THE SHRIMP HANDBOOK



For decades, shrimp farming has thrived on its reputation as a risky yet highly profitable activity. Since the emergence of professional hatchery practices in the early ‘90s, the industry has attracted hundreds of thousands, if not millions, of smallholder farmers to convert their lands in the hope of achieving better livelihoods and a higher quality of life.
In recent years, however, shrimp farming has been confronted with a series of new challenges that threaten its traditional model of success. The industry is indeed facing issues such as oversupply, leading to low farm gate prices, reduced demand due to inflation, higher production costs from intensified farming and recurrent disease outbreaks.
These challenges have forced the industry to face a new reality – one that demands innovation, adaptation, and more durable practices. While farmers may not control the market dynamics, such as global supply and demand, they hold the power to manage their costs and operations more effectively to sustain their businesses in this evolving landscape.
In this new reality, a renewed focus on health and a revised approach to functional feed management are paramount.

Despite substantial progress in feed quality and performance, there is still considerable room for improvement in health management and functional nutrition. With the absence of vaccines and the compounding effects of climate change and intensification, strategies that prioritize prevention through holistic microbiota management, immunity, and stress mitigation have become essential
The program Aquasaf Shrimp is a comprehensive technical package that aims to guide the application of fermentation solutions from yeast and bacteria.
It provides crafted recommendations to support shrimp producers in addressing some of the most impacting challenges, including mortalities caused by bacterial infections from Vibrio spp., reduced growth associated with oxidative stress, and dysbiosis linked to the replacement of marine ingredients in feeds. Importantly, the program encourages sustainable practices by reducing the need for antimicrobial and chemical substances. To build the program, Phileo by Lesaffre draws on knowledge from more than 20 R&D projects carried out across Asia, Europe and LATAM using different products and their combination, both in laboratory and field conditions
Enjoy the read.

Foreword
Yeast: one of humankind’s oldest ingredients
Our production process: from primary fermentation
To functional ingredients
Fermentations solutions: supporting health & Nutrition
1. Gut health & microbiota management
Introduction to shrimp gut health
Probiotics in shrimp farming
Association of proprietary bacillus strains to optimize gut microbial balance
Gut health management with probiotics
2. Diseases & stress management
Disease outbreaks: main production challenge faced by farmers
Shrimp defenses against pathogens & possible routes of intervention
Safmannan®: measurable benefits for producers
3. Health & performance in plant-based diets
Yeast, a naturally occurring fungus, has played a pivotal role in human cultures for millennia, being used in baking, brewing, and fermentation. Its historical use traces back to ancient civilizations, such as the Egyptians, who unknowingly incorporated yeast into their bread-making processes, and the Babylonians, who employed it in the production of beer.
In the 19th century, Louis Pasteur’s experiments illuminated the role of yeast in fermentation, revealing its ability to convert sugar into carbon dioxide, water, and ethanol. This discovery, pivotal in beer, wine, and bread production, marked a significant turning point and strongly influenced our founder Louis Lesaffre (1835-1888), shaping his vision for the company’s direction in its early years.
Later on in the 20th century, yeast was further studied and developed as a commercial product. It is during this period that Lesaffre created Saf-Instant, the world’s first instant dry yeast. This product revolutionized bread-making, making it faster and more efficient.
Today, yeast has become indispensable in various industries, including food and beverage production, biofuels, and healthcare (Figure 1). Advances in genetic engineering have led to the development of new, more environmentally resilient yeast strains, allowing for more reliable and consistent results in industrial applications. As a result, yeast remains a pivotal force in shaping human history and maintains its relevance in our modern society.
1680
First observation of yeast by Antonie Van Leeuwenhoek
1857
Louis Pasteur discovers the fermentation process in Lille, France
1863 Lesaffre starts to develop research about yeast near to Lille

Figure 1. The broad spectrum of yeast applications.
The microorganism: Saccharomyces cerevisiae
Yeast has been naturally present on Earth for millions of years. Among the diverse yeast families, the Saccharomyces genera stands out for its unique role in both human development and various industries. Within this group, several commercially significant species, including Saccharomyces cerevisiae, S. boulardii, S. pastorianus, and S. bayanus, play pivotal roles. These species have diversified through natural processes in their DNA like mutations (i.e., random DNA changes), genome assortment (i.e., shuffling of genetic material), or hybridization (i.e., mixing of genes from two species).
S. cerevisiae is the most recognized yeast species, as it is extensively used in baking, beer and wine fermentation, and healthcare, thus playing a vital role in our everyday lives.

In its natural state, S. cerevisiae consists of approximately 49% protein, 40% carbohydrates, 7% minerals, and 4% lipids, along with other functional compounds. Its thick cell wall is rich in polysaccharides like 1.3 / 1.6 -beta-glucans, mannoprotein, and chitin, which are critical to support its structural integrity and facilitate interactions with its external environment. Within the cell, the cytoplasm contains essential amino acids, nucleic acids, and enzymes necessary for its biological functions. These attributes make yeast a rich source of biologically active compounds, making it valuable across diverse industries.
NUCLEUS

Yeast is produced and multiplied through fermentation, a natural process initiated when yeast consumes sugar. This metabolic activity results in the production of carbon dioxide and water, with alcohol formation being a specific outcome observed in anaerobic conditions like beer brewing. Yeast products can be sourced from two main manufacturing processes, often referred to as primary and secondary fermentation. Primary fermentation is a process specifically designed to cultivate yeast as the main product, with the manufacturing process designed to optimize its growth and quality. Certain products are derived from secondary fermentation in brewing or bioethanol production, where yeast is primarily viewed as a by-product. These often result in lower quality products with less stability than those
obtained from primary fermentation. Hence, for applications aiming at probiotic or postbiotic benefits, especially in the realm of animal health, utilizing yeast from primary fermentation is essential.
At Lesaffre, two-thirds of our employees work in our 77 industrial facilities, transforming tiny microorganisms into several hundred tons of yeast in less than two weeks. The production of our live yeast cultures and functional ingredients follows three key steps: fermentation, breakage, and separation (Figure 3).
A specific yeast strain is selected based on the intended use and expected final product tailored to fit customers’ expectations.
The selected strain is then cultivated in fermentation tanks under regular growing conditions: a glucose source, a controlled temperature (around 30 °C), and a sufficient oxygen supply. These conditions are essential for yeast growth and multiplication and custom adjustments to the culture conditions may be performed to cater to the unique requirements of the selected strain and its final intended use. Fermentation followed by centrifugation yields a juice called “yeast cream”, rich in live yeast cells, which can be dried to produce a storage probiotic or further processed to extract metabolites from the yeast’s cell wall and cytoplasm, also called postbiotics.
The yeast cream is cooled to stop yeast cells from multiplying, and it is then transferred into large tanks where the temperature is elevated to 45 –55 °C. This controlled heating triggers autolysis, a process during which the yeast’s own enzymes, naturally present in the yeast cell, begin to break down cell walls, internal proteins, and other macromolecules into smaller components. Breakage occurs without any external additives.
Centrifugation is used to separate the cell wall from the inner cell nutrients. The separated components are then concentrated through gentle evaporation (60 °C) to preserve all organoleptic properties and qualities of the products. At the end of this step, water is evaporated, leaving behind the separated and concentrated yeast cell wall and cytosol.

Based on the production process described earlier, Lesaffre’s facilities are equipped to produce a large array of yeast-based probiotics and postbiotics solutions. These products are meticulously crafted to meet various husbandry objectives, encompassing both health and nutrition. In 2017, our capabilities expanded to include the production of bacterial probiotics, thereby enriching our portfolio of beneficial microorganisms.
Phileo by Lesaffre, a division of the Lesaffre group dedicated to animal health and nutrition, offers a suite of solutions for shrimp farming. These solutions are organized into three over-arching themes: enhancing gut health, managing pathogens and stress, and providing functional proteins to support alternative diets.

The shrimp industry encounters persistent challenges, including disease outbreaks, unpredictable production outcomes, adverse environmental conditions leading to stress and dysbiosis, and a substantial dependence on marine proteins. The introduction of live yeast and bacteria into shrimp diets helps support a healthy gut ecosystem, which is fundamental for efficient digestion and strong immune response. Yeast fractions and active ingredients can also play a pivotal role in regulating the physiological responses of shrimp, enabling them to better cope with stress and diseases. Conversely, functional proteins act as a functional supplement for shrimp, providing essential amino acids and bioactive compounds that contribute to optimal growth and health, especially in plant-based diets.
In the subsequent chapters, we will dive deeper into these industry challenges and the solutions offered by yeast and bacteria.
Through a blend of diverse perspectives, insightful case studies, and clarification of the underlying mechanisms, we aim to provide practical recommendations for effective implementation in the field.


CHAPTER 1
The gut plays a vital role in shrimp well-being and performance, serving as the central hub for digestion, nutrient absorption, and immune defense. Maintaining gut health is therefore pivotal for optimal growth, immunity, and overall shrimp welfare. The digestive system in shrimp consists of three main parts: the foregut, comprising the esophagus and the anterior and posterior stomachs; the midgut, extending to the 6th abdominal segment (Figure 4); and the hepatopancreas (HP), which envelops both the posterior stomach and the anterior section of the midgut. The hepatopancreas plays a significant dual role, contributing not only to digestion but also to the shrimp’s immune system.
Once ingested, the feed begins its journey in the anterior stomach which is located directly behind the mouth. The anterior stomach is covered by a hard layer called cuticle, and contains calcified cuticular ossicles which function as teeth. These, along with the action of the surrounding muscles, break down the feed into smaller particles for easier digestion further along the gastrointestinal tract. Once food shredding is complete, the resulting particles move to the posterior stomach where a brush-like apparatus filters them. Particles that are small enough to pass through this filter continue their journey through the digestive tract, while larger ones remain in the stomach for further size reduction.
In shrimp, the hepatopancreas is the organ responsible for producing and releasing digestive enzymes (e.g., trypsin, lipase, amylase, protease (Ye et al. 2023)) essential for breaking down feed particles into absorbable nutrients. It also performs the bulk of nutrient uptake and storage in the form of lipid droplets (Silva et al. 2018; Vogt 2019). Additionally, this organ plays a crucial role in the immune response, aiding in pathogen elimination.
The hepatopancreas of decapod crustaceans like shrimp is a complex organ with an extensive network of tubules critical for digestion and nutrient absorption (Silva et al. 2018). The inner surfaces of these tubules are lined with microvilli –microscopic, finger-like extensions that increase the surface area available for absorbing nutrients into the hemolymph (Figure 5). The greater their number and length, the more efficient the nutrient absorption process. Similarly, the anterior midgut is also lined with a well-developed border of microvilli, further contributing to the shrimp’s ability to absorb nutrients effectively.
Recent advances in shrimp farming underscore the importance of understanding gut functions, particularly the hepatopancreas and midgut, due to their key roles in nutrient absorption and immunity. Strategies for optimal feeding, water quality management, and disease control now incorporate insights into shrimp digestive physiology, emphasizing the importance of maintaining a healthy and functional digestive system. The strategic use of probiotics to optimize feed digestion and absorption can significantly boost shrimp growth and health. This approach not only enhances the overall efficiency of feed utilization but also contributes to the reduction of the environmental impact associated with shrimp farming.
Additionally, a comprehensive understanding of the immune functions of the hepatopancreas is critical for developing disease management strategies that focus on enhancing shrimp innate immune response, thereby reducing the need for antibiotics or other chemical treatments.
Overall, a deeper comprehension of the functions and significance of the hepatopancreas and midgut in shrimp holds the potential to enhance the sustainability and profitability of shrimp farming, while concurrently promoting the health and welfare of the farmed animals.
Gut health in shrimp farming is affected by a range of factors that can compromise the overall health and productivity of shrimp. These include pathogenic infections, the use of treatments to eradicate pathogens, environmental stressors such as changes in water quality and unpredictable weather conditions, as well as the nutritional quality and composition of shrimp diets. These stressors can lead to a series of internal changes impacting multiple organs and systems, disturbing the natural balance of gut microbiota, altering gut structure, and weakening physical barriers that protect against disease (Figure 6).
Pathogens and parasites are key contributors to gut-related complications. The digestive tract is often the main entry point for several harmful microorganisms, including Enterocytozoon hepatopanaei (EHP) and other agents responsible for severe diseases like Early Mortality Syndrome (EMS) and White Feces Syndrome (WFS). The anterior midgut and hepatopancreas are especially prone to parasitic and bacterial infections due to their delicate structure. Lacking a protective cuticle, these regions only rely on a thin layer of cells to facilitate nutrient absorption, rendering them more vulnerable to pathogen invasion.
Once in the gut, pathogens can induce significant damage by secreting harmful metabolites and toxins. These substances target the epithelial cells of the anterior midgut and hepatopancreas, including the specialized F, R, B, and E cells (Figure 7). These cells play a vital role in nutrient absorption and storage, as well as in maintaining the osmotic balance within the shrimp. This intricate balance is crucial for ensuring the shrimp’s survival and fostering its optimal growth.


Figure 7. Cross sections of a hepatopancreas at (a) 10X objective and (b) 40X objective showing B cells with a large vacuole (black arrow), R cells with lipid droplets (white arrow), F cells (orange arrow) and microvilli (yellow arrow).
Pathogens not only attack the shrimp directly but also disrupt the balance of beneficial gut microbiota, which can trigger immune responses that may result in chronic inflammation and additional tissue damage. Overtime, such chronic damage can degrade the epithelial cells and intensify inflammation, leading to a diminution in the shrimp’s capacity to absorb nutrients, slow growth, and impaired immune function.
Early detection of gut issues in shrimp remains a challenging task. Initial signs are often subtle, such as a slight discoloration of the hepatopancreas

(Figure 8a), incomplete filling of the gut, and reduced feed intake. Visual inspection of the hepatopancreasunder a microscope is necessary for a conclusive diagnosis. Shrimp with affected gut typically display signs of deterioration of the HP, including thin, deformed tubules, which may be crooked towards their tips (Figure 8b). Incomplete filling of the tubules with lipid droplets can also indicate limited energy storage and compromised hepatopancreas function. Additionally, the presence of aggregated transformed microvilli (ATM) –damaged cell materials aggregated within the tubules – signals an underlying health issue (Figure 8c).
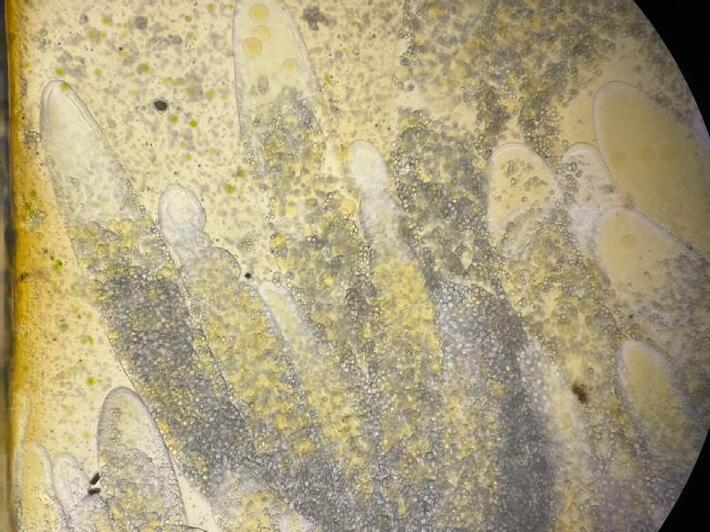

Figure 8. Signs of gut disruption in shrimp, including (a) hepatopancreas discoloration, (b) crooked hepatopancreas tubules, and (c) the formation of aggregated transformed microvilli (ATM) inside tubules.
Comprehensive histopathological analyses conducted at the laboratory can unveil further damage to the hepatopancreas, characterized by irregularly shaped tubules and a peeling epithelial barrier (Figure 9c). An unhealthy hepatopancreas may show accumulations of immune cells (i.e., hemocytes) which cluster around foreign objects (Figure 9c), indicative of severe tissue impairment and infection.



Figure 9. (a, b) Cross-section of healthy hepatopancreas tubules under the microscope, showing regular shape, against (c) a cross-section of an unhealthy hepatopancreas showing hemocytic infiltrations (black arrow), nodules of hemocytes and trapped microorganisms (red arrow), tubule atrophy (double black arrow) and sloughed epithelial cells in lumen (white arrow).
If
left untreated, the deterioration
of the hepatopancreas will escalate which can have two significant
outcomes.
Firstly, the functionality of epithelial cells responsible for digestion and immune defenses (F, R, B, E) significantly wanes, hindering the shrimp’s ability to process nutrients and combat pathogens.
Secondly, the microvilli within the hepatopancreas tubules and the anterioir midgut reduce in length and eventually detach from their base to be discarded through the gut lumen along with dead cells, indigested feed, and pathogens (Figure 10). Advanced stages of this condition are marked by the release of white feces, visible to the naked eye within the shrimp’s digestive tract and often found floating on the pond’s water surface.

WFS has become a major concern for shrimp farmers due to its impact on shrimp health, growth, and farm productivity. While an underlying infection by pathogens can initiate these gut issues, contributing factors like environmental changes or poor feed quality can exacerbate them. Shrimp are highly sensitive to environmental fluctuations, including water temperature, pH levels, and salinity. These changes can cause oxidative stress and cellular damage to the gut, particularly during key production stages such as post-stocking or peak biomass. Therefore, stable environmental conditions are essential to minimize the occurrence of WFS
The composition and quality of feed, especially when incorporating plant-based proteins as a substitute for fish meal (FM), are also critical for gut health. Especially, amino acid balance, digestibility, fiber content and the presence of anti-nutritional factors must be carefully considered. Too often, the pursuit of cost-effective feed overlooks these crucial health determinants.
It is also important to note that the impact of gut health issues varies across different shrimp production systems (Figure 11). Intensive farming models face greater challenges due to their increased reliance on commercial diets, their exposure to water quality variations and pathogen prevalence from high stocking densities.
11. Incidence of gut health issues in Vietnamese shrimp farms during site visits (n = 89). Extensive: 0.15 to 1 t./ha/year; semi-intensive: 1 to 20 t./ha/year; intensive: 20 to 80 t./ha/year; super-intensive: 80 to 150 t./ha/year.
With the shrimp industry’s shift toward more intensive production systems, these issues are likely to become more acute, emphasizing the need for effective management strategies that promote optimal shrimp gut health and overall well-being.
Probiotics are live and beneficial microorganisms extensively used in shrimp farming, both in feed and water, to promote the growth of beneficial microbiota in the gut and surrounding aquatic environment. Probiotics can stem from both bacterial and yeast sources, presenting a modern, convenient, and efficient alternative to traditional antimicrobial methods.
Employing both bacterial and yeast probiotics in the digestive system plays an essential role in promoting the health, growth, and resilience of farmed shrimp. These microorganisms increase feed efficiency, bolster immunity, and contribute to the profitability of shrimp farming operations by decreasing disease prevalence and gut health problems. Their beneficial impacts stem from a variety of actions and interactions with their host.
The major mechanisms of probiotic action include:
• Exclusion and competition by colonizing the shrimp gut and outcompeting harmful bacteria for resources and space, making pathogen establishment more difficult.
• Antimicrobial effects by producing antimicrobial substances such as acids and bacteriocins that inhibit the growth of pathogenic bacteria.
• Nutritional support by releasing enzymes that break down complex feed components, enhancing the shrimp’s ability to digest and absorb nutrients.
• Immune modulation by activating specific immune cells that identify and destroy harmful pathogens, thereby reducing the severity of diseases.
• Anti-inflammatory effects by reducing the production of harmful metabolites, toxins, and pro-inflammatory molecules from pathogens. Additionally, promoting gut mucus production and enhancing the proliferation and integrity of intestinal epithelial cells contribute to these positive effects.
The complementary actions of bacterial and yeast probiotics can be harmoniously combined in commercial products. This integration capitalizes on their natural complementarity, providing shrimp with a comprehensive probiotic treatment that fosters optimal gut health.
Historically, aquaculture farmers have used probiotics like Bacillus spp., Lactobacillus spp., Paracoccus spp., and Saccharomyces spp. to enhance water quality and overall culture environment. These probiotics help reduce water levels of organic matter, toxic ammonia and nitrites. By creating a less favorable environment for pathogenic bacteria, they effectively mitigate the risk of disease outbreaks.
Originally, probiotics have been applied directly to pond water to manage environmental conditions. Water probiotics are easy for farmers to apply, largely contributing to their popularity in shrimp farming. However, these essentially impact the production environment and as such, their contribution to shrimp health can largely vary depending on pond conditions and management practices.
Recently, the scope of probiotic use in aquaculture has expanded to include oral administration through feed, aimed specifically at enhancing gut health. This method is gaining popularity as a valuable complement to water-based applications, offering a more targeted approach to managing shrimp health.
Oral and water-based
applications can synergistically address various aspects of shrimp health.
It’s crucial to recognize that these practices form part of a broader, more intricate strategy aimed at effectively managing the microbiota within shrimp farming (Figure 12).
Oral probiotics applied on feed or through pondside fermentation differ from water probiotics both in their mode of action and their ability to affect shrimp health and performance. These probiotics have a direct interaction with the host gastrointestinal system, leading to more targeted effects on the gut microbiome and its structural integrity which in turn can enhance nutrient absorption and immunity. Oral probiotics also offer application versatility, allowing farmers to adjust supplementation according to various growth stages or seasonal requirements.
Despite these advantages, the industrial incorporation of probiotics in aquafeed formulas remains limited, particularly due to challenges such as high heat and pressure during extrusion and pelleting processes. This issue was verified in a study conducted by Phileo in partnership with INRAE (France), where significant spore loss was recorded during extrusion even under controlled thermic conditions (Figure 13). Although incorporating probiotics after pelleting holds promise, its adoption has been limited, largely due to the substantial investment required from feed millers.
Application in extrusion > 80 ºC
80 to 115 ºC 42 bars Few seconds
BC45
Application in extrusion < 80 ºC
to 80 ºC 42 bars Few seconds Clextral BC45
Application via top coating (enrobage)
13. Endurance of bacterial probiotics during extrusion and post-extrusion 3 weeks after application (INRAE/Phileo 2013).
Consequently, much like water probiotics, the predominant method for administering oral probiotics in shrimp farming involves applying them to feed at the farm level. However, several factors can impact their effectiveness. For example, probiotics commercialized on farms are often prone to wild repacking and may be blended with other products, which can compromise the viability of the spores and introduce contaminations in the process. Additionally, the thorough identification and characterization of the incorporated strains are crucial to ensure their efficacy and safety in aquaculture settings.
The method used to apply probiotics – often referred to as top coating – plays a crucial role in the viability and effectiveness of probiotics.
Farmers commonly use binding agents such as oils or other hydrophobic substances to manually coat commercial feed, although water is often employed as well. The choice of application and coating agent is paramount to ensure the creation of a protective layer around probiotics, safeguarding them from humidity and air before being fed to shrimp, and from the aquatic environment once in the ponds.
Leaching in the water can be particularly concerning if the top coating is not carried out properly. For instance, studies by Phileo revealed that improperly coated feed could result in up to 99% of the probiotic leaching out within the first 30 minutes of immersion (Figure 14). This particular outcome was observed when using water as a sole coating agent, highlighting the importance of selecting the right procedure to effectively deliver the supplemented dose of probiotics to the shrimp’s gut.
To ensure their efficacy, feed probiotics must reach their target location, the gut, alive and active.
However, this can be challenging to achieve since shrimp have a very short digestive transit time – approximately 3 to 4 hours (Limsuwan and Ching 2012) - which is similar to chicken (Hughes 2008) but much shorter than swine (40 hours; (Wilfart et al. 2007)) or ruminants (50 hours; (Peyraud and Mambrini 1992)) (Table 1).
Most commercial probiotics used in shrimp farming are based on spore-forming bacteria, which can survive extended periods on store shelves or in the gut but need an average of 4 to 8 hours to germinate from their dormant state. This delay can mean that probiotics may not yet be active by the time they reach critical areas like the hepatopancreas and midgut, potentially being excreted in shrimp feces before they can influence shrimp health.
Phileo has addressed this challenge by developing a technology that optimizes the germination of spore-forming bacteria probiotics.
Known as the GO-technology, this patented innovation enriches and primes the germination receptors of spore forming probiotics, allowing them to activate and germinate more rapidly when exposed to the required stimulus.
Efficient germination of most spores depends on three main conditions. Firstly, spores require activation or priming, often achieved through heat exposure. Subsequently, the spores’ germinant receptors must come into contact with germinant activators, specific molecules such as amino acids and sugars (Figure 15). Lastly, the presence of water is essential to facilitate exchanges within the spore content and liberate the spore’s enzymatic machinery.
Our proprietary GO-technology primes spores by pre-exposing them to heat and positioning germinant activators near the germination receptors in advance (Figure 16). Once the third element, water, is added, germination proceeds immediately and rapidly. The priming of the GO-spores significantly reduces the activation time, ensuring the spores are ready to function effectively and promptly once introduced to the aquatic environment, even under sub-optimal conditions
Translated into shrimp farming practices, the GO-technology simplifies the probiotic application process by removing the need for pre-activation steps before feed application. This not only streamlines operations but also boosts cost efficiency by ensuring the delivery of active spores in the gut before the feed is entirely digested.
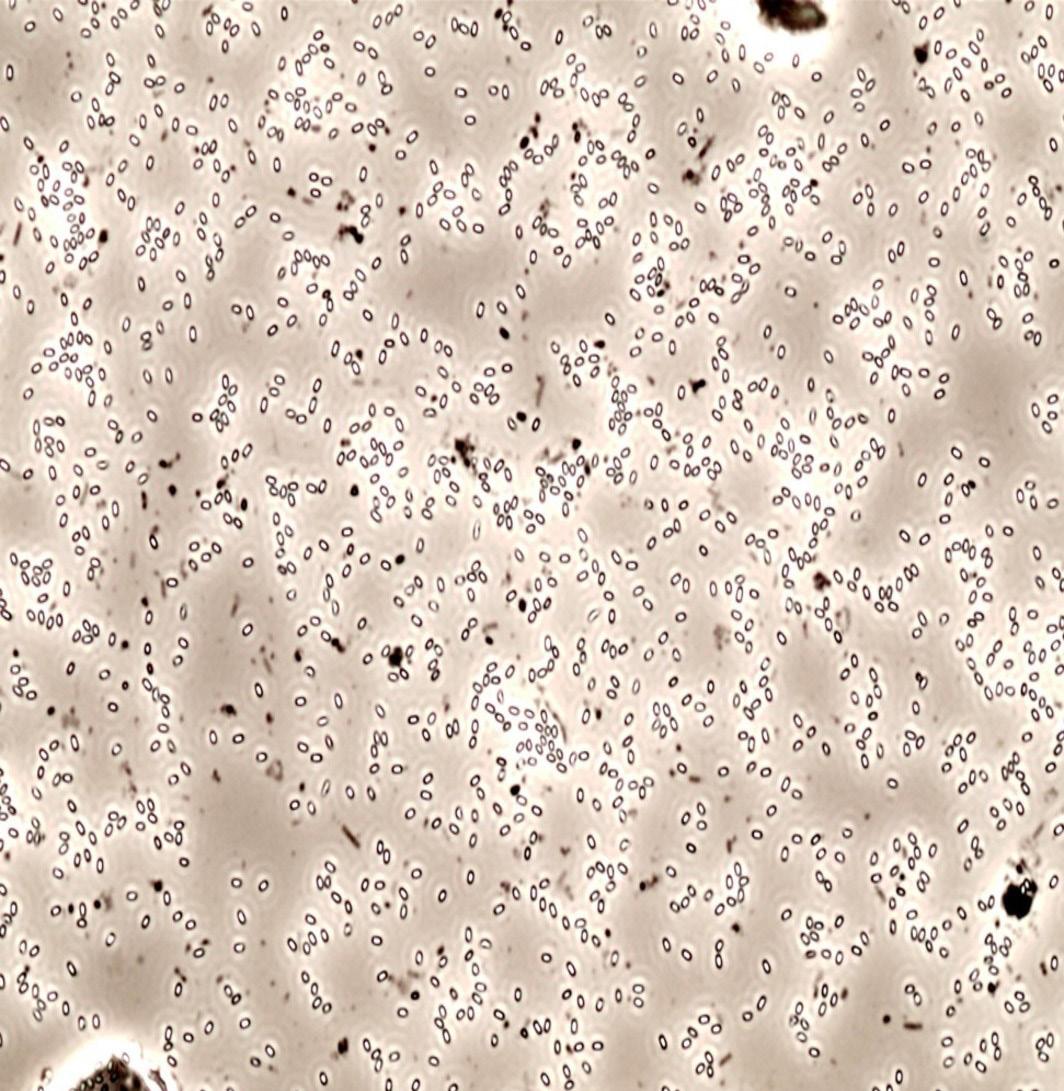

Figure 16. Spores after 90-minute incubation (a) without and (b) with the GO-technology. White cells are spores, which turn black once in vegetative state.
The GO-technology offers consistent performance across variable environmental conditions that probiotics may encounter in the digestive tract of animals or aquatic ecosystems. To verify its efficacy, in vitro trials were performed to evaluate the improvement of germination of Bacillus licheniformis strains with or without the GOtechnology under variable conditions. Notably, these trials demonstrated the potency of the GO-technology in activating bacterial spores within minutes after their introduction to a medium when non-treated spores would take several hours (Figure 17). Additionally, variations in conditions did not significantly impair the early priming of GO-technology spores. For instance, an accelerated germination rate was observed at temperatures as low as 10 °C and as high as 37 °C, demonstrating the GO-technology’s reliability for both warm and cold water aquaculture species. Additionally, an acceleration of germination was observed across pH levels ranging from 4 to 7, indicating that the diverse gut conditions of shrimp may have minimal impact on the activity of GO-technology spores.
ermination as a function of temperature A
Germination as a function of pH


Figure 17. Germination rates of GO-technology spores at (a) different temperatures and (b) different pH levels.
As the shrimp farming industry moves towards more intensive and consolidated production systems, there is a growing need to develop more cost-effective and accurate methods of application of oral probiotics. In this context, the results presented above highlight the potential of GO-technology probiotics to deliver active bacteria more effectively to the shrimp gut, leading to higher performance at similar dosages compared to conventional probiotics thereby improving cost-effectiveness. This is particularly relevant as gut health issues become increasingly prevalent on shrimp farms and probiotics can account for approximately 5 to 10% of total production costs on average.
It is generally considered that a diverse microbiota, constituted by multiple species and strains at high concentrations, is a guarantee of superior quality and higher performance at harvest. While this can contribute to enhanced production outcomes, the value of probiotics also lies in the rigorous characterization and selection process of species and strains based on their pathogen inhibitory and metabolic activity. Equally critical is the ability of probiotics to thrive under nutrient limitations and to proliferate rapidly, countering the competitive advantages of disease-causing Vibrio spp. which are highly prevalent in farm systems.
In this context, probiotics that can quickly establish themselves in the shrimp gut and efficiently utilize nutrients to compete with other bacteria for limited resources are likely to perform better. Rapid colonization is particularly relevant, as probiotics prove highly beneficial in the post-transfer phase early in the growth cycle or when addressing gut dysbiosis. Additionally, the ability of bacteria to compete in nutrient-limited environments is crucial, given that high inclusion rates of probiotics applied to feed can induce nutrient limitations in the gut, leading to the exclusion of less competitive bacterial species and strains.
Phileo’s Microsaf® is a unique association of Bacillus pumilus, B. licheniformis, and B. amyloliquefaciens, each carefully characterized and selected for their strain-specific activities.
These bacteria, with their complementary modes of action and distinct contributions to the probiotic blend, collectively work towards the overarching goal of optimizing farm performance. Bacillus pumilus was selected due to its proficiency in stimulating the immune response. Bacillus amyloliquifaciens was chosen for its capacity to produce enzymes, thereby improving feed digestion and enhancing the overall nutritional status of the animal. Additionally, Bacillus licheniformis was specifically identified for its robust lactic acid production, known to effectively inhibit gut pathogens (Figure 18).
The benchmark and selection of these strains from an extensive internal library of over 3,000 bacteria involved rigorous technical and functional criteria aligned with targeted production objectives. In addition, safety criteria are also applied to safeguard public health, ensuring the exclusion of pathogenic and toxigenic strains. Strains that show antibiotic resistance markers are avoided, thus ensuring the probiotic application is safe and effective.
The positive impact of Microsaf® on farm performance was clearly demonstrated in a trial conducted at Labomar-UFC in Brazil on L. vannamei shrimp (internal data, 2020). The trial took place in outdoor tanks of 6 m3 with a stocking density of 280 individuals of 0.8 grams per square meter to simulate the systemic stress conditions typical of commercial shrimp farming.
The trial evaluated the efficacy of Microsaf® over a 10-week period, at inclusion rates of 105 and 106 CFU per gram of feed with 5 replicates per treatment group. The goal was to determine if low inclusion rates of Microsaf® could match or exceed the performance typically seen with competitor products recommended at 106 to 108 CFU per gram of feed.
Remarkable improvements in zootechnical parameters on shrimp were observed after 10 weeks, with significant statistical differences achieved on all measured technical parameters at a concentration of 106 CFU per gram of feed (Figure 19). Survival rates improved by 27.5% compared to the control group, without compromising productivity per cubic meter – an impressive feat considering the high survival rates observed in the control.

Figure 19. Shrimp performance as evaluated by (a) survival and (b) biomass gain.
Different letters indicate statistically significant differences; Tukey’s HSD test, p < 0.05.
The improvements in survival rates had a positive effect on the Feed Conversion Ratio (FCR) of the two groups supplemented with Microsaf®, which translated to significant economic gains ranging from USD 20,000 to 35,000 per hectare annually (Figure 20). Such results not only underline the biological advantages of using Microsaf® but also its substantial contribution to optimizing the economic viability of shrimp farming, even under high-performing and systematized systems.
Figure 20. Microsaf® benefits as indicated by (a) feed conversion ratio (FCR) and (b) feed costs compared to the control. Different letters indicate statistically significant differences; Tukey’s HSD test, p < 0.05.
Additional improvements in production performance can also be achieved through the application of yeast probiotics. These are being increasingly combined with commercial bacterial probiotics as their mechanisms of action tend to complement each other.
To explore this possibility, an initial validation of the production benefits of Actisaf® was conducted through a 60-day growth trial under laboratory conditions at the University of Can Tho in Vietnam (internal data, 2021). The weekly addition of 2g of Actisaf® per cubic meter of water (resulting in approximately 2.6 x 104 CFU / mL) led to an improvement of L. vannamei performances in both open and recirculating aquaculture systems. The trial resulted in significantly better final weights, FCR, and survival rates in the tanks supplemented with Actisaf® compared to control tanks (Figure 21).
Figure 21. (a) Final weight, (b) FCR, and (c) survival rates in shrimp tanks supplemented with Actisaf® (2.6x104 CFU / mL) compared to control tanks. Different letters indicate statistically significant differences; Student’s t-test, p < 0.05.
After the successful completion of a controlled trial in Vietnam, the efficacy of Actisaf® in enhancing farm performances has been validated through a larger scale trial performed in 50 m3 commercial nursery tanks in Brazil on L. vannamei, this time over 12 production cycles (internal data). A daily Actisaf® water application of 0.5 g / m³ / day resulted in a statistically significant 14.9% increase in survival and a 21.8% improvement in growth rates compared to the control tanks (Figure 22).
These results provide insights into the potential production improvements achievable using high-quality yeast probiotics which can be readily added to shrimp ponds during periods of high stress to complement their bacterial counterparts.
Furthermore, yeast gut probiotics like Actisaf® offer notable benefits for shrimp farming, including the development of natural resistance to commonly used antibiotics such as tetracyclines (Avery et al. 2000). The introduction of these probiotics into water can have a range of effects on shrimp, involving the modulation of water microbiota (del Valle, Bonadero, and Gimenez 2023).
Shrimp may ingest these probiotics through drinking yeast-containing water, as well as through direct ingestion of yeast particles, thereby influencing the composition of the shrimp gut microbiota. Additionally, yeast may colonize external surfaces such as gills and the exoskeleton.
Once ingested, yeast can colonize shrimp digestive tract and compete with potentially pathogenic bacteria for adherence to the intestinal mucosa, boosting gut functionality and consequently improving both feed efficiency and health status (del Valle, Bonadero, and Gimenez 2023). Additionally, and as shown in internal and published trials with Phileo’s products on other aquatic species, yeast can have a direct and beneficial effect on gut morphometry, enhancing microvilli length and density (Ran et al. 2015). In the gut, the presence of parietal fractions from digested yeast, containing beta-glucans and mannanoligosaccharides (MOS), can also stimulate shrimp immune response (del Valle, Bonadero, and Gimenez 2023).
In shrimp farming, probiotics also play an integral role in enhancing feed fermentation and supporting symbiotic systems, offering a range of benefits that notably contribute to promoting gut health throughout the production cycle.
Fermentation of feed and raw materials in shrimp farming uses both bacterial and yeast probiotics which represents a popular practice in some producing countries, allowing for greater inclusion of both plant-based and low-quality ingredients in the feed ration. During fermentation, probiotics break down complex carbohydrates, proteins, and other organic compounds into simpler, more digestible forms. Besides increasing the nutritional value of feed materials, fermentation also generates organic acids, enzymes, vitamins, and other beneficial metabolites for cultured organisms. Since these fermented products are often not subjected to heat treatments in farms, they can also confer a probiotic benefit once ingested by shrimp.
In this context, a trial was conducted with Labomar-UFC in Brazil (Leite, Diógenes, and Nunes 2022) to evaluate the effect of replacing conventional feed with pellets made from fermented plant grains under zero water exchange conditions and high stocking densities of whiteleg shrimp juveniles. The plant-based pellets were primarily composed of grain by-products (> 50%) such as broken rice and wheat middlings (Table 2). Pellets underwent solid-state fermentation, based on the methodology of Yabaya et al. (2009), using Procreatin 7 (25 kg per ton of pellet) as the main fermenting agent. A 24-hour fermentation period was selected for its optimal enhancement of nutritional value. Subsequently, shrimp were fed the fermented pellets at substitution rates ranging from ranging from 0% in the control group to 25%, 50%, 75%, and 100% over a period of 67 days. Throughout this duration, key performance indicators including growth, survival, and FCR were closely monitored.
Broken rice
Soybean meal
Wheat flour
Wheat middlings
Salmon meal
Rice meal
Sugar cane molasses
Soy protein concentrate
Cassava starch
Salmon oil
Calcium carbonate
Soy lecithin
Wheat gluten
Soybean oil
Sodium monophosphate
Magnesium sulfate
NaCI
KCI
Vitamin/Mineral premix
Caolin
Synthetic binder
L-lysine
L-threonine
The incorporation of Procreatin 7 in the fermentation process resulted in a substantial rise in the crude protein content, from 20.9% to 25.2% (Table 3). Additionally, there was a notable reduction in nitrogen-free extract (i.e., the portion of feed that does not contain nitrogen) indicating a conversion of carbohydrates - a vital energy source for microorganisms - into more bioavailable products like organic acids, vitamins, and microbial biomass. This shift not only showcases the yeast’s ability to break down and qualitatively improve feed materials but also highlights the rise in essential amino acid content (Table 4), further demonstrating the potential of feed fermentation.
Table 3. Nutritional composition (g / kg, dry matter) of fermented versus non-fermented plant-based feed pellets.

Amino acids Fermented vs non-fermented (%)
Essential amino acids (EAA) Arginine
Fermented Non-fermented Plant-based pellets
Met + Cys
EAA
Non essential amino acids (NEAA)
Table 4. Amino acid content (g / kg, dry matter) of fermented versus non-fermented plant-based feed pellets.
Fermentation with Procreatin 7 not only enhanced the nutritional quality and protein content of grain-based pellets but also proved to be technically feasible for replacing up to 25% of the total feed ration. This replacement rate effectively maintained performance metrics at levels comparable to those achieved with conventional feeds, all the while realizing cost savings of USD 0.08 per kilogram of shrimp (Table 5) (Leite, Diógenes, and Nunes 2022).
Feed replacement by fermented plant-based pellets (%)
Initial bod y weight (g)
Final body weight (g)
Survival (%)
Growth rate (g / week)
Biomass gain (g / m2)
Feed intake (g / shrimp)
Table 5. Comparative performance parameters of L. vannamei shrimp fed with different diets replacing conventional feed with fermented grain-based pellets (mean ± S.E.).

Low quality grain by-products, such as bran and husk, are also now emerging as cost-effective carbon sources to maintain water quality and promote shrimp growth. These alternatives to molasses can function as nutrients for beneficial microorganisms, playing a crucial role in organic matter decomposition and nutrient cycling. The beneficial effects of water enrichment from fermented rice by-products (bran, husk, kernels) with Procreatin 7 were demonstrated in a trial conducted with L. vannamei at Labomar-UFC in Brazil (internal data, 2018). Performed over 70 days in 1.5 m3 tanks with a stocking density of 120 individuals / m2, the experiment aimed to assess the benefits of fermenting traditional carbon sources added to pond water to boost productivity and water quality. The trial included four experimental groups: control tanks with no carbon enrichment, tanks supplemented with sugar cane molasses, unfermented rice by-products, and fermented rice fertilizer (FYR) with yeast (Procreatin 7 at 0.06 g / m3) and premium yeast cell wall (Safmannan®) for 24 hours. This enrichment process was conducted for 24 hours, three times per week, at a rate of 4.5 grams / m3 .
The results affirmed the potential for significant enhancements in production conditions through the enrichment of pond systems with a fermented carbon source. This enrichment contributes to rebalancing the carbon-to-nitrogen ratio, particularly in systems employing intensive feeding, and fosters optimal conditions for the proliferation of beneficial microorganisms. The trial also revealed that fermenting carbon sources such as rice coproducts with Procreatin 7 could effectively increase their nutrient value and bioavailability, leading to superior shrimp growth compared to supplementation with non-fermented rice byproducts (Figure 23).
Figure 23. Comparison of final body weights of shrimp non-supplemented, supplemented with yeast-fermented rice by-products, and supplemented with unfermented sugar cane molasses or rice by-products. Different letters indicate statistically significant differences; Tukey’s HSD test, p < 0.05.
Therefore, incorporating Actisaf® or Procreatin 7 into shrimp farming practices shows promising potential in enhancing performance by improving multiple aspects of gut health, nutrient availability in low-cost feeds, and water quality.
Yeast probiotics play a pivotal role in gut health optimization, thereby supporting digestion, nutrient absorption, and overall gut function. Additionally, these probiotics can contribute to the fermentation of various agricultural by-products, enhancing their bioavailability for shrimp consumption and effectiveness in supporting the growth of beneficial microorganisms in aquaculture systems.

CHAPTER 2
The shrimp industry faces numerous challenges, but perhaps the most significant and recurrent one has been and still is the threat of mass mortalities due to disease outbreaks. According to a survey conducted by Hatch Blue in 2020, disease outbreaks consistently emerge as one of the most predominant production challenges cited by farmers globally (Table 6). The progressive intensification of production systems, driven by growing market demand and water and land constraints, has inadvertently created conditions in which pathogens can easily thrive. Among these, the White Spot Syndrome Virus (WSSV) has proven to be the most devasting, causing catastrophic losses for shrimp farmers.
Other infections such as vibriosis, the EMS or Acute Hepatopancreatic Necrosis Disease (AHPND), and EHP, further compound the struggles faced by the industry. Overall, the shrimp industry contends with a wide range of pathogens, including bacteria, viruses, fungi, and parasites which are linked to intricate disease dynamics and symptoms, making disease diagnosis and treatment complex. The resulting diseases often lead to high mortalities, significantly impacting the productivity and profitability of shrimp farming.
Amidst these challenges, optimal health management practices are crucial for cost control and production success.
Despite their important economic impacts, there are currently no effective treatments against most shrimp diseases, rendering prevention measures the best viable strategy for farmers. Efforts are directed essentially at managing the three components of the epidemiological triad – the interplay between the host (shrimp), the pathogens, and the environment surrounding the host where pathogens can thrive –to mitigate risks (Figure 24).
Historically, farmers have focused on environmental and pathogen control, investing in various measures such as disease-free stocks and resistant genetics, water disinfection and conditioning, biosecurity barriers, etc. Yet, even with these integrated into intensive systems, they do not always eradicate the risk of a disease outbreak. Pathogens continue to find their way into shrimp farming systems through various means, such as improper disinfection protocols or accidental introduction of carriers, and the efficacy of disease-resistant stocks can be compromised by the evolution of strains or the emergence of new pathogens.
Once pathogens have infiltrated the farm, their rapid spread is facilitated by the tropical temperatures necessary for shrimp farming and the ubiquity of water, which serves as a crucial medium for pathogen transmission. Moreover, unlike fish, shrimp live on the pond bottom, where they are in constant contact with substrates and potentially pathogenic microorganisms. Shrimp also undergo regular molting to grow, which makes them more susceptible to infections and parasites during this period. Furthermore, shrimp have limited adaptive immune abilities when compared to fish, and there are currently no commercial vaccines available to protect against future infections.
In light of these circumstances, there has been growing interest in implementing preventive strategies that enhance the natural defense mechanisms of shrimp – the host – enabling them to combat infections and better cope with the conditions that may lead to disease.
The advancement of intensive practices and the development of high-quality compound diets extend beyond basic nutrition, influencing immune function and stress resistance to address specific challenges or production objectives. Over the past two decades, farmers have also progressively shifted and professionalized their management practices, enabling the development of more advanced functional feed concepts. These approaches aim to strengthen disease prevention and minimize disease impacts for both farmers and feed producers. This requires, however, a good understanding of the physiological mechanisms associated with the desired functionality and the use of the appropriate functional ingredient.
Shrimp have a rudimentary immune system primarily relying on innate responses, sometimes also referred to as non-specific responses. This innate immunity helps provide a generalized defense against pathogens and invaders, unlike the more complex adaptive immune systems found in other organisms like fish. Despite this, shrimp are still able to mount some degree of specific immune response, although not as robust or long-lasting as in more advanced water species.
The innate immune system of shrimp is organized into three successive layers of defense (Figure 25):
Physical barriers: the cuticle, along with the epithelial layers of the gills and gastrointestinal tract, act as the first line of defense, effectively preventing pathogen entry. The mucus covering some of these surfaces also acts as a physical barrier, trapping pathogens. This mucus contains various antimicrobial enzymes and peptides (AMPs) which neutralize and inhibit pathogen growth. These barriers also possess various receptors that ensure efficient pathogen recognition which triggers appropriate immune responses.
Cellular responses: if pathogens evade the primary barrier and enter the body cavity, the humoral and cellular components of the innate immune system respond, preventing the infection from progressing. Cellular responses, also known as cellmediated responses, are carried out by hemocytes – the primary immune cells found in shrimp (Rajendran et al., 2022). These are located in the hemolymph and within tissues such as gills, lymphoid organ, and the hepatopancreas. Hemocytes perform crucial immune-related functions such as phagocytosis – engulfment and digestion of foreign particles or pathogens – and pathogen encapsulation – encirclement of the invader to neutralize it and prevent its spread (Aguirre-Guzman et al., 2009; Kulkarni et al., 2021; Rajendran et al., 2022).
Humoral response: this involves a diverse range of antimicrobial substances, including enzymes, peptides, and reactive oxygen species, that either directly destroy pathogens or mark them for destruction by other components of the immune system. Shrimp produce several antimicrobial substances, including penaeidins, lysozymes, crustins, stylicins, and the anti-lipopolysaccharide factor (ALF) (Rajendran et al., 2022). These are synthesized and stored by the hemocytes and released in concert with cellular responses to effectively eliminate pathogens.
Biosecurity at farm
Water quality, management, nutrition, etc.
Epithelial barriers
Integrity of the cuticula r layer mucus, gills, gut
Pathogen breach
Pathogen recognition
Number of immune cells and receptors
Cellular responses

Functionalit y of hemocytes
Humoral responses
Maintaining the integrity and functionality of the shrimp’s physical barriers and the hemocytes is therefore key for a strong and effective defense system. Strategic interventions can be implemented to support and enhance these natural defenses, providing a more effective safeguard against pathogenic threats.
As evoked in chapter 1, the gut is the main route of infection for several important pathogens leading to major diseases, including EMS, EHP, and WFS. Infections caused by these microorganisms are particularly common in regions such as the hepatopancreas and the anterior midgut, inducing damage mainly by colonizing and releasing toxins that harm the epithelial cells. Moreover, damage can be exacerbated by prolonged inflammatory reactions from the shrimp’s own immune system.
Probiotics are widely employed in shrimp farming to stimulate the growth of beneficial bacteria in the gut and prevent the proliferation of harmful ones. However, their application, especially when targeting the gut through feed, faces complexities due to challenges associated with feed extrusion and pelleting processes. Their germination can also be variable, and spores may not always be activated before the feed is digested and excreted.
To complement probiotic use, aquafeeds are increasingly being supplemented with components that directly impede pathogenic bacteria from binding and interacting with the gut epithelium.
Bacteria also utilize fimbriae, commonly known as pili, which are hair-like appendages on their surface. These structures play a crucial role in adhering to surfaces, such as gut cells during infection, and in the formation of biofilm, which is essential for evading host immune responses (Telford et al., 2006; Soonthornchai et al., 2015) (Figure 26).
components of a Gram-negative bacteria.
Bacterial fimbriae have a strong affinity for mannose, a sugar molecule commonly found on host cell surfaces, as part of glycoproteins or glycolipids forming their wall. Yeast cells, composed largely of mannose-rich polysaccharides (approximately 40% of the yeast cell wall mass), can be hydrolyzed to yield MOS. Phileo’s in vitro trials conducted at the IMAqua laboratory at Ghent University in Belgium demonstrated that Safmannan®, a Phileo solution containing high levels of mannans, has a high affinity for binding with Vibrio campbelli, a pathogen implicated in EMS in shrimp (Tang et al., 2020) (Figure 27).




Trial performed at IMAqua laboratory, Univ. of Ghent, Belgium.
27. Safmannan®’s binding effect with Vibrio campbelli after 30-minute interaction.
Given that most aquaculture pathogens are Gram-negative bacteria (Table 7), which possess fimbriae, Safmannan® is aptly poised to protect against a broad spectrum of pathogens commonly encountered on shrimp farms. Furthermore, the consistent concentration in mannans (≥ 20%) in Safmannan® and the highly controlled manufacturing process ensure consistent and reliable biological performances.



Gram +
GramVibrio parahaemolyticus
Vibrio harveyi
Vibrio alginolyticus
Streptococcus agalactiae
Aeromonas hydrophila
Francisella noatunesis
Flavobacterium columnare
Edwarsiella ictalurii
Aeromonas hydrophila

Moritella viscosa
Tenacibaculum maritimum
Flavobacterium columnare
Vibrio anguillarum
Table 7. Overview of predominant bacterial pathogens in major aquaculture species, highlighting the prevalence of Gram-negative bacteria in shrimp and other fish.
The efficacy of Safmannan® has also been validated through in vivo trials. For instance, in Nile tilapia (Oreochromis niloticus), which is Phileo’s species of reference for research, the consistent inclusion of Safmannan® in feed at mild dosages of 0.5 and 1 kg per ton resulted in a significant reduction of gut pathogen levels after infection with Streptococcus agalactiae. This led to increased survival rates over a three-month period (Figure 28) (internal data, Thailand, 2006).
Concentration (CFU / ml) of Streptococcus inside the gut 4 days after challenge
ortality after challenge by Streptococcus
Figure 28. Status of Nile tilapia after challenge with Streptococcus agalactiae as evaluated by (a) gut concentration of S. agalactiae 4 days post-challenge and (b) mortality rates in control groups versus Safmannan® inclusion at 0.5 and 1 kg / ton.
Once ingested through feed, mannans bind and saturate the fimbriae of pathogenic bacteria, thereby reducing their adhesion to the digestive tract.
This not only exposes them to the shrimp’s immune responses but also facilitates their expulsion through gut fluids and feces (Gainza & Romero, 2020). Additionally, mannans serve a dual protective role by binding to mannose receptors in the intestinal tract. Mannose receptors are a specific type of protein present on the surface of shrimp cells involved in recognizing and binding to mannose-containing structures present on the surface of potential pathogens. When mannans engage with these receptors, they block potential sites for bacterial attachment and trigger a series of signaling events leading to the activation of the innate immune response. Overall, these mechanisms importantly contribute to preserving the epithelial barrier of the gut and the integrity of its cells.
A challenge trial was organized at the University of Can Tho in Vietnam to verify the effectiveness of Safmannan® in preventing alterations of the gut epithelium caused by EMS in shrimp (Oanh and Tacon, 2015). Earthen ponds were used and
Safmannan® was incorporated into the feed at pulse concentrations of 1 to 2 kg per ton. To induce pathogens in the gut, shrimp previously infected with Vibrio parahaemolyticus were introduced with healthy stocked shrimp at a ratio of 1:20. Since EMS typically occurs 30-35 days after stocking, shrimp with an average body weight of 1 g were selected. Following infection, a histopathological analysis was performed on the hepatopancreas of both the control and the Safmannan®-treated groups to evaluate gut morphological changes.
At the end of the trial, the Safmannan®-treated groups exhibited a milder loss of differentiated cells in the hepatopancreas compared to the control one (Figure 29). Additionally, in the Safmannan® groups, the hemocytic infiltration between the tubules of the hepatopancreas was less profuse, and interstitial space appeared more fibrous. These findings were consistent with a substantial improvement in the overall health of the shrimp (Figure 30) and a remarkable increase in survival rates from 1.6% in the control group to 42% in the group supplemented with Safmannan® (1 kg / ton) after 21 days. These findings suggest that the incorporation of Safmannan® supports the maintenance of the hepatopancreas epithelial tissue integrity and may enhance the immune and digestive functionality of the gut.

Tubule morphology was preserved and loss of cell type differentiation was less pronounced (mainly less B-cells) compared to the control. Hemocytic infiltration was less profuse, with interstitial space appearing more fibrous.
Control group

Typical hepatopancreas in Control group shrimp at 3 days post challenge: palecolored, atrophied, empty gut.
Safmannan® group

Typical hepatopancreas in Safmannan® group shrimp at 3 days post challenge: normal color and size, full gut.
Preserving the integrity of the gut epithelium and optimizing immune cell functionality are essential aspects of successful disease prevention strategies in shrimp farming.
Figure 30. Comparison of the gut and hepatopancreas conditions in shrimp from the control group versus those treated with Safmannan® 3 days post-challenge.
As immunostimulants become more prevalent in feed formulations, it is critical to recognize that robust immune responses depend not only on the quality of the feed but also on the health of the shrimp’s digestive and immune systems. The latter must be preserved to ensure that shrimp can properly process and utilize nutrients, thereby mounting a potent immune defense.
Despite the important economic impacts caused by disease-related mortalities, there are currently no effective treatments against many shrimp pathogens apart from antibiotics, highlighting the need for alternative approaches.
The use of antibiotics in recent times has become customary to treat recurrent diseases in shrimp farming. In some cases, their usage has also shifted from being exclusively curative to being used preventatively to control the emergence of pathogens. Such practice is particularly prevalent in the first weeks of the production cycle and among intensive farms (Figure 31), where gut health is crucial yet vulnerable. Ultimately, the overuse of antibiotics raises substantial concerns about bioaccumulation, antibiotic-resistant pathogens (Pham et al., 2018; Li et al., 2021), and environmental impacts. Moreover, the preventive use of antibiotics can weaken the immune system, notably by eliminating beneficial bacteria in the gut (Chen et al., 2023), making them more dependent on the substance.
Conversely, vaccines can be efficiently used to make the host immune-competent. However, unlike mammals and other vertebrates, shrimp do not possess an adaptive immune system and consequently developing vaccines for shrimp remains a complex, costly, and ongoing area of investigation.
Given these challenges, discontinuing the use of antibiotics altogether is not a viable option, as it would leave shrimp vulnerable to diseases and farmers to significant financial losses associated with high mortalities. This dilemma further emphasizes the urgent need for alternatives that help to reduce the dependence on antibiotics in shrimp aquaculture.
In this context, immunostimulants have emerged as one feasible approach to preventing and fighting disease outbreaks, compensating for the discrepancies of antibiotics and vaccine usage. By stimulating key immune cells and pathways, immunostimulants offer producers the opportunity to “hack” the shrimp’s defense machinery, triggering a robust and effective response. These molecules are used proactively to prepare the shrimp’s immune system before pathogen exposure, resulting in heightened immune response that can reduce the severity and duration of infections.
Figure 31. Proportion of shrimp farmers using antibiotics to prevent diseases per production system in Vietnam (n = 57) Extensive: 0.15 to 1 t./ha/year; Semi-intensive: 1 to 20 t./ha/year; Intensive: 20 to 80 t./ha/year; Super-intensive: 80 to 150 t./ha/year
Using immunostimulants as part of a regular health management regime provides shrimp with ongoing protection, potentially averting disease outbreaks and minimizing the need for antibiotics.
This strategic preemptive approach utilizes the natural pathway of the shrimp’s innate immune response, which is normally triggered upon pathogen detection by pattern recognition receptors (PRRs) – sensor-like proteins that continuously survey the shrimp’s environment to identify the presence of pathogens (Rajendran et al., 2022). PRRs are distributed across shrimp tissues and detect pathogen-associated molecular patterns (PAMPs) such as bacterial and fungal cell wall components (e.g., lipopolysaccharides, peptidoglycans, beta-glucans) as well as viral nucleic acids (Song and Huang, 2000; Lee and Söderhäll, 2002; Kulkarni et al., 2021; Aguirre-Guzman et al., 2009). Upon binding with the shrimp’s PRRs, PAMPs trigger a cascade of immune signaling events leading to the activation of immune cells and the release of immune effectors from the humoral and cellular responses (Figure 32).
beta-glucans from fungi
PRR on epithelial barrier of shrimp & various tissues and cells
Double-stranded RNA (dsRNA) from viruses
LPS from gram-negati ve bacteria
Most molecular “hacking” strategies to stimulate this pathway utilize beta-glucans as a triggering factor. These are particularly effective because they are naturally found on the cell surfaces of diseasecausing pathogens, making them recognizable to the immune system. Beta-glucans largely pass through the shrimp’s digestive system undigested, given the limited enzymatic ability of shrimp to break down such complex components. Upon reaching the gut, they function as PAMPs, activating immune cells.
Beta-glucans, extracted from the cell wall of yeast, fungi, seaweed, and bacteria, vary in their immunestimulating efficacy based on their molecular structure (e.g., molecular weight, solubility, branching proportion, and types of linkages). Yeastderived beta-glucans are generally considered superior due to their 1.3 backbone linkage and 1.6 side chains (Table 8). These unique characteristics enhance their structural diversity and therefore their ability to interact with the PRRs.
In aquaculture, yeast beta-glucans are primarily sourced from yeast cell wall (YCW) extracts, which are rich in both beta-glucans and MOS. Yet, the biological efficacy of this source can significantly vary due to factors such as the yeast strain origin, production processes (primary production vs. secondary by-products), extraction, and purification methods. These factors dictate the beta-glucans’ structure, composition, and properties, ultimately impacting their effectiveness as immunostimulants and leading to a large variety of qualities (Figure 33). Additionally, discrepancies between yeast beta-glucan sources on the market, both in terms of analytical methods and guarantees provided on beta-glucans’ concentrations, add complexity to the identification and selection of an appropriate source for aquaculture use.
Phileo by Lesaffre ensures the potency of its beta 1.3 / 1.6 glucans by controlling the yeast strain selection, extraction, and processing methods to maintain the desired conformation, composition, and properties.
This rigorous control guarantees that products like Safmannan® consistently provide a high concentration of bioactive beta 1.3 / 1.6 glucans and mannans, establishing it as a reliable and effective immunostimulant option for aquaculture use. Furthermore, Safmannan® sets itself apart by providing clear and accurate guarantees of active component concentrations and batch-to-batch consistency (Figure 34). This provides transparency and reassurance to aquaculture producers seeking a dependable beta-glucan source and more biological benefits than YCW by-products from other industries.

Analysis of Mannans in 16 batches of Safmannan®
Analysis of beta-glucans in 16 batches of Safmannan®
(1,3 and 1,6)
ch number
ch number
Activation of shrimp immune signaling pathways through PPRs can lead to various defense responses, primarily implemented by hemocytes – the immune cells at the forefront of the shrimp’s immune response.
Hemocytes play a crucial role in coordinating and executing diverse humoral and cellular defense mechanisms in response to specific pathogen types.
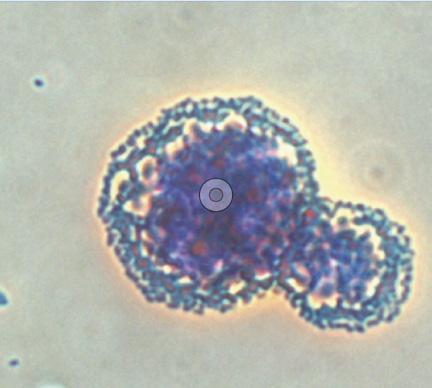
They serve as critical indicators of the success of immune stimulation strategies and their biological impact. Shrimp possess different types of hemocytes, each with specific functions within the immune system. These include hyalinocytes (agranular), semi-granulocytes (small granule), and granulocytes (large granule) (Sun et al., 2020; Aguirre-Guzman et al., 2009) (Figure 35). These hemocytes are commonly distinguished by their cell size, nuclear to cytoplasmic ratio, and the number of intracellular granules (Rajendran et al., 2022) .
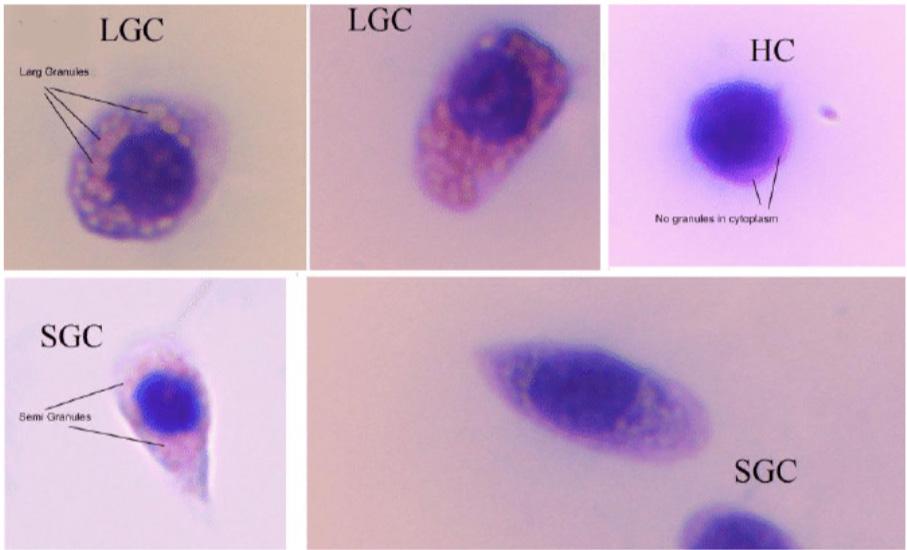
Recent research revealed the distinct roles of each hemocyte type. Hyalinocytes are primarily involved in phagocytosis, a cellular response, while semi-granulocytes and granulocytes are more active in the humoral immune signaling and effector pathways, such as the activation of the prophenoloxidase (proPO) system. Yet, the full understanding of each hemocyte type’s role in immunity remains limited and there are functional overlaps between them. For instance, hyalinocytes, semi-granulocytes, and granulocytes can all participate in cellular responses such as encapsulation (Sun et al., 2020; Rajendran et al., 2022).
Cell-mediated responses directly involve hemocyte interventions to prevent pathogen spread and proliferation via phagocytosis, encapsulation, and nodule formation (Figure 36).
The type of response adopted by hemocytes will usually vary depending on the nature and extent of the pathogenic threat.
Extension of hemocyte membrane around invading particle (e.g., bacteria), enclosing it within a phagocytic vacuole or phagosome. Inside the phagosome, a humoral response consisting of enzymes and other antimic robial factors is released to destroy the engulfed particle.
Isolates and neutralizes large foreign particles (e.g., parasites). Upon detection, hemocy tes are triggered to cluster around it, forming a multi-l ayered capsule and minimizing its impact on shrimp tissues. The hemocytes then release various humoral substances to immobilize the pathogen
Hemocytes form aggregates or clusters around a foreign object, particularl y when they are numerous. Alike encapsulation, these clusters isolate the pathogen. However, they can be formed without a well-defined structure as seen in encapsulation. However, they involve the release of humoral effectors and can recruit other immune cells.
Shrimp also exhibit humoral responses, mediated by soluble factors in the hemolymph produced and stored by hemocytes. These responses work in harmony with cellular mechanisms. After isolating pathogens through processes like phagocytosis or encapsulation, the hemocytes deploy humoral factors to completely eradicate them. Similarly to cellular responses, the specific humoral response and effector molecules produced can vary depending on the type of pathogen encountered and the signaling cascade initiated by the PAMPs via the PRRs. Some common immune effectors include antimicrobial peptides, lysozymes, and proPO enzymes which work in diverse ways to neutralize or eliminate pathogens (Table 9).
Periods of sudden and chronic stress can significantly influence the abundance of hemocytes in shrimp, thus affecting their capability to produce strong cellular and humoral responses against pathogens
Research by Jiang et al. (2005) examined the impact of different water oxygen concentrations (7.5, 5.5, 3.5, and 2.0 ppm) on the hemocyte concentration in shrimp. The results revealed a strong correlation between dissolved oxygen and hemocyte levels. Notably, short-term exposure (24 h) to suboptimal oxygen concentrations of 5.5 ppm (70% saturation), 3.5 ppm (45% saturation), and 2.0 ppm (20% saturation) resulted in a significant loss of hemocytes (up to 40%), diminishing the shrimp’s immune capacity (Figure 37). These findings emphasize the significance of maintaining optimal dissolved oxygen levels in shrimp aquaculture, particularly in the context of intensified farming where oxygen fluctuations are frequent and challenging to manage within short time frames. Such variations can heighten the vulnerability of shrimp to infections, particularly in intensive farming environments where factors like algal blooms or the accumulation of organic matter rapidly impact water quality by increasing bacterial activity and, consequently, oxygen consumption.
In another study conducted by Wang and Cheng (2005), shrimp were stocked at 25 parts per thousand (ppt) salinity and injected with the pathogenic V. alginolyticus (1.0x104 CFU). Subsequently, shrimp were transferred to varying salinity levels (i.e., 5, 15, 25, and 35 ppt) for 96 hours. The results indicated that the highest shrimp mortality occurred in shrimp abruptly transferred from 25 to 5 ppt, as opposed to those transferred from 25 to 35 ppt. Total hemocyte count (THC) analysis revealed that the abrupt salinity change compromised the shrimp’s immune defenses and resistance to Vibrio (Figure 38). In contrast, transferring shrimp from low (25 ppt) to high salinity (35 ppt) did not significantly impact their survival.
These results hold particular relevance, considering that a substantial portion of global shrimp production occurs in estuarine regions, such as parts of Ecuador, Vietnam, India, and China. In these areas, salinity levels are inherently variable, influenced by factors like tides, river flow, and rainfall. Moreover, these fluctuations are exacerbated during monsoon seasons with heavy rainfall. Such environmental stressors like fluctuating oxygen and salinity levels can weaken shrimp’s immune defenses. Prolonged infections can also exhaust the shrimp’s immune system, rendering it unable to mount an effective defense against pathogens. This leaves shrimp stocks susceptible to secondary infections, often resulting in sudden and severe mass mortality events. Recovering shrimp from such infections may also require significant time and effort, resulting in substantial financial losses for farmers.
Yeast beta-glucans stand out for their ability to supplement a high-quality diet by providing the immune system with the necessary stimulation to maintain consistent and high levels of hemocytes during periods of sudden environmental stress and throughout the course of an infection (Meena et al., 2013).
Incorporating beta-glucans into shrimp feed primes the immune system for heightened resilience against adverse conditions and more efficient infection management.
An experimental challenge trial was conducted at the University of Can Tho in Vietnam to assess the effects of yeast beta-glucans on the immune system of shrimp (Oanh and Tacon, 2015). Specifically, the trial aimed to evaluate the ability of Safmannan® on shrimp immune system prior to and after infection with EMS. Healthy L. vannamei shrimp with an average weight of 2.5 grams were fed Safmannan® at a low concentration (0.5 kg per ton of feed) for two weeks and then challenged with the pathogenic V. parahaemolyticus. The total hemcoytes count (THC) was measured on the day of the challenge and three weeks after, to determine the impact of the Vibrio on shrimp’s immune cell levels throughout the course of infection and assess the influence of Safmannan® supplementation.
Three weeks after the challenge, results revealed a 24.2% decline in THC in the infected group fed the standard diet, while the non-challenged group’s THC remained relatively stable, illustrating the pathogen’s detrimental effect on immune health (Figure 39). Notably, unlike previous studies conducted by Wang and Cheng (2005) and Jiang et al. (2005), the shrimp used in this trial were initially healthy and maintained under optimal conditions, underscoring the extent to which pathogens can affect even healthy and well-maintained shrimp stocks.
Figure 39. Total hemocyte counts (THC) pre and post EMS challenge in shrimp fed a control diet or a diet supplemented with Safmannan®. Adapted from Oanh and Tacon (2015).
Crucially, the trial demonstrated that dietary inclusion of Safmannan® allowed the challenged shrimp to maintain consistent hemocyte concentration throughout the three-week challenge, comparable to that of the non-challenged control group. This outcome suggests that Safmannan® not only supports immune activation in shrimp but also upholds the optimal number of immune cells during disease exposure
To complement the results, the proPO activity – one of the humoral responses hemocytes can mount as part of the immune response – was measured to evaluate the capability of Safmannan® to not only activate and stimulate immune cells but also to improve their functionality and response. Indeed, while an increase in immune cell count may suggest an activation of the immune system, it does not guarantee its full functionality. To assess the immunostimulant impact accurately, it is crucial to evaluate parameters that reflect the immune system’s functionality, such as the production of
immune molecules during the responses performed by hemocytes (e.g., phagocytosis, encapsulation). In the study, a two-week Safmannan® application (at 0.5 kg / ton of feed) before the challenge more than doubled proPO activity (Figure 40). This illustrates Safmannan®’s capability to stimulate immune cells and enhance their functionality prior to infection, offering shrimp significantly heightened defenses and increased chances of survival in the event of an infection. Additionally, a significant 60% decline of the proPO activity in the control group (Figure 40) was observed three weeks after EMS challenge. This vulnerability is critical, as it not only leads to a reduction in the population of immune cells but also compromises their functionality within a short time span, leaving shrimp susceptible to secondary infections and hindering their ability to recover. Conversely, continuous application of Safmannan® throughout the infection period effectively mitigated this decline, with only a 37% reduction in proPO activity.
In the broader context of shrimp health management, where effective treatments for major diseases are lacking and considering the relatively primitive immune defenses in shrimp, the strategic stimulation of various immune components assumes particular importance. A proper understanding of the physiological mechanisms desired and the modes of action of health additives is key to achieving tangible results. The timing and dosage of such supplements are also decisive factors that contribute to measurable benefits in shrimp health and benefits to farmers.

Timely application of YCW fractions containing MOS and beta-glucans is critical for the activation of the shrimp’s immune system and managing gut pathogen levels, especially during periods when shrimp are more prone to infections.
There are different strategies for the application of YCW fractions, combining time and dose, tailored to specific technical objectives, including the following:
Prophylactic use: for ongoing immune support and pathogen resistance, a lower dose is administered consistently over an extended period. This strategy aims to prime the immune system and enhance its readiness to combat pathogens.
Therapeutic use: aimed at boosting the immune response during active infections and aiding in recovery. Higher doses may be used initially to provide a strong response within a short period of time, followed by a rapid reduction to avoid an over-stimulation of the immune system.
Seasonal / stress-induced use: recognizing times or conditions when shrimp are known to be more susceptible to infections, YCW fractions can be applied preventively to maintain immune system functionality and reduce the impact of opportunistic pathogens.
The selection of dose and timing for application is influenced by various factors, such as shrimp species, age, health status, and environmental conditions on the farm.
Given the ubiquity of pathogens and their increasing threat to shrimp farming, there is a growing interest in the adoption of therapeutic-like applications of immunostimulants for rapid and effective immune activation.
A study at the ShrimpVet laboratory in Vietnam explored the impact of various Safmannan® application methods on shrimp (L. vannamei) survival after exposure to the pathogenic V. parahaemolyticus. The trial compared the effects of Safmannan® supplementation for 7 or 14 days before the challenge with doses ranging from 0.5 to 3 kg per ton of feed. Lower doses (0.5 to 1 kg per ton of feed) simulated prophylactic use, while higher doses (2 to 3 kg per ton of feed) mimicked therapeutic-like applications. The effectiveness of each application method was evaluated by measuring the survival rates 10 days post-challenge.
The results showed that the highest Safmannan® dosages generally produced better outcomes, but the duration of supplementation (7 or 14 days) was not a decisive factor in enhancing shrimp survival after challenge. Specifically, a 14-day supplementation at 2 kg per ton of feed resulted in the highest survival rate of 63%. Interestingly, similar results could be achieved with a shorter, 7-day supplementation at a higher dose of 3 kg per ton of feed, achieving a 61% survival rate (Table 10).
Treatment 1 –prophylactic
Treatment 2 –prophylactic
Treatment 3 –therapeutic
Treatment 4 –therapeutic
Treatment 5 –therapeutic
Table 10. Shrimp survival rates post-Vibrio challenge with varying Safmannan® application methods.
A notable correlation emerged between the total quantity of Safmannan® administered pre-challenge and shrimp’s survival rates observed at the end of the trial, suggesting that doses of 1 kg / ton of feed or higher are crucial for significant protection, irrespective of the application period (Figure 41). Lower doses, under 1 kg / ton of feed, failed to offer substantial protection within the 7 to 14-day timeframe.
These results suggest the potential for rapid immune activation in shrimp through adequate Safmannan® supplementation. Such observations are in line with previous studies conducted by Xu et al. (2019), showing that shrimp’s immune system could be activated within a few hours following sudden and activating stimuli induced by a combination of temperature shock and air exposure. This underlines the speed at which the immune system of shrimp and its various defense mechanisms can be mobilized in time to counteract infections.
Figure 41. Correlation between the total dose of Safmannan® provided pre-challenge and shrimp survival rate post-challenge across different treatment groups.
In urgent scenarios, where there is an immediate threat of pathogens and a sense of emergency, implementing booster doses of Safmannan® could be a crucial strategy to mitigate the risk of mass mortality and increase the chances of survival for the shrimp.
To enhance the effectiveness of disease management in shrimp farming, early intervention is key. On the first day after exposure, pathogens attempt to establish a position within the host, a period during which the shrimp’s immune response may not be fully activated. This period is crucial as it gives pathogens the opportunity to multiply rapidly before the immune system mounts a full response. For example, a study monitoring shrimp (L. vannamei) challenged with V. parahaemolyticus for 10 days showed that more than half of the mortalities occur within the first 48 hours of infection (Tran, Richard, and Tacon 2017). The onset of mortalities typically escalated after a short incubation period of around 24 hours (Figure 42), marking the beginning of an acute phase of the disease. This phase can extend for several days, during which symptoms become distinctly visible.
Taking effective measures to impede the development of pathogens during this crucial early phase can yield significant differences in the overall outcome.
By intervening early, it becomes possible to disrupt the pathogen’s ability to take hold and proliferate within the shrimp. This can involve the use of appropriate treatments to effectively suppress the growth or activity of the pathogens. Applications of Safmannan® have demonstrated a significant effect on the rate at which pathogens spread during the initial stages of infection, resulting in dramatic reductions in mortality within the first 48 hours (Figure 42). This early impact of Safmannan® is particularly valuable as it provides producers with
additional time to implement emergency measures such as aeration, water exchange, or therapeutic treatments. Moreover, Safmannan® not only helps during the initial response but also contributes to reducing the duration and severity of the acute infection phase. This leads to stronger performance outcomes at harvest, such as better growth rates and FCR, resulting in tangible benefits for shrimp producers.
In addition to the previous scientific studies conducted in controlled conditions, Safmannan® has also undergone several field trials to further understand its mode of action. These trials were performed to assess its effectiveness in addressing pathogenic challenges under various conditions, including disease pressure, environmental stress, and different production practices.
The first trial of this kind was conducted in the province of Rio Grande do Norte, located on Brazil’s northeastern tip. This region is renowned for its vast coastal sand dunes and salt production areas, which are also utilized for the semi-extensive production of whiteleg shrimp. The farm where the trial took place encompassed over 100 hectares and faced various environmental challenges, including water infiltration caused by sandy soil, strong winds, and sunlight, and a high baseline salinity of the source water recorded around 50 ppt. The farm was also grappling with frequent White Spot virus outbreaks and a high incidence of pathogenic Vibrio strains, slashing average survival rates from 65% down to 32%. Consequently, the farmer aimed to recover the losses in survival and yield experienced, without incurring a significant increase in production costs.
To address these challenges, the farm integrated Safmannan® into shrimp feed at 1 kg per ton, beginning from the nursery phase and continuing throughout the three-month culture period in the grow-out ponds. In addition, Selsaf®, Phileo’s organic selenium-enriched yeast, was included in the later phase at 0.1 kg per ton to mitigate potential negative effects caused by the saline stress conditions.
To assess the benefits of Safmannan® and Selsaf®, a comparative trial was conducted in four commercial ponds covering an average area of 3.8 hectares which were compared to a control group consisting of four similar ponds. The objective of this trial was to validate the technical advantages of the combination of Safmannan® and Selsaf® against the prevalent pathogens and environmental stressors.
Although the crop did not experience severe pathogenic challenges, the strategic addition of Safmannan® and Selsaf® led to a significant increase in survival rates, up to 59.8% in the supplemented group compared to 53% in the control (Table 11). This highlights the effectiveness of Safmannan® and Selsaf® in reducing mortalities throughout the production cycle. Furthermore, the use of Safmannan® and Selsaf® resulted in numerical improvements in harvest size, productivity per hectare, FCR, and in shorter production cycle (Table 11). Implementing this supplementation strategy also led to a 12.5% increase in revenue per hectare while increasing feeding costs by a mere 1.13%. The impressive return on investment (ROI) of USD 113 for every dollar invested underscores the dual benefit of implementing Safmannan® and Selsaf® i.e., bolstering shrimp health while optimizing farm profitability.
Table 11. Comparative performance indicators in shrimp farming with and without the addition of Safmannan® and Selsaf®
A second large-scale trial was conducted with a cluster of corporate farms in the region of Guayaquil, Ecuador. Although the environmental challenges experienced on these farms were somewhat less severe compared to the one in Brazil, the Ecuadorian farms presented a challenge due to their semi-intensive nature and the considerable variability in production outcomes. This heterogeneity is illustrated by the significant variability in some of the production parameters from the 12 L. vannamei ponds, covering 101 hectares in surface area, that were part of the trial (Figure 43).



The primary objective of the trial was to assess the effectiveness of Safmannan® and Selsaf® in optimizing production performances and revenue under the highly variable conditions encountered in semi-intensive shrimp culture. This type of shrimp cultivation remains dominant on a global scale, even with the rapid shift towards more intensive practices. Furthermore, the study also aimed to validate the efficacy of the combination of Safmannan® and Selsaf® on larger shrimp – typical of the Ecuadorian market – often weighing over 25 g.
Given the absence of a clear pathogenic challenge, Safmannan® was incorporated into the feed at a mild dosage of 0.5 kg per ton from stocking to harvest and in combination with Selsaf® at a dose of 0.1 kg per ton to mitigate the potential negative effects of stress. Notably, survival rates varied significantly across the farm sites in the control group, ranging from 43.0% to 56.9% due to the heterogeneity of practices and conditions. The addition of Safmannan® and Selsaf® numerically improved survival, from an average of 50.3% in the control group to 54.3% in the supplemented group (Figure 44a). It should be noted that challenges arose in accurately assessing the enhanced survival rate and adjusting feeding, potentially due to the adoption of the blind feeding method and the large size of production units. Despite the supplemented group exhibiting higher survival compared to the control, there was a 20% reduction in total feed given per hectare compared to the control group. Consequently, the supplemented group experienced an average growth rate 15% slower than the control, indicating potential room for improvement in responding to increased animal biomass in supplemented groups.
It is believed that the better survival rates achieved with Safmannan® and Selsaf® supplementation reflect the improved overall health condition and robustness of the shrimp populations. This was further supported by improved FCR, which was 10% lower in the supplemented ponds than in the control ones (Figure 44b). This improvement translated into remarkable economic benefits for farmers, as production costs decreased from an average of USD 4 per kg of shrimp in the control group to USD 3.6 per kg of shrimp in the supplemented one (Figure 44c). Consequently, farmers benefited from an increased profit margin of USD 409 per hectare (Figure 44d) and a ROI of USD 25 for every dollar invested in Safmannan® and Selsaf® supplementation.

Production cost Production ma rgin
Figure 44. Enhanced shrimp performance and productivity after Safmannan® and Selsaf® supplementation compared to the control as indicated by (a) survival, (b) feed conversion raito (FCR), (c) production costs, and (d) production margins. Mean ± S.E. (Control: n=7, Safmannan® 0.5 kg / ton + Selsaf® 0.1
/ton: n = 5).

CHAPTER 3
Feed represents the main operational cost of shrimp farms, making efficient feeding key for economic sustainability. This becomes especially crucial in the prevailing conditions of low market prices, elevated production costs, and increasingly narrow and variable profit margins. Moreover, feed efficiency is of the utmost importance for the environmental sustainability of shrimp farming. Reduced leaching and improved FCR are linked to lower nutrient discharge into the environment, thereby enhancing water quality and promoting better animal welfare.
Historically, aquafeed millers have been heavily relying on fish meal (FM) for its ideal nutrient composition, high attractability and digestibility. However, overfishing and high demands have led to growing FM prices and market volatility over the years, promoting the search for cheaper and more sustainable protein sources. As a result, FM has gradually switched from being a major dietary component to a strategic, low-inclusion ingredient
Plant-based ingredients have become the most common alternative to substitute FM in aquafeeds. The most popular one in shrimp diets is soybean meal, due to its favorable protein level, amino acid profile and digestibility. Plant-based ingredients represent a more economical and consistent protein source than FM (Tantikitti 2014), although their increased utilization faces challenges that hinder their inclusion at higher rates. The drawbacks of low-FM diets in shrimp have been demonstrated in several studies, encompassing nutritional deficiencies, poor feed efficiency, compromised growth performance, and potential impacts on shrimp health (Figure 45).
Figure 45. Final body weight of L. vannamei fed diets at increasing substitution levels of fish meal (FM). Adapted from Sá et al. (2013). Different letters indicate statistically significant differences (p < 0.05).
First, plant-based ingredients often present lower concentrations of bioavailable nutrients than FM and can lack essential amino acids (EAA) like threonine, lysine, and methionine, and minerals Substitution of FM with plant protein derivatives can also slow down growth rates due to unidentified deficiencies in essential nutrients for penaeid shrimp (Zhou et al. 2013). Second, anti-nutritional factors (ANFs) such as protease inhibitors, phytic acid, and tannins in plant proteins lead to large variations in EAA digestibility compared to FM (Richard et al. 2011) (Figure 46).
Additionally, plant-based ingredients may contain heat-resistant mycotoxins such as aflatoxins, trichothecenes and ochratoxins, which can affect protein, lipid, and starch digestibility and result in increased gut inflammation and dysbiosis. The action of both ANFs and mycotoxins can impair shrimp’s immune defenses and antioxidant response, leading to an increased susceptibility to pathogens (Amaya, Davis, and Rouse 2007; Tantikitti 2014; Sookying, Davis, and Soller Dias da Silva 2013; Naylor et al. 2009) (Figure 47).

Figure 46. Effect of fish meal (FM) replacement on apparent digestibility of P. monodon diets. Different letters indicate statistically significant differences (p < 0.05). Adapted from Richard et al. (2011).
47. Effect of partial fish meal (FM) replacement on (a) total hemocyte counts and (b) lysozyme activity in L. vannamei. Adapted from Yarahmadi et al. (2022).
Furthermore, plant-based ingredients are often characterized by poor attractability and palatability (Naylor et al. 2009), thereby affecting feed intake and subsequent shrimp growth. This is particularly relevant in shrimp farming, where chemosensory cues – chemical signals detected by shrimp’s sensory systems – are vital for locating feed at the bottom of ponds (Darodes de Tailly et al. 2021).
Given these considerations, while plant-based diets offer an economically and environmentally sustainable alternative to FM, their use must be carefully managed to ensure that health and productivity of shrimp are not adversely affected.
Yeast-derived alternative proteins offer a large array of nutritional solutions to bridge the gaps incurred by the replacement of traditional ingredients such as FM in commercial shrimp diets.
Attractability & palatability: critical factors to maintaining optimal feed intake and performance in plantbased diets
The attractability and palatability of feed are crucial for optimal intake and performance, especially in plant-based diets where the inherent flavors and odors of vegetable proteins like soybean meal and corn gluten meal may be less appealing to shrimp compared to FM. The varying digestibility of vegetable proteins can also impact the release of soluble compounds that contribute to the feed’s attractability. Additionally, the presence of ANFs in some vegetable proteins can negatively affect palatability. Overall, these factors contribute to offflavor, bitterness, or gastric irritation that can deter shrimp from consuming the feed
In shrimp farming, feed management often relies on a blind-feeding method where feed rations are distributed in specifically designated areas for consumption monitoring, such as ‘feeding trays’ (Ullman, Rhodes, and Allen Davis 2019; Reis et al. 2020). This method requires shrimp to swiftly detect the feed from any part of the pond and move towards it. Nevertheless, when immersed in water, feed pellets experience nutrient leaching—a phenomenon linked to a decline in the growth of L. vannamei over immersion time spans exceeding 30 minutes (Ullman, Rhodes, and Davis 2019).
From serving as a rich source of EAA and highly bio-assimilable peptides to providing vitamins, minerals, and immune-boosting compounds, yeast proteins offer wide-ranging functionalities, enabling a holistic approach to enhance the health and performance of shrimp fed with plant-based diets

The effectiveness of this feeding strategy depends on the feed’s attractability and palatability. Discrepancies in these qualities can induce selective and inconsistent feeding behavior within shrimp populations, leading to suboptimal growth and significant size disparities. Moreover, the relationship between water quality and feed attractability and consumption is crucial in shrimp feeding management. Poor water quality can reduce feed attractability and palatability, while over-feeding can deteriorate water quality over the production cycle.
As omnivorous and benthic feeders, shrimp rely mostly on chemical cues to locate food at the bottom of ponds (Hindley 1975). Those are detected by chemoreceptors located over the body, namely on the antennules, legs, and mouthparts (Derby and Sorensen 2008) (Figure 48).

Enhancing feed palatability and attractability is therefore crucial, especially in plant-based diets. This involves utilizing the natural abilities of shrimp to detect substances from feed, for instance through the incorporation of feed palatants and attractants to mask or neutralize undesirable flavors and odors while enhancing the overall appeal of the feed to shrimp. Additionally, optimizing the feed formulation by balancing amino acid profiles, incorporating highly digestible protein sources, and ensuring the proper inclusion of essential nutrients, can further contribute to improving feed palatability and attractability.
In this context, peptides and amino acids play essential roles. Certain free amino acids can contribute to enhancing the taste of feed, notably the umami flavor, and its attractability to shrimp. Amino acids like glutamic acid, alanine, and arginine are polar and more soluble in water compared to others which enhances their detection by shrimp’s sensory organs. Furthermore, water-soluble amino acids can dissolve more readily in digestive fluids, facilitating their absorption through the cellular membrane. This process not only improves shrimp vitality and health but also increases the likelihood of shrimp finding the feed palatable and appetizing.
In a similar fashion, short-chain peptides, valued for their bioactive potential, play a pivotal role in shrimp feed acceptance. These peptides not only possess potential flavor-enhancing properties but also trigger physiological responses that stimulate the shrimp’s appetite. Much like amino acids, the water solubility of short-chain peptides is influenced by their chemical structure and the presence of hydrophilic molecules, such as arginine and glutamic acid, which have an affinity for water. This enhanced solubility facilitates the improved detection and consumption of feed by shrimp, contributing to their overall vitality and growth.
Prosaf® is a purified soluble yeast extract obtained from the primary culture of a unique proprietary S. cerevisiae strain. The yeast cells are autolyzed and then centrifuged to separate the yeast core from the YCW, yielding an extract rich in proteins (> 63%) mainly consisting of free amino acids, small-sized peptides, and nucleotides.
Prosaf® only contains low molecular weight compounds, with over 88% of its peptides being below 3.6 kDa and 38% under 1 kDa (Figure 49). These low molecular weight peptides, known for their antimicrobial and palatability-enhancing properties, make Prosaf® a rich source of highly bioassimilable peptides with great potential to improve bioactivity and feed palatability
Moreover, Prosaf® is rich in EAA and boasts a high protein digestibility coefficient of 89%, making it an excellent supplement to plant-based ingredients especially in low FM scenarios. The autolysis process releases a substantial amount of free amino acids, constituting approximately 26% of Prosaf®. This figure is roughly ten times higher than that found in traditional sources such as FM and fish hydrolysates. These free amino acids can be more easily and rapidly absorbed by living organisms and are therefore of prime interest for young life stages or animals with compromised digestive systems. Prosaf® also includes a large proportion of free amino acids known to trigger feeding behavior in penaeid shrimp, such as glutamic acid (5.5%), alanine (3.4%), arginine (1.3%), and glycine (0.5%) (Table 12).
Table 12. Free amino acid/proximate composition of different attractants/palatants commonly used in shrimp diets (%, as-is) (Suresh, Kumaraguru vasagam, and Nates 2011; Lee and Meyers 1996).
To verify Prosaf®’s ability to improve attractability and palatability, a feeding preference trial in reduced FM diets with Prosaf® was conducted at LabomarUFC (Brazil) and compared to other common shrimp feed supplements such as squid and krill meal. Five diets were tested two-by-two, in quadruplicates: a standard diet with 12% FM (high FM), a low FM diet with 3% FM (low FM), a diet containing 3% FM + 2% squid meal, a diet containing 3% FM + 2% krill meal, and a diet containing 3% FM + 2% Prosaf® . Shrimp (L. vannamei) with an initial weight of 13 ± 2 g, were stocked at a density of 140 individuals per m². The groups were subjected to a 15-day testing period with two meals provided each day, ensuring equal feed amounts at each meal. To eliminate potential behavioral bias, the position of feeding trays was switched daily (Figure 50).

50. Representation of the feeding tray layout.
Table 13. Comparative composition of shrimp diets with varying levels of fish meal (FM) and alternative protein sources.
The initial round of two-by-two tests showed a significant decrease in total feed intake, dropping from 82.9% to 76.5% of distributed feed when FM content was reduced from 12% to 3% (Figure 51a). The high FM diet led to increased feed intake for over 80% of the daily feeding period and 76.7% of the meals (Figure 51b), emphasizing the importance of developing plant-based diets that not only meet nutritional requirements but also maintain palatability for high feed intake.
51. (a) Total and (b) daily feed intake of shrimp fed low and high fish meal (FM) diets. Different letters indicate statistically significant differences; paired Student’s t-test, p < 0.05, n = 4.
Introducing squid meal (2%) in a low FM diet resulted in a modest and non-statistically significant increase in feed intake, from 75.7% to 78.2% (Figure 52a). Squid meal inclusion led to a numerically higher feed intake for 53.3% of the daily feeding period and 60.0% of the meals compared to the low FM diet without squid meal (Figure 52b).
52. (a) Total and (b) daily feed intake of shrimp fed a low fish meal (FM) diet compared to a low FM diet with 2% squid meal. Different letters indicate statistically significant differences; paired Student’s t-test, p < 0.05, ns: not statistically significant, p > 0.05; n = 4.
However, the addition of 2% Prosaf® led to a significant improvement in the feed intake in the low FM diet, increasing from 76.8% to 82% (Figure 53a). The incorporation of Prosaf® numerically increased feed intake for over 80.0% of the daily feeding period and 76% of the meals (Figure 53b). Overall, at 2% inclusion, Prosaf® elicited similar feed intakes to the high FM diet, demonstrating its capacity to mitigate any adverse effects of plant-based ingredients on the shrimp diet’s attractability and palatability.
0.05, n = 4.
To further underscore the efficacy of Prosaf®, its performance was compared with krill meal, another highquality marine protein source recognized for its palatability benefits. The incorporation of 2% Prosaf® effectively equaled the consumption levels observed with 2% krill meal (Figure 54). This reaffirms its capacity to restore feed consumption to levels comparable to traditional FM diets. These results position Prosaf® not only as an equal to high-quality marine ingredients in terms of performance but also as a sustainable choice that circumvents the environmental concerns tied to marine ingredient sourcing, such as the depletion of marine resources and by-catch.
To comprehensively assess the influence of different Prosaf® inclusion rates, an in vivo trial was conducted at Prince of Songkla University. Prosaf® was integrated at varying rates, spanning from 0.5% to 2.5%, accompanied by a reduction in FM content from 15% to 5%. The five diets were isonitrogenous (crude protein: 26%), isolipidic (8%) and with balanced amino acid profiles (Table 14). Shrimp (L. vannamei) with an average weight of 1.0 ± 0.1 g were stocked at a density of approximately 100 individuals per m² and meticulously monitored throughout the trial period.
Table 14. Comparative composition of shrimp diets with increasing levels of Prosaf® alongside decreasing levels of fish meal (FM). F

Substituting significant amounts of FM with plant-based ingredients not only affected feed intake but also impacted production performance. The reduction of FM from 15% to 5% affected shrimp survival rates, growth, and FCR. Incorporating Prosaf® effectively mitigated the negative effects on growth and feed conversion, especially at an inclusion rate of 2.5% (Figure 55). Meanwhile, lower dosages of 0.5% and 1.5% delivered equivalent performances to the high FM diet.
55. (a) Body weight gain and (b) feed conversion ratio (FCR) in shrimp fed low fish meal (FM) diets supplemented with varying inclusion levels of Prosaf® compared to a low FM diet without supplementation and a high FM diet. Different letters indicate statistically significant differences; Tukey’s HSD test, p < 0.05.
Additionally, a trend towards lower THC was observed for the 5% FM diet compared to the 15% FM one, indicating reduced immune potential and, consequently, lower disease resistance. However, all inclusion levels of Prosaf® led to more balanced hemocyte counts, even exceeding those observed in the high FM diet with 2.5% inclusion level (Figure 56), suggesting improved immunity potential. These results highlight Prosaf®’s ability to maintain and improve the health of shrimp on diets with reduced key ingredients like FM. Further trials conducted in Brazil at Labomar-UFC corroborated Prosaf®’s ability to improve performance in low FM diets at even lower inclusion rates, such as 0.25%, delivering significant growth benefits at harvest.
Total hemocyte count ab ab ab



Figure 56. Total hemocyte count (THC) in shrimp fed low fish meal (FM) diets supplemented with varying inclusion levels of Prosaf® compared to a low FM diet without supplementation and a high FM diet. Different letters indicate statistically significant differences; Tukey’s HSD test, p < 0.05.
Substituting FM in shrimp feed requires careful consideration to ensure nutritional requirements are met without compromising animal health and welfare.
Maintaining the nutritional content of the feed is one of the most important considerations, as is the quality of proteins, amino acids, vitamins, and minerals. It is therefore crucial to assess and adjust the nutritional profile of substitutes to provide
shrimp with essential nutrients for optimal growth and health.
Notably, it is important to look for protein sources that are easily digestible and provide a balanced profile of EAA (Table 15); alternatives with lower digestibility can affect nutrient absorption. Furthermore, the substitution also needs to be economically viable given the large incorporation rates applied. The sustainability and safety of the alternative ingredients are also important considerations.

Once processed into protein fractions, yeast offer numerous advantages compared to other alternative protein sources, particularly in diets that include high levels of plant-based ingredients. These advantages encompass nutritional value, sustainability, safety, and versatility
Prosaf®, Selsaf® and Nutrisaf® offer a complete set of solutions to counteract the nutritional and functional losses incurred by the substitution of FM with plantbased ingredients.
Nutrisaf® is obtained from a primary grown S. cerevisiae strain which undergoes autolysis to release EAA and functional compounds such as mannans and beta-glucans. As a result, Nutrisaf® emerges as a cost-effective functional protein source in feed formulation, allowing for the rebalancing of EAA in plant-based diets to enhance performance and boost immunity to sustain optimal health. The added value of Nutrisaf® on both growth performance and health in shrimp fed reduced FM diets was demonstrated on L. vannamei in a study conducted at the Ocean University of China, Qingdao (Ma et al. 2020). The experiment aimed to evaluate substituting FM from 25% to 20% with a combination of peanut meal and Nutrisaf® , examining their effects on production performance and animal health. The trial consisted of four groups: a positive control with 25% FM and 8% peanut meal, a negative control with reduced FM (20%) substituted with peanut meal (13%), and two treatments corresponding to the negative control supplemented with 1% and 2% of Nutrisaf® (Table 16). The study was structured into two successive phases including a 56-day growth trial followed by an 8-day challenge with V. parahaemolyticus.
Fish meal
Squid visceral meal
Peanut meal
Soybean meal
Whea
Proximate analysis (% diet)
Table 16. Comparative composition of shrimp diets with varying levels of fish meal (FM) and peanut meal with Nutrisaf® inclusion.
The trial demonstrated that substituting a mere 5% of FM with plant-based ingredients could significantly impair harvest size and FCR. Notably, even minor additions of Nutrisaf® to diets containing 20% FM and 13% peanut meal resulted in a significant performance boost, comparable to the control diet featuring 25% FM. Moreover, the inclusion of 2% Nutrisaf® numerically outperformed the positive control regarding survival and final body weight, and significantly reduced FCR (Figure 57). This efficiency gain was accentuated by a 3% reduction in total feed formulation costs, underpinning the economic viability of Nutrisaf® in FM-replacing strategies. Additional trials corroborate these findings, indicating that Nutrisaf® inclusion rates of up to 5% can deliver substantial production and economic benefits.
After the growth trial, the study was followed by a challenge test at day 56 with the pathogenic V. parahaemolyticus. The inclusion of Nutrisaf® at both dosages significantly improved hemolymph THC (Figure 58a, assessed before the challenge test), demonstrating the product’s ability to mobilize shrimp immune response to potential infections. A notable enhancement in Total Antioxidant Capacity TAOC was observed with the application of Nutrisaf®, reaching statistical significance at a 2% inclusion rate (see Figure 58b). This suggests that shrimp exhibit an increased reservoir of antioxidants, thereby being better equipped to withstand oxidative stress. Most importantly, this bolstered immunity translated into tangible survival benefits, as evidenced by significantly reduced mortality rates at the end of the V. parahaemolyticus challenge (Figure 58c).
Figure 57. (a) Survival, (b) final body weight, and (c) feed conversion ratio (FCR) in shrimp fed low fish meal (FM) diets supplemented with varying inclusion levels of Nutrisaf® compared to a low and high FM diets without supplementation (adapted from Ma et al. (2020)). Different letters indicate statistically significant differences; Duncan’s test, p < 0.05.
The trial findings highlight the nutritional and health advantages of integrating Nutrisaf® as an alternative and functional protein source in shrimp diets. While the partial substitution of FM had adverse effects on shrimp performance, supplementation with Nutrisaf® resulted in significant improvements in both feed efficiency and post-infection survival – critical factors for profitability in shrimp farming. Moreover, the strategic inclusion of Nutrisaf® resulted in notable economic advantages by reducing overall feed formulation costs.
Figure 58. (a)Total hemocyte count (THC), (b) TAOC, and (c) cumulative mortality of shrimp fed low fish meal (FM) diets supplemented with varying inclusion levels of Nutrisaf® compared to a low and high FM diets without supplementation (adapted from Ma et al. (2020)). Different letters indicate statistically significant differences; Duncan’s test, p < 0.05.
Overall, Prosaf® and Nutrisaf® offer comprehensive solutions to address the nutritional and functionality challenges associated with replacing FM with plantbased ingredients.
These innovative alternatives not only enhance performance and animal health, but also help improve the environmental sustainability of aquafeed formulations.
Ensuring the sustainability of aquafeeds is imperative given the expanding environmental impact of aquaculture. As an industry heavily reliant on finite resources, including land, water, wild fish stocks, and critical ecosystems like estuaries and mangroves, shrimp farming stands at the forefront of the sustainability challenge. The sector is also under scrutiny for its contributions to pollution, stemming from the release of organic effluents from ponds and cages, incidents of animal escapes, and the use of pharmaceuticals in open systems.
In this critical scenario, alternative proteins can play a pivotal role in redefining aquaculture production systems to be more resourceefficient and environmentally friendly.
These can be part of viable substitution strategies for key ingredients such as FM and fish oil. These resources, often derived from environmentally stressed marine ecosystems, can be replaced without compromising the health and welfare of farmed animals or the safety of end consumers.
Through the strategic use of Prosaf® and Nutrisaf® , the dependency of FM in shrimp diets can be significantly reduced, thereby minimizing the strain on wild fish stocks. Numerous trials have demonstrated that incorporating Prosaf® at 0.5% and Nutrisaf® at 2% can lead to a notable 40.1% reduction in the Fish In – Fish Out ratio (FIFO), according to the IFFO method (Figure 59). This translates to a decrease of 420 kg of pelagic fish utilization per ton of live shrimp produced, down from an initial average of 1,050 kg.
In essence, for every ton of Prosaf® and Nutrisaf® incorporated in aquafeeds, up to 3 tons of FM, or an equivalent of 13 MT of pelagic fish, can be spared.
FIFO ratio in whiteleg shrimp
Figure 59. Estimated FIFO ratio in whiteleg shrimp fed Prosaf® or Nutrisaf® compared to the control group fed a high FM diet and the negative control fed a low FM diet.
Furthermore, the environmental benefits of alternative yeast proteins extend beyond FIFO ratios. A comprehensive life cycle analysis of Nutrisaf® (conducted in accordance with ISO 14044 and with support of INRAE, France), assessed its ecological impact relative to other protein sources commonly used in aquafeed. This analysis encompassed a wide range of environmental factors, including greenhouse gas emissions and the consumption of oceanic, land, and water resources throughout the production cycle.
Yeast-derived proteins offer several environmental benefits compared to their counterparts from traditional aquafeed. The results of the lifecycle analysis demonstrated that Nutrisaf® requires less land use, thanks to the efficient nature of yeast cultivation (Figure 60a). As singlecell microorganisms, yeast can be cultivated in controlled fermentation processes within spaceefficient facilities, rapidly converting biomass into protein. Moreover, Nutrisaf® represents a competitive alternative to reduce dependency on FM and the extraction of natural resources and materials, thereby reducing the environmental impact of alternative diets (Figure 60).
Figure 60. (a) Land use, (b) water consumption, (c) greenhouse gas emissions, and (d) primary production utilization of different protein sources used in aquafeeds. Insect meal (black soldier fly) and Nutrisaf® are from European origin.
Our continuous efforts to curtail emissions have led to a comprehensive review and data collection across our factories in 2021, aimed at precisely quantifying our carbon footprint and identifying the main sources of emissions.
This critical analysis has allowed us to identify CO2 emissions as an important point for improvement, and has paved the way for a tailored decarbonization roadmap, targeting energy efficiency enhancements, diversification of electric energy sources, and sustainable utilization of biomass and biogas.
We are implementing these strategies vigorously at our historical and largest site in Marcq-en-Baroeul, France, with an ambitious pilot project to decarbonize two-thirds of its activities by 2025.

Yeast and bacteria-derived products such as Actisaf®, Procreatin 7®, Microsaf®, Safmannan® , Selsaf®, Prosaf®, and Nutrisaf®, each serve specific roles, from improving gut health and immune response to enhancing feed palatability and efficiency.
Our meticulously crafted technical recommendations offer specific applications and key benefits of each product in the Aquasaf Shrimp program range (Table 17). These recommendations are derived from extensive research and field trials, providing guidelines for dosage and application to maximize their benefits.
Their precise use according to the recommended dosages is crucial for achieving the desired outcomes and aiding producers in overcoming the unique challenges of their shrimp farming operations.

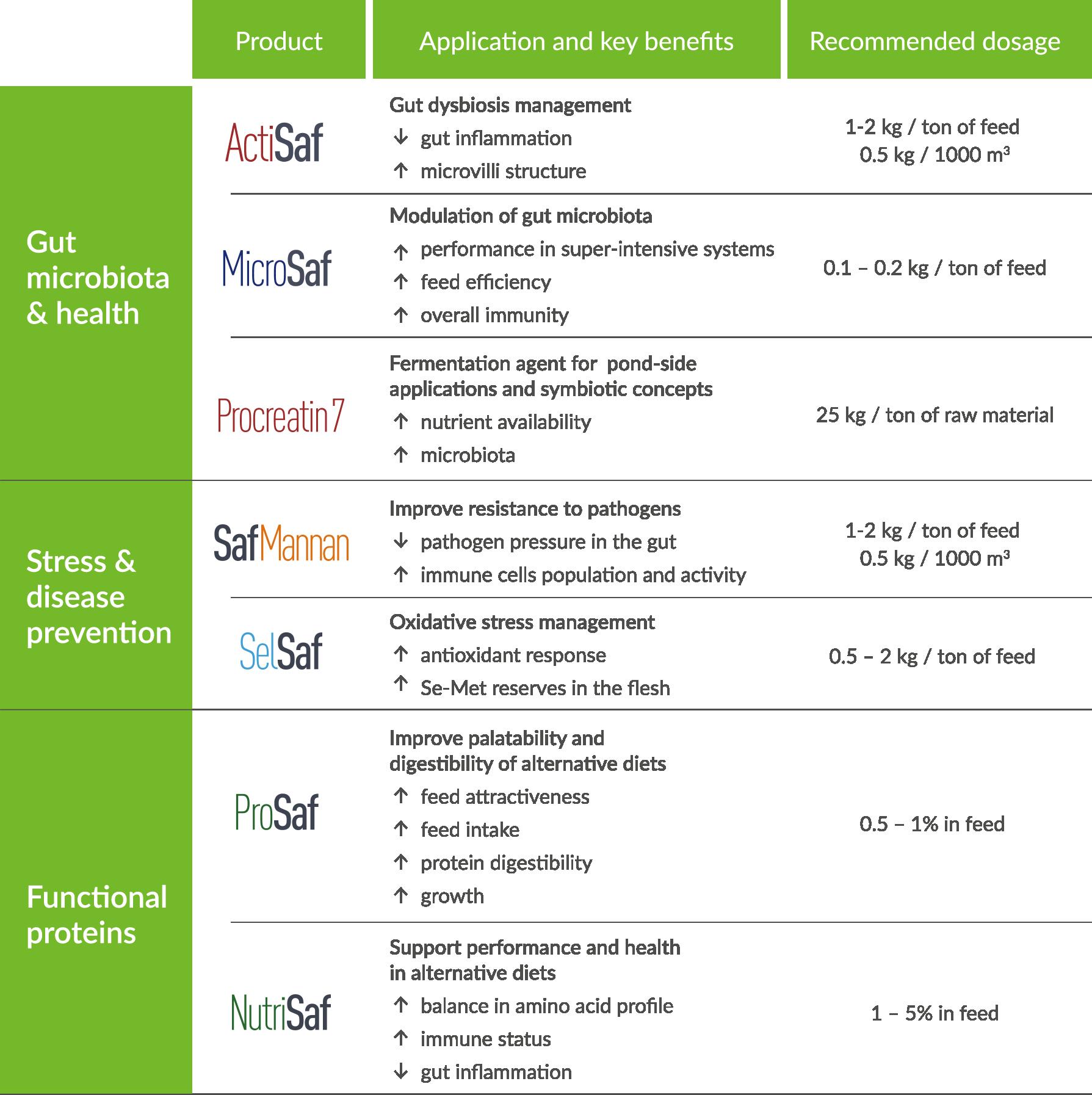


For tailored guidance and to explore how our innovative solutions can enhance your shrimp farming operations, please reach out to Phileo. Our team of experts is ready to provide personalized support and share our comprehensive knowledge on optimizing aquaculture practices with both probiotics and postbiotics.
The in-depth exploration of yeast and bacteria-based solutions in shrimp aquaculture, as detailed in this white book, emphasizes the pivotal role these innovative products play in addressing multifaceted industry challenges. From enhancing gut health and immunity to improving feed efficiency and reducing reliance on scarce resources like FM, our solutions propose a holistic approach to fostering sustainable, efficient, and profitable shrimp farming practices. The incorporation of these solutions into shrimp diets or their application at the farm level can assist producers in striking a delicate balance between economic viability and environmental stewardship, ensuring the long-term sustainability of the aquaculture sector.
In summary, the Aquasaf Shrimp White Book aims to serve as a valuable resource for shrimp producers, nutritionists, and industry stakeholders, providing actionable insights and practical guidance for leveraging yeast and bacteria-based solutions. These innovations not only address the immediate needs of shrimp farming but also pave the way for a more sustainable, resilient, and prosperous future for the global aquaculture industry.
Aguirre-Guzman, Gabriel, Jesus Sánchez-Martínez, Ángel Campa-Córdova, Antonio Luna, and Felipe Ascencio. 2009. “Penaeid Shrimp Immune System.” The Thai Veterinary Medicine 39: 205–15.
Amaya, Elkin, D. Allen Davis, and David B. Rouse. 2007. “Alternative Diets for the Pacific White Shrimp Litopenaeus Vannamei.” Aquaculture 262 (2): 419–25.
Avery, S. V., S. Malkapuram, C. Mateus, and K. S. Babb. 2000. “Copper/Zinc-Superoxide Dismutase Is Required for Oxytetracycline Resistance of Saccharomyces Cerevisiae.” Journal of Bacteriology 182 (1): 76–80.
Chen, Yunsong, Li Zhou, Qiuran Yu, Erchao Li, and Jia Xie. 2023. “Effects of Sulfamethoxazole and Florfenicol on Growth, Antioxidant Capacity, Immune Responses and Intestinal Microbiota in Pacific White Shrimp Litopenaeus Vannamei at Low Salinity.” Antibiotics (Basel, Switzerland) 12 (3). https:// doi.org/10.3390/antibiotics12030575.
Darodes de Tailly, Jean-Benoît, Jonas Keitel, Matthew A. G. Owen, Jose M. Alcaraz-Calero, Mhairi E. Alexander, and Katherine A. Sloman. 2021. “Monitoring Methods of Feeding Behaviour to Answer Key Questions in Penaeid Shrimp Feeding.” Reviews in Aquaculture 13 (4): 1828–43.
Derby, Charles D., and Peter W. Sorensen. 2008. “Neural Processing, Perception, and Behavioral Responses to Natural Chemical Stimuli by Fish and Crustaceans.” Journal of Chemical Ecology 34 (7): 898–914.
Graubaum, Hans-Joachim, Regina Busch, Heike Stier, and Joerg Gruenwald. 2012. “A Double-Blind, Randomized, Placebo-Controlled Nutritional Study Using an Insoluble Yeast Beta-Glucan to Improve the Immune Defense System.” Food and Nutrition Sciences 3 (6).
Hindley, J. P. R. 1975. “Effects of Endogenous and Some Exogenous Factors on the Activity of the Juvenile Banana Prawn Penaeus Merguiensis.” Marine Biology 29 (1): 1–8.
Hughes, R. J. 2008. “Relationship between Digesta Transit Time and Apparent Metabolisable Energy Value of Wheat in Chickens.” British Poultry Science 49 (6): 716–20.
Jiang, Ling-Xu, Lu-Qing Pan, and Fang-Bo. 2005. “Effect of Dissolved Oxygen on Immune Parameters of the White Shrimp Litopenaeus Vannamei.” Fish & Shellfish Immunology 18 (2): 185–88.
Kakoolaki, Shapour, Mehdi Soltani, Hossein Mousavi, I. Sharifpour, Seyed Saeed Mirzargar, Mohammad Afsharnasab, and Abbasali Motallebi. 2011. “The Effect of Different Salinities on Mortality and Histopathological Changes of SPF Imported Litopenaeus Vannamei, Experimentally Exposed to White Spot Virus and a New Defferential Hemocyte Staining Method.” Iranian Journal of Fisheries Sciences 10 (3): 447–60.
Kulkarni, Amod, Sreedharan Krishnan, Deepika Anand, Shyam Kokkattunivarthil Uthaman, Subhendu Kumar Otta, Indrani Karunasagar, and Rajendran Kooloth Valappil. 2021. “Immune Responses and Immunoprotection in Crustaceans with Special Reference to Shrimp.” Reviews in Aquaculture 13 (1): 431–59.
Lee, P. G., and S. P. Meyers. 1996. “Chemoattraction and Feeding Stimulation in Crustaceans.” Aquaculture Nutrition 2 (3): 157–64.
Lee, So Young, and Kenneth Söderhäll. 2002. “Early Events in Crustacean Innate Immunity.” Fish & Shellfish Immunology 12 (5): 421–37.
Leite, Jordana, Alexandre Diógenes, and Alberto Nunes. 2022. “Dietary Contribution of Fermented Grain Pellets to the Growth of Juvenile Litopenaeus Vannamei Raised in an Intensive Biofloc-Based Rearing System.” Aquaculture International: Journal of the European Aquaculture Society 31: 1–22.
Li, Feipeng, Jiong Huang, Mingzhu Wang, Ling Chen, and Yihua Xiao. 2021. “Sources, Distribution and Dynamics of Antibiotics in Litopenaeus Vannamei Farming Environment.” Aquaculture 545 (December): 737200.
Limsuwan, C., and C. Ching. 2012. “Temperature Affects Feeding Behavior of Pacific White Shrimp.” Global Aquacultre Advocate, 2012.
Ma, Shuoli, Xiaoxia Wang, Weihua Gao, Weiqi Xu, Wenbing Zhang, and Kangsen Mai. 2020. “Effects of Yeast Autolysate in the Practical Diet on the Growth Performance, Immune Response, and Disease Resistance of Pacific White Shrimp.” Journal of Aquatic Animal Health 32 (3): 109–15.
Meena, D. K., Pronob Das, Shailesh Kumar, S. C. Mandal, A. K. Prusty, S. K. Singh, M. S. Akhtar, et al. 2013. “Beta-Glucan: An Ideal Immunostimulant in Aquaculture (a Review).” Fish Physiology and Biochemistry 39 (3): 431–57.
Naylor, Rosamond L., Ronald W. Hardy, Dominique P. Bureau, Alice Chiu, Matthew Elliott, Anthony P. Farrell, Ian Forster, et al. 2009. “Feeding Aquaculture in an Era of Finite Resources.” Proceedings of the National Academy of Sciences 106 (36): 15103–10.
Oanh, D., and P. Tacon. 2015. “Yeast Parietal Fractions Enhance the Immune Response of Shrimp towards AHPND Infections.” Aquatic Asia Pacific Magazine.
Peyraud, Jean-Louis, and Muriel Mambrini. 1992. “Direct Measurement of Transit Time in the Stomachs and Intestine of Dairy Cows.” Annales De Zootechnie - ANN ZOOTECH 41: 55–55.
Pham, Thi Thu Hang, Pierre Rossi, Hoang Dang Khoa Dinh, Ngoc Tu Anh Pham, Phuong Anh Tran, To Thi Khai Mui Ho, Quoc Tuc Dinh, and Luiz Felippe De Alencastro. 2018. “Analysis of Antibiotic Multi-Resistant Bacteria and Resistance Genes in the Effluent of an Intensive Shrimp Farm (Long An, Vietnam).” Journal of Environmental Management 214 (May): 149–56.
Rajendran, K. V., K. Sreedharan, A. Deepika, and Amod Kulkarni. 2022. “Shrimp Immune System and Immune Responses.” In Fish Immune System and Vaccines, edited by Makesh M. and Rajendran K. V., 17–43. Singapore: Springer Nature Singapore.
Ran, Chao, Lu Huang, Zhi Liu, Li Xu, Yalin Yang, Philippe Tacon, Eric Auclair, and Zhigang Zhou. 2015. “A Comparison of the Beneficial Effects of Live and Heat-Inactivated Baker’s Yeast on Nile Tilapia: Suggestions on the Role and Function of the Secretory Metabolites Released from the Yeast.” PloS One 10 (12): e0145448.
Reis, João, Romi Novriadi, Anneleen Swanepoel, Guo Jingping, Melanie Rhodes, and D. Allen Davis. 2020. “Optimizing Feed Automation: Improving TimerFeeders and on Demand Systems in Semi-Intensive Pond Culture of Shrimp Litopenaeus Vannamei.” Aquaculture 519 (734759): 734759.
Richard, Lenaïg, Anne Surget, Vincent Rigolet, Sadasivam J. Kaushik, and Inge Geurden. 2011. “Availability of Essential Amino Acids, Nutrient Utilisation and Growth in Juvenile Black Tiger Shrimp, Penaeus Monodon, Following Fishmeal Replacement by Plant Protein.” Aquaculture 322–323 (December): 109–16.
Sá, M. V. C., H. Sabry-Neto, E. Cordeiro-Júnior, and A. J. P. Nunes. 2013. “Dietary Concentration of Marine Oil Affects Replacement of Fish Meal by Soy Protein Concentrate in Practical Diets for the White Shrimp, Litopenaeus Vannamei.” Aquaculture Nutrition 19 (2): 199–210.
Silva, M. A. S., M. E. Almeida Neto, B. O. Ramiro, I. T. F. Santos, and R. R. Guerra. 2018. “Histomorphologic Characterization of the Hepatopancreas of Freshwater Prawn Macrobrachium Rosenbergii (De Man, 1879).” Arquivo Brasileiro de Medicina Veterinaria e Zootecnia 70 (5): 1539–46.
Song, Y. L., and C. C. Huang. 2000. “Aplications of Immunostimulant to Prevent Shrimp Diseases.” In Recent Advances in Marine Biotechnology, edited by M. Fingerman and R. Negabhusanam. Playmouth: Science Publishers Inc.
Sookying, D., D. A. Davis, and F. Soller Dias da Silva. 2013. “A Review of the Development and Application of Soybean-Based Diets for Pacific White Shrimp Litopenaeus Vannamei.” Aquaculture Nutrition 19 (4): 441–48.
Soonthornchai, Wipasiri, Sage Chaiyapechara, Padermsak Jarayabhand, Kenneth Söderhäll, and Pikul Jiravanichpaisal. 2015. “Interaction of Vibrio Spp. with the Inner Surface of the Digestive Tract of Penaeus Monodon.” PloS One 10 (8): e0135783.
Sun, Mingzhe, Shihao Li, Xiaojun Zhang, Jianhai Xiang, and Fuhua Li. 2020. “Isolation and Transcriptome Analysis of Three Subpopulations of Shrimp Hemocytes Reveals the Underlying Mechanism of Their Immune Functions.”
Developmental and Comparative Immunology 108 (July): 103689.
Suresh, Arul Victor, K. P. Kumaraguru vasagam, and Sergio Nates. 2011. “Attractability and Palatability of Protein Ingredients of Aquatic and Terrestrial Animal Origin, and Their Practical Value for Blue Shrimp,
Litopenaeus Stylirostris Fed Diets Formulated with High Levels of Poultry Byproduct Meal.” Aquaculture https://doi.org/10.1016/j.aquaculture.2011.06.039.
Tang, K. F. J., M. G. Bondad-Reantaso, J. R. Arthur, B. MacKinnon, B. Hao, V. Alday-Sanz, Y. Liang, and X. Dong. 2020. “Shrimp Acute Hepatopancreatic Necrosis Disease Strategy Manual.” FAO. Tantikitti, Chutima. 2014. “Feed Palatability and the Alternative Protein Sources in Shrimp Feed.” Songklanakarin Journal of Science and Technology 36: 51–55.
Telford, John L., Michèle A. Barocchi, Immaculada Margarit, Rino Rappuoli, and Guido Grandi. 2006. “Pili in Gram-Positive Pathogens.” Nature Reviews. Microbiology 4 (7): 509–19.
Ullman, Carter, Melanie A. Rhodes, and D. Davis. 2019. “Feed Management and the Use of Automatic Feeders in the Pond Production of Pacific White Shrimp Litopenaeus Vannamei.” Aquaculture 498: 44–49.
Tran, Loc, Nadège Richard, and Philippe Tacon. 2017. “Immunostimulants Need Appropriate Dosages and Administration Time to Mitigate AHPND Infections in White Shrimp.” AQUA Culture Asia Pacific Magazine
Valle, Cristina del, María Bonadero, and Analía Gimenez. 2023. “Saccharomyces Cerevisiae as Probiotic, Prebiotic, Synbiotic, Postbiotics and Parabiotics in Aquaculture: An Overview.” Aquaculture 569: 739342.
Vogt, Günter. 2019. “Functional Cytology of the Hepatopancreas of Decapod Crustaceans.” Journal of Morphology 280 (9): 1405–44.
Wang, Long-Uong, and Jiann-Chu Chen. 2005. “The Immune Response of White Shrimp Litopenaeus Vannamei and Its Susceptibility to Vibrio Alginolyticus at Different Salinity Levels.” Fish & Shellfish Immunology 18 (4): 269–78.
Wilfart, A., L. Montagne, H. Simmins, J. Noblet, and J. van Milgen. 2007. “Effect of Fibre Content in the Diet on the Mean Retention Time in Different Segments of the Digestive Tract in Growing Pigs.” Livestock Science 109 (1): 27–29.
Xu, Zihan, Weiliang Guan, Dandan Xie, Wenjing Lu, Xingchen Ren, Jiajia Yuan, and Linchun Mao. 2019. “Evaluation of Immunological Response in Shrimp Penaeus Vannamei Submitted to Low Temperature and Air Exposure.” Developmental and Comparative Immunology 100 (November): 103413.
Yabaya, A., J. A. Akinyanju, and E. D. Jatou. 2009. “Yeast Enrichment of Soybean Cake.” World J. Dairy Food Sci 4: 141–44.
Yarahmadi, Peyman, Ali Taheri Mirghaed, and Seyed Pezhman Hosseini Shekarabi. 2022. “Zootechnical Performance, Immune Response, and Resistance to Hypoxia Stress and Vibrio Harveyi Infection in Pacific White Shrimp (Litopeneaus Vannamei) Fed Different Fishmeal Diets with and without Addition of Sodium Butyrate.” Aquaculture Reports 26 (October): 101319.
Ye, Yucong, Bihong Zhu, Junya Zhang, Ying Yang, Jiangtao Tian, Wenyue Xu, Xinglin Du, Yizhou Huang, Yiming Li, and Yunlong Zhao. 2023. “Comparison of Growth Performance and Biochemical Components between Low-SalinityTolerant Hybrid and Normal Variety of Pacific White Shrimp (Penaeus Vannamei).” Animals: An Open Access Journal from MDPI 13 (18). https://doi. org/10.3390/ani13182837.
Zhou, Qi-Cun, Yong-Li Wang, Hua-Lang Wang, and Bei-Ping Tan. 2013. “Dietary Threonine Requirements of Juvenile Pacific White Shrimp, Litopenaeus Vannamei.” Aquaculture 392–395 (May): 142–47.
