Implications of lipids and modified lipoproteins in atherogenesis: From mechanisms towards novel diagnostic and therapeutic targets
Edited by Alexander Akhmedov, Chu-Huang Chen and Tatsuya Sawamura
Published in Frontiers in Cardiovascular Medicine

The copyright in the text of individual articles in this ebook is the property of their respective authors or their respective institutions or funders. The copyright in graphics and images within each article may be subject to copyright of other parties. In both cases this is subject to a license granted to Frontiers.
The compilation of articles constituting this ebook is the property of Frontiers.
Each article within this ebook, and the ebook itself, are published under the most recent version of the Creative Commons CC-BY licence. The version current at the date of publication of this ebook is CC-BY 4.0. If the CC-BY licence is updated, the licence granted by Frontiers is automatically updated to the new version.
When exercising any right under the CC-BY licence, Frontiers must be attributed as the original publisher of the article or ebook, as applicable.
Authors have the responsibility of ensuring that any graphics or other materials which are the property of others may be included in the CC-BY licence, but this should be checked before relying on the CC-BY licence to reproduce those materials. Any copyright notices relating to those materials must be complied with.
Copyright and source acknowledgement notices may not be removed and must be displayed in any copy, derivative work or partial copy which includes the elements in question.
All copyright, and all rights therein, are protected by national and international copyright laws. The above represents a summary only. For further information please read Frontiers’ Conditions for Website Use and Copyright Statement, and the applicable CC-BY licence
ISSN 1664-8714
ISBN 978-2-8325-2973-7
DOI 10.3389/978-2-8325-2973-7
About Frontiers
Frontiers is more than just an open access publisher of scholarly articles: it is a pioneering approach to the world of academia, radically improving the way scholarly research is managed. The grand vision of Frontiers is a world where all people have an equal opportunity to seek, share and generate knowledge. Frontiers provides immediate and permanent online open access to all its publications, but this alone is not enough to realize our grand goals.
Frontiers journal series
The Frontiers journal series is a multi-tier and interdisciplinary set of openaccess, online journals, promising a paradigm shift from the current review, selection and dissemination processes in academic publishing. All Frontiers journals are driven by researchers for researchers; therefore, they constitute a service to the scholarly community. At the same time, the Frontiers journal series operates on a revolutionary invention, the tiered publishing system, initially addressing specific communities of scholars, and gradually climbing up to broader public understanding, thus serving the interests of the lay society, too.
Dedication to quality
Each Frontiers article is a landmark of the highest quality, thanks to genuinely collaborative interactions between authors and review editors, who include some of the world’s best academicians. Research must be certified by peers before entering a stream of knowledge that may eventually reach the public - and shape society; therefore, Frontiers only applies the most rigorous and unbiased reviews. Frontiers revolutionizes research publishing by freely delivering the most outstanding research, evaluated with no bias from both the academic and social point of view. By applying the most advanced information technologies, Frontiers is catapulting scholarly publishing into a new generation.
What are Frontiers Research Topics?
Frontiers Research Topics are very popular trademarks of the Frontiers journals series: they are collections of at least ten articles, all centered on a particular subject. With their unique mix of varied contributions from Original Research to Review Articles, Frontiers Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area.
Find out more on how to host your own Frontiers Research Topic or contribute to one as an author by contacting the Frontiers editorial office: frontiersin.org/about/contact
August 2023 1 frontiersin.org Frontiers in Cardiovascular Medicine
FRONTIERS EBOOK COPYRIGHT STATEMENT
Implications of lipids and modified lipoproteins in atherogenesis: From mechanisms towards novel diagnostic and therapeutic targets
Topic editors
Alexander Akhmedov — University of Zurich, Switzerland
Chu-Huang Chen — Texas Heart Institute, United States
Tatsuya Sawamura — Shinshu University, Japan
Topic coordinator
Simon Kraler — University of Zurich, Switzerland
Citation
Akhmedov, A., Chen, C.-H., Sawamura, T., eds. (2023). Implications of lipids and modified lipoproteins in atherogenesis: From mechanisms towards novel diagnostic and therapeutic targets. Lausanne: Frontiers Media SA.
doi: 10.3389/978-2-8325-2973-7
August 2023 2 frontiersin.org Frontiers in Cardiovascular Medicine
Table of contents
05 Editorial: Implications of lipids and modified lipoproteins in atherogenesis: from mechanisms towards novel diagnostic and therapeutic targets
Simon Kraler, Tatsuya Sawamura, Grace Yen-Shin Harn, Chu-Huang Chen and Alexander Akhmedov
09 Differential Effects of Genetically Determined Cholesterol Efflux Capacity on Coronary Artery Disease and Ischemic Stroke
Aoming Jin, Mengxing Wang, Weiqi Chen, Hongyi Yan, Xianglong Xiang and Yuesong Pan
17 U-Shaped Relationship of Non-HDL Cholesterol With All-Cause and Cardiovascular Mortality in Men Without Statin Therapy
Rui-Xiang Zeng, Jun-Peng Xu, Yong-Jie Kong, Jia-Wei Tan, Li-Heng Guo and Min-Zhou Zhang
27 The accuracy of four formulas for LDL-C calculation at the fasting and postprandial states
Jin Xu, Xiao Du, Shilan Zhang, Qunyan Xiang, Liyuan Zhu and Ling Liu
36 Diabetic dyslipidemia impairs coronary collateral formation: An update
Ying Shen, Xiao Qun Wang, Yang Dai, Yi Xuan Wang, Rui Yan Zhang, Lin Lu, Feng Hua Ding and Wei Feng Shen
47 Physical activity to reduce PCSK9 levels
Amedeo Tirandi, Fabrizio Montecucco and Luca Liberale
54 Pinocytotic engulfment of lipoproteins by macrophages
Takuro Miyazaki
60 Association of remnant cholesterol and lipid parameters with new-onset carotid plaque in Chinese population
Bo Liu, Fangfang Fan, Bo Zheng, Ying Yang, Jia Jia, Pengfei Sun, Yimeng Jiang, Kaiyin Li, Jiahui Liu, Chuyun Chen, Jianping Li, Yan Zhang and Yong Huo
70 Spotlight on very-low-density lipoprotein as a driver of cardiometabolic disorders: Implications for disease
progression and mechanistic insights
Hsiang-Chun Lee, Alexander Akhmedov and Chu-Huang Chen
83 Crosstalk between high-density lipoproteins and endothelial cells in health and disease: Insights into sex-dependent modulation
Elisa Dietrich, Anne Jomard and Elena Osto
102
Independent association of PCSK9 with platelet reactivity in subjects without statin or antiplatelet agents
Shuai Wang, Di Fu, Huixing Liu and Daoquan Peng
August 2023 3 frontiersin.org Frontiers in Cardiovascular Medicine
111 Correlation of cardiovascular risk predictors with overweight and obesity in patients with familial hypercholesterolemia
Yaodong Wang and Jinchun He
123 Bayesian network analysis of panomic biological big data identifies the importance of triglyceride-rich LDL in atherosclerosis development
Szilard Voros, Aruna T. Bansal, Michael R. Barnes, Jagat Narula, Pal Maurovich-Horvat, Gustavo Vazquez, Idean B. Marvasty, Bradley O. Brown, Isaac D. Voros, William Harris, Viktor Voros, Thomas Dayspring, David Neff, Alex Greenfield, Leon Furchtgott, Bruce Church, Karl Runge, Iya Khalil, Boris Hayete, Diego Lucero, Alan T. Remaley and Roger S. Newton
139 Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis
Christin G. Hong, Elizabeth Florida, Haiou Li, Philip M. Parel, Nehal N. Mehta and Alexander V. Sorokin
150 Prognostic value of remnant cholesterol in patients with coronary heart disease: A systematic review and meta-analysis of cohort studies
Yun Tian, Wenli Wu, Li Qin, Xiuqiong Yu, Lin Cai, Han Wang and Zhen Zhang
160 Paraoxonase 1 and atherosclerosis
Paul N. Durrington, Bilal Bashir and Handrean Soran
August 2023 4 frontiersin.org Frontiers in Cardiovascular Medicine
EDITEDANDREVIEWEDBY
ElenaOsto, MedicalUniversityofGraz,Austria
*CORRESPONDENCE
Chu-HuangChen cchen@texasheart.org AlexanderAkhmedov alexander.akhmedov@uzh.ch
RECEIVED 23June2023
ACCEPTED 14July2023
PUBLISHED 24July2023
CITATION
KralerS,SawamuraT,HarnGY-S,ChenC-H andAkhmedovA(2023)Editorial:Implications oflipidsandmodifiedlipoproteinsin atherogenesis:frommechanismstowardsnovel diagnosticandtherapeutictargets. Front.Cardiovasc.Med.10:1245716. doi:10.3389/fcvm.2023.1245716
COPYRIGHT
©2023Kraler,Sawamura,Harn,Chenand Akhmedov.Thisisanopen-accessarticle distributedunderthetermsofthe Creative CommonsAttributionLicense(CCBY).Theuse, distributionorreproductioninotherforumsis permitted,providedtheoriginalauthor(s)and thecopyrightowner(s)arecreditedandthatthe originalpublicationinthisjournaliscited,in accordancewithacceptedacademicpractice. Nouse,distributionorreproductionis permittedwhichdoesnotcomplywiththese terms.
SimonKraler1,TatsuyaSawamura2,GraceYen-ShinHarn3, Chu-HuangChen3,4* andAlexanderAkhmedov1*
1CenterforMolecularCardiology,UniversityofZurich,Schlieren,Switzerland, 2DepartmentofMolecular Pathophysiology,ShinshuUniversitySchoolofMedicine,ShinshuUniversity,Matsumoto,Japan, 3HEART, HealthResourceTechnology,LLC,Houston,TX,UnitedStates, 4VascularandMedicalResearch,TheTexas HeartInstitute,Houston,TX,UnitedStates
KEYWORDS
low-densitylipoprotein,inflammation,biomarkers,riskstratification,residual cardiovascularrisks,high-densitylipoproteins,triglycerides
EditorialontheResearchTopic

Implicationsoflipidsandmodifiedlipoproteinsinatherogenesis:from mechanismstowardsnoveldiagnosticandtherapeutictargets
1.Introduction
Atherosclerosisistheleadingcauseofmorbidityandmortalityworldwide.Despitethe unprecedentedgainsoverthepastdecades,patientswithestablishedatherosclerotic cardiovasculardisease(ASCVD)remainathighriskofrecurrentischemiceventsdespite optimalmanagement(1–3).Deepeninginsightsintotheunderlyingmechanismsprovide uniqueopportunitiestorefinepreviousconceptsofatherosclerosispathobiologywiththe ultimategoaltoimproveitspreventionandtreatmentandeventuallypatientoutcomes (4).Theatherogenicityofapolipoprotein-Bcontaininglipoproteinsiswellestablished,but recentstudieshaveshednewlightontheimportanceoflipidqualityinthedevelopment ofatherosclerosisanditsclinicalcomplications(5, 6).
WiththisResearchTopic,wehaveassembledanissueofcompellingarticles,comprising originalresearch,casereports,editorials,andstate-of-the-artreviews,withtheaimtogivethe readersof FrontiersinCardiovascularMedicine acomprehensiveoverviewoftheroleof modifiedlipoproteinsinthedifferentphasesofatherogenesis.Indeed,thisResearchTopic notonlydeepensourunderstandingoflipid-drivenmechanismsunderpinningASCVDbut alsoprovidesinsightsintonovelconceptstoaddressthehighburdenofASCVD.
2.Low-densitylipoproteins,high-density lipoproteins,andtriglycerides
Withtheaccumulationofevidenceonsex-specificdifferencesinatherosclerosis pathobiology,clinicaltoolstoguidesex-specificpatientcarearegainingincreasing
Editorial:Implicationsoflipidsand modifiedlipoproteinsin atherogenesis:frommechanisms towardsnoveldiagnosticand therapeutictargets
TYPE Editorial PUBLISHED 24July2023 | DOI 10.3389/fcvm.2023.1245716 Frontiersin CardiovascularMedicine 01 frontiersin.org 5
attentioninrecentyears(7).Thenarrativereviewarticleby Wang andHe highlightsspecificdifferencesinlipoproteinmetabolism andassociatedriskfactorsinwomenandmen.Forexample, womentendtohavehigherhigh-densitylipoproteincholesterol (HDL-C)levels,whichareobservationallylinkedtolower ASCVDrisk;however,atthesametime,womentendtohave highertriglyceridelevels,whichareassociatedwithhigher cardiovascularrisk.Furthermore,sexhormonesandreproductive factorsmayaffectlipoproteinmetabolismand,thus,theriskof majoradversecardiovascularevents(MACE).Gainingabetter understandingofsex-specificdifferencesmayopennovelavenues forclinicalinterventionsthatcouldimprovethepreventionand treatmentofASCVD.
Takingthisconceptastepfurther, Dietrichetal. have emphasizedtheimportanceofsexhormonesandsex-specificeffects ofHDLanditsinteractionwithendothelialcells.Inadditiontotheir immunomodulatory,anti-inflammatory,andanti-oxidative properties,HDLparticlesarethoughttoprovidevasculoprotective effectsbypromotingvasorelaxationandregulatingvascularlipid metabolism.Animprovedunderstandingofhowsex-specificfactors affecttheseinteractionsmaybeusefulindevelopingpersonalized approachesforpreventingandtreatingASCVD.
Inthereviewarticleby Tirandietal. thepotentialroleof physicalactivityinregulatingtheexpressionofproprotein convertasesubtilisin/kexintype9(PCSK9),akeyregulatorof LDL-Clevels,isdiscussed.Inarelatedstudy, Wangetal. investigatedpotentialassociationsbetweenPCSK9levelsand plateletreactivityinindividualsnotreceivingstatinor antiplatelettherapy.Theauthorsreportedasignificantpositive correlationbetweenPCSK9andplateletreactivity,suggestingthat inhibitingPCSK9mayattenuateplateletreactivityandthus MACEriskinpatientsathighASCVDrisk.Thisdiscovery encouragesfurtherresearchonthepleiotropiceffectsofPCSK9 inhibitionincaringforpatientswithestablishedASCVD.
Theretrospectivestudyby WangandHe assessedthe correlationbetweencardiometabolicriskfactorsandobesityin 103patientswithfamilialhypercholesterolemia.Their findings demonstratethepotentialofusingnovelbiomarkersforrisk stratificationandpersonalizedmanagementofmoderatetohighriskpatients,suchasthosewithfamilialhypercholesterolemia. Similarly,thestudyby Zengetal. emphasizestheimportanceof consideringnon-HDL-Casariskfactorinmennotreceiving statintherapy.TheauthorsdescribeaU-shapedrelationship betweennon-HDL-Clevelsandall-causeandcardiovascular mortality,suggestingthatbothlowandhighlevelsofnon-HDLCareassociatedwithincreasedmortalityrisk,a findingthatwas similarlyreportedforLDL-C(8).
Xuetal.’sstudyfocusedoncomparingestablishedformulas usedforcalculatingLDL-Clevelsinfastingandpostprandial statesintheChinesepopulation.Notably,theFriedewald formulaprovidedthehighestaccuracyfordeterminingfasting LDL-Clevels,whereastheSampsonformulaperformedbetterfor measuringpostprandialLDL-Clevels.These findingsmay stimulatefurtherresearchinthisdirectiontoaccuratelyassess cardiovascularriskandeventuallyrefineinterventionsinthe Chinesepopulation.
Inanimportantreviewonmacrophage-mediatedpinocytotic engulfmentoflipoproteins, Miyazaki highlightsareceptorindependentendocyticpathwayforfoamcellformation.This processappearstooccurwhenlipoproteinsaccumulatearound inflammatorycellsandinvolvesplasmamembraneruffling, small GTPases,andcytoskeletalrearrangement.AlthoughnativeLDL maynotbethemaindriveroffoamcellformation,further experimentalstudiesarenecessarytoidentifythemaster regulatoroflipoproteinengulfmentbymacrophagestoimprove ourunderstandingofitsroleinASCVDpathogenesis.
3.Qualityoflipoproteins
Oneareaofresearchthathasgainedincreasingattentioninrecent yearsistheroleofmodifiedlipoproteinsinthelifecycleof atheroscleroticplaque.Modifiedlipidsincludeoxidizedlow-density lipoprotein(oxLDL),small-denseLDL,triglyceride-richLDL, electronegativeLDL,andverylow-densitylipoprotein(VLDL)(5, 9–11).
Leeetal.’sreviewfocusesonVLDL,apotentialdriverof cardiometabolicdiseases.ThemostelectronegativeVLDL subclassexertscytotoxiceffectsontheendotheliumandhasbeen linkedtochroniccoronarysyndromesandatrialremodelingin patientswithmetabolicsyndrome.Thereviewarticlehighlights thesignificanceofpostprandialVLDLmodificationandtheneed forfurtherinvestigationintotheroleofVLDLsubclassesinthe pathobiologyofcardiometabolicdiseases.
InaBayesiannetworkanalysisfocusedonbiomarkersofcoronary atherosclerosis, Vorosetal. havehighlightedtheimportanceof triglyceride-richLDLparticlesinASCVDdevelopment.Inthe665 patientsincludedintheanalysis,LDL-triglyceridesweredirectly linkedtocarotidatherosclerosisinover95%ofthemodels. Interestingly,geneticvariantsintheLIPCgene(encodinghepatic lipase)wereassociatedwithLDL-triglyceridelevelsandthepresence ofatheroscleroticplaque.These findingssuggestthattriglyceriderichLDLparticlesmayplayacrucialroleinatherosclerosis development,thusprovidingapotentialtargetforfuturestudies.
InaChinesepopulation, Liuetal. exploredpotential associationsbetweenremnantcholesterol(RC)andnew-onset carotidplaques.TheauthorsconcludedthatelevatedRClevels arestronglyassociatedwiththepresenceofnew-onsetcarotid plaquesrelativetootherlipidparameters,particularlyinthose withlowLDL-Clevels.Thisstudyhighlightstheimportanceof consideringRCinpredictingresidualcardiovascularrisk,suchas inthosewithlowlevelsofLDL-C.Ameta-analysisby Tianetal. furtherevaluatedtheprognosticvalueofRCinpatientswith chroniccoronarysyndromes.Theyreportedanincreasingriskof MACEwithhigherRClevels,althoughnosignificantassociation withall-causemortalitywasidentified.
Thereviewarticleby Shenetal. providesinsightsintotheimpact ofdyslipidemiaoncoronarycollateralformationduringdiabetic states.Theauthorsnotethatbothalteredserumlipidprofilesand glycoxidativemodificationoflipoproteinscontributetoendothelial dysfunctionandbluntendothelialprogenitorcellresponsesand interferewiththegrowthandmaturationofcollateralvesselsin diabeticpatientswithchroniccoronarysyndromes.
Kraleretal. 10.3389/fcvm.2023.1245716 Frontiersin CardiovascularMedicine 02 frontiersin.org 6
Theabovereviewarticleandtheaforementionedworkdemonstrate thesignificanceoflipidsinatherosclerosisanditsacuteandchronic clinicalsequelae(Miyazaki, Vorosetal.Shenetal.).Furtherresearch inthisareamayleadtoinnovativestrategiesforpreventingand treatingASCVDandultimatelyimprovingpatientoutcomes.
Hongetal. conductedasystematicreviewandmeta-analysisto evaluatetherelationshipbetweenoxLDLandcardiovascular diseaseinpatientswithchronicinflammatoryconditions.The analysisofthreeobservationalstudiescomprisingatotalof1,060 participantsshowedthatcirculatingoxLDLlevelsareincreased inparticipantswithASCVDduringchronicinflammatory conditions.Indeed,theirstudysuggeststhatoxLDLmaybea usefulbiomarkerforriskstratificationofpatientswith establishedcardiovasculardiseasedrivenbychronicinflammation.
Sincetheintroductionofthetraditionalriskfactorconceptin theFraminghamStudy(12),cholesterol-richlipoproteinshave beenextensivelyexamined,withinstrumentalvariableapproaches nowallowingforcausalinferenceusingobservationaldata.Inthe innovativestudyby Jinetal.,thecausalroleofcholesterolefflux capacityinchroniccoronarysyndromes,acutemyocardial infarction,andischemicstrokewasexaminedusingMendelian randomization.Consideringthepotentiallimitationsoftheir approach,these findingssuggestthatincreasedcholesterolefflux capacityreducestheriskofchroniccoronarysyndromesand myocardialinfarctionbuthasaweakercausaleffectonischemic stroke,likelybecauseofitsmoreheterogeneouspathobiology.
Collectively,thestudiesby Wangetal.Zengetal.Xuetal.Liu etal.Tianetal. and Jinetal. highlighttheimportanceof consideringmultiplefactorsinassessingcardiovascularriskwith
potentialfuturetherapeuticimplications.Inadditionto traditionalriskfactors,non-HDL-CandRCconcentrationsmay providevaluableinsightsintoapatient’soverallcardiovascularrisk.
Thesestudiesalsohighlighttheinterplayoflipoproteins,physical activity,sex-specificdeterminants,andemergingbiomarkersin cardiovascularhealthanddisease(WangandHe).Althoughthe traditionalriskfactorconcepthaslongprevailed,itmighthave resultedinanoversimplifiedunderstandingofASCVD.Animproved understandingofASCVDpathobiologyoffersuniqueopportunitiesto furtherimprovepatientcareandtomitigateresidualcardiovascularrisk.
Intheirreviewarticle, Durringtonetal. highlightacriticalrole ofHDL-containedserumparaoxonase-1(PON1)inprotecting againstharmfulLDLoxidation,amechanismthatdrivesearly phasesofASCVD.Accordingly,reducedserumPON1activityis associatedwithdyslipidemic,diabetic,andinflammatorystates. LowPON1levelsarefurtherlinkedtoadversecardiovascular events,particularlyinpatientswithdiabetesandestablished ASCVD,providingaconceptualframeworktostudyfunctional determinantsofHDLtoreduceresidualcardiovascularrisk.
Thearticlesby WangandHe, Dietrichetal., Tirandietal., Lee etal., Hongetal.,and Durrington et al. underscorethesignificance ofbiomarkersincardiovasculardiseasepreventionand personalizedpatientcare.Theidentificationofnovelbiomarkers canaidinpersonalizedriskassessmentandprovideabasisfor targetedinterventionsinhigh-riskpatients.Moreover,these studiessuggestthatnovelbiomarkers,includingoxLDL,PON1, andelectronegativeVLDL,mayserveascomplementarytoolsfor theidentificationofpatientsatparticularlyhighASCVDrisk whomaybenefitfromintensivesecondarypreventionmeasures.
Lipidsandtheirmodificationscontributeimportantlytothepathogenesisofatheroscleroticcardiovasculardisease(ASCVD).High-throughput technologies,includinglipidomics,nowallowthein-depthcharacterizationoflipoproteinstructureandfunction.Bothphysicalandchemical characteristicsmodulatetheatherogeniceffectsofbloodlipids,butgeneticsusceptibility,sexandgender,physicalactivity,andreceptor(in-) dependentmechanismscandeterminetheir(cardio-)vasculareffects.AbetterunderstandingofhowthesebiomarkerscontributetoASCVD pathogenesismayallowforpersonalizedriskassessmentandtheidentificationofnoveltherapeutictargets,aimedatreducingresidualcardiovascular riskandthusimprovingoutcomesofpatientsatparticularlyhighcardiovascularrisk.
 FIGURE1
FIGURE1
Kraleretal. 10.3389/fcvm.2023.1245716 Frontiersin CardiovascularMedicine 03 frontiersin.org 7
However,itisessentialtorecognizethatthesebiomarkersshould notbeusedinisolationbutshouldbeconsideredalongsideother clinicaltools,includingriskscores.Moreover,theclinicalutilityof novelbiomarkersforriskassessmentmustundergorigorous validationindifferentpopulationsandsettings,takingintoaccount potentiallimitationsinherentinthedesignofthestudiesnotedabove.
Together,thestudiespresentedinthisResearchTopichighlight theimportanceofbiomarkersinthepreventionandmanagement ofASCVDanditscomplications(Figure1).Theidentificationand rigorousvalidationofnovelbiomarkerscouldassistinpersonalized riskassessmenttoguidetargetedinterventionsinhigh-risk populations.Tothatend,high-throughputassaysareneededfor qualitativelyassessingchangesinthestructureandfunctionof lipoproteinstoworktowardtheclinicalimplementationof multidimensionallipidprofiling.Thehereinproposedresearch pursuitiscrucialfordevelopingeffectivepreventionand managementstrategiesforpatientsatriskfororwithestablished ASCVDwiththeultimategoalofreducingtheburdenof residualcardiovascularrisk.
Authorcontributions
Allauthorslistedhavemadeasubstantial,direct,andintellectual contributiontotheworkandapproveditforpublication.
References
1.KralerS,WenzlFA,LuscherTF.Repurposingcolchicinetocombatresidual cardiovascularrisk:theLoDoCo2trial. EurJClinInvest.(2020)50:e13424.doi:10. 1111/eci.13424
2.SabatineMS,GiuglianoRP,KeechAC,HonarpourN,WiviottSD,MurphySA, etal.Evolocumabandclinicaloutcomesinpatientswithcardiovasculardisease. NEnglJMed.(2017)376:1713–22.doi:10.1056/NEJMoa1615664
3.SchwartzGG,StegPG,SzarekM,BhattDL,BittnerVA,DiazR,etal.Alirocumab andcardiovascularoutcomesafteracutecoronarysyndrome. NEnglJMed.(2018) 379:2097–107.doi:10.1056/NEJMoa1801174
4.KralerS,LibbyP,EvansPC,AkhmedovA,SchmiadyMO,ReinehrM,etal. Resilienceoftheinternalmammaryarterytoatherogenesis:shiftingfromriskto resistancetoaddressunmetneeds. ArteriosclerThrombVascBiol.(2021) 41:2237–51.doi:10.1161/ATVBAHA.121.316256
5.KralerS,WenzlFA,VykoukalJ,FahrmannJF,ShenM-Y,ChenD-Y,etal.Lowdensitylipoproteinelectronegativityandriskofdeathafteracutecoronarysyndromes: acase-cohortanalysis. Atherosclerosis.(2023)376:43–52.doi:10.1016/j.atherosclerosis. 2023.05.014
6.RuuthM,NguyenSD,VihervaaraT,HilvoM,LaajalaTD,KondadiPK,etal. Susceptibilityoflow-densitylipoproteinparticlestoaggregatedependsonparticle lipidome,ismodifiable,andassociateswithfuturecardiovasculardeaths. EurHeart J.(2018)39:2562
73.doi:10.1093/eurheartj/ehy319
Acknowledgments
TheauthorsthankRebeccaBartow,ofTheTexasHeart InstituteinHouston,TX,foreditorialassistance.
Conflictofinterest
GHandCH-HwereemployedbyHEART,HealthResource Technology,LLC.
Theremainingauthorsdeclarethattheresearchwasconducted intheabsenceofanycommercialor financialrelationshipsthat couldbeconstruedasapotentialconflictofinterest.
ThehandlingeditorEOdeclaredapastcollaborationwiththe authorAA.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
7.WenzlFA,KralerS,AmblerG,WestonC,HerzogSA,RaberL,etal.Sex-specific evaluationandredevelopmentoftheGRACEscoreinnon-ST-segmentelevationacute coronarysyndromesinpopulationsfromtheUKandSwitzerland:amultinational analysiswithexternalcohortvalidation. Lancet.(2022)400:744–56.doi:10.1016/ S0140-6736(22)01483-0
8.JohannesenCDL,LangstedA,MortensenMB,NordestgaardBG.Association betweenlowdensitylipoproteinandallcauseandcausespecificmortalityinDenmark: prospectivecohortstudy. BrMedJ.(2020)371:m4266.doi:10.1136/bmj.m4266
9.ChanHC,KeLY,ChuCS,LeeAS,ShenMY,CruzMA,etal.Highlyelectronegative LDLfrompatientswithST-elevationmyocardialinfarctiontriggersplateletactivation andaggregation. Blood.(2013)122:3632–41.doi:10.1182/blood-2013-05-504639
10.KralerS,WenzlFA,GeorgiopoulosG,ObeidS,LiberaleL,vonEckardsteinA, etal.Solublelectin-likeoxidizedlow-densitylipoproteinreceptor-1predicts prematuredeathinacutecoronarysyndromes. EurHeartJ.(2022)43:1849–60. doi:10.1093/eurheartj/ehac143
11.ShenMY,ChenFY,HsuJF,FuRH,ChangCM,ChangCT,etal.PlasmaL5 levelsareelevatedinischemicstrokepatientsandenhanceplateletaggregation. Blood.(2016)127:1336–45.doi:10.1182/blood-2015-05-646117
12.KannelWB,DawberTR,KaganA,RevotskieN,StokesJ3rd.Factorsofriskin thedevelopmentofcoronaryheartdisease–sixyearfollow-upexperience.The framinghamstudy. AnnInternMed.(1961)55:33–50.doi:10.7326/0003-4819-55-1-33
–
Kraleretal. 10.3389/fcvm.2023.1245716 Frontiersin CardiovascularMedicine 04 frontiersin.org 8
Editedby: Chu-HuangChen, TexasHeartInstitute,UnitedStates
Reviewedby: C.M.Schooling, CityUniversityofNewYork, UnitedStates AdriaArboix, SacredHeartUniversity Hospital,Spain
*Correspondence: YuesongPan yuesongpan@ncrcnd.org.cn
†Theseauthorshavecontributed equallytothisworkandsharefirst authorship
Specialtysection: Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine

Received: 07March2022
Accepted: 14June2022
Published: 04July2022
Citation:
JinA,WangM,ChenW,YanH, XiangXandPanY(2022)Differential EffectsofGeneticallyDetermined CholesterolEffluxCapacityon CoronaryArteryDiseaseandIschemic Stroke. Front.Cardiovasc.Med.9:891148. doi:10.3389/fcvm.2022.891148
DifferentialEffectsofGenetically DeterminedCholesterolEfflux CapacityonCoronaryArteryDisease andIschemicStroke
Background: Observationalstudiesindicatedthatcholesteroleffluxcapacity(CEC) ofhigh-densitylipoprotein(HDL)isinverselyassociatedwithcardiovascularevents, independentlyoftheHDLcholesterolconcentration.Theaimofthestudyistoexamine thecasualrelevanceofCECforcoronaryarterydisease(CAD)andmyocardialinfarction (MI),andcompareitwiththatforischemicstrokeanditssubtypesusingaMendelian randomizationapproach.
Methods: Weperformeda2-sampleMendelianrandomizationtoestimatethecasual relationshipofCECwiththeriskofCAD,MI,andischemicstroke.ACEC-related geneticvariant(rs141622900)andotherfivegeneticvariantswereusedasthe instrumentalvariables.AssociationofgeneticvariantswithCADwereestimatedina GWASinvolving60,801CADcasesand123,504controls.Theywerethencompared withtheassociationsofthesevariantswithischemicstrokeanditssubtypes(large vessel,smallvessel,andcardioembolic)involving40,585ischemicstrokecasesand 406,111controls.
Results: UsingtheSNPofrs141622900astheinstrument,a1-SDincreaseinCEC wasassociatedwith45%lowerriskforCAD(oddsratio[OR]0.55,95%confidence interval[CI]0.44–0.69, p < 0.001)and33%lowerriskforMI(oddsratio[OR]0.67,95% CI0.52–0.87, p = 0.002).Bycontrast,thecausaleffectofCECwasmuchweakerfor ischemicstroke(oddsratio[OR]0.79,95%CI0.64–0.97, p = 0.02; p forheterogeneity = 0.03)and,inparticular,forcardioembolicstroke(p forheterogeneity = 0.006)when comparedwiththatforCAD.Resultsusingfivegeneticvariantsastheinstrumentalso indicatedconsistentlyweakereffectsonischemicstrokethanonCAD.
Conclusion: GeneticpredictedhigherCECmaybeassociatedwithdecreasedriskof CAD.However,thecasualassociationofCECwithischemicstrokeandspecificsubtypes wouldneedtobevalidatedinfurtherMendelianrandomizationstudies.
Keywords:cholesteroleffluxcapacity,Mendelianrandomization,coronaryarterydisease,stroke,genetics
ORIGINALRESEARCH published:04July 2022
FrontiersinCardiovascularMedicine|www.frontiersin.org 1 July2022 | Volume9|Article891148
doi:10.3389/fcvm.2022.891148
AomingJin 1,2†,MengxingWang 1,2†,WeiqiChen 1,2,HongyiYan 1,2,XianglongXiang 1,2 and YuesongPan 1,2*
1 DepartmentofNeurology,BeijingTiantanHospital,CapitalMedicalUniversity,Beijing,China, 2 ChinaNationalClinical ResearchCenterforNeurologicalDiseases,BeijingTiantanHospital,CapitalMedicalUniversity,Beijing,China
9
INTRODUCTION
Epidemiologicstudieshaveshownaninverserelationship betweenhigh-densitylipoprotein(HDL)cholesterollevelsand cardiovasculardisease(1);however,recentclinicaltrials(2, 3) andMendelianrandomization(MR) studies(4, 5)failedto established aclearcausalassociationbetweenHDLcholesterol andcardiovasculardisease.Thisledtothehypothesisthatthe atheroprotectiveroleofHDLliesinitsfunctionratherthanin itsconcentrations(6).
Themostimportant measureofHDLfunctionischolesterol effluxcapacity(CEC),theabilityofHDLtoreversecholesterol transportfromperipheralcells(7).PreviouscohortandcasecontrolstudiesshowedthatCECwasinverselyassociatedwith atherosclerosisandtheincidenceofcardiovasculareventsin thegeneralpopulation,independentlyoftheHDLcholesterol concentration(8–11).However,observationalepidemiological studiesmaysuffer fromconfoundingandselectionbiasthat representobstaclestovalidcausalinference(12, 13).Thecausal associationbetweenCEC andcardiovasculardiseasesisstill controversial.Furthermore,ischemicstrokehadaheterogeneous mechanismandmayhavedifferentcauseandriskfactorsfrom coronaryarterydisease(CAD)(14).PreviousMRstudieshave showedaweaker effectonischemicstrokethanonCADfor somelipidmetabolicfactors,suchaslow-densitylipoprotein cholesterolandproproteinconvertasesubtilisin/kexintype9 (PCSK9)variants(15, 16).Therefore,therelativeeffectsofCEC onCADandischemicstrokeneedsfurtherinvestigation.
MRstudy,usinggeneticvariantsasinstrumentalvariables, isamethodthatcancontrolpotentialconfoundersandreverse causationthatmaybiasobservationalstudies,andmakestronger causalinferencesbetweenanexposureandriskofdiseases(12). Inthepresentstudy,weaimedtouseMRanalysistoexaminethe causalrelevanceofCECforCADandmyocardialinfarction(MI), andcomparesitwiththatforischemicstrokeanditssubtypes.
MATERIALSANDMETHODS StudyDesign
Atwo-sampleMRanalysisusingCEC-relatedgeneticvariants asinstrumentalvariablewasdesignedtoevaluatethecausal effectbetweenCECandriskofCADandischemicstroke (SupplementaryFigure1).Summary-leveldataontheexposure (CEC)werederivedfromarecentpublishedgenome-wide associationstudy(GWAS)ofupto5,293Europeanindividuals (17)anddataontheoutcome(CADandischemicstroke) wereobtainedfrom GWASsofupto446,696European individuals(18, 19). Table1 and SupplementaryTable1 shows thecharacteristicsoftheseGWASs.Approvalofethicscommittee andwritteninformedconsentwereobtainedbeforedata collectionintheoriginalGWASs.
GeneticInstrumentalVariables
Weused6singlenucleotidepolymorphisms(SNPs)associated withCECidentifiedthroughGWASbyLow-Kametal.(17) asthe instrumentalvariables.Low-Kametal.(17)testedthe geneticassociation between4CECmeasuresandgenotypesat
>9millioncommonautosomalDNAsequencevariantsin5,293 FrenchCanadians.Theyidentified10genome-widesignificant signals(P < 6.25 × 10 9)representing7loci.Amongthe7loci, 2loci(nearthe PPP1CB/PLB1 and RBFOX3/ENPP7 genes)only reachedgenome-widesignificanceinthemodelfurtheradjusted forHDL-Candtriglyceridelevelswhichmayleadtofalsepositive associationsintheGWAScontext(i.e.,colliderbias).Other5 loci(CETP, LIPC, LPL, APOA1/C3/A4/A5,and APOE/C1/C2/C4) harboredgeneswithimportantrolesinlipidbiologyandreached genome-widesignificanceinthemodeladjustedforsex,age squared,coronaryarterydiseasestatus,experimentalbatches, statintreatment,andthefirst10principalcomponents.Except forthe APOE/C1/C2/C4 variant,associationofother4loci disappearedwhencorrectingforHDL-Candtriglyceridelevels. OnlytheSNPofrs141622900in APOE/C1/C2/C4 locusreached genome-widesignificanceinbothtwomodelsandwasusedasthe instrument.Insensitivityanalysis,weusedthemostsignificant SNPineachofthe5loci(rs77069344,rs2070895,rs247616, rs964184,andrs445925)astheinstrument.These5SNPswere indifferentgenomicregionsandnotinlinkagedisequilibrium (r2 < 0.1).The1SNP(rs141622900)instrumentexplained0.9% andthe5SNPsinstrumentexplained5.3%ofthevarianceinCEC (F statistic = 59.2and45.9,respectively,indicatingsufficient strengthoftheinstruments). Table2 showsthecharacteristics andassociationsoftheseincludedSNPswithCEC.
Outcomes
SummarystatisticsfortheassociationofeachCEC-relatedSNP withtheCADandMIwereextractedfromtheCoronaryARtery DIseaseGenome-wideReplicationAndMeta-AnalysisPlus CoronaryArteryDiseaseGenetics(CARDIoGRAMplusC4D) 1000Genomes-basedGWAS(17).TheCARDIoGRAMplusC4D 1000Genomes-basedGWASinterrogated9.4millionvariantsin upto60,801CADcasesand123,504controlsfrom48studies ofpredominantlyEuropeanancestry.Summarystatisticsfor theassociationoftheincludedSNPswithischemicstrokeand the3mainsubtypesofischemicstroke(largearterystroke [LAS],smallvesselstroke[SVS],cardioembolicstroke[CES]) wereextractedfromtheGWASofMultiancestryGenome-wide AssociationStudyofStroke(MEGASTROKE)consortium(19). TheMEGASTROKE consortiumtested ∼8millionSNPsand indelswithminor-allelefrequency ≥0.01inupto67,162stroke casesand454,450controlsfrom29studies,predominantly Europeanancestry(40,585cases;406,111controls).ThisGWAS involved34,217caseswithLAS,5,386caseswithSVSand7,193 caseswithCESofEuropeanancestry.Theassociationsofthe6 individualSNPsforCECwithCADandMI,andischemicstroke anditssubtypesarepresentedin Tables3, 4,respectively.
StatisticalAnalysis
Per-alleleeffectsoftheselectedSNPsonCECanddisease outcomeswereextractedfromtheGWASsandusedtoestimate thecausaleffectofCEConoutcomesusingtwo-sampleMR analyses.UsingtheSNPofrs141622900astheinstrument,Wald ratiomethodwereusedtoobtaineffectestimatebydividing theSNP-outcomeestimatebytheSNP-CECestimate.Standard errorwereestimatedusingtheDeltamethodbydividing
Jinetal. CholesterolEffluxCapacityandVascularDiseases
FrontiersinCardiovascularMedicine|www.frontiersin.org 2 July2022| Volume9|Article891148 10
TABLE1| CharacteristicsoftheGWASstudiesusedinthisstudy.
TABLE2| Characteristicsoftheincluded SNPlociassociatedwithcholesteroleffluxcapacity.
theSNP-outcomestandarderrorbytheSNP-CECestimate (20).Whenusingthe5SNPsastheinstruments,weuseda conventionalinverse-variance weighted(IVW)MRanalysiswith multiplicativerandomeffectsassumingallgeneticvariantsare validinstruments.InIVWmethod,theSNP-outcomeestimate isregressedontheSNP-CECestimate,weightedbytheinversevarianceofSNP-outcomeestimateandwiththey-axisinterceptis fixedtozero(21).Wefurtherconductedmethodologicsensitivity analysesusingMR-Egger,simplemedian,weightedmedian methodsusingthe5SNPsastheinstruments,whicharemore robusttotheinclusionofpleiotropicorinvalidinstruments.MREggermethodcanassessandcontrolfordirectionalpleiotropic biasandprovideanpleiotropy-correctedeffectestimatein whichgeneticvariantsarepermittedtobeinvalidinstrumental variables(22).Themedianmethodscanprovideaconsistent effectestimate usingthemedianoftheempiricaldistribution functionofindividualSNPratioestimatesinwhichupto50%of thegeneticvariantsarepermittedtobeinvalidinstruments(23).
Presencesof heterogeneitybetweencausaleffectsofindividual variantsandcomparisonsbetweenthecausaleffectsofCECon CADvs.ischemicstrokeweretestedusingtheCochranQstatistic and I2 indexintheIVWanalysis(23).Evidenceofpleiotropic effectswereassessedusinginterceptsoftheMR-Eggerregression (22).Moreover,multivariabletwo-sampleMRwereperformed toadjustfor majorcausesofsurvival(smoking,bodymass index,andbloodpressure)usingthe5SNPsastheinstruments (24).MultivariableMRanalysiswasusedtoassesswhetherthe associationsbetweengenetic predispositiontoCECandischemic strokemaybeaffectedbyselectionbias(25).Theaboveanalysis
TABLE3| Geneticassociationofcholesteroleffluxcapacityrelatedgenetic variantswithcoronaryarterydiseaseandmyocardialinfarctioninthe CARDIoGRAMplusC4Dconsortium.
SNPsEA/OACoronaryarterydiseaseMyocardialinfarction
EA,effectallele;OA,otherallele;SE,standarderror;SNP,singlenucleotidepolymorphism. Theunitofbetacoefficientsislog-oddsperallele.Theoddsratio = exp(Beta),upper boundofoddsratio = exp(Beta+1.96 * SE),andlowerboundofoddsratio = exp(Beta1.96 * SE).
wereconductedintheUKBiobankGWASandFinnGenGWAS assensitivityanalyses.
ThepercentageofvarianceexplainedinCECwasestimated by2× (effectonCEC)2 × minorallelefrequency × (1-minor allelefrequency)(16).Apoweranalysiswasperformedusing aweb-based application(https://sb452.shinyapps.io/power/). EffectestimatesofCEC-outcome(CAD,MI,ischemicstrokeand itssubtypes)arepresentedasoddsratios(ORs)withtheir95% confidenceintervals(CIs)ofoutcomeper1-SDgeneticallyhigher CEC.Toaccountformultipletesting,aBonferroni-corrected significancelevelof p < 0.0083(i.e.,0.05/6for6outcomes)
Jinetal. CholesterolEffluxCapacityandVascularDiseases
Phenotype Consortium N EthnicityGenotypedata PMID Exposure Cholesterolefflux capacity(CEC) Upto5,293individualsEuropean GWASarray 30369316 Outcomes Coronaryarterydisease(CAD) CARDIoGRAMplusC4DUpto184,305individualsEuropean GWASarray 26343387 Myocardialinfarction(MI) CARDIoGRAMplusC4DUpto171,876individualsEuropean GWASarray 26343387 Ischemicstroke(IS) MEGASTROKE Upto446,696individualsEuropean GWASarray 29531354 Largearterystroke(LAS) MEGASTROKE Upto440,328individualsEuropean GWASarray 29531354 Smallvesselstroke(SVS) MEGASTROKE Upto411,497individualsEuropean GWASarray 29531354 Cardioembolicstroke(CES) MEGASTROKE Upto413,304individualsEuropean GWASarray 29531354
SNP LocusChromosome(Position)(hg19)EA/OAEAF CEC BetaSE p rs77069344 LPL 8(19821782) G/T 0.099J774basal0.20080.03277.96 × 10 10 rs2070895 LIPC 15(58723939) A/G0.230J774basal0.14240.02328.49 × 10 10 rs247616 CETP 16(56989590) T/C0.314J774basal0.14660.02114.08 × 10 12 rs964184 APOA1/C3/A4/A5 11(116648917) C/G0.857J774ABCA1dependent0.20190.02816.78 × 10 13 rs445925 APOE/C1/C2/C4 19(45415640) A/G0.114J774ABCA1dependent0.21550.03031.20 × 10 12 rs141622900 APOE/C1/C2/C4 19(45426792) A/G0.058BHKstimulated0.28330.04171.03 × 10 11
SNP,singlenucleotidepolymorphism;EA,effectallele;OA,otherallele;EAF,effectallelefrequency;CEC,cholesteroleffluxcapacity;SE,standarderror.Theunitofbetacoefficientsis SDincreaseofCECperallele.
BetaSE p BetaSE p rs77069344G/T 0.05140.0158 0.001 0.06510.01760.000 rs2070895A/G0.03720.01080.0010.04140.01210.001 rs247616T/C 0.03120.01030.002 0.02800.01140.014 rs964184C/G 0.05000.01240.000 0.04880.01390.000 rs445925A/G 0.08580.01870.000 0.06640.02140.002 rs141622900A/G 0.14210.02780.000 0.09630.03150.002
FrontiersinCardiovascularMedicine|www.frontiersin.org 3 July2022| Volume9|Article891148 11
EA,effectallele;OA,otherallele;LAS,largearterystroke;SVS,small vesselstroke;CES,cardioembolicstroke;SE,standarderror;SNP,singlenucleotidepolymorphism.Theunitof betacoefficientsislog-oddsperallele.Theoddsratio = exp(Beta),upperboundofoddsratio = exp(Beta+1.96 * SE),andlowerboundofoddsratio = exp(Beta-1.96* SE).
waspredefinedasstatisticallysignificantevidenceforacausal association.AllanalyseswereconductedwithR3.5.1(R DevelopmentCoreTeam).
RESULTS
Geneticallydetermined1-SDincreaseinCECwascasually associatedwithasubstantialdecreaseinriskofCAD(OR = 0.55, 95%CI:0.44–0.69, p < 0.001)andMI(OR = 0.67,95%CI:0.52–0.87, p = 0.002);but,bycontrast,wasnotcausallyassociated withischemicstroke(OR = 0.79,95%CI:0.64–0.97, p = 0.02) oranyseparatesubtypeofischemicstroke(LAS:OR = 0.67, 95%CI:0.39–1.17, p = 0.16;SVS:OR = 1.08,95%CI:0.71–1.63, p = 0.73;CES:OR = 0.72,95%CI:0.44–1.17, p = 0.18)at theBonferroni-adjustedlevelofsignificance(p < 0.0083)using theSNPofrs141622900astheinstrument(Figure1).Theeffect ofCEConischemicstrokewasweakerthanthatonCAD(p forheterogeneity = 0.03, I2 = 80%),andinparticularonCES (p forheterogeneity = 0.006, I2 = 87%),whereastheeffectsof CEConLASandSVSwerecompatiblewiththemagnitudeof theeffectobservedforCAD(p forheterogeneity = 0.53and 0.34,respectively).TheeffectsofCEConischemicstrokeandits subtypeswerecompatiblewiththemagnitudeoftheeffectforMI (p forheterogeneity = 0.34,1.00,0.80,and0.06,respectively). Theseanalyseshada >99, >99,70,and82%powertodetect a30%decreaseinriskofischemicstroke,LAS,SVS,andCES (equivalenttotheupperlimitoftheCIforCAD),respectively; thiscanexcludeacausaleffectofCEConischemicstrokeand CESofthesamemagnitudeasonCAD,andindicatecomparable effectsofCEConLAS,SVS,andCAD.Whereas,thepowerto detecta13%decreaseinriskofischemicstroke,LAS,SVS,and CES(equivalenttotheupperlimitoftheCIforMI)was72,65,16, and20%,respectively;thisindicatedcomparativelylittlepower forcomparableeffectsofCEConischemicstroke,particular strokesubtypesandMI.
SimilardisparateassociationsofCECwereobservedwiththe riskofCAD(OR = 0.85,95%CI:0.79–0.90, p < 0.001)andMI (OR = 0.86,95%CI:0.80–0.92, p < 0.001),comparedtoischemic stroke(OR = 1.02,95%CI:0.95–1.09, p = 0.58)anditssubtypes (LAS:OR = 0.90,95%CI:0.76–1.07, p = 0.25;SVS:OR = 1.00, 95%CI:0.85–1.17, p = 0.95;CES:OR = 1.04,95%CI:0.91–1.19, p = 0.60),usingtheIVWmethodswiththe5-SNPsinstrument
(Figure1).TheeffectofCEConischemicstrokewasweakerthan thatonCAD(p forheterogeneity <0.001, I2 = 93%)andMI(p forheterogeneity <0.001, I2 = 91%),andinparticularonCES (p forheterogeneity = 0.008, I2 = 86%;pforheterogeneity = 0.01, I2 = 83%),whereastheeffectsofCEConLASandSVSwere compatiblewiththemagnitudeoftheeffectobservedforCAD (p forheterogeneity = 0.49and0.07,respectively)andMI(p for heterogeneity = 0.58and0.10,respectively).Theseanalyseshad a >99,99,42,and53%powertodetecta10%decreaseinrisk ofischemicstroke,LAS,SVS,andCES(equivalenttotheupper limitoftheCIforCADandMI),respectively;thiscanexcludea causaleffectofCEConischemicstrokeofthesamemagnitudeas onCADandMI,andindicatecomparableeffectsofCEConLAS, CAD,andMI.
SignificantassociationforCADandMIandinsignificant associationforischemicstrokeanditssubtypeswerealso observedinsensitivityanalysesusingtheMR-Egger,simple medianandweightedmedianmethodsusingthe5-SNPs instrument(Table5).Evidenceofheterogeneitywasobservedfor theoutcomeofCADandMI(Q = 42.5, p < 0.001; Q = 35.4, p < 0.001)butnotfortheoutcomeofischemicstrokeoritssubtypes (Q = 5.3, p = 0.26; Q = 6.6, p = 0.16; Q = 2.8, p = 0.59; Q = 2.0, p = 0.74)intheIVWanalysis.MR-Eggerregressionshowedno evidenceofdirectionalpleiotropyfortheassociationofCECwith alldiseaseoutcomes(all p valueforintercept >0.05)(Table5). InsensitivityanalysesusingFinnGenGWASdata,significant associationsforischemicheartdiseaseandMIandinsignificant associationforischemicstrokeandCESwereobservedusingthe SNPofrs141622900astheinstrument.However,nosignificant associationsfortheoutcomeswasobservedintheIVWanalysis usingthe5-SNPsinstrument(SupplementaryTable2).Inthe multivariableMRadjustingformajorcausesofsurvivalusingthe 5-SNPsinstrument,theassociationsofCECwithischemicstroke anditssubtypesremainedinsignificant,whichweresimilarto theestimatesbytheIVWmethod(SupplementaryTable3). Similarresultsofinsignificantassociationsforischemicstroke wereobservedintheUKBiobankdata(SupplementaryTable4).
DISCUSSION
Thepresentstudyisthefirstlarge-scaleassessmentand comparisonofcausalrelevanceofCECandtheriskofvascular
Jinetal. CholesterolEffluxCapacityandVascularDiseases
SNPs EA/OAIschemicstroke LAS SVS CES BetaSE p BetaSE p BetaSE P BetaSE p rs77069344G/T0.01530.01600.3390.04500.03960.2560.00620.03710.868 0.02070.03160.514 rs2070895A/G 0.00330.01210.783 0.05870.03040.0540.03670.02780.1870.01360.02350.563 rs247616T/C0.00820.01100.455 0.01680.02760.542 0.01190.02580.6450.01080.02120.609 rs964184C/G0.01810.01520.2330.00600.03730.872 0.00710.03490.8380.01220.02970.681 rs445925A/G 0.02980.01840.106 0.07230.04610.117 0.03650.04130.378 0.03620.03500.301 rs141622900A/G 0.05790.02540.023 0.09630.06790.156 0.07930.05980.1850.01780.05090.727
TABLE4| GeneticassociationofcholesteroleffluxcapacityrelatedgeneticvariantswithischemicstrokeanditssubtypesintheMEGASTROKEconsortium.
FrontiersinCardiovascularMedicine|www.frontiersin.org 4 July2022| Volume9|Article891148 12
diseaseusingMendelianrandomizationapproach.Theresults showedthat geneticpredictedhigherCECmaybeassociated withdecreasedriskofCAD.However,thecasualassociationof CECwithischemicstrokeandspecificsubtypeswouldneedtobe validatedinfurtherMendelianrandomizationstudies.

OurfindingsofinverserelationshipbetweenCECandCAD andMIareconsistentwithseveralmeta-analysissummarizing previousobservationalstudies(10, 26, 27).Althoughresults fromthemajorityofstudieswereinlinewiththehypothesis thathigherCECisassociatedwithlowerriskofCAD(9, 11, 28–31),astudybyLishowedthatincreasedHDLmediatedCECwas paradoxicallyassociatedwithincreased riskforincidentmyocardialinfarctionorstroke,whichbased onthestudypopulationundergoingcoronaryangiography (32).Moreover,theGermanDiabetesDialysisStudy(4D Study)failedto observeansignificantassociationofCEC withthecompositeoutcome(cardiacdeath,nonfatalMI,and stroke)inpatientswithend-stagerenaldisease(33).The CECwasquantified usinghumanTHP-1-derivedmacrophage foamcellsloadedwithcholesterol,whichwasdifferent fromcAMP(cyclicadenosinemonophosphate)-stimulated murineJ774macrophagesemployedbyotherstudiesinthe generalpopulation(8, 11).Thereasonsfortheapparent discrepanciesamong previousstudiesintherelationship betweenCECandCADareunclearbutcouldbeascribedto differenceinsamplesize,studypopulation,studydesign,and
methodsforCECmeasurementsacrossstudies.Considering theheterogeneitybetweenobservationalstudiesandpotential confoundersthatwarrantcaution,MRstudiesusinggenetic variantsasinstrumentalvariablescouldprovidemorerobust evidenceforthecausalrelationshipofCECandthehealth outcomeofinterest.Thepresentstudyshowedgeneticpredicted higherCECwasassociatedwithlowerCADrisk,whichsupports thedirectcausalassociationbetweenCECandCAD.
OurstudydoesnotsupportacausalroleofCECinischemic stroke.Fewstudieshaveinvestigatedtheassociationbetween CECandischemicstrokeanditssubtypesexpecttwocohort studieswithinconsistentresults(9, 29).Resultsfromthe MESA(Multi-EthnicStudy ofAtherosclerosis)cohortshowed norelationshipofcholesterolmasseffluxcapacitywithstroke orwithnon-hemorrhagicstroke.However,asmallsubgroup(n = 37)oftheDallasHeartstudyreportedaninverseassociation betweenCECandstroke.UsinggeneticvariantsrelatedtoCEC astheinstrument,theassociationbetweenCECandischemic strokewasexamineddirectlyinourstudy.However,wefound noevidenceofsignificantcausalrelationshipsbetweenCEC andischemicstrokeanditssubtypes.InthepresentMendelian randomizationstudy,theestimatesofCECwithischemicstroke mightbebiasedbysampleselectiononsurvivingexposureof interestandonsurvivingcompetingriskoftheoutcome(24). However,theresultsofmultivariableMRanalysesconducted byadjustingforpotentialcausesofsurvivalwereconsistent
Jinetal. CholesterolEffluxCapacityandVascularDiseases
FIGURE1| Causaleffectestimatesofgeneticallypredictedcholesteroleffluxcapacityoncoronaryarterydiseaseandstroke.Estimatesrepresentedoddsratio(95% CI)perSDgeneticallyhighercholesteroleffluxcapacityderivedfromWaldratiomethodusingrs141622900astheinstrumentandinverse-varianceweightedmethod using5-SNPs(rs77069344,rs2070895,rs247616,rs964184,andrs445925)astheinstrument.CI,confidenceinterval;SNP,singlenucleotidepolymorphism;CAD, coronaryarterydisease;MI,myocardialinfarction;IS,ischemicstroke;LAS,largearterystroke;SVS,smallvesselstroke;CES,cardioembolicstroke.
FrontiersinCardiovascularMedicine|www.frontiersin.org 5 July2022| Volume9|Article891148 13
nucleotidepolymorphism;CAD,coronaryarterydisease;MI,myocardialinfarction;IS,ischemicstroke; LAS,largearterystroke;SVS,smallvesselstroke;CES,cardioembolicstroke.
withthemainresults.Aswithanyselectionbiascorrectionby multivariableadjustment,itmaynotbefeasibletorecoverthe validestimate.WerepeatedtheanalysesintheUKBiobank dataandFinnGendata,respectively.Bothresultsweresimilar tothemainresultsinthepresentstudy.LargerscaleMendelian randomizationstudiesarestillneededtoclarifythegenetic effectsofCEConischemicstrokeandassessanyheterogeneity betweenischemicstrokesubtypes,whichmayshedlightonthe relationshipofCEConischemicstrokefurther.Furthermore, inthesefuturestudies,theeffectsofgeneticallydetermined CEConischemicstrokecouldbecomparedbetweenpatients withvascularcognitiveimpairmentvs.patientswithnoncognitiveimpairment.
TheHDL-mediatedCECistheabilitytoremoveexcess cholesterolfromlipid-ladenmacrophagesrepresentingthefirst crucialstepwithintheprocessofreversecholesteroltransport (7).Reversecholesteroltransportplaysanimportantrole inatheroprotective mechanismbyfacilitatingtheremovalof cholesterolinthearterialwallandthesubsequentdecreasein theproinflammatoryresponse(34, 35).Ourstudyfoundthatthe effectofCEC wasweakeronischemicstrokethanCAD,which wereconsistentlyobservedinsuchcomparisonsintheeffects ofotherbloodlipidonvasculardiseaseinrecentMendelian studies.AMendelianstudysuggestedthattheeffectsofLDL cholesterolonischemicstrokewasweakerthanthatoncoronary heartdisease(16).Moreover,anotherMendelianstudyshowed PCSK9geneticvariants hadsmallerassociationswithriskof ischemicstrokethanwithriskofcoronaryheartdisease(15).
Thepotentialexplanation forthedifferencebetweentheeffects ofCEConCADandischemicstrokeisthebiologicaldifferences inthesediseaseprocess.Ischemicstrokeinvolvesphenotypic heterogeneity,withdifferentbiologicalpathwaysforLAS,SVS, andCES,comparedtothemorehomogenousCADphenotype (36).Moreover,areviewreportedthathematologicaldisorders werethemost frequentetiologyofcerebralinfarctsofunusual cause(37).Exceptfortheusualcerebrovascularriskfactors suchas hypertension,diabetesmellitus,anddyslipidemia,other newlyfactorscouldbeconsideredofthecausalrelevancefor ischemicstroke.Furthermore,differencesinthedistribution ofriskfactorsaswellaspatientcharacteristicsbetween CARDIoGRAMPlusC4DandMETASTROKEconsortiummay partlyexplainthedifferenteffectsforCADandISobservedin
thestudy.Additionally,insufficientstatisticalpowerduetosmall samplesize,especiallyforstrokesubtypes,maybeconsidered asanotherreason.Anacetrapib,anCETP(cholesterylester transferprotein)inhibitorthatwasdevelopedforincreasing HDLcholesterollevelsandpromotingCECtoagreaterdegree, wasshownasignificantreductioninCADintheREVEAL trial(38).Althoughitmetitsprimaryendpoint,thesmall improvementagainst themaingoalandthesafetyofCETP inhibitorhadbecomeapointofcontentionconsistently.Finally, AnacetrapibwasnotfiledforapprovalwiththeUSFoodand DrugAdministration.Ourstudyprovidedsupportingevidence forthecausalrelationshipbetweenCECandriskofCAD, indicatingpotentialinterventiontargetstotheincreaseofCEC forimprovingcardiovascularoutcomes.
ThepresentMendelianrandomizationanalysesreliesonthree underlyingassumptions.First,weidentified6CEC-relatedSNPs (P < 6.25 × 10 9)servedasinstrumentsintheMRanalysis thatsatisfiedthefirstassumption.Second,wedidnotfindthat the5-SNPsinthestudywereassociatedwithotherkeylipids includinglow-densitylipoprotein,triglycerides,totalcholesterol, andapolipoproteinBbasedonGWASdatasets.Andtheresultsof multivariableMRanalysesandsensitivityanalysesusingtheUK BiobankdataandFinnGendatawereconsistentwiththemain results.Thus,theseresultsincreaseconfidenceinthevalidityof thesecondassumptionthatthereisnoconfounding(measured orunmeasured)ofthegeneticvariantswiththeoutcome.Third, allthegeneticvariantswerenotdirectlyassociatedwiththe outcomes(all P > 5 × 10 8),whichsuggestedthatthethird assumptionwasnotviolated.
Ourstudyhasseverallimitations.First,thestudywas conductedbasedondatasetsofpredominantlyEuropean ancestryandgeneralizationoftheresultstootherpopulations ofnon-Europeanancestrywaslimited.However,theuniformity oftheincludedsubjectsmayminimizetheriskofbiasby populationadmixture.Second,thesamplesizesofischemic strokesubtypeswerestillrelativelysmall,specificallyforSVS andCES.InsignificantassociationbetweenCECandischemic strokesubtypescouldbeattributedtoinsufficientstatistical power.However,mostestimateswereconsistentusingdifferent MRapproaches,whichsuggeststhattheobservedassociations arenotbychance.Third,althoughtheGWASthatweused toidentifyallCEC-relatedSNPsrepresentsthefirstandlargest
Jinetal. CholesterolEffluxCapacityandVascularDiseases
Outcome MR-Egger Simplemedian Weightedmedian OR(95%CI) P Intercept (95%CI) p valueforinterceptOR(95%CI) P OR(95%CI) p CAD(60,801/123,504)0.35(0.14–0.89)0.030.156( 0.007,0.319) 0.06 0.78(0.71–0.86) <0.0010.79(0.71–0.87) <0.001 MI(43,677/128,199)0.34(0.13–0.89)0.030.161( 0.004,0.326) 0.056 0.79(0.70–0.88) <0.0010.79(0.71–0.88) <0.001 IS(40,585/406,111)0.97(0.57–1.65)0.920.008( 0.083,0.100) 0.86 1.06(0.96–1.17)0.261.06(0.97–1.16)0.21 LAS(34,217/406,111)1.60(0.43–6.00)0.49 0.101( 0.330,0.128) 0.39 0.89(0.69–1.16)0.390.92(0.72–1.17)0.49 SVS(5,386/406,111)0.66(0.26–1.64)0.370.074( 0.086,0.233) 0.37 0.97(0.78–1.19)0.740.96(0.78–1.19)0.72 CES(7,193/406,111)0.79(0.36–1.71)0.550.048( 0.086,0.182) 0.48 1.08(0.90–1.28)0.411.08(0.91–1.28)0.41 MR,mendelianrandomization;OR,oddsratio;CI,confidenceinterval;SNP,single
*FiveSNPsintheinstrumentincludedrs77069344,rs2070895,rs247616,rs964184,andrs445925.
TABLE5| MRstatisticalsensitivityanalyses using5SNPsastheinstrumentalvariables*
FrontiersinCardiovascularMedicine|www.frontiersin.org 6 July2022| Volume9|Article891148 14
efforttoidentifygenome-widesignificantlociassociatedwith CEC,samplesize remainsmodest(N = 5,293participants), andthereforethereisalimitationofpowertofindweakeffect variants.Inthemainanalysis,onlyoneSNP(rs141622900)was usedastheinstrument.TheSNPisstronglyassociatedwith manykeylipidsrelevanttocardiovasculardisease,suchaslowdensitylipoproteinandapolipoproteinB.Thepleiotropiceffects oftheCEC-relatedSNPsonotherkeylipidswasunabletobe assessedbytwo-samplemultivariableMRinthepresentstudy becauseofthelackofdata.However,wehaveconductedMREggerregressionusing5CEC-relatedSNPsastheinstrumental variablestoassessevidenceofpleiotropiceffectsinthestudy. Thoughtheresultsshowednoevidenceofdirectionalpleiotropy, furtherstudiesareneededtovalidatetheassociationsofCEC withdiseaseoutcomes.Forth,weperformedmultivariableMR andsensitivityanalysestocorrectselectionbias.However, recoveringthevalidestimatesofCECforischemicstrokehas notbeenfullyaddressed.Finally,Mendelianrandomizationhas beenconsideredasanalternativeapproachforcausalinferences withalotofadvantagescomparedtorandomizedcontrolled trials.However,itcannotreplacerandomizedcontrolledtrials inestablishingaclaimofcausality(39).Futureclinicaltrialsare stillneededwith sufficientstatisticalpowertovalidatethecausal relationshipofCEC.
ThestudyexaminedcausalrelationshipsbetweenCECand riskofvasculardiseaseusingMRanalysis,andsuggeststhat geneticpredictedhigherCECmaybeassociatedwithdecreased riskofCAD.However,thecasualassociationofCECwith ischemicstrokeandspecificsubtypeswouldneedtobevalidated infurtherMendelianrandomizationstudies.
DATAAVAILABILITYSTATEMENT
Theoriginalcontributionspresentedinthestudyareincluded inthearticle/SupplementaryMaterial,furtherinquiriescanbe directedtothecorrespondingauthors.
ETHICSSTATEMENT
Ethicalreviewandapprovalwasnotrequiredforthestudy onhumanparticipantsinaccordancewiththelocallegislation andinstitutionalrequirements.Writteninformedconsentfor
REFERENCES
1.HuxleyRR,BarziF,LamTH,CzernichowS,FangX,WelbornT,etal. Isolatedlowlevelsofhigh-densitylipoproteincholesterolareassociatedwith anincreasedriskofcoronaryheartdisease:anindividualparticipantdata meta-analysisof23studiesintheAsia-Pacificregion. Circulation. (2011) 124:2056–64.doi:10.1161/CIRCULATIONAHA.111.028373
2.KeeneD,PriceC,Shun-ShinMJ,FrancisDP.Effectoncardiovascularriskof highdensitylipoproteintargeteddrugtreatmentsniacin,fibrates,andCETP inhibitors:meta-analysisofrandomisedcontrolledtrialsincluding117,411 patients. BMJ. (2014)349:g4379.doi:10.1136/bmj.g4379
3.BoekholdtSM,ArsenaultBJ,HovinghGK,MoraS,Pedersen TR,LarosaJC,etal.LevelsandchangesofHDLcholesteroland
participationwasnotrequiredforthisstudyinaccordancewith thenationallegislationandtheinstitutionalrequirements.
AUTHORCONTRIBUTIONS
AJandMWperformedthestudy,analyzedthedata,wrotethe paper,andrevieweddraftsofthepaper.WCandXXwrotethe paperandrevieweddraftsofthepaper.HYanalyzedthedata, preparedfiguresandtables,andrevieweddraftsofthepaper. YPconceivedanddesignedthestudy,performedthestudy, analyzedthedata,wrotethepaper,andrevieweddraftsofthe paper.Allauthorscontributedtothearticleandapprovedthe submittedversion.
FUNDING
ThisworkwassupportedbygrantsfromtheNationalNatural ScienceFoundationofChina(81971091and81901177), BeijingHospitalsAuthorityYouthProgramme(QML20190501 andQML20200501),YoungEliteScientistSponsorship ProgramfromChinaAssociationforScienceandTechnology (2019QNRC001),BeijingTiantanHospital,CapitalMedical University(2018-YQN-1and2020MP01)andOutstanding YoungTalentsProjectofCapitalMedicalUniversity(A2105).
ACKNOWLEDGMENTS
Dataoncholesteroleffluxcapacitywerederivedfromthe GWASperformedbyLow-Kametal.Dataoncoronary arterydisease/myocardialinfarctionhavebeencontributed byCARDIoGRAMplusC4Dinvestigatorsandobtainedfrom www.CARDIOGRAMPLUSC4D.ORG.Dataonstrokehave beencontributedbyMEGASTROKEinvestigatorsandobtained fromhttp://megastroke.org.TheMEGASTROKEproject receivedfundingfromsourcesspecifiedathttp://megastroke. org/acknowledgements.html.
SUPPLEMENTARYMATERIAL
TheSupplementaryMaterialforthisarticlecanbefound onlineat:https://www.frontiersin.org/articles/10.3389/fcvm. 2022.891148/full#supplementary-material
apolipoproteinA-Iinrelationtoriskofcardiovascularevents amongstatin-treatedpatients:ameta-analysis. Circulation. (2013) 128:1504–12.doi:10.1161/CIRCULATIONAHA.113.002670
4.VoightBF,PelosoGM,Orho-MelanderM,Frikke-SchmidtR,Barbalic M,JensenMK,etal.PlasmaHDLcholesterolandriskofmyocardial infarction:amendelianrandomisationstudy. Lancet. (2012)380:572–80.doi:10.1016/S0140-6736(12)60312-2
5.HolmesMV,AsselbergsFW,PalmerTM,DrenosF,LanktreeMB,NelsonCP, etal.Mendelianrandomizationofbloodlipidsforcoronaryheartdisease. Eur HeartJ. (2015)36:539–50.doi:10.1093/eurheartj/eht571
6.AnastasiusM,KockxM,JessupW,SullivanD,RyeKA,KritharidesL. Cholesteroleffluxcapacity:anintroductionforclinicians. AmHeartJ. (2016) 180:54–63.doi:10.1016/j.ahj.2016.07.005
Jinetal. CholesterolEffluxCapacityandVascularDiseases
FrontiersinCardiovascularMedicine|www.frontiersin.org 7 July2022 | Volume9|Article891148 15
7.RosensonRS,BrewerHBJr,DavidsonWS,FayadZA,FusterV, GoldsteinJ,etal.Cholesteroleffluxandatheroprotection:advancingthe conceptofreversecholesteroltransport. Circulation. (2012)125:1905–19.doi:10.1161/CIRCULATIONAHA.111.066589
8.KheraAV,CuchelM,delaLlera-MoyaM,RodriguesA,Burke MF,JafriK,etal.Cholesteroleffluxcapacity,high-density lipoproteinfunction,andatherosclerosis. NEnglJMed. (2011) 364:127–35.doi:10.1056/NEJMoa1001689
9.RohatgiA,KheraA,BerryJD,GivensEG,AyersCR,WedinKE,etal.HDL cholesteroleffluxcapacityandincidentcardiovascularevents. NEnglJMed. (2014)371:2383–93.doi:10.1056/NEJMoa1409065
10.YeH,XuG,RenL,PengJ.Cholesteroleffluxcapacityin coronaryarterydisease:ameta-analysis. CoronArteryDis. (2020) 31:642–9.doi:10.1097/MCA.0000000000000886
11.SaleheenD,ScottR,JavadS,ZhaoW,RodriguesA,PicataggiA,etal. AssociationofHDLcholesteroleffluxcapacitywithincidentcoronaryheart diseaseevents:aprospectivecase-controlstudy. LancetDiabetesEndocrinol. (2015)3:507–13.doi:10.1016/S2213-8587(15)00126-6
12.LawlorDA,HarbordRM,SterneJA,TimpsonN,DaveySmithG.Mendelian randomization:usinggenesasinstrumentsformakingcausalinferencesin epidemiology. StatMed. (2008)27:1133–63.doi:10.1002/sim.3034
13.BareinboimE,PearlJ.Causalinferenceandthedata-fusionproblem. Proc NatlAcadSciUSA. (2016)113:7345–52.doi:10.1073/pnas.1510507113
14.BoehmeAK,EsenwaC,ElkindMS.Strokeriskfactors, genetics,andprevention. CircRes. (2017)120:472–95.doi:10.1161/CIRCRESAHA.116.308398
15.HopewellJC,MalikR,Valdés-MárquezE,WorrallBB,CollinsR; METASTROKECollaborationoftheISGC.DifferentialeffectsofPCSK9 variantsonriskofcoronarydiseaseandischaemicstroke. EurHeartJ. (2018) 39:354–9.doi:10.1093/eurheartj/ehx373
16.Valdes-MarquezE,ParishS,ClarkeR,StariT,WorrallBB;METASTROKE ConsortiumoftheISGC,etal.RelativeeffectsofLDL-Conischemicstroke andcoronarydisease:aMendelianrandomizationstudy. Neurology. (2019) 92:e1176–87.doi:10.1212/WNL.0000000000007091
17.Low-KamC,RhaindsD,LoKS,BarhdadiA,BouléM,AlemS,etal. VariantsattheAPOE/C1/C2/C4locusmodulatecholesteroleffluxcapacity independentlyofhigh-densitylipoproteincholesterol. JAmHeartAssoc. (2018)7:e009545.doi:10.1161/JAHA.118.009545
18.NikpayM,GoelA,WonHH,HallLM,WillenborgC,KanoniS,etal.A comprehensive1,000Genomes-basedgenome-wideassociationmeta-analysis ofcoronaryarterydisease. NatGenet. (2015)47:1121–30.doi:10.1038/ng.3396
19.MalikR,ChauhanG,TraylorM,SargurupremrajM,OkadaY,MishraA,et al.Multiancestrygenome-wideassociationstudyof520,000subjectsidentifies 32lociassociatedwithstrokeandstrokesubtypes. NatGenet. (2018)50:524–37.doi:10.1038/s41588-018-0058-3
20.ThomasDC,LawlorDA,ThompsonJR.Re:estimationofbiasinnongenetic observationalstudiesusing“Mendeliantriangulation”byBautistaetal. Ann Epidemiol. (2007)17:511–3.doi:10.1016/j.annepidem.2006.12.005
21.BurgessS,ButterworthA,ThompsonSG.Mendelianrandomizationanalysis withmultiplegeneticvariantsusingsummarizeddata. GenetEpidemiol. (2013)37:658–65.doi:10.1002/gepi.21758
22.BowdenJ,DaveySmithG,BurgessS.Mendelianrandomizationwithinvalid instruments:effectestimationandbiasdetectionthroughEggerregression. IntJEpidemiol. (2015)44:512–25.doi:10.1093/ije/dyv080
23.BowdenJ,DaveySmithG,HaycockPC,BurgessS.Consistent estimationinmendelianrandomizationwithsomeinvalidinstruments usingaweightedmedianestimator. GenetEpidemiol. (2016) 40:304–14.doi:10.1002/gepi.21965
24.SchoolingCM,LopezPM,YangZ,ZhaoJV,AuYeungSL,Huang JV.Useofmultivariablemendelianrandomizationtoaddressbiases duetocompetingriskbeforerecruitment. FrontGenet. (2020) 11:610852.doi:10.3389/fgene.2020.610852
25.SmitRAJ,TrompetS,DekkersOM,JukemaJW,leCessieS.Survivalbiasin mendelianrandomizationstudies:athreattocausalinference. Epidemiology. (2019)30:813–6.doi:10.1097/EDE.0000000000001072
26.QiuC,ZhaoX,ZhouQ,ZhangZ.High-densitylipoproteincholesterol effluxcapacityisinverselyassociatedwithcardiovascularrisk:
asystematicreviewandmeta-analysis. LipidsHealthDis. (2017) 16:212.doi:10.1186/s12944-017-0604-5
27.Soria-FloridoMT,SchroderH,GrauM,FitoM,LassaleC.High densitylipoproteinfunctionalityandcardiovasculareventsandmortality: asystematicreviewandmeta-analysis. Atherosclerosis. (2020)302:36–42.doi:10.1016/j.atherosclerosis.2020.04.015
28.Soria-FloridoMT,CastanerO,LassaleC,EstruchR,Salas-SalvadoJ,MartinezGonzalezMA,etal.Dysfunctionalhigh-densitylipoproteinsareassociated withagreaterincidenceofacutecoronarysyndromeinapopulationat highcardiovascularrisk:anestedcase-controlstudy. Circulation. (2020) 141:444–53.doi:10.1161/CIRCULATIONAHA.119.041658
29.SheaS,SteinJH,JorgensenNW,McClellandRL,TascauL,ShragerS,et al.Cholesterolmasseffluxcapacity,incidentcardiovasculardisease,and progressionofcarotidplaque. ArteriosclerThrombVascBiol. (2019)39:89–96.doi:10.1161/ATVBAHA.118.311366
30.EbtehajS,GruppenEG,BakkerSJL,DullaartRPF,TietgeUJF.HDL(HighDensityLipoprotein)cholesteroleffluxcapacityisassociatedwithincident cardiovasculardiseaseinthegeneralpopulation. ArteriosclerThrombVasc Biol. (2019)39:1874–83.doi:10.1161/ATVBAHA.119.312645
31.ZhangJ,XuJ,WangJ,WuC,XuY,WangY,etal.Prognosticusefulnessof serumcholesteroleffluxcapacityinpatientswithcoronaryarterydisease. Am JCardiol. (2016)117:508–14.doi:10.1016/j.amjcard.2015.11.033
32.LiXM,TangWH,MosiorMK,HuangY,WuY,MatterW,etal. Paradoxicalassociationofenhancedcholesteroleffluxwithincreased incidentcardiovascularrisks. ArteriosclerThrombVascBiol. (2013)33:1696–705.doi:10.1161/ATVBAHA.113.301373
33.KopeckyC,EbtehajS,GenserB,DrechslerC,KraneV,AntlangerM,etal. HDLcholesteroleffluxdoesnotpredictcardiovascularriskinhemodialysis patients. JAmSocNephrol. (2017)28:769–75.doi:10.1681/ASN.2016030262
34.Vallejo-VazAJ,RayKK.Cholesteroleffluxcapacityasanovel biomarkerforincidentcardiovascularevents:hashigh-density lipoproteinbeenresuscitated? CircRes. (2015)116:1646–8.doi:10.1161/CIRCRESAHA.115.305938
35.FeigJE,HewingB,SmithJD,HazenSL,FisherEA.High-densitylipoprotein andatherosclerosisregression:evidencefrompreclinicalandclinicalstudies. CircRes. (2014)114:205–13.doi:10.1161/CIRCRESAHA.114.300760
36.MendelsonSJ,PrabhakaranS.Diagnosisandmanagementoftransient ischemicattackandacuteischemicstroke:areview. JAMA. (2021)325:1088–98.doi:10.1001/jama.2020.26867
37.ArboixA,JiménezC,MassonsJ,ParraO,BessesC.Hematologicaldisorders: acommonlyunrecognizedcauseofacutestroke. ExpertRevHematol. (2016) 9:891–901.doi:10.1080/17474086.2016.1208555
38.BowmanL,HopewellJC,ChenF,WallendszusK,StevensW,CollinsR,etal. Effectsofanacetrapibinpatientswithatheroscleroticvasculardisease. NEngl JMed. (2017)377:1217–27.doi:10.1056/NEJMoa1706444
39.PlotnikovD,GuggenheimJA.Mendelianrandomisationandthegoal ofinferringcausationfromobservationalstudiesinthevisionsciences. OphthalmicPhysiolOpt.(2019)39:11–25.doi:10.1111/opo.12596
ConflictofInterest: Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcouldbeconstruedasa potentialconflictofinterest.
Publisher’sNote: Allclaimsexpressedinthisarticlearesolelythoseoftheauthors anddonotnecessarilyrepresentthoseoftheiraffiliatedorganizations,orthoseof thepublisher,theeditorsandthereviewers.Anyproductthatmaybeevaluatedin thisarticle,orclaimthatmaybemadebyitsmanufacturer,isnotguaranteedor endorsedbythepublisher.
Copyright©2022Jin,Wang,Chen,Yan,XiangandPan.Thisisanopen-access articledistributedunderthetermsoftheCreativeCommonsAttributionLicense(CC BY).Theuse,distributionorreproductioninotherforumsispermitted,provided theoriginalauthor(s)andthecopyrightowner(s)arecreditedandthattheoriginal publicationinthisjournaliscited,inaccordancewithacceptedacademicpractice. Nouse,distributionorreproductionispermittedwhichdoesnotcomplywiththese terms.
Jinetal. CholesterolEffluxCapacityandVascularDiseases
FrontiersinCardiovascularMedicine|www.frontiersin.org 8 July2022| Volume9|Article891148 16
Editedby: AlexanderAkhmedov, UniversityofZurich,Switzerland
Reviewedby: YangPeng, TheUniversityof Queensland,Australia DongfengZhang, QingdaoUniversity,China
*Correspondence: Min-ZhouZhang minzhouzhang@gzucm.edu.cn
Specialtysection:
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine

Received: 24March2022
Accepted: 06June2022
Published: 07July2022
Citation:
ZengR-X,XuJ-P,KongY-J,TanJ-W, GuoL-HandZhangM-Z(2022)
U-ShapedRelationshipofNon-HDL CholesterolWithAll-Causeand CardiovascularMortalityinMen WithoutStatinTherapy. Front.Cardiovasc.Med.9:903481. doi:10.3389/fcvm.2022.903481
U-ShapedRelationshipofNon-HDL CholesterolWithAll-Causeand CardiovascularMortalityinMen WithoutStatinTherapy
Rui-XiangZeng 1,2,Jun-PengXu 1,2,Yong-JieKong 1,2,Jia-WeiTan 1,2,Li-HengGuo 1,2 and Min-ZhouZhang 1,2*
1 TheSecondClinicalCollegeofGuangzhouUniversityofChineseMedicine,Guangzhou,China, 2 DepartmentofCriticalCare Medicine,GuangdongProvincialHospitalofChineseMedicine,Guangzhou,China
Background: Non-HDL-Ciswellestablishedcausalriskfactorfortheprogressionof atheroscleroticcardiovasculardisease.However,thereremainsacontroversialpatternof hownon-HDL-Crelatestoall-causeandcardiovascularmortality,andtheconcentration ofnon-HDL-Cwheretheriskofmortalityislowestisnotdefined.
Methods: Apopulation-basedcohortstudyusingdatafromtheNationalHealthand NutritionExaminationSurvey(NHANES)from1999to2014.Maleparticipantswithout statintherapyweredividedintothesixgroupsaccordingtonon-HDL-Clevels(<100, 100–129,130–159,160–189,190–219, ≥220mg/dl).MultivariableCoxproportional hazardsmodelswereconductedwithahazardratio(HR)andcorresponding95% confidenceinterval(CI).Tofurtherexploretherelationshipbetweennon-HDL-Cand mortality,Kaplan–Meiersurvivalcurves,restrictedcubicsplinecurves,andsubgroup analysiswereperformed.
Results: Among12,574individuals(averageage44.29 ± 16.37years),1,174(9.34%) deathsduringamedianfollow-up98.38months.Bothlowandhighnon-HDL-Clevels weresignificantlyassociatedwithincreasedriskofall-causeandcardiovascularmortality, indicatingaU-shapedassociation.Thresholdvaluesweredetectedat144mg/dlforallcausemortalityand142mg/dlforcardiovascularmortality.Belowthethreshold,per 30mg/dlincreaseinnon-HDL-Creduceda28and40%increasedriskofall-cause (p < 0.0001)andcardiovascularmortality(p = 0.0037),respectively.Inversely,above thethreshold,per30mg/dlincreaseinnon-HDL-Cacceleratedriskofbothall-cause mortality(HR1.11,95%CI1.03–1.20, p = 0.0057)andcardiovascularmortality(HR 1.30,95%CI1.09–1.54, p = 0.0028).
Conclusions: Non-HDL-CwasU-shapedrelatedtoall-causeandcardiovascular mortalityamongmenwithoutstatintherapy.
Keywords:non-HDLcholesterol,all-causemortality,cardiovascularmortality,U-shapedrelationship,men
ORIGINALRESEARCH published:07July 2022
FrontiersinCardiovascularMedicine|www.frontiersin.org 1 July2022 | Volume9|Article903481
doi:10.3389/fcvm.2022.903481
17
INTRODUCTION
Non-high-densitylipoproteincholesterol(non-HDL-C),which representsthetotalcholesterolcontentofapolipoprotein B-containinglipoproteins,includesverylow-densitylipoproteins (VLDL)andtheirmetabolicremnants,intermediate-density lipoproteins(IDL),lipoprotein(a),andlow-densitylipoproteins (LDL)(1, 2).Non-HDL-Ciswellestablishedcausalriskfactor forthedevelopmentofatheroscleroticcardiovasculardisease (1, 3, 4).Twodecadesago,non-HDL-Cwashighlightedasan importantsecondarylipidtherapeuticgoalintheUnitedStates NationalCholesterolEducationProgram’sAdultTreatment Panel(5).Furthermore,theNationalLipidAssociationand InternationalAtherosclerosis Societyrecentlyrecommendedthat non-HDL-CshouldbeanequaltargettoLDL-cholesterol(LDLC)inpatientswithatheroscleroticcardiovasculardisease(6). Preponderantly,non-HDL-Ctrajectoriesremainfairlyflat,and non-HDL-Cbetweenage25and40yearsissufficientto confidentlycategoriseindividualsashighorlownon-HDL-C forthenext25to30years(3).Moreover,non-HDL-Ccan beaccurately calculatedinanon-fastingspecimen,without incurringadditionalexpense(7).
Asystematicreviewand meta-analysisbyourteampreviously demonstratedthattheincreasedlevelsofnon-HDL-Cwere significantlyassociatedwithanincreasedriskofmortalityin patientswithcoronaryheartdisease(CHD)(8),whichwas similartoprevious researchfindingsinpopulationwithout cardiovasculardiseases(9)orpatientswithdiabetes(10). Interestingly,aU-shapedrelationshipwasalsorecentlyidentified indifferentpopulations(11, 12).StudiesdetectedtheU-shaped associationofnon-HDL-Cwithall-causeandcardiovascular mortalityamongpatientswithhypertensionorchronickidney disease(CKD)stages3–5(11, 12),non-HDL-CwasU-shaped associatedwith mortalityamongmalehypertensioninsubgroup analysis,butnotinfemale(12).Differently,onestudyfocused onthemen population,fromtheIsraeliNationalDeathRegistry demonstratedapositiveassociationthatnon-HDL-Casauseful predictorofcardiovasculardiseasemortalityin22yearsfollowup(13).Hence,formoreclearlyunderstandhownon-HDLCrelatestoall-causeandcardiovascularmortalityinmen,the aimofthepresentstudyuseddatafromthelargepopulation representativesurveystodeterminetherelationshipofnonHDL-Cwithall-causeandcardiovascularmortality,andthe concentrationofnon-HDL-Cassociatedwiththelowestriskof mortalityinmenwithoutstatintherapy.
MATERIALSANDMETHODS StudyPopulation
Weperformedapopulation-basedcohortstudyusingdata fromtheNationalHealthandNutritionExaminationSurvey (NHANES),anddatawerecombinedacross8continuous NHANEScycles:1999–2000,2001–2002,2003–2004,2005–2006, 2007–2008,2009–2010,2011–2012,and2013–2014.NHANES isaseriesofnationalsurveystoevaluatethehealthstatus oftheUnitedStatespopulationwithacomplex,stratified, multistage,probabilitysamplingmethod.TheCentresfor
DiseaseControlandPreventionratifiedthestudyprotocols,and alltheparticipantsprovidedwritteninformedconsent.Detailed abouttheNHANEShasbeenpublishedelsewhere(14, 15).
Thetotalnumber ofparticipantsinprimarysurveywas 82,091.Afterexcludingparticipantsforage <18(n = 34,735), female(n = 24,534)orwithoutfollow-updata(n = 1,085), andthoseinbaselinewithcancerormissingdata(n = 3,534), acutemyocardialinfarction(AMI)ormissingdata(n = 952), heartfailure(HF)ormissdata(n = 331)andexcluding becausecovariateswereunavailable(n = 2,455),finallyexcluding forbaselinewithstatintherapy(n = 1,891).Hence,12,574 individualswereincludedinourfinalanalysis(Figure1).
Covariates
Fastingsamplesobtainedfromperipheralvenousbloodwere storedat 20◦Candshippedweeklyforlaboratoryanalyses. Non-HDL-Clevelswerecalculatedfromtotalcholesterol (TC)minusHDLcholesterol(HDL-C).Themeasurementof TCusedwithanenzymaticassaymethod,andHDL-Cwas usedwithaheparin-manganeseprecipitationmethodora directimmunoassaytechnique.Furtherdetailedinformation aboutthecollectionofbloodsamplesandlipidconcentration measurementisavailableinanotherstudy(16).Inaddition, creatinine,haemoglobin,andglycatedhaemoglobinA1c (HbA1c)measurementswerebasedonstandardisedprocedures. Demographicvariablessuchasage,gender,bodyweight, height,race/ethnicity(MexicanAmerican,otherHispanic, non-HispanicWhite,non-HispanicBlack,otherrace),education (Lowerthanhighschool,highschool,morethanhighschool), wereacquiredaccordingtothehouseholdinterview.Information onsmokingstatus(currentsmoker,formersmoker,andnever smoked),andhistoryofdiseasehadbeenassessedatbaselineby standardexaminations,andquestionnaireswereadministered bytrainedhealthtechnicians,interviewers,andphysicians.The meanbloodpressurewascalculatedastheaverageofthreevalid measurements.Nutritionalstatus(e.g.,energyintake,protein intake,carbohydrateintake,totalfatintake)wasacquired accordingtothedietaryinterview.A“multiplepass”24-hdietary interviewformatwasusedtocollectdetailedinformationabout allfoodsandbeverages,whichwereusedtoestimatethetotal intakeofenergy,nutrients,andnon-nutrientfoodcomponents. Moreinformationisavailableatwww.cdc.gov/nchs/nhanes.
Outcomes
Theprimarydeterminationofmortalityforeligibleparticipants isbaseduponmatchingsurveyrecordstotherecordsfromthe NationalDeathIndex(NDI),andothersourcesincludingthe SocialSecurityAdministration,theCentresforMedicareand MedicaidServices,datacollection,andforthefollow-upsurveys oftheNationalCentreforHealthStatistics,ascertainment ofdeathcertificatesarealsoincorporated.Participantswere eligibleformortalityfollow-upbasedonmatchingidentifying informationduringtheirNHANESinterviews,suchasthelast 4digitsofsocialsecuritynumber,fullname,dateofbirth,state ofbirth,stateofresidence,maritalstatus,race,andsex(17).Allcausemortality and cardiovascularmortalityweretheendpoints ofthepresentstudy.Themortalitystatusofindividualswas
Zengetal. Non-HDL-CandMortalityinMen
FrontiersinCardiovascularMedicine|www.frontiersin.org 2 July2022| Volume9|Article903481 18
obtainedfromdatafromtheNDIthroughDecember31,2015. Thisstudyclassifiedcausesofmortalityreferredtothecodesof theinternationalstatisticalclassificationofdiseases,10threvision (ICD-10).CardiovascularmortalitywasdefinedbytheICD10codesforasI00-I09,I11,I13,andI20-I51.Whentreating cardiovascularmortalityasanoutcome,thedeathsduetoother causeswerecensored.

StatisticalAnalysis
Thedatawerepresentedasmeanvalueswithstandarddeviation (SD),themedianwithinterquartileranges,orfrequencieswith percentages,asappropriate.Comparisonsofthedifferences betweengroupsweremadewithone-wayANOVA,chi-square tests,orKruskal–WallisH-testsbytheclassificationofnon-HDLClevels(<100,100–129,130–159,160–189,190–219, ≥220 mg/dl).Survivalanalysisaccordingtonon-HDL-Cstratification wasperformedusingstandardisedKaplan–Meiercurves.The proportionalhazardassumptionwasexaminedandmetby plottingthelogminuslogsurvivalcurvesandsurvivaltimes. ThemultivariableCoxproportionalhazardsmodelswereused
forexploringtheassociationofnon-HDL-Cwithall-causeand cardiovascularmortality.Inmodel1,therewasnoadjustment. Inmodel2,weadjustedforageandrace.Inmodel3,we adjustedforage,race,education,bodymassindex(BMI),systolic bloodpressure,diastolicbloodpressure,smoking,diabetes, hypertension,CHD,stroke,creatinine,haemoglobin,HbA1c, triglycerides,energyintake,proteinintake,carbohydrateintake, andtotalfatintake.Restrictedcubicsplinemodelswereused fornonlinearrelationshipswithknotsat5,35,65,and95 percentilesofnon-HDL-C.Iftherelationshipswerenon-linear, thedifferenceofrelationshipsatthethresholdwasdetected bytwopiecewiselinearregressionmodels.Thepointwiththe highestlikelihoodamongallthepossiblevalueswaschosento definethethresholdvalue.Thedifferencesintheresultswhen applyingone-lineortwopiecewiselinearregressionmodelswere comparedbyalogarithmiclikelihoodratiotest.Furthermore, thesubgroupanalysisincludesage(<65or ≥65years),race (White,Black,orotherrace),education(lowerthanhighschool, highschool,morethanhighschool),BMI(<25or ≥25kg/m2), smoking(yesorno),diabetes(yesorno),hypertension(yesor
Zengetal. Non-HDL-CandMortalityinMen
FIGURE1| Studyflowchart.NHANES,Nationalhealthandnutritionexaminationsurvey;AMI,acutemyocardialinfarction;HF,heartfailure.
FrontiersinCardiovascularMedicine|www.frontiersin.org 3 July2022| Volume9|Article903481 19
Non-HDL-C,non-high-densitylipoproteincholesterol;N,number;SBP,systolicbloodpressure;DBP,diastolicbloodpressure;HbA1c,glycatedhaemoglobinA1c;TC,totalcholesterol;HDL-C,high-densitylipoproteincholesterol; LDL-C,low-densitylipoproteincholesterol.
Valuesareexpressedasthemean ± SD,themedianwithinterquartilerangeorn(%).
Zengetal. Non-HDL-CandM ortalityinMen
Non-HDL-C,mg/dl P-value Total <100100–129 130–159 160–189 190–219 ≥220 N 12,574 1,298 2,690 3,644 2,786 1,400 756 Age,years 44.29 ± 16.3736.87 ± 16.9841.77 ± 17.4245.24 ± 16.47 46.79 ± 15.43 46.93 ± 14.10 47.26 ± 12.92 <0.001 Race <0.001 White 5,562(44.23%)534(41.14%)1,138(42.30%)1,671(45.86%) 1,230(44.15%) 626(44.71%) 363(48.02%) Black 2,539(20.19%)437(33.67%)663(24.65%) 685(18.80%) 461(16.55%) 185(13.21%) 108(14.29%) Other 4,473(35.57%)327(25.19%)889(33.05%) 1,288(35.35%) 1,095(39.30%) 589(42.07%) 285(37.70%) Education <0.001 Lowerthanhigh school 3,565(28.35%)322(24.81%)738(27.43%) 977(26.81%) 863(30.98%) 417(29.79%) 248(32.80%) Highschool 3,027(24.07%)330(25.42%)645(23.98%) 863(23.68%) 644(23.12%) 361(25.79%) 184(24.34%) Morethanhigh school 5,982(47.57%)646(49.77%)1,307(48.59%)1,804(49.51%) 1,279(45.91%) 622(44.43%) 324(42.86%) Bodymassindex, kg/m2 28.09 ± 5.6925.30 ± 5.2126.92 ± 5.94 28.47 ± 5.84 29.04 ± 5.37 29.29 ± 4.96 29.44 ± 4.91 <0.001 SBP,mmHg 124.19 ± 16.20120.82 ± 15.59122.57 ± 15.83124.33 ± 16.35125.22 ± 16.35 126.02 ± 15.49 127.92 ± 16.89 <0.001 DBP,mmHg 72.83 ± 11.8668.65 ± 11.7770.76 ± 11.6072.77 ± 11.89 74.34 ± 11.25 75.41 ± 11.65 77.21 ± 11.86 <0.001 Smoking 6,673(53.07%)660(50.85%)1,378(51.23%)1,888(51.81%) 1,456(52.26%) 810(57.86%) 481(63.62%) <0.001 Diabetes 765(6.08%)68(5.24%) 171(6.36%) 208(5.71%) 171(6.14%) 85(6.07%) 62(8.20%) 0.116 Hypertension2,921(23.23%)217(16.72%)568(21.12%) 867(23.79%) 695(24.95%) 359(25.64%) 215(28.44%) <0.001 Coronaryheart disease 99(0.79%) 8(0.62%) 26(0.97%) 22(0.60%) 27(0.97%) 9(0.64%) 7(0.93%) 0.428 Stroke 178(1.42%) 9(0.69%) 33(1.23%) 57(1.56%) 41(1.47%) 23(1.64%) 15(1.98%) 0.133 Creatinine,mg/dl86.46 ± 34.0787.25 ± 33.3186.99 ± 31.7585.80 ± 26.28 86.57 ± 30.04 86.60 ± 55.42 85.77 ± 38.440.689 Haemoglobin,g/l15.23 ± 1.1814.82 ± 1.3015.07 ± 1.24 15.24 ± 1.16 15.35 ± 1.11 15.46 ± 1.05 15.54 ± 1.14 <0.001 HbA1c,% 5.58 ± 0.995.33 ± 0.72 5.46 ± 0.84 5.55 ± 0.89 5.63 ± 0.94 5.75 ± 1.18 6.08 ± 1.67 <0.001 Triglyceride,mg/dl124.04 (82.04–198.02) 66.01 (49.00–92.06) 92.06(66.98–131.04)122.00(86.03–176.05)156.02(108.00–231.07)196.07(134.05–293.09)265.58(178.09–456.62) <0.001 TC,mg/dl 199.05 ± 42.07139.13 ± 20.04167.38 ± 16.61192.14 ± 15.25218.15 ± 14.29 245.97 ± 13.75 290.67 ± 42.03 <0.001 HDL-C,mg/dl47.84 ± 14.0055.01 ± 17.0451.57 ± 15.0547.56 ± 13.19 44.94 ± 11.96 43.74 ± 11.35 41.96 ± 10.90 <0.001 LDL-C,mg/dl121.54 ± 35.3769.56 ± 12.9695.66 ± 12.49118.73 ± 14.48142.30 ± 14.99 165.13 ± 16.73 202.66 ± 38.87 <0.001 Non-HDL-C, mg/dl 151.21 ± 43.5484.13 ± 12.14115.81 ± 8.54144.57 ± 8.65 173.22 ± 8.48 202.23 ± 8.50 248.70 ± 41.75 <0.001 Energyintake,kcal2,562.21 ± 1,148.91 2,698.28 ± 1,208.16 2,599.52 ± 1,201.382,554.34 ± 1,129.652,497.82 ± 1,080.052,537.78 ± 1,172.342,516.30 ± 1,127.45 <0.001 Proteinintake,gm98.00 ± 49.24100.23 ± 53.7599.25 ± 51.7297.09 ± 47.13 96.43 ± 47.57 99.31 ± 48.32 97.44 ± 49.750.088 Carbohydrate intake,gm 307.40 ± 147.76326.10 ± 155.89310.65 ± 152.37308.76 ± 148.86299.71 ± 137.13301.92 ± 151.54295.73 ± 138.69 <0.001 Totalfatintake,gm94.34 ± 53.2797.27 ± 55.1195.99 ± 55.4893.91 ± 52.47 92.72 ± 50.79 93.45 ± 53.11 93.17 ± 54.830.073 All-causemortality1,174(9.34%)115(8.86%) 258(9.59%) 312(8.56%) 277(9.94%) 134(9.57%) 78(10.32%)0.381 Cardiovascular mortality 184(1.46%)21(1.62%) 39(1.45%) 41(1.13%) 41(1.47%) 23(1.64%) 19(2.51%) 0.101
TABLE1| Baselinecharacteristicsaccordingtonon-HDL-Clevels.
FrontiersinCardiovascularMedicine|www.frontiersin.org 4 July2022| Volume9|Article903481
100mg/dl(P = 0.3687).
no).AllstatisticalanalyseswereperformedusingRversion3.3.2 (RFoundationfor StatisticalComputing,Vienna,Austria),and p < 0.05wasconsideredstatisticallysignificant.
RESULTS
BaselineCharacteristics
Finally,12,574individuals(averageage44.29 ± 16.37years) wereincludedinthisanalysis. Table1 demonstratesbaseline characteristicsaccordingtonon-HDL-Clevels.Therewere significantsubgroupdifferencesinage,race,education,BMI, systolicbloodpressure,diastolicbloodpressure,smoking, hypertension,haemoglobin,HbA1C,triglycerides,TC,HDL-C, LDL-C,energyintake,andcarbohydrateintake(all p <0.001), exceptfordiabetes,CHD,stroke,creatinine,proteinintake,total fatintake,all-cause,andcardiovascularmortality.Wefound 1,174(9.34%)all-causedeathsand184(1.46%)cardiovascular deathsduringamedianfollow-upof98.38 ± 53.78months.
RelationshipsofNon-HDL-CWith All-CauseandCardiovascularMortality
Asdemonstratedin Figure2,Kaplan–Meiersurvivalcurveswere divergedaccordingtonon-HDL-Cstratification.Thehighestrisk forall-causemortalitywasobservedwhennon-HDL-C <100 mg/dl,comparedtoothergroups(all p < 0.005).Besides,more riskforcardiovascularmortalitywasonlyobservedwhennonHDL-C <100mg/dl,comparedtowhennon-HDL-C = 130159or160-189mg/dl(all P < 0.005).Theoptimalnon-HDL-C
concentrationrangewasbetween130and159mg/dlforalower riskofall-causeandcardiovasculardeath.
Table2 summarisedthemultivariableCoxregressionresults. Whennon-HDL-Cwastreatedasacontinuousvariable,per 30mg/dlincrementinnon-HDL-Ccorrespondedtothehazard ratio(HR)(95%confidenceinterval,CI)as0.94(95%CI0.90–0.99, p = 0.0193)forall-causemortalityand1.03(95%CI0.91–1.16, p = 0.6826)forcardiovascularmortalityinmodel3.When non-HDL-Cwastreatedasacategoricalvariable,non-HDL-C = 130-159mg/dlasareference,thefullyadjustedHRsforallcausemortalitywere1.98(95%CI1.59–2.48, p < 0.0001),1.38 (95%CI1.17–1.62, p = 0.0002),1.11(95%CI0.95–1.31, p = 0.2007),1.12(95%CI0.91–1.38, p = 0.2807)and1.04(95%CI 0.79–1.36, p = 0.8018)fornon-HDL-Clevels <100,100–129, 160–189,190–219,and ≥220mg/dl,respectively.Meanwhile,for cardiovascularmortality,thefullyadjustedHRswere2.99(95% CI1.73–5.16, p < 0.0001),1.72(95%CI1.10–2.69, p = 0.0169), 1.36(95%CI0.88–2.11, p = 0.1636),1.61(95%CI0.94–2.73, p = 0.0811),and2.16(95%CI1.17–3.98, p = 0.0134)fornon-HDLClevels <100,100–129,160–189,190–219,and ≥ 220mg/dl, respectively.
Non-LinearRelationshipsofNon-HDL-C WithAll-CauseandCardiovascular Mortality
Asshownin Figure3,themultivariableadjustedrestrictive cubiccurvesconfirmedthattherelationshipsofnon-HDL-C

Zengetal. Non-HDL-CandMortalityinMen
FIGURE2| Kaplan-Meiersurvivalcurves ofnon-HDL-Cwithall-cause (A) andcardiovascular (B) mortality.Non-HDL-C,non-high-densitylipoproteincholesterol. (A) Othergroupsvs.non-HDL-C <100mg/dl(all P < 0.005). (B) non-HDL-C = 130–159or160–189mg/dlvs.non-HDL-C <100mg/dl(all P < 0.005),non-HDL-C = 100–129mg/dlvs.non-HDL-C <100mg/dl(P = 0.0443),non-HDL-C = 190–219mg/dlvs.non-HDL-C <100mg/dl(P = 0.0546),non-HDL-C ≥220mg/dlvs. non-HDL-C <
FrontiersinCardiovascularMedicine|www.frontiersin.org 5 July2022| Volume9|Article903481 21
TABLE2| Multivariablecoxregressionanalysisofnon-HDL-Cwithall-causeandcardiovascularmortality.
Non-HDL-C,non-high-densitylipoproteincholesterol;HR,hazardratio;CI, confidenceinterval.
Model1:noadjustment;Model2:adjustedforageandrace;Model3:adjustedforage,race,education,bodymassindex,systolicbloodpressure,diastolicbloodpressure,smoking, diabetes,hypertension,coronaryheartdisease,stroke,creatinine,haemoglobin,glycatedhaemoglobinA1c,triglycerides,energyintake,proteinintake,carbohydrateintake,andtotal fatintake.
TABLE3| Theresultsof twopiecewiselinearregressionmodelofnon-HDL-Cwithall-causeandcardiovascularmortality.
Non-HDL-C,non-high-densitylipoproteincholesterol;HR,hazardratio;CI, confidenceinterval.
SubgroupsAnalysisoftheRiskof All-CauseandCardiovascularMortality
Thestratifiedanalysesaredemonstratedin Figure4 (Detail dataasshownin SupplementaryTableS1).Thenon-linear relationshipsforall-causemortalitywithstatisticalsignificance werefoundamongparticipantswhowereaged <65years old,andrace(White).Besides,thenon-linearrelationshipsfor cardiovascularmortalitywithstatisticalsignificancewerefound amongparticipantswhowererace(White),BMI ≥ 25kg/m2, andwithoutdiabetes.Whennon-HDL-C ≥ 142mg/dl,per30 mg/dlincreaseinnon-HDL-Cincreasedriskofcardiovascular mortalitywere1.41-foldforaged <65yearsold(p = 0.0040), 1.38-foldforrace(White)(p = 0.0182)and1.68-foldforrace
Thetwopiecewiselinearregressionmodelswereadjustedforadjustedforage,race,education,bodymassindex,systolicbloodpressure,diastolicbloodpressure,smoking,diabetes, hypertension,coronaryheartdisease,stroke,creatinine,haemoglobin,glycatedhaemoglobinA1c,triglycerides,energyintake,proteinintake,carbohydrateintake,andtotalfatintake. withall-causeandcardiovascularmortalitywereU-shaped(All p forlikelihoodratiotest < 0.0001).Thethresholdvalues ofnon-HDL-Crelatedtothelowestriskinmultivariable adjustedanalyseswere144mg/dlforall-causemortality and142mg/dlforcardiovascularmortality.Asshownin Table3,belowthethreshold,per30mg/dlincreaseinnonHDL-Creduceda28%increasedriskofall-causemortality (p < 0.0001)anda40%increasedriskofcardiovascular mortality(p = 0.0037).Inversely,abovethethreshold,per30 mg/dlincreaseinnon-HDL-Cacceleratedriskofbothallcausemortality(HR1.11,95%CI1.03–1.20, p = 0.0057) andcardiovascularmortality(HR1.30,95%CI1.09–1.54, p = 0.0028).
Zengetal. Non-HDL-CandMortalityinMen
Model1HR(95%CI), P Model2 HR(95%CI), P Model3HR(95%CI), P All-causemortality Non-HDL-C(per30 mg/dlincrement) 1.01(0.97,1.05)0.6087 0.95(0.91,0.99)0.0207 0.94(0.90,0.99)0.0193 Non-HDL-Cgroup,mg/dl <100 1.20(0.97,1.49)0.0926 2.18(1.76,2.71) <0.0001 1.98(1.59,2.48) <0.0001 100–129 1.18(1.00,1.39)0.0465 1.45(1.22,1.71) <0.0001 1.38(1.17,1.63)0.0002 130–159 Reference Reference Reference 160–189 1.13(0.96,1.32)0.1518 1.12(0.95,1.31)0.1758 1.11(0.95,1.31)0.2007 190–219 1.06(0.87,1.30)0.5705 1.16(0.95,1.42)0.1493 1.12(0.91,1.38)0.2807 ≥220 1.09(0.85,1.40)0.4902 1.24(0.97,1.60)0.0857 1.04(0.79,1.36)0.8018 P fortrend 0.3574 <0.0001 <0.0001 Cardiovascularmortality Non-HDL-C(per30mg/dlincrement) 1.08(0.98,1.19)0.1076 1.05(0.94,1.18)0.3504 1.03(0.91,1.16)0.6826 Non-HDL-Cgroup,mg/dl <100 1.64(0.97,2.77)0.0667 3.15(1.85,5.36) <0.0001 2.99(1.73,5.16) <0.0001 100–129 1.36(0.88,2.11)0.1699 1.66(1.07,2.58)0.0241 1.72(1.10,2.69)0.0169 130–159 Reference Reference Reference 160–189 1.27(0.83,1.96)0.2726 1.32(0.86,2.04)0.2039 1.36(0.88,2.11)0.1636 190–219 1.38(0.83,2.31)0.2128 1.63(0.97,2.72)0.0633 1.61(0.94,2.73)0.0811 ≥220 2.03(1.18,3.50)0.0107 2.52(1.45,4.35)0.0010 2.16(1.17,3.98)0.0134 Pfortrend 0.5151 0.8498 0.5035
All-causemortality HR(95%CI) P Cardiovascular mortality HR(95%CI) P Cut-offvalue 144 142 <Cut-offvalue (ascontinuousvariables,per30mg/dlincrement) 0.72(0.62,0.82) <0.0001 0.60(0.42,0.85) 0.0037 ≥Cut-offvalue(ascontinuousvariables,per30mg/dlincrement) 1.11(1.03,1.20) 0.0057 1.30(1.09,1.54) 0.0028
FrontiersinCardiovascularMedicine|www.frontiersin.org 6 July2022| Volume9|Article903481 22
(Black)(p = 0.0017),1.57-foldforhighschooleducation(p = 0.0002),1.67-foldforsmoking(p = 0.0002),1.31-foldfor participantswithoutdiabetes(p = 0.0054)and1.41-foldfor participantswithhypertension(p = 0.0038).
DISCUSSION
ThenovelfindingofthestudyisthatbothlowandhighnonHDL-Clevelsweresignificantlyassociatedwithincreasedriskof all-causeandcardiovascularmortalityamongmenwithoutstatin therapyinU-shapedrelationships.Furthermore,weconfirmed thatthenon-HDL-Clevelwasrelatedtothelowestriskofallcauseandcardiovascularmortalityatthresholdvaluesof144and 142mg/dl,respectively.Thesenewresultsareprobabletohave implicationsfortheinterpretationoflevelsofnon-HDL-Cin clinicalpractise.
Globalage-standardisedmeannon-HDL-Cremainedalmost unchangedfrom1980to2018,andhighnon-HDL-Cwas responsibleforanestimated3.9millionworldwidedeathsfrom ischemicheartdiseaseandischemicstrokein2017,accounting forathirdofdeathsfromthesecauses(18).Undoubtedly, itisparticularlyurgenttoclearlyunderstandtherelationship ofnon-HDL-Cstratificationwithdeath,andlocatethebest thresholdvaluesofnon-HDL-C.Oneofourmajorfindings isthatoncenon-HDL-Clevelsaregreaterthanthreshold values,iscloselycontributedtohighermortality.Thisfinding issimilartopreviousseveralstudiesindifferentpopulations (8, 9, 11, 13, 19–21).Thepotentialexplanationforthis findingisthatextremelyhighnon-HDL-Clevelsplayarole inacceleratedatherosclerosis,leadingtoanincreasedriskof death(22).InUnitedStatespopulationstudy,non-HDL-C maybebest suitedforthepredictionoffuturecoronaryartery calcium(CAC)progression,especiallysincenon-HDL-Clevels ≥ 190mg/dlareconsistentlyassociatedwithsignificantCAC progressionintheoverallpopulation(β 16.4,95%CI 5.63 to27.2, p = 0.003)(23).Onegeneticstudyfindingisthat levelsofnon-HDL-Careassociatedwiththeextentofcoronary atherosclerosis.Besides,themutationsofsomegeneslikeLDLR, apolipoproteinB,andproproteinconvertasesubtilisin/kexin type9(PCSK9),canresultinhypercholesterolemia,and guidelinessuggestedthatnon-HDL-C ≥ 220mg/dlcould possiblyimplyhereditarygenetichypercholesterolemia(11, 24). Patientswithhypercholesterolemiahaveincreasednon-HDL-C andaremorepronetosufferfromatheroscleroticcardiovascular andcardiovasculardeath(11, 23).
Moreover,the presentstudycontributedevidencethatlower non-HDL-Cisalsocloselyrelatedtohighermortalityinmen withoutstatintherapy,andindicatedaU-shapedassociation. Althoughthedisparityinthestudypopulation,inaccordance withourresults,severalpreviousstudieshaveobservedthe U-shapedassociationbetweennon-HDL-Candmortality(11, 12).OnestudybyChengandcolleaguesanalysingdatafrom NHANESdemonstratedthatrelativelyhigherorlowernonHDL-Cconcentrationswerelinkedtoincreasedmortality,and thelowestriskwasfoundatthresholdvaluesof158and190 mg/dlforall-causeandcardiovascularmortality,respectively.
Thedifferenceinthresholdestimatesmightbeattributedto differentstudypopulations,ofwhichallpatientsinthestudy byChengetal.werehypertension,andhadrelativelyhigher non-HDL-Clevels(25).Likewise,theU-shapedrelationships betweennon-HDL-Cand theriskofall-causeandcardiovascular mortalityhavebeenshowninpatientswithCKDandthe optimalnon-HDL-Cconcentrationrangewasbetween116.2 and143.9mg/dl(11).Similarly,astudyofageneralpopulation cohortalsofounda U-shapedassociationbetweenlevelsofLDLCandtheriskofall-causemortality(26).Inpatientswith CHD,aparadoxicalassociationexistedbetweenbaselinenonHDL-Candlong-termall-causemortality,butdisappearedafter takingintoaccounttheeffectsofmalnutrition,indicatingthat theworselong-termprognosisinthelownon-HDL-Cgroup (<2.2mmol/L)wasmainlymediatedbytheunderlyingeffect ofmalnutrition(27).Apartfromthat,aninverseassociation betweencholesterol andmortalityhasbeendemonstratedin theelderly(28, 29).Anotherpopulation-basedregisterstudy including118,160subjectswithoutstatintherapyfoundthathigh lipoproteinlevelswereassociatedwithlowermortalityindicating thathighlipoproteinlevelsdonotseemtobedefinitelyharmful inthegeneralpopulation(29).Similarly,participantswithlow serumTCseem tohavealowersurvivalratethanparticipants withanelevatedcholesterollevel,irrespectiveofconcomitant diseasesorhealthstatus(28).Unexpectedly,thefindingofaUshapedassociationinourstudyisinconsistentwithapositive associationinanotherstudy(13).Thedifferencesbetweenthe twostudiesin thepopulation(UnitedStatesorIsrael),sample size(13,562or4,832),andfollow-uptime(98.38 ± 53.78months or22.1 ± 3.2years)mayresultindifferentconclusions.
However,theunderlyingmechanismofU-shapedassociation isnotclear.First,onepossiblereasonisthattheparticipants withthelowestcholesterollevelshadapoorerhealthstatus (28),ordebilitationandillnesshavebeenhypothesisedto causeadecreaseinlevelsofcholesterol(26, 30, 31).Second, higherHDL-C equalstolownon-HDL-Clevelsaccording tothecalculationformula,extremelyhighHDL-Cincreases mortalityinthegeneralpopulationbyanalysingthedatafrom NHANES(12).Thegeneticvariationofparticulargenesand variationofthe sizeorfunctionofHDLparticlesmaybethe underlyingmechanism(12).Finally,theU-shapedassociation betweenlipoproteinlevels andmortalitymaybesimilartothe obesityparadox,whichislargelyexplainedbymethodological issues,includingreversecausation.Nomatterhow,more studiesareneededtoclarifytheexactmechanismoftheUshapedassociation.
Theadvantageofthepresentstudyliesinitsrelatively largesamplesizeandlong-termfollow-up.Regardingclinical importance,ournovelfindingsareconducivetounderstanding theriskstratificationofnon-HDL-Candremindusthat wheninitiatinglipid-loweringtherapyinclinicalpractise, attentionshouldbepaidtoassessingtheabsoluteriskof atheroscleroticcardiovasculardisease(26, 32, 33),ratherthan startingtreatment basedsolelyonamoderateincreasein levelsofaspecificlipidmarker.Anyway,therearestillsome limitationstothisstudy.First,duringlong-termfollow-up,only asinglemeasurementofserumnon-HDLCconcentrationat
Zengetal. Non-HDL-CandMortalityinMen
FrontiersinCardiovascularMedicine|www.frontiersin.org 7 July2022| Volume9|Article903481 23
FIGURE3| Restrictedcubicspinemodelsofnon-HDL-Cwithall-cause (A) andcardiovascular (B) mortality.Non-HDL-C,non-high-densitylipoproteincholesterol. Restrictedcubicspinemodelswereadjustedforadjustedforage,race,education,bodymassindex,systolicbloodpressure,diastolicbloodpressure,smoking, diabetes,hypertension,coronaryheartdisease,stroke,creatinine,haemoglobin,glycatedhaemoglobinA1c,triglycerides,energyintake,proteinintake,carbohydrate intake,andtotalfatintake.

FIGURE4| Subgroupanalysis.Non-HDL-C,non-high-densitylipoproteincholesterol;HR,hazardratio;CI,confidenceinterval;BMI,bodymassindex.Resultsare expressedasmultivariable-adjustedHRincontinuousanalyses(Non-HDL-Cper30mg/dlincrement).Whenanalysingasubgroupvariable,age,race,education, BMI,systolicbloodpressure,diastolicbloodpressure,smoking,diabetes,hypertension,coronaryheartdisease,stroke,creatinine,haemoglobin,glycated haemoglobinA1c,triglycerides,energyintake,proteinintake,carbohydrateintake,andtotalfatintakewerealladjustedexceptthevariableitself.
baselineisavailable,leadingtopotentialbiasandfailureto evaluatetheaffectionofnon-HDL-Ctrajectoriesonmortality. Second,althoughweadjustedmanyrelevantconfounding

Zengetal. Non-HDL-CandMortalityinMen
FrontiersinCardiovascularMedicine|www.frontiersin.org 8 July2022| Volume9|Article903481 24
variablesthatwereconsideredtoinfluencemortality,residual confoundersandhiddencomorbiditiesmighthavebeennot eliminated.Finally,ourstudywasperformedinanationally
representativesampleofmenintheUnitedStates,soour resultsmaynot beeasilyextrapolatedtothepopulationin otherregions.
CONCLUSION
Fromapopulation-basedcohortstudybaseonthenational representativedatabase,ourstudydemonstratedthatnon-HDLCwasU-shapedandrelatedtoall-causeandcardiovascular mortalityamongmenwithoutstatintherapy.Themore clearriskstratificationofnon-HDL-Candcomprehensive strategicmanagementtodealwithdyslipidemiadeservesfurther investigationforconfirmation.
DATAAVAILABILITYSTATEMENT
Thedatasetspresentedinthisstudycanbefoundinonline repositories.Thenamesoftherepository/repositoriesand accessionnumber(s)canbefoundbelow:https://www.cdc.gov/ nchs/nhanes/index.htm.
ETHICSSTATEMENT
Thestudiesinvolvinghumanparticipantswerereviewed andapprovedbytheUSCentresforDiseaseControland Preventionratifiedthestudyprotocols.Thepatients/participants
REFERENCES
1.BrunnerFJ,WaldeyerC,OjedaF,SalomaaV,KeeF,SansS,etal.Applicationof non-HDLcholesterolforpopulation-basedcardiovascularriskstratification: resultsfromtheMultinationalCardiovascularRiskConsortium. Lancet. (2019)394:2173–83.doi:10.1016/S0140-6736(19)32519-X
2.EmergingRiskFactorsC,DiAngelantonioE,SarwarN,PerryP,Kaptoge S,RayKK,etal.Majorlipids,apolipoproteins,andriskofvasculardisease. JAMA. (2009)302:1993–2000.doi:10.1001/jama.2009.1619
3.PencinaKM,ThanassoulisG,WilkinsJT,VasanRS,NavarAM, PetersonED,etal.TrajectoriesofNon-HDLCholesterolAcrossMidlife: implicationsforcardiovascularprevention. JAmCollCardiol. (2019) 74:70–9.doi:10.1016/j.jacc.2019.04.047
4.YusufS,JosephP,RangarajanS,IslamS,MenteA,HystadP,et al.Modifiableriskfactors,cardiovasculardisease,andmortalityin155 722individualsfrom21high-income,middle-income,andlow-income countries(PURE):aprospectivecohortstudy. Lancet. (2020)395:795–808.doi:10.1016/S0140-6736(19)32008-2
5.NationalCholesterolEducationProgramExpertPanelonDetection E,TreatmentofHighBloodCholesterolinA.ThirdReportofthe NationalCholesterolEducationProgram(NCEP)ExpertPanelon Detection,Evaluation,andTreatmentofHighBloodCholesterolin Adults(AdultTreatmentPanelIII)finalreport. Circulation.(2002) 106:3143–421.doi:10.1161/circ.106.25.3143
6.ExpertDyslipidemiaP,GrundySM.AnInternationalAtherosclerosisSociety PositionPaper:globalrecommendationsforthemanagementofdyslipidemia. JClinLipidol. (2013)7:561–5.doi:10.1016/j.jacl.2013.10.001
7.ZengRX,LiXL,ZhangMZ,GuoYL,ZhuCG,GuoLH,etal.NonHDLcholesterolisabettertargetforpredictingperiproceduralmyocardial injuryfollowingpercutaneouscoronaryinterventionintype2diabetes. Atherosclerosis. (2014)237:536–43.doi:10.1016/j.atherosclerosis.2014.10.030
8.LiaoP,ZengR,ZhaoX,GuoL,ZhangM.Prognosticvalueofnon-highdensitylipoproteincholesterolformortalityinpatientswithcoronaryheart
providedtheirwritteninformedconsenttoparticipatein thisstudy.
AUTHORCONTRIBUTIONS
R-XZ,J-PX,Y-JK,L-HG,andM-ZZ:conceivedanddesignedthe study.R-XZ,J-PX,andJ-WT:collectedandanalysedthedata.RXZ:draftedthepaper.M-ZZ:revisedthemanuscript.Allauthors havereviewedthefinalmanuscript.Allauthorscontributedto thearticleandapprovedthesubmittedversion.
FUNDING
ThisworkwaspartiallysupportedbytheNationalNatural ScientificFoundation(No.82004135toR-XZ),theResearch FundforZhaoyangTalentsofGuangdongProvincialHospital ofChineseMedicine(No.ZY2022KY03toR-XZ),andthe SpecificResearchFundforTCMScienceandTechnology ofGuangzhouProvincialHospitalofChineseMedicine (YN10101915toM-ZZ).
SUPPLEMENTARYMATERIAL
TheSupplementaryMaterialforthisarticlecanbefound onlineat:https://www.frontiersin.org/articles/10.3389/fcvm. 2022.903481/full#supplementary-material
disease:asystematicreviewandmeta-analysis. IntJCardiol. (2017)227:950–55.doi:10.1016/j.ijcard.2016.10.106
9.CuiY,BlumenthalRS,FlawsJA,WhitemanMK,LangenbergP,Bachorik PS,etal.Non-high-densitylipoproteincholesterollevelasapredictor ofcardiovasculardiseasemortality. ArchInternMed. (2001)161:1413–9.doi:10.1001/archinte.161.11.1413
10.LiC,FordES,TsaiJ,ZhaoG,BalluzLS.GiddingSS.Serumnon-high-density lipoproteincholesterolconcentrationandriskofdeathfromcardiovascular diseasesamongUSadultswithdiagnoseddiabetes:theThirdNational HealthandNutritionExaminationSurveylinkedmortalitystudyCardiovasc Diabetol.(2011)10:46.doi:10.1186/1475-2840-10-46
11.ChiuH,WuPY,HuangJC,TuHP,LinMY,ChenSC,etal.ThereisaUshaped associationbetweennonhighdensitylipoproteincholesterolwithoveralland cardiovascularmortalityinchronickidneydiseasestage3-5. SciRep. (2020) 10:12749.doi:10.1038/s41598-020-69794-2
12.ChengQ,LiuXC,ChenCL,HuangYQ,FengYQ,ChenJY.TheU-Shaped AssociationofNon-High-DensityLipoproteinCholesterolLevelsWithAllCauseandCardiovascularMortalityAmongPatientsWithHypertension. FrontCardiovascMed. (2021)8:707701.doi:10.3389/fcvm.2021.707701
13.HarariG,GreenMS,MagidA,Zelber-SagiS.UsefulnessofNon-HighDensityLipoproteinCholesterolasaPredictorofCardiovascularDisease MortalityinMenin22-YearFollow-Up. AmJCardiol. (2017)119:1193–98.doi:10.1016/j.amjcard.2017.01.008
14.FordES,GilesWH,DietzWH.Prevalenceofthemetabolicsyndromeamong USadults:findingsfromthethirdNationalHealthandNutritionExamination Survey. JAMA. (2002)287:356–9.doi:10.1001/jama.287.3.356
15.PalmerMK,TothPP.Trendsinlipids,obesity,metabolicsyndrome,and diabetesmellitusintheUnitedStates:AnNHANESAnalysis(2003-2004to 2013-2014). Obesity(SilverSpring). (2019)27:309–14.doi:10.1002/oby.22370
16.YanYQ,ChenJ,HuangYQ.ANon-linearassociationofhighdensitylipoproteincholesterolwithall-causeandcause-specific mortalityindiabeticpatients. DiabetesMetabSyndrObes. (2021) 14:2851–62.doi:10.2147/DMSO.S313006
Zengetal. Non-HDL-CandMortalityinMen
FrontiersinCardiovascularMedicine|www.frontiersin.org 9 July2022 | Volume9|Article903481 25
17.NationalCenterforHealthStatistics.OfficeofAnalysisandEpidemiology. TheLinkageofNationalCenterforHealthStatisticsSurveyDatatotheNational DeathIndex–2015LinkedMortalityFile(LMF):MethodologyOverview andAnalyticConsiderations. (2019).Hyattsville,Maryland.Availableonline at:https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm(accessed May11,2022).
18.CollaborationNCDRF.Repositioningoftheglobalepicentreofnon-optimal cholesterol. Nature. (2020)582:73–7.doi:10.1038/s41586-020-2338-1
19.EchidaY,OgawaT,OtsukaK,AndoY,NittaK.Serumnon-highdensitylipoproteincholesterol(non-HDL-C)levelsandcardiovascular mortalityinchronichemodialysispatients. ClinExpNephrol. (2012)16:767–72.doi:10.1007/s10157-012-0615-5
20.HuangYQ,LiuXC,LoK,LiuL,YuYL,ChenCL,etal.TheUShaped RelationshipBetweenHigh-DensityLipoproteinCholesterolandAll-Cause orCause-SpecificMortalityinAdultPopulation. ClinIntervAging. (2020) 15:1883–96.doi:10.2147/CIA.S271528
21.LeeEY,YangY,KimHS,ChoJH,YoonKH,ChungWS,etal.Effectof visit-to-visitLDL-,HDL-,andnon-HDL-cholesterolvariabilityonmortality andcardiovascularoutcomesafterpercutaneouscoronaryintervention. Atherosclerosis. (2018)279:1–9.doi:10.1016/j.atherosclerosis.2018.10.012
22.StoneNJ,RobinsonJG,LichtensteinAH,BaireyMerzCN,Blum CB,EckelRH,etal.2013ACC/AHAguidelineonthetreatment ofbloodcholesteroltoreduceatheroscleroticcardiovascularriskin adults:areportoftheAmericanCollegeofCardiology/AmericanHeart AssociationTaskForceonPracticeGuidelines. Circulation. (2014)129:S1–45.doi:10.1161/01.cir.0000437738.63853.7a
23.ZalawadiyaSK,VeerannaV,PanaichS,KottamA,AfonsoL.Nonhigh-densitylipoproteincholesterolandcoronaryarterycalcium progressioninamultiethnicUSpopulation. AmJCardiol. (2014) 113:471–4.doi:10.1016/j.amjcard.2013.10.027
24.DefescheJC,GiddingSS,Harada-ShibaM,HegeleRA,SantosRD, WierzbickiAS.Familialhypercholesterolaemia. NatRevDisPrimers. (2017) 3:17093.doi:10.1038/nrdp.2017.93
25.ContrerasF,LaresM,CastroJ,VelascoM,RojasJ,GuerraX,etal. Determinationofnon-HDLcholesterolindiabeticandhypertensivepatients. AmJTher. (2010)17:337–40.doi:10.1097/MJT.0b013e3181c1233c
26.JohannesenCDL,LangstedA,MortensenMB,NordestgaardBG. Associationbetweenlowdensitylipoproteinandallcauseandcause specificmortalityinDenmark:prospectivecohortstudy. BMJ. (2020) 371:m4266.doi:10.1136/bmj.m4266
27.WangB,GuoZ,LiH,ZhouZ,LuH,YingM,etal.Non-HDL cholesterolparadoxandeffectofunderlyingmalnutritioninpatientswith coronaryarterydisease:a41,182cohortstudy. ClinNutr. (2022)41:723–30.doi:10.1016/j.clnu.2022.01.027
28.TuikkalaP,HartikainenS,KorhonenMJ,LavikainenP,KettunenR, SulkavaR,etal.Serumtotalcholesterollevelsandall-causemortality inahome-dwellingelderlypopulation:asix-yearfollow-up. Scand JPrimHealthCare. (2010)28:121–7.doi:10.3109/02813432.2010. 487371
29.BathumL,DepontChristensenR,EngersPedersenL,Lyngsie PedersenP,LarsenJ,NexoeJ.Associationoflipoproteinlevels withmortalityinsubjectsaged50 + withoutpreviousdiabetesor cardiovasculardisease:apopulation-basedregisterstudy. ScandJ PrimHealthCare. (2013)31:172–80.doi:10.3109/02813432.2013. 824157
30.JacobsD,BlackburnH,HigginsM,ReedD,IsoH,McMillanG,etal.Report oftheconferenceonlowbloodcholesterol:mortalityassociations. Circulation. (1992)86:1046–60.doi:10.1161/01.CIR.86.3.1046
31.RanieriP,RozziniR,FranzoniS,BarbisoniP,TrabucchiM.Serumcholesterol levelsasameasureoffrailtyinelderlypatients. ExpAgingRes. (1998) 24:169–79.doi:10.1080/036107398244300
32.BurgessS,DaviesNM,ThompsonSG,ConsortiumEP-I.Instrumental variableanalysiswithanonlinearexposure-outcomerelationship. Epidemiology. (2014)25:877–85.doi:10.1097/EDE.0000000000 000161
33.MachF,BaigentC,CatapanoAL,KoskinasKC,CasulaM,BadimonL,et al.2019ESC/EASGuidelinesforthemanagementofdyslipidaemias:lipid modificationtoreducecardiovascularrisk. EurHeartJ. (2020)41:111–88.doi:10.1093/eurheartj/ehz455
ConflictofInterest: Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcouldbeconstruedasa potentialconflictofinterest.
Publisher’sNote: Allclaimsexpressedinthisarticlearesolelythoseoftheauthors anddonotnecessarilyrepresentthoseoftheiraffiliatedorganizations,orthoseof thepublisher,theeditorsandthereviewers.Anyproductthatmaybeevaluatedin thisarticle,orclaimthatmaybemadebyitsmanufacturer,isnotguaranteedor endorsedbythepublisher.
Copyright©2022Zeng,Xu,Kong,Tan,GuoandZhang.Thisisanopen-access articledistributedunderthetermsoftheCreativeCommonsAttributionLicense(CC BY).Theuse,distributionorreproductioninotherforumsispermitted,provided theoriginalauthor(s)andthecopyrightowner(s)arecreditedandthattheoriginal publicationinthisjournaliscited,inaccordancewithacceptedacademicpractice. Nouse,distributionorreproductionispermittedwhichdoesnotcomplywiththese terms.
Zengetal. Non-HDL-CandMortalityinMen
FrontiersinCardiovascularMedicine|www.frontiersin.org 10 July2022| Volume9|Article903481 26
OPENACCESS
EDITEDBY Chu-HuangChen, TexasHeartInstitute,UnitedStates
REVIEWEDBY TongyuMa, FranklinPierceUniversity, UnitedStates DickC.Chan, UniversityofWestern Australia,Australia
*CORRESPONDENCE LingLiu feliuling@csu.edu.cn
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine
RECEIVED 14May2022
ACCEPTED 19July2022
PUBLISHED 18August2022
CITATION
XuJ,DuX,ZhangS,XiangQ,ZhuL andLiuL(2022)Theaccuracyoffour formulasforLDL-Ccalculationatthe fastingandpostprandialstates. Front.Cardiovasc.Med. 9:944003. doi:10.3389/fcvm.2022.944003
COPYRIGHT
© 2022Xu,Du,Zhang,Xiang,Zhuand Liu.Thisisanopen-accessarticle distributedunderthetermsofthe CreativeCommonsAttributionLicense (CCBY).Theuse,distributionor reproductioninotherforumsis permitted,providedtheoriginal author(s)andthecopyrightowner(s) arecreditedandthattheoriginal publicationinthisjournaliscited,in accordancewithacceptedacademic practice.Nouse,distributionor reproductionispermittedwhichdoes notcomplywiththeseterms.
PUBLISHED 18August2022
DOI 10.3389/fcvm.2022.944003
Theaccuracyoffourformulas forLDL-Ccalculationatthe fastingandpostprandialstates
1 DepartmentofCardiovascularMedicine,TheSecondXiangyaHospital,CentralSouthUniversity, Changsha,China, 2 ResearchInstituteofBloodLipidandAtherosclerosis,CentralSouthUniversity, Changsha,China, 3 ModernCardiovascularDiseaseClinicalTechnologyResearchCenterofHunan Province,Changsha,China, 4 CardiovascularDiseaseResearchCenterofHunanProvince, Changsha,China, 5 DepartmentofGastroenterology,TheSecondXiangyaHospital,CentralSouth University,Changsha,China
Background: Elevatedleveloflow-densitylipoproteincholesterol(LDL-C)is concernedasoneofthemainriskfactorsforcardiovasculardisease,inboth thefastingandpostprandialstates.Thisstudyaimedtocomparethemeasured LDL-CwithLDL-CcalculatedbytheFriedewald,Martin–Hopkins,Vujovic, andSampsonformulas,andestablishwhichformulacouldprovidethemost reliableLDL-CresultsforChinesesubjects,especiallyatthepostprandialstate.
Methods: Twenty-sixsubjectswereenrolledinthisstudy.Thebloodsamples werecollectedfromallthesubjectsbeforeandaftertakingadailybreakfast. ThecalculatedLDL-CresultswerecomparedwithLDL-Cmeasuredbythe verticalautoprofilemethod,atboththefastingandpostprandialstates. Thepercentagedi erencebetweencalculatedandmeasuredLDL-C(total error)andthenumberofresultsexceedingthetotalerrorgoalof12% wereestablished.
Results: ThecalculatedLDL-CF levelsshowednosignificantdi erencefrom LDL-CVAP levelsatthefastingstate.ThecalculatedLDL-CS weresignificantly higherthanLDL-CVAP atthefastingstate(P < 0.05),whilethecalculated LDL-Cs wereveryclosetoLDL-CVAP levelsafteradailymeal.Atthefasting state,themediantotalerrorofcalculatedLDL-CF was0(quartile: 3.8to 6.0),followedbyLDL-CS,LDL-CMH,andLDL-CV.Atthepostprandialstates, themediantotalerrorsofLDL-CS werethesmallest,1.0( 7.5,8.5)and 0.3 ( 10.1,10.9)at2and4h,respectively.ThecalculatedLDL-CF levelsshowed thehighestcorrelationtoLDL-CVAP andaccuracyinevaluatingfastingLDL-C levels,whiletheSampsonformulashowedthehighestaccuracyatthe postprandialstate.
Conclusion: TheFriedewaldformulawasrecommendedtocalculatefasting LDL-C,whiletheSampsonformulaseemedtobeabetterchoicetocalculate postprandialLDL-ClevelsinChinesesubjects.
KEYWORDS
LDL-C,postprandial,Friedewaldformula,Vujovicformula,Martin–Hopkinsformula, Sampsonformula,verticalautoprofilemethod
TYPE OriginalResearch
JinXu1,2,3,4,XiaoDu1,2,3,4,ShilanZhang1,2,3,4,5 , QunyanXiang1,2,3,4,LiyuanZhu1,2,3,4 andLingLiu1,2,3,4*
Frontiersin CardiovascularMedicine 01 frontiersin.org 27
Background
Cardiovasculardisease(CVD)hasbeentheleading causeofdeath worldwide (1).Theelevatedleveloflowdensitylipoproteincholesterol (LDL-C)isconcerned asoneofthemainriskfactorsofCVD,especiallyfor atheroscleroticCVD (2).Inclinicalpractice,itisthe mainlaboratoryparameterused forcardiovascularrisk assessmentandtheprimarytargetforcholesterolcontrol (3).Therefore,itiscrucialtoensurereliablemeasurement ofLDL-Clevels
ThereareseveralmethodstomeasureLDL-Clevels, includingultracentrifugation,formulamethods,directmethod, andnuclearMR(NMR)method (4).Amongthem,the ultracentrifugationmethod isrecommendedasthereference methodforLDL-Cmeasurement.Duetocomplexoperations andexpensiveequipment,ultracentrifugationisdifficulttobe widelyusedinclinicalpractice.Verticalautoprofile(VAP) methodology,oneoftheultracentrifugationmethods,wasused tomeasurelipidprofilesasareferencemethod,commonlyatthe fastingstate (5).However,astudyinvolvingthemeasurement oflipidprofilesby VAPatthepostprandialornon-fastingstate isveryrareworldwide (6,7),andtherewasnosimilarstudy inChina.
Incomparison,the formulamethodissimplerandcheaper. TheFriedewaldmethoddevelopedin1972isthemain mathematicalformulaforLDL-Ccalculation (8).Itusesa fixedcoefficient,2.2 (formmol/l),todescribetherelationship betweentriglyceride(TG)andvery-low-densitylipoprotein cholesterol(VLDL-C) (8).WhentheTGlevelwasabove 4.5mmol/l,theaccuracyofthisformulawilldecline.Thus, otherformulaswereproposed.In2003,theVujovicformula, whichuses3.0(formmol/l)asaratioofTGtoVLDL-C, wasproposedforLDL-Ccalculation (9).Then,theMartin–Hopkinsformulawasdevelopedwithanadjustableratio basedonTGandnon-high-densitylipoproteincholesterol (non-HDL-C)levels (10).In2020,Sampsonandcolleagues (11) proposedanewformula,whichwasprovedtobe suitableforsampleswithTGlevelsupto9.0mmol/l.Those novelformulaswereprovedtobemoreaccuratethanthe Friedewaldformula (12)
Recently,avariety ofexpertrecommendationshave supportednon-fastinglipidassessment (13–15),aselevatednonfastingTGandLDL-Clevelshadbeenregardedasindependent riskfactorsofatheroscleroticCVD (16,17).Weoncereported thatthedirectmeasuredLDL-Clevelsweresignificantlyhigher thancalculatedLDL-ClevelsbytheFriedewaldformulaatboth thefastingandnon-fastingstatesinChinesesubjects (18).Three novelformulamethods andtheVAPmethodwerenotinvolved inthisstudy.Thus,thisstudyaimedtoestablishwhichformula methodcouldprovidethemostreliableLDL-Cresultswhen comparedwiththeVAPmethodforChinesesubjects,especially inthepostprandialstate.
Methods Studysubjects
Therewere26subjects(in-patient)includedinthisstudy intheDepartmentofCardiovascularMedicineoftheSecond XiangyaHospital,CentralSouthUniversity.Allthesubjects wereinvitedtofilloutaquestionnaireonmedicalhistoryand useofmedicationbeforeparticipation.SubjectswithfastingTG levelsabove4.5mmol/lwereexcluded.Nosubjecthadahistory ofthyroiddiseases,liverandkidneydiseases,autoimmune diseases,cancer,orotherseveremedicalillnesses.Thestudy wasapprovedbytheEthicsCommitteeoftheSecondXiangya HospitalofCentralSouthUniversityandinformedconsentwas gainedfromalltheparticipants.
Specimencollection
Afteratleast12hofovernightfasting,venousbloodsamples werecollectedinallthesubjectsbefore(i.e.,0h)andat2and 4haftertakingadailybreakfastaccordingtotheirdailyhabits, suchassteamedbread,riceporridge,ornoodles (19).Allthe subjectswererequired tocompletethemealin15min.Allthe bloodsampleswerecentrifugedat4◦Cfor3,000rpmfor15min andstoredat 80◦Crefrigeratoruntilanalysis.
Laboratoryassays
Bloodlipidsweredetectedintwoways.First,allthe bloodsamplesweremeasuredinamedicallaboratoryin SecondXiangyaHospitalbyalaboratorytechnicianwhohad noknowledgeofthisstudyasdescribedbefore (20).Serum levelsoftotalcholesterol(TC)andTGweremeasuredby automatedenzymaticassays,andtheconcentrationofHDL-C wasmeasuredbyadirectmethod,i.e.,theselectiveprotection method.LDL-Clevelwasmeasureddirectlybythechemical masking(CM)method(i.e.,LDL-CCM)regardlessofTGlevel. Then,theVAPmethodwasusedtomeasureallthelipid profiles,includingLDL-C(i.e.,LDL-CVAP),asareference method (5).Inbrief,itsimultaneouslymeasurescholesterol concentrationsofallthe fivelipoproteinclassesin <1h.After centrifugation,thecontentsofthecentrifugetube(separated layersoflipoproteins)wereanalyzedforcholesterolusingthe continuousflowVAPanalyzer (5)
Low-densitylipoproteincholesterol calculation
Foreachsample,theLDL-Clevelwascalculatedusing mathematicalformulaswithCMmeasuredlipidsasfollows:
Xuetal. 10.3389/fcvm.2022.944003
Frontiersin CardiovascularMedicine 02 frontiersin.org 28
Friedewald (8):LDL-CF = TC –HDL-C– TG/2.2(mmol/l)
Vujovic (9):LDL-CV = TC–HDL-C– TG/3(mmol/l)
Martin–Hopkins (10):LDL-CMH = TC–HDL-C –TG/adjustablefactor(mg/dl)
Sampson (11):LDL-CS = TC/0.948–HDL-C/0.971 –(TG/8.56 + TG × non-HDL-C/2,140–TG2/16,100)–9.44(mg/dl).
Thefactorof0.026wasusedtoconvertLDL-Cfrommg/dl intommol/l,ifnecessary.
Statisticalanalysis
Allthecontinuouslevelswereexpressedasmedian (interquartilerange)andqualitativevariableswereexpressed asnumbersandpercentages.ThemeasuredTC,HDL-C,LDLC,andthecalculatedLDL-Cweretestedtobedistributed normally,whilethemeasuredTGwasproventobenon-normal distribution.Theparametricandnon-parametricstatisticaltests wereusedforcorrespondingdata,respectively.Differences betweendifferentgroupswereanalyzedby one-way ANOVA, whiledifferencesamongdifferenttimepointswithinthe samegroupwereanalyzedbyrepeatedmeasure one-way ANOVAanalysis.Categoricalvariableswerecomparedusing the chi-squared statistictests.TheBland–Altmandifference plotswereusedtocomparecalculatedLDL-Clevelsand theLDL-CVAP.CorrelationbetweencalculatedLDL-Clevels andtheLDL-CVAP wasconductedwithPearsoncorrelation analyses.Foreachsample,thetotalerror(%)betweenthe mathematicallycalculatedLDL-CandLDL-CVAP wasestimated asfollows:[(LDL-Cformula –LDL-CVAP)/LDL-CVAP] × 100%. Theaccuracyofestimationwasdefinedasthetotalerror ± 12% (21).Allthestatisticalanalyseswereperformedwith SPSSversion25.0.All the P-levelswere2-tailed,and P < 0.05wasconsideredstatisticallysignificant.Fordifferences betweenLDL-CVAP andLDL-Cformula, P < 0.01wasconsidered statisticallysignificant,aswereplaced post-hoc analysisinthe repeatedmeasureone-wayANOVAanalysesusingone-way ANOVAanalyses.
Results Populationcharacteristics
Therewere26subjectswhoparticipatedinthisstudy, including17(65.4%)menand9(34.6%)women.Theirages rangedfrom46to73years,withamedianageof62.5 years.Fiveofthemgotabodymassindex(BMI)ofover
TABLE1Studypopulationcharacteristics.
Valuesarerepresentedasmedian(interquartilerange)andn(%)asappropriate. BMI,bodymassindex;CHD,coronaryheartdisease.
28kg/m2 andthemedianBMIofallthesubjectswas25.5 kg/m2.Thepatientswithcoronaryheartdisease,hypertension, anddiabetesaccountedfor69.2,69.2,and30.8%,respectively. Therewere42.3%ofsmokersand46.2%ofsubjectstaking statins (Table1)
Postprandialchangesinserumlevelsof bloodlipidsmeasuredbydi erent methods
ItwasobviousthatthelevelsofTC,TG,andLDL-C measuredbyCMweresignificantlyhigherthanthosemeasured byVAP,whileHDL-Clevelsmeasuredbythetwomethodswere similaratboththefastingandpostprandialstates (Figure1).No matterwhichmethod wasused,boththeTCandLDL-Clevels decreasedsignificantlyafteradailymealcomparedtothefasting state(P < 0.05, Figures1A,C),whileTGshowedtremendously increaseatthepostprandialtimepoints(P < 0.05, Figure1B). ThelevelsofHDL-Ckeptstableafteradailymealnomatter whichmethodwasused (Figure1D)
Thecalculatedlevels ofLDL-Cwereacquiredbyfour differentformulaswithbloodlipidsmeasuredbyCM,andthey showedasimilardecreaseafteradailymealandthepostprandial changesreachedastatisticdifference(P < 0.05, Figure2).Itis worthnotingthattherewasnosignificantdifferencebetween calculatedLDL-Clevelsat2and4h,nomatterwhichformula wasused.
ThecalculatedLDL-Clevels via Friedewald,Martin–Hopkins,andSampsonformulasshowednosignificant differencewhencomparedtoLDL-CVAP levelsatboththe fastingandpostprandialstates (Figures2A,C,D).However,the calculatedLDL-CV levelswere significantlyhigherthanLDLCVAP levelsatthefastingstate(P < 0.05 Figure2B),whilethe calculatedLDL-CV levelswere veryclosetoLDL-CVAP levels afteradailymeal (Figure2B).ThecalculatedLDL-CF levels seemtobelowerthanLDL-CVAP levelsatthepostprandial
Xuetal. 10.3389/fcvm.2022.944003
Parameters N = 26 Male, n (%) 17(65.4) Age,y 62.5 (54.75,66.5) BMI,Kg/m2 25.51(23.2,27.0) Currentsmoking, n (%) 11(42.3) CHD, n (%) 18(69.2) Hypertension, n (%) 18(69.2) Diabetes, n (%) 8(30.8) Historyofstatins, n (%) 12(46.2)
Frontiersin CardiovascularMedicine 03 frontiersin.org 29
states,whilethedifferencedidnotreachstatisticalsignificance (P < 0.05, Figure2A).
Thecalculated LDL-CF, LDL-CS,andLDL-CMH levels wereallsignificantlylowerthanLDL-CCM levelsatthe fastingandpostprandialstates(P < 0.05, Figures2A,C,D).
TheLDL-CV levelswere lowerthanLDL-CCM levels;however, thedifferencereachedstatisticalsignificanceonlyat4h postprandially (Figure2B)
Consistencyandcorrelationbetween estimatedandmeasuredlow-density lipoproteincholesterol

TheBland–Altmandifferenceplotsshowedgreat consistencyinestimatedandmeasuredLDL-C (SupplementaryFigure1).ThemeasuredLDL-CCM alsohada goodconsistency withLDL-CVAP (SupplementaryFigure1)
ThePearsoncorrelation analysesshowedastrongandpositive correlationbetweenLDL-CVAP levelsandthecalculatedLDL-C levelsbyfourformulas,andthe r levelsrangedfrom0.836to
0.961atthefastingandpostprandialstates(P < 0.05, Table2). Atthefastingstate,thestrongestcorrelationwasfoundbetween LDL-CVAP levelsandLDL-CF levels(r 0.870, P < 0.05).Atthe postprandialstates,thestrongestcorrelationwasfoundbetween LDL-CVAP levelsandtheLDL-CV levels(2h: r 0.961,4h: r 0.956, P < 0.05).
ApositivecorrelationwasalsofoundbetweenLDL-CVAP andLDL-CCM atthefastingandpostprandialstates(0h: r 0.780,2h: r 0.883,4h:r0.859, P < 0.05, Table2).However, thecorrelationbetweenLDL-CVAP andLDL-CCM wasweaker thanthecorrelationbetweenLDL-CVAP andthecalculatedLDLClevelsbyfourformulasatboththefastingandpostprandial states (Table2)
Distributionofthetotalerroratthe fastingandpostprandialstates
TodeterminethereliabilityofcalculatedLDL-Clevels bydifferentformulas,wecalculatedthetotalerrorsbetween
Xuetal. 10.3389/fcvm.2022.944003
FIGURE1 ChangesinserumlevelsofbloodlipidsviaVAPandchemicalmaskingmethodafteradailymeal.*P < 0.05whencomparedwithVAPmeasured valuesatthesametimepoint. #P < 0.05whencomparedwithfastingvalueusingthesamemeasuremethod.
Frontiersin CardiovascularMedicine 04 frontiersin.org 30
calculatedLDL-CandLDL-CVAP levels.Atthe fastingstate,the mediantotalerrorofcalculatedLDL-CF was0(quartile: 3.8to 6.0; Figure3A;SupplementaryTable1).Themediantotalerrors ofcalculatedfastingLDL-CV,LDL-CMH,andLDL-CS were11.2 (3.2,18.9),7.0( 2.5,15.3),and3.4( 1.7,10.0),respectively (Figures3B–D;SupplementaryTable1).
ThemediantotalerrorsofLDL-CF were 3.9( 14.1, 2.4)and 9.9( 15.3,0)at2and4h,respectively(Figure3A; SupplementaryTable 1),whichsuggestedthattheFriedewald formulacouldunderestimateLDL-Clevelswhencomparedwith theVAPmethodatthepostprandialstate.Themediantotal errorsofLDL-CV andLDL-CMH rangedfrom2.6and6.5at

thepostprandialstates(Figures3B,C;SupplementaryTable1), whichindicatedthatVujovicandMartin–Hopkinsformulas couldoverestimateLDL-Clevelswhencomparedwiththe VAPmethodatthepostprandialstate.Themediantotal errorsofpostprandialLDL-CS weresmall,i.e.,1.0( 7.5,8.5) and 0.3( 10.1,10.9)at2and4h,respectively (Figure3D; SupplementaryTable 1)
Percentageoftheaccuracyofestimated low-densitylipoproteincholesterol
TheaccuracyofcalculatedLDL-Clevelsbyformulaswas consideredasthepercentageofthetotalerrorbetween 12 and12%whencomparedwithLDL-CVAP levels.TheFriedewald formulashowedthehighestaccuracy,80.8%,atthefasting state,followedbySampson,Martin–Hopkins,andVujovic formulas (Figure3E).TheSampsonformulashowedthehighest accuracy,80.8%,at 2hpostprandially,followedbyFriedewald, Martin–Hopkins,andVujovicformulas (Figure3E).At4h afteradailymeal,theMartin–HopkinsformulaandSampson formulashowedhigheraccuracythanVujovicandFriedewald formulas (Figure3E).
Xuetal. 10.3389/fcvm.2022.944003
FIGURE2 ChangesinLDL-Clevelsviadi erentcalculatedmethodsafteradailymeal.*P < 0.05whencomparedwithcalculatedvaluesatthesame timepoint.
LDL0LDL2LDL4 Friedewald 0.870 0.920 0.913 Vujovic 0.85 0.961 0.956 Martin/Hopkins 0.837 0.927 0.902 Sampson 0.836 0.939 0.928 CM 0.780 0.883 0.859
TABLE2Correlationinchemicalmaskingmethodmeasuredand formulaestimatedvs.VAPmeasuredLDL-C.
Frontiersin CardiovascularMedicine 05 frontiersin.org 31
Discussion
ThisisthefirststudytocomparethecalculatedLDL-C levelsbydifferentformulas toLDL-CVAP levelsatboththe fastingandpostprandialstatesinChinesesubjects.Wefound thatthecalculatedLDL-CF levelsshowedthehighestcorrelation toLDL-CVAP andaccuracyinevaluatingfastingLDL-Clevels, whiletheSampsonformulashowedthehighestaccuracyat
thepostprandialstate.Therefore,theFriedewaldandSampson formulasseemedtobeabetterchoicetocalculatefastingand postprandialLDL-Clevels,respectively,inChinesesubjects.
SimilartothepostprandialchangeinLDL-CCM levels,LDLCVAP levelssignificantlydecreasedat2and4hafteradaily mealinthisstudy,whichwasdifferentfromtheresultsreported byHuetal. (22) whomeasuredlipidprofilesbyenzymaticandNMR-based methodsin87Chinesesubjectsandreported

Xuetal. 10.3389/fcvm.2022.944003
FIGURE3
AccuracyofestimatedLDL-Cviadi erentformulas. (A–D) Di erencebetweenLDL-CVAP andLDL-Cformula atfastingandpostprandialstates. (E) Accuracypercent(totalerror ±12%)ofcalculatedvalueviafourformulasatfastingandpostprandialstates. # P < 0.05whencomparedwith fastingstate.
Frontiersin CardiovascularMedicine 06 frontiersin.org 32
thatthere wasnosignificantreductioninLDL-Clevelsand LDLparticlesdeterminedby NMRafteradailymeal.However, theyfoundcholesterolcontentinlargeLDLparticlesthat significantlydecreasedat2and4hcomparedtothefastingone (22).Americanresearcherscomparedlipidprofilesdetectedby theVAPmethod between10,135fastingand5,262non-fasting (<8hsincelastmeal)subjects,andfoundsignificantlylower LDL-ClevelsandLDLparticlesinnon-fastingsubjects,although percentdifferencesintheseparametersweresmall (6).Chinese subjectsshowed amoreobviousreductioninLDL-Clevelsat2 and4hpostprandially,forexample,about18%afteradailymeal and28%afterahigh-fatmeal (18,23).Moreover,considering thatultracentrifugation isthereferencemethodforLDL-C measurement,andtheVAPmethodisrapidultracentrifugation, thereductionofLDL-Clevelsafteradailymealcannotbe ignored,especiallyinChinesesubjects.
ItwasreportedthatLDL-CCM levelswerehigherthanLDLCmeasuredbyNMRinChinesesubjectswithdifferentdiseases (22).Inthisstudy,boththeLDL-CVAP levelsandLDL-C levels calculatedbyformulaswerelowerthanLDL-CCM levelsatboth thefastingandpostprandialstates,whichpromptedustopay moreattentiontothedifferencebetweenLDL-Clevelscalculated byformulasandLDL-CVAP levels.
TheFriedewaldformulaisrecommendedtocalculateLDLClevelswhenTGlevelsarenotveryhigh (8).Inthisstudy, therewasnosubjectwithfastingTG ≥ 4.5mmol/l,which maycontributetothestrongestcorrelationbetweenLDL-CF andLDL-CVAP atthefastingstate.However,theTG/VLDL ratiovarieswithTGincreasingatthefastingandnon-fasting states,whichdecreasestheaccuracyoftheFriedewaldformula inLDL-Cestimation.Itisworthnotingthatthelowest accuracywasfoundinLDL-CF at4hpostprandiallywhenTG reachedthepeaklevel.Therefore,otherformulaswereproposed forhigheraccuracywhenTGincreased,especiallyatthe postprandialstate.
ComparedtothestableratioofTG/VLDL-Cinthe Friedewaldformula(2.2formmol/lor5.0formg/dl),those intheVujovicandMartin–Hopkinsformulaswerechanged (9,10).TheratiointheVujovicformulawasstillfixed,but relativelygreater,presentingas3formmol/lor6.85formg/dl (9).ItsaccuracyhadbeendemonstratedinwholeTC,TG,and LDL-Cranges (9) WithTGlevelsincreased,thepostprandial correlationcoefficientsbetweenLDL-CV andLDL-CVAP were strongerthanthefastingstate,andhigherthanthoseoftheother threeformulas.However,theaccuracyoftheVujovicformula seemedtoberelativelylow,especiallyatthefastingstateand 2hpostprandially,althoughitincreasedafteradailymeal,and seemedtobebetterthanFriedewaldformulaat4hafteradaily meal.Thelowaccuracymayberesultedfromitsoverestimation ofLDL-CcomparedtoLDL-CVAP
TheratioofTGandVLDL-CintheMartin–Hopkins formulabecomescomplicatedanddependentonTGand non-HDL-Clevels,varyingfrom3.1to11.9(formg/dl) (10) WithTGincreasingandnon-HDL-Cdecreasing,itelevates
correspondently.TheaccuracyofMartin–Hopkinsformula wasmoderateonthewhole,butthedifferenceinaccuracy betweenthefastingandpostprandialstateswassmall,which wasconsistentwiththefindingsofSathiyakumaretal.,which foundthattheMartin–Hopkinsformulawaslessaffectedbydiet thantheFriedewaldformula (7).However,thecomplexityofthe Martin–Hopkinsratiocouldreduce theconvenienceinclinical practicetoacertainextent.Afterall,clinicianscannotremember somanynumbers.
OtherthantheFriedewald,Vujovic,andMartin–Hopkins formulas,thenovelSampsonformulauseshigher-order mathematicaltermsintheformofabivariatequadraticequation thatshouldbetterreflecttheamountofTGinthecore ofthelipoproteins (24).TheSampsonformulaisbasedon thedataof 8,656Americanadultswithahighfrequencyof hypertriglyceridemia,anditwasconfirmedtobesuitablefor LDL-CcalculationofsampleswithTGover9mmol/l (11), whichmaycontributeto thehighestaccuracyatthepostprandial statesafteradailymeal.Actually,atthebeginningofthe establishmentoftheSampsonformula,acomparisonwasmade betweenfastingandnon-fastingsamples,whichsuggestedthat thisformulawasalsoapplicabletonon-fastingsamples (11)
TheLDLparticlescould bedividedintodifferent subfractionsaccordingtotheirsize.ThesizeofLDLparticles hadbeensuggestedasareliableassessmentofatherogenicity (25).ThesubfractionsofLDLparticlesatthepostprandial stateswerereported tobelowerbyadifferentdegreethanthose infastingstates (6).ThismaycontributetotheFriedewald andSampsonformulas beingthebestchoiceforfastingand postprandialstates,respectively.
Thisstudyisassociatedwithseverallimitations.First,the samplesizeinthisstudywassmallcomparedtootherclinical studies (7).Second,therewere46.2%ofsubjectsgotastatin historywhichmaycausevariationwiththosewithoutstatinuse. Third,weanalyzedoursubjectsasawholeotherthanstratified analysis,whichmaymakeamorepreciseresult.
Conclusion
Inconclusion,amongfourformulas,theFriedewaldformula wasrecommendedto calculatefastingLDL-C,whilethe Sampsonformulaseemedtobeabetterchoicetocalculate postprandialLDL-ClevelsinChinesesubjects.
Dataavailabilitystatement
Therawdatasupportingtheconclusionsofthisarticlewill bemadeavailablebytheauthors,withoutunduereservation.
Ethicsstatement
Thestudiesinvolvinghumanparticipantswerereviewedand approvedbyEthics CommitteeofTheSecondXiangyaHospital
Xuetal. 10.3389/fcvm.2022.944003
Frontiersin CardiovascularMedicine 07 frontiersin.org 33
ofCentralSouthUniversity.Thepatients/participantsprovided theirwritteninformedconsentto participateinthisstudy.
Authorcontributions
Allauthorshaveacceptedresponsibilityfortheentire contentofthismanuscriptandapproveditssubmission.
Funding
ThisstudywassupportedbygrantsfromtheNational NaturalScienceFoundation ofChina(Nos.81270956and 81470577toLL),theNaturalScienceFoundationofHunan Province(2022JJ70136),andtheProjectoftheHealth CommissionofHunanProvince,China(No.202203012938 toLL).
Acknowledgments
WewouldliketoacknowledgeprofessorDengin XiangyaSchoolof PublicHealthforprovidinghelpwith statisticalanalyses.
References
1.ReamyBV,WilliamsPM,KuckelDP.Preventionofcardiovasculardisease. PrimCare. (2018)45:25–44.doi:10.1016/j.pop.2017.11.003
2.DomanskiMJ,TianX,WuCO,ReisJP,DeyAK,GuY,etal.Timecourseof LDLcholesterolexposureandcardiovasculardiseaseeventrisk. JAmCollCardiol. (2020)76:1507–16.doi:10.1016/j.jacc.2020.07.059
3.TomlinsonB,PatilNG,FokM,LamCWK.Managingdyslipidemiain patientswithType2diabetes. ExpertOpinPharmacother. (2021)22:2221–34.doi:10.1080/14656566.2021.1912734
4.WolskaA,RemaleyAT.MeasuringLDL-cholesterol:whatisthebestwayto doit? CurrOpinCardiol. (2020)35:405–11.doi:10.1097/HCO.0000000000000740
5.KulkarniKR.Cholesterolprofilemeasurementbyverticalautoprofilemethod. ClinLabMed. (2006)26:787–802.doi:10.1016/j.cll.2006.07.004
6.FarukhiZM,DemlerOV,CaulfieldMP,KulkarniK,WohlgemuthJ,Cobble M,etal.Comparisonofnon-fastingandfastinglipoproteinsubfractionsandsize in15,397apparentlyhealthyindividuals:ananalysisfromtheVITaminDand OmegA-3TriaL. JClinLipidol. (2020)14:241–51.doi:10.1016/j.jacl.2020.02.005
7.SathiyakumarV,ParkJ,GolozarA,LazoM,QuispeR,GuallarE,etal.Fasting versusnon-fastingandlow-densitylipoproteincholesterolaccuracy. Circulation. (2018)137:10–9.doi:10.1161/CIRCULATIONAHA.117.030677
8.FriedewaldWT,LevyRI,FredricksonDS.Estimationoftheconcentration oflow-densitylipoproteincholesterolinplasma,withoutuseofthepreparative ultracentrifuge. ClinChem. (1972)18:499–502.doi:10.1093/clinchem/18.6.499
9.VujovicA,Kotur-StevuljevicJ,SpasicS,BujisicN,MartinovicJ,VujovicM,et al.EvaluationofdifferentformulasforLDL-Ccalculation. LipidsHealthDis. (2010) 9:27.doi:10.1186/1476-511X-9-27
10.MartinSS,BlahaMJ,ElshazlyMB,TothPP,KwiterovichPO,Blumenthal RS,etal.Comparisonofanovelmethodvs.theFriedewaldequationforestimating low-densitylipoproteincholesterollevelsfromthestandardlipidprofile. JAmMed Assoc. (2013)310:2061–8.doi:10.1001/jama.2013.280532
11.SampsonM,LingC,SunQ,HarbR,AshmaigM,WarnickR,etal.Anew equationforcalculationoflow-densitylipoproteincholesterolinpatientswith normolipidemiaand/orhypertriglyceridemia. JAmMedAssocCardiol. (2020) 5:540–8.doi:10.1001/jamacardio.2020.0013
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedin theabsenceof anycommercialorfinancialrelationships thatcouldbeconstruedasapotentialconflict ofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddo notnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
Supplementarymaterial
TheSupplementaryMaterialforthisarticlecanbe foundonlineat: https://www.frontiersin.org/articles/10.3389/ fcvm.2022.944003/full#supplementary-material
12.PianiF,CiceroAFG,VenturaF,DormiA,FogacciF,Patrono D,etal.EvaluationoftwelveformulasforLDL-Cestimationin alarge,blinded,randomItalianpopulation. IntJCardiol. (2021) 330:221–7.doi:10.1016/j.ijcard.2021.02.009
13.NordestgaardBG,LangstedA,MoraS,KolovouG,BaumH,BruckertE,et al.Fastingisnotroutinelyrequiredfordeterminationofalipidprofile:clinical andlaboratoryimplicationsincludingflaggingatdesirableconcentrationcutpoints-ajointconsensusstatementfromtheEuropeanAtherosclerosisSocietyand EuropeanFederationofClinicalChemistryandLaboratoryMedicine. EurHeartJ. (2016)37:1944–58.doi:10.1093/eurheartj/ehw152
14.KolovouGD,MikhailidisDP,KovarJ,LaironD,NordestgaardBG,Ooi TC,etal.Assessmentandclinicalrelevanceofnon-fastingandpostprandial triglycerides:anexpertpanelstatement. CurrVascPharmacol. (2011)9:258–70.doi:10.2174/1570211213146321611
15.KolovouGD,WattsGF,MikhailidisDP,Perez-MartinezP,MoraS,Bilianou H,etal.Postprandialhypertriglyceridaemiarevisitedintheeraofnon-fastinglipid profiletesting:a2019expertpanelstatement,maintext. CurrVascPharmacol. (2019)17:498–514.doi:10.2174/1570161117666190507110519
16.DellaPepaG,VetraniC,VitaleM,BozzettoL,CostabileG,CiprianoP, etal.Effectsofadietnaturallyrichinpolyphenolsonlipidcompositionof postprandiallipoproteinsinhighcardiometabolicriskindividuals:anancillary analysisofarandomizedcontrolledtrial. EurJClinNutr. (2020)74:183–92.doi:10.1038/s41430-019-0459-0
17.KolovouG,OoiTC.Postprandiallipaemiaandvasculardisease. CurrOpin Cardiol. (2013)28:446–51.doi:10.1097/HCO.0b013e3283606971
18.LinQZ,ChenYQ,GuoLL,XiangQY,TianF,WenT,etal.Comparison ofnon-fastingLDL-ClevelscalculatedbyFriedewaldformulawiththose directlymeasuredinChinesepatientswithcoronaryheartdiseaseaftera dailybreakfast. ClinChimActa. (2019)495:399–405.doi:10.1016/j.cca.2019. 05.010
19.XuJ,ChenYQ,ZhaoSP,LiuL.Determinationofoptimalcut-off pointsafterahigh-fatmealcorrespondingtofastingelevationsoftriglyceride andremnantcholesterolinChinesesubjects. LipidsHealthDis. (2019) 18:206.doi:10.1186/s12944-019-1146-9
Xuetal. 10.3389/fcvm.2022.944003
Frontiersin CardiovascularMedicine 08 frontiersin.org 34
20.XiangQY,TianF,LinQZ,DuX,ZhangSL,GuiYJ,etal.Comparisonof remnantcholesterollevelsestimatedbycalculatedandmeasuredLDL-Clevels inChinesepatientswithcoronaryheartdisease. ClinChimActa. (2020)500:75–80.doi:10.1016/j.cca.2019.09.020
21.BachorikPS,RossJW.NationalCholesterolEducationProgram recommendationsformeasurementoflow-densitylipoproteincholesterol: executivesummary.TheNationalCholesterolEducationProgram WorkingGrouponlipoproteinmeasurement. ClinChem. (1995) 41:1414–20.doi:10.1093/clinchem/41.10.1414
22.HuD,MaoL,TangX,ChenJ,GuoX,LuoQ,etal.Nuclearmagnetic resonancerevealspostprandiallow-densitylipoproteincholesteroldetermined byenzymaticmethodcouldbeamisleadingindicator. ClinChimActa. (2021) 514:59–65.doi:10.1016/j.cca.2020.12.013
23.ZhuLY,WenXY,XiangQY,GuoLL,XuJ,ZhaoSP,etal.Comparisonof thereductionsinLDL-Candnon-HDL-Cinducedbytheredyeastriceextract Xuezhikangbetweenfastingandnon-fastingstatesinpatientswithcoronary heartdisease. FrontCardiovascMed. (2021)8:674446.doi:10.3389/fcvm.2021. 674446
24.CwiklinskaA,WieczorekE,GliwinskaA,MarcinkowskaM,CzaplinskaM, MickiewiczA,etal.Non-HDL-C/TGratioindicatessignificantunderestimation ofcalculatedlow-densitylipoproteincholesterol(LDL-C)betterthanTGlevel:a studyonthereliabilityofmathematicalformulasusedforLDL-Cestimation. Clin ChemLabMed. (2021)59:857–67.doi:10.1515/cclm-2020-1366
25.SuperkoHR,GadesamRR.IsitLDLparticlesizeornumberthat correlateswithriskforcardiovasculardisease? CurrAtherosclerRep. (2008)10:377–85.doi:10.1007/s11883-008-0059-2
Xuetal. 10.3389/fcvm.2022.944003
Frontiersin CardiovascularMedicine 09 frontiersin.org 35
PUBLISHED 22August2022
DOI 10.3389/fcvm.2022.956086
OPENACCESS
EDITEDBY
TatsuyaSawamura, ShinshuUniversity,Japan
REVIEWEDBY JinxingLiu, FuwaiHospital,China HiroyukiItabe, ShowaUniversity,Japan
*CORRESPONDENCE
FengHuaDing ruijindfh@126.com
WeiFengShen rjshenweifeng@126.com
† Theseauthorshavecontributed equallytothiswork
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine
RECEIVED 29May2022
ACCEPTED 01August2022
PUBLISHED 22August2022
CITATION
ShenY,WangXQ,DaiY,WangYX, ZhangRY,LuL,DingFHandShenWF (2022)Diabeticdyslipidemiaimpairs coronarycollateralformation:An update. Front.Cardiovasc.Med. 9:956086. doi:10.3389/fcvm.2022.956086
COPYRIGHT
©2022Shen,Wang,Dai,Wang,Zhang, Lu,DingandShen.Thisisan open-accessarticledistributedunder thetermsofthe CreativeCommons AttributionLicense(CCBY).Theuse, distributionorreproductioninother forumsispermitted,providedthe originalauthor(s)andthecopyright owner(s)arecreditedandthatthe originalpublicationinthisjournalis cited,inaccordancewithaccepted academicpractice.Nouse,distribution orreproductionispermittedwhich doesnotcomplywiththeseterms.
Diabeticdyslipidemiaimpairs coronarycollateralformation: Anupdate
YingShen1†,XiaoQunWang1†,YangDai1†,YiXuanWang1 , RuiYanZhang1,2,LinLu1,3,FengHuaDing1* and WeiFengShen1,3*
1 DepartmentofCardiovascularMedicine,SchoolofMedicine,RuijinHospital,ShanghaiJiaoTong University,Shanghai,China, 2 ShanghaiClinicalResearchCenterforInterventionalMedicine, Shanghai,China, 3 InstituteofCardiovascularDisease,ShanghaiJiaoTongUniversitySchool ofMedicine,Shanghai,China
Coronarycollateralizationissubstantiallyimpairedinpatientswithtype2 diabetesandocclusivecoronaryarterydisease,whichleadstoaggravated myocardialischemiaandamoredismalprognosis.Inadiabeticsetting,altered serumlipidprofilesandprofoundglycoxidativemodificationoflipoprotein particlesinduceendothelialdysfunction,bluntendothelialprogenitorcell response,andseverelyhampergrowthandmaturationofcollateralvessels. Theimpactofdyslipidemiaandlipid-loweringtreatmentsoncoronary collateralformationhasbecomeatopicofheightenedinterest.Inthisreview, wesummarizedtheassociationoftriglyceride-basedintegrativeindexes, hypercholesterolemia,increasedLp(a)withitsglycoxidativemodification,as wellasquantityandqualityabnormalitiesofhigh-densitylipoproteinwith impairedcollateralformation.Wealsoanalyzedtheinfluenceofinnovative lipid-modifyingstrategiesoncoronarycollateraldevelopment.Therefore, clinicalmanagementofdiabeticdyslipidemiashouldtakeintoaccountof itseffectoncoronarycollateralizationinpatientswithocclusivecoronary arterydisease.
KEYWORDS
dyslipidemia,type2diabetesmellitus,coronarycollateralcirculation,coronaryartery disease,lipid-loweringtherapy
Abbreviations:AGE,advancedglycationendproduct;Apo,apolipoprotein;CAD,coronaryartery disease;CEC,cholesteroleffluxcapacity;CETP,cholesterolestertransferprotein;CTRP,C1q tumornecrosisfactorrelatedprotein;DPP4,dipeptidylpeptidase-4;eNOS,endothelialnitric oxidesynthase;FGF,fibroblastgrowthfactor;GLP-1,glucagon-likepeptide-1;HDL,high-density lipoprotein;HDL-C,HDLcholesterol;HGF,hepatocytegrowthfactor;HIF,hypoxia-inducible factor;LCAT,lecithincholesterolacyltransferase;LDL,low-densitylipoprotein;LDL-C,lowdensitylipoproteincholesterol;Lp(a),lipoprotein(a);MCP,monocytechemotacticprotein;NO, nitricoxide;oxLDL-C,oxidizedlow-densitylipoproteincholesterol;PCSK9,proproteinconvertase subtilisin/kexintype9;PON,paraoxonase;PUFA,polyunsaturatedfattyacid;RAGE,receptorfor advancedglycationendproduct;ROS,reactiveoxidespices;sdLDL-C,smalldenselow-density lipoproteincholesterol;SGLT2,sodium-glucoseco-transporter-2;T2DM,type2diabetesmellitus; TG/HDL-C,triglyceride/high-densitylipoproteincholesterol;TRL,triglyceride-richlipoprotein;TyG index,triglyceride-glucoseindex;VEGF,vascularendothelialgrowthfactor;VEGFR,VEGFreceptor; VLDL,verylowdensitylipoprotein.
TYPE Review
Frontiersin CardiovascularMedicine 01 frontiersin.org 36
Introduction
Type2diabetesmellitus(T2DM)isincreasinglyprevalent worldwide,andcardiovasculardiseaserepresentstheleading causeofmorbidityandmortalityinpatientswithT2DM(1).
Aclusteroflipidmetabolicabnormalities,collectivelyreferred toasdiabeticdyslipidemia,havebeenwellestablishedas amajorriskfactorforadversecardiovascularoutcomesin diabeticpatients.Thepatternofdiabeticdyslipidemiaconsists ofhypertriglyceridemiaassociatedwithincreasedtriglyceriderichlipoproteinsandtheirremnants,decreasedhigh-density lipoproteincholesterol(HDL-C),andelevatedlow-density lipoproteincholesterol(LDL-C)levelswithpredominanceof smalldenseLDL-C(sdLDL-C).Meanwhile,hyperglycemiaand chronicinflammationindiabeticconditionspromoteglycation andoxidativemodificationoflipoproteinparticles,leadingto changesinconformationandfunction,alteredinteractionwith membranereceptorsanddownstreamsignaling,andswitchof thephenotypetowardamoreatheropronestate(2).
Coronarycollateralshavebeenrecognizedasanimportant compensatorymechanisminsalvageofischemicmyocardium, preservationofleftventricularfunction,andimprovementof prognosisforpatientswithobstructivecoronaryarterydisease (CAD)(3–5).Duringthedevelopmentofcoronarycollaterals, twodistinctprocesses,arteriogenesisandangiogenesis,are involved.Theformerpertainstotheremodelingofpreexisting arterialvesselsthroughanatomicincreaseinlumenareaand wallthickness.Thelatterisdefinedasgrowthofnewcapillaries thatstemfromthebuddingofpreexistingcapillaryvessels(6). Theseprocessesarefinelytunedbyavarietyofbiomechanical andbiochemicalfactors,includingperfusionpressure,wall shearstress,systematichypoxia,oxidativestress,inflammatory responseandendothelialfunction.
Numerousclinicalobservationsrevealsubstantially impairedcollateralcirculationinocclusiveCADpatients withdiabetes.Giventhegenerallymoresevereatherosclerotic lesionsandmicrocirculationdysfunctionindiabeticpatients, poorcollateralizationmayprovideasignificantadd-oneffect toaggravatemyocardialischemiaandcontributetoamore dismalprognosis(7).Acoupleofunderlyingmechanisms forthepoorcollateralformationindiabeticpatientshave beenidentified.Chronichyperglycemiaandtheengagement ofadvancedglycationend-productswiththeirreceptors (AGE-RAGEaxis)adverselyaffectscollateraldevelopment byinhibitingvesselgrowthandmaturation(8).Ontheother hand,disturbedlipidmetabolismalsoplaysacriticalrole andisregardedasahallmarkofimpairedangiogenesis(9, 10).Currently,theimpactofdyslipidemiaandlipid-lowering treatmentsoncoronarycollateralformationhasbecomeatopic ofheightenedinterest.Thisreviewisthefirsttosummarize therecentliterature,incombinationwithourstudyfindings, toelucidatetheassociationofdifferentcomponentsofdiabetic dyslipidemiawithcoronarycollateralizationandhighlighttheir
potentialclinicalimplicationsinT2DMpatientswithCAD.The relevantclinicalstudiesinvestigatingtheassociationbetween lipidprofilesandcoronarycollateralizationaresummarizedin Table1
Impactofdiabeticdyslipidemiaon collateralformation
Hypertriglyceridemia
Inpatientswithimpairedglucosetolerance,bluntinsulin sensitivityleadstocompensatoryhyperinsulinemiaand increasessecretionoftriglycerideandtriglyceride-rich lipoproteins.Hypertriglyceridemiaconfersanincreasedrisk ofCADandadverseoutcomesinpatientswithT2DM,by promotingreleaseofexcessivefreefattyacidsandstimulating productionofproinflammatorycytokines,fibrinogen,and coagulationfactors(11).Previousstudieshaveshownthat certainconditionswithaclusterofriskcomponentsincluding hypertriglyceridemia(e.g.,metabolicsyndrome,overweight, orobesity)aremorelikelytobeassociatedwithendothelial dysfunctionandreducednewvesselgrowth(10, 12),but theindependentroleofhypertriglyceridemiaincoronary collateralformationremainsdifficulttobeprovenlargelydueto concomitantchangesinotherlipoproteinsandrelevantfactors, particularlyinpatientswithT2DM.
Inrecentyears,severalnovelindexesbyintegrating triglyceridewithsomerelatedmetabolicmeasurements(suchas HDL-Candglucose)havebeenproposedtobetterstratifythe statusofcoronarycollateralization.Triglyceride-glucose(TyG) index,calculatedaslog[fastingtriglycerides(mg/dL) × fasting bloodglucose(mg/dL)/2],hasbeensuggestedasasurrogate markerofinsulinresistance(13).ElevatedTyGindexcorrelates wellwithhigharterialstiffnessandmicrovasculardamage(14), whichisassociatedwithdecreasedcoronaryperfusion,reduced shearstressandarteriogenesis(15).Inalargeobservational study,chronictotalocclusionpatientswithpoorcoronary collateralshadhigherTyGindexcomparedtothosewithgood collaterals.TyGwassignificantlyassociatedwithpoorcollateral formationevenafteradjustingforvariousconfounders(16).
TriglyceridetoHDL-Cratio(TG/HDL-Cratio)andatherogenic indexofplasma(logarithmictransformationofTG/HDL-C ratio)reflectthecomprehensivesituationofbloodlipidsand severityofinsulinresistance(17).Anobservationalstudyof elderlypatientswithacutemyocardialinfarctionshowedthatan elevatedTG/HDLratiowasindependentlyassociatedwithpoor developmentofcoronarycollateralcirculation(18).Ofnote, althoughanumberofreportshavesuggestedtheprognosticrole ofatherogenicindexofplasmabeyondtraditionalriskfactors (19, 20),furtherprospectivestudiesareneededtoexamineifthis indexisapplicabletopredictcoronarycollateralizationintype2 diabeticpatientswithCAD.
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 02 frontiersin.org 37
TABLE1Summaryofclinicalstudiesinvestigatingtheassociationbetweenlipidprofilesandcoronarycollateralization. AuthorsStudy
Liuetal.(10)Consecutivepatients withCTO undergoingCAG
Gaoetal.(16)Consecutivepatients withacutecoronary syndromeandCTO
Observational study
Observational study
1,653patients(poor CC:355;goodCC: 1298)
1,093patients(poor CC:775;goodCC: 318)
TGAftermultipleadjustment,thequartilesofTG(adjusted OR=1.267,95%CI1.088–1.474, P =0.002)remainedan independentfactorofpoorCC.
TyGindexTyGindexwassignificantlyhigherinpatientswithpoorCC comparedtothosewithgoodCC(9.3 ± 0.65vs.8.8 ± 0.53, P < 0.001).
TheproportionofpoorCCincreasedstepwisefromthelowest tothehighestTyGindextertile(15.3%vs.22.8%vs.49.2%, P < 0.001).
Afteradjustingforconfoundingfactors,TyGindexremained correlatedwiththeoccurrenceofimpairedCC(OR1.59,95% CI1.07–2.36andOR5.72,95%CI3.83–8.54formiddleand highesttertilegroupsvs.lowesttertilegroup,all P < 0.001)
TheimprovementoftheAUCforassessingpoorCCwasmost significantwhenaddingTyGindextobaselinemodel,witha bestcut-offvalueof9.105.
Themostsignificantenhancementinriskreclassificationand discriminationwasfoundafterinclusionofTyGindexinto baselinemodel,withaNRIof0.238(P < 0.001)andanIDIof 0.103(P < 0.001).
Liuetal.(18)Consecutivepatients (≥60years)with ST-elevationMI undergoingprimary PCI
Retrospective case-control study
346patients(poor CC:238;goodCC: 108)
TG/HDLTG/HDLratiowassignificantlyhigherinpatientswithpoorly developedCCthaninthosewithwell-developedCC (2.88 ± 2.52vs.1.81 ± 1.18, P < 0.001).
Inmultivariatelogisticregressionanalysis,higherTG/HDL ratioservedasanindependentpositivepredictorofpoor developmentofCC(OR1.789,95%CI1.346–2.378, P < 0.001).
TheAUCofTG/HDLratioforpredictingpoorCCwas0.716 (95%CI0.654–0.778, P < 0.001)withanoptimalcut-offvalue of1.58,sensitivityof55.7%andspecificityof71.9%.
Arasetal.(33)Stableangina pectoriswithCTOof onemajorcoronary artery
Retrospective study 60patients(poor CC:31;goodCC:29)
Lp(a)Lp(a)levelsweresignificantlyhigherandvascularendothelial growthfactorlevelsweresignificantlylowerinpatientswith poorCCthaninthosewithgoodCC(34 ± 19vs. 20 ± 12mg/dl, P < 0.001,and2.5 ± 0.7vs.3.4 ± 0.8ng/dl, P < 0.001,respectively).
PoorlydevelopedCCweremoreprevalentinpatientswithLp (a)levels ≥30mg/dlthaninthosewithLp(a)levels <30mg/dL (72vs.37%, P =0.008).
AstrongnegativecorrelationwasobservedbetweenLp(a)and vascularendothelialgrowthfactor(r = 0.708, P < 0.0001). HighlevelsofLp(a)negativelyaffectedthedevelopmentofCC (adjustedOR0.92,95%CI0.88–0.96, P =0.009).
Fanetal.(34)Chronicstable coronarydisease withatleastone majorcoronary occlusionora stenosisof ≥95% withTIMIgrade1
Shenetal.(35)Consecutivestable CADpatientswith CTOofatleastone majorepicardial coronaryartery
Observational study 654patients (Rentropscore0,1, 2,and3in44,91, 232,and287 patients, respectively)
Observational study 1284patients(DM: 706;non-DM:578; poorCC:505;good CC:779)
Lp(a)Lp(a)levelsweresignificantlydecreasedacrossRentropscore 0–3(25.80 ± 24.72,18.99 ± 17.83,15.39 ± 15.80,and 8.40 ± 7.75mg/dL, P < 0.001).
Inmodel1,theriskofpoorCC(Rentrop0)wasgreaterinthe thirdLp(a)tertilecomparedtothefirstLp(a)tertile(OR3.34, 95%CI2.32–4.83, P < 0.001).Inmodel2,theriskofpoorCC (Rentrop0)wasgreaterinLp(a) >30mg/dLgroupcompared toLp(a) <30mg/dLgroup(OR6.77,95%CI4.44–10.4, P < 0.001).
Lp(a) TC
LDL-C
Non-HDL
HDL-C TG
Fordiabeticandnon-diabeticpatients,Lp(a),totalcholesterol, LDL-C,andnon-HDL-Clevelswerehigherinpatientswith poorCCthaninthosewithgoodCC,whereasHDL-CandTG levelsweresimilar.
Afteradjustmentforpotentialconfoundingfactors,tertilesof Lp(a),totalcholesterol,LDL-Candnon-HDL-Cremained independentdeterminantsforpoorCC.Asignificant interactionbetweenLp(a)andtotalcholesterol,LDL-Cor non-HDL-Cwasobservedindiabeticpatients(allPinteraction <0.001)butnotinnon-diabetics.
Athightertileoftotalcholesterol(≥5.35mmol/L),LDL-C (≥3.36mmol/L)andnon-HDL-C(≥4.38mmol/L),diabetic patientswithhightertileofLp(a)(≥30.23mg/dL)hadan increasedriskofpoorCCcomparedtothosewithlowtertileof Lp(a)(<12.66mg/dL)(adjustedOR=4.300,3.970and4.386, respectively,all P < 0.001). (Continued)
Shenetal. 10.3389/fcvm.2022.956086
population DesignNumberof patients Parameterof lipidprofiles
Mainrelevantresults
Frontiersin CardiovascularMedicine 03 frontiersin.org 38
TABLE1Continued
AuthorsStudy population DesignNumberof patients
Youetal.(36)Consecutiveacute MIundergoing interventionalCAG
Observational study 409patients(poor CC:277:goodCC 132)
Parameterof lipidprofiles
Mainrelevantresults
Lp(a)PatientswithpoorCChadahigherLp(a)levelthanthosewith goodCC(219.1[98.0–506.9]vs.122.0[64.5–215.6]mg/L, P < 0.001).
TheAUCofLp(a)forpredictingpoorCCwas0.647(95%CI: 0.592–0.702)withthecut-offvalueof199.0mg/L,sensitivityof 55.7%andspecificityof71.9%.
RegressionanalysesrevealedthatpatientswithhighLp(a)levels hadagreaterriskofpoorCCthanthosewithlowLp(a)levels (unadjustedOR2.924,95%CI1.900–4.501;adjustedOR2.929, 95%CI1.863–4.604,both P < 0.001).PatientswithLp(a) ≥30mg/dLalsohadagreaterriskofpoorCCthanthosewith Lp(a) <30mg/dL(unadjustedOR3.394,95%CI2.042–5.640; adjustedOR4.232,95%CI1.400–12.797,both P < 0.001).
Kadietal.(38)Consecutivepatients withCTOofatleast onemajorepicardial coronaryartery
Case-control study 151patients(poor CC:49;goodCC: 102)
HDL-CSerumHDL-CwaslowerinpoorCCgroupcomparedtogood CCgroup(34.9 ± 8mg/dLvs.43.7 ± 9.4mg/dL, P < 0.001). TheproportionofpatientswithlowHDL-Cwassignificantly higherinthepoorCCgroupcomparedwiththegoodCCgroup (P < 0.001).SerumTGlevelsandpercentageofMIhistorywere higherinthepoorCCgroupcomparedwithgoodCCgroup (P =0.015and P =0.026,respectively).Therewasapositiveand strongcorrelationbetweenRentropgradeandserumHDL-C level(r =0.503, P < 0.001).Multivariateregressionanalysis showedthatreducedHDL-Clevelwasanindependent predictorforpoorCC(OR4.3,95%CI1.964–9.369, P < 0.001).
Hsuetal.(39)Consecutivepatients undergoingCAG
Leeetal.(44)Consecutivepatents undergoingCAG
Case-control study
Case-control study
501patients(poor CC:311;good CC:190)
226patients(poor CC:71;good CC:155)
HDL-C TherewasnosignificantdifferenceinHDL-Candother variablesbetweengoodandpoorCC.Multivariateanalysis showedonlynumberofdiseasedvesselswasasignificant predictorofpoorcollateraldevelopment(OR0.411, p < 0.001).
CEC CECwashigherinthegoodthaninthepoorCCgroup (22.0 ± 4.6%vs.20.2 ± 4.7%, P =0.009).Inmultivariable analyses,CECwasidentifiedasanindependentpredictorof goodCCafteradjustmentforage,sex,HDL-C(OR,1.10,95% CI1.03–1.18, P =0.004).Itremainedsignificantafteradditional adjustmentforDM,acutecoronarysyndrome,andGensini score(OR1.09,95%CI1.02–1.17, P =0.011).
Wangetal.(46)Patientswithstable anginaand angiographicCTOof atleastonemajor coronaryartery
Case-control study 437patients(DM: 102;non-DM:355; poorCC:210;good CC:227)
CEC Comparedtogoodcollateralizationgroup,CECinpoor collateralizationgroupwassignificantlyhigherinnon-diabetic patients(17.54 ± 11.86%vs.13.91 ± 9.07%, P =0.002).In contrast,CECwasimpairedintype2diabetesirrespectiveof collateralizationstatus(14.66 ± 10.47%vs.13.26 ± 8.64%, P =0.462).CECcorrelatedcloselywithRentropscorein non-diabeticsubjects,whereasnosuchassociationwaspresent forHDL-CorapolipoproteinA-I.Afteradjustingfor conventionalriskfactorsincludingapolipoproteinA-Iin logisticregressionanalysis,elevatedCECwasindependently associatedwithhigherriskofpoorcollateralizationin non-diabeticbutnotindiabeticsubjects.
AUC,areaunderthecurve;CAD,coronaryarterydisease;CAG,coronaryarteryangiography;CC:coronarycollaterals;CEC,cholesteroleffluxcapacity;CI,confidenceinterval;CTO: chronictotalocclusion;DM,diabetesmellitus;HDL-C,high-densitylipoproteincholesterol;IDI,integrateddiscriminationimprovement;LDL,low-densitylipoprotein;Lp(a):lipoprotein (a);MI:myocardialinfarction;NRI,netreclassificationimprovement;OR,oddsratio;PCI,percutaneouscoronaryintervention;TC,totalcholesterol;TG,triglyceride;TyGindex, triglyceride-glucoseindex.
Hypercholesterolemia
Chronicexposuretohighlevelsofcholesteroland LDL-Cresultsinfunctionalandstructuralabnormalities ofthevasculature,includingendothelialdysfunction, subendotheliallipiddeposition,plaqueprogressionand compromisedcollateralvesselgrowth(21, 22).Indiabetic dyslipidemia,hypercholesterolemiaandapredominant increaseinsdLDLparticlesplaynegativerolesincoronary collateralformation.
Underhypercholesterolemia,angiogenesisand arteriogenesisinresponsetotissuehypoxiaaremarkedly attenuated.Hypercholesterolemiadecreasesendothelialnitric oxide(NO)bioavailabilityandNOsynthase(eNOS)expression andactivitywhichareessentialforendothelialprogenitorcell (EPC)migration(23).Inanimalmodels,cholesterolathigh concentrationresultedindelayednativearteriolargrowth causedbyreducedearlymonocyte/macrophageinfluxand migration,andevenmildlyelevatedcholesterolsignificantly decreasedexpressionoffibroblastgrowthfactor(FGF)receptor
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 04 frontiersin.org 39
1,vascularcelladhesionmolecule-1,andmacrophagescavenger receptor-1,mimickingrelativechangesinarteriogenesisand tissueperfusion(24).Theextentofthesealterationswasrelated tothedurationofhypercholesterolemia.Inpatientswith hypercholesterolemia,thenumberandactivityofcirculating EPCsweredecreasedcomparedtonormocholesterolemic subjects(25).CirculatingEPCshavespecialcellularmachinery thatisresistanttovarioustypesofstress,whichallowthemto participateintissuerepair.Interestingly,hypercholesterolemia reducedarteriogenesismoredominantlythanhyperglycemiaor hyperinsulinemia(26).
Theinhibitoryeffectofhypercholesterolemiaon angiogenesis/arteriogenesiscouldbeattributedtothenegative effectofLDL-Conendothelialcellresponsivenesstogrowth factors(25).T2DMisusuallyaccompaniedbyoxidationor glycationofLDL,andglycoxidativelymodifiedLDLposesmore pro-atherogenicandantiangiogenicpropertiesthannative LDL(27).Inaddition,thepredominanceofsdLDL-Cconfers athreefoldincreasedriskforCAD,owningtotheirgreater propensityforendothelialpenetrationintoarterialwall,lower bindingaffinityforLDLreceptor,longercirculationtime, andhighersusceptibilitytoglycation,oxidativemodification, anduptakebyscavengerreceptors(28).Theprospective Framinghamoffspringstudyandlargecohortstudiessuggest thatsdLDLissuperiortoLDL-Candotherbiomarkersin predictingfuturecardiovasculareventsinstableCADpatients withT2DMorhypertriglyceridemia(29–31).Nevertheless, thereisapaucityofclinicaldataregardingtheimpactof elevatedLDL-CandsdLDL-ConcollateralformationinT2DM patientswithCAD.
Increasedcirculatinglipoprotein(a)
Lipoprotein(a)isageneticallydeterminedlipoprotein, whichcontainsprincipallyacholesterolrichLDLparticle,one moleculeofapoB-100,andanapo(a).Noteworthy,Lp(a) isknowntohaveatherothrombogenicpropertybyinhibiting fibrinolysissystemandpromotingthrombusformation.Inspite ofaveryskeweddistribution,elevatedcirculatingLp(a)has emergedasanindependentpredictorofadverseoutcomesfor bothgeneralandhigherriskpopulations,especiallywhenLDLClevelsareelevated(32).Inobservationalstudiesofpatients withstableCAD,serumLp(a)levelsdecreasedstepwiseacross angiographiccoronarycollateralgrade,andelevatedLp(a) predictedpoorcollateraldevelopment(33, 34).Intriguingly,a robustassociationbetweenLp(a)interactionswithcholesterolcontaininglipidsandcoronarycollateralformationwas suggestedinpatientswithT2DM,whichwasnon-linearand limitedtohighLp(a)andLDL-Cornon-HDL-Clevels(35).
Athightertilesoftotalcholesterol(≥5.35mmol/L),LDL-C (≥3.36mmol/L)andnon-HDL-C(≥4.38mmol/L),patients withhightertileofLp(a)(≥30.23mg/dL)hadasignificantly
increasedriskofpoorcollateralizationcomparedwiththose withlowtertileofLp(a)(<12.66mg/dL)(all P < 0.001). Furthermore,theadditionalinclusionofinteractionofLp(a) withtotalcholesterol,LDL-Candnon-HDL-Cprovidedbetter riskpredictionofpoorcoronarycollaterals.However,no interactionofLp(a)withHDL-Candtriglycerideoncoronary collateralizationwasobserved.Inpatientswithacutemyocardial infarction,increasedLp(a)inserumwascloselycorrelated withpoorcoronarycollaterals(36).OverexpressionofLp(a)in transgenicmiceresultedinmarkedlyreducednaturalrecovery ofbloodflowinhindlimbischemiaanimalmodelsinadosedependentmanner.Lp(a)wasfoundtostimulatethegrowthof vascularsmoothmuscle,whichwasreversedbyintramuscular injectionofhepatocytegrowthfactor(HGF)(37).Overall,these resultshighlightthatLp(a)mayreflectcoronarycollateralstatus.
Lipoprotein(a)ishighlyconcentratedinthearterial wall,carriescholesterolandbindsoxidizedphospholipids. ElevatedcirculatingLp(a)inhibitstransforminggrowthfactorβ activityandattenuatessynthesisand/orreleaseofvascular endothelialgrowthfactor(VEGF)anddecreasesproduction ofendothelium-derivedNO,leadingtoimpairedangiogenesis (33).Moreover,oneofourongoingstudiesindicatesthat circulatingLp(a)canalsoundergoglycationmodification.As acharacteristicproteinofLp(a),apo(a)isalargeprotein containingmanykringledomains.Basedonmassspectrometry results,theglycationmodificationsitesofapo(a)aremainly concentratedonthekringleIVdomain,whereasonlyafew glycationmodificationsitesaredistributedinotherdomains. Phenotypicexperimentsconfirmedthatglycatedapo(a)and glycatedapo(a)-kIVcanconsistentlyinduceinflammatory factorexpressionandRAGEpathwayactivation.Inadiabetic mousemodelwithhindlimbischemia,intraperitonealinjection ofglycatedapo(a)andglycatedapo(a)-kIV,respectively,resulted inasubstantialinhibitionofangiogenesis.Furtherstudies havedemonstratedthatglycatedapo(a)andglycatedapo(a)kIVpromotedtheexpressionofadhesionmolecules,decreased theactivitiesofeNOSandproductionofNO,andinhibited endothelialproliferation,migration,andtubularformation. Glycatedapo(a)andglycatedapo(a)-kIVinducedendothelial dysfunctionmainlythroughup-regulationofnuclearcorepressorNR0B1,whichbindsandinhibitsthetranscriptional activityofcardiovascularprotectivenuclearreceptorssuchas LXR,NR4A1,andestrogenreceptor.
Subnormalhigh-densitylipoprotein cholesterollevelandhigh-density lipoproteindysfunction
ReducedHDL-Cinserumisoneofthetypical manifestationsofdiabeticdyslipidemia.Therelationship betweenserumlevelsofHDL-Candcoronarycollateral formationremainscontroversial.InpatientswithstableCAD,
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 05 frontiersin.org 40
onestudyshowedthatdecreasedHDL-Clevelspredicted poorcoronarycollateralization(38),butsuchresultswerenot replicatedinotherstudies(35, 39).Theseobservationssupport anotionthatHDLfunctionalityratherthanquantityalone maymorereliablyreflectitsoverallpropertiesandhasabetter clinicalrelevance(40).
High-densitylipoproteinparticleiscomposedofanouter layerofapolipoproteinsandphospholipids,surroundingacore ofesterifiedcholesterol,andhaspluripotenteffects.Itprimarily mediatesreversecholesteroltransportbycarryingcholesterol fromperipheraltissuestotheliverformetabolismand excretion.Inadditiontoitsantioxidative,anti-inflammatory, antithromboticandanti-apoptoticfeatures,HDLitselfhas proangiogenicpropertiesandregulatesischemia-induced angiogenesisinmultipleways(41).Cholesteroleffluxcapacity ofHDL(HDL-CEC)isessentialinmaintainingcholesterol balanceinendothelialcells,anditregulatesangiogenesis via modulationoflipidraftsandVEGFreceptor(VEGFR)2signaling(42).Largecohortstudiesandmeta-analysis indicatedthatelevatedHDL-CECwasassociatedwithfavorable clinicaloutcomesindependentofcirculatingHDL-Clevels (43).Acase-controlstudyreportedahigherHDL-CEC inchronictotalocclusionpatientswithgoodcoronary collateralscomparedtothosewithpoorcollaterals,and highHDL-CECpredictedthepresenceofgoodcoronary collaterals(44).Thedegreeofcoronarycollateralization fromthecontra-lateralvessel(usually via connectionsof theepicardialsurfaceorintraventricularseptum)wasoften visuallyestimatedusingtheRentropgradingsystem(45): 0=novisiblefillingofanycollateralchannel;1=fillingof sidebranchesofthearterytobeperfusedbycollateralvessels withoutvisualizationofepicardialsegment;2=partiallyfilling oftheepicardialarterybycollateralvessels;3=complete fillingoftheepicardialarterybycollateralvessels.Patients werecategorizedintopoor(grade0or1)orgood(grade 2or3)coronarycollateralizationgroup.Thisangiographic assessmentofcoronarycollateralsisroutinelyappliedinclinical practice.Wangetal.foundthatHDL-CECcorrelatedclosely withangiographicRentropcollateralscoreinnon-diabetic patients,whereasHDL-CECwasimpairedinpatientswith T2DMirrespectiveofcollateralizationstatus.Furthermore, thisfindingissupportedby invitro experimentalresults, showingthatalthoughHDLisolatedfromnon-diabetics withpoorcollateralshadsignificantlygreaterpotentialin promotingendothelialtubularformationinMatrigelcompared toHDLisolatedfromthosewithgoodcollateralization, theproangiogeniccapacityofHDLisolatedfromdiabetic patientswasmarkedlyimpairedwhichwasnotinfluenced bycollateralconditions(46).TheseresultsimplythatwellfunctioningHDLisbiologicallycardioprotective,contributing tocoronarycollateralformation.Nevertheless,thefunctional capacityofHDLisseverelycompromisedintype2diabetic patientswithCAD.
Glycationandoxidativemodificationarekeyunderlying mechanismsthatleadtoHDLdysfunctionandtransforms thelipoproteinintoaproinflammatoryproteinunderdiabetic conditions(47).Shenetalfoundthatrelativeintensity ofglycationofapoA-I(apredominantproteinmoietyin HDL)correlatedpositively,whileHDL-associatedparaoxonase (PON)1andPON3activitiesinversely,withtheseverityof coronaryatherosclerosis(48),andwasrelatedtodecreased lecithin:cholesterolacyltransferase(LCAT)activityandplaque progressionintype2diabeticpatientsundergoingpercutaneous coronaryintervention(49).Similarly,abundanceofapoA-IV glycationwasalsocorrelatedwiththepresenceandseverity ofCADinpatientswithT2DM.GlycosylatedapoA-IV inducespro-inflammatoryresponse invitro andincreasesthe expressionoftumornecrosisfactor(TNF)-α andadhesion moleculesbythenuclearreceptorNR4A3,therebypromoting atherosclerosisinapoE-/-mice(50).Recently,thedeleterious effectsofapoA-IandapoA-IVglycationonvesselgrowthin diabeteswereassessed.Inadiabetichindlimbischemiamouse model,bloodreperfusionwasdeterminedbylaserDoppler perfusionimagingaftertreatmentwithintraperitonealinjection ofglycatedapoA-IorglycatedapoA-IV,andthegastrocnemius andsoleusmuscleswerecollectedforpathologicalanalysisand molecularbiologyevaluation.Theresultsshowedthatboth glycatedapoA-IandglycatedapoA-IVinducedinflammatory reactionsinendothelialcellsanddecreasednewvesselgrowth. FurthermechanisticalstudiesrevealedthatglycatedapoA-I skewedmacrophagepolarizationtowardM1phenotypeby activatingSHP2,whereasglycatedapoA-IVdown-regulated cardiovascularprotectivenuclearreceptorNR4A1expression (51),allofwhicharerecognizedasimportantstepsto theinhibitionofangiogenesis.Thesefindingspointtoa notionthatHDLdysfunctionandsubnormalHDL-Clevels actsynergisticallytodecreasecollateralformationinT2DM patientswithCAD.
Clinicalrelevance
Sincediabeticdyslipidemiahamperscollateralvesselgrowth throughinhibitingangiogenesis/arteriogenesisinitsspecific mannerandcoronarycollateralizationisofimportantclinical significance,thechoicebetweentheavailablemanagement optionsforT2DMpatientswithCADshouldaccountforits effectoncollateralformation(Figure1).
Intermsofnon-pharmacologicalintervention,intensive lifestylemodification(includinglivinghabitatchange,exercise, anddiet)exertsbeneficialimpactonhomeostasis,lipidprofiles andcoronarycollateralcirculation(52),thusshouldbethe maininitialstrategy.Cessationofcigarettesmokingisproven todecreaseinflammatoryresponse,increasethenumber andfunctionofEPCs,andimproveVEGFactivities,which arebeneficialfornewvesselgrowth(53).Regularphysical
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 06 frontiersin.org 41
Impactofdiabeticdyslipidemiaoncoronarycollateralformation.Indiabeticconditions,triglyceride(TG)andTG-richlipoproteins(TRL)levels areincreased,high-densitylipoproteincholesterol(HDL-C)levelisreduced,andlow-densitylipoproteincholesterol(LDL-C)leveliselevated withpredominanceofsmalldenseLDL-C(sdLDL-C).Meanwhile,glycationandoxidativemodificationoflipoproteinparticlesoccur,promoting inflammatoryreaction,productionofreactiveoxidespecies(ROS),andendothelialprogenitorcell(EPC)dysfunction.Thesechangeshamper collateralformationthroughinhibitingtheprocessofangiogenesisandarteriogenesis.Exercise,lipid-loweringtherapy,andantidiabeticagents mayimprovecoronarycollateralformation. Representscholesterolesters.GLP-1:glucagon-likepeptide-1;IDL:intermediatedensity lipoprotein;Lp(a):lipoprotein(a);PCSK9:proproteinconvertasesubtilisin/kexintype9;SGLT2:sodium-glucosecotransporter2;VLDL:very low-densitylipoprotein.

exerciseimproveslipidprofile(54),andaugmentsmyocardial oxygendemandandbloodflow,actingasadrivingforcefor arteriogenesis,whichhelpsincoronarycollateralformation inpatientswithstableCAD,exceedingtheeffectofany
drugtreatment(55).Similarly,optimalbloodpressurecontrol (especiallydiastolicbloodpressure)iscrucialinachieving maximalcoronarycollateralflow(56, 57).Whiledietaryquality isimportantforoverallhealth,thetotaldailycaloricintake

Shenetal. 10.3389/fcvm.2022.956086
FIGURE1
Frontiersin CardiovascularMedicine 07 frontiersin.org 42
perse shouldbeakeydeterminantofhyperlipidemiainwhich ahypocaloricplanisfavorableforreducingoverweightand improvinglipidprofileandinsulinsensitivity(58).
Hypertriglyceridemiashouldbetreatedtoeliminateresidual cardiovascularrisk.Fibrates,aputativeagonistligandfor peroxisomeproliferatoractivatedreceptor-alpha,reducethe secretionofvery-low-densitylipoprotein(VLDL)-triglyceride, enhanceremovalofLDL,andincreaseHDL-Clevels(59).
High-doseofomega-3polyunsaturatedfattyacids(PUFA), mainlyeicosapentaenoicacid(EPA)anddocosahexaenoicacid (DHA),isaworthwhileadd-ontreatment,especiallyinstatintreatedpatientswithT2DMandCADinwhomtriglyceride levelsremainelevated(60).Inaddition,PUFAcouldattenuate inflammation,improveendothelialfunction,anddecrease thrombusformation(61).TheREDUCE-ITtrialevaluateda highlypurifiedEPApreparation(4g/day)inpatientswith hypertriglyceridemiaandhighcardiovascularrisk.Theresults wereextraordinary,astherewasa25%relativereduction incardiovascularevents,totalcoronaryrevascularizationas wellasplaqueburden(62).However,theSTRENGTHtrial failedtoobtainsimilarfavorableresultsbytreatmentwith EPA/DHApreparation,andincontrast,itwasassociated withaslightlyhigherrateofatrialfibrillation(63).This discrepancymaybeexplainedbydifferentstudydesignand variousdegreesofchangeintriglyceride-richlipidsaswellas differentialeffectsofEPAandDHAonmembranestructure, inflammatorybiomarkers,endothelialfunction,andtissue distribution(64).Arecentstudydemonstratedanegative correlationbetweenperi-coronaryadiposetissueattenuation assessedbyCTangiographyandtreatmentwithPUFA, suggestingalowerextentofcoronaryinflammation(65, 66). AdipokineC1qtumor-necrosisfactor-relatedprotein(CTRP)
1hasbeenshowntobeinvolvedininflammatoryreaction anddiseasedevelopment(67).ElevatedcirculatingCTRP1 wasassociatedwithpoorcoronarycollateralizationinT2DM patientswithstableanginapectoris.Notably,stimulationof EPCswithCTRP1decreasesbothcordlengthandbranchpoint numberandVEGFR-2levels(68).Whetherthebeneficialeffect offibratesaloneorincombinationwithPUFAoncollateral formation via affectingadipokinesinT2DMpatientswithCAD meritsfurtherconfirmation.
Cholesterol-loweringtherapyisthemainstayinprimary andsecondarypreventionofcardiovasculardiseases(69, 70).StatinseffectivelydecreaseserumLDL-CandsdLDL-C whileincreasingHDL-Clevelsandreducethesusceptibility ofapoBofLDLtoundergooxidationandglycation.
Theyalsodisplaysignificantanti-inflammatorypropertiesand improveendothelialfunction(so-calledpleiotropiceffects) (71).Robustevidencesupportsthefactthatuseofstatins enhancesangiogenesisaswellasarteriogenesisindependentofa cholesterol-loweringmechanism(72, 73).Collateralformation benefitsfromstatintreatmentinT2DMpatientswithCAD, duepartlytoreducedapoptosisanddecreasedreleaseof
solubleVEGFR-1inducedbyproinflammatorycytokines(74, 75).Proproteinconvertasesubtilisin/kexintype9(PCSK9) inhibitorshavebeenincreasinglyusedinthemanagementof dyslipidemiainindividualswithT2DM(76, 77).Theresults of post-hoc subgroupanalysisofrandomizedclinicaltrials indicatedthatalirocumabandevolocumabsignificantlyreduced circulatingLDL-CandLp(a),andincreasedHDL-C,without affectingglycemiclevelsinpatientswithT2DM(78, 79).Current dataconcerningPCSK9inhibitorsoncollateralformation arescarce.An invivo studydemonstratedaproangiogenic activityofevolocumabthroughpromotingcellproliferation, migration,tubulogenesis,andVEGFsecretion(80).Given theirunambiguouslipid-loweringproperties,suchaspecific roleofPCSK9inhibitorsforneo-angiogenesisshouldbe clinicallyattractive.
Severalnewhypoglycemicagents,suchasglucagon-like peptide-1(GLP-1)receptoragonistsandsodium-glucose cotransporter-2(SGLT2)inhibitors,havebeenshown tofavorablyaffectlipoproteinmetabolism(81, 82). Nevertheless,furtherstudiesareneededtoexamineifthey canimprovecollateralformationespeciallyfortype2diabetic patientswithCAD.
Conclusion
Intype2diabeticpatientswithCAD,theroleof hypertriglyceridemiaincollateralformationisnotclearlikely duetotheconcomitantchangesinotherlipoproteins.Elevated circulatingcholesterolandLp(a)andtheirglycoxidative modificationhampertheprocessofnewvesselgrowth. SubnormalHDL-Clevelsand,moreimportantly,deficientHDL functionmayactsynergisticallytodecreasecollateralformation. Thechoicebetweentheavailablemanagementoptionsshould accountforitseffectoncoronarycollateralization.Inthe future,muchmoreresearchneedstobedonetofocusonthe benefitsofinnovativelipid-modifyingstrategies,includinguse ofPCSK9andnewtriglyceride-andLp(a)-loweringtreatment, anti-diabeticagentsaswellastherapeuticnormalizationof attenuatedproangiogenicandantiatherogenicHDLfunction,in theimprovementofcoronarycollateralformationandclinical outcome.Novelinformationassuchshouldaddnewknowledge oncoronarypathophysiologyandprovideusefulguidanceof patientcareforclinicians.
Authorcontributions
YSandFDwrotethemanuscript,substantiallycontributed todiscussionofthecontent,andeditedthemanuscript.YD, XW,andYWresearcheddataforthemanuscript.RZand LLsubstantiallycontributedtodiscussionofthecontentand
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 08 frontiersin.org 43
reviewedthemanuscript.WSreviewedthemanuscriptbefore submission.Allauthorsreadandapprovedthefinalmanuscript.
Funding
ThisstudywassupportedbytheNationalNatural ScienceFoundationofChina(81870179,81870357,81970362, 81970293,82170417,and82170423),ChinaPostdoctoral ScienceFoundation(2018M640408),ShanghaiMunicipal EducationCommission-GaofengClinicalMedicineGrant Support(20181801),TechnologyTransferProjectofShanghai JiaoTongUniversitySchoolofMedicine(ZT202103),MedicoengineeringResearchProjectofShanghaiJiaoTongUniversity (YG2021ZD04),andShanghaiClinicalResearchCenterfor InterventionalMedicine(19MC1910300).
References
1.EinarsonTR,AcsA,LudwigC,PantonUH.Economicburdenof cardiovasculardiseaseintype2diabetes:asystematicreview. ValueHealth. (2018) 21:881–90.doi:10.1016/j.jval.2017.12.019
2.BahiruE,HsiaoR,PhillipsonD,WatsonKE.Mechanismsandtreatmentof dyslipidemiaindiabetes. CurrCardiolRep. (2021)23:26.doi:10.1007/s11886-02101455-w
3.BiglerMR,SeilerC.Thehumancoronarycollateralcirculation,Itsextracardiac anastomosesandtheirtherapeuticpromotion. IntJMolSci. (2019)20:3726.doi: 10.3390/ijms20153726
4.DaiY,ChangS,WangS,ShenY,LiC,HuangZ,etal.Thepreservationeffectof coronarycollateralcirculationonleftventricularfunctioninchronictotalocclusion anditsassociationwiththeexpressionofvascularendothelialgrowthfactorA. Adv ClinExpMed. (2020)29:493–7.doi:10.17219/acem/104535
5.ShenY,AihemaitiM,ShuXY,YangCD,ChenJW,DaiY,etal.Circulating chromograninBisassociatedwithleftventricularfunctionalrecoveryafter successfulrecanalizationofchronictotalocclusion. FrontCardiovascMed. (2021) 8:756594.doi:10.3389/fcvm.2021.756594
6.ZimarinoM,D’AndreamatteoM,WaksmanR,EpsteinSE,DeCaterinaR.The dynamicsofthecoronarycollateralcirculation. NatRevCardiol. (2014)11:191–7. doi:10.1038/nrcardio.2013.207
7.YangZK,ShenY,DaiY,WangXQ,HuJ,DingFH,etal.Impactofcoronary collateralizationonlong-termclinicaloutcomesintype2diabeticpatientsafter successfulrecanalizationofchronictotalocclusion. CardiovascDiabetol. (2020) 19:59.doi:10.1186/s12933-020-01033-4
8.ShenY,DingFH,DaiY,WangXQ,ZhangRY,LuL,etal.Reducedcoronary collateralizationintype2diabeticpatientswithchronictotalocclusion. Cardiovasc Diabetol. (2018)17:26.doi:10.1186/s12933-018-0671-6
9.BalakrishnanS,SenthilKumarB.Factorscausingvariabilityinformationof coronarycollateralsduringcoronaryarterydisease. FoliaMorphol(Warsz). (2021). doi:10.5603/FM.a2021.0110
10.LiuT,WuZ,LiuJ,LvY,LiW.Metabolicsyndromeanditscomponents reducecoronarycollateralizationinchronictotalocclusion:anobservationalstudy. CardiovascDiabetol. (2021)20:104.doi:10.1186/s12933-021-01297-4
11.PackardCJ,BorenJ,TaskinenMR.Causesandconsequencesof hypertriglyceridemia. FrontEndocrinol(Lausanne). (2020)11:252.doi:10.3389/ fendo.2020.00252
12.diSommaM,VlioraM,GrilloE,CastroB,DakouE,SchaafsmaW,etal.Role ofVEGFsinmetabolicdisorders. Angiogenesis. (2020)23:119–30.doi:10.1007/ s10456-019-09700-1
13.LiuXC,HeGD,LoK,HuangYQ,FengYQ.Thetriglyceride-glucose index,aninsulinresistancemarker,wasnon-linearassociatedwithall-causeand cardiovascularmortalityinthegeneralpopulation. FrontCardiovascMed. (2021) 7:628109.doi:10.3389/fcvm.2020.628109
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
14.GuoW,ZhuW,WuJ,LiX,LuJ,QinP,etal.Triglycerideglucoseindex isassociatedwitharterialstiffnessand10-yearcardiovasculardiseaseriskina chinesepopulation. FrontCardiovascMed. (2021)8:585776.doi:10.3389/fcvm. 2021.585776
15.BaykanAO,GürM,AceleA,¸SekerT,QuisiA,YildirimA,etal.Coronary collateraldevelopmentandarterialstiffnessinpatientswithchroniccoronarytotal occlusions. ScandCardiovascJ. (2015)49:228–34.doi:10.3109/14017431.2015. 1062130
16.GaoA,LiuJ,HuC,LiuY,ZhuY,HanH,etal.Associationbetweenthe triglycerideglucoseindexandcoronarycollateralizationincoronaryarterydisease patientswithchronictotalocclusionlesions. LipidsHealthDis. (2021)20:140. doi:10.1186/s12944-021-01574-x
17.OuchiG,KomiyaI,TairaS,WakugamiT,OhyaY.Triglyceride/low-densitylipoproteincholesterolratioisthemostvaluablepredictorforincreasedsmall, denseLDLintype2diabetespatients. LipidsHealthDis. (2022)21:4.doi:10.1186/ s12944-021-01612-8
18.LiuGY,MengXX,ZhangZ.TriglyceridetoHDL-cholesterolratioas anindependentriskfactorforthepoordevelopmentofcoronarycollateral circulationinelderlypatientswithST-segmentelevationmyocardialinfarctionand acutetotalocclusion. Medicine(Baltimore). (2018)97:e12587.doi:10.1097/MD. 0000000000012587
19.FuL,ZhouY,SunJ,ZhuZ,XingZ,ZhouS,etal.Atherogenicindexof plasmaisassociatedwithmajoradversecardiovasculareventsinpatientswithtype 2diabetesmellitus. CardiovascDiabetol. (2021)20:201.doi:10.1186/s12933-02101393-5
20.WuJ,ZhouQ,WeiZ,WeiJ,CuiM.Atherogenicindexofplasmaand coronaryarterydiseaseintheadultpopulation:ameta-analysis. FrontCardiovasc Med. (2021)8:817441.doi:10.3389/fcvm.2021.817441
21.BogachkovYY,ChenL,LeMasterE,FancherIS,ZhaoY,AguilarV,etal.LDL inducescholesterolloadingandinhibitsendothelialproliferationandangiogenesis inMatrigels:correlationwithimpairedangiogenesisduringwoundhealing. AmJ PhysiolCellPhysiol. (2020)318:C762–76.doi:10.1152/ajpcell.00495.2018
22.DeindlE,QuaxPH.Arteriogenesisandtherapeuticangiogenesisinits multipleaspects. Cells. (2020)9:1439.doi:10.3390/cells9061439
23.DuanJ,MuroharaT,IkedaH,KatohA,ShintaniS,SasakiK,etal. Hypercholesterolemiainhibitsangiogenesisinresponsetohindlimbischemia: nitricoxide-dependentmechanism. Circulation. (2000)102(19Suppl.3):III370–6. doi:10.1161/01.cir.102.suppl_3.iii-370
24.TirziuD,MoodieKL,ZhuangZW,SingerK,HelischA,DunnJF, etal.DelayedarteriogenesisinhypercholesterolemicMice. Circulation. (2005) 112:2501–9.doi:10.1161/CIRCULATIONAHA.105.542829
25.LuoY,YanQN,WuWZ,LuoFY.Decreasedcountanddysfunctionof circulatingEPCsinpostmenopausalhypercholesterolemicfemalesviareducing
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 09 frontiersin.org 44
NOproduction. StemCellsInt. (2018)2018:2543847.doi:10.1155/2018/254
3847
26.vanWeelV,deVriesM,VosholPJ,VerloopRE,EilersPHC,van HinsberghVWM,etal.Hypercholesterolemiareducescollateralarterygrowth moredominantlythanhyperglycemiaorinsulinresistanceinmice. Arterioscler ThrombVascBiol. (2006)26:1383–90.doi:10.1161/01.ATV.0000219234.78165.85
27.LinFY,TsaoNW,ShihCM,LinYW,YehJS,ChenJW,etal.The biphasiceffectsofoxidized-lowdensitylipoproteinonthevasculogenicfunctionof endothelialprogenitorcells. PLoSOne. (2015)10:e0123971.doi:10.1371/journal. pone.0123971
28.JinX,YangS,LuJ,WuM.Small,denselow-densitylipoprotein-cholesterol andatherosclerosis:relationshipandtherapeuticstrategies. FrontCardiovascMed. (2022)8:804214.doi:10.3389/fcvm.2021.804214
29.IkezakiH,LimE,CupplesLA,LiuCT,AsztalosBF,SchaeferEJ.Small denselow-densitylipoproteincholesterolisthemostatherogeniclipoprotein parameterintheprospectiveFraminghamoffspringstudy. JAmHeartAssoc. (2021)10:e019140.doi:10.1161/JAHA.120.019140
30.JinJL,ZhangHW,CaoYX,LiuHH,HuaQ,LiYF,etal.Associationof smalldenselow-densitylipoproteinwithcardiovascularoutcomeinpatientswith coronaryarterydiseaseanddiabetes:aprospective,observationalcohortstudy.
CardiovascDiabetol. (2020)19:45.doi:10.1186/s12933-020-01015-6
31.HuangJ,GuJX,BaoHZ,LiSS,YaoXQ,YangM,etal.Elevatedserumsmall denselow-densitylipoproteincholesterolmayincreasetheriskandseverityof coronaryheartdiseaseandpredictcardiovasculareventsinpatientswithtype2 diabetesmellitus. DisMarkers. (2021)2021:5597028.doi:10.1155/2021/5597028
32.ZhangY,JinJL,CaoYX,ZhangHW,GuoYL,WuNQ,etal.Lipoprotein (a)predictsrecurrentworseoutcomesintype2diabetesmellituspatientswith priorcardiovascularevents:aprospective,observationalcohortstudy. Cardiovasc Diabetol. (2020)19:111.doi:10.1186/s12933-020-01083-8
33.ArasD,GeyikB,TopalogluS,ErgunK,AyazS,MadenO,etal.Serum leveloflipoprotein(a)isinverselyassociatedwiththedevelopmentofcoronary collateralcirculation. CoronArteryDis. (2006)17:159–63.doi:10.1097/00019501200603000-00010
34.FanY,HuJS,GuoF,LuZB,XiaH.Lipoprotein(a)asapredictorofpoor collateralcirculationinpatientswithchronicstablecoronaryheartdisease. BrazJ MedBiolRes. (2017)50:e5979.doi:10.1590/1414-431X20175979
35.ShenY,ChenS,DaiY,WangXQ,ZhangRY,YangZK,etal.Lipoprotein (a)interactionswithcholesterol-containinglipidsonangiographiccoronary collateralizationintype2diabeticpatientswithchronictotalocclusion. Cardiovasc Diabetol. (2019)18:82.doi:10.1186/s12933-019-0888-z
36.YouXD,JinJL,ZhangH,GuoN,HouBJ,GuoYL,etal.Lipoprotein(a) asamarkerforpredictingcoronarycollateralcirculationinpatientswithacute myocardialinfarction. PerMed. (2020)17:67–78.doi:10.2217/pme-2018-0127
37.MorishitaR,SakakiM,YamamotoK,IguchiS,AokiM,YamasakiK,etal. Impairmentofcollateralformationinlipoprotein(a)transgenicmice:therapeutic angiogenesisinducedbyhumanhepatocytegrowthfactorgene. Circulation. (2002) 105:1491–6.doi:10.1161/01.cir.0000012146.07240.fd
38.KadiH,OzyurtH,CeyhanK,KocF,CelikA,BurucuT.Therelationship betweenhigh-densitylipoproteincholesterolandcoronarycollateralcirculation inpatientswithcoronaryarterydisease. JInvestigMed. (2012)60:808–12.doi: 10.2310/JIM.0b013e31824e980c
39.HsuPC,SuHM,JuoSH,YenHW,VoonWC,LaiWT,etal.Influenceofhighdensitylipoproteincholesteroloncoronarycollateralformationinapopulation withsignificantcoronaryarterydisease. BMCResNotes. (2013)20:105.doi:10. 1186/1756-0500-6-105
40.Soria-FloridoMT,SchröderH,GrauM,FitóM,LassaleC.Highdensity lipoproteinfunctionalityandcardiovasculareventsandmortality:asystematic reviewandmeta-analysis. Atherosclerosis. (2020)302:36–42.doi:10.1016/j. atherosclerosis.2020.04.015
41.RohatgiA,WesterterpM,vonEckardsteinA,RemaleyA,RyeKA.HDLin the21stcentury:amultifunctionalroadmapforfutureHDLresearch. Circulation. (2021)143:2293–309.doi:10.1161/CIRCULATIONAHA.120.044221
42.EbtehajS,GruppenEG,BakkerSJL,DullaartRPF,TietgeUJF.HDL (high-densitylipoprotein)cholesteroleffluxcapacityisassociatedwithincident cardiovasculardiseaseinthegeneralpopulation. ArteriosclerThrombVascBiol. (2019)39:1874–83.doi:10.1161/ATVBAHA.119.312645
43.LeeJJ,ChiG,FitzgeraldC,KazmiSHA,KalayciA,KorjianS,etal.Cholesterol effluxcapacityanditsassociationwithadversecardiovascularevents:asystematic reviewandmeta-analysis. FrontCardiovascMed. (2021)8:774418.doi:10.3389/ fcvm.2021.774418
44.LeeS,ParkJM,AnnSJ,KangM,CheonEJ,AnDB,etal.Cholesterolefflux andcollateralcirculationinchronictotalcoronaryocclusion:effect-circstudy. J AmHeartAssoc. (2021)10:e019060.doi:10.1161/JAHA.120.019060
45.RentropKP,CohenM,BlankeH,PhillipsRA.Changesincollateralchannel fillingimmediatelyaftercontrolledcoronaryarteryocclusionbyanangioplasty ballooninhumansubjects. JAmCollCardiol. (1985)5:587–92.doi:10.1016/s07351097(85)80380-6
46.WangXQ,ShenY,ChenJW,ShuXY,LiC,YangCD,etal.Relationship ofHDL-associatedcholesteroleffluxcapacitytocoronarycollateralizationin diabeticandnon-diabeticpatientswithstableanginaandchronictotalocclusion. Circulation (2019)140(Suppl.1):A10762.
47.ErtekS.High-densitylipoprotein(HDL)dysfunctionandthefutureofHDL. CurrVascPharmacol. (2018)16:490–8.doi:10.2174/1570161115666171116164612
48.ShenY,DingFH,SunJT,PuLJ,ZhangRY,ZhangQ,etal.Association ofelevatedapoA-Iglycationandreducedparaoxonase1,3activity,andtheir interactionwithangiographicseverityofcoronaryarterydiseaseinpatientswith type2diabetes. CardiovascDiabetol. (2015)14:52.doi:10.1186/s12933-015-0221-4
49.PuLJ,LuL,ZhangRY,DuR,ShenY,ZhangQ,etal.Glycationofapoprotein A-Iisassociatedwithcoronaryarteryplaqueprogressionintype2diabeticpatients. DiabetesCare. (2013)36:1312–20.doi:10.2337/dc12-1411
50.DaiY,ShenY,LiQR,DingFH,WangXQ,LiuHJ,etal.Association ofapolipoproteinA-IVglycationwithCADinT2DMpatients:glycated apolipoproteinA-IVinducesatherogenesis. JAmCollCardiol. (2017)70:2006–19. doi:10.1016/j.jacc.2017.08.053
51.ZhaoY,BruemmerD.NR4Aorphannuclearreceptors:transcriptional regulatorsofgeneexpressioninmetabolismandvascularbiology. Arterioscler ThrombVascBiol. (2010)30:1535–41.doi:10.1161/ATVBAHA.109.191163
52.Möbius-WinklerS,UhlemannM,AdamsV,SandriM,ErbsS,LenkK,etal. Coronarycollateralgrowthinducedbyphysicalexercise:resultsoftheimpactof intensiveexercisetrainingoncoronarycollateralcirculationinpatientswithstable coronaryarterydisease(EXCITE)trial. Circulation. (2016)133:1438–48;discussion 1448.doi:10.1161/CIRCULATIONAHA.115.016442
53.KidaN,NishigakiA,Kakita-KobayashiM,TsubokuraH,HashimotoY, YoshidaA,etal.Exposuretocigarettesmokeaffectsendometrialmaturation includingangiogenesisanddecidualization. ReprodMedBiol. (2021)20:108–18. doi:10.1002/rmb2.12360
54.KolwiczSCJr.An“exercise”incardiacmetabolism. FrontCardiovascMed. (2018)5:66.doi:10.3389/fcvm.2018.00066
55.NickolayT,NicholsS,IngleL,HoyeA.Exercisetrainingasamediatorfor enhancingcoronarycollateralcirculation:areviewoftheevidence. CurrCardiol Rev. (2020)16:212–20.doi:10.2174/1573403X15666190819144336
56.ShenY,DingFH,WuF,LuL,ZhangRY,ZhangQ,etal.Associationofblood pressureandcoronarycollateralizationintype2diabeticandnondiabeticpatients withstableanginaandchronictotalocclusion. JHypertens. (2015)33:621–6; discussion626.doi:10.1097/HJH.0000000000000455
57.ShenY,DaiY,WangXQ,ZhangRY,LuL,DingFH,etal.Searchingfor optimalbloodpressuretargetsintype2diabeticpatientswithcoronaryartery disease. CardiovascDiabetol. (2019)18:160.doi:10.1186/s12933-019-0959-1
58.IkezakiH,FurusyoN,YokotaY,AiM,AsztalosBF,MurataM,etal. Smalldenselow-densitylipoproteincholesterolandcarotidintimalmedial thicknessprogression. JAtherosclerThromb. (2020)27:1108–22.doi:10.5551/jat.5 4130
59.IdaS,KanekoR,MurataK.Efficacyandsafetyofpemafibrateadministration inpatientswithdyslipidemia:asystematicreviewandmeta-analysis. Cardiovasc Diabetol. (2019)18:38.doi:10.1186/s12933-019-0845-x
60.LazarteJ,HegeleRA.Dyslipidemiamanagementinadultswithdiabetes. Can JDiabetes. (2020)44:53–60.doi:10.1016/j.jcjd.2019.07.003
61.BhattDL,StegPG,MillerM,BrintonEA,JacobsonTA,KetchumSB,etal. Cardiovascularriskreductionwithicosapentethylforhypertriglyceridemia. NEngl JMed. (2019)380:11–22.doi:10.1056/NEJMoa1812792
62.SheikhO,VandeHeiAG,BattishaA,HammadT,PhamS,ChiltonR. Cardiovascular,electrophysiologic,andhematologiceffectsofomega-3fattyacids beyondreducinghypertriglyceridemia:asitpertainstotherecentlypublished REDUCE-ITtrial. CardiovascDiabetol. (2019)18:84.doi:10.1186/s12933-0190887-0
63.DoiT,LangstedA,NordestgaardBG.Apossibleexplanationforthe contrastingresultsofREDUCE-ITvs.STRENGTH:cohortstudymimickingtrial designs. EurHeartJ. (2021)42:4807–17.doi:10.1093/eurheartj/ehab555
64.PirilloA,CatapanoAL.Omega-3forcardiovasculardiseases:wheredowe standafterREDUCE-ITandSTRENGTH? Circulation. (2021)144:183–5.doi:10. 1161/CIRCULATIONAHA.121.053144
65.BittnerDO,GoellerM,DeyD,ZopfY,AchenbachS,MarwanM.High levelsofeicosapentaenoicacidareassociatedwithlowerperi-coronaryadipose tissueattenuationasmeasuredbycoronaryCTA. Atherosclerosis. (2021)316:73–8. doi:10.1016/j.atherosclerosis.2020.10.006
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 10 frontiersin.org 45
66.ChaitA,denHartighLJ.Adiposetissuedistribution,inflammationand itsmetabolicconsequences,includingdiabetesandcardiovasculardisease. Front CardiovascMed. (2020)7:22.doi:10.3389/fcvm.2020.00022
67.LuL,ZhangRY,WangXQ,LiuZH,ShenY,DingFH,etal.C1q/TNFrelatedprotein-1:anadipokinemarkingandpromotingatherosclerosis. EurHeart J. (2016)37:1762–71.doi:10.1093/eurheartj/ehv649
68.ShenY,LuL,LiuZH,WuF,ZhuJZ,SunZ,etal.Increasedserumlevelof CTRP1isassociatedwithlowcoronarycollateralizationinstableanginapatients withchronictotalocclusion. IntJCardiol. (2014)174:203–6.doi:10.1016/j.ijcard. 2014.03.205
69.RotturaM,ScondottoG,BarbieriMA,SorbaraEE,NassoC,Marino S,etal.Managementofhighcardiovascularriskindiabeticpatients:focus onlowdensitylipoproteincholesterolandappropriatedruguseingeneral practice. FrontCardiovascMed. (2021)8:749686.doi:10.3389/fcvm.2021.74 9686
70.TokgozogluL,OrringerC,GinsbergHN,CataponoAL.Theyearin cardiovascularmedicine2021:dyslipidemia. EurHeartJ. (2022)43:807–17.doi: 10.1093/eurheartj/ehab875
71.Jansen-ChaparroS,López-CarmonaMD,Cobos-PalaciosL,Sanz-Cánovas J,Bernal-LópezMR,Gómez-HuelgasR.Statinsandperipheralarterialdisease:a narrativereview. FrontCardiovascMed. (2021)8:777016.doi:10.3389/fcvm.2021. 777016
72.DehnaviS,KianiA,SadeghiM,BireganiAF,BanachM,AtkinSL.Targeting AMPKbystatins:apotentialtherapeuticapproach. Drugs. (2021)81:923–33.doi: 10.1007/s40265-021-01510-4
73.AnnexBH,CookeJP.Newdirectionsintherapeuticangiogenesisand arteriogenesisinperipheralarterialdisease. CircRes. (2021)128:1944–57.doi: 10.1161/CIRCRESAHA.121.318266
74.DincerI,OngunA,TurhanS,OzdolC,ErtasF,ErolC.Effectofstatin treatmentoncoronarycollateraldevelopmentinpatientswithdiabetesmellitus. AmJCardiol. (2006)97:772–4.doi:10.1016/j.amjcard.2005.09.124
75.GuptaM,TummalaR,GhoshRK,BlumenthalC,PhilipK,Bandyopadhyay D,etal.Anupdateonpharmacotherapiesindiabeticdyslipidemia. ProgCardiovasc Dis. (2019)62:334–41.doi:10.1016/j.pcad.2019.07.006
76.FelekosI,KaramasisGV,PavlidisAN.PCSK9inhibitorsforthemanagement ofdyslipidemiainpeoplewithtype2diabetes:howlowistoolow? CurrPharmDes. (2021)27:1008–14.doi:10.2174/1381612826666200617170252
77.BardoliaC,AminNS,TurgeonJ.Emergingnlon-statintreatmentoptions forloweringlow-densityipoproteincholesterol. FrontCardiovascMed. (2021) 8:789931.doi:10.3389/fcvm.2021.789931
78.RayKK,DelPratoS,Müller-WielandD,CariouB,ColhounHM, TinahonesFJ,etal.Alirocumabtherapyinindividualswithtype2diabetes mellitusandatheroscleroticcardiovasculardisease:analysisoftheODYSSEYDMDYSLIPIDEMIAandDM-INSULINstudies. CardiovascDiabetol. (2019)18:149. doi:10.1186/s12933-019-0951-9
79.LorenzattiAJ,MonsalvoML,LópezJAG,WangH,RosensonRS.Effects ofevolocumabinindividualswithtype2diabeteswithandwithoutatherogenic dyslipidemia:ananalysisfromBANTINGandBERSON. CardiovascDiabetol. (2021)20:94.doi:10.1186/s12933-021-01287-6
80.SafaeianL,VaseghiG,JabariH,DanaN.Evolocumab,aproprotein convertasesubtilisin/kexintype9inhibitor,promotesangiogenesisinvitro. Can JPhysiolPharmacol. (2019)97:352–8.doi:10.1139/cjpp-2018-0542
81.BerberichAJ,HegeleRA.Lipideffectsofglucagon-likepeptide1receptor analogs. CurrOpinLipidol. (2021)32:191–9.doi:10.1097/MOL.0000000000000750
82.SzekeresZ,TothK,SzabadosE.TheEffectsofSGLT2inhibitorsonlipid metabolism. Metabolites. (2021)11:87.doi:10.3390/metabo11020087
Shenetal. 10.3389/fcvm.2022.956086
Frontiersin CardiovascularMedicine 11 frontiersin.org 46
PUBLISHED 25August2022
DOI 10.3389/fcvm.2022.988698
OPENACCESS
EDITEDBY
TatsuyaSawamura, ShinshuUniversity,Japan
REVIEWEDBY ChiaraMacchi, UniversityofMilan,Italy
*CORRESPONDENCE
LucaLiberale luca.liberale@unige
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal
FrontiersinCardiovascularMedicine
RECEIVED 07July2022
ACCEPTED 08August2022
PUBLISHED 25August2022
CITATION
TirandiA,MontecuccoFandLiberaleL (2022)Physicalactivitytoreduce PCSK9levels.
Front.Cardiovasc.Med. 9:988698. doi:10.3389/fcvm.2022.988698
COPYRIGHT
© 2022Tirandi,Montecuccoand Liberale.Thisisanopen-accessarticle distributedunderthetermsofthe CreativeCommonsAttributionLicense (CCBY).Theuse,distributionor reproductioninotherforumsis permitted,providedtheoriginal author(s)andthecopyrightowner(s) arecreditedandthattheoriginal publicationinthisjournaliscited,in accordancewithacceptedacademic practice.Nouse,distributionor reproductionispermittedwhichdoes notcomplywiththeseterms.
Physicalactivitytoreduce PCSK9levels
AmedeoTirandi1 , FabrizioMontecucco1,2 andLuca Liberale1,2*
1 FirstClinicofInternalMedicine,DepartmentofInternalMedicine,UniversityofGenoa,Genoa,Italy, 2 IRCCSOspedalePoliclinicoSanMartinoGenoa-ItalianCardiovascularNetwork,Genoa,Italy
Theamountofphysicalactivity(PA)peoplepracticeeverydayhasbeen reducinginthelast decades.Sedentarysubjectstendtohaveanimpaired lipidplasmaprofilewithahigherriskofatherosclerosisandrelated cardio-andcerebrovascularevents.RegularPAhelpsinbothprimaryand secondarycardiovascularpreventionbecauseofitsbeneficiale ectonthe wholemetabolism.Severalstudiesreportedlowerlevelsofplasmalipids intrainedsubjects,buttheprecisemechanismsbywhichPAmodulates lipoproteinsremainonlypartiallydescribed.Thereupon,proproteinconvertase subtilisin/kexintype9(PCSK9)isaserinproteasewhosemainfunction istoreducetheamountoflow-densitylipoproteincholesterol(LDL-C) receptors,withthedirectconsequenceofreducingLDL-Cuptakebytheliver andincreasingitscirculatingpool.Accordingly,recentlydevelopedPCSK9 inhibitorsimprovedcardiovascularpreventionandareincreasinglyusedto reachLDL-CgoalsinpatientsathighCVrisk.WhetherPAcanmodulate thelevelsofPCSK9remainspartiallyexplored.RecentstudiessuggestPA asanegativemodulatorofsuchadeleteriousCVmediator.Yetthelevelof evidenceislimited.Theaimofthisreviewistosummarizetherecentreports concerningtheregulatoryroleofPAonPCSK9plasmalevels,highlightingthe beneficialroleofregularexerciseonthepreventionofatherosclerosisand overallCVhealth.
KEYWORDS proproteinconvertasesubtilisin/kexintype9,physicalactivity,exercise,inflammation, cardiovascular
Introduction
Regularexercisehasbeenrecommendedtoimprovebothqualityandquantity oflifeindifferentclinicalsettings.Theimportanceoffitnessforahealthierlifeis particularlyimportantnowadays,sincethemodernsocietytendstounderestimate thetimespentsittinginfrontofdevicescreens,withalmosttwobillionof physicallyinactivesubjectsworldwide(1).Frombeingnomadichuntergatherers, webecamesettled agriculturalists.Nowadays,wetendtospendevenlesstime outdoorwithmanyworksarenowavailableonlineandcanbedoneathome usinganinternetconnection.Inthiscontext,therecentsevereacuterespiratory syndromecoronavirus2(SARS-CoV2)pandemicdidnothelp.Epidemiologicaldata reportthatthetotalamountofphysicalactivity(PA)hasprogressivelyreduced inthelastdecades(2),meanwhilethenumberofobesesubjectsalmosttripled
TYPE MiniReview
Frontiersin CardiovascularMedicine 01 frontiersin.org 47
worldwide(3).Obesity isawell-knowncardiovascularrisk factorandisassociatedwithahighnumberofcomorbidities involvingmostorgansofourbodies.
However,physicalactivitymightbeaerobicoranaerobic. Theaerobictrainingconsistsinexercisesinvolvinglargegroup ofmusclesinarhythmicanddynamicmannerforalongtime, whiletheanaerobictraininginamoreintenseactivity,exerted inashortedtime,andinvolvingarestrictedgroupofmuscles (4).Eventhough,theaerobicphysicalexercisehasalwaysbeen regardedas healthierandthereforepreferable,recentevidence showsthatbothaerobicandanaerobicphysicalactivitymay havebeneficialeffectonthecardiovascularsystem(4).Regular PAhasprotectiveeffectsagainstcardiovasculardiseasesandallcausemortality(5)andatherosclerosisisnegativelymodulated byregularexercise(6).Evenslightincreaseofdailyamountof PA,inthemagnitudeof30minoflight-tovigorous-intensity PAperdaycansignificantlyimprovecardiovascularhealth(7). Greaterresultsare obtainedwithregularhigh-intensityexercise training(8).
Ourbodyhas agreaterabilitytofacePAandstarvation periodsratherthananexcessofcaloricintakeandsedentarism. Wehavemanyhormonalpathwaysthatcanmobilizedepots andincreasecirculatingglucoseandlipidlevels,insteadthe machinerytoreducetheirlevelsisratherlimited.Asaresult, theexcessofcaloricintakeassociatedwithlowexercisetraining favorthedevelopmentofmetabolismimpairment.Thedirect consequenceistheslowlyprogressiontowardtheso-called “Xsyndrome,”betterknownasthemetabolicsyndrome.This syndromeisassociatedwithseveralcardiovasculardiseases (9)andsuddencardiacdeath(10).CVpreventiontherefore findsacornerstonein strategiesaimingatregulatinglipid levelsbyreducinglow-densitylipoproteincholesterol(LDL-C) andincreasinghigh-densitylipoproteincholesterol(HDL-C). PAwasshowntohavebeneficialeffectsonlipidprofile (withsex-relateddifferences),especiallywhencoupledwith betterdietaryhabits(11, 12).Whenthisisnotenoughto reachthetargetlipoproteinlevelssuggestedbytheguidelines, pharmacologicalapproachesincludingstatinsandPCSK9 inhibitorsaresuggested.Inconsiderationoftherecentparadigm forcholesterollevels“thelowerthebetter,”theuseofproprotein convertasesubtilisin/kexintype9(PCSK9)inhibitorsdrugssuch asalirocumabandevolocumabtoreducecirculatingLDL-Cin patientatintermediatetoveryhighriskisgettingmoreand morecommonintheclinicalpractice.
PCSK9isaserineproteasewhosemainroleistofavoritethe catabolismofLDL-Creceptoronthehepatocytesurfacethereby reducingLDL-Cinternalizationandincreasingitscirculating pool(13, 14).ReducingthePCSK9plasmalevelstherefore leadstoreducedcirculatingcholesterollevelswithdirect inhibitoryeffectsontheatheroscleroticprocess.Yet,PCSK9 recentlyshowedpleiotropic,non-LDL-C-mediated,effectsthat arenowadaysknowntomediatesomeoftheanti-atherosclerotic effectsofPCSK9inhibitors(i.e.,throughinflammation)(15,
16).Infact,lowgradeinflammationisassociatedwithPCSK9 transcription,especially inmetabolicsyndromepatients(17). Furthermore,inflammationfavoritetheexpressionofPCSK9in theendothelialcellsandvascularsmoothmusclecells(18).
WhetherPCSK9mediates someofthebeneficialeffectsof PAonplasmalipoproteinsremainstobefullyinvestigated. RecentevidenceshowedthatPCSK9plasmalevelsareregulated byregularexerciseinbothanimalmodelsandhumans,as reportedin Table1.Although,thestrengthofthisassociation andthepossible pathwaysarenotpreciselydescribed.The purposeofthisreviewistohighlighttheroleofexercise trainingonPCSK9plasmalevels,asapreventionstrategyagainst atherosclerosis.WesearchedPubMedandWebofScience forthefollowingkeywords:“proproteinconvertase/subtilisin kexintype,”“PCSK9”incombinationwith“exercise”and “physicalactivity.”Additionally,alistofrecentpre-clinical andclinicalstudiesconcerningthistopicisreportedasatable anddiscussed.
Thee ectofexercisetrainingon low-densitylipoproteincholesterol
ExercisetrainingconsistsinastructuredandrepetitivePA thatispracticed regularly.Aslongtimeknown,theamountand thequalityofexercisesandtrainingscandeterminedifferent resultsrangingfrombeneficialtoevennegativeresultsin differentsettings(29).Asaresult,oneofthemajorpitfallsin comparingdifferenttrialsisdue tothedifferenttype,intensity, anddurationoftheexerciseprotocol.Concerningtheeffectof exerciseoncholesterol,severalpreclinical(30–32)andclinical studies(33–35)foundanameliorationofthelipidplasmaprofile via regularexercise,especiallyaerobicone.
ThemodulationofcirculatingLDL-Cintrainedanimals andhumansrelatestotheadaptationofthebodytowarda highermetabolicstate.Accordingly,theeffectsofexercisecan beseenevenacutely.ImmediatelyafterPA,LDL-Cplasmalevels decreases(36, 37).Aftertermination,LDL-Clevelstendto returntotheirbasallevels,yetwithregularexercisetheyremain loweronthelong-term(38).Concerningtheabovementioned adaptations,ahighermetabolicstateassociateswithareduction ofPCSK9.Consequently,thereducedcatabolismofLDL receptor,increasestheuptakeofcirculatingLDLthereby reducingcirculatingcholesterollevels.Accordingly,recent evidenceshowedthatPAincreasestheamountofhepaticLDL-C receptor(19, 39). Table1 summarizesthemainevidenceproving aneffectof PAonPCSK9levels.Asmoststudiesagreeona beneficialloweringeffect,anaugmentationofPCSK9levelsmay coexisttogetherwithLDL-Creductioninwell-trainedpeople (27).Suchmixedeffectmightbeduetomanylimitationsin theanalysisandinterpretation ofthestudies.Furthermore,the amountofevidenceconcerningthistopicremainsquitelimited.
Tirandietal. 10.3389/fcvm.2022.988698
Frontiersin CardiovascularMedicine 02 frontiersin.org 48
Pre-clinicalresearch
AuthorYearModel Intervention Findings
Wenetal. (19)
NgoSock etal.(20)
Farahnak etal.(21)
Lietal. (22)
Wolf etal.(23)
2013 Mouse AET ontreadmilland differenttypesofdietlasting upto8weeks
TreadmillexerciseincreaseshepaticPCSK9 mRNAwhilereducingcirculatingPCSK9. Reducedlipidlevelsinmicefedwithhigh-fatdiet
2014 Rat Treadmill AETfor8weeksExercisetraininghasnoeffectoncirculating PCSK9evenifitreestablishestheexpressionof sterolregulatoryelementbindingprotein2
2018 Rat AET via voluntarywheel runningfor6weeks
IncreasingofintestinalLDL-RandPCSK9 transcriptsinbothintactandovariectomized animals,indicatingapossibleroleinthe trans-intestinalcholesterolexcretion
2020 Rat AETon treadmillfor8weeksIncreaseofhepaticLDL-R;inhibitionof neointimalformation via PCSK9andLOX-1 reduction
2021 Rat AET via voluntarywheel runningfor10months
Clinical
ExercisefavorstheexpressionofPCSK9inthe musclesofnormotensiveratswithoutaffecting circulatingpool.
AuthorYearPopulation Intervention Findings
Arsenault etal.(24)
Kamani etal.(25)
Boyer etal.(26)
Sponder atal.(27)
2014 Obese,sedentarymenaged between30and65yearsold
2015 Hospitalemployees agedmore than18yearsold
ModerateAET(160 min/week),more occupationalPA,anddiet
Useofstairsinsteadof elevatoratworkplaceforup to6months
2016 Men39–80yearsoldundergoing coronaryarterybypassgraft 150min/weekofPA,anddiet programfor1year
2017 Subjectsagedbetween30and65 yearsoldwithatleastone cardiovascularriskfactor
Moderate-vigorousAETfor8 months,from75min/weekof high-intensityto150 min/weekof moderate-intensityPA
ModestreductionofPCSK9levelsafter1year. PCSK9isslightlyassociatedwithinsulinresistance butnotwithLDL-Cplasmalevels
ReductionofcirculatinglevelsofPCSK9upto 20%at3rdmonthbutsimilarlevelstothebaseline at6thmonth
IncrementofPCSK9inrelationwithfitnessand visceralfatmobilization;noLDL-Cmodification
ReductionofLDL-Clevelswithincreased circulatingPCSK9
Makela etal.(28)
2019 Sedentary,pre-diabetic,middle agedpatients AETfor60minthreetimesin aweekforupto3months
ReductionofPCSK9plasmalevelseventhough lowintensityPAseemsnottoinfluencePCSK9 levels
AET,aerobicexercisetraining;LDL-r,low-densitylipoproteincholesterolreceptor;LOX-1,low-densitylipoproteinreceptor-1;mRNA,messengerribonucleicacid;PCSK9,proprotein
MediatorsofPCSK9modulationin trainedsubjects
Trainedpeopletendtoshowhigheramountofleanmass andlowerlevelsofvisceralfat(40–42).Asknowntoday,visceral fatisnotonlya storagetissue,butitplaysacrucialrolein thepathophysiologyofdifferentdiseases(e.g.,atherosclerosis)
byreleasingseveralmediatorswithlocalandsystemiceffects (i.e.,adipocytokines)(Figure1).
Thehypertrophicvisceraladipose tissueofobesepatients undergoesdegenerativeremodelingincludinghypoxia andmacrophageinfiltration,favoringthedevelopmentof inflammationwithdirecteffectsonthequalityandquantity ofreleasedadipocytokines.Thereductionofatheroprotective
Tirandietal. 10.3389/fcvm.2022.988698
TABLE1 Summaryofrecentpre-clinicalandclinicalstudiesevaluatingthee ectofexercisetrainingonPCSK9.
convertasesubtilisin/kexintype9.
Frontiersin CardiovascularMedicine 03 frontiersin.org 49
EvidencesuggestthatregularphysicalactivitycanregulatethePCSK9plasmalevelseventhoughfewevidenceisavailableatthemoment.As possibleexplanations,thereductionofresistin,annexinA2,andLOX-1arereportedwiththemostconcreteresults.Although,PCSK9canbealso augmentedintrainedsubjects.Thisisprobablyrelatedtoahighermetabolism,apossibleundiscoveredmechanism,orconfoundingfactors. Whileitislessknownifexercisetraininga ectstheplasmalevelsofSREBP2,bothfastingandstatintreatmentrepresenttwoconfounding factorswhenevaluatingthepossibleroleofphysicalactivityonPCSK9levels.LOX-1,lectin-typeoxidizedlow-densitylipoproteinreceptor-1; Proproteinconvertasesubtilisin/kexintype9;SREBP2,sterolregulatoryelement-bindingprotein2.
adiponectin(43)andtheincreasedlevelsofresistinfacilitate lipolysiswithdirect effectsonvesselhealth(44).Asexercisewas showntoreduceresistin levels(45, 46),suchadipokinecanbe afurtherlinkbetweenexerciseandPCSK9sinceresistinwas reportedtoincreasetheexpressionofPCSK9inhepatocyte therebymodulatingLDLRlevelsandindirectlyatherogenesis (47).Furthermore,activationofadiponectinreceptorswas showntoregulatePCSK9 expressioninexperimentalmodel ofatherosclerosiswithdirectimpactonthediseaseburden (48).Also,leptininterfereswithPCSK9expression via the activationof STAT3andp38MAPKpathways(49, 50).Among, adipocytokines,fibroblastgrowthfactor21isapeptide impliedinfattyacidsandglucosemetabolism(51)under bothphysiologicaland pathologicalconditionsincluding obesityandmetabolicsyndrome(52).Acuteexercisewas showntoincrease thecirculatinglevelsoffibroblastgrowth factor21(53),whichinturnimpairstheexpressionof PCSK9 via thesuppressionofthehepaticsterolregulatory elementbindingprotein2(54).Expressedbyavarietyof celltypesincludingadipocytes,annexinA2isananionic phospholipid-bindingproteinsoftheCa2+ dependentfamily thatwasreportedtoreducePCSK9levelsbyfavoringthe modificationofitscatalyticsubunit(55–57).Ofinterest, annexinA2levels areelevatedinpeoplewhoregularly practiceexercise(25),therebyindicatinganotherpossible molecularlink.
Recentlydescribedasanimportantmediatorof atherogenesis,lectin-typeoxidizedlow-densitylipoprotein receptor-1(LOX-1)maybeanothermediatoroftheeffectofPA
onPCSK9levels.LOX-1isascavengerreceptorwithimportant functioninoxidizedLDL-Cuptakebyendothelialcells(58). PCSK9andLOX-1arebothinvolvedintheatherosclerotic process(59)andrecentevidenceshowedthatPCSK9and LOX-1influenceeachotherinthevasculartissue(18, 60) andareco-relatedtotheatheromaformation.Pre-clinical evidenceshowedthatPAcanreducecholesterolaccumulation inatheroscleroticplaques via thereductionofLOX-1gene expression(61).BothPCSK9andLOX-1arereducedupon exercise(22).

Sterolregulatoryelementbinding protein2(SREBP2)isa moleculeimplicatedinthesynthesisofcholesterol(62)aswell asatranscriptionalactivatorofPCSK9(63, 64).Forsuchreason, SREBP2isoftenusedto explaintheparadoxicalassociationof elevatedPCSK9levelsandreducedLDL-Cthatisseenduring fastingperiods.
EventhoughitisnotclearwhetherPAcanmodulate SREBP2levels,regularexercisereducestheexpressionofseveral enzymesthatregulateslipidmetabolisminanimalmodels(65, 66).ThereductionofSREBP2levelswouldendinlowerPCSK9 plasmalevels,as ithappensduringfasting(67, 68).Indeed,few daysoffasting reducetheamountofcirculatingPCSK9butwith theunexpectedresultsofincreasingthequantityofcirculating LDL-C(69).
Furthermore,regular PAcanaugmentthecirculatinglevels ofinterferongamma,acytokinewithcriticalroleinregulationof immunesystem,asshowedinbothpre-clinical(70)andclinical (71, 72)settings.Recentstudiessuggestedaroleforinterferon gammainPCSK9regulation (73).
Tirandietal. 10.3389/fcvm.2022.988698
FIGURE1
Frontiersin CardiovascularMedicine 04 frontiersin.org 50
Anexcessofbodyfatisrelatedtoseveraldetrimental isassociatedwith higherriskofinsulinresistance(74),and insulinresistanceis oneofthekeyelementofthemetabolic syndrome.Also,higherlevelsofinsulinareassociatedwith ahigherexpressionofPCSK9 via sterolregulatoryelement bindingprotein1c(SREBP-1c)(17).Ontheotherhand,regular physicalactivitycanimproveinsulinsensitivity(75–77).
Pitfallsindeterminingthee ectofPAon PCSK9
Firstofall,thevariationsofLDL-Ccirculatinglevelscan eitherbeaconsequenceoracauseofPCSK9variations.Infact, whenPCSK9modulatetheamountofLDL-CalsoLDL-Ccan directlybindthePCSK9molecule,causingadirectimpairment ofitsfunctionality(78).Furthermore,manylimitationsreside intheevaluation ofcirculatinglevelsofthismediatorasitis notclearwhetherthisisafaircounterpartofitshepaticlevels. Also,asmoststudiesinvestigatethelevelsofthismolecule,they mightnotdirectlyreflectitsactivity.Underthispointofview, thepresenceofstudiesshowingaugmentationofPCSK9along withreductionofLDL-Ccirculatinglevelsmightbeexplained bydifferentPCSK9functionality.Furthermore,mostofthe analyticmethodfordetectingPCSK9useantibodiesbindingto itsmatureform.However,theactivityofPCSK9residesinits catalyticprocesses(79).
Aspreviouslymentioned, anothergreatlimitationresides inthedifficultstandardizationofexerciseprotocols.Also,with regardtoclinicalresearch,samplesizesaregenerallysmall, andsomestudieshasnon-negligibleunbalancedgenderand/or comorbiditiesdifferences.Theinterpretationoftherelatively smallnumberofrecentclinicaltrialsisalsohamperedbythe factthatoftentheyuseholisticinterventionalprogramwith prescriptionofbothPAanddiet.Lastly,theconcomitantuseof statintherapycaninterferewiththeinterpretationoftheresults. Infact,statintreatmentaugmentstheamountofcirculating PCSK9(80–82),probablybecausetheyincreasethelevelsof SREBP2(83).
Conclusions
Regularexerciseisknowntoamelioratelipidprofileand representthecornerstoneofcardiovascularprevention.Patient
References
thatpracticeregularexerciseshowanon-negligiblereduced riskofdevelopingatherosclerosisandpossiblecardiovascular events.TherelationshipbetweenPCSK9andPAcanbea possibleexplanationwithexercisetrainingenvisaginganonpharmacologicalPCSK9inhibitor.Indeed,thereductionof adiposityandmoleculeslikeLOX-1,annexinA2,fibroblast growthfactor21,andresistinsecondarytoexercisefavorthe reductionofPCSK9inthebloodstream.Yet,atpresent,several limitationsimpactontheinterpretationoftheresultsincluding thedifficultstandardizationofexerciseprotocols.
Authorcontributions
ATconceivedanddraftedthemanuscript.FMandLL reviseditforimportantintellectualcontent.Allauthorsreadand approveditsfinalversion.
Funding
LLreceivedaresearchgrantfromtheSwiss HeartFoundation.
Conflictofinterest
AuthorLLisco-inventorontheInternationalPatent WO/2020/226993filedinApril2020; thepatentrelatestotheuse ofantibodieswhichspecificallybindinterleukin-1α toreduce varioussequelaeofischemia-reperfusioninjurytothecentral nervoussystem.
Theremainingauthorsdeclarethattheresearchwas conductedintheabsenceofanycommercialorfinancial relationshipsthatcouldbeconstruedasapotentialconflict ofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddo notnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
Tirandietal. 10.3389/fcvm.2022.988698
1.HallalPC,AndersenLB,BullFC,GutholdR,HaskellW,EkelundU,etal. Globalphysicalactivitylevels:surveillanceprogress,pitfalls,andprospects. Lancet. (2012)380:247–57.doi:10.1016/S0140-6736(12)60646-1
Frontiersin CardiovascularMedicine 05 frontiersin.org 51
2.ArcherE,LavieCJ,McDonaldSM,ThomasDM,HebertJR,TavernoRossSE, etal.Maternalinactivity:45-yeartrendsinmothers’useoftime. MayoClinProc. (2013)88:1368–77.doi:10.1016/j.mayocp.2013.09.009
3.NCDRiskFactorCollaboration.Worldwidetrendsinbody-massindex, underweight,overweight,andobesityfrom1975to2016:apooledanalysisof2416 population-basedmeasurementstudiesin128.9millionchildren,adolescents,and adults. Lancet. (2017)390:2627–42.doi:10.1016/S0140-6736(17)32129-3
4.PatelH,AlkhawamH,MadaniehR,ShahN,KosmasCE,VittorioTJ.Aerobic vsanaerobicexercisetrainingeffectsonthecardiovascularsystem. WorldJCardiol. (2017)9:134–8.doi:10.4330/wjc.v9.i2.134
5.Ekblom-BakE,HalldinM,VikstromM,StenlingA,GiganteB,deFaire U,etal.Physicalactivityattenuatescardiovascularriskandmortalityinmen andwomenwithandwithoutthemetabolicsyndrome-a20-yearfollow-upof apopulation-basedcohortof60-year-olds. EurJPrevCardiol. (2021)28:1376–85.doi:10.1177/2047487320916596
6.LazarosG,OikonomouE,VogiatziG,ChristoforatouE,TsalamandrisS, GoliopoulouA,etal.Theimpactofsedentarybehaviorpatternsoncarotid atheroscleroticburden:implicationsfromthecorinthiaepidemiologicalstudy. Atherosclerosis. (2019)282:154–61.doi:10.1016/j.atherosclerosis.2019.01.026
7.GermanC,MakaremN,FanningJ,RedlineS,ElfassyT,McClainA,etal.Sleep, sedentarybehavior,physicalactivity,andcardiovascularhealth:MESA. MedSci SportsExerc. (2021)53:724–31.doi:10.1249/MSS.0000000000002534
8.KarlsenT,AamotIL,HaykowskyM,RognmoO.Highintensityinterval trainingformaximizinghealthoutcomes. ProgCardiovascDis. (2017)60:67–77.doi:10.1016/j.pcad.2017.03.006
9.TuneJD,GoodwillAG,SassoonDJ,MatherKJ.Cardiovascular consequencesofmetabolicsyndrome. TranslRes. (2017)183:57–70.doi:10.1016/j.trsl.2017.01.001
10.TirandiA,CarboneF,MontecuccoF,LiberaleL.Theroleofmetabolic syndromeinsuddencardiacdeathrisk:recentevidenceandfuturedirections. Eur JClinInvest. (2022)52:e13693.doi:10.1111/eci.13693
11.SkoumasJ,PitsavosC,PanagiotakosDB,ChrysohoouC,ZeimbekisA, PapaioannouI,etal.Physicalactivity,highdensitylipoproteincholesteroland otherlipidslevels,inmenandwomenfromtheATTICAstudy. LipidsHealthDis. (2003)2:3.doi:10.1186/1476-511X-2-3
12.PitangaFJG,GriepRH,AlmeidaMDC,FonsecaM,SouzaAR,SilvaRC,etal. AssociationbetweenleisuretimephysicalactivityandHDL-Cintheelsa-brasil studyparticipants:arethereanygenderdifferencesinthedose-responseeffect? ArqBrasCardiol. (2021)117:494–500.doi:10.36660/abc.20200438
13.GlerupS,SchulzR,LaufsU,SchluterKD.Physiologicalandtherapeutic regulationofPCSK9activityincardiovasculardisease. BasicResCardiol. (2017) 112:32.doi:10.1007/s00395-017-0619-0
14.LuqueroA,BadimonL,Borrell-PagesM.PCSK9functionsinatherosclerosis arenotlimitedtoplasmaticLDL-cholesterolregulation. FrontCardiovascMed. (2021)8:639727.doi:10.3389/fcvm.2021.639727
15.GiunzioniI,TavoriH,CovarrubiasR,MajorAS,DingL,ZhangY,etal. LocaleffectsofhumanPCSK9ontheatheroscleroticlesion. JPathol. (2016) 238:52–62.doi:10.1002/path.4630
16.MacchiC,FerriN,SirtoriCR,CorsiniA,BanachM,Ruscica M.Proproteinconvertasesubtilisin/kexintype9:aviewbeyond thecanonicalcholesterol-loweringimpact. AmJPathol. (2021) 191:1385–97.doi:10.1016/j.ajpath.2021.04.016
17.FerriN,RuscicaM.Proproteinconvertasesubtilisin/kexintype 9(PCSK9)andmetabolicsyndrome:insightsoninsulinresistance, inflammation,andatherogenicdyslipidemia. Endocrine. (2016) 54:588–601.doi:10.1007/s12020-016-0939-0
18.DingZ,PothineniNVK,GoelA,LuscherTF,MehtaJL.PCSK9and inflammation:roleofshearstress,pro-inflammatorycytokines,andLOX-1. CardiovascRes. (2020)116:908–15.doi:10.1093/cvr/cvz313
19.WenS,JadhavKS,WilliamsonDL,RideoutTC.Treadmillexercisetraining modulateshepaticcholesterolmetabolismandcirculatingPCSK9concentrationin high-fat-fedmice. JLipids. (2013)2013:908048.doi:10.1155/2013/908048
20.NgoSockET,ChapadosNA,LavoieJM.LDLreceptorandPcsk9transcripts aredecreasedinliverofovariectomizedrats:effectsofexercisetraining. Horm MetabRes. (2014)46:550–5.doi:10.1055/s-0034-1370910
21.FarahnakZ,ChapadosN,LavoieJM.Exercisetrainingincreasedgene expressionofLDL-RandPCSK9inintestine:linktotransintestinalcholesterol excretion. GenPhysiolBiophys. (2018)37:309–17.doi:10.4149/gpb_2017047
22.LiW,ParkH,GuoE,JoW,SimKM,LeeSK.Aerobicexercise traininginhibitsneointimalformationviareductionofPCSK9andLOX-1in atherosclerosis. Biomedicines. (2020)8:92.doi:10.3390/biomedicines8040092
23.WolfA,KutscheHS,AtmanspacherF,KaradedeliMS,SchreckenbergR, SchluterKD.Untypicalmetabolicadaptationsinspontaneouslyhypertensive ratstofreerunningwheelactivityincludesuncouplingprotein-3(UCP-3)and
proproteinconvertasesubtilisin/kexintype9(PCSK9)expression. FrontPhysiol. (2021)12:598723.doi:10.3389/fphys.2021.598723
24.ArsenaultBJ,Pelletier-BeaumontE,AlmerasN,TremblayA, PoirierP,BergeronJ,etal.PCSK9levelsinabdominallyobese men:associationwithcardiometabolicriskprofileandeffectsof aone-yearlifestylemodificationprogram. Atherosclerosis. (2014) 236:321–6.doi:10.1016/j.atherosclerosis.2014.07.010
25.KamaniCH,GencerB,MontecuccoF,CourvoisierD,VuilleumierN,Meyer P,etal.StairsinsteadofelevatorsattheworkplacedecreasesPCSK9levelsina healthypopulation. EurJClinInvest. (2015)45:1017–24.doi:10.1111/eci.12480
26.BoyerM,LevesqueV,PoirierP,MaretteA,MathieuP,DespresJP,etal. Impactofa1-yearlifestylemodificationprogramonplasmalipoproteinand PCSK9concentrationsinpatientswithcoronaryarterydisease. JClinLipidol. (2016)10:1353–61.doi:10.1016/j.jacl.2016.08.014
27.SponderM,CampeanIA,DalosD,EmichM,Fritzer-SzekeresM,Litschauer B,etal.Effectoflong-termphysicalactivityonPCSK9,high-andlow-density lipoproteincholesterol,andlipoprotein(a)levels:aprospectiveobservationaltrial. PolArchInternMed. (2017)127:506–11.doi:10.1093/eurheartj/ehx502.P1516
28.MakelaKA,LeppaluotoJ,JokelainenJ,JamsaT,Keinanen-KiukaanniemiS, HerzigKH.EffectofphysicalactivityonplasmaPCSK9insubjectswithhighrisk fortype2diabetes. FrontPhysiol. (2019)10:456.doi:10.3389/fphys.2019.00456
29.HalbertJA,SilagyCA,FinucaneP,WithersRT,HamdorfPA.Exercise trainingandbloodlipidsinhyperlipidemicandnormolipidemicadults:ametaanalysisofrandomized,controlledtrials. EurJClinNutr. (1999)53:514–22.doi:10.1038/sj.ejcn.1600784
30.HeerenMV,DeSousaLE,MostardaC,MoreiraE,MachertH,RigattoKV, etal.Exerciseimprovescardiovascularcontrolinamodelofdislipidemiaand menopause. Maturitas. (2009)62:200–4.doi:10.1016/j.maturitas.2008.12.011
31.MeissnerM,LombardoE,HavingaR,TietgeUJ,KuipersF,GroenAK. Voluntarywheelrunningincreasesbileacidaswellascholesterolexcretionand decreasesatherosclerosisinhypercholesterolemicmice. Atherosclerosis. (2011) 218:323–9.doi:10.1016/j.atherosclerosis.2011.06.040
32.DonattoFF,NevesRX,RosaFO,CamargoRG,RibeiroH,Matos-Neto EM,etal.Resistanceexercisemodulateslipidplasmaprofileandcytokine contentintheadiposetissueoftumour-bearingrats. Cytokine. (2013)61:426–32.doi:10.1016/j.cyto.2012.10.021
33.KnightS,BerminghamMA,MahajanD.Regularnon-vigorousphysical activityandcholesterollevelsintheelderly. Gerontology. (1999)45:213–9.doi:10.1159/000022090
34.RiglaM,Sanchez-QuesadaJL,Ordonez-LlanosJ,PratT,CaixasA,Jorba O,etal.Effectofphysicalexerciseonlipoprotein(a)andlow-densitylipoprotein modificationsintype1andtype2diabeticpatients. Metabolism. (2000)49:640–7.doi:10.1016/S0026-0495(00)80041-4
35.Ruiz-RamieJJ,BarberJL,SarzynskiMA.Effectsof exerciseonHDLfunctionality. CurrOpinLipidol. (2019)30:16–23.doi:10.1097/MOL.0000000000000568
36.FergusonMA,AldersonNL,TrostSG,DavisPG,MosherPE,DurstineJL. Plasmalipidandlipoproteinresponsesduringexercise. ScandJClinLabInvest. (2003)63:73–9.doi:10.1080/00365510310000510
37.RahnamaN,YounesianA,MohammadionM,BambaeichiE.A90minute soccermatchdecreasestriglycerideandlowdensitylipoproteinbutnothighdensitylipoproteinandcholesterollevels. JResMedSci. (2009)14:335–41.
38.KujalaUM,AhotupaM,VasankariT,KaprioJ,TikkanenMJ.LowLDL oxidationinveteranenduranceathletes. ScandJMedSciSports. (1996)6:303–8.doi:10.1111/j.1600-0838.1996.tb00475.x
39.PintoPR,RoccoDD,OkudaLS,Machado-LimaA,CastilhoG,daSilva KS,etal.Aerobicexercisetrainingenhancestheinvivocholesteroltrafficking frommacrophagestotheliverindependentlyofchangesintheexpressionof genesinvolvedinlipidfluxinmacrophagesandaorta. LipidsHealthDis. (2015) 14:109.doi:10.1186/s12944-015-0093-3
40.KaySJ,FiataroneSinghMA.Theinfluenceofphysicalactivityon abdominalfat:asystematicreviewoftheliterature. ObesRev. (2006)7:183–200.doi:10.1111/j.1467-789X.2006.00250.x
41.Dahl-PetersenIK,BrageS,BjerregaardP,TolstrupJS,JorgensenME.Physical activityandabdominalfatdistributioningreenland. MedSciSportsExerc. (2017) 49:2064–70.doi:10.1249/MSS.0000000000001337
42.JungHC,JeonS,LeeNH,KimK,KangM,LeeS.Effectsofexercise interventiononvisceralfatinobesechildrenandadolescents. JSportsMedPhys Fitness. (2019)59:1045–57.doi:10.23736/S0022-4707.18.08935-1
43.NeelandIJ,RossR,DespresJP,MatsuzawaY,YamashitaS,Shai I,etal.Visceralandectopicfat,atherosclerosis,andcardiometabolic
Tirandietal. 10.3389/fcvm.2022.988698
Frontiersin CardiovascularMedicine 06 frontiersin.org 52
disease:aposition statement. LancetDiabetesEndocrinol. (2019) 7:715–25.doi:10.1016/S2213-8587(19)30084-1
44.NeelandIJ,PoirierP,DespresJP.Cardiovascularandmetabolicheterogeneity ofobesity:clinicalchallengesandimplicationsformanagement. Circulation. (2018) 137:1391–406.doi:10.1161/CIRCULATIONAHA.117.029617
45.KadoglouNP,PerreaD,IliadisF,AngelopoulouN,LiapisC,AlevizosM. Exercisereducesresistinandinflammatorycytokinesinpatientswithtype2 diabetes. DiabetesCare. (2007)30:719–21.doi:10.2337/dc06-1149
46.JungSH,ParkHS,KimKS,ChoiWH,AhnCW,KimBT,etal.Effectofweight lossonsomeserumcytokinesinhumanobesity:increaseinIL-10afterweightloss. JNutrBiochem. (2008)19:371–5.doi:10.1016/j.jnutbio.2007.05.007
47.MeloneM,WilsieL,PalyhaO,StrackA,RashidS.Discoveryofanew roleofhumanresistininhepatocytelow-densitylipoproteinreceptorsuppression mediatedinpartbyproproteinconvertasesubtilisin/kexintype9. JAmColl Cardiol. (2012)59:1697–705.doi:10.1016/j.jacc.2011.11.064
48.SunL,YangX,LiQ,ZengP,LiuY,LiuL,etal.Activationofadiponectin receptorregulatesproproteinconvertasesubtilisin/kexintype9expressionand inhibitslesionsinApoE-deficientmice. ArteriosclerThrombVascBiol. (2017) 37:1290–300.doi:10.1161/ATVBAHA.117.309630
49.DuY,LiS,CuiCJ,ZhangY,YangSH,LiJJ.Leptindecreasestheexpression oflow-densitylipoproteinreceptorviaPCSK9pathway:linkingdyslipidemiawith obesity. JTranslMed. (2016)14:276.doi:10.1186/s12967-016-1032-4
50.MacchiC,GrecoMF,BottaM,SperandeoP,DongiovanniP,ValentiL, etal.Leptin,resistin,andproproteinconvertasesubtilisin/kexintype9:therole ofSTAT3. AmJPathol. (2020)190:2226–36.doi:10.1016/j.ajpath.2020.07.016
51.FisherFM,Maratos-FlierE.UnderstandingthephysiologyofFGF21. Annu RevPhysiol. (2016)78:223–41.doi:10.1146/annurev-physiol-021115-105339
52.XieT,LeungPS.Fibroblastgrowthfactor21:aregulatorofmetabolic diseaseandhealthspan. AmJPhysiolEndocrinolMetab. (2017)313:E292–302.doi:10.1152/ajpendo.00101.2017
53.KhalafiM,AlamdariKA,SymondsME,NobariH,Carlos-VivasJ. Impactofacuteexerciseonimmediateandfollowingearlypost-exerciseFGF-21 concentrationinadults:systematicreviewandmeta-analysis. Hormones. (2021) 20:23–33.doi:10.1007/s42000-020-00245-3
54.HuangZ,XuA,CheungBMY.Thepotentialroleoffibroblastgrowth factor21inlipidmetabolismandhypertension. CurrHypertensRep. (2017) 19:28.doi:10.1007/s11906-017-0730-5
55.LyK,SaavedraYG,CanuelM,RouthierS,DesjardinsR,HamelinJ,etal. AnnexinA2reducesPCSK9proteinlevelsviaatranslationalmechanismand interactswiththeM1andM2domainsofPCSK9. JBiolChem. (2014)289:17732–46.doi:10.1074/jbc.M113.541094
56.MayerG,PoirierS,SeidahNG.AnnexinA2isaC-terminalPCSK9-binding proteinthatregulatesendogenouslowdensitylipoproteinreceptorlevels. JBiol Chem. (2008)283:31791–801.doi:10.1074/jbc.M805971200
57.SeidahNG,PoirierS,DenisM,ParkerR,MiaoB,MapelliC,etal. AnnexinA2isanaturalextrahepaticinhibitorofthePCSK9-inducedLDLreceptor degradation. PLoSONE. (2012)7:e41865.doi:10.1371/journal.pone.0041865
58.PirilloA,NorataGD,CatapanoAL.LOX-1,OxLDL,andatherosclerosis. MediatorsInflamm. (2013)2013:152786.doi:10.1155/2013/152786
59.TamJ,ThankamF,AgrawalDK,RadwanMM.CriticalroleofLOX-1-PCSK9 axisinthepathogenesisofatheromaformationanditsinstability. HeartLungCirc. (2021)30:1456–66.doi:10.1016/j.hlc.2021.05.085
60.DingZ,LiuS,WangX,DengX,FanY,ShahanawazJ,etal.Cross-talk betweenLOX-1andPCSK9invasculartissues. CardiovascRes. (2015)107:556–67.doi:10.1093/cvr/cvv178
61.PintoPR,daSilvaKS,IborraRT,OkudaLS,Gomes-KjerulfD,Ferreira GS,etal.Exercisetrainingfavorablymodulatesgeneandproteinexpressionthat regulatearterialcholesterolcontentinCETPtransgenicmice. FrontPhysiol. (2018) 9:502.doi:10.3389/fphys.2018.00502
62.WeberLW,BollM,StampflA.Maintainingcholesterolhomeostasis:sterol regulatoryelement-bindingproteins. WorldJGastroenterol. (2004)10:3081–7.doi:10.3748/wjg.v10.i21.3081
63.HortonJD,ShahNA,WarringtonJA,AndersonNN,ParkSW,BrownMS, etal.Combinedanalysisofoligonucleotidemicroarraydatafromtransgenicand knockoutmiceidentifiesdirectSREBPtargetgenes. ProcNatlAcadSciUSA. (2003) 100:12027–32.doi:10.1073/pnas.1534923100
64.JeongHJ,LeeHS,KimKS,KimYK,YoonD,ParkSW.Steroldependentregulationofproproteinconvertasesubtilisin/kexintype9expression bysterol-regulatoryelementbindingprotein-2. JLipidRes. (2008)49:399–409.doi:10.1194/jlr.M700443-JLR200
65.GriffithsMA,FiebigR,GoreMT,BakerDH,EsserK,OscaiL,etal.Exercise down-regulateshepaticlipogenicenzymesinfood-deprivedandrefedrats. JNutr. (1996)126:1959–71.
66.FiebigRG,HollanderJM,JiLL.Exercisedown-regulateshepatic fattyacidsynthaseinstreptozotocin-treatedrats. JNutr. (2001)131:2252–9.doi:10.1093/jn/131.9.2252
67.HortonJD,BashmakovY,ShimomuraI,ShimanoH.Regulationofsterol regulatoryelementbindingproteinsinliversoffastedandrefedmice. ProcNatl AcadSciUSA. (1998)95:5987–92.doi:10.1073/pnas.95.11.5987
68.PerssonL,CaoG,StahleL,SjobergBG,TrouttJS,KonradRJ,etal.Circulating proproteinconvertasesubtilisinkexintype9hasadiurnalrhythmsynchronous withcholesterolsynthesisandisreducedbyfastinginhumans. ArteriosclerThromb VascBiol. (2010)30:2666–72.doi:10.1161/ATVBAHA.110.214130
69.BrowningJD,HortonJD.Fastingreducesplasmaproproteinconvertase, subtilisin/kexintype9andcholesterolbiosynthesisinhumans. JLipidRes. (2010) 51:3359–63.doi:10.1194/jlr.P009860
70.HeidarianpourA,VahidianRezazadehM,ZamaniA.Effectof moderateexerciseonseruminterferon-gammaandinterleukin-17levels inthemorphinewithdrawalperiod. IntJHighRiskBehavAddict. (2016) 5:e26907.doi:10.5812/ijhrba.26907
71.VijayaraghavaA,KR.Alterationofinterferongamma(IFN-gamma)in humanplasmawithgradedphysicalactivity. JClinDiagnRes. (2014)8:BC05–7.doi:10.7860/JCDR/2014/9502.4440
72.ZamaniA,SalehiI,Alahgholi-HajibehzadM.Moderateexerciseenhances theproductionofinterferon-gammaandinterleukin-12inperipheralblood mononuclearcells. ImmuneNetw. (2017)17:186–91.doi:10.4110/in.2017.17.3.186
73.PiraultJ,PolyzosKA,PetriMH,KetelhuthDFJ,BackM,Hansson GK.Theinflammatorycytokineinterferon-gammainhibitssortilin-1expression inhepatocytesviatheJAK/STATpathway. EurJImmunol. (2017)47:1918–24.doi:10.1002/eji.201646768
74.PatelP,AbateN.Bodyfatdistributionandinsulinresistance. Nutrients. (2013)5:2019–27.doi:10.3390/nu5062019
75.YamanouchiK,NakajimaH,ShinozakiT,ChikadaK,KatoK,OshidaY,etal. Effectsofdailyphysicalactivityoninsulinactionintheelderly. JApplPhysiol. (1992)73:2241–5.doi:10.1152/jappl.1992.73.6.2241
76.Macias-CervantesMH,MalacaraJM,Garay-SevillaME,Diaz-CisnerosFJ. EffectofrecreationalphysicalactivityoninsulinlevelsinMexican/Hispanic children. EurJPediatr. (2009)168:1195–202.doi:10.1007/s00431-008-0907-7
77.CaiC,ZhangZ,McDonaldS,StromC,SkowRJ,MayLE,etal.LeisureTimephysicalactivitybeforeandduringpregnancyisassociatedwithimproved insulinresistanceinlatepregnancy. IntJEnvironResPublicHealth. (2021) 18:4413.doi:10.3390/ijerph18094413
78.KosenkoT,GolderM,LeblondG,WengW,LagaceTA.Lowdensity lipoproteinbindstoproproteinconvertasesubtilisin/kexintype-9(PCSK9)in humanplasmaandinhibitsPCSK9-mediatedlowdensitylipoproteinreceptor degradation. JBiolChem. (2013)288:8279–88.doi:10.1074/jbc.M112.421370
79.FarnierM.Theroleofproproteinconvertasesubtilisin/kexintype9in hyperlipidemia:focusontherapeuticimplications. AmJCardiovascDrugs. (2011) 11:145–52.doi:10.2165/11590330-000000000-00000
80.CareskeyHE,DavisRA,AlbornWE,TrouttJS,CaoG, KonradRJ.Atorvastatinincreaseshumanserumlevelsofproprotein convertasesubtilisin/kexintype9. JLipidRes. (2008)49:394–8.doi:10.1194/jlr.M700437-JLR200
81.MayneJ,DewpuraT,RaymondA,CousinsM,ChaplinA,LaheyKA,etal. PlasmaPCSK9levelsaresignificantlymodifiedbystatinsandfibratesinhumans. LipidsHealthDis. (2008)7:22.doi:10.1186/1476-511X-7-22
82.SahebkarA,Simental-MendiaLE,Guerrero-RomeroF,GolledgeJ,WattsGF. Effectofstatintherapyonplasmaproproteinconvertasesubtilisinkexin9(PCSK9) concentrations:asystematicreviewandmeta-analysisofclinicaltrials. Diabetes ObesMetab. (2015)17:1042–55.doi:10.1111/dom.12536
83.RoglansN,VerdJC,PerisC,AlegretM,VazquezM,AdzetT,etal.Highdoses ofatorvastatinandsimvastatininducekeyenzymesinvolvedinVLDLproduction. Lipids. (2002)37:445–54.doi:10.1007/s11745-002-0916-0
Tirandietal. 10.3389/fcvm.2022.988698
Frontiersin CardiovascularMedicine 07 frontiersin.org 53
OPENACCESS
EDITEDBY
TatsuyaSawamura, ShinshuUniversity,Japan
REVIEWEDBY
MasayukiYoshida, TokyoMedicalandDental University,Japan
*CORRESPONDENCE
TakuroMiyazaki taku@pharm.showa-u.ac.jp
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal
FrontiersinCardiovascularMedicine
RECEIVED 31May2022
ACCEPTED 11August2022
PUBLISHED 29August2022
CITATION
MiyazakiT(2022)Pinocytotic engulfmentoflipoproteinsby macrophages.
Front.Cardiovasc.Med. 9:957897. doi:10.3389/fcvm.2022.957897
COPYRIGHT
© 2022Miyazaki.Thisisan open-accessarticledistributedunder thetermsoftheCreativeCommons AttributionLicense(CCBY).Theuse, distributionorreproductioninother forumsispermitted,providedthe originalauthor(s)andthecopyright owner(s)arecreditedandthatthe originalpublicationinthisjournalis cited,inaccordancewithaccepted academicpractice.Nouse,distribution orreproductionispermittedwhich doesnotcomplywiththeseterms.
PUBLISHED 29August2022
DOI 10.3389/fcvm.2022.957897
Pinocytoticengulfmentof lipoproteinsbymacrophages TakuroMiyazaki*
DepartmentofBiochemistry,ShowaUniversitySchoolofMedicine,Tokyo,Japan
Atherosclerosisisamajorcauseofacutecoronarysyndromeandstroke.Foam cellformationinmacrophagesisinvolvedincontrollingplaquestabilityandthe pathogenesisofatherosclerosis.Accordingly,manystudieshaveexaminedthe processesoflipidincorporation,suchasscavengerreceptor-mediateduptake ofoxidizedlow-densitylipoprotein,incells.Inadditiontoreceptor-mediated machinery,growingevidencehassuggestedthatpinocytosis,whichis areceptor-independentendocyticpathway,isassociatedwithfoamcell formationwhenasu cientnumberoflipoproteinsisaccumulatedaround cells.Pinocytoticengulfmentofnanoparticlesisinitiatedbyplasmamembrane ru inginaphosphatidylinositol-3kinase-dependentmanner.Subsequent topinosomeclosure,themajorityofpinosomesareinternalizedthrough endocyticprocesses,andtheycanberecycledintotheplasmamembrane. ThesepinocytoticprocessesaremodulatedbysmallGTPasesandtheir cytoskeletalrearrangement.Moreover,pinocytoticabilitiesmayvarybetween immunologicalsubsetsincells.Accordingly,macrophagesmayshowdiverse pinocytoticabilitiesdependingonthesurroundingmicroenvironment.This reviewsummarizesthecurrentunderstandingofpinocytoticengulfmentof lipoproteininmacrophages,anddiscusseshowthisendocyticprocessis governedunderhypercholesterolemicconditions.
KEYWORDS
CWC22,calpain-6,Rac1,Cdc42,Akt,liverXreceptors,macrophage-colony stimulatingfactor
Introduction Ischemicheartdiseaseistheleadingcauseofdeathglobally.Atherosclerosis isamajorriskfactorforcoronarydiseasebecauseexpansionofsucheccentric thickeningofthearterialwallcanleadtothromboembolismandthromboticocclusion. Numerousbasicandepidemiologicalstudieshaveshownthatanabnormalityof cholesterolhandling,excessiveadaptiveresponsetovascularinsults,andinflammation burdenareassociatedwiththepathogenesisofatherosclerosis(1, 2).Amongthese events,cholesterolaccumulationinmacrophagesisanessentialprocesstoexpand atheroscleroticplaquesandisamajorfactorfordefiningplaquestability(3, 4).Several endocyticpathwaysforinternalizing low-densitylipoprotein(LDL)cholesterolhave beenreported.Scavengerreceptors,suchasscavengerreceptor-A,CD36,andlectinlikeoxidizedLDLreceptor1(5, 6),inmacrophagesrecognizeoxidizedLDL,but notnativeLDL,tointernalize LDLthroughtheconventionalendocyticprocess.In additiontothereceptor-mediatedpathways,macrophagesenabletheincorporation
TYPE MiniReview
Frontiersin CardiovascularMedicine 01 frontiersin.org 54
ofnanoparticlesthroughpinocytosis.Pinocytosisisa fundamentalcellularprocessthatengulfsextracellular fluids.Pinocytosisiscategorizedintomicropinocytosisand macropinocytosis,whichareendocyticprocessesthatengulf smallparticles(typically < 0.1 µm)andlargeparticles(typically > 0.2 µm),respectively(7).Suchpinocytoticengulfment inmacrophages, whichoccursspontaneouslyandcanbe furthermodifiedbyenvironmentalfactors,arerelatedto theinnateimmunesystemtomonitorsurroundingantigens andmicrobial-associatedmolecules.Growingevidencehas suggestedthatpinocytosisismediatedthroughincorporationof nativeLDL,therebyinducingformationoffoamymacrophages (8).Thisminireviewsummarizestherecentunderstanding ofinternalizationandtrafficking ofpinosomesandtheir regulatorymechanisms.
Membraneregulationandendocytic tra ckingduringpinocytotic incorporationofLDLin macrophages
ThepinocytoticdepositionofnativeLDLissufficientto convertculturedmacrophagesintofoamcells(9).Pinocytotic uptakeisindependentofthedegreeofLDLoxidationanddoes notsaturate(7),andthemolecularmechanismsunderlying pinocytoticLDLuptakeare distinctfromthoseofreceptormediatedpathways.Indeed,targeteddeletionofthemacrophage scavengerreceptorsscavengerreceptor-AandCD36does notinhibitpinocytoticLDLuptakeinmacrophage-colony stimulatingfactor(M-CSF)-differentiatedmacrophages(10).
Generally,macropinocytoticinternalizationof nanoparticlesis dividedintoplasmamembranerufflingandpinosomeclosure, whichareregulatedbysubmembranousactinorganization. SmallGTPaseshavecentralrolesinthiscytoskeletalregulation. Ras-GTPdrivestheactivationofphosphoinositide3-kinases (PI3Ks),whichgeneratespatchesofPtdIns(3,4,5)P3(11).The RhofamilyGTPaseRacandactin-nucleation-promotingfactors, suchasSCAR/WAVEcomplex,enablebindingtothesepatches, whichnucleatesactinfilaments.Inadditiontoactin-regulating factors,phospholipaseCγ canbeactivatedbyPtdIns(3,4,5)P3, therebygeneratingdiacylglycerolandsubsequentactivationof proteinkinaseC,whichisinvolvedinthepositiveregulationof pinocytosis(12).Kruthandcolleaguesinvestigatedmechanisms underlyingpinocytosisusingpharmacologicalapproachesinMCSF-differentiatedmacrophages,andfoundthatpinocytosiswas inhibitedbyabroad-rangePI3Kinhibitors(10).Incontrast, inhibitionoftheclassIPI3Kisoforms β , γ ,or δ didnotaffect micropinocytosisinM-CSF-stimulatedmacrophages.Similarly, macrophagesfrommiceexpressingdominant-negativeclassI PI3K β , γ ,or δ isoformshadnoinhibitoryeffects.Therefore, PI3Ks,excludingclassIisoforms,drivemacropinocytosisin
macrophages.Pharmacologicalscreeningofsignalingpathways hasshownthatdynamin,microtubules,actin,andvacuolartype H(+)-ATPaseappeartobeassociatedwithpinocytoticuptake (10).Accordingly,phosphoinositidemetabolismaccompanied bycytoskeletalregulation areindispensableevenforpinocytotic LDLuptakeinmacrophages(Figure1).
Inadditiontothe moleculesnotedabove,thecontribution ofRhoGTPasestopinocytoticregulationhasbeenwell–documented.Anzingeretal.reportedthattheRhoGTPase inhibitortoxinBsubstantiallyinhibitedpinocytoticLDL uptakeinM-CSF-differentiatedhumanmacrophages(13). Usingtime-lapsemicroscopy,theyfoundthatthisinhibitor almostcompletelyinhibitedmacropinocytosis,although cholesteroldepositionincellswasnotcompletelyinhibited. Theirfindingssuggestthecontributionofanotherendocytic process,suchasmicropinocytosis,toLDLcholesteroluptake. Incontrast,pharmacologicalinhibitionofRac1failedto inhibitpinocytoticLDLuptakeinM-CSF-differentiated macrophages(10).Ourpreviousstudyshowedthatexpression oftheRho GTPasesRhoAandRac1inbonemarrowcellswas imperceptible,whileitwasdramaticallyinducedduringthe differentiationofcellsintomacrophagesinthepresenceofMCSF(14).Treatmentofbonemarrowcellswithtumornecrosis factor(TNF)-α suppressedmaturationof Rac1 mRNAand potentiatedmacropinocytosis.Deficiencyofcalpain-6,which isanon-proteolyticisoformofthecalpainproteasefamily,in TNF-α-stimulatedmurinemacrophagesshowedinterrupted macropinocytosisconcomitantlywiththenormalizationof Rac1 splicing.Macropinocytosisincalpain-6-deficientmacrophages wasrestoredbysmallinterferingRNA-basedsilencingof Rac1 Therefore,aberrant Rac1 mRNAregulationexertsinhibitory actionsinpinocytoticLDLuptakeinTNF-α-stimulated macrophages.WhilethemechanismsbywhichRac1orits splicevariantsinterruptLDLuptakeareunclear,Rac1-mediated regulationoftheendosomalrecyclingpathwaymaycontribute totheinhibitoryactions.Indeed,pinosomesinRac1-expressing macrophagesfrequentlyexpresstherecyclingendosomemarker Rab11.Moreover,macropinosomevelocityincellsisdecelerated bypharmacologicalinhibitionofRac1.Therefore,pinocytotic LDLuptakeislikelytobeduetoRac1-dependentvesicle traffickingratherthanRac1-dependentmembraneruffling. RhoGTPases,suchasRhoD,Rac1,Cdc42,TCL,andTC10, arethoughttobeessentialfactorsforendocytictrafficking (15).Indeed,overexpressionofdominantactiveformsof Rac1accelerate macropinocytosisactivityinratfibroblasts (16).However,notably,prolongedRac1activityimpairsthe maturationofRab21-positive pinosomesinmacrophages (17),suggestingthatoptimalRac1regulationcanmaximize pinocytosis.However,Ding etal.showedpositiveregulation ofpinocytoticLDLuptakebyCdc42,whichisasmallGTPase, inhumanandmurinemacrophages(18).Indeed,thelossof Akt3inmurineandhuman M-CSF-differentiatedmacrophages upregulateswith-no-lysinekinase1andsubsequentlyactivates
Miyazaki 10.3389/fcvm.2022.957897
Frontiersin CardiovascularMedicine 02 frontiersin.org 55
Overviewofpinocytoticdepositionoflow-densitylipoproteincholesterolinmacrophages.Phosphoinositide3-kinasedrivespinocytoticplasma membraneru ing.SmallGTPasesareassociatedwithtra ckingandrecyclingofpinosomes,aswellaswithpinocytoticplasmamembrane regulation,andhavediverseactionsinpinocytoticlow-densitylipoproteincholesteroluptakeinmacrophages.Whilepinocytosisappearstobe optimizedinanalternative(M2)macrophagesubset,certainelements,suchasToll-likereceptor4(TLR4)-mediatedsignaling,enablethe restorationofpinocytosis,eveninaninflammatory(M1)subset.TLR4driverlipopolysaccharide(LPS)canpolarizemacrophagedi erentiation towardM1subset,andactivatespinocytoticactivationthroughunknownmechanisms.Chemokine(C-Cmotif)ligand19reportedlypossesses similarpinocytotice ectsinthecells.InthecaseofmmLDL-di erentiatedmacrophages,cytoskeletalrearrangementappearstobedriven throughTLR4/Ras/Raf/ERK/MEKaxisindependentlyofPI3Ksignaling.Furthermore,pro-inflammatorycytokineTNF-α upregulatescalpain-6,a non-proteolyticisoformofcalpainproteasefamily,toinhibitCWC22-mediatedRac1splicing.Thisinterfereswithendosomalrecyclingpathway, andinturnincreaseslysosomalprocessingofendosome-derivedlipoproteincholesteroltogeneratecytosoliclipiddroplets.IncontrasttoM1 subset,macrophage-colonystimulatingfactor(M-CSF)-di erentiatedM2macrophagesexhibitsphosphatidylinositol3-kinase(PI3K)-dependent cytoskeletalrearrangementandmembraneru ing.Similarly,thrombospndin-1enablestoelicitCD47-mediatedactivationofPI3Kand subsequentpinocytoticuptakeofnativeLDL.Inthiscase,itislikelythatAkt3negativelyregulatesCdc42andacyl-CoAcholesterol acyltransferase1todeceleratepinocytoticcholesteroldepositioninthecells.ACAT1,acyl-CoAcholesterolacyltransferase1;CCL19, Chemokine(C-Cmotif)ligand19;EE,earlyendosome;LE,lateendosome;LPS,lipopolysaccharide;M-CSF,macrophage-colonystimulating factor;mmLDL,minimallymodifiedlowdensitylipoprotein;PI3K,phosphatidylinositol3-kinase;PIP3,phosphatidylinositol3-phosphate;Rac1, RacfamilysmallGTPase1;RE,recyclingendosome;TLR4,Toll-likereceptor4;TNF-α,tumornecrosisfactor-α;TSP-1,thrombospndin-1.
serumandglucocorticoid-induciblekinase1.Serumand glucocorticoid-induciblekinase1promotesexpressionofthe RhofamilyGTPaseCdc42,therebyacceleratingcytoskeletal rearrangementandpinocytosis.Similarly,Akt3-dependent negativeregulationofpinocytosisisdetectableinmurine peritonealmacrophages.Thisregulationisaccompanied bylimitedreceptor-dependentuptakeofacetylatedLDL anddownregulationofacyl-CoAcholesterolacyltransferase (19).Acyl-CoAcholesterolacyltransferaseconvertsfree cholesterolintocholesterolestertoformcytosoliclipid dropletsinperitonealmacropahges(20).Therefore,Akt3 hasaprotective roleinfoamcellformationinmacrophages andatherogenesis.HowAkt3simultaneouslydownregulates receptor-dependentandreceptor-independentpathwaysis currentlyunclear,butAkt3mightinterferewithacommon signalingpathway,suchasendocyticvesicletraffickingor theexocytoticprocess.Thispossibilityisconsistentwitha
previousstudy,whichshowedthatoverexpressionofdominantnegativeCdc42counteractedmacropinocytosisinvascular endothelialcells(21).Collectively,smallGTPasesareassociated withvesicletraffickingandpinocytoticplasmamembrane regulation(Figure1),andhavediverseactionsregarding pinocytoticLDLuptakein macrophages.Therefore,defining pinocytoticactivitybyexpressionlevelsofsmallGTPasesalone isdifficult.
Regulatorymechanismsunderlying pinocytoticLDLincorporation
Macrophagescanbedividedintoavarietyof subpopulations,whichcomprisepro-inflammatory, immunosuppressive,andtissue-repairingtypes,andthey aredynamicallyinterconverteddependingonthetissue

Miyazaki 10.3389/fcvm.2022.957897
FIGURE1
Frontiersin CardiovascularMedicine 03 frontiersin.org 56
microenvironment(22).During bacterialinfection,monocytemacrophages canbeactivatedbyinflammatoryelements,such asToll-likereceptor(TLR)ligandsandinterferon-γ,which facilitatetheskewingofmacrophagesintotheM1subset (classicallyactivatedmacrophages).M1macrophagesproduce inflammatorycytokines,suchasTNF-α,interleukin(IL)-6, andIL-12,reactiveoxygenspecies,andreactivenitrogen species,andinduceaTh1-typeimmuneresponse(23). Furthermore,M1macrophagesexertstrongantibacterialor antiviralactivityandantitumoreffects.IncontrasttotheM1 subset,macrophagesactivatedbyIL-4andIL-13produced byTh2cells,basophils,mastcells,andinnatelymphoid cellsareconvertedintoM2macrophages(alternatively activatedmacrophages)(23).Thisactivationexertshost defenseagainstparasites,tissuerepair,angiogenesis,andtumor growth,andbecomesimmunosuppressive.M2macrophages stronglyexpressarginaseandmannosereceptors(24).
Tumor-associatedmacrophagesthatareinfiltratedintotumor tissuearethoughttobeconvertedfromM1toM2subsets, therebyacceleratingtumorprogression(25).Tumor-associated macrophages showinglowIL-12expressionlevelsandhigh IL-10expressionlevels,possessweakantitumoractivity, anddrivematrixremodelingandangiogenesis(26).In additiontotheprogressionofcancer,aberrantregulation ofmacrophagesubsetscanbeinvolvedinvariousdiseases. Therefore,phenotypicregulationofmacrophagesubsetscan beregardedasatherapeutictarget.Arteriosclerosis,whichisa Th1-dominantvasculardisorder,canbetreatedbyskewingM1 toM2macrophages(27).Incontrast,bronchialasthma,which isaTh2-dominantdisease,mightbetargetedbytheopposite strategy(28).
M2macrophagesshowconstitutivepinocytoticactivityand canbefurtherstimulatedwithrelatedcytokinessuchasMCSF.Notably,themajorityoftheabove-mentionedobservations onpinocytoticLDLuptakewerefrominvestigationsusing M-CSF-differentiatedhumanmonocyte-derivedmacrophages. Redkaetal.showedthatM-CSF/IL-4-differentiatedM2 macrophageshadrobustmicropinocytosisactivity,while thatingranulocyteM-CSF/interferon-γ /lipopolysaccharidedifferentiatedM1macrophageswasnegligible(29).Theyfound thatRhoGTPaseexpressionlevelsinM1macrophageswere lowerthanthoseintheM2subset.Inadditiontosmall GTPases,insufficientPI3Kactivityislikelyresponsiblefor modestpinocytoticabilityintheM1subset(29).Calciumsensingreceptorsappeartobenecessaryforsustaining smallGTPasesandPI3KinM2macrophages.However, notably,M1macrophagesshowrobustpinocytoticactivity whenthecellsarestimulatedwithcertainpro-inflammatory substances.Indeed,acutetreatmentofpro-inflammatory macrophageswithlipopolysaccharideorCCchemokineligand 19markedlyacceleratesmacropinosomeformationinhuman monocyte-derivedmacrophages(29).Moreover,minimally oxidizedlow-densitylipoprotein(mmLDL),whichisan
alternativeTLR4ligand,andcholesterylesterhydroperoxide, anactivecomponentofmmLDL,induceTLR4-dependent macropinocytoticincorporationofoxidizedLDLandnative LDL(30).Inthiscase,mmLDLelicitsanassociation ofspleentyrosinekinase withaTLR4complex,TLR4 phosphorylation,activationoftheVav1-Ras-Raf-MEK-ERK1/2 axis,phosphorylationofpaxillin,andactivationofRac,Cdc42, andRhoinmurineperitonealmacrophages.Thesefindings suggestthatmmLDL-inducedTLR4signalingnormalizesthe disparityofsmallGTPases.Collectively,thestatusofsmall GTPasesislikelytodependontypesofextracellularstimuli ratherthansynchronizingwiththephenotypicstatusofM1/M2 subsets.Accordingly,themasterregulatorofpinocytosisis currentlyunclear.
Whiletheregulatorymechanismsunderlyingpinocytosis arepoorlyunderstood,severalstudieshavefocusedonthe upstreammodulatorofthisprocess(Figure1).Agonistsof liverXreceptors (LXRs)areinvolvedinthedownregulation ofpinocytoticuptakeofnativeLDLinM-CSF-differentiated macrophages(31).LXRsareligand-activatedtranscription factorsinvolvedin thecontroloflipidmetabolismand inflammation.BecausetargetingLXRsinbonemarrow cellsfacilitatesatherosclerosis(32),LXRsmayinterfere withpinocytoticcholesteroldepositioninmacrophagesand subsequentpathogenesisofatherosclerosis.Csányietal.found thatthrombospndin-1(TSP-1)anditscytoskeletalregulation potentiatedpinocytosis(33).Indeed,treatmentofTSP-1 withhumanandmurine M-CSF-differentiatedmacrophages stimulatedmembraneruffleformationandpericellularsolute internalizationbymacropinocytosis.TheTSP1cognate receptorCD47,NADPHoxidase1(Nox1)signaling,PI3K, andmyotubularin-relatedprotein6appeartobeassociated withTSP1-inducedmacropinocytosis.Ourpreviousstudy showedthatCWC22,whichisanessentialloadingfactor ofexonjunctioncomplex,wasassociatedwithpinocytotic incorporationofnativeLDLinmurinebonemarrow-derived macrophages(14).CWC22inmacrophagesshowsnuclear localizationinhumanmild atheroscleroticlesions,while itshowscytosoliclocalizationinadvancedatherosclerotic lesions.Macrophagesinadvancedlesionssimultaneously expresscalpain-6,whichpotentiatesformationofacalpain6/CWC22complexinthecytoplasmandinhibitsnuclear localizationofCWC22.BecauseCWC22hasadirectrolein Rac1 mRNAsplicing,calpain-6counteractsCWC22-mediated maturationof Rac1 mRNA.Indeed,calpain-6-deficient macrophagesshowlowRac1proteinexpressionlevelsand insufficientmacropinocytosisactivity.Knockdownof Cwc22 incalpain-6-deficientmacrophagesleadstotherecovery macropinocytosis,concomitantlywithrestoring Rac1 mRNA maturation.Asnotedabove,Rac1facilitatesthedynamics ofrecyclingendosomes.Therefore,CWC22andrelated splicingfactorsarethoughttobenegativeregulatorsofthe pinocytoticprocess.
Miyazaki 10.3389/fcvm.2022.957897
Frontiersin CardiovascularMedicine 04 frontiersin.org 57
Doespinocytosisdrive atherogenesis?
While fluidphasepinocytosisisaconstitutiveprocessin macrophages andothercellsunderthephysiologicalconditions, thisprocessisreportedlypotentiatedinformercelltypewhen theyarelocalizedinatheroscleroticlesions. Invivo experiments using Apoe / hypercholesterolemicmicehaveindicated thatfluorescentnanobeads,whichissimilarinsizetoLDL andwereinjectedintobloodcirculation,weremassively accumulatedinCD68-positivemacrophagesinatherosclerotic lesions,butnotinnon-atheromaregionsinarteries(34).
Wealsoinvestigated theuptakeofnanobeadsusing Ldlr / hypercholesterolemicmice.Asaresult,similardeposition ofnanoparticlewasreproducedinfoamymacrophagesin atheroscleroticlesions(14).Theseobservationssuggestthat macrophages inatheroscleroticlesionspreferentiallyengulf environmentalLDL-likeparticlesunderhypercholesterolemia. Interestingly,targetingcalpain-6suppressednanobeads incorporationinfoamymacrophagesaswellasatherogenesis inhypercholesterolemicmicewithoutalteringplasmalipid profiles.Notably,overexpressionofcalpain-6canupregulate pinocytoticincorporationofLDLinbonemarrow-derived macrophageswithoutmodifyingreceptor-mediateduptake ofoxidativeLDLandphagocyticuptakeofaggregated LDL.Consideringthatcalpain-6isexclusivelyexpressedin macrophagesinatheroscleroticlesions,itisinterpretedthatthe pinocytoticincorporationofLDLinlesionalmacrophages,at leastoftheircalpain-6-mediatedportion,mayberesponsiblefor thepathogenesisofatherosclerosis.Nevertheless,sincecalpain6contributetotheotheratherogenicprocessesinmacrophages suchascellularmotility(14),thepathophysiologicalimportance ofthisendocyticprocessinthepathogenesisofatherosclerosis hasnotbeenfullydetermined.
Discussion
Asnotedabove,thecontributionofpinocytosisto thepathogenesisof atherosclerosisiscurrentlysketchy. Thisisbecauseofthelackofresponsibleregulatory element(s)ofthisprocesses.Cell-basedexperiments suggestthedominantrolesofsmallGTPasesinpinocytotic membraneruffling,whiletheyalsocontributetothe otherfundamentalprocessesofcells,includingmitosis andcellmotility.Identificationofthepinocytoticmaster regulatorenablestoperforminterventionstudyinanimal modelstodeterminethepathogenicsignificanceof thisprocesses.Itwasreportedthattargeteddeletionof scavengerreceptors,CD36,andscavengerreceptor-Ain
hypercholesterolemicmiceinhibitsthepathogenesisof atherosclerosiswithoutalteringoxidizedLDLuptakein macrophages(35).Therefore,pinocytoticuptakeofLDLin thecellsisworthy tobefurtherinvestigated.Toidentifythe masterregulatorofpinocytoticLDLuptake,comprehensive studiesevaluatingmembraneregulation,vesicletrafficking, exocytosis/efflux,andsubsequentcholesteroldeposition arenecessary.
Authorcontributions
TMconceivedanddesignedthereview,appraisedthe literature,andwrotethemanuscript.
Funding
ThisstudywassupportedinpartbytheJapanSociety forthePromotionof ScienceKAKENHIgrants[grantnos.: JP19K08590andJP22H03520(toTM)],aresearchgrant fromBristol-MyersSquibb(toTM),aresearchgrant fromtheMochidaMemorialFoundationforMedical andPharmaceuticalResearch(toTM),andaresearch grantfromtheNaitoMemorialFoundation(toTM).
Acknowledgments
WethankEllenKnapp,PhD,fromEdanz (https://jp.edanz.com/ac)foreditingadraftof thismanuscript.
Conflictofinterest
Theauthordeclaresthattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythose oftheauthorsanddonotnecessarilyrepresentthose oftheiraffiliatedorganizations,orthoseofthepublisher, theeditorsandthereviewers.Anyproductthatmaybe evaluatedinthisarticle,orclaimthatmaybemadeby itsmanufacturer,isnotguaranteedorendorsedbythe publisher.
Miyazaki 10.3389/fcvm.2022.957897
Frontiersin CardiovascularMedicine 05 frontiersin.org 58
References
1.Miyazaki T,MiyazakiA.Dysregulationofcalpainproteolyticsystems underliesdegenerativevasculardisorders. JAtherosclerThromb. (2018)25:1–15.doi:10.5551/jat.RV17008
2.RossR.Atherosclerosis–aninflammatorydisease. NEnglJMed. (1999) 340:115–26.doi:10.1056/NEJM199901143400207
3.MiyazakiT,MiyazakiA.Impactofdysfunctionalproteincatabolism onmacrophagecholesterolhandling. CurrMedChem. (2019)26:1631–43.doi:10.2174/0929867325666180326165234
4.MiyazakiT,MiyazakiA.Emergingrolesofcalpainproteolyticsystems inmacrophagecholesterolhandling. CellMolLifeSci. (2017)74:3011–21.doi:10.1007/s00018-017-2528-7
5.TianK,OguraS,LittlePJ,XuSA-O,SawamuraT.TargetingLOX-1in atherosclerosisandvasculopathy:currentknowledgeandfutureperspectives. Ann NYAcadSci. (2019)1443:34–53.doi:10.1111/nyas.13984
6.ChistiakovDA,BobryshevYV,OrekhovAN.Macrophage-mediated cholesterolhandlinginatherosclerosis. JCellMolMed. (2016)20:17–28.doi:10.1111/jcmm.12689
7.KruthHS.Fluid-phasepinocytosisofLDLbymacrophages:anoveltarget toreducemacrophagecholesterolaccumulationinatheroscleroticlesions. Curr PharmDes. (2013)19:5865–72.doi:10.2174/1381612811319330005
8.KruthHS.Receptor-independentfluid-phasepinocytosismechanismsfor inductionoffoamcellformationwithnativelow-densitylipoproteinparticles. Curr OpinLipidol. (2011)22:386–93.doi:10.1097/MOL.0b013e32834adadb
9.KruthHS,JonesNL,HuangW,ZhaoB,IshiiI,ChangJ,etal. Macropinocytosisistheendocyticpathwaythatmediatesmacrophagefoamcell formationwithnativelowdensitylipoprotein. JBiolChem. (2005)280:2352–60.doi:10.1074/jbc.M407167200
10.BarthwalMJ,AnzingerJJ,XuQ,BohnackerT,WymannMP,Kruth HS.Fluid-phasepinocytosisofnativelowdensitylipoproteinpromotesmurine M-CSFdifferentiatedmacrophagefoamcellformation. PLoSONE. (2013) 8:e58054.doi:10.1371/journal.pone.0058054
11.HoellerO,BolouraniP,ClarkJ,StephensLR,HawkinsPT,WeinerOD,et al.TwodistinctfunctionsforPI3-kinasesinmacropinocytosis. JCellSci. (2013) 126:4296–307.doi:10.1242/jcs.134015
12.SinglaB,GhoshalP,LinH,WeiQ,DongZ,CsányiG.PKCδ-MediatedNox2 activationpromotesfluid-phasepinocytosisofantigensbyimmaturedendritic cells. FrontImmunol. (2018)9:537.doi:10.3389/fimmu.2018.00537
13.AnzingerJJ,ChangJ,XuQ,BuonoC,LiY,LeyvaFJ,etal.Nativelow-density lipoproteinuptakebymacrophagecolony-stimulatingfactor-differentiatedhuman macrophagesismediatedbymacropinocytosisandmicropinocytosis. Arterioscler ThrombVascBiol.(2010)30:2022–31.doi:10.1161/ATVBAHA.110.210849
14.MiyazakiT,TonamiK,HataS,AiuchiT,OhnishiK,LeiXF,etal.Calpain-6 confersatherogenicitytomacrophagesbydysregulatingpre-mRNAsplicing. JClin Invest. (2016)126:3417–32.doi:10.1172/JCI85880
15.PhuyalS,FarhanH.MultifacetedRhoGTPaseSignalingatthe Endomembranes. FrontCellDevBiol. (2019)7:127.doi:10.3389/fcell.2019.00127
16.AhramM,SameniM,QiuRG,LinebaughB,KirnD,SloaneBF. Rac1-inducedendocytosisisassociatedwithintracellularproteolysisduring migrationthroughathree-dimensionalmatrix. ExpCellRes. (2000)260:292–303.doi:10.1006/excr.2000.5031
17.FujiiM,KawaiK,EgamiY,ArakiN.DissectingtherolesofRac1activation anddeactivationinmacropinocytosisusingmicroscopicphoto-manipulation. Sci Rep. (2013)3:2385.doi:10.1038/srep02385
18.DingL,ZhangL,KimM,ByzovaT,PodrezE.Akt3kinase suppressespinocytosisoflow-densitylipoproteinbymacrophagesvia anovelWNK/SGK1/Cdc42proteinpathway. JBiolChem. (2017) 292:9283–93.doi:10.1074/jbc.M116.773739
19.DingL,BiswasS,MortonRE,SmithJD,HayN,ByzovaTV,etal.Akt3 deficiencyinmacrophagespromotesfoamcellformationandatherosclerosisin
mice. CellMetab. (2012)15:861–71.doi:10.1016/j.cmet.2012.04.020
20.HuangL-H,MeltonEM,LiH,SohnP,RogersMA,Mulligan-KehoeMJ, etal.MyeloidAcyl-CoA:cholesterolacyltransferase1deficiencyreduceslesion macrophagecontentandsuppressesatherosclerosisprogression. JBiolChem. (2016)291:6232–44.doi:10.1074/jbc.M116.713818
21.TkachenkoE,LutgensE,StanRV,SimonsM.Fibroblastgrowthfactor2 endocytosisinendothelialcellsproceedviasyndecan-4-dependentactivationof Rac1andaCdc42-dependentmacropinocyticpathway. JCellSci. (2004)117:3189–99.doi:10.1242/jcs.01190
22.MurrayPJ,AllenJE,BiswasSK,FisherEA,GilroyDW,GoerdtS, etal.Macrophageactivationandpolarization:nomenclatureandexperimental guidelines. Immunity. (2014)41:14–20.doi:10.1016/j.immuni.2014.06.008
23.Shapouri-MoghaddamA,MohammadianS,VaziniH,TaghadosiM,Esmaeili SA,MardaniF,etal.Macrophageplasticity,polarization,andfunctioninhealthand disease. JCellPhysiol. (2018)233:6425–40.doi:10.1002/jcp.26429
24.GenselJC,ZhangB.Macrophageactivationanditsrolein repairandpathologyafterspinalcordinjury. BrainRes. (2015) 1619:1–11.doi:10.1016/j.brainres.2014.12.045
25.MiyazakiT,AkasuR,MiyazakiA.Calpain-associatedproteolytic regulationofthestromalmicroenvironmentincancer. Curr PharmDes. (2021)27:3128–38.doi:10.2174/1381612827666210311 143053
26.YamaguchiT,FushidaS,YamamotoY,TsukadaT,Kinoshita J,OyamaK,etal.Tumor-associatedmacrophagesoftheM2 phenotypecontributetoprogressioningastriccancerwithperitoneal dissemination. GastricCancer. (2016)19:1052–65.doi:10.1007/s10120-01 5-0579-8
27.deGaetanoM,CreanD,BarryM,BeltonO.M1-andM2-typemacrophage responsesarepredictiveofadverseoutcomesinhumanatherosclerosis. Front Immunol. (2016)7:275.doi:10.3389/fimmu.2016.00275
28.OgulurI,PatY,ArdicliO,BarlettaE,CevhertasL,Fernandez-Santamaria R,etal.Advancesandhighlightsinbiomarkersofallergicdiseases. Allergy. (2021) 76:3659–86.doi:10.1111/all.15089
29.RedkaDS,GütschowM,GrinsteinS,CantonJ.Differentialability ofproinflammatoryandanti-inflammatorymacrophagestoperform macropinocytosis. MolBiolCell. (2018)29:53–65.doi:10.1091/mbc.E1706-0419
30.ChoiS-H,HarkewiczR,LeeJH,BoullierA,AlmazanF,LiAC,etal. Lipoproteinaccumulationinmacrophagesviatoll-likereceptor-4-dependentfluid phaseuptake. CircRes. (2009)104:1355–63.doi:10.1161/CIRCRESAHA.108.1 92880
31.BuonoC,LiY,WaldoSW,KruthHS.LiverXreceptors inhibithumanmonocyte-derivedmacrophagefoamcellformation byinhibitingfluid-phasepinocytosisofLDL. JLipidRes. (2007) 48:2411–8.doi:10.1194/jlr.M700170-JLR200
32.TangiralaRK,BischoffED,JosephSB,WagnerBL,WalczakR,LaffitteBA,et al.IdentificationofmacrophageliverXreceptorsasinhibitorsofatherosclerosis. ProcNatlAcadSciUSA. (2002)99:11896–901.doi:10.1073/pnas.182 199799
33.CsányiG,FeckDM,GhoshalP,SinglaB,LinH,NagarajanS,etal.CD47 andNox1mediatedynamicfluid-phasemacropinocytosisofnativeLDL. Antioxid RedoxSignal. (2017)26:886–901.doi:10.1089/ars.2016.6834
34.BuonoC,AnzingerJJ,AmarM,KruthHS.Fluorescentpegylated nanoparticlesdemonstratefluid-phasepinocytosisbymacrophagesinmouse atheroscleroticlesions. JClinInvest. (2009)119:1373–81.doi:10.1172/JCI
35548
35.Manning-TobinJJ,MooreKJ,SeimonTA,BellSA,SharukM,Alvarez-Leite JI,etal.LossofSR-AandCD36activityreducesatheroscleroticlesioncomplexity withoutabrogatingfoamcellformationinhyperlipidemicmice. Arterioscler ThrombVascBiol. (2009)29:19–26.doi:10.1161/ATVBAHA.108.176644
Miyazaki 10.3389/fcvm.2022.957897
Frontiersin CardiovascularMedicine 06 frontiersin.org 59
PUBLISHED 30August2022
DOI 10.3389/fcvm.2022.903390
OPENACCESS
EDITEDBY
AlexanderAkhmedov, UniversityofZurich,Switzerland
REVIEWEDBY YulingZhang, SunYat-senMemorialHospital,China AleksandraKlisic, PrimaryHealthCareCenterPodgorica, Montenegro
*CORRESPONDENCE
YanZhang drzhy1108@163.com
YongHuo huoyong@263.net.cn
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal
FrontiersinCardiovascularMedicine
RECEIVED 24March2022
ACCEPTED 09August2022
PUBLISHED 30August2022
CITATION
LiuB,FanF,ZhengB,YangY,JiaJ, SunP,JiangY,LiK,LiuJ,ChenC,LiJ, ZhangYandHuoY(2022)Association ofremnantcholesterolandlipid parameterswithnew-onsetcarotid plaqueinChinesepopulation. Front.Cardiovasc.Med. 9:903390. doi:10.3389/fcvm.2022.903390
COPYRIGHT ©2022Liu,Fan,Zheng,Yang,Jia,Sun, Jiang,Li,Liu,Chen,Li,ZhangandHuo. Thisisanopen-accessarticle distributedunderthetermsofthe CreativeCommonsAttributionLicense (CCBY).Theuse,distributionor reproductioninotherforumsis permitted,providedtheoriginal author(s)andthecopyrightowner(s) arecreditedandthattheoriginal publicationinthisjournaliscited,in accordancewithacceptedacademic practice.Nouse,distributionor reproductionispermittedwhichdoes notcomplywiththeseterms.
Associationofremnant cholesterolandlipidparameters withnew-onsetcarotidplaque inChinesepopulation
BoLiu1,FangfangFan1,2,BoZheng1,2,YingYang1,3,JiaJia1,2 , PengfeiSun1,YimengJiang1,KaiyinLi1,JiahuiLiu1 , ChuyunChen1,JianpingLi1,2,YanZhang1,2* andYongHuo1,2*
1 DepartmentofCardiology,PekingUniversityFirstHospital,Beijing,China, 2 Instituteof CardiovascularDisease,PekingUniversityFirstHospital,Beijing,China, 3
Background: Remnantlipoproteincholesterol(RC)isanindependentrisk factorforcardiovasculardisease(CVD).However,therelationshipsofremnant cholesterolandotherconventionallipidparameterswithnew-onsetcarotid plaquearenotfullyunderstoodintheChinesecommunity-basedpopulation.
Materialsandmethods: Atotalof872plaque-freeparticipants(51.39 ± 4.96yearsold)withnohistoryofCVDwereincludedinthisstudy.Theplasma concentrationsofRCwerecalculatedbysubtractinglow-densitylipoprotein cholesterol(LDL-C)andhigh-densitylipoproteincholesterol(HDL-C)from totalcholesterol(TC).Multivariateregressionmodelswereusedtoevaluate andcomparetheassociationsbetweenRCandotherlipidparametersand new-onsetcarotidplaque.
Results: Afteramean6.77-yearfollow-up,theincidenceofnew-onsetcarotid plaquewas188(21.56%).RCwassignificantlyassociatedwithnew-onset carotidplaque[Oddratio(OR) = 1.57per1mmol/Lincrease,95%confidence interval(CI):1.03–2.41, p = 0.038].ThehighesttertileofRC(T3group)had thehighestriskofnew-onsetcarotidplaque(OR = 2.53,95%CI:1.63–3.95).
Similarresultswereseenforincreasedotherlipidparameters,butdecreased HDL-Clevels.Whenaddinganotherlipidparameterintotheadjustedmodel withRCsimultaneously,onlyRCremainedsignificantlyassociatedwith new-onsetcarotidplaqueafteradjustingforotherlipidparameters(all p value < 0.005).Furthermore,RCwasstronglyassociatedwithnew-onset carotidplaqueinparticipantswithlowerbaselineLDL-Clevels.
Conclusion: IncreasedRClevelsweresuperiortootherconventional lipidparameterstobeassociatedwithnew-onsetcarotidplaquein theChinesecommunity-basedpopulation.Furthermore,RCshouldbe consideredinparticipantswithlowerLDL-Clevelsforthepurposeofearly atherosclerosisprevention.
KEYWORDS
TYPE OriginalResearch
EchocardiographyCore Lab,InstituteofCardiovascularDisease,PekingUniversityFirstHospital,Beijing,China
remnantcholesterol,carotidplaque,lipids,atherosclerosis,populationstudy Frontiersin CardiovascularMedicine 01 frontiersin.org 60
Introduction
Thereisconsiderableresidualriskofarteriosclerotic cardiovasculardisease(ASCVD)afterreductionoflowdensitylipoproteincholesterol(LDL-C)totherecommended concentrationachievedbystatinregimens,andevenafter managingothermodifiableriskfactors,suchashypertension (1–3).Overthepastmanyyears,numerousclinicalstudies havefocusedonhighlevelsoftriglyceride-richlipoproteins cholesterol,whichindicatesincreasedconcentrationsof potentiallyremnantcholesterolandmayhelptoexplainthe residualrisk(4–6).
Remnantcholesterolisthecholesterolcontentof triglyceride-richlipoproteins,andiscomposedofVLDL andIDLinthefastingstate,andchylomicronremnantsin thenon-fastingstate(2).Whenthereisanexcessofremnant lipoproteinsintheplasma,remnantscancarrylargeamounts ofcholesterolandhavethesamepotentialabilityasLDLto penetrateandbecometrappedintheintimaofthearterialwall, resultingintheformationoffoamcells,atherosclerosis,and low-gradeinflammation(7–12).
Thepresenceofnew-onsetcarotidplaquefrequentlyserves asariskpredictorintheassessmentofCVD/Strokerisk,and carotidplaqueformationisasurrogatemarkerofahighriskofcarotidatheroscleroticdisease(13–15).Therelationship betweenremnantlipoproteinscholesterolandcardiovascular eventshasbeendemonstratedfordecades(4, 8, 16–20). However,fewstudieshavefocusedoncomparingthedifferences betweenRCandotherconventionallipidparametersin atheroscleroticdisease,evenothersurrogatemarkers,suchas carotidplaqueformation(21–24).Inotherwords,thelackof developmentintheevidencebasefortheassociationsbetween RCandconventionallipidparametersandtheriskofnewonsetcarotidplaquehasbeenmoreimportant,especiallyin theChinesecommunity-basedpopulationwithnohistoryof cardiovasculardisease(25).
Thepresentstudyaimedtolongitudinallyevaluatethe relationshipsbetweenRCandotherconventionallipid parametersandnew-onsetcarotidplaque,andfurtherassess thecomparisonsofRCandotherparametersinrelationto new-onsetcarotidplaquewhenbothlipidswereputintothe modelsimultaneously.
Materialsandmethods Studypopulation
Allparticipantsincludedinthisstudywereenrolled fromacommunity-basedatherosclerosiscohortsetupin 2011inBeijing,China.Detaileddescriptionsofthestudy procedureshavebeendescribedpreviously(26).Initially, atotalof4,431participantsaged ≥ 40yearsunderwent
thebaselinesurveyin2012andrespondedon-siteduring thefollow-upvisitin2018.Forthepresentstudy,1,960 participantswithcarotidplaque-freestatusatbaselinewere selected,andthen988participantswithquantitativecarotid arterymeasurementsatthefollow-upvisitwereincluded. Afterstepwiseexclusion,116participantsincludedusinglipidloweringmedications(n = 80),historyofcardiovascular disease(n = 33),andmissingdataforlipidprofiles(n = 3). Ultimately,thisanalysisincluded872eligibleparticipants withamean6.77-yearfollow-up(SupplementaryFigure1). ThisstudywasapprovedbytheethicscommitteeofPeking UniversityFirstHospital,andconfirmedtotheprovisions oftheDeclarationofHelsinki.Allparticipantssigned informedconsent.
Datacollection
Baselineandfollow-updatawerecollectedbytrained researcherstaffaccordingtostandardoperatingprocedures.All participantswereinterviewedusingastandardquestionnaire thatwasspecificallydesignedforthepresentstudy,to obtaininformationondemographiccharacteristics,education, occupation,lifestyle,personal,andmedicalhistory.Current smokingmeanssmokingatleastonecigaretteperdayfor atleast6months.Currentdrinkingmeansdrinkingalcohol atleastonceperweekforatleast6months.Thebody massindex(BMI)wascalculatedbythefollowingformula: BMI = weight(kg)/height(m2).Theperipheralsystolic(SBP) anddiastolicbloodpressure(DBP)readingsusedthemean valueofthesethreesuccessfulmeasurementsusingastandard method(26).
Avenousbloodsamplewasobtainedfromtheforearm ofeachparticipantafteranovernightfast(atleast12h)at thebaselinesurvey.Subsequently,theRocheC8000Automatic Analyzerwasusedtoexamineallbiochemistryparametersin serum,includingfastingbloodglucose(FBG),2-hglucosein thestandard75-goralglucosetolerancetest(OGTT),total cholesterol(TC),triglycerides(TG),high-densitylipoprotein cholesterol(HDL-C),andlow-densitylipoproteincholesterol (LDL-C),whichwerealsodirectlymeasuredbyachemical method;serumcreatinine(Scr, µmol/L)levelsweremeasured enzymatically.Non-HDL-CwascalculatedbysubtractingHDLCfromTC.RCwascalculatedbysubtractingLDL-CandHDLCfromTC,asdonepreviously(21, 27, 28).Inaddition,the estimatedglomerularfiltrationrate(eGFR)wasdeterminedby theCKD-EPIequation(26).
Hypertensionwasdefinedasanyself-reportedhistory, SBP ≥ 140mmHgorDBP ≥ 90mmHg,ortaking anti-hypertensivemedication.Diabetesmellituswas definedasanyself-reportedhistoryofdiabetes,useof hypoglycemicmedication,FBG ≥ 7.0mmol/L,and/or OGTT ≥ 11.1mmol/L.
Liuetal. 10.3389/fcvm.2022.903390
Frontiersin CardiovascularMedicine 02 frontiersin.org 61
Carotidultrasonography
Allparticipantsunderwentcarotidultrasonography bytrainedandcertifiedsonographersbothatthebaseline surveyin2012usingthehigh-resolutionB-modeultrasound system(GEVivid7,8∼10MHzlinear-arrayvascular transducer;Milwaukee,WI,UnitedStates)andatthe follow-upvisitin2018usingaTerasonEchoUltrasound System(Burlington,MA,UnitedStates).Briefly,carotid ultrasoundwasperformedaccordingtostandardscanning andreadingprotocolsatthebaselinesurveyandfollowupvisit.Intima-mediathickness(IMT)wasdetectedas thedistancebetweenthelumen-intimaandthemediaadventitiaultrasoundinterfaces.CarotidIMT(cIMT) wasdefinedasthemeanIMTmeasuredat1cmlengths ofthefarwallofthebilateraldistalcommoncarotid artery.Carotidplaquewasdefinedasfocalstructures encroachingintothearteriallumenofatleast0.5mmor 50%ofthesurroundingcIMTvalue,ordemonstratinga thickness > 1.5mmasmeasuredfromtheintima–lumen interfacetothemedia-adventitiainterfaceatanylevelofthe bilateralcommoncarotidartery,internalcarotidartery,and/or bifurcation(29).
Statisticalanalysis
Descriptivestatisticswereexpressedasthemean ± standard deviation(SD)ormedian(interquartilerange)forcontinuous variablesandnumber(percentage)fordichotomousvariables. Normallydistributedcontinuousvariableswerecompared usingStudent’s t-test,whereasKruskal-Wallistestwasused forvariableswithaskeweddistribution.Pearson’s χ2-testor Fisher’sexacttestwasappliedtoallcategoricalvariablesas appropriate.Univariateandmultivariateregressionmodels wereusedtoevaluatetherelationshipsbetweenbaselinelipid parameters(bothasacontinuousandcategoricalvariable) andnew-onsetcarotidplaque,afteradjustingforsexandage (Model1),andfurtheradjustingforBMI,currentsmoking, currentdrinking,estimatedglomerularfiltrationrate,diabetes mellitus,hypertension,andtheuseofantihypertensiveand hypoglycemicmedications(Model2).Regardingpossible collinearity,thevarianceinflationfactor(VIF)wascalculated fortheincludedvariablesineachmultivariableregressionmodel (SupplementaryTable1).Wefurtherassessedthecomparisons ofRCandotherconventionallipidparametersinrelationto new-onsetcarotidplaquewhenbothlipidparameterswere putintothemodelsimultaneously.Inaddition,weconducted
< 0.001
Diabetesmellitus113(12.96%)30(10.34%)28(9.66%)55(18.84%)0.001
Treatment,N(%)
Antihypertensive114(13.07%)35(12.07%)33(11.38%)46(15.75%)0.242
Hypoglycemic34(3.90%)12(4.15%)7(2.41%)15(5.14%)0.229
Dataareshownasmean ± standarddeviation(SD)ormedian(IQR,Q1-Q3)forcontinuousvariablesandnumber(percentage)fordichotomousvariables. BMI,bodymassindex;RC,remnantcholesterol;TC,totalcholesterol;TG,triglycerides;HDL-C,high-densitylipoproteincholesterol;LDL-C,low-densitylipoproteincholesterol;NonHDL-C,non-high-densitylipoproteincholesterol;FBG,fastingbloodglucose;eGFR,estimatedglomerularfiltrationrate.
Liuetal. 10.3389/fcvm.2022.903390
TotalRemnantcholesterol,mmol/L P-value Tertile1(< 0.42)Tertile2(0.42- < 0.64)Tertile3(≥ 0.64) N872290290292 Age,year51.39 ± 4.9650.86 ± 5.0151.01 ± 5.1152.30 ± 4.62 < 0.001 Female,N(%)642(73.62%)227(78.28%)213(73.45%)202(69.18%)0.045 BMI,kg/m2 25.62 ± 3.3124.35 ± 3.1225.84 ± 3.2526.67 ± 3.15 < 0.001 Totalcholesterol,mmol/L5.27 ± 0.904.83 ± 0.715.21 ± 0.805.76 ± 0.92 < 0.001 Triglycerides,mmol/L1.22(0.88,1.77)0.79(0.63,1.02)1.23(1.00,1.55)2.07(1.54,2.71) < 0.001 HDL-C,mmol/L1.49 ± 0.401.74 ± 0.411.46 ± 0.331.26 ± 0.29 < 0.001 LDL-C,mmol/L3.20 ± 0.742.79 ± 0.573.23 ± 0.653.57 ± 0.78 < 0.001 Non-HDL-C,mmol/L3.78 ± 0.913.09 ± 0.603.76 ± 0.664.50 ± 0.83 < 0.001 Remnantcholesterol,mmol/L0.52(0.37–0.70)0.32(0.24–0.37)0.52(0.47–0.58)0.81(0.70–0.97) < 0.001 FBG,mmol/L5.84 ± 1.495.64 ± 1.435.71 ± 1.056.17 ± 1.83 < 0.001 eGFR,mL/min/1.73◦ m2 100.23 ± 9.42101.75 ± 8.82101.03 ± 9.2797.92 ± 9.73 < 0.001 Currentdrinking,N(%)196(22.48%)57(19.66%)69(23.79%)70(23.97%)0.370 Currentsmoking,N(%)133(15.25%)33(11.38%)41(14.14%)59(20.21%)0.010 Disease,N(%) Hypertension235(26.95%)57(19.66%)67(23.10%)111(38.01%)
TABLE1Baselinecharacteristicsstratifiedbyremnantlipoproteincholesterol(RC)tertiles.
Frontiersin CardiovascularMedicine 03 frontiersin.org 62
thresholdeffectanalysisforlipidparametersiftherelationships werenon-linear(SupplementaryTable2),andinvestigated themodificationofbaselineLDL-Clevelsfortheeffectof RConnew-onsetcarotidplaque.Inthisstudy,a P-value of < 0.05(two-sided)wasconsideredstatisticallysignificant foralltests.Allstatisticalanalyseswereperformedusing Empower(R)(X&Ysolutions,Inc.,Boston,MA,UnitedStates) andRsoftware.1
Results
Baselinepatientcharacteristics
Table1 showsthebaselinecharacteristicsofeligible participants,bothoverallandstratifiedbyRCtertiles.Among the872subjects,73.62%werefemale,withanaverageage of51.39 ± 4.96yearsoldandamean(SD)BMIof 25.62 ± 3.31kg/m2.Thosewithhypertensionanddiabetes accountedfor26.95%(235),and12.96%(113),respectively.The mean(SD)baselinelipidparameterswere5.27 ± 0.90mmol/L forTC,3.20 ± 0.74mmol/LforLDL-C,1.49 ± 0.40mmol/Lfor
HDL-C,and3.78 ± 0.91mmol/Lfornon-HDL-C,respectively. Themedian(interquartilerange,IQR)RCwas0.52(0.37, 0.70)◦mmol/,andTGwas1.22(0.88,1.77)◦mmol/L.The participantswithhigherRC(thetoptertile)hadhigherlevelsof BMI,TC,LDL-C,TG,non-HDL-C,FBG,lowerlevelsofHDLC,andahigherprevalenceofhypertension,diabetesmellitus (p < 0.05).Therewasnosignificantdifferencebetweenthe differentRCtertilesforcurrentdrinking,ortheuseofantihypertensiveandhypoglycemicmedication.





Associationsofremnantlipoprotein cholesterolandotherlipidparameters withnew-onsetcarotidplaquewhen consideredindividually

Ofthe872eligibleplaque-freeparticipantsatbaseline inthisstudy,188(21.56%)individualsdevelopednew-onset carotidplaqueafteramean6.77-yearfollow-up.Asshown in Figure1,therewasmainlypositiveassociationbetween lipidparametersandnew-onsetcarotidplaque,exceptfora negativelinearassociationwithHDL-C. Table2 demonstrates theassociationsofRCandotherconventionallipidparameters withnew-onsetcarotidplaque.RC(per1mmol/Lincrease) wassignificantlyassociatedwithincreasesof65%(95%CI:
*Adjustedfor:sex,age,bodymassindex,currentdrinking,currentsmoking,estimatedglomerularfiltrationrate,diabetesmellitus,hypertension, antihypertensive,andhypoglycemicdrugs.

Liuetal. 10.3389/fcvm.2022.903390
1 www.R-project.org
FIGURE1
Therelationshipbetweenlipidparametersandnew-onsetcarotidplaque*. (A) Remnantlipoproteincholesterol(RC); (B) totalcholesterol(TC); (C) triglycerides(TG); (D) low-densitylipoproteincholesterol(LDL-C); (E) high-densitylipoproteincholesterol(HDL-C);and (F) Non-HDL-C.
Frontiersin CardiovascularMedicine 04 frontiersin.org 63
TABLE2Logisticregressionsfortheeffectsofbaselinelipidparametersandnew-onsetcarotidplaque.
Model1:adjustedforageandsex.Model2:adjustedforage,sex,bodymassindex,currentdrinking,currentsmoking,estimatedglomerularfiltrationrate,diabetesmellitus,hypertension, antihypertensive,andhypoglycemicdrugs.
OR,oddsratio;CI,confidenceinterval;Ref.,referencevalue;RC,remnantcholesterol;TC,totalcholesterol;TG,triglycerides;HDL-C,high-densitylipoproteincholesterol;LDL-C, low-densitylipoproteincholesterol;Non-HDL-C,non-high-densitylipoproteincholesterol.
1.10–2.48; p = 0.016)fortheriskofnew-onsetcarotidplaque.In theadjustedmultivariableregressionmodels,increasedRCwas stronglyassociatedwithnew-onsetcarotidplaque(OR = 1.57 per1mmol/Lincrease;95%CI:1.03–2.41; p = 0.038).Similar resultsappearedinlipidparametersascategoricalvariablesin tertiles,andshowedagradientrelationshipexceptforHDL-C(p fortrend < 0.05).
Associationsofremnantlipoprotein cholesterolandotherlipidparameters withnew-onsetcarotidplaquewhen
consideredsimultaneously
WhenRCandanotherconventionallipidparameterwere putintothemultivariableregressionmodelsimultaneously,
Liuetal. 10.3389/fcvm.2022.903390
LipidparametersN(%)OR(95%CI) P-value CrudeAdjustedmodel1Adjustedmodel2 RC,per1mmol/Lincrease188(21.56%)1.65(1.10–2.48)0.0161.52(1.02–2.28)0.0421.57(1.03–2.41)0.038 TertilesofRC T1(< 0.42)46(15.86%)Ref.Ref.Ref. T2(0.42–< 0.64)50(17.24%)1.11(0.71–1.71)0.6551.07(0.69–1.67)0.7641.23(0.78–1.95)0.376 T3(≥ 0.64) 92(31.51%) 2.44(1.64–3.64) < 0.0012.18(1.45–3.28) < 0.0012.53(1.63–3.95) < 0.001 p fortrend < 0.001 < 0.001 < 0.001 TC,per1mmol/Lincrease188(21.56%)1.28(1.08–1.54)0.0061.30(1.08–1.56)0.0061.28(1.06–1.55)0.011 TertilesofTC T1(< 4.87)50(17.30%)Ref.Ref.Ref. T2(4.87–< 5.60)59(20.21%)1.21(0.80–1.84)0.3701.25(0.81–1.91)0.3131.23(0.79–1.90)0.355 T3(≥ 5.60) 79(27.15%) 1.78(1.19–2.66)0.005 1.81(1.19–2.76)0.005 1.81(1.18–2.78)0.007 p fortrend 0.004 0.005 0.006 TG,per1mmol/Lincrease 188(21.56%) 1.15(1.01–1.31)0.040 1.12(0.97–1.28)0.115 1.12(0.97–1.30)0.128 TertilesofTG T1(< 0.99) 45(15.68%) Ref. Ref. Ref. T2(0.99–< 1.55) 64(21.92%) 1.51(0.99–2.30)0.056 1.49(0.97–2.29)0.070 1.55(0.99–2.41)0.053 T3(≥ 1.55) 79(26.96%) 1.99(1.32–2.99)0.001 1.79(1.18–2.72)0.006 1.92(1.22–3.00)0.004 p fortrend 0.001 0.007 0.005 HDL-C,per1mmol/Lincrease 188(21.56%) 0.63(0.41–0.96)0.032 0.73(0.46–1.15)0.175 0.64(0.39–1.05)0.079 TertilesofHDL-C T1(< 1.28) 69(23.88%) Ref. Ref. Ref. T2(1.28–< 1.60) 69(23.79%) 1.00(0.68–1.46)0.982 1.03(0.69–1.55)0.867 0.96(0.64–1.46)0.863 T3(≥ 1.60) 50(17.06%) 0.66(0.44–0.99)0.042 0.74(0.48–1.15)0.183 0.65(0.41–1.05)0.077 p fortrend 0.046 0.188 0.0780 LDL-C,per1mmol/Lincrease 188(21.56%) 1.43(1.15–1.78)0.001 1.40(1.12–1.76)0.003 1.40(1.11–1.77)0.004 TertilesofLDL-C T1(< 2.85) 50(17.42%) Ref. Ref. Ref. T2(2.85–< 3.46) 58(19.80%) 1.17(0.77–1.78)0.463 1.10(0.72–1.69)0.666 1.14(0.73–1.76)0.567 T3(≥ 3.46) 80(27.40%) 1.79(1.20–2.67)0.004 1.70(1.13–2.57)0.012 1.75(1.14–2.67)0.010 p fortrend 0.004 0.010 0.009 Non-HDL-C,per1mmol/Lincrease188(21.56%)1.39(1.16–1.65) < 0.001 1.35(1.12–1.61)0.001 1.36(1.13–1.65)0.001 TertilesofNon-HDL-C T1(< 3.37) 49(16.96%) Ref. Ref. Ref. T2(3.37–< 4.10) 57(19.52%) 1.19(0.78–1.81)0.424 1.09(0.71–1.68)0.696 1.14(0.73–1.77)0.571 T3(≥ 4.10) 82(28.18%) 1.92(1.29–2.87)0.001 1.81(1.20–2.73)0.005 1.89(1.23–2.89)0.004 p fortrend 0.001 0.003 0.003
Frontiersin CardiovascularMedicine 05 frontiersin.org 64
TABLE3Comparisonsofremnantlipoproteincholesterol(RC)andanotherlipidparameterinrelationtonew-onsetcarotidplaque.
ComparisonI† (whenconsideredRCandTCsimultaneously)
† (whenconsideredRCandTGsimultaneously)
† (whenconsideredRCandHDL-Csimultaneously)
† (whenconsideredRCandNon-HDL-Csimultaneously)
† RCandotherlipidparametersweresimultaneouslyaddedintothemultivariableregressionmodel.Themodelwasadjustedforage,sex,bodymassindex,currentdrinking,current smoking,estimatedglomerularfiltrationrate,diabetesmellitus,hypertension,antihypertensive,andhypoglycemicdrugs. OR,oddsratio;CI,confidenceinterval;Ref.,referencevalue;RC,remnantcholesterol;TC,totalcholesterol;TG,triglycerides;HDL-C,high-densitylipoproteincholesterol;LDL-C, low-densitylipoproteincholesterol;Non-HDL-C,non-high-densitylipoproteincholesterol.
onlyRCremainedsignificantlyassociatedwithnew-onset carotidplaque,evenafteradjustingforotherlipidparameters respectivelyindifferentcomparisons.Comparedwiththe bottomtertile(T1),theeffectofhigherRC(thetoptertile) fornew-onsetcarotidplaquewasincreasedby2.26(95%CI: 1.40–3.65)afteradjustingforTC,2.55(95%CI:1.41–4.16)after adjustingforTG,2.54(95%CI:1.55–4.15)afteradjustingfor HDL-C,2.25(95%CI:1.39–3.63)afteradjustingforLDL-C, and2.28(95%CI:1.31–3.97)afteradjustingfornon-HDL-C, respectively(Table3).
Associationofremnantlipoprotein cholesterolfornew-onsetcarotid plaquemodifiedbybaseline low-densitylipoproteincholesterol levels
Furthermore,weinvestigatedthemodificationofbaseline LDL-ClevelsfortheeffectofRConnew-onsetcarotidplaque
inparticipantswithbaselineLDL-Clevels.Afteradjusting forpossiblecovariates, Figure2 displaysthesmoothcurves showingtherelationshipsbetweenRCandnew-onsetcarotid plaquestratifiedbybaselineLDL-C. Table4 showsthatbaseline LDL-ClevelsmodifiedtheassociationofRCfornew-onset carotidplaque,withanincreasedORto1.95(95%CI:1.06–3.56)inparticipantswithlowerbaselineLDL-Clevels(p for interaction = 0.044).
Discussion
Themajorfindingsofthisstudyarethatconventional lipidparameters,especiallyRC,weresuperiorlyassociated withnew-onsetcarotidplaque,independentofother lipids,inChinesecommunity-basedpopulationwithno historyofcardiovasculardisease.Additionally,among participantswithlowerbaselineLDL-Clevels,RCshould beconsideredanimportantbiomarkertoassesscarotidartery atherosclerosisrisk.
Liuetal. 10.3389/fcvm.2022.903390
ComparisonsOR(95%CI) P-value OR(95%CI) P-value
RC,mmol/L TC,mmol/L T1(< 0.42)Ref.T1(< 4.87) Ref. T2(0.42–< 0.64) 1.16(0.73–1.86)0.525 T2(4.87–< 5.60) 1.12(0.72–1.75)0.611 T3(≥ 0.64) 2.26(1.40–3.65) < 0.001 T3(≥ 5.60) 1.33(0.83–2.12)0.230 ComparisonII
RC,mmol/L TG,mmol/L T1(< 0.42) Ref. T1(< 0.99) Ref. T2(0.42–< 0.64) 1.16(0.70–1.94)0.564 T2(0.99–< 1.55) 1.26(0.77–2.07)0.363 T3(≥ 0.64) 2.55(1.41–4.61)0.002 T3(≥ 1.55) 1.01(0.55–1.85)0.963 ComparisonIII
RC,mmol/L HDL-C,mmol/L T1(< 0.42) Ref. T1(< 1.28) Ref. T2(0.42–< 0.64) 1.21(0.75–1.94)0.428 T2(1.28–< 1.60) 1.22(0.79–1.88)0.377 T3(≥ 0.64) 2.54(1.55–4.15) < 0.001 T3(≥ 1.60) 1.02(0.60–1.74)0.929 ComparisonIV
RC-mmol/L LDL-C,mmol/L T1(< 0.42) Ref. T1(< 2.85) Ref. T2(0.42–< 0.64) 1.14(0.71–1.84)0.578 T2(2.85–< 3.46) 1.07(0.68–1.67)0.772 T3(≥ 0.64) 2.25(1.39–3.63) < 0.001 T3(≥ 3.46) 1.33(0.84–2.11)0.230 ComparisonV
RC,mmol/L Non-HDL-C,mmol/L T1(< 0.42) Ref. T1(< 3.37) Ref. T2(0.42–< 0.64) 1.19(0.72–1.96)0.494 T2(3.37–< 4.10) 0.94(0.59–1.52)0.811 T3(≥ 0.64) 2.28(1.31–3.97)0.004 T3(≥ 4.10) 1.17(0.68–2.00)0.569
† (whenconsideredRCandLDL-Csimultaneously)
Frontiersin CardiovascularMedicine 06 frontiersin.org 65
Previousstudieshavealreadyinvestigatedtherelationship betweenremnantlipoproteincholesterolandcardiovascular diseases(8, 16–20, 30–34).Remnantcholesterolwasconsidered ariskfactorforvariouscardiovascularevents.Varboand colleaguesfoundthatelevatedRCcouldcausesischemicheart disease,independentofreducedHDL-C(8).Remnant-like particle(RLP)cholesterolhasalsosimilarlybeenshowntobe anindependentriskfactorforcardiovasculardiseaseamong 1,567womenfromtheFraminghamHeartStudy(30),and inelderlyJapanesecoronaryarterydisease(CHD)patients (31).Inaddition,someprospectivestudieshavebeenpresented supportingtheprognosticvalueofremnantlipoproteinfor cardiovasculardisease,theresultsfromtheJacksonHeartStudy andFraminghamOffspringCohortStudydemonstratedthat RCwaspositivelyassociatedwithincidentCHDevents,but theassociationwasnotsignificantafteradjustmentsforHDLCandLDL-C(16).Somestudieshavereportedthesignificant associationbetweenremnantlipoproteincholesterolandthe riskofcoronaryeventsinCHDorACSpatientswithorwithout diabetes(32–36).
However,fewstudieshavefocusedoncarotid atherosclerosisassessedbycarotidplaque.Massonetal. conductedacross-sectionalstudyandconcludedthathigher RCwasassociatedwiththepresenceofcarotidatherosclerotic plaque(21).Inthepresentstudy,asuperiorindependent associationofincreasedRClevelswithnew-onsetcarotid plaquecomparedtootherconventionallipidparameters wasdemonstrated.
Severalpotentialmechanismsmayaccountfortheeffectof elevatedlevelsofRConnew-onsetcarotidplaque.LikeLDL-C passingtheendotheliallayerandtrappingintothearterial
intima,thiswouldleadtotheaccumulationofcholesterol, theoccurrenceofatherosclerosisandcardiovascularevents(3). UnlikeLDL,remnantlipoproteincholesterolcouldbetakenup directly(noneedtobemodified:oxidation)bymacrophagesto causefoamcellformationandatheroscleroticplaqueformation (37).Additionally,ithasbeenshownthatRCisanindicator ofendothelialvasomotordysfunction(38)thatcanupregulate theexpressionofpro-inflammatoryfactors(facilitatemonocyte movementintothearterialwall),adhesionmolecules(promote theformationofthrombus)(39),andcoagulationfactors (enhancetheaggregationofplatelets)(40).ElevatedRCwas causallyassociatedwithlow-gradeinflammationatawholebodylevel,with37%higherC-reactiveproteinlevelsfor 1-mmol/LhigherlevelsofRC(12),andrelatedtocarotid macrophagecontent,amarkerforplaqueinstability(24).Taken together,thedirectandindirectroles(pro-inflammatoryand pro-atherothrombotic)ofremnantlipoproteincholesterolcould partiallyexplainincreasedriskofnew-onsetcarotidplaque.


TABLE4Associationofremnantlipoproteincholesterol(RC)for new-onsetcarotidplaquemodifiedbybaselinelow-density lipoproteincholesterol(LDL-C)levels.
VariablesN(%) OR(95%CI) P-valuep interaction

LDL-C,mmol/L
< 3.4105(18.72%)1.95(1.06–3.56)0.0310.044 ≥ 3.483(26.69%)0.97(0.40–2.34)0.952
Modeladjustedforage,sex,bodymassindex,currentdrinking,currentsmoking, estimatedglomerularfiltrationrate,diabetesmellitus,hypertension,antihypertensive, andhypoglycemicdrugs.
OR,oddsratio;CI,confidenceinterval;LDL-C,low-densitylipoproteincholesterol.
Liuetal. 10.3389/fcvm.2022.903390
FIGURE2
Effectofnew-onsetcarotidplaquebasedonremnantlipoproteincholesterol(RC)modifiedbylow-densitylipoproteincholesterol(LDL-C) levels*. (A) BaselineLDL-C < 3.4mmol/L; (B) baselineLDL-C ≥ 3.4mmol/L.*Adjustedfor:sex,age,bodymassindex,currentdrinking,current smoking,estimatedglomerularfiltrationrate,diabetesmellitus,hypertension,antihypertensive,andhypoglycemicdrugs.
Frontiersin CardiovascularMedicine 07 frontiersin.org 66
Inaddition,Nakamuraetal.reportedthatRCwas superiortonon-HDL-Cforpredictingcardiovascularevents withLDL-Clevels < 2.6mmol/Ltreatedwithstatinsin patientswithcoronaryarterydisease(41).Consistently,our studydemonstratedthatincreasedRClevelsweremore stronglyassociatedwiththeriskofnew-onsetcarotidplaque whencomparingtwolipidparametersinthesamemodel simultaneously.StudieshavereportedthatincreasedRCcan explainpartoftheresidualriskofcardiovasculardiseasewith lowerorwell-controlledlevelsofLDL-Cgoal(4, 42).Lin etal.foundthathigherRCconcentrationsweresignificantly associatedwithcoronaryatheroscleroticburden,evenwithan optimallevelofLDL-C(23).Inanotherstudy,theinvestigator reportedthatsubjectswithhigherbaselineRChadahigher riskofmajoradversecardiovascularevents(MACEs)than thoseatlowerconcentrations,especiallylowerLDL-Clevels, withahighestHRof2.69(p = 0.001)(20).Similarly,our studyfoundthatthestrongerassociationofRCwiththerisk ofnew-onsetcarotidplaquewasdemonstratedinparticipants withlowerbaselineLDL-Clevels(< 3.4mmol/L),which indicatedthatRCremainedaresidualriskfactorforASCVD fornew-onsetcarotidplaquewhenLDL-Cachievedtogoal (< 3.4mmol/L).Asimilarstudydemonstratedthatthehigh RC/lowLDL-Cgroup,wasassociatedwithincreasedASCVD risk(43).
Thepresentstudy,tothebestofourknowledge,is thefirsttoevaluatetheassociationsbetweenRCandnewonsetcarotidplaque,andtocompareRCandotherlipid parametersinrelationtonew-onsetcarotidplaqueinthe Chinesepopulation.Additionally,differentbaselineLDL-C levelsmodifiedtheassociationofRCforcarotidplaque. Thereareseverallimitationsthatneedtobeaddressed.First, allparticipantswerefromacommunity-basedcohort,and thereforeexternalgeneralizabilityislimited.Second,theuse offastingsamplesmayunderestimatethecontributionof chylomicron,duetoVLDLarethedominantconstituents ofcirculatingremnants(44),andcalculatedRCcannot beasaccurateasdirectmeasurement,whileit’seasierto calculateRCbyotherconventionallipidparametersto savecosts,andtheassociationwasremarkablyconsistent (27, 45–47).Third,datasuchasinflammatorybiomarkers, dietaryhabits,fattyliver,vascularultrasoundinother territories,etc.,werenotcollectedatbaseline,whichmayaffect atherosclerosisformation.Finally,carotidplaqueformation isamarkerforcarotidarterydamagetoevaluatethe riskofcardiovascularevents,andtheneedtoobserve theriskofMACEsduringcontinuousfollow-upshould beconsidered.
Inconclusion,remnantcholesterolwassuperiorand independentofotherconventionallipidparameters,and wassignificantlyassociatedwithnew-onsetcarotidplaque whenconsideredsimultaneously.Remnantcholesterolcould behelpfultopredictcarotidarterydamageinparticipants
withlowerbaselineLDL-Clevelsforthepurposeofearly atherosclerosisprevention.
Dataavailabilitystatement
Therawdatasupportingtheconclusionsofthisarticlewill bemadeavailablebytheauthors,withoutunduereservation.
Ethicsstatement
Thestudiesinvolvinghumanparticipantswerereviewed andapprovedbyPekingUniversityFirstHospitalEthics Committee.Thepatients/participantsprovidedtheirwritten informedconsenttoparticipateinthisstudy.
Authorcontributions
YZandYHwereresponsibleforthestudyconceptand design.BZ,YY,andJLhelpedwiththedesignandcoordination ofthestudy.PS,YJ,KL,JhL,andCCcollectedandrechecked thedata.FF,JJ,andBLanalyzedandinterpretedthedata.BL draftedthemanuscript.FFandYZrevisedthemanuscript.All authorsreviewedandapprovedthemanuscriptandagreedtobe accountableforallaspectsofthework.
Funding
ThisstudywassupportedbygrantfromNational KeyResearchandDevelopmentProgramofChina (2021YFC2500500and2021YFC2500503),ProjectsofNational NaturalScienceFoundationofChina(Grants81703288and 82170452),UMHS-PUHSCJointInstituteforTranslational andClinicalResearchandtheFundamentalResearchFundsfor theCentralUniversities(BMU20110177andBMU20160530), ChineseCardiovascularAssociation-AccessFund(2019-CCAACCESS-112),KeyLaboratoryofMolecularCardiovascular Sciences(PekingUniversity),andNationalHealthCommission KeyLaboratoryofCardiovascularMolecularBiologyand RegulatoryPeptides.
Acknowledgments
Wethankthestaffandparticipantsofthepresentstudyfor theirimportantcontributions.Weareespeciallygratefultothe sitemanagersofGuchengandPingguoyuanCommunityHealth Centersfortheirsupports.
Liuetal. 10.3389/fcvm.2022.903390
Frontiersin CardiovascularMedicine 08 frontiersin.org 67
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated
References
1.VarboA,NordestgaardBG.Remnantlipoproteins. CurrOpinLipidol. (2017) 28:300–7.doi:10.1097/MOL.0000000000000429
2.NordestgaardBG,VarboA.Triglyceridesandcardiovasculardisease. Lancet. (2014)384:626–35.doi:10.1016/S0140-6736(14)61177-6
3.NordestgaardBG.Triglyceride-richlipoproteinsandatherosclerotic cardiovasculardisease:newinsightsfromepidemiology. GenetBiolCircRes. (2016)118:547–63.doi:10.1161/CIRCRESAHA.115.306249
4.JepsenAM,LangstedA,VarboA,BangLE,KamstrupPR,NordestgaardBG. Increasedremnantcholesterolexplainspartofresidualriskofall-causemortality in5414patientswithischemicheartdisease. ClinChem. (2016)62:593–604.doi: 10.1373/clinchem.2015.253757
5.SchwartzGG,AbtM,BaoW,DeMiccoD,KallendD,MillerM,etal.Fasting triglyceridespredictrecurrentischemiceventsinpatientswithacutecoronary syndrometreatedwithstatins. JAmCollCardiol. (2015)65:2267–75.doi:10.1016/ j.jacc.2015.03.544
6.SampsonUK,FazioS,LintonMF.Residualcardiovascularriskdespiteoptimal ldlcholesterolreductionwithstatins:theevidence,etiology,andtherapeutic challenges. CurrAtherosRep. (2012)14:1–10.doi:10.1007/s11883-011-0219-7
7.TwicklerTB,Dallinga-ThieGM,CohnJS,ChapmanMJ.Elevatedremnantlikeparticlecholesterolconcentration:acharacteristicfeatureoftheatherogenic lipoproteinphenotype. Circulation. (2004)109:1918–25.doi:10.1161/01.CIR. 0000125278.58527.F3
8.VarboA,BennM,Tybjaerg-HansenA,JorgensenAB,Frikke-SchmidtR, NordestgaardBG.Remnantcholesterolasacausalriskfactorforischemicheart disease. JAmCollCardiol. (2013)61:427–36.doi:10.1016/j.jacc.2012.08.1026
9.VarboA,BennM,NordestgaardBG.Remnantcholesterolasacauseof ischemicheartdisease:evidence,definition,measurement,atherogenicity,highrisk patients,andpresentandfuturetreatment. PharmacolTher. (2014)141:358–67. doi:10.1016/j.pharmthera.2013.11.008
10.NordestgaardBG,BennM,SchnohrP,Tybjaerg-HansenA.Nonfasting triglyceridesandriskofmyocardialinfarction,ischemicheartdisease,anddeath inmenandwomen. JAMA. (2007)298:299–308.doi:10.1001/jama.298.3.299
11.IzumidaT,NakamuraY,HinoY,IshikawaS.Combinedeffectofsmalldense low-densitylipoproteincholesterol(Sdldl-C)andremnant-likeparticlecholesterol (Rlp-C)onlow-gradeinflammation. JAtherosclerThromb. (2020)27:319–30.doi: 10.5551/jat.49528
12.VarboA,BennM,Tybjaerg-HansenA,NordestgaardBG.Elevatedremnant cholesterolcausesbothlow-gradeinflammationandischemicheartdisease, whereaselevatedlow-densitylipoproteincholesterolcausesischemicheart diseasewithoutinflammation. Circulation. (2013)128:1298–309.doi:10.1161/ CIRCULATIONAHA.113.003008
13.MitchellC,KorcarzCE,GepnerAD,KaufmanJD,PostW,TracyR,etal. Ultrasoundcarotidplaquefeatures,cardiovasculardiseaseriskfactorsandevents: themulti-ethnicstudyofatherosclerosis. Atherosclerosis. (2018)276:195–202.doi: 10.1016/j.atherosclerosis.2018.06.005
14.HollanderM,BotsML,DelSolAI,KoudstaalPJ,WittemanJC,GrobbeeDE, etal.Carotidplaquesincreasetheriskofstrokeandsubtypesofcerebralinfarction inasymptomaticelderly:theRotterdamstudy. Circulation. (2002)105:2872–7. doi:10.1161/01.cir.0000018650.58984.75
organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
Supplementarymaterial
TheSupplementaryMaterialforthisarticlecanbe foundonlineat: https://www.frontiersin.org/articles/10.3389/ fcvm.2022.903390/full#supplementary-material
15.YangY,FanF,KouM,YangY,ChengG,JiaJ,etal.Brachial-anklepulse wavevelocityisassociatedwiththeriskofnewcarotidplaqueformation:datafrom aChinesecommunity-basedcohort. SciRep. (2018)8:7037.doi:10.1038/s41598018-25579-2
16.JoshiPH,KhokharAA,MassaroJM,LiretteST,GriswoldME,MartinSS,etal. Remnantlipoproteincholesterolandincidentcoronaryheartdisease:theJackson heartandFraminghamoffspringcohortstudies. JAmHeartAssoc. (2016)5:2765. doi:10.1161/JAHA.115.002765
17.VarboA,NordestgaardBG.Remnantcholesterolandtriglyceride-rich lipoproteinsinatherosclerosisprogressionandcardiovasculardisease. Arterioscler ThrombVascBiol. (2016)36:2133–5.doi:10.1161/ATVBAHA.116.308305
18.SaeedA,FeofanovaEV,YuB,SunW,ViraniSS,NambiV,etal.Remnantlikeparticlecholesterol,low-densitylipoproteintriglycerides,andincident cardiovasculardisease. JAmCollCardiol. (2018)72:156–69.doi:10.1016/j.jacc. 2018.04.050
19.JorgensenAB,Frikke-SchmidtR,WestAS,GrandeP,NordestgaardBG, Tybjaerg-HansenA.Geneticallyelevatednon-fastingtriglyceridesandcalculated remnantcholesterolascausalriskfactorsformyocardialinfarction. EurHeartJ. (2013)34:1826–33.doi:10.1093/eurheartj/ehs431
20.CastanerO,PintoX,SubiranaI,AmorAJ,RosE,HernaezA,etal.Remnant cholesterol,notldlcholesterol,isassociatedwithincidentcardiovasculardisease. J AmCollCardiol. (2020)76:2712–24.doi:10.1016/j.jacc.2020.10.008
21.MassonW,LoboM,MolineroG,SiniawskiD.Discordantlipidpatternand carotidatheroscleroticplaque. ImportRemnantCholesArqBrasCardiol. (2017) 108:526–32.doi:10.5935/abc.20170069
22.GaoY,LouY,LiuY,WuS,XiZ,WangX,etal.Therelationshipbetween residualcholesterolriskandplaquecharacteristicsinpatientswithacutecoronary syndrome:insightsfromanopticalcoherencetomographystudy. Atherosclerosis. (2021)317:10–5.doi:10.1016/j.atherosclerosis.2020.11.033
23.LinA,NerlekarN,RajagopalanA,YuvarajJ,ModiR,MirzaeeS,etal. Remnantcholesterolandcoronaryatheroscleroticplaqueburdenassessedby computedtomographycoronaryangiography. Atherosclerosis. (2019)284:24–30. doi:10.1016/j.atherosclerosis.2019.02.019
24.ZambonA,PuatoM,FagginE,GregoF,RattazziM,PaulettoP.Lipoprotein remnantsanddenseLdlareassociatedwithfeaturesofunstablecarotid plaque:aflagfornon-Hdl-C. Atherosclerosis. (2013)230:106–9.doi:10.1016/j. atherosclerosis.2013.06.024
25.JointcommitteeissuedChineseguidelineforthemanagementof dyslipidemiainadults.[2016Chineseguidelineforthemanagementof dyslipidemiainadults]. ZhonghuaXinXueGuanBingZaZhi. (2016)44:833–53. doi:10.3760/cma.j.issn.0253-3758.2016.10.005
26.FanF,QiL,JiaJ,XuX,LiuY,YangY,etal.Noninvasivecentralsystolicblood pressureismorestronglyrelatedtokidneyfunctiondeclinethanperipheralsystolic bloodpressureinaChinesecommunity-basedpopulation. Hypertension. (2016) 67:1166–72.doi:10.1161/HYPERTENSIONAHA.115.07019
27.VarboA,FreibergJJ,NordestgaardBG.Extremenonfastingremnant cholesterolvsextremeLdlcholesterolascontributorstocardiovasculardiseaseand all-causemortalityin90000individualsfromthegeneralpopulation. ClinChem. (2015)61:533–43.doi:10.1373/clinchem.2014.234146
Liuetal. 10.3389/fcvm.2022.903390
Frontiersin CardiovascularMedicine 09 frontiersin.org 68
28.VarboA,BennM,SmithGD,TimpsonNJ,Tybjaerg-HansenA,Nordestgaard BG.Remnantcholesterol,low-densitylipoproteincholesterol,andbloodpressure asmediatorsfromobesitytoischemicheartdisease. CircRes. (2015)116:665–73. doi:10.1161/CIRCRESAHA.116.304846
29.TouboulPJ,HennericiMG,MeairsS,AdamsH,AmarencoP,Bornstein N,etal.Mannheimcarotidintima-mediathicknessandplaqueconsensus(20042006-2011).Anupdateonbehalfoftheadvisoryboardofthe3rd,4thand 5thwatchingtherisksymposia,atthe13th,15thand20thEuropeanstroke conferences,Mannheim,Germany,2004,Brussels,Belgium,2006,andHamburg, Germany,2011. CerebrovascularDis(BaselSwitzerland). (2012)34:290–6.doi: 10.1159/000343145
30.McNamaraJR,ShahPK,NakajimaK,CupplesLA,WilsonPW,Ordovas JM,etal.Remnant-likeparticle(Rlp)cholesterolisanindependentcardiovascular diseaseriskfactorinwomen:resultsfromtheFraminghamheartstudy. Atherosclerosis. (2001)154:229–36.doi:10.1016/s0021-9150(00)00484-6
31.InoueT,UchidaT,KamishiradoH,TakayanagiK,HayashiT,MorookaS, etal.Remnant-likelipoproteinparticlesasriskfactorsforcoronaryarterydiseasein elderlypatients. HormMetabRes. (2004)36:298–302.doi:10.1055/s-2004-814486
32.KugiyamaK,DoiH,TakazoeK,KawanoH,SoejimaH,MizunoY,etal. Remnantlipoproteinlevelsinfastingserumpredictcoronaryeventsinpatients withcoronaryarterydisease. Circulation. (1999)99:2858–60.doi:10.1161/01.cir. 99.22.2858
33.FukushimaH,SugiyamaS,HondaO,KoideS,NakamuraS,SakamotoT, etal.Prognosticvalueofremnant-likelipoproteinparticlelevelsinpatientswith coronaryarterydiseaseandtypeiidiabetesmellitus. JAmCollCardiol. (2004) 43:2219–24.doi:10.1016/j.jacc.2003.09.074
34.NguyenSV,NakamuraT,UematsuM,FujiokaD,WatanabeK,WatanabeY, etal.Remnantlipoproteinemiapredictscardiovasculareventsinpatientswithtype 2diabetesandchronickidneydisease. JCardiol. (2017)69:529–35.doi:10.1016/j. jjcc.2016.04.011
35.ShaoQ,YangZ,WangY,LiQ,HanK,LiangJ,etal.Elevatedremnant cholesterolisassociatedwithadversecardiovascularoutcomesinpatientswith acutecoronarysyndrome. JAtherosclerThromb. (2022)2022:63397.doi:10.5551/ jat.63397
36.ChenY,LiG,GuoX,OuyangN,LiZ,YeN,etal.Theeffectsofcalculated remnant-likeparticlecholesterolonincidentcardiovasculardisease:insightsfrom ageneralChinesepopulation. JClinMed. (2021)10:15.doi:10.3390/jcm10153388
37.NakajimaK,NakanoT,TanakaA.Theoxidativemodificationhypothesisof atherosclerosis:thecomparisonofatherogeniceffectsonoxidizedLdlandremnant lipoproteinsinplasma. ClinChimActa. (2006)367:36–47.doi:10.1016/j.cca.2005.
12.013
38.KugiyamaK,DoiH,MotoyamaT,SoejimaH,MisumiK,KawanoH, etal.Associationofremnantlipoproteinlevelswithimpairmentofendotheliumdependentvasomotorfunctioninhumancoronaryarteries. Circulation. (1998) 97:2519–26.doi:10.1161/01.cir.97.25.2519
39.DoiH,KugiyamaK,OkaH,SugiyamaS,OgataN,KoideSI,etal.Remnant lipoproteinsinduceproatherothrombogenicmoleculesinendothelialcellsthrough aredox-sensitivemechanism. Circulation. (2000)102:670–6.doi:10.1161/01.cir.
102.6.670
40.SaniabadiAR,UmemuraK,ShimoyamaM,AdachiM,NakanoM, NakashimaM.Aggregationofhumanbloodplateletsbyremnantlikelipoprotein particlesofplasmachylomicronsandverylowdensitylipoproteins. Thromb Haemost. (1997)77:996–1001.
41.NakamuraT,ObataJE,HiranoM,KittaY,FujiokaD,SaitoY,etal.Predictive valueofremnantlipoproteinforcardiovasculareventsinpatientswithcoronary arterydiseaseafterachievementofLdl-cholesterolgoals. Atherosclerosis. (2011) 218:163–7.doi:10.1016/j.atherosclerosis.2011.04.040
42.FujiharaY,NakamuraT,HorikoshiT,ObataJE,FujiokaD,WatanabeY,etal. Remnantlipoproteinsareresidualriskfactorforfuturecardiovasculareventsin patientswithstablecoronaryarterydiseaseandon-statinlow-densitylipoprotein cholesterollevels <70Mg/Dl. CircJOffiJJapaneseCircSoc. (2019)83:1302–8. doi:10.1253/circj.CJ-19-0047
43.QuispeR,MartinSS,MichosED,LambaI,BlumenthalRS,SaeedA, etal.Remnantcholesterolpredictscardiovasculardiseasebeyondldlandapob:a primarypreventionstudy. EurHeartJ. (2021)2021:432.doi:10.1093/eurheartj/ ehab432
44.WangT,NakajimaK,LearyET,WarnickGR,CohnJS,HopkinsPN,etal. Ratioofremnant-likeparticle-cholesteroltoserumtotaltriglyceridesisaneffective alternativetoultracentrifugalandelectrophoreticmethodsinthediagnosisof familialtypeIIIhyperlipoproteinemia. ClinChem. (1999)45:1981–7.
45.ZhaoQ,ZhangTY,ChengYJ,MaY,XuYK,YangJQ,etal.Prognostic impactofestimatedremnant-likeparticlecholesterolinpatientswithdiffering glycometabolicstatus:anobservationalcohortstudyfromchina. LipidsHealthDis. (2020)19:179.doi:10.1186/s12944-020-01355-y
46.ChenJ,KuangJ,TangX,MaoL,GuoX,LuoQ,etal.Comparisonof calculatedremnantlipoproteincholesterollevelswithlevelsdirectlymeasuredby nuclearmagneticresonance. LipidsHealthDis. (2020)19:132.doi:10.1186/s12944020-01311-w
47.VarboA,NordestgaardBG.Directlymeasuredvs.calculatedremnant cholesterolidentifiesadditionaloverlookedindividualsinthegeneralpopulation athigherriskofmyocardialinfarction. EurHeartJ. (2021)2021:293.doi:10.1093/ eurheartj/ehab293
Liuetal. 10.3389/fcvm.2022.903390
Frontiersin CardiovascularMedicine 10 frontiersin.org 69
PUBLISHED 04October2022
DOI 10.3389/fcvm.2022.993633
OPENACCESS
EDITEDBY
KailashGulshan, ClevelandStateUniversity, UnitedStates
REVIEWEDBY Chia-FengLiu, ClevelandClinic,UnitedStates BabunageswararaoKanuri, TheOhioStateUniversity, UnitedStates
*CORRESPONDENCE
Chu-HuangChen cchen@texasheart.org
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine
RECEIVED 13July2022
ACCEPTED 12September2022
PUBLISHED 04October2022
CITATION
LeeH-C,AkhmedovAandChenC-H (2022)Spotlightonvery-low-density lipoproteinasadriver ofcardiometabolicdisorders: Implicationsfordiseaseprogression andmechanisticinsights. Front.Cardiovasc.Med. 9:993633. doi:10.3389/fcvm.2022.993633
COPYRIGHT
©2022Lee,AkhmedovandChen.This isanopen-accessarticledistributed underthetermsofthe Creative CommonsAttributionLicense(CCBY) Theuse,distributionorreproductionin otherforumsispermitted,provided theoriginalauthor(s)andthecopyright owner(s)arecreditedandthatthe originalpublicationinthisjournalis cited,inaccordancewithaccepted academicpractice.Nouse,distribution orreproductionispermittedwhich doesnotcomplywiththeseterms.
Spotlightonvery-low-density lipoproteinasadriverof cardiometabolicdisorders: Implicationsfordisease progressionandmechanistic insights
Hsiang-ChunLee1,2,3,4,5,AlexanderAkhmedov6 and Chu-HuangChen7*
1 DepartmentofInternalMedicine,DivisionofCardiology,KaohsiungMedicalUniversityHospital, KaohsiungMedicalUniversity,Kaohsiung,Taiwan, 2 DepartmentofInternalMedicine,Schoolof Medicine,CollegeofMedicine,KaohsiungMedicalUniversity,Kaohsiung,Taiwan, 3 LipidScience andAgingResearchCenter,CollegeofMedicine,KaohsiungMedicalUniversity,Kaohsiung,Taiwan, 4 Institute/CenterofMedicalScienceandTechnology,NationalSunYat-senUniversity,Kaohsiung, Taiwan, 5 GraduateInstituteofAnimalVaccineTechnology,NationalPingtungUniversityofScience andTechnology,Pingtung,Taiwan, 6 CenterforMolecularCardiology,UniversityofZurich, Schlieren,Switzerland, 7 VascularandMedicinalResearch,TexasHeartInstitute,Houston,TX, UnitedStates
Very-low-densitylipoprotein(VLDL)istheonlylipoproteincontaining apolipoproteinBthatissecretedfromtheliver,whereVLDLisassembled fromapolipoproteins,cholesterol,andtriglycerides.Theprimaryfunctionof VLDListotransportcholesterolandotherlipidstoorgansandcellsfor utilization.Apartfromitsroleinnormalbiologicprocesses,VLDLisalsoknown tocontributetothedevelopmentofatheroscleroticcardiovasculardisease. LargeVLDLparticles,whicharesubclassifiedaccordingtotheirsizebynuclear magneticresonancespectrometry,aresignificantlycorrelatednotonlywith atherosclerosis,butalsowithinsulinresistanceanddiabetesincidence.VLDL canalsobesubclassifiedaccordingtosurfaceelectricalchargebyusing anion-exchangechromatography.ThemostelectronegativeVLDLsubclass ishighlycytotoxictoendothelialcellsandmaycontributetocoronaryheart disease.Inaddition,electronegativeVLDLcontributestothedevelopmentof atrialremodeling,especiallyinpatientswithmetabolicsyndrome,whichis anestablishedriskfactorforatrialfibrillation.Inthisreview,wefocusonthe VLDLsubclassesthatareassociatedwithapolipoproteinalterationsandare involvedincardiometabolicdisease.ThepostprandialenhancementofVLDL’s pathogenicityisacriticalmedicalissue,especiallyinpatientswithmetabolic syndrome.Therefore,thesignificanceofthepostprandialmodificationof VLDL’schemicalandfunctionalpropertiesisextensivelydiscussed.
KEYWORDS
very-low-densitylipoprotein,cardiovasculardisease,triglycerides,metabolic syndrome,apolipoproteins,cardiometabolicdisorders
TYPE Review
Frontiersin CardiovascularMedicine 01 frontiersin.org 70
Introduction
Compositionofvery-low-density lipoprotein
Very-low-densitylipoprotein(VLDL)isaprecursorto intermediate-densitylipoprotein(IDL),whichsubsequently formslow-densitylipoprotein(LDL).Density-gradient ultracentrifugationisthestandardmethodusedtoisolate VLDLandothermajorlipoproteins,includingchylomicrons, IDL,LDL,andhigh-densitylipoprotein(HDL)fromserumor plasma(1, 2).ThelipidcoreofVLDLconsistsoftriglycerides (TGs,50–70%ofparticlemass),cholesterolester(10–25%), andfattyacids(<10%).ThemajorcoreproteinofVLDL isapolipoprotein(apo)B100;otherproteinsincludeapoCI, apoCII,apoCIII,andapoE.Thesesurfaceapolipoproteinsalso serveasligandsforcell-surfacereceptorsandcoordinatorsfor lipolysis(3).
VLDLsubfractionsbyparticlediameter,moststudieshave usedasimplifiedclassificationsystemwithdifferentcategories ofaveragediameter.Thequantitativeanalysisofserumor plasmalipoproteinsubfractionsrequireshighreproducibility. Suchreproducibilityhasbeenexaminedbypoolingquality controlplasmalipoproteinsamplesandcomparingNMR resultsamong11spectrometersand5laboratories.Intotal, 16subclasseswereidentified:6forVLDL,6forLDL,and 4forHDL(8).However,aconsensushasnotbeenreached withrespecttostandarddiameterrangesforclassifyingVLDL subfractions.Forinstance,inthestudybyGarveyetal. (9),threecategoriesweredefinedasfollows:largeVLDL (>60nm),intermediateVLDL(35–60nm),andsmallVLDL (<35nm).InthestudybyPhillipsetal.(10),thecategories weredefinedasfollows:largeVLDL(includingchylomicrons, ifpresent, >60nm),mediumVLDL(42–60nm),andsmall VLDL(<42nm).Wangetal.(11)usedsixcategoriesof VLDLasfollows:largest(includingchylomicrons, ± 75nm), verylarge(averagediameter,64.0nm),large(53.6nm), medium(44.5nm),small(36.8nm),andverysmall(31.3nm) VLDL.
VLDLfunctionsasacargocarrier,transportingcholesterol, TGs,andproteinstoperipheralcellsforessentialbioactivities. Intheliver,TGsandcholesterolareincorporatedwithapoB100, whichaffectsthelipidabundanceandsizeofsecretedVLDL (4).AfterVLDLissecreted,itishydrolyzedbylipoprotein lipase(LPL),whichispresentinthecapillaryendotheliumor associatedwithVLDLreceptors,andtransformedintoVLDL remnantandIDL.HDLthentakesupapoCIIfromVLDL remnantandIDL,andcholesterolestertransferprotein(CETP) exchangestheirTGsandphospholipidswithcholesterol.IDL canbetakenupbytheliverviatheLDLreceptoror afterbeingtransformedintoLDLuponlosingapoEand TGs(3).VLDLisaTG-richlipoprotein,anditsassembly andmetabolismareaffectedbyinsulinresistanceandlongtermnutrientexcess(5).VLDLalsomodulatesnitricoxide signaling,whichisessentialforvascularsmoothmuscle relaxationandbloodpressurecontrol(6).Inaddition,VLDL enhancesphospholipaseDactivitybyincreasingcytosolic calciumlevelsandstimulatesaldosteronesynthesisinthe adrenalgland(7).Therefore,VLDLdoesnotonlyserveasa lipidcargocarrier,butitalsomodulateslipid-relatedblood pressureregulation.
Theclassificationofvery-low-density lipoproteinbyparticlesize
ThediameterofVLDLparticlescanbemeasuredusing nuclearmagneticresonance(NMR)spectrometry.Toclassify
Theclassificationofvery-low-density lipoproteinbyparticlecharge
In1988,Avogaroetal.(12)firstcharacterizedLDL onthebasisofsurfaceelectricalchargeratherthan particlesizebyusinganion-exchangechromatography toseparateLDLintoLDL(+)andLDL(–).Inaddition, Yangetal.(13)andChenetal.(14)dividedLDL intofivesubfractionsaccordingtoelectricalcharge, calledL1-L5.Similarly,Chenetal.alsousedthe samemethodofanion-exchangechromatographyto separateVLDLintofivesubfractions,calledV1-V5(15) (Table1).
Immunochemicalisolationof very-low-densitylipoprotein accordingtoapolipoproteincontent
Apolipoproteinsarechemicallyunique,maintainingthe structuralintegrityandfunctionalspecificityofdifferent lipoproteinparticlesinlipidtransportprocesses.Therefore, lipoproteinscanbeclassifiedimmunochemicallyaccording totheirapolipoproteincomposition(16).Thetwomajor classesofapolipoprotein-basedfamiliesareapoA-containing andapoB-containinglipoproteins.VLDL,alongwithIDLand LDL,isanapoB-containinglipoproteinfamily.TheapoBcontaininglipoproteinscanbedividedintoseveralsubfamilies, includingcholesterolester-richlipoprotein(LP-B)andTG-rich lipoproteins(16).
Leeetal. 10.3389/fcvm.2022.993633
Thephysiologicfunctionsof very-low-densitylipoprotein–More thanacargocarrierforlipids
Frontiersin CardiovascularMedicine 02 frontiersin.org 71
TABLE1VLDLsubclassifiedbysizeandelectricalchargeandtheeffectsofVLDLsubclassesonatheroscleroticCVD,MetS,andotherconditions.
ClassificationPatientsFasting/postprandialEffectsReferences
NMR-basedVLDLsubclassesandatheroscleroticCVD
Large,medium,andsmallVLDL particles
Large,medium,andsmallVLDL particles
Adultswithincidentcoronary arterycalcium(n =6814;age, 45–85years)
Healthypostmenopausalwomen (n =286;meanage,61.7years)
NMR-basedVLDLsubclassesandMetSorotherconditions
LargeVLDL,mediumVLDL,and smallVLDL Irishadults(n =1834, middle-aged)
LargestVLDL(including chylomicrons)andfivedifferent VLDLsubclasses
Large,intermediate,andsmall VLDLparticles
Large,medium,andsmallVLDL particles
Overnightfasting(12h)LargeVLDLwaspositivelyassociated withincidentcoronaryartery calcificationinamodeladjustedfor scannertype,age,gender,andrace
Fasting(12h)LargeVLDLwaspositivelyassociated (p < 0.05)withhighercoronaryartery calcificationafteradjustingforage, systolicbloodpressure,current smokingstatus,LDLcholesterol,HDL cholesterol,andtriglycerides
OvernightfastingMetabolicallyhealthypatientswith smaller(belowmedian)VLDLsize
Zebetal.(25)
Mackeyetal.(26)
SixVLDLsubfractions(V1-V6, increasingdensity)
Finnishmenwithorwithout glucoseintolerance(n =9399; meanage,56.8 ± 6.9)
Patientswithorwithoutdiabetes (n =148;meanage, 36.8 ± 11.8years)
Healthywomen(n =26,836; age ≥ 45years)
Womenwithtype1diabetes mellitus(n =112;meanage, 44.9 ± 7.8years)
Adults,freeofclinically detectableCVD(n =6814;age, 44–84years)
OvernightfastingTheconcentrationsofalllipid componentsintheVLDLsubclasses wereincreasedasglucosetolerance decreased
OvernightfastingProgressiveinsulinresistancewas associatedwithincreasedVLDLsize andanincreaseinlargeVLDLparticle concentrations
75.8%without-diabetesand 78.6%withdiabeteswere fasting
LargeVLDLimpartedahigherrisk forincidenttype2diabetesmellitus thandidsmallparticles
Overnightfasting(10–12h)MediumVLDLwasassociatedwith previouspre-eclampsia
Fasting(12h)SeveralVLDLsubfractions(V1-V4) wereassociatedwithabdominalbody compositionandintra-musclefat infiltration
Phillipsetal.(10)
Wangetal.(11)
Garveyetal.(9)
Moraetal.(27)
Amoretal.(28)
Marronetal.(91)
Anion-exchangechromatography–basedVLDLsubclassesandMetS VLDLsubfractionswith increasingnegativecharge (V1-V5)
LDLandVLDLsubfractionswith increasingnegativecharge (L1-L5,V1–V5)
Mostelectronegativelycharged VLDLsubfraction(VLDL-χ)
PatientswithorwithoutMetS (n =26)
Asymptomaticindividuals (n =33;age,32–64years)
PatientswithorwithoutMetS (n =167;age,23–74years)
Pathogenicvery-low-density lipoprotein
Overnightfasting V5,ahighlynegativelychargedVLDL subfraction,directlydamagedthe endothelium
Fasting CombinedelectronegativityofL5and V5plasmaconcentrationwas significantlycorrelatedwithcoronary heartdiseaserisk
Overnightfastingand postprandial
PlasmaconcentrationofVLDL-χ (%) at2hpostprandialwaspositively correlatedwithatrialenlargementin patientswithMetS
Chenetal.(15)
Shenetal.(31)
Thephysiologicbasisforthedifferencesincomposition, structure,andfunctionamongVLDLparticlesisimportant becausethesedifferencescanstronglyinfluencethe atherogenicpropertiesofVLDL.Moreover,abnormal
VLDLcanadverselyaffectvascularorcardiaccells(see below),whichhasimportantimplications.Inthisreview, wepresentasummaryoftheemergingevidenceforVLDL inpromotingcardiometabolicdiseasesandhighlighthow thesubclassificationofVLDLcanbeusedtodistinguish VLDLparticlesthatarepathogenicfromthosethatare physiologicallynecessary.
Leeetal. 10.3389/fcvm.2022.993633
Leeetal.(71)
CVD,cardiovasculardisease;LDL,low-densitylipoprotein;NMR,nuclearmagneticresonance;VLDL,very-low-densitylipoprotein.
Frontiersin CardiovascularMedicine 03 frontiersin.org 72
PlasmaLDL-cholesterol(LDL-C)aloneisnotsufficient topredictallnon-atheroscleroticandatherosclerotic cardiovasculardisease(ASCVD).AsidefromLDL-C,VLDL cholesterol(VLDL-C)isalsoknowntocontributetothe developmentofASCVD.PlasmaVLDL-Cistheprimary componentofnon–HDL-cholesterol(HDL-C)(17)andisa predictorofASCVDindependentofLDLcholesterol(LDL-C) (18–20).
Prenneretal.(18)usedcardiacelectronbeamcomputed tomographyscanningtoassesscoronaryarterycalcification, whichisanindependentpredictorofCVDrisk,ina populationofhigh-riskpatientswithtype2diabetes.Their resultsshowedthatVLDL-Cisanindependentriskfactor forcoronaryarterycalcification,particularlyinwomen. Furthermore,thisassociationwasindependentofcirculatory TGlevels(18).Inpatientswithtype2diabeteswhopreviously underwentcoronarystentimplantation,anelevatedVLDL-C level >0.52mmol/Lwasindependentlyassociatedwithinstentrestenosis(hazardratio=3.01)(21).Iannuzzietal.(22) usedultrasoundtomeasurecarotidintima–mediathickness inpostmenopausalwomenandshowedthatVLDL-Cwas thelipoproteinmoststronglyassociatedwithsubclinical atherosclerosis.Inaddition,evidencefromclinicalstudieshas consistentlyindicatedacausalroleforTG-richlipoproteins suchasVLDLinASCVD.Anupdatedconsensusstatement regardingthecurrentunderstandingoftheroleofTG-rich lipoproteinsandtheirremnantsinASCVDhasbeenpublished recently(5).
Thesizeofvery-low-density lipoproteinaffectsitsatherogenicity
LargeVLDLparticleshaveagreaterassociationwiththe incidenceofatherosclerosisthandosmallerVLDLparticles (Table1).Thesize-basedsubclassificationoflipoproteinsis performedbytheNMRanalyzer,whichusescharacteristic signalsoflipoproteinsubclasseswithdifferentsizesasthebasis forquantification.Asetofpurifiedstandardsisrequiredfor convertingsignalamplitudestospecificparticleconcentrations (23).ThestandardsforVLDLareisolatedbyusinga combinationofultracentrifugationandagarosegelfiltration,
andthesizedistributionisdeterminedbyusingelectron microscopy(2).
VLDLcirculatesinthebloodforabout4hbeforeitis convertedtoIDLandthenLDL(24).Lipolyticremodelingis responsibleforthedown-sizingofthelargestVLDLparticles andtheirconversiontoIDLandLDL.UnlikesmallLDL,large VLDLwasassociatedwithanincreasedriskofincidentcoronary arterycalcificationandcalciumscoreprogressionduringfollowup(25).Likewise,inrelativelyhealthypostmenopausalwomen, largeVLDLwaspositivelyassociatedwithcoronaryartery calcification,suggestingthatthemeasurementoflipoprotein subclassesmayimprovethepredictionofcoronaryartery diseasebeyondusingtheconventionallipidpanel(26).
Inaddition,thesizeofVLDLwasshowntobecorrelated withinsulinresistanceanddiabetesmellitus(11, 27)(Table1). InaprospectivestudybyMoraetal.(27)of26,836initially healthywomenfollowedfor13years,largeVLDLparticles werefoundtopredicttype2diabetes.Likewise,Wangetal. (11)reportedinapopulationstudyof9399Finnishmenthat abnormalglucosetoleranceandnewonsettype2diabetes wereassociatedwithanincreaseinVLDLparticles,withthe exceptionofverysmallVLDL.Conversely,alowernumber oflargeVLDLparticleswasshowntobethemostsignificant predictorofmetabolichealthinadults,regardlessofbody massindexandobesitystatus(10).Garveyetal.(9)described theeffectsofinsulinresistanceandtype2diabetesonthe particlesizeandconcentrationoflipoproteinsubclasses.Their resultsshowedthatprogressiveinsulinresistancewasassociated withincreasedVLDLsize.Comparedwithindividualswho havenormalinsulinsensitivity,patientswithinsulinresistance ordiabetesshowedincreasedconcentrationsoflargeVLDL particles,butnochangeinmediumVLDLorsmallVLDL particleconcentrations.Forpatientswithtype1diabetes, mediumVLDLparticleconcentrationwasindependently associatedwithpreviouspre-eclampsiaduringpregnancyafter adjustingforageandstatinuse(28).
Thecharge-basedelectronegativityof very-low-densitylipoprotein determinesitsatherogenicity
Lipoproteinparticlescanbeseparatedaccordingtocharge byusinganion-exchangechromatography.L5,whichisthe mostelectronegativelychargedsubfractionofLDL,induces endothelialapoptosisthroughthelectin-likeoxidizedLDL receptor-1(LOX-1)intheabsenceoftheLDLreceptor (LDLR)(29).Similarly,themostelectronegativesubfraction ofVLDL,V5,wasshowntoinduceendothelialapoptosis andwasthesubfractionmostrapidlyinternalizedinto endothelialcells(15).Inaddition,patientswithmetabolic syndrome(MetS)werefoundtohaveincreasedlevelsof electronegativeVLDL.VLDLisolatedfrompatientswith MetSinducedbraininflammationwithglialcellactivation
Leeetal. 10.3389/fcvm.2022.993633
Independentoflow-density lipoprotein,very-low-density lipoproteinisassociatedwith cardiometabolicdisorders
Cholesterolscarriedbyboth low-densitylipoproteinand very-low-densitylipoproteinare associatedwithatherosclerosis
Frontiersin CardiovascularMedicine 04 frontiersin.org 73
TABLE2ClinicalstudiesshowingalteredVLDLapolipoproteinsinpatientswithmetabolicandatherogenicdiseases.
ApoCIHumanCross-sectionalstudies(age,56–80years)Fastingand postprandial(4h)
ApoCIIIHumanLudwigshafenRiskandCardiovascularHealth Study(LURIC; n =3041)
ApoC1positively correlatedwithcarotid atherosclerosis
NotspecifiedSevencommonvariantsof APOC3 (rs734104,rs4520, rs5142,rs5141,rs5130, rs5128,andrs4225)were associatedwithmodestly raisedapoC-IIIand elevatedVLDL/TGbut werenotassociatedwith CAD
HumanMiddle-agedpatients(n =688;averageage, 66years;52%women)
ApoAVHumanPatientswithnon-alcoholicfattyliverdisease (n =17)vs.healthyliver(n =6)
ApoE HumanTwoindependentcohorts:women(n =322; age,30–55years)andmen(n =418;age, 40–75years)
Angiopoietin-like protein(ANGPTL)-3
Humanand mice
Humansandmice(e.g., Angptl3
inmice,suggestingthatelectronegativeVLDLcanpromote cognitivedysfunction(30).Furthermore,Shenetal.(31) furtherconfirmedthatthemostelectronegativehumanplasma LDL(i.e.,L5)andVLDL(i.e.,V5)arehighlyatherogenic. Intheirstudy,thecombinedelectronegativityofL5and plasmaconcentrationofV5wassignificantlycorrelatedwith coronaryheartdiseaseriskinanage-adjustedanalysesof asymptomaticindividuals.Moreover,whenhumanaortic endothelialcellsweretreatedwithL5+V5andL1+V1, L5+V5inducedsignificantlygreatersenescence-associated–β-galactosidaseactivitythandidL1+V1.In ApoE / mice,aorticlipidaccumulationandcellularsenescence wereassociatedwiththeelectronegativityofLDLand VLDL(31).
Alteredapolipoproteincontentin
By1972,theprimarystructures,includingproteinandDNA sequences,hadbeendeterminedforalmostallapolipoproteins
FastingApoCII,apoCIII,andapoE wereassociatedwith compositeCVD(fataland non-fatalmyocardial infarction,ischemicstroke, andsuddencardiacdeath)
VLDLandLDLwith apoCIIIwereassociated withalowerriskofCHD
(AI,AIV,B,CI,CII,CIII,D,E,I,andJ)(16).VLDL particlescontainingapoE,apoCI,apoCIII,andapoAVhave beenshowntoaffectVLDLmetabolism,siteutilization,and atherogenicity.Inthefollowingsections,eachlipoproteinis brieflydescribed.
ApoE
Emergingevidencesupportsthatthecompositionalchange ofapolipoproteinsinVLDLaffectsitsatherogenicity(Table2). VLDLisoneofseveralmajorlipoproteinscontainingapoE, whichisaspecificligandforcysteine-bindingrepeatsof theVLDLreceptor(VLDLR).VLDLRiswidelyexpressed throughoutthebody,includingtheheart,skeletalmuscle, adiposetissue,andbrain,andithasanimportantrole intheuptakeandmetabolismofapoE-containingTG-rich lipoproteins.ApoEisapolymorphicproteinarisingfromthree allelesatasinglegenelocus(32).TheenrichmentofapoE contentinVLDLhasbeenshowntoprotectagainstcoronary heartdisease(33).
ApoCI
PrimarilyassociatedwithHDLinthefastingstate,apoCI transientlyattachestothesurfaceofTG-richlipoproteinssuch
Leeetal. 10.3389/fcvm.2022.993633
ApolipoproteinStudy type PatientsFasting/ postprandial EffectsReferences
(35–37)
(92)
(39)
Fasting ApoA5mRNAlevelwas associatedwith hepatosteatosis (46)
NotspecifiedIncreasedapoEcontentin
(33)
Fasting ANGPTL-3inhibition reducesthecontentand sizeoflipidsinVLDL (54)
/ ,Ldlr / , Lipg / )withhyperlipidemia
CAD,coronaryarterydisease;CHD,coronaryheartdisease;CVD,cardiovasculardisease;LDL,low-densitylipoprotein;TG,triglyceride;VLDL,very-low-densitylipoprotein.
very-low-densitylipoproteinaffectsits atherogenicity
Frontiersin CardiovascularMedicine 05 frontiersin.org 74
aschylomicronsandVLDLpostprandially.ApoCImodulates severalenzymesinvolvedinlipoproteinmetabolismand canreducetheuptakeofVLDLbyinhibitingitsbinding toVLDLR(34).Theincreasedintima-mediathicknessof thecommoncarotidarteryindicatesearlyatherosclerosis andwasfoundtobeassociatedwithapoCIcontentin postprandialTG-richlipoproteins(35, 36).Inaddition,the numberofapoCImoleculesperVLDLparticleinthe fastingstatewasassociatedwiththeplaquesizeofcarotid atherosclerosis(37).ApoCIwasalsoshowntobecorrelated withcholesterolenrichmentinVLDLparticlesandthedelayed clearanceofTG-richlipoproteins(37).Inhypercholesterolemic rabbits,theconstitutiveexpressionofhumanapoCIprovided protectionagainstseriousatherosclerosis(38).Thisbenefitwas foundtoberelatedtotheinhibitionofplasmacholesteryl estertransferprotein(CETP)activity(38).Thesefindings supportthatapoCIenrichmentattenuatestheatherogenicity ofVLDLparticles.
ApoCIII
ApoCIIIhasbeensuggestedtobeacentralregulatorof TG-richlipoproteinmetabolism(39).Adirectassociation ofapoCIIIwithatherosclerosiswasrevealedbyclinical geneticstudiesandstudiesshowingthatloss-of-function mutationsin APOC3 areassociatedwithlowTGlevels (40)andareducedincidenceofischemicCVD(41). IncreasedplasmalevelsofapoCIIIareassociatedwith increasedlevelsofVLDL,IDLparticles,andTGs(42).In humanmonocyticTHP-1cells,apoCIIIactivatedprotein kinaseCalpha(PKCα)andtransformingproteinRhoA, whichresultedin β1-integrinactivationandpromoted endothelialcelladhesion.Theseresultssuggestedthat apoCIIInotonlymodulateslipoproteinmetabolism,but mayalsocontributetoatherosclerosisdevelopment(43).
TheantisenseapoCIIIinhibitorvolanesorsen,whichreduces apoCIIIlevelsby >75%andplasmaTGslevels,inhibits apoCIIIsynthesisintheliver(44).However,theindication fortheclinicaluseofvolanesorsenislimitedtopatients withfamilialchylomicronaemiasyndromeforpreventing pancreatitis;therefore,itseffectonreducingCVDremains undetermined(39).
ApoAV
Incontrastto APOC3,genotypecombinationsofcommon APOA5 variants(c.-1131T > C,S19W,andc.∗31C > T) areassociatedwithelevatedTGlevelsandincreasedCHD risk(45).Inaddition,patientswithnon-alcoholicfattyliver diseasehaveelevatedapoAVexpression,whichpromotes hepaticTGstorageinlipiddropletsbutdecreasesVLDL secretionbytheliver(46).ApoAValsoacceleratesTG-rich lipoproteinuptakebytheliver(47).However,themechanismby whichapoAVregulatescirculatoryVLDLmetabolismremains largelyunknown.
Mechanismsofmodified very-low-densitylipoproteinin cardiometabolicdisorders
OverproductionofTGsintheliverand non-alcoholicfattyliverdisease
AkeyfeatureoflargeVLDListheoverproductionofTGs intheliver,whichmayoccurforseveralyearsbeforetheonset oftype2diabetes(27).Intheliver,thebiogenesisofVLDLs andtheassemblyofapolipoproteinsarecomplexandhighly regulatedprocesses(4).AmajorsourceofTGsynthesisisthe endoplasmicreticulum(ER)lumen,whereTGsareassembled withapoB100toformlipid-poorprimordialVLDLparticles. Thisprocessisfacilitatedbymicrosomaltriglyceridetransfer protein(MTP)(4),whichtransfersbothneutralandpolarlipids toformVLDLparticles(Figure1).WhetherandhowMTP ismodulatedinpatientswithinsulinresistanceanddiabetes remainunclear.
Becauseofitslargesize(averagediameter >60nm),VLDL isshiftedfromtheERmembranetothecisGolgiforcargo selectionandvesicleformation.However,theutilizationof vesicularcarrierproteinsforVLDLremainsanongoingsubject ofinvestigation(4).IthasbeensuggestedthatVLDLexitsthe hepaticERinaspecializedvesicle(i.e.,theVLDLtransport vesicle),whichcanaccommodateaparticlediameterofupto 100–200nm(48).
Patientswithnon-alcoholicfattyliverdiseasehaveincreased hepaticstearol-CoAdesaturase(SCD)-1activity,whichconverts saturatedfattyacidstomonosaturatedfattyacidsthatserveas amajorsubstrateforthesynthesisof denovo TGsandother lipids(49).HowtheabundanceofTGsandthedegreeofTG desaturationarecontrolledorregulatedduringVLDLsynthesis remainundetermined.
HepaticapoAIVexpression,whichisregulatedbynuclear transcriptionfactorcAMP-responsiveelement-bindingprotein H(CREBH),iscorrelatedwithhepaticTGcontentinpatients withchronicliversteatosis(50).CREBHactivationplayskey rolesinhepaticsteatosisbyupregulatingapoAIVduringVLDL assemblyintheERandpromotestheassemblyoflargeand TG-enrichedVLDLparticles(50)(Figure1).Inadditionto itsexpressionintheliver,apoAIVispredominantlyexpressed inhumanenterocytestofacilitateintestinalchylomicron assemblyandishighlyupregulatedafterafattymeal (51).
Regulationoflipolysis
TheutilizationofVLDLandthebreakdownofTGs inorgansrequirethekeyenzymelipoproteinlipase (LPL)togeneratefreefattyacids.Theinhibitionof
Leeetal. 10.3389/fcvm.2022.993633
Frontiersin CardiovascularMedicine 06 frontiersin.org 75
Mechanismsoflargevery-low-densitylipoprotein(VLDL)innon-alcoholicfattyliverdiseaseandgutmicrobiomeimbalance.The overproductionoftriglycerides(TGs)isrelatedtoincreasedactivityofhepaticstearol-CoAdesaturase(SCD)-1,whichconvertssaturatedfatty acidstomonosaturatedfattyacidsthatserveasthesubstrateforthesynthesisof denovo TGs.TheassemblyofTGswithapolipoprotein (apo)B100isfacilitatedbymicrosomaltriglyceridetransferprotein(MTP).Innon-alcoholicfattyliverdisease,thenucleartranscriptionfactor cAMP-responsiveelement-bindingproteinH(CREBH)isupregulated,inturnincreasingexpressionofhepaticapoAIV,whichpromotesthe assemblyofTG-rich,largeVLDL.Angiopoietin-likeproteinfamily3(ANGPTL3)inhibitstheenzymeactivityoflipoproteinlipase(LPL),whichis essentialforbreakdownofTGsinVLDLutilization.Bothintermediate-densitylipoprotein(IDL)andLDLparticlesarerecognizedbyLDLreceptor (LDLR)expressedintheliver.LPLactivityisalsoinhibitedbyapoCIII.LargeVLDLpromotesplasmaCETP-inducedremodelingofTG-richHDL. Ahigh-carbohydratedietandobesityimpairmicrobiomediversity,whichisrelatedtoreducedplasmaHDLlevelsandincreasedhepaticapoCIII productionthatinturninhibitLPLactivityandenhancetheabundanceoflargeVLDLinthecirculation.

lipolysisincreasesthesizeofcirculatingVLDL.Several membersoftheangiopoietin-likeprotein(ANGPTL)family regulatetheactivityofLPL.ANGPTL3,ANGPTL4,and ANGPTL8areupregulatedinpatientswithtype2diabetes andobesity(52).Inagroupofpatientswhoreceived RNAinhibitiontherapywithantisenseoligonucleotides targeting ANGPTL3,proteinlevelsofANGPTL3were reducedbyasmuchas84.5%frombaseline6weeksafter injection,whilelevelsofTGswerereducedby63.1%, VLDLcholesterolby60.0%,andapoCIIIby58.8%(53).
Inmice,ANGPTL3inhibitionreducedTGcontentin theliverandretardedatherosclerosisprogression(53). Endotheliallipase,whichreducesLDL-CviaanLDLRindependentmechanism,isessentialforphospholipid reductioninVLDLandLDL(54).In LDLR / mice, ANGPTL3inhibitioncausedamarkedreductioninthe TGcontentofVLDL.Furthermore,in ApoE / mice, ANGPTL3inhibitionpromotedVLDLclearancewiththe involvementofmultipleremnantreceptors(54).However, intheliver,ANGPTL3didnotperturbateapoBlipidation andhepaticVLDLassembly(54).Thesefindingssuggest thatANGPTL3governsVLDLcatabolismandlargely affectsVLDLlipidcontentandsize.Ontheotherhand, endotheliallipaseexertsanti-atherogeniceffectsbyenhancing thecatabolismof β-VLDLs(55),whicharecholesterol-rich chylomicronandVLDLremnantsthataccumulateinthe plasmaofpatientswithtypeIIIdysbetalipoproteinemia
(56).Inelderlypatients,theremovalofTG-richlipoprotein remnantsisdelayed,butTGbreakdownisunchanged. WhetherVLDLreceptorfunctionisimpairedandwhether ANGPTL3isinvolvedinaging-related,delayedVLDL removalremainunknown.
Interactionofvery-low-density lipoproteinwithhigh-density lipoprotein
Thereverse-remnantcholesteroltransportmechanism, whichistheacquisitionofVLDLsurfacecomponentsby HDLduringLPL-mediatedlipolysis,playsanimportant roleinVLDLcatabolism(57).HDLaffectsthelipolysisof VLDLTGsandthereleaseofsurfacelipids,freecholesterol, phospholipids,andexchangeableapoE,apoCII,andapoCIII fromVLDLduringlipolysis(58).HDLcanalsobeclassified intosubpopulationsaccordingtosize,apolipoproteincontent, charge,mass,anddensity.Althoughsubpopulationsofboth largeandsmallHDLparticlesincreasedVLDLTGlipolysis efficiencyandsurfacematerialremovalfromVLDL,the small,protein-enrichedHDLparticlesexhibitedagreater effectonthisprocessandpromotedamoreefficientrelease ofsurfacecomponents,therebyaffectingtheproperties ofthegeneratedremnants.LossofapoCproteinsfrom VLDLduringlipolysispromotedthemetabolismof
Leeetal. 10.3389/fcvm.2022.993633
FIGURE1
Frontiersin CardiovascularMedicine 07 frontiersin.org 76
apoB-containinglipoproteinbecausebothapoCIIand apoCIIIinhibitthebindingofapoBlipoproteinstothe LDLR(58).
IncreasedTGcontenthasbeensuggestedtodecreasethe stabilityofHDL,VLDL,andLDLviaseveralmechanisms. First,TGshaveadirectdestabilizingeffectonlipoprotein particlesfromtheCETP-inducedremodelingofTG-richHDL. Second,TGshaveindirecteffectsthatenhancespontaneous andenzymatichydrolysisandoxidation.Third,productsof theaforementionedprocesses,particularlyfreefattyacids, furtheraugmentlipoproteindestabilizationandfusion.TGs arealsoinvolvedinthesubstantialreleaseofproteinsfrom lipoproteins.Finally,thecombinationofdestabilizedLDLand VLDLenhancestheirretentioninthearterialwall,triggering atherosclerosis(59).
Geneticvariantsassociatedwith very-low-densitylipoproteinparticles
Geneticvariantshavebeenassociatedwithlipoprotein subclasses.Amongthose,thecommonvariantrs73059724 resultedinsmallVLDLparticleswithfewerphospholipids (60).Thevariantrs73059724islocatedonchromosome19 andisassociatedwiththepromoterandintronof HIF3A, whichregulatesthecellularuptakeofcholesterolestersand VLDLbypromotinghypoxicconditions.Inaddition, HIF3A hypermethylationisassociatedwithincreasedadiposityinAsian infantsandchildren(61, 62).Thesefindingssuggestthat HIF3AmayregulateVLDLparticlesize.Furthermore,DNA methylationat HIF3A mayexplaintheprenatalinfluenceson adiposity.Inanotherrecentgeneticstudy,Li-Gaoetal.(63) investigatedpostprandialmetabolomicsandfoundthatthe ANKRD55 locusledbythers458741:Cvariantwasstrongly associatedwithextremelylargeVLDL,bodycomposition, andtheincidenceofdiabetes.Thisfindingilluminates thestronggeneticlinkagebetweenVLDLmodificationand insulinresistance.
Gutmicrobiomeimbalance
Vojinovicetal.(64)showedinaprospectivepopulationbasedcohortof2309individualsthat32microbialfamilies andgeneraingutmicrobiotawereassociatedwithsize-defined subfractionsofVLDL,HDL,serumlipidvalues,andglycolysisrelatedmetabolites.Amongthe32,18microbialfamiliesand generaweresignificantlyassociatedwithVLDLparticlesof varioussizes(extrasmall,small,medium,large,verylarge, andextremelylarge)(64).Anotherrecentstudyshowedthat, inhealthyindividuals,lowmicrobiotadiversitywasassociated withobesity,abdominalobesity,andlowHDL-Clevel(65). Thesereportssuggestthatgutmicrobiotaimbalancemaybe
involvedinthealterationofVLDLparticlesize.Thus,thesource ofalteredVLDLparticlesispresumablytheintestines,although therealoriginofalteredVLDLparticlesmaybediet.Inanimals andhumans,ahigh-carbohydratedietresultsintheelevationof largeTG-enrichedVLDLparticles,alongwiththeenrichmentof apoCproteins.Carbohydrateintakeincreaseshepaticsecretory ratesofVLDLTGswithoutchangingthesecretionofapoB, whichtogetherleadtolargeanddenseVLDLparticles(66).
Very-low-densitylipoprotein particlesinthenon-fastingstate carryariskforatherosclerosisand atrialfibrillation
Very-low-densitylipoproteinparticle changesinfastingandpostprandial states
Postprandialhypertriglyceridemiaisahallmarkof dyslipidemiainpatientswithtype2diabetes.Recently,it hasbeensuggestedthatpostprandialdyslipidemiaisequally asimportantastheestimationoflipidsinthefastingstate, particularlyforpatientswithtype2diabetes(67).Moraetal. (27)characterizedlipoproteinparticlesaccordingtosizein fastingandnon-fastingstatesbyusingNMR,notingsimilar resultsbetweenLDLandHDLparticles.However,compared withfastingVLDL,non-fastinglargeVLDLparticlescarried muchhigherriskfordiabetes.IntheCopenhagenGeneral PopulationStudy,inwhichNMRspectrometrywasused toanalyzethelipidsof9293individuals,theresultsshowed thatVLDLandIDLparticlescontainedone-thirdofplasma cholesterolinthenon-fastingstate(68).PostprandialTGsare carriedbyprimarilychylomicronandVLDLremnants,which areligandsoftheVLDLreceptorinvolvedinmacrophagefoam cellformationduringthedevelopmentofatherosclerosis(69).
Correlationofpostprandial very-low-densitylipoproteinrather
thanfastingvery-low-density lipoproteinwithatrialcardiopathy
VLDLutilizationservesasthemajorenergysourceforthe heart.Underphysiologicconditions,approximately70%ofthe heart’senergyisderivedfromfattyacidoxidation(70).Lee etal.(71)showedthatpostprandialVLDLisindependently correlatedwithatrialenlargement,indicatingthatpostprandial VLDLisariskfactorforatrialfibrillation(Table1).Ina prospectivestudyofindividualswithMetS(n =87)andwithout MetS(n =80),theyfoundthatnegatively-chargedVLDL(2hpostprandialVLDL-χ,concentrationin%),waistandhip
Leeetal. 10.3389/fcvm.2022.993633
Frontiersin CardiovascularMedicine 08 frontiersin.org 77
circumferences,bodymassindex,andbloodpressurewere positivelycorrelatedwithleftatrialdiameter.Afteradjusting forobesityandbloodpressure,2-hpostprandialVLDL-χ,but notfastingVLDL,wasindependentlycorrelatedwithleftatrial diameter.Each1%increaseinVLDL-χ correlatedwithan incrementalleftatrialdiameterincreaseof0.23cm.Nakajima etal.(72)showedthatpostprandialVLDLhasahigheraffinity totheVLDLreceptor,withbetterinternalizationintocells thannon-postprandialVLDL.Withthesefindingsinmind, postprandialmodifiedVLDLhasbeensuggestedasatherapeutic targetforatrialremodelinginpatientswithMetS(54).
VLDLcomposition,especiallyinthepostprandialstate,is influencedbymealsandeatinghabits.Guerreroetal.(73) describedtheeffectsofasucrose-enricheddietonelevatedlevels ofVLDL-cholesterolandTGs,insulinresistance,andhepatic steatosisinmaleWistarrats.Inaddition,Drornaetal.(74) reviewedtheavailableevidencefortheimpactofhigh-fructose intakeonhealth.Inhealthyindividuals,theconsumptionofup to1.5gfructose/kgbodyweightperdayfor4weeksresulted inincreasedplasmaTGconcentrations(74).Inadditionto elevatingTGlevels,highfructoseintakecaninducehepatic steatosis,insulinresistance,andhyperuricemia(74).Itisvery likelythathighfructoseintakecanalterVLDLparticleswith respecttosizeandTGrichness.Afterasinglehigh-fatmeal, postprandialchangesinTGsandVLDLcanbesignificantin menwithabdominalobesitycomparedwithnon-obesemen (75).However,nosuchdifferencewasobservedbetweenobese andnon-obesewomen(75),suggestingsex-baseddifferencesin postprandialVLDLsecretionduringthereproductivestage.
Therapeuticimplications
Nutritionalintervention
Inpatientswithexistingcardiometabolicrisks,8-week nutritionalinterventionwithahighpolyphenoldietcan significantlyreducethepostprandiallipidcontentoflarge VLDLafterahigh-fattestmeal(76).Anotherstudyshowed thattheconsumptionofadietcomposedoffruit,avocado, wholegrains,andtroutfor8weekscanreducefasting insulinandVLDLandlowerthepostprandialincreasein TGsandVLDL(77).Withrespecttotheintakeoffish, notabledifferenceswereseenintheNMRlipoproteinprofile ofthethreemainn-3fattyacidsubtypes:eicosapentaenoic acid(EPA),docosahexaenoicacid(DHA),anda-linolenicacid (ALA).OnlyahighintakeofEPAsignificantlyreducedVLDL particlesandVLDLTGs(78).Inaddition,thereduction ofapoCIIIexpressionisbelievedtobethemechanism underlyingtheTG-loweringeffectsofomega-3carboxylic acids,whichcontain50–60%EPAand15–25%DHA,as wellasotheractiveomega-3freefattyacids(79).Fasting perse isbeneficialforVLDLmodification.Inastudy of40relativelyhealthy,middle-agedindividuals,long-term fastingimprovedthepostprandiallipidprofile,especially withrespecttotheconcentrationsoflargeVLDLparticles, whicharesignificantlydecreasedafter7and14days offasting(80).Nevertheless,theimpactofnutritional interventiononclinicalcardiovascularoutcomeswarrantslongtermobservationandfollow-up.
Size-andcharge-definedsubfractionsofVLDLandtheirassociationwithcardiometabolicdiseases.Thesize-definedclassificationofVLDL accordingtoparticlediameterisperformedusingnuclearmagneticresonance(NMR)spectrometry.LargeVLDL,whichhasadiameterlarger than60nm,isassociatedwithinsulinresistance,type2diabetesmellitus,andcoronaryarterycalcification.VLDL-χ orV5,themost negatively-chargedsubfractionofVLDL,isisolatedandmeasuredusinganion-exchangechromatography.VLDL-χ orV5causesdirectdamage totheendotheliumandassociatedwithcoronaryheartdiseaseriskandatrialmyopathyinmetabolicsyndrome(MetS).

Leeetal. 10.3389/fcvm.2022.993633
FIGURE2
Frontiersin CardiovascularMedicine 09 frontiersin.org 78
Potentialofothervery-low-density lipoprotein-targetedtherapies
Inadditiontonutritionalintervention,synbioticand probioticsupplementsthatimprovegutmicrobiomeimbalance haveshownpotentialfordecreasingserumVLDL-Clevels (81).Inaddition,severaloralanti-diabeticdrugshavebeen identifiedthatpromotebeneficialeffectsonVLDLmetabolism.
Pioglitazone,aPPAR-γ activator,wasshowntofacilitateLPL activityandpromotetheclearanceofVLDL(82).Furthermore, glucagon-likepeptide1(GLP-1)agonistreducedTGlevelsin theliverandtheVLDLsecretionrate(83).
Commonlyusedlipid-loweringdrugs,althoughnot specificallyVLDL-targeted,havealsobeenshowntohelp reduceVLDL.HMG-CoAreductaseinhibitors(i.e.,statins) reduceone-thirdofVLDL-TGsandmorethan40%of apoCIIIlevels(84).Inaddition,peroxisomeproliferatoractivatedreceptor-α (PPAR-α)agonists(i.e.,fibrates),whichare prescribedprimarilyformanaginghypertriglyceridemia,reduce VLDL-apoCIIIlevels,aswell(84).Similartoselectiveestrogen receptormodulators,thefirstselectivePPAR-α modulator (SPPARMα)LY-518674,whichtargetsthereceptor–cofactor bindingprofileofthePPARα ligand,modulatestissue-and gene-selectiveresponses.InclinicalphaseII/IIItrials,this SPPARMα agonistreducedTGandapoCIIIlevelsbyabout50% (85).Proproteinconvertasesubtilisin-kexintype9(PCSK9) inhibitors,whichreducethedegradationofLDLreceptors andpromoteLDLuptakeintheliver,alsoupregulateVLDL receptorsandreduceVLDLlevels.PCSK9inhibitorshavealso beenshowntopreferentiallymodifythesizeandapolipoprotein compositionofVLDLparticles(86).
Severallipid-loweringagentsareunderdevelopment, includingCETPinhibitor(87),microsomaltriglyceridetransfer protein(MTTP)inhibitor(88),andantisenseoligonucleotides targetingthegenesencodingapoB100(88)andapoCIII(89). Thesetherapeuticsarecurrentlybeingtestedinclinicaltrials. MonoclonalantibodytargetingANGPTL3hasbeenshownto robustlyreduceVLDLlevelsbutattheexpenseofelevating LDLlevels(90).Inaddition,ARO-ANG3isansiRNA-based medicationthatinhibitsthehepatictranslationof ANGPTL3 mRNA[102].Thesenewmedicationshavethepotentialto producefavorableeffectsonVLDLstructureandmetabolism.
Concludingremarks
IndependentofLDL-C,VLDL’satherogenicproperties areassociatedwithTGabundance,whichlargelyaffects particlesize,apolipoproteincontentalteration,electricalcharge, andlipidcomposition,especiallyinthepostprandialstate (Figure2).Withadversemodification,VLDLfacilitates
ectopiclipidaccumulation,whichhasbeenobservedin theliver,heart,andskeletalmuscles.Toelucidatethe pathogenicrolesofVLDLincardiovasculardiseases,the issuesofmodification,inbothfastingandpostprandialstates, shouldbetakenintoconsideration.Toimproveadversely modifiedVLDL,nutritionalintervention,especiallythrough thereductionoffructosecontentinfood,shouldbewidely recommended,especiallyforpatientswithinsulinresistance andcardiometabolicrisks.However,interpretingdatafrom onlythesize-based,charge-based,orapolipoprotein-based classifiedVLDLdoesnotprovidecompleteknowledgeor informationaboutlipidsinhealthanddiseases.Toobtaina morecomprehensiveunderstandingofthelipidtransportand metabolismprocess,methodologiesareneededthatcanreflect thecompleximmunochemicalandfunctionalpropertiesofall apolipoprotein-containinglipoproteinsintheblood.
Authorcontributions
H-CLcontributedtotheconceptualizationofthestudy, participatedinfundingacquisition,andwrotethemanuscript. AAandC-HCreviewedandeditedthemanuscript.Allauthors haveapprovedthesubmittedversionandagreedtobepersonally accountablefortheirowncontributions.
Funding
ThisstudywassupportedbyKaohsiungMedicalUniversity Hospital(KMUH109-9R11andKMUH110-0R11),Taiwan MinistryofScienceandTechnologyGrants(MOST1092314-B-037-111-MY3),KMUGlobalNetworkingTalentPlan (110KMUOR01),andNationalHealthResearchInstitutes NHRI(NHRI-EX107-10724SC,NHRI-EX108-10724SC,NHRIEX109-10724SC,andNHRI-EX110-10724SC).
Acknowledgments
WethankMs.Yi-LinShiaoforherartworkshowninthe schematicfigure,andNicoleStancel,Ph.D.,ELS(D),ofScientific PublicationsattheTexasHeartInstitute,forhercontributions totheeditingofthismanuscript.
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Leeetal. 10.3389/fcvm.2022.993633
Frontiersin CardiovascularMedicine 10 frontiersin.org 79
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated
References
1.Niimi,M,YanH,ChenY,WangY,FanJ.Isolationandanalysisofplasma lipoproteinsbyultracentrifugation. JVisExp. (2021).doi:10.3791/61790
2.ChapmanMJ,GoldsteinS,LagrangeD,LaplaudPM.Adensitygradient ultracentrifugalprocedurefortheisolationofthemajorlipoproteinclassesfrom humanserum. JLipidRes. (1981)22:339–58.
3.HuangJK,LeeHC.Emergingevidenceofpathologicalrolesofvery-lowdensitylipoprotein(VLDL). IntJMolSci. (2022)23:4300.
4.TiwariS,SiddiqiSA.IntracellulartraffickingandsecretionofVLDL. Arterioscl ThrombVascBiol. (2012)32:1079–86.
5.GinsbergHN,PackardCJ,ChapmanMJ,BorenJ,Aguilar-SalinasCA,Averna M,etal.Triglyceride-richlipoproteinsandtheirremnants:metabolicinsights, roleinatheroscleroticcardiovasculardisease,andemergingtherapeuticstrategiesaconsensusstatementfromtheEuropeanAtherosclerosisSociety. EurHeartJ. (2021)42:4791–806.doi:10.1093/eurheartj/ehab551
6.MagnificoMC,OberkerschRE,MolloA,GiambelliL,GrootenY,SartiP,etal. VLDLinducedmodulationofnitricoxidesignallingandcellredoxhomeostasisin HUVEC. OxidMedCellLongev. (2017)2017:2697364.doi:10.1155/2017/2697364
7.TsaiYY,RaineyWE,BollagWB.Verylow-densitylipoprotein(VLDL)inducedsignalsmediatingaldosteroneproduction. JEndocrinol. (2017)232:R115–
29.
8.JimenezB,HolmesE,HeudeC,TolsonRF,HarveyN,LodgeSL,etal. Quantitativelipoproteinsubclassandlowmolecularweightmetaboliteanalysisin humanserumandplasmaby(1)HNMRspectroscopyinamultilaboratorytrial. AnalChem. (2018)90:11962–71.doi:10.1021/acs.analchem.8b02412
9.GarveyWT,KwonS,ZhengD,ShaughnessyS,WallaceP,HuttoA,etal. Effectsofinsulinresistanceandtype2diabetesonlipoproteinsubclassparticle sizeandconcentrationdeterminedbynuclearmagneticresonance. Diabetes. (2003) 52:453–62.
10.PhillipsCM,PerryIJ.Lipoproteinparticlesubclassprofilesamong metabolicallyhealthyandunhealthyobeseandnon-obeseadults:doessizematter? Atherosclerosis. (2015)242:399–406.doi:10.1016/j.atherosclerosis.2015.07.040
11.WangJ,StancakovaA,SoininenP,KangasAJ,PaananenJ,KuusistoJ, etal.Lipoproteinsubclassprofilesinindividualswithvaryingdegreesofglucose tolerance:apopulation-basedstudyof9399Finnishmen. JInternMed. (2012) 272:562–72.doi:10.1111/j.1365-2796.2012.02562.x
12.AvogaroP,BonGB,CazzolatoG.Presenceofamodifiedlowdensity lipoproteininhumans. Arteriosclerosis. (1988)8:79–87.
13.YangCY,RayaJL,ChenHH,ChenCH,AbeY,PownallHJ,etal.Isolation, characterization,andfunctionalassessmentofoxidativelymodifiedsubfractions ofcirculatinglow-densitylipoproteins. ArteriosclerThrombVascBiol. (2003) 23:1083–90.doi:10.1161/01.ATV.0000071350.78872.C4
14.ChenCH,JiangT,YangJH,JiangW,LuJ,MaratheGK,etal.Low-density lipoproteininhypercholesterolemichumanplasmainducesvascularendothelial cellapoptosisbyinhibitingfibroblastgrowthfactor2transcription. Circulation. (2003)107:2102–8.doi:10.1161/01.CIR.0000065220.70220.F7
15.ChenCH,LuJ,ChenSH,HuangRY,YilmazHR,DongJ,etal.Effectsof electronegativeVLDLonendotheliumdamageinmetabolicsyndrome. Diabetes Care. (2012)35:648–53.doi:10.2337/dc11-1623
16.AlaupovicP.Significanceofapolipoproteinsforstructure,function,and classificationofplasmalipoproteins. MethodsEnzymol. (1996)263:32–60.
17.JohannesenCDL,MortensenMB,LangstedA,NordestgaardBG. ApolipoproteinBandNon-HDLcholesterolbetterreflectresidualriskthan LDLcholesterolinstatin-treatedpatients. JAmCollCardiol. (2021)77:1439–50.
18.PrennerSB,MulveyCK,FergusonJF,RickelsMR,BhattAB,ReillyMP.Very lowdensitylipoproteincholesterolassociateswithcoronaryarterycalcificationin type2diabetesbeyondcirculatinglevelsoftriglycerides. Atherosclerosis. (2014) 236:244–50.doi:10.1016/j.atherosclerosis.2014.07.008
organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
19.RenJ,GrundySM,LiuJ,WangW,WangM,SunJ,etal.Long-termcoronary heartdiseaseriskassociatedwithvery-low-densitylipoproteincholesterolin Chinese:theresultsofa15-YearChineseMulti-ProvincialCohortStudy(CMCS). Atherosclerosis. (2010)211:327–32.doi:10.1016/j.atherosclerosis.2010.02.020
20.LiuJ,SemposCT,DonahueRP,DornJ,TrevisanM,GrundySM.Nonhigh-densitylipoproteinandvery-low-densitylipoproteincholesterolandtheirrisk predictivevaluesincoronaryheartdisease. AmJCardiol. (2006)98:1363–8.
21.QinZ,ZhengFW,ZengC,ZhouK,GengY,WangJL,etal.Elevatedlevels ofverylow-densitylipoproteincholesterolindependentlyassociatedwithin-stent restenosisindiabeticpatientsafterdrug-elutingstentimplantation. ChinMedJ. (2017)130:2326–32.doi:10.4103/0366-6999.213575
22.IannuzziA,GiallauriaF,GentileM,RubbaP,CovettiG,BrescianiA, etal.AssociationbetweenNon-HDL-C/HDL-Cratioandcarotidintima-media thicknessinpost-menopausalwomen. JClinMed. (2021)11:78.doi:10.3390/ jcm11010078
23.PetersenM,DyrbyM,ToubroS,EngelsenSB,NorgaardL,PedersenHT,etal. Quantificationoflipoproteinsubclassesbyprotonnuclearmagneticresonancebasedpartialleast-squaresregressionmodels. ClinChem. (2005)51:1457–61.doi: 10.1373/clinchem.2004.046748
24.NakajimaK,NakanoT,TokitaY,NagamineT,InazuA,KobayashiJ,etal. Postprandiallipoproteinmetabolism:VLDLvschylomicrons. ClinChimActa. (2011)412:1306–18.
25.ZebI,JorgensenNW,BlumenthalRS,BurkeGL,Lloyd-JonesD,BlahaMJ, etal.Associationofinflammatorymarkersandlipoproteinparticlesubclasseswith progressionofcoronaryarterycalcium:themulti-ethnicstudyofatherosclerosis. Atherosclerosis. (2021)339:27–34.doi:10.1016/j.atherosclerosis.2021.11.003
26.MackeyRH,KullerLH,Sutton-TyrrellK,EvansRW,HolubkovR,Matthews KA.Lipoproteinsubclassesandcoronaryarterycalciuminpostmenopausalwomen fromthehealthywomenstudy. AmJCardiol. (2002)90:71i–6i.doi:10.1016/s00029149(02)02636-x
27.MoraS,OtvosJD,RosensonRS,PradhanA,BuringJE,RidkerPM. Lipoproteinparticlesizeandconcentrationbynuclearmagneticresonanceand incidenttype2diabetesinwomen. Diabetes. (2010)59:1153–60.
28.AmorAJ,VinagreI,ValverdeM,UrquizuX,MelerE,LopezE,etal.Nuclear magneticresonancelipoproteinsareassociatedwithcarotidatherosclerosisintype 1diabetesandpre-eclampsia. DiabetesMetabResRev. (2021)37:e3362.doi:10. 1002/dmrr.3362
29.LuJ,YangJH,BurnsAR,ChenHH,TangD,WalterscheidJP,etal. Mediationofelectronegativelow-densitylipoproteinsignalingbyLOX-1:a possiblemechanismofendothelialapoptosis. CircRes. (2009)104:619–27.
30.LinYS,LiuCK,LeeHC,ChouMC,KeLY,ChenCH,etal. Electronegativevery-low-densitylipoproteininducesbraininflammationand cognitivedysfunctioninmice. SciRep. (2021)11:6013.doi:10.1038/s41598-02185502-0
31.ShenMY,HsuJF,ChenFY,LuJ,ChangCM,MadjidM,etal.Combined LDLandVLDLelectronegativitycorrelateswithcoronaryheartdiseaseriskin asymptomaticindividuals. JClinMed. (2019)8:1193.doi:10.3390/jcm8081193
32.MahleyRW,RallSCJr.ApolipoproteinE:farmorethanalipidtransport protein. AnnuRevGenomicsHumGenet. (2000)1:507–37.
33.MendivilCO,RimmEB,FurtadoJ,SacksFM.ApolipoproteinEinVLDL andLDLwithapolipoproteinC-IIIisassociatedwithalowerriskofcoronaryheart disease. JAmHeartAssoc. (2013)2:e000130.doi:10.1161/JAHA.113.000130
34.FuiorEV,GafencuAV.ApolipoproteinC1:itspleiotropiceffectsinlipid metabolismandbeyond. IntJMolSci. (2019)20:53.doi:10.3390/ijms20235939
35.HamstenA,SilveiraA,BoquistS,TangR,BondMG,deFaireU,etal. TheapolipoproteinCIcontentoftriglyceride-richlipoproteinsindependently predictsearlyatherosclerosisinhealthymiddle-agedmen. JAmCollCardiol. (2005) 45:1013–7.doi:10.1016/j.jacc.2004.12.049
Leeetal. 10.3389/fcvm.2022.993633
Frontiersin CardiovascularMedicine 11 frontiersin.org 80
36.HansenJB,FernandezJA,NotoAT,DeguchiH,BjorkegrenJ,Mathiesen EB.TheapolipoproteinC-Icontentofvery-low-densitylipoproteinsisassociated withfastingtriglycerides,postprandiallipemia,andcarotidatherosclerosis. JLipids. (2011)2011:271062.doi:10.1155/2011/271062
37.NotoAT,MathiesenEB,BroxJ,BjorkegrenJ,HansenJB.TheApoC-I contentofVLDLparticlesisassociatedwithplaquesizeinpersonswithcarotid atherosclerosis. Lipids. (2008)43:673–9.doi:10.1007/s11745-008-3193-2
38.GautierT,DeckertV,AiresV,LeGuernN,ProukhnitzkyL,Patoli D,etal.HumanapolipoproteinC1transgenesisreducesatherogenesisin hypercholesterolemicrabbits. Atherosclerosis. (2021)320:10–8.doi:10.1016/j. atherosclerosis.2021.01.011
39.PechlanerR,TsimikasS,YinX,WilleitP,BaigF,SanterP,etal.Very-lowdensitylipoprotein–associatedapolipoproteinspredictcardiovasculareventsand areloweredbyinhibitionofAPOC-III. J.Am.Coll.Cardiol. (2017)69:789–800. doi:10.1016/j.jacc.2016.11.065
40.WulffAB,NordestgaardBG,Tybjaerg-HansenA.APOC3loss-offunctionmutations,remnantcholesterol,low-densitylipoproteincholesterol, andcardiovascularrisk:mediation-andmeta-analysesof137895individuals. ArteriosclThrombVascBiol. (2018)38:660–8.doi:10.1161/ATVBAHA.117.310473
41.JorgensenAB,Frikke-SchmidtR,NordestgaardBG,Tybjaerg-HansenA. Loss-of-functionmutationsinAPOC3andriskofischemicvasculardisease. NEngl JMed. (2014)371:32–41.
42.vanCapelleveenJC,BernelotMoensSJ,YangX,KasteleinJJP,WarehamNJ, ZwindermanAH,etal.ApolipoproteinCIIIlevelsandincidentcoronaryartery diseaserisk:theEPIC-Norfolkprospectivepopulationstudy. ArteriosclerThromb VascBiol. (2017):doi:<PMID<PMID:NOPMID</PMID<
43.KawakamiA,AikawaM,LibbyP,AlcaideP,LuscinskasFW,SacksFM. ApolipoproteinCIIIinapolipoproteinBlipoproteinsenhancestheadhesionof humanmonocyticcellstoendothelialcells. Circulation. (2006)113:691–700.doi: 10.1161/CIRCULATIONAHA.105.591743
44.GrahamMJ,LeeRG,BellTAIII,FuW,MullickAE,AlexanderVJ, etal.AntisenseoligonucleotideinhibitionofapolipoproteinC-IIIreducesplasma triglyceridesinrodents,nonhumanprimates,andhumans. CircRes. (2013) 112:1479–90.doi:10.1161/CIRCRESAHA.111.300367
45.DaiW,ZhangZ,YaoC,ZhaoS.Emergingevidencesfortheoppositeroleof apolipoproteinC3andapolipoproteinA5inlipidmetabolismandcoronaryartery disease. LipidsHealthDis. (2019)18:220.doi:10.1186/s12944-019-1166-5
46.FengQ,BakerSS,LiuW,ArbizuRA,AljomahG,KhatibM,etal. IncreasedapolipoproteinA5expressioninhumanandratnon-alcoholicfatty livers. Pathology. (2015)47:341–8.doi:10.1097/PAT.0000000000000251
47.ZhengXY,ZhaoSP,YanH.TheroleofapolipoproteinA5inobesityandthe metabolicsyndrome. BiolRevCambPhilosSoc. (2013)88:490–8.
48.GusarovaV,BrodskyJL,FisherEA.ApolipoproteinB100exitfromthe endoplasmicreticulum(ER)isCOPII-dependent,anditslipidationtoverylow densitylipoproteinoccurspost-ER. JBiolChem. (2003)278:48051–8.doi:10.1074/ jbc.M306898200
49.KotronenA,Seppanen-LaaksoT,WesterbackaJ,KiviluotoT,ArolaJ, RuskeepaaAL,etal.Hepaticstearoyl-CoAdesaturase(SCD)-1activityand diacylglycerolbutnotceramideconcentrationsareincreasedinthenonalcoholic humanfattyliver. Diabetes. (2009)58:203–8.
50.ChengD,XuX,SimonT,BoudyguinaE,DengZ,VerHagueM,etal.Very lowdensitylipoproteinassemblyisrequiredforcAMP-responsiveelement-binding proteinHprocessingandhepaticapolipoproteinA-IVexpression. JBiolChem. (2016)291:23793–803.doi:10.1074/jbc.M116.749283
51.BlackDD.Developmentandphysiologicalregulationofintestinallipid absorption.I.Developmentofintestinallipidabsorption:cellulareventsin chylomicronassemblyandsecretion. AmJPhysiolGastrointestLiverPhysiol. (2007) 293:G519–24.doi:10.1152/ajpgi.00189.2007
52.Abu-FarhaM,Al-KhairiI,CherianP,ChandyB,SriramanD,Alhubail A,etal.IncreasedANGPTL3,4andANGPTL8/betatrophinexpressionlevels inobesityandT2D. LipidsHealthDis. (2016)15:181.doi:10.1186/s12944-0160337-x
53.GrahamMJ,LeeRG,BrandtTA,TaiL-J,FuW,PeraltaR,etal.Cardiovascular andmetaboliceffectsofANGPTL3antisenseoligonucleotides. NEnglJMed. (2017) 377:222-32.
54.AdamRC,MintahIJ,Alexa-BraunCA,ShihanianLM,LeeJS,BanerjeeP,etal. Angiopoietin-likeprotein3governsLDL-cholesterollevelsthroughendothelial lipase-dependentVLDLclearance. JLipidRes. (2020)61:1271–86.doi:10.1194/jlr.
RA120000888
55.YanH,NiimiM,WangC,ChenY,ZhouH,MatsuhisaF,etal.Endothelial lipaseexertsitsanti-atherogeniceffectthroughincreasedcatabolismofbetaVLDLs. JAtherosclerThromb. (2021)28:157–68.doi:10.5551/jat.55244
56.HopkinsPN,WuLL,SchumacherMC,EmiM,HegeleRM,Hunt SC,etal.TypeIIIdyslipoproteinemiainpatientsheterozygousforfamilial hypercholesterolemiaandapolipoproteinE2.Evidenceforagene-geneinteraction. ArteriosclerThromb. (1991)11:1137–46.doi:10.1161/01.atv.11.5.1137
57.KontushA.HDLandreverseremnant-cholesteroltransport(RRT):relevance tocardiovasculardisease. TrendsMolMed. (2020)26:1086–100.
58.WieczorekE,CwiklinskaA,KuchtaA,Kortas-StempakB,GliwinskaA, JankowskiM.ThedifferentialeffectsofHDLsubpopulationsonlipoprotein lipase(LPL)-mediatedVLDLcatabolism. Biomedicines. (2021)9:1839.doi:10.3390/ biomedicines9121839
59.JayaramanS,BaveghemsC,ChavezOR,Rivas-UrbinaA,Sanchez-Quesada JL,GurskyO.Effectsoftriacylglycerolonthestructuralremodelingofhuman plasmaverylow-andlow-densitylipoproteins. BiochimBiophysActaMolCellBiol Lipids. (2019)1864:1061–71.
60.DavisJP,HuygheJR,LockeAE,JacksonAU,SimX,StringhamHM, etal.Common,low-frequency,andraregeneticvariantsassociatedwith lipoproteinsubclassesandtriglyceridemeasuresinFinnishmenfromthe METSIMstudy. PLoSGenet. (2017)13:e1007079.doi:10.1371/journal.pgen.1007 079
61.WangS,SongJ,YangY,ZhangY,WangH,MaJ.HIF3ADNAmethylation isassociatedwithchildhoodobesityandALT. PLoSOne. (2015)10:e0145944. doi:10.1371/journal.pone.0145944
62.PanH,LinX,WuY,ChenL,TehAL,SohSE,etal.HIF3Aassociation withadiposity:thestorybeginsbeforebirth. Epigenomics. (2015)7:937–50.doi: 10.2217/epi.15.45
63.Li-GaoR,HughesDA,vanKlinkenJB,deMutsertR,RosendaalFR,MookKanamoriDO,etal.Geneticstudiesofmetabolomicschangeafteraliquidmeal illuminatenovelpathwaysforglucoseandlipidmetabolism. Diabetes. (2021) 70:2932–46.doi:10.2337/db21-0397
64.VojinovicD,RadjabzadehD,KurilshikovA,AminN,WijmengaC,Franke L,etal.Relationshipbetweengutmicrobiotaandcirculatingmetabolitesin population-basedcohorts. NatCommun. (2019)10:5813.doi:10.1038/s41467-01913721-1
65.KashtanovaDA,KlimenkoNS,TkachevaON,StrazheskoID,Metelskaya VA,GomyranovaNV,etal.Subfractionalspectrumofserumlipoproteinsand gutmicrobiotacompositioninhealthyindividuals. Microorganisms. (2021)9:1461. doi:10.3390/microorganisms9071461
66.WitztumJL,SchonfeldG.Carbohydratediet-inducedchangesinvery lowdensitylipoproteincompositionandstructure. Diabetes. (1978)27: 1215–29.
67.ChahalJ,GuptaS,ChawlaSPS,GrewalH.Comparativestudyonfasting andpostprandiallipidprofileintype2diabetesmellitus. JFamilyMedPrimCare. (2021)10:1288–93.
68.BallingM,LangstedA,AfzalS,VarboA,DaveySmithG,NordestgaardBG. Athirdofnonfastingplasmacholesterolisinremnantlipoproteins:lipoprotein subclassprofilingin9293individuals. Atherosclerosis. (2019)286:97–104.
69.TakahashiS.Triglyceriderichlipoprotein-LPL-VLDLreceptorandLp(a)VLDLreceptorpathwaysformacrophagefoamcellformation. JAtheroscler Thromb. (2017)24:552–9.doi:10.5551/jat.RV17004
70.NiuYG,EvansRD.Very-low-densitylipoprotein:complexparticlesin cardiacenergymetabolism. JLipids. (2011)2011:189876.
71.LeeHC,ShinSJ,HuangJK,LinMY,LinYH,KeLY,etal.Theroleof postprandialvery-low-densitylipoproteininthedevelopmentofatrialremodeling inmetabolicsyndrome. LipidsHealthDis.(2020)19:210.doi:10.1186/s12944-02001386-5
72.NakajimaK,TokitaY,TanakaA,TakahashiS.TheVLDLreceptorplaysa keyroleinthemetabolismofpostprandialremnantlipoproteins. ClinChimActa. (2019)495:382–93.doi:10.1016/j.cca.2019.05.004
73.PlazasGuerreroCG,AcostaCotaSJ,CastroSanchezFH,VergaraJimenez MJ,RiosBurguenoER,SarmientoSanchezJI,etal.Evaluationofsucrose-enriched dietconsumptioninthedevelopmentofriskfactorsassociatedtotype2diabetes, atherosclerosisandnon-alcoholicfattyliverdiseaseinamurinemodel. IntJ EnvironHealthRes. (2021)31:651–69.doi:10.1080/09603123.2019.1680817
74.DornasWC,deLimaWG,PedrosaML,SilvaME.Healthimplicationsof high-fructoseintakeandcurrentresearch. AdvNutr. (2015)6:729–37.
75.SabakaP,KruzliakP,GasparL,CaprndaM,BendzalaM,BalazD,etal. Postprandialchangesoflipoproteinprofile:effectofabdominalobesity. Lipids HealthDis. (2013)12:179.
76.DellaPepaG,VetraniC,VitaleM,BozzettoL,CostabileG,CiprianoP,etal. Effectsofadietnaturallyrichinpolyphenolsonlipidcompositionofpostprandial lipoproteinsinhighcardiometabolicriskindividuals:anancillaryanalysisofa
Leeetal. 10.3389/fcvm.2022.993633
Frontiersin CardiovascularMedicine 12 frontiersin.org 81
randomizedcontrolledtrial. EurJClinNutr. (2020)74:183–92.doi:10.1038/ s41430-019-0459-0
77.Munoz-PerezDM,Gonzalez-CorreaCH,Astudillo-MunozEY,PorrasHurtadoGL,Sanchez-GiraldoM,Lopez-MirandaJ,etal.Alternativefoodsin cardio-healthydietarymodelsthatimprovepostprandiallipemiaandinsulinemia inobesepeople. Nutrients. (2021)13:2225.doi:10.3390/nu13072225
78.AmigoN,AkinkuolieAO,ChiuveSE,CorreigX,CookNR,MoraS.Habitual fishconsumption,n-3fattyacids,andnuclearmagneticresonancelipoprotein subfractionsinwomen. JAmHeartAssoc. (2020)9:e014963.doi:10.1161/JAHA. 119.014963
79.MortonAM,FurtadoJD,LeeJ,AmerineW,DavidsonMH,SacksFM.The effectofomega-3carboxylicacidsonapolipoproteinCIII-containinglipoproteins inseverehypertriglyceridemia. JClinLipidol. (2016)10:1442–51.e4.doi:10.1016/j. jacl.2016.09.005
80.GrundlerF,PlonneD,MesnageR,MullerD,SirtoriCR,Ruscica M,etal.Long-termfastingimproveslipoprotein-associatedatherogenicrisk inhumans. EurJNutr. (2021)60:4031–44.doi:10.1007/s00394-021-025 78-0
81.ShakeriH,HadaeghH,AbediF,Tajabadi-EbrahimiM,MazroiiN,Ghandi Y,etal.ConsumptionofsynbioticbreaddecreasestriacylglycerolandVLDLlevels whileincreasingHDLlevelsinserumfrompatientswithtype-2diabetes. Lipids. (2014)49:695–701.doi:10.1007/s11745-014-3901-z
82.RodriguezV,NewmanJD,SchwartzbardAZ.Towardsmorespecific treatmentfordiabeticdyslipidemia. CurrOpinLipidol. (2018)29:307.
83.PatelVJ,JoharapurkarAA,ShahGB,JainMR.EffectofGLP-1 basedtherapiesondiabeticdyslipidemia. CurrDiabetesRev. (2014)10: 238–50.
84.OoiEM,NgTW,WattsGF,ChanDC,BarrettPHR.Effectoffenofibrateand atorvastatinonVLDLapoEmetabolisminmenwiththemetabolicsyndrome. J LipidRes. (2012)53:2443–9.doi:10.1194/jlr.P029223
85.FruchartJC,SantosRD,Aguilar-SalinasC,AikawaM,AlRasadiK, AmarencoP,etal.Theselectiveperoxisomeproliferator-activatedreceptoralpha modulator(SPPARMalpha)paradigm:conceptualframeworkandtherapeutic potential:aconsensusstatementfromtheInternationalAtherosclerosisSociety (IAS)andtheResidualRiskReductionInitiative(R3i)Foundation. Cardiovasc Diabetol. (2019)18:71.doi:10.1186/s12933-019-0864-7
86.HollsteinT,VogtA,GrenkowitzT,StojakovicT,MärzW,LaufsU,etal. TreatmentwithPCSK9inhibitorsreducesatherogenicVLDLremnantsina real-worldstudy. VascPharmacol. (2019)116:8–15.doi:10.1016/j.vph.2019.0
3.002
87.GroupHTRC.Effectsofanacetrapibinpatientswithatheroscleroticvascular disease. NEnglJMed. (2017)377:1217–27.
88.ButtWZ,YeeJK.Theroleofnon-statinlipid-loweringmedicationsinyouth withhypercholesterolemia. CurrAtherosclerRep. (2022)24:379–89.doi:10.1007/ s11883-022-01013-x
89.ZhangJ,deAlbuquerqueRochaN,McCulloughPA.ContributionofApoCIII todiabeticdyslipidemiaandtreatmentwithvolanesorsen. RevCardiovascMed. (2018)19:13–9.
90.AhmadZ,PordyR,RaderDJ,GaudetD,AliS,Gonzaga-JaureguiC,etal. Inhibitionofangiopoietin-likeprotein3withevinacumabinsubjectswithhigh andseverehypertriglyceridemia. JAmCollCardiol. (2021)78:193–5.doi:10.1016/ j.jacc.2021.04.091
91.MarronMM,AllisonM,KanayaAM,LarsenB,WoodAC,Herrington D,etal.Associationsbetweenlipoproteinsubfractionsandareaanddensityof abdominalmuscleandintermuscularadiposetissue:themulti-ethnicstudyof atherosclerosis. FrontPhysiol. (2021)12:713048.doi:10.3389/fphys.2021.713048
92.SilbernagelG,ScharnaglH,KleberME,HoffmannMM,DelgadoG, StojakovicT,etal.CommonAPOC3variantsareassociatedwithcirculatingApoCIIIandVLDLcholesterolbutnotwithtotalapolipoproteinBandcoronaryartery disease. Atherosclerosis. (2020)311:84–90.doi:10.1016/j.atherosclerosis.2020.0 8.017
Leeetal. 10.3389/fcvm.2022.993633
Frontiersin CardiovascularMedicine 13 frontiersin.org 82
OPENACCESS
EDITEDBY
TatsuyaSawamura, ShinshuUniversity,Japan
REVIEWEDBY
MasatsuneOgura, EasternChibaMedicalCenter,Japan
FabriziaBonacina, UniversityofMilan,Italy
*CORRESPONDENCE
ElenaOsto elena.osto@uzh.ch
SPECIALTYSECTION
Thisarticlewassubmittedto AtherosclerosisandVascularMedicine, asectionofthejournal
FrontiersinCardiovascularMedicine
RECEIVED 08July2022
ACCEPTED 16September2022
PUBLISHED 06October2022
CITATION
DietrichE,JomardAandOstoE(2022)
Crosstalkbetweenhigh-density lipoproteinsandendothelialcellsin healthanddisease:Insightsinto sex-dependentmodulation. Front.Cardiovasc.Med. 9:989428. doi:10.3389/fcvm.2022.989428
COPYRIGHT
© 2022Dietrich,JomardandOsto. Thisisanopen-accessarticle distributedunderthetermsofthe CreativeCommonsAttributionLicense (CCBY).Theuse,distributionor reproductioninotherforumsis permitted,providedtheoriginal author(s)andthecopyrightowner(s) arecreditedandthattheoriginal publicationinthisjournaliscited,in accordancewithacceptedacademic practice.Nouse,distributionor reproductionispermittedwhichdoes notcomplywiththeseterms.
PUBLISHED 06October2022
DOI 10.3389/fcvm.2022.989428
Crosstalkbetweenhigh-density lipoproteinsandendothelial cellsinhealthanddisease:
Insightsintosex-dependent modulation
1 InstituteforClinicalChemistry,UniversityofZurichandUniversityHospitalZurich,Zurich, Switzerland, 2 DepartmentofCardiology,HeartCenter,UniversityHospitalZurich,Zurich,
Atheroscleroticcardiovasculardiseaseistheleadingcauseofdeathworldwide. Intenseresearchinvascularbiologyhasadvancedourknowledgeofmolecular mechanismsofitsonsetandprogressionuntilcomplications;however, severalaspectsofthepatho-physiologyofatherosclerosisremaintobe furtherelucidated.Endothelialcellhomeostasisisfundamentaltoprevent atherosclerosisastheappearanceofendothelialcelldysfunctionisconsidered thefirstpro-atheroscleroticvascularmodification.Physiologically,highdensity lipoproteins(HDLs)exertprotectiveactionsforvesselsandinparticularfor ECs.Indeed,HDLspromoteendothelial-dependentvasorelaxation,contribute totheregulationofvascularlipidmetabolism,andhaveimmune-modulatory, anti-inflammatoryandanti-oxidativeproperties.Sex-andgender-dependent di erencesareincreasinglyrecognizedasimportant,althoughnotfully elucidated,factorsincardiovascularhealthanddiseasepatho-physiology.In thisreview,wehighlighttheimportanceofsexhormonesandsex-specific geneexpressionintheregulationofHDLandECcross-talkandtheir contributiontocardiovasculardisease.
KEYWORDS
HDL,endothelialcells,sexdi erences,cardiovasculardisease,HDL-endothelial crosstalk
Introduction
Therelationshipbetweenhigh-densitylipoproteins(HDLs)andcardiovascular disease(CVD)is atopicofintenseinvestigationsincedecades(1).
EpidemiologicalstudieshaveshownacorrelationbetweenlowlevelsofHDLcholesterol(HDL-C)andincreasedincidenceofCVD(2).Indeed,aU-shapecorrelation hasbeenrecently reportedwherebybothlow(<50mg/dlinwomenand <40mg/dlin men;0.8and1.3mmol/L,respectively)andhigh(>80to90mg/dl; >2.3mmol/L)levels
TYPE Review
ElisaDietrich 1,AnneJomard 1 andElenaOsto 1,2
*
Switzerland
Frontiersin CardiovascularMedicine 01 frontiersin.org 83
ofHDLhave beenassociatedtoincreasedall-causeandCV mortalityinboth menandwomenwithoutpreviousCVD(3–5).
Increasingevidence suggeststhatratherthancholesterol levelspresentonHDL,HDLparticlenumber,lipid andproteincompositionplayakeyprotectiverolein reducingCVDrisk(6–8).HDLparticlecomposition directlyinfluencesHDL vaso-protectivefunctions(i.e. reversecholesteroltransport(RCT),nitricoxide(NO) productionfromendothelialcells(ECs),anti-oxidativeand anti-inflammatoryproperties).
ECsareaphysicalbarrierbetweenbloodandbodytissues, whichactasgatekeepersofcardiovascularhomeostasis. Indeed,EC-releasedvasoactivesubstances(inparticular NO)regulatehemostasis,controlvascularpermeability andmodulatebothacuteandchronicimmuneresponses toinjuries(9).Inlightofitsstrongvasodilatory,antiinflammatoryandanti-oxidativeproperties,NOplays acentralroleinthemaintenanceofvascularhealth (10).ReductioninNObioavailabilityisthehallmarkof endothelialcelldysfunction (ECD),whichinturnfavors atherosclerosis(11).
Sex-relatedinter-individualvariability(hormonallevels, hormonetherapies,geneexpressionprofilesetc.)caninfluence CVDriskbyactingonbothHDLsandECs(12–14).
Increasingevidence suggeststhatsexualhormone levels—inparticulartestosteroneandestradiol—andsexspecificcellulargeneexpressionprofilecaninfluence notonlyHDL-ClevelsbutalsoHDLsubclassesand function.Indeed,mendisplayreducedlevelsofHDLCandamorepro-atherogenicphenotypecomparedto women(15–17).
Furthermore,estrogens arewell-recognizedECprotective molecules,abletostimulateNOproduction,ECgrowthand woundhealingmechanisms(18, 19).Ofnote,differencesin geneexpression profilebetweenfemaleandmaleECsappear toinfluenceECsusceptibilitytoinsults,withtheactivation infemaleECsofmoreefficientstress-responsemechanisms comparedtomaleECs(20, 21).Thesedifferencescouldexplain, atleastin part,whypre-menopausalwomenhavelesserCVD riskthanage-matchedmenandcouldgiveusefulhintsfor personalizedtherapydevelopment.
InthisReview,wemainlyfocusedontheinfluenceofsexspecificfactorsonbothHDLandECfunctionandhowsexdependentdifferencesmodulatingHDL-ECcross-talkmay contributetotheCVprotectionofpre-menopausalwomen comparedtoage-matchedmen(22–24).Indeed,sexclosely interactswithgender inthedevelopmentofatherosclerosis therefore,althoughnotsystematicallyaddressed,somegenderspecificaspects(i.e.,pertainingtothesocio-economicand culturalsphere)havebeenalsomentionedincaseoftheir knowninfluenceonHDLandECfunctionandpotentialCV patho-physiologicalimpact(23–26).
HDL-targetingdrugs:Thefailureof cholesterylestertransferprotein inhibitors
TheconceptthatHDListhe“goodcholesterol”first originatedfromtheFraminghamHeartStudy,whichshowed stronginverseassociationbetweenHDL-Candcoronaryheart disease(CHD)(27).However,thisconcepthasbeenchallenged byresultsoffollowing clinicaltrialsinwhichcholesterylester transferprotein(CETP)inhibitors,despiteraisingHDL-C levels,failedtoreduceCVmorbidityandmortality.These resultssuggestedthatbeyondthesimpleincreaseofHDLCplasmalevels,themodulationofHDLcompositioncould bemoreimportanttoachievecardiovascularbenefits(28–30). CETPisaplasmaproteinthattransferscholesterylesterfrom HDLtoapolipoproteinB(ApoB)-containinglipoproteinsin exchangefortriglyceride(TG).TheinhibitionofCETPleads tohighercholesterollevelsinHDLs.Indeed,specieslacking CETPandpatientswithCETPdeficiencyarecharacterizedby increasedHDL-ClevelsandreducedriskforCVD(31–34).In theInvestigationof LipidLevelManagementtoUnderstand itsImpactinAtheroscleroticEvents(ILLUMINATE)trial,the CETPinhibitorTorcetrapibincreasedHDL-Clevelsasexpected, butthisincreasewasnotparalleledbydecreasedCHDand thetrialwasstoppedduetoelevatedriskofcardiacanddeath events(35).
InlinewiththenotionthatHDL-Calonemaynotbe areliablemarkerofthecardio-protectivequalityofHDLs, ithasbeenrecentlyshowninasex-mixedpoolofpatients thatCETPinhibitors,TorcetrapibandEvacetrapib,notonly increasedHDL-Cbutalsoenhancedtheconcomitantcontent ofapoC3/apoEinHDLs.ThesetwoproteinsrenderedHDLs dysfunctionalandwereassociatedwithhigherCHD(36). DifferentCETPinhibitors, suchasDalcetrapibandAnacetrapib slightlyreducedCHDrisk,althoughthiseffectcouldhavebeen influencedbytheconcomitantreductioninnon-HDL-Cin treatedpatients(37–42).
Geneticpolymorphismsassociated withincreasedHDLClevelsalsodidnotinfluencetheriskscoreformyocardial infarction(1, 43).PopulationstudiescarriedoutinCopenhagen highlightedadramaticenhancementofCHDriskinwomen withCETPdeficiency,inspiteoftheelevatedHDL-Clevels (44).Furthermore,asresultofraregeneticvariantsonscavenger receptorBI(SR-BI) geneandreducedabilityofHDLstodeliver cholesteroltotheliver,theconsequentincreasedHDL-Clevels werelinkedtohigherratherthanlowerriskofCHDriskinboth menandwomen(45, 46).Indeed,theincreasedcholesterolin HDLinthese specificcircumstanceswaslinkedtoanimpaired HDL-mediatedRCT.Takentogether;thisevidencequestioned therationaleofusingCETPinhibitorsastreatmentforCVD andhighlightedtheneedforabettercharacterizationofthe
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 02 frontiersin.org 84
complexityofHDL,in particularfocusingonHDLcomposition askeydeterminantoffunction inhealthanddisease.
Sex-andage-relateddi erencesin HDLmeasurements
HDL-Cis commonlyusedasapredictormarkerfor CVDrisk,asreported inSCORE(SystematicCoronary RiskEvaluation)riskchartsandtheASCVDPooledCohort Equations(47, 48).Sofar,referencevaluesforlipidprofiles, includingHDL,usedinclinicalpracticearethesameforboth menandwomen,despitegrowingevidenceontheinfluence ofsexdifferencesinthediscriminativeperformanceofCVD riskscores.Indeed,pre-menopausalwomenhavehigherHDLClevelsandlowerriskofCVDcomparedtomen(Figure1) (12, 49–51).Phasesofmenstrualcyclemayinfluencelipid profile.Whilepost-prandialserumTG werehigherinwomen duringthefollicularcomparedtothelutealphaseofmenstrual cycle,HDLandApoBlevelswerestableinbothphases(54).In anotherstudy,asignificantdecreaseinthemeanlevelsofTC, LDL-C,TC/HDL-C,LDL/HDLandTG/HDLwasobservedin thelutealcomparedtothefollicularphaseofmenstrualcycle (55).Somestudiessuggestedassessingfemaleparametersduring thefollicularphase ofmenstrualcyclecouldhelptominimize differencesduetosexualhormonesfluctuations(55, 56).
Patientageisanotherimportantfactorinfluencingsexdependentdifferences,sinceHDL-Clevelscanvaryduring individuallifetime.Healthypre-pubertalchildrenhadhigh levelsofHDL-Cindependentlyfromtheirsex(56).HDL-C levelsthendrasticallydecreasedinboysafterpuberty(∼45 mg/dL/1.16mmol/L),whileremaininghigheringirls(∼55 mg/dL/1.42mmol/L)(57).Thesedifferenceswerelostinpostmenopausalwomen,independentlyfrom menopausalage(58, 59).
Hormonaltherapiescanalsoalterlipidparameters. Transgendermen(i.e.,femaletomale)displayedaclear reductioninHDLcomparedtowomen,buthigherlevelsthan cis-gendermen(25, 60).
Thesesex-and age-dependentdifferencesneedtobetaken intoaccountwhenHDL-ClevelsareusedasaCVDprognostic marker.Moreover,whenconsideringtherelationshipbetween highHDL-ClevelsandincreasedallcauseandCVmortality, relevantfactorstobeevaluatedaresexdifferencestogether withthepresenceofCVDandothercomorbidities.Infact, theincreasedcardiovascularriskassociatedwithhighHDLCinitiallyreportedinasex-mixedpoolofpatientswithout previouscardiovascularconditionsbytheCANHEARTStudy andothers(3, 5)hasnotbeenconfirmedafterwardsinwomen withhypertension(61).Anotherstudyanalyzedsixcommunitybasedcohortsandshowedthatinmentheinverselinear associationbetweenHDL-CandCHDeventshasabroader spancomparedtowomen.ForHDL-Cvalues >90mg/dL
(>2.33mmol/L)inmenandHDL-Cvalues >75mg/dL(>1.94 mmol/L)inwomen,theassociationbetweenHDL-CandCHD eventsreachedaplateauwithnofurtherreductionsinCHD risk(62).
All-causemortalityin healthy,smoking,middle-aged(50–59years)andolder(>60years)Finnishmenwaspositively associatedwithHDL-Cinthemiddle-agedgroup,whilethere wasaU-shapedpatterninoldermen.Ofnote,themiddleagedgrouphadahigherreportedalcoholintakethantheolder individuals.Moreover,alcohol-andviolence-relatedmortality wasstronglypositivelyassociatedwithHDL-Cspecificallyinthe middleagegroup(63).Thus,alcoholmayhaveinfluencedthe associationofHDL-C andmortalitythroughitsHDLraising effectandbeingariskfactorforbehavioral-relatednon-natural aswellasalcohol-relateddeathsbeyondcoronarydisease,such ascancer,cardiomyopathy,stroke(5, 63).
Insightsintosex-dependentand independentdi erencesinHDL structureandcomposition
HDLsareheterogeneouslipoproteinsformedbya cholesterolesterand TGenrichedhydrophobiccoreand asurfacelipidbilayercontainingmainlyfreecholesterol, phospholipidsandvariousproteins(6, 64).Thebiogenesisof HDLsstartsfromthesynthesisandsecretionofapolipoproteinAI(ApoA-I)intheliverandintestine(65).Theinteraction betweensecretedApoA-I andcellmembraneproteinATPbindingcassettetransporterA1(ABCA1),expressedby hepatocytesandenterocytes(66),allowstheacquisitionof lipidsandformationofnascentHDLs.NascentHDLsare convertedintomatureparticles via cholesterolesterification performedbylecithin-cholesterol-acyltransferase(LCAT)(67).
Endotheliallipaseand hepaticlipaseareinvolvedinthelipolysis ofphospholipidsandTGsinHDLs,leadingtosmallerHDL particles(68).Phospholipidtransferproteinfurtherexchanges lipidsbetweenHDLs(69). HDLclearanceisorchestratedby SR-BIandCETP,whichregulatethetransferofcholesteryl-ester fromHDLstotheliverandtheexchangeofApoB-containing lipoproteinswithTGs(70).
WomenhaveincreasedHDL-CandApoA-Ilevelsandlower ApoBcomparedtomen.Thesesex-relateddifferencesinplasma lipoproteinsstarttobeevidentduringpuberty,inconcomitance withtheincreaseintestosteroneinmalesandestrogensin females(Figure1)(52).
EstrogensincreasedApoA-Iexpressionintheliverand HDL-Clevelsinpre-menopausalwomenbymodulatingthe expressionofSR-BIandhepaticlipase(71–73).Onthecontrary, testosteroneadministrationenhanced hepaticlipaseactivity, increasingHDLcatabolism(Figure1)(52).Androgentherapy wasalsoassociatedwithanunfavorableshifttowardan atherogeniclipidprofilecharacterizedbyreducedApoA-Iand
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 03 frontiersin.org 85
towomen,whichinturnhavehigherSR-BIexpressionlevels.Thesedi erencescorrelatewithestrogensandtestosteronelevelsandresultina reducedriskforCVDandCHDinwomencomparedtomen(53).Sex-independentfactorsassociatedwithincreasedriskforCVDandchanges inlipidprofilehavealsobeenreportedinthisfigure (bottom)
increasedapo-Blevelsinmen(74).Suppressionofandrogens inmen,infact,leadedtoanincreaseinHDL-C,ApoA-I andreducedApoBlevels(75).Ithasalsobeenshownthat hyperandrogenism,whichisa commonfeatureofpolycystic ovarysyndrome,wasassociatedwithlowerHDL-Clevelsand dyslipidemia(76, 77).

Differencesinlipidprofile havealsobeenassociatedwith sex-specificgeneexpressionprofile.TheKDM6Ageneencodes forahistone-demethylaseproteinhighlyexpressedinthefemale liveranditsexpressionlevelspositivelycorrelatedwithHDLC(78, 79).Inturn,KDM6Asilencinginhepatocytesleadto downregulationofgenes regulatingHDL-Clevels(13).
Singlenucleotidepolymorphisms(SNPs) ontheCETPgene havebeenassociatedwithhigherHDL-CandApoA-Ilevels(80, 81).TaqIBisthemostcommonSNPvariantoftheCETPgene andtheTaqIB genotypecanbeexpressedaseitherdominant B1B1homozygote,B1B2heterozygoteorrecessiveB2B2.In particular,B2B2carriershadhigherHDL-Cplasmalevelsand 20%lowerriskofCHDvs.theB1B1carriers(82).Ofnote,the increaseinHDL-ClevelsinCETP-TaqIB,B2B2carriersseemed tobeindependentfromsexualhormones(81)andwaslostin
obesityandtype2 diabetes(T2D).Indeed,otherCETPSNP variantsinbothsexeswerenotassociatedwithHDL-Clevelsnor withmetabolicsyndromeandobesity(83).A16%increasein HDL-ClevelshasbeenreportedinmenwithB2TaqIBvariant affectedbyT2DcomparedwiththosehomozygousfortheB1 allele(83).
ApoE,encodedbyAPOEgene,isthemajorligandfor clearanceofTG-richlipoproteinsandhasanti-atherogenic function(84, 85).APOE-e2polymorphismhasasex-specific effectonlipidprofile andhasbeenassociatedwithhighHDL-C levelsinwomanandincreasedTGlevelsinmen(86).Thereare nosex-differencesreportedfor ApoEisoform4inthecontext ofCVDrisk,whiletheApoE4alleleseemstoconferamemory advantageinmidlifemenandanincreasedriskofAlzheimerin women(87, 88).
HDL-associatedLCATincreasedmassconcentrationand higherLCATactivityhavebeencorrelatedwithCHDriskin womenbutnotinmen(89, 90).However,mechanismsof thesex-specificassociationofLCATandCVriskneedfurther investigationgiventheconflictingresultssofaravailable,for instanceinpatientswithsicklecellanemiaandproteinuria
Dietrichetal. 10.3389/fcvm.2022.989428
FIGURE1
Sex-specificdi erencesinlipoproteinsandtheire ectoncardiovascularriskfactors.Women (left) displayincreasedlevelsofHDL-Cand ApoA-IandreducedlevelsofTGandApoBcomparedtomen (right) (12,49–52).Menalsodisplayincreasedlevelsofhepaticlipasecompared
Frontiersin CardiovascularMedicine 04 frontiersin.org 86
wherealess pronouncedreductionofLCATactivityinwomen comparedtomenhasbeenconsideredprotectiveagainst acceleratedkidneydiseaseprogressioninthispatientpopulation (91).Moreover,LCATdeficiencyledtothedevelopmentof spontaneousatherosclerotic lesionssimilarlyinagedmaleand femalemice(92)andafemalespecificprotectionagainstdietinducedobesityandinsulin resistancehasbeendescribedin micewithcombinedLCATandLDLreceptordeficiency(93).
InflammationdecreasesHDL-ClevelsandalteredHDL compositioninasex-independentmanner(Figure1)(94, 95).
ChangesintheHDL-associatedlipidsincludeadecreasein cholesterolesterandanincreaseinfreecholesterol,TG,free fattyacidsandceramide-enrichedlipoproteins.Dysfunctional HDLsshowmarkedalterationsinproteincompositionand becomepro-atherogenic.Thesechangesincludeanincreaseof serumamyloidA(SAA),adecreaseapoA-Ibutalsovariations inenzymesandtransferproteins,suchasLCAT,CETP,PON-1, andapolipoprotein-M(apoM)(94).
CentraladipositydirectlycorrelateswithCVDrisk(96, 97).IncreaseincentraladipositywasabletoalterHDL subclasses distribution,butoverallHDL-Clevelsseemednot affectedbythisparameter(98).ObesityalsoaffectsHDL composition,functionandsubclassesdistribution(99, 100).
Obesityinduces,most prominentlyinwomencomparedto men,apro-atherogenicdyslipidemiacharacterizedbyincreased LDLandTGandreducedHDL-C,ApoA-IandApoA-IIlevels. Weandothersshowedthatinmorbidlysevereobesepatients bariatricsurgeryrestoresHDLendothelial-protectiveproperties bymodulatingHDLcomposition(101–104).Bariatricsurgery improvesCVmorbidity andmortalityregardlessofsexand gender(105, 106).Indeed,inasmallpatientcohort,wealso showedafterRoux-en-YgastricbypasssimilarbenefitsonHDL endothelialprotectivefunctionforbothsexes(103).Circulating HDL-ClevelsincreasedinourpatientsafterRYGBinagreement withotherstudies(107)howeverconcentrationsusuallyremains wellbelowcutoffs (80–90mg/dL;2.06–2.33mmol/L)thatare associatedwithhigherCVDrisk(53, 108).Finally,itisworth toconsiderthat,inthecontextofobesityandbariatricsurgery, gender-dependentdifferences(e.g.,differencesbetweenwomen andmenintheperceptionoftheirbodyweightinrelationto esthetic,healthandtherapeuticperspective)areveryimportant andneedtobeappraisedwhenevaluatingstudyresultsand identifyinggapsofexistingknowledge(106).
Insightintosex-dependent regulationofECfunction
Womenbeforemenopausehavelowerriskofdeveloping CHDandendothelial-protective propertiesofestrogenscan beimportantcontributors(Figure1)(109).Aging-driven reductionofflow-mediateddilationappearsattheage ofmenopause,morethanadecadelaterthaninmen,in
concomitancewiththelossofcirculatingestrogens(110). Chronictreatmentwith estrogensimprovedendothelialdependentvasodilationinbothovariectomizedanimal modelsandpost-menopausalwomen(111–113).Moreover, clinicalstudiessuggestedthatestradioltreatmentwasableto revertendothelialdysfunctioninpost-menopausalwomen withatherosclerotic,non-stenoticarteriesbypreventing acetylcholine-inducedcoronaryvasospasm(114).Atpresent however,controversiesstillexistonthecardio-protective effectofhormonalreplacementtherapyinpost-menopausal women(115).
Atthemolecular level,estrogenscanstimulateECs primarilythroughestrogenreceptors(ERα andErβ,GPER1) onECsurface.ActivationofERsincreaseseNOSactivity andNOproduction,thuspromotingEC-mediatedvasodilation (Figure2)(116, 123).ECglycocalyxprotectsECsfromsecond insultsaftertraumahemorrhagicshock(T/SH)(117).Recently, ithasbeenshownthatestrogenadministrationafterT/SH protectstheECglycocalyxfromdegradationbyregulatingtPA andPAI-1levels,makingECsmoreresistanttoadditional damages(124).Furthermore,estradiolsignalingattenuated endothelialinflammatoryresponse byreducingcytokineand chemokinerelease(suchasmonocyteschemoattractantprotein 1(MCP-1)andIL-8)aswellasECadhesionmoleculeexpression (125, 126).Prolongedexposuretoestradiolalsocreatedanew homeostaticstatusin whichimmunecellswerepotentiated andECswerelesssensibletopro-inflammatorystimuliand apoptosis(Figure2)(109).
WhiletheECprotectiveroleofestrogenshavebeenwell established,theeffectofandrogensonECsisstillunderdebate. MonocytesbindingtoaorticECsseemedtobehigherin malethanfemalerabbitswithhypercholesterolemia,suggesting acorrelationbetweenandrogenlevelsandECinflammation. However,thissex-dependentdifferencewasalsoevidentin non-hypercholesterolemicrabbits(127).Testosteronehasalso beenassociatedwithimpairedvascularfunctioninwomen (122, 128).Thiscouldbeduetothefactthat,although testosteroneproductionis10 timeshigherinmencompared towomen,womenmaybemoresensitivetothishormone (129).Indeed,severalstudiespointedoutanimportant sex-independentroleofandrogen receptorsinregulating ECviability,proliferation,andangiogenesis/repairlikely via upregulationoftheVEGF-A,cyclinA,andcyclinD1expression (Figure2)(121).Ofnote,abundanceoftestosteroneinmale micemayfavor itsconversionintoestradiolmediatedby aromataseP450,causinghyper-activationofERspromoting atherosclerosis(130, 131).
Anoverallmarkerofearlyatherosclerosisisthe transformationofmacrophagesintofoamcellsthrough intracellularlipidaccumulation.Treatmentwithtestosterone promotedfoamcellformationinmen(butnotinwomen)by increasinglipidloading,thuscontributingtothedevelopment ofatherosclerosis(Figure2)(132).
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 05 frontiersin.org 87
Sex-specificgeneexpressionpatternsandsexualhormonesinfluenceECresponsetostimuliandEC-HDLcrosstalk.ECsderivedfromfemale donors (left) displayreducedsensibilitytostressandinflammationthankstotheactivationofsex-specificpathwaysinvolvedinstressresponse. EstradiolisabletoreduceinflammationbyreducingMCP-1releaseandVCAM-1expression.Furthermore,estradiolboundtoHDLsisableto stronglyactivateSR-BIpathwayincreasingtheNOproduction(53,114,116,117).Onthecontrary,ECsderivedfrommaledonors (right) display astrongersusceptibilitytoinflammation,increasedlevelsofoxidativestressandautophagy(118–120).Testosteronealsocontributestocreatea pro-inflammatoryenvironmentbyfavoringthetransformationofmacrophagesintofoamcells(121).EstrogensstimulateNOproductionin bothsexesthroughtheactivationofERreceptors(114).Incontrast,androgenreceptorsareabletoincreasetheexpressionofgenesinvolvedin cellviability,proliferationandangiogenesis/repairbothinmenandwomenECs(122).
Interestingly,transgendermentreatedwithtestosterone for12weeksdisplayedincreasedleukocytes-endothelium interactions,expressionofadhesionmoleculesonEC surface,pro-inflammatorycytokinerelease,decreasedHDL-C levelsanddyslipidemia(23, 26).Furthermore,thelevelsof polymorphonucleateadhesion toECsintransgendermenwere similartodiabeticmenwithsilentmyocardialischemia,which highlighttheneedofaclosermonitoringofcardiovascular riskinthesepatients(26).Progesterone,instead,protected ECsaftercerebrovascularocclusioninmalerats(133)andhas beenassociatedwithincreasedNOproductioninwomen(134). However,administrationof syntheticprogesteroneanalogs, suchasmedroxyprogesterone,correlatedwithincreasedrisk ofcoronarydiseaseandstrokeinwomenunderhormonal replacementtherapy(115, 135).Indeed,ithasbeenshownthat estrogenorprogesteroneand itssyntheticanalogsdifferently affectplasmalipoproteins,inparticular,estrogenincreases whereasprogesteroneanditssyntheticanalogsdecreaseHDL-C

concentrations(136).Accordingly,while17beta-estradiolhad noeffects,progesterone andthreesyntheticanalogssuppressed ApoA-I-mediatedcellularcholesterolreleasefromhuman fibroblastsresultingingenerationoflessHDLparticles(137).
Sexisakey variableinvascularbiologyandinparticular, ECfunctionisinfluencedbysexualhormones,butalsoby chromosomesresultinginsex-specificdifferencesingene expressionprofile.Humanumbilicalveinendothelialcells (HUVECs)derivedfromfemalesandmaleswerefound intrinsicallydifferentindependentlyfromtheirexposureto sexualhormones,implicatingaroleforgenomicsexual dimorphismsinCVsystem(14, 138).Transcriptomicperformed inHUVECsinboy-girltwinsor innon-twinadultECsshowed thatsex-differenceswerepresenteitheratbirthandmaintained throughoutlifeoracquiredoverlife(118).Asexpected,sex differencesinadultEC transcriptomeinvolvedmanygenes influencedbyestrogensorandrogens.Interestingly,androgen andestrogenreceptorswerenotdifferentiallyexpressedin
Dietrichetal. 10.3389/fcvm.2022.989428
FIGURE2
Frontiersin CardiovascularMedicine 06 frontiersin.org 88
adultECs.Intriguingly,half ofthegenesshowingsex-specific differencesinHUVECswere sexchromosomalgenes.Moreover, coronaryarterydiseasetargets(derivedusingmultiplegenomewideassociationstudies)werealsoenrichedinthegene setshowingsexdifferenceinHUVECs,makingpossibleto speculateaboutsexdifferencesinCADrootedindifferential geneexpressioninECsalreadyatbirth(118).Genehallmark analysisshowedincreasedexpressionofgenesinvolvedin endothelialtomesenchymaltransition,NF-kBpathwayand hypoxiainfemales,whileincreasedexpressioninMYC targets,oxidativephosphorylationandmTORpathwaywere reportedinmales(118).Otherstudiesreportedtarget-specific differencescomparingmaleand femalenon-twinHUVECs, whichmaycontributetosexdifferencesbetweenmalesand femalesinendothelialfunction.Highercellproliferation, migratorypropertiesandendothelialNOsynthaseexpression wereobservedinfemaleHUVECs,whileinthemalecellsbeclin1andtheLC3-II/LC3-Iratio,twowidelyacceptedmarkersof autophagy,werehigher(119).Notably,cellularsize,shapeas wellasmRNA andproteinexpressionofestrogenandandrogen receptorsweresimilaramongsexes(119).Proteomicanalysis ofthesecretome ofserum-deprivedHUVECsisolatedfrom healthyfemaleandmalenewbornsrevealedhigherexpression ofproteinsinvolvedincellularresponsetostress(e.g.,several membersofAnnexinandHeatShockProteinfamilies)and apoptosis(e.g.,PTX3)inmalecells(120).Theseresultsare inagreementwithreports obtainedindifferentcells(e.g., neuronsorcardiomyocytes)challengedwithstressorstimuli andoverallsuggestlowerresistancetooxidativestressand higherpropensityofmalecellstoundergoapoptosis.Onthe contrary,femaleneurons/cardiomyocytesmaybemoreresistant tooxidativestresswithapro-autophagypredisposition(139, 140);thelattercharacteristicwillneedtobefurtherinvestigated inECsasthe abovementionedstudyonHUVECtranscriptome inboy-girlstwinsshowsamaleandnotafemalepro-autophagy genesignature(118).
FemaleHUVECsshoweda strongertranscriptional responseaftershearstressexposurecomparedtomalecells involving,forinstance,upregulationofgenessuchaseNOS, heme-oxygenase1(HO-1)downregulationofNADPHoxidase 4(Nox4),endothelin-1(ET-1)orvascularcelladhesion molecule1(VCAM-1),thelatterdownregulatedby22.2-foldin femalevsonly3.5-foldinmales(141).
RegardingECenergysupply,similarbaselineratiosof glycolysisvs.mitochondrialrespirationwereobservedin HUVECsobtainedfrommale/femaletwins,butfemalecells performedbetterunderstarvationorunderVEGFstimulation withhigherATPandmetabolitelevelscomparedtomalecells, suggestingamoreflexiblemodulationofenergyproductionin females(120, 142).
Furtherstudieswill needtoelucidatewhetherthedescribed higheradaptabilityoffemaleECstostressmayconfer themprotectionagainstCVDrisk.Conversely,astronger
transcriptionalresponseinfemaleECsmight,inspecificcases, favordiseaseonsetandprogression(e.g.,inthecontextof thehigherprevalenceofautoimmunediseasesinthefemale population)(143–145).
Collectively,increasing evidencehighlightsthepresence ofsexdependentdifferencesinECsatdifferentstagesof life.However,thereareveryfewstudiesinadultECs(i.e., HAECs)comparedtothestudiesinHUVECs,whichmakes difficulttoadequatelyinvestigateorcomparechangesinEC geneexpressionacquiredlaterinlife.Moreover,itisimportant toconsidersexasacrucialbiologicalvariablenotonlyin cardiovascularclinicalresearchbutalsoinexperimentalstudies onECbiologytoincreasethequalityandtranslationalvalue ofresults.
Insightsintosex-dependent di erencesinHDLandECcrosstalk
LifestyleandCVD:Sex-dependent di erences
Smoking,alcoholconsumption, dietandexerciseare modifiableCVDriskfactors(Table1).
Thenumberofsmokedcigarettespositivelycorrelatedwith increasedCHDriskinbothsexes.Infact,smokinginduced endothelialdysfunctionanddamage,increasinglipidoxidation, decreasingHDL,andpromotinginflammation,andaprothromboticstate(146, 147).Furthermore,aworselipidprofile characterizedbyincreasedApoBandreducedApoA-Iand ApoA-IIwasreportedinsmokerscomparedtonon-smokers independentlyfromtheirsex(148).Interestingly,smokingwas reportedasastronger riskfactorforCVDinwomenthaninmen accordinglytotheFinnmarkStudy(149).Thiscouldbepartially attributedtotheabilityofsmokingtoalterestradiolmetabolism leadingtotheformationofinactivecatechols(150, 151), thusinhibitingestradiolvaso-protectiveproperties.Moreover, exposuretopassivesmokingfrombirthwasassociatedwith reducedHDL-Clevelsinadolescentgirlsbutnotinboys(152). Theanti-estrogeniceffectofsmokingpositivelycorrelateswith increasedCHDriskandstrongreductioninHDL-Clevelsin youngcomparedtoolderwomenandmen(150, 153).The evidencethatex-smokershadhigherHDL-Clevelscompared tosmokersofbothsexesfurtherconfirmtheseresults(154). Furthermore,the CopenhagenCityHeartStudyreportedthat smokingwomenhad9.4higherriskofmyocardialinfarction comparedtonon-smokingwomen,whiletheriskscorewasonly 2.9timeshigherinsmokingmencomparedtonon-smokers (155).Onthecontrary,reducedlevelsofendothelialprogenitor cells(EPCs)have beenreportedinmencomparedtowomen. SmokingfurtherdecreasedEPCsinmen,whilenodifferencewas foundbetweensmokingandnon-smokingwomen.Inthiscase,
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 07 frontiersin.org 89
TABLE1 Comparativedescriptionofthee ectoflifestylehabitsonHDLsandECsinmenandwomen.
Men
Smoking IncreasesCHDrisk(147, 148)
InducesECdysfunction(147, 148)
ReducesHDLnumberandfunctionality[147,148]
Promotesinflammation(147, 148)
ReducesEPCsnumber(157)
Alcohol Increases HDL-Clevels(162)
PreventsECactivationandinflammation(170)
Diet Mediterranean diet:reducessmalldenseLDLand increasesmediumLDL,reducesECinflammationand oxidativestress(174, 175)
Physicalactivity
PreventsECdysfunctionandatherosclerosis(194, 195)
Increases HDL-Clevels(198, 199)
Women
Increases CHDrisk(147, 148)
InducesECdysfunction(147, 148)
ReducesHDLnumberandfunctionality(147, 148)
Promotesinflammation(147, 148)
Altersestrogen metabolism(151, 152)
Increases riskofCVDandMI(152)
Increases HDL-Clevels(162)
Reducesstrokerisk(169)
Increases overallmortality(169)
PreventsECactivationandinflammation(170)
Mediterraneandiet: reducesmediumdenseLDLandincreases smallLDL,reducesECinflammationandoxidativestress. IncreaseseNOSactivityandreducesCVDrisk.(174, 175, 192)
Dairydiet:Reducesinsulinsensitivity(176, 177)
PreventsECdysfunctionandatherosclerosis(194, 195)
IncreasesHDL-Clevels(198, 199)
CHD,CoronaryHeartDisease;EC,EndothelialCells;HDL,High-DensityLipoproteins; HDL-C,HDL-Cholesterol;CVD,CardiovascularDisease;MI,MyocardialInfarction;LDL, Low-DensityLipoproteins;eNOS,endothelialNitricOxideSynthase;EPCs,EndothelialPrecursorCells.
sex-differencesontheeffectofsmokingweremostlyattributed toaprotectiveeffectofestradiolonEPCs(156).
Lowtomoderatealcoholconsumption didnotaffectCVD riskinbothsexes(157).TheCoLausStudyreportedno differencesinexpressionofHDL-relatedgenes(i.e.,ABCA1, APOE5,CETP,hepaticlipaseandlipoproteinlipase)basedon alcoholconsumeinasex-mixedpoolofCaucasianpatients (158).CHDriskcouldperhapsvarydependingontheethnicity ofthepatients.TheAtherosclerosisRiskinCommunities (ARIC)studyreportedreducedCHDinwhitesbutincreased diseaseinblackalcoholconsumermenindependentlyfrom levelsofalcoholconsumed(159).Thiswaspartiallyattributedto differenthepaticgenevariants andexpressionlevels(i.e.,CETP, hepaticlipase,LPL,andPON1)betweenthesetwoethnicities (159, 160).Meta-analysisdatasuggestedthatHDL-Clevels increasedanaverageof0.06mmol/Lper23g/dayofalcohol consumed(161).TheincreaseofHDL-Clevelsinalcohol consumershave beenattributedtoenhancedHDLproduction (hepaticandextra-hepatic),decreasedCETPactivityandlower HDL-Cclearance(162).However,thisincreaseisstrongly influencedbyalcohol-geneinteractions (163).Asanexample, menandpost-menopausalwomen carryingthehomodimeric γ2variantoftheADHICgenehadaslowerrateofalcohol clearance,whichwasassociatedwithelevatedHDL-Clevels (164, 165).Itisworthspecifyingthatsexdifferencesinalcohol consumptionaredifficulttodetect,sincegenerallywomen cantoleratelesseramountofalcoholduetotheirsex-specific absorption,bodyfat/waterratio,reducedlevelsofenzymes involvedinalcoholmetabolismandglomerularfiltrationrate
(166).Furthermore,studiesinthegeneralpopulationindicated thatamongallalcohol consumers/abusers,only1/3were women(167).Nevertheless,moderatealcoholconsumptionwas associatedwithlower riskofstrokeinwomencomparetomen butalsowitha10%increasedriskofoverallmortality(168).
Alcoholhasalso adirecteffectonECs.Indeed,moderate levelsofalcoholwereabletopreventendothelialactivation andpro-inflammatorycytokinereleaseinhumancoronary arteryECsstimulatedwiththepro-inflammatorySAA(169). Furthermore,areduction inmonocyteadhesiontoTNF-αstimulatedECswasreportedinmoderatealcoholconsumer mencomparedtonon-consumers(170).Ontheotherhand, heavyalcoholconsumption(bothmeasuredandself-reported) wasassociatedwithincreasedcirculatingvascularadhesion molecules(i.e.,E-selectin,intracellularadhesionmolecule1 (ICAM-1)andVCAM-1)andreducedflow-mediateddilationin sex-mixedcohortsofpatients,independentlyofalcoholicliver disease(171, 172).
Dietalsoaffects womenandmeninadifferentmanner. Mediterraneandietwasabletoreducetotalcholesterol,LDLC,ApoBandApoA-1plasmalevelsinbothsexes.However, menunderMediterraneandietexperiencedareductionin smalldenseLDLandanincreaseinmediumLDL,whilethe oppositetrendwasobservedinwomen(173, 174).Furthermore, a comparisonbetweenredmeat anddairydiethighlighteda reductionininsulinsensitivityinwomenfollowingthedairy diet.Instead,nodifferencesbetweenthetwodietswerereported inmen(175, 176).ABCA1isoneofthemostsex-influencedgene intheliverandits expressionishigherinfemales(177).Indeed,
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 08 frontiersin.org 90
estrogenlevelsand dietarycomponentswereabletoregulate ABCA1expressioninmacrophages,leukocytesandliverin humanandrodents,increasingApoE-positiveHDLparticles andimprovingcholesterolefflux(178–181).Amongallthe geneticvariants,ABCA1/R230C wasassociatedwithlowHDL-C (182).Ithasbeenshownthatdietarymacronutrientproportions regulatedtheeffectofABCA1/R230Cinpremenopausalwomen bydirectlyinteractingwithABCA1gene(183).Inparticular, metabolicallyunfavorablepatternwasfoundinABCA1/R230C premenopausalwomenfollowinghighcarbohydratesandlow fatdiet,whiletheoppositepatternwasfoundinwomen followinghighfatandlowcarbohydratesdiet(183).
Onthecontrary,lowering dietaryfatintakewasable torestoreHDLfunctionality(inparticularHDL-CEC)in hypercholesterolemicfemalepigsbyreducingcholesterol plasmalevels(184).
Thereissomeevidence thatdietcoulddirectlyaffect ECfunction.Transitorydisruptionofendothelialfunction andreductioninvasorelaxationhavebeenreportedafter acuteadministrationofhigh-fatmeal,inconcomitancewith increasedtriglyceride-richlipoproteinsinplasma(185, 186). Onthecontrary,chronicconsumptionoflow-fatdiets (i.e.,Mediterraneandiet)wasassociatedwithimproved endothelialfunctionandreducedmarkersofendothelial activationinplasmainmen(187–189),mostlikelythrough changesincholesterolmetabolismandthepresenceofoleate anddecosahexanoicacid,whichwereabletoreduceproinflammatorymoleculeexpressionandmonocyteadhesionin ECs invitro (190).Sameresultswereshownalsoinwomen.
Indeed,apilotstudy demonstratedthatspecificcomponentsof Mediterraneandiet(i.e.,legumes,redmeat,andoverallproteins) wereassociatedwithreducedinflammationandoxidativestress, increasedeNOSactivityandreducedCVDriskinacohortof ethnicallymixedwomen(191).
Physicalactivityis wellknownasprotectivefactoragainst CVD(192).Evidenceshowedthatphysicalactivitywasableto slowdownECdysfunction andatherosclerosisprogressionin bothsexes(193, 194).However,howphysicalactivitydifferently influenceCVDriskinwomen andmenisstillunderdebate. Asystematicreviewfocusedonphysicalactivityandstroke incidenceclaimingthat,amongalltheanalyzedstudies,35% ofthemreportedastrongassociationbetweenphysicalactivity andstrokeincidenceinwomen,whilethesamecorrelation inmenwasevidentonlyinfewstudies(195).Evenso,the FraminghamStudyreported thatphysicalactivityconferred protectionagainststrokeinmen,butnotinwomen(196).
Thecorrelationbetweenphysical activityandreductionin CVDriskcouldbepartiallyattributedtoincreasedHDL-C levelsinphysicallyactiveindividualscomparedtosedentary ones.However,theincreaseinHDL-Cseemedtobesignificant onlywhenathresholdvolumeofphysicalactivitywasreached (197, 198).Evenifthethresholdlevelhasnotbeenaccuratelyand systematicallyestimatedyet,epidemiologicalandcross-sectional
studiessuggestedthatthethresholdvaluewas1,500kcal/week inmenand1,200kcal/weekinwomenindependentlyfromtheir menopausalstatus(199–201).
Reversecholesteroltransport
The best-knownpropertyofHDLisRCT,whichconsistsin theabilityofHDLtoacceptexcesscholesterolfromperipheral cells,inparticularmacrophages,andtransportittotheliver forexcretionorre-utilization.RCTisconsideredthemost importantanti-atherogenicfunctionofHDLs.Componentsof cholesteroleffluxincludethepassivediffusionofcholesterol fromcellsaswellastheactivecellularcholesteroltransferby ABCA1,ABCG1,andSR-BI.Inthiscontext,ECsmayrepresent apotentialbarriertoHDLinreachingmacrophageswithin thevesselwall.However,HDLandlipid-freeApoA-Iareable tocrossintactaorticECmonolayersfromtheapicaltothe basolateralcompartmentinatranscytosisprocess,involving ABCG1andSR-BI(202).Contrarytoothercellsthatformthe atheroscleroticplaque(smoothmusclecellsandmacrophages), ECsdonotaccumulatecholesterol(203)andhaveastrong abilitytoeffluxcellular cholesteroltoHDLsindependently ofABCA1,ABCG1,andSR-BIexpressionoractivity(204).
Ontheother hand,ithasbeenshownthatinconditions ofhyperlipidemiaECscanmetabolizeLDLsintocholesterol crystals,whichaccumulateintracellularlyandconferafoam cell-likemorphologytoECs(205).
HDLsderivedfromhealthynormolipidemicmenand womenhaddifferentRCTcapacitiesduetotheactivationofsexspecificmechanismsforcholesterolefflux(206).Thers1799837 (APOA1)andrs1800588(LIPC) SNPvariantsrepresented themajordeterminantsofHDLcholesteroleffluxcapacity inwomen,whilers2230806(ABCA1)andrs5082(APOAII) variantswerekeydeterminantsinmen(207).Furthermore, serumisolatedfromwomendisplayed anenrichmentinlargeHDL-particles(L-HDL-P)andincreasedcapacitytomediate cholesteroleffluxthroughSR-BIreceptors.Ontheotherhand, serumisolatedfrommenshowedincreasedpreβ-HDLparticles andcholesteroleffluxthroughABCA1receptors(206).Both lowandhighHDL-Clevelswereassociatedwithreduced freecholesteroltransferonHDLsinbothsexes,especiallyin women,inpatientswithacutemyocardialinfarctionandTangier disease(208).
Thesefindingsnot onlysuggestthatHDLcomposition differsinmencomparedtowomen,butalsothatthese differencesinHDLpoolmayhaveanimpactintheir abilitytostimulatecholesterolefflux.Estradiollevels,infact, positivelycorrelatedwithHDLcholesteroleffluxcapacity (HDL-CEC)frommacrophagesinpre-menopausalwomenand wereassociatedwithincreasedconcentrationofL-HDL-Pand lowerconcentrationofsmall-HDL-particles(S-HDL-P)(209). However,HDL-CEC decreasedintransgenderwomen(men
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 09 frontiersin.org 91
towomen)underestradiol hormonetherapy,suggestingthat reductionoftestosteroneand increaseinestradiolmayact synergisticallyinreducingHDL-CEC(24).Thishypothesisis inlinewiththe evidencethattestosteronedeprivationinmen increasedHDL-ClevelsbutnotHDL-CEC,whileestradiol treatmenthadtheoppositeeffect(210).Thecorrelationbetween androgenlevelsandCVD inmeniscontroversial.Lowlevelsof androgenswereassociatedtoincreasedCVDinoldermen(211). Ontheother hand,testosteroneadministrationinhypogonadal mencanbluntEC-mediatedvasorelaxation(212, 213).Thus, ageandtypeofandrogen usedareimportantfactorsto beconsidered.
Inflammation
Increasedexpressionlevelsofspecificadhesionmolecules— suchasVCAM-1andICAM-1andE-selectin—areawellrecognizedmarkerofECinflammationandoxidativestress. HDLparticleconcentrationisinverselycorrelatedwith theexpressionofcellularadhesionmolecules,aswellas inflammatorymediatorsC-reactiveprotein(CRP)andTNF-α TheunderlyingmechanismcanbepartlyattributedtoHDLassociatedsphingosine1phosphate(S1P).S1Psignalingthrough S1PreceptorhasbeenshowntoprotectagainstTNF-α-induced monocytebindingtoECspreventingtheactivationofNFkBandc-Junpathwaysaswellasreducingthesecretionof pro-inflammatorychemokines(214).
OxLDLscaninduceMCP-1,whichisinvolvedinthe recruitmentofmonocytesintothesub-endothelialspace andtheirdifferentiationintofoamcells.Itisanimportant inflammatoryprocessintheinitialstagesofatherosclerosis (215). Invitro and invivo experimental studiessuggestedthat HDLassociated-PON1inhibitedLDL-oxidationbycatalyzing thebreakdownofoxidizedphospholipids,thusabolishingthe productionofpro-inflammatorycytokines(MCP-1,IL-8and macrophagecolonystimulatingfactor)fromECs(216–220).
HDLsarealsoable totransfermicroRNAtoECs(221).Itwas shownthatHDL-transferredmicroRNA-223directlytargeted ICAM-1geneat3’UTRsitessuppressinggeneexpressionand functioninHUVECsstimulatedwithTNF-α thusreducing leukocyteadhesion.RegulationofTNFα-inducedICAM-1 expressionbyHDLswasnotfoundinfibroblasts,suggestinga specificmiR-223deliveryonECs(222–224).
HDL-inducedNOproductionalso playsanimportant roleinthereductionofECinflammation.Theinduction ofPI3K-Akt-eNOSsignalingmediatedbythebindingof ApoA-1andSR-B1up-regulatescyclooxygenase(COX-2) expressionandprostaglandinI2(PGI2)releaseinECs (225).PGI2 isapotentinhibitorofinflammation, which limitsimmunecellproliferationaswellasinhibitsplatelet aggregation,thusaffectingsmoothmusclerelaxationand vasodilation(226).
HDL-Clevels also correlatedwithriskofinfection.Similarly towhatwasshowedregardingall-causemortalityrisk(3, 4), bothlowand highlevelsofHDL-Cwereassociatedwith increasedriskofinfection(227).Theincreasedriskofinfection associatedwithlow levelsofHDL-Ccouldbeinpartduetothe lossofleucopoieticcontrolandimmunecellmodulationfrom HDLs(228, 229).ThemechanismincaseofhighlevelsofHDLCislessclear,butparticulargeneticmutationsassociatedwith increasedHDLlevelsmayalsoaffectdiseasesusceptibility.For instance,incaseofLIPCandSCARB1(encodingforhepatic lipaseandSR-BI,respectively),whosemutationswereassociated withincreasedriskofCAD(46, 230).Interestingly,severalSNP variantslocatedinthepromoterandintron1ofLIPCgene wereassociatedwithchangesinHDL-Clevelsinwomenbutnot inmen(231).SCARB1rs5888SNPvariant,instead,hasbeen associatedwitha greaterreductionintotalcholesterol,LDL-C andApoBinwomentreatedwithatorvastatincomparedtomen inpatientswithacutecoronarysyndrome(232).
Peri-menopausalandmenopausal womenhadincreased levelsofTNF-α,CRP,TGandLDLcomparedtotheirpremenopausalcounterparts,whichmayunderlineareductionon HDLfunctionalitydrivenbythecollapseinestrogenlevels(233).
HDLanti-inflammatorypropertiesare impairedorlost inchronicinflammatoryconditions.Concomitantlywith adecreaseofHDLparticlelevelsalsochangesinthe structureandfunctionofHDLsareobserved.Patients withchronicinflammatorydisorders,includingrheumatoid arthritis,systemiclupuserythematosus,andpsoriasis,whichare associatedwithanincreasedriskofatheroscleroticCVD,exhibit aconsistentdecreaseinHDLparticlesandApoA-1levelsand HDLvaso-protectivepropertiesinasex-independentmanner (234–236).However,inotherdiseasesasintheantiphospholipid syndrome,studiesreporting astrongreductioninHDL functionalitywereconductedcomparingonlyaffectedand healthywomen(237).Thus,itwillbeimportanttofurther elucidatewithsex-matchedcomparisonswhetherornotmen andwomenHDLsaresimilarlyaffectedalsointhoseimmuneinflammatorydiseases.
Interestingly,ithasbeenrecentlyshownthatHDL-C andApoA-IlevelsmeasuredbeforeSARS-CoV-2infection negativelycorrelatedwithCOVID-19mortalityand hospitalization,independentlyofage,sex,comorbidities, orstatintreatment(238–240).Furthermore,HDLcargowas profoundlyalteredinsevere COVID-19patients,withincreased abundanceofSAA-1and 2,SFTPB,ApoF,andinter-alphatrypsininhibitorheavychainH4(241).Thesefindingsare corroboratedbytheevidencethattreatmentwithreconstituted HDLsinCOVID-19patientsreducedSAA-1,SFTPB,andApoF inHDLs(242).Ofnote,menwithCOVID-19weremoreprone todevelopintothesevereconditionanddiecomparedtowomen (Figure2)(243, 244).Indeed,nostrikingdifferenceswerefound betweenpre-andpost-menopausal women,suggestingthat reductioninfemalemortalitymaybeindependentfrom
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 10 frontiersin.org 92
estrogenlevels(243, 244). Invitro experimentsconducted onECsexposedtoSARS-CoV-2S1spikeproteinshoweda significantincreaseintheoverallinflammatorystatusincells treatedwithandrogens(245).Recentfindingsshowedthatmale sexclinical-biological characteristics,ratherthanmalegenderrelateddifferences(i.e.,pertainingtothesocio-economic sphere,suchaseducation)wereindependentlyassociatedwith intensivecareunitadmission,invasiveventilation,and/ordeath inCOVID-19(246).
Anti-apoptoticandanti-oxidative properties
EChomeostasisreliesonthebalancebetweenpro-andantiapoptoticstimulicomingfrombloodstreamandneighboring cells(247, 248).Oncethebalanceisdisrupted,(pro)-apoptotic ECsfavorplatelet aggregationandcoagulation,creatingaproatherogenicenvironment(249, 250).OxLDLcanpromoteEC apoptosisbyincreasing intracellularCa2+ levels(251–253), favoringtheonset andprogressionofCVD(114).Incontrast toLDLs,HDLsprotect ECsfromapoptosisbypreserving mitochondrialintegrityandinhibitingtheactivationofthe caspase-downstreamcascade(254–256).Indeed,HDLsisolated fromamixedsex poolofhealthydonorswasabletoreduceEC apoptosisboth invivo and invitro,whilesex-matchedHDLs isolatedfromCADpatientsshowedoppositeresults(257).
Inparticular,HDLparticlescontainingApoA-Iseemedtobe morecytoprotectivethanotherHDLsubclasses(258, 259).
MoreoverEPCscanquickly differentiateintomatureECs torescuevascularintegrityinconditionsofhighcellturnover (260).HDLscanpromoteendothelialrepairbyincreasingEPC numberandfunctioninmale mice(261, 262).
Inadditiontotheir anti-apoptoticproperties,HDLscanalso reduceoxidativestress.PON-1isanaccessoryproteinofHDL that,incoordinationwithApoA-I,protectlipoproteins,ECsand intimalmacrophagesfromoxidativeinsultsbyhydrolyzinglipolactones(263–266).Highlevelsofoxidativestresscanincrease HDLlipidperoxideloading, decreasingtheirprotectiveactivity againstLDLoxidation(267, 268).SignificantdifferencesinHDL peroxidelevelsbetweenmen andwomenhavebeenreported asareadoutofsex-specificreductioninHDLanti-oxidative functionsinmen(269).
DecreasedHDL anti-oxidativepropertiesdrivenbyhigh glycemicburdenhavealsobeenreportedinbothtype1and type2diabeticmenandpost-menopausalwomen(270, 271).In thiscontext,it hasbeenshownthatPON-1activitywasmore stronglyimpairedinT2Dwomencomparedtomen(272).
Furthermore,womenwithhypertension,metabolic syndromeorperi-menopausenotonlyhadhigherlevels ofoxLDLscomparedtohealthymiddle-ageorpremenopausalwomen,butalsoreduceddefensesagainst
oxLDLs.However,thecontributionofestrogensinthiscontext isstillunclear(273–275).
HDLscanserve ascarriersforothermolecules,forinstance estrogens.Ithasbeenshownthatthebindingofestrogens withHDLsincreasestheiranti-oxidativepropertiesdueto estrogenesterificationperformedbyLCAT(276).Indeed,LCAT wasableto esterifyHDL-boundedE2.EsterifiedE2wasthen transferredfromHDLtoLDLthankstoCETP(276).Incubation experimentsdemonstratedthat E2esterificationandfurther associationwithLDLwasabletoincreaseLDLresistanceto oxidation(276, 277).Onthecontrary,hyperandrogenismis associatedwithincreasedoxidativestressandreducedHDL anti-oxidativefunctionalityinwomen(278, 279).Tothebest ofourknowledgeHDLs havenotbeenreportedascarriers forandrogens.
HDL-mediatedendothelial NO-production
SeveralmechanismsaccountfortheendothelialNOstimulatingcapacityofHDLs.InculturedECs,HDLsdirectly activatetheproductionandreleaseofNObybindingof ApoA-ItoSR-BI,leadingtoincreasedintracellularceramide levelsandphosphorylationofendothelialNO-synthase(eNOS) (280).CholesteroleffluxfromECstoHDLs via ABCA1also promotesNO synthesisbymodulatingcholesterol-binding proteincaveolin-1andeNOS(281).Mechanistically,thebinding ofHDLstoSR-B1 leadstotyrosinekinaseSrc-mediated activationofphosphoinositide3-kinase(PI3K),whichinturn stimulatesAktandErkpathways.TheactivationofAktdirectly stimulateseNOSbyphosphorylationatSer-1179(282)(see Figure2).
Estradiolisableto inducerapidarterialvasodilationby stimulatingeNOSactivitybyacting via ERs(18).HDLsisolated fromfemalemiceandhealthywomen(butnotmalemiceor men)wereabletoenhanceNOproduction.InadditiontoERs, estradiolboundtoHDLswasalsoabletoenhanceeNOSactivity throughtheactivationofSR-BIreceptors(Figure2)(283).
Specificlipidandprotein componentsofHDLshavea strongimpactonNOproduction.S1Pisalipidcarried mainlyonapoM-containingHDLs(>50%ofcirculatingS1P).
S1P-apoMHDLsareinvolvedinpersistentactivationof Akt/eNOSpathway,thusleadingtoNOdependentvasodilation throughS1Preceptorstimulation(284–286).Decreasedlevelsof ApoM/HDLcorrelatedwithincreasedCVDriskandhavebeen reportedintype2diabetesinbothsexesandinwomen(butnot inmen)withtype1diabetes(287–289).
ReducedHDL-associated PON-1activityleadstothe activationofendotheliallectin-likeoxidizedLDLreceptor (LOX-1)andPKCαII,thusinhibitingtheactivityofeNOS(290). Inflammationandmetabolic syndromescanalsodrastically
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 11 frontiersin.org 93
affectthe abilityofHDLstostimulateNOproduction(291, 292), thuspromotingatherosclerosis (293).
Conclusions
Sexhormonesandsex-specificgeneexpressionare importantalthough stillincompletelyunderstooddeterminants intheregulationofHDLandECcrosstalkandtheir contributiontocardiovascularhealthanddisease.Despite increasingevidencepointingoutthatsex-originofculturedcells andinparticularECscanstronglyaffectscientificresults(294–296),mostofthearticlesdonotreportanyinformationabout thesexofthecellsoroftheanimalsusedintheirexperiments. Theuseofsex-mixedcohortsofpatientsorimbalancednumber ofwomenandmenduringclinicaltrialsalsorepresentsa potentialsourceofbias.AshighlightedinthisReview,itisof foremostimportancetoalwaysconsidertheinfluenceofsexas biologicalvariableineachstepofresearchandtoclearlyreport andanalyzethisinformation.
Authorcontributions
ED,AJ,andEOwrotethemanuscript.EDdesignedthe figuresunder BioRender commoncreative license.Allauthors contributedtothearticle andapprovedthesubmittedversion.
References
1.VoightBF,PelosoGM,Orho-MelanderM,Frikke-SchmidtR, BarbalicM, JensenMK,etal.PlasmaHDLcholesterolandriskof myocardialinfarction:amendelianrandomisationstudy. Lancet. (2012) 380:572–80.doi:10.1016/S0140-6736(12)60312-2
2.CasulaM,ColpaniO,XieS,CatapanoAL,BaragettiA.HDLin atheroscleroticcardiovasculardisease:insearchofarole. Cells.(2021) 10:1869.doi:10.3390/cells10081869
3.MadsenCM,VarboA,NordestgaardBG.Extremehighhigh-density lipoproteincholesterolisparadoxicallyassociatedwithhighmortalityinmen andwomen:twoprospectivecohortstudies. EurHeartJ. (2017)38:2478–86.doi:10.1093/eurheartj/ehx163
4.YuY,LiM,HuangX,ZhouW,WangT,ZhuL,etal.AU-shapedassociation betweentheLDL-cholesteroltoHDL-cholesterolratioandall-causemortalityin elderlyhypertensivepatients:aprospectivecohortstudy. LipidsHealthDis. (2020) 19:238.doi:10.1186/s12944-020-01413-5
5.KoDT,AlterDA,GuoH,KohM,LauG,AustinPC,etal.High-density lipoproteincholesterolandcause-specificmortalityinindividualswithoutprevious cardiovascularconditions:theCANHEARTstudy. JAmCollCardiol. (2016) 68:2073–83.doi:10.1016/j.jacc.2016.08.038
6.KontushA,LhommeM,ChapmanMJ.Unravelingthecomplexitiesofthe HDLlipidome. JLipidRes. (2013)54:2950–63.doi:10.1194/jlr.R036095
7.Birner-GruenbergerR,SchittmayerM,HolzerM,MarscheG.Understanding high-densitylipoproteinfunctionindisease:recentadvancesinproteomicsunravel thecomplexityofitscompositionandbiology. ProgLipidRes. (2014)56:36–46.doi:10.1016/j.plipres.2014.07.003
8.KontushA,LhommeM,CalabresiL,ChapmanMJ,DavidsonWS,Davidson WS.StructureofHDL:particlesubclassesandmolecularcomponents. HandbExp Pharmacol.(2015)224:3–15.doi:10.1007/978-3-319-09665-0_1
9.SturtzelC.Endothelialcells. AdvExpMedBiol. (2017)1003:71–91.doi:10.1007/978-3-319-57613-8_4
Funding
ThisresearchwasfundedbytheSwissNational ScienceFoundation(SNSF), grantnumberPRIMA: PR00P3_179861/1andtheSwissLifeFoundation,the AlfredandAnnemarievonSickGrantsforTranslational andClinicalResearchCardiologyandOncology,the HeubergstiftungandtheSwissHeartFoundation,Switzerland alltoEO.
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddo notnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
10.CyrAR,HuckabyLV,ShivaSS,ZuckerbraunBS.Nitricoxideandendothelial dysfunction. CritCareClin. (2020)36:307–21.doi:10.1016/j.ccc.2019.12.009
11.GimbroneMA,García-CardeñaG.Endothelialcelldysfunction andthepathobiologyofatherosclerosis. CircRes. (2016)118:620–36.doi:10.1161/CIRCRESAHA.115.306301
12.DavisCE,WilliamsDH,OganovRG,TaoSC,RywikSL,SteinY,etal.Sex differenceinhighdensitylipoproteincholesterolinsixcountries. AmJEpidemiol. (1996)143:1100–6.doi:10.1093/oxfordjournals.aje.a008686
13.García-CalzónS,PerfilyevA,deMelloVD,PihlajamäkiJ,LingC. Sexdifferencesinthemethylomeandtranscriptomeofthehumanliverand circulatingHDL-cholesterollevels. JClinEndocrinolMetab. (2018)103:4395–408.doi:10.1210/jc.2018-00423
14.HuxleyVH,KempSS,SchrammC,SievekingS,BingamanS,YuY,etal.Sex differencesinfluencingmicro-andmacrovascularendothelialphenotypeinvitro. J Physiol. (2018)596:3929–49.doi:10.1113/JP276048
15.AgirbasliM,AgaogluNB,OrakN,CagliozH,OcekT,KarabagT,etal. Sexhormones,insulinresistanceandhigh-densitylipoproteincholesterollevelsin children. HormResPaediatr. (2010)73:166–74.doi:10.1159/000284357
16.MorrisonJA,BartonBA,BiroFM,SprecherDL.Sexhormonesandthe changesinadolescentmalelipids:longitudinalstudiesinabiracialcohort. JPediatr. (2003)142:637–42.doi:10.1067/mpd.2003.246
17.ElKhoudarySR,BrooksMM,ThurstonRC,MatthewsKA.Lipoprotein subclassesandendogenoussexhormonesinwomenatmidlife. JLipidRes. (2014) 55:1498–504.doi:10.1194/jlr.P049064
18.MendelsohnME.Protectiveeffectsofestrogenonthecardiovascularsystem. AmJCardiol. (2002)89:12E 7E.doi:10.1016/S0002-9149(02)02405-0
19.MendelsohnME.Mechanismsofestrogenactioninthe cardiovascularsystem. JSteroidBiochemMolBiol. (2000)74:337–43.doi:10.1016/S0960-0760(00)00110-2
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 12 frontiersin.org 94
20.JamesBD,AllenJB.Sex-specificresponsetocombinationsofshearstress andsubstratestiffnessbyendothelialcellsinvitro. AdvHealthcMater. (2021) 10:e2100735.doi:10.1002/adhm.202100735
21.BoscaroC,TrentiA,BaggioC,ScapinC,TrevisiL,CignarellaA, etal.Sexdifferencesinthepro-angiogenicresponseofhumanendothelial cells:focusonPFKFB3andFAKactivation. FrontPharmacol. (2020) 11:587221.doi:10.3389/fphar.2020.587221
22.PoldermanTJC,KreukelsBPC,IrwigMS,BeachL,ChanYM,DerksEM, etal.Thebiologicalcontributionstogenderidentityandgenderdiversity:bringing datatothetable. BehavGenet. (2018)48:95–108.doi:10.1007/s10519-018-9889-z
23.MarakaS,SinghOspinaN,Rodriguez-GutierrezR,Davidge-PittsCJ, NippoldtTB,ProkopLJ,etal.Sexsteroidsandcardiovascularoutcomesin transgenderindividuals:asystematicreviewandmeta-analysis. JClinEndocrinol Metab. (2017)102:3914–23.doi:10.1210/jc.2017-01643
24.vanVelzenDM,AdorniMP,ZimettiF,StrazzellaA,SimsekS, SirtoriCR,etal.Theeffectoftransgenderhormonaltreatmentonhigh densitylipoproteincholesteroleffluxcapacity. Atherosclerosis. (2021)323:44–53.doi:10.1016/j.atherosclerosis.2021.03.008
25.HumbleRM,GreeneDN,SchmidtRL,WinstonMcPhersonG,Rongitsch J,ImborekKL,etal.Referenceintervalsforclinicalchemistryanalytesfor transgendermenandwomenonstablehormonetherapy. JApplLabMed. (2022).doi:10.1093/jalm/jfac025
26.IannantuoniF,SalazarJD,Martinezde.MarañonA,BañulsC,LópezDomènechS,RochaM,etal.Testosteroneadministrationincreasesleukocyteendotheliuminteractionsandinflammationintransgendermen. FertilSteril. (2021)115:483–9.doi:10.1016/j.fertnstert.2020.08.002
27.KannelWB,DawberTR,FriedmanGD,GlennonWE,McNamara.PMRISK factorsincoronaryheartdiseaseanevaluationofseveralserumlipidsaspredictors ofcoronaryheartdisease,theframingham.study. AnnInternMed. (1964)61:888–99.doi:10.7326/0003-4819-61-5-888
28.CheungMC,BrownBG,WolfAC,AlbersJJ.Alteredparticlesizedistribution ofapolipoproteinA-I-containinglipoproteinsinsubjectswithcoronaryartery disease. JLipidRes. (1991)32:383–94.doi:10.1016/S0022-2275(20)42061-9
29.RobertJ,OstoE,vonEckardsteinA.Theendotheliumisboth atargetandabarrierofHDL’sprotectivefunctions. Cells.(2021) 10:1041.doi:10.3390/cells10051041
30.AsztalosBF,CupplesLA,DemissieS,HorvathKV,CoxCE,BatistaMC, etal.High-densitylipoproteinsubpopulationprofileandcoronaryheartdisease prevalenceinmaleparticipantsoftheFraminghamOffspringStudy. Arterioscler ThrombVascBiol. (2004)24:2181–7.doi:10.1161/01.ATV.0000146325.93749.a8
31.SmithJD,BreslowJL.Theemergenceofmousemodelsofatherosclerosis andtheirrelevancetoclinicalresearch. JInternMed. (1997)242:99–109.doi:10.1046/j.1365-2796.1997.00197.x
32.CalabresiL,GomaraschiM,FranceschiniG.Endothelialprotectionbyhighdensitylipoproteins:frombenchtobedside. ArteriosclerThrombVascBiol. (2003) 23:1724–31.doi:10.1161/01.ATV.0000094961.74697.54
33.InazuA,BrownML,HeslerCB,AgellonLB,KoizumiJ,TakataK, etal.Increasedhigh-densitylipoproteinlevelscausedbyacommoncholesterylestertransferproteingenemutation. NEnglJMed. (1990)323:1234–8.doi:10.1056/NEJM199011013231803
34.BarterPJ,BrewerHB.Jr,ChapmanMJ,HennekensCH,RaderDJ, TallARCholesterylestertransferprotein:anoveltargetforraisingHDL andinhibitingatherosclerosisArterioscler. ThrombVascBiol. (2003)23:160–7.doi:10.1161/01.ATV.0000054658.91146.64
35.BarterPJ,CaulfieldM,ErikssonM,GrundySM,KasteleinJJ,KomajdaM, etal.Effectsoftorcetrapibinpatientsathighriskforcoronaryevents. NEnglJ Med. (2007)357:2109–22.doi:10.1056/NEJMoa0706628
36.FurtadoJD,RuotoloG,NichollsSJ,DulleaR,Carvajal-GonzalezS,SacksFM. PharmacologicalinhibitionofCETP(CholesterylEsterTransferProtein)increases HDL(High-DensityLipoprotein)thatcontainsApoC3andotherHDLsubspecies associatedwithhigherriskofcoronaryheartdisease. ArteriosclerThrombVascBiol. (2022)42:227–37.doi:10.1161/ATVBAHA.121.317181
37.SchwartzGG,OlssonAG,BarterPJ.Dalcetrapibinpatients withanacutecoronarysyndrome. NEnglJMed. (2013)368:869–70.doi:10.1056/NEJMc1300057
38.TardifJC,RhéaumeE,LemieuxPerreaultLP,GrégoireJC, FerozZadaY,AsselinG,etal.Pharmacogenomicdeterminantsof thecardiovasculareffectsofdalcetrapib. CircCardiovascGenet. (2015) 8:372–82.doi:10.1161/CIRCGENETICS.114.000663
39.MiyaresMA.Anacetrapibanddalcetrapib:twonovelcholesteryl estertransferproteininhibitors. AnnPharmacother. (2011)45:84–94.doi:10.1345/aph.1P446
40.BowmanL,ChenF,SammonsE,HopewellJC,WallendszusK,Stevens W,etal.RandomizedEvaluationoftheEffectsofAnacetrapibthroughLipidmodification(REVEAL)-Alarge-scale,randomized,placebo-controlledtrialofthe clinicaleffectsofanacetrapibamongpeoplewithestablishedvasculardisease:Trial design,recruitment,andbaselinecharacteristics. AmHeartJ. (2017)187:182–90.doi:10.1016/j.ahj.2017.02.021
41.GroupTHT-RC,CommitteeW,SammonsE,HopewellJC,ChenF, StevensW,etal.Long-termsafetyandefficacyofanacetrapibinpatientswith atheroscleroticvasculardisease. EurHeartJ. (2021)43:1416–24.
42.KrishnaR,GheyasF,LiuY,HagenDR,WalkerB,ChawlaA,etal.Chronic administrationofanacetrapibisassociatedwithaccumulationinadiposeandslow elimination. ClinPharmacolTher. (2017)102:832–40.doi:10.1002/cpt.700
43.PalmerTM,LawlorDA,HarbordRM,SheehanNA,TobiasJH,TimpsonNJ, etal.Usingmultiplegeneticvariantsasinstrumentalvariablesformodifiablerisk factors. StatMethodsMedRes. (2012)21:223–42.doi:10.1177/0962280210394459
44.Agerholm-LarsenB,Tybjaerg-HansenA,SchnohrP,SteffensenR, NordestgaardBG.Commoncholesterylestertransferproteinmutations, decreasedHDLcholesterol,andpossibledecreasedriskofischemicheart disease:TheCopenhagenCityHeartStudy. Circulation. (2000)102:2197–203.doi:10.1161/01.CIR.102.18.2197
45.VergeerM,KorporaalSJ,FranssenR,MeursI,OutR,HovinghGK,etal. GeneticvariantofthescavengerreceptorBIinhumans. NEnglJMed. (2011) 364:136–45.doi:10.1056/NEJMoa0907687
46.ZanoniP,KhetarpalSA,LarachDB,Hancock-CeruttiWF,MillarJS, CuchelM,etal.RarevariantinscavengerreceptorBIraisesHDLcholesterol andincreasesriskofcoronaryheartdisease. Science. (2016)351:1166–71.doi:10.1126/science.aad3517
47.ArnettDK,BlumenthalRS,AlbertMA,BurokerAB,GoldbergerZD, HahnEJ,etal.(2019).ACC/AHAguidelineontheprimarypreventionof cardiovasculardisease:executivesummary:areportoftheamericancollegeof cardiology/americanheartassociationtaskforceonclinicalpracticeguidelines. J AmCollCardiol. (2019)74:1376–414.
48.ConroyRM,PyöräläK,FitzgeraldAP,SansS,MenottiA,DeBackerG,etal. Estimationoften-yearriskoffatalcardiovasculardiseaseinEurope:theSCORE project. EurHeartJ. (2003)24:987–1003.doi:10.1016/S0195-668X(03)00114-3
49.KimHJ,ParkHA,ChoYG,KangJH,KimKW,KangJH,etal.Gender differenceinthelevelofHDLcholesterolinKoreanadults. KoreanJFamMed. (2011)32:173–81.doi:10.4082/kjfm.2011.32.3.173
50.KlingelSL,RokeK,HidalgoB,AslibekyanS,StrakaRJ,AnP,etal.Sex differencesinbloodHDL-c,thetotalcholesterol/HDL-cratio,andpalmitoleic acidarenotassociatedwithvariantsincommoncandidategenes. Lipids. (2017) 52:969–80.doi:10.1007/s11745-017-4307-5
51.YiS-W,ParkH-B,JungM-H,YiJ-J,OhrrH.High-densitylipoprotein cholesterolandcardiovascularmortality:aprospectivecohortstudyamong15.8 millionadults. EurJPrevCardiol. (2021)29:844–54.doi:10.1093/eurjpc/zwab230
52.BagatellCJ,BremnerWJ.Androgenandprogestageneffectsonplasmalipids. ProgCardiovascDis. (1995)38:255–71.doi:10.1016/S0033-0620(95)80016-6
53.JomardA,LiberaleL,DoytchevaP,ReinerMF,MüllerD,VisentinM,etal. EffectsofacuteadministrationoftrimethylamineN-oxideonendothelialfunction: atranslationalstudy. SciRep. (2022)12:8664.doi:10.1038/s41598-022-12720-5
54.TzeraviniE,TentolourisA,EleftheriadouI,ChaviarasN,Kolovou G,Apostolidou-KioutiF,etal.Comparisonofpostprandialserum triglycerideandapolipoproteinBconcentrationsbetweenthetwophases ofmenstrualcycleinhealthywomen. CurrVascPharmacol. (2021) 19:411–22.doi:10.2174/1573406416666200611105113
55.VashishtaS,GahlotS,GoyalR.Effectofmenstrualcyclephasesonplasma lipidandlipoproteinlevelsinregularlymenstruatingwomen. JClinDiagnRes. (2017)11:Cc05–cc7.doi:10.7860/JCDR/2017/26031.9799
56.López-SimónL,deOyaM,LasunciónMA,RiestraP,BenaventeM, deOyaI,etal.GeneticdeterminantsofplasmaHDL-cholesterollevelsin prepubertalchildren. ClinChimActa. (2009)403:203–6.doi:10.1016/j.cca.2009. 03.002
57.ChoKH,KimJR.RapiddecreaseinHDL-Cinthepubertyperiod ofboysassociatedwithanelevationofbloodpressureanddyslipidemiain Koreanteenagers:anexplanationofwhyandwhenmenhavelowerHDLClevelsthanwomen. MedSci(Basel). (2021)9:35.doi:10.3390/medsci90 20035
58.InarajaV,ThuissardI,Andreu-VazquezC,JodarE.Lipid profilechangesduringthemenopausaltransition. Menopause. (2020) 27:780–7.doi:10.1097/GME.0000000000001532
59.CollinsP,HDL-C.inpost-menopausalwomen:animportanttherapeutic target. IntJCardiol. (2008)124:275–82.doi:10.1016/j.ijcard.2007.06.009
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 13 frontiersin.org 95
60.Jacobson TA,ItoMK,MakiKC,OrringerCE,BaysHE,JonesPH,etal. NationalLipid Associationrecommendationsforpatient-centeredmanagement ofdyslipidemia:part1-executivesummary. JClinLipidol. (2014)8:473–88.doi:10.1016/j.jacl.2014.07.007
61.TrimarcoV,IzzoR,MoriscoC,MoneP,MariaVirginiaM,FalcoA,etal. HighHDL(high-densitylipoprotein)cholesterolincreasescardiovascularrisk inhypertensivepatients. Hypertension.2022:101161hypertensionaha12219912. doi:10.1161/HYPERTENSIONAHA.122.19912
62.WilkinsJT,NingH,StoneNJ,CriquiMH,ZhaoL,GreenlandP,etal. CoronaryheartdiseaserisksassociatedwithhighlevelsofHDLcholesterol. JAm HeartAssoc. (2014)3:e000519.doi:10.1161/JAHA.113.000519
63.PaunioM,HeinonenOP,VirtamoJ,KlagMJ,ManninenV,AlbanesD,etal. HDLcholesterolandmortalityinFinnishmenwithspecialreferencetoalcohol intake. Circulation. (1994)90:2909–18.doi:10.1161/01.CIR.90.6.2909
64.RachedFH,ChapmanMJ,KontushA.HDLparticlesubpopulations:focus onbiologicalfunction. Biofactors. (2015)41:67–77.doi:10.1002/biof.1202
65.ZannisVI,FotakisP,KoukosG,KardassisD,EhnholmC,JauhiainenM, etal.HDLbiogenesis,remodeling,andcatabolism. HandbExpPharmacol. (2015) 224:53–111.doi:10.1007/978-3-319-09665-0_2
66.ParksJS,ChungSFau-ShelnessGS,ShelnessGS.HepaticABC transportersandtriglyceridemetabolism. CurrOpinLipidol.(2012)23:14736535.doi:10.1097/MOL.0b013e328352dd1a
67.NorumKR,RemaleyAT,MiettinenHE,StrømEH,BalboBEP,SampaioC, etal.Lecithin:cholesterolacyltransferase:symposiumon50yearsofbiomedical researchfromitsdiscoverytolatestfindings. JLipidRes. (2020).61:15397262.doi:10.1194/jlr.S120000720
68.DuongM,PsaltisM,RaderDJ,MarchadierD,BarterPJ,RyeK-A. Evidencethathepaticlipaseandendotheliallipasehavedifferentsubstrate specificitiesforhigh-densitylipoproteinphospholipids. Biochemistry. (2003) 42:13778–85.doi:10.1021/bi034990n
69.AlbersJJ,VuleticS,CheungMC.Roleofplasmaphospholipidtransfer proteininlipidandlipoproteinmetabolism. BiochimBiophysActaMolCellBiol Lipids. (2012)1821:345–57.doi:10.1016/j.bbalip.2011.06.013
70.SchaeferEJ,AnthanontP,AsztalosBF.High-densitylipoproteinmetabolism, composition,function,anddeficiency. CurrOpinLipidol. (2014)25:194–9.doi:10.1097/MOL.0000000000000074
71.Lamon-FavaS,MicheroneD.RegulationofapoA-Igeneexpression: mechanismofactionofestrogenandgenistein. JLipidRes. (2004)45:106–12.doi:10.1194/jlr.M300179-JLR200
72.LopezD,SanchezMD,Shea-EatonW,McLeanMP.Estrogenactivatesthe high-densitylipoproteinreceptorgeneviabindingtoestrogenresponseelements andinteractionwithsterolregulatoryelementbindingprotein-1A. Endocrinology. (2002)143:2155–68.doi:10.1210/endo.143.6.8855
73.JonesDR,SchmidtRJ,PickardRT,FoxworthyPS,EachoPI.Estrogen receptor-mediatedrepressionofhumanhepaticlipasegenetranscription. JLipid Res. (2002)43:383–91.doi:10.1016/S0022-2275(20)30144-9
74.HartgensF,RietjensG,KeizerHA,KuipersH,WolffenbuttelBH.Effectsof androgenic-anabolicsteroidsonapolipoproteinsandlipoprotein(a). BrJSports Med. (2004)38:253–9.doi:10.1136/bjsm.2003.000199
75.GoldbergRB,RabinD,AlexanderAN,DoelleGC,GetzGS.Suppression ofplasmatestosteroneleadstoanincreaseinserumtotalandhighdensity lipoproteincholesterolandapoproteinsA-IandB. JClinEndocrinolMetab. (1985) 60:203–7.doi:10.1210/jcem-60-1-203
76.WekkerV,vanDammenL,KoningA,HeidaKY,PainterRC,Limpens J,etal.Long-termcardiometabolicdiseaseriskinwomenwithPCOS:a systematicreviewandmeta-analysis. HumReprodUpdate. (2020)26:942–60.doi:10.1093/humupd/dmaa029
77.SpałkowskaM,MrozinskaS,Gałuszka-BednarczykA,GosztyłaK, PrzywaraA,GuzikJ,etal.ThePCOSpatientsdifferinlipidprofile accordingtotheirphenotypes. ExpClinEndocrinolDiabetes. (2018)126:437–44.doi:10.1055/s-0043-121264
78.HallE,VolkovP,DayehT,EsguerraJL,SalöS,EliassonL,etal.Sexdifferences inthegenome-wideDNAmethylationpatternandimpactongeneexpression, microRNAlevelsandinsulinsecretioninhumanpancreaticislets. GenomeBiol. (2014)15:522.doi:10.1186/s13059-014-0522-z
79.MayneBT,Bianco-MiottoT,BuckberryS,BreenJ,CliftonV, ShoubridgeC,etal.Largescalegeneexpressionmeta-analysisreveals tissue-specific,sex-biasedgeneexpressioninhumans. FrontGenet. (2016) 7:183.doi:10.3389/fgene.2016.00183
80.RussoGT,HorvathKV,DiBenedettoA,GiandaliaA,CucinottaD, AsztalosB.Influenceofmenopauseandcholesterylestertransferprotein(CETP) TaqIBpolymorphismonlipidprofileandHDLsubpopulationsdistribution
inwomenwithandwithouttype2diabetes. Atherosclerosis. (2010)210:294–301.doi:10.1016/j.atherosclerosis.2009.11.011
81.Lamon-FavaS,AsztalosBF,HowardTD,ReboussinDM,Horvath KV,SchaeferEJ,etal.Associationofpolymorphismsingenesinvolvedin lipoproteinmetabolismwithplasmaconcentrationsofremnantlipoproteins andHDLsubpopulationsbeforeandafterhormonetherapyinpostmenopausal women. ClinEndocrinol(Oxf). (2010)72:169–75.doi:10.1111/j.1365-2265.2009. 03644.x
82.BoekholdtSM,SacksFM,JukemaJW,ShepherdJ,FreemanDJ,McMahon AD,etal.CholesterylestertransferproteinTaqIBvariant,high-densitylipoprotein cholesterollevels,cardiovascularrisk,andefficacyofpravastatintreatment: individualpatientmeta-analysisof13,677subjects. Circulation. (2005)111:278–87.doi:10.1161/01.CIR.0000153341.46271.40
83.HeilbronnLK,NoakesM,CliftonPM.AssociationbetweenHDL-cholesterol andtheTaq1Bpolymorphisminthecholesterolestertransferproteingenein obesewomen. Atherosclerosis. (2002)162:419–24.doi:10.1016/S0021-9150(01) 00733-X
84.RaderDJ.MolecularregulationofHDLmetabolism andfunction:implicationsfornoveltherapies. J ClinInvest. (2006)116:3090–100.doi:10.1172/JCI 30163
85.ReillyM,RaderDJ.ApolipoproteinEandcoronarydisease:apuzzling paradox. PLoSMed. (2006)3:e258.doi:10.1371/journal.pmed.0030258
86.MosherMJ,LangeLA,HowardBV,LeeET,BestLG,FabsitzRR,etal. Sex-specificinteractionbetweenAPOEgenotypeandcarbohydrateintakeaffects plasmaHDL-Clevels:theStrongHeartFamilyStudy. GenesNutr. (2008)3:87–97.doi:10.1007/s12263-008-0075-4
87.ZokaeiN,GiehlK,SillenceA,NevilleMJ,KarpeF,NobreAC,etal.Sexand APOE:AmemoryadvantageinmaleAPOE ε4carriersinmidlife. Cortex. (2017) 88:98–105.doi:10.1016/j.cortex.2016.12.016
88.AltmannA,TianL,HendersonVW,GreiciusMD.Sexmodifiesthe APOE-relatedriskofdevelopingAlzheimerdisease. AnnNeurol. (2014)75:563–73.doi:10.1002/ana.24135
89.TanakaS,YasudaT,IshidaT,FujiokaY,TsujinoT,MikiT,etal.Increased serumcholesterolesterificationratespredictcoronaryheartdiseaseandsudden deathinageneralpopulation. ArteriosclerThrombVascBiol. (2013)33:1098–104.doi:10.1161/ATVBAHA.113.301297
90.HolleboomAG,KuivenhovenJA,VergeerM,HovinghGK,vanMiertJN, WarehamNJ,etal.Plasmalevelsoflecithin:cholesterolacyltransferaseandriskof futurecoronaryarterydiseaseinapparentlyhealthymenandwomen:aprospective case-controlanalysisnestedintheEPIC-Norfolkpopulationstudy. JLipidRes. (2010)51:416–21.doi:10.1194/P900038-JLR200
91.EmokpaeMA,UwumarongieOH,OsadolorHB.Sexdimorphisminserum lecithin:cholesterolacyltransferaseandlipoproteinlipaseactivitiesinadultsickle cellanaemiapatientswithproteinuria. IndianJClinBiochem. (2011)26:57–61.doi:10.1007/s12291-010-0096-9
92.GuoM,LiuZ,XuY,MaP,HuangW,GaoM,etal.Spontaneous atherosclerosisinagedLCAT-deficienthamsterswithenhanced oxidativestress-briefreport. ArteriosclerThrombVascBiol. (2020) 40:2829–36.doi:10.1161/ATVBAHA.120.315265
93.LiL,HossainMA,SadatS,HagerL,LiuL,TamL,etal.Lecithincholesterol acyltransferasenullmiceareprotectedfromdiet-inducedobesityandinsulin resistanceinagender-specificmannerthroughmultiplepathways. JBiolChem. (2011)286:17809–20.doi:10.1074/jbc.M110.180893
94.KhovidhunkitWKMM,MemonRR,ShigenagaJK,MoserAH,Feingold KR,GrunfeldC.Effectsofinfectionandinflammationonlipidandlipoprotein metabolism:mechanismsandconsequencestothehost. JLipidRes.(2004) 45:1169–96.doi:10.1194/jlr.R300019-JLR200
95.MemonRA,HolleranWM,MoserAH,SekiT,UchidaY,FullerJ,etal. Endotoxinandcytokinesincreasehepaticsphingolipidbiosynthesisandproduce lipoproteinsenrichedinceramidesandsphingomyelin. ArteriosclerThrombVasc Biol. (1998)18:1257–65.doi:10.1161/01.ATV.18.8.1257
96.PrasadDS,KabirZ,DashAK,DasBC.Abdominalobesity,an independentcardiovascularriskfactorinIndiansubcontinent:aclinico epidemiologicalevidencesummary. JCardiovascDisRes. (2011)2:199–205.doi:10.4103/0975-3583.89803
97.SelvarajS,MartinezEE,AguilarFG,KimKY,PengJ,ShaJ,etal. Associationofcentraladipositywithadversecardiacmechanics:findingsfromthe hypertensiongeneticepidemiologynetworkstudy. CircCardiovascImaging.(2016) 9:e004396.doi:10.1161/CIRCIMAGING.115.004396
98.WoudbergNJ,LecourS,GoedeckeJH.HDLsubclass distributionshiftswithincreasingcentraladiposity. JObes. (2019) 2019:2107178.doi:10.1155/2019/2107178
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 14 frontiersin.org 96
99.StadlerJT, LacknerS,MörklS,TrakakiA,ScharnaglH,BorenichA, etal. ObesityaffectsHDLmetabolism,compositionandsubclassdistribution. Biomedicines.(2021)9.doi:10.3390/biomedicines9030242
100.TalbotCPJ,PlatJ,JorisPJ,KoningsM,KustersY,SchalkwijkCG, etal.HDLcholesteroleffluxcapacityandcholesterylestertransferare associatedwithbodymass,butarenotchangedbydiet-inducedweightloss: Arandomizedtrialinabdominallyobesemen. Atherosclerosis. (2018)274:23–8.doi:10.1016/j.atherosclerosis.2018.04.029
101.LorkowskiSW,BrubakerG,RotroffDM,KashyapSR,BhattDL,Nissen SE,etal.BariatricsurgeryimprovesHDLfunctionexaminedbyapoa1exchange rateandcholesteroleffluxcapacityinpatientswithobesityandtype2diabetes. Biomolecules.(2020)10:551.doi:10.3390/biom10040551
102.ThakkarH,VincentV,ShuklaS,SraM,KangaU,AggarwalS, etal.ImprovementsincholesteroleffluxcapacityofHDLandadiponectin contributetomitigationincardiovasculardiseaseriskafterbariatric surgeryinacohortwithmorbidobesity. DiabetolMetabSyndr. (2021) 13:46.doi:10.1186/s13098-021-00662-3
103.OstoE,DoytchevaP,CortevilleC,BueterM,DörigC,StivalaS,etal. Rapidandbodyweight-independentimprovementofendothelialandhigh-density lipoproteinfunctionafterRoux-en-Ygastricbypass:roleofglucagon-likepeptide1. Circulation. (2015)131:871–81.doi:10.1161/CIRCULATIONAHA.114.011791
104.AsztalosBF,SwarbrickMM,SchaeferEJ,DallalGE,HorvathKVAiM,etal. Effectsofweightloss,inducedbygastricbypasssurgery,onHDLremodelingin obesewomen. JLipidRes. (2010)51:2405–12.doi:10.1194/jlr.P900015-JLR200
105.vanVeldhuisenSL,GorterTM,vanWoerdenG,deBoerRA, RienstraM,HazebroekEJ,etal.Bariatricsurgeryandcardiovascular disease:asystematicreviewandmeta-analysis. EurHeartJ. (2022) 43:1955–69.doi:10.1093/eurheartj/ehac071
106.AlyS,HacheyK,PernarLIM.Genderdisparitiesinweightlosssurgery. Mini-invasiveSurgery. (2020)4:21.doi:10.20517/2574-1225.2019.57
107.AminianA,ZeliskoA,KirwanJP,BrethauerSA,SchauerPR.Exploringthe impactofbariatricsurgeryonhighdensitylipoprotein. SurgObesRelatDis. (2015) 11:238–47.doi:10.1016/j.soard.2014.07.017
108.GenuaI,RamosA,CaimariF,BalaguéC,Sánchez-QuesadaJL,PérezA, etal.EffectsofbariatricsurgeryonHDLcholesterol. ObesSurg. (2020)30:1793–8.doi:10.1007/s11695-020-04385-8
109.ArnalJF,FontaineC,Billon-GalésA,FavreJ,LaurellH,LenfantF, etal.Estrogenreceptorsandendothelium. ArteriosclerThrombVascBiol. (2010) 30:1506–12.doi:10.1161/ATVBAHA.109.191221
110.CelermajerDS,SorensenKE,SpiegelhalterDJ,GeorgakopoulosD,Robinson J,DeanfieldJE.Agingisassociatedwithendothelialdysfunctioninhealthymen yearsbeforetheage-relateddeclineinwomen. JAmCollCardiol. (1994)24:471–6.doi:10.1016/0735-1097(94)90305-0
111.SaderMA,CelermajerDS.Endothelialfunction,vascularreactivityand genderdifferencesinthecardiovascularsystem. CardiovascRes. (2002)53:597–604.doi:10.1016/S0008-6363(01)00473-4
112.SimonciniT.Mechanismsofactionofestrogenreceptorsinvascular cells:relevanceformenopauseandaging. Climacteric. (2009)12(Suppl1):6–11.doi:10.1080/13697130902986385
113.MendelsohnME,KarasRH.Molecularandcellularbasisofcardiovascular genderdifferences. Science. (2005)308:1583–7.doi:10.1126/science.1112062
114.CollinsP,RosanoGM,SarrelPM,UlrichL,AdamopoulosS,BealeCM,etal. 17beta-Estradiolattenuatesacetylcholine-inducedcoronaryarterialconstriction inwomenbutnotmenwithcoronaryheartdisease. Circulation. (1995)92:24–30.doi:10.1161/01.CIR.92.1.24
115.RossouwJE,AndersonGL,PrenticeRL,LaCroixAZ,Kooperberg C,StefanickML,etal.Risksandbenefitsofestrogenplusprogestin inhealthypostmenopausalwomen:principalresultsFromthe Women’sHealthInitiativerandomizedcontrolledtrial. JAMA. (2002) 288:321–33.doi:10.1001/jama.288.3.321
116.FredetteNC,MeyerMR,ProssnitzER.RoleofGPERinestrogen-dependent nitricoxideformationandvasodilation. JSteroidBiochemMolBiol. (2018)176:65–72.doi:10.1016/j.jsbmb.2017.05.006
117.TumaM,CanestriniS,AlwahabZ,MarshallJ.Traumaand endothelialglycocalyx:themicrocirculationhelmet? Shock. (2016) 46:352–7.doi:10.1097/SHK.0000000000000635
118.HartmanRJG,KapteijnDMC,HaitjemaS,BekkerMN,MokryM, PasterkampG,etal.Intrinsictranscriptomicsexdifferencesinhumanendothelial cellsatbirthandinadultsareassociatedwithcoronaryarterydiseasetargets. Sci Rep. (2020)10:12367.doi:10.1038/s41598-020-69451-8
119.AddisR,CampesiI,FoisM,CapobiancoG,DessoleS,FenuG,etal. Humanumbilicalendothelialcells(HUVECs)haveasex:characterisation
ofthephenotypeofmaleandfemalecells. BiolSexDiffer. (2014) 5:18.doi:10.1186/s13293-014-0018-2
120.CattaneoMG,BanfiC,BrioschiM,LattuadaD,VicentiniLM. Sex-dependentdifferencesinthesecretomeofhumanendothelial cells. BiolSexDiffer. (2021)12:7.doi:10.1186/s13293-020-0 0350-3
121.Torres-EstayV,CarreñoDV,SanFranciscoIF,SotomayorP,GodoyAS, SmithGJ.Androgenreceptorinhumanendothelialcells. JEndocrinol. (2015) 224:R131–7.doi:10.1530/JOE-14-0611
122.LiuPY,DeathAK,HandelsmanDJ.Androgensandcardiovasculardisease. EndocrRev. (2003)24:313–40.doi:10.1210/er.2003-0005
123.KimKH,BenderJR.Membrane-initiatedactionsofestrogenonthe endothelium. MolCellEndocrinol. (2009)308:3–8.doi:10.1016/j.mce.2009. 03.025
124.DiebelLN,WheatonM,LiberatiDM.Theprotectiveroleofestrogenon endothelialandglycocalyxbarriersaftershockconditions:amicrofluidicstudy. Surgery. (2021)169:678–85.doi:10.1016/j.surg.2020.08.006
125.RodríguezE,LópezR,PaezA,MassóF,MontañoLF.17Beta-estradiol inhibitstheadhesionofleukocytesinTNF-alphastimulatedhumanendothelial cellsbyblockingIL-8andMCP-1secretion,butnotitstranscription. LifeSci. (2002)71:2181–93.doi:10.1016/S0024-3205(02)01999-9
126.Caulin-GlaserT,WatsonCA,PardiR,BenderJR.Effectsof17beta-estradiol oncytokine-inducedendothelialcelladhesionmoleculeexpression. JClinInvest. (1996)98:36–42.doi:10.1172/JCI118774
127.HolmP,AndersenHL,ArrøeG,StenderS.Gendergapinaortic cholesterolaccumulationincholesterol-clampedrabbits:roleoftheendothelium andmononuclear-endothelialcellinteraction. Circulation. (1998)98:2731–7.doi:10.1161/01.CIR.98.24.2731
128.UsselmanCW,YarovinskyTO,SteeleFE,LeoneCA,TaylorHS,Bender JR,etal.Androgensdrivemicrovascularendothelialdysfunctioninwomenwith polycysticovarysyndrome:roleoftheendothelinBreceptor. JPhysiol. (2019) 597:2853–65.doi:10.1113/JP277756
129.LopesRA,NevesKB,CarneiroFS,TostesRC.Testosteroneand vascularfunctioninaging. FrontPhysiol. (2012)3:89.doi:10.3389/fphys.2012. 00089
130.VillablancaA,LubahnD,ShelbyL,LloydK,BartholdS. Susceptibilitytoearlyatherosclerosisinmalemiceismediatedby estrogenreceptorα. ArteriosclerThrombVascBiol. (2004) 24:1055–61.doi:10.1161/01.ATV.0000130467.65290.d4
131.VillablancaAC,TetaliS,AltmanR,NgKF,RutledgeJC.Testosteronederivedestradiolproductionbymaleendotheliumisrobustanddependent onp450aromataseviaestrogenreceptoralpha. Springerplus. (2013) 2:214.doi:10.1186/2193-1801-2-214
132.McCrohonJA,DeathAK,NakhlaS,JessupW,HandelsmanDJ,StanleyKK, etal.Androgenreceptorexpressionisgreaterinmacrophagesfrommalethanfrom femaledonors.Asexdifferencewithimplicationsforatherogenesis. Circulation. (2000)101:224–6.doi:10.1161/01.CIR.101.3.224
133.RemusEW,SayeedI,WonS,LyleAN,SteinDG.Progesterone protectsendothelialcellsaftercerebrovascularocclusionbydecreasingMCP1-andCXCL1-mediatedmacrophageinfiltration. ExpNeurol. (2015)271:401–8.doi:10.1016/j.expneurol.2015.07.010
134.YouY,TanW,GuoY,LuoM,ShangFF,XiaY,etal.Progesterone promotesendothelialnitricoxidesynthaseexpressionthroughenhancingnuclear progesteronereceptor-SP-1formation. AmJPhysiolHeartCircPhysiol. (2020) 319:H341–h8.doi:10.1152/ajpheart.00206.2020
135.GradyD,HerringtonD,BittnerV,BlumenthalR,DavidsonM,HlatkyM, etal.Cardiovasculardiseaseoutcomesduring6.8yearsofhormonetherapy:heart andestrogen/progestinreplacementstudyfollow-up(HERSII). JAMA. (2002) 288:49–57.doi:10.1001/jama.288.1.49
136.JiangY,TianW.Theeffectsofprogesteronesonblood lipidsinhormonereplacementtherapy. LipidsHealthDis. (2017) 16:219.doi:10.1186/s12944-017-0612-5
137.KojimaK,Abe-DohmaeS,ArakawaR,MurakamiI,SuzumoriK,Yokoyama S.Progesteroneinhibitsapolipoprotein-mediatedcellularlipidrelease:aputative mechanismforthedecreaseofhigh-densitylipoprotein. BiochimBiophysActa. (2001)1532:173–84.doi:10.1016/S1388-1981(01)00124-X
138.WangJ,BingamanS,HuxleyVH.Intrinsicsex-specificdifferencesin microvascularendothelialcellphosphodiesterases. AmJPhysiolHeartCircPhysiol. (2010)298:H1146–54.doi:10.1152/ajpheart.00252.2009
139.DuL,BayirH,LaiY,ZhangX,KochanekPM,WatkinsSC,etal.Innate gender-basedproclivityinresponsetocytotoxicityandprogrammedcelldeath pathway. JBiolChem. (2004)279:38563–70.doi:10.1074/jbc.M405461200
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 15 frontiersin.org 97
140.DuL,HickeyRW,BayirH,WatkinsSC,TyurinVA,GuoF,etal. Starvingneuronsshowsexdifferenceinautophagy. JBiolChem. (2009)284:2383–96.doi:10.1074/jbc.M804396200
141.LorenzM,KoschateJ,KaufmannK,KreyeC,MertensM,Kuebler WM,etal.Doescellularsexmatter?Dimorphictranscriptionaldifferences betweenfemaleandmaleendothelialcells. Atherosclerosis. (2015)240:61–72.doi:10.1016/j.atherosclerosis.2015.02.018
142.LorenzM,BlaschkeB,BennA,HammerE,WittE,Kirwan J,etal.Sex-specificmetabolicandfunctionaldifferencesinhuman umbilicalveinendothelialcellsfromtwinpairs. Atherosclerosis. (2019) 291:99–106.doi:10.1016/j.atherosclerosis.2019.10.007
143.McDonaldG,CabalN,VannierA,UmikerB,YinRH,OrjaloAV, etal.Femalebiasinsystemiclupuserythematosusisassociatedwiththe differentialexpressionofX-linkedtoll-likereceptor8. FrontImmunol. (2015) 6:457.doi:10.3389/fimmu.2015.00457
144.KhanD,AnsarAhmedS.Theimmunesystemisanaturaltargetforestrogen action:opposingeffectsofestrogenintwoprototypicalautoimmunediseases. Front Immunol. (2015)6:635.doi:10.3389/fimmu.2015.00635
145.KeselmanA,HellerN.Estrogensignalingmodulatesallergicinflammation andcontributestosexdifferencesinasthma. FrontImmunol. (2015) 6:568.doi:10.3389/fimmu.2015.00568
146.MessnerB,BernhardD.Smokingandcardiovasculardisease:mechanisms ofendothelialdysfunctionandearlyatherogenesis. ArteriosclerThrombVascBiol. (2014)34:509–15.doi:10.1161/ATVBAHA.113.300156
147.HeBM,ZhaoSP,PengZY.EffectsofcigarettesmokingonHDLquantity andfunction:implicationsforatherosclerosis. JCellBiochem. (2013)114:2431–6.doi:10.1002/jcb.24581
148.KaussAR,AntunesM,deLaBourdonnayeG,PoulyS,HankinsM, HeremansA,etal.Smokingandapolipoproteinlevels:ameta-analysisofpublished data. ToxicologyReports. (2022)9:1150–71.doi:10.1016/j.toxrep.2022.05.009
149.NjølstadI,ArnesenE,Lund-LarsenPG.Smoking,serumlipids,blood pressure,andsexdifferencesinmyocardialinfarction.A12-yearfollow-upofthe FinnmarkStudy.Circulation. (1996)93:450–6.doi:10.1161/01.CIR.93.3.450
150.BolegoC,PoliA,PaolettiR.Smokingandgender. CardiovascRes. (2002) 53:568–76.doi:10.1016/S0008-6363(01)00520-X
151.BaronJA,LaVecchiaC,LeviF.Theantiestrogeniceffect ofcigarettesmokinginwomen. AmJObstetGynecol. (1990) 162:502–14.doi:10.1016/0002-9378(90)90420-C
152.Le-HaC,BeilinLJ,BurrowsS,HuangR-C,OddyWH,HandsB, etal.Genderdifferenceintherelationshipbetweenpassivesmokingexposure andHDL-cholesterollevelsinlateadolescence. JClinEndocr. (2013)98:2126–35.doi:10.1210/jc.2013-1016
153.CriquiMH,WallaceRB,HeissG,MishkelM,SchonfeldG,JonesGT. Cigarettesmokingandplasmahigh-densitylipoproteincholesterol.Thelipid researchclinicsprogramprevalencestudy. Circulation. (1980)62:Iv70–6.
154.ForeyBA,FryJS,LeePN,ThorntonAJ,CoombsKJ.Theeffectofquitting smokingonHDL-cholesterol-areviewbasedonwithin-subjectchanges. Biomark Res. (2013)1:26.doi:10.1186/2050-7771-1-26
155.NyboeJ,JensenG,AppleyardM,SchnohrP.Smokingandthe riskoffirstacutemyocardialinfarction. AmHeartJ. (1991)122:438–47.doi:10.1016/0002-8703(91)90997-V
156.ShaoY,LuoL,RenZ,GuoJ,XiaoX,HuangJ,etal.Smoking-induced inhibitionofnumberandactivityofendothelialprogenitorcellsandnitricoxide inmaleswerereversedbyestradiolinpremenopausalfemales. CardiolResPract. (2020)2020:9352518.doi:10.1155/2020/9352518
157.PianoMR,ThurLA,HwangCL,PhillipsSA.Effectsof alcoholonthecardiovascularsysteminwomen. AlcoholRes. (2020) 40:12.doi:10.35946/arcr.v40.2.12
158.Marques-VidalP,BochudM,PaccaudF,WaterworthD,Bergmann S,PreisigM,etal.NointeractionbetweenalcoholconsumptionandHDLrelatedgenesonHDLcholesterollevels. Atherosclerosis. (2010)211:551–7.doi:10.1016/j.atherosclerosis.2010.04.001
159.FuchsFD,ChamblessLE,FolsomAR,EigenbrodtML,DuncanBB,Gilbert A,etal.Associationbetweenalcoholicbeverageconsumptionandincidence ofcoronaryheartdiseaseinwhitesandblacks:theAtherosclerosisRiskin CommunitiesStudy. AmJEpidemiol. (2004)160:466–74.doi:10.1093/aje/kwh229
160.VolcikK,BallantyneCM,PownallHJ,SharrettAR,Boerwinkle E.Interactioneffectsofhigh-densitylipoproteinmetabolismgene variationandalcoholconsumptiononcoronaryheartdiseaserisk:the atherosclerosisriskincommunitiesstudy. JStudAlcoholDrugs. (2007) 68:485–92.doi:10.15288/jsad.2007.68.485
161.HataY,NakajimaK.Life-styleandserumlipidsandlipoproteins. J AtherosclerThromb. (2000)7:177–97.doi:10.5551/jat1994.7.177
162.HannukselaM,MarcelYL,KesäniemiYA,SavolainenMJ.Reductioninthe concentrationandactivityofplasmacholesterylestertransferproteinbyalcohol. J LipidRes. (1992)33:737–44.doi:10.1016/S0022-2275(20)41437-3
163.WilliamsPT.Quantile-dependentexpressivityandgene-lifestyle interactionsinvolvinghigh-densitylipoproteincholesterol. LifestyleGenom. (2021)14:1–19.doi:10.1159/000511421
164.HinesLM,HunterDJ,StampferMJ,SpiegelmanD,ChuNF,RifaiN, etal.Alcoholconsumptionandhigh-densitylipoproteinlevels:theeffectof ADH1Cgenotype,genderandmenopausalstatus. Atherosclerosis. (2005)182:293–300.doi:10.1016/j.atherosclerosis.2005.02.005
165.HinesLM,StampferMJ,MaJ,GazianoJM,RidkerPM,HankinsonSE, etal.Geneticvariationinalcoholdehydrogenaseandthebeneficialeffectof moderatealcoholconsumptiononmyocardialinfarction. NEnglJMed. (2001) 344:549–55.doi:10.1056/NEJM200102223440802
166.GreavesL,PooleN,BrabeteAC.Sex,gender,andalcohol use:implicationsforwomenandlow-riskdrinkingguidelines. Int JEnvironResPublicHealth.(2022)19:4523.doi:10.3390/ijerph190 84523
167.WilliamsGD,GrantBF,HarfordTC,NobleJ.Populationprojectionsusing DSM-IIIcriteria:alcoholabuseanddependence,190-2000. AlcoholResHealth (1989)1989:366.
168.ZhengYL,LianF,ShiQ,ZhangC,ChenYW,ZhouYH,etal.Alcoholintake andassociatedriskofmajorcardiovascularoutcomesinwomencomparedwith men:asystematicreviewandmeta-analysisofprospectiveobservationalstudies. BMCPublicHealth. (2015)15:773.doi:10.1186/s12889-015-2081-y
169.RajendranNK,LiuW,ChuCC,CahillPA,RedmondEM.Moderate dosealcoholprotectsagainstserumamyloidproteinA1-inducedendothelial dysfunctionviabothnotch-dependentandnotch-independentpathways. Alcohol ClinExpRes. (2021)45:2217–30.doi:10.1111/acer.14706
170.BadíaE,SacanellaE,Fernández-SoláJ,NicolásJM,AntúnezE,RotilioD, etal.Decreasedtumornecrosisfactor-inducedadhesionofhumanmonocytes toendothelialcellsaftermoderatealcoholconsumption. AmJClinNutr. (2004) 80:225–30.doi:10.1093/ajcn/80.1.225
171.TanakaA,CuiR,KitamuraA,LiuK,ImanoH,YamagishiK,etal.Heavy alcoholconsumptionisassociatedwithimpairedendothelialfunction. JAtheroscler Thromb. (2016)23:1047–54.doi:10.5551/jat.31641
172.SacanellaE,EstruchR,BadíaE,Fernández-SolaJ,NicolásJM,UrbanoMárquezA.Chronicalcoholconsumptionincreasesserumlevelsofcirculating endothelialcell/leucocyteadhesionmoleculesE-selectinandICAM-1. Alcohol Alcohol. (1999)34:678–84.doi:10.1093/alcalc/34.5.678
173.BédardA,RiverinM,DodinS,CorneauL,LemieuxS.Sexdifferencesinthe impactoftheMediterraneandietoncardiovascularriskprofile. BrJNutr. (2012) 108:1428–34.doi:10.1017/S0007114511006969
174.BédardA,CorneauL,LamarcheB,DodinS,LemieuxS.Sexdifferences intheimpactofthemediterraneandietonLDLparticlesizedistributionand oxidation. Nutrients. (2015)7:3705–23.doi:10.3390/nu7053705
175.TurnerKM,KeoghJB,CliftonPM.Redmeat,dairy,andinsulinsensitivity: arandomizedcrossoverinterventionstudy. AmJClinNutr. (2015)101:1173–9.doi:10.3945/ajcn.114.104976
176.BrennanL,GibbonsH.Sexmatters:afocusontheimpactofbiological sexonmetabolomicprofilesanddietaryinterventions. ProcNutrSoc. (2020) 79:205–9.doi:10.1017/S002966511900106X
177.ZhangY,KleinK,SugathanA,NasseryN,DombkowskiA,ZangerUM, etal.Transcriptionalprofilingofhumanliveridentifiessex-biasedgenesassociated withpolygenicdyslipidemiaandcoronaryarterydisease. PLoSONE. (2011) 6:e23506.doi:10.1371/journal.pone.0023506
178.DarabiM,RabbaniM,AniM,ZareanE,Panjehpour M,MovahedianA.IncreasedleukocyteABCA1geneexpression inpost-menopausalwomenonhormonereplacementtherapy. GynecolEndocrinol. (2011)27:701–5.doi:10.3109/09513590.2010.5 07826
179.ShinohataR,ShibakuraM,AraoY,WatanabeS,HirohataS,UsuiS, etal.high-fat/high-cholesteroldiet,butnothigh-cholesterolalone,increasesfree cholesterolandapoE-richHDLserumlevelsinratsandupregulateshepatic ABCA1expression. Biochimie. (2022)197:49–58.doi:10.1016/j.biochi.2022.01.011
180.MauererR,EbertS,LangmannT.Highglucose,unsaturatedandsaturated fattyacidsdifferentiallyregulateexpressionofATP-bindingcassettetransporters ABCA1andABCG1inhumanmacrophages. ExpMolMed. (2009)41:126–32.doi:10.3858/emm.2009.41.2.015
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 16 frontiersin.org 98
181.SingarajaRR,JamesER,CrimJ,VisscherH,ChatterjeeA,HaydenMR. Alternatetranscriptsexpressedinresponsetodietreflecttissue-specificregulation ofABCA1. JLipidRes. (2005)46:2061–71.doi:10.1194/jlr.M500133-JLR200
182.Villarreal-MolinaMT,Aguilar-SalinasCA,Rodríguez-CruzM,Riaño D,Villalobos-ComparanM,Coral-VazquezR,etal.TheATP-bindingcassette transporterA1R230CvariantaffectsHDLcholesterollevelsandBMIinthe Mexicanpopulation:associationwithobesityandobesity-relatedcomorbidities. Diabetes. (2007)56:1881–7.doi:10.2337/db06-0905
183.Jacobo-AlbaveraL,Posadas-RomeroC,Vargas-AlarcónG,RomeroHidalgoS,Posadas-SánchezR,González-SalazarMdelC,etal.Dietaryfatand carbohydratemodulatetheeffectoftheATP-bindingcassetteA1(ABCA1) R230Cvariantonmetabolicriskparametersinpremenopausalwomenfromthe GeneticsofAtheroscleroticDisease(GEA)Study. NutrMetab(Lond). (2015) 12:45.doi:10.1186/s12986-015-0040-3
184.SchochL,SutelmanP,SuadesR,CasaniL,PadroT,BadimonL,etal. Hypercholesterolemia-inducedHDLdysfunctioncanbereversed:theimpactof dietandstatintreatmentinapreclinicalanimalmodel. IntJMolSci. (2022) 23:8596.doi:10.3390/ijms23158596
185.VogelRA,CorrettiMC,PlotnickGD.Effectofasinglehigh-fatmeal onendothelialfunctioninhealthysubjects. AmJCardiol. (1997)79:350–4.doi:10.1016/S0002-9149(96)00760-6
186.OngPJ,DeanTS,HaywardCS,DellaMonicaPL,SandersTA,CollinsP. Effectoffatandcarbohydrateconsumptiononendothelialfunction. Lancet. (1999) 354:2134.doi:10.1016/S0140-6736(99)03374-7
187.Pérez-JiménezF,CastroP,López-MirandaJ,Paz-RojasE,Blanco A,López-SeguraF,etal.Circulatinglevelsofendothelialfunctionare modulatedbydietarymonounsaturatedfat. Atherosclerosis. (1999)145:351–
8.doi:10.1016/S0021-9150(99)00116-1
188.FuentesF,López-MirandaJ,SánchezE,SánchezF,PaezJ,PazRojasE,etal.Mediterraneanandlow-fatdietsimproveendothelial functioninhypercholesterolemicmen. AnnInternMed. (2001) 134:1115–9.doi:10.7326/0003-4819-134-12-200106190-00011
189.CuevasAM,GermainAM.Dietandendothelialfunction. BiolRes. (2004) 37:225–30.doi:10.4067/S0716-97602004000200008
190.MataP,AlonsoR,Lopez-FarreA,OrdovasJM,LahozC,GarcesC,etal. EffectofdietaryfatsaturationonLDLoxidationandmonocyteadhesionto humanendothelialcellsinvitro. ArteriosclerThrombVascBiol. (1996)16:1347–55.doi:10.1161/01.ATV.16.11.1347
191.ShahR,MakaremN,EminM,LiaoM,JelicS,AggarwalB.Mediterranean dietcomponentsarelinkedtogreaterendothelialfunctionandlowerinflammation inapilotstudyofethnicallydiversewomen. NutrRes. (2020)75:77–84.doi:10.1016/j.nutres.2020.01.004
192.YusufS,JosephP,RangarajanS,IslamS,MenteA,HystadP,etal.Modifiable riskfactors,cardiovasculardisease,andmortalityin155722individualsfrom21 high-income,middle-income,andlow-incomecountries(PURE):aprospective cohortstudy. Lancet. (2020)395:795–808.doi:10.1016/S0140-6736(19)32008-2
193.GambardellaJ,MorelliMB,WangXJ,SantulliG.Pathophysiological mechanismsunderlyingthebeneficialeffectsofphysicalactivityinhypertension. J ClinHypertens(Greenwich). (2020)22:291–5.doi:10.1111/jch.13804
194.AlevizosA,LentzasJ,KokkorisS,MariolisA,Korantzopoulos P.Physicalactivityandstrokerisk. IntJClinPract. (2005)59:922–30.doi:10.1111/j.1742-1241.2005.00536.x
195.MadsenTE,SamaeiM,PikulaA,YuAYX,CarcelC,MillsapsE,etal.Sex differencesinphysicalactivityandincidentstroke:asystematicreview. ClinTher. (2022)44:586–611.doi:10.1016/j.clinthera.2022.02.006
196.KielyDK,WolfPA,CupplesLA,BeiserAS,KannelWB.Physicalactivity andstrokerisk:theFraminghamStudy. AmJEpidemiol. (1994)140:608–20.doi:10.1093/oxfordjournals.aje.a117298
197.SuperkoHR,HaskellWH.Theroleofexercisetrainingin thetherapyofhyperlipoproteinemia. CardiolClin. (1987)5:285–310.doi:10.1016/S0733-8651(18)30552-6
198.KokkinosPF,FernhallB.Physicalactivityandhighdensitylipoprotein cholesterollevels:whatistherelationship? SportsMed. (1999)28:307–14.doi:10.2165/00007256-199928050-00002
199.DrygasW,JeglerA,KunskiH.Studyonthresholddoseofphysical activityincoronaryheartdiseaseprevention.PartIrelationshipbetweenleisure timephysicalactivityandcoronaryriskfactors. IntJSportsMed. (1988)9:275–8.doi:10.1055/s-2007-1025021
200.KokkinosPF,HollandJC,NarayanP,ColleranJA,DotsonCO, PapademetriouV.Milesrunperweekandhigh-densitylipoproteincholesterol levelsinhealthy,middle-agedmen.Adose-responserelationship. ArchInternMed. (1995)155:415–20.doi:10.1001/archinte.1995.00430040091011
201.DurstineJL,HaskellWL.Effectsofexercisetrainingon plasmalipidsandlipoproteins. ExercSportSciRev. (1994)22:477–521.doi:10.1249/00003677-199401000-00017
202.RohrerL,OhnsorgPascaleM,LehnerM,LandoltF,RinningerF,von EckardsteinA.High-densitylipoproteintransportthroughaorticendothelialcells involvesscavengerreceptorBIandATP-bindingcassettetransporterG1. CircRes. (2009)104:1142–50.doi:10.1161/CIRCRESAHA.108.190587
203.HassanHH,DenisM,KrimbouL,MarcilM,GenestJ.Cellularcholesterol homeostasisinvascularendothelialcells. CanJCardiol.(2006)22(Suppl B):35b 40b.doi:10.1016/S0828-282X(06)70985-0
204.O’ConnellBrianJ,DenisM,GenestJ.Cellularphysiology ofcholesteroleffluxinvascularendothelialcells. Circulation. (2004) 110:2881–8.doi:10.1161/01.CIR.0000146333.20727.2B
205.BaumerY,McCurdyS,WeatherbyTM,MehtaNN,HalbherrS, HalbherrP,etal.Hyperlipidemia-inducedcholesterolcrystalproduction byendothelialcellspromotesatherogenesis. NatCommun. (2017) 8:1129.doi:10.1038/s41467-017-01186-z
206.CatalanoG,DucheneE,JuliaZ,LeGoffW,BruckertE,ChapmanMJ, etal.CellularSR-BIandABCA1-mediatedcholesteroleffluxaregender-specificin healthysubjects. JLipidRes. (2008)49:635–43.doi:10.1194/jlr.M700510-JLR200
207.VillardEF.PEIK,FrisdalE,BruckertE,ClementK,Bonnefont-RousselotD, etal.Geneticdeterminationofplasmacholesteroleffluxcapacityisgender-specific andindependentofHDL-cholesterollevels.ArteriosclerThrombVascBiol. (2013) 33:822–8.doi:10.1161/ATVBAHA.112.300979
208.MaF,DarabiM,LhommeM,TubeufE,CanicioA,Brerault J,etal.Phospholipidtransfertohigh-densitylipoprotein(HDL)upon triglyceridelipolysisisdirectlycorrelatedwithHDL-cholesterollevelsand isnotassociatedwithcardiovascularrisk. Atherosclerosis. (2021)324:1–8.doi:10.1016/j.atherosclerosis.2021.03.002
209.ElKhoudarySR,HutchinsPM,MatthewsKA,BrooksMM,OrchardTJ, RonseinGE,etal.CholesteroleffluxcapacityandsubclassesofHDLparticlesin healthywomentransitioningthroughmenopause. JClinEndocrinolMetab. (2016) 101:3419–28.doi:10.1210/jc.2016-2144
210.RubinowKB,VaisarT,ChaoJH,HeineckeJW,PageST.Sexsteroidsmediate discreteeffectsonHDLcholesteroleffluxcapacityandparticleconcentrationin healthymen. JClinLipidol. (2018)12:1072–82.doi:10.1016/j.jacl.2018.04.013
211.HolmboeSA,VradiE,JensenTK,LinnebergA,HusemoenLL,Scheike T,etal.Theassociationofreproductivehormonelevelsandall-cause,cancer, andcardiovasculardiseasemortalityinmen. JClinEndocrinolMetab. (2015) 100:4472–80.doi:10.1210/jc.2015-2460
212.BerniniG,VersariD,MorettiA,VirdisA,GhiadoniL,BardiniM, etal.Vascularreactivityincongenitalhypogonadalmenbeforeandafter testosteronereplacementtherapy. JClinEndocrinolMetab. (2006)91:1691–7.doi:10.1210/jc.2005-1398
213.StanhewiczAE,WennerMM,StachenfeldNS.Sexdifferencesinendothelial functionimportanttovascularhealthandoverallcardiovasculardiseaserisk acrossthelifespan. AmJPhysiolHeartCircPhysiol. (2018)315:H1569–h88.doi:10.1152/ajpheart.00396.2018
214.BolickDavidT,SrinivasanS,KimKyuW,HatleyMelissaE,ClemensJeremy J,WhetzelA,etal.Sphingosine-1-phosphatepreventstumornecrosisfactor-αmediatedmonocyteadhesiontoaorticendotheliuminmice. ArteriosclerThromb VascBiol. (2005)25:976–81.doi:10.1161/01.ATV.0000162171.30089.f6
215.RossR.Thepathogenesisofatherosclerosis:aperspectiveforthe(1990s). Nature. (1993)362:801–9.doi:10.1038/362801a0
216.NavabM,BerlinerJudithA,SubbanagounderG,HamaS,LusisAldons J,CastellaniLawrenceW,etal.HDLandtheinflammatoryresponseinduced byLDL-derivedoxidizedphospholipids. ArteriosclerThrombVascBiol. (2001) 21:481–8.doi:10.1161/01.ATV.21.4.481
217.MacknessB,DurringtonP,McElduffP,YarnellJ,AzamN,WattM,etal.Low paraoxonaseactivitypredictscoronaryeventsintheCaerphillyProspectiveStudy. Circulation. (2003)107:2775–9.doi:10.1161/01.CIR.0000070954.00271.13
218.MacknessB,HineD,LiuY,MastorikouM,MacknessM.Paraoxonase1inhibitsoxidisedLDL-inducedMCP-1productionbyendothelialcells. BiochemBiophysResCommun. (2004)318:680–3.doi:10.1016/j.bbrc.2004. 04.056
219.GharaviNM,GargalovicPS,ChangI,AraujoJA,ClarkMJ,Szeto WL.etal.High-densitylipoproteinmodulatesoxidizedphospholipidsignaling inhumanendothelialcellsfromproinflammatorytoanti-inflammatory ArteriosclerThrombVascBiol.(2007)27:1346–53.doi:10.1161/ATVBAHA.107.1 41283
220.NichollsSJ,DustingGJ,CutriB,BaoS,DrummondGR,Rye K-A,etal.Reconstitutedhigh-densitylipoproteinsinhibittheacute
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 17 frontiersin.org 99
pro-oxidantandproinflammatoryvascularchangesinducedbya periarterialcollar innormocholesterolemicrabbits.Circulation.(2005) 111:1543–50.doi:10.1161/01.CIR.0000159351.95399.50
221.VickersKC,PalmisanoBT,ShoucriBM,ShamburekRD,RemaleyAT. MicroRNAsaretransportedinplasmaanddeliveredtorecipientcellsbyhighdensitylipoproteins. NatCellBiol. (2011)13:423–33.doi:10.1038/ncb2210
222.MichellDL,VickersKC.LipoproteincarriersofmicroRNAs. Biochimicaetbiophysicaacta.(2016)1861:2069–74.doi:10.1016/j.bbalip.2016. 01.011
223.CockerillGillianW,RyeK-A,GambleJenniferR,VadasMathewA, BarterPhilipJ.High-densitylipoproteinsinhibitcytokine-inducedexpression ofendothelialcelladhesionmolecules. ArteriosclerThrombVascBiol. (1995) 15:1987–94.doi:10.1161/01.ATV.15.11.1987
224.TabetF,VickersKC,CuestaTorresLF,WieseCB,ShoucriBM,LambertG, etal.HDL-transferredmicroRNA-223regulatesICAM-1expressioninendothelial cells. NatCommun. (2014)5:3292.doi:10.1038/ncomms4292
225.ZhangQ-H,ZuX-Y,CaoR-X,LiuJ-H,MoZ-C,ZengY,etal.An involvementofSR-B1mediatedPI3K–Akt–eNOSsignalinginHDL-induced cyclooxygenase2expressionandprostacyclinproductioninendothelialcells. BiochemBiophysResCommun. (2012)420:17–23.doi:10.1016/j.bbrc.2012.02.103
226.DorrisSL,PeeblesRS.PGI2asaregulatorofinflammatorydiseases. MediatorsInflamm.(2012)2012:926968.doi:10.1155/2012/926968
227.MadsenCM,VarboA,Tybjærg-HansenA,Frikke-SchmidtR,Nordestgaard BG.U-shapedrelationshipofHDLandriskofinfectiousdisease:two prospectivepopulation-basedcohortstudies. EurHeartJ. (2017)39:1181–90.doi:10.1093/eurheartj/ehx665
228.CatapanoAL,PirilloA,BonacinaF,NorataGD,HDL.ininnateandadaptive immunity. CardiovascRes. (2014)103:372–83.doi:10.1093/cvr/cvu150
229.ShorR,WainsteinJ,OzD,BoazM,MatasZ,FuxA,etal.LowHDL levelsandtheriskofdeath,sepsisandmalignancy. ClinResCardiol. (2008) 97:227–33.doi:10.1007/s00392-007-0611-z
230.AndersenRV,WittrupHH,Tybjaerg-HansenA,SteffensenR, SchnohrP,NordestgaardBG.Hepaticlipasemutations,elevatedhighdensitylipoproteincholesterol,andincreasedriskofischemicheart disease:theCopenhagenCityHeartStudy. JAmCollCardiol. (2003) 41:1972–82.doi:10.1016/S0735-1097(03)00407-8
231.FeitosaMF,MyersRH,PankowJS,ProvinceMA,BoreckiIB,LIPC.variants inthepromoterandintron1modifyHDL-Clevelsinasex-specificfashion. Atherosclerosis. (2009)204:171–7.doi:10.1016/j.atherosclerosis.2008.09.007
232.WuDF,LinD,LuF,LiaoQC,WuYJ,WangZ,etal.Sex-specificinfluence oftheSCARB1Rs5888SNPontheserumlipidresponsetoatorvastatininpatients withacutecoronarysyndromeundergoingpercutaneouscoronaryintervention. PharmgenomicsPersMed. (2020)13:553–61.doi:10.2147/PGPM.S273346
233.Taleb-BelkadiO,ChaibH,ZemourL,FatahA,ChafiB,MekkiK.Lipid profile,inflammation,andoxidativestatusinperi-andpostmenopausalwomen. GynecolEndocrinol. (2016)32:982–5.doi:10.1080/09513590.2016.1214257
234.FeingoldKR,GrunfeldC.Effectofinflammationon HDLstructureandfunction. CurrOpinLipidol. (2016)27:521–30.doi:10.1097/MOL.0000000000000333
235.ChungCP,OeserA,RaggiP,SokkaT,PincusT,SolusJF,etal.Lipoprotein subclassesdeterminedbynuclearmagneticresonancespectroscopyandcoronary atherosclerosisinpatientswithrheumatoidarthritis. JRheumatol. (2010)37:1633–8.doi:10.3899/jrheum.090639
236.SchulteDM,PaulsenK,TürkK,BrandtB,Freitag-WolfS,Hagen I,etal.SmalldenseLDLcholesterolinhumansubjectswithdifferent chronicinflammatorydiseases. NutrMetabCardiovascDis. (2018)28:1100–5.doi:10.1016/j.numecd.2018.06.022
237.CharakidaM,BeslerC,BatucaJR,SangleS,MarquesS, SousaM,etal.Vascularabnormalities,paraoxonaseactivity,and dysfunctionalHDLinprimaryantiphospholipidsyndrome. JAMA. (2009) 302:1210–7.doi:10.1001/jama.2009.1346
238.MostazaJM,Salinero-FortMA,Cardenas-ValladolidJ,Rodriguez-Artalejo F,Díaz-AlmironM,Vich-PérezP,etal.Pre-infectionHDL-cholesterollevels andmortalityamongelderlypatientsinfectedwithSARS-CoV-2. Atherosclerosis. (2022)341:13–9.doi:10.1016/j.atherosclerosis.2021.12.009
239.HilserJR,HanY,BiswasS,GukasyanJ,CaiZ,ZhuR,etal.Association ofserumHDL-cholesterolandapolipoproteinA1levelswithriskofsevereSARSCoV-2infection. JLipidRes. (2021)62:100061.doi:10.1016/j.jlr.2021.100061
240.PengF,LeiS,ZhangQ,ZhongY,WuS.Triglyceride/highdensitylipoproteincholesterolratioisassociatedwiththemortality ofCOVID-19:aretrospectivestudyinChina. IntJGenMed. (2022) 15:985–96.doi:10.2147/IJGM.S346690
241.SouzaJuniorDR,SilvaARM,Rosa-FernandesL,ReisLR,AlexandriaG, BhosaleSD,etal.HDLproteomeremodelingassociateswithCOVID-19severity. J ClinLipidol. (2021)15:796–804.doi:10.1016/j.jacl.2021.10.005
242.TanakaS,BegueF,VeerenB,Tran-DinhA,RobertT,TashkP,etal.First recombinanthigh-densitylipoproteinparticlesadministrationinasevereICU COVID-19patient,amulti-omicsexploratoryinvestigation. Biomedicines.(2022) 10:754.doi:10.3390/biomedicines10040754
243.ShaJ,QieG,YaoQ,SunW,WangC,ZhangZ,etal.Sexdifferences onclinicalcharacteristics,severity,andmortalityinadultpatientswith COVID-19:amulticentreretrospectivestudy. FrontMed(Lausanne). (2021) 8:607059.doi:10.3389/fmed.2021.607059
244.GebhardC,Regitz-ZagrosekV,NeuhauserHK,MorganR,KleinSL.Impact ofsexandgenderonCOVID-19outcomesinEurope. BiolSexDiffer. (2020) 11:29.doi:10.1186/s13293-020-00304-9
245.KumarN,ZuoY,YalavarthiS,HunkerKL,KnightJS,KanthiY,etal.SARSCoV-2spikeproteinS1-mediatedendothelialinjuryandpro-inflammatorystateis amplifiedbydihydrotestosteroneandpreventedbymineralocorticoidantagonism. Viruses.(2021)13:2209.doi:10.3390/v13112209
246.GebhardCE,HamoudaN,GebertP,Regitz-ZagrosekV,GebhardC,Bengs S,etal.Sexversusgender-relatedcharacteristics:whichpredictsclinicaloutcomes ofacuteCOVID-19? IntensiveCareMed. (2022).doi:10.1007/s00134-022-06836-5
247.MallatZ,TedguiA.Apoptosisinthevasculature:mechanisms andfunctionalimportance. BrJPharmacol. (2000)130:947–62.doi:10.1038/sj.bjp.0703407
248.WinnRK,HarlanJM.Theroleofendothelialcellapoptosisin inflammatoryandimmunediseases. JThrombHaemost. (2005)3:1815–24.doi:10.1111/j.1538-7836.2005.01378.x
249.BombeliT,SchwartzBR,HarlanJM.Endothelialcellsundergoing apoptosisbecomeproadhesivefornonactivatedplatelets. Blood. (1999)93:3831–8.doi:10.1182/blood.V93.11.3831
250.BombeliT,KarsanA,TaitJF,HarlanJM.Apoptoticvascularendothelialcells becomeprocoagulant. Blood. (1997)89:2429–42.doi:10.1182/blood.V89.7.2429
251.Escargueil-BlancI,SalvayreR,Nègre-SalvayreA.Necrosisand apoptosisinducedbyoxidizedlowdensitylipoproteinsoccurthrough twocalcium-dependentpathwaysinlymphoblastoidcells. FASEBJ. (1994) 8:1075–80.doi:10.1096/fasebj.8.13.7926374
252.HesslerJR,MorelDW,LewisLJ,ChisolmGM.Lipoprotein oxidationandlipoprotein-inducedcytotoxicity. Arteriosclerosis. (1983) 3:215–22.doi:10.1161/01.ATV.3.3.215
253.Escargueil-BlancI,MeilhacO,PieraggiMT,ArnalJF,SalvayreR, Nègre-SalvayreA.OxidizedLDLsinducemassiveapoptosisofcultured humanendothelialcellsthroughacalcium-dependentpathway.Prevention byaurintricarboxylicacid. ArteriosclerThrombVascBiol. (1997)17:331–9.doi:10.1161/01.ATV.17.2.331
254.NoferJR,LevkauB,WolinskaI,JunkerR,FobkerM,vonEckardstein A,etal.Suppressionofendothelialcellapoptosisbyhighdensitylipoproteins (HDL)andHDL-associatedlysosphingolipids. JBiolChem. (2001)276:34480–5.doi:10.1074/jbc.M103782200
255.WalterM.InterrelationshipsamongHDLmetabolism,aging, andatherosclerosis. ArteriosclerThrombVascBiol. (2009)29:1244–50.doi:10.1161/ATVBAHA.108.181438
256.NoferJR,KehrelB,FobkerM,LevkauB,AssmannG,vonEckardstein A,etal.andarteriosclerosis:beyondreversecholesteroltransport. Atherosclerosis. (2002)161:1–16.doi:10.1016/S0021-9150(01)00651-7
257.RiwantoM,RohrerL,RoschitzkiB,BeslerC,MocharlaP,Mueller M,etal.Alteredactivationofendothelialanti-andproapoptoticpathways byhigh-densitylipoproteinfrompatientswithcoronaryarterydisease:role ofhigh-densitylipoprotein-proteomeremodeling. Circulation. (2013)127:891–904.doi:10.1161/CIRCULATIONAHA.112.108753
258.SucI,Escargueil-BlancI,TrolyM,SalvayreR,Nègre-SalvayreA,HDL. andApoApreventcelldeathofendothelialcellsinducedbyoxidizedLDL. ArteriosclerThrombVascBiol. (1997)17:2158–66.doi:10.1161/01.ATV.17. 10.2158
259.deSouzaJA,VindisC,Nègre-SalvayreA,RyeKA,CouturierM, TherondP,etal.Small,denseHDL3particlesattenuateapoptosisinendothelial cells:pivotalroleofapolipoproteinA-I. JCellMolMed. (2010)14:608–20.doi:10.1111/j.1582-4934.2009.00713.x
260.Opden.BuijsJ,MustersM,VerripsT,PostJA,BraamB,vanRielN. Mathematicalmodelingofvascularendotheliallayermaintenance:theroleof endothelialcelldivision,progenitorcellhoming,andtelomereshortening. Am JPhysiolHeartCircPhysiol. (2004)287:H2651–8.doi:10.1152/ajpheart.0033
2.2004
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 18 frontiersin.org 100
261.TsoC,MartinicG,FanWH,RogersC,RyeKA,BarterPJ.High-density lipoproteinsenhanceprogenitor-mediatedendotheliumrepairinmice. Arterioscler ThrombVascBiol. (2006)26:1144–9.doi:10.1161/01.ATV.0000216600.37436.cf
262.PuDR,LiuL,HDL.slowingdownendothelialprogenitorcellssenescence: anovelanti-atherogenicpropertyofHDL. MedHypotheses. (2008)70:338–42.doi:10.1016/j.mehy.2007.05.025
263.CervellatiC,VignaGB,TrentiniA,SanzJM,ZimettiF,DallaNora E,etal.Paraoxonase-1activitiesinindividualswithdifferentHDLcirculating levels:Implicationinreversecholesteroltransportandearlyvasculardamage. Atherosclerosis. (2019)285:64–70.doi:10.1016/j.atherosclerosis.2019.04.218
264.FurlongCE,MarsillachJ,JarvikGP,CostaLG.Paraoxonases1, 2and 3:whataretheirfunctions? ChemBiolInteract.(2016) 259:51–62.doi:10.1016/j.cbi.2016.05.036
265.RosenblatM,KarryR,AviramM.Paraoxonase1(PON1)isamorepotent antioxidantandstimulantofmacrophagecholesterolefflux,whenpresentinHDL thaninlipoprotein-deficientserum:relevancetodiabetes. Atherosclerosis. (2006) 187:74–81.doi:10.1016/j.atherosclerosis.2005.08.026
266.TavoriH,KhatibS,AviramM,VayaJ.CharacterizationofthePON1active siteusingmodelingsimulation,inrelationtoPON1lactonaseactivity. BioorgMed Chem. (2008)16:7504–9.doi:10.1016/j.bmc.2008.06.008
267.KelesidisT,RobertsCK,HuynhD,Martínez-MazaO,Currier JS,ReddyST,etal.Ahighthroughputbiochemicalfluorometric methodformeasuringlipidperoxidationinHDL. PLoSONE. (2014) 9:e111716.doi:10.1371/journal.pone.0111716
268.FarbsteinD,LevyAP,HDL.dysfunctionindiabetes:causesandpossible treatments. ExpertRevCardiovascTher. (2012)10:353–61.doi:10.1586/erc.11.182
269.FlahertySM,WoodEK,RyffCD,LoveGD,KelesidisT,Berkowitz L,etal.RaceandsexdifferencesinHDLperoxidecontentamong Americanadultswithandwithouttype2diabetes. LipidsHealthDis. (2022) 21:18.doi:10.1186/s12944-021-01608-4
270.GomezRossoL,LhommeM,MeroñoT,DellepianeA,SorrocheP,Hedjazi L,etal.Poorglycemiccontrolintype2diabetesenhancesfunctionaland compositionalalterationsofsmall,denseHDL3c. BiochimBiophysActaMolCell BiolLipids. (2017)1862:188–95.doi:10.1016/j.bbalip.2016.10.014
271.ChapmanMJ,HDL.functionalityintype1andtype2 diabetes:newinsights. CurrOpinEndocrinolDiabetesObes. (2022) 29:112–23.doi:10.1097/MED.0000000000000705
272.RostaV,TrentiniA,PassaroA,ZulianiG,SanzJM,BosiC,etal.Sex differenceimpactsontherelationshipbetweenparaoxonase-1(PON1)andtype 2diabetes. Antioxidants(Basel). (2020)9:683.doi:10.3390/antiox9080683
273.Garrido-SánchezL,García-FuentesE,CardonaF,Rojo-Martínez G,SoriguerF,TinahonesFJ.Anti-oxidizedLDLantibodylevelsare reducedinwomenwithhypertension. EurJClinInvest. (2009) 39:800–6.doi:10.1111/j.1365-2362.2009.02156.x
274.LapointeA,CouillardC,PichéME,WeisnagelSJ,BergeronJ,Nadeau A,etal.CirculatingoxidizedLDLisassociatedwithparametersofthe metabolicsyndromeinpostmenopausalwomen. Atherosclerosis. (2007)191:362–8.doi:10.1016/j.atherosclerosis.2006.03.036
275.ChaeJS,KimOY,PaikJK,KangR,SeoWJ,JeongTS,etal.Associationof Lp-PLA(2)activityandLDLsizewithinterleukin-6,aninflammatorycytokineand oxidizedLDL,amarkerofoxidativestress,inwomenwithmetabolicsyndrome. Atherosclerosis. (2011)218:499–506.doi:10.1016/j.atherosclerosis.2011.06.036
276.TikkanenMJ,VihmaV,JauhiainenM,HöckerstedtA,HelistenH, KaamanenM.Lipoprotein-associatedestrogens. CardiovascRes. (2002)56:184–8.doi:10.1016/S0008-6363(02)00535-7
277.ShwaeryGT,VitaJA,KeaneyJF.AntioxidantprotectionofLDL byphysiologicconcentrationsofestrogensisspecificfor17-beta-estradiol. Atherosclerosis. (1998)138:255–62.doi:10.1016/S0021-9150(98)00020-3
278.GonzálezF,NairKS,DanielsJK,BasalE,SchimkeJM,BlairHE. Hyperandrogenismsensitizesleukocytestohyperglycemiatopromoteoxidative stressinleanreproductive-agewomen. JClinEndocrinolMetab. (2012)97:2836–43.doi:10.1210/jc.2012-1259
279.SunY,LiS,LiuH,BaiH,HuK,ZhangR,etal.Oxidativestresspromotes hyperandrogenismbyreducingsexhormone-bindingglobulininpolycysticovary syndrome. FertilSteril. (2021)116:1641–50.doi:10.1016/j.fertnstert.2021.07.1203
280.LiX-A,TitlowWB,JacksonBA,GiltiayN,Nikolova-KarakashianM, UittenbogaardA,etal.Highdensitylipoproteinbindingtoscavengerreceptor, classb,typeIactivatesendothelialnitric-oxidesynthaseinaceramide-dependent manner∗ JBiolChem. (2002)277:11058–63.doi:10.1074/jbc.M110985200
281.TerasakaN,WesterterpM,KoetsveldJ,Fernández-HernandoC,YvanCharvetL,WangN,etal.ATP-bindingcassettetransporterG1andhighdensitylipoproteinpromoteendothelialNOsynthesisthroughadecreaseinthe interactionofcaveolin-1andendothelialNOsynthase. ArteriosclerThrombVasc Biol. (2010)30:2219–25.doi:10.1161/ATVBAHA.110.213215
282.MineoC,YuhannaIS,QuonMJ,ShaulPW.Highdensitylipoproteininducedendothelialnitric-oxidesynthaseactivationismediatedbyAktandMAP Kinases∗ JBiolChem. (2003)278:9142–9.doi:10.1074/jbc.M211394200
283.GongM,WilsonM,KellyT,SuW,DressmanJ,KincerJ,etal.HDLassociatedestradiolstimulatesendothelialNOsynthaseandvasodilationinan SR-BI-dependentmanner. JClinInvest. (2003)111:1579–87.doi:10.1172/JCI16777
284.RuizM,OkadaH,DahlbäckB.HDL-associatedApoMisanti-apoptotic bydeliveringsphingosine1-phosphatetoS1P1&S1P3receptorsonvascular endothelium. LipidsHealthDis. (2017)16:36.doi:10.1186/s12944-017-0429-2
285.ChristoffersenC,ObinataH,KumaraswamySB,GalvaniS,Ahnström J,SevvanaM,etal.Endothelium-protectivesphingosine-1-phosphate providedbyHDL-associatedapolipoproteinM. ProcNatAcadSci. (2011) 108:9613.doi:10.1073/pnas.1103187108
286.WilkersonBA,GrassGD,WingSB,ArgravesWS,Argraves KM.Sphingosine1-phosphate(S1P)carrier-dependentregulation ofendothelialbarrier:highdensitylipoprotein(HDL)-s1pprolongs endothelialbarrierenhancementascomparedwithalbumin-s1pvia effectsonlevels,trafficking,andsignalingofS1p1∗ JBiolChem. (2012) 287:44645–53.doi:10.1074/jbc.M112.423426
287.MemonAA,BennetL,ZöllerB,WangX,PalmérK,Dahlbäck B,etal.TheassociationbetweenapolipoproteinMandinsulinresistance varieswithcountryofbirth. NutrMetabCardiovascDis. (2014)24:1174–80.doi:10.1016/j.numecd.2014.05.007
288.BrinckJW,ThomasA,LauerE,JornayvazFR,Brulhart-Meynet MC,ProstJC,etal.Diabetesmellitusisassociatedwithreducedhighdensitylipoproteinsphingosine-1-phosphatecontentandimpairedhigh-density lipoproteincardiaccellprotection. ArteriosclerThrombVascBiol. (2016)36:817–24.doi:10.1161/ATVBAHA.115.307049
289.FrejC,MendezAJ,RuizM,CastilloM,HughesTA,DahlbäckB, etal.AShiftinApoM/S1PbetweenHDL-particlesinwomenwithtype 1diabetesmellitusisassociatedwithimpairedanti-inflammatoryeffectsof theApoM/S1Pcomplex. ArteriosclerThrombVascBiol. (2017)37:1194–205.doi:10.1161/ATVBAHA.117.309275
290.BeslerC,HeinrichK,RohrerL,DoerriesC,RiwantoM,ShihDM, etal.MechanismsunderlyingadverseeffectsofHDLoneNOS-activating pathwaysinpatientswithcoronaryarterydisease. JClinInvest. (2011)121:2693–708.doi:10.1172/JCI42946
291.DenimalD,MonierS,BrindisiMC,PetitJM,BouilletB, NguyenA,etal.ImpairmentoftheabilityofHDLfrompatientswith metabolicsyndromebutwithoutdiabetesmellitustoactivateeNOS: correctionbyS1Penrichment. ArteriosclerThrombVascBiol. (2017) 37:804–11.doi:10.1161/ATVBAHA.117.309287
292.GomaraschiM,OssoliA,FavariE,AdorniMP,SinagraG,CattinL,etal. InflammationimpairseNOSactivationbyHDLinpatientswithacutecoronary syndrome. CardiovascRes. (2013)100:36–43.doi:10.1093/cvr/cvt169
293.ErenE,YilmazN,AydinO.Functionallydefectivehigh-densitylipoprotein andparaoxonase:acoupleforendothelialdysfunctioninatherosclerosis. Cholesterol. (2013)2013:792090.doi:10.1155/2013/792090
294.ShahK,McCormackCE,BradburyNA.Doyouknowthesexofyourcells? AmJPhysiolCellPhysiol. (2014)306:C3–18.doi:10.1152/ajpcell.00281.2013
295.TaylorKE,Vallejo-GiraldoC,SchaibleNS,ZakeriR,MillerVM.Reporting ofsexasavariableincardiovascularstudiesusingculturedcells. BiolSexDiffer. (2011)2:11.doi:10.1186/2042-6410-2-11
296.WoitowichNC,WoodruffTK.Opinion:Researchcommunity needstobetterappreciatethevalueofsex-basedresearch. Proc NatlAcadSciUSA. (2019)116:7154–6.doi:10.1073/pnas.19035 86116
Dietrichetal. 10.3389/fcvm.2022.989428
Frontiersin CardiovascularMedicine 19 frontiersin.org 101
OPENACCESS
EDITEDBY
Chu-HuangChen, TexasHeartInstitute,UnitedStates
REVIEWEDBY
ZenengWang, ClevelandClinic,UnitedStates
MariaGiovannaLupo, UniversitàdegliStudidiPadova,Italy
*CORRESPONDENCE
DaoquanPeng Pengdq@csu.edu.cn
† Theseauthorshavecontributed equallytothisworkandsharefirst authorship
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal
FrontiersinCardiovascularMedicine
RECEIVED 03May2022
ACCEPTED 12September2022
PUBLISHED 17October2022
CITATION
WangS,FuD,LiuHandPengD(2022) IndependentassociationofPCSK9 withplateletreactivityinsubjects withoutstatinorantiplateletagents. Front.Cardiovasc.Med. 9:934914. doi:10.3389/fcvm.2022.934914
COPYRIGHT
© 2022Wang,Fu,LiuandPeng.Thisis anopen-accessarticledistributed underthetermsoftheCreative CommonsAttributionLicense(CCBY)
Theuse,distributionorreproduction inotherforumsispermitted,provided theoriginalauthor(s)andthecopyright owner(s)arecreditedandthatthe originalpublicationinthisjournalis cited,inaccordancewithaccepted academicpractice.Nouse,distribution orreproductionispermittedwhich doesnotcomplywiththeseterms.
PUBLISHED 17October2022
DOI 10.3389/fcvm.2022.934914
Independentassociationof PCSK9withplateletreactivityin subjectswithoutstatinor antiplateletagents
ShuaiWang†,DiFu†,HuixingLiu andDaoquanPeng*
DepartmentofCardiovascularMedicine,SecondXiangyaHospitalofCentralSouthUniversity, Changsha,China
Backgroundandaims: Proproteinconvertasesubtilisin/kexintype9(PCSK9) levelscouldpredictcardiovasculareventinpatientswithwell-controlled LDL-Clevels,suggestinganLDL-independentmechanismofPCSK9onthe cardiovascularsystem.AccumulatingevidencesuggestsPCSK9mightbe associatedwithincreasedplateletreactivity.Thisstudyaimedtoassessthe relationshipbetweenPCSK9levelsandplateletreactivityinsubjectsnottaking statinsorantiplateletagents.
Methods: Across-sectionalstudywasconductedtoinvestigatethe independentcontributionofPCSK9toplateletactivitybycontrollingforthe potentialconfoundingfactors.Thestudypopulationincluded89subjectsfrom ahealthexaminationcentrewhounderwentroutineannualhealthcheck-ups orhadanexaminationbeforeaselectiveoperation.Subjectstakingstatins orantiplateletagentswereexcluded.Adenosinediphosphate(ADP)-induced plateletaggregationwasdeterminedbyPL-11plateletanalyzerusing impedanceaggregometryandplasmaPCSK9levelsweredeterminedusing anELISA.SerumLipidprofilewasassessedbymeasuringtheconcentration oftotalcholesterol(TC),high-densitylipoproteincholesterol(HDL-C),and triglyceride(TG),withlow-densitylipoproteincholesterol(LDL-C)being directlymeasuredusingenzymatictechniques.Theassociationbetween PCSK9andplateletreactivitywasinvestigated.
Results: Thestudysubjectswerecomposedof53malesand36females withanaverageageof55(±11)yearsold.Theunivariatecorrelationanalysis showedsignificantcorrelationbetweenADP-inducedmaximalaggregation rate(MAR)andPCSK9(r = 0.55, p < 0.001)aswellasTC(r = 0.23, p = 0.028), LDL-C(r = 0.27, p < 0.001),andPLT(r = 0.31, p = 0.005).Beingmale(41.2% vs.46.6, p = 0.04)andsmoking(37.4vs.46.2%, p = 0.016)wereassociated withlowerADP-inducedMARthanbeingfemaleandnon-smoking.However, thereisnocorrelationbetweenPCSK9andAA-inducedplateletmaximal aggregationrate(r = 0.17, p = 0.12).Multipleregressionanalysissuggestedthat PCSK9contributedindependentlytoADP-inducedmaximalaggregationrate (β = 0.08, p = 0.004)aftercontrollingforthee ectofTC,LDL-C,PLT,being male,andsmoking.
TYPE OriginalResearch
Frontiersin CardiovascularMedicine 01 frontiersin.org 102
Conclusions: PCSK9ispositivelyassociatedwithplateletreactivity,whichmay partlyaccountforthebeneficiale ectofPCSK9inhibitioninreducingtherisk ofmajoradversecardiovasculareventsafteracutecoronarysyndrome(ACS).
KEYWORDS
proproteinconvertasesubtilisin/kexintype9,plateletaggregation,ADP, crosssectionalstudy,impedanceaggregometry
Introduction
Atheroscleroticcardiovasculardisease(CVD)isamajor causeofdisease burden.Plateletshaveanimportantrolein coronarythrombosispathogenesisandatherogenesis.
Studieshaveshownthatplateletactivityvariesgreatlyamong individuals.Itcouldexplainthevariabilityintheriskfor CVD(1–4).Priorclinicalstudiesfoundanassociationbetween plateletactivityandincident cardiovascularmorbidityand mortality(5, 6).Hypercholesterolemiaanditsinducedreactive oxygenspeciesproduction canactivateplatelets(7–9).However, themoleculesthroughwhichplateletsbecomehyperactive remainnotfullyunderstood.
ProproteinConvertaseSubtilisin/KexinType9(PCSK9), mainlysynthesizedbytheliver,kidney,andsmallintestine, bindandinhibitlowdensitylipoproteinreceptor(LDLR) recircularizationbypromotingitsdegradationinthelysosomes andconsequentlyincreaselowdensitylipoprotein(LDL) particlesinthecirculation(10).Regulationofcholesterol-rich LDLlevelisnot theonlyrolethatPCSK9hasinatherosclerosis pathogenesis.Inprospectivecohortstudies,plasmaPCSK9 levelwascorrelatedwithenhancedatherosclerosisprogression andelevatedprobabilityoffuturecardiovascularevents independentlyofLDLplasmalevels,suggestingalternativeroles forPCSK9inthepathogenesisofatherosclerosis(11, 12).
ArelationshipbetweenPCSK9plasmalevelsandtotal numberofcirculatingplateletshasbeenreportedin patientswithstablecoronaryarterydisease(13).Astrong correlationbetweenPCSK9levels andplateletreactivity wasalsorevealedinpatientswithrecentacutecoronary syndromeswhounderwentcoronaryinterventionand receivedP2Y12 inhibitors(14).However,statinusecould increasePCSK9levelsandantiplateletdrugscouldaffect plateletactivityinCADpatients(15, 16).Inanotherstudy, humanrecombinantPCSK9added tohealthyhumanplasma significantlyincreasedplateletaggregationwhenstimulated withepinephrine(17).Buttheconcentrationofhuman recombinantPCSK9usedin an in-vitro studywasmuchhigher thanthephysiologicalconcentrationinhumans.Therefore, thenaivecorrelationbetweenplasmaPCSK9andplatelet reactivityinsubjectswithoutlipidloweringorantiplateletdrugs isnotknown.
Inthepresentstudy,werevealedacorrelationbetween plasmaPCSK9andplateletreactivitywhenstimulatedbyagonist adenosinediphosphate(ADP) invitro inhealthysubjects withoutlipid-loweringorantiplateletdrugs.TheADP-induced maximalaggregationrateofplateletswas15.8%higherin patientswithhighesttertilePCSK9valuethanthepatients inthelowesttertile.Additionally,wefoundthatPCSK9was independentlycorrelatedtoplateletactivityafteradjustingfor lowdensitylipoproteincholesterol(LDL-C)andplatelet(PLT) count.Theresultsofthisstudyprovideanotherpieceofevidence onthecorrelationbetweenPCSK9andplateletactivityin healthysubjects.
Methods Studypopulationanddesign
Thisstudyisacross-sectional,singlecenterclinicalstudy. Eighty-ninesubjectswhounderwentroutineannualhealth check-upsorhadanexaminationbeforeaselectiveoperation wereenrolledfromahealthexaminationcenter.Inclusion criteriawere:1)agedbetween18and80yearsand2)obtained signedinformedconsent.Theexclusioncriteriaweresubjects withatheroscleroticcardiovasculardisease(ASCVD),malignant tumor,renaldysfunction,liverdysfunction,thyroiddisease, autoimmunedisease,orcoagulationdisorders.Subjectswhohad lipidloweringdrugsorantiplateletdrugsinthepast3months beforescreeningwereexcluded.Hypertensionanddiabeteswere definedasbeingpresentwhenanindividualself-reporteda healthprofessional’sdiagnosisandwasusingassociateddrugs. Writteninformedconsentwasobtainedfromeachpatient includedinthestudy.Thestudyprotocolconformstotheethical guidelinesofthe1975DeclarationofHelsinkiandthestudy protocolhasbeenpriorlyapprovedbytheethicscommitteeof SecondXiangyaHospitalonresearchonhumans.
Assessmentofplateletreactivity
Abloodsamplewaswithdrawnafterovernightfasting andanalyzedforplateletreactivitywithin2h.Wholeblood
Wangetal. 10.3389/fcvm.2022.934914
Frontiersin CardiovascularMedicine 02 frontiersin.org 103
aggregation wasdeterminedusingPL-11plateletanalyzer (SINNOWA,Nanjing).The systemdetectstheelectrical impedancechangeduetotheadhesionandaggregationof plateletontwoindependentelectrode-setsurfaces.Sodium citratewasusedasananticoagulant;adenosinediphosphateand arachidonicacidwereusedasagonists.A1:9dilutionofwhole bloodanticoagulatedwithsodiumcitrateand0.9%NaClwas stirredat25◦C.ADP50µmol/Landarachidonicacid(AA)2 mg/mLwereadded.
AssessmentofPCSK9serumlevels
Bloodsampleswerecollectedafterovernightfastingand thesampleswerestoredat 80◦Cuntilanalysis.Plasma PCSK9concentrationwasdeterminedbyasandwichenzymelinkedimmunosorbentassay(ELISA).CommercialPCSK9 (QuantikineELISA,R&DsystemsInc.)ELISAkitswereusedto quantifytheconcentrationsofthem.
Statisticalanalysis
Forclinicaldata,continuousdataareexpressedasmean andstandarddeviationfornormallydistributeddataormedian andinterquartilerangefornon-Gaussiandatadistribution.For comparisonofvariablesbetweendifferentgroupsoftertile PCSK9values,one-wayANOVAtestwasusedfornormally distributeddata,withLSDperformedformultiplecomparisons. Kruskal-Wallistestwasusedfornon-Gaussiandistributeddata. ThedistributionofdatawasexaminedwiththeKolmogorovSmirnovtest.Categoricalvariablesarepresentedaspercentages ofsubjectsandwerecomparedusingPearson X2 orFisher’s exacttests,asappropriate.ThecorrelationbetweenPCSK9, otherlipidparameters,PLT,andplateletactivitywasevaluated bySpearman’sranktest.ContinuousPCSK9levelswere categorizedintotertilesofequalsizetoassesstheassociation withADP-inducedmaximalaggregationrate(MAR).Platelet reactivityabovemeanvaluewasclassifiedashigher.Multivariate linearregressionwasusedtoassesstheassociationbetween PCSK9levelsandADP-inducedMAR.Unstandardized B and p-valueswereusedtopresentresultsofthelinearregression model.AdjustedRsquareand p-valueswereusedtopresent forgoodnessoffitofthemultivariatelinearregression.All testsweretwo-sided;a p < 0.05wasconsideredstatistically significant.CalculationswereperformedusingSPSSversion26.0 (IBMCorporation,Chicago,USA).
Results Studypopulation
Between2020and2021,89 subjects,including53maleand 36femalewithanaverageageof55(±11)years,wererecruited
atSecondXiangyahospital.Allsubjectshadnottakenstatins orantiplateletagentsbefore.Baselineclinicalcharacteristics, comorbidities,andlaboratorytestsofparticipantsaccordingto tertileofPCSK9aresummarizedin SupplementaryTable1
Factorscorrelatewithplateletreactivity
Correlationanalysisincludingallcharacteristicparameters revealedasignificantcorrelationofADP-inducedMARwith totalcholesterol(TC)(r = 0.23, p = 0.028),LDL-C(r = 0.27, p <0.001),andPLT(r = 0.312, p = 0.005).Nosignificant correlationwasfoundbetweenADP-inducedMARwithageand otherlipidparametersincludingplasmatriglyceride(TG),highdensitylipoproteincholesterol(HDL-C),nonesterifiedfatty acid(NEFA),apolipoproteinA,[apo(A)],andapolipoproteinB [apo(B)](Figure1).
AsignificantlyhigherADP-inducedMARwasalsoobserved infemalescomparedtomales(46.6 ± 11.1%vs.41.2 ± 11.7%, p = 0.039)andinnon-smokingsubjects(46.2 ± 11.4%vs.37.4 ± 11.4%, p = 0.016)incomparisontosmokers.Nosignificant differenceinADP-inducedMARwasfoundinsubjectswithor withouthypertensionanddiabetes(Figure2).
Plasmatotalcholesterol,lowdensity lipoproteincholesterol,andplatelet reactivity
Inaccordancewithcorrelationanalysis,asignificantly higherADP-inducedMARwasobservedinsubjectswithhigher TClevel(TC≥3.64mmol/L:46.8 ± 10.3%vs.TC<3.64mmol/L: 40.7 ± 12.1%, p = 0.013; Figure3A).Ontheotherhand, subjectswith ADP-inducedMARhigherthanthemeanvalue hadsignificantlyhigherTClevel(3.88 ± 0.90mmol/Lvs.3.44 ± 0.72mmol/L, p = 0.013; Figure4A).
AlthoughnosignificantdifferenceofADP-inducedMAR wasobservedbetweentertileLDL-Cgroupsorbetweenhigh vs.lowLDL-Cgroupsaccordingtomeanvalue(Figure3B), thosewhohadhigherplateletreactivityhadsignificantlyhigher LDL-Clevels(2.51 ± 0.72mmol/Lvs.2.13 ± 0.57mmol/L; Figure4B).
Plateletcountandplateletreactivity
Inlinewiththecorrelationanalysis,ADP-inducedMARwas significantlyhigherinsubjectswithhigherPLT(PLT≥220.6 × 1012/L:47.6 ± 12.3%vs.PLT<220.6 × 1012/L:39.4 ± 9.8%; Figure3C).Viseversa,thosewhohadhigherplateletreactivity alsohadsignificantly higherPLT[ADP-inducedMAR≥mean (43.7%):248.4 ± 71.9 × 1012/Lvs.ADP-inducedMAR<43.7%: 201.7 ± 93.0 × 1012/L](Figure4C).
Wangetal. 10.3389/fcvm.2022.934914
Frontiersin CardiovascularMedicine 03 frontiersin.org 104
ADP-inducedplateletaggregationstratifiedbySexandComorbidities.ADP-inducedplateletmaximalaggregationrate(MAR)inhealthysubjects stratifiedbySex (A),Hypertension (B),Smoking (C),andDiabetes (D).*: P < 0.0332,ns: P > 0.1234.


Wangetal. 10.3389/fcvm.2022.934914
FIGURE1 Correlationanalysis.Univariatelinearcorrelationanalysisofsubjectcharacteristicsincludingage (A),TC (B),TG (C),LDL-C (D),HDL-C (E),NEFA (F),apoA (G),apoB (H),andPLT (I) withADP-inducedplateletmaximalaggregationrate(MAR).
FIGURE2
Frontiersin CardiovascularMedicine 04 frontiersin.org 105
PlasmaPCSK9concentrationandplatelet reactivity
Analysisofthecorrelation betweenplasmaPCSK9 concentrationandbaselinecharacteristicsrevealedthatPCSK9 concentrationwasnotcorrelatedwithage.ExceptforLDL-C(r = 0.23, p = 0.03),PCSK9concentrationwasnotsignificantly correlatedwithotherlipidparameters.AhigherplasmaPCSK9 wasobservedinsubjectswithhypertension(246.1 ± 53.8vs. 207.6 ± 57.0, p = 0.03)andinnon-smokingsubjects(221.8 ± 58.6vs.185.8 ± 43.3, p = 0.043).Nosignificantdifferencein PCSK9levelwasobservedbetweenfemalesandmales,norin subjectswithandwithoutdiabetes(SupplementaryFigure1).
Adirectlinearcorrelation wasfoundbetweenincreased plasmaPCSK9levelsandadenosinediphosphate(ADP)inducedmaximalaggregationrate(MAR)(r = 0.555, p<0.001; Figure5A).Thiscorrelationwasalsoobservedinbothfemale

andmalesubgroups despitedifferenceinADP-inducedMAR betweensex(Figures5B,C).
ThedistributionofADP-induced MARbetweensubgroups ofsubjectswithdifferentPCSK9valueexhibitedatrendof incrementwhenPCSK9increase(SupplementaryFigure2A). Viceversa,thedistributionofPCSK9insubjectswithhigher ADP-inducedMARabovethemeanvaluecomparedtosubjects withlowADP-inducedMARshowedacorrelationwithhigh PCSK9level(SupplementaryFigure2B).
WhenassessedaccordingtotertilevaluesofPCSK9,there wasasignificantincreaseinADP-inducedMARinthe2ndtertilecomparedto1st-tertile(43.5 ± 11.7%vs.35.0 ± 7.6%; p<0.001).Inaddition,anincreaseofADP-inducedMARwas observedinthe3rd-tertilecomparedto2nd-tertile(48.7 ± 9.3% vs.43.5 ± 11.7%; p = 0.035; Figure5D).Ontheotherhand, subjectswith highADP-inducedMARhadsignificantlyhigher plasmalevelofPCSK9(Figure5E).

Wangetal. 10.3389/fcvm.2022.934914
FIGURE3
ADP-inducedplateletaggregationstratifiedbyTC,LDL-C,andPLT.ADP-inducedplateletmaximalaggregationrate(MAR)inhealthysubjects stratifiedbyTC (A),LDL-C (B),andPLT (C).*: P < 0.033,**: P < 0.002,ns: P > 0.1234.
FIGURE4 TC,LDL-C,andPLTstratifiedbylowandhighADP-inducedplateletaggregationrate.Comparisonof (A) SerumTClevel, (B) serumLDL-Clevel, and (C) PLTinsubjectswithADP-inducedmaximalplateletaggregationrate(MAR)lowerandhigherthanmeanvalue.*: P < 0.033, **: P < 0.002,ns: P > 0.1234.
Frontiersin CardiovascularMedicine 05 frontiersin.org 106
FIGURE5
Associationbetweenproproteinconvertasesubtilisin/kexintype9(PCSK9)levelsandADP-inducedplateletmaximalaggregationrate(MAR). (A) UnivariatelinearcorrelationanalysisofserumPCSK9withADP-inducedMAR. (B,C) ThecorrelationofserumPCSK9withADP-inducedMARwas stratifiedbysex. (D) ComparisonofADP-inducedMARinsubjectwithdi erentPCSK9accordingtotertilevalue. (E) Comparisonofserum PCSK9insubjectswithADP-inducedMARlowerandhigherthanmeanvalue.****: P < 0.0001.
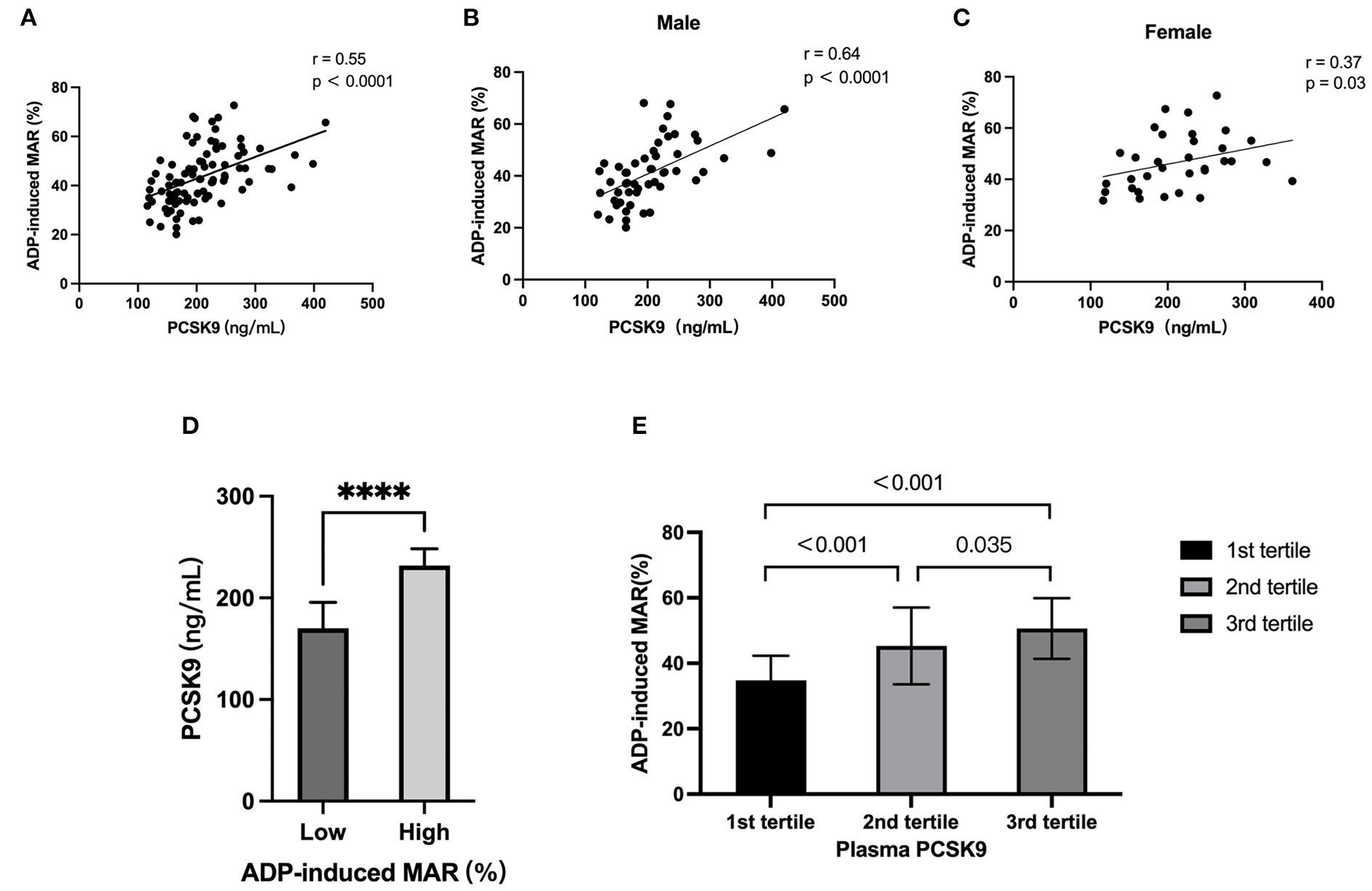
TABLE1Univariatelinearregressionanalysisregardingthe associationofADP-inducedMARandcharacteristics.
Univariateandmultivariatelinear regressionanalysis
ADP,Adenosinediphosphate;PCSK9,ProproteinConvertaseSubtilisin/KexinType9; MAR,maximalaggregationrate;TC,totalcholesterol;LDL-C,lowdensitylipoproteincholesterol;PLT,platelet.
PlasmaPCSK9levelwasnotassociatedwithAA-induced plateletaggregationinthecorrelationanalysis.Inaddition,AAinducedplateletMARwasnotdifferentaccordingtotertilevalue ofPCSK9.PlasmaPCSK9concentrationinsubjectswithhigh ADP-inducedMARwassimilarcomparedtothosewithlow ADP-inducedMAR(SupplementaryFigure3).
Inunivariatelinearregressionanalysis,PCSK9(β = 0.089, p<0,001),TC(β = 3.254, p =0.028),LDL-C(β = 4.779, p = 0.009),PLT(β = 0.041, p = 0.005),andsmoking(β =−8.832, p = 0.016)werefactorsthatcouldsignificantlypredictthe valueofADP-inducedMAR(Table1).Inmultivariateregression analysis,onlyparameters of covariatesthatwereretainedinthe modelduringstepwiseeliminationprocedureareincludedin model2.PCSK9(β = 0.09, p = 0.001),LDL-C(β = 4.81, p = 0.046),andPLT(β = 0.05, p = 0.005)werefoundtopredict ADP-inducedMAR.TheadjustedR2 ofthemultivariatemodel was0.284, p<0.001(Table2).
Discussion
TheroleofPCSK9inalteringplasmaLDL-C via PCSK9LDLRaxishas beenwellestablished;recentstudieshave suggestedapossibleroleofPCSK9inregulatingplatelet function.Ourstudyisthefirsttoconfirmanindependentand
Wangetal. 10.3389/fcvm.2022.934914
Variate Univariate β p-value PCSK9 0.089 <0.001 TC 3.254 0.028 LDL-C 4.779 0.009 PLT 0.041 0.005 Sex 4.026 0.096 Smoking 8.832 0.016
Frontiersin CardiovascularMedicine 06 frontiersin.org 107
positivecorrelationbetweenPCSK9levelandplateletreactivity inpopulationswithoutestablishedCVD,whodidnottakestatin orantiplateletagents.
Plateletreactivityreferstothedegreeoftheresponse ofbloodplateletstoanexternalstimulus.Agonistssuchas ADPandcollagenactivateplateletsbybindingtospecific receptorsthatarepresentedontheplateletsurfacemembrane.
Plateletactivationleadstoanincreaseofintra-cytoplasmatic concentrationofcalciumandplateletshapechange,enabling plateletstointeractwitheachotherandaggregate(18).On theotherhand,plateletactivationinducesconformational changesinGPIIb/IIIathattransformitintoitsfibrinogen bindingformthroughactivatingphospholipaseCβ (PLCβ)or phospholipaseCγ (PLCγ)(19).Thereceptor-boundfibrinogen connectstwoGPIIb/IIIamoleculesonnearbyplatelets.This processisthefinalcommonpathwayofagonist-inducedplatelet aggregation(20).
Plateletaggregationismodifiedbyactivatingand inactivatingbiomoleculesandconditions.Somecomponents circulatinginthebloodcanpotentiatetheactivation processinthepresenceofastrongagonist.Forexample, adrenalinelowerscytosoliccAMPlevelsandaugmentsplatelet activation;insulin-likegrowthfactorIandthrombopoietin enhanceplateletactivation via phosphatidylinositol3kinase(PI3K)signalingpathway(21).Diabetesmellitus andstatesof increasedvascularstressmightincreasethe responsivenessofplateletstoagonists(22).Ontheother hand,bioactivemediatorsreleasedfromendothelialcells, suchasprostaglandinI2 (PGI2),prostaglandinE2 (PGE2), andnitricoxide(NO),werenegativeplatelet-priming substances(23).Astudyindicatedthatpolyunsaturated fattyacidproductsof 12-lipoxygenasecanalsohamper plateletactivation(24).
Measurementofplatelet aggregationinplatelet-richplasma usinglighttransmissionaggregometry(LTA)isconsidered the“goldstandard”formeasurementbutiscomplexand technicallydemanding.Newerapproachestomeasuringplatelet aggregationusesimpedanceaggregometry,whichisbasedonthe measurementoftheelectricalresistancebetweentwoelectrodes immersedinstirredwholeblood.Asplateletsaggregateand bindtotheelectrodes,thereisachangeinelectricalimpedance thatcorrespondstothedegreeofaggregationthathasoccurred. Therefore,itisdeemedtobemorephysiologicalthanstudies performedinplatelet-richplasma(25).
ADPisoneofthemajorcomponentsreleasedfrom activatedplateletsanditactsasanagonistattwoplatelet purinergicG-proteincoupledreceptors—theGq-coupledP2Y1 andGi-coupledP2Y12 receptor.WhileP2Y1 activationis responsibleforintracellularcalciummobilization,shapechange, andinitiationofaggregation,theP2Y12 receptorisresponsible forthecompletionoftheaggregationtoADP(26).Inthe presentstudywefound thatADP-inducedplateletaggregation wasassociatedwithPLT,PCSK9,andLDL-Cinmultivariate regressionmodel.
Inmultivariateregressionanalysis,ADP-inducedplatelet aggregationwasassociatedwithPLT,whichhasbeendescribed previouslyinpatientsafterrecentcoronarystent-implantation andondual-antiplatelettherapy(27).
Previousstudieshavefoundacorrelationbetweenplasma PCSK9andplateletreactivityinpatientswithcoronaryartery diseaseandhypercholesterolemia.Inacohortofstablecoronary arterydiseasepatients,plasmaPCSK9levelswerepositively correlatedwiththeplateletcountandplateletcrit(13).The PCSK9-REACTstudyfoundthat,inpatientswitharecent acutecoronarysyndrome(ACS)undergoingpercutaneous coronaryinterventionandreceivingP2Y12inhibitor,there wasadirectassociationbetweenPCSK9plasmaleveland high-on-treatmentplateletreactivity(14).Inpatientswith hypercholesterolemiawho receivedbackgroundstatinand acetylsalicylicacidtherapy,plateletfunctionparameterswere significantlyreducedafter12monthsoftreatmentwith themonoclonalantibody(mAb)anti-PCSK9alirocumabor evolocumab(28).However,inthesestudies,useofstatinand aspirinwillaffect boththeplasmaPCSK9levelandplatelet activity(15, 16).Inourstudy,asignificantassociationof PCSK9withincreasedADP-inducedMARwasdetectedin healthysubjectswithoutstatinandantiplatelets.Importantly, thisassociationwassignificantevenafteradjustingfor othercovariates.
SimilartoapreviousstudyinCAD,plateletreactivity correlatedwithplasmaLDL-Clevelinthepresentstudy (29, 30).Hypercholesterolemiamayinfluenceplateletreactivity throughseveralmechanisms.1)Formationofox-LDL, whichwasinducedbyhighLDL-C,couldactivateplatelets bybindingwithCD36andLOX-1receptors(31–33).2)
Wangetal. 10.3389/fcvm.2022.934914
VariablesModel1 Model2 (adjustedR2 0.27,(adjusted R2 0.284, p = 0.002) p = 0.001) β p-value β p-value PCSK90.080.0040.09 0.001 TC 2.920.591 / / LDL-C7.58 0.2364.81 0.046 PLT 0.040.0730.05 0.005 Sex 0.630.83 / / Smoking 4.730.262 / /
TABLE2 Multivariatelinearregressionanalysisoftheassociationof ADP-inducedMARandvariables.
ADP,Adenosinediphosphate;PSCK9,ProproteinConvertaseSubtilisin/kexinType9; MAR,maximalaggregationrate;TC,totalcholesterol;LDL-C,lowdensitylipoproteincholesterol;PLT,platelet.
Frontiersin CardiovascularMedicine 07 frontiersin.org 108
Cholesterolincorporationin plasmamembranesinduces platelethypersensitivitytostimuli, whereasitsdepletion strikinglyreducesplateletreactivity(34–36).However,PCSK9 correlatedwithplateletreactivityafteradjustingLDL-Clevel inmultivariatelinearregressioninourstudy,suggesting PCSK9mayaffectplateletactivitythroughalipid-lowering independentmechanism.ArecentstudyfoundPCSK9 candirectlyenhanceagonist-inducedplateletactivationby bindingtoplateletCD36andthusactivatingSrckinaseand MAPK-extracellularsignal-regulatedkinase5andc-Jun Nterminalkinase,increasingthegenerationofreactive oxygenspeciesandactivatingthep38MAPK/cytosolic phospholipaseA2/cyclooxygenase-1/thromboxaneA2 signalingpathway(10).
AnotherfindingofthisstudyisAA-inducedplatelet aggregationwasnotcorrelatedtoplasmaPCSK9.Arachidonic acidisderivedfrommembranephospholipidsthrough phospholipaseA2(PLA2).AAistransformedintoprostaglandin G2andprostaglandinH2bycyclooxygenase-1(COX-1), thentransformedintoTXA2,whichisastrongactivator ofplatelets.OurfindingsuggestsPCSK9doesnotaffect PLA2/Cox-1/TXA2pathway.
Thelimitationofthepresentstudyisitscross-sectional nature.Thefindingsofourstudycouldonlyindicate associations,notcausality.Anotherlimitationistherelatively smallsamplesizeofthepopulation.
Conclusions
Insummary,PCSK9levelsareassociatedwithplatelet activationinsubjectsnottakingstatinsorantiplatelet agents.SubjectswithincreasedconcentrationofPCSK9have significantlyhigherplateletactivation.Thefindingofthisstudy providesadditionalevidenceofthecorrelationbetweenPCSK9 levelandplateletactivationbeyondCVDpatients.Future studiesarewarrantedtofurtherelucidatetheroleofPCSK9asa riskfactorforASCVD.
Dataavailabilitystatement
Therawdatasupportingtheconclusionsofthisarticlewill bemadeavailablebytheauthors,withoutunduereservation.
References
(2013)109:85–92.doi:10.1160/TH12-03-0202
Ethicsstatement
Thestudiesinvolvinghumanparticipantswerereviewedand approvedbytheEthicsCommitteeofSecondXiangyaHospital onresearchonhumans.Thepatients/participantsprovidedtheir writteninformedconsenttoparticipateinthisstudy.
Authorcontributions
SWanalyzedtheresultandwrotethemanuscript.DFand HLcollectedthe datasets.DPconceivedthestudyandrevised themanuscript.Allauthorscontributedtothearticleand approvedthesubmittedversion.
Funding
ThisworkwassupportedbygrantsfromtheNationalNature ScienceFoundationYouth Project(81600359)andtheNational NatureScienceFoundationGeneralProject(81870336).
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddo notnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
Supplementarymaterial
TheSupplementaryMaterialforthisarticlecanbe foundonlineat: https://www.frontiersin.org/articles/10.3389/ fcvm.2022.934914/full#supplementary-material
8:369–78.doi:10.1111/j.1538-7836.2009.03700.x
Wangetal. 10.3389/fcvm.2022.934914
1.BergerJS,BeckerRC,KuhnC,HelmsMJ,OrtelTL,WilliamsR. Hyperreactive plateletphenotypes:relationshiptoalteredserotonintransporter number,transportkineticsandintrinsicresponsetoadrenergicco-stimulation. ThrombHaemost.
Frontiersin CardiovascularMedicine 08 frontiersin.org 109
2.KondkarAA,BrayMS,LealSM,NagallaS,LiuDJ,JinY,etal. VAMP8/endobrevinisoverexpressedinhyperreactivehumanplatelets: suggestedroleforplateletmicroRNA. JThrombHaemost. (2010)
3.MontenontE,EchagarrugaC,AllenN,AraldiE,SuarezY,BergerJS.Platelet WDR1suppressesplateletactivityandisassociatedwithcardiovasculardisease. Blood. (2016)128:2033–42.doi:10.1182/blood-2016-03-703157
4.SharmaG,BergerJS.Plateletactivityandcardiovascularriskinapparently healthyindividuals:areviewofthedata. JThrombThrombolysis. (2011)32:201–8.doi:10.1007/s11239-011-0590-9
5.TripMD,CatsVM,vanCapelleFJ,VreekenJ.Platelethyperreactivityand prognosisinsurvivorsofmyocardialinfarction. NEnglJMed. (1990)322:1549–54.doi:10.1056/NEJM199005313222201
6.ToflerGH,BrezinskiD,SchaferAI,CzeislerCA,RutherfordJD,Willich SN,etal.Concurrentmorningincreaseinplateletaggregabilityandtheriskof myocardialinfarctionandsuddencardiacdeath. NEnglJMed. (1987)316:1514–8.doi:10.1056/NEJM198706113162405
7.FuentesF,PalomoI,FuentesE.Plateletoxidativestressasanoveltarget ofcardiovascularriskinfrailolderpeople. VasculPharmacol. (2017)93-95:14–9.doi:10.1016/j.vph.2017.07.003
8.ElHaouariM.Plateletoxidativestressanditsrelationshipwithcardiovascular diseasesintype2diabetesmellituspatients. CurrMedChem. (2019)26:4145–65.doi:10.2174/0929867324666171005114456
9.WangN,TallAR.Cholesterolinplateletbiogenesisandactivation. Blood. (2016)127:1949–53.doi:10.1182/blood-2016-01-631259
10.QiZ,HuL,ZhangJ,YangW,LiuX,JiaD,etal.PCSK9(proprotein convertasesubtilisin/kexin9)enhancesplateletactivation,thrombosis,and myocardialinfarctexpansionbybindingtoplateletCD36. Circulation. (2021) 143:45–61.doi:10.1161/CIRCULATIONAHA.120.046290
11.LeanderK,MalarstigA,Van’tHooftFM,HydeC,HelleniusML,Troutt JS,etal.Circulatingproproteinconvertasesubtilisin/kexintype9(PCSK9) predictsfutureriskofcardiovasculareventsindependentlyofestablishedrisk factors. Circulation. (2016)133:1230–9.doi:10.1161/CIRCULATIONAHA.115. 018531
12.XieW,LiuJ,WangW,WangM,QiY,ZhaoF,etal.Association betweenplasmaPCSK9levelsand10-yearprogressionofcarotid atherosclerosisbeyondLDL-C:acohortstudy. IntJCardiol. (2016) 215:293–8.doi:10.1016/j.ijcard.2016.04.103
13.LiS,ZhuCG,GuoYL,XuRX,ZhangY,SunJ,etal.Therelationshipbetween theplasmaPCSK9levelsandplateletindicesinpatientswithstablecoronaryartery disease. JAtherosclerThromb. (2015)22:76–84.doi:10.5551/jat.25841
14.NavareseEP,KolodziejczakM,WinterMP,AlimohammadiA,LangIM, BuffonA,etal.AssociationofPCSK9withplateletreactivityinpatientswith acutecoronarysyndrometreatedwithprasugrelorticagrelor:thePCSK9REACTstudy. IntJCardiol. (2017)227:644–9.doi:10.1016/j.ijcard.2016. 10.084
15.SahebkarA,Simental-MendiaLE,Guerrero-RomeroF,GolledgeJ,WattsGF. Effectofstatintherapyonplasmaproproteinconvertasesubtilisinkexin9(PCSK9) concentrations:asystematicreviewandmeta-analysisofclinicaltrials. Diabetes ObesMetab. (2015)17:1042–55.doi:10.1111/dom.12536
16.NennaA,NappiF,LusiniM,SatrianoUM,SchiliroD,SpadaccioC,etal. Effectofstatinsonplateletactivationandfunction:frommolecularpathways toclinicaleffects. BiomedResInt. (2021)2021:6661847.doi:10.1155/2021/66
61847
17.CameraM,RossettiL,BarbieriSS,ZanottiI,CancianiB,TrabattoniD,etal. PCSK9asapositivemodulatorofplateletactivation. JAmCollCardiol. (2018) 71:952–4.doi:10.1016/j.jacc.2017.11.069
18.Varga-SzaboD,BraunA,NieswandtB.Calciumsignalinginplatelets. J ThrombHaemost. (2009)7:1057–66.doi:10.1111/j.1538-7836.2009.03455.x
19.Varga-SzaboD,PleinesI,NieswandtB.Celladhesion mechanismsinplatelets. ArteriosclerThrombVascBiol. (2008) 28:403–12.doi:10.1161/ATVBAHA.107.150474
20.KoltaiK,KesmarkyG,FeherG,TiboldA,TothK.Plateletaggregometry testing:molecularmechanisms,techniquesandclinicalimplications. IntJMolSci. (2017)18:1803.doi:10.3390/ijms18081803
21.vanderMeijdenPEJ,HeemskerkJWM.Plateletbiologyandfunctions: newconceptsandclinicalperspectives. NatRevCardiol. (2019)16:166–79.doi:10.1038/s41569-018-0110-0
22.KraakmanMJ,LeeMK,Al-ShareaA,DragoljevicD,BarrettTJ,Montenont E,etal.Neutrophil-derivedS100calcium-bindingproteinsA8/A9promote reticulatedthrombocytosisandatherogenesisindiabetes. JClinInvest. (2017) 127:2133–47.doi:10.1172/JCI92450
23.NaseemKM,RobertsW.Nitricoxideataglance. Platelets. (2011)22:148–52.doi:10.3109/09537104.2010.522629
24.TourdotBE,AdiliR,IsingizweZR,EbrahemM,Freedman JC,HolmanTR,etal.12-HETrEinhibitsplateletreactivityand thrombosisinpartthroughtheprostacyclinreceptor. BloodAdv.(2017) 1:1124–31.doi:10.1182/bloodadvances.2017006155
25.AlgahtaniM,HeptinstallS.Novelstrategiesforassessingplateletreactivity. FutureCardiol. (2017)13:33–47.doi:10.2217/fca-2016-0054
26.GremmelT,FrelingerIIIAL,MichelsonAD.Plateletphysiology. Semin ThrombHemost. (2016)42:191–204.doi:10.1055/s-0035-1564835
27.OlivierCB,MeyerM,BauerH,SchnabelK,WeikP,ZhouQ,etal. TheratioofADP-toTRAP-inducedplateletaggregationquantifiesP2Y12dependentplateletinhibitionindependentlyoftheplateletcount. PLoSONE. (2016)11:e0149053.doi:10.1371/journal.pone.0149053
28.BaraleC,BonomoK,FrascaroliC,MorottiA,GuerrasioA,CavalotF, etal.Plateletfunctionandactivationmarkersinprimaryhypercholesterolemia treatedwithanti-PCSK9monoclonalantibody:a12-monthfollow-up. NutrMetab CardiovascDis. (2020)30:282–91.doi:10.1016/j.numecd.2019.09.012
29.Petersen-UribeA,KremserM,RohlfingAK,CastorT,KolbK,DicentaV, etal.Platelet-derivedPCSK9isassociatedwithLDLmetabolismandmodulates atherothromboticmechanismsincoronaryarterydisease. IntJMolSci. (2021) 22:1179.doi:10.3390/ijms222011179
30.DiMinnoG,SilverMJ,CerboneAM,RainoneA,Postiglione A,ManciniM.Increasedfibrinogenbindingtoplateletsfrom patientswithfamilialhypercholesterolemia. Arteriosclerosis. (1986) 6:203–11.doi:10.1161/01.ATV.6.2.203
31.MagwenziS,WoodwardC,WraithKS,AburimaA,RaslanZ,JonesH,etal. OxidizedLDLactivatesbloodplateletsthroughCD36/NOX2-mediatedinhibition ofthecGMP/proteinkinaseGsignalingcascade. Blood. (2015)125:2693–703.doi:10.1182/blood-2014-05-574491
32.ChenK,FebbraioM,LiW,SilversteinRL.AspecificCD36-dependent signalingpathwayisrequiredforplateletactivationbyoxidizedlow-density lipoprotein. CircRes. (2008)102:1512–9.doi:10.1161/CIRCRESAHA.108.172064
33.HofmannA,BrunssenC,MorawietzH.Contributionoflectin-like oxidizedlow-densitylipoproteinreceptor-1andLOX-1modulatingcompounds tovasculardiseases. VasculPharmacol. (2017)19:S1537-1891(17)30171-4. doi:10.1016/j.vph.2017.10.002
34.ShattilSJ,Anaya-GalindoR,BennettJ,ColmanRW,CooperRA.Platelet hypersensitivityinducedbycholesterolincorporation. JClinInvest. (1975)55:636–43.doi:10.1172/JCI107971
35.TomizukaT,YamamotoK,HiraiA,TamuraY,YoshidaS.Hypersensitivity tothromboxaneA2incholesterol-richhumanplatelets. ThrombHaemost. (1990) 64:594–9.doi:10.1055/s-0038-1647364
36.KorporaalSJ,MeursI,HauerAD,HildebrandRB,HoekstraM,CateHT, etal.Deletionofthehigh-densitylipoproteinreceptorscavengerreceptorBIin micemodulatesthrombosissusceptibilityandindirectlyaffectsplateletfunction byelevationofplasmafreecholesterol. ArteriosclerThrombVascBiol. (2011) 31:34–42.doi:10.1161/ATVBAHA.110.210252
Wangetal. 10.3389/fcvm.2022.934914
Frontiersin CardiovascularMedicine 09 frontiersin.org 110
PUBLISHED 28November2022
DOI 10.3389/fcvm.2022.1026243
OPENACCESS
EDITEDBY
AlexanderAkhmedov, UniversityofZurich,Switzerland
REVIEWEDBY FedericaFogacci, UniversityofBologna,Italy
ŽeljkoReiner, UniversityHospitalCentreZagreb, Croatia
*CORRESPONDENCE
JinchunHe hejch@lzu.edu.cn
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine
RECEIVED 23August2022
ACCEPTED 07November2022
PUBLISHED 28November2022
CITATION
WangYandHeJ(2022)Correlation ofcardiovascularriskpredictorswith overweightandobesityinpatients withfamilialhypercholesterolemia. Front.Cardiovasc.Med. 9:1026243. doi:10.3389/fcvm.2022.1026243
COPYRIGHT
©2022WangandHe.Thisisan open-accessarticledistributedunder thetermsofthe CreativeCommons AttributionLicense(CCBY).Theuse, distributionorreproductioninother forumsispermitted,providedthe originalauthor(s)andthecopyright owner(s)arecreditedandthatthe originalpublicationinthisjournalis cited,inaccordancewithaccepted academicpractice.Nouse,distribution orreproductionispermittedwhich doesnotcomplywiththeseterms.
Correlationofcardiovascular riskpredictorswithoverweight andobesityinpatientswith familialhypercholesterolemia
YaodongWang1,2 andJinchunHe3*
1 LaboratoryMedicineCenter,LanzhouUniversitySecondHospital,LanzhouUniversity,Lanzhou, Gansu,China, 2 TheFirstSchoolofClinicalMedicine,LanzhouUniversity,Lanzhou,Gansu,China, 3 DepartmentofLaboratoryMedicine,TheFirstHospitalofLanzhouUniversity,TheFirstSchool ofClinicalMedicine,LanzhouUniversity,Lanzhou,Gansu,China
Purpose: Weaimedtoanalyzethecorrelationbetweenoverweightand obesity-relatedindicatorsandcardiovascularriskpredictorsinpatients withfamilialhypercholesterolemia(FH)andtoevaluatetheirmutual predictiveproperties.
Methods: Atotalof103patientswithFHincludedfrom2004to2017 wereretrospectivelyanalyzed.Pearsoncorrelationanalysisandmultiple linearregressionanalysiswereusedtoassessthecorrelationbetween overweightandobesity-relatedindicatorsandcardiovascularriskpredictorsin FHpatients.Subjectoperatingcharacteristic(ROC)curvewasusedtoanalyze theirreciprocalpredictiveperformance.
Results: (1)Atherogenicindexofplasma(AIP)(β =0.020)andApoB/ApoA1 Ratio(BAR)(β =0.015)wereindependentlycorrelatedwithbodymass index(BMI)(P < 0.05);AIP(β =1.176)wasindependentlycorrelatedwith waist-to-hipratio(WHR)(P < 0.01);AIP(β =1.575),BAR(β =0.661) andatherogeniccoefficient(AC)(β =0.427)wereindependentlycorrelated withwaist-to-heightratio(WHtR)(P < 0.05).(2)TheareaundertheROC (AUC)foroverweightcorrespondingtoAIP,BAR,andACwere0.695(95% CI =0.593–0.797, P < 0.01),0.660(95% CI =0.555–0.766, P < 0.01),and 0.632(95% CI =0.525–0.740, P < 0.05),respectively;andAUCsforcentral obesitycorrespondingtoAIP,BARandACwere0.757(95% CI =0.656–0.857, P < 0.001),0.654(95% CI =0.536–0.771, P < 0.05)and0.651(95% CI =0.538–0.764, P < 0.05),respectively.TheAUCsformoderateriskofAIP correspondingtoBMI,WHR,andWHtRwere0.709(95% CI =0.608–0.811, P < 0.001),0.773(95% CI =0.678–0.867, P < 0.001),0.739(95% CI =0.641–0.836, P < 0.001),respectively,andBMI,WHRandWHtRcorrespondedto anAUCof0.691(95% CI =0.585–0.797, P < 0.01),0.734(95% CI =0.632–0.835, P < 0.001),and0.706(95% CI =0.603–0.810, P < 0.01)forhighriskof AIP,respectively.
TYPE OriginalResearch
Frontiersin CardiovascularMedicine 01 frontiersin.org 111
Conclusion: AIPhasindependentpositivelinearcorrelationwithindicators relatedtooverweightandobesityinFHpatients;AIPhasgoodpredictive performanceforoverweightandobesityinFHpatients,andWHRhasgood performanceforidentifyingmoderateandhighriskofAIPinFHpatients.
KEYWORDS
Introduction
Familialhypercholesterolemia(FH)isanautosomal dominantdisorderwithanestimatedprevalenceof1/300 to1/500inheterozygouspopulationsandatleast20 millionpeopleworldwidewithFH(1–5).Itiswellknown thathypercholesterolemiapredisposestotheformationof atheroscleroticplaquesinthevascularwallandhasahigh riskofcardiovasculardisease(CVD)events.Theprevalence ofFHishigherinpatientswhohaveexperiencedaCVD event,andcontrolofotherCVDriskfactorsappearsto belessoptimalthaninotherpatients(6).Theresultsof across-sectionalsurveyofFHinChinashowedthatthe prevalenceofFHintheChinesepopulationissimilartothat inothercountries;however,FHinChinaismainlyfound inpatientswithearly-onsetcoronaryheartdiseaseandtheir lipidlevelsarepoorlycontrolledandathigherriskofCVD (7).Togetherwiththefactthatcholesterolisinvolvedinthe formationofcellularbarriersformanybasicphysiological processesandactsasanimportantcomponentofsignal transduction(1),manystudieshaveemphasizedthatpatients withFHshouldbeidentifiedearlyandgivenearlyintervention (8–11).
Thedetectionoftraditionallipidprofilesandtheir associatedcalculatedindicesarethemainmethodscurrently usedtoassesstheriskofCVD.However,intheabsenceofan abnormallipidprofile,thepossibilityofcoronaryarterydisease (CAD)cannotbeexcluded.Therefore,ithasbeensuggestedthat differentcombinationsoftheselipidprofileparameterscould beusedtoidentifysuchhigh-riskindividuals.Theatherogenic indexofplasma(AIP),ApoB/ApoA1Ratio(BAR)andthe atherogeniccoefficient(AC)havebeenconsideredashighqualitypredictorsofcardiovascularrisk(12, 13).Inrecentyears, AIPhasgainedwidespreadinterestasascreeningtoolfor dyslipidemiaandisconsideredamajorcardiometabolicrisk factorandanemergingindicatortopredictCVDrisk(14), reflectingthebalancebetweenatherogenicandantiatherogenic factorsinanintegratedmanner(15, 16).BARhasalsobeen proposedtobebetterthanLDL-Candsuperiortonon-highdenselipoproteincholesterol(non-HDL-C)asamarkerof CVDrisk(17–20).Thisratiohasalsobeenconsideredasa potentialmarkerofcardiovascularriskbecauseitcanoften
beabnormalinthepresenceofnormalconventionallipid levels(21).AstudybyLuetal.indicatedthatBARisa validpredictorofcoronaryheartdiseaseriskinoverweight andobesepeople(22).AnotherratioindexthatisHDLCdependentandhassignificanceinpredictingCADriskis AC,calculatedas[(TC-HDL-C)/HDL-C](23).Ithasbeen demonstratedthatACreflectstheatherogenicpotentialof theentirelipoproteinfractionprofileandcanbeusedfor therapeuticmanagement(12).
Itiswellknownthatoverweightandobesity-related indicators,includingbodymassindex(BMI),waist-to-height ratio(WHtR)andwaist-to-hipratio(WHR),areamong thegoodcriteriatoreflectthedegreeofbodyfatness andhealthiness,andarewidelyusedtoscreenoverweight andcentrallyobesepeople.Alargeepidemiologicalsurvey showedthatmorethantwo-thirdsofdeathsassociatedwith highBMIwereduetoCVD(24).Abdominalobesity(also centralobesity)involvestheaccumulationofabdominalfat andisconsideredanindependentriskfactorforobesityrelateddiseasesanddeath(25).Ithasbeenreportedthat whenAIPvaluesof0.12–0.21and > 0.21indicatethe likelihoodofcriticalabdominalobesityandabdominalobesity, respectively,whilethecombinationofwaistcircumferenceand AIPmayincreasethespecificityandsensitivityofabdominal obesitydetectioninclinicalpractice,thussuggestingthat AIPmaybeusedasareferenceforestimatingabdominal obesity(26).
Inthisstudy,todeterminewhetheratherogeniclipid indicatorssuchasAIPareindependentlyassociatedwith overweightandobesity-relatedindicatorssuchasBMI, weanalyzedthecorrelationbetweenlipidparameters(i.e., lipidcalculationindicators)suchasAIP,BAR,andAC withoverweightandobesity-relatedindicators,andfinally evaluatedthepredictiveperformanceofcardiovascularrisk predictorsforoverweightandoverweightandobesity-related indicatorsforAIPinriskandhighriskidentification performance.Itisalsohopedthatthesefindingswill highlightthethreatofoverweightandevenobesityinFH patientsandpromotethebenefitsofweightcontrolinFH patients,thusreducingtheriskofatherogenicdiseasein thispopulation.
WangandHe 10.3389/fcvm.2022.1026243
hypercholesterolemia,cardiovascular,obesity,atherogenic,correlation
Frontiersin CardiovascularMedicine 02 frontiersin.org 112
Patientsandmethods
Inclusionofstudysubjects
TheoriginaldataoftheFHstudysampleswereobtained fromthesubproject“Collectionandclinicalepidemiologyof hereditaryhyperlipidemiabloodspecimensinfamilylines” underthe“Collection,preservationandutilizationofgenetic resourcesofmajordiseases”ofthe“TenthFive-YearPlan” ofChina.Theoriginaldatawasinitiallyscreenedfromthe populationwhoattendedtheoutpatientclinicoftheFirst HospitalofLanzhouUniversityfrom2004to2017basedon theinitialfastinglipidlevels,andtheninvitedthemandtheir first-degreerelativestoundergoaphysicalexaminationagain onaspecifieddate,whichincludedbiochemicaltestssuchas lipidpanel,physicalexamination,electrocardiogramandfaceto-facequestionnaires,andallstudysubjectssignedaninformed consentformtovoluntarilyparticipateinthisstudy.Inaddition, twoofthefollowingthreecriteriaweremetandincludedin theproject:(1)Atleasttwofamilymembersineachfamilyline withdyslipidemia,asdeterminedbytheNationalCholesterol EducationProgram(NCEP)(27),withTC ≥ 6.20mmol/L and/orLDL-C ≥ 4.10mmol/Lwithoutsecondarycauses;(2)at least2generationsofinvolvementperfamilyline;and(3)atleast 1memberofeachfamilylinewithhypercholesterolemiawithan ageofonsetnoolderthan50years.
Inordertomaketheaboveinformationmeettheneedsof thecurrentstudy,weinitiallyscreenedoutcasesinwhichthe aboveinformationmightbiastheresults,includingnon-Han, non-first-degreerelatives,age < 18years,andTG > 5.6mmol/L. Finally,wefollowedtheDutchLipidClinicNetwork(DLCN) (28)forclinicallipidmonitoringguidelinesandincluded Patientswithscoresof6andabove(i.e.,definiteFHand probableFH)wereincludedinthecurrentstudythescreening processforthestudysampleisshownin Figure1

Questionnairesurveyandclinical evaluation
Questionnaire
Thequestionnaireforthehyperlipidemiafamilyblood specimencollectionprojectwaspre-designedbythe researcherstoobtaingeneraldemographicinformation, lifeanddietaryhabits,diseasehistoryandmedicationhistory oftheparticipants.Thesurveyinvolvedinthisstudymainly includedthefollowingaspects:(1)generaldemographic information:suchasethnicity,gender,age,etc.;(2)lifestyle habits:suchassmoking,alcoholconsumption,physicalexercise, etc.;(3)dietaryhabits:dietarypreferences;(4)diseasehistory: pasthistory,currentdiseasehistoryandfamilyhistory,etc.;(5) medicationhistory:typeofmedication,nameofmedication, startdateofmedication,doseofmedication,etc.
Clinicalassessment
Clinicalassessmentswereperformedbyuniformlytrained clinicians.BMIwascalculatedbydividingweight(kg)by thesquareofheight(m2),usinganoverweightcut-off pointof24kg/m2 suitableforBMIinAsianpopulations (29),andthestudypopulationwasdividedintooverweight andnon-overweightgroups.WHRwasobtainedbydividing waistcircumference(cm)byhipcircumference(cm),and WHtRcalculatedaswaistcircumference(cm)/height(cm.)
WHtR ≥ 0.5andWHR ≥ 0.9inmenand ≥ 0.85inwomen wereconsideredcentrallyobese(30).Xanthomaincluded tendinousxanthoma(whichcouldbelocatedthebackofthe fingers,elbows,knees,orelsewhereandalsoincludedthe thickeningoftheAchillestendon)andtuberousxanthoma, aswellasrashandflatxanthoma.Fastingbloodglucose (FBG) ≥ 7.0mmol/Lorthosewhohadbeentreatedwith hypoglycemictherapywereidentifiedashavingdiabetes.With regardtofamilyhistoryofdisease,itwasdefinedasa familyhistoryofappropriatediseaseinfirst-degreerelatives (i.e.,children,parents,andsiblings)andgrandparentsin second-degreerelativesofstudysubjects,includingdiabetes, hypertension,hypercholesterolemia,andCVD(coronaryheart disease,stroke).Systolicbloodpressure(SBP) ≥ 140 mmHg or diastolicbloodpressure(DBP) ≥ 90 mmHg measuredthree timesonnon-samedayortakingantihypertensivemedication wasdefinedashypertension.Meanarterialpressure(MAP)was calculatedas(SBP+2DBP)/3.Thosewhosmokedwithinthe
WangandHe 10.3389/fcvm.2022.1026243
FIGURE1 Flowchartofthestudysample.FH,familialhypercholesterole mia;TG,triglycerides.
Frontiersin CardiovascularMedicine 03 frontiersin.org 113
past6monthsandreached1cigarette/daywereclassifiedas smokers,thosewhoneversmokedorsmokedoccasionallybut didnotmeetthesmokingcriteriaorquitsmokingformorethan 1yearwereclassifiedasnon-smokers.Thosewhodrankalcohol continuouslyformorethan6monthsanddrankalcoholatleast onceaweekonaveragewereclassifiedasalcoholdrinkers,and thosewhoneverdrankalcoholordrankoccasionallybutdid notmeetthecriteriafordrinkingalcoholwereclassifiedasnondrinkers.TheAIPriskwasdividedintothreegroups:(1)low risk,AIP < 0.11;(2)moderaterisk,AIP ≥ 0.11and ≤ 0.21;and (3)highrisk,AIP > 0.21(31, 32).
Laboratorytests
Theresultsofthelaboratoryanalyseswereobtainedfrom thesubjects’dataprofiles.Theanalysisofbiochemicalitems suchasthefulllipidpanelwasperformedattheLaboratory DepartmentoftheFirstHospitalofLanzhouUniversity,and allbloodsamplingwasperformedonthefollowingmorning after8–12hoffasting,withappropriatequalitycontrol, usingthesamefullyautomatedbiochemicalanalyzer.Serum TC,HDL-C,LDL-C,andTGconcentrationsweremeasured byapplyingenzymecolorimetricmethod,serumApoA1, ApoBandLipoprotein(a)[Lp(a)]levelsweremeasuredby applyingimmunoturbidimetricmethod,andserumFBGlevels weremeasuredbyapplyinghexokinasemethod.Theabove biochemicalitemswereperformedinanautomatedbiochemical analyzer(BeckmanCoulter,Brea,CA,USA).Basedon independentlipidparameters,thefollowingclinicalindicators werecalculated:non-HDL-C,AIP,BAR,LDL-C/ApoBratio, HDL-C/ApoA1ratio,LDL-C/HDL-Cratio,andAC.non-HDLCvalueswereobtainedbysubtractingHDL-CvaluesfromTC values.AIPwascalculatedasLog10 (TG/HDL-C)(15).
Statisticalanalysis
DatawereanalyzedusingSPSSversion20.0(SPSS, Inc.,Chicago,IL,USA).Forcontinuousvariables,normal distributionwasexpressedasmean ± standarddeviation (x ± s)andnon-normaldistributionwasexpressedasmedian andquartiles[M(P25,P75)];forcategoricalvariables,it wasexpressedasnumberandpercentage(N/%).Normality ofcontinuousvariableswastestedusingShapiro-Wilktest andQ-Qplottest.Inclinicalcharacteristicsandbiochemical parametersbetweengroups,forsomephysicalandblood indicatorssuchasMAP,BMI,WHR,whicharenormally distributedcontinuousvariables,theStudent’s t-testwasapplied toanalyzethedifferencesbetweentwoindependentvariables, andthechi-squaretestwasperformedbeforethe t-test;forTG andLp(a),whicharetwocontinuousvariablesthatdonotobey normaldistribution,theMann-Whitney U-testwasapplied
toanalyzethedifferencesbetweentwoanalysisofvariance betweenindependentvariables.Fortheanalysisofvariance ofcategoricalvariablessuchasmales,smokers,andalcohol drinkers,weappliedthechi-squaretest.Pearsoncorrelation analysiswasusedtodeterminetherelationshipbetweenBMI, WHR,andWHtRandthelevelsofAIP,BAR,andAC.Multiple stepwiselinearregressionanalysiswasusedtodeterminethe independentcorrelationsbetweenBMI,WHR,andWHtRand thelevelsofAIP,BAR,andAC.Receiveroperatingcharacteristic (ROC)analysiswasusedtoexploretheperformanceof cardiovascularriskpredictorsinidentifyingoverweightand centralobesity. P < 0.05wasconsideredstatisticallysignificant, andalltestsweretwo-sided.
Results
Basicinformationoffamilial hypercholesterolemiapatients
Afterexcludingsampleswithmissingkeydatasuchasbasic information,overweightandobesity-relatedindicatorsandlipid indicators,atotalof103patientswithFHfrom17familylines werefinallyincludedinthisstudy.Asshownin Table1,the studysubjectswereallHanChinese,including39(37.9%)males and64(62.1%)females,withanagerangeof18–85years, anoverallmeanageof(46.12 ± 14.29)years,andanoverall meanBMIof(23.63 ± 3.39)kg/m2.Thestudysubjectswere classifiedaccordingtoBMIintooverweightgroup(53,51.4%) andnon-overweightgroup(50,48.6%),andtheresultswere comparedbetweenthetwogroupsforbasicconditionsshowing age(P =0.019),xanthoma(P =0.027),hypertension(P < 0.001), MAP(P < 0.001),BMI(P < 0.001),WHR(P < 0.001), WHtR(P < 0.001),FBG(P =0.012),TG(P =0.002),HDLC(P =0.016),ApoA1(P =0.007),AIP(P =0.001),BAR (P =0.003),AC(P =0.029),andLDL-C/HDL-Cratio(P =0.035) werestatisticallysignificantbetweenthetwogroups,while gender,smoking,alcoholconsumption,dietaryoiliness,dietary saltiness,historyofcoronaryheartdisease,TC,LDL-C,ApoB, Lp(a),non-HDL-C,LDL-C/ApoB,andHDL-C/ApoA1werenot statisticallysignificantbetweenthetwogroups(P > 0.05).The studysubjectsweredividedintocentralobesitygroup(59cases, 59%)andnon-centralobesitygroup(41cases,41%)according toWHRandWHtR,andthebasicconditionswerecompared betweenthetwogroups,whichshowedthatage(P =0.001), male(P =0.023),smoking(P =0.024),xanthoma(P =0.014), hypertension(P =0.003),MAP(P < 0.001),BMI(P < 0.001), WHR(P < 0.001),WHtR(P < 0.001),FBG(P =0.007), TG(P < 0.001),HDL-C(P =0.010),ApoA1(P =0.007), andAIP(P < 0.001)werestatisticallysignificantbetweenthe twogroups.statisticallysignificant,whereasthedifferencesin alcoholconsumption,dietaryoiliness,dietarysalinity,history
WangandHe 10.3389/fcvm.2022.1026243
Frontiersin CardiovascularMedicine 04 frontiersin.org 114
TABLE1BasicClinicalfeaturesofpatientswithfamilialhypercholesterolemia.
Dataarepresentedasmean ± standarddeviation,median(25th–75thpercentile)or n (%).Boldvaluesindicatestatisticalsignificance.
ofcoronaryheartdisease,TC,LDL-C,ApoB,Lp(a),non-HDLC,LDL-C/ApoB,BAR,AC,LDL-C/HDL-C,LDL-C/ApoB,and HDL-C/ApoA1werenotstatisticallysignificantbetweenthetwo groups(P > 0.05).
Relationshipbetweenoverweightand obesity-relatedindicatorsand cardiovascularriskpredictorsin patientswithfamilial hypercholesterolemia
Simplecorrelationanalysis
Furtheranalysisoflinearrelationshipsbetweenoverweight andobesity-relatedindicatorsandconventionallipidprofiles andlipid-relatedcalculatedparametersinpatientswithFH,as shownin Table2,revealedthatBMIwassignificantlynegatively
correlated(P < 0.01)withHDL-C(r = 0.284)andApoA1 (r = 0.269),andwithAIP(r =0.385),BAR(r =0.348) andAC(r =0.256)weresignificantlypositivelycorrelated (P < 0.01);WHRwassignificantlynegativelycorrelatedwith HDL-C(r = 0.303)andApoA1(r = 0.361)(P < 0.01) andpositivelycorrelatedwithTG(r =0.329),AIP(r =0.501) andBAR(r =0.287)(P < 0.01).WHtRshowedsignificant negativecorrelations(P < 0.05)withHDL-C(r = 0.196) andApoA1(r = 0.203),andsignificantpositivecorrelations (P < 0.01).Theoverallsignificantcorrelationsofoverweightand obesity-relatedindicatorswithAIP,BARandACamonglipid parametersinFHpatientswereshown,andthescatterplotsof correlationsbetweenBMI,WHRandWHtRandAIP,BAR,and ACwereplottedin Figure2
Independentcorrelationanalysis
Giventheabovecorrelations,independentcorrelation analysesbetweenBMI,WHRandWHtRandAIP,BARandAC
WangandHe 10.3389/fcvm.2022.1026243
VariablesAll n =103BMI ≥ 24kg/m2 P Centralobesity P No(n =53)Yes(n =50)No(n =59)Yes(n =41) Age(years)46.12 ± 14.2942.94 ± 16.2749.48 ± 11.04 0 019 42.19 ± 15.8051.54 ± 10.24 0 001 Male(N/%) 39(37.9%) 17(32.1%) 22(44%)0 21217(28.8%) 21(51.2%) 0 023 Smokers(N/%) 28(27.2%) 12(22.6%) 16(32%)0 28611(18.6%) 16(39%) 0 024 Alcoholdrinkers(N/%)45(43.7%) 23(43.4%) 22(44%)0 28626(44.1%) 16(39%)0 615 Dietaryoiliness(N/%)21(20.4%) 12(22.6%) 9(18%)0 55912(20.3%) 9(22%)0 846 Saltydiet(N/%) 27(26.2%) 10(18.9%) 17(34%)0 08111(18.6%) 15(36.6%)0 063 CHDhistory(N/%) 5(4.8%) 2(3.8%) 3(6%) 0 5993(5.1%) 2(4.9%)0 925 Xathoma(N/%) 18(17.5%) 5(9.4%) 13(26%) 0 027 6(10.2%) 12(29.3%) 0 014 Hypertension(N/%) 34(33%) 8(15.1%) 26(52%) <0 001 12(20.3%) 20(48.8%) 0 003 MAP(mmHg) 91.24 ± 14.1185.77 ± 12.4097.03 ± 13.58 <0 001 86.47 ± 12.4997.19 ± 13.83 <0 001 BMI(kg/m2) 23.63 ± 3.3920.94 ± 1.9126.48 ± 1.99 <0 001 21.85 ± 2.7225.97 ± 2.73 <0 001 WHR 0.87 ± 0.08 0.83 ± 0.07 0.91 ± 0.08 <0 001 0.82 ± 0.060.94 ± 0.06 <0 001 WHtR 0.51 ± 0.06 0.47 ± 0.04 0.55 ± 0.04 <0 001 0.47 ± 0.040.56 ± 0.04 <0 001 FBG(mmol/L) 4.96 ± 0.79 4.77 ± 0.68 5.16 ± 0.85 0 012 4.78 ± 0.625.21 ± 0.93 0 007 TC(mmol/L) 5.86 ± 1.41 5.82 ± 1.21 5.92 ± 1.590 7245.95 ± 1.275.74 ± 1.610 483 TG(mmol/L) 1.48(0.90–2.34)1.10(0.76–1.88)1.78(1.10–2.61) 0 002 1.1(0.77–1.72)1.92(1.38–2.78) <0 001 HDL-C(mmol/L) 1.33 ± 0.27 1.39 ± 0.28 1.26 ± 0.25 0 016 1.39 ± 0.261.25 ± 0.27 0 010 LDL-C(mmol/L) 3.95 ± 1.30 3.82 ± 1.12 4.08 ± 1.460 3133.95 ± 1.203.90 ± 1.460 876 ApoA1(g/L) 1.43 ± 0.27 1.50 ± 0.29 1.36 ± 0.24 0 007 1.49 ± 0.281.34 ± 0.25 0 007 ApoB(g/L) 0.93 ± 0.50 0.86 ± 0.22 0.91 ± 0.250 2620.89 ± 0.250.87 ± 0.220 658 Lp(a)(mg/L) 249.5(168–309)227.5(175–324)256.5(156–304)0 905238(168–329)256.5(147–286)0 762 AIP 0.05 ± 0.30-0.05 ± 0.290.15 ± 0.28 0 001 0.06 ± 0.270.21 ± 0.29 <0 001 BAR 0.63 ± 0.17 0.58 ± 0.15 0.68 ± 0.18 0 003 0.61 ± 0.160.66 ± 0.190 103 AC 3.50 ± 1.17 3.26 ± 0.90 3.76 ± 1.35 0 029 3.35 ± 0.943.68 ± 1.340 168 LDL-C/HDL-C 3.05 ± 1.14 2.82 ± 0.92 3.28 ± 1.29 0 035 2.91 ± 0.963.19 ± 0.160 227 LDL-C/ApoB 4.49 ± 0.95 4.53 ± 1.04 4.44 ± 0.860 6494.50 ± 1.034.42 ± 0.860 693 HDL-C/ApoA1 0.93 ± 0.12 0.93 ± 0.12 0.93 ± 0.130 9110.94 ± 0.120.93 ± 0.130 845 Non-HDL-C(mmol/L)4.54 ± 1.32 4.42 ± 1.11 4.65 ± 1.510 3854.55 ± 1.194.49 ± 1.520 822
Frontiersin CardiovascularMedicine 05 frontiersin.org 115
TABLE2Correlationanalysisofoverweightandobesityindicators andlipidparametersinpatientswithfamilialhypercholesterolemia.
AIPhasthebestpredictiveperformanceforoverweightand obesityamongcardiovascularriskpredictors,whilethearea underthecurvesuggeststhepossibilitythatAIPitspredictive performanceforobesityisbetterthanthatforoverweight;in addition,thecombinedAIP,BAR,andACthreeindicators haveamoderatepredictiveperformanceforoverweightinFH patients.
Analysisoftheidentification performanceofoverweightand
obesity-relatedindicatorsinfamilial hypercholesterolemiapatientsfor moderateandhighriskofatherogenic indexofplasma
wereperformedbyapplyingmultiplestepwiselinearregression, asshownin Tables3–5.Afteradjustingforage,sex,smoking, xanthoma,MAPandFBG,theresultsshowedthatindependent correlationswithBMIwereAIP(β =0.020, P =0.013)and BAR(β =0.015, P =0.003),AIP(β =1.176, P =0.001) independentlyassociatedwithWHR,andAIP(β =1.575, P =0.001),BAR(β =0.661, P =0.024)andAC(β =0.427, P =0.035)independentlyassociatedwithWHtR.Itcanbeseen thatoverweightandobesity-relatedindicatorsBMI,WHR,and WHtRallhadindependentpositivelinearcorrelationswith AIP.
Predictiveperformanceanalysisof cardiovascularriskpredictorsfor overweightandobesityinpatientswith familialhypercholesterolemia
TofurtherassesstheroleofAIP,BARandACinidentifying overweightaswellascentralobesityconditionsinFHpatients, weplottedROCcurves,whichshowedthattheareaunder theROC(AUC)foroverweightwhenAIP,BAR,ACand combinedtripleindicatorswere0.695(95% CI =0.593–0.797, P =0.001),0.660(95% CI =0.555–0.766, P =0.005),0.632 (95% CI =0.525–0.740, P =0.021)and0.710(95% CI =0.611–0.810, P < 0.001),respectively,asshownin Figure3A; theAUCsforcentralobesitywithAIP,BARandACand thecombinationofallthreewere0.757(95% CI =0.656–0.857, P < 0.001),0.654(95% CI =0.536–0.771, P =0.012), 0.651(95% CI =0.538–0.764, P =0.013)and0.762(95% CI =0.666–0.858, P < 0.001), Figure3B.Itcanbeseenthat
AIPisknowntohavethebestpredictiveperformance foroverweightandobesitybasedonBMI,WHRandWHtR judgments,however,toexplorewhichindicatorismore accurateforidentifyingtherisklevelofAIP,theAUCwas furtherusedtocomparethethreeoverweightandobesity relatedindicatorsforidentifyingmoderateriskofAIPand highriskofAIP,respectively,andtheresultsshowedthat theAUCforBMI,WHR,andWHtRformoderaterisk were0.709(95% CI =0.608–0.811, P < 0.001),0.773(95% CI =0.678–0.867, P < 0.001),and0.739(95% CI =0.641–0.836, P < 0.001),respectively,asshownin Figure4A;the AUCsofBMI,WHR,andWHtRforAUCforhighriskof AIPwere0.691(95% CI =0.585–0.797, P =0.002),0.734(95% CI =0.632–0.835, P < 0.001),and0.706(95% CI =0.603–0.810, P =0.001),respectively,asshownin Figure4B.It canbeseenthatthethreeoverweightandobesity-related indicatorsBMI,WHR,andWHtRhavegoodidentification performanceforbothmoderateandhighriskofAIP,with WHRhavingthelargestAUC,followedbyWHtR,andBMI havingthesmallest.ItissuggestedthatWHRhasbetterand morerobustperformanceinidentifyingmoderateandhigh riskofAIP.Asforthecombineddiagnosticeffectiveness,the AUCofcombiningBMI,WHR,andWHtRwas0.782(95% CI =0.689–0.874, P < 0.001)formoderateriskofAIPand 0.749(95% CI =0.648–0.850, P < 0.001)forhighriskofAIP. ItshowedthatthecombinationofBMI,WHR,andWHtR hadamoderatelevelofdiscriminationabilityformoderate andhighriskofAIPalthoughthediscriminationperformance wasnotsignificantlyimprovedcomparedtotheindividual indicators.
Discussion
Inthisstudy,byanalyzingthecorrelationbetween cardiovascularriskpredictorsandoverweightandobesityrelatedindicatorsinpatientswithFH,theresultsshowed
WangandHe 10.3389/fcvm.2022.1026243
BMIWHRWHtR rPrPrP TC(mmol/L)0 0480 634 0 1120 2720 1360 173 TG(mmol/L) 0 2330 0180 329 0 001 0 310 0 002 HDL-C(mmol/L) 0 284 0 004 0 303 0 002 0 196 0 048 LDL-C(mmol/L)0 1260 207 0 0170 8650 216 0 029 ApoA1(g/L) 0 269 0 006 0 361 <0 001 0 203 0 041 ApoB(g/L) 0 1610 1050 0310 7600 1860 061 Lp(a)(mg/L) 0 0580 6890 0350 810 0 0690 633 AIP 0 385 <0 001 0 501 <0 001 0 465 <0 001 BAR 0 348 <0 001 0 287 0 004 0 327 0 001 AC 0 256 0 009 0 1210 2330 275 0 005 LDL-C/HDL-C0 2470 0120 1230 2240 2820 004 LDL-C/ApoB 0 0590 554 0 1280 2060 0220 823 HDL-C/ApoA1 0 0500 6150 0560 584 0 0090 929 Non-HDL-C(mmol/L)0 1090 274 0 0570 5770 1850 062
Pearsoncorrelationanalyseswereused.Boldvaluesindicatestatisticalsignificance.
Frontiersin CardiovascularMedicine 06 frontiersin.org 116
BMI(un-adjusted)BMI(adjusted)
WHR(un-adjusted)WHR(adjusted)
Multivariablestepwiselinearregressionmodelsareshown.Adjustedconfounders includedage,sex,smoking,xanthoma,MAPandFBG.Boldvaluesindicate statisticalsignificance. a “/”denotesnoindependentcorrelation.
thatAIPwasindependentlyassociatedwithBMI,WHR andWHtR,BARwasindependentlyassociatedwithBMI andWHtR,andACwasindependentlyassociatedwith WHtR.AlthoughindependentcorrelationsbetweenAIPand BMIhavebeenreported(33)andbetweenBARandwaist circumference(34),uptonow,inpatientswithFH,the
//
Multivariablestepwiselinearregressionmodelsareshown.Adjustedconfounders includedage,sex,smoking,xanthoma,MAPandFBG.Boldvaluesindicate statisticalsignificance.
presentstudyisthefirsttoreportcorrelationsregardingthe groupofcardiovascularriskpredictorsinpatientswithFH withthegroupofoverweightandobesity-relatedindicators. Thesignificanceofthisstudyisthat(1)byanalyzing thecorrelationbetweencardiovascularriskpredictorsand overweightandobesity-relatedindicatorsinpatientswith

WangandHe 10.3389/fcvm.2022.1026243
FIGURE2 Scatterplotsofcorrelationbetweenoverweightandobesityindicatorsandcardiovascularriskpredictors.
TABLE3Independentcorrelationanalysisofcardiovascularrisk predictorswithBMI.
Constant β P Constant β P AIP 0 7650 035 <0 001 1.1690.020 0 013 BAR0 2100 018 <0 001 0.1380.015 0 003 AC1 4120 088 0 009 /a //
TABLE4Independentcorrelationanalysisofcardiovascularrisk predictorswithWHR.
Constant β P Constant β P AIP 1 5571 848 <0 001 1.6921.176 0.001 BAR0 1050 605 0 004 /
AC1 9911 7160 233/
//
Frontiersin CardiovascularMedicine 07 frontiersin.org 117
TABLE5Independentcorrelationanalysisofcardiovascularrisk predictorswithWHtR.
WHtR(un-adjusted)WHtR(adjusted)
Multivariablestepwiselinearregressionmodelsareshown.Adjustedconfounders includedage,sex,smoking,xanthoma,MAPandFBG.Boldvaluesindicate statisticalsignificance.
FHfamilies,itprovidesatheoreticalbasisforactively controllingoverweightandobesity-relatedindicatorsinFH patientsandthusreducingCVDrisk,whichhasimportant publichealthimplications;(2)amongcardiovascularrisk predictors,AIPwasfoundtohavethestrongestpredictive effectonoverweight,especiallycentralobesity,whichprovides abasisforidentifyingoverweight,especiallyobesity,through cardiovascularriskpredictors.

AIPisamorecomprehensiveindicatorofthebalanced relationshipbetweenatheroprotectiveandatherogenicfactors thanasimplelipidindex.Theresultsofthisstudyalso showedthatAIPamongcardiovascularriskpredictorshas abetterperformancethanBARandACbothintermsof independentcorrelationwithoverweightandobesity-related indicatorsandintermsofidentificationofoverweightand obesity.Infact,Shenetal.havereportedthatAIPcanbe avalidindicatorfortheassessmentofabdominalobesity (26),andarecentcross-sectionalstudyfromaChinese populationalsoconcludedthatAIPwasanovelandgood biomarkerassociatedwithabdominalobesity(35).Thisis consistentwiththeresultsoftheROCcurveanalysisin thepresentstudy.GiventhesuperiorityofAIPoverlipid indicesalone,coupledwiththefactthatitcanbeeasily calculatedfromconventionallipidprofiles,AIPhasgradually beenincreasinglyfavoredandusedinclinicalpracticetoguide theassessmentofCVDriskanddiseaseprognosisinrecent years(16, 13, 36–38).Therefore,itisreasonabletoassume thatCVDriskisfurtherelevatedinthoseobesepopulations inFHpatientsbytheanalysisofthisstudy.Asforthe studyonAIPinFH,TomášFreiberger’steamcomparedthe levelsoflipid-relatedparametersbetweenFHpatientswith andwithoutahistoryofCVDandfoundthatAIPandTG weresignificantlyhigherinthosewithCVDeventsinFH, concludingthatAIPisassociatedwithahistoryofCVD inFHpatients,whichreflectstheatheroscleroticsmallLDL andsmallHDLparticlespresence,whichmaybeassociated withtheriskofCVDinFHpatients(34).Theresultsof thepresentstudyshowedsignificantlyhigherTGandAIPas wellassignificantlylowerHDL-Cinoverweightindividuals withFH,suggestinganimbalancebetweenatherogenicand anti-atherogenicfactors.Moreover,infurthermultifactorial
regressionanalysisinthisstudy,BMIwasindependently associatedwithAIP,andBMIwasanindependentriskfactor forincreasedrisklevelofAIP,suggestingthatoverweight patientswithFHareathigherriskofCVD(33).Itiswell knownthatthemainclinicalmanifestationsofFHpatientsare significantlyelevatedatherogeniclipidindicatorsLDL-Cand TC,andCVDeventsarethemaincauseofdeathinFHpatients, andifBMI,anindicatorassociatedwithoverweightandobesity, isnoteffectivelycontrolledinthesepatients,itwilladdto theirhighCVDrisk.
BARrepresentsthebalancebetweenApoB-richatherogenic particlesandApoA1-richanti-atherogenicparticles(17, 39)and


WangandHe 10.3389/fcvm.2022.1026243
Constant β P Constant β P AIP 1 1242 314 <0 001 0 9671 575 0 001 BAR0 1600 927 0 001 0 0460 661 0 024 AC0 8415 244 0 005 1 2880 427 0 035
FIGURE3
(A) PredictiveperformanceanalysisofAIP,BAR,andACfor overweight. (B) PredictiveperformanceanalysisofAIP,BAR,and ACforcentralobesity.
Frontiersin CardiovascularMedicine 08 frontiersin.org 118
isalsoconsideredasapotentialmarkerofcardiovascularrisk duetothefactthatthisratiocanoftenbeabnormalinthe presenceofnormalconventionallipidlevels(21).Itisgenerally acceptedthataBARabove0.9isassociatedwithahighrisk ofCVD(40),alongwithhigherTGlevels,AIPvaluesand lowerHDL-Clevels(17).ShowingthatBARwassignificantly andpositivelycorrelatedwithAIP,whichisalsoconsistent withwhatweobservedinourresults,somestudieshavealso pointedoutthatAIPreflectsthequalitativecompositionof lipoproteins,whileBARshowstheirquantity.Sincetheyhave differentbutcomplementaryemphases,wesuspectthatthey areintrinsicallylinkedandhypothesizethatthereshouldbe consistencyinthemanifestationofsomediseases,andthat combiningthesetwoindicestopredictcertaindiseasesmaybe promising.Currently,althoughtherelationshipbetweenBMI
andBARisunclear,theresultsofsomestudiessuggestthat theremaybeanintrinsicassociationbetweenBMIandBAR. AcohortstudyfromChinashowedthatbothBMIandBARwere significantlyelevatedinpatientswithlactinoma(41),suggesting thattheremaybesomeintrinsicassociationbetweenBMIand BARinthediseasestate.Consistentwiththis,theresultsofthe presentstudyshowedthatBMIwasindependentlyassociated withBARinFHpatients,suggestingthatBMIisanindependent influenceontheelevatedBARinFHpatients,andthepresent studyalsofoundthatBARindicatorsweresignificantlyhigher inoverweightindividualsthaninnon-overweightindividuals, suggestingthatoverweightfactorsfurtherincreasetheriskof CVDeventsinFHpatients.
Severalotherlipid-relatedparameters,including non-HDL-C(42),AC(12),LDL-C/HDL-C(43),LDL-C/ApoB ratio(44, 45),andHDL-C/ApoA1ratio(46, 47),havealsobeen reportedtobeassociatedwithCVDrisk.However,theresults ofthecurrentstudydidnotshowanindependentcorrelation betweenBMIandtheseindicators.Althoughtheresultsofthe presentstudyshowedasignificantcorrelationbetweenBMI andLDL-C/HDL-CandACinpatientswithFH,thecorrelation betweenthemwasfoundtodisappearafteradjustingfor confoundingfactors.


Itisgenerallyacceptedthatage,maleandbloodpressureare importantriskfactorsforcardiovascularevents,andthisisalso trueinpatientswithFH.Consistently,theresultsofthecurrent studyalsoshowedthatAIPwassignificantlycorrelatedwithage, maleandMAPinadditiontoBMIindependently,suggestinga higherriskofCVDinmenwithFHthaninwomen,andthe possibilitythatbloodpressureisalsoariskfactorforCVDin patientswithFH(27, 36, 48).
TheresultsofthisstudyshowedthatbothAIPandBAR hadsignificantindependentcorrelationsforBMI.However,by plottingROCcurves,itwasshownthatAIPwasslightlybetter thanBARinpredictingoverweight.Ourresultsalsoshowed thatcardiovascularriskpredictorsAIP,BAR,andACwere allindependentlycorrelatedwithWHtRamongoverweight andobesity-relatedindicators,butcomparativeanalysisof ROCcurvesrevealedthatAIPwasthestrongestidentifierof centralobesityamongthethreecardiovascularriskpredictors. Consistentwiththisresult,onestudyfoundthatAIPwas significantlyassociatedwithBMIbutnotBARbyanalyzing changesincardiometabolicmarkersinoverweight/obese childrenbeforeandafterlifestyleinterventions;theirresults alsoshowedthatAIPwasstronglyassociatedwithobesity, whereasBARwasnotsignificantlyassociatedwithobesity(49). AlthoughAIPisacalculatedvalue,itisasensitiveindicatorof dyslipidemiaandmayindirectlyreflectthediameterofLDL-C particles(50).Therefore,wehypothesizedthatthecombination ofBMIandAIPcouldincreasethespecificityandsensitivity ofoverweightandevenobesitydetectioninclinicalpractice. FromShenetal.showedthatanAIPof0.11–0.21or > 0.21 suggestedthepossibilityofborderlineabdominalobesityor

WangandHe 10.3389/fcvm.2022.1026243
FIGURE4
(A) PerformanceanalysisofBMI,WHR,andWHtRforidentifying moderateriskofAIP. (B) PerformanceanalysisofBMI,WHR,and WHtRforidentifyinghighriskofAIP.
Frontiersin CardiovascularMedicine 09 frontiersin.org 119
abdominalobesity,respectively,byexaminingtherelationship betweenwaistcircumferenceandAIP,suggestingthatAIPcan beusedasareferenceforestimatingabdominalobesity(26). Similarly,ourresultsshowalinearcorrelationbetweenBMI andAIP,accordingtoourderivedmathematicalexpression fortherelationshipbetweenAIPandBMI,anincreasein BMIof1.0kg/m2 causesanincreaseinAIPof0.035,anAIP valueof0.110whenBMIis25kg/m2,andanAIP ≥ 0.215 whenBMI ≥ 28kg/m2,whichisessentiallyconsistentwith BMI ≥ 25and ≥ 28kg/m2 correspondtomoderaterisk (≥ 0.11)andhighrisk(≥ 0.21)forAIP,respectively,which alsoindicatesthatmoderateriskAIPindicatesoverweight,while highriskAIPindicatesthepresenceofobesity.WHtR ≥ 0.5 and/orWHR ≥ 0.9inmenand ≥ 0.85inwomenare knowntobeconsideredcentrallyobese(30).Accordingtothe mathematicalexpressionofthepresentstudyresultsAIPand WHR,AIP=1.848WHR-1.557,bringingtheAIPvaluesof0.11 and0.24intotheformula,theresultingWHRvaluesare0.90 and0.97,respectively,indicatingthatcentralobesityjudged basedonWHRcorrespondstoamoderateriskofAIP,and whenWHRexceeds0.97,patientswithFHareathighrisk ofAIP.AccordingtothemathematicalexpressionofAIPand WHtRoftheresultsofthisstudy,AIP=2.314WHtR-1.124, bringingtheAIPvaluesof0.11and0.24intotheformula,the resultingWHtRvaluesare0.53and0.59,respectively,andit canbebasicallyconcludedthatcentralobesityjudgedbased onWHtRcorrespondstomoderateriskofAIP,andwhen WHtRexceeds0.59FHpatientswouldhaveahighriskof AIP.Thus,itcanbeseenthatifobesityjudgedbasedonBMI hasahighriskofAIP,whilecentralobesityjudgedbasedon WHtRandWHRhasamoderateriskofAIP.Ontheother hand,theassessmentofAIPriskbasedonBMImaybemore sensitivethantheassessmentofAIPriskbasedonWHtRand WHR.However,theAIPrisklevelcorrespondingtoobesity judgedbasedonBMIandtheAIPrisklevelcorrespondingto centralobesityjudgedbasedonWHRcombinedwithWHtR derivedfromthisstudycontradicteachother.Inviewofthis, whichofBMI,WHtRandWHRidentifiesthemorereliable AIPrisk,thepresentstudyagaincomparedtheidentification ofthesethreeoverweightandobesity-relatedindicatorsfor intermediateAIPriskandhighAIPrisk,respectively,using AUC,andtheresultsshowedthatWHRhadthelargest AUC,WHtRthesecondlargest,andBMIthesmallestfor bothintermediateAIPriskandhighAIPrisk.Itissuggested thatWHRmaybeabetterandmorerobustidentifierof overweightandobesity-relatedindicatorsformoderateand highriskofAIP.
However,therearestillsomeshortcomingsinthis study:(1)Althoughourconclusionswereobtainedbased onretrospectivedataanalysis,thecausalrelationship betweenBMIandcardiovascularriskpredictorssuchas
AIPhasnotbeenclearlyansweredinthisstudy,anddeeper mechanismsbasedongeneticdiagnosisneedtobefurther explored.(2)WiththeacceleratedurbanizationinChina, theincreaseof“smallfamily”hasmadethecollectionof FHfamilycasesmoredifficult.Althoughthesamplesize ofthisstudyiseligibleforthissmallincidencegenetic disease,alargersamplesizestudyisstillnecessaryto improvetherobustnessoftheresultsandthereliabilityof theconclusions.
Conclusion
(1)Overweightandobesity-relatedindicatorsBMI,WHR andWHtRinFHpatientsallhadindependentpositive linearcorrelationswithAIP;(2)amongcardiovascular riskpredictors,AIPhasbetterperformanceforpredicting overweightandobesity;(3)overweightandobesity-related indicatorshadbetterperformanceinidentifyingbothmedium andhighriskforAIP,amongwhichWHRhadthebest performanceinidentifyingmediumandhighriskforAIPin patientswithFH.
Dataavailabilitystatement
Thedataanalyzedinthisstudyissubjecttothefollowing licenses/restrictions:Thedatapresentedinthisstudyare availableonreasonablerequestfromthecorrespondingauthor. RequeststoaccessthesedatasetsshouldbedirectedtoJH, hejch@lzu.edu.cn
Ethicsstatement
Writteninformedconsentwasobtainedfromthe individual(s)forthepublicationofanypotentiallyidentifiable imagesordataincludedinthisarticle.
Authorcontributions
JH:conceptualization,datacuration,fundingacquisition, projectadministration,resources,andsupervision.YW:formal analysis,investigation,methodology,software,andwriting— originaldraft.JHandYW:validationandwriting—reviewand editing.Bothauthorscontributedtothearticleandapprovedthe submittedversion.
WangandHe 10.3389/fcvm.2022.1026243
Frontiersin CardiovascularMedicine 10 frontiersin.org 120
Funding
ThisworkwassupportedbytheNationalScience andTechnologyMajorProjectoftheministryofScience andTechnologyofChinaduringthe“10thFive-Year Plan”(Grantno.2002DA711A028-17)andtheNatural ScienceFoundationofGansuProvince(Grantno. 1308RJZA218),China.
Acknowledgments
WewouldappreciatethefamilieswithFH fortheirpositivecontributionandwillingnessto participate.
References
1.Benito-VicenteA,UribeKB,JebariS,Galicia-GarciaU,OstolazaH,MartinC. Familialhypercholesterolemia:themostfrequentcholesterolmetabolismdisorder causeddisease. IntJMolSci. (2018)19:3426.doi:10.3390/ijms19113426
2.HuP,DharmayatKI,StevensCAT,SharabianiMTA,JonesRS,WattsGF, etal.Prevalenceoffamilialhypercholesterolemiaamongthegeneralpopulation andpatientswithatheroscleroticcardiovasculardisease:asystematicreviewand meta-analysis. Circulation. (2020)141:1742–59.doi:10.1161/circulationaha.119. 044795
3.KitaharaH,MoriN,SaitoY,NakayamaT,FujimotoY,KobayashiY.Prevalence ofachillestendonxanthomaandfamilialhypercholesterolemiainpatientswith coronaryarterydiseaseundergoingpercutaneouscoronaryintervention. Heart Vessels. (2019)34:1595–9.doi:10.1007/s00380-019-01400-6
4.BellDA,HooperAJ,WattsGF,BurnettJR.Mipomersenandothertherapies forthetreatmentofseverefamilialhypercholesterolemia. VascHealthRiskManag. (2012)8:651–9.doi:10.2147/vhrm.S28581
5.WattsGF,GiddingS,WierzbickiAS,TothPP,AlonsoR,BrownWV,etal. Integratedguidanceonthecareoffamilialhypercholesterolaemiafromthe internationalFHfoundation. IntJCardiol. (2014)171:309–25.doi:10.1016/j.ijcard. 2013.11.025
6.DeBackerG,BesselingJ,ChapmanJ,HovinghGK,KasteleinJJ,KotsevaK, etal.Prevalenceandmanagementoffamilialhypercholesterolaemiaincoronary patients:ananalysisofEUROASPIREIV,astudyoftheEuropeansocietyof cardiology. Atherosclerosis. (2015)241:169–75.doi:10.1016/j.atherosclerosis.2015. 04.809
7.Tomlinson,B,HuM,ChowE.Currentstatusoffamilialhypercholesterolemia inChinesepopulations. CurrOpinLipidol. (2019)30:94–100.doi:10.1097/MOL. 0000000000000580
8.DanielsSR.Howtoidentifychildrenwithfamilialhypercholesterolemia. J Pediatr. (2017)183:2.
9.AlonsoR,MataP,ZambónD,MataN,Fuentes-JiménezF.Earlydiagnosisand treatmentoffamilialhypercholesterolemia:improvingpatientoutcomes. Expert RevCardiovascTher. (2013)11:327–42.
10.PlanaN,Rodríguez-borjabadC,IbarretxeD,FerréR,FeliuA,CasellesA,etal. Lipidandlipoproteinparametersfordetectionoffamilialhypercholesterolemia inchildhood.TheDECOPINproject. ClinInvestigArterioscler. (2018)30:170–8. doi:10.1016/j.arteri.2017.12.003
11.CiceroAFG,FogacciF,GiovanniniM,BoveM,DebellisG,BorghiC.Effectof quantitativeandqualitativedietprescriptiononchildrenbehaviorafterdiagnosis ofheterozygousfamilialhypercholesterolemia. IntJCardiol. (2019)293:193–6. doi:10.1016/j.ijcard.2019.05.069
12.KaleliogluT,GencA,KaramustafaliogluN,EmulM.Assessment ofcardiovascularriskviaatherogenicindicesinpatientswithbipolar disordermanicepisodeandalterationswithtreatment. Diabetes
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
MetabSyndr. (2017)11(Suppl.1):S473–5.doi:10.1016/j.dsx.2017. 03.038
13.CaoB,FanZ,ZhangY,LiT.Independentassociationofseverityof obstructivesleepapneawithlipidmetabolismofatherogenicindexofplasma(AIP) andapoB/apoAIratio. SleepBreath. (2020)24:1507–13.doi:10.1007/s11325-02002016-1
14.NiroumandS,KhajedalueeM,Khadem-RezaiyanM,AbrishamiM,JuyaM, KhodaeeG,etal.Atherogenicindexofplasma(AIP):amarkerofcardiovascular disease. MedJIslamRepubIran. (2015)29:240.
15.DobiásováM,FrohlichJ.Theplasmaparameterlog(TG/HDL-C)asan atherogenicindex:correlationwithlipoproteinparticlesizeandesterificationrate inapoB-lipoprotein-depletedplasma(FER(HDL)). ClinBiochem. (2001)34:583–8. doi:10.1016/s0009-9120(01)00263-6
16.DobiásováM,FrohlichJ,SedováM,CheungMC,BrownBG.Cholesterol esterificationandatherogenicindexofplasmacorrelatewithlipoproteinsizeand findingsoncoronaryangiography. JLipidRes. (2011)52:566–71.doi:10.1194/jlr. P011668
17.KanevaAM,PotolitsynaNN,BojkoER,OdlandJ.Theapolipoprotein B/apolipoproteinA-Iratioasapotentialmarkerofplasmaatherogenicity. Dis Markers. (2015)2015:591454.doi:10.1155/2015/591454
18.WalldiusG,JungnerI,HolmeI,AastveitAH,KolarW,SteinerE.High apolipoproteinB,lowapolipoproteinA-I,andimprovementinthepredictionof fatalmyocardialinfarction(AMORISstudy):aprospectivestudy. Lancet. (2001) 358:2026–33.doi:10.1016/s0140-6736(01)07098-2
19.LamarcheB,MoorjaniS,LupienPJ,CantinB,BernardPM,DagenaisGR, etal.ApolipoproteinA-IandBlevelsandtheriskofischemicheartdiseaseduring afive-yearfollow-upofmenintheQuébeccardiovascularstudy. Circulation. (1996) 94:273–8.doi:10.1161/01.cir.94.3.273
20.Long-TermInterventionwithPravastatininIschaemicDisease(Lipid)Study Group.Preventionofcardiovasculareventsanddeathwithpravastatininpatients withcoronaryheartdiseaseandabroadrangeofinitialcholesterollevels. NEnglJ Med. (1998)339:1349–57.doi:10.1056/nejm199811053391902
21.WalldiusG,JungnerI.TheapoB/apoA-Iratio:astrong,newriskfactor forcardiovasculardiseaseandatargetforlipid-loweringtherapy–areviewof theevidence. JInternMed. (2006)259:493–519.doi:10.1111/j.1365-2796.2006. 01643.x
22.LuM,LuQ,ZhangY,TianG.ApoB/apoA1isaneffectivepredictorof coronaryheartdiseaseriskinoverweightandobesity. JBiomedRes. (2011) 25:266–73.doi:10.1016/s1674-8301(11)60036-5
23.IgharoOG,AkinfenwaY,IsaraAR,IdomehFA,NwobiNL,AnetorJI, etal.Lipidprofileandatherogenicindicesinnigeriansoccupationallyexposed toe-waste:acardiovascularriskassessmentstudy. Maedica. (2020)15:196–205. doi:10.26574/maedica.2020.15.2.196
WangandHe 10.3389/fcvm.2022.1026243
Frontiersin CardiovascularMedicine 11 frontiersin.org 121
24.AfshinA,ForouzanfarMH,ReitsmaMB,SurP,EstepK,LeeA,etal.Health effectsofoverweightandobesityin195countriesover25years. NEnglJMed. (2017)377:13–27.doi:10.1056/NEJMoa1614362
25.MatsuzawaY.Establishmentofaconceptofvisceralfatsyndromeand discoveryofadiponectin. ProcJpnAcadSerBPhysBiolSci. (2010)86:131–41. doi:10.2183/pjab.86.131
26.ShenSW,LuY,LiF,YangCJ,FengYB,LiHW,etal.Atherogenicindexof plasmaisaneffectiveindexforestimatingabdominalobesity. LipidsHealthDis. (2018)17:11.doi:10.1186/s12944-018-0656-1
27.NationalCholesterolEducationProgram(NCEP)ExpertPanelonDetection, Evaluation,andTreatmentofHighBloodCholesterolinAdults(AdultTreatment PanelIII).Thirdreportofthenationalcholesteroleducationprogram(NCEP) expertpanelondetection,evaluation,andtreatmentofhighbloodcholesterolin adults(adulttreatmentpanelIII)finalreport. Circulation. (2002)106:3143–421.
28.NordestgaardBG,ChapmanMJ,HumphriesSE,GinsbergHN,Masana L,DescampsOS,etal.Familialhypercholesterolaemiaisunderdiagnosedand undertreatedinthegeneralpopulation:guidanceforclinicianstopreventcoronary heartdisease:consensusstatementoftheEuropeanAtherosclerosisSociety. Eur HeartJ. (2013)34:3478–90a.doi:10.1093/eurheartj/eht273
29.WHOExpertConsultation.Appropriatebody-massindexforAsian populationsanditsimplicationsforpolicyandinterventionstrategies. Lancet. (2004)363:157–63.doi:10.1016/s0140-6736(03)15268-3
30.BrowningLM,HsiehSD,AshwellM.Asystematicreviewofwaist-to-height ratioasascreeningtoolforthepredictionofcardiovasculardiseaseanddiabetes: 0 5couldbeasuitableglobalboundaryvalue. NutrResRev. (2010)23:247–69. doi:10.1017/s0954422410000144
31.DobiásováM.Atherogenicindexofplasma[log(triglycerides/HDLcholesterol)]:theoreticalandpracticalimplications. ClinChem. (2004)50:1113–5. doi:10.1373/clinchem.2004.033175
32.SapunarJ,Aguilar-FaríasN,NavarroJ,AranedaG,Chandía-PobleteD, ManríquezV,etal.[Highprevalenceofdyslipidemiaandhighatherogenicindex ofplasmainchildrenandadolescents]. RevMedChil. (2018)146:1112–22.doi: 10.4067/s0034-98872018001001112
33.RašlováK,DobiášováM,HubáèekJA,BencováD,SivákováD,DankováZ, etal.Associationofmetabolicandgeneticfactorswithcholesterolesterification rateinHDLplasmaandatherogenicindexofplasmaina40yearsoldSlovak population. PhysiolRes. (2011)60:785–95.doi:10.33549/physiolres.932069
34.SoškaV,Jarkovskı J,RavèukováB,Tichı L,FajkusováL,FreibergerT.The logarithmofthetriglyceride/HDL-cholesterolratioisrelatedtothehistoryof cardiovasculardiseaseinpatientswithfamilialhypercholesterolemia. ClinBiochem. (2012)45:96–100.doi:10.1016/j.clinbiochem.2011.11.001
35.ZhuX,YuL,ZhouH,MaQ,ZhouX,LeiT,etal.Atherogenicindexofplasma isanovelandbetterbiomarkerassociatedwithobesity:apopulation-basedcrosssectionalstudyinChina. LipidsHealthDis. (2018)17:37.doi:10.1186/s12944-0180686-8
36.TautuOF,DarabontR,OnciulS,DeaconuA,ComanescuI,AndreiRD,etal. Newcardiovascularriskfactorsandtheiruseforanaccuratecardiovascularrisk assessmentinhypertensivepatients. Maedica. (2014)9:127–34.
37.ZhouK,QinZ,TianJ,CuiK,YanY,LyuS.Theatherogenicindexof plasma:apowerfulandreliablepredictorforcoronaryarterydiseaseinpatients
withtype2diabetes. Angiology. (2021)72:934–41.doi:10.1177/000331972110
12129
38.GargR,KnoxN,PrasadS,ZinzuwadiaS,RechMA.Theatherogenicindex ofplasmaisindependentlyassociatedwithsymptomaticcarotidarterystenosis. J StrokeCerebrovascDis. (2020)29:105351.doi:10.1016/j.jstrokecerebrovasdis.2020. 105351
39.ThompsonA,DaneshJ.AssociationsbetweenapolipoproteinB, apolipoproteinAI,theapolipoproteinB/AIratioandcoronaryheartdisease: aliterature-basedmeta-analysisofprospectivestudies. JInternMed. (2006) 259:481–92.doi:10.1111/j.1365-2796.2006.01644.x
40.WalldiusG,JungnerI.ApolipoproteinBandapolipoproteinA-I: riskindicatorsofcoronaryheartdiseaseandtargetsforlipid-modifying therapy. JInternMed. (2004)255:188–205.doi:10.1046/j.1365-2796.2003. 01276.x
41.JiangXB,HeDS,MaoZG,FanX,LeiN,HuB,etal.BMI,apolipoprotein B/apolipoproteinA-Iratio,andinsulinresistanceinpatientswithprolactinomas: apilotstudyinaChinesecohort. TumourBiol. (2013)34:1171–6.doi:10.1007/ s13277-013-0660-z
42.BrunnerFJ,WaldeyerC,OjedaF,SalomaaV,KeeF,SansS,etal.Application ofnon-HDLcholesterolforpopulation-basedcardiovascularriskstratification: resultsfromthemultinationalcardiovascularriskconsortium. Lancet. (2019) 394:2173–83.doi:10.1016/s0140-6736(19)32519-x
43.NairD,CarriganTP,CurtinRJ,PopovicZB,KuzmiakS,Schoenhagen P,etal.Associationoftotalcholesterol/high-densitylipoproteincholesterol ratiowithproximalcoronaryatherosclerosisdetectedbymultislicecomputed tomography. PrevCardiol. (2009)12:19–26.doi:10.1111/j.1751-7141.2008.00 011.x
44.KanevaAM,PotolitsynaNN,BojkoER.UsefulnessoftheLDL-C/apoBratio intheoverallevaluationofatherogenicityoflipidprofile. ArchPhysiolBiochem. (2017)123:16–22.doi:10.1080/13813455.2016.1195411
45.ChangTY,ChenJD.Low-densitylipoproteincholesterol/apolipoprotein BratioissuperiortoapolipoproteinBaloneinthediagnosisofcoronary arterycalcification. CoronArteryDis. (2021)32:561–6.doi:10.1097/mca.
0000000000001004
46.MazerNA,GiulianiniF,PaynterNP,JordanP,MoraS.Acomparisonof thetheoreticalrelationshipbetweenHDLsizeandtheratioofHDLcholesterolto apolipoproteinA-IwithexperimentalresultsfromtheWomen’shealthstudy. Clin Chem. (2013)59:949–58.doi:10.1373/clinchem.2012.196949
47.JieniL,YazhiX,XiaorongZ,DanL,YushengM,JiahuanR,etal.Effectof renalfunctiononhigh-densitylipoproteinparticlesinpatientswithcoronaryheart disease. BMCCardiovascDisord. (2021)21:534.doi:10.1186/s12872-021-02354-2
48.PaquetteM,DufourR,BaassA.Themontreal-FH-SCORE:anewscore topredictcardiovasculareventsinfamilialhypercholesterolemia. JClinLipidol. (2017)11:80–6.doi:10.1016/j.jacl.2016.10.004
49.VrablíkM,DobiášováM,ZlatohlávekL,UrbanováZ,ÈeškaR.Biomarkersof cardiometabolicriskinobese/overweightchildren:effectoflifestyleintervention. PhysiolRes. (2014)63:743–52.doi:10.33549/physiolres.932895
50.DobiásováM.[AIP–atherogenicindexofplasmaasasignificantpredictor ofcardiovascularrisk:fromresearchtopractice]. VnitrLek. (2006)52: 64–71.
WangandHe 10.3389/fcvm.2022.1026243
Frontiersin CardiovascularMedicine 12 frontiersin.org 122
PUBLISHED 04January2023
DOI 10.3389/fcvm.2022.960419
OPENACCESS
EDITEDBY
Chu-HuangChen, TexasHeartInstitute,UnitedStates
REVIEWEDBY NardosAbebe, EötvösLorándResearchNetwork (ELKH)ELKH-DEPublicHealth ResearchGroup,Hungary RobertHegele, WesternUniversity,Canada
*CORRESPONDENCE
SzilardVoros szilard.voros@globalgenomicsgroup.com
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine
RECEIVED 02June2022
ACCEPTED 23November2022
PUBLISHED 04January2023
CITATION
VorosS,BansalAT,BarnesMR, NarulaJ,Maurovich-HorvatP, VazquezG,MarvastyIB,BrownBO, VorosID,HarrisW,VorosV, DayspringT,NeffD,GreenfieldA, FurchtgottL,ChurchB,RungeK, KhalilI,HayeteB,LuceroD, RemaleyATandNewtonRS(2023) Bayesiannetworkanalysisofpanomic biologicalbigdataidentifies theimportanceoftriglyceride-rich LDLinatherosclerosisdevelopment. Front.Cardiovasc.Med. 9:960419. doi:10.3389/fcvm.2022.960419
COPYRIGHT
©2023Voros,Bansal,Barnes,Narula, Maurovich-Horvat,Vazquez,Marvasty, Brown,Voros,Harris,Voros,Dayspring, Neff,Greenfield,Furchtgott,Church, Runge,Khalil,Hayete,Lucero,Remaley andNewton.Thisisanopen-access articledistributedunderthetermsof the CreativeCommonsAttribution License(CCBY).Theuse,distribution orreproductioninotherforumsis permitted,providedtheoriginal author(s)andthecopyrightowner(s) arecreditedandthattheoriginal publicationinthisjournaliscited,in accordancewithacceptedacademic practice.Nouse,distributionor reproductionispermittedwhichdoes notcomplywiththeseterms.
atherosclerosisdevelopment
SzilardVoros1*,ArunaT.Bansal2,MichaelR.Barnes2 , JagatNarula3,PalMaurovich-Horvat4,GustavoVazquez5 , IdeanB.Marvasty1,BradleyO.Brown1,IsaacD.Voros1 , WilliamHarris6,ViktorVoros1,7,ThomasDayspring5 , DavidNeff1,AlexGreenfield8,LeonFurchtgott8 , BruceChurch8,KarlRunge8,IyaKhalil8,BorisHayete8 , DiegoLucero9,AlanT.Remaley9 andRogerS.Newton1
1 GlobalGenomicsGroup,Atlanta,GA,UnitedStates, 2 Acclarogen,Ltd,Cambridge,UnitedKingdom, 3 MountSinaiSchoolofMedicine,NewYork,NY,UnitedStates, 4 MTA-SECardiovascularImaging ResearchGroup,HeartandVascularCenter,SemmelweisUniversity,Budapest,Hungary, 5 Global InstituteforResearch,LLC,Richmond,VA,UnitedStates, 6 OmegaQuant,SiouxFalls,SD, UnitedStates, 7 DepartmentofPsychiatry,MedicalSchool,UniversityofPécs,Pécs,Hungary, 8 GNS Healthcare,Somerville,MA,UnitedStates, 9 LipoproteinMetabolismLaboratory,NationalHeart, LungandBloodInstitute,NationalInstitutesofHealth,Bethesda,MD,UnitedStates
Introduction: Wesoughttoexplorebiomarkersofcoronaryatherosclerosisin anunbiasedfashion.
Methods: Weanalyzed665patients(mean ± SDage,56 ± 11years;47%male) fromtheGLOBALclinicalstudy(NCT01738828).Casesweredefinedbythe presenceofanydiscernableatheroscleroticplaquebasedoncomprehensive cardiaccomputedtomography(CT).DenovoBayesiannetworksbuiltoutof 37,000molecularmeasurementsand99conventionalbiomarkersperpatient examinedthepotentialcausalityofspecificbiomarkers.
Results: Mosthighlyrankedbiomarkersbygradientboostingwereinterleukin6,symmetricdimethylarginine,LDL-triglycerides[LDL-TG],apolipoprotein B48,palmitoleicacid,smalldenseLDL,alkalinephosphatase,andasymmetric dimethylarginine.InBayesiananalysis,LDL-TGwasdirectlylinkedto atherosclerosisinover95%oftheensembles.Geneticvariantsinthegenomic regionencodinghepaticlipase(LIPC)wereassociatedwithLIPCgene expression,LDL-TGlevelsandwithatherosclerosis.
Discussion: Triglyceride-richLDLparticles,whichcannowberoutinely measuredwithadirecthomogenousassay,mayplayanimportantrolein atherosclerosisdevelopment.
TYPE OriginalResearch
Bayesiannetworkanalysisof panomicbiologicalbigdata identifiestheimportanceof triglyceride-richLDLin
Frontiersin CardiovascularMedicine 01 frontiersin.org 123
Clinicaltrialregistration: GLOBALclinicalstudy(GeneticLociand theBurdenofAtheroscleroticLesions);[https://clinicaltrials.gov/ct2/show/ NCT01738828?term=NCT01738828&rank=1],identifier[NCT01738828].
KEYWORDS
triglyceride-richLDL,LDL-triglycerides,cardiovascularrisk,Bayesiannetwork analysis,omics,hepaticlipase
Highlights
-InourBayesiananalysis,LDL-TGwasdirectlyupstreamfrom atherosclerosis.
-LDL-TGwasassociatedwithatherosclerosisindependentlyof well-knownfactors.
-Hepaticlipase’sgeneticvariantscorrelatedwithLDL-TGlevels andatherosclerosis
-LDL-TGwaspositivelylinkedtotriglycerides,sd-LDL,and inflammatorymarkers.
Introduction
Cardiovasculardiseaseremainstheleadingcauseof mortalityandmorbidityworldwide(1).Acutemanifestations ofcoronaryarterydisease(CAD)arecausedbyatleast threerelevantbiologicalprocesses:underlyingcoronaryarterial atherosclerosisthatdevelopsoverdecades(2),acuteplaque rupture/erosion(3)followedbycoronaryarterialthrombosis (4).Manylong-termstudiesofcardiovascularoutcomes haveidentifiedlow-densitylipoproteincholesterol(LDL-C) andapolipoprotein-B(Apo-B)askeycausalriskfactorsfor cardiovascularevents(5, 6).
Suchlong-termcardiovascularoutcomesstudiesarevery helpfulinestablishingclinicallyrelevantriskfactorsthatcan bemonitoredandmodifiedinclinicalpractice,suchasLDLCandApo-B.Alimitationofthecurrentcardiovascular biomarkerstudiesisthattheyprimarilyrelyonclinicalevents, whichisacombinationofthethreeunderlyingbiological processeswithdifferenttimescales,namelyatherogenesis, plaquerupture/erosionandthrombosis.Asaconsequence, thecurrentcardiovascularbiomarkerstudiesdonotefficiently identifyanddiscriminatewhichofthesethreespecificbiological processesisassociatedwithandcausallylinkedtoariskfactor.
Non-invasivecoronaryarterialimagingwithcardiac CTpresentsauniqueopportunitytoisolatecausalfactors ofatherosclerosis perse.Accordingly,wedesigneda nestedcase-controlanalysiswithintheGeneticLociand theBurdenofAtheroscleroticLesions(GLOBAL)clinical study(ClinicalTrials.gov numberNCT01738828)(7)to identifyadditionalcausalfactors.Weused denovo Bayesian
networkanalysis,ahypothesis-freeapproach(8),toenrich forassociationswithriskofCTforcausalrelevancetothe developmentofatherosclerosis.Inordertoexaminecausal relevanceofrelationshipsamongthemulti-modalcovariates ofCAD(genetics,geneexpression,proteomics,etc.),we usedaspecifictechniquesuccessfullyemployedtoinfer biologicalpathwaysfromsteady-statecross-sectionaldata, namelyBayesianbeliefnetworks(8, 9).Inaddition,our networkanalysisincorporatedwholegenomesequencingdata andotherdatamodalitiestoavoidlatentconfoundingand tostudypotentialcausalbiomarkersrevealedbyBayesian networkanalysis.
Materialsandmethods Patients
Theanalysesforthepresentstudywereperformedin asubgroupfromtheGeneticLociandtheBurdenof AtheroscleroticLesions(GLOBAL)multicentricclinicalstudy (ClinicalTrials.gov numberNCT01738828).Thepresentnested Case-Controlstudywasperformedinthepre-specifiedPilot Discovery(340patients)andPilotValidation(340patients) cohorts,which,incombination,included680patients.Entirely completeclinical,imaging,multiomicandgeneticdatawithzero missingnessthatisrequiredtobuildtheintegrateddataframe forBayesiananalysiswasavailablein665patients.Ofthese,317 subjectshadnodiscernableatherosclerosisoncomprehensive CTandwerethereforedesignatedas“Controls”and348 subjectshaddiscernableplaqueonCTandweredesignatedas “Cases.”TheGLOBALstudyincludedsubjectsof18–90years ofageandself-referredasCaucasian,withtheindicationofor undergoingcoronarycomputerizedtomography(CT).Subjects underimmunosuppressiveorimmunomodulatorytherapyor chemotherapywereexcludedfromthestudy.Thosewithmajor surgeryandbloodtransfusionwithinthelasttwomonths, contraindicatedCT,orpreexistingcardiacaffectionswerealso excludedfromthestudy.Blooddrawforallblood-based biomarkeranalysis,“omics”testingandgenetictestingwas performedatthetimeoftheCTimagingprocedure.Forfurther detailsabouttheGLOBALstudydesign,pleasegotoVoros etal.(7).Thestudywasconductedaccordingtothecriteria
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 02 frontiersin.org 124
setbythedeclarationofHelsinkiandallincludedsubjects signedinformedconsentfortheuseofgeneticmaterialfor researchpurposes.Thestudywasapprovedbyinstitutional reviewboardsandethicscommitteeasappropriate.Cardiac CTwasevaluatedaspreviouslydescribed(7).Subjectswith anyevidenceofatheroscleroticplaqueincoronaryCTwere consideredcases,andthosewithout,controls.Peripheralblood sampleswereobtainedfromenrolledsubjects,andplasma, serum,wholeblood,andbuffycoatwereadequatelystoredfor furtheranalysis.
Dataanalysisapproach
Inordertoexaminecausalrelevanceofrelationshipsamong themulti-modalcovariatesofCAD(genetics,geneexpression, proteomics,etc.),weusedBayesianbeliefnetworks(8, 9). AlthoughBayesianBeliefnetworksmaynotalwaysbeableto establishauniquerelationshipbetweencovariates,wereliedon Markovequivalencetotakeadvantageofadditionalinformation inordertobreaksynonymousprobabilisticrelationships.This approachcantaketheformofintentionalperturbations(8)or ofgeneticconstraints,theuseofwhichinBayesiannetworks permitsanalysisthatisstatisticallyrelatedtomendelian randomization(10).Furthermore,weinvestigatedournetworks ofcausallyenrichedprobabilisticrelationshipsbymeansof per-patientcounterfactualsimulations(11),wherethegenetic constraintsplayedarolesimilartothatofinstruments ininstrumentalvariableanalysis.Inaddition,ournetworks incorporatedourwholegenomesequencingdataandotherdata modalitiestoavoidlatentconfoundingandtostudypotential causalbiomarkersrevealedbyBayesiannetworkanalysis.
Detailedsamplesizecalculationsfor Bayesiannetworkanalysis
TannerandDonohohavepioneeredacompressedsensing approachwhichboundsinferablecomplexitygivenavailable dataandassumedsparseness(12).Intheirsimulations,for example,ifnumberofsamples, n =300,numberofuseful predictors, k,is3onaverage,andnumberofvariables, p,is 100,000(asinourcase),thex-axis–delta–in Figure1 is n/p =300/100000 ∼ 0,theworstpossiblecase,butthey-axis–rho–is k/n =3/300 ∼ 0 << 0.15.Whilethespecificnumbersdo notmaptoourproblemdomain,theyillustratethatstatistical inferencedependson k, n,and p,andinourcaseisexpected tobeveryhard.Inordertoensurethatwedonotsufferfrom overfitting,wehaveappliedanumberofpriors,asdocumented inthemanuscript.Inparticular,theseincludetheprobability ofthelocalmodel(modeledbyBIC,orpenalizedlikelihood) multipliedbythepriorprobabilityofthemodelofagiven complexityandhasbeendescribedbyusinthesupplement
toapriorwork(13).Inthecaseofasingleclass(e.g.,only geneexpression),thetotaloverallpenaltysimplifiestoE-BIC withgamma=1/2,orsimplyBIC+log(|S|),where|S|isthe numberofallpossiblemodels(networkfragments)ofthesame sizeasS.Whenmultipledatatypesarepresent,ourincremental penaltyforaddingatermofclassCtoamodelisdefinedas deltaBIC+log(|C|)+log(|S_c|),wheredeltaBICisthechange inBICduetothisaddition,|C|isthenumberofclasses,and |S_c|isthenumberofelementsinclassC.Effectively,this formulacomputesE-BICsubjecttotheBayesianbeliefthatall classesareequallyinformative apriori,beforeanydataisseen, thuspenalizinglargeclasses,e.g.,genetics,morethansmall ones,e.g.,clinicaldata.Subjecttothisregularizationstrategy, thenetwork’sdefaultstateistobefullydisconnected,anditcan onlybecomeconnectedthroughthepreponderanceofevidence thatovercomesthesetwopenalties.Further,theuseoflarge modelensemblesmakesitvirtuallyimpossiblethatthenetwork overtrainssystematically;inawaythatwouldberepeatablein simulations.Anyovertrainingwouldbedilutedbytheentropy oftheensemble.Performingsimulationsonaper-networkbasis andaveragingtheirpredictionsallowsustoshrinktheoverall standarderroroftheestimatebytheaforementioneddilution oferrors.Thispropertyofensemblemethodsiswell-studied andhasbeenreflectedinanumberofpopularapproachesto classificationandregression,asdescribedinthemanuscripts citedabove.
Conventionalbiomarkeranalysis
Apanelofconventionalbiomarkerswereevaluated usingcommerciallyavailablekitsandreagents,aslistedin SupplementaryTable2
Isolationofgenomicdeoxyribonucleic acid
IsolationofgenomicDNAwasperformedusingthe QIAampDNABloodMidiKit(Qiagenpartno.51185).Starting with0.3–1mlofwholeblood,alysisbufferandproteasewere addedtoeachsampleforcelllysis.Afterlysis,thelysatewas loadedontoaQIAampspincolumn.DNAremainedboundto theQIAampmembrane,whileimpuritieswerewashedawayin 2vacuumsteps.Upondryingthemembrane,DNAwaseluted in200 µlofelutionbuffer.TheyieldofgenomicDNAwas subsequentlydeterminedbyPicoGreenquantitationorbyusing theQubitfluorometer.
Wholegenomesequencing(Illumina ServiceLaboratory)
Whole-genomesequencingwasperformedbytheIllumina ServiceLaboratory.
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 03 frontiersin.org 125
ClustersofcirculatingbiomarkersagainstCTmeasuresofatheroscleroticplaque,stenosis,anddiseaseburden.Thisfiguredisplaystheresultsof unsupervisedhierarchicalclusteringofcirculatingbiomarkersagainstCTmeasuresofatheroscleroticplaque,stenosis,anddiseaseburden. Eachrowrepresentsabiomarkerthatwasnominallyassociatedwithatherosclerosis,andeachcolumnrepresentsaCTmeasurementof atherosclerosis.Thedendrogramsonbothaxesshowtheresultsofhierarchicalclusteranalysis.Insidetheheatmap,positivecorrelationsare showninredandnegativecorrelationsareshowninblue;theintensityofthecolorrepresentsthestrengthofassociation,asquantifiedby Kendall’stau.TherearesixmajorclustersofCTmeasurementsofatherosclerosis:complexplaque,calcifiedplaque,non-calcifiedplaque, calcification/stenosis,diseaseburden,andasecondclusterofcomplexplaque.Therearefourclustersofcirculatingbiomarkerswithdifferent patternsofassociationwithdifferentmeasuresofatherosclerosis:Cluster1includestriglycerides,fattyacids,calcification,endothelial dysfunction,fibrosis,andinflammation.LDL-TGisstronglyassociatedwithearly-stage,non-calcifiedplaqueandcomplexplaque,andIL-6is stronglyassociatedwithlater-stage,calcifiedplaqueandatherosclerosisburden.Cluster2includesApoB-containinglipoproteins,lipoprotein (a),andbiomarkersofinsulinresistance;Cluster3includeshepaticbiomarkersandmarkersofhepaticcholesterolsynthesis;andCluster4 containsvitaminDalone.
Genomicdeoxyribonucleicacidquantitation
GenomicDNAwasquantifiedpriortolibraryconstruction usingPicoGreen(Quant-iTTM PicoGreen R dsDNAReagent, Invitrogen,Catalog#:P11496).Quantswerereadwith SpectromaxGeminiXPS(MolecularDevices).
Libraryconstruction–Polymerasechain reaction-free
Paired-endlibrariesweremanuallygeneratedfrom500ngto 1 µgofgenomicDNAusingtheIlluminaTruSeqDNASample PreparationKit(Catalog#:FC-121-2001),basedontheprotocol intheTruSeqDNAPCR-freeSamplePreparationGuide.
PrefragmentationgenomicDNAcleanupwasperformedusing paramagneticsamplepurificationbeads(Agencourt R AMPure R XPreagents,BeckmanCoulter).Sampleswerefragmented andlibrariesweresize-selectedfollowingfragmentationand end-repairusingparamagneticsamplepurificationbeads, targeting300bpinserts.Finallibrarieswerequalitycontrolled forsizeusingagelelectrophoreticseparationsystemand werequantified.

Clusteringandsequencing–v3chemistry
Followinglibraryquantitation,DNAlibrarieswere denatured,diluted,andclusteredontov3flowcellsusingthe
Vorosetal. 10.3389/fcvm.2022.960419
FIGURE1
Frontiersin CardiovascularMedicine 04 frontiersin.org 126
IlluminacBotTM system.cBotrunswereperformedbasedon thecBotUserGuide,usingthereagentsprovidedinIllumina TruSeqClusterKitv3.Clusteredv3flowcellswereloadedonto HiSeq2000instrumentsandsequencedon100bppaired-end, non-indexedruns.Allsamplesweresequencedonindependent lanes.SequencingrunswereperformedbasedontheHiSeq 2000UserGuide,usingIlluminaTruSeqSBSv3Reagents. IlluminaHiSeqControlSoftwareandReal-timeAnalysiswere usedonHiSeq2000sequencingrunsforreal-timeimage analysisandbasecalling.
Genotyping
SampleswereprocessedusingInfiniumchemistry, basedontheInfiniumLCGAssayGuide,andrunonthe HumanOmni2.5-8array.Resultingintensity.idatfileswere loadedintoGenomeStudio R softwaretoexportgenotypingcalls.
Ribonucleicacidisolationfrom PAXGenetubes
RNAisolationwascompletedusingthePAXgeneBlood miRNAKit(Qiagen,Venlo,TheNetherlands).PAXgeneBlood RNATubeswerefirstcentrifugedtopelletthesamples,then washedwithwaterandresuspended.Afterdigestionwith proteinaseK,thesampleswerehomogenizedbycentrifugation throughPAXgeneShredderspincolumns.Isopropanolwas addedtothesamplestooptimizebindingconditions,andthe sampleswerethencentrifugedthroughPAXgeneRNAspin columns,wheretotalRNA >18nucleotides(includingmiRNA) wasboundtothesilicamembrane.TheboundRNAwas treatedwithDNasetoremovegenomicDNAcontamination andwashed.PureRNAwastheneluted.
Smallribonucleicacidsequencing methodsandmaterials
LibrarieswerepreparedforsmallRNAsequencingusing theTruSeqSmallRNASamplePrepKit(Illumina).Prior tolibrarypreparation,RNAsampleswerequantitated byspectrophotometryusingaNanodropND-8000 spectrophotometerandassessedforRNAintegrityusing anAgilent2100BioAnalyzerorCaliperLabChipGX.RNA sampleswithA260/A280ratiosrangingfrom1.6to2.2,with RNAintegritynumbervalues ≥7.0,andforwhichatleast1,000 ngoftotalRNAwasavailableproceededtolibrarypreparation. TotalRNAsamplesmusthavebeenpreparedusingextraction chemistrythatdoesnotexcludesmallRNAspecies(e.g.,the QIAGENmiRNeasyKit).
Librarypreparationbeganwith1,000ngoftotalRNAin 5 µlofnuclease-freewater,towhichanadapteroligonucleotide wasaddedthatwasthenligatedtothe3 hydroxylpresenton
miRNAspeciesusingT4RNAligase(NewEnglandBiolabs). Similarly,adifferentadaptersequencewasligatedtothe5 end ofRNAsthatpossesseda5 phosphate,inordertocreatea single-strandedmoleculewithdefinedsequencesatboththe 5 and3 ends.Thismoleculewasreverse-transcribedand amplifiedusing14cyclesofPCRwithprimersthatinclude sequencescomplementarytothe5 and3 adaptersequences, aspecificindexsequence,andIlluminasequencingadapter sequences.TheresultingproductwasanalyzedusinganAgilent 2100BioAnalyzer,andthemolaramountofmaturemiRNA presentinthelibrarywasestimatedbyintegratingthearea underthecurveinthe145–160bprange.Individuallibraries weremixedtocreatemultiplexedpools,andthemixturewas purifiedbygelelectrophoresis,whereinthe145–160bprange wasexcisedfromthegel,crushedusingaGelBreakertube(IST Engineering),elutedintonuclease-freewater,andconcentrated byprecipitationwithethanol.Theconcentrationofthefinal librarypoolwasdeterminedusingPicoGreen(Invitrogen),and thesizedistributionofthepoolwasdeterminedusinganAgilent 2100BioAnalyzer.Librarypoolswerenormalizedto2nMin preparationforsequencing.
mRNAsequencing
Priortolibrarypreparation,alphaandbetaglobinmRNA wasreducedusingtheGLOBINclearTM-HumanKit(Life Technologies,Carlsbad,CA),followingthemanufacturers protocol.TotalRNAsampleswereconvertedintocDNA librariesusingtheTruSeqStrandedmRNASamplePrep Kit(Illumina,#RS-122-2103).Startingwith100ngoftotal RNA,polyadenylatedRNA(primarilymRNA)wasselected andpurifiedusingoligo-dTconjugatedmagneticbeads. ThismRNAwaschemicallyfragmentedandconverted intosingle-strandedcDNAusingreversetranscriptaseand randomhexamerprimers,withtheadditionofActinomycin DtosuppressDNA-dependentsynthesisofthesecond strand.Double-strandedcDNAwascreatedbyremoving theRNAtemplateandsynthesizingthesecondstrandin thepresenceofdUTPinsteadofdTTP.AsingleAbase wasaddedtothe3 endtofacilitateligationofsequencing adapters,whichcontainedasingleTbaseoverhang.AdapterligatedcDNAwasamplifiedbypolymerasechainreaction toincreasetheamountofsequence-readylibrary.During thisamplification,thepolymerasestallswhenitencounters aUbase,renderingthesecondstrandapoortemplate. Accordingly,amplifiedmaterialusedthefirststrandasa template,therebypreservingthestrandinformation.Final cDNAlibrarieswereanalyzedforsizedistributionusingan AgilentBioAnalyzer(DNA1000Kit,Agilent#5067-1504), quantitatedbyqPCR(KAPALibraryQuantKit,KAPA Biosystems#KK4824),andthennormalizedto2nMin preparationforsequencing.
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 05 frontiersin.org 127
Mass-spectrometry–basedproteomics methods
Weperformedproteomicsdiscoveryexperimentsin2 stages;thefirststagewasperformedusingnon-targetedmass spectrometry,followedbythesecondstageoftargetedmass spectrometryusingmultiplereactionmonitoring.
Discoveryexperimentsusing non-targetedmassspectrometry
Sampleswereprocessedessentiallyasdescribedpreviously (14).Briefly,each30 µlsamplewasdepletedofhighabundance proteinsusinganaffinityresin(IgY14/Supermix,Sigma). Allcolumnswerepreparedwiththesamemanufacturing batchofaffinityresinandtestedforconsistentperformance priortouse.Controlsamples,consistingofaliquotsofa pooledhumanplasmasample,wereinsertedatthestart, middle,andendofeachsetof20pairedstudysamples, resultinginabatchsizeof23.Afterdepletion,samples werefrozen,freeze-dried,digestedwithtrypsin(1:10,w:w, Promega),anddesaltedonEmporeC18plates(3MBioanalytical Technologies).Resultingpeptideswereseparatedbystrong cationexchange(SCX,Waters)chromatographyinto6fractions withalinearsaltgradientanddesaltedonOasisHLBplates (Waters).Samplesweredistributedintotwo96-wellplates(one testplateandonebackupplate).Sampleswerethendriedand resuspendedin96.25/3.75(v/v)water/acetonitrileand0.1% formicacid,containing19internalstandardpeptides.Mass spectrometryanalysiswasperformedbynanoflowreversed phaseliquidchromatography(NanoAcquityUPLC,Waters), coupledbyelectrospray(MichromADVANCECaptiveSpray MSSource)toahigh-resolutionmassspectrometer(Q Exactive,ThermoScientific)inliquidchromatographymass spectrometry(LC-MS)andliquidchromatography/tandem massspectrometry(LC-MS/MS)mode.TheLCcolumnwas usedataflowrateof1.8 µl/min(WatersnanoAcquityUPLC columnBEH130C18,150 µm × 100mm,1.7 µm).Eachof the6fractionswasrunasaseparatesetof338samplesplus controlsamples.
IntensitydatafilesforeachLC-MSrunwithinaSCXfraction werealignedusingElucidator(RosettaBiosoftware).Peak intensitiesforeachpeptideionwerethenextractedacrossall files.LC-MS/MSfileswereanalyzedbyMascot(MatrixSciences) andtheUniprothumanproteindatabase(version2013_08) toassignhighconfidencepeptidesequencestotheobserved peptideions.Allsequencedpeptideswerethenclusteredbytheir parentproteins.Potentialintensitybiasintroducedbysample processingand/orlossofsensitivityofthemassspectrometer overthetimeoftheexperimentwascorrectedbynormalization. Thenormalizationprocedurewasbasedonaregressionmodel, whichpredictedlog-intensitylevelonaper-peptidebasis.First,
themeanrawlog-intensityforeachpeptidewascalculated.Then theregressionmodel(linearregressionornaturalcubicspline smoothing)forsampleprocessingvariableswasfittothedata. Finally,thenormalizedlog-intensitywascomputedastheraw log-intensityminustheregression-predictedlog-intensityplus themeanrawlog-intensity.
Thestatisticalsignificanceoftheintensitydifferences betweenthevariousclinicalgroupswasassessedusinga paired t test,whichwasperformedindependentlyoneach peptideandeachprotein,forthematchedcaseandcontrol samples.Ananalysisofvariancemodelwasalsousedto comparethesametwogroupstoaccountfordyslipidemia, hypertension,anddiabetesstatuscovariates,whichwerenot matchedbetweensamplepairs.Allstatisticaltest P valueswere adjustedformultipletestingbyconversionto Q valuesusing Storey’smethod.
Metabolomicsandlipidomicsmethods bymassspectrometry
Samplepreparationforglobalmetabolomics Sampleswerestoredat–70◦Cuntilprocessed.Sample preparationwascarriedoutasdescribedpreviously(15)at Metabolon,Inc.Briefly,recoverystandardswereaddedprior tothefirststepintheextractionprocessforqualitycontrol purposes.Toremoveprotein,dissociatesmallmoleculesbound toproteinortrappedintheprecipitatedproteinmatrix, andtorecoverchemicallydiversemetabolites,proteinswere precipitatedwithmethanolundervigorousshakingfor2min (GlenMillsGenogrinder2000),followedbycentrifugation.The resultingextractwasdividedinto4fractions:1foranalysisby ultra-highperformanceliquidchromatographytandemmass spectrometry(UPLC-MS/MS;positivemode),1foranalysis byUPLC-MS/MS(negativemode),1foranalysisbygas chromatography–massspectrometry(GC-MS),and1sample wasreservedforbackup.
Threetypesofcontrolswereanalyzedinconcertwith theexperimentalsamples:samplesgeneratedfromapool ofhumanplasma(extensivelycharacterizedbyMetabolon, Inc.)servedastechnicalreplicatesthroughoutthedataset; extractedwatersamplesservedasprocessblanks;andacocktail ofstandardsspikedintoeveryanalyzedsampleallowedfor instrumentperformancemonitoring.Instrumentvariability wasdeterminedbycalculatingthemedianrelativestandard deviation(RSD)forthestandardsthatwereaddedtoeach samplepriortoinjectionintothemassspectrometers(median RSD=5%; n =30standards).Overallprocessvariabilitywas determinedbycalculatingthemedianRSDforallendogenous metabolites(i.e.,non-instrumentstandards)presentin100% ofthepooledhumanplasmasamples(medianRSD=11%; n =610metabolites).Experimentalsamplesandcontrolswere randomizedacrosstheplatformrun.
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 06 frontiersin.org 128
Massspectrometryanalysis
Non-targetedMSanalysiswasperformedatMetabolon, Inc.ExtractsweresubjectedtoeitherGC-MS(16)orUPLCMS/MS(15).Thechromatographywasstandardizedand,once themethodwasvalidated,nofurtherchangesweremade.As partofMetabolon’sgeneralpractice,allcolumnswerepurchased fromasinglemanufacturer’slotattheoutsetoftheexperiments. Allsolventsweresimilarlypurchasedinbulkfromasingle manufacturer’slotinsufficientquantitytocompleteallrelated experiments.Foreachsample,vacuum-driedsampleswere dissolvedininjectionsolventcontaining8ormoreinjection standardsatfixedconcentrations,dependingontheplatform. Theinternalstandardswereusedtoassurebothinjection andchromatographicconsistency.Instrumentsweretunedand calibratedformassresolutionandmassaccuracydaily.
TheUPLC-MS/MSplatformutilizedaWatersAcquity UPLCwithWatersUPLCBEHC18-2.1 × 100mm, 1.7 µmcolumnsandaThermoScientificQ-Exactivehigh resolution/accuratemassspectrometerinterfacedwithaheated electrosprayionizationsourceandOrbitrapmassanalyzer operatedat35,000massresolution.Thesampleextractwas driedandthenreconstitutedinacidicorbasicLC-compatible solvents,eachofwhichcontained8ormoreinjectionstandards atfixedconcentrationstoensureinjectionandchromatographic consistency.Onealiquotwasanalyzedusingacidic,positive ion–optimizedconditions,andtheotherusingbasic,negative ion–optimizedconditionsin2independentinjectionsusing separatededicatedcolumns.Extractsreconstitutedinacidic conditionsweregradientelutedusingwaterandmethanol containing0.1%formicacid,whilethebasicextracts,which alsousedwater/methanol,contained6.5mMammonium bicarbonate.TheMSanalysisalternatedbetweenMSand data-dependentMS2 scansusingdynamicexclusion,andthe scanrangewasfrom80to1,000 m/z
ThesamplesdestinedforanalysisbyGC-MSweredried undervacuumdesiccationforaminimumof18hpriorto beingderivatizedunderdriednitrogenusingbistrimethylsilyltrifluoroacetamide.Derivatizedsampleswereseparatedon a5%phenyldimethylsiliconecolumnwithheliumascarrier gasandatemperaturerampfrom60to340◦Cwithina17-min period.AllsampleswereanalyzedonaThermo-FinniganTrace DSQMSoperatedatunitmassresolvingpowerwithelectron impactionizationanda50–750atomicmassunitscanrange.
Compoundidentification,quantification,and datacuration
Metaboliteswereidentifiedbyautomatedcomparisonof theionfeaturesintheexperimentalsamplestoareference libraryofchemicalstandardentriesthatincludedretention time,molecularweight(m/z),preferredadducts,andin-source fragmentsaswellasassociatedMSspectraandcuratedby visualinspectionforqualitycontrolusingsoftwaredeveloped atMetabolon.Identificationofknownchemicalentitieswas
basedoncomparisontometabolomiclibraryentriesof purifiedstandards.Over2,500commerciallyavailablepurified standardcompoundshavebeenacquiredandregisteredintothe LaboratoryInformationManagementSystemfordistribution toboththeLC-MSandGC-MSplatformsfordetermination oftheirdetectablecharacteristics.Anadditional250mass spectralentrieshavebeencreatedforstructurallyunnamed biochemicals,whichhavebeenidentifiedbyvirtueoftheir recurrentnature(bothchromatographicandmassspectral). Thesecompoundshavethepotentialtobeidentifiedbyfuture acquisitionofamatchingpurifiedstandardorbyclassical structuralanalysis.Peakswerequantifiedusingarea-under-thecurve.Rawareacountsforeachmetaboliteineachsamplewere normalizedtocorrectforvariationresultingfrominstrument inter-daytuningdifferencesbythemedianvalueforeachrunday;therefore,themediansweresetto1.0foreachrun.This preservedvariationbetweensamplesbutallowedmetabolites ofwidelydifferentrawpeakareastobecomparedonasimilar graphicalscale.Missingvalueswereimputedwiththeobserved minimumafternormalization.
TrueMass R lipomicpanel
Lipidswereextractedinthepresenceofauthentic internalstandardsbythemethodofFolchetal.(17)using chloroform:methanol(2:1v/v).Fortheseparationofneutral lipidclasses[FFA,TAG,DAG,CE],asolventsystemconsisting ofpetroleumether/diethylether/aceticacid(80:20:1)was employed.Individualphospholipidclasseswithineachextract [PC,PE]wereseparatedusingtheAgilentTechnologies1100 SeriesLC.Eachlipidclasswastransesterifiedin1%sulfuric acidinmethanolinasealedvialunderanitrogenatmosphere at100◦Cfor45min.Theresultingfattyacidmethylesters wereextractedfromthemixturewithhexanecontaining0.05% butylatedhydroxytolueneandpreparedforGCbysealingthe hexaneextractsundernitrogen.Fattyacidmethylesterswere separatedandquantifiedbycapillaryGC(AgilentTechnologies 6890SeriesGC)equippedwitha30mDB88capillarycolumn (AgilentTechnologies)andaflameionizationdetector.
Evaluationofassociationsbetween lowdensitylipoproteintriglycerides andplasmalipoproteins
Foraconfirmatorystudy,eighthundredandsix subjectswereincludedfromtheNationalInstitutesof HealthCTstudy.Thecohortincludedbothmalesand femalesthatwereatleast18yearsofageandwithclinical indicationforacoronaryCTangiography.Therewere noadditionalinclusioncriteria.Exclusioncriteriawere currentpregnancyandseverelydecreasedrenalfunction (estimatedglomerularfiltrationrate < 30mL/min/1.73m2 bodysurfacearea).Thestudyprotocolwasapprovedby
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 07 frontiersin.org 129
theNationalHeart,Lung,andBloodInstitute’sInstitutional ReviewBoardandallsubjectsprovidedinformedconsent atenrolment. ClinicalTrials.gov identifier:NCT01621594. PlasmaLDL-TGwasdeterminedbyhomogeneousassay (DenkaSeikenCo.,Ltd.,Tokyo,Japan),andsubjectswere dividedaccordingtoLDL-TGterciles.Fastinglipidpanel wasdeterminedbystandardenzymaticmethodsona Cobas6000analyzer(RocheDiagnostics,Indianapolis,IN, USA).LDLcholesterolandverylow-densitylipoprotein (VLDL)cholesterolwerecalculatedusingSampson’s formula(18).SmalldenseLDLcholesterolwasmeasured byahomogeneousassay(DenkaSeikenCo,Ltd.,Tokyo, Japan).Lipoproteinsubclassprofilewasdeterminedin VanteraClinicalNMRAnalyzer(Labcorp,Burlington, NC,USA).TheLipoProfile-3or4algorithmwasusedto determinetheparticlenumberoflipoproteinsubclasses: Numberoftriglyceride-richlipoproteinparticles(TRLP)andthefollowingsubclasses:verysmall-,small-, medium-andlarge-TRL-P;LDLparticlenumber(LDL-P), anditssubclasses:small-,medium-,large-LDL-P;HDL particlenumber(HDL-P),aswellasHDLsubclasses: small-,medium-,andlarge-HDL-P.GlycAlevelswere determinedinaVanteraClinicalNMRAnalyzer(Labcorp, Burlington,NC,USA).Plasmahighsensitivity(hs-CRP)was measuredontheCobas6000analyzer(RocheDiagnostics, Indianapolis,IN,USA).
Results
Demographicfeaturesand atherosclerosisinthepatient population
Atotalof665patientswereincludedinouranalysis; generaldemographicfeaturesareshownin Supplementary
Table1.Typicalangina(62vs.64%)andatypicalangina (36.5vs.36%)weresimilarincasesandcontrols.Ingeneral, themean ± SDageintheoverallstudypopulationwas 56 ± 11years,47%ofpatientsweremale,andthemean Diamond-Forresterscorewas26%(range,0–94%).LDL-C, high-densitylipoproteincholesterol(HDL-C),andtriglycerides incasesandcontrolsarealsoshownin Supplementary
Table1.Theprevalenceofatherosclerosis(i.e.,cases)was 52%intheoverallcohort.Sevenpercentofpatientshad acoronarycalciumscoreofzerobuthadanon-calcified plaque.Predominantlynon-calcified,partiallycalcified,and calcifiedplaqueswerepresentin7,36,and57%ofcases, respectively.Napkinringsign,ahigh-riskfeaturebyCT, wasobservedin10%ofpatients.Moderatestenosis(50–69%)wasthehighestdegreeofstenosisin7%ofpatients, and16%ofpatientshadmoderate-to-severestenosis(≥50% luminalstenosis).Mean ± SDsegmentinvolvementscore
andsegmentinvolvementscoreindexwere2.2 ± 3.1and 2.4 ± 3.3%,respectively.
Biomarkerassociationswith atherosclerosis
Inapreliminarycoarsefilterofthebiomarkers,nominal univariateassociations(raw P < 0.05)withatherosclerosiswere identifiedfor30ofthe99conventionalbiomarkers;theseare illustratedinaheatmapin Figure1.Thedendrogramonthe leftoftheplotwasgeneratedbyunsupervisedhierarchical clusteringandindicatesfour(4)clusters.Cluster1included totalplasmatriglyceridesandLDL-TG,aswellasfattyacidsand measuresofendothelialdysfunction,inflammation,andfibrosis. Cluster2includedApoB-containinglipoproteinmeasurements, lipoprotein(a),andmeasuresofinsulinresistance.Cluster3 includedhepaticmeasurementsofbilirubinmetabolismand amarkerofcholesterolbiosynthesis.Cluster4contained vitaminDalone.
Thethirtybiomarkersidentifiedbyunivariateanalysiswere furthersubjectedtogradientboostinganalysistoidentifythe strongestpredictorsofatherosclerosis Figure2A indicates therelativeinfluenceoftheeightbiomarkersrankedmost highly.Interleukin-6[IL-6],symmetricdimethylarginine,and LDL-TGemergedasthetop3predictorsofcase-control status,witharelativeinfluenceofover ∼30%forIL6andsymmetricdimethylarginineand ∼15%forLDLTG.Asdescribedbelow,oftheseeight(8)biomarkers stronglyassociatedwithatherosclerosis,onlyLDL-TGwas directlyconnectedtoatherosclerosisintheBayesiannetwork analysis.
Bayesiannetworkanalysisusing reverseengineeringwithforward simulation
Theprimaryresultandoutputfromthehypothesis-free Bayesiannetworkanalysisisshownin Figure2B.Theensemble ofBayesiannetworksidentifiedconsistedof24,929nodes and110,350edges,whichoccurredin >5%ofthemodels intheensemble.LDL-TGwastheonlybiomarkerdirectly upstreamfromthepresenceofatheroscleroticCAD(ASCAD), whichoccurredin95%ofnetworksintheensemble.This suggestsapotentialcausalroleoftriglyceride-richLDLparticles, asmeasuredbyLDL-TGlevels,inthedevelopmentand progressionofatherosclerosis.GiventhecentralroleofLDLTGintheBayesiannetworks,wefurtherexploredthepotential contributionofLDL-TGtoatherosclerosis.Itisimportantto pointoutthatclinicalfeatures,suchasageandgender,werealso includedintheBayesiananalysisandtherefore,theBayesian findingsdonormalizeourfindingsforageandgender.
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 08 frontiersin.org 130
Gradientboostinganalysisidentifiedthestrongestpredictorsofatheroscleroticcoronaryarterydisease.Hypothesis-freeBayesiannetwork analysisusingreverseengineeringwithforwardsimulation(REFSTM )Suggestsapotentialcausalroleoftriglyceride-richLDLparticles,as measuredbyLDL-TGlevels,inthedevelopmentandprogressionofatherosclerosis. (A) Outofthe30conventionalbiomarkersincludedinthe multivariateanalysis,thetop8analytesareshowninthebargraph.Thelengthofthebarcorrespondstotherelativeinfluencethebiomarkerin predictingatheroscleroticcoronaryarterydisease.ThebiomarkersincludeIL-6(inflammation),symmetricdimethylarginine(endothelial dysfunction),andLDL-TG(ApoB-containinglipoproteincluster). (B) Thisfigureisnotanillustration;itisanactualoutputfromthe hypothesis-freeBayesiannetworkanalysis.Atotalof24,929nodesand110,350edgeswerediscoveredinmorethan5%ofthenetworksinthe ensemble;shownisthesubnetworkofmeasurementswith1degreeofseparationfromLDL-TG.Arrowthicknessindicatesthefractionof networksinwhichthecausaledgeappears;differentcoloredboxesrepresentdifferenttypesofmeasurements(yellow: mass-spectrometry–basedlipidomics;pink:mass-spectrometry–basedmetabolomics;green:geneexpression[mRNA];gray:conventional biomarkermeasurements).Notably,amongallofthebiomarkersthatweremeasuredandincludedinthemodel,LDL-TGwastheonly biomarkerwithadirectconnectiontoASCAD(seebluearrowspointingtotheedgeconnectingLDL-TGtoASCAD).Thissuggeststhat triglyceride-richlipoproteinparticles,asmeasuredbyLDL-TGlevels,mayhaveacausalroleinatherosclerosis.Interestingly,inthiscausal model,sd-LDL,ApoB,andC-reactiveproteinaredownstreamfromLDL-TG,whilepalmitoleicacidandtotaltriglyceridelevelsappearupstream fromLDL-TG.Fibrinogenandgalectin-3aredownstreamfromC-reactiveprotein.ADMA,asymmetricdimethylarginine;ALP,alkaline phosphatase;ApoB,apolipoproteinB;IL-6,interleukin-6;LDL-TG,low-densitylipoprotein-triglycerides;SDMA,symmetricdimethylarginine, ASCAD,atheroscleroticcoronaryarterydisease;CAD,coronaryarterydisease;CRP,C-reactiveprotein;POA,palmitoleicacid;sd-LDL,small, denselow-densitylipoprotein;TG,triglycerides.



Vorosetal. 10.3389/fcvm.2022.960419
FIGURE2
Frontiersin CardiovascularMedicine 09 frontiersin.org 131
Lowdensitylipoproteintriglycerides andatherosclerosis
Asanindependentbiomarker,LDL-TGlevelswere significantlyhigherincasesversuscontrols(mean ± SE, 20.19 ± 0.93vs17.21 ± 0.40mg/dL; P < 0.001).The fourquartilesofLDL-TGmeasurementswereexamined;odds ratiosinthesecond,third,andfourthquartileswere1.38 (95%CI,0.86–2.24),1.43(95%CI,0.89–2.31),and2.84 (95%CI,1.75–4.64),respectively,comparedtothefirst(i.e., reference)quartile(Table1).Adjustingthemodelforage, sex,LDL-CandApoBlevelsdemonstratedthattheassociation ofLDL-TGwithatherosclerosiswasindependentofthese well-knownfactors.
Cumulativeincidencecurves
Toexaminetherelativecontributionofeachkeybiomarker toatherosclerosis,weconstructedcumulativeincidencecurves forApoB,LDL-C,andLDL-TGlevelsagainstthecumulative incidenceofatherosclerosisinallpatientsandinpatients whowerenotonstatins(Figure3).Ourdataconfirmed thewell-describedrelationshipbetweenLDL-Clevelsand theincidenceofatherosclerosis,whichwasmostapparent inpatientswhowerenotonstatintherapy(Figure3E). AsimilarpatternwasalsoobservedforApoB(Figure3D). Itisacknowledgedthatthelefttailsofthecumulative incidencecurvesarelikelytobehighlyinfluencedbypatients withknownASCADwhoseLDL-CandApoBlevelswere likelyloweredbyrecentstatintherapy.Importantly,statin therapyappearstohavelittleinfluenceonthecumulative incidenceofASCADasafunctionofLDL-TGmeasurements (Figures3C,F).
Hepaticlipase(LIPC),lowdensity lipoproteintriglyceridesand atherosclerosis
Sincepreviouspublications(19–21)have demonstrated anassociation betweenhepaticlipase(encodedbytheLIPC gene),LDL-TGandatherosclerosis,weperformedagenomic screenoftheLIPCgeneregion.TheSNPrs261336was associatedwithbothhigherlevelsofLDL-TGandhigherodds ofatherosclerosis,whilers12898984,rs12900448,rs4774301, rs4775064andrs4775065wereassociatedwithlowercirculating levelsofLDL-TGandloweroddsofatherosclerosis(Table2 and Figure4).
Inaddition,LIPCgeneexpressionincirculating mononuclearcellswassignificantlylowerinCasesthan inControls(meanexpression:1.20(0.08)vs.1.49(0.07); p =0.015).
Associationsoflowdensitylipoprotein triglycerideswithotherknownrisk markers
Weanalyzedlipidpaneltestresultsandlipoproteinsubclass profile,determinedbyprotonnuclearmagneticresonance (1H-NMR)spectroscopy,in800patientsfromtheNational InstitutesofHealthCTcohort,subdividedbyLDL-TGterciles (SupplementaryTable3).TotalCholesterolandLDL-C increasedfromlowtohighLDL-TGterciles(p < 0.0001for trend).Thesametrendwasobservedfortriglyceridesand calculatedVLDL-C(18).SmalldenseLDL(sd-LDL)cholesterol asmeasuredbyDenkaassayincreasedalongtheLDL-TG tertials.Lipoproteinsubclassanalysisby 1H-NMRspectroscopy revealedthattotal,large,medium,andverysmalltriglyceriderichlipoprotein(TRL)particlenumberincreasedfromlowto highLDL-TGterciles(p < 0.0001fortrend).Furthermore,the numberofLDLparticleswashigherinhighLDL-TGtercilesat theexpenseofsmallerLDLparticles(p < 0.0001),probablydue tothepoorersdLDLrecognitionbyLDLreceptor(22),leading toitsaccumulationintheplasma.
Finally,LDL-TGwaspositivelyassociatedwithGlycA (p < 0.0001fortrend),arecentlyidentifiedsystemic inflammationmarkerderivedfromthe 1H-NMRsignalof N-acetylgroupsontheglycanportionofacute-phaseproteinsin plasma(23).Overall,theseresultssuggestthatincreasedLDLTGislinkedtoamorepro-inflammatoryandpro-atherogenic phenotypeandare,therefore,alignedwiththefindingsfromthe BayesianNetworkAnalysis.
Discussion
Ourresultssuggestthat,whileseveralserumbiomarkers areassociatedwithhumanASCAD,triglyceride-richLDL particles,asmeasuredbyLDL-TGlevels,mayhave animportant central role,potentiallyasaresultofabnormalhepaticlipase function.Ourstudydesignprovidedauniqueopportunity toassessbiomarkerassociationsinthecontextoftheimpact ofgeneticpredispositiononatherosclerosis.Wecompleted preciseanddetailedquantitativephenotypingmeasurements ofhumancoronaryarterialatherosclerosisinaprospective studyusingcomprehensivecardiacCT,analyzedinacentral corelaboratory.Thisprecisionphenotypingwascoupled withmeasuringandranking99circulatingbiomarkersand 37,000“omics”measurements.Webuilthypothesis-free,causal Bayesiannetworksofbiologicalpathwaystoexaminethe potentialrole ofserumbiomarkersinacomprehensivemanner.
Ourinitialanalysisidentifiedfourmainbiomarkerclusters, thusprovidinguniquehigh-levelinsightsintothepathogenesis ofASCAD(Figure1).Thecontentoftheseclustersis consistentwithprevailinghypothesesofthedevelopment ofatherosclerosisasaresultofatherogeniclipoproteins,
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 10 frontiersin.org 132
TABLE1Oddsratios(95%CI)foratherosclerosisagainstthelowestquartileofLDL-TG.
CI,confidenceinterval;LDL-TG,low-densitylipoprotein–triglycerides.
a Adjustedforageandgender.
b AdjustedforLDL-C.






c Adjustedforage,gender,andLDL-C.
d AdjustedforAPOB.
e Adjustedforage,gender,andAPOB.
FIGURE3
CumulativeincidencecurvesforthepresenceofcoronaryatherosclerosisasafunctionofApoB,LDL-C,andLDL-TG.Cumulativeincidence curvesdemonstratethewell-describedrelationshipbetweenApoBandLDL-Clevelsandtheincidenceofatherosclerosis,whichisprimarily apparentintheApoBrangeof50–150mg/dlandintheLDL-Crangeof60–200mg/dl.Thelefttailsofthecurvesaredistortedbystatin-treated patients (A,B),inwhichyouseeahighcumulativeincidenceofatherosclerosisdespiteverylowlevelsofApoB(panelA)andLDL-C (B).This likelyrepresentspatientswithknownASCADwhoseLDL-CandApoBlevelshavebeenloweredbyaggressivestatintherapy.Thesigmoid relationshipforApoBandLDL-Cismoreapparentwhenstatin-treatedpatientsareexcluded (D,E).Ontheotherhand,aclear,near-exponential relationshipisseenfortheincidenceofatherosclerosisasafunctionofserumLDL-TGlevels,withnoapparenteffectofstatintherapy (C,F) ApoB,apolipoproteinB;LDL,lowdensitylipoprotein.
inflammation,andendothelialdysfunction,insomeinstances inthecontextofinsulinresistanceanddiabetes(24–26).
Furthermore,inunivariate(Figure1)andmultivariable analyses(Figure2A),wealsofoundthatApoB-containing
lipoproteins,insulinresistance,endothelialdysfunction, inflammation,andfibrosisareallstronglyassociatedwith humancoronaryatherosclerosis,consistentwithdecadesof hypothesis-drivendata.

Vorosetal. 10.3389/fcvm.2022.960419
Quartile1 [8.5–14.1mg/dl] Quartile2 [14.1–16.9mg/dl] Quartile3 [16.9–22.1mg/dl] Quartile4 [22.1–45.7mg/dl] P-valueversus fourthquartile P-valuefor trend LDL-TG Unadjusted Reference1.38 (0.86–2.24) 1.43 (0.89–2.31) 2.84 (1.75–4.64) 2.67e-055.35e-05 LDL-TG Model1a Reference1.31 (0.80–2.13) 1.39 (0.86–2.27) 3.00 (1.84–4.95) 1.38e-052.50e-05 LDL-TG Model2b Reference1.43 (0.88–2.33) 1.56 (0.95–2.56) 3.36 (1.95–5.85) 1.50e-053.88e-05 LDL-TG Model3c Reference1.34 (0.82–2.20) 1.48 (0.89–2.46) 3.37 (1.94–5.91) 1.86e-054.49e-05 LDL-TG Model4d Reference1.44 (0.89–2.35) 1.56 (0.94–2.60) 3.42 (1.88–6.29) 6.31e-051.99e-04 LDL-TG Model5e Reference1.34 (0.82–2.20) 1.46 (0.87–2.47) 3.32 (1.81–6.16) 1.19e-043.55e-04
Frontiersin CardiovascularMedicine 11 frontiersin.org 133
TABLE2GeneticassociationbetweenLIPCgenevariants,LDL-TGandatherosclerosis.
Variant(SNP)LDL-TGASCAD(“Case”)
SNP,singlenucleotidepolymorphism.LDL-TG,lowdensitylipoproteintriglycerides;ASCAD,atheroscleroticcoronaryarterydisease;SE,standarderror;CI,confidenceinterval; Ln,naturallog.



GeneticvariantsintheLIPCGene,CirculatingLevelsofLDL-TGandAtherosclerosis.Thegeneticvariantrs4774301intheLIPCgenomicregionis associatedwithsignificantlylowercirculatinglevelsofLDL-TG (A) andsimultaneously,withsignificantlylowerprevalenceofatherosclerosis (B) Jitterplot (C) demonstratesthesameconceptinasinglegraph,demonstratingthatthenumberofthe“T”allelesatrs4774301isassociatedwith lowercirculatinglevelsofLDL-TGandwithlowerprevalenceofASCAD.LIPC:hepaticlipasegene;LDL-TG:lowdensitylipoproteintriglycerides.

Akeyfindingwasthat,outoftensofthousandsof blood-basedmoleculesandbiomarkers,LDL-TGemergedwith apotentialcentralroleinhumancoronaryatherosclerosis, potentiallyasafunctionofabnormalhepaticlipaseactivity. Thiswasseeninourhypothesis-free,causal,Bayesiannetwork analysis,whichincluded24,929variablesand110,350significant edgesinthemodels.LDL-TGwasdirectlyconnectedtohuman coronaryatherosclerosisin95%ofthemodelsintheensemble.
TheoutputoftheBayesiannetworkanalysisshownin Figure2B (notanillustration)indicatesthepotentialcentralroleof triglyceride-richLDLparticles,asmeasuredbyLDL-TGlevels. Inthismodel,triglyceridelevelsandpalmitoleicacidwere upstreamfromLDL-TG,whilesmall,denseLDL(sd-LDL) inflammatorymarkers(e.g.,C-reactiveprotein,fibrinogen, andlipoprotein-associatedphospholipaseA2),andfibrosis markers(e.g.,galectin-3)weredownstream.Inadditiontothese potentially“positive”controls,theabsenceofHDL-C,ApoAI, CETPandvitamin-Dmayserveasrelevant“negativecontrols” intheBayesiannetworks.
Thehierarchalorganizationofthelipid/lipoprotein-related biomarkers,inflammatorybiomarkersandfibrosis-related biomarkersintheBayesiannetworksareconsistentwitha mechanistichypothesisinwhichtriglyceride-richLDLparticles
drivedownstreaminflammationandafibroticresponse,directly contributingtotheinitiationandprogressionofhuman coronaryatheroscleroticplaques(Figure2B).Itisimportant toemphasize,however,thatourfindingsdonotsuggestthat triglyceride-richLDLparticlesthemselvesphysicallylocalizein thecoronaryvesselwalltoinitiatetheatherosclerosisprocess. TheoveralllipoproteinmilieuinpatientswithelevatedLDL-TG mayleadtotheincreasedformationofsd-LDLparticles,which thenmayphysicallylocalizetothearterialwall.
Ourpanomicdatasetoffersauniqueandunprecedented opportunitytoassesscausalityofcertainbiomarkers,aswe canassess simultaneous associationsbetweengenotypes,gene expressionlevels,circulatingbiomarkersandtheatherosclerotic phenotypeviacomprehensivecardiovascularCT.Overall,our dataisconsistentwiththepotential central hypothesisthat loss-of-functionvariantsinthehepaticlipasegenemaybe associatedwithlowerhepaticlipaseactivity,higherLDLTGlevelsresultinginatherosclerosis.Inourdata,LIPC geneexpressionlevelsweresignificantlylowerandLDL-TG levelsweresignificantlyhigherinpatientswithatherosclerosis. Furthermore,singlenucleotidepolymorphisms(SNP’s)inthe LIPCgenethatwereassociatedwithelevatedLDL-TGlevels weresimultaneouslyassociatedwithincreasedprevalenceof
Vorosetal. 10.3389/fcvm.2022.960419
BetaSECI P-valueORLn(SE)CI P-value rs2613360 070.02970.01_0.13 0.0223 1.5 0.20111.01_2.230.0434 rs12898984 0 060.0285–0.11to0.000.04240.670.18990.46_0.970.0342 rs12900448 0 060.0285–0.11_–0.00 0.04240.670.18990.46_0.970.0342 rs4774301 0 060.0285–0.11_–0.00 0.04240.670.18990.46_0.970.0342 rs4775064 0 060.0285–0.12_–0.00 0.03860.670.18990.46_0.980.0374
FIGURE4
Frontiersin CardiovascularMedicine 12 frontiersin.org 134
atherosclerosis,suggestingthe potentialrole ofLDL-TGbased ontheprinciplesofnaturalrandomization(Figure4)(27).Our dataisconsistentwithhistoricalfindingsthatpolymorphismsin theLIPCgeneareassociatedwithcirculatinglevelsofLDL-TG (28–30).
Fromamechanisticpointofview,ourresultsareconsistent withapotentialhypothesiswherebylowerhepaticlipaseactivity mayresultindecreasedlipolysis,decreasedremodeling,and decreasedinitialclearanceofTRL’s,suchasverylow-density lipoprotein(VLDL)particles.Theincreasedresidencetime ofTRL’smayleadtoprolongedexposureofTRL’stoCETP activity,resultinginmoreTG-richIDLandLDLparticles, asreflectedinelevatedLDL-TGlevels.ThepresenceofTGrichIDLandLDLparticlesfavorthegenerationofsd-LDL particles,whichphysicallymaylocalizetothearterialwall, resultingintheretentionoftheseatherogeniclipoprotein particles,triggeringaninflammatoryreactionandendothelial dysfunction,culminatingintheinitiationandpropagationof atherosclerosis.
Ingeneral,ourfindingsareconsistentwiththeliterature butalsoaddtothosefindingsbydemonstratingapossible central roleofLDL-TG,potentiallyasafunctionofabnormal hepaticlipaseactivity,asrevealedbyouruniquecausalBayesian networkanalysisandthroughgeneticvalidation.Alarge epidemiologicstudyhasexaminedtheassociationofLDL-TG withangiographicASCAD(31).Inthatstudy,LDL-TGlevels weremeasuredbytheultracentrifugation-precipitationmethod (“beta-quantification”),acumbersomereferenceprocedurenot usedforroutinediagnostictesting.Themainfindingwas thatLDL-TGwasastrongerpredictorofASCADcompared toLDL-CandwasindependentofLDL-C,withanoverall oddsratioof1.3(95%CI,1.19–1.43; P < 0.001).Although consistentwithourfindings,theoddsratioinourstudy wasmuchhigherat3.41(95%CI,1.94–6.01),likelydue totheuseofprecisionphenotypinginourapproach.Also consistentwithourfindings,theyalsoidentifiedsignificant correlationsbetweenLDL-TGandIL-6andbetweenLDLTGandC-reactiveprotein.Inasmallermoremechanistic sub-studyof114patients,ithasalsobeenreportedthatin patientswithhighLDL-TGlevels,LDLparticlesareenrichedin triglyceridesanddepletedincholesterolesters.VLDLparticles showedtheoppositetrend;theywereenrichedincholesterol estersanddepletedintriglycerides.Theseobservationsare inlinewiththeassociationofLDL-TGwithverysmallTRL particlenumberthatweobserved.Itisalsoconsistentwithour mechanistichypothesisonthecentralroleoftheremodeling ofapoB-containinglipoproteinparticlesinthedevelopment ofatherosclerosis.
Severalotherlargeclinicaltrialshavealsoprovided importantinformationrelatedtotheassociationofLDL-TG withatherosclerosis.Albersetal.examinedthepotentialrole ofLDL-TG,sd-LDLandHDLsubclassesin3,094subjectsin theAIM-HIGHclinicaltrial(19),whichwasevaluatingthe
effectofextended-releaseniacininasecondaryprevention populationonstatinbackground.Theprimaryendpointwas thecompositeofdeathfromcoronaryarterydisease,nonfatalmyocardialinfarction,ischemicstroke,hospitalization foracutecoronarysyndromeorsymptom-drivencoronary orcerebrovascularrevascularization.Intheirstudy,sd-LDL andLDL-TGwerenoteventrelated.Theadvantageofour studyisaveryclearphenotypeofcoronaryatherosclerosis basedoncomprehensivecardiovascularCT.Inaddition,the AIM-HIGHstudywasasecondarypreventionpopulation onthebackgroundofstatintherapy,differentfromour patientpopulation.
Saeedetal.alsoexaminedthepotentialroleofLDL-TG in9,334subjectswithoutprevalentCADtheARICstudy(20), usingadirecthomogenousassaythatcanberoutinelyappliedin clinicallaboratories.TheyfoundthatLDL-TGweresignificantly associatedwithcardiovasculardisease,evenafteradjustingfor traditionalriskfactors,includinglipids.Thisisconsistentwith ourownfindingsinasimilarpatientpopulation.Similarly,the authorsalsofoundthatvariantsinthepromoterregionofthe LIPCgenewereassociatedwithlowerhepaticlipaseactivity, consistentwithourownfindings.
Finally,Silbernageletal.(21)demonstratedthatLDLTGwasassociatedwithcardiovascularmortalityin3,140 subjects.Genome-wideassociationstudyinthiscohort demonstratedthatvariantsintheLIPCgeneweresignificantly associatedwithcirculatingLDL-TGlevels,consistentwith ourownfindings.Furthermore,inatwo-sampleMendelian randomizationanalysis,theauthorsfoundthatlowhepatic lipaseactivitymaybethecausalfactorbehindelevatedLDL-TG levels,drivingatheroscleroticcardiovascularrisk.Theauthors suggestedthatLDL-TGmaybeonthecausalpathwayrelatedto cardiovasculardisease.Ourcombinedunbiased,causalBayesian networkanalysisandgenomicanalysis isconsistentwith thesefindingsand propose amoredetailedbiologicalnetwork explainingthehepaticlipase/LDL-TGaxisofatherosclerosis (Figure5).
Insummary,weperformedanunbiased,causalBayesian networkanalysistoidentifypotentialnovelcausalfactorsin humancoronaryatherosclerosis,revealingthepotentialkeyrole ofTG-richlipoproteinparticles.Wethenusedourpanomic data,includinggeneticvalidation,tofurtherexplorethe potential central roleofLDL-TG,demonstratingthatthehepatic lipase/LDL-TGaxismaybe animportant pathwayinASCAD.
Limitations
Althoughthiswasaprospective,multicenterstudywith centralcorelaboratoryanalysisofallimagingandbiochemical measurements,ithassomelimitations.First,weonlyincluded Caucasiansubjectsinourstudy,asitwaspoweredforgenomewideassociationanalysesbasedonasingleethnicbackground,
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 13 frontiersin.org 135
CentralIllustration.MultiomicValidationoftheHepaticLipase(LIPC)/LDL-TGAxisinAtheroscleroticCADalongtheCentralDogmaofBiology. Geneexpressionlevelsofhepaticlipase(LIPC)aresignificantlylower (A) andcirculatingLDL-TGlevelsaresignificantlyhigher (B) inpatientswith atheroscleroticCAD,presumablyduetoLOFvariantsintheLIPCgene(upperpartoftheFigure).Ontheotherhand,GOFvariantsintheLIPC genearesimultaneouslyassociatedwithlowercirculatingLDL-TG (C) levelsANDwithlowerprevalenceofatheroscleroticCAD (D).Exceptfor hepaticlipaseactivitymeasurements(indicatedbydottedlinearoundhepaticlipaseintheFigure),theGLOBALstudydatabasecontainsallother multiomicmeasurementsalongthehepaticlipase/LDL-TG/atherosclerosisaxis.Theoveralldatapresentedhereisconsistentwithour hypothesisthatcirculatingtriglyceride-richLDLparticlesmayhaveapotentialcausalroleinatherosclerosis,duetoabnormalhepaticlipase activity.LIPC,hepaticlipase;LDL-TG,lowdensitylipoproteintriglycerides;CAD,coronaryarterydisease;LOF,loss-of-function;GOF, gain-of-function.




requiringatleast6,700subjects(7).Second,wehavelimited longitudinalfollow-upofthepatients.Nevertheless,akey featureofBayesiannetworkanalysiswiththeimplementation ofREFSisitsabilitytogeneratecausalbiologicalmodels, evenintheabsenceoflongitudinaloutcomes.Inaddition, sincewehadwholegenomesequencedata,wewerealso abletodemonstratecausalitythroughgeneticmethods.Third, althoughthegeneticanalysisisconsistentwithapotential centralroleofthehepaticlipase/LDL-TGaxis,wedidnothave functionalmeasurementsofhepaticlipase.Finally,although wehadin apriori DiscoveryandValidationdatasetinour ownGLOBALclinicalstudy,wedidnotvalidateourfindings inexternaldatasets,technicallylimitingourfindingstothe GLOBALclinicalstudypopulation.
Summaryandconclusion
WhileApoB-containinglipoproteins,inflammatory biomarkers,andmarkersofendothelialdysfunctionandfibrosis wereallassociatedwithhumancoronaryatherosclerosis, triglyceride-richLDLparticles,asmeasuredbyLDL-TGlevels, emergedasapotentiallykeyfactor,withinasub-network thatincludesapoBandLDL-C.Furthermore,geneticanalysis revealedthepotential central roleofthehepaticlipase/LDL-TG axisinatherosclerosis.Withtherecentintroductionofa simpleandfullyautomatedmethodforthequantificationof LDL-TGlevels(32),thisbiomarkermaybecomeanimportant toolintheclinicalassessmentofpatientsatriskfor,orwith, atherosclerosis.Furthermore,theresultsfromthisstudyhave

Vorosetal. 10.3389/fcvm.2022.960419
FIGURE5
Frontiersin CardiovascularMedicine 14 frontiersin.org 136
confirmed apossibleroleofhepaticlipaseinhumancoronary atherosclerosis,whichinthefuturecanbeexploredasatarget fordrugdevelopment.Itisalsoalreadyknownthatseveral approvedlipid-loweringdrugs,suchasfibrates,andstatins,have adifferentialeffectonLDL-TGversusLDL-C(33),whichwill beusefultofurtherinvestigatetobetterunderstandtheiroverall impactincardiovasculareventreduction.
Dataavailabilitystatement
TheGlobalGenomicsGroup,LLCcompanyhasspentin excessof$30MUSDtocollecttherawdatathatwasusedin thepresentanalysis.Valuecreationinthecompanyoccursby commercializinginsightsfromtherawdata,andthatishow investedcapitalisreturnedtoinvestors.Publicdisclosureofthe rawdatawouldcompromisethefullpotentialofvaluecreation bythecompanyatthepresentstage.Oncethecompanyreturns investedcapitaltoitsinvestors,therawdatamaybedeposited inpublicdatasources.Inaddition,underappropriatenondisclosureagreements,thecompanymaydiscloserawdatato interestedparties.Enquirersregardingrawdataaccessaretobe directedtoBB, bbrown@g3therapeutics.com
Ethicsstatement
Thestudiesinvolvinghumanparticipantswerereviewed andapprovedbyWesternInstitutionalReviewBoard,Inc.The patients/participantsprovidedtheirwritteninformedconsentto participateinthisstudy.
Authorcontributions
SV,AB,DL,AR,andRN:studydesign,dataanalysis andmanuscriptpreparation.MB:studydesignandstatistical analysis.IMandBB:studyexecution.JN,PM-H,GV,IV, WH,VV,TD,DN,andBH:dataanalysisandmanuscript preparation.AG,LF,BC,KR,andIK:dataanalysis. Allauthorscontributedtothearticleandapprovedthe submittedversion.
References
1.BenjaminE,MuntnerP,AlonsoA,BittencourtM,CallawayC,CarsonA,etal. Heartdiseaseandstrokestatistics-2019update:areportfromtheAmericanheart association. Circulation. (2019)139:e56–528.
2.TabasI,WilliamsK,BorenJ.Subendotheliallipoproteinretentionas theinitiatingprocessinatherosclerosis:updateandtherapeuticimplications. Circulation. (2007)116:1832–44.
Funding
GlobalGenomicsGroupsponsoredandfundedthestudy andhasfiledpatentsonbiomarkerdiagnosisofcoronary arterydisease.DenkaSeikenprovidedfinancialsupportfor manuscriptpreparation.ResearchbyDLandARwassupported byintramuralDIRfundsfromtheNationalHeart,Lungand BloodInstitute,NIH,Bethesda,MD.
Conflictofinterest
SVwasFounder,CEO,andafull-timeemployeeofGlobal GenomicsGroup.PM-HandLFreportgrantsfromGlobal GenomicsGroupduringtheconductofthestudy.IMwas afounderofGlobalGenomicsGroup.BBwasaco-founder, VPofClinicalAffairs,andafull-timeemployeeofGlobal GenomicsGroup.IVwasthesonoftheFounderandCEO ofGlobalGenomicsGroup.BC,KR,andBHweresalaried employeesofGNSHealthcare.WHwasashareholderofGlobal GenomicsGroup.ABandMBwereemployedbyAcclarogen, Ltd,Cambridge,UnitedKingdom.
Theremainingauthorsdeclarethattheresearchwas conductedintheabsenceofanycommercialorfinancial relationshipsthatcouldbeconstruedasapotentialconflictof interest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
Supplementarymaterial
TheSupplementaryMaterialforthisarticlecanbe foundonlineat: https://www.frontiersin.org/articles/10.3389/ fcvm.2022.960419/full#supplementary-material
3.BentzonJ,OtsukaF,VirmaniR,FalkE.Mechanismsofplaqueformationand rupture. CircRes. (2014)114:1852–66.
4.LibbyP,TherouxP.Pathophysiologyofcoronaryarterydisease. Circulation. (2005)111:3481–8.
5.FerenceB,GinsbergH,GrahamI,RayK,PackardC,BruckertE,etal. Low-densitylipoproteinscauseatheroscleroticcardiovasculardisease.1.Evidence
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 15 frontiersin.org 137
fromgenetic,epidemiologic,andclinicalstudies.Aconsensusstatementfromthe Europeanatherosclerosissocietyconsensuspanel. EurHeartJ. (2017)38:2459–72. doi:10.1093/eurheartj/ehx144
6.ImesC,AustinM.Low-densitylipoproteincholesterol,apolipoprotein B,andriskofcoronaryheartdisease:fromfamilialhyperlipidemiato genomics. BiolResNurs. (2013)15:292–308.doi:10.1177/10998004124
36967
7.VorosS,Maurovich-HorvatP,MarvastyI,BansalA,BarnesM,Vazquez G,etal.Precisionphenotyping,panomics,andsystem-levelbioinformaticsto delineatecomplexbiologiesofatherosclerosis:rationaleanddesignofthe“Genetic lociandtheburdenofatheroscleroticlesions”study. JCardiovascComputTomogr. (2014)8:442–51.doi:10.1016/j.jcct.2014.08.006
8.SachsK,PerezO,Pe’erD,LauffenburgerD,NolanG.Causalprotein-signaling networksderivedfrommultiparametersingle-celldata. Science. (2005)308:523–9. doi:10.1126/science.1105809
9.ZhuJ,ZhangB,SmithE,DreesB,BremR,KruglyakL,etal.Integrating large-scalefunctionalgenomicdatatodissectthecomplexityofyeastregulatory networks. NatGenet. (2008)40:854–61.doi:10.1038/ng.167
10.HoweyR,ShinS,ReltonC,DaveySmithG,CordellH.Bayesiannetwork analysisincorporatinggeneticanchorscomplementsconventionalMendelian randomizationapproachesforexploratoryanalysisofcausalrelationshipsin complexdata. PLoSGenet. (2020)16:e1008198.doi:10.1371/journal.pgen.1008198
11.PearlJ. Causality:Models,Reasoning,andInference.Cambridge:Cambridge UniversityPress(2000).384p.
12.DonohoD,TannerJ.Observeduniversalityofphasetransitionsinhighdimensionalgeometry,withimplicationsformoderndataanalysisandsignal processing. PhilosTransAMathPhysEngSci. (2009)367:4273–93.doi:10.1098/ rsta.2009.0152
13.LatourelleJ,BesteM,HadziT,MillerR,OppenheimJ,ValkoM,etal. Large-scaleidentificationofclinicalandgeneticpredictorsofmotorprogressionin patientswithnewlydiagnosedParkinson’sdisease:alongitudinalcohortstudyand validation. LancetNeurol. (2017)16:908–16.doi:10.1016/S1474-4422(17)30328-9
14.GendelmanR,XingH,MirzoevaO,SardeP,CurtisC,FeilerH,etal. BayesiannetworkinferencemodelingidentifiesTRIB1asanovelregulatorof cell-cycleprogressionandsurvivalincancercells. CancerRes. (2017)77:1575–85. doi:10.1158/0008-5472.CAN-16-0512
15.XingH,McDonaghP,BienkowskaJ,CashoraliT,RungeK,MillerR, etal.Causalmodelingusingnetworkensemblesimulationsofgeneticandgene expressiondatapredictsgenesinvolvedinrheumatoidarthritis. PLoSComputBiol. (2011)7:e1001105.doi:10.1371/journal.pcbi.1001105
16.HayeteB,WuestD,LaramieJ,McDonaghP,ChurchB,EberlyS,etal.A BayesianmathematicalmodelofmotorandcognitiveoutcomesinParkinson’s disease. PLoSOne. (2017)12:e0178982.doi:10.1371/journal.pone.0178982
17.FolchJ,LeesM,SloaneStanleyG.Asimplemethodfortheisolationand purificationoftotallipidesfromanimaltissues. JBiolChem. (1957)226:497–509.
18.SampsonM,LingC,SunQ,HarbR,AshmaigM,WarnickR,etal.Anew equationforcalculationoflow-densitylipoproteincholesterolinpatientswith normolipidemiaand/orhypertriglyceridemia. JAMACardiol. (2020)5:540–8.doi: 10.1001/jamacardio.2020.0013
19.AlbersJ,SleeA,FlegJ,O’BrienK,MarcovinaS.Relationshipofbaseline HDLsubclasses,smalldenseLDLandLDLtriglyceridetocardiovascularevents
intheAIM-HIGHclinicaltrial. Atherosclerosis. (2016)251:454–9.doi:10.1016/j. atherosclerosis.2016.06.019
20.SaeedA,FeofanovaE,YuB,SunW,ViraniS,NambiV,etal.Remnantlikeparticlecholesterol,low-densitylipoproteintriglycerides,andincident cardiovasculardisease. JAmCollCardiol. (2018)72:156–69.doi:10.1016/j.jacc. 2018.04.050
21.SilbernagelG,ScharnaglH,KleberM,DelgadoG,StojakovicT,Laaksonen R,etal.LDLtriglycerides,hepaticlipaseactivity,andcoronaryarterydisease: anepidemiologicandMendelianrandomizationstudy. Atherosclerosis. (2019) 282:37–44.doi:10.1016/j.atherosclerosis.2018.12.024
22.DiffenderferM,SchaeferE.Thecompositionandmetabolismoflarge andsmallLDL. CurrOpinLipidol. (2014)25:221–6.doi:10.1097/MOL. 0000000000000067
23.BalloutR,RemaleyA.GlycA:anewbiomarkerforsystemicinflammationand cardiovasculardisease(CVD)riskassessment. JLabPrecisMed. (2020)5:17.doi: 10.21037/jlpm.2020.03.03
24.HopkinsP.Molecularbiologyofatherosclerosis. PhysiolRev. (2013) 93:1317–542.
25.LibbyP,BuringJ,BadimonL,HanssonG,DeanfieldJ,BittencourtM,etal. Atherosclerosis. NatRevDisPrimers. (2019)5:56.
26.PetersenM,ShulmanG.Mechanismsofinsulinactionandinsulinresistance. PhysiolRev. (2018)98:2133–223.doi:10.1152/physrev.00063.2017
27.FerenceB,RobinsonJ,BrookR,CatapanoA,ChapmanM,NeffD, etal.VariationinPCSK9andHMGCRandriskofcardiovasculardisease anddiabetes. NEnglJMed. (2016)375:2144–53.doi:10.1056/NEJMoa16 04304
28.HegeleR,LittleJ,VezinaC,MaguireG,TuL,WoleverT,etal.Hepaticlipase deficiency.Clinical,biochemical,andmoleculargeneticcharacteristics. Arterioscler Thromb. (1993)13:720–8.doi:10.1161/01.atv.13.5.720
29.RuelI,CoutureP,GagneC,DeshaiesY,SimardJ,HegeleR,etal. Characterizationofanovelmutationcausinghepaticlipasedeficiencyamong FrenchCanadians. JLipidRes. (2003)44:1508–14.doi:10.1194/jlr.M200479JLR200
30.HegeleR,BreckenridgeW,CoxD,MaguireG,LittleJ,ConnellyP.Elevated LDLtriglycerideconcentrationsinsubjectsheterozygousforthehepaticlipase S267Fvariant. ArteriosclerThrombVascBiol. (1998)18:1212–6.doi:10.1161/01. atv.18.8.1212
31.MarzW,ScharnaglH,WinklerK,TiranA,NauckM,BoehmB,etal.Lowdensitylipoproteintriglyceridesassociatedwithlow-gradesystemicinflammation, adhesionmolecules,andangiographiccoronaryarterydisease:theludwigshafen riskandcardiovascularhealthstudy. Circulation. (2004)110:3068–74.doi:10.1161/ 01.CIR.0000146898.06923.80
32.ItoYO,IkezakiH,HiraoY,MachidaA,SchaeferE,FurusyoN. Developmentandpopulationresultsofafullyautomatedhomogeneousassay forLDLtriglyceride. JApplLabMed. (2018)2:11.doi:10.1373/jalm.2017.02
4554
33.Hirano,T,KoderaR,HirashimaT,SuzukiN,AokiE,HosoyaM,etal. Metabolicpropertiesoflowdensitylipoprotein(LDL)triglyceridesinpatients withtype2diabetes,comparisonwithsmalldenseLDL-cholesterol. JAtheroscler Thromb. (2021)29:762–74.doi:10.5551/jat.62789
Vorosetal. 10.3389/fcvm.2022.960419
Frontiersin CardiovascularMedicine 16 frontiersin.org 138
PUBLISHED 16January2023
DOI 10.3389/fcvm.2022.1023651
OPENACCESS
EDITEDBY Chu-HuangChen, TexasHeartInstitute,UnitedStates
REVIEWEDBY FedericaFogacci, UniversityofBologna,Italy
XianweiWang, XinxiangMedicalUniversity,China
*CORRESPONDENCE
AlexanderV.Sorokin alexander.sorokin2@nih.gov
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal
FrontiersinCardiovascularMedicine
RECEIVED 20August2022
ACCEPTED 30December2022
PUBLISHED 16January2023
CITATION
HongCG,FloridaE,LiH,ParelPM,MehtaNN andSorokinAV(2023)Oxidizedlow-density lipoproteinassociateswithcardiovascular diseasebyaviciouscycleofatherosclerosis andinflammation:Asystematicreviewand meta-analysis. Front.Cardiovasc.Med. 9:1023651. doi:10.3389/fcvm.2022.1023651
COPYRIGHT
© 2023Hong,Florida,Li,Parel,Mehtaand Sorokin.Thisisanopen-accessarticle distributedunderthetermsoftheCreative CommonsAttributionLicense(CCBY).Theuse, distributionorreproductioninotherforumsis permitted,providedtheoriginalauthor(s)and thecopyrightowner(s)arecreditedandthat theoriginalpublicationinthisjournaliscited,in accordancewithacceptedacademicpractice. Nouse,distributionorreproductionis permittedwhichdoesnotcomplywiththese terms.
Oxidizedlow-densitylipoprotein associateswithcardiovascular diseasebyaviciouscycleof atherosclerosisandinflammation: Asystematicreviewand meta-analysis
ChristinG.Hong,ElizabethFlorida,HaiouLi,PhilipM.Parel, NehalN.Mehtaand AlexanderV.Sorokin*
SectionofInflammationandCardiometabolicDiseases,NationalHeart,Lung,andBloodInstitute,National InstitutesofHealth,Bethesda,MD,UnitedStates
Background: Low-densitylipoproteincholesterol (LDL-C)isanestablishedmarker forcardiovasculardisease(CVD)andatherapeutictarget.OxidizedLDL(oxLDL) isknowntobeassociatedwithexcessiveinflammationandabnormallipoprotein metabolism.Chronicinflammatorydiseasesconferanelevatedriskofpremature atherosclerosisandadversecardiovascularevents.WhetheroxLDLmayserveasa potentialbiomarkerforCVDstratificationinpopulationswithchronicinflammatory conditionsremainsunderstudied.
Objective: Toperformasystematicreviewandmeta-analysisevaluatingthe relationshipbetweenoxLDLandCVD(definedbyincidentCVDevents,carotid intima-mediathickness,presenceofcoronaryplaque)inpatientswithchronic inflammatorydiseases.
Methods: Asystematicliteraturesearchwasperformedusingstudiespublished between2000and2022fromPubMed,CochraneLibrary,Embase(Elsevier),CINHAL (EBSCOhost),Scopus(Elsevier),andWebofScience:CoreCollection(Clarivate Analytics)databasesontherelationshipbetweenoxLDLandcardiovascularriskon inflamedpopulation.Thepoolede ectsizewascombinedusingtherandome ect modelandpublicationbiaswasassessedif P < 0.05fortheEggerorBeggtestalong withthefunnelplottest.
Results: Atotalofthreeobservationalstudieswith1,060participantswere ultimatelyincludedinthefinalmeta-analysis.TheresultsdemonstratedthatoxLDLis significantlyincreasedinparticipantswithCVDinthesettingofchronicinflammatory conditions.Thismeta-analysissuggeststhatoxLDLmaybeausefulbiomarkerinrisk stratifyingcardiovasculardiseaseinchronicallyinflamedpatients.
KEYWORDS cardiovasculardisease,atherosclerosis,inflammation,oxidizedlow-densitylipoprotein,lipids
TYPE SystematicReview
Frontiersin CardiovascularMedicine 01 frontiersin.org 139
Introduction
Atherosclerosisis acomplexpathophysiologicalprocessdriven bymetabolicderangements,lipid accumulation,andinflammation (1–3).Whileitmaybeclinicallysilentatearlystages,atherosclerotic lesionsoftentransforminto vulnerableplaquespronetoruptureand incitesubsequentadverseevents,includingmyocardialinfarction, strokeanddeath(4, 5).Coronaryarterydisease(CAD)isan atheroscleroticcardiovasculardisorderandcontinuestobethe leadingcauseofmortalityworldwide,despiteadvancementsin treatments(6, 7).Whiletraditionalcardiovascular(CV)riskfactors contributetothepathogenesisofCAD,othernovelriskfactors maybeinvolved.Inparticular,systemicinflammationhasthought toplayaroleinthedevelopmentandprogressionofCAD(1, 8). Growingbodyofevidencehasshownthatchronicinflammatory diseases,suchaspsoriasis(PSO),rheumatoidarthritis(RA),human immunodeficiencyvirus(HIV),andsystemiclupuserythematosus (SLE)areassociatedwithacceleratedatherosclerosisandpremature adverseCVevents(9–14).Infact,suchconditionsarenow consideredindependentriskfactorsforcardiovasculardisease(CVD) (15).However,traditionalCVriskstratificationusingFramingham riskscoreandage issuboptimalinassessingCVDriskinpatients withchronicinflammatoryconditions(16–18).Forexample,severe psoriasishasbeen showntoconferanadditional6.2%increasein long-termriskofCVDbasedonFraminghamRiskscore(19).Given theelevatedCVD riskandcurrentchallengesinevaluatingCVDin theseinflamedpopulations,itisnecessarytoidentifyprognostictools thatwilladequatelycaptureandassessCVDrisk.
Low-densitylipoproteincholesterol(LDL-C)isaknown biomarkerofcardiovasculardisease(CVD)(20, 21).Pharmacological reductionofLDL-Cisconsidered amaintoolintheprimary preventionofatheroscleroticcardiovasculardisease(ASCVD); however,theissueofresidualatheroscleroticriskthatremains inpatientswithdecreasedLDL-Candelevatedhighdensity lipoprotein-cholesterol(HDL-C)isofadditionalclinicalconcern (22).Alternativly,otherLDL-relatedlipoproteinspecies,suchas small-dense LDL(sdLDL),lipoprotein(a)[Lp (a)],andoxLDL,have beenshowntobereliablemarkersofCVDriskprognosisaswell(23–25).Oxidativestresscontributestoatheroscleroticplaqueformation bystimulatingactivationof macrophagesandvascularsmooth musclecells,increasingextracellularcholesterolaccumulationwithin vesselwalls,andtransformingmacrophagesintopro-inflammatory andpro-thromboticphenotypes(26).Theobservedcriticalstepin atheroscleroticplaquebuild-up,thefoamcellformation,istriggered bytheuptakeofoxLDLbymacrophagesthroughscavengerreceptors, suchasCD36,aswellaslectin-likeoxLDLreceptor(LOX-1)(27–29).PreviousstudieshavefoundthatcirculatingoxLDLassociates witheverystageofatherosclerosis,fromsubclinicalatherosclerosis toovertcardiovasculardisease,includinghypertension,coronaryand peripheralarterialdisease,acutecoronarysyndromes,andischemic cerebralinfarction,andhasprognosticvalueinestimatingCVD risk(25, 30, 31).Indeed,elevatedlevelsofoxLDLwereshownto predictmyocardialinfarction intheHealthABCcohort,evenafter adjustingforage,gender,race,smoking,andmetabolicsyndrome (30).OxLDLmayevenbeassociatedwitharterialaging,asarecent studyfoundthatoxLDL demonstratedpredictivevalueofarterial stiffness,asmeasuredbypulse-wavevelocity,inpatientswithnormal tomildlyreducedrenalfunction(32).Further,oxLDLislinkedwith
metabolicallydysfunctional pathologiesfrequentlyassociatedwith CVD,includingobesity,metabolicsyndrome,anddiabetesmellitus (25).Thus,oxLDLhasrecentlybecomeanimportanttherapeutic targetforCVD and hasbeenrecognizedasabiomarkerforCAD andotherage-relatedatheroscleroticprocesses(24, 31, 33, 34). However,towhat extentoxLDLcontributestoCVDwithinsystemic inflammationandwhetherithasanyclinicalutilityinCVDrisk stratificationforsuchpopulationsremainunderstudied.Therefore, weconductedasystematicreviewtoexaminetheavailableevidence andaimedtoinvestigatetheassociationbetweenoxLDLlevelsand CVDinthesettingofchronicinflammationbymeta-analysis.
Methods
Searchstrategy
Thesystematicreviewand meta-analysiswereconducted accordingwiththePreferredReportingItemsforSystematicreviews andMeta-Analysesguidelines(35)andtheprotocolwasregistered withthePROSPEROInternationalProspectiveRegisterofSystematic Reviews(PROSPERO2022CRD42022354525).Thismeta-analysis wasnotbasedontheindividualparticipantdata,thusethicalapproval wasnotapplicable.
Asystematicsearchofstudiespublishedbetween2000and 2022wasconductedthroughPubMed,CochraneLibrary,Embase (Elsevier),CINHAL(EBSCOhost),Scopus(Elsevier),andWeb ofScience:CoreCollection(ClarivateAnalytics)databases.The initialsearchstrategieswereperformed:“oxidizedphospholipid” OR“oxPLs”OR“oxidizedLDL-C”OR“oxidizedlow-density lipoprotein”OR“oxLDL”OR“low-densitylipoproteinreceptor-1” OR“LOX-1”OR“sLOX-1”OR“apoA-I”OR“ApolipoproteinAI”OR“ApolipoproteinsE”OR“apolipoproteinE”OR“ApoE”OR “ApoC2”OR“ApoC3”OR“oxHDL”OR“Lipoproteins,LDL”OR “Lipoproteins,HDL”OR“modifiedlipoprotein”and(“Myocardial Infarction”OR“Stroke”OR“Cerebral”OR“AnginaPectoris” OR“Arteriosclerosis”OR“atherogenes”OR“atherosclerotic”OR “coronaryarterydisease”OR“Psoriasis”)and(“PatientOutcome Assessment”OR“RiskAssessment”OR“TreatmentOutcome”). Whileweinitiallyplannedtoincludealloxidizedlipidsinour systematicreview,theresultsofthesearchstrategywereultimately focusedonoxidizedlow-densitylipoproteinasthissearchterm yieldedthegreatestnumberofrelevantstudies.Wealsoconsidered referencelistsandreviewarticlesforotherpotentiallyrelevant citations.Thereferencesofretrievedarticleswerealsoreviewed toidentifyanyrelevantstudy.LanguagerestrictionofEnglish wasapplied.WeusedEndnotesoftware(ClarivateAnalytics, Philadelphia,PA)formanagementofthestudies.
Studyselectioncriteria
A2-stepselectionprocesswasconductedusingCovidence (Covidence,Melbourne,Victoria,Australia)screeningsoftware. Inthefirststep,titlesandabstractsgeneratedfromthesearch strategywerereviewedbytwoindependentresearchers.Studies thatdidnotexaminetheassociationbetweenoxidizedlow-density lipoprotein,chronicinflammatoryconditions,andcardiovascular diseasemeasureswereexcluded.Inthesecondstep,studies
Hongetal. 10.3389/fcvm.2022.1023651
Frontiersin CardiovascularMedicine 02 frontiersin.org 140
successfully screenedinafterthefirststepwerereviewedinfulltextto confirmiftheyreportedthemeanwithstandarddeviation,ormedian withinterquartilerangeforobservationalstudies.
Inclusionandexclusioncriteria
Inclusioncriteriawere:observationalstudiesinvestigatingthe relationshipbetweenoxLDLandCVDinpatientpopulations withchronicinflammatorydiseases,includingpsoriasis, systemiclupuserythematosus,rheumatoidarthritis,andhuman immunodeficiencyvirus.
Exclusioncriteriawere:incorrectstudydesign;literaturereviews, discussions,editorials,opinionpieces,andabstracts-onlytexts; wrongcomparators;incorrectsetting;wrongpatientpopulation; studiesthatdidnotreportmeanandstandarddeviationormedian withinterquartilerangeforobservationalstudies;unavailablefull textarticles.
Dataextractionandqualityassessment
Afterthe47availablefull-textarticleswereselected,3full-text sourceswereexaminedforrepresentativedatacontainingeffectsize (ES)ofoxLDLmeasuredbymeanandstandarddeviationormedian andinterquartilerange.Forthesestudies,Covidencesoftwarewas usedtoextractthedata.Thefollowingdatawereextractedfrom eachincludedstudy:firstauthor’sname,publicationyear,number ofsubjects,participantpopulation,typeofpublication,patient characteristics(meanormedianageinyears,percentageofmen, baselinebodymassindex),effectsizeofoxLDL(representedbymean withstandarddeviation,medianwithinterquartilerange),andstudy outcomes[definedasincidentCVDevent,carotidintima-media thickness,orcoronaryplaquepresenceasmeasuredbycoronary computedtomographicangiography(CCTA)].Tostandardizethe differentmeasurementsandunitsofoxLDLreportedinthestudies usedinouranalysis,weutilizedthestandardizedmeandifference with95%confidenceintervaltoconsistentlycompareoxLDLacross studies.Anystudiesrepresentingresultsthroughmedianwith interquartilerange(IQR)wereconvertedtomeanwithstandardized meandifferencebasedonmethodsfromWanetal.(36).Alldata extractionswerecompletedby tworeviewers(EF,HL)andchecked byanotherreviewer(CGH).
Statisticalanalyses
Thepooledstandardizedmeandifferencewithits95%confidence interval(CI)wascalculatedforoxLDLtoaccountforthe differentunitsofoxLDLmeasurementacrossallstudies.Statistical heterogeneitywasidentifiedifthe P valueforCochranQwas <0.05 orthe I2 statisticswas >50%(37).TheHedgesrandomeffects modelwaschosenif heterogeneitywasdetected(38).Otherwise,an inversevariancefixedeffectmodelwasused.Publicationbiaswas consideredifP<0.05fortheEggerorBeggtestalongwiththefunnel plotmethod(SupplementaryFigure1).Allstatisticalanalyseswere performedusingRStatisticalSoftware(version4.2.0,RFoundation forStatisticalComputing,Vienna,Austria).
Results
Studyselection
Thescreeningand selectionprocessisdemonstratedusing aflowchartdiagramin Figure1.Initially,atotalof7,309 relevantstudieswere importedintoCovidencewith846duplicates immediatelyremoved.Ofthe6,463remainingreferences,6,416were excludedinthefirststepoftheselectionstrategybasedontitleand abstractscreening.Reviewoftheremaining47studiesinfulltext formduringthesecondstepoftheselectionstrategyyielded3final studies(39–41)with1,060participantsthatwereincludedinthe meta-analysis.Whileexcluded fromthemeta-analysis,4additional studiesfromthe47full-textsourceswereincludedinourdiscussion fortheirfindingsonotherpromisingbiomarkersofLDLoxidation, includingLDL-conjugateddienes,solublelectin-likeoxidizedLDL receptor-1(sLOX-1),oxidizedphospholipids(Ox-PLs),andother oxidation-modifiedlipoproteins(OMLs)(27, 33, 42, 43).
Studycharacteristics
Thestudies includedinourmeta-analysisareshownin Table1 Thesamplesizeof individualstudyrangedfrom105to755 participants.Ofthethreestudies,onewascross-sectional(40)and therestwerecohort studies(39, 41).Allthestudieswerepublished between2010and2021andonly includedparticipantswithout knownCVDhistory.Theenrolledparticipantshadameanagerange from38.96to51years.Thesexbypercentmaleinthestudies rangedfrom31.5to77%.Thebaselinebodymassindex(BMI)ranged from23.3to28.0.AllthreestudiesmeasuredoxLDLconcentration usinganenzyme-linkedimmunosorbentassay(ELISA)(Mercodia, Uppsala,Sweden).OnestudyreportedoxLDLasperthechangein oxLDLlevels( oxLDL)(39),onestudyreportedoxLDLlevelsin U/L(40),andonestudy reportedoxLDLlevelsinmU/L(41).Thus, toaccountforthedifferentmeasurementsofoxLDLreportedin thesestudies,thepooledstandardizedmeandifferencewithits95% confidenceinterval(CI)wascalculated.Inordertoassesstheeffect size(ES)ofoxLDLandCVD,twostudiesutilizedtheoddsratio(OR) (40, 41)andonestudyusedthehazardratio(HR)(39)(Table1).All qualityscoresoftheincludedstudieswerecalculatedas >5according totheNewcastle-OttawaScale(NOS)(44).NOSscoresofthestudies includedinthemeta-analysis arepresentedin Table2
ElevatedoxLDLsignificantlyassociateswith CVDininflamedpopulations
Theindividualstudiesandpooledmeta-analysisresultsare demonstratedin Figure2.Ofthethreestudies,twoassessed oxLDLlevelsin468participants withHIVdiseaseandassociated CVD(definedbycarotidintima-mediathicknessorcoronary plaquepresenceoncoronaryCTA)vs.487participantswithHIV diseasewithoutCVDandfoundthatincreasedoxLDLlevelswere significantlyassociatedwithCVDcomparedtothosewithoutCVD (ESforParra:0.75(0.46,1.03];ESforHoffman:0.34[0.20,0.48]). Inonestudyofparticipantswithrheumatoidarthritis,therewas nosignificantassociationbetweenoxLDLESandCVD.Asshown
Hongetal. 10.3389/fcvm.2022.1023651
Frontiersin CardiovascularMedicine 03 frontiersin.org 141
in Figure2, thepooledtotaleffectsizeofelevatedoxLDLindicated thatparticipantswith chronicinflammatorydiseaseswithassociated CVDhadasignificantincreaseinoxLDLcomparedtothose withoutCVD(0.44[95%CI:0.11,0.77]).CochranQand I2 index indicatedthattherewasheterogeneityobservedforthemarginal analysis.Theheterogeneitymaybesecondarytodifferentmethodsof measurementsandstudyparticipants. P-valueoftheEgger’stestfor funnelplotasymmetrywas0.86,whichdoesnotsuggestthepresence ofpublicationbias.

Discussion
Chronicinflammatoryconditions,suchaspsoriasis,RA,HIV, andSLE,haveincreasedoxLDLlevels,acceleratedatherosclerosis, andprematureadversecardiovascularoutcomes(9–13, 45).Thus, these pathologies providesuitablehumanmodelstostudythe mechanismsofinflammatoryatherosclerosisandassociatedCVD inhumans.Inoursystematicreviewwithmeta-analysis,weaimed tousethesediseasestobetterunderstandoxLDLasaCVrisk biomarkeranditsrelationshipwithatheroscleroticCVDwithin thecontextofchronicinflammation.Wefoundthatcomparedto
chronicallyinflamedsubjectswithoutCVD,elevatedoxLDLlevels weresignificantlyassociatedwithhigherCVDpresenceinpatients withchronicinflammatoryconditions(EStotal:0.44[95%CI:0.11, 0.77]).Theseresultsextendthecurrentunderstandingoftheclinical utilityofoxLDLasapotentialbiomarkerforCVriskassessmentin chronicallyinflamedpopulations.
OxidizedLDLisknowntohavepro-inflammatoryandproatherogenicproperties(46)andcanpredictincreasedriskof myocardialinfarction(MI) (47–49).Additionally,manystudieshave demonstratedelevatedlevels ofoxLDLinchronicinflammatory populations.AutoantibodiesagainstoxLDL(auAb-oxLDL)were showntobeelevatedinpatientswithpsoriasiscomparedto matchedcontrols,with42%ofpsoriasispatientsand3.3%ofcontrol subjectshavinghigherauAb-oxLDLlevelsthanthecut-offpoint (352mU/mL)(50).Theautoantibodylevelswerealsofoundto significantlycorrelatewiththePsoriasisAreaSeverityIndexscore,a toolusedtoassesstheseverityandextentofpsoriasis(50).OxLDL alsosignificantlyassociated withnoncalcifiedcoronaryburden,a markerofsubclinicalatherosclerosis,inpatientswithpsoriasis(33). Arecentstudycomparingfemale lupuspatientswithandwithout CVDfoundthatoxLDLwassignificantlyhigherinthosewithCVD (14).However,toourknowledge,thisisthefirstmeta-analysis
Hongetal. 10.3389/fcvm.2022.1023651
FIGURE1 FlowchartofthestudyselectiongeneratedbyPRISMA.
Frontiersin CardiovascularMedicine 04 frontiersin.org 142
TABLE1Baselinecharacteristicsofstudiesincludedinthemeta-analysis.
BARFOOT,BetterAnti-RheumaticPharmaco-Therapy;REPRIEVE,RandomizedTrialtoPreventVascularEventsinHIV;SBP,systolicbloodpressure;DBP,diastolicbloodpressure;LDL-C,low-densitylipoproteincholesterol;HDL-C,high-densitylipoproteincholesterol; TG,triglycerides;BMI,bodymassindex;HIV-1,humanimmunodeficiencyvirus-1;NRTI,nucleosidereversetranscriptaseinhibitors;NNRTI,Non-nucleosidereversetranscriptaseinhibitors;ASCVD,atheroscleroticcardiovasculardisease;ART,anti-retroviraltherapy; HTN,hypertension;MI,myocardialinfarct;HF,heartfailure;FRS,Framinghamriskscore;CAD,coronaryarterydisease;CTA,computedtomographyangiography.
Hongetal. 10.3389/fcvm.2022.1023651
Reference Design Study Population Age Sex, male% BMI oxLDL mean (SD) oxLDL assay Adjustedvariables CVD outcome E ectsize (95%CI) oxLDL exposure Othervariables Ajeganova etal.(38) Cohort (hospitalbased) 114RApatients fromtheBARFOT trial 50.6 ±11.2 31.5 24.93 ± 4 Case:56.4 (12.69); Control: 54.2 (15.5) Mercodia oxLDL Age Occurrenceof MI,Angina pectoris, congestiveHF, ischemic cerebrovascular event HR1.03 [1.0–1.06] 0.035 Parraetal.(39) Crosssectional 187HIVpatientsat Hospital UniversitarideSant Joan 38.96 ± 0.61 68.8 23.31 ± 0.27 Case:97.8 (29.22); Control: 76.22 (28.43) Mercodia U/L Age,gender,smoking status,SBP,DBP, glucose,LDL-C, HDL-C,TG,BMI, HIV-1basalviralload, basalCD4cellcount, lipodystrophy, exposuretimeto NRTI,NNRTIand(PI) treatments, inflammatorymarkers,
Atherosclerosis evaluatedby carotid intima-media thickness (CIMT).CVD riskestimated usingFRS,low risk(<10%), moderate (10–20%)and highrisk (>20%) OR1.026 [1.001–1.05] Hoffmann etal.(40) Cohort (communitybased) 755HIV-positive participantsfrom theREPRIEVE study 51 ± 6 77 28.0 ± 6.0 Case: 382.27 (138.09) Control: 340.03 (116.48) Mercodia mU/L ASCVDrisk,HIV Parameters(ART duration,CD4,nadir CD4),age,sex,and race,LDL-Clevel, HTN,andcurrent smoking Prevalenceand compositionof CAD measuredas coronary plaqueon coronaryCTA OR1.01 [0.90–1.15]
andoxidativemarkers
Frontiersin CardiovascularMedicine frontiersin.org
E ectsizebetweencirculatingoxidizedlow-densitylipoproteinandatheroscleroticcardiovasculardiseaserisk.Forestplotsdescribinge ectsize(ES), alsoknownasthestandardmeandi erence,with95%confidenceinterval(CI)forallincludedobservationalstudies.Squaresrepresentstudy-specific estimates;boxsizesrepresentindividualstudyweight;horizontallinerepresent95%CIs;diamondsrepresentthetotalmeandi erencewithits95%CI.
toobservetheassociationbetweenoxLDLandCVDonlywithin chronicinflammatorydisease populations.Thus,ouraimforthis systematicreviewandmeta-analysiswasto:(1)tosummarize currentliteratureontherelationshipbetweenoxLDLandCVDin chronicinflammatorypopulations,and(2)toprovideastandardized representationofmeasuredoxLDLlevelsacrossvariousstudies. Currently,nostandardizedunitsorreferencelevelsexistforreporting oxLDLmeasuredbydifferentbiochemicalassays(51–54).Thus, our findingsutilizedthe standardizedmeandifferencetounifythe reportingofoxLDLacrossdifferentstudies.
PharmacologicalperspectiveonoxLDLasa potentialtherapeutictarget
Low-densitylipoproteincholesterolisaprognosticcirculating biomarkerforstratifyinggeneralcardiovascularrisk(20, 21). Consequently,lipid-loweringtreatments,such asstatinsandfibrates, arethemainstaytreatmentsofloweringLDL-Clevelsaswellas decreasingtriglycerides,increasingHDL-Clevels,andreducing hepaticcholesterolbiosynthesis(55, 56).However,thereisstill aneedformorespecific biomarkerswithpathologicalrelevance, especiallyinchronicallyinflamedpopulations,toimprovethe riskstratificationofcardiovascularevents(57).OxLDLmaybe apromisingcandidate, asincreasedoxLDLlevelsarecentralto atheroscleroticplaqueformationandthusmaybemorecausally associatedwithCVDoutcomesthanLDL-C(31, 58).Several studieshaveillustrated theassociationbetweenelevatedcirculating oxLDLandadverseCVDoutcomes(31, 58, 59).Moreimportantly, Tsimikasetal.demonstrated thathighdoseatorvastatinreduced totalplasmaoxidizedphospholipidscomplexedwithapolipoprotein B-100(ApoB-100),theprimaryproteinoftheLDLparticle, suggestingthatstatinsmaypartlyexertprotectivecardiovascular effectsthroughmobilizationofpro-inflammatoryoxidationspecies fromatheroscleroticlesions(60).Further,the“Standardvs.high-dose therApywithRosuvastatinforlipiDlowering”(SARD)randomized clinicaltrialfoundthathighdoserosuvastatinsignificantlyreduced levelsofoxLDLwhencomparedtolowdoserosuvastatin(61).Inthe settingofHIV,several studiesshowedthatstatintherapyreduced noncalcifiedcoronaryplaquevolume,totalplaquevolume,and positivelyremodeledplaqueinpatientswithHIV(62, 63)(Table1). Thus,usingcurrentstatin therapytotreatelevatedoxLDLlevels

inadditiontoLDL-Cmayprovideincreasedbenefitsinpotentially reducingtheriskofadverseCVDeventsinsuchpopulations.
Whilestatinsarethemainstaylipid-loweringtherapy,many peoplearestatinintolerantorcannotachievegoalLDL-C levelsonstatintherapyaloneandthusrequirealternative therapy.Injectablelipid-loweringtherapycurrentlyusedfor inheritedhypercholesterolemiaandhigh-riskCVpatientshave demonstratedgreatbenefitforsuchpatients(64).Proprotein convertasesubtilisin/kexintype 9(PCSK9)inhibitorsareatype ofinjectablelipid-loweringtherapytargetingPCSK9,aprotease enzymeproducedinhepatocytesinvolvedinLDLbinding, internalization,anddegradation(65, 66).Interestingly,PCSK9is alsoimplicatedinthe oxLDL-inducedinflammatorypathway.In adultratventricularcardiomyocytes,oxLDLsignificantlyimpaired contractilefunction via inductionofPCSK9(67).Tangetal. foundthatPCSK9small interferingRNAsuppressedoxLDLinducedinflammatoryresponseinTHP-1-derivedmacrophages(68). Thus,PCSK9inhibitorsoffer anoveltherapeuticopportunityin targetingoxLDL-relatedatheroscleroticoutcomes(67).Evolocumab isaPCSK9inhibitorthatwhenaddedtomaximallytolerated statintherapywasfoundtoreducetheriskofcardiovascular outcomesinpatientswithatheroscleroticCVD,whiledatafrom the“ODYSSEYOUTCOMES”trialdemonstrateddecreasedriskof recurrentischemiccardiovasculareventsinpatientswithprevious acutecoronarysyndrometreatedwithalirocumabinaddition tohigh-intensitystatins(65, 69, 70).Thesefindingsillustrate theimportanceofdiscoveringadditionaltreatmentmodalitiesfor patientsathighriskforCVDcomplications.
InadditiontooxLDLasacandidatebiomarkerforCVD, thereisagrowingbodyofliteratureshowingthepotentialrole ofanti-oxLDLantibodiesandotheroxLDL-relatedmoietiesfor CVDriskstratificationandpromisingtherapeutictargets(71). Autoantibodiestooxidation-specific epitopesonLDL,suchas MDA-modifiedLDL(MDA-LDL),arefoundinatherosclerotic lesionsofhumansandanimals(72, 73)andthereissignificant researchon theclinicalcorrelatesoftheseantibodies(74–76). Karvonenetal.demonstrated thatIgMautoantibodiestoMDALDLepitopehadaninverseassociationwithcarotidatherosclerosis inapopulationcohortstudyof1,022middle-agedmenand women(77).Solublelectin-likeoxLDLreceptor1(sLOX-1)isan inflammation-inducedreceptorforoxLDLthathasbeenshown toinducemyocardialischemiathroughunstableatherosclerotic
Hongetal. 10.3389/fcvm.2022.1023651
FIGURE2
Frontiersin CardiovascularMedicine 06 frontiersin.org 144
plaqueformation,suggestinganimportantroleofLOX-1inthe pathogenesisofoxLDL-relatedCVD(78, 79).Interestingly,increased sLOX-1levelshave beenassociatedwithsystemicinflammatory diseases.sLOX-1levelswerehigherinpatientswithRAwithpositive rheumatoidfactorandanti-citrullinatedproteinantibodyserology thanthosewithout,andcontinuedtoremainathighlevelsinnonremissionpatientscomparedtothoseinremissionirrespectiveof treatment,highlightingthepotentialutilityofsLOX-1asabiomarker fordiseaseactivityandremissioninRA(80).SLEpatientshadtwofold higherlevelsof sLOX-1,whichpositivelyassociatedwithhighsensitivityCRPlevels,oxLDL,proinflammatoryHDL,andimpaired HDLefflux,insteadoftraditionalriskfactorsandSLEdiseaseactivity (79).Further,theauthorsfoundthatSLEpatientswithhigher sLOX-1levelswereyoungerthanthosewithlowlevels,whichis concerninggiventhatSLEpatientsareatgreaterriskofCVDand isparticularlyevidentintheyoungerfemaleSLEpopulation,as 54%ofcardiaceventsthatoccurinfemaleSLEpatientsareunder theageof44(81).Thus,elevatedsLOX-1levelsmayserveasan usefulbiomarkerofincreasedCVDrisk,andsLOX-1inhibition maybeatherapeuticopportunityfordecreasingatherosclerosis inthesepatients.Additionally,geneticmodulationhasbecomea promisingtherapeuticapproachforoxLDLtreatment.Forinstance, overexpressionofthelongnon-codingRNALINC00452hasbeen showntoreverseoxLDLinjuryinhumanumbilicalveinendothelial cells(HUVECS)byregulatingthemiR-194-5p/IGF1Raxis(82). Additionally, miR-214-3pinHUVECSregulatesoxLDL-initiated macrophageautophagy,thussuggestingapotentialtherapeuticrole formiRNAsinatherosclerosis(83).Furtherstudiesarenecessaryto elucidatemoretherapeutictargetsaimedatthefunctionandquantity ofoxLDLinthepathogenesisofcardiovasculardisease.
Severalarticlesexcludedbasedonourexclusioncriteriafor themeta-analysisweredeemedimportanttoincludeherefor theirdiscussionofotheroxLDL-relatedpotentialbiomarkersfor optimizationofCVDriskstratificationininflamedpopulations. Inamulticenterobservationalstudy,Nyyssönenetal.foundthat increasedLDL-conjugateddieneconcentrations,identifiedasone ofthefirststagesofLDLoxidationandsubclinicalatherosclerosis, exhibitedapositiverelationshipwithincreasedCIMTinhighrisksubjectspresentingwithatleastthreevascularriskfactors (VRF)(27).Oxidation-specificbiomarkersprimarilyoxidized phospholipids(Ox-PLs)onapolipoproteinB-100-containing lipoproteins(oxPL/ApoB-100),havebeendemonstratedasessential inidentifyingtheriskofperipheralarterydisease(43).Other studieshavefocused ontheuptakepathwayofoxLDLthrough solublelectin-likeoxidizedLDLreceptor-1(sLOX-1)tobetter understandatherosclerosis.Deyetal.showedthatinpatientswith psoriasis,sLOX-1associatedwithimagingmarkersofsubclinical atherosclerosisandincreasedpsoriasisseverity(42).Moreover, patients withpsoriasishaddecreaseinplasmalevelsofoxidationmodifiedlipoproteins,includingoxLDLunderspecificbiologic treatment(33, 42).
OtherpotentialoxLDL-relatedbiomarkersof CVDandatherosclerosis
OtherLDL-relatedlipoproteins,includingsdLDLandLp(a),are pronetooxidationandassociatedwithelevatedcardiovascularrisk
Hongetal. 10.3389/fcvm.2022.1023651 TABLE2Qualityassessmentofstudiesincludedinmeta-analysis. Reference Isthecasedefinitionadequate? Representativenessofthe cases Selectionofcontrols Definitionofcontrols Comparabilityofgroupsofbasisofdesignoranalysis Ascertainmentofexposure Ascertainmentofbothgroupswithsamemethod OverallNOS scores Ajeganova etal.( 38 ) ⋆ ⋆⋆ ⋆ ⋆ ⋆ 6 Parraetal.( 39 ) ⋆ ⋆⋆ ⋆⋆ ⋆ ⋆ 7 Hoffmann etal.( 40 ) ⋆ ⋆ ⋆ ⋆ ⋆⋆ ⋆ ⋆ 8 NOS,Newcastle-OttawaScale.
Frontiersin CardiovascularMedicine 07 frontiersin.org 145
(84–86). Becauseoftheirphysicalandcompositionalcharacteristics, theyhavehigheraffinityforextracellularmatrix,reducedbinding toLDLreceptor,andincreasedresidencetimeinthecirculation comparedtolarge(buoyant)LDLparticles(23, 87, 88).Inpatients withpsoriaticarthritis,sdLDL concentrationwasincreased independentlyofthepresenceofmetabolicsyndrome,suggesting apotentialmediationbysdLDLofatherosclerosisdevelopment inpsoriaticarthritis(89).Furthermore,astudycomparing HIV-positivewithHIV-negativeparticipantsfoundincreased sdLDLlevelsinthosewithHIV(63).BothHIVinfectionand combinationantiretroviraltherapyarethoughttoinduceendothelial dysfunctionthroughendothelialcellactivation,oxidativestress, andinflammationthatleadstoincreasedcardiovasculardiseasein thesepatients(90).Indeed,inastudybyPostetal.,theauthors foundthatsuboptimalHIV RNAsuppressionandcombined antiretroviraltherapyadherencewerethemaindeterminantsof coronaryarterystenosisprogressionduringamedianfollowupof4.5years(91).Lp(a)isalsoacandidatebiomarker andhasbeendemonstrated topredictCIMTinHIV-positive females(92).Whilethesefindingsarepromising,meta-analyses investigatingtherole ofoxidizedlipidswithinsystemically inflamedpopulationsarelackingandthusfuturestudieswill continuouslybenecessarytofurtherelucidatetheserelationships (93, 94).
Ourmeta-analysishadseverallimitations.Firstly,thecausal associationbetweenoxLDLandCVDoutcomesinourpopulations ofinterestcouldnotbedefinedbecauseofthecohortorcrosssectionalnatureoftheincludedstudies.Anotherlimitationis thatstudiesusingothertechniquestoestimateCVDoutcomes werenotincludedinthismeta-analysis.Asobservationalstudies showmoreheterogeneitythanrandomizedcontroltrialsand severaloftheincludedstudieswereobservationalstudies,this factormustalsobeconsideredgiventhatheterogeneityinterferes withthedetectionofpublicationbias(95, 96).Theheterogeneity sourcesmaycorrelatewithstudydesign,participantages,and whetherpatientshaveatheroscleroticriskfactors.WhileoxLDL isapromisingbiomarkerforCVDriskstratification,oxLDL isnotyetusedintheclinicasadiagnostictoolforCVD. Finally,wewereunabletodeterminetheeffectsofpopulational characteristicsorpharmacologictherapyontheprogressionof CVDoutcomesinrelationtooxLDLinpatientswithchronic inflammatorydiseases.
Conclusion
Oursystematicreviewandmeta-analysisdemonstratethat patients withchronicinflammatory diseases,particularlyRA andHIV,havesignificantlyhigherlevelsofcirculatingoxLDLas measuredbyeffectsizeinrelationtoincreasedcardiovascular risk.Thus,oxLDLmayofferinsightintooptimizingCVD riskstratificationinchronicallyinflamedpopulations.We alsodiscussedadditionalatherogeniclipoproteinparameters associatedwithoxLDLthatofferamorenuancedunderstanding oflipoproteinmodificationslinkedwithCVDinthesetting ofinflammation.Largermeta-analysisandfuturemechanistic studiesarenecessarytofurtherelucidatetherelationshipbetween oxidizedlipoproteinsandcardiovasculardiseaseinpatientswith long-standinginflammatoryconditions.
Dataavailabilitystatement
Theoriginalcontributionspresentedinthestudyarepublicly available.This datacanbefoundhere: https://www.crd.york.ac.uk/ prospero/#searchadvanced,accessionnumber:CRD42022354525.
Authorcontributions
CH,EF,HL,andASwereinvolvedindesigningthe conceptofthereview andoversightandintheliterature search,summationoftheliterature,andrevisionsofthe manuscript.CH,EF,HL,PP,NM,andASdraftedthe manuscript.Allauthorsread,reviewed,andapprovedthe finalmanuscript.
Funding
ThisstudywassupportedbytheNationalHeart,Lung,and Blood InstituteIntramuralResearchProgram(HL006193-07).This researchwasmadepossiblethroughtheNIHMedicalResearch ScholarsProgram,apublic-privatepartnershipsupportedjointlyby theNIHandcontributionstotheFoundationfortheNIHfrom theDorisDukeCharitableFoundation,Genentech,theAmerican AssociationforDentalResearch,theColgate-PalmoliveCompany, andotherprivatedonors.Thefundingsourceshadnorolein thedesignandconductofthestudy;collection,management, analysis,andinterpretationofthedata;preparation,review,or approvalofthemanuscript;anddecisiontosubmitthemanuscript forpublication.
Acknowledgments
WewouldliketothanktheNationalInstitutesofHealth Library ofMedicineforthe resourcestomakethissystematic reviewpossible.Wewouldespeciallyliketoacknowledge GiselaButera,MEd,MLIS,BiomedicalandInformationist Librarian,forhervaluableexpertiseandhelpthroughoutthis entireprocess.
Conflictofinterest
NMhasservedasaconsultantforAmgen,EliLilly,andLeo Pharmareceivinggrants/otherpayments,asaprincipalinvestigator and/orinvestigatorforAbbVie,Celgene,AstraZeneca,Janssen Pharmaceuticals,Inc.,Novartis,andAbcentrareceivinggrants and/orresearchfunding,andasaprincipalinvestigatorfortheNIH receivinggrantsand/orresearchfunding.
Theremainingauthorsdeclarethattheresearchwasconducted intheabsenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythose oftheauthors anddonotnecessarilyrepresentthoseof
Hongetal. 10.3389/fcvm.2022.1023651
Frontiersin CardiovascularMedicine 08 frontiersin.org 146
theiraffiliatedorganizations,orthoseofthepublisher, theeditorsandthereviewers.Anyproductthatmaybe evaluatedinthisarticle,orclaimthatmaybemadeby itsmanufacturer,isnotguaranteedorendorsedbythe publisher.
References
1.LibbyP,RidkerPM,HanssonGK.Inflammationinatherosclerosis: frompathophysiologyto practice. JAmCollCardiol. (2009)54:2129–38.doi:10.1016/j.jacc.2009.09.009
2.HarringtonRA.Targetinginflammationincoronaryarterydisease. NEnglJMed. (2017)377:1197–8.doi:10.1056/NEJMe1709904
3.LusisAJ.Atherosclerosis. Nature. (2000)407:233–41.doi:10.1038/35025203
4.Arbab-ZadehA,FusterV.Themythofthe“vulnerableplaque”:transitioningfroma focusonindividuallesionstoatheroscleroticdiseaseburdenforcoronaryarterydisease riskassessment. JAmCollCardiol. (2015)65:846–55.doi:10.1016/j.jacc.2014.11.041
5.ChenYC,HuangAL,KyawTS,BobikA,PeterK.Atheroscleroticplaquerupture: identifyingthestrawthatbreaksthecamel’sback. ArteriosclerThrombVascBiol. (2016) 36:e63–72.doi:10.1161/ATVBAHA.116.307993
6.ViraniSS,AlonsoA,AparicioHJ,BenjaminEJ,BittencourtMS,CallawayCW, etal.Heartdiseaseandstrokestatistics-2021update:areportfromtheAmericanheart association. Circulation. (2021)143:e254–743.doi:10.1161/CIR.0000000000000950
7.AhmadFB,AndersonRN.TheleadingcausesofdeathintheUsfor2020. Jama. (2021)325:1829–30.doi:10.1001/jama.2021.5469
8.ElluluMS,PatimahI,Khaza’aiH,RahmatA,AbedY,AliF.Atherosclerotic cardiovasculardisease:areviewofinitiatorsandprotectivefactors. Inflammopharmacology. (2016)24:1–10.doi:10.1007/s10787-015-0255-y
9.MasonJC,LibbyP.Cardiovasculardiseaseinpatientswithchronicinflammation: mechanismsunderlyingprematurecardiovasculareventsinrheumatologicconditions. EurHeartJ. (2015)36:482–9c.doi:10.1093/eurheartj/ehu403
10.FunderburgNT,MehtaNN.LipidabnormalitiesandinflammationinHIV inflection. CurrHIV/AIDSRep. (2016)13:218–25.doi:10.1007/s11904-016-0321-0
11.AksentijevichM,LateefSS,AnzenbergP,DeyAK,MehtaNN.Chronic inflammation,cardiometabolicdiseasesandeffectsoftreatment:psoriasisasahuman model. TrendsCardiovascMed. (2020)30:472–8.doi:10.1016/j.tcm.2019.11.001
12.HarringtonCL,DeyAK,YunusR,JoshiAA,MehtaNN.Psoriasisasahumanmodel ofdiseasetostudyinflammatoryatherogenesis. AmJPhysiolHeartCircPhysiol. (2017) 312:H867–h73.doi:10.1152/ajpheart.00774.2016
13.McMahonM,GrossmanJ,SkaggsB,FitzgeraldJ,SahakianL,RagavendraN, etal.Dysfunctionalproinflammatoryhigh-densitylipoproteinsconferincreasedriskof atherosclerosisinwomenwithsystemiclupuserythematosus. ArthritisRheum. (2009) 60:2428–37.doi:10.1002/art.24677
14.KhairyN,EzzatY,NaeemN,TahaR,WesamR.Atherosclerosisbiomarkersin femalesystemiclupuserythematosuspatientswithandwithoutcardiovasculardiseases. EgyptRheumatol. (2017)39:7–12.doi:10.1016/j.ejr.2016.03.003
15.GrundySM,StoneNJ,BaileyAL,BeamC,BirtcherKK,BlumenthalRS,etal. 2018Aha/Acc/Aacvpr/Aapa/Abc/Acpm/Ada/Ags/Apha/Aspc/Nla/Pcnaguidelineonthe managementofbloodcholesterol:areportoftheamericancollegeofcardiology/american heartassociationtaskforceonclinicalpracticeguidelines. JAmCollCardiol. (2019) 73:e285–350.doi:10.1016/j.jacc.2018.11.003
16.FinckhA,CourvoisierDS,PaganoS,BasS,Chevallier-RuggeriP,Hochstrasser D,etal.Evaluationofcardiovascularriskinpatientswithrheumatoidarthritis:do cardiovascularbiomarkersofferaddedpredictiveabilityoverestablishedclinicalrisk scores? ArthritisCareRes(Hoboken). (2012)64:817–25.doi:10.1002/acr.21631
17.Lloyd-JonesDM,WilsonPW,LarsonMG,BeiserA,LeipEP,D’AgostinoRB,etal. Framinghamriskscoreandpredictionoflifetimeriskforcoronaryheartdisease. AmJ Cardiol. (2004)94:20–4.doi:10.1016/j.amjcard.2004.03.023
18.ChungCP,OeserA,AvalosI,GebretsadikT,ShintaniA,RaggiP,etal.Utilityofthe framinghamriskscoretopredictthepresenceofcoronaryatherosclerosisinpatientswith rheumatoidarthritis. ArthritisResTher. (2006)8:R186.doi:10.1186/ar2098
19.TorresT,SalesR,VasconcelosC,MartinsdaSilvaB,SeloresM.Framinghamrisk scoreunderestimatescardiovasculardiseaseriskinseverepsoriaticpatients:implications incardiovascularriskfactorsmanagementandprimarypreventionofcardiovascular disease. JDermatol. (2013)40:923–6.doi:10.1111/1346-8138.12267
20.PackardC,ChapmanMJ,SibartieM,LaufsU,MasanaL.Intensivelow-density lipoproteincholesterolloweringincardiovasculardiseaseprevention:opportunitiesand challenges. Heart. (2021)107:1369–75.doi:10.1136/heartjnl-2020-318760
TheSupplementaryMaterialforthisarticlecanbefound online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022. 1023651/full#supplementary-material
21.BorénJ,ChapmanMJ,KraussRM,PackardCJ,BentzonJF,BinderCJ, etal.Low-densitylipoproteinscauseatheroscleroticcardiovasculardisease: pathophysiological,genetic,andtherapeuticinsights:aconsensusstatementfrom theEuropeanatherosclerosissocietyconsensuspanel. EurHeartJ. (2020)41:2313–30. doi:10.1093/eurheartj/ehz962
22.RidkerPM,GenestJ,BoekholdtSM,LibbyP,GottoAM,NordestgaardBG, etal.Hdlcholesterolandresidualriskoffirstcardiovasculareventsaftertreatment withpotentstatintherapy:ananalysisfromthejupitertrial. Lancet. (2010)376:333–9. doi:10.1016/S0140-6736(10)60713-1
23.PackardCJ.Smalldenselow-densitylipoproteinanditsroleasan independentpredictorofcardiovasculardisease. CurrOpinLipidol. (2006)17:412–7. doi:10.1097/01.mol.0000236367.42755.c1
24.TsimikasS,A.Testincontext:lipoprotein(a):diagnosis,prognosis, controversies,andemergingtherapies. JAmCollCardiol. (2017)69:692–711. doi:10.1016/j.jacc.2016.11.042
25.TrpkovicA,ResanovicI,StanimirovicJ,RadakD,MousaSA,Cenic-MilosevicD, etal.Oxidizedlow-densitylipoproteinasabiomarkerofcardiovasculardiseases. CritRev ClinLabSci. (2015)52:70–85.doi:10.3109/10408363.2014.992063
26.DiPietroN,FormosoG,PandolfiA.Physiologyandpathophysiologyofoxldl uptakebyvascularwallcellsinatherosclerosis. VasculPharmacol. (2016)84:1–7. doi:10.1016/j.vph.2016.05.013
27.NyyssönenK,KurlS,KarppiJ,NurmiT,BaldassarreD,VegliaF,etal.Ldloxidative modificationandcarotidatherosclerosis:resultsofamulticenterstudy. Atherosclerosis. (2012)225:231–6.doi:10.1016/j.atherosclerosis.2012.08.030
28.FebbraioM,SilversteinRL.Cd36:Implicationsincardiovasculardisease. IntJ BiochemCellBiol. (2007)39:2012–30.doi:10.1016/j.biocel.2007.03.012
29.BarretoJ,KarathanasisSK,RemaleyA,SpositoAC.RoleofLox-1(lectinlikeoxidizedlow-densitylipoproteinreceptor1)asacardiovascularriskpredictor: mechanisticinsightandpotentialclinicaluse. ArteriosclerThrombVascBiol. (2021) 41:153–66.doi:10.1161/ATVBAHA.120.315421
30.HolvoetP.OxidizedLdlandcoronaryheartdisease. ActaCardiol. (2004)59:479–84. doi:10.2143/AC.59.5.2005219
31.HolvoetP,MertensA,VerhammeP,BogaertsK,BeyensG,VerhaegheR,etal. Circulatingoxidizedldlisausefulmarkerforidentifyingpatientswithcoronary arterydisease. ArteriosclerThrombVascBiol. (2001)21:844–8.doi:10.1161/01.ATV.21. 5.844
32.CiceroAFG,KuwabaraM,JohnsonR,BoveM,FogacciF,RosticciM, etal.Ldl-oxidation,serumuricacid,kidneyfunctionandpulse-wavevelocity: datafromthebrisighellaheartstudycohort. IntJCardiol. (2018)261:204–8. doi:10.1016/j.ijcard.2018.03.077
33.SorokinAV,KotaniK,ElnabawiYA,DeyAK,SajjaAP,YamadaS,etal.Association betweenoxidation-modifiedlipoproteinsandcoronaryplaqueinpsoriasis. CircRes. (2018)123:1244–54.doi:10.1161/CIRCRESAHA.118.313608
34.MeisingerC,BaumertJ,KhuseyinovaN,LoewelH,KoenigW.Plasmaoxidized low-densitylipoprotein,astrongpredictorforacutecoronaryheartdiseaseeventsin apparentlyhealthy,middle-agedmenfromthegeneralpopulation. Circulation. (2005) 112:651–7.doi:10.1161/CIRCULATIONAHA.104.529297
35.PageMJ,MoherD,BossuytPM,BoutronI,HoffmannTC,MulrowCD,etal. Prisma2020explanationandelaboration:updatedguidanceandexemplarsforreporting systematicreviews. BMJ. (2021)372:n160.doi:10.1136/bmj.n160
36.WanX,WangW,LiuJ,TongT.Estimatingthesamplemeanandstandarddeviation fromthesamplesize,median,rangeand/orinterquartilerange. BMCMedResMethodol. (2014)14:135.doi:10.1186/1471-2288-14-135
37.HigginsJP,ThompsonSG,DeeksJJ,AltmanDG.Measuringinconsistencyin meta-analyses. BMJ. (2003)327:557–60.doi:10.1136/bmj.327.7414.557
38.BorensteinM,HedgesLV,HigginsJPT,RothsteinHR. IntroductiontoMetaAnalysis.Hoboken,NJ:Wiley(2021).doi:10.1002/9781119558378
39.AjeganovaS,HuizingaT.Sustainedremissioninrheumatoidarthritis:latest evidenceandclinicalconsiderations. TherAdvMusculoskeletDis. (2017)9:249–62. doi:10.1177/1759720X17720366
Hongetal. 10.3389/fcvm.2022.1023651
Supplementarymaterial
Frontiersin CardiovascularMedicine 09 frontiersin.org 147
40.Parra S,CollB,AragonésG,MarsillachJ,BeltránR,RullA,etal.Nonconcordance betweensubclinicalatherosclerosisandthecalculatedframinghamriskscoreinHIVinfectedpatients:relationshipswithserummarkersofoxidationandinflammation. HIV Med. (2010)11:225–31.doi:10.1111/j.1468-1293.2009.00766.x
41.HoffmannU,LuMT,FoldynaB,ZanniMV,KaradyJ,TaronJ,etal. Assessmentofcoronaryarterydiseasewithcomputedtomographyangiography andinflammatoryandimmuneactivationbiomarkersamongadultswithHIV eligibleforprimarycardiovascularprevention. JAMANetwOpen. (2021)4:e2114923. doi:10.1001/jamanetworkopen.2021.14923
42.DeyAK,GaddipatiR,ElnabawiYA,OngstadE,GoyalA,ChungJH,etal. Associationbetweensolublelectinlikeoxidizedlow-densitylipoproteinreceptor1andcoronaryarterydiseaseinpsoriasis. JAMADermatol. (2020)156:151–7. doi:10.1001/jamadermatol.2019.3595
43.BertoiaML,PaiJK,LeeJH,TalebA,JoostenMM,MittlemanMA,etal.Oxidationspecificbiomarkersandriskofperipheralarterydisease. JAmCollCardiol. (2013) 61:2169–79.doi:10.1016/j.jacc.2013.02.047
44.WellsGA,WellsG,SheaB,SheaB,O’ConnellD,PetersonJ,etal. TheNewcastleOttawaScale(Nos)forAssessingtheQualityofNonrandomisedStudiesinMeta-Analyses (2014).
45.CollB,ParraS,Alonso-VillaverdeC,AragonésG,MonteroM,CampsJ,etal.The roleofimmunityandinflammationintheprogressionofatherosclerosisinpatientswith HIVinfection. Stroke. (2007)38:2477–84.doi:10.1161/STROKEAHA.106.479030
46.NavabM,BerlinerJA,WatsonAD,HamaSY,TerritoMC,LusisAJ,etal.TheYin andYangofoxidationinthedevelopmentofthefattystreak:areviewbasedonthe1994 georgelymanduffmemoriallecture. ArteriosclerThrombVascBiol. (1996)16:831–42. doi:10.1161/01.ATV.16.7.831
47.VaaralaO,MänttäriM,ManninenV,TenkanenL,PuurunenM,AhoK,etal. Anti-cardiolipinantibodiesandriskofmyocardialinfarctioninaprospectivecohortof middle-agedmen. Circulation. (1995)91:23–7.doi:10.1161/01.CIR.91.1.23
48.HamstenA,BjörkholmM,NorbergR,DeFaireU,HolmG.Antibodiesto cardiolipininyoungsurvivorsofmyocardialinfarction:anassociationwithrecurrent cardiovascularevents. Lancet. (1986)327:113–6.doi:10.1016/S0140-6736(86)92258-0
49.PalinskiW,Ylä-HerttualaS,RosenfeldME,ButlerSW,SocherSA,Parthasarathy S,etal.Antiseraandmonoclonalantibodiesspecificforepitopesgeneratedduring oxidativemodificationoflowdensitylipoprotein. Arteriosclerosis. (1990)10:325–35. doi:10.1161/01.ATV.10.3.325
50.OremA,Cim¸sitG,DegerO,OremC,VanizorB.Thesignificanceofautoantibodies againstoxidativelymodifiedlow-densitylipoprotein(Ldl)inpatientswithpsoriasis. Clin ChimActa. (1999)284:81–8.doi:10.1016/S0009-8981(99)00062-5
51.ElkanAC,SjöbergB,KolsrudB,RingertzB,HafströmI,FrostegårdJ.Glutenfreevegandietinducesdecreasedldlandoxidizedldllevelsandraisedatheroprotective naturalantibodiesagainstphosphorylcholineinpatientswithrheumatoidarthritis:a randomizedstudy. ArthritisResTher. (2008)10:R34.doi:10.1186/ar2388
52.TsimikasS,WitztumJL.Measuringcirculatingoxidizedlow-densitylipoproteinto evaluatecoronaryrisk. Circulation. (2001)103:1930–2.doi:10.1161/01.CIR.103.15.1930
53.HolvoetP,CollenD,VandeWerfF.Malondialdehyde-modifiedldlasamarkerof acutecoronarysyndromes. JAMA. (1999)281:1718–21.doi:10.1001/jama.281.18.1718
54.ItabeH,YamamotoH,ImanakaT,ShimamuraK,UchiyamaH,KimuraJ,etal. Sensitivedetectionofoxidativelymodifiedlowdensitylipoproteinusingamonoclonal antibody. JLipidRes. (1996)37:45–53.doi:10.1016/S0022-2275(20)37634-3
55.PahanK.Lipid-loweringdrugs. CellMolLifeSci. (2006)63:1165–78. doi:10.1007/s00018-005-5406-7
56.StoneNJ,RobinsonJG,LichtensteinAH,BaireyMerzCN,BlumCB,EckelRH,etal. 2013Acc/Ahaguidelineonthetreatmentofbloodcholesteroltoreduceatherosclerotic cardiovascularriskinadults:areportoftheamericancollegeofcardiology/American HeartAssociationtaskforceonpracticeguidelines. Circulation. (2014)129:S1–45. doi:10.1161/01.cir.0000437738.63853.7a
57.SandhuPK,MusaadSM,RemaleyAT,BuehlerSS,StriderS,DerzonJH, etal.Lipoproteinbiomarkersandriskofcardiovasculardisease:alaboratory medicinebestpractices(Lmbp)systematicreview. JApplLabMed. (2016)1:214–29. doi:10.1373/jalm.2016.021006
58.ChoiSH,ChaeA,MillerE,MessigM,NtaniosF,DeMariaAN,etal.Relationship betweenbiomarkersofoxidizedlow-densitylipoprotein,statintherapy,quantitative coronaryangiography,andatheromavolume:observationsfromthereversal(reversalof atherosclerosiswithaggressivelipidlowering)study. JAmCollCardiol. (2008)52:24–32. doi:10.1016/j.jacc.2008.02.066
59.GaoS,ZhaoD,WangM,ZhaoF,HanX,QiY,etal.Associationbetweencirculating oxidizedLdlandatheroscleroticcardiovasculardisease:ameta-analysisofobservational studies. CanJCardiol. (2017)33:1624–32.doi:10.1016/j.cjca.2017.07.015
60.TsimikasS,WitztumJL,MillerER,SasielaWJ,SzarekM,OlssonAG,etal. High-doseatorvastatinreducestotalplasmalevelsofoxidizedphospholipids andimmunecomplexespresentonapolipoproteinb-100inpatientswith acutecoronarysyndromesinthemiracltrial. Circulation. (2004)110:1406–12. doi:10.1161/01.CIR.0000141728.23033.B5
61.NishikidoT,OyamaJ,KeidaT,OhiraH,NodeK.High-dosestatintherapy withrosuvastatinreducessmalldenseldlandmda-ldl:thestandardvs.high-dose
therapywithrosuvastatinforlipidlowering(sard)trial. JCardiol. (2016)67:340–6. doi:10.1016/j.jjcc.2015.05.017
62.NouE,LoJ,GrinspoonSK.Inflammation,immuneactivation,andcardiovascular diseaseinHIV. Aids. (2016)30:1495–509.doi:10.1097/QAD.0000000000001109
63.DuongM,PetitJM,MarthaB,GallandF,PirothL,WalldnerA,etal. ConcentrationofcirculatingoxidizedLdlinHIV-infectedpatientstreatedwith antiretroviralagents:relationtohiv-relatedlipodystrophy. HIVClinTrials. (2006)7:41–7. doi:10.1310/7381-M1YD-RTV5-4RYT
64.StrilchukL,FogacciF,CiceroAF.Safetyandtolerabilityofinjectablelipidloweringdrugs:anupdateofclinicaldata. ExpertOpinDrugSaf. (2019)18:611–21. doi:10.1080/14740338.2019.1620730
65.SteffensD,BramlageP,ScheeffC,KasnerM,HassaneinA,FriebelJ,etal. Pcsk9inhibitorsandcardiovascularoutcomes. ExpertOpinBiolTher. (2020)20:35–47. doi:10.1080/14712598.2020.1677604
66.RosensonRS,HegeleRA,FazioS,CannonCP.Theevolvingfutureofpcsk9 inhibitors. JAmCollCardiol. (2018)72:314–29.doi:10.1016/j.jacc.2018.04.054
67.SchlüterKD,WolfA,WeberM,SchreckenbergR,SchulzR.Oxidizedlow-density lipoprotein(Oxldl)affectsload-freecellshorteningofcardiomyocytesinaproprotein convertasesubtilisin/kexin9(Pcsk9)-dependentway. BasicResCardiol. (2017)112:63. doi:10.1007/s00395-017-0650-1
68.TangZ,JiangL,PengJ,RenZ,WeiD,WuC,etal.Pcsk9sirnasuppressesthe inflammatoryresponseinducedbyoxldlthroughinhibitionofNf-?bactivationinThp-1derivedmacrophages. IntJMolMed. (2012)30:931–8.doi:10.3892/ijmm.2012.1072
69.SchwartzGG,StegPG,SzarekM,BhattDL,BittnerVA,DiazR,etal.Alirocumab andcardiovascularoutcomesafteracutecoronarysyndrome. NEnglJMed. (2018) 379:2097–107.doi:10.1056/NEJMoa1801174
70.SabatineMS,GiuglianoRP,KeechAC,HonarpourN,WiviottSD,MurphySA,etal. Evolocumabandclinicaloutcomesinpatientswithcardiovasculardisease. NEnglJMed. (2017)376:1713–22.doi:10.1056/NEJMoa1615664
71.HartleyA,HaskardD,KhamisR.OxidizedLdlandanti-oxidizedLdlantibodies inatherosclerosis-novelinsightsandfuturedirectionsindiagnosisandtherapy. Trends CardiovascMed. (2019)29:22–6.doi:10.1016/j.tcm.2018.05.010
72.HammerA,KagerG,DohrG,RablH,GhassempurI,JürgensG.Generation, characterization,andhistochemicalapplicationofmonoclonalantibodiesselectively recognizingoxidativelymodifiedapob-containingserumlipoproteins. Arterioscler ThrombVascBiol. (1995)15:704–13.doi:10.1161/01.ATV.15.5.704
73.PalinskiW,OrdVA,PlumpAS,BreslowJL,SteinbergD,WitztumJL.Apoe-deficient miceareamodeloflipoproteinoxidationinatherogenesis.Demonstrationofoxidationspecificepitopesinlesionsandhightitersofautoantibodiestomalondialdehyde-lysinein serum. ArteriosclerThromb. (1994)14:605–16.doi:10.1161/01.ATV.14.4.605
74.TsimikasS,BrilakisES,LennonRJ,MillerER,WitztumJL,McConnellJP, etal.RelationshipofIggandIgmautoantibodiestooxidizedlowdensitylipoprotein withcoronaryarterydiseaseandcardiovascularevents. JLipidRes. (2007)48:425–33. doi:10.1194/jlr.M600361-JLR200
75.GrönlundH,HallmansG,JanssonJH,BomanK,WikströmM,deFaireU, etal.Lowlevelsofigmantibodiesagainstphosphorylcholinepredictdevelopmentof acutemyocardialinfarctioninapopulation-basedcohortfromNorthernSweden. Eur JCardiovascPrevRehabil. (2009)16:382–6.doi:10.1097/HJR.0b013e32832a05df
76.RavandiA,BoekholdtSM,MallatZ,TalmudPJ,KasteleinJJP,WarehamNJ,etal. RelationshipofIggandIgmautoantibodiesandimmunecomplexestooxidizedLdl withmarkersofoxidationandinflammationandcardiovascularevents:resultsfromthe epic-norfolkstudy. JLipidRes. (2011)52:1829–36.doi:10.1194/jlr.M015776
77.KarvonenJ,PäivänsaloM,KesäniemiYA,HörkköS.Immunoglobulin Mtypeofautoantibodiestooxidizedlow-densitylipoproteinhasaninverse relationtocarotidarteryatherosclerosis. Circulation. (2003)108:2107–12. doi:10.1161/01.CIR.0000092891.55157.A7
78.PothineniNVK,KarathanasisSK,DingZ,ArulanduA,VarugheseKI,MehtaJL. Lox-1inatherosclerosisandmyocardialischemia:biology,genetics,andmodulation. J AmCollCardiol. (2017)69:2759–68.doi:10.1016/j.jacc.2017.04.010
79.SagarD,GaddipatiR,OngstadEL,BhagrooN,AnLL,WangJ,etal.Lox-1:a potentialdriverofcardiovascularriskinslepatients. PLoSONE. (2020)15:e0229184. doi:10.1371/journal.pone.0229184
80.IshikawaM,ItoH,FuruM,HashimotoM,FujiiT,OkahataA,etal.Plasmaslox-1is apotentbiomarkerofclinicalremissionanddiseaseactivityinpatientswithseropositive Ra. ModRheumatol. (2016)26:696–701.doi:10.3109/14397595.2015.1128871
81.ManziS,MeilahnEN,RairieJE,ConteCG,MedsgerTA,Jansen-McWilliamsL,etal. Age-specificincidenceratesofmyocardialinfarctionandanginainwomenwithsystemic lupuserythematosus:comparisonwiththeframinghamstudy. AmJEpidemiol. (1997) 145:408–15.doi:10.1093/oxfordjournals.aje.a009122
82.YuanL,WangD,ZhouZ.Linc00452overexpressionreversesoxldl-inducedinjury ofhumanumbilicalveinendothelialcells(Huvecs) via regulatingMir-194-5p/Igf1raxis. FrontCardiovascMed. (2022)9:975640.doi:10.3389/fcvm.2022.975640
83.WangJ,WangWN,XuSB,WuH,DaiB,JianDD,etal.Microrna-214-3p:alink betweenautophagyandendothelialcelldysfunctioninatherosclerosis. ActaPhysiol(Oxf). (2018)222:3.doi:10.1111/apha.12973
Hongetal. 10.3389/fcvm.2022.1023651
Frontiersin CardiovascularMedicine 10 frontiersin.org 148
84.GentileM,IannuzzoG,MattielloA,MarottaG,IannuzziA,PanicoS,etal. Association betweenLp(a)andatherosclerosisinmenopausalwomenwithoutmetabolic syndrome. BiomarkMed. (2016)10:397–402.doi:10.2217/bmm.16.4
85.St-PierreAC,CantinB,DagenaisGR,MauriègeP,BernardPM,DesprésJP,etal. Low-densitylipoproteinsubfractionsandthelong-termriskofischemicheartdiseasein men:13-yearfollow-updatafromtheQuébeccardiovascularstudy. ArteriosclerThromb VascBiol. (2005)25:553–9.doi:10.1161/01.ATV.0000154144.73236.f4
86.LamarcheB,TchernofA,MoorjaniS,CantinB,DagenaisGR,LupienPJ,etal.Small, denselow-densitylipoproteinparticlesasapredictoroftheriskofischemicheartdisease inmen.Prospectiveresultsfromthequébeccardiovascularstudy. Circulation. (1997) 95:69–75.doi:10.1161/01.CIR.95.1.69
87.GardnerCD,FortmannSP,KraussRM.Associationofsmalllow-densitylipoprotein particleswiththeincidenceofcoronaryarterydiseaseinmenandwomen. Jama. (1996) 276:875–81.doi:10.1001/jama.1996.03540110029028
88.StampferMJ,KraussRM,MaJ,BlanchePJ,HollLG,SacksFM,etal.Aprospective studyoftriglyceridelevel,low-densitylipoproteinparticlediameter,andriskof myocardialinfarction. JAMA. (1996)276:882–8.doi:10.1001/jama.1996.03540110036029
89.GentileM,PelusoR,DiMinnoMND,CostaL,CasoF,deSimoneB, etal.Associationbetweensmalldenseldlandsub-clinicalatherosclerosisinpatients withpsoriaticarthritis. ClinRheumatol. (2016)35:2023–9.doi:10.1007/s10067-0163344-4
90.KovacsL,KressTC,Belinde.ChantemèleEJ.HIV,combinationantiretroviral therapy,andvasculardiseasesinmenandwomen. JACC. (2022)7:410–21. doi:10.1016/j.jacbts.2021.10.017
91.FogacciF,BorghiC,GrassiD,CiceroAFG.Peoplelivingwithhuman immunodeficiencyvirus:cardiovascularriskscreeningforanearlyandeffectiverisk management. Atherosclerosis. (2022)353:28–9.doi:10.1016/j.atherosclerosis.2022.06.001
92.EnkhmaaB,AnuuradE,ZhangW,LiCS,KaplanR,LazarJ,etal.Lipoprotein(a) andHIV:allele-specificapolipoprotein(a)levelspredictcarotidintima-mediathicknessin HIV-infectedyoungwomeninthewomen’sinteragencyHIVstudy. ArteriosclerThromb VascBiol. (2017)37:997–1004.doi:10.1161/ATVBAHA.117.309137
93.PelusoR,CasoF,TassoM,SabbatinoV,LupoliR,DarioDiMinnoMN,etal. Biomarkersofsubclinicalatherosclerosisinpatientswithpsoriaticarthritis. OpenAccess Rheumatol. (2019)11:143–56.doi:10.2147/OARRR.S206931
94.ColacoK,OcampoV,AyalaAP,HarveyP,GladmanDD,PiguetV,etal.Predictive utilityofcardiovascularriskpredictionalgorithmsininflammatoryrheumaticdiseases:a systematicreview. JRheumatol. (2020)47:928–38.doi:10.3899/jrheum.190261
95.EggerM,SchneiderM,DaveySmithG.Spuriousprecision?Meta-analysisof observationalstudies. BMJ. (1998)316:140–4.doi:10.1136/bmj.316.7125.140
96.Delgado-RodríguezM.Systematicreviewsofmeta-analyses: applicationsandlimitations. JEpidemiolCommunityHealth. (2006)60:90–2. doi:10.1136/jech.2005.035253
Hongetal. 10.3389/fcvm.2022.1023651
Frontiersin CardiovascularMedicine 11 frontiersin.org 149
OPENACCESS
EDITEDBY
Chu-HuangChen, TheTexasHeartInstitute,UnitedStates
REVIEWEDBY RizandaMachmud, AndalasUniversity,Indonesia Chueh-LungHwang, TheUniversityofTexasatArlington, UnitedStates
*CORRESPONDENCE
HanWang wanghan@swjtu.edu.cn
ZhenZhang zhangzhen@swjtu.edu.cn
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal
FrontiersinCardiovascularMedicine
RECEIVED 24May2022
ACCEPTED 30December2022
PUBLISHED 19January2023
CITATION
TianY,WuW,QinL,YuX,CaiL,WangHand ZhangZ(2023)Prognosticvalueofremnant cholesterolinpatientswithcoronaryheart disease:Asystematicreviewandmeta-analysis ofcohortstudies.
Front.Cardiovasc.Med. 9:951523. doi:10.3389/fcvm.2022.951523
COPYRIGHT
©2023Tian,Wu,Qin,Yu,Cai,WangandZhang. Thisisanopen-accessarticledistributedunder thetermsofthe CreativeCommonsAttribution License(CCBY).Theuse,distributionor reproductioninotherforumsispermitted, providedtheoriginalauthor(s)andthe copyrightowner(s)arecreditedandthatthe originalpublicationinthisjournaliscited,in accordancewithacceptedacademicpractice. Nouse,distributionorreproductionis permittedwhichdoesnotcomplywith theseterms.
PUBLISHED 19January2023
DOI 10.3389/fcvm.2022.951523
Prognosticvalueofremnant cholesterolinpatientswith coronaryheartdisease:A systematicreviewand meta-analysisofcohortstudies
YunTian,WenliWu,LiQin,XiuqiongYu,LinCai,HanWang* and ZhenZhang*
DepartmentofCardiology,TheAffiliatedHospitalofSouthwestJiaotongUniversity,TheThirdPeople’s HospitalofChengdu,Chengdu,Sichuan,China
Background: Therelationshipbetweenabnormallipidlevelsandatherosclerotic cardiovasculardiseasesiswellestablished,buttheassociationbetweenremnant cholesterol(RC)andcoronaryheartdisease(CHD)remainsuncertain.Theaimofthis meta-analysisistosystematicallyevaluatetheprognosticvalueofRCconcentration inpatientswithCHD.
Methods: PubMed,EMBASE,Cochrane,andWebofSciencedatabaseswere reviewedtoidentifyrelevantobservationalcohortstudiespublishedinEnglish uptoDecember2021.Random-effectsmeta-analysiscomparedthehighestand lowestRCconcentration.Theprimaryoutcomewasacompositeofmajoradverse cardiovascularevents(MACEs)andall-causemortalityinpatientswithCHD.
Results: Atotalof10studiesrecruiting30,605patientswithCHDwereselectedto beincludedinthismeta-analysis.PatientswithCHDwithelevatedRCconcentration hadanincreasedriskofthecompositeendpointevents(RR=1.54,95%CI:1.26–1.87)andMACEs(RR=1.70,95%CI:1.54–1.88),buttheriskofall-causemortality wasnotstatisticallysignificant(RR=1.16,95%CI:0.79–1.69, P =0.44).Subgroup analysisshowedconsistentresults.
Conclusion: OurresultssuggestthatelevatedconcentrationRCmayindependently predictMACEsinpatientswithCHD.DeterminationofRCconcentrationmay improveriskstratificationofprognosisinpatientswithCHD.However,morehighqualitystudiesarenecessarytoconfirmthisassociation.
KEYWORDS
remnantcholesterol,coronaryheartdisease,prognosis,dyslipidemia,meta-analysis
Introduction
Coronaryheartdisease(CHD)isthemostcommontypeoforgandiseasecausedby atherosclerosis,whichisseriouslythreateningpeople’slifeandhealth(1, 2).Theprevalence ofCHDintheworldisapproximately4.6–9.2%,andin2019,thediseasecaused9.14million deathsworldwide(1).DyslipidemiaisoneoftheimportantriskfactorsforCHD.Currently,
TYPE SystematicReview
Frontiersin CardiovascularMedicine 01 frontiersin.org 150
loweringlow-densitylipoproteincholesterol(LDL-C)isoneof themaininterventiontargetsinthetreatmentofCHD.However, previousstudiesfoundthatevenafterreducingLDL-Ctoan appropriatelevelandcontrollingotherriskfactors,theremaybe significantdifferencesintheprognosisofpatientswithCHD(3). Therefore,itisimportanttofindmorereliableprognosticindicators toevaluatethelong-termprognosisofCHDandformulatethe besttreatmentplan.
Inrecentyears,remnantcholesterol(RC)hasbeenreported tohaveacriticalroleinatherosclerosisandCHD,whichmight indicateitmayalsobeacriticalcomponentoftheresidualriskof patientswithCHD(3, 4).RCisthecholesterolcontentofallnonlow-densitylipoprotein(LDL)andnon-high-densitylipoprotein (HDL).ComparedwithLDL-C,RChadastrongeratherogenicability becauseitpossessesalargerquantityandvolume,carriesmore cholesterol,anddoesnotneedoxidativemodification(5, 6).Some observationalcohortstudieshavelinkedhighRCconcentrations withanincreasedriskofCHD(7, 8).Furthermore,RCwasfound tobecausallyassociatedwithCHDdevelopmentinpreviously healthyindividuals(9).However,theprognosticvalueofplasma RClevelsinsecondarypreventionsettingsisstillundefinedbecause previousstudiesshowedinconsistentandcontroversialresults(10–12).Meanwhile,itisyettobeestablishedwhethertheprognostic valueofRCvariesamongpopulations,ages,ortheclassificationof CHDs.Inparticular,thereisstillalackofastandardizedmethod forRCmeasurement,withstrikinglydifferentRCconcentrations acrossstudies(10, 12, 13),whichmayalsohavecontributedtothe discrepancyintheoutcomes(14–16).
Therefore,ourstudyaimstosystematicallyreviewandcompile meta-analysesoftheevidenceontherelationshipbetweenRC concentrationandCHDoutcome,byidentifyingthepotential confoundersandinvestigatingtheprognosticvalue,whichmay provideanovelperspectiveforriskassessmentandtreatmentin patientswithCHD.
Materialsandmethods
Ourmeta-analysiswasperformedaccordingtothe recommendationofthemeta-analysisofObservationalStudies inEpidemiology(MOOSE)(17)andthePreferredReportingItems forSystematicReviewsandMeta-Analyses(PRISMA)statement(18).
Searchstrategy
Wecomprehensivelysearchedfourmedicaldatabases,including PubMed,EMBASE,Cochrane,andWebofScience,toidentifycohort studiesassessingtherelationshipbetweenRCconcentrationand cardiovascularoutcomespublishedinEnglishfrominceptionupto 25December2021.ThequerysyntaxwassetusingMedicalSubject Headings(MeSH)andthesaurussearchterms,including(“remnant cholesterol”OR“remnant-likeparticlecholesterol”OR“triglyceriderichlipoproteincholesterol”)AND(“coronaryheartdisease”OR “coronarydisease”OR“coronaryarterydisease”).Thedetailedsearch strategywaspresentedinthe Supplementarymaterial.References retrievedfromthestudies,aswellasrelevantreports,werealso hand-searchedtoreducethelikelihoodofmissinganypublications.
Inclusionandexclusioncriteria
Theinclusioncriteriaforthisstudywerepresentedasfollows: (1)cohortstudies;(2)aninceptioncohortinvolvingadultswith CHD;CHDincludedstableorunstableangina,andmyocardial infarction(MI);(3)theexposurefactorwasRCconcentration;(4) theendpointwasmajoradversecardiovascularevents(MACEs)and all-causemortality.MACEsincludedcardiacdeath,MI,ischemic stroke,myocardialischemia,heartfailure,unstableanginarequiring readmission,andcoronaryrevascularization;and(5)thehighest andlowestRCconcentrationgroupsofmultivariate-adjustedrelative risks(RRs),oddsratios(ORs),orhazardratios(HRs)andtheir 95%confidenceintervals(95%CI)ortheaboveindicatorscouldbe calculatedwiththecompletedata(19).
Exclusioncriteriaincluded(1)casereports,commentary, andconferenceabstracts;(2)animal,cross-sectionalstudies,or randomizedclinicaltrials;(3)studiescarriedoutamongpregnant womenorchildren;and(4)examinednon-relevantoutcomes.
YTdidthescreeningofthetitlesandabstractsoftheidentified articles,andpertinentarticleswereindependentlyreviewedinfull textbytwoinvestigators(YTandWW).Thus,disagreementwas resolvedthroughconsensus.
Datacollectionandqualityassessment
Dataextractionwasinastandardizedstyle.Twoinvestigators (YTandLQ)independentlyextractedthefollowingdata:thefirst author,publicationyear,population,studydesign,typeofCHD, samplesize,percentageofwomen,age,exposureassessmentmethod, fastingstatus,follow-upduration,outcomeassessment,categoricalor continuous,adjustedriskestimates,andadjustmentforvariables.
Thequalityofobservationalcohortstudieswasassessedusingthe Newcastle–OttawaScale(NOS)(20),whichwasrankedaspoor(score 1–3),fair(score4–6),orgood(score7–9)accordingtothequalityof studyparticipantselection,comparability,andoutcome.Studieswith NOS ≥7pointswereconsideredhighquality.Anydisagreements werediscussedandresolvedbyachiefinvestigator(HW),anda consensuswasreachedinallcases.
Statisticalanalysis
ReviewManager5.4software(TheCochraneCollaboration, Oxford,UK)andStata16.0(StataCorporation,CollegeStation,TX, USA)wereemployedforstatisticalanalysis.The I2 statisticsandchisquareCochran’s Q-testwereusedtoassesstheheterogeneityacross studies.If P ≥ 0.05and I2 < 50%,suggestingthatnosignificant heterogeneitycouldbefound,afixed-effectmodelwasalsoapplied. Inaddition,if P < 0.05and I2 ≥ 50%,arandom-effectmodelwas cautiouslyapplied,andthensubgroupanalysiswasusedtoexplore thesourceofheterogeneity(21).Theeliminationofindividualstudies onebyonewasalsoperformedforsensitivityanalysisinorderto exploretheheterogeneityandassessthestabilityofthemeta-analysis. AfunnelplotcombinedwithEgger’stestwasemployedtoinvestigate thepotentialpublicationbiasoftheinvolvedstudies.Finally,theRR with95%CIwasemployedfortheeffectestimationmetric,andHR andORwereconvertedintoRR(22–24).The p-valueof < 0.05meant thedifferencewasstatisticallysignificant.
Tianetal. 10.3389/fcvm.2022.951523
Frontiersin CardiovascularMedicine 02 frontiersin.org 151
Results
Literaturesearchresults
Atotalof2,308articleswereretrieved,andafter381duplicates wereremoved,1,927uniquerecordsremained.Afterscreeningthe titlesandabstractsofthearticles,38recordswereconsideredfor adetailedfull-textscreening.Ofthesestudies,28articleswere excluded:studieswithanon-CHDpopulation(n =11)(16, 25–34), thosewithnointerestoutcome(n =5)(35–39),thosewithpotential patients’duplicationwithotherarticles(n =5)(8, 9, 40–42),and studieswithineligiblestudydesign(n =7)(43–49).Finally,10articles (4, 10–13, 50–54)covering12cohortsenrolling30,605subjectsmet theselectioncriteria. Figure1 depictstheliteraturescreeningprocess andresultsindetail.
Studycharacteristicsandquality assessment
Themaincharacteristicsoftheincludedstudiesaresummarized in Table1.Ofthe10includedstudies,threewereperformedinChina
(11, 50, 54),threeinJapan(4, 13, 51),twoinDenmark(52, 53), oneintheUnitedStates(12),andonecollaborativestudyinvolving multiplecountries(10).Thestudieswerepublishedfrom1999(13) to2021(11),ofwhichninewereprospectivecohortdesigns(4, 11–13, 50–54)andonewasretrospectivecohortdesign(10).Inaddition, twostudiesincludedtwocohorts(50, 52).Thesamplesizeranged from120(51)to6723(11).Thefollow-uptimerangedfrom1.7 (51)to7.0(52)years,andparticipants’agevariedfrom57.7(11)to 68.0(53)years.TheaverageNOSscoresforthesestudiesincluded were7.3,demonstratingthatthequalityofthecohortstudywas good.

Meta-analysisresults
TheresultsdemonstratedthatelevatedRCconcentrationwas relatedtoanincreasedriskofcompositeendpointevents(MACEs andall-causedeath)(RR=1.54,95%CI:1.26–1.87, P < 0.0001)ina random-effectmodel(Figure2).Significantheterogeneitybetween studieswasobserved(I2 =85%, P < 0.0001),andsensitivity analysisindicatedthatthetotalcombinedeffectsizedidnotchange significantlyineachstep,demonstratingthatthemeta-analysis resultswererelativelystable.However,ifthestudyconductedby
Tianetal. 10.3389/fcvm.2022.951523
FIGURE1
Frontiersin CardiovascularMedicine 03 frontiersin.org 152
Flowdiagramofthestudysearchandselectionprocess.FromMoheretal.(65).
TABLE1Maincharacteristicsofthe includedstudies.
NP,notprovided;HR,hazardratio;OR,oddsratio;RR,riskratio;CI,confidenceintervals;CAD,coronaryarterydisease;DM,diabetesmellitus;IHD,ischemicheartdisease;MI,myocardialinfarction;IS,ischemicstroke;AMI,acutemyocardialinfarction;NSTE-ACS, non-STsegmentelevationacutecoronarysyndrome;MACEs,majoradversecardiovascularevents;T,tertile;Q4,quartile;Q5,quintile;NOS,Newcastle–OttawaScale.
1 Adjustments:age(1),sex(2),bodymassindex(3),smoking(4),diabetes(5),statinuse(6),familyhistoryofCAD(7),TC(8),LDL-C(9),HDL-C(10),triglyceride(11),hsCRP(12),HbA1c(13),ApoB(14),lipoprotein(a)(15),hypertension(16),three-vesseldisease(17), leftventricularejectionfraction(18),stenosisofleftmaincoronaryartery(19),numberofdiseasedcoronaryarteries(20),creatinine(21),lipid-loweringtherapy(22),theGRACE1.0score(23),site(24),race(25),insurance(26),education(27),alcoholuse(28),physical activity(29),kidneydisease(30),heartfailure(31),priorMI(32),ezetimibe(33),niacin(34),fibrate(35),andfishoil(36).
Tianetal. 10.3389/fcvm.2022.951523
First author, year Population Typeof study Participation Sample size (number) % females Age (year) RC concentration (mg/dl) Exposure assessment Fasting status Followup duration (year) Outcome assessment OR,RR, orHR (95%CI) Categorical or continuous Variables adjusted1 NOS score Caoetal. (50) Chinese prospective cohortstudy CAD 4355 28.9 58.2 ± 9.7 5.0(2.7–9.7) Automatedassay Fasting 5.1 MACEs HR:1.53 (1.16–2.02) Q5vs.Q1 1,2,3,4,5,6, 7,8, 9,10,11, 12 9 Caoetal. (50) Chinese prospective cohortstudy CAD 4355 28.9 58.2 ± 9.7 9.0(6.5–12.4) Immunoseparation Fasting 5.1 MACEs HR:1.49 (1.12–2.09) Q5vs.Q1 1,2,3,4,5,6, 7,8, 9,10,11, 12 9 Elshazly etal.(10) NorthandSouth American,etc. retrospective cohortstudy CAD 5754 28.0 58.1 ± 9.2 23.8(19.1–30.8) Calculation Fasting 2.0 MACEs HR1.62 (1.27–2.07) Q4vs.Q1 – 6 Fujihara etal.(4) Japanese prospective cohortstudy CAD 247 9.0 67(60–74) 3.6(2.5–5.5) Immunoseparation Fasting 3.2 MACEs HR1.62 (1.26–2.07) ≥ 3.9mg/dl vs. < 3.9mg/dl 4,11,13,14,15 8 Fukushima etal.(51) Japanese prospective cohortstudy CAD+DM 120 37.5 65.6 ± 8.4 5.8(3.1–6.2) Immunoseparation Fasting 1.7 MACEs OR2.2 (1.2–6.4) > 4.7mg/dl vs. ≤ 4.7mg/dl 1,4,7,8,9,10, 11,13, 16,17, 18 6 Jepsenetal. (52) Danish prospective cohortstudy IHD 5414 30.3 64.4 14.4(9.0–21.6) Calculation non-fasting 7.0 mortality HR:1.5 (1.2–2.0) Q4vs.Q1 1,2,4,6,16 8 Jepsenetal. (52) Danish prospective cohortstudy IHD 5414 30.3 64.4 1.4(0.7–3.4) Automatedassay non-fasting 7.0 mortality HR:1.2 (1.0–1.5) Q4vs.Q1 1,2,4,6,16 8 Kugiyama etal.(13) Japanese prospective cohortstudy CAD 135 34.0 65.0 ± 9.7 3.4 Immunoseparation Fasting 2.2 MACEs OR6.38 (2.3–17.6) highestvs.lowest tertile 1,2,4,5,8,10, 11,16, 19,20 7 Langsted etal.(53) Danish prospective cohortstudy MI/IS 2973 32.0 68(61–74) NP Calculation non-fasting NR MACEs HR1.71 (1.24–2.36) Q4vs.Q1 4,9,15,16,22 7 Liuetal. (11) Chinese prospective cohortstudy CAD 6723 26.2 57.7 ± 10.8 9.2 ± 5.0 NP NP 4.9 MACEs HR1.79 (1.18–2.71) Q4vs.Q1 1,2,3,4,5,6, 7,9, 10,11,12, 16,18,21,33 8 Martin etal.(12) American prospective cohortstudy AMI 2465 32.0 58 ± 12 20(14–27) VLDL3-C+IDLC NP 2.0 mortality HR0.76 (0.64–0.91) T3vs.T1 1,2,3,4,5,6, 9,10, 16,20, 23,24,25,26, 27,28,29,30, 31,32,33,34, 35,36 7 Zhaoetal. (54) Chinese prospective cohortstudy NSTE-ACS 2419 28.2 60.08 ± 8.97 12.4 ± 7.6 Calculation Fasting 3.0 MACEsand mortality MACEs:HR 1.960 (1.558–2.465); mortality:HR 2.207 (0.612–7.959); highestvs.lowest – 7
Frontiersin CardiovascularMedicine frontiersin.org
Martinetal.(12)waseliminated,theheterogeneitydecreased significantly(I2 =44%,RR=1.61,95%CI:1.43–1.80).
Inaddition,asshownin Figure3,eightof10studiesreportedthe MACEsasanoutcome,andtheothertwostudiesaddressedtheallcausemortalityoutcome,inwhichonestudyreportedbothMACEs riskandall-causemortalityrisk.Furthermore,patientswithCHD withelevatedRCconcentrationhadanincreasedriskofMACEs (RR=1.70,95%CI:1.54–1.88, P < 0.0001)withoutsignificant heterogeneity(I2 =0%, P =0.56)inafixed-effectmodel.However,the riskofall-causemortalitywasnotstatisticallysignificant(RR=1.16, 95%CI:0.79–1.69, P =0.44),withsignificantheterogeneity(I2 =89%, P < 0.0001)inarandomeffectmodel.Inaddition,sensitivityanalysis showedthatafterremovingthestudyofMartinetal.(12),the
heterogeneitydecreasedsignificantly,andthetotalcombinedeffect sizechangedaswell(I2 =32%,RR=1.34,95%CI:1.10–1.63, P =0.003).
Subgroupanalysis


Inthesubgroupanalysis,theassociationremainedconstant, suggestingapositiveassociationbetweenRCconcentrationandCHD risksinstudiesconductedintheAsianregion(RR=1.72,95%CI: 1.53–1.95, P < 0.0001)forthosewiththediagnosisofMI(RR=1.64, 95%CI:1.46–1.85, P < 0.0001)andwitholderage(≥65yearsold) (RR=1.78,95%CI:1.46–2.15, P < 0.0001).Inaddition,thisconstant


Tianetal. 10.3389/fcvm.2022.951523
FIGURE2
ForestplotsshowingthepooledRRwith95%CIofcompositeendpointeventsforthehighestversuslowestremnantcholesterolconcentration.
FIGURE3
Frontiersin CardiovascularMedicine 05 frontiersin.org 154
ForestplotsshowingthepooledRRwith95%CIofmajoradversecardiovascularevents(MACEs) (A) andall-causemortality (B) forthehighestversus lowestremnantcholesterolconcentration.
associationcontinuedinselectedstudiesforthosepublicationswith samplesize <1,000cases(RR=1.88,95%CI:1.38–2.55, P < 0.0001), thosethatusedtheimmunoseparationmethod(RR=1.72,95%CI: 1.39–2.12, P < 0.0001)andfastingstatustest(RR=1.70,95%CI: 1.51–1.91, P < 0.0001)forRCassessment,andstudieswithalong follow-uptime(≥3years)(RR=1.54,95%CI:1.35–1.76, P < 0.0001) (Table2).
Publicationbiastest
Asshownin Figure4,thefunnelplotwasasymmetrical,andfor furtherquantitativeanalysisusingEgger’stest,apublicationbiaswas suggested(P < 0.05).
Discussion
Inthepresentstudy,therelationshipbetweenRCconcentration andtheprognosisofthepatientswithCHDwasevaluatedby meta-analysisforthefirsttime.Theresultsillustratedthatelevated RCconcentrationwassignificantlycorrelatedwithanincreased riskofthecompositeendpointeventsandMACEsinpatients withCHD,buttheriskofall-causemortalitywasnotstatistically significant.Inaddition,theprognosticsignificanceofhigherRC concentrationonCHDriskswasalsoconfirmedinthesubgroup
analysis.Thismeta-analysiscontributestotheincreasingevidence thathigherRCconcentrationmaybeanindependentpredictorof poorcardiovascularoutcomesinpatientswithCHD.
Remnantcholesterol,alsoknownastriglyceride-richlipoprotein cholesterol,isthecholesterolcontentofallnon-LDLandnon-HDL. Inthefastingstate,RCiscomposedofliver-derivedverylowdensitylipoprotein(VLDL)andintermediate-densitylipoprotein (IDL)inthefastingstate,aswellasintestinal-derivedchylomicron remnants(CM)(27).Recently,anincreasingnumberofstudies havedemonstratedthatRCconcentrationhadarelationshiptothe occurrenceanddevelopmentofatherosclerosis(27, 53).Particularly, whenLDL-Cwascontrolledatanappropriatelevel,RCwasassumed tobethemainreasonformediatingresidualrisksinthepatientswith CHDandevenabetterpredictorofriskthanLDL-C(52).Unlike LDL-C,RCcouldeasilypenetratethevesselwallandisdirectly takenupbythescavengingreceptorsonmacrophageswithout oxidativemodification,leadingtoformingfoamcellsandpromoting atheroscleroticplaqueformation(55, 56).Inaddition,itcouldalso increasetheproductionofreactiveoxygenspeciesfreeradicals,cause endothelialcelldysfunction(57),andinducetheexpressionofproinflammatorymediators,aswellastheproductionofcytokines, interleukin,andatheroscleroticadhesionmolecules(58).Allofthe earliermechanismscanleadtoplaqueformationandprogressive ruptureandpromotetheoccurrenceofMACEs,whichinturn influencestheprognosisofthepatients.
Tianetal. 10.3389/fcvm.2022.951523
Subgroup No.ofstudies Pooledriskration 95%confidence interval P-value Heterogeneity betweenstudies Region Asian 6 1.72 1.53–1.95 <0 0001 I2 =8.9%, P =0.361 No-Asian 4 1.55 1.25–1.93 <0 0001 I2 =63%, P =0.030 Participation CAD 6 1.64 1.46–1.85 <0 0001 I2 =0%, P =0.55 MI 3 1.36 0.70–2.64 0 37 I2 =96%, P < 0.001 Samplesize ≥ 1000 7 1.44 1.15–1.81 0 0020 I2 =87%, P < 0.001 < 1000 3 1.88 1.38–2.55 <0 0001 I2 =44%, P =0.17 Age ≥ 65yearsold 4 1.78 1.46–2.15 0 005 I2 =88%, P < 0.001 < 65yearsold 6 1.42 1.11–1.81 0 04 I2 =91%, P < 0.001 RCassessment Calculation 4 1.69 1.49–1.91 <0 0001 I2 =0%, P =0.42 Immunosepa-ration 4 1.72 1.39–2.12 <0 0001 I2 =33%, P =0.21 Automatedassay 2 1.32 1.05–1.67 0 0200 I2 =52%, P =0.15 Fastingstatus Fasting 6 1.70 1.51–1.91 <0 0001 I2 =11%, P =0.35 Non-fasting 2 1.41 1.15–1.73 0 0009 I2 =55%, P =0.11 Follow-upduration ≥ 3year 5 1.54 1.35–1.76 <0 0001 I2 =50%, P =0.06 < 3year 4 1.54 0.87–2.74 0 14 I2 =93%, P < 0.001 Frontiersin CardiovascularMedicine 06 frontiersin.org 155
TABLE2Subgroupanalysisoncompositeendpointevents.
Thepreviousclinicalstudiesdemonstratedsimilarconclusions betweenRCconcentrationandprognosisinthegeneralpopulation butnotinpatientswithCHD.ThelateststudybyWadstrometal.(8) revealedthatintheCopenhagenGeneralPopulationStudy,during the15-yearfollow-upof106,937people,elevatedRCconcentration wasrelevanttoanincreasedriskofMIuptomultivariable-adjusted HRof4.2,aswellascorrespondingHRswere1.8forischemic stroke,and4.8forperipheralarterydisease(PAD).Inaddition,inthe CopenhagenCityHeartStudy,correspondingHRswere2.6forMI, 2.1forischemicstroke,and4.9forPAD(8).Castañeretal.(27)also reportedthatinthePREDIMEDcohortstudyofhighcardiovascularriskgroups,every10mg/dlincreaseinRCconcentrationwould increasetheriskofcardiovasculareventsby21%.Aftermultivariateadjustedanalysis,itwasconcludedthatthelevelsoftriglycerideand RCratherthanLDL-CwererelatedtotheoccurrenceofMACEsin thepopulationwhowereoverweightorobeseandhadahighrisk ofcardiovasculardiseases,whichwereindependentoflifestyleand otherriskfactors.Afewotherstudieshavealsoreachedasimilar conclusion(32, 33, 59).However,inourstudy,RCconcentration hadnoeffectontheriskofall-causemortality,possiblyduetothe availablesmallsamplesizeandhighheterogeneity.Furthermore,it seemstheresultswereconsistentacrossthepopulationsandagesin oursubgroupanalysis.Wangetal.(31)alsoindicatedtheimportance ofpreventiveeffortsacrosstheadultlifecourse.Obviously,these resultsneedfurtherconfirmationinmorestratifiedcases.
Inaddition,geneticevidencehasalsobeenfoundthatRCwas theriskfactorforatherosclerosis.Varboetal.(9)performedthe Mendelianrandomizationmethodbydetectingthegenesof73,513 peoplefromtheCopenhagenstudyandselected15genotypesto observetheincidenceofischemicheartdisease(IHD)foreachtype ofgene.Theresultsindicatedthatforevery1mmol/lincreaseofnonfastingRCconcentration,theriskofIHDincreasedby2.8times.

InanotherMendelianrandomizedtrial,Varboetal.(60)found thatelevatedRCconcentrationsinnon-fastingstatuswerecausally relatedtoinflammationandIHD,whereasincreasedLDL-Cwasonly relatedcausallytoIHDwithoutinflammation.Jørgensenetal.(61)
alsoindicatedthatgeneticvariationinApoA5relatedtostepwise increasesoftheRCconcentrationandwithcomparableincreasesin theriskofMI.Thus,theseresultsillustratedthatexposuretoelevated RCconcentrationscausedbygeneticabnormalitiescouldbringa greaterriskofcardiovasculardiseases.
Inourstudy,sensitivityanalysisfoundthatthesourceof heterogeneitymightbetheresearchconductedbyMartinetal.(12). Inthisstudy,theRCevaluationmethodwassignificantlydifferent fromothers,inwhichthesumofVLDL3-CandIDL-Cwasused tocalculateRCandfastingstatewasunknown.Atpresent,no uniformmethodtomeasureRCconcentrationhasbeenprovided, andaccuratemeasurementisstillchallenging,whichmightbethe mainreasonforconflictinthefindings(14–16).Thiswasmainly becauseRCwascomposedofdifferentlipidsandlipoproteins.Then, itsrapidandcontinuouscatabolism,thesize,quantity,density, andcompositionoflipoproteinresidueswerehighlydynamic, whichwasdifficulttodistinguishfromitsprecursors(non-remnant lipoproteins)(47).Currently,thesimplestwaytoestimateRC concentrationisthroughcalculationmethod(62);thatis,RCwas calculatedastotalcholesterol(TC)minusLDL-CminusHDL-C[i.e., RC=(TC)–(LDL-C)–(HDL-C)].Althoughitwasnotasaccurate asthemethodtodirectthedetectionofRC,ithasbeenwidely appliedatpresentduetoitsconvenienceandsimplicity(10, 52–54). Apartfromthecalculation,therewerealsoseveraldirectmethods toidentifyandquantifyRCdependingontheirspecificingredients, suchasimmunoseparation(4, 13, 50, 51),directhomogenous assays(50, 52),preparativeultrafiltration(63),andnuclearmagnetic resonance(64).Inourstudy,subgroupanalysisindicatedthat elevatedRCconcentrationmeasuredbytheimmunoseparation methodhadhighercardiovascularrisk.Furthermore,whetherina fastingstateduringthedetectionalsohadanimpactontheRC measurement.Thesubgroupanalysisofourstudyindicatedthat theCHDpatientswithelevatedRCconcentrationsinthefasting statehadahigherriskofpoorprognosis.Apparently,nooptimal wayofaccuratelyquantifyingRCmeasurementcurrentlyexists,so therewasalackofuniformRCcut-offlevelstodefinehighRC
Tianetal. 10.3389/fcvm.2022.951523
FIGURE4
Funnelplotsfortheanalysisofremnantcholesterolconcentrationandcompositeendpointevents.Resultscompareparticipantsinthehighestversus lowestremnantcholesterolconcentration.
Frontiersin CardiovascularMedicine 07 frontiersin.org 156
concentration.However,asincreasingimportancehasbeenattached toRC,aconsensusdefinitionofRCwithaccurateandreproducible quantitativemeasurementapproachesiseagerlyrequired.
Limitations
Thisstudyalsohadpotentiallimitations.First,thestudies includedinthisstudywereallpublishedinEnglish,whichmight havealanguagebias.Second,thedifferencesinthetypesof CHDandRCmeasurementapproachesineachstudymayleadto clinicalheterogeneity.Third,theincludedstudiesadjustedsome confounders,butotherunadjustedriskfactorsmayexist.Some traditionalCHDfactorscannotbeextractedadequatelyfromthe includedstudies,whichmightalsoleadtobias.Thus,furtherstudies ofstratifiedanalysisfortheriskfactorsofCHDoutcomeare necessary.Fourth,therewereinsufficientrelevantdatatocompare theprognosticeffectbetweenLDL-CandRCfromtheincluded studies.Then,itisworthansweringthisvaluablequestioninfuture studies.Finally,therewasasignificantpublicationbiasinour study,suggestingthepossiblepresenceofnegativeresultsthatwere notpublished.Therefore,futurestudiesareneededbeforeafirm conclusioncanbedrawnconcerningtheassociationbetweenRC concentrationandCHDoutcome.
Conclusion
Thismeta-analysisof10cohortstudiesshowedthatCHD patientswithelevatedRCconcentrationshadahigherriskofadverse cardiovascularoutcomes.MeasurementofRCconcentrationhas thepotentialtoimproveriskclassificationinpatientswithCHD. However,futurelargersamplesizesandhigherqualitystudiesarestill requiredtoconfirmthefindings.
Dataavailabilitystatement
Theoriginalcontributionspresentedinthisstudyareincluded inthisarticle/Supplementarymaterial,furtherinquiriescanbe directedtothecorrespondingauthors.
References
1.RothG,MensahG,JohnsonC,AddoloratoG,AmmiratiE,BaddourL,etal.Global burdenofcardiovasculardiseasesandriskfactors,1990-2019:updatefromtheGBD2019 Study. JAmCollCardiol. (2020)76:2982–3021.doi:10.1016/j.jacc.2020.11.010
2.FusterV,KellyB,VedanthanR.Promotingglobalcardiovascularhealth:moving forward. Circulation. (2011)123:1671–8.doi:10.1161/CIRCULATIONAHA.110.0 09522
3.SalinasC,ChapmanM.Remnantlipoproteins:aretheyequaltoormoreatherogenic thanLDL? CurrOpinLipidol. (2020)31:132–9.doi:10.1097/mol.0000000000000682
4.FujiharaY,NakamuraT,HorikoshiT,ObataJ,FujiokaD,WatanabeY,etal.Remnant lipoproteinsareresidualriskfactorforfuturecardiovasculareventsinpatientswithstable coronaryarterydiseaseandon-statinlow-densitylipoproteincholesterollevels < 70 mg/dL. CircJ. (2019)83:1302–8.doi:10.1253/circj.CJ-19-0047
5.MillerY,ChoiS,FangL,TsimikasS.Lipoproteinmodificationandmacrophage uptake:roleofpathologiccholesteroltransportinatherogenesis. SubcellBiochem. (2010) 51:229–51.doi:10.1007/978-90-481-8622-8_8
Authorcontributions
YT,WW,andLQperformedtheliteraturesearch,dataextraction, andqualityassessment.YTperformeddataanalysisanddraftedthe manuscript.XYandLCcriticallyrevisedthestudy.HWandZZ designedthestudy,interpretedthedata,andrevisedthemanuscript. Allauthorscontributedintellectuallytothismanuscriptandhave approvedthisfinalversion.
Funding
ThisstudywassupportedbytheChengduHigh-level KeyClinicalSpecialtyConstructionProject,SichuanProvince, China,No.202112001.
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcouldbe construedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthereviewers. Anyproductthatmaybeevaluatedinthisarticle,orclaimthatmay bemadebyitsmanufacturer,isnotguaranteedorendorsedbythe publisher.
Supplementarymaterial
TheSupplementaryMaterialforthisarticlecanbefoundonline at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.951523/ full#supplementary-material
6.RosensonR,DavidsonM,HirshB,KathiresanS,GaudetD.Geneticsandcausalityof triglyceride-richlipoproteinsinatheroscleroticcardiovasculardisease. JAmCollCardiol. (2014)64:2525–40.doi:10.1016/j.jacc.2014.09.042
7.HolmesM,MillwoodI,KartsonakiC,HillM,BennettD,BoxallR,etal.Lipids, lipoproteins,andmetabolitesandriskofmyocardialinfarctionandstroke. JAmColl Cardiol. (2018)71:620–32.doi:10.1016/j.jacc.2017.12.006
8.WadströmB,WulffA,PedersenK,JensenG,NordestgaardB.Elevatedremnant cholesterolincreasestheriskofperipheralarterydisease,myocardialinfarction,and ischaemicstroke:acohort-basedstudy. EurHeartJ. (2021)43:3258–69.doi:10.1093/ eurheartj/ehab705
9.VarboA,BennM,Tybjærg-HansenA,JørgensenA,Frikke-SchmidtR,Nordestgaard B.Remnantcholesterolasacausalriskfactorforischemicheartdisease. JAmCollCardiol. (2013)61:427–36.doi:10.1016/j.jacc.2012.08.1026
10.ElshazlyM,ManiP,NissenS,BrennanD,ClarkD,MartinS,etal.Remnant cholesterol,coronaryatheromaprogressionandclinicaleventsinstatin-treatedpatients
Tianetal. 10.3389/fcvm.2022.951523
Frontiersin CardiovascularMedicine 08 frontiersin.org 157
withcoronaryarterydisease. EurJPrevCardiol. (2020)27:1091–100.doi:10.1177/ 2047487319887578
11.LiuH,LiS,CaoY,GuoY,ZhuC,WuN,etal.Associationoftriglyceride-rich lipoprotein-cholesterolwithrecurrentcardiovasculareventsinstatin-treatedpatients accordingtodifferentinflammatorystatus. Atherosclerosis. (2021)330:29–35.doi:10. 1016/j.atherosclerosis.2021.06.907
12.MartinS,FaridiK,JoshiP,BlahaM,KulkarniK,KhokharA,etal.Remnant lipoproteincholesterolandmortalityafteracutemyocardialinfarction:furtherevidence forahypercholesterolemiaparadoxfromtheTRIUMPHRegistry. ClinEardiol. (2015) 38:660–7.doi:10.1002/clc.22470
13.KugiyamaK,DoiH,TakazoeK,KawanoH,SoejimaH,MizunoY,etal.Remnant lipoproteinlevelsinfastingserumpredictcoronaryeventsinpatientswithcoronaryartery disease. Circulation. (1999)99:2858–60.doi:10.1161/01.cir.99.22.2858
14.WilsonP,JacobsonT,MartinS,JacksonE,LeN,DavidsonM,etal.Lipid measurementsinthemanagementofcardiovasculardiseases:practicalrecommendations ascientificstatementfromthenationallipidassociationwritinggroup. JClinLipidol. (2021)15:629–48.doi:10.1016/j.jacl.2021.09.046
15.TothP,BaysH,BrownW,CatapanoA,DavidsonM,FarnierM,etal. Comparingremnantlipoproteincholesterolmeasurementmethodstoevaluateefficacy ofezetimibe/statinvsstatintherapy. JClinLipidol. (2019)13:997–1007.e8.doi:10.1016/j. jacl.2019.09.001
16.JoshiP,KhokharA,MassaroJ,LiretteS,GriswoldM,MartinS,etal.Remnant lipoproteincholesterolandincidentcoronaryheartdisease:thejacksonheartand framinghamoffspringcohortstudies. JAmHeartAssoc. (2016)5:e002765.doi:10.1161/ jaha.115.002765
17.StroupD,BerlinJ,MortonS,OlkinI,WilliamsonG,RennieD,etal.Metaanalysisofobservationalstudiesinepidemiology:aproposalforreporting.meta-analysis ofobservationalstudiesinepidemiology(MOOSE)group. JAMA. (2000)283:2008–12. doi:10.1001/jama.283.15.2008
18.MoherD,LiberatiA,TetzlaffJ,AltmanD,PRISMAGroup.Preferredreporting itemsforsystematicreviewsandmeta-analyses:thePRISMAstatement. PLoSMed. (2009) 6:e1000097.
19.TierneyJ,StewartL,GhersiD,BurdettS,SydesM.Practicalmethodsfor incorporatingsummarytime-to-eventdataintometa-analysis. Trials. (2007)8:16.doi: 10.1186/1745-6215-8-16
20.StangA.CriticalevaluationoftheNewcastle-Ottawascalefortheassessmentof thequalityofnonrandomizedstudiesinmeta-analyses. EurJEpidemiol. (2010)25:603–5. doi:10.1007/s10654-010-9491-z
21.HigginsJ,ThompsonS,DeeksJ,AltmanD.Measuringinconsistencyinmetaanalyses. BMJ. (2003)327:557–60.doi:10.1136/bmj.327.7414.557
22.ZhangJ,YuK.What’stherelativerisk?Amethodofcorrectingtheoddsratioin cohortstudiesofcommonoutcomes. JAMA. (1998)280:1690–1.doi:10.1001/jama.280.
19.1690
23.McNuttL,WuC,XueX,HafnerJ.Estimatingtherelativeriskincohortstudiesand clinicaltrialsofcommonoutcomes. AmJEpidemiol. (2003)157:940–3.doi:10.1093/aje/ kwg074
24.RonksleyP,BrienS,TurnerB,MukamalK,GhaliW.Associationofalcohol consumptionwithselectedcardiovasculardiseaseoutcomes:asystematicreviewand meta-analysis. BMJ. (2011)342:d671.doi:10.1136/bmj.d671
25.QuispeR,MartinS,MichosE,LambaI,BlumenthalR,SaeedA,etal.Remnant cholesterolpredictscardiovasculardiseasebeyondLDLandApoB:aprimaryprevention study. EurHeartJ. (2021)19:4324–32.doi:10.1093/eurheartj/ehab432
26.ChenY,LiG,GuoX,OuyangN,LiZ,YeN,etal.Theeffectsofcalculatedremnantlikeparticlecholesterolonincidentcardiovasculardisease:insightsfromageneralChinese population. JClinMed. (2021)10:3388.doi:10.3390/jcm10153388
27.CastañerO,PintóX,SubiranaI,AmorA,RosE,HernáezÁ,etal.Remnant cholesterol,notLDLcholesterol,isassociatedwithincidentcardiovasculardisease. JAm CollCardiol. (2020)76:2712–24.doi:10.1016/j.jacc.2020.10.008
28.BonfiglioC,LeoneC,SilveiraL,GuerraR,MisciagnaG,CarusoM,etal.Remnant cholesterolasariskfactorforcardiovascular,cancerorothercausesmortality:a competingrisksanalysis. NutrMetabCardiovDis. (2020)30:2093–102.doi:10.1016/j. numecd.2020.07.002
29.SaeedA,FeofanovaE,YuB,SunW,ViraniS,NambiV,etal.Remnant-likeparticle cholesterol,low-densitylipoproteintriglycerides,andincidentcardiovasculardisease. J AmCollCardiol. (2018)72:156–69.doi:10.1016/j.jacc.2018.04.050
30.VarboA,NordestgaardB.Remnantcholesterolandriskofischemicstrokein 112,512individualsfromthegeneralpopulation. AnnNeurol. (2019)85:550–9.doi:10. 1002/ana.25432
31.WangA,TianX,ZuoY,ChenS,MengX,ChenP,etal.Agedependentassociation betweenremnantcholesterolandcardiovasculardisease. AtherosclerPlus. (2021)45:18–24.doi:10.1016/j.athplu.2021.09.004
32.IkezakiH,LimE,CupplesL,LiuC,AsztalosB,SchaeferE.Smalldenselow-density lipoproteincholesterolisthemostatherogeniclipoproteinparameterintheprospective framinghamoffspringstudy. JAmHeartAssoc. (2021)10:e019140.doi:10.1161/jaha.120.
019140
33.HussainA,SunC,SelvinE,NambiV,CoreshJ,JiaX,etal.Triglyceride-rich lipoproteins,apolipoproteinC-III,angiopoietin-likeprotein3,andcardiovascularevents
inolderadults:atherosclerosisriskincommunities(ARIC)study. EurJPrevCardiol. (2021)14:e53–64.doi:10.1093/eurjpc/zwaa152
34.VarboA,NordestgaardB.Directlymeasuredvs.calculatedremnantcholesterol identifiesadditionaloverlookedindividualsinthegeneralpopulationathigherriskof myocardialinfarction. EurHeartJ. (2021)42:4833–43.doi:10.1093/eurheartj/ehab293
35.ImkeC,RodriguezB,GroveJ,McNamaraJ,WaslienC,KatzA,etal.Areremnantlikeparticlesindependentpredictorsofcoronaryheartdiseaseincidence?TheHonolulu HeartStudy. ArteriosclerThrombVascBiol. (2005)25:1718–22.doi:10.1161/01.ATV. 0000173310.85845.7b
36.CaoY,ZhangH,JinJ,LiuH,ZhangY,GaoY,etal.Thelongitudinal associationofremnantcholesterolwithcardiovascularoutcomesinpatientswith diabetesandpre-diabetes. CardiovascDiabetol. (2020)19:104.doi:10.1186/s12933-020-0 1076-7
37.HongL,YanX,LuZ,FanY,YeF,WuQ,etal.Predictivevalueofnon-fasting remnantcholesterolforshort-termoutcomeofdiabeticswithnew-onsetstablecoronary arterydisease. LipidsHealthDis. (2017)16:7.doi:10.1186/s12944-017-0410-0
38.WinterM,WiesbauerF,BlessbergerH,PavoN,SulzgruberP,HuberK,etal.Lipid profileandlong-termoutcomeinprematuremyocardialinfarction. EurJClinInvest. (2018)48:e13008.doi:10.1111/eci.13008
39.SakaiK,KobaS,NakamuraY,YokotaY,TsunodaF,ShojiM,etal.Smalldense low-densitylipoproteincholesterolisapromisingbiomarkerforsecondaryprevention inoldermenwithstablecoronaryarterydisease. GeriatrGerontolInt. (2018)18:965–72. doi:10.1111/ggi.13287
40.VarboA,FreibergJ,NordestgaardB.Extremenonfastingremnantcholesterolvs extremeLDLcholesterolascontributorstocardiovasculardiseaseandall-causemortality in90000individualsfromthegeneralpopulation. ClinChem. (2015)61:533–43.doi: 10.1373/clinchem.2014.234146
41.VarboA,FreibergJ,NordestgaardB.Remnantcholesterolandmyocardialinfarction innormalweight,overweight,andobeseindividualsfromthecopenhagengeneral populationstudy. ClinChem. (2018)64:219–30.doi:10.1373/clinchem.2017.279463
42.JorgensenA,Frikke-SchmidtR,WestA,GrandeP,NordestgaardB,TybjaergHansenA.Geneticallyelevatednon-fastingtriglyceridesandcalculatedremnant cholesterolascausalriskfactorsformyocardialinfarction. EurHeartJ. (2013)34:1826–33.
43.WangK,DingY,GaoW,WangR,YangJ,LiuX,etal.Associationofincreased remnantcholesterolandtheriskofcoronaryarterydisease:aretrospectivestudy. Front CardiovascMed. (2021)8:740596.doi:10.3389/fcvm.2021.740596
44.KimJ,ParkJ,JeongS,SchellingerhoutD,ParkJ,LeeD,etal.Highlevelsofremnant lipoproteincholesterolisariskfactorforlargearteryatheroscleroticstroke. JClinNeurol. (2011)7:203–9.doi:10.3988/jcn.2011.7.4.203
45.McNamaraJ,ShahP,NakajimaK,CupplesL,WilsonP,OrdovasJ,etal.Remnantlikeparticle(RLP)cholesterolisanindependentcardiovasculardiseaseriskfactorin women:resultsfromtheFraminghamHeartStudy. Atherosclerosis. (2001)154:229–36. doi:10.1016/s0021-9150(00)00484-6
46.GoliaschG,WiesbauerF,BlessbergerH,DemyanetsS,WojtaJ,HuberK, etal.Prematuremyocardialinfarctionisstronglyassociatedwithincreasedlevels ofremnantcholesterol. JClinLipidol. (2015)9:801–6.doi:10.1016/j.jacl.2015. 08.009
47.DuranE,PradhanA.Triglyceride-richlipoproteinremnantsandcardiovascular disease. ClinChem. (2021)67:183–96.doi:10.1093/clinchem/hvaa296
48.WulffA,NordestgaardB,Tybjaerg-HansenA.APOC3loss-of-functionmutations, remnantcholesterol,low-densitylipoproteincholesterol,andcardiovascularrisk: mediation-andmeta-analysesof137895individuals. ArteriosclerThrombVascBiol. (2018)38:660–8.doi:10.1161/atvbaha.117.310473
49.FengX,GuoQ,ZhouS,SunT,LiuY,ZhouZ,etal.Couldremnant-likeparticle cholesterolbecomeariskfactorindiabeticmenopausalwomenwithcoronaryartery disease?Across-sectionalstudyofsingleacademiccenterinChina. LipidsHealthDis. (2020)19:44.doi:10.1186/s12944-020-01224-8
50.CaoY,ZhangH,JinJ,LiuH,ZhangY,XuR,etal.Prognosticutilityoftriglyceriderichlipoprotein-relatedmarkersinpatientswithcoronaryarterydisease. JLipidRes. (2020)61:1254–62.doi:10.1194/jlr.RA120000746
51.FukushimaH,SugiyamaS,HondaO,KoideS,NakamuraS,SakamotoT,etal. Prognosticvalueofremnant-likelipoproteinparticlelevelsinpatientswithcoronary arterydiseaseandtypeIIdiabetesmellitus. JAmCollCardiol. (2004)43:2219–24.doi: 10.1016/j.jacc.2003.09.074
52.JepsenA,LangstedA,VarboA,BangL,KamstrupP,NordestgaardB.Increased remnantcholesterolexplainspartofresidualriskofall-causemortalityin5414patients withischemicheartdisease. ClinChem. (2016)62:593–604.doi:10.1373/clinchem.2015. 253757
53.LangstedA,MadsenC,NordestgaardB.Contributionofremnantcholesterolto cardiovascularrisk. JInternMed. (2020)288:116–27.doi:10.1111/joim.13059
54.ZhaoQ,ZhangT,ChengY,MaY,XuY,YangJ,etal.Prognosticimpactofestimated remnant-likeparticlecholesterolinpatientswithdifferingglycometabolicstatus:an observationalcohortstudyfromChina. LipidsHealthDis. (2020)19:179.doi:10.1186/ s12944-020-01355-y
55.SandesaraP,ViraniS,FazioS,ShapiroM.Theforgottenlipids:triglycerides, remnantcholesterol,andatheroscleroticcardiovasculardiseaserisk. EndocrRev. (2019) 40:537–57.doi:10.1210/er.2018-00184
Tianetal. 10.3389/fcvm.2022.951523
Frontiersin CardiovascularMedicine 09 frontiersin.org 158
56.BorenJ,OlinK,LeeI,ChaitA,WightT,InnerarityT.Identificationoftheprincipal proteoglycan-bindingsiteinLDL.Asingle-pointmutationinapo-B100severelyaffects proteoglycaninteractionwithoutaffectingLDLreceptorbinding. JClinInvest. (1998) 101:2658–64.doi:10.1172/JCI2265
57.WangL,GillR,PedersenT,HigginsL,NewmanJ,RutledgeJ.Triglyceride-rich lipoproteinlipolysisreleasesneutralandoxidizedFFAsthatinduceendothelialcell inflammation. JLipidRes. (2009)50:204–13.doi:10.1194/jlr.M700505-JLR200
58.ShinH,KimY,KimK,LeeJ,HongK.Remnantlipoproteinparticlesinduce apoptosisinendothelialcellsbyNAD(P)Hoxidase-mediatedproductionofsuperoxide andcytokinesvialectin-likeoxidizedlow-densitylipoproteinreceptor-1activation: preventionbycilostazol. Circulation. (2004)109:1022–8.doi:10.1161/01.CIR.0000117403. 64398.53
59.DuranE,AdayA,CookN,BuringJ,RidkerP,PradhanA.Triglyceride-rich lipoproteincholesterol,smalldenseLDLcholesterol,andincidentcardiovasculardisease. JAmCollCardiol. (2020)75:2122–35.doi:10.1016/j.jacc.2020.02.059
60.VarboA,BennM,Tybjaerg-HansenA,NordestgaardB.Elevatedremnant cholesterolcausesbothlow-gradeinflammationandischemicheartdisease,whereas
elevatedlow-densitylipoproteincholesterolcausesischemicheartdiseasewithout inflammation. Circulation. (2013)128:1298–309.doi:10.1161/CIRCULATIONAHA.113. 003008
61.JørgensenA,Frikke-SchmidtR,WestA,GrandeP,NordestgaardB,TybjærgHansenA.Geneticallyelevatednon-fastingtriglyceridesandcalculatedremnant cholesterolascausalriskfactorsformyocardialinfarction. EurHeartJ. (2013)34:1826–33.
62.JacobsonT,ItoM,MakiK,OrringerC,BaysH,JonesP,etal.Nationallipid associationrecommendationsforpatient-centeredmanagementofdyslipidemia:part 1–fullreport. JClinLipidol. (2015)9:129–69.doi:10.1016/j.jacl.2015.02.003
63.KulkarniK.Cholesterolprofilemeasurementbyverticalautoprofilemethod. Clin LabMed. (2006)26:787–802.doi:10.1016/j.cll.2006.07.004
64.OtvosJ.Measurementoftriglyceride-richlipoproteinsbynuclearmagnetic resonancespectroscopy. ClinCardiol. (1999)22:II21–7.doi:10.1002/clc.4960 221405
65.MoherD,LiberatiA,TetzlafJ,AltmanDG,ThePRISMAGroup.Preferredreporting itemsforsystematicreviewsandmeta-analyses:ThePRISMAstatement. PLoSMed 6:e1000097doi:10.1371/journal.pmed.1000097
Tianetal. 10.3389/fcvm.2022.951523
Frontiersin CardiovascularMedicine 10 frontiersin.org 159
OPENACCESS
EDITEDBY TatsuyaSawamura, ShinshuUniversity,Japan
REVIEWEDBY MattiSakariJauhiainen, MinervaFoundationInstituteforMedical Research,Finland JerzyBeltowski, MedicalUniversityofLublin,Poland CorinaRosales, HoustonMethodistResearchInstitute, UnitedStates
*CORRESPONDENCE PaulN.Durrington pdurrington@manchester.ac.uk
SPECIALTYSECTION
Thisarticlewassubmittedto LipidsinCardiovascularDisease, asectionofthejournal FrontiersinCardiovascularMedicine
RECEIVED 10October2022
ACCEPTED 30January2023
PUBLISHED 16February2023
CITATION
DurringtonPN,BashirBandSoranH(2023) Paraoxonase1andatherosclerosis. Front.Cardiovasc.Med. 10:1065967. doi:10.3389/fcvm.2023.1065967
COPYRIGHT
©2023Durrington,BashirandSoran.Thisisan open-accessarticledistributedundertheterms ofthe CreativeCommonsAttributionLicense (CCBY).Theuse,distributionorreproductionin otherforumsispermitted,providedtheoriginal author(s)andthecopyrightowner(s)are creditedandthattheoriginalpublicationinthis journaliscited,inaccordancewithaccepted academicpractice.Nouse,distributionor reproductionispermittedwhichdoesnot complywiththeseterms.
PUBLISHED 16February2023
DOI 10.3389/fcvm.2023.1065967
Paraoxonase1andatherosclerosis
PaulN.Durrington1*,BilalBashir1,2 andHandreanSoran1,2
1 CardiovascularResearchGroup,FacultyofBiology,MedicineandHealth,TheUniversityofManchester, Manchester,UnitedKingdom, 2 DepartmentofDiabetes,EndocrinologyandMetabolism,PeterMount Building,ManchesterUniversityNHSFoundationTrust,Manchester,UnitedKingdom
Paraoxonase1(PON1),residingalmostexclusivelyonHDL,wasdiscoveredbecause ofitshydrolyticactivitytowardsorganophosphates.Subsequently,itwasalsofound tohydrolyseawiderangeofsubstrates,includinglactonesandlipidhydroperoxides.
PON1iscriticalforthecapacityofHDLtoprotectLDLandoutercellmembranes againstharmfuloxidativemodification,butthisactivitydependsonitslocation withinthehydrophobiclipiddomainsofHDL.Itdoesnotpreventconjugated dieneformation,butdirectslipidperoxidationproductsderivedfromtheseto becomeharmlesscarboxylicacidsratherthanaldehydeswhichmightadduct toapolipoproteinB.SerumPON1isinverselyrelatedtotheincidenceofnew atheroscleroticcardiovasculardisease(ASCVD)events,particularlyindiabetesand establishedASCVD.ItsserumactivityisfrequentlydiscordantwiththatofHDL cholesterol.PON1activityisdiminishedindyslipidaemia,diabetes,andinflammatory disease.Polymorphisms,mostnotablyQ192R,canaffectactivitytowardssome substrates,butnottowardsphenylacetate.Geneablationorover-expression ofhuman PON1 inrodentmodelsisassociatedwithincreasedanddecreased atherosclerosissusceptibilityrespectively.PON1antioxidantactivityisenhanced byapolipoproteinAIandlecithin:cholesterolacyltransferaseanddiminishedby apolipoproteinAII,serumamyloidA,andmyeloperoxidase.PON1losesthisactivity whenseparatedfromitslipidenvironment.Informationaboutitsstructurehas beenobtainedfromwatersolublemutantscreatedbydirectedevolution.Such recombinantPON1may,however,losethecapacitytohydrolysenon-polar substrates.Whilstnutritionandpre-existinglipidmodifyingdrugscaninfluence PON1activitythereisacogentneedformorespecificPON1-raisingmedicationto bedeveloped.
KEYWORDS
paraoxonase1,paraoxonase1activity,cardiovasculardisease,highdensitylipoprotein,lipid peroxidation,PON1polymorphism
Introduction
Itis20yearssinceourlastreviewoftheroleofparaoxonaseinatherogenesis(1).Inthat timemuchhasbeenlearntregardingthestrengthoftherelationshipofserumparaoxonase activitywithatheroscleroticcardiovasculardisease(ASCVD).Despitethis,alltoofrequentlythe involvementofparaoxonaseinatherogenesisisstillregardedascontroversial.However,whilst someaspectsoftheroleofparaoxonasemaybeasyetpoorlyunderstood,agreatdealhasbeen clearlyestablished.Thatwillbethesubjectofthisreview.
Review
TYPE
Frontiersin CardiovascularMedicine 01 frontiersin.org 160
Thedevelopmentoftheconceptthat PON1isanti-atherosclerotic
ParaoxonasewasidentifiedbyAldridgein1953asanenzyme presentinserumwiththecapacitytohydrolysediethyl paranitrophenylphosphate(2).Itwasoriginallytermed“A”esterase todistinguishitfrom“B”esterases,suchasacetylcholinesterase andbutyrylcholinesterase,whichareinhibitedbydiethyl para-nitrophenylphosphate,anorganophosphate.Diethyl paranitrophenylphosphate,whichisnowmorecommonlyreferredto asparaoxon,isthepotentneurotoxinproducedbythemetabolism oftheorganophosphatepesticide,parathion.The“A”enzymes hydrolysingparaoxon(EC.3.1.8.1,aryldialkylphosphatase)havethus cometobeknownasparaoxonases.Paraoxonase,circulatingin bloodandtissuefluidnowdesignatedasparaoxonase1(PON1), wasfoundtoconstitutethefirstlineofdefenceagainstapanoplyof organophosphatetoxins,includinginsecticidesandmilitarynerve gasses.Becauseofitsimportanceintoxicology,itwasintensively studiedmostnotablybytheresearchgroupsheadedbyFurlong(3)in SeattleandDraganovandLaDu(4)inAnnArbor.However,itwas notuntilthemid-1980’sthatMackness,workinginthetoxicology departmentattheUniversityofReading,demonstratedthatthe locationofparaoxonasewasalmostexclusivelyonHDL(5).Because oftheknownassociationoflowHDLwithASCVD,itwasbuta shortsteptodiscoveringthattheserumactivitywasdiminishedin myocardialinfarction(MI)survivors(6).Towardstheendofthat decadeMacknessjoinedthelipoproteingroupinManchesterand webegantostudyPON1indiseasesassociatedwithaccelerated atherosclerosis.Wesoondiscoveredthatitsactivitywasdiminished bothindiabetes(7, 8)andfamilialhypercholesterolaemia(8).
Theprevailingdogmatoexplaintheepidemiologicallyobserved inverserelationshipbetweenHDLandASCVDwasthatHDLwas criticalforreversecholesteroltransport.TheevidencethatHDL israte-limitingforthisprocessintypicalhumanatherosclerosis wasandremainsscant(9).However,therewerereportsthatHDL mightprotectLDLagainstpotentiallyatherogenicmodificationsto itsstructure.Asearlyas1979ithadbeenfoundthatthecytotoxicity ofLDLtohumanvascularsmoothmuscleandendothelialcellsin tissueculturecouldbeabolished,ifHDLwasalsopresent(10).Later itwasfoundthat:
(a)HDLdecreasedlipidperoxidationproductsmeasuredas thiobarbituricacidreactingsubstancesaccumulatingonLDL duringCu2+-orendothelialcell—inducedoxidation(11).
(b)HDLdiminishedtheincreaseinelectrophoreticmobilityofLDL followingCu2+-inducedoxidation(12).
(c)HDLdecreasedtheaccumulationofmalondiadehydeinLDL duringoxidationinducedbyFe2+ orbyprolongedincubation (13).Fogelman’sgroupinLosAngelesthenfoundthatHDL preventedtheminimallyoxidisedLDL-inducedmigrationof humanbloodmonocytesthroughalayerofculturedendothelial cells(14).
Itwasuncertainwhethertheseeffects(11–14)weredueto transferoflipidperoxidesfromLDLtoHDL(probablyfor subsequentdisposalbytheliver)orwasduetotheirbreakdownby anenzymepresentonHDL.Lecithin:cholesterolacyltransferase (LCAT)wasconsideredforthelatterrole,butnoneofthegroupswas awareofthepresenceofPON1onHDL.Webegantospeculatethat
PON1,whichhadnoknownphysiologicalrole,mightbeinvolved (15).WestudiedCu2+-induced invitro oxidationofLDLinthe presenceandabsenceofHDL,usingthemethodofEl-Saadanietal. tomeasurelipidperoxides(16)onLDLandHDL.Wediscovered in1991thatCu2+-inducedLDLlipidperoxidationwasnotonlyless whenco-incubatedwithHDL,butthatitwasalsounaccompanied byanyincreaseinlipidperoxidesontheHDLandthatthetotal lipidperoxidesinthesystemwerelesswhenHDLwaspresent (17; Figure1).ItwasthuslikelythatHDLdidnotsimplyreceive lipidperoxidationproductsfromLDL,butthatitalsocatalysed theirconversiontoproductsnotdetectedaslipidperoxidesinour assay.Toexplainthisphenomenon,PON1freeofLCATactivitywas isolatedfromHDLinlipidmicellesandfoundtobeapotentinhibitor oftheaccumulationoflipidperoxidesonLDLwhenincubated withCu2+ (17).Aseriesofpublicationsfromourgroupfollowed supportingthehypothesisthatPON1wascriticalfortheprotection affordedtoLDLagainstoxidativemodificationandextendingthisto includetheconceptthatHDL,whichisthepredominantlipoprotein intissuefluid,protectedcellmembranesagainstoxidativeandother damagingprocesses(18–20).Thisseemedtoprovideanattractive anti-atheroscleroticroleforPON1onHDL.Interestintheoxidative theoryofatherogenesiswas,however,waningbecauseofthefailureof fat-solubleantioxidantvitaminstosuppressatherosclerosisinclinical trials(seelater)despiteevidencethattheypreventtheformation ofconjugateddienesintheinitialstageofLDLlipidperoxidation bybeingmoresusceptibletooxidationthanunsaturatedfattyacyl groups.However,oncetheythemselvesareoxidisedtheyareprooxidantandfurthermoretheyincreasecholesterylestertransfer protein(CETP)activity(21, 22).PON1ontheotherhanddecreases theaccumulationofthelipidperoxidesgeneratedafterconjugate dieneformationanddoessooveralongertimeperiod.
In1995independentconfirmationthatpreventionofoxidative modificationofLDLbyHDLwasduetoPON1wasprovided

Durringtonetal. 10.3389/fcvm.2023.1065967
FIGURE1
Frontiersin CardiovascularMedicine 02 frontiersin.org 161
TheaccumulationoflipidperoxidesonLDLandHDLwhenincubated aloneandtogetherinthepresenceofCu2 + .LDL+HDLsignificantly differentfromLDLalone. ∗ P < 0.05; ∗∗ P < 0.001.
byFogelman’sgroupinLosAngeles(23).Itwasreported thattheminimallyoxidisedLDL-inducedmigrationofhuman bloodmonocytesthroughalayerofculturedendothelialcells wasdiminishedinthepresenceofPON1purifiedfromHDL. Furthermore,byamassspectrometrymethod,whenpurifiedPON1 wasincubatedwithminimallyoxidisedLDL,itwasfoundthat oxidationproductsofphosphatidylcholineweredecreased.Later, usingelectrosprayionisationmassspectrometry,weconfirmedthe markeddecreaseinhistidineresiduesmodifiedby4-hydroxy-2non-enal(HNE)(abreak-downproductofperoxidisedlinoleicacid typicallypresentattheSn2positionofphosphatidylcholine)inthe trypticfragmentsofLDL,whichhadbeensubjecttoCu2+-induced oxidationwhenco-incubatedwithHDLpossessingPON1(24).
TheoxidationproductsobservedbytheLosAngelesgroupto bepresentonminimallyoxidisedLDLintheabsenceofPON1 couldthemselvesstimulatemonocytemigration.Theeffectof PON1indecreasingmonocytemigrationandphosphatidylcholine peroxidationproductswasenhancedbyplateletactivatingfactor acetylhydrolase(PAFAH)(23).However,aswediscusslatermuch ofthePAFAHactivityonHDLisprobablyduetoPON1.Other factorsproposedtoexplaintheprotectiveeffectofHDLagainst lipidperoxideaccumulationwereLCATandapoA1(11, 25, 26).Experimentsreportedbyourgroupshowedthatneitherof theseactingalonewereeffectiveinprotectingLDLagainstlipid peroxidationincomparisontoPON1(27).However,enhancement oftheeffectofPON1wasobservedfromtheadditionofeither LCATorapoA1topurifiedPON1(27).Thiswasgreaterafter 4–8hofco-incubationwithLDLandPON1thaninthefirst4h (28).Theseobservationsweremadeunderexperimentalconditions designedtodeterminewhetherPON1hasthecapacitytoprevent oxidativemodificationofLDLand,aswediscusslater,theintensely hydrophobicenvironmentcreatedonHDLproducedbythepresence ofapoA1andtheactionofLCATinconvertingpre-betaHDLto morematureHDL(29)islikelytobemorecritical invivo forPON1 toexertitsanti-oxidative,anti-atherogenicrole.Theseandother HDLcomponentswhichmaycontributetoanti-oxidantactivity haverecentlybeenreviewedbyus(30).However,insupportof animportantroleforPON1,geneticdeficiencyofneitherapoA1 norLCATinhumansis,unlikePON1deficiency,conspicuously associatedwithprematureatherosclerosis(31, 32).Susceptibility toexperimentalatherosclerosisin APOA1 or LCAT ablatedmouse modelsrequiresanadditionalmutation,suchasapoEdeficiencyor LDLreceptordeficiency(33, 34)andeventhenmayinvolvedecreased PON1,whereas PON1 knock-outmicearepronetoatherosclerosis inducedsimplywithatherogenicchowevenwithoutcross-breeding withapolipoproteinE(apoE)ablatedmice(35).
Underoxidisingconditions,regardlessofthepresenceorabsence ofLDL,lipidperoxidesbegintoformonHDLatanearlystage, buttheiraccumulationthenceasesremainingatalowlevelrelative toLDL(Figure1).In1998aconsortiuminAnnArborandHaifa (36)showedinexperiments,involvingenrichmentofHDLwith purifiedPON1andspecificinhibitionofPON1presentinHDL,that theresistanceofHDLtolipidperoxidationwasduetoitsPON1 component.
TotestourtheorythatHDLbyvirtueofitsPON1component mighthaveawiderrolebyprovidingasystemtoprotect cellmembranesagainstoxidativedamage,wedeterminedthe concentrationofPON1inexperimentalblisterfluidasasurrogate forinterstitialfluid(37).PON1concentrationwasapproximatelyone fifththatinserumand,althoughitwasstillassociatedwithapoA1,
theratiobetweenthetwohaddecreasedwhichwasinterpretedas likelytobeduetosequestrationofPON1bythetissues.LaterJames’ groupinGenevashowedthatPON1couldexchangebetweenHDL andoutercellmembraneswhereitdecreasedcellularsusceptibility tolossoffunctioninducedbyoxidisingconditions(38).Thisfitted wellwithourearlierimmunohistochemicalstudyofatheromainthe humanaorta(39).ApoA1,clusterin(apoJ),andPON1werefound tobepresentinhealthycoronaryarteries,stainingwithincreasing intensityasatheromaprogressed(39).
Itwouldbewrongtocreatetheimpressionthattheconceptthat PON1canexplaintheanti-oxidativeactivityofHDLhasnotbeen withoutcriticism.Firstly,apersistingeffectofHDLtopreventLDL oxidationevenwhennoPON1activitycanbedetected,forexample inthepresenceofEDTA(40)andsecondly,failureofhighlypurified orrecombinantPON1toprotectLDLagainstlipidperoxidation(41–43)havebeeninterpretedasdenyingthetheory.Theseassertions havebeenchallengedbydirectexperimentation(44).Furthermore, theevidencethatPON1activitywasabsentduetoinhibitionby EDTAwasbasedonmeasurementsmadeusingphenylacetateas thesubstrate(40).HydrolysisofphenylacetatebyPON1ishighly Ca2+-dependentwhereasPON1anti-oxidantactivitycanpersist eveninthepresenceofEDTA(45).Also,inexperimentswherehighly purifiedorrecombinantPON1didnotprotectLDLagainstoxidative modification,itcanbearguedthatinpurifyingPON1toahigh degree,whetherfromserumortissueculturefluid,itisextremely difficulttomaintainalipidenvironmentinwhichtheconformation ofPON1necessaryforitsanti-oxidantactivitycanbemaintained (41).Water-solublePON1mutantsareevenlesslikelytointeract withthelipidenvironmentphysiologicallytoprovidehydrolytic activityagainsthighlyhydrophobicsubstrates.Interestinglytoo, recombinantPON1hascytotoxicproperties(44)mostlikelydue toitsPAFAH-likeactivityinproducinglysophosphatidylcholine (46),which,whenitoccursoutsidethesafeenvironmentofHDL,is intenselydamagingtotissues.
PONgenefamilyandPON1 polymorphisms
WhilstthemajorparaoxonaseinserumisPON1,itwasfound thattherearetwoothermembersofitsfamilywhosegenesclusteron chromosome7(47).Paraoxonase2(PON2)isawidelydistributed, highlyexpressedintracellularenzyme,whichcontributestothe intracellularanti-oxidantdefences.Paraoxonase3(PON3)isanother memberoftheparaoxonasefamilylocatedonHDL,butatmuch lowerconcentrationthanPON1.Ithasverylimitedarylesterase andpracticallynoorganophosphataseactivity.Aslactonases,the substratespecificityofPON1andPON3overlap,butdifferbydegree withPON3showingapreferenceforhighermolecularmasslactones.
Paraoxonase1ishighlypolymorphicanditwasfoundeven beforetheadventofgenesequencingtechnologythatatleastone ofthesepolymorphismsconferredvariationinactivitytodifferent substrates(48).Thisvariationinactivitydidnotapplywhenphenyl acetate,whichhasahighmolarrateofhydrolysiscomparedtoother substratesincludingparaoxon.Inthecaseofparaoxon,however, thefrequencydistributionofPON1activityinEuropidpopulation revealedthatalmosthalfhavealowactivity,around8–9%high activityandtherestformanintermediatepeak.Theheritability oftheseactivitiesledtothediscoverythatPON1wasallelicwith
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 03 frontiersin.org 162
lowactivityandhighactivityhomozygotesinHardy-Weinberg equilibriumwithheterozygotes.Bytheearly1990’ssequencingof PON1and PON1 revealedthatthisdifferenceinactivitywaslargely determinedbyasubstitutionofglycine(Q)forarginine(R)at position192resultingina192QisoenzymewithlowPON1activity anda192Risoenzymewithhighactivity.Thisgenevariantis alsoknownasrs662.Itshouldbenotedthattheactivityofthese isoenzymeswasreversedwithsomesubstratesotherthanparaoxon, suchasdiazoxon(49).Althoughnaturallyhumansareunlikelyto beexposedtoparaoxonordiazoxon,theseexamplessuggestedthe polymorphismmayhaveevolvedtopermitapopulationtowithstand awiderrangeoftoxinsthanwouldotherwisebethecase(see later).Theprevalenceof192RandQalloenzymesvariesindifferent populationsandtendstoreflecttheirtypicalhydrolyticactivity towardsparaoxon(48).
In1995Ruizandcolleaguesreportedthatintype2diabetesthe 192RisoenzymeofPON1wasassociatedwithcoronaryheartdisease (50).Initiallythisseemedcounter-intuitive,becausethehydrolytic activityofthisisoenzyme,atleastwithparaoxonassubstrate,is higherthanthatof192Q.Theexplanationprovedtobethat,per mgofHDLprotein,192Rwasslightlylesseffectivethanthe192Q alloenzymeinprotectingLDLagainstlipidperoxidation(51, 52). Inotherwords,theanti-atheroscleroticactivityofPON1isgreater fortheisoenzymewhichislesseffectiveinhydrolysingparaoxon. Nonethelessthe192Risoenzymedoeshaveanti-oxidativeactivity and,ifpresentathighconcentration,itwillprotectperhapsmore thaninanindividualexpressingthe192Qpolymorphismatlower concentration.Althoughthepolymorphismatposition192doesnot affectPON1serumconcentration,othersforexampleatposition55 (53)andinthepromoterregion(54)doandthereareahugenumber ofepigeneticandacquiredfactors,includingdiabetes,inflammatory disorders,infectionsandnutrition,reviewedelsewhere(55, 56) thatcontributetovariationinconcentrationandactivitywhichin differentindividualscanvarybyasmuchas16-foldand40-fold, respectively(57).
Epidemiology:Mendelian randomisationandserumPON1 activities
InepidemiologicalstudiesEDTAplasmaistypicallystored,butto testtheassociationbetweenPON1activityandatherosclerosiswhen phenylacetate,paraoxon,diazoxon,oralactoneareemployedas substrate,serumisrequired,becausealltheseactivitiesarehighly Ca2+-dependent(1).Genotyping,however,whichcanbedone onanystoredmateriallikelytoyieldDNA,hassincethe1990’s becomeincreasinglyeasy.Wewereamongstthegroupswhoseresults werenegative(58)withrespecttoanassociationbetweentheQ alleleandASCVDrisk,butothersreportedpositively.By2001our meta-analysisshowedaweakassociationbetweentheQalleleand ASCVD,whichwasapproximatelywhatwouldbeexpectedfromour experiments(51, 52).Inasubsequentmeta-analysisbyWheeleretal., producingsimilarfindings(59),itwasconsideredthattheassociation couldalsobeexplainedbypublicationbias,withwhichweagreed (58).However,theseauthorsconcludedthattheirfindingsmadeit unlikelythatPON1wascriticalinatherogenesis.Thiswasbased ontheunrealisticassumptionthatagenecodingforvariationin PON1activitytowardsparaoxoncouldprovideresultsinterpretable
accordingtotheprinciplesofMendelianrandomisation.Forthis tobethecasetheinfluenceofthe192variantsonatherosclerosis shouldhavebeensimilarinmagnitudetoitseffecton invitro paraoxonhydrolysis.Theconclusionfrommeta-analysisofthe 192polymorphismsmustbethatitdoesnotdenythehypothesis thatlowPON1isassociatedwithatherosclerosis:eitherthe192 genotypecontributesnothing(publicationbias)oritissupportive. Subsequently,meta-analyses,somewithoutpublicationbias(60) havecontinuedtoshowasmallcontributionofthe192Qalleleto ASCVDrisk,mostobviouslywhendiabetesisalsopresent(60–62).
Aftertheinitialcase-controlstudyshowinganassociation betweenPON1activityandmyocardialinfarction(6),weperformed anotherstudyinwhichitwasfoundthatserumPON1activitywas alreadylowinsamplestakenwithin2hoftheonsetofsymptomsof acutemyocardialinfarction(63).Therefollowedothercase-control studiesinwhichserumPON1activitywas,asexpected,moreclosely relatedtothepresenceofASCVDthanPON1genotypes(58, 64).The criticalepidemiologicaltestofwhetherPON1activitywasrelevant tofutureatherosclerosis,however,camewithreportsofprospective studies.SerumPON1wasfoundtobeindependentlyassociated withthelikelihoodoffutureASCVDevents,generallycontributing tovariationinriskwithasimilarmagnitudetoestablishedrisk factors,includingHDLcholesterol(65–70).Thefirstprospective resultscamefromtheCaerphillyHeartStudy(65)ofmiddle-aged men(Figure2).Kunutsoretal.performedameta-analysis(70) oftheseresultscombinedwiththoseofanadditionalfivereports (65–69).Therewere15,064participantsand2,958incidencesof ASCVD.Inthreestudiesparaoxonwasemployedassubstrate(65, 66, 69)andinthreephenylacetate(67, 68, 70).Theage-adjusted pooledrelativeriskforASCVDper1SDhigherPON1activity wasa0.87,whichwashighlystatisticallysignificant.Therewas noevidenceofpublicationbias.Theliteratureingeneraldoes notrevealaclosecorrelationbetweenPON1activityandHDL cholesterolorapoA1.Kunutsoretal.(70),however,didnotfind thatPON1activitycouldcontributemorethanHDLcholesterolto amultivariateequationtopredictthelikelihoodoffutureASCVD events.Thatcouldhavebeen,becauseindividualvariationin serumPON1activityisgreaterthanforHDLforcholesteroland thusregressiondilutionbiaswouldfavourHDLcholesterol.The potentiallygreaterbiologicalsignificanceofPON1anddiscordance betweenHDLcholesterolandPON1wasemphasisedbythereportof Corsettietal.ofdecreasedPON1activityinpeoplewithpremature ASCVDdespitehighHDLcholesterollevels(71).InthemetaanalysisbyKunutsoretal.(70)PON1measuredasarylesterase activity(phenylacetatehydrolysis),whichisunaffectedbythe192 polymorphism,predictedASCVDmorestronglythanparaoxonase (paraoxonhydrolysis)activity.PON1activitypredictednewASCVD eventsparticularlystronglyinpeoplewithestablishedASCVD, suchasthosewhohadundergonecoronaryrevascularisation.The studybyBhattacharyyaetal.(72)wasofparticularinterestinthe lattercontext.Theystudied1,399peoplewhounderwentcoronary angiographyattheClevelandClinic.PON1measuredbothasaryl esteraseandparaoxonaseactivitywasstronglyinverselyassociated withtheincidenceofnewASCVDeventoveraminimumfollowupof3years(Figure3).Inasubgroupof150participants matchedforthePON1192polymorphism(equalnumberswith theQQ,QR,andRR)serumPON1activitywasstronglycorrelated (P < 0.001)withconcentrationsoffattyacidoxidationproducts (hydroxyeicosatetraenoicacid,HETE;hydroxyloctadecadienoicacid, HODE,and8-isoprostaneprostaglandinF2α,8-isoPGF2 α ).
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 04 frontiersin.org 163
BecauseofthetheorythatPON1hasevolvedasalactonase (seelater),ithasbeenproposedthatuseofalactonesubstrate, suchashomocysteinethiolactoneordihydroxycoumarin(73, 74) mightprovideamorebiologicallyrelevantestimateofPON1 activity.Thereisasyetnoprospectiveepidemiologicalevidence thatthelactonaseactivityofPON1providesasuperiorindicator ofASCVDrisk(75).ThesignificanceofPON1lactonaseactivityin humandiseaseisemergentterritory(76).Specifically,inthecase ofatherosclerosissomeevidencesuggestshomocysteinethiolactone, which,likeglucoseandlipidperoxides,canbindtoapoB,may beassociatedwithASCVDincidence(77).Urinaryhomocysteine thiolactoneexcretionhasbeenreportedtobeincreasedinpeople withlowserumPON1,particularlyincarriersofthePON1-192R allele(73).

Evolutionandbiologicalplausibility

PolymorphismsofPON1,bybroadeningtherangeof organophosphateneurotoxinresistance,willincreasethesurvivalof anexposedpopulationwithouttheneedtoawaitanewmutationwith greaterdetoxifyingproperties,albeitattheexpenseofindividuals withthelessfavourablevariant(78).Darwinianevolutiontakes vastlylongerandextinctionmayoccurbeforeamoresuccessful mutationoccurs.Inthiscontext,wehavereportedthatagricultural workersinvolvedinsheep-dippingwithdiazinon(activemetabolite diazinoxon)arelesslikelytoexperienceneuropsychiatricsymptoms iftheypossessthe192QPON1alleleassociatedwithhigherserum PON1measuredasdiazoxonaseactivity(79).Theorganophosphates whichledtothediscoveryofPON1weresynthetic,butavastarrayof organophosphatesisnaturallyproduced.Thehabitatoftheearliest hominidswasontheshoresofthegreatlakesofAfrica,where cyanobacteria(blue-greenalgae)(80),whichcanproducelarge quantitiesofneurotoxicorganophosphates,attimes,wouldhave threatenedhumansurvival.However,modernmanhasbeenpresent foramere6millionyears,notlongenoughtoexplaintheevolution oftheparaoxonasefamilyofproteins,theancestralproteinforwhich mayhaveexistedhundredsofmillionsorevenbillionsofyearsago. ItislikelythatPON1evolvedfromPON2,itsintracellularrelative (49).Beyondthatweknowthatparaoxonasesarenothomologous toserineesterases,carboxyesterases,orarylesterasesandthusdonot havesimilarancestry(49).Thecapacitytosynthesisecholinesterases
andtorespondtoacetylcholinedatestobeforemetazoaemerged andtheevolutionofanyrecognisablenervoussystem(81).The potentialfororganophosphatetoxicitymusthavebeenpresent foratleastaslong.Organophosphatesproducedintheanaerobic conditionsarounddeepseahydrothermalventsmusthavebeen incorporatedintotheearliestlifeforms.Soitmaynotbetoofanciful toconsiderthattheancestralproteingivingrisetoparaoxonases mayhaveexistedmanyaeonsagoandhavelonghadarolein organophosphatemetabolism.Otherexamplesofenzymeswith organophosphataseactivityconservedacrossthedomainsofliving organismsare:diisopropylfluorophosphatase(DFPase)(eukaryocyte squid)(82),organophosphatehydrolase,organophosphateacid anhydrolaseandphosphotriesterase(bacteria)(83)andSsoPoxan organophosphatase/lactonasefrom Sulfolobussolfataricus (archea) (84).OfthesethestructureofrePON1resemblesthatofsquid DFPase.Botharesix-bladedpropellerswitheachbladeconsistingof four β-sheets.Moreover,inbothstructurestwocalciumionscanbe foundintheircentraltunnel(82).
Withtheadventofphotosyntheticorganismsanatmosphererich inoxygenwascreatedandthusthescenewassetfortheevolutionof lifewithmorerapidmetabolism(energisedbyoxidativerespiratory chainphosphorylation)thancouldbesustainedbysimpleglycolysis. However,simultaneouslythenecessityforprotectionagainstthe toxicityofoxygenalsobecameessential.Paraoxonasesandtheother anti-oxidativeenzymeswouldhavecontributedtothat(85).

EliasandTawfikinafascinatingreviewhavestronglyarguedthat paraoxonasesmayhaveevolved,notasesterases,butaslactonases withapromiscuousesteraseactivity(86).Althoughnotrelated inotheraspectsoftheirstructure,theiractivesitehasfeatures moreincommonwithlactonasesthanesterases.Manysinglecelledorganismssignaltoeachotherbyproducinglactones,such as N-acylhomoserinelactones,usuallywhentheircolonysizehas reachedsomecriticalpoint(quorumsensing),alteringexpression ofgenesregulatingsuchprocessesasbioluminescence,biofilm formation,virulencefactorexpression,andmotility.Justasfora hormonetoexciteratherthaninhibittheremustbeaprocess todestroyitafterreceptorbinding(ironically,forexample,acetyl cholineandacetylcholinesterase),soalactonasecouldhavearolein quorumsensing.PON1hasthecapacitytometabolisehomocysteine thiolactone,whichhasbeenimplicatedinatherogenesis(87).

Durringtonetal. 10.3389/fcvm.2023.1065967
FIGURE2
TheriskofASCVDrelativetothelowestriskquartileorquintile(RR)asafunctionofserumparaoxonase1(PON1)activitystudiedprospectivelyin (A) CaerphillyandSpeedwell(CHDonly)(65), (B) clevelandclinic(ASCVDandall-causemortality)(72),and (C) meta-analysisbyKunutsoretal.(ASCVD) (70).ClosedcirclesareRRunadjustedforotherriskfactorsandopencirclesafteradjustmentforsomeofthese(seereferencesfordetails).
Frontiersin CardiovascularMedicine 05 frontiersin.org 164
SymbolicrepresentationoftheperoxidationofphosphatidylcholinewithlinoleateinSn2byreactiveoxygenspecies(hydroperoxyradicalinthis example).Asuperscriptdotprecedinganatomindicatesanunpairedelectron.(1)Hydrogenabstractionfromthehydrocarbonchainleadsto(2) rearrangementinwhichthedoublebondsbetweencarbon6and9arenolongerseparatedby2singlebondsbutbyonlyone(conjugateddiene formation).(3)Additionofoxygenthenleadstoperoxyradicalformationwiththepotentialforinitiatingachainreactionand(4)spontaneousbreakdown toaldehydesandketoneswhichcanadducttoapolipoproteinBofLDLalteringitsreceptor-binding.WhenPON1inHDLispresentbreakdownto carboxylicacidsratherthanaldehydesandketonesisbelievedtooccur.

However,thisactivityisdwarfedbesidethatofbiphenylhydrolaselikeprotein,makingacriticalroleforancestralPON1inthatregard lesslikely(73, 76, 77, 88).
TheviewthatPON1mayhaveamoregeneralisedroleinthe immunesystemhasbeenproposedbyCampsetal.(89)basedon theirfindingofanincreaseinchemokine(C-Cmotif)ligand2 (CCL2)productioninPON1deficiency.CCL2inducesmigration andinfiltrationofimmunecellsintotargettissuesinarangeof inflammatorydisorders,whichcouldincludethearterialwall.
AnapparentlyquitedifferentroleforPON1whichmight haveselectiveadvantageisitscapacitytoinactivategramnegative bacterialendotoxin(90).Thisendotoxinisalipopolysaccharide,
whichintroducesyetanotherclassofsubstrateswhichPON1can hydrolysewithimportantbiologicalconsequences.
Thusparaoxonasesappeartohavedivergedfromotherenzymes earlyinevolution.Theydisplaygreatsubstratepromiscuityand theirprimaryfunction(organophosphatase,anti-oxidant,lactonase, lipopolysaccharidase)mayhavebeendifferentatvarioustimesin evolutionaryhistoryandindifferentclassesorevenordersofliving organisms.Myocardialinfarctionwasunknownbeforethe20th century(91).ItisthusinconceivablethatPON1hasevolvedto combatatherosclerosis.Nonethelessitisverypossiblethatanenzyme whichhasprovidedsurvivalsuccessinsomeothercontextmight byvirtueofitspromiscuityprotectagainstASCVD(“widesubstrate
Durringtonetal. 10.3389/fcvm.2023.1065967
FIGURE3
Frontiersin CardiovascularMedicine 06 frontiersin.org 165
specificity”mightbebetterterminologythan“promiscuity”when consideringvirtue).
Evidencefromanimalexperiments thatPON1protectsagainst atherosclerosis
BirdsdonotexpressserumPON1andtheyarenotonly susceptibletoorganophosphatepoisoning,buttheirHDLlacksthe capacitytoimpedetheaccumulationoflipidperoxidesinhuman LDLunderoxidisingconditions(92).Mammalsdisplayserum PON1activity,althoughwithconsiderablespeciesvariation(93). Experimentalatherosclerosisinrodentshasprovidedconsistent evidencethatPON1infusionorover-expressioncansuppress atherogenesisorthatinhibitionorablationofthePON1gene promotesatherogenesis.Thusthe PON1 knockoutmouse,which, likebirdsissusceptibletoorganophosphatetoxicity,alsoproduces HDLwhichhasadiminishedcapacitytoprotectLDLagainst oxidativemodification.Itissusceptiblebothtoatherosclerosis inducednutritionallyandbyapoEdeficiency(35).Consistentwith this,rabbitsfedanatherogenicdietdevelopedmoreadvanced atheroscleroticlesionswhenPON1activitywasinhibitedwith nandrolone(94).Aslongagoas2002aUSpatentwasregistered(95) forthepreventionofatherosclerosisbyinjectionofapreparation ofPON1192Qisoformbasedonamousemodel.Over-expression ofPON1hasbeenachievedinmouse(96–100),rabbit(101–103),andrat(104)models.Suchexperimentshaveconsistently revealeddecreasedsusceptibilitytoatherosclerosisandenhanced HDLfunctionality.Ablationorover-expressionofthePON1gene causeslittleifanyeffectonlipoproteinmetabolism,unlikeknock outoroverexpressionofmajorgenessometimesconsideredto beimportantinatherogenesis,suchasapoA1andLCAT.The smalldecreaseinbloodpressureinPON1deficiency(105)would beexpectedtoopposeratherthanincreaseatherogenesis.Thus, amajorroleforPON1inatherogenesisistheonlyplausible explanationfortheresultsofanimalexperiments.Furthermore,in experimentstotestthecontributionofriskfactorsotherthanPON1 toatherosclerosis,theatherogenicdietusedin,forexamplerabbits, wouldhavedecreasedPON1andcontributedtoatherogenesisfrom whateverothercausewasbeingexamined(106).
Physicalandstructuralpropertiesof PON1
Foritsantioxidativeactivitytowardslipidhydroperoxides, PON1requiresanintenselyhydrophobicenvironment.Itsmolecular structurecontainslongsequencesofhydrophobicaminoacids creatingregionseschewingwaterandallowingPON1toexist withinthelipid-richdomainsofHDL.Thestrongdetergent propertiesofapoA1(107)arelikelytobecrucialinthisrespect. ThehydrophobicityofPON1makesitresistanttocrystallisation. Removedfromitslipidenvironment,naturallyoccurringPON1is unstableandtendstoaggregateintheabsenceofdetergents.This istruewhetherwild-typePON1(wtPON1)isisolatedfromserum orfromtheculturemediumof Ecoli expressingwtPON1.Thishas hadtwomajoreffectsonprogressinresearchintotheinvolvementof
PON1inatherosclerosis.Firstly,itsstructureremainedthesubject ofspeculation,whichwasunresolveduntilitwassubmittedto directedevolutioninordertoincreaseitssolubility(108).Secondly, asmethodsweredevelopedforincreasingpurificationofwtPON1, disputeensuedabouthowmuchofitscapacitytopreventthe accumulationoflipidperoxidesonLDLwasretained(41–44).
FamilyshufflingoffourPON1genes(human,mouse,rabbit,and rat)resultedinmanyvariantsthatcouldbeexpressedin E.coli.One ofthemproducedcrystalsofaqualitysuitableforX-raydiffraction studies(108).Thiswastherecombinant-PON1(rePON1)G2E6 variant,whichexhibits91%homologytothewtrabbitPON1and 86%homologytothehumanPON1(109).Structuralanalysisusing X-raycrystallographyrevealedthesix-bladed β-propellerstructureof PON1withacentraltunnelthathousestwocalciumions.Thereisa uniqueadditiontothe β-propellerscaffoldintheformofthree αhelices,whicharelocatedonthetopofthepropeller.Thesehelices arelikelytobeinvolvedinanchoringofPON1toHDLparticles.
Eachcalciumion,dependingonitslocationwithintheenzyme, playsanimportantpartintheactivityofPON1.Thecalciumion locatedclosertothetunnelentrancehasastructuralrolethatmay benecessaryforsomeconformationalaspectsofPON1important forsomeofitssubstrates,suchasorganophosphates.Itmaybeless criticalto,sayphospholipids,withwhichitissurroundedwithin HDL.Theothercalciumionwhichliesdeepintheactivesitecavity hasacatalyticroleandisimportantforsubstratepositioningand esterbondactivation.Differencesintheactivesiteconfigurationand positioningofacalciumionarelikelytobeimportantinexplaining thedifferentialsubstratespecificityofthe192polymorphisms,but withsuchawidespectrumofsubstratesnosinglemechanismmay exist(110).Engineeredvariantswithincreasedaqueoussolubility andtaggedtosimplifypurificationhaveatthetimeofwritingled to21rePON1productsbeingavailablefrom8suppliers.Generally, theevidencethattheseretainenzymicactivityininitialscreening hasbeenbasedonphenylacetatehydrolysis.Thecommercial incentivehasbeentoproducerePON1variantsforthetreatmentof organophosphatepoisoningorprevention.Theaimhasthusbeento createrePON1’sthataremoreactiveinhydrolysingorganophosphate neurotoxinsthanwtPON1,whichislessactivethansquidDFPase, arivaltargetforbio-engineering.ThehydrophobicityofrePON1 mustnecessarilyhavebeendiminished.Thismaynotimpairits capacitytohydrolysemoleculessuchasphenylacetateandsimple organophosphates,buthydrolysisofmoreintenselyhydrophobic longchainfattyacyllipidsubstratesmaybeabolished.
MechanismbywhichHDLandPON1 acttoprotectagainstlipidperoxide accumulation
Polyunsaturatedfattyacylgroupsaresusceptibletoperoxidation duetooxygenfreeradicalsleakingfromcellsordeliberatelyproduced inthetissuefluidbyinflammatorycells(myeloperoxidase,NADPH oxidase).Inthehuman,linoleate(C18:2)isthemajorcirculating polyunsaturatedfattyacid,typicallyoccurringattheSn2position ofphosphatidylcholine.Theearliestphaseoflipidperoxidation ishydrogenabstraction.Thisleadstoconjugateddieneformation detectablebyultravioletspectroscopy(111).ThedoubleC=Cbonds (outerorbitalsoccupiedbyanelectronfromasinglehydrogen) inlinoleateareseparatedbytwosingleC-Cbonds(outerorbitals
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 07 frontiersin.org 166
occupiedbyelectrosfromtwohydrogens).Aconjugateddieneis formedwhenoneofthedoublebondsflipsover,becauseoneofits hydrogenatomsisattractedbyhydroxylradicals(OH• createdbythe reactionofO2 • withwater)withtheirunpairedelectrons(Figure3). Thenewlycreateddoublebondisseparatedfromthenextdouble bondbyasinglebond(conjugateddiene),anassemblagewhich resonateswithultravioletlight.Thisearlyphaseoflipidperoxidation isopposedbychain-breakinglipidsolubleantioxidants,suchas αtocopherol(vitaminE), β-carotene,andubiquinol-10,whichoffer themselvesaselectrondonorsinpreferencetolinoleate,butitis largelyunaffectedbyPON1.ThemajoreffectofPON1presentin HDLisindecreasingtheaccumulationoflipidperoxidesinLDL derivedfromtheseconjugateddienefreeradicalsinthelaterphase oftheoxidationoflinoleate(21).Thefailureofclinicaltrialswith, forexample,vitaminE(112)isfrequentlyseenasdismissingthe oxidativemodificationofLDLtheoryofatherosclerosis.However,it isnotattheearlystageofconjugateddieneformationthatLDLis chemicallymodified,allowingarterialwallmacrophageandsmooth musclecellreceptor-mediateduptake(111).Thisoccurslaterwhen oxygenbecomesboundtolinoleatecausingitsdecompositionfirstly into,forexample,9-hydroperoxy-10,12-octodecadienoicacid(9HPODE),andthencealdehydes(e.g.,propanedial,hexanal,nonanal), unsaturatedaldehydes(e.g.,hexenal,nonenal)andtheirvarious hydroperoxyderivatives.Itisthesealdehydeswhichadducttothe sidechainsofprolineandarginineresiduesofapoB,leadingto fragmentationoftheapoBmolecule,whichtherebybecomesaligand formacrophageandtransformedsmoothmusclecellscavenger receptors,suchasscavengerreceptorclassBtype1(SCARB1)(111, 113–119).Possiblysomederivativesofoxidisedpolyunsaturatedfatty acidshaveastericresemblancetolactones(120).
ForPON1toprotectLDLorcellmembranesagainstaldehyde adductionitmustcomeintophysicalcontactwiththeirphosphatidyl cholineandcholesterylestercomponents.Intissuefluidthismaybe achievedthroughtheengagementofHDLparticlewithoutercell membranes(38, 121).Inthecirculation,particularlyinhumansin whomcholesterolesterificationoccursonHDLduetotheaction ofLCAT,hugeamountsofphospholipidandoffreeandesterified cholesterolaretransferredbetweenHDLandapoB-containing lipoproteins.Thisprocessisgreatlyfacilitatedbycholesterylester transferprotein(CETP)andphospholipidtransferprotein(PLTP) (122–124).EsterificationofcholesterolbyLCATyieldsonemolecule ofthehighlycytotoxiclysophosphatidylcholineforeverymolecule ofcholesterolesterified(125).HDLretainsthislysophosphatidyl cholinesafelyuntilitpassesthroughthehepaticsinusoidswhereit isreleasedtohepatocytesforre-esterification.
ArrivingonHDL,phosphatidylcholinewith,astheconsequence ofoxygenfreeradicalattack,fattyacylhydroperoxide/conjugated dienesatSn2,theinitialphaseofdetoxificationislikelytobethe releaseofthesefattyacylmoleculesfromSn2bythePAFAH(platelet activatingfactor;synphospholipaseA2,PLA2)-likeactivityofPON1. Quitepossiblythisisfacilitatednotatthelactonaseactivesitedeep inthecatalytictunnelofPON1,butperhapsmoresuperficiallyand mighteventakeplacetosomeextentspontaneously.Itis,however, likelythattheoxidisedlinoleateproductsreleasedcanreachthe deeperPON1catalyticsite(whateveritsteleology)(45)wherethey areconvertedtoharmlesscarboxylicacidsasopposedtoreactive aldehydes(20, 23, 126; Figure4).ThisconceptofPON1activity hasbeenchallengedbyareportthathighlypurifiedPON1lacks bothPAFAHactivityandthecapacitytopreventtheaccumulation ofphosphatidylcholineoxidationproducts(41).Nonetheless,ashas

SchematicrepresentationofthemechanismbywhichHDLimpedes theatherogenicmodificationofLDL.ApoA1,apolipoproteinAI; ApoAII,apolipoproteinAII;apoB,apolipoproteinB;ApoM, apolipoproteinM;PON1,paraoxonase1;PLA2,phospholipaseA2(syn: PAFAH,plateletactivatingfactorhydrolase);LCAT,lecithincholesterol acyltransferase;SAA,serumamyloidA;PLTP,phospholipidtransfer protein;CETP,cholesterylestertransferprotein;SCARB1,scavenger receptorclassB1;oxLDL,oxidativelymodifiedLDL;LDLR,LDL receptor;L,lipid; • LOOH,hydroxylipidradical;ROS,reactiveoxygen species(containingoxygenwithanunpairedelectrongivingitan outershellresemblingfluorine).
beenpreviouslydiscussed,PON1divorcedfromHDLmaynotbe abletohydrolyseintenselyhydrophobicsubstratesandthereisno denyingtheantioxidantactivityofintactHDLortheevidencefrom genemanipulationthatPON1makesacrucialcontributiontothis.
Epigeneticfactorsandmodulatorsof PON1activity
Ahostofdiseasesandnutritionalfactorsareassociatedwith variationinPON1activity.Theseandpotentialmechanismsfor theirassociationswithPON1haverecentlybeenreviewed(55, 56).Bothdyslipidaemia(8, 127–134)anddiabetesmellitus(8, 135–145)areassociatedwithdecreasedactivity(seelater).The compositionofHDLhasamajoreffectonPON1activity.In inflammationHDLhasdecreasedPON1activity(146–148).Atthe sametimetheapolipoproteinAIandclusterin(syn.apolipoproteinJ) contentofHDLalsodiminishwhereasapoliporoteinAII(apoAII) andserumamyloidA(SAA)increase(146–149).Theresulting pro-inflammatoryHDLhasdecreasedantioxidantcapacity.SAA increasesininflammation.ExperimentallySAAcandisplacePON1 fromHDL(148)butthemechanismforthereplacementofPON1 bySAAinpro-inflammatoryHDLmayalsoinvolveinhibitionof hepaticPON1expressionandstimulationofthatofSAA(146–150). TheantioxidantactivityofPON1isalsolimitedbymyeloperoxidase
Durringtonetal. 10.3389/fcvm.2023.1065967
FIGURE4
Frontiersin CardiovascularMedicine 08 frontiersin.org 167
(MPO),apro-oxidantenzymesecretedbyneutrophils(151–155)to killbacteriabyshoweringthemwithoxygenfreeradicals.MPOis takenupbyHDLwhereitmayformacomplexwithPON1.TheantioxidativeactivityofHDLmaythusreflectabalancebetweenthose two.BearinginmindthatHDListhedominantlipoproteinintissues, itmaynotbefancifultospeculatethatitmayoperatetoconfineprooxidantactivitytositesofinflammationandtolimitthespreadof oxygenfreeradicalssystemically.Wheninfectionorinflammation becomesgeneralised,asin,say,septicaemia(90, 156)orsystemic lupuserythematosus(157)PON1activityhasdeclined.
Diabetesandmetabolicsyndrome
Bothtype1andtype2diabetesareassociatedwithdecreased PON1activityandlowPON1activityisrelatedtobothmacro-and microvascularcomplications(8, 135–145).FurthermorelowPON1 maypredisposetothedevelopmentoftype2diabetes(143, 158, 159).
Itisreportedthat invitro glycationofPON1byincubationwith glucoseathighconcentrationdecreasesitsactivity(160, 161).This, ofcourse,cannotexplainthelowserumPON1activityinmetabolic syndrome(prediabetes)beforetheonsetofhyperglycaemia.What isofmuchgreaterinterestistheoccurrenceofglycatedapoBin thecirculation.Innon-diabeticpeoplesome4%ofserumapoB isglycatedandindiabetesthepercentageistypicallytwicethis (162).TheconcentrationofglycatedapoBisalsoraisedwhenLDL isincreasedevenintheabsenceofdiabetes,forexampleinfamilial hypercholesterolaemia.TheconcentrationofglycatedapoB,whether indiabeticornon-diabeticpeople,ishigherthanoxidativelymodified apoB(162, 163).ApoBinthesmaller,densersubfractionsofLDL (SD-LDL)ismoreheavilyglycatedthaninVLDLandlessdense LDL(164–166).ThismaybebecausemoreoftheapoBmoleculeis exposedtoglucoseinSD-LDLorbecauseithasalongerresidence timeinthecirculationorboth.GlycationofapoBoccursatthe arginineandprolineresiduestowhichketonesandotherderivatives oflipidperoxidationadduct(167).GlycatedapoB,likeoxidatively modifiedapoB,istakenupbymacrophagestoformfoamcells(168).
Invitro apoBinLDLisresistanttoglycation.Prolongedincubation ofnormalLDLwithhighconcentrationsofglucosedoesraiseits level,butnotusuallytothesameextentasisfoundindiabetes (165, 168).Theexplanationmaybethatthemorehighlyoxidative environment invivo allowsprioradductionof,sayaldehydesto arginine,whichisthenreplacedbyglucose(166, 169)oramore reactivederivativeofglucose,suchasgluconolactoneormethyl glyoxal,isgeneratedduringglycolysis(170–172).Eithermechanism mightsuggestapossibleeffectofHDLinprotectingLDLapoBagainst glycationwhichhasbeenreported invitro withHDLfrompeople withabovemedianserumPON1activityopposingglycationmore thanHDLfromthosewithloweractivity(173).Moreworkisneeded toexplorethepossibilitythatHDLmightprotectagainstatheroma andmorespecificallydiabeticcomplicationsbythismechanism.
Futuredirections:Therapeuticand diagnosticpotential
ThereisaplethoraofnutritionalstudiesofPON1activity. Unsurprisingly,giventhedifferentsubstratesusedtomeasure PON1activity,thesmallsizeandtheinadequatedesignofmany, findingsoftenappearconflictingorunconvincing.Noamount
ofmeta-analysiscanprovideclarification.Theimpressiongained isthatobesityisoftenassociatedwithdecreasedPON1activity, albeitmostobviouslywhentriglyceridesareraisedordiabetes ispresent(127, 128, 132–145, 174).ItisalsoclearthatHDL cholesterolconcentrationisoftendiscordantwithchangesin PON1activity.TheMediterraneandietmayincreasePON1activity (175)andvariousfruitjuices,mostconspicuouslypomegranate juice,canraisePON1activity(176).Dyslipidaemia,whether duetofamilialhypercholesterolaemiaorhypertriglyceridaemia, isassociatedwithdiminishedPON1activity(8, 127, 128, 130–134)withperhapsitsmostprofounddecreasesoccurringin familialdysbetalipoproteinaemia[unpublishedobservation].Statin (177),fibrate(178),ezetimibe(179),probucol(128),niacin(180), andmetformin(181)drugsraiseserumPON1activitywhilst sulphonamidesmaydecreaseit(182).
ApharmacologicalapproachtoraisingPON1activityis attractive,buttraditionallyitiseasiertoblockratherthanactivate enzymes.RaisingHDLbyCETPinhibitionwasineffectivein preventingatherosclerosisexceptbyitsLDLloweringeffect(183). CETPmaybenecessaryforthetransferofoxidisedphospholipid andcholesterylestertoHDLforPON1toactonthemandtheHDL particlescreatedarelarge(184)andnotthesmaller,desirableparticles richinPON1capableoffacilitatingcholesterolefflux.PON1-rich HDLinfusionisprobablynotapracticalpossibility,particularly asrePON1,whichiseasilyproducedmayhavelittleanti-oxidant capacity[seeearlier].EvidencesuggeststhatHDLmimetics,some ofwhichcouldbegivenorally,canraisePON1activityinparticles resemblingphysiologicalHDL(185, 186).Itwillalsobeimportantto beawareofeffectsonPON1ofthevariousantisenseoligonucleotides forloweringLDLandtriglyceridesastheyemerge.Therealsoexists thetheoreticalpossibilityofraisingPON1activitybypromotionofits geneorexpressionofagain-of-functionvariant(butwithoutapolar tagsothatitisincorporatedphysiologicallyintoHDL)(187).
Paraoxonase1hasthepotentialtocontributetotheclinical assessmentofASCVDrisk.However,continuinguncertaintiesabout identificationofthesubstratecriticalinitsanti-atherosclerotic activityhaveslowedprogressinthatdirection.Isitimportant,for example,toemployalongchainfattyacidperoxideorlactone ratherthan,say,phenylacetateorparaoxonasthesubstratein anassay?However,whilstdiscoveryofthekeysubstrate(s)in themechanismbywhichPON1protectsagainstatherosclerosisis essentialforourunderstandingofitsrole,thismaynotbecritical tomakeuseofitclinically.Alkalinephosphataseisoneofthe mostfrequentlyrequestedandinformativebiochemicaltestsin clinicalpractice,butitsphysiologicalroleremainsobscure,and thesubstrateusedinitsmeasurementartificial(188).Currently, measurementofPON1hydrolyticactivityhasgenerallybeenmore closelyassociatedwithASCVDthanPON1proteinconcentration, becausethespecificactivityofPON1isvariable,forexamplein diabetes(seeearlierdiscussion).PON1hydrolysesphenylacetateata muchhigherratethanparaoxon.ThePON1192polymorphismdoes notaffectthehydrolysisofphenylacetatebutdoesthatofparaoxon. Ontheotherhand,ifthetruephysiologicalroleofPON1isits lactonaseactivity,thenpotentiallytherecouldbeadvantagestousing lactones,suchasdihydrocoumarinorhomocysteinethiolactone, asassaysubstrates(73–75).However,thishasyettobeproven. Undoubtedlytoo,mistakes,suchastheuseofplasmaratherthan serumandinclusionofBesteraseandnon-specifichydrolysisinsome methodsfordeterminationofAesterase(PON1)activity,haveled tosomeconfusion.Acarefullyconductedlaboratoryinvestigation
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 09 frontiersin.org 168
usingavarietyofcandidatesubstrates(189–192)linkedtoan epidemiologicalstudyisrequired.
MeasurementofserumPON1activityalsoprovidesanindication ofwheretheHDLpresentinindividualsisinthespectrumof pro-toanti-inflammatoryandpro-toanti-atheroscleroticcapacity (100, 193).Cholesteroleffluxcapacityisanotherindicator,butits measurementismoredifficultandmorepronetoerror(194).Because decreasedPON1activityisfrequentlyassociatedwithincreasedSAA inHDLtheratioofSAAconcentrationtoPON1activityhasbeen proposedasbetterindexofthetypeofHDLpresentthaneither measurementsingly(148).
Conclusion
SerumPON1activityisinverselyassociatedwithASCVD incidencebothinhumanandanimalstudies.Althoughthiswas discoveredduetothepresenceonHDLofPON1anditscontribution totheanti-oxidativecapacityofHDL,thelactonaseactivityofPON1 mayalsocontributetothemechanismbywhichitreducesASCVD risk.PON1providesapotentialadditionalmeansofclinicalASCVD riskassessmentandisanindicatoroftheextenttowhichHDLhas retaineditsanti-atherogenicandanti-inflammatoryproperties.Ithas thepotentialfortherapeuticexploitation.
References
1.DurringtonP,MacknessB,MacknessM.Paraoxonaseandatherosclerosis. ArteriosclerThrombVascBiol. (2001)21:473–80.doi:10.1161/01.ATV.21.4.473
2.AldridgeW.Serumesterases.II.Anenzymehydrolysingdiethylp-nitrophenyl phosphate(E600)anditsidentitywiththeA-esteraseofmammaliansera. BiochemJ. (1953)53:117–24.doi:10.1042/bj0530117
3.FurlongC.Paraoxonase:anhistoricalperspective.In:RidleyA,FramptonJ editors. Theparaoxonases:theirroleindiseasedevelopmentandxenobioticmetabolism Dordrecht:Springer(2008).
4.DraganovD,LaDuB.Pharmacogeneticsofparaoxonases:abriefreview. Naunyn SchmiedebergsArchPharmacol. (2004)369:78–88.doi:10.1007/s00210-003-0833-1
5.MacknessM,HallamS,PeardT,WarnerS,WalkerC.Theseparationofsheepand humanserum“A”-esteraseactivityintothelipoproteinfractionbyultracentrifugation. CompBiochemPhysiolB. (1985)82:675–7.doi:10.1016/0305-0491(85)90506-1
6.McElveenJ,MacknessM,ColleyC,PeardT,WarnerS,WalkerC.Distributionof paraoxonhydrolyticactivityintheserumofpatientsaftermyocardialinfarction. Clin Chem. (1986)32:671–3.doi:10.1093/clinchem/32.4.671
7.PatelB,MacknessM,HartyD,ArrolS,Boot-HandfordR,DurringtonP.Serum esteraseactivitiesandhyperlipidaemiainthestreptozotocin-diabeticrat. Biochim BiophysActa. (1990)1035:113–6.doi:10.1016/0304-4165(90)90182-V
8.MacknessM,HartyD,BhatnagarD,WinocourP,ArrolS,IsholaM,etal.Serum paraoxonaseactivityinfamilialhypercholesterolaemiaandinsulin-dependentdiabetes mellitus. Atherosclerosis. (1991)86:193–9.doi:10.1016/0021-9150(91)90215-O
9.vonEckardsteinA.LDLContributestoreversecholesteroltransport. CircRes. (2020) 127:793–5.doi:10.1161/CIRCRESAHA.120.317721
10.HesslerJ,RobertsonAJr,ChisolmGIII.LDL-inducedcytotoxicityandits inhibitionbyHDLinhumanvascularsmoothmuscleandendothelialcellsinculture. Atherosclerosis. (1979)32:213–29.doi:10.1016/0021-9150(79)90166-7
11.KlimovA,NikiforovaA,PleskovV,Kuz’minA,KalashnikovaN.[Protectiveeffect ofhighdensitylipoproteins,theirsubfractionsandlecithin-cholesterol-acyltransferases ontheperoxidationmodificationoflowdensitylipoproteins]. Biokhimiia. (1989)54: 118–23.
12.OhtaT,TakataK,HoriuchiS,MorinoY,MatsudaI.Protectiveeffectof lipoproteinscontainingapoproteinA-IonCu2+-catalyzedoxidationofhumanlow densitylipoprotein. FEBSLett. (1989)257:435–8.doi:10.1016/0014-5793(89)81590-X
13.ParthasarathyS,BarnettJ,FongL.High-densitylipoproteininhibitstheoxidative modificationoflow-densitylipoprotein. BiochimBiophysActa. (1990)1044:275–83. doi:10.1016/0005-2760(90)90314-N
Authorcontributions
PDconceptualisedthestudy,performedtheliteraturesearch, wrotethedraft,andfinalisedthemanuscript.BBandHSperformed literaturesearches,contributedtowriting,revisedthedraft, proofreadthemanuscript,anddesignedthefigures.Allauthors contributedtothearticleandapprovedthesubmittedversion.
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcouldbe construedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthereviewers. Anyproductthatmaybeevaluatedinthisarticle,orclaimthatmay bemadebyitsmanufacturer,isnotguaranteedorendorsedbythe publisher.
14.NavabM,ImesS,HamaS,HoughG,RossL,BorkR,etal.Monocytetransmigration inducedbymodificationoflowdensitylipoproteinincoculturesofhumanaorticwall cellsisduetoinductionofmonocytechemotacticprotein1synthesisandisabolishedby highdensitylipoprotein. JClinInvest. (1991)88:2039–46.doi:10.1172/JCI115532
15.DurringtonP.NewideasabouttheroleofHDL.In:ShepherdJ,PackardC,Brownlie Seditors. Lipoproteinsandthepathogenesisofatherosclerosis.Amsterdam:Excerpta Medica(1991).
16.el-SaadaniM,EsterbauerH,el-SayedM,GoherM,NassarA,JürgensG.A spectrophotometricassayforlipidperoxidesinserumlipoproteinsusingacommercially availablereagent. JLipidRes. (1989)30:627–30.doi:10.1016/S0022-2275(20)38354-1
17.MacknessM,ArrolS,DurringtonP.Paraoxonasepreventsaccumulationof lipoperoxidesinlow-densitylipoprotein. FEBSLett. (1991)286:152–4.doi:10.1016/ 0014-5793(91)80962-3
18.MacknessM,ArrolS,AbbottC,DurringtonP.Protectionoflow-densitylipoprotein againstoxidativemodificationbyhigh-densitylipoproteinassociatedparaoxonase. Atherosclerosis. (1993)104:129–35.doi:10.1016/0021-9150(93)90183-U
19.MacknessM,ArrolS,AbbottC,DurringtonP.Isparaoxonaserelatedto atherosclerosis. ChemBiolInteract. (1993)87:161–71.doi:10.1016/0009-2797(93) 90038-Z
20.MacknessM,DurringtonP.HDL,itsenzymesanditspotentialtoinfluencelipid peroxidation. Atherosclerosis. (1995)115:243–53.doi:10.1016/0021-9150(94)05524-M
21.MacknessM,AbbottC,ArrolS,DurringtonP.Theroleofhigh-densitylipoprotein andlipid-solubleantioxidantvitaminsininhibitinglow-densitylipoproteinoxidation. BiochemJ. (1993)294:829–34.doi:10.1042/bj2940829
22.KlimovA,GurevichV,NikiforovaA,ShatilinaL,KuzminA,PlavinskyS,etal. Antioxidativeactivityofhighdensitylipoproteins invivo Atherosclerosis. (1993)100:13–8.doi:10.1016/0021-9150(93)90063-Z
23.WatsonA,BerlinerJ,HamaS,LaDuB,FaullK,FogelmanA,etal.Protective effectofhighdensitylipoproteinassociatedparaoxonase.Inhibitionofthebiological activityofminimallyoxidizedlowdensitylipoprotein. JClinInvest. (1995)96:2882–91. doi:10.1172/JCI118359
24.SangvanichP,MacknessB,GaskellS,DurringtonP,MacknessM.Theeffectof high-densitylipoproteinsontheformationoflipid/proteinconjugatesduring invitro oxidationoflow-densitylipoprotein. BiochemBiophysResCommun. (2003)300:501–6. doi:10.1016/S0006-291X(02)02849-8
25.GarnerB,WaldeckA,WittingP,RyeK,StockerR.Oxidationofhighdensity lipoproteins.II.Evidencefordirectreductionoflipidhydroperoxidesbymethionine
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 10 frontiersin.org 169
residuesofapolipoproteinsAIandAII. JBiolChem. (1998)273:6088–95.doi:10.1074/ jbc.273.11.6088
26.HayekT,OiknineJ,DanknerG,BrookJ,AviramM.HDLapolipoproteinA-I attenuatesoxidativemodificationoflowdensitylipoprotein:studiesintransgenicmice. EurJClinChemClinBiochem. (1995)33:721–5.doi:10.1515/cclm.1995.33.10.721
27.ArrolS,MacknessMI,DurringtonPN.Highdensitylipoprotein-associatedenzymes andthepreventionoflowerdensitylipoproteinoxidation. EurJLabMed. (1995)4:33–8.
28.HineD,MacknessB,MacknessM.CoincubationofPON1,APOA1,andLCAT increasesthetimeHDLisabletopreventLDLoxidation. IUBMBLife. (2012)64:157–61. doi:10.1002/iub.588
29.NearyR,BhatnagarD,DurringtonP,IsholaM,ArrolS,MacknessM.An investigationoftheroleoflecithin:cholesterolacyltransferaseandtriglyceride-rich lipoproteinsinthemetabolismofpre-betahighdensitylipoproteins. Atherosclerosis. (1991)89:35–48.doi:10.1016/0021-9150(91)90005-N
30.SoranH,SchofieldJ,LiuY,DurringtonP.HowHDLprotectsLDLagainst atherogenicmodification:paraoxonase1andotherdramatispersonae. CurrOpin Lipidol. (2015)26:247–56.doi:10.1097/MOL.0000000000000194
31.JamesR,BlatterGarinM,CalabresiL,MiccoliR,vonEckardsteinA,Tilly-Kiesi M,etal.Modulatedserumactivitiesandconcentrationsofparaoxonaseinhighdensity lipoproteindeficiencystates. Atherosclerosis. (1998)139:77–82.doi:10.1016/S00219150(98)00058-6
32.HolleboomA,DaniilG,FuX,ZhangR,HovinghG,SchimmelA,etal.Lipid oxidationincarriersoflecithin:cholesterolacyltransferasegenemutations. Arterioscler ThrombVascBiol. (2012)32:3066–75.doi:10.1161/ATVBAHA.112.255711
33.FurbeeJJr,SawyerJ,ParksJ.Lecithin:cholesterolacyltransferasedeficiencyincreases atherosclerosisinthelowdensitylipoproteinreceptorandapolipoproteinEknockout mice. JBiolChem. (2002)277:3511–9.doi:10.1074/jbc.M109883200
34.MooreR,NavabM,MillarJ,ZimettiF,HamaS,RothblatG,etal.Increased atherosclerosisinmicelackingapolipoproteinA-Iattributabletobothimpairedreverse cholesteroltransportandincreasedinflammation. CircRes. (2005)97:763–71.doi:10. 1161/01.RES.0000185320.82962.F7
35.ShihD,XiaY,WangX,MillerE,CastellaniL,SubbanagounderG,etal. Combinedserumparaoxonaseknockout/apolipoproteinEknockoutmiceexhibit increasedlipoproteinoxidationandatherosclerosis. JBiolChem. (2000)275:17527–35. doi:10.1074/jbc.M910376199
36.AviramM,RosenblatM,BisgaierC,NewtonR,Primo-ParmoS,LaDuB. Paraoxonaseinhibitshigh-densitylipoproteinoxidationandpreservesitsfunctions. Apossibleperoxidativeroleforparaoxonase. JClinInvest. (1998)101:1581–90.doi: 10.1172/JCI1649
37.MacknessM,MacknessB,ArrolS,WoodG,BhatnagarD,DurringtonP.Presence ofparaoxonaseinhumaninterstitialfluid. FEBSLett. (1997)416:377–80.doi:10.1016/ S0014-5793(97)01243-X
38.DeakinS,BiolettoS,Bochaton-PiallatM,JamesR.HDL-associatedparaoxonase-1 canredistributetocellmembranesandinfluencesensitivitytooxidativestress. FreeRadic BiolMed. (2011)50:102–9.doi:10.1016/j.freeradbiomed.2010.09.002
39.MacknessB,HuntR,DurringtonP,MacknessM.Increasedimmunolocalization ofparaoxonase,clusterin,andapolipoproteinA-Iinthehumanarterywallwiththe progressionofatherosclerosis. ArteriosclerThrombVascBiol. (1997)17:1233–8.doi: 10.1161/01.ATV.17.7.1233
40.GrahamA,HassallD,RafiqueS,OwenJ.Evidenceforaparaoxonaseindependentinhibitionoflow-densitylipoproteinoxidationbyhigh-densitylipoprotein. Atherosclerosis. (1997)135:193–204.doi:10.1016/S0021-9150(97)00162-7
41.ConnellyP,DraganovD,MaguireG.Paraoxonase-1doesnotreduceormodify oxidationofphospholipidsbyperoxynitrite. FreeRadicBiolMed. (2005)38:164–74. doi:10.1016/j.freeradbiomed.2004.10.010
42.TeiberJ,DraganovD,LaDuB.PurifiedhumanserumPON1doesnotprotectLDL againstoxidationinthe invitro assaysinitiatedwithcopperorAAPH. JLipidRes. (2004) 45:2260–8.doi:10.1194/jlr.M400213-JLR200
43.DraganovD,TeiberJ,SpeelmanA,OsawaY,SunaharaR,LaDuB.Human paraoxonases(PON1,PON2,andPON3)arelactonaseswithoverlappinganddistinct substratespecificities. JLipidRes. (2005)46:1239–47.doi:10.1194/jlr.M400511-JLR200
44.LiuY,MacknessB,MacknessM.Comparisonoftheabilityofparaoxonases1and3 toattenuatethe invitro oxidationoflow-densitylipoproteinandreducemacrophage oxidativestress. FreeRadicBiolMed. (2008)45:743–8.doi:10.1016/j.freeradbiomed. 2008.05.024
45.AviramM,BilleckeS,SorensonR,BisgaierC,NewtonR,RosenblatM,etal. ParaoxonaseactivesiterequiredforprotectionagainstLDLoxidationinvolvesitsfree sulfhydrylgroupandisdifferentfromthatrequiredforitsarylesterase/paraoxonase activities:selectiveactionofhumanparaoxonaseallozymesQandR. ArteriosclerThromb VascBiol. (1998)18:1617–24.doi:10.1161/01.ATV.18.10.1617
46.RodrigoL,MacknessB,DurringtonP,HernandezA,MacknessM.Hydrolysisof platelet-activatingfactorbyhumanserumparaoxonase. BiochemJ. (2001)354(Pt.1):1–7. doi:10.1042/bj3540001
47.ChistiakovD,MelnichenkoA,OrekhovA,BobryshevY.Paraoxonaseand atherosclerosis-relatedcardiovasculardiseases. Biochimie. (2017)132:19–27.doi:10. 1016/j.biochi.2016.10.010
48.BrophyV,JarvikGP,FurlongCE. Paraoxonase(PON1)inhealthanddisease:basic andclinicalaspects.Boston:KluwerAcademicPublishers(2002).
49.LaDuB,AviramM,BilleckeS,NavabM,Primo-ParmoS,SorensonR,etal.Onthe physiologicalrole(s)oftheparaoxonases. ChemBiolInteract. (1999)119–120:379–88. doi:10.1016/S0009-2797(99)00049-6
50.RuizJ,BlanchéH,JamesR,GarinM,VaisseC,CharpentierG,etal.Gln-Arg192 polymorphismofparaoxonaseandcoronaryheartdiseaseintype2diabetes. Lancet. (1995)346:869–72.doi:10.1016/S0140-6736(95)92709-3
51.MacknessB,MacknessM,ArrolS,TurkieW,DurringtonP.Effectofthehuman serumparaoxonase55and192geneticpolymorphismsontheprotectionbyhighdensity lipoproteinagainstlowdensitylipoproteinoxidativemodification. FEBSLett. (1998) 423:57–60.doi:10.1016/S0014-5793(98)00064-7
52.MacknessM,ArrolS,MacknessB,DurringtonP.Alloenzymesofparaoxonaseand effectivenessofhigh-densitylipoproteinsinprotectinglow-densitylipoproteinagainst lipidperoxidation. Lancet. (1997)349:851–2.doi:10.1016/S0140-6736(05)61755-2
53.DeakinS,LevievI,NicaudV,BrulhartMeynetM,TiretL,JamesR.Paraoxonase1L55Mpolymorphismisassociatedwithanabnormaloralglucosetolerancetest anddifferentiateshighriskcoronarydiseasefamilies. JClinEndocrinolMetab. (2002) 87:1268–73.doi:10.1210/jcem.87.3.8335
54.LevievI,PoirierO,NicaudV,EvansA,KeeF,ArveilerD,etal.Highexpressor paraoxonasePON1genepromoterpolymorphismsareassociatedwithreducedriskof vasculardiseaseinyoungercoronarypatients. Atherosclerosis. (2002)161:463–7.doi: 10.1016/S0021-9150(01)00668-2
55.SoranH,YounisN,Charlton-MenysV,DurringtonP.Variationinparaoxonase1activityandatherosclerosis. CurrOpinLipidol. (2009)20:265–74.doi:10.1097/MOL. 0b013e32832ec141
56.MahroozA,MacknessM.Epigeneticsofparaoxonases. CurrOpinLipidol. (2020) 31:200–5.doi:10.1097/MOL.0000000000000687
57.CostaL,VitaloneA,ColeT,FurlongC.Modulationofparaoxonase(PON1)activity. BiochemPharmacol. (2005)69:541–50.doi:10.1016/j.bcp.2004.08.027
58.MacknessB,DaviesG,TurkieW,LeeE,RobertsD,HillE,etal.Paraoxonasestatus incoronaryheartdisease:areactivityandconcentrationmoreimportantthangenotype? ArteriosclerThrombVascBiol. (2001)21:1451–7.doi:10.1161/hq0901.094247
59.WheelerJ,KeavneyB,WatkinsH,CollinsR,DaneshJ.Fourparaoxonasegene polymorphismsin11212casesofcoronaryheartdiseaseand12786controls:metaanalysisof43studies. Lancet. (2004)363:689–95.doi:10.1016/S0140-6736(04)15 642-0
60.AshiqS,AshiqK.Theroleofparaoxonase1(PON1)genepolymorphismsin coronaryarterydisease:asystematicreviewandmeta-analysis. BiochemGenet. (2021) 59:919–39.doi:10.1007/s10528-021-10043-0
61.ZengQ,ZengJ.Ameta-analysisonrelationshipbetweenparaoxonase1 polymorphismsandatheroscleroticcardiovasculardiseases. LifeSci. (2019)232:116646. doi:10.1016/j.lfs.2019.116646
62.LuoZ,PuL,MuhammadI,ChenY,SunX.AssociationsofthePON1rs662 polymorphismwithcirculatingoxidizedlow-densitylipoproteinandlipidlevels:a systematicreviewandmeta-analysis. LipidsHealthDis. (2018)17:281.doi:10.1186/ s12944-018-0937-8
63.AyubA,MacknessM,ArrolS,MacknessB,PatelJ,DurringtonP.Serum paraoxonaseaftermyocardialinfarction. ArteriosclerThrombVascBiol. (1999)19:330–5. doi:10.1161/01.ATV.19.2.330
64.JarvikG,RozekL,BrophyV,HatsukamiT,RichterR,SchellenbergG,etal. Paraoxonase(PON1)phenotypeisabetterpredictorofvasculardiseasethanis PON1(192)orPON1genotype. ArteriosclerThrombVascBiol. (2000)20:2441–7.doi: 10.1161/01.ATV.20.11.2441
65.MacknessB,DurringtonP,McElduffP,YarnellJ,AzamN,WattM,etal.Low paraoxonaseactivitypredictscoronaryeventsinthecaerphillyprospectivestudy. Circulation. (2003)107:2775–9.doi:10.1161/01.CIR.0000070954.00271.13
66.BirjmohunR,VergeerM,StroesE,SandhuM,RickettsS,TanckM,etal.Both paraoxonase-1genotypeandactivitydonotpredicttheriskoffuturecoronaryartery disease;theEPIC-Norfolkprospectivepopulationstudy. PLoSOne. (2009)4:e6809. doi:10.1371/journal.pone.0006809
67.TangW,HartialaJ,FanY,WuY,StewartA,ErdmannJ,etal.Clinicalandgenetic associationofserumparaoxonaseandarylesteraseactivitieswithcardiovascularrisk. ArteriosclerThrombVascBiol. (2012)32:2803–12.doi:10.1161/ATVBAHA.112.253930
68.vanHimbergenT,vanderSchouwY,VoorbijH,vanTitsL,StalenhoefA,Peeters P,etal.Paraoxonase(PON1)andtheriskforcoronaryheartdiseaseandmyocardial infarctioninageneralpopulationofDutchwomen. Atherosclerosis. (2008)199:408–14. doi:10.1016/j.atherosclerosis.2007.11.018
69.TroughtonJ,WoodsideJ,YarnellJ,ArveilerD,AmouyelP,FerrièresJ,etal. Paraoxonaseactivityandcoronaryheartdiseaseriskinhealthymiddle-agedmales:the PRIMEstudy. Atherosclerosis. (2008)197:556–63.doi:10.1016/j.atherosclerosis.2007.0 8.019
70.KunutsorS,BakkerS,JamesR,DullaartR.Serumparaoxonase-1activityandriskof incidentcardiovasculardisease:thePREVENDstudyandmeta-analysisofprospective populationstudies. Atherosclerosis. (2016)245:143–54.doi:10.1016/j.atherosclerosis. 2015.12.021
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 11 frontiersin.org 170
71.CorsettiJ,SparksC,JamesR,BakkerS,DullaartR.Lowserumparaoxonase-1 activityassociateswithincidentcardiovasculardiseaseriskinsubjectswithconcurrently highlevelsofhigh-densitylipoproteincholesterolandC-reactiveprotein. JClinMed. (2019)8:1357.doi:10.3390/jcm8091357
72.BhattacharyyaT,NichollsS,TopolE,ZhangR,YangX,SchmittD,etal.Relationship ofparaoxonase1(PON1)genepolymorphismsandfunctionalactivitywithsystemic oxidativestressandcardiovascularrisk. JAMA. (2008)299:1265–76.doi:10.1001/jama. 299.11.1265
73.Perła-KajánJ,BorowczykK,GłowackiR,NygårdO,JakubowskiH.Paraoxonase1 Q192Rgenotypeandactivityaffecthomocysteinethiolactonelevelsinhumans. FASEBJ. (2018)doi:10.1096/fj.201800346R[Epubaheadofprint].
74.Murillo-GonzálezF,Ponce-RuizN,Rojas-GarcíaA,RothenbergS,BernalHernándezY,Cerda-FloresR,etal.PON1lactonaseactivityanditsassociationwith cardiovasculardisease. ClinChimActa. (2020)500:47–53.doi:10.1016/j.cca.2019.09.016
75.PetricB,KunejT,BavecAA.Multi-omicsanalysisofPON1lactonaseactivityin relationtohumanhealthanddisease. Omics. (2021)25:38–51.doi:10.1089/omi.2020. 0160
76.JakubowskiH.Homocysteinemodificationinproteinstructure/functionand humandisease. PhysiolRev. (2018)99:555–604.doi:10.1152/physrev.00003.2018
77.BorowczykK,PiechockaJ,GłowackiR,DharI,MidtunØ,TellG,etal.Urinary excretionofhomocysteinethiolactoneandtheriskofacutemyocardialinfarctionin coronaryarterydiseasepatients:theWENBITtrial. JInterMed. (2019)285:232–44. doi:10.1111/joim.12834
78.SmithJ. Thetheoryofevolution.3rded.Cambridge:CambridgeUniversityPress (1975).
79.CherryN,MacknessM,DurringtonP,PoveyA,DippnallM,SmithT,etal. Paraoxonase(PON1)polymorphismsinfarmersattributingillhealthtosheepdip. Lancet. (2002)359:763–4.doi:10.1016/S0140-6736(02)07847-9
80.ZanchettG,Oliveira-FilhoE.Cyanobacteriaandcyanotoxins:fromimpactson aquaticecosystemsandhumanhealthtoanticarcinogeniceffects. Toxins. (2013)5:1896–917.doi:10.3390/toxins5101896
81.VenterJ,diPorzioU,RobinsonD,ShreeveS,LaiJ,KerlavageA,etal.Evolution ofneurotransmitterreceptorsystems. ProgNeurobiol. (1988)30:105–69.doi:10.1016/ 0301-0082(88)90004-4
82.BlumM,ChenJ.Structuralcharacterizationofthecatalyticcalcium-bindingsite indiisopropylfluorophosphatase(DFPase)–comparisonwithrelatedbeta-propeller enzymes. ChemBiolInteract. (2010)187:373–9.doi:10.1016/j.cbi.2010.02.043
83.ThakurM,MedintzI,WalperS.Enzymaticbioremediationoforganophosphate compounds-progressandremainingchallenges. FrontBioengBiotechnol. (2019)7:289. doi:10.3389/fbioe.2019.00289
84.HiblotJ,GotthardG,ChabriereE,EliasM.Characterisationoftheorganophosphate hydrolasecatalyticactivityofSsoPox. SciRep. (2012)2:779.doi:10.1038/srep00779
85.LeiX,ZhuJ,ChengW,BaoY,HoY,ReddiA,etal.Paradoxicalrolesofantioxidant enzymes:basicmechanismsandhealthimplications. PhysiolRev. (2016)96:307–64. doi:10.1152/physrev.00010.2014
86.EliasM,TawfikD.Divergenceandconvergenceinenzymeevolution:parallel evolutionofparaoxonasesfromquorum-quenchinglactonases. JBiolChem. (2012) 287:11–20.doi:10.1074/jbc.R111.257329
87.McCullyK.Homocysteineandthepathogenesisofatherosclerosis. ExpertRevClin Pharmacol. (2015)8:211–9.doi:10.1586/17512433.2015.1010516
88.MarsillachJ,SuzukiS,RichterR,McDonaldM,RademacherP,MacCossM, etal.Humanvalacyclovirhydrolase/biphenylhydrolase-likeproteinisahighlyefficient homocysteinethiolactonase. PLoSOne. (2014)9:e110054.doi:10.1371/journal.pone.
0110054
89.CampsJ,CastañéH,Rodríguez-TomàsE,Baiges-GayaG,Hernández-AguileraA, ArenasM,etal.Ontheroleofparaoxonase-1andchemokineligand2(C-Cmotif)in metabolicalterationslinkedtoinflammationanddisease.A2021update. Biomolecules. (2021)11:971.doi:10.3390/biom11070971
90.CampsJ,IftimieS,García-HerediaA,CastroA,JovenJ.Paraoxonasesandinfectious diseases. ClinBiochem. (2017)50:804–11.doi:10.1016/j.clinbiochem.2017.04.016
91.HerrickJ.Landmarkarticle(JAMA1912).Clinicalfeaturesofsuddenobstruction ofthecoronaryarteries.ByJamesB.Herrick. JAMA. (1983)250:1757–65.doi:10.1001/ jama.250.13.1757
92.MacknessB,DurringtonP,MacknessM.Lackofprotectionagainstoxidative modificationofLDLbyavianHDL. BiochemBiophysResCommun. (1998)247:443–6. doi:10.1006/bbrc.1998.8803
93.MeyerW,JamisonJ,RichterR,WoodsS,ParthaR,KowalczykA,etal.Ancient convergentlossesofparaoxonase1yieldpotentialrisksformodernmarinemammals. Science. (2018)361:591–4.doi:10.1126/science.aap7714
94.AmaniM,DarbinA,PezeshkianM,AfrasiabiA,SafaieN,JodatiA,etal.Theroleof cholesterol-enricheddietandparaoxonase1inhibitioninatherosclerosisprogression. J CardiovascThoracRes. (2017)9:133–9.doi:10.15171/jcvtr.2017.23
95.RadtkeK. MethodofusingPON-1todecreaseatheromaformation.USApatentUS 6,391,298B1.Pittsburgh,PA:BayerCorporation(2002).
96.ZhangC,PengW,WangM,ZhuJ,ZangY,ShiW,etal.Studiesonprotectiveeffects ofhumanparaoxonases1and3onatherosclerosisinapolipoproteinEknockoutmice. GeneTher. (2010)17:626–33.doi:10.1038/gt.2010.11
97.MacknessB,QuarckR,VerrethW,MacknessM,HolvoetP.Humanparaoxonase1overexpressioninhibitsatherosclerosisinamousemodelofmetabolicsyndrome. ArteriosclerThrombVascBiol. (2006)26:1545–50.doi:10.1161/01.ATV.0000222924. 62641.aa
98.GunsP,VanAsscheT,VerrethW,FransenP,MacknessB,MacknessM,etal. Paraoxonase1genetransferlowersvascularoxidativestressandimprovesvasomotor functioninapolipoproteinE-deficientmicewithpre-existingatherosclerosis. BrJ Pharmacol. (2008)153:508–16.doi:10.1038/sj.bjp.0707585
99.SheZ,ZhengW,WeiY,ChenH,WangA,LiH,etal.Humanparaoxonasegene clustertransgenicoverexpressionrepressesatherogenesisandpromotesatherosclerotic plaquestabilityinApoE-nullmice. CircRes. (2009)104:1160–8.doi:10.1161/ CIRCRESAHA.108.192229
100.IkhlefS,BerrouguiH,KamtchuengSimoO,ZerifE,KhalilA.Humanparaoxonase 1overexpressioninmicestimulatesHDLcholesteroleffluxandreversecholesterol transport. PLoSOne. (2017)12:e0173385.doi:10.1371/journal.pone.0173385
101.BurilloE,TarinC,Torres-FonsecaM,Fernandez-GarcíaC,Martinez-Pinna R,Martinez-LopezD,etal.Paraoxonase-1overexpressionpreventsexperimental abdominalaorticaneurysmprogression. ClinSci. (2016)130:1027–38.doi:10.1042/ CS20160185
102.BaiJ,ZhouH,YangX,LiuH,MengY.Inhibitoryeffectoftheparaoxonasegeneon theformationofrabbitcoronaryatherosclerosis. AsianPacJTropMed. (2013)6:544–7. doi:10.1016/S1995-7645(13)60093-0
103.MiyoshiM,NakanoY,SakaguchiT,OgiH,OdaN,SuenariK,etal.Genedelivery ofparaoxonase-1inhibitsneointimalhyperplasiaafterarterialballoon-injuryinrabbits fedahigh-fatdiet. HypertensRes. (2007)30:85–91.doi:10.1291/hypres.30.85
104.WangF,XueJ,WangD,WangX,LuS,TanM.Treatmentofatherosclerosis bytransplantationofboneendothelialprogenitorcellsover-expressedparaoxonase-1 genebyrecombinantadeno-associatedvirusinrat. BiolPharmBull. (2010)33:1806–13. doi:10.1248/bpb.33.1806
105.Gamliel-LazarovichA,AbassiZ,KhatibS,TavoriH,VayaJ,AviramM, etal.Paraoxonase1deficiencyinmiceisassociatedwithhypotensionandincreased levelsof5,6-epoxyeicosatrienoicacid. Atherosclerosis. (2012)222:92–8.doi:10.1016/j. atherosclerosis.2012.01.047
106.MacknessM,BoullierA,HennuyerN,MacknessB,HallM,TailleuxA,etal. Paraoxonaseactivityisreducedbyapro-atheroscleroticdietinrabbits. BiochemBiophys ResCommun. (2000)269:232–6.doi:10.1006/bbrc.2000.2265
107.Lund-KatzS,PhillipsM.Highdensitylipoproteinstructure-functionandrolein reversecholesteroltransport. SubcellBiochem. (2010)51:183–227.doi:10.1007/978-90481-8622-8_7
108.HarelM,AharoniA,GaidukovL,BrumshteinB,KhersonskyO,MegedR,etal. Structureandevolutionoftheserumparaoxonasefamilyofdetoxifyingandantiatheroscleroticenzymes. NatStructMolBiol. (2004)11:412–9.doi:10.1038/nsmb767
109.HarelM,BerchanskyA,SussmanJL,PriluskyJ,HodisE,BrumshteinB,etal. SerumParaoxonase.(2020).Availableonlineat: https://proteopedia.org/wiki/index.php/ Serum_Paraoxonase (accessedSeptember14,2022).
110.KarabulutS,MansourB,Casanola-MartinG,RasulevB,GauldJ.Thehydrolysis rateofparaoxonase-1QandRisoenzymes:aninsilicostudybasedon invitro data. Molecules. (2022)27:6780.doi:10.3390/molecules27206780
111.EsterbauerH,GebickiJ,PuhlH,JürgensG.Theroleoflipidperoxidationand antioxidantsinoxidativemodificationofLDL. FreeRadicBiolMed. (1992)13:341–90. doi:10.1016/0891-5849(92)90181-F
112.MyungS,JuW,ChoB,OhS,ParkS,KooB,etal.Efficacyofvitaminand antioxidantsupplementsinpreventionofcardiovasculardisease:systematicreviewand meta-analysisofrandomisedcontrolledtrials. BMJ. (2013)346:f10.doi:10.1136/bmj.f10
113.SpitellerP,KernW,ReinerJ,SpitellerG.Aldehydiclipidperoxidationproducts derivedfromlinoleicacid. BiochimBiophysActa. (2001)1531:188–208.doi:10.1016/ S1388-1981(01)00100-7
114.FrankelE. Lipidoxidation.Sawston:WoodheadPublishing(2003).
115.YoshidaY,UmenoA,AkazawaY,ShichiriM,MurotomiK,HorieM.Chemistryof lipidperoxidationproductsandtheiruseasbiomarkersinearlydetectionofdiseases. J OleoSci. (2015)64:347–56.doi:10.5650/jos.ess14281
116.AhmedZ,RavandiA,MaguireG,EmiliA,DraganovD,LaDuB,etal.Multiple substratesforparaoxonase-1duringoxidationofphosphatidylcholinebyperoxynitrite. BiochemBiophysResCommun. (2002)290:391–6.doi:10.1006/bbrc.2001.6150
117.PoznyakA,NikiforovN,MarkinA,KashirskikhD,MyasoedovaV,Gerasimova E,etal.OverviewofOxLDLanditsimpactoncardiovascularhealth:focuson atherosclerosis. FrontPharmacol. (2020)11:613780.doi:10.3389/fphar.2020.613780
118.SteinbergD,WitztumJ.Oxidizedlow-densitylipoproteinandatherosclerosis. ArteriosclerThrombVascBiol. (2010)30:2311–6.doi:10.1161/ATVBAHA.108.179697
119.GlassC,WitztumJ.Atherosclerosis.Theroadahead. Cell. (2001)104:503–16. doi:10.1016/S0092-8674(01)00238-0
120.GiladD,AtiyaS,Mozes-AutmazginZ,Ben-ShushanR,Ben-DavidR,AmramE, etal.Paraoxonase1inendothelialcellsimpairsvasodilationinducedbyarachidonic
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 12 frontiersin.org 171
acidlactonemetabolite. BiochimBiophysActaMolCellBiolLipids. (2019)1864:386–93. doi:10.1016/j.bbalip.2018.12.008
121.BerrouguiH,LouedS,KhalilA.Purifiedhumanparaoxonase-1interactswith plasmamembranelipidraftsandmediatescholesteroleffluxfrommacrophages. Free RadicBiolMed. (2012)52:1372–81.doi:10.1016/j.freeradbiomed.2012.01.019
122.ChristisonJ,RyeK,StockerR.Exchangeofoxidizedcholesteryllinoleatebetween LDLandHDLmediatedbycholesterylestertransferprotein. JLipidRes. (1995) 36:2017–26.doi:10.1016/S0022-2275(20)41119-8
123.HineD,MacknessB,MacknessM.Cholesteryl-estertransferproteinenhancesthe abilityofhigh-densitylipoproteintoinhibitlow-densitylipoproteinoxidation. IUBMB Life. (2011)63:772–4.doi:10.1002/iub.508
124.KimD,BurtA,RanchalisJ,VuleticS,VaisarT,LiW,etal.PLTPactivityinversely correlateswithCAAD:effectsofPON1enzymeactivityandgeneticvariantsonPLTP activity. JLipidRes. (2015)56:1351–62.doi:10.1194/jlr.P058032
125.MyantN. Thebiologyofcholesterolandrelatedsteroids.Oxford:ButterworthHeinemann(1981).doi:10.1016/B978-0-433-22880-6.50009-6
126.RosenblatM,GaidukovL,KhersonskyO,VayaJ,OrenR,TawfikD,etal.The catalytichistidinedyadofhighdensitylipoprotein-associatedserumparaoxonase-1 (PON1)isessentialforPON1-mediatedinhibitionoflowdensitylipoproteinoxidation andstimulationofmacrophagecholesterolefflux. JBiolChem. (2006)281:7657–65. doi:10.1074/jbc.M512595200
127.BritesF,VeronaJ,SchreierL,FruchartJ,CastroG,WikinskiR.Paraoxonase1and platelet-activatingfactoracetylhydrolaseactivitiesinpatientswithlowhdl-cholesterol levelswithorwithoutprimaryhypertriglyceridemia. ArchMedRes. (2004)35:235–40. doi:10.1016/j.arcmed.2004.02.002
128.InagakiM,Nakagawa-ToyamaY,NishidaM,NakataniK,NakaokaH,KawaseM, etal.EffectofprobucolonantioxidantpropertiesofHDLinpatientswithheterozygous familialhypercholesterolemia. JAtherosclerThromb. (2012)19:643–56.doi:10.5551/jat. 12807
129.ItabeH,ObamaT,KatoR.ThedynamicsofoxidizedLDLduringatherogenesis. J Lipids. (2011)2011:418313.doi:10.1155/2011/418313
130.RoestM,JansenA,BarendrechtA,LeusF,KasteleinJ,VoorbijH.Variationat theparaoxonasegenelocuscontributestocarotidarterialwallthicknessinsubjects withfamilialhypercholesterolemia. ClinBiochem. (2005)38:123–7.doi:10.1016/j. clinbiochem.2004.10.005
131.RoestM,RodenburgJ,WiegmanA,KasteleinJ,VoorbijH.Paraoxonasegenotype andcarotidintima-mediathicknessinchildrenwithfamilialhypercholesterolemia. Eur JCardiovascPrevRehabil. (2006)13:464–6.doi:10.1097/00149831-200606000-00025
132.RosenblatM,WardS,VolkovaN,HayekT,AviramM.VLDLtriglyceridesinhibit HDL-associatedparaoxonase1(PON1)activity: invitro and invivo studies. Biofactors. (2012)38:292–9.doi:10.1002/biof.1021
133.RozekL,HatsukamiT,RichterR,RanchalisJ,NakayamaK,McKinstryL,etal.The correlationofparaoxonase(PON1)activitywithlipidandlipoproteinlevelsdifferswith vasculardiseasestatus. JLipidRes. (2005)46:1888–95.doi:10.1194/jlr.M400489-JLR200
134.vanHimbergenT,vanTitsL,HectorsM,deGraafJ,RoestM,StalenhoefA. Paraoxonase-1andlinoleicacidoxidationinfamilialhypercholesterolemia. Biochem BiophysResCommun. (2005)333:787–93.doi:10.1016/j.bbrc.2005.05.176
135.AbbottC,MacknessM,KumarS,OlukogaA,GordonC,ArrolS,etal. Relationshipbetweenserumbutyrylcholinesteraseactivity,hypertriglyceridaemiaand insulinsensitivityindiabetesmellitus. ClinSci. (1993)85:77–81.doi:10.1042/cs0850077
136.AbbottC,MacknessM,KumarS,BoultonA,DurringtonP.Serumparaoxonase activity,concentration,andphenotypedistributionindiabetesmellitusandits relationshiptoserumlipidsandlipoproteins. ArteriosclerThrombVascBiol. (1995) 15:1812–8.doi:10.1161/01.ATV.15.11.1812
137.MacknessB,MacknessM,ArrolS,TurkieW,JulierK,AbuashaB,etal. Serumparaoxonase(PON1)55and192polymorphismandparaoxonaseactivity andconcentrationinnon-insulindependentdiabetesmellitus. Atherosclerosis. (1998) 139:341–9.doi:10.1016/S0021-9150(98)00095-1
138.MacknessB,DurringtonP,AbuashiaB,BoultonA,MacknessM.Lowparaoxonase activityintypeIIdiabetesmellituscomplicatedbyretinopathy. ClinSci. (2000)98:355–63.doi:10.1042/cs0980355
139.MacknessB,DurringtonP,BoultonA,HineD,MacknessM.Serumparaoxonase activityinpatientswithtype1diabetescomparedtohealthycontrols. EurJClinInvest. (2002)32:259–64.doi:10.1046/j.1365-2362.2002.00977.x
140.WuD,WuC,ZhongY.Theassociationbetweenparaoxonase1activity andthesusceptibilitiesofdiabetesmellitus,diabeticmacroangiopathyanddiabetic microangiopathy. JCellMolMed. (2018)22:4283–91.doi:10.1111/jcmm.13711
141.SrikanthanK,FeyhA,VisweshwarH,ShapiroJ,SodhiK.Systematicreviewof metabolicsyndromebiomarkers:apanelforearlydetection,management,andrisk stratificationinthewestvirginianpopulation. IntJMedSci. (2016)13:25–38.doi: 10.7150/ijms.13800
142.HashemiM,MousaviE,Arab-BafraniZ,NezhadebrahimiA,MarjaniA.Themost effectivepolymorphismsofparaoxonase-1geneonenzymeactivityandconcentrationof paraoxonase-1proteinintype2diabetesmellituspatientsandnon-diabeticindividuals: asystematicreviewandmeta-analysis. DiabetesResClinPract. (2019)152:135–45.doi: 10.1016/j.diabres.2019.05.007
143.Koren-GluzerM,AviramM,MeilinE,HayekT.TheantioxidantHDL-associated paraoxonase-1(PON1)attenuatesdiabetesdevelopmentandstimulates β-cellinsulin release. Atherosclerosis. (2011)219:510–8.doi:10.1016/j.atherosclerosis.2011.07.119
144.ShokriY,VarijiA,NosratiM,Khonakdar-TarsiA,KianmehrA,KashiZ,etal. Importanceofparaoxonase1(PON1)asanantioxidantandantiatherogenicenzyme inthecardiovascularcomplicationsoftype2diabetes:genotypicandphenotypic evaluation. DiabetesResClinPract. (2020)161:108067.doi:10.1016/j.diabres.2020. 108067
145.VaisarT,KanterJ,WimbergerJ,IrwinA,GauthierJ,WolfsonE,etal.High concentrationofmedium-sizedHDLparticlesandenrichmentinHDLparaoxonase1 associatewithprotectionfromvascularcomplicationsinpeoplewithlong-standingtype 1diabetes. DiabetesCare. (2020)43:178–86.doi:10.2337/dc19-0772
146.BarterP,NichollsS,RyeK,AnantharamaiahG,NavabM,FogelmanA. AntiinflammatorypropertiesofHDL. CircRes. (2004)95:764–72.doi:10.1161/01.RES. 0000146094.59640.13
147.RibasV,Sánchez-QuesadaJ,AntónR,CamachoM,JulveJ,Escolà-GilJ,etal. HumanapolipoproteinA-IIenrichmentdisplacesparaoxonasefromHDLandimpairs itsantioxidantproperties:anewmechanismlinkingHDLproteincompositionand antiatherogenicpotential. CircRes. (2004)95:789–97.doi:10.1161/01.RES.0000146031. 94850.5f
148.KotaniK,YamadaT,GugliucciA.Pairedmeasurementsofparaoxonase1and serumamyloidAasusefuldiseasemarkers. BiomedResInt. (2013)2013:481437.doi: 10.1155/2013/481437
149.YangN,QinQ.ApolipoproteinJ:anewpredictorandtherapeutictargetin cardiovasculardisease? ChinMedJ. (2015)128:2530–4.doi:10.4103/0366-6999.164983
150.HanC,ChibaT,CampbellJ,FaustoN,ChaissonM,OrasanuG,etal. ReciprocalandcoordinateregulationofserumamyloidAversusapolipoproteinA-Iand paraoxonase-1byinflammationinmurinehepatocytes. ArteriosclerThrombVascBiol. (2006)26:1806–13.doi:10.1161/01.ATV.0000227472.70734.ad
151.CastellaniL,ChangJ,WangX,LusisA,ReynoldsW.Transgenicmiceexpress humanMPO–463G/Aallelesatatheroscleroticlesions,developinghyperlipidemiaand obesityin–463Gmales. JLipidRes. (2006)47:1366–77.doi:10.1194/jlr.M600005-JLR200
152.LiuC,DesikanR,YingZ,GushchinaL,KampfrathT,DeiuliisJ,etal.Effectsof anovelpharmacologicinhibitorofmyeloperoxidaseinamouseatherosclerosismodel. PLoSOne. (2012)7:e50767.doi:10.1371/journal.pone.0050767
153.HuangY,WuZ,RiwantoM,GaoS,LevisonB,GuX,etal.Myeloperoxidase, paraoxonase-1,andHDLformafunctionalternarycomplex. JClinInvest. (2013) 123:3815–28.doi:10.1172/JCI67478
154.KhineH,TeiberJ,HaleyR,KheraA,AyersC,RohatgiA.Association oftheserummyeloperoxidase/high-densitylipoproteinparticleratioandincident cardiovasculareventsinamulti-ethnicpopulation:observationsfromthedallasheart study. Atherosclerosis. (2017)263:156–62.doi:10.1016/j.atherosclerosis.2017.06.007
155.BacchettiT,FerrettiG,CarboneF,MinistriniS,MontecuccoF, JamialahmadiT,etal.Dysfunctionalhigh-densitylipoprotein:theroleof myeloperoxidaseandparaoxonase-1. CurrMedChem. (2021)28:2842–50. doi:10.2174/0929867327999200716112353
156.ScavoneD,SgorbiniM,BorgesA,Oliveira-FilhoJ,VitaleV,PaltrinieriS.Serial measurementsofParaoxonase-1(PON-1)activityinhorseswithexperimentallyinduced endotoxemia. BMCVetRes. (2020)16:422.doi:10.1186/s12917-020-02629-4
157.GaálK,TarrT,L˝orinczH,BorbásV,SeresI,HarangiM,etal.High-density lipopoproteinantioxidantcapacity,subpopulationdistributionandparaoxonase-1 activityinpatientswithsystemiclupuserythematosus. LipidsHealthDis. (2016)15:60. doi:10.1186/s12944-016-0229-0
158.MenesesM,SilvestreR,Sousa-LimaI,MacedoM.Paraoxonase-1asaregulator ofglucoseandlipidhomeostasis:impactontheonsetandprogressionofmetabolic disorders. IntJMolSci. (2019)20:4049.doi:10.3390/ijms20164049
159.ManandharB,CochranB,RyeK.Roleofhigh-densitylipoproteinsincholesterol homeostasisandglycemiccontrol. JAmHeartAssoc. (2020)9:e013531.doi:10.1161/ JAHA.119.013531
160.MastorikouM,MacknessB,LiuY,MacknessM.Glycationofparaoxonase-1 inhibitsitsactivityandimpairstheabilityofhigh-densitylipoproteintometabolize membranelipidhydroperoxides. DiabetMed. (2008)25:1049–55.doi:10.1111/j.14645491.2008.02546.x
161.YuW,LiuX,FengL,YangH,YuW,FengT,etal.Glycationofparaoxonase1by highglucoseinstigatesendoplasmicreticulumstresstoinduceendothelialdysfunction invivo SciRep. (2017)7:45827.doi:10.1038/srep45827
162.TamesF,MacknessM,ArrolS,LaingI,DurringtonP.Non-enzymaticglycationof apolipoproteinBintheseraofdiabeticandnon-diabeticsubjects. Atherosclerosis. (1992) 93:237–44.doi:10.1016/0021-9150(92)90260-N
163.HolvoetP,VanhaeckeJ,JanssensS,VandeWerfF,CollenD.OxidizedLDLand malondialdehyde-modifiedLDLinpatientswithacutecoronarysyndromesandstable coronaryarterydisease. Circulation. (1998)98:1487–94.doi:10.1161/01.CIR.98.15.1487
164.YounisN,SoranH,SharmaR,Charlton-MenysV,GreensteinA,ElseweidyM, etal.Small-denseLDLandLDLglycationinmetabolicsyndromeandinstatin-treated andnon-statin-treatedtype2diabetes. DiabVascDisRes. (2010)7:289–95.doi:10.1177/ 1479164110383063
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 13 frontiersin.org 172
165.YounisN,Charlton-MenysV,SharmaR,SoranH,DurringtonP.Glycationof LDLinnon-diabeticpeople:smalldenseLDLispreferentiallyglycatedboth invivo and invitro Atherosclerosis. (2009)202:162–8.doi:10.1016/j.atherosclerosis.2008.04.036
166.YounisN,SoranH,PembertonP,Charlton-MenysV,ElseweidyM,Durrington P.SmalldenseLDLismoresusceptibletoglycationthanmorebuoyantLDLinType2 diabetes. ClinSci. (2013)124:343–9.doi:10.1042/CS20120304
167.PietzschJ,JuliusU.Differentsusceptibilitytooxidationofprolineandarginine residuesofapolipoproteinB-100amongsubspeciesoflowdensitylipoproteins. FEBS Lett. (2001)491:123–6.doi:10.1016/S0014-5793(01)02181-0
168.YounisN,SoranH,SharmaR,Charlton–MenysV,DurringtonP.Lipoprotein glycationinatherogenesis. ClinLipidol. (2009)4:781–90.doi:10.2217/clp.09.61
169.SobalG,MenzelJ,SinzingerH.WhyisglycatedLDLmoresensitivetooxidation thannativeLDL?acomparativestudy. ProstaglandinsLeukotEssentFattyAcids. (2000) 63:177–86.doi:10.1054/plef.2000.0204
170.RohatgiN,NielsenT,BjørnS,AxelssonI,PagliaG,VoldborgB,etal.Biochemical characterizationofhumangluconokinaseandtheproposedmetabolicimpactofgluconic acidasdeterminedbyconstraintbasedmetabolicnetworkanalysis. PLoSOne. (2014) 9:e98760.doi:10.1371/journal.pone.0098760
171.SchalkwijkC,StehouwerC.Methylglyoxal,ahighlyreactivedicarbonylcompound, indiabetes,itsvascularcomplications,andotherage-relateddiseases. PhysiolRev. (2020) 100:407–61.doi:10.1152/physrev.00001.2019
172.LindsayR,SmithW,LeeW,DominiczakM,BairdJ.Theeffectofdeltagluconolactone,anoxidisedanalogueofglucose,onthenonenzymaticglycationof humanandrathaemoglobin. ClinChimActa. (1997)263:239–47.doi:10.1016/S00098981(97)00067-3
173.YounisN,SoranH,Charlton-MenysV,SharmaR,HamaS,PembertonP,etal. High-densitylipoproteinimpedesglycationoflow-densitylipoprotein. DiabVascDis Res. (2013)10:152–60.doi:10.1177/1479164112454309
174.KjellmoC,KarlssonH,NestvoldT,LjunggrenS,CederbrantK,Marcusson-Ståhl M,etal.Bariatricsurgeryimproveslipoproteinprofileinmorbidlyobesepatientsby reducingLDLcholesterol,apoB,andSAA/PON1ratio,increasingHDLcholesterol, buthasnoeffectoncholesteroleffluxcapacity. JClinLipidol. (2018)12:193–202.doi: 10.1016/j.jacl.2017.10.007
175.Lou-BonafonteJ,Gabás-RiveraC,NavarroM,OsadaJ.PON1andmediterranean diet. Nutrients. (2015)7:4068–92.doi:10.3390/nu7064068
176.Lou-BonafonteJ,Gabás-RiveraC,NavarroM,OsadaJ.Thesearchfordietary supplementstoelevateoractivatecirculatingparaoxonases. IntJMolSci. (2017)18:416. doi:10.3390/ijms18020416
177.FerrettiG,BacchettiT,SahebkarA.Effectofstatintherapyonparaoxonase-1 status:asystematicreviewandmeta-analysisof25clinicaltrials. ProgLipidRes. (2015) 60:50–73.doi:10.1016/j.plipres.2015.08.003
178.SahebkarA,Hernández-AguileraA,AbellóD,SanchoE,CampsJ,JovenJ. Systematicreviewandmeta-analysisdecipheringtheimpactoffibratesonparaoxonase-1 status. Metabolism. (2016)65:609–22.doi:10.1016/j.metabol.2016.01.002
179.TurfanerN,UzunH,BalciH,ErcanM,KarterY,CanerM,etal.Ezetimibe therapyanditsinfluenceonoxidativestressandfibrinolyticactivity. SouthMedJ. (2010) 103:428–33.doi:10.1097/SMJ.0b013e3181d83374
180.YadavR,LiuY,KwokS,HamaS,FranceM,EatoughR,etal.Effectofextendedreleaseniacinonhigh-densitylipoprotein(HDL)functionality,lipoproteinmetabolism, andmediatorsofvascularinflammationinstatin-treatedpatients. JAmHeartAssoc. (2015)4:e001508.doi:10.1161/JAHA.114.001508
181.EsteghamatiA,EskandariD,MirmiranpourH,NoshadS,MousavizadehM, HedayatiM,etal.Effectsofmetforminonmarkersofoxidativestressandantioxidant reserveinpatientswithnewlydiagnosedtype2diabetes:arandomizedclinicaltrial. Clin Nutr. (2013)32:179–85.doi:10.1016/j.clnu.2012.08.006
182.DemirY,KöksalZ.Theinhibitioneffectsofsomesulfonamidesonhumanserum paraoxonase-1(hPON1). PharmacolRep. (2019)71:545–9.doi:10.1016/j.pharep.2019. 02.012
183.BowmanL,HopewellJ,ChenF,WallendszusK,StevensW,CollinsR,etal.Effects ofanacetrapibinpatientswithatheroscleroticvasculardisease. NEnglJMed. (2017) 377:1217–27.
184.ChenY,DongJ,ZhangX,ChenX,WangL,ChenH,etal.Evacetrapib reducespreβ-1HDLinpatientswithatheroscleroticcardiovasculardiseaseordiabetes. Atherosclerosis. (2019)285:147–52.doi:10.1016/j.atherosclerosis.2019.04.211
185.DunbarR,MovvaR,BloedonL,DuffyD,NorrisR,NavabM,etal.Oral apolipoproteinA-ImimeticD-4FlowersHDL-inflammatoryindexinhigh-riskpatients: afirst-in-humanmultiple-dose,RandomizedControlledTrial. ClinTranslSci. (2017) 10:455–69.doi:10.1111/cts.12487
186.VakiliL,HamaS,KimJ,TienD,SafarpoorS,LyN,etal.TheeffectofHDLmimetic peptide4FonPON1. AdvExpMedBiol. (2010)660:167–72.doi:10.1007/978-1-60761350-3_15
187.BrandesR,DueckA,EngelhardtS,KaulichM,KupattC,DeAngelisM,etal. DGKandDZHKpositionpaperongenomeediting:basicscienceapplicationsandfuture perspective. BasicResCardiol. (2021)116:2.doi:10.1007/s00395-020-00839-3
188.SillerA,WhyteM.Alkalinephosphatase:discoveryandnamingofourfavorite enzyme. JBoneMinerRes. (2018)33:362–4.doi:10.1002/jbmr.3225
189.Charlton-MenysV,LiuY,DurringtonP.Semiautomatedmethodfordetermination ofserumparaoxonaseactivityusingparaoxonassubstrate. ClinChem. (2006)52:453–7. doi:10.1373/clinchem.2005.063412
190.MarsillachJ,RichterR,CostaL,FurlongC.Paraoxonase-1(PON1)statusanalysis usingnon-organophosphatesubstrates. CurrProtoc. (2021)1:e25.doi:10.1002/cpz1.25
191.MohammedC,LamichhaneS,ConnollyJ,SoehnlenS,KhalafF,MalhotraD, etal.APONforallseasons:comparingparaoxonaseenzymesubstrates,activityand actionincludingtheroleofPON3inhealthanddisease. Antioxidants. (2022)11:590. doi:10.3390/antiox11030590
192.CampsJ,MarsillachJ,JovenJ.Theparaoxonases:roleinhumandiseasesand methodologicaldifficultiesinmeasurement. CritRevClinLabSci. (2009)46:83–106. doi:10.1080/10408360802610878
193.LouedS,IsabelleM,BerrouguiH,KhalilA.Theanti-inflammatoryeffectof paraoxonase1againstoxidizedlipidsdependsonitsassociationwithhighdensity lipoproteins. LifeSci. (2012)90:88–92.doi:10.1016/j.lfs.2011.10.018
194.AnastasiusM,KockxM,JessupW,SullivanD,RyeK,KritharidesL.Cholesterol effluxcapacity:anintroductionforclinicians. AmHeartJ. (2016)180:54–63.doi:10. 1016/j.ahj.2016.07.005
Durringtonetal. 10.3389/fcvm.2023.1065967
Frontiersin CardiovascularMedicine 14 frontiersin.org 173

+41 (0)21 510 17 00 frontiersin.org/about/contact Avenue du Tribunal-Fédéral 34 1005 Lausanne, Switzerland frontiersin.org Contact us Frontiers Innovations and improvements in cardiovascular treatment and practice Focuses on research that challenges the status quo of cardiovascular care, or facilitates the translation of advances into new therapies and diagnostic tools. Discover the latest Research Topics See more Frontiers in Cardiovascular Medicine


 FIGURE1
FIGURE1


















































