




you will need
• clear glue and vinyl glue
• shaving foam
• baking soda
• food coloring
• liquid laundry detergent
• a bowl
• a spoon

Now, let’s mix!
1
Pour 3 tablespoons of clear glue and 3 tablespoons of vinyl glue into a bowl. Add a handful of shaving foam and food coloring and start mixing with a spoon.
DIFFICULTY:










DIRTINESS: TIME: do it with: 10-15 minutes +10-15min.resting

2
Add 1 tablespoon of baking soda and 1 tablespoon of detergent and continue stirring vigorously.
You can add glitter, to make your slime even brighter.

3
Leave the mixture to rest for 15 minutes, then start mixing again. Add another spoonful of detergent if it doesn’t thicken in a short time.
4
Finally, knead the slime with your hands, as if it were dough.
Whenaddingbakingsodaanddetergent, thereactionthatturnsliquidglue intoagelatinousandslimypaste istriggered.Remember:restingtime iscritical.Tryseveraltimesifyou don’t succeed the first time. Go for it!
A part from slime, there are other examples of NON-NEWTONIAN FLUIDS that are easy to find in everyday life. These include: ketchup, blood, paints or toothpaste. Others are more dangerous and difficult to find, like quicksand!





you will need
• Corn starch (or potato starch)
• Water
• Bowl
• Glass
• Food coloring (if you want)
Take a bowl and pour 2 glasses of starch in it.
Slowly pour 2 glasses of water in, mixing the substance until you obtain a thick mixture.
Find your favorite consistency, and add a bit more water if needed.




1 2 3 DIFFICULTY:


TIME: do it with: 5-10 minutes

Quicklytaponthesubstance, usingyourfingeroraspoon.What happens?Andwhatifyoutouch thesubstanceslowly,instead?
Ifyouhitithardandfast, the substance it will become hard, while,ifyouwet itgently,itwill behave more like aliquid.




you will need
• Kitchen scale
• 0.1oz(3g)ofborax
• water
• 0.7oz(20g)ofclearglue
• 0.7oz(20g)ofshavingfoam
• 0.3oz(10g)ofdishdetergent
• foodcoloring

3
Pour the clear glue, foam and dish detergent into a bowl. Mix very well and add food coloring as desired.




DIFFICULTY: DIRTINESS: TIME: do it with: 10-15 minutes


1
Heat 2.8 oz (80 g) of water in a saucepan and then add 0.1 oz (3 g) of borax. Mix well: your activator is ready!

2
When the mixture becomes smooth, add 3 teaspoons of activator and start stirring for a few minutes.
4
In a short time, the mixture becomes more and more filamentous. When it is no longer sticky, take it in your hand and start kneading it as if you were to make a ball. Continue for a few minutes, until the slime reaches the desired consistency.
Theboraxthatyouaddedasanactivatorfitsbetween thefilamentchainsofthegluetoformthegelatinousnet, typicalofaslime.

Among the many metals that surround us, some are a potent force against rust!
Stainless steel contains a small amount of chromium, which makes it stainless, i.e. better resistant to OXIDATION and corrosion, the phenomena that cause rust. Chromium, reacting with oxygen, is able to coat itself in a whitish layer that protects the underlying metal from the corrosive action of external agents.
Steel is a METAL ALLOY, mainly composed of iron and a small percentage (2%) of carbon.
TYPES OF ALLOYS
An alloy is a mixture of two or more elements, one of which is a metal.
Iron + carbon = steel
Iron + carbon + chromium = stainless steel
Copper + zinc = brass
Copper + tin = bronze
The new material obtained has metallicpropertiesthatdiffer from those of the individual components.
Forexample,steelismore resistant than iron; brass is harder thancopperandshinierthanzinc.

Fats and oils are part of a large family that chemists call LIPIDS.
Meat, fish, eggs contain fat, because they are all derived from animals, which, like us, use LIPIDS for their vital functions. Animals use fats as an energy reserve, for thermal insulation and in making cellular structures.
Lipidsarenotsoluble,thatis,theydonotdissolve wellinwater.However,theyaresolubleinother substances similar to them, such as acetone, alcoholsandhydrocarbons(gasoline).Thisiswhy wemustusespecificproductstoremovelipids frompots,pansandclothes!
Waxes are also lipids. They constitute the thin layer that covers leaves and fruits of some plants to limit the dispersion of water, and as a protection against pests. Waxes are part of the skeleton of many insects, and cover the plumage of waterfowl.
The worst enemy of the galaxy of dirt is soap. It is the product of a CHEMICAL REACTION, called SAPONIFICATION.
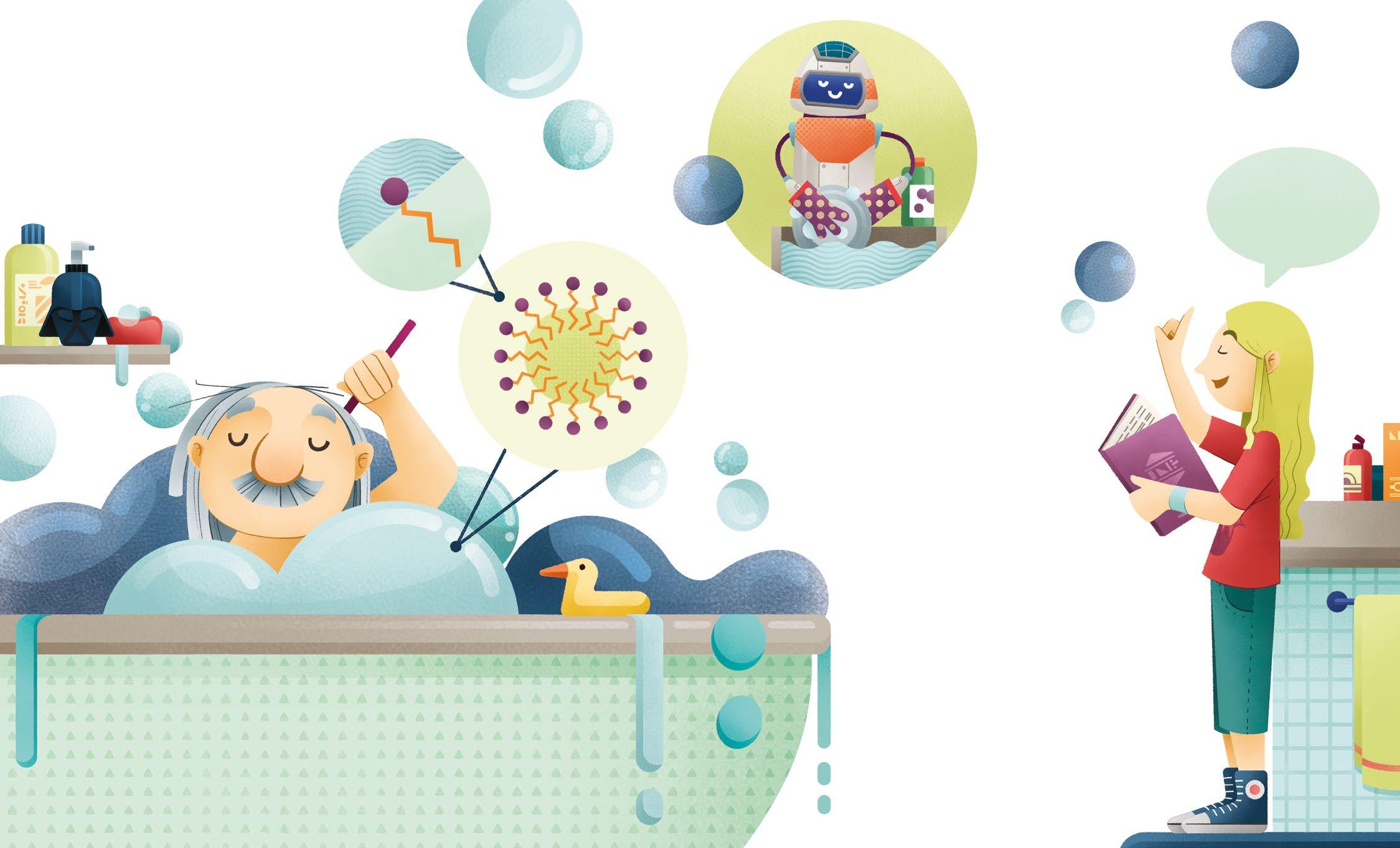
The MOLECULES that compose soap have a double nature:
• a hydrophilic head, which binds well to water;
• a hydrophobic tail, which repels water and attracts fatty and oily substances.
When the MOLECULES of soap meet dirt, they arrange themselves in a circular way, with the tails turned inwards – in contact with the dirt –and the heads turned outwards, in contact with the water. This way, a small sphere called “MICELLE” is created.

The secret of the cleaning power of soap is in the MICELLES! This particular arrangement allows soap to trap dirt.
Soap is able to break the SURFACE TENSION, that is. the invisible and elastic “membrane” that forms on the surface of a liquid. For this reason, soap is also called a SURFACTANT.
Some insects manage to float and walk on water using SURFACE TENSION, that is, this COHESION FORCE between MOLECULES.
CLEAN! OR CLEAN NOT. THERE IS NO RINSING.

In addition to dressing salads, vinegar also has other talents! It is an ACID substance, and this characteristic makes it a good detergent.
If it is too concentrated, vinegar can even corrode some surfaces such as marble, stone and rubber. Vinegar can also be very aggressive on some metals such as cast iron, aluminum and steel, releasing irritants for the skin. The word “vinegar” comes from the Old French vinaigre , meaning “sour wine.”
Have you ever tried mixing vinegar with baking soda?
The mixture releases water and carbon dioxide, accompanied by a magnificent, effervescent foam!
However, it is not a useful reaction to fight dirt: if we mix acid and basic substances together, they cancel each other out and are not very effective.
What is that whitish layer that forms on the surface of sinks, tiles and appliances in contact with water?
THAT'S LIMESCALE.
Limescale is a rock, whose main component is calcium carbonate.

During its cycle, water comes into contact with the rocks, bringing with it the minerals contained in them such as CALCIUM CARBONATE, MAGNESIUM and BICARBONATES. These minerals are then deposited during evaporation, forming limescale incrustations.
Day after day, drop by drop, the calcium carbonate present in running water accumulates, creating small grains that can clog and damage pipes. Limescale is dissolved by acids, which is why vinegar is excellent for removing it!
It may seem incredible, but mold gave ALEXANDER FLEMING, a British physician and a biologist, the Nobel Prize in Medicine in 1945.
In 1928, Fleming was conducting research on bacteria grown in special containers.He went away for a few days on vacation, and, when he returned, he noticed that one of the bacteria had been contaminated by a fungus, and that bacteria did not grow in that area. Fleming sensed that mold could be the cause of the death of bacteria, and therefore he started studying mold, obtaining a substance that he called Penicillin.
PENICILLIN IS THE ANCESTOR OF ANTIBIOTICS, AND HAS ALLOWED US TO DEFEAT VARIOUS DISEASES.

Thirty-five years earlier, an Italian doctor, Vincenzo Tiberio, had already discovered the beneficial effects of this mold. He noticed that, every time a well near a house was cleaned of mold, people who drank that water had bowel problems that stopped only when the molds reappeared. Tiberio published his results in a scientific journal, but unfortunately his discovery was ignored.
Soil is a mixture of inorganic and organic substances that covers the surface of the earth. The INORGANIC PART (about 45-50%) is formed by minerals contained in the rocks. The ORGANIC PART (about 5-10%), called humus, is made of leaves, seeds and animal remains, which make the soil very fertile. The remaining 50% of the soil is made up of IDLE SPACE, which is filled with air and water according to the type of soil and the environmental conditions – temperature, humidity, precipitation –in which it is found.
INORGANIC PART IDLE SPACE
Permeability is the ability of the soil to let water pass through it. The more the ground contains voids between one space and another, the more permeable it is.

GRAVELLY water flows through it easily. SANDY permeable, that is, it allows water to pass through. CLAY impermeable, that is, it does not allow water to pass through.
MIXED (gravel, sand and clay), more or less fertile and suitable to be cultivated.

you will need
•three67floz(2l) plasticbottles
•1glassofsand
•1glassofclay
•1glassofgardensoil
• water
• gauze
• 3 elastic bands
• scissors
1
For each bottle, cut the upper part to obtain a funnel about 1/3 of the height of the whole bottle.
2






DIFFICULTY: DIRTINESS: TIME: do it with:





4
5 15-20 minutes
Fill the funnel with 1 glass of soil (bottle A: sand, bottle B: clay, bottle C: gardening soil).
Coat the neck of each bottle with the same amount of gauze (three layers per bottle are enough), and secure them with an elastic band to create a cap.
3
Place the funnel you obtained on each bottle.
The soil retain water in different ways: water passes quicklythrough the sand, slower throughthegardening soil and even slower throughtheclay.
Pour 1 cup of water in each funnel and wait for 10 minutes, observing what happens.