Cleanroom Conceptual Design Paradigm:
Presented by: Matt Dean
September 26, 2024
AES Clean Technology
Advancing Scientific Innovations


About Us
We go where you go





Philadelphia Headquarters
-Engineering
-Project Management
Irvine Office
-Project Management
-Project Development
Atlanta Manufacturing
Manheim Warehouse
-Phased Shipping
-Future Manufacturing


Meet the Presenter...

Matt Dean RA, NCARB, LEED GA
Director of Process Architecture
Process Technology Team Leader
15 years Biopharma/Life Science industry
Specialized in Biopharma/Life Science design
BS. of Architecture, M. Architecture
Registered Architect, NCARB certified, LEED certified

Today’s Agenda

Philosophical Differences

Operations & Level of Compliance

Procedural controls
Commercial vs Industrial Facilities
Production Regimen & Process Platforms
GMP Flows & Access Controls

Utilities Infrastructure & Construction Challenges

Systems & Logistics
Questions?

Cleanroom Infrastructure Considerations
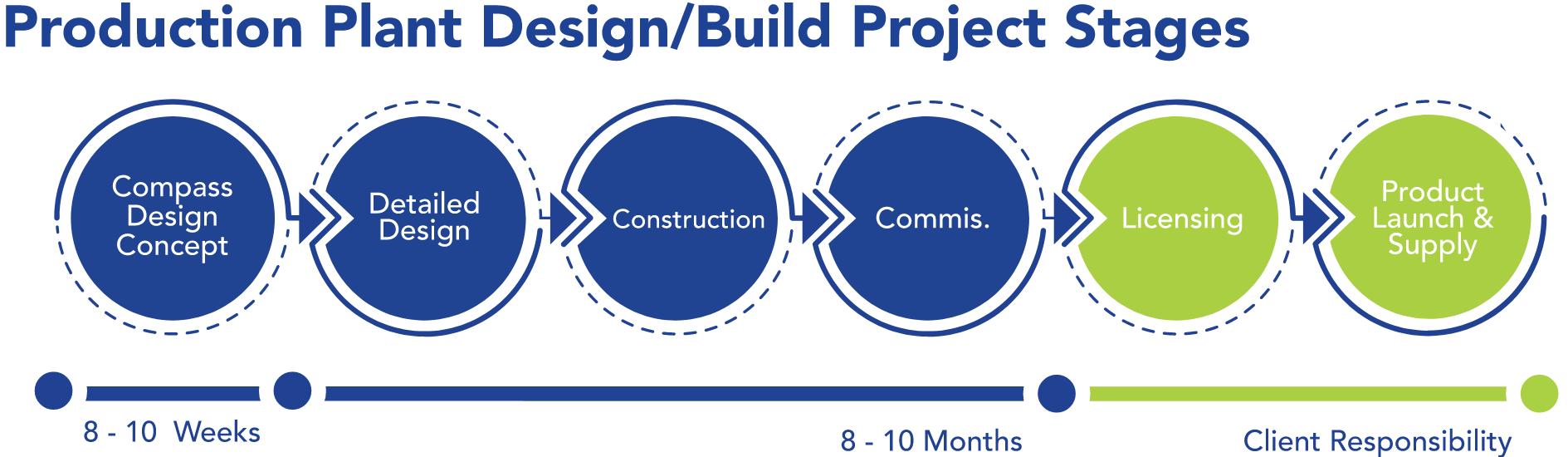
Compass Project Roadmap™


• Warehouse/Logistics
Centers may not exist or be limited or remote
• Site access availability
• Project area access for operations (multi-level configuration?)
• Mechanical systems suitability and availability
• Existing corridor system constraints
• Warehouse/Logistics
Centers upgrading for project suitability
• Site access reconfigurations
• Project area accessible
• Mechanical systems upgrades
• Potential mechanical platforms


• Manual process operations
• Small scale processes
• Limited process automation
• Production regimen higher criticality
• Potentially separate upstream and downstream suites operations
• Process utilities required vs existing
• Process utility local cylinders
• Manual or automated process operations
• Small or large scale processes
• Process automation may be implemented
• Ballrooms for upstream & downstream operations
• Central process utility generation potential


• Opportunities constrained by space or access to project area
• Fit-for-purpose consideration
• GMP operational impacts
• Mechanical system location may be remote
• Impacts of multiple tenants in single facility
• Investigate “bolt on” concepts for expansion
• Opportunities vetted with facility & operations impacts
• Fit-for-purpose vs futureproof
• GMP operational impacts
• Mechanical system location may be remote or on platform


• Minimized interstitial space
• Walkable ceiling access could be compromised
• Mechanical maintenance remote
• Increased interstitial space
• Walkable ceiling access possible
• Mechanical maintenance provided above ceiling

Cleanroom Design Attribute Trends
1 2
Flexibility
Cleanrooms accommodating to operational changes under similar process platforms.
Adaptability
Cleanrooms accommodating to similar process unit operations with minimized shutdown periods utilizing equipment changeover.
3 4 5
Segregation (Room vs Equipment)
Implementing segregation in multiproduct operations via control at highrisk zones in the same room or designing independent suites.
Phased
Implementation
Opportunities to tailor cleanroom design and installation into phases for strategic build-out.
Future Growth
Responsive to business drivers and process development plans.

Cleanroom Design Practices
Efficient Material & Personnel Flows
Room Design Supports Secondary Process Containment
Optimized Process Equipment Adjacencies Mechanical Design Coordinated with Process Design
Plan for the Future

Deciding Operational and Compliance Alignment

cGMP & Compliance Keys: Multi-Product Operations
ATMP Manufacturing, US-FDA Compliance
Independent Suites, Closed Process

ATMP Manufacturing, EU Compliance
Ballroom with Physical Work Zones, Open Process


cGMP & Compliance Keys: Process Design & Scale
Manual Operations




Open Processing Manual Filling
cGMP & Compliance Keys: Process Design & Scale
Automated & Closed
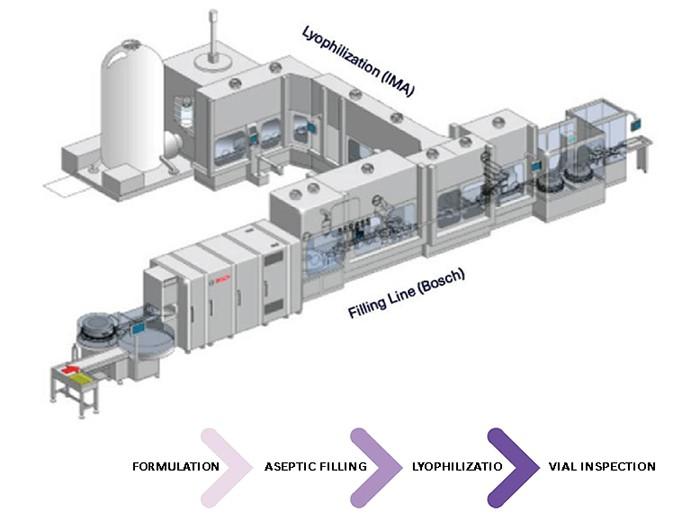

cGMP & Compliance Keys: Room Containment

PROCESS ROOM

PAL MIST
Compliance Guidance Highlights
COMPLIANCE CATEGORY DESIGN COMPLIANCE TOPICS HIGHLIGHTS
Eudrolex Vol 4 Annex 1
Eudrolex Part IV (ATMPs)
US-FDA: Sterile Drug
Products Produced by Aseptic Processing – cGMP
Cross-Contamination
CCS
Aseptic Filling background environment
Pass-through Chambers
Unidirectional flow
Airflow Visualization Studies
Containment
CCS
Multi-product facility
Concurrent production
Closed systems
Containment
Multi-product operations
Drains
Closed systems
ISPE ATMPs – Autologous Cell Therapy
Containment
CCS
Multi-product operations
Aseptic Processing
EM
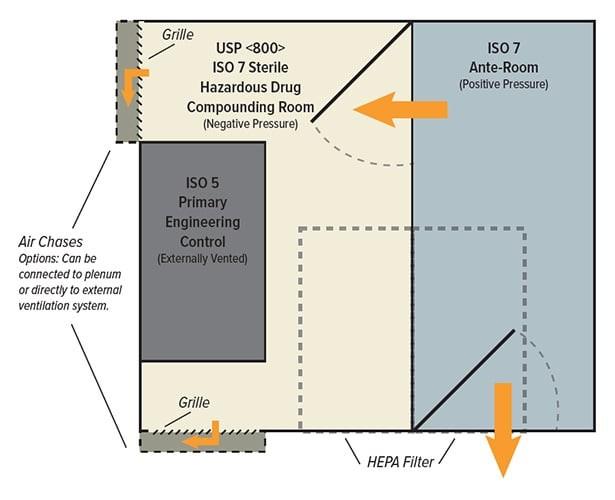
USP <797>, updated 2022
USP <800>, updated 2019
Pressurization scheme + DPs
P-PEC + P-SEC
Revised CSPs
BUDs
Reference to USP <800>
Pressurization scheme + DPs
P-PEC + P-SEC
Containment & Ventilation
Once-through air system (room or containment zone) in high-risk zone, AHUs prevent cross-contamination
Grade B for manual filling, Grade C or D when closed isolator is used
Pass-through chambers with active filtered air supply flush. interlocked
Recommended physically segregated personnel and material flows (temporal acceptable) and unidirectional
personnel flow (Grade B specifically or result of CCS)
Final stage AL to be same classification as process room into which it leads
Sink and Bubble pressurizations for BSL2 aerosolization, AHUs designed to prevent cross-contamination
Once-through air system (room or containment zone) in high-risk zone
AHUs designed to prevent cross-contamination
Segregated sterile and non-sterile flows
Recommend unidirectional flows
ISO 7 background adjacent to aseptic processing, ISO 5 equipment environment
Segregated personnel entry & exit flows, AHUs designed to prevent cross-contamination
Inappropriate in aseptic processing areas other than ISO 8 with rare exceptions
LPZ for stopper vials prior to capping
Protocols at critical aseptic areas
Open processing conducted in BSC with ISO 5 environment in ISO 7 background
Physical segregations, temporal requirements
Sink and Bubble pressurizations for BSL2 aerosolization, AHUs designed to prevent cross-contamination
Electronic barcoding
Segregated personnel entry & exit flows
Positive pressure for sterile compounding (non-HD), 30ACH, 0.01-0.03wc
Non-sterile compounding
CSP 1,2,3 - CSP 1 only may be in C-PEC in C-SCA in unclassified area
BSCs, RABS (CACI or CAI), LAFW
PPE and protocols
Negative pressure for sterile compounding (HD), 30ACH, 0.01-0.03wc
C-PEC & C-SEC externally ventilated, BSCs Class II A2, B1 or B2 or CACI for sterile HDs
Antineoplastic compounding and storage segregation
Designing for SOPs & Controls in Cleanrooms
Architectural control systems are intended to:
• Control access to critical areas
• Accommodate frequent cleaning & equipment
• Segregate personnel from processing exposure
SOPs are intended to:
• Prevent cross-contamination
• Align production schedule with operations
• Enable cleanroom operator occupancy
• Segregate flows where required
• Protect operators
Architecture Controls:
• Personnel, materials, waste
• Classified area access
• secure IP, critical materials & product

Environmental Controls:
• Temperature
• Humidity
• Pressurization
• Cleanliness (particle removal)
• Surface cleaning

cGMPs for Access Controls



cGMP for Airlock Scheme
Pressure Bubble Pressure Sink Pressure Cascade

cGMP & Compliance Keys: Gowning SOPs
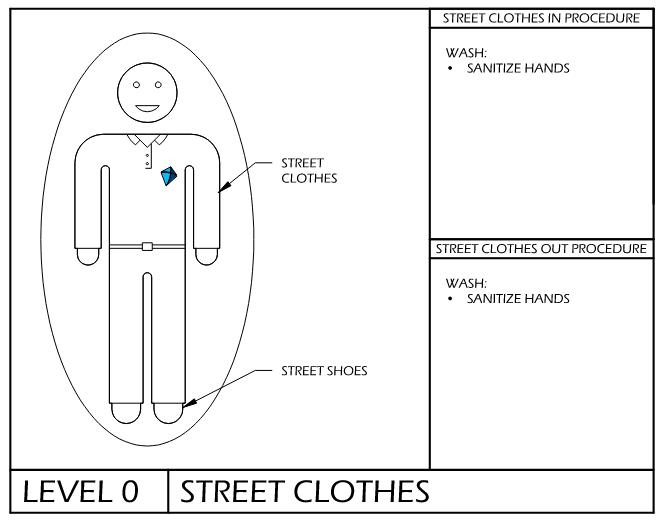
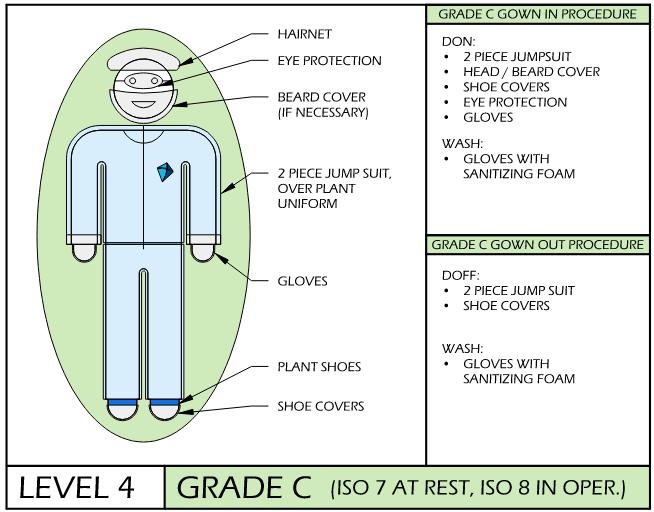

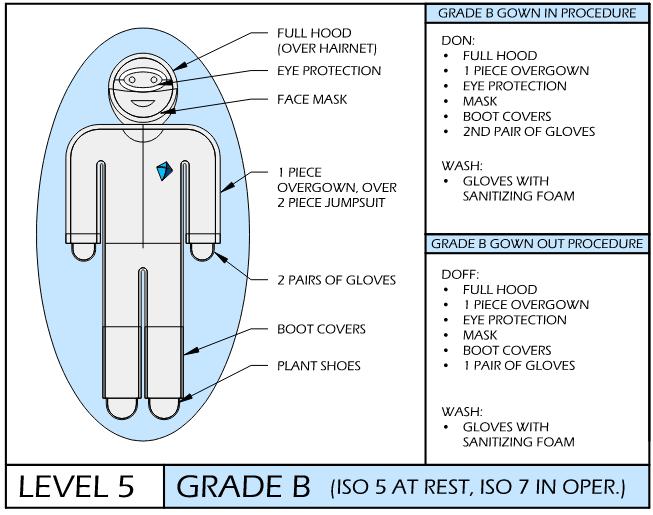



Temporal Procedures

cGMP & Compliance Keys: Process Design
PEOPLE PRODUCT

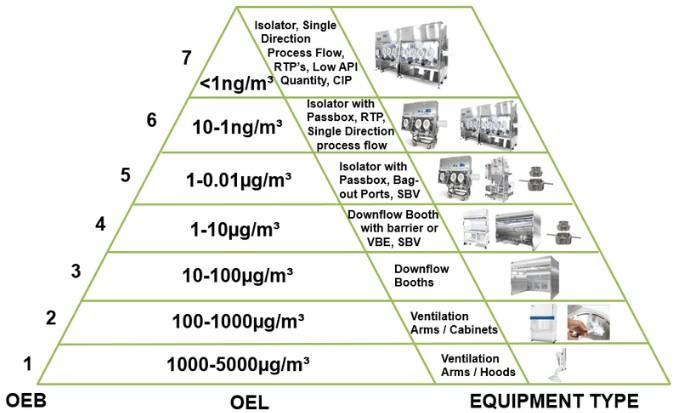



Cleanroom HVAC Design Practices





AHU Zoning
• 1 AHU per FlexSuite
• Cell & Gene Therapy
• BSL-2 or lower
• Recirculation (10-30%OA)
• Optional once thru (100%OA)
Environment al
• 66°F Grade B
• 68°F Grade C
• 70°F Grade D/CNC
• Temp +/- 4°F
• Summer Humidity max 60%
• Winter
Humidity min 30%
Classificatio n
• Grade B –60ACH
• Grade C –45ACH
• Grade D –25ACH
• CNC –15ACH
Containment Strategy
• Supply / Return Corridors
• ++Bubble MAL/ PAL in
• + Processing room
• - Sink MAL/PAL out

Cleanroom HVAC Design Schematic

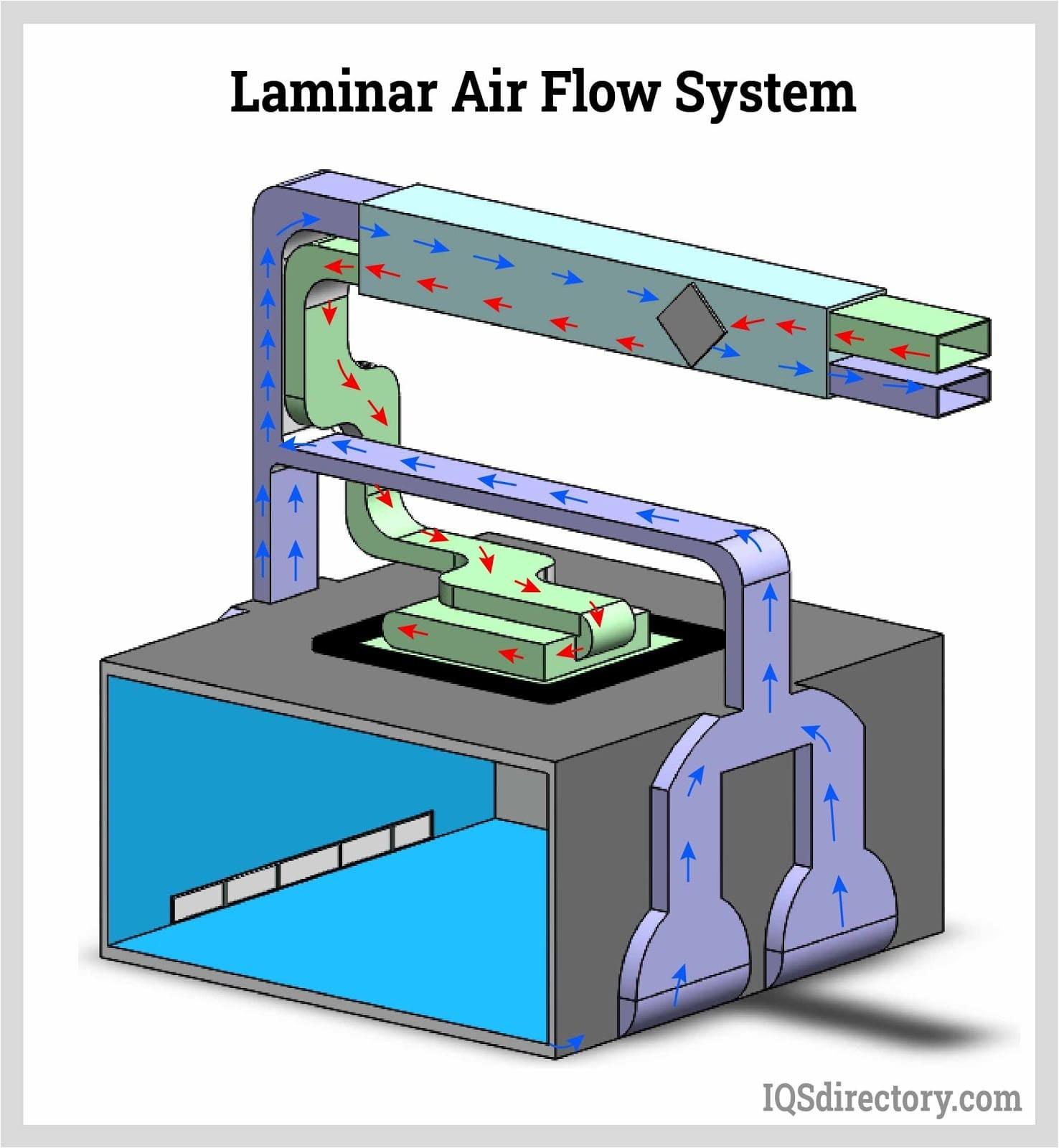

Cleanroom HVAC Design Practices



Critical Zone Airflow Design Example



Cleanroom Mechanical Design Considerations
Cleanroom AHU Cooling & Heating Source
Cleanroom AHU

Cooling / Heating Options
• Chilled Water + Heating Hot Water AHUs
• Central Chiller / Boiler
• Hydronic piping
• Precise temperature control
• Best heating/cooling performance for large loads
• Lowest energy costs
• Direct Expansion + Elec Re-Heat / Heat Pump
• Packaged units mounted outside or Split System
• Refrigerant piping for split systems
• Less precise temperature control
• Higher energy costs
Total Cost of Ownership

HVAC Equipment location options
C• Mezzanine Supported
• Structural additions
• Space under Mezz. can be utilized
• Risk of leaks from Chilled/Heating water
• AHUs protected from elements, easier maintenance
• Roof Supported
• Structural reinforcement/additions
• Consumes no interior space
• Risk of leaks from Chilled/Heating water
• Packaged DX RTU’s are cost effective
• RTU’s exposed to elements
• Floor Supported
• No structural additions / seismic
• Consumes floor space
• Lower risk of leaks from Chilled/Heating water
• AHUs protected from elements
•Hung in Interstitial space
•Requires structural additions
•Uses no space at perimeter
•Shorter duct runs
•Higher risk of leaks from Chilled/Heating water
•Can be difficult to install/maintain (requires access)
•AHUs protected from elements

Construction Challenges in GMP Space Conversion
Access

• Location for construction material entry relative to project area
• Corridor system
• Project area above grade can affect constructability
• Proximity to project area impacts efficiency
• Exterior points of entry
• Shared corridors/access points with other facility tenants

Rigging
• Rigging equipment impacted by project area location (central or exterior access)
• Mechanical equipment highest impact
• Site or facility restrictions on equipment used
Material Staging

• Interior or Exterior location
• Protection of materials is important
• Constraints on material movement through occupied spaces
• Proximity to project area impacts efficiency
Building Volume

• Potentially limited interstitial space above cleanroom
• Height to structure above impacts sequencing and modular system design
Sequencing

• Installation starting point (build-out concept)
• Interface with other trades
• Work schedule with other trades
• Safety & Logistics in work area
• Intent to avoid disruption of current ongoing operations
Future Phasing

• Phased expansion internal or external
• Intent to avoid disruption of current ongoing operations
• Consider “bolton” concept

Construction Phasing Strategies in Spatial Conversions


Roadmap to Project Success
CONSTRAINT IDENTIFICATION REGULATORY & cGMP ALIGNMENT

PROCESS
ARCHITECTURE & MECHANICAL ENGINEERING
PHASED IMPLEMENTATIO N STRATEGIES

Capital Projects Start with Conceptual Design

Prepare for strategic review with scope, schedule, and cost schematic design data