

armacy N | S U M M E R 2 0 2 3 Remedying the Missing Clotting Factors 2023 Annual Convention Highlights In This Edition: Celebrating the Achievements of Arizona Pharmacy Professionals 2023 Legislative Recap
BoardofDirectors2022-2023

OFFICERS
PresidentDawnGerber
PresidentElectKimberlyLangley
PastPresidentDarrenClonts
TreasurerJacobSchwarz
SecretaryNancyCostlow
Director/CEOKellyFine
DIRECTORSATLARGE
CommunityPhillipIeng
HealthSystemChristopherEdwards
TechnicianMelindaBrowning
ReasolChino
RyanGries
BrandyDeChellis
MistyBrannon
NinaVadiei
LIASIONS
UniversityofArizona
StudentChapterJoseEspinoza
Dean'sDesignatedRepresentativeNancyAlvarez
MidwesternUniversity
StudentChapterLyndyAbdelsayed
Dean'sDesignatedRepresentativeMichaelDietrich
CreightonUniversity
StudentChapterSharonRuditser
Dean'sDesignatedRepresentativeJaneStein
LegalCounsel
RogerMorris
AzPAStaff
ChiefExecutiveOfficer
KellyFine
Education&ProfessionalDevelopment
DawnGerber
Events&StrategicPartnerships
CindyEsquer
Membership&VolunteerServices
MarquesBottorf
StrategicPrograms
KristinCalabro
AdministrativeServices
MelinaEsquer
Editor KellyFine
CreativeCoordinator
ElizabethNelson
TheinteractivedigitalversionoftheArizonaJournalofPharmacyisavailableformembers onlyonlineinyourmemberportal
(480)838-3385|admin@azpharmacy.org
Editor'sNote:Anypersonalopinionsexpressedinthismagazinearenotnecessarilythose heldbytheArizonaPharmacyAssociation."ArizonaJournalofPharmacy"(ISSN1949-0941) ispublishedquarterlybytheArizonaPharmacyAssociationat:1845E.SouthernAvenue, Tempe,AZ85282-5831

President’s Message 4 Contents COVER STORY
pg. 11 AzPA News Welcome New Members 5 Editorial 2023 Fall Conference Preview 6 Advocacy 2023 Legislative Session Recap 36 Continuing Education Remedying the Missing Clotting Factors 19 Az-ASHP State Affiliate News 14 University & Alumni News 29 Arizona Board of Pharmacy Update 47 Preceptors Corner 15 Rx & the Law: Computerized Provider Order Entry 27 Audit Target – Pre-Filled Injectable Pens & Syringes 35
U P C O M I N G E V E N T S
Fall 2023 | Virtual

August 26 | Virtual
September 23 - 24 | Glendale


September 16 | Virtual
February 24-25 | Phoenix, AZ

June 6 | Phoenix, AZ
June 6-9 | Phoenix, AZ


3
Dawn Gerber, PharmD, BCGP, FASCP, FAzPA Associate Professor of Pharmacy Practice at Midwestern University College of Pharmacy-Glendale, Arizona, earned her Doctor of Pharmacy degree from Drake University, Des Moines, Iowa She completed a pharmacy practice residency at the Creighton University Medical Center, Omaha, Nebraska. Dr. Gerber is a Board Certi�ed Geriatric Pharmacist (BCGP) and recognized as Fellow of the American Society of Consultant Pharmacists (ASCP) and Fellow of the Arizona Pharmacy Association (AzPA). She collaborates with the Banner Geriatric Medical Fellowship Multidisciplinary Rounds She teaches geriatric pharmacotherapy topics and the required Complementary and Alternative Medicine course She has held leadership positions with AzPA, ASCP, and American Society of Health-System Pharmacists (ASHP) She is a Pharmacy Residency Accreditation Practitioner Surveyor with ASHP
Dear AzPA Members,
As my term as President of the Arizona Pharmacy Association from 2022 to 2023 comes to a close, I wanted to take a moment to express my deepest gratitude for the opportunity to serve and lead your association
As I re�ect on the past year, it has been a privilege to witness �rsthand the unwavering commitment and passion exhibited by members of our association I also want to remind us of the critical importance of embracing technology in our profession. The healthcare system continues to undergo rapid digital transformation, and it is imperative that we, as pharmacy professionals, embrace technology in all capacities to remain relevant and provide the highest level of care to our patients Technology can streamline our work�ows, enhance patient safety, and improve medication management, ultimately leading to better health outcomes
Let us seize the opportunities presented by technology and continue to explore innovative ways it can support our profession. In addition to embracing technology, I urge each of you to become more involved in advocacy at the state level this coming year. The Arizona Pharmacy Association has been instrumental in promoting and protecting the interests of our profession, and your active engagement in advocacy e�orts is crucial for our continued success By working together, we can amplify our collective voice and drive positive change that bene�ts both pharmacy professionals and the patients we serve
Lastly, I want to acknowledge the challenging times we have faced and continue to navigate. The ongoing "do more with less" environment has placed immense pressure on all of us, and it is essential that we give ourselves and our pharmacy colleagues grace during these trying times. Let us support one another and show empathy as we strive to deliver exceptional patient care while navigating the challenges of limited resources
In closing, I extend my deepest appreciation to each and every one of you for your unwavering dedication, hard work, and commitment to advancing the pharmacy profession in Arizona It has been an honor to serve as your President I have full con�dence that the Arizona Pharmacy Association will continue to thrive under the leadership of the incoming President, Kim Langley, and the board of directors. I encourage you to extend your unwavering support to them as they guide the association towards a strong future.
Thank you once again for your trust and support Together, we will shape the future of pharmacy in Arizona and make a lasting impact on the health and well-being of our patients and pharmacy community
With deepest gratitude,
Dawn Gerber, PharmD, BCGP, FASCP, FAzPA AzPA President 2022-2023

EDITORIAL PRESIDENT'S MESSAGE 4
WELCOME NEW MEMBERS!
1st Year Practitioner
Shean Benares
Amanda Mikhail
Pharmacist
Trisha Chandler
Courtney Coombe
Daniel DeGarmo
Kristen Ellis
Susan Follis
Tatum Hamilton
Susan Hopkins
Timothy Ivers
Chernery Kinemond
Pooyan Mesdaghi
Nicoleta Nedelcu
Nicholas Palm
Jolene Patterson
Alberto Ranjel
Linda Savarese
Liz Sche�el
Caitlin Shelar
Christopher Sweeney
Kimberly Tran
Cuong Truong
Thalia Vega
Lawrence York
Alden Carter
Erin Carter
Resident
Aya Alshamaa
Alexis Altmaier
Lourva Begay
Breanna Brungardt
Ashley Burke
Victor Camargo
Jamie Chichester
Corwin Coppinger
Danielle DeCuir
David Do
Miriam Easo
Denisse Garcia Zavala
Megan Hellwege
Megan Lai
Brandi Lee
Weston Lewis
Gloria Lo
Benjamin Lowry
Sydni MArtinez
Melissa McCrary
Derek Rodriguez
Emma Rolfes
Garrett Rueda
Haley Sedo
Jonathan Shin
Amanda Smith
Christina Tamou
Eric Taylor
Luis Tinoco
Yen Tran
Peter Triggiani iV
Shemual Tsai
Scott Volker
Student Pharmacist
Diyana Ahmad
Sheilla Ahmadi
Zulfa Alaaf
Haya Albazzaz
Dhuha Alrubie
Dyanah Altameemi
Tabarak Altameemi
Zahra Bandehyazdani
Maximilian Bezzegh
Breanna Cesare
Julia Czarnik
Kaela Eller
Justin Enriquez
Bianca Garrow
Janae Hagen
Deanna Harvey
Nhu Ho
Dona John
Anjali Kumar
Sabrina Lamere
Hanna Loxtercamp
Kunal Mistry
Danielle Noble
Christoper Palting
Andrew Pham
Megan Phillips
Waleed Riaz
Leah Rios
Jeehan Sami
Clarissa Sarmiento
Yaharim Satterwhite
Nicole Unwin
Matthew Wood
Mahdokht Ghahraman
Kory Muto
Retired
David Narayan
Kristine Wells
Larissa Worster
Dillon Yup
Technician
Shelby Clem
Stacy Cochran
Sheena Lee
Vanessa Leyvas
Everette Pailzote Jr
Roxalinne Shannon
Cheryl Yu
Associate
Lisa Morris
Christopher Santarone
Premium Pharmacist
AZPA NEWS 5
2023 FALL CONFERENCE PREVIEW

12:15PM – 12:30PM – NON-ACCREDITED EDUCATION
12:30PM-1:00PM – BREAK
1:00PM-2:00PM - HORMONAL CONTRACEPTION – PATIENT CASES
Speaker-TBD
Learning Objectives: PENDING
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Women's Health | Activity Type: Knowledge-Based
CPE Hours: 1.0 | ACPE UAN: PENDING
This session is pending accreditation
8:00AM-9:00AM - 2023 LEGISLATIVE & REGULATORY UPDATE
Kelly Fine, RPh, FAzPA
Learning Objectives (Pharmacists and Technicians):
1 List the AzPA bills that passed during the 2023 Legislative Session
2. Discuss the impact of the new bills on pharmacy practice.
3. Identify opportunities to get more involved in AzPA’s advocacy e�orts
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Law | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: PENDING
This session is pending accreditation
9:05AM-10:05AM - IMMUNIZATIONS IN THE IMMUNOCOMPROMISED PATIENT POPULATION
Holly Van Lew, PharmD, BCPS, AAHIVP
Learning Objectives (Pharmacists and Technicians):
1. Describe the e�ects of immunocompromise on recommended vaccine schedules, including indications and contraindications that should be considered in immunocompromised populations
2 Identify common immunocompromising conditions and immunocompromising medications that may a�ect indications, timing, or e�cacy of vaccines.
Level: Intermediate | Audience: Pharmacists & Technicians
Topic: Immunizations | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: PENDING
This session is pending accreditation
10:10AM-11:10AM - TBD
11:15AM-12:15PM - CARDIOLOGY UPDATES FROM THE NEW 2023 BEERS CRITERIA
Dawn Gerber, PharmD, BCGP, FASCP, FAzPA; Andrea Calles, PharmD; Jeannie K Lee, PharmD, BCPS, BCGP, FASHP
Learning Objectives: PENDING
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Geriatrics | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: PENDING
This session is pending accreditation
2:05PM-3:05PM - MEDICATION ERROR: I DIDN’T DO THAT! Eman Kirolos, PharmD, MBA
Learning Objectives (Pharmacists and Technicians):
1. Describe organizational risk models.
2. Review Reason’s model of error causation.
3 Identify the role of Just Culture in medication safety
4 List common examples of medication errors
5 Discuss Human Factors approach to error prevention

6. Recognize the role of practitioner’s well-being.
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Patient Safety | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: PENDING
This session is pending accreditation
3:10PM-4:10PM - PREVENTING NARCOTIC DIVERSION A HEALTH SYSTEM APPROACH
Alexa Myers, PharmD
Learning Objectives (Pharmacists and Technicians):
1 Discuss the impact of the opioid epidemic on the healthcare system
2. Discuss federal law regarding controlled substances in an acute care setting.
3. Identify common medications involved in diversion
4 Identify common methods involved in diversion
5 Explain the consequences that may result from diversion
Level: General Interest| Audience: Pharmacists & Technicians
Topic: Opioid | Activity Type: Knowledge-Based
CPE Hours: 1.0 | ACPE UAN: PENDING
This session is pending accreditation
EDITORIAL FALL CONFERENCE
REGISTER 6
ON-DEMAND SESSIONS
TheseCPEprogramshavebeenpreviouslyofferedatAzPAConferences.Ifyouhavealreadyclaimedcredit fortheseCPEsessionsalreadyyouwillNOTbeabletoclaimcreditagain
OPIOID CPE- THE HIGHS AND LOWS OF ADDICITION
Daniel DeGarmo, PharmD
Pharmacist Learning Objectives:
1. Describe opioid addiction's disease pathophysiology.
2. List available opioid addiction treatments
3 Describe impact of pharmacy operated opioid addiction clinics
Technician Learning Objectives:
1 De�ne opioid addiction
2. List available opioid addiction treatments.
3. Describe impact of pharmacy operated opioid addiction clinics.
Level: General | Audience: Pharmacists & Technicians
Topic: Opioids | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: 0100-0000-23-039-H01-P/T
Previously o�ered live at the 2023 AzPA Annual Convention
IMMUNIZATION UPDATE
Holly Van Lew, PharmD, BCPS, AAHIVP; Vanessa Leyvas, CPhT
Pharmacist Learning Objectives:
1 Discuss recently authorized vaccines incorporated into the Advisory Committee on Immunization Practices routine vaccination schedule.
2. Recommend the most appropriate pneumococcal vaccination for adults and pediatrics
3 Summarize the current and anticipated changes in the COVID-19 vaccines
Technician Learning Objectives:
1. Discuss recently authorized vaccines incorporated into the Advisory Committee on Immunization Practices routine vaccination schedule
2 Utilize the ACIP vaccine schedule to identify the most appropriate pneumococcal vaccinations for adults and pediatrics
3. Summarize the current and anticipated changes in the COVID-19 vaccines.
Level: Intermediate | Audience: Pharmacists & Technicians
Topic: Immunization | Activity Type: Knowledge-Based
CPE Hours: 1 5 | ACPE UAN: 0100-0000-23-055-H06-P/T
Previously o�ered live at the 2023 AzPA Annual Convention
2023 PHARMACY LAW UPDATE

Roger Morris, RPh, JD; Michael French, JD; Katie Lavigne, JD
Pharmacist & Technician Learning Objectives:
1 Describe rami�cations of recent pharmacy related court cases
2 List recent changes in Federal Pharmacy Law
3. List recent changes in State Pharmacy Law.
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Law | Activity Type: Knowledge-Based
CPE Hours: 1 25 | ACPE UAN: 0100-0000-23-045-H03-P/T
Previously o�ered live at the 2023 AzPA Annual Convention
DEMYSTIFYING PAIN MANAGEMENT – OVERVIEW OF THE 2022 CDC OPIOID GUIDELINES UPDATE
Christopher Edwards, PharmD, BCPS; FASHP, FAzPA; Amy Kennedy, PharmD, BCACP; Jaclyn Juarez, PharmD Pharmacist Learning Objectives:
1 Given a patient case, develop a plan to taper the patient o� of long-term opioid therapy
2 Evaluate a patient case to determine if co-prescribing of naloxone is appropriate.
3. Di�erentiate between substances detectable and not detectable on commonly available opioid screening assays
Technician Learning Objectives:
1 Identify long-term opioids used in pain management
2 Discuss the purpose of co-prescribing naloxone
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Opioids | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: 0100-0000-23-014-H08-P/T
Previously o�ered live at the 2023 AzPA Spring Clinical Conference
IMMUNIZATION UPDATE - JEOPARDY STYLE!
Holly Van Lew, PharmD, BCPS, AAHIVP Pharmacist Learning Objectives:
1 Discuss the most recent updates for routine vaccinations, including pneumococcal, Hepatitis B and other vaccines with updated indications and expanded age ranges
2. Apply the current recommendations for COVID-19 vaccinations to case-based scenarios and review planned commercialization activities for COVID-19 vaccinations
3 Assess current immunization practices at your practice site and determine implementation strategies for expanding vaccination e�orts
4. Discuss vaccine administration best practices, including injection technique and documentation.
Technician Learning Objectives:
1 Describe tools and resources available for immunization schedules and recommendations
2 Identify the appropriate age-based recommendations for immunizations that impact dose and route of administration.
3. Determine ways to support increased vaccine administration and improve work�ow to support vaccinations
4 Discuss vaccine administration best practices, including injection technique and documentation
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Immunizations | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: 0100-0000-23-017-H06-P/T
Previously o�ered live at the 2023 AzPA Spring Clinical Conference
EDITORIAL FALL CONFERENCE
7
EDITORIAL
FALL CONFERENCE
ON-DEMAND SESSIONS
TheseCPEprogramshavebeenpreviouslyofferedatAzPAConferences.Ifyouhavealreadyclaimedcredit fortheseCPEsessionsalreadyyouwillNOTbeabletoclaimcreditagain
TREATMENT OF OPIOID USE DISORDER
Steven Wright, MD
Pharmacist Learning Objectives:
1. Describe the diagnosis of OUD.
2. Discuss non-pharmacologic treatment of OUD
3 Discuss pharmacologic treatment of OUD
4 Describe integrating treatments for patients with OUD
Technician Learning Objectives:
1. De�ne OUD.
2. List non-pharmacologic treatment of OUD.
3. List pharmacologic treatment of OUD
Level: Intermediate | Audience: Pharmacists & Technicians
Topic: Opioid/SUD | Activity Type: Knowledge-Based
CPE Hours: 1 5 | ACPE UAN: 0100-0000-22-146-H08-P/T
Previously o�ered live at the 2022 AzPA Fall Conference
UPDATES ON TREATMENTS TO QUIT E-CIGARETTES
Jing Li, PharmD, BCPS
Learning Objectives:
1 Describe the risks versus bene�ts in using e-cigarettes
2. Identity the long-term e�ect of e-cigarettes on a user.
3. List the treatment options based o� the current evidence-based recommendations for e-cigarette cessation
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Tobacco Cessation | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: 0100-0000-22-147-H01-P/T
Previously o�ered live at the 2022 AzPA Fall Conference
REGISTRATIONFEES
AzPAMemberPrices
PremiumPharmacist:$109
Pharmacist:$119
Technician:$59
IMPLEMENTING A TOBACCO CESSATION COLLABORATIVE PRACTICE AGREEMENT

Ryan Gries, PharmD, BCPS, BC-ADM; Alejandro Vazquez, PharmD
Pharmacist Learning Objectives:
1. De�ne the pharmacist’s role in tobacco cessation
2 Identify the settings in which a pharmacist can perform tobacco cessation
3 Summarize the items necessary to develop a comprehensive Collaborative Practice Agreement (CPA).
4. Explain the nonpharmacological and pharmacological methods used in tobacco cessation
Technician Learning Objectives:
1 Describe the importance of tobacco cessation
2 Identify the settings in which a pharmacist can perform tobacco cessation.
3. Discuss the available types of tobacco.
Level: General Interest | Audience: Pharmacists & Technicians
Topic: Tobacco Cessation | Activity Type: Knowledge-Based
CPE Hours: 1 0 | ACPE UAN: 0100-0000-22-148-H99-P/T
Previously o�ered live at the 2022 AzPA Fall Conference
Non-MemberPrices
Pharmacist:$189
Technician:$79
RefundPolicy:Norefundswillbeissuedforthisevent.Allsessionswillberecordedandmade availableon-demand.
REGISTER
8



COVER STORY ANNUAL CONVENTION THANK YOU FOR ATTENDING! 36 CE Sessions 292 Attendees 42 Sponsors & Exhibitors 29 Award Winners 9



COVER STORY ANNUAL CONVENTION 10
CONGRATULATIONS TO OUR 2023 AWARD RECIPIENTS!
Distinguished Young Pharmacist
Yousef Toma
Excellence in Innovation
Jenny Bingham
Exemplary Patient Care
Brianne Spaeth
Student of the Year
Alex Brown
Technician of the Year
Kristine Smith
Pharmacist of the Year
Reasol Chino
Pharmaceutical Rep of the Year

Angili Arora
Hall of Fame
Nancy Alvarez
Tom van Hassel
Kevin Boesen
Jon Glover
Ken Bykowski
Elias Schlossberg
Melissa Duke
Bowl of Hygeia
Shareen El-Ibiary
Pharmacy Appreciation
Nicki Scovis
Nick Ruiz
MaryJo Zunic
Laura “Michelle” Vaughn
Lindsay Davis
Jose Espinoza
Sharon Ruditser
Lyndy Abdelsayed
AzPA Fellows

Ryan Gries
Sophia Galloway
Kimberly Langley
Corporate Appreciation
Pharmacy Technician Certi�cation Board
Residency Director/Preceptor of the Year
Kellie Goodlet
Outstanding Leadership Award
Dawn Gerber
Incoming President Award

Kimberly Langley
NCPA Pharmacy Leadership
Kimberly Langley
The Gavel & Block
Kimberly Langley
WELCOME
TO THE 2023-2024
OFFICERS
President: Kimberly Langley
President-Elect Pro Tempore: Jacob Schwarz
Past President: Dawn Gerber

Treasurer: Ryan Gries
Secretary: Brandy DeChellis
CEO: Kelly Fine
DIRECTORS AT LARGE
Community Pharmacy: Brianne Spaeth
Health System Pharmacy: Mary Manning
Technician: Melinda Browning
Director at Large: Misty Brannon
Director at Large: Reasol Chino
Director at Large: Jimmy Stevens
Director at Large: Joey Pellerit
Director at Large: Danielle Gilliam
BOARD OF DIRECTORS!
LIAISONS
University of Arizona
Student Chapter: David Campa
Dean’s Designated Representative: Nancy Alvarez
Midwestern University
Student Chapter: Shams Rehman
Dean’s Designated Representative: Michael Dietrich
Creighton University
Student Chapter: TBA
Dean’s Designated Representative: Jane Stein
Legal Counsel
Roger Morris
COVER STORY 2023 AWARD WINNERS
11



12

13
AzPA HEALTH SYSTEM SPECIAL INTEREST GROUP (AZ-ASHP)



This year’s annual convention marked the end of my term on AzPA’s Board of Directors Serving on the board provided several wonderful opportunities, including developing and strengthening relationships with some incredible people, learning the inner workings of an organization doing great work for pharmacists and patients in the state of Arizona, and leading our state a�liate chapter of ASHP While I am no longer in a leadership role, I plan to stay engaged with AzPA and the Health System Special Interest Group (SIG), and if you are reading this, I encourage you to do the same.
Pharmacy practice is predominantly regulated at the state level, and AzPA is the organization that ensures all pharmacists in Arizona have a voice in this process This includes health systems pharmacists Unfortunately, health systems pharmacists are less visible than our colleagues in the community, and as such, less understood by regulators and the public. We are also under-represented on the Board of Pharmacy and in AzPA, but you can help change this By engaging with AzPA and the Health System SIG, you can help to ensure that AzPA is meeting the needs of health systems pharmacists in the state of Arizona in terms of not only advocacy and education, but also research and networking opportunities We quite literally can’t do it without you!
I am eternally grateful for the opportunity to have served on the board and I look forward to continuing to work with this organization for years to come. I hope to see you on a future HS SIG call, at the HS SIG’s spring clinical meeting, or at annual convention!
All the best,
Learn More Here
Christopher J. Edwards, PharmD, BCPS, FASHP , FAzPA AzPABoardofDirectors-HealthSystem
AZ-ASHP AFFILIATE NEWS 14
Christopher J. Edwards, PharmD, BCPS, FASHP,FAzPA AzPA Board of Directors-Health System
Learning Styles in Pharmacy

Precepting
AUTHORS/CONTRIBUTORS
LauraHanson,PharmD,BCGP,QualityAssuranceSiteVisitor,Department ofPharmacyPractice,MidwesternUniversityCollegeofPharmacy
DISCLOSURE
Theauthor(s)declarenorealorpotentialconflictsorfinancialinterestinanyproduct orservicementionedinthemanuscript,includinggrants,equipment,medications, employment,gifts,andhonorarium.
FUNDING
Thisresearchwasnotfunded.
ACKNOWLEDGEMENT
TheauthorgratefullyacknowledgesSuzyLarson,PharmDandJanetCooley,PharmD, BCACPforinspiration,supportandeditorialreviews.
EDITORIAL PRECEPTOR CORNER 15
CONT. PRECEPTOR CORNER
What is your current understanding of learning style/ preferences in classroom and experiential settings?
The concept that learners have a distinct learning style or preference (e g auditory, visual and kinesthetic) is relatively well accepted within the current educational landscape According to learning style theory, every learner has a de�ned learning style that works best for them, and optimal learning occurs when material is presented in a manner congruent to these styles or preferences Culture, personal history, and relationship to technology can all play a role in determining an individual’s learning style
Many assessment tools and associated products have been developed to ascertain and cater to various learning styles from early childhood to adult learners A strong commercial market has developed within this space, including the pharmacy speci�c Pharmacists’ Inventory of Learning Styles (PILS) However, there is some question as to how learning style theory should be implemented and assessed. Some believe that students should adjust their learning style to the way information is presented while others may modify their teaching approach to match de�ned learning preferences through a practice known as meshing. This article will explore the practice of meshing and the evidence supporting its use.
•
While meshing is a popular method typically well received by educators and learners, there is minimal high quality evidence to support its use which may be surprising given the broad commercial market and general acceptance of the concept. It is inherently challenging to measure the success of this method due to the real-world educational environment in which the intervention is applied, di�culty in objectively assessing learning, and the need for a relatively complex crossover design to truly assess the method. A brief review of several well-designed studies assessing the e�cacy of meshing is as follows:
• 6
Massa and Meyer utilized a well-designed crossover study comparing meshing for visual vs verbalizer learners with over twenty individual- di�erence measures and concluded no di�erence in results when meshing was incorporated
Cook et al looked speci�cally at medical students and compared meshing vs no meshing in the presentation of ambulatory care modules and subsequent testing relating to concrete vs abstract learning types No di�erence in performance was found for meshing vs no meshing groups
• 7 Constantinidou and Baker focused on task completion vs learning environment and looked at the use of meshing for visual vs verbalizer learners to complete de�ned tasks involving recall and task performance Overall, visual presentation led to greater recall and performance in all participants regardless of de�ned learning style, with no support for the use of meshing
found
Other studies with less stringent design o�er con�icting results The existing body of evidence does not currently give a strong conclusion to support the use of meshing as an educational approach
Were you surprised to find that learning style theory is not strongly evidence based?
Given the widespread popularity of learning style and meshing theories, many are surprised to learn that there is not a strong consensus of evidence supporting their use There are several theories as to why existing studies have not resulted in the expected positive results Research design challenges play a role as well as di�culty in de�ning a unique and distinct learning style for a given individual There is imperfection inherent in all learning style assessment tools and study participants may have combined learning styles which confound results Further research is needed in this area.
The debate regarding the validity of learning style and meshing theory can be heated. Proponents of learning styles and meshing claim the practice “just feels right” and cite the large commercial market and widespread adoption in all levels of education. Critics of learning style theory and meshing state the whole concept has been “debunked” and is completely invalid. As is often the case with polarized arguments, the middle ground o�ers a reasonable perspective that individuals may indeed have learning methods or styles that they �nd to work best to promote learning; however, the existing body of evidence does not support meshing as an e�ective educational intervention and the practice does not mimic real world environments.
1,2,3,4 1,2, 5 1,2,3
8 1
1,2,3 1,2,9
What is your current understanding of learning style/ preferences in classroom and experiential settings?
Do you modify instructional approaches based on learning style/preferences?
16
While the existing body of evidence does not support the strict use of meshing, discussion and re�ection around the concept of learning styles can be a method to support metacognition and self-awareness while honoring the individual identity of learners. Educators (including pharmacy preceptors) may better use limited time and resources by o�ering opportunities to re�ect upon and discuss learning style theory and its place in didactic and experiential education as well as the workplace Learners should be discouraged from becoming stuck in a �xed mindset around learning styles (e g that they can only learn then information is presented in a speci�c way) with preceptors instead facilitating discussions about how to best integrate learning preferences with the real world practice environment Conversations regarding didactic exam preparation vs the complexities of real world patient care may also be bene�cial to help learners become more openminded regarding the processing of information presented in di�erent ways
REFERENCES
1 Pashler et al (2009) Learning Styles: Concepts and Evidence, Psychological Science in the Public Interest, 9(3): 105-119.
2 Romanelli F, Bird E, Ryan M (2009) Learning styles: a review of theory, application, and best practices American Journal of Pharmaceutical Education;73(1):9.
3 Willingham D T (2005, Summer) Do visual, auditory, and kinesthetic learners need visual, auditory, and kinesthetic instruction? American Educator, 29 (2), 31–35
4 Learning Styles as a Myth https://poorvucenter yale edu/LearningStylesMyth ,accessed 5 2 2023)
5 Austin, Z (2004) Development and Validation of the Pharmacists' Inventory of Learning Styles (PILS) American Journal of Pharmaceutical Education, 68(2).

6 Massa L J , Mayer R E (2006) Testing the ATI hypothesis: Should multimedia instruction accommodate verbalizer-visualizer cognitive style? Learning and Individual Di�erences, 16, 321–336.
7 Cook D A , Thompson W G , Thomas K G , Thomas M R (2009) Lack of interaction between sensing-intuitive learning styles and problem-�rst versus information-�rst instruction: A randomized crossover trial Advances in Health Science Education, 14, 79–90
8 Constantinidou F , Baker S (2002) Stimulus modality and verbal learning performance in normal aging Brain and Language, 82, 296–311
9 An D, Carr M (2017) Learning styles theory fails to explain learning and achievement: Recommendations for alternative approaches, Personality and Individual Di�erences, 116, 410-416
In lieu of a strong focus on meshing, other teaching approaches utilizing methods appealing to a variety of learning styles may be preferable Providing information through a variety of sensory representations may allow learners to integrate concepts more easily and may allow easier connections with individuals with speci�c learning styles Other practices with a stronger basis in evidence vs meshing include building on prior knowledge, making conceptual connections, transferring knowledge, active learning and group work Learning styles and their place in designing/delivering educational content is an area of strong interest with additional discussion and more research forthcoming
2, 9 9
The next article in this series will build on the concepts discussed here by elaborating on ways to tailor pharmacy experiential education rotations to individual learners This content will provide practical tips with a focus beyond learning styles- stay tuned!
Will the information shared in this article change your teaching approaches?
1 2
1 CONT. PRECEPTOR
CORNER How can this information be utilized in the didactic vs experiential space?
February 24-25 | Phoenix, AZ save the date! 17


18
Remedying the Missing Clotting Factors
AUTHORS/CONTRIBUTORS
BreannaBrungardt,PharmD,PGY-1PharmacyResident,BannerBoswellMedicalCenter
MaeleeBrown,PharmD,BCPS,PharmacyClinicalCoordinator,BannerBoswellMedical Center
ACKNOWLEDGEMENT- None
FUNDING - Thisresearchwasnotfunded
DISCLOSURES - Theauthorshavenorelevantfinancialrelationshiptodisclose
CONTINUING EDUCATION INFORMATION
Target Audience: Pharmacists
Activity Type: Knowledge
Learning Objectives:
1 Explain the pathophysiology of hemophilia A and B and its implications on the clotting cascade
2 Identify an appropriate treatment agent for hemophilia A and B in the event of an acute bleed.
3 Describe how the acute bleeding management of hemophilia may differ when a patient has an inhibitor present.
CONTINUING
19
CONT. CONTINUING EDUCATION
Introduction
Hemophilia is a X-linked recessive bleeding disorder characterized by the de�ciency or complete absence of a clotting protein There are two main types of hemophilia: Hemophilia A (also known as classic hemophilia), which involves a de�ciency of coagulation factor VIII, and Hemophilia B (or Christmas disease), which involves a de�ciency of coagulation factor IX Hemophilia A is four times more prevalent than Hemophilia B. Males are more susceptible to hemophilia due to their possession of one X chromosome and one Y chromosome. Male patients only need to inherit the a�ected X chromosome from their mother to develop hemophilia. According to the Centers for Disease Control and Prevention (CDC), hemophilia A a�ects 1 in 5,000 male births annually in the United States, resulting in an estimated 400 male infants born each year with hemophilia A Although it is rare, females can also have hemophilia Since females possess two X chromosomes, they can develop hemophilia if both chromosomes are a�ected or if one chromosome is a�ected and the other is missing or inactive.
The Clotting Cascade
The clotting cascade describes the process by which the body forms a clot It consists of three pathways: the intrinsic pathway, extrinsic pathway, and common pathway The extrinsic pathway is initiated by tissue damage. Tissue factor III is released into the blood and binds to factor VIIa, leading to the activation of factor X. In the intrinsic pathway, factor XII interacts with highmolecular-weight kininogen and plasma prekallikrien, resulting in the conversion of factor XII to factor XIIa Factor XIIa then activates factor XI, which in turn activates factor XIa The intrinsic pathway culminates in the activation of factor IXa, aided by factor VIIIa, which converts factor X into factor Xa The common pathway occurs when both the extrinsic and intrinsic pathways converge, with factor Xa converting prothrombin to thrombin. Thrombin subsequently triggers the conversion of �brinogen into a �brin clot. Figure 1 illustrates the clotting cascade and identi�es the speci�c points where a de�ciency in clotting factors would result in hemophilia ⁵
Pathophysiology of Hemophilia

As mentioned in the introduction, hemophilia is a bleeding disorder caused by a defect in the production of clotting factors. Hemophilia A occurs due to insu�cient production of factor VIII, while hemophilia B occurs due to insu�cient production of factor IX In both types of hemophilia, this de�ciency prevents the conversion of factor X to Xa, resulting in the inadequate formation of �brin from �brinogen and leading to weak clot formation
The normal coagulation cascade requires factor levels greater than 50% of the normal range. Without the replacement of these factors, patients are at risk of experiencing uncontrolled bleeding events ¹
Hemophilia Diagnosis
The diagnosis of hemophilia relies on the ability to obtain clotting factor tests. The results of these blood tests determine the presence of hemophilia and also indicate the severity of the disease. The results of the blood test will show a prolonged aPTT and allude to a de�ciency in factors VIII, IX, XI, and XII If any of these clotting factors are low, the body will not be able to produce a clot as quickly The PT test will be normal as this evaluates factors I, II, V, VII, and X abilities to form a clot Since these are not low in hemophilia, the PT will be normal. If the factors VIII or IX level in the blood shows 50 to 100%, the patient has normal factor levels, thus no hemophilia. If the factor VIII level is between 5% and 40%, the patient has mild hemophilia A If that level is the case with factor IX, the patient has mild hemophilia B Moderate hemophilia is factor levels of 1% to 5%, while severe hemophilia is less than 1% ⁶
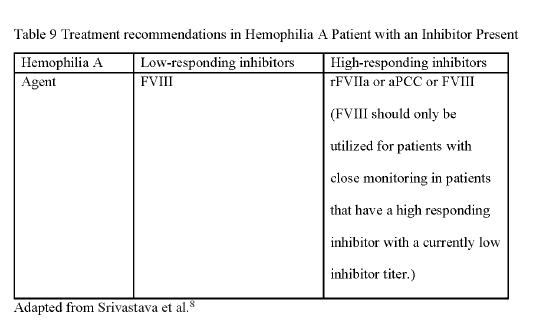
Treatment Agents
Various treatment agents are utilized for management of hemophilia. This section will discuss some of the agents utilized for the treatment of bleeding for hemophilia A and hemophilia B patients
Factor VIII (FVIII) Clotting Factor Concentrates (CFC) Factor VIII clotting factor concentrates are utilized for the treatment of hemophilia A as they replace the de�ciency
continued on next page
¹ ² ‚ ³ ⁴
20
in FVIII. ⁷ FVIII CFCs are available in vials with variable number of international units (IU) per vial. Typically, each vial ranges from 250 to 3,000 IU per vial. A useful guide for determining the required number of IU is that each IU per kilogram of body weight will increase the FVIII level by 2 IU/dL This information can be applied in an equation to calculate the necessary IU dosage for a patient with hemophilia

The desired levels are determined by indication and dependent on practice patterns. ⁸ A recent publication evaluated changes in prescribing practices. A noteworthy observation is the shift towards higher doses of CFC, speci�cally exceeding 40 units per kilogram, which had become more prevalent between 1999 and 2021 ⁹ One consideration for the administration of FVIII includes the rate of the infusion which varies based on the individual product FVIII comes in many di�erent brand names by di�erent manufacturers, thus review the package insert for the average rate of infusion for the speci�ed product. A peak level is drawn around 30 minutes after the infusion to ensure an adequate dose was given to raise the factor VIII level If repeat doses are needed, utilize the half-life of the product to determine when the next dose should be given For example, a patient requiring a second dose of a FVIII product that has a 10 7-hour half-life would require another dose either 8 or 12 hours after the �rst dose depending on the severity of bleed. If the bleed is severe, a second dose around 8 hours after the �rst would be appropriate. This contrasts a moderate bleed that would require a second dose at around 12 hours after the �rst dose. Appendix A contains common dosing regimens based on the severity of bleed
Factor IX CFCs
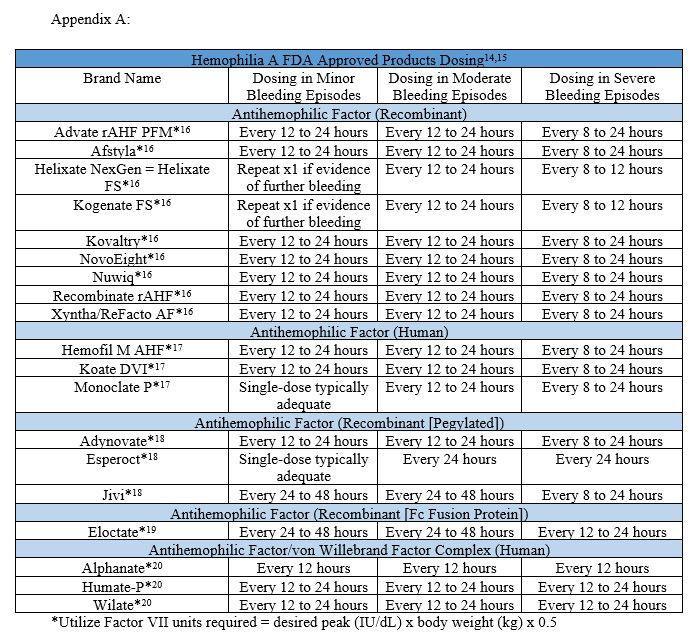
Factor IX CFCs are utilized for the treatment of hemophilia
B FIX CFCs are broken down into two categories: pure FIX CFCs and prothrombin complex concentrates (PCC). The pure FIX CFC is often preferred over PCC as it reduces the risk of thromboembolism formulation. PCC contains additional factors beyond IX including factors II, VII, and X. Higher doses of PCC would be required to increase the factor IX level, which puts the patient at a high risk for thrombus formation FIX CFCs are available in vials containing 250-4,000 IU per vial If the patient doesn’t have an inhibitor, each IU of factor IX per kilogram of body weight will raise the plasma level by 1 IU/dL. If utilizing the recombinant FIX (rFIX) CFC, each IU of rFIX given per kilogram of body weight, will raise the level by 0.8 IU/dL in adult patients. ⁸
⁸
A ⁸ continued on next page IU per dose = body weight (kg) x desired factor level (IU/dL) x 0 5
⁸
The same administration considerations for FVIII are applied to FIX including verifying the rate of administration with the package insert of the speci�ed product The peak level should be drawn around 30 minutes after the end of the infusion and utilize the half-life of the product for repeat dosing if the patient continues to bleed. Appendix B contains common dosing regimens based on severity of bleed One additional point includes allergic reactions, including anaphylaxis, occur in two to four percent of cases for either rFIX or FIX ⁸ CONT. CONTINUING EDUCATION IU per dose = body weight (kg) x desired factor level (IU/dL) rFIX IU per dose = body weight (kg) x desired factor level (IU/dL) x 1 25 21
CONTINUING EDUCATION
Bypassing Agents
Bypassing agents are utilized for patients that develop inhibitors or antibodies against the factor VIII or IX. The inhibitors or antibodies are produced by the patient’s immune system as a protective measure against foreign substances. The inhibitor production makes treatment of hemophilia harder as the body will attack the clotting factors rendering them inactive. These agents are utilized to bypass the need for factor VIII or IX administration by attaining hemostasis through other coagulation pathways ⁸
Recombinant Activated Factor VIIa (rFVIIa)
As seen in Figure 1, rFVIIa will allow for the conversion of factor X to Xa by utilization of the extrinsic pathway The activation of factor X to Xa allows the coagulation cascade to resume and ultimately achieve clot formation and resolution of the bleed This would allow rFVIIa to be utilized in either hemophilia A or B as it bypasses the intrinsic clotting cascade The dosing of rFVIIa is based on the speci�c product, so check the speci�c product’s package insert for dosing recommendations Unlike FVIII and FIX, rFVIIa does not have any dependable laboratory monitoring to assess the e�cacy Coagulation tests such as INR, aPTT, and factor assays have not shown bene�t Monitoring of the hemoglobin, hematocrit, and signs or symptoms of bleeding is most useful when determining if rFVIIa was e�ective in stopping the acute bleeding episode ⁸
Other Pharmacological Options
Even though CFCs are considered the �rst line option for bleeding associated with hemophilia, a few alternatives to CFC exist Desmopressin is a vasopressin analogue that will increase FVIII and Von Willebrand Factor, thus can be e�ective for hemophilia A ⁸ Desmopressin has shown an increase in factor levels above the target in patients that received a single dose factor concentrate ¹⁰ Caution should be utilized in young patients due to an increased risk of hyponatremia-induced seizures, history of cardiovascular disease, or thrombosis Tranexamic acid is �brinolytic and utilized as another alternative agent. Tranexamic acid is contraindicated in patients with hematuria as it may raise the risk of obstructive uropathy. See Table 1. ⁸
Management of Acute Bleeding


Patients may get routine infusions as prophylaxis for bleeding episodes However, bleeding events may still occur and knowing how to stop the bleed may just save someone’s life A couple factors to consider when determining treatment of an acute bleed in a patient with hemophilia is the location and severity of the bleed. The goal of treatment is to stop the bleed as soon as possible With any bleeding event, an important consideration regarding pain management is to avoid non-steroidal antiin�ammatory medications such as ibuprofen, naproxen, and aspirin as these agents increase the risk of having an acute bleed This section will discuss some of treatment considerations for a few di�erent types of bleeds
Joint Hemorrhage
A joint hemorrhage is de�ned as having any combination of the following: increasing swelling or warmth of the skin over the joint, increase pain, progressive loss of range of motion or di�culty using the limb The plan of care starts with initiating CFCs immediately with a dose high enough to stop the bleed. Current guidelines recommend giving one intravenous infusion of the clotting factor concentrate and repeat if clinically indicated Table 2 shows target peak actor levels that can be utilized with the calculation to determine an appropriate factor dose. If the bleeding ontinues over the following 6-12 hours, further diagnostics are needed including factor assay A repeat dose will ypically be 12 hours after the initial dose if utilizing a tandard factor VIII (FVIII) product for hemophilia A or 24 hours if utilizing a standard factor IX (FIX) product for hemophilia B However, this is variable depending on the half-life of the product utilized (see Appendix A and B) ⁸ See Table 2
CONT. continued on next page
22
A patient responding to treatment will include a decrease in symptoms and an increase in the range of motion of the limb. The patient’s response to the CFC may be classi�ed as none, moderate, good, or excellent. None indicates no or very small improvements during the �rst 8 hours after the infusion Moderate relief indicates some improvement; however the patient requires another infusion within 72 hours and does not have complete resolution of symptoms Good indicates a patient has pain relief and/or decrease in signs of bleeding within the �rst 8 hours after the infusion, however, does require another dose in the next 72 hours to achieve resolution of symptoms The excellent outcome indicates the patient has complete resolution of symptoms in the �rst 8 hours after the �rst infusion and would not require subsequent doses ⁸
Gastrointestinal (GI)/ Abdominal Hemorrhage
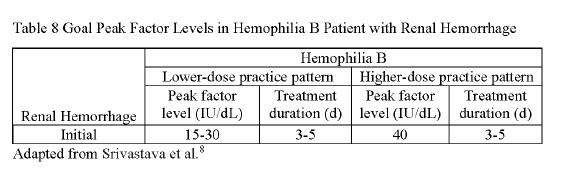
In a hemophilia patient presenting with a GI bleed, a clotting factor level should be obtained and subsequently raised, if needed The factor levels should be maintained until the cause of the bleeding is known. Tables 3 and 4 should be utilized to determine an appropriate peak factor level, which can be utilized when calculating an appropriate CFC dose Current guidelines recommend the use of an anti�brinolytic agents such as tranexamic acid in hemophilia patients with a GI bleed If utilized with PCC, caution is advised due to increased risk of thrombosis. The management of the GI bleed itself, however, is the same as it is with a nonhemophilia patient Some steps include monitoring of hemoglobin and utilizing endoscopy to visualize and potentially stop the bleed. See Table 3 and Table 4. ⁸





Intracranial Hemorrhage
⁸
Hematuria
Patients experiencing hematuria should undergo evaluation for potential causes of persistent bleeding or recurrent episodes of hematuria In many cases hematuria is mild, and hydration and rest are often enough. CFCs can be given if bleeding persists In moderate and severe cases, CFCs should be administered, and the site of bleeding will need to be identi�ed. Tables 7 and 8 can be utilized to �nd the appropriate peak factor level. This level will then be utilized to �nd an appropriate CFC dose in a patient with hematuria Hydration and rest should be utilized until the bleeding ceases. See Tables 7 and 8.
Inhibitors
⁸ 23
When any patient presents with a suspicion for an intracranial hemorrhage, a computed tomography (CT) scan of the brain should be performed as soon as possible along with the administration of clotting factor replacements The utilization of Tables 5 and 6 can help determine an appropriate peak factor level that can be utilized within the CFC dosing equations The blood levels of the factors should be obtained and maintained at an appropriate factor level for the following 10 to 14 days. In those patients that have an intracranial hemorrhage, secondary prophylaxis is recommended. Duration of the secondary prophylaxis is dependent on patient bleeding risk factors and may be short term for 3 to 6 months up to lifelong See Tables 5 and 6 CONT. CONTINUING EDUCATION continued on next page
In the context of hemophilia, the term "inhibitors" in the medical literature refers to the antibodies produced by patients to neutralize the factor VIII or IX agents
CONTINUING EDUCATION
administered to them This will negate the e�ects that the supplemental clotting factors will have in that individual ⁸ ‚ ¹² Although it is largely unknown why the immune system reacts to create antibodies to the clotting factors, some possible reasons include a genetic predisposition or environmental factors including clotting factor received, patient’s age of �rst clotting factor treatment, the intensity of the treatment regimen, surgical history, and/or infection
If a patient fails to respond to the clotting factor therapy being administered, development of an inhibitor should be suspected ¹²
Hemophilia A Inhibitors


Approximately 30% of hemophilia A patients receiving clotting factor concentrate (CFC) replacement therapy are known to develop inhibitors Roughly 80% of these patients will develop an inhibitor in the �rst 20 administrations of clotting factor therapy The other 20% develop the inhibitor in the �rst 75 exposures of factor VIII therapy Common risk factors for hemophilia A patients to develop an inhibitor include severe hemophilia disease, familial history of inhibitor production, African American or Hispanic ancestry, increase exposure to factor VIII therapy, and high intensity factor replacement. ⁸ Treatment recommendations di�er based on the inhibitor titer The inhibitor titer can be ordered as part of the blood draw A low responding inhibitor shows a lab result of 5 NBU/BU or less, while a high responding inhibitor shows a lab result of greater than 5 0 NBU/BU ¹² Table 9 refers to di�erent treatment recommendations in patients with inhibitors present When utilizing FVIII for the treatment in a patient with a lowresponding inhibitor, a loading dose is needed to help neutralize the inhibitor The utilization of the below equation should be utilized to ensure the FVIII replacement is su�cient to overcome the inhibitor
Hemophilia B Inhibitors
Inhibitors for hemophilia B typically occur only in severe cases. Most inhibitors will develop during the �rst 9 to 11 exposures of CFC, typically before the age of 2 years One major complication associated with hemophilia B inhibitor formation is the increased risk of anaphylaxis This may occur in up to 50% of hemophilia B patients taking CFCs and could be the �rst indication of inhibitor development Treatment recommendations di�er based on low- or highresponding inhibitors and can be seen in Table 10 If the patient has a history of anaphylaxis, utilization of the aPCC shouldn’t be used as it contains factor IX ⁸
Conclusion
Hemophilia is a complex bleeding disorder characterized by insu�cient clotting factors VIII or IX Bleeding is a common manifestation of hemophilia if the clotting factors are not replaced Treatment approaches involve the administration of clotting factor concentrates (CFCs), either FVIII or FIX
Alternative treatment options include recombinant activated factor VII (rFVIIa), desmopressin, tranexamic acid, and activated prothrombin complex concentrate (aPCC). The speci�c treatment for bleeding events depends on factors such as the severity of hemophilia, the site of the bleed, and the presence of inhibitors
CONT.
¹³ IU loading dose = {body weight (kg) x 80 x [(1-hematocrit) x antibody titer (BU)]} + [50 x body weight (kg)]
24
References
1 Moake JL. Hemophilia [Internet]. New Jersey: Merck & Co.; 2022 [cited 2023 Feb 28] Available from:
https://www merckmanuals com/professional/hematology-andoncology/coagulation-disorders/hemophilia
2 Centers for Disease Control and Prevention [Internet] Atlanta: U S
Department of Health& Human Services; c2022 [cited 2023 Feb 28]
Hemophilia Available from:
https://www.cdc.gov/ncbddd/hemophilia/facts.html.
3 Centers for Disease Control and Prevention [Internet] Atlanta: U S
Department of Health & Human Services; c2022 [cited 2023 Feb 28]
Data & Statistics Available from:
https://www cdc gov/ncbddd/hemophilia/data html
4 Smith SA, Travers RJ, Morrissey JH How it all starts: initiation of the clotting cascade Crit Rev Biochem Mol Biol 2015 May 28; 50 (4): 326-336
5 Norris B Haemostasis and Clotting SimpleMed [Internet] 2023 [cited 2023 Mar 2]; Available from
https://simplemed.co.uk/subjects/pathology/haemostasis-andclotting
6 Centers for Disease Control and Prevention [Internet] Atlanta: U S
Department of Health & Human Services; c2022 [cited 2023 Feb 28]
Diagnosis Available from:
https://www cdc gov/ncbddd/hemophilia/diagnosis html
7 Centers for Disease Control and Prevention [Internet] Atlanta: U S
Department of Health & Human Services; c2022 [cited 2023 Feb 28].
Treatment Available from:
https://www cdc gov/ncbddd/hemophilia/treatment html
8 Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al WFH Guidelines for the Management of Hemophilia, 3rd edition Haemophilia 2020 Aug;26 Suppl 6:1-158
9 Curtis R, Roberts JC, Crook N, Decker-Palmer M, Khainar R, Baker JR, et al Trends in prescribing practices for management of haemophilia: 19992021 Haemophilia 2023;1-9
10 Zwagemaker AF, Kloosterman FR, Coppens M, Gouw SC, Boyce S, Bagot CN, et al Desmopressin for bleeding in non-severe hemophilia A: Suboptimal use in real-world setting Res Pract Thromb Haemost 2022;6:e12777
11 Desmopressin [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 13).
12 Centers for Disease Control and Prevention [Internet] Atlanta: U S Department of Health & Human Services; c2022 [cited 2023 Feb 28]
Inhibitors Available from: https://www cdc gov/ncbddd/hemophilia/inhibitors html
13 National Hemophilia Foundation [Internet] New York: U S National Hemophilia Foundation; c2023 [cited 2023 Mar 16] Why do Some Patients Develop Inhibitors?. Available from: https://www hemophilia org/bleeding-disorders-az/overview/inhibitors/why-do-some-patients-develop-inhibitors
14 World Federation of Hemophilia; c2023 [cited 2023 Feb 28] WFH Online Registry of Clotting Factor Concentrates Available from: https://elearning wfh org/resource/online-cfc-registry/
15 U S Food & Drug Administration; c2023 Feb 27 [cited 2023 Feb 28]
Licensed Biological Products with Supporting Documents Available from: https://www fda gov/vaccines-blood-biologics/licensed-biologicalproducts-supporting-documents
16 Antihemophilic Factor (Recombinant) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
17 Antihemophilic Factor (Human) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar2
18 Antihemophilic Factor (Recombinant [Pegylated]) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2).
19 Antihemophilic Factor (Recombinant [Fc Fusion Protein]) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
20 Antihemophilic Factor/von Willebrand Factor Complex (Human) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-
https://www fda gov/vaccines-blood-biologics/licensed-biologicalproducts-supporting-documents
16 Antihemophilic Factor (Recombinant) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
17 Antihemophilic Factor (Human) [monograph]. In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar2
18 Antihemophilic Factor (Recombinant [Pegylated]) [monograph]. In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
19 Antihemophilic Factor (Recombinant [Fc Fusion Protein]) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
20 Antihemophilic Factor/von Willebrand Factor Complex (Human) [monograph]. In: Lexicomp Online [online database]. Hudson, OH: LexiComp (accessed 2023 Mar 2)
21 Factor IX (Human) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
22 Factor IX (Recombinant) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
23 Factor IX (Recombinant [Fc Fusion Protein]) [monograph] In: Lexicomp Online [online database]. Hudson, OH: Lexi-Comp (accessed 2023 Mar 2).
24 Factor IX (Recombinant [Albumin Fusion Protein]) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
25 Factor IX (Recombinant [Glycopegylated]) [monograph] In: Lexicomp Online [online database] Hudson, OH: Lexi-Comp (accessed 2023 Mar 2)
CE Assessment Questions
1) Which of the following statements accurately describes hemophilia?
a. Hemophilia A is more common than Hemophilia B, and it is characterized by a de�ciency of coagulation factor IX.
b Hemophilia is a dominant bleeding disorder caused by the overproduction of a clotting protein
c Hemophilia is an X-linked recessive bleeding disorder caused by the de�ciency or absence of a clotting protein.
d. Hemophilia primarily a�ects females due to their possession of two X chromosomes.
2) What clotting factor production is lacking in hemophilia B?
a Factor IX
b. Factor X
c. Factor XI
d. Factor XII
3) In assessing the need for CFC replacement in a patient with hemophilia A, which factor level is expected to be low?
a. Factor IX
b. Factor VII
c Factor VIII
d Factor XI
4) NB, a 59-year-old male, arrives at the emergency department with a joint hemorrhage. His medical history reveals hemophilia B with a clotting factor IX level of 3% and no inhibitor. He has a height of 60 inches and weighs 70 kg Considering the provider's intention to follow a high-dose practice pattern treatment plan, which CFC agent and dose would be suitable for this patient?
a. FIX 1,750 IU per dose
b. FIX 3,500 IU per dose
c. FVIII 1,750 IU per dose
d rFIX 2,800 IU per bdose
continued on next page
25
CONT. CONTINUING EDUCATION
CONT.
CONTINUING EDUCATION
5) BB, a 70-year-old male, arrives at the emergency department with a gastrointestinal bleed. He reports having hemophilia A and a history of highresponding inhibition The inhibitor titer reveals a result of 8 NBU/BU. Which of the following options is NOT a treatment option for this patient?
a aPCC
b FVIII
c. rFVIIa
6) Is it appropriate for a provider to use desmopressin as adjunct therapy for a patient with hemophilia A?
a. No, desmopressin will decrease factor IX and Von Willebrand Factor
b No, desmopressin will decrease factor VIII and Von Willebrand Factor
c. Yes, desmopressin will increase factor IX and Von Willebrand Factor
d Yes, desmopressin will increase factor VIII and Von Willebrand Factor
8) A patient with hemophilia A was hospitalized due to a moderate joint hemorrhage. After receiving one dose of FVIII, he experienced pain relief, but his symptoms did not completely resolve at that time. Following another dose of FVIII, he achieved complete resolution of symptoms. What is the classi�cation of his Clotting Factor Concentrates (CFC) response?
a. None
b. Moderate
c Good
d Excellent
9) Which of the following agents can be used in the treatment of hemophilia A or B as it facilitates the conversion of X to Xa through the extrinsic clotting pathway?
a FIX
b FVIII
c FXI
d. rFVII
7) DD, a 35-year-old female, presents to the hospital with her �rst case of mild hematuria. She has a history of hemophilia B with no inhibitor present. Which of the following is the most correct initial treatment for her mild hematuria?
a. FVIII
b. PCC
c Rest and hydration
d rFVII
CONTINUING EDUCATION INFORMATION
AzPA Members may retrieve FREE CE for this article up to one year after the program release date

This program provides 0 5 contact hours of continuing education credit.
Accredited Date: 07/24/2023
Expiration Date: 07/24/2026
Universal Activity Number (UAN) is: 0100-0000-23-156-H01-P
Apply for credit here: https://www.lecturepanda.com/a/AJPSummer23
The Arizona Pharmacy Association is accredited by the Accreditation Council for Pharmacy Education as providers of continuing education.
26
Computerized Provider Order Entry
Thisseries,PharmacyandtheLaw,ispresentedbyPharmacistsMutualInsuranceCompanyandtheArizonaPharmacy AssociationthroughPharmacyMarketingGroup,Inc.,acompanydedicatedtoprovidingqualityproductsandservicestothe pharmacycommunity.
Roberta, the pharmacist at Anytown Pharmacy, received an electronic prescription for bacitracin ointment The amount ordered was one tube with the directions to apply the ointment as directed Roberta �lled the prescription with a 30-gram tube of topical bacitracin. Mrs. Partridge was carrying her one-year old son, Chris, when she came into the pharmacy to pick up the prescription. Mrs. Partridge was not o�ered any counseling, paid for the prescription, and left with the prescription and her child in hand
What Roberta did not know was that young Chris had been diagnosed with an eye infection and the prescription should have been for the ophthalmic ointment dosage form. Unfortunately, this went undiscovered as no counseling session took place. The pharmacy was later noti�ed of the error when Mrs. Partridge had taken Chris back to the doctor because his eye was not improving and now was covered with gobs of the topical ointment Computerized Provider Order Entry (CPOE) was intended to cut down on prescription errors, especially those caused by illegible handwriting. What went wrong here?
The early promotion of CPOE touted a reduction of prescription errors. This was to be achieved through the elimination of illegible handwriting and better information on products and strengths available

Pharmacists Mutual has been tracking prescription errors since 1989 through its Claims Study The Claims Study has been published a couple of times during its history The Claims Study tracks Mechanical error claims (i.e., patients received the wrong drug, the wrong strength of the right drug, or the wrong directions) as one component of the study. One would expect that more legible prescriptions would decrease these types of errors In 2001, the study showed that 50 4% of the claims reported involved the wrong drug, 24 4% involved the wrong strength, and 7 8% involved the wrong directions These three categories totaled 82 6% of the reported claims If CPOE delivered on its promise, one should see a decrease in those numbers as the use of CPOE has become more common place in the years since 2001. In 2023, those three categories were 48.2% (wrong drug), 24.6% (wrong strength), and 8 4% (wrong directions) for a total of 81 2% Why so little improvement?
The bacitracin example was from a few years ago when systems tended to leave more �elds as free-form, allowing the entry of "one tube" and "use as directed". Fewer free-form �elds decrease the potential number of entry mistakes by forcing the order provider to actively choose from sizes and strengths available. The knowledge level of the person entering the prescription also impacts its accuracy
EDITORIAL RX AND THE LAW 27
Did the input person here know this order was for an eye infection? If so, did they know the di�erence between an ophthalmic ointment and a topical ointment?
Systems are continually improving and one of those e�orts is changing systems to minimize order entry mistakes This applies to the pharmacy dispensing systems as well. Programs today have more hard-stops and alerts to help users avoid common errors. It is important to know and understand what your system can and cannot do for you. The pharmacist and technician must be also be diligent and not fall prey to the idea that computer output is always correct
In Roberta's case, a short interaction with Mrs Partridge would have avoided this situation with one question, what did your doctor tell you this prescription was for? Once Roberta heard the answer, an eye infection, she would have known the topical ointment was the incorrect product. This is an example of a prescription that might be facially correct, but therapeutically incorrect We should see fewer of
these as systems improve. However, many times, changes implemented to correct one type of error open a door to a di�erent type of error. Any process in which humans work will never be perfect That is why pharmacists should not let their guard down and remain diligent to the possibility of errors In the words of Juan Manuel Fangio, "You must always strive to be the best, but you must never believe that you are."
© Don R. McGuire Jr., R.Ph., J.D., is General Counsel, Senior Vice President, Risk Management & Compliance at Pharmacists Mutual Insurance Company
This article discusses general principles of law and risk management It is not intended as legal advice Pharmacists should consult their own attorneys and insurance companies for speci�c advice. Pharmacists should be familiar with policies and procedures of their employers and insurance companies, and act accordingly.

CONT. RX AND THE LAW 28
Rick G Schnellmann, PhD Dean, University of Arizona College of Pharmacy

R. Ken Coit College of Pharmacy Celebrate 195 Graduates

The University of Arizona R. Ken Coit College of Pharmacy honored its spring 2023 graduating class at Centennial Hall on May 12

The Coit College of Pharmacy celebrated its second graduating class of its Bachelor of Science in Pharmaceutical Sciences by conferring 62 bachelor degrees The four-year degree was launched in fall 2018 and o�ers a curriculum aimed at preparing graduates for employment in a variety of biomedical research settings, as well as preparing them for postbaccalaureate education in graduate or healthprofessional studies.
Ali Robinson gave the undergraduate response and will pursue her doctor of pharmacy degree with the Coit College of Pharmacy in fall 2023
"To my fellow graduates, I want to remind you that you deserve to be proud of yourselves and everything you accomplished and remember tomorrow is another day to continue on and do the best we can," she said.
The graduate programs celebrated and hooded four Master of Science, two Master of Science in Pharmacology and Toxicology graduates, six Doctor of Philosophy in Pharmaceutical Sciences graduates, four Doctor of Philosophy in Pharmacology and Toxicology graduates.
Mavis Obeng-Kusi, a doctor of philosophy in pharmaceutical sciences, gave the doctoral student response during the ceremony and is currently employed with Merck and Co
"We owe a debt of gratitude to the faculty, sta�, an administrators whose mentorship, support, and expertise have been invaluable to our success," she said. "We also appreciate our fellow graduate students with whom we have formed friendships that we hope will last a lifetime "
The College conferred doctor of pharmacy degrees to 117 graduates. Some of the graduates choose to accept positions in community or health-system settings Others continue their education by pursuing a graduate degree Among the Class of 2023, 30 students have applied and successfully matched for postgraduate year on (PGY1) pharmacy residency training
Stormmy Boettcher and Daniel Tellez were selected to give the PharmD response during the ceremony. Both will continue their clinical training with residencies at the Henry Ford Hospital in Detroit and the Southern Arizona VA Health Care System, respectively.
"To the faculty and sta�, I speak for all students when I say that we are blessed to have studied under some of the �nest pharmacy faculty on the planet," Tellez said.
Dean Rick Schnellmann, PhD, encouraged graduates to expect change and to seek out new opportunities
"New responsibilities and challenges can lead to promotions, new opportunities and greater job satisfaction Then, if you don’t immediately see a new opportunity, create one. Be the change you wish to see," Schnellmann said.
EDITORIAL UNIVERSITY & ALUMNI 29
Midwestern University College of Pharmacy
 Mitchell R. Emerson, PhD
Dean, Midwestern University College of Pharmacy
Mitchell R. Emerson, PhD
Dean, Midwestern University College of Pharmacy

Greetings from the College of Pharmacy at Midwestern University!
As we welcome summer, we also celebrate the many accomplishments of our students, faculty, and alumni.
In March we hosted the APhA Alumni and Friends reception in downtown Phoenix. The room was �lled with smiling faces and over�owing with conversations. Thank you to everyone that stopped by and joined us
On May 12, we celebrated the Class of 2023 at the Graduation Awards Ceremony. Following the awards ceremony, the class celebrated with a delicious BBQ on campus
Congratulations to all the students, preceptors and faculty who received awards for their excellence
Excellence in Pharmacy Award
Ryan Brower
Excellence in Patient Communication Award
Timothy Green
Excellence in Pharmacology Award
Christine Wolesensky
Excellence in Pharmacy Administration Award
Katrina Henry- Boudreaux
Excellence in Therapeutics Award
India Bhatia
Excellence in Professional Skills Development Award
Heather Hotchkiss
Excellence in Medicinal Chemistry Award

Megan Rauschnot
Excellence in Pharmaceutics/Pharmacokinetics Award
Mycah Martens
Excellence in Service Award
Amal Basset
Excellence in Research Collaboration Award
Sarah Lira
Excellence in Public Health Award
Desiree Greenberg
Excellence in Evidence-Based Healthcare Award
Luis Mejia- Nieto
Robert C. Johnson Leadership Award
Mary Robinson
CPG Alumni Council Scholarship Award
Marsa Esmaeili Koosej
Merck Manual Award for Academic Excellence
Razel Mosquito
India Bhatia
Mycah Martens
Viatris Excellence in Pharmacy Award
Breanne Boyette
Wolters Kluwer Award for Excellence in Clinical Communications
Peter Huynh
2023 Mentor of the Year
Je�rey Barletta, PharmD, FCCM
EDITORIAL UNIVERSITY & ALUMNI continued on next page 30
2023 Preceptor of the Year
Kumar Swamy, PharmD, BCPS
2023 Rookie Preceptor of the Year
Pamela Allison, PharmD, BCACP, BC-Adm, AAHIVP
2023 Faculty Preceptor of the Year

Je�rey Barletta, PharmD, FCCM
On May 31, the Class of 2023 hosted it’s First Annual Hooding ceremony Over 500 guests attended this special ceremony. Hooding is especially meaningful to those families and friends who were able to join us and hood their graduate

On June 1, we o�cially celebrated the Class of 2023 at Graduation. We welcome the Class of 2023 to the ranks of alumni and encourage each and everyone to keep in touch with your CPG family We wish you continued success as you start your next chapter
On June 23 at 7pm, in conjunction with AZPA, we’ll be hosting the Alumni and Friends Dessert Reception in Tucson at the Loews Ventana Resort If you’re able to join us, please reach out to Kimberly at KHastings@midwestern.edu and look for details in your email

CONT.
& ALUMNI
As we said goodbye to the Class of 2023, we welcomed the Class of 2026 to campus on May 30 Orientation and classes begin June 5 We welcome our newest class and wish them success as they take steps to becoming a future pharmacist. The Class of 2026 is extra special as we welcome students from all over the nation, but 40% are coming from Arizona and California with 21%
We are looking forward to catching up with all of you and connecting at a future event. If you’re ever back in the Glendale area, please reach out and stop by the campus So much has changed, but still remains the same welcoming place.
If you’ve recently moved or relocated, please ensure we have your updated contact information Please email updates to your Manager of Alumni Relations, Kimberly Hastings at KHastings@midwestern.edu
To follow us and learn more about our events and wins, join the MWU Pharmacy social media community:
Like us on Facebook: Midwestern University-College of Pharmacy
Follow us on Twitter: @MWUpharmacy
Follow us on Instagram: @MWUpharmacy
UNIVERSITY
31
Creighton University College of Pharmacy
Jane Stein, PharmD Professor, Creighton University College of Pharmacy

Students Work, Learn Together in Interprofessional Health Program
The days when healthcare providers worked in a bubble, unaware of what other healthcare professionals were doing with the same patient, are long gone at Creighton University where interprofessional education has become a mainstay of academic learning and clinical training.
Creighton's approach to interprofessional education means health sciences students learn alongside students from other professional programs, preparing them to better serve patients and giving them hands-on work experience that prepares Bluejays for what they'll face in their careers
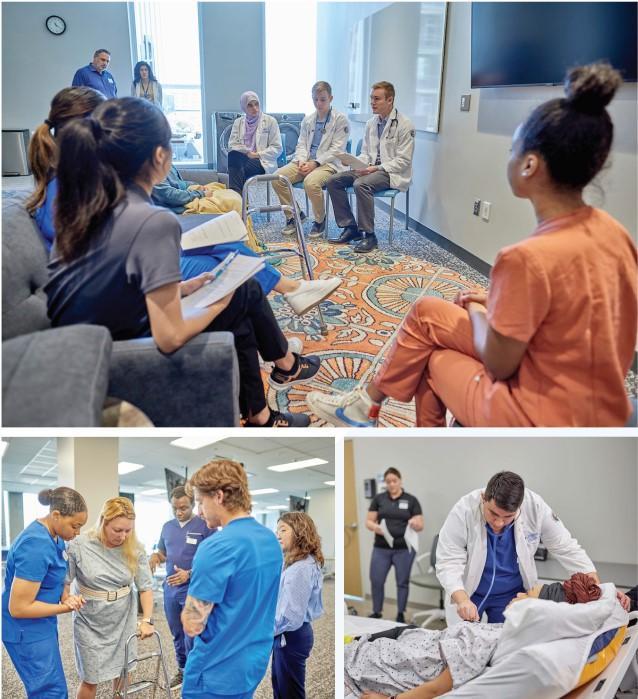

That commitment is strong both on Creighton's 145-yearold Omaha campus and its 18-month-old campus in Phoenix, opened in 2021at a cost of approximately $100 million
A day-long event dubbed Interprofessional Education Day was the latest expression of Creighton's commitment Held largely on the Phoenix campus with virtual participation from Omaha's paramedicine program, about 36 students drawn from medicine, paramedicine, nursing, pharmacy, physical therapy and occupational therapy combined their skills to treat an unfolding, simulated case of a 35-year-old woman who has su�ered a stroke.
It is a pilot program that is expected to grow in future years and to include the Phoenix physician assistant program.
Students demonstrate understanding of the roles and responsibilities of each of the professions represented within the event; that they will respect the roles, responsibilities and expertise of team members as they collaborate with students from other professions to develop a patient care plan; that they will recognize one's limitations as a team member; and will use e�ective communication tools and techniques with the simulated patients, actors and team members, said Gianluca Del Rossi, PhD, ATC, professor of physical therapy
As for this inaugural Interprofessional Education Day: This IPE Day is such an amazing opportunity for all future health care providers to get exposure to other
professions by working alongside them and seeing them in action before they even get into hospitals and clinics, says Sara Dahlhauser, OTD, OTR/L, assistant professor in the Department of Occupational Therapy on the Phoenix campus Students have a safe space to experience one another's specialties and to ask questions of each other in a way that helps them develop a much deeper level of understanding about what each profession brings to the table
EDITORIAL UNIVERSITY & ALUMNI 32
During the event, the groups, with each he group Each of the gr scenarios involving b actors. Debrie�ngs a debrie�ng after all fo joined with an hourhas had a stroke des working with a healt Interprofessional Ed the beginning Collea physician assistant, p occupational therap paramedicine along department collaborated every step of the way


Interprofessional education, through the Center for Interprofessional Practice, Education and Research, is very well established at Creighton," says Jaime Nesbit PT, DPT, assistant professor of physical therapy ?So, while this is by no means a unique event, to our knowledge this is the �rst IPE event that includes representation from all of the health professions in a single event "

UNIVERSITY & ALUMNI 33
NEW ON-DEMAND PROGRAM!
Pharmacist-Directed
Hormonal Contraception Training

The Arizona Pharmacy Association is happy to announce that 2 years after the passage of SB1082 Arizona pharmacists can now dispense Self-Administered Hormonal Contraceptives to women 18 years and older pursuant to the newly adopted ADHS Statewide Standing Order!
Any pharmacist wishing to dispense self-administered hormonal contraceptives pursuant to this statewide Standing Order must be prepared to do the following:
Complete a 3-hour Training -AzPA has created one that is compliant with ARS 32-1979.01 and AAC R4-23-407 and R4-23-408-409.
Obtain necessary equipment to measure blood pressure. Review the Standing Order, Standard Procedures, and Self-Screening Questionnaire. Establish SOP’s to ensure your pharmacy is compliant with all state laws.
REGISTER NOW 34
Audit Target – Pre-Filled Injectable Pens and Syringes

•
Pre-�lled injectable medication such as insulin, Invega , Humira , Enbrel and Ozempic remain a big target for audit risk due to their high cost and potential billing pitfalls One claim error can cost you thousands of dollars PAAS National often sees prescriptions for pre-�lled injectable medications �agged for recoupment due to one or more of the following reasons:
® ® ® ® ®
1 Missing a unit of measure (UOM) or written for a UOM that does not make sense
2 Missing dosage or quantity to inject
3 Missing the route of administration (ROA)
4 Missing calculable instructions/frequency
PAAS Tips: Here is a Humira prescription example:

The SIG does not contain calculable instructions = how much to inject, where to inject (ROA) or how often to inject
If the pharmacy received this prescription and did not clarify any elements, it would be marked discrepant upon audit.
Upon receiving this prescription, ideally, the pharmacy would clarify the following elements with the prescriber’s o�ce and make a clinical notation
•
The quantity of #1 could be interpreted as 1 pen when the box comes as a kit and contains 2 pen
• There is no UOM, so does the prescriber want 1 box, 1 mL, 1 pen, 1 kit?
• SIG – Inject 40 mg subcutaneously once a week – this clari�es how much to inject, the ROA and how often to inject
Quantity of 1 = 1 kit or 2 pens
• Pay special attention to pre-�lled injectable pens and syringes to avoid thousands of dollars of chargebacks upon an audit
PAAS National® is committed to serving community pharmacies and helping keep hard-earned money where it belongs Contact PAAS today at (608) 873-1342 or info@paasnational.com to see why PAAS Audit Assistance membership might be right for you.

• ®
By Trenton Thiede, PharmD, MBA, President at PAAS National , expert third party audit Assistance and FWA/HIPAA compliance.
Copyright © 2023 PAAS National, LLC Unauthorized use or distribution prohibited All use subject to terms at https://paasnational com/terms-of-use/
EDITORIAL PAAS NATIONAL 35


ADVOCACY LEGISATIVE UPDATE 36
The �rst regular session of the 56th Legislature has not adjourned “sine die” at this point. We expect that to happen when the legislature returns on July 31. Although we don’t expect any further legislation at that time, things can obviously change. Normally we would wait for the �nish, but given the unique schedule this year, we decided not to wait on this report
A total of 1,671 bills and 109 memorials and resolutions were introduced. At this time, 346 of those bills gained the approval of both chambers and made it to the Governor’s desk. Of those 346 bills, Governor Katie Hobbs signed 202 into law and vetoed 143, a 41% veto rate and more than double the record for the most bills vetoed in a session of 58, which was formerly held by Governor Janet Napolitano. One bill has not been acted on at this time. SB 1131 residential lease; municipal tax exemption is still in the possession of the Senate and has not been sent to the Governor.
Speculation is that the bill may be used as part of a possible deal on the Maricopa County transportation sales tax extension. Read more about both issues in the Notable Legislation and Issues section below.
Having a split government was something most of these members had never experienced before In addition, more than a third of the Legislature were brand new legislators, not knowing the process at all. Those two factors and the general political ideological di�erences of the two caucuses made the goal of the 100-day session unlikely from the start. The Republicans wanted to send up several bills dealing elections, transgender issues, critical race theory, and other political statement issues which contributed to the record-breaking veto number
Below are the bills that the Arizona Pharmacy Association were proactively running or supporting.
HB 2207 insurance; reimbursement rates; pharmacists (Rep. Wilmeth – R)
SUMMARY: Requires health and disability insurers that issue, amend, deliver, or renew a subscription contract, policy, or evidence of coverage after the e�ective date of this legislation to provide reimbursement coverage to a pharmacist at a rate not less than the rate provide to a licensed physician, nurse practitioner, or physician assistant. Stipulates that for the service or procedure to be covered, the pharmacist must act within their scope of practice and the service or procedure must otherwise be covered under the policy.

ACTION: Held awaiting hearing in committee.
P3 NOTE: This bill was one that we had run on behalf of the Arizona Pharmacy Association. However, after the introduction of the bill, we chose to hold this bill to continue the discussions with BCBS. Those conversations have been ongoing and productive. We will likely expand to other insurers or consider running the bill in a future session
CONT. LEGISATIVE UPDATE
37
CONT.
LEGISATIVE UPDATE
Below are the bills that the Arizona Pharmacy Association was active on during this legislative session:
HB 2529 scope of practice; process; repeal. (Rep. Montenegro – R)
SUMMARY: Repeals the sunrise process for health care providers seeking an increase in their scope of practice.
ACTION: SB 1248 was substituted on Third Reading SB 1248 was subsequently vetoed on 3/3/23
P3 NOTE: This was a priority bill for the Association since any time we want to add items to the statutes that fall under the practice of a pharmacist, a scope of practice sunrise application must be �led These applications are no longer really reviewed by the committees anymore, which is why the health chairs sponsored the bills. The Arizona Medical Association, and the smaller doctor groups, all came out opposed to the bill. Ultimately, the bill was vetoed It is likely that some unrelated drama dealing with the CRNAs had some impact on the decision to veto There is potential to amend the scope of practice sunrise application in future sessions to just have to �le the education/training quali�cations when the bill is �led.
SB 1248 scope of practice; process; repeal. (Sen. Shope – R)
SUMMARY: Repeals the sunrise process for health care providers seeking an increase in their scope of practice.
ACTION: Vetoed on 3/3/23
P3 NOTE: This was a priority bill for the Association since any time we want to add items to the statutes that fall under the practice of a pharmacist, a scope of practice sunrise application must be �led These applications are no longer really reviewed by the committees anymore, which is why the health chairs sponsored the bills The Arizona Medical Association, and the smaller doctor groups, all came out opposed to the bill. Ultimately, the bill was vetoed It is likely that some unrelated drama dealing with the CRNAs had some impact on the decision to veto
SB 1382 pharmacy bene�ts managers; certi�cate requirements (Sen. Shamp – R)
SUMMARY: Requires pharmacy bene�t managers to obtain a valid certi�cate of authority to operate as a pharmacy bene�t manager in Arizona from the Department of Insurance and Financial Institutions (DIFI). Outlines information that must be included in an application for a certi�cate of authority. Permits DIFI to issue a cease-anddesist order if a pharmacy bene�t manager does not hold a valid certi�cate, and to suspend or revoke a certi�cate in certain circumstances. Establishes civil penalties for violations. Requires DIFI to establish a record retention schedule for all data related to enforcement of these requirements. Contains an e�ective date of January 1, 2025.
ACTION: Signed (Chapter 74)
P3 NOTE: This was a bill the Arizona Independent Pharmacist Coalition were lead on. However, the Association strongly supported bringing PBMs under the regulation of DIFI since there was previously no agency that had jurisdiction over the PBMs After several stakeholder meetings, we were able to get the PBMs to neutral and still able to have a good regulatory bill.
SB 1460 pharmacists; independent testing; treatment; requirements (Sen. Shope – R)
SUMMARY: Authorizes pharmacists to independently order testing and initiate and perform treatment for eligible persons who test positive for in�uenza, a respiratory infection, or a condition related to an emerging or existing public health threat identi�ed by the Arizona Department of Health Services (ADHS) for which a statewide standing order, rule or executive order is issued. Establishes requirements for a pharmacist who conducts tests or provides patient treatment in these circumstances
ACTION: Held awaiting hearing in committee.
P3 NOTE: This bill was the Arizona Retailers Association bill that the Arizona Pharmacy Association supported. Senator Shope held a stakeholder meeting on this issue where the Arizona Medical Association shared several concerns with how the language was drafted. Senator Shope asked the groups to try to work together over the interim to come back with a solution next year.
38
HB 2290 insurance; claims;
appeals;
provider credentialing (Rep. Cook – R)
SUMMARY: Requires a health care insurer who denies a health care services claim, in whole or in part, the insurer to provide the health care provider with contact information for an individual who can respond to questions about the denial. Requires an insurer, upon request, to provide a detailed reason why the health care service was not medically necessary and the provider’s right to appeal a denial based on lack of medical necessity, if applicable, and the provider’s right to dispute a decision using the insurer’s internal grievance process Establishes timeless for the insurer to respond to the provider Authorizes a health care provider to submit a written request for a hearing to the Arizona Department of Insurance and Financial Institutions if the provider’s grievance is unresolved after the speci�ed process and timeframes. Additionally, requires a health insurer to conclude the process of credentialing and loading the applicant’s information in the insurer’s billing system within 45 calendar days, rather than 100 calendar days, after the date to insurer receives a complete credentialing application
ACTION: Held in Senate GOV committee
P3 NOTE: The Association supported this bill as it put a process around appeals and denials, including allowing DIFI to intervene when necessary. Additionally, with working with the insurers on payment for services, pharmacists will have to be credentialed as providers, which would make that section applicable also.
HB 2344 pharmacy board; duties; regulation (Rep. Shah
– D)
SUMMARY: Makes a variety of changes to statutes relating to the Board of Pharmacy (Board). Expands the list of prohibited acts to include wholesaling or distributing a prescription drug or device, a controlled substance, a nonprescription drug, medical gas or durable medical equipment without a valid Board-issued permit, and in the case of a manufacturer, manufacturing, possessing or shipping into Arizona a manufactured prescription drug or device, a controlled substance, a nonprescription drug, a precursor chemical or any other regulated chemical without a valid Board-issued permit. Requires Board permittees are to notify the Board within 15 days of various information changes, including a change to hours of operation. Expands the list of acts constituting unprofessional conduct for Board permittees to include failing to routinely operate according to the permittee’s hours of operation and failing to report an unexpected serious adverse drug event from a compounded prescription that resulted in a patient being hospitalized or that resulted in death and that meets other speci�ed conditions. Authorizes the Board to delegate to the Executive Director the authority to issue a subpoena to solicit information about a complaint or investigation and the authority to enter into agreements between a state or federal regulatory agency
ACTION: Held awaiting hearing in committee
P3 NOTE: This was the Board of Pharmacy bill. The Association has serious concerns with how broadly the bill language was drafted and the powers of the Executive Director being expanded. Unfortunately, they did not work with the Association prior to session. The chair of the Health committee chose not to hear the bill due to the concerns
HB 2426 technical correction; prior authorization; timelines (Rep. Gress – R)
SUMMARY: Makes a minor change in Title 20 (Insurance) related to prior authorization.
ACTION: Held awaiting hearing in committee
P3 NOTE: This bill was supposed to be used for ArMA’s Gold Card prior authorization language, which the Association supported. Ultimately, the Chair did not hear the bill. While there were attempts to add this language in other locations, it never made it on to another bill. They plan to reintroduce something similar to address the issue in a future session
HB 2564 hospitals; physicians; dispensing opioids (Rep. Shah – D)
SUMMARY: Allows a hospital or a “health professional” (de�ned) who is working in a hospital that is not within 50 miles of a 24-hour pharmacy, when discharging a patient with an acute illness or injury after regular pharmacy business hours, to dispense a 12-hour supply of a schedule II-controlled substance that is an opioid to the patient.
ACTION: Signed (Chapter 42)
P3 NOTE: The Association was neutral on this legislation It ensures that patients have access to the medications they need.
CONT. LEGISATIVE UPDATE
39
HB 2344 pharmacy board; duties; regulation (Rep. Shah – D)
SUMMARY: Makes a variety of changes to statutes relating to the Board of Pharmacy (Board) Expands the list of prohibited acts to include wholesaling or distributing a prescription drug or device, a controlled substance, a nonprescription drug, medical gas or durable medical equipment without a valid Board-issued permit, and in the case of a manufacturer, manufacturing, possessing or shipping into Arizona a manufactured prescription drug or device, a controlled substance, a nonprescription drug, a precursor chemical or any other regulated chemical without a valid Board-issued permit Requires Board permittees are to notify the Board within 15 days of various information changes, including a change to hours of operation Expands the list of acts constituting unprofessional conduct for Board permittees to include failing to routinely operate according to the permittee’s hours of operation and failing to report an unexpected serious adverse drug event from a compounded prescription that resulted in a patient being hospitalized or that resulted in death and that meets other speci�ed conditions. Authorizes the Board to delegate to the Executive Director the authority to issue a subpoena to solicit information about a complaint or investigation and the authority to enter into agreements between a state or federal regulatory agency
ACTION: Held awaiting hearing in committee.
P3 NOTE: This was the Board of Pharmacy bill. The Association has serious concerns with how broadly the bill language was drafted and the powers of the Executive Director being expanded. Unfortunately, they did not work with the Association prior to session The chair of the Health committee chose not to hear the bill due to the concerns
HB 2426 technical correction; prior authorization; timelines (Rep. Gress – R)
SUMMARY: Makes a minor change in Title 20 (Insurance) related to prior authorization.
ACTION: Held awaiting hearing in committee
P3 NOTE: This bill was supposed to be used for ArMA’s Gold Card prior authorization language, which the Association supported Ultimately, the Chair did not hear the bill While there were attempts to add this language in other locations, it never made it on to another bill. They plan to reintroduce something similar to address the issue in a future session
HB 2564 hospitals; physicians; dispensing opioids (Rep. Shah – D)
SUMMARY: Allows a hospital or a “health professional” (de�ned) who is working in a hospital that is not within 50 miles of a 24-hour pharmacy, when discharging a patient with an acute illness or injury after regular pharmacy business hours, to dispense a 12-hour supply of a schedule II-controlled substance that is an opioid to the patient.
ACTION: Signed (Chapter 42)
P3 NOTE: The Association was neutral on this legislation It ensures that patients have access to the medications they need
HB 2622 cost sharing; health coverage; report (Rep. Hendrix – R)
SUMMARY: Requires an organization or individual advocating a legislative proposal that would place a restriction on the form or amount of cost sharing applied to a health plan bene�t issued by an insurer to submit a written report to the Joint Legislative Audit Committee (JLAC) by September 1 before the start of the legislative session for which the legislation is proposed, and JLAC must assign the report to the appropriate legislative committee of reference for review. Requires information that must be included in the mandated health coverage or cost sharing restriction reports submitted to JLAC to include the impact on other policyholders that do not use the treatment or service subject to the mandated coverage or cost
CONT. LEGISATIVE UPDATE
40
HB 2622 cost sharing; health coverage; report (Rep. Hendrix – R)
SUMMARY: Requires an organization or individual advocating a legislative proposal that would place a restriction on the form or amount of cost sharing applied to a health plan bene�t issued by an insurer to submit a written report to the Joint Legislative Audit Committee (JLAC) by September 1 before the start of the legislative session for which the legislation is proposed, and JLAC must assign the report to the appropriate legislative committee of reference for review. Requires information that must be included in the mandated health coverage or cost sharing restriction reports submitted to JLAC to include the impact on other policyholders that do not use the treatment or service subject to the mandated coverage or cost sharing restriction, and an analysis of whether the state will be required to defray the costs that a treatment or service may add to the federal marketplace subsidies under speci�ed federal code.
ACTION: Held awaiting Third Reading.
P3 NOTE: The Association was opposed to this bill due to the fact that insurers were trying to �nd additional roadblocks to patients getting the access to the medications necessary. We were able to pull at least two Republicans o� the bill and the Democratic caucus was opposed. Therefore, the bill never received a full vote of the House
SB 1087 professional licensure fees; waiver; reduction (Sen. Kern – R)
SUMMARY: Requires, by September 1 of each year, each regulatory board and agency to review the costs it incurs, the monies it has in its funds, and the fee revenues it collects to determine whether the regulatory board or agency expects ending balances in its licensing fund to exceed 50 percent of the appropriations from that fund in the current �scal year. States that if the regulatory board or agency determines that the ending balance in its licensing fund would exceed 50 percent of its appropriation, the regulatory board or agency must provide a onetime waiver or reduction from licensure or certi�cation renewal fees to reduce the balance in its licensing fund to below 50 percent of its appropriation from that fund in the upcoming �scal year, or within the board’s or agency’s normal schedule for renewing licenses or certi�cates if longer than one year.
ACTION: Held awaiting hearing in House Rules committee
P3 NOTE: The Association supported this bill due to the large surplus the Board of Pharmacy has from fees. However, due to the �scal impact of the bill on many other agencies, the bill was passing party line. Additionally, it would have ongoing �scal impacts Therefore, it was not included in the budget
SB 1254 opioids; containers; labeling; requirements; repeal (Sen. Shamp – R)
SUMMARY: Permits, rather than requires, the container of a schedule II controlled substance that is an opioid that is directly dispensed by a pharmacist and that is not for the immediate administration to the ultimate user to have a red cap.
ACTION: Failed House Third Reading (20-38-1-0-1).
P3 NOTE: The Association supported this bill at the request of the Senator The bill would have permitted a pharmacist to use a red cap instead of requiring However, some members had concerns over the impact on seniors accidentally taking opioids. Others wanted to make the decision an opt-out process for the patient. There was not an agreement reached and the hostile opt-out amendment did not get adopted during Committee of the Whole However, that also made it where the bill failed, after the Freedom Caucus joined the Democrats in opposing the bill.
SB 1280 prescriptions; public health emergencies (Sen. Shamp – R)
SUMMARY: Requires a pharmacist during a public health emergency to dispense all prescription orders written by a medical practitioner for the o�-label use of a prescription drug. Grants a pharmacist immunity from criminal prosecution and adverse board action or discipline if the pharmacist, in good faith, refuses to dispense a prescription because this action would be contrary to law, contrary to the health and safety of the patient, or impossible or inappropriate because of one of a list of speci�ed circumstances
ACTION: Held awaiting hearing in committee.
P3 NOTE: The Association had concerns about this bill as drafted It was very similar to a bill Senator Townsend had previously run where there were issues We spoke to the sponsor on behalf of the Association and shared with her the concerns about the language. She decided to ask that the bill not be moved.
CONT. LEGISATIVE UPDATE
41
CONT. LEGISATIVE UPDATE
HB
2001 department of health services; rulemaking (Rep. Cook
– R)
SUMMARY: Exempts rules made by the Arizona Department of Health Services (ADHS) to regulate an accredited hospital from all statutory requirements if the rules reduce a regulatory burden without jeopardizing health and safety, do not increase costs to regulated persons, and the public is given at least 15 days to comment on the rules prior to their adoption. Contains an emergency clause.
ACTION: The bill was awaiting Senate Committee of the Whole P3 NOTE: ADHS had major issues with the bill so a comprise was made between Rep Cook and the Governor’s o�ce. However, the bill never moved.
HB 2126 contraception;
cost sharing prohibition
(Rep. Salman – D)
SUMMARY: Prohibits health and disability insurers from imposing deductibles, coinsurance, copayments, or other cost containment measures for contraceptive drugs, intrauterine devices, prescription barrier methods, or male sterilization. Speci�es that religiously a�liated employers are no longer exempt from the requirement to provide coverage for contraceptives if the contract provides coverage for prescription drugs.
ACTION: This bill was never heard in committee.
HB
2139 medical services; purchase; study committee (Rep. Salman
– D)
SUMMARY: Establishes the 15-member Medical Services Purchase Program Study Committee (Committee) to research and make recommendations for establishing and implementing a medical services purchase program. The Committee is required to submit a report of its �ndings and recommendations to the Governor and the Legislature by March 1, 2024. Repeals the Committee on July 1, 2024.
ACTION: This bill was never heard in committee
HB 2157
medical malpractice; statute of limitations (Rep. Diaz – R)
SUMMARY: Increases the statute of limitations on a cause of action for medical malpractice to �ve years, from two years, after the cause of action accrues.
ACTION: This bill was never heard in committee
HB 2190
health professions; �ngerprint cards; websites (Rep. Longdon – D)
SUMMARY: Requires, beginning January 1, 2024, an applicant for licensure or license renewal from any of the following health profession regulatory boards to have a valid �ngerprint clearance card: the Arizona Medical Board, the Arizona Board of Osteopathic Examiners in Medicine and Surgery, the Naturopathic Physicians Medical Board, the Board of Homeopathic and Integrated Medicine Examiners, the Board of Behavioral Health Examiners, the Board of Chiropractic Examiners, the Arizona Regulatory Board of Physician Assistants, the Board of Nursing, the Board of Respiratory Care Examiners, the State Board of Dispensing Opticians, and the State Board of Optometry. Requires, beginning January 1, 2024, an applicant for licensure as a midwife, hearing aid dispenser, audiologist, or speech-language pathologist, and an applicant for certi�cation as a radiologic technologist from the Department of Health Services to have a valid �ngerprint clearance card. Additionally, requires each health profession regulatory board’s public website to have a list of licensees or certi�cate holders that includes speci�ed information, including the status of the license or certi�cate and a list of o�cial actions taken by the board against each licensee or certi�cate holder. Contains a delayed e�ective date of January 1, 2024.
ACTION: This bill was never heard in committee.
HB
2243 insulin; health insurance coverage (Rep. De Los Santos – D)
SUMMARY: Requires health and disability insurers to limit the total amount that a subscriber or enrollee must pay for a covered “prescription insulin drug” (de�ned) to $25 per 30-day supply of insulin, regardless of the amount or type of insulin required to �ll the prescription. Additionally, requires drug manufacturers or distributors of insulin operating in Arizona to make insulin available through local pharmacies to person who are uninsured or underinsured for a cost of no more than $30 for a 30-day supply
ACTION: This bill was never heard in committee
42
HB 2254 rulemaking; regulatory costs; legislative rati�cation (Rep. Wilmeth – R)
SUMMARY: Prohibits a proposed rule that is estimated to increase regulatory costs in Arizona more than $500,000 within �ve years after implementation or have an adverse impact on economic growth in Arizona in excess of $500,000 within �ve years after implementation from becoming e�ective until the Legislature enacted rati�es the proposed rule through legislation Prohibits an agency from �ling a �nal rule with the Secretary of State before obtaining legislative approval of the rule through legislation Exempts emergency rules from the bill’s requirements.
ACTION: Vetoed on 5/19/23.
HB 2316 federal government; mandatory vaccinations; prohibition (Rep. Jones – R)
SUMMARY: Prohibits the federal government from requiring an Arizona resident to receive a vaccination for COVID-10 or any variant of COVID-19 Except as otherwise required by federal law, the prohibition on any government entity requiring an Arizona resident to receive a vaccination for COVID-10 or any variant of COVID-19 applies to a health care institution that is owned or operated by a government entity in Arizona.
ACTION: Failed in HHS committee by a vote of 4-5-0-0.
HB 2347 AHCCCS; continuation. (Rep. Shah – D)
SUMMARY: Continues AHCCCS for eight years.
ACTION: This bill was never heard in committee.
P3 NOTE: AHCCCS was continued with the signing of HB 2826 (health boards; AHCCCS; continuation).
HB 2370 minors; consent; venereal disease prevention (Rep. Sun – D)
SUMMARY: Permits a minor to give consent to medical care and prescription usage related to the prevention of a venereal disease, and the consent is not subject to disa�rmance because of minority. States that the consent of the minor’s parent or legal guardian is not necessary to authorize medical or pharmaceutical care.
ACTION: This bill was never heard in committee.
HB 2388 health care workers;
employment rights (Rep. M Hernandez – D)
SUMMARY: Establishes a new article in Title 23 (Labor) relating to essential “health care workers” (de�ned)
Requires a health care employer to pay hazard pay of �ve percent above “base pay” (de�ned) to each health care worker for each hour of work performed outside the health care worker’s home to serve a COVID-19 essential function with some exceptions. Requires a health care employer to supply appropriate personal protective equipment to each health care worker the health care employer employs at no cost to the health care worker Requires a health care employer to provide a health care worker with at least three weeks of paid sick leave at the health care worker’s regular rate of pay if the health care worker is unable to work because of any of a list of speci�ed circumstances related to COVID-19. Establishes whistleblower protections for health care workers.
ACTION: This bill was never heard in committee.
HB 2608 pharmacists; prescription orders; requirements (Rep. Seaman – D)
SUMMARY: Stipulates that if a person presents a valid prescription order to a pharmacist and the pharmacy has the prescription medication in stock, the pharmacist must �ll the prescription order. States if the pharmacy does not have the prescription medication in stock, the pharmacist must return the prescription order to the person who presented the prescription order or the patient or, on request, electronically transfer the prescription order to a pharmacy that is able to �ll the prescription order.
ACTION: This bill was never heard in committee
CONT. LEGISATIVE UPDATE
43
HB 2706 unborn children; homicide; assault; jurisdiction. (Rep. McGarr – R)
SUMMARY: Expands the de�nition of “person” to include a living human unborn child at every stage of development from fertilization until birth for the purpose of homicide, including negligent homicide, manslaughter, second degree murder, and �rst-degree murder, and for the purpose of assault and related o�enses. States that the Attorney General and the county attorney have concurrent jurisdiction to prosecute homicide violations and assault violations if the victim is an unborn child. Precludes a person from being charged with a crime for medical care or treatment that is provided with the requisite consent by a licensed physician to avert the death of a pregnant female if the medical care or treatment results in the accidental or unintentional injury to or death of the pregnant female’s unborn child and all reasonable alternatives to save the life of the unborn child were attempted or none were available. Applies only to conduct that occurs after the e�ective date of this legislation. Contains an emergency clause.
ACTION: This bill was never heard in committee
HB 2720 contraception; coinsurance (Rep. Ortiz – D)
SUMMARY: Prohibits, beginning January 1, 2025, health and disability insurers that issue subscription contracts or insurance policies from imposing copayment or coinsurance requirements for emergency contraception that is prescribed by a health care provider or that is issued through a standing prescription drug order authorizing the dispensing of emergency contraception.
ACTION: This bill was never heard in committee.
HB 2742 emergency contraception; standing order (Rep. Shah – D)
SUMMARY: Permits a licensed physician or nurse practitioner who is authorized by law to prescribe drugs to issue a standing prescription drug order authorizing the dispensing of emergency contraception. Requires the Arizona Department of Health Services to designate emergency contraception that may be used with a standing prescription drug order based on an evaluation of the drug’s safety and e�cacy
ACTION: This bill was never heard in committee
HB 2775 worker’s rights; public health emergency (Rep. Austin – D)
SUMMARY: Prohibits employers from discriminating or retaliating against any worker based on the worker raising any reasonable concern about workplace violations of government health and safety rules, from requiring a worker to sign a contract or other agreement that would limit or prevent the worker from disclosing information about workplace health and safety practices related to a public health emergency, and from discriminating or retaliating against any worker based on the worker voluntarily wearing at the workplace the worker’s own personal protective equipment with certain exceptions Establishes penalties for violations Appropriates an unspeci�ed amount (blank in original) from the General Fund in FY2023-24 to the Employment Support Fund Applies to conduct occurring from and after the e�ective date of this legislation. Contains an emergency clause.
ACTION: This bill was never heard in committee.
SB 1065 appropriations; widening; I-10 (Sen. Shope – R)
SUMMARY: Appropriates $360 million from the General Fund in FY2023-24 to the Arizona Department of Transportation (ADOT) to widen Interstate 10 between Chandler and Casa Grande. Requires ADOT to use the monies for construction-related activities, including drawing down federal matching monies for the project Prohibits ADOT from accepting federal monies if the acceptance is conditioned on the design and construction of a bicycle path or pedestrian walkway as a component of the project
ACTION: Discussed and Held in House Transportation and Infrastructure committee.
P3 NOTE: HB2209 language o�ered as a striker in Senate TI committee. This deals with credentialing and denials. Chairman Cook held the bill after Sen Shope objected to his bill being used as a vehicle
SB 1081 AHCCCS; continuation (Sen. Shope – R)
SUMMARY: Continues AHCCCS for eight years.
ACTION: Held awaiting Committee of the Whole
P3 NOTE: AHCCCS was continued with the signing of HB 2826 (health boards; AHCCCS; continuation)
CONT. LEGISATIVE UPDATE
44
SB1250 employers; vaccines; religious exemption (Sen. Shamp – R)
SUMMARY: Requires employers to allow employees to claim a religious exemption from taking the COVID-19 vaccination, in�uenza A or B vaccination or �u vaccination, or any vaccination approved by the U.S. Food and Drug Administration for emergency use. Prohibits employers from inquiring into the veracity of an employee’s religious beliefs, and from discriminating against an employee regarding employment, wages, or bene�ts based on the employee’s vaccination status. Allows employees to �le a complaint with the Attorney General (AG) if the employer did not o�er the employee a religious exemption form or improperly applied or denied the employee’s religious exemption and their employment was terminated. Requires the AG to investigate all complaints. States that if the AG validates a complaint, the AG must notify the employer and allow the employer an opportunity to correct the noncompliance within 10 days Additionally, requires the AG to assess an employer a civil penalty of $5,000 for not coming into compliance.
ACTION: Vetoed on 3/30/23.
SB 1285 obstetric services; rural communities; recommendations (Sen. Shamp – R)
SUMMARY: Requires the Arizona Department of Health Services (ADHS) to convene stakeholders and sta� to develop recommendations to ensure that obstetrics and gynecology services are provided in low-volume, high-risk rural communities in Arizona. Requires ADHS to report their recommendations to the Executive and the Legislature by December 31, 2024
ACTION: Discussed and held in House Appropriations committee
P3 NOTE: The bill that was discussed and held in House Appropriations committee was a strike-everything amendment related to international medical graduates
SB 1333 database; health professionals; license revocations (Sen. Shamp – R)
SUMMARY: Requires each “health profession regulatory board” (de�ned) to report to the Arizona Department of Health Services (ADHS) all license and certi�cation revocations that occurred between July 1, 2019 through July 1, 2024, and the revocation of a health professional’s license or certi�cation. Requires ADHS, by July 1, 2024, to create a searchable online database that is posted on the ADHS public website that contains the names of the health professionals who have had a license or certi�cation revoked in the preceding �ve years, the date of the revocation, and the health profession regulatory board that revoked the license or certi�cation
ACTION: Signed (Chapter 158)
P3 NOTE: The Association was neutral on this bill since it is already public information.
SB 1386 occupational licensure; fee waiver; appropriation (Sen. Kavanagh – R)
SUMMARY: Prohibits a “regulatory board or agency” that regulates a “licensee” (both de�ned) from charging a fee for applications, initial licensure, licensure renewal, temporary licensure or examinations relating to the license in FY2023-24 and FY2024-25. Establishes the Occupational Licensure Fee Waiver Fund (Fund), to be administered by the State Treasurer and used to transfer to regulatory boards and agencies that demonstrate a �nancial need in FY2023-24 and FY2024-25 Repeals all bill provisions on July 1, 2026, and speci�es that any monies remaining in the Fund revert to the General Fund. Appropriates $32 million from the General Fund in FY2023-24 to the Fund.
ACTION: Held awaiting Committee of the Whole
SB 1433 employer liability; COVID-19 vaccine requirement (Sen. Wadsack – R)
SUMMARY: States that if an employer denies a religious exemption and requires a person to receive a COVID-19 vaccination as a prerequisite to or requirement for maintaining employment, the employer is liable to the person for damages that result from a signi�cant injury that is caused by receiving the COVID-19 vaccination. Asserts that a claimant who prevails under this provision must be awarded actual damages, court costs, and reasonable attorney fees or statutory damages of $1 million, whichever is greater, and may also recover exemplary damages. Speci�es that these rights supplement any other rights and remedies provided by law.
ACTION: Held awaiting Committee of the Whole
CONT. LEGISATIVE UPDATE
45
CONT. LEGISATIVE UPDATE
SB 1633 adult immunizations; reporting system (Sen. Gonzales – D)

SUMMARY: Expands the Child Immunization Reporting System to include adult vaccination records. Renames the Immunization Reporting System, and requires health care professionals licensed to provide immunizations to report information on adult patients receiving a vaccine.
ACTION: This bill was never heard in committee.
SB 1701 type 1 diabetes; drugs; devices (Sen. Wadsack – R)


SUMMARY: Requires the Arizona Health Care Cost Containment System Administration (AHCCCS) to establish a diabetes treatment program to provide medically necessary drugs and devices to treat type 1 diabetes, including insulin and insulin pumps, for Arizona residents who are younger than 24 years of age, have been diagnosed with type 1 diabetes, are uninsured or underinsured, and are not eligible under title XIX or XXI of the federal Social Security Act Prohibits AHCCCS from imposing cost sharing requirements for the drugs and devices, and from considering the family income of a person when determining eligibility Additionally, requires AHCCCS to act as payor of last resort for persons who are eligible under these provisions Appropriates an unspeci�ed amount (blank in original) from the General Fund in FY2023-24 to the AHCCCS for the diabetes treatment program
ACTION: This bill was never heard in committee.
YOUR TEAM
 Dianne McCallister AzPA Lobbyist
Kelly Fine AzPA CEO
Ken Bykowski AzPA Legislative A�airs Committee Co-Chair
Dianne McCallister AzPA Lobbyist
Kelly Fine AzPA CEO
Ken Bykowski AzPA Legislative A�airs Committee Co-Chair
46
Mark Boesen AzPA Legislative A�airs Committee Co-Chair
BOARD OF PHARMACY
Arizona State Board of Pharmacy Update

BOARD MEMBERS
Attention!TechnicianTrainees:
OTCNaloxoneApprovedbyFDA
The Arizona Board of Pharmacy has drafted a FAQ addressing the recent FDA approval of OTC naloxone. Please see the new OTC Naloxone FAQ.
Pharmacist-DirectedHormonalContraception
• ADHS Standing Order, Self-Screening Questionnaire, and Standard Procedures
Notice of Final Rule Making-Dispensing a SelfAdministered Hormonal Contraceptive and CE Requirements
• ADHS Women’s Health-Family Planning Program
• Improving access to birth control for Arizona patients: Making contraceptives available without a prescription at your local pharmacy
Senate Bill (“SB”) 1569 was signed into law in 2022 and will take e�ect on July 1, 2023. SB 1569 created a means for prospective pharmacy technician trainees to obtain a registration, as opposed to licensure, with the Arizona State Board of Pharmacy. Individuals who are currently licensed as pharmacy technician trainees with this Board are not impacted by SB 1569 and will remain licensees of this Board. Beginning July 1, 2023, eligible pharmacy technician trainee applicants will be issued a registration by this Board. In order to prepare for this change in law, the online application for Pharmacy Technician Trainee License was disabled on June 16, 2023. If you applied for a Pharmacy Technician Trainee License on or before June 16, 2023, your application for licensure will be processed as normal.
Please note: You may not apply for a Pharmacy Technician Trainee Registration if you previously held a Pharmacy Technician Trainee license.
Pharmacy Technician Trainee Registration applicants must submit:
•
• Frequently Asked Questions
UPCOMING MEETINGS
1 $25 00 application fee (this fee is nonrefundable)
2. Proof of legal residency and birth date
3. Government-issued photo ID (if the document you submit to prove your legal residency does not contain a photograph).
4. Documentation of any name changes.
To view application: CLICK HERE
Mailing Address: P.O. Box 18520 Phoenix, AZ 85005
Physical Address: 1110 W. Washington St., Suite 260 Phoenix, AZ 85007
EDITORIAL L o r r i W a l m s l e y R P h , P r e s i d e n t T e n i l l e D a v i s , P h a r m D , V i c e P r e s i d e n t C e d a r L a h a n n , P h a r m D , R P h , M e m b e r T h e o d o r e T o n g , P h a r m D , R P h , M e m b e r K e v i n D a n g , P h a r m D , R P h , M e m b e r K r i s t e n S n a i r , C P h T , M e m b e r J o s e p h L e y b a , R P h , M e m b e r F r a n k T h o r w a l d , M e m b e r ( P u b l i c )
NEWS
MEETING DATE Complaint Review August 8, 2023 Board Meeting August 16-17, 2023 Complaint Review October 10, 2023 Board Meeting October 18-19, 2023 Complaint Review November 28, 2023 Board Meeting December 6-7, 2023
47

48



















































 Mitchell R. Emerson, PhD
Dean, Midwestern University College of Pharmacy
Mitchell R. Emerson, PhD
Dean, Midwestern University College of Pharmacy


















 Dianne McCallister AzPA Lobbyist
Kelly Fine AzPA CEO
Ken Bykowski AzPA Legislative A�airs Committee Co-Chair
Dianne McCallister AzPA Lobbyist
Kelly Fine AzPA CEO
Ken Bykowski AzPA Legislative A�airs Committee Co-Chair

