STUDENT
WORKBOOK

WORKBOOK


Published by Accelerate Learning Inc., 5177 Richmond Ave, Suite 800, Houston, TX 77056. Copyright © 2025, by Accelerate Learning Inc. All rights reserved. No part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without prior written consent of Accelerate Learning Inc., including, but not limited to, in any network or other electronic storage or transmission, or broadcast for distance learning.
To learn more, visit us at www.stemscopes.com.
This Student Notebook is designed to be used as a companion piece to our online curriculum.
The pages of this book are organized and follow the 5E model.
A short activity to grab students’ interest
EXPLORE Student Journal
Hands-on tasks, including scientific investigations, engineering solutions, and problem-based learning (PBL)
Claim-Evidence-Reasoning (CER)
A formative assessment in which students write a scientific explanation to show their understanding
ELABORATE
A reference material that includes parent connections, technology, and science news
Reading Science
A reading passage about the concept that includes comprehension questions
Claim-Evidence-Reasoning (CER)
A summative assessment in which students write a scientific explanation to show their understanding
Open-Ended Response (OER)
A short answer and essay assessment to evaluate mastery of the concept
Only student pages are included in this book and directions on how to use these pages are found in our online curriculum. Use the URL address and password provided to you by your district to access our full curriculum.




Draw a diagram of carbon cycling through ecosystems to organisms.

Plants, algae, and other photosynthetic organisms get energy from the Sun to make their own food through the process of photosynthesis. In this activity you will be simulating photosynthesis in various photosynthetic organisms such as plants, algae, and photosynthetic bacteria. You will work through each lab station and collect the raw materials needed to complete the investigation. A plastic bag will act as your photosynthetic organism.
1. Travel to each lab station and follow the directions on the Station Card.

2. Return to your seat and remove the contents of your bag. Put the resources you have collected together to make energy.
3. Pull cube combinations apart and put back together as needed. Some combinations may become large.
4. Connect 6 carbon + 6 oxygen + 12 hydrogen cubes together. This model represents glucose, which is the potential chemical energy in a plant. Add the photons of light to represent stored energy.
5. Your “oxygen” model will enter the atmosphere as a by-product from this process.
6. The glucose will be stored in the bag (photosynthetic organism) until needed.
Answer the following questions.
1. What are the raw materials an organism needs from nature to carry out photosynthesis?
2. What cell part was used for this process to take place?
3. What are the results of photosynthesis?

4. What happens to carbon in the process of photosynthesis?
5. What is the path or flow of energy in the process of photosynthesis?
6. How can the energy stored in the photosynthetic organism be used?
Unlike plants, animals are unable to make their own food through the process of photosynthesis. Write a scientific explanation describing how a rabbit gets energy to live and grow.
Claim:
Evidence:
Reasoning:

Both animals and plants carry out cellular respiration. Cells in both animals and plants contain organelles called mitochondria where cellular respiration happens. Mitochondria are sometimes called the powerhouse of the cell because this is where food, water, and oxygen are converted to energy for life.
Cellular respiration uses glucose and oxygen to produce energy in the form of ATP molecules, along with water and carbon dioxide. ATP, or adenosine triphosphate (C10H16N5O13P3), powers the organism’s life activities, such as movement, excretion, and circulation. ATP molecules capture the energy released when chemical bonds break. This process of producing ATP can be done using oxygen, which is called aerobic respiration, or done without the use of oxygen, which is called anaerobic respiration.
Use snap-together cubes to model the process of cellular respiration.
Procedure
1. Your teacher will give each group a large picture of a mitochondrion.
2. Obtain three different colors of snap cubes. You will need the following:
• 12 of one color, which will be the element hydrogen;
• 6 of another color, which will be the element carbon; and
• 18 of another color, which will be the element oxygen.
3. As a group, make a molecule of glucose out of the snap cubes. The formula for glucose is C6H12O6.
4. As a group, make six molecules of oxygen. The formula for oxygen is O2
5. Place the molecule of glucose and the molecules of oxygen on the picture of the mitochondrion in the labeled area.
6. Move all of the molecules into the mitochondrion and break all the bonds apart. The breaking of the bonds creates energy that is captured by ATP molecules and used by the cell for cellular activity.
7. Using the snap cubes in the mitochondrion, make six water molecules and six carbon dioxide molecules. The formula for water is H2O and the formula for carbon dioxide is CO2
8. Take the new molecules out of the mitochondrion and place them in the labeled area.

Answer the following questions.
1. How many carbon atoms did you start with?
2. How many carbon atoms were left in the mitochondrion?
3. How many hydrogen atoms did you start with?
4. How many hydrogen atoms were left in the mitochondrion?
5. How many oxygen atoms did you start with?
6. How many oxygen atoms were left in the mitochondrion?
7. Since you used oxygen during this process, did you produce ATP using aerobic or anaerobic respiration?
8. Where does the energy that ATP molecules capture come from?

Carbohydrates, proteins, and lipids (fats) have molecules that are too large to be absorbed into our bloodstreams. Enzymes and other substances chemically react with the foods to break the large molecules down into smaller molecules of simple sugars, amino acids, and fatty acids that can enter the bloodstream and be used for energy.
Complete the three puzzles to model how the large molecules found in carbohydrates, proteins, and lipids can be broken down to form smaller, more absorbable molecules.
1. Locate the puzzle pieces on the Puzzle Pieces. Color the large carbohydrate molecule orange, the enzyme-engaged molecule orange, and the sugar molecules pink.
2. Cut out each of the pieces for the carbohydrate puzzle.
3. Color the enzymes associated with the breakdown of carbohydrates green.
4. Glue the pieces to complete the carbohydrate puzzle.
CARBOHYDRATE PUZZLE
Starches, sugars, and cellulose are types of carbohydrates. They are digested in the mouth, stomach, and small intestine. Digestion of carbohydrates requires the presence of certain enzymes and water. Large carbohydrate molecules must be broken down into small molecules called simple sugars through digestion before your body can use them as an energy source.
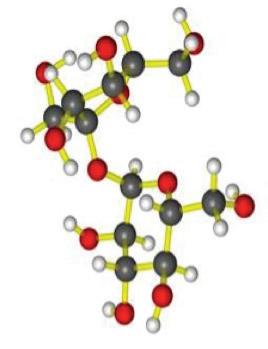
molecule
Before Carbohydrate and Enzyme Reaction

During Carbohydrate and Enzyme Reaction

After Carbohydrate and Enzyme Reaction

5. Repeat steps 1 through 4 to complete the protein puzzle and the lipid puzzle, but color the pieces using the following coloring key:
Large protein molecules—red
Enzyme-engaged protein molecules—red
Amino acids—yellow
Enzymes associated with the breakdown of proteins—brown
Bile—gray
Large fat (lipid) molecules—blue
Enzyme-engaged lipid molecules—blue
Fatty acids—white
Enzymes associated with the breakdown of lipids—purple
PROTEIN PUZZLE
protein molecule
Just as with the digestion of carbohydrates, enzymes are involved in protein digestion. The acid in the stomach is also required to help the enzymes break the proteins into smaller molecules called amino acids. Digestion continues in the upper portion of the small intestine, where the amino acids are absorbed by the capillaries of the small intestines and are carried through the liver and into the bloodstream.
Before Protein and Enzyme Reaction
During Protein and Enzyme Reaction
After Protein and Enzyme Reaction

Fat digestion and absorption require that the complex fat molecules be broken down into smaller, more manageable molecules by another enzyme. However, because fat does not dissolve in water, the fat molecules enter the small intestines stuck together in a mass, which makes it impossible for the enzyme to attack them.
To overcome this problem, the digestive system uses a substance called bile, which is produced in the liver but stored in the gallbladder. Bile separates the fat into tiny droplets, thus making it easier for the enzyme to access the fat molecules. Enzymes chop up large, complex lipid molecules into smaller molecules called fatty acid molecules.
Before Lipid and Enzyme Reaction
During Lipid and Enzyme Reaction
After Lipid and Enzyme Reaction

6. Use the digestion models and your knowledge of cellular respiration to describe how food molecules are processed through chemical reactions involving oxygen to form new molecules.

Earth is a complex system. Everything in Earth’s system can be classified into one of several subsystems that are often referred to as spheres. The biosphere is the combination of all of the planet’s ecosystems and is where all living organisms may be found. The biosphere includes the atmosphere (air), the lithosphere (land), and the hydrosphere (all of the water on the planet, including oceans, lakes, rivers, and the water vapor in the air). As long as water is available, living organisms can be found from many meters below ground to several kilometers above ground. Life is also found several kilometers below the surface of the oceans. Matter and energy cycle throughout the biosphere from one sphere to another according to the law of conservation of matter. This law states that matter can neither be created nor destroyed, but may only change form. There are four very important substances that are cycled through Earth’s spheres: carbon, oxygen, nitrogen, and water.
Procedure
1. Analyze the diagrams for each of the ways matter is cycled through Earth’s system.
Evidence of Importance to the Ecosystem
Evidence of Importance to Organisms
Interaction with Other Cycles

2. Write an evidence-supported description of the importance of each cycle to the health of organisms and ecosystems.

Humans depend on ecosystems for various services the systems provide. Ecosystem services can be categorized as provisioning, regulating, cultural, and supporting. Provisioning services are assets that can be collected from the ecosystem. Examples include drinking water, timber, fuels, plant fibers, food, and medicines. Regulating services are described as those that control or support natural phenomena. Examples include pollination, decomposition, water purification, erosion and flood control, carbon storage, and climate regulation. Cultural services benefit humans by adding to the quality of life and the advancement of cultures. Supporting services include the natural processes that provide energy, nutrients, and resources needed for an ecosystem to function. Examples include photosynthesis, nutrient cycling, soil formation, and the water cycle. Disruptions to the supporting services can result in changes to the other services provided by an ecosystem.
Using all group members, create and act out a skit to teach your classmates how a disruption in the cycling of matter affects ecosystem services and biodiversity.
1. Your teacher will assign your group a disruption scenario.
2. Collaborate with your group members to discuss how your ecosystem is affected.
3. Record your disruption and a brief description of its effect on the cycling of matter. Include how the change to the affected cycle(s) results in changes to the ecosystem services and the biodiversity or the number of different types of organisms in the system. Record how the changes could affect humans.
Disruption:
Possible

4. Plan your skit. Use any supplies that your teacher has provided for visual aids. Be sure to show how biodiversity and ecosystem services are affected, and indicate which cycles are affected by the disruption. Discuss how the changes may impact human life.
5. While watching the other groups perform their skits, record their disruptions and effects as well.
Effect on Cycles
Effect on Ecosystem Services
Effect on Biodiversity
Effect on Human Life

6. Use your collected information to write a letter to the editor of your local paper to explain how disruptions to the cycling of matter affect the sustainability of human life on Earth and should be limited.

Humans benefit from ecosystems in multiple ways. These benefits are called ecosystem services. Examples of these services include water purification, nutrient recycling, and prevention of erosion. When humans disturb ecosystems, the loss of these ecosystem services can have a great impact on the surrounding ecosystems.
Wetlands are some of the most biodiverse ecosystems on Earth. Not only do they contain many different species of organisms, but they also may provide many ecological services that humans have come to depend on. When a developer fills in a wetland for housing or some other project, the developer must rebuild a similar wetland in an alternative location. This is called wetland mitigation. The new wetlands should be able to provide all the ecosystem services that the original wetlands did. You will design a wetland mitigation project.
Create a new design solution for a wetland mitigation that can carry as many ecosystem services as the original wetland.
• Design must be roughly the same size as the wetland that has been lost, which is 10 square miles.
• Incorporate as many wetland services as possible into your model from the list below:
• Flood control

• Buffering ocean waves in coastal areas to prevent beach erosion
• Reproductive areas for many species
• Filtering water for water purification
• Nursery area for the young of many species
• Stopover for migrating birds
• Erosion control
• Prevention of the introduction of invasive species
• Wetland must be built in a location that has the resources that will help maintain it, such as rainfall.
• Students must provide a detailed to-scale diagram of the prototype.
• Students must provide a list of needed materials.
• Students must provide instructions for constructing the wetland.
• Students must provide instructions for maintaining the wetland.

Use your lab journal to record all of your steps and observations.
A. What is the problem?
1. State the problem in your own words.
2. Work as a class to develop an agreed-upon set of criteria to be used to evaluate the proposed solutions in regard to maintaining biodiversity and ecosystem services in a wetland ecosystem.
B. Explore and research the problem. List what you know and what you need to know.
1. Research to gather all needed information on how the chosen wetland provides the identified ecosystem services.
2. Identify the biodiversity of the chosen wetland.
3. Identify the criteria.
4. Identify any constraints you encounter.
5. Identify possible effects a small change may have on biodiversity or ecosystem services in a wetland ecosystem.
C. Brainstorm and design a solution to the problem.
1. Brainstorm and diagram possible designs for the model.
2. Select the best design. Show the design to your teacher for approval.
3. Identify what materials you will use. Create some sketches or diagrams of your approach. You may need to brainstorm with other people on other teams to share ideas.
D. Build, test, and analyze your solution.
1. What information will you include in your model?
2. How will you organize the information?
3. Create a to-scale diagram of your design.
4. Build a prototype model of your design.
5. How will you determine if your design is effective or not? What data will you provide to show your design meets the agreed-upon criteria?
E. Improve or redesign and retest your solution.
1. What errors could have been made during designing of the model, and how can you improve this technique?
2. Were you able to create an effective wetlands mitigation project for your local area? If not, what changes do you need to make to your model?
3. Finalize your solution and draw a detailed diagram of the proposed mitigation project with an explanation of how the design maintains biodiversity and ecosystem services.

F. Present and share your solution.
1. Decide how you will share your model with the teacher or class.
2. Discuss who will talk about what you discovered.
3. Observe the presentation of each solution.
4. Ask questions to clarify why each solution was chosen.
5. Use the agreed-upon criteria to evaluate each presented solution.
6. Evaluate if portions of the presented solutions could be combined to create the best possible solution to maintain biodiversity and ecosystems in a wetland.

Certain conditions within an ecosystem cannot tilt too far to one or another extreme. Ecosystems thrive when conditions are balanced, or “just right.” In the picture to the right, what are some of the components—living and nonliving—of this ecosystem? How might conditions in the ecosystem—the amount of rainfall, the nutrients in the soil, the number of organisms within each population—fall out of balance? How might such changes affect the ecosystem? How does matter cycle between living and nonliving things?

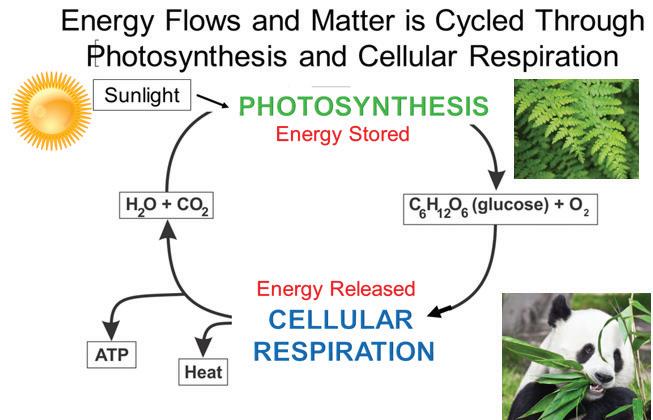
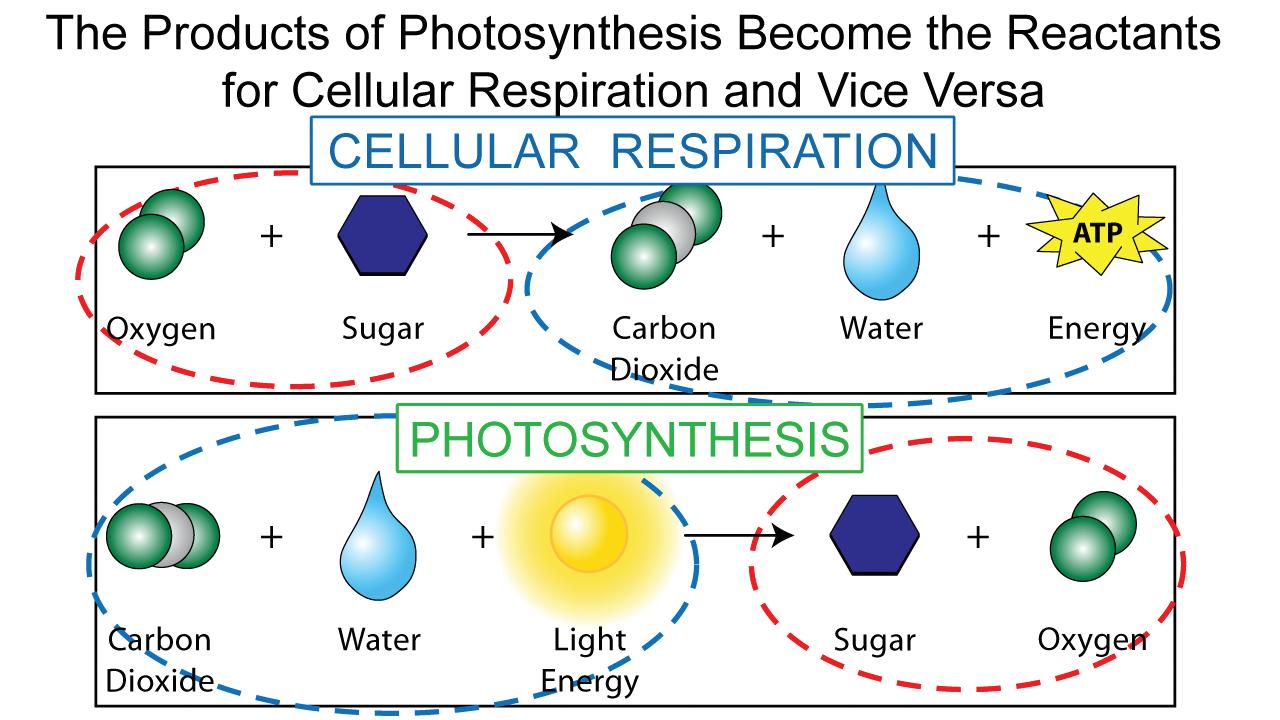
Elements such as carbon and oxygen cycle through the atmosphere and biosphere. Systems on Earth are healthiest when their various components are balanced. Two important elements that contribute to this balance are carbon and oxygen. Carbon and oxygen move between living and nonliving things on Earth. These movements make up the carbon cycle and the oxygen cycle.
Like all cycles, the carbon cycle has neither beginning nor end. Instead, it consists of a number of related processes. One part of the carbon cycle centers on photosynthesis. Carbon exists in Earth’s atmosphere primarily within molecules of carbon dioxide (CO2). During the Calvin cycle of photosynthesis, plants use the carbon (C) from CO2 to make organic compounds called carbohydrates. One of the most important of these carbohydrates is glucose (C6H12C6), a sugar that plants use for food. Plants can store glucose for later consumption in the form of starch, another type of carbohydrate. Oxygen is a by-product of photosynthesis and is used by animals in cellular respiration.
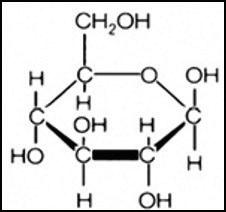

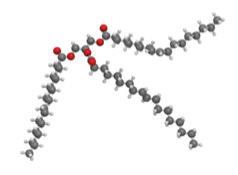
Carbohydrates such as this molecule of glucose (left) have a carbon backbone. Foods made from grains, such as bread and pasta (right), contain complex forms of carbohydrates.
Oxygen, in addition to carbon dioxide, is cycled through photosynthesis and cellular respiration
Another important part of the carbon cycle occurs when organisms consume carbohydrates produced by plants to fuel their cellular activities. For example, in an animal’s cells, carbohydrates undergo a series of chemical reactions that break down the molecule to release energy during cellular respiration. One of the waste products of cellular respiration is carbon dioxide, which animals exhale into the atmosphere where plants can once again use it to make carbohydrates. In this way, carbon cycles continually between the atmosphere (as carbon dioxide) and the

biosphere (as carbohydrates). Oxygen cycles through both processes too.
Carbon in biomass—the living (or recently living) matter in an ecosystem—is also returned to the environment through decomposition. The bodies of organisms are primarily made up of carbon. When an organism dies, worms, bacteria, and other decomposers break down the body into its component elements. In this way, carbon moves from biomass into the ground, where it can remain buried for thousands of years.

Carbon cycles from biomass, to fossil fuels, to the atmosphere, and back to plants. Over millions of years, extreme heat and pressure from Earth’s interior transformed buried plants and animals from the Carboniferous Period (approximately 300–350 million years ago) into fossil fuels such as coal, petroleum, and natural gas. When humans burn fossil fuels—to power machines, generate electricity, and heat buildings—the carbon cycles back to the atmosphere, where it can be used again by plants during photosynthesis.
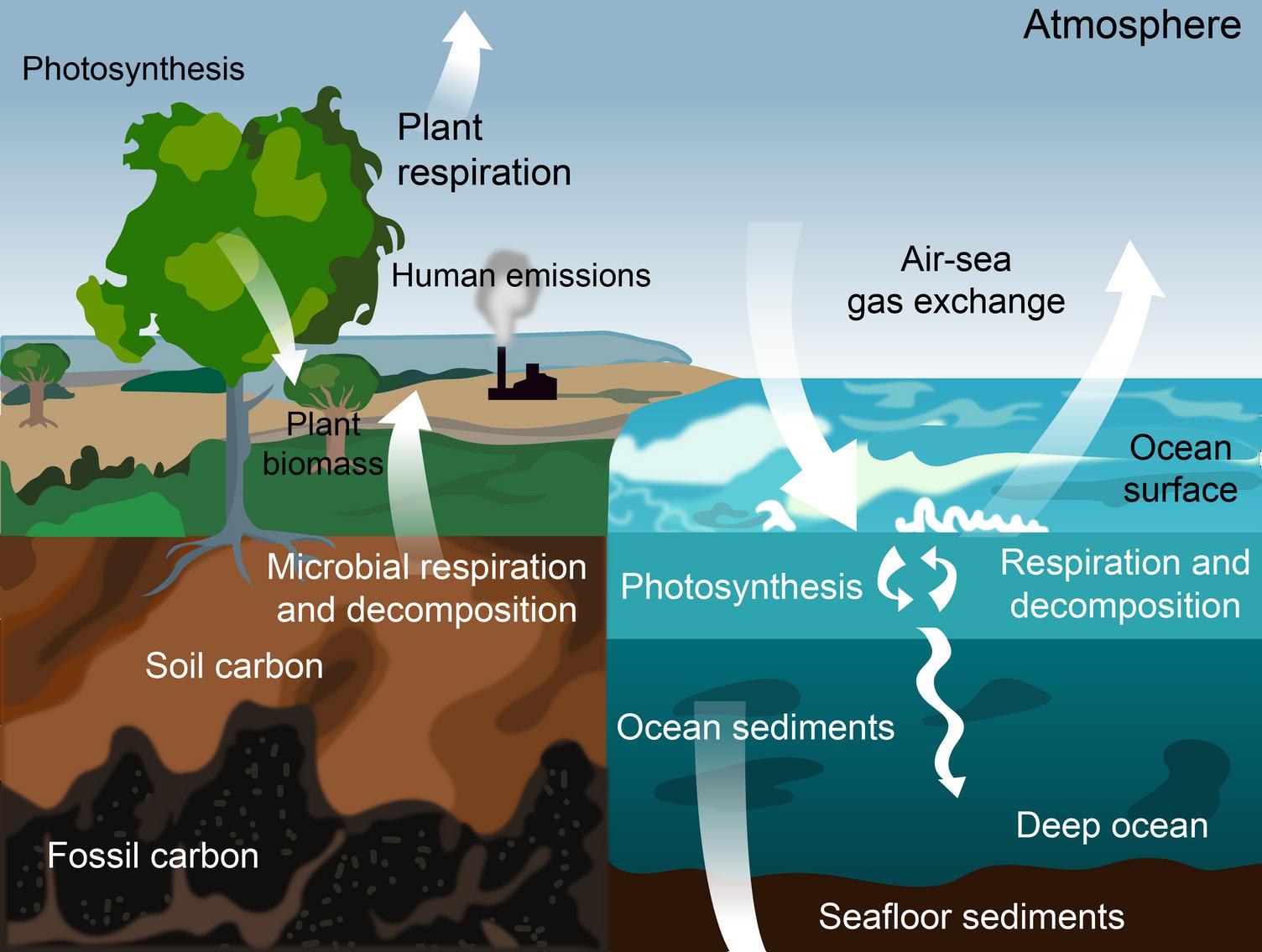
Photosynthesis, respiration, and decomposition happen in the oceans as well as on land. In the oceans, carbon cycles between the surface ocean, the deep ocean, and the seafloor. These movements are part of the carbon cycle.

Humans contribute to the carbon cycle by burning fossil fuels and other forms of biomass, such as wood. (Biomass also burns through natural processes. For example, a bolt of lightning can spark a forest fire.) However, over the past few centuries, humans have released huge quantities of carbon into the atmosphere—more than can be absorbed through photosynthesis and other natural processes. Scientists have evidence this excess carbon dioxide is trapping heat at Earth’s surface, leading to global climate change.
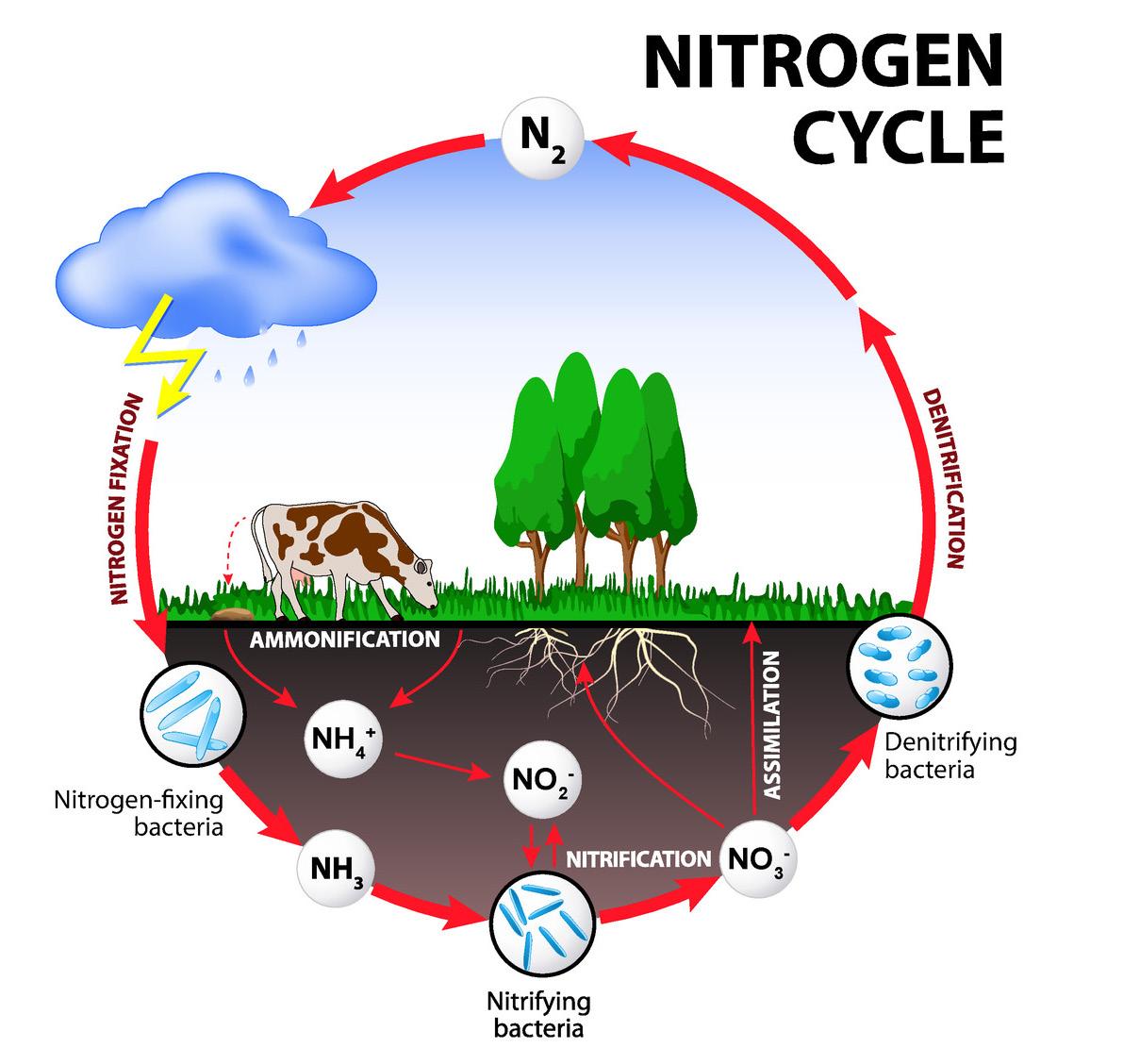
Nitrogen also cycles between Earth’s atmosphere and biosphere

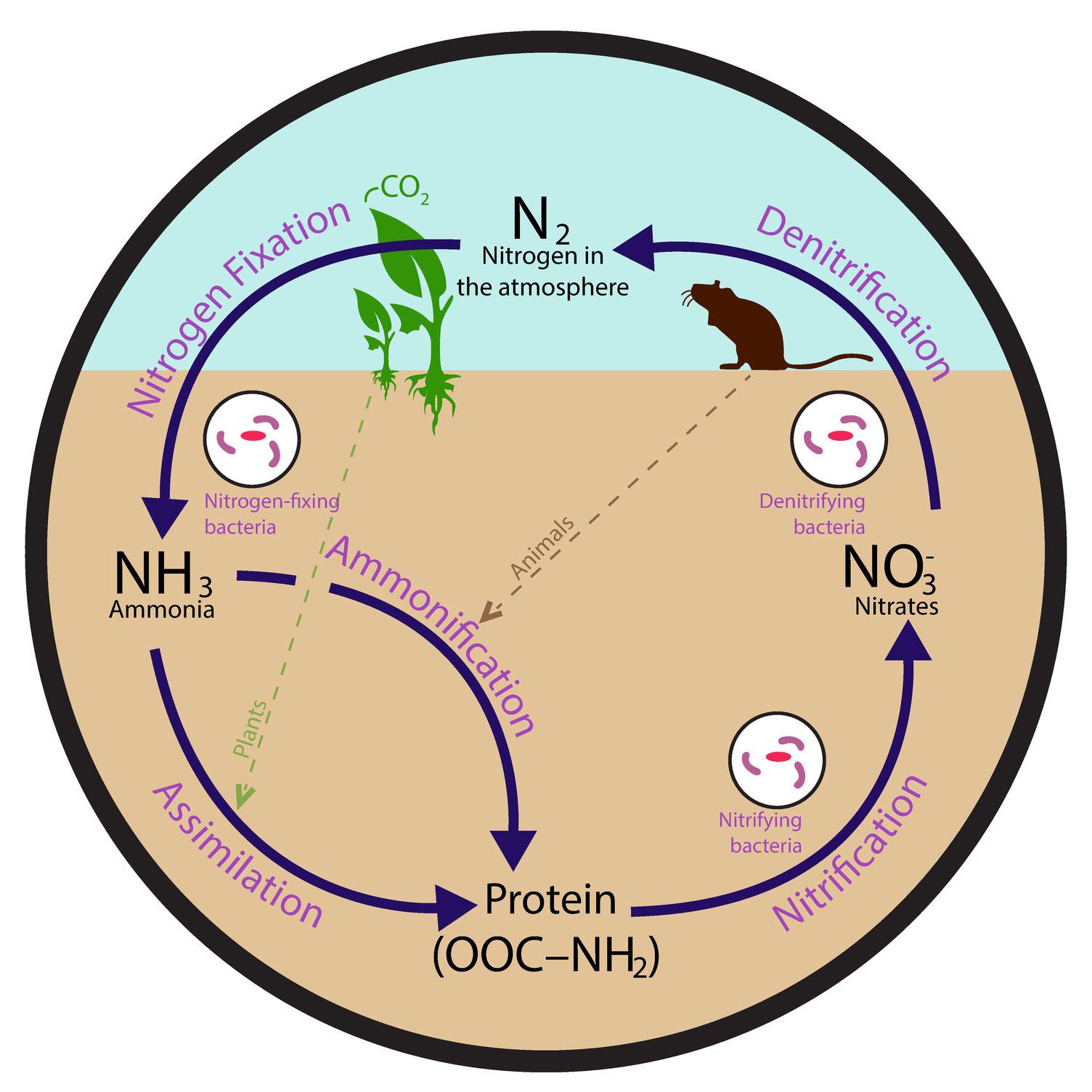
Both plants and animals require nitrogen to survive. It is a key component of DNA, RNA, and amino acids, which are used to form proteins in living organisms. Approximately 78% of Earth’s atmosphere is nitrogen gas (N2), but most living things cannot use nitrogen in this form. Only certain types of bacteria can take nitrogen directly from the atmosphere. For this reason, if it were not for these bacteria, nitrogen would not cycle through the biosphere for other organisms to use.

Nitrogen fixation: Many legumes—plants such as peas, soybeans, peanuts, and alfalfa—coexist with bacteria called rhizobia. The bacteria live in the soil on the roots of the legumes. These bacteria are able to take nitrogen directly from the atmosphere and convert it into ammonia (NH3); this process is called nitrogen fixation
Nitrification: Other bacteria in the soil change the ammonia into nitrites (NO2 ) and nitrates (NO3 ) in a process called nitrification
Assimilation: Plants absorb the nitrogen compounds through their roots and use them to synthesize amino acids; this process is called assimilation
Denitrification: As consumers eat plant producers, nitrogen passes up through the food chain for other living things to use. In addition, some bacteria in the soil convert nitrates into nitrogen gas through a process called denitrification. The nitrogen gas is then released back into the atmosphere.
Ammonification: The decomposition of organic material is also a crucial part of the nitrogen cycle. When organisms die, nitrogen compounds held in their bodies are broken down by detritivores (organisms that feed on dead matter) and returned to the soil as nitrates and nitrites. Bacteria in the soil can also convert nitrogen compounds from decaying matter into ammonia through a process called ammonification. The nitrogen cycle continues as plants reabsorb these compounds into their bodies.

As mentioned previously, ecosystems thrive when conditions are balanced. The carbon and nitrogen cycles are crucial to maintaining this balance. Disruptions to these cycles can have devastating effects on an ecosystem. Unfortunately, too often these disruptions result from human activities.
A large problem, however, is excessive carbon in the atmosphere. Recall that burning fossil fuels releases carbon dioxide gas into the air. In the last 150 years, there has been a dramatic increase in the burning of coal, oil, and natural gas for factories, cars, airplanes, heat, and other technologies invented by humans. Not only does this excessive use deplete Earth of its natural fossil fuel resources, but it also pollutes the air and creates unbalance in the carbon cycle.
During the last few decades, governments and individuals in the United States and other countries have worked at reducing the amount of CO2 emissions from refineries, factories, and automobiles. Additionally, efforts are being made to make homes and office buildings more energy efficient using “green” technology.

This algae bloom—the over-reproducing of algae in a water body at the expense of other organisms—is the result of eutrophication.
Look Out! Reflect

Exhaust from vehicles releases lots of carbon dioxide into the atmosphere.
Burning fossil fuels also results in excessive nitrogen buildup in the atmosphere, which leads to destructive acid rain. Overusing nitrogen-type fertilizers in agriculture also disturbs the nitrogen cycle. The availability of nitrogen can be a limiting factor to the growth of crops. To combat this, farmers use fertilizers with high concentrations of nitrogen and other nutrients. The fertilizers may help with farming, but the nitrogen compounds in the soil get carried away by rainwater. The runoff causes nitrogen to build up in estuaries, lakes, and streams.
This change in water composition is called eutrophication. Algae thrive on the excess nitrogen concentrations and reproduce uncontrollably, resulting in an algal bloom. As the algae die, the decaying matter and decomposers use up most of the available oxygen, choking out other organisms in the water such as fish.
You and every other known organism on the planet need water to survive. Specifically, all living things need a supply of clean drinking water. Where does your drinking water come from? What is the risk that your drinking water could run out or become contaminated? What can you do to help protect your drinking water supply?


About 70% of Earth’s surface is covered with water. This water is stored in many different places, or reservoirs. These include liquid reservoirs on Earth’s surface, such as oceans, lakes, ponds, streams, and rivers. There is also water in frozen reservoirs, such as glaciers and polar ice caps. There is water in and below the ground, in the atmosphere, and in the bodies of organisms, including you! Water circulates between these reservoirs through the water cycle. Water evaporates and rises as vapor into the atmosphere.
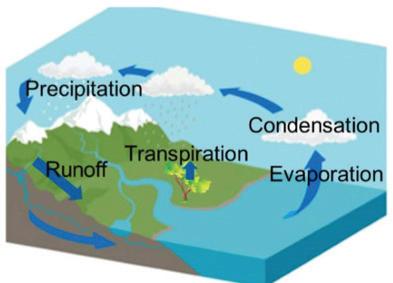
Water vapor in the atmosphere condenses to form clouds. Liquid and solid water then fall back to Earth’s surface as rain, snow, or other precipitation. Some water flows across Earth’s surface as runoff; other water is absorbed into the ground. The areas on or below Earth’s surface where this water collects are called watersheds. For example, when rainwater runs off your backyard into a stream, your backyard is part of a watershed.
Look Out! Reflect
Humans disrupt the biodiversity of life and ecosystem services with the misuse of water and aquatic life. Pesticide runoff can poison marine organisms, whereas fertilizer runoff can cause bacterial and algal populations to explode, crowding out other organisms and reducing biodiversity. Spills from ships such as oil tankers also pollute the oceans.
Humans impact the oceans through overharvesting. This involves harvesting a resource at an unsustainable level. Marine life is sometimes fished until populations are nearly or completely wiped out. Changing the population sizes of different species also disturbs the balance of marine food webs. If prey animals die off, the predators that depend on them also die. On the other hand, if predators die off, prey animal populations may grow out of control.

ship was
One way that humans attempt to compensate for overharvesting is to use artificial reefs. Artificial reefs are human-made underwater structures that promote marine life by providing surfaces for marine plants and animals to attach to on the ocean floor. These human-made ecosystems can support complex food webs. However, there are concerns with artificial reefs. Often they are made out of old objects such as oil rigs, train cars, and boats. Such materials may release toxic chemicals into the ocean. Humans also modify ocean systems by introducing new species into non-native ecosystems. Such invasive species disrupt the biodiversity and food webs by competing with or harming species native to that ecosystem. Invasive species can also promote the spread of disease, because they may carry germs to which native species have never been exposed. Invasive species can be introduced in a number of ways. Ships unintentionally pick up various marine plants, animals, bacteria, and viruses and transport them to other locations. Humans also dump exotic aquarium species into waterways that lead to the ocean, competing with or harming species native to that ecosystem. Public education and changing local, state, and federal laws aimed at controlling invasive species are several ways to counteract these practices.

Matter is also cycled through the synthesis of food molecules that use oxygen to form new molecules. An elaborate city could be made of these small, simple building blocks. Organisms are built in much the same way. Despite their complexity, organisms are made of relatively simple building blocks. How are these building blocks assembled into complex organisms? What role does each main type of molecule play?

Macromolecules are molecules that are made by organisms and are essential for performing life functions. They range in size and perform specific functions in and among cells. Their function is often determined by their structure. If the structure is disrupted, the macromolecule can no longer function properly.
Macromolecules are made of building-block monomers. A monomer is a small molecule that can be combined chemically with other monomers to form larger molecules. Monomers are made up of relatively simple elements. The most abundant elements in biological monomers are carbon, hydrogen, and oxygen. A polymer is a group of monomers linked to form a much larger molecule. The prefix mono- means “one,” and poly- means “many.” Think of monomers as the building blocks and polymers as the final products. The process of making a polymer is called polymerization
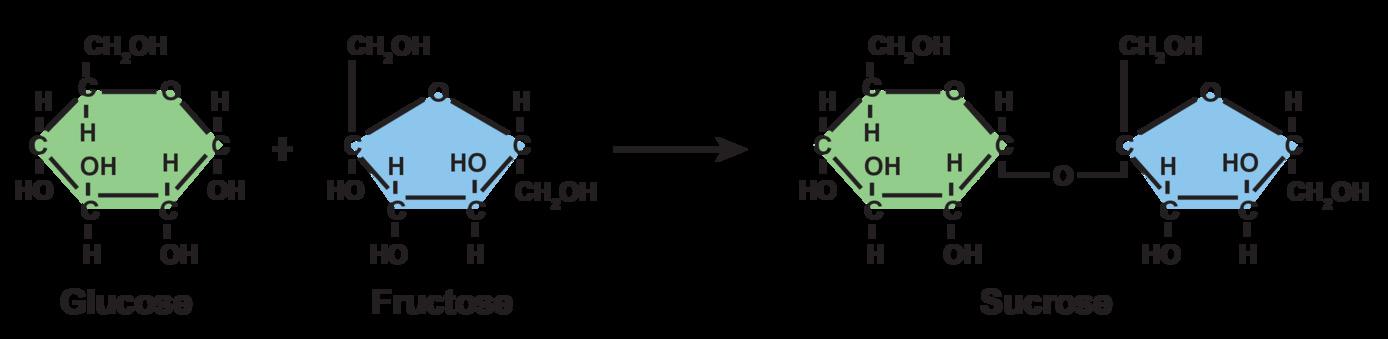
The linking of monosaccharide monomers to form lactose is an example of polymerization.
Carbohydrates: Polysaccharides are polymers of monosaccharides linked together by dehydration synthesis reactions. Carbohydrate polysaccharides can be made from the same type of monomers or from different monosaccharides linked together. Look at the example of sucrose in the diagram above. Sucrose is a polysaccharide, made up of two different monosaccharides, glucose and fructose. Carbohydrates are important energy storage molecules in cells.
In the human diet, carbohydrates are found in flour, sugar, pasta, potatoes, and other “starchy” foods. Carbohydrates also play a number of important structural and signaling roles in all living cells. They form part of the molecular backbone of nucleic acids, and they are critical for maintaining life. Animals store sugars as glycogen, which is made of glucose molecules linked together. Plants store sugars as starch

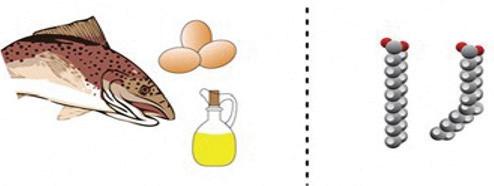
Fatty acids are the building blocks of fats found in fish, eggs, and oil.
unsaturated: having at least one double or triple bond between carbon atoms
Lipids are a diverse group. Fats, a common type of lipid, are combinations of fatty acids and glycerol. Fatty acids are long chains of carbon and hydrogen linked together into a hydrocarbon chain. Some chains are straight, while others bend wherever there is an unsaturated carbon in the hydrocarbon chain (carbon is not saturated with hydrogen). A double bond forms between two adjacent unsaturated carbons. The carbons on either side of the double bond have one fewer hydrogen than other carbons in the chain. The term unsaturated is used to describe this type of fatty acid. Fatty acids are used to store energy. These monomers are linked together with glycerol to form diglycerides (two monomers) or triglycerides (three monomers).
Lipids are the main structural component of the cell membranes of all organisms. Similar to carbohydrates, lipids are used for long-term energy storage. They are nonpolar, which makes them hydrophobic, or water-repellent. They do not dissolve in water. Plant lipids are usually liquids, such as olive oil, while animal lipids are usually solids, like the fat in beef.
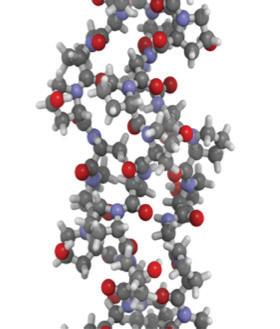
Amino acids are the building blocks of proteins. There are hundreds of types of amino acids, but just 20 of these make up our proteins. Each amino acid has a common core of a central carbon, an amine group containing nitrogen, a carboxyl group made of carbon and oxygen, and a side chain (labeled R on amino acid diagrams). The side chain is different for each of the 20 amino acids. Some side chains are hydrophobic, while others are hydrophilic, or water-soluble. Some side chains are charged, while others are neutral. The different properties of the side chains give each amino acid different properties.
Amino acids are linked together by covalent bonds called peptide bonds. This type of bond forms only between amino acids. The reaction to form a peptide bond is a dehydration synthesis reaction. One hydrogen atom and one hydroxyl group (–OH) are removed from the amino acids to form one water molecule for each peptide bond that is formed.
Amino acids are linked together to form a polypeptide chain. Inside the cell, an organelle called the ribosome is responsible for linking together amino acids to form the polypeptide chain. When a chain contains more than about 50 amino acids arranged in a biologically functional way, it is called a protein
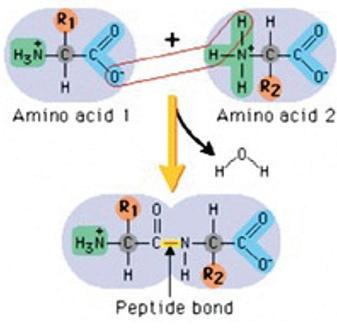
Amino acids are linked together by strong peptide bonds.
amine: a functional group with the general formula R—NH2
carboxyl: a functional group with the general formula R—COOH
Proteins are essential macromolecules in all cells. They give a cell its structure, communicate information, synthesize molecules, transport molecules, and make up enzymes, which are molecules that speed up the chemical reactions necessary for life.

Nucleotides are small molecules made of a sugar (monosaccharide), one or more phosphate groups, and a nitrogenous base. The nucleotides ATP (adenosine triphosphate) and GTP (guanosine triphosphate) are important for energy transport within cells. The nitrogenous base of ATP is adenosine, and the phosphate group is a triphosphate (three phosphates linked together). GTP is similar to ATP, with guanosine replacing adenosine as the nitrogenous base. Other nucleotides are enzyme cofactors and signaling molecules.
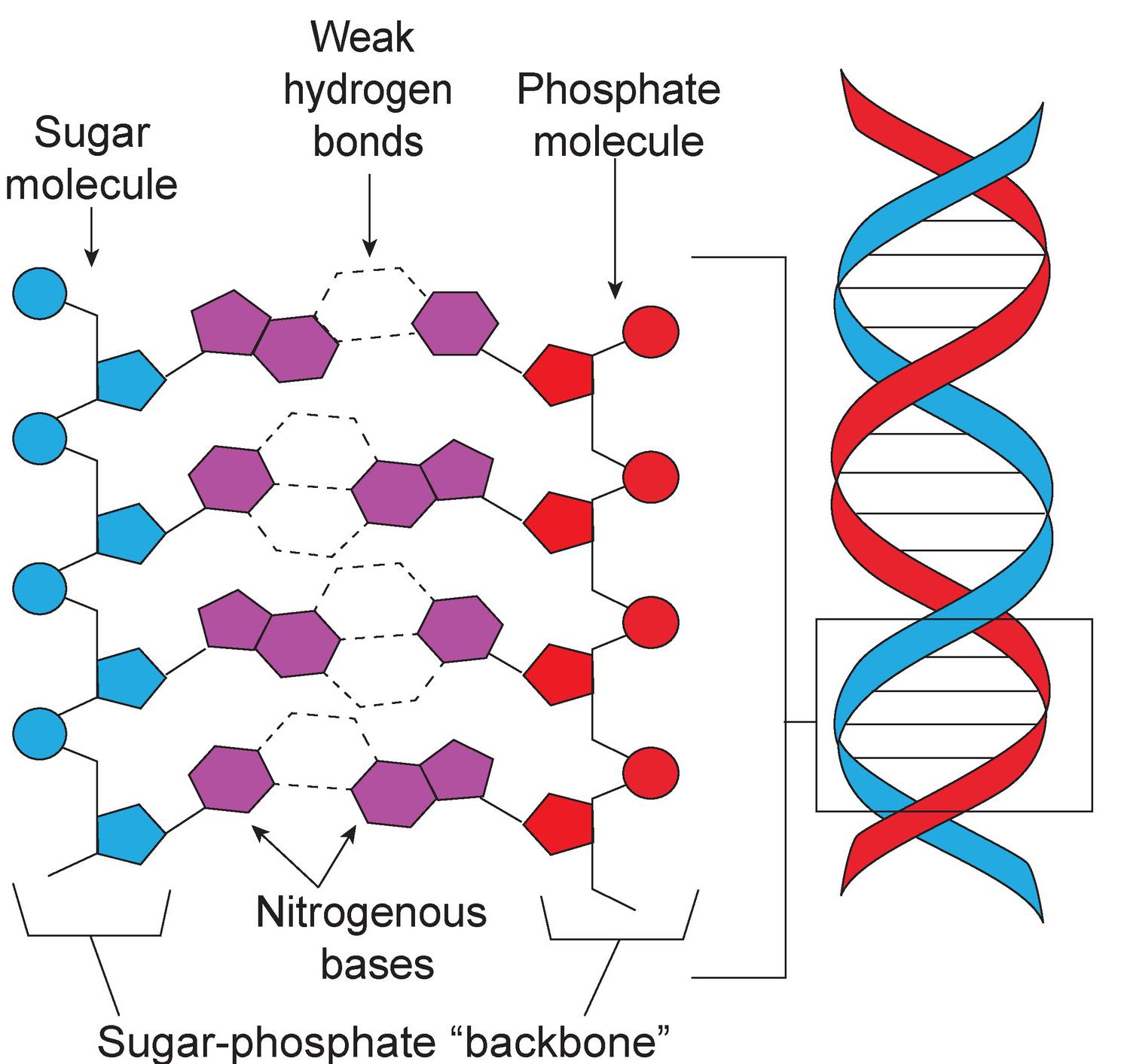
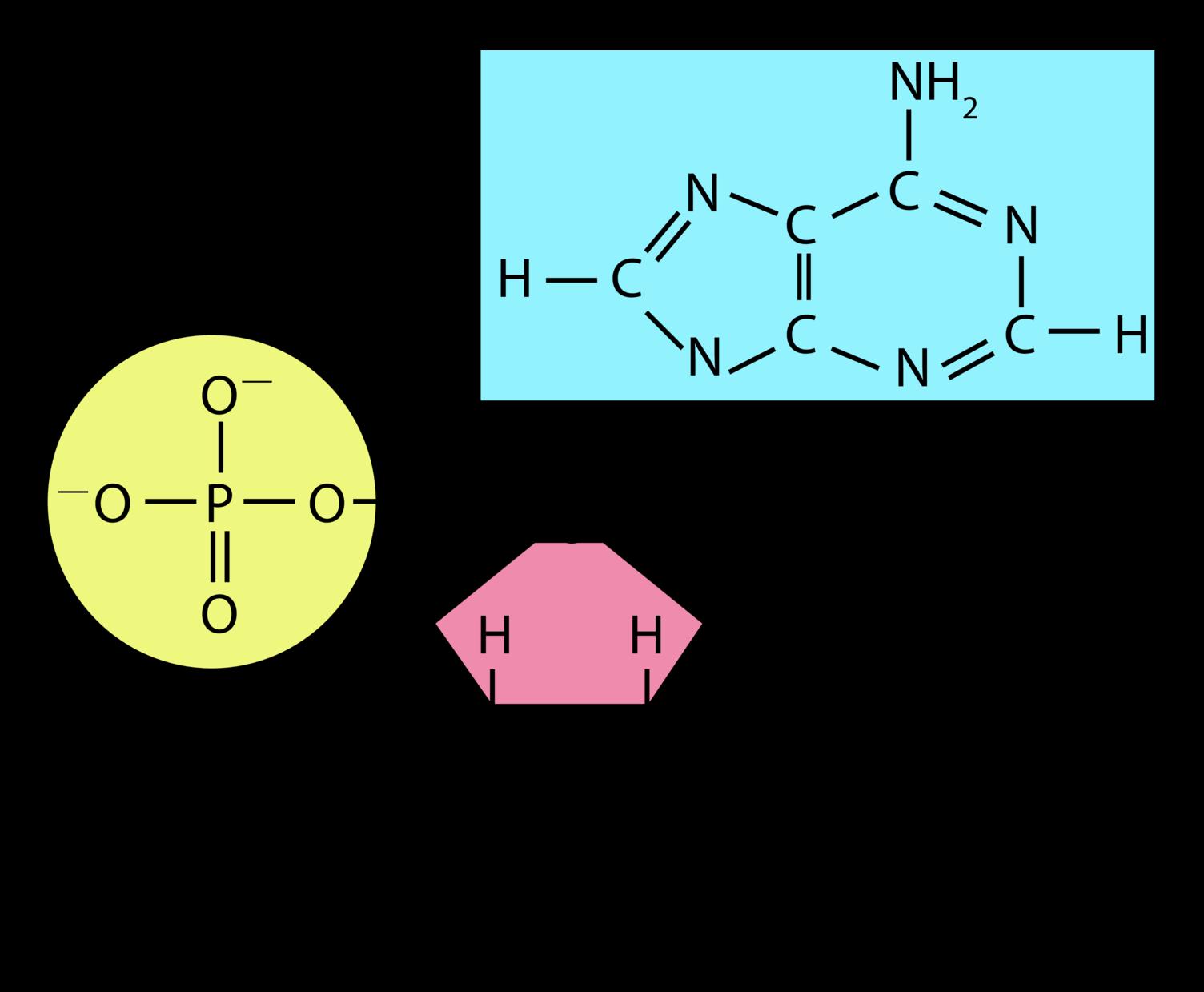
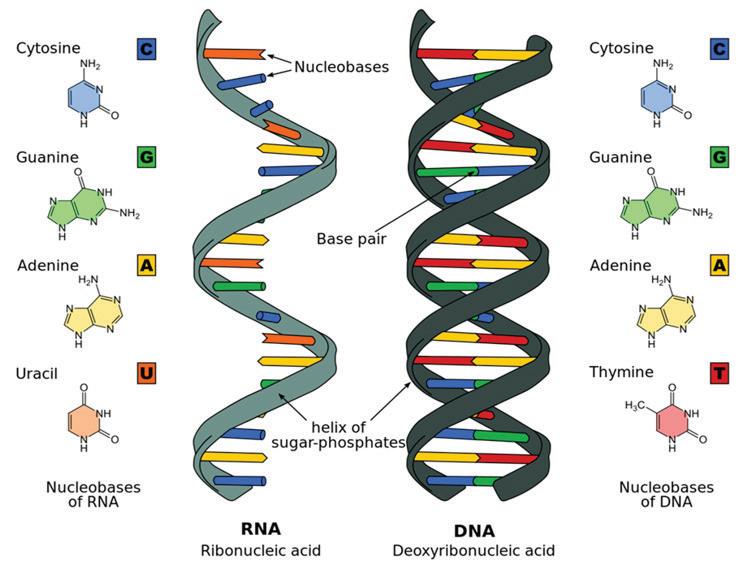
Nucleotides are the building blocks of nucleic acids. Nucleotides build nucleic acids, including DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). DNA includes four nucleotides—guanine, adenine, thymine, and cytosine. In RNA, uracil replaces thymine as a nucleotide. DNA and RNA are essential for storing and utilizing genetic information. DNA and RNA work together to create proteins. As you can see in the diagram on the left, alternating bonds between sugar and phosphate molecules of adjacent nucleotides link the nucleotides that make up DNA. This forms the sugar-phosphate backbone of a strand of DNA. Two DNA strands typically join together via weak hydrogen bonds between nitrogenous bases. The DNA strands twist around further to form the familiar double-helix configuration. In contrast, RNA is typically single stranded and does not form a double helix. How does DNA encode protein? The arrangement of nucleotides in DNA stores the code by which amino acids should be brought together in the protein. RNA helps by transferring amino acids to ribosomes for protein creation and by helping to build new proteins.
Look Out!
DNA and RNA
Be careful not to confuse the function of DNA with its structure. A DNA molecule provides information about which amino acids are needed to produce certain proteins.
Amino acids are not, however, part of a DNA molecule.

The box below lists some of the characteristics of the four main types of large macromolecules: carbohydrates, lipids, proteins, and nucleic acids. Match each characteristic in the box to the correct macromolecule in the table. Write your answers in the right column of the table.
• Made of nucleotides
• Make up oils and fats
• Made of amino acids
• Store and utilize genetic information
• Main structural component of cell membranes
• Made of monosaccharides
• Make up enzymes
• Main component of bread and pasta
• Made of fatty acids linked with glycerol
• Stored in plants as starch
Macromolecule
Characteristics Carbohydrate

Try Now
Use the Venn diagram to compare and contrast the carbon and nitrogen cycles. Add three to five responses that show how each cycle is different and three to five responses that show how the cycles are similar. Be sure to include consequences that result from human activities or other disruptions.

To help your child learn more about the carbon and nitrogen cycles and the effects of human activities on them, work together to develop carbon and nitrogen recycling programs around your home, school, or community.
You may begin by creating a place to store a compost pile. Have your child choose a convenient place that is shaded, well drained, and not too close to any tree roots or wooden fences.
For a home compost pile, one square meter is an acceptable size. A larger area may be appropriate for compost piles at school or in a community location. Begin the compost pile with a thick layer of organic materials such as dead leaves, yard clippings, or vegetable scraps. When ready, add a thin layer of fertilized soil to activate the compost. Then layer a thin covering of topsoil, which may include microorganisms.

You can recycle biomass at home by making a compost pile.
All organic waste from uneaten food including leftovers, eggshells, and peelings can be added to a compost pile. Important: Never include human or animal waste products in compost used on food plants.
You will need to water the compost and turn it every couple of weeks. To turn the compost, use a garden tool to move the inside material to the outside and vice versa—all parts should be exposed to oxygen. Within a couple of months, the compost pile should be ready to use.
You can also research your carbon footprint and find ways to reduce it. Encourage your child to think about ways he or she can personally reduce fossil fuel emissions into the atmosphere. Suggestions include walking or riding a bike instead of driving and recycling materials made in factories such as plastics. Encourage your child to expand a personal program to the entire family, the school, or the community.
Here are some questions to discuss with your child:
• How do the carbon and nitrogen cycles keep nutrients balanced between the atmosphere and biosphere?
• What roles do humans play in the carbon and nitrogen cycles?
• What can you do to help maintain balance in the nutrient cycles?

1 The element carbon is one of the most important elements for biological life, as it is able to be used in so many ways. Some form of carbon is found in all forms of life, and most organisms need some form of carbon in the food that they eat. But where does all the carbon come from, and where does it go? Let us take a journey and follow a single carbon atom through the many stages and forms in which it may find itself. This carbon atom will be referred to as “our carbon atom.”

2 Our carbon atom begins its journey in the atmosphere as an element in carbon dioxide gas (CO₂). Carbon dioxide is a naturally occurring gas and is a source of carbon for a huge number of biological organisms. This chemical formula for carbon dioxide, CO₂, means that there are two oxygen atoms bound to one carbon atom. The CO₂ containing our carbon atom will remain in the atmosphere until it reaches the outer surface of a leaf of a tree. Here, the CO₂ gas will enter the leaf through an opening called a stoma.
3 An amazing chemical reaction happens once the CO₂ molecule enters the leaf. The CO₂ molecule enters a chloroplast within the leaf. A chloroplast is (most often) the green part of the leaf and is where the chemical reactions in photosynthesis take place. Here, the carbon atom from the CO₂ molecule is added to a sugar molecule that is created during this process. In the leaf of a plant, the carbon is moved from a CO₂ molecule to a glucose (sugar) molecule, or C₆H₁₂O₆.
4 On this journey, our carbon atom will remain attached to the glucose sugar and will move to a fruit on the tree. When the fruit drops to the ground, our carbon atom goes with it. In this case, an animal eats the fruit that contains our carbon atom attached to the sugar. The fruit is digested in the stomach and intestines of the animal. The nutrients from the fruit are then absorbed and carried to the cells of the animal.
5 Our carbon atom then goes through another incredible change. The carbon in the sugar (C₆H₁₂O₆) enters a cell of an animal where it is broken into smaller molecules. Our carbon atom can then enter into the mitochondrion, the energy-processing organelle of cells. Here is where cellular respiration happens. In the mitochondrion, our carbon atom again becomes part of a CO₂ molecule, which is released back into the atmosphere with the animal’s next breath.

6 Our carbon atom travels over the surface of a shallow ocean and is soon taken in by a small plant-type plankton that performs photosynthesis. Our carbon atom is bound to a sugar molecule again during photosynthesis. It is carried by this plankton until a small fish eats the plankton, which is then eaten by a salmon. Once inside the salmon, our carbon atom is chemically bound to a protein. The carbon atom is used over and over inside this salmon. For instance, once the original protein is broken into amino acids, it can become yet another protein. Eventually, our carbon becomes part of a fat molecule, a hydrocarbon (a CH chain). Here, the carbon stays for quite some time.
7 Our carbon atom is carried up a river in the body of the salmon during the salmon mating run. The salmon eventually dies after spawning in the stream in which it was born. The salmon’s body washes ashore and starts to break down, or decompose. Our carbon atom from the decomposing fish now goes into the soil for microorganisms to use. Eventually, the carbon will return to the atmosphere as either CO₂ or CH₄ (methane), and the carbon atom will begin another incredible journey. Our carbon atom may have been traveling for millions of years, and, over the next million years, it will have many new and exciting adventures. The movement from atmosphere to plant to animal to another location is all a part of what we refer to as the carbon cycle.

1 Based on this reading, which of the following statements is true?
A Carbon atoms move between the atmosphere and organisms, connecting Earth’s ecosystems.
B Carbon atoms remain in the atmosphere for their long journey.
C Once a carbon atom becomes a part of a sugar, it will always be a sugar.
D Carbon can only enter an animal’s body when it takes in sugars from photosynthesis in plants.
2 Which of the following statements is NOT true regarding the element carbon?
A Carbon can only form a few types of chemical compounds.
B Carbon may be found in many types of molecules.
C Carbon can bond to other molecules in many ways.
D Carbon is an important element found in all biological organisms.
3 What happened to the carbon atom when it entered the plant through the stoma on the leaf?
A It moved from a sugar molecule to a carbon dioxide molecule.
B It moved from a gas molecule to a protein molecule.
C It moved from a carbon dioxide molecule to a sugar molecule.
D It remained a carbon dioxide molecule in the leaf.

4 Carbon may appear in more than one form on Earth. Several of those forms were discussed in the reading. What is one form that was not discussed?
A Carbon dioxide (CO₂)
B Glucose sugar (C₆H₁₂O₆)
C Calcium carbonate (limestone) (CaCO₃)
D A hydrocarbon (a CH chain)
5 Carbon is an important element for biological life because it–
A is always attached to oxygen atoms.
B can form many important molecules.
C does not easily go through changes.
D is only found in plants, such as trees.
6 Which of the following statements is false?
A Carbon can be stored in the body fat of a salmon.
B Carbon can move within the cells of an organism.
C Carbon is broken down by amino acids.
D Carbon can stay in soil bacteria for a long time.

1. Look at the diagram of the nitrogen cycle. Why is the cycling of nitrogen important to organisms on Earth?

2. Look at the diagram and explain how it demonstrates that cellular respiration and photosynthesis work together to meet the needs of both plants and animals.

3. Explain how human activities have disrupted the carbon and nitrogen cycles on Earth. How do these disruptions affect ecosystem services like water, food, and medications that people need?
4. Introducing a non-native plant species can reduce the biodiversity of an area because invasive species can kill or damage the native species in an area. Reduction in biodiversity threatens the existence of resources needed by humans for survival. Choose a specific ecosystem in Mississippi and design a system to prevent invasive species from entering that ecosystem.

Of the more than 100 known elements, carbon and nitrogen are two of the most important and widely used in living systems.

Write a scientific explanation describing the processes by which carbon is moved through Earth’s spheres. Include a diagram of this process in your reasoning. Claim: Evidence: Reasoning:
Rebuttal:


Working with a partner, sort the scenario cards based on whether the properties described are physical or chemical properties.
Record your answers in the table below.
Physical Properties Chemical Properties

Station 1: Magnetism
Organize your observations using the chart below.
Data:
1. How did you determine if an object was magnetic or not?
2. What common physical property do the magnetic objects share?
3. Are all objects made of metal also magnetic?

Follow the steps posted at the station and write your answers below.
Data:
Use the results of this activity to answer the questions below.
1. Did you use the same method to calculate the volume of each object? Explain.
2. If two objects were made of the same material but were different sizes, would the density be the same or different? Explain.

Follow the steps posted at the station and write your answers below.
Data:
1. What does it mean for a substance to be “soluble” in water?
2. Which objects would you classify as “soluble” and which would you classify as “not soluble”?
3. What physical property do the soluble substances have in common?

Write your answers in the chart below.
Data:
1. Which cup kept the ice from melting the most?
2. In which cup did the ice melt the most?
3. Which cup is the best conductor of thermal energy?
4. Which cup is the worst conductor of thermal energy?
5. If you wanted to keep a drink cold or warm, which is the best insulator?

Write your answers for what happens when you create a simple circuit, and identify which materials are conductors and insulators.
1. What physical property is shared by the items you observed to be conductors?
2. What physical properties are shared by the items labeled as insulators?
3. Are all metallic substances conductors of electrical energy? Explain.

Answer the questions below.
1. What state of matter will boil?
2. Which substance has the highest boiling point? What is its boiling point?
3. Which substance has the lowest boiling point? What is its boiling point?
4. If liquid samples of each substance were placed in glass beakers on a hot plate, which substance would boil first? How can you tell?
5. Which state of matter will melt?
6. Which substance has the highest melting point? What is its melting point?
7. Which substance has the lowest melting point? What is its melting point?
8. If solid samples of each substance were placed in glass beakers on a hot plate, which substance would melt first? How can you tell?
9. How do melting points and boiling points help scientists identify and classify substances?

Characteristics of matter used to describe or identify a substance are called properties. Properties of matter can be classified as physical or chemical. Physical properties can be observed without changing the composition of a substance. In contrast, chemical properties are characteristics that can only be observed or measured when atoms of matter rearrange during a chemical reaction. For example, when something is flammable, it will react with oxygen, producing new products and releasing energy in the form of heat and light. Can you think of other examples of chemical properties?
• DO NOT mix any items unless instructed to do so.
• DO NOT taste any of the materials; some of them are poisonous.
• Wear goggles and an apron at all times.
• Wash your hands after completing the experiments.
Record your observations:

Analyze and interpret your data to answer the following questions.
1. Examine your data from station 1. Based on your observations, what is one chemical property of baking soda?
2. Examine your data from station 2. Based on your observations, what is one chemical property of steel wool?
3. Examine your data from station 3. Based on your observations, what is one chemical property of a match head?
4. Examine your data from station 4. Based on your observations, what is one chemical property of effervescent tablets?
5. Examine your data from station 5. Based on your observations, what is one chemical property of iodine?

Scientific Investigation
Pure substances can be identified and distinguished from each other by investigating their physical and chemical properties. Each pure substance has a characteristic set of properties that gives it a unique identity.
Procedure
Plan an investigation to determine the identities of four liquids by testing their properties.
Step 1: Question

Step 2: Relevance
Step 3: Variables, if applicable
Step 4: Hypothesis

Step 5: Materials
Step 6: Safety Considerations
Step 7: Procedure
1. Determine the density of each of the four unknown liquids by measuring the mass of 25 mL of liquid. Record the results in the data table on the following page.
2. Obtain samples of salt, sugar, and oil from your teacher. Investigate the solubility properties of the four liquids by observing how readily each of these samples dissolves in each liquid. Use 10 mg of salt, 10 mg of sugar, and 10 mg of oil in 25 mL of liquid for each test. Record your observations in your data table.
3. Carefully note whether each liquid has a distinctive odor. CAUTION: DO NOT PUT YOUR NOSE TO THE CHEMICAL AND INHALE. Instead, hold the beaker at a distance from your face. Gently wave your hand over the top of the beaker to waft a little of the vapors toward your nose. You will notice any distinctive smell in this way in a safe manner. Enter your observations in your data table.
4. Obtain samples of the four liquids, which have been in a freezer overnight, from your teacher. Some may not be frozen. Note the temperature of any samples that are still liquid in your data table. For frozen samples, use a beaker and hot plate to warm the sample. Note the temperature at which each sample begins to melt and record this in your data table.
5. Heat each sample until it begins to boil, and record the temperature in your data table.
6. Conduct a flammability test for each unknown liquid by dipping a scrap of paper in the liquid and carefully holding it over a candle flame. Remember to use tongs, fire mitts, and safety goggles.

Step 8: Data Collection
Use the table to record your data.
Density
Solubility
Odor
Melting Point
Boiling Point
Flammability
Identity of Liquid

Step 9: Data Analysis
Create a graph based upon the data, if needed. Make a general statement about the results.

Step 10: Conclusion and Scientific Explanation
Write a scientific explanation of how comparing and contrasting the chemical and physical properties of substances can help you identify one from the other.
Claim:
Evidence:
Reasoning:
Name: ____________________________ Date: ___________

Earth contains a number of different materials that we use to meet specific needs. We breathe air, drink water, and use different rocks and stones for construction and transportation. Early humans used rocks and minerals that they took from the ground to help them hunt and travel and provide them with shelter. Humans could remove materials such as copper, gold, and silver from Earth rather easily. They found these materials had properties that made them useful.
Over time, the way that humans used these materials changed their lives. Iron, silicon, and wood are only a few of the many materials that have made building, traveling, and communicating much easier. How do you use these materials to make your life easier? What properties of these materials make them so useful?
How can matter be classified using physical properties?
There is matter all around us that makes up our world. All matter has physical properties that can be used for classification.

Can you name some specific physical properties of these ice cubes?


Iron can be found beneath Earth’s surface. What physical properties does iron have?
matter: anything that has mass and takes up space
Based on the physical properties you see in this picture, how would you describe the matter shown?
Matter can be described, compared, and classified by its physical properties. Matter has both physical and chemical properties. Physical properties can be observed using our senses and measured using various tools.
We can classify matter based on physical properties, such as density (ability to float or sink), attraction to a magnet, melting and boiling points, solubility in water, and ability to conduct heat or electricity. We will learn about each of these below.
property: a characteristic or feature of a substance or an object
Density is the amount of matter in a given space. In other words, it is an object’s mass per unit of volume. One way we can observe the density of objects is by seeing if they have the ability to float or sink in water. Density can also be calculated using this equation:
D = m/v
D = an object’s density; m = an object’s mass; v = an object’s volume

When determining how packed or compact the mass of an object or a substance is, you are calculating density. It does not matter if the total amount of the object or the substance changes; the density always stays the same.
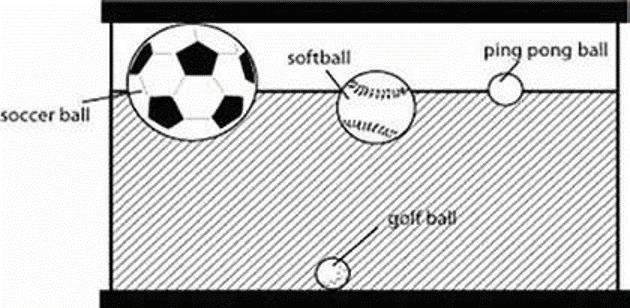
Observing whether something sinks or floats as compared to something else is known as relative density. In the picture above, the soccer ball is less dense than the golf ball because it floats. The particles of the golf ball are tightly packed, so its density is greater than the soccer ball’s. That is why the soccer ball is floating and the golf ball has sunk to the bottom. Can you name anything else that is less dense than the golf ball?
Wood is classified with a lower density than water because a piece of wood floats in water. Other examples of relative density include rocks sinking in water and helium balloons floating in air.
Density can be measured and calculated.
In order to find an object’s density, you first need to find the mass and volume of the object. The units for density are grams per cubic centimeter (g/cm3) or grams per milliliter (g/mL). A cubic centimeter is equivalent to a milliliter. For example, water has a density of 1 g/mL or 1 g/cm3. In other words, for every 1 gram of water you have, it takes up 1 milliliter, or 1 cubic centimeter, of space. If you were to put 1 milliliter of water in a test tube and measure its mass, it would be exactly 1 gram.
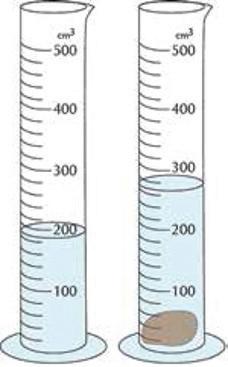
Let us investigate how to determine the density of a rock. To find the mass of the rock, you can use a balance. Let us say that the rock has a mass of 25 grams. To find the volume of the rock, you can use a graduated cylinder with a specific amount of water. Graduated cylinders measure milliliters (mL), which can be converted to cubic centimeters (cm3). When you put the rock in the graduated cylinder, the water level will rise. Find the difference between the volume of water before the rock was added and after the rock was added. Let us say the water rose 4 mL. This would mean that the volume of the rock is 4 mL, or 4 cm3. Now let us use the equation we learned on the previous page to calculate the density:
D = m/v
D = 25 grams/4 cubic centimeters = 6.25 grams/cubic centimeter
To find the volume of the rock, measure the amount of water in a graduated cylinder, and then place the rock inside. Check how much the water rises. The difference between the volume of water before the rock was added and after is the volume of the rock.

Magnetism
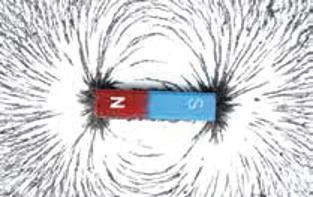
Magnetism is a force between certain kinds of objects. This force can be either a push or a pull. The force can act between two magnets or between a magnet and something made of iron or steel. Magnets are also made of iron. You can use magnetism to classify matter.
A magnet, or magnetized material, is an object with a north and south pole that produces a magnetic field. Certain objects, particularly certain metals such as iron, that enter the magnetic field are attracted to the magnet. Be careful! Not all metals are magnetic, including aluminum, copper, and gold.
The magnetic force of a magnet forms a pattern called a magnetic field. A magnetic field is made up of magnetic lines of force. The lines of force are invisible, but there is a way to see their shape. Try putting a magnet under a piece of paper and sprinkling small bits of iron filings on the paper. The iron bits will line up with the lines of force. You can see this in the picture on the right.
Boiling and Melting Points

Magnets come in many shapes and sizes.
The boiling point is the temperature at which a substance changes to a gas when heated. Have you ever seen boiling water in a pot? When you heat water on the stove, and the temperature reaches 100°C, it will begin to bubble up or boil as it changes to a gas. This is an example of a substance reaching its boiling point! Various substances have different boiling points, allowing this to be another way to classify matter.
The melting point is the temperature at which a substance melts, changing from a solid to a liquid. Have you ever seen melting crayons? When you heat crayons in a pan, and the temperature reaches 48.89°C, the crayons begin to melt. This is an example of a substance reaching its melting point! Just like the boiling point, various types of matter have different melting points. This is another way to classify matter.
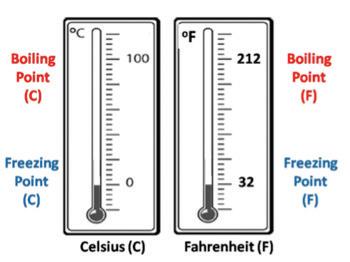
All substances have specific boiling and melting points. They are important because they help you distinguish and classify different kinds of matter. Boiling and melting points are constants, meaning they do not change unless the substance itself is changed. Whether you have a large pot of water or a small droplet of water, the boiling point does not change.
Once the liquid starts to boil, the temperature remains constant until all of the liquid has been converted to a gas. If you add salt to water, though, you are changing pure water into a different substance. This new substance will have a different boiling point.

Solubility is another way to classify matter. Solubility is the ability of a substance to dissolve when placed in a liquid. The substance that dissolves in a solution is called the solute. The substance that does the dissolving is called the solvent. For a given solvent, some solutes have greater solubility than others. For example, sugar is more soluble in water than salt. Yet sugar does have a limit on how much can be dissolved in water. Sugar will not dissolve if you add more than 1,000 grams to a half liter of water at 20°C. If you add more sugar than this, the extra sugar will not dissolve in it.
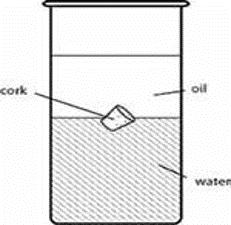
Water is one of the most abundant liquids on Earth. It is also one of the best solvents. Water dissolves many different types of substances. However, substances such as vegetable oil do not dissolve in water.
Instead, substances with a high density will sink beneath the less dense substance. In this example, water is more dense, so it will sink below the vegetable oil.
The temperature of a solution can also affect the solubility of a solute. Typically, the solubility of a solid in a liquid solute increases with temperature. Have you ever noticed that you can dissolve more lemonade mix in hot water than in cold water? On the other hand, gases are less soluble in liquids at higher temperatures. If you were to leave a can of soda in a car on a very warm day, it might explode. The soda is a mixture of gas (carbon dioxide) and liquid (water). At warmer temperatures the gas particles move more quickly, so it is easier for them to escape from solution. As they collide with greater force against the walls of the can, pressure builds inside the can. Eventually, the pressure could cause the can to explode!
Have you ever put a metal spoon in hot soup and then touched the spoon to your mouth? What do you think might be happening between the molecules in the soup and the atoms in the spoon to make the spoon get hot? Energy passes through some materials easily. These materials are called conductors. Conducted energy can be in the form of heat or electricity. Thermal or electrical conductivity is another way to classify matter.

To understand how heat and electricity move, we must understand that the atoms inside of matter are always moving. Heat is the energy of these atoms when they move. If the atoms in an object move faster, the object feels hotter. Picture a pot that is put on a hot stove. The atoms closest to the stove burner begin to move faster and faster. Faster atoms bump into slower atoms nearby and cause them to speed up. This is how heat spreads. The best conductors of heat or electricity are metals. Silver, copper, gold, aluminum, and iron are some of the best conductors of heat. Silver and gold are very expensive; therefore, most pots and pans are made out of copper, aluminum, and iron. Metals are also good conductors of electricity. Lamps, toasters, TVs, and other electrical devices are connected to electrical cords. Inside electrical cords are metal wires usually made of copper.

Look Out!
Extensive properties are physical properties of matter that depend on the amount of matter found within a substance. The amount of matter may be represented by mass, number of particles, or the amount of energy within the substance:
• Mass—a measurement of the amount of matter in an object; measured on a balance scale in units of grams
• Weight—a measurement of the gravitational force of attraction of Earth acting on an object; measured by a spring scale in units of newtons
• Volume—a measurement of the amount of space a substance occupies. Liquid volume is measured in units of liters using a graduated cylinder or beaker. Solid volume is measured using units of cubic meters or cubic centimeters.
• Length, width, height—measurements of linear dimensions in units of meters
Intensive physical properties are physical properties of matter that do not depend on the amount of matter found within a substance. Instead, intensive properties depend on the type of matter found within a substance and are represented by properties such as these:
• Color— the shade you see (Example: Sulfur is yellow, while the carbon in coal is black.)
• Odor—a smell, such as musty, sweet, etc. (Example: Hydrogen sulfide smells like rotten eggs.)
• Luster—how shiny a substance is, or its reflectivity (Example: Metals are shiny, while baking soda is dull.)
• Crystal shape—the geometric form of a solid (Example: Quartz crystals are shaped like hexagonal prisms.)
• Malleability—the ability of a substance to be beaten into thin sheets (Example: The malleability of gold can form it into thin sheets called gold leaf.)
• Ductility—the ability of a substance to be drawn into thin wires (Example: The ductility of copper makes it a common metal used as wire.)
• Conductivity—the ability to allow the flow of thermal energy or electricity (Example: Metals are good conductors of both heat and electricity.)
• Hardness—how easily a substance can be scratched (Example: The mineral talc is so soft it can be scratched with a fingernail, while diamond is so hard you can cut it only with another diamond.)
• Solubility—how easily a substance can dissolve in water (Example: Sugar dissolves in water.)
• Magnetism—the ability to attract iron (Example: Magnetite is naturally magnetic.)
• Melting/freezing point—the temperature at which a solid becomes a liquid and at which a liquid becomes a solid, measured in degrees Celsius
• Density—the amount of matter per unit of volume measured in g/cm3 (Example: Metals are denser than plastics.)



The physical properties of matter are measured without changing a substance’s chemical identity. Extensive physical properties such as mass, weight, volume, and density do depend on the amount of matter. Intensive physical properties such as freezing/melting point, boiling point, and conductivity, for example, do not depend on the amount of matter.
The chemical properties of matter (such as flammability, ability to oxidize, reactivity to water, heat of combustion, pH, and reactivity) are measured only by changing a substance’s chemical identity. A chemical property defines whether a chemical reaction will or will not take place. Chemical properties of matter include all of the possible chemical changes that a sample of matter can undergo. Scientists use chemical properties to predict whether a sample will have a certain chemical reaction.
Chemical properties may be used to classify compounds and find applications for them. Understanding a material’s chemical properties helps in its purification, in its separation from other chemicals, or in identifying an unknown sample. Chemical properties include the following:
• Flammability—the ability to ignite in the presence of a flame; how quickly a material will catch on fire (Example: Wood and paper are highly flammable, while rocks are not.)
• Combustibility—the ability to burn
• Ability to oxidize—the ability to gain oxygen, lose hydrogen, or gain electrons (Example: Iron corrodes into rust easily.)
• Reactivity to water—whether a material produces violent bubbles or explodes in the presence of water (Example: Alkali metals react violently with water.)
• pH—reactivity with water to see how acidic or basic a substance is (Example: Citric juices are very acidic [have a low pH], while detergents are very basic [have a high pH].)


Try Now
When a substance boils at a certain temperature and changes to a gas, this is called its boiling point. When a substance melts at a certain temperature and changes to a liquid, this is called the melting point. Every substance has a unique boiling and melting point. Use the examples listed in the table below to answer the questions.
Answer these questions:
1. What is the melting point of ammonia?
2. What is the melting point of olive oil?
3. What is the boiling point of salty ocean water?
4. What is the boiling point of petroleum?
5. Which substance boils first: pure water or petroleum? Why?
6. Which substance melts first: salty ocean water or sodium chloride? Why?
7. What is the boiling point of a substance?
8. What is the melting point of a substance?
9. How do the boiling point and melting point differ?

Try Now
You learned about many physical properties that can be demonstrated, measured, and used to classify and compare matter. These physical properties were density, thermal or electrical conductivity, solubility, magnetic properties, and melting and boiling points. Look at the columns below. Match each property below with its definition by writing the letter of the definition that matches in the blank by each word.
___ 1. Melting point ___ 2. Thermal conductivity ___ 3. Density ___ 4. Magnetic property ___ 5. Solubility
___ 6. Boiling point
___ 7. Electrical conductivity
___ 8. Flammability
___ 9. Combustion
___10. Oxidation
___11. pH
___12. Solubility
A. The amount of matter in a given volume
B. A material’s ability to conduct an electric current
C. The temperature at which a substance changes from a liquid to a gas
D. The property of a material to conduct heat
E. The temperature at which a substance changes from a solid to a liquid
F. A force between two magnets or between a magnet and something made of iron
G. The ability to dissolve in another substance
H. The ability to dissolve in water
I. How acidic or basic a substance is
J. How quickly a material will burn
K. Will the material burn?
L. Will the substance combine with oxygen (rust)?

Try Now
Think about how to classify these different materials. Which physical property are you going to use to classify them? Put them in different groups.
Materials
Glass - Iron - Silver - Plastic - Salt - Steel - Wood - Styrofoam - Sugar - Cloth
Group 1
Physical Property:
Materials: Why?
Group 2
Physical Property:
Materials: Why?
Group 3
Physical Property:
Materials: Why?
You have learned that density can be calculated from this equation: D = m/v, where D is an object’s density, m is an object’s mass, and v is an object’s volume. Let us use your knowledge about density to solve these problems:
• A piece of aluminum has a volume of 12 cm3 and a mass of 32 g. What is its density? Do not forget the units.
Solution:
• A gold nugget has a density of 38.6 g/cm3 and a mass of 270.2 g. What is its volume?
This problem is a little different. It gives you the density and the mass. You need to find the volume. We need to rearrange the formula D = m/v. Move the volume and density to the other side: v = m/D.
Solution:

Take a few minutes to examine the physical properties of everyday objects with your child. To complete this activity, you will need the following materials:
• A bar magnet
• A beaker filled halfway with water
• 1 D battery and electrical tape
• Bulb holder with light bulb or alternate insulated bulb holder
• 12" pieces of number 22 insulated copper bell wire with approximately 1" of insulation stripped off all ends
• Several small objects such as an iron nail, a marble, a wooden block, and a piece of aluminum foil. (Each object should fit completely in the beaker.)
Follow these steps:
1. Record the physical state of each object.
2. Predict which object has the greatest mass and which object has the least amount of mass.
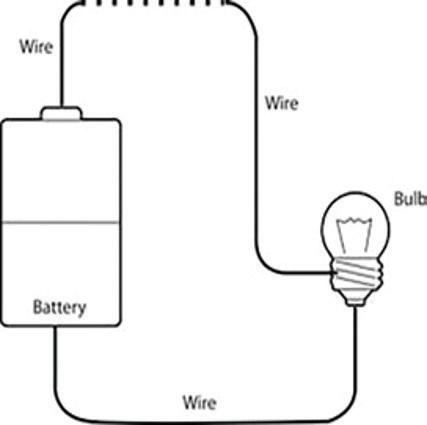
Place the various objects in the dotted-line section of the circuit to test for conductivity.
3. Predict whether each object is magnetic. Then place the magnet next to each object and record what happens.
4. Predict whether each object is denser than the water in the beaker. Then place each object in the water and record what happens.
5. Predict whether each object is conductive. To test electrical conductivity, take one wire and attach it to the positive (+) side of the battery with electrical tape and attach the other side to one side of the bulb holder. Take another wire and attach it to the negative (-) side of the battery with electrical tape and leave the other side clear. This side will be used to touch the different objects. Take another wire and attach it to the other side of the bulb holder and leave the other side of the wire clear. This side will be used to touch the different objects. Look at the diagram above for a possible circuit arrangement. If the light turns on, the object is a good electrical conductor. If the light does not turn on, the object is not an electrical conductor and is an insulator.
Note: DO NOT use household electrical current for this experiment. This experiment should be conducted under adult supervision.
Here are some questions to discuss with your child:
1. What is a physical property of matter?
2. Name some physical properties of matter.
3. How are physical properties used to classify matter?
4. Which objects are magnetic?
5. Which objects are denser than the water?
6. Which objects are good electrical conductors?

1 All matter is made of tiny particles called atoms. It is the number of protons within the nucleus of atoms that gives each element its own identity. In a pure element, every atom within that element has the exact same number of protons. Elements cannot be broken down (decomposed) into parts smaller than the atoms they are made of. Each element is known as a pure substance.
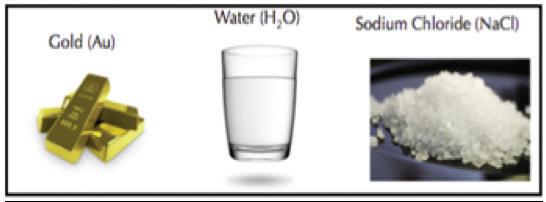
2 A pure substance is simply one type of matter with its own physical and chemical properties. We know that a pure substance can be one type of element, such as gold. However, pure substances may also be compounds (substances made of more than one element) such as water and table salt. Water (H₂O) and table salt (NaCl) are also pure substances. Water is made of two hydrogen atoms (H) and one oxygen atom (O). Table salt is made of one sodium atom (Na) and one chlorine atom (Cl). Pure substances cannot be separated by physical changes such as heating or freezing. Pure substances that are compounds may be separated or created by chemical changes.
3 All matter has both physical and chemical properties. Physical properties include smell, color, the ability to conduct electricity or act as a magnet, melting point, and boiling point. Density (the mass per volume of a substance) is also a physical property. Density, however, may change as a substance changes its state. For example, in water, changes of state, such as freezing or turning to steam (water vapor), are physical changes. The water stays water no matter what state it is in. Other examples of physical changes include when large salt crystals are ground into smaller grains or when gold is shaped into a ring.
4 Chemical changes result in the breakdown of substances or the formation of new substances. The starting compound is changed and a new compound is created. A chemical property describes how a substance reacts with other materials. This includes all of the possible chemical changes that a substance can go through. Some examples include whether a metal will rust or if a substance is flammable. Some substances rust and some do not. Some substances burn while others do not. In fact, some substances explode violently.
5 Gold is an element, and all of the atoms found in gold contain 79 protons. Gold cannot be broken down into anything other than individual gold atoms, making gold a pure substance. It cannot be changed by physical means such as breaking it into smaller pieces or melting it. It will still be gold. Gold will combine with other elements and compounds in very predictable ways, giving gold its own unique chemical properties. Each time gold reacts with another substance to form a new compound, this shows that a chemical change has taken place.

6 Even though salt and water are compounds, we can show that they are also pure substances. Pure liquid water will have the same freezing point and melting point as it undergoes physical changes. However, its density will change as it changes state. Frozen water (ice) is less dense than liquid water, which is why ice floats in your glass of iced water. Both states of water (ice and liquid) are still water. Water also reacts with other substances in predictable ways. It can either be broken apart or created in a chemical reaction. Pure sodium chloride (table salt) works in the same way. You can change table salt by physical means, but pure sodium chloride will always have its salty taste and a melting point of 801°C no matter how you physically change it.
7 These physical and chemical properties change if you combine salt and water, as you will have a mixture of two pure substances. The mixture will not have set physical and chemical properties, since the chemical makeup of the mixture depends on the amount of salt and water that are mixed together. For example, if you add a pinch of salt to the water, this will change the freezing point of the solution (the salt and water combination.) The more salt you add, the lower the mixture’s freezing temperature will be. Also, you can separate the salt and water mixture by physical means. If you gently heat the saltwater solution, the water will evaporate in the form of steam (water vapor), leaving you with salt in the pan.

1 Paragraph 2 is about pure substances. Which one of the following statements is true?
A Pure substances can be separated by physical changes.
B Pure substances have their own physical and chemical properties.
C Elements are the only true pure substances.
D Compounds can never be pure substances.
2 The element gold will have the same color regardless of the physical changes that it goes through. This identifies gold as what type of substance?
A A mixture
B A compound
C A pure substance
D A physical property
3 Water and salt are compounds made of the combination of more than one type of atom. Which statement is false?
A They cannot be pure substances because they are compounds.
B Both compounds are considered pure substances.
C These compounds have specific physical properties that never change.
D These compounds have specific chemical properties.

4 When pure water and sodium chloride combine, what do they create?
A Another pure substance
B A mixture
C A chemical change
D Not enough information is given
5 Pure water can be chemically broken down in a chemical reaction, resulting in the formation of a new product. What type of change is this?
A A physical change
B A chemical change
C A mixture change
D A compound change
6 Iron filings are placed in powdered sulfur. Both iron and sulfur are elements. The iron and sulfur are stirred together until they are evenly mixed. Then, the iron filings are removed from the sulfur with the use of a magnet. What type of substance is the iron and sulfur combination?
A A pure substance
B A compound
C A solution
D A mixture

1. Explain why some objects float in water and others sink.
2. Look at the following table and decide which materials will sink and which will float. Explain your reasoning. The density of water is 1 g/cm3

3. Describe the physical properties of gold.

4. Look at the two pictures of a steel chain and describe a chemical property of steel.



5. Sort the following properties as either chemical or physical. What is the difference between a chemical property and a physical property?
• conducts heat
• conducts electricity
• flammability
• magnetic
• color
• density
• soluble in water
• oxidizes
• reactivity
• volume
• mass or weight
• melting point, freezing point, boiling point
• combustion
• pH

Jess, Lara, and Sue are students working on a lab assignment. Their task is to identify which of five substances is baking soda. They know that baking soda has an immediate reaction with some liquids.
Based on the data table, which of the substances is baking soda?
Observations before Substances Interact
Texture Granular Granular Fine powder Fine powder Fine powder
Color White White White/yellow White White
Observations after Substances Interact
Interaction with H2O Dissolves Dissolves Does not dissolve Does not dissolve Dissolves
Interaction with Vinegar Dissolves Dissolves Forms bubbles Forms bubbles Forms bubbles

Write a scientific explanation that justifies your selection.
Claim:
Evidence:
Reasoning: Prompt 3
Rebuttal:

1. Using arrows to represent pressure, draw the air inside of the can before, during, and after the activity.
2. What does this activity show about the relationship between temperature and pressure?
3. What type of proportional relationship is it?
4. What does this activity show about the relationship between volume and temperature?
5. What type of proportional relationship is it?
6. How could you perform this activity so that the can crushes at a slower speed?
1. How does this activity show the relationship between temperature and density?
2. What type of proportional relationship is it?
3. How could you perform this activity so that the spinner spins at a slower speed?

Station 3: Chill Out, Warm Up
1. After two minutes, record the temperature of the ice water and the volume of air in the syringe.
Temperature: ______________ Volume: _________________
2. After two minutes, record the temperature of the warm water and the volume of air in the syringe.
Temperature: ______________ Volume: _________________
3. What does this activity show you about the relationship between temperature and volume? What type of proportional relationship is it?
4. How could you perform this activity so that the volume increases at a faster speed?
Station 4: Steak Sauce Gas?
1. What does this activity show about the relationship between temperature and pressure? What type of proportional relationship is it?
2. How could you perform this activity so that the pressure changes at a faster speed?
Station 5: Not So Squishy
1. Draw the appearance of the marshmallow once you pulled back on the plunger, and then after the plunger was released.
2. What does this activity show about the relationship between volume and pressure?

Answer the following questions using arrows or using the words increases or decreases
1. When the pressure of a gas ↑, density____________.
2. When density of a gas ↓, the pressure ____________.
3. When the temperature of a gas ↑, volume ____________.
4. When the temperature of a gas ↓, volume ____________.
5. When the volume of a gas ↑, density ____________.
6. When the volume of a gas ↓, density ____________.
7. In a fixed volume, when temperature ↑, pressure ____________.
8. In a fixed volume, when temperature ↓, pressure ____________.
Based on your observations, complete the chart to show what will happen to other properties of the gas in a balloon as the density, pressure, temperature, and volume of particles in a balloon increase (+) or decrease (-).
Answer the following questions using the words inversely or directly
9. In gases, pressure and density are ____________ related.
10. Temperature and volume are ____________ related.
11. Volume and density are ____________ related.
12. Temperature and pressure are ____________ related.
13. Do these same answers hold true for liquids? Explain.
14. Do these same answers hold true for solids? Explain.

Observations
Record your direct observations during the demonstration:
Questions
Working with your team, develop 10 questions to ask your teacher that will help you to explain your observations using scientific concepts.
Scientific Explanation
Use your observations to write an explanation of how the density of matter was affected on the molecular level during this demonstration.

Observations
Record your direct observations during the demonstration.
Questions
Working with your team, develop 10 questions to ask your teacher that will help you to explain your observations using scientific concepts.
Scientific Explanation
Use your observations to write an explanation of how the density of matter was affected on the molecular level during this demonstration.

Materials science is the study of matter and its arrangement and function. Materials science combines chemistry, the study of matter, with engineering in order to create new materials to improve our lives. In the modern age, materials are either naturally occurring or synthetic (man-made). Understanding naturally occurring materials can lead to breakthroughs in the engineering of synthetic materials.
You are composed of naturally occurring materials, while much of everything else you encounter in daily life is a combination of natural and synthetic materials. Examine your clothes; look around this room; think of your ride to school. What examples of natural and synthetic materials do you see? What understanding of matter and its properties led to the usefulness of the materials?
This Explore examines a variety of materials along with their chemical properties and applications. In Part I, you will investigate the material rubber. Rubber is a naturally occurring polymer found in rubber trees. In Part II, you will work with candy fibers and consider the science behind recent breakthroughs in glass technology. Finally, in Part III, you will consider the structure and function of concrete.
A polymer is a chain of molecules connected by covalent bonds. There are many different types of polymers both natural and synthetic (man-made). DNA, found in all living things, is a naturally occurring polymer. DNA is composed of repeating monomers called nucleotides that are connected in long chains. Cellulose is found in the cell walls of plants. It is composed of monomers of glucose. This polymer makes up the cell walls of plants.
Can you think of other naturally occurring polymers? You may have noticed your list of polymers is very diverse. Polymers serve a variety of purposes in nature. Many naturally occurring polymers are structural. Polymers may be strong, flexible, or sticky, among other things.
Because naturally occurring polymers have so many functions in nature, scientists have worked to create a variety of synthetic polymers in the laboratory.

Nylon is a synthetic polymer manufactured to have properties similar to silk. Kevlar® is a very strong polymer used in bulletproof vests.
Glue or adhesive is a product manufactured from different combinations of monomers. Can you think of other synthetic polymers?
In this activity you will investigate the effects of temperature on the properties of rubber. Considering what you know about the effect of temperature on the motion of molecules, make a prediction about the effect of temperature on the elasticity of a rubber band.
Prediction:
Procedure
1. Tie the room-temperature rubber band to the ring clamp attached to a ring stand.
2. Tie the weight to the opposite end of the rubber band.
3. Place the ring stand near the edge of the table so that the weight can hang over the edge if needed.
4. Use a meterstick to measure the distance of the weight from the ring clamp.
5. Record measurements in the data table.
6. Obtain a cold rubber band from your teacher.
7. Repeat steps 1–5 using the cold rubber band.
8. Obtain a hot rubber band from your teacher.
9. Repeat steps 1–5.
Analysis
Based on your observations, what can you conclude about the molecular relationship between temperature and the elasticity of rubber?

1. Was your prediction correct? Explain.
2. Think about products made from synthetic polymers. Can you think of a product that uses an understanding of polymers and temperatures to solve an everyday problem?
3. Why is it important for scientists to understand the behavior of molecules in polymers to create products used every day?
4. Working with your group, consider a current problem that you think can be solved by technology that utilizes an understanding of the behavior of molecules in a polymer. What is the problem, and how would you propose to solve it?

Glass is a common material that has been manufactured and used by man for hundreds of years. Early in its history, glass was used to make containers, dishes, windows, and lenses. More recently, scientists have applied their understanding of glass fibers to create high-speed communication cables, medical devices, and of course, electronics.
In this activity, you will investigate the properties of hard candy. You should find that many of the properties of candy are similar to those of glass.
Considering what you know about the effect of temperature on the motion of molecules, make a prediction about the length and strength of candy glass fibers.
Prediction:
Procedure
1. Begin with a clean, dry beaker.
2. Place three unwrapped hard candies in the beaker.
3. Place the beaker on the hot plate on a medium temperature setting. Do not heat on high. Sugar will burn.
4. Heat the candy 5 to 10 minutes until it is melted into a liquid. Stir with a popsicle stick.
5. Note: Once the candy is in liquid form, use the wooden popsicle stick to pull one fiber from the beaker by dipping the skewer into the melted candy.
6. Each group member should take a turn pulling a fiber. See who can pull the longest fiber.
7. Make some long fibers and some short fibers.
8. See who can make the strongest fiber.
9. Record your observations in the data table.

Based on your observations, what can you conclude about the molecular relationship between temperature and the length and strength of fibers?
Questions
1. Was your prediction correct? Explain.
2. Think about products made from glass. Can you think of a product that uses an understanding of the motion of the molecules in glass to solve an everyday problem?
3. Why is it important for scientists to understand the behavior of the molecules in glass to create products used every day?
4. Working with your group, consider a current problem that you think can be solved by technology that utilizes an understanding of the behavior of the molecules in glass. What is the problem, and how would you propose to solve it?

Concrete is a material that has been used by man since ancient times. As nations have industrialized, scientists and engineers have studied the physical and chemical properties of the components of concrete to formulate mixtures to perform specific structural and functional tasks.
In recent years, concern about the environmental impact of the use of concrete in cities has led to new interest in understanding the science of concrete. Scientists are now engineering a material that is strong but porous.
In this activity, you will investigate the properties of concrete.
Considering your personal experiences with concrete, make a prediction about the strength of concrete vs. its ability to absorb water.
Prediction:
Procedure
Day 1: Making Concrete
1. In your plastic cup, measure sand, gravel, cement, and water according to the ratio card provided by your teacher.
2. If your mixture is very dry and you cannot stir it, add additional water very sparingly until all parts are wet and you can stir the concrete.
3. Transfer your concrete to the Petri dish using a popsicle stick.
4. Smooth the concrete to create a level disk in the dish.
5. Allow the concrete to cure overnight in a warm, dry location.
Day 2: Experimenting with Concrete Water Runoff Test
1. Remove the concrete from the Petri dish.
2. Place the concrete on a paper towel.
3. Pour 25 mL of water on the concrete.
4. Record your observations in the data table.
Strength Test
1. Place your concrete disk in the C-clamp so that the clamp is just touching the concrete.
2. Tighten the clamp one turn at a time, counting the turns until the concrete cracks.
3. Record your observations in the data table.

Use the table below to record your observations about the properties of the concrete.
Analysis
Based on your observations, what can you conclude about the molecular relationship between the concrete formula and its properties?
Questions
1. Was your prediction correct? Explain.

2. Think about products made from concrete. How has the need for concrete changed through the years?
3. Why is it important for scientists to understand the behavior of the molecules in concrete when creating new products?
4. Working with your group, consider a current problem that you think can be solved by technology that utilizes an understanding of the physical properties of concrete. What is the problem, and how would you propose to solve it?

Your teacher will show you two different kinds of soap. As a class, you will perform a series of experiments on the soap to determine what differences are present. You will examine the soap samples, try to float them in water, and heat them in the microwave. Make observations about the soap samples in the spaces below.
Observations
Prior to Experiments: Smell, Appearance, Texture, etc.
Prediction: Will It Float When Placed in Water?
Result When Placed in Water
Prediction: What Will Happen When Heated?
Result When Heated
Prediction: What Will Happen When the Soap Is Cooled?
Result When Cooled

1. What differences were present between the soap samples in your initial observation?
2. Why do you think one soap floated while the other soap sank?
3. The floating soap contained small air bubbles. What happens to air bubbles, like balloons, when they are heated?
4. Both soaps contain water. When water is heated, what happens?
5. Describe the movement of the air molecules in the bubbles in the expanding soap.
6. The bubbles in the soap contained steam as the soap was heated. Once the microwave was turned off, the bubbles began to contract. Describe the movement of the steam molecules as the bubbles began to contract.
7. Many of the materials in soap are also present in self-healing polymers, an example of which is homemade slime. However, if you microwave slime, it will get hard instead of expanding into foamy bubbles. Create an explanation for why the materials behave so differently when heated.

Reflect
Imagine rafting with a group of friends along a winding river on a hot summer day. Occasionally you hit some exciting rapids, but mostly you cruise along at a leisurely pace. At noon, the group decides to take a lunch break. Everyone jumps out and helps pull the raft up on shore. It is very hot, so your friends find a shady spot a few yards away to eat lunch. Later, when you return to the raft, you are dismayed to find that the raft burst while sitting in the sunlight. What caused that to happen?
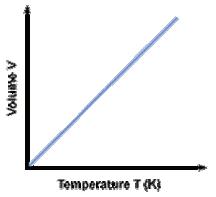
As the temperature of a gas increases, its volume increases when all other conditions are kept constant.

The volume of a gas varies directly with temperature
The rafting friends did not realize that the temperature and volume of a gas are directly proportional. The raft burst because the volume of air inside increased as it sat in the hot sunlight. Much earlier that day, when it was cooler, the raft had been inflated with air. As the day continued, conditions got hotter. The temperature of the air inside the raft increased quite a bit, reaching a very high temperature when it was left in the sunlight at noon.
The volume of the air increased in response to this temperature increase. However, because the canvas walls of the raft were not flexible, the expanding air exerted enough pressure to cause a hole to develop in a weak spot of the canvas.
The rafters have discovered an important gas law: as a gas’s temperature increases, the gas’s volume also increases. This is true of all gases, not just air. The graph above shows data collected for a gas as its temperature increased. Notice there is a direct, linear relationship between volume and temperature. In other words, as one variable increases, the other variable increases at the same rate. All other variables must be constant in order to observe this.
Charles’s Law
Mathematically, we can write the following equation to show this direct, linear relationship: V = kT
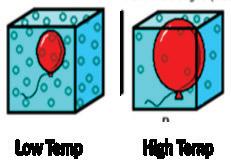
In this equation, V is volume, T is temperature, and k is a constant representing all other variables (such as pressure). This mathematical relationship can be rearranged and expanded to show how a gas’s volume changes when its temperature changes.


In this equation, V1 and T1 are the starting volume and temperature, and V2 and T2 are the ending volume and temperature. This mathematical relationship is known as Charles’s law, in honor of the French scientist Jacques Charles, whose experimental work supported its discovery.
Suppose you inflated a balloon with air at room temperature, and then you placed it in a freezer. What do you think you would observe about the balloon if you took it out of the freezer 30 minutes later? Does this fit the relationship expressed by Charles’s law? Explain.
Boyle’s Law
The volume of a gas varies inversely with pressure. Volume and temperature are not the only two properties of a gas that vary in a predictable way. The volume and pressure of a gas also show a mathematical relationship.
To think about this, consider how it would feel if you tried squeezing a balloon to decrease its volume. It is difficult to do, because you have to exert a great deal of pressure to force the balloon to assume a smaller size. From this thought experiment, you should be able to say that volume decreases as pressure is increased.
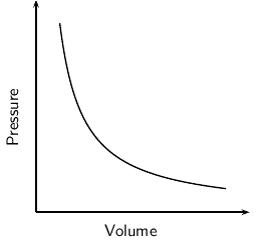
The pressure and volume of a gas vary inversely and in a nonlinear fashion. Pressure is measured in units called atmospheres (atm).

This means that volume and pressure are inversely related. If you actually performed an experiment to measure the change in volume as you increased the pressure, you would generate data similar to that shown in the graph above. Notice that the line drawn through the data points shows a downward trend, as you would expect from an inverse relationship. However, the data fit a curved rather than a straight line. All other variables must be constant in order to observe this relationship. Mathematically, the data can be expressed according to the following equation:
In this equation, V is volume, P is pressure, and k is a constant representing all other variables (such as temperature). The English scientist Robert Boyle discovered this relationship and published his discovery in 1662. In his honor, the relationship between pressure and volume of a gas is now widely known as Boyle’s law. Mathematically, Boyle’s law can be written to show how the pressure and volume of the same gas, taken at two different times, are related:
Think about a can of carbonated beverage. The carbon dioxide bubbles that were pumped under pressure into that soda at the factory are ready to escape. When you open a shaken can, the pressure in the liquid drops rapidly, and the volume of billions of CO2 bubbles increases instantly. Remember: as pressure decreases, volume increases proportionately. Spritz! The quickly expanding carbon dioxide bubbles instantly push any liquid above them out of the can as a foaming geyser.

The pressure of a gas varies directly with temperature. When the volume of a container is fixed, the pressure and temperature have a direct relationship. As the temperature increases, the kinetic energy of the particles increases, causing them to hit the sides of the container, increasing the pressure of the gas on the container.
The pressure and temperature of a gas vary directly and in a linear fashion. Pressure is measured in units called atmospheres (atm).
Gay-Lussac’s Law
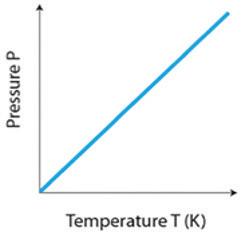
The graph above shows data collected for a gas as its temperature increased. Notice there is a direct, linear relationship between pressure and temperature. In other words, as one variable increases, the other variable increases at the same rate. All other variables must be constant in order to observe this relationship.
Mathematically, we can write the following equation to show this direct, linear relationship: P = kT
In this equation, V is volume, T is temperature, and k is a constant representing all other variables (such as pressure). This mathematical relationship can be rearranged and expanded to show how a gas’s volume changes when its temperature changes:
P1 P2
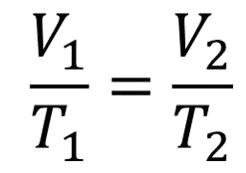
In this equation, P1 and T1 are the starting pressure and temperature, and P2 and T2 are the ending pressure and temperature. This mathematical relationship is known as Gay-Lussac’s law, in honor of the French chemist Joseph Gay-Lussac, who studied the pressure-temperature relationship between gas particles.
Try using Boyle’s law to calculate the pressure of a gas that expands to a volume of 20.0 liters (L) if the gas’s initial pressure is 2.50 atm and its initial volume is 5.00 L.

Look Out!
Would you leave your spray can of suntan lotion sitting out in the hot Sun? Of course not, but why not? The molecules of gas are under pressure at a fixed volume inside the can. As the Sun heats the can, the molecules move faster, thus increasing temperature. As temperature increases, the gas increases pressure against the sides of the can. If the gases become too hot, the can can explode!

Reflect
Effects of temperature and pressure on the physical state, molecular motion, and molecular interactions of polymers is a new field of research. A polymer is a large molecule (macromolecule) made of a chain of repeating structural units connected by covalent chemical bonds. In everyday language, polymers refer to plastics. Actually, polymers are a large group of natural and synthetic materials with a variety of unique properties.
Long-chained polymer molecules can be natural, such as proteins, DNA, and cellulose, or man-made, such as Styrofoam (made out of polypropylene). However, polymers are not just limited to having carbon backbones. Silicon is another element that is part of polymers like Silly Putty and waterproof plumbing sealant. Polymers that are stretchy and return to their original shape are called elastomers. Thermoset elastomers do not melt when heated, while thermoplastic elastomers do melt when heated. Elastomers have interesting applications:

• Natural rubber—used in the manufacture of gaskets, shoes, tires, etc.
Cross-linked polymer fibers bounce back to their original form.
• Polyurethanes—used in the textiles for elastic clothing such as lycra; also used as foam, wheels, etc.
• Polybutadiene—used on wheels or tires for wear resistance
• Neoprene—used primarily in wetsuits, wire insulation, industrial belts, etc.
• Silicone—used in a wide range of materials due to its excellent thermal and chemical resistance and in the manufacture of computer chips (silicon operates at higher temperatures than other semiconductors), pacifiers, medical prostheses, lubricants, industrial molds, and cookware.

Chemists have discovered new uses for “self-healing” elastomers that naturally spring back to their shape after being deformed, cracked, or scratched in the presence of heat. For example, car shops are using car-paint sealants made of a self-healing protective film that repairs itself after cracks or scratches if left for a few minutes in the Sun or a warm garage. The process is similar to your skin self-healing after a cut.

Chemists have also developed special concretes that allow water to pass through so standing water does not collect. This “pervious” or “porous” concrete is more friendly to the environment.
Consider the behavior of molecules of gas and the relationship between volume, temperature, and pressure in the three gas laws below. Next, apply them correctly to the three scenarios below. Answer each question and identify which gas law would be the best explanation and why.
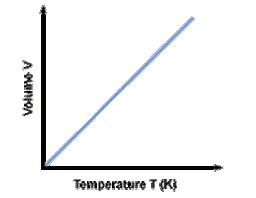
Charles’s Law

Boyle’s Law
Gay-Lussac’s Law
1. Consider a sealed glass bottle. If the bottle is thrown into a fire, what will happen to the bottle and why? Which law is the best explanation?
2. Why are SCUBA tanks stored in the shade rather than the Sun? Use a gas law to explain your answer.
3. Why do tire inflation alarms read high pressure in the summer and low pressure in the winter?

Construct and Test a Solar Balloon
To help your child learn more about Charles’s law, research hot-air balloons to learn how they operate. (A flame heats air inside the balloon, causing the air’s volume to increase; as the air expands, it lifts the balloon off the ground.) Basically, Charles’s law states that as the temperature of a gas increases, so does the volume.
Then, have your child research solar balloons to learn how radiant energy from the Sun can be used as a source of energy for heating air molecules inside a balloon to achieve lift.
Finally, have your child choose a design he or she finds in research to construct a solar balloon out of black, plastic trash bags.
Your child can test his or her balloon by attempting to launch it on a sunny day.
Here are some questions to discuss with your child:
• What allows a traditional hot-air balloon to achieve lift?
• What gas law applies to explain the behavior of a solar balloon?
• How does a solar balloon achieve lift, and how is it similar to and different from a traditional hot-air balloon?


1 As you know, all matter is made up of very small particles. What you may not know is that these are in constant motion. The particles in liquids are constantly moving, as are the particles in solids, such as rocks or frozen water (ice). You may know the term kinetic from kinetic energy, or the energy of motion. The state that matter exists in (solid, liquid, or gas) depends on something known as kinetic molecular theory.
2 In very simple terms, this theory states that all particles are very small, they are in constant motion, and they have an average kinetic energy that changes with temperature. This theory states that the particles in matter will move faster as energy is added (heated) or slower as energy is removed (cooled). For example, as energy in the form of heat is added to matter, that matter will change from a solid to a liquid to a gas.

3 The particles of solids are in fixed positions, making the solid hold a definite shape and volume. Generally, the particles in solids are so close to each other that there is very little room for movement. The particles in liquids are not in fixed positions. They are free to move past each other, but they are still bound to each other. Liquids take the shape of the container they are in, but they cannot hold that shape without the container. The particles in gases are very far apart from each other and are constantly moving. This allows gases to expand to fill any container size. There is no definite shape or volume for matter in the gas phase.
4 One way to compare the three states is to look at the density of matter (mass-to-volume ratio) in each state. The solid and liquid states have a higher density compared to the gas state. In the illustration above, notice how the mass-to-volume ratio (density) is different for each of the three states. The particles in the solid state are packed tightly together, making solids relatively hard to compress (push together). The liquid state has more space between particles than the solid state, but less than the gas state. This makes liquids more compressible than solids but less compressible than gases. Finally, the gas state has a lot of space between particles, making most gases highly compressible.

5 In looking at both volume and density, let us compare water in its various states of matter. Imagine it is in a closed container. Below 0°C, the molecules within ice (water in its solid state) are moving, but only vibrating in place. This holds the solid form of water in a fixed position. As energy (in the form of heat) is added to the ice cube, the kinetic energy increases in the ice particles, making the particles within the ice cube move faster. Eventually, the particles will gain enough energy to loosen the bonds holding them in the solid state, and they will begin to slide past each other.
6 Above 0°C, the ice changes from a solid state to a liquid state. At 100°C, or the boiling point of water, water changes from a liquid state to a gaseous state. In this state, the kinetic energy of the particles increases so much that the individual water particles break free of each other, forming individual gas particles (steam). The gaseous water particles will be free to move wherever they can, and the gas particles will now occupy the entire volume of a closed container. The more the water vapor is heated in a closed container, the greater the pressure will be in the container. This is because as the water vapor is heated, the kinetic energy of each particle is increased. Therefore, each particle will hit the sides of the closed container with greater force, thereby increasing the pressure within the container. This means that as the temperature of a gas increases in a closed container, the pressure in that container will increase as well.

1 Which particles are very far apart from each other, are constantly moving, and have no definite shape or volume?
A Solids
B Liquids
C Gases
D Water
2 What is the best definition of density?
A The change from a solid to a liquid state
B The mass-to-volume ratio of matter
C The kinetic molecular theory
D The constant motion of particles
3 Which particles are not in fixed positions, so they are free to move past each other but are still bound to each other?
A Solids
B Liquids
C Gases
D Ice

4 Which of the following is NOT a part of kinetic molecular theory?
A All particles are very small.
B All particles are in constant motion.
C All particles stay in fixed positions.
D All particles have an average kinetic energy that changes with temperature.
5 Which particles are in fixed positions and hold a definite shape and volume?
A Solids B Liquids
C Gases D Steam
6 Which of the following statements is NOT true?
A Gaseous water particles are free to move wherever they can.
B As energy (heat) is added to ice, the particles within the ice cube move faster.
C Solids, liquids, and gases all have the same density no matter how much energy is added.
D As the temperature of a gas increases in a closed container, the pressure increases as well.

1. How could the distance between molecules in a liquid be increased? How could it be decreased?

2. Observe the balloon in the picture. How could you increase the volume of gas in the balloon? What would that do to the density of the gas in the balloon? Why?
3. Look at the balloon in the picture again. How could you decrease the volume of gas in the balloon? What would that do to the density of gas in the balloon? Why?


4. How are pressure and volume related in a gas? How are temperature and volume related in a gas?


A balloon is filled with air. Suzie wants to understand how changes in temperature would affect the air in the balloon.

Using scientific reasoning, describe what would happen to the balloon if the temperature in the balloon were to increase and decrease.


Match the scientist’s name with the correct model and statement. Then write the information in the corresponding spaces in the timeline below.
Name
Model of Atom

Statement
E. An atom has a positively charged nucleus and negative electrons around it.
F. An atom is made up of negative particles evenly scattered in a cloud of positive charge.
G. The electrons in the atom can only occupy certain energy levels around the nucleus.
H. All matter is made up of particles called atoms that cannot be divided into smaller particles.

Hydrogen Atom Helium Atom
Key
Symbol

1. Fill in the data table below.
Subatomic Particle Proton Neutron
Electron
Properties of Subatomic Particles
2. Where is the mass of an atom found? Explain.
3. Look at the diagrams to determine whether the nucleus or the electron cloud contributes the most to the volume of an atom. Explain.
4. Since protons (p+) with a positive charge and neutrons (n0) without a charge are located in the nucleus, what is the overall charge of the nucleus portion of an atom: positive, negative, or no charge at all? Explain.
5. What is the overall charge of the electron cloud of the atom? Explain.
6. What similarities do you see when comparing the hydrogen atom diagram with the helium atom diagram?
7. Compare the numbers of each subatomic particle found in both diagrams, and then list what makes hydrogen different from helium.


1. Use all four atom diagrams to complete the data table below. Use your diagrams and the information in the Student Guide to answer the questions. Hydrogen has been completed for you.
Characteristics
2. Explain why the charge is 0 (zero) for each of the four atoms that you built on the diagrams.
3. Rewrite the following statement so that is it TRUE: The number of protons and neutrons for these atoms increases sequentially by one.
4. Use the periodic table to look up the atomic numbers for the elements of hydrogen, helium, lithium, and beryllium. Complete the data table below and answer the follow-up question below the table.
5. What statement can be made about an element’s atomic number and the number of protons in one atom of the element?

6. Use the trend that you see in the previous table to predict how many protons will be found in an atom of each of the following elements. Then predict the number of electrons found in a single neutral atom of each element.
7. What statement can be made about an electrically neutral atom’s number of protons and electrons?
8. Look at the example, using the atoms that you built on the diagrams. Then complete the rest of the data table below.

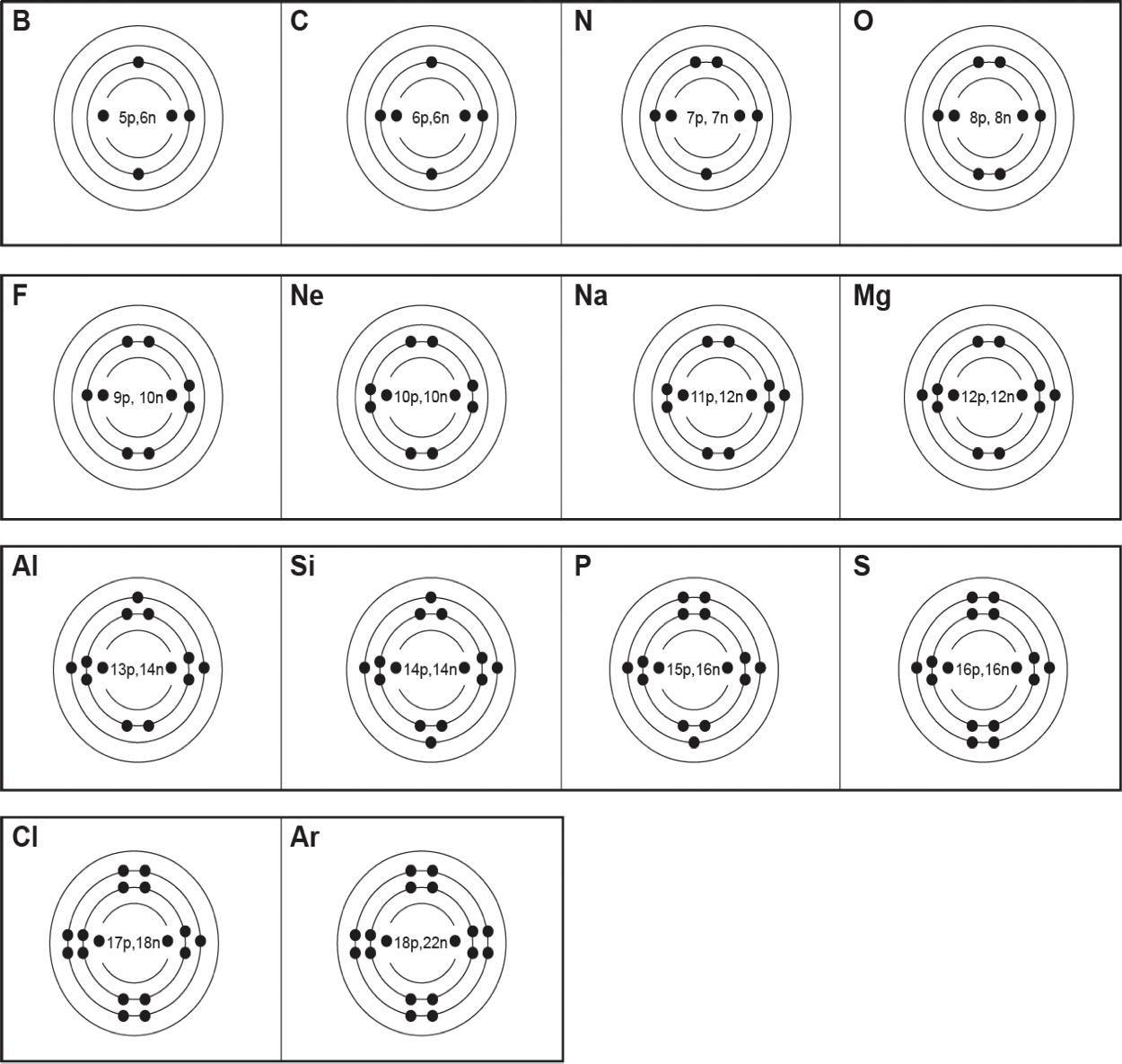

Atomic number = Atomic mass = 11
Number of protons = Number of neutrons =
Number of electrons =
Is the last energy level full?
Number of energy levels =
6.
Atomic number = Atomic mass = 12
Number of protons =
Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?
Atomic number = Atomic mass = 14
Number of protons = Number of neutrons =
Number of electrons =
Is the last energy level full?
Number of energy levels =
Atomic number = Atomic mass = 16
Number of protons =
Number of electrons =
Is the last energy level full?
Number of neutrons =
Number of energy levels =

Atomic number = Atomic mass = 19
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels = Is the last energy level full?
10.
Atomic number = Atomic mass = 20
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?
Atomic number = Atomic mass = 23
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?
Atomic number = Atomic mass = 24
Number of protons =
Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?

Atomic number = Atomic mass = 27
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels = Is the last energy level full?
14.
Atomic number = Atomic mass = 28
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?
Atomic number = Atomic mass = 31
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?
Atomic number = Atomic mass = 32
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?

Atomic number = Atomic mass = 35
Number of protons = Number of neutrons =
Number of electrons = Number of energy levels = Is the last energy level full?
Atomic number = Atomic mass = 40
Number of protons =
Number of neutrons =
Number of electrons = Number of energy levels =
Is the last energy level full?

Elements, Compounds, and Mixtures, Oh My!
Description
Definition
How to Separate
Properties
A mixture of different substances; contains elements, compounds, or both in various amounts
Can be separated into its parts by physical methods such as sorting, evaporation, filtering, or magnetic attraction
Properties depend upon the elements and compounds present and their amounts
Representation Not represented on the periodic table
Examples Salt water Trail mix
1. How are elements and compounds alike?
2. How are elements and compounds different?


Use colored pencils to draw your four models in the correct columns.
Compound
Analyzing Compounds
Element Name and Element Symbols Present in the Compound
Model (glue in place after teacher approves)
1. How is the periodic table useful?
2. How is a compound different from a mixture?

1. Determine which substances listed below are elements and which are compounds. Write an “E” for element and a “C” for compound.
2. What is a compound?
3. Explain why a compound is a pure substance although it is made from more than one element.
4. What are the advantages of using a plastic snap-together toy, like the one shown in the picture, as a model for elements and compounds?

5. What are the disadvantages of using a plastic snap-together toy, like the one shown in the picture, as a model for elements and compounds?

The periodic table is a very useful tool for scientists and research because it allows us to make predictions about the properties of elements. Design an investigation to determine the trends or patterns observed in properties of elements moving from left to right across the periodic table. Elements to be tested are sulfur, silicon, aluminum, and magnesium for the properties of appearance (color, luster), malleability, hardness, conductivity, reaction with HCl (acid), and reaction with NaOH (base). Then predict the properties of sodium and phosphorus based on scientific principles and evidence from the investigation.
Procedure
In your lab journal, address the following investigation.
Step 1: Question
Step 2: Relevance
Step 3: Variables, if applicable
Independent variable (also known as the manipulated variable):
Dependent variable (also known as the responding variable):
Control variable(s) or group (also known as constants):
Step 4: Hypothesis. Is a hypothesis needed? If so, what is it?
Step 5: Materials
Step 6: Safety Considerations

Step 7: Procedures
Sample procedure:
1. Use the back of a spatula to apply a small amount of force to each substance. Use the steel wool or sandpaper to see how each substance responds when it is rubbed with one of these. (Hardness)
2. Observe color. If necessary, use a magnifying glass. (Appearance)
3. Combine each substance with a few drops of distilled water in a spot well plate. (Make sure to first test the conductivity tester in distilled water to make sure it is working correctly.) Place a conductivity tester in each sample to determine if the substance will conduct electricity. Make sure to rinse with distilled water in between testing each sample. (Conductivity)
4. Place a small sample of each substance into a test tube. Add about four drops of HCl to each test tube and observe for two to three minutes. Record observations and clean out test tubes. Repeat using NaOH. (Reactivity with HCl and NaOH)
Step 8: Data Collection
Step 9: Data Analysis
Step 10: Conclusion
Write a conclusion and scientific explanation about the trend or pattern in properties of elements going from left to right across the periodic table.

1. What are the starting substances (molecules) in a chemical equation called?
2. What are the final or new substances (molecules) in a chemical equation called?
3. In your own words, describe what happens to the hydrogen and oxygen molecules when forming water. Something must be recombined. How does this happen?

1. Explain what types of elements form a covalent bond.
2. Explain what types of elements form an ionic bond.
3. Look at each molecular formula to determine if the compound represents an ionic or a covalent bond. Record your answers in the table below. Molecular

Imagine a piece of aluminum foil about the size of a sheet of paper. If you cut this piece of foil in half, have you changed the identity of the matter making up the foil? What happens if you cut the foil in half again? And if you cut this sample in half, what do you have? You know that cutting aluminum foil in half does not change its chemical identity. You still have aluminum foil every time you cut a piece in half. But suppose you could keep cutting the foil in half indefinitely. Would you ever reach a point where you could not cut any further without destroying the aluminum’s identity?


The Greeks hypothesized that matter is not infinitely divisible. Can matter be divided into infinitely smaller portions? Democritus used the idea of tiny, indivisible particles called atoms to explain the properties and behavior of matter. (In Greek, atom literally means “uncuttable.”) Democritus hypothesized that these particles are always moving, and therefore their arrangement in space must be constantly changing. According to Democritus, the movements and changing arrangements of atoms determine all of the phenomena that we observe in the natural world.
John Dalton, an English schoolteacher, carried out experiments that were the first to provide indirect evidence of atoms. He showed that oxygen combined with another gas, nitric oxide, in specific volume and weight ratios. In other words, no matter how much oxygen and nitric oxide he combined, the two always reacted with one another in a constant weight ratio. Dalton concluded that this could occur only if the gases were made up of atoms reacting with one another in whole number combinations. He proposed his atomic theory in 1803 as a series of statements:
• All matter is composed of indivisible particles that cannot be created or destroyed.
• Atoms of the same element are identical, but atoms of different elements have different properties Reflect

Dalton explained the differences between elements. He proposed that each element is composed of the same type of atoms but that the atoms in one element differ from the atoms in another element. A chief difference is atomic weight—different types of atoms have different weights.
• Atoms group together in whole number ratios to form compounds. Dalton’s experiments confirmed what other scientists had discovered about chemical compounds—they are made up of two or more different elements. He also confirmed that, in any compound, the same weight ratio of the elements is always present.
• Chemical reactions are the result of the rearrangement of atoms. By the time Dalton began his work, several scientists had already published results of their experiments involving chemical reactions. These results indicated that two substances combined in constant proportions.
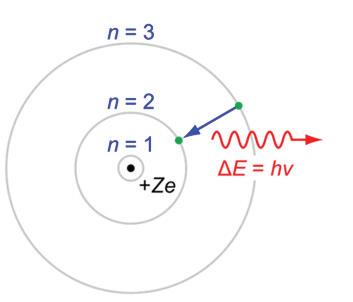
J. J. Thomson discovered the first subatomic particle: the electron
In 1897, English scientist J. J. Thomson used a device called a cathode ray tube to explore some mysterious rays that caused a fluorescent glow inside the tube. He discovered the first elementary particle, the electron.
In 1913, Niels Bohr developed a model of the hydrogen atom using quantum theory in which a negatively charged electron orbits a small, positively charged nucleus. The electron makes a quantum jump between orbits, emitting or absorbing a packet of electromagnetic radiation. Reflect
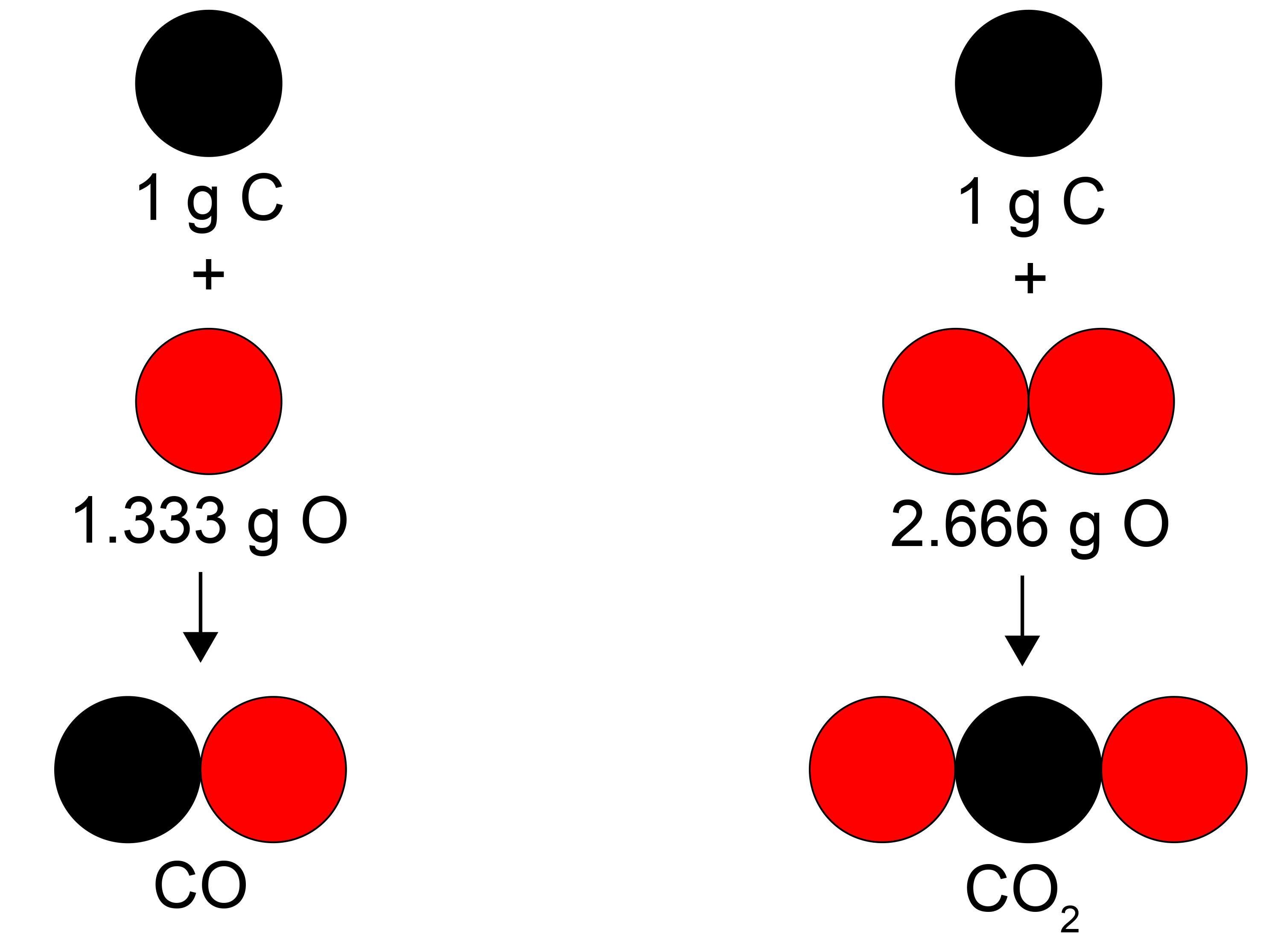
Rutherford discovered the atomic nucleus.
In 1911, Ernest Rutherford discovered that atoms contained a small, dense, positively charged nucleus and that the area around the nucleus was empty space with negatively charged electrons.

The electron cloud model of an atom is different from the older atomic model developed by Niels Bohr in 1913, which described electrons orbiting the nucleus in a flat plane like planets in our solar system. The electron cloud model has the nucleus in the center containing positively charged protons and neutral neutrons. The nucleus is surrounded by a negatively charged electron cloud. Electrons travel within that cloud in high-probability areas called orbitals.
The location and charge of subatomic particles determine structure. Protons, neutrons, and electrons differ from each other in their locations in an atom and their electrical charges. Electrical attractions and repulsions between charged particles (i.e., atomic nuclei and electrons) in matter explain the structure of atoms.
Protons (shown as red spheres) are positively charged particles found in the nucleus of an atom. Since protons are the only charged particles in the nucleus, an atom’s nucleus is always positively charged. Atoms of each element contain a characteristic number of protons. In fact, the number of protons or atomic number determines what atom we are looking at. For example, all nitrogen atoms have only seven protons and thus an atomic number of 7. Sulfur atoms have 16 protons, while iron has 26 protons in each atom.
Neutrons (shown as yellow spheres) do not have an electrical charge. They are neutral. Neutrons are found in the nucleus of the atom.
Electrons (shown as blue spheres) are negatively charged particles. They travel within the electron cloud surrounding the nucleus. Electrons are constantly moving. The Bohr models above emphasize the electron energy levels that influence how the atom can react with other atoms.

Each proton and neutron has a mass of approximately 1.67 x 10–27 kg. The mass of one electron is negligible at 9.11 x 10–31 kg. Most of the atom’s mass is located in the nucleus. Atomic mass is the average mass of all of the protons and neutrons in an atom. The average atomic mass, where P is the number of protons and N is the number of neutrons, is P + N. Electrons are so small that they do not make a significant contribution to the mass of an atom. The electron cloud makes up a large amount of the atom’s volume.
1. If the number of protons equals the number of electrons, the atom does not have an electrical charge and is neutral. In other words, the positives and negatives balance out.
2. If there are more protons than electrons, the atom is positively charged.
3. If there are more electrons than protons, the atom is negatively charged.
4. The number of neutrons in an atom does not affect the overall charge of the atom because neutrons have no charge.
Suppose a carbon atom has six protons and seven neutrons and is neutrally charged overall. How many electrons are in this atom of carbon? We know that the seven neutrons do not affect the overall charge of the atom. We also know that since the atom is neutral, it must have the same number of protons and electrons. Therefore, we can conclude that this carbon atom has six electrons.

A pure substance made up of the same atom is called an element. Atoms can exist by themselves (i.e., helium gas) or combine in a molecule with the same type of atom (i.e., hydrogen gas). Atoms can also combine (bond) with different kinds of atoms to form new substances called compounds (i.e., water). Shorthand equations for writing the reactions that form compounds use chemical letter symbols for each element in a molecular formula. Subscripts indicate how many atoms of each element are in the compound. Coefficients show how many molecules are involved.
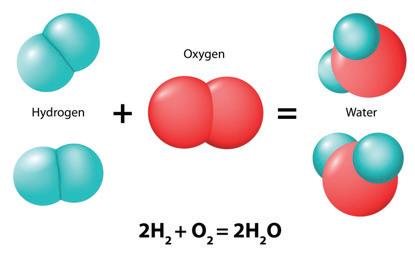
molecule: the simplest unit of a chemical compound that can exist, formed when two or more atoms join together chemically
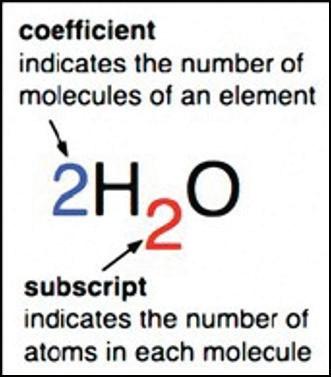
A compound is assigned a special chemical name, a common name if the substance is well known, and a formula. This information reveals its chemical composition. For example, chalk is calcium carbonate, and its chemical formula is CaCO3, which means every molecule of chalk has one calcium atom (Ca), one carbon atom (C), and three oxygen atoms (O). The subscripts tell how many of each atom there are in the formula. No subscript infers there is only one atom.


Pure substances can be a single substance—either an element or a compound—with definite composition and properties.
Type of Pure Substance
Example with Formula and Molecular Structure
Individual atoms of same type connect to form extended structure Graphite (carbon) C (repeated)
Individual atoms of different type connect to form extended structure Salt crystal (sodium chloride) NaCl


Individual atoms that are not attracted to each other Helium He
Molecules of different types of atoms that are not attracted to each other
Molecules of different types of atoms that are attracted to each other to form extended structures
Carbon dioxide CO2
Nylon (C12H22N2O2)n
Molecules of same type of atom that are not attracted to each other Oxygen gas O2

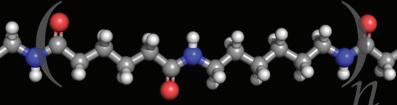

A compound is two or more different atoms bonded together. The ratio is important. Just because two compounds have the same elements does not mean they are the same substance. Look at the compounds on the side. The one on the left is water. On the right is hydrogen peroxide, the bubbly stuff you apply to cuts. They both have hydrogen and oxygen but in different ratios. Water has two hydrogen atoms and one oxygen atom, and hydrogen peroxide has two of each.
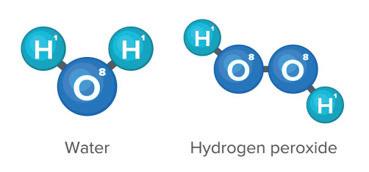

Two major types of bonds form compounds. Ionic bonds: If a compound contains a metal and a nonmetal, the bond is ionic. In ionic bonds, valence electrons move from one metal atom to a nonmetal atom. The atom that loses electrons becomes a positively charged ion (called a cation). The atom that gains electrons becomes a negatively charged ion (called an anion). The attraction between the newly formed cation and anion results in the formation of an ionic bond. In salt, sodium loses an electron to chlorine. The sodium becomes the positive cation, and the chlorine becomes the negative anion. Together they form an ionic bond to form NaCl (table salt).
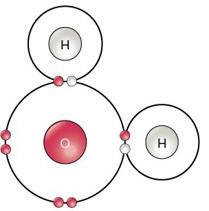
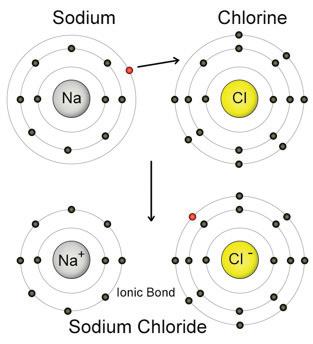
Covalent bonds: If two or more nonmetals are bonded in a compound, the bond will be covalent. In covalent bonds, valence electrons are shared between atoms. A covalent compound, or molecule, may contain two or more atoms. In molecules with more than two atoms, one atom is the central atom. Electron pairs are placed around all atoms to fulfill the octet rule. A molecule of water represents a covalent bond in which the electrons from two hydrogen atoms are shared with the oxygen atom to make eight electrons in the outer shell. In the molecule of methane, four hydrogen atoms share their outer electron with a carbon atom to make an octet.


mixture: a combination of two or more substances in which no new matter is formed
ingredients: the separate parts of a mixture
physical change: a change to matter in which no new kinds of matter are formed
Every day, we interact with many different kinds of matter. Some are mixtures. We look at matter, feel it, taste it, and even breathe it. Sometimes different types of matter are combined. For example, a salad might have several types of matter, or ingredients, such as lettuce, tomatoes, and onions. Or, a sample of soil might include sand, leaves, and pebbles.
In these examples, you can see each ingredient individually. It is pretty easy to pick out each part of a salad mixture. With a little more effort, you could separate the parts of a soil mixture. But think about lemonade, a drink that contains water, lemon juice, and sugar. You cannot see each ingredient, so is lemonade really a mixture?
To scientists, a mixture is a combination of two or more substances in which no new kinds of matter are formed. Making a mixture results in physical changes only. In the example of a salad, all of the ingredients are combined, but they do not form any new substances. Similarly, sand, leaves, and pebbles are combined to make a soil mixture, but the combination does not result in any new substances. The same is even true about lemonade. Even though you are not able to see each ingredient, once the mixture has been made, new substances are not formed. In contrast, if you took eggs, flour, a little oil, baking soda, and some spices, combined them, and baked them, the ingredients would become a new substance—a cake! A cake is not a mixture because it is a new substance. You cannot go in reverse and separate those ingredients anymore.
Matter can be composed of either a pure substance or a mixture. When matter is made of the same molecules, such as gold (Au) or water (H2O), that material is called a pure substance. When elements and/or compounds are physically combined and no new substances are formed (no chemical change occurs), this matter is called a mixture. The different paper clips and the gravel with the sand are examples of mixtures because all the substances keep their physical properties in the mixtures.


What properties could you use to separate the ingredients in these mixtures?

Look Out!
Solutions are special mixtures.
Not all mixtures are unevenly mixed like a salad or trail mix. When a mixture has an even distribution of one ingredient dissolved in another, that mixture is called a solution Sugar dissolved in coffee is an example of a solution. Not all solution mixtures are liquid! Solution mixtures can be a combination of solids, liquids, or gases:
• Solid dissolved in liquid—salt water
• Gas dissolved in liquid—carbonated soft drink
• Solid dissolved in solid—brass and zinc evenly blended in copper
• Gas dissolved in gas—oxygen and nitrogen evenly blended in air
Uneven and Even Mixtures

A heterogeneous mixture (unevenly mixed) is called just a mixture. However, when you have a homogeneous mixture (evenly mixed), it is called a solution.


Heterogeneous mixtures have an uneven distribution of parts and are physically separate. The parts are easily seen and easily separated. Think about eating trail mix. Each handful you grab will not have an identical amount of peanuts, raisins, and chocolate. Each bite will not be uniform. This makes trail mix a heterogeneous mixture. Some other examples of heterogeneous mixtures include cereal, pizza, salad, Italian salad dressing, and vegetable soup. You can just pick out the pieces. In the case of the salad dressing, the oil sits on top of the water, and you can pour it off.
A homogeneous mixture (solution) has an even distribution of its parts. A solution is always homogeneous. This type of mixture is usually more difficult to separate because you cannot always see the separate parts. Consider drinking a glass of lemonade. Each sip you take is the same. It is not extremely sour one sip and only sweet the next. Lemonade is homogeneous. Some other examples of homogeneous mixtures include milk, air, vanilla ice cream, Jell-O, mouthwash, and tomato soup.

To show the relationships between various atoms that make up the elements, the periodic table was created in 1869 by Dmitri Mendeleev. Since its creation, the table has undergone a variety of evolutions, eventually morphing into the chart with which we are familiar today. We know that the atom has various parts. The nucleus consists of protons and neutrons, which make up its mass. Orbiting that in a “cloud” are all of the electrons, with a neutral atom having the same number of electrons as protons. On the outermost layer, a special energy level of electrons, known as the “valence level,” exists. These electrons help determine the reactivity of the individual elements.

• Metals are found on the left side of the table. They lose electrons to form cations (positive ions). Most are shiny, solid, silver, ductile, and malleable. They are good electrical/heat conductors.
• Metalloids share properties of metals and nonmetals.
• Nonmetals are found on the right side of the table. They gain electrons to form anions (negative ions). Most are dull, brittle nonconductors that exist in all three states of matter.

Reflect
Periods, Groups, and Trends
Periods/Series:
Horizontal rows
Share the same number of energy levels
Groups/Families:
Vertical columns
Have similar properties
Share the same number of valence electrons
Trends of the Periodic Table:
Atomic number: the number of protons in an atom

Atomic mass: the mass of an atom; calculated by using the ratios of how often the mass numbers (protons + neutrons) of naturally occurring isotopes of an atom are found in nature
Atomic radii: size of the atom, measured from the nucleus to the outer electron level
Metallic character: how strong the characteristics of a metal are present; is also an indicator of conductivity
Nonmetallic character: how strong the characteristics of a nonmetal are present; increases diagonally up and to the right; is also an indicator of poor conductivity
Electron affinity: a neutral atom’s likelihood of gaining an electron
Ionization energy: amount of energy an atom must absorb to discharge an electron
Reactivity: Metals react by losing electrons, so reactivity decreases from left to right on the table and increases down each group. Nonmetals react by gaining electrons, so reactivity increases from left to right, excluding the noble gases.

Groups
Alkali Metals
• Most active metals
• Stored under oil
• 1+ oxidation number
• Because of their reactivity, alkali metals do not exist as free elements.
• All Group 1 metals react vigorously with water.
Hydrogen
• Unique element; most abundant in the universe (75%)
• Nonmetal on the left side of the periodic table (found in metal column)
• Gas state
• One valence electron
Halogens
• Most active nonmetals
• 1- oxidation number
Noble Gases
• Relatively inactive (inert)
• Contain eight valence electrons
• All gases

Take a look at the diagram of the periodic table below. It shows metals in orange, metalloids in green, and nonmetals in gray. Metals are shiny solids that are ductile and malleable, and they are good conductors of heat and electricity. Nonmetals are often gases or brittle solids that are typically insulators of heat and electricity. The metalloids are along a diagonal line that separates the metals from the nonmetals. Their properties are intermediate between metals and nonmetals. Based on their location on the periodic table, what types of properties would you expect of alkali metals? Alkaline earth metals? Halogens? Noble gases?
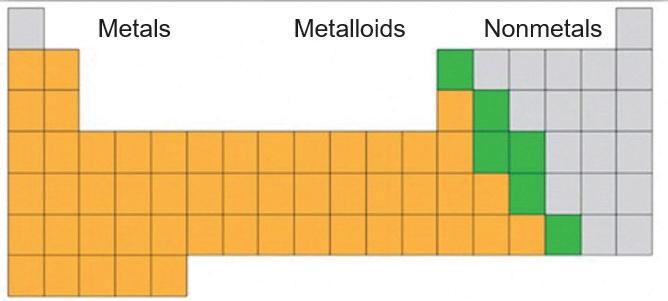
Two groups of elements not in the main group are the transition elements in the middle region and the inner transition elements in the lower region. (Sometimes both are called transition elements.) All elements in these groups are metals, have high melting points, and often form colored compounds. They can react with other elements in various ways. Some are also radioactive, which means their atoms are unstable. Let us look at these two locations on the periodic table in more depth.
• Transition elements (in blue): These elements are metals, tend to lose electrons to form stable ions, and have very similar properties common to many metals. Unlike most main group elements, they do not always use the same number of valence electrons in chemical reactions. This leads to a variety of possible charges for each element.
• Lanthanide and actinide series: Two rows of elements lie below the main periodic table, shown in the image to the right in yellow, called the inner transition elements. The lanthanides are 15 metals often called the rare earth elements. They are all silvery-white metals often found in ores. The actinides are mostly man-made elements, except for uranium and thorium.

Order the following from greatest (5) to least (1) according to atomic number, atomic mass, atomic radius, electron affinity, ionization energy, metallic character, and nonmetallic character: oxygen, magnesium, carbon, iron, lead.
Lead
Sketch all known trends on the periodic table below:

The Periodic Table of the Elements categorizes elements based on their chemical and physical properties. Study the list of properties in the box below. Decide if each represents an element that is part of the following chemical families: an alkali metal, an alkaline earth metal, a halogen, a noble gas, a transition element, or an inner transition element. Write your answers in the table below the list. Some of the answers may appear in multiple families.
• Contains elements that do not typically react
• Elements donate two electrons to produce a cation with a 2+ charge.
• Elements are mostly found in the solid state.
• Elements donate one electron to produce a cation with a 1+ charge.
• Elements are found as a solid, a liquid, or a gas.
• Elements are all gases.
• Elements typically accept one electron to produce an anion with a 1- charge.
• Contains many elements that are radioactive
• Elements can be found in different ionic forms.

To help your child learn more about the chemical and physical properties among elements of the same family on the periodic table, work together to create a brochure describing one of the following chemical families: alkali metal, alkaline earth metal, halogen, noble gas, transition element, or inner transition element.
Use an 8½" × 11" piece of paper and fold the paper either in half or in thirds. Your child should research—using books or the Internet—the chemical family that was chosen and include the following information in the brochure:
• Names and symbols of all the elements in the family
• Abundance of all elements
• Melting/boiling points of the elements
• Uses of the elements
• Any special properties of the elements
Have your child record the sources of the information and include pictures as appropriate.
Here are some questions to discuss with your child:
• Why did you choose the particular elemental family?
• How are the elements within the family you chose similar? How are they different?
• How are the elements within the family you chose organized on the periodic table? In other words, why are these elements included in this particular order?

1 What exactly is the periodic table? And why is it so important to chemistry? Once you learn how to use it, you will find that it contains a wealth of information. Almost 3,000 years ago, there were people who studied nature. They were known as natural philosophers, and they tried to understand how natural systems work. Aristotle was one of these natural philosophers. In 330 BC, he stated that all matter is made of four elements: earth, air, fire, and water. That sounds simple to us now, but he actually was not that far off. Those four “elements” relate to the state of matter and the energy needed for chemical reactions.

2 It wasn’t until the 1700s that modern studies in science began. A scientist named Antoine Lavoisier is often thought of as the “father of modern chemistry.” In the late 1700s, Lavoisier was able to describe the difference between metals and nonmetals. This became an important part of the modern periodic table. He also was able to identify and name more than 30 elements. In 1789, he published the first modern chemistry textbook. In 1828, another scientist named Jöns Jakob Berzelius created a table based upon the atomic weights of the known elements. He also gave letters to each element. In 1829, a scientist named Johann Döbereiner saw patterns of elements with similar properties. He grouped elements into groups of three, which were known as triads.
3 In 1864, a man named John Newlands used the 56 elements to build on Döbereiner’s triads. Newlands saw that there were even more things in common between the elements, specifically between the first and ninth elements. He called these similarities the law of octaves. (In the modern table, these elements are in the same period.) He organized these elements into 11 groups, all based on similar characteristics. Also in 1864, a scientist named Lothar Meyer developed a version of the table using the pattern of the outer electrons in 28 of the 56 known elements.
4 Then, in 1869, Meyer took all this information and created a periodic table of the 56 known elements based on other elemental properties, such as atomic weight. In the same year, Dmitri Mendeleev created his own table. His table was also based on atomic weights, but it was the method in which he arranged this information that made his table so valuable. He arranged the elements in a periodic way. This means that elements with similar properties were placed underneath each other. The elements with lower atomic weights were placed on the top row. The elements with heavier atomic weights were placed below them.

This led to the first periodic table in the form like the one you use today.
5 There were gaps in Mendeleev’s table, but those gaps would soon be filled by future discoveries. In 1894, William Ramsay discovered the noble gases. In 1913, Henry Moseley calculated the atomic number of each of the known elements. The atomic number is the number of protons in the nucleus of each element, or atom. He discovered that the properties of elements rely only in part on their atomic number. Even though elements had been placed in the periodic table in order of increasing atomic number, he showed that the organization of the periodic table is based on electron properties. There were two final pieces of the puzzle. In 1945, Glenn Seaborg discovered lanthanides and actinides. The truly interesting thing is that as modern science was able to fill these gaps, the form of Mendeleev’s table did not need to be altered. Mendeleev had gotten it right, and he is the scientist given credit for laying the foundation for the periodic table as we know and use it today.

1 Who created the first chemistry textbook and was known as the “father of modern chemistry”?
A Jöns Jakob Berzelius
B Dmitri Mendeleev
C Antoine Lavoisier
D Lothar Meyer
2 Dmitri Mendeleev created an early version of the periodic table based on atomic mass. The modern periodic table has elements placed because of–
A atomic number.
B electron properties.
C atomic size.
D the date the elements were discovered.
3 Dmitri Mendeleev and Lothar Meyer created similar elemental tables in 1869. Why was Mendeleev’s table “more valuable” to modern science than Meyer’s table?
A Mendeleev ordered his table in a periodic way.
B Meyer did not include atomic weights.
C Mendeleev used more elements.
D There were gaps in Meyer’s table.

4 In 1913, a scientist named Henry Moseley discovered that the properties of elements rely partially on–
A their color.
B their atomic mass.
C the properties of electrons.
D their atomic size.
5 Which scientist discovered that elements could be grouped into triads, or elements with similar properties?
A James Chadwich
B Aristotle
C William Ramsay
D Johann Döbereiner
6 A scientist named John Newlands discovered an early pattern in the periodic table regarding similarities between the first and ninth elements. What did he call this pattern?
A The law of periodicity
B The law of octaves
C The law of elements
D The law of trends

1. What are atoms? What sub-particles do atoms contain? Draw an atom.
2. A major component of modern atomic theory is based on models that illustrate the structure of an atom. Describe two changes that happened to the atomic model based on the experiments of Dalton, Rutherford, Thomson, or Bohr.

3. Sulfur has an atomic number of 16 and belongs in group 16 on the periodic table. Calcium has an atomic number of 20 and belongs in group 2 on the periodic table. Which element has a greater reactivity and why?
4. This is the chemical formula for glucose:
C6H12O6
Describe the structure of the chemical compound identified by the formula. Be sure to include the number of elements, the number of atoms of each element, and the number of molecules in the compound.
5. How are ionic and covalent bonds different?

A Mars rover has collected and analyzed several samples from the surface and atmosphere of Mars. NASA has processed most of the data but is looking for help in identifying two of the remaining elements from the samples. They know that both elements are main group elements. The following information about unknown Element J and unknown Element Q has just arrived for you to examine.
Reaction with Oxygen* Yes (forms XO) No
Reaction with Chlorine* Yes (forms XCI2) No
*X was used to represent the unknown elements in the compounds listed in the table.

Prompt 3
Use your knowledge of the periodic table to write a scientific explanation to justify the group in which the elements belong. Be sure to state a claim, present your evidence, and give your reasoning in a way that is accessible to both scientists and laypeople.
Claim:
Evidence:
Reasoning:
Rebuttal:


1. Measure 50 mL of baking soda in a beaker.
2. Measure 50 mL of water in a second beaker.
3. Add the water to the baking soda.
4. Stir until the baking soda is dissolved. This is your invisible ink.
5. Use a cotton swab to write a message or draw a picture on white paper.
6. Let the ink dry.
7. Measure 50 mL of grape juice in a beaker.
8. Use a cotton swab to brush the grape juice over the paper, and observe what happens.
9. Record your observations.
Observations:

A chemical reaction is a process by which one or more substances change to produce one or more new substances with different properties. Chemical reactions are represented by chemical equations. A chemical equation uses chemical formulas and chemical symbols to represent the reactants and products in a chemical reaction. A chemical equation is balanced when the same number and type of atoms appear on each side of the equation.
Chemical reactions are taking place all around you all the time. When a chemical reaction occurs, new substances with different properties form. Some chemical reactions take place quickly, and others take place over time. It is sometimes difficult to determine if a new substance(s) formed, so observations such as the following provide evidence that a chemical reaction has occurred:
• A gas is produced (observe bubbling, smoke, fizzing, foaming, expanding container, change in odor).
• A precipitate is formed (observe a solid forming in a liquid).
• A change in energy occurs (observe a temperature change or light being released).
• An unexpected color change occurs (observe a color change that is not expected).
Consider the everyday chemical reactions pictured on the Student Reference Sheet. What are the reactants? What are the products? How do you know that new substances have formed?

1. Have you ever cooked out using a gas grill? The flame in your grill is produced by burning the gas propane. The following is the equation for the combustion of propane:
C3H8 + 5O2 --------> 3CO2 + 4H2O
A. Is this equation balanced? How do you know?
B. Circle the reactants and place a box around the products.
C. Provide evidence that combustion of propane is a chemical reaction.
2. Plants convert the Sun’s energy into a chemical compound called glucose using the process of photosynthesis. The following is the equation for photosynthesis:
6CO2 + 6H2O --------> C6H12O6 + 6O2
A. Is this equation balanced? How do you know?
B. Circle the reactants and place a box around the products.
C. Provide evidence that photosynthesis is a chemical reaction.

3. Animals get energy from the foods they eat. When you eat a candy bar, your body converts the food into energy during cellular respiration. The following is the equation for cellular respiration:
C6H12O6 + 6O2 --------> 6CO2 + 6H2O
A. Is this equation balanced? How do you know?
B. Circle the reactants and place a box around the products.
C. Provide evidence that cellular respiration is a chemical reaction.
4. You have probably noticed rusty bridges, pipes, nails, fences, or even cars in your daily life. Rusting occurs when iron reacts with oxygen in the air. The following is the equation for rust formation:
4Fe + 3O2 --------> 2Fe2O3
A. Is this equation balanced? How do you know?
B. Circle the reactants and place a box around the products.
C. Provide evidence that rusting of iron is a chemical reaction.

1. Read the experimental procedure below.
2. Before beginning the experiment, make a prediction as to how your solid will react with the water and the vinegar. What evidence of chemical change do you expect to see in each reaction?
Predictions
Reaction with water:
Reaction with vinegar:
Your teacher will assign you one of the following solids:
Calcium chloride
Magnesium sulfate
Sodium carbonate
Citric acid
3. Follow the procedure below.
• First go to the chart and circle your assigned solid.
• Place a small amount of your assigned solid on the wax paper.
• Examine with the hand lens and write down your observations.
• Labels two plastic zip-top bags as follows: #1 and #2.
• Add one leveled teaspoon of your assigned solid to bag #1.
• Take the covered condiment cup labeled 1-Water and place it in the bag.
• Press as much air out of the bag as possible and seal the bag.
• Use your fingers to take the top off the condiment cup, and mix the solid with the liquid (WITHOUT opening the bag).
• Make sure you keep your hand at the bottom of the bag to check whether the mixture shows any temperature change.
• Note any other signs of a chemical change taking place.
• Record your observations on the chart where you circled your assigned solid earlier.
• Repeat this process using bag #2, your assigned solid, and the liquid in the condiment cup labeled 2-Vinegar.
• Record all shared group information on the chart.
4. Consider your predictions from number 2 above. Did the actual experiment produce the expected results as you predicted? Explain.

5. Fill in your own information first, and then collect the rest of the data from your lab group (and share your own).
Physical Properties (observed with hand lens)
Observations during a Chemical Reaction When Mixed with Water
Observations during a Chemical Reaction When Mixed with Vinegar

Scientific Investigation
Your group will be assigned an everyday chemical reaction to investigate. Use the informational card from your teacher to design an investigation to provide evidence that a chemical reaction has occurred.
Step 1: Question
Step 2: Variables, if applicable
Independent variable (also known as the manipulated variable)
Dependent variable (also known as the responding variable)
Control variable(s) or group, also known as constants
Step 3: Hypothesis
Is a hypothesis needed? If so, what is it? How will the responding variable change when the manipulated variable changes?

Materials
Step 5: Safety Considerations
Step 6: Procedure

Step 7: Data Collection
Develop a data table to record your observations and measurements.
Step 8: Data Analysis
Create a graph based upon the data, if needed. Make a general statement about the results.

1. Prepare a poster to share your procedures, data, and conclusions for your investigation.
2. Display your poster as directed by your teacher.
3. Participate in a gallery walk to collect data from each investigation. Record your data in the data table. Investigation Observed Changes
Evidence that a Chemical Reaction Occurred

Everyday substances all around us can be classified as acids or bases based on their chemical and physical properties. For example, acids taste sour, while bases taste bitter and feel slippery.
We encounter weak acids and weak bases in our everyday lives without them causing us any harm. However, strong acids and strong bases can be dangerous, burning your skin, eyes, or respiratory passages. It is important to be careful when handling even household chemicals. When working with acids and bases, it is important to understand how to identify their strength.
Procedure
Set Up the Experiment
1. Carefully tear the red and blue litmus papers and pH paper into four pieces approximately the same size.
2. Label five empty clear plastic cups
A. Indicator + cream of tartar
B. Indicator + detergent
C. Indicator + vinegar
D. Indicator + water
E. Indicator only
Investigate
1. Beginning with the cream of tartar, test each sample using the indicators and litmus papers.
A. Carefully pour about two tablespoons of indicator solution into each cup. Place the five labeled cups on a white piece of paper. Using the dropper, add a stream of liquid to the solution. Gently swirl to mix. Observe any color change in the cup and record observations in the data table. Leave the cup sitting on the white paper for comparison later.
B. Using the dropper, place two to three drops of the cream of tartar solution on the small section of blue litmus paper. Observe any color change and record observations in the data table.
C. Using the dropper, place two to three drops of the cream of tartar solution on the small section of red litmus paper. Observe any color change and record observations in the data table.
D. Using the dropper, place two to three drops of the cream of tartar solution on the small section of pH paper. Observe any color change and record observations in the data table.
2. Repeat steps A through D using the remaining four samples.

Record your data in the table below.
Substance
Cream of Tartar
Detergent
Vinegar Water
Indicator Alone
Questions
1. Which substances did you classify as acids? Bases?
2. Which method of determining pH did you find to be easiest. Why?
3. Why do scientists need multiple methods of determining the same information?

Procedure
Set Up the Experiment
1. Label three empty clear plastic cups
A. Indicator + detergent
B. Indicator + cream of tartar
C. Control
D. Indicator alone
Investigate
1. Carefully pour two tablespoons of indicator solution into each cup and place the cups on a white piece of paper. Record your observations.
2. Use the dropper to add three to five drops of cream of tartar solution to the indicator + cream of tartar cup. Gently swirl to mix. Record your observations.
3. Use the dropper to add three to five drops of detergent solution to the indicator + detergent cup. Gently swirl to mix. Record your observations.
4. Using the solutions at your table, devise a method to return the samples from steps 3 and 4 to the color of the control. Complete the chart provided.
Substance
Indicator Alone
Cream of Tartar + Indicator
Detergent + Indicator
Observations

5. Using the solutions at your table, devise a method to return the samples from steps 3 and 4 to the color of the control. Complete the chart provided.
Proposed Procedure
Reasoning
Observations
Proposed Modifications to Procedure
Questions
1. What was the control in this experiment? Why was it necessary to have a control?
2. Were you able to return the samples to the original color of the cabbage juice indicator? If yes, explain the science of neutralization. If not, where do you think you went wrong?

When a chemical reaction occurs, atoms are neither created nor destroyed; instead, the atoms are rearranged. Energy is stored in the bonds of chemical compounds.
Bonds present in the reactants are broken, and new bonds are formed in the products. Energy is required to break the bonds of the reactants. Energy is released when new bonds are formed in the products. If it takes more energy to break the bonds of the reactants than is released when the bonds of the products are formed, the reaction is endothermic, absorbing heat. If more energy is released when the bonds of the products are formed than was required to break the bonds in the reactant, the reaction is exothermic, releasing heat.
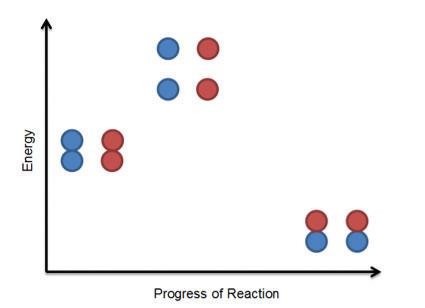
Procedure
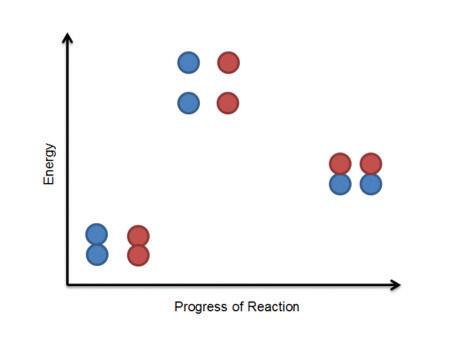
In this activity you will use a model to explore the breaking and formation of bonds during a chemical reaction.
1. Lay the blank energy diagram provided by your teacher on the lab table.
2. Select a color of chips to represent each of the atoms in the balanced equation.
3. Use the chips to form the chemical formulas of the reactants.
4. Using colored pencils, draw the model of the reactants in the space provided (see next page).
5. Separate the atoms to model the breaking of the bonds holding the reactants together. Draw the separating atoms in the space between the reactants and products sections in the diagram.
6. In the products section of the energy diagram, use the chips to form the chemical formulas of the products.
7. Pay attention to the energy of the reactants vs. the energy of the products.
8. Using colored pencils, draw the model of the reactants in the space provided.
9. Summarize your model to explain how the modeled reaction stores and releases energy.
10. Repeat steps 2–9 for each of the four reactions.

Animals get energy from the food they eat. When you eat a candy bar, your cells convert the food into energy during cellular respiration. There is energy stored in the bonds of the glucose molecule, C6H12O6. The energy is released during the reaction. The following is the equation for cellular respiration:
+ 6O2 -> 6CO2 + 6H2O
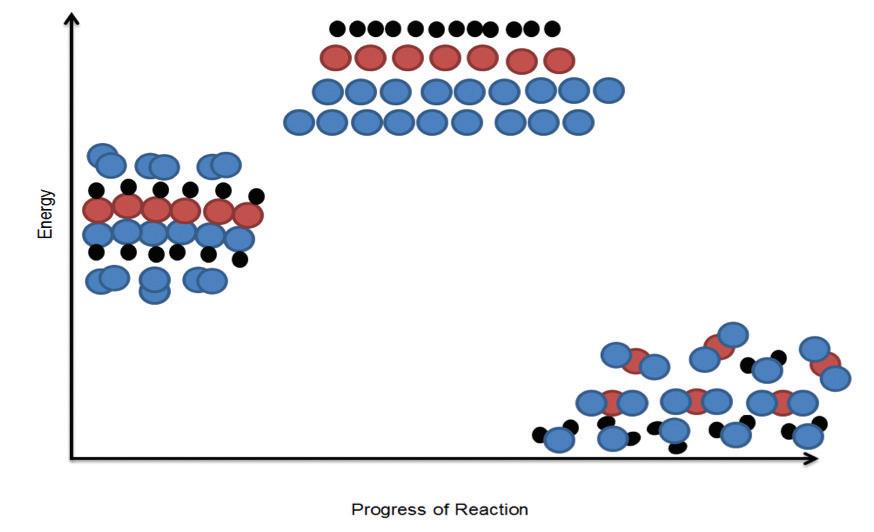
1. Using appropriate science vocabulary, describe your graph in two to three complete sentences. Be sure to explain the energy stored and released from the chemical bonds.

Plants convert the Sun’s energy into a chemical compound called glucose, C6H12O6, using the process of photosynthesis. Energy is stored in the bonds of the glucose molecule. The following is the equation for photosynthesis:
6CO2 + 6H2O ------> C6H12O6 + 6O2
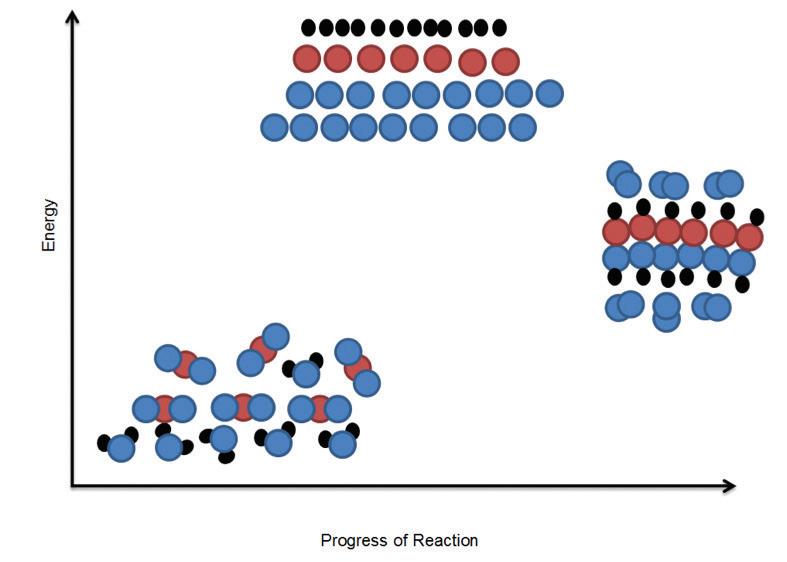
2. Using appropriate science vocabulary, describe your graph in two to three complete sentences. Be sure to explain the energy stored and released from the chemical bonds.

Sports medicine first aid kits often include cold packs. Before it is activated, a cold pack contains sodium bicarbonate and acetic acid. When you crack the cold pack, the chemicals mix and sodium acetate and carbonic acid are formed. The reaction absorbs energy.
NaHCO3 + HC2H3O2 → NaC2H3O2 + H2CO3
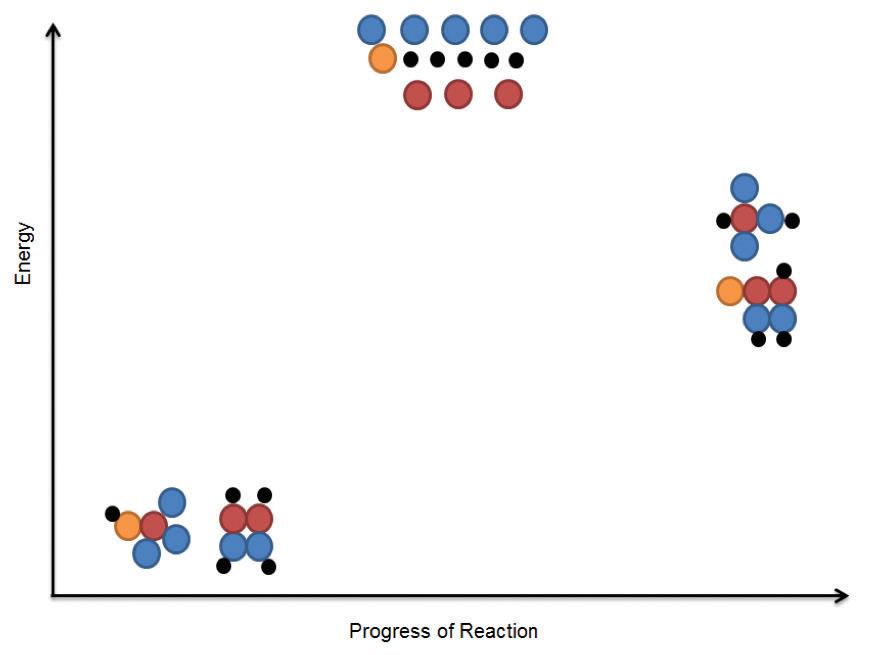
3. Using appropriate science vocabulary, describe your graph in two to three complete sentences. Be sure to explain the energy stored and released from the chemical bonds.

During the winter months you can purchase hand warmers at the sporting goods store. Similar to cold packs, hand warmers contain chemicals in separate compartments that are activated when the pack is twisted and the compartments are mixed. The reaction in the hand warmer releases energy. Sodium bicarbonate reacts with calcium chloride, forming calcium carbonate, carbon dioxide, sodium chloride, and water.
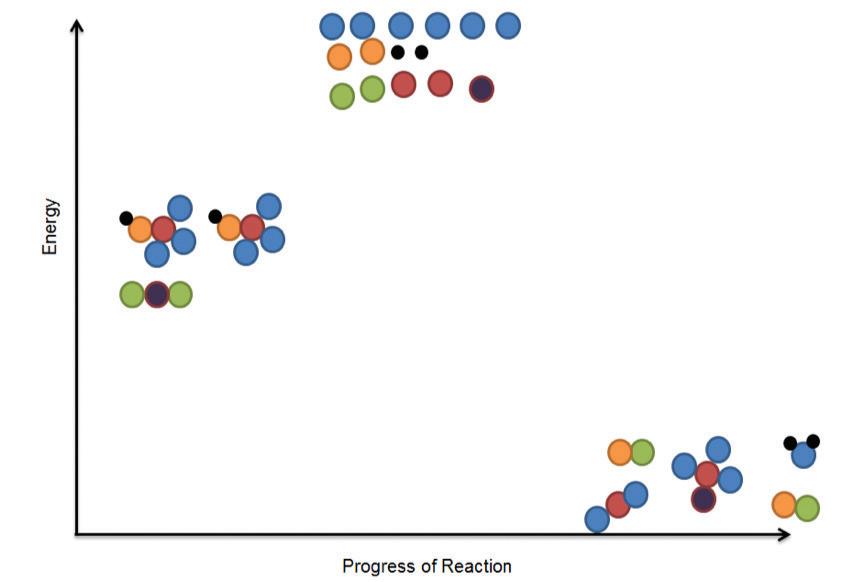
4. Using appropriate science vocabulary, describe your graph in two to three complete sentences. Be sure to explain the energy stored and released from the chemical bonds.

Reflect
Have you ever seen fireworks explode in the sky? If so, you may have observed a variety of different colors and shapes in the sky and watched with amazement as the colors changed overhead. These displays are not just entertainment. When each firework is ignited, one or more chemical reactions take place. The color of the display in the sky is determined by each chemical reaction. Each burst of color depends on the elements and compounds that are present in the firework.
What can you observe during a fireworks display? Perhaps you hear loud sounds, see bright lights, and feel the warmth of the explosion. Do any of these indicate that a chemical reaction has taken place?
Chemical reactions produce new molecules.

Fireworks are chemical reactions. The color of the firework depends on the reactants that are used.
In a chemical reaction, molecules can change through the rearrangement of their atoms. More specifically, atoms of the reactants rearrange to form new molecules. A chemical equation can be written to show what happens to the atoms in a chemical reaction. A chemical equation is written like this: (reactants) (products)
The arrow in a chemical reaction means “produce” or “are converted to.” If you have ever seen water, you have seen the result of a chemical reaction. In this reaction, oxygen gas (O2) and hydrogen gas (H2) rearrange to form water (H2O). The balanced chemical equation for this reaction is written like this:
O2 + 2H2 2H2O
In this reaction, the reactants have different properties from the product. Oxygen gas and hydrogen gas have different properties from water. In the gas form, water vapor may look similar to both hydrogen gas and oxygen gas. They are all colorless gases, yet they have different chemical properties. Oxygen is very reactive compared to both hydrogen gas and water vapor.
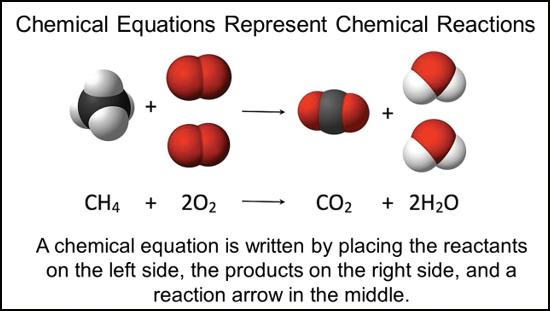
Evidence of a chemical reaction can be seen when elements regroup to form molecules, and the same number of atoms of each type of molecule is on both sides of the equation.

During a chemical reaction, the atoms in one group of molecules (the reactants) rearrange to form new molecules (the products). According to the law of conservation of mass, the same atoms must be present in both reactants and products. Therefore, in a chemical equation, the same atomic symbols must appear to the left of the arrow and to the right of the arrow. Look at the chemical reaction in the following example, in which calcium carbonate decomposes into calcium oxide and carbon dioxide:
s → CaO s + CO2 g
On the reactants side of the equation, there are one atom of calcium (Ca), one atom of carbon (C), and three atoms of oxygen (O). Likewise, on the products side of the equation, there are one calcium atom, one carbon atom, and three oxygen atoms. The equation is balanced. This is a relatively straightforward reaction. One molecule of reactant equals one molecule of one product and one molecule of another product. Most reactions are more complicated, however, involving different numbers of molecules on both sides of the equation. To balance the equation for such a reaction, you will need to use coefficients: numbers placed in front of a chemical formula to indicate the number of specific molecules present during a reaction.
Let us look at an example involving the combustion (burning) of propane gas. In this reaction, propane (C3H8) reacts with oxygen (O2) to produce water vapor (H2O) and carbon dioxide (CO2):
C3H8 g + O2 g → H2O g + CO2 g
How can we tell if this equation is balanced? Begin by counting the atoms of each element on both sides of the equation. You can do this by making a table, as shown on the following page.

The combustion of propane allows this

We will need to use coefficients to balance this equation. Where should we begin? Balancing an equation often involves trial and error, but here are some guidelines:
1. First try to balance elements that appear in only one molecule on each side of the equation. 2. If several elements appear in only one molecule, begin with the element that has the fewest number of atoms.
Let us take another look at the equation for the combustion of propane:
C3H8 g + O2 g → H2O g +CO2 g
Both carbon (C) and hydrogen (H) appear in one reactant molecule and one product molecule. Because the unbalanced equation contains fewer carbon atoms, let us begin with this element. The reactants side of the equation contains three carbon atoms, and the products side contains one carbon atom. To balance the carbon atoms, add a coefficient of 3 to the CO2 molecule on the products side of the equation:
C3H8 g + O2 g → H2O g + 3CO2 g
This coefficient means the products side of the equation contains three molecules of CO2 As a result, both sides of the equation now contain three carbon atoms. In addition, the products side of the equation now contains seven oxygen atoms. (H2O contains one oxygen atom, and 3CO2 contains six oxygen atoms.) Because it appears in several products, however, let us wait to balance oxygen until we have balanced hydrogen.
The reactants side of the equation contains eight hydrogen atoms, and the products side contains two hydrogen atoms. To balance the hydrogen atoms, add a coefficient of 4 to the H2O molecule on the products side of the equation:
C3H8 g + O2 g → 4H2O g + 3CO2 g
This coefficient means the products side of the equation now contains four molecules of H2O. As a result, both sides of the equation now contain eight hydrogen atoms. In addition, the products side of the equation now contains 10 oxygen atoms. (4H2O contains four oxygen atoms, and 3CO2 contains six oxygen atoms.)

We may now balance oxygen. The reactants side of the equation contains only two oxygen atoms. Fortunately, they appear together in a molecule with no other atoms (O2). We can add a coefficient of 5 to O2 without affecting the balance of other atoms in the equation. Here is the balanced equation for the combustion of propane:
C3H8 g + 5O2 g → 4H2O g + 3CO2 g
To confirm, count the atoms of each element on both sides of the equation. The products side contains 3 carbon atoms, 8 hydrogen atoms, and 10 oxygen atoms. The reactants side also contains 3 carbon atoms, 8 hydrogen atoms, and 10 oxygen atoms. The equation is balanced. If, after working with each element, you have still not balanced the equation, you will need to change your coefficients. As before, begin with the element that appears in the fewest number of atoms. Continue to try new combinations of coefficients until you have balanced the equation.

Do not confuse coefficients with subscripts.
• When you change a subscript in a chemical formula, you are changing the type of molecule involved in the reaction. Never change subscripts to balance a reaction!
• When you change a coefficient in a chemical formula, you are changing the number of molecules involved in the reaction.
For example, consider the reaction of oxygen gas (O2) and hydrogen gas (H2) to produce water vapor (H2O).

Look Out!
The products side of the equation contains two hydrogen atoms and two oxygen atoms. The reactants side of the equation contains two hydrogen atoms and one oxygen atom. What happens if you add a subscript of 2 to the oxygen atom in the product?
H2 g + O2 g → H2O2 g
By changing the subscript, you have changed the molecule. Instead of water (H2O), the product is now hydrogen peroxide (H2O2). Instead, add a coefficient of 2 in front of the water molecule:
H2 g + O2 g → 2H2O g
Hydrogen gas is much lighter than both oxygen gas and water vapor. The molecular structure of water is different than the structures of oxygen and hydrogen gas. These properties can distinguish the reactants from the products, and the differences allow scientists to identify when a chemical reaction occurs.
Take a look at the following photographs. The picture on the left shows rusty nails, the middle shows a lit matchstick, and the right shows a rotting pumpkin. These images are all examples of chemical reactions taking place. In each image, how do you know a chemical reaction is taking place?




We can observe evidence of chemical reactions. Scientists confirm that a chemical reaction occurs by determining whether a new substance with new properties is formed. Scientists may perform additional chemical reactions and use instrumentation to confirm these new substances. However, there are signs or indicators to suggest that a chemical reaction has occurred. The only way to know for sure that a chemical reaction has occurred is to identify the new substance. If you observe one or more of these signs, this provides evidence that a chemical reaction may have taken place. Remember that not all chemical reactions will produce one of these signs. Let us look at the five signs that provide evidence of a chemical reaction.
1. Production of a gas: One very common reaction that involves the production of a gas is mixing sodium bicarbonate, also known as baking soda (NaHCO3), with an acid such as vinegar (CH3COOH). The products of this reaction are water (H2O), carbon dioxide (CO2), and a substance called sodium acetate (CH3COONa). During this reaction, you can see bubbles in the solution, as shown in the picture to the right. These bubbles are caused by the carbon dioxide gas escaping into the air as the reaction takes place. The chemical equation for this reaction is CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 (Vinegar) + (Baking soda)→ (Sodium acetate) + (Water) + (Carbon dioxide)
2. Production of light: The burning of logs in a fireplace is the reaction of the wood and oxygen along with a heat initiation source. Wood is made of cellulose, a combination of different substances that contain carbon, hydrogen, and oxygen. When this reaction occurs, a large amount of energy is produced. This energy is in the form of both heat and light. This type of reaction is a combustion reaction. It is similar to the reaction that produces the bright light and heat in fireworks.
3. Change in temperature: Chemical reactions can either give off heat (exothermic) or use heat (endothermic). Perhaps you have had an injury and applied a chemical heat pack to the area. A chemical heat pack is an example of a reaction that produces heat. A common substance in a heat pack is magnesium sulfate (MgSO4). When the heat pack is activated, the magnesium sulfate reacts with water. The result is the production of heat, which you use to soothe your injury. Chemical cold packs work in an opposite way to use heat when they mix with water. They may feel very cool to the touch. These temperature changes are evidence of a chemical reaction.
endothermic reaction: a chemical reaction in which energy is absorbed (uses heat)
exothermic reaction: a chemical reaction in which energy is released (gives off heat, light, sound, etc.)

Look Out!
Photosynthesis is an example of an endothermic chemical reaction in which plants absorb energy from the Sun and use carbon dioxide and water to form glucose and oxygen as byproducts. The equation for this reaction is 6CO2 + 6H2O ------> C6H12O6 + 6O2
Other examples of an endothermic reaction are cooking an egg, baking a cake, or activating a chemical ice pack. In each of those processes, energy is absorbed to form new substances.
The most obvious example of an exothermic reaction is any combustion reaction where heat is released, such as a burning match, fireworks, airbag-release mechanisms, etc. However, slow exothermic processes may not be so obvious, such as the oxidation of iron into rust, the breaking down of food molecules in digestion that releases energy, or cellular respiration in which the slow combustion of glucose and oxygen releases carbon dioxide, water, and energy. The equation for cellular respiration is C6H12O6 + 6O2 -----> 6CO2 + 6H2O
Reflect
4. Formation of a precipitate: A precipitate is a solid substance that forms and separates from a solution. A precipitate often settles to the bottom of a liquid reaction. One common chemical reaction that forms a precipitate is the reaction of solutions of lead nitrate (Pb(NO3)2) and potassium iodide (KI).
Each of these substances in a solution is clear and colorless. But if you mix a solution of each substance, lead iodide (PbI2) and potassium nitrate (2KNO3) form as products. Lead iodide is insoluble, so it separates from the solution as a yellow precipitate (shown in the image on the right). The potassium nitrate remains in the solution. The chemical equation for this reaction is Pb(NO3)2 + PbKI → PbI2 + 2KNO3

5. Change in color: You may have seen rust form on a steel object, such as a chain or an automobile. In this chemical reaction, iron (Fe) in the steel reacts with oxygen (O2) in the air, as well as with water (H2O), to produce rust (Fe(OH)3).
The properties of steel are different from the properties of rust. Steel is a shiny, silver metal made from iron and other elements. Rust is a flaky, reddish-colored substance. The change in color from silver to red provides evidence that a chemical reaction has happened. Rusting is a complex reaction that happens in stages. The overall reaction may be written as follows: 4Fe + 6H2O + 3O2 → 4Fe(OH)3




The process of fermentation—the conversion of sugar to alcohol and carbon dioxide—causes bread to rise.
Chemical reactions occur all around us: in rotting fruit (decay), rusting nails (oxidation), and baking bread. Bread dough is a mixture of ingredients, including flour, salt, sugar, warm water, and yeast. When these ingredients are combined, the ball of dough begins to expand, or rise. The rising happens because a chemical reaction is taking place inside the dough.
This reaction is caused by yeast, a living organism that becomes active in warm water. Once activated, the yeast converts the sugar (C6H12O6) in the dough into alcohol (CH3CH2OH) and carbon dioxide (CO2) through a chemical reaction. This process is called fermentation. The balanced chemical equation for this reaction is C6H12O6 2CH3CH2OH + 2CO2 (glucose) → (alcohol) + (carbon dioxide)
The release of carbon dioxide, which is a gas, causes the bread to rise. The carbon dioxide also gives the bread its light, airy texture. The alcohol produced by the reaction gives the bread its final taste. During baking, this alcohol evaporates, and the yeast cells cease to be active.
Acids are ionic compounds that break apart in water to form a hydrogen ion (H+). The strength of an acid is based on the concentration of H+ ions in the solution. The more H+, the stronger the acid. Bases are ionic compounds that break apart to form a negatively charged hydroxide ion (OH-) in water. Scientists use a pH scale to measure how acidic or basic a liquid is that focuses on concentrations of hydrogen ions (H+) and hydroxide ions (OH-). The scale measures values from 0 up to 14. Acids are between 0 and 7. Bases are from 7 to 14.
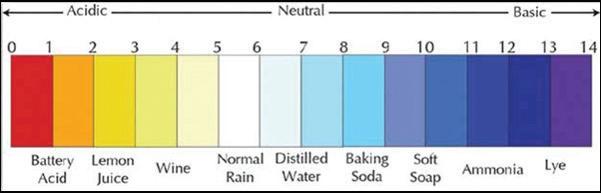

Litmus paper can also be used to indicate acids and bases. Blue litmus paper will turn red in an acidic solution, while red litmus paper will turn blue in a basic solution. Concentrated red cabbage juice will turn red in an acid, purple in a neutral solution, and yellowish-green in a base. To neutralize means to move the solution to the middle of a pH scale. If a base is added to an acidic solution, the solution becomes less acidic; if an acid is added to a basic solution, the solution becomes less basic and moves toward the middle of the pH scale. This is called neutralizing
Certain evidence suggests that a chemical reaction occurred. This evidence includes the production of a gas, the production of light, a change in temperature, a change in color, and the formation of a precipitate. Look at the types of reactions given below. For each reaction, determine which of the five types of evidence would be observed and write your answer in the space below that evidence in the table below. More than one type of evidence may be possible for each example.
• Exploding fireworks
• Baking a cake
• Burning paper
• Mixing an antacid tablet and water
• Making chalk from two liquids
• Blue litmus paper turning red in acid
• Folding a heat pack to activate it, which causes an increase in temperature
• A copper penny tarnishing
• Oxygen gas and hydrogen gas producing water and heat
• Testing for carbon dioxide by bubbling a gas in limewater to produce a milky-white solution

To help your child learn more about chemical reactions, work together to determine how to identify evidence that may be observed when a chemical reaction occurs. Begin by gathering the following materials:
• Three glasses of water
• Two effervescent tablets
• A tablespoon of sugar
• A tablespoon of Epsom salt
• A thermometer
While performing the chemical reactions, encourage your child to record all observations. Let the first glass contain the control sample in which no chemical reaction occurs. Add a tablespoon of sugar to the water in the glass and stir until the sugar dissolves completely. Record all observations until the sugar dissolves.
Remember that this control sample does not involve a chemical reaction because sugar dissolving in water is only a physical change. Then have your child add both effervescent tablets to the second glass of water. Record any observations for at least two minutes while the tablets dissolve.
Finally, place the thermometer in the third glass and record the initial water temperature. If a thermometer is not available, feel the outside of the glass and record if it feels hot, warm, or cold. Then add a tablespoon of Epsom salt and gently stir the liquid using the thermometer. Make sure to watch the temperature closely and determine how the temperature changes when the salt is added.
After performing the reactions, discuss the following questions with your child:
• In which of the glasses did a chemical reaction take place? How do you know?
• Why can you determine that a chemical reaction did not take place in all three glasses? How can you confirm that a chemical reaction took place in some glasses?
• Can you write a chemical equation to describe each chemical reaction that occurred?

1 Each element has its own, unique chemical symbol. The chemical symbol for hydrogen is H. The chemical symbol for helium is He. The chemical symbol for sodium is Na. During chemical reactions, elements come together or break apart to form different compounds. Scientists represent these compounds with chemical formulas. In a chemical formula, chemical symbols identify the elements in a compound. Numbers called subscripts show the number of each type of element in the compound.
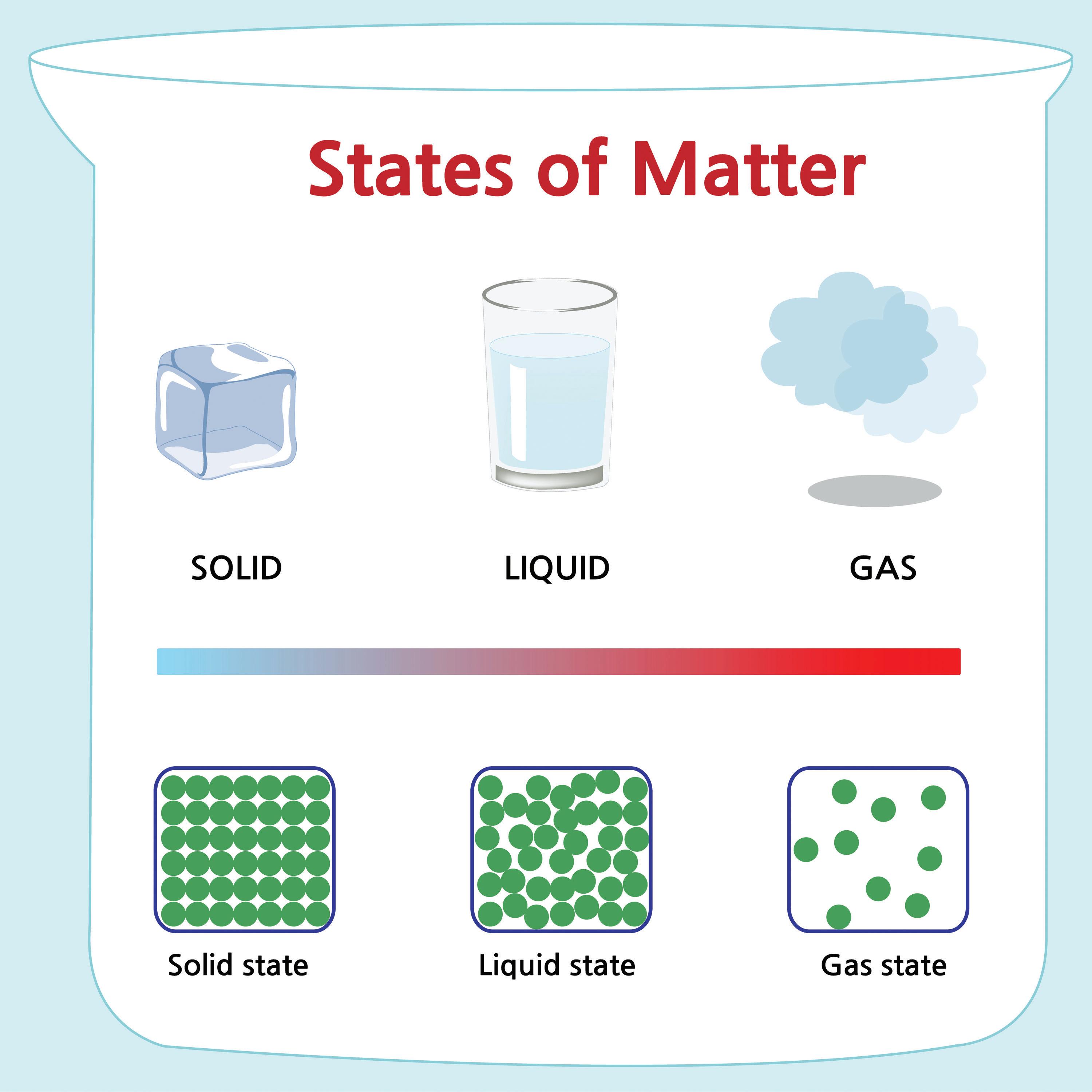
2 Chemical formulas are used for all compounds. For example, the chemical formula for water is H₂O. This formula means that a water molecule has both hydrogen (H) and oxygen (O). The subscript “2” (to the right of the hydrogen symbol) means that each molecule of water has two hydrogen atoms. There is no subscript beside the letter “O.” This means each water molecule contains only one oxygen atom. In other words, if there is only one atom of an element in a compound, then a subscript is not placed to the right of the chemical symbol for that element. There are two main types of compounds: ionic compounds and covalent compounds.
3 Ionic compounds are formed when positively charged ions (cations) “donate” electrons to negatively charged ions (anions) in a bond known as an ionic bond. An ion is any atom or compound with an electric charge. In ionic bonds, the total number of electrons lost by the ionic compound is equal to the number of electrons gained so that the charge is always balanced. The smallest unit of an ionic compound is called a formula unit. A familiar ionic compound is sodium chloride, or table salt. Each formula unit of sodium chloride is made from one sodium cation (Na+) and one chlorine anion (Cl-). The chemical formula is written NaCl. Notice that neither symbol has a subscript. This means each formula unit of sodium chloride is composed of one atom of each element. The one positive ion of sodium balances the one negative ion of chlorine, making a neutral sodium chloride compound.
4 A covalent compound forms when atoms bond by sharing electrons. This type of compound usually forms between two or more nonmetal elements. Most nonmetals are found on the upper right side of the periodic table. Common nonmetals include carbon (C), nitrogen (N), oxygen (O), and chlorine (Cl). Each atom in a covalent compound shares one or more electrons with a neighboring atom, forming a covalent bond. The smallest unit of a covalent compound is called a molecule.

5 An example of a covalently bound molecule is methane. Methane is composed of one atom of carbon (C) and four atoms of hydrogen (H). The chemical formula of methane is CH₄. This formula shows us that the carbon atom shares its electrons with four hydrogen atoms and that each hydrogen atom shares its electron with the carbon atom. Another common covalently bound molecule is glucose. This molecule is a very important part of the chemical reactions of photosynthesis and cellular respiration. Its formula is C₆H₁₂O₆. This means there are 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen sharing electrons in this molecule.
6 A metallic bond is a third type of chemical bond. In metallic bonds, electrons are not held tightly by one atom’s nucleus, nor are they shared between atoms. In metallic bonds, electrons are free to move from one nucleus to another. Do not confuse metallic bonds with ionic bonds. Metallic bonds form only among metal atoms, while ionic bonds form between metal and nonmetal atoms.

1 What types of bonds are formed when positively charged ions (cations) “donate” electrons to negatively charged ions (anions)?
A Metallic bonds
B Covalent bonds
C Ionic bonds
D Chemical formulas
2 Calcium sulfite is an ionic compound. Its chemical formula is CaSO₃. This means this compound has the elements calcium (Ca), sulfur (S), and oxygen (O). How many calcium atoms and how many oxygen atoms does this compound have?
A 0 Ca atoms and 1 O atom
B 1 Ca atom and 1 O atom
C 3 Ca atoms and 3 O atoms
D 1 Ca atom and 3 O atoms
3 What type of bond is formed when atoms bond by sharing electrons, usually between two or more nonmetal elements?
A Metallic bond
B Covalent bond
C Ionic bond
D Chemical formula

4 Dinitrogen trioxide is a covalently bonded compound that contains both nitrogen (N) and oxygen (O). This compound has two nitrogen atoms and three oxygen atoms. Which would be the correct formula for this covalent compound?
A NO
B N₃O₂
C N₂O₃
D N₆O₆
5 What do scientists use to represent the elements and compounds that are either broken apart or created during chemical reactions?
A Metallic bonds
B Covalent bonds
C Ionic bonds
D Chemical formulas
6 An ionic bond is formed between atoms of magnesium (Mg) and chlorine (Cl). The chemical formula for this compound, magnesium chloride, is MgCl₂. Magnesium is a cation with a positive charge of 2+. What must the negative charge on chlorine be? Remember, MgCl₂ must be a neutral compound.

1. An experiment was performed in which a crystalline substance was added to a beaker filled with room temperature water. The following observations were made:
• The crystals dissolved.
• A precipitate was formed.
• The bottom of the beaker felt warm to the touch.
• Bubbles began to form within the water.
Which observations are evidence that a chemical reaction took place?
2. A major component of modern atomic theory is based on models that illustrate the structure of an atom. Describe two changes that happened to the atomic model based on the experiments of Dalton, Rutherford, Thomson, or Bohr.

3. On pH paper, bleach is a base that has a pH of 12. What could be added to the bleach to neutralize it? Explain how the process of neutralization works.


4. Sometimes bases are added to a place in the ecosystem that has been affected by acid rain. For example, limestone is often added to soil to combat the effects of acid rain. Explain why this might be an effective method of solving the problem created by acid rain.
5. During a chemical reaction, energy that enters or leaves a system comes from the building or breaking of chemical bonds. How can you tell whether the system absorbed or released thermal energy?


Chemical reactions are constantly occurring in the natural world. One very common chemical reaction occurs during the process of photosynthesis as sunlight strikes the leaves of plants.
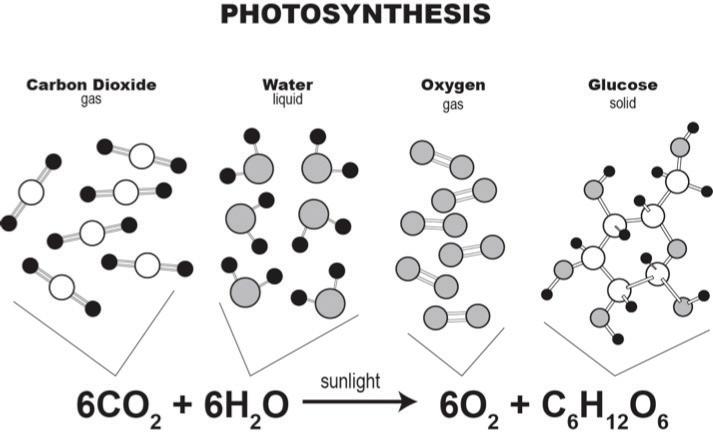
Write a scientific explanation that describes how the carbon dioxide and water molecules change after being exposed to sunlight.


1. Obtain a glow stick from your teacher. Be careful not to activate it!
2. Use the balance to find the mass of the glow stick, and record its mass in your lab journal.
3. Bend the glow stick to activate it.
4. Observe what happens, and record your observations in your lab journal.
5. Find the mass of the glow stick again after it has been activated. Record the mass in your lab journal.


Background
A chemical reaction is a process by which one or more substances change to produce one or more new substances with different properties. Reactants are the parts of the experiment participating in the reaction. The products are the parts that are produced as a result of the reaction. The law of conservation of mass states that mass is neither created nor destroyed. Investigate this law in the following activity.
For safety, students need to wear their safety goggles.
1. Pour 200 mL of water into the plastic water bottle. Be careful not to drip!
2. Find the mass of the water, the plastic water bottle, the Alka-Seltzer tablet, and the balloon. Record your data in the data table below.
3. Gently break up the Alka-Seltzer tablet and put the pieces into the balloon.
4. Place the balloon around the neck of the water bottle and tip the Alka-Seltzer into the water bottle. Record your observations below.
5. Measure and record the mass of the entire system of the water, the plastic water bottle, the dissolved Alka-Seltzer tablet, and the balloon in the data table.
Object (Reactants)
Water and Water Bottle
Alka-Seltzer Tablet
Balloon
Total System (Water, Water Bottle, Alka-Seltzer, and Balloon)
Observations
Initial Mass (g)
Final Mass (g)

1. How is the mass of the reactants related to the mass of the products in the experiment?
2. What is the purpose of the balloon in this experiment? How might the results differ if the student forgot to attach the balloon?
3. Compare the results of your investigation to Antoine-Laurent Lavoisier’s statement of the law of conservation of mass.

Scientific Investigation
Chemical reactions can take place in either a closed or an open system. A closed system, such as a glow stick, is one in which the reaction is isolated from the surrounding environment. An open system, such as a beaker, is one in which the reaction is not fully enclosed but is open to the surrounding environment. What we can observe about a chemical reaction may be different depending on whether it is done in an open or closed system.

Plan an investigation to compare the masses of reactants and products of a chemical reaction between an open and a closed system.
Procedure
Step 1: Question
Step 2: Variables, if applicable
Independent variable (also known as the manipulated variable):
Dependent variable (also known as the responding variable):
Control variable(s) or group (also known as constants):

Step 3: Hypothesis
Is a hypothesis needed? If so, what is it? How will the responding variable change when the manipulated variable changes?
Step 4: Materials
Step 5: Safety Considerations
Step 6: Procedure Open System Closed System

Step 7: Data Collection
Use a table to record your data.
Step 8: Data Analysis
Create a graph based upon the data, if needed. Make a general statement about the results shown in the graph. Be sure your statement references the masses in both the open and closed systems.

Step 9: Conclusion and Scientific Explanation
Write a scientific explanation on how conservation of mass can be observed.
Claim:
Evidence:
Reasoning:

Activity
Chemical reactions can be written as equations using either words or symbols.
Hydrogen + Oxygen → Water
2H2 + O2 → 2H2O
The letters represent the elements in the equation. Groups of symbols represent molecules. The subscripts represent the number of atoms of each element in the molecule. The number before a molecule (the coefficient) represents the number of molecules in the equation. An equation is “balanced” when the number of atoms of each element on the reactant side of the equation equals the number of atoms of that element on the product side.
Procedure
1. Follow steps 2–5 for each of the equations listed below. Identify which equations are balanced and which are not balanced chemical reaction equations.
2. Count the number of atoms on the left side of the arrow (reactants) and place that number of paper clips in the pan on the left side of the apparatus.
3. Count the number of atoms on the right side of the arrow (products) and place that number of paper clips in the pan on the right side of the apparatus.
4. If the equation has the same number of atoms in the products and the reactants, then the apparatus should be perfectly balanced. If there is a difference between the numbers of atoms, then the apparatus will lean toward the side with the most atoms. In this circumstance, the equation is not balanced, indicating that the equation does not reflect a valid chemical reaction.
5. Record whether each equation is balanced or does not reflect a valid chemical reaction next to the equation.
A. 4Fe + 3O2 → 2Fe2O3
B. CH4 + 2O2 → CO2 + 2H2O
C. CaBr2 + Na2CO3 → CaCO3 + NaBr
D. CH3COOH + NaHCO3 → NaCH3COO + CO2 + H2O

The idea that mass is conserved in a chemical reaction was not always known. In chemistry, a balanced chemical equation is the expression of the law of conservation of mass. In the late 1700s, a scientist named Antoine Lavoisier confirmed the law of conservation of mass.
Lavoisier found that during the oxidation of metal, the mass gained by the metal came from the reaction of oxygen with the metal. Using a closed system, Lavoisier was able to demonstrate that the mass of the products equaled the mass of the reactants.
Chemical formulas describe atoms held together by chemical bonds.

A compound is a group of atoms of different elements joined together by sharing or transferring electrons. The atoms are then held together by a chemical attraction, called a bond. A covalent compound forms when two or more atoms combine by sharing electrons.
The smallest unit of a covalent compound is called a molecule. You may be familiar with a molecule of water or carbon dioxide. An example of atoms held together by transferring electrons is sodium chloride. You may also have seen the chemical formulas for these compounds. A chemical formula is a representation of the smallest unit of a compound using elemental symbols to show the type of elements in the unit. Subscripts are used in the chemical formula to show the number of each type of element. Each element is represented in the chemical formula. The number of each type of element is represented by a subscript after the symbol.
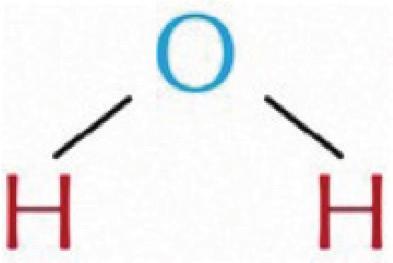
A molecule of water contains two atoms of hydrogen and one atom of oxygen. So its chemical formula is H2O.
Let us look at how to write the chemical formulas of some common substances. Some substances contain only two types of atoms. An example is sodium chloride, which you may also know as salt. Its chemical formula uses the symbols for sodium (Na) and chloride (Cl): NaCl. Water also contains two different atoms. Water is formed from hydrogen (H) and oxygen (O). It has the chemical formula H2O. The subscript after the H in the formula is written to identify the number of hydrogen atoms in the molecule. So a molecule of water contains two hydrogen atoms. When the smallest unit of a compound contains only one atom of an element, such as oxygen in water or sodium and chloride in salt, a subscript is not needed.

Some compounds may contain only a few atoms, such as sodium chloride and water. Others may contain multiple numbers and types of atoms. In some cases, atoms of the same element join together. Take a look at the chemical formulas of glucose (red), oxygen (blue), and carbon dioxide (green). How do you know which elements are in each compound? Identify the types of elements and the number of atoms of each element in each compound below.
Chemical equations describe chemical reactions. In a chemical reaction, molecules undergo a change. Atoms rearrange to form new substances.
Chemical equations are written to show what happens to the atoms in a chemical reaction. A chemical equation shows the changes in the arrangement of atoms.
Parts of a chemical equation:
• Reactants: In a chemical equation the reactants, which are the starting substances, are usually written on the left side of the equation.
• Products: The products of the reaction are the substances that result from the reaction. Products are usually written on the right side of the equation. The reactants are separated from the products by an arrow. The direction of the arrow points to the products in the reaction.
An example of a chemical reaction occurs when oxygen and hydrogen gas rearrange to form water. Oxygen gas (O2) and hydrogen gas (H2) are the reactants. Water (H2O) is the product of the reaction. During the reaction, the atoms of the reactants rearrange into a new substance, the product. Here is the reaction with the reactants circled:
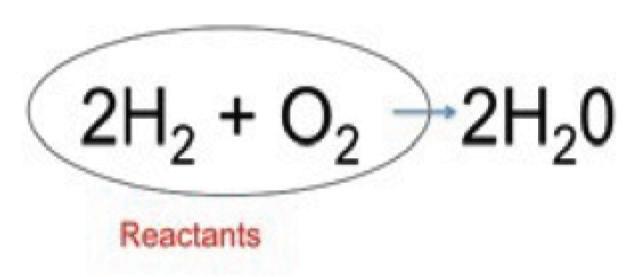


Reflect
Here is the same reaction with the products circled:
In the reaction to the right, one molecule of O2 reacts with two molecules of H2 to form two molecules of water. You know this from the number written before each substance in the reaction. This number is called a coefficient

A coefficient is used in a chemical equation to show how many molecules take part in the reaction. H2 and H2O each have a coefficient of 2. This means that two molecules of hydrogen gas are needed to form two molecules of water. A coefficient of 1 is never written in front of a substance. This is why there is no coefficient in front of the molecule O2. Only one molecule of oxygen gas is needed to form two molecules of water.
Chemical equations obey the law of conservation of mass.
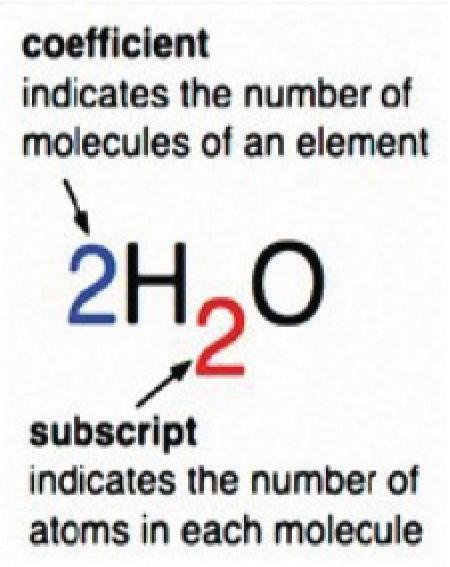
The law of conservation of mass states that mass is not created or destroyed in a chemical reaction. This law must be followed when you write a chemical equation. In the same way that you cannot create a mushroom pizza from a pepperoni pizza, the same atoms must be included in both the reactants and products of a chemical equation.
Each side of the chemical equation—reactants and products—must be balanced. This means that the number and type of elements on the side of the reactants must be the same as or equal to those on the products’ side.

Look at the equation in the top diagram on the right (“Unbalanced”). This equation shows the reaction of methane (CH4) with oxygen gas (O2). The product is carbon dioxide (CO2) and water (H2O). This equation is not balanced. There is one carbon atom on each side of the equation, so the carbon atom is balanced. However, there are four atoms of hydrogen on the side of the reactants, and only two atoms of hydrogen on the side of the products. There are also two atoms of oxygen on the reactants’ side and three atoms of oxygen on the products’ side of the equation.
Look Out!
In order to balance an equation, coefficients can be added or removed from a formula in a reaction. In the previous equation, the hydrogen atoms can be balanced by adding a coefficient of 2 to H2O. Then the oxygen atoms can be balanced by adding a coefficient of 2 to O2. The diagram on the right shows the balanced equation for this reaction.
Unbalanced:
O = 2 O = 3
Balanced: CH4 + 2O2 → CO2 + 2H2O
C = 1 C = 1
H = 4 H = 4
O = 4 O = 4
Balanced:
+ 2O2 → CO2 + 2H2O C
At this point, there are equal numbers of carbon, hydrogen, and oxygen atoms on both the reactants’ and products’ sides of the equation. There are four atoms of hydrogen, one atom of carbon, and four atoms of oxygen on both sides of the equation. To balance a reaction, you must confirm that all atoms are balanced. In this way, the equation is written so that it follows the law of conservation of mass. When balancing equations, do not confuse the coefficient with the subscript of a formula. Remember that the coefficient describes the number of molecules of each substance that are present during a reaction. You can change this number to balance a chemical reaction. The subscript in a chemical formula determines the number of atoms that are present during a chemical reaction. This number cannot be changed to balance the chemical reaction. Getting Technical: Chemical


Airbags inflate to protect you in a car crash, The following chemical equation represents the primary reaction that causes an airbag to inflate:
NaN3 is called sodium azide. It is a solid substance that is a reactant in the reaction. It is stored in a very small space such as the steering wheel of the car. When a crash occurs, the reactions begin. N2 is known as nitrogen gas. The production of this gas causes the airbag to inflate. The nitrogen gas takes up a large volume that inflates the airbag. It is important that scientists understand the chemical equation to know how much sodium azide is needed to produce a quantity of nitrogen gas that will inflate the airbag properly. How many of each type of atom are present in the unbalanced and balanced chemical equations?
In the unbalanced equation on the right, you can add coefficients to balance the equation, but the subscripts do not change in any reaction. Fill in the blanks in the diagram for the unbalanced and balanced equations to show how adding coefficients balances the number of each type of atom.
Part II: Balanced or Unbalanced?
Chemical equations can be written to describe chemical reactions. Look at the chemical equations in the table below. For each equation, count the number of each type of atom in the reactants and the products. Then write “Balanced” or “Unbalanced” beside each equation.
Chemical Equation
H2 + I2 → 2HI (Hydrogen reacts with iodine.)
2Al + 3O2 → 2Al2O3 (Aluminum reacts with oxygen.)
2Zn + 2HCl → ZnCl2 + H2 (Zinc reacts with hydrogen chloride.)
Unbalanced: H2 + N2 NH3 H = N = H = N = ≠ Balanced: 3H2 + N2 2NH3 H = N = H = N = =
Balanced or Unbalanced?

In each equation below, a blank line shows where one term is missing. The term may be an elemental symbol, a coefficient, or a subscript. Decide which term would balance the equation. Write the missing term in the second column. Then determine the reactants (column 3) and products (column 4) of the reaction. Some reactions may have only one reactant or product, and other reactions may have multiple reactants or products. Write your answers in the spaces in the table. (The first reaction involves sodium chloride, not sodium, carbon, and iodine.)
2NaCl → 2Na + Cl__
C___4 + 2O2 → CO2 + 2H2O
6___O2 + 6H2O → C6H12O6 + 6O2
To help your child learn more about chemical formulas and equations, work with him or her to explain how equations are similar to a recipe that might be used while cooking. Interestingly, there are many different ways that chemical reactions and chemical equations are used in cooking. For example, when you bake a cake, one of the chemical reactions that occurs is the baking soda reacting with water to produce carbon dioxide gas. This gas produces the “holes” in the cake that gives the cake its light, fluffy texture. A similar type of reaction occurs when baking soda is mixed with vinegar.
Work with your child to investigate, either online or via textbook, the chemical formula of baking soda and vinegar. (Hint: The substance in vinegar that reacts is called acetic acid.) Then work with your child to try to balance the reaction that occurs when baking soda and vinegar are mixed. Remember that carbon dioxide (CO2) is a product of the reaction.
After your investigation, work closely with your child to perform this reaction. In a sink or outdoors, use a funnel to place one tablespoon of baking soda in a deflated balloon. Next, pour ¼ cup of water and ¼ cup of vinegar into a clear, empty, plastic water bottle. Carefully stretch the neck of the balloon over the lip of the bottle, being careful not to allow the baking soda to fall into the bottle. Finally, hold the bottle securely as you lift the end of the balloon so that the baking soda falls into the water and vinegar. The balloon will inflate from the carbon dioxide that is produced in the reaction.
After performing the reaction, discuss the following questions with your child:
• Why are chemical formulas used to represent vinegar and baking soda?
• Which elements are in the initial reactants—the baking soda and the vinegar? How does this compare to the elements in the products?
• How do you know a chemical reaction occurred? How do you know that carbon dioxide (a gas) formed in the reaction? Why is a chemical equation used to represent the reaction?

1 Chemical reactions are occurring both inside us and all around us every second of every day. As a matter of fact, there is never a time when some type of chemical reaction is not occurring. In these processes, the starting chemical compounds (reactants) are destroyed and new chemical compounds (products) are created.
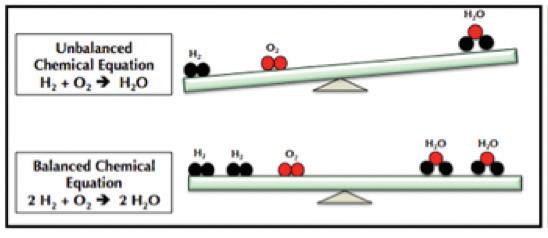
2 However, during all these trillions of chemical reactions that occur every day, matter is neither destroyed nor created. For all of these chemical reactions, the total mass of the chemicals that reacted will always equal the total mass of the products created. This is the most important law for all chemical reactions. It is called the law of conservation of mass.
3 The simple definition of the law of conservation of mass is as follows: The mass found in matter is neither created nor destroyed during a nonnuclear chemical reaction; it simply changes forms. The total mass of the reactants always equals the total mass of the products. The picture on this page illustrates that oxygen atoms on both sides of the equation were neither created nor destroyed during the reaction—they simply changed forms.
4 How did early chemists discover that mass is neither created nor destroyed? Many early scientists, known at the time as alchemists, worked with various elements and combined them to investigate what was produced. However, the conservation of mass was difficult for early scientists to conceptualize, as many of the gases required for or produced during chemical reactions were difficult, if not impossible, to measure. The gases were invisible in most cases and would either come from or escape back into the atmosphere.
5 A classic example of this issue happens with the burning of wood. As the product ash has less mass than the reactant wood, it seemed that mass was lost. It was not until experiments were conducted in sealed containers that scientists began to understand that the masses of the starting and ending substances did not change during chemical reactions. In other words, scientists began to understand that during chemical reactions, mass was “conserved.” Understanding that mass is conserved during chemical reactions was a very important discovery. This scientific discovery changed the study of alchemy into what we know as modern chemistry.
6 One of these early and influential modern chemists was Antoine Lavoisier (1743–1794). He is given the credit for being the first scientist to discover the principle of mass conservation. At the time, both water and air were considered elements. Lavoisier wanted to better understand what water and air were made of, so he conducted very precise experiments. He was careful to measure the masses of both the reactants and products of the chemical reactions that he was working with. Due to his precise measurements, he concluded that although the matter may have changed forms during the reaction, no mass was lost. As a result of his experiments, Lavoisier identified and named the elements hydrogen and oxygen. Due to his experiments, he became known as the father of modern chemistry.

1 The law of conservation of mass tells us, in part, that matter is neither created nor destroyed. How can we see this by using chemical reactions?
A By measuring the mass of the reactants and products in a closed system
B By writing a balanced chemical equation
C By counting the number of atoms on each side of the equation
D All of the above
2 Early chemists, known at the time as alchemists, had a difficult time understanding the law of conservation of mass. Using the burning of wood as an example, what property of matter made this law difficult for early scientists to understand?
A Wood is heavier than ash.
B The fire was too bright to observe the reaction.
C The gases produced escaped to the atmosphere.
D Different types of wood burned differently.
3 Which of the following is NOT true about the law of conservation of mass?
A The products always have less mass than the reactants.
B The mass found in matter is neither created nor destroyed.
C The total mass of the reactants always equals the total mass of the products.
D The mass in matter changes form during chemical reactions.

4 Early scientists did experiments with mass and products for many years, but had a hard time seeing that mass was conserved. This changed when they did what with these experiments under what specific conditions? Look to Paragraph 5.
A They did experiments with certain gases.
B They did experiments with certain types of wood.
C They created scales that gave more precise measurements.
D The experiments were done in sealed containers.
5 One scientist stands out with regard to the law of conservation of mass and is often called the “father of modern chemistry.” Who is this scientist?
A Albert Einstein
B Henry Cavendish
C Antoine Lavoisier
D Amedeo Avogadro
6 In the picture found on the first page, how many total hydrogen atoms (H) are on both sides of the balanced chemical equation?
A 1
B 2
C 3
D 4

1. According to the law of conservation of mass, matter is–
2. Hydrogen peroxide, H2O2, is unstable under certain conditions and decomposes as shown below. What will be the result when an initial 40 g of hydrogen peroxide decomposes? 2H2O2 → 2H2O + O2
g ?
3. Students are designing an experiment to test the law of conservation of mass. They will use the following materials:
• 10 g baking soda
• 30 mL vinegar
• Electronic balance
• Resealable plastic bag
Describe the basic procedure the students should follow to model this law.

4. A chemical equation is shown below.
Is this equation balanced? Explain your answer and include the characteristics of a balanced equation.

For science homework, a student was responsible for creating a model that accurately represented the law of conservation of mass. The model below depicts this student’s attempt at creating this model by using the chemical reaction that creates water.
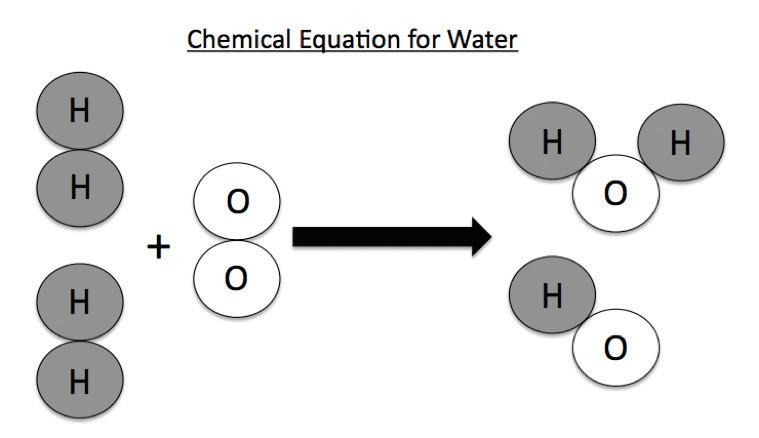
Write a scientific explanation that describes why the model pictured above does not follow the law of conservation of mass.
PEER EVALUATION
Peer Name: Rebuttal:


1. Fill a metal container with room-temperature water.
2. Add a thermometer, and wait for it to adjust to the temperature of the water.
3. Put your hand next to the container, and notice how it feels.
4. Record your observation below.
5. Add ice cubes to the container.
6. Observe and record what happens to the sides of the container and to the temperature of the water.
7. Put your hand next to the container again, and make observations.
8. Record your final observations below.

Part I: Weather Photos
Photo
Description of the Environment and Weather Conditions
Is This an Example of Weather or Climate?
Choose one of the photos and use it to answer the following questions:
What weather conditions do you see in this picture?
Does this seem like normal weather for this area?
What is the weather usually like there?

Discuss with your group if the descriptions are examples of weather or climate. Circle the keyword(s) that helped you decide. Glue under the proper heading.

The movement of water and air masses in the atmosphere is caused by thermal energy transferred to Earth’s surface from the Sun. This energy-driven atmospheric movement causes the weather we see day to day. The movement of water and air masses leads to changes in atmospheric conditions such as wind speed and direction, temperature, humidity, and precipitation, which can be tracked over time. Weather stations exist all over the world and measure these conditions daily, hourly, or even every minute. By knowing the atmospheric conditions today and for the past week, month, year, or decade, meteorologists can use this information to predict what the weather will do tomorrow and next week.
Procedure
1. Use the chart to record your data.

Temperature
Precipitation Symbols
Probability of Rain (high or low)
Miles Traveled by Cold Front
Miles Traveled by Warm Front
2. Using the weather map provided by your teacher, you will be plotting the cold and warm fronts for the next week and predicting the weather of Omaha, Nebraska. Looking at your weather map, fill in the chart for Monday.
3. Using the information on your weather map, calculate how far the fronts will move each day. To calculate how far they will move, take the speed at which they are moving and multiply it by the number of hours in a day. Record the distance each front will travel each day in the data table.
4. Using the map scale and the ruler, draw in the new location of each front for the remaining four days of the week.
5. With your fronts now drawn, predict the weather in Omaha, Nebraska, for Tuesday through Friday. Write your predictions of the daily temperature, the probability of rain, and the predicted precipitation symbol in the data table.

Convection is a process of thermal energy transfer in fluids, like air and water. When thermal energy is added to a fluid, the matter in the fluid gains kinetic energy and spreads out. As the matter spreads, the fluid becomes less dense (same amount of mass but larger volume). Less-dense fluids rise above fluids with greater density, causing movement within the liquid or gas called a current.
1. Look at the Deep Ocean Currents Map.
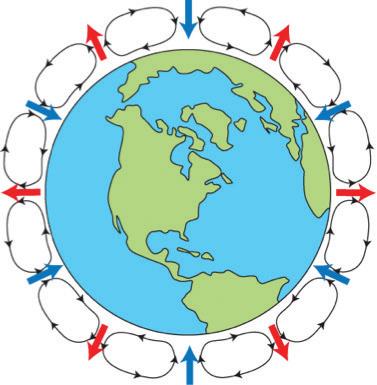
2. Follow the flow of the cold water indicated in blue and the warm water indicated in red.
3. Write an explanation of where and why the cold-water current becomes a warm-water current.
4. Write an explanation of where and why the warm-water current becomes a cold-water current.
1. Look at the Ocean Surface Currents Map.
2. Follow the flow of the cold water indicated in blue and the warm water indicated in red.
3. Write a statement to compare the flow patterns of the deep ocean and surface currents.
4. On the Teacher Printout: Ocean Surface Currents Diagram, use red and blue colored pencils to indicate possible convection currents.
5. Explain how these convection currents affect the weather at different locations on Earth. Pick at least five locations to research, create a data table, and record your data in your table. Write a brief summary about what you found and how convection impacts the climate and weather patterns of a particular area.

Predicting the weather is tricky business. Many factors can affect the movement of air masses. Meteorologists use multiple computer-generated models when making weather predictions. Computer models are also used to predict the paths of tropical storms such as hurricanes.
1. Research to gather information about the various types of computer models meteorologists use to predict what types of weather (thunderstorms, hurricanes, and tornadoes) result from the movement and interactions of air masses, high- and low-pressure systems, and frontal boundaries. Use both online and print resources.
2. Write a summary of the information gathered from each source and an assessment of the credibility of each source in your lab journal. Include a bibliography of the sources.
3. Compare and contrast the information gathered about each type of weather using models to predict weather by making a Venn diagram in your lab journal.
1. Look at the provided hurricane spaghetti model.

2. Probability is calculated by dividing the number of events by the number of outcomes. According to the model, the probability of the storm making landfall in Florida is calculated by dividing the one event of a model showing the storm striking Florida by 14 (the total number of predicted paths) 1/14 = .07 There is a 7% probability of the storm making landfall (first striking land) in Florida.
3. Use the information from the model to calculate the probability that the storm will make landfall in Texas.
4. Record your calculation and write an explanation of how you calculated the probability in your lab journal.
5. Choose two more states and calculate the probability that the storm will pass through each of the selected states. Record your calculations in your lab journal.

Complete the Venn diagram comparing satellite, radar, and weather maps.
1. After looking at all three types of maps (radar, satellite, and weather), what type of phenomenon do you think is most likely predicted using the following maps?
Radar:
Satellite: Weather:
2. Look at the radar map over Mississippi on accuweather.com. What type of weather is your city experiencing?
3. Click on the satellite tab and determine what additional feature you can see on this type of map. Record your answer below.
4. Looking at the satellite map, what type of cloud cover is your city currently experiencing?
5. Click on the forecast weather map. Look at the temperature maps and interpret the current temperature for your city. Record your answer below.

1. What do the lines on the map represent?
2. What information do the contour lines give the map reader?
3. What is the highest elevation of the mountain according to this map?
4. What do you call the elevation at the 0 elevation level?

A rain shadow is a dry or arid region on the back side or leeward side of a mountain range. The climate is desertlike because most of the moisture from the nearby ocean is released on the front side of the mountain range. The windward or ocean side of the mountain range has a wet climate caused by the rapid cooling of the moisture-laden air as it moves up the mountain.
1. Observe the map of the state of Oregon.
2. Locate the cities listed below.
3. Next to the name of each city, place the amount of yearly rainfall in inches. In order to find this information you will need to type each of the following cities in the Google search bar. For example, you would type, “what is the average yearly rainfall in Salem, Oregon?” Record the information and go on to the next city.

Answer the following questions. Prepare to discuss with your classmates.
4. What pattern do you notice?
5. What might cause the pattern to repeat itself year after year?
6. Why are the rainfall amounts in Medford and Lakeview similar?
7. Which side of a mountain range would receive the most precipitation, the windward or leeward? Explain.
8. How does what you observed compare to the information found in the introductory paragraph?


Plan and investigate how wind causes global ocean surface currents and how landmasses affect the direction of the flow.
Question
Hypothesis
Materials
1 Aluminum pan, 9 x 13
3 Rocks, various sizes
1 Ball of clay, 50 g
1 Set of colored pencils
1 Safety goggles
Water, 500 mL
Italian spices, 5 g
4 Bendable straws
1 Sheet Aluminum foil, 15 cm x 15 cm

Procedure
Task 1
1. Fill the container half full of water.
2. Sprinkle Italian seasoning in the water carefully so it is floating on the surface.
3. Blow gently through the straw across the surface of the water from two different corners opposite each other. Make sure you blow across, not down on, the water.
4. Make a drawing of what you observe in the diagram on the next page.
Task 2
1. Place rocks or clay landmasses in the model.
2. Repeat blowing through the straws, and make observations of what happens. Make sure you blow in the same spots as before.
3. Make a drawing of what you observe in the diagram on the next page.
Task 3
1. Make a small boat out of foil.
2. Launch the boat in your model with the landmasses and again blow exactly as you did in Tasks 2 and 3.
3. Make a drawing of what you observe in the diagram on the next page.

Fill in the boxes below with your drawings and observations from Tasks 1, 2 and 3.
Task 1 Diagram
Task 2
Diagram
Task 3
Diagram

Compare the diagrams of your investigation results as a class. Make a general statement about the results shown in the diagrams.

You are going on vacation in a week, and you have to start thinking about what clothes you are going to pack for your trip. You have read the weather reports for your vacation spot, but you know that the weather can change from day to day. You decide that the best way to pack is to choose clothes that work best for the climate you are going to. Is that a wise decision? What exactly is the difference between weather and climate?
When we talk about weather, we mean the daily conditions in the atmosphere of a local area. Many conditions make up the weather. A few are cloud cover, wind, humidity, and temperature, which is how hot or cold the air is. One condition that is important for planning a vacation is rainfall.

weather: daily conditions of the atmosphere in a local area
climate: type of weather in an area averaged over a long period of time
Weather reports can include information about the temperature, cloud cover, winds, humidity, and precipitation. Precipitation has many forms, including rain, snow, sleet, and hail. They are all slightly different based on the temperature of the air as the water falls through it. Rain is liquid water that falls in droplets. Snow and hail, on the other hand, are particles of ice that fall when it is colder outside. Sleet is a mixture of rain and snow. Weather is an important part of daily life. It describes the changing conditions of the environment around us. What is the weather like where you are today?
All weather is caused by the Sun heating Earth. When the Sun’s energy heats the atmosphere unevenly, it causes different air pressures. Pressure is the weight of the air. Cold air weighs more than warm air because it is denser. Low-pressure air and highpressure air cause different weather conditions. Low-pressure air often brings rain, thunderstorms, and hurricanes. High-pressure air usually means clear skies and sunshine. The uneven heating of the atmosphere is the reason there is different weather in most places on Earth during spring, summer, autumn, and winter.

What is climate? What are some characteristics of climate?
Weather is constantly changing. Scientists who predict, or forecast, the weather cannot usually make forecasts beyond 10 days. Even weather reports cannot guarantee that the forecasts will be accurate. However, climate in a particular area is consistent. Climate is the type of weather in an area averaged over a long period of time, such as 30 years or more.
For example, when most people think of Hawaii, they picture sunshine, high temperatures, and warm rainfall. Hawaii has a tropical climate. The weather there is usually warm and humid with cool breezes, and it has been that way for many years. But that does not mean that Hawaii does not have days with cold temperatures and storms. The climate of an area describes its average temperatures, precipitation, humidity, wind, cloud cover, and other weather conditions over long periods of time.
An area’s climate is affected by several factors.
These may include its distance from water (like oceans or lakes), latitude on the globe, surface features, shape of land, ocean currents, and elevation above sea level. There are several different climate zones in the world. Tropical climate zones are found closest to the equator. The climate in a tropical zone is hot and humid with lots of rain. Rain forests are found mostly in tropical climates.
Farther away from the equator is the temperate climate zone. Most of the United States has a temperate, or subtropical, climate. The temperatures are neither very high nor very low, and there are moderate amounts of precipitation. Temperate climates usually have different weather for each of the four seasons.
Past the temperate climate zones are the polar zones. They are found close to the North and South Poles. These zones are well known for their extremely cold temperatures and snow. However, polar climate zones are also quite dry, with little precipitation during the year.

In what climate zone is this place located?
temperate: mild, moderate; not extreme

Weather is one of those things that affects everybody. The clothes we wear, our travel plans, our daily activities—all of these depend on the weather.
In order to plan our day, we need to know what the weather will be. For this we depend on the work of special scientists known as meteorologists. How do meteorologists do their work? How do they predict the weather?
Predicting the weather is like predicting anything. To make a good prediction, it helps to know about what is going on right now and how things usually turned out in the past.
Let us take an example from baseball. Say a batter steps to the plate, and we want to predict if he will get a hit or strike out. It helps to know some things. What is the batting average of the player? How is the player batting today? What about the pitcher? Has the pitcher been striking out many previous batters? Suppose we know that the batter has a low batting average and has struck out both previous times at bat this game. Suppose also that we know the pitcher strikes out seven of every ten left-handed batters and that the player at bat is left-handed.
We might predict the batter will strike out, but will he? Maybe yes and maybe no. So many other things might affect the outcome that we cannot be certain.
Predicting the weather is a lot like predicting the outcome of the baseball player at the plate. To predict the weather, we need to know current conditions. What is the current temperature? How about the humidity? What are the current conditions of air pressure, wind, and solar radiation?
It also helps to know what weather was produced in the past when conditions were similar.
Meteorologists help to create the weather maps we might see in the newspaper or on television.
meteorologist: a scientist that studies weather

Predicting the weather is like making other kinds of predictions.

How does the water cycle affect weather and climate?
As water cycles, the movement of the water frequently takes the form of weather. The heat from the Sun causes the water to move upward, but it also causes winds, and those winds push the water molecules around. Winds are caused because the Sun’s rays do not land equally all over the surface of Earth. Some areas heat up more than others. When the molecules of air are heated, it causes them to spread out more from each other. Hotter masses of air sometimes bump into cooler ones. In the cooler air masses, the molecules have been heated less, so they are closer together.
When a hotter, less dense (with molecules that are farther apart from each other) mass moves next to a cooler, more dense (with molecules that are closer together because less heat energy has reached them) mass, the warmer mass cannot move the cool one out of the way. This difference in density causes clouds, thunderstorms, and even tornadoes to form as the masses try to move around and past each other. This is why we see weather that changes from day to day and, sometimes, hour to hour. The same principles apply to climate, except the conditions are averaged for a longer period of time for a region.
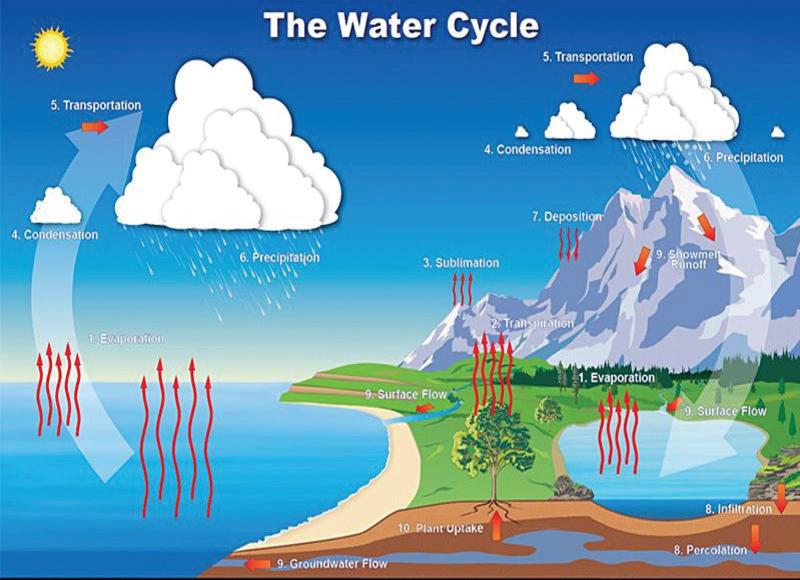

Hurricanes: In the United States, National Oceanic and Atmospheric Administration (NOAA) meteorologists and support personnel are charged with flying into hurricanes to determine their characteristics, such as degree of organization, wind speed, and precise location of the eye. Information from flights is used by scientists at the hurricane center to make predictions about a storm’s course and strength. Hurricanes are strong, rotating, low-pressure weather systems that have strong winds and thunderstorms but no fronts. When a storm’s maximum sustained winds reach 74 mph, it is called a hurricane.


Tornadoes: Tornadoes are the violent storms produced when warm, humid, lower-atmosphere winds meet cooler, upper-atmosphere winds in a rotating thunderstorm supercell. Tornadoes occur frequently during spring and summer thunderstorms in the central United States in an area called “Tornado Alley.”
Jet streams are relatively narrow bands of strong winds in the upper levels of the atmosphere. These jet streams can bring weather extremes called El Niño (brings repeated thunderstorms to the southern states) and La Niña (brings heavy winter storms to the northern states). Look Out!

Look Out!
Without the Sun, Earth would not experience weather as we know it. The Sun’s radiation is electromagnetic waves, mostly visible light, UV, and infrared (heat).
Some of this heat is reflected back into space by Earth’s atmosphere and surface. Earth’s atmosphere and surface absorb some of this energy from the Sun, with Earth’s surface absorbing almost half of incoming solar radiation. The heat that is absorbed by Earth produces convection currents in the air. Convection refers to the transfer of heat through a liquid or gas, such as air. Convection currents in the atmosphere refer to the flow of air in circular patterns: air rises, flows parallel to Earth’s surface, falls, flows parallel to Earth’s surface, and rises again. What causes the air to move like this?
The ground heats up as it absorbs sunlight, warming air near Earth’s surface. As this air heats up, it becomes less dense than the cooler air above. This causes the warm air near Earth’s surface to rise. As warm air rises, denser, cooler air rushes to fill in the area. The cooler air sinks toward the ground, where it too becomes warmer. Eventually it becomes less dense than the air above it and begins to rise. As this cycle repeats itself, convection currents form.
Rising warm air creates a wind called an updraft. Sinking cool air creates a wind called a downdraft. Air in convection currents also moves parallel to Earth’s surface. This produces surface winds as well as winds higher in the atmosphere, including the jet stream.
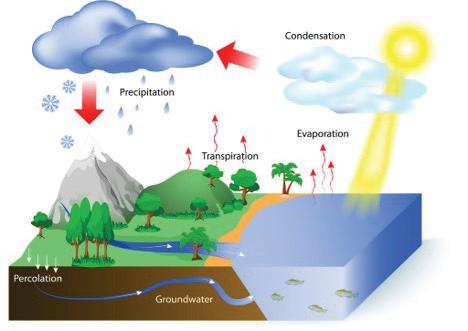
radiation: energy that travels from a source
The ground absorbs sunlight. Convection currents form as warm air rises (red arrows), cools in the atmosphere, and sinks (blue arrows) toward the ground.
As air warms and cools, water in the air changes between gas (water vapor), liquid (water droplets), and solid (ice). These phase changes produce other weather events. Heat near Earth’s surface causes liquid water to evaporate from oceans, lakes, and rivers. As the water vapor rises and cools, it condenses to form clouds of water droplets or ice pellets. When the droplets or pellets become heavy enough, the water falls back to Earth as precipitation like rain, snow, or hail.

Air moves across Earth’s surface in huge volumes called air masses. Each air mass possesses a characteristic temperature (cold—arctic; polar or warm— tropical), pressure (high or low), and moisture content (humid or dry). In various combinations, these properties can change the weather where the air mass flows. The leading edge of an air mass is called a front. A front is also the boundary between two air masses where weather often changes. Weather scientists, or meteorologists, identify three major kinds of fronts: cold fronts, warm fronts, and stationary fronts.
A cold front is the leading edge of a cold air mass that pushes against a mass of warm air. Because cool air is denser, it pushes the warm air up, usually very quickly. This collision between cold and warm air masses often produces strong storms. On a weather map, a cold front is usually indicated by blue triangles, as you can see on the bottom of the first page of this companion. The triangles point in the direction of the front’s movement.
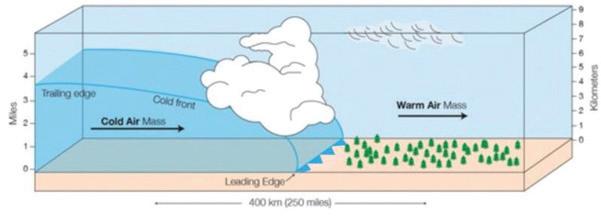
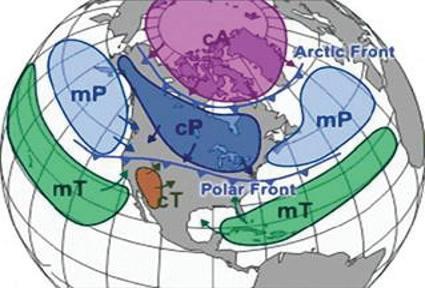
Air masses that form over water are called maritime (m). They tend to be more humid than continental air masses (c), which form over land. Air masses that form over the equator are called tropical (T). They tend to be warmer than polar (P) or arctic (A) air masses, which form nearer the poles.
A warm front is the leading edge of a warm air mass that pushes against a mass of cold air. Because warm air is less dense, it flows over the cold air at a warm front. This collision between warm and cold air typically produces overcast skies and rain. On a weather map, a warm front is represented by red half-circles. The rounded portions of the half-circles point in the direction of the front’s movement.
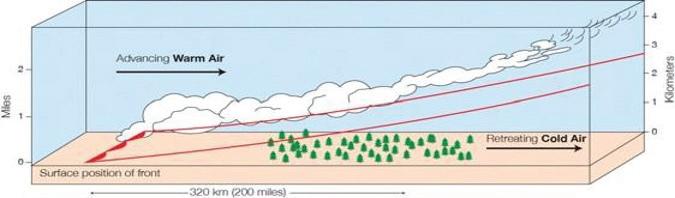
At a warm front, a warm air mass rises above a cold air mass.

Sometimes a front may stall over an area. Such a front is called a stationary front. On a weather map, a stationary front is represented by alternating red half-circles and blue triangles that point in opposite directions. The weather at such a front tends not to change for as long as the front stays in place. Stationary fronts usually produce long periods of rain.
Air pressure refers to the weight of a column of air over a particular location on Earth. Denser air masses exert greater pressure because they contain more particles of air per unit volume. On a weather map, high-pressure air masses are labeled with the word high or the letter H. Low-pressure air masses are labeled with the word low or the letter L. Each type of air mass is associated with certain kinds of weather:
• Low-pressure air masses usually produce stormy weather and contain winds that flow counterclockwise in the Northern Hemisphere and upward toward the center of the air mass.
• High-pressure air masses usually produce calm, clear weather and contain winds that flow outward from the center of the air mass in a clockwise direction in the Northern Hemisphere.
At sea level and 0°C, the average air pressure is 760 mm Hg. In other words, the column of mercury in the barometer will reach a graduation on the tube of 760 mm (approximately 30 inches). As air pressure increases, the column of mercury rises. As air pressure decreases, the column of mercury falls. Look Out!
Particles of cool air sink toward Earth’s surface, creating areas of high pressure. Particles of warm air rise into the atmosphere, creating areas of low pressure. The air pressure at the top of Mount Everest (measured in kPa, or kilopascals) is more than three times lower than at Earth’s surface.
The mercury barometer, once used by scientists to measure air pressure, was invented in 1643 by an Italian physicist named Evangelista Torricelli. It consists of a graduated glass tube with a scale typically in millimeters. The top end of the tube is closed, and the bottom end is open. The tube is emptied of air, and then filled with mercury and placed open-end-down in an open container, or reservoir, of mercury. The weight of air pressing down on the mercury forces the mercury up to a certain height in the tube. This measurement gives the air pressure in millimeters of mercury (mm Hg). (The chemical symbol for the element mercury is Hg.)
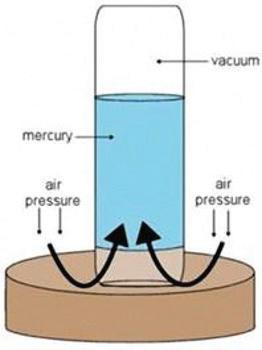
A mercury barometer is an instrument for measuring air pressure. Today many meteorologists use new aneroid barometers, partly because mercury is toxic and partly because the aneroid barometer can take multiple measurements more easily.

We can try to make our own weather prediction by using historical weather data and comparing it to current weather data.
Below is a table of various weather conditions for the same location over many days, along with the weather that resulted (basically, whether it rained that day or not).
Use the historical weather data to assign a probability of rain for the day we are trying to predict.
Day 1
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers
RAIN
Day 6
Winds: Westerly
Air Pressure: Steady Clouds: Cumulus towers RAIN
Day 11
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers RAIN
Day 16
Winds: Westerly
Air Pressure: Falling Clouds: Cirrostratus RAIN
TODAY
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers
Day 2
Winds: Westerly
Air Pressure: Falling Clouds: Cumulus NO RAIN
Day 7
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers NO RAIN
Day 12
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers RAIN
Day 17
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers RAIN
Day 3
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers RAIN
Day 8
Winds: Easterly
Air Pressure: Rising Clouds: Stratus NO RAIN
Day 13
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers NO RAIN
Day 18
Winds: Easterly
Air Pressure: Steady Clouds: Stratus RAIN
Day 4
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers RAIN
Day 9
Winds: Northerly
Air Pressure: Falling
Clouds: Cumulus NO RAIN
Day 14
Winds: Westerly
Air Pressure: Rising Clouds: Cumulus towers RAIN
Day 19
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers NO RAIN
Day 5
Winds: Easterly
Air Pressure: Rising Clouds: Stratus RAIN
Day 10
Winds: Easterly
Air Pressure: Rising Clouds: Cumulus towers NO RAIN
Day 15
Winds: Northerly
Air Pressure: Falling Clouds: Cirrus NO RAIN
Day 20
Winds: Easterly
Air Pressure: Falling Clouds: Cumulus towers RAIN
What is your best prediction for the probability of rain today?

The first step to predicting weather is just paying attention to weather. With a simple spiral-bound notebook and a pencil, your child can become an amateur meteorologist.
Encourage your child to record weather conditions he or she observes every day or maybe even more than once a day—say, in the morning and in the evening.
Some of these observations need no special tools. Your child can see the sky conditions just by looking up. If your child happens to know which direction is north, he or she can easily record wind direction. (Note: meteorologists record wind direction by the direction from which the wind is coming. An easterly wind blows from the east to the west.)
Other observations may require simple tools, such as a thermometer (temperature) or a barometer (air pressure). If your child does not have access to special tools, he or she can always get information like this from several sources, either online or from television or newspapers.
After your child records observations for several weeks, he or she can begin to look for patterns. What time of day is warmest? Coolest? What kind of weather usually follows a drop in temperature?
Here are some ideas and questions to discuss with your child:
• What kinds of data are easiest to collect? What kinds of data are hardest? Which observations are most helpful for making predictions?
• What other kinds of data might be helpful? Do animals behave differently when there are different weather conditions?

1 Climate is defined as the main weather conditions in one area over a long period of time. These conditions include temperature, humidity, rainfall, and wind. Climate around the world can be divided into five basic types: tropical, dry, moderate, continental, and polar. Have you ever wondered what creates the climate where you live? Latitude, terrain, altitude, and nearby bodies of water all play a role in the climate of a given location. Let us find out more about how latitude and air circulation affect climate and weather.
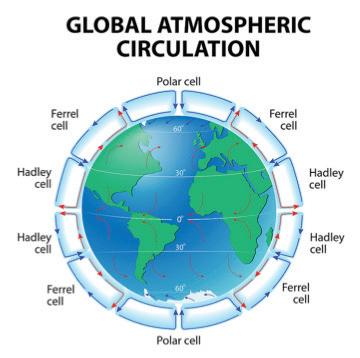
2 Lines of latitude are imaginary horizontal lines drawn around the globe parallel to the equator. These lines mark the distance of a place either north or south of the equator. Zero latitude is located at the equator. The north pole is 90° (degrees) latitude, as is the south pole. Miami, Florida, is located at 25° north, while Anchorage, Alaska, is located at 61° north. Latitude also controls the amount of sunlight a given area receives. Within 30° of the equator, the Sun shines nearly perpendicular (in a straight line at a 90° angle) to Earth’s surface. This means that the areas around the equator take in most of the energy from the Sun. This also means that these areas take in the most heat. At higher latitudes, the angles of the Sun’s rays are shallower, so these areas receive less energy from the Sun. The farther north or south you go, the less energy Earth receives.
3 The rotational speed of Earth also changes with latitude. Differences in the speed of Earth’s rotation affect air circulation. The rotation of Earth prevents the wind from blowing in a straight line. In the northern hemisphere, wind is “pushed” to the right. In the southern hemisphere, wind is “pushed” to the left. The force responsible for the deflection (the “pushing” of winds) is called the Coriolis effect, which is a result of Earth’s rotation. All of this may sound pretty confusing, so let us look at several examples where latitude and air circulation come together to create weather.
4 There are three bands of air masses circulating in each hemisphere. Each band of air is generally kept around 30° latitude. This means that one band may be found between 0° and 30°, another between 30° and 60°, and a third between 60° and 90°. Close to the equator, climate and weather are known for their stability. Temperature varies little between day and night and between the seasons. Warm humid air rises, producing low pressure. High in the atmosphere, the air starts to flow toward the poles. This is because air moves from an area of high pressure to an area of low pressure.
5 High pressure comes from cooler air sinking toward the ground, while low pressure comes from warm air rising from the ground. The movement of air from high to low pressure is known as wind. In the northern hemisphere, wind deflects to the right due to the Coriolis effect. As the air mass moves northward, it cools and a portion of it sinks, flowing back toward the equator along Earth’s surface. This happens in both hemispheres between 20° and 30° latitudes.

6 The rising air at the equator creates clouds and rain. In some land areas near the equator, tropical rain forests thrive. The air that sinks back to the ground near 30° latitude is very stable and makes a constant high-pressure system. This band of high pressure circling Earth is known as the subtropical ridge. In areas which lie along this latitude, few clouds and little rain create major deserts, such as the Sahara Desert in Africa and the Mojave Desert in North America.
7 In the midlatitudes, another mass of air circulates, generally creating winds that move from west to east. These winds are known as westerlies in both hemispheres. They blow between 30° and 60° latitudes. The westerlies are strongest in winter and weakest in summer. The climate in these midlatitudes tends to be temperate, meaning that temperature and precipitation (rainfall) change with the seasons.
8 Above 60° latitudes are the polar regions, where the weather is dominated by cold air sinking at the poles. This produces very high pressure, so strong winds are common near the poles. These winds blow south at the north pole and north at the south pole. Sinking air and the resulting high pressure create very little precipitation at the poles.
9 At each latitude, landforms also influence climate and weather. In general, temperature decreases with altitude. At higher altitude, air pressure is lower, and this lower pressure leads to lower temperatures. Precipitation depends on both the altitude and the direction of wind. As air rises, it gets colder. When this happens, moisture trapped in the air falls as rain. In addition to latitude, climate and weather strongly depend on how close an area is to the ocean.
10 Let us look at Seattle, Washington, as an example of how landforms can affect weather. Wind blows from west to east near Seattle, picking up moisture from the Pacific Ocean and dropping it over Seattle, giving the city its rainy reputation. Higher elevations on the western side of the mountains in Washington still receive lots of rain, but as the air rises over the top of the mountains, most moisture is left behind. Areas on the east of the mountains receive little rain. Land further inland tends to receive less precipitation because the wind cannot pick up moisture as it travels.

1 At which latitude range would a student most likely find an area that has all four seasons?
A Between 0° and 30°
B Between 30° and 60°
C Between 60° and 90°
D All latitudes have all four seasons.
2 Which of the following variables influences climate the least?
A Latitude
B Longitude
C Altitude
D Terrain
3 City A is located at 30° north latitude, while City B is located at 30° south latitude. Which city is closest to the equator?
A City A
B City B
C Both cities are equally close.
D City A in winter, City B in summer

4 Which statement correctly describes the relationship between air temperature and air pressure?
A Warm air rises, creating an area of low pressure.
B Cool air sinks, creating an area of low pressure.
C Warm air sinks, creating an area of low pressure.
D Cool air rises, creating an area of low pressure.
5 The westerlies blow toward the equator and from west to east. What factor is most responsible for the westto-east direction?
A The strong solar radiation at the equator
B The high pressure at around 30° latitude
C The low air pressure at the equator
D The rotation of Earth
6 At which latitude would tropical rain forests be most likely?
A 10° north
B 30° south
C 60° north
D 90° south

1. Describe the differences between weather and climate in terms of temperature and precipitation.

2. Write a statement that describes the current weather in your area. How does this current weather relate to the normal climate of the region?
3. How can the geography of a region affect its weather and climate?

4. What are the main factors that influence the patterns of movement of Earth’s air and water?
5. The jet stream is a global pattern of atmospheric movement that influences patterns in local weather. Rain and even severe thunderstorms are often associated with the passage of a cold front. According to this information and the weather map below, which city will most likely experience precipitation in the next few days and why?
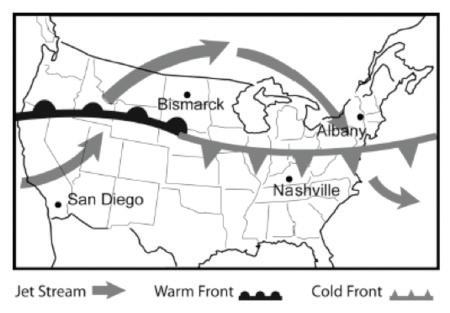
After learning about weather and climate in class, José is looking at the global climate zone graphic he pasted into his science journal. Where he lives in Mississippi, it snowed last week.
Write a scientific explanation describing why where José lives is still considered a tropical climate even though it sometimes snows there. Use the graphic to support your claim.


Description
Earth receives energy from the Sun as both heat and light. The atmosphere also absorbs energy as part of the process of the greenhouse effect. As energy radiates from Earth’s surface back toward space, greenhouse gases such as carbon dioxide, methane, nitrous oxide, and fluorinated gases work as a blanket to trap some of the radiation within the atmosphere. The trapping of heat is an important part of Earth’s natural system that maintains a livable temperature. However, scientists are concerned that the current trend of increasing levels of carbon dioxide in the atmosphere will result in trapping too much energy and lead to higher than average temperatures.
Procedure
1. Break your effervescent tablet(s) in half. Add the tablet halves one at a time to ONE of the water cups. Wait about a minute between adding the tablets, giving them time to react.
2. Make certain that your light is positioned eight inches from the top of the bottle and your thermometer is centered in the bottle.
3. Turn on the light.
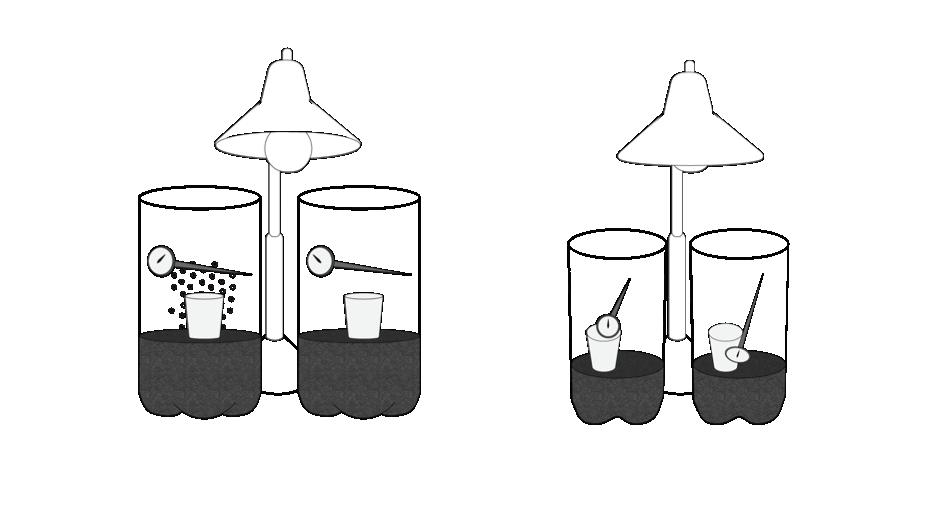
4. Take the temperature at time 0 and begin timing the experiment.

5. Beginning at time 0, check the temperature in each bottle every minute for 10 minutes. Add the results to your data table.
6. Share your results with the class. Record data for 0, 1, 2, and 3 tablets.

7. Plot your data and the data of other groups below. You should have four lines on your plot.
8. Describe the relationship between the number of effervescent tablets and the change in temperature. How is the number of tablets related to the amount of carbon dioxide in the bottles?

9. Consider the graph below and your answer from step 8. Predict what a graph of atmospheric carbon dioxide during the Industrial Revolution would look like. Sketch your graph below.
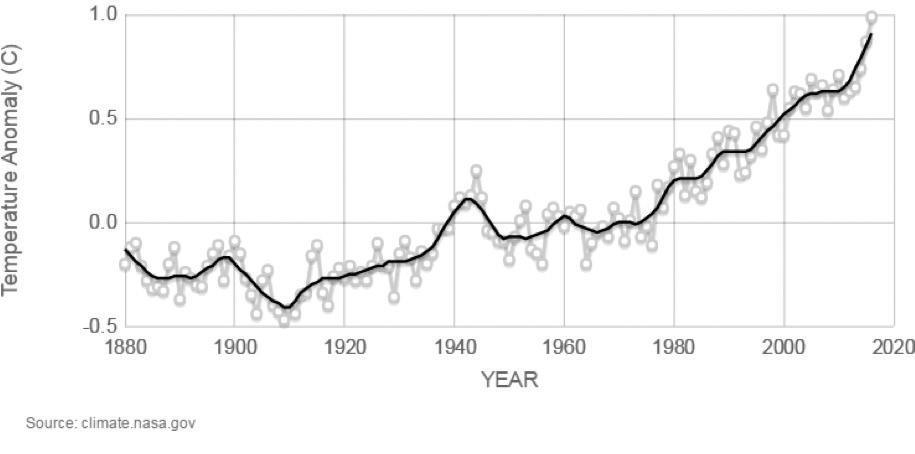

10. The graph below reflects global carbon emissions from burning coal, oil, and gas, and producing cement (1850–2009). Compare the graph below to the graph from step 9. What conclusion can you draw about carbon emissions and atmospheric carbon dioxide?
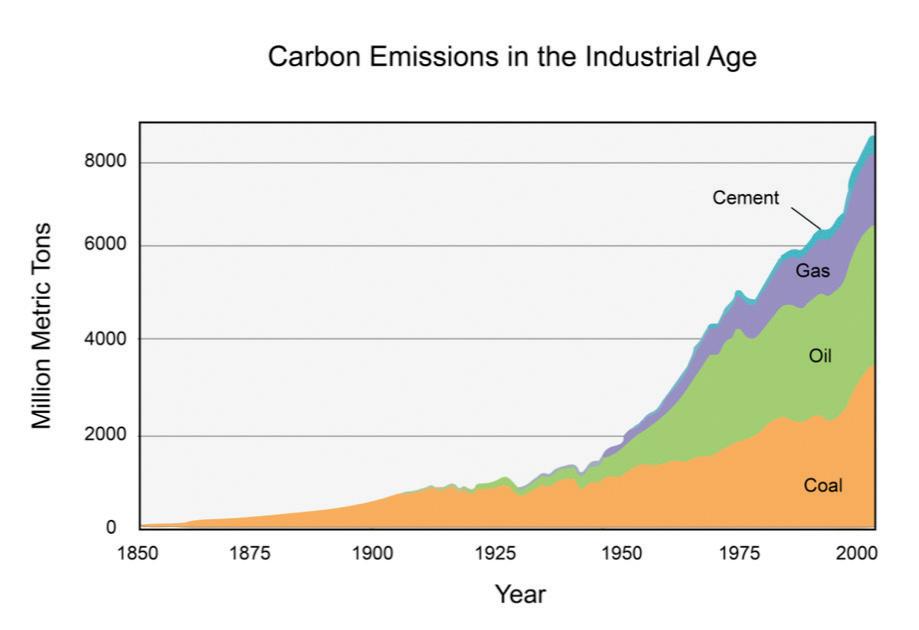

Activity
Scientific knowledge and understanding is sparked by thoughts of why and how. These questions can be answered through research and scientific investigation. Research findings must be supported by valid and reliable sources in order to be accepted by the scientific community.
Procedure
1. As a group, choose one of the posted questions to research.
2. Research the question to

A. find the major factors and natural processes concerned with the question; B. identify any human activities involved with the factors and processes; and C. review a scientific model based on the question.
3. Create a documentary that includes the above information based on your research. Include at least two direct quotations that support your research from at least two different reliable sources. Be sure to cite the resources properly. Ask your teacher if APA or MLA standards are required.
Answer the following questions.
1. How has Earth’s climate changed over time?
2. What human activities have had an effect on Earth’s climate?

3. What natural processes have had an effect on Earth’s climate?
4. What evidence is there of changes in Earth’s climate over the past 100 years?
5. How have humans affected Earth’s climate in the past 100 years?
6. What actions can humans take to lessen their effect on Earth’s climate in the next 100 years?
7. Does climate change occur naturally, or is it being accelerated through the influence of man?

Claim:
Evidence:
Reasoning:
Rebuttal:

How do human activities influence global climate change? Activities such as the release of greenhouse gases from burning fossil fuels are major factors in the current rise in Earth’s mean (average) surface temperature. This is more commonly referred to as global warming
Human activities continue to add carbon to the atmosphere. Plants take up carbon from the atmosphere and store it in their tissues. This is why plants are called a carbon sink. The oceans and rain forests are huge carbon sinks since they contain so much plant life. The introduction of increased levels of carbon back into the atmosphere through the burning of fossil fuels is a big problem. Through the process of decay, carbon is returned to the lithosphere.
Global climate is the range of weather that varies from region to region but is impacted by natural processes and human activity.

global warming: the rise in Earth’s average surface temperature
Climate is the range of a region’s weather and varies from region to region. Climate of a region can be affected by oceans, land, elevation, and atmosphere. Climate change is defined by extreme or recurring changes in an area’s average weather conditions. Some changes occur rapidly, like volcanic eruption or meteor impact. A large amount of matter is added to the atmosphere and causes change in ocean currents. Other change is gradual. Gradual change occurs with the shift in Earth’s axis or a change in response to plant or human life on Earth.

Global climate zones are based on global circulation patterns.
Cities have climate patterns that are based on average conditions over periods of time. These climate patterns help us to break areas down into climate zones. Earth is also divided into climate zones created based on global circulation patterns:
• Tropical climates are warm, wet regions.
• Subtropical climates are dry, high-pressure zones at about 30° latitude north and south.
• Polar climates are low temperature and low pressure due to year-round cold and dry air.
The Sun’s energy interacts with Earth’s systems (hydrosphere, geosphere, atmosphere, and biosphere) locally and globally to produce global climate.
External causes forcing climate change are changes in plate tectonics, changes in Earth’s orbit, or changes in the Sun’s strength. Within the climate system, internal interactions among the atmosphere, vegetation, ice, land surfaces, and oceans affect climate variations.
The foundation for Earth’s global climate system is the electromagnetic radiation (energy) from the Sun as well as its reflection, absorption, storage, and redistribution among the atmosphere, ocean, and land systems and this energy’s reradiation into space. Weather and climate are shaped by interactions involving sunlight, the ocean, wind, the atmosphere, ice, landforms, and living things. Sunlight heats Earth’s surface, which in turn heats the atmosphere. Winds release energy through atmospheric circulation. Both the ocean and wind moderate and stabilize the global climate.


As the Sun warms Earth, water, especially from oceans, evaporates into the atmosphere. As this moisture rises ever higher, it begins to cool and condense, even as it is being blown by the wind to a new location. The droplets eventually become heavy enough and fall back to Earth as precipitation, which eventually makes its way back into the large bodies of water. The ocean absorbs most of the Sun’s energy and releases it slowly through ocean currents.
Ocean currents act as conveyor belts of warm and cold water, sending heat toward the polar regions and helping tropical areas cool off. These interactions drive the daily weather patterns and changes in climate over time.
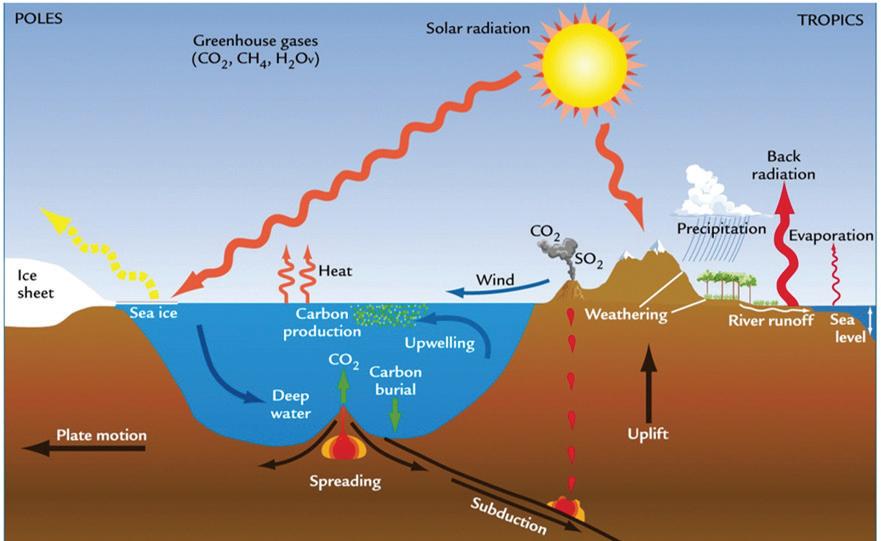
Past global climate change is evidenced in the geologic record and points to future changes.
Climate change can occur when certain parts of Earth’s systems are altered. Geological evidence indicates that past climate changes were either sudden changes caused by alterations in the atmosphere (e.g., volcanic eruptions or meteoric impacts that suddenly put a large amount of particulate into the atmosphere); longerterm changes (e.g., ice ages) due to variations in solar output, Earth’s orbit, or the orientation of its axis; or even more gradual atmospheric changes due to plants and other organisms that captured carbon dioxide and released oxygen. The timescales of these changes varied from a few to millions of years. Climate changes are significant and persistent changes in an area’s average or extreme weather conditions that can occur if any of Earth’s systems change (e.g., composition of the atmosphere, reflectivity of Earth’s surface, etc.). Positive feedback loops can amplify the impacts of these effects and trigger relatively abrupt changes in the climate system; negative feedback loops tend to maintain stable climate conditions. Scientists can infer these changes from geological evidence.


The greenhouse effect warms Earth. Earth’s surface stays warmer than it otherwise would due to the greenhouse effect. To maintain average temperature, certain gases in the atmosphere absorb and retain energy that radiates from Earth’s surface and insulates the planet. Examples of these are carbon dioxide, methane, and nitrous oxide. Without this, Earth’s surface would be too cold to be inhabited. So it is a good thing. However, changes in the atmosphere, such as increases in these greenhouse gases, occur due to human activities and can cause global warming that makes Earth too hot for other species.
Some
The Greenhouse E ect
Greenhouse E ect



Prior to the Industrial Revolution in the 1700s, climate change could be explained by natural causes like changes in solar energy, volcanic eruptions, and natural greenhouse gas concentrations. After that time frame, human activity has contributed substantially more by adding carbon dioxide and other heat-trapping gases to the atmosphere. These gases have increased the greenhouse effect and have caused Earth’s surface temperature to rise, primarily due to the burning of fossil fuels. Humans have now overpopulated Earth and are a resource-dependent species. With the increase in urban population and the use of concrete, industrialized centers have become heat islands. Their activity affects every part of the environment from the oceans to the stratosphere to outer space.

Modeling climate change helps to predict trends.
Climate models are created by scientists to simulate the physics and chemistry of Earth systems and their complex interactions. These models summarize the existing data and evidence, are tested for patterns, and are then used to forecast how the future is affected by human activity. Climate models are helping us to determine how weather patterns will change and the consequences, when and where new water supplies will be affected.
Global climate change evidence includes sea level rises, global temperature rise, warming oceans, shrinking ice sheets, Arctic sea ice decline, glacial retreat, increased extreme weather events, and ocean acidification.




Sea level rise: Global sea level rose 6.7 inches in the last century. The rate in the last decade is nearly double that of the last century. Melting ice sheets add water globally and increase sea level. Coastal cities have the most threat from this change in sea level.
Global temperature rise: All global surface temperature reconstructions show that Earth has warmed since the 1880s. Most warming has occurred since the 1970s, with the twenty warmest years having occurred since 1981 and with all ten of the warmest years occurring in the last dozen years. Even though the last century had a solar output decline resulting in an unusually deep solar minimum in 2007–2009, surface temperatures continue to rise.
Increased ocean heat: The oceans absorb much of the increased heat, with the top 700 meters of ocean water showing warming of 0.302 degrees Fahrenheit since 1969.
of ice caps: The warming ocean causes most Antarctic ice shelf loss. The Greenland and Antarctic ice sheets have decreased in mass in data from NASA’s Gravity Recovery and Climate Experiment. Greenland lost 150 to 250 cubic kilometers (36 to 60 cubic miles) of ice per year between 2002 and 2006, while Antarctica lost about 152 cubic kilometers (36 cubic miles) of ice between 2002 and 2005.

Look Out!




Try Now
Declining Arctic sea ice: Both the size and thickness of Arctic sea ice has declined quickly over the last several decades.
Glacial retreat: Glaciers are retreating (melting) at a rapid rate almost everywhere around the world—including in the Alps, Himalayas, Andes, Rockies, Alaska, and Africa.
Extreme weather events: The number of record high temperatures in the United States has increased, while the number of record low temperatures has been decreasing since 1950. The United States has also had increasing numbers of intense rainfall events.
Ocean acidification increases from carbon dioxide pollution: Since the Industrial Revolution, the acidity of surface ocean waters has increased by about 30 percent, resulting from humans releasing excess carbon dioxide into the atmosphere, which is being absorbed into the oceans. The amount of carbon dioxide absorbed is increasing by about two billion tons per year.
Due to Earth’s rotation, the atmosphere is deflected to the right in the Northern Hemisphere and to the left in the Southern Hemisphere. Try this experiment to see how the Coriolis effect moves the atmosphere. You will need a piece of cardboard, scissors, a ruler, and a marker.
Cut a large circle from the cardboard; this represents Earth. Make a dot in the center of the circle to represent the North Pole. Using a ruler and a marker, draw a straight line from the center of the cardboard to one edge. This will represent wind direction unaffected by Earth’s rotation. Slowly turn the cardboard counterclockwise. While turning the cardboard, use the ruler and marker to try to draw a straight line from the center of the cardboard to the edge. This will represent the winds moving from the North Pole to the equator. Examine the line you have drawn. Are the lines straight? How would the wind move and change?

Your child has been learning about natural and human-made effects on local and global temperatures. Areas that have buildings tend to be warmer than areas with trees, especially at night. Have your child gather his or her own data on this urban heat island effect.
Decide with your child which areas around your neighborhood or town would be safe to go to at night. Try to choose at least one location surrounded by lots of buildings and one location without buildings, such as a park. Bring a thermometer that can measure air temperature, along with a piece of paper and pen or pencil. Thermometers used in cooking should work, while a thermometer used to measure body temperature will not.
Accompany your child. In each location, have your child measure the air temperature at the same height above ground. Have your child record the temperature along with the location.
Here are some questions to discuss with your child:
• Was the air temperature different in the different places?
• If your city/town became larger, what effect do you think that would have on the local temperature?
• If nighttime temperatures in the city are higher than in the countryside, what effect could that have on energy consumption in the summer? In the winter?

1 Earth’s climate seems to be changing. To understand this environmental trend, scientists are studying data from the past and present. They must be able to tell the difference between global climate change (broad average trends) and regional weather patterns (local trends). Each region of the world is controlled by its own local weather patterns. These patterns may change from hour to hour or from day to day. Collected weather data include temperature, precipitation, humidity, cloud cover, wind velocity, and atmospheric pressure. Climate, on the other hand, is defined as the average weather conditions of an area, including extremes (or records) over a long span of time.
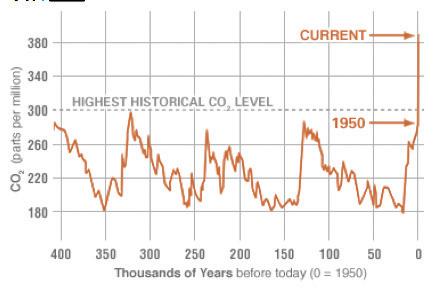
2 Why is Earth undergoing global climate change at this time? There are actually several possible reasons. Earth’s climate responds to changes in its orbit, the Sun’s energy, and greenhouse gas levels. Over the last several decades, scientists have been recording significant global climate changes in temperature, precipitation, and wind patterns. This data seems to indicate a warming trend on Earth’s surface, which some have termed global warming. However, warming is only one part of global climate change. Climate change is a more accurate term to use than global warming.
3 The data also reveal a slow and steady rise of carbon dioxide (CO₂) and other greenhouse gases in the atmosphere since 1880, or the time of the Industrial Revolution. These gases, which include carbon dioxide (CO₂), nitrous oxide (NO), and methane (CH₄), are causing more heat to be trapped in the atmosphere. The result is a rise in Earth’s surface temperature, causing warming oceans, melting of Arctic ice, increases in land-ice runoff, and rising sea levels.
4 Why is CO₂ such an important factor when it comes to a rise in average global temperatures? CO₂ is a colorless, odorless gas that has properties that allow it to trap heat energy. This means that the CO₂ in our atmosphere prevents some of the heat that radiates from Earth from going off into space. This is not a bad thing in and of itself. We actually need CO₂ in our atmosphere. It has been in our atmosphere for a very long time. If we did not have naturally occurring CO₂ and other greenhouse gases in our atmosphere, all of the heat from Earth would escape into space. If we need CO₂ in our atmosphere, why has it been a source of concern lately? One factor has to do with fossil fuels. As fossil fuels are burned, the carbon in them combines with the oxygen in the atmosphere and creates more “artificial” CO₂. We cannot say with absolute certainty that human use of fossil fuels is the main cause for global warming. What we do know from scientific data is that the rise in CO2 concentrations in the atmosphere relates to the increased burning of fossil fuels. Therefore, there is concern that rising CO₂ levels could be a large factor in rising global temperatures.

5 For the 800,000 years before the Industrial Revolution, the average CO₂ concentrations in the atmosphere shifted between 170 and 300 parts per million (ppm). Since the Industrial Revolution, CO₂ concentrations have increased, with atmospheric data as of 2017 showing levels over 400 ppm. How do scientists know this? They were not taking scientific recordings 800,000 years ago. One of the ways they are able to study past climates is by looking at something known as ice cores.
6 Scientists will drill into glaciers and pull out long cores of ice. There are some glaciers, such as in Greenland and Antarctica, that have been around for hundreds of thousands of years. Scientists can look at these ice cores, layer by layer, and find interesting data. They can look at the pollen that existed at different periods of time. More pollen points to warmer weather. They can also look at the gases trapped in the cores. These gases give the scientists a good idea of what gases existed in the atmosphere in the past. Then they measure the amounts of each gas found and can determine the percentage of each type of gas that existed in the atmosphere. The data collected from the ice cores show that the current CO₂ concentrations are higher than they have been in the past 800,000 years.
7 Some of the most reliable measurements of current atmospheric CO₂ levels are taken at the Mauna Loa Observatory in Hawaii. Scientists have discovered that the trend found in the ice core samples is supported by the current readings from Mauna Loa. The Keeling Curve is a graph that charts the changes in atmospheric CO₂ concentrations based on these readings. The readings on the Keeling Curve have steadily increased since 1958, with today’s readings being over 400 ppm.
8 In conclusion, climate change is a very broad term. It includes global warming as well as weather changes at the regional and global level. How fast will our global climate change? What are the consequences of this warming trend? As of now, scientists are not sure, so the studies must go on. In the meantime, weather prediction models are now being used to prepare for what lies ahead at the global and regional levels. Using satellites, scientists have measured changes in the Sun’s energy. These variations are small when compared to the warming properties of greenhouse gases. Most of the warming has occurred since 1981. In fact, the past 12 years have produced the 10 warmest years recorded.

1 Based on the information provided in this reading, what is the best conclusion that can be reached?
A Human activity is the cause of increasing global temperatures.
B Increased CO₂ concentrations are the only cause for increasing global temperatures.
C Past and present data show a trend in increasing CO₂ concentrations.
D There is nothing that can be done to reduce global temperatures.
2 Why are scientists so concerned about rising levels of CO₂? What is it about this particular gas that makes it concerning?
A It produces a bad odor.
B It can trap heat.
C It has an unpleasant color.
D It is only made by humans.
3 Which of the following statements about CO₂ is NOT true?
A CO₂ is just one type of greenhouse gas.
B CO₂ is harmful to the planet.
C Life as we know it could not exist without CO₂.
D The CO₂ in our atmosphere has been around a long time.

4 Why do climatologists study ice cores from ancient glaciers?
A To find out what ancient glaciers are made of
B To find out how long humans have lived on Earth
C To find out what existed in the ancient atmosphere
D None of the above
5 The climate of Earth is affected by which of the following factors?
A The Sun’s energy
B Greenhouse gas emissions
C Earth’s orbit
D All of the above
6 The past 12 years contained the 10 hottest years on record. But scientific data indicate that carbon dioxide levels in the atmosphere have been increasing since–
A 1780.
B 1880.
C 1981.
D 2001.

1. Some people argue that human activities do not contribute to global climate change. What do they believe is responsible for global climate change instead? What evidence do they use to support their beliefs?
2. What are some indicators that the overall climate of Earth is getting warmer?
3. Look at the graph below. What could be documented to link human activities to global climate change?
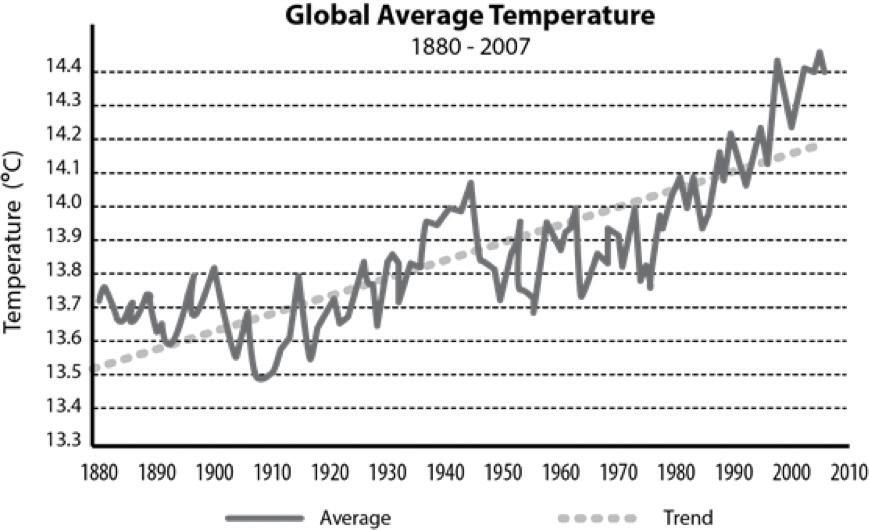

4. How does burning of fossil fuels affect the concentration of carbon dioxide in the atmosphere? What is one consequence of carbon dioxide buildup in Earth’s atmosphere?

5. Over the past 100 years, there has been a significant increase in atmospheric temperature. Which human activity has contributed the greatest amount of greenhouse gases during this period?
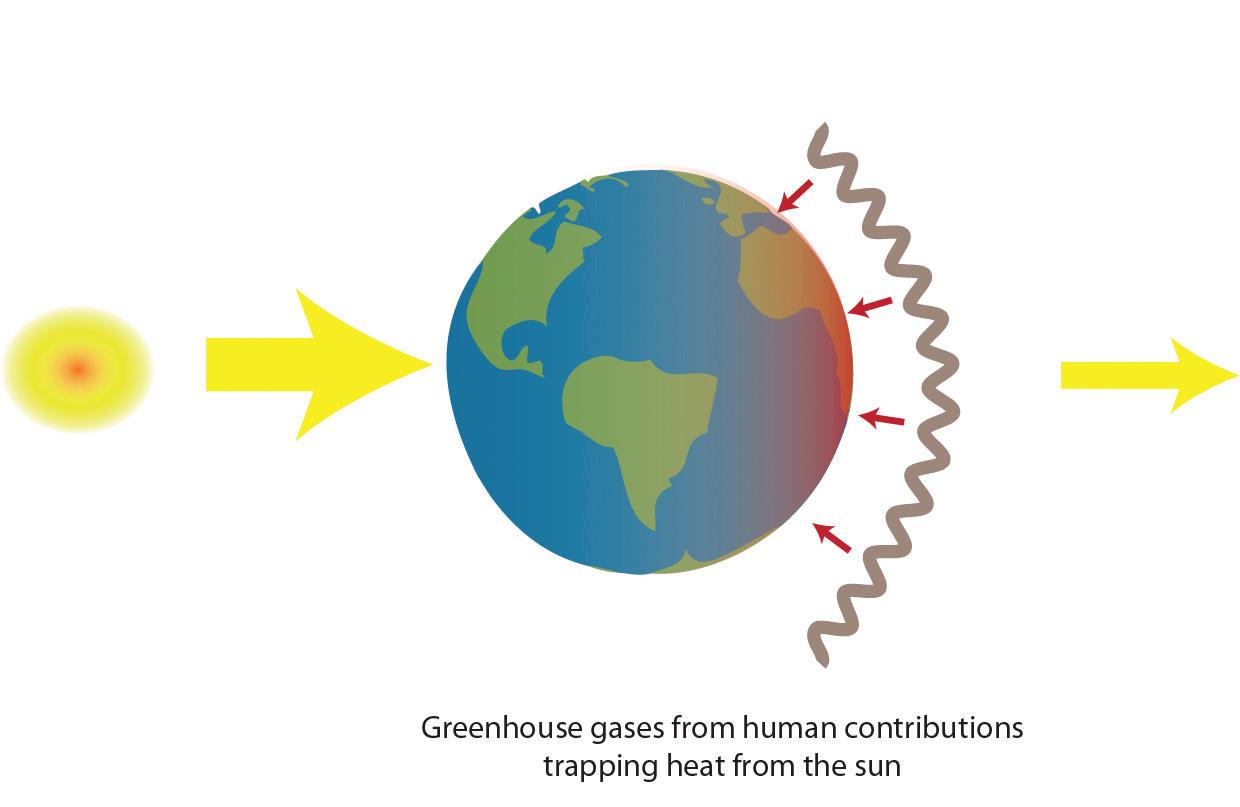

Scientists use different models, charts, and graphs to predict the effects of human behaviors on global climate change. The first graph is just one of many that scientists have created to display past, present, and possible future fossil fuel emissions data, while the second graph displays similar information regarding sea level changes.
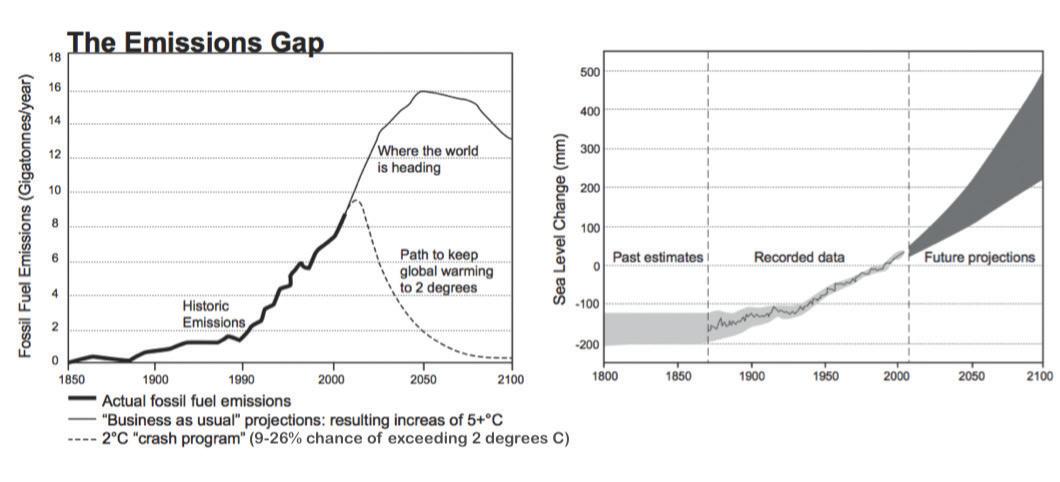
Prompt 3
Write a scientific explanation to describe what would happen to Earth’s sea levels if fossil fuel emissions reached a point similar to that of 1850.
PEER EVALUATION
Peer Name:
Rebuttal:


The axis is the center of Earth’s rotation. Earth has a measurable tilt of 23.5° on its axis, orienting it in space so the North Pole always points toward the North Star as it moves along its orbital path around the Sun. As a result of the fixed tilt of Earth’s axis, the areas of Earth’s surface exposed to rays from the Sun change seasonally as Earth moves through its orbital path. The tilt of Earth on its axis is directly responsible for the changing number of daylight hours we receive.
Procedure
1. Use the table to record your data.
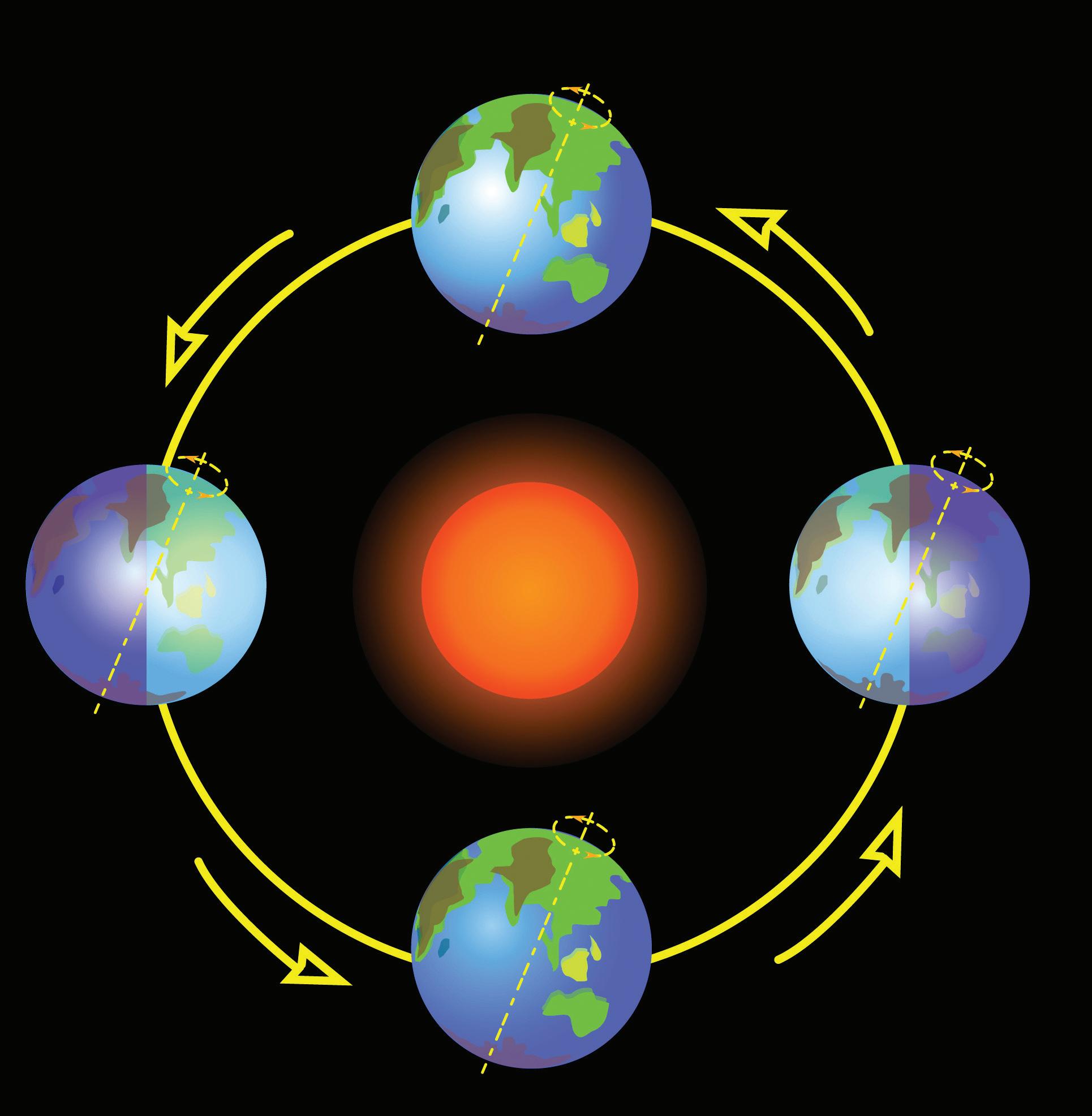
2. Use the model to answer the questions on the following pages.
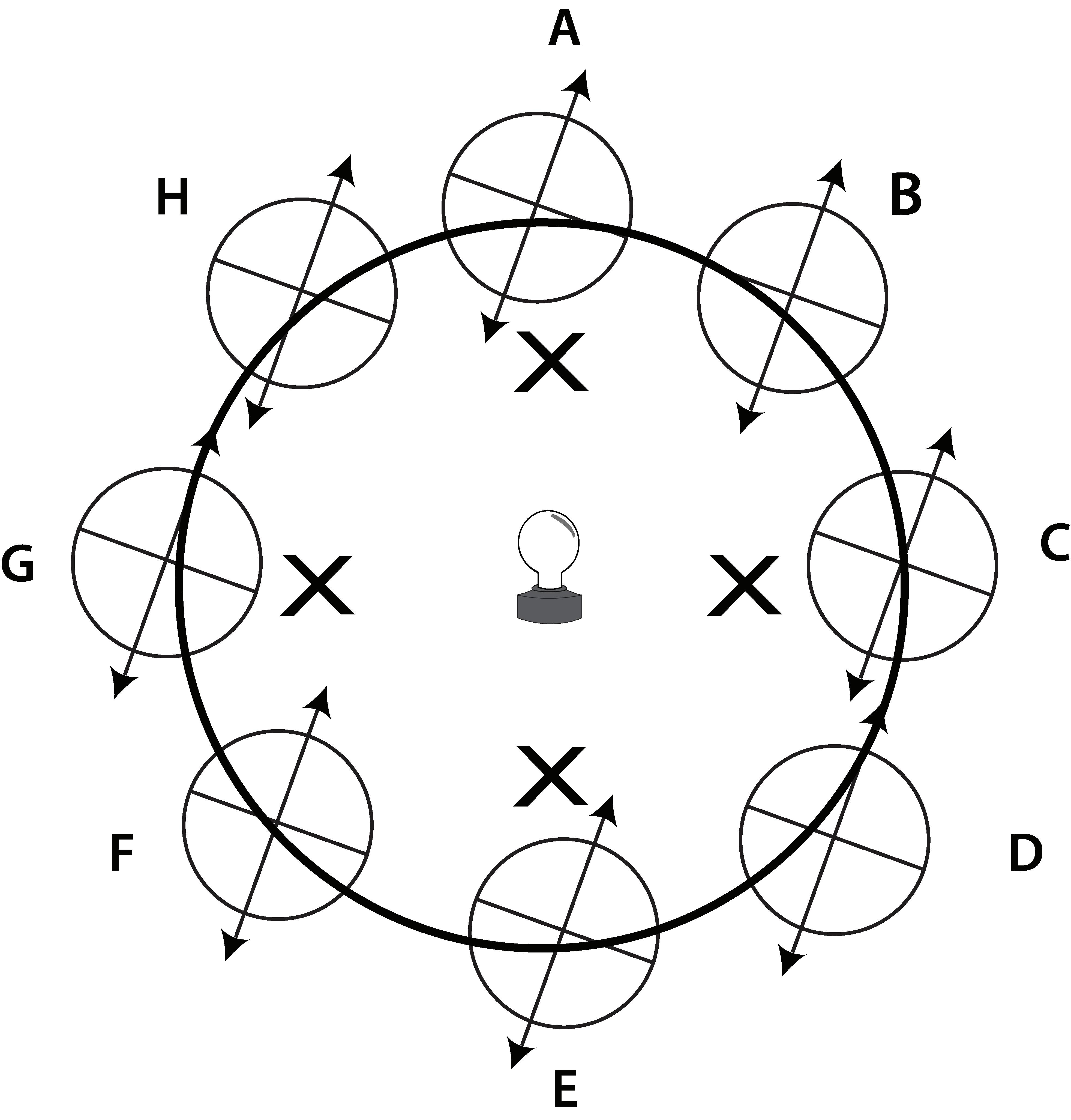

3. Write your prediction of what the positions marked with large Xs represent. Then go to your first Earth position with your group and begin. Prediction:
4. You have four minutes at each position to make and record observations in the data table.
5. When your teacher gives the signal, move counterclockwise (to the left) around the lamp, stopping at each of the eight locations on the path of Earth’s revolution.
6. Make observations of how the model of the Sun (lamp) and Earth (globe) shows a relationship based on how the light strikes Earth in each of the eight designated positions in Earth’s revolution around the Sun.
7. Complete the following questions after observing Earth in all eight positions.
A. At position C, how was the amount of light different in the two hemispheres?
B. Position C shows Earth in the month of December. What season would it be in December in the Southern Hemisphere?
C. At Position G, how was the amount of light different in the two hemispheres?

D. What month is Earth in at Position G? How do you know?
E. Position A and Position E show Earth receiving equal amounts of sunlight in the Northern and Southern Hemispheres. What seasons occur at these points? Why?
F. At which location does the South Pole receive 24 hours of daylight?
G. At which location does the North Pole receive 24 hours of darkness?
H. Is there any point at which Earth is closer to the Sun than at other points?
I. What two things cause seasons on Earth?

As a group, brainstorm about household items you could use to design and construct a model to help explain to your classmates why seasons occur. You have one day to develop your design ideas and one day to construct your model. On the third day, your group will use your model to explain seasons to your classmates.
You must also develop three questions to ask your classmates about the phenomena that cause seasons to occur. Your questions must be approved by your teacher.
Use the space below to draw a diagram of your concept design. Once complete, have your teacher approve your design before you start building your model.

When the Sun is directly overhead, the rays are perpendicular as they strike Earth. When the Sun is lower in the sky, the rays strike the ground at an angle, causing the energy to “spread out.”
Procedure
1. Observe as your teacher stands about .5 m from the wall and directs a flashlight beam on a sheet of graph paper from cheek level.

2. Observe as a student volunteer outlines the even glow of light produced by the flashlight beam using a red marker, and then counts and records the number of squares inside the shape on the graph paper or whiteboard.
3. Observe as your teacher stands about .5 m from the wall and directs the flashlight beam on the graph paper, this time holding the flashlight as high overhead as possible instead of at cheek level.
4. Observe as a student volunteer outlines the even glow of light produced by the flashlight beam using a blue marker, and then counts and records the number of squares inside the shape on the graph paper or whiteboard.
5. Draw the two shapes and note the corresponding number of squares counted for each.

6. Decide which shape should be labeled direct light and which shape should be labeled indirect light.
7. Explain why winter is colder than summer. Include (i) the tilt of Earth’s axis and how the tilt effects (ii) the angle and intensity of light and (iii) the number of hours of daylight.
8. Answer the following questions.
A. Which illuminated shape models a situation where a cone of ice cream would melt the fastest, the direct rays or indirect rays?
B. Which illuminated shape would create the longest shadow for a dart placed in the center of the outline?
C. Do you expect that the Sun’s rays are more direct in the summer or the winter?

Earth’s axis is on a tilt. What does this mean for the differences in weather around the world? Does this affect the amount of sunlight different locations receive?
Investigate the relationship of day length and seasons by comparing the number of daylight hours and minutes that five cities received on the 21st of each month in 2017.
Procedure
Step 1: Question

Step 2: Relevance
Step 3: Variables
Independent variable (also known as the manipulated variable):
Dependent variable (also known as the responding variable):
Control variable:
Step 4: Hypothesis Is a hypothesis needed? If so, what is it?
How will the responding variable change when the manipulated variable changes?

Step 5: Materials
Step 6: Safety Considerations
Step 7: Procedure
1. Locate the latitudes of Quito, Ecuador; West Palm Beach, Florida; Boise, Idaho; Johannesburg, South Africa; and Christchurch, New Zealand on the world map.
2. Record the latitudes in the data table.
3. Use military time (24-hour clock) to calculate the number of daylight hours and minutes for each city using the Student Reference Sheet: Sunrise and Sunset Data Table. An example has been completed for the month of January.
4. Record the number of daylight hours and minutes in the data table.

Step 8: Data Collection
Use the tables to record your data.
Quito, Ecuador
West Palm Beach, Florida Boise, Idaho
Johannesburg, South Africa Christchurch, New Zealand
Hours of Daylight Data Table: Calculated for the 21st of each month in 2017
Date Quito, Ecuador
1/21/2017
2/21/2017
3/21/2017
4/21/2017 5/21/2017 6/21/2017 7/21/2017 8/21/2017 9/21/2017 10/21/2017 11/21/2017 12/21/2017
West Palm Beach, Florida Boise, Idaho
Johannesburg, South Africa Christchurch, New Zealand

Input the data in a spreadsheet program of your choice and generate a graph. Attach your graph in the space below. Make a general statement about the results shown in the graph.

Step 10: Conclusion and Scientific Explanation
Write a scientific explanation to describe why seasons occur.
Claim:
Evidence:
Reasoning:


You have noticed that the seasons change in a regular pattern: spring brings new growth, summer brings hot weather, fall brings colorful autumn leaves, and winter brings cold weather and snow. What causes those changes? Do the polar and equatorial regions have the same seasons? In order to understand seasons, we will look at Earth’s tilted motion on its axis (rotation) and its orbit around the Sun (revolution).
Earth has two motions: rotation and revolution.
Rotation: Rotation is a term that describes the motion of a spinning object. Each of the planets and moons in our solar system rotates about an axis. An axis is an imaginary line about which each planet or moon spins. This imaginary line marks the center of a planet’s or moon’s rotation.
In space, there is no such thing as up or down. An object’s position can be measured only relative to other objects. The Sun is the center of our solar system. Therefore, the motion of the planets and other objects in our solar system can be measured relative to the Sun. Like the other planets, Earth rotates about an axis. Earth’s axis is not a perfectly vertical, or perpendicular, line. Instead, our planet tilts at an angle of 23.5° relative to its path around the Sun. The northern end of Earth’s axis, known as the geographic North Pole, always points at the North Star.

This clementine orange is an oblate spheroid. Earth is more rounded than this clementine, but it is still an oblate spheroid.
A day is the amount of time a planet takes to complete one full rotation. Earth takes 24 hours to complete one full rotation, so one day on Earth is 24 hours. Because Earth rotates, different parts of the planet face the Sun at different times. When the Western Hemisphere is facing the Sun, it is daytime there and nighttime in the Eastern Hemisphere. When the Western Hemisphere is facing away from the Sun, it is nighttime there and daytime in the Eastern Hemisphere.

Rotation can describe the motion of a spinning top (left). You can think of an axis as the center peg of a spinning top. This top’s axis is tilted like Earth’s. A globe (right) is a model of Earth that represents how the planet rotates about a tilted axis.
Even though you cannot feel it, Earth rotates very fast. Earth’s rotation is so fast that it causes the planet to bulge out slightly at the equator and shrink slightly at the poles. Therefore, Earth is not a perfect sphere. Earth’s circumference is slightly wider at the equator than it is at the poles. This shape is called an oblate spheroid. In most photographs and diagrams that you will see, Earth probably looks like a perfect sphere.

Revolution: Revolution is a term that describes the motion of one object as it moves around another object. While each planet rotates about its axis, it is also revolving around the Sun. Even though the Sun appears to move across the sky each day, Earth is actually moving around the Sun.
For many years, people believed that the Sun and other planets revolved around Earth. This was called the geocentric model of the solar system. Geo- means “Earth,” so geocentric means “Earth-centered.” Later, scientists discovered the motion of the planets makes much more sense if the Sun is the center of the solar system.
The Sun is the center of the solar system. All of the planets revolve around the Sun.
This is called the heliocentric model of the solar system. Helio- means “Sun,” so heliocentric means “Sun-centered.” This is now the accepted model of the solar system. The term solar means “of the Sun,” so solar system means a system driven by the Sun. A year is the amount of time a planet takes to complete one full revolution around the Sun. Earth takes 365.25 days to revolve around the Sun.
Because a full Earth year is actually 365.25 days, every four years we have a “leap year.” During a leap year, we make up for this extra quarter-day by adding an extra day to the calendar. A leap year has 366 days instead of the normal 365 days.
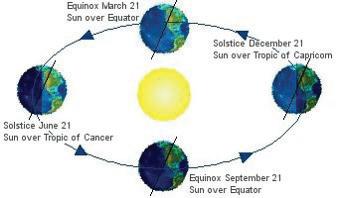
Because the Sun is so massive, it has very strong gravity. The force of the Sun’s gravitational pull holds all of the objects in the solar system—including the planets—in its orbit. An orbit is the path an object takes as it revolves around another object. Each planet in our solar system revolves around the Sun in a predictable orbit. These orbits are not perfect circles—they are elliptical, or oval shaped. This means that each planet is sometimes closer to and sometimes farther from the Sun. The diagram of seasons in the Northern Hemisphere (shown above) shows an example of an elliptical orbit.

Look Out!
The fact that Earth’s distance from the Sun changes throughout the year might seem like a good explanation for the seasons. You might think Earth is colder when it is farther from the Sun and warmer when it is closer to the Sun. However, this explanation is incorrect. Not every part of Earth experiences the same seasons at the same time. When it is summer in the Northern Hemisphere, it is winter in the Southern Hemisphere. What, then, causes the seasons?
If Earth’s axis were not tilted, we would not experience different seasons. However, Earth’s axis is tilted at a 23.5° angle. As Earth revolves around the Sun, sometimes the Northern Hemisphere is tilted toward the Sun. When the Northern Hemisphere is tilted toward the Sun, it receives more direct rays of sunlight. It is summer. In summer, days are longer, and weather is warmer in the Northern Hemisphere. Plants there have plenty of sunlight for photosynthesis, and animals have plenty to eat.
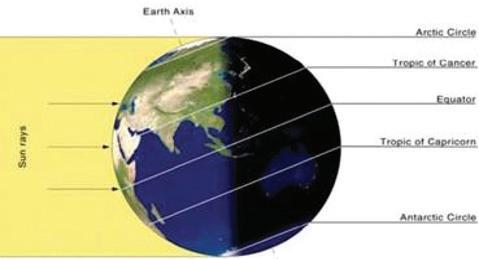
When the Northern Hemisphere is tilted toward the Sun, it receives more direct rays of sunlight than the Southern Hemisphere. During this time, the Northern Hemisphere experiences summer, and the Southern Hemisphere experiences winter.
At the same time, the Southern Hemisphere is tilted away from the Sun and receives fewer direct rays of sunlight. As a result, days are shorter, and weather is cooler in the Southern Hemisphere. It is winter. Plants there grow less actively, and many lose their leaves. Animals are also less active; some hibernate, or sleep through, the winter.
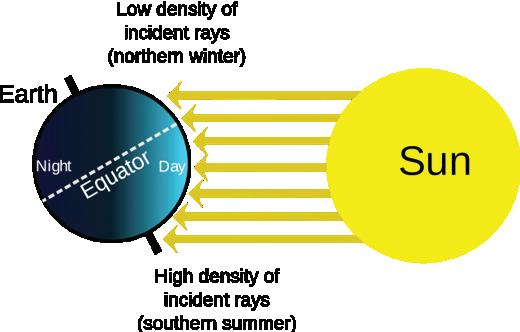

When Earth reaches the opposite side of its orbit— a process that takes about six months—the Southern Hemisphere will be tilted toward the Sun. It will receive more-direct rays of sunlight, and the Northern Hemisphere will receive less-direct rays of sunlight. As a result, the Southern Hemisphere will experience summer, and the Northern Hemisphere will experience winter. Therefore, Earth’s tilted axis and Earth’s revolution around the Sun—not Earth’s distance from the Sun—cause seasons.
Polar seasons: Seasons are more noticeable in places that are farther from the equator—the imaginary line around the horizontal center of the planet. Although the midlatitudes experience four seasons, the polar regions do not. Due to Earth’s tilt, the poles receive less sunlight. This creates only two polar seasons: summer, when the Sun does not set, and winter, when the Sun does not rise.

When the Southern Hemisphere is tilted toward the Sun, it receives more direct rays of sunlight than the Northern Hemisphere. During this time, the Southern Hemisphere experiences summer, and the Northern Hemisphere experiences winter.
Equatorial seasons: No matter where Earth is along its orbit, the equator is never tilted away from the Sun. It receives direct rays of sunlight year-round. This is why climates are generally warmer near the equator.
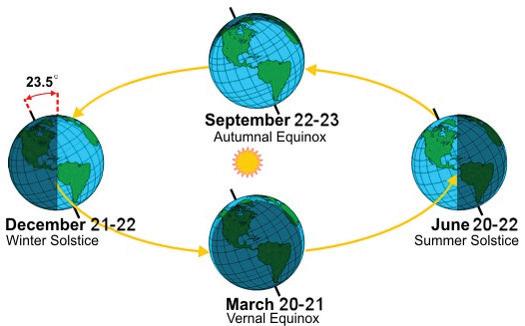
While there is some variation, temperatures near the equator stay relatively constant from month to month.
You can see how Earth’s orbit is elliptical in this diagram. As Earth revolves around the Sun, the planet’s tilted axis causes the seasons. When the Northern Hemisphere is tilted away from the Sun, it experiences winter. As the planet moves to the opposite end of its orbit, the Northern Hemisphere passes through spring into summer. During summer, the Northern Hemisphere is tilted toward the Sun. It then passes through autumn (or fall), making its way back to winter. Remember: The Northern Hemisphere and the Southern Hemisphere are opposites. When the Northern Hemisphere is experiencing summer in the diagram above, the Southern Hemisphere is experiencing the opposite season, which is winter.

Daylight hours change with the seasons.
Not only does the temperature change with the season, but the number of daylight hours changes significantly with each season depending on the observer’s latitude on Earth.
Midlatitudes: Due to Earth’s tilt, those living in midlatitudes, such as 50°N, experience four seasons. The Sun’s apparent path is the highest in the sky and takes the longest to rise and set, with more than 12 hours of sunlight during summer.
Equator: Equatorial regions experience the longest daylight hours because the apparent path of the Sun is overhead. Because the angle of sunlight remains fairly direct all year long, there are no seasonal changes at the equator, just “dry” and “wet” seasons.
Polar regions: Due to Earth’s tilt, the angle of sunlight is quite low at the poles, resulting in only two seasons: summer when it is constantly daylight (the Sun remains above the horizon for many months) and winter when it is constantly nighttime (the Sun remains below the horizon for many months).
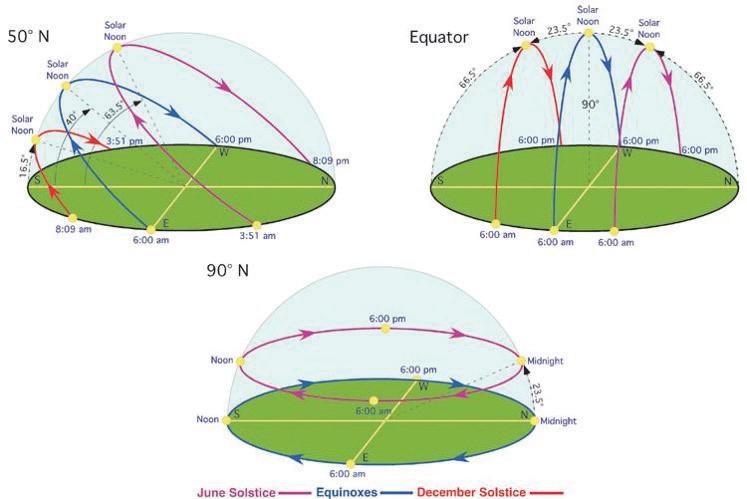

Try Now
Earth’s rotation and revolution affect day/night cycles as well as the seasons. The following diagram shows Earth at two positions in its orbit around the Sun. (This diagram is not drawn to scale.)
For each position, decide whether each hemisphere is experiencing day or night and winter or summer. Write your answers in the charts below.
Position 1
Day or Night? Winter or Summer? Day or Night? Winter or Summer? Day or Night?
Position 2
or Summer? Day or Night? Winter or Summer?
Position 1
Position 2
Day or Night? Winter or Summer? Day or Night? Winter or Summer? Day or Night? Winter or Summer? Day or Night? Winter or Summer?

To help your child learn more about rotation and revolution, try a few simple experiments. First, gather a flashlight and a round object such as a globe or ball (preferably about the size of a basketball or beach ball). Create an axis for the ball by taping two drinking straws, pencils, or similar objects to opposite ends of the ball.
In a darkened room, hold the ball a few feet away from your child, and have your child shine the flashlight on the ball. Hold the ball so that the axis is pointing up and down at a slight tilt toward the flashlight. Holding the ball steady at this tilt, walk in a circle around your child, who should keep the flashlight aimed at the ball. As you revolve around your child, discuss how the ball represents Earth on its tilted axis and the flashlight represents the Sun. Stop periodically at different points in the “orbit,” and ask your child to explain which season each hemisphere is experiencing and where it is day and where it is night.
Try the exercise again, this time holding the ball so that the axis is perfectly straight up and down rather than at a tilt. Ask your child how this changes the effect of Earth’s orbit on each hemisphere. (Earth will still experience day and night as it rotates, but without a tilted axis, different hemispheres will not experience different seasons.)
Here are some questions to discuss with your child:
• How does Earth’s tilted axis affect each hemisphere as the planet revolves?
• Point to different spots on the ball. What would people living here experience when Earth is at this point in its orbit?
• If Earth’s axis were not tilted, what would change?

1 What causes seasons? In order to answer this question, we need to look at how Earth rotates each day and how it revolves around the Sun. Both of these factors are very important to the creation of seasons on Earth.
2 Rotation is a term that describes the motion of a spinning object. The Sun, the planets, and moons in our solar system rotate about an axis. An axis is an imaginary line about which the Sun, planets, and moons spin. This imaginary line marks the center of rotation. A day is the amount of time a planet takes to complete one full rotation. Earth takes 24 hours to complete one full rotation.
3 Revolution is a term that describes the motion of one object as it moves around another object. While each planet rotates about its axis, each planet is also revolving around the Sun. A year is the amount of time any planet takes to complete one full revolution around the Sun. Earth takes 365.25 days to complete one full revolution. We usually define one Earth year as 365 days.
4 Earth does not revolve around the Sun in a perfect circle. It has the pattern of an ellipse, or oval, shape. This means that at different times of the year, Earth is either closer to or farther away from the Sun. During summer solstice, Earth is closest to the Sun. During winter solstice, Earth is farthest from the Sun. During the spring and fall equinoxes, Earth is the same distance from the Sun.
5 The fact that Earth’s distance from the Sun changes throughout the year might seem like a good explanation for the seasons. You might think that Earth is colder when it is farther from the Sun and warmer when it is closer to the Sun; however, this explanation is incorrect. Not every part of Earth experiences the same seasons at the same time. When it is summer in the northern hemisphere, it is winter in the southern hemisphere. What, then, causes the seasons?
6 Earth’s axis is tilted at a 23.5° angle. If Earth’s axis were not tilted, we would not experience different seasons. As Earth revolves around the Sun, sometimes the northern hemisphere is tilted toward the Sun. When the northern hemisphere is tilted toward the Sun, it receives more direct rays of sunlight. It is summer. In summer, days are longer and weather is warmer in the northern hemisphere. Plants there have plenty of sunlight for photosynthesis, and animals have plenty to eat.

7 When the southern hemisphere is tilted away from the Sun, it receives less direct rays of sunlight. As a result, days are shorter and weather is cooler in the southern hemisphere. It is winter. When Earth reaches the opposite side of its orbit—a process that takes about six months—the southern hemisphere is tilted toward the Sun. It then receives more direct rays of sunlight, and the Northern Hemisphere receives less direct rays of sunlight. As a result, the southern hemisphere experiences summer and the northern hemisphere experiences winter. Earth’s tilted axis and its revolution around the Sun—not Earth’s distance from the Sun— cause the seasons.
8 Seasons are more noticeable in places that are a greater distance from the equator, the imaginary line around the horizontal center of the planet. No matter where Earth is along its orbit, the equator is never tilted away from the Sun. It receives direct rays of sunlight year-round. This is why climates are generally warmer near the equator. While there is some variation, temperatures near the equator stay relatively constant from month to month.
9 Although Earth’s axis is currently tilted at 23.5°, this angle is not always constant. Earth wobbles slightly over time as it revolves around the Sun. This wobble causes the angle of Earth’s tilted axis to shift between 22° and 24.5°. This does not happen fast enough for humans to notice. Rather, the shift between 22° and 24.5° takes about 40,000 years. This process is called the Milankovitch cycle, named after the astronomer who theorized that changes in Earth’s tilted axis affect the seasons.
10 According to Milankovitch’s theory, when Earth’s tilt increases, there is a greater difference between summer and winter. A strong winter can lead to significant buildup of ice at the poles. This ice can reflect the Sun’s radiation, blocking it from warming Earth’s surface. This can cause temperatures to drop even more. Eventually, this can lead to an ice age. When Earth’s tilt decreases, the seasons become milder. It is important to realize that many factors are involved with climate on Earth. Although Milankovitch cycles likely play a role in climate change, scientists do not think their effects will be strong enough to produce a new ice age during the next 50,000–100,000 years, and possibly longer.

1 What is the best explanation as to why certain places on Earth experience four seasons?
A Earth is closer to and farther away from the Sun at certain times of the year.
B Earth rotates every day.
C Earth’s axis is tilted.
D Earth revolves around the Sun.
2 What type of shape would best describe Earth’s orbit around the Sun?
A A triangle
B A perfect circle
C A long rectangle with rounded edges
D An ellipse
3 When will the northern hemisphere of Earth experience winter?
A When it is tilted toward the Sun
B When it is tilted away from the Sun
C When it is closest to the Sun
D When it is farthest from the Sun

4 Which of the following statements is NOT true?
A Earth’s axis is always tilted at 23.5°.
B Earth wobbles as it revolves around the Sun.
C When Earth’s tilt decreases, the seasons become milder.
D Many factors are involved with climate on Earth.
5 What is the Milankovitch cycle?
A The cycle of the seasons in the northern and southern hemispheres
B The wobble that causes Earth’s axis to shift between 22° and 24.5°
C The pattern that Earth takes as it revolves around the Sun
D The cycle Earth takes as it revolves each day
6 What is a term that describes the motion of a spinning object?
A Revolution
B Cycle
C Rotation
D Axis

1. Construct a diagram to illustrate how Earth is tilted on its axis as it orbits the Sun for an entire year. Explain how this tilting creates the seasons on Earth.

2. Look at the diagram of Earth tilted on its axis. What season is it in the northern hemisphere? What season is it in the southern hemisphere? How can you tell?
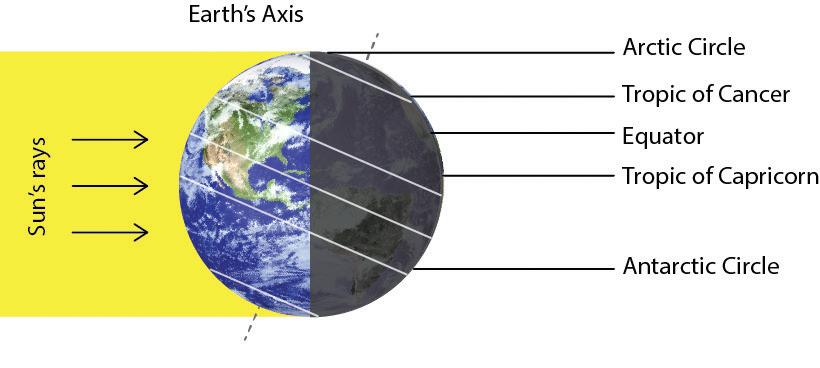
3. How does the intensity of sunlight along the equator differ from the intensity of sunlight at the north pole? What causes this difference?
4. Why are summer days “longer” than winter days?



The sweet gum and other trees like it are indigenous trees to the Mississippi habitat. The climate of Mississippi is mild and warm most of the year; however, most plants and trees do react to the changing of the seasons.


Thinking like a scientist, write an explanation describing how the sweet gum and other trees change throughout the seasons in Mississippi. Claim:
acid
acid – a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts; acids yield hydrogen ions (H+) or hydronium ions (H3O+) when dissolved in water
air mass – body of air extending over large areas (1,000 miles or more) that develops and retains specific characteristics of pressure, temperature, and humidity
atmosphere – the whole mass of air surrounding Earth, made up of 78% nitrogen, 21% oxygen, and other trace gases
atomic mass – the mass of an atom; approximately equal to the number of protons and neutrons in the atom
balanced chemical equation
atomic number – the number of protons in the nucleus of one atom of an element atom – the smallest particle of an element, made of electrons, protons, and neutrons
balanced chemical equation – a symbolic representation of a chemical reaction in which both sides of the equation contain equivalent numbers of atoms of each element; the mass and the charge must be balanced on both sides of the reaction
bases – substances that, in aqueous solution, are slippery to the touch, taste bitter, change the color of indicators (e.g., turning red litmus paper blue), react with acids to form salts, and promote certain chemical reactions; they produce hydroxide ions in solution, accept hydrogen ions, or donate an electron pair
biodiversity – the number of different species of plants and animals in an area
biosphere – the sum of all living matter, made of a limited number of elements, including oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus
carbon cycle – the continuous movement of carbon among the abiotic environment and living things
carbon dioxide – a gas that is a natural component of the atmosphere; produced by cells during cellular respiration and used by plants and other organisms for photosynthesis
catastrophic event –extreme weather events such as floods, hurricanes, and tornadoes; classified by the extent and intensity of their impact on the ecosystem
cellular respiration –the process of obtaining energy from the breaking of chemical bonds in nutrients
chemical change – a change that alters the identity of a substance, resulting in a new substance or substances with different properties
chemical energy color change
chemical energy – energy stored in the particles that make up food, fuel, and other matter, which is released during a chemical reaction such as burning wood or during the reactions that occur in the cells of organisms
chemical equation –
chemical formulas and symbols written to represent a reaction
chemical formula – a shorthand notation that uses chemical symbols and numbers as subscripts to represent the type and number of atoms that are present in the smallest unit of the substance
chemical property –characteristics that can only be observed or measured when atoms of matter rearrange during a chemical change
chemical reaction – a
process by which two or more chemical substances interact and are chemically changed, producing different chemical substances
climate change – a longterm change in the prevailing environmental conditions in a part of Earth
climate patterns – any recurring characteristic of the climate in the form of patterns that can last tens of thousands of years, or repeat each year; a climatic pattern may come in the form of regular and irregular cycles
coefficient – a number placed in front of a chemical symbol or formula in order to balance the equation
color change – a change in the way something reflects light can indicate a chemical change
compound geosphere
compound – a substance made of two or more elements that are chemically combined in fixed amounts
condensation – the change from a gas state to a liquid state
corrosion – the process of destroying a solid material by a chemical reaction
cycle of matter – the continuous movement of different types of matter, such as water, phosphorus, nitrogen, and carbon, through different parts of the hydrosphere, lithosphere, atmosphere, and biosphere
density – the amount of matter in a given space or volume, a relationship between mass and volume; less-dense matter will form layers above dense matter
electron – a negatively charged subatomic particle that orbits the atomic nucleus
element – a pure substance composed of the same type of atom throughout
endothermic reaction – a chemical reaction in which the absorption of heat occurs
exothermic reaction – a process that releases heat
expansion – when a substance grows in size due to particles separating from each other; can be caused by adding heat
geosphere – portion of Earth system that includes Earth’s interior, rocks and minerals, landforms, and the processes that shape Earth’s surface
global warming jet stream
global warming –continuing rise in the average temperature of Earth’s climate system
global wind system – winds that blow from the poles and act as a heat transfer system due to the unequal heating of Earth’s surface
greenhouse effect – the process by which gases in Earth’s lower atmosphere trap heat emitted by the Sun
groups – the columns on a periodic table that arrange the elements by the number of electrons that are in the outermost shell; also called families
hazardous weather –severe or dangerous weather phenomena that threaten life and property
hemisphere – half of a sphere; Earth and the celestial sphere can be divided into northern and southern or eastern and western hemispheres
high-pressure air mass – an air mass with greater atmospheric pressure than the surrounding air masses; air moves away from the center of the high pressure, traveling in a clockwise direction in the northern hemisphere and a counterclockwise direction in the southern hemisphere
human activity – thing that humans do
jet stream – a narrow zone of strong winds in the upper level of the atmosphere
lithosphere non-metal
law of conservation of matter – the mass of all reactants must equal the mass of all products; Matter is neither created nor destroyed
lithosphere – the rigid, outer part of Earth
low-pressure air mass – an air mass with less atmospheric pressure than the surrounding air masses; air moves toward the low pressure, traveling in a counterclockwise direction in the northern hemisphere and a clockwise direction in the southern hemisphere
metalloids – elements that have properties of both metals and nonmetals, sometimes referred to as semiconductors
metal – most elements are metals; they are typically solid, shiny, malleable, and good conductors of heat and electricity
molecule – the simplest unit of a chemical compound that can exist, formed when two or more atoms join together chemically
motion – the change in an object’s position with respect to time and in comparison with the position of other objects used as reference points
neutron – a subatomic particle of the nucleus of an atom that is without charge that contributes to the mass of an atom
nitrogen cycle – the process by which nitrogen is converted among various chemical forms as it cycles among the soil, biosphere, lithosphere, and atmosphere
non-metal production of a gas
non-metal – elements that are typically not shiny, not malleable, and poor conductors of heat and electricity; usually gases or brittle solids
ocean currents – a directional movement of ocean water; surface currents result from steady winds over the ocean surface; deep currents result from density variations due to temperature and salinity differences
orbital path – the gravitationally curved path of an object around a point in space
oxygen – an element that exists in the atmosphere as a gas; atmospheric oxygen is produced by photosynthesis and consumed by aerobic cellular respiration
oxygen cycle – the movement of carbon dioxide and oxygen on Earth by the processes of respiration and photosynthesis
– a table in which all the known elements are arranged by properties and are represented by one or two letters, referred to as chemical symbols
physical property –characteristics that can be observed or measured without changing the substance; for example, color, melting point, or conductivity
pressure – force exerted on matter through contact with other matter; affects melting and boiling points
production of a gas –evidence of a new substance formed from a chemical change
production of a precipitate temperature
production of a precipitate – evidence of a new substance formed from a chemical change, resulting in solid particles that form or separate out of a liquid
products – a substance produced during a chemical reaction
proton, p+ – a positively charged subatomic particle of the nucleus of an atom that contributes to the mass of the atom
radar – a system for bouncing electromagnetic waves off of objects to locate them; used to track weather patterns
reactant – a substance that takes part in and undergoes change during a reaction
reactivity – rate at which a chemical substance tends to undergo a chemical reaction; significantly influenced by valence electrons of the reacting substances
seasons – the four natural divisions of the year based on changes in temperature due to the varied amounts of sunlight (both intensity and number of daylight hours received); caused by the tilt of Earth during revolution
substance – any form of matter that is uniform throughout and has consistent properties
temperature – a measure of the amount of heat energy; average kinetic energy of all the particles in a material; measured by a thermometer in degrees (usually Celsius or Fahrenheit)
temperature change wind
temperature change –increase or decrease of heat energy in a substance may be evidence of a new substance formed during chemical change
tilt – the slant of Earth’s axis, which is 23.5 degrees from vertical compared to Earth’s orbital plane around the Sun; as a result, the north pole always points toward the North Star
toxic – harmful to life
volume – a measure of the space that matter occupies
water cycle – the constant movement of water through the land, air, oceans, and living things
weather system – a specific set of weather conditions occurring in the lowest levels of the atmosphere, reflecting the configuration of air movement
wind – a natural movement of air, sometimes with considerable force, from an area of high density and pressure to an area of low density and pressure
MISSISSIPPI
ISBN: 979-8-3308-1901-0


