




Published by Accelerate Learning Inc., 5177 Richmond Ave, Suite 800, Houston, TX 77056. Copyright © 2025, by Accelerate Learning Inc. All rights reserved. No part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without prior written consent of Accelerate Learning Inc., including, but not limited to, in any network or other electronic storage or transmission, or broadcast for distance learning.
To learn more, visit us at www.stemscopes.com.

By analyzing data and designing a solution for sustaining biodiversity, the student is expected to demonstrate an understanding of the importance that matter cycles between living and nonliving parts of the ecosystem to sustain life. Student Expectations
How do plants, animals, and even tiny microbes work together to keep our planet full of life and variety?
Key Concepts
• Matter cycles through the atmosphere and biosphere. Matter is ultimately recycled by decomposers. These cycles are important for nutrient availability in ecosystems. Disruptions of the cycling of matter result in the disequilibrium of nutrients in an ecosystem; this can ultimately lead to the destruction of the ecosystem itself.
• Photosynthesis and cellular respiration cycle carbon, moving it through the ecosystem. The carbon cycle links the atmosphere and biosphere. The carbon cycle can be disrupted in various ways.
• Animals obtain food from eating plants or eating other animals. Within individual organisms, food moves through a series of chemical reactions in which it is broken down and rearranged to form new molecules to support growth or to release energy.
• Nitrogen exists in different forms, and only a small portion of it is available for plants to absorb (in the form of ammonium or nitrate-containing compounds). Nitrogen availability is a limiting factor in ecosystems, and increasing its availability disrupts the ecological balance.
• Water is cycled through its many states within the geosphere, atmosphere, and biosphere.
• Biodiversity can be protected through preservation, reforestation, sustainable agriculture and fishing practices, composting instead of landfills, use of green products, etc.
This unit develops students’ understanding of how matter cycles between living and nonliving components and sustains life. Learners trace carbon through food webs and decomposition, model photosynthesis, cellular respiration, and digestion to connect energy flow with conservation of matter, and compare major biogeochemical cycles. They analyze data on disruptions, synthesize patterns in impacts on biodiversity and ecosystem services, and communicate evidence-based claims. Students then apply their learning in a design challenge, creating and refining a mitigation plan that balances biodiversity with human needs.
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Atmosphere
The layer of gas surrounding a planet that is held in place by gravity
Biodiversity
The number of different species of plants and animals in an area
Biosphere
The sum of all living matter on Earth
Carbon Cycle
The continuous movement of carbon in and between the abiotic and biotic environments
Carbon Dioxide
A gas that is a natural component of the atmosphere; produced by cells during cellular respiration and used by plants and other organisms for photosynthesis
Carbon Dioxide–Oxygen Cycle
The movement of carbon dioxide and oxygen on Earth by the processes of respiration and photosynthesis
Cellular Respiration
The process of obtaining energy from the breaking of chemical bonds in nutrients
Cycle of Matter
The continuous movement of different types of matter, such as water, phosphorous, nitrogen, and carbon, through different parts of the hydrosphere, lithosphere, atmosphere, and biosphere
Geosphere
Portion of the system of Earth that includes Earth’s interior, rocks and minerals, landforms, and the processes that shape Earth’s surface
Lithosphere
The cool, rigid, outermost layer of Earth that consists of the crust and the uppermost part of the mantle; broken into pieces or segments called plates
Nitrogen Cycle
The process by which nitrogen is converted among various chemical forms as it cycles among the soil, biosphere, lithosphere, and atmosphere
Oxygen
A gas produced by plants during photosynthesis that animals use for respiration
Water Cycle
The constant movement of water through the land, air, oceans, and living things
Students revisit food chains to trace how carbon moves through living and nonliving parts of ecosystems.
• Analyze slides of a food chain and a compost bin to discuss carbon transfer among organisms, decomposers, soil, and plants.
• Create and refine diagrams showing carbon flow through food chains and composting, including uptake by plants via photosynthesis.
• Identify and add pathways that return carbon to the atmosphere (organism respiration, burning of fossil fuels).
• Briefly compare carbon cycling to other element or compound cycles.
Making a Model - Cycling Through Organisms
Students investigate how energy flows and matter cycles through photosynthesis, cellular respiration, and digestion using hands-on models.
• Model photosynthesis with station-based snap cubes to simulate inputs, products, and energy transfer in chloroplasts.
• Construct and break apart molecular models to represent cellular respiration in mitochondria, emphasizing conservation of matter and energy release from bonds.
• Complete enzyme-focused puzzles to model digestion of carbohydrates, proteins, and lipids into smaller molecules, connecting breakdown products to respiration.
Students examine how matter cycles sustain ecosystems and how disruptions alter biodiversity, ecosystem services, and human wellbeing.
• Analyze diagrams of the water, carbon, nitrogen, and oxygen cycles and write evidence-based explanations of their importance to organisms and ecosystems.
• In groups, investigate assigned disruption scenarios and create skits showing which cycles are affected and how biodiversity, ecosystem services, and humans are impacted.
• Record impacts from their own and peers’ scenarios to synthesize patterns across disruptions.
• Write a letter to the editor explaining why limiting disruptions to matter cycling is essential for sustaining human life.
Students engage in an engineering design challenge to create a wetland mitigation plan that maintains biodiversity and supports human needs.
• Research a local wetland context and collaborate in teams to define criteria and constraints.
• Brainstorm and draft a to-scale diagram or model, including materials, safety, and procedures.
• Build, test, analyze, and iteratively refine the design to meet all criteria and constraints.
• Present solutions, field peer questions, and evaluate designs to identify the strongest features.

Estimated 15 min - 30 min
In this brief Engage activity, students revisit food chains to discuss how carbon cycles through living systems.
Materials
Printed
● 1 Carbon—It's a Cycle (per student)
● 1 Carbon—It's a Cycle Answer Key (per teacher)
● 1 Slide Show (per class)
Set up a computer and projector to show a slide show to the class.
Developing and Using Models, and Constructing Explanations and Designing Solutions, and Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict how carbon cycles through living systems, illustrating the interactions and energy flows within ecosystems. By drawing diagrams of carbon flow through food chains and compost bins, students will evaluate the limitations of their models and modify them based on evidence to account for changes in variables, such as the introduction of compost as fertilizer. This process will help them construct explanations supported by scientific ideas and principles, demonstrating the interconnectedness of plants, animals, and microbes in maintaining life and biodiversity on Earth.
Notes
Cause and effect: Mechanism and explanation
Systems and system models
Energy and matter: Flows, cycles, and conservation
During this activity, students will explore the phenomenon of how plants, animals, and microbes work together to sustain life on Earth by examining the carbon cycle. They will classify relationships as causal or correlational, recognizing that correlation does not imply causation, and use cause and effect relationships to predict how carbon cycles through natural systems. By creating models of food chains and compost systems, students will understand how systems interact and how energy and matter flow within these systems, illustrating the conservation of matter and the transfer of energy that drives the cycling of carbon.
● Print one Carbon—It's a Cycle document for each student.
● Show the slide of a food chain.
● Ask students to describe the relationship of the element carbon in a food chain.
● The element carbon is a component of all living organisms. When a cow eats a plant, carbon from the plant is passed on to the cow.
● Show the slide of a compost bin.
● Ask students to describe the relationship of carbon in a compost bin.
● Carbon is passed on to the decomposers and into the compost of the bin. When the compost is used as fertilizer, the carbon in the compost is passed on to the soil and then the plants.
● Have students draw a diagram of carbon flowing through a food chain and compost bin.
● Ask students where plants get carbon.
● From the air during photosynthesis
● Ask students how carbon flows from Earth materials and organisms back into the atmosphere.
● Burning of fossil fuels, respiration of organisms
● Have students add the flow of carbon from Earth materials and organisms into the atmosphere in their diagrams.
● Discuss the following:
○ Carbon is one element that cycles through ecosystems to organisms. What are other elements or compounds that flow through similar cycles? Accept all ideas.
Phenomenon Connection
How do the interactions and processes within ecosystems ensure the continuous cycling of carbon, and what role do these cycles play in maintaining life on Earth?
1. In what ways do plants, animals, and microbes contribute to the cycling of carbon within an ecosystem, and how does this process support the diversity of life?
2. How might changes in one part of the carbon cycle, such as increased fossil fuel burning, impact the balance and health of ecosystems globally?
3. What are some other essential cycles, like the carbon cycle, that involve the interaction of living organisms and Earth materials, and how do they contribute to sustaining life on our planet?
FACILITATION TIP
While showing the compost slide, ask: “Does anyone compost at home or know someone who does? What happens to food scraps over time?” This helps students connect science to personal experience.
FACILITATION TIP
As students draw their diagrams, encourage them to use arrows and labels that clearly show processes (photosynthesis, respiration, decomposition, combustion). This builds diagram literacy.
FACILITATION TIP
When discussing other cycles (like nitrogen, water, or oxygen), guide students to compare similarities: matter cycles continuously, energy flows one way.

Estimated 1 hr - 2 hrs
In Part I, students model the process of photosynthesis and demonstrate the transfer of energy in a system. In Part II, students model the breaking down of glucose and oxygen to water and carbon dioxide during cellular respiration. In Part III, students use a puzzle to model how large molecules are broken down into small usable molecules during the digestion of carbohydrates, proteins, and lipids.
Materials
Printed
● 1 Student Journal (per student)
● 1 Station Cards (per class)
● 1 Mitochondrion (per group)
● 1 Puzzle Pieces (per student)
Reusable
Part I
● 3 snap cubes, yellow (per group)
● 3 snap cubes, orange (per group)
● 12 snap cubes, blue (per group)
● 18 snap cubes, white (per group)
● 6 snap cubes, black (per group)
● 1 bag, plastic, ziplock, quart-size (per group)
● 6 bags, paper, brown, lunch-size (per class)
● 1 marker, black (per teacher)
Part II
● 12 snap cubes of one color (per group)
● 6 snap cubes of another color (per group)
● 18 snap cubes of a different color (per group)
Part III
● 1 scissors (per student)
● 1 glue stick (per student)
Consumable
Part I
● 5 note cards, 5 x 7 inch (per class)
*Note if using STEMscopes kits the colors of the snap cubes may vary.
1. Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
2. Review the CER Key prior to conducting the CER with students.
3. Print one Station Cards. Cut the cards for each station and place at the appropriate station.
4. Colors of cubes may be added or substituted if needed. The math department often has snap cubes you can borrow.
5. Label the quart-sized plastic bag as "Photosynthetic Organism." Label one brown paper bag for each station as follows: "Chloroplasts," "Energy," "Hydrogen and Oxygen," and "Oxygen and Carbon."
6. Make a card for each station below with the name and phrase. The number of snap cubes needed for each station should be placed by the Station Card.
Chloroplast Station Supplies: Each group will need three orange cubes. Place all of the cubes needed for the class in the bag labeled "Chloroplasts."
Chloroplast Station Card: To be able to capture the energy from sunlight, you will need to go get chloroplasts for cells. Place three cubes in the "Photosynthetic Organism" bag (the plastic bag).
Energy Station Supplies: Each group will need three yellow cubes. Place all of the cubes needed for the class in the bag labeled "Energy."
Energy Station Card: Collect sunlight. The sunlight will hit the chlorophyll in the chloroplast and start the process. Get three photons (light waves) of light. Snap them to the chloroplasts. Place in the "Photosynthetic Organism" bag (the plastic bag).
Water Station Supplies: Each group will need six oxygen/hydrogen molecules. You may need to use shades of blue due to the high number needed.
Water Station Card: Collect at least six water molecules for the process. You will need two snap cubes for hydrogen (blue) and one white snap cube (oxygen) together to represent one molecule of water. Place the completed water molecules in the "Photosynthetic Organism" bag (the plastic bag).
Carbon Dioxide Station Supplies: You will need two white snap cubes for oxygen and one black snap cube for carbon. Each group will need six molecules of carbon dioxide.
Carbon Dioxide Station Card: The photosynthetic organism will gather carbon dioxide from the atmosphere. You will need six molecules of carbon dioxide (six carbon atoms and 12 oxygen atoms) to make food (chemical energy) for the plant. Place the needed atoms in the "Photosynthetic Organism" bag (the plastic bag).
Print one Student Journal for each student in your class.
Obtain materials. The math department may have snap cubes available, or you may use snap beads.
Each group does not need to have the same colors to represent the three elements, but within each group, all of the snap cubes for the element carbon should be the same color and so on.
Print one Puzzle Pieces for each student.
Developing and Using Models, and Constructing Explanations and Designing Solutions, and Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict how plants, animals, and microbes interact to sustain life on Earth. By modeling photosynthesis, cellular respiration, and digestion, students will evaluate the limitations of these models and modify them based on evidence to understand the cycling of energy and matter. This process allows students to construct explanations and design solutions for the phenomenon of how these organisms work together to maintain biodiversity and life on our planet.
Cause and effect: Mechanism and explanation
Systems and system models
Energy and matter: Flows, cycles, and conservation
During this activity, students will explore the phenomenon of how plants, animals, and microbes work together to sustain life on Earth by modeling photosynthesis and cellular respiration. They will use cause and effect relationships to predict and understand these processes, recognizing that multiple factors contribute to these natural systems. Students will also use system models to represent interactions within and between systems, such as energy and matter flows, and learn that these models have limitations. Through this, they will gain insights into the conservation of matter and the transfer of energy, which drives the cycling of matter in Earth’s systems.
Notes

Part I
Students work in groups of four to complete this task.
Pre-Activity Discussion
1. What do plants need to live? Plants need water, air, soil, and sunlight.
2. What materials do plants need for growth? Plants need water, air, and sunlight.
3. How do plants get energy? Plants get energy from the Sun. Have students complete Part I of their Student Journal.
Post-Activity Discussion
1. How does photosynthesis cycle carbon into and out of organisms? The carbon begins as gas in the air and is absorbed into the leaves. It is then changed into a solid and part of the plant as glucose. When an animal eats the plant, the carbon becomes part of the animal.
2. How does photosynthesis cycle oxygen into and out of organisms? Oxygen enters the plant in two different ways. Through water molecules, it enters the plant through its roots from the soil. Through carbon dioxide molecules, it enters the leaves from the atmosphere. Photosynthesis causes some of the oxygen to remain in the plant as part of the glucose molecule. The rest of the oxygen is released into the atmosphere as oxygen gas. Animals can get the oxygen either by breathing in the oxygen gas or by eating the plant and absorbing the oxygen in the water and carbohydrates of the plant.
3. What is the path or flow of energy in the process of photosynthesis? The energy flows from the Sun in the form of sunlight. The energy is absorbed by the organism into the chloroplasts. The process of photosynthesis transforms, or changes, the light energy into chemical energy or stored energy.
Pre-Activity Discussion
1. What is an element? A pure chemical substance consisting of a single type of atom
2. What is a molecule? The smallest particle in a chemical element or compound that has the chemical properties of that element or compound
3. How are molecules held together? Molecules are held together by chemical bonds.
4. What does a molecule of oxygen look like? It has two oxygen atoms bonded together.
Notes
5. What is the chemical formula for carbon dioxide? CO2
6. What would a molecule of carbon dioxide look like? It would have one carbon atom and two oxygen atoms bonded together. By the end of the Pre-Activity Discussion, students should be thinking about the molecules and their bonds.
Have students complete Part II of their Student Journal.
Post-Activity Discussion
1. Was matter created or destroyed in the cellular respiration process? No
2. How do you know? We ended with the same atoms with which we started, and no atoms were left in the mitochondrion.
3. What did you do to model the release of energy during the cellular respiration process? I broke the bonds holding the molecules together by unsnapping the cubes.
Part III
Students work individually to complete this task.
Pre-Activity Discussion
1. What are the three basic molecular categories of nutrients from food? Carbohydrates, proteins, and lipids
2. Molecules of carbohydrates, proteins, and lipids are too large to enter our bloodstream. How are the molecules broken down into a usable size? Accept all ideas.
Work the Carbohydrate Puzzle as a class so students understand the correct placement of the pieces. Discuss what is occurring in each step. (Example: Before the reaction, the large molecule is intact. During the reaction, the large molecule begins to break apart. After the reaction, the large molecule has broken down into the smaller molecules. Throughout, the enzyme remains unchanged.)
Notes
FACILITATION TIP
Prompt students to compare their cube models to the actual chemical formulas written on the board, reinforcing the link between the physical model and symbolic notation.
If students struggle with molecular bonding, remind them that the snapping cubes represent bonds that can be broken and re-formed.
FACILITATION TIP
Nutrients may be new vocabulary for students. You can refer to nutrition labels to help students make sense of the terms.

Procedure
1. Locate the puzzle pieces on the Puzzle Pieces. Color the large carbohydrate molecule orange, the enzyme-engaged molecule orange, and the sugar molecules pink.
2. Cut out each of the pieces for the carbohydrate puzzle.
FACILITATION TIP
Have students work with a partner to check that their puzzle diagrams match the correct sequence before gluing them down.
3. Color the enzymes associated with the breakdown of carbohydrates green.
4. Glue the pieces to complete the carbohydrate puzzle.
5. Repeat steps 1 through 4 to complete the protein puzzle and the lipid puzzle, but color the pieces using the following coloring key:
○ Large protein molecules: red
○ Enzyme-engaged protein molecules: red
○ Amino acids: yellow
○ Enzymes associated with the breakdown of proteins: brown
○ Bile: gray
○ Large fat (lipid) molecules: blue
○ Enzyme-engaged lipid molecules: blue
○ Fatty acids: white
○ Enzymes associated with the breakdown of lipids: purple
6. Use the digestion models and your knowledge of cellular respiration to describe how food molecules are processed through chemical reactions involving oxygen to form new molecules.
Post-Activity Discussion
1. How are food molecules broken down into molecules usable by the human body? The large molecules are broken down by chemical reactions involving oxygen aided by enzymes and other substances such as water for carbohydrates, stomach acid for proteins, and bile for lipids.
2. How is the process of digestion part of the cycling of matter through Earth’s systems? Digestion involves the molecules of glucose moving from plants to a human, with some molecules of carbon dioxide being expelled into the air and some molecules remaining in the human.
This activity requires much writing, which may be difficult for some students. Since the goal is not to measure the ability to read and write, and since there are multiple questions to answer about the stations, students could dictate the answers to a scribe or orally answer the questions from the handout. Learn more strategies to help students with difficulty writing in the Intervention Toolbox.
Notes
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to students completing the activity, say the words and have the students repeat them.
When teaching the words, concentrate on using them repeatedly and with examples. This will also facilitate students' ability to communicate with other students using academic vocabulary.
To check their understanding and to keep for future reference, students can draw pictures and complete the following sentence stems in their journals after the group activity. You can give students the option of using a word bank to label their pictures or of labeling them without a word bank. Students may also read the sentences out loud with a partner or in their groups.
Possible questions and sentence stems could be the following:
● Level 1 Knowledge Question: What are the starting chemicals in photosynthesis?
○ Stem: The starting chemicals in photosynthesis are _______.
● Level 2 Comprehension Question: Can you explain what a product is?
○ Stem: A product is ________.
● Level 3 Application Question: How would you show your understanding of the energy source of photosynthesis?
○ Stem: I would show my understanding of the energy source by ________.
● Level 4 Analysis Question: Can you categorize the differences between photosynthesis and cellular respiration?
○ Stem: The differences are _________.
● Level 5 Synthesis Question: Can you create a model that shows how cellular respiration works?
○ Stem: My model looks like ________.
● Level 6 Evaluation Question: How could you determine the types of energy transformations that occur during photosynthesis or cellular respiration?
○ Stem: I could determine the types of energy transformations that occur by __________.
How do these biological processes ensure the continuous cycling of matter and energy within ecosystems?
1. In what ways do photosynthesis and cellular respiration complement each other in the cycling of carbon and oxygen through ecosystems?
2. How does the digestion of food in animals contribute to the cycling of nutrients and energy in the environment?
3. How might disruptions in one of these processes affect the balance of life and variety on our planet?

Estimated 1 hr - 2 hrs
In this activity, students analyze diagrams of the water, carbon, nitrogen, and oxygen cycles to determine the importance of each to ecosystems and organisms. Students develop and perform skits addressing how disruptions in the cycling of matter could affect biodiversity and ecosystem services.
Materials
Printed
● 1 Student Journal (per student)
● 1 Diagram Cycles (per group)
● 1 Disruption Scenarios (per class)
Reusable
● 1 marker, set (per group)
Consumable
● 1 board, poster, white (per group)
1. Print one set of Diagram Cycles per group. As this is a large packet, it is recommended that you print this packet double-sided and then laminate it for repeated use.
2. Print one Disruption Scenarios and cut the cards apart to distribute one card to each group.
3. Plan to divide the class into six groups for Part II.
4. Students can use props to help with the visual aid portion of their skits. Some props might include construction paper for students to write or draw their roles. Students may choose to bring props from home.
Developing and Using Models, and Constructing Explanations and Designing Solutions, and Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict how disruptions in the cycling of matter, such as water, carbon, nitrogen, and oxygen cycles, affect biodiversity and ecosystem services. By analyzing diagrams and performing skits, they will construct explanations based on evidence to understand the interconnectedness of plants, animals, and microbes in maintaining Earth’s life and variety. Students will also communicate their findings through written and oral presentations, evaluating the impact of these disruptions on human sustainability and the natural world.
Cause and effect: Mechanism and explanation
Systems and system models
Energy and matter: Flows, cycles, and conservation
During this activity, students will explore the cause and effect relationships within the cycles of matter, such as water, carbon, nitrogen, and oxygen, to understand how disruptions can impact biodiversity and ecosystem services. By analyzing these cycles and performing skits, students will classify relationships as causal or correlational, recognizing that correlation does not necessarily imply causation. They will use models to represent systems and their interactions, understanding that systems may interact with other systems and have sub-systems. Additionally, students will track the transfer of energy and matter, learning how these flows drive the motion and cycling of matter within ecosystems, ultimately affecting the sustainability of human life on Earth.
1. Have students analyze the diagrams for each of the ways matter is cycled through Earth’s system.
2. Have them write an evidence-supported description of the importance of each cycle to the health of organisms and ecosystems.
1. Divide the class into six groups and assign each group one of the disruption scenarios.
2. Instruct students to collaborate with their group members to discuss how their ecosystem is affected.
3. Ask students to record their disruption and a brief description of its effect on the cycling of matter. They should include how the change to the affected cycle(s) results in changes to the ecosystem services and the biodiversity or the number of different types of organisms in the system. Students should also record how the changes could affect humans.
4. Have students plan their skit, using any supplies you provide for visual aids. Make sure students show how biodiversity and ecosystem services are affected and indicate which cycles are affected by the disruption. Have students discuss how the changes may impact human life.
5. While students watch the other groups perform their skits, have them record their disruptions and effects as well.
6. Instruct students to use their collected information to write a letter to the editor of their local paper to explain how disruptions to the cycling of matter affect the sustainability of human life on Earth and should be limited.
Disruption Scenarios
● An extended period of extreme drought
● Deforestation in a local forest to build a large shopping mall with parking lots
● Increase in fossil fuel emissions of carbon dioxide due to added power plants in an area
● Overuse of pesticides resulting in an almost total removal of the bacterial population living within the soil
● Increase in chemical fertilizers for plants, causing runoff to carry fertilizer to plants in natural areas
● Massive volcanic eruption resulting in global dimming from ash remaining in the atmosphere
Before starting, remind students of the law of conservation of matter and ask: “If matter can’t be created or destroyed, what must be happening when it moves through cycles?”
If students struggle with the “Interaction with Other Cycles” column, provide examples such as:
The carbon cycle interacts with the oxygen cycle during photosynthesis and respiration.
The water cycle impacts the nitrogen cycle through precipitation moving nitrogen into soils.
Wrap up with a reflection question: “Which ecosystem services do you personally depend on most, and how would your life change if they were disrupted?”

Sentence Stems
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After the students have time to explore with the animal and plant cards, give them the opportunity to write down their self-reflections by using the following sentence stems:
● The plant and animal exchange _____________ and _____________________.
● The plant releases _____________, while the animal releases ______________.
● The plant uses __________________, and the animal uses _____________________.
Have the students write the sentence stems in their journals. Group them into partners so they can share their sentence stems with each other.
How do disruptions in natural cycles impact the collaboration between plants, animals, and microbes to maintain biodiversity and ecosystem services on Earth?
1. How do the water, carbon, nitrogen, and oxygen cycles contribute to the balance and health of ecosystems?
2. In what ways might a disruption in one of these cycles affect the biodiversity within an ecosystem?
3. How could changes in these cycles impact human life and what actions can we take to mitigate these effects?

Estimated 2 hrs - 3 hrs
Students use criteria and constraints to design a wetland mitigation project with the goal of maintaining biodiversity and the resources humans need for survival.
Materials
Printed
● 1 Student Journal (per student) The following materials are suggested per group. Other materials may be utilized in this Engineering Solution as available.
Reusable, suggested
● Ruler, metric (per group)
● Computer with Internet connection (per group)
● 1 box or container (per group)
● 1 set of markers (per group)
Consumable, suggested
● Paper, graph, 1 cm2, 8.5 x 11 (per group)
● Paper, notebook (per group)
● 6 straws (per group)
● Variety of cardboard scraps (per group)
● Construction paper, various colors (per group)
● Clay (per group)
● Duct tape (per group)
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
● Students will request materials to create their models. Select and display materials that are readily available.
Technology Suggestion
You may want to review the process for using different search engine tools and the procedure for verifying sources of online information. You can also set up a web page with links for the assignment.
● String or wire (per group) Notes
Developing and Using Models, and Constructing, Explanations and Designing Solutions, and Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict how plants, animals, and microbes interact within ecosystems to maintain biodiversity and support life on Earth. By designing a wetland mitigation project, students will evaluate the limitations of their models, modify them based on evidence, and construct explanations using scientific principles. This process will help them understand the complex interactions and energy flows within ecosystems, and how these systems can be designed to optimize biodiversity and resource availability.
Cause and effect: Mechanism and explanation
Systems and system models
Energy and matter: Flows, cycles, and conservation
During this activity, students will engage in designing a wetland mitigation project, which will allow them to explore the cause and effect relationships within ecosystems. They will classify these relationships as causal or correlational, recognizing that correlation does not necessarily imply causation. By using models to represent the interactions within the wetland system, students will understand how systems interact with other systems and how energy and matter flow within these systems. This hands-on experience will help them predict how plants, animals, and microbes work together to sustain biodiversity and maintain the resources necessary for life on Earth.
The deliverable for this design challenge is a detailed to-scale diagram or model of a wetland built as a mitigation project. If you choose to have the students actually build the wetland, be prepared to provide the materials students request, or set additional constraints based on the available materials. Select a local wetland to be the one replaced.
21st Century Skills addressed: Innovation
Students create a new and original solution to a problem by creating a new design for a wetland mitigation.
The skills used by engineers to identify and solve problems are useful well beyond the science classroom and an important part of being able to function in and contribute meaningfully to society. When we say “developing engineering solution,” we are describing the intentional immersion of students in the reiterative engineering design process. For further information regarding developing engineering solutions, please click the provided link. A. Introduce the design challenge.
● Discuss the criteria for the design challenge.
● Remind students that their design solution must include all needed materials, safety precautions, and procedures used so that anyone can replicate the investigation.
● Have students test their design as needed.
● Instruct students to have a method for determining that all criteria and constraints have been met.
Clarify the constraints visually.
Post a one-page “Challenge Card” that lists the 10-mi² size constraint, required services, resources requirement (rainfall), deliverables (to-scale diagram, materials list, construction, and maintenance instructions). Keep it visible throughout work time.

FACILITATION TIP
Use low-cost modelling constraints to teach trade-offs.
Limit the number of “premium” items (e.g., only two pumps or one waterproof liner per group). This forces trade-offs and mirrors real engineering constraints.
A. Research and explore the problem.
● Remind students that engineers usually work in groups or teams so that many different ideas can be generated and combined to come up with one great idea. Divide the class into groups.
● Give each group a copy of the design challenge along with the rubric.
● Make sure the materials needed to complete the project are in a central location where groups can access them as needed.
B. Brainstorm and design a solution to the problem.
● Give students time to brainstorm and design.
● Make sure students have created a materials list, safety precautions list, and a procedure before they proceed.
● Assist students as needed.
C. Build, test, and analyze your solution.
FACILITATION TIP
Provide design templates and a scale primer.
Give a simple scale worksheet (e.g., 1 cm = 0.5 mi) and a blank grid map so groups can quickly convert 10 square miles into a classroom drawing. Model one example conversion and a quick sketch.
● Monitor as the students complete the design process.
● Ask questions and redirect thinking as necessary.
● Allow sufficient in-class time for groups to complete their project.
D. Improve or redesign and retest your solution.
● Give the groups time to analyze their criteria on the design process.
● Assist the students in redesigning and retesting the solution as needed.
E. Present and share your solution.
● Allow time for each group to present their results.
● Let other groups ask questions. Discuss as desired.
● Lead class discussion of evaluations of each solution and possible combinations to create a best solution.
● Complete the group rubric for each group.
● Discuss with students.
● Hold a Post-Activity Discussion, which includes the post-activity discussion questions for students to evaluate their solution and make notes for improvement.
1. How many of the ecosystem services were you able to design into your wetland mitigation project? Answers will vary. Example: Our solution addresses all of the wetland services except for buffering ocean waves.
2. What services were you not able to use in your project? Explain why you could not put them in your project. Answers will vary. Example: Our solution does not address buffering ocean waves as our wetland does not share a coastline with the ocean.
3. After listening to each presentation, what solution to the design problem was the best? Explain. Answers will vary but should show how the solution meets the criteria.
4. If you could choose aspects of each of the designs presented, which could be combined to form the best possible solution? Answers will vary but should show how the chosen aspects best meet the criteria.
Connection Sticky Notes
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
As students research about wetlands and ways to conserve their biodiversity, they will run across many new terms. Instruct the students to create three columns in their lab journals with these labels:
● New Key Terms
○ Students should create at least four sticky notes for the New Key Terms section.
● Similar To
○ The Similar To section requires at least two sticky notes, on which students compare the results of their research to something that has occurred in their home state.
● Human Actions
○ The Human Actions section should include the ideas students uncover in their research, with one or two examples on each, either positively or negatively affecting wetlands.
Phenomenon Connection
How do the interactions between plants, animals, and microbes in a wetland ecosystem contribute to maintaining biodiversity and supporting human needs?
1. How does your wetland mitigation design ensure the survival and interaction of different species within the ecosystem?
2. In what ways does your project demonstrate the interdependence of plants, animals, and microbes in maintaining a healthy ecosystem?
3. How can the principles used in your wetland design be applied to other ecosystems to enhance biodiversity and resource availability for humans?
Notes

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Arborist
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - Carbon’s Long Journey
A reading passage about the concept, which includes five to eight comprehension questions.
Notes
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
Student Learning Objectives
Matter cycles through the atmosphere and biosphere. Matter is ultimately recycled by decomposers. These cycles are important for nutrient availability in ecosystems. Disruptions of the cycling of matter result in the disequilibrium of nutrients in an ecosystem; this can ultimately lead to the destruction of the ecosystem itself.
Photosynthesis and cellular respiration cycle carbon, moving it through the ecosystem. The carbon cycle links the atmosphere and biosphere. The carbon cycle can be disrupted in various ways.
Animals obtain food from eating plants or eating other animals. Within individual organisms, food moves through a series of chemical reactions in which it is broken down and rearranged to form new molecules to support growth or to release energy.
Nitrogen exists in different forms, and only a small portion of it is available for plants to absorb (in the form of ammonium or nitratecontaining compounds). Nitrogen availability is a limiting factor in ecosystems, and increasing its availability disrupts the ecological balance.
Water is cycled through its many states within the geosphere, atmosphere, and biosphere.
Biodiversity can be protected through preservation, reforestation, sustainable agriculture and fishing practices, composting instead of landfills, use of green products, etc.

The student is expected to demonstrate an understanding of the physical and chemical properties of matter through analysis of qualitative data.
• The matter that makes up our world has physical and chemical properties that can be used for classification.
• The physical properties of matter include properties that describe the substance, such as color, smell, boiling point, and density.
• Chemical properties of matter include all the possible chemical changes that a sample of matter can undergo, such as corrosion, toxicity (the degree to which a substance is poisonous to an organism or the environment), and the ability to burn.
Scope Overview
This unit builds conceptual understanding of matter by contrasting physical and chemical properties through observation, discussion, and evidence-based classification. Students qualitatively analyze magnetism, density-related behavior, solubility, thermal and electrical conductivity, and melting/boiling points, then examine reactivity, rusting, combustion, and flammability using rotating stations. Culminating investigations have students plan tests to distinguish and identify unknown liquids, record observational data, compare to reference properties, and justify claims with evidence. Emphasis is on analyzing qualitative data to demonstrate understanding of matter’s properties.
Scope Vocabulary
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Chemical Energy
Energy stored in chemical bonds and released through chemical reactions
Chemical Property
A characteristic that can be observed or measured only when atoms rearrange during a chemical change
Corrosion
The process of destroying a solid material by a chemical reaction
Density
The amount of matter in a given space or volume
Physical Property
A characteristic that can be observed or measured without changing the substance
Toxic
Harmful to lif
Notes
Students explore how to distinguish between physical and chemical properties through sorting and discussion.
• Engage in a brief discussion on how observable characteristics and tests help identify substances.
• Work in pairs to sort scenario cards into physical vs. chemical properties using evidence from examples.
• Participate in a class review to confirm classifications and connect scenarios to key vocabulary (e.g., flammability, solubility, density).
Activity - Physical Properties
Students investigate the states and properties of matter through hands-on measurement and testing across six stations.
• Test household items for magnetism and classify materials as magnetic or nonmagnetic.
• Measure mass, volume, and displacement to calculate density and predict sinking or floating; compare solubility of common solutes in water over time.
• Compare thermal conductivity by observing ice melt in different cup materials and analyze which materials transfer heat best.
• Build a simple circuit to identify electrical conductors and insulators; organize substances by melting and boiling points using reference cards and a thermometer.
Activity - Observing Chemical Properties
Students investigate common chemical properties through rotating lab stations.
• Rotate through five stations to test materials: vinegar with baking soda, steel wool with vinegar/oxygen, match combustion, effervescent tablet with water, and iodine with potato starch.
• Observe and record evidence of chemical change in their journals.
• Identify and categorize chemical properties such as reactivity with acids and water, rusting, and flammability.
• Share and synthesize findings in a post-lab discussion.
Scientific Investigation - Identifying Liquids
Students plan and conduct an investigation to identify four unknown clear liquids using characteristic physical and chemical properties.
• Design and carry out tests (e.g., melting/boiling point, density, solubility, odor, flammability) to generate distinguishing data.
• Use lab tools to measure and record observations, then compare results to reference properties to determine each liquid’s identity.
• Justify identifications by constructing a concise conclusion and scientific explanation connecting evidence to claims.
Notes

Estimated 15 min - 30 min
In this activity, students determine whether properties are physical or chemical properties.
Material
Printed
● 1 Sorting Properties (per student)
● 1 Card Sort Cards (per pair)
● You may choose to either print out a page for each student, or you could also project the page on the board.
● Print Card Sort Cards on card stock and consider laminating them for reuse.
Planning and Carrying Out Investigations, and Analyzing and Interpreting Data, and Using Mathematics and Computational Thinking
During this activity, students will plan and carry out investigations to explore the phenomenon of a solid ice cube melting into liquid water and then disappearing into the air as gas. They will identify and analyze the physical and chemical properties involved in each phase change, such as melting point and boiling point, and use these investigations to provide evidence for the changes in properties. By analyzing and interpreting data, students will distinguish between physical and chemical changes, using mathematical and computational thinking to support their explanations and understand the underlying processes.
Conservation
During this activity, students will identify and analyze patterns in the physical and chemical properties of substances to understand the phenomenon of how a solid ice cube melts into liquid water and then disappears into the air as gas. They will recognize that macroscopic patterns, such as changes in state, are related to the microscopic and atomic-level structure of matter. Additionally, students will explore how the transfer of energy drives these changes, tracking how energy flows through the system as matter cycles between solid, liquid, and gas states. Activity
Pre-Activity Discussion
1. If you have a pencil and a pen in front of you, and you are instructed to pick up the pencil, how do you know which of the two objects to pick up? What tells you which one is the pen and which one is the pencil? Accept all ideas.
2. How do we tell the difference between all of the objects we encounter in our everyday lives? They look and feel different. They have different shapes, colors, sizes, and textures. They have different temperatures and scents. Each object has a group of characteristics that distinguish it from other objects.
3. What if the substances are colorless, odorless gases? You could perform tests on the gases.
Post-Activity Discussion
1. Discuss correct answers to the sort.
2. Introduce vocabulary related to scenarios.
Ice melts. (melting point)
Water evaporates. (boiling point)
A skillet conducts heat. (conductivity)
A rock sinks. (density)
Sugar dissolves in water. (solubility)
A penny is shiny. (luster)
Metals can be pulled and stretched. (ductility)
Iron filings are attracted to a magnet. (magnetism)
Phenomenon Connection
Chemical
Carbon dioxide extinguishes a fire. (flammability, not flammable)
Hydrogen gas lights on fire. (flammability)
Gasoline burns in an engine. (combustibility)
Iron rusts. (reacts with oxygen)
Baking soda and vinegar bubble when mixed. (chemical reactivity)
Uranium is radioactive. (radioactivity)
Sugar burns when heated. (flammability)
Sodium bursts into flames in water. (reacts with water)
How do the physical and chemical properties of substances help us understand the changes that occur when a solid ice cube melts into liquid water and then disappears into the air as gas?
1. What physical properties of ice and water can help us predict the conditions under which ice will melt and water will evaporate?
2. How do the chemical properties of water remain unchanged during the phase transitions from solid to liquid to gas?
3. In what ways can understanding the properties of substances help us manipulate the rate at which an ice cube melts or water evaporates?
FACILITATION TIP
Monitor for misconceptions. Listen for students confusing physical change (like melting) with chemical property. Redirect by asking: “Does the substance stay the same after this property is observed, or does it form something new?”
FACILITATION TIP
Show one example card to the whole class and think aloud: “This property describes how something looks/feels, so it’s physical. This one describes how it reacts with another substance, so it’s chemical.”

Estimated 1 hr - 2 hrs
In this activity, students use various measurement tools to explore states and properties of matter. The activity is divided into six stations: Magnetism, Density, Solubility, Thermal Conductivity, Electrical Conductivity, and Phase Changes.
Materials
Printed
● 1 Student Journal (per student)
● 1 Station Signs (per class)
● 1 Station 6: Thermometer (per class)
● 1 Station 6: Melting and Boiling Point Cards (per class)
● 2 Station 2: Density (per class)
● 1 Station Instruction Cards (per class)
Reusable
Station 1: Magnetism
● 20 common household items (mix of magnetic and nonmagnetic items) (per class)
● 1 container for the items (per class)
● 4 strong magnets (per student in each group)
Station 2: Density
● 1 triple-beam balance (per class)
● 1 graduated cylinder, plastic, 250 mL (per class)
● 1 metric ruler (per class)
● 1 calculator (per class)
● 2 regularly shaped objects (per class)
● 2 irregularly shaped objects (per class)
● 1 water source (per class)
● 4 sheet protectors (per class)
Station 3: Solubility
● 5 containers, labeled A, B, C, D, and E (per class)
● 5 timers or student watches (per class)
● 5 cups, clear, plastic, 9 oz (per class)
● 5 spoons, plastic (per class)
● 5 graduated cylinders, 100 mL (per class)
● 5 measuring spoons (per class)
● 1 goggles, pair (per student)
Station 4: Thermal Conductivity
● 3 aluminum baking pans, 2 in. deep (per class)
● 3 cups, clear, plastic, 9 oz (per class)
● 3 foam cups, 9 oz (per class)
● 3 cups, paper, 8 oz (per class)
● 1 cooler for ice cubes (per class)
● 1 timer (per class)
Station 5: Electrical Conductivity
● 1 piece of thick cloth, felt (per class)
● Several coins (penny, nickel, dime, quarter) (per class)
● 1 strip of aluminum foil (per class)
● 1 craft stick (per class)
● 1 plastic spoon (per class)
● Ceramic tiles (per class)
● Rubber erasers (per class)
● Materials to build a simple circuit: battery, battery holder, 3–6 in. wires, light bulbs, and bulb holder (per class)
Station 6: Melting and Boiling Points
● 1 resealable plastic bag (per class)
Consumable
Station 3: Solubility
● Solutes: salt, sugar, drink powder, baking soda, cornstarch (per class)
● Water, 500 mL (per class)
Station 4: Thermal Conductivity
● Ice cubes (per class)
Preparation
● Print and post the Station Signs.
● Print one Station Instruction Cards. Cut apart and place at the appropriate station.
Station 1: Magnetism
1. Place about 20 common household items in a container on the table. Examples of items include children’s toys, kitchen utensils or tools, office or school supplies, nature items, etc. Be sure that the materials from which these objects are made include plastic, rubber, cloth, wood, stone, magnetic, and nonmagnetic materials. About half the items should be magnetic.
Station 2: Density
1. Print and place in sheet protectors two copies of the Student Reference Sheet: Station 2: Density.
2. The irregularly shaped objects used should fit in the graduated cylinder without getting stuck. You can adjust the size of the graduated cylinder to be compatible with the size of the object.
3. Determine the density of each object before you implement this activity. If the density is greater than 1 g/cm3, the object will sink; if the density is less than 1 g/cm3, the object will float.
4. You may want students to rotate objects to decrease the number of items you have to deal with.
Station 3: Solubility
1. Caution! Remind students to wear goggles and waft scents at all times.
2. Fill each of the five labeled (A–E) containers with one of the solutes. Remember to record which solute is in which container for your reference.
Station 4: Thermal Conductivity
1. This activity may be done with partners in each group. Place a foam cup, a plastic cup, and a paper cup in the aluminum pan. Keep a small cooler to hold the ice during the class period.
2. You may determine that a longer time period—for example, 4 or 5 minutes—yields better observations for this station activity. If so, tell students to make the adjustment on their Station 4 procedure.
Station 5: Electrical Conductivity
1. Provide materials for students to create a simple electric circuit.
2. Each student can have his or her own circuit, or one circuit can be used by multiple students to test different materials.
Station 6: Melting and Boiling Points
1. Print and laminate the Melting and Boiling Point Cards.
2. Place the cards in a resealable plastic bag. Print, laminate, and cut out the Thermometer, and tape the two pieces together.
Planning and Carrying Out Investigations, and Analyzing and Interpreting Data, and Using Mathematics and Computational Thinking
During this activity, students will plan and carry out investigations to explore the phenomenon of why a solid ice cube melts into liquid water and then disappears into the air as gas. By using various measurement tools at different stations, they will identify independent and dependent variables, collect and analyze data, and evaluate the accuracy of their methods. This hands-on approach will help them understand the changes in properties during each phase transition and provide evidence to support scientific explanations for the observed phenomena.
Patterns and Energy and matter: Flows, cycles, and conservation
During this activity, students will explore the states and properties of matter through various measurement tools, allowing them to recognize patterns in the rates of change and numerical relationships at both macroscopic and microscopic levels. As they investigate phase changes, they will understand how energy transfer drives the motion and cycling of matter, and how matter is conserved during physical processes, providing insights into the phenomenon of an ice cube melting and transitioning into gas.

1. Review with students how to find the density of an object before they begin stations.
2. Station 2: You may want to have the students round their answers if they have trouble working with decimals.
3. Station 6: Tell students that not all the cards fit on the thermometer. They can place cards outside the thermometer range.
Safety Goggles
When using any form of very small particles or powder, it is safest for students to protect their eyes by wearing goggles.
Wafting
Students should waft in order to smell substances rather than directly inhaling.
Electric Circuit
When testing conductivity, tell students not to touch wires to objects, especially body parts or wet materials, unless directed to do so.
Think, Draw, Explain
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After the students have had a chance to explore through the investigation, give them an opportunity to show their understanding. Write the following question on the board: What is the difference between density and mass?
● First, ask your students to think.
● Then ask them to draw their response on paper.
● After they are finished with the drawing, ask them to explain their answer.
● Provide them with the following sentence stem:
○ The difference between density and mass is ___________. The above process can be duplicated for any other term from the Explore activity that is giving students trouble.
When an ice cube melts and eventually turns into gas, what happens to its properties, and how can we be sure that all the matter is still present in a different form?
1. Based on your observations at the thermal conductivity station, how does the material of a container affect the rate at which an ice cube melts, and what implications does this have for the conservation of matter?
2. After comparing the density of different objects, how can we use density to predict whether the liquid from a melted ice cube will occupy the same volume when refrozen, and what does this tell us about the conservation of mass?
3. Considering the solubility station, how might the solubility of a substance change as it transitions from solid to liquid to gas, and how does this relate to the properties of matter during phase changes?

Estimated 30 min - 45 min
Students test objects to observe chemical properties.
Materials
Printed
● 1 Student Journal (per student)
● 1 Station Instruction Cards (per class)
Reusable
● 2 large test tubes (per class)
● 2 sets of forceps (per class)
● 1 small beaker or plastic cup (per class)
● 1 dropper bottle (per class)
Consumable
● 50 mL vinegar (per group)
● 1 tbsp baking soda (per group)
● 1 piece steel wool, 2 cm x 2 cm (per group)
● 3–4 drops iodine (per group)
● 1 match (per group)
● ½ effervescent tablet (per group)
● 1 wooden coffee stir stick (per class)
● 1 potato
● Tap water
● Paper towels
● 1 disposable dropper pipette (per group)
● Print one Station Instruction Cards, cut the cards, and place at the appropriate station.
● Place materials for each station in a container for easy distribution and storage.
Station 1: Vinegar and Baking Soda
1. Set up station with vinegar and baking soda in separate small beakers or clear plastic cups.
2. Provide a dropper pipette for transferring the vinegar and a wooden coffee stirring stick for transferring the baking soda.
3. Provide a large test tube in a rack for performing the experiment.
Station 2: Steel Wool and Vinegar
1. Tear steel wool into small pieces. Set up station with vinegar in an easy-to-pour bottle and steel wool pieces in a small beaker or clear plastic cup.
2. Provide forceps for handling steel wool.
3. Provide a small beaker or clear plastic cup for the experiment.
4. Provide paper towels for drying the steel wool.
Station 3: Matches with Oxygen (May be performed by teacher as demonstration)
1. Provide one match per group. Do not distribute matches in advance!
Station 4: Effervescent Tablet with Water
1. Break effervescent tablets into small pieces.
2. Set up station with water in an easy-to-pour bottle (or access to faucet) and tablet pieces in a small beaker or clear plastic cup.
3. Provide a large test tube in a rack for performing the experiment.
Station 5: Iodine with Starch
1. Cut potato into thin slices and store underwater.
FACILITATION TIP
Ask: “Why does iodine change color in the presence of starch?”
Reinforce that color change is evidence of chemical change.
2. Provide forceps for handling potato.
3. Provide paper towel to prevent students handling potato after iodine is applied.
4. Provide iodine in a dropper bottle.
5. Caution students that iodine may stain fingers or clothing.
Notes
Planning and Carrying Out Investigations, and Analyzing and Interpreting Data, and Using Mathematics and Computational Thinking
During this activity, students will plan and carry out investigations to explore the phenomenon of phase changes in matter, such as why a solid ice cube melts into liquid water and then disappears into the air as gas. They will identify independent and dependent variables, use appropriate tools to gather data, and analyze and interpret this data to provide evidence for the changes in properties during each phase transition. By testing objects to observe chemical properties, students will gain insights into the molecular interactions and energy changes involved in phase changes, supporting their understanding of the phenomenon.
Procedure and Facilitation
During this activity, students will identify patterns in the rates of change and numerical relationships as they observe chemical reactions, such as the reaction of vinegar with baking soda or steel wool with oxygen. These patterns help them understand the cause and effect relationships at a macroscopic level, which are related to the microscopic and atomic-level structures involved in the changes of state from solid to liquid to gas. Additionally, students will explore how energy transfer drives the motion and cycling of matter, observing that matter is conserved as atoms are rearranged during these chemical processes.
This activity is designed as a laboratory exercise with lab stations.
1. Assign students to five groups.
2. Distribute a Student Journal to each student.
3. Discuss this question: Can you think of other examples of chemical properties? Guide students toward properties such as reaction with acids, reactions with water, tendency to rust, and explosiveness.
4. Go over safety precautions found in the Student Journal.
5. Give groups instructions on rotating.
6. Set a timer and have students rotate every three to five minutes.
Post-Lab Discussion
1. Upon completing the Student Journal, students should have identified chemical properties including reactivity with acids, reactivity with water, rusting, and flammability.
2. At Station 2, the steel wool is not reacting with the vinegar. The vinegar removes the protective coating and allows the iron in the steel wool to react with the oxygen.
3. At Station 4, water is one of the reactants. Students may not realize water can be involved in chemical reactions. Group 1 metals react violently with water. If time permits, you can show students video clips of alkali metals reacting with water.
Notes

Safety Guide
Safety Goggles
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Gloves
When working with chemicals, students should wear gloves to protect their skin.
Aprons
Have students wear aprons or lab coats when handling chemicals.
Do Not Eat or Drink Materials
Students should be reminded not to eat or drink any materials unless directed to do so.
Do Not Mix Materials
Students should be reminded not to mix materials unless directed to do so.
Roadblock: Unable to Generalize
Students may not be able to generalize the chemical changes seen in this activity to other chemical changes. Identify the parts of the demonstrations that are signs of a chemical change. Compare each to another real-world example and encourage students to think of their own. Speak in terms of analogies or make direct comparisons. Read more strategies for generalization in the Interventions Toolbox.
Notes
Think Time/Talk Time
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After students complete the Explore lesson, allow them to form groups by numbering off from one to four. Allow them think time to answer the questions in their journals. Then give them talk time to discuss their answers with each other. When you call a number, the student with that number reports for their group.
Possible questions and sentence stems may include the following:
● Level 1 Knowledge Question: How would you describe a chemical reaction?
○ Stem: A chemical reaction is ______.
● Level 2 Comprehension Question: What can you say about a chemical change?
○ Stem: I can say that a chemical change is ______.
● Level 3 Application Question: How would you show your understanding of a chemical property?
○ Stem: I would show my understanding of a chemical property by ______.
● Level 4 Analysis Question: What is the difference between a chemical property and a physical property?
○ Stem: The difference between physical and chemical properties is ______.
● Level 5 Synthesis Question: What facts can you compile to determine whether a chemical reaction has occurred?
○ Stem: I can determine whether a chemical reaction has occurred by ______.
● Level 6 Evaluation Question: How could you use an object’s physical or chemical properties to identify it?
○ Stem: I could use an object’s physical or chemical properties to identify it by ______.
Phenomenon Connection
When observing the transformation of an ice cube from solid to liquid to gas, what chemical and physical changes are occurring, and how do these relate to the chemical reactions observed in the lab activity?
1. How do the chemical reactions observed in the lab (such as vinegar with baking soda or steel wool with oxygen) compare to the physical changes of an ice cube melting and evaporating?
2. In what ways do the properties of substances change during a chemical reaction, and how is this similar or different from the changes in properties when an ice cube melts and evaporates?
3. How can understanding the chemical properties of substances help us predict and explain the changes that occur when an ice cube transitions from solid to liquid to gas?

Estimated 1 hr - 2 hrs
Students plan an investigation to identify four unknown clear liquids as water, alcohol, vinegar, or hydrogen peroxide by testing their properties.
Materials
Printed
● 1 Student Journal (per student, group, or class)
● 1 Chemical and Physical Properties (per group)
Reusable
● 9 beakers, 100 mL (per group)
● 3 cups, plastic, 250 mL (per group)
● 1 graduated cylinder (per group)
● 1 balance, triple-beam or electronic scale (per group)
● 1 hot plate (per group)
● 1 thermometer or temperature probe (per group)
● 1 tongs, pair (per group)
● 1 mitts, safety, fire, pair (per group)
● 1 goggles, safety, pair (per student)
● 1 pan, aluminum, round, 9 in. (per group)
● 1 marker, black (per class)
Consumable
● Vinegar, 125 mL (per group)
● Water, 125 mL (per group)
● Alcohol, 125 mL (per group)
● Hydrogen peroxide, 125 mL (per group)
● Salt, 40 g (per group)
● Sugar, 40 g (per group)
● Oil, corn, 37 mL (~40 g) (per group)
● 1 candle (per group)
● 1 matches, box (per group)
● 4 paper, scraps (per group)
● 1 tape, masking, roll (per class)
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
● Review the Writing a Scientific Explanation Rubric Key for Teacher prior to conducting the CER with students.
● Prepare the four unidentified liquids by pouring 100 mL of each liquid into a separate beaker for each group. Label the beakers as follows: 1 for the water, 2 for the vinegar, 3 for the alcohol, and 4 for the hydrogen peroxide. Plastic cups may be substituted for beakers if necessary.
● The night BEFORE the activity, freeze 25 mL samples of each liquid in beakers, labeled as above, for each group. These will be used to investigate melting point and boiling point of the liquids. Plastic cups may be substituted for beakers if necessary.
● Prepare and label plastic cups of salt, sugar, and corn oil for each group to be used for solubility testing.
● Lay out the rest of the materials and equipment where they can be available for student use as needed throughout the activity.
● Print one Chemical and Physical Properties for each group. Do not distribute until students complete their testing. Students will compare their results to the reference sheet.
During this activity, students will plan and carry out investigations to identify unknown clear liquids by testing their properties, which will help them understand the phenomenon of phase changes in substances like ice melting into water and evaporating into gas. By identifying independent and dependent variables, selecting appropriate tools, and collecting data, students will gather evidence to explain the changes in properties during each phase transition. They will analyze and interpret data to distinguish between causal and correlational relationships, using mathematical and computational thinking to support their conclusions and understand the underlying principles of the phenomenon.
During this activity, students will identify patterns in the properties of different liquids to understand the phenomenon of phase changes in matter, such as why a solid ice cube melts into liquid water and then disappears into the air as gas. By recognizing macroscopic patterns related to microscopic and atomic-level structures, students will explore how energy transfer drives the motion and cycling of matter, and how matter is conserved in these processes. Through their investigation, they will use graphs and charts to identify patterns in data, helping them to understand the cause and effect relationships in natural systems.
1. The following investigation is a sample investigation tightly aligned to the Mississippi College- and Career-Readiness Standards for Science with sample materials, procedures, and anticipated student answers provided.
2. All investigations are inquiry-based, so teachers guide students through differentiated science inquiry events within their comfort level.
3. A set of suggested procedures is given in the Student Journal. These procedures are to be used as an example. You may choose to guide the students in planning their own investigation by going through each of the suggested 10 steps before distributing the Student Journal, or you may have the students plan their investigations using the Student Journal as a guide.
The investigation is written to encourage students to plan and implement their own investigation with your guidance. You can provide appropriate grouping/ differentiated inquiry with the following scaffolding suggestions:
● Group students who need more guided practice together and spend more time with them. Let the other groups work more independently.
● Group students with mixed needs and have them work together. Monitor all groups equally.
1. Probeware may require a data interface to connect to a graphing calculator or computer, so students may need to review how to set up this connection beforehand, as well as find out if any system updates need to be performed.
2. Flammability tests can be done as a teacher demonstration depending on the school/district guidelines for safety in labs and classrooms.

FACILITATION TIP
Many students assume that clear equals water. Pause and highlight that appearance alone is not reliable.
Reinforce that multiple tests are always needed for identification.
Pre-Investigation Discussion
1. We know that pure substances are elements or compounds that cannot be broken down physically into smaller components. We also know that each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. What are some of these properties? Color, density, solubility, and so on
2. If you were given a set of unidentified pure substances, what kind of tests might you do to identify the substances? Test for different properties, such as melting point, boiling point, flammability, observations of color and odor, etc.
3. If you had two unidentified clear liquids, which properties do you think would be most helpful in identifying them and distinguishing them from each other? Odor, boiling point, melting point, density, flammability. These are easy to observe and measure in liquids and would therefore be a good starting point.
FACILITATION TIP
Create a large class chart with columns for each property (odor, solubility, density, etc.). As groups test, ask them to add one observation to the shared tracker. This builds a collective data set and lets students see patterns emerge.
4. Tell the students their challenge is to plan an investigation to identify four clear liquids. They will be given unlabeled samples of water, vinegar, alcohol, and hydrogen peroxide.
Post-Investigation Discussion
1. Which liquid sample did you identify as water? Explain your reasoning. Sample 1; water has a density of 1 g/mL and is odorless.
2. Which liquid sample did you identify as alcohol? Explain your reasoning. Sample 3; alcohol is flammable and has a distinct odor.
3. Which liquid sample did you identify as vinegar? Explain your reasoning. Sample 2; vinegar is not flammable and has a distinct odor.
4. Which liquid sample did you identify as hydrogen peroxide? Explain your reasoning. Sample 4; hydrogen peroxide is the densest.
5. Which properties, if any, were unique to one liquid?
Conclusion and Scientific Explanation
1. To complete Step 10, students write a conclusion and a scientific explanation based on the following prompt: How can the properties of a substance help you identify it?
This is an electrical device. Ensure that it is working properly and that the cord is in good condition. Never reach over a hot plate that is on, and never leave it unattended. Use caution when heating materials and wear protective gear such as safety goggles and heat-resistant gloves.
Fire Extinguisher or Blanket
Have access to a fire extinguisher or blanket when burning materials.
Secure Hair and Loose Items
Ask students to tie back long hair and to secure loose clothing or jewelry when working with heat sources.
Safety Goggles
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Gloves
When working with chemicals, students should wear gloves to protect their skin.
Heat-Resistant Mitts
Students should wear hand protection when working with hot materials.
Do Not Eat or Drink Materials
Students should be reminded not to eat or drink any materials unless directed to do so.
Do Not Mix Materials
Students should be reminded not to mix materials unless directed to do so.
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
This activity is a closure technique that encourages students to reflect upon the content of the just-completed lesson. This can be completed as a class, with a peer, or as individuals via a journal entry.
Write the following acronym on the board, or display it using a document camera:
● C—Communicate what you have learned.
● R— React to what you have learned.
● O—Offer one sentence that sums up the lesson/activity.
● W—Write a way you can use what you have learned.
● N—Note how well you did today.
Have students complete a short writing about the lesson using the CROWN technique.
When matter changes states, how do we know all the matter is still there, just in a different form?
1. Based on your comparison with other classmates, how could you make the ice cube in the video melt faster using the properties of the liquids you tested?
2. If you took all of the liquid from the melted ice cube and froze it, would it make the same size of ice cube, and how does this relate to the concept of conservation of mass?
3. How could you make all of the matter from the melted ice cube become a gas, and what role do the properties of the substances play in this transformation?

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Heat Shield Engineer
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - Physical or Chemical
A reading passage about the concept, which includes five to eight comprehension questions.
Notes
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
Student Learning Objectives
The matter that makes up our world has physical and chemical properties that can be used for classification.
The physical properties of matter include properties that describe the substance, such as color, smell, boiling point, and density.
Chemical properties of matter include all the possible chemical changes that a sample of matter can undergo, such as corrosion, toxicity (the degree to which a substance is poisonous to an organism or the environment), and the ability to burn.
Does Student Mastery Look Like?
The student is expected to use evidence to demonstrate an understanding about the effects of temperature and pressure on physical state, moleular motion, and molecular interactions.
• The movement of atoms and molecules depends on the amount of energy in the system.
• When temperature increases, movement of particles increases proportionally.
• An increase in pressure will result in a decrease in volume.
• When the pressure is held constant, a decrease in temperature results in a decrease in volume.
• When the volume is held constant, a decrease in temperature results in a decrease in pressure.
Scope Overview
This unit develops evidence-based understanding of how temperature and pressure affect physical state, molecular motion, and interactions. Students model particle behavior across solids, liquids, and gases; observe phase changes; and test gas behavior under heating, cooling, compression, and expansion to identify variable relationships. They investigate how temperature and pressure influence density and buoyancy, refine questions and explanations through discourse, and analyze material properties in varied contexts. Journaling and data synthesis emphasize connecting observations to particle-level mechanisms and accurate scientific vocabulary.
Scope Vocabulary
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Atom
The smallest particle of an element; made of electrons, protons, and neutrons
Condensation
The change from a gas state to a liquid state
Density
The amount of matter in a given space or volume
Expansion
When a substance grows in size due to particles separating from each other; can be caused by adding heat
Molecule
The simplest unit of a chemical compound that can exist, formed when two or more atoms join together chemically
Motion
The change in an object’s position with respect to time and in comparison with the position of other objects used as reference points
Pressure
Force exerted on matter through contact with other matter; affects melting and boiling points
Temperature
Average kinetic energy of all the particles in a material; measured by a thermometer in degrees (usually degrees Celsius or degrees Fahrenheit)
Volume
A measure of the space that matter occupies
Notes
Students investigate how particle arrangement and motion change across states of matter through modeling and observation.
• Collaboratively model molecular position and motion for ice, liquid water, and steam using whole-body movement, determining representations without direct instruction.
• Optionally sketch their models and descriptions in lab journals.
• Predict, observe, and describe changes in molecular motion as wax is heated to melt and then cools back to a solid, connecting particle behavior to phase changes.
Scientific Investigation - Gas Laws
Students investigate how changes in temperature, pressure, volume, and density affect gases through hands-on station activities.
• Rotate through five stations (crushing a can, heat-driven spinner, syringe in hot/cold water, sealed bottle with dime, marshmallow in syringe) to manipulate and observe gas behavior.
• Make real-time observations of how heating, cooling, compression, and expansion change particle spacing and motion.
• Record procedures, observations, and explanations in the Student Journal for each station.
• Synthesize findings by identifying direct and inverse relationships among gas variables using increase/decrease statements and a comparison chart.
Students investigate how changes in heat and pressure affect the density of matter through observation, questioning, and class discourse.
• Observe demonstrations or conduct hands-on explorations with hot/cold water “bottle” setups and Cartesian divers, recording detailed observations in journals.
• Collaborate in small groups to generate targeted questions that probe the role of temperature, pressure, molecular motion, and density in the observed phenomena.
• Engage in a teacher-led “20 questions” sequence to refine explanations, then synthesize findings into a class scientific explanation using appropriate vocabulary.
Students investigate how temperature and pressure influence material properties by observing molecular motion in polymers, candy, concrete, and soap.
• Predict, test, and compare rubber strips at cold, room, and heated conditions to observe changes in elasticity and length.
• Heat hard candy to examine glass-like behavior and fiber formation, connecting outcomes to molecular structure.
• Formulate concrete with varied ratios, cure samples, then test water ponding and strength to analyze mixture-performance relationships.
• Compare floating behavior and microwave heating of different soaps to observe expansion and texture changes tied to trapped air and molecular motion.
Estimated 15 min - 30 min
In this activity, students model the movement of molecules of substances at different states.
Materials
Reusable
● 1 microwave (per class)
● 1 beaker of liquid water (per class)
● 1 beaker of ice (per class)
● 1 empty beaker (per class)
Consumable
● 1 bar of wax or wax candle (per class)
● 1 paper plate (per class)
● 1 lab journal (per student, optional)
● Either perform this activity in the room with the student desks cleared out, or do this lab activity in a large open area.
● The microwave portion of the activity can be done either immediately after the physical activity or once students are back in the classroom.
Asking Questions and Defining Problems, and Planning and Carrying Out Investigations, and Analyzing and Interpreting Data
During this activity, students will engage in modeling the movement of molecules in different states to explore the phenomenon of why a balloon shrinks in the freezer and expands when taken out. They will ask questions and define problems related to molecular motion and temperature changes, plan and carry out investigations to observe these changes, and analyze and interpret data to understand the relationship between temperature and molecular behavior. This hands-on experience will help clarify the principles underlying the phenomenon and provide empirical evidence to support their explanations.
Patterns
Cause and effect: Mechanism and explanation
During this activity, students will recognize patterns in the behavior of molecules at different states and use these patterns to identify cause and effect relationships. By modeling the molecular movement in ice, liquid water, and steam, students will understand how macroscopic patterns, such as the shrinking and expanding of a balloon, are related to microscopic molecular motion. This will help them predict and explain the phenomenon of a balloon shrinking in the freezer and expanding when taken out, recognizing the causal relationships between temperature changes and molecular behavior.
● As students run around, be careful that they do not collide with each other. Make sure there is enough space for students to safely move without harming each other or the school space.
● Wax heated in a microwave will be very hot; be careful while handling.
1. Show students the container of ice. Ask them what water is made up of.
2. Tell students they are responsible for modeling the molecules in the ice. They have to, as a class, show the position and motion of the molecules. Do not tell the students how to do this; have them work together to determine the correct model.
3. Show students the container of liquid water. Tell them to model the molecules in the liquid water. Again, do not tell them how to model this; let them figure it out on their own.
4. Show students the container of “steam,” which is the empty beaker. Tell them to model the steam molecules.
5. Optional: have students draw their molecule models in their lab journals.
6. Show students the block of wax. Have them describe the molecules using words.
7. Explain to students you are going to heat the wax up in the microwave. Ask students to predict what will happen to the wax molecules as the wax is heated.
8. Using the microwave, melt the wax. Ask students to describe the molecular motion in the new state.
9. Let the wax cool, and have students generate an explanation for why the wax is returning to the solid state as it cools.
10. Optional: have students write their descriptions, predictions, and explanations in their lab journals.
When substances change states, how does the movement of their molecules explain the changes we observe, such as a balloon shrinking in the freezer and expanding when taken out?
1. Based on your observations of the wax and water, how does the movement of molecules differ between solid, liquid, and gas states, and how might this relate to the balloon’s behavior in different temperatures?
2. How does the concept of molecular motion help explain why a balloon shrinks in the freezer and expands when it is removed?
3. Considering the activity with the wax and water, what predictions can you make about the molecular behavior of the air inside the balloon when it is subjected to temperature changes?
FACILITATION TIP
As students model, introduce terms such as: “rigid structure,” “loosely packed,” “free movement,” “kinetic energy.”
This makes their body-models connect directly to science language.
FACILITATION TIP
If students model differently, pause and ask:
“Do we agree molecules move in place but don’t slide past each other?”

Estimated 1 hr - 2 hrs
Students move through stations to develop an understanding of the relationships between the temperature, pressure, volume, and density of a gas.
Materials
Printed
● 1 Student Journal (per student)
● 1 Station Instruction Cards (per class)
Reusable
● 1 goggles (per student)
Station 1
● 1 Bunsen burner or alcohol burner (per Station 1)
● 1 striker (per Station 1)
● 1 pie plate (per Station 1)
● 1 beaker tongs (per Station 1)
Station 2
● 1 paper plate (per Station 2)
● 1 scissors (per Station 2)
● 1 roll of tape (per Station 2)
● String
● 1 hot plate (per Station 2)
Station 3
● 1 hot plate (per Station 3)
● 1 sealed syringe (per Station 3)
● 1 thermometer (per Station 3)
● 3 beakers (per Station 3)
● 1 timer (per Station 3)
Station 4
● 3 beakers (per Station 4)
● 1 empty steak sauce bottle (per Station 4)
● 1 dime (per Station 4)
Station 5
● 1 syringe (per Station 5)
● 1 marker, black (per Station 5)
Consumable
● 1 soda can, empty (per group) (Station 1)
● Water (Station 1)
● Ice (Stations 1, 3, and 4)
● Marshmallows, mini, several (Station 5)
● Paper towels (per station)
● Set up each station before class begins. You may want to set up two of each since there are only five stations.
Station 1
● Collect soda cans beforehand.
● Have the ice water prepared. You will need to add ice as the day progresses.
Station 2
● Premake the spinner by cutting a spiral out of the paper plate similar to the one pictured below.
● Tape a length of string to the center of the spiral.
● The higher the setting of the hot plate, the faster the spiral will spin.
Station 3
● Have the water already warmed since this station takes a little longer than the others.
Station 4
● Have the bottle already chilling before the lab begins.
Station 5
● Draw faces on the marshmallows beforehand.
Asking Questions and Defining Problems
Planning and Carrying Out Investigations
Analyzing and Interpreting Data
During this activity, students will ask questions and define problems related to the phenomenon of why a balloon shrinks in the freezer and expands when taken out. They will plan and carry out investigations at various stations to explore the relationships between temperature, pressure, volume, and density of gases. By analyzing and interpreting data collected from these investigations, students will develop a deeper understanding of the causal relationships and apply scientific principles to explain the observed phenomenon.
Patterns and Cause and Effect: Mechanism and Explanation
During this activity, students will explore the relationships between temperature, pressure, volume, and density of gases to understand the phenomenon of why a balloon shrinks in the freezer and expands when taken out. By recognizing patterns in the behavior of gases at different temperatures, students will identify cause and effect relationships at the macroscopic level that are related to the microscopic and atomic-level structure of gases. They will use these patterns to predict and explain the observed changes in the balloon’s size, classifying the relationships as causal and understanding that multiple factors may contribute to the observed phenomenon.
1. Review the concepts of pressure, density, temperature, and volume.
2. Discuss with students how the length of an arrow shows strength (pressure).
3. Discuss proportional relationships.
4. Review stations, emphasizing safety, before students perform the activities.
Station 1: Crush the Can
Facilitation
● Goggles are recommended for this station.
● Have students recycle the soda cans.
Procedure
1. Pour enough water in the empty soda can to barely cover the bottom.
2. Using the beaker tongs, hold the can over the burner until the water in the can starts boiling.
3. Quickly invert the can in the ice water. Do not submerge the entire can, only the open end!
4. Answer the questions for Station 1 in your Student Journal.
Station 2: The Spinner
Facilitation
● The higher the setting of the hot plate, the faster the spiral will spin.
● Warn students that the hot plate can catch the plate on fire and that they should NOT touch the spinner to the hot plate coils.
Procedure
1. Holding the end of the string, allow the spiral to hang over the hot plate. DO NOT allow the end of the spiral to touch the hot plate coils.
2. Answer the questions for Station 2 in your Student Journal.
FACILITATION TIP
Pause and ask: “What is happening inside the can before it’s inverted?” to reinforce pressure differences.
Encourage students to describe the event using pressure, volume, and temperature language instead of just saying “it got crushed.”

Station 3: Chill Out, Warm Up
Facilitation
● Warn students to keep the syringe sealed.
Procedure
FACILITATION TIP
Emphasize that the plunger moves by itself—a clear sign of changing gas pressure inside.
Prompt students to consider: “What is happening to the motion of gas molecules in the syringe in cold vs. warm water?”
1. Place the sealed syringe in the beaker with ice water for 2 minutes.
2. Answer question 1 for Station 3 in your Student Journal.
3. Move the syringe to the beaker with warm water for an additional 2 minutes.
4. Answer the remaining questions for Station 3.
Station 4: Steak Sauce Gas?
Procedure
1. Remove the chilled bottle from the ice water.
2. Place a layer of water around the rim and put a dime on top, sealing the bottle.
3. Warm the bottle with your hands and observe the dime.
4. Answer the questions for Station 4 in your Student Journal.
5. Put the bottle back in the ice water when you are done.
Station 5: Not So Squishy
FACILITATION TIP
Let students draw silly faces to show “squished” vs. “stretched” molecules to help make it memorable.
Facilitation
● You can draw faces on the marshmallows beforehand or allow students to draw them.
Procedure
1. Place the marshmallow in the syringe and replace the plunger.
2. Push the plunger down until it just barely touches the top of the marshmallow.
3. Put your finger over the open end of the syringe to form a seal.
4. Pull up on the plunger without breaking the seal from your finger.
5. Do not break the seal, but let go of the plunger.
6. Answer the questions for Station 5 in your Student Journal.
Answer the following questions using the word increases or decreases.
1. When the pressure of a gas ↑, the density increases.
2. When the density of a gas ↓, the pressure decreases.
3. When the temperature of a gas ↑, the volume increases
4. When the temperature of a gas ↓, the volume decreases
5. When the volume of a gas ↑, the density decreases.
6. When the volume of a gas ↓, the density increases
7. In a fixed volume, when temperature ↑, pressure increases
8. In a fixed volume, when temperature ↓, pressure decreases
Based on your observations in the Explore, complete the chart regarding what will happen to other properties of the gas in a balloon as the density, pressure, temperature, and volume of particles in the balloon increase (+) or decrease (–).
Answer the following questions using the word inversely or directly.
9. In gases, pressure and density are directly related.
10. Temperature and volume are directly related.
11. Volume and density are inversely related.
12. Temperature and pressure are directly related.
13. Do these same answers hold true for liquids? Explain. No, because you cannot compress liquids
14. Do these same answers hold true for solids? Explain. No, because you cannot compress solids
Safety Guide
Hot Plate
This is an electrical device. Ensure that it is working properly and that the cord is in good condition. Never reach over a hot plate that is on, and never leave it unattended. Use caution when heating materials and wear protective gear such as safety goggles and heat-resistant gloves.
Fire Extinguisher or Blanket
Have access to a fire extinguisher or blanket when burning materials.
Secure Hair and Loose Items
Ask students to tie back long hair and secure loose clothing or jewelry when working with heat sources.
Safety Goggles
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Gloves
When working with chemicals, students should wear gloves to protect their skin.
Heat-Resistant Mitts
Have students wear hand protection when working with hot materials.
Do Not Eat or Drink Materials
Students should be reminded not to eat or drink any materials unless directed to do so.

For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After the students have completed the investigation, allow them to show you what they have learned from the investigation by taking part in Ticket Out, a short written reflection about the lesson. Hand the students an index card. Write the following prompt on the board or display it using a document camera:
The molecules in a gas are constantly moving, bouncing off each other or the walls. When pressure goes up, this could be due to the fact that the molecules are hitting the walls of their container harder. When temperature goes up, pressure goes up. What does this tell you about the connection between the movement of molecules and temperature?
After the students complete the explanation, allow them to switch index cards with another student. They can read their explanations and discuss what they observed.
Why does a balloon shrink when you put it in the freezer and expand when you take it out?
1. How does the behavior of gases in the stations you explored relate to the shrinking and expanding of a balloon when exposed to different temperatures?
2. In what ways do the concepts of pressure, volume, and temperature observed in the activity help explain the phenomenon of a balloon’s size changing in different environments?
3. How might the principles of gas behavior observed in the activity be applied to understand other real-world phenomena involving temperature changes?

Estimated 1 hr - 2 hrs
Following demonstrations (or student explorations) of Bewildered Bottles and Cartesian Divers, students ask questions to explain how density of matter is affected by a change in heat and/or pressure in a teacher-led game of 20 questions.
Materials
Printed
● 1 Student Guide: 20 Questions of Density (per student)
● 1 Student Journal: 20 Questions of Density (per student)
Part I: Bewildered Bottles
Reusable Material
● 4 Erlenmeyer flasks (per class)
Consumable
● 2 index cards (per class)
● White sheet of paper (per class)
● Cold water (per class)
● Hot water (per class)
● Red food coloring (per class)
● Blue food coloring (per class)
Part II: Cartesian Diver
Reusable
● 1 empty 2 L bottle, clear, with a cap (per class)
● 1 eyedropper (per class)
Consumable
● Water (per class)
● 6 paper towels (per class)
SEP Connection
● Print one Student Journal for each student.
● Prepare the water for the Bewildered Bottles demonstration. You will need enough hot water to completely fill two Erlenmeyer flasks. You will also need the same amount of cold water. Use food coloring to tint the hot water red and the cold water blue.
Asking Questions and Defining Problems, and Planning and Carrying Out Investigations, and Analyzing and Interpreting Data
During this activity, students will engage in asking questions and defining problems by observing the phenomenon of a balloon shrinking in the freezer and expanding when taken out. They will develop questions based on their observations of the Bewildered Bottles and Cartesian Divers demonstrations to explore how changes in heat and pressure affect the density of matter. This process will help them specify relationships between variables and clarify their understanding of molecular motion and density changes, ultimately leading to a scientific explanation of the observed phenomenon.
CCC Connection
Patterns and Cause and Effect: Mechanism and Explanation
During this activity, students will explore the phenomenon of why a balloon shrinks in the freezer and expands when taken out by recognizing patterns in the behavior of gases under different temperatures. They will use the demonstrations of Bewildered Bottles and Cartesian Divers to identify cause and effect relationships related to changes in density and pressure, and connect these observations to the macroscopic patterns of gas behavior, thereby understanding the underlying microscopic and atomic-level structures that cause these changes.
The goal of this activity is to observe molecular motion in a pair of teacher demonstrations or student explorations and generate questions to explain how density of matter (observable in various objects) is affected by a change in heat and/or pressure.
1. Either perform the explorations as demonstrations or provide students with instructions, allowing exploration to take place in small groups.
2. Hand out Student Journal: 20 Questions of Density.
3. If students are performing an exploration, hand out Student Guide: 20 Questions of Density.
4. Instruct students to record observations in the Student Journal.
5. Perform the demonstration.
6. Have students work in small groups to develop questions leading to an explanation for their observations. Unlike traditional 20 questions, these do not have to be yes/no questions.
7. Engage in a game of 20 questions with groups taking turns asking you questions.
8. After 20 questions have been asked, write a scientific explanation as a class. Part I: Bewildered Bottles
To perform the demonstration, do the following:
1. Fill two flasks with cold water. Add 3 to 4 drops of blue food coloring to tint the water blue.
2. Fill the other two flasks with hot water. Add 3 to 4 drops of red food coloring to tint the water red.
3. Cover the mouth of one of the hot (red) flasks with half an index card and place this flask on TOP of one of the cold (blue) flasks.
4. Carefully and quickly, slip the paper away so nothing is between the two flasks. Hold a white sheet of paper behind the flasks. Have students observe carefully and record observations in their Student Journals.
5. Repeat the procedure with the other two flasks. But this time leave the hot (red) flask of water on the BOTTOM and place the cold (blue) flask on the top.
Notes
Ask students to sketch what they see before writing explanations—this often reveals misunderstandings. If students struggle, guide them by asking, “What do you notice about the movement of the colors? Why might heat matter?”

Part II: Cartesian Diver
FACILITATION TIP
Encourage students to predict before squeezing: “What do you think will happen?” and compare with actual results.
FACILITATION TIP
Encourage students to write not only “what happened” but also “why it happened” using evidence from their observations.
You may choose to build one or more Cartesian Divers for a whole-class demonstration or a hands-on exploration in groups. If class time permits, students can build the Cartesian Divers.
To build a Cartesian Diver, do the following:
1. Fill the entire 2 L bottle with water and place on two paper towels.
2. Fill the eyedropper about ¼ full with water.
3. Place the eyedropper into the 2 L bottle. The 2 L bottle should be so full that water overflows out of the bottle.
4. Place the cap firmly back on the 2 L bottle. For the exploration, do the following:
1. Explain that the students should make observations of the water-to-air ratio inside the eyedropper.
2. Have students squeeze the bottle (which increases the pressure inside the bottle) and observe what happens to the eyedropper, also known as a diver. At this point, students should realize that the diver fell to the bottom of the bottle and that the volume of air inside the diver decreased.
3. Have students release the bottle and notice the diver rise to the top of the bottle. The volume of air inside the diver increased with the decrease in pressure.
4. Have students repeat the process and record their observations and conclusions in their Student Journals.
Direct students to work with their group to develop 10 questions about each demonstration to ask that will help them to explain their observations using scientific concepts.
Possible Questions and Answers
1. Why are they mixing?
DO NOT ANSWER THIS QUESTION! This is what the students need to figure out.
2. What is the red stuff? Water
3. Are they two different liquids? They are both water.
4. Is there anything different about the liquids other than the color?
One is hot, and one is cold.
5. Is there anything added to them like sugar or salt? No, just water
6. Are the red/blue liquids the same in both containers? No, one is hot water, and one is cold water.
7. What would it look like tomorrow? They would be mixed.
8. What would happen if they stayed that way for an hour? They would be mixed.
9. What would happen if I shook it? They would be mixed.
10. Is the volume the same? Yes
11. Are they the same temperature? No, one is hot, and one is cold.
12. Is the red/blue one hot or cold? Red is hot; blue is cold.
13. Does it have to do with convection currents? Yes
14. Are the red and blue the same density? No
15. Are they the same pressure? Yes
16. What would happen if it were sideways? It would mix but in a different pattern.
17. What makes molecules move? When they are heated
18. What is density?
19. Which is denser?
1. What makes it sink?
The amount of matter in a given space or volume; a relationship between mass and volume
What do you think? Are hotter liquids more or less dense than cooler liquids? Yes, hotter liquids are less dense.
Answer
DO NOT ANSWER THIS QUESTION! This is what the students need to figure out.
2. What is in the dropper? Water and air
3. Is the dropper all the way full? No, it has water and air.
4. Is the bottle all the way full? Yes
5. What does squeezing the bottle do?
It squeezes the dropper, making the volume smaller.
6. Would it matter if the bottle were a different size? No
7. Would it matter if it were a different liquid? No
8. Is it getting hotter when you squeeze it? No
9. Why is it called a Cartesian diver?
10. What is changing when you squeeze the bottle?
11. When the diver is moving, what is changing about the diver?
It is named after French philosopher and scientist René Descartes.
The volume of the dropper is decreasing. Water may enter the dropper.
The dropper volume is decreasing. Water may enter the dropper and displace the air.

12. Can you make it go up by squeezing it?
13. What would happen if I turned it upside down and squeezed it?
14. Does it make a difference where you squeeze the bottle?
15. Is the same thing in the dropper as in the bottle?
16. Is air more or less dense than water?
17. Does the amount of air/water in the dropper change?
18. How does the dropper get heavier?
19. Why does the dropper not float?
FACILITATION TIP
End with a summary connection: heat and pressure both change density by changing the motion and spacing of particles.
No, it goes down.
The diver would move down toward the lid of the bottle.
It might if you do not squeeze as strongly.
Sort of. The bottle has only water; the dropper has air and water.
Less
Yes. Air moves out and water moves in when the bottle is squeezed.
When water replaces air
After the bottle is squeezed, water enters the dropper, making it denser.
20. What makes something sink? Increasing density
Post 20 Questions Discussion
Based on the questions and answers, the class should be able to explain what happened in each demonstration. They may arrive at the answer before 20 questions and ask, "Is it ____________________?"
Students should write the scientific explanation using appropriate vocabulary in their Student Journals.
Notes
Think, Draw, Explain
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After students explore the investigation, give them an opportunity to show their understanding.
Write the following question on the board:
If energy is absorbed by a gas, then how will the gas density change?
● Ask students to think about their response before drawing it on paper.
● After they are finished with the drawing, ask them to explain their answer.
● Provide students with the following sentence stem:
This drawing accurately represents the atoms after a gas absorbed energy because ________________________.
Phenomenon Connection
When heat or pressure changes, how does it affect the density of matter, and what implications does this have for the behavior of gases, such as the air in a balloon?
1. How does the change in temperature affect the density of the air inside the balloon when it is placed in the freezer and then taken out?
2. In what ways are the principles observed in the Bewildered Bottles and Cartesian Divers demonstrations similar to the phenomenon of the shrinking and expanding balloon?
3. How might the concepts of molecular motion and density help explain why a balloon expands when warmed and contracts when cooled?
Notes

Estimated days 3 - 5
Students examine the movement and behavior of molecules in polymers, hard candy, concrete, and soap to show the effect of temperature and pressure on these materials. As part of this activity, students make predictions and analyze outcomes in terms of molecular movement.
Materials
Printed
● 1 Student Journal: Molecules and Material Science (per student)
● 1 Concrete Recipe Cards (per teacher)
Reusable
● Computer with Internet access
Part I: Rubber Band Science
Reusable
● 1 freezer (per class)
● 1 heating pad (per class)
● 1 hand towel, cloth (per class)
● 1 ring stand with ring (per group)
● 1 hooked weight 100 (per group)
● 1 meterstick (per group)
Consumable
● 3 rubber bands ( per group)
Part II: Candy Glass
Reusable
● 1 beaker (per group)
● 1 beaker tongs (per group)
● 1 hot plate (per group or 2)
● 1 meterstick (per group)
Consumable
● 3 hard candies (per group)
● 1 popsicle stick (per student)
Part III: Concrete Innovations
Reusable
● 1 measuring tablespoon or small scoop (per group)
● 1 C-clamp (per class)
● 1 Petri dish (per group)
● 1 beaker (per group)
Consumable
● 1 large disposable cup (per group)
● 1 pair of gloves (per student)
● 1 popsicle stick (per group)
● 3 tablespoons of sand (per group)
● 3 tablespoons of cement (per group)
● 3 tablespoons of gravel (per group)
● 3 tablespoons of water (per group)
Part IV: Soap Bubbles
Reusable
● 1 microwave (per class)
● 1 knife to cut bar of soap into cubes (per class)
● 1 container of water (per class)
● 2 magnifying glasses (per group)
● Glass stirring rod or other tools for manipulation (per group)
● 1 balance (optional, per class)
Consumable
● 1 bar of Ivory™ soap, cut into 4 squares (per class)
● 1 bar of Ivory™ soap (per class)
● 1 bar of brand of soap that does not float in water (per class)
● 1 bar of brand of soap that does not float in water, cut into 4 squares (per class)
● 2 paper plates (per class)
● Print one Student Journal: Molecules and Material Science for each student.
● Print and laminate a set of Concrete Ratio Cards for reuse.
● Prior to the lesson, search the Internet for appropriate videos relating to recent breakthroughs in material science. Recommended videos can be found by searching the following:
○ Part I: Scientists Make Self-Healing Rubber ACS Headline Science
○ Part II: Future Technology of 2020: Our Digital World Made of Glass
○ Part III: New "Thirsty" Concrete Absorbs Water
● Prepare a bin of supplies for each part of the Explore for each group of students.
Part I
● Cut three rubber bands per group to make strips of rubber versus bands of rubber. Groups use a frozen band, a roomtemperature band, and a heated band.
● Freeze one rubber band and one strip of rubber per group for Part I of the investigation.
● Remove cold rubber bands from the freezer as close to lab time as possible.
● Heat hot rubber bands evenly using a heating pad. Place the rubber bands on a pad set to high. Cover the bands with a cloth towel.
Part IV
● This is best done with a fresh bar of Ivory™ soap. A bar that has been on the shelf for a long time will not have the same effect as one that is “new.”
● The high air content in the Ivory™ brand is what makes this experiment different from other brands of soap. If a brand other than Ivory™ is used, it literally will not work (will melt and flatten) and will make a mess in the microwave.
● If students are very observant, they might observe the bubbles present in the Ivory soap.
● The Ivory soap should be sample 1, while the non-Ivory soap should be sample 2.
● Ivory brand soap does NOT harm the microwave and actually produces a clean scent.
● It is important that the bar of soap that does not float is similar in size, shape, and color to the Ivory™ soap.
● It is important to note that you should NOT use a wet piece of Ivory™ in the microwave.
● Do NOT leave the microwave unattended while heating the soap cube.
● Every microwave is different, and you will want to perform a trial run to see about how long it takes for the Ivory™ cube to expand.
● Have students sit relatively close to the microwave so they can actually see the soap expanding because it happens quickly. It will start expanding at approximately 15 seconds.
● Microwaving the Ivory™ soap will not affect food that is heated afterward, but soap other than Ivory™ may leave a lingering scent and could possibly harm the microwave.
● After students examine the soap, have them wash their hands so they will not accidentally rub flakes of the soap into their eyes.
Pre-Activity Discussion Questions
1. Describe how water molecules move in a bottle of water. The molecules are constantly moving, bumping into each other and the walls of the bottle.
2. What happens to the water molecules as the temperature of the water is heated by a stove or microwave? The molecules move faster and faster, hitting the walls and each other harder and harder.
3. What happens to the water molecules as the temperature of the water is lowered by a freezer? The molecules move slower and slower, hitting the walls and each other with less force.

Asking Questions and Defining Problems, and Planning and Carrying Out Investigations, and Analyzing and Interpreting Data
During this activity, students will engage in asking questions and defining problems by observing the phenomenon of a balloon shrinking in the freezer and expanding when removed. They will specify relationships between temperature and molecular movement, clarifying arguments and models related to thermal expansion and contraction. Students will plan and carry out investigations to collect data on how temperature affects the behavior of materials, using tools like freezers and heating pads to manipulate variables. By analyzing and interpreting data, students will provide evidence for the phenomenon, distinguishing between causal and correlational relationships, and applying statistical concepts to characterize the data. This hands-on exploration will deepen their understanding of molecular motion and its impact on material properties.
After the polymer science video, ask students to identify one real-world application of polymers and connect it back to their experiment.
Patterns and Cause and Effect: Mechanism and Explanation
During this activity, students will explore the phenomenon of why a balloon shrinks in the freezer and expands when taken out by examining the movement and behavior of molecules in various materials. They will identify patterns in molecular motion and relate these to macroscopic changes, recognizing that the expansion and contraction of materials are due to changes in temperature and pressure. By analyzing cause and effect relationships, students will predict how molecular motion affects material properties, understanding that these phenomena may have multiple causes and that some relationships can be described using probability.
1. Lead the class in a pre-activity discussion using the provided questions.
2. Divide students into six groups.
3. Pass out a copy of Student Journal: Molecules and Material Science to each student.
4. Before beginning each part of the activity, have students make predictions in their Student Journals.
5. Refer to the procedures provided in the Student Journals.
6. Once all parts are completed, lead the class in a debrief using the postactivity questions.
1. Have students follow the procedure in the Student Journal
2. Following the investigation of the effect of temperature on polymers, have students watch a video about recent breakthroughs in polymer science and answer a series of analysis questions.
1. Safety Precautions: Be careful when handling the beaker, as it will be very hot. Allow the beaker and hot plate to cool before handling.
2. Have students follow the procedure in the Student Journal
3. Following the investigation of candy fibers, have students watch a video about recent breakthroughs in glass technology and answer a series of analysis questions.
1. Using various combinations of sand, gravel, cement, and water, have students create a sample of concrete according to their group's ratio card. These mixtures should be poured into empty Petri dishes and cured overnight.
2. Review the concept of ratios with students.
3. Have students mix concrete according to the instructions in the Student Journal.
4. Instruct students to start with one part water and add any additional water sparingly until they have a spreadable concrete mixture to pour into the petri dish.
5. The minimum amount of time to cure the concrete is 24 hours. Longer times are acceptable.
6. When the concrete is cured, have students test the concrete for ponding of water and strength.
7. Have groups perform the tests one at a time as a whole-class demonstration.
8. Instruct students to record observations for each ratio of concrete in their Student Journals.
9. Following the investigation of the concrete, have students watch a video about recent breakthroughs in pervious concrete technology and answer a series of analysis questions.
Make sure every student in a group has a role (measuring, mixing, recording, cleaning).
Pause to highlight how ratios in concrete mixing connect to algebraic reasoning.

1. Have the students examine the bars of soap and have them write down their observations in their Student Journal, including texture, smell, size, shape, color, etc. Have them also examine the cut edges of the blocks of soap to note the internal composition. If you choose, you may have the students weigh the soap as well.
2. Based on the students' observations, have them predict whether the bars of soap will float in water and have them write down their predictions in their Student Journal.
3. Place both bars of soap in water one at a time and have the students write down their observations. Make sure students know which sample is which.
4. Give students an opportunity to predict what will happen when the soap samples are heated. Encourage discussion among group-mates.
Connect to everyday life: Ask, “Where have you seen foamy or bubbly materials like this before? How is this property useful?”
5. Place a cube of soap in the microwave and heat on high for between 1–2 minutes. Depending on the sample, it will either melt into a puddle or expand into a foamy mass.
6. Have the students record their observations.
7. Remove the soap, either in a foamy mass or a puddle, from the microwave. Caution: it will be warm, so let it cool for a couple of minutes before letting the students examine the result.
8. Repeat this process with the other sample. Have students record their observations.
9. Let the students examine both samples by touch so that they can feel how light and fluffy, yet rigid and still brittle the foam appears, while the puddle of soap feels similarly brittle without feeling light and foamy.
10. Have the students complete the observations and questions in their Student Journal.
Post-Activity Questions
1. What was the connection between material expansion and temperature? As the temperature went up, the materials expanded.
2. How does the expansion or contraction of materials depend on molecular motion? When molecules move faster, the material expands; when they move slower, the material contracts.
3. Would materials expand and contract the same if the external air pressure increased while the temperature changed? Answers will vary but should conclude that the material will not expand/contract in the same fashion.
This is an electrical device. Ensure that it is working properly and that the cord is in good condition. Never reach over a hot plate that is on or leave one unattended. Use caution when heating materials and wear protective gear, such as safety goggles and heat-resistant gloves.
Heat-Resistant Mitts
Wear hand protection when working with hot materials.
Safety Goggles
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Gloves
When working with chemicals, students should wear gloves to protect their skin.
The group work needed may cause some conflict between students. Assign jobs within groups so that everyone is involved and held accountable. This can be teacher directed or group directed. Find more strategies for students that are argumentative with peers in the Interventions Toolbox.

Graphic Organizer
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After students have time to explore the lesson, provide them with a sheet of construction paper. Have students fold the paper in half hamburger style and draw a line in the middle to create two sections labeled as follows:
1. Polymer
2. Molecular Motion
Ask students to draw a diagram under each tab that best represents the term and label the diagram using the following key terms:
1. Material Science
2. Temperature and Pressure
Then, have students use the provided sentence stems to explain their diagrams. Ask them to share their graphic organizer with the class, and encourage them to read their sentences out loud.
Sentence Stems
1. Stem: My diagram represents atom motion because __________________.
2. Stem: My diagram represents material science because
How does temperature affect the behavior of molecules in different materials, and what implications does this have for understanding phenomena such as the shrinking and expanding of a balloon in a freezer?
1. How does the movement of molecules in polymers, like rubber bands, change with temperature, and how does this relate to the shrinking and expanding of a balloon?
2. In what ways do the experiments with candy, concrete, and soap illustrate the effects of temperature on molecular motion, and how can these observations help explain the behavior of gases in a balloon?
3. How might changes in external pressure, in addition to temperature, influence the expansion and contraction of materials, and what does this suggest about the conditions inside a balloon when it is placed in a freezer?
Notes

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Storm Chaser
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - Particle Motion
A reading passage about the concept, which includes five to eight comprehension questions.
PhET: Simulation Practice
Student activities using the PhET Interactive Simulations from the University of Colorado Boulder.
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
The movement of atoms and molecules depends on the amount of energy in the system.
When temperature increases, movement of particles increases proportionally.
An increase in pressure will result in a decrease in volume.
When the pressure is held constant, a decrease in temperature results in a decrease in volume.
When the volume is held constant, a decrease in temperature results in a decrease in pressure.

Student Expectations
The student is expected to demonstrate an understanding of the proper use of the periodic table to predict and identify elemental properties and how elements interact.
This unit builds conceptual and practical fluency with atomic structure and the periodic table to support prediction of elemental properties and interactions. Students connect historical models to modern evidence, use periods and groups to locate elements, and relate subatomic structure to states of matter. They distinguish elements, compounds, and mixtures; interpret chemical formulas; and classify basic bond types. Through hands-on testing of representative elements, students analyze patterns and trends (e.g., reactivity, conductivity) to explain and predict properties across the table using evidence-based reasoning.
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Atom
• Atoms are made of subatomic particles called protons, neutrons, and electrons.
• Matter is composed of atoms that have physical properties, including subatomic particles that have characteristic masses, charges, and locations. Atoms also exhibit chemical characteristics such as reactivity to other atoms. The physical and chemical properties of atoms can be interpreted by their arrangement on the periodic table of elements.
• All matter on Earth can be classified as either a pure substance (an element or compound with its own definite composition and properties) or a mixture (combination of two or more pure substances not chemically bonded, with variable composition and properties).
• Elements are arranged on the periodic table of elements in increasing order of the atomic number or number of protons in the nucleus of the atom in an element. Elements within the same column (group) have similar chemical properties.
• The three main types of elements are classified and shown on the periodic table of elements as metals, nonmetals, and metalloids.
• A chemical formula provides information regarding how many of each type of atom are present in the compound.
• Molecular compounds are composed of two nonmetals held together by a covalent bond. Ionic compounds are composed of a metal and a nonmetal.
The smallest particle of an element; made of electrons, protons, and neutrons
Atomic Mass
The mass of an atom, approximately equal to the number of protons and neutrons in the atom
Atomic Number
The number of protons in the nucleus of one atom of an element
Chemical Formula
A shorthand notation that uses chemical symbols and numbers as subscripts to represent the type and number of atoms that are present in the smallest unit of a substance
Compound
A new substance with unique chemical and physical properties formed when two or more elements are chemically bonded during a chemical reaction
Electron
A negatively charged subatomic particle located in the electron cloud; involved in the formation of chemical bonds
Element
A pure substance composed of the same type of atom throughout
Groups
The columns on a periodic table that arrange the elements by the number of electrons that are in the outermost shell; also called families
Metalloids
Elements that have properties of both metals and nonmetals; sometimes referred to as semiconductors
Metals
Elements that are typically solid, shiny, malleable, and good conductors of heat and electricity; includes most elements
Neutron
A neutrally charged subatomic particle located in the nucleus of an atom; contributes to the mass of the atom
Nonmetal
Elements that are typically not shiny, not malleable, and poor conductors of heat and electricity; usually gases or brittle solids
Periodic Table of Elements
A table in which all the known elements are arranged by properties and are represented by chemical symbols
Proton
A positively charged subatomic particle located in the nucleus of an atom; contributes to the mass of the atom
Reactivity
The ability of a chemical substance to undergo a chemical reaction; significantly influenced by valence electrons of the reacting substances
Students investigate how the periodic table is organized by using group and period information to locate specific elements.
• Review atomic structure and how elements are ordered by atomic number into periods (energy levels) and groups (valence electrons).
• Use a reference sheet and element cards to place missing elements in their correct positions on a blank periodic table.
• Record the period and group for each placed element and participate in a class discussion to confirm placements.
Making a Model - The Atom
Students explore the development and structure of the atom through historical analysis, modeling, and pattern-finding.
• Construct a timeline of key atomic theorists (Dalton, Thomson, Rutherford, Bohr) and connect their models to experimental evidence.
• Infer unseen properties using a sealed-box analogy, then build and annotate atomic models with protons, neutrons, and electrons using provided materials.
• Use the periodic table to determine atomic number, mass relationships, and particle counts (neutral atoms), identifying patterns such as protons ≈ neutrons for many elements.
• Compare particle arrangement and motion in solids, liquids, and gases to relate subatomic structure to states of matter.
Students investigate the distinctions between elements, compounds, and mixtures through sorting, modeling, and analysis.
• Sort cut-and-paste descriptors into categories for elements, compounds, and mixtures, then respond in journals.
• Build snap-cube models of O2, CO2, H2O, and copper; classify each as element or compound and document observations.
• Combine pure-substance models to create mixtures and compare their varied compositions to uniform pure substances.
• Construct and analyze paper models of compounds and complete reflective journal questions.
Activity - Periodic Trends and Reactivity
Students investigate periodic trends by testing element properties and using evidence to make predictions and communicate reasoning.
• Plan and conduct tests on sulfur, silicon, aluminum, and magnesium for appearance, hardness, conductivity, and reactions with HCl and NaOH.
• Analyze results to identify patterns across a period and discuss connections to ionization energy, atomic radius, and electronegativity.
• Use data to predict properties of sodium and phosphorus.
• Develop a CER explanation and participate in peer review with rebuttal or reflection.
Students explore chemical formulas and basic bond types through guided analysis and classification.
• Identify element symbols and interpret chemical formulas to count atoms of each element.
• Use the periodic table to classify compounds as ionic or covalent based on metal/nonmetal/metalloid composition.
• Record reasoning in a journal and explain the differences between ionic and covalent bonds with examples.

Estimated 15 min - 30 min
In this activity, students explore the periodic table by determining the location of missing elements based on group and period.
Materials
Printed
● 1 Where Do I Live? (per student)
● 1 Periodic Table, available in Teacher Toolbox: Resources (per teacher)
● 1 Where Do I Live? Student Reference Sheet (per group)
● 1 Slide Show (per class)
● 1 Missing Elements (per group)
Reusable
● 1 computer with projector (per teacher)
SEP Connection
Developing and Using Models
Planning and Carrying Out Investigations Engaging in Argument from Evidence
● Print one Periodic Table so you can project the completed table for the students.
● Print one Missing Elements page for each group. Cut out the pieces and laminate for durability.
● Print one Where Do I Live? Student Reference Sheet for each group and laminate for durability.
● Print one Where Do I Live? for each student.
During this activity, students will develop and use models to describe and predict the behavior of elements based on their position in the periodic table, which helps explain why some elements explode in water while others do nothing at all. By placing missing elements in their correct locations, students will modify models to provide a mechanistic account of this natural phenomenon, considering the number of valence electrons and energy levels as key variables.
Notes
CCC Connection
Patterns
Scale, proportion, and quantity Structure and function
During this activity, students will explore the periodic table to identify patterns in the arrangement of elements, which are related to their atomic structure. By determining the location of missing elements based on group and period, students will recognize how the macroscopic patterns of element behavior, such as reactivity with water, are linked to microscopic and atomic-level structures. This understanding will help them identify cause and effect relationships and use these patterns to explain why some elements explode in water while others do not.
1. Display the slide show with an illustration of helium. Before beginning the activity, encourage students to discuss the element.
2. Start your class discussion by reviewing what an element is. Have students describe the atom and the protons and electrons that are in that atom. Then, tell students that we organize the elements in a chart. Put the periodic table on a projector so they can see the completed table. Refresh students’ memories of the key on the periodic table. Point out that the elements are ordered based on their atomic number and show students this on the screen. Explain that each element has a home on the table. Point out that rows are horizontal and columns are vertical. In the table, rows have a special name called a period. Explain to students that all elements in a period have something in common; they have the same number of energy levels. The elements at the top, hydrogen and helium, have one energy level, while the elements at the bottom of the chart, like francium or radium, have seven energy levels. Moving on to the columns, which are called groups, all of the elements in a group have the same number of valence electrons. All of the elements under hydrogen in group 1 have only one valence electron.
3. Instruct students to use the Where Do I Live? Student Reference Sheet and the cards from the Missing Elements to complete the periodic table. Make sure to take the completed periodic table off the screen and explain to students that they need to place the cards in their proper locations on the Where Do I Live? Student Reference Sheet. After their elements are on the chart, pass out the Student Handout and have students list the period and group of each of the elements.
Compare the periodic table to a “neighborhood map” where each element has a “home.” This helps students visualize why placement matters.
When explaining valence electrons, pause and have students turn to a partner to explain in their own words what makes elements in the same group similar.

The missing elements on the Where Do I Live? Student Reference Sheet are the following:
Sodium
Dubnium
Where Do I Live?
Hydrogen
Period 1
Group 1
Aluminum
Period 3
Group 13
Krypton
Period 4
Group 18
Gold Period 6
Group 11
Sodium Period 3
Group 1
Dubnium
Period 7
Group 5
As a class, discuss the results.
Notes
The periodic table can seem overwhelming and be hard to track visually. Suggest using bookmarks or cut out boxes to help track the print while looking at the periodic table. This can help students who have difficulty processing visual information focus and reduce the amount of print in their field of vision. Find more strategies for dealing with difficulty processing visual information in the Intervention Toolbox.
How does the arrangement of elements on the periodic table help us predict their reactions with water, such as why some elements explode while others do nothing at all?
1. Based on your understanding of the periodic table, what characteristics of elements in Group 1 might explain their explosive reactions with water compared to elements in other groups?
2. How does the number of valence electrons in an element influence its reactivity with water, and why might this lead to explosive reactions for some elements?
3. Considering the energy levels of elements, how might the position of an element in a period affect its interaction with water, and why do some elements remain unreactive?
Notes

Estimated 3 - 5 days
In Part I, students review what they know about atoms and establish a time line for the development of the atom. In the time line, they are introduced to the four major scientists who contributed to the development of modern atomic theory.
In Parts II–IV, students investigate the structure of atoms by creating models and then looking for patterns to learn about their composition and the attributes of subatomic particles and to determine the motion of particles in solids, liquids, and gases.
Materials
Printed
● 1 Student Guide (per group)
● 1 Student Journal (per student)
● 1 Scientists’ Quotes (per teacher)
● 1 Slide Show (per teacher)
● 1 Protons and Neutrons (per group)
● 1 Periodic Table of Elements (per student), available in Teacher Toolbox: Resources
● 1 Atomic Structure (per student)
Reusable
● 1 box, cardboard, small (per teacher)
● 1 tape, mailing, roll (per teacher)
● 1 dice, pair (per teacher)
● 1 scissors (per student)
● 1 pencil, red (per student)
● 1 glue, stick (per student)
● 1 marker, yellow (per student)
● 1 marker, blue (per student)
● 1 computer with Internet access (per group)
Consumable
● 22 beans, navy (per student)
● 20 beans, black (per student)
● 20 lentils (per student)
● 1 pencil, colored, red (per student)
● 1 plastic, bag, ziplock, sandwichsize (per student
● Put the pair of dice in the cardboard box and seal the box with the tape. Make sure to seal the box extremely well so the students cannot pry it open. Pull up the slide show for the class.
● Copy the Scientists' Quotes and cut out the quotations from each scientist. Randomly give the quotations to four students before class starts and tell them to hold onto them.
● Print the Protons and Neutrons, single-sided. Each Protons and Neutrons provides enough paper protons and neutrons for four students. Cut the pages into strips and distribute two strips of protons and neutrons, a glue stick, a pair of scissors, a yellow marker, a blue marker, and a red pencil to each student.
● Students can complete their own models, but they should work cooperatively in groups or pairs.
● Print out the Periodic Table of Elements and laminate or print on card stock if possible, as this resource is used in many activities. Periodic Tables are available in the Teacher Toolbox: Resources.
Developing and Using Models, and Planning and Carrying Out Investigations, and Engaging in Argument from Evidence
During this activity, students will develop and use models to describe and predict the phenomenon of why some elements explode in water while others do nothing at all. By investigating the structure of atoms and creating models, students will gain insights into the composition and attributes of subatomic particles, allowing them to evaluate the limitations of models and modify them based on evidence. This process will help students understand the interactions and energy flows within systems, providing a mechanistic account of the natural phenomenon. Through planning and carrying out investigations, students will collect data to support explanations, and by engaging in argument from evidence, they will construct arguments to support or refute explanations for the observed phenomena.
Scale, proportion, and quantity
Structure and function
During this activity, students will explore the phenomenon of why some elements explode in water while others do nothing at all by investigating the atomic structure and identifying patterns in the composition and behavior of elements. They will recognize that macroscopic patterns, such as reactivity with water, are related to the microscopic and atomic-level structure of elements. By creating models and analyzing data, students will identify patterns in atomic structure that explain the cause and effect relationships underlying the phenomenon. Through this process, they will understand how the structure and function of atoms influence the properties and behaviors of elements at different scales.
Part I: A Moment in Time
The goal of this activity is for students to understand the experimental design and conclusions used in the development of the theory of atoms, or modern atomic theory. These include John Dalton’s postulates, J. J. Thomson’s discovery of electron properties, Ernest Rutherford’s components of atoms, and Niels Bohr’s nuclear atom.
1. Have students review the basic structure of an atom, including all of the subatomic particles. Have students look at their hands; remind them that their hands are composed of atoms, as are their chairs, their desks, and the air they breathe.
2. Ask students if they have any idea when the concept of the atom was first proposed. Allow students to provide any answers they may know. When they are done, tell them that the ancient Greeks were the first to discuss the atom over 2,000 years ago. A Greek philosopher named Democritus first proposed the concept of the atom around 400 BC. He stated that there was matter that could not be divided into smaller parts. Early philosophers debated this idea. Alchemists sought ways to harness the properties of the different elements they discovered. Not until scientists started using experiments that followed the scientific method was the notion of the atom more fully understood.
3. Pass around the sealed box with the dice inside. Ask students to tell you as much as they can about what is in the box. They cannot open the box; they can use only their senses to guess the characteristics of the item. Do not reveal what is inside the box! Let them keep guessing until the end of class. Tell students that early scientists went through a similar guessing game when trying to discover the nature of matter.
As students handle the mystery box, encourage them to share not just guesses, but what evidence led to their guess (sound, weight, movement). Link this directly to how early scientists made inferences from indirect evidence.

4. Project the slide show onto the board. Tell students that the four scientists shown on the time line made major contributions toward the development of modern atomic theory. The time line shows when their research and model development took place. Have students read each of the ideas proposed by the scientists in chronological order. Then, have the students match the scientist to his model and his postulate or theory in their Student Journal.
5. John Dalton, J. J. Thomson, Ernest Rutherford, and Niels Bohr all contributed to the modern view of atomic theory. However, the experimental design of these four scientists in particular led to the conclusions that advanced modern atomic theory. Explain that these scientists each contributed a piece of the puzzle. Each new piece was built on existing pieces, and these pieces together furthered science’s understanding of the atom.
6. Choose four students to read each scientist’s idea to the class, but do not have them read anything yet. The scientists are color-coded to show the chronological order. The scientist, his model, and his statement about his model are listed on the Student Journal. Each student must match the scientist, model, and statement in chronological order on the Student Journal.
FACILITATION TIP
You can also encourage students to summarize the scientist’s contribution in their own words before reading the quotation.
7. Once the students have completed their Student Journals, ask the four students who have the quotations from the scientists to stand up. Have each student read his or her quotation out loud, and have the class decide which scientist to attribute that quote to.
Four Corners Game (if time allows)
Students pick a corner based on facts as they correspond to the scientist.
1. Have four posters with the following names on each of the posters: John Dalton, J. J. Thomson, Ernest Rutherford, and Niels Bohr.
2. Place each of the four posters in a different corner of the room.
3. Either read different facts out loud or create cards that have different facts about the atomic ideas associated with each scientist.
4. Instruct students to then go to the corner of the scientist who was responsible for the idea/theory/fact that was read.
5. Have students discuss why they chose the corner they did.
1. According to modern atomic theory, the Bohr model (the old model of the electrons orbiting the nucleus like the planets in our solar system) is no longer used by scientists. Ensure students understand that they are placing electrons in Part II of their Student Journal to visualize where the electrons are found. Mention that the exact location of an electron is impossible to determine at any given point in time. Instead, modern atomic theory states that there are regions in which there is a high probability of finding the electrons. These regions are referred to as orbitals. It is important that students do not associate the word orbitals with the motion of planets in our solar system. Emphasize that electrons do not orbit the electron cloud; instead, they have unpredictable movement from one position to another at extremely high speeds.
2. Explain that everything on Earth is made of matter. All this matter is made from tiny particles that we cannot see with the naked eye or even with the most powerful optical microscope. These tiny particles are called atoms. Everything on this planet, including us, is made from unique combinations of atoms.
3. Time-saver: Suggest that students use the yellow marker to broadly color over the protons and then use the blue marker to do the same for the neutrons. The color does not need to be within the outline of the circle because students will cut the circles out.
Part III: Atom Patterns
1. If the pattern is not apparent, help students see that for mass in amu, number of protons = number of neutrons.
Part IV: Protons, Electrons, and Neutrons
Note: At this time, you could include the concept of APE MAN, stressing that APE works only in neutral atoms. (Tell your students that APE represents this: the Atomic number equals the number of Protons, which equals the number of Electrons. MAN represents this: the atomic Mass minus the Atomic number equals the number of Neutrons.)
A = Atomic number
P = Protons
E = Electrons (in neutral atoms)
M = Atomic mass
A = Atomic number
N = Neutrons
1. Have students look at the example diagrams in the Student Journal as they go through the instructions in the Student Guide so that they understand what to do. This process takes a little practice.
2. Check for understanding of the process. You may want to write the steps on the board and walk students through the first three atom examples as a class. Students will use the same process to complete the remaining 17 elements.
3. It is important that students go through the process of building and answering the questions for each diagram. The repetition gives students time to absorb these abstract concepts. Check to see that students are correctly completing the diagrams as they are working. As students build larger atoms, direct their attention to the increasing mass in the nucleus and the volume expansion with the addition of more electrons. Remind students that matter is anything that has mass and takes up space.
4. Consider stopping the lab after students complete the diagrams and going over each one to allow students the opportunity to correct inaccuracies and ask questions. The remaining activities refer to these diagrams and are based on student understanding.
Notes
Work through the first atom as a class while verbalizing your thought process step by step, then gradually release responsibility to students.

5. Note that students are given mass numbers for the first 18 atom diagrams. Atomic mass, as shown on the periodic table, is not in whole numbers. Some students may question this. The concept of average mass numbers is due to the occurrence of isotopes.
6. A separate Student Journal KEY is provided for correctly completed atom diagrams. Some teachers copy and laminate the Student Journal KEY for each student group so that they can self-correct as they complete the investigation.
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After the students have a chance to explore the investigation, play a quick game of Wall to Wall. Place a sheet of chart paper on all four walls of your room and write down the following numbers and terms:
1. Dalton versus Rutherford
2. Atomic Number versus Atomic Mass
3. Proton versus Electron
4. Bohr versus Thomson
(Optional: You can place a picture under each word as a reference.)
● Have your students form groups by counting off one to four.
● Instruct students to go to the chart paper that has their corresponding number.
● Give them one minute to write down anything they remember about the terms.
● Then have them rotate to the next wall, moving in numerical order (1–4).
● Instruct students to place a check mark next to an idea a student before them has written down if they agree with it.
● After they have moved through all the groups, have students go back to their original spots. While there, ask them to share one to two key points they read from the chart paper to the class, beginning with group 4.
Why do some elements explode in water while others do nothing at all?
1. How do the number and arrangement of electrons in an atom affect its reactivity with water?
2. In what ways do the subatomic particles and their arrangement contribute to the stability or instability of an element when it comes into contact with water?
3. How can understanding the atomic structure of elements help predict their behavior in chemical reactions, such as those involving water?

Estimated 2 hrs - 3 hrs
Activity Preparation P.7.5C
In Part I, students sort characteristics of elements and compounds while using mixtures as a reference point. In Part II, students use plastic cubes to construct and compare models of elements, compounds, and mixtures. In Part III, students construct and analyze paper models of compounds.
Materials
Printed
● 1 Student Guide (per student or group)
● 1 Student Journal (per student)
● 1 Elements, Compounds, and Mixtures (per group)
● 1 Analyzing Compounds (per group)
● 1 Periodic Table (per student), available in Teacher's Toolbox: Resources.
Reusable
Part II
● 1 scissors (per teacher)
● 1 glue stick (per student)
● 20 snap cubes, blue (per group)
● 4 snap cubes, red (per group)
● 8 snap cubes, yellow (per group)
● 4 snap cubes, green (per group)
● 4 index cards (per group)
● 1 beaker, 500 mL (per group)
● 1 marker, black (per teacher)
● 1 pencil, colored, set (per student)
Preparation
Part I Preparation
● Print one Elements, Compounds, and Mixtures for every four students in your class. Cut apart each page on the dotted lines so each student in the group receives one part of the page.
Part III Preparation
● Print one Analyzing Compounds for every student in your class.
● 1 bag, plastic, ziplock, sandwichsized (per group) Notes
Developing and Using Models, and Planning and Carrying Out Investigations, and Engaging in Argument from Evidence
During this activity, students will develop and use models to describe and predict the phenomenon of why some elements explode in water while others do nothing at all. They will construct and analyze models of elements and compounds, allowing them to evaluate the limitations of these models and modify them based on evidence. This hands-on experience will help students understand the interactions and energy flows within systems, providing a mechanistic account of the natural phenomenon. By planning and carrying out investigations, students will gather evidence to support explanations, enhancing their ability to engage in argument from evidence and construct convincing arguments about the behavior of elements in water.
Patterns, and Scale, proportion, and quantity, and Structure and function
During this activity, students will explore the phenomenon of why some elements explode in water while others do nothing at all by constructing and analyzing models of elements, compounds, and mixtures. They will recognize patterns in the atomic-level structure that relate to macroscopic behaviors, such as reactivity with water. By using models, students will observe how the structure and composition of elements and compounds influence their function and reactivity, identifying cause and effect relationships. They will also understand how scale, proportion, and quantity affect the properties and behaviors of substances, using proportional relationships to represent scientific concepts.
Procedure and Facilitation
Have students read Part I of the Student Guide, or read aloud as a class. Distribute one square from the Elements, Compounds, and Mixtures to each student.
Instruct students to cut apart each box. Then, have them sort the boxes into the correct columns on the chart in their Student Journal and glue them into place. Have students answer the questions in Part I of their Student Journal.
● Have students read Part II of the Student Guide, or read aloud as a class.
● Have students follow the steps listed and check models as outlined below:
1. First, make oxygen gas, or O2, by connecting two blue cubes. Is O2 an element or compound? Recall that an element is a pure substance made of only one kind of atom, so O2 is an element. Use the blocks to make three more individual models of O2 and set these models aside to use later.
Check student models. Each of the four individual models should be made from two blue cubes to represent oxygen gas.
2. Next, use the blocks to make a model of carbon dioxide, or CO2. Is carbon dioxide an element or a compound? Carbon dioxide is a pure substance made from two different elements, so it is a compound. Use the blocks to make three more individual models of carbon dioxide, CO2, and set these models aside to use later.
Check student models. Each of the four individual models should be made from one red block to represent one atom of carbon and two blue blocks to represent two atoms of oxygen.
FACILITATION TIP
Before sorting, ask students to share everyday examples of elements, compounds, and mixtures (e.g., oxygen, salt, trail mix).
If students struggle, guide them by asking: “How many types of atoms do you see here?”

3. Now, use the blocks to make a model of the compound water, H2O. How do you know by just looking at this model that it is a compound? Compare the single model of water to the models of oxygen you made earlier. These models clearly represent when a substance is an element or a compound simply by their colors. Make three more models of water.
Check student models. Each model should be made from two yellow cubes to represent the hydrogen and one blue cube to represent the oxygen.
4. Leave the individual atom models of the element copper in a pile.
Check to see that the green cubes remain unconnected.
5. Identify the models you created as elements or compounds by placing a labeled index card next to the appropriate set.
Check index cards to see that students identify the following:
Oxygen gas = element
Carbon dioxide = compound
Water = compound
Copper = element
6. Complete the chart in Part II of your Student Journal using colored pencils. All four sets of models that you made are pure substances! Recall that a pure substance is uniform throughout and has consistent properties such as melting or freezing points, hardness, and flammability (whether or not the substance burns).
7. Use any combination of the pure substances that you modeled to create a mixture by placing them in a beaker. Your mixture will probably not look like the mixtures created by other groups. Recall that mixtures can vary in the amounts and types of matter present, which explains why mixtures do not have consistent properties.
The focus of this activity is not mixtures. Mixtures are used to as a reference point, and they demonstrate a non-example of a pure substance. Emphasize that elements and compounds are pure substances. Students may be confused because compounds contain different elements. Remind them that the ratio of the elements in a compound is fixed and remains the same throughout the matter.
Wrap up with a quick formative check. Give an unlabeled cube model and ask, “Element, compound, or mixture?”
As a final activity, have students use their experience with the snap cubes to model atoms in elements and compounds.
Have students complete this section of their Student Journal.
Review the reflection questions as a class.
Notes
Think, Draw, Explain
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After students explore the investigation, give them an opportunity to show their understanding.
Write the following instruction on the board:
Create a diagram of atoms inside a compound.
Ask students to think about their response before drawing it on paper. After they are finished with the drawing, ask them to explain their answer. Provide students with the following sentence stem:
This drawing accurately represents atoms in a compound because
How do the properties and interactions of elements and compounds explain why some elements react explosively with water while others do not?
1. Based on your models of elements and compounds, what characteristics might make an element more likely to react with water?
2. How do the fixed ratios of elements in compounds affect their reactivity compared to individual elements?
3. Considering the periodic table, what trends or patterns might help predict an element’s reactivity with water?

Estimated 1 hr - 2 hrs
Students design an investigation to determine the trends or patterns observed in properties of elements moving from left to right across the periodic table. These patterns may be observed and studied to provide evidence for causality in explanations of phenomena. Elements to be tested are sulfur, silicon, aluminum, and magnesium for the properties of appearance (color, luster, malleability), hardness, conductivity, reaction with HCl (acid), and reaction with NaOH (base). Students then predict the properties of sodium and phosphorus based on scientific principles and evidence from the investigation.
Materials
Printed
● 1 Student Guide (per group)
● 1 CER Rubric Key (per student)
Reusable
● 8 test tubes (per group)
● 1 test tube rack (per group)
● 1 conductivity tester (per group)
● 2 small beakers (per group)
● 2 droppers (per group)
● 4 scoopulas or spatulas (per group)
● 1 test tube brush (per group)
● 1 magnifying glass (optional) (per group)
● 1 well plate (per group)
Consumable
● Distilled water (per group)
● 3M HCl, 20 mL (per group)
● 3M NaOH, 20 mL (per group)
● 5 grams silicon (large chips if possible) (per group)
● 5 grams sulfur (per group)
● 1/8 strip magnesium ribbon (3–4 per group)
● 1/8 strips/pieces of aluminum (may be foil or solid) (3–4 per group)
● 1 sandpaper sheet or steel wool wad (per group)
● 1 lab journal (per student)
Set up each lab station with materials. Make sure there is easy access to a sink with water and soap for ease of clean up.
You can provide appropriate grouping/differentiated inquiry with the following scaffolding suggestions:
● Group together students who need more guided practice and spend more time with them to develop their procedures. Let the other groups work more independently.
● Group students with mixed needs and have them work together to develop a procedure. Monitor all groups equally.
Developing and Using Models, and Planning and Carrying Out Investigations, and Engaging in Argument from Evidence
During this activity, students will develop and use models to describe and predict the phenomena of why some elements explode in water while others do nothing at all. By designing and conducting investigations to observe trends in elemental properties across the periodic table, students will evaluate the limitations of their models and modify them based on evidence. This process will help them understand the mechanistic accounts of natural phenomena and predict the behavior of elements like sodium and phosphorus. Through engaging in argument from evidence, students will construct and critique explanations, using empirical data to support their claims about the reactivity of elements with water and other substances.
Patterns, and Scale, proportion, and quantity, and Structure and function
During this activity, students will identify and analyze patterns in the properties of elements as they move across the periodic table, which are related to the microscopic and atomic-level structure of the elements. By observing these patterns, students will explore the phenomenon of why some elements explode in water while others do not, using their understanding of atomic structure and reactivity. They will use models and data to recognize cause and effect relationships, and apply this knowledge to predict the properties and behaviors of sodium and phosphorus, thereby deepening their understanding of the structure and function of elements at different scales.
1. Lead a discussion with students using the following prompt:
○ Does the periodic table have any sort of system or pattern to its design? If so, give some examples. Yes, it has many trends. These include increase in atomic number, increase in number of valence electrons, and decrease in atomic radius as you go from left to right across the periodic table.
2. Tell students that today they will conduct an investigation. The investigation is written to encourage the students to plan and implement their own investigations with your guidance.
3. The following is a sample investigation tightly aligned to the Mississippi standards with sample materials, procedures, and anticipated student answers provided. All investigations are inquiry-based so the teacher can guide the students through differentiated science inquiry events to their comfort level.
4. Magnesium does not react with NaOH unless it is subjected to heat and/or pressure. Magnesium reacts with water at elevated temperatures, not with NaOH itself, and pressure does not affect magnesium reactivity at all. Metals must be sanded to see their true appearance and to make sure conductivity can be properly observed and recorded. Under the right conditions (large enough piece, good contact, good conductivity meter), a slight conductivity can be observed in silicon. This may not always happen.
○ Use the back of a spatula to apply a small force to each substance. Use the steel wool or sandpaper to see how substance responds when it is rubbed with one of these. (Hardness)
○ Observe color. If necessary, use a magnifying glass to observe. (Appearance)
FACILITATION TIP
Remind students how to look for subtle changes (bubbling, color change, temperature shift, etc.) so they can plan what data they may want to collect.

○ Combine each substance with a few drops of distilled water in a spot well plate. (Make sure to test conductivity tester first in distilled water to make sure it is working correctly.) Place a conductivity tester in each sample to determine if the substance will conduct electricity. Make sure to rinse with distilled water in between testing each sample. (Conductivity)
○ Place a small sample of each substance into a test tube. Add about 4 drops of HCl to each test tube and observe for 2–3 minutes. Record observations and clean out test tubes.
○ Repeat using NaOH. (Reactivity with HCl and NaOH)
As results come in, ask guiding questions like, “How does this observation connect to what you know about metals versus nonmetals?” or “Does your result support or challenge the idea of periodic trends?”
5. When students have finished their discussion, hold a discussion to review their findings:
○ Out of the four characteristic properties of elements, which characteristic has to do with the amount of energy that is required to remove an electron from an atom? Is this property the atomic radius, the ionic radius, the ionization energy, or the electronegativity of an atom? Identify the periodic trend that this property follows. This property describes the ionization energy of an atom. This property generally increases as you move from left to right across a period on the periodic table and decreases as you move down a group on the periodic table.
○ In your own words, explain why the atomic radii of atoms decrease as you move from left to right across a period on the periodic table. With each increase in atomic number, a proton is added to the nucleus of the atom. This increase in the positive charge within the nucleus causes the surrounding negatively charged electrons to draw closer into the nucleus. This is why each atom is smaller moving across a period in the periodic table. This increasing positive charge will have a stronger pull on the electrons of the atom, pulling them closer to the nucleus and thereby decreasing the radii of the atoms.
○ The trends for ionization energy and electronegativity of the elements on the periodic table are slightly different. Compare and contrast these two trends. Then, using the representation of the periodic table given, use an arrow and sketch the trends for increasing ionization energy and electronegativity across a period from left to right and moving down a group. The trend for ionization energy has to do with the amount of energy that it takes to remove an electron from an atom, where the trend for electronegativity has to do with the ability of an atom to attract electrons in a chemical bond.
6. After students complete their task, have them use their data and the observations they gathered in the investigation to write a scientific explanation that includes a claim, evidence, and reasoning (CER).
7. After completing the CER, tell students to trade, read another student’s CER, and ask questions to ensure they understand what was written. Then, ask students to write a rebuttal or reflection based on the other student’s CER response.
Notes
Safety Goggles
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Gloves
When working with chemicals, students should wear gloves to protect their skin.
Four-Square Vocabulary
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
Have students create a set of index cards about the following terms:
● Hardness
● Reactivity
● Metal
● Nonmetal
● Give each student four index cards.
● Instruct students to draw four equal sections on each card. The four sections will represent a vocabulary term, the definition, the term used in a sentence, and an illustration.
● A visual reminder can be sketched on the back of the card.
● Have students create one card for each term.
● After completing all four cards, give students a chance to switch cards or discuss with a partner.
Phenomenon Connection
How do the properties of elements across the periodic table influence their reactions with water, and why do some elements explode in water while others remain inert?
1. Based on your investigation of element properties, what patterns did you observe that might explain why some elements react violently with water while others do not?
2. How do the trends in ionization energy and atomic radius across the periodic table help us predict the reactivity of elements with water?
3. Considering the properties you tested, how might the reactivity of sodium and phosphorus with water differ, and what evidence supports your prediction?
Notes

Estimated 1 hr - 2 hrs
In this activity, students learn about the chemical formulas and equations. In Part I, students learn how to recognize how many atoms of each element are found in chemical formulas. In Part II, students look at a chemical formula to determine if it represents an ionic or covalent bond.
Materials
Printed
● 1 Student Journal (per student)
● 1 Student Guide (per group)
● 1 Periodic Table, available in Teacher Toolbox: Resources (per student)
SEP Connection
Preparation
● Print two Student Guides for each group in your class. Note that some pages of the Student Guide need color printing. Alternately, plan to project these pages so students can see the color references, or have students use red and blue colored pencils to highlight the black and white copies as you direct on page 1 of the Student Guide.
● Print one Student Journal for each student in your class.
● Print one Periodic Table for each student in your class.
Developing and Using Models, and Planning and Carrying Out Investigations, and Engaging in Argument from Evidence
During this activity, students will develop and use models to describe and predict the phenomenon of why some elements explode in water while others do nothing at all. By analyzing chemical formulas and determining the type of bonds present, students will evaluate the limitations of models and modify them based on evidence to understand the interactions and energy flows within systems. This will help them construct arguments supported by empirical evidence to explain the differences in elemental reactions with water.
Patterns
Scale, proportion, and quantity
Structure and function
During this activity, students will explore the chemical formulas and equations to understand why some elements explode in water while others do nothing at all. By recognizing patterns in atomic-level structures and identifying the types of bonds (ionic or covalent), students will connect macroscopic patterns to microscopic structures. They will use these patterns to identify cause and effect relationships, such as the reactivity of elements with water, and represent these scientific relationships through models and equations. This will help them understand how the structure and function of elements and compounds influence their behavior in natural systems.
Notes
1. Before students begin reading the Student Guide, remind them that atomic symbols are always represented by a capital letter that is sometimes followed by a lowercase letter. Point out the examples of H for hydrogen, He for helium, and I for iodine. Make sure students understand that the symbol for iodine (I) is an uppercase i, not a lowercase L. Also, point out that many elements have Latin origins, such as gold (Au from Latin aurum), and thus will not be represented by their English letter(s) (e.g., G or Go for gold). Another common element that students will be expected to identify is Cl for Chlorine; the second letter is a lowercase L, not an uppercase i.
2. Have students review and follow the instructions in Part I of their Student Guide.
3. Have students answer the questions in Part I of their Student Journal.
1. During this part of the lesson, you are simply introducing the concept of ionic and covalent bonds. You are not going to get into the transferring or sharing of electrons. Just focus on the type of element in each compound (metal, nonmetal, or metalloid) to classify what type of bond is occurring.
2. Have students review and follow the instructions in Part II of their Student Guide.
3. Have students answer the questions in Part II of their Student Journal.
Notes

Think, Draw, Explain
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After students explore the investigation, give them an opportunity to show their understanding.
Write the following question on the board:
What is the difference between ionic and covalent bonds?
● Ask students to think about their response before drawing it on paper.
● After they are finished with the drawing, ask them to explain their answer.
● Provide students with the following sentence stem:
The difference between ionic and covalent bonds is ________.
Answers should provide an argument as to how bonds are different and why. This argument should include at least three examples.
When elements interact with water, why do some react explosively while others remain inert?
1. Based on your understanding of chemical formulas and bonds, how might the type of bond (ionic or covalent) influence an element’s reaction with water?
2. Considering the periodic table, what properties of elements might predict their reactivity with water?
3. How do the number and type of atoms in a compound’s chemical formula affect its potential to react violently with water?
Notes

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Nuclear Plant Expert
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - What Is So Important about the Periodic Table?
A reading passage about the concept, which includes five to eight comprehension questions.
Data Literacy
Student analyze data sets and interpret information related to the scope’s content.
PhET: Simulation Practice
Student activities using the PhET Interactive Simulations from the University of Colorado Boulder.
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
Student Learning Objectives
Atoms are made of subatomic particles called protons, neutrons, and electrons.
Matter is composed of atoms that have physical properties, including subatomic particles that have characteristic masses, charges, and locations. Atoms also exhibit chemical characteristics such as reactivity to other atoms. The physical and chemical properties of atoms can be interpreted by their arrangement on the periodic table of elements.
All matter on Earth can be classified as either a pure substance (an element or compound with its own definite composition and properties) or a mixture (combination of two or more pure substances not chemically bonded, with variable composition and properties).
Elements are arranged on the periodic table of elements in increasing order of the atomic number or number of protons in the nucleus of the atom in an element. Elements within the same column (group) have similar chemical properties.
The three main types of elements are classified and shown on the periodic table of elements as metals, nonmetals, and metalloids.
A chemical formula provides information regarding how many of each type of atom are present in the compound.
Molecular compounds are composed of two nonmetals held together by a covalent bond. Ionic compounds are composed of a metal and a nonmetal.

Scope Overview
This unit develops students’ ability to identify, model, and predict chemical reactions using common substances. Through hands-on investigations with indicators, pH, and neutralization, students gather evidence of chemical change, distinguish it from physical change, and classify reactions (acid–base, combustion, rusting, fermentation, endo/exothermic). Modeling bond breaking and formation with energy diagrams, they connect temperature change to energy transfer and verify conservation of atoms. Students use observations, data, and chemical formulas to infer reactants, products, and likely outcomes of reactions.
The student is expected to demonstrate an understanding of chemical formulas and common chemical substances to predict the types of reactions and possible outcomes of reactions through investigations and models.
What happens when you mix baking soda and vinegar, and why does it fizz and bubble?
Key Concepts
• When a chemical reaction occurs, new substances are formed that have different properties than the original substances.
• Common evidences of a chemical reaction are formation of a gas, production of light, change in temperature, formation of a precipitate, or change in color.
• The pH scale is used to determine the strength of an acid or base.
• Neutralization occurs when acids and bases react with each other, usually forming a salt and water.
• Temperature changes indicate the formation of new substances. Chemical reactions can either give off heat (exothermic reaction) or use heat (endothermic reaction).
Scope Vocabulary
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Acid
A compound that produces hydrogen ions in solution, donates hydrogen ions, or is an electron-pair acceptor
Base
A compound that releases hydroxide ions in solution, accepts hydrogen ions, or donates an electron pair
Chemical Change
A change that involves one substance being pulled apart or combined with another substance to form a completely different substance or substances
Chemical Equation
Chemical formulas and symbols written to represent a reaction
Chemical Formula
A shorthand notation that uses chemical symbols and numbers as subscripts to represent the type and number of atoms that are present in the smallest unit of a substance
Chemical Reaction
The process by which one or more substances change to produce one or more different substances
Color Change
A change in the way something reflects light; can indicate a chemical change
Endothermic Reaction
A chemical reaction in which heat is absorbed
Exothermic Reaction
A chemical reaction in which heat is released
Production of a Gas
Evidence of a new substance formed from a chemical change: includes bubbles, fizzing, or odor change
Production of a Precipitate
Evidence of a new substance formed from a chemical change: solid particles formed or separated out of a liquid
Temperature Change
Increase or decrease of heat energy in a substance; may be evidence of a new substance formed during a chemical chang
Students investigate chemical reactions by creating and revealing messages using an acid–base indicator.
• Write messages with a baking soda solution as “invisible ink” on white paper using cotton swabs.
• Brush grape juice over the paper to reveal the writing and observe the resulting color change.
• Discuss evidence of chemical change versus physical change, connecting the color change to a pH indicator reaction.
Scientific Investigation - Signs of a Chemical Reaction
Students explore evidence of chemical reactions in everyday contexts through discussion, guided testing, and student-designed investigations.
• Analyze images of everyday phenomena to identify reactants, products, and signs of chemical change.
• Mix powdered solids with water and vinegar to observe indicators (gas, color, temperature, precipitate) and make predictions about reactions.
• Plan and conduct investigations at stations (e.g., photosynthesis, combustion, rusting, fermentation, acid–base, endothermic) to gather evidence that reactions occur.
• Record observations and draw conclusions to support claims that new substances formed.
Students investigate pH, acids, and bases using indicators and apply neutralization concepts.
• Test clear solutions with cabbage juice, litmus, and pH paper; record and compare color changes to determine acidity or basicity.
• Organize data and interpret indicator responses to classify substances by pH.
• Design and implement a method to neutralize an acidic and a basic sample, using indicator color to identify when they approach neutral.
Making a Model - Modeling Energy in Reactions
Students model how energy changes during chemical reactions by representing bond breaking and formation.
• Build and manipulate counter-chip models to represent reactants and products, then translate them into Energy Diagrams.
• Compare endothermic and exothermic reactions by analyzing where energy is absorbed or released.
• Complete and interpret multiple Energy Diagrams, then respond to targeted discussion questions.
• Use the models to verify conservation of atoms and connect temperature change to energy stored in chemical bonds.
Notes

Estimated 30 min - 45 min
Students explore chemical reactions by writing in invisible ink.
Materials
Printed
● 1 Invisible Ink (per student, group, or class)
Reusable
● 2 beakers, 250 mL (per group)
● 2 cups, plastic, 250 mL (per group)
● 1 spoon (per class)
● 1 stirring rod or spoon (per group)
Consumable
● Water, 50 mL (per group)
● Baking soda, 50 mL (~110 g) (per group)
● Grape juice, purple, concentrate preferred, 50 mL (per group)
● 1 paper, white, plain, sheet (per student)
● 2 swabs, cotton (per student)
Developing and Using Models
Analyzing and Interpreting Data
1. Put 50 mL or more of baking soda in a cup for each group.
2. Put 50 mL or more of grape juice or grape juice concentrate in a cup for each group.
3. Lay out the rest of the materials where students can retrieve them.
4. Exact quantities are not important for this activity.
5. It is recommended that the baking soda and water be mixed approximately one-to-one.
Constructing Explanations and Designing Solutions
During this activity, students will develop and use models to describe and predict the chemical reaction between baking soda and grape juice, which serves as a pH indicator. By analyzing the visible change in color, students will evaluate the limitations of their models and modify them based on evidence to better understand the underlying mechanisms of the reaction. This process will help them construct explanations supported by scientific principles, demonstrating the interaction of inputs and outputs within the system.
Cause and effect: Mechanism and explanation and Structure and function
During this activity, students will explore cause and effect relationships by observing the chemical reaction between baking soda and grape juice, which causes a color change. This will help them understand how chemical reactions can be used to predict phenomena in natural or designed systems. Additionally, students will analyze the structure and function of the materials involved, understanding how the composition and relationships among the components lead to the observed changes.
Pre-Activity Discussion
1. What distinguishes a chemical reaction from a physical reaction? There is a change in properties of the substances after the reaction, aside from just size or shape.
2. What do you think some of those changes might be? Accept all ideas.
Post-Activity Discussion
1. What happened when you spread the grape juice over your invisible ink? It became visible.
2. What do you think caused this result? A chemical reaction between the baking soda and the grape juice caused a change in color. A similar reaction is used frequently in laboratory experiments as an indicator.
3. Can you guess what that might be? Accept all ideas. Grape juice contains a chemical component that can be used as a pH indicator. The alkalinity of the baking soda causes it to change color when they are mixed.
Do Not Eat or Drink Materials
When baking soda and vinegar are mixed, why does it fizz and bubble, and how does this relate to the chemical reaction observed when using invisible ink?
Guide students to notice that the paper itself didn’t change color—only the areas where baking soda was applied. Reinforce that this difference is evidence of a chemical interaction, not just “wet paper.”
1. How does the reaction between baking soda and grape juice in the invisible ink activity compare to the reaction between baking soda and vinegar in terms of observable changes?
2. What role does the pH indicator play in the invisible ink activity, and how might a similar concept explain the fizzing and bubbling when baking soda and vinegar are mixed?
3. In what ways can we identify that a chemical reaction has occurred in both the invisible ink activity and the baking soda-vinegar reaction, beyond just visual changes?
Students should be reminded not to eat or drink any materials unless directed to do so. Notes
P.7.5D

Estimated 1 hr - 2 hrs
In Part I, students discuss evidence of chemical changes in everyday life. In Part II, students observe the characteristics of a powdered solid when it is mixed with two known liquids and predict likely outcomes of the reactions. In Part III, students design and conduct scientific investigations to support evidence that chemical reactions have occurred in everyday situations.
Materials
Printed
● 1 Student Journal: Signs of a Chemical Reaction (per student)
● 1 Images of Everyday Reactions (per group)
● 1 Chemical Reactions Experimental Design Cards (per group)
Reusable
Part II
● 1 rolling pin (per teacher)
● 1 hand lens (per student)
● 4 cups, condiment, 2 oz, with lids (per group)
● 1 goggles, chemical splash, safety (per group)
● 1 apron, laboratory, safety (per group)
● 8 spoons, plastic, teaspoon (per group)
● 8 knives, plastic (per group)
● 1 marker, permanent (per group)
● 8 cups, plastic, 8 oz (per group)
Part III
● 1 light source
● 1 small Erlenmeyer flask
● 1 stopper to fit flask
● 5 beakers or clear plastic cups
● 1 roasting stick for marshmallows
● 1 candle, alcohol burner or Bunsen burner
● 2 eyedroppers
● 3 thermometers
● 1 pencil
● 1 marker, set (per group)
Consumable
Part II
● 4 plastic bag, ziplock, sandwich (per group)
● Water, 100 mL (per group)
● Vinegar, white 100 mL (per group)
● 1 paper, wax, sheet, 30 cm (per group)
● Calcium chloride, anhydrous, 20 g (per group)
● Magnesium sulfate, heptahydrate, 20 g (per group)
● Sodium carbonate, monohydrate, 20 g (per group)
● Citric acid, 20 g (per group)
● Indicator, phenol red, 15 mL (per class)
Part III
● 2 sprigs of Elodea (found at pet stores)
● Bromothymol blue 0.1% solution
● Drinking straw
● Marshmallow
● 50 mL yeast solution
● 25 g flour
● 1 g sugar
● 1 piece of steel wool
● 2 antacid tablets, active ingredient calcium carbonate
● Small disposable paper plate
● 2 droppers or pipettes
● 100 mL of water
● 50 mL of vinegar
● 2 bags, ziplock, snack-size
● 1 tsp baking soda
● 1 bag, ziplock, sandwich-sized
● 1 poster board (per group)
Part I
● Print one Student Journal for each student in your class.
● Print one Images of Everyday Reactions (per group)
Part II
● Print one Possible Observations for each group in the class and laminate for reuse.
● Add 5 mL of phenol red indicator to every 500 mL of vinegar prepared for distribution; a class of 30 requires 1500 mL.
● Prepare the liquids by labeling the condiment cups as follows:
○ Water
○ Vinegar for each student
● Place 50 mL of each liquid in the labeled condiment cups and seal each with a lid.
● Make a set of labeled plastic cups, spoons, and knives for each group in your class as follows:
○ Calcium chloride
○ Magnesium sulfate
○ Sodium carbonate
○ Citric acid
● Prepare the four solids by using a rolling pin to smash them into finer grains to make them less distinguishable.
● Put the matching spoon and knife into the labeled cup and add the appropriate solid. For teachers with multiple classes, expect to replenish as needed throughout the day. Prepare to distribute one set of labeled cups containing the four solids to each group.
Part III
1. Print one Chemical Reactions Experimental Design Cards and laminate for reuse. Cut the cards apart and place at the appropriate station.
2. Set up six stations.
Remember that students are designing their own experiments. At each station, provide the indicated materials. You may choose to provide optional materials for more student choice.
Station 1: Photosynthesis in Elodea
1. Tear off two sprigs of Elodea.
2. Prepare a 0.1% solution of Bromothymol blue by adding 15 drops to 50 mL of water.
3. Provide enough additional water to fill the flask provided.
4. Place the reagents and a variety of equipment for the lab including goggles, straw, flask, stopper, and light source at the station.
Station 2: Roasting a Marshmallow
1. Determine heat source easily available in your classroom. You may use a candle, an alcohol burner, or a Bunsen burner.
2. Place the reagents (marshmallow) and a variety of equipment for the lab including goggles, roasting stick, and heat source at the station.
Station 3: Rusting of Steel Wool
1. Take a piece of steel wool and pull to separate and feather in order to maximize surface area.
2. Measure 50 mL water into a plastic cup or beaker.
3. Place the reagents and a variety of equipment for the lab including goggles, ziplock sandwich bags, thermometer, and beaker or plastic cup at the station.

Station 4: Rising of Bread Dough
1. Measure 25 grams of plain flour and 1 gram of sugar into a snack-size ziplock bag.
2. Prepare 50 mL yeast suspension according to package directions. Be sure to use warm water.
3. Place the reagents and a variety of equipment for the lab including goggles, wax paper, and mixing bowl at the station.
Station 5: Acid Rain and Statues
1. Draw a face on two antacid tablets to represent statues.
2. Measure 20 mL of vinegar into a small cup or beaker.
3. Place the reagents and a variety of equipment for the lab including goggles, ziplock sandwich bags, eyedroppers, and beaker or plastic cup at the station.
Station 6: Cold Pack Reaction
1. Place about 20 mL of vinegar in a snack-sized ziplock bag.
2. Place about 1 teaspoon of baking soda dissolved in 10 mL of water in a snack-sized ziplock bag.
3. Place the reagents and a variety of equipment for the lab including goggles, ziplock sandwich bags, thermometer, and beaker or plastic cup at the station.
Developing and Using Models
Analyzing and Interpreting Data
Constructing Explanations and Designing Solutions
During this activity, students will develop and use models to describe and predict the phenomenon of fizzing and bubbling when baking soda and vinegar are mixed. They will evaluate the limitations of their models and modify them based on evidence to account for changes in variables or components of the system. Through analyzing and interpreting data, students will construct explanations supported by evidence to understand the chemical reactions occurring, applying scientific reasoning to ensure the adequacy of their conclusions.
Cause and effect: Mechanism and explanation
Structure and function
During this activity, students will explore the cause and effect relationships by observing and predicting the outcomes of mixing baking soda and vinegar, understanding that the fizzing and bubbling are due to a chemical reaction. They will classify these relationships as causal, recognizing that the production of gas is a direct result of the interaction between the substances. Additionally, students will model the structure and function of the reactants and products, analyzing how the composition and relationships among the parts lead to the observable phenomena. This will help them understand that phenomena may have more than one cause and that some cause and effect relationships can be described using probability.
Notes
Part I: Chemical Reactions and Equations
Students may work individually or in small groups to review information and discuss the photos in Part I and follow the instructions in Part I of their Student Guide.
Questions for Discussion
Consider the everyday chemical reactions pictured below. What are the reactants? What are the products? How do you know new substances are formed?
Steaks on a Grill
Students may discuss the cooking of the steak. In the Student Journal they will discover the question being asked is about the combustion of propane. Guide the discussion to include the definition of reactants (starting material) and products (ending material). Evidence of chemical change includes a change in color for the steak and production of heat and light for the burning of the propane.
Boy Eating Pizza
Students may discuss digestion of the pizza. In the Student Journal, they will discover the question being asked is about cellular respiration. Evidence of chemical change includes color change and change in composition of the food.
Leaves
Students should be familiar with photosynthesis as a chemical process. Evidence of chemical change includes change in energy and change in composition.
Rusty Chain
Students should be familiar with rust. Evidence of chemical change includes color change and change in composition of the iron.
Part II: Signs of a Chemical Reaction Using Known Reactants
Have students review and follow the instructions in Part II of their Student Guide. Students should answer the questions in Part II of their Student Journal.
Part III: Investigating
The following investigation is a sample investigation tightly aligned to the Mississippi College- and Career-Readiness Standards for Science with sample materials, procedures, and anticipated student answers provided. All investigations are inquiry-based, so teachers guide students through differentiated science inquiry events within their comfort levels. You may choose to guide students in planning their own investigation by going through each of the suggested eight steps before distributing the Student Journal, or you may have students plan their investigations using the Student Journal as a guide.
Notes
Prompt students to justify their answers: “What evidence did you see (color change, odor, gas, energy)?”
Remind them of the signs of a chemical reaction and have them reference those when giving answers.
FACILITATION
Remind students to use all their senses safely (sight, touch for temperature, smell only if instructed—never directly sniff). Encourage them to write down exactly what they see, not what they think is happening.

The investigation is written to encourage students to plan and implement their own investigation with your guidance. You can provide appropriate grouping/ differentiated inquiry with the following scaffolding suggestions:
● Group students who need more guided practice together and spend more time with them. Let the other groups work more independently.
● Group students with mixed needs and have them work together. Monitor all groups equally.
Experiment 1, photosynthesis of Elodea, is the most difficult to investigate. This experiment may be assigned to a more advanced group of students.
Post-Activity Discussion
1. When making observations in daily life, what observations are evidence that a chemical change has occurred?
○ Observe a bubbling, smoking, fizzing, foaming, expanding container or a change in odor.
○ Observe a solid forming in a liquid.
○ Observe a temperature change or light released.
○ Observe a color change that is not expected.
2. Discuss examples of chemical change you have observed today. What evidence leads you to believe a chemical change occurred?
○ Possible answers include these: food being cooked, digestion, combustion of gasoline. Evidence is provided from the list in question 1.
3. What evidence of chemical change was observed in Part II of the investigation? Some produced a gas, some produced color changes, some produced a temperature change, and others produced a precipitate.
Give students a purpose during the gallery walk/presentation review. For example, ask each group to identify one similarity, one difference, and one new question after viewing another group’s work.
You may choose to have students create posters of their investigation for a gallery walk or to share online through class websites or digital presentations.
Safety Guide
Safety Goggles
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Gloves
When working with chemicals, students should wear gloves to protect their skin.
Aprons
Have students wear aprons or lab coats when handling chemicals.
Fire Extinguisher or Blanket
Have access to a fire extinguisher or blanket when burning materials.
Secure Hair and Loose Items
Ask students to tie back long hair and secure loose clothing or jewelry when working with heat sources.
Students should be reminded not to eat or drink any materials unless directed to do so.
Students should be reminded not to mix materials unless directed to do so.
The stations in this activity may be hard to complete for students who have trouble managing time. Help those who need to better manage their time by posting a visual timer and creating an assignment chart. This chart can help students and groups track which criteria they have met and determine what still needs to be finished with the time remaining. Learn more strategies for time management in the Intervention Toolbox.
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
● Have students think about the Post-Activity Discussion.
● Separate students into pairs.
● Have the pairs discuss answers to each question, justifying their answers with evidence from the activity.
● Once pairs are ready, bring the class back together and have students share their answers.
When baking soda and vinegar are mixed, why do they fizz and bubble, and what does this tell us about chemical reactions?
1. What are the observable signs that a chemical reaction has occurred when baking soda and vinegar are mixed?
2. How do the products of the baking soda and vinegar reaction differ from the reactants, and what evidence supports this change?
3. In what ways can the reaction between baking soda and vinegar be accelerated or slowed down, and what factors influence these changes?

In this activity, students are introduced to the concept of pH and acid base indicators. In Part I, students collect and organize data to determine pH of a variety of clear solutions using cabbage juice indicator, litmus paper, and pH paper. In Part II, students devise a method to neutralize an acid and a base and then test their plans.
Materials
Printed
● 1 Student Journal: Acids and Bases (per student)
● 1 Strength Indicators of Acids and Bases (per student)
Reusable
● 4 glass bottles with droppers (per group)
● 1 permanent marker (per group)
Consumable
● 250 mL cabbage juice indicator (per group)
● 10 mL cream of tartar solution (per group)
● 10 mL detergent solution (per group)
● 5 mL vinegar solution (per group)
● 5 mL water (per group)
● 8 cups, clear plastic (per group)
● 1 strip blue litmus paper (per group)
● 1 strip red litmus paper (per group)
● 1 strip pH paper (per group)
1. Print one Student Journal for each student.
2. Print one Strength Indicators of Acids and Bases in color for each group and laminate for reuse.
3. Label dropper bottles or beakers and transfer pipettes for distribution of samples to students. Samples include cream of tartar, detergent, vinegar, and water.
4. Prepare cabbage juice indicator:
○ Tear 2–4 fresh red cabbage leaves into small pieces and place them in a large ziplock plastic bag.
○ Fill the bag half-full with warm water. Squeeze out any air; then firmly seal the bag.
○ Hold the bag and repeatedly squeeze the cabbage leaves and water until the solution is dark blue.
○ Open one corner of the bag and pour the indicator out of the bag.
○ If you need additional indicator, you may be able to add warm water to the leaves and repeat. Alternatively, start with fresh leaves and water.
○ If you have a blender available, you may choose to make the indicator in the blender. To make the indicator, add a 2-inch slice of cabbage to the blender along with 1 L of warm water. Blend until liquid appears dark blue; then pour through a sieve to separate the leaves from the liquid. You may prepare the cabbage juice a day in advance and store in the refrigerator.
5. Prepare samples for testing:
○ Add 3 tablespoons of powder to 100 mL of water and stir. Solutions may be poured into labeled dropper bottles or beakers with accompanying transfer pipettes. If using transfer pipettes, label to avoid mix-ups during the investigation.
○ You may choose to dilute vinegar 50:50 with water. This will not change the outcome of the experiment and will require less vinegar to perform Part I of the experiment.
Notes
Developing and Using Models
Analyzing and Interpreting Data
Constructing Explanations and Designing Solutions
During this activity, students will develop and use models to describe and predict the chemical reactions and interactions between acids and bases, such as the fizzing and bubbling observed when mixing baking soda and vinegar. They will evaluate the limitations of their models by testing different variables, such as the concentration of solutions, to understand the mechanisms behind the neutralization process. Through analyzing and interpreting data from their experiments, students will construct explanations based on evidence to describe the phenomenon of acid-base reactions and the resulting changes in pH.
Cause and Effect: Mechanism and Explanation and Structure and Function
During this activity, students will explore the cause and effect relationships by observing the chemical reaction between baking soda and vinegar, which results in fizzing and bubbling due to the production of carbon dioxide gas. This will help them classify relationships as causal, understand that phenomena may have more than one cause, and recognize that some cause and effect relationships can only be described using probability. Additionally, students will model the structure and function of acids and bases, using indicators to visualize how their function depends on their composition and relationships among their parts.
Procedure and Facilitation
Facilitation
1. In Part I, students work in groups of three or four to collect, organize, and interpret data using various indicators including litmus paper, pH paper, and cabbage juice. In Part II, students neutralize samples from Part I, using cabbage juice indicator to determine the equivalence point.
2. Students should wear safety goggles.
3. Ensure students do not mix up droppers or pipettes.
4. Ensure students tear the litmus and pH strips into smaller pieces to avoid waste.
5. Be prepared to review with students proper lab behavior and safety.
Differentiation Points
● Group students who need more guided practice together and spend more time with them. Let the other groups work more independently.
● Group students with mixed needs and have them work together. Monitor all groups equally.
Notes

FACILITATION TIP
If students say “All acids are dangerous,” redirect by contrasting strong acids (battery acid) with weak, everyday acids (lemon juice, vinegar). Use examples they eat or drink to normalize the concept.
Pre-Activity Discussion
1. You may have heard of acids before. Can you name a common substance that is an acid? What are some properties of this substance? Answers will vary. Students may know citrus juices are acids and taste sour. Students might believe all acids are dangerous and talk about battery acid.
2. Acid base indicators change color in the presence of acids or bases. Do you think this is a chemical or physical change? Why? Color change is an indicator of chemical change.
Set Up the Experiment
1. Carefully tear the red and blue litmus papers and pH paper into four pieces approximately the same size.
FACILITATION TIP
Model careful labeling of cups before students begin. Mislabeling is the most common error.
2. Label five empty clear plastic cups as follows:
○ Indicator + cream of tartar
○ Indicator + detergent
○ Indicator + vinegar
○ Indicator + water
○ Indicator only
Investigate
1. Beginning with the cream of tartar, test each sample using the indicators and litmus papers.
○ Carefully pour about 2 tablespoons of indicator solution into each cup. Place the four labeled cups on a white piece of paper. Using the dropper, add a stream of liquid to the solution. Gently swirl to mix. Observe any color change in the cup and record observations in the data table. Leave the cup sitting on the white paper for comparison later.
○ Using the dropper, place 2–3 drops of the cream of tartar solution on the small section of blue litmus paper. Observe any color change and record observations in the data table.
○ Using the dropper, place 2–3 drops of the cream of tartar solution on the small section of red litmus paper. Observe any color change and record observations in the data table.
○ Using the dropper, place 2–3 drops of the cream of tartar solution on the small section of pH paper. Observe any color change and record observations in the data table.
2. Repeat steps a–d using the remaining three samples.
Part II: Neutralization
Pre-Activity Discussion
1. When people and countries are in a disagreement, they may meet at a neutral place to work through their differences. What do you think the word neutral means? Neutral means not having a positive or negative opinion, not taking sides.
2. When considering acids and bases, what do you think the term neutralize means? To neutralize means to bring toward the middle. In the case of pH, it means to bring toward 7.
Set Up the Experiment
1. Label three empty clear plastic cups as follows:
○ Indicator + detergent
○ Indicator + cream of tartar
○ Control
Investigate
1. Carefully pour 2 tablespoons of indicator solution into each cup and place the cups on a white piece of paper. Record your observations.
2. Using the dropper, add 3–5 drops of cream of tartar solution to the indicator + cream of tartar cup. Gently swirl to mix. Record your observations.
3. Using the dropper, add 3–5 drops of detergent solution to the indicator + detergent cup. Gently swirl to mix. Record your observations.
4. Using the solutions at your table, devise a method to return the samples from steps 2 and 3 to the color of the control. Complete the chart provided.
Safety Goggles
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Gloves
When working with chemicals, students should wear gloves to protect their skin.
Do Not Eat or Drink Materials
Students should be reminded not to eat or drink any materials unless directed to do so.
Let students share different methods at the end so they see that multiple approaches may work, mirroring real scientific problem-solving.

Before and After
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
Students create a graphic organizer titled Before and After. The organizer can be completed as an additional worksheet or in Student Journals. Students can create the organizer either after the teacher demonstration or after the student activity. Students may draw pictures to represent the changes along with completing the sentence stems.
Before
The litmus looked ___________ before the experiment.
I think the litmus will ___________ (change/not change) when we put it in the solutions.
After
Acids cause ___________ to happen.
The litmus looked ___________ after the experiment. I know acid is present because ___________.
When baking soda and vinegar are mixed, they fizz and bubble due to a chemical reaction. How does this reaction compare to the changes observed when acids and bases interact with indicators?
1. How does the color change in the cabbage juice indicator help us understand the reaction between baking soda and vinegar?
2. In what ways do the concepts of acids, bases, and neutralization relate to the fizzing and bubbling observed in the baking soda and vinegar reaction?
3. How can we use our understanding of pH and indicators to predict or explain the outcome of mixing baking soda and vinegar?
Notes

Estimated 1 hr - 2 hrs
Students use a model to explore the breaking and formation of bonds during a chemical reaction.
Materials
Printed
● 1 Student Journal: Modeling Energy in Reactions (per student)
● 1 blank Energy Diagram (per group)
Reusable
● Set of colored counting chips containing at least 20 chips each of six different colors (per group).
● Colored pencils
SEP Connection
Developing and Using Models
Analyzing and Interpreting Data
● Print a Student Journal for each student or assign online.
● Print a class set of the Student Guide. (Optional: laminate for repeated use.)
● Print an 11 x 17 copy of the blank Energy Diagram for each group. (Optional: print on card stock and laminate for repeated use.)
● Fill a plastic resealable bag with 20 counting chips of six different colors. Have extra counting chips available for replacement as needed.
Constructing Explanations and Designing Solutions
During this activity, students will develop and use models to explore the breaking and formation of bonds during a chemical reaction, allowing them to describe and predict the phenomenon of fizzing and bubbling when baking soda and vinegar are mixed. By evaluating the limitations of their models and modifying them based on evidence, students will gain a mechanistic understanding of the energy changes involved, including the release of carbon dioxide gas. They will analyze and interpret data from their models to construct explanations of the chemical reaction, applying scientific reasoning to demonstrate the adequacy of their evidence and conclusions.
CCC Connection
Cause and Effect: Mechanism and Explanation and Structure and Function
During this activity, students will explore the cause and effect relationships by modeling the breaking and formation of bonds during a chemical reaction, such as the reaction between baking soda and vinegar. They will classify the reaction as exothermic, where the energy released as heat is a result of the bonds in the reactants being greater than those in the products. This will help them understand that the fizzing and bubbling observed is due to the release of carbon dioxide gas, a direct effect of the chemical reaction. Additionally, students will analyze the structure and function of the reactants and products, visualizing how the rearrangement of atoms leads to the observed phenomena, and how energy is stored and released in chemical bonds.
Notes
This activity may be differentiated to meet student needs.
For those students who may need more help, you may provide them a copy of the first Energy Diagram model completed to use as an example.
For more advanced students, you may have them predict the Energy Diagrams for reactions from previous scientific investigations.
1. What do you think the prefix thermo- means? Thermo- refers to "heat."
2. What do you think the prefixes endo- and exo- mean? Endo- means "inside" while exo- means "outside."
3. Can you think of a chemical reaction that releases heat? Students should know combustion reactions are exothermic. Other possible answers include hand warmers and cellular respiration.
4. Can you think of a chemical reaction that absorbs heat? Students should remember the endothermic reaction from Explore 1. Other possible answers include cold packs and photosynthesis.
5. In an exothermic reaction, where does the energy that is released come from? The energy comes from breaking the bonds of the reactants.
6. In an endothermic reaction, where does the energy go? The energy is stored in the bonds of the products.
1. Assign students to groups of three or four.
2. Pass out the class set of the Student Guide.
3. Have the class read the Student Background information that will help them to model the breaking and forming of chemical bonds during chemical reactions. The Student Guide provides the exact procedure to follow to make counter chip models and explains how to collect data on the Energy Diagrams. Chips on an overhead projector or magnetic circles on a whiteboard can be used for large group instruction.
4. Walk through the first reaction and then allow students to finish the three additional models. Circulate during this activity to assist those needing guidance with counter chip models or with completing the Energy Diagrams in their Student Journals.
5. After completing the Energy Diagrams, students should answer the discussion questions.
Challenge students to explain why some reactions feel “cold” or “hot” to the touch in terms of particle collisions and energy transfer.
Relate terms to real-world experiences: “Have you ever used a hot pack after sports? Was it exo or endo?” This anchors vocabulary in everyday life.

1. How do the types and numbers of atoms in the reactants compare to the types and numbers of atoms in the products? The types and numbers of atoms in the reactants are identical to the types and numbers of atoms in the products. Atoms are neither created nor destroyed in a chemical reaction but are rearranged to form new chemical compounds.
2. Provide evidence that energy is stored in the bonds of chemical compounds. In an endothermic reaction, energy in the form of heat is absorbed during the formation of the products. The bonds are storing the energy. During an exothermic reaction, heat is released when the energy stored in the bonds of the reactants is greater than the energy stored in the bonds of the products. The change in temperature is evidence of energy stored in the bonds.
3. Provide evidence that energy is released in an exothermic reaction. The energy is released as heat.
FACILITATION TIP
Connect back to Law of Conservation of Energy by explaining that energy isn’t lost, it just changes form (stored in bonds vs. released as heat).
4. What do you know about the amount of energy required to break the bonds of the reactants compared to the amount of energy released when the products are formed in endothermic versus exothermic reactions? In an endothermic reaction, more energy is stored in the bonds of the products than the bonds of the reactants. In an exothermic reaction, more energy is stored in the bonds of the reactants than the bonds of the products, and the excess energy is released as heat.
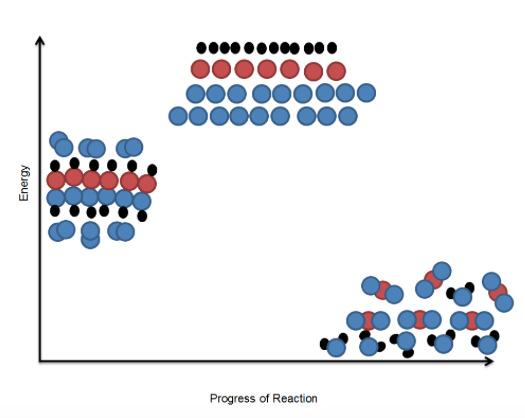
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
At the end of class, have students write a short description of bond breaking and forming in a chemical reaction. Encourage students to use vocabulary terms from the lesson, including endothermic and exothermic. Hold a discussion about what they wrote either immediately after they finish or at the start of next class.
When baking soda and vinegar are mixed, why does the reaction fizz and bubble, and how is this related to the breaking and formation of chemical bonds?
1. How does the rearrangement of atoms during the reaction between baking soda and vinegar lead to the release of gas?
2. In the context of the baking soda and vinegar reaction, how can we determine if the reaction is endothermic or exothermic?
3. What evidence from the reaction between baking soda and vinegar supports the idea that energy is stored in chemical bonds?

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Microbiologist
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - Ways to Bond
A reading passage about the concept, which includes five to eight comprehension questions.
PhET: Simulation Practice
Student activities using the PhET Interactive Simulations from the University of Colorado Boulder.
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
PhET: Simulation Practice
Student activities using the PhET Interactive Simulations from the University of Colorado Boulder.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
When a chemical reaction occurs, new substances are formed that have different properties than the original substances.
Common evidences of a chemical reaction are formation of a gas, production of light, change in temperature, formation of a precipitate, or change in color.
The pH scale is used to determine the strength of an acid or base.
Neutralization occurs when acids and bases react with each other, usually forming a salt and water.
Temperature changes indicate the formation of new substances. Chemical reactions can either give off heat (exothermic reaction) or use heat (endothermic reaction).

Student Expectations
The student is expected to demonstrate an understanding of the law of conservation of mass through investigation and analysis.
• According to the law of conservation of matter, matter is neither created nor destroyed in any ordinary chemical reaction. Therefore, the mass of substances produced (products) by a chemical reaction is always equal to the mass of the reacting substances (reactants).
• A chemical equation is written by placing the reactants on the left side, the products on the right side, and a reaction arrow in the middle between the reactant side and the product side. Due to the law of conservation of mass, the same type and number of elements must be present in the reactants and products of a chemical reaction.
• To balance a chemical equation, count the number of atoms of each element on the reactant side and compare that to the number of atoms of the same elements on the product side of the reaction. Use coefficients in front of the chemical formulas of the reactants or products to make the number of atoms on each side of the arrow equal.
Scope Overview
This unit builds conceptual and empirical understanding of the law of conservation of mass. Students conduct hands-on reactions in closed and open systems, observe evidence of chemical change (e.g., light production, gas formation), and use balances to compare masses before and after reactions. They analyze discrepancies due to escaping gases, evaluate data quality, and refine measurement practices. Through modeling with particle representations and balanced equations, students connect atom counting to mass constancy, culminating in a shared, evidence-based articulation of conservation of mass.
Scope Vocabulary
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Balanced Chemical Equation
A symbolic representation of a chemical reaction in which both sides of the equation contain equal numbers of atoms of each element
Chemical Reaction
The process by which one or more substances change to produce one or more different substances
Coefficient
A number placed in front of a chemical symbol or formula that represents the number of molecules
Law of Conservation of Matter
Scientific law stating that the mass of all reactants must equal the mass of all products;
Product
A substance produced during a chemical reaction
Reactant
A substance that takes part in and undergoes change during a chemical reaction
Reactivity
The ability of a chemical substance to undergo a chemical reaction; significantly influenced by valence electrons of the reacting substances
Notes
Students investigate chemical reactions and conservation of matter using glow sticks in a closed system.
• Activate glow sticks and observe light production as evidence of a chemical reaction.
• Measure and compare mass of glow sticks before and after activation using a balance.
• Record observations and reflections in lab journals.
• Collaboratively develop and write a class definition of the law of conservation of matter.
Scientific Investigation - The Evidence is in the Balloon
Students investigate whether mass changes during a chemical reaction by measuring a closed system before and after the reaction.
• Measure and record the initial mass of the bottle, water, balloon, and effervescent tablet.
• Combine the tablet with water while the balloon captures produced gas; observe fizzing, bubbling, and balloon inflation.
• Measure and compare the final mass of the complete system and reflect on what the results indicate about conservation of mass.
Scientific Investigation - Closing in on Reactions
Students investigate conservation of mass by comparing chemical reactions in open and closed systems.
• Plan and conduct a reaction-based investigation, modeling unobservable mechanisms and identifying variables and controls.
• Measure and record masses before and after in open versus closed setups to examine apparent mass changes due to gas escape.
• Analyze data to argue that mass is conserved in both physical and chemical processes, distinguishing physical properties from chemical changes.
• Evaluate the accuracy of data-collection methods and discuss sources of error.
Activity - Keeping the Balance
Students model chemical reactions to understand conservation of mass.
• Represent atoms with equal-mass units on a double-pan balance to model reactants and products and observe that total mass remains constant.
• Analyze word and symbolic chemical equations to count atoms, determine if equations are balanced, and connect balanced equations to the balance model.
• Record and explain the law of conservation of mass in their journals, citing evidence that atoms are rearranged but neither created nor destroyed.

Estimated 15 min - 30 min
Students use glow sticks to observe a chemical reaction and conservation of matter in a closed system.
Materials
Printed
● 1 Glow Sticks (per student, group, or class)
Reusable
● 1 balance, triple beam or electronic scale (per group)
Consumable
● 1 stick, glow (per student or group)
● 1 lab journal (per student)
Be prepared to write a class definition of the law of conservation of matter on the board.
Developing and Using Models
Planning and Carrying Out Investigations
Using Mathematics and Computational Thinking
During this activity, students will develop and use models to describe and predict the phenomenon of conservation of matter in a closed system, as demonstrated by the glow stick experiment. They will evaluate the limitations of their models and modify them based on evidence to match what happens when the chemical reaction occurs. By planning and carrying out investigations, students will collect data to support their understanding of the conservation of mass, using mathematical and computational thinking to analyze the data and support scientific conclusions.
Energy and matter: Flows, cycles, and conservation
During this activity, students will explore the phenomenon of what happens to the total weight of a cake when you mix all the ingredients together and then bake it by observing a chemical reaction in glow sticks. This will help them understand the CCC statement that matter is conserved because atoms are conserved in physical and chemical processes. They will see that the mass of the glow stick remains the same before and after the reaction, illustrating the conservation of matter, and relate this to the baking process where the total mass of the cake ingredients remains constant, despite changes in form and energy transfer.
Pre-Activity Discussion
1. What do you think makes a glow stick glow? Encourage all answers. A glow stick contains a glass capsule inside that holds a solution of phenyl oxalate and fluorescent dye. Outside the capsule is a hydrogen peroxide solution. When the capsule is broken, the solutions mix, causing a chemical reaction that produces light.
2. What do we know about the number of atoms inside the glow stick before and after the reaction? The number of atoms does not change.
3. If we were to weigh a glow stick before and after it is activated, what do you predict we would find? The mass would probably be the same.
Post-Activity Discussion
1. What caused the glow stick to glow when it was bent? Bending the glow stick caused a chemical reaction to occur.
2. How do you know a chemical reaction occurred? Light was produced.
3. What did you observe about the mass of the glow stick before and after the chemical reaction? The mass did not change.
4. If you were to write a law of conservation of matter for chemical reactions, how would you express it in your own words? Answers should express the idea that matter (or mass) is not gained or lost as a result of a chemical reaction. Write the class definition of the law of conservation of matter on the board and have students write the definition in their lab journals.
When a cake is baked, what happens to the total weight of the cake compared to the combined weight of its ingredients, and how does this relate to the law of conservation of matter?
1. How does the chemical reaction in a glow stick demonstrate the law of conservation of matter, and how might this principle apply to baking a cake?
2. If the mass of a glow stick remains constant before and after activation, what might you predict about the mass of a cake before and after baking?
3. Considering the process of baking a cake, what changes occur to the ingredients, and how can we account for these changes in terms of matter conservation?
Notes
Have students jot down their own predictions about mass on sticky notes before sharing aloud. This increases participation and gives you a quick formative check.

Estimated 30 min - 45 min
In this activity, students conduct an investigation to find out if substances lose or gain mass after a chemical reaction takes place.
Materials
Printed
● 1 Student Journal (per student)
Reusable (per group)
● Triple beam balance or digital scale
● Empty plastic water bottle, 16–20 oz.
● 1 balloon, 9 in.
● Graduated cylinder
● Beaker of water, large (or water source)
● Safety goggles
Consumable (per group)
● Water, 200 mL
● 1 effervescent tablet
SEP Connection
Developing and Using Models
Planning and Carrying Out Investigations
● If you have limited access to water, place a large beaker of water at each table. (Students will measure out 200 mL of water using the graduated cylinder and pour it into their water bottles.)
Using Mathematics and Computational Thinking
During this activity, students will develop and use models to describe and predict the phenomenon of mass conservation in a chemical reaction, such as what happens to the total weight of a cake when ingredients are mixed and baked. They will evaluate the limitations of their models by comparing the initial and final mass of the system, and modify their models based on evidence to account for changes in mass distribution and gas production. Students will plan and carry out investigations to gather data, identify variables, and use mathematical concepts to support their conclusions about the conservation of mass in closed systems.
CCC Connection
Energy and matter: Flows, cycles, and conservation
During this activity, students will explore the phenomenon of what happens to the total weight of a cake when you mix all the ingredients together and then bake it. They will investigate the conservation of matter by observing that the mass of the system remains constant before and after the chemical reaction, aligning with the CCC statement that matter is conserved because atoms are conserved in physical and chemical processes. Additionally, students will understand how the transfer of energy, such as thermal energy during baking, drives the motion and cycling of matter within a system.
Safety: Students need to wear their safety goggles.
Disposal: All materials may be rinsed down the sink.
1. Have students find the mass of the balloon, water, plastic water bottle, and effervescent tablet and record it in their Student Journals.
2. Instruct students to pour 200 mL of water into the plastic water bottle. Encourage them to be careful not to drip any water.
3. Have students gently break the effervescent tablet up and put the pieces into the balloon.
4. Ask students to carefully place the balloon around the neck of the water bottle and tip the effervescent tablet into the water bottle.
5. Instruct students to record their observations. Students may say they hear fizzing, observe bubbles, and see the balloon inflating.
6. Have students find the mass of the entire system of water bottle, water, balloon, and effervescent tablet.
7. Ask students to reflect on their results.
Model the setup. Demonstrate how to attach the balloon securely to the bottle neck to prevent premature spills of the tablet.

CER Sentence Stems
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them. Allow students to complete the following sentence stems before the discussion portion:
My claim is ________.
My evidence is ________.
My reasoning is ________.
I heard you say ________, and I have not thought about that before. However, I think ________.
Let students work in pairs to develop their CERs. After completing the CER, have students trade CERs with their partner, read that student’s reasoning, and ask questions to make sure they understand what was written. Then, instruct students to write either a rebuttal to or a reflection on their partner’s CER responses.
When a cake is baked, what happens to the total weight of the cake compared to the combined weight of its raw ingredients, and how does this relate to the conservation of mass during chemical reactions?
1. Based on your observations, how does the mass of the system (water bottle, water, balloon, and effervescent tablet) change after the reaction, and what does this tell us about the conservation of mass?
2. How can the results of the effervescent tablet experiment help us understand what happens to the mass of a cake before and after baking?
3. In what ways can we ensure that all matter is accounted for when substances undergo a chemical reaction, such as baking a cake or reacting an effervescent tablet with water?
Notes

Estimated 1 hr - 2 hrs
Students plan and conduct an investigation to model and describe unobservable mechanisms, such as the conservation of mass, in physical and chemical processes in both open and closed systems, to limit possible solutions, and to evaluate the accuracy of methods used to collect data.
Materials
Printed
● 1 Student Journal: Closing in on Reactions (per student, group, or class)
Reusable
● 2 flasks, 250 mL (per group)
● 1 cylinder, graduated, 100 mL (per group)
● 1 scoop, measuring, 5 mL (per group)
● 1 balance, triple beam or electronic scale (per group)
● 1 goggles, safety (per student)
Consumable
● Vinegar, 100 mL (per group)
● Baking soda, 10 mL (~22 g) (per group)
● 1 balloon, latex, 9 in. (per group)
● 1 cup, paper, 3 oz. (per group)
● 1 funnel, paper (per group)
● 1 lab journal (per student)
● 2 plastic, ziplock bags, gallon-size (per group)
● 2 small plastic cups (per group)
● It is important to note that some carbon dioxide may leak out of the balloon. Carbon dioxide effuses through latex, and some mass may be lost. Try this investigation prior to classroom implementation. If loss of mass in the closed system proves to be a problem, try the alternate procedure as follows:
○ Perform the closed system investigation with a gallon plastic bag.
○ Place 1 gram of vinegar and 1 gram of baking soda into two small plastic cups, carefully put them into the plastic bag, and seal it closed.
○ Without spilling the cup contents, find the initial mass of the sealed bag.
○ Tip the cups to mix the contents. (If the seal is good, the bag will inflate but not pop.) Obtain the final mass of the sealed bag plus contents.
○ Repeat for the open system but leave the bag unsealed.
○ Obtain the initial mass of the bag, plus contents, and then the final mass. It is entirely possible that such a small amount of carbon dioxide will be effusing from the balloon that you can disregard this alternate procedure and complete the lab as described below and in the Student Journal.
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online. Review the CER Key prior to conducting the CER with students.
● If possible, have students use an electronic balance. This avoids the tendency of students who have the misconception that the mass will change to incorrectly measure the closed system with a triple beam balance. As an option, the experiment may be completed while the flask is on the balance. This allows students to immediately see the mass fluctuate or not as the reaction takes place.
● At the end of the investigation, all chemicals can be washed down the drain.
Notes
Developing and Using Models
Planning and Carrying Out Investigations
Using Mathematics and Computational Thinking
During this activity, students will develop and use models to describe and predict the phenomenon of mass conservation in both open and closed systems when baking a cake. They will evaluate the limitations of their models and modify them based on evidence to match what happens when variables, such as the system being open or closed, are changed. This will help them understand the unobservable mechanisms of mass conservation during physical and chemical processes, allowing them to plan and carry out investigations, collect data, and use mathematical and computational thinking to support their conclusions.
During this activity, students will plan and conduct an investigation to model and describe the conservation of mass in physical and chemical processes, referencing the phenomenon of what happens to the total weight of a cake when you mix all the ingredients together and then bake it. This aligns with the CCC statement that matter is conserved because atoms are conserved in physical and chemical processes, and within a natural or designed system, the transfer of energy drives the motion and/or cycling of matter. Through this investigation, students will observe that despite the transformation of ingredients and the energy transfer during baking, the total mass remains constant, illustrating the conservation of matter.
1. The following investigation is a sample investigation tightly aligned to the NGSS standards with sample materials, procedures, and anticipated student answers provided. All investigations are inquiry-based, so teachers guide students through differentiated science inquiry events within their comfort level.
2. A set of suggested procedures is given in the Student Journal. These procedures are to be used as an example. You may choose to guide the students in planning their own investigation by going through each of the suggested 10 steps before distributing the Student Journal, or you may have the students plan their investigations using the Student Journal as a guide. Differentiation Points
● The investigation is written to encourage the students to plan and implement their own investigation with your guidance. You can provide appropriate grouping/differentiated inquiry with the following scaffolding suggestions:
○ Group students who need more guided practice together and spend more time with them to develop their procedure. Let the other groups work more independently.
○ Group students with mixed needs and have them work together to develop a procedure. Monitor all groups equally.
1. What do we know about the arrangement of atoms during a chemical reaction? Atoms are rearranged during the reaction, but no atoms are either gained or lost.
2. We want to compare the results of a chemical reaction in a closed system with those in an open system. A glow stick is an example of a closed system. What do you think it means to have a closed system? With a glow stick, the chemical reaction takes place inside the stick. A closed system is one in which the reaction is isolated from the surrounding environment.
Guide student inquiry with questions like:
“What do you want to keep the same in both systems so the comparison is fair?”
“How could we make sure we capture all products?”

3. What do you think an open system is? An open system is one where the reaction is not fully enclosed but is open to the surrounding environment.
1. Why was a change in mass observed during the reaction in the open system, but not in the closed system? In the closed system, all of the products of the reaction were measured. In the open system, the gas product escaped.
2. Was the change in mass a result of a physical process or a chemical process? Although the gas was produced due to a chemical process (reaction), the change in mass was a physical process because the mass of a substance is considered a physical property.
3. Did conservation of mass occur during both reactions? Yes. We just could not observe and measure the mass of all of the products in the open system.
4. Is a closed system always required to measure conservation of mass? No, only when a gas is involved in the reaction. When all of the reactants are solids and liquids, the mass will be contained in an open system.
5. How does this investigation show that mass is conserved in both physical and chemical processes? By determining the mass of the reactants and the mass of the products of a chemical reaction in a closed system, it can be observed that the physical property of mass stays the same so mass is conserved. Observing the chemical changes that occur during the chemical process of the reaction shows that, even though new products were made in the form of a gas, the mass did not change and so mass was conserved.
FACILITATION TIP
Promote metacognition by asking:
“How did you know whether your procedure gave reliable data?”
6. You used either a digital scale or a triple beam balance in this investigation. Which of these methods of collecting data do you think is the most accurate? Explain your thinking. The triple beam balance is probably less accurate due to human error of moving the riders back and forth. The digital scale is more accurate because no human error is involved unless someone fails to zero the balance before weighing the flask.
When using any form of chemicals, it is safest for students to protect their eyes by wearing goggles.
Students may find the materials in this investigation tempting to use inappropriately, including vinegar, baking soda, and balloons. Create a nonverbal signal with students to let them know when they are not behaving appropriately. Strategically create groups that will limit distraction and include a positive peer model. Provide positive reinforcement when students are working correctly. Find more strategies for impulsive behavior in the Intervention Toolbox.
Notes
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them. After students complete the different parts of the Explore activity, students can play a game that will encourage them to put their heads together.
1. Divide students into groups of equal numbers and number off one to four.
2. Give out cards containing a different question on each one (eight or fewer). Give each group one card.
3. Have students in each group put their heads together to identify how to answer their question. All students are responsible for knowing and explaining the information.
4. Choose a number at random. A game spinner is recommended.
5. Call on the number that the spinner indicates. For example, if the spinner lands on number 2, all the students in the class who counted off two must present the answer to their question.
6. Have each group help their selected group member prepare to present his or her answers.
7. Instruct students with that number to raise their hand and call on one of them to present his or her answer. Possible questions are as follows:
● Level 1 Knowledge Question: Define the conservation of mass.
● Level 2 Comprehension Question: How would you contrast open and closed systems?
● Level 3 Application Question: How does your body represent an open system?
● Level 4 Analysis Question: What is the difference between ending mass in an open system and in a closed system?
● Level 5 Synthesis Question: What elements would you need to design a reaction in a closed system?
● Level 6 Evaluation Question: Based on what you know, how would you explain why no mass is lost in a closed system?
Phenomenon Connection
When baking a cake, what happens to the total weight of the cake from mixing the ingredients to the final baked product, and how does this relate to the conservation of mass in both open and closed systems?
1. How does the concept of a closed system apply to the baking process, and what might be the challenges in ensuring all mass is accounted for in a realworld scenario like baking a cake?
2. In what ways does the baking of a cake resemble the chemical reaction observed in the investigation, and how does this help us understand the conservation of mass?
3. Considering the investigation’s results, what factors could lead to a perceived loss of mass in the cake after baking, and how can we determine if mass is truly conserved?

Estimated 30 min - 45 min
Students develop models with mass units and a double pan balance to describe unobservable mechanisms such as conservation of mass.
Materials
Printed
● 1 Student Journal: Keeping the Balance (per student, group, or class)
Reusable
● 1 double pan balance (per group)
● 25 paper clips or mass units (per group)
Consumable
● 1 Lab Journal (per student)
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
● Although the lab calls for paper clips, you may use math manipulatives for mass units that represent the atoms of the chemical equations (such as counting bears or any other units that are of equal mass). Test the mass units on the double pan balance prior to implementing the activity to make sure that the balance is sensitive enough to detect the addition/subtraction of a single mass unit. Some double pan balances are not sensitive enough to detect gram cubes and/or paper clips.
● Before starting the activity, tell students that each mass unit represents one atom. In this activity, colors do not represent a specific type of atom. If your mass units are different colors, be sure students understand to disregard colors.
Developing and Using Models
Planning and Carrying Out Investigations
Using Mathematics and Computational Thinking
During this activity, students will develop and use models to describe and predict the phenomenon of mass conservation in a chemical reaction, such as when mixing and baking a cake. They will evaluate the limitations of their models and modify them based on evidence to match changes in the system, using a double pan balance and mass units to represent atoms. This hands-on experience will help them understand that the total mass remains constant, even as ingredients are combined and baked, aligning with the law of conservation of mass.
Energy and matter: Flows, cycles, and conservation
During this activity, students will develop models to understand the conservation of mass in chemical reactions, illustrating how matter is conserved because atoms are conserved in physical and chemical processes. This aligns with the CCC statement on Energy and Matter, as students will explore how the transfer of energy during baking drives the motion and cycling of matter, while tracking energy flow through the system.
1. Have students work in groups of four to complete this task.
2. After discussing Post-Activity Discussion question 4, introduce the law of conservation of mass and have students write it in their lab journals.
○ Law of conservation of mass: During a chemical reaction, mass cannot be destroyed or created in a closed system.
Differentiation Points
● Group students who need more guided practice together and spend more time with them. Let the other groups work more independently.
● Group students with mixed needs and have them work together. Monitor all groups equally.
Pre-Activity Discussion
Show students the chemical equation in words and symbols for the reaction between hydrogen and oxygen gas to form water:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
1. We know that chemical reactions can be expressed as equations. What do you think the H₂ stands for in the lower equation? Two hydrogen atoms (which is a molecule of hydrogen gas)
2. What do you think the O₂ stands for? A molecule of oxygen gas
3. What does the 2H₂O stand for? Two molecules of water
4. How do you think we can tell from looking at the equation that mass is conserved during this reaction? The number of each kind of atom in all of the reactants equals the number of each kind of atom in all of the products.
5. What do you think it means when we say that an equation is balanced? The number of atoms for the reactants shown in the equation equals the number of atoms shown for the products, just like the mass of the reactants measured on a physical balance equals the mass of the products.
Post-Activity Discussion
For future content reference, have students record their answers in complete sentences in their lab journals throughout the discussion.
1. What does a balanced equation tell us about the atoms in the reactants and products of a chemical reaction? The number of each kind of atom in the reactants equals the number of each kind of atom in the products. No new atoms are added, and no atoms are lost; the atoms are simply rearranged.
2. If you were to weigh all of the atoms in the reactants of a chemical reaction and then weigh all of the atoms in the products of the reaction, what would you expect to find? They would weigh the same.
3. How is a chemical equation related to the mass of the substances in a chemical reaction? In a chemical reaction, the total mass of the reactants and products does not change. The chemical equation shows exactly how mass is conserved for each element in the reaction.
Keep this balanced equation posted throughout the activity to refer to as students complete the activity.
Compare balancing equations to balancing a see-saw or a recipe (same ingredients in, same amount out).

4. Is mass always conserved in a chemical reaction? Yes, because the number (and mass) of the individual atoms is always conserved
5. Which of the given equations is not a valid chemical reaction as it does not follow the law of conservation of mass? The third equation, as there is an unequal number of atoms on each side of the equation
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
While giving students time to explore with their group through research, have them play Tell Someone. Provide students with materials as stated in the teacher directions and inform them of their task. Students should use the sentence stems below to communicate with their partners about the project.
Provide a sheet of paper with the following sentence stems:
● We could use ________ and ________ to explain our answer.
● ________ is an example of conservation of mass because ________.
● I agree because ________.
When they are finished, allow ELP students to finish the activity with their group.
When baking a cake, how does the total weight of the cake ingredients before baking compare to the weight of the cake after it is baked?
1. How can we use the concept of conservation of mass to explain the change in weight of a cake before and after baking?
2. If some ingredients in the cake batter evaporate during baking, how does this affect the total mass of the cake?
3. In what ways does the process of baking a cake illustrate the rearrangement of atoms, similar to a chemical reaction?
Notes

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Welder
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - It Is a Balancing Act
A reading passage about the concept, which includes five to eight comprehension questions.
PhET: Simulation Practice
Student activities using the PhET Interactive Simulations from the University of Colorado Boulder.
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
PhET: Simulation Practice
Student activities using the PhET Interactive Simulations from the University of Colorado Boulder.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
Student Learning Objectives What Prompts Will Be Used?
According to the law of conservation of matter, matter is neither created nor destroyed in any ordinary chemical reaction. Therefore, the mass of substances produced (products) by a chemical reaction is always equal to the mass of the reacting substances (reactants).
A chemical equation is written by placing the reactants on the left side, the products on the right side, and a reaction arrow in the middle between the reactant side and the product side. Due to the law of conservation of mass, the same type and number of elements must be present in the reactants and products of a chemical reaction.
To balance a chemical equation, count the number of atoms of each element on the reactant side and compare that to the number of atoms of the same elements on the product side of the reaction. Use coefficients in front of the chemical formulas of the reactants or products to make the number of atoms on each side of the arrow equal.
Does Student Mastery Look Like?

Student Expectations
The student is expected to demonstrate an understanding of how complex changes in the movement and patterns of air and water molecules caused by the Sun, winds, landforms, ocean temperatures, and currents in the atmosphere are major determinants of local and global weather patterns.
Student Wondering of Phenomenon
How does the Sun’s energy create the weather we see every day?
Key Concepts
• The unequal heating of Earth’s surface and the rotation of Earth create patterns of atmospheric and oceanic circulation that determine regional climates.
• Unequal heating of land and water surfaces and Earth’s rotation form large global wind systems and bring severe weather events such as thunderstorms and tornadoes.
• Ocean currents are important in regulating weather patterns around the globe.
• Climate zones have different characteristics based on latitude, elevation, and proximity to water.
• We use the information on weather maps to make predictions about weather changes.
• Severe weather events result from the combination of low pressure systems, high winds, and rising moist air. Tornadoes and hurricanes can occur when those stormy air masses spin.
This unit develops students’ understanding of how energy from the Sun drives the movement of air and water, shaping local and global weather patterns. Through investigations and data analysis, students connect heat transfer, condensation, convection, air masses, winds, and ocean currents—along with landforms and elevation—to changes in temperature, moisture, and precipitation. Students interpret and compare maps, build and revise models of interacting systems (including unobservable variables), and apply probabilistic reasoning with real-world datasets to explain and predict weather while distinguishing short-term weather from long-term climate patterns.
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Air Mass
A body of air extending over a large area (1,000 miles or more) that develops and retains specific characteristics of pressure, temperature, and humidity
Catastrophic Events
Extreme weather events such as floods, hurricanes, and tornadoes; classified by the extent and intensity of their impact on the ecosystem
Global Winds
Winds that act as a heat transfer system due to the unequal heating of Earth’s surface
Hazardous Weather
Severe or dangerous weather phenomena that threaten life and property
High-Pressure Air Mass
An air mass with greater atmospheric pressure than the surrounding air masses; air moves away from the center of the high pressure, traveling in a clockwise direction in the northern hemisphere and a counterclockwise direction in the southern hemisphere
Jet Stream
A narrow zone of strong winds in the upper level of the atmosphere
Low-Pressure Air Mass
An air mass with less atmospheric pressure than the surrounding air masses; air moves toward the area of low pressure, traveling in a counterclockwise direction in the northern hemisphere and a clockwise direction in the southern hemisphere
Ocean Currents
Directional movements of ocean water; surface currents result from steady winds over the ocean surface; deep currents result from density variations due to temperature and salinity differences
Radar
A system for bouncing electromagnetic waves off objects to locate them; used to track weather patterns
Weather Pattern
Similar weather for a certain number of days
Wind
A natural movement of air, sometimes with considerable force, from an area of high density and pressure to an area of low density and pressure
Students investigate condensation and heat transfer using an ice-water setup and connect their observations to ocean influences on local climate.
• Use a metal can with ice and water and a thermometer to cool the container and nearby air.
• Observe the drop in air temperature near the can and the formation of condensation on the outside.
• Explain results through thermal energy transfer and reduced moisture-holding capacity of cooled air.
• Relate the model to how cold and warm ocean currents affect adjacent land and air temperatures.
Students investigate the distinction between weather and climate and identify global patterns that influence them.
• Analyze weather photos in groups, describing observed conditions and discussing whether they represent weather or climate.
• Record observations in a student journal and individually respond to guiding questions about a selected image.
• Sort “Weather or Climate?” situation cards by identifying time-scale keywords and justifying placement on a T-chart.
• Compare maps of global winds and ocean surface currents to identify large-scale patterns related to climate.
Students investigate how air masses and modeling support weather prediction and apply probabilistic reasoning to forecast outcomes.
• Analyze weather maps to track fronts, collect data, and predict a week of changing conditions using cause-and-effect relationships among air masses.
• Develop and revise models over several days to represent interactions (including unobservable variables) and connect convection and ocean currents to local weather and climate.
• Research severe weather modeling and use spaghetti-model outputs to calculate probabilities (e.g., hurricane landfall) and answer data-driven questions.
Students investigate how different maps and landforms inform weather predictions.
• Research and compare radar, satellite, and weather maps for a local area, analyzing patterns and conditions.
• Learn basics of topographic maps (elevation, contour spacing) and connect elevation to weather impacts.
• Examine how mountain ranges and prevailing winds create effects like rain shadows using regional and global examples.
• Record analyses in a Student Journal and synthesize findings through class discussion.
Students investigate how wind and landmasses influence ocean surface currents through hands-on modeling.
• Use a water-filled pan with floating spices to visualize surface movement as they gently blow across the water with straws and record observations with drawings.
• Add rock/clay landmasses to observe deflection and changes in flow direction, comparing results to the initial setup.
• Launch a small foil boat to trace current pathways, complete data charts, and synthesize conclusions about wind-driven currents and landmass effects.

Estimated 15 min - 30 min
Students observe condensation and heat transfer using ice water.
Materials
Printed
● 1 Temperature Sensitive (per student)
Reusable
● 1 can, metal, empty, 10.75 oz (per group)
● 1 thermometer (per group)
Consumable
● Ice, 50 mL (per group)
● Water, room temperature, 250 mL (per group)
● Have ice available in a freezer or ice chest.
Developing and Using Models
Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict the phenomenon of how the Sun’s energy creates the weather we see every day. By observing condensation and heat transfer using ice water, students will evaluate the limitations of their models, modify them based on evidence, and represent systems and interactions such as inputs, processes, and outputs. This will help them understand the mechanistic account of natural phenomena, such as how ocean temperatures influence climate and weather patterns, similar to how the Sun’s energy affects atmospheric conditions.
Notes
Patterns
Systems and system models
During this activity, students will explore the phenomenon of how the Sun’s energy creates the weather we see every day by observing patterns in heat transfer and condensation. They will identify macroscopic patterns related to microscopic interactions, such as thermal energy transfer and condensation, to understand cause and effect relationships. By using a simple model of ice water and metal cans, students will represent systems and their interactions, recognizing how energy flows within these systems and how they can influence larger systems like weather and climate.
1. Begin the activity with a discussion:
○ How do you think the ocean can influence the weather or climate of an area? Accept all answers for now.
○ What do you think happens to warm air if it is suddenly cooled? Accept all answers for now.
2. Have students gather their materials.
3. Ask students to follow the directions on the Temperature Sensitive document to complete the activity.
4. When student groups are finished, discuss the results:
○ What two effects did you observe when the ice cubes suddenly cooled the water? The temperature of the air close to the container dropped, and condensation formed on the outside of the container.
○ What caused the air adjacent to the container to cool down? Thermal energy transfer from the warm air to the now-cold water caused the air to cool off.
○ What caused the condensation? When the warm air suddenly cooled, it could no longer hold the moisture it contained, and it condensed on the outside of the container.
○ What does this experiment have to do with the ocean and climate? Accept all ideas. The temperature of the ocean water can affect the temperature of a landmass and air in the same way as the ice in the container. For example, a cold water current, such as the one that flows past San Francisco, cools the adjacent land and air, giving it an average summer temperature of 67°F. A warmer water current, such as the one that flows past New York City, warms the land and air, giving it an average summer temperature of 84°F. This causes the two coastal cities to have two very different climates. San Francisco is also known for its fog (condensation), while New York City is known for its humidity in the summertime (the warmer air holding more moisture).
Phenomenon Connection
How does the transfer of thermal energy from the Sun influence the weather patterns we observe daily?
1. How does the process of condensation observed in the activity relate to the formation of clouds and precipitation in the atmosphere?
2. In what ways does the Sun’s energy drive the movement of air masses and ocean currents, and how does this affect local weather conditions?
3. How can understanding the principles of heat transfer and condensation help us predict and prepare for weather changes in different regions?
Notes
FACILITATION TIP
Use a think-pair-share strategy so students can discuss their ideas with a partner before sharing with the class.
FACILITATION TIP
Ask students to note how quickly condensation forms and where it forms on the container.
FACILITATION TIP
Use a class diagram or chart to show the relationship between water temperature, air temperature, and condensation for visual learners.

Estimated 30 min - 45 min
In this activity, students analyze and interpret information to help them differentiate between weather and climate. Students also compare maps of the global winds and global ocean surface currents to identify patterns.
Materials
Printed
● 1 Weather Picture Cards, set (per group)
● 1 Weather or Climate? Situation Cards, set (per student)
● 1 Student Guide (per student)
● 1 Student Journal (per student, group, or class set)
Reusable
● 1 scissors (per student)
● 1 glue stick or bottle (per student)
Consumable
● 1 Lab Journal (per student)
SEP Connection
Developing and Using Models
Preparation
● Copy Weather Photos in color and laminate for durability.
● Copy Weather or Climate? Situation Cards, one set per group.
● Copy the Student Journal pages. Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict the phenomenon of how the Sun’s energy creates the weather we see every day. By analyzing and interpreting information to differentiate between weather and climate, students will evaluate the limitations of models and modify them based on evidence to match changes in variables or components of the system. They will use models to represent systems and interactions, such as inputs, processes, and outputs, and energy and matter flows within systems, providing a mechanistic account of the natural phenomenon of weather formation. Additionally, students will obtain, evaluate, and communicate information to describe patterns and evidence about the natural world, integrating scientific information from multiple sources to clarify claims and findings.
Notes
CCC Connection
Patterns
Systems and system models
During this activity, students will analyze and interpret data to identify patterns in weather and climate, recognizing how the Sun’s energy influences these systems. They will use models to understand the interactions between atmospheric and oceanic systems, identifying cause and effect relationships and recognizing the limitations of models in representing complex systems.
Students work in groups to study the weather and climate information throughout this lesson, guided by the discussion questions. Weather describes the daily atmospheric and environmental conditions in a specific location. (Key words: today, tomorrow, last week, current, last month, May 5th, an hour ago, etc.) Climate is the average weather conditions of a region over a longer period of time. (Key words: average, typical, usually, year, etc.)
Pre-Activity Discussion
1. How does climate differ from weather? The difference between weather and climate is usually described as a measure of time. Atmospheric conditions (temperature, humidity, air pressure, wind speed) over a short period of time (minutes to months) are considered weather conditions for a local area. Climate conditions are more general and describe typical conditions for a given area over relatively long periods of time for a larger region.
Part I: Weather and Climate Photos
1. Have students observe and describe the environments in the pictures in detail.
2. Ask students to record their information in the table in Part I on the Student Journal page.
3. Help them to identify specific features and weather conditions, such as sunny skies, green grass, snow, etc.
4. Pick one environment and discuss the guiding questions as a class:
○ Does the weather you see in the picture seem normal for the environment?
○ What is weather usually like there?
○ Is the picture an example of weather or climate?
5. Have students individually select one picture and answer the questions at the bottom of the Student Journal page.
Part II: Weather or Climate?
1. Have groups select and cut out one situation card at a time. Cards can be used in any order.
2. Ask students to identify and circle the key words in each phrase. Teamwork is important to debate, discuss, and identify the key words.
3. Have students glue the cards on the appropriate side of the T chart on the Student Journal page.
4. Check for accuracy and clear up any misconceptions.
Post-Activity Discussion
1. What climate zones can you name and what are the typical weather conditions that are associated with it?
(Students may be familiar with all or some of the following:)
Polar and Tundra - long, dark winters that are cold and dry
Boreal Forest - temperatures fall below freezing for 4–6 months a year in these cold coniferous forests
Mountain - temperatures decreases with altitude increases, usually wetter and windier than lowland areas
FACILITATION TIP
Pick one photo and demonstrate how to identify key weather and environmental features before letting students do it individually. Use descriptive vocabulary for weather conditions: temperature, wind, precipitation, cloud cover, etc.
FACILITATION TIP
Use a world map or climate map to visually locate the zones being discussed.

Temperate Forest - year-round rain or snow with warm summers and cool winters
Mediterranean - cool, wet winters and hot, dry summers
Desert - hot and dry year-round
Dry Grassland - little rainfall, cold winters and hot summers
Tropical grassland - distinct wet season and dry season, and hot year-round
Tropical rainforest - wet and hot year-round
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
● Have students think about the activity to learn about the difference between weather and climate.
● Ask them to jot down some examples of the difference in these terms.
● Remind them that anytime they hear someone talking about climate, they should consider whether the person is using the word correctly.
● Now ask students to get with a partner to discuss their examples.
● Instruct students to challenge their partners by asking the following question: “How does your example demonstrate ______________?”
How does the Sun’s energy influence the weather patterns we observe daily, and how can we differentiate these patterns from long-term climate trends?
1. How do global wind patterns and ocean currents, driven by the Sun’s energy, affect the weather in different regions?
2. In what ways can we observe the immediate effects of the Sun’s energy on daily weather conditions, and how do these differ from the long-term effects on climate?
3. How can understanding the difference between weather and climate help us predict and prepare for changes in our environment?

Estimated 3 - 5 days
In Part I, students use a weather map model to collect data that provides evidence for how motions and complex interactions of air masses result in changing weather conditions and how cause-and-effect relationships can be used to predict phenomena in natural systems.
In Part II, students develop and revise the model to show relationships among variables over a five-day period, including those that are not observable but predict observable phenomena such as weather and climate conditions at a particular location.
In Part III, students research the use of computer-generated weather models (thunderstorms, hurricanes, and tornadoes) based on a complex range of interacting air masses, high- and low-pressure systems, and frontal boundaries and use collected data from a spaghetti model to produce statistical data as the basis for evidence to answer scientific questions, such as the probability that a hurricane will make landfall at a specific location.
Materials
Printed
● 1 Student Journal: Predicting Weather (per student, group or class)
● 1 Weather Map for Omaha (per group)
● 1 Ocean Currents Maps and Ocean Surface Currents Diagram (per group)
● 1 Ocean Surface Currents Diagram (per student)
● 1 Hurricane Spaghetti Model (per student)
Reusable
● 1 pencil, colored, set (per student)
● 1 ruler, metric (per student)
● 1 pencil, colored, blue (per student)
● 1 pencil, colored, red (per student)
● 1 scissors (per group)
● 1 glue, stick (per group)
● 1 calculator (per student)
● 1 Internet device (per student)
Consumable
● 1 lab journal (per student)
Part I
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online. Print one copy of the Weather Map for Omaha per pair of students.
● Cut the two weather maps apart and give each student one weather map. It is very important that, when printed, the map KEY cm scale is exactly equal to 1 cm as measured using classroom metric rulers. Do not resize the maps or the scale of the activity will change and will not match sample answers.
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online. Print two Ocean Surface Currents Maps and Ocean Surface Currents Diagram in color for each group. Print one Convection Currents for each student.
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online. Print one Hurricane Spaghetti Model for each student.
Developing and Using
Obtaining, Evaluating, and Communicating
During this activity, students will develop and use models to describe and predict the phenomenon of how the Sun’s energy creates the weather we see every day. They will evaluate the limitations of weather map models, modify these models based on evidence to reflect changes in variables such as air masses and pressure systems, and use these models to represent systems and interactions, including energy and matter flows within weather systems. Through this process, students will critically obtain, evaluate, and communicate information to describe patterns and evidence about natural weather phenomena.
During this activity, students will use weather map models to identify patterns in the movement of air masses and predict weather changes, thereby understanding the cause-andeffect relationships in natural systems. They will also explore how the Sun’s energy drives these patterns and interactions, allowing them to recognize the interconnectedness of systems and system models, and how these models can be used to represent and predict weather phenomena.
1. Have students complete this activity as a group or as individuals. Instruct them to use a ruler, map scale, and their knowledge of fronts to track the path of fronts and predict the weather for one week.
2. Begin with a discussion:
○ Meteorologists are scientists who study weather patterns and make forecasts about upcoming weather. What would a meteorologist analyze when predicting weather? The movement of fronts
○ The meteorologist is predicting cooler weather tomorrow. What must be happening to support that prediction? A cold front must be near the area and likely to move in our direction.
○ Why do meteorologists track weather over long periods of time? They track and collect weather data so they can make predictions about the weather before it happens.
○ Why are meteorologists' predictions sometimes incorrect? They are predictions based on patterns observed over long periods of time. Sometimes things change due to unforeseen events. Weather is affected by so many factors that it is impossible to accurately predict every time.
3. At this point, pass out the weather maps to the students. If you want students in groups, place them in groups now.
4. Go over the chart and discuss with students the information you are looking for. They will predict the temperature and precipitation symbols based on the movement of the fronts.
5. Discuss with students what probability means and have them list the probability of rain as high or low. As a way to double-check math, have students record the number of miles the fronts move each day. The map as printed should be used for Monday.
Walk students through one example of tracking a front and predicting temperature/precipitation before independent work.

6. An optional ending to this activity is to project the weather map on the board and have students come to the board and act the part of meteorologists to share their predictions and path of the fronts.
7. For future content reference, have students record their answers in complete sentences in their lab journals throughout the discussion.
○ Why did the weather conditions change for Omaha? The movement of warm and cold air masses along frontal boundaries caused Omaha’s weather conditions to change.
○ What cause-and-effect relationship allowed you to predict the weather for Omaha? The movement of warm or cold fronts into an area results in typical changes in weather conditions. The effect of a warm front moving into an area is typically an increase in temperature and moisture in the air with cloud cover. The effect of a cold front moving into an area is typically a decrease in temperature and moisture in the air with clear skies. The typical cause-and-effect relationship allows me to make a prediction.
○ What data was most important in making the prediction? Answers may vary, such as the rate of movement of the air masses or fronts, the air temperature on each side of the front, and the moisture level (rain or no rain) behind the front.
1. Have students work individually for this task. Allow students to discuss content with their groups if appropriate.
2. Begin with a discussion:
○ How does air move from Earth’s surface into the atmosphere and back? Convection currents caused by the Sun heating the air at the surface and the air rising and cooling in the atmosphere
○ What causes deep ocean currents? Convection currents from the sinking colder and denser melted sea ice is pushed along by a current of warmer water filling in the space behind the sinking water.
○ What is convection? The transfer of energy in fluids that occurs when thermal-energy-heated, less dense fluid rises in the fluid column away from the heat source. The thermal energy transfers from the warmer fluid to the surrounding cooler fluid. Fluid that is cool has less thermal energy and is denser. Fluid that has transferred its thermal energy sinks in the column back toward the heat source where the fluid is reheated.
3. Have students complete Part II of the Student Journal using the Ocean Surface Current Map and the Deep Ocean Current Map.
4. Have students select five locations to research and describe the weather conditions and climate as it relates to convection.
FACILITATION TIP
Ask students to explain why equatorial and polar regions are key to deep ocean currents.
5. For future content reference, have students record their answers in complete sentences in their lab journals throughout the discussion.
○ Where did the changes in deep ocean current temperatures take place? At the equator and near the North Pole
○ What do the two types of currents have in common? Both are convection currents.
○ How is the thermal energy absorbed in the ocean along the equator distributed around the globe? The deep and surface ocean currents work as convection cells or currents to transfer thermal energy throughout the globe.
1. Have students work individually on this activity, but allow them to discuss the procedures with their group.
2. Begin with a discussion:
○ What tools do meteorologists use to predict the weather? Data collected from weather stations, historical weather data, weather maps, patterns of atmospheric movements
○ The interactions of air pressure, winds, humidity, and temperature are very complex. How does a meteorologist blend all of these factors to make a prediction? Accept all ideas. Lead students to the idea of computer models.
○ If more than one model is used, would you expect all of the results to be the same? Accept all ideas.
3. For Part A, allow students to research severe weather and how it can be predicted. Have students summarize this information and compare each in their lab journals.
4. Have students predict the probability of landfall for a hurricane based on a computer model.
5. Instruct students to complete Part III of the Student Journal.
6. For future content reference, have students record their answers in complete sentences in their lab journals throughout the discussion.
○ How would you determine the probability of the storm first striking land in Louisiana? Fourteen models predict different possible pathways of the storm. Three of the models predict the storm will strike Louisiana. 3/14 = .21; according to the models, there is a 21% chance the storm will strike Louisiana.
○ Why can weather be predicted only probabilistically? Meteorologists use multiple computer models to predict the weather. Atmospheric conditions are complex and affected by changes in air pressure, winds, surface and air temperatures, movement of air masses, and the amount of moisture in the air. Different models focus on different aspects of possible atmospheric conditions. An average of the predictions is used to determine the probability of occurrence of predicted weather.
Students may need assistance completing this research project. Provide a list of websites for reference for students who struggle reading through large pieces of information and distinguishing between good and poor sources of information. Allow students extra time to complete this research project since it could require time to complete outside the classroom. In addition, provide a writing graphic organizer for students to utilize when organizing their thoughts before writing. Learn more strategies for helping students conduct research in the Intervention Toolbox.
Notes
FACILITATION TIP
Encourage students to research multiple reliable sources (NOAA, National Hurricane Center) and summarize concisely.

Guess Your Corner
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
● After the students have had time to explore the investigation, it is time to play a game called Guess Your Corner. Place one sign with one of the following terms in each corner of the classroom:
○ Weather
○ Atmospheric movement
○ Air pressure
○ Barometer
○ Humidity
○ Air masses
○ High-pressure air mass
○ Low-pressure air mass
○ Warm front
○ Cold front
○ Weather map
● Choose one student to be blindfolded.
● Using a timer, give your students five seconds to go stand in one of the corners.
● Have the student who is blindfolded describe one of the vocabulary words out loud. For example, “This term represents an air mass with greater atmospheric pressure than the surrounding air masses.”
● Instruct any students standing in the corner with the vocabulary word described to sit down.
● Give the students another five seconds with the option to move to another corner.
● Continue playing until everyone is seated.
How does the Sun’s energy drive the movement of air masses and ocean currents to create the weather patterns we observe daily?
1. How do the interactions between warm and cold air masses, influenced by the Sun’s energy, lead to changes in weather conditions?
2. In what ways do convection currents in the atmosphere and oceans, powered by solar energy, contribute to the formation of weather phenomena like hurricanes and tornadoes?
3. How can understanding the role of the Sun’s energy in weather systems help meteorologists improve the accuracy of their weather predictions?

Estimated 2 hrs - 3 hrs
In this activity, students analyze and interpret information from radar maps, satellite maps, and weather maps to help them predict weather patterns and conditions. Students also learn about how meteorologists use topographic maps to help them predict the weather.
Materials
Printed
● 1 Student Journal (per student)
● 1 Map of Oregon (per group)
● 1 Topographic Map (per class)
Reusable
● Internet access (per group)
● 1 large United States map (per class)
● 1 computer with projector (per class)
● Copy the Student Journal pages. Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
● Copy the Map of Oregon for each group. They will be writing on this document.
Developing and Using Models
Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict the phenomenon of how the Sun’s energy creates the weather we see every day. By analyzing and interpreting information from radar, satellite, and weather maps, students will evaluate the limitations of these models and modify them based on evidence to understand the impact of variables such as topography on weather patterns. This process will help them represent systems and interactions, including energy and matter flows, and provide a mechanistic account of natural phenomena, enhancing their ability to communicate scientific information effectively.
Notes
Patterns
Systems and system models
During this activity, students will analyze and interpret various types of maps to identify patterns in weather conditions and understand how the Sun’s energy influences these patterns. By examining radar, satellite, and topographic maps, students will explore the interactions within weather systems and recognize the cause-and-effect relationships that contribute to daily weather phenomena. Through this process, they will gain insights into how landforms and elevation impact local weather, using models to represent these complex systems and their interactions.
After students have gotten familiar with reading these types of weather maps, introduce topographic maps. Students just need to understand the basics of a topographic map. Your focus should be on the fact that these maps show us the elevation of different areas. Lastly, have students look at land elevations to see how the different elevations impact the weather in a particular area.
1. Have students work in groups to research the difference between radar, satellite, and weather maps. They will need Internet access to do this portion of the lesson.
2. Make sure students understand that weather describes the daily atmospheric and environmental conditions in a specific location. Remind students of the terms' meanings.
3. Begin with a discussion:
○ What are some types of maps meteorologists use to predict the weather? Radar, weather (temperature, snowfall, precipitation), satellite
○ What are some differences between these types of maps? Answers will vary. Accept all plausible answers.
4. Have students complete Part I of their Student Journal using the Internet.
5. Instruct students to use computers and go to the following website: Accuweather.com. Have students click on the search menu and type in the city in Mississippi where they live; for example, "Jackson, Mississippi."
6. Show the students how to switch between the three different maps—radar, satellite, and weather—by clicking on the different map tabs on the screen.
7. Ask students to analyze each of the maps and then answer the questions on their Student Journal page.
8. When students are finished, review with a class discussion:
○ Why would meteorologists want to look at so many different maps before predicting the weather? Answers will vary. Accept all plausible answers.
1. Project a copy of the topographic map on your projector and ask students the following questions:
○ What types of maps do scientists look at to determine the elevation of an area? Topographic maps
○ What can you tell by looking at this map? Answers will vary. Accept all plausible answers.
○ What does it mean when the lines are closer together? It means the area is steeper.
2. Check for accuracy and clear up any misconceptions.
3. Have students complete Part II of their Student Journal
4. Discuss how meteorologists use a topographic map when predicting the weather.
Clarify Overlaps. Guide students to think about what information each map provides uniquely and what is common among the three.
Ask, “Which map would be best to predict severe storms and why?”

1. Group students who need more guided practice together and spend more time with them. Let the other groups work more independently.
2. As you work through the pre-discussion questions, have a large United States map available for the students to look at. If you do not already have one in your room, find one on the Internet that you can project during your discussion.
○ Name some mountain ranges in the United States. Rocky Mountains, Appalachian Mountains, Cascade Mountains, Sierra Nevada
○ Seattle, Washington, is sometimes called the Rain City. Why do you think the Seattle area gets so much rain? It is close to the Pacific Ocean.
○ The major mountain range that runs through both Washington and Oregon State is the Cascades. What are the Cascades known for? Volcanoes. Mount St. Helens and Mount Rainer are both in this range.
Emphasize that topography and atmospheric circulation are stable factors affecting climate annually.
○ What effect could the mountains have on the climate in Washington and Oregon? It is colder in the upper reaches of the mountains.
3. Have students complete Part III in their Student Journal. Students need to type in each city from the chart in a google search bar to find out the yearly rainfall.
4. When students are finished, have a class discussion:
○ The Andes mountain range runs along the coast of Chile in South America. Warm, moisture-laden trade winds blow from east to west over the continent of South America. What climate conditions do you predict occur on the east side of the Andes mountain range? A wet climate because the moisture-laden air is cooled rapidly as it flows up the mountain, causing the water vapor to condense and fall as precipitation
○ What cause-and-effect relationship assists in making such a prediction? The cause-and-effect relationship allows a prediction of a wet climate on the east side of the Andes mountain range.
○ What climate conditions do you predict occur on the west side of the Andes mountain range? The west side of the mountain range will show a rain shadow and will have a dry, arid climate as the air releases all of its moisture on the windward side of the mountains.
○ There are no mountains in Mississippi. What could you predict about the climate in your state? All of the state should have about the same amount of rainfall and a similar climate.
○ How do landforms affect local weather patterns? Landforms such as mountains can affect local weather patterns by directing the moisturefilled air upward into the cooler atmosphere where the cooled water vapor condenses to form rain, creating a wet climate for one side of the mountain range. This also causes the area on the other side of the mountains to receive little rainfall as the air that flows over the mountains has released its moisture. The direction of wind flow determines which side of the mountain range receives moisture and which side has dry conditions.
Notes
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
● After students have completed the activity, pass out blank pieces of notebook paper.
● Have students write a question, draw a picture representing a concept they feel comfortable with, or write a sentence about a concept they feel comfortable with.
● Then have students crumple up their notebook paper.
● Before allowing students to toss their papers, go over rules for the game and demonstrate appropriate throwing of the paper.
● When you say, “Snowball fight!” have students toss their paper and others that are flying toward them around the room.
● When you say, “Stop!” have students pick up a paper near them and check to make sure it is not their paper.
● Ask students to sit in small groups and discuss the information that is on their paper. Students may answer the question or talk about the concept shown in the picture or written about in the sentence.
How does the Sun’s energy interact with Earth’s surface features to influence the weather patterns we observe daily?
1. How do different types of maps (radar, satellite, weather, topographic) help meteorologists understand the influence of the Sun’s energy on weather patterns?
2. In what ways do landforms like mountains impact the distribution of the Sun’s energy and consequently affect local weather conditions?
3. How might the absence of significant landforms, such as mountains, in a region like Mississippi influence the uniformity of weather patterns and climate across the area?

Estimated 45 min - 1 hr
Students develop and use a model to determine how wind and landmasses affect ocean surface currents.
Materials
Printed
● 1 Student Journal: Ocean Surface Currents (per student)
Reusable
● 1 Aluminum pan, 9 x 13 (per group)
● 3 Rocks, various sizes (per group)
● 1 Ball of clay, 50 g (per group)
● 1 Set of colored pencils (per group)
● 1 Safety goggles (per student)
Consumable
● Water, 500 mL (per group)
● Italian spices, 5 g (per group)
● 4 Bendable straws (per group)
● 1 Sheet Aluminum foil, 15 cm x 15 cm (per group)
● Print Student Journal: Ocean Surface Currents for each student.
● Arrange all materials so that students may easily access them.
● Safety tip: Remind students to blow very gently on the water. Be careful not to blow spices in someone’s face. Be careful of spills and the slipping hazard they create.
Developing and Using Models and Obtaining, Evaluating, and Communicating Information
During this activity, students will develop and use models to describe and predict the phenomenon of how the Sun’s energy creates the weather we see every day by examining how wind and landmasses affect ocean surface currents. They will evaluate the limitations of their models, modify them based on evidence, and represent systems and interactions such as inputs, processes, and outputs, as well as energy and matter flows within these systems. Through this process, students will gain a mechanistic understanding of natural phenomena and communicate their findings effectively.
Patterns
Systems and system models
During this activity, students will develop and use models to explore how the Sun’s energy creates the weather we see every day by examining the interaction between wind, landmasses, and ocean surface currents. They will identify patterns in the movement of water and use these patterns to understand cause and effect relationships within the system. By observing how wind and landmasses affect ocean currents, students will recognize that these macroscopic patterns are related to the underlying processes driven by the Sun’s energy. Through this exploration, students will gain insight into how systems interact and how models can represent the flow of energy and matter within these systems, while acknowledging the limitations of models in capturing all aspects of the phenomenon.
1. As a class, discuss:
● What causes ocean surface currents? Answers will vary but could include wind, Earth’s rotation, and the shape of ocean basins.
● Define ocean currents: A directional movement of ocean water; surface currents result from steady winds over the ocean surface; deep currents result from density variations due to temperature and salinity differences.
● How could we test our ideas? Accept all ideas.
2. Students will work through Ocean Surface Currents in groups.
a. Plan and investigate how wind causes global ocean surface currents and how landmasses affect the direction of the flow.
b. Students should be guided into creating their testable question.
i. You can accept all ideas and begin writing them on the board.
ii. Begin to lead the students into thinking about how to frame the question about landmasses, currents, and wind.
iii. Guide them to a question like this: “What factors affect global ocean surface currents?”
c. Allow students time to come up with their hypothesis. If the wind blows on the water, then the water will move in the direction of the wind.
d. Students will follow the following procedure:
Task 1
1. Fill the container half full of water.
2. Sprinkle Italian seasoning in the water carefully so it is floating on the surface.
3. Blow gently through the straw across the surface of the water from two different corners opposite each other. Make sure you blow across, not down on, the water.
4. Make a drawing of what you observe in the diagram on the next page.
Task 2
1. Place rocks or clay landmasses in the model.
2. Repeat blowing through the straws and make observations of what happens. Make sure you blow in the same spots as before.
3. Make a drawing of what you observe in the diagram on the next page.
Notes
FACILITATION TIP
Provide partially completed diagrams or guiding questions to help them interpret water movement.
FACILITATION TIP
Prompt students to note differences between blowing from two corners—this will help them see converging currents.

FACILITATION TIP
Have studets note if the boat moves faster or slower along certain paths to help connect current speed with real-world effects.
Task 3
1. Make a small boat out of foil.
2. Launch the boat in your model with the landmasses and again blow exactly as you did in Tasks 2 and 3.
3. Make a drawing of what you observe in the diagram on the next page.
4. Students will complete their data collection charts and answer the questions after the investigation is complete.
5. When students complete their investigation, lead the class in the following discussion:
a. From this investigation, what are ocean currents? A directional movement of ocean water; surface currents result from steady winds over the ocean surface
b. How did landmasses affect the movement of water in the pan? Landforms cause the water to be deflected from its current path, causing it to form circles around the landmass.
c. What determines how ocean surface currents move? Surface currents are formed by Earth’s rotation and wind moving across the surface of the ocean. Landmasses deflect the currents and cause them to change direction.
6. Students should compare the diagrams and come up with a conclusion statement based on their results. Students should compare their lab drawings to draw a conclusion. For example: based on the observations of the lab drawings, I can conclude that the water moves in the direction of the wind and will break in opposite directions when it hits a landmass. When the wind runs along the surface of the water, the water will also flow in that direction. When the moving water hits a landmass, it will separate and run along the coastline.
How does the interaction between wind, landmasses, and ocean currents help us understand the role of the Sun’s energy in creating weather patterns?
1. How does the Sun’s energy influence the movement of wind and ocean currents, and how do these movements contribute to weather formation?
2. In what ways do landmasses alter the path of ocean currents, and how might this affect local weather conditions?
3. How can understanding the relationship between wind, ocean currents, and landmasses help us predict weather changes and patterns?
Notes

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Meteorlogist
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - Coriolis Effect
A reading passage about the concept, which includes five to eight comprehension questions.
Data Literacy
Student analyze data sets and interpret information related to the scope’s content.
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
The unequal heating of Earth’s surface and the rotation of Earth create patterns of atmospheric and oceanic circulation that determine regional climates.
Unequal heating of land and water surfaces and Earth’s rotation form large global wind systems and bring severe weather events such as thunderstorms and tornadoes.
Ocean currents are important in regulating weather patterns around the globe.
Climate zones have different characteristics based on latitude, elevation, and proximity to water.
We use the information on weather maps to make predictions about weather changes.
Severe weather events result from the combination of low pressure systems, high winds, and rising moist air. Tornadoes and hurricanes can occur when those stormy air masses spin.

Scope Overview
This unit engages students in interpreting real-world and experimental data to connect natural phenomena, human activity, and global climate change. Learners analyze visual evidence of glacier change, conduct a hands-on model of the greenhouse effect to quantify CO2–temperature relationships, and synthesize findings through collaborative research. Students communicate conclusions and construct evidence-based arguments about the drivers of climate change, considering human relevance such as sea-level rise and biodiversity impacts, and reflecting on mitigation ideas.
The student is expected to demonstrate an understanding of the relationship between natural phenomena, human activity, and global climate change by interpreting data and engaging in scientific argument.
Key Concepts
• Global climate is the range of weather that varies from region to region but is impacted by natural processes and by human activity.
• Past global climate change is evidenced in the geologic record and points to future changes.
• The greenhouse effect naturally warms Earth. Human activity increases the greenhouse effect and impacts global climate change.
• Modeling climate change helps to predict trends.
• Global climate change evidence includes sea level rise, global temperature rise, warming oceans, shrinking ice sheets, Arctic sea ice decline, glacial retreat, increased extreme weather events, and ocean acidification.
Scope Vocabulary
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Climate Change
Long-term change in the prevailing weather patterns
Climate Patterns
Any recurring characteristic of the climate in the form of patterns that can last tens of thousands of years or repeat each year; may come in the form of regular and irregular cycles
Global Warming
Continual rise in the average temperature of Earth’s climate system
Greenhouse Effect
The increase in temperature of the surface and lower atmosphere of Earth due to the trapping of heat by an accumulation of greenhouse gases
Human Activity
Things that humans do
Notes
Students analyze visual evidence of glacier change to explore the human relevance of global warming.
• Activate prior knowledge by discussing what glaciers are and why monitoring their growth or shrinkage matters.
• View selected documentary clips of glacier melt and collaborate to explain causes of accelerated melting.
• Evaluate implications for humans, including signals about warming rates, sea-level rise, and biodiversity impacts.
• Brainstorm and record potential mitigation actions in lab journals, then share with peers.
Scientific Investigation - Greenhouse Gases
Students investigate how carbon dioxide levels influence temperature as a model of the greenhouse effect.
• Build a bottle setup that generates different amounts of carbon dioxide and expose it to a heat source.
• Measure and record temperature changes over time, then compile and graph class data to compare CO2 levels.
• Analyze results to identify the relationship between carbon dioxide concentration and temperature and connect findings to greenhouse gases in Earth’s atmosphere.
Research - Researching Global Climate Change
Students investigate the causes and effects of global climate change through collaborative research and evidence-based argumentation.
• Elicit prior knowledge and develop researchable questions about climate change.
• Collaborate in groups to research credible sources and produce a communication product to share findings.
• Present to peers, then individually craft a scientific argument on whether climate change is natural or human-accelerated, citing evidence.
• Reflect on answers found and new questions that emerged.
Notes

Estimated 15 min - 30 min
Students watch clips from a video and explore the importance of global warming for humans.
Materials
Reusable
● 1 video, Chasing Ice (per class)
Consumable
● 1 lab journal (per student)
● Chasing Ice is a documentary available for purchase or rent. This video shows images taken at regular intervals over time to show the rapid melting of multiple glaciers. Select clips from the video to show. The entire video is 75 minutes. Not all portions are appropriate for student viewing (language).
Planning and Carrying Out Investigations
Obtaining, Evaluating, and Communicating Information
During this activity, students will plan and carry out investigations to explore the phenomenon of how the melting of ice in the Arctic affects weather patterns around the world and impacts our daily lives. They will identify variables and controls, gather and evaluate data, and communicate their findings. By watching selected clips from “Chasing Ice” and engaging in discussions, students will critically analyze the evidence of glacier melting and its implications for global warming, integrating information from various sources to support their claims and proposed solutions.
Notes
Cause and effect: Mechanism and explanation
Stability and change
During this activity, students will explore the cause and effect relationships between the melting of Arctic ice and global weather patterns, recognizing that while correlation does not necessarily imply causation, understanding these relationships can help predict phenomena in natural systems. They will also examine how changes in glacier stability can lead to significant changes in global systems, considering both immediate and gradual impacts on the environment and human life.
1. Begin the activity with a discussion:
○ What are glaciers? Large accumulations of snow and ice that do not melt away in the summertime and can move
○ Why do you think it might be important to know whether glaciers are growing or shrinking? Accept all answers for now.
2. Show the class the clips from the video Chasing Ice.
3. With their group, have students discuss why the glaciers are melting at a faster rate.
4. Ask students to consider why this phenomenon is important to humans.
○ What do glaciers tell us about global warming? How fast global warming is happening and what the impact might be on sea levels
○ Why is awareness of global warming important for human beings? It will cause extinctions as the climate becomes too warm for some plants and animals to live.
How does the rapid melting of glaciers, as observed in the video, relate to the broader impacts of ice melting in the Arctic on global weather patterns and our daily lives?
1. How might the melting of Arctic ice influence weather patterns in different parts of the world?
2. In what ways could changes in sea levels, due to melting glaciers, affect human populations and ecosystems?
3. What actions can individuals and communities take to reduce the impact of global warming and slow down the melting of glaciers?
5. Challenge students to brainstorm what humans might be able to do to mitigate the effects of global warming. Tell them to make a list in their lab journal. Share as desired. Use less petroleum products; reduce waste; reduce the use of energy; walk, bicycle, or use public transportation; limit population growth; protect habitats and food sources of essential plants and animals, such as honeybees. Notes
FACILITATION TIP
You can pose questions such as “What do you notice about the ice over time?” or “How quickly is this glacier changing?”

Estimated 1 hr - 2 hrs
In this activity, students collect and interpret data related to carbon dioxide production, temperature, and the fossil fuel burning.
Materials
Printed
● 1 Student Journal (per student)
Reusable
● 2 Bi-Therm thermometers (per group)
● 1 lamp with 150-watt bulb (per group)
● 1 stopwatch (per group)
Consumable
● 2 empty plastic 2 L soda bottles
● 4 cups potting soil
● 2 paper cups
● 6 effervescent tablets
Cut off the tops of the empty 2 L bottles to create open-mouthed bottles approximately 8 inches tall.
● Cut a hole in each bottle 5 inches from the bottom to hold the thermometer.
● Fill the bottom of each plastic bottle with about 2 inches of soil.
● Insert a Bi-Therm thermometer through the holes in the bottles.
● Position the light 4 to 5 inches from the top of the bottles and equal distance from both. Fill each cup halfway with water.
● Place the cup on top of the soil.
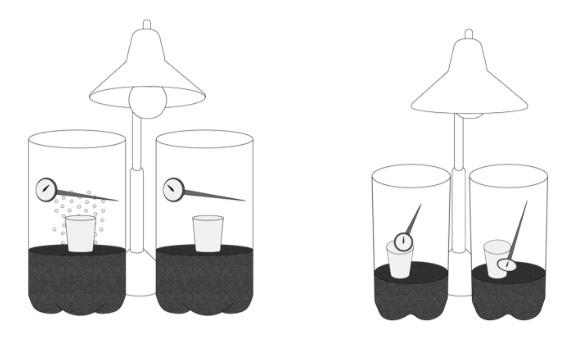
● To use fewer bottles and materials, you may choose to set up one control bottle instead of having each group set up an individual control.
● If the number of lamps is limited, two or more groups may set up bottles under a single lamp.
● Digital thermometers or probeware may be used instead of analog thermometers.
Notes
Planning and Carrying Out Investigations
Obtaining, Evaluating, and Communicating Information
During this activity, students will plan and carry out investigations by identifying independent and dependent variables, using tools to gather data, and evaluating the accuracy of their methods to understand the relationship between carbon dioxide levels and temperature changes. This investigation will help them explore how the melting of ice in the Arctic affects weather patterns globally and impacts daily life. By obtaining, evaluating, and communicating information, students will critically analyze data and integrate scientific information to describe patterns and evidence about the natural world, enhancing their understanding of the phenomenon.
Cause and effect: Mechanism and explanation
Stability and change
Procedure and Facilitation
1. This activity simulates the greenhouse effect by creating carbon dioxide inside a 2 L bottle. To compare results from varying amounts of carbon dioxide, divide students into groups and assign groups different numbers of effervescent tablets. Dividing students into six groups allows two sets of data for each of the carbon dioxide levels (one, two, and three tablets).
2. Begin the activity with a discussion:
○ Do you know what a greenhouse is? What is the purpose of a greenhouse? What is it like inside a greenhouse? A greenhouse is a glass building used to grow flowers or vegetables even in the winter. The greenhouse traps heat from the Sun and is warmer than the outdoors. It can be very warm and humid in a greenhouse.
○ How is Earth’s atmosphere like a greenhouse? Carbon dioxide and other gases can collect in the atmosphere and trap heat.
3. Have student groups work together to complete the directions listed in the Student Journal.
4. Have students share results at the end of the experiment.
During this activity, students will explore cause and effect relationships by examining how varying levels of carbon dioxide, a greenhouse gas, impact temperature, simulating the greenhouse effect. This understanding will help them predict how the melting of ice in the Arctic, which releases additional greenhouse gases, can affect weather patterns globally and impact daily life. Students will classify relationships as causal or correlational and recognize that changes in one part of a system, such as increased carbon dioxide levels, can lead to significant changes in another, such as global weather patterns, illustrating the concepts of stability and change. Notes
Prompt students to note other observations, like bubbling or condensation, as this connects the chemical reaction to gas production.

5. Review students' answers as a class and end with a discussion:
Additional facilitation questions include:
“Why did more CO₂ result in a larger temperature increase?”
“How does this relate to the greenhouse gases in Earth’s atmosphere?”
○ According to your graph of class data, what is the relationship between temperature and amount of carbon dioxide? As the amount of carbon dioxide increases, the change in temperature increases.
○ In this investigation, you created carbon dioxide using an effervescent tablet. How does carbon dioxide enter Earth’s atmosphere? Carbon dioxide is created when living things exhale and when fossil fuels are burned.
○ What are other greenhouse gases? How do these gases enter the atmosphere? Methane, nitrous oxide, and fluorinated gases are greenhouse gases. There are natural and human sources of these gases. Methane is produced naturally in wetlands and the oceans. Human activities including farming and landfills also create methane. Nitrous oxide is produced in soil naturally but also comes from human activities including agriculture and industrial processes. Fluorinated gases enter the atmosphere from products like refrigerators and air conditioners.
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
Ask students to write down three questions they have during the Explore to ask their group members afterward.
Give students the following sentence stems if needed:
● Why did ________?
● What do you think will happen if ________?
● When did ________ happen?
How does the increase in carbon dioxide and other greenhouse gases, as demonstrated in our activity, relate to the melting of ice in the Arctic and its subsequent effects on global weather patterns and our daily lives?
1. How might the increase in carbon dioxide levels, as observed in our experiment, contribute to the melting of Arctic ice?
2. In what ways could the melting of Arctic ice, influenced by greenhouse gases, alter weather patterns globally?
3. How do changes in weather patterns due to Arctic ice melt impact our daily lives, and what actions can we take to mitigate these effects?

Estimated 3 - 5 days
Students research information about the causes and effects of global climate. Students think critically by asking questions to identify or clarify evidence and/or the premise of an argument as they search for answers to the selected question.
Materials
Printed
● 1 Student Journal: Research Global Climate Change (per student, group, or class)
● 1 Lab Journal (per student)
Reusable
● 1 computer, with Internet access and presentation software (per group)
● 1 camera, video (per group)
SEP Connection
Planning and Carrying Out Investigations
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
● You may want to review the process for using different search engine tools and the procedure for verifying sources of online information if students will be using computers for research. You can also set up a web page with links for the assignment.
Obtaining, Evaluating, and Communicating Information
During this activity, students will plan and carry out investigations to explore how the melting of ice in the Arctic affects weather patterns globally and impacts daily life. They will identify variables and controls, gather and evaluate data, and communicate their findings through various media. By critically reading and synthesizing information from multiple sources, students will develop a scientific argument regarding the natural occurrence of climate change versus human acceleration, thereby deepening their understanding of the phenomenon.
CCC Connection
Cause and effect: Mechanism and explanation
Stability and change
During this activity, students will explore the phenomenon of how the melting of ice in the Arctic affects weather patterns globally and impacts daily life by examining cause and effect relationships. They will classify these relationships as causal or correlational, recognizing that correlation does not necessarily imply causation. Students will use their research to predict phenomena in natural systems, understanding that these phenomena may have multiple causes. Additionally, they will explain stability and change in these systems by examining changes over time and considering forces at different scales, recognizing that changes in one part of a system might cause significant changes in another part.
Notes
1. Have students work collaboratively to research climate change and share research with their peers. Following the presentation of information, have students work individually to write a scientific argument.
2. Start the activity with a class discussion:
a. Scientists use the practice of making observations and asking questions to study phenomena of the natural world. What do you know about the phenomena of climate change? Accept all ideas.
3. Tell students the statements are their observations of other people’s ideas about global climate change.
4. Tell students to use their observations to develop questions about global climate change.
5. Assist students in narrowing or broadening their topics to answer the questions related to the causes and effects of global climate change. Write these questions on the board for later use by students.
a. Ask students how can we find answers to our questions. Design investigations; research; talk to scientists.
6. Tell the students their challenge is to find an answer to their questions by researching what scientists have learned about global climate change and to communicate their findings. Groups should select questions from the board or through a teacher-approval process.
7. You may choose to give students a choice or assign the product. Possible choices include documentaries, informational websites, brochures, slide shows, poster presentations, newscasts, or dramatic productions.
8. Have student groups share their product with the class.
9. Following presentation of group research, have students individually engage in scientific argument based on their research and presentation to answer this question: Does climate change occur naturally, or is it being accelerated through the influence of humans?
10. End the activity with a discussion:
a. Were you able to answer your question about global climate change? Accept all answers. Most likely students were able to find some answers but now have more questions.
b. Does climate change happen naturally, or is it being accelerated through the influence of humans? Global climate change is the result of an accumulation of events, such as the release of greenhouse gases from burning fossil fuels. In terms of human life, the release has been accumulating over the last 100 years, mirroring the increase in human population and industry, resulting in an increase in the use of fossil fuels. In terms of geologic time, this event could be considered sudden, since 100 years is a relevantly insignificant amount of time.
Notes
Provide a curated list of reliable sources (websites, documentaries, articles) to guide student research and prevent misinformation. You can also partner with the school librarian to find databases for research.
Provide sentence starters or graphic organizers to help students organize research.

Students who struggle with oral articulation may have difficulty completing group work or the presentation. Assign a role specifically with the students’ strengths and abilities in mind. Some students may not be able to fulfill the group presentation portion due to anxiety when speaking in front of a large group. Therefore, allow them to create the PowerPoint slides for the presentation. If you would still like them to present, allow the presentation to be done in a small group or one-on-one. Assess students on content rather than speech quality or clarity. Learn more strategies for students who have difficulty with oral articulation in the Intervention Toolbox.
The Token Game
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After the students have experienced Explore, provide them with the following sentence stems to guide them as they play the game:
● Humans have affected the climate by ___________.
● The effects of climate change were __________.
Token Game Rules
● Group students into groups of four.
● Provide each student with two tokens of one color. You can use blue, red, green, and yellow for the group of four.
● Instruct each student to read his or her sentence stem to the group members.
● As each student shares the sentence stem, have him or her place one token in the middle of the table.
● Make sure each student has a turn and continue on to the second sentence stem until all students in the group have placed their tokens down.
● Have them record their sentence stems in their journals after the game.
How does the melting of ice in the Arctic influence global weather patterns and impact our daily lives?
1. How might the melting of Arctic ice contribute to changes in global weather patterns?
2. In what ways could the melting of Arctic ice affect ecosystems and human communities worldwide?
3. How can understanding the impact of Arctic ice melt help us develop strategies to mitigate climate change effects?

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Political Leader
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - Ice Cores and Scientific Data
A reading passage about the concept, which includes five to eight comprehension questions.
Data Literacy
Student analyze data sets and interpret information related to the scope’s content.
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
Global climate is the range of weather that varies from region to region but is impacted by natural processes and by human activity.
Past global climate change is evidenced in the geologic record and points to future changes.
The greenhouse effect naturally warms Earth. Human activity increases the greenhouse effect and impacts global climate change.
Modeling climate change helps to predict trends.
Global climate change evidence includes sea level rise, global temperature rise, warming oceans, shrinking ice sheets, Arctic sea ice decline, glacial retreat, increased extreme weather events, and ocean acidification.

Expectations
The student is expected to demonstrate an understanding that the seasons are the direct result of Earth’s tilt and the intensity of sunlight on Earth’s hemispheres by creating models.
Why do we have different seasons, and how does the tilt of the Earth affect the amount of sunlight different parts of the world receive throughout the year?
Key Concepts
• As a result of the fixed tilt of Earth’s axis, the areas of Earth’s surface exposed to the direct rays of the Sun change seasonally as Earth moves through its orbital path.
• Known as Earth’s seasons, these cycles of temperature and length of day affect many of Earth’s other cycles such as the weather and plant growth.
This unit develops conceptual understanding of seasons through data analysis and modeling. Students collect and graph daylight patterns across latitudes and months, compare hemispheres, and connect phenomena like polar day to Earth’s tilt and revolution. Using globe–lamp and flashlight investigations, they explore solar angle, direct vs. indirect sunlight, day length, and energy per area. Students synthesize findings by designing and presenting models that explain equinoxes, solstices, and cyclical seasonal changes, demonstrating that seasons result from Earth’s tilt and varying sunlight intensity on each hemisphere.
Scope Vocabulary
The terms below and their definitions can be found in Picture Vocabulary and are embedded in context throughout the scope.
Hemisphere
Half of a sphere; Earth and the celestial sphere can be divided into northern and southern or eastern and western hemispheres
Orbit
A curved path followed by a satellite as it revolves around an object
Seasons
The four natural divisions of the year based on changes in temperature due to varying amounts of sunlight received (both intensity and number of daylight hours vary); caused by the tilt of Earth during its revolution
Temperature
Average kinetic energy of all the particles in a material; measured by a thermometer in degrees (usually degrees Celsius or degrees Fahrenheit)
The slant of Earth’s axis, which is 23.5 degrees from vertical compared to Earth’s orbital plane around the Sun; results in the North Pole always pointing toward the North Star
Notes
Students investigate how daylight hours vary by location and season to understand Earth’s tilt and its effects.
• Gather and record daylight-hour data for three cities (near the North Pole, near the South Pole, and local) across January, April, July, and October.
• Compare patterns to determine which months represent summer for each location based on maximum daylight.
• Use a globe-and-lamp model to visualize Earth–Sun geometry and explain seasonal differences in daylight.
• Connect the model to phenomena such as 24-hour daylight at high latitudes during summer.
Making a Model - The Seasonal Tilt and Whirl
Students investigate how Earth’s tilt and revolution cause seasonal changes in daylight and solar angle.
• Rotate through eight globe-and-lamp stations to observe light/shadow patterns at equinoxes and solstices, recording observations at each position.
• Discuss and identify the cause of seasons, relate hemisphere tilt to seasonal patterns, and recognize the cyclical nature of these changes.
• Collaboratively design, build, and present a student-created model explaining seasons, including developing teacher-approved questions for peers.
Scientific Investigation - Intensity of Sunlight
Students explore how sunlight intensity and day length relate to seasonal patterns.
• Model direct vs. indirect sunlight using a flashlight setup to observe how angle affects energy per area and surface warming.
• Analyze daylight-hours data for cities at different latitudes using a spreadsheet to create graphs and identify patterns across the year.
• Compare hemispheres and the equator to explain opposite seasonal trends and write a conclusion about the relationship between day length and seasons.

Estimated 15 min - 30 min
Students observe and compare the daylight hours in various cities.
Materials
Printed
● 1 Daylight Hours (per student)
Reusable
● 1 computer with Internet access (per class)
● 1 projector (per class)
● 1 globe (per class)
● 1 lamp (per class)
In advance, plan which cities you will look up as a class (suggested cities: Utqiagvik, Alaska, your home city, and Vostok, Antarctica). To find the data, conduct an Internet search for “Weather Underground”; the amount of daylight can be found under the “Astronomy” tab.
Developing and Using Models
Planning and Carrying Out Investigations
Analyzing and Interpreting Data
During this activity, students will develop and use models to describe and predict the phenomenon of why we have different seasons and how the tilt of the Earth affects the amount of sunlight different parts of the world receive throughout the year. By observing and comparing daylight hours in various cities, students will evaluate limitations of their models and modify them based on evidence to match changes in Earth’s tilt and position. They will use a globe and lamp to represent systems and interactions, such as inputs, processes, and outputs, to provide a mechanistic account of this natural phenomenon. Additionally, students will plan and carry out investigations by collecting and analyzing data to support their explanations, using graphical displays to identify temporal and spatial relationships, and distinguishing between causal and correlational relationships in the data.
Notes
Cause and effect: Mechanism and explanation Stability and change
During this activity, students will use cause and effect relationships to predict the phenomenon of why we have different seasons and how the tilt of the Earth affects the amount of sunlight different parts of the world receive throughout the year. By observing and comparing daylight hours in various cities, students will classify relationships as causal, recognizing that the Earth’s tilt causes variations in sunlight, leading to seasonal changes. They will also explore how changes in one part of the Earth’s system, such as its axial tilt, can cause large changes in another part, like the distribution of sunlight, thereby explaining stability and change in natural systems.
1. Look up the number of hours of daylight for three cities for one day in January, April, July, and October.
2. Find data for a city near the North Pole, a city near the South Pole, and the city where you live.
3. Have students record the data in their data table.
4. During the summer, we get a lot more daylight. Ask the students to identify what month represents summer for each place. July has the most hours of daylight for the city near the North Pole and our city, so July must be summer for those places. The city near the South Pole must have summer in January, since that is when it gets the most daylight.
5. Use a globe and a lamp to demonstrate the position of Earth and the Sun during these months to show why certain places get more daylight than others. Based on the model, ask students why they think some places would get 24 hours of daylight. When I look at the model, I can see that some places near the North Pole are tilted far enough toward the Sun to always receive sunlight in the summer.
How does the tilt of the Earth influence the amount of daylight different parts of the world receive, and how does this relate to the changing seasons?
1. Based on your observations of daylight hours in different cities, how does the Earth’s tilt affect the amount of sunlight each location receives throughout the year?
2. How does the position of the Earth in relation to the Sun explain why some places experience 24 hours of daylight or darkness at certain times of the year?
3. Considering the data you collected, how do the changes in daylight hours impact the seasons experienced in each of the cities we studied?
FACILITATION TIP
Before researching data, ask students to predict which city will have the most daylight in each month.
FACILITATION TIP
Reinforce that latitude determines how extreme the daylight variation is. Poles have the most extreme, and the equator, the least.

Estimated 30 min - 45 min
Students discover seasons as a result of changes in the number of daylight hours and the angle of incidence of the Sun’s light rays on Earth’s surface due to the tilt of Earth on its axis and Earth’s revolution around the Sun.
Materials
Printed
● 1 Student Journal: The Seasonal Tilt and Whirl (per student)
Reusable
● 1 lamp, shadeless (per class)
● 1 light bulb, 100-watt incandescent or equivalent brightness fluorescent (per class)
● 1 cord, extension (per class)
● 1 clipboard (per student)
● 8 globes, inflatable (per class)
● 1 globe with stand (per class)
● 1 star, cutout, to represent North Star (per class)
● 8 tape, masking, rolls (per class)
Consumable
● 1 tape, masking, roll (per class)
Part I
1. Student Journals can be printed individually for student use, as a reusable class set, or assigned online.
2. Inflate eight inflatable globes.
3. Draw or print out an illustration of a star to represent the North Star. Use a tilted globe to determine where the star should be positioned on the wall to create the 23.5° tilt. (The star placed on the wall will represent where the North Star is positioned in the sky.) Place the tilted globe on the floor and estimate the ray from the axis to the wall, since this will be a floor activity. Place the North Star at that point on the wall.
4. Make large Xs on the floor with masking tape to mark the points of the spring and fall equinox and the summer and winter solstice. (Note that these equinoxes and solstices are named as they occur in the Northern Hemisphere.)
5. Mark four small Xs halfway between each of the large Xs. All marks are directly on the path that represents Earth’s revolution around the Sun. Use the diagram below to label the positions A–H.
6. Plan to darken the room and keep the main lights on until performing the activity. Plan to place one of the eight rolls of masking tape at each position to hold the inflatable globes in place. The bulb in the lamp must be at the same level as the inflatable globes for the shadows to be correct.
Developing and Using Models
Planning and Carrying Out Investigations
Analyzing and Interpreting Data
During this activity, students will develop and use models to describe and predict the phenomenon of why we have different seasons. They will evaluate the limitations of their models and modify them based on evidence to match changes in Earth’s tilt and orbit. By planning and carrying out investigations, students will identify variables and gather data to support their explanations. They will analyze and interpret data to provide evidence for the effects of Earth’s tilt on sunlight distribution, using graphical displays to identify temporal and spatial relationships. This hands-on experience will help students understand the cyclical nature of seasons and the role of Earth’s axial tilt and revolution around the Sun.
Cause and effect: Mechanism and explanation
Stability and change
During this activity, students will explore the cause and effect relationships that explain the phenomenon of Earth’s seasons. By examining how the tilt of Earth’s axis and its revolution around the Sun affect the distribution of sunlight, students will classify these relationships as causal, recognizing that the tilt causes variations in sunlight intensity and duration, leading to seasonal changes. This understanding will help them predict and explain the cyclical nature of seasons and how changes in one part of Earth’s system can lead to significant changes in another, demonstrating the principles of stability and change.
When the activity begins, explain the following to students: when directed, each student group will gather around one of the globes, make observations, and record them in their Student Journals. Tell the students to be aware of their body shadows. Explain that their focus should be on how the Sun’s light falls on the globe. They will explain what parts of Earth are in shadow based on each position during Earth’s year. Explain that they will have four minutes at each position to make and record observations. Then, on your signal, they will move counterclockwise to Earth’s next position until all groups complete the eight positions.
Students work in groups to complete this task.
Pre-Activity Discussion
Gather the students in a circle around the lamp. Explain that this will be a floor activity, and they will bring their clipboards to different positions, sit on the floor, and make observations. Show the students a classroom globe and point out the North Star that you taped to the wall. Hold the globe so that the North Pole is pointed toward the North Star. Discuss the 23.5° tilt of Earth toward the North Star.
Ask each group to place its inflated globe on the roll of masking tape on the position marked in the circular path. Then position each globe so that the North Pole points at the North Star; check the positions. Explain that the model represents Earth’s position in space relative to the Sun and the tilt of its axis during a one-year period.
1. What do you think the four large Xs represent? Accept all ideas.
2. Does this model demonstrate that the orbital path Earth takes around the Sun is nearly circular or elliptical? Nearly circular; it is a common misconception that Earth makes an exaggerated elliptical orbit.
Post-Activity Discussion
1. What does this model indicate is the cause of seasons? The tilt of Earth’s axis causes sunlight to strike Earth more intensely in one hemisphere than another depending upon where Earth is located in its orbital path around the Sun.
2. What do the locations marked with an X represent? Equinoxes and solstices. These are the times when Earth receives equal amounts of daylight and nighttime (equinoxes), as well as the start of new seasons and the days with the most or least amount of sunlight (solstices).
3. What pattern did you observe using the model? There is a pattern to the location of the direct sunlight striking Earth as Earth orbits the Sun. When the Northern Hemisphere is tilted toward the Sun and receives the most sunlight, it is summer for that hemisphere and winter for the Southern Hemisphere. When the Southern Hemisphere is tilted toward the Sun and receives the most sunlight, it is summer for that hemisphere and winter for the Northern Hemisphere.
4. Would you describe the pattern as cyclical or occasional? The pattern is cyclical since it repeats on a yearly basis as Earth orbits the Sun.
Assign groups to specific positions at the start to prevent confusion during movement between stations.
Students may need clarification on how an elliptical path is different from a circular path.
Student can include notes on their models to highlight that when it is summer in the Northern Hemisphere, it is winter in the Southern Hemisphere, and vice versa.

Divide the class into groups to brainstorm ideas about household items they could use to design and construct a model to help them explain why seasons occur. Students will get one day to develop their design ideas and one day to construct their model. On the third day, groups take turns using their models to explain seasons to their classmates.
Procedure
FACILITATION TIP
If needed, provide examples of household items that could be used, such as: Balls, balloons, or globes for Earth Lamps or flashlights for the Sun Rulers or sticks to show tilt
FACILITATION TIP
Require students to draw a diagram of their proposed model with labels for tilt, Sun, and Earth’s position.
Approve diagrams before construction to prevent misconceptions or incomplete designs.
As a group, brainstorm ideas about household items you could use to design and construct a model to help explain to your classmates why seasons occur. You have one day to develop your design ideas and one day to construct your model. On the third day, your group will use your models to explain seasons to your classmates.
You must also develop three questions to ask your classmates about the phenomena that cause seasons to occur. Your questions must be approved by your teacher.
Questions:
Teacher approval ________
Use the space below to draw a diagram of your concept design. Once complete, have your teacher approve your design before you start building your model.
Understanding seasons in relation to the tilt of Earth’s axis is a crucial concept for students to learn. There are many misconceptions that slower processing students may need to have addressed. Consider as an exit ticket, in a one-on-one setting, having students orally explain how and why seasons exist to determine if students fully mastered and understood the expectations in this activity. Read more strategies to assist students who have slow information processing in the Intervention Toolbox.
Notes
Guess the Word
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
Provide students with a set of cards that have the below terms on one side and their descriptions on the other.
○ Hemisphere: half of a ball or sphere
○ Equator: a line that circles Earth from east to west, dividing it into two hemispheres
○ Axis: an imaginary line that Earth seems to rotate around
○ Rotate: spin in place
○ Revolve: travel in a circular path
○ Year: the time it takes Earth to revolve once around the Sun
● Place students into pairs. (This could also be done in small groups or as a class activity.)
● Have students take turns getting their partner to guess a term.
● Instruct students to draw a picture or write clues, so long as they avoid writing the actual term or sharing the definition.
● Once a partner guesses the correct word, have the two students switch roles.
Phenomenon Connection
How does the tilt of Earth’s axis and its orbit around the Sun lead to the different seasons we experience, and what role does the angle of sunlight play in this process?
1. How does the tilt of Earth’s axis affect the intensity and distribution of sunlight on different parts of the Earth throughout the year?
2. In what ways do the equinoxes and solstices mark significant changes in the amount of daylight and the transition between seasons?
3. How can we use models to better understand the cyclical nature of Earth’s seasons and predict seasonal changes in different hemispheres?
Notes

Estimated 1 hr - 2 hrs
In Part I, students discover seasons as a result of changes due to differential intensity of the Sun’s light rays on Earth’s surface. In Part II, students investigate and compare cause-and-effect relationships demonstrated by patterns of daylight hours received at various latitudes during a one-year time period.
Students use digital tools (computer and spreadsheet program) to analyze patterns and trends found in the number of daylight hours at various latitudes during different seasons.
Printed
● 1 Student Journal: Intensity of Sunlight (per student)
● 1 Daylight Hours Data (per student)
Reusable
● 1 flashlight (per class)
● 1 marker, red (per class)
● 1 marker, blue (per class)
● 1 meterstick (per class)
● 1 computer with Internet access (per group)
● 1 software, spreadsheet program (per group)
● 1 ruler, metric (per student)
● 1 pencil, colored, set (per student)
● 1 calculator (per student)
Consumable
● 1 paper, graph, chart size (per class)
● Batteries (per class)
1. Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
2. Tape a chart-sized sheet of graph paper to the wall, at eye level, in a central viewing area.
3. Place a meterstick on the floor so that you can maintain a constant distance when demonstrating the two light beams.
4. Try out flashlights to find one that directs an even glow in the illuminated area and does not cast an odd shadow due to the reflector.
5. Determine how far you will stand from the wall during the demonstration.
6. Plan to darken the room.
● Tape, clear, 6 cm (per student) Notes
Developing and Using Models
Planning and Carrying Out Investigations
Analyzing and Interpreting Data
During this activity, students will develop and use models to describe and predict the phenomenon of different seasons and the effect of Earth’s tilt on sunlight distribution. By analyzing patterns of daylight hours at various latitudes, students will evaluate model limitations and modify models based on evidence to understand how changes in Earth’s tilt and position affect seasonal sunlight intensity. Through planning and carrying out investigations, students will collect and analyze data to provide evidence for the relationship between day length and seasons, ultimately developing a mechanistic account of how Earth’s tilt leads to seasonal variations in sunlight and temperature.
Cause and effect: Mechanism and explanation
Stability and change
During this activity, students will explore the cause and effect relationships between the Earth’s tilt and the varying intensity of sunlight received at different latitudes, which in turn affects the seasons. By analyzing patterns in daylight hours and using digital tools to graph these changes, students will classify these relationships as causal, understanding that the tilt of the Earth is a primary cause of seasonal changes. They will also recognize that while correlation does not imply causation, the consistent patterns observed provide strong evidence for the causal relationship between Earth’s tilt and seasonal variations.
This activity can also be done using a growing lamp with a thermometer or temperature probe to investigate how the temperature varies according to the angle of sunlight.
Pre-Activity Discussion
1. When is sunlight the most intense? Sample answers include midday or during the summer.
2. When is sunlight the least intense? Sample answers include early morning, late evening, or during the winter.
3. What causes these changes of intensity? Accept all answers.
Post-Activity Discussion
1. Which system, direct light or indirect light, provided evidence that the most energy was delivered per square? The direct light system; 4 joules per square as compared to indirect 2.4 joules per square (actual values will vary)
2. What do these squares represent on Earth? Surface area
3. Refer to the diagram on the Student Journal to discuss and answer the following:
○ Which hemisphere receives the most direct light on June 21st? What season is this hemisphere experiencing on June 21st? Northern; summer
○ Which hemisphere receives the most direct light on December 21st? What season is this hemisphere experiencing on December 21st? Southern; summer
○ Which hemisphere receives the most indirect light on June 21st? What season is this hemisphere experiencing on June 21st? Southern; winter
○ Which hemisphere receives the most indirect light on December 21st? What season is this hemisphere experiencing on December 21st? Northern; winter
As you adjust the flashlight, ask:
“What do you notice about the shape of the beam? Is the light tightly focused or spread out?”

FACILITATION TIP
Help make the connection to seasons by asking students:
“If the Sun’s rays are angled like the wide beam for months at a time, what season do you think that location is in?”
4. What causes the sunlight that obviously comes from the same source to be more or less intense? When rays are more direct, they deliver more energy to a specific surface area. When the Sun is lower in the sky, the rays strike the ground at an angle causing the energy to spread out. These rays are less direct and deliver less energy to a specific surface area.
5. How does the tilt of Earth affect the intensity of sunlight in each hemisphere? When a hemisphere is tilted toward the Sun, the sunlight is more direct and intense. When a hemisphere is tilted away from the Sun, the sunlight is indirect and less intense.
Have students complete Part I of the Student Journal.
Preparation
● Student Journals can be printed individually for student use, printed as a reusable class set, or assigned online.
● Print one Daylight Hours Data for each student.
Facilitation
The following investigation is a sample investigation tightly aligned to the Mississippi College- and Career-Readiness Standards for Science with sample materials, procedures, and anticipated student answers provided. All Investigations are inquiry-based so the teacher can guide the students through differentiated science inquiry events to their comfort level.
A set of suggested procedures is given in the Student Journal. These procedures are to be used as an example. You may choose to guide the students in planning their own investigation by going through each of the suggested 10 steps before distributing the Student Journal, or you may have the students plan their investigations using the Student Journal as a guide.
Differentiation Points
The investigation is written to encourage the students to plan and implement their own investigations with your guidance. You can provide appropriate grouping/differentiated inquiry with the following scaffolding suggestions:
● Group students who need more guided practice and spend more time with them to develop their procedure. Let the other groups work more independently.
● Group students with mixed needs and have them work together to develop a procedure. Monitor all groups equally.
Notes
Pre-Investigation Discussion
1. What causes day and night on Earth? When Earth rotates on its axis, half of Earth is illuminated by sunlight and experiences day. The dark side, in shadow, experiences night.
2. What happens in the Earth-Sun system that explains sunrise? As Earth rotates on its axis, the Sun becomes visible on the eastern horizon at sunrise.
3. And at sunset? As Earth rotates on its axis, the last part of the Sun departs from view on the western horizon at sunset.
4. During what seasons are there an equal number of hours of day and night? Spring and autumn at the equinoxes
5. In what seasons are the numbers of daylight hours more nearly equal to the number of hours when the Sun is not present? Spring and autumn Plan to discuss the 24-hour clock/military method of keeping time as this is used in the data table. Plan to discuss how to calculate total daylight, as it is not always appropriate to simply subtract one time from another. Save time by having students calculate the number of daylight hours and minutes using military time for the two rows of data for dates 2/21/2017 and 3/21/2017. Then provide the rest of the data in hours and minutes (from the second page of Student Reference Sheet: Daylight Hours Data) for students to simply copy into their chart. Student understanding that derived data provides a total number of daylight hours and minutes at specific latitudes (locations) on specific dates is foundational. Have students input the data into a spreadsheet program to create computergenerated graphs.
Have students locate the following cities and note the latitude for each on a classroom globe or map: Quito, Ecuador (0.0° latitude); West Palm Beach, Florida (26.7° North latitude); Boise, Idaho (43.6° North latitude); Johannesburg, South Africa (26.2° South latitude); and Christchurch, New Zealand (43.53° South latitude).
Have students complete all parts of their Student Journal.
Conclusion and Scientific Explanation
To complete Step 10, students write a conclusion and a scientific explanation based on the following prompt: Is there a relationship between day length and seasons?
Post-Investigation Discussion
1. What does the graphed data show for the number of daylight hours throughout the year in the city of Quito, Ecuador? The city located on the equator had the same amount of sunlight due to its location on the equator.
2. Does the data provide evidence for a lack of seasonal changes in Quito? Explain. Yes, the data provides evidence that the number of daylight hours does not vary much for this city on the equator. The constant amount of daylight hours contributes nearly the same amount of radiant energy every day, so there is little variation in temperature.
Have students record raw daylight data before jumping to graphing to help avoid errors in interpretation.

FACILITATION TIP
Map Connection: Show latitudes on a globe while pointing out data cities. Students can see why Quito (0°) doesn’t change much, while Boise (43°N) has wide swings.
3. What relationship is shown between seasons as they are experienced in the Northern Hemisphere and the graphed data for the cities of Boise, Idaho, and West Palm Beach, Florida? Boise, Idaho, and West Palm Beach, Florida, experience seasons at the same time of year as shown by the highest number of daylight hours on the June 21st summer solstice (15:26 for Boise and 13:49 for West Palm Beach) and the lowest number of daylight hours on the December 21st winter solstice (8:56 for Boise and 10:28 for West Palm Beach).
4. How can the opposite patterns be explained in the graphed data for the cities of Boise, Idaho, and Christchurch, New Zealand? Boise, Idaho, at 43.6° North latitude, has the longest periods of daylight during the months of June, July, and August and the shortest periods of daylight during November, December, and January. Christchurch, New Zealand, at 43.53° South latitude, has the shortest periods of daylight during the months of June, July, and August and the longest periods of daylight during November, December, and January. They are both at similar latitudes in opposite hemispheres.
5. What do the data patterns suggest about how the number of daylight hours affects seasons? Longer periods of daylight hours occur during the summer season; shorter periods of daylight hours occur during the winter season. The amount of radiant energy received increases with longer days and contributes to warmer temperatures.
FACILITATION TIP
Stress the role of Earth’s tilt (23.5°) as the driver of day length variation, not just rotation.
6. What evidence does the data provide to explain why the Northern and Southern Hemispheres experience opposite seasons? The tilt of Earth changes the amount of daylight hours experienced in each season. It also causes the seasonal pattern to be opposite in each hemisphere. The cities located at the 26° North and South latitudes had a similar pattern of the amount of sunlight received for each season, but the seasons occurred at opposite times in the year. The amount of daylight ranged from a low of 10:28 at the winter solstice to a high of 13:49 during the summer solstice. The same seasonal pattern occurred for the cities located at the 43° North and South latitudes, except the amount of daylight received each day had a greater range from a low of 8:56 at the winter solstice and a high of 15:26 during the summer solstice.
Notes
Think-Pair-Share
For beginner and intermediate ELPs, have the materials translated into their native language as a reference for them to use during the activity. Prior to the students completing the activity, say the words and have the students repeat them.
After the students have gone through the Explore part of the investigation, allow them to regroup with their 3 o'clock partner (the partner to their immediate right). Give them time to think about their questions, answer them in the journals, and then discuss their answers.
Possible questions and sentence stems could be the following:
● Level 1 Knowledge Question: What is Earth’s axis?
Stem: Earth’s axis is ___________.
● Level 2 Comprehension Question: What can you say about the relationship between Earth’s revolution and the seasons?
Stem: The relationship between Earth’s revolution and the seasons is __________.
● Level 3 Application Question: What facts would you select to show that Earth’s revolution causes the changes between seasons?
Stem: The facts I would select to show that Earth’s revolution causes the changes between seasons are ___________.
● Level 4 Analysis Question: How would you distinguish between rotations and revolutions?
Stem: I would distinguish between rotations and revolutions by _________.
● Level 5 Synthesis Question: What would happen if Earth's axis were not tilted?
Stem: If Earth's axis were not tilted, then __________.
● Level 6 Evaluation Question: How would you evaluate the season based on the location of Earth in its revolution around the Sun?
Stem: I would evaluate the season based on the location of Earth in its revolution around the Sun by __________.
Phenomenon Connection
How does the tilt of the Earth affect the intensity and duration of sunlight received at different latitudes, and how does this relate to the changing seasons?
1. How does the angle of sunlight impact the temperature and climate experienced in different regions throughout the year?
2. In what ways do the patterns of daylight hours at various latitudes provide evidence for the occurrence of different seasons?
3. How do the data patterns from cities in the Northern and Southern Hemispheres illustrate the concept of opposite seasons?

STEMscopedia
Reference materials that includes parent connections, career connections, technology, and science news.
Linking Literacy
Strategies to help students comprehend difficult informational text.
Picture Vocabulary
A slide presentation of important vocabulary terms along with a picture and definition.
Content Connections Video
A video-based activity where students watch a video clip that relates to the scope’s content and answer questions.
Career Connections - Astronomer
STEM careers come to life with these leveled career exploration videos and student guides designed to take the learning further.
Math Connections
A practice that uses grade-level appropriate math activities to address the concept.
Reading Science - Why Do We Have Seasons?
A reading passage about the concept, which includes five to eight comprehension questions.
Notes
Claim-Evidence-Reasoning
An assessment in which students write a scientific explanation to show their understanding of the concept in a way that uses evidence.
Multiple Choice Assessment
A standards-based assessment designed to gauge students’ understanding of the science concept using their selections of the best possible answers from a list of choices
Open-Ended Response Assessment
A short-answer and essay assessment to evaluate student mastery of the concept.
Guided Practice
A guide that shows the teacher how to administer a smallgroup lesson to students who need intervention on the topic.
Independent Practice
A fill in the blank sheet that helps students master the vocabulary of this scope.
Extensions
A set of ideas and activities that can help further elaborate on the concept.
Use this template to decide how to assess your students for concept mastery. Depending on the format of the assessment, you can identify prompts and intended responses that would measure student mastery of the expectation. See the beginning of this scope to identify standards and grade-level expectations.
As a result of the fixed tilt of Earth’s axis, the areas of Earth’s surface exposed to the direct rays of the Sun change seasonally as Earth moves through its orbital path.
Known as Earth’s seasons, these cycles of temperature and length of day affect many of Earth’s other cycles such as the weather and plant growth.

ISBN: 979-8-3308-1921-8
