation. It should also ensure that no major studies on the topic have been missed in the review. The authors acknowledge the potential that they have missed some trials and that this could influence the outcome of the review. They state that their search was rigorous and that missed trials are highly unlikely. As the authors have been rigorous in their literature search, it is likely that any robust literature relevant to the treatment of acne scarring will have been included. It is important, however, to note that this review was published in 2016, and any studies since that date will consequently not be included. Although this review is from 2016, it is still the most relevant piece of evidence found in the literature search.
The authors of this review were rigorous in their assessment of the quality of the included studies. Each study was reviewed by each author to consider risk of bias relating to allocation concealment and blinding of participants and investigators.
Summaries of these were given in graphic forms as well as a more detailed description for each trial. They also report using the GRADE approach to summarising findings and measuring the quality of outcomes.9 The authors also commented on the low total number of trial participants considering the prevalence of acne. The authors state that their paper looks at seven different interventions spread across a total of 24 trials with a total of 789 participants. Of these, 16 trials include a laser intervention, with a total of 395 participants. The remaining eight trials are not relevant to the question of efficacy of laser treatment in acne scarring. Hay et al.1 comment on the impact of the large number of interventions being that each intervention only has a small cohort of participants, leaving it open to greater potential bias. They have concluded that the combination of low participant numbers for each intervention, and high risk
of bias in several areas of the studies, has resulted in these studies providing a very low quality of evidence for these interventions in the treatment of acne scarring. This rigorous approach to the evaluation of each study has enabled Hay et al.1 to provide a high-quality review of the available evidence for the use of treatments for acne scarring.
The results of Hay et al.1 are clear. They answer their initial question by stating that the review does not provide support for the use of any intervention in the treatment of acne scars as a gold standard. It found only limited support for the use of any lasers in the treatment of atrophic or hypertrophic acne scarring. They also provide clear breakdown of the results for each of their primary and secondary outcomes with risk ratios and confidence intervals linked to each intervention. Two included studies showed an investigator-assessed improvement in skin texture from the use of CO2 laser compared to a placebo. This was shown to be statistically significant, but there was no statistically significant improvement in patient reported scarring or in patient satisfaction.
There is some indication that fractional laser is more effective at improving scarring than non-fractional non-ablative laser, but this is not good quality evidence. This may be due to the small numbers included in each of the trials, and further clarity may be gained from larger studies. Hay et al.1 were not able to compare data using meta-analysis as the data collected from the different trials was not comparing similar interventions. Whilst it is disappointing that it was not possible for a metaanalysis to be carried out, it highlights the need for more
Selection bias – Refers to differences created in trial groups created by the system used to allocate them.
Sequence generation – The method by which trial participants are allocated to different trial arms. Can impact selection bias.
Allocation concealment – Concealing the sequence generation from those involved in trial enrolment. Can impact selection bias.
Performance bias – Differences in the care provided to participants, other than a difference in the intervention being studied. Blinding of participants and trial personnel can overcome this.
Detection bias – Differences in how outcomes are perceived by those measuring the outcomes. This can be overcome by blinding of participants and of those measuring the outcomes.
Attrition bias – Attrition relates to those trial participants who withdraw or are withdrawn from the study but where the reasons are not clearly documented.
GRADE – A system for measuring the quality and certainty of evidence.
robust studies into the use of laser for treating acne scarring. Overall, this review identified that without adequate trials comparing an intervention to a placebo, it is not possible to assess the efficacy of a treatment. The trials included in this review identified improvements in acne scarring with laser treatment, but the comparison groups also demonstrated improvement in acne scarring. Since we cannot determine the cause of this improvement without a control, Hay et al.1 suggest the review does not provide evidence to support the use of laser for the treatment of acne scarring.
At this point we have answered our original clinical question. We asked: Is laser treatment effective in the treatment of acne scarring in young adults?
The answer we have found is that there is not good quality evidence to support the use of laser in acne scarring in young adults. So, what now? In the final article in this series, we will consider how we apply this evidence to our practice.
1. Hay AR, Shalaby K, Zaher H, Hafez V, Chi CC, Dimitri S et al. Interventions for acne scars. The Cochrane database of systematic reviews 2016. 4(4)
2. NMC. The Code. [online] The Nursing and Midwifery Council 2018. Available at: https://www.nmc.org.uk/standards/code/read-the-codeonline/ [Accessed November 2024]
3. Critical Appraisal Skills Programme (CASP). What is critical appraisal? CASP - Critical Appraisal Skills Programme 2024. Available at: https://casp-uk.net/what-is-critical-appraisal/ [Accessed November 2024].
4. Critical Appraisal Skills Programme. Systematic Reviews Checklist – CASP. CASP - Critical Appraisal Skills Programme 2018. Available at: https://casp-uk.net/casp-tools-checklists/systematic-review-checklist/ [Accessed November 2024]
5. Cavaleri R, Bhole S, Arora A. Critical Appraisal of Quantitative Research. ResearchGate 2018. Available at: https://www.researchgate. net/publication/327261434_Critical_Appraisal_of_Quantitative_ Research [Accessed November 2024]
6. Cochrane. Master critical appraisal with Cochrane Evidence Essentials module 6. Cochrane 2024. Available at: https://www.cochrane. org/news/master-critical-appraisal-cochrane-evidence-essentialsmodule-6 [Accessed November 2024]
7. Long H, French D, Brookes J. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. ResearchGate 2020. Available at: https://www. researchgate.net/publication/343504166_Optimising_the_value_of_ the_critical_appraisal_skills_programme_CASP_tool_for_quality_ appraisal_in_qualitative_evidence_synthesis [Accessed November 2024]
8. Davies A. Carrying out systematic literature reviews: an introduction. British Journal of Nursing 2019. Available at: https:// www.britishjournalofnursing.com/content/professional/carrying-outsystematic-literature-reviews-an-introduction/ [Accessed November 2024]
9. Schünemann H, Broek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations Updated October 2013. The GRADE Working Group, 2013. Available at: www.guidelinedevelopment.org/handbook [Accessed November 2024].





Jing Husaini
Psoriasis and psoriatic arthritis (PsA) are both chronic autoimmune diseases. According to the National Institute for Health and Care Excellence (NICE)1 psoriasis affects approximately 1.3-2.2% of the UK population, and at least 2030% of individuals with psoriasis will develop PsA. One hallmark feature of PsA is dactylitis, an inflammation of the digits (fingers or toes), which occurs in around 40% of affected patients. The underlying pathophysiology of PsA involves an immune response to biomechanical stress or injury, triggering inflammation in the bone marrow, entheses, and joints. This can potentially lead to joint ankylosis. Vaso-occlusion in the metacarpal and metatarsal bones contributes to joint stiffness and painful swelling, particularly in the dorsal surfaces of the hands and feet, ultimately reducing the range of motion.2 While the precise biomolecular mechanisms of PsA remain incompletely understood, it is known to be associated with the B27.05 haplotype and the TNFα, IL-23, and IL-17A cytokine axis.
Dactylitis is notable not only for its clinical presentation but also for its association with radiographic changes, which makes it an important feature in the disease’s pathophysiology, diagnosis, and prognosis. However, research indicates that there is often a delay in the early recognition of joint involvement and symptoms in PsA, which can lead to delayed diagnosis and treatment.3 Despite the prevalence of dactylitis and its distinctive clinical features, comprehensive literature and case studies on effective treatments remain limited. This case study explores early detection of PsA with dactylitis and aims to provide evidence supporting timely referral and treatment strategies.
Summary:
A 46-year-old female patient presented to the severe psoriasis clinic with a longstanding diagnosis of psoriasis affecting highimpact areas, including the scalp, sub-mammary folds, and peri-anal region. She also reported experiencing nail changes, specifically onycholysis (separation of the nail from the nail bed). The patient had been managing her psoriasis with topical therapies for the past six years; however, in the past year, these treatments had become progressively ineffective, prompting her visit to the clinic.
In addition to her psoriasis, the patient complained of pain and swelling in her right index finger and left third toe for the past six-seven weeks. She described the pain as severe, rating it 8/10 on the Visual Analogue Scale (VAS). The pain was significant enough to impair her ability to use a keyboard, which in turn interfered with her ability to carry out her daily work tasks. Despite undergoing two courses of antibiotics prescribed by her GP, who initially suspected an infection, there was no improvement, prompting further investigation with an MRI.
The patient also disclosed experiencing high levels of personal stress related to running her own charity, which she felt might be exacerbating her condition. Additionally, she had a history of migraines and used sumatriptan as needed. Family history revealed that her mother had psoriasis, which is effectively managed with adalimumab. The patient did not smoke and only consumed alcohol occasionally. She had two children and had no plans to have more.
Upon examination, there was tenderness and fusiform swelling around the right index finger, particularly at the
Keywords:
This article provides a case history of a patient with psoriasis who presented with pain in fingers and toes. It considers the diagnosis and treatment of dactylitis and enthesitis, including telltale signs and areas for misdiagnosis.
Citation:
Husaini J. Management of a patient living with PsA exhibiting symptoms of dactylitis and enthesitis: A case study. Dermatological Nursing 2024. 23(4):45-49.
Case history, Psoriasis, Psoriatic arthritis, Dactylitis, Enthesitis
Author info:
Jing Husaini is an Advanced Nurse Practitioner at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust.
metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints. There was also notable swelling in the left third toe, specifically involving the distal interphalangeal (DIP) joint. These findings were highly suggestive of dactylitis (often referred to as ‘sausage digit’), which is a hallmark feature of PsA.
In line with NICE guidelines1 for psoriasis assessment and management, we identified clinical signs suggestive of PsA and arranged a referral to a rheumatologist to prevent long-term joint damage. At our clinic, we offer a combined dermatology-rheumatology service, providing streamlined care for patients with overlapping dermatologic and rheumatologic conditions. As an Advanced Nurse Practitioner specialising in dermatologic inflammatory diseases, I conducted a thorough assessment of the patient’s psoriasis, noting a Psoriasis Area and Severity Index (PASI) score of nine, indicating moderate disease severity. Her Dermatology Life Quality Index (DLQI) score was 14/30, reflecting a significant impact on her quality of life. Psychological screening revealed a Generalised Anxiety Disorder-7 (GAD-7) score of 10, indicating moderate anxiety, and a Patient Health Questionnaire-9 (PHQ-9) score of five, suggestive of mild depression. The patient attributed her mental health concerns to a combination of her busy lifestyle and the recent worsening of her disease. She declined to meet with our clinical psychologist, but was advised to self-refer to the NHS talking therapies service, which she is considering.
After reviewing the case with our rheumatology team, we established a working diagnosis of PsA with dactylitis, pending confirmation from the MRI results.
Given the clinical suspicion of PsA, we conducted comprehensive blood tests to assess for markers of inflammation and autoimmune diseases, including Rheumatoid Factor (RF), antinuclear antibodies (ANA), and C-reactive protein (CRP). Importantly, the negative RF result helped rule out rheumatoid arthritis (RA), a common differential diagnosis. Despite normal inflammatory markers (i.e., CRP and ESR), which suggested an absence of
an acute inflammatory process like RA or gout, these markers do not always correlate with PsA, where inflammatory markers may be normal in some patients.
We conducted a series of screening tests to assess the patient’s suitability for methotrexate treatment. This included a baseline liver evaluation to identify any risk factors for liver disease (such as fatty liver disease or alcohol use), along with liver function tests, hepatitis B core antibody (Hep B cAb), hepatitis B surface antigen (Hep B sAg), and hepatitis C IgG serology. We also performed a fibroscan to evaluate liver stiffness. Additionally, a full blood count (FBC), renal profile, and HIV testing were conducted, in accordance with local guidelines,4 to identify any contraindications or risk factors associated with methotrexate therapy.
The blood test results revealed a negative RF, ruling out rheumatoid arthritis. Other inflammatory markers, including CRP and ESR, were normal, suggesting that the patient did not have an acute inflammatory arthritis like RA or gout. The remaining test results were unremarkable.
The MRI confirmed our clinical suspicion of PsA, showing dactylitis and enthesitis. Specifically, the MRI revealed a focally increased marrow signal within the middle phalanx of the third ray on STIR imaging, with a corresponding signal decrease on T1-weighted images. This finding was consistent with inflammatory changes at the affected joints. The MRI also revealed articular irregularity of the middle phalangeal surface at the distal interphalangeal (DIP) joint of the left third toe, confirming inflammatory joint involvement. Additionally, there was loss of joint space at the first metatarsophalangeal (MTP) joint, with intact plantar plates and plantar bursitis at the second, fourth, and fifth MTP joints. There was also mild fatty infiltration of the forefoot musculature, a common feature seen in enthesitis (inflammation of tendon or ligament insertion points).
Based on the clinical examination and MRI findings, we confirmed the diagnosis of PsA with dactylitis. The patient’s PEST score of 3 indicated that a referral to rheumatology was appropriate.5
Additionally, the patient’s CASPAR (Classification Criteria for PsA) score was four, which meets the diagnostic threshold for PsA, as a score of three or higher is required for diagnosis.6
In accordance with NICE1 and the European Alliance of Associations for Rheumatology (EULAR) guidelines,7 methotrexate was recommended as the first-line conventional synthetic disease-modifying antirheumatic drug (csDMARD) for patients with high-impact psoriasis when topical treatments are no longer effective. We discussed the option of initiating methotrexate therapy via subcutaneous injection at a dose of 15mg once weekly with the patient. During the discussion, we emphasised the importance of close monitoring and the potential side effects, including mucositis and liver toxicity.
The patient was also prescribed folic acid (5mg daily), except on the day of methotrexate administration, to help mitigate the risk of methotrexate-related side effects. In addition, the rheumatologist administered a 40mg Depo-Medrol (methylprednisolone acetate) injection with lidocaine suspension into the right index finger second flexor tendon sheath, which provided significant pain relief. The patient reported improvement within hours, with her VAS score decreasing from 8/10 to 5/10.
The patient was monitored in our combined dermatology-rheumatology clinic. At her three-month follow-up, she reported significant pain relief, with her VAS score dropping to 2/10. The swelling had resolved, and her skin had improved markedly, with a PASI score of three and DLQI of six, indicating a moderate impact on her quality of life.
At the six-month follow-up, the patient continued to improve. She was able to resume her daily activities, including work, without difficulty. This improvement led to a significant reduction in her anxiety levels, as evidenced by a reduction in her GAD-7 and PHQ-9 scores to two, reflecting a decrease in both anxiety and depression symptoms.
Psychological screening was an ongoing part of her management plan, reflecting the importance of addressing both the physical and mental health aspects of managing chronic inflammatory diseases. The reduction in the patient’s GAD-7 and PHQ-9 scores further demonstrated the positive impact of controlling her psoriasis and psoriatic arthritis on her overall wellbeing.
Due to the multifaceted nature of PsA, both dermatological and rheumatological expertise are essential for accurate diagnosis and effective management. PsA often develops 5-10 years after a diagnosis of psoriasis,8 though recent updates to the CASPAR criteria suggest that PsA may be diagnosed even before the onset of noticeable skin symptoms. In this case, the patient had high-impact psoriasis, which could have easily been mistaken for other conditions such as eczema or mycosis fungoides, highlighting the importance of careful differential diagnosis. The dermatologist in this case played a pivotal role in correctly identifying the skin manifestations of psoriasis, which ultimately guided the rheumatological assessment.9
One of the key clinical features of PsA is dactylitis, which affects up to 49% of patients and is often an early indicator of the disease. Dactylitis presents with swelling of one or more fingers or toes. In this case, the patient exhibited this classic manifestation, with significant swelling in the right index finger and left third toe, both of which were tender and swollen, supporting the diagnosis of PsA. Additionally, the patient’s nail changes, including onycholysis – which is commonly seen in PsA – further reinforced the clinical suspicion.6
The pathophysiology of dactylitis is not fully understood, but is believed to involve an immunological response affecting both soft tissues and subcutaneous oedema. This inflammation is typically localised at the entheses, the sites where tendons, ligaments, and joint capsule fibres attach to bone, and is considered a hallmark of PsA. Enthesitis, along with dactylitis, is rarely seen in RA.10 Acute dactylitis in PsA can serve as a marker of disease severity and often correlates with more aggressive forms of PsA.
Imaging studies, including X-rays and MRI, are critical for confirming the diagnosis and assessing the severity of dactylitis and enthesitis. MRI, in particular, can reveal bone remodelling and enthesopathy, indicating irreversible joint damage that can occur without early intervention. Imaging is essential not only for confirming the diagnosis but also for monitoring disease progression. In this case, the MRI findings of focal marrow signal changes and articular irregularities were consistent with PsA and highlighted the need for urgent intervention to prevent further joint destruction.11
The NICE guidelines1 recommend early referral to a rheumatologist as soon as PsA is suspected. Early diagnosis and treatment are critical to prevent permanent joint damage, reduce deformities, improve joint function, and enhance the patient’s quality of life (QoL). Addressing PsA early can also reduce the psychological impact of the disease, which is particularly important for patients like this one, whose PsA symptoms have interfered significantly with their daily life, including work and social activities.
To streamline care and ensure comprehensive management, our institution has established a combined dermatology-rheumatology clinic, where both dermatologists and rheumatologists assess patients simultaneously. This integrated approach allows for more efficient diagnosis and treatment, reducing referral waiting times and ensuring that patients receive timely care. This collaborative model has shown promising results, with studies indicating that combined clinics lead to earlier diagnoses and more appropriate treatment.12 Though challenges exist in terms of clinician numbers and logistical coordination, the benefits of such clinics in improving patient outcomes are clear. Our patient, for example, benefited from both reassurance and a structured management plan developed jointly by the dermatology and rheumatology teams.
Another significant issue in this case was the initial misdiagnosis by the patient’s GP, who prescribed two courses of antibiotics based on a suspected infection. This is a common pitfall in PsA, as dactylitis
can be confused with an infection or other inflammatory conditions. While infection-related dactylitis can occur due to conditions like syphilis, tuberculosis, or sickle cell disease, PsA remains one of the most frequent causes of dactylitis.13 This underscores the importance of thorough history taking, clinical assessment, and appropriate laboratory investigations to rule out differential diagnoses before initiating treatment.
There is a well-documented delay in diagnosing PsA, particularly in patients with psoriasis. Studies show that the diagnosis of PsA is often delayed, sometimes by several years, which can lead to irreversible joint damage.3 The clinical link between psoriasis and PsA is often not fully recognised, especially in cases where musculoskeletal symptoms are subtle or develop gradually. Therefore, education of primary and secondary care physicians is crucial to improving early recognition and referral. Tools such as the CASPAR and PEST criteria should be more widely utilised in clinical practice to facilitate the early identification of PsA.
The primary goals in treating PsA are to prevent structural joint damage, optimise functional status, and improve the patient’s QoL. The patient had only used topical steroids in the past and never started any systemic therapy. Following NICE guidelines, a conventional synthetic csDMARD would our first choice, so the patient was started on methotrexate. Methotrexate was chosen because it has a proven track record in treating PsA, and is generally well tolerated by patients. Alongside methotrexate, the patient received local corticosteroid injections to provide rapid symptom relief. This combination of therapies was effective in controlling disease progression without the need for biologic agents, at least initially.
According to the GRAPPA guidelines, early initiation of anti-TNFα, IL-12/23 inhibitors, and IL-17A inhibitors is recommended for managing dactylitis and enthesitis in PsA.14,15 These biologic agents are highly effective in controlling inflammation and preventing disease progression. Although the patient’s
psoriasis affects high-impact areas and her PASI score is below 10, she would meet the criteria for starting biologic treatment should methotrexate therapy fail.16 While biologics are typically reserved for patients who fail conventional treatments, the introduction of biosimilars has significantly reduced the cost of biologic therapies, making them a more accessible option for long-term management. This shift toward biosimilars helps alleviate the financial burden of treating chronic conditions like PsA, particularly within healthcare systems such as the NHS, which must balance treatment efficacy with cost-effectiveness.17
PsA is a distinct form of inflammatory arthritis closely associated with psoriasis, with dactylitis being a hallmark clinical feature. Early recognition and intervention are crucial to prevent destructive joint changes and improve patient quality of life. The NICE guidelines1 recommend early referral to a rheumatologist for patients with suspected PsA to prevent long-term joint damage.
This case highlights the importance of early diagnosis and the multidisciplinary approach to managing PsA, involving both dermatology and rheumatology teams. Additionally, psoriasis and PsA treatment strategies, including methotrexate and corticosteroid injections, effectively controlled the patient’s symptoms and prevented disease progression.
The patient’s positive response to methotrexate and local corticosteroid
injections eliminated the need for biologic therapies or long-term steroid use. However, biologic agents remain an important treatment option for patients who do not respond to conventional therapies, and they are considered the most effective treatment for PsA with dactylitis.
Lastly, routine psychological support and screening should be an integral part of managing patients with chronic inflammatory conditions like PsA, as mental health plays a crucial role in overall disease management.
References:
1. NICE. Psoriasis: assessment and management. NICE 2012 (updated 2017). Available at: https:// www.nice.org.uk/guidance/cg153 [Accessed November 2024]
2. Gibson LE. Spondyloarthropathy pathophysiology. WiKi Doc: The living textbook of medicine 2020. Available at: https://www. wikidoc.org/index.php/Spondyloarthropathy_ pathophysiology [Accessed November 2024]
3. Lebwoh M, Kavanaugh A, Armstrong A, Voorhees A. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. American journal of clinical dermatology 2016. 17(1): 87-9
4. Guy’s and St Thomas’ NHS foundation Trust 2020. Clinical Guideline: Use of methotrexate in adult patient in dermatology.
5. NICE. How should I assess a person with suspected psoriasis. NICE 2023. Available at: https://cks.nice.org.uk/topics/psoriasis/diagnosis/ assessment/ [Accessed November 2024]
6. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. CASPAR
Key learning points from the case study
1. Early diagnosis of PsA especially in patients with high-impact psoriasis, is essential to prevent joint damage.
2. Dactylitis (sausage digit) and enthesitis are hallmark features of PsA that can be diagnosed with MRI.
3. Methotrexate is the first-line treatment for moderate-to-severe PsA, with corticosteroid injections providing rapid symptom relief.
4. Multidisciplinary care, combining dermatology and rheumatology, ensures comprehensive management of PsA and psoriasis.
5. Psychological screening and management of anxiety and depression are crucial in improving the quality of life for patients with chronic inflammatory diseases.
6. Biologic therapies should be considered for patients who do not respond to conventional treatments for PsA.
Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheumatology 2006. 54(8):2665-2673
7. European Alliance of Associations for Rheumatology (EULAR). EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Annals of the Rheumatic Diseases 2020. 79(6):700-712
8. National Health Service (NHS). Psoriatic arthritis. NHS 2022. Available at: https://www. nhs.uk/conditions/psoriatic-arthritis/ [Accessed November 2024]
9. Yamamoto T. Optimal management of dactylitis in patient with psoriatic arthritis. Open Access Rheumatology: Research and Reviews 2015. 7:55-62
10. Mease P. Psoriatic arthritis: update on pathophysiology, assessment and management. Annals of the Rheumatic Diseases 2011. 70:77-84.
11. Kaeley G, Eder L, Aydin S, Gutierrez M, Bakewell C. Dactylitis: A hallmark of psoriatic arthritis. Seminar in Arthritis and Rheumatism 2018. 48(2):263-273
12. Forand R. Multiple aspect of psoriatic disease addressed with combined clinic model. Healio Psoriatic Disease 2021. Available at: https:// www.healio.com/news/dermatology/20210819/ multiple-aspects-of-psoriatic-disease-addressedwith-combined-clinic-model [Accessed November 2024]
13. Bagel J, Schwartzman S. Enthesitis and dactylitis in psoriatic disease: A guide for dermatologists. American Journal of Clinical Dermatology 2018. 19(6): 839-852
14. Coates L, Soriano E, Corp N, Bertheussen H, Duffin K, Campanholo C et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): update treatment recommendations for psoriatic arthritis 2021. Nature Reviews, Rheumatology. 2022. 18(8):465479
15. Gossec L, Baraliakos X, Kerschbaumer A, Wit M, Mclnnes I, Dougados M et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Annals of the Rheumatic Diseases 2020. 79(6):700-712
16. SEL IMOC (South East London Integrated Medicines Optimisation Committee) Treatment Pathway for Adults with Moderate to Severe Psoriasis 2024. Available at: https://www. selondonics.org/wp-content/uploads/dlm_ uploads/PSO-pathway-August-2024-FINAL.pdf [Accessed November 2024]
17. Cohen P, Blauvelt A, Rifkin M, Danese S, Gokhale B, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs 2018. 78(4):463-78.

“Working at the Centre for Appearance Research, we focus on the impact appearance plays in people’s lives”
Summary:
Robert Mair (RM): Hi Ella, to start, it would be great to get a little background, by way of an introduction, to you career and your interest in the psychological aspect of skin conditions.
Ella Guest (EG): I’m a health psychologist, and I’m also a senior research fellow, based at the Centre for Appearance Research, which is at the University of the West of England, in Bristol. We’re a research centre, and there’s about 30 of us now, so we’re growing in size.
I originally did a psychology degree – quite a while ago now – and was really interested in health psychology. Health psychology relates to managing the psychosocial aspects of illnesses, and also understanding how we can promote wellbeing and promote healthy behaviours and healthy choices.
For example, if you take dermatology, it’s thinking about how we can help people manage the psychological impact that comes along with having a dermatological condition, and also things like how we can make it easier for people to actually adhere to treatment and medication.
I did an MSc in health psychology. And then I started working at the Centre for Appearance Research, and then ended up doing a professional doctorate in health psychology, which is the standard route to become a health psychologist. This is a professional training doctorate. You do a piece of research and a
In this issue, we meet health psychologist and researcher Ella Guest, who is based at the Centre for Appearance Research at the University of the West of England. Ella’s work is to understand the psychological impact of skin diseases on people, as well as impact of stigma and discrimination.
Author info:
Ella Guest is a Registered Health Psychologist and Senior Research Fellow at the Centre for Appearance Research, University of the West of England. Rob Mair is the Managing Editor of Dermatological Nursing.
Keywords:
Dermatology, Psychology, Psychological burden, Stigma, Appearance
Citation:
Guest E, Mair R. Working to understand the psychological impact of skin disease. Dermatological Nursing 2024. 23(4):49-51.
thesis, but you also have to do other competencies to become registered as a health psychologist. Part of this involves teaching and training to do psychological interventions with patients.
RM: And how is this background and interest reflected in your current work?
EG: Working at the Centre for Appearance Research, we focus on the impact appearance plays in people’s lives. Within this, there are two main themes. One of them is body image, and developing interventions, preventing the development of negative body image or body dissatisfaction and promoting positive body image.
Then the other side, which is the area I’m more involved with –although there’s overlap – is what we call visible difference. So that’s any condition that affects how someone looks. So, skin conditions come under that umbrella, as do some of the chronic health conditions which affect or alter someone’s appearance.
We know that people with conditions that affect their appearance often, unfortunately, can experience stigma and discrimination from members of the general population. Particularly when thinking of skin conditions, there’s often this misconception about them being contagious. Sometimes people might ask inappropriate questions, we know that children can experience bullying and teasing. Then, from the research, we know how managing a chronic condition and having symptoms which affect your appearance can lead to things like depression and anxiety or self-esteem issues. In turn, things like social anxiety and depression can lead to social isolation.
So, my research, essentially, is looking at the impact of living with different conditions. I’ve looked at various skin conditions, including eczema, psoriasis,
vitiligo, and congenital melanocytic navi (CMN), and then looking at the impact of those diseases. This might be through qualitative research – so interviewing people to understand their lived experiences – but also through quantitative research, such as developing and evaluating interventions.
For example, we’ve just evaluated, using a randomised controlled trial, an intervention called Expand Your Horizon that’s based on writing and reflecting on what your body can do, rather than how it looks. This is called body functionality appreciation, which can be used to reinforce positive body image.1
“Literature shows us that if we really focus on how we look, which society teaches us to do, we start to evaluate ourselves and base our worth on our appearance”
Literature shows us that if we really focus on how we look, which society teaches us to do, we start to evaluate ourselves and base our worth on our appearance and compare ourselves to unrealistic appearance ideals (e.g., perfect skin). So, if we can get people to think more about what their body can do, rather than just how it looks, that can have a positive impact.
Then, finally, the other branch of research I’m interested in is around the societal side of appearance and the impact of stigma. This is about trying to understand how we can use
social media to educate the general population about different conditions and reduce stigma, and make people more accepting. It’s a really interesting area of research, but it’s quite new and rather difficult. We’re very much at the start of that journey.
RM: Was there any point that you realised this was the research path you wanted to follow?
EG: I think it was quite a natural journey. I was interested in doing health psychology and started doing an MSc, and during that time I had to do a placement. I’ve also always been interested in doing research, and was lucky to get a placement at the Centre for Appearance Research, which is where I’m based now.
I’m definitely learning much more about the area of dermatology and skin diseases, and I’m finding it really interesting. I think one of my areas of interest, which plays into this, is that there’s a very practical psychological aspect about managing a condition, but there’s also this intersection with appearance and the psychology that comes with that. Also, from a personal perspective, I’ve done quite a lot of research around eczema, which is something I’ve had my whole life.
RM: One thing I have found eye-opening, while editing the journal, is just how important understanding the psychological aspect of skin disease is. We see it in the patient voice articles every issue, and even if it’s not discussed so evidently, there’s always an element of ‘this is how this skin condition made me feel, and how feeling like this prevented me from doing something’. Like, it’s not the skin condition per se that’s the issue, but the psychological burden of it. And often, the severity of the disease itself is entirely disconnected from that psychological impact.
EG: Yeah, it’s an important point, and it’s something we see a lot as well. When people are referred for treatment and psychological support, and they take an assessment based on how severe – how noticeable and visible it is – there really isn’t any real correlation between that objective severity and how bothered somebody is by it. Often, people who have what you might perceive objectively to be quite minor, or not very visible conditions, may struggle more with their appearance.
“In some dermatological conditions, stress and anxiety can exacerbate them, which means we’re used to looking at things holistically”
One of the suggestions put forward for this is that when you have a condition that is very visible, you can’t really disguise it, so you have to come to terms with it. Whereas, people who conceal conditions, it can make it a bigger thing for them, particularly in situations where their skin is visible.
It is an interesting discussion though, and it shows how people perceive their conditions differently.
For professionals though, there is an important message here, and it’s that we can’t really guess at this sort of thing – we have to ask people about the impact.
RM: Does whether it’s a sudden change play into this? If people have been living with a condition
for a long time, are they better prepared to deal with it?
EG: Sometimes. I did a study a couple of years ago with some adolescent girls who have CMN, which is a birthmark condition. 2 In some cases, it covered quite a lot their body, but because they had been born with it, it was part of their identity. Some of the girls had embraced it – they’d grown up with it and saw that it was something that made them unique. If people develop conditions later on, it can be more difficult for them to come to terms with, because it comes down to that idea of how your appearance has changed or altered from what you were used to.
RM: One of the things I’ve been most struck with, in terms of working with professionals in dermatology, is how they’re really switched onto investigating this psychological aspect of the impact of skin disease. It often feels much more advanced compared to other chronic disease. Is that the case?
EG: I think so – definitely in terms of dermatology, there’s much more consideration given to the wider impact. I think one of the things to consider here is that, in some dermatological conditions, stress and anxiety can exacerbate them, which means we’re used to looking at things holistically. You especially see this when you think of dermatology specialists – certainly more so than if you just went to a GP, where it might not be so recognised.
I think, broadly, how we respond to people is important here too. It can take some time to get a diagnosis, and often there’s a focus on treating the physical symptoms, rather than looking at that wider, holistic picture.
In fact, I’m thinking of some research I did on eczema with young people. Some of them were worried about how they looked, but responses like
‘It will probably go away’ and ‘You’ll probably grow out of it’ were really unhelpful. People didn’t want their experiences minimised. Whereas, if that response was sensitive and more considered, there was a greater satisfaction. So it’s really important that psychoeducation is integrated.
It’s also worth saying that Eczema Outreach Support have created some care plans for eczema which look at both the physical and psychosocial aspects of the condition. These are available at: https://eos.org.uk/healthcare-plans/.
RM: I guess the danger with that ‘It will go away’ response – which tries to minimise or trivialise the condition – if it doesn’t go away, I imagine that risks doing some damage to that person’s trust?
EG: Well, exactly – you simply don’t know if someone will grow out of it. Lots of people do, but lots of people continue to have eczema into adulthood.
But also, someone wouldn’t bring this up in a consultation unless it was a big concern for them. Especially for teenagers, where this is such a big time for them –friendships, romantic relationships and so on – it’s really important. We know that having these kinds of concerns can lead to things like low self-esteem, and it can also affect how they engage in school. So, there can be a bigger impact longer term.
References
1. Guest E, Halliwell E, Mathews A, Alleva JM, Harcourt D. More than my Appearance: a pilot evaluation of the Expand Your Horizon online functionality-based writing programme for adults with visible differences. Health Psychology and Behavioral Medicine 2024. 12(1):2349004
2. Guest E, Williamson H, Harcourt D. Congenital melanocytic naevus (CMN) through the lens: Using photo-elicitation interviews to explore adjustment in adolescents with a rare birthmark condition. Body Image 2024. 48:101656.
For your eligible patients with moderate to severe plaque psoriasis (PsO) and concomitant psoriatic arthritis (PsA)1,2

+1 million patients treated globally across indications and counting*3
Cosentyx has a consistent safety profile with over 8 years’ experience across indications1,2 The most frequently reported adverse reactions are URTIs (17.1%) (most frequently nasopharyngitis, rhinitis)1,2
Therapeutic indications
Cosentyx®️ (secukinumab) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy, and for the treatment of active psoriatic arthritis in adult patients, alone or in combination with methotrexate, when the response to previous DMARD therapy has been inadequate. Please refer to the Summary of Product Characteristics for the complete list of therapeutic indications.1,2
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated.
This promotional material has been created and funded by Novartis for UK healthcare professionals only. Please see SmPC for full product information before prescribing. The images do not depict real patients. For illustrative purposes only. Prescribing Information can be found on the next page.
*Refers to people who have been prescribed Cosentyx for any indication since launch. This is an estimated number at December 2022.3
†GOES FURTHER = FUTURE 5 was a randomised, double blind, placebo-controlled, multicentre, 2-year, phase 3 study in patients with PsA (N=996). Subjects were stratified by prior anti-TNF use (naïve or inadequate response), and randomised to receive Cosentyx 300 mg, 150 mg or placebo. At 104 weeks, the proportion of patients with no radiographic progression (change from baseline in vdH-mTSS ≤0.5) receiving Cosentyx 300 mg (n=222) was 89.5%.4
‡SKIN CLEARANCE = STEPIn was a randomised, open-label, multicentre study in adults with new onset (≤12 months) moderate to severe PsO (N=160). Subjects were randomised to receive Cosentyx 300 mg or nb-UVB phototherapy for 52 weeks. The primary endpoint was to demonstrate that early treatment with secukinumab 300 mg is superior to nb-UVB in patients with respect to the proportion of patients achieving a ≥90% improvement in PASI (PASI 90 response) at Week 52, which was met (PASI 90 response: 91.1% [70/77] vs 42.3% [32/76] for Cosentyx 300 mg vs nb-UVB phototherapy, respectively; p<0.0001). PASI 100, an exploratory endpoint, was achieved in 68.8% of patients receiving Cosentyx 300 mg vs 22.4% (p<0.0001) of patients receiving nb-UVB phototherapy at Week 52.5 DMARD, disease-modifying anti-rheumatic drugs; nb-UVB, narrow-band ultraviolet B; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, plaque psoriasis; TNF, tumour necrosis factor; URTI, upper respiratory tract infection; vdH-mTSS, van der Heijde-modified total Sharp score.
References: 1. Cosentyx (secukinumab) GB Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/11973/smpc (Accessed November 2023); 2. Cosentyx (secukinumab) NI Summary of Product Characteristics. Available at: https://www.emcmedicines.com/en-gb/northernireland/medicine?id=627fbcd7-1ca5-458c-9841-9103cb457911&type=smpc (Accessed November 2023); 3. Novartis Data on File. Secukinumab (Sec008). February 2023; 4. Mease PJ et al. RMD Open 2021;7(2):e001600; 5. Iversen L et al. J Eur Acad Dermatol Venereol 2023;37(5):1004–1016.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis via uk.patientsafety@novartis.com or online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report. If you have a question about the product, please contact Medical Information on 01276 698370 or by email at medinfo.uk@novartis.com.
Cosentyx® (secukinumab) Great Britain Prescribing Information.
Please refer to the Summary of Product Characteristics (SmPC) before prescribing.
Indications: Treatment of: moderate to severe plaque psoriasis in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis in adults (alone or in combination with methotrexate) who have responded inadequately to disease-modifying anti-rheumatic drug therapy; active ankylosing spondylitis in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI) evidence in adults who have responded inadequately to non-steroidal antiinflammatory drugs; active enthesitis-related arthritis and juvenile psoriatic arthritis in patients 6 years and older (alone or in combination with methotrexate) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active moderate to severe hidradenitis suppurativa (acne inversa) in adults with an inadequate response to conventional systemic HS therapy. Presentations: Cosentyx 75 mg solution for injection in pre-filled syringe; Cosentyx 150 mg solution for injection in pre-filled syringe; Cosentyx 150 mg solution for injection in pre-filled pen; Cosentyx 300 mg solution for injection in prefilled pen. Dosage & Administration: Administered by subcutaneous injection at weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Consider discontinuation if no response after 16 weeks of treatment. Each 75 mg dose is given as one injection of 75 mg. Each 150 mg dose is given as one injection of 150 mg. Each 300 mg dose is given as two injections of 150 mg or one injection of 300 mg. If possible avoid areas of the skin showing psoriasis. Plaque Psoriasis: Adult recommended dose is 300 mg. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher. Adolescents and children from the age of 6 years: if weight ≥ 50 kg, recommended dose is 150 mg (may be increased to 300 mg as some patients may derive additional benefit from the higher dose). If weight < 50 kg, recommended dose is 75 mg. Psoriatic Arthritis: For patients with concomitant moderate to severe plaque psoriasis see adult plaque psoriasis recommendation. For patients who are anti-TNFα inadequate responders, the recommended dose is 300 mg, 150 mg in other patients. Can be increased to 300 mg based on clinical response. Ankylosing Spondylitis: Recommended dose 150 mg. Can be increased to 300 mg based on clinical response. nr-axSpA: Recommended dose 150 mg. Enthesitis-related arthritis and juvenile psoriatic arthritis: From the age of 6 years, if weight ≥ 50 kg, recommended dose is 150 mg. If weight < 50 kg, recommended dose is 75 mg. Hidradenitis suppurativa: Recommended dose is 300 mg monthly. Based on clinical response, the
Cosentyx® (secukinumab) Northern Ireland Prescribing Information.
Please refer to the Summary of Product Characteristics (SmPC) before prescribing.
Indications: Treatment of: moderate to severe plaque psoriasis in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis in adults (alone or in combination with methotrexate) who have responded inadequately to disease-modifying anti-rheumatic drug therapy; active ankylosing spondylitis in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI) evidence in adults who have responded inadequately to non-steroidal antiinflammatory drugs; active enthesitis-related arthritis and juvenile psoriatic arthritis in patients 6 years and older (alone or in combination with methotrexate) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active moderate to severe hidradenitis suppurativa (acne inversa) in adults with an inadequate response to conventional systemic HS therapy. Presentations: Cosentyx 150 mg solution for injection in pre-filled pen; Cosentyx 300 mg solution for injection in pre-filled pen. Dosage & Administration: Administered by subcutaneous injection at weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Consider discontinuation if no response after 16 weeks of treatment. Each 150 mg dose is given as one injection of 150 mg. Each 300 mg dose is given as two injections of 150 mg or one injection of 300 mg. If possible avoid areas of the skin showing psoriasis. Plaque Psoriasis: Adult recommended dose is 300 mg monthly. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher. Adolescents and children from the age of 6 years: if weight ≥ 50 kg, recommended dose is 150 mg (may be increased to 300 mg as some patients may derive additional benefit from the higher dose). If weight < 50 kg, recommended dose is 75 mg. However, 150mg solution for injection in pre-filled pen is not indicated for administration of this dose and no suitable alternative formulation is available. Psoriatic Arthritis: For patients with concomitant moderate to severe plaque psoriasis see adult plaque psoriasis recommendation. For patients who are anti-TNFα inadequate responders, the recommended dose is 300 mg, 150 mg in other patients. Can be increased to 300 mg based on clinical response. Ankylosing Spondylitis: Recommended dose 150 mg. Can be increased to 300 mg based on clinical response. nraxSpA: Recommended dose 150 mg. Enthesitis-related arthritis and juvenile psoriatic arthritis: From the age of 6 years, if weight ≥ 50 kg, recommended dose is 150 mg. If weight < 50 kg, recommended dose is 75 mg. However, 150mg solution for injection in pre-filled pen is not
maintenance dose can be increased to 300 mg every 2 weeks. Contraindications: Hypersensitivity to the active substance or excipients. Clinically important, active infection. Warnings & Precautions: Infections: Potential to increase risk of infections; serious infections have been observed. Caution in patients with chronic infection or history of recurrent infection. Advise patients to seek medical advice if signs/symptoms of infection occur. Monitor patients with serious infection closely and do not administer Cosentyx until the infection resolves. Non-serious mucocutaneous candida infections were more frequently reported for secukinumab in the psoriasis clinical studies. Should not be given to patients with active tuberculosis (TB). Consider anti-tuberculosis therapy before starting Cosentyx in patients with latent TB. Inflammatory bowel disease (including Crohn’s disease and ulcerative colitis): New cases or exacerbations of inflammatory bowel disease have been reported with secukinumab. Secukinumab, is not recommended in patients with inflammatory bowel disease. If a patient develops signs and symptoms of inflammatory bowel disease or experiences an exacerbation of pre-existing inflammatory bowel disease, secukinumab should be discontinued and appropriate medical management should be initiated. Hypersensitivity reactions: Rare cases of anaphylactic reactions have been observed. If an anaphylactic or serious allergic reactions occur, discontinue immediately and initiate appropriate therapy. Vaccinations: Do not give live vaccines concurrently with Cosentyx; inactivated or nonlive vaccinations may be given. Paediatric patients should receive all age appropriate immunisations before treatment with Cosentyx. LatexSensitive Individuals: The removable needle cap of the 75mg and 150 mg pre-filled syringe and 150mg pre-filled pen contains a derivative of natural rubber latex. Concomitant immunosuppressive therapy: Combination with immunosuppressants, including biologics, or phototherapy has not been evaluated in psoriasis studies. Cosentyx was given concomitantly with methotrexate, sulfasalazine and/or corticosteroids in arthritis studies. Caution when considering concomitant use of other immunosuppressants. Interactions: Live vaccines should not be given concurrently with secukinumab. No interaction between Cosentyx and midazolam (CYP3A4 substrate) seen in adult psoriasis study. No interaction between Cosentyx and methotrexate and/or corticosteroids seen in arthritis studies. Fertility, pregnancy and lactation: Women of childbearing potential: Use an effective method of contraception during and for at least 20 weeks after treatment. Pregnancy: Preferably avoid use of Cosentyx in pregnancy. Breast feeding: It is not known if secukinumab is excreted in human breast milk. A clinical decision should be made on continuation of breast feeding during Cosentyx treatment (and up to 20 weeks after discontinuation) based on benefit of breast feeding to the child and benefit of Cosentyx therapy to the woman. Fertility: Effect on human fertility not evaluated. Adverse Reactions: Very Common (≥1/10): Upper respiratory tract infection. Common (≥1/100 to <1/10): Oral herpes, headache, rhinorrhoea, diarrhoea, nausea, fatigue. Uncommon
indicated for administration of this dose and no suitable alternative formulation is available. Hidradenitis suppurativa: Recommended dose is 300 mg monthly. Based on clinical response, the maintenance dose can be increased to 300 mg every 2 weeks. Contraindications: Hypersensitivity to the active substance or excipients. Clinically important, active infection. Warnings & Precautions: Infections: Potential to increase risk of infections; serious infections have been observed. Caution in patients with chronic infection or history of recurrent infection. Advise patients to seek medical advice if signs/symptoms of infection occur. Monitor patients with serious infection closely and do not administer Cosentyx until the infection resolves. Non-serious mucocutaneous candida infections were more frequently reported for secukinumab than placebo in the psoriasis clinical studies. Should not be given to patients with active tuberculosis (TB). Consider anti-tuberculosis therapy before starting Cosentyx in patients with latent TB. Inflammatory bowel disease (including Crohn’s disease and ulcerative colitis): New cases or exacerbations of inflammatory bowel disease have been reported with secukinumab. Secukinumab, is not recommended in patients with inflammatory bowel disease. If a patient develops signs and symptoms of inflammatory bowel disease or experiences an exacerbation of pre-existing inflammatory bowel disease, secukinumab should be discontinued and appropriate medical management should be initiated. Hypersensitivity reactions: Rare cases of anaphylactic reactions have been observed. If an anaphylactic or serious allergic reactions occur, discontinue immediately and initiate appropriate therapy. Vaccinations: Do not give live vaccines concurrently with Cosentyx; inactivated or nonlive vaccinations may be given. Paediatric patients should receive all age appropriate immunisations before treatment with Cosentyx. LatexSensitive Individuals: The removable needle cap of the 150mg pre-filled pen contains a derivative of natural rubber latex. Concomitant immunosuppressive therapy: Combination with immunosuppressants, including biologics, or phototherapy has not been evaluated in psoriasis studies. Cosentyx was given concomitantly with methotrexate, sulfasalazine and/or corticosteroids in arthritis studies. Caution when considering concomitant use of other immunosuppressants. Interactions: Live vaccines should not be given concurrently with secukinumab. No interaction between Cosentyx and midazolam (CYP3A4 substrate) seen in adult psoriasis study. No interaction between Cosentyx and methotrexate and/or corticosteroids seen in arthritis studies. Fertility, pregnancy and lactation: Women of childbearing potential: Use an effective method of contraception during and for at least 20 weeks after treatment. Pregnancy: Preferably avoid use of Cosentyx in pregnancy. Breast feeding: It is not known if secukinumab is excreted in human breast milk. A clinical decision should be made on continuation of breast feeding during Cosentyx treatment (and up to 20 weeks after discontinuation) based on benefit of breast feeding to the child and benefit of Cosentyx therapy to the woman. Fertility: Effect on human fertility not evaluated. Adverse Reactions: Very Common (≥1/10): Upper
(≥1/1,000 to <1/100): Oral candidiasis, lower respiratory tract infections, neutropenia, inflammatory bowel disease. Rare (≥1/10,000 to <1/1,000): anaphylactic reactions, exfoliative dermatitis (psoriasis patients), hypersensitivity vasculitis. Not known: Mucosal and cutaneous candidiasis (including oesophageal candidiasis). Infections: Most infections were non-serious and mild to moderate upper respiratory tract infections, e.g. nasopharyngitis, and did not necessitate treatment discontinuation. There was an increase in mucosal and cutaneous (including oesophageal) candidiasis, but cases were mild or moderate in severity, non-serious, responsive to standard treatment and did not necessitate treatment discontinuation. Serious infections occurred in a small proportion of patients (0.015 serious infections reported per patient year of follow up). Neutropenia: Neutropenia was more frequent with secukinumab than placebo, but most cases were mild, transient and reversible. Rare cases of neutropenia CTCAE Grade 4 were reported. Hypersensitivity reactions: Urticaria and rare cases of anaphylactic reactions were seen. Immunogenicity: Less than 1% of patients treated with Cosentyx developed antibodies to secukinumab up to 52 weeks of treatment. Other Adverse Effects: The list of adverse events is not exhaustive, please consult the SmPC for a detailed listing of all adverse events before prescribing. Legal Category: POM. MA Number & List Price: PLGB 00101/1205 – 75 mg pre-filled syringe x 1 - £304.70; PLGB 00101/1029 - 150 mg pre-filled pen x2 £1,218.78; PLGB 00101/1030 - 150 mg pre-filled syringe x2 £1,218.78; PLGB 00101/1198 – 300 mg pre-filled pen x 1 £1218.78. PI Last Revised: June 2023. Full prescribing information, (SmPC) is available from: Novartis Pharmaceuticals UK Limited, 2nd Floor, The WestWorks Building, White City Place, 195 Wood Lane, London, W12 7FQ. Telephone: (01276) 692255. UK | 290802 | June 2023
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis via uk.patientsafety@novartis.com or online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report
If you have a question about the product, please contact Medical Information on 01276 698370 or by email at medinfo.uk@novartis.com
respiratory tract infection. Common (≥1/100 to <1/10): Oral herpes, headache, rhinorrhoea, diarrhoea, nausea, fatigue. Uncommon (>1/1,000 to <1/100): Oral candidiasis, lower respiratory tract infections, neutropenia, inflammatory bowel disease. Rare (≥1/10,000 to <1/1,000): anaphylactic reactions, exfoliative dermatitis (psoriasis patients), hypersensitivity vasculitis. Not known: Mucosal and cutaneous candidiasis (including oesophageal candidiasis). Infections: Most infections were non-serious and mild to moderate upper respiratory tract infections, e.g. nasopharyngitis, and did not necessitate treatment discontinuation. There was an increase in mucosal and cutaneous (including oesophageal) candidiasis, but cases were mild or moderate in severity, non-serious, responsive to standard treatment and did not necessitate treatment discontinuation. Serious infections occurred in a small proportion of patients (0.015 serious infections reported per patient year of follow up). Neutropenia: Neutropenia was more frequent with secukinumab than placebo, but most cases were mild, transient and reversible. Rare cases of neutropenia CTCAE Grade 4 were reported. Hypersensitivity reactions: Urticaria and rare cases of anaphylactic reactions were seen. Immunogenicity: Less than 1% of patients treated with Cosentyx developed antibodies to secukinumab up to 52 weeks of treatment. Other Adverse Effects: The list of adverse events is not exhaustive, please consult the SmPC for a detailed listing of all adverse events before prescribing. Legal Category: POM. MA Number & List
Price: EU/1/14/980/005 - 150 mg pre-filled pen x2 £1,218.78; EU/1/14/980/010 – 300 mg pre-filled pen x 1 £1218.78. PI Last Revised: May 2023. Full prescribing information, (SmPC) is available from: Novartis Pharmaceuticals UK Limited, 2nd Floor, The WestWorks Building, White City Place, 195 Wood Lane, London, W12 7FQ. Telephone: (01276) 692255. UK | 284832 | May 2023
Adverse Event Reporting:
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis via uk.patientsafety@novartis.com or online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report
If you have a question about the product, please contact Medical Information on 01276 698370 or by email at medinfo.uk@novartis.com
Rod Tucker, Emma Button

Emma Button
Emma didn’t set out to be a dermatology nurse. Her first role was in Accident and Emergency (A&E), but after 18 months she wanted to find a more specialist area of focus. Having always been interested in public health, Emma took a degree in the subject, but decided to train as a health visitor. Initially, she found the job to be rewarding.
“I very much enjoyed this role when we had a Labour government because the narrative was more to prevent rather than deal with things afterwards,” she said.
Remaining unfulfilled, Emma did a stint of nursing in Australia. When she returned home, she opted to work back in emergency care. Within this role, she found dermatology an area of practice which was particularly challenging.
“A patient [with a skin problem] would come in and you would deliberate over a management plan, concerned you were missing something acute, because you never had enough education on how to deal with these conditions,” she said.
This lack of understanding was a key driver in her decision to become more familiar with dermatology. She joined Kingston Hospital’s dermatology department in December 2020 and has remained there ever since.
Summary:
Having recently completed a three-year Master’s degree in advanced clinical practice, Emma Button spoke with Rod Tucker about the course and how it has helped in her role as a lead clinical specialist for inflammatory dermatology at Kingston Hospital.
Citation:
Tucker R, Button E. The advantages of taking a Master’s degree in advanced clinical practice. Dermatological Nursing 2024. 23(4):54-55.
For Emma, advanced practice means “being able to start an episode of care and cohesively look after someone autonomously and finish that care.”
An advanced practitioner works at an enhanced level of autonomy with the capacity for decision-making. Emma says her Master’s degree made her aware that clinical acumen is just one aspect of advanced practice. Nurses working at this level are required to have a good understanding of the other three key pillars: leadership, research and education. Just how much time is allocated to each of these pillars varies considerably.
“In some job roles like A&E, if you did advanced practice, probably 90% of your pillars would be clinically focused and only a small percentage of your time allocated to leadership, research or team education,” she explains.
However, this is not necessarily the same for other specialities where a greater focus on research may be needed due to the faster rate of change in clinical practice.
Generally, the advanced practice course is not designed to equip nurses with a better grounding in their chosen speciality, but there are anomalies. For example, the Royal College of Emergency Physicians (RCEP) has mandated a national portfolio of the educational and skillset requirements for nurses and allied healthcare professionals that are required in addition to doing the Master’s
Keywords:
Advanced clinical practice, Advanced practitioner, Inflammatory dermatology, Career pathways
Author info:
Emma Button is the lead clinical specialist for inflammatory dermatology at Kingston Hospital as well as the events educational lead for the British Dermatology Nursing Group (BDNG). Rod Tucker is a pharmacist with a special interest in dermatology, and a member of the Dermatological Nursing Editorial Board.
programme. Unfortunately, such a mandate is yet to be widely accepted by other speciality organisations, particularly where advanced practice is relatively new.
“Having spoken to NHS England and an advancing practice lead, they have made it very clear that any such mandate remains the responsibility of the individual trusts,” Emma said.
The BDNG is in the process of creating a guidance piece to help nurses, but this will not be prescriptive, as Emma explains: “This wouldn’t state that you have to have seen a particular number of skin conditions, because, in reality, there appears to be considerable variation in practice across trusts as to the roles nurses carry out.”
As an example, Emma cites how there is currently substantial variation in what is expected of nurses within dermatology departments across the UK. Moreover, she is also aware that the clinical expectation of a junior CNS and a senior CNS can be similar and there are even recognised disparities in banding and responsibilities.
The clinical role of an advanced practitioner
Emma believes that although the growth of advanced nurse practitioners has arisen partly in response to a shortage of doctors, in practice, the advanced nurse practitioner role is more complementary. For instance, following an initial diagnosis from a doctor, the advanced nurse practitioner maintains responsibility for the ongoing care and monitoring of patients. Emma has found that at Kingston, this approach works well, but it does mean that her clinical role is very demanding.
She describes how clinics can be very busy and how she has “a mixture of biologic and systemic patients experiencing disease flares contacting the department and who need their treatment switching.”
Once clerked in by a dermatologist, Emma is often the first, consistent, clinician for patients with inflammatory conditions, such as atopic eczema. While
some need to be started on oral or light therapy, she feels that many simply benefit from more treatment education.
Ideally, Emma thinks that it would be possible to reduce many of these inappropriate referrals, particularly in children, by “supporting an individual with a good, manageable, realistic regime, and then, if they fail, have them referred onwards quickly. You don’t delay it and leave them to have five years or so because it affects their sleep, nutrition, mental health and even their ability to learn. Such a delay would risk the child’s development and ability to reach normal milestones.”
Fortunately, at Kingston, Emma has some degree of flexibility with the management of patients. This can involve referral for psychological input, habit reversal or nutritional support.
Emma is a firm believer in the importance of understanding patients holistically and she often finds herself simply listening to patients, which they generally appreciate.
“The rapport takes a while to build up, but once it comes through, you can start to see and understand the bigger picture,” she explains.
An advanced nurse practitioner’s role extends far beyond clinical work. In fact, Emma has come to realise how leadership is an essential part of her role. This requires an understanding of the differences between leadership and effective people-management skills, something she initially found to be one of the more challenging aspects of her role.
Her leadership role is all embracing. It ranges from logistical matters such as staff rostering, supervision, dealing with long-term sickness, service planning and delivery, through to clinical governance, caseload management and occasionally the provision of additional clinics.
She also contends with the added pressures facing the department to ensure that it remains on track to achieve its cancer targets. This, she says, can involve a lot of staff juggling and
organising additional clinics. One area where Emma has taken the lead is the development of policies for nurse-led clinics in response to a project she carried out as part of her MSc called ‘Work-Based Learning’. Here, research indicates that nurses who either felt pressured to or had worked outside their scope of practice had experienced unwanted scenarios. This means a need to develop policies which contribute to clinical governance and which have the ability to mitigate risk. To support nurses working within scope of practice, she feels role descriptors, such as those developed by the BDNG, help other staff (such as doctors) to better understand the roles and limitations of nursing staff within the department.
Emma says that training to become an advanced practitioner offers nurses an alternative career pathway, rather than solely focusing on becoming a nurse consultant. She says the course she undertook provided her with the skills to think more creatively about ways of working, especially improving service delivery. This has included the development of a remote monitoring service for those who are prescribed isotretinoin.
Emma’s advice to nurses wishing to undertake the advance practitioner course is to first build their skillset in all aspects of dermatological care within the department, including shadowing more experienced colleagues wherever possible. She also recommends joining the BDNG which has a wide range of dermatology educational material to help equip those new to dermatology with the basics.
Finally, Emma adds a note of caution for nursing colleagues wishing to enrol on the advanced practice course. While it can be professionally rewarding, she says the challenge for those interested is to work closely with the management team to develop a strong business case that demonstrates tangible benefits to the department in terms of improvements to service delivery and more importantly, that it will provide value for money.








Recommend Nystaform HC Cream – now available and ready to prescribe
Reference: 1. Perspectus Global; omnibus survey of 1,000 adults; July 2023. Job code: 1/NYS/12/2404/970. Date of preparation: April 2024. Abridged Prescribing Information Nystaform HC Cream. See Nystaform HC Cream Summary of Product Characteristics (SmPC) prior to prescribing. Presentation: Cream containing nystatin (100,000 I.U./g), chlorhexidine hydrochloride (1.0% w/w) and hydrocortisone (0.5% w/w) for topical administration. Indications: Treatment of infected dermatoses where fungal (particularly monilial) and/or bacterial infections are present. Posology and Method of Administration: Adults and children: For topical application only. Apply to the infected area 2-3 times daily. Treatment should be for a maximum of 7 days. If the condition does not improve within seven days, return to doctor. Contraindications: Known sensitivity to the active substances, especially those with a history of chlorhexidine-related allergic reactions. Tuberculous lesions of the skin. Special Warnings and Precautions for Use: For external use only. Avoid contact with the eyes. If sensitivity occurs or if new infection appears, discontinue use and institute alternative therapy. Cetostearyl alcohol may cause local skin reactions (e.g. contact dermatitis). Chlorhexidine is known to induce hypersensitivity, including generalised allergic reactions and anaphylactic shock. Should not be administered to anyone with a potential history of an allergic reaction to a chlorhexidine-containing compound. In infants, long-term continuous topical steroid therapy should be avoided. Adrenal suppression can occur when extensive areas are treated, particularly under occlusion. Long term continuous or inappropriate use of topical steroids can result in the development of rebound flares after stopping treatment (topical steroid withdrawal syndrome). A severe form of rebound flare can develop which takes the form of a dermatitis with intense redness, stinging and burning that can spread beyond the initial treatment area. It is more likely to occur when delicate skin sites such as the face and flexures are treated. Should there be a reoccurrence of the condition within days to weeks after successful treatment a withdrawal reaction should be suspected. Reapplication should be with caution and specialist advise is recommended in these cases or other treatment options should be considered. Pregnancy and Lactation: Nystatin and corticosteroids should be administered with caution during the early months of pregnancy and its use requires that the anticipated benefits outweigh the possible risks. Undesirable E ects: Skin disorders: Allergic skin reactions such as dermatitis,

Rod Tucker, a pharmacist with a special interest in dermatology, answers more questions submitted by DN readers. If you have a burning question that you’d like answered in a future issue, please email it to susan. maguire@bdng.org.uk entitling your email: Ask the Pharmacist.
Antibiotics can give rise to serious cutaneous reactions, and while rare, these can be life-threatening. But which particular classes of antibiotics are more likely to cause such reactions and how great is the risk?
According to the World Health Organization, an adverse drug reaction (ADR) represents ‘a response to a medicine which is noxious and unintended, and which occurs at doses normally used in man’.1 Cutaneous ADRs are fairly common, with one estimate suggesting that between 30% and 45% of all ADRs affect the skin.2 Clinically, skinrelated ADRs can present in several different ways. The most common type is an exanthematous or maculopapular eruption, which is often referred to as the typical ‘drug rash’, and arises due to a T-cell mediated type IV hypersensitivity reaction.3 Maculopapular eruptions consist of erythematous macules or papules with a symmetrical distribution and invariably develop anywhere between four and 14 days after commencing the medicine. Other forms of cutaneous ADRs include an urticarial
rash (the second most common reaction); photosensitivity; vasculitis and a serum sickness-like reaction.4
Luckily, in the majority of cases, once the causative medicine has been stopped, the cutaneous reaction will slowly disappear.5
“Skin related ADRs are regularly caused by a small range of drugs including antibiotics, nonsteroidal antiinflammatories and anti-convulsant agents”
In theory, any medicine has the potential to cause a cutaneous ADR; in practice, skin-related ADRs are regularly caused by a small range of drugs including antibiotics, nonsteroidal anti-inflammatories and anticonvulsant agents. The most frequently responsible class of drugs are antibiotics, as illustrated in a 2019 Indian study. Researchers collected data on the types of cutaneous ADRs reported between 2013 and 2016. From a total of 2,171 ADRs, 24.78% affected the skin, most commonly a maculopapular eruption (58.92%), with antimicrobials responsible for nearly half (46%) of cases.6 In a more recent but similar analysis of 125 cutaneous ADRs, antimicrobials were responsible for almost two-thirds
(63.2%) of adverse reactions, with a maculopapular rash again being the most common clinical presentation (33.6%).7 But some antibiotic-related adverse skinrelated reactions prompt an emergency department (ED) visit. In one US study, for instance, of 142,505 ED visits, 19.3% were due to antibiotics and of these visits, 78.7% were for an allergic reaction.8
The most serious and potentially life-threatening cutaneous ADRs are Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Others include a drug rash with eosinophilia and systemic symptoms (DRESS) and acute generalised exanthematous pustulosis. Both SJS and TEN are variants of the same condition, and the definition depends on the extent of skin detachment. For instance, SJS is defined by a less than 10% skin detachment, whereas TEN involves a detachment exceeding 30%. Medication is thought to be the cause in over 80% of cases.9 Typically, SJS and TEN occur within four to 28 days following drug exposure, and both give rise to painful lesions on the skin followed by the development of haemorrhagic erosions of mucous membranes in around 95% of cases.5 Acute stage mortality is around 10% for cases of SJS and up to 40% for TEN, although the overall in-hospital European mortality for both is 22%.10
Which antibiotics are most likely to cause serious cutaneous ADRs?
Several analyses have strongly implicated antibiotics as the causative agents in the development of serious cutaneous ADRs. In one retrospective analysis of electronic medical records, from a total of 596 cases of a drug rash, 35 were
deemed to be serious including SJS, TEN and DRESS, with antibiotics identified as being responsible in 84.2% of SJS and TEN cases.11 A second retrospective review of 74 cases specifically focusing on serious cutaneous ADRs once again identified antibiotics as the most common culprit.12 Finally, an in-patient study of 377 adults identified that nearly 90% of SJS and TEN cases were due to oral antibiotics.13
“Both SJS and TEN are variants of the same condition, and the definition depends on the extent of skin detachment”
But while it is clear that antibiotics can be responsible for severe cutaneous ADRs, what remains uncertain is the level of risk posed by the different antibiotic classes. Fortunately, a recent study in JAMA has shed light on which types of antibiotics are more liable to give rise to serious cutaneous ADRs.14 This Canadian study focused on elderly adults (66 years and older) who received at least one antibiotic prescription between 2002 and 2022. Researchers focused on those who required an ED visit for a serious cutaneous ADR (including SJS and TEN) within 60 days of being prescribed their antibiotic. The risk for different classes of antibiotics were compared against macrolides, because this group has been
shown to give rise to the lowest risk of serious cutaneous ADRs.15 Over a 20year period, 21,758 adults with a median age of 75 years visited an ED or were hospitalised for a serious cutaneous ADR. The results showed that the risk of a serious cutaneous ADR (based on the adjusted odds ratio) was 2.9 for sulfonamides, 2.6 for cephalosporins and 2.2 for nitrofurantoin. This was followed by penicillins (odds ratio = 1.4) and fluoroquinolones (odds ratio = 1.3). The researchers calculated that the rate of causing a serious ADR for cephalosporins was 4.92 per 1,000 prescriptions and 3.22 for sulfonamides.
The JAMA paper serves as an important reminder that commonly used antibiotics can result in serious cutaneous ADRs –but these findings do need to be seen in context. The authors calculate that among older adults, for every 1,000 antibiotic prescriptions, there would be at least two visits to an ED because of a cutaneous ADR. Whilst this figure might seem low, nurses still need to be aware and remain vigilant of this potential risk, not just with sulfonamides and cephalosporins but for most antibiotics.
References
1. World Health Organization. Safety of Medicines: A guide to detecting and reporting adverse drug reactions. 2002. Available at: https://www.who.int/publications/i/item/WHOEDM-QSM-2002-2 [Accessed October 2024]
2. Das S, De, A. Recent Advances in Severe Cutaneous Adverse Drug Reaction. Indian J Dermatol 2018. 63:16-17
3. Lee AY. Immunological Mechanisms in Cutaneous Adverse Drug Reactions. Biomol Ther (Seoul) 2024. 32(1):1-12
4. Valeyrie-A Allanore L, Sassolas, B, Rouheau JC. Drug-Induced Skin, Nail and Hair Disorders. Drug Safety 2007. 30:1011-1030


5. Marzano AV, Borghi A, Cugno M. Adverse drug reactions and organ damage: The skin. Eur J Intern Med 2016. 28:17-24
6. Modi A, Desai M, Shah, S et al. Analysis of Cutaneous Adverse Drug Reactions Reported at the Regional ADR Monitoring Center. In J Dermatol 2019. 64:250-261
7. Chindhalore CA, Gupta AV, Dakhale GN, Srivastava A. Analysis of Cutaneous Adverse Drug Reactions (ADR) Reported at an ADR Monitoring Center of a Tertiary Care Teaching Institute in Central India. Cureus 2024. 16(2):e53706
8. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibioticassociated adverse events. Clin Infect Dis 2008. 47(6):735-743
9. Oakley AM KK. Stevens-Johnson Syndrome. StatPearls Publishing 2023. Available at: https:// www.ncbi.nlm.nih.gov/books/NBK459323/ [Accessed October 2024]
10. Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet 2017. 390(10106):1996-2011
11. Zhang C, Van DN, Hieu C, Craig T. Drug-induced severe cutaneous adverse reactions: Determine the cause and prevention. Ann Allergy Asthma Immunol 2019. 123(5):483487
12. Lin YF,Yang CH, Sindy H, Lin JY, Rosaline Hui CY, Tsai YC, et al. Severe cutaneous adverse reactions related to systemic antibiotics. Clin Infect Dis 2014. 58(10):1377-1385
13. Micheletti RG, Chiesa-Fuxench Z, Noe MH, Stephen S, Aleshin M, Agarwal A, et al. StevensJohnson Syndrome/Toxic Epidermal Necrolysis: A Multicenter Retrospective Study of 377 Adult Patients from the United States. J Invest Dermatol 2018. 138(11):2315-2321
14. Lee EY, Gomes T, Drucker AM, Daneman N, Asaf A, Wu F, et al. Oral Antibiotics and Risk of Serious Cutaneous Adverse Drug Reactions. JAMA 2024. 332(9):730-737
15. Lee EY, Knox C, Phillips EJ. Worldwide Prevalence of Antibiotic-Associated StevensJohnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Metaanalysis. JAMA Dermatol 2023. 159(4):384-392.



SUITABLE FOR USE ON FACE & BODY
Full range allows personalised approach
Cost efficient, quality products
UK’s #1 prescription emollient company1
Proven patient satisfaction2




The entire Zeroderma® range is approved by the British Skin Foundation
Prescribing Information

Zerobase Emollient Cream. Manufacturer: Thornton & Ross Ltd, Linthwaite, Huddersfield, HD7 5QH, UK. Code # of Notified Body: N/A. Legal classification: Class I. Product name: Zerobase Emollient Cream. Ingredients: Purified Water, Liquid Paraffin 11% w/w, White Soft Paraffin, Cetostearyl Alcohol, Macrogol Cetostearyl Ether, Sodium Dihydrogen Phosphate, Chlorocresol, Phosphoric Acid. Indications: Zerobase Emollient Cream softens, moisturises and protects the skin, providing symptomatic relief for red, inflamed or dry skin. If you have eczema, the cream can also be applied before a bath to stop the skin drying further. It can be used as an alternative to soap. Adverse reactions: Zerobase contains cetostearyl alcohol and chlorocresol which may cause local skin reactions (e.g. contact dermatitis) or allergic reactions. Rarely, mild allergic reactions such as a skin rash may occur. If you notice any side effects; or your skin condition worsens, stop use and tell your doctor or pharmacist, they will tell you what to do. Precautions: For external use only. Do not use if you or your child have an allergy to any of the ingredients listed. If the skin is badly cracked, infected or bleeding do not apply; seek medical advice before use. Avoid contact with the eyes. Use during pregnancy and breastfeeding is unlikely to have any ill effects when used as directed. If unsure, talk to your doctor or pharmacist. Do not use if tamper evident collar is broken or missing at first use (500g pack). Do not use if tube seal is broken or appears tampered with at first use (tubes). Fire hazard. Do not smoke or go near naked flames; clothing & bedding with this product dried on them can catch fire easily. Washing clothing and bedding may reduce product build-up but not totally remove it. If you accidentally swallow some see a doctor straight away. Take the pack with you to show which product you have swallowed. Use within three months of opening. Keep out of the sight and reach of children. Keep in the original carton (tube packs). Use by the date shown on the label (500g pack). Use by the date shown on the side of the carton (tube packs). Contra-indications: Hypersensitivity to any of the ingredients. Method of administration: Pumps: Prime the pump before first use by depressing fully until cream appears. If the pump stops working do not dismantle the pack. Re-start by covering the nozzle, then fully depress the pump several times to expel air. Adults, the elderly and children: Apply to the affected areas of skin as often as required. Smooth gently into the skin, following the direction of the hair growth. Date of preparation: 14/11/2023.
Zerocream Emollient. Manufacturer: Thornton & Ross Ltd, Linthwaite, Huddersfield, HD7 5QH, UK. Code # of Notified Body: N/A. Legal classification: Class I. Product name: Zerocream Emollient. Ingredients: Purified Water, White Soft Paraffin 14.5% w/w, Liquid Paraffin 12.6% w/w, Cetyl Alcohol, Glyceryl Monostearate, Sodium Cetostearyl Sulfate, Lanolin, Citric Acid Monohydrate, Carbomer, Sodium Hydroxide, Sodium Methyl Hydroxybenzoate, Sodium Propyl Hydroxybenzoate, BHT. Indications: Zerocream Emollient is used for the relief of symptoms of flaking, dry skin, ichthyosis, dermatitis, the dry stage of eczema and dry cases of psoriasis. It can be used as an alternative to soap. Adverse reactions: Zerocream contains methyl and propyl parahydroxybenzoates, cetyl alcohol and lanolin which may cause local skin reactions (e.g. contact dermatitis) or allergic reactions which may not appear immediately. Rarely, mild allergic reactions such as a skin rash may occur. If you notice any side effects; or your skin condition worsens, stop use and tell your doctor or pharmacist, they will tell you what to do. Precautions: For external use only. Do not use if you or your child have an allergy to any of the ingredients listed. Avoid contact with the eyes. If the skin is badly cracked, infected or bleeding do not apply; seek medical advice before use. Use during pregnancy is unlikely to have any ill effects when used as directed. If unsure, talk to your doctor or pharmacist. Wash and dry hands before use. Do not use if tamper evident collar is broken or missing at first use. Fire hazard. Do not smoke or go near naked flames; clothing & bedding with this product dried on them can catch fire easily. Washing clothing and bedding may reduce product build-up but not totally remove it. If you accidentally swallow some see a doctor straight away. Take the pack with you to show which product you have swallowed. Treated feet may be slippery. Take extra care when walking on smooth surfaces. Use within 12 months of opening. Keep out of the sight and reach of children. Keep in the original carton (tube packs). Do not use after expiry date shown on the side of the label (500g pack). Do not use after expiry date shown on the side of the carton (tube packs). Contra-indications: Hypersensitivity to any of the ingredients. Method of administration: Pumps: Prime the pump before first use by depressing fully until cream appears. If the pump stops working do not dismantle the pack. Re-start by covering the nozzle, then fully depress the pump several times to expel air. Adults, the elderly and children: Apply as required to the affected areas of skin, two or three times daily. Smooth gently into the skin, following the direction of hair growth. Date of preparation: 14/11/2023. Zeroveen Emollient. Manufacturer: Thornton & Ross Ltd, Linthwaite, Huddersfield,
HD7 5QH, UK. Code # of Notified Body: N/A. Legal classification: Class I. Product name: Zeroveen Emollient. Ingredients: Purified Water, Glycerol 12.6% w/w, Liquid Paraffin 5% w/w, Isopropyl Palmitate, Distearyldimonium Chloride, Avena Sativa Kernal Flour, Cetyl Alcohol, Dimeticone, White Soft Paraffin, Benzyl Alcohol, Allantoin, Stearyl Alcohol, Microcrystalline Wax, Myristyl Alcohol, Sodium Chloride. Indications: Fragrance-free 2-in-1 moisturising cream and wash with natural oatmeal (avena sativa kernal flour) for use in the management of dry skin conditions as may be found in eczema, psoriasis, dermatitis, ichthyosis, elderly pruritus and other dry skin conditions. Adverse reactions: Rarely, mild allergic reactions such as skin rash can occur. If you notice any side effects or the condition worsens while using the product, stop use and tell your doctor or pharmacist. They will tell you what to do. Zeroveen contains benzyl alcohol and cetyl, myristyl and stearyl alcohols which may cause local skin reactions (e.g. contact dermatitis) or allergic reactions which may not appear immediately. Precautions: Do not use if you or your child have an allergy to any of the ingredients listed. If the skin is badly cracked, infected or bleeding do not apply; seek medical advice before use. Avoid contact with the eyes. Use during pregnancy or breastfeeding is unlikely to have any ill effects when used as directed. If unsure, talk to your doctor or pharmacist. Wash and dry hands before use. Do not use if tube seal is broken or appears tampered with at first use (tubes). Fire hazard. Do not smoke or go near naked flames; clothing or bedding with this product dried on them can catch fire easily. Washing clothing and bedding may reduce product build-up but not totally remove it. Always replace the cap after use (tubes). If you accidentally swallow some see a doctor straight away. Take the pack with you to show which product you have swallowed. Treated feet may be slippery. Take extra care when walking on smooth surfaces. Keep out of sight and reach of children. Keep in the original carton (tubes). Do not use after expiry date shown on the side of the label (500g pack). Do not use after expiry date shown on the side of the carton (tubes). Contra-indications: Hypersensitivity to any of the ingredients. Method of administration: Pumps: Prime the pump before first use by depressing fully until cream appears. If the pump stops working do not dismantle the pack. Re-start by covering the nozzle, then fully depress the pump several times to expel air. All packs: Apply cream to the skin. Adults, the elderly and children: Apply as required to the affected areas of skin. Smooth gently into the skin, following the direction of hair growth. Date of preparation: 14/11/2023.
Zerodouble Gel. Manufacturer: Thornton & Ross Ltd., Linthwaite, Huddersfield, HD7 5QH, UK. Code # of Notified Body: N/A. Legal classification: Class I. Product name: Zerodouble Gel. Ingredients: Purified Water, Isopropyl Myristate 15% w/w, Liquid Paraffin 15% w/w, Glycerol, Triethanolamine, Phenoxyethanol, C10-C30 Alkyl Acrylate Cross Copolymer, Sorbitan Laurate. Indications: Highly moisturising emollient gel for dry skin conditions, as may be found in eczema, psoriasis, dermatitis, ichthyosis, elderly pruritus, and other dry skin conditions. It can also be used as an alternative to soap. Adverse reactions: Rarely, mild reactions such as skin rash may occur, or allergic skin reactions on extremely sensitive skin. These effects tend to occur during the first few uses. If you notice any side effects, stop use and tell your doctor or pharmacist, they will tell you what to do. Precautions: For external use only. Do not use if you or your child have an allergy to any of the ingredients listed. If the skin is badly cracked, infected or bleeding do not apply; seek medical advice before use. Avoid contact with the eyes. Use during pregnancy and breastfeeding is unlikely to have any ill effects when used as directed. The ingredients have been in widespread use for many years, without reports of problems. However, safety trials have not been conducted. If unsure, talk to your doctor or pharmacist. The ingredients in this product are not considered likely to affect or be affected by other medicines. Fire hazard. Do not smoke or go near naked flames; clothing & bedding with this product dried on them can catch fire easily. Washing clothing and bedding may reduce product build-up but not totally remove it. Check that the tube seal is not broken before first use (tubes). Do not use if tamper evident collar is broken or missing at first use (pump pack). If you accidentally swallow some, see a doctor straight away. Take this pack with you to show which product you have swallowed. Keep out of the sight and reach of children. Keep in the original carton (tubes). Use within 12 months of opening (100g tube & 500g pump pack). Use within 28 days of opening (20g tube). Use by the expiry date shown on the side of the carton (tubes). Use by the expiry date shown on the side of the label (pump pack). The expiry date refers to the last day of that month. Contraindications: Hypersensitivity to any of the ingredients. Method of administration: Tubes: Unscrew the cap, check and remove the foil seal, then replace the cap. Pump pack: Prime the pump before first use by depressing fully until gel appears. If the pump stops working do not dismantle the pack. Re-start by covering the nozzle, then fully depress the pump several times to expel air. All: Apply the gel only to the skin. Make sure your hands are clean and dry before applying the gel. Adults,
Pump format in line with NICE guidance3
Expert dermatology training & education
Request FREE Patient Evaluation Products!
Allow your patients to try our range of emollients and check their acceptability before a prescription is written. Simply scan the QR code or visit zeroderma.co.uk/pep
the elderly and children: Apply the gel to the affected area on a regular basis and as often as required. Smooth gently into the skin following the direction of the hair growth. Alternatively apply as directed by your doctor or pharmacist. Zerodouble Gel may also be applied before washing or bathing in order to prevent further drying of the skin. Date of preparation: 14/11/2023.
Zeroderm Ointment. Manufacturer: Thornton & Ross Ltd, Linthwaite, Huddersfield, HD7 5QH, UK. Code # of Notified Body: N/A. Legal classification: Class I. Product name: Zeroderm Ointment. Ingredients: Liquid Paraffin 40% w/w, White Soft Paraffin 30% w/w, Cetearyl Alcohol, Polysorbate 60. Indications: Zeroderm Ointment is a rich emollient used to moisture and soften dry skin in eczema, dry cases of psoriasis and other dry skin conditions. It is suitable for all ages and may also be used as a skin cleanser or bath additive. Adverse reactions: The product contains cetearyl alcohol which may cause local skin reactions (e.g. contact dermatitis). If you notice any side effects, stop use and tell your doctor or pharmacist. They will tell you what to do. Precautions: For external use only. Do not use the ointment if you or your child have an allergy to any of the ingredients listed. If the skin is badly cracked, infected or bleeding do not apply; seek medical advice before use. Avoid contact with the eyes. Using Zeroderm Ointment during pregnancy and breastfeeding is unlikely to have any ill effects. If unsure, talk to your doctor or pharmacist. Baths and showers may become slippery. Take care not to slip. Clean away any residues with household cleaner using warm water. Fire hazard. Do not smoke or go near naked flames; clothing & bedding with this product dried on them can catch fire easily. Washing clothing and bedding may reduce product build-up but not totally remove it. Do not use if tear-tab is broken or appears tampered with at first use. If you accidentally swallow some see a doctor straight away. Show this label or pack. Use within 12 months of opening. Keep out of the sight and reach of children. Do not use after the expiry date. Contra-indications: Hypersensitivity to any of the ingredients. Method of administration: Adults, the elderly and children: As an emollient: Apply to the affected area as often as required. Smooth gently into the skin, following the direction of the hair growth. As a bath additive: Melt about 4g (roughly a teaspoonful) of Zeroderm Ointment in hot water in a suitable container. Then add to the bath ensuring that the water has sufficiently cooled before getting in. As a soap substitute: Take a small amount of Zeroderm Ointment from the tub, lather it under warm water and use as required when washing or in the shower. Pat skin dry. Date of preparation: 14/11/2023.
Do not smoke or go near naked flames – risk of severe burns. Fabric (clothing, bedding, dressings etc) that has been in contact with this product burns more easily and is a serious fire hazard. Washing clothing and bedding may reduce product build-up but not totally remove it.
Medical advice should be sought before use of emollients if the skin is broken, badly cracked, infected or bleeding.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Thornton and Ross Limited by emailing thorntonross@medinformation. co.uk or by calling 01484 848164.
Zeroderma, Zerobase, Zerocream, Zeroveen, Zerodouble and Zeroderm are registered trade marks of Thornton & Ross Ltd.
References: 1. IQVIA RXA Unit Sales ending March 2024. 2. Thornton & Ross, data on file. 3. NICE, Which emollient product should I prescribe? Available at: https://cks.nice. org.uk/topics/eczema-atopic/prescribing-information/emollients/#choice-of-product accessed August 2024.
STADA UK, Thornton & Ross Ltd., The Globe (3rd floor), Bridge Street, Slaithwaite, Huddersfield, HD7 5JN
Telephone: +44 (0)1484 500283 www.zeroderma.co.uk UK-ZERO-303(1) | August 2024.



1,3






BIMZELX was well tolerated, the most frequently reported adverse reactions were: upper respiratory tract infections and oral candidiasis . Other common reported adverse reactions include tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis, headache, rash, dermatitis, eczema, acne, injection site reactions, fatigue, and vulvovaginal mycotic infection (including vulvovaginal candidiasis).
Please refer to the SmPC for further information. 2
¥co-primary endpoints PASI 90 and IGA 0/1 at Week 16 were met *secondary endpoints



Stay connected with UCB by scanning the QR code and set your digital preferences
Use this QR code to access Bimzelx.co.uk
This is a promotional UCB website
§N= modified non-responder imputation (mNRI), missing data were imputed with mNRI (patients with missing data following treatment discontinuation due to lack of efficacy or a treatment-related adverse event were counted as non-responders; multiple imputation methodology was used for other missing data) BIMZELX® (Bimekizumab) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. Bimzelx, alone or in combination with methotrexate, is indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumatic drugs (DMARDs). Please refer to the SmPC for further information. 2
(Please consult the Summary of Product Characteristics (SmPC) before prescribing)
Bimzelx® (Bimekizumab)
Active Ingredient: Bimekizumab – solution for injection in pre-filled syringe or prefilled pen: 160 mg of bimekizumab in 1 mL of solution (160 mg/mL).
Indications: Moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. Alone or in combination with methotrexate, for active psoriatic arthritis in adults who have had an inadequate response or intolerant to one or more disease-modifying antirheumatic drugs (DMARDs). Adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI) who have responded inadequately or are intolerant to non-steroidal anti-inflammatory drugs (NSAIDs). Adults with active ankylosing spondylitis who have responded inadequately or are intolerant to conventional therapy. Active moderate to severe hidradenitis suppurativa (acne inversa) in adults with an inadequate response to conventional systemic HS therapy. Dosage and Administration: Should be initiated and supervised by a physician experienced in the diagnosis and treatment of conditions for which Bimzelx is indicated. Recommended dose: Plaque Psoriasis: 320 mg (given as two subcutaneous injections of 160 mg each) at week 0, 4, 8, 12, 16 and every 8 weeks thereafter. Psoriatic arthritis: 160 mg (given as 1 subcutaneous injection of 160 mg) every 4 weeks. For psoriatic arthritis patients with coexistent moderate to severe plaque psoriasis, the recommended dose is the same as for plaque psoriasis. After 16 weeks, regular assessment of efficacy is recommended and if a sufficient clinical response in joints cannot be maintained, a switch to 160 mg every 4 weeks can be considered. Axial spondyloarthritis (nr-axSpA and AS): 160 mg (given as 1 subcutaneous injection) every 4 weeks. For patients with plaque psoriasis (including psoriatic arthritis with coexistent moderate to severe psoriasis) and a body weight ≥ 120 kg who did not achieve complete skin clearance at week 16, 320 mg every 4 weeks after week 16 may further improve treatment response. Consider discontinuing if no improvement by 16 weeks of treatment. Hidradenitis suppurativa: 320 mg (given as 2 subcutaneous injections of 160mg each) every 2 weeks up to Week 16 and every 4 weeks thereafter. Renal or hepatic impairment: No dose adjustment needed. Elderly: No dose adjustment needed. Administer by subcutaneous injection to thigh, abdomen or upper arm. Rotate injection sites and do not inject into psoriatic plaques or skin that is tender, bruised, erythematous or indurated. Do not shake pre-filled syringe or pre-filled pen. Patients may be trained to self-inject.
Contraindications: Hypersensitivity to bimekizumab or any excipient; Clinically important active infections (e.g. active tuberculosis).
Warnings and Precautions: Record name and batch number of administered product.
Infection: Bimekizumab may increase the risk of infections e.g. upper respiratory tract infections, oral candidiasis. Caution when considering use in patients with a chronic infection or a history of recurrent infection. Must not be initiated if any clinically important active infection until infection resolves or is adequately treated. Advise patients to seek medical advice if signs or symptoms suggestive of an infection occur. If a patient develops an infection, the patient should be carefully monitored. If the infection becomes serious or is not responding to standard therapy do not administer bimekizumab until infection resolves. TB: Evaluate for TB infection prior to initiating bimekizumab – do not give if active TB. While on bimekizumab, monitor for signs and symptoms of active TB. Consider anti-TB therapy prior to bimekizumab initiation if past history of latent or active TB in whom adequate treatment course cannot be confirmed. Inflammatory bowel disease: Bimekizumab is not recommended in patients with inflammatory bowel disease. Cases of new or exacerbations of inflammatory bowel disease have been reported. If inflammatory bowel disease signs/symptoms develop or patient experiences exacerbation of preexisting inflammatory bowel disease, discontinue bimekizumab and initiate medical management. Hypersensitivity: Serious hypersensitivity reactions including anaphylactic reactions have been observed with IL-17 inhibitors. If a serious hypersensitivity reaction occurs, discontinue immediately and treat. Vaccinations: Complete all age appropriate immunisations prior to bimekizumab initiation. Do not give live vaccines to bimekizumab patients. Patients may receive inactivated or non-live vaccinations.
Interactions: A clinically relevant effect on CYP450 substrates with a narrow therapeutic index in which the dose is individually adjusted e.g. warfarin, cannot be excluded. Therapeutic monitoring should be considered.
Fertility, pregnancy and lactation: Women of child-bearing potential should use an effective method of contraception during treatment and for at least 17 weeks after treatment. Avoid use of bimekizumab during pregnancy. It is unknown whether bimekizumab is excreted in human milk, hence a risk to the newborn/infant cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from Bimzelx therapy. No data available on human fertility.
Driving and use of machines: No or negligible influence on ability to drive anduse machines.
Adverse Effects: Refer to SmPC for full information Very Common (≥ 1/10): upper respiratory tract infection; Common (≥ 1/100 to < 1/10): oral candidiasis, tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis; headache, rash, dermatitis and eczema, acne, injection site reactions, fatigue; vulvovaginal mycotic infection (including vulvovaginal candidiasis); Uncommon(≥ 1/1,000 to < 1/100): mucosal and cutaneous candidiasis (including oesophageal candidiasis), conjunctivitis, neutropenia, inflammatory bowel disease.
Storage precautions: Store in a refrigerator (2ºC – 8ºC), do not freeze. Keep in outer carton to protect from light. Bimzelx can be kept at up to 25ºC for a single period of maximum 25 days with protection from light. Product should be discarded after this period or by the expiry date, whichever occurs first.
Legal Category: POM
Marketing Authorisation Numbers: PLGB 00039/0802 (Pre-filled Syringe), PLGB 00039/0803 (Pre-filled Pen).
UK NHS Costs: £2,443 per pack of 2 pre-filled syringes or pens of 160 mg each.
Marketing Authorisation Holder: UCB Pharma Ltd, 208 Bath Road, Slough,Berkshire, SL1 3WE, United Kingdom.
Further information is available from: UCBPharma Ltd, 208 Bath Road, Slough, Berkshire, SL1 3WE. Tel: 0800 2793177
Email: ucbcares.uk@ucb.com
Date of Revision: June 2024 (GB-BK-2400297)
Bimzelx is a registered trademark.
Adverse events should be reported. Reporting forms and information can be found at http://www.mhra.gov.uk/yellowcard. Adverse events should also be reported to UCB Pharma Ltd at ucbcares.uk@ucb.com or 0800 2793177

Rod Tucker
Does a higher intake of vitamin E protect against eczema?
Vitamin E might offer a degree of protection against atopic eczema (AE) according to the findings of a Mendelian randomisation (MR) study by researchers from Beijing. 1 An MR study makes use of genetic variants, termed single nucleotide polymorphisms (SNPs), to explore whether exposure to antioxidants, such as vitamin E, might be causally associated with a lower risk of AE. The team looked at SNPs associated with vitamin C, E, carotene and retinol. They identified a total of 108 SNPs related to antioxidant intake. Of these, 31 related to vitamin E, 25 to vitamin C, 30 to carotene and 22 to retinol. The results showed that there was a significant causal relationship only for vitamin E and AE (p = 0.038).
“Although the study offers tentative evidence for the benefits of vitamin E, why the other antioxidants failed to show a similar effect is unclear”
To some extent, the findings are biologically plausible. It is already known that oxidative stress leads to upregulation of pro-inflammatory cytokines and expression of genes related to oxidative stress is enhanced in eczematous skin. Furthermore, lipid peroxidation, caused by an excess of oxidant products, damages and impairs the skin’s barrier. As an antioxidant, vitamin E would be expected to counteract these
Summary:
In this new regular feature, Rod Tucker casts his eye over the dermatology literature to find articles that might be of interest to readers.
oxidant compounds. In fact, one small randomised trial has already shown that patients with AE given 400 IU/ day of vitamin E saw improvements in disease severity.
Although the study offers tentative evidence for the benefits of vitamin E, why the other antioxidants failed to show a similar effect is unclear, bringing into question the robustness of the finding. Until further trials provide more definitive evidence, perhaps nurses shouldn’t rush to tell their patients with eczema to start popping vitamin E pills.
Topical ivermectin could help rosacea patients with inflamed rhinophyma, according to a small, randomised trial by Italian researchers.2 The study compared ivermectin (IVM) 1% cream, applied either once or twice daily and metronidazole 0.75% gel, applied twice daily, in naïve patients with mild to moderately inflamed rhinophyma. Assessments were performed at baseline and at weeks 8 and 12 using three separate indices, with the same 5-point scale, ranging from 0 (no erythema) to 4 (severe erythema). The researchers used an investigator erythema severity assessment (IESA) score, an investigator global assessment (IGA) of efficacy score and an erythemadirected digital photography score.
At week eight, the mean baseline score for IESA reduced from 2 to 1.45. Similarly, the mean baseline digital photography score changed from 2.35 to 1.5 in those applying IVM twice daily, compared to the other two treatments (changes not specified). By week 12, the mean IESA score dropped slightly further to 1.1, although none of the other indices reduced. Based on the IGA score, 25% of patients using IVM twice daily achieved a score of 0 (clear) versus 10% for once daily use and 20% for metronidazole. The improvements for IVM twice daily were maintained for longer (36 days
Author info:
Rod Tucker is a pharmacist with a special interest in dermatology, and a member of the Dermatological Nursing Editorial Board.
versus 25 days compared to the once daily application and 18 days with metronidazole). The statistical significance of the results was not reported, which probably suggests that it wasn’t.
Topical IVM may help those with mild to moderately inflamed rhinophyma, but the effect seemed to be small. Just how well IVM compares with the currently recommended treatment, oral doxycycline, remains to be seen.
“The authors made clear they are not claiming that a CBD-based cream could serve as a sunscreen against UVA”
Cannabis cream reduces UVA-associated skin damage
In the first human trial, US researchers showed that a nano-encapsulated cannabidiol (CBD) cream reduced markers of UVA-induced damage in the skin.3 After determining patients’ minimal erythema dose (MED) of UVA, 20 healthy volunteers, with a mean age of 48.6 years and Fitzpatrick skin types I to III, applied the CBD cream or its vehicle to buttock sites twice daily for 14 days. Neither the participants or investigators were aware of which treatment was being used as both were supplied in identical tubes. After the treatment period, the skin was irradiated with UVA at < 3 x MED and 24 hours later, sites were visually inspected to compare the extent of erythema. In addition, punch biopsies were collected from irradiated and non-irradiated sites to explore any differences in markers of UVA-induced DNA damage.
Overall, 21% of participants had less observable erythema on CBD-treated sites compared to vehicle-treated areas. But the result wasn’t really that impressive, as 47% of participants showed no difference in erythema, 21% had no observable erythema and 11% (2 participants), had less erythema on the vehicle-treated site. The biopsy samples showed that the CBD cream reduced UVA-induced epidermal hyperplasia and a number of DNA markers associated with UVA damage, such as mitochondrial DNA damage, which is linked to skin photo-ageing.
These results were not surprising, given the well-known antioxidant effects of CBD. The authors made clear they are not claiming that a CBD-based cream could serve as a sunscreen against UVA. This was strange because in the abstract, they noted that current photo-protectant strategies are limited by the availability/utilisation of UVA-filters.
The visible nature of most dermatological conditions is invariably associated with a psychological burden. Following treatment, as the cutaneous symptoms resolve there is often a tandem improvement in patient’s psychological wellbeing. But according to a recent commentary in the British Journal of Dermatology, this isn’t always the case, with a delay observed in the improvement in psychological sequalae, which they refer to as ‘psycholag’.4
This type of delay in the resolution of psychological wellbeing has already been observed in both psoriasis and atopic eczema patients, despite a rapid improvement in skin symptoms. Just why this happens remains unclear. It is known, for example, that the pro-inflammatory cytokines interleukins 1, 6, 17 and 23 are implicated in the pathways leading to depression, and that despite effective therapies, symptom improvement in depression is not immediate. As these same cytokines are targeted in skin diseases, it is reasonable to assume that there will also be an improvement in depressive symptoms, which is often the case in clinical practice.
The authors ponder whether there are inherent differences in psycholag for inflammatory diseases and if it might be possible to identify parameters that are predictive of psycholag. Meanwhile, from a practical perspective, nurses need to recognise the potential for psycholag, especially when reviewing patients whose cutaneous symptoms have resolved yet who have still not achieved positive psychological outcomes.
“From a practical perspective, nurses need to recognise the potential for psycholag”
References
1. Wang S, et al. Causal relationships between dietary antioxidant vitamin intake and atopic dermatitis: A two-sample Mendelian randomization study. Skin Res Technol 2024. 30:11111
2. Dall’Oglio F et al. Inflamed rhinophyma treated with topical ivermectin: A randomized controlled study. J Am Acad Dermatol 2024. 91(3):519-521
3. McCormick E et al. Topical nanoencapsulated cannabidiol cream as an innovative strategy combating UV-A induced nuclear and mitochondrial DNA injury: A pilot randomized clinical study. J Am Acad Dermatol 2024. 91(5):855 - 862
4. Ahmed, A et al. ‘Psycholag’: a new term to describe the delay between physical and psychological improvement in patients with skin disease, British Journal of Dermatology 2024. ljae333.
Think PDT first for treating AK; think METVIX as a first option for proven clinical outcomes 1–3

METVIX® is indicated in adults (≥18 years) for the treatment of: Thin or non-hyperkeratotic and non-pigmented actinic keratoses on the face and scalp · Superficial and/or nodular basal cell carcinoma (BCC) only when unsuitable for other available therapies due to possible treatment related morbidity and poor cosmetic outcome; such as lesions on the mid-face or ears, lesions on severely sun damaged skin, large lesions, or recurrent lesions · Squamous cell carcinoma (SCC) in situ (Bowen’s disease) when surgical excision is considered less appropriate1
PI Metvix 160 mg/g cream Prescribing Information (UK & IRE)
Presentation: Cream containing 160mg/g of methyl aminolevulinate (as hydrochloride). Indications: Treatment in adults over 18 years of thin or non-hyperkeratotic and non-pigmented actinic keratoses (AK) on the face and scalp. Only for treatment of superficial and/or nodular basal cell carcinoma (BCC) unsuitable for other available therapies due to possible treatment related morbidity and poor cosmetic outcome; such as lesions on the mid-face or ears, lesions on severely sun damaged skin, large lesions, or recurrent lesions.Treatment of squamous cell carcinoma in situ (Bowen´s disease) when surgical excision is considered less appropriate. Dosage and administration: Please refer to summary of product characteristics before use. Treatment of AK lesions and/or field cancerization, BCC and Bowen’s disease using red-light lamp: For treatment of AK, one session of photodynamic therapy should be administered. Treated lesions should be evaluated after three months and if there has been an incomplete response, a second treatment may be given. For treatment of BCC and Bowen’s disease, two sessions should be administered with an interval of one week between sessions. Before applying Metvix cream, the surface of AK and superficial BCC lesions should be prepared to remove scales and crusts and roughen the surface of the lesions. Exposed tumour material should be removed gently without any attempt to excise beyond the tumour margins. Using a spatula, apply the cream (about 1mm thick) to the lesion area (for field cancerization, up to 20 cm², approximately) and approximately 5-10mm of the surrounding area. Cover the treated area with an occlusive dressing for 3 hours. Remove dressing and clean with saline. Immediately after cleaning the lesions, illuminate the treatment area with a red-light source (must be CE-marked), either with a narrow spectrum around 630 nm and light dose of approximately 37 J/cm² or a broader and continuous spectrum in a range between 570-670 nm with a light dose of 75 J/cm2. Healthy, untreated skin surrounding the lesions does not need to be protected during illumination. Multiple lesions may be treated during the same treatment session. Lesion response should be assessed after three months, and it is recommended to confirm the response of BCC and Bowen’s disease lesions by histological biopsy. AK and BCC lesion sites that show incomplete response may be retreated if desired. Treatment of AK lesions and/or field cancerization with natural daylight: Daylight treatment may be used to treat mild to moderate AK lesions. One treatment should be given. Treatment lesions should be evaluated after three months and if there is incomplete response, a second treatment may be given. Daylight treatment can be used if the temperature conditions are suitable to stay comfortably outdoors for 2 hours. If the weather is rainy, or is likely to become so, daylight treatment should not be used. A sunscreen should be applied. Once sunscreen has dried, scales and crusts should be removed from the lesion(s) or field of cancerization and the skin surface roughened before applying a thin layer of Metvix to the treatment areas. No occlusion is necessary. Patients should go outside after Metvix application or, at the latest, 30 minutes later in order to avoid excessive protoporphyrin IX accumulation which would lead to greater pain on light exposure. In order to minimize pain and ensure maximum efficacy the patient should then stay outdoors for 2 continuous hours in full daylight and avoid going indoors. On sunny days, should the patient feel uncomfortable in direct sunlight, shelter in the shade may be taken. Following the 2-hour exposure period, Metvix should be washed off. Multiple lesions may be treated during the same treatment session. Treatment of AK lesions and/or field cancerization using artificial daylight device (CE-marked): Scales and crusts should be removed, and the skin surface roughened before applying a thin layer of Metvix to the areas to be treated. Occlusion is not necessary. Sunscreen is not needed, as patients are not exposed to ultraviolet light. Lesion should be exposed to artificial daylight after Metvix application or, at the latest, 30 minutes later in order to avoid excessive protoporphyrin IX accumulation which would lead to greater pain on light exposure. In order to minimize pain and ensure maximum efficacy, the patient should be exposed to artificial daylight for 2 continuous hours in a comfortable position. Following the 2-hour exposure period, Metvix should be washed off. The devices should have a continuous light spectrum of 400-750 nm and an illuminance greater than 12,000 lux at the lesion surface. Healthy, untreated skin surrounding the lesions does not need to be protected during illumination. Multiple lesions may be treated during the same treatment session. Lesion responses should be assessed after three months, and at this response evaluation, lesion sites showing incomplete response may be retreated. Contraindications: Hypersensitivity to the active or excipients, including arachis oil or peanut or soya. Morpheaform basal cell carcinoma. Porphyria. Precautions and Warnings: Photodynamic therapy, when using red light or an artificial daylight lamp, should only be administered in the presence of a health care professional trained in the use of photodynamic therapy with Metvix. When using daylight treatment, a sunscreen should be applied to all areas exposed to daylight, prior to lesion preparation. Sunscreen used should offer adequate protection (SPF30 or higher) and must not include physical filters (e.g. titanium dioxide, zinc oxide, iron oxide). Only sunscreens with chemical filters should be used with daylight treatment. Not recommended during pregnancy. Only limited experience is available in relation to the treatment of actinic keratosis and/or Bowen’s disease amongst transplant patients on concomitant
immunosuppressive therapy. A close monitoring of these patients, with re-treatment if necessary is recommended in this population.There is no experience in treating Bowens disease in patients with a history of arsenic exposure.There is no experience of treating pigmented or highly infiltrating lesions with Metvix cream. Thick (hyperkeratotic) actinic keratoses should not be treated with Metvix. Methyl aminolevulinate may cause sensitization by skin contact resulting in angioedema, application site eczema or allergic contact dermatitis. The excipient cetostearyl alcohol may cause local skin reactions (e.g. contact dermatitis), methyl and propyl parahydroxybenzoate (E218, E216) may cause allergic reactions (possibly delayed). Any UV-therapy should be discontinued before treatment. As a general precaution, sun exposure on the treated lesion sites and surrounding skin has to be avoided for a couple of days following treatment. Direct eye contact with Metvix cream should be avoided. Metvix cream should not be applied to the eyelids and mucous membranes. Pain during illumination with red light may induce increased blood pressure. Measure blood pressure in patients prior to treatment with red light. If severe pain occurs during treatment, the blood pressure should be checked. In case of severe hypertension, the illumination with red light should be interrupted in addition to taking appropriate symptomatic measures. Conventional Photodynamic Therapy (PDT) with a red-light lamp may be a precipitating factor for transient global amnesia in very rare instances. If signs of confusion or disorientation are observed, PDT must be discontinued immediately Pregnancy and lactation: There is no or limited data from the use of methyl aminolevulinate in pregnant women. Metvix is not recommended during pregnancy and in women of childbearing potential not using contraception. It is unknown whether methyl aminolevulinate /metabolites are excreted in human milk. A risk to the newborns/infants cannot be excluded. A decision must be made whether to discontinue breastfeeding or to discontinue/abstain from Metvix therapy taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman. Undesirable effects: Metvix with red light in AK, BCC and Bowen’s disease: Approximately 60% of patients experience reactions localised to the treatment site that are attributable to toxic effects of the photodynamic therapy (phototoxicity) or to preparation of the lesion.The most frequent symptoms are painful and burning skin sensation typically beginning during illumination or soon after and lasting for a few hours with resolving on the day of treatment.The symptoms are usually of mild or moderate severity and rarely require early termination of illumination. The most frequent signs of phototoxicity are erythema and scab. The majority are of mild or moderate severity and persist for 1-2 weeks or occasionally longer. Repeated treatment with Metvix is associated with reduced frequency and severity of local phototoxic reactions.The incidence of adverse reactions in a clinical trial population of 932 patients receiving the standard treatment regimen with red light and from post marketing surveillance are as follows. Very common (≥1/10): pain of skin, skin burning sensation, scab, erythema; common (≥1/100, <1/10): skin infection, skin ulcer, skin oedema, skin swelling, blister, skin haemorrhage, pruritus, skin exfoliation, skin warm, paraesthesia, headache, application site discharge, feeling hot; uncommon (≥1/1000, ≤1/100): fatigue, eye swelling, eye pain, wound haemorrhage, nausea, urticaria, rash, skin irritation, photosensitivity reaction, skin hypopigmentation, skin hyperpigmentation, heat rash, skin discomfort; not known: transient global amnesia (including confusional state and disorientation), eyelid oedema, hypertension, angioedema, face oedema (swelling face), application site eczema, allergic contact dermatitis, rash pustular (application site pustule). Metvix with daylight in AK: No new local adverse reactions were reported in the two phase III Metvix daylight studies compared to the already known local adverse reactions with Metvix red light. In the two Phase III studies, including a total of 231 patients, local related adverse events were reported less frequently on Metvix DL-PDT than on c-PDT treated sides (45.0% and 60.1% of subjects, respectively). Prescribers should consult the summary of product characteristics in relation to other side effects. MA Numbers: PL 10590/0048 (UK) & PA22743/010/001 (Ireland). Packaging Quantities and Cost: Tube of 2 gram, UK - £171.50 (NHS), Ireland - €227.00. Legal Category: POM. Marketing Authorisation Holder: UK: Galderma (UK) Limited, Galderma (UK) Limited, Evergreen House North, Grafton Place, London, NW1 2DX, United Kingdom,Tel: +44 (0)300 3035674; IE: Galderma International S.A.S.,Tour Europlaza, La Défense 4, 20 Avenue André Prothin, 92927, France. Date of Revision: October 2022.
Adverse events should be reported.
For the UK: Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. For Ireland: Suspected adverse events can be reported via HPRA Pharmacovigilance, Website: www.hpra.ie; Adverse events should also be reported to Galderma (UK) Ltd.
E-mail: Medinfo.uk@galderma.com; Tel: +44 (0)300 3035674












Molly Connolly
“There’s always a story. It’s all stories, really. The sun coming up every day is a story. Everything’s got a story in it. Change the story, change the world.”
Dungeons and Dragons, commonly known as DnD, is a multiplayer role-playing game. Created in 1974, DnD was initially created to be played in person, but it has now evolved to be played anywhere at any time using virtual platforms.
Put simply, DnD is a way to engage and craft stories with others. It allows you to create new worlds where anything (however weird, wild or funny) is possible. Although players can create stories together, they are bound by certain rules – and an element of chance or fate – using a variety of dice to determine the outcomes of a character’s decisions.
DnD has increased in popularity over recent years, featuring in popular shows like Stranger Things and podcasts like The Adventure Zone. Shows centred around DnD such as Dimension 20 and Critical Role have also spread the game to a wider audience. It became particularly popular during the pandemic as it enabled people to keep in touch with friends scattered across the world.
Usually, DnD is played by a group of players and a General Manager. Each player creates a character – whether that be a spell-slinging sorcerer, a master thief planning their
Summary:
final heist, or a fighter rebelling against an evil empire. Players can create characters of any design, encompassing features like species, gender, and role or archetype. Depending on these features, characters will have specific abilities or traits. Often, players will use character sheets to help keep track of the characters’ abilities.
Some players will decide to give their characters unique voices or they will mimic popular characters or celebrities (which can be easier for those trialling roleplay for the first time). For example, one of the first characters I created was based on Dwayne “The Rock” Johnson and I used many of his catchphrases in fights against enemies.
A player may also shape their characters to the type of game or story – often called a ‘campaign’ – that is played out (see Box 1). Campaigns are often controlled by a General Manager (known as a GM), who manages and helps set rules and expectations for players. GMs help run and co-create the story together with players while also determining the outcome of the characters’ decisions with the aid of multiple dice.
There are many settings that campaigns can take play – from the traditional Lord of the Rings style fantasies
Dungeons and Dragons (or DnD) is a multiplayer role-playing game which allows players to craft and engage in fictional worlds. For Molly, it serves as a way to bring friends together, expanding and challenging world views through the safe place of a story.
Author info:
Molly Connolly is Clinical Nurse Specialist, Dermatology Outpatients at NHS Lothian and the BDNG’s Education Nurse
Citation:
Connolly M. Dungeons and Dragons: Where stress lessens and adventure beckons. Dermatological Nursing 2024. 23(4):65-67.
Keywords: Dungeons and Dragons, DnD, Roleplay, Storytelling

to a Mission Impossible-style heist, searching hidden temples for treasure, or protecting your town from an alien invasion.
A GM will keep track of not only the various players, but other characters, maps of the location, enemies, traps or any other story element to ensure an engaging experience for players. Campaigns are often co-created by players and the GM.
There are various systems to play DnD, the most popular and commonplace being Wizards of the Coast 5th Addition (frequently referred to as ‘5E’), which
Campaign 1


provides players with a basic ruleset. The game is fleshed out through additional resources and books from the central Player’s Handbook outlining the basics of creating a character and playing within this world.
There are various companion books that players or GMs can use as prewritten campaigns or containing additional elements to the game. Various
This story is set in a world where a cruel vampire lord has taken over the country, and our characters have been tasked with the secret mission to try finally defeat him once and for all.
‘A small dwarven lady enters the tavern, her armour battered and beaten and hanging oddly from her body, a bit too big for her small frame.
‘You notice a small hammer pin on her cloak, which glimmers in the torchlight. She grins, placing her hammer on the bar table, raises an eyebrow at you all – “Let’s cook that vampire,” she says.’
Campaign 2
The story is set in a small valley with various towns. Rumours of weird things – earthquakes in towns not on fault lines, snowstorms in the middle of summer, fires burning across rivers – have reached your ears.
‘You all see a half-elf, fiddling with their multi-coloured robe.You notice arrowshaped tattoos covering their entire forehead and on their arms, which have sinewy muscles. Flames wreath their hands as they face down your attackers.’
books both official and unofficial, which provide simpler explanations for newer GMs or players – filled with template characters for players to use.
DnD 5E uses a variety of different dice to help determine both the players’ abilities and actions, but also the reactions of non-playable characters roleplayed by the GM. Some players will collect dice or make their own.
However, there are other systems, like Kids on Bikes or Powered by the Apocalypse, that newer players might find easier to get into, especially if they are not used to roleplaying.
When playing face-to-face together at a table, whiteboards are often used to help keep track of where characters are placed in the environment. Many players
will use figurines to represent their characters in the game or they may use tokens to represent them on maps.
During the pandemic, virtual platforms became more popular as many countries were locked down. DnD Beyond and Roll 20 are both very popular virtual platforms that can keep track of character sheets, allow GMs and players to annotate or create maps, as well as send virtual messages.
Often, the fun part of DnD is simply roleplaying with other players. Although the memorable parts of DnD are often the funny scenarios characters might get into (for example, our party’s decision to dress in disguises to infiltrate a temple led to hilarious consequences), sometimes the most meaningful parts are the small moments between characters.
Quote from The Adventure Zone



“Strength is a tool, Magnus, it’s a commodity . You can spend it and spend it, but everyone’s got some, and lots of folks are gonna have more than you. But if you ask for it, Magnus, other folks’ strength can become your own. That is what strength is, Magnus. Who gives you strength, how willing are you to ask for it? Pride and glory are the enemies of true strength, Magnus. In every warrior’s life, there comes a moment where they are overpowered by a superior fighter. But you keep friends nearby, and you ask for help when you need it, Magnus, and you won’t just be strong. You’ll be unbeatable. ”
Although campaigns are often played between friends, sometimes they will be played with acquaintances or people you may have never met. DnD draws people together to a specific time or place allowing players to simply be with each other.
Our most recent campaign has been going for several years, combating evil cultists who threaten to use elemental powers to raze towns and villages. We have disguised ourselves to infiltrate cults, taken down pirates and saved


entire towns from attack – all within the stories we have created together. We have also had our share of losses as a party, but we have shared those moments together.
DnD is a great way to bring people together, to spend time in different worlds – but also to expand and challenge world views through the safe place of a story.
Our most recent campaign has been going for several years, drawing a
consistent group of friends together every week to relax, create stories with a blend of seriousness and hilarity. From a wellbeing perspective, DnD is a valuable social routine with a clear structure, light-heartedness, creativity, and friendship. On a personal level, once I got past the complicated exterior of the game, it has become one of the most vital wellbeing activities of my week, and I encourage anybody longing for social routine and low-pressure creativity to give it a try.
Created and funded by LEO Pharma. Intended for UK healthcare professionals only.
For the treatment of moderate to severe atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy.1,2

Adtralza® has demonstrated up to 3 years of skin clearance: 73% of patients achieved a 75% improvement in Eczema Area and Severity Index (EASI-75) at 3 years (n=29/40).1-3 Based on a 3-year subgroup interim analysis of ECZTEND.3
The most common adverse reactions with Adtralza® are upper respiratory tract infections (23.4%; mainly reported as common cold), injection site reactions (7.2%), conjunctivitis (5.4%) and conjunctivitis allergic (2.0%).1,2

Not an actual patient. For illustrative purposes only. Individual results may vary. There is limited amount of data from the use of Adtralza® in pregnant women.1,2 As a precautionary measure, it is preferable to avoid the use of Adtralza during pregnancy.1,2
Prescribing Information for Adtralza® (tralokinumab) 150 mg solution for injection in pre-filled syringe and Adtralza® (tralokinumab) 300 mg solution for injection in pre-filled pen
Please refer to the full Summary of Product Characteristics (SmPC) (www.medicines.org.uk/emc) before prescribing.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Indications: Treatment of moderate-to-severe atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy. Active ingredients: Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL). Each pre-filled pen contains 300 mg of tralokinumab in 2 mL solution (150 mg/mL). Dosage and administration: Posology: The recommended dose of tralokinumab for adult and adolescent patients 12 years and older is an initial dose of 600 mg (four 150 mg injections by pre-filled syringe or two 300 mg injections by pre-filled pen) followed by 300 mg (two 150 mg injections by pre-filled syringe or one 300 mg injection by pre-filled pen) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. The probability of maintaining clear or almost clear skin may be lower with every fourth week dosing. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. Missed dose: If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. Special populations: No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 12 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe and pen should not be shaken. After removing the pre-filled syringes or pre-filled pens from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting a pre-filled syringe or 45 minutes before injecting a prefilled pen. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections or two 300 mg tralokinumab injections, should be administered consecutively in different injection sites within
the same body area. It is recommended to rotate the injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Interactions: See SmPC for full details on interactions. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology. Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Please see SmPC for full list of side effects. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: Pre-filled syringe: PLGB 05293/0182, EU/1/21/1554/002. Pre-filled pen: PLGB 05293/0189, EU/1/21/1554/004. LEO Pharma A/S, Ballerup, Denmark. Basic NHS price: 4 pre-filled syringes: £1,070 (each syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL)). 2 pre-filled pens: £1,070 (each pen contains 300 mg of tralokinumab in 2 mL solution (150 mg/mL)). Last revised: January 2024. Reference number: MAT-70774.
Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be found at: www.mhra.gov.uk/yellowcard or search for MHRA Yellow card in the Google Play or Apple App Store.
Adverse events should also be reported to Drug Safety at LEO Pharma by calling +44 (0)1844 347333 or e-mail: medical-info.uk@leo-pharma.com
Further information can be found in the Summary of Product Characteristics or from: LEO Pharma, Building 5, Foundation Park, Roxborough Way, Maidenhead, Berkshire SL6 3UD, UK. e-mail: medical-info.uk@leo-pharma.com ® Registered trademark
Lynne Skrine

Lynne
Well, conference had a great turn out; speakers went down a storm and it was great to welcome two delegates from Singapore, who have written a few words which you will find on p age 71
My quest to build a roadmap of all the services in England, Wales, Scotland, Northern Ireland, Ireland and the isles around the UK is well under way, and my findings can be seen in Table 1.
I am still a long way off and I am always looking for leads, so please get in touch if you don’t feel you are on the service road map.
Table 1. Services in the UK and Ireland
Just after our 33rd BDNG annual conference there was the European Academy of Dermatology and Venereology (EADV) in Amsterdam. The nurse day was held on the Saturday, and as part of the nurse’s taskforce, I was able to attend their meeting via Teams, where they discussed the programme for the next EADV in 2025, which will also be on the Saturday for half a day. The task force has already had approval for their wound care and phototherapy sessions which are being held virtually, so please watch this space for more information and the confirmed dates.
“Social media engagement over the past few months has gone up, with Faceook up by 63% and Instagram up by 14%”
Social media engagement over the past few months has gone up, with Faceook up by 63% and Instagram up by 14%. Please carry on liking and following on Facebook, X, Instagram and LinkedIn.
We will be using these platforms to promote all the educational events and other activities you might be interested in. Our aim is to do this in a timely manner, ensuring you have enough notice to book time off work, as we realise that most members are required to give at least six-eight weeks’ notice – if not longer – to attend our face-to-face meetings, especially when having to rearrange clinics.



Lim Chay Mien
Attending the 33rd annual British Dermatology Nursing Group (BDNG) Conference from 17-19 September 2024, at the Harrogate Convention Centre, was an inspiring and insightful experience that I am excited to reflect upon.
As participants, we had the opportunity to engage with the industry leaders in pharmaceuticals and others, and expand our knowledge on the use of the biologic secukinumab for hidradenitis suppurativa and dupilumab for urticaria. We were also able to learn about the newer treatments that are not currently available in Singapore, such as the biologic cream ruxolitinib for vitiligo and fludroxycortide (steroid-impregnated tape) for scars and prurigo. We were also able to network with professionals from diverse backgrounds.
One of the most insightful moments for us was the sharing from Community Dermatology Advance Practice Nurse Valerie Anderson, who talked about the structure and workflow of referral between general practitioners. It is apparent that harmonising the referral structure and flow may be necessary. Additionally, different states within the UK may have differences in practices and legislation for different grades of dermatology nurses; this is seemingly less of an issue in Singapore’s referral system. Learning about the use of artificial intelligence (AI) as part of a dermatology nursing assessment is relatively new in practice, but it may benefit patients in the long term.
Beyond the sessions, we had the chance to connect with fellow attendees who share similar interests and goals.
“Attending the BDNG conference is where I recharge my enthusiasm and gain fresh motivation. They allow me to step away from day-to-day demands of work, and help me to look at the bigger picture for future dermatological nursing in Singapore”, said Ms Brenda Lim, Head of Nursing, Chair of Dermatology Chapter of Singapore Nurses Association and The Asian Dermatological Nursing Group.
Overall, the BDNG annual conference was not just an event but a valuable learning opportunity. It provided us with a platform to delve deeper into the expanded scope of practice of dermatology nurses and broaden our understanding of the demands and needs of population health. We left the conference with a renewed sense of motivation and new ideas to explore in our work.
We are grateful to Lynne Skrine, International Dermatology Nursing liaison officer at the BDNG, for constantly keeping us updated of the BDNG’s activities, and to Susan Maguire, Chief Executive Officer of BDNG, for the warm hospitality, and for her excellent team for curating such a well-rounded and impactful event. We look forward to attending future conferences that continue to push the boundaries of learning and collaboration, and we hope to develop greater recognition for nurses working in the field of dermatology.

Summary:
In this article, the author provides a summary of attending the BDNG’s annual conference, held in Harrogate in September.

Author info:
Lim Chay Mien is Assistant Secretary of the Singapore Nurses Association – Dermatology Chapter, in the Asian Dermatological Nursing Group


PRESCRIBING INFORMATION
Please consult the Summary of Product Characteristics (SmPC) before prescribing
Name: Wynzora, 50 micrograms/g + 0.5 mg/g cream
Active Ingredient: One gram of Wynzora Cream contains 50 micrograms of calcipotriol and betamethasone dipropionate equivalent to 0.5 mg betamethasone. Excipients with known effect: Butylated hydroxyanisole (E 320) 1.0 micrograms/g cream Macrogolglycerol hydroxystearate 3.4 micrograms/g cream
Indication: Wynzora is indicated for topical treatment of mild to moderate psoriasis vulgaris, including scalp psoriasis, in adults. Dosage and Administration: Rub a thin layer of Wynzora Cream to affected areas once daily for up to 8 weeks. Discontinue when control is achieved. Treatment should be continued only after medical review and under regular medical supervision. For calcipotriol containing medicinal products, do not exceed the maximum daily dose of 15 g; the body surface area treated should not exceed 30%. Wynzora Cream should not be applied directly to the face or eyes. Allow 8 hours between the application and showering or bathing. Hands must be washed after use. Consult SmPC and package leaflet for full method of administration.
Contraindications, Precautions and Warnings: Contraindications: Hypersensitivity to the active substances or to any of the excipients listed in section 6.1 of SmPC. Wynzora Cream is contraindicated in erythrodermic, exfoliative and pustular psoriasis; in patients with known disorders of calcium metabolism; viral (e.g. herpes or varicella) lesions of the skin, fungal or bacterial skin infections, parasitic infections, skin manifestations in relation to tuberculosis, perioral dermatitis, atrophic skin, striae atrophicae, fragility of skin veins, ichthyosis, acne vulgaris, acne rosacea, rosacea, ulcers and wounds. Precautions: Avoid application under occlusive dressings, large areas of damaged skin or on mucous membranes or in skin folds due to increased corticosteroids systemic absorption and potential adrenocortical suppression or impact on the metabolic control of diabetes mellitus. Hypothalamic–pituitary–adrenal axis suppression was evaluated in adult subjects (N=27) with extensive psoriasis (including scalp). Adrenal suppression was seen in 1 out of 27 subjects (3.7%) after 4 weeks of treatment, and in one additional patient after 8 weeks of treatment. Visual disturbance may be reported with systemic and topical corticosteroid use. Patients presenting with blurred vision or other visual disturbances should be considered for a referral to an ophthalmologist. Hypercalcaemia may occur however the risk is minimal if maximum daily dose (15 g) is not exceeded. Serum calcium is normalised upon discontinuation. Avoid concurrent treatment with other steroids on the same treatment area. Do not use on the face and genital areas. Patients should wash hands after each application. Treat secondarily infected lesions with antimicrobiological therapy. Stop treatment with corticosteroids if the infection worsens. Continue medical supervision in the post-treatment period in case of rebound effects or development of generalised pustular psoriasis. Discontinue treatment in case of adverse reactions related to long-term use of corticosteroid. Limit or avoid excessive exposure to either natural or artificial sunlight during treatment. Consider risk/benefits balance of the use of topical calcipotriol with ultra-violet radiation. Butylhydroxyanisole (E320) contained in Wynzora Cream may cause local skin reactions (e.g. contact dermatitis) or irritation to the eyes and mucous membranes. Macrogolglycerol hydroxystearate contained in Wynzora Cream may cause skin reactions. Do not smoke or go near naked flames due to the risk of severe burns. Fabric (clothing, bedding, dressings etc) that has been in contact with this product burns more easily and is a serious fire hazard. Washing clothing and bedding may reduce product build-up but not totally remove it. Consult SmPC and package leaflet for more information.
Fertility, pregnancy and lactation: Fertility: No impairment of male and female fertility (animal studies). Pregnancy: No adequate data available. The potential risk for humans is uncertain. Carefully consider benefit/risk balance during pregnancy. Lactation: Exercise caution in breast-feeding women. Do not use Wynzora Cream on the breast when breast-feeding. Consult SmPC and package leaflet for more information.
Adverse Reactions: All reported adverse reactions were seen at a frequency below 1%. Uncommon: Application site reactions including application site irritation, pain, pruritus, eczema, exfoliation, telangiectasia and folliculitis. Rash, urticaria and pruritus. Insomnia. Not known (cannot be estimated from available data):Vision, blurred. Adverse reactions considered to be related to the pharmacological classes of calcipotriol and betamethasone. Calcipotriol: application site reactions, pruritus, skin irritation, burning and stinging sensation, dry skin, erythema, rash, dermatitis, eczema, psoriasis aggravated, photosensitivity and hypersensitivity reactions including very rare cases of angioedema and facial oedema. Very rarely cases of hypercalcaemia or hypercalciuria. Betamethasone (as dipropionate): Skin atrophy, telangiectasia, striae, folliculitis, hypertrichosis, perioral dermatitis, allergic contact dermatitis, depigmentation and colloid milia. Generalised pustular psoriasis. Systemic reactions are rare in adults, but can be severe. Long-term treatment may cause adrenocortical suppression, cataract, infections, impact on the metabolic control of diabetes mellitus and increase of intraocular pressure. Systemic reaction may occur when applied under occlusion (plastic, skinfolds), on large areas and during long-term treatment. Consult SmPC and package leaflet for further information. Legal Category: Ireland: POM Subject to prescription which may not be renewed (A). United Kingdom: POM Price: Ireland: Price to wholesaler United Kingdom: UK NHS Cost: £35.66 (excluding VAT). Marketing Authorisation Numbers: Ireland: PA 0968/006/001; United Kingdom: PL 16973/0044 Marketing Authorisation Holder: Almirall, S.A., Ronda General Mitre, 151 08022 Barcelona, Spain Further information available from: Almirall Limited, Harman House, 1 George Street, Uxbridge, Middlesex, UB8 1QQ, UK. Date of First Issue: March 2022 Item code: IE-WYN-2200007
UK-Adverse events should be reported. Reporting forms and information can be found at MHRA https://yellowcard.mhra.gov.uk Adverse events should also be reported to Almirall Ltd. Tel. 0800 0087 399
IE-Adverse events should be reported. Reporting forms and information can be found at HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse events should also be reported to Almirall Ltd. Tel. +353 (0) 1431 9836


Emma Button
he BDNG has been busy planning our 2025 events and would like to give you a sneak peek of what is to come:
On the 24 March 2025 at the IET Birmingham, the BDNG will be hosting an atopic eczema masterclass day with support from Sanofi. Topics will include: TCS withdrawal, managing eczema in skin of colour, the psychological burden of living with eczema, nurse-led eczema clinics, and information from support services.
The following day, 25 March 2025, at the IET Birmingham, a day dedicated to HS will be hosted by the BDNG. We have listened to our delegates and understand the need for further education for nurses. The day includes, what is upcoming in HS, what does the nurse-led HS clinic look like, wound care information and the impact of living with HS.
This will be held in Manchester on 12-13 May 2025 at the Mercure Manchester Hotel, Portland Street, M1 4PH. After a huge success with our most recent aesthetic events, the subgroup will be delivering ‘Advancing in aesthetics for dermatology nurses’. This would be a great opportunity for delegates practising in both areas to gain more knowledge in the subject area. The subgroup is led by incredibly experienced practitioners with dual skillsets.
Rare disease day on the 12 May 2025 will have a unique focus, involving support groups including DEBRA and
the Ichthyosis Support Group. Professor Andrew Thompson and Olivia Hughes will be discussing the Toolkit for Parents of Children with Epidermolysis Bullosa.
We are planning a PDT Day for the 13 May 2025, with a view to support delegates new to PDT and for those requiring a refresher. A podcast will be available hosted by Emma Button, the BDNG’s Events Education Lead, speaking with Paula Oliver, Nurse Consultant, and David Clements, Skin Cancer Clinical Nurse Specialist, discussing an initiative that has taken place to support the demands on a current service. Podcasts are available via Spotify or Apple if you search for BDNG podcasts.
‘Advancing knowledge and skills in phototherapy’ will be running over two days on 12-13 May 2025. This will be led by Dermatology Clinical Nurse Specialist Stephanie Greenleaf, who has significant experience in running a nurse-led service and has a wealth of knowledge to share. The two-day event would be suitable for those practising in phototherapy and looking to enhance, refresh and gain new knowledge and skills. The BDNG will be hosting an introductory session to phototherapy in the autumn in Birmingham. The date for this is the 11 November 2025.
As mentioned above, this is just a sneak peak. There are more days to come and other supportive educational streams including webinars, podcasts and e-learning!
Summary
What: Atopic eczema masterclass day, Sanofi
When: 24 March 2025
Where: IET Birmingham, Austin Court, 80 Cambridge Street, Birmingham, B1 2NP
What: HS day
When: 25 March 2025
Where: IET Birmingham, Austin Court, 80 Cambridge Street, Birmingham, B1 2NP
What: Manchester Spring Meeting
When: 12-13 May 2025
Where: Mercure Manchester Hotel, Portland Street, M1 4PH
What: BDNG Awards ceremony
When: 16 May 2025
Where: Invite only – nominations are open now
What: BDNG Annual Conference
When: 30 September - 2 October 2025
Where: Harrogate Convention Centre, King’s Road, Harrogate, HG1 5LA
What: Birmingham Autumn Meeting
When: 10-11 November 2025
Where: Leonardo Royal Hotel, 245 Broad Street, Birmingham, B1 2HQ
Please check your BDNG email updates for future events and educational opportunities.
Follow us on X @BDNG / Instagram / Facebook
For more information on these events, please use the QR code. above




Teena Mackenzie
Welcome back to the BDNG podcast update where the education team are bringing you the latest insights and updates in dermatology. Our episodes are designed to keep you informed and inspired. Whether you’re a long-term listener or tuning in for the first time, we have some incredible topics and there’s something for everyone.
Clinically, many of us work closely with rheumatology colleagues. Therefore, I was delighted to record a podcast with Julie Begum, who is the lead nurse for rheumatology in her area. Julie has a passion for education and sharing rheumatology knowledge. In this BDNG podcast episode, she discusses the importance of collaboration between the two specialties and why dual education is important. On the topic of collaboration, the BDNG has been working with the British Association of Medical Aesthetic Nurses (BAMAN), hosting the first BDNG/BAMAN Instagram live, a very successful aesthetic focused regional day in Birmingham and the first BDNG/ BAMAN podcast. Please listen to the latest BDNG podcast, as Emma Button and I discuss various training and education opportunities for aesthetic nurses with Rachel Goddard and Anna Barker from BAMAN.
Gretchen Robertson, who many of you may know, is now a dedicated psychotherapist and recorded a previous BDNG podcast focusing on wellness tips for the workforce which proved very popular. The second BDNG podcast Gretchen and I have recorded highlights depressive illness with a focus on skin of colour. We hope to record further podcasts with Gretchen in the future, as she has many more topics of interest.
The valuable work Sandra Lawton, Amanda Roberts, Professor Kim Thomas and Dr Miriam Santer have done to develop resources and evidence available via Eczema Care Online (ECO) has been well received. A recent BDNG webinar highlighting this work was extremely popular and the BDNG now have a podcast discussing ECO. Further information on ECO can be obtained from www.eczemacareonline.org.uk . This podcast is well worth a listen to ensure as healthcare professionals we can signpost patients confidently. Thank you to the team and Eczema Outreach Support for being so inspiring.
On World Urticaria Day, the BDNG launched a podcast with Dr John Reed, a leading specialist in Oxford in the field of urticaria. Having had the fortune of working alongside Dr Reed in the past, he leads the way in urticaria, and if you work with this type of patient I would recommend listening.
Dr Reed fully explains pathophysiology, types, assessments, treatments and resources. You can also visit Urticaria Centres of Reference and Excellence at https://ga2len-ucare.com/ for urticaria educational resources.
Epidermolysis bullosa (EB) is a rare skin condition and the BDNG have been highlighting the valuable work Professor Andrew Thompson and Dr Olivia Hughes at Cardiff University have been working on to develop a toolkit for parents of children with EB. Listen to the BDNG podcast highlighting this work, and if you work with EB patients, please contact Olivia at hughesoa@cardiff.ac.uk as you could help in this project.
The Education Team have many podcasts recorded and they will be going live soon. If you have a topic you would like to talk about, or if you know someone with a story to tell that would make a great podcast, please email me, Teena Mackenzie, at EDLead@bdng.org.uk. We arrange a time that suits you and the interview is recorded over Teams and then edited so we make it as comfortable as we can. Thank you for now.
Derma UK podcasts
Managing a Scaly Scalp with Sebco™ Scalp Treatment
A Derma UK podcast, led by Dr Angelika Razzaque – GP, Associate Specialist in Dermatology, in conversation with Teena Mackenzie – Consultant Nurse Dermatology and Education and Development Lead, BDNG.
Menthoderm ® – Managing Itch with Menthol
A Derma UK podcast, led by Valerie Anderson, Dermatology Advanced Nurse Practitioner, in conversation with Teena Mackenzie – Consultant Nurse Dermatology and Education and Development Lead, BDNG.
Both podcasts are available at https://bdng.org.uk/derma-uk/ or via the QR code right. This is a member’s only page.

New BRaCE clinical study demonstrates CeraVe cream with MVE & Ceramides*:



Resolves skin dryness faster than a reference O/W + Glycerin cream
Strengthens the skin barrier over time in individuals with dry, eczema-prone skin
Reduces skin sensitivity to common household irritants


*Moisturiser with skin identical restores barrier function in eczema-prone skin and reduces skin susceptibility to irritants’ poster presented at the 2024 AAD Annual Meeting.
Carron Layfield (UK DCTN Network Manager)
Please note all studies mentioned below are funded by a range of National Institute for Health and Social Care (NIHR) funding programmes. These are just a selection of studies developed with UK DCTN support – for a full list please see http://www.ukdctn.org/ourclinicaltrials/index.aspx.
ALPHA hand eczema study – full NIHR HTA report published
Involving 441 participants, ALPHA compared alitretinoin and PUVA therapy in adults aged 18 years and over with severe hand eczema. The study demonstrated that as a first-line therapy, alitretinoin showed more rapid improvement and superiority to ultraviolet therapy at week 12, although this difference was not observed at later time points. A limitation of the study was poor compliance with ultraviolet therapy as most patients in this arm of the study did not receive regular twice weekly therapy. See https://pubmed.ncbi.nlm. nih.gov/39364555/ for the full NIHR Health Technology Assessment (HTA) report.
Newly funded studies
EXCISE study – £1.5 million NIHR award for skin cancer surgery study
The EXCISE study, led by Consultant Dermatologist Dr Rachel Abbott (Cardiff), will compare the clinical and cost effectiveness of oral flucloxacillin versus no antibiotic (placebo) in adults undergoing ulcerated skin cancer excision under local anaesthetic with planned wound closure. This randomised double-blind trial aims to recruit 380 adult patients undergoing surgical excision of ulcerated skin cancer from eight to 10 hospital sites across the UK. Recruitment will begin in September 2025.
Patients will be followed up by telephone (on days 5-10, 1520, and 30) and asked to monitor their wounds for infection. Should they develop any symptoms suggestive of infection, they will be asked to contact their local recruiting centre to arrange an in-person clinical assessment. The study’s primary outcome will be Surgical Site Infection within 30 days and clinical assessors will be trained in the diagnosis of wound infections and be blinded to the randomised allocation. Secondary outcomes include adverse events within 30 days, antibiotic resistance in infected wounds, quality of life, time to return to normal activities, resource use related to costs of infection, and cost of hospital visits/stays with patient
wound burden as a tertiary outcome. A qualitative process evaluation of acceptability, facilitators and barriers will be conducted with patients and healthcare professionals. The study team are currently identifying sites to get involved. If your hospital has capacity, please contact us on ukdctn@ nottingham.ac.uk and we’ll put you in touch with the study team.
PEARLS, HEALS2 and COAT – studies celebrating recruitment success
PEARLS – LS study reaches internal pilot recruitment target
We’re delighted to let you know that the PEARLS study has reached its internal pilot recruitment target, recruiting 74 patients in six months with 14 sites now open across the UK. The study is registered with the NIHR Associate PI scheme with 10 centres actively involved in this initiative. Led by Dr Rosalind Simpson and Prof Kim Thomas (Nottingham), PEARLS is investigating proactive versus reactive therapy for the prevention of lichen sclerosus exacerbation and progression of disease. The study will test using steroid flare creams twice a week, regardless of whether symptoms are experienced, or using the flare creams to treat symptoms when they re-appear. The study aims to recruit 400 female participants aged five years and above. Further information is available at https://www.nottingham.ac.uk/pearls/.
COAT study – over 120 patients now recruited to cellulitis study
COAT is a randomised controlled trial (RCT) assessing the effectiveness and safety of five-day treatment with oral flucloxacillin for the treatment of cellulitis in primary care vs the standard seven-day treatment. COAT has seen a real boost to recruitment over the past few months with 130/356 participants now recruited. To date there are 64 sites including nine Urgent Treatment Centres, but the team are keen to enlist new General Practice or Urgent Treatment Centre sites that have experience of delivering RCTs and regularly see patients presenting with cellulitis as first point
of contact. The Chief Investigator for the study is Prof Nick Francis (Southampton) and you can contact the study team directly on coat@soton.ac.uk to get involved. Further details are available on the study website www.southampton. ac.uk/ctu/coat-study.page#home.
HEALS2 – skin surgery study celebrates moving forward to the next recruitment phase
This pragmatic RCT has now recruited 60/396 participants, meeting its internal pilot recruitment target. The trial will evaluate the clinical and cost effectiveness of compression therapy in the healing of surgical wounds (healing by secondary intention) following excision of lower limb keratinocyte cancers. The study team (Chief Investigators Dr David Veitch and Dr Aaron Wernham) have had great feedback from the NIHR and have moved to the next recruitment phase with 19 secondary care centres now open across the UK.
A total of 32 sites are needed, so please do get in touch with the study team on HEALS2@leeds.ac.uk if your secondary care site has capacity to get involved. The study is registered with the NIHR Associate PI scheme, with 15 dermatology trainees and nurses involved to date. Find out more on the study website https:// ctru.leeds.ac.uk/heals2/
TIGER and BEACON – two very different eczema studies need your support
TIGER – help promote the self-referral pathway for this paediatric study of IgE food allergy tests
The TIGER study, investigating if dietary advice based on food allergy tests improves disease control in children with eczema, has now recruited 296/493 participants. Children less than two years of age with eczema are randomised into two groups: “usual care” or “test guided dietary advice”. Those in the testing group will have skin prick tests to common allergy-causing foods (milk, egg, wheat, and soya). Parents will then be advised to exclude or include one or more foods for one month with participants followed up for nine months. Subject to funder approval, the study is now looking to extend its recruitment period to August 2025.
TIGER is recruiting children through GP practices in Bristol, North and Northeast Somerset, South Gloucestershire, Bath, Dorset, Hampshire, Wiltshire, Swindon, West Berkshire, Oxfordshire, and Greater Manchester. If you work in these areas and see children aged three-months to under two years with eczema, please ask their parents/carers if they are aware of the study and encourage them to take part. Parents can self-refer by emailing tiger-study@bristol.ac.uk, but they must be registered at one of the participating GP surgeries. The list of GP surgeries, eligibility criteria and further information about participation can be found at www.bristol. ac.uk/tiger-study.
BEACON – additional sites needed for systemic treatment study
BEACON is a UK-wide, assessor blinded RCT in adults with moderate-severe eczema requiring systemic therapy. The aim is to assess the efficacy, tolerability, and cost-effectiveness of the key systemic treatments for moderate-severe adult eczema (ciclosporin, methotrexate and dupilumab). The study is utilising a platform design to incorporate emerging systemic therapies; the first addition will be abrocitinib in collaboration with Pfizer towards the end of 2024.
Recruitment is well underway at eight sites across the UK with more in set up, and the team are looking for further sites across the UK to take part to help meet the recruitment target of 402 participants. If you are interested in taking part or would like to discuss the trial with the central team, please contact the trial managers on BEACON@kcl.ac.uk. Further information is available at https://www.beacontrial.org/.
2024 UK DCTN Nurse/Pharmacist Fellowship Outcome
We’re delighted to let you know that the 2024 UK DCTN Nurse/Pharmacist Fellowship has been awarded to Lucy Moorhead, a Nurse Consultant in Inflammatory Skin Disease who is based at St John’s Institute of Dermatology, London. Lucy will undertake a two-year programme with us including attending the ‘Better Evaluation of Evidence and Statistics’ course, joining the UK DCTN Steering Committee and embarking
on a three-day visit to the UK DCTN Co-ordinating Centre in Nottingham. Please remember this award is offered on an annual basis – if you’re interested in applying next year, please contact Carron Layfield for more information or see http://www.ukdctn.org/fellowships/ index.aspx.
We received nine applications for our 2024 Themed Call on ‘Supporting Dermatology Research Priorities Identified by Priority Setting Partnerships’ which was co-funded with the British Society for Paediatric and Adolescent Dermatology (BSPAD). Five applications were short-listed and presented to the UK DCTN Steering Committee in October, with two studies being awarded funding. These were ‘Pain Management in HS’ (Dr Hannah Wainman, £8,530) and ‘Feasibility work to support the development of an RCT investigating the role of spironolactone to treat acne in younger girls’ (Dr Fiona Sexton on behalf of 2024 UK DCTN Paediatric Dermatology Trainee Group, £10,000). Our 2025 Themed Call will focus on skin of colour – please see https://www. ukdctn.org/funding-awards/ukdctnfunding-awards.aspx for details.
Membership of the UK DCTN is free and open to anyone with an interest in dermatology research. You can join via the website www. ukdctn.org, and can keep up to date with what is happening at https://x.com/UK_DCTN.
Carron Layfield (UK DCTN Network Manager)
UK DCTN Co-ordinating Centre, Centre of Evidence Based Dermatology, Applied Health Research Building, Main University Campus, University of Nottingham, Nottingham NG7 2RD. Email carron.layfield@nottingham.ac.uk

Start prescribing QV for your patients with dry skin conditions.


Dermatologically tested emollients


Sources:
In-line with NICE Guideline recommendations first-line atopic eczema treatment1–4


Suitable for a range of dry and sensitive skin conditions, including eczema, dermatitis, and psoriasis


Available both on prescription and over the counter
1. National Institute for Health and Care Excellence (NICE). Eczema — atopic. Emollients. [Internet]. London: National Institute for Health and Care Excellence, April 2022 [cited August 2023]. Available from: https://cks.nice.org.uk/topics/ eczema-atopic/prescribing-information/emollients/emollients/.
2. National Institute for Health and Care Excellence (NICE). Eczema — atopic. Scenario: Mild eczema. [Internet]. London: National Institute for Health and Care Excellence, April 2022 [cited August 2023].Available from: https://cks.nice.org. uk/topics/eczema-atopic/management/mild-eczema/.
3. National Institute for Health and Care Excellence (NICE). Eczema — atopic. Scenario: Severe eczema. [Internet]. London: National Institute for Health and Care Excellence, April 2022 [cited August 2023]. *Fifth Quadrant, Sponsored by Ego Pharmaceuticals, OTC Skincare, n=374 (Australian Dermatologists, GPs and Pharmacists). October 2023 www.qvskincare.co.uk

It has been a busy few months for the BDNG education team. With the new members, like myself, settling into our roles, we are now planning our future events and education based on the feedback and discussions held with our delegates at our autumn events.
In this article, I’ll provide a short summary of what we’ve been doing.
In September 2024 we had our 33rd annual conference in Harrogate, with roughly 450 nurses and associate members attending. Derm School’s interactive workshop proved to be very popular, with the opportunity to become hands-on with emollients and applications.
The Wednesday evening banquet bash went down well, with delegates having the chance to try different cuisines and meet up with friendly faces. I have to say, personally, that’s one of the most enjoyable and important moments at conference — it’s so great to have the opportunity to speak with likeminded colleagues. Throughout the year, there may be a few email exchanges asking for support, advice or opinions, but there’s rarely the opportunity for general chit-chat.
We have collated feedback from our delegates and are on our way to planning next year’s event, so watch this space! The dates for our annual conference next year are 29 September to 2 October 2025. Again, this will be held in Harrogate.
Shortly after conference, we held our London meeting. The days included: an update on dermatology in primary care; menopause and the skin; research; and patch testing. Members of the BDNG research subgroup hosted a full agenda aiming to address the research needs of our nurses.
Speakers from the Dermatology Clinical Trials Network (UKDCTN) presented ‘Centralising the patient voice and the power of PPI’ and ‘UK DCTN Nurse Fellowship awards – Experience’. A presentation was also given on qualitative and quantitative research, while a representative from NHS England provided an over-arching update on the four pillars of advanced practice.
I would like to say a particular thank you to Liza Benfield for hosting the patch testing update day. This had a high number of attendances, with nurses keen to ensure they are aligned with best practice. It is important to highlight that there is a patch testing practice educational e-learning module available on the website which was produced by Jodie Newman, a BDNG educational nurse. In addition, the BDNG website provides a best practice document, supporting those undertaking this practice.
The primary care subgroup hosted another jam-packed day with representation from primary care-based nurses working with a specialist interest in dermatology. Acknowledging that the first presentation for patients with dermatological conditions is often in primary care, the need for up-to-date, evidenced-based information is essential to make appropriate decisions and management plans. To meet the educational needs of the BDNG delegates, topics included: understanding the importance of maintaining good skin integrity; an update on acne; and the assessment and management of the various types of eczema, with a particular focus on better supporting children and their families. The day continued with the assessment and management of psoriasis, paying particular attention to how to optimise therapy in primary care prior to specialised secondary care input. Identification of common skin lesions in primary care and how to become more confident in reassuring patients about their benign, non-skin cancer lesions were also presented and discussed.
Menopause day was a huge success with fantastic consultant speakers joining us to share their knowledge and highlight how nurses play such an important role when interacting with patients who may be experiencing menopausal symptoms. It is an emerging topic, with clinicians advocating for more of a holistic approach to address the gaps in practice.
The agenda included an array of talks: Dr Archana Rao spoke about the pathophysiology of certain hair loss conditions and common diagnoses seen in dermatological practice; Dr Thivi Maruthappu, author of Skin Food, presented nutrition and the skin connection; Dr Momotaj Islam kindly joined us to discuss menopause management techniques, with resources for patients including her new book Coping with Menopause. Our GP guest speakers Dr Siobhan Carver and Dr Maryam Rafique presented on HRT and lifestyle medicine.
Our London event was a huge success, and included a fabulous lunch. I would like to acknowledge the hard work of our events co-ordinator Katherine Mansfield alongside Rachel McCutchen our administrator and, of course, our CEO, Susan Maguire, for supporting delegates with arrangements for the day and for ensuring such a fabulous range of refreshments were available.
Following on from our London study days, we kicked off our Birmingham winter meeting with a collaboration with Medac Pharma, talking all things subcutaneous methotrexate. Dr Tom King started the day with a discussion around the role of subcutaneous methotrexate and when to use it in psoriasis patients. This led to an opportunity for a practical demonstration with a dummy pen.
An in-depth session on blood monitoring was hosted by Emmanuel Toni, Dermatology Nurse and BDNG Trustee, who aimed to make the session light and engaging, despite the heavy (and important) topic. Teena Mackenzie, BDNG Events Education Lead, and I dived into common questions and how to address shared care. Case studies and a panel discussion were held with a fantastic dinner hosted by Medac Pharma to conclude the day.
On 12 November, we held sessions providing an aesthetics update, service development, and inclusivity and neurodiversity.
The aesthetics subgroup hit the ground running with an overview of aesthetic medicine. A panel discussion followed, involving experienced practitioners from across the dermatological and aesthetics fields.
There was a presentation on safety in aesthetics, which is a really hot topic currently, especially with aesthetic emergencies presenting in secondary care. Another presentation focused on lesions presenting in aesthetic practice, while a presentation on the patient journey, with a case study discussion, created a vast amount of discussion.
I have been working closely with the aesthetics subgroup, with a particular interest myself, and would like to say a big thank you to our two subgroup leads, Melissa Pugh and Alison Mahon. I also want to thank David Clements, Skin Cancer Nurse Specialist and Aesthetic Practitioner, who also made huge contributions to the day. I am excited for our future events, including our recent collaboration with BAMAN, and our plan to expand on this day offering more advanced knowledge.
The service development day was hosted by myself and Val Anderson, and focused on understanding what service development is and how to write a service development plan. It was great to see delegates attending who wanted to support positive changes in practice with brilliant ideas emerging and knowledge being shared in the room. Nicola Housam spoke about her experience of developing a nurse-led atopic dermatitis clinic and GIRFT, sparking lots of conversations. We closed with a panel discussion; I would like to thank the delegates that attended, particularly our paediatric colleagues.
The inclusivity and neurodiversity day was hosted by Molly Connolly and was chaired by Teena Mackenzie. It started with Molly presenting on an introduction to neurodiversity and access to services for those with learning disabilities.
Dr Bernard Ho joined us via Microsoft Teams and presented ‘Taking care of our LGBTQ+ patients’. Orla Duncan, a psychosocial nurse practitioner in Scotland, joined us to discuss ‘Paediatric Psychology and Liaison Service – Impact of differences’. Dr Bilal Malik focused on the importance of including mental health assessments, and he is keen to support BDNG nurses further providing education. Recognising common inflammatory diseases in skin of colour was presented by Modupe Olubunmi Soji-Adeyemo. The day concluded with Q&A’s.
Alongside these days, the two-day skin surgery skills course was held and hosted by Carrie Wingfield and Kate Davies. The course is often oversubscribed, with nurses keen to learn and develop
surgical skills. The first morning reinforced the importance of clinical governance, reminding nurses of best and safe practice, before moving onto skin lesion recognition. Day two brought the theory into practice, giving delegates the opportunity to practice on pork belly, including punch biopsies, curettage/ shaves, ellipse excisions, suturing and lidocaine infiltration. Q&A sessions ran throughout, allowing delegates to gain as much insight and knowledge as possible. Special thanks to Diane Rolland and our experienced surgical nurses who helped facilitate the day.
It is safe to say that the year has been a huge success, and we have already started planning our in-person events next year. We will shortly be sharing all our future in-person events dates; they will appear in the events section under ‘In person meetings’ on the BDNG website.
Thank you to our wonderful members for your continued support. We have thoroughly enjoyed interacting with you this year. Many of our members continue to contact us for advice and support in career progression and for further education. Support has been provided in ways such as Teams meetings, placing you in touch with other members, supporting academic publications, webinars, sharing emerging evidence, and developing our educational work streams. There has been a huge increase in many of you joining subgroups, writing journal articles, supporting with podcasts and speaking at events. If you would like to get involved, please do not hesitate to contact us.
Podcasts are also available via Spotify or Apple if you search for BDNG podcasts. The live webinars have proved successful, so thank you to everyone who has attended any of our webinars. For future events, please check your BDNG email updates and follow us on X, Instagram and Facebook.
For those of you celebrating Christmas, we wish you an enjoyable time and hope you can have a break over the festive period. We look forward to seeing you in the new year.
Sandra Lawton
It was a real privilege to attend the Q uality in Care (QiC) awards to catch up with so many dermatology colleagues and friends, old and new – many of whom I had not seen for a few years.
Over my long dermatology career I have seen what a difference everyone has made, from a service, patient, research and education perspective. Dermatology is an ever-evolving speciality with healthcare professionals, patient groups, educators and researchers working together to make a difference.
Author info:
Sandra Lawton OBE, Queen’s Nurse, MSc, RN,OND, RN Diploma (Child), ENB 393.
EThis was especially apparent at the awards, and everyone deserved to win, be commended and be on the finalist list.
I had nominated Eczema Outreach Support (EOS) family workers and youth panel for the Team Award, and it was an honour to accept the award for the youth panel on their behalf (see photo). Seeing Nicola Housam win the Healthcare Hero Award for her work with emollients and fire risk, and Mandy Aldwin-Easton for winning the People’s Award for all her amazing work with the Ichthyosis Support Group was an added bonus. It’s so important to work together share ideas, look at different ways of providing care and support for our patients and not be afraid to shout about what you have done. Please continue to do so and keep the dermatology word out there. Congratulations everyone!
ach year, the Dermatological Nursing Editorial Board actively seeks and encourages colleagues to write up their work and publish. As Clinical Editor, I support this and would like to highlight this call for papers.
l Are you interested in having your work published?
l Have you completed some work or a project which enhances knowledge, skills or dermatology nursing?
l Do you have a patient case study that would be of interest to our readership?
l Have you recently completed an audit that has informed practice development or service delivery?
l Have you recently completed a dissertation or a literature review?
l Is there a particular clinical area that interests you and you wish to share?
l Do you have a medical or nursing colleague who would like to write for publication?
If so, please consider submitting a paper to Dermatological Nursing
Support and advice is available to guide you through the writing process, so please, do not hesitate to contact myself, Jackie, Rob or any member of the Editorial Board for advice. (Email addresses are listed in the journal).
Polly Buchanan Clinical Editor, BDNG Dermatological Nursing Journal























The BDNG would like to thank all our corporate sponsors for their support and continued investment with the BDNG
A NICE-recommended treatment for moderate-to-severe atopic dermatitis (AD) in eligible patients1,2
EBGLYSS is indicated for the treatment of moderate-to-severe AD in adults and adolescents 12 years of age and older with a body weight of at least 40 kg who are candidates for systemic therapy.1
NICE has recommended EBGLYSS for treating moderate-to-severe AD that is suitable for systemic treatment in people 12 years and over with a body weight of 40 kg or more, only if:2
• the AD has not responded to at least 1 systemic immunosuppressant or these treatments are not suitable, and
• dupilumab or tralokinumab would otherwise be offered, and
• the company provides it according to the commercial arrangement
Contact Almirall today for further information and evidence to support inclusion of EBGLYSS on your hospital formulary or local guideline


and adverse event reporting information.
AD, atopic dermatitis; NICE, National Institute for Health and Care Excellence.
References: 1. EBGLYSS (lebrikizumab). Summary of Product Characteristics. 2. National Institute for Health and Care Excellence. Lebrikizumab for treating moderate to severe atopic dermatitis in people 12 years and over. Available at: https://www.nice.org.uk/guidance/ta986/ chapter/3-Committee-discussion [Last accessed: November 2024].