INNOVATION REPORT 20

23
As cardiothoracic surgeons we have always been at the forefront of medicine with truly and literally cutting-edge technology. Most of the operations that we now do every day were previously thought to be impossible, or surgeons were discouraged to do these operations as the risk was considered to be too high.
As cardiothoracic surgeons we have always been at the forefront of medicine with truly and literally cutting-edge technology. Most of the operations that we now do every day were previously thought to be impossible, or surgeons were discouraged to do these operations as the risk was considered to be too high.

Cardiothoracic surgery is a relatively young specialty and the road towards where we are now has never been an easy one. To put it simply: we have come a long way – a journey that was full of obstacles – but we now have an impressive arsenal of techniques. Millions of patients that would die some decades ago can now be saved by cardiothoracic surgeons.
The caveat of our success is that we may take our knowledge and techniques for granted. Impressive steps, such as percutaneous valve implantation, have been made by interdisciplinary teams and allow the treatment of patients with many co-morbidities.
As cardiothoracic surgeons, our knowledge must be used to further evolve our treatments. Many of our techniques can be improved and can even be used to the benefit of patients with other diseases. We should not lose our momentum and must continuously strive for innovation. Better outcomes for patients also contribute to the sustainability of health systems.


At EACTS we have made ‘Innovation’ our highest priority, leading to the first ‘EACTS Innovation Summit’ in April 2023. In a very interactive setting, representatives from many disciplines (basic science, cell and tissue engineering, radiology, mechanical engineering, chemistry and others) were joined by experts from cardiothoracic surgery and representatives from industry.
Topics that were discussed ranged from ‘organs on a chip’ to a contracting tube from cultured myocardial cells, from new fibre technologies for CPB and ECMO to total artificial heart, from sophisticated imaging modalities to a simple device to visualise the aortic valve.
Everything that could possibly benefit our patients was open for discussion. Bringing an innovative idea to a prototype and then to clinical use is a complex and lengthy process. EACTS does not want to promote innovation for its own sake, but to assist and bring innovations forward so that they can be used to cure cardiothoracic disease. Yearly ‘Innovation Summits,’ an innovation programme at the Annual Meetings, a web-based ‘Innovation Forum’ and support for young investigators are just a few examples we are planning in order to restore innovation as a core value in our specialty.
We hope this special Innovation Report will inspire you to consider how you can make a positive difference through innovation, to foster debate, to share ideas and insights, and remove barriers.
EACTS / INNOVATION REPORT 3 2 INNOVATION REPORT 20 23
Friedhelm Beyersdorf Mark Hazekamp
FOREWORD
CONTENTS Foreword 3 EACTS Innovation Summit 4 Innovation Timeline 6 Innovation in Action 7 The 37th EACTS Annual Meeting 17
EACTS INNOVATION SUMMIT PARIS, 2023
Innovation is part of the DNA of cardiothoracic surgery. The inaugural EACTS Innovation Summit, which took place in April 2023, reaffirmed our organisation’s renewed commitment to fostering a culture of discovery and development to bring forward the very best technologies and techniques for the benefit of patients.
An invited audience of more than 60 surgeons, engineers, scientists, cardiologists and industry leaders attended the two-day summit in Paris and responded positively to a call to embrace innovation in surgery and the development of new ideas and concepts.
The first Innovation Summit was led by two Past Presidents of EACTS, Friedhelm Beyersdorf and Mark Hazekamp. The expectation is that the summit will become an annual event, as well as contributing to the EACTS Annual Meeting.
We must incorporate innovation thinking into our blood and bring innovations to the clinic. It is really important; there is no future if we do not do that.
Mark Hazekamp
In Paris, experts from the cardiothoracic surgery field, as well as other scientific specialties, shared new science and thinking on technologies and
techniques that could drive better outcomes for patients with heart and lung disease. There were 36 presentations, with a focus on innovative approaches to surgery that are already having a positive impact on care or hold out the promise of improvements in the near future.

Friedhelm Beyersdorf and Mark Hazekamp asked the audience to take inspiration from the pioneers of heart surgery from the 1950s and 1960s, a period of extraordinary innovation. The techniques and instruments that they developed are still present today. “Our grandfathers achieved the unachievable,” said Friedhelm.
The mission of cardiothoracic surgery all over the world is to improve the treatment for our patients. We are here to serve our patients as best we can.
Friedhelm Beyersdorf
Mark said: “In the beginning there was a lot of work to be done and progress was immense. In 10 or 20 years we almost went to the moon.” He cited the caged ball and the biological valve prosthesis as examples of innovations from decades ago that are still in daily use in 2023.
As well as showcasing some of the most promising developments on the cardiothoracic landscape, the innovation summit invited those in attendance to consider what obstacles currently discourage innovation and how these can be overcome. Mark highlighted regulatory hurdles in Europe, including the EU’s Medical Devices Regulation,
which was creating challenges for innovators looking to bring forward new solutions. The environment in the United States was more conducive to innovation, he said.
The Paris summit put forward four principles that would drive EACTS’ commitment to innovation going forward:
• Cardiothoracic surgery has to offer innovative treatments for patients
• Cardiothoracic surgery will lose without innovation
• Cardiothoracic surgeons have the capacity to embrace innovation
• Cardiothoracic surgeons must be innovative Attendees were also asked to consider the best way to ensure that innovation became embedded across EACTS’ activities.
One option under discussion is the creation of a new Innovation Domain, alongside the four existing domains of Acquired Cardiac Disease, Congenital Heart Disease, Thoracic Disease and Vascular Disease. The new domain would also complement the Techno-College and work with the prestigious Francis Fontan Fund for Education.
The Innovation Domain would become a vehicle for the cross-fertilisation of ideas, bringing together senior and junior professionals, including residents, surgeons, scientists, engineers and representatives from industry.
Prof Sir Bruce Keogh, former Medical Director of the NHS in the UK, congratulated EACTS for its ambitious approach to innovation and urged members to harness the opportunities this presented. “There is no better place to try to incubate innovation than in the sort of organisation with the intellectual capacity that EACTS has at its disposal,” he said.
If we don’t use this opportunity to think about how we can use some of the collective brains in the room to focus EACTS on the intellectual capacity of the combined membership to bring innovation to improve the quality of care for our patients, then all of this has been a bit irrelevant.
Prof Sir Bruce Keogh
EACTS / INNOVATION REPORT 5 4
20 23 PARIS
INNOVATION TIMELINE
1896
First successful suture of a heart wound by Ludwig Rehn, Frankfurt
1952
INNOVATION IN ACTION
ASD closure using deep hypothermic arrest by Lewis and Taufic
1953
First successful heart operation using a heart lung machine (ASD closure) by John Gibbon, Philadelphia
1954
First successful cross-circulation (Perfusion by one parent, “living heart lung machine”) by Walt Lillehei, Minnesota
1960
In this section you can read a summary of a selection of the presentations that were given at the Innovation Summit in Paris.
First successful aortic valve replacement by Dwight Harken, Boston
1967
Coronary bypass surgery by René Favaloro, Cleveland Clinic
1967
First heart transplantation by Christiaan Barnard, Cape Town
1969
Mechanical circulatory support (“artificial heart”) by Denton Cooley, Houston

1983
Isolated lung transplantation by Joel Cooper and Alec Patterson, Toronto

6
20 23 PARIS
INNOVATIVE SOLUTIONS THAT WILL IMPROVE CARDIOTHORACIC SURGERY IMAGING TECHNOLOGIES
Jutta Arens
Professor in Engineering Organ Support Technologies at University of Twente, The Netherlands
New hollow fibre technologies have the potential to change the world of extracorporeal membrane oxygenation. With this new technology a single ECMO device provides cardiac, respiratory and renal support. Oxygenators play a key role when a surgical procedure may necessitate the interruption or cessation of blood flow in the body, a critical organ or great blood vessel. As the blood passes through the oxygenator, it comes into intimate contact with the fine surfaces of the device itself. Gas containing oxygen and medical air is delivered to the interface between the blood and the device, permitting the blood cells to absorb oxygen molecules directly.
Research is taking place into a new generation of oxygenators, for higher efficiency and greater long-term stability, with more applications to address changing clinical needs, such as combining kidney and lung treatment, the ambulation of patients and implantability.
Research is taking place into a new generation of oxygenators, for higher efficiency and greater long-term stability
Work is taking place in the development of new membranes. In the early days, oxygenators used flat sheet membranes. Today fine hollow fibres are employed. In Aachen, Germany, there are two groups researching 3D printed membranes which mimic the alveolar structure of the lung, with new oxygenator member geometries with high flux composite membranes. Researchers seek to multiply these structures as part of the process towards the design of artificial lungs that meet opposing requirements with regard to handling the blood, the gas exchange and pressure loss.
Amir Hossein Sadeghi
Cardiothoracic surgery, Erasmus Medical Center, Rotterdam, The Netherlands
The membranes would be highly efficient, with locally adjusted permeability for ideal flow distribution. Looking towards implanting these lungs, it should be possible to overcome geometric restrictions associated with hollow fibre membranes and help develop an artificial lung that really fits in the thorax.

Existing printing technology and resolution quality is not yet suitable for this purpose but the technology is evolving very quickly, holding out the possibility of this becoming available in five to 10 years.
Other groups of researchers are working on so-called micro fluidic devices, which can mimic the pulmonary capillary bed, as a step towards the development of artificial organs. But many barriers need to be overcome, a major challenge is how you scale up to a full organ, with collective channels. Research is taking place at McMasters University in Canada and at the University of Michigan on scaling and on production technology.
Virtual and augmented reality help the surgeon to improve planning the procedure. Minimised damage, shorter operative times and a more personalised treatment strategy are advantages for the patient.
Three types of extended reality are available to assist surgeons.
Virtual reality creates a fully digital environment that enables interaction in the virtual world by wearing a head-mounted display; augmented reality expands the physical reality with a digital projection; and mixed reality is the combination of the real environment with digital projections.
Pulmo VR is a software platform that harnesses the possibilities available through extended reality for the benefit of surgical patients. It was co-developed by the Department of Cardiothoracic Surgery at Erasmus and a start-up in Amsterdam.
The Pulmo VR platform places a digital twin of the patient in front of the surgeon. This twin is made up of patient CT scans and segmentations which visualise anatomic structures as airways, arteries, veins and segments.
The user has complete control over the position of the digital twin, meaning it can be placed in any position. Likewise, the user has full control over the CT scan and segmentised structures, which can be adjusted in opacity, brightness, colour or values.
The structures can be selected individually from each other. For example, within the presets function with a single click, the surgeon can decide to only show the airways and segments or veins and airways, or decide their own presets. Users have even more freedom than in the operating room, by combining images and creating a 3D reconstruction of the patient. It also enhances preoperative planning, making it possible to rehearse a procedure before surgery.
Validation studies included a technical and clinical feasibility trial in 10 patients, followed by a study of 50 consecutive patients undergoing segmentectomies at the Erasmus Medical Center. These showed that plans were changed in 52% of cases, based on the clearer images provided by Pulmo VR. Lung sparing increased by 10% and 14% used surgery on a different segment, including locating the tumour in a different section.
A multicentre trial has been set up in nine hospitals in the Netherlands to validate the results of the single centre trial. Already 30 patients are included, with an aim to reach 100 patients within a year.
8 EACTS / INNOVATION REPORT 9
01 02 INNOVATION SUMMIT INNOVATION SUMMIT
“ 6.1% 8.2% 2019 3.8% 3.8% 2018 21.9% 2020 8.2% PulmoVR 7.7% 18% 2021 PulmoVR 9.8% 19.4% 2022 PulmoVR ? 28% 2023 (Apr) PulmoVR Nationwide (Netherlands) average ErasmusMC >130 PULMO VR SEGMENTECTOMIES PLANNED & PERFORMED SINCE APRIL 2020 % segmentectomy for primary lung cancer in the NL
Pulmo VR is a software platform that harnesses the possibilities available through extended reality for the benefit of surgical patients
3D MEMBRANES
From 3D membranes to oxygenator
INNOVATIONS THAT WILL REALLY EXPAND THE FIELD OF CARDIOTHORACIC SURGERY
Georg Trummer
Department of Cardiovascular Surgery, Universitäts-Herzzentrum Freiburg
Expanded resuscitation and multi organ repair are the next level of ECPR: controlled and automated whole-body reperfusion highly increases the chances of cardiac arrest patients while minimising damage to brain and other organs.
CARL therapy is a new method of resuscitation, which was developed in an interdisciplinary collaboration of physicians and perfusionists at the Department of Cardiovascular Surgery at Freiburg University Hospital.
In an initial pilot study, the doctors were able to save many of the patients treated with CARL therapy even though resuscitation time was very long, ranging from 50 to 120 minutes.
The standard response to cardiac arrest – compressions of the chest – has been with us since the 1960s. Nothing has changed since then, and the outcomes have not changed either.
The pathophysiologic mechanism of injury after CA is ischemia reperfusion, not only in vital organs but also to the whole body. After ischemia induced by CA, low-flow reperfusion is usually established during the first phase by CPR (basic life support and advanced life support).
CPR may result in the return of spontaneous circulation or (in selected cases) in the use of extracorporeal circulation. Even if return of spontaneous circulation can be established, intermittent phases of additional CA may occur.
CARL, based on cardiac surgical techniques developed over the past 20 to 30 years, was designed to minimise this whole-body ischemia–reperfusion injury.

As a result of cardiac arrest, blood vessels in the brain swell, making them less permeable to gas exchange. CARL therapy helps to maintain a high, pulse-like blood pressure during controlled whole-body reperfusion, creating conditions more quickly to resuscitate the brain.
Oxygen levels must be low and increased only slowly. Otherwise, free radicals are generated in the tissues. These very aggressive molecules can then attack, among other
Pedro del Nido
Chairman, Department of Cardiac Surgery. William E. Ladd Professor of Child Surgery, Harvard Medical School.
things, the mitochondria, the power plants of the cells. A reduced calcium concentration in the blood also helps to protect the mitochondria.
CARL treats the damage caused by cardiac arrest and the associated lack of oxygen, because it is possible to measure and control all the important parameters, such as blood values, that are necessary for successful resuscitation.
A unique dual-pump control system enables the necessary high pulsatile blood flow and realises a high blood pressure, oxygen levels can be precisely controlled, and a mobile cooling unit allows the patient’s body to be cooled down quickly and safely. The device is relatively small and light, so it fits in the ambulance and can be carried directly to the patients.

Mitochondrial transplantation is a promising technique that transfers healthy autologous mitochondria from non-ischemic tissue into damaged cardiac cells and thus allows for recovery of myocardium.
The uptake and cellular functional integration of the transplanted mitochondria appears to occur in all cell types. Efficacy and safety have been demonstrated in cell culture, isolated perfused organ, in vivo large animal studies and in a first-human clinical study.
If mitochondria are so important and we know that they can enter the cell, can we transplant mitochondria to damaged tissue? The one Achilles heel of ischemia reperfusion is supporting mitochondrial. You can support organs for a very long time but any organ that is very dependent on energy production, mitochondrial damage is the key to deterioration.
Efficacy and safety have been demonstrated in cell culture, isolated perfused organ, in vivo large animal studies and in a firsthuman clinical study
Experiments have demonstrated that the transplanted mitochondria are taken up rapidly by the cells by actindependent endocytosis. Mitochondrial uptake by clathrinmediated endocytosis, caveolae-mediated endocytosis and tunnelling nanotubes has been shown not to play a role in mitochondria uptake in the initial and early phases of mitochondrial transplantation.
Doctors were able to save many of the patients treated with CARL therapy even though resuscitation time was very long
Studies have also demonstrated that both direct injection and intravascular delivery of mitochondria to the heart are safe. When compared to control hearts, studies have shown that there is no short- or long-term proarrhythmia associated with mitochondrial transplantation.
The first clinical application of mitochondrial transplantation in paediatric patients who suffered myocardial ischemia–reperfusion injury were performed under an Innovative Therapies process developed by the Boston Children’s Hospital Institutional Review Board. This was a single-centre retrospective study of 24 patients.
To date, only autologous mitochondria, isolated from the patient’s own body have been used. However, it has been shown that there is no direct or indirect, acute or chronic alloreactivity, allo-recognition or DAMPs reaction to single or serial injections of allogeneic mitochondria. These results greatly expand the use of mitochondrial transplantation by allowing for serial mitochondrial transplantations and the use allogeneic tissue as a source of mitochondria.
10 EACTS / INNOVATION REPORT 11
03 04 INNOVATION SUMMIT INNOVATION SUMMIT
INNOVATIVE SOLUTIONS THAT WILL IMPROVE CARDIOTHORACIC SURGERY

Bardia Arabkhani
Cardiothoracic surgeon, Leiden University Medical Center
NEW HORIZONS IN ROBOTIC SURGERY
Franca Melfi
Vice Chair of EACTS and Professor of Thoracic Surgery at the Medical School of the University of Pisa and Chair of Robotic Multidisciplinary Centre for Surgery – Thoracic MIS and Robotic surgery at the University Hospital of Pisa
A simple device to see and test the aortic valve after repair. Before the aorta is closed and the clamp is removed, we can now measure aortic regurgitation and visualise the valve. This allows for better understanding (and correcting) of an imperfect repair. It saves bypass and clamp time and provides better insight into the finesses of aortic valve repair.
Aortic valve repair procedures can be technically challenging, and there are no tools to evaluate the valve intraoperatively. To meet this need, the Aortic valve Visualisation and Pressurisation device (AVP) has been developed.
The AVP is an intraoperative device that enables valve inspection and evaluation under physiological conditions, including visualisation of the valve before reimplantation of the coronary arteries. The device also allows measurements of potential aortic valve regurgitation and targeted adjustments on the valve. It is easy to use and makes valve-sparing procedures more accessible.
IN 22 PATIENTS POST OPERATIVE VALVULAR REGURGITATION WAS
GRADE 1
AND THE MEDIAN LEAKAGE MEASURED WAS 90 MIL/MIN, IQR 55 – 120 ML/MIN
The AVP is a cylindrical device with a few openings. One opening is for the introduction of an endoscopic camera to visualise the valve. There is a two-way canula, usually a cardioplegic canula, attached to the heart lung machine and a pump to pressurise the root. Measurements of potential valvular leakage are taken. Clinical results are promising. So far, measurements are available for use of the AVP device in 24 patients undergoing valve-sparing root replacement (reimplantation techniques). In 22 patients post operative valvular regurgitation was < grade 1 and the median leakage measured was 90 mil/min, IQR 55 – 120 ml/min. In two patients with complex anatomy the valve was replaced after evaluation with the AVP device.

The next step in the development of the AVP is a clinical trial. The latest generation of the device is easy and intuitive to apply, taking only a few minutes. It should be applicable to many types of valve-sparing techniques (and SCAR/Ross procedures). It is important to gather more data for validation with echocardiography.
Robotic surgery is being transformed by the rise of new technologies like AI and 5G. Even though the technology has been around for more than 20 years, in many ways it has remained in the early stages of adoption. But now, with more companies entering the market and new technologies enabling better robotic systems, a transformation is taking place. Robotic surgery is already well-established. Around the world more than 12 million patients have undergone robotic surgery procedures in all specialties. In Europe more than 900 robotic systems are in place in surgical theatres and more are being commissioned. Europe pioneered robotic surgery, although progress has stalled more recently because of regulatory hurdles.
Nonetheless, the advance of robotic surgery has been remarkable. To put this progress into context, in 2001 Prof Melfi was the first surgeon to perform a robotic lobectomy. This was considered an experimental procedure, with a team with no experience of robotic surgery, with no guidelines and using a platform with three surgical arms that was already old.
Today there is a substantial body of research to reassure patients over the safety of robotic surgery. We know that rates of long-term survival are higher for robotic surgery than for open surgery; recovery is quicker so people spend less time in hospital; and the risk of complications, such as blood clots is lower. Robotic surgery is better for patients because it is less intrusive. It is easy for surgeons to switch from open surgery to robotic surgery, using the same movements and techniques.
Now we are in a new phase of the development of robotic surgery. The robot is just one element in a vast system designed to assist the surgeon. Research is ongoing into augmented reality platforms for robot-assisted laparoscopic surgery; 3D deformable object tracking and extraction in medical imaging; AI for image processing; new surgical instruments; and new sensing devices.
The images now available to surgeons during procedures are remarkably clear and getting even better as the technology advances. The 3D virtual reconstructions are really helping surgery. When you apply the AI algorithm it gives you the opportunity to study and understand the shape of the tumour and the aortic arch. This manoeuvre can be performed in a safe way for the patient.
Robotic surgery is already well-established. Around the world more than 12 million patients have undergone robotic surgery procedures in all specialties.
We are seeing many new platforms and new approaches, which can only be good for the future of robotic surgery. There are exciting advances in robotic navigation; for example, two robotic bronchoscopes have been approved by the FDA (Ion and Monarch).
We have new sensing devices, including sensors for surgical robotic tools to provide information to surgeons, and a new class of sensors to acquire vital chemical information from the field. There are new surgical instruments for telerobotic surgery, such as mechatronic design of tools with integrated sensors and control strategies to improve the surgeon’s sensorimotor skills.
12
05 06 INNOVATION SUMMIT INNOVATION SUMMIT EACTS / INNOVATION REPORT 13
<
MEDICAL ENGINEERING TECHNOLOGY
Andre Vincentelli
MD, PhD, Centre Hospitalier Régional Universitaire de Lille
Heart transplantation remains the gold standard therapy for heart failure. However, this therapy is constrained by the shortage of donors, which limits the number of transplants. The emergence of total artificial hearts offers new hope to patients.
Heart failure occurs when the heart can no longer carry out its essential function as a blood pump to provide a sufficient cardiac output to satisfy the metabolic needs of the organism. It primarily affects the left chamber of the heart, then the right chamber, leading to biventricular heart failure. At this stage, vital organs such as the brain, liver and kidneys do not receive enough nutrients and oxygen to function.
Heart failure is the leading cause of death in the Western world and is increasing in prevalence. Despite advances in therapies and prevention, the prognosis is poor: a survival rate of less than 50% five years after diagnosis.
A shortage of donors for heart transplants has encouraged the development of mechanical circulatory support (MCS) systems, including total artificial hearts. These are placed in the chest to replace damaged heart ventricles and valves, usually as a temporary measure (often called a “bridge to transplant”) to keep blood pumping while a person waits for a donor heart.
Aeson, the world’s most advanced artificial heart, has been developed by France’s CARMAT. This device is electrohydraulically driven with a shape close to a human heart. Once the Aeson is connected, it duplicates the action of a normal heart, providing mechanical circulatory support and restoring normal blood flow through the body.
Aeson is composed of three parts: the implanted prosthesis; external equipment, including a controller and batteries lasting up to four hours; and a hospital care console for the hospital medical team to operate the prosthesis during implantation and track how the device is functioning.

CARMAT announced the first implantation of the Aeson artificial heart in January 2023. The procedure was performed during the last week of December 2022 by Prof André Vincentelli and his team at Lille Regional University Hospital. The implantation was within the framework of the EFICAS clinical study in France. In addition to the Lille hospital, five other medical centres take part in this study: Pitié Salpêtrière University Hospital and Georges Pompidou European Hospital in Paris, Rennes University Hospital, Strasbourg University Hospital and Lyon University Hospital (Hospices Civils de Lyon).
This prospective study will involve a total of 52 patients eligible for a heart transplant in France and will allow CARMAT to collect both additional data on the efficacy and safety of its artificial heart and medico-economic data to support its value proposition and the device’s reimbursement, notably in France. The study’s primary endpoint is survival for 180 days after implantation of the device without a disabling stroke, or a successful heart transplant within 180 days of implantation.
MITIGATING THE NEGATIVE SIDE EFFECTS OF CARDIAC SURGERY
Vincent Portero
Leiden, The Netherlands
Optogenetic engineering can terminate atrial fibrillation and ventricular tachycardias. Arrhythmias can be treated from within the cells themselves after introducing light-gated depolarising ion channels with viral vector technology. Arrhythmias will trigger a light pulse from an implanted LED and then stop the rhythm disturbance.
Ventricular arrhythmias are a large and growing problem worldwide, with high annual mortality and morbidity rates. Despite progress in therapeutic strategies, the current treatment options remain suboptimal. Drug treatment can be ineffective while catheter ablation may cause irreversible complications and generally has a modest-long-term efficiency. Electroshock therapy is effective in terminating ventricular arrhythmias and has shown reduced mortality. However, the high voltage shocks delivered by these devices are traumatising, especially when given inappropriately, as they are associated not only with severe pain, anxiety and depression but also with myocardial tissue damage. So there is an unanswered need for ambulatory acute termination of atrial fibrillation.
Optogenetics, however, is a novel biological technique allowing electrical modulation in a specific, reversible and trauma-free manner using light-gated ion channels. The technique allows researchers to turn a specific cell or cellular region on and off with precision and high resolution by using a protein called opsin. This makes it possible to control animals’ specific behaviour (such as pain and fear) and demystifies the involvement of specific cells in controlling those behaviours.

This may provide a new incentive for the development of biological and pain-free treatment options for cardiac arrhythmias. Recent studies have shown that cardiac optogenetics allows for optical pacing of the whole heart and light-induced arrhythmia termination in cell cultures.
Francis Crick first proposed the concept of optogenetics in 1979 where light can be used to obtain rapid spacetime dependent control over targeted neurons. However, the idea wasn’t applied in neuroscience due to the unavailability of advanced tools and methods.
The development of new tools and processes has accelerated further research, particularly over the past 10 years. Results indicate that optogenetics can be deployed efficiently to terminate atrial fibrillation by making use of bioelectrical current and could be used for ambulatory acute termination of AF in patients.
Further research is needed to address a number of challenges, including the immune response, cytotoxicity, stable expression over a long period, non-invasive delivery, the LED system and biocompatible materials that will be needed.
14
07 08 INNOVATION SUMMIT INNOVATION SUMMIT EACTS / INNOVATION REPORT 15
THE CARMAT TOTAL ARTIFICIAL HEART
LIGHT SOURCE
AESON®
IMPLANTABLE
THE FUTURE OF CT AND MRI IN CV MEDICINE AND SO, TO VIENNA!
Fabian Bamberg
Freiburg, Germany
Quantum physics is essential for photon-counting CT scan technology with a much higher spatial resolution and lower radiation dose. Coronary angiography will be replaced by this technique in the coming years.
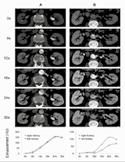
For nearly 50 years, CT has proven to be a vital imaging tool used by clinicians for the diagnosis of disease, trauma, or abnormality and for planning, guiding and monitoring therapy. CT is able to detect cancers at early, more treatable stages and effectively evaluate many heart conditions.
Photon counting technology has the potential to be a substantial step forward for CT imaging that will benefit millions of patients worldwide. It promises to further improve the capabilities of traditional CT, including the visualisation of minute details of organ structures, improved tissue characterisation, more accurate material density measurement (or quantification) and lower radiation dose.
For nearly 50 years, CT has proven to be a vital imaging tool used by clinicians for the diagnosis of disease, trauma, or abnormality and for planning, guiding and monitoring therapy

This can help to provide clinicians with more detailed images of small blood vessels, vascular pathologies and malignant changes at an earlier stage. As a result, photon counting CT can significantly increase imaging performance for oncology, cardiology, neurology and many other CT applications.
Photon counting CT uses new, energy-resolving x-ray detectors to count the number of incoming photons and quantify photon energy. This technology results in higher contrast-to-noise ratio, improved spiral resolution and optimised spectral imaging, compared to conventional energy-integrating techniques.
4D INFORMATION: VASCULAR
A photon counting detector is made from a semiconducting material that allows for the direct conversion of the x-ray photon to an electrical signal. In this case, the many photons hitting the detector can be counted individually, creating a more accurate signal to generate images. In addition, the energy level of each photon can be quantified, producing high quality spectral information.
The UK’s first photon counting CT scanner was installed at John Radcliffe Hospital in Oxford in 2022. It replaced an existing MRI at the AVIC, which took up to an hour to complete a single cardiac study. The new scanner reduces cardiac scanning time to just a few minutes, enabling the expansion of services to include vascular imaging for acute patients and the provision of a routine cardiac CT service. Specialists can also chart disease progression.
The reduction in the radiation dose means that the technology has a much wider range of applications. Over the next five years or so we shall see an increase in the use of imaging, which will detect disease earlier over time.
The 37th EACTS Annual Meeting takes place in Vienna from October 4 to October 7 2023. Innovation will be one of the themes of the year’s event, and members will have an opportunity to further discuss and develop proposals put forward in Paris earlier in the year. In particular, there will be an opportunity to build on the proposed creation of a new Innovation Domain.
In addition, four presentations from Paris have been selected to feature at the annual meeting. The aim is to disseminate new ideas to the wider cardiothoracic surgery community. Topics include opto-electronic implants, an arrhythmia treatment that takes place at a cellular level using small-scale implanted light-emitting diodes (LEDs); mitochondrial transplantation, which sees healthy autologous mitochondria transferred into damaged cardiac cells; myocardial regeneration; and automated reperfusion of the whole body.

There will be two full-day Techno-College streams featuring the latest innovation and technological breakthroughs in cardiovascular and thoracic surgery as well as interventional cardiology, and an extensive exhibition featuring the latest innovations from industry.
eacts.org
16
09 INNOVATION SUMMIT EACTS / INNOVATION REPORT 17
INNOVATION REPORT 20 23

















