
Arizona Journal of Pharmacy O F F I C I A L P U B L I C A T I O N O F A R I Z O N A P H A R M A C Y A S S O C I A T I O N | S P R I N G 2 0 2 4 Not Just a Pediatric Illness: Updates on Respiratory Syncytial Virus (RSV) Prevention In This Edition: The Drug Supply Chain Security Act Continuous Glucose Monitor (CGM) Billing and Supply Allowance
BoardofDirectors2023-2024
OFFICERS

PresidentKimberlyLangley
President-ElectPro-TemporeJacobSchwarz
ImmediatePastPresidentDawnGerber
TreasurerRyanGries
SecretaryBrandyDeChellis
Director/CEOKellyFine
DIRECTORSATLARGE
CommunityBrianneSpaeth
HealthSystemMaryManning
TechnicianMelindaBrowning
JosephPellerito
JimmyStevens
ReasolChino
MistyBrannon
DanielleGilliam
LIASIONS
UniversityofArizona
StudentChapterDavidElias-Campa
Dean'sDesignatedRepresentativeNancyAlvarez
MidwesternUniversity
StudentChapterShamsRehman
Dean'sDesignatedRepresentativeMichaelDietrich
CreightonUniversity
StudentChapterHaleyDeMartinis
Dean'sDesignatedRepresentativeJaneStein
LegalCounsel
RogerMorris
ChiefExecutiveOfficer
KellyFine
Education&ProfessionalDevelopment
DawnGerber
Events&StrategicPartnerships
CindyEsquer
Membership&VolunteerServices
MarquesBottorf
StrategicPrograms
KristinCalabro AdministrativeServices
MelinaEsquer

CindyEsquer
ElizabethNelson TheinteractivedigitalversionoftheArizonaJournalofPharmacyisavailableformembers onlyonlineinyourmemberportal
Editor'sNote:Anypersonalopinionsexpressedinthismagazinearenotnecessarilythose heldbytheArizonaPharmacyAssociation."ArizonaJournalofPharmacy"(ISSN1949-0941) ispublishedquarterlybytheArizonaPharmacyAssociationat:1845E.SouthernAvenue, Tempe,AZ85282-5831 President’s Message 4 Contents AzPAStaff pg. 6 AzPA News Welcome New Members 5 Editorial Annual Convention 6 Advocacy Pharmacy Day at the Capitol 38 Continuing Education Not Just a Pediatric Illness: Updates on Respiratory Syncytial Virus (RSV) Prevention 21 Az-ASHP State Affiliate News 15 University & Alumni News 30 Preceptors Corner 16 The Drug Supply Chain Security Act 19 COVER STORY Continuous Glucose Monitor (CGM) Billing and Supply Allowance 36
Editor KellyFine Co-Editor
CreativeCoordinator
(480)838-3385|admin@azpharmacy.org

April 20, 2024 | Virtual

June 6, 2024 | Phoenix, AZ

June 6, 2024 | Phoenix, AZ

April 20, 2024 | Glendale

June 6, 2024 | Phoenix, AZ

June 6-9, 2024 | Phoenix, AZ
U P C O M I N G E V E N T S
3

CAPTKimberly “Kim” Langley, PharmD, MBA, BCPS, FAzPA
CAPTKimberly Langley is the Chief Financial Officer for the DoD Federal Electronic Health Record Modernization office responsible for implementing a single, common federal electronic health record system for Department of Defense, Department of VeteranAffairs, Department of Homeland Security, and the National Oceanic and AtmosphericAdministration. CAPT Langley is a pharmacist in the U.S. Public Health Service (USPHS) Commissioned Corps and has been on continuous active duty since 2009. She has completed previous USPHS assignments at multiple duty stations across New Mexico and Arizona with Indian Health Service. During this time, CAPTLangley served in several diverse leadership positions including Pharmacy Manager, PGY-1 Residency Program Director, Cardiovascular Clinic Director, and IHSAgencyRepresentative to the Million Hearts Initiative. In 2015, CAPTLangley served as the Chief Pharmacy Officer for her team in Liberia as a part of the Ebola crisis response in WestAfrica.
CAPTLangley holds a Bachelor of Science in MedicalTechnology from Georgia Southern University; a Doctor of Pharmacy degree from Medical University of South Carolina; a Master of BusinessAdministration fromThe Citadel; and completed a Certificate in Business Process Management from Villanova University. She is also board-certified in Pharmacotherapy and served on multiple technical expert panels for Pharmacy QualityAlliance for the development of cardiovascular and diabetes quality measures. CAPTLangley is actively engaged inAzPA activities including serving on the Board of Directors as President, Director-at-Large,APhA House of Delegates Representative, member on several committees, faculty for 2 certificate programs, abstract peer reviewer, and conference speaker. In 2023, she was recognized as a fellow of theArizona PharmacyAssociation. Her leadership experience extends to various roles on multiple USPHS committees, workgroups, and mentoring programs.
DearAzPAMembers,
Spring is here and I find myself reflecting on the recent encounters, challenges, and triumphs within the pharmacy profession. It is a time of both excitement and concern, as we navigate the ever-evolving landscape of healthcare and pharmacy practice.
One of the highlights of this season was undoubtedly theAzPASpring Clinical Conference. Pharmacy professionals from acrossArizona as well as out of state converged to share knowledge, expertise, and insights at this yearly conference. This gathering of dedicated and passionate pharmacy professionals filled me with pride and optimism for the future of our profession as I witnessed interprofessional collaboration and a sense of community among our healthcare colleagues from other professional organizations. This conference served as a reminder of the crucial role pharmacists play in team-based healthcare delivery that improves patient outcomes.
However, amidst the camaraderie and exchange of ideas, recent statements from the American MedicalAssociation (AMA) regarding pharmacist training have left many of us disheartened.The assertion that pharmacists lack the necessary training to provide certain healthcare services undermines the years of education, training, and experience that pharmacists bring to their practice. It is imperative that we stand united in advocating for the value of pharmacist-led care and dispel misconceptions about our capabilities. Education and collaboration remain key in fostering mutual respect and understanding among healthcare professionals.
Advancements in healthcare technology provides significant opportunities for improved patient care and efficiency. However, it also exposes us to new vulnerabilities, with sensitive patient data at risk of exploitation by malicious actors. The recent cyberattack on Change Healthcare serves as a wakeup call for investing in robust cybersecurity measures and staying abreast of emerging threats to minimize risk and ensure the trust of our patients.As guardians of patient health information, pharmacy professionals have a role in safeguarding our patient’s data and privacy
Despite these challenges for the profession, I am filled with excitement and anticipation for the upcomingAzPAAnnual Convention.This event promises to be a culmination of learning, networking, and celebration of our profession's achievements. I look forward to seeing you in Phoenix!.
4
CAPTKimberly “Kim” Langley, PharmD, MBA, BCPS, FAzPA 2023-2024AzPAPresident
EDITORIAL PRESIDENT'S MESSAGE
WELCOME NEW MEMBERS!
2nd Year Practitioner
Alexis O’Neill
Jessica Severin
Pharmacist
Roseanna Borst
Monique DallAgnol
Michonne Dietrich
David Gurule
Elizabeth Holmgren
Yvonne Huckleberry
Tincy Maroor
Bradley Nash
Yolimar Perez
John Robinson
Kerry Rodriguez
Edward Spark
Resident
Tara Bunni
Adrian Chavez Serrato
Michael Constant
Jules Fuentebella
Brian Gange
J'kiya Jackson
Allyson Prichard
Josalynn Rightnour
Florencio Robles
Retired
Shelly Swalley
Student Pharmacist
JesseAbah
HaneenAlkaylanie
CheleenAn
Mona Bayat
Lorin Deese
Keval Desai
Ejemen Eichie
Jaime Elliott-Shields
Maha Elrakaiby
Halie Erwin
EricaTherese Esteban
Juliette Feigal
Candice Flinner
Spencer Hemmingsen
Thomasine James
Rxyl Jade Jinon
Ramandeep Kaur
Heleen Khan
Amit Lakha
Jenny Le
Paola Lugo
Rania Mamo
Andrew Marcelo
Ali Mohsin
Masha Mosavi
Michelle Nguyen
Minh Nguyen
Kyra OkinAyushi Patel
Uprami Patel-Dimitrijevic
Megan Paul
Stormi Rich
Keri Scheidt
AyaTayeb
LoretoTena Gutierrez
TaylorTokach
AlinaTorres
Vanessa Virgen
Noelle Walton
Mona Zegar
Jenna Dalina
Jeremy Gwizdalski
Derek Jordan
Maxwell Kukowski
Benjamin Romero
Harriett Samaroo
Cassie Watson Technician
Associate
Marc Crouch
Davin Deb
Erin Fry
NEWS
AZPA
5
EDITORIAL
ANNUAL CONVENTION
Thursday, June 6
8:00AM - 5:00PM | SOUTHWESTERN STATES RESIDENCY CONFERENCE
(Separateregistrationandfee)
8:00AM - 5:00PM | AzPAANTICOAGULATION CERTIFICATE TRAINING PROGRAM
(Separateregistrationandfee)
ElizabethPogge,PharmD,MPH,BCPS,FASCP,FAzPA; LindsayDavis,PharmD,BCPS,BCCP,ASH-CHC,TTS,FAzPA, FCCP;KellyErdos,PharmD,BCACP,CACP;ElisabethPalmer, PharmD

8:00AM - 9:00AM | BREAKOUT SESSION 2: LIFESTYLE MEDICINE (LM) IN PHARMACYPRACTICE: WHATIS LMALLABOUTAND HOW CAN PHARMACISTS BE PREPAREDTO ENGAGE INA RAPIDLYGROWINGAND EFFECTIVE DISCIPLINE
EdwardStein,PharmD,MPH,DipACLM;Jane E. Stein, PharmD
Learning Objectives for Pharmacists and Technicians:

8:00AM - 12:00PM |APhA’sTHE PHARMACIST & PATIENTCENTERED DIABETES CARE CERTIFICATETRAINING PROGRAM
(Separateregistrationandfee)
CAPTKimberlyLangley,PharmD,MBA,BCPS;AnthonyAlbert, PharmD,BC-ADM,BCACP
12:00PM - 1:00PM | NON-ACCREDITED SYMPOSIUM
5:30PM - 7:00PM |AZPABOARD OF DIRECTORS MEETING
Opentoallattendees
7:30PM - 8:30PM | WELCOME RECEPTION
OpentoAllAttendees
Friday, June 7
6:00AM - 5:00PM | REGISTRATION
7:00AM - 7:45AM | COFFEE & NETWORKING ROUNDTABLES: Topics to be determined.
7:45AM - 8:00AM | WELCOME, OVERVIEW OF CONVENTION,AND CE CLAIM PROCESS
KellyFine,RPh,FAzPA&DawnGerber,PharmD,BCGP,FASCP , FAzPA
8:00AM - 9:00AM |BREAKOUT SESSION 1:ANTIOBESITY MEDICINES (AOMS): PATIENT-CENTRIC BEST PRACTICES
KelseyLeite,PharmD
Learning Objectives for Pharmacists:
1. Identify patient populations for whomAOMs may be suitable.
2. Develop strategies to mitigate potential risks associated withAOMs.
3. Describe potential barriers to adherence and compliance with AOMs.
1. Describe Lifestyle Medicine (LM) and the Six Pillars for lifestyle.
2. Discuss differences between Lifestyle Medicine and Integrative and Functional Medicine and appropriate applications as a foundation for conventional health care practice.
3. Identify three major challenges or barriers to integrating Lifestyle Medicine interventions in an interprofessional practice setting.
4. Identify areas within the continuum of pharmacy education curricula (from undergraduate, to pharmacy school, to postgraduate residency training and beyond) in which to integrate Lifestyle Medicine learning to prepare pharmacists to engage in these evidence-proven health interventions.
9:10AM - 10:10AM | BREAKOUT SESSION 1: INK & INSIGHTS: MEDICALWRITING FOR PHARMACY PROFESSIONALS
KathleenFairman,PhD,MA;ElizabethPogge,PharmD,MPH, BCGP,BCPS,FASCP,FAzPA
Learning Objectives for Pharmacists and Technicians:
1. Identify topics that may be appropriate for publication.
2. Describe how to implement clinical research into the pharmacy professional's practice.
3. Summarize existing research information efficiently to maximize the value of the pharmacy professional's work.
4. Work effectively with peer reviewer comments to produce a publishable article.
6
9:10AM - 10:10AM | BREAKOUT SESSION 2: HIVANDAGING:
FOCUS ON CARDIOVASCULAR RISK REDUCTION
CassandraAnderson,PharmD,MS,BCGP,CSP ,AAHIVP,CAPM
Learning Objectives for Pharmacists:
1. Identify current numbers and trends for HIV infection in the geriatric population in the United States.
2. Describe how HIV contributes to cardiovascular disease risk.
3. Discuss current treatment regimens for CVD risk reduction and their safety and effectiveness profiles in people living with HIV
4. List population-specific factors to target for CVD risk reduction in people living with HIV
10:20AM - 11:20AM | BREAKOUT SESSION 1: HEPATITIS C-YA LATER- GUIDANCE ON SCREENINGAND CURATIVETREATMENT OPTIONS FOR HEPATITIS C
JosalynnRightnour,PharmD;JacobNorthrup,PharmD,BC-ADM, CDCES
Learning Objectives for Pharmacists:
1. Identify eligible patients for HCV Screening.
2. List appropriate lab work for initial HCV screening.
3. Summarize pangenotypic treatment options’counseling points.
10:20AM - 11:20AM | BREAKOUT SESSION 2:THE MONOCLONAL MOSAIC: NAVIGATINGALZHEIMER'S TREATMENTFRONTIERS
AndreaCallas,PharmD;DawnGerber,PharmD,BCGP,FASCP , FAzPA
Learning Objectives for Pharmacists:
1. Discuss anti-amyloid-β monoclonal antibodies' FDAapproval process history
2. Compare anti-amyloid-β monoclonal antibodies' and cholinesterase inhibitors' mechanism of actions.
3. Contrast anti-amyloid-β monoclonal antibodies' and cholinesterase inhibitors' adverse effect profiles.



10:20AM - 11:20AM | BREAKOUT SESSION 3:AHISTORICAL EXPLORATION OF VETERINARYCOMPOUNDING PHARMACY
REGULATION INTHE UNITED STATES
MichaelBlaire,RPh
Learning Objectives for Pharmacists and Technicians:
1. List key legislation for veterinary compounding pharmacy in the United States.
2. Identify key legislation's implications for veterinary compounding pharmacy in the United States.
3. Describe the evolution of compounding standards and guidelines specific to veterinary pharmacy practice, recognizing their impact on ensuring the safety and efficacy of compounded medications.
4. Describe case studies illustrating notable events that have influenced regulatory decisions and shaped the landscape of veterinary compounding pharmacy

Accreditation Information
The Arizona Pharmacy Association is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education (CPE)
Continuing Education
Sessions approved for CE credits are indicated by the CE icon, an ACPE number, and the number of CEUs in the session listing
Learning Level: Varies
Activity Type: Knowledge and Application-based
Target Audience: Pharmacists and Technicians
To obtain CPE credit for any of the LIVE sessions, learners must attend the session live, enter the session code, and complete the assessment quiz and evaluation for each session attended. The session code and further instructions will be provided at the end of each LIVE session. Disclosures Disclosures will be announced at the beginning of each session and can be found in the Event Program under each speaker(s)
interest have been resolved through content review by the AzPA CE Committee Department and Committee.
EDITORIAL
ANNUAL CONVENTION
declare
Disclosures Con�icts of
biography AzPA CE sta�
no con�icts of interest or �nancial interests in any product or service mentioned in this activity View
Register Today! 7

11:30AM - 12:30PM | NON-ACCREDITED SYMPOSIUM
12:40PM - 1:40PM | BREAKOUT SESSION 1: HOW DID MY TUESDAYCHANGE?THE CRUCIALROLE OFACTIVE POLITICAL INVOLVEMENTIN SAFEGUARDING YOUR PHARMACY PROFESSION
MarkBoesen,PharmD,JD,FAzPA
Learning Objectives for Pharmacists & Technicians:
1. Develop effective strategies for engaging in political advocacy
2. Define the key principles and concepts of political advocacy
3. Apply ethical considerations in political advocacy efforts within the pharmacy profession.
12:40PM - 1:40PM | BREAKOUT SESSION 2: HEY SUGAR SUGAR
SamanthaZimmerman,PharmD,MLS,BS;JacobNorthrup,PharmD, BC-ADM,CDCES,LeahRios,PharmDCandidate
Learning Objectives for Pharmacists:
1. Identify updates to the 2024American DiabetesAssociation (ADA) Standards of Care in Diabetes guidelines.
2. Choose an appropriate Continuous Glucose Monitoring (CGM) device based on patient preferences.
3. Compare CGM device characteristics for various patient populations.
Learning Objectives forTechnicians:
1. Summarize Continuous Glucose Monitoring (CGM) device and insulin pump options for patients with diabetes.
2. Identify necessary components of Continuous Glucose Monitoring (CGM) device and insulin pump dispensing.
12:40PM - 1:40PM | BREAKOUT SESSION 3: TRANSITION CONSIDERATIONS FOR PROVIDERS, PAYORS,AND PATIENTS IN THE EMERGENCE OF BIOSIMILARS
AlexisSmith,PharmD;HannahHenderson,PharmD;LillianneDo, PharmD,BS;PilarGaggin,PharmD,CSP ,AAHIVE
Learning Objectives for Pharmacists:
1. Define biologics and biosimilars.
2. Identify interchangeable adalimumab.
3. Discuss the differences between each adalimumab biosimilar including indications/dosing, side effects, efficacy, and cost.
4. Describe the advantages of using an adalimumab biosimilar over the reference product.
1:50PM - 2:50PM | BREAKOUT SESSION 1: THREATSAND OPPORTUNITIES:ACOMPOUNDING PUBLIC POLICY UPDATE
TenilleDavis,PharmD,RPh,BCSCP,FACP Learning Objectives for Pharmacists and Technicians:
1. Evaluate how proposed regulation that affects the practice of compounding should be assessed.
2. Describe Pharmacy Compounding's Regulatory Framework.
3. Identify some regulatory challenges that compounding pharmacists face from the state and federal level.
ThissessionisunderwrittenbyagrantfromthePharmacy CompoundingFoundation.
1:50PM - 2:50PM | BREAKOUT SESSION 2: DIABETES REGIMEN
PERSONALIZATIONAND INTRA-CLASS MEDICATION SELECTION
AnthonyAlbert,PharmD,BCACP,BC-ADM
Learning Objectives for Pharmacists:
1. Apply pharmacotherapy guidelines for diabetes (ADA,AACE) to a patient case.
2. Contrast agents within the same class with respect to safety, effectiveness, pharmacokinetics, and pharmacodynamics.
3. Design a diabetes medication regimen with respect to patient characteristics.
1:50PM - 2:50PM | BREAKOUT SESSION 3: EMBRACING RESILIENCE:APERSONALJOURNEYTHROUGHADDICTION AND LIFELONG SOBRIETY INAPROFESSIONALSETTING
RyanGries,PharmD,BCPS,BC-ADM
Learning Objectives for Pharmacists & Technicians:
1. Develop a deeper understanding of the challenges faced by individuals with addiction by actively listening to the personal story of addiction and recovery shared by the pharmacy professional.
2. Identify key factors and triggers that contribute to addiction.
3. Recognize signs of potential addiction in patients in a pharmacy setting.
4. Explore strategies for providing empathetic and non-judgmental support to individuals on their journey to recovery
8
EDITORIAL ANNUAL CONVENTION

3:00PM - 4:00PM | GENERALSESSION: PURDUE PHARMA
SUPREME COURTDECISION: UNRAVELING PAST, PRESENT,AND FUTURE IMPLICATIONS IN HEALTHCAREAND JUSTICE
MarkBoesen,PharmD,JD,FAzPA
Learning Objectives for Pharmacists and Technicians:
1. Describe the December 2023 Purdue Pharma Supreme Court decision.
2. Describe the historical timeline leading to the December 2023 Purdue Pharma Supreme Court decision.
3. Examine the implications of the December 2023 Purdue Pharma Supreme Court decision.
4:10PM - 5:10PM | BREAKOUT SESSION 1: PRECEPTOR
WORKSHOP: CHOOSE YOUR OWNADVENTURE
JanetCooley,PharmD,BCACP;SuzanneLarson,PharmD
Learning Objectives for Pharmacists:
1. Compare and contract different approaches to precepting situations (e.g. student orientation, feedback, wellness).
2. Modify approach to precepting and student concerns based on evidence regarding precep.
3. Recommend a plan to incorporate precepting practices back into their practice sites.
3:10PM - 4:10PM | BREAKOUT SESSION 2: HELLO INSULIN, MY OLD FRIEND,CATCHING UPON NEW FORMULATIONS WITH REAL-WORDAPPLICATION
LisaBeckett,PharmD,BCACP,CDCES,AAHIVP;SaraShahdoost Moghadam,PharmD,BCACP,CDCES,AAHIVP
Learning Objectives for Pharmacists:
1. Review the implications of new insulin products on patient care.
2. Review legal landscape around biosimilar and interchangeable biosimilars with specific applications to insulin products.
3. Describe when a new insulin product may be preferred.
4. Describe how to perform insulin dose conversions.
5. Summarize anticipated next developments in the insulin therapy landscape with a focus on once weekly insulin(s).
Learning Objectives forTechnicians:
1. Identify brand and generic names of new insulin products.
2. ExplainArizona state rules for substitution with interchangeable biosimilar insulin(s).
3. Discuss upcoming advances in insulin therapy such as once weekly insulin.
ANNUAL CONVENTION
3:10PM - 4:10PM | BREAKOUT SESSION 3: THE NEW PRESCRIPTION DRUG LAW; INFLATION REDUCTIONACT
EmanKirolos,PharmD,MS,DASPL
Learning Objectives for Pharmacists and Technicians:
1. Identify the major milestones of the Inflation ReductionAct implementation.
2. Differentiate Part-B and Part-D insulin and vaccine coverage.
3. Describe how the new prescription drug law helps millions of Medicare beneficiaries.
4. List valuable resources to be shared with Medicare beneficiaries.
5:15PM - 7:00PM | EXHIBIT HALL: WELCOME RECEPTION
Support our Convention Sponsors and Exhibitors while you mingle and enjoy appetizers!
7:00PM - 8:00PM | FRIENDS &ALUMNI MIDWESTERN UNIVERSITYRECEPTION
InvitationOnly
8:00PM - 10:00PM | ENTERTAINMENTNIGHT: NAMETHATTUNE
Saturday, June 8
6:00AM - 5:00PM | REGISTRATION
7:00AM - 7:45AM | NON-ACCREDITED SYMPOSIUM
7:00AM - 5:00PM | STUDENT LEADERSHIP& LEGACYTRACK*
7:00AM - 5:00PM | TECHNICIANTRACK*
7:45AM - 8:00AM | WELCOME, OVERVIEW OF CONVENTION, AND CE CLAIM PROCESS
KellyFine,RPh,FAzPA&DawnGerber,PharmD,BCGP,FASCP , FAzPA
EDITORIAL
9

8:00AM - 9:00AM | GENERALSESSION: PHARMACY BOWL
Moderator:JacobSchwarz,PharmD,MBA,BCIDP,BCCCP,BCPS, FAzPA
Learning Objectives for Pharmacists & Technicians:
1. Recall the brand or generic name of medications.
2. Perform pharmacy calculations needed in different practice settings.
9:15AM - 10:15AM | BREAKOUTSESSION 1: UNMASKING IMPOSTER SYNDROME: HOW TO CONVINCEYOUR INNER FRAUDYOU'RETHE REALDEAL!
BiancaGlab,PharmD,MC,RPh;JacobNorthrup,PharmD,BC-ADM, CDCES
Learning objectives for Pharmacists & Technicians:
1. Define imposter syndrome (IS).
2. List the subtypes of imposters.
3. Discuss effective strategies to recognize imposter syndrome.
4. Discuss effective strategies to manage imposter syndrome.
9:15AM - 10:15AM | BREAKOUT SESSION 2: GOLDEN INSIGHTS: NAVIGATING CHRONIC OBSTRUCTIVE PULMONARYDISEASE GUIDELINES
CourtneyCoombe,PharmD,CDCES;AdrienneWaibel,PharmD, CDCES
Learning Objectives for Pharmacists:
1. List the most up to date recommendations for management of COPD per GOLD 2024 Guidelines.
2. List the most up to date recommendations for management of COPD exacerbations per GOLD 2024 Guidelines.
3. Describe the recommended vaccine schedule in COPD patients.
9:15AM - 10:15AM | BREAKOUT SESSION 3:ANTIMICROBIAL STEWARDSHIPUPDATESACROSSTHEARIZONAHEALTHCARE SPECTRUM
ThoPham,PharmD,BCIDP;VanthidaHuang,BSPHM,PharmD
Learning Objectives for Pharmacists:
1. Discuss important updates on the CDC Priority Core Elements of HospitalAntibiotic Stewardship Programs.
2. List stewardship updates from theArizona Department of Health Services.
3. Review the CDC Core Elements ofAntibiotic Stewardship for Nursing Homes.
4. Review stewardship updates fromArizona Department of Health Services.
5. Describe CDC Core Elements of OutpatientAntibiotic Stewardship, including outpatient and dental healthcare settings.
10:30AM - 12:00PM | BREAKOUT SESSION 1: BIOSTATS 101: BASIC PRINCIPLESAND EVALUATION OF STUDIES
JacobSchwarz,PharmD,MBA,BCIDP,BCCCP,BCPS,FAzPA
Learning Objectives for Pharmacists:
1. Differentiate between types of data (nominal, ordinal, interval/ratio), measures of central tendency, and appropriate statistical tests.
2. Interpret hypothesis testing, p values, confidence intervals, odds and risk ratios, and both statistical and clinical significance.
3. Define the absolute risk, relative risk, number needed to treat given data from a study
4. Calculate the absolute risk, relative risk, number needed to treat given data from a study
5. Review the hierarchy of evidence and discuss both the advantages and disadvantages of various study designs.
10:30AM - 12:00PM | BREAKOUT SESSION 2: TRENDS IN HORMONALCONTRACEPTION
ErinRaney,PharmD,BCPS,BC-ADM,FCCP;ShareenEl-Ibiary, PharmD,BCPS,FCCP,FAzPA
Learning Objectives for Pharmacists:
1. Compare and contrast recently approved hormonal contraceptives.
2. Choose individualized hormonal contraceptive regimens based upon a statewide standing order for pharmacists.
3. Summarize the recommendations for the new nonprescription progestin-only contraceptive.
10:30AM - 11:30AM | BREAKOUT SESSION 3: CANDIDAAURIS: THE PATHWAYOFYEASTRESISTANCE
ScottVolker,PharmD;VanthidaHuang,BSPHM,PharmD
Learning Objectives:
1. Discuss the key principles in the management of C. auris infection.
2. Identify infection prevention and control measures for C. auris transmission and outbreaks.
3. Describe the epidemiology and transmission of C.auris as an emerging pathogen.
4. Describe the transmission of C.auris as an emerging pathogen.
12:15PM - 2:00PM | EXHIBITHALL| RECEPTION
Support our Convention Sponsors and Exhibitors while you mingle and enjoy lunch.
EDITORIAL ANNUAL
10
CONVENTION

2:15PM - 3:15PM | KEYNOTE GENERALSESSION: CONNECTING TOYOUR PASSION FOR PHARMACYAFTER BURNOUT
JerricaDodd,PharmD
3:30PM - 5:00PM | BREAKOUT SESSION 1: IMMUNIZATION UPDATE
CAPTHollyVanLew,PharmD,BCPS,AAHIVP;SophiaGalloway, PharmD,BCACP,FAzPA
Learning Objectives for Pharmacists:
1. Discuss recent updated indications and expanded age ranges for routine vaccinations, including pneumococcal, RSV, and meningococcal.
2. Apply the current recommendations for routine vaccinations.
3. Assess current immunization practices for their practice site.
4. Determine implementation strategies for expanding vaccination efforts.
5. Discuss vaccine administration best practices, including injection technique and updated injection routes for certain live vaccines.
Learning Objectives forTechnicians:
1. Describe tools and resources available for immunization schedules and recommendations.
2. Utilize the 2024Adult & Pediatric Immunization Schedules to select the correct dose and route of administration.
3. Determine ways to support increased vaccine administration.
4. Describe ways to improve workflow support vaccinations.
5. Discuss vaccine administration best practices, including injection technique and updated injection routes for certain live vaccines.
3:30PM - 4:30PM | BREAKOUT SESSION 2: SPECIALOLYMPICS
ARIZONAINCLUSIVE HEALTH PROGRAMS
AshkanRastegar,PharmDCandidate2024,ErinRaney,PharmD, BCPS,BC-ADM,FCCP
Learning Objectives for Pharmacists & Technicians:
1. Describe the structure of the SOAZ, including its mission, goals, and key functions.
2. Identify opportunities for pharmacy professionals within the SOAZ.
3. Discuss the practical applications of pharmacy professionals' experiences and perspectives within the context of the SOAZ.
3:30PM - 4:30PM | BREAKOUTSESSION 3: TO EXTEND OR NOT TO EXTEND: PROLONGED INFUSION BETA-LACTAMS
KayleeWhitenack,PharmD;JacobSchwarz,PharmD,MBA,BCIDP , BCCCP,BCPS,FAzPA
Learning Objectives for Pharmacists:
1. Summarize the current state of practice regarding extendedinfusion beta.
2. Evaluate the evidence supporting the use of extended-infusion beta.
3. Identify the appropriate clinical scenarios to employ an extended infusion.
5:00PM - 5:45PM | PASTPRESIDENT'S RECEPTION
-InvitationOnly-
7:00PM - 9:00PM | 2024ANNUALAWARDS PRESENTATION
Join us for a celebration ofAzPA’s prestigious 2024 awardees.This evening is a not-to-be-missed event!
Sunday, June 9
6:00AM - 1:00PM | REGISTRATION
7:00AM - 8:00AM | NON-ACCREDITED SYMPOSIUMBREAKFAST
7:00AM - 7:50AM | HEADING TOWARDS OUR FUTURE:AZPA TOWN HALL
JacobSchwarz,PharmD,MBA,BCIDP,BCCCP,BCPS,FAzPA; CAPTKimberlyLangley,PharmD,MBA,BCPS;DawnGerber, PharmD,BCGP,FASCP,FAzPA;KellyFine,RPh,FAzPA
Learning Objectives for Pharmacists & Technicians:
1. Describe the key components of the new strategic plan forAzPA.
2. Discuss the implications of the new strategic plan forAzPAand its stakeholders.
3. Discuss the practical implementation of the new strategic plan.
EDITORIAL
CONVENTION
ANNUAL
11

8:00AM - 9:00AM | BREAKOUTSESSION 1: THE PHARMACIST'S ROLE IN MANAGINGANXIETY, INSOMNIA,AND DEPRESSION IN OLDERADULTS
MarthaFankhauser,BS(Pharmacy),MS(Pharmacy),BCPP,FASHP , FAzPA
Learning Objectives for Pharmacists:
1. Identify risk factors for mental health conditions in later life.
2. Describe treatment options for anxiety, insomnia, and depression in older adults.
3. List prevention strategies for older adults that focus on healthy aging.
4. Recommend how clinicians can recognize, assess, treat, and monitor mental health disorders in older adults.
8:00AM - 9:00AM | BREAKOUT SESSION 2: NAVIGATING GENERATIVEAI: RISKS, BENEFITS,AND PRACTICAL APPLICATIONS
RyanGries,PharmD,BCPS,BC-ADM;DawnGerber,PharmD,BCGP , FASCP,FAzPA
Learning Objectives for Pharmacists & Technicians:
1. List the types of generative artificial intelligence.
2. Articulate the potential uses of generative artificial intelligence.
3. Discuss the limitations of generative artificial intelligence.
4. Describe strategies for optimizing the use of generative.
8:00AM - 9:00AM | BREAKOUT SESSION 3: VALUE BASED CARE: WHATPROVIDERS WISH PHARMACISTS KNEW
CassandraRichardson,PharmD,BCACP ,TTS;AdamS.Chesler, PharmD,MBA
Learning Objectives for Pharmacists and Technicians:
1. Describe a brief history of Medicare and value-based care (VBC).
2. Define of VBC terminology
3. List real-life examples of VBC.
4. Explain the measures and metrics used to evaluate physicians/advanced practice practitioners (APPs).
5. Explain the areas of overlap between pharmacists and physicians/Advanced Practice Providers (APPs) concerning Healthcare Effectiveness Data and Information Set (HEDIS), Star Ratings (STAR), ConsumerAssessment of Healthcare Providers and Systems (CAHPS), and various other Value-Based Care (VBC) measurement metrics.
9:10AM - 10:10AM | BREAKOUTSESSION 1: DIVERSION WITHIN THE PHARMACY; WHATCAN I DO?
KristenSnair,CPhT,MSJ;SabrinaHernandez,CPhT
Learning Objectives for Pharmacists and Technicians:
1. Identify red flags that are cause for a level of awareness for potential diversion.
2. Discuss strategies that can be deployed to mitigate diversion from pharmacy staff.
3. List and create an awareness of what can and should be done if pharmacy staff diversion is suspected along with potential consequences of non-compliance.
9:10AM - 10:10AM | BREAKOUT SESSION 2: MENTORSHIPAND SPONSORSHIP-APANELPERSPECTIVE
BroughttoyoubytheAzPAMCPCommittee
CaseyOrton,PharmD,MBA
Learning Objectives for Pharmacists & Technicians:
1. Describe the difference between mentorship and sponsorship.
2. List the benefits of mentorship.
3. Summarize the outcomes of sponsorship.
4. Discuss methods to sponsor talent in workplace and professional network settings.
10:20AM - 11:20AM | BREAKOUT SESSION 1: CARING BEYOND THE COUNTER: EMOTIONALCHALLENGES IN THE OPIOID CRISIS
JimmyStevens,PharmD
Learning Objectives for Pharmacists & Technicians:
1. Analyze the ethical dilemmas faced by the protagonist in "The Pharmacist."
2. Explore the impact of opioid crisis portrayed in "The Pharmacist" on communities and healthcare systems.
3. Discuss the emotional toll on healthcare providers dealing with societal challenges.
10:20AM - 11:20AM | BREAKOUT SESSION 2: TAKINGTHE LEAP: NAVIGATING CAREER TRANSITIONS IN PHARMACY
BroughttoyoubytheAzPAEducationCommittee
ArianeGuthrie,PharmD,BCGP,BCPS
Learning Objectives for Pharmacists & Technicians:
1. Discuss the landscape of pharmacy and workforce projections.
2. Identify sources of job dissatisfaction and burnout among pharmacy professionals.
3. Identify resources to assist with career transitions within pharmacy
12
EDITORIAL ANNUAL CONVENTION
ANNUAL CONVENTION

10:20AM - 11:20AM | BREAKOUT SESSION 3: BREAKING BARRIERS: ENHANCING PREPACCESSANDADHERENCE IN UNDERSERVED POPULATIONS
YousefToma,PharmD,BCPS,CSP ,AAHIVP
Learning Objectives for Pharmacists:
1. Identify communities at increased risk of HIV transmission who also experience disparities in accessing PrEP
2. Describe the influence of social determinants of health and healthcare barriers on the uptake of PrEP
3. Identify trends in PrEP use and persistence among different populations.
4. Identify effective strategies for healthcare professionals to enhance PrEPwithin vulnerable populations.
11:30AM - 12:30PM | GENERALSESSION: 2024 PHARMACY LAW UPDATE
KaitlynFydenkevez,JD;AlexSnyder,JD
Learning Objectives for Pharmacists & Technicians:
1. Describe ramifications of recent pharmacy-related court cases.
2. List recent changes in Federal Pharmacy Law.
3. List recent changes in State Pharmacy Law.
Technician Track - Saturday
Note:Technicians have an opportunity to attend any other CE session accredited for technicians (UAN ends in T)
7:00AM - 7:50AM | COFFEE & NETWORKING ROUNDTABLE
MelindaBrowning,MSc,CPhT;KristenSnair,CPhT,MSJ
8:00AM - 9:00AM | PHARMACY BOWL
Moderator:JacobSchwarz,PharmD,MBA,BCIDP,BCCCP,BCPS, FAzPA
Learning Objectives for Pharmacists and Technicians:
1 Recall the brand or generic name of medications.
2 Perform pharmacy calculations needed in different practice settings.
9:15AM - 10:15AM | BREAKOUT SESSION: UNMASKING IMPOSTER SYNDROME: HOW TO CONVINCEYOUR INNER
FRAUDYOU'RETHE REALDEAL!
BiancaGlab,PharmD,MC;JacobNorthrup,PharmD,BC-ADM, CDCES
Learning Objectives for Pharmacists & Technicians:
1. Define imposter syndrome (IS).
2. List the subtypes of imposters.
3. Discuss effective strategies to recognize imposter syndrome.
4. Discuss effective strategies to manage imposter syndrome.
10:30AM - 11:30AM |ARTICULATING LEADERSHIP PROFICIENCY INTHE 'TELLMEABOUTYOURSELF' MOMENT
JeremyGerber,PharmD,MBA,BCPS,BCOP,CNSC
Learning Objectives for Pharmacists & Technicians:
1. List common pitfalls and challenges related to incorporating leadership skills into introductory responses.
2. Describe strategies for overcoming common pitfalls and challenges related to incorporating leadership skills into introductory responses.
3. Apply strategies for overcoming common pitfalls and challenges related to incorporating leadership skills into introductory responses.
12:15PM - 2:00PM | EXHIBIT HALL| LUNCH
Support our Convention Sponsors and Exhibitors while you mingle and enjoy lunch.
2:15PM - 3:15PM | KEYNOTE GENERALSESSION: CONNECTING TOYOUR PASSION FOR PHARMACYAFTER BURNOUT
JerricaDodd,PharmD
3:30PM - 4:30PM | CREATINGACULTURE OF POSITIVE FEEDBACKAND RECOGNITION FOR PHARMACYTECHNICIANS
KristineSmith,B.S.,CPhT-Adv,CSPT;NickRuiz,CPhT-Adv,CSPT , FAPC;MatthewAlvarado,CPhT,CSPT
Learning Objectives for Pharmacists & Technicians:
1. List various feedback models.
2. Describe how to effectively give feedback.
3. Explain how feedback directly impacts engagement.
EDITORIAL
13
EDITORIAL
ANNUAL CONVENTION
Student Track - Saturday
8:00AM - 9:00AM | ELEVATEYOUR RESIDENCY CANDIDACY: STRATEGIESTO SHINE INTHE PHARMACYRESIDENCY SELECTION
YousefToma,PharmD,BCPS,CSP ,AAHIVP
9:15AM - 10:15AM | UNMASKING IMPOSTER SYNDROME: HOW TO CONVINCEYOUR INNER FRAUD YOU'RETHE REALDEAL!
BiancaGlab,PharmD,MC,RPh;JacobNorthrup,PharmD,BCADM,CDCES
Learning Objectives for Pharmacists & Technicians:
1 Define imposter syndrome (IS).
2 List the subtypes of imposters.
3 Discuss effective strategies to recognize imposter syndrome.
4 Discuss effective strategies to manage imposter syndrome.
10:30AM - 11:15AM | INTERVIEWTIPSANDTRICKS FOR STUDENTS
DawnGerber,PharmD,BCGP,FASCP,FAzPA

11:20AM-12:20PM |TAKING CARE OF YOU: LIFEAFTER SCHOOL
LilianeMoforPharmD,MBA
Learning Objectives for Pharmacists & Technicians:
1 Define financial literacy
2. Identify challenges to providing care caused by financial stress.
3. List the components of a budget.
4. Assess your situation and be able to write 2-3 personal financial goals with action steps.
12:15PM - 2:00PM | EXHIBIT HALL| LUNCH
Support our Convention Sponsors and Exhibitors while you mingle and enjoy lunch.
2:15PM - 3:15PM | KEYNOTE GENERALSESSION: CONNECTING TOYOUR PASSION FOR PHARMACYAFTER BURNOUT
JerricaDodd,PharmD
3:30PM - 5:00PM | MOCK INTERVIEWS
ValerieRichards,PharmD

14
AzPA HEALTH SYSTEM SPECIAL INTEREST GROUP (AZ-ASHP)
Springisintheairandthisyearisofftoagreatstart!Timeseemstoflybyveryquicklyand 2030isjustaroundthecorner. WhydidIpicktheyear2030youask…?Well,theASHP PracticeAdvancementInitiative(PAI)2030hasbeenonmymind. Thisworkservesasan aspirationalroadmaptopharmacypracticeadvancementwithfuture-focusedconceptsthat lookbeyondtoday’sbarrierstochange. PAI2030has59recommendationsonproviding optimal,safe,andeffectivemedicationusethatfallintosixthemesforpracticechange.
• Integratepharmacyenterpriseforconvenientandcost-effectivecare.
Optimizecareviapharmacist-providedcomprehensivemedicationmanagement.
• Harnessdatatoimprovepatienthealth.
• Adoptpersonalized,targetedtherapies.
• Increasepublichealthopportunitiesinsocialdeterminants,chronicillness,andaddiction.
•
• Advancepharmacytechnicianroles.
ASHP’sPAI2030isaforward-thinkinginitiativethatenvisionsthefutureroleofpharmacistsin healthcare. Byembracinginnovation,prioritizingpatient-centeredcare,andinvestingin professionaldevelopment,ASHPaimstopositionpharmacistsasintegralmembersofthe healthcareteamintheyearstocome.IfyouwouldliketolearnmoreaboutPAI2030youcan findadditiondetailsatAboutPAI2030-ASHP
Intheveinofpracticeadvancement,wehadaverysuccessfulSpringClinicalConferencein February,andwearenowgearingupfortheAnnualConventionwhichwillbeheldatthe HiltonPhoenixResortatThePeakonJune6–9,2024. Thisisanothergreatopportunityfor gettingCEaswellasnetworkingwithcolleaguesandreunitingwithfriends.
Asareminder,theHealthSystemsPharmacySIGisactivelyrecruitingnewmembers. Ifyouareinterestedingettinginvolved,pleasesignupusingthelinkbelow
WemeetmonthlytodiscussissuesrelevanttohealthsystemspharmacistsinArizona.The moreperspectiveswehaveonthisgroup,themorewecanmakesureAzPAisservingthe needsofitshealthsystemsmembers.Hopetoseeyouonthe nextcall!
MBA,BCPS

 Mary Manning, PharmD, MBA, BCPS AzPABoardofDirectors-HealthSystem
Mary Manning, PharmD, MBA, BCPS AzPABoardofDirectors-HealthSystem
Learn More Here AZ-ASHP AFFILIATE NEWS
Mary Manning, PharmD, MBA, BCPS AzPABoardofDirectors-HealthSystem
15
Mary Manning, PharmD,

"Sharingourfailureswith
studentscannormalize thediscussionabout failureanddemonstrate theconceptthatfailureis oftentimesacatalystfor growthandthesparkthat canpropelustowards success."
AUTHORS/CONTRIBUTORS
MelindaJ.Burnworth,PharmD,FASHP,FAzPA,BCPS,ProfessorofPharmacyPractice,Midwestern UniversityCollegeofPharmacy
SuzanneLarson,PharmD,DirectorofExperientialEducation,Departmentof PharmacyPractice,MidwesternUniversityCollegeofPharmacy
JanetCooley,PharmD,BCACPDirectorofExperientialEducation,DepartmentofPharmacy PracticeandScience,R.KenCoitCollegeofPharmacy,UniversityofArizona
DISCLOSURE
Theauthor(s)declarenorealorpotentialconflictsorfinancialinterestinanyproductorservice mentionedinthemanuscript,includinggrants,equipment,medications,employment,gifts,and honorarium.
FUNDING
Nofundingwasprovided.
ACKNOWLEDGEMENT
Theauthorgratefullyacknowledgesallpharmacypracticepreceptorsdedicatedto providinghighqualitylearningexperiences.
No,
We Don’t Talk About Failure...
No, No
EDITORIAL PRECEPTOR CORNER
16
Introduction
The 2021 Disney musical “Encanto” features a catchy song about a misunderstood and rarely discussed uncle, Bruno. The chorus describing the family’s relationship to Bruno proclaims, “We don’t talk about Bruno, no, no, no.” The Madrigal family misunderstood Bruno’s gift of seeing the future, and instead thought Bruno’s visions were omens that caused uncomfortable or unfortunate events. This song highlights the human reflex of avoiding people, situations, and discussions that make us uncomfortable. In the end, the Madrigal family comes to a healthier and more accurate conclusion about Bruno, confronted their erroneous perceptions, and embraced this family member, leading to a happy ending for the entire family
The authors of this commentary have noticed a similar parallel to how educators and students can sometimes neglect discussing the concept of failure in academic settings. We don’t talk about failure, no, no, no. When educators speak to students about failure, these conversations often involve a box of tissues, intense emotions, and are hidden behind closed office doors.There may be an element of shame and embarrassment. Neither student nor educator typically enjoys discussions about failure, and this important topic can be left neglected.This commentary builds on previous discussions initiated by Brazeau and DiPiro to normalize and destigmatize failures in an academic setting. The purpose of this commentary is to continue dialog about how educators can promote healthy perspectives about this uncomfortable topic. In other words, we are going to talk about Bruno.
Discussion
Educatorandpreceptorperspectivesaboutfailure
Pharmacy preceptors fill many roles including student advisors, mentors, and coaches, and in these roles, should be equipped to handle discussions about student failure.Anatural starting place for pharmacy preceptors to prepare for these discussions can include some self-reflection.Are we comfortable with the topic of failure? How do we feel about our own failures?Are we comfortable sharing our own shortcomings with our supervisors and mentors?
As preceptors work through their own reflections on the topic of failure, it can be helpful to remember that failure is an evitable part of the human condition.As Brazeau noted, many pharmacy educators have listened to keynote speakers tell of their successes and often, their stories begin with challenges they had to overcome. Each one of us will experience failure in some capacity. Some failures that may feel familiar to pharmacy preceptors
include poor rotation evaluations, rejected manuscripts, denial of promotion or advancement, or not being selected for a job. Reflecting on our own failures can help us recognize that failure can be an important accelerator of the learning process and a step in developing resilience. Oftentimes, failure and success seem like opposite ends of the same continuum. However, a more realistic and accurate way to view failure is as a steppingstone on the road to success. In other words, we can remember that failures are an essential part of our own hero’s journey. Often, a failure can prompt us to make necessary adjustments that are required for subsequent successes.
LiteratureonEducationalFailures
Literature is emerging on failure in pharmacy education. Regarding professional development, Nohria and McBane thoughtfully address the need for educators to reflect on how their own failures can be seen as a step toward innovation with an appropriate mindset and support. Additionally, there are examples of educators intentionally building opportunities into pharmacy curricula to help equip students to discuss and address different types of failures. Ragucci and Poirier both describe interprofessional error disclosure educational programming. Frenzel describes pharmacy educational simulations in which students apply methods of root cause analysis to determine the cause of medication errors. Cain and Piascik describe the use of serious gaming in pharmacy education to promote opportunity for risk-taking and acceptable failure. Mattingly advocates for the inclusion of curricular opportunities for students to take responsible risks without the possibility of jeopardizing or delaying their career, such as an elective course in entrepreneurship. While some of these examples introduce students to concepts around medication errors and root cause analysis, others showcase that it is possible to provide students educational opportunities to take risks and talk about some of the potential areas for failures that they may encounter in their future careers while still in a safe learning environment.
Carr and colleagues directly interviewed medical students who had to repeat a unit of study to explore the student experience. Their research showed that students felt disappointed in themselves, shame, and sometimes guilt, feeling that they were letting people down. Other students reported relief as they recognized that they weren’t ready for the next stage in their learning or that they needed to make changes as they were overextended. Importantly, some students reported that they tried to keep the failure to themselves, making the students feel isolated. Students have much to tell 4 5,6
1 1 1 1 1 2,3 2
7 8
10 10 10 CONT.
CORNER 17
9
PRECEPTOR
PRECEPTOR CORNER
educators about how to help them through failure when they are provided a safe space to share. Apharmacy student recounted the story of how she encountered several challenges and failures while trying to complete a research project during pharmacy school. Her positivity offers insight both to struggling pharmacy students and preceptors hoping to coach students through a failure.
CalltoAction
The authors of this article propose a few suggestions to spark ideas and discussions about handling failures in a productive manner. Some of these ideas can be implemented immediately by individual educators; others may require thoughtful consideration and implementation at the college or university level.
In a one-on-one setting, individual preceptors can begin by discussing failures with students openly and without fear. We can model healthy attitudes about failure and speak positively about how our failures have helped us reach new levels of development. According to Brazeau, as we are all working to ensure our students meet key elements of accreditation, “we, collectively, as a group of educators, can provide student pharmacists with our vulnerable stories too.” Sharing our failures with students can normalize the discussion about failure and demonstrate the concept that failure is oftentimes a catalyst for growth and the spark that can propel us towards success. Finally, reinforce positive attitudes about failure in conversations with students on an individual level. We can reframe the discussion about an academic failure or remediation opportunities from “failed” to the more growth mindset-oriented term “not yet.” Individual conversations and mentoring provide an opportunity to reinforce that pharmacy education is a safe space to learn how to become a pharmacist.
In smaller groups of students, such as with student advising, mentoring, and precepting, these concepts can be reinforced and supplemented. Student advising can be a safe space to normalize discussions about a variety of setbacks, from not getting elected to a student leadership position to having a course failure. This might be a space where a small group would feel comfortable sharing their failures, such as a wall of failures or creating a group CV of failures. Students can learn together how to shape challenges and failures into life lessons. These lessons may even be helpful stories for future situational questions that could be encountered during job or Residency interviews. Reflecting on failures with a small group of students and a mentor can be a positive experience for all.
College, school, or institutional strategies to normalize productive discussions about failure may take more time, strategy, and organization to implement but have the potential for widespread benefit. It may be helpful to have discussions about failure with students during orientation or during the first months of pharmacy school.This information could also be covered annually during a town hall, lunch and learn, or retreat. Imagine the impact of teaching first year pharmacy students about the power of embracing a growth mindset, that their challenges can be embraced, and with persistence, they can grow and improve. Educators can create a culture where students understand that learning is messy, non-linear, and may take a few detours, bumps, and bruises to get to our destinations. By setting an expectation that educators understand the challenges of the professional training program, students may feel more empowered to come to educators before challenges become failures. Pharmacy school should be a place where learning occurs, where students are challenged and encounter setbacks that may ultimately allow them to grow into the professionals they aspire to be.Academic detours are part of the process.
12,15
Institutionally, we can strive to be proactive and recognize that academic setbacks can trigger students to feel shame and embarrassment. This sentiment is articulated by Elmore, “Each failure seemed a desecration of my work and each rejection a dismissal of my personal worth.” Imagine a culture of pharmacy education where students receive coaching that can help them work through these emotions. We can help students recognize how failures can propel them to a better place and may even be a “blessing in disguise.”
Conclusion
In this article, we talked about Bruno, er, failure. It wasn’t too awkward, was it?The more we can have healthy, productive dialog about this topic, the better equipped we will be as we navigate this uncomfortable yet inevitable element of learning. The authors have sought to continue the discussion started by Brazeau, DiPiro, and others by talking about failure. We hope to normalize discussions like this so we can continue the work of reframing how failure is viewed in pharmacy education. Let’s talk about failure.
10,15 16 17 1
2, 3
11 11 11 2 12 13,14 CONT.
continued on page 29 18

The Drug Supply Chain Security Act
Thisseries,PharmacyandtheLaw,ispresentedbyPharmacistsMutualInsuranceCompanyandtheArizonaPharmacy AssociationthroughPharmacyMarketingGroup,Inc.,acompanydedicatedtoprovidingqualityproductsandservicestothe pharmacycommunity.
There is a new regulatory date looming that is very important to pharmacies; November 27, 2024. That is the date that the Food and DrugAdministration (FDA) will begin enforcing the requirements of the Drug Supply Chain SecurityAct (DSCSA). DSCSAwas signed into law on November 27, 2013 and requires the ability to track and trace drug products from manufacturers downstream to the ultimate users. The law was implemented in a step-wise fashion over the last ten years. How did we get here and what happens on November 27, 2024?
The DSCSAcreates a drug product history, starting with the manufacturer that must be passed on with the product as it is sold or distributed down the supply chain. This encompasses wholesalers, third-party logistics providers, manufacturers, repackagers, and dispensers. The drug product history is not required to be provided by the dispenser to the prescribed patient. Pharmacies must do business withAuthorized Trading Partners (ATP). Pharmacies can check the FDAwebsite to see if their trading partners are authorized. Manufacturers and repackagers must register with FDA, so pharmacies can check registrations on FDA's website. Wholesalers and third-party logistics providers must file an annual report with FDAand pharmacies can verify the report has been filed.
The pedigree provision of the Food, Drug, and Cosmetic Act added by the Prescription Drug MarketingAct of 1987 was replaced on January 1, 2015 by the Product Tracing
requirements of the DSCSA. These requirements mandate the transaction information, transaction history, and transaction statement associated with each drug transaction be captured and provided for most human prescription drugs in finished form. This implementation was not enforced until March 1, 2016 under guidance from FDA.
The next major implementation step was the requirement that a product identifier be affixed to the packaging of prescription drugs. This identifier was to be readable by both humans and machines. The product identifier includes the NDC number, serial number, lot number, and expiration date. This requirement was implemented for manufacturers as of November 27, 2017 and repackagers as of November 27, 2018.
For pharmacies, the next implementation date was November 27, 2020. Dispensers would be required to verify the product identifier of at least three packages or ten percent of a suspect product, whichever is greater, or all packages, if there are fewer than three. FDAguidance provides many reasons why a pharmacy may suspect a product is illegitimate. Examples include product sold at extremely low prices, signs of tampering with the packaging, shipping labels indicating the package came from an unexpected source, misspelled words on the packaging, or the product name differs from the FDAapproved name.
EDITORIAL Rx AND THE LAW
19
RX AND THE LAW
Dispensers would also have to verify product in this manner (three packages/ten percent) in response to a notification of illegitimate product from FDAor a trading partner Again, enforcement of this requirement was deferred until November 27, 2023.
Other requirements from 2020 are in effect now Dispensers must still only transact products encoded with a product identifier and must comply with other suspect and illegitimate verification requirements. Pharmacies must have systems and processes in place to address three situations; 1) determining whether a product is suspect, 2) investigating suspect products and quarantining them if found to be counterfeit, or unfit for distribution and potentially dangerous, and 3) notifying FDAif the drug is illegitimate or has a high risk of being illegitimate. This last notification must occur within 24 hours after the determination.
The last piece of the puzzle was to be effective November 17, 2023. Among these requirements, manufacturers, distributors, and dispensers were to provide and receive transaction information and transaction statements in a secure, electronic manner. ATP's must have systems in place to verify product identifiers at the package level. ATP's must be able to respond to tracing requests and trace products at a package level. OnAugust 25, 2023, FDAannounced a one-year stabilization period intended to giveATP's additional time to stabilize their systems and become fully interoperable for secure and timely electronic data exchange. With this announcement, FDA intends to use enforcement discretion until November 17, 2024.
What does all of this mean for pharmacies? Pharmacies should have implemented all of the requirements of the DSCSAby now and be working to test and verify their system is working correctly If your pharmacy is not in this position today, there is time to work on your implementation before the non-enforcement period ends. However, depending on where your pharmacy is in the process, completing and testing your system could be a tall order in a short period of time. Resources are available through national and state pharmacy associations and third-party vendors to assist with implementation. Once the non-enforcement period ends, FDAand/or state regulators can bring enforcement actions for violations. Violations could result in seizures of products, court-ordered injunctions, license suspensions or revocations, and civil and criminal fines and penalties, including imprisonment.
The old saying goes that the way to eat an elephant is to do it in small bites. The DSCSAtimeline has given pharmacies the chance to implement the requirements in small bites. If your pharmacy is behind, there is still time, but your bites are going to be much larger
© Don R. McGuire Jr., R.Ph., J.D., is General Counsel, Senior Vice President, Risk Management & Compliance at Pharmacists Mutual Insurance Company
Thisarticlediscussesgeneralprinciplesoflawandrisk management. Itisnotintendedaslegaladvice. Pharmacistsshouldconsulttheirownattorneysand insurancecompaniesforspecificadvice. Pharmacists shouldbefamiliarwithpoliciesandproceduresoftheir employersandinsurancecompanies,andactaccordingly

CONT.
20

Not Just a Pediatric Illness: Updates on Respiratory Syncytial Virus (RSV) Prevention
AUTHORS/CONTRIBUTORS
ArianeGuthrie,PharmD,BCPS,BCGP , ClinicalPharmacistPractitioner,NorthernArizonaVA HealthCareSystem
IvanaKreso,PharmD2024Candidate,UniversityofArizonaR.KenCoitCollegeofPharmacy
FUNDING - Thisresearchwasnotfunded
DISCLOSURES - Theauthorshavenorelevantfinancialrelationshiptodisclose
CONTINUING EDUCATION INFORMATION
Target Audience: Pharmacists
Activity Type: Knowledge
Pharmacist Learning Objectives:
1 List high risk patient populations that may benefit from RSV prophylaxis.
2 Discuss the changes observed in the RSV season.
3 Describe available RSV agents.
4 Explain key clinical trials assessing the safety of RSV agents.
5 Identify appropriate counseling information regarding RSV infection prevention.
CONTINUING EDUCATION
21
Abstract
Respiratory syncytial virus (RSV) is a respiratory infection that can lead to serious complications, especially in high-risk individuals.There has been a recent increase in agents approved by the United States Food and DrugAdministration (FDA) for RSV prophylaxis. The following article will provide a review of the currently available RSV agents, their indications for use, available evidence that supported their approval, and clinical considerations for pharmacists and prescribers.
I. Background of RSV
RSV is a single-stranded ribonucleic acid (RNA) virus. It can bind to epithelial cells in the nasal cavity and the respiratory tract to cause infections. RSV has two major glycoproteins on its surface that help in the attachment to host cells: the attachment glycoprotein (G) and the fusion (F) glycoprotein. RSV works by attaching to the host cell and releasing its genetic content into the cell. This allows for the spread, replication, and ultimately leads to illness. RSV spreads through respiratory droplets and direct contact. For most people without risk factors for RSV complications, it presents like the common cold and symptoms usually resolve within two weeks.
Children aged two years and younger face heightened risk from RSV infections, especially those with underlying conditions such as prematurity, chronic lung or heart disease, weakened immune systems, neuromuscular disorders, congenital anomalies, or cystic fibrosis. RSV risk factors for infants and children include being in childcare or having a sibling who attends childcare or school. Symptoms that are commonly seen in infants and children include rhinorrhea, decreased appetite, cough, sneezing, fever, wheezing, lethargy, irritability, decreased activity, and apnea. It has been postulated that infants are at a higher risk for RSV infection due to their small bronchioles and narrow luminal diameter, leading to airway obstruction due to cell accumulation.Another possible factor contributing to the heightened risk of RSV infection in infants is their immature immune system.
In addition to infants and children, older adults who are 65 years and older face an increased risk of RSV infections. Common conditions that place older adults at a higher risk for developing severe illness from RSV include having underlying lung and cardiovascular disease; being immunocompromised; having diabetes and/or neurologic conditions; and having kidney, liver, or hematologic disorders. Other risk factors include frailty, as well as residence in a nursing home or long-term care facility Symptoms that are commonly seen in adults include rhinorrhea, pharyngitis, cough, headache, fatigue, and fever
Proposed explanations for the elevated risk of RSV infections
among older adults include a higher incidence of comorbidities, immunosenescence, and decreased production of RSV-specific serum and nasal immunoglobulin A(IgA).
Older adults were previously overlooked with regards to RSV infections, however the need for a prophylactic agent in this population is evident when examining annual RSV-related infection rates and hospitalizations. RSV is estimated to account for 5.0-7.8% of respiratory infections annually among adults. While incidence of RSV is lower in adults compared to children and infants younger than six months old, risk of RSV-related complications is relatively higher for older adults (20 per 1,000 versus 27 per 1,000, respectively). According to the Centers for Disease Control and Prevention (CDC), there are about 58,000-80,000 hospitalizations for RSV in children less than five years of age annually and about 60,000-160,000 hospitalizations in adults 65 years and older
Regarding RSV-related mortality, CDC reports there are about 6,000 to 10,000 deaths annually among adults 65 years of age and older compared to approximately 100 to 300 deaths annually for children younger than five years old.
Comparing RSV-related mortality to influenza-related mortality emphasizes the importance of the new RSV agents and their expanded indications.Across-section study evaluating U.S. death certificates between 1999 to 2018 found that a mean of 23 underlying respiratory deaths annually were associated with influenza among children younger than one year old, versus 96 annual deaths secondary to RSV in this age group (a five-fold increased risk). Both viruses, however, were associated with significant mortality among the elderly Among adults aged 65 years and older, the mortality rate per 100,000 people was 14.7 for RSV and 20.5 for influenza.
II. Changes to the RSV Season
According to the CDC, the typical RSV season occurs from September to May, with peak activity occurring during the winter season. It is interesting to note that RSV may not follow the “normal” seasonal patterns in all states. In Florida and Hawaii, for example, RSV onset occurs from September to November with peak onset from December to February More recently, changes to the RSV season have been observed as a result of the Coronavirus 2019 (COVID-19) pandemic. Cases of RSV were being reported during the spring season with peaks seen in the middle of July. This shift was not only observed with RSV, but with other respiratory diseases as well.The CDC continues to surveil RSV activity to assess whether these peaks will revert to prepandemic trends.
continued on next page
1 2 3 4 5 6
CONT. CONTINUING EDUCATION
6 7 8 9 9 10 9 9 22
It is also important to note that many of trials leading to FDA approval of newer RSV agents were conducted during the COVID-19 pandemic. Preventative measures (i.e., social distancing, quarantining, and wearing masks in public) likely impacted the spread of not only COVID-19, but other respiratory illnesses such as RSV. It is difficult to account for the impact that these measures played in preventing RSV compared to these studied therapies.
III. RSV Detection and Testing
There are multiple methods that can be used to test for RSV, including real-time reverse transcriptionpolymerase chain reaction (rRT-PCR), antigen testing, viral cultures/panels, and serology testing. The most commonly used methods for RSV testing include rRT-PCR and antigen testing. Nasal samples should be taken during the first few days of symptom onset because the amountof RSV virus decreases over time.
Figure 1: RSV Agent FDA Approval Timeline
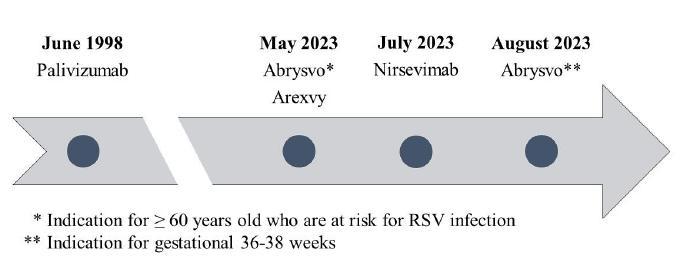
i Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants (IMpact-RSV).14
Molecular testing using rRT-PCR is the most sensitive test in both children and adults, since this method uses RSV genetic material within the testing sample. rRT-PCR testing is usually sent to a laboratory for processing andinterpretation which can take several hours to complete. One advantage of rRT-PCR testing is that it can be combined with additional respiratory testing so other causes of illness can be ruled out.
Antigen testing has shown to be less sensitive in adults, presumably because adults tend to have less virus located in their nostrils compared to younger children. Some advantages of antigen testing include that it can provide results within an hour, tests are simple to interpret, and they do not have to be sent to a laboratory for processing.
IV. RSV PreventativeAgents
It is important to note that while there are currently four agents approved by the FDAfor RSV prophylaxis, there are no FDA-approved treatments specifically for RSV Available agents are either monoclonal antibodies (mAb), palivizumab and nirsevimab, or bivalent protein subunit vaccines,Arexvy andAbrysvo.These agents are approved for use in two main populations: infants/children and adults 65 years and older
a Palivizumab (Synagis)
Before 2023, palivizumab was the only RSV agent on the market (Figure 1). Palivizumab is a F protein inhibitor mAb, that works by providing passive immunity to infants and children through the inhibition of the RSV fusion to host cells and as a result it prevents viral replication. Palivizumab is indicated for use in high-risk infants and children for RSV (Table 1). This mAb is administered once a month for up to five months, given its half-life of 20-24.5 days.
The IMpact-RSV trial aimed to evaluate the safety and efficacy of palivizumab in reducing hospitalizations due to RSV among high-risk infants. The trial was a randomized, double-blind, placebo-controlled multicenter study conducted during the 1996-1997 RSV season. The study randomized 1,502 children with prematurity or bronchopulmonary dysplasia to receive either palivizumab or placebo.
This study found that palivizumab prophylaxis reduced hospitalizations among high-risk infants (4.8% in the palivizumab group compared to 10.6% in the placebo group). The most commonly reported adverse effects were transient erythema and mild-moderate increases in aspartate aminotransferase (3.6% in the palivizumab group compared to 1.6% in the placebo group) and injection-site reactions (2.7% in the palivizumab group compared 1.8% in the placebo group).
One limitation of this study was that there were slightly more households with at least one active smoker in the palivizumab group compared to the placebo group. It should also be noted that this study excluded immunocompromised individuals, those who had seizure disorders, and children with congenital heart disease (except individuals with patent ductus arteriosus or uncomplicated and hemodynamically insignificant septal defect).
4 11 11
12
CONT. CONTINUING EDUCATION
13
23
Table 1: RSV Agent Indications and Eligibility
Agent and Manufacturer
Palivizumab (Synagis) Sobi 13
• RSVPreF3 OA(Arexvy) GSK 26,31
• Nirsevimab-alip (Beyfortus) AstraZeneca
RSVpreF (Abrysvo) Pfizer
Indication and Eligibility
• Infants or children with BPD that need medical therapy within the last 6 months and who are ≤24 months of age at the beginning of RSV season
Infants born prematurely (≤ 35 weeks) and who are aged 6 months or less at the beginning of RSV season
• Infants or children who are born with certain types of heart disease (such as hemodynamically significant CHD) and who are ≤24 months of age at the beginning of RSV
Individuals ≥60 years who are at risk with shared clinical decision-making
33 Neonates and infants born during or entering their first RSV season
•
• Children ≤24 months who remain vulnerable to severe RSV disease through their second RSV season
31, 35 Individuals ≥60 years who are at risk with shared clinical decision-making
•
• Individuals at 32-36 weeks gestational age of pregnancy
Abbreviations: Sobi, Swedish Orphan Biovitrum AB; GSK, GlaxoSmithKline Pharmaceuticals; RSV, respiratory syncytial virus; CDL, chronic liver disease; BPD, bronchopulmonary dysplasia; CHD, congenital heart disease
b. RSVPreF3OA(Arexvy)
InMayof2023,theFDAapprovedGlaxoSmithKline Pharmaceutical’s(GSK)RSVvaccine,Arexvy,fortheusein individuals60yearsandolder ArexvyisanRSVF glycoproteinstabilizedinitsprefusionform,whichisafusion glycoproteinthathasbeenextractedandstabilizedwithan AS01 adjuvant.TheAS01 adjuvantisamixtureof monophosphoryllipidA(MPLA)andasaponinderivedfrom QuillajaSaponaria(QS-21). Arexvyisabletocreateapotent immuneresponseoncetakenupbyhostantigenpresenting cells.Arexvyiscurrentlyrecommendasaone-timevaccine. Ongoingtrialsareassessingthedurationofprotectionprovided byArexvyagainstRSVandthepotentialneedforboosterdoses.
i. RespiratorySyncytialVirusPrefusionFProteinVaccinein OlderAdults(AReSVi-006)Trial17
GSK’sAReSVi-006trialinvestigatedtheefficacyandsafetyof Arexvycomparedtoplaceboinpreventinglowerrespiratorytract diseaseinadults60yearsandolderduringoneRSVseason. Thetrialwasadouble-bind,placebo-controlled,phasethree studythatrandomized24,966adultsovertheageof60years oldwhoweredeemedmedicallystabletoreceiveeitherArexvy orplacebo. 15 B B 16
The study found thatArexvy had an efficacy of 82.6% (96.95% CI, 57.9 to 94.1) in preventing RSV-related lower respiratory tract disease, with 7 cases in the vaccine group and 40 cases in the placebo group.This study examined solicited reactions (injection site pain and fatigue) within four days of treatment administration and found that 71.9% of individuals in the Arexvy group reported a solicited reaction compared to 27.9% of individuals in the placebo group.This study also examined unsolicited reactions within 30 days of treatment administration within a prespecified safety population and found that 14.9% of individuals in theArexvy group reported an unsolicited reaction while 14.6% of individuals in the placebo reported an unsolicited reaction.They also examined unsolicited reactions within 30 days of treatment administration within the entire study population and found that 33% of individuals in the Arexvy group reported an unsolicited reaction while 17.8% of individuals in the placebo group reported an unsolicited reaction.The study examined serious events, such as atrial fibrillation, that occurred within six months of treatment administration and found that 4.2% of individuals in theArexvy group reported a serious event compared to 4.0% of individuals in the placebo group.There were three fatal adverse events reported in this study (one associated with cardiopulmonary failure, one associated with a pulmonary embolism, and one associated with an unknown cause).
CONT. CONTINUING EDUCATION
24
Table 2: Summary of RSV Agents
Agent Dosing
Palivizumab (Synagis)13
15 mg/kg IM once monthly
RSVPreF3 OA (Arexvy)26 0.5 mLIM once
Mechanism ofAction
Monoclonal antibody (half-life 20-24.5 days)
Considerations
Dosage forms
• 50 mg/0.5 mL(0.5 mL)
• 100 mg/mL(1 mL)
• 12
• Use is currently recommended by theAAPbased on available data
First dose should be administered before the start of the current RSV season
• There are separate dosing recommendations for individuals who undergo CPB due to reported decreased in palivizumab serum concentrations
• 12 Not recommended for use against healthcare associated RSV disease
•
• 12 Manufactured as vialed solution
Dosage forms
• 120 mcg/0.5 mL(0.5 mL)
Bivalent protein subunit withAS01 adjuvant B
• Contains the same adjuvant found in Shingrix
• Some formulations contain polysorbate 80
•
• Manufactured as a 2-vial formulation: the powder vial must be reconstituted with adjuvant (liquid) vial
Dosage forms
• 100 mcg/mL(1 mL)
• 50 mg/0.5 mL(0.5 mL)
Nirsevimab-alip (Beyfortus)33
First RSV season:
Weight <5 kg: 50 mg once
Weight ≥5 kg:100 mg once
Second RSV season: <24 months: 200 mg once
RSVpreF (Abrysvo)35 0.5 mLIM once
YTE-modified monoclonal antibody (half-life 70-100 days)
• Provides protection for about 5 months
• 34
• AAPrecommends use toAmerican Indian andAlaska Native children 8-19 months who are not eligible for palivizumab and who live in remote regions
• 12
There are separate dosing recommendations for individuals who undergo CPB due to reported decreased in palivizumab serum concentrations
•
Manufactured as prefilled syringes
Dosage forms
• 120 mcg/0.5 mL(0.5 mL)
Bivalent protein subunit
• Some formulations contain polysorbate 80
•
• Manufactured in vials that must be diluted with the provided diluent
Abbreviations: IM, intramuscular; RSV, respiratory syncytial virus; CPB, cardiopulmonary bypass; AAP, American Academy of Pediatrics
CONTINUING EDUCATION CONT.
25
Some strengths of this trial include a high completion rate of 91% in the six-month follow-up period and the examination of immunogenicity for both RSVAand B subtypes. Limitations of this study included the exclusion of immunocompromised individuals and a limited representation of participants aged 80 years or older or those considered frail.Additionally, vaccine protection was only examined for one RSV season and the study was conducted during the COVID-19 pandemic, the latter of which likely explains the relatively low incidence of RSV (only 47 participants reported an episode of RSV-related lower respiratory tract disease).
c. Nirsevimab-alip (Beyfortus)
In July of 2023, the FDAapprovedAstraZeneca’s Beyfortus (nirsevimab-alip) for infants and children born during or entering their first RSV season and for children up to 24 months who remain vulnerable during their second RSV season (Table 1). Nirsevimab is aYTE-modified recombinant human long-acting mAb, which extends the agent’s half-life to 70-100 days. It acts by directly fusing to the RSV F protein epitope that inhibits membrane fusion of the virus into the host cell.This agent is given once and provides protection against RSV for several months.
i Nirsevimab for Prevention of RSV in Health LatePreterm andTerm Infants – (MELODY)Trial20
MedImmue/AstraZeneca and Sanofi’s recent MELODYtrial investigated the efficacy and safety of nirsevimab in healthy late-preterm and term infants entering their first RSV season. The trial was a randomized, phase three, placebo-controlled trial that randomized 1,490 infants born at a gestational age of at least 35 weeks to receive either nirsevimab or a placebo.
There were 12 infants (1.2%) in the nirsevimab group and 25 infants (5.0%) in the placebo group who developed medically attended RSV-associated lower respiratory tract infections, corresponding to an efficacy of 74.5% (95% CI, 49.6 to 87.1) for nirsevimab.Additionally, nirsevimab demonstrated 62.1% (95% CI, 8.6-86.8) efficacy in preventing hospitalizations for RSV-associated lower respiratory tract infections (0.6% incidence of hospitalizations versus 1.6%). Severe adverse events, defined as events that resulted in death, were lifethreatening, required hospitalization, or caused persistent or significant disability or incapacity, occurred in 3.6% of infants in the nirsevimab group and in 4.3% of infants in the placebo group. In total, three deaths occurred but none were thought to be associated with nirsevimab or placebo. Of note, there was one report of a nirsevimab participant having a grade three generalized macular rash without any systemic features that resolved without treatment after 20 days.
Astrength of this study was that it examined antidrug antibodies after injection, however the impact of these antibodies on subsequent nirsevimab administration is still being investigated. Limitations of this study include that target enrollment was not reached, the study duration coincided with the COVID-19 pandemic, participants were healthy term infants, and the study excluded individuals with renal impairment, hepatic dysfunction, congenital anomaly of the respiratory tract, seizure disorders, congenital heart disease (except uncomplicated), and immunocompromised infants.
d. RSVpreF (Abrysvo)
In May 2023, the FDAapproved Pfizer's vaccine,Abrysvo, for use in individuals aged 60 years and older. Subsequently, in August 2023, the indication was expanded to include pregnant individuals at 32-36 weeks gestation (Table 1). Abrysvo is a bivalent protein subunit vaccine similar toArexvy, however it does not contain an adjuvant. With its dual indication, the vaccine provides active immunity to adults as well as passive immunity for infants.Abrysvo is currently recommend as a onetime vaccine, however there are ongoing trials to examine the duration of protection against RSV and the potential need for additional boosters.
i Efficacy and Safety of a Bivalent RSV Perfusion F Vaccine in OlderAdults (RENOIR)Trial22
The RENOIR trial evaluated the immunogenicity, efficacy, and safety ofAbrysvo in preventing lower respiratory tract illnesses in adults 60 years and older during one RSV season. The trial was a randomized, phase three, multicentered, placebocontrolled study The trial randomized 34,284 adults 60 years and older to received eitherAbrysvo or placebo.
RSV-associated lower respiratory tract illness with at least two signs or symptoms occurred in 11 participants in theAbrysvo group compared to 33 participants in the placebo group, demonstrating a vaccine efficacy of 66.7% (96.66% CI, 28.885.8). Vaccination withAbrysvo also demonstrated 62.1% (95% CI, 37.1-77.9) efficacy in preventing RSV-associated acute respiratory illness (22 cases in theAbrysvo compared to 58 cases in the placebo group). More local site reactions were reported in theAbrysvo group compared to placebo (12% and 7%, respectively), with the most common reaction being injection site pain. Reported systemic events within seven days of treatment were similar between the groups (27% in the Abrysvo group and 26% in the placebo groups).The most commonly reported systemic events were headache and fatigue.Three serious adverse events were considered by the investigators to be related to the trial intervention: one delayed allergic response seven hours after injection, one case of Miller-Fisher syndrome (a variant form of Guillain-Barre syndrome characterized with ophthalmoplegia, ataxia, and areflexia), and one myocardial infarction which occurred six days after injection.
continued on next page
CONT. CONTINUING
EDUCATION
21
18 19 19
26
One of the strengths of this trial was that most participants had at least one high-risk condition (i.e. tobacco use, diabetes, lung disease, heart disease, liver disease, or renal disease). Limitations of the study included that protection was only examined for one RSV season, the study was conducted during the COVID-19 pandemic, and immunocompromised individuals were excluded.
ii. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants (MATISSE)Trial
The MATISSE study assessed the efficacy and safety in preventing RSV-associated lower respiratory tract illness in infants with maternalAbrysvo vaccination compared to placebo.The trial was an international, randomized, phase three, placebo-controlled study The study randomized 7,392 women at 24-36 weeks gestation with an uncomplicated singleton pregnancy to receive eitherAbrysvo or a placebo. There were two primary efficacy end points: medically attended severe RSV-associated lower respiratory tract illness and medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
Abrysvo only met criterion for vaccine efficacy in regards to medically attended severe lower respiratory tract illness. Severe lower respiratory illness occurred within 90 days after birth in 6 infants of women in theAbrysvo group compared to 33 infants of women in the placebo group (vaccine efficacy, 81.8%; 99.5% CI, 40.6 to 96.3) and 19 infants in theAbrysvo group compared to 62 infants in the placebo group, after 180 days (vaccine efficacy 69.4% (97.58 CI, 44.3-84.1). Regarding rates of medically attended RSV-associated lower respiratory tract illness, the results of this trial did not show statistical significance. Regarding safety data for mothers, the most frequently reported local reaction within seven days was injection site pain (41% in theAbrysvo group and 10% in the placebo group).The most commonly reported systemic effects were slightly greater in theAbrysvo group compared to placebo (muscle pain reported in 27% and 17%, respectively; and headache reported in 31% and 28%, respectively). Serious maternal adverse events were found to be similar between both the groups.There were four serious adverse events associated with vaccines with mothers (pain in arm followed by bilateral lower-extremity pain, premature labor, systemic lupus erythematosus, and eclampsia). One mother who received the vaccine died from postpartum hemorrhage and hypovolemic shock. Stillbirths occurred in 10% of participants who received the vaccine and in 8% of participants who received placebo. Infant adverse event rates were similar between the two groups, and there were no serious events in infants considered to be associated with theAbrysvo.
One of the strengths of this trial was that it was the first trial to examine passive immunity to infants from a vaccine given
to their mother with regards to RSV. Some limitations of this study include the exclusion of individuals characterized as having high-risk pregnancies, those at current risk of preterm birth, those with multiple pregnancies, and infants with clinically significant congenital anomalies. This study was also not powered enough to examine differences in efficacy in RSV antigen subgroups.
V. Clinical Pearls for Pharmacists
When considering RSV prophylaxis, it's important to be aware of the differences between agents to select the best one for each individual (Table 2).As with many other vaccines, individuals experiencing any acute illness should not receive RSV vaccines until they are no longer ill. RSV vaccines should be avoided in individuals who have experienced severe allergic reactions, such as anaphylaxis to any component of the vaccines. For example, if an individual experienced an allergic reaction to the Shingrix vaccine then they should avoid theArexvy vaccine since both contain the same adjuvant (AS01 ).
Another important consideration that has yet to be elucidated is co-administration of RSV agents with other vaccines. Data are currently limited, however the CDC has stated that the RSV vaccines may be administered with other adult vaccines during the same visit. When examining co-administration with influenza vaccines, there has been some data showing noninferiority with regards to immunogenicity. Lower antibody titers were observed with co-administration with influenza and RSV vaccines, but the clinical significance of this finding is not fully understood. When theArexvy vaccine was administered with the adjuvanted quadrivalent inactivated influenza vaccine (FLUAD QUADRIVALENT), there was no significant difference in regards to immunogenicity with influenzaAsubtype H3N2.
Individuals should be counseled about the heightened risk of both local and systemic reactions when multiple vaccines are administered simultaneously When deciding if an individual should receive the RSV vaccine, several considerations are important. These include assessing if they are up to date on all other vaccines, determining if it is feasible for them to return for additional doses, evaluating their risk of contracting RSV, reviewing the vaccine's reactogenicity profiles, and taking into account the individual's preference regarding vaccination. Caution should be taken with all individuals who are at an increased risk for bleeding due to the risk of hematoma development.
It is important to note that ongoing trials for the new RSV agents will offer more insights into booster requirements and provide additional safety information. Recommendations are subject to change as more data and post-marketing surveillance becomes available.
24 25
26, 27
B
25
29, 30 25
25, 28, 29 28,30
23 CONT CONTINUING EDUCATION 27
CONTINUING EDUCATION
Non-pharmacologic options should be encouraged with all cases of RSV This includes frequent handwashing, avoiding exposure to individuals who have confirmed or suspected RSV, keeping common areas clean, not sharing drinking glasses and utensils with others, washing toys or frequently touched surfaces, and avoiding smoking.
5, 31
VI. Conclusion
There has been an increase in the number of different RSV prophylactic agents available, accompanied by expansions of FDA indications and eligibility criteria for these agents. Pharmacists can play a vital role in educating patients and other healthcare professionals about RSV prophylactic agents.They can significantly contribute to combating RSV infections by identifying high-risk individuals who may benefit from RSV prophylaxis, interpreting the clinical evidence supporting FDAapprovals of these agents, and staying updated on newly available options.
References
1 McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. CurrTopMicrobiolImmunol. 2013;372:83-104. doi:10.1007/978-3-642-38919-1_4
2 Center for Disease Control and Prevention. Transmission. https://www.cdc.gov/rsv/about/transmission.html (Accessed 2023 Nov 2).
3 Center for Disease Control and Prevention. People at High Risk for RSV https://www.cdc.gov/rsv/high-risk/index.html (Accessed 2023 Nov 2).
4 Centers for Disease Control and Prevention. For healthcare professionals: RSV (respiratory syncytial virus). https://www.cdc.gov/rsv/clinical/index.html#antibody-product-for-children (Accessed 2023 Sep 23).
5 Mayo Clinic. Respiratory syncytial virus (RSV). https://www.mayoclinic.org/diseasesconditions/respiratory-syncytial-virus/symptoms-causes/syc-20353098 (Accessed 2023Aug 28).
6 Hu M, Bogoyevitch MA, Jans DA. Impact of Respiratory Syncytial Virus Infection on Host Functions: Implications forAntiviral Strategies.PhysiolRev. 2020;100(4):1527-1594. doi:10.1152/physrev.00030.2019
7 AJMC. Incidence and prevalence of RSV https://www.ajmc.com/view/incidence-and prevalenceof-rsv (Accessed 2023 Oct 11).
8 TinTin Htar M,Yerramalla MS, Moïsi JC, Swerdlow DL. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis.EpidemiolInfect. 2020;148:e48. Published 2020 Feb 13. doi:10.1017/S0950268820000400
9 Centers for Disease Control and Prevention. RSV surveillance & research.
5 Mayo Clinic. Respiratory syncytial virus (RSV). https://www.mayoclinic.org/diseasesconditions/respiratory-syncytial-virus/symptoms-causes/syc-20353098 (Accessed 2023Aug 28).
6 Hu M, Bogoyevitch MA, Jans DA. Impact of Respiratory Syncytial Virus Infection on Host Functions: Implications forAntiviral Strategies.PhysiolRev. 2020;100(4):1527-1594.
doi:10.1152/physrev.00030.2019
7 AJMC. Incidence and prevalence of RSV https://www.ajmc.com/view/incidence-and prevalenceof-rsv (Accessed 2023 Oct 11).
8 TinTin Htar M,Yerramalla MS, Moïsi JC, Swerdlow DL. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis.EpidemiolInfect. 2020;148:e48. Published 2020 Feb 13. doi:10.1017/S0950268820000400
9 Centers for Disease Control and Prevention. RSV surveillance & research. https://www.cdc.gov/rsv/research/index.html (Accessed 2023 Sep 16).
10 Hansen CL, Chaves SS, Demont C, Viboud C. MortalityAssociated With Influenza and Respiratory Syncytial Virus in the US, 1999-2018. JAMANetwOpen. 2022;5(2):e220527.
Published 2022 Feb 1. doi:10.1001/jamanetworkopen.2022.0527
11 U.S. National Library of Medicine. (n.d.).Respiratorysyncytialvirus(RSV)tests:Medlineplus medicaltest. MedlinePlus. https://medlineplus.gov/lab-tests/respiratory-syncytial-virus-rsv-tests/
12 AmericanAcademy of Pediatrics Committee on Infectious Diseases;AmericanAcademy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):e620-e638. doi:10.1542/peds.2014-1666
13 Synagis [package insert]. Gaithersburg, MD: MedImmune, LLC.; 2014. (Accessed 2023 Oct 14).
14 Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531-537.
15 U.S. Food and DrugAdministration. FDAapproves first respiratory syncytial virus (RSV) vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytialvirus-rsv-vaccine (Accessed 2023Aug 28).
16 FacciolàA, Visalli G, LaganàA, Di PietroA.An Overview of VaccineAdjuvants: Current Evidence and Future Perspectives. Vaccines(Basel). 2022;10(5):819. Published 2022 May 22. doi:10.3390/vaccines10050819
17 Papi,A., Ison, M., Langley, J., Lee, D., Leroux-Roels, I., Martinon-Torres, F., . . . Hulstrøm, V (2023). Respiratory Syncytial Virus Prefusion F Protein Vaccine in OlderAdults. TheNew EnglandJournalofMedicine,388(7), 595-608.
18 U.S. Food and DrugAdministration. FDAnew drug to prevent RSV in babies and toddlers. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsvbabies-and-toddlers (Accessed 2023Aug 28).
19 Zhu Q, McLellan JS, Kallewaard NL, et al.Ahighly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. SciTranslMed. 2017;9(388):eaaj1928. doi:10.1126/scitranslmed.aaj1928
20 Hammitt LL, Dagan R, YuanY, et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm andTerm Infants.NEnglJMed. 2022;386(9):837-846. doi:10.1056/NEJMoa2110275
21 U.S. Food and DrugAdministration. FDAapproves first vaccine for pregnant individuals to prevent RSV in infants. https://www.fda.gov/news-events/press-announcements/fda-approvesfirst-vaccine-pregnant-individuals-prevent-rsv-infants (Accessed 2023Aug 28).
22 Walsh, E., Pérez Marc, G., Zareba,A., Falsey A., Jiang, Q., Patton, M., . . . Schmoele-Thoma, B. (2023). Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in OlderAdults. TheNew EnglandJournalofMedicine,388(16), 1465-1477
23 2Kampmann B, Madhi SA, Munjal I, et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants.NEnglJMed. 2023;388(16):1451-1464. doi:10.1056/NEJMoa2216480
24 Centers for Disease Control and Prevention. Respiratory Syncytial Virus Infection (RSV) VIS. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html (Accessed 2023 Oct 13).
25 Centers for Disease Control and Prevention. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of theAdvisory Committee on Immunization Practices — United States, 2023. https://www.cdc.gov/mmwr/volumes/72/wr/mm7229a4.htm (Accessed 2023 Oct 16).
26 Arexvy [package insert]. Durham, NC: GlaxoSmithKline Biologicals.; 2023. (Accessed 2023 Oct 14).
27 Shingrix [package insert]. Durham, NC: GlaxoSmithKline Biologicals.; 2023. (Accessed 2023 Nov 15).
. MedlinePlus. https://medlineplus.gov/lab-tests/respiratory-syncytial-virus-rsv-tests/

Adjuvants: Current Evidence
28 Friedland L. GSK’s RSVPreF3 OAvaccine (AREXVY) [Presentation slides]. Presented at the Advisory Committee on Immunization Practices meeting,Atlanta, GA; June 21, 2023.
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-RSV-AdultsFriedland-508.pdf (Accessed 2023 Nov 5).
29 Gurtman S. RSVpreF older adults clinical development program updates [Presentation slides]. Presented at theAdvisory Committee on Immunization Practices meeting,Atlanta, GA; June 21, 2023. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-RSVAdults-Gurtman-508.pdf (Accessed 2023 Nov 5).
30 Centers for Disease Control and Prevention. Weekly U.S. Influenza Surveillance Report. https://www.cdc.gov/flu/weekly/index.htm (Accessed 2023 Nov 5).
31 Centers for Disease Control and Prevention. RSV Prevention.
https://www.cdc.gov/rsv/about/prevention.html (Accessed 2023 Nov 4).
32 Centers for Disease Control and Prevention. CDC Recommends RSV Vaccine For OlderAdults.
https://www.cdc.gov/media/releases/2023/s0629-rsv.html (Accessed 2023 Oct 16).
33 Beyfortus [package insert]. Sodertalje, Sweden:AstraZenecaAB.; 2023. (Accessed 2023 Oct 14).
34 Nirsevimab frequently asked questions. Home.Accessed November 15, 2023.
https://www.aap.org/en/patient-care/respiratory-syncytial-virus-rsv-prevention/nirsevimabfrequently-asked-questions/ (Accessed 2023 Nov 15).
35 Abrysvo [package insert]. New York, NewYork: Pfizer Labs.; 2023. (Accessed 2023 Oct 14).
Synagis [package insert]. Gaithersburg, MD: MedImmune, LLC.; 2014. (Accessed 2023 Oct 14). approves first respiratory syncytial virus (RSV) vaccine. .fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-
22 23 allewaard NL, et al.Ahighly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. SciTranslMed.
2017;9(388):eaaj1928. doi:10.1126/scitranslmed.aaj1928 24 Hammitt LL, Dagan R, YuanY, et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm andTerm Infants NEnglJMed 2022;386(9):837-846 doi:10 1056/NEJMoa2110275
CONT.
28
Assessment Questions
1. Which of the following accurately lists risk factors for infants and children in developing severe illness from Respiratory Syncytial Virus (RSV)?
A. Full-term birth, chronic lung disease, congenital heart disease
B. Homeschooled, full-term birth, immunosuppressed
C. Prematurity, congenital heart disease, immunosuppressed
D. Prematurity, homeschooled, congenital heart disease
2. Which of the following RSV agents are vaccines?
A. Abrysvo,Arexvy
B. Arexvy, Beyfortus
C. Beyfortus, Synagis
D. Synagis,Abrysvo
3. Which RSV agent is FDA-approved for use during pregnancy?
A. Abrysvo
B. Arexvy
C. Beyfortus
D. Synagis
4. Which of the following individuals should receive RSV prophylaxis?
A. 4-year-old with cystic fibrosis
B. 12-year-old with seasonal allergies
C. 28-year-old with chronic obstructive pulmonary disease
D. 55-year-old with benign prostatic hyperplasia
5. Which RSV agent would be appropriate for use in a 72-year-old with a reported allergy to the recombinant zoster vaccines?
A. Abrysvo
B. Arexvy
C. Shingrix
D. Synagis
Continued from page 18
REFERENCES
1 MirandaL.“Wedon’ttalkaboutBruno”inEncanto.Disney;2021.
2 BrazeauGA.Shareallyourstories.AmJPharmEduc.2018;82(10):7434.DOI: https://doi.org/10.5688/ajpe7434.
3 DiPiroJT,Chisholm-BurnsMA.Failfast.AmJPharmEduc.2013;77(8):159. DOI:https://doi.org/10.5688/ajpe778159
4 NohriaR,McBaneS.Theimpactoffailureonfacultydevelopment.CurrPharm TeachLearn.2022;14(2):123-126.DOI:https://doi.org/10.1016/j.cptl.2021.11.016
5 RagucciKR,KernDH,ShraderSP.Evaluationofinterprofessionalteam disclosureofamedicalerrortoasimulatedpatient.AmJPharmEduc. 2016;80(8):138.DOI:https://doi.org/10.5688/ajpe808138
6 PoirierTI,PaildenJ,JhalaR,RonaldK,WilhelmM,FanJ.Studentselfassessmentandfacultyassessmentofperformanceinaninterprofessionalerror disclosuresimulationtrainingprogram.AmJPharmEduc.2017;81(3):54 [publishedcorrectionappearsinAmJPharmEduc.2017Aug;81(3):S7].DOI: https://doi.org/10.5688/ajpe81354
7 FrenzelJE,SkoyET,EukelHN.Useofsimulationstoimprovepharmacy studentsknowledge,skills,andattitudesaboutmedicationerrorsandpatient safety AmJPharmEduc.2018;82(8):6644.DOI: https://doi.org/10.5688/ajpe6644
8 CainJ,PiascikP Areseriousgamesagoodstrategyforpharmacyeducation? AmJPharmEduc.2015;79(4):47.DOI:https://doi.org/10.5688/ajpe79447
9 MattinglyTJ2nd,AbdelwadoudM,MullinsCD,EddingtonND.Pharmapreneurdefiningaframeworkforentrepreneurshipinpharmacyeducation.AmJPharm Educ 2019;83(10):7548.DOI:https://doi.org/10.5688/ajpe7548
10 CarrSE.WearnA,CannyBJ,CarmodyD,BalmerD,CelenzaA,DiugB,Leech M,WilkinsonTJ.Whenthewheelsfalloff–Medicalstudents’experiencesof
6. What is the primary mechanism of action of Nirsevimab in preventing RSV infection?
A. Enhancing antibody production
B. Inducing passive immunity
C. Inhibiting RSV fusion to host cells
D. Stabilizing RSV glycoproteins
7. Which FDA-approved RSV prophylactic agent is administered once a month for up to five months?
A. Abrysvo
B. Arexvy
C. Nirsevimab
D. Palivizumab
8. During the Nirsevimab for Prevention of RSV in Health LatePreterm andTerm Infants – (MELODY)Tria, which group had a higher percentage of infants experiencing severe adverse events, defined as events that resulted in death, were life-threatening, required hospitalization, or caused persistent or significant disability or incapacity?
A. Both groups had the same percentage
B. Nirsevimab group
C. Placebo group
REFERENCES
1 MirandaL.“Wedon’ttalkaboutBruno”inEncanto.Disney;2021.
9. Which of the following is NOTa recommended nonpharmacologic measure to encourage in cases of RSV?
2 BrazeauGA.Shareallyourstories.AmJPharmEduc.2018;82(10):7434.DOI: https://doi.org/10.5688/ajpe7434.
A. Avoiding exposure to individuals with confirmed or suspected RSV
B. Avoiding smoking
3 DiPiroJT,Chisholm-BurnsMA.Failfast.AmJPharmEduc.2013;77(8):159. DOI:https://doi.org/10.5688/ajpe778159
C. Sharing drinking glasses and utensils with others
4 NohriaR,McBaneS.Theimpactoffailureonfacultydevelopment.CurrPharm TeachLearn.2022;14(2):123-126.DOI:https://doi.org/10.1016/j.cptl.2021.11.016
D. Washing toys or frequently touched surfaces
5 RagucciKR,KernDH,ShraderSP.Evaluationofinterprofessionalteam disclosureofamedicalerrortoasimulatedpatient.AmJPharmEduc. 2016;80(8):138.DOI:https://doi.org/10.5688/ajpe808138
10. Which statement best describes the shift in peak cases of RSV due to the COVID-19 pandemic?
A. Fall instead of winter
6 PoirierTI,PaildenJ,JhalaR,RonaldK,WilhelmM,FanJ.Studentselfassessmentandfacultyassessmentofperformanceinaninterprofessionalerror disclosuresimulationtrainingprogram.AmJPharmEduc.2017;81(3):54 [publishedcorrectionappearsinAmJPharmEduc.2017Aug;81(3):S7].DOI: https://doi.org/10.5688/ajpe81354
B. Spring season instead of winter
C. Winter season instead of fall.
D. Winter season instead of spring.
7 FrenzelJE,SkoyET,EukelHN.Useofsimulationstoimprovepharmacy studentsknowledge,skills,andattitudesaboutmedicationerrorsandpatient safety AmJPharmEduc.2018;82(8):6644.DOI: https://doi.org/10.5688/ajpe6644
8 CainJ,PiascikP Areseriousgamesagoodstrategyforpharmacyeducation? AmJPharmEduc.2015;79(4):47.DOI:https://doi.org/10.5688/ajpe79447
9 MattinglyTJ2nd,AbdelwadoudM,MullinsCD,EddingtonND.Pharmapreneurdefiningaframeworkforentrepreneurshipinpharmacyeducation.AmJPharm Educ 2019;83(10):7548.DOI:https://doi.org/10.5688/ajpe7548
10 CarrSE.WearnA,CannyBJ,CarmodyD,BalmerD,CelenzaA,DiugB,Leech M,WilkinsonTJ.Whenthewheelsfalloff–Medicalstudents’experiencesof interruptedacademicprogression.MedTeach.2022;1-8.DOI: https://doi.org/10.1080/0142159X.2022.2055455
11 TilleyM.Realityversusexpectation:lessonslearnedthroughafailureduring pharmacyschool.https://www.idstewardship.com/reality-versus-expectationlessons-learned-failure-pharmacy-school/PublishedMay24,2020.Accessed May20,2022.
12 DweckCS.Mindset,TheNewPsychologyofSuccess.RandomHouseDigital; 2008.
13 StefanM.ACVoffailures.Nature.2010:468;467.DOI: https://doi.org/10.1038/nj7322-467a
14 OrsiniP.‘Failurewall’inspiressuccess.https://www.cnbc.com/id/46101756 PublishedJanuary23,2012.UpdatedJanuary27,2012.AccessedMay20, 2022.
15 BynumWE,VarpioL,LagooJ,TeunissenPW.‘I'munworthyofbeinginthis space’:Theoriginsofshameinmedicalstudents.MedEduc.2021;55:185-197. DOI:https://doi.org/10.1111/medu.14354
16 ElmoreL.Onfailuresandrejections.AmJHealth-SystPharm.2012;69:924-926.
DOI:https://doi.org/10.2146/ajhp110441
17 GoodmanM,GauthierTP.Fivereasonswhypeoplefailinpharmacyschool. https://www.idstewardship.com/five-reasons-people-fail-pharmacy-school/ PublishedNovember7,2017.AccessedMay20,2022.
AZPA CONTINUING EDUCATION
29
 Rick G. Schnellmann, PhD Dean, University of Arizona College of Pharmacy
Rick G. Schnellmann, PhD Dean, University of Arizona College of Pharmacy
Computational drug discovery could open new doors
Imagine you have a key chain with four keys on it, and the locksmith down the road has a key chain with 500 keys. You would be able to open some doors and the locksmith would be able to open more, but neither of you will be able to open every door you come across. This is similar to the practical challenges pharmaceutical researchers have faced when discovering new therapeutics.
Historically, scientists physically tested a collection of thousands of candidate drugs to see what effect they might have. Over time, computational approaches have made the process more efficient, but advancement has needed to be faster, and drug discovery is still considered to be time consuming and expensive.
Travis Wheeler, PhD, associate professor in the R. Ken Coit College of Pharmacy, and his team at the University ofArizona Health Sciences are developing cutting-edge machine learning techniques that they expect will bring costs down and make new discoveries possible. He uses the analogy of finding a key for a locked door
“You and the locksmith might find a company that could provide you with 500,000 keys, but that would take a long time to try one-by-one manually,” Dr. Wheeler said. “Our technology is like being able to take a picture of the lock you want to open and having a computer rapidly scan through hundreds of thousands of keys to provide you with a few worth trying on the door.”
Providing researchers with computational tools
Most people only think about protein when looking at a nutrition label, but every human can thank tens of thousands of proteins for making their life possible.All the cells in the body stay alive and perform their necessary functions by producing and organizing proteins. Proteins are also key players in different stages of disease progression and are critical to the functions of viruses.
When these proteins do not work as expected, or when bacteria or viruses produce proteins that interfere with the workings of our cells, drugs can be used to set things right.Adrug is simply a small molecule that fits into a targeted protein, much like a key might fit into a lock.

“Aprotein folds into a three-dimensional structure with little pockets or cavities that allow it to bind to something. The key-in-lock interaction is called drug binding and produces results by changing something about how the protein works,” Dr. Wheeler said. “This is where drug discovery comes in. For example, if you find a pocket that a virus needs for replicating itself, you might be able to pour something into it and stop it from replicating.”

TravisWheeler,PhD,andhisteamattheR.KenCoitCollegeofPharmacy aredevelopingsoftwaretoaidinidentifyingdrugsfortargetproteinsandfor predictingpossiblesideeffects.
Unlike some health sciences researchers, Dr. Wheeler’s group concerns itself more with computation than with the human body They develop algorithms, machine learning methods and software implementations. However, they do direct these tools toward problems that often arise when analyzing biological data.
“Alot of what we do is build tools that biologists of all stripes can use to understand what are the contents of a genome or what are the functional parts of proteins. One natural offshoot of that work is drug discovery,” said Dr Wheeler
To make drug discovery a more efficient process, the group is developing software to aid in identifying drugs for target proteins and for predicting possible side effects. Similar to how an advanced search engine works, the computer learns from hundreds of thousands of examples to gain insight into the process of drug binding. The software will be able to rapidly
UNIVERSITY & ALUMNI
EDITORIAL
continued on next page 30
scan billions of small molecules for expected interactions and to recognize relationships between chunks of protein sequences.This work could lead to novel treatments and address issues related to infection, immunity and inflammation.
“We hear a lot about machine learning and artificial intelligence and how this will change many fields of life, including health and cures for diseases,” said Janko Nikolich, MD, PhD, head of the UArizona College of Medicine –Tucson’s Department of Immunobiology and the UArizona Health Sciences’Personalized Defense initiative, which is helping fund some of Dr. Wheeler’s drug discovery efforts.
“Dr Wheeler’s work will put this into practice, and help us fast-discover new drugs to, for example, stop the next pandemic or treat currently untreatable chronic diseases.”
Helping more patients
According to Dr. Wheeler, advancements in computational drug discovery are particularly important for diseases that do not produce profitable revenue streams for pharmaceutical companies. For very large drug searches, physical discovery can cost tens of millions of dollars to find a potential drug target. When dealing with a condition like hypertension that effects millions of people, pharmaceutical
companies have incentive to spend that upfront cost. But for rarer conditions, or for conditions mainly afflicting poor populations, that upfront cost can be a barrier to finding therapeutics. Dr. Wheeler explains what a binding pocket is and how the process of drug binding helps identify new drug targets.
“These computational approaches explore a much wider diversity of candidate molecules. That increases the chance that you will be able to find something that otherwise would not have been found, and it can be done much cheaper and much faster,” said Dr. Wheeler. “Potential cures might be identified for smaller groups, and the price of those drugs could be more affordable.”
Dr. Wheeler says his work in computer science has always been driven by intellectual curiosity and the rewarding feeling of solving complex puzzles. His computational drug discovery work delivers those feelings with the added benefit of being able to help people.
“I’ve got the best job in the world,” he said. “Alot of people buy books filled with word or number puzzles. But instead of needing to buy those, I get paid to work out solutions to really complicated problems.And it’s also rewarding, because I’m able to use these computational and statistical methods that I have learned to potentially make an impact on someone’s life or on the state of human health in general.”

CONT UNIVERSITY & ALUMNI
31

Midwestern University College of Pharmacy
Mitchell R. Emerson, PhD
Dean, Midwestern University College of Pharmacy
Seasons Greetings from the College of Pharmacy at Midwestern University!
Greetings from the College of Pharmacy at Midwestern University.As we welcome 2024, we are reminded of the many accomplishments of our faculty, staff, students, alumni, and friends.As always, we want to highlight and celebrate all our milestones.
Campus life has been busy with preparation plans for the Spring Quarter, celebrating Residency Match and the Class of 2024 Graduation, and welcoming the Class of 2027 this summer Things can never be planned too early! We are excited to get a refresh in Cholla Hall (home to much of the pharmacy program) this spring and early Summer Residency Match Day was March 13th, and we are proud of the Class of 2024 as we had the highest percentage of any class to Match. CPG had 28 students match in Phase I and we expect several others to match in Phase II.
Practice sites range from Community Pharmacies to HealthSystems including several VeteransAffairs facilities, Teaching and Community Hospitals, and clinics. Many of the class will remain inArizona to complete their training; however, they will be distributed all over as this dynamic class is going coast-to-coast.
On Saturday, March 23rd we hosted the College of PharmacyAlumni and Friends reception in Orlando in conjunction with theAmerican PharmacistsAssociations Annual Meeting (APhA). We had an incredible reception atThe Hampton Social- Orlando. Thank you to our faculty, staff, alumni, students, and friends who stopped by to join us. I hope you had the chance to enjoy the incredible meeting and all of what Orlando has to offer.
As noted above, we’re counting down until we celebrate our newest graduates.The final day of rotations is May 10th and we will host the class for anAwards Ceremony and BBQ. Graduation is scheduled on May 30th at 12pm with a festive reception following the ceremony All are welcome to celebrate the Class of 2024. The events continue as we host our annual College of Pharmacy Dessert Reception in conjunction with the annualAzPAconference at the Hilton Phoenix Resort at the Peak. We hope you will plan to join us on Friday, June 7th. Look for more details as the date draws near

We are looking forward to catching up with all of you and connecting at a future event. If you’re ever in the Glendale area, please reach out and stop by the campus. So much has changed, but still remains the same welcoming place.
Did you know we have a Midwestern University Job Board? If you’re looking to hire or looking for a new opportunity, please click here for more information
https://www.midwestern.edu/alumni/alumni-job-finder
If you’ve recently moved or relocated, please ensure we have your updated contact information. Please email updates to your Manager ofAlumni Relations, Kimberly Hastings at KHastings@midwestern.edu
To follow us and learn more about our events and wins, join the MWU Pharmacy social media community:
Like us on Facebook: Midwestern University-College of Pharmacy
Follow us onTwitter: @MWUpharmacy
Follow us on Instagram: @MWUpharmacy



EDITORIAL UNIVERSITY & ALUMNI 32

Creighton University College of Pharmacy
Jane Stein, PharmD Professor, Creighton University College of Pharmacy
Creighton addresses healthcare needs in Southwest
Arizona is one of the fastest growing states in the U.S., and Creighton University’s infusion of health professionals comes at a crucial time for the state that is experiencing a population boom and shortfall of healthcare providers simultaneously
It’s estimated that by 2030,Arizona will have a shortage of 50,000 nurses and a physician-patient ratio of 1:1,500. It’s a potential quality care crisis that Creighton is addressing now
“The Creighton UniversityArizona Health EducationAlliance is an important addition to Phoenix’s healthcare community, which faces a critical shortage of healthcare professionals to service the region’s diverse population,” says Kevin Howard, director of recruitment for the School of Pharmacy and Health Professions. “The CreightonAlliance provides students essential hands-on patient-care experience at mission-focused Phoenix healthcare facilities.”
Creighton University’s campus in Omaha, Nebraska, joins its Phoenix counterpart to address this shortage through innovative programming, pipelines between the two campuses and community partnerships to graduate healthcare professionals exceptional in their expertise and compassionate in their care.


Creighton’s proactive approach includes:
Doctor of Pharmacy EarlyAssurance Program
What:High school seniors who meet certain criteria for admission and progression can apply for admission to the University’s Doctor of Pharmacy Program.
How:Students who begin their academic careers at Creighton’s Omaha campus can complete their doctorate in Omaha or transfer to the University’s Phoenix campus to finish their PharmD degree, for a total of two years of undergrad in Omaha and four years of pharmacy at either campus.
“We will be graduating our first PharmD class at the Phoenix campus in 2025, and the majority of these students are planning to remain inArizona,” says Timothy Ivers, PharmD, assistant professor of pharmacy practice at Creighton’s School of Pharmacy and Health Professions.
Innovative and Flexible Entry-level PharmD Program
What: Housed in the 195,000-square foot, state-of-the-art Creighton-Phoenix campus, this hybrid pathway combines distance learning for the didactic portion of the curriculum and cutting-edge technology and equipment for the clinical lab component of the curriculum.
How: Students benefit from a program that includes distance learning opportunities that can be tailored their schedules as well as necessary hands-on instruction “designed to promote interactive and interdisciplinary learning for future clinicians in pharmacy,” says Howard. The program’s structure is appealing to PharmD students and thus helps to draw more to the program, which is good for the Greater Phoenix area.
EDITORIAL UNIVERSITY & ALUMNI 33
Community Partnerships
What: Partnerships provide students with hands-on learning and service opportunities and fill a need within the community Partnerships include Shoo the Flu Clinics on Creighton’s campus; First Place, an organization dedicated to fostering independence for adults with autism and other neurodiversity conditions; and the Virginia G. Piper Medical Clinic.
How:As students integrate into local populations, they form connections that create a sense of community and belonging.
Experiential Learning
What: Foundational skills acquired in the classroom are reinforced with time spent in a variety of pharmacy settings – community pharmacy, acute pharmaceutical care, inpatient hospital and outpatient clinic – throughout the Greater Phoenix area. In fact, it comprises 30 % of the curriculum, providing practice that is completed in a realworld pharmacy setting.
How: Such interaction between local pharmacists and Creighton’s PharmD students provides students invaluable skill development – patient counseling and client interaction, for instance – and employers the chance to meet future employees. “We receive great feedback from pharmacists who work with our pharmacy interns,” says Ivers.
3+4 Dual Degree PharmD Program withASU
What: Students can earn a bachelor’s degree atArizona State University and then complete their PharmD degree Creighton in Phoenix. Students typically apply to the PharmD program during their junior year atASU and then start the program during their senior year, receiving their bachelor’s after the fourth year
How: Partnerships withArizona institutions such asASU strengthens ties to their home state forArizonians as well as draws new inhabitants to the area.
Creighton University is one of the largest Catholic health professions educators in the U.S. And with its focus on academic excellence and the Jesuit value ofcurapersonalis – care for the whole person – it brings quality of care to the Southwest.


34
EDITORIAL UNIVERSITY & ALUMNI

NEW ON-DEMAND PROGRAM!
Pharmacist-Directed Hormonal Contraception Training
The Arizona Pharmacy Association is happy to announce that 2 years after the passage of SB1082 Arizona pharmacists can now dispense Self-Administered Hormonal Contraceptives to women 18 years and older pursuant to the newly adopted ADHS Statewide Standing Order!
Any pharmacist wishing to dispense self-administered hormonal contraceptives pursuant to this statewide Standing Order must be prepared to do the following:
Complete a 3-hour Training -AzPA has created one that is compliant with ARS 32-1979.01 and AAC R4-23-407 and R4-23-408-409.
Obtain necessary equipment to measure blood pressure. Review the Standing Order, Standard Procedures, and Self-Screening Questionnaire.
Establish SOP’s to ensure your pharmacy is compliant with all state laws.
REGISTER NOW 35


Continuous Glucose Monitor (CGM) Billing and Supply Allowance
CMS has issued an update on the Glucose MonitorPolicyArticle effective as of January 1, 2024.Asupplier now has the option to bill and dispense up to a 90-day supply for procedure codesA4238 (Adjunctive CGM) and A4239 (Non-Adjunctive CGM).
The policy article states:
“Uptoamaximumofthree(3)months,ninety(90)daysof thesupplyallowancemaybebilledforcodeA4238or A4239totheDMEMACatatimeandsuppliersmaynot dispensemorethananinety(90)daysupply.”
Below is a helpful chart to categorize the Dexcom and FreeStyle CGM products, their respective NCPDP billing units, and corresponding days’supply
1 EDITORIAL PAAS NATIONAL
Product NDC NCPDPBilling Unit Day's Supply Dexcom G6 Receiver 08627-0091-11 1 Each Once a year Dexcom G6 Transmitter 08627-0016-01 1 Each Every 3 months Dexcom G6 Sensor 08627-0053-03 1 Each Every 30 days Dexcom G7 Receiver 08627-0078-01 1 Each Once a year Dexcom G7 Sensor 08627-0077-01 1 Each Every 10 days FreeStyle Libre 14-day Sensor 57599-0001-01 1 Each Every 14 days FreeStyle Libre 14-day Reader 57599-0002-00 1 Each Every 3 years FreeStyle Libre Reader 57599-0000-21 1 Each Every 3 years
36
PAASTips:
• The NCPDPbilling units shown in the chart above would apply to non-Medicare Part B claims
When billing Medicare Part B claims for procedure codesA4238 orA4239, claims are billed as 1 unit of service (UOS) per 30 days
• ACGM supply allowance includes all items necessary for the use of the device and includes, but is not limited to, CGM sensors and transmitters
• For adjunctive CGMs , the supply allowance code (A4238), does not include supplies for a Blood Glucose Monitor (BGM.).Any required BGM supplies must be billed separately
• For non-adjunctive CGMs, the supply allowance code (A4239), also includes a home BGM and related supplies in the bundled payment (test strips, lancets, lancing device, calibration solution, and batteries)
• The supplier must monitor usage and verify the beneficiary has sufficient supplies to last for each 30day billing period
• Utilize the CGM SupplyAllowance Billing
• Calculator found on your DME MAC website to help determine when the next date of service can be billed
• CGS
• 2
Noridian
• 3
• 4
Check the same/similar tool in myCGS for a complete claim history to be sure the patient has not received supplies from another supplier in the last 30 or 90 days·
• Beware that any PBM, including Medicare B, will recoup or deny a claim if it is refilled too soon based on days’ supply guidelines above
If the patient requires a replacement sensor, transmitter, or receiver due to a product failure, be sure to document and replace the item while reaching out to the manufacturer
• ®
ByTrentonThiede, PharmD, MBA, President at PAAS
National , expert third party audit assistance, FWA/HIPAA and USP800 compliance.
References:
1. https://www.cms.gov/medicare-coveragedatabase/view/article.aspx?articleId=52464&ContrID=140
1. https://www.cgsmedicare.com/medicare_dynamic/jc/ k0553/index.aspx
3. https://med.noridianmedicare.com/web/jadme/searc h-result/-/view/2230703/continuous-glucose-monitor-cgmsupply-allowance-calculator
4. https://mycgsportal.com/mycgs/
Copyright © 2024 PAAS National, LLC. Unauthorized use or distribution prohibited.All use subject to terms at https://paasnational.com/terms-of-use/

37
CONT. PAAS NATIONAL

Pharmacy Day at the Capitol
March 20th was our annual Pharmacy Day at the Capitol!
We had over 100 pharmacy professionals including pharmacists, pharmacy technicians and pharmacy students from all threeArizona colleges of pharmacy! We met with over 40 legislators to communicate the education need to obtain a Doctor of Pharmacy degree and the specialized certifications available. During the meetings, our volunteers discussed Payment for Pharmacist Services, PBMAbuses, andTest andTreat.
In addition to the meetings, over 75 legislators and staffers joined us for lunch at Wesley Bolin Plaza.
Thank you to everyone who came out to make this day a success!



ADVOCACY PHARMACY DAY
LOCATION Arizona State Capitol Complex 1700 W. Washington St
38
| Phoenix, AZ 85007

PHARMACY DAY CONT.





ADVOCACY
MeetingwithRepresentativeLeoBiasiucci Righttoleft:Dawn,Jarrod,Shams,Ashkan
MeetingwithRepresentativeLiguori Lefttoright:Jeffrey,Glory,Kelly,Jacob,Marcus
MeetingwithSenatorBravo Lefttoright:Gabriela,Denise,Sarah,Michael,Melinda
MeetingwithRepresentativeSeaman Lefttoright:Ken,Joseph,Miranda,Ivan,Josalynn
39
MeetingwithRepresentativePatriciaContreras Lefttoright:Lillianne,Mariphil,Jan,Sammy

40















 Mary Manning, PharmD, MBA, BCPS AzPABoardofDirectors-HealthSystem
Mary Manning, PharmD, MBA, BCPS AzPABoardofDirectors-HealthSystem






 Rick G. Schnellmann, PhD Dean, University of Arizona College of Pharmacy
Rick G. Schnellmann, PhD Dean, University of Arizona College of Pharmacy



























