



Pharmacologist A Publication by The American Society for Pharmacology and Experimental Therapeutics Vol. 65 • Number 2 • June 2023 the
the 2023-2024 Council INSIDE Herceptin: A Breast Cancer Breakthrough
Leadership Profile: Namandjé Bumpus 2023 Annual Meeting Recap Meet
The
March Issue: February 1
June Issue: May 1
September Issue: August 1
December Issue: November 1
THE PHARMACOLOGIST PRODUCTION TEAM
Catherine L. Fry, PhD
Lynne Harris, MA, APR
Dave Jackson, MBA, CAE
Maria Pasho
COUNCIL President
Michael F. Jarvis, PhD
President Elect
Namandjé Bumpus, PhD
Past President
Margaret Gnegy, PhD
Secretary/Treasurer
Kathryn A. Cunningham, PhD
Secretary/Treasurer Elect
Xinxin Ding, PhD
Past Secretary/Treasurer
Carol L. Beck, PhD
Councilors
Nina Isoherranen, PhD
Randy A. Hall, PhD
John R. Traynor, PhD
Chair, Publications Committee
Kenneth Tew, PhD
Chair, Program Committee
Michael W. Wood, PhD
FASEB Board Representative
Catherine M. Davis, PhD
Inclusion, Diversity, Equity & Accessibility Representative
Martha I. Dávila-Garcia, PhD
Chair, Young Scientists Committee
Dianicha Santana, PhD
Executive Officer
Dave Jackson, MBA, CAE
The Pharmacologist (ISSN 0031-7004) is published quarterly in March, June, September, and December by the American Society for Pharmacology and Experimental Therapeutics, 1801 Rockville Pike, Suite 210, Rockville, MD 20852-1633. Annual subscription rates: $25.00 for ASPET members; $75.00 for U.S. nonmembers and institutions; $100.00 for nonmembers and institutions outside the U.S. Single copy: $25.00. Copyright © 2023 by the American Society for Pharmacology and Experimental Therapeutics Inc. All rights reserved.
GST number for Canadian subscribers: BN:13489 2330 RT.
ASPET assumes no responsibility for the statements and opinions advanced by contributors to The Pharmacologist.
Postmaster: Send address changes to: The Pharmacologist, ASPET, 1801 Rockville Pike, Suite 210, Rockville, MD 20852-1633.

The Pharmacologist is published and distributed by
for Pharmacology
Experimental
Contents
the American Society
and
Therapeutics
1 Message from the President 3 Leadership Profile: Namandjé Bumpus, ASPET President-Elect 5 2023 Annual Meeting Recap 6 Call for 2023 Award Nominations 12 Meet the 2023-2024 Council 14 Feature Article: Herceptin 25 Science Policy News 28 Education News 31 Journals News 34 Membership News 38 Members in the News 39 Division News
Pharmacologist
Content Deadlines
The President
Dear ASPET members,
This is my last message to the ASPET membership as President. I am amazed at how quickly my time in this role has passed and the many milestones ASPET has experienced as we have collectively worked to build a stronger Society.
ASPET 2023, our first stand-alone meeting in over three decades, was held a few weeks ago at the St. Louis Union Station Hotel in St. Louis, Mo. It was a delight to see so many friends and colleagues, as well as meet many new ASPET members and first-time meeting attendees. Based on the feedback I have received thus far, our annual meeting was a smashing success! More than 1,000 attendees enjoyed the engaging venue, numerous social/networking activities and compelling scientific programming during the meeting. Our new meeting format, that was designed based on member feedback, facilitated standing-room-only attendance at all the oral symposia and vibrant poster sessions. The timely and thoughtful keynote presentations by Holden Thorp, William Kaelin, and Yasmin Hurd were also very well received and will likely become a recurring feature of our annual meetings going forward. Melissa Huston, ASPET Meetings Director and the entire ASPET staff are to be congratulated for all their hard work to put on this truly remarkable meeting. Their efforts have also built a great foundation for future meetings. As we discussed during the meeting in St. Louis, a key long-term strategy for ASPET is to provide a home for the science and profession of pharmacology. This year’s meeting fully embodied this aspirational goal, and I am very much looking forward to ASPET 2024 (May 16-19) at the Hyatt Regency Crystal City in Arlington, Va.
I also want to remind everyone about the upcoming IUPHAR World Congress of Basic and Clinical Pharmacology, occurring July 2-7, 2023 in Glasgow, Scotland, United Kingdom. A link to the meeting program is provided here: https://wcp2023.org/programme/. This important international meeting is hosted by our British Pharmacological Society colleagues this year and will feature many cutting-edge topics covering all aspects of pharmacology and translational medicine. ASPET is a platinum sponsor for the event, and I invite those attending to stop by and say hello at the ASPET Booth D31.

During the past year, ASPET has accomplished several key milestones that will serve to further strengthen the Society and facilitate greater opportunities for engagement with our membership. Our revised Bylaws modernize the operation of ASPET and provide greater transparency for our members. The 2023-2027 Strategic Plan is both aspirational and mission-driven with a focus on advancing all aspects of pharmacology through research, education, innovation, and advocacy. Fundamental to this new plan is the commitment to enhance our collective sense of belonging to ASPET as the home for pharmacology. The evolution of ASPET’s IDEA Taskforce into a standing committee with representation on the ASPET Council, the Call for Volunteers Initiative, and our Volunteers Appreciation Recognition event at the annual meeting serve as great examples of ASPET’s focus in ensuring diversity and membership involvement in all of ASPET’s activities.
I would like to take this opportunity to acknowledge outgoing and incoming ASPET Council members. Peggy Gnegy (Past-President) and Randy Hall (Councilor) are leaving Council while Carol Beck is changing roles

1 The Pharmacologist • June 2023 Message from
from Past-Secretary/Treasurer to President-Elect. Additionally, Pamela Hornby (Secretary/Treasurer-Elect) and Amy Arnold (Councilor) join Council effective July 1, 2023. Please join me in thanking these individuals as well as all our volunteers who serve on our committees and taskforces, Division Executive Committees, Division Communications Officers and our journal editorial boards. ASPET’s strength as a society is fully dependent on the dedicated contributions of all our volunteers, and I encourage our entire membership to actively seek opportunities to contribute to ASPET’s journey.
It has been an honor and privilege to serve as President of ASPET. I am truly grateful for all the energy and wisdom of ASPET’s expert staff and our volunteer leaders for all their hard work to build such a great Society. I am confident, under the direction of ASPET’s next president, Namandjé Bumpus, that all our collective efforts will effectively build upon our past successes and serve our membership in good stead in the year ahead and beyond.


Sincerely,
 Michael F. Jarvis, PhD ASPET President
Michael F. Jarvis, PhD ASPET President
The Pharmacologist • June 2023 2
Leadership Profile



A Conversation with ASPET President-Elect Namandjé Bumpus, PhD
Dr. Namandjé Bumpus is the President-Elect for 2022-2023. She begins her term as ASPET President July 2023. She was named as the FDA’s Chief Scientist on June 30, 2022. As the Chief Scientist, Dr. Bumpus supports the research foundation, science, and innovation that underpins the FDA’s regulatory mission. Prior to her appointment as the FDA Chief Scientist, she served as an associate professor of medicine and the associate dean for institutional and student equity at Johns Hopkins University School of Medicine in Baltimore, Md. A recipient of ASPET’s John J. Abel Award in Pharmacology, Dr. Bumpus has co-authored numerous peer-reviewed publications and has previously served on the editorial board of ASPET’s journal Drug Metabolism and Disposition. She has been a member of ASPET since 2008.

How did you get started in pharmacology?
As an undergrad I participated in the University of Michigan Department of Pharmacology Charles R. Ross Summer Undergraduate Fellowship Program.
I learned that Dr. Charles Richard Ross was the first African American to receive a Ph.D. in Pharmacology from the Department of Pharmacology at the University of Michigan Medical School. Participating in a fellowship program established to honor his legacy inspired me. During that summer, I worked in the lab of Dr. Rick Neubig. I had a very fulfilling and rewarding experience through having the opportunity to perform exciting research and work with an encouraging and supportive mentor in Rick Neubig. It was my first experience with pharmacology, and I knew from then that I wanted to pursue graduate training in pharmacology.
How did you first get involved with ASPET?
In graduate school I joined the laboratory of Dr. Paul Hollenberg at the University of Michigan. Paul had been president of ASPET and encouraged his students to attend the annual meeting and to become active in ASPET. I followed his example and continue to do so in all aspects of my career.
3 The Pharmacologist • June 2023
What do you want the ASPET membership to know about you and your ideas on how to move the organization forward during your term?

I would like people to know that I am accessible and always happy to talk with our members about how we can continue to strengthen ASPET. I would like to continue to widen our range of volunteer opportunities within ASPET and ensure that all of our members feel included and empowered.
What has been your proudest accomplishment in your career so far?
My proudest accomplishment is having contributed to the development of students/fellows/emerging scientists through various roles over my career including laboratory principal investigator, associate dean for graduate and institutional equity, associate dean for basic research, and chair of the Department of Pharmacology all at the Johns Hopkins University School of Medicine. In my current role as FDA Chief Scientist, I similarly work to
cultivate an environment that enables our scientists to thrive, and it brings me great joy.
What advice would you give young scientists who are just starting out in their careers?
Identify and engage a team of mentors who will be honest with you, but always productive in helping you to determine what next steps are needed for you to achieve your goals including concrete guidance about skills to develop and areas to strengthen. Stay true to what you would like to contribute to the world through your life’s work, and lead with that. During the tough times, remember all that you have accomplished and where you are trying to go with your career.
What do you want your legacy to be as the first Black President for ASPET?
I would like for my legacy to be that I used my voice and platform to create opportunities for others, and widened the circle of people who view themselves as future leaders of ASPET.
The Pharmacologist • June 2023 4
"I would like for my legacy to be that I used my voice and platform to create opportunities for others, and widened the circle of people who view themselves as future leaders of ASPET."
ASPET 2023 Annual Meeting Wrap-Up by the Numbers



Attendees Declare the Annual Meeting a Success
97% of survey respondents expressed high satisfaction levels about the “overall experience” of the ASPET 2023 Annual Meeting
Scientists With a Passion for Pharmacology Gather from Across the Globe Registrants traveled from 33 countries and 44 U.S. states.
5 The Pharmacologist • June 2023
Total Registrations (as of 6/8/2023)
1,030
(virtual-only packages are still being sold)
42% of registrants were young scientists
45% had never attended the ASPET Annual Meeting as part of EB
Exceeded attendance expectations by 27%
Thank you to our exhibitors and career table sponsors!
62% of attendees were either the purchasing decision-maker or they research/recommend purchasing decisions.
Gene Tools
Mayo Clinic - OPART
ACS Publications
Warren Center for Neuroscience Drug Discovery, Vanderbilt School of Medicine
British Pharmacological Society
RayBiotech
Inotiv
Case Western Reserve University
Med Associates
Bentham Sciences
National Institute of Neurological Disorders and Stroke
University of Michigan Department of Pharmacology
Boston University
University of Texas Health Science Center at San Antonio
Divisional Interests of Registrants
(Attendees were allowed to select as many as were applicable.)
The Pharmacologist • June 2023 6
0 100 200 300 400 500 600 Behavioral Pharmacology Cancer Pharmacology Cardiovascular Pharmacology Drug Discovery and Development Drug Metabolism and Disposition Molecular Pharmacology Neuropharmacology Pharmacology Education Translational and Clinical Pharmacology Toxicology
96%
Highest Attended Concurrent Sessions
1. Evolution of Psychedelics: Molecules Inspired by 5-HT2 Receptor Biology
2. Recent Advances in Orphan GPCR Structure, Signaling and Pharmacology
3. Pharmacology of Opioid/Stimulant Co-abuse: From the Lab to the Clinic
4.
Transporter Regulations and
Their
Role in Drug Disposition and Interaction
Average attendance at sessions increased by 26% compared to EB 2022 and exceeded pre-COVID ASPET sessions at EB 2019 by 18% 93% of survey respondents expressed satisfaction with the “scientific excellence of speakers/sessions”
49 sessions and 198 speakers
Social / Networking
25 networking events over 4 days
Pharmacologists enjoyed 1,220 hosted beverages and explored St. Louis’s 120,000 square-foot Aquarium with over 13,000 animals across 44 gallery exhibits.

ASPET attendees volunteered over 64 hours of time at the St. Louis Dream Center. In addition, ASPET donated $2,000 and an in-kind donation of our leftover meetings bags.

7 The Pharmacologist • June 2023 Abstracts 608 abstracts submitted 585 abstracts presented as posters 181 young scientists invited to be part of the Poster Competition 126 young scientists offered travel awards 69 abstract authors selected for oral presentations 30 abstract authors invited to present a teaser of their poster in the popular ASPET Daily Datablitz
of survey respondents expressed satisfaction with the “scientific excellence of
posters”
the
Call for 2024 Award Nominations
Each year ASPET recognizes the best research in, contributions to, and accomplishments in all areas of pharmacology. We encourage members to nominate deserving scientists to raise awareness of the outstanding work being done in the field of pharmacology.
ASPET is strongly committed to diversity. Nominations for members of underrepresented groups, women, and persons with disabilities are particularly encouraged.
Who can submit a nomination?
You must be an ASPET member to submit nominations.
Who is eligible to receive awards?
Scientists, educators, and others who have impacted the field of pharmacology from all over the world and at all career stages are eligible for ASPET’s various awards. Learn more about the specific eligibility details for each award at http://www.aspet.org/ awards.
How do I submit a nomination?



To nominate someone or yourself, visit: http://www.aspet.org/awards. Review the award criteria and nomination requirements. Nomination forms can be accessed via the Awards Portal.
When are nominations due?
The deadline for nominations is Friday, October 31, 2023, at 5:00 PM EDT.
What happens after a nomination is submitted?
Each nomination is reviewed by the members of a designated committee. Scores and rankings are given, and compiled results are discussed by the committee, leading to the final selection of the 2024 awardee.
ASPET Scientific Achievement Awards
JOHN J. ABEL AWARD IN PHARMACOLOGY

This award is presented for original, outstanding research in the field of pharmacology and/or experimental therapeutics by a candidate who is younger than 45. Named after the founder of ASPET, the award aims to stimulate fundamental research in pharmacology and experimental therapeutics by young investigators.
JULIUS AXELROD AWARD IN PHARMACOLOGY
This award is presented for significant contributions to understanding the biochemical mechanisms underlying the pharmacological actions of drugs and for contributions to mentoring other pharmacologists. It honors the memory of the eminent American pharmacologist who shaped the fields of neuroscience, drug metabolism, and biochemistry and who served as a mentor for numerous eminent pharmacologists around the world.
GOODMAN & GILMAN AWARD IN


RECEPTOR PHARMACOLOGY
This award was established in 1980 to recognize and stimulate outstanding research in the pharmacology of biological receptors. Such research might provide a better understanding of the mechanisms of biological processes and potentially provide the basis for the discovery of drugs useful in the treatment of diseases.
The Pharmacologist • June 2023 8
OTTO KRAYER AWARD IN PHARMACOLOGY
This award is presented to commemorate the enduring legacy of Otto Krayer’s personal qualities: his ethical behavior, his commitment to teaching, his high standards of scientific scholarship, publication and editorship, his promotion of interdisciplinary research to reveal the actions of drugs or other chemicals, and his guidance and support of younger scientists. The award recognizes an individual whose character and career contributions to pharmacology are in accord with those exemplified by Dr. Krayer.

ROBERT R. RUFFOLO CAREER ACHIEVEMENT AWARD IN PHARMACOLOGY

This award honors the scientific achievements of scientists who are at the height of their careers (typically mid-to late-career) and who have made significant contributions to any area of pharmacology. It recognizes the contributions made to drug discovery and development by Dr. Ruffolo.
PHARMACIA-ASPET AWARD IN EXPERIMENTAL THERAPEUTICS



This award recognizes and stimulates outstanding research in pharmacology and experimental therapeutics, basic laboratory, or clinical research that has had, or potentially will have, a major impact on the pharmacological treatment of disease.
DAVID LEHR RESEARCH AWARD
This award is intended to extend funding for preclinical or clinical research directed toward improving human health. This award is made possible by an endowment to ASPET from Mrs. Lisa Lehr in honor of her husband, the late Dr. David Lehr, former chair of the Department of Pharmacology of New York Medical College. It includes two years of funding at $50,000 per year.
Are you an ASPET emeritus member in need of travel funding to attend the ASPET 2024 Annual Meeting?
E. LEONG WAY EMERITUS TRAVEL


AWARD
The E. Leong Way award provides financial support to defray the expenses for an ASPET emeritus member to attend the ASPET Annual Meeting. The award honors Edward Leong Way (1916-2017), who is remembered for his contributions to drug metabolism research, opioid pharmacology, and a western understanding of Chinese traditional medicine, as well as the numerous scientists he mentored over 75 years of his professional life. Selfnominations are permitted.
ASPET Division-Sponsored Awards
EARLY CAREER AWARDS
Division-sponsored early career awards are intended for ASPET members who are past the postdoc or trainee career stage but still early in their careers (no more than 15 years after receiving their doctorate). Applications and nominations are welcome from members in academia, industry, government, or other organizational affiliations.
Sponsored by the ASPET Division for Behavioral Pharmacology
The JH Woods Early Career Award in Behavioral Pharmacology recognizes outstanding original research by early career investigators in behavioral pharmacology.
Sponsored by the ASPET Division for Cardiovascular Pharmacology
The Benedict R. Lucchesi Young Scientist Travel Award in Cardiac Pharmacology honors Dr. Lucchesi’s lifelong scientific contributions to our better understanding and appreciation of the pharmacological treatment and prevention of cardiovascular disease and for his mentoring of countless prominent
9 The Pharmacologist • June 2023
cardiovascular pharmacologists in translational approaches. The award recognizes a member early in their career whose research interest is related to cardiovascular pharmacology.
Sponsored by the ASPET Division for Molecular Pharmacology
The Division for Molecular Pharmacology Early Career Award recognizes scholarly achievements of investigators early in their independent careers in molecular pharmacology.
Sponsored by the ASPET Division for Neuropharmacology
The Division for Neuropharmacology Early Career Award recognizes and honors a young independent investigator who is working in any area of neuropharmacology. Preference is given to candidates who hold an independent position. An independent position is considered to be one that is responsible for securing and administering their own budgets for research (traditionally a faculty position, or a team leader in a non-university setting).
Sponsored by the ASPET Division for Pharmacology Education Pharmacology Educators Awards defray costs to participate in the ASPET Annual Meeting. They are available for pharmacology educators who have relatively less experience as a pharmacology educator and/or junior faculty members (e.g., assistant professor). In addition to promoting participation in the ASPET meeting by pharmacology educators, this award is intended to foster career development in pharmacology education. Applicants must have significant teaching responsibilities in pharmacology, either graduate, undergraduate college classes, or professional schools.
Sponsored by the ASPET Division for Toxicology
The Division for Toxicology Early Career Award recognizes excellent original research by early career investigators in toxicology.
Sponsored by the ASPET Division for Translational and Clinical Pharmacology





The Division for Translational and Clinical Pharmacology Early Career Awards recognize excellence in translational and clinical pharmacology research that comes from early career scientists. The purpose is to provide travel support to defray costs for two members to participate at the ASPET Annual Meeting.
Other Division-Sponsored Awards
ASPET is strongly committed to diversity. Nominations for members of underrepresented groups, women, and persons with disabilities are particularly encouraged.

Sponsored by the ASPET Division for Cancer Pharmacology
The Susan B. Horwitz Award Lecture in Cancer Pharmacology recognizes excellent original research by established investigators in cancer pharmacology. The award honors Dr. Horwitz who has been a pioneer in understanding the mechanism of action of cancer chemotherapy drugs many of which have been and remain mainstays of cancer therapy.
Sponsored by the ASPET Division for Cardiovascular Pharmacology
The Division for Cardiovascular Pharmacology Mid-Career Award recognizes and honors those who are working in any area of cardiovascular science. The award is open to mid-career stage, independent investigators from all types of organizations including academia, industry, private or government institutes who are primary members of the division.

The Pharmacologist • June 2023 10
Sponsored by the ASPET Division for Drug Discovery and Development

The Scientific Achievement Award in Drug Discovery and Development recognizes outstanding investigators who have made significant contributions in drug discovery, translational and/ or drug development science. This can include investigators who have developed technologies, methods or processes that have enhanced the process of drug discovery or enabled accelerated drug development. Contributions to any therapeutic area or therapeutic modality (small molecule, oligonucleotide, gene therapy, biologic or drug-device combination) will be considered.
Sponsored by the ASPET Division for Drug Metabolism and Disposition
The B.B. Brodie Award in Drug Metabolism and Disposition honors the fundamental contributions of Bernard B. Brodie in the field of drug metabolism and disposition. The award is presented biennially in even years to recognize outstanding original research contributions in drug metabolism and disposition, particularly those having a major impact on future research in the field.
Sponsored by the ASPET Division for Drug Metabolism and Disposition

The James R. Gillette Awards are presented each year to two outstanding papers published in Drug Metabolism and Disposition, one each in the broad categories of a) drug metabolism and b) disposition and pharmacokinetics. No additional application is needed. All articles published in DMD 2023 issues will be considered.




Sponsored by the ASPET Division for Neuropharmacology
The Division for Neuropharmacology Diversity, Equity and Inclusion Recognition Award (DEI Recognition Award) honors an individual who has made important contributions to the enhancement and promotion of diversity, equity and inclusion in the field of neuropharmacology. Applicant should have mentored trainees from diverse, underrepresented backgrounds as defined by NIH
Sponsored by the ASPET Division for Pharmacology Education Pharmacology Educators Awards defray costs to participate in the ASPET Annual Meeting, are available for pharmacology educators at all career levels who are faculty members. Applicants must have significant teaching responsibilities in pharmacology: either graduate, undergraduate college classes, or professional schools.
Sponsored by the ASPET Division for Toxicology
The Division for Toxicology Career Award recognizes outstanding original research contributions to toxicology by an established investigator.
Recognize someone who has made an impression on you and your career. Submit your nomination by October 31, 2023. Awards will be presented at the ASPET 2024 Annual Meeting. Visit http://www.aspet.org/awards to learn more.

11 The Pharmacologist • June 2023
Submit Your Nomination
Meet the 2023-2024 Council








ASPET’s newly elected Council members start their new positions July 1, 2023. ASPET is governed by an elected council consisting of the President, the President-Elect, the immediate Past President and the Secretary/ Treasurer, the Secretary/Treasurer-Elect and the immediate Past Secretary/Treasurer (each serving for a oneyear term in that elected office). Each of the three Councilors serves a three-year term in that elected office, with one Councilor retiring each year. In addition to the elected members of Council, the Executive Officer, the chair of the Board of Publications Trustees, the chair of the Program Committee, and the FASEB Board representative are non-voting ex officio members of Council during their terms in the respective offices.

The Pharmacologist • June 2023 12
Namandjé N. Bumpus, PhD President
Carol L. Beck, PharmD, PhD President-Elect
Michael F. Jarvis, PhD, FBPhS Past President
Xinxin Ding, PhD Secretary/Treasurer
Pamela Janet Hornby, PhD Secretary/Treasurer-Elect
Kathryn A. Cunningham, PhD Past Secretary/Treasurer
Get to Know ASPET’s Leadership








Reach out and connect with ASPET’s Council members on ASPETConnect. https://connect.aspet.org

13 The Pharmacologist • June 2023
Amy C. Arnold, PhD Councilor
Nina Isoherranen, PhD Councilor
John R. Traynor, PhD Councilor
Carol Paronis, PhD Ex Officio Chair, Program Committee
Jerry Madukwe, PhD Ex Officio FASEB Board Representative
Ashim Malhotra, PhD Ex Officio Chair, IDEA Committee
Kenneth Tew, PhD Ex Officio Chair, Publications Committee
David Jackson Ex Officio Executive Officer
Dianicha Santana, PhD Ex Officio Chair, Young Scientists Committee
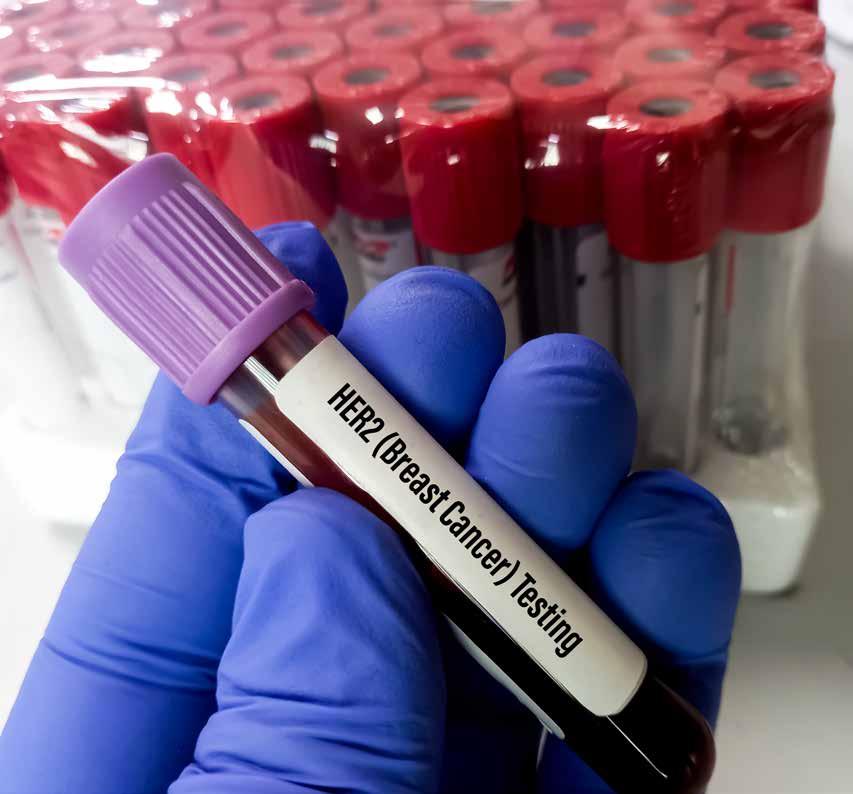
Herceptin: A Breast Cancer Breakthrough

Rebecca J. Anderson, PhD
In the summer of 1990, Barbara Bradfield, a 48-yearold former school teacher in Burbank, Calif., discovered a mass in her breast and a lump under her arm (1, 2). Only a few months earlier, her mammogram had failed to detect them “It grew that fast” (2). A biopsy confirmed she had breast cancer, and it had spread to her lymph nodes.
Lacking confidence in her original doctors’ approach, Barbara took matters into her own hands. She searched and found a more amiable oncologist in Burbank. He prescribed six months of chemotherapy to shrink the tumor, followed by a bilateral mastectomy. Then, more chemotherapy and radiation for nearly seven months. The treatment ended in the spring of 1991 (1, 2). In August 1992,
Barbara felt a spongy grape-sized mass above her collarbone (1, 2). A biopsy confirmed that her cancer had returned, and a CAT scan revealed another lesion in her lung. Her Burbank doctor suggested high-dose chemotherapy. But chemotherapy had not worked the first time, and there was no reason to think it would work now. “Done with that,” she said. “If I’m going to die, I don’t want to die bald and throwing up” (2)
Instead, Barbara considered alternative treatments, like nutritional supplements, psychotherapy, and homeopathy. She enrolled in a herbal therapy program, drank vegetable juices, and planned a trip to a clinic in Mexico (1, 2).

Something neu
A decade earlier, Robert Weinberg at the Massachusetts Institute of Technology perfected a technique for isolating cancer-causing genes (called oncogenes) from cancer cells. In 1982, one of his postdoctoral fellows, Lakshmi Padhy, found an interesting oncogene in a rat neuroblastoma tumor (1, 2). Because it came from neuroblastoma, Weinberg named the
The Pharmacologist • June 2023
Barbara Bradfield
oncogene “neu.”
Most oncogenes coded for proteins that were sequestered inside the cell, but neu was different. The neu-generated protein was on the cell membrane surface, making it much more accessible to drug treatment. As part of his research, Padhy had created an antibody against this neu protein (1, 3). It would have been easy for Padhy and Weinberg to take the next step: to see if this antibody bound to the neu protein and hampered growth of the cancer cells. But they didn’t.
Likewise, other researchers failed to see the significance of Padhy’s results, which were published in the prestigious journal, Cell. Weinberg later said, “It would have been an overnight test…If I had been more studious and more focused…I would have made that connection” (1).
They Saw HER Standing There
Axel Ullrich, a universally recognized master gene cloner, received his doctorate in Germany. In 1975, he joined the Biochemistry Department of the University of California San Francisco. In neighboring UCSF labs, Harold Varmus (later, Director of the National Institutes of Health (NIH)) and Michael Bishop were conducting their Nobel Prize research on oncogenes, and Herb Boyer (later, a co-founder of Genentech) was developing innovative genetic engineering techniques (2, 3).
One of Ullrich’s early successes at UCSF was to isolate the gene that produces insulin (2). By incorporating this human gene into a bacterial genome, the bacteria became little insulin-producing factories, and that greatly facilitated the production of human insulin.
In 1977, Ullrich and several of his UCSF colleagues moved to South San Francisco to work at newly founded Genentech (2). Ullrich’s first cancer research involved epidermal growth factor (EGF). Mike Waterfield, a protein chemist in London, asked Ullrich to work backwards from the EGF receptor protein to


identify the gene that codes for it (2, 3)
Ullrich and Waterfield continued collaborating, and in 1984, they offered the first evidence connecting growth factors to cancer (2, 3). In fact, almost all oncogenes are mutated forms of the genes that regulate cell growth and division.
Ullrich and his Genentech team looked for DNA sequences that were similar to Waterfield’s human EGF receptor gene (which was designated as EGFR, or Her-1). The first look-alike gene they discovered was named Her-2 (human EGF receptor gene 2) (2-4).
When Ullrich cloned the protein that was produced by the Her-2 gene, it turned out to be the same protein that Padhy had identified on the mouse neuroblastoma cells. Ullrich had independently rediscovered the gene corresponding to Weinberg’s neu oncogene (1, 2, 4)
In the early years after this discovery, the gene was commonly known as Her-2/neu, in deference to both Weinberg and Ullrich’s work (2). Now, most researchers and clinicians simply call it HER2.
In the summer of 1986, Ullrich presented a seminar at UCLA on his HER2 findings and acknowledged Weinberg’s prior work. Among those in the audience was Dennis Slamon, a UCLA oncologist who was already searching for ways to cure cancer (1, 2).
Mining Coal, Then Cancer
Dennis Slamon came from a family of Appalachian coal miners. Scholarships allowed him to attend college and the MD-PhD program at the University of Chicago. His 1975 doctoral dissertation was in cell biology, and after his residency, in 1979, he joined the Department of Hematology-Oncology at UCLA (2).
A tall, gentle man, Slamon was a down-to-earth and accessible physician (5). His patients described him as caring and attentive. In 1982, a medical school friend referred a patient to him. The patient, Brandon Tartikoff, was 30 years old, head of television entertainment at NBC, and had been unsuccessfully
The Pharmacologist • June 2023 15
Axel Ullrich
Dennis Slamon
treated for Hodgkin’s disease for four years (2)
Slamon was just a junior faculty member with limited experience and was reluctant to see Tartikoff. But he thought he could handle a “simple” case of Hodgkin’s disease. He prescribed an aggressive course of experimental chemotherapy. After a year and 9 cycles of drug treatment, Tartikoff went into remission and remained healthy for the next 15 years (2).
Clinical Proof
Slamon treated patients with all forms of cancer, but he was most energized and animated when talking about his laboratory research and its clinical applications. One reporter called him a “velvet jackhammer” for his combination of smoothness and tenacity (1). His active research team of technicians, students, postdoctoral fellows, and colleagues were laser-focused on finding a superior cancer treatment (2, 6).
In 1982 (the same year that Weinberg’s lab isolated the neu oncogene), Slamon submitted a grant proposal to NIH for funds to build a bank of human tumor cells (5). He wanted to screen the cells for specific genetic alterations. NIH turned down his proposal, but Slamon pressed ahead anyway. With support from the Jonsson Cancer Center Foundation and UCLA, he collected tumor cells that had been discarded after surgery for breast, prostate, colon, and lung cancer (1, 5)
Slamon was screening specifically for genes that regulate cell growth in those tumors (2, 5, 6). So, Ullrich’s seminar at UCLA in 1986 particularly piqued his interest.
That evening at a Thai restaurant in Santa Monica, Slamon and Ullrich forged a unique but very productive collaboration (2). Ullrich would provide samples of DNA from his gene collection, and Slamon would try to match them to the DNA he had extracted
from his banked tumor cells. The goal was to identify which growth factor genes, if any, caused cancer (1, 2).
Ullrich did not inform Genentech of this collaboration, but he protected the company’s patent rights by coding the samples that he sent to Slamon (2). After several months of systematic research, Slamon found a match with certain breast and ovarian cancers (1-3). Ullrich then revealed that the gene was HER2.
In most cases, a gene mutation causes the production of either a defective protein or no protein at all. To Ullrich and Slamon’s surprise, the breast and ovarian cancer cells produced the normal HER2 protein, but it was produced in abnormally high amounts. This overexpression amplified the protein’s effect, and the cells grew out of control. That is, they became cancerous. When copies of the HER2 gene were incorporated into normal cells in tissue culture, they were transformed into malignant cells (2)
Slamon had over 30 breast cancer tumors in his collection, but he did not know the patients’ outcomes. On the other hand, William McGuire in San Antonio had a sizeable collection of tumors and the corresponding women’s medical histories (2, 3)
Slamon and McGuire collaborated and found that the HER2 gene was amplified in 25-30% of the human breast cancer tumors in their collection (7, 8). Also, the women whose tumors carried multiple copies of the HER2 gene relapsed more quickly and died sooner than those whose tumors contained only one copy (7, 8).
Making a Drug
Although their results appeared in Science in 1987, the scientific community, for the most part, was not interested. Nevertheless, Ullrich and his small group of Genentech researchers continued their work, hoping to develop an anti-cancer drug (2). A compound (such as an antibody) that bound to the HER2 receptor on the cell’s surface would impede signal transduction and, hopefully, suppress the tumor’s growth (4).
At Ullrich’s request, scientists in Genentech’s Immunology Division produced a series of mouse monoclonal antibodies against the HER2 protein (2, 3). Their top pick was 4D5. It bound selectively to the HER2 receptor protein and not to other closely related human EGF receptors (3-6)
When Ullrich and Slamon added 4D5 to HER2-cancer
The Pharmacologist • June 2023 16
"In most cases, a gene mutation causes the production of either a defective protein or no protein at all."
cells in tissue culture, the cells stopped growing and became normal (1, 2, 5). When they washed away the monoclonal antibody, the cancerous growth returned. Also, as they hoped, 4D5 had no effect on other cells in the tissue culture.
The next step was testing the antibody in an animal model. In collaboration with Pepino Giovanella in Houston, Slamon injected 4D5 into mice, and their overexpressed HER2 tumors shrank (1, 2). There was no effect in mice whose tumors did not overexpress HER2. Other researchers confirmed their findings.
Genentech Hesitates
Genentech owned the rights to this promising 4D5 antibody, but the company’s executives were not interested in investing in another cancer drug (2).
In 1982 (while Padhy was discovering the neu oncogene and Slamon was submitting his failed grant proposal to NIH), Genentech perfected Ullrich’s genetically engineered insulin. This recombinant human insulin was the company’s first major accomplishment. But rather than marketing it, they licensed the technology to Eli Lilly, a major insulin manufacturer (2).
The company had invested heavily in gene-based cancer treatments, but the clinical trials failed (2). Other biotech companies had investigated monoclonal antibodies to treat cancer, but their attempts also failed. Genentech executives doubted that any monoclonal antibody could penetrate a solid tumor (4). And at best, the 4D5 antibody would benefit only one-quarter of breast cancer patients—those whose tumors overexpressed HER2.
Genentech had disbanded its oncology clinical trial staff and pulled funding from most of its cancer projects. There was no interest in pouring millions of dollars into another long-shot cancer drug. Frustrated by Genentech’s decision, Ullrich left the company to pursue his research elsewhere (1, 2).
“It’s Real”
Mike Shepard took over the HER2 lab program, and his small cadre of ardent Genentech scientists persevered. Despite limited resources, they improved the production and purification of 4D5 (1-3). By 1989, Shepard’s team and Slamon knew this was a promising new therapy for breast cancer.

While Shepard kept lobbying unsuccessfully for support from Genentech’s senior management, Slamon pushed even harder from outside the company (1, 2) . He did not have the resources to develop the drug himself. A persistent gadfly, he repeatedly phoned the Genentech executives and often flew to San Francisco, using all his skills to charm them (1, 2) . “You’ve got something real here. If you don’t want to make it, license it out” (2)
Mike Shepard
Along with his persistent lobbying, Slamon forged ahead in his own lab. And his professional stature was growing. In 1988, he became director of clinical research at the Jonsson Comprehensive Cancer Center at UCLA, one of NIH’s research centers. In 1991, he was named chief of the Division of HematologyOncology at UCLA School of Medicine (6). And money was no longer a problem.
Lilly Tartikoff, Brandon’s wife, was grateful for Slamon’s care of her husband and wanted to contribute to his research. For several years, Slamon resisted, not wanting to take advantage of a wealthy patient. But Lilly persisted and Slamon finally relented (2).
Lilly reached out to Ronald Perelman, chairman of the cosmetic firm Revlon, which prided itself on appealing to ordinary American women. She thought it was reasonable and a public service for Revlon to support research of a disease that primarily affects women (5). Revlon’s executives did their due diligence by visiting Slamon and were impressed.
In 1989, Revlon pledged $800,000 per year for three years, a total of $2.4 million ($5.8 in today’s currency). It was an unprecedented level of support from an American corporation for a single scientific group, and the funds were unrestricted (5, 6).
The Revlon Women’s Cancer Research program was established at UCLA with Slamon as its director (5) Lilly also launched a series of fundraisers, tapping into her extensive network of Hollywood entertainers, who all made generous donations. Slamon was now
The Pharmacologist • June 2023 17
bringing in more research money than any other UCLA faculty member (2).
Flipping the Switch
In late 1989, Shepard and Slamon finally convinced a vice president on Genentech’s development committee (whose mother had developed breast cancer) to champion the HER2 project. “Just like that, one man flipped the switch on HER2” (2).
Because 4D5 was a mouse monoclonal antibody, it would be recognized as foreign by the patient’s immune system. Even if it successfully inhibited the HER2 receptor on the tumor cells, it would be rapidly destroyed. Repeated administration would trigger a stronger immune response and probably inactivate 4D5 before it reached the tumor.
Coincidently, Genentech and a few other companies were developing a new technology to “humanize” monoclonal antibodies (2). Paul Carter, a 29-year-old Englishman, learned the technique in England and had recently joined Genentech.
In January 1990, Carter began work to produce a hybrid gene that coded for a “humanized” version of 4D5. It would retain just the bits of 4D5 that bind to the HER2 receptor and replace the rest with human antibody sequences (3, 4). Hopefully, the humanized antibody would not cause an inflammatory response.
Proving the Concept
Unfortunately, Genentech’s revenues were not keeping pace with its research expenses. The company marketed three products. Human growth factor, which prevented dwarfism in children, was never a big seller. Pulmozyme, a treatment for cystic fibrosis, was another specialty product. And Activase
(t-PA) dissolved blood clots to prevent heart attacks, but studies were showing that t-PA was no better than a competing drug that sold for one-tenth the price (2)
In February 1990, Roche Holding, Ltd., paid $2.1 billion for 60% of Genentech’s stock, with an option to buy the remaining shares over several years. One of the new owner’s actions was to cut the HER2 budget (2).
Genentech’s senior management defied that budget cut and continued to support HER2 (2). They authorized $3 million, which supplemented Slamon’s Revlon funds, to begin clinical testing. Slamon had already established a network of doctors in southern California who would refer patients to him. He thought he could conduct the bulk of the HER2 clinical trials at UCLA (2)
As part of the planning for the clinical trials, Genentech and Slamon developed clinical diagnostic tests to detect HER2. They needed to confirm that the women’s tumors overexpressed HER2, which was a requirement for enrollment (4)
While awaiting delivery of Carter’s humanized antibodies, they tested 4D5 in volunteers. Because of the anticipated immune response, 4D5 could be given to each woman only once. The trial enrolled 20 women with highly advanced breast or ovarian cancer, and they all overexpressed HER2 (2).
This “proof-of-concept” trial confirmed that a radiolabeled form of 4D5 bound selectively to the women’s HER2 cancer cells and caused no side effects (2, 4, 5).
Humanizing HER
Paul Carter made a series of humanized antibodies. One of them bound to the HER2 receptor three-times more tightly than 4D5. In tissue culture, it blocked proliferation of the HER2-positive breast cancer cells as effectively as 4D5 (3, 4). Preclinical tests showed that this humanized antibody, which was named trastuzumab, was non-toxic to animals.
Genentech wanted influential oncologists to conduct the Phase I trial and administer trastuzumab as a single treatment. Slamon, unfortunately, was not yet considered “influential,” and he wanted to give trastuzumab in combination with cisplatin. Cisplatin was a harsh drug and useless against breast cancer, but Slamon had shown that it enhanced the effect of
The Pharmacologist • June 2023 18
"This 'proof-of-concept' trial confirmed that a radiolabeled form of 4D5 bound selectively to the women’s HER2 cancer cells and caused no side effects."
the HER2 antibody in lab tests. At Genentech, Shepard confirmed those results, and he also advocated using the cisplatin-trastuzumab combo (2)
The HER2 clinical team compromised. The influential oncologists at Sloan Kettering and UCSF tested trastuzumab alone in women with advanced HER2positive breast cancer. At UCLA, Slamon was allowed to test trastuzumab in combination with cisplatin (2)
Barbara Agrees
In the summer of 1992, when Barbara Bradfield rejected her oncologist’s suggestion for a second round of chemotherapy, he asked her permission to send samples of her cancer cells to Slamon at UCLA (1). She said, “I don’t care. Go ahead” (2). She had plans to spend three weeks at a cancer clinic in Tijuana.
Shortly before she left, Slamon called and said that her tumor had one of the highest levels of amplified HER2 that he had ever seen. She was an ideal candidate for the Phase I trial, but she politely turned him down (1, 2)
The next morning, Slamon called again. Her decision had troubled him all night, and he urged her to reconsider (1, 2). He invited her to his lab at UCLA, where he convincingly described his research and the Phase I protocol in detail. Women would receive a three-month regimen of trastuzumab and cisplatin. Barbara decided to sign up.
At this point, Barbara’s lung tumor had metastasized to 16 new lesions, in addition to her neck tumor. The Phase I trial was designed to assess only safety, but within two weeks, Barbara’s neck tumor visibly shrank. After two months of treatment, it disappeared completely, and the lesions in her lungs had been reduced from 16 to about five (1, 2)
At the end of the three-month trial, Slamon and Genentech decided that the drug combination worked well enough to extend treatment for another three months, at least for some of the participants, including Barbara. Her extraordinary response continued. She eventually discontinued cisplatin because it caused nerve pain and hearing loss (2). Repeated tests showed her to be totally cancer-free (1, 2)
Not everyone was as fortunate as Barbara. Although half of the women experienced benefits, if temporarily, only five of the original 15 volunteers completed the 6-month trial. The results at Sloan Kettering and UCSF
with trastuzumab alone were similar (2). Considering that these women were all thought to be beyond any treatment, the temporary remissions were powerful evidence of efficacy (1, 2).
The Phase I trial also demonstrated that repeated administration of trastuzumab did not trigger an immune response. And, it lacked the harsh side effects of chemotherapy drugs.
Things Get Active
By the summer of 1993, as Genentech was planning the Phase II trial, news of the Phase I results raced through the community of breast cancer patients, especially in San Francisco. Desperate women pressed Genentech for access to this new drug (1, 2)

Genentech resisted the pressure. Allowing women who did not fit the protocol’s strict enrollment criteria could severely compromise the data and might even invalidate the results. The Phase II trial was kept small and focused: 27 patients at Sloan Kettering, 16 at UCSF, and 39 at UCLA (1, 2)
But the activists’ protests and demonstrations created a public relations headache. Genentech realized they were dealing with an emotional and political issue that would not be appeased or deflected with intellectual and scientific responses. In early 1995, they acknowledged that the activists had a valid point of view and invited representatives to join the HER2
The Pharmacologist • June 2023 19
Barbara Bradfield
team as advisors (1, 2). They also agreed to permit compassionate drug access.
The problem was that supplies of trastuzumab were limited, and the clinical testing was already highly complex (2). The solution was a lottery. HER2-positive patients who had failed other treatment applied to the lottery, and about a dozen designated hospitals across the country treated the lottery “winners,” who were chosen at random. In exchange for the drug, the participating hospitals agreed to follow a specific protocol, guaranteeing that Genentech would get data on each woman (1, 2).
To avoid ethically difficult decision-making, Genentech outsourced management of this compassionate access program to an independent company (1). More than 300 women got the drug through the lottery, in parallel with Genentech’s clinical trials. In fact, data from the lottery patients ultimately helped Genentech win FDA approval (2)
The Critical Trials
While the Phase III trials were being planned, Genentech’s marketing department settled on a market-friendly trade name for trastuzumab. They named it Herceptin®: “Her” from the gene’s name and the obvious connection to a woman’s disease, “cept” for the drug’s ability to intercept the HER2 target protein, and “in” for its inhibitory action (1, 2)
The Phase III clinical program consisted of three trials. Trial 648 was the largest and would provide definitive data. As such, it would be randomized, double-blind, and placebo controlled.
Two smaller trials of 200 patients each would provide supporting data and were open-labeled. Trial 649 enrolled women whose metastatic disease had failed to respond to chemotherapy, and they received Herceptin alone. Trial 650 enrolled women who had newly diagnosed metastatic disease but did not want any chemotherapy. They were also offered Herceptin alone.
Because Genentech lacked experience in running Phase III clinical trials and had no oncologists on staff, Covance (a clinical research organization) was contracted to establish the trial infrastructure, recruit investigators, and monitor the clinical sites. By June 1995, Covance had set up 99 clinical sites in the US, seven in Canada, 33 in Europe, 10 in Australia, and
one in New Zealand (2)
In February 1995, Ginger Empey, a 50-year-old nurse in Bakersfield, CA, was diagnosed with breast cancer, and it had metastasized to her liver, ribs, and spine (2, 9). Unfortunately, surgery and chemotherapy in Bakersfield had no effect. Then, with Stage Four cancer, she received several rounds of chemotherapy at UCLA. There was no improvement, and the paclitaxel chemotherapy caused permanent nervous system damage (9). In June, her UCLA oncologist said, “Get your affairs in order” (9). A few days later, she called Dennis Slamon’s group.

Through her cancer support group, Ginger had learned about Slamon’s research and clinical trials (9). She submitted her tumor cells, which were highly HER2positive and qualified her for Trial 649. Unfortunately, arrangements for starting the trial were progressing slowly that summer. Ginger persisted. “I got real frisky” (9). She repeatedly called Slamon’s clinical coordinator and pushed the clinic to implant the infusion port, so she would be ready for her first treatment (9).
Finally, on August 31, 1995, Ginger received her first Herceptin infusion, the first patient in Trial 649. With that, Genentech’s Phase III program officially began (2). After 12 weeks, Ginger’s tumors had shrunk by 25%, and each following checkup showed them shrinking further (9)
Fairly quickly, other women around the world also enrolled in Trial 649 (2). Unfortunately, the pivotal 648 trial was having serious problems.
Problems and Solutions
At the end of 1995, only 21 of the required 480 patients had enrolled in Trial 648 (2, 10). The original protocol randomly divided women who were newly diagnosed with metastatic breast cancer into two groups. Half of them got chemotherapy plus Herceptin. The other half received chemotherapy plus a placebo infusion (11). The treating physicians
The Pharmacologist • June 2023 20
Ginger Empey
monitored the patients for evidence of disease progression, at which point they were guaranteed access to Herceptin (10)
The FDA had insisted on a placebo arm and blinded evaluations, to ensure that the tumors were assessed impartially. But many patients disliked being subjected to the onerous infusion procedures when they might not be getting this new and promising drug (2).
Genentech also worried that doctors would be tempted to prematurely declare disease progression, so that the patient could be switched to Herceptin (10). Individual patients might benefit from the premature switch, but this could endanger Genentech’s ability to draw definitive conclusions about Herceptin’s efficacy.
To encourage enrollment, the Genentech team, in conjunction with Covance, made a number of changes to the 648 protocol. The biggest changes (in the summer of 1996) were to make the trial essentially open-label and to ensure that patients received a placebo for the shortest possible time. To eliminate bias by the treating physicians (who now knew the treatments of each patient), tumor evaluations were handled by an independent panel (2, 10).
This independent Tumor Response Evaluation Committee consisted of 10 radiologist-oncologist pairs, who did not know which patients were receiving Herceptin (2). The radiologists read the X-ray films, and the oncologists evaluated the lesions and assigned a response category using standardized definitions. Covance established procedures for checking inter-reader variation and ensuring that each pair of reviewers used the same criteria for assessing disease progression (10)
Covance also handled all of the logistics regarding shipment of materials and communications. The Committee’s assessments were fully integrated into the treating physicians’ activities, and information and decisions flowed seamlessly between the reviewers and the clinical sites (10)
Genentech and Covance managed to convince FDA officials, who were initially cool to the protocol changes (2) Those changes increased the enrollment rate by 500%, and all patients were enrolled by March 1997. Because of its success, this innovative solution inspired use of similar Committees in clinical trials of other drugs (10).
Race to The Finish
At that time, the average woman treated for metastatic breast cancer would suffer a recurrence 9 months after beginning treatment. Based on that, the Genentech statisticians calculated that they would need to follow all the women for 12 months to determine whether Herceptin offered any meaningful benefit (2, 10).
But because HER2-positive tumors were particularly aggressive, the women in the placebo group were experiencing cancer recurrence far more rapidly. As a result, the trial reached the time-to-progression endpoint faster. Trial 648 concluded five months earlier than the statisticians had originally calculated (2, 10)
The day after Thanksgiving 1997, Steven Shak, Genentech’s Herceptin team leader, flew to Los Angeles carrying a bulky briefcase. In the cocktail lounge of the Burbank Airport, Shak showed Slamon the final Phase III clinical results (2, 5).
Nearly twice as many women given the combination of Herceptin and chemotherapy saw their tumors shrink or disappear, compared to those taking chemotherapy alone (11). The Herceptin combination increased the time to disease progression by 65% (11).
For women who were taking paclitaxel (the most powerful breast cancer drug available at that time) as their chemotherapy agent, the results were especially impressive. Clinicians knew that when paclitaxel was given alone, it shrank tumors in only 16% of women (2) But nearly 50% of the women taking the combination of Herceptin and paclitaxel benefitted, a response rate unheard of in the investigators’ clinical experience (1,
The Pharmacologist • June 2023 21
"Nearly twice as many women given the combination of Herceptin and chemotherapy saw their tumors shrink or disappear, compared to those taking chemotherapy alone."
11). And unlike chemotherapy, Herceptin had relatively few and comparatively mild side effects.
Shak also briefed investigators at the US National Cancer Institute (NCI). Throughout Genentech’s clinical trials, NCI had shown little interest in Herceptin, but they were impressed with the Phase III results. NCI agreed to take over management of the Herceptin compassionate access program. Its network of 32 comprehensive cancer care centers and several military hospitals offered women a broader choice of locations for receiving Herceptin until the FDA approved the drug (2).
The ASCO Show
Genentech submitted the Herceptin application to the FDA on May 1, 1998. Two weeks later, Slamon and Genentech’s Herceptin team gathered in Los Angeles for the 34th annual meeting of the American Society of Clinical Oncology (ASCO) (2)
On Sunday afternoon, May 17, 1998, most of the 18,000 ASCO delegates packed into the convention center’s massive amphitheater for a special session, “Her-2/ neu in breast cancer” (1, 2). A panel of four speakers presented the preclinical and clinical results. Slamon spoke last and summarized the results of the pivotal 648 trial, along with anecdotes about Herceptin’s lifesaving effect in patients like Ginger Empey and Barbara Bradfield.
Ginger had paid her own way to attend the ASCO meeting. For her, it was thrilling to mingle among the scientists and doctors and thank them personally. She was approaching 3 years of Herceptin treatment and remained cancer-free. “I had gotten my life back again” (2)
Barbara had taken Herceptin for only 18 months and was the longest survivor among all the trial
participants, now more than 6 years (1, 2, 5). Like Ginger, she had been getting phone calls from desperate women who were unable to get into the clinical trials. With Herceptin’s approval, she said, “At least now it’s going to be possible to tell them…what to ask for, and they’ll be able to get it” (2).
Five months later, on September 25, 1998, the FDA approved Herceptin.
It Gets Better
Like most cancer clinical trials, Genentech’s Herceptin trials enrolled patients with metastasized and refractory tumors, where even small benefits of a drug would outweigh the risks. But the real value of Herceptin would come from treating women with early-stage breast cancer and who had never received any prior treatment (1).
In 2003, NCI launched two large multinational trials to examine this question (12). When combined, the results of the two trials showed that the overall survival of women treated with Herceptin was increased by 33%, an unprecedented benefit in the history of chemotherapy for HER2-positive cancer (1, 12). And Herceptin cut the rates of recurrence in half (12).
In 2006, FDA approved Herceptin for use in patients with early-stage breast cancer, before or after surgery, and either alone or in conjunction with adjuvant (radiation or chemotherapy) treatment (3). From the beginning, investigators noted that patients with the highest HER2 overexpression (like Barbara and Ginger) benefitted most from Herceptin treatment (11). Unfortunately, in some cases, women saw their cancer disappear in the lungs, liver, and bones, only to spring up as brain tumors (2). Recently, new small molecule HER2 inhibitors such as tucatinib penetrate the brain more effectively than Herceptin and have shown some efficacy against brain metastases (13)
Another strategy has also enhanced the efficacy against HER2-positive tumors: linking Herceptin to a “payload” chemotherapy drug. Herceptin seeks out the tumor and binds to the HER2 receptors on the cell surface. The chemotherapy drug is then uncoupled from Herceptin and kills the tumor cells (14, 15). This dual-action compound provides a large therapeutic index with limited systemic toxicity (3).
Herceptin-payload drugs like Enhertu® have proven effective against both advanced metastatic HER2-
The Pharmacologist • June 2023 22
"Herceptin-payload drugs like Enhertu® have proven effective against both advanced metastatic HER2positive breast cancer and 'HER2-low' tumors."
positive breast cancer and “HER2-low” tumors (3, 14, 15) These newly defined “HER2-low” tumors have HER2 expression that is intermediate between the traditional definitions of HER2-positive and HER2-negative, and account for 50-60% of all breast cancers (13-15).
Turning Point
Herceptin marked several major milestones in medicine. It was the first humanized monoclonal antibody approved for clinical use. For the first time, a drug had successfully attacked a genetic alteration in cancer, and it served as a model for many subsequent targeted therapies (3, 5).
This also marked the first time that the FDA had coordinated and simultaneously approved two products that were dependent on each other: Herceptin and the HER2 diagnostic test kit, HercepTest®. HercepTest was needed to identify women whose breast cancer tumors overexpressed HER2, because only they would benefit from Herceptin treatment.
Today, it is possible to profile every patient’s tumor for genomic alterations. Investigators and regulatory authorities now expect clinical trials of a molecularly
targeted therapy to screen patients using genomic diagnostic tests (3).
Because Herceptin lacked the horrendous side effects (hair loss, nausea, infertility, etc.) that limits the use of chemotherapy drugs, it could theoretically be administered indefinitely. Ginger, for example, took Herceptin for more than 10 years and was then considered “cured” (9)
Finally, Herceptin opened the door for a new class of biologic drugs: monoclonal antibodies that were directed against diseases requiring long-term treatment. Newer monoclonal antibodies such as pertuzumab were developed with broader HER2/HER3 binding efficacy. And other types of non-immunogenic monoclonal antibodies have been developed to treat inflammatory diseases, osteoporosis, high cholesterol, infectious diseases, and many other conditions.
Over the years, Barbara and Ginger appeared in news articles about Herceptin, inspiring women to seek treatment (2). They have now remained cancerfree for more than 25 years. In 2019, Dennis Slamon, Mike Shepard, and Axel Ullrich received the LaskerDeBakey Clinical Medical Research Award for their invention of Herceptin (4).
The Pharmacologist • June 2023 23
"Because Herceptin lacked the horrendous side effects (hair loss, nausea, infertility, etc.) that limits the use of chemotherapy drugs, it could theoretically be administered indefinitely."
References
1. Mukherjee S (2010) The emperor of all maladies. Scribner, New York.
2. Bazell R (1998) Her-2: The making of Herceptin, a revolutionary treatment for breast cancer. Random House, New York.
3. Sawyers CL (2019) Herceptin: A first assault on oncogenes that launched a revolution. Cell 179: 8-12.
4. Strauss E (2019) Herceptin—a targeted antibody therapy for breast cancer. Lasker Award for 2019; available from: https://laskerfoundation.org/ winners/herceptin-a-targeted-antibody-therapy-for-breast-cancer/.
5. Gable M (January 1, 2000) The culprit is cancer. UCLA Magazine; available from: https://newsroom.ucla.edu/magazine/cancer-dennis-slamonherceptin-her-2.
6. Marsa L (October 20, 1991) One last chance: Ovarian cancer is killing Diane Hinton, conventional treatments have failed, now her life depends on an experimental therapy that would block the action of a deadly gene. Los Angeles Times; available from: https://www.latimes.com/archives/la-xpm1991-10-20-tm-534-story.html.
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, and McGuire WL (1987) Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785): 177-182.
8. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905): 707-712.
9. Ginger Empey, personal correspondence, March 21, 2023.
10. Robbins-Roth C (April 1999) Genentech, Covance, and Herceptin: Biotech’s unsung heroes. Bioventure View 14(4): 1-4.
11. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med 344(11): 783-792.
12. Romond EH, Perez EA, Bryant J, Suman VJ, GeyerCE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New Engl J Med 353(16): 1673-1684.
13. Med Lett (November 16, 2020) Two drugs for advanced HER2-positive breast cancer. Med Lett 62(1611): 182-184.
14. Ben-Ari E (July 5, 2022) Enhertu improves survival for metastatic Her-2-low breast cancer. National Cancer Institute blog; available from: https://www. cancer.gov/news-events/cancer-currents-blog/2022/enhertu-her2-lowbreast-cancer?cid=eb_govdel
15. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al for the DESTINY-Breast04 Trial Investigators (2022) Trastuzumab deruxtecan in previously treated HER2low advanced breast cancer. New Engl J Med 387(1): 9-20.
Biosketch:
Rebecca J. Anderson holds a bachelor’s in chemistry from Coe College and earned her doctorate in pharmacology from Georgetown University. She has 25 years of experience in pharmaceutical research and development and now works as a technical writer. Her most recent book is Nevirapine and the Quest to End Pediatric AIDS. Email rebeccanderson@msn.com
In the next issue of The Pharmacologist…
Dr. Anderson will feature veterinary pharmacology. Don't miss the September 2023 issue.

The Pharmacologist • June 2023 24
Science Policy News
ASPET Provides Comments to NIH Open


Access Plan
ASPET submitted comments to the National Institutes of Health (NIH) on its Plan to Enhance Public Access to the Results of NIH-Supported Research (Plan). The Plan outlines how NIH will be implementing the Office of Science and Technology Policy’s (OSTP) open access plan which was released in August 2022. The OSTP provided guidance for agencies to update their public access policies as soon as possible to make publications and research funded by taxpayers publicly accessible, without an embargo or cost. All agencies will fully implement updated policies, including ending the optional 12-month embargo, no later than December 31, 2025.
ASPET is generally supportive of the Plan; however, as noted in its comments has concerns about the implementation of the open access plan and its effects on scholarly publications. ASPET encouraged NIH to include supplemental information covering all allowable paths for charging publishing costs, including from indirect costs
and other university general or restricted funds. ASPET also encouraged NIH to include in this guidance coverage for all costs, such as open access fees, page charges, and submission fees among other costs. ASPET encourages NIH to permit CC BY-NC license options that allow for the free reuse of content by the public (in line with the goals of NIH) but not for commercial purposes.
ASPET recommended that NIH not monitor publication fees, which could lead to a system that favors quantity over quality. Any “one-size fits all” pricing structure which is the logical result of this type of monitoring does not enhance the publication’s quality; it just streamlines the bookkeeping. Ultimately, NIH should allow the marketplace and competition between publishers to determine the reasonable publication costs.
ASPET commended NIH for engaging to improve the plan for public access and to develop a policy that allows researchers to comply more readily.
ASPET Responds to NIH Requests for Information
ASPET spent the spring responding to several requests for information (RFI) from NIH that would improve research grant applications and the postdoc training experience.

Proposed Simplified Review Framework for NIH Research Project Grant Applications
In the RFI, Proposed Simplified Review Framework for NIH Research Project Grant Applications, NIH proposed streamlining the factors for reviewers
for NIH grant applications into three main areas: Importance of the Research, Rigor and Flexibility, and Expertise and Resources.
ASPET commended NIH on its proposal and goal to reduce implicit bias and minimize workload within the grant review process. While maintaining the necessary rigor that NIH holds its grantees to, the transition from nine areas of review to three main areas with additional areas shows a willingness to streamline the review process.
25 The Pharmacologist • June 2023
ASPET highlighted that any change to the grant application, review, and award process will take time to become commonplace. During this time of change, ASPET strongly encouraged NIH to work with its stakeholders to prepare the community for this change. NIH is encouraged to provide detailed timelines for not only alerting the community of the change, but also when the change is going to occur. NIH should work with stakeholders on educational materials to train all members of the grant application process so that when the changes are implemented, there is little to no disruption.
Re-envisioning of U.S. Postdoc Training within Biomedical Research Enterprise
In the RFI, Re-envisioning of U.S. Postdoc Training within Biomedical Research Enterprise, the NIH sought information regarding the current state of postdoctoral research training and career progression within the biomedical research enterprise. NIH is particularly interested in understanding the perspective and experience of recent and current postdoctoral trainees, postdoctoral office leaders, as well as graduate students considering becoming postdoctoral trainees within the academic sector.
ASPET shared its belief that an academic postdoc position should be an established and temporary period that has defined goals for the advancement and enhancement of professional skills needed to pursue his or her chosen path whether it is in academia, industry, government, or another area.
ASPET highlighted that the current system needs close oversight that will assist in mitigating some of the concerns outlined below. Along with oversight, better surveying on the following areas will allow NIH
to observe trends and take proactive measures before any pertaining issues escalate. Inequities in postdoc pay create significant financial barriers that can lead to physical and mental health difficulties. Currently, accurate guidance on how postdocs can be paid is lacking from the NIH, which enables institutions to treat postdocs not as employees but independent contractors depriving them of standardized pay, benefits, and employment protection. This creates a barrier, such that only those that have the means to become a postdoc without suffering financial hardships pursue that career path.
ASPET shared that without clear guidance from the NIH on standardized benefits for postdocs, inequities in benefits have emerged within institutions and across institutions that create discrepancies in terms of quality of life. To sustain and enhance quality of life, more and more early career scientists are looking at careers outside of academia for a healthier work/life balance and a better paycheck that they believe they deserve.
ASPET commented that the treatment as a “trainee” is degrading and disingenuous as much of the workforce looks at new PhDs as top candidates and employees and far from a “trainee.” These issues in a work environment manifest as prolonged work hours impacting work/life balance, strained menteementor relationships, with bullying and harassment that can deter and drive postdocs to careers outside of academia. New PhDs would need to be recognized for their professional strengths and skills, rewarded with competitive salaries, enabling work/life balance.
The Pharmacologist • June 2023 26
Washington Fellows Visit Capitol Hill



In April, ASPET hosted its Hill Day for its 2023 Washington Fellows class. This year’s program was the first in person event following COVID. The Washington Fellows met with legislators and staff to advocate for increased funding for biomedical research and the importance of the use of animal models. This year’s class of ten were accompanied by four former fellows
who served as guides. Working in groups of three (two fellows and one guide), the Washington Fellows met with nine House offices and eight Senate offices over the course of the day. The Fellows also were able to attend FASEB’s Congressional briefing on The Value of Large Animal Research in Biomedical, Veterinary, & Agricultural Research Progress.
2023-2024 Washington Fellows Program
27 The Pharmacologist • June 2023
TO REMEMBER Program Applications Open AUGUST 1, 2023 Program Applications Close SEPTEMBER 29, 2023 Program Activities DECEMBER 1, 2023 -- MAY 30, 2024
DATES
Education News



Report from Annual Meeting: Association of American Medical Colleges Council of Faculty and Academic Societies
The Association of American Medical Colleges Council of Faculty and Academic Societies (AAMC-CFAS) held its annual meeting in Salt Lake City, Utah, on April 11-13, 2023. ASPET is a AAMC-CFAS member society. Joe Blumer (Medical University of South Carolina) as senior faculty society member represented ASPET at the meeting. CFAS provides a platform for faculty at academic medical centers to identify critical issues facing medical school faculty and academic societies and provide a voice for faculty in the creation and implementation of AAMC programs. This was the first in-person CFAS meeting since 2019.
Major topics discussed included mission alignment within academic medical centers; leveraging wellbeing to support researchers, educators and clinicians; unintended bias; faculty salary equity; and sustaining faculty for success as medical educators.

A new CFAS committee, Faculty as Medical Educators, was recently established and held its inaugural meeting during the 2023 CFAS spring meeting. Action items for the committee included: sharing best practices regarding how faculty are recognized and compensated for teaching; tension between preclerkship medical educators and increasing student disengagement; the impact of reducing numbers of basic science departments and a concomitant increase in departments of medical education on promotion and tenure policies of educators; and the development of an educator portfolio. In a separate but related plenary entitled “Mission Accepted: Sustaining Faculty for Success as Medical Educators,” the attendees discussed the perspectives of educators, department chairs and deans on medical education.
Three troubling trends were identified regarding sustaining the success of faculty as medical educators: 1) the devolving relationship between students and faculty, 2) student preference for communicating with administrators rather than teaching faculty, and 3) how medical educators are valued. Chairs of basic science departments often feel powerless to make changes in medical education because they have largely been taken out of the planning decisions. From the dean’s perspective, there must be institutional transparency in how departments and individuals get funded for teaching.
The newly renamed Biomedical Research and Training committee held a session entitled, “’I Couldn’t Sleep at All Last Night’: Challenges to Biomedical Researchers and Educators” moderated by Neil Osheroff, PhD (Vanderbilt University) with Martha AlexanderMiller, PhD (Wake Forest) and ASPET member Kelly Quesnelle, PhD (USC School of Medicine-Greenville)
The Pharmacologist • June 2023 28
as speakers. The committee called for increase advocacy to promote the value of research at academic medical centers (AMCs) in a way that would be most persuasive to AMC leadership, particularly those involved in financial decisions who point out that “research costs the medical school money.” Many basic scientists have reported feeling undervalued even though most medical schools are recognized by their research. Committee members also called for action regarding the evolving role of basic scientists in graduate student education, including the structure of graduate education and updating a framework for the relationship between faculty and their students. The AAMC is developoing a framework for aligning the graduate student mentor-mentee relationship, which was last updated in 2016 (https://www.aamc.org/whatwe-do/mission-areas/medical-research/grad-compact).
Mission alignment among the pillars of academic medicine – education, research and patient care – was a consistent theme among many of the plenaries and sessions during the meeting. The opening plenary, “Faculty Thriving in Academic Medicine: From Mission Impossible to Mission Accomplished” pointed out that the strength of AMCs is having faculty participate in a mix of all three of these missions; however, it can often seem like the missions are in conflict from an individual standpoint. An oft-used phrase during the meeting was, “No mission, no margin; no margin, no mission.” Workforce shortages across the clinical enterprise combined with continued financial fallout from the
COVID-19 pandemic and adjustments to the Medicare fee schedule has stretched the patient care mission near its limit. One of the recommendations to address the patient care pillar was for AMCs to better realign for translational science and encourage clinicians to partner with data and quality scientists to get better patient care.

Medical educators face one of the biggest challenges for the education mission, including the lack of a sense of community and a perceived lack of value placed on educators. Institutions should ensure promotion tracks reflect the educational activities faculty do, including mentoring and coaching. AMCs should provide for robust faculty development for teaching and education research, support teaching academies, peer coaching, and educational consults, as well as recognition of exceptional educators with teaching awards.
ASPET member and invited speaker Dr. Kent Vrana (Pennsylvania State University College of Medicine) identified numerous challenges regarding the research mission and called for a re-valuing of research in the tripartite mission. Some of the challenges to research include the widespread recognition that research is expensive, greater emphasis on clinical revenue generation over research and discovery, competition for institutional resources, creation of medical schools with minimized research emphases, and recruitment of non-educator basic scientists. Dr. Vrana pointed out that research crosses all three missions of AMCs and that faculty should find ways to bind these missions together through research.
29 The Pharmacologist • June 2023
Some of the potential solutions included establishing and nurturing clinical and translational science institutes (CTSAs), using team science to support clinician involvement in research (beyond simply being a source of samples), engagement of research scientists in education and development of centers for medical education research.
AAMC Chief Scientific Officer, Ross McKinney, provided an update on research funding. Dr. McKinney pointed out that with the power and control of AMCs shifting away from deans to CEOs of health systems and an increasing focus on margins, academic medicine must keep the focus on our missions of research, education, and patient care. There is the possibility of a 25% cut to the NIH budget, and it may not have a permanent director for another two years due to the current political climate. The funding rate for NIH is still ~30%, but a challenge is that NIH funding is set as criteria for promotion. Inflation is eroding the value of NIH grants (current NIH funding is almost at 2003 levels in inflation-adjusted dollars); if the average grant size increases, then fewer grants will be funded if the NIH budget remains flat. Faculty engaged in these policy conversations will help ensure sufficient appropriations to the NIH budget.
ASPET representatives will continue to engage with CFAS to ensure AMC and the general public hears the voices and concerns of pharmacologists voices, including at CFAS meetings.
Do you have issues you would like raised at the next CFAS meeting? Email your comments to ASPET representatives. Visit the CFAS website for more information and resources: https://www.aamc.org/members/cfas/.

Joe Blumer, PhD
Department of Cell and Molecular Pharmacology and Experimental Therapeutics


Medical University of South Carolina
Charleston, SC blumerjb@musc.edu
Marieke Kruidering-Hall, PhD
Department of Cellular and Molecular Pharmacology
Make ASPET your professional home.
Join ASPET as an undergraduate student for $10/yr or graduate student for $40/yr and take advantage of tools and resources available to advance your career, promote your research and discipline, and build lasting relationships with fellow scientists.
A growing ASPET means greater recognition for the field of pharmacology, more resources and support for our members, and a louder voice with policy makers.
Some benefits include:
• Awards and recognition opportunities
• Networking and mentoring opportunities
Make ASPET your professional home.
• Free membership in ASPET Divisions





Make ASPET your professional home.
University of California San Francisco San Francisco, CA marieke.kruidering@ucsf.edu Join


• Free or reduced registration fees for ASPET webinars or meetings
• Free full-text access to ASPET journals
Join ASPET as an undergraduate student for $10/yr or graduate student for $40/yr and take advantage of tools and resources available to advance your career, promote your research and discipline, and build lasting relationships with fellow scientists.


build lasting relationships with fellow scientists.
For a full listing of our benefits visit www.aspet.org or contact the membership department at membership@aspet.org.
To learn more about ASPET member benefits, visit www.aspet.org or contact the membership department at membership@aspet.org.
A growing ASPET means greater recognition for the field of pharmacology, more resources and support for our members, and a louder voice with policy makers.
Some benefits include:
A growing ASPET means greater recognition for the field of pharmacology, more resources and support for our members, and a louder voice
The Pharmacologist • June 2023 30
ASPET as an undergraduate student for $10/yr or graduate student for $40/yr and take advantage of tools and resources available to advance your career, promote your research and discipline, and
Awards and recognition
Journals News
Drug Metabolism and Disposition Marks Its 50th Anniversary with Celebration
ASPET’s journal Drug Metabolism and Disposition (DMD) is celebrating 50 years of publication in 2023. The journal has published nearly 9,000 papers since 1973. For this anniversary, many special sections and commissioned articles are planned and will be published in a special 50th Anniversary Celebration Collection.
The January 2023 issue contains an editorial by Editor-in-Chief Dr. Xinxin Ding, titled “Celebrating 50 Years of Excellence in DMD Science.” In addition to providing his perspectives on the history and importance of DMD, Ding honored four individuals who have each published 80 or more papers in the
Collection



journal. These individuals, who are recognized as “Most Prolific Author in DMD”, include: Drs. Curtis Klaassen, Miki Nakajima, Scott Obach, and Yuichi Sugiyama. Ding urges all Associate Editors, Editorial Advisory Board members, authors, and the DMD community at large to redouble their efforts to support the journal.
Five commissioned articles were published in the January issue. Articles for the collection will continue to be published in DMD throughout 2023. All content in the DMD 50th Anniversary Celebration Collection is freely accessible through June 2024.
New 2023 JPET Editorial Fellowship Program
We are excited to welcome the inaugural 10 JPET Editorial Fellows! This is a one-year program for individuals with a relevant background to build important skills as a peer reviewer and editor. The program will provide an in-depth opportunity to work interactively with a JPET editor learning important elements of the editorial process including triaging, peer review, and editorial decisions.
Fellows will also participate in JPET editorial board meetings and meet quarterly together to discuss aspects of the editorial process, develop special projects, and write viewpoints on JPET manuscripts.

31 The Pharmacologist • June 2023
Call for Papers on Nanotherapeutics in Cancer Research for JPET
A special section on Nanotherapeutics in Cancer Research is being planned for publication in the March 2024 issue of The Journal of Pharmacology and Experimental Therapeutics
Original research pertaining to innovative systems based on nanotherapeutics used in preclinical cancer research and their emerging clinical applications will be considered for this special section. Manuscripts describing efforts in demonstrating the role of nanotherapeutics regarding functionality and in their ability to target tumors to treat various cancers are especially welcome. Research papers describing innovative in vitro/ex vivo/in vivo, bioanalytical, -omics,
modeling, and/or clinical research approaches to advance the understanding of the biological properties of nanotherapeutics are highly encouraged. Reports on animal models addressing any of these topics will also be considered. Of benefit, but not essential, a path describing translation is also encouraged. Review articles addressing any aspects of the aforementioned topics will be considered for publication.
Proposals for articles should be sent to the guest editors, Dr. Rheal Towner and/or Dr. Marya Ahmed, for approval prior to submission. All submissions must refer to the journal Instructions to Authors. The submission deadline is July 30, 2023.
Consider submitting your work to an ASPET Journal!
ASPET members gain access to all content in the journals, along with reduced or waived fees if they publish.
ASPET publishes four highly respected and widely read journals that provide rapid publication and easy access on mobile devices, tablets and desktop computers. The society co-publishes a wholly open access journal with the British Pharmacological Society and Wiley. Since the launch of JPET in 1909, ASPET has provided scientists in pharmacology and related fields with leading primary research and review articles. ASPET members have access to all journal content as a member benefit.
Visit https://www.aspet.org/aspet/journals to learn more about ASPET journals.
The Pharmacologist • June 2023 32
Highlighted Trainee Authors
Congratulations to the latest Highlighted Trainee Authors selected for Drug Metabolism and Disposition, The Journal of Pharmacology and Experimental Therapeutics, and Molecular Pharmacology:
Drug Metabolism and Disposition
■ Dr. Nicolò Milani (Hoffmann-La Roche) – March
JPET
■ Ubong Ekperikpe (Univ.of Mississippi Medical Center) – March

■ Dr. Behnaz Lahooti (Sanofi) – April
■ Erika Carlson (Univ. of Texas at Austin) – May


Molecular Pharmacology
■ Ivan Kristell Domingo (Univ. of Alberta) – March



■ Chaoling (Linda) Chen (Virginia Commonwealth Univ.) – April

■ Dr. Wallace Chan (Pfizer) – May
A brief description of their areas of research, current projects, the anticipated impact of their work, and what they enjoy when not in the lab is online at https://bit.ly/2yX1YeH. We congratulate all of them for being selected.
33 The Pharmacologist • June 2023
Ubong Ekperikpe
Dr. Behnaz Lahooti Erika Carlson
Dr. Nicolò Milani
Ivan Kristell Domingo Chaoling (Linda) Chen
Dr. Wallace Chan
Membership News
ASPET welcome new members. The Society offers a variety of member benefits, including: publications, ASPET meetings, ASPETConnect online community,
New Members
Affiliate Members


Josiline Chigwada, Chinhoyi Univ of Technology, Zimbabwe
Joshua Cooper, Inotiv, MO
Tu Dang, Philadelphia Col of Osteopathic Med/NERL, GA
Lauren A. Klaskala, Inotiv, MO
Roger Melton, Inotiv, MO
Michael Shockley, Charles River Laboratories, MA
Postdoctoral Members
Pooja Acharya, Univ of Cincinnati, OH
Mariam K. Alamoudi, Prince Sattam bin Abdulaziz Univ, MA
Maral Budak, Univ of Michigan
Lobna Elkhadragy, Univ of Illinois at Chicago
Imoh Etim, Merck, PA
Ceren Eyileten-Postula, Med Univ of Warsaw, Poland
Umida Ganieva, Rosalind Franklin University, IL
Juliana Giacomini, Univ of Texas
Peter W. Halcrow, Univ of North Dakota School of Medicine and Health Sciences
Andrew D. Huber, St Jude Children’s Res Hosp, TN

Ying Li, Saint Louis University, MO
Karyn Alicia Olascuaga Castillo, Univ Privada Antenor Orrego, Peru
Saba Omer, Shifa Coll of Dentistry, Pakistan
Rohini Ople, Sr, Univ of Health Science and Pharmacy, MO
Thiele Osvaldt Rosales, Temple Univ Health Science Center, PA
Julia A. Schulz Pauly, AbbVie Inc., IL
Zijing Song, The Chinese Univ of Hong Kong
ASPET Career Center, public affairs and advocacy, ASPET awards, ASPET divisions and more.
Regular Members
Shaquria Adderley, Touro Univ Nevada
Tauseef Ahmad, Univ of Tasmania, Australia
Ream Al-Hasani, Washington Univ in St Louis, MO
Mohammad Ali, SUNY Binghamton Univ, NY
Olivera Antic, Abbvie, IL
Dinesh Aryal, Edward via College of Osteopathic Med, LA
Jelena Baranovic, Univ of Edinburgh, United Kingdom
Bruce P. Bean, Harvard Univ Medical School, MA
Dylan Burger, Ottawa Hospital Research Institute, Canada
Adriana Caballero, Univ of Illinois Chicago
Todd Camenisch, Saint John Fisher, NY
Joanna Dabrowska, Rosalind Franklin Univ of Medicine and Science, IL
Wei Du, Univ of Pittsburgh, PA
Wael Eldahshan, Southwestern Oklahoma State Univ
Bahaa Elgendy, Washington Univ in St. Louis and UHSP, MO
Wilber Escorcia, Xavier Univ, OH
Robert Evans, CA
Susan Farr, Saint Louis Univ, MO
Stanley Fernandez, Univ at Buffalo, NY
Su Guan, Univ of California Davis Health
Mary Guaraldi, NH
Yi Huang, Univ of Iowa
Sally L. Huskinson, The Univ of Mississippi Medical Center
Helen Ibeawuchi, Kentucky Coll of Osteopathic Med
Mark E. Issa, Massachusetts General Hosp BHX-1
Hongmei Jiang, Envigo RMS, MO
Kevin Johnson, Inotiv, MO
Ajith Karunarathne, Saint Louis Univ, MO
The Pharmacologist • June 2023 34
Islam Khan, Kuwait Univ
Pallavi Kompella, Stanford Univ Med Ctr, CA
Jordan McCall, Washington Univ in St Louis, MO
Erin Messer, A. T. Sill Univ School of Osteopathic Medicine in Arizona
Swarup Mitra, Marshall Univ, WV
Seiji Miyauchi, Toho Univ, Japan
Brian Muntean, Augusta Univ, GA
Detlef Obal, Stanford Univ, CA
Bongkyun Park, Korea Institute of Oriental Medicine
Karson Putt, Purdue Univ, IN
Li Qiang, Columbia Univ, NY
Vidhya Rao, Loyola Univ, IL
Hong-can Ren, China
Thomas Rich, Univ of South Alabama
Rajika Roy, Temple Univ, PA
Flori R. Sari, Univ Islam Negeri Syarif Hidayatullah Jakarta, Indonesia
Charles P. Scott, Thomas Jefferson Univ, PA
John J. Shacka, Univ of Alabama at Birmingham
Savita Sharma, Charles River Laboratories, MA
Rakesh Singh, Touro University Nevada
Harald H. Sitte, Medical University of Vienna, Austria
Youssef I. Soliman, Geisinger Commonwealth school of Med, PA
Su Jeong Song, Korea Inst of Oriental Med, Republic Of Korea
Sandhya Subash, Washington State University
Artur Swierczek, Jagiellonian Univ Medical College, Poland
Serge Thierry Omouessi, Univ des Sciences de la Sante, Gabon
Anita Thyagarajan, Wright Univ, OH
Yajing Wang, Univ of Alabama, Birmingham
Craig T. Werner, Oklahoma State Univ Center for Health Sciences
Daniel Wesson, Univ of Florida
Rongxue Wu, Univ of Chicago, IL
Jianqiu Xiao, Inotiv, MO
Hani Zaher, Frontage Laboratories, PA
Asad Zeidan, Qatar Univ
Yong Zhang, Texas Tech Univ Health Sciences Center
Yongjie Zhang, China Pharmaceutical Univ, China
Student Members
Israa G. Abdelr, Univ of Minnesota
Tolulope Adebusuyi, Texas Southern Univ
Daniel Afolabi, Creighton Univ, NE
Jhoan Aguilar, Washington Univ in St. Louis, MO
Cemal Akmese, Univ of Florida
Justin D. Anair, Northwestern Univ, IL
Jeffrey Angell, Univ of the Incarnate Word, TX
Kala Babb, Virginia Commonwealth Univ
Amna K. Bhatti, Howard Univ, VA
Mike P. Boeckman, Ohio State Univ
Noelle Cataldo, Univ of Wisconsin Madison
Hao Chen, Univ of Michigan
Alexia Dalton, Northern Michigan Univ
Han-lin Ding, II, Wuhan Univ Sch of Med China
Engie S. El Sawaf, Univ of Minnesota
Jyotsna Devi Godavarthi, Texas Southern Univ
Ronghui Han, China
Taylor Henry, Univ of Miami, FL
Mason Hochstetler, Oklahoma State Univ-Tulsa Ctr for Hlth Sci
Kenneth F. Horner, Rosalind Franklin Univ, WI
Chunping Huang, DePaul Univ, IL
Lu Huang, Shanghai Jiao Tong Univ, China
Katelin Hubbard, Univ of Tennessee Knoxville
Yuxin Jiang, Guangdong Med Univ, China
Santina Johnson, Univ of South Alabama
Spencer Jones, Saint Louis Univ, MO
Vivian Jones, Vanderbilt Univ, TN
Nikhil Joseph, Univ of the Incarnate Word, TX
Juliann A. Jugan, Univ of California-Davis
Sun Min Jung, Univ of Michigan Coll of Pharmacy
Samuel Jurek, Northeastern Univ, MA
Joyce Kariuki, Emory Univ, GA
Jacob D. Layne, Oklahoma State Univ Center for Health Sciences
Hyejin Lee, Univ of Science & Technology, Korea
Kyutae Lee, Univ of Miami, FL
Alex Lekander, Univ of Michigan
Xiyu Li, Emory Univ, GA
Danyong Liu, Southern Medical Univ, China
Kassandra Looschen, Marshall Univ Sch of Med, WV
Vanessa Lopez, Univ of Washington
Ayma F. Malik, Rutgers, The State Univ of New Jersey
Elizaveta L. Mangutov, Univ of Washington at St Louis, MO
Daniel K. Marri, Michigan State Univ MI
Anuoluwapo A. Mattix, Univ of Wisconsin
Srujith Medharametla, Midwestern Univ, IL
Emily J. Miller, Univ of Florida
Kyle Miller, IN
Uzoamaka A. Nwagbo, Univ of Utah
35 The Pharmacologist • June 2023
Dimitri U. Nwankwo, Rosalind Franklin Univ, IL
Emmanuel S. Ojo, Southern Illinois Univ Sch of Med
Yueyang Pan, The Chinese Univ of Hong Kong
Rupesh Chandra Panta, Southern Illinois Univ
Ji Youn Park, Univ of Pittsburgh, PA
Anika Patel, Paul L Foster School of Medicine, TX
Rebecca M. Peter, Rutgers Univ, NJ
Leah Phan, Univ of Florida
Gabrielle Pittman, Augusta Univ, GA
Andres M. Prieto Trujillo, Indiana Univ School of Medicine
Jacinda M. Pujols, Univ of Miami, FL
Darius N. Quansah, Univ of North Dakota
Mohammad Atiqur Rahman, Univ of Houston, TX
Morgan Reeves, Univ of Florida
Kareem Sawali, Jordan Univ of Sci and Tech, Kuwait
Ally J. Su, Emory University, GA
Reniel Suarez Gonzalez, Georgetown University, VA
Bovornpat Suriyapakorn, Univ of Minnesota
Ira Tandon, Univ of Wisconsin Madison
Sam Taylor, Univ of Miami, FL
Laura Teal, Vanderbilt Univ, TN
Rhea Temmermand, Drexel Univ, PA
Waruna Thotamune, Saint Louis Univ, MO
Luis E. Tron Esqueda, Univ of Cincinnati, OH
Sithurand Ubeysinghe, Saint Louis Univ, MO
Udomsak Udomnilobol, Chulalongkorn Univ, Thailand
M. Frances Vest, Louisiana State Univ Hlth Sci Ctr
Jennifer Vigliaturo, Univ of Minnesota
Maria Vishnyakova, Univ of Washington
Yuyin Wang, Univ of Pittsburgh, PA
Xin Wen, Emory Univ, GA
Dhanusha Wijayaratna, Saint Louis Univ, MO
Abie E. Williams-Villalobo, Texas Southern Univ
Bo J. Wood, Louisiana State Univ Health Shreveport
Daniel P. Woods, Univ of Tennessee Knoxville
Postbaccalaureate Member
Cecilia Lee, Univ of San Diego, CA
Undergraduate Members
Taylor Aldrich, Pacific Univ, OR
Tessa Allen, Oglethorpe Univ, GA
Liudmyla Arifova, Univ of South Florida
Annika Baker, Calvin Univ, MI
Atheeva Bansal, Southwest Christian, TX
Alexander D. Bartkowiak, Univ of North Florida
Carley E. Beeman, Oregon State Univ
Ramsey R. Beladia, Univ of Virginia
Beau B. Benoit, Univ of Louisiana Monroe
Joshua L. Berkowitz, Univ of Pittsburgh, PA
Olivia M. Buchweitz, Albion College, MI
Hien M. Bui, Middlebury College, VT
Amy Castillo, Univ of Michigan
Zhen-Hin E. Chan, Northern Kentucky Univ
Hubert Chen, West Windsor-Plainsboro High School South, NJ
Jeremy Y. Cho, Washington Univ in St Louis
Anand Chundi, Duke Univ, NC
Emilia Cole, Aston Univ, United Kingdom
Madeline E. Collins, Loyola Univ Chicago
Karenna J. Collins-Thompson, Smith College, MI
Mauricio Dominguez, Xavier Univ, OH
Julia Driggers, Xavier Univ, OH
Santiago G. Fernandez, Univ of Florida
Andrew L. Ferns, Western Oregon Univ
Joseph R. Figura, Univ of Pittsburgh, PA
Taylor A. Finkelstein, Univ of Michigan
Laurens Fontaine, Univ of Florida
Angelica Gatica-Gomez, Pacific Univ, OR
Emma E. Gevelhoff, Univ of Arizona
Katelyn A. Harris, Univ of Findlay, PA
Ryan Herzog, Univ of Tennessee
Hannah Hoffmann, The Ohio State Univ
Jose J. Jarquin, Georgia State Univ
Christopher E. Koch, Villanova Univ, OH
Kai Hui Grace Koh, Case Western Reserve Univ, NC
Lance Kuo-Esser, Xavier Univ, OH
Drew Kwitchoff, Rowan Univ, NJ
Avery J. Kyle, Belmont Univ, NC
Isabelle L. Lamug, Univ of Michigan
Giulia Lelli, Univ of Wisconsin - Eau Claire
Brian S. Liu, New York Univ
Joseph A. Mancuso, Univ of Arizona
Chaniya Miller, Univ of Florida
Ciara J. Miller, Univ of Virginia
Cecille M. Montes James, Univ de Puerto Rico, Río Piedras
Jocelyn Nichols-Lockett, Univ of Michigan Ann Arbor
Francis D. Ongkodjojo, Univ of Chicago
Maria Jose Orozco Fuentes, Lake Forest College, IL
Kaleb M. Ott, Univ of Michigan
Yari Parada, Univ of Delaware
Sydney L. Patton, Pacific University Oregon
Nguyen Pham, Univ of Arizona
Vivian Pho, Pacific Univ, OR
The Pharmacologist • June 2023 36
Rafael Pluguez, Univ of Puerto Rico
Sarah C. Posewitz, Univ of Colorado
Ram Prajapati, Univ of New Hampshire
Evelyn Qi, Williams College, NY
Elaine Qu, Univ of Michigan
Lakshmi Rao, CUNY John Jay College of Criminal Justice, NY
Ashutosh Reddy, Princeton Univ, AL
Kenneth C. Rogers, III, Michigan State Univ
Nathanyal D. Ross, Washington Univ, MO
Shelby Roy, Centenary College of Louisiana
Elian N. Ruiz-Arevalo, Univ of South Florida
Samuel D. Sanderson, Michigan State Univ
Adam M. Shaibat, Univ of Illinois
Michelle Shanguhyia, Syracuse Univ, NY
Beonka Ruth C. Sharpe, Fayetteville State Univ, NC
In Memoriam
Aruna Sreenivasan, Univ of Arizona
Hannah Stewart, Univ of Michigan
Grace Su, Smith College, MA
Nora Sypkens, Univ of Kentucky
Dionne Torres, Hollins Univ, VA
Colleen M. Tytler, State Univ of New York
Brynn Urban, DePauw Univ, IN
Liliane Vasquez, Michigan State Univ
Ruoyang Wang, Univ of Illinois-Urbana
Alyssa M. Welborn, Univ of Arizona
Daryna Yakovleva, Case Western Reserve Univ, OH
Yuhui Yang, Guangdong Medical Univ, China
Celina Zhao, Univ of Pittsburgh
Shuaitong Zhao, Indiana Univ
Shijie Zhou, Case Western Reserve Univ, OH
ASPET notes with sympathy the passing of the following members:
Salvatore Joseph Enna, PhD (1944-2023) was ASPET Past President (2000-2001) and also selected as an ASPET Fellow in the inaugural Class 2019. Dr. Enna served for six years as editor of The Journal of Pharmacology and Experimental Therapeutics and also served as co-editor of Current Protocols in Pharmacology,


Editor-in-Chief of Biochemical Pharmacology, Executive
Editor-in-Chief of Pharmacology and Therapeutics, and Series Editor of Advances in Pharmacology. His honors received included ASPET’s John Jacob Abel and Torald Sollmann Awards, the Daniel H. Efron Award from the American College of Neuropsychopharmacology, a PhARMA Foundation Excellence Award, and the Paoletti Medal from the European Pharmacology Society. Elective offices have included Secretary-Treasurer and President of ASPET, as well as Secretary General and President of the International Union of Basic and Clinical Pharmacology (IUPHAR).
L. Jackson Roberts II, MD (1943-2023) was professor emeritus, pharmacology and an internationally known clinical pharmacologist in the Vanderbilt University School of Medicine. His research focused on the role of lipid peroxidation and oxidative stress in human disease. Dr. Roberts received the 2001 prestigious National Institutes of Health MERIT Award. He is the recipient of the Pharmacia-ASPET Award for Experimental Therapeutics. Dr. Roberts became a member of ASPET in 1980.
37 The Pharmacologist • June 2023
Salvatore Joseph Enna, PhD, FASPET
L. Jackson Roberts II, MD
Members in the News



The Division for Pharmacology Education gathered at the DPE Academy of Pharmacology Educator’s meeting for a reception at ASPET 2023 titled “Fellows of the Academy.”

M. Nabeel Ghayur, PhD Receives Sankofa Culture Award

M. Nabeel Ghayur, PhD, was recently (Mar 2023) presented the Sankofa Culture Award 2023 from the Office of Diversity at the Kentucky College of Osteopathic Medicine (KYCOM) in Pikeville, K.Y. This Award is given in recognition of significant leadership and endeavors delivered in raising awareness about the important issues of diversity and inclusion across the University campus. Dr. Ghayur is serving as an Assistant Professor and Director of Pharmacology for KYCOM. He also serves on the Institutional Resources and Research committees at KYCOM. In addition, he is also the faculty advisor for multiple student clubs on campus.
Dr. Ghayur has an undergrad degree in Pharmacy, and master’s and PhD degrees in Pharmacology. He also completed a pre-doctoral fellowship in Neuropharmacology and Pharmacognosy from King’s College London, England and a post-doctoral fellowship in Physiology and Pharmacology from McMaster University in Hamilton, Canada. Dr. Ghayur is a licensed pharmacist in Ontario, Canada.
He has been a member of ASPET since 2001 when he joined as a graduate student member. Since that time, Dr. Ghayur has served twice (2002-2003 & 2004-2005) as a Student Councilor with the ASPET Student Chapter and was also affiliated in the past with one of ASPET’s special interest groups on Herbal Medicines and Medicinal Plants from 20032005. Dr. Ghayur is a member of ASPET Divisions for Pharmacology Education, Toxicology, Drug Discovery and Development, Cardiovascular Pharmacology and Molecular Pharmacology.
The Pharmacologist • June 2023 38
Division News



DPE Inducts Three New Fellows into the Academy of Pharmacology Educators

The Academy of Pharmacology Educators was established in 2010 to recognize individuals who have made exemplary contributions to pharmacology education in one or more of the following areas: studentteacher interaction, innovative contributions, scholarly endeavors, professional development, and service. Three new Fellows were inducted into the Academy this year by the Division for Pharmacology Education. Additional information about the Academy, including application instructions and a roster of inductees, can be found here: http://www.aspet.org/Education/Academy/.
Dr. Diptiman Bose holds the Associate Professor of Pharmacology position at the Western New England College of Pharmacy and Health Science. He obtained his Ph.D. from the University of Pacific and holds an M.Ed. from Georgia Southern University. Within the PharmD curriculum, he leads various integrated pharmacotherapeutics course modules. Additionally, he serves as the Program Director for the PharmD Distance Learning Pathway in the College of Pharmacy and Health Sciences. Alongside his academic roles, Dr. Bose actively engages in clinical pharmacy practice and dedicates his time to volunteering in healthcare and immunization clinics for the community. Dr. Bose’s research primarily focuses on instructional methods that facilitate meaningful learning. His contributions have greatly enhanced our understanding of the

effectiveness of graphical representations, such as flowcharts or simulation-based pharmacology instructions. Notably, his research on scaffolded notetaking has revealed the profound impact of visual imagery on retention and learning. Furthermore, his investigations have shed light on the factors influencing motivation and distractions in both classroom and online learning environments.
Dr. Jorge Iñiguez-Lluhí is an associate professor of pharmacology at the University of Michigan Medical School. A native of Mexico, he is a classically trained pharmacologist that obtained his doctoral degree from the University of Texas Southwestern Medical Center under the guidance of the Nobel Laureate Dr. Alfred. G. Gilman. He joined the faculty at the University of Michigan after completing postdoctoral training on nuclear receptors in the laboratory of Dr. Keith Yamamoto at the University of California San Francisco. In addition to a fruitful research career, Dr. Iñiguez-Lluhí has developed a wide range of experience as a pharmacology educator spanning undergraduate, graduate and professional levels that include pharmacology Ph.D. as well as nursing, dental, pharmacy and medical school learners. He is also the director of the ASPET-funded Pharmacology Summer Undergraduate Research Program at the University of Michigan.

39 The Pharmacologist • June 2023
As an early developer of technology and curricular innovations, his experience establishing online interactive mixed-mode flipped classroom approaches years before COVID-19, proved prescient and allowed him to provide timely guidance to colleagues transitioning to these instruction modes. Serving as the Pharmacology Discipline Lead, he has contributed to pharmacology instruction reforms at the University of Michigan Medical School. Dr. Iñiguez-Lluhí has developed and implemented novel, evidence-based pedagogical approaches to pharmacology instruction that incorporate engaging analogies and impactful simulation tools. He is a contributor to the classical pharmacology textbook Goodman and Gilman’s the Pharmacological basis of therapeutics and has received multiple teaching awards at the University of Michigan. Dr. Iñiguez-Lluhí enjoys the outdoors and performing choral works with the University Musical Society Choral Union, with which he was nominated for a Grammy in 2014.
Dr. John L. Szarek is Professor and Director of Clinical Pharmacology and Vice Chair of Curriculum in the Department of Medical Education and Education Director for Simulation at Geisinger Commonwealth School of Medicine in Scranton PA. He received his Ph.D. in Pharmaceutical Sciences from the University of Kentucky College of Pharmacy, a B.S. in Pharmacy from the University of Illinois Chicago
College of Pharmacy, and a B.S. in Biology from the University of Illinois Urbana. He completed his postdoctoral training in the Department of Physiology and Biophysics at the University of Vermont. Prior to joining GCSOM as a founding faculty member in 2009, Dr. Szarek spent 16 years at Marshall University School of Medicine in Huntington WV, five years as Chair of Pharmacology at Ross University School of Medicine in Dominica and two years at A. T. Still University School of Osteopathic Medicine in Arizona.
In addition to teaching pharmacology to the medical students at GCSOM, he has been actively involved in curriculum development. He has served on the Executive Committees of the IUPHAR Education Section and ASPET Division of Pharmacology Education. He is a founding codirector of the Pharmacology Education Project, pharmacologyeducation.org, a key project of the IUPHAR Education Section. He is involved in various education-related activities and his work has been shared with peers through regional, national and international presentations at multiple professional conferences plus several peer reviewed publications. Dr. Szarek was recognized by the International Association of Medical Science Educators with the Master Teacher Award in 2015.
The Division for Pharmacology Education considers it a privilege to add these educator-scholars to the roster of the Academy of Pharmacology Educators and appreciates their many contributions to the discipline.

2023 ASPET Young Scientist Award Winners
The ASPET Student/Postdoctoral Poster Competition was held at the ASPET 2023 Annual Meeting held in St. Louis, Mo. Of the nearly 600 posters presented, the following are the winners. Additionally, several divisions held oral competitions for young scientists, and those winners are also recognized.
2023 Dolores C. Shockley Poster Awards
The 2023 Dolores C. Shockley Competition took place at the Student /Postdoctoral Poster Competition in St.
Louis, Mo. Dr. Shockley was the first African American woman to earn a PhD in pharmacology and the first to be appointed to chair a pharmacology department in the United States. The following are the winners in each category.
Undergraduate Students
1st place: “Defining the Role of Mitochondrial Dynamics in Regulating PDAC Cell Sensitivity to KRAS and ERK Inhibition,” Amber Amparo, UNC Lineberger Comprehensive Cancer Center
The Pharmacologist • June 2023 40
2nd place: “Antinociceptive Effects of Fentanyl and Non-opioid Drugs in Methocinnamox-treated Rats” Saba Ghodrati, University of Texas Health Science Center at San Antonio
Graduate and Post-baccalaureate Students
1st place: “An Elastin-like Polypeptide-fused Peptide Inhibits MMP-2 Activity at nM Concentrations with High Specificity”, Adesanya Akinleye, University of Mississippi Medical Center
2nd place: “N-cadherin Signaling via PI3K -Akt3 Stabilizes Occludin Tight Junctions in the Blood-Brain Barrier,” Quinn Lee, University of Illinois Chicago
3rd place: “The Plasma Membrane Monoamine Transporter (PMAT) is Highly Expressed in Neuroblastoma and Functions as a Mitochondrial mIBG Transporter”, Leticia Vieira, University of Washington
Postdoctoral Scientists
1st place: “Evaluating Serotonin 2C and Dopamine D3 Receptor Ligands, Alone and in Mixtures, as Candidate Medications for Substance Use Disorders,” Briana Mason, University of Texas Health Science Center at San Antoni
2nd place: “Sexual Dimorphism of the Fasting Adipose: Mitigation of Metabolic and Cardiovascular Dysfunction in a Non-Obese Prediabetic Rat Model,” Haneen Dwaib, Palestine Ahliya University
3rd place: “Organic Cation Transporter 3 is a Crucial Modulator of Serotonin Clearance in Basolateral Amygdala and Sex-Dependently Modulates Fear Behaviors,” Nikki Clauss, University of Texas Health Science Center at San Antonio
2023 ASPET Division Award Winners
Division for Behavioral Pharmacology

Affects Cocaine Related Behaviors,” Rishik Bethi, Vanderbilt University
2nd place: “Antinociceptive Effects of Fentanyl and Non-opioid Drugs in Methocinnamox-treated Rats,” Saba Ghodrati, University of Texas Health Science Center at San Antonio
Graduate and Post-baccalaureate Students
1st place: “Effects of Cannabinoids on Fentanyl Antinociception,” Dalal Alkhelb, Northeastern University
2nd place: “Fentanyl-Targeting Monoclonal Antibodies Block Fentanyl-Induced Respiratory Depression in Non-Human Primates,” Emily Burke, McLean Hospital
3rd place: “Modifying the Conditioned Reinforcing Properties of Cocaine in the New Response Acquisition Procedure,” Lauren Rysztak, University of Michigan
Postdoctoral Scientists
1st place: “Targeting A3 Adenosine Receptor (A3AR) Attenuates Paclitaxel-induced Cognitive Impairment”, Silvia Squillace, Saint Louis University School of Medicine
2nd place: “Evaluating Serotonin 2C and Dopamine D3 Receptor Ligands, Alone and in Mixtures, as Candidate Medications for Substance Use Disorders,” Briana Mason, University of Texas Health Science Center at San Antonio
3rd place: “Microglia in the Locus Coeruleus Mediate the Behavioral and Neuronal Consequences of Social Stress in Females,” Cora Smiley, University of South Carolina
Division for Cancer Pharmacology

Undergraduate Student
Undergraduate Students
1st place: “Characterizing How Lesioning of Cocaine Activated Ensembles in the Nucleus Accumbens
1st place: “Defining the Role of Mitochondrial Dynamics in Regulating PDAC Cell Sensitivity to KRAS and ERK Inhibition,” Amber Amparo, UNC Lineberger Comprehensive Cancer Center
41 The Pharmacologist • June 2023
Graduate and Post-baccalaureate Students
1st place: “Direct Targeting of Menin-MLL1 Interaction Inhibits Cancer Stem Cells to Inhibit Neuroblastoma Growth,” Rameswari Chilamakuri, St. John’s University
2nd place: “Sotorasib, a KRAS-G12C Inhibitor, Induces Chemoresistance by Activating Human Pregnane Xenobiotic Receptor,” Julia Salamat, Auburn University
3rd place: “Direct Targeting of the Cell cycle Regulator WEE1 is a Novel Therapeutic Approach for Neuroblastoma,” Parul Suri, St. John’s University
Division for Cardiovascular Pharmacology

Division for Drug Discovery and Development Undergraduate Student
1st place: “Effects of Simultaneous Inhibition of Fatty Acid Amide Hydrolase and Soluble Epoxide Hydrolase on Acute and Persistent Pain in Male Rats,” Cassandra Yuan
Graduate and Post-baccalaureate Students
1st place: “Structure, Function, and Pharmacology of Delta Opioid Receptor Bitopics,” Sarah Bernhard, Washington University School of Medicine
Undergraduate Student
1st place: “Clopidogrel Induces More Bleeding in P2Y12deficient Mice: Optimization of Murine Tail Bleeding Assay,” Taylor Rabanus, Michigan State University
Graduate and Post-baccalaureate Students
1st place: “Resolving Unchecked Inflammation in Atrial Fibrillation”, Deanna Sosnowski, McGill University
2nd place: “A Convolutional Neural Network Model classifies Beat-to-Beat Arterial Pressure Time-Series: Sex Stratification and Cardiovascular Risk Prediction”, Nour Mounira Bakkar, American University of Beirut
3rd place: “Effects of Dapagliflozin as an Adjunct Therapy to Insulin in Streptozotocin-induced Diabetic Rats,” Abdul-Azeez Lanihun, Southern Illinois University
Postdoctoral Scientists
1st place: “Mitochondrial CaMKII as a Novel Regulator of Cardiometabolic Dysfunction,” Kimberly Ferrero, Johns Hopkins University School of Medicine
2nd place: “Cardiomyocyte Signaling Factors are Responsible for Heart-Fat Communication and Mediate the Development of Cardiometabolic Disease,” Stephanie Kereliuk, Temple University
2nd place: “An Elastin-like Polypeptide-fused Peptide Inhibits MMP-2 Activity at nM Concentrations with High Specificity” Adesanya Akinleye, University of Mississippi Medical Center
3rd place: “Efficacy of LSD1 Directed Agents is Enhanced with Kinase Signaling Inhibition in Glioblastoma Stem Cells,” Lea Stitzlein, University of Texas MD Anderson Cancer Center
Postdoctoral Scientists
1st place: “Pharmacological Rescue of Human Disease Mutations in KCNJ10 and KCNJ16 by Novel Kir4.1/5.1 Potentiator, VU0493206,” Samantha McClenahan, Vanderbilt University
2nd place: “Pharmacological and Genetic Preclinical Models of Ghrelin Receptor Functional Selectivity to Investigate Metabolic Disease Pathophysiology,” Joshua Gross, Duke University School of Medicine
3rd place: “Profiling Context-Dependent Activity of Allosteric Modulators at mGlu7,” Xia Lei, Vanderbilt University
Division for Drug Metabolism and Disposition
Graduate and Post-baccalaureate Students
1st place: “Tenofovir Activation in Brain and Liver is Diminished in Creatine Kinase Brain-Type Knockout Mice,” Colten Eberhard, Johns Hopkins University
The Pharmacologist • June 2023 42
2nd place: “The Plasma Membrane Monoamine Transporter (PMAT) is Highly Expressed in Neuroblastoma and Functions as a Mitochondrial mIBG Transporter,” Leticia Vieira, University of Washington
2nd place: “Human Cytochrome P450 Interactions with Redox Partner Cytochrome P450 Reductase”, Sarah Burris-Hiday, University of Michigan
3rd place: “Development of Novel DIA Based Proteomics Tools for Quantification of Drug-protein Adducts,” Ellen Riddle, University of Washington
Postdoctoral Scientists
1st place: “High-Resolution Mass Spectrometry Coupled with XCMS Online for High-throughput Detection and Identification of Drug Metabolites,” Dilip Singh, Washington State University
2nd place: “Metabolism of (-)-∆9-Tetrahydrocannabinol (THC) in Chicken and Potential Exposure of THC and Its Metabolites to Humans,” Ziteng Wang, National University of Singapore
Division for Molecular Pharmacology

Undergraduate Students
1st place: “Structural and Functional Characterization of Phospholipase C β 3”, Kennedy Outlaw, Purdue University
2nd place: “Urothelial Knock-in of the Engineered Chloride Channel EG3RF as a Potential Treatment for Cystitis-Induced Bladder Inflammation,” Grace Ward, Emory University
Graduate and Post-baccalaureate Students
1st place: “N-cadherin Signaling via PI3Kβ-Akt3 Stabilizes Occludin Tight Junctions in the Blood-Brain Barrier,” Quinn Lee, University of Illinois Chicago
2nd place: “Fluorescence Resonance Energy Transfer (FRET) Spatiotemporal Mapping of Atypical P38 Reveals an Endosomal and Cytosolic Spatial Bias,” Jeremy Burton, University of Georgia Athens
3rd place: “The Adhesion GPCR GPR114/ADGRG5 is Activated by its Tethered-Peptide-Agonist Following Receptor Fragment,” Tyler Bernadyn, University of Michigan
Division for Neuropharmacology
Undergraduate Students
1st place: “Characterization of Novel Tryptamines’ Anxiolytic and Anti-Inflammatory Effects in Relation to their Psychoactive,” Catharine Carfagno, Louisiana State University
2nd place: “Characterizing the Role of Nicotine on SexSpecific Neural Circuit Mechanisms Underlying Sensory Reinforcement,” Grace Bailey, Vanderbilt University
Graduate and Post-baccalaureate Students
1st place: “Nucleus Accumbens Single Nucleus RNA Sequencing Identifies Cell Subtype-Specific Maladaptations after Prolonged Spared Nerve Injury,” Randal Serafini, Icahn School of Medicine at Mt. Sinai
2nd place: “Sex Differences in GABA Regulation of Dopamine Release in the Nucleus Accumbens and its Role in Cocaine Use Disorder,” Brooke Christensen, Vanderbilt University
3rd place: “Butyrate Prevents Opioid Induced Peripheral Hypersensitivity via a Gut Dependent Mechanism,” Donald Jessup, Virginia Commonwealth University
Postdoctoral Scientists
1st place: “Antidepressants and Dopamine Levels Induced by Substance Misuse Regulate Inflammation and HIV in Myeloid Cells,” Stephanie Matt, Drexel University College of Medicine
2nd place: “Effect of Inhibition of Xenobiotic Transporter MRP5/ABCC5 on Neurite Elongation: Screening for MRP5 Inhibitors among Clinically Used Drugs,” Takahiro Ishimoto, Kanazawa University
3rd place: “Chronic Morphine and Fentanyl Induce Intestinal Dysbiosis by Decreasing the Antimicrobial Activity of the Ileum,” Karan Hitesh Muchhala, Virginia Commonwealth University
43 The Pharmacologist • June 2023
Division for Pharmacology Education

Graduate and Post-baccalaureate Students
1st place: “Identifying Students’ Causal Mechanistic Reasoning (CMR) in Medical Pharmacology,” Rosalyn Bloch, Michigan State University
2nd place: “Acute and Subchronic GPR171 Agonism Affects Anxiety and Depression in Female Mice,” Megan Amber Lim, Carle Illinois College of Medicine
Division for Toxicology

Undergraduate Students
1st place: “Eugenol Protects Against DiclofenacInduced Hepatocellular Injury in an In Vitro Mouse Model,” Jaiden Martin, Pacific University
2nd place: “Cutaneous Exposures to Nitrogen Mustard in C57BL/6 Mice Cause Hematologic Toxicity that could lead to Acute and Long-term Effects,” Ellen Kim, Michigan State University
Graduate and Post-baccalaureate Students
1st place: “The Influence of Arsenic on Selenium Uptake by Red Blood Cell Anion Exchanger 1/SLC4A1 Variants,” Serena Li, University of Alberta
2nd place: “Mitragynine Pretreatment Prevents
Morphine-Induced Respiratory Depression,” Julio Zuarth Gonzalez, Texas Tech University Health Sciences Center
3rd place: “Nrf2-dependent and independent effects of tBHQ on dendritic cell function,” Saamera Awali, Michigan State University
Postdoctoral Scientist
1st place: “Differential Induction of Microsomal and Soluble Epoxide Hydrolases by Arsenic in Drinking Water,” Hui Li, Hui Li, University of Arizona
Division for Translational and Clinical Pharmacology

Undergraduate Students
1st place: “3D Reconstruction Reveals Changes in Mitochondrial Morphology in Mouse Skeletal Muscle Across Aging and Upon Loss of the MICOS Complex,” Kit Neikirk, University of Hawaii at Hilo
2nd place: “Excessive exercise increases metabolites associated with pathophysiologic changes in ultramarathon runners,” Mikolaj Marszalek, Medical University of Warsaw
Graduate and Post-baccalaureate Students
1st place: “Chronic Exposure to Organophosphate Pesticides and Elevated Markers of Systemic Inflammation: Possible Neuroinflammatory and Genotoxic Effects,” Dina El-Gameel, Alexandria University Hospitals
2nd place: “Non-alcoholic Fatty Liver Disease (NAFLD) causes Erythropoietin Hyporesponsiveness,” Huixi Zou, The Chinese University of Hong Kong
3rd place: “Social stress causes the emergence of functional Histamine H3 Receptors in urinary bladder smooth muscle,” B Malique Jones, Michigan State University
Postdoctoral Scientist
1st place: “Acute restoration of respiratory function in mice with botulism after treatment with aminopyridines,” William McClintic, University of Tennessee
Oral Competition Award Winners
Division for Cancer Pharmacology

Young Investigator Symposium (Oral Sessions)
In the postdoctoral category, “finalist” prizes were awarded to Jingwen Zhu - St. Jude Children’s Research Hospital, Maria Voronkova - West Virginia University, Vrushank Bhatt - Rutgers Cancer
The Pharmacologist • June 2023 44
Institute of New Jersey, Gavin Traber - UC Davis School of Medicine
Division for Cardiovascular Pharmacology

Graduate and Post-baccalaureate Students
1st place: “Opposing Effects of ß2-ARs on ß1-ARs on Phospholipase C-mediated Cardiac Hypertrophic Signaling,” Wenhui Wei, University of Michigan
2nd place: “Delivery of Human ACE2 Across the Blood Brain Barrier Attenuated Development of Neurogenic Hypertension Using An Engineered Liposome-Based Delivery System,” Yue Shen, North Dakota State University
3rd place: “Angiotensin-(1-7) and Gut-Bone Marrow Axis in Aging,” Kishore Chittimalli, North Dakota State University
Postdoctoral Scientist
1st place: “Sexual Dimorphism of the Fasting Adipose: Mitigation of Metabolic and Cardiovascular Dysfunction in a Non-Obese Prediabetic Rat Model,” Haneen Dwaib, Palestine Ahliya University
2nd place: “ANGPTL4 attenuates hypoxia/ reoxygenation-induced cardiomyocyte apoptosis via activation of Akt signaling,” Weiyi Xia, The Hong Kong Polytechnic University
Division for Molecular Pharmacology

Division for Neuropharmacology
Postdoctoral Scientists
1st place: “Metabotropic mGlu1 Receptor Regulation of Cortical Inhibition and Cognitive Function: Implications in Adolescent Cocaine Exposure” Deborah Luessen, Vanderbilt University
2nd place: “Role and Regulation of BNST GluN2Dcontaining NMDARs in a Continuous Access Ethanol Task,” Marie Doyle, Vanderbilt University
3rd place: “Antidepressants and Dopamine Levels Induced by Substance Misuse Regulate Inflammation and HIV in Myeloid Cells,” Stephanie Matt, Drexel University College of Medicine
Honorable Mention- “Impact of Maternal Overnutrition in Central Nervous System Circuits That Regulate Feeding,” Sai Shilpa Kommaraju, Barrow Neurological Institute
Division for Translational and Clinical Pharmacology

Young Investigator Awards Platform (Oral Sessions)
In the Graduate Student category, prizes were awarded to Shams Osman – Alexandria University, Mikolaj Marszalek – Medical University of Warsaw, Yin Zhu – University of Pittsburgh, Kennedy Kuchinski –Xavier University
Postdoctoral Scientists
1st place: “Optimization of a Novel D2 Dopamine Receptor-Selective Antagonist into Lead Candidates for the Treatment of Neuropsychiatric Disorders,” Ashley Nilson, National Institute of Neurological Disorders and Stroke and “Identification and Pharmacological Characterization of a Novel β-arrestin-biased Negative Allosteric Modulator of the β<-adrenergic Receptor,” Francesco De Pascali, Thomas Jefferson University
45 The Pharmacologist • June 2023
American Society for Pharmacology and Experimental Therapeutics (ASPET)
1801 Rockville Pike, Suite 210 Rockville, Maryland 20852-1633

PRSRT STD US POSTAGE PAID MANASSAS VA PERMIT #250







 Michael F. Jarvis, PhD ASPET President
Michael F. Jarvis, PhD ASPET President












































































