8 minute read
Me and My Microbes
from Aston in Touch 2018
by Aston Alumni
ME AND MY MICROBES
Advertisement
Professor Anthony Hilton is explaining a theory called competitive exclusion. “The analogy that’s been used is a car park,” he says. “If you’re a bad bacterium and you enter an empty car park, you can park wherever you want; if everything is full of cars you just drive around and can’t get in anywhere. In other words, if your gut is rammed full of good bacteria, there’s no real space for the bad bacteria to get a foothold.”
This neat and vivid example is typical of Professor Hilton’s engaging style of communication. Well known at Aston and in the wider world for his scholarly work on bacterial survival and control, he is also an award-winner when it comes to public engagement. He has made numerous TV appearances, notably in BBC3’s documentary series Grime Scene Investigation, and has commissioned a series of ‘microbial’ plays including last year’s The Drugs Don’t Work: a collaboration with Hobgoblin Theatre Company about the misuse of antibiotics. He has a passion for sharing his insights with young learners and can sometimes be spotted with giant cuddly-toy microbes, which he uses as props for talks in schools (“When you’re trying to enthuse others about things that are essentially invisible you need all the help you can get!”) His research on the ‘five second rule’, about the relative cleanliness of food dropped onto different surfaces, went viral in the media last year: an appropriate outcome for a microbiologist, you might say.
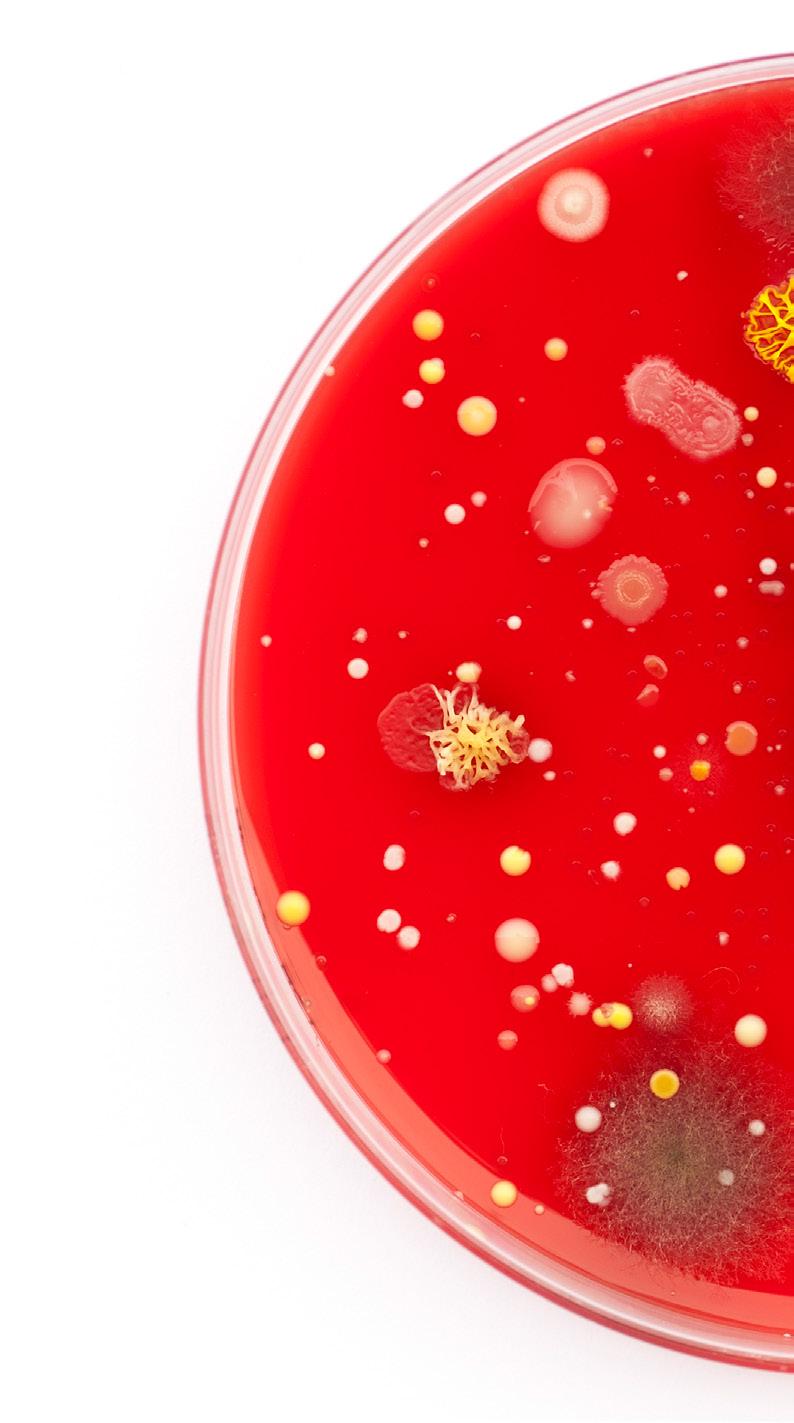
Clostridium difficile bacteria on a blood agar plate. Wikimedia Commons.
His enthusiasm for the invisible world of microbes is also highly infectious. Whether good, bad or neutral the astonishing fact is that the human body has more microbes than it has human cells. These typically range from bacteria and fungi to protozoans (mostly single-celled animal-like organisms) and, collectively, these tiny tenants are known as the microbiome. We are not born with this mass of microbes - though we do receive an initial dose of them when passing through the birth canal - rather, we acquire them in the first few years of life. In recent years the microbiome has become a hot scientific topic and current thinking is that it can even influence our eating habits and risk behaviours.
“There’s a parasitic element to microbes. The parasite will always drive its host to be consumed by the next host up, so a parasitic infection drives us to take risks, such as running across the road when we shouldn’t. We’re just vessels for these bacteria in our gut and on our skin. To some extent, the fact that they’re calling the shots doesn’t surprise me one bit,” says Professor Hilton cheerfully.
“At one time the mantra was that we should just kill all microbes because they’re all bad. What has become obvious is that some are actually beneficial. We need to understand how we can apply control in a way that maximises the effect of killing the bad bacteria without harming the good ones.”
Hence the idea of our gut as a microbial carpark, and the ongoing research into probiotics and prebiotics, which are useful methods of encouraging populations of good bacteria to grow. But the work undertaken at Aston extends much further than the microbiome. One of Professor Hilton’s key interests is antimicrobial resistance (AMR) or the ability for a microorganism to resist the effects of antibiotics, antivirals and antimalarials. The issue is complex and far-reaching, affecting the treatment of diseases such as cancer and HIV, right through to the problem of controlling hospital ‘superbugs’.
“At Aston we see our work as a continuum,” he explains. “The pathway to antimicrobial resistance is very long and starts with a person becoming ill because of a microorganism which is itself resistant to antibiotics. Everywhere along that continuum there’s probably a place where we can intervene.” His own interests lie ‘upstream’ of the AMR continuum in a less publicised area - illness prevention. He is particularly focussed on the antimicrobial properties of metals and how we can harness them to ward off undesirable bacteria in our everyday lives.
“The idea of using silver, gold and other metals like titanium and gallium is not a new idea, it’s actually quite an old technology, some of which is embedded within urban myths. People used to claim that if you had a sty on your eye you should rub it with a gold ring, for example. I’m interested in the idea of targeting the application of metal into places it normally wouldn’t be. It’s one thing rubbing a silver or gold ring on an infection but it’s another thing having an item that’s got silver within it, such as a carrier bag or a disposable coffee cup.”
Professor Hilton’s work frequently takes him from microbiology into areas such as engineering, where the health benefits of metals can be translated into usable products. When the UK Government introduced a levy on plastic carrier bags in 2015, for example, people diligently started reusing bags but the potential for cross-contamination with foodstuffs like fish and poultry led to an increased risk of infection. Thanks to his collaboration with a company called Addmaster, however, the antimicrobial reusable-carrier-bag was born and has since been adopted by many large retailers.
“One thing to say is that these plastic bags are not self-sterilising surfaces,” he warns. “People think of antimicrobial activity as: you spray something on, leave it a few minutes and it’s clean. It’s not like that with these surfaces. If your bag did become contaminated, say with chicken juices - which we know carry harmful bacteria - you could clean it when you discovered it and the antimicrobial surface would give you some added protection. It’s a safety net rather than an instant cure.”
Another way that scientists are using this technology is in hospitals. Peter Lambert, Emeritus Professor of Microbial Chemistry at Aston, was involved in a study looking at copper fittings in the ward of a working hospital. Half of the ward was provided with touch surfaces made from copper alloys, while half remained standard. The results were astonishing. After a series of daily tests it was shown that, compared to the regular surfaces, the copper contained 98 to 100 per cent fewer harmful organisms. Professor Hilton has since built some of his own work on these findings.
“One of the problems with copper is that it’s very expensive, particularly when you’re creating door architecture like handles. It’s quite a soft metal and not particularly durable. I’ve been working on a project to create architectural ironwork which is antimicrobial on its surface yet iron throughout its core. That way you can take a structurally sound, cheap metal and make it functional as well as beautiful.”

The good, the bad and the cuddly: Professor Anthony Hilton with a soft-toy microbe (Salmonella).
Antibiotic resistance is a natural phenomenon. Over time bacteria adapt to the drugs developed to kill them and mutate in order to survive. However, human behaviour can accelerate these changes in many ways - by mishandling food, living in unsanitary conditions or overusing antibiotics. Another high-risk scenario is when patients are admitted to hospital with serious illnesses. Treatments may have wiped out their normal gut bacteria - the good bacteria that regulate their immune systems - and they become vulnerable to the so-called hospital ‘superbugs’ such as MRSA and Clostridium difficile (or C. diff).
This area of the microbial continuum is Dr Tony Worthington’s specialist interest. He started his career in medical microbiology, working for the National Health Service, but moved to Aston University in 2004, having collaborated with Professor Peter Lambert since 1996. For the last 14 years he has worked on - amongst other things - methods of eradicating C. diff: a bacterium that affects the bowel and is endemic in hospitals.
“C. diff is a spore-forming bacteria, so when the environment for survival isn’t favourable for the bacterial cells it produces spores which are hardy little dormant, survival structures of C. diff that can live in the healthcare environment for many months - on work surfaces; patient bedside tables; curtains; bedding,” he explains. “They need to be physically removed through appropriate cleaning and disinfection otherwise hospitalised patients with a sterile gut, for example those who have been on broad spectrum antibiotics, can ingest them. The ingested spores will germinate into bacterial cells in their gut and produce very potent proteinaceous toxins that cause explosive diarrhoea and a condition called PMC or pseudomembranous colitis. C. diff infection can kill some patients.”
The problem is that C. diff spores are resistant to many common disinfectants because the spores have hard outer coatings that can’t be penetrated without the use of harsh disinfectants like bleach. But when the spores enter the gut, they begin to germinate into bacterial cells, and at this point they become more sensitive to antimicrobials. “So we thought, could we con the spores by inventing a germination solution which could be applied to the environmental spores through a spray or wipe for example, while they are on a hard surface, so they would ‘wake up’ and think they’re in the gut? Then clinical staff could use everyday disinfectants to eliminate them alongside other infection control procedures.”
In the gut the main germinant for C. diff is sodium taurocholate. Dr Worthington and colleagues took this ingredient and added five amino acids to develop a formulation that would rapidly wake the spores. They also found that, once germinating, they could easily be destroyed with alcohol and even natural products like tea tree oil and eucalyptus oil. Building on Professor Lambert’s work, they placed C. diff spores on copper surfaces - a spray of the germination solution woke them, then the antimicrobial properties of the metal killed them off. “Then we thought, if we put an antimicrobial into the spray, it could be a one-stage process. So we worked with colleagues at Aston and found that benzylkonium chloride works in a germination solution, eliminating spores of C. diff in one move.” More work is underway to try to incorporate an alternative to sodium taurocholate into plastic, while the original germination solution is now patented worldwide. A London-based company, Insight Health, is helping to bring it to market.
Aston’s work on healthcare-associated infections also touches on surgery itself. When preparing a patient for an operation, clinicians currently use a formulation called ChloraPrep (a combination of chlorhexidine and alcohol) which eliminates bacteria on the surface of the skin. However, when a cut is made in the skin bacteria lying much further down - out of the reach of the ChloraPrep - can enter the patient’s bloodstream and cause an infection. “Bugs will take advantage of wounds if they can; even the friendly bacteria on and in your skin will take advantage of you if you’re debilitated or in a compromised position,” says Dr Worthington. However, he has discovered that eucalyptus oil can improve the penetration of chlorhexidine into the skin, acting as “a second-generation antiseptic for people undergoing surgery”. As with the C. diff spray, the formulation is patented and there is work underway getting it into clinical practice.
“My true belief is that we have to work with industry, in harmony, to come up with solutions for the problems that we have in the world, whether that’s in the food industry, diagnostics or, in my case, healthcare,” says Dr Worthington. “I think the C. diff solution on hard surfaces and the chlorhexidine with eucalyptus are definitely two areas that would contribute to reducing healthcare infections. They won’t eliminate them, of course, but they’re intended to form part of a healthcare package.” Microbes might be invisible, but their influence is felt by every one of us.

Professor Anthony Hilton

Dr Tony Worthington
Anthony Hilton is a Professor of Applied Microbiology and Deputy Executive Dean of the School of Life & Health Sciences at Aston University. Tony Worthington is an Associate Professor in Clinical Microbiology and Infectious Disease at Aston University.



