What

How Does a Wound Dressing With Sensors Monitor the Healing Process?

What is the Role of Fish Skin Graft in Combat Injuries in Austere Conditions?

Volume 2: Issue 4 - March 2023 woundmasterclass.com Limitations of Applying Summary Results of Clinical Trials to Individual Patients
is the Role of Point-Of-Care Fluorescence Imaging for Bacterial Load Identification in Diabetic Foot Ulcers? A Novel Concept for Treating Large Soft Tissue Defects After Necrotizing Soft Tissue Infection
Hydro-Responsive
Fish Skin Technology: Burns
Science: Eyes
Prize Lymphedema in Clinical Practice Critical Limb Ischaemia Open Access | Peer Reviewed | International | Quarterly ISSN 2753-6963 Official Journal of the Association for the Advancement of Wound Care®
Masterclass GUIDES
Wound Dressing Decellularized Fish Skin Technology Decellularized
Technology vs
on the




































Middle East Prof Amit Gefen Professor of Biomedical Engineering, Tel Aviv University Tel Aviv, Israel Editorial Board North America Mr Frank Aviles Wound Care Clinical Coordinator, Natchitoches Regional Medical Center Natchitoches LA, United States Dr Windy Cole Director of Wound Care Research, Kent State University of Podiatric Medicine National Director of Clinical Safety, Quality and Education, Woundtech Streetsboro OH, United States Ms Kara Couch President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies George Washington University Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States Dr Kenneth Burhop Life Sciences Advisor and Consultant San Diego CA, United States Mr Tobe Madu Data Scientist, Net Health Atlanta GA, United States Dr M. Mark Melin Medical Director of the M Health Wound Healing Institute Adjunct Associate Professor, University of Minnesota Surgical Department Mineapolis MN, United States Dr Leo Nherera Director, Global Head of Health Economics & Outcomes Research Fort Worth TX, United States Dr Mitch Sanders CSO and EVP Alira Health. CEO of WoundForce Inc. and Firefly Innovations LLC. Boston MA, United States Dr Brandon Bosque Foot and Ankle Surgeon Philadelphia PA, United States Prof David Armstrong Professor of Surgery and Director, Southwestern Academic Limb Salvage Alliance (SALSA), Keck School of Medicine of USC Los Angeles CA, United States Dr Aliza Lee Clinical Research Investigator, Department of Veterans Affairs Salem VA, United States Dr Alton R. Johnson Podiatric Surgeon Wound Care Physician Ann Arbor MI, United States Dr Jonathan Johnson Surgical Director, Comprehensive Wound Care Services Washington DC, United States Dr David Alper Trustee Board of Trustees, American Podiatric Medical Association Board Member American Diabetes Association (New England) Surgical staff (Emeritas) Mount Auburn Hospital Cambridge, MA, United States Boston MA, United States
Prof Dimitri Beeckman Professor of Nursing Science, Ghent University (Belgium) and Vice-Head of the School of Health Sciences, Örebro University (Sweden) Ghent, Belgium Prof Dr C. Can Cedidi Clinic Director for Plastic, Reconstructive & Aesthetic Surgery Bremen, Germany Dr Paul Chadwick National Clinical Director, Royal College of Podiatry Manchester, United Kingdom Dr Przemysław Lipiński Wound Surgeon, National Representative of Poland in D-Foot International Łódź, Poland Prof Declan Patton Director of Nursing and Midwifery Research and Deputy Director of SWaT Research Center, RCSI University of Medicine and Health Sciences Dublin, Ireland Mr Harm Jaap Smit Wound Biologist, Erasmus MC Academy Rotterdam Rotterdam, Netherlands Ms Lian Stoeldraaijers President, Dutch Association of Diabetes Podiatrists Valkenswaard, Netherlands Dr Negin Shamsian Consultant Plastic & Reconstructive Surgeon (Locum) Chief Editor of Wound Masterclass London, United Kingdom Prof Jan Kottner Professor of Nursing Science, Charité Berlin University of Medicine Berlin, Germany Prof Dr Luca Dalla Paola Specialist in Endocrinology, Metabolic Diseases and Diabetology Expert in medical and surgical treatment of Diabetic Foot Ferrara, Italy Dr Sebastian Probst EWMA President Professor of Tissue Viability and Wound Care at the School of Health Sciences, University of Applied Sciences and Arts Western Switzerland, Geneva Genf, Switzerland Africa Sr Trish Idensohn Wound Nurse Specialist, Consultant and Educator Durban, South Africa Dr Joon Pio Hong Professor of Plastic and Reconstructive Surgery at the University of Ulsan College of Medicine and Asan Medical Center Seoul, South Korea Prof Dr Harikrishna K. R. Nair President Elect, WUWHS - World Union of Wound Healing Societies President, Asia Pacific Association of Diabetic Limb Problems Kuala Lumpur, Malaysia Australia Dr Ross D Farhadieh Cosmetic Plastic & Reconstructive Surgeon Sydney, Australia South & Central America Dr Luis Alejandro Boccalatte Head and Neck Surgeon, Associate Professor Instituto Universitario Hospital Italiano Buenos Aires, Argentina Dr Eduardo Camacho Plastic and Reconstructive Surgeon Mexico City, Mexico East Asia Ms Terry Swanson Vice Chair, International Wound Infection Institute Victoria, Australia Dr Honda Hsu Plastic Surgeon and Associate Professor, Tzu Chi General Hospital Hualien, Taiwan Dr Ruth Bryant Nurse Scientist and WOC nurse, Abbott Northwestern Hospital Minneapolis MN, United States
United Kingdom & Europe
Chief Editor
Miss Negin Shamsian
Commercial Director
Mr Alec Wright
Contact Editor editor@woundmasterclass.com Commercial Inquiries commercial@woundmasterclass.com

Humans vs Machines: Are we Becoming Replaceable? | Dr Negin Shamsian
How Does a Wound Dressing With Sensors Monitor the Healing Process? | Mr Armin Haas, Prof Kai-Uwe Zirk
Limitations of Applying Summary Results of Clinical Trials to Individual Patients: The Need for Risk Stratification | Mr Zwelithni Tunyiswa
The Dawning of a New Horizon For Venous Leg Ulcer Treatment | Mr Bernard Ross
Synergistic Effects of Vashe and Ovine Forestomach in Chronic Venous Disease | Dr Monika Gloviczki, Dr M. Mark Melin, Dr Peter Gloviczki, Dr Abigail Chaffin, Dr Lee Ruotsi
What is the Role of Fish Skin Graft in Combat Injuries in Austere Conditions? | Dr Fouad Reda, Dr Hilmar Kjartansson, Dr Steven Jeffery
Clinical Experience of Omega 3 Fish Graft in Full Thickness Wounds | Dr Ariel M. Aballay
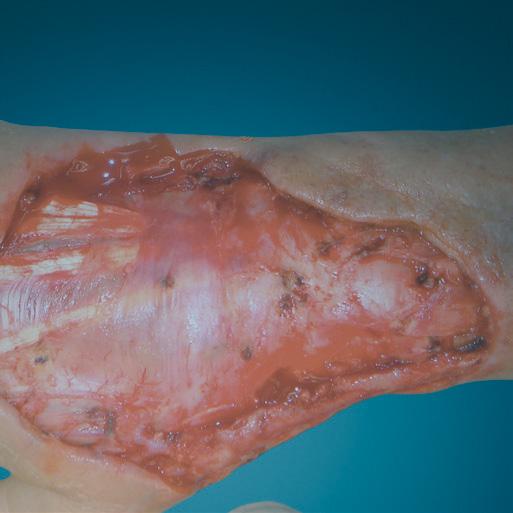
A Novel Concept for Treating Large Soft Tissue Defects After Necrotizing Soft Tissue Infection of the Back | Dr Marcus F. Yarbrough
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition | Dr Tristan Pennella, Dr Karen Andrews, Dr Heather Hettrick, Dr Joseph Raffetto, Dr Wei Chen, Dr Daniel Miller, Dr Michael Shao, Dr M. Mark Melin
What is the Role of Point-Of-Care Fluorescence Imaging for Bacterial Load
Identification in Diabetic Foot Ulcers? | Dr David G. Armstrong, Dr Michael E. Edmonds, Dr Thomas E. Serena
MasterSeries: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro | Dr Monika Gloviczki, Dr M. Mark Melin, Dr Peter Gloviczki, Dr Abigail Chaffin, Dr Lee Ruotsi
MasterSeries: Topical Oxygen Therapy: All Your Questions Answered | Mr Frank Aviles, Ms Kara Couch, Dr Paul Haser, Dr Matthew G. Garoufalis, Dr Anil Hingorani
MasterSeries: Surgical Site Infection: All Your Questions Answered | Dr Jonathan Johnson, Dr M. Mark Melin, Dr Hüseyin Kemal Raşa, Dr Windy Cole, Dr Michael Magro
Masterclass GUIDES

Hydro-Responsive Wound Dressing
Decellularized Fish Skin Technology: Burns
Decellularized Fish Skin Technology
Article
Published
Clarus Communications Ltd., Oxford, United Kingdom No part of this issue is to be copied or reproduced without permission of the publisher © Clarus Communications Ltd. This publication is intended for online distribution and this issue is not suitable for print in this form To inquire about obtaining a printable version of this issue or any article therein, please contact the editor March 2023
Submissions submissions@woundmasterclass.com
by
3 4 - 5 15 - 17 20 - 25 30 - 41 44 - 49 50 - 53 60 - 62 68 - 83 92 - 96 106 - 114 116 - 120 122 - 130
Lymphedema in Clinical Practice Critical Limb Ischaemia 28 - 31 54 - 57 64 - 67 86 - 89 98 - 101 Cover image: Licenced from Adobe Stock Credit: McKinney Photography
Virtual Reality innovates Wound Care Training
Three-dimensional VR training modules provide healthcare professionals with an interactive and realistic clinical learning experience that allows collaborative learning without the involvement of the patient.
hartmann.info

Humans vs Machines: Are We Becoming Replaceable?
The future of AI is both exciting and uncertain. AI is poised to transform many industries, from healthcare to transportation to finance, and could bring about unprecedented advances in scientific discovery and problem-solving. However, there are also concerns about the impact of AI on security, privacy, and jobs. Our cover image for this issue was generated by AI. Will there come a time when we will all be replaced by technology? Just today, ChatGPT managed to pass a medical licensing exam diagnosing a rare condition affecting 1 in 100,000 individuals in a mere few seconds.
Artificial intelligence (AI) has the potential to revolutionize the field of wound care by improving diagnosis and treatment outcomes for patients through the use of machine learning algorithms to analyze and interpret large amounts of data, including medical records, images, and other clinical data.
Machine learning algorithms can improve the accuracy of wound diagnosis, and recognize different types of wounds, such as pressure ulcers, diabetic foot ulcers, and venous ulcers, based on their visual appearance and other clinical characteristics, providing more targeted treatment for patients.
AI can also be used to develop predictive models that can help healthcare professionals anticipate the course of a wound and determine the best course of treatment. Machine learning algorithms can be trained to analyze data from wound images and predict the likelihood of healing based on factors such as wound size, depth, and the presence of infection. This can help clinicians determine the most appropriate treatment plan for each patient, including the use of advanced wound care products, such as wound dressings and skin substitutes.
Another potential benefit of AI in wound care is its ability to improve patient outcomes by reducing the risk of complications and promoting faster healing. Machine learning algorithms can be used to identify patients at high risk of developing complications, such as infections or delayed healing, and provide targeted interventions to reduce this risk. This can lead to better outcomes for patients, including reduced pain, faster healing times, and improved quality of life.
In addition to improving diagnosis and treatment outcomes, AI can also help to streamline the wound care process by reducing the workload for healthcare professionals, as machine learning algorithms can be used to automate tasks such as wound measurements and documentation, allowing clinicians to spend more time on patient care.
There are also some challenges associated with the use of AI in wound care, such as the need for high-quality data and the potential for algorithm bias, which can be addressed through careful data collection and analysis, and the use of transparent and unbiased algorithms.
Overall, AI has the potential to significantly improve the diagnosis and treatment of wounds, leading to better outcomes for patients and a more efficient and effective wound care process. As AI technology continues to evolve, it is likely that we will see more widespread adoption of these tools in wound care and other areas of healthcare.
We are excited to bring you this packed spring issue of Wound Masterclass with excellent content from global wound care leaders. I also invite you to register to enjoy all our free content including Wound MasterSeries 60 Minutes, The Wound Masterclass Podcast (woundmasterclass.com/Events) and find out how to get the best outcomes for your patients at the Global Innovation in Wound Care Summit ( woundmasterclass.com/innovation-summit ). Hope you enjoy the issue!
Could there come a day when my editorial could be written by a computer? If so, I’m already looking forward to putting my feet up and sitting by the beach while AI puts together a Wound Masterclass issue for me!

Wound Masterclass - Vol 2 - March 2023 3 Dr Negin Shamsian Consultant Plastic & Reconstructive Surgeon (Locum)
of
Masterclass
Chief Editor
Wound
London, United Kingdom
© Copyright. Wound Masterclass. 2023
How Does a Wound Dressing With Sensors Monitor the Healing Process?
Editorial Summary
This article describes and considers new technology which uses sensors integrated into wound dressings to provide data on the wound healing status. Discussed is the development of this technology, how it is constructed and used, and the possibilities for future implementation in wound care.
Introduction
For clinicians, what is happening under a dressing is a mystery that can only be solved by uncovering the wound. Since this can be as different as the wound is healing or is infected, technology that could provide information on the wound status could be revolutionary. This is precisely what a collaboration between code’n’ground AG and the Private University of Applied Sciences for Business and Technology (phwt) has been working towards since 2020.
What are the goals?
The goal is to ‘elevate primary wound healing to a new digital level using sensor technology and artificial intelligence’. To expand further, this would require evidence that sensor technology assisted with connected AI could measure and provide information such as temperature and moisture under a dressing, giving the clinician an indication of wound healing status. By dividing the project between the technical challenge involved with the wound dressing, which was undertaken by the Private University of Applied Sciences for Business and Technology (phwt), and the software component to develop the AI, which was lead by code’n’ground, this goal has been achieved.
What is the technology?


The sensor would need to capture the relevant parameters that would indicate a disruption in wound healing and/ or infection, and would have to be easily incorporated into the wound dressing. This was approached by incorporating
conductive structures within a plastic film, which is then combined with the dressing allowing monitoring of the temperature and humidity underneath; a significant increase in either parameter could be an indication of inflammation, infection and/ or disruption of healing.
The sensor structure was comprised of an ultrathin layer of copper measuring a few micrometers; the properties of this mean that if there is a change in temperature, the conductivity of this component will also change. The moisture is monitored by changes in electrical capacitance.
The plastic film is designed to be flexible and elastic, and crucially non-irritating or harmful to skin. The sensor structure is incorporated between two of these films, and combined with a thermal connection to the dressing. The dressing is contained in sterile packaging as any other; nursing staff may use it routinely by opening the packaging, applying the dressing and covering as usual with bandages.
The dressing collects, stores and sends data continuously; the AI then processes the data which is read by an app. Nursing staff can then access a wound assessment based on this data with the app. There is also an intention to make it possible for patients themselves to transmit the data, which again would be done with an app; the technology was developed with this in mind, and therefore the app is an integral part of the AI architecture.
Currently, in the hospital setting wounds are assessed when the dressing is changed; this
4 Wound Masterclass - Vol 2 - March 2023
Prof Kai-Uwe Zirk
Center for Mechatronics and Electrical Engineering, phwt (Private Hochschule für Wirtschaft und Technik gGmbH)
Vechta, Germany
Mr Armin Haas
Member of the Executive Board, code’n’ground AG
© Copyright. Wound Masterclass. 2023
Heidenheim an der Brenz, Germany
is the only time when clinicians can get any information on the healing status of the wound, before the wound is covered again. The smart dressing in comparison measures the temperature and moisture parameters throughout the healing process, which can amount to 30,000 measuring points. Parameters that relate to the correlation between wound healing and duration are also collected, as well as previously mentioned, moisture and temperature; an alarm will sound when the parameters are out of sync, indicating a problem. The nursing staff will then be able to assess the wound; so the sensor is actually the primary source of data.
The concept for the patient being able to transmit data themselves would work as follows: using the proposed app, the patient will be able to report any pain, feelings of tightness, or any other signs and symptoms of a problem with the wound healing process, infection, etc. This adds another facet to the approach of constant assessment of the wound; the implementation of this has always been part of the modelling, though this has not been done yet.
In this multi-faceted approach to assessment, feedback from healthcare professionals is another source of information. In the event that a wound has become infected,
the interventions performed (changing the bandage, administering antibiotics, etc.) would be recorded.
This would ultimately make available hugely valuable data, and it will be possible to analyze the success of interventions and perhaps draw conclusions on the most successful treatments and interventions in the future; ultimately, the goal is to support the medical team.
An obvious problem was to avoid making the dressing too cumbersome or uncomfortable, in that it has to contain a sensor. The technology can be made much smaller if in major production; currently the dressing is not as small as is ideal, due to the huge undertaking of development. In major production the data device could realistically be as small as 1.5cm in diameter and around 5 - 6mm in height.
In terms of this technology being introduced into the industry, and when and how this could happen, the function of the dressing has been proven with tests on technical models in the lab. An effort is underway to find interested partners who could support the project, and allow us to move onto the next step, which is to use the dressings on humans and process the data with AI.

Wound Masterclass - Vol 2 - March 2023 5
How Does a Wound Dressing With Sensors Monitor the Healing Process?
© Copyright. Wound Masterclass. 2023
Figure 1: Wound dressing with sensor and data logger.
How
Global Wound Care Leaders
Including:
Prof Sebastian Probst | EWMA President; Professor of Tissue Viability and Wound Care at the School of Health Sciences, Geneva, Switzerland
Prof Amit Gefen | Professor of Biomedical Engineering, Tel Aviv University, Israel
Dr M. Mark Melin | Medical Director of the M Health Wound Healing Institute; Minnesota, USA
Dr Mitch Sanders | CSO and EVP Alira Health. CEO of WoundForce Inc. and Firefly Innovations LLC, Boston, USA
Dr Windy Cole | Director of Wound Care Research, Kent State University of Podiatric Medicine, Ohio
Ms Terry Swanson | Vice Chair, International Wound Infection Institute, Victoria, Australia
Ms Trish Idensohn | Wound Nurse Specialist, Consultant and Educator
Dr Honda Hsu | Plastic Surgeon and Associate Professor, Tzu Chi General Hospital
Mr Frank Aviles | Wound Care Clinical Coordinator, Natchitoches Regional Medical Center
Dr Aliza Lee | Clinical Research Investigator, Department of Veterans Affairs
woundmasterclass.com/Events woundmasterclass.com/Register
Improving Patient Outcomes: Evidence-Based Technology
Advances in Dressings
Wound Bed Prep and Debridement
Surgical Site Infection
Venous Leg Ulcer (VLU)
Diabetic Foot Ulcer (DFU)
Pressure Injuries
Lymphedema
Obstetric Wounds
Biofilm
Oxygen Therapy
Skin Substitutes
Three Dimensional Printing
Advanced Modalities

31st
Global Innovation in Wound Care Summit | May
2023
to Improve Patient Outcomes: Evidence-Based Technology







Flexible solutions for complex wound reconstruction Native collagen fibers Elastin No chemical cross-linking
GUIDES
Introduction
This Masterclass Guide is a concise overview of Moisture Associated Skin Damage (MASD). MASD occurs when skin is exposed to moisture for prolonged periods of time, resulting in over-hydrated or eroded skin. This leads to trans epidermal water loss (TEWL) and an elevated skin pH that reduces the skin’s ability to maintain its barrier function.4,5 The end result is separation of the skin layers, which is also known as maceration.
What Are the Risk Factors for MASD?
Body/ Body Fluids
■ Direct skin contact with urine and/ or (liquid) faeces
■ Sweat on the skin surface
■ (Increased) wound secretions on the skin surface
■ Other body fluids such as mucus, (tracheal) secretions, or saliva on the skin surface
■ Increased dermal metabolism, elevated local temperature, abnormal skin pH, history of atopy, genetic susceptibility to contaminants, irritants, deep body folds, dermal atrophy, and inadequate sebum production
■ Pressure related injuries, such as immersion foot
Skin cleansing procedures and products
■ Repeated or excessive skin cleansing, strong friction or abrasive drying procedures, use of rough materials such as coarse towels
■ Repeated use of harsh skin cleansers
■ Ingredients in skin cleansers such as anionic tensides, fragrances, alcohol, preservatives, essential oils
Mechanical factors
■ Mechanical irritation (friction) from clothing or in skin folds
■ Occlusion, for example due to long periods of lying on non-breathable materials, wearing non-breathable clothing, incontinence pads
■ Pressure or shear forces
■ Skin damage from adhesive products, such as band-aids
Indirect risk factors
■ Old age
■ Care dependency
■ Immobility
■ Malnutrition
■ Obesity
■ Atopic diathesis
■ Microangiopathy and/ or macroangiopathy
■ Reduced sensory functions such as blindness, polyneuropathy, dementia
■ Immunosuppression
Moisture Associated Skin Damage
Keywords
■ Wound
■ Wounds
■ Wound care
■ Moisture associated skin damage (MASD)
■ Trans epidermal water loss (TEWL)
■ Epidermal injury
■ Medical-adhesive-related skin injury (MARSI)
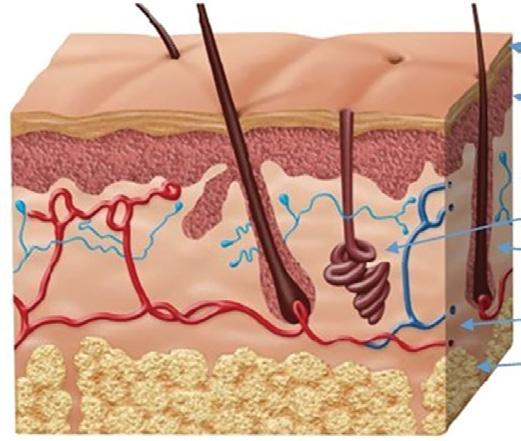
Stratum Corneum
Epidermis
Dermis
Sweat Gland
Hair Follicle
Blood Vessels
Subcutaneous Layer
How Can MASD Be Prevented?
■ As the onset of MASD often goes undetected, it may first present as basic inflammation of the skin with or without skin breakdown7,8
■ It is often only when significant inflammation, maceration and/ or skin breakdown emerges that clinicians are able to notice and intervene
■ Protection from MASD can be achieved via the application of natural moisturizers containing pyrrolidone carboxylicacid, urocanicacid, propylene glycol, lactic acid, urea, dimethicone, and petrolatum9,10
■ Preventing MASD with barrier ointments and cyanoacrylates is key for at-risk skin and managing alterations to skin integrity such as IAD, ITD, and peri wound skin damage9,10
■ Preventing MASD requires replenishing the natural moisture of the skin with moisturizing products such as skin barrier ointments. The skin should be kept clean and free of excess moisture, and a moisturizer or skin barrier cream should be applied daily
8 Wound Masterclass - Vol 2 - March 2023
Masterclass
Figure 1: Human skin layers
Vehicle Consistency Water/ lipid Content Advantages for at-risk skin Disadvantages for at-risk skin Lotion Light and non-greasy High concentration of water May have a role in end-of-life skin care and very fragile skin Increases TEWL; may contain more dehydrating ingredients Cream Viscous and nongreasy Similar parts of oil and water Spreads easily; creams with quality ingredients can decrease TEWL; aesthetically pleasing Washes off easily; creams with medicalgrade silicones may not prevent TEWL as well as ointments Ointment Thick and greasy 8 parts oil to 2 parts water Can hold moisture in the skin for prolonged periods; can protect open skin More difficult to spread; can stain clothing; feels greasy; nonadherence regarding application is possible Table 1:
© Copyright. Wound Masterclass. 2023
Comparison of different vehicles used as moisturizers for at-risk skin.
Moisture Associated Skin Damage Masterclass GUIDES
Overview of MASD Types
Incontinence-associated dermatitis (IAD):
■ Incontinence-associated dermatitis is a type of irritant contact dermatitis found in patients with faecal and/ or urinary incontinence. The urea present in urine is transformed into ammonia by urease present on human skin
■ This reaction causes an elevation of pH that consequently compromises the skin’s acid mantle, thus reducing the chemical barrier effect of the skin. Faeces contains proteolytic and lipolytic enzymes highly corrosive to the epidermis, with liquid faeces having a higher concentration of these enzymes than formed faeces
■ These cofactors in combination with excessive exposure to moisture increase the risk of epidermal injury. Earlier literature supports a prevalence range of 5.6 - 50%, with the higher being related to faecal or dual incontinence (both faecal and urinary5,12
■ The reporting of MASD is often inconsistent, as many clinicians mistakenly document IAD as Stage 1 or 2 pressure injuries (PIs)7
Intertriginous dermatitis (intertrigo or ITD):
■ Intertrigo is the result of friction in the presence of moisture. Areas of the body most susceptible to intertrigo are those where the skin is warm, where moisture can accumulate, and where the skin is prone to friction. These areas include, but may not be limited to, the axilla, inframammary, abdominal and inguinal folds
■ For patients dealing with incontinence-related issues, the presence of lower body folds in the lower pelvic region may also contribute significantly to morbidity. Obesity and diabetes are two conditions considered to be related to an increased risk for ITD as they are both prone to physiological skin changes, including higher rates of TEWL and increased sweat gland activity
■ ITD tends to be more prevalent in geographic regions with hot and humid climates. In one acute care setting, ITD was prevalent in 2.66% of all reported cases of MASD13
■ The prevalence of ITD falls across a variety of sectors, with 20% of patients living in community dwellings, 17% in long-term care homes and only 6% in acute care settings10
Periwound MASD:
■ Periwound skin damage is multifactorial and often associated with irritant or allergenic contact dermatitis of the surrounding wound skin secondary to moisture. Literature pertaining to the prevalence of periwound MASD is low, and the exact burden remains elusive. It has been hypothesized that the impact of periwound skin MASD is substantial10
■ Wound exudate is created during the natural process of the inflammatory phase of wound healing due to infection, inflammation, or systemic edema. When a wound is stalled, the concentration of metalloproteinases (a proteolytic enzyme) present in wound exudate increases, resulting in periwound skin damage and increasing the opportunity for maceration to occur. Times in which factors such as inadequate compression or inappropriate selection of wound dressings are present, wound exudate may not be well contained and can accumulate on the surface of the skin
■ When moisture is trapped under a dressing there are two factors to consider: the length of time between cleansing the skin and applying the dressing may not be sufficient, or the dressing selected may not have adequate capacity to handle the amount of exudate present
■ Inadequate cover dressings may cause the wound exudate to seep back out of the dressing, especially as the level of compression over the dressing increases
Peristomal MASD:
■ The major determinant of skin damage around a stoma is the enzymatic-containing effluent, although other contributory factors can also play a major role. These include mechanical trauma or medical-adhesive-related skin injury (MARSI) from appliances, bacteria, underlying skin disorders such as psoriasis or eczema, and the possibility of allergies to chemicals or fabrics
■ A multifactorial aetiology is common, with mechanical trauma, moisture and stomal effluent all working in tandem to break down the epidermal barrier.
■ Peristomal MASD affects 17.4% of people with colostomies and 34% with ileostomies, as appliance leakage occurs in more than 50% of the ostomates10
■ A more recent study out of Japan concurs that those living with an ileostomy were more likely to experience peristomal MASD than those with a colostomy14
Wound Masterclass - Vol 2 - March 2023 9
© Copyright. Wound Masterclass. 2023
Skin Damage
Overview of MASD Types (cont.)
Immersion Foot (IF): Incontinence-Associated Dermatitis:
■ Immersion/ trench foot is a syndrome secondary to prolonged foot exposure to moisture and had initially been described by soldiers practising trench warfare in the early part of the 20th century. Recently, a rise in incidence has been noted among individuals who are homeless and those living with untreated serious mental health issues15
■ In IF, moisture damage to the stratum corneum, the outermost layer of skin, compromises barrier function. Prolonged exposure to wet conditions at temperatures above freezing results in peripheral neuropathy and microvascular damage
■ Progression to severe pain is most often associated with tissue ischemia. In more severe injury, cyanosis with significant swelling of the extremities has been well documented in published literature16
Intertriginous Dermatitis or Intertrigo:

■ Intertriginous dermatitis occurs from moisture trapped between skin folds. Air is not circulated well in these areas, and therefore the moisture, usually as perspiration, remains trapped. Due to this, the skin is macerated, and friction damage from skin surfaces rubbing together may occur
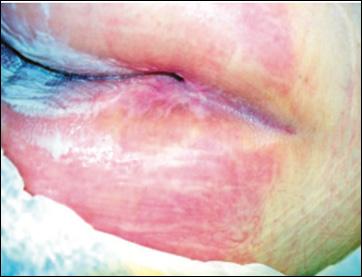
■ This damage is mirrored on both sides of the skin fold. When the outer layer of the skin (stratum corneum) becomes macerated, the results of friction are increased. Consequently, this further erodes the epithelium and can progress to inflammation and breakdown. Thus, the area becomes a potential entry point for microorganisms and may lead to a secondary infection (Figure 2)
■ Moisture from urine and/ or stool leads to what is commonly called IAD (Figure 1)
■ This is predominately a chemical irritation caused by urine and/ or stool coming in direct contact with the skin. The alkaline nature of urine increases the skin’s pH, changing it from acidic (pH <7) to alkaline (pH >7)
■ In addition, the alkaline urine may promote the enzymatic activity of proteinases and lipases when faecal incontinence is present and further erode the skin’s surface. Maceration of the skin takes place, making the area prone to friction or shear damage. This is especially problematic in older adults with fragile skin that are subjected to sliding for transfer from bed to chair and similar activities
Periwound-Associated Dermatitis:
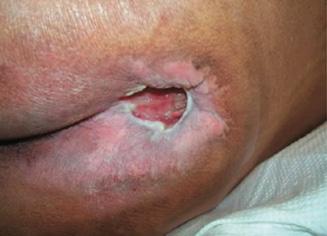
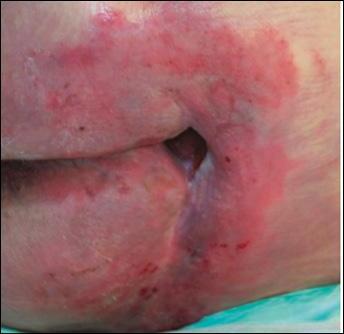
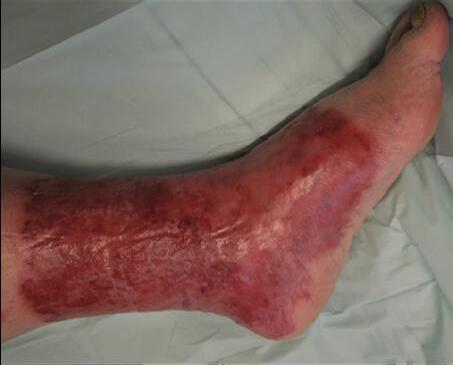
■ Skin surrounding a wound can develop toxic or allergic contact eczema, called periwound dermatitis (Figure 3). Periwound dermatitis may occur under wound dressings, due to insufficient management of exudation and longterm contact with the wound secretions.19 This eczema is limited to the areas that come into contact with moisture
■ Chronic wounds, usually stalled in the inflammatory stage, hold higher levels of proinflammatory cytokines and proteases and lower levels of growth factors. This causes an elevated pH (pH >7), and this alkaline environment makes the skin more susceptible to pathogens, resulting in extensive areas of redness surrounding the wound and more tissue destruction. Aggressive or frequent dressing removal, including any adhesive products, can also damage this fragile skin (Figures 4 and 5)
Breast
Moisture Associated
Masterclass
10 Wound Masterclass - Vol 2 - March 2023
GUIDES
Figure 1: Incontinence associated dermatitis on sacrum of older adult after protective cream application.
Figure 2: Early intertriginous dermatitis without infection as seen in redness.
Figure 3: Clinical example of toxic periwound dermatitis.
Figure 4: MASD secondary to wound exudate and urinary incontinence.
© Copyright. Wound Masterclass. 2023
Figure 5: White tissue around wound edge as result of maceration from wound exudate.
Moisture Associated Skin Damage Masterclass GUIDES
Peristomal Moisture-Associated Dermatitis:

■ Peristomal dermatitis is a (usually toxic) eczema around the site of a colostomy (stoma). This occurs in 30 - 67% of all stoma patients22
■ It may result from a poor seal around the stoma, allowing stool or urine to collect under the seal. Inflammation and erosion (an incomplete loss of the epidermis caused by moisture that is circumscribed, and usually depressed) of the moisture-damaged skin can extend outward in a 10-cm radius. This can occur due to the fit of the pouch not being correct, or the person has a stoma in a difficult area to allow for adherence
■ The ostomy drainage is urine or stool, so the mechanisms of skin irritation are the same as that of IAD, but treatment is difficult because of pouching issues. Frequent removal of the skin barrier needed for pouch placement can further complicate skin issues
Medical Adhesive–Related Skin Injury:
■ Medical adhesive–related skin injury is tissue trauma related to the use of medical adhesive products or devices. Adhesive is found within tapes, dressings, stoma barriers, electrocardiogram electrodes, and also medication patches. This includes any product that is used to approximate wound edges or affix a device to the skin
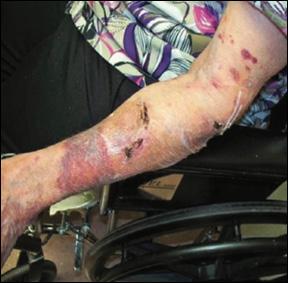
■ If correct placement and removal of such adhesive-containing items do not occur, then superficial layers of the skin are removed with the adhesive product
■ Despite cases where there is no visible irritation, some skin cell detachment still occurs, and a repeated process of application and removal compromises skin barrier function, initiating inflammation and the wound healing response. Medical adhesive–related skin injury is suspected if erythema or other forms of skin injury persist for 30 minutes or more after adhesive removal (Figure 6)
■ Shear, friction, or trauma can result in skin tears, caused by a separation of the skin layers. It usually presents as the epidermis is pulled away, resulting in a partial thickness wound, but in some cases it may be full thickness. Skin tears are classified by the International Skin Tear Advisory Panel (ISTAP) classification system as having no skin loss (type 1), partial flap loss (type 2), or total flap loss (type 3)24
■ Skin tears may occur during the removal of adhesive-based products, and also any maceration makes the skin more susceptible to friction, related to tearing of the epidermis (Figures 7 and 8)
■ Skin tears should be closely monitored and accurately described. Older persons or anyone with fragile skin should be taught prevention measures
■ If a skin tear is present, it should be carefully cleansed following assessment to remove debris. Skin tears are acute wounds and should be closed with primary intention. The skin flap (pedicle) should be approximated when possible, and a nonadherent dressing applied. Any dressing must be removed with caution to avoid additional skin injury

■ The skin injury takes place when skin-to-adhesive attachment is stronger than skin-to-skin attachment. This causes seperation of the epidermal layers or seperation of the entire epidermis from the dermis. Repeated application and removal of adhesive products may lead to skin injury. Trauma may be mechanical and can range from skin stripping, to tension or blisters, or to a skin tear. Irritant or allergic dermatitis may develop under the product, and maceration from trapped moisture or folliculitis can also occur
■ Risk factors for medical adhesive-related skin injury (or medical device-related pressure injury (MDRPI)) also include dry skin, and use of harsh cleaning agents. The use of moisturizers and skin barrier products helps mitigate the risk of skin damage caused by intrinsic and extrinsic factors. It has been demonstrated that a cyanoacrylate barrier film can protect at-risk skin from MDRPI caused by friction.23 When applied to hydrated skin, a cyanoacrylate barrier film significantly reduced the coefficient of friction between the skin and a simulated bed linen
Wound Masterclass - Vol 2 - March 2023 11
Skin Tears:
Figure 6: Damage around wound after frequent dressing removal; possible allergy to adhesive.
Figure 7: Skin tear with film dressing shows some scabbing starting and superficial redness.
© Copyright. Wound Masterclass. 2023
Figure 8: Full thickness skin tear.
Moisture Associated Skin Damage
References
Overview of MASD Types (cont.)
1. North American Nursing Diagnosis Association. NANDA diagnoses. Risk for impaired skin integrity. 2022. Accessed March17, 2023. https://nandadiagnoses.com/risk-for-impaired-skin-integrity
2. DowsettDAL.Moisture associated skin damage made easy. 2013. www.wounds-uk.com/ made-easy/moisture-associated-skin-damage-made-easy. Last accessed June 9, 2017.
3. Gray M, Black JM, Baharestani MM, et al. Moisture-associated skin damage: overview and pathophysiology. J Wound Ostomy Continence Nurs 2011;38:233-41.
4. Bender JK, Faergemann J, Sköld M. Skin health connected to the use of absorbent hygiene products: A review. Dermatol Ther (Heidelb). 2017;7(3):319–330.
5. Voegeli D. Moisture-associated skin damage: Aetiology, prevention and treatment. Br J Nurs. 2012;21(9):517–518, 520–521.
6. Baranoski S, Ayello EA. Wound Care Essentials: Practice Principles. Philadelphia: Lippincott Williams & Wilkins; 2008.
7. Black JM, Gray M, Bliss DZ, Kennedy-Evans KL, Logan S, Baharestani MM, et al. MASD Part 2: Incontinence-associated dermatitis and intertriginous dermatitis: A consensus. J Wound Ostomy Continence Nurs. 2011;38(4):359–370; quiz 371–372.
8. Gray M, Black JM, Baharestani MM, Bliss DZ, Colwell JC, Goldberg M, et al. Moisture- associated skin damage: Overview and pathophysiology. J Wound Ostomy Continence Nurs. 2011;38(3):233–241.
9. Beeckman D, Campbell J, LeBlanc K, et al. Best practice recommendations for holistic strategies to promote and maintain skin integrity. February 28, 2020. Wounds International. Accessed March
17, 2023. https://www.woundsinternational.com/resources/details/best-practice-recommendations-holistic-strategies-promote-and-maintain-skin-integrityWoo KY, Beeckman D, Chakravarthy D. Management of moisture-associated skin damage: a scoping review. Adv Skin Wound Care. 2017;30(11):494-501. doi:10.1097/01. ASW.0000525627.54569.da
10. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. Haesler E, ed. EPUAP/ NPIAP/PPPIA; 2019.
11. Nobles T, Miller RA. Intertrigo. Treasure Island, FL: StatPearls Publishing; 2018 [cited 2019 Feb 5]. Retrieved from: www.ncbi.nlm.nih.gov/books/NBK531489.
12. Werth SL, Justice R. Prevalence of moisture-associated skin damage in an acute care setting: Outcomes from a quality improvement project. J Wound Ostomy Continence Nurs. 2019;46(1):51.
13. Nagano M, Ogata Y, Ikeda M, Tsukada K, Tokunaga K, Iida S. Peristomal moisture-associated skin damage and independence in pouching system changes in persons with new faecal ostomies. Wound Ostomy Continence Nurs. 2019;46(2):137.
14. Kuhnke JL, Wright G, Kapteyn R. Wound care in a drop-in and rehabilitation centre: A Calgary perspective. Wound Care Canada. 2015;13(2):6.
15. Bush JS, Watson S. Trench Foot. Treasure Island, FL: StatPearls Publishing; 2018. Retrieved from: www.ncbi.nlm.nih.gov/books/NBK482364.
16. Dissemond J, Bültemann A, Gerber V et al. WeitereDefini- tionen und Schreibweisenfür die Wundbehandlung. Hautarzt 2017; 68: 415–7.
17. Voegeli D. Incontinence-associated dermatitis: new insights into an old problem. Br J Nurs 2016;25:256, 258, 260-2.
18. Dini V, Janowska A, Oranges T et al. Surrounding skin man- agement in venous leg ulcers: A systematic review. J Tissue Viability 2020; 29: 169–75.
19. Rippon MG, Ousey K, Cutting K. Wound healing and hyper-hydrationVa counter intuitive model. J Wound Care 2016;25(2):68-75.
20. Gray M, Colwell JC, Doughty D, et al. Peristomal moisture–associated skin damage in adults with fecal ostomies: a comprehensive review and consensus. J Wound Ostomy Continence Nurs 2013;40:389-99.
21. Almutairi D, LeBlanc K, Alavi A. Peristomal skin complications: what dermatologists need to know. Int J Dermatol 2018; 57: 257–64.
22. Bernatchez SF, Mengistu GE, Ekholm BP, Sanghi S, Theiss SD. Reducing friction on skin at risk: the use of 3MTM CavilonTM No Sting Barrier Film. Adv Wound Care (New Rochelle). 2015;4(12):705710. doi:10.1089/ wound.2015.0628
23. LeBlanc K, Baranoski S, Christensen D, et al. International Skin Tear Advisory Panel: a tool kit to aid in the prevention, assessment, and treatment of skin tears using a simplified classification system. Adv Skin Wound Care 2013;26:459-76.
24. LeBlanc K, Baranoski S. Skin tears: state of the science: consensus statements for the prevention, prediction, assessment, and treatment of skin tears. Adv Skin Wound Care 2011;24(9 Suppl):2-15.
25. Arndt JV, Kelechi TJ. An overview of instruments for wound and skin assessment and healing. J Wound Ostomy Continence Nurs 2014;41:17-23.
26. Baranoski S, LeBlanc K, Gloeckner M. CE: preventing, assessing, and managing skin tears: a clinical review. Am J Nurs 2016;116(11):24-30.
27. International Skin Tear Advisory Panel (ISTAP). 2023. Available from: www.skintears.org.

How
Masterclass Guide: Moisture Associated Skin Damage. Wound Masterclass. Volume 2. No 4. March 2023.
Masterclass
12 Wound Masterclass - Vol 2 - March 2023
GUIDES
to Cite this Article © Copyright. Wound Masterclass. 2023
the Wound Masterclass website
Visit
to transform wound care.
To learn more about MIRRAGEN®, the only bioactive glass wound martrix on the market, contact us today.


etswoundcare.com

engineered













woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register Lessons From Space for Wound Care: All Your Questions Answered Concise. Interactive. Accredited. MasterSeries 60 Minutes Interactive June 14th: 1pm EST | 6pm GMT woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register MasterSeries 60 Minutes Interactive Better wound care. Better content. Better clinical articles. Get accredited. June 14th: 1pm EST | 6pm GMT Lessons From Space for Wound Care: All Your Questions Answered Supported by Moderator Dr Negin Shamsian Consultant Plastic & Reconstructive Surgeon (Locum) Global expert Dr M. Mark Melin Medical Director of the M Health Wound Healing Institute Global expert Dr Alan R. Hargens Professor and Director Emeritus of the Orthopaedic Clinical Physiology Lab at the University of California Previously Chief, Space Physiology at NASA Ames and Consulting Prof at Stanford University Global expert Ms Kelli Kedis Ogborn Space Economy Strategist, Technology Commercialization Expert Vice President of Space Commerce and Entrepreneurship at Space Foundation Global expert Dr Danielle Carroll General Surgery Resident, TRISH-funded Space Health researcher, USAF Veteran, and Instructor Pilot President of the AsMA Space Surgery Association Founder, Orbital Biodesign Global expert Dr Rowena Christiansen Fellow of the Aerospace Medical Association (AsMA) Member of the Australian Space Agency Space Medicine and Life Sciences Technical Advisory Group Research Director, Health Law for the Jus Ad Astra Global expert Dr Heather Barnhart Professor, Dept of Physical Therapy, Nova Southeastern University Member of the Academy of Clinical Electrophysiology and Wound Management Special Interest Group Past President of Global expert Dr Christine Mehner Assistant Professor of Biomedical Engineering Research Scientist Department of Physiology and Biomedical Engineering, Mayo Clinic
Limitations of Applying Summary Results of Clinical Trials to Individual Patients: The Need for Risk Stratification
Editorial Summary
One of the limiting factors in contemporary wound care research is the elidation and ignorance of the pathologies with wound care data. This leads to the use of statistical tools that cannot deal with the nature of the data. This article explores some of the problems and solutions with the application of wound care data, and outlines the need for risk stratification.

Introduction
When I began writing this article I originally titled it ‘The Pathology of Wound Care Data’, but the title did not seem quite right. Pathology refers to the ‘study of causes and effects of the disease or injury’; this was not quite what I wanted to write about regarding wound care data. The more I thought about it, pathophysiology seemed to fit better. Pathophysiology is the study of a ‘disordered physiological processes that cause, result from, or otherwise are otherwise associated with a disease or injury’.
Wound care data in particular, but data in general, can be thought of as the result of a data generating process in the real world. In a perfect world, researchers design experiments where the data generating process is not one of these ‘disordered physiological processes’; however, in the real world, wound care data is often ‘diseased or injured’. Wound care data possesses many statistical pathologies that make analysis and inference difficult, and if not identified and treated, these pathologies can cause analysts and researchers to arrive at conclusions that range from completely wrong, to less correct than they could be.
I have often thought of creating something like a DSM-4 (The Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) (American Psychiatric Association [APA], 2000) is a compendium of mental disorders, a listing of the criteria used to diagnose them, and a detailed system for their definition, organization, and classification) of wound care data; a guide on how to identify
these statistical pathologies, and how to attend to them in a principled and ethical manner. This article is an abridged version of such a guide, where we focus mostly on the diagnosis and description of the pathologies that I come across most often in my work: censoring and truncation, measurement error, fat tails, and zero-inflation.
Censoring and Truncation
Censoring occurs when the value of an observation is only partly known. An example of censoring, when the number of observations is equal to one, would be an unstageable pressure ulcer. As there is an obstruction to viewing the underlying tissue, the clinician cannot determine whether the wound is actually a stage 3 or 4 pressure ulcer, and thus the ‘unstageable moniker’ is used, which explicitly indicates that censoring has occurred. Carrying this idea forward, one could think of a deep tissue injury as a censored stage 1, 2, 3, or 4 pressure ulcer.
Censoring most often occurs in the longitudinal analysis of wound care data; longitudinal wound care data comprises repeated measurements of the same wounds over time. The most often used longitudinal design is a pre-post test (one repeat measurement), where there is one initial baseline measurement, and then one single follow-up measurement. When there is more than one repeat measurement, we call this a longitudinal cohort study. In the context of longitudinal data, there are 3 types of censoring: left-censoring, right-censoring, and interval-censoring. Interval-censoring can combine with left or right censoring, and a wound can have multiple occurrences of
15 Wound Masterclass - Vol 2 - March 2023
University of Pennsylvania
Mr Zwelithini Tunyiswa Open Wound Research,
Concord NC, United States
© Copyright. Wound Masterclass. 2023
interval-censoring. Truncation is related to but not the same as censoring, insofar as there is no missing information about the wound itself, only the absence of data during the period of interest. When it relates to longitudinal data, truncation occurs when the wound heals prior to the start of the study window. For example, let’s say that our study has two screening visits, and the wound is initially observed at the first screening visit and heals by the second screening visit; that wound would be truncated, relative to the study window.
Censoring can be attended to by using imputation methods to estimate the true value of the measurement(s) at the missing time step(s). Methods range from the naive, such as linear interpolation, to the sophisticated such as hierarchical models, Gaussian processes, or neural nets.1
Measurement Error
Measurement error occurs when the recorded values of an observation are different from the true value. The most common example is a simple wound measurement; any wound has an empirical value for its area, however, there are many ways to measure the wound, both analog and digital. Inherent in all of them is the fact that there is a gap between the empirical (true value) and what is measured. Even if the same method is used to measure the wound, say measurement tape, the measurements will be slightly different depending on who is performing the measurement.
It is good practice to assume the omnipresence of measurement error and to be reasonably skeptical of data. Measurement error can be attended to by using exploratory data analysis, and Bayesian inference, including but not limited to Bayesian Hierarchical models, to estimate true values.2
Fat Tails
Fat tails occur when the empirical distribution of data exhibits a large skewness to one or both sides. Generally, in wound care, the skewness is to the right, such as in the representation of a cohort’s wound time-to-heal (TTH) in days. Fat-tailed distributions, unlike normal distributions, cannot be described fully using just the mean (or average) and standard
deviation.
In fact, the notion of mean and standard deviation in fat-tailed distribution does not connote the same thing as in a normal distribution; in a normal distribution, the mean describes the central point of the data, and the standard deviation (or sigma) is the spread of the data around that distribution. Of values, 68% will fall within one sigma of the mean, 95% within 2 sigmas, and 99.7% within 3 sigmas. These implications don't hold if the empirical distribution of the data is fat-tailed, and pretending as if they do leads to a myriad of inferential problems.
Fat tailed distributions abound in wound care data. We see it not only in TTH, but in the starting area of wounds in a cohort, proportion of high risk patients who develop state 2 - 4 pressure ulcers, total cost of wound care, daily cost of wound care, etc. We even routinely run into our friend, the Pareto distribution, or as it is commonly referred to, the ‘80 - 20 rule’, when describing the share of costs related to a large population of wounds. For example, 20% of wounds result in 80% of wound care costs to an insurer, or 2% of wound care cases result in 30% of a nursing home’s medical malpractice costs.
Fat tails are not amenable to the traditional way of analyzing data. That is to say, the mean and standard deviation are meaningless in describing and analyzing this type of data. The mean is not the parameter of interest, but the extremes, as they generate most of the consequences. The best approach to understanding fat tailed distributions is through probabilistic models that estimate parameters or values of interest.3
Zero-Inflation
Zero-inflation occurs when data exhibits a disproportionately large number of zeros in its empirical distribution. Zero-inflation generally suggests one of two things; first, that there are two processes captured in the data, or second, that left-censoring is occurring in a significant part of the sample.
Let’s discuss the first case; say a nursing home collects data for a weekly count of new nosocomial pressure ulcers. They find that in 20 of 52 weeks there were no new nosocomial pressure ulcers, and in the remaining weeks between 1
Limitations of Applying Summary Results of Clinical Trials to Individual Patients: The Need for Risk Stratification 16 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
Limitations of Applying Summary Results of Clinical Trials to Individual Patients: The Need for Risk Stratification
and 10 new nosocomial pressure ulcers were counted. In effect, there are two processes; the case where there is no new pressure ulcer, where the value is always 0, and the case where there are new nosocomial pressure ulcers, and the count is greater than 0.
In the second case, let’s say that we have a retrospective observational study of 1,000 pressure ulcers from 700 patients where we are looking at TTH in days. As we examine the data, we notice that 20% of the data is zero; a skeptic might ask how it is possible for a wound to occur and heal all on the same day? That would seem highly unlikely, and would make one wonder about the data generating process; there might be systemic left-censoring occurring as a result of the TTH being computed from the start of the study window, rather than the date of injury; not only would this result in zeroinflation, but systemically would pull the data leftwards, resulting in an undercounting of the actual TTH of all wounds.
Zero-inflated data is best modelled using a class of tools called mixture models. These models are able to model the parameters of both processes and result in good approximations of the data. In the case of systemic left-censoring, as described in the second case of zero-inflation above, Bayesia mixture models can estimate the true length of TTH of the wounds by systematically adjusting TTH upwards based on the model and observed data, and pooling of information across wounds.
Conclusion
Acknowledgement of the pathologies of wound care data allows us to identify ways that we can deal with them in a principled and ethical manner. One of the limiting factors in contemporary wound care research is the elidation and ignorance of the pathologies with wound care data; this leads to the use of statistical tools that cannot deal with the nature of the data. This is unfortunate, as we are in a golden age of statistical flexibility, statistical software, and computational power. The combination of these 3 factors allows us to deal with many of these pathologies, and arrive at better insights that allow clinicians to deliver better care, decrease the cost of care to payers, drive innovation in wound care modalities, and drive forward the field of wound care.
References
1. Colchero F, Clark JS. Bayesian inference on age-specific survival for censored and truncated data. J Anim Ecol. 2012 Jan;81(1):139-49. doi: 10.1111/j.1365-2656.2011.01898.x. Epub 2011 Aug 26. PMID: 21883202
2. Chapter 4. Measurement error and bias. Epidemiology for the uninitiated. [Internet]. Available from: https://www.bmj.com/about-bmj/resources-readers/publications/epidemiologyuninitiated/4-measurement-error-and-bias. Accessed 11/04/2023
3. Haas, M., Pigorsch, C. (2009). Financial Economics, Fat-Tailed Distributions. In: Meyers, R. (eds) Encyclopedia of Complexity and Systems Science. Springer, New York, NY. https://doi. org/10.1007/978-0-387-30440-3_204
Wound Masterclass - Vol 2 - March 2023 17 © Copyright. Wound Masterclass. 2023
largerBrandnew size To know more about our Debrisoft® family and other L&R products visit lohmann-rauscher.co.uk Debrisoft is registered to L&R. ® 2019. The whole Debrisoft® Family is now
NICE
Debrisoft®
recommended by
NICE concluded that
is more clinically and cost effective than other debridement methods
 Mr Bernard Ross
Mr Bernard Ross
 CEO of Sky Medical Technology
CEO of Sky Medical Technology
Liverpool, United Kingdom
The Dawning of a New Horizon For Venous Leg Ulcer Treatment
Editorial Summary
A high percentage of patients present with chronic or recurrent venous leg ulcers that are associated with a reduction in health-related quality of life and an increased economic burden to healthcare systems. The current challenge of generating high quality evidence for potential treatments is detrimental to patient outcomes. Implementing a self-controlled trial design in addition to using more achievable trial endpoints may provide a solution to the current lack of evidence. Furthermore, the use of a neuromuscular electrical stimulator as well as standard wound care may improve ulcer management. This article provides an overview of a study implementing a randomised self-controlled study design to assess the efficacy of a wearable device neuromuscular electrostimulator.
Introduction
Venous leg ulcers (VLU) inflict a substantial burden on healthcare services, demanding significant resources.1-3
It is estimated that 3% of the adult population worldwide are affected by VLUs.4 In the United Kingdom alone, approximately 3.8 million adults are affected by a wound. Many become chronic and difficult to manage, despite following treatment guidelines. More than 50% of VLUs do not heal within 12 months,5 and that number is predicted to grow.6 The cost of VLUs to overburdened healthcare systems is also staggering, higher than that of both cancer and cardiovascular disease. Annually, the cost to the NHS of managing wounds is £8.3 billion, £5.6 billion of which is for the treatment of wounds that fail to heal.5 Nurse visits in addition to dressings and compression bandages contribute to the huge financial burden faced by healthcare providers when managing patients with recurrent and difficult to treat VLUs.7 Most individuals with leg ulceration have venous disease, however around 20% have arterial disease. There are some patients for whom both arterial and venous disease is a factor.8 Because chronic venous insufficiency (CVI) is not diagnosed until VLUs have developed, treatment is often complex and a large proportion of patients experience recurrent ulceration. 7 Although VLUs can arise spontaneously, they often appear following minor trauma and exist clinically as part of CVI.2
Challenges to Proving Treatment Efficacy
Generally, when investigating the effectiveness of treatments for healing wounds, the outcome of the study is complete wound resolution. There are numerous problems associated with this strategy. Given that a large percentage of leg ulcers become chronic, taking potentially months to resolve fully, using complete healing as the primary outcome means lengthy trial periods would be needed to reach any statistical significance. The adoption of conventional randomised controlled trials (RCT) introduces a supplementary problem. The heterogeneity of ulcers makes matching trial control and intervention participant groups impossibly challenging. 12
Adopting alternative trial endpoints like speed of wound healing presents a viable option to assess the efficacy of possible treatments. In addition, modifying trial design to a self-controlled model, collecting data post-intervention, and comparing it with data from the same individual’s control data pre-intervention, removes confounding heterogeneity present in typical RCT models. This technique has been approved by opinion leaders in a consensus document who have concluded that self-controlled study designs might be more suitable for investigating treatment efficacy than more usual RCTs.12
20 Wound Masterclass - Vol 2 - March 2023
© Copyright. Wound Masterclass. 2023
The Neuromuscular Electrostimulator
The geko™ device (Firstkind Ltd, Daresbury) is a small, self-adhesive, wearable neuromuscular electro stimulator (NMES). It is placed on the lateral aspect of the leg just below the knee, over the head of the fibula. The NMES is designed to sit on the skin’s surface and deliver a gentle electrical pulse once each second to the common peroneal nerve. This augments the venous, arterial and microvascular flow of the leg and foot by activating the muscle pumps, essentially mimicking the effects of exercise.13
Since leg ulcers form as a result of venous insufficiency, it follows that compression provides an effective treatment by opposing hydrostatic pressures in the leg and increasing venous flow. However, most venous blood flow in the lower limb is driven by muscle pumps. Therefore, immobility and muscle pump dysfunction can exacerbate venous insufficiency. Exercise has previously proven effective when combined with compression in patients with VLUs. Therefore, the benefits of using neuromuscular electrical stimulation to activate muscle pumps in the legs are implied.12
The innovative application of non-invasive neuromuscular electrostimulation has been developed into a ground-breaking NMES platform called OnPulse™. This has then been integrated into its geko™ device, an industryleading product.14
Publication of a landmark multi-centre randomised self-controlled trial (RCT. Sky Medical Technology work to develop products specifically tailored to suit different medical needs. The company delivers products in a range of clinical settings with an interest in chronic wounds, treating and preventing oedema, and the prevention of venous thromboembolism (VTE). The overarching aim in any setting is to work collaboratively with healthcare professionals to achieve better patient outcomes
while delivering cost-effective treatment.14
Overview of Study
The study to assess the effectiveness of the NMES (geko™ device) was conducted using the self-controlled trial design to combat the challenge of collecting sufficient evidence, figure 1. This was combined with the use of two metrics of short-term intermediate determinants of efficacy. Healing rate outcomes were used in place of complete healing. This offered the opportunity to assess the efficacy of the novel treatment swiftly, with greater sensitivity and with more nuance. The primary endpoint of the study was the rate of Wound Margin Advance (WMA). The study compared standard of care (SoC), which consisted of multi-layer compression bandaging or hosiery kits, with SoC supplemented with the use of NMES (geko™ device).12
Interim analysis of the first 20 participants was performed to determine sample size. Ultimately, 60 patients participated.12 Participants were randomised to receive either SoC alone or NMES (geko™) for 12 hours each day in addition to SoC. Randomisation was 1:1 using the Castor EDC platform using variable block size and differences in group sizes were due to patient exclusion post-randomisation. 12 To be included in the trial, participants had to meet various inclusion criteria. This included being 18 years or older, being able to give written informed consent and having a chronic venous leg ulcer due to underlying venous disease that had been present for at least 6 weeks, but not more than five years before entry into the study. The size of the ulcer was also specified at between 3 cm2 and 39 cm 2, with an anklebrachial pressure index (ABPI) of 0.8-1.2. No active wound infection could be present for at least 48 hours before commencing the study and no systemic antimicrobial for a minimum of 7 days prior to the start of the study was permitted.12
The Dawning of a New Horizon For Venous Leg Ulcer Treatment Wound Masterclass - Vol 2 - March 2023 21 © Copyright. Wound Masterclass. 2023
“The study to assess the effectiveness of the NMES was conducted using the selfcontrolled trial design, to combat the challenge of collecting sufficient evidence.”
Overall, there were 22 participants in the SoC arm and 29 in the NMES arm. Nine patients were withdrawn post-randomisation because they failed to meet the study criteria, withdrew consent, or did not comply. There was no difference in the ratio of male to female patients between the groups and no significant differences were found between the two cohorts according to unpaired t-tests.12
Patients in both arms spent 4 weeks on a runin phase where they received only SoC. This phase was then able to be used as within-patient control for those on the NMES arm of the trial. After the initial run-in phase, the 29 patients on the NMES randomised arm went on to receive 4 weeks of NMES with the geko™ device, in addition to SoC. The 22 patients in the SoC cohort continued to receive SoC alone for a further 4 weeks.
The study was powered to allow comparison between the run-in and trial phases within each cohort but not between the SoC and NMES cohorts.12 Wounds were photographed prior to debridement at day 0 and at every weekly visit. The Aranz SilhouetteStar™, a digital camera which is part of the Silhouette™ wound assessment system, was used. This is a portable, non-contact device which can image and measure ulcers. The collected images were sent in a random order to be assessed by an international independent wound expert for delineation of the wound perimeter. From this, the wound area and perimeter could be calculated. The independent assessor was blinded to the image date as well as trial arm.12 Patients were followed up for 3 months following the 4-week intervention phase. However, complete wound healing during this time was only reported using patient self-report with no clinical measurements or examinations performed. Patients in both arms of the study were provided with SoC only during this time.12
The Dawning of a New Horizon For Venous Leg Ulcer Treatment 22 Wound Masterclass - Vol 2 - March 2023
“Patients in both arms spent 4 weeks on a run-in phase where they received only SoC. This phase was then able to be used as within-patient control for those on the NMES arm of the trial.”
Screening Recruitment (Day 0) Run-in Phase Standard of Care (Day 0 to 28) Self Controlled Paired Comparisons Randomisation Self Controlled Paired Comparisons Standard of Care (SOC) SOC plus NMES 12hr daily Treatment Phase (Day 28 - 56) Treatment Phase (Day 28 - 56) Long Term Follow-Up Phase (3 Months) SOC subjects continue Standard Care NMES subjects revert to Standard of Care Subject Journey © Copyright. Wound Masterclass. 2023
Figure 1: Trial design schematic.
Assessed Endpoints
A prerequisite for the use of speed of healing as a primary endpoint when comparing the control and intervention arm, is that the endpoint is linear in its trajectory. A mathematical method for calculating wound margin advancement (WMA) has previously been developed. The advancement of the wound margin in leg ulcers treated with compression therapy has subsequently been shown to follow a linear trajectory over a 4-week period.15-16 Therefore, a 4-week run-in phase and 4-week trial phase was appropriate to assess the efficacy of the NMES device.
WMA, the primary endpoint of the study, is powerful predictor of wound healing. Vidal’s method was used to calculate WMA ,15 where area/perimeter of the wound was calculated and then regressed against time. Rate of wound healing was also calculated using the metric of Percentage Area Reduction (PAR). PAR was represented by the reduction in total wound area as a percentage of the initial wound area at the beginning of the 4-week trial period.12
The study also assessed several secondary endpoints for descriptive analysis. These included adherence, rates of infection, percentage complete healing, quality of life scores EQ-5D-5L, Cardiff Wound Impact Schedule (CWIS), Venous Clinical Severity Score (VCSS), and Visual Analog Score (VAS) for pain.

Success of NMES (geko™ device):
The study described demonstrated that the NMES device, when used in combination with SoC, was successful in increasing the rate of wound healing. In comparison to SoC alone, the intervention arm saw a statistically significant (p=0.016) two-fold increase in the rate of healing, as measured by the primary outcome, WMA. The control arm saw no significant difference in the rate of healing between each trial phase.12
PAR of the ulcer was also analysed. This too showed a two-fold acceleration in healing rate (p=0.011) during the treatment phase for the intervention arm of the trial. The rate of healing remained the same throughout the run-in and treatment study phases for the SoC cohort.12
The Dawning of a New Horizon For Venous Leg Ulcer Treatment Wound Masterclass - Vol 2 - March 2023 23
“The study described demonstrated that the NMES device, when used in combination with SoC, was successful in increasing the rate of wound healing.”
Reproduced with the kind permission of International Wound Journal and Wiley Publishers. © Copyright. Wound Masterclass. 2023
Figure 2: A typical trajectory of a single wound over the 8 weeks.
Images of each wound were taken throughout the trial to be independently assessed. For the patients in the intervention group, the size and perimeter of the wound was largely unchanged for the run-in phase of the trial when only SoC was provided. The wound margin is then seen to progressively advance and the wound area reduce during the second half of the trial, when patients received treatment with the NMES device (geko™).
Regardless of efficacy, the device would not be suitable for clinical application without the approval of patient populations. Concordance with treatment plays an important role in treatment outcome. In the trial, the NMES device (geko™) was reported to be well tolerated with a concordance rate of 94.1% among study participants. Self-administration was also reported to be simple and without issue.12 Other important findings among patients in the treatment arm was a greater reduction in Visual Analogue Score (VAS) for pain and improved Venous Clinical Severity Score (VCSS). More patients in the treatment arm saw their wound completely heal from the start of the trial to the end of the follow-up period.12 The descriptive reporting of secondary outcomes is most likely linked to the reduction in wound size that was seen in patients who were treated with the NMES device (geko™). As ulcers healed more quickly, it is reasonable that they would report a greater reduction in pain and more frequent complete wound healing.
Clinical Implications
This study was the first of its kind to demonstrate a statistically significant increase in rate of healing. The use of an NMES device (geko™) to stimulate the common peroneal nerve in patients with chronic, difficult to treat VLUs has proven effective in significantly accelerating the rate of healing. The rate of wound healing in these patients more than doubled, 12 which if applied on a wider scale could prove beneficial in improving the quality of life of the patient, in addition to being hugely cost-effective it appears to shorten the time for complete wound resolution which will reduce costs associated with
nursing visits, dressings, and associated co-morbidities. Rate of wound healing is a clinically important parameter when treating patients with VLUs. Chronic and recurrent ulceration is associated with a reduction in mobility, pain, and quality of life.2 Treatments which accelerate the healing process can therefore have a positive effect on patient outcomes. The NMES device (geko™) appears to demonstrate this potential.
“As a clinician in wound care.. the results of this RCT are extremely impressive. NonhealingVLUsstoppatientslivingtheirlives and robs them of hope. The geko™ device consistently accelerates VLU healing in the patients I treat.” Agnes Juguilon Collarte Tissue Viability Specialist Nurse Lead, Inner Northwest Division (Central London, Hammersmith & Fulham, West London) .12
The device demonstrated simplicity of use with high rates of compliance seen among participants. The study was also able to continue throughout the pandemic even with the enforcement of COVID-19 protocols.12 This again demonstrates the ease of use of these remote devices and the potential for their implementation into clinical practice to relieve pressure on burdened healthcare systems. Empowering patients to deliver their own care remotely will not only be cost-efficient but enable patients to receive high quality and efficacious treatment without the need for repeated hospital appointments. Using a self-controlled study model with the introduction of short-term endpoints in place of full wound healing not only allowed for statistically significant results to be achieved quickly, but also eliminated confounding heterogeneity between cohorts.12 Assessing WMA and PAR as endpoints enabled clinicians to determine more nuanced and sensitive improvements in wound healing in patients treated with NMES when compared to the SoC group. Using this trial design also enables researchers to achieve results with fewer study participants. 12 This study sets a precedence for the implementation of this trial design in future to gather more substantial evidence. It also begins to address the current lack of evidence base surrounding treatments for VLUs.
The Dawning of a New Horizon For Venous Leg Ulcer Treatment 24 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
Acknowledgements
Dr. Thomas E. Serena MD FACS FACHM MAPWCA., independent wound care expert responsible for the blinded assessment of anonymised wound images.
Ms M Bowden for preparation of editorial manuscript.
Participating centres that enrolled subjects:
Sarah Bradbury, Welsh Wound Innovation Centre, Rhodfa Marics, Ynysmaerdy, Pontyclun, Rhondda Cynon Taf, Wales, CF728UX.
Isaac Nyamekye, Worcestershire Acute Hospitals NHS Trust, Worcestershire Royal Hospital, Charles Hastings Way, Worcester, WR5 1DD.
Janice Tsui, Royal Free London NHS Foundation Trust, Royal Free Hospital, Pond Street, London, NW3 2QG.
Karen Reay, South Tyneside and Sunderland NHS Foundation Trust, Clarendon House, Windmill Way, Hebburn, NE31 1AT.
Juliet Price, Royal Devon and Exeter NHS Foundation Trust, Royal Devon and Exeter Hospital, Barrack Road, Exeter, EX2 5DW.
Richard Bull, Accelerate CIC, Centenary Wing, St Joseph’s Hospice, Mare St, Hackney, London, E8 4SA.
Kay Baxter, Barnsley Hospital NHS Foundation Trust, Barnsley Hospital, Gawber Road, Barnsley, S75 2EP.
Chandana Wijewardena, Queen Elizabeth Hospital NHS Foundation Trust, The Queen Elizabeth Hospital, Gayton Road, Kings Lynn, PE30 4ET.
James Coulston, Somerset NHS Foundation Trust, Musgrove Park Hospi-tal, Taunton, TA1 5DA. Richard Gaunt, Rowden Medical Partnership, Rowden Hill, Chippenham, SN15 2SB.
Sam Davis, West Walk Surgery, Yate West Gate Centre, 21 West Walk, Yate, BS37 4AX.
Amardeep Heer, Lakeside Healthcare, Lakeside Surgery, Cottingham Road,Corby, Northants, NN17 2UR.
N’Jaimeh Asamoah-Owusu, The Crouch Oak Family Practice, 45 Station Rd, Addlestone, KT15 2BH.
Gordon Irvine, The Brekland Alliance, Grove Lane, Thetford, IP24 2HY.
Patrick Moore, The Adam Practice, 306 Blandford Road, Poole, BH15 4JQ.
Stephanie Howard, Norfolk Community Health and Care NHS Trust, Norwich Community Hospital, Bowthorpe Rd, Norwich, NR2 3TU.
Katherine Morgan, Lancashire Care NHS Trust Foundation, Leyland Clinic, Yewlands Drive, Leyland, PR25 2TN.
Agnes Collarte, Central London Community Healthcare NHS Trust, St Charles Centre for Health & Wellbeing, Exmoor St, London, W10 6DZ.
Shruti Singh, Trafalgar Group Medical Practice, 25 Osbourne Road, Southsea, Ports-mouth PO5 3ND.
Heart of Bath: Tim Johnson, Heart of Bath, Oldfield Surgery, 45 Upper Oldfield Park, Bath BA23HT.
This article was funded by Firstkind Ltd, Daresbury, Cheshire, UK.
References
1. Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12):e009283.
2. Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. Journal of the European Academy of Dermatology and Venereology. 2016;30(11):1843-75.
3. Harding KG. Chronic wounds: a clinical problem requiring ownership and coordination. British Journal of Dermatology. 2022;187(2):133-4.
4. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: Incidence and prevalence in the elderly. Journal of the American Academy of Dermatology. 2002;46(3):381-6.
5. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10(12):e045253.
6. Probst S, Weller CD, Bobbink P, Saini C, Pugliese M, Skinner MB, et al. Prevalence and incidence of venous leg ulcers—a protocol for a systematic review. Systematic Reviews. 2021;10(1):148.
7. Phillips CJ, Humphreys I, Thayer D, Elmessary M, Collins H, Roberts C, et al. Cost of managing patients with venous leg ulcers. International Wound Journal. 2020;17(4):1074-82.
8. Nelson EA, Adderley U. Venous leg ulcers. BMJ Clin Evid. 2016;2016.
9. Briggs M, Flemming K. Living with leg ulceration: a synthesis of qualitative research. J Adv Nurs. 2007;59(4):319-28.
10. Green J, Jester R. Health-related quality of life and chronic venous leg ulceration: part 2. British journal of community nursing. 2010;15(Sup1):S4-S14.
11. Maddox D. Effects of venous leg ulceration on patients’ quality of life. Nursing standard. 2012;26(38):42-50.
12. Bull RH, Clements D, Collarte AJ, Harding KG. The impact of a new intervention for venous leg ulcers: A within-patient controlled trial. Int Wound J.2023;1-9.
13. Tucker A, Maass A, Bain D, Chen LH, Azzam M, Dawson H, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol. 2010;19(1):e31-7.
14. Sky Medical Technology Ltd. Sky Medical Technology: New study demonstrates double the rate of healing of venous leg ulcers with the geko™ device 2023 [cited 02 April 2023].
Available from: https://www.prnewswire.co.uk/news-releases/sky-medical-technology-new-study-demonstrates-double-the-rate-of-healing-of-venous-leg-ulcers-with-the-gekodevice-301784552.html.
15. Vidal A, Zerón HM, Giacaman I, Romero MdSC, López SP, Trillo LEM, et al. A simple mathematical model for wound closure evaluation. Journal of the American College of Clinical Wound Specialists. 2015;7(1-3):40-9.
16. Bull RH, Staines KL, Collarte AJ, Bain DS, Ivins NM, Harding KG. Measuring progress to healing: A challenge and an opportunity. International Wound Journal. 2022;19(4):734-40.
17. Image: Sky Medical Technology Ltd. Sky Medical Technology: New study demonstrates double the rate of healing of venous leg ulcers with the geko™ device. 2023 [cited 02 April 2023]. Available from: https://www.prnewswire.co.uk/news-releases/sky-medical-technology-new-study-demonstrates-double-the-rate-of-healing-of-venous-leg-ulcers-with-the-gekodevice-301784552.html.
Venous
Ulcer Treatment Wound Masterclass - Vol 2 - March 2023 25
The Dawning of a New Horizon For
Leg
© Copyright. Wound Masterclass. 2023

Hartmann Ad
Masterclass GUIDES
Introduction
This Masterclass Guide provides a concise overview of a hydroresponsive wound dressing (HRWD), and how to incorporate this into your clinical practice.
Evidence suggests a number of barriers to the healing of a wound, including: devitalized tissue, necrotic tissue, biofilm, and an imbalance of wound biomarkers such as matrix metalloproteinases (MMPs).
What Are Hydro-Responsive Dressings?
Hydro-responsive wound dressings are versatile dressings with the ability to adjust moisture levels depending on the wounds requirements, providing moisture when debridement is needed and absorbing moisture when exudate is present.


They are non-medicated wound dressings with a pivotal role of reducing bacterial load by:
• Absorptive properties leading to reduction of bioburden and removal of bacteria
• Aiding in removal of devitalised and necrotic tissue
What Is HydroClean®?
■ HydroClean® offers a multidimensional mechanism incorporating properties of:
■ Cleansing
■ Debriding
■ Exudate absorption
■ Binding and retention of wound biomarkers that may delay wound progression
■ The dressing is comprised of a soft, conforming pad containing Super Absorbant Polymers (SAP) particles which regulate the excess matrix metalloproteinases (MMP) which may impair wound healing
■ A variety of sizes and shapes are available that are designed for and applicable to wounds on multiple areas of the body
Keywords
■ Wounds
■ Wound
■ Chronic wounds
■ Wound bed
■ Debridement
Hydro-Responsive Wound Dressing: HydroClean®
■ Autolytic debridement
■ Hydroresponsive
■ HydroClean®/ HydroClean® Advance
■ Sequestration
How Does HydroClean® Work?
■ HydroClean® has a unique mechanism of action encompassing several key and pivotal steps:
■ Softening: this is optimized by a continuous release of Ringer’s solution thereby enhancing and regulating the body’s own enzymes and inflammatory process to rehydrate, soften and liquify devitalized tissue and aid removal from the wound bed
■ Absorption of wound exudate, slough and bacteria into the dressing and removal of fibrin and necrotic tissue
■ Sequestration and retention: the dressing binds devitalized tissue and bacteria reducing bacteria load and biofilm
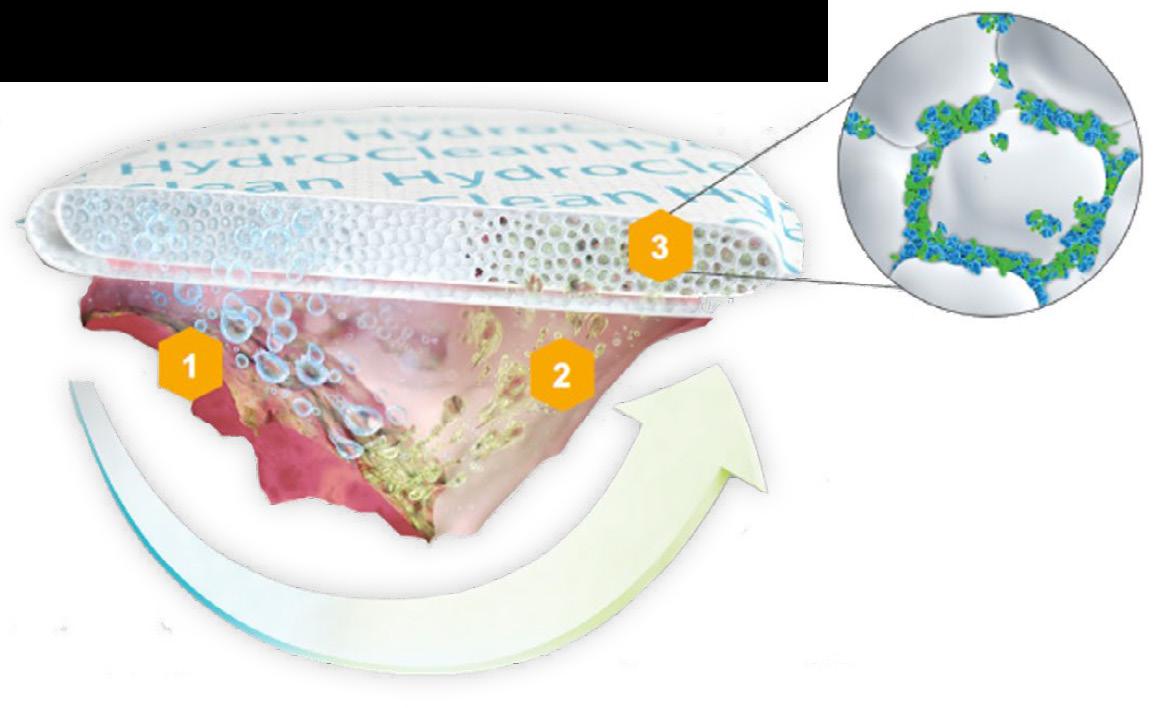
■ Removal: When changed, the devitalized tissue and bacteria are removed with the dressing
1
2
3
Debridement
Rehydrate, soften and liquify devitalized tissue and aid removal from the wound bed
Absorption
Continuously releases Ringer’s solution facilitating autolysis of necrosis
Sequestration
Bacteria are locked-in effectively inside the dressing core
4 Retention
Elements of the SAP core trap and immobilise bacteria, MMPs and other impediments
5 Removal Removes fibrin and necrotic tissue within which bacteria may reside; absorbs wound exudate
28 Wound Masterclass - Vol 2 - March 2023
Figure 2: HydroClean® dressing types
Figure 1: HydroClean
1 2 3
4 5
© Copyright. Wound Masterclass. 2023
Hydro-Responsive Wound Dressing: HydroClean®


What Types of Wounds Are Suitable?
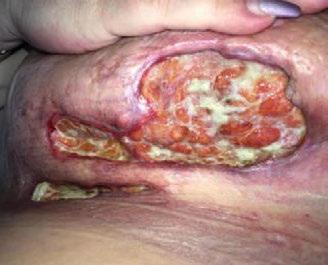
VIDEO LINK
Scope of Usage
Figure 3
VENOUS 3a 3b 3c 3d
ULCERATION
3a, 3b: Day 0: Start of treatment
This 89 year old diabetic female presented with bilateral venous ulceration as a result of chronic venous insufficiency, poor nutritional status and an elevated BMI. She presented with multiple exudating wounds of the bilateral lower limbs associated with extensive oedema. There were high levels of pain associated with each dressing change.
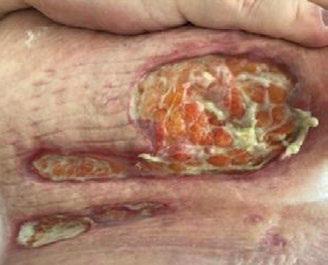


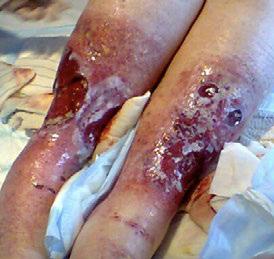
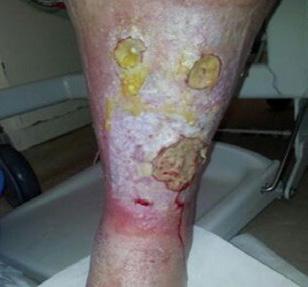
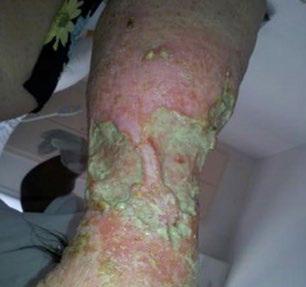
4a
4b
3c, 3d: Day 38
4c: Week 4. HydroClean was selected in order to remove fibrin and to reduce the characteristic pain of PG within a short period of time. The patient’s pain was reduced 3-4 points after 2 weeks.
4d: Week 12: a significant increase of granulation tissue and a reduction of fibrin. HydroClean® was crucial for reduction of pain and itching and is effective management when used with a systemic immunomodulatory therapy – e.g. biologicals under clinical supervision.
© Copyright. Wound Masterclass. 2023
Wound Masterclass - Vol 2 - March 2023 29
Masterclass
GUIDES
The patient, with underlying dementia, was initially very agitated during dressing changes. After effective analgesia 1 hour prior to the treatment, the wound was cleaned daily with soap and water and HydroClean was applied with adapted venous compression. After granulation tissue formed, HydroTac® dressing was utilized to absorb the remaining fibrin and exudate, while protecting the granulation tissue. By day 38, the wounds on both legs had reduced in size, with increased granulation tissue and epithelialisation. The rapid autolytic debridement produced by HydroClean allowed mechanical debridement to be avoided which would have been high risk in this patient. PYODERMA GANGRENOSUM
4a: Week 0: A 52-year-old female patient presented with pyoderma gangrenosum (PG).
4b: Week 1: The wounds, at 2.5 x 7cm and 8 x 12cm, were painful (VAS 7/10). Different types of dressings were used, but were found to result in erythema, oedema and itching.
4c
4d
Figure 4
HydroClean® is suitable for usage in healing chronic and hard-to-heal wounds
HydroClean® in action:
GUIDES
Hydro-Responsive Wound Dressing: HydroClean®
HydroClean® is a hydro-responsive, non-medicated wound dressing with specific important properties. The combination mode of action of cleansing, debriding, exudate absorption, and bacterial binding and retention confer an advantage of adaptive versatility in the types of chronic and hard-to-heal wounds that can be treated effectively with this dressing. This therapeutic approach aligns well with the TIME framework. This is an overview of the strong evidence base under these three set parameter metrics below.
Efficacy Pain
What Is the Evidence? Healing Progression
■ HydroClean’s® efficacy in preparing a clean wound bed to support the next stage of wound healing has been established3
■ Over a 12 week treatment period wound exudate composition in venous leg ulcer wounds was compared with wound exudates composition of acute wounds (split-thickness donor sites)3
■ The venous leg ulcer cohort had a large proportion of wounds with low healing tendencies, such as a wound area of more than 10cm2 and a duration of more than 6 months3
■ Biochemical markers in the venous leg ulcer wound exudates showed a significant change in the expression pattern during the first 14 days3
■ Biochemical markers in the venous leg ulcer exudates started to resemble those patterns shown by acute wounds at the height of granulation tissue formation and epithelialisation3
■ HydroClean® performed well in the management of exudate production, was effective in promoting wound cleansing and debridement, and supported good wound bed preparation. 86 patients participated in this study, with a mean age of 67.7 ± 21.7 years. At the end of the study, at 20 weeks, 18.6% (16 of 86 wounds) achieved closure. Clinicians were able to then utilize alternative dressings to facilitate healing outcomes3
■ The use of HydroClean® was assessed according to several clinical parameters (wound size, exudate production, tissue type); a positive wound healing trajectory was seen, with a 44% reduction in mean wound area3
■ A robust response was shown with relative wound area reduction reaching 48.9% ± 51.9%, and 61.4% of the patients achieved relative wound area reduction of ≥40%3
■ This pattern was stable throughout the remaining 10 weeks of the study and suggests that HydroClean® rapidly changed the biochemical marker pattern in this type of chronic wound to a pattern observed in acute healing3
■ Pain levels (assessed by VAS) were low at dressing change in most patients in an open-labelled non-comparative study. In all of the follow-up visits where pain was assessed and noted, mean pain score was 15.1 ± 22.6mm. Only 20% of the dressing removals led to pain >30mm3
■ Pain scores were reduced, with patients reporting low levels of pain experienced at and between dressing changes. Both patients and caregivers rated the dressing positively in terms of pain and atraumatic dressing change, ease of use, acceptability and assessment of wound response
■ General wound pain and pain at dressing change were both assessed over the evaluation period. Overall, mean pain levels were low at the start of the assessment period. There was a decrease in the level of pain experienced by patients in both wound pain (visual analogue score (VAS): 30 ± 2 – 13 ± 9mm, and pain at dressing change (VAS score: 18 ± 2 – 7 ± 5mm) over the assessment period1
Wound Bed Preparation
■ Hydroresponsive wound dressings are highly effective in preparing a clean wound bed to support the next stage of wound healing3
■ As a result of a open-labelled and noncomparative observational study involving patients with acute and hard-to-heal wounds, the use of Ringer’s solutionpreactivated polyacrylate dressings in daily practice was found to have the potential to improve clinical outcomes, including healing1
■ Patients with a range of wounds of varying severity were observed. To enable healing to progress, the wounds required debridement in order to remove devitalized tissue3
■ This hydroresponsive wound dressing, when used on wounds with a significant level of devitalised tissue, leads to a decrease in the percentage of predominantly fibrinous and necrotic wounds, and a corresponding increase in wounds with healthy granulation tissue and wounds showing reepithelialisation3
■ The first phase in this open-labelled and noncomparative study used HydroClean® 3
■ HydroClean® has the unique quality of rapidly changing the biochemical marker pattern in this type of chronic wound to a pattern observed in acute healing.3 This unique property may contribute to healing progression
■ In an analysis of 278 patients, it was found that during the course of the treatment wound size decreased from a median of 3.6cm2 at baseline to a median of 2.6cm2 at the fifth visit1
■ At the final visit that the level of the new epithelium significantly increased from 0.0% to 10.0%1
■ An increase in granulation tissue was noted during the assessment period, with the proportion of healthy granulation tissue increasing to 50% from 25% at the baseline1
■ The effect of hydroresponsive wound dressings on wound bed preparation and healing progression in a variety of wounds, shows that there is a decrease in the overall PUSH score3
■ Showing a similar magnitude of PUSH score reduction indicates that the hydroresponsive wound dressing enabled wound healing progression3
The PUSH Tool monitors three parameters: surface area of the wound, wound exudate and type of wound tissue. Wounds are measured using a centimeter ruler. The scores are rated from 0 to 10 according to the size of the wound. Tissue type is noted as necrotic, slough, granulation, epithelial or closed/resurfaced.
■ *HydroClean® can be integrated into the daily routine of wound therapies, assisting with normalising the wound environment and blocking excessive protesase levels associated with impaired wound healing1
Video case studies: tinyurl.com/33k2c2r8 tinyurl.com/yc49t2zf
Masterclass
30 Wound Masterclass - Vol 2 - March 2023
© Copyright. Wound Masterclass. 2023
Hydro-Responsive Wound Dressing: HydroClean®
Key Points
■ Suitable as a first-line dressing in a wide variety of wounds
■ Supports wound bed preparation, enabling wounds to progress to healing
■ Promotes autolytic debridement and removal of devitalised tissue and microorganisms
■ Hydro-responsive, so can be used on wounds requiring exudate management or dry wounds
■ Easy to use by non-specialist staff and across care settings
■ Easy to remove, reducing pain at dressing change
■ Do not use HydroClean on patients with an intolerance to any of its components
“These dressings can help to normalise the local wound environment and block excessive protease levels associated with impaired wound healing.” 1
“The hydroresponsive wound dressing was highly effective in preparing a clean wound bed such that the next stage of wound healing could be supported.” 3
“A significant healing response was achieved in patients with a variety of wound types.” 1
References
1. Goedecke F. et al, (2022). An Observational Study of Chronic Wounds Treated with Hydro-Responsive Wound Dressings. Journal of Wound Care, Vol 31(12) p.1029-1038
2. Atkin L, Bucko Z, Conde Montero E et al (2019) Implementing TIMERS: The race against hard-to-heal wounds. J Wound Care 28(3 Suppl 3): S1-S49
3. Sterpione F, Mas K, Rippon M, Rogers A, Mayeux G, Rigaudier F, Chauvelot P, Robilliart L, Juhel C, Lecomte Y. The clinical impact of hydroresponsive dressings in dynamic wound healing: Part I. J Wound Care. 2021 Jan 2;30(1):15-24. doi: 10.12968/jowc.2021.30.1.15. PMID: 33439084.
4. HydroClean Made Easy. Wounds International (April 2021). Available from www.woundsinternational.com
5. HydroClean plus mini. [Internet]. Hartmann. [cited 05 Nov 2022]. Available from: https://www.hartmann.info/en-gb/brands/l/gb/for-wound-management/hydroclean-plus-mini
6. Bjarnsholt, T. et al. (2020). The role of non-medicated dressings for the management of wound infection. Wounds International.
7. Leaper DJ,Schultz G, Carville K et al. Extending the TIME concept: what we learned in the past 10 years. Int Wound J 2012; 9 (supp 2): 1-19. http://doi.org/10.1111/j.1742-481X.2012.01097.
Useful Links

Sponsored by HARTMANN. All production resources provided by HARTMANN.
Guide: Hydro-Responsive Wound Dressing. Wound Masterclass. Volume 1. No 3.
Masterclass
Dec 2022
Masterclass GUIDES Wound Masterclass - Vol 2 - March 2023 31 Use your device to scan this QR code for more information about HydroClean® Visit the HARTMANN website
How to Cite this Article
© Copyright. Wound Masterclass. 2023
Synergistic Effects of Pure Hypochlorous Acid and Ovine Forestomach in Chronic Venous Disease
Editorial Summary
The prevalence of chronic venous disease (CVD) can vary depending on the population studied and the diagnostic criteria used, but is estimated to affect up to 30% of adults worldwide. There is an increasing prevalence with age, with rates as high as 50% in people over the age of 50. Females are more likely than men to develop CVD, as are patients who are smokers, overweight or obese. In severe cases, CVD can lead to complications such as venous leg ulcers (VLU), deep vein thrombosis (DVT), and pulmonary embolism (PE). Wound bed preparation and irrigation are important steps in wound care that help to remove debris and promote healing. The options for wound bed preparation and irrigation include normal saline irrigation, antiseptic solutions containing agents that can kill bacteria and other microorganisms in the wound bed. Examples include povidone-iodine, chlorhexidine, and hydrogen peroxide. These should be used with caution. Enzymatic debridement agents break down and remove necrotic tissue and debris from the wound bed. Examples include collagenase and papain-urea. Mechanical debridement involves physically removing debris from the wound bed using methods such as wet-to-dry dressings or irrigation with a syringe. Negative pressure wound therapy (NPWT) is a specialized wound dressing to promote wound healing. It can help to remove excess fluid from the wound bed and promote the growth of healthy tissue. The choice of wound bed preparation depends on the type and severity of the wound, as well as individual patient factors. This article examines the potential for synergistic use of pure hypochlorous acid (pHA) with ovine forestomach matrix for patients with venous leg ulceration.

Introduction
Chronic venous disease is a huge spectrum of medical conditions, and is one of the most prevalent medical conditions in the world affecting 25 - 40 million Americans; however, there are 2 - 4 million, or even more, that have advanced chronic venous insufficiency; 500,000 - 1 million patients have venous ulceration.



Chronic wounds progress from hemostasis to inflammatory, proliferative, and remodelling phases, but wounds become a chronic wound if they are stalled in the inflammation phase and have not entered the proliferation phase after 4 weeks of standard of care (SOC) therapy.
Venous ulceration is associated with older age groups, however many of these individuals are still actively working, resulting in significant economic cost due to time taken off work due to the condition, or as happens frequently, the condition leading to a decision to take early retirement, which will often involve a reduced quality of life.1
 Dr Peter Gloviczki
Dr Peter Gloviczki
32 Wound Masterclass - Vol 2 - March 2023
Dr Abigail Chaffin
Dr Lee Ruotsi
Dr Monika Gloviczki
Dr M. Mark Melin
Minneapolis
United
Editor-in-Chief
Minnesota Scottsdale AZ, United States Medical
Medicine Saratoga Springs NY, United
Division
-
Surgery Medical
University New Orleans LA, United States Scientist and Artist
Vascular Science & Art Scottsdale AZ, United States © Copyright. Wound Masterclass. 2023
Medical Director of the M Health Wound Healing Institute
MN,
States
of the Journal of Vascular Surgery Publications. Professor of Surgery (Emeritus), Mayo Clinic, Rochester,
Director at the Saratoga Hospital Center for Wound Healing and Hyperbaric
States Associate Professor of Surgery,
Chief
Plastic
Director, MedCentris Wound Healing Institute at Tulane
at
“Micronutrient components can maximize nitric oxide production which improves lymphatic function, micro arterial vasodilation, venous tone, and immune function which are all incredibly beneficial to these patients who may be micronutrient deficient.”
Venous Leg Ulcer
Of the 2.5 million leg ulcer cases per year, 80% are venous leg ulcers (VLUs), and with an everincreasing elderly population, there is increasing incidence. The costs involved are substantial, on an individual basis and also considering the wider economic impact, including:
Reduced quality of life
High frequency of hospitalizations
Wound clinic visits
Home healthcare
Decreased work capacity
Annual US cost of $14.9 billion
The Typical VLU Checklist
In the 4 weeks of SOC, there should be a 50% reduction in the wound size; if not, it may be necessary to signal for a change, or additional therapies, or advanced extracellular matrices (ECM). This is important, because generally wound area reduction after 4 weeks is predictive of complete wound healing by 24 weeks.
It is important to run a typical VLU checklist, like an airplane pilot’s pre-flight checks, to maximize outcomes. Local and national guidelines should be followed, with incorporation of evidence-based modalities that lead to the highest quality outcomes with the most appropriate resource utilization. The goals are to alleviate pain, rapidly heal the ulcer, and to prevent long term sequelae.
Investigation with venous competency ultrasound is critical, identifying both saphenous and non-saphenous reflux, as well as perforator status. Studies suggest that axial and non-axial venous reflux should be treated
early, managing the venous hypertension as a basis for wound healing.
A nutrition evaluation typically, in practical terms, ranks low on the checklist. Micronutrient components can maximize nitric oxide production which improves lymphatic function, micro arterial vasodilation, venous tone, and immune function which are all incredibly beneficial to these patients who may be micronutrient deficient. Focusing on the B vitamins (B12, B6 folate), and high dose vitamin C can help with decreasing reactive oxygen species and antioxidants. A caveat on vitamin C is that if the patient has a history of renal stones, it may be necessary to be careful and discuss with the primary care physician. Vitamin D can also have an impact, not just on bone health, but on microvascular perfusion. Arginine certainly contributes to nitric oxide production and Micronized Purified Flavonoid Fraction (MPFF).
Certainly, looking at the medication list is important, because medications like amlodipine or other calcium channel blockers, can contribute directly to lower extremity edema; typically amlodipine needs to be stopped to maximize edema control. Oncology patients undertaking chemotherapy or who are on hydroxyurea are certainly at a high risk of inhibited long term healing.
It is prudent to consider biopsy; with atypical vs typical ulcerations, if an atypical is missed such as a malignancy, or pyoderma gangrenosum, and it is treated as a typical venous leg ulcer, then there will not be a good outcome.
There is of course a focus on reducing wound pH; peer reviewed validated data confirms that decreasing the wound pH can help with decreasing bacteria population, improve angiogenesis, and improve accelerated epithelialization, combined with other types of adjunctive modalities.
Forestomach in Chronic Venous Disease Wound Masterclass - Vol 2 - March 2023 33
Synergistic Effects of Vashe and Ovine
© Copyright. Wound Masterclass. 2023
Another important consideration is compression, and it is pivotal to get certified lymphedema therapists involved and begin utilizing inelastic Velcro; this is applied and removed over the top of dressings, and it is vital not to forget about both the foot component and the calf component.
Cost effective wound bed modulation, as shown with ovine forestomach later in this article, should also be opted for. Also, smoking has to be ceased, including vaping.
To conclude, the steps should include the checklist for VLUs with special attention to avoiding missing the identification of Peripheral Arterial Disease (PAD); it has been observed in a growing demographic with diabetes and metabolic syndrome in the population. Another focus point is avoiding undertreating lymphedema; recognizing malnutrition and treating it appropriately, focusing not just on the protein component but on the micronutrient component. In terms of investigations, venous ultrasounds must be done early. It is necessary to be vigilant with medications contributing to lower extremity edema and to modify them. Of course, tobacco cessation is another vital component.
Pure hypochlorous acid can be utilized to manage microbial adherent aggregates, decreasing the pH to 5.5. Ovine forestomach matrix can be incorporated for extracellular matrix modulation; for both there is supporting evidence, both micro to macro, for wound modification and healing.


Pure Hypochlorous Acid (pHA) Preserved Cleanser
Pure Hypochlorous Acid (pHA) preserved cleanser (Vashe®, Urgo Medical North America, Fort Worth, TX), is manufactured at 300 ppm (parts per million), it has a pH range of 3.5 - 5.5, which is conducive to healing.2
It is not cytotoxic, unlike standard sodium hypochlorite solutions.3 This product is consumed by organic matter in the wound and dissipates in seconds, so it is safe to use with ECM in the same setting.4 For wounds that have microbial adherent aggregates, 5 - 8 minutes of wound contact with the solution should be considered, in combination with sharp debridement.5 This mechanical disruption of germs and associated necrotic debris is a rapid process, and does not require long exposure as some other modalities may.
Some very recent consensus documents and guidelines suggest discontinuation of use of cytotoxic cleansing agents such as Dakin’s, or solutions that contain cytotoxic agents, such as chlorhexidine gluconate.6
A paper published in 2022 also considers the use of hypochlorous acid versus sodium hypochlorite.7 The issue of margin of safety is discussed at length.
Another paper looks at the cytotoxicity of cleansers via the lens of the therapeutic indices of the cleansing agent preservative
“Another important consideration is compression, and it is pivotal to get certified lymphedema therapists involved.”
34 Wound Masterclass - Vol 2 - March 2023
Synergistic Effects of Vashe and Ovine Forestomach in Chronic Venous Disease
Figure 1: Oxidative Burst Pathway.
© Copyright. Wound Masterclass. 2023
Figure 2: Effects of Hypochlorous Acid.
“Hypochlorous acid has a higher therapeutic index compared to the hypochlorite present in Dakin’s, offering a higher effectiveness with a lower toxicity to the tissue.”
molecule.8 Hypochlorous acid has a higher therapeutic index compared to the hypochlorite present in Dakin’s, offering a higher effectiveness with a lower toxicity to the tissue.
The hypochlorous acid molecule, a naturally evolving substance inside the human tissue, forms part of the cellular barrier immunity; humans are designed to live with hypochlorous acid in their tissue, which explains the lack of cytotoxicity of the pHA based cleanser.
The pHA based cleanser has been studied extensively in its ability to disrupt microbial adherent aggregates. Laboratory studies have shown that hypochlorous acid has the ability to mechanically disrupt 90% of microbial adherent aggregates after a short exposure.
Microbial adherent aggregates, the exopolymeric substances secreted by and surrounding bacteria, has presented many problems in the healing of wounds. Estimates suggest they are present and potentially delaying healing in 60% of chronic wounds; disrupting them is a crucial element of any wound care clinician’s armamentarium in treating chronic wounds.
Hypochlorous acid has a stable shelf life of approximately 18 months, a pH of 5.5, and it is non-cytotoxic in wound and peri-wound environments; it decreases inhibitory wound bed pathogens. In many institutions, it is SOC with Negative Pressure Wound Therapy (NPWT) after surgical debridement, in order to maximize lowering of the wound pH, and not having a recurrence of microbial adherent aggregates. It is applicable to all wounds, including pyoderma, diabetic foot ulcerations and traumatic wounds.
On sulfamylon or mafenide and bacterial adherent aggregates, Murphy et al. report that the mafenide solution had a flat curve; very little effects occurred on the bacterial adherent aggregates, as compared
to the pHA based cleanser solution.17
Chronic wounds tend to be deficient in a good quality extracellular matrix (ECM), which is comprised of a complex process of cells, blood vessels, and macromolecules.10 The ECM is important for structural and biochemical support. Chronic wounds often have deficient ECM, senescent fibroblasts and increased Matrix Metalloproteinases (MMPs). Enhancing and optimizing the ECM with a replacement ECM to heal these ulcers more effectively should be considered.
It is an important consideration to try to focus on decreasing interstitial edema, and by maximizing lymphatic functionality resulting in immune function enhancement, as well as decreasing subcutaneous edema, microvascular arterial perfusion can be maximized, resulting in
Wound Masterclass - Vol 2 - March 2023 35
Synergistic Effects of Vashe and Ovine Forestomach in Chronic Venous Disease
Figure 3: Pure Hypochlorous acid versus Mafenide profile.
© Copyright. Wound Masterclass. 2023
Figure 4: Composition of Extracellular Matrix.
Synergistic Effects of Vashe and Ovine Forestomach in Chronic Venous Disease
immune function enhancement, and when also decreasing subcutaneous edema, microvascular arterial perfusion can be maximized at the 5-micron level. Peri-wound lymphatic stasis occurs in almost every wound; the epiboly seen in wounds is actually built-up lymphatic stasis, and when lymphatics are static, the immune system doesn’t function well. By maximizing peri-wound lymphatic stasis resolution, we can improve overall wound healing.11,14,15
The matrisome is defined as the ensemble of more than 1000 genes encoding ECM and ECM-associated proteins vital for numerous processes in living tissue, including homeostasis, wound healing, growth, and development. The sheer number of proteins and the differences in their abundance in different tissue types demonstrates how complex a system is being orchestrated between cells and the many proteins of the ECM.
Ovine Forestomach for Skin Grafts
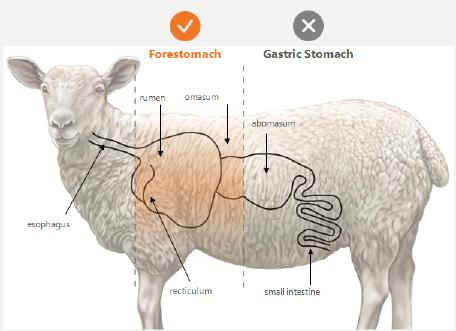
Overall, the ideal skin substitute graft would have the following criteria:
An effective advanced ECM for use in VLUs is Ovine Forestomach Matrix (OFM) (Endoform™ Antimicrobial; Endoform™ Natural; Myriad Matrix™, Aroa Biosurgery, Auckland, New Zealand). After years of research, the forestomach of sheep in New Zealand was identified as an ideal starting tissue to construct advanced ECM technologies for soft tissue repair. This source tissue from juvenile sheep (less than 12 months of age) is highly abundant in New Zealand, and the animals are disease free. The forestomach, even from these young animals, is very large, meaning large sheets of ECM can be easily manufactured. The forestomach is a highly vascular tissue, designed for digestion that is integral for rapid nutrient absorption. This also means that residual vascular channels in the graft help it to revascularize more quickly, and then this tissue undergoes more rapid remodelling, where soft tissues are continuously renewed in the body; it has a high concentration of secondary macromolecules, which are known to be important for healing.12
“By maximizing peri-wound lymphatic stasis resolution, we can improve overall wound healing.”
36 Wound Masterclass - Vol 2 - March 2023 Ideal Skin Substitute Graft 100% wound closure Single application Rapid wound closure Long term tissue durability Ideally inexpensive Easy to apply within an office setting Readily available, easy storage and transport
© Copyright. Wound Masterclass. 2023
Figure 5: Ovine forestomach.
Effects
“A study of OFM usage in a military veteran hospital in 2017 shows that in 9 months, cellular or tissue-based graft unit usage decreased by 60%; expenditures on these grafts decreased by 66%, with a higher and faster rate of healing.”
In terms of the ECM, OFM is recommended for use in the operating room, and Endoform™ Antimicrobial or Endoform™ Natural (Aroa Biosurgery, Auckland, New Zealand) in the clinic. Myriad Morcells™ (Aroa Biosurgery, Auckland, New Zealand) is another option that is available; it is a structured retention of architecture from the forestomach of young sheep, typically less than 12 months old. The stomach of sheep has to heal very quickly to maintain the health of the animal, so the OFM has a diverse array of collagens including 1, 2, 3, and 5, as well as an abundance of glycoproteins and proteoglycans. All these are preserved through a very specific process and retention of vascular channels, and it is cost effective.
A study of OFM usage in a military veteran hospital in 2017 shows that in 9 months, cellular or tissue-based graft unit usage decreased by 60%; expenditures on these grafts decreased by 66%, with a higher and faster rate of healing.13
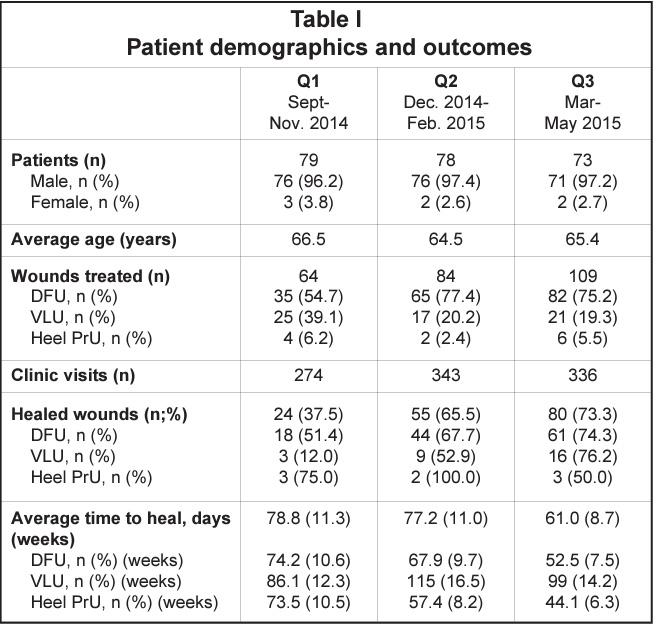
Using Ovine Forestomach for VLUs
General treatment algorithms for VLUs include commencing with standard compression, leg elevation, and then a thorough wound bed preparation with sharp excisional debridement; a high consideration for bacterial control with appropriate wound cleansers (a pure hypochlorous acid preserved wound cleanser is recommended); early use of advanced ECMs when appropriate for coverage of vital structures, dermal regeneration and accelerating primary healing of small VLUs (preferred option would be to use an OFM graft).
In patients that have failed healing of large venous ulcers with standard of care in conjunction with a granulated wound base, with no bone or tendon exposure, and where a high quality of skin coverage is desired, a Split Thickness Skin Graft (STSG) may be considered.
Utilizing Synergistic Effects of Both Modalities
An ideal operative algorithm for STSGs could be considered as the following: a combination of performing a thorough intra-operative ultrasonic debridement with pure hypochlorous acid solutions; sharp debridement with a curette, and OFM coverage. The graft can then be stapled or sutured in place with a thrombin sealant. A standard dressing protocol can be used, which can include: a non-adherent silver silicone dressing with Negative Pressure Wound Therapy (NPWT); appropriate 4 or 5 layer compression wrap dressings, and then immobilization at the ankle or knee as indicated. The most complex patients are often admitted, put on bed rest, and some require a stay for optimal take of the graft, as well as compression and post-operative patient education.
Disease Wound Masterclass - Vol 2 - March 2023 37
Synergistic
of Vashe and Ovine Forestomach in Chronic Venous
© Copyright. Wound Masterclass. 2023
Figure 6: Patient Demographics and Outcomes with OFM.13
Synergistic Effects of Vashe and Ovine Forestomach in Chronic Venous Disease
Mini-case Series
Patient 1: VLU
This patient was a 67-year-old male with chronic venous disease, 3 failed Cellular and Tissuebased Product (CTP) applications for VLU and a failed skin graft (failed STSG placement). He was referred to the center.
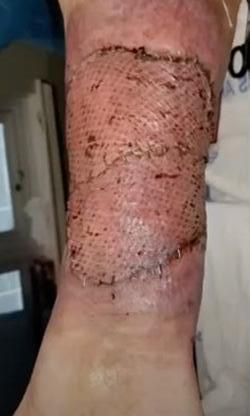



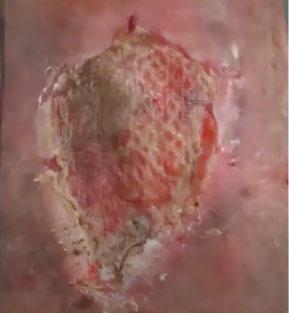
7a: Presentation after the failed STSG. Intra-clinic weekly debridement and placement for OFM was planned.

7c: Application of the OFM treatment. There is clearly residual OFM as shown by the arrow. At one week there is residual graft in the wound, this should not be debrided. The weekly application of the graft continued.
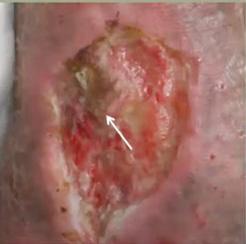
7d: Week 24: the wound that had previously been so recalcitrant is now fully 100% epithelialized and healed.

8a:
8b:
8c:
Patient 2: VLU
This second patient was a 66-year-old female with a 4 year history of VLU, compliant with compression and weekly debridement. She also had irrigation with Polyhydroxy Acid (PHA) solution.
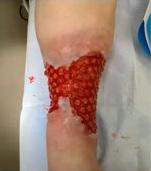
38 Wound Masterclass - Vol 2 - March 2023
Figure 7:
7b 7a 7c 7d
Figure 8:
A clean granulated wound after minimal epithelium.
Application of the skin graft following protocol.
8a 8c 8b © Copyright. Wound Masterclass. 2023
At 3 weeks, healed for staple removal.
Synergistic Effects of Vashe and Ovine Forestomach
Patient 3: VLU and Necrotizing Soft-tissue Infections (NSTI)
The next patient was a 65-year-old male with a catfish spine through his leg. He had a history of VLU, hypertension and diabetes, and had developed a necrotizing soft tissue injury.
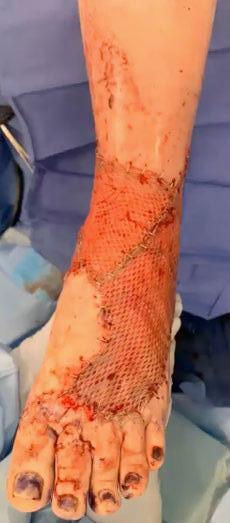
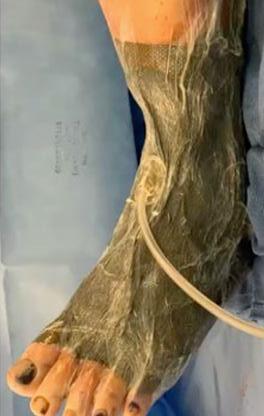
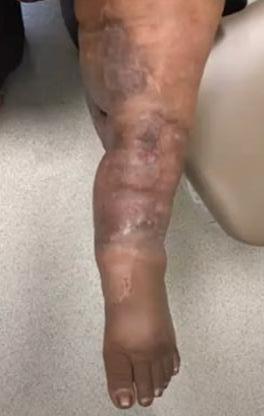

9a, 9b: Referred from the Long-term Acute Care Hospital (LTACH) with extensive injury, loss of soft tissue and crucial structures on the foot and leg, and necrotic fat on the dorsal foot.
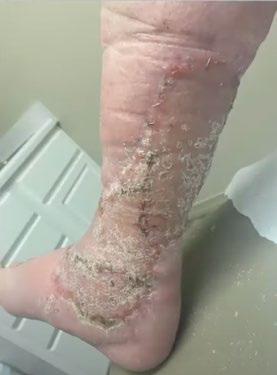
9c: After debridement, application of OFM to obtain coverage over the exposed dorsal extensor tendons region.
9d, 9e: Application of OFM product over the remainder of the wound to help achieve this dermal regeneration coverage.
9f, 9g: 2 weeks postoperative: early granulation tissue showing through the graft.
9h, 9i: 13 weeks postoperative: fully granulated with an excellent contour restoration.
Patient 4: Venous Gangrene
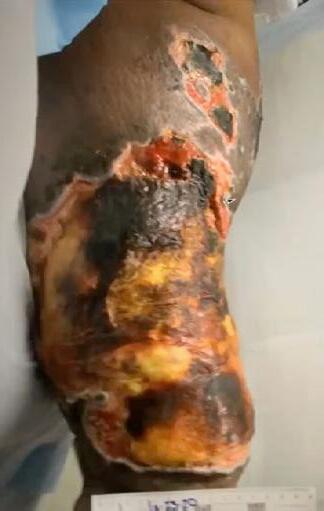
This patient had been immediately admitted to hospital with an inpatient wound team consultation, vascular surgery performed, and a venoplasty for improved outflow.
10b: An operative debridement of 1800mm2 wound had been performed twice with a pHA solution and sharp debridement.


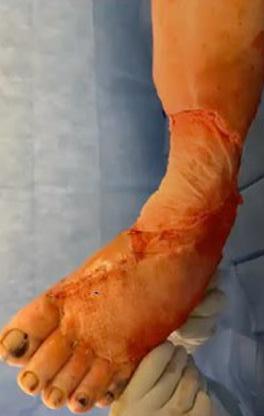
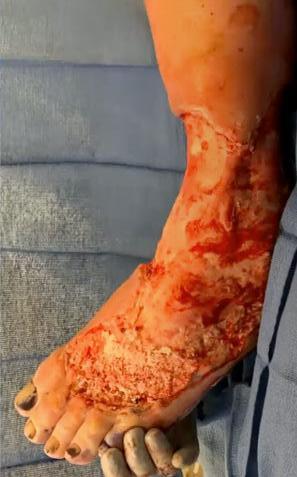
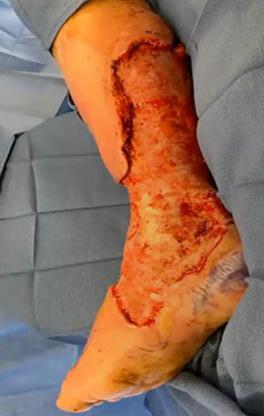
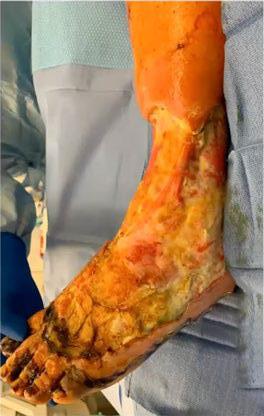
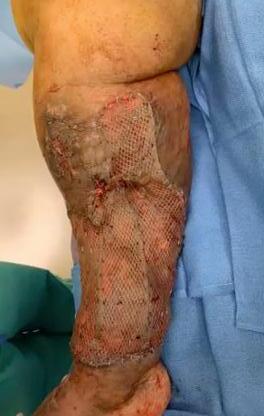
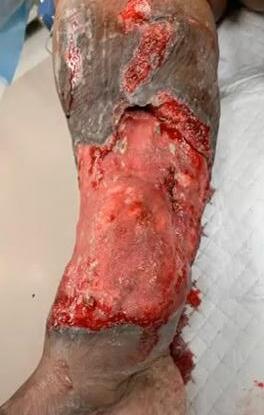
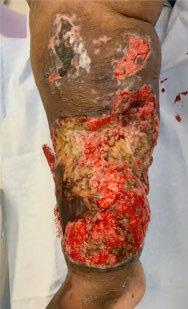
10c: Inpatient installation of NPWT with the pHA preserved wound cleanser solution.
10d, 10e: The patient was transferred again to the long-term acute care hospital (LTACH) for a further 3 weeks after application of graft. The wound became resolved, and the patient transitioned to outpatient care, following a comprehensive team-based approach.
10g: 3 month follow up. Quality of life improved for this patient due to the success of treatment.
in Chronic
Disease Wound Masterclass - Vol 2 - March 2023 39
Venous
Figure 9:
Figure 10:
10a 10b 10c 10d 10e 10f 10g 9a 9b 9c 9d 9e 9f 9g 9h 9i 9j 9k © Copyright. Wound Masterclass. 2023
Synergistic Effects of Vashe and Ovine Forestomach in Chronic Venous Disease
Pure Hypochlorous Acid is a non-cytotoxic, pH-balanced solution topical wound cleanser used to clean and irrigate acute and chronic wounds, formulated with hypochlorous acid (HOCl), a naturally occurring molecule that is produced by white blood cells to fight infection. Evidence shows that pHA is effective in reducing wound bioburden, promoting wound healing, and reducing the risk of infection. It is well-tolerated by patients and has a low risk of adverse reactions. It is effective against a broad range of microorganisms, including bacteria, viruses, and fungi. It can be used on a variety of wound types, including pressure ulcers, diabetic foot ulcers, venous stasis ulcers, surgical wounds, and burns.
Utilizing pHA in conjunction with ovine forestomach can lead to excellent results compared to STSG alone. For patients with these exposed structures such as tendons, or a significant volumetric defect, the ovine forestomach ECM helps achieve a robust granulation; this is likely due to the source of the tissue being highly vascular with the residual channels. It is a structure that is very used to a hostile environment of digestive enzymes, and so it seems to work well in these wounds that have significant microbial adherent aggregates and are difficult to heal; it may just be the ideal tissue for this difficult problem.
Considering the importance of maintaining volume and edema reduction of the leg following grafting, the compressive therapy and aftermath of the skin graft is vital. Immediately after surgery, after the negative pressure is applied, it is advisable to apply a 4 or 5 layer compression wrap; the Urgo K-Two wrap (Urgo K-Two, Urgo Medical North America, Fort Worth, TX) is an excellent option that is typically used in the clinical setting. A comproflex wrap can be applied on top, potentially as layer 5 for their long term compressive control, and also a venous edema leg pump can be used.
The biggest struggle must be considered recurrent ulcers due to recurrent edema, and so patient education is crucial, or they could be in the same condition again; an integrated approach to making sure that message is very clear is important.
Again, comparing using STSG alone to using
the ECM layer, the latter has clearly given a better contour on the overall end result of the leg. Considering case 3, this patient had significant 1cm contour deficit and although that tissue was viable, and could have tolerated an early skin graft, much more robust coverage and an excellent cosmetic result was achieved by utilizing a staged approach with the ECM with negative pressure.
Finally, it is worth noting that working with vascular surgeons and having evaluations conducted by the vascular team can be very beneficial.
This allows the patients to undergo evaluation by a vascular team and maybe some interventions, to treat the underlying venous problems. For the venous gangrene patient (case 4), the vascular team was consulted on admission, leading to an endovascular venoplasty; the expression ‘no flow no go’ is appropriate; improve the inflow and improve the outflow, and occasionally that is done outpatient or inpatient depending on the appropriateness of the setting, but this must be optimized before grafting, or there may be a failure of the graft.
New Venous Ulcer Treatments
The Society for Vascular Surgery and the American Venous Forum have published excellent guidelines, although it must be said that more recent venous ulcer guidelines are needed.
These guidelines need upgrades frequently, not only because there are a large number of new venous ulcer treatments that are not interventional, but because there have been tremendous changes in vascular reconstruction because of the endovascular revolution; during the last 2 decades, vein care has been changed forever.
References
1. Gloviczki ML, Kalsi H, Gloviczki P, Gibson M, Cha S, Heit JA. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for estimating the prevalence of venous ulcer. J Vasc Surg Venous Lymphat Disord. 2014 Oct;2(4):362-7. doi: 10.1016/j.jvsv.2014.03.002. Epub 2014 May 10. PMID: 26993538.
2. Nagoba BS et al. Acidic environment and wound healing: a review, Wounds 2015;27(1):5-11
3. Hidalgo E et al. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness. Chem Biol Interact. 2002 Mar 20;139(3):265-82.
4. C. Winterbourn et al. Modeling the Reactions of Superoxide and Myeloperoxidase in the Neutrophil Phagosome. Implications for Microbial Killing. J Biol Chem Volume 281. No. 52. Dec. 29, 2006. 39860 - 39869;
5. Robson MC. Treating chronic wounds with hypochlorous acid disrupts biofilm. Wound Prevention and Management 2020;66 (5):9-10
6. Swanson, T., Ousey, K., Haesler, E., Bjarnsholt, T., Carville, K., Idensohn, P., Kalan, L., Keast, D. H., Larsen, D., Percival, S., Schultz, G., Sussman, G., Waters, N., & Weir, D. (2022). IWII Wound Infection in Clinical Practice Consensus Document: 2022 Update. Journal of wound care, 31(S12), S10-S21. https://doi.org/10.12968/jowc.2022.31.Sup12.S10
7. Eriksson E, Liu PY, Schultz GS, Martins-Green MM, Tanaka R, Weir D, Gould LJ, Armstrong DG, Gibbons GW, Wolcott R, Olutoye OO, Kirsner RS, Gurtner GC. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022 Mar;30(2):156-171. doi: 10.1111/ wrr.12994. Epub 2022 Feb 7. Erratum in: Wound Repair Regen. 2022 Jul;30(4):536. PMID:
Discussion
40 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
35130362; PMCID: PMC9305950. 9. Wound Repair and Regeneration – the international journal of tissue repair and regeneration. Available from: https:// onlinelibrary.wiley.com/journal/1524475x

10. Melphine M. Harriott, Nayan Bhindi, Salam Kassis, Blair Summitt, Galen Perdikis, Blair A. Wormer, Timothy M. Rankin, Christodoulos Kaoutzanis, Mario Samaha, Charles Stratton, Jonathan E. Schmitz. Comparative Antimicrobial Activity of Commercial Wound-Care Solutions on Bacterial and Fungal Biofilms, Ann Plast Surg. 83(4): 404-410, 2019.
11. Optical Microscopy and the Extracellular Matrix Structure: A review, Cells 2021, 10(7),1760; https://doi.org/10.3390/ cells10071760
12. Sandi G. Dempsey, Christopher H. Miller, Ryan C. Hill, Kirk C. Hansen, and Barnaby C. H. May, Journal of Proteome Research 2019 18 (4), 1657-1668, DOI: 10.1021/acs.jproteome.8b00908
13. Topps J, Kay et al (1968). Digestion of concentrate and of hay diets in the stomach and intestines of ruminants. Br J Nutr
22, 261-280, 2. Engelhardt W and Hales J (1977). Partition of capillary blood flow in rumen, reticulum, and omasum of sheep.
American Journal of Physiology- Endocrinology and Metabolism 232(1 ): E53. 3. Baldwin, R. L; McLeod, K. R; Klotz, J. L.;
Heitmann, R. N.Rumen development, intestinal growth and hepatic metabolism in the pre- and post weaning ruminant. J. Dairy Sc. 2004, 87 (Suppl. E), E55-E65
14. Ferreras, D. T., S. Craig and R. Malcomb (2017). “Use of an Ovine Collagen Dressing with Intact Extracellular Matrix to Improve Wound Closure Times and Reduce Expenditure in a US Military Veteran Hospital Outpatient Wound Center”. Surg Technol Int 30:61-69
15. Dempsey SG, Miller CH, Schueler J, Veale RWF, Day DJ, May BCH. A novel chemotactic factor derived from the extracellular matrix protein decorin recruits mesenchymal stromal cells in vitro and in vivo. PLoS One. 2020 Jul 13;15(7):e0235784. doi: 10.1371/journal.pone.0235784. Erratum in: PLoS One. 2020 Sep 3;15(9):e0238964. PMID: 32658899; PMCID: PMC7357784.
16. Suami H, Scaglioni MF. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin Plast Surg. 2018 Feb;32(1):5-11. doi: 10.1055/s-0038-1635118. Epub 2018 Apr 9. PMID: 29636647; PMCID: PMC5891651.
17. 1. Murphy RC, Kucan JO, Robson MC, Heggers JP. The effect of 5% mafenide acetate solution on bacterial control in infected rat burns. J Trauma. 1983 Oct;23(10):878-81. doi: 10.1097/00005373-198310000-00006. PMID: 6415293.
Wound Masterclass - Vol 2 - March 2023 41
Image licenced from Adobe Stock. Credit: Rawpixel.com Sponsored by Urgo Medical and Aroa Biosurgery. All production resources provided by Urgo Medical and Aroa Biosurgery. © Copyright. Wound Masterclass. 2023

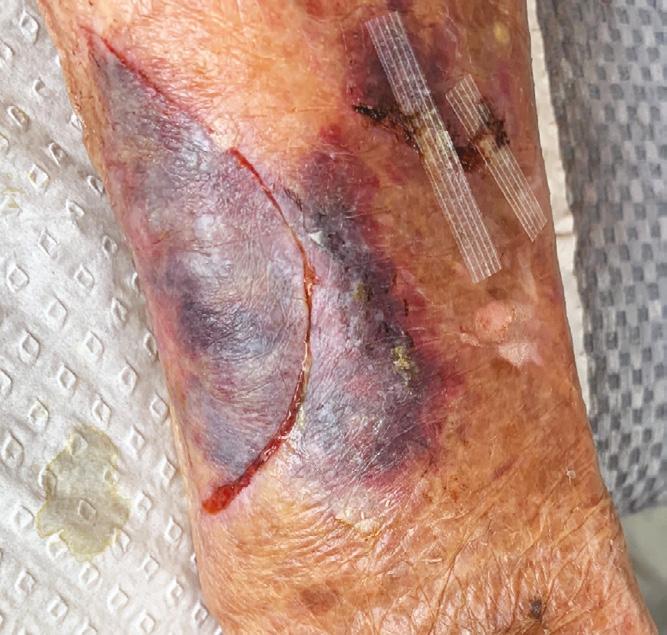



Week 0 Week 1 Week 2 Results may vary. Not all products available in all countries. Consult your local rep or distributor for more information.
Advanced extracellular matrix (ECM) available from Day 1 for all wound care professionals Simple to apply and use Available in Antimicrobial and Natural formats, as well as a range of sizes CASE STUDY: Endoform in skin tear - 96-year-old female MKT.1807.00 | ©May 2022 AROA™, AROA ECM™, Endoform™ Natural and Endoform™ Antimicrobial are trademarks of Aroa Biosurgery Limited. For product questions, sampling needs or to contact a sales representative, please email – customerservice@aroabio.com. www.aroabio.com SCAN THE QR CODE to request an overview, or a product sample
Case
Study courtesy of Cecelia Chote, NZRN Comp BHSc



woundmasterclass.com/Register Prevention of Pressure Injury Concise. Interactive. Accredited. MasterSeries 60 Minutes Interactive November 17th 2022: 1pm EST | 6pm GMT
What is the Role of Fish Skin Graft in Combat Injuries in Austere Conditions?


Editorial Summary
Military conflict presents a series of challenges for clinicians when treating injured individuals. Blast injuries and burns are common, however access to standardized treatment remains limited in remote and resource scarce environments. Fish skin grafts (FSG) present a unique opportunity to treat both burns and blast injuries incurred in conflict where access to more usual treatments is more logistically challenging. This article will provide an overview of the potential use of FSG in military field hospitals to treat wounds.
Introduction
Blast injuries and burns are prevalent presentations in the context of conflict. Blast injuries result from exposure to a violent release of energy caused by explosions leading to widespread injury and disruption to multiple body systems. Thermal and chemical burns can be component features.1 The use of white phosphorus in combustibles contributes to the severity of chemical burns, which most commonly affect exposed skin on the face and hands. These wounds can result in significant morbidity and mortality. There is also an association with lengthy stays in hospital.2
Managing such injuries during active conflict presents logistical challenges; they can often be highly complex and potentially fatal, requiring urgent action by the clinician to avoid loss of life or life changing injury. Circumstances themselves are challenging with potential ongoing enemy action against both civilians and military personnel.1 Additionally, conditions for storage of equipment for the treatment of injured soldiers is often sub-optimal.

Cadaver skin is the favoured cover for severe burns in the hospital environment; however, this is not practical in austere settings like combat zones and so attempts have been made to address the problem. Cellular derived alternatives are similarly unsuitable due to their storage requirements.3 For example, ultra-low cold storage of allograft is unlikely to be available.4 Therefore, alternative treatments which provide readily accessible and low maintenance options for medical application must be sought.
Fish Skin Grafts
Fish skin has the potential for use in the management of combat related wounds. Acellular matrix derived from the skin of wild-caught Atlantic cod has been developed as a patented graft material, or FSG, by Kerecis™ (Kerecis Omega3, Kerecis, Isafjordur, Iceland). FSG is supplied as a thick sheet with the indications for use given by the manufacturer including partial
44 Wound Masterclass - Vol 2 - March 2023
Dr Fouad Reda
Plastic Surgeon, Ajapnyak Medical Center
Yerevan, Armenia
Dr Steven Jeffery
Consultant Plastic Surgeon
Birmingham, United Kingdom
Dr Hilmar Kjartansson
Kerecis LLC, Staff Specialist Landspitali University Hospital
© Copyright. Wound Masterclass. 2023
Reykjavic, Iceland
“To explore the suitability of FSG for use in field-like hospital environments, it was used to treat injured soldiers in the Nagorno-Karabakh War in 2020.”
properties of fish skin coupled with its compliant structure allowing it to yield to irregular surfaces reduces infection rates by providing good wound coverage and preventing bacterial entry of the wound bed.3
and full-thickness wounds, in addition to trauma wounds.5 FSG is comparatively robust; the relative thickness of the graft material safeguards against environmental irritants which in turn reduces the risk of infection.3,6,7
The biological material collected contains epidermis and dermis layers which remain fully intact.5 Acellular fish skin is advantageous as skin replacement because its basic physical macrostructure is comparable to that of human skin.
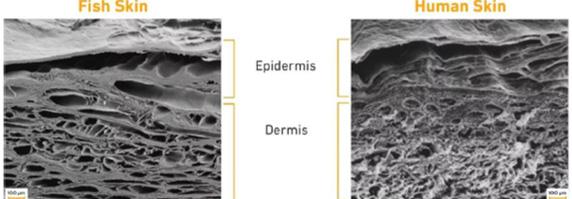
The matrix is also composed of mainly type 1 collagen allowing it to bind efficiently with proinflammatory cytokines. The microstructure of acellular fish skin is equally suitable for use on human wounds, with pores in the same size range as human epithelial tissue.8
As the product undergoes only minimal processing, it remains high in natural Omega-3 fatty acids.3 Omega-3 plays an important role in injury resolution. Previous literature has highlighted its bacteriostatic properties and its ability to accelerate wound healing.3,6 FSGs can act as a barrier against bacterial invasion of the wound site. They are also superior to allografts for stimulating cell ingrowth, a fundamental initial step of tissue regeneration.3 Burn injuries and injuries resulting from explosions are associated with significant complications including infection, incomplete healing, and supplementary injury from fragment wounds. The bacteriostatic
The extraction and production process of fish skin is cost effective and eco-friendly. There is no requirement for the use of toxic chemicals, meaning not only does the material retain its structure, but patients treated with FSG are unlikely to suffer hypersensitivity reactions, and the risk of virus transmission is negligible. Its low cost makes it more accessible in areas where high-cost advanced treatments are unobtainable.8
Storage of FSG is relatively low maintenance. It requires only clean, dry storage at room temperature.5 A 3-year shelf life at this temperature also makes the product advantageous for use in field-like hospital conditions. FSG requires just minimal training for use by clinicians with only access to basic sterile medical field equipment and sterile saline needed.4
Efficacy Testing
To explore the suitability of FSG for use in fieldlike hospital environments, it was used to treat injured soldiers in the Nagorno-Karabakh War in 2020. This was an armed conflict between Azerbaijan and Armenia, fought over a region of ethnic and historical significance. Beginning on September 27, 2020, it lasted until a ceasefire on November 10, 2020.4
As the situation in field hospitals worsened, the Armenian government called upon Iceland (Kerecis) for aid in treating injured soldiers, as Armenia and Iceland share historical links.
Physicians Dr. H. Kjartansson from Iceland and Dr S. Jeffery from the United Kingdom travelled to Yerevan near the centre of the
What is the Role of Fish Skin Graft in Combat Injuries in Austere Conditions? Wound Masterclass - Vol 2 - March 2023 45
© Copyright. Wound Masterclass. 2023
Figure 1: A comparison between fish skin vs. human skin.
conflict, in order to deliver FSG and train field medics on its use in injury management.4 Their primary goal was to use FSG to stabilize and improve the wound bed if needed before skin grafting could be performed. Other aims included improving wound healing time, achieving earlier skin grafting and attaining better cosmetic outcomes.4
The nature of wounds seen in combat has changed as military tactics have progressed. Improvised explosive devices used commonly in modern warfare has increased the number of blast injuries and large surface area burns. This is a shift from penetrating injuries caused by more traditional combat.3 The NagornoKarabakh War was infamous for its use of drones, used for reconnaissance and air-strikes. Long-range weapons were also used. Drone warfare is yet another advancement in modern military strategy.4 The number of casualties from both sides was high, with many injured soldiers suffering burn and flash burn injuries. This was only compounded by reports of the use of white phosphorus as drone ammunition.4
Most burns seen during the conflict were deeppartial or full-thickness burns; however, due to the large numbers of casualties, Armenia’s military hospitals were overwhelmed. This led to an increasing reliance on civilian hospitals. Ultimately, demand for treatment led to extended wait times of more than a week, during which time burns were managed with regular dressings. Although the usual management of burns involves total excision of the burn wound, the increasing demand on hospitals meant patients were treated with partial excision. This can result in further complications including common infections requiring antibiotics, and further surgery.4
Only minimal training is required to use FSG, and it is suitable to cover large surface areas. The material is typically held in place with staples. The use of negative pressure wound therapy (NPWT), or a bolster dressing is also commonplace. Dressings were changed every 3 - 5 days.4
During their time at the hospital, the clinicians consecutively selected several patients requiring treatment. Injuries including largearea full thickness burn and blast injuries were treated using fish skin.4 Most wounds had been managed with initial debridement and simple wet-to-dry dressings before FSG application. Wounds were typically 3 - 5 days old by the time FSG was able to be applied. If further debridement was needed, this was performed, and FSG was applied in combination with NPWT. Follow-up was done 7 days post application of FSG. In some circumstances, more than one FSG was applied.4 Efficacy was ultimately assessed depending on whether the wound was sufficiently prepared for skin grafting.4
Clinical Cases
The following provide examples of three of the injuries treated using FSG during the conflict and the outcomes of each case. They offer evidence of the success of wound treatment with FSG in the field-hospital setting for speeding up the process of wound healing, and in one case serving as a substitute for further skin graft.
What is the Role of Fish Skin Graft in Combat Injuries in Austere Conditions? 46 Wound Masterclass - Vol 2 - March 2023
© Copyright. Wound Masterclass. 2023
Clinical Cases
Case 1 provides an example of the successful use of FSG used in combination with NPWT to treat wounds when access to treatment has been delayed. In this case, the wound was 8 days old by the time FSG was applied. Non-viable tissue was debrided prior to the application of FSG.4
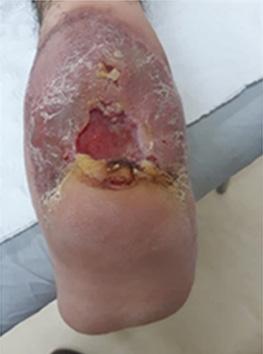
Case 2 is of a 32-year-old male with a right lower open tibial bone fracture. External fixation was used to stabilise the fracture. An open wound (15cm x 21cm) was debrided 3 times to remove non-viable tissue. In total, 3 applications of FSG were used before split-thickness skin grafting (STSG).4 This case presents evidence for the use of FSG on complex wounds and the ability of FSG to temporize and prepare the wound bed for further grafting.
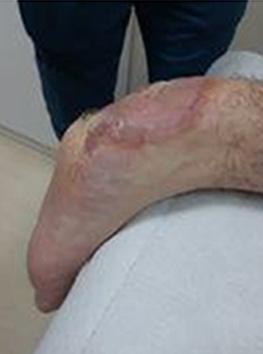
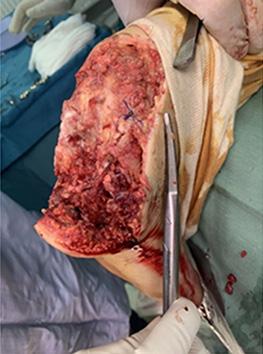
Case 3 presents a 28-year-old male with approximately 75% burns due to an explosion containing white phosphorous. A total of 10 debridements following water jet pressure
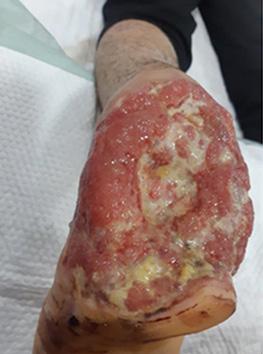
cleaning were needed. FSG was then able to be used in place of skin grafting in this case. This demonstrates the successful use of FSG as a substitute to further skin grafting.4
Success of FSG
The use of FSG for treating combat induced burns and blast injuries was highly successful during two trips made by Dr. H. Kjartansson and Dr S. Jeffery. The necessary training of other medics was also minimal. Nominal storage requirement gives FSG an advantage over other skin graft material, in areas which may be remote and storage facilities limited.4 This study documented the first successful deployment of FSG for use in field-hospitals.


Use of FSG was found to induce granulation of the wound bed several days, if not weeks, sooner in all cases; this allowed stepdown in the reconstruction ladder. Earlier skin grafting procedures could be performed and flap surgery was less likely to be required.4
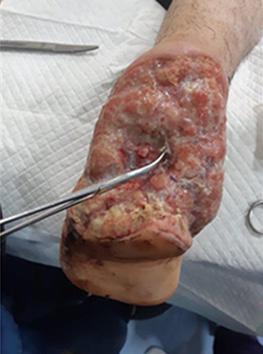
What is the Role of Fish Skin Graft in Combat Injuries in Austere Conditions? Wound Masterclass - Vol 2 - March 2023 47 © Copyright. Wound Masterclass. 2023
Images available from article: Reda F, Kjartansson H, Jeffery SLA. Use of Fish Skin Graft in Management of Combat Injuries Following Military Drone Assaults in Field-Like Hospital Conditions. Military Medicine. 2023.
Figure 2: Case 1 demonstrates the use of FSG used to manage a large full-thickness injury resulting from blast injury in a 19-year-old male. The wounds were 8 days old upon presentation. Of note was a large left heel injury where the bone was exposed.4
What is the Role of Fish Skin Graft in Combat Injuries in Austere Conditions?
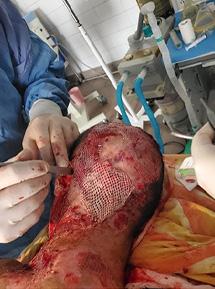


Training was endorsed by field clinicians at the four other hospitals that the physicians visited in the region.4 This supports other literature which has reported the ease of use of FSG.
Pain levels were not assessed, which must be addressed with further investigation; however, no infections were reported.4
Future Clinical Applications
While the current intention of treatment in military field-like hospitals is to temporize wounds until better treatment can be sought, the use of FSG marks a shift in this strategy. FSG not only temporizes the wound but actively accelerates the healing process, and in some circumstances can be used in place of an alternative graft.3,4,6 This has the potential to make permanent treatment more portable, easily stored, and accessible in field-like military
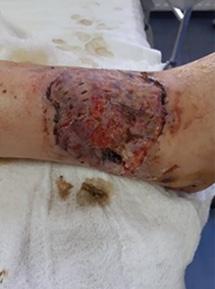
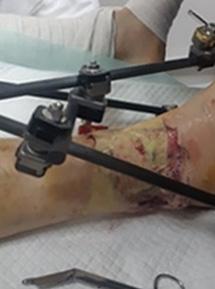
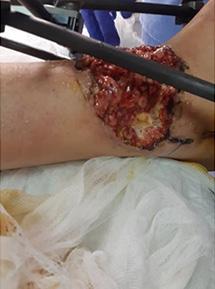

hospitals and other austere environments. The use of FSG in the cases provided above is, however, an unusual clinical application of the product and there are some limitations to the evidence produced. Given the environment, the study was not controlled and there is no welldocumented assessment of each wound prior to the application of FSG. Sufficient follow-up of each patient is also lacking. NPWT was also used which is the accepted treatment of wounds. It is therefore difficult to ascertain the true efficacy of FSG for each individual case.4
Changes in war tactics, such as the use of drones and targeted air strikes, inevitably produces more burn and complex blast injury victims. The need for a strategy to treat these injuries is woefully unmet by current approaches which are impractical in such austere environments and when local military field hospitals become overwhelmed. FSGs offer an opportunity to
48 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
Figure 3: Case 2 shows a large open wound over a lower right tibial fracture. The wound is debrided and temporized with FSG. STSG was then used.
4
Images available from article: Reda F, Kjartansson H, Jeffery SLA. Use of Fish Skin Graft in Management of Combat Injuries Following Military Drone Assaults in Field-Like Hospital Conditions. Military Medicine. 2023. Images available from article: Reda F, Kjartansson H, Jeffery SLA. Use of Fish Skin Graft in Management of Combat Injuries Following Military Drone Assaults in Field-Like Hospital Conditions. Military Medicine. 2023.
Figure 4: Case 3 is a male with 75% TBSA burns successfully treated with FSG in place of further skin grafting.4
What is the Role of Fish Skin Graft in Combat Injuries in Austere
address this unmet clinical need. FSG is a CEmarked and FDA approved product which is suitable for use in other contexts. Therefore, while its use on this mission is unusual, the use of the product to treat wounds is not experimental.4
Treatment of soldiers in the Nagorno-Karabakh War has demonstrated that FSG has the potential to become the first line treatment of combat wounds. Fish skin can accelerate recovery times, mitigate the need for flap surgery and reduce infection rates, as well as other associated morbidities. Further exploration of FSG which addresses the limitations of this mission is needed; however, the implementation of FSG in austere environments appears to be associated with improved outcomes.4
Key Points
Acellular matrix from the skin of wildcaught Atlantic cod can be used as graft material or FSG
• FSG has demonstrated antibacterial properties and accelerates wound healing
• FSG requires minimal training and is easily stored and transported
• FSG was exploratively used to treat injured soldiers in the Nagorno-Karabakh War
• The product demonstrated success in accelerating wound healing and reducing the requirement for flap surgery.
References
1. Plurad DS. Blast injury. Mil Med. 2011;176(3):276-82.
2. Davis KG. Acute Management of White Phosphorus Burn. Military Medicine. 2002;167(1):83-4.
3. Magnusson S, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson GF. Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Military Medicine. 2017;182(34):383-8.
4. Reda F, Kjartansson H, Jeffery SLA. Use of Fish Skin Graft in Management of Combat Injuries Following Military Drone Assaults in Field-Like Hospital Conditions. Military Medicine. 2023.
5. Kerecis®. Kerecis® Omega3 OR Instructions for Use [Cited 30 Mar 2023]. Available from: https://www.kerecis.com/wp-content/uploads/2021/07/KM-20-0079_v2-IFU-Kerecis-Omega3OR-US.pdf.
6. Magnusson S, Winters C, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson GF. Acceleration of wound healing through utilization of fish skin containing omega-3 fatty acids. Today’s Wound Clinic. 2016;10(5):26–9.
7. Kotronoulas A, López García de Lomana A, Karvelsson ST, Heijink M, Stone R, Giera M, et al. Lipid mediator profiles of burn wound healing: Acellular cod fish skin grafts promote the formation of EPA and DHA derived lipid mediators following seven days of treatment. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2021;175:1-8.
8. Fiakos G, Kuang Z, Lo E. Improved skin regeneration with acellular fish skin grafts. Engineered Regeneration. 2020;1:95-101.
9. Image: Magnusson S, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson GF. Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Military Medicine. 2017;182(3-4):383-8.
Conditions?
Wound Masterclass - Vol 2 - March 2023 49 Sponsored By Kerecis®. All production resources provided by Kerecis® © Copyright. Wound Masterclass. 2023
Dr Ariel M. Aballay Director, West Penn Burn Center Pittsburgh PA, United States

Clinical Experience of Omega 3 Fish
Graft in Full Thickness Wounds
Editorial Summary
This article is an overview of a case series which examines how decellularized intact fish skin grafts facilitate the healing process in full thickness burn injuries, and describes over time its integration into the wound during follow up after application. Historically, options for reconstruction include various xenografts as well as allografts. Omega3 fatty acids (EPA and DHA) are known for their anti-inflammatory properties. Fish skin grafts have been well established in the management of other challenging clinical scenarios.1-5
Introduction
Current treatments of partial and full thickness burns include various xeno and allografts. Allografts (cadaveric skin) will need to be removed and mammalian derived acellular grafts need harsh processing to decrease the risk of viral disease transmission. Recent advances in technology have made it possible to process fish sourced tissue with a more gentle process, making it safe for medical applications while preserving its intrinsic structure and biological benefits.
Previously published clinical trials showed that full-thickness acute wounds treated with fish skin grafts (FSGs) healed significantly faster than wounds treated with porcine tissue.6-8
Methods
In this case series, three burn patients aged 2271 of various ethnicities, with burn wound sizes ranging from 3-19% Total Body Surface Area (TBSA), received FSGs (Omega3 GraftGuide, Kerecis, Ísafjörður, Iceland (Figure 1)) applied post debridement and/ or excision. The fish skin graft effects on wound healing were evaluated regarding time to wound closure, restoration of barrier function, and complications; level of pain was recorded.
50 Wound Masterclass - Vol 2 - March 2023
© Copyright. Wound Masterclass. 2023
Figure 1: Kerecis Omega3 GraftGuide.
Patient 1: 22 year old male, thermal burn to the back.
The patient, with a past medical history of hydronephrosis, presented with a 19% TBSA thermal burn.
The mechanism of the burn was bonfire-induced, with clothing set alight. The patient presented to the burn center two days post initial injury having had an initial treatment with silver sulfadiazine cream. A single application of fish skin graft resulted in good secondary intention healing for the patient, without the need for an autograft.
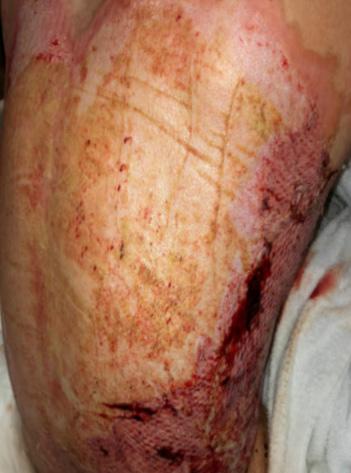
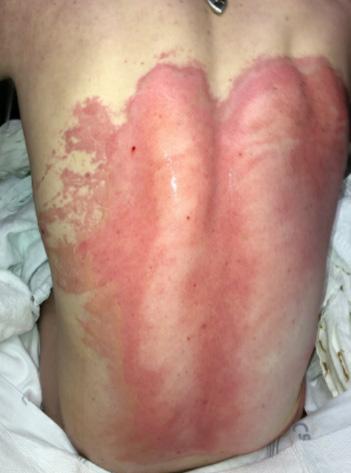
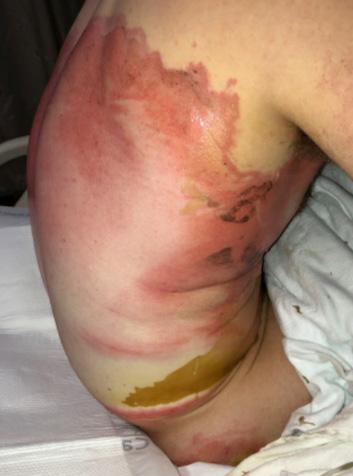
2a, 2b: Initial presentation images .
2c: Day 2 pre-treatment images.
2d, 2e: Day 2 post dermabrasion and fish skin graft application.
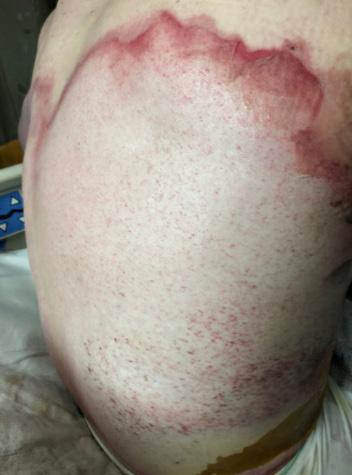
2f: Post-operative day 4.
2g, 2h: Post-operative day 15.
2i: Post-operative day 21.
Patient 2: 71 year old male, thermal burn to the right arm.
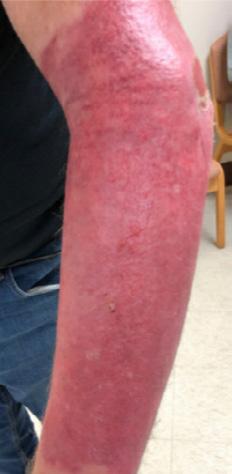
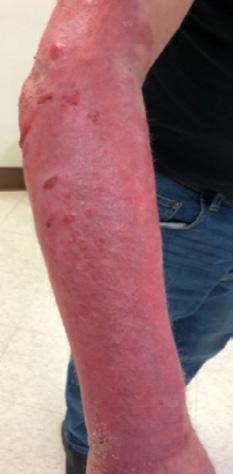
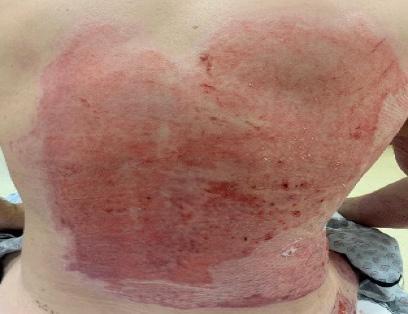
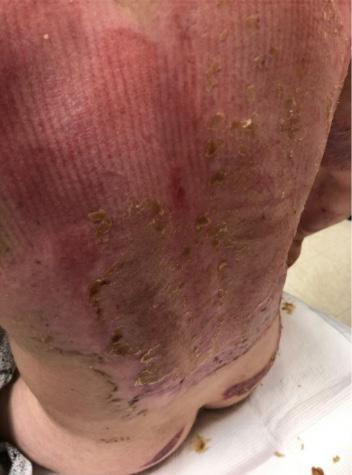
The patient, with a past medical history of hypertension and hyperlipidemia, presented with a 17% TBSA thermal burn due to a lawn tractor.
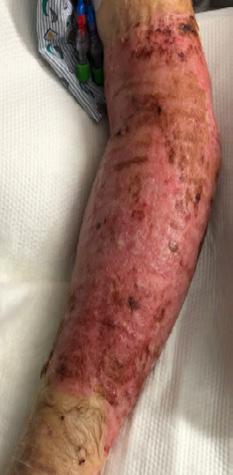
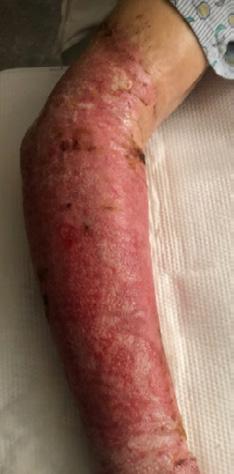
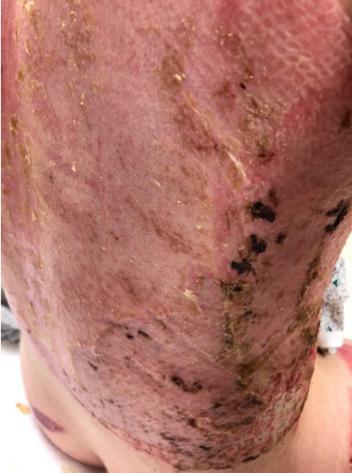
The patient presented on day 4 post injury after sustaining an explosion after hitting a gas line.
At the 1 year follow up visit, skin matching is significantly improved; the skin quality is soft, flat and pliable, with minimum itching. There was excellent range of movement with no limitation.
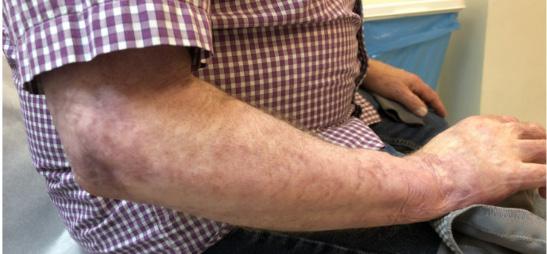
3a, 3b: Initial presentation 4 days post burn injury, prior to fish skin graft application.
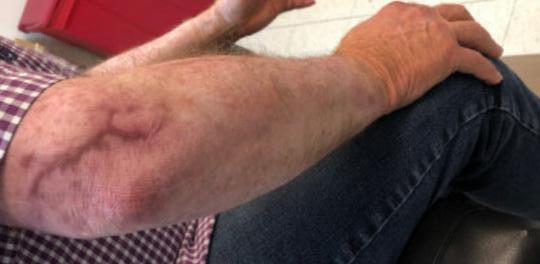
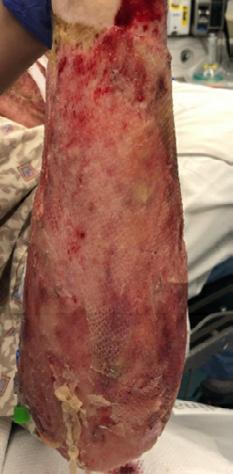
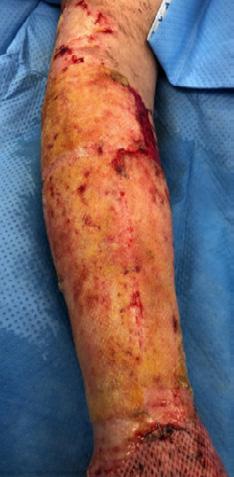
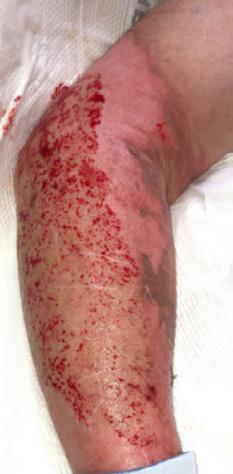

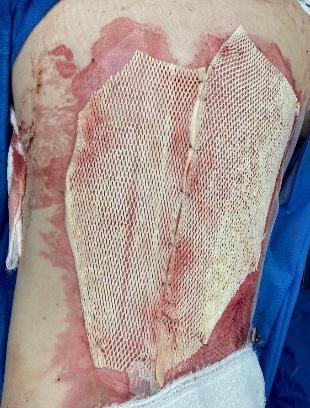
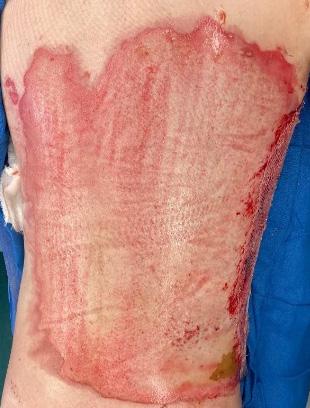
3c, 3d: Post-operative day 3.
3e, 3f: Post-operative day 10.
3g, 3h: Post-operative day 60.
3i, 3j: Follow up, 9 months; no itching or pain, flat with small areas of hypertrophy.
Clinical Experience of Omega 3 Fish Graft in Full Thickness Wounds Wound Masterclass - Vol 2 - March 2023 51
2a 2b 2c 2d 2e 2f 2g 2h 2i 3a 3b 3c 3d 3e 3f 3g 3h 3i 3j © Copyright. Wound Masterclass. 2023
Clinical Experience of Omega 3 Fish Graft in Full Thickness Wounds
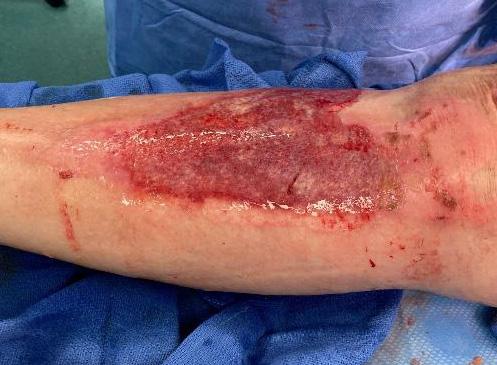
Patient 3: 65 year old female, scald burn.
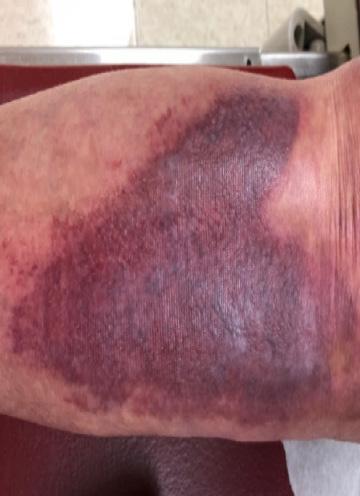
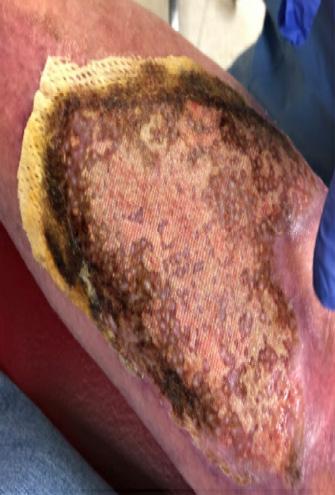
The patient had inflammatory arthritis and rheumatoid arthritis, chronic kidney disease stage 3, obesity and pulmonary valve dysfunction, and concurrent small cell carcinoma stage IV, and was undergoing chemotherapy.
The patient presented on day 10 post injury, with a 3% TBSA scald burn, previous treatment was standard of care and Collagenase SantylTM (Smith & Nephew, London, United Kingdom). Patient was discharged from the practice due to follow up limitations and chemotherapy treatments. During follow up (1 year after treatment), patient had improved pigment matching, skin was soft and pliable with no itching, and showed a full range of motion without limitation.
4a: Appearance of wound on day 10.
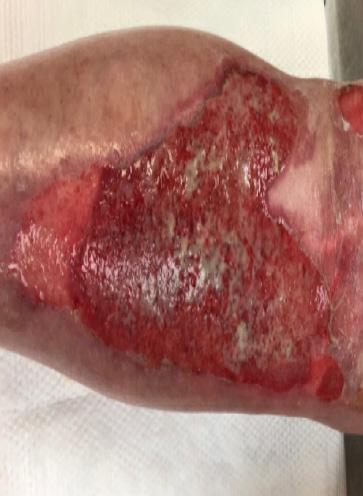
4b: Post debridement.
4c: Fish skin graft application.
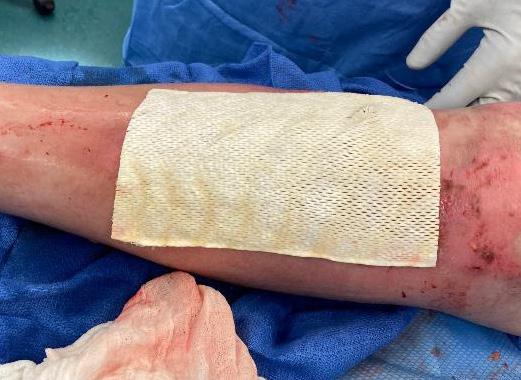
4d: Post-operative day 8.
4e: Post-operative 15.
4f: Post-operative 25.
Results
Overall, the cohort of patients treated with FSG had an observed decrease in healing time, complications, and patient-reported pain. The clinical challenges encountered are outlined. Integration of the product ranged from day 8 - 15, with a phase of caramelization from day 3 - 7.
Conclusions
In this case study, the fish skin graft demonstrated comparable or better results to historic xenograft usage. Certain characteristics observed during and after wound closure indicate higher speed of product integration compared to standard of care products, reaching the same healing objectives in a shorter time. This allows for earlier physio therapy, hospital discharge or application of split thickness skin graft (STSG). Animal studies have shown that Omega-3 fatty acids in the fish skin graft have an inhibitory effect on bacterial growth, improve epithelial cell migration, angiogenesis and accelerated blood perfusion in the wound bed.4,5 The fish skin graft used has shown to be a safe, effective alternative, and has promising results as a non-mammal xenograft in burn care management. The almost complete preservation of its tissue characteristics during biologic processing may offer further benefits not yet identified in mammal alternatives and should be trialled in larger scale studies.
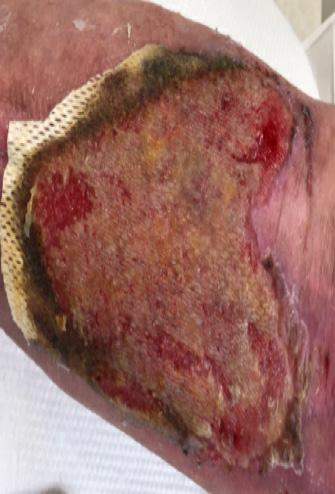
52 Wound Masterclass - Vol 2 - March 2023
4a
4b 4c
4d
4e
© Copyright. Wound Masterclass. 2023
4f
1. Huang, R., Chan, A., Wu, S. The Impact of HbA1c Levels on Diabetic Foot Ulcers Using Fish Skin Grafts. Wound Masterclass. Volume 1. No 2. Sept 2022.
2. Statkus, R. P., Darji, D., McEneaney, P. A., Rundell, J. D. Surgical Applications of Intact and Fragmented Fish Skin Grafts and NPWT for Lower Extremity Wounds: A Case Report. Wound Masterclass. Volume 1. No 2. Sept 2022.
3. Romberg, M. S. Hidradenitis Suppurativa: A Guide to Surgical Management With Omega3-Rich Fish Skin Grafts. Wound Masterclass. Volume 1. No 2. Sept 2022.
4. Wahab, N. The Application of Omega3 Fish Skin Graft in Nutritional Skin Failure Syndrome: Two Case Studies. Wound Masterclass. Volume 1. No 2. Sept 2022.
5. Masterclass Guide: Kerecis® Omega3. Wound Masterclass. Volume 1. No 2. Sept 2022.

6. Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis Ii JC. A Multicenter, Blinded, Randomized Controlled Clinical Trial Evaluating the Effect of Omega-3-Rich Fish Skin in the Treatment of Chronic, Nonresponsive Diabetic Foot Ulcers. Wounds. 2021 Jul;33(7):169-177. doi: 10.25270/wnds/2021.169177. Epub 2021 Apr 14. PMID: 33872197.
7. Magnusson S, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson GF. Regenerative and Antibacterial Properties of
Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Mil Med. 2017 Mar;182(S1):383-388. doi: 10.7205/MILMED-D-16-00142. PMID: 28291503.
8. Kirsner RS, Margolis DJ, Baldursson BT, Petursdottir K, Davidsson OB, Weir D, Lantis JC 2nd. Fish skin grafts compared to human amnion/chorion membrane allografts: A doubleblind,prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2020 Jan;28(1):75-80. doi: 10.1111/wrr.12761. Epub 2019 Oct 25. PMID: 31509319; PMCID: PMC6972637.
9. Kotronoulas A, Jónasdóttir HS, Sigurðardóttir RS, Halldórsson S, Haraldsson GG, Rolfsson Ó. Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. J Tissue Eng Regen Med. 2020 Mar;14(3):441-451. doi: 10.1002/term.3005. Epub 2019 Dec 30. PMID: 31826323.
10. Stone R 2nd, Saathoff EC, Larson DA, Wall JT, Wienandt NA, Magnusson S, Kjartansson H, Natesan S, Christy RJ. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int J Mol Sci. 2021 Feb 4;22(4):1590. doi: 10.3390/ijms22041590. PMID: 33557424; PMCID: PMC7915828.
Wound Masterclass - Vol 2 - March 2023 53
Clinical Experience of Omega 3 Fish Graft in Full Thickness Wounds
Sponsored By Kerecis®. All production resources provided by Kerecis® © Copyright. Wound Masterclass. 2023
References
GUIDES
Introduction
This is a Masterclass Guide to decellularized fish skin technology specifically designed to address the challenges of burn healing, providing a concise overview of these innovative new treatments; what they are, how to incorporate them into your practice and what evidence we have for them.
Decellularized Fish Skin Technology: Burns Kerecis® GraftGuide™

Keywords
■ Kerecis® GraftGuide™
■ Decellularized fish skin technology
■ Regenerative healing
■ Wound management
■ Natural intact fish skin
■ Wound
■ Wounds
■ Burns
■ Decellularized intact fish skin specifically developed to address the challenges of burn healing and burn wound management. Intact fish skin is used to regenerate tissue on surgical, traumatic, and acute wounds 1-5
■ It contains lipids and proteins that (in a concerted manner), help the body to regenerate damaged tissue 3-5
■ Derived from decellularized Icelandic cod skin. Its protein composition closely resembles that of human skin and the porous microstructure provides a scaffold for efficient ingrowth of dermal cells and capillaries 1-5
■ GraftGuide™ Mano (Figure 2a) is designed for burns on the hand; it covers the hand in such a way that the need for fixation and multiple grafts is reduced 3,6,7

■ GraftGuide™ Micro (Figure 2b) is the fragmented form of this technology, designed for use in burns with uneven surfaces and in deeper spaces 3,6,7
1 When applying for the first time, debride the wound bed to obtain a fresh tissue surface by removing necrotic tissue or tangential excision
2 Irrigate to remove debris and exudates. Control bleeding after applying the fish skin graft
3 Remove product from the pouch using aseptic technique

4 Fit the product roughly to the size of the area to be covered. Prehydrate in sterile solution
5 Apply product directly into the wound. The product should not overlap the wound edges
6 More than one product may be necessary for complete coverage. Overlap product edges slightly to assure coverage of the entire wound
7 To maintain direct device contact with wound bed/surface the device can be secured with steristrips, sutures, or staples
8 Apply an appropriate secondary, non-adherent wound dressing to maintain a moist wound environment
9 Inspect wound every two days. The duration between inspections may be extended up to seven days as the healing progresses
10 Insert a new product into the wound if the previously applied product has been absorbed and is no longer visible. Change the cover dressing as needed to maintain a moist, clean wound area
8
54 Wound Masterclass - Vol 2 - March 2023
What Is Kerecis® GraftGuide™?
Masterclass
Ten Point
How Kerecis® GraftGuide™ Works: The
Guide
Figure 1: Kerecis® GraftGuide™ standard
Figure 2a: Kerecis® GraftGuide™ Mano
Figure 2b: Kerecis® GraftGuide™ Micro
© Copyright. Wound Masterclass. 2023
Decellularized Fish Skin Technology: Burns
Kerecis® GraftGuide™
What Types of Wounds Are Suitable?
■ Partial and full thickness wounds
■ Trauma wounds
■ Surgical wounds (donor sites, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence) 1-3
■ Burns
THERMAL BURN
Contraindications
■ Contraindicated in patients with a known allergy or other sensitivity to fish 3-5

Scope of Usage
22 year old male, thermal burn to the back.
The patient with a past medical history of hydronephrosis presented with a 19% total body surface area thermal burn.

The mechanism of the burn was bonfire-induced, with clothing set alight.
The patient presented to the burn center two days post initial injury having had an initial treatment with silver sulfadiazine cream.
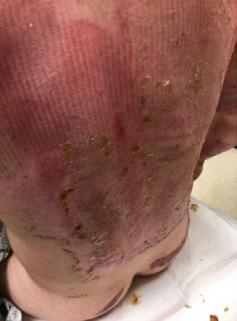
A single application of fish skin graft resulted in good secondary intention healing for the patient, without the need for an autograft.


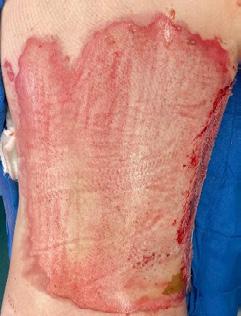
3a, 3b: Initial presentation images.
3c: Day 2 pre-treatment images.
3d, 3e: Day 2 – post dermabrasion and fish skin graft application.
3f: Post-operative day 4.
3g, 3h: Post-operative day 15.
3i: Post-operative day 21.
SCALD BURN
Patient 3: 65 year old female, scald burn to the lower leg.

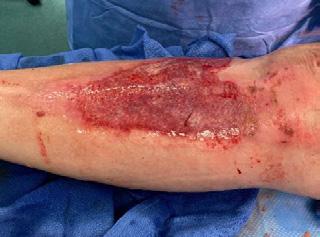
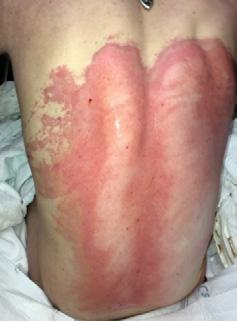
The patient had inflammatory arthritis and rheumatoid arthritis, chronic kidney disease stage 3, obesity and pulmonary valve dysfunction and concurrent small cell carcinoma stage IV and was undergoing chemotherapy.
The patient presented on day 10 post injury, with a 3% total body surface area scald burn, previous treatment was standard of care and Collagenase Santyl . Patient was discharged from the practice due to follow up limitations and chemotherapy treatments. During follow up (1 year after treatment), patient had improved pigment matching, skin was soft and pliable with no itching, and showed a full range of motion without limitation.

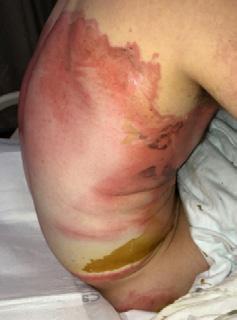
4a: Appearance of wound on day 10.
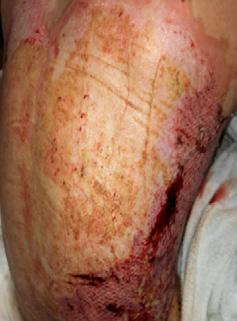
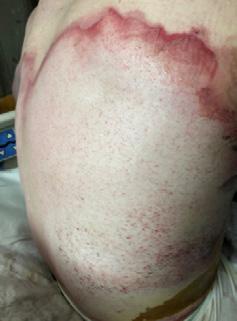
4b: Post debridement.
4c: Fish skin graft application.
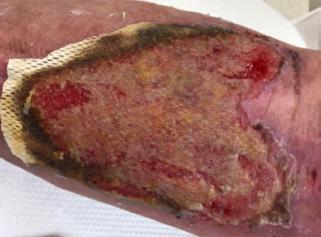
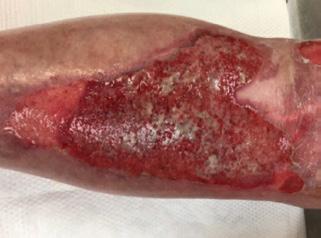
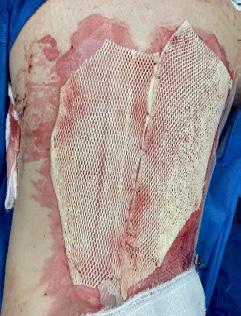
4d: Post-operative day 8.
4e: Post-operative day 15.
4f: Post-operative day 25.
Wound Masterclass - Vol 2 - March 2023 55
Masterclass
GUIDES
Figure 3
3a 3b 3c 3d 3e 3f 3g 3h 3i 4a 4b 4c 4d 4e 4f © Copyright. Wound Masterclass. 2023
Figure 4
GUIDES
What Is the Evidence?
Decellularized Fish Skin Technology: Burns Kerecis® GraftGuide™
Kerecis® Omega3 has had numerous trials supporting its efficacy in healing rates. Decellularized fish skin technology also has significant positive supporting evidence in the healing and wound management of burns.
Efficacy
■ Other tissue transplant products that are based on tissues of human and porcine origin are not ideal substitutes because of the heavy processing needed to eliminate the risk of disease transmission. This harsh, anti-viral treatment removes most of the material’s natural components, making it too dissimilar to human skin.
Kerecis® Omega3 is not subject to this treatment, leaving a more naturally intact product 4,6
■ Kerecis® has demonstrated its proficiency in creating lipidcontaining tissue matrices from fish skin, and it has been shown that the material is safe, non-toxic and structurally sound 4,5
■ In a systematic review on the use of decellularized fish skin technology in burn wound management, Luze et al. write that the use of this technology may represent an effective, low-cost alternative for the treatment of deep partial thickness burns and superficial partial-thickness burns, claiming the evidence indicates accelerated wound healing, reduction of pain and necessary dressing changes as well as improved long-term outcomes 10
Quality of Wound Healing
■ Compared to mammalian-based skin substitutes, Kerecis® Omega3 offers improved economics and clinical performance, as well as reduced disease transfer risk 4,5
■ Mammalian tissue carries the risk of disease transmission to humans. This is not a risk with Kerecis® Omega3 4,5
■ Kerecis® Omega3 fish skin contains lipids and proteins that, in a concentrated form, help the body regenerate damaged tissue 4,5
■ Kerecis® GraftGuide™ Mano allows patients to begin physical therapy after application, meaning that healing can be faster and loss of hand motion is less likely 3,6,7
Costs
■ Kerecis® is focused on developing medical device applications with a predicated high return on investment and a low to medium cost of development. The company focuses exclusively on tissue regeneration and maintenance, utilizing its core Omega3 fish skin technology 4,5
“I have seen first-hand the benefits of using fish skin grafts for burns, and GraftGuide Mano takes it to the next level with its innovative design. I am excited to incorporate it into my practice and see the positive impact it can have on my patients.”
“GraftGuide Micro has exceeded my expectations in terms of its handling properties.”
Lt. Col Steven Jeffery, Burn and plastic surgeon, Royal Army Medical Corps (RAMC); Professor, Birmingham City University, UK 7
“The Kerecis® Omega3 Wound matrix is a decellularized skin matrix derived from fish skin and represents an innovative concept to achieve wound healing... in this study (it) represented an effective treatment option in 25 complicated wounds.” Dorweiler, et al. 2018 14
Masterclass
56 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
Key Points
■ Kerecis® GraftGuide™ is a product line designed to address the problem of burn healing and management
■ Kerecis® GraftGuide™ Mano is designed for burns on the hand
■ Kerecis® GraftGuide™ Micro is designed for use in burns with uneven surfaces and in deeper spaces
■ No cultural or religious barriers to clinician/ patient acceptance
■ Non allergenic and bio-compatible ■ No known risk of disease transfer ■ Improved infection control ■
References
1. Magnusson, S. et al. Decellularized fish skin: characteristics that support tissue repair. Laeknabladid 101, 567–573 (2015).
2. Baldursson, B. T. et al. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a non-inferiority study. Int. J. Low. Extrem. Wounds 14, (2015).
3. Kerecis® GraftGuide™ Fish skin application for burns. Kerecis®, 2023. [Internet]. https://www.kerecis.com/omega3-burn/ [accessed 20/03/2023].
4. Kerecis® Fish-Skin Technology. Kerecis®, 2023. [Internet]. https://www.kerecis.com/omega3-fishskin/ [accessed 20/03/2023].
5. Masterclass Guide: Kerecis® Omega3. Wound Masterclass. Volume 1. No 2. Sept 2022.
6. Kerecis® introduces GraftGuide™ Mano and GraftGuide™ Micro. Medical Device Network, 2023. [Internet]. https://www.medicaldevice-network.com/news/kerecis-graftguide-mano-micro/ [Accessed 20/03/2023].
7. Kerecis Announces New Fish-Skin Burn Products. Business Wire, 2023. [Internet]. https://www.businesswire.com/news/home/20230120005442/en/Kerecis-Announces-New-Fish-Skin-Burn-Products/ [Accessed 20/03/2023].
8. Kerecis® Omega3 GraftGuide™ Instructions for use. [Internet, PDF]. Available from: https://www.kerecis.com/wp-content/uploads/2023/01/KM-21-0111_v3-IFU-Kerecis-Omega3-GraftGuide-US-EO-US.pdf [accessed 20/03/2023].

9. Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis Ii JC. A Multicenter, Blinded, Randomized Controlled Clinical Trial Evaluating the Effect of Omega-3-Rich Fish Skin in the Treatment of Chronic, Nonresponsive Diabetic Foot Ulcers. Wounds. 2021 Jul;33(7):169-177. doi: 10.25270/wnds/2021.169177. Epub 2021 Apr 14. PMID: 33872197.
10. Luze H, Nischwitz SP, Smolle C, Zrim R, Kamolz LP. The Use of Acellular Fish Skin Grafts in Burn Wound Management-A Systematic Review. Medicina (Kaunas). 2022 Jul 9;58(7):912. doi: 10.3390/medicina58070912. PMID: 35888631; PMCID: PMC9323726.
10. Huang, R., Chan, A., Wu, S. The Impact of HbA1c Levels on Diabetic Foot Ulcers Using Fish Skin Grafts. Wound Masterclass. Volume 1. No 2. Sept 2022.
11. Statkus, R. P., Darji, D., McEneaney, P. A., Rundell, J. D. Surgical Applications of Intact and Fragmented Fish Skin Grafts and NPWT for Lower Extremity Wounds: A Case Report. Wound Masterclass. Volume 1. No 2. Sept 2022.
12. Romberg, M. S. Hidradenitis Suppurativa: A Guide to Surgical Management With Omega3-Rich Fish Skin Grafts. Wound Masterclass. Volume 1. No 2. Sept 2022.
13. Wahab, N. The Application of Omega3 Fish Skin Graft in Nutritional Skin Failure Syndrome: Two Case Studies. Wound Masterclass. Volume 1. No 2. Sept 2022.
14. Dorweiler B, Trinh TT, Dünschede F, Vahl CF, Debus ES, Storck M, Diener H. The marine Omega3 wound matrix for treatment of complicated wounds: A multicenter experience report. Gefasschirurgie. 2018;23(Suppl 2):46-55. doi: 10.1007/s00772-018-0428-2. Epub 2018 Aug 1. PMID: 30147244; PMCID: PMC6096721.
15. Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139:804S-819S.
16. Feingold KR. Thematic review series: Skin lipids. The role of epidermal lipids in cutaneous Permeability barrier homeostasis. J Lipid Res. 2007;48:2531-2546.
17. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1-13.
18. Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605-614.
19. Lin CC, Ritch R, Lin SM, Ni MH, Chang YC, et al. A new fish-scale-derived scaffold for corneal regeneration. Eur Cell Mater. 2010;19:50-57.
20. Hawkes JW. The structure of fish skin. I. General Organization. Cell Tissue Res. 1974;149:147-158.
21. Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int J Dev Biol. 2004;48:217-231.
22. Rakers S, Gebert M, Uppalapati S, Meyer W, Maderson P, et al. ‘Fish Matters’: the relevance of fish-skin biology to unvestigative dermatology. Exp Dermatol. 2010;19:313-324.
Sponsored By Kerecis®. All production resources provided by Kerecis® Masterclass Guide: Decellularized Fish Skin Technology: Burns: Kerecis® GraftGuide™. Wound Masterclass. Volume 2. No 4. March 2023
Adjustable rate of absorption into the
tissue 3,4,6,7 Useful Links How to Cite this Article Decellularized Fish Skin Technology: Burns Kerecis® GraftGuide™ Masterclass GUIDES Wound Masterclass - Vol 2 - March 2023 57 Use your device to scan this QR code for more information about Kerecis® GraftGuide™ Click here to visit the Kerecis® website © Copyright. Wound Masterclass. 2023
surrounding
Pre-meshed 2:1
fish-skin graft
Expands to cover wounds over 100 cm²
A novel solution for chronic wounds that are larger than 100cm2 and treated in the outpatient setting
Scan the QR code for more information
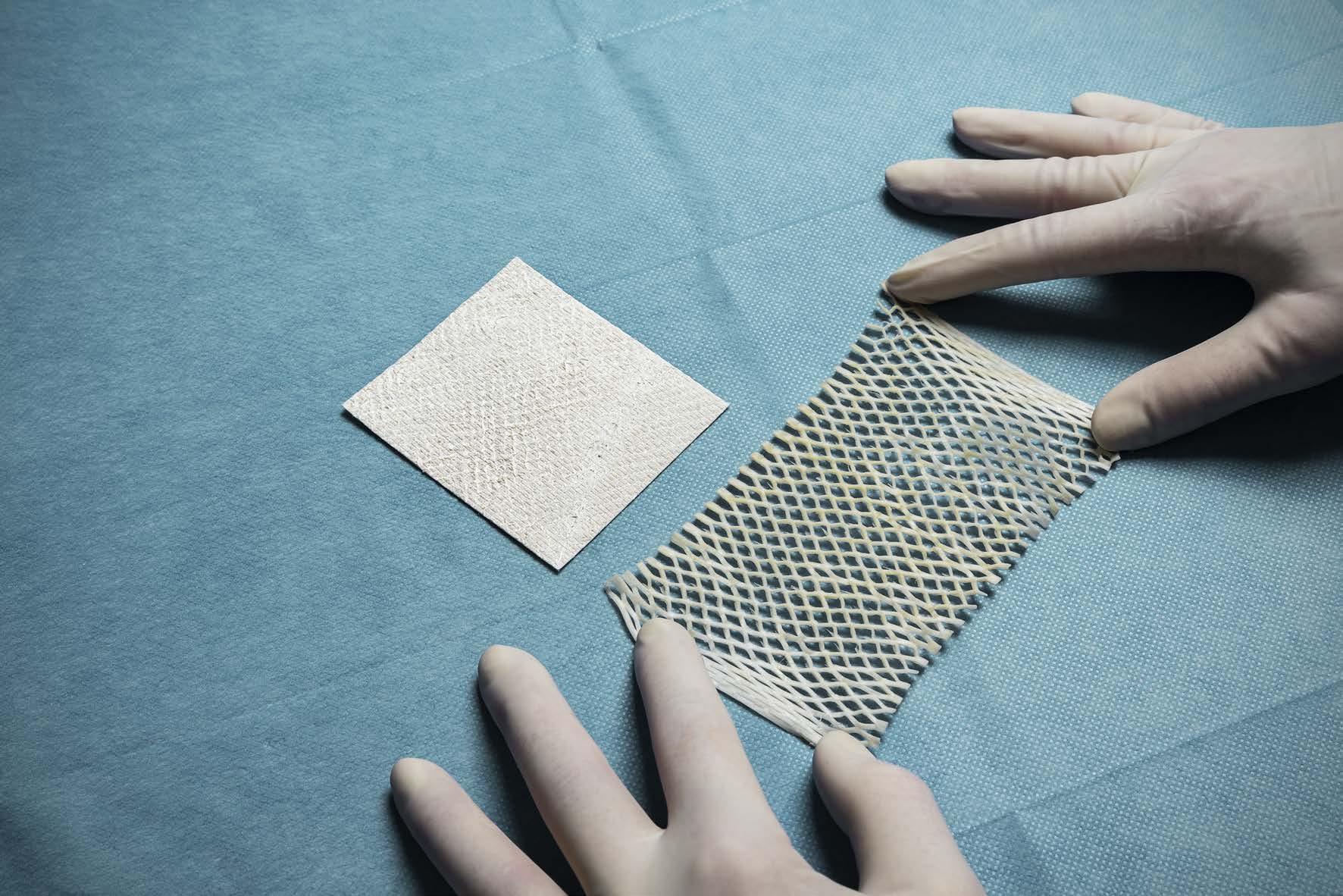
Services Offered
woundmasterclass.com/Register


Preparation of Publications: Randomized Controlled Trials
Consensus Documents

Clinical Reviews
Case Series
Publishing Events
MasterSeries 60 minutes Interactive: Global, interactive, focused webinar with global experts interacting live with audience on specialised wound care topics

Masterclass Guides
Bespoke Masterclass Guides for Clinicians: Have an innovative product in the field of wound care? Let us produce a Masterclass Guide for you!

woundmasterclass.com
 Dr Marcus F. Yarbrough Alpha Wound Care Solutions and Wellness
Dr Marcus F. Yarbrough Alpha Wound Care Solutions and Wellness

A Novel Concept for Treating Large Soft Tissue Defects After Necrotizing Soft Tissue Infection of the Back
Editorial Summary
Necrotizing fasciitis, a life-threatening subcutaneous soft-tissue infection, requires a high index of suspicion for diagnosis. It is vital to consider the diagnosis wherever there is a soft-tissue infection that appears to have rapidly progressing characteristics presenting with edema and erythema, or systemic signs of infection. However, necrotizing fasciitis can be easily missed because the patient may present earlier in the disease process with non-specific signs and severe pain (disproportionate to the clinical findings or anaesthesia over the site of infection). If necrotizing fasciitis is suspected, the patient should immediately be referred for urgent surgical debridement. Necrotizing fasciitis is a clinical diagnosis. Surgical debridement should be repeated as necessary until the patient has no necrotic tissue remaining. Adjunctive antibiotic therapy and supportive care are crucial, as well as starting intravenous empirical antibiotics as soon as blood cultures have been taken. Once culture results are available, antibiotics can be modified to target the causative organism. This article explores the surgical strategy of acellular fish skin in wound bed restoration post necrotizing fasciitis in a case of a diabetic patient diagnosed with extensive necrotizing fasciitis.
Introduction
Necrotizing fasciitis (NF) is defined as a severe and lethal bacterial infection that occurs with a rapid onset resulting in extended soft tissue and fascial necrosis with relative sparing of skin and muscle.1 Necrotizing fasciitis is a destructive infection of the skin and subcutaneous tissues associated with significant mortality and morbidity.2 Survival from the condition often necessitates patient referral for appropriate reconstructive surgery and supportive medical management. The mortality rate is high, ranging from 11% to 22%; this rate is even higher with mortalityassociated risk factors such as diabetes mellitus. Management is complex and multimodal, beginning with early identification and multiple surgical debridement with resultant large soft tissue defects.2,3
Post necrotizing fasciitis reconstruction can prove a challenge for the reconstructive surgeon. With a large amount of tissue debrided during the early process, the challenges vary depending on the anatomical location.
Methods
This case is that of a 64-year-old AfricanAmerican female with diabetes mellitus type 2 who was initially treated at a hospital for a back wound, developed NF, and was diverted and transferred to the burn unit with involvement extending from bilateral upper back to the bilateral gluteal area. The wound was initially treated with wide surgical debridement and reconstruction of the large back and gluteal soft tissue defect with intact
fish skin in combination with hyperbaric oxygen therapy and negative pressure wound therapy (NPWT). On assessment the total body surface area (TBSA) of the posterior back was approximately 18%. She underwent early debridement on day 2 of admission, with application of negative pressure wound VAC therapy for 3 days to stimulate a granulating wound bed. Subsequent surgical debridement ensued with the first application of intact fish skin graft (FSG). A method of a 3 layer ‘stacked’ intact FSG was used to ensure adequate coverage and to minimize tissue deficits. A ‘rolled’ configuration of the FSG was placed between the wound bed and skin flaps located on the superior, lateral and inferior edges, which was sutured in place with PDS® II (polydioxanone) Suture, (Ethicon (Johnson and Johnson), Raritan, New Jersey, United States).
A NPWT was put in place for 7 days. After the 4th debridement and fish skin application with NPWT, debridement and excision of the sacral tubercules was completed due to a concern for osteomyelitis. The intact FSG was then stacked on the sacrum. On the 45th hospital day, a split thickness skin graft (STSG), meshed at a ratio of 4:1 was performed along with autologous skin cell suspension. This was buttressed in place with burn mesh gauze and inspected on post-operative day 7. The intact fish skin was re-applied to residual areas where the graft had not taken. On day 63 of hospitalization, a STSG meshed at 2:1 was placed on the sacrum and bilateral gluteal defect.
60 Wound Masterclass - Vol 2 - March 2023
Virginia Beach VA, United States
© Copyright. Wound Masterclass. 2023
A Novel Concept for Treating Large Soft Tissue Defects After Necrotizing Soft Tissue Infection of the Back
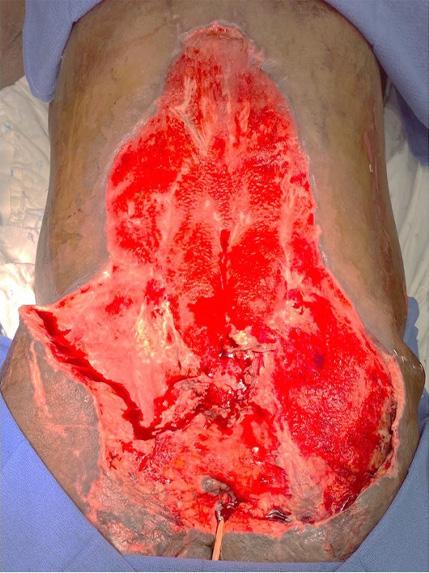
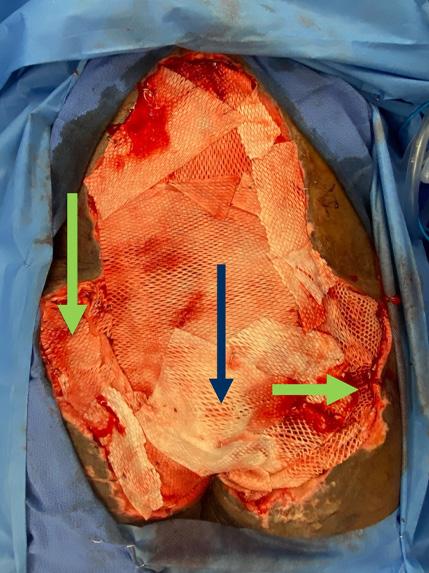

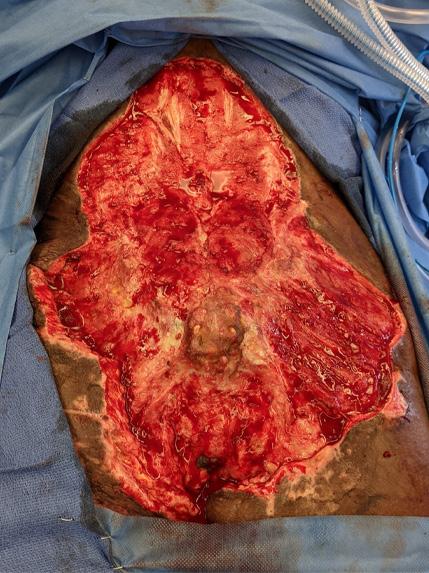
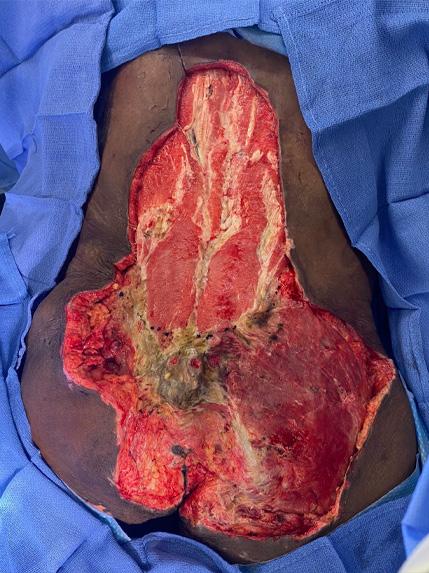

Figure 1: 64 year old female presenting with necrotizing fasciitis of the back TBSA of 18%.
1a: Initial presentation.
1b: Pre-operative appearance: pre-excision and debridement.
1c: Post-debridement.
1d: Placement of wound VAC to stimulate granulation tissue.
1e: Pre-operative: pre-excision.
1f: Post excision: area of concern was the sacrum which was debrided using a rongeur until punctate bleeding was observed.
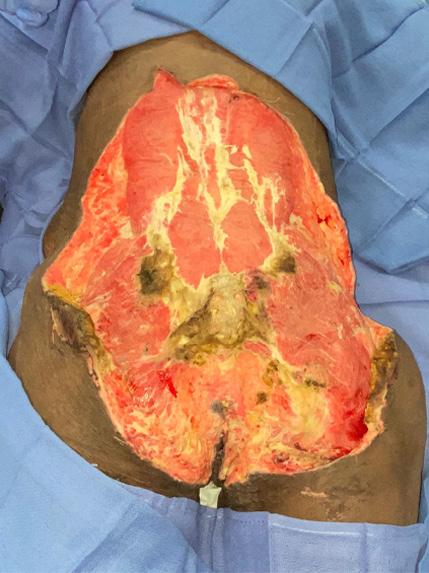
1g: Application of the intact fish skin using the multilayered stacked approach on the sacrum 3 layers (blue arrow), as well as rolled product to tunneling areas (green arrows, NPWT applied).
1h: Healthy granulation and ready for grafting. Note the sacrum with granulation tissue. Tunneling incorporated from ‘rolling the intact fish skin’ between lateral skin flaps and primary closure, although one area on the right remains.
Results
After multiple surgical debridements, Application of intact FSG resulted in a viable granulation and neo-dermis. Utilization of intact FSG allowed for reduction in fluid, protein loss and minimized microbial invasion. The ‘stack and roll’ method allowed for contraction of the large soft tissue defect from 45cm x 50cm to 35cm x 30cm.
The adjuvant use of NPWT allowed for over 90% intact fish graft incorporation and good wound bed formation. Incorporation of the intact fish skin over the sacral bone was observed on day 36 of the hospital stay. On the 45th day of hospitalization the incorporation of autologous skin cell with a ratio of 4:1 STSG was applied. The graft was inspected on the 51st hospital day and over 80% take was observed to the gluteal, upper and lower back areas. Despite our efforts, the patient unfortunately succumbed to her injuries.
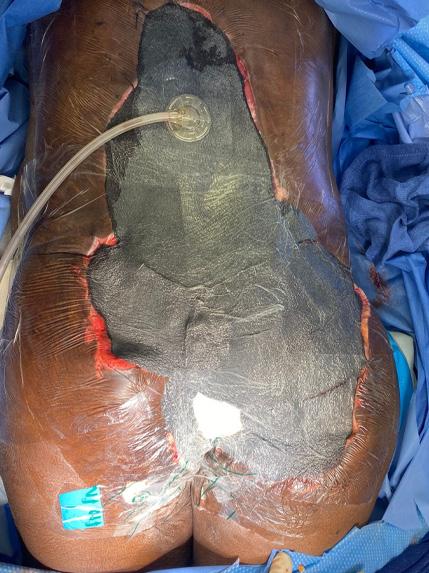
Wound Masterclass - Vol 2 - March 2023 61
1a 1b 1c 1d 1e 1f 1g 1h © Copyright. Wound Masterclass. 2023
A Novel Concept for Treating Large Soft Tissue Defects After Necrotizing Soft Tissue Infection of the Back
Conclusion
To conclude, intact fish skin should be considered a safe and efficient treatment modality to achieve a viable wound bed, as well as wound contraction and ultimately autograft, in patients with soft tissue defects caused by necrotizing fasciitis.
As demonstrated in published clinical trials, full-thickness acute wounds show faster healing time when treated with FSGs compared to those treated with porcine tissue.4-6
Research studies involving animals have demonstrated an inhibitive effect on bacterial growth due to the Omega-3 fatty acids in the FSG; also shown was improvement of angiogenesis, epithelial cell migration, as well as accelerated blood perfusion in the wound bed.7-8
By the incorporation of aggressive wound debridement and/ or preparation and application of NPWT, when combined with standard practice in wound management aiming for reduction of bioburden and infection, and appropriate offloading, healing time in these wounds can be increased significantly compared to conventional wound care.9
References
1. Wallace HA, Perera TB. Necrotizing Fasciitis. 2023 Feb 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 28613507.
2. Pasternack MS, Swartz MN. Cellulitis, necrotizing fasciitis, and subcutaneous tissue infections. In Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia (PA): Churchill Livingstone Elsevier; 2015:1:1195–216.
3. Hodgins N, Damkat-Thomas L, Shamsian N, Yew P, Lewis H, Khan K. Analysis of the increasing prevalence of Necrotizing fasciitis referrals to a regional plastic surgery unit: a retrospective case series. J Plast Reconstr Aesthet Surg. 2015 Mar;68(3):304-11. doi: 10.1016/j. bjps.2014.11.003. Epub 2014 Nov 20. PMID: 25444278.
4. Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis Ii JC. A Multicenter, Blinded, Randomized Controlled Clinical Trial Evaluating the Effect of Omega-3-Rich Fish Skin in the Treatment of Chronic, Nonresponsive Diabetic Foot Ulcers. Wounds. 2021 Jul;33(7):169-177. doi: 10.25270/wnds/2021.169177. Epub 2021 Apr 14. PMID: 33872197.
5. Magnusson S, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson GF. Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Mil Med. 2017 Mar;182(S1):383-388. doi: 10.7205/MILMED-D-16-00142. PMID: 28291503.
6. Kirsner RS, Margolis DJ, Baldursson BT, Petursdottir K, Davidsson OB, Weir D, Lantis JC 2nd. Fish skin grafts compared to human amnion/chorion membrane allografts: A doubleblind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2020 Jan;28(1):75-80. doi: 10.1111/wrr.12761. Epub 2019 Oct 25. PMID: 31509319;
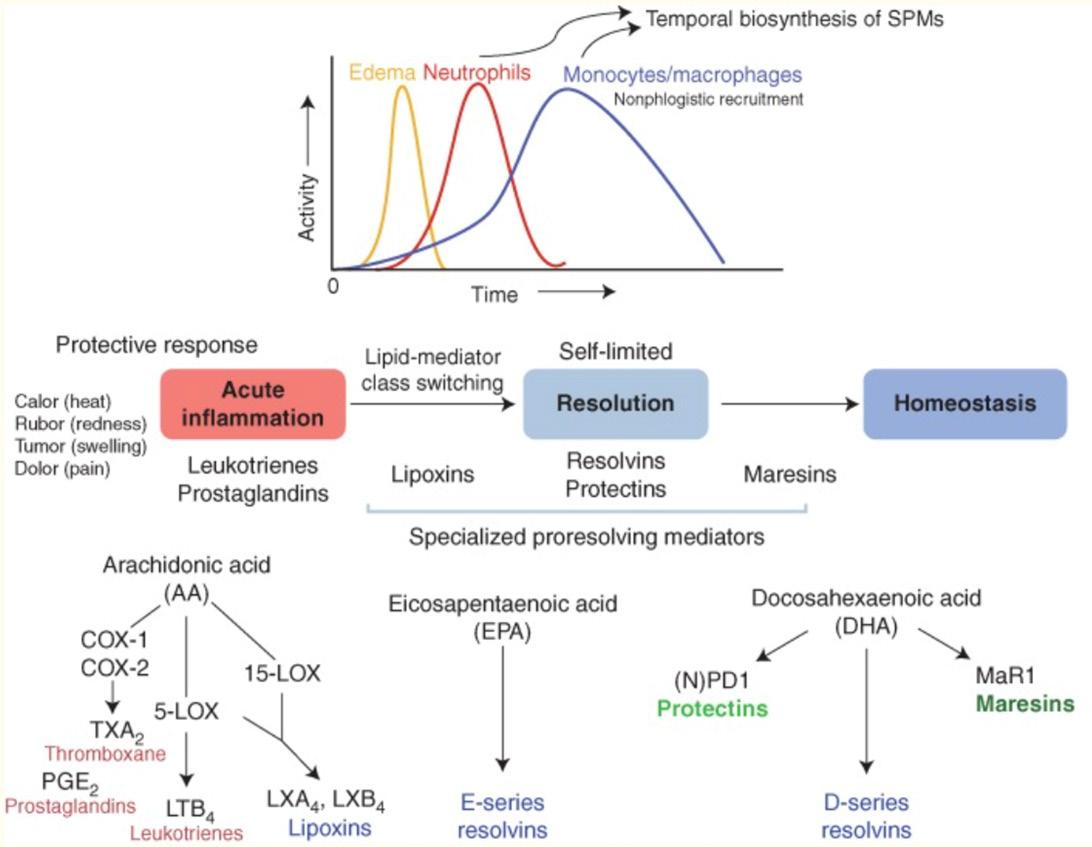
PMCID:
7. Kotronoulas A, Jónasdóttir HS, Sigurðardóttir RS, Halldórsson S, Haraldsson GG, Rolfsson
Ó. Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. J Tissue Eng Regen Med. 2020 Mar;14(3):441-451. doi: 10.1002/term.3005. Epub 2019 Dec 30. PMID: 31826323.
8. Stone R 2nd, Saathoff EC, Larson DA, Wall JT, Wienandt NA, Magnusson S, Kjartansson H, Natesan S, Christy RJ. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int J Mol Sci. 2021 Feb 4;22(4):1590. doi: 10.3390/ijms22041590.

PMID: 33557424; PMCID: PMC7915828.
9. Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. Kotronoulas A, Jonasdotir H, Siguroardottir, Halldorsson S, Haraldsson G, ROlfsson O. J Tissue Eng and Regen Medicine. 2019 Dec 11.
10. Patel et al (2019). Fish skin acellular dermal matrix: potential in the treatment of chronic wounds. Chronic Wound Care Management and Research.
11. Mortality in patients with necrotizing fasciitis. GolgerA, Goldsmith CH, Pennie RA, Bain JR. Plastic Recontr Surg. 2007; 119: 1803-1807. (PubMed)
12. Cheng NC, Tai HC, Chang SC, Lai HS. Necrotizing fasciitis in patient with diabetes mellitus; clinical characteristics and risk factors for morthality. BMC Infec Dis. 2015 Oct 13; 15: 417. doi: 10.1186/s12879-015-1144-0 PMID: 2643900.
13. Hua C, Sbidian E, Hemery F, et al. Prognostic factor in necrotizing soft-tissue infections (NSTI): A cohort study. J Am Acad Dermatol. 2015; 73(6): 1006-12.e8. doi:10.1016/j. jaaad.2015.08.054
62 Wound Masterclass - Vol 2 - March 2023
Sponsored By Kerecis®. All production resources provided by Kerecis® © Copyright. Wound Masterclass. 2023
Figure 2: STSG meshed at 4:1 and recell and re-application of fish skin graft Figure 3:
Pre-meshed 2:1
fish-skin graft
Expands to cover wounds over 100 cm²
A novel solution for chronic wounds that are larger than 100cm2 and treated in the outpatient setting
Scan the QR code for more information

GUIDES
Introduction
This Masterclass Guide is a concise overview of decellularised fish skin technology and the application in clinical practice for the wound care clinician. An overview of the technology and a guide on incorporation into clinical practice is provided.

What is Kerecis® Marigen™?
■ Intact decellularized fish skin sterilized with ethylene oxide
■ Used for management of chronic wounds such as diabetic wounds, pressure ulcers, vascular ulcers, and draining wounds commonly treated in the private office and wound care centers
■ Fat, protein, elastin, glycans and other natural skin elements comprises the fish skin sheet and is supplied in multiple shapes and sizes

■ Forms a scaffold for epithelial cells to attach to and promote healing1
■ Utilised for cases with limited donor skin availability
■ The graft is rich in omega-3 fatty acids which are antiinflammatory and help with the healing of chronic wounds2
Decellularized Fish Skin Technology
Kerecis® Marigen™
Keywords
■ Kerecis® Marigen™
■ Burns
■ Regenerative healing
■ Fish-skin technology
■ Wounds
■ Chronic wounds
How to use Kerecis® Marigen™:
■ Ensure there is a clean wound bed by debriding the wound to remove necrotic tissue
■ Clean the wound with normal saline irrigation to remove debris and exudate
■ Measure the defect of the wound and pre-hydrate product in saline solution
■ The product should be applied to the lightly bleeding wound bed ensuring the sheet does not overlap the wound edges
■ More than one sheet may be necessary for complete coverage�� overlap product edges slightly
■ A non-adherent wound dressing should be applied
■ Inspect the wound every 48 hrs
■ The duration may be extended up to seven days as healing progresses
■ Insert a new product into the wound if the previously applied product has been absorbed and is no longer visible
■ Change the outer dressing as needed to maintain a moist, clean wound
64 Wound Masterclass - Vol 2 - March 2023
Masterclass
Figure 2: 2a: MariGen™ Standard. 2b: MariGen™ Micro.
Figure 1: MariGen™ Expanse.
2a 2b
Decellularized Fish Skin Technology
Kerecis® Marigen™
What Types of Wounds Are Suitable?
■ Partial and full thickness
■ Pressure ulcers
■ Chronic vascular ulcers
■ Diabetic ulcers
■ Draining wounds
■ Trauma wounds, including abrasions, lacerations and skin tears
■ Surgical wounds
■ Donor site/grafts
■ Mohs surgery
■ Post- LASER surgery
■ Podiatric
■ Wound dehiscence
Contraindications
■ This product should not be used in people with fish allergies or sensitivities
Product Image Description Uses
MariGen™ Standard
MariGen™ Micro
MariGen™ Expanse
Fish skin graft in sheets Thin sheets for most wound types

Fragmented fish skin Uneven, irregular wound surfaces
2:1 pre-meshed intact fish skin graft Can expand and cover wounds 100cm2 and larger

Wound Masterclass - Vol 2 - March 2023 65
Masterclass GUIDES
Figure 1: MariGen™ product range.
Decellularized Fish Skin Technology
Kerecis® Marigen™
Evidence
Decellularised fish skin possesses several characteristics that make it a promising option for wound management. A natural three-dimensional structure promotes cell proliferation, migration, and differentiation. It also contains an extracellular matrix that helps to facilitate tissue regeneration and angiogenesis. Additionally, decellularised fish skin has been shown to possess antimicrobial properties, which can help to reduce the risk of infection in the wound bed. Several clinical studies have reported favorable outcomes following the use of decellularised fish skin in the treatment of chronic wounds, burns, and surgical incisions. Overall, decellularised fish skin technology shows great potential as a safe and effective approach to promoting wound healing.
Efficacy
■ Evidence supports lipid-containing tissue matrices created from fish skin
■ safe, non-toxic and structurally sound
■ A double-blind, prospective, randomized clinical trial compared the efficacy of fish skin graft with dehydrated human amnion/ chorion membrane allograft (dHACM) for full-thickness wound reconstruction3
■ Full thickness wounds were created using punch biopsies on 85 healthy volunteers aged between 19 and 51 years excluded if they were on immunosuppression, anticoagulation,or peripheral vascular disease
■ Full thickness acute wounds treated with fish skin grafts healed faster than wounds treated with dHACM, with a p-value of 0.00143
■ Wounds treated with fish skin grafts heal faster than those treated with porcine small-intestine submucosa4
Infection
■ There is no documented risk of viral or prion disease transmission between humans and Atlantic cod
■ Mammalian tissue requires a level of processing that fish skin does not5
■ The gentle processing of the cod skin does not include viral deactivation3
■ Less processing of the tissue preserves the natural three dimensional extracellular matrix and porosity aiding in cellular recognition3
■ Chronic wounds that undergo debridement begin to physiologically resemble the characteristics of acute wounds3
Costs
■ A retrospective comparative cohort study examined the cost of fish skin grafts versus standard of care for the treatment of diabetic foot ulcers (DFUs)7
■ Fish skin treatment could result in lower costs
■ More wounds healing (83.2% vs. 63.4%)
■ Fewer amputations (4.6% vs. 6.9%)
■ Higher quality of life (0.676 vs. 0.605 quality-adjusted life year (QALY) than the standard of care7
Fish skin grafts “offers improved economics and clincial performance, as well as reduced disease transfer risk"
Winters et al.7
There were “promising results in a 18-patient prospective study with 5 weekly applications of the fish skin with a 40% decrease in wound surface area (P < 0.05) and a 48% decrease in wound depth seen with fish-skin graft and secondary dressing.”
Yang et a.10
“Management with fish skin has shown faster granulation rates in burn wounds for skin grafting, resulting in improved patient outcomes with no documented infections.”
Reda et al.9
66 Wound Masterclass - Vol 2 - March 2023
Masterclass GUIDES
Decellularized Fish Skin Technology
Key Points
■ Provides versatility for wound defects with a variety of product shapes and sizes
■ In head to head comparison with commonly utilised wound products, has improved time to closure
■ Cost-effective and safe for wound reconstruction
■ Application over any viable tissue bed

References
1. Biazar, E, Heidari Keshel, S, Rezaei Tavirani, M, Kamalvand, M. Healing effect of acellular fish skin with plasma rich in growth factor on full-thickness skin defects. Int Wound J. 2022; 19( 8): 21542162. doi:10.1111/iwj.13821
2. Magnusson, S. et al. (2016) Acceleration of Wound Healing Through Utilization of Fish Skin Containing Omega-3 Fatty Acids, Hmpgloballearningnetwork.com. Today’s Wound Clinic. Available at https://www.hmpgloballearningnetwork.com/site/twc/articles/acceleration-wound-healing-through-utilization-fish-skin-containing-omega-3-fatty-acids
(Accessed: March 30, 2023)
3. Kirsner, R.S., Margolis, D.J., Baldursson, B.T., Petursdottir, K., Davidsson, O.B., Weir, D. and Lantis, J.C., II (2020), Fish skin grafts compared to human amnion/chorion membrane allografts: A double-blind, prospective, randomized clinical trial of acute wound healing. Wound Rep Reg, 28: 75-80. https://doi.org/10.1111/wrr.12761
4. Baldursson BT, Kjartansson H, Konrádsdóttir F, Gudnason P, Sigurjonsson GF, Lund SH. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Low Extrem Wounds. 2015 Mar;14(1):37-43. doi: 10.1177/1534734615573661. Epub 2015 Mar 9. PMID: 25759413.
5. Magnusson, S., Baldursson, BT., Kjartansson, H., Rolfsson, O., Sigurjonsson, GF., Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care, Military Medicine, Volume 182, Issue suppl_1, March 2017, Pages 383–388, https://doi.org/10.7205/MILMED-D-16-00142
6. Fiakos, Gabriella & Kuang, Zeming & Lo, Evan. (2020). Improved skin regeneration with acellular fish skin grafts. Engineered Regeneration. 1. 95-101. 10.1016/j.engreg.2020.09.002.
7. Winters C, Kirsner RS, Margolis DJ, Lantis JC. Cost Effectiveness of Fish Skin Grafts Versus Standard of Care on Wound Healing of Chronic Diabetic Foot Ulcers: A Retrospective Comparative Cohort Study. Wounds. 2020 Oct;32(10):283-290. PMID: 33370245.
8. Luze H, Nischwitz SP, Smolle C, Zrim R, Kamolz LP. The Use of Acellular Fish Skin Grafts in Burn Wound Management-A Systematic Review. Medicina (Kaunas). 2022 Jul 9;58(7):912. doi: 10.3390/medicina58070912. PMID: 35888631; PMCID: PMC9323726.
9. Reda F, Kjartansson H, Jeffery SLA. Use of Fish Skin Graft in Management of Combat Injuries Following Military Drone Assaults in Field-Like Hospital Conditions. Mil Med. 2023 Feb 15:usad028. doi: 10.1093/milmed/usad028. Epub ahead of print. PMID: 36794813.
10. Yang et al. A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds.2016;28(4):112-18.
Masterclass GUIDES
Masterclass Guide: Decellularized Fish Skin Technology: Kerecis® Marigen™ Wound Masterclass. Volume 2. No 4. March 2023 How to Cite this Article Wound Masterclass - Vol 2 - March 2023 67 Useful Links Use your device to scan this QR code for more information about Kerecis® Marigen™ Click here to visit the Kerecis® website Sponsored By Kerecis®. All production resources provided by Kerecis® © Copyright. Wound Masterclass. 2023
Kerecis® Marigen™
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition




Editorial Summary
Lower extremity lymphedema is an underrecognized and undermanaged clinical condition. Educational endeavors at the medical school level continue to lag behind the progressive and robust research and peer reviewed publication rate present over the past decade. The resulting inadequate medical student and care provider education regarding the lymphatic vasculature, clinical pathophysiology and available treatment modalities impairs both the delivery of care and the prevention of progression and poor outcomes.1
Introduction


Too often unrecognized, the correct diagnosis of lymphedema is essential for appropriate clinical management.2,3 Often undermanaged, or potentially mismanaged through the liberal use of diuretics, the result can lead to secondary unintended consequences and result in progressive adipose deposition, tissue fibrosis, increasing limb volume, heaviness, functional difficulties, increased susceptibility to recurrent episodes of cellulitis, and overall higher healthcare utilization. These associated comorbidities carry significant tangible and intangible burdens for patients, families, and the medical community alike. Due to the propagated medical education gaps, significant diagnostic barriers exist, resulting in unacceptable treatment initiation and management.4 The diagnostic gap also impairs determination of true epidemiologic analysis of lymphedema prevalence.5 Performing accurate epidemiologic analysis, integration of medical school and health provider education, and patient-level education will increase all facets of care for the patient with lymphedema. This ultimately will improve payor education, coverage, and a realized societal cost saving and demographic health improvement.

Traditional and dogmatic medical education continues to propagate the now antiquated understanding that 90% of interstitial fluid is reabsorbed in the venous capillary.6 The perpetuation of the dogmatic ‘Classical Starling Curve’ only impedes good clinical care for the patient with lower extremity edema. The sum of all the Starling forces, including capillary lumen hydrostatic and tissue osmotic, is now recognized to be a continuous net filtration force. This is now effectively characterized as the Revised Starling Principle, which not only illustrates the continuous net filtration force of the lymphatics, but also highlights the importance of the glycocalyx (GCX) in facilitating the movement of fluid and particles as seen in Figure 1.
 Dr Michael Shao
Dr M. Mark Melin
Dr Michael Shao
Dr M. Mark Melin
68 Wound
- Vol 2 - March 2023
Dr Tristan Pennella
Masterclass
Dr Daniel Miller
Dr Heather Barnhart
Dr Joseph Raffetto
Dr Wei Chen
Professor
Surgeon
Cleveland Clinic Cleveland OH, United States Vascular Surgeon and Chief, Section of Vascular Surgery, Swedish Hospital, Chicago, Illinois Chicago IL, United States Department of Dermatology, University of Minnesota Minneapolis MN, United States Nova Southeastern University, Department of Physical Therapy Fort Lauderdale FL, United States Department of Family Medicine, Mayo Clinic Health System Mankato, MN, United States M Health Fairview, Medical Director Wound Healing Institute Minneapolis MN, United States Vascular Surgeon Boston MA, United States
Department of Physical Medicine and Rehabilitation, Mayo Clinic Rochester MN, United States © Copyright. Wound Masterclass. 2023
and Attending
of Plastic Surgery,
Dr Karen Andrews
Providing care and proper management for patients with lymphedema within an up to date, evidence-based algorithms will improve clinical outcomes, maximize cost effective management, and potentially decrease related complications. This is a prudent task as lymphedema causes significant physical and psychosocial morbidity, and when left untreated can lead to recurrent infections, hospitalization, limb deformity, employment difficulties, and reduced quality of life.3,7,8 The aim of this article is to describe the anatomical and physiological basis for lymph accumulation, discuss the importance of the correct clinical diagnosis of lower extremity lymphedema, and emphasize the critical need for a multidisciplinary approach to management to obtain effective edema reduction and maintenance for improved patient quality of life.
Anatomy and Physiology of the Lymphatic Vasculature
As an integral component of the circulation, the lymphatic vasculature serves to maintain tissue fluid homeostasis and mediate the local and regional immune response.

Lymphatic endothelial cells have their origin in the cardinal vein of the 3 - 4 week embryo, thereby linking the venous and lymphatic system genetically and phenotypically together.9 The lymphatic system is a closed network originating within the interstitial tissues, as lymphatic buds have low resistance to the passage of fluid, hydrophilic molecules, cells, bacteria, and viruses from the interstitial space into the lumen of the lymphatics.10 A critical component of dermal lymphatic functionality are the anchoring filaments, composed of type VII collagen. The regular occurrence and extensive presence of anchoring filaments (Figure 2) aids to bind the outer lymphatic endothelial cell surface to surrounding collagen and connective tissues. Structurally, this facilitates the transfer of applied mechanical forces from the dermis to the lymphatics thereby separating lymphatic endothelial cells. The inter-endothelial cell gaps subsequently allow flow into the lymphatic vasculature lumen which allows for the clearance of interstitial fluid.11 The reabsorption of lymph fluid from the interstitium into the lymphatic vasculature occurs in part due to the subatmospheric environment of tissue beds.12,13,14 This concept
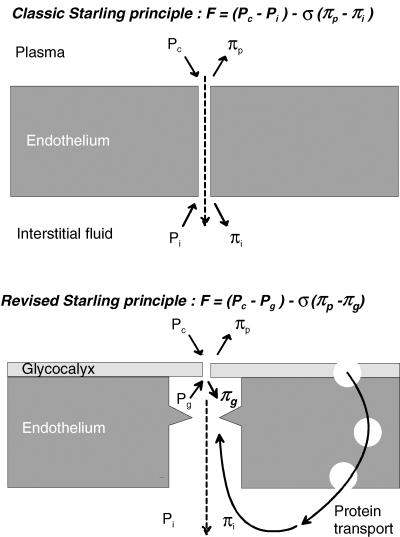
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition Wound Masterclass - Vol 2 - March 2023 69
“As an integral component of the circulation, the lymphatic vasculature serves to maintain tissue fluid homeostasis and mediate the local and regional immune response.”
© Copyright. Wound Masterclass. 2023
Figure 1: Importance of the glycocalyx (GCX) in facilitating the movement of fluid and particles. © The Physiological Society 2004.
The lymphatic segment between lymphatic valves is termed a lymphangion and is considered the functional unit of the lymphatics.15
is known as the ‘suction theory’ of lymph reabsorption and can be considered as a constant ‘pull’ of fluid from interstitial spaces into the lymphatic vasculature.16 The ‘pull’ of fluid into the lymphatic system is maintained by flowmediated dilation which has been shown to be disrupted in animal models following lymphatic injury.17
The lymphatic segment between lymphatic valves is termed a lymphangion and is considered the functional unit of the lymphatics (Figure 3).15,18,19 The lymphatic vasculature contains elements of both striated, cardiac-like and smooth muscle cells that result in lymphangion contractility. Intraluminal shear forces result in the production of nitric oxide (NO) from lymphatic endothelial cells, a positive feedback to lymphangion contractility, and also contribute to the immune responsiveness of the lymphatic vasculature.6,20-22
The anatomical construction of the lymphatic and venous system is clinically important for understanding, preventing, and managing lower extremity lymphedema. The lymphatic system is structurally comprised of lymphatic
capillaries, precollectors, superficial lymphatic collectors, and deep lymphatic collectors (Figure 4).23
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition 70 Wound Masterclass - Vol 2 - March 2023
“The anatomical construction of the lymphatic and venous system is clinically important for understanding, preventing, and managing lower extremity lymphedema.”
23 Image available from article: Suami, H., & Scaglioni, M. F. (2018). Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Seminars in plastic surgery, 32(1), 5–11. © Copyright. Wound Masterclass. 2023
Figure 4: Structure of the lymphatic system.
11 Basal
fibrils Fibroblast Lumen Anchoring filaments Image available from article: Leak, L. V., and J. F. Burke. 1968. “ULTRASTRUCTURAL STUDIES ON THE LYMPHATIC ANCHORING FILAMENTS.” The Journal of Cell Biology 36 (1): 129–49. Basal membrane Lymphatic endothelial cells Lymphangion Lympathic valves Lympathic muscle cell Glycocalyx
Figure 2: The regular occurrence and extensive presence of anchoring filaments aids to bind the outer lymphatic endothelial cell surface to surrounding collagen and connective tissues.
lamina Collagen
Image available from article: Hansen, Kirk C., Angelo D’Alessandro, Cristina C. Clement, and Laura Santambrogio. 2015. “Lymph Formation, Composition and Circulation: A Proteomics Perspective.” International Immunology 27 (5): 219–27.
Figure 3:
“In lymphedema, there is disruption of the normal ‘lymphosome’ arrangement as lymph begins to flow backwards. This concept, known as ‘dermal backflow’, can be visualized with intradermal injection of a tracer material.”
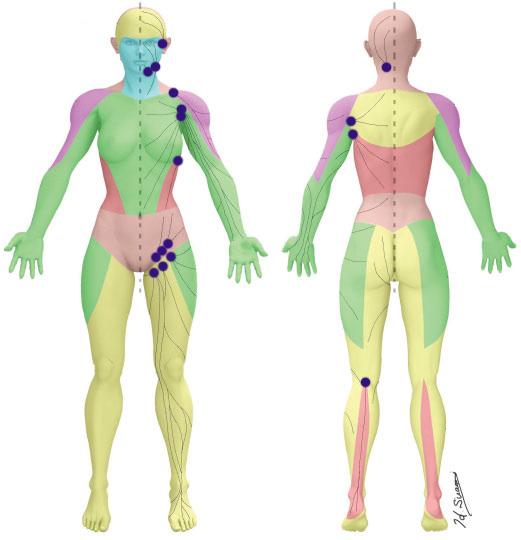
The lower extremity lymphatic system is divided into the ventromedial and dorsolateral lymphatic bundles, with the first draining lymph nodes of the ventromedial bundle located in the groin.24 Kubik and Manestar’s work in 1995 was an early triumph in highlighting this important topographical alignment of the lymphatic and venous systems of the ventromedial bundle. They found that only the great saphenous vein is overcrossed by lymph collectors, which can be easily damaged with surgical interventions near the saphenous opening.25 This is also true at the dorsum of the foot, where lymphatic collectors lie superficially within the corium of the supramalleolar region.25 More recently, Suami and Scaglioni’s cadaveric work has led to the discovery of ‘lymphosomes’, which is an anatomical mapping connecting local lymphatic vessels to regional lymph nodes (Figure 5).23
Their work highlights the anatomical and physiological doctrine of the lymphatics (Figure 6), as follows: 23
1. Superficial lymphatics travel in the straight path towards its corresponding lymph node
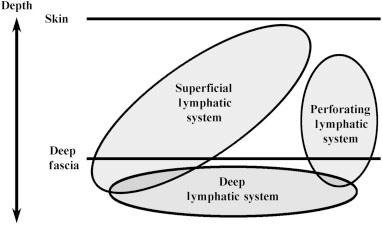
2. No perforating lymphatic vessels connect the superficial and deep lymphatics
3. Superficial lymphatics do not overlap and are arranged in plane
In lymphedema, there is disruption of the normal ‘lymphosome’ arrangement as lymph begins to flow backwards. This concept, known as ‘dermal backflow’, can be visualized with intradermal injection of a tracer material. Dermal backflow is prevented in healthy lymphatics in part by the arrangement of bicuspid valves. Mutations in the genes for lymphatic valve development have been implicated in primary lymphedema and more recently in the development of cancer related lymphedema.26 Iyer et al. found that
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition Wound Masterclass - Vol 2 - March 2023 71
23 Image available from article: Suami, H., & Scaglioni, M. F. (2018). Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Seminars in plastic surgery, 32(1), 5–11. Temporal Submental Subclavicular Lateral axillary Superior inguinal Inferior inguinal Occipital Subscapular Pectoral Superior inguinal Popliteal
Figure 5: Lymphosomes: an anatomical mapping connecting local lymphatic vessels to regional lymph nodes.
23 Image available from article: Suami, H., & Scaglioni, M. F. (2018). Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Seminars in plastic surgery, 32(1), 5–11.
Figure 6: Anatomical and physiological doctrine of the lymphatics.
© Copyright. Wound Masterclass. 2023
when there is decreased lymph flow, there is reduced vascular endothelial-cadherin signaling required for lymphatic valve maintenance.26 This results in loss of lymphatic valves and further reduction in lymph flow leading to dermal backflow and stagnant lymph.
Vital to the ability of lymphatic endothelial cells to maintain fluid homeostasis, immune modulation, and anti-inflammatory capabilities is the lymphatic endothelial glycocalyx (GCX). The GCX is a negatively charged, polysaccharide brush-like layer, attached to endothelial cells lining the entirety of the arterial, venous, and lymphatic systems.27 The GCX composition consists of glycoproteins and proteoglycan ‘backbones’ which attach variable glycosaminoglycan chains. At least five types of glycosaminoglycan (GAG) chains have been identified, including heparan sulfate, chondroitin sulfate, dermatan sulfate, keratan sulfate, and hyaluronic acid. Variation exists in the proteoglycan core proteins with regards to size, the glycosaminoglycan chains that are attached, and cell membrane ‘bound’ status. Other proteoglycans (mimecan, perlecan, biglycan) are attached after being secreted from cytosol bodies, and are subsequently assembled as GAG modified chains leading to soluble aspects of proteoglycans which diffuse into the blood stream.
The functions of the GCX are:
1. Modulate inflammatory responses through cytokine binding
2. Act as 'quenchers' of oxygen free radicals and maintenance of nitric oxide availability
3. Maintain anticoagulant components that contribute to the thromboresistant nature of healthy endothelium
4. Prevent leukocyte and platelet adhesion
5. Improve rheology of red blood cells in the microvasculature
6. Acts as a mechano-sensor and transducer responding to shear stress resulting in the production of nitric oxide (NO) and other cytokines28-30
7. Regulates vascular molecular and biofluid permeability, thereby preventing reabsorption of interstitial fluid into the venules27,29-34
Consequently, both venous and arterial hypertension have been associated with glycocalyx alterations, shedding, and microcirculatory dysfunction, thereby inhibiting these important functions of the GCX.35,36
The lymphatic system is integrally connected to the functional immune response. Unfortunately, lymphatic vessels are often thought of as a mere passageway to the lymphoid tissues instead of an integral component of immune activation. Lymphatic endothelial cells exhibit important immunomodulatory roles, with circulating dendritic and T cells, and exhibit both MHC I and MHC II molecules for antigen processing and presentation.37 In addition, lymphatic vessels maintain the appropriate gradients for cytokine and chemokine signaling, aid in lymphocyte rolling, maintain fluid balance of tissue and vasculature, and facilitate pathogen clearance.31,38,39 In states of high inflammation, lymphatic endothelial cells can coordinate immunosuppression by decreasing dendritic cell maturation.40 In a recent model study, high inflammatory states were found to initiate shedding of GCX components, thereby allowing for rapid drainage of inflamed tissue beds without subsequent neutrophil recruitment.41 The integrity of the GCX is therefore vital for modulating inflammation and facilitating the immune responses of the lymphatic system.
As we age so does our lymphatic system; aging-associated changes to the lymphatic system include decreased collecting vessels, decreased collateral conduits within local lymphatic arcades, aneurism-like formations, impaired contractile function, decreased nitric oxide production, and significant loss of the GCX.42 The age-associated loss of the GCX layer includes a reduction in both the size and continuity of this protective layer and the production of pro-inflammatory cytokines, causing increased lymphatic permeability.43
Endothelial nitric oxide synthase (eNOS) and subsequent nitric oxide (NO) production are essential to maintain native lymphatic pumping and increase the diastolic filling of lymphangions.44 As we age, there is a decrease in eNOS activity and a subsequent increase in inducible NO synthase (iNOS), leading to an increased lymphatic vessel load without the ability of the lymphatic system to adapt its pumping capabilities (Figure 7).43
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition 72 Wound Masterclass - Vol 2 - March 2023
© Copyright. Wound Masterclass. 2023
Of interest, similar alterations to eNOS have been found in obesity-induced endothelial dysfunction due to direct and indirect alterations in regulatory microRNA expression.Ait-Aissa2020 This results in decreased NO bioavailability and subsequently decreased endothelial vasodilatory capacity. As approximately onethird of our population is affected by obesity, and with the aging population, the clinical utility of evaluating microRNA gene regulation may hold promising therapeutic value.45,46
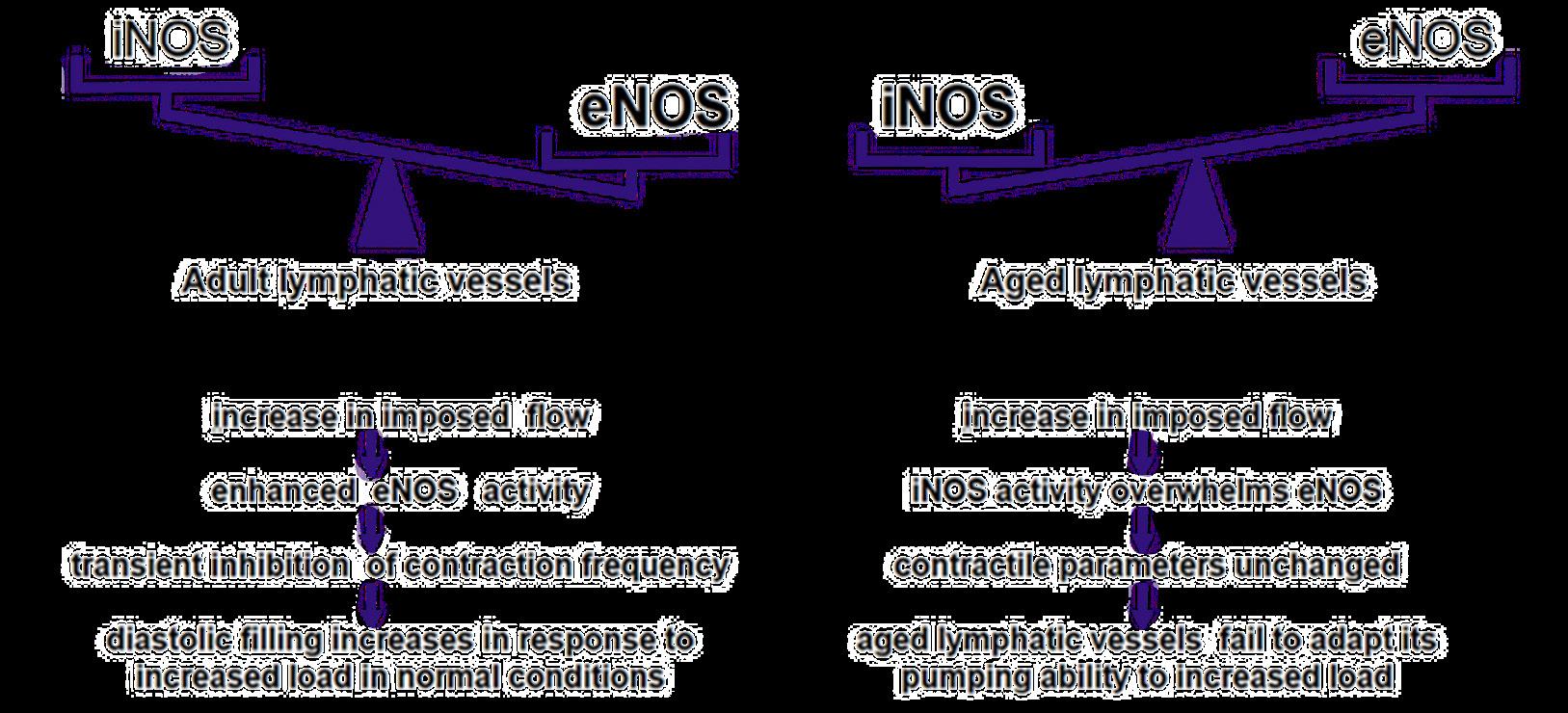
Starling Principles of Lymphatic Function
The recognition of decreased post-venule reabsorption of interstitial fluid as a result of the permeability function of the GCX resulted in a paradigm shift of a new and profound relevance of the lymphatic system, due to the modification of the dogmatic Starling curve. In 1896, E.H. Starling, working in the Physiological Laboratory of Guy’s Hospital, developed the Classic Starling Curve. The Classic Starling curve evaluated luminal hydrostatic pressures and the balance with tissue osmotic pressures.47 Based upon his data, Starling proposed that~90% of fluid shed into the interstitium is
reabsorbed into the venule, with the remaining 10% being collected by the lymphatic system. For decades, the dogmatic Starling curve relegated the lymphatics to a minor vascular system component, only of major significance in clinical lymphedema and surgical procedures gone awry that resulted in quality of life altering large vessel lymphatic leaks. The origins of the modification of the Starling curve, recognizing that all interstitial fluid is returned to the central venous system via the lymphatic system, and the recognition of the GCX to be a major determinant of vascular permeability, began in 1987 with the work of Michel and Phillips, demonstrating only transient venule reabsorption of interstitial lymphatic fluid.48 Further details were defined by Michel and Levick in 2004, with the introduction of the fully revised Starling principle recognizing the GCX layer of endothelial cells direct impact on reabsorption of interstitial lymphatic fluid into the lumen of venules. This resulted in the revised Starling Principle application to the treatment of lower extremity edema, expressly advocating for no diuretic use.49
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition Wound Masterclass - Vol 2 - March 2023 73
Further investigation has characterized the
© Copyright. Wound Masterclass. 2023
“The recognition of decreased post-venule reabsorption of interstitial fluid as a result of the permeability function of the GCX resulted in a paradigm shift of a new and profound relevance of the lymphatic system, due to the modification of the dogmatic Starling curve.”
Figure 7: NO-dependent regulatory mechanisms in aged lymphatic vessels.43
7a:
In
adult lymphatic vessels, the enhanced eNOS activity mediates the transient inhibition of contraction frequency to adapt the load by the increase of imposed flow.
Image available from article: Shang T, Liang J, Kapron CM, Liu J. Pathophysiology of aged lymphatic vessels. Aging (Albany NY). 2019; 11:6602-6613. 7a 7b
7b: In aged lympathic vessels, increased iNOS level renders the contractile parameters unchanged in response to increased imposed flow.
GCX as a semipermeable layer coating the endoluminal side of the capillary wall, recognizing the lymphatic system as the dominant force responsible for interstitial fluid homeostasis, and promoting interest in the molecular and genetic underpinnings of lymphatic dysfunction.39
In 2014, Drs. Mortimer and Rockson brought the Modified Starling Curve to international attention and clinical applicability, by recognizing that edema develops when the capillary and venule filtration rate exceeds lymph drainage either because the microfiltration rate is too high, lymphatic flow is impaired, or both exist; hence, all chronic edema indicates an inadequacy or failure of lymphatic drainage. Therefore, a clinical approach to subcutaneous edema of the extremities should begin with a consideration and assessment of lymphatic function to evaluate whether primary lymphatic impairment exists, or if lymphatic circulation is normal but overloaded by elevated microvascular filtration.6
The GCX is now thoroughly recognized in peer reviewed literature as critical to endothelial cells, micro/macro vascular and lymphatic function, and bioreactivity. Pioneering work by Bjork and Hettrick led to one of the first connections tying the intricacies of the GCX to the functional lymphatic system.50 Pathophysiologic conditions are associated with variable alterations at the local, regional and systemic levels, resulting in GCX thinning, shedding, and restoration. GCX dysfunction is directly correlated with endothelial cell dysfunction; such conditions include acute and chronic hyperglycemia in diabetes, events resulting in ischemic/ reperfusion injury, trauma, sepsis, tobacco use, arterial hypertension and venous hypertension.35,36 Persistent venous hypertension is thought to alter microvascular shear forces, resulting in shedding of the GCX, permitting leukocyte adhesion and migration through the endothelial cell lining, triggering
an inflammatory cascade that contributes to the pathophysiology of venous changes noted in chronic venous disease.35 Chronic venous insufficiency is an important secondary cause of lymphedema, a condition known as phlebolymphedema. Phlebolymphedema has been reported to be the predominant cause of lower extremity lymphedema, in a 440 patient cohort treated in a cancer-affiliated physical therapy clinic.51
Assessment of Lymphedema
Lymphedema is the result of a loss of the finely tuned balance of microvascular tissue fluid production and recovery through the lymphatic vasculature, chronic inflammation, and loss of integrity of the lymphatic endothelial cell GCX.6,15,31,38,50,52
The etiology of lymphedema is generally described as primary or secondary. Primary lymphedema is due to a genetic mutation, resulting in abnormal lymphatic vascular development causing either a structural or functional abnormality that impairs proper drainage of lymphatic fluid; primary lymphedema is further categorized as congenital (identification based upon abnormalities identified shortly after birth), praecox (abnormalities identified most often during teenage years or early adulthood) or tarda (typically occurring after age 35).6 It is important to note that genetic alterations resulting in clinical lymphedema can manifest and present later in life, though this can be a point of confusion and a missed opportunity to correctly diagnose the patient.6 If an accurate phenotypic profile is performed, the correct diagnosis of primary lymphedema can be established, despite the absence of the genetic identification.53 Due to some forms of primary lymphedema presenting later in life, this can hinder the ability of a clinician to accurately differentiate primary vs secondary etiology; as such, the presence of potentiating secondary
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition 74 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
“The GCX is now thoroughly recognized in peer reviewed literature as critical to endothelial cells, micro/macro vascular and lymphatic function, and bioreactivity.”
“Accurate clinical staging of lymphedema is also critical for treatment, documentation, and to gain appropriate insurance coverage to support patient participation in the treatment plan.”
factors associated with secondary lymphedema, such as chronic venous insufficiency, obesity, lipedema, trauma, cellulitis, surgery, and cancer must be considered, but also the possibility of primary etiology should not be mutually excluded. An accurate diagnosis through genetic and phenotypic evaluation and an understanding of the complete disease process will result in the most appropriate treatment algorithm.
Accurate clinical staging of lymphedema is also critical for treatment, documentation, and to
The 2016 International Society of Lymphology classifies 4 stages:
Stage 0: Latent or subclinical; no evidence of swelling; subjective symptoms.
Stage I: Early accumulation of fluid; usually pitting; subsides with elevation.
Stage II: Swelling rarely reduced with elevation; pitting still present in early stage II, whereas pitting is absent in later stages as fibrosis and fat deposition begin.
Stage III: Lymphostatic elephantiasis; nonpitting with trophic skin changes, further deposition of fat and fibrosis, and warty overgrowths.54
gain appropriate insurance coverage to support patient participation in the treatment plan. Due to the challenges associated with accurately diagnosing lymphedema, clinicians should carefully consider the differential diagnosis. Patients can be broadly segregated by those with unilateral asymmetric or bilateral leg edema, and by acuity of onset. For patients presenting with acute onset unilateral leg edema, it is imperative to consider deep vein thrombosis (DVT) and evaluate using duplex ultrasonography. If DVT has been excluded, patients should be evaluated for musculoskeletal injury or cellulitis, which should be evident based on history and physical exam findings.
Class
Antidepressants
Specific medications
Monoamine oxidase inhibitors, trazodone
Antihypertensives Beta-adrenergic blockers, calcium channel blockers, clonidine (Catapres), hydralazine, methyldopa, minoxidil
Antivirals
Acyclovir (Zovirax)
Chemotherapeutics Cyclophosphamide, cyclosporine (Sandimmune), cytosine arabinoside, mithramycin
Cytokines Granulocyte colonystimulating factor, granulocyte-macrophase colony-stimulating factor, interferon alfa, interleukin-2, interleukin-4
Hormones Androgen, corticosteroids, estrogen, progesterone, testosterone
Nonsteroidal antiinflammatory drugs
Celecoxib (Celebrex), ibuprofen
For patients presenting with bilateral leg edema, the differential diagnosis includes medicationinduced edema, acute heart failure, end-stage renal disease, and bilateral DVT. Common medication-induced edema is often forgotten within the differential, yet well described in the literature. Clinicians should closely examine medication lists as a significant proportion of patients now take antihypertensive medications commonly associated with edema (Table 1).55
The Wells score or Modified Wells score may be used to calculate clinical suspicion for DVT.56 If clinical suspicion is moderate or high, duplex ultrasound should be done to evaluate for DVT. Patients with a history of dyspnea, orthopnea, or paroxysmal nocturnal dyspnea; or signs of tachypnea, tachycardia, rales, or jugular venous distention, should undergo echocardiography to evaluate for acute heart failure. Urinalysis or urine dipstick can screen for the presence of proteinuria, and if present,
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition Wound Masterclass - Vol 2 - March 2023 75
© Copyright. Wound Masterclass. 2023
Table 1: Medications commonly associated with edema.
quantitative urine protein-to-creatinine ratio and serum albumin should be obtained.
For patients presenting with unilateral leg edema, the differential diagnosis includes chronic venous insufficiency, chronic lymphedema, Baker’s cyst, MayThurner syndrome, pelvic tumor, complex regional pain syndrome, syndromic limb hypertrophy (Klippel-Trenaunay syndrome and Proteus syndrome), and poor calf contractility (radiculopathy, stroke). Duplex ultrasonography can be helpful to identify chronic venous insufficiency and Baker’s cyst. Complex regional pain syndrome and chronic lymphedema can generally be diagnosed based on clinical features in the history and physical examination. Complex regional pain syndrome usually presents with skin hyperesthesia, allodynia, and/ or temperature or skin color asymmetry, 4 - 6 weeks following trauma. Syndromic limb hypertrophy is typically associated with other clinical features, such as capillary malformations (port wine stains), in the case of Klippel-Trenaunay syndrome. Primary lymphedema patients may report positive family history of lymphedema or early onset lymphedema (congenital lymphedema or lymphedema praecox); secondary lymphedema patients may report a history of cancer, inguinal or pelvic lymphadenectomy, radiation therapy, infection, obesity, chronic venous insufficiency, or inflammatory disorders.
Microdermal Changes Associated With Lymphedema
Cutaneous changes associated with lymphedema
As noted above, the early phases of lymphedema present with soft tissue infiltration which clinically resembles the edema of venous insufficiency; however over time, the inflammatory milieu of chronic lymphedema induces vascular and connective tissue changes
within the dermis and subcutis. The clinical and microscopic cutaneous findings of chronic lymphedema can be both benign/ reactive and malignant, as lymphedema is a known risk factor for skin and soft tissue malignancies.57
The benign, reactive cutaneous changes associated with chronic lymphedema include erythema, skin thickening and fibrotic, scarlike changes with hyperkeratosis, and a distinctive ‘cobblestone’ pattern referred to as ‘lymphostasisverrucosa cutis’, or ‘elephantiasis nostrasverrucosa’ (ENV).58 ENV presents as a diffusely infiltrated, typically hyperpigmented, firm plaque of the distal lower extremities, with a verruous, ‘pebbly’ surface and hyperkeratosis, which is sometimes described as ‘mossy’ in appearance.58 Microscopically, early lymphedema results in ectatic, thin-walled or slitlike small vascular channels within the upper dermis. The histopathologic changes of chronic lymphedema, including biopsies of ENV, include reactive (pseudoepitheliomatous) epidermal hyperplasia, dilated dermal lymphatic channels (which can be highlighted by immunostains such as D2-40/LYVE-1), and pronounced, scarlike fibrosis of the dermis and subcutis.59 These chronic fibrotic changes are believed to result from the induction of fibroblast proliferation by protein-rich lymph fluid.58
Chronic lymphedema is a significant risk factor for angiosarcoma (AS); this phenomenon is referred to as Stewart-Treves syndrome, and was first described in 1948.57 Stewart-Treves is classically associated with lymphedema resulting from breast cancer surgery and lymphadenectomy, but has also been observed in chronic lower extremity lymphedema. The clinical presentation typically involves rapidly-expanding purpuric plaques, nodules and tumors, commonly with ulceration. The microscopic features of AS include extensive replacement of dermal connective tissue by irregular and anastomosing vascular channels,
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition 76 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
“Chronic lymphedema is a significant risk factor for angiosarcoma (AS).”
which dissect between collagen fibers, encircle normal dermal vessels, and adnexal structures, and exhibit endothelial cell atypia and multlayering. Chronic lymphedema is also a risk factor for Kaposi’s sarcoma (KS), especially in patients from areas with human herpesvirus-8 (HHV-8) endemicity.57 Cutaneous KS presents with purpuric, purplehued papules and plaques, and microscopically demonstrates slitlike vascular channels with spindled endothelial cells, stromal fibrosis and a lymphoplasmacytic infiltrate.
Lymphatic Examination
After other etiologies for generalized edema have been excluded, the diagnosis of chronic lymphedema is usually established based on typical clinical features found by thorough history and physical examination:
• Slowly progressive edema affecting one or both lower extremities
• History of surgery, lymph node dissection, or radiation therapy
• Non-pitting edema is suggestive, although pitting edema may be present in early-stage lymphedema until further deposition of fat and fibrosis causes the characteristic non-pitting presentation
• Positive Stemmer sign: inability to pinch the skinfold at the base of the second toe (Figure 8)60
• Positive Bjork Bow Tie Test: gently pinched skin when lifted and rolled is thickened, less pliable, less able to be pinched and lifted off, and produce limited 'bow tie' of wrinkles (Figure 9)61
• Bier spots are the presence of multiple irregular white macules along extensor surfaces and have been associated with lower extremity lymphedema62
• Characteristic skin changes: hypertrophic nodules, hyperkeratotic, verrucous and vesicular skin lesions
• Dorsal hump and squaring of toes: this distinguishes lymphedema from lipedema which often spares the foot and toes. Lipedema can be further distinguished from lymphedema due to its characteristic adiposity distribution from hips to ankles while sparing the feet in a distinct 'step off' appearance.63
• 'Ski-jump' nails evident by anup-turned concavity can be clinically useful as a phenotypic manifestation of primary lymphedema64
Lymphatic Imaging
Imaging modalities for chronic lymphedema include lymphoscintigraphy and indocyanine green lymphangiography, but are not usually necessary and are generally reserved for diagnostic dilemmas. Duplex ultrasound (DUS) has become a practical first-line modality for the evaluation of lymphedema, due to its availability and low-cost. DUS not only provides diagnostic value, but has also been shown to allow for classifying edema severity and monitoring response to treatment.
DUS can be used to differentiate dependent edema from secondary lower extremity edema, by visualization of subcutaneous echogenicity and echo-free space.65 DUS can also aid in differentiating lipidema from lymphedema, as lipidema characteristically demonstrates normal dermal thickness and echogenicity, whereas lymphedema often demonstrates increased dermal thickness and reduced echogenicity.66 Newer modalities, although not available currently in the United States, is the use of magnetic resonance lymphography (MRL). MRL has been shown to identify superficial lymphatic vessels down to the 0.5 mm level, with a high sensitivity and specificity for illustrating abnormal lymphatics and drainage patterns.67 Similarly, the use of multifrequency bioimpedance analysis has been shown to be reliable and reproducible for accurately documenting the presence of lymphedema in a quick, cost-effective manner.68,69
Management
Although there is currently no cure for lymphedema, it is a manageable disease by implementing the components of Complete Decongestive Physiotherapy (CDPT). The objective of CDPT is to achieve a reduction in limb volume and improve the integrity and quality of the skin. This is attained through a two-phase approach: phase one is the intensive or decongestion phase that is clinician-driven, and phase two is the maintenance phase that is patient-driven. The intensive phase is best performed daily (or as frequently as possible), until maximal volume reduction is achieved. Once the lymphedematous limb has plateaued and is no longer achieving a reduction in volume, the patient is transitioned into the maintenance phase, which is continued for life.
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition Wound Masterclass - Vol 2 - March 2023 77
© Copyright. Wound Masterclass. 2023
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition

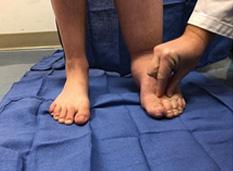
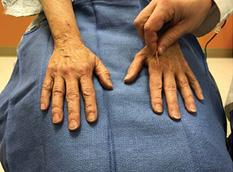
The components of CDPT are implemented and facilitated by a trained lymphedema specialist during the intensive phase and include:
1. Meticulous skin and nail care to the affected areas
2. Manual lymphatic drainage (MLD), which is a gentle manual technique that redirects lymph flow
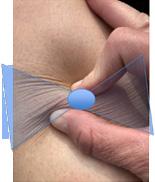
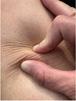
3. Multilayer, short-strech compression bandaging during active decongestion, and over-the-counter or custom garments once the limb has decongested

4. Therapeutic exercise to enchance and promote lympathic pumping mirrors the effects of MLD, with patient reports of ease of use and comfort, without an increase in pain scores70
60
9a: Negative Bjork ‘bow tie’ test.
9b: Positive Bjork ‘bow tie’ test.
9c: ‘Bow tie’ of wrinkles in negative test.61
Additionally, during the maintenance phase, the utilization of intermittent pneumatic compression (IPC) may be employed as an adjunctive device to help maintain fluid reduction.
Of note is the growing understanding of the effects of manual lymph drainage beyond the reduction of fluid volume. Emerging evidence is highlighting the impact MLD has on soft tissue remodeling, and the resolution and prevention of soft tissue dysfunction. To appreciate this, it is important to understand the inter-relationship between the lymphatic and integumentary systems.
When lymphatic fluid is stagnant, a pathohistological state of chronic inflammation results.71 This chronic inflammatory state induces a cascade of events that results in connective tissue proliferation and excess collagen tissue formation, culminating in the thickened, fibrotic skin and wart-like projections (papillomatosis and verrucous) commonly seen with chronic lymphedema.72 Further, disorders of the lymph system, whether systemic (macro-lymphedema) or localized (micro-lymphedema), produce cutaneous regions susceptible to infection, inflammation and carcinogenesis.72 The chronic inflammation resulting from lymphedema creates a region of cutaneous immune deficiency, or a localized skin barrier failure; the associated abnormalities are called lymphostatic dermopathy, which is the failure of the skin as an immune organ.57,72,73 Areas of lymphostatic dermopathy are often associated with the presence of chronic wounds and chronic infections.
Manual lymph drainage helps to re-route stagnant lymph fluid to functional lymphatics, thereby mobilizing the fluid and reducing limb volume. This is coupled with the fact that mobilizing the stagnant lymph breaks the chronic inflammatory cycle and the deleterious integumentary sequelae that ensues. A growing body of evidence is shedding light on the positive effects MLD has on many structures and conditions. Although beyond the scope
78 Wound Masterclass - Vol 2 - March 2023
“Manual
lymph drainage helps to re-route stagnant lymph fluid to functional lymphatics, thereby mobilizing the fluid and reducing limb volume.”
Figure 8:
8a: True-negative Stemmer sign (examiner is able to pinch the skin in a patient without lymphedema).
8b: True-positive Stemmer sign (skin is unable to be pinched in an individual with lymphedema).
Images available from article: Goss JA, Greene AK. Sensitivity and Specificity of the Stemmer Sign for Lymphedema: A Clinical Lymphoscintigraphic Study. Plast Reconstr Surg Glob Open. 2019 Jun 25;7(6):e2295. 8a 8b 8c
8c: False-negative Stemmer sign (the skin is able to be pinched in a subject with lymphedema).
Figure 9:
Images available from article: Bjork, R., Hettrick, H. Lymphedema: New Concepts in Diagnosis and Treatment. Curr Derm Rep 8, 190–198 (2019).
9a 9b 9c
© Copyright. Wound Masterclass. 2023
of this article, the following highlights the current evidence in support of MLD: the evidence supports MLD for influencing soft tissue remodeling;74,75 synergistic effects when combined with exercise;76 positive effects on the veins and venous ulceration;77-81 improving orthopedic outcomes such as total knee replacements;82-84 and MLD is beneficial for other diagnoses such as orthopedic injuries,85 neurological diseases, autoimmune diseases, autism, when combined with Proprioception Neuromuscular Facilitation.86-90 The benefits of MLD have been assessed using near-infrared fluorescence imaging evaluating pre- and posttherapy sessions, and illustrating improved contractile lymphatic function.91
It is well understood that lymphedema can be a complex and challenging condition to manage. This is further evidenced by a report that lymphedema patients seen in an outpatient wound clinic had an average of 7.3 comorbidities and took 8.4 concomitant medications.3 To assist healthcare providers in managing these complex patients, the Lower Extremity Lymphedema Complexity Score (LLCS) has been created.92
The tool is meant to help clinicians identify the various contributing factors and complexities of patients presenting with lymphedema and skin impairments. This could help drive proper examination and differential diagnosis, treatment planning, as well as utilization of proper resources. The LLCS contains 12 domains: comorbidities (referring to any copresenting condition that impacts overall health), limb edema, tissue texture, scars, skin integrity, skin changes, fat disorders (lipedema), BMI, mobility, activities of daily living (ADLs), pain/ discomfort and the Lymphedema Life Impact Scale (LLIS, used with permission from the author).
The 12 established domains reflect common presentations ranging in complexity that can contribute to lymphedema severity and complications. Each domain is to be scored based upon the complexity of the condition, ranging from nominal complexity (0), mild complexity (1), moderate complexity (2), severe complexity (3) or extreme complexity (4). If a patient has bilateral lymphedema, the score is based on the most complex or involved limb. Descriptions under each domain and section are provided for additional clarity. A
cumulative score will indicate if the patient presents with mild, moderate, severe or extreme complexity.
The next phase in the development of the LLCS is to create algorithms of suggested interventions and/ or referrals based upon standards of practice and evidence-based medicine. Though not prescriptive, the algorithms are meant to provide guidance and suggestions for healthcare providers as to how best manage the common associated complications and underlying pathologies widespread in this patient population.
Intermittent pneumatic compression pumps (IPCs) are available as simple (leg sleeves) and advanced (leg sleeves with an abdominal component). In addition, available IPCs have varying mechanisms of action, including push and squeeze, push-squeeze-pull, and pull. Push and squeeze IPC devices are typically high pressure and show a dose-dependent relationship between pressure and total fluid flow rates. At times, this can be beneficial as patients with later stage lymphedema often have epidermal and dermal thickening and fibrotic changes often requiring more force to move fluid.93 Consequently, high flow and pressure rates can also lead to collateral channel formation lacking lymphatic endothelial lining, therefore lacking the clinical benefits of the glycocalyx. Push-squeeze-pull IPC devices often cycle between pressures while completely deflating between compression phases. Pull IPC devices most closely mimic the suction theory and MLD by facilitating the subatmospheric environment of tissue beds and allowing interstitial fluid to be drawn into the lymphatic vasculature. The goal with IPC devices and conventional therapy on fluid redistribution is to promote lymphangiogenesisvia stimulation of local mediators and promote native lymphatic function.94-96
The push-squeeze-pull mechanism commonly seen in advanced IPC devices have been evaluated with Near InfraRed Florescence Imaging (NIRFLI) to effectively move lymphatic fluid proximally, similar in manner in which NIRFLI demonstrated the effectiveness of MLD.97-99
The purpose of the abdominal component in advanced IPC devices is to ‘prime’ the receiving abdominal and upper thigh lymphatics,
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition Wound Masterclass - Vol 2 - March 2023 79 © Copyright. Wound Masterclass. 2023
creating receptive capacitance as the pump ‘cycles’ through the abdominal chambers, initially to clear the underlying lymphatic vasculature with subsequent initiation of the leg sleeve chambers propagating distally from the ankles to the thighs. The advanced pumps mimic the cycling treatments of MLD therapy, maximizing extremity treatment.
For patients deemed to be appropriate candidates for an IPC, outcomes are best when used in conjunction with a physical medicine and rehabilitation/ vascular/ lymphatic specialist, and/ or a certified lymphedema therapist (CLT), providing treatments and education; MLD, CDPT, stretching, exercise, nutrition, and skin care remain critical elements of daily treatment. Also, because patients with lymphedema may present in various clinical settings, it is prudent for education awareness on the appropriate referral and management options that exist.
Multiple benefits of IPC devices are recognized, including patient access to use on a daily basis, utilization in locations where access to healthcare may be limited, and peer reviewed data demonstrating improved medical outcomes associated with decreasing overall medical resource utilization.100-102 In addition, advanced pneumatic compression devices have been shown to have significant economic and quality of life benefits when compared to conservative treatment. Advanced IPC use has been shown to reduce phlebolymphedema and phlebolymphedema sequelae related costs annually by 69%, including 59% reduction in hospitalizations, 82% lower inpatient costs, 55% lower outpatient costs, and reduction in physical and occupational therapy costs.102 In addition to the economic benefit, utilization of IPC can also lead to improved quality of life for patients.
Surgical Management of Lymphedema
Pharmacotherapy
Multiple over the counter medications tout a venotonic or lymphotonic effect; regarding many components present in over the counter medications, quality peer reviewed publications are limited. Flavonoids are a phenolic plant derived compound found in over 8000 different forms, with multiple biological properties and are among the most studied medications for management of lymphedema associated with chronic venous insufficiency. The specific flavonoids, hesperidin and diosmin, were evaluated in a 2017 meta-analysis, identifying moderate quality evidence demonstrating benefit for management of venous ulcerations and edema (phlebolymphedema).103 Diosmin, as a component of a hybrid medication, was found to improve outcomes when used in conjunction with CDT as compared to CDT alone.104 Diosmin formulations are commercially available in the U.S., including Vasculera’s diosmiplex, a proprietary micronized blend of purified diosmin glycoside and alkyline granules. Use of the FDA regulated Diosmiplex has been shown to have therapeutic value, although initially mislabeled in a recent publication in Wounds 2020 that has been since corrected.105
Micronized purified flavonoid fraction (MPFF) has been shown to decrease the inflammatory cascade and leukocyte-endothelial activation, thereby discouraging edema formation.106 MPFF consists of 90% diosmin and 10% hesperidin and supplementary flavonoids, and comes from extracts of rutaceaeaurantiae, a type of small orange that is micronized for improved bioavailability.106,107 MPFF has not been associated with known major side effects, while simultaneously showing significant improvement in quality of life in patients with chronic venous insufficiency, and accelerated healing of leg ulcers.106,108-110 Similarly,
Lower Extremity Lymphedema; An Often Overlooked and Undermanaged Condition 80 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
“Micronized purified flavonoid fraction (MPFF) has been shown to decrease the inflammatory cascade and leukocyte-endothelial activation, thereby discouraging edema formation.”
selenium has been noted to have a positive impact on lymphedema symptoms in a recent literature review.111 Often considered for patients with lymphedema undergoing long-distance travel or flight is the use of pinus pinaster bark extract (PBE), which goes by the tradename Pycnogenol. PBE possesses vasorelaxant activity, and enhances microcirculation through increasing capillary permeability. PBE has been shown to be effective in improving leg heaviness, subcutaneous edema, and venous pressure in patients with chronic venous insufficiency.112
Patients should also be educated in skin care, including use of a ceramide based hydrating cream to maintain dermal integrity and hydration, in order to decrease micro-cracks that leave the patient susceptible to episodes of cellulitis and erysipelas. As lymphatic dysfunction progresses, the immune function of the lymphatics is also impaired, thereby increasing patient risk of infection. Immediate access to antibiotics via an up to date prescription is generally recommended to minimize the intensity and duration of an episode of cellulitis. For patients with recurrent cellulitic episodes, chronic prophylaxis may be the best management option to prevent persistent demise of already compromised lymphatics. It is well established that with every additional cellulitic episode there is further damage to the lymphatic system, causing a degree of secondary lymphedema.113,114 This creates a cyclical problem, as the already compromised lymphatics are susceptible to infection, which in return potentiates further lymphatic damage.115 It should be noted that there is no current recommended diet for the management of lymphedema; some patients are instructed to limit water or protein intake, however this is not supported by the literature.116,117
Conclusion
Current medical and clinical education regarding the anatomical and pathophysiological state of lymphedema have far lagged behind the rapidly advancing field of lymphatic medicine. Consequently, the accurate and evidencedbased approach to lymphedema assessment and management are not unanimously and universally employed. This paper seeks to outline the basic fundamental structure of the lymphatic vasculature and its importance in lymphatic function. As patients with
lymphedema often present in a myriad of different clinical settings, it is important to deploy accurate clinical assessment and readily offer best practice management options. These management options should include meticulous skin and nail care, manual lymphatic drainage, compression, therapeutic exercise, and review of pharmacologic options. As chronic lymphedema will likely require chronic management, the use and application of IPC devices is also discussed. The use of IPC devices as adjuncts to traditional therapy can be used to decrease lymphedema related complications and improve patient quality of life. In addition, IPC devices have the ability to be used in regions where limited lymphedema therapists or specialists exist, thereby continuing to provide patients with lymphatic stimulation. The goal of MLD or IPC devices is to stimulate the underlying lymphatic system, to redistribute stagnant lymph as outlined by the revised starling curve; by redistributing stagnant lymph back into circulation the not uncommon lymphedema sequelae of recurrent infection, ulceration, deformity, and dermal/ epidermal skin changes can be minimized.
References
1. Vuong, D.; Nguyen, M.; Piller, N. Medical education: A deficiency or a disgrace. J. Lymphoedema 2011, 6, 44–49.
2. Maclellan RA, Couto RA, Sullivan JE, Grant FD, Slavin SA, Greene AK. Management of Primary and Secondary Lymphedema: Analysis of 225 Referrals to a Center. Ann Plast Surg. 2015 Aug;75(2):197-200. doi: 10.1097/SAP.0000000000000022. PMID: 24691335.
3. Wang W, Keast D. Prevalence and characteristics of lymphedema at a wound-care clinic. J Wound Care. 2016;25(4):S11-S15.
4. Son A, O’Donnell TF Jr, Izhakoff J, Gaebler JA, Niecko T, Iafrati MA. Lymphedemaassociated comorbidities and treatment gap. J Vasc Surg Venous Lymphat Disord. 2019 Sep;7(5):724-730. doi: 10.1016/j.jvsv.2019.02.015. Epub 2019 Jun 24. PMID: 31248833.
5. Rockson, Stanley G., and Kahealani K. Rivera. 2008. “Estimating the Population Burden of Lymphedema.” Annals of the New York Academy of Sciences 1131: 147–54. https://doi. org/10.1196/annals.1413.014.
6. Mortimer, Peter S., and Stanley G. Rockson. 2014. “New Developments in Clinical Aspects of Lymphatic Disease.” The Journal of Clinical Investigation 124 (3): 915–21. https://doi. org/10.1172/JCI71608.
7. Szuba, A., and S.G. Rockson. 1998. “Lymphedema: Classification, Diagnosis and Therapy.” Vascular Medicine 3 (2): 145–56. https://doi.org/10.1191/135886398674160447.
8. Morgan PA, Franks PJ, Moffatt CJ. Health-related quality of life with lymphoedema: a review of the literature. Int Wound J. 2005 Mar;2(1):47-62. doi: 10.1111/j.1742-4801.2005.00066.x.
PMID: 16722853; PMCID: PMC7951421.
9. Srinivasan, R. Sathish, Miriam E. Dillard, Oleg V. Lagutin, Fu-Jung Lin, Sophia Tsai, MingJer Tsai, Igor M. Samokhvalov, and Guillermo Oliver. 2007. “Lineage Tracing Demonstrates the Venous Origin of the Mammalian Lymphatic Vasculature.” Genes & Development 21 (19): 2422–32. https://doi.org/10.1101/gad.1588407.
10. Negrini, Daniela, and Andrea Moriondo. 2011. “Lymphatic Anatomy and Biomechanics: Biomechanics of Initial Lymphatics.” The Journal of Physiology 589 (12): 2927–34. https://doi. org/10.1113/jphysiol.2011.206672. “(PDF) Role of Flavonoids as Wound Healing Agent.” n.d. ResearchGate. Accessed April 26, 2020. http://dx.doi.org/10.5772/intechopen.79179.
11. Leak, L. V., and J. F. Burke. 1968. “ULTRASTRUCTURAL STUDIES ON THE LYMPHATIC ANCHORING FILAMENTS.” The Journal of Cell Biology 36 (1): 129–49. https://doi.org/10.1083/jcb.36.1.129.
12. Clough, G. &Smaje, L. H. Simultaneous measurement of pressure in the interstitium and the terminal lymphatics of the cat mesentery. The Journal of Physiology283, 457–468 (1978).
13. Granger, H. J., Lane, G. A., Barnes, G. E. & Lewis, R. E. Dynamics and control of transmicrovascular fluid exchange. Edema8, 189–224 (1984).
14. Hogan, R. D. The initial lymphatics and interstitial fluid pressure. In “Tissue Fluid Pressure and Composition” (A. R. Hargens, ed.). Williams and Wilkins, Baltimore., 155–163 (1981).
15. Hansen, Kirk C., Angelo D’Alessandro, Cristina C. Clement, and Laura Santambrogio. 2015. “Lymph Formation, Composition and Circulation: A Proteomics Perspective.” International Immunology 27 (5): 219–27. https://doi.org/10.1093/intimm/dxv012.
16. Guyton, A. C. & Coleman, T. G. Regulation of interstitial fluid volume and pressure. Annals of the New York Academy of Sciences150, 537–547, https://doi.org/10.1111/j.1749-6632.1968. tb14705.x (1968).
17. Nelson TS, Nepiyushchikh Z, Hooks JST, Razavi MS, Lewis T, Clement CC, Thoresen M, Cribb MT, Ross MK, Gleason RL Jr, Santambrogio L, Peroni JF, Dixon JB. Lymphatic remodelling in response to lymphatic injury in the hind limbs of sheep. Nat Biomed Eng. 2020 Jun;4(6):649-661. doi: 10.1038/s41551-019-0493-1. Epub 2019 Dec 23. PMID: 31873209; PMCID: PMC7549051.
18. Borisov, A. V. 1997. “[The theory of the design of the lymphangion].” Morfologiia (Saint Petersburg, Russia) 112 (5): 7–17.
19. Gashev, A. A. 1991. “[The mechanism of the formation of a reverse fluid filling in the lymphangions].” Fiziologicheskiizhurnal SSSR imeni I. M. Sechenova 77 (7): 63–69.
20. Liao, Shan, Gang Cheng, David A. Conner, Yuhui Huang, Raju S. Kucherlapati, Lance
L. Munn, Nancy H. Ruddle, Rakesh K. Jain, Dai Fukumura, and Timothy P. Padera. 2011. “Impaired Lymphatic Contraction Associated with Immunosuppression.” Proceedings of the National Academy of Sciences of the United States of America 108 (46): 18784–89. https://doi. org/10.1073/pnas.1116152108.
21. Rockson SG. The lymphatics and the inflammatory response: lessons learned from human lymphedema. Lymphat Res Biol. 2013 Sep;11(3):117-20. doi: 10.1089/lrb.2013.1132. Epub 2013
Lower Extremity Lymphedema; An
Overlooked and Undermanaged Condition Wound Masterclass -Vol 2 - March 2023 81 © Copyright. Wound Masterclass. 2023
Often


83 Wound Masterclass - Vol 2 - March 2023 Have an innovative product in the field of wound care? Feature it in our commercial@woundmasterclass.com Masterclass GUIDES




Masterclass GUIDES
Introduction
Lower extremity lymphedema is an under recognized and under managed clinical condition. Too often unrecognized, the correct diagnosis of lymphedema is essential for appropriate clinical management.1,2 Often undermanaged, or potentially mismanaged through the liberal use of diuretics, the result can lead to secondary unintended consequences and result in progressive adipose deposition, tissue fibrosis, increasing limb volume, heaviness, functional difficulties,increased susceptibility to recurrent episodes of cellulitis, and overall higher healthcare utilization.
This Masterclass Guide provides an overview of this condition in clinical practice.
What is Lymphedema?
Assessment of Lymphedema
■ Lymphedema is the result of a loss of the finely tuned balance of microvascular tissue fluid production and recovery through the lymphatic vasculature, chronic inflammation, and loss of integrity of the lymphatic endothelial cell GCX 3-7
■ Primary lymphedema is due to a genetic mutation resulting in abnormal lymphatic vascular development causing either a structural or functional abnormality that impairs proper drainage of lymphatic fluid, and is further categorized as congenital (identification based upon abnormalities identified shortly after birth), praecox (abnormalities identified most often during teenage years or early adulthood), or tarda (typically occurring after age 35)3
■ Due to the challenges associated with accurately diagnosing lymphedema, clinicians should carefully consider the differential diagnosis. Patients can be broadly segregated by those with unilateral asymmetric or bilateral leg edema, and by acuity of onset
Acute onset unilateral leg edema
■ Consider DVT and evaluate using duplex ultrasonography. If DVT has been excluded, patients should be evaluated for musculoskeletal injury or cellulitis, which should be evident based on history and physical exam findings
Bilateral leg edema
■ The differential diagnosis includes medication-induced edema, acute heart failure, end-stage renal disease, and bilateral DVT. Common medicationinduced edema is often forgotten within the differential, yet is well described in the literature. Clinicians should closely examine medication lists as a significant proportion of patients now take antihypertensive medications commonly associated with edema (Table 1)8
Unilateral leg edema
■ The differential diagnosis includes chronic venous insufficiency, chronic lymphedema, Baker’s cyst, May-Thurner syndrome, pelvic tumor, complex regional pain syndrome, syndromic limb hypertrophy (Klippel-Trenaunay syndrome and Proteus syndrome), and poor calf contractility (radiculopathy, stroke). Duplex ultrasonography can be helpful to identify chronic venous insufficiency and Baker’s cyst
Lymphedema in Clinical Practice
Keywords
■ Lymphedema
■ Chronic edema
■ Phlebolymphedema
■ Chronic venous insufficiency
■ Lymphedema
■ Glycocalyx
Table 1: Medications commonly associated with edema.
Class
Antidepressants
Antihypertensives
Antivirals
Chemotherapeutics
Cytokines
Hormones
Nonsteroidal anti-inflammatory drugs
Specific medications
Monoamine oxidase inhibitors, trazodone
Beta-adrenergic blockers, calcium channel blockers, clonidine (Catapres), hydralazine, methyldopa, minoxidil
Acyclovir (Zovirax)
Cyclophosphamide, cyclosporine (Sandimmune), cytosine arabinoside, mithramycin
Granulocyte colony-stimulating factor, granulocyte-macrophase colony-stimulating factor, interferon alfa, interleukin-2, interleukin-4
Androgen, corticosteroids, estrogen, progesterone, testosterone
Celecoxib (Celebrex), ibuprofen
What Are the Stages of Lymphedema?
Stage 0
■ Latent or subclinical; no evidence of swelling; subjective symptoms
Stage I
■ Early accumulation of fluid; usually pitting; subsides with elevation
Stage II
■ Swelling rarely reduced with elevation; pitting still present in early stage II, whereas pitting is absent in later stages as fibrosis and fat deposition begin
Stage III
■ Lymphostatic elephantiasis; non pitting with trophic skin changes, further deposition of fat and fibrosis, and warty overgrowths9
86 Wound Masterclass - Vol 2 - March 2023
© Copyright. Wound Masterclass. 2023
Temporal Submental Subclavicular Lateral axillary Superior inguinal Inferior inguinal Occipital Subscapular Pectoral Superior inguinal Popliteal Image available from Suami, H., & Scaglioni, M. F. (2018). Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Seminars in plastic surgery, 32(1), 5–11.
Figure 1: Lymphosomes of the body.10
Lymphedema in Clinical Practice Masterclass GUIDES
Assessment of Lymphedema
Cutaneous changes
■ The benign, reactive cutaneous changes associated with chronic lymphedema include erythema, skin thickening and fibrotic, scar-like changes with hyperkeratosis and a distinctive ‘cobblestone’ pattern referred to as ‘lymphostasis verrucosa cutis’ or ‘elephantiasis nostras verrucosa’ (ENV)11
■ ENV presents as a diffusely infiltrated, typically hyperpigmented, firm plaque of the distal lower extremities, with a verruous, ‘pebbly’ surface and hyperkeratosis which is sometimes described as ‘mossy’ in appearance11
Lymphatic imaging
■ Imaging modalities for chronic lymphedema include lymphoscintigraphy and indocyanine green lymphangiography, but are not usually necessary and are generally reserved for diagnostic dilemmas
■ Duplex ultrasound has become a practical first-line modality for the evaluation of lymphedema due to its availability and low cost. DUS not only provides diagnostic value, but has also been shown to allow for classifying edema severity and monitoring response to treatment12
■ DUS can be used to differentiate dependent edema from secondary lower extremity edema by visualization of subcutaneous echogenicity and echo-free space, and can also aid in differentiating lipidema from lymphedema, as lipidema characteristically demonstrates normal dermal thickness and echogenicity whereas lymphedema often demonstrates increased dermal thickness and reduced echogenicity13
■ A newer modality, although not available currently in the United States, is the use of magnetic resonance lymphography (MRL). MRL has been shown to identify superficial lymphatic vessels down to the 0.5 mm level with a high sensitivity and specificity for illustrating abnormal lymphatics and drainage patterns14
■ Similarly, the use of multi-frequency bioimpedance analysis has been shown to be reliable and reproducible for accurately documenting the presence of lymphedema in a quick, costeffective manner 15,16

Figure 2:
2a: True-negative Stemmer sign (examiner is able to pinch the skin in a patient without lymphedema).

2b: True-positive Stemmer sign (skin is unable to be pinched in an individual with lymphedema).
2c: False-negative Stemmer sign (the skin is able to be pinched in a subject with lymphedema).17


■ After other etiologies for generalized edema have been excluded, the diagnosis of chronic lymphedema is usually established based on typical clinical features found on thorough history and physical examination:
■ Slowly progressive edema affecting one or both lower extremities
■ History of surgery, lymph node dissection, or radiation therapy
■ Non-pitting edema is suggestive, although pitting edema may be present in early-stage lymphedema until further deposition of fat and fibrosis causes the characteristic nonpitting presentation


■ Positive Stemmer sign: inability to pinch the skin fold at the base of the second toe (Figure 2)17
■ Positive Bjork Bow Tie Test: gently pinched skin when lifted and rolled is thickened, less pliable, less able to be pinched and lifted off, and produce limited ‘Bow Tie’ of wrinkles (Figure 3)18
■ Bier spots are the presence of multiple irregular white macules along extensor surfaces and have been associated with lower extremity lymphedema19
■ Characteristic skin changes: hypertrophic nodules, hyperkeratotic, verrucous and vesicular skin lesions
■ Dorsal hump and squaring of toes; this distinguishes lymphedema from lipedema which often spares the foot and toes. Lipedema can be further distinguished from lymphedema due to its characteristic adiposity distribution from hips to ankles while sparing the feet in a distinct ‘step off’ appearance20
3a: Negative Bjork ‘bow tie’ test.
3b: Positive Bjork ‘bow tie’ test.
3c: ‘Bow tie’ of wrinkles in negative test.18
Wound Masterclass - Vol 2 - March 2023 87
Lymphatic examination
Images available from article: Goss JA, Greene AK. Sensitivity and Specificity of the Stemmer Sign for Lymphedema: A Clinical Lymphoscintigraphic Study. Plast Reconstr Surg Glob Open. 2019 Jun 25;7(6):e2295. 2a 2b 2c
Figure 3:
Images available from article: Bjork, R., Hettrick, H. Lymphedema: New Concepts in Diagnosis and Treatment. Curr Derm Rep 8, 190–198 (2019). 3a 3b 3c © Copyright. Wound Masterclass. 2023
Management of Lymphedema
■ Although there is currently no cure for lymphedema, it is a manageable disease by implementing the components of Complete Decongestive Physiotherapy (CDPT)
■ The objective of CDPT is to achieve a reduction in limb volume and improve the integrity and quality of the skin. This is attained through a two-phase approach:
■ Phase I is the intensive or decongestion phase that is clinician-driven, and Phase II is the maintenance phase that is patient-driven
■ The intensive phase is best performed daily (or as frequently as possible) until maximal volume reduction is achieved. Once the lymphedematous limb has plateaued and is no longer achieving a reduction in volume, the patient is transitioned into the maintenance phase, which is continued for life
■ The components of CDPT are implemented and facilitated by a trained lymphedema specialist during the intensive phase and include:
■ Meticulous skin and nail care to the affected areas
■ Manual lymphatic drainage (MLD) which is a gentle manual technique that redirects lymph flow
■ Multilayer, short-stretch compression bandaging during active decongestion and over-the-counter or custom garments once the limb has decongested
■ Therapeutic exercise to enhance and promote lymphatic pumping
■ During the intensive phase, patient education is paramount as all aspects of CDPT continue as part of the maintenance phase directed by the patient or a caregiver for lifelong lymphedema management. The major difference between the two phases of CDPT is the form of compression utilized
■ Once the limb volume is stable, compression garments are worn during the day followed by short-stretch bandaging or an alternative compression system at night
■ Alternative systems utilize Velcro closure mechanisms that assist the patient with donning and doffing often improving adherence with use. Compression systems are available in both elastic or inelastic material
■ Multiple over the counter medications tout a venotonic or lymphotonic effect. Quality peer reviewed publications are limited regarding many components present in over the counter medications
■ Flavonoids are a phenolic plant derived compound found in over 8000 different forms with multiple biological properties and among the most studied medications for management of lymphedema associated with chronic venous insufficiency21
■ The specific flavonoids, hesperidin and diosmin, were evaluated in a 2017 meta-analysis, identifying moderate quality evidence demonstrating benefit for management of venous ulcerations and edema (phlebolymphedema)22
■ Diosmin, as a component of a hybrid medication, was found to improve outcomes when used in conjunction with CDPT as compared to CDPT alone23
■ Micronized purified flavonoid fraction (MPFF) has been shown to decrease the inflammatory cascade and leukocyteendothelial activation thereby discouraging edema formation24
■ MPFF consists of 90% diosmin and 10% hesperidin and supplementary flavonoids and comes from extracts of rutaceaeaurantiae, a type of small orange that is micronized for improved bioavailability24,25
■ MPFF has not been associated with known major side effects while simultaneously showing significant improvement in quality of life in patients with chronic venous insufficiency and accelerated healing of leg ulcers24,26-28
■ Similarly, selenium has been noted to have a positive impact on lymphedema symptoms in a recent literature review29
■ Often considered for patients with lymphedema undergoing long-distance travel or flight is the use of pinus pinaster bark extract (PBE) which goes by the trade name, Pycnogenol. PBE possesses vasorelaxant activity and enhances microcirculation through increasing capillary permeability
■ PBE has been shown to be effective in improving leg heaviness, subcutaneous edema, and venous pressure in patients with chronic venous insufficiency30
Lymphedema in Clinical Practice Masterclass
88 Wound Masterclass - Vol 2 - March 2023
GUIDES
© Copyright. Wound Masterclass. 2023
Complete Decongestive Physiotherapy (CDPT) Pharmacotherapy
Lymphedema in Clinical Practice Masterclass GUIDES
Key Points
■ Current medical and clinical education regarding the anatomical and pathophysiological state of lymphedema have far lagged behind the rapidly advancing field of lymphatic medicine. Consequently, the accurate and evidenced-based approach to lymphedema assessment and management are not unanimously and universally employed

■ As patients with lymphedema often present in a myriad of different clinical settings, it is important to deploy accurate clinical assessment and readily offer best practice management options
■ These management options should include meticulous skin and nail care, manual lymphatic drainage, compression, therapeutic exercise, and review of pharmacologic options


■ The use of IPC devices as adjuncts to traditional therapy can be used to decrease lymphedema related complications and improve patient quality of life. In addition, IPC devices have the ability to be used in regions where limited lymphedema therapists or specialists exist thereby continuing to provide patients with lymphatic stimulation
■ The goal of MLD or IPC devices is to stimulate the underlying lymphatic system to redistribute stagnant lymph as outlined by the revised starling curve
■ By redistributing stagnant lymph back into circulation, the not uncommon lymphedema sequelae of recurrent infection, ulceration, deformity, and dermal/ epidermal skin changes can be minimized
124 (3): 915–21. https://doi.org/10.1172/JCI71608.

4. Ghanta, Swapna, Daniel A. Cuzzone, Jeremy S. Torrisi, Nicholas J. Albano, Walter J. Joseph, Ira L. Savetsky, Jason C. Gardenier, David Chang, Jamie C. Zampell, and Babak J. Mehrara. 2015. “Regulation of Inflammation and Fibrosis by Macrophages in Lymphedema.” American Journal of Physiology. Heart and Circulatory Physiology 308 (9): H1065-1077. https://doi.org/10.1152/ajpheart.00598.2014.

5. Reitsma, Sietze, Dick W. Slaaf, Hans Vink, Marc A. M. J. van Zandvoort, and Mirjam G. A. oudeEgbrink. 2007. “The Endothelial Glycocalyx: Composition, Functions, and Visualization.” PflugersArchiv: European Journal of Physiology 454 (3): 345–59. https://doi.org/10.1007/s00424-007-0212-8.
6. Zolla V, Nizamutdinova IT, Scharf B, Clement CC, Maejima D, Akl T, Nagai T, Luciani P, Leroux JC, Halin C, Stukes S, Tiwari S, Casadevall A, Jacobs WR Jr, Entenberg D, Zawieja DC, Condeelis J, Fooksman DR, Gashev AA, Santambrogio L. Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell. 2015 Aug;14(4):582-94. doi: 10.1111/acel.12330. Epub 2015 May 15. PMID: 25982749; PMCID: PMC4531072.
7. Bjork, R., Hettrick, H. 2018. “Endothelial glycocalyx layer and interdependence of lymphatic and integumentary systems.” Wounds International. Vol 9.

8. Trayes KP, Studdiford JS, Pickle
Qureshi OS, et al. Role of Flavonoids as Wound Healing Agent. Phytochemicals Source of Antioxidants and Role in Disease Prevention [Internet]. 2018 Nov 7; Available from: http://dx.doi.org/10.5772/intechopen.79179
22. Bush, Ronald, Anthony Comerota, Mark Meissner, Joseph D. Raffetto, Steven R. Hahn, and Katherine Freeman. 2017. “Recommendations for the Medical Management of Chronic Venous Disease: The Role of Micronized Purified Flavanoid Fraction (MPFF).” Phlebology 32 (1_suppl): 3–19. https://doi.org/10.1177/0268355517692221.
23. Cacchio, Angelo, Rosa Prencipe, Marina Bertone, Luciana De Benedictis, Luciano Taglieri, Erika D’Elia, CesidiaCentoletti, and Giancarlo Di Carlo. 2019. “Effectiveness and Safety of a Product Containing Diosmin, Coumarin, and Arbutin (Linfadren®) in Addition to Complex Decongestive Therapy on Management of Breast Cancer-Related Lymphedema.” Supportive Care in Cancer 27 (4): 1471–80. https://doi.org/10.1007/s00520-018-4514-5.
24. Katsenis K. Micronized purified flavonoid fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. CurrVascPharmacol. 2005;3(1):1–9. doi:10.2174/1570161052773870
25. Nair B. Venous leg ulcer: systemic therapy. Indian Dermatol Online J. 2014;5(3):374–377. doi:10.4103/2229-5178.137821
26. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J VascEndovascSurg. 2005;30(2):198–208. doi:10.1016/j.ejvs.2005.04.017


27. Danielsson G, Jungbeck C, Peterson K, Norgren L. A randomized controlled trial of micronized purified flavonoid fraction vs placebo in patients with chronic venous disease. Eur J VascEndovasc Surg. 2002;23(1):73–76. doi:10.1053/ejvs.2001.1531
28. Scallon C, Bell-Syer SE, Aziz Z. Flavonoids for treating venous leg ulcers. Cochrane Database Syst Rev. 2013;(5):CD006477.

Wound Masterclass - Vol 2 - March 2023 89
References 1. Maclellan RA, Couto RA, Sullivan JE, Grant FD, Slavin SA, Greene AK. Management of Primary and Secondary Lymphedema: Analysis of 225 Referrals to a Center. Ann Plast Surg. 2015 Aug;75(2):197-200. doi: 10.1097/SAP.0000000000000022. PMID: 24691335. 2. Wang W, Keast D. Prevalence and characteristics of lymphedema at a wound-care clinic. Wound Care. 2016;25(4):S11-S15. 3. Mortimer, Peter S., and Stanley G. Rockson. 2014. “New Developments in Clinical Aspects of Lymphatic Disease.” The Journal of Clinical Investigation
S, Tully AS. Edema: diagnosis and management. Am Fam Physician. 2013 Jul 15;88(2):102-10. PMID: 23939641. 9. Executive Committee. 2016. “The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology.” Lymphology 49 (4): 170–84. 10. Suami, H., & Scaglioni, M. F. (2018). Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Seminars in plastic surgery, 32(1), 5–11. https://doi.org/10.1055/s-0038-1635118 11. Sisto K, Khachemoune A. Elephantiasis nostrasverrucosa: a review. Am Clin Dermatol 2008;9(3):141-6. 12. O’Donnell, Thomas F Jr et al. “New diagnostic modalities in the evaluation of lymphedema.” Journal of vascular surgery. Venous and lymphatic disorders vol. 5,2 (2017): 261-273. doi:10.1016/j.jvsv.2016.10.083 13. Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, Miller LT, Fox KR, DeMichele A, Solin LJ. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010 Nov;19(11):2734-46. doi: 10.1158/1055-9965.EPI-09-1245. Epub 2010 Oct 26. PMID: 20978176; PMCID: PMC2976830. 14. Miseré RML, Wolfs JAGN, Lobbes MBI, van der Hulst RRWJ, Qiu SS. A systematic review of magnetic resonance lymphography for the evaluation of peripheral lymphedema. J Vasc Surg Venous Lymphat Disord. 2020 Sep;8(5):882-892.e2. doi: 10.1016/j.jvsv.2020.03.007. Epub 2020 May 13. PMID: 32417145. 15. Rockson, S.G. (2007). bioimpedance analysis in the assessment of lymphoedema diagnosis and management. 16. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg. 2007 Oct;59(4):464-72. doi: 10.1097/01.sap.0000257149.42922.7e. PMID: 17901744. 17. Goss, Jeremy A. MD; Greene, Arin K. MD, MMSc Sensitivity and Specificity of the Stemmer Sign for Lymphedema: A Clinical Lymphoscintigraphic Study, Plastic and Reconstructive Surgery - Global Open: June 2019 - Volume 7 - Issue 6 - p e2295 doi: 10.1097/GOX.0000000000002295 18. Bjork, R., Hettrick, H. 2018. “Endothelial glycocalyx layer and interdependence of lymphatic and integumentary systems.” Wounds International. Vol 9. 19. Dean SM, Starr J. An unusual case of familial lymphedema. Ann Vasc Surg. 2014 Jul;28(5):1314.e1-3. doi: 10.1016/j.avsg.2013.09.009. Epub 2013 Dec 11. PMID: 24333525. 20. Warren Peled A, Kappos EA. Lipedema: diagnostic and management challenges. Int Womens Health. 2016;8:389-395. Published 2016 Aug 11. doi:10.2147/IJWH.S106227 21. Aslam MS, Ahmad MS, Riaz H, Raza SA, Hussain S,
doi:10.1002/14651858.CD006477.pub2 29. Forte, Antonio J., Daniel Boczar, Maria T. Huayllani, Xiaona Lu, Sarah A. McLaughlin, Forte A. J, Boczar D, Huayllani M. T, Lu X, and Mclaughlin S. A. 2019. “Pharmacotherapy Agents in Lymphedema Treatment: A Systematic Review.” Cureus Journal of Medical Science 11 (12). https://doi.org/10.7759/cureus.6300. 30. Iravani S, Zolfaghari B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res Pharm Sci. 2011;6(1):1-11 Bjork R, Hettrick H (2019) Introducing the Leg Lymphedema Complexity Score. The Journal of Lymphoedema 15(1): 8–13 Masterclass Guide: Lymphedema in Clinical Practice. Wound Masterclass. Volume 2. No 4. March 2023. Useful Links How to Cite this Article Authors Dr Tristan Pennella Department of Family Medicine, Mayo Clinic Health System Mankato MN, United States Dr Karen Andrews Department of Physical Medicine and Rehabilitation, Mayo Clinic Rochester MN, United States Dr Wei Chen Professor and Attending Surgeon of Plastic Surgery, Cleveland Clinic Cleveland OH, United States Dr Heather Barnhart Nova Southeastern University, Department of Physical Therapy Fort Lauderdale FL, United States Dr Joseph Raffetto Vascular Surgeon Boston MA, United States Dr Daniel Miller Department of Dermatology, University of Minnesota Minneapolis MN, United States Dr Michael Shao Vascular Surgeon and Chief, Section of Vascular Surgery, Swedish Hospital, Chicago, Illinois Chicago IL, United States Dr M. Mark Melin M Health Fairview, Medical Director Wound Healing Institute Minneapolis MN, United States © Copyright. Wound Masterclass. 2023 Visit the Wound Masterclass website











woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register Join global wound care experts to answer all your questions about Surgical Site Infection (SSI) Concise. Interactive. Accredited. Supported by MasterSeries 60 Minutes Interactive December 13th 2022: 1pm EST | 6pm GMT woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register MasterSeries 60 Minutes Interactive Better wound care. Better content. Better clinical articles. Get accredited. December 13th 2022: 1pm EST | 6pm GMT Supported by All your questions answered: Surgical Site Infection (SSI) Moderator Miss Negin Shamsian Consultant Plastic & Reconstructive Surgeon (Locum) London, United Kingdom Global expert Dr Michael Magro Consultant Obstetrician & Gynaecologist London, United Kingdom Global expert Dr M. Mark Melin Medical Director of the M Health Wound Healing Institute Mineapolis MN, United States Global expert Dr Hüseyin Kemal Raşa Surgical Infections Society, European President Kocaeli, Turkey Global expert Dr Windy Cole Director of Wound Care Research, Kent State University of Podiatric Medicine Streetsboro OH, United States Global expert Dr Jonathan Johnson Surgical Director, Comprehensive Wound Care Services Washington DC, United States



If you think... ...have a look at the VACOcast Diabetic Boot Contact us at sales@opedmedical.com or call (800) 334-1906. . ● to the innovative Vacuum Technology*1-5 ● Fast, safe & easy to apply ● Non-removable, but removable for dressings ● as TCC*2-4 *1 Biomechanics Study by BASiS Biomechanics Institute at Munich University J. Mitternacht et.al 08/09 1994 *2 *3 Evaluating a removable knee high cast walker within the diabetic foot pathway G Bowen P Spruce 2019 Diabetic Foot Journal 22(3): 52–9 *4 *5 Vacuum cushioned removable cast walkers reducing foot loading in patients with diabetes mellitus A Nagel D Rosenbaum; Gait & Posture Volume 30 Issue 1, July 2009, p11-15. opedmedical.com
London, United Kingdom
What is the Role of Point-Of-Care Fluorescence Imaging for Bacterial Load Identification in Diabetic Foot Ulcers?

Editorial Summary
We recently published 'Point-Of-Care Fluorescence Imaging Reveals Extent of Bacterial Load in Diabetic Foot Ulcers' in the International Wound Journal, which explores point-of-care fluorescence imaging using a prospective, single-blind, multi-center and cross-sectional clinical trial.1 In this editorial we discuss the relevance of this trial and the impact of new terminology: chronic inhibitory bacterial load (CIBL), and how it intends to help define the presence of bacteria at high loads that is distinct from infection, but may inhibit wound healing.


Introduction
Worldwide, there are almost half a billion people with diabetes. Of these, 1 in 3 will develop diabetic foot ulcer (DFU), which has an associated 1.89 increased mortality risk.1 The most important clinical complication is diabetic foot infection.2 In diabetic patients, 60% of foot ulcers will become infected during clinical management.3 Diabetic foot infections are associated with increased economic costs, in addition to significant morbidities. They are the most frequent diabetes related indication for hospital admission and the most common reason for lower extremity amputation in diabetic patients. Overall, long-term healing rates and outcomes in these patients are poor.2
In addition to infection, other local factors influencing the healing rate of ulcers are pressure at the wound site and adequate blood supply to the site. When treating ulcers, it is often difficult to establish whether the wound is infected or not. High bacterial loads are often found in ulcers that do not appear clinically remarkable.4 Therefore, another important factor to consider in determining the rate of ulcer healing is bacterial infiltration of the wound, with or without overt clinical infection. The presence of a high burden of bacteria at the site of a wound has previously been shown to disrupt wound healing. Left unaddressed, bacterial endotoxins released from Gram-negative bacteria e.g., Pseudomonas aeruginosa, attract immune cells that perpetuate the inflammatory response, stall the healing cascade and force the wound to remain chronically open.5,6 Polymicrobial biofilms, present in 68% - 100% of DFUs, similarly contribute to wound chronicity by stimulating inflammation.8,9 The degree of bacterial infiltration varies and is dependent upon bacterial virulence and species.4,7,8
Cambridge MA, United States
The presence of high numbers of bacteria and formation of biofilms at the site of DFUs perpetuates the chronic wound cycle and increases the risk of infection. Earlier and more accurate detection of bacteria, with appropriate management, could reduce the risk of associated morbidity.1
92 Wound Masterclass - Vol 2 - March 2023
Dr David G. Armstrong
Department of Surgery, Keck School of Medicine of University of Southern California
Los Angeles CA, United States
Dr Thomas E. Serena SerenaGroup® Research Foundation
Dr Michael E. Edmonds
Diabetic Foot Clinic, King’s College Hospital Foundation Trust
Chronic inhibitory bacterial load (CIBL) is defined as:
“The chronic presence of bacterial microorganisms in a wound or its surrounding tissue at loads which can damage tissues and be inhibitory to healing, as well as require clinical intervention, with or without the presence of clinical symptoms.”
Point-of-Care Fluorescence Imaging
There are few reliable diagnostic tools to directly detect the presence of bacteria in wounds. Of those available, most do not provide immediate results. Point-of-care fluorescence imaging (FL-imaging) of bacteria is a biotechnology well positioned to address this clinical concern.
Under current guidelines, sampling and microbial analysis of a wound is done only if clinical infection is suspected.2 Although this allows treatment with appropriate antibiotics, superficial sampling techniques are suboptimal and are impeded by the presence of bacterial biofilms.1 This also misses the opportunity to treat patients with subclinical infection, where high levels of bacteria are present at the wound site, delaying healing and increasing the risk of future clinical infection.
Point-of-care FL-imaging uses endogenous FL signals, produced by bacterial metabolites and virulence factors to identify bacterial loads in wounds. It is objective, sensitive and noninvasive. FL-imaging is able to identify most bacterial species at clinically relevant levels, considered to be >104 colony-forming unit per gram (CFU/g), and crucially can detect biofilmencased bacteria.1
Since many patients who do not have clinically overt infection do have high levels of bacteria present at the wound site, the term infection is not particularly helpful in characterizing the degree of bacterial infiltration. This value is clinically important, as the presence of high levels of bacteria may inhibit wound healing, even in the absence of infection. Therefore, a new terminology, chronic inhibitory bacterial load (CIBL), helps to make the distinction between infection and high levels of bacterial infiltration requiring intervention.
The FLAAG Clinical Trial
In summary, the FL-Imaging Assessment and Guidance (FLAAG) clinical trial was a prospective, single-blind, multi-center, and cross-sectional study. It included 350 adults (>18 years) presenting with wounds of unknown infection status.10 Patients were recruited from 14 outpatient wound care centres across the United States between May 2018 and April 2019. An independent third party (Ironstone Product Development, Toronto, ON, Canada) was used to control for bias and to ensure appropriate blinding. The study aim was to compare the performance of standard clinical signs and symptoms (CSS) assessment using International Wound Infection Institute (IWII) guidelines, to CSS in combination with FLimaging, to detect clinically relevant bacterial loads. The clinicians received relevant training prior to commencing the study. They reviewed the patient’s history and inspected the wounds for all signs and symptoms of covert, overt, and spreading infection, identified by the IWII 2016 guidelines.11 Each sign and symptom was recorded when detected, including delayed healing beyond expectations; this was identified if the wound area had not reduced by at least 30% during the prior 4 weeks of care.
Post-hoc analysis of the FLAAG was conducted. The recorded CSS was reviewed to identify wounds that fulfilled the International Working Group of the Diabetic Foot (IWGDF) criteria for infection. Immediately following clinical assessment, standard and FL images were taken using the FL-imaging device (MolecuLight i:X, Toronto, Canada). This advanced imaging technology creates a map of high bacterial loads in and around wounds, without the use of contrast agents.10,12 Clinicians participating in the trial underwent didactic and hands-on training on use of the device; they were also trained on image interpretation and were required to pass an image interpretation certification test with a score of >80%.
What is the Role of Point-Of-Care Fluorescence Imaging for Bacterial Load Identification in Diabetic Foot Ulcers? Wound Masterclass - Vol 2 - March 2023 93
Point of care testing (POCT) is defined as diagnostic testing that is performed at or near to the site of the patient with the result leading to a potential change in the care of that patient.
Red FL on images indicates the presence of endogenously produced porphyrins from most common wound pathogens, while cyan FL indicates pyoverdine virulence factors from Pseudomonas aeruginosa specifically, both at loads >104 CFU/g.13,14
Up to 3 punch biopsies were collected from each DFU after cleansing of the wound with saline and gauze. More than 1 biopsy was only taken if an area of interest was identified or red or cyan FL was detected outside the wound centre.
Quantitative culture was subsequently performed, ensuring optimal conditions for difficult-to-culture bacteria were maintained.10 Diluted biopsy samples were cultured on various agars in conditions to support both aerobic and anaerobic growth.10,15 To identify specific bacterial species, matrix assisted laser desorption ionisation-time of flight mass spectrometry (Bruker Daltonics) was used.
Statistical analysis of data calculating both sensitivity and specificity at various bacterial thresholds was performed using MedCalc© Version 19.1.5. This was calculated with 95% confidence intervals (exact Clopper-Pearson). Bacterial loads were compared between the wound centre and periwound biopsies using a 2-sided paired student t-test.1
Results
Data regarding participant characteristics was noted. Importantly, participants with skin tones from across the Fitzpatrick scale (I to VI) were included. Most had a DFU of >3 months duration.1
In this study, 138 DFUs were examined; the majority of these (131) had some bacterial presence. Most ulcers that had some bacterial presence were also found to have high bacterial loads. Of the 131 ulcers where bacteria were found, only 6.1% (8/131) had bacterial loads below 104 CFU/g, while 93.9% (123/131) had bacterial loads exceeding 104 CFU/g, and 83.2% (109/131) had bacterial loads exceeding 105 CFU/g.1 The average bacterial load of DFUs with confirmed bacterial presence was 1.44 x 108 CFU/g. The number of bacterial species varied between wounds, with some ulcers having as many as 8 different species. The most common species found was Staphylococcus
aureus (52.4%) and the average number of bacterial species per biopsy was 2.74.1
Although red and/ or cyan FL was detected in the majority of DFUs, as the bacterial load increased so did the proportion of DFUs with FL indicating bacterial loads. Red or cyan FL was identified in 92.3% of DFUs with the highest bacterial loads. In wounds where high volumes of bacteria were identified, IWGDF infection criteria were largely missing.
Five CSS were included in the present study, following those in the IWGDF criteria; these were swelling, erythema, warmth, and purulent discharge. In ulcers where there were very high levels of bacteria (>108 CFU/g), the most common IWGDF criteria detected were swelling (11.5%), erythema (15.4%), pain (11.5%), and local warmth (11.5%). Where bacterial levels were very low, only swelling was detected. Purulent discharge was consistently the least commonly observed. Of the clinically assessed signs and symptoms, none proved better than chance at predicting high bacterial loads of greater than 104 CFU/g.
Sensitivity did not improve at higher bacterial loads for 3 out of 5 IWGDF criteria. The only criteria which did show a high sensitivity at higher bacterial loads were pain and purulent discharge, however their prevalence was low. The low sensitivity and 1-specificity values indicate a poor predictive value of all 5 IWGDF criteria when assessing high bacterial load.1
What is the Role of Point-Of-Care Fluorescence Imaging for Bacterial Load Identification in Diabetic Foot Ulcers? 94 Wound Masterclass - Vol 2 - March 2023
Bacterial load (CFU/g) n CSS sensitivity (%) CSS + FL sensitivity (%) P-value 104–105 14 9.8 71.6 P < .0001 105–106 25 11.0 75.2 P < .0001 106–107 24 10.7 78.6 P < .0001 107–108 34 11.7 86.7 P < .0001 >108 26 11.1 92.6 P < .0001
Table 1: Sensitivity in detection of bacterial load using CSS according to the IWGDF criteria, compared to CSS with FL-imaging.
In the study, the most commonly observed clinical sign was delayed wound healing beyond expectations. Generally, as bacterial load increased, the time taken for the wound to heal also increased. In over 50% of cases (52%), DFUs with bacterial loads of >104 CFU/g were healing delayed. This increased to 64.7% of wounds with bacterial loads of 107 - 108 CFU/g. Most wounds demonstrating a delay in healing were FL positive (70.0% - 95.5%).1
Sensitivity of delayed healing beyond expectations for predicting high bacterial loads was high across all bacterial thresholds, but specificity was lower than IWGDF criteria.1
In this study, 84.2% of the DFUs that exhibited red or cyan bacterial FL had FL indicating bacteria outside of the wound bed; this was mostly confined to callused tissue in the periwound (2cm radius extending out from the wound edge).16 Red FL signals within this region usually appeared blush pink to yellow due to the bacteria being below the surface. Bright red FL signals observed were attributed to the presence of higher bacterial loads at or near the callus surface.
If an additional biopsy was taken, the number of species and bacterial load at the wound centre was compared to the biopsy from outside the wound bed. The mean bacterial load from regions of red or cyan FL in the periwound region was significantly higher than the mean bacterial load of biopsies collected from the wound centre, implying that higher bacterial loads are present at the edge of the wound.1
Chronic Inhibitory Bacterial Load
The findings of this study led to a proposal of new clinical terminology: chronic inhibitory bacterial load (CIBL). This term refers to the state of bacterial infiltration within a wound that resists healing, but the process is not linked to any known clinically perceptible sign or symptom.1 CIBL is a state that is both CSS independent and is characterized by an inhibition of normal healing processes.
The DFUs in this study experiencing delayed healing were likely to be FL-positive, regardless of the bacterial threshold. Most DFUs that were FL-positive had signals indicating bacteria in the peri-wound region (84.2%). In addition, of the clinically assessed signs and symptoms,
none were better than chance at predicting high bacterial loads.1 These findings suggest that a state exists where there are high bacterial loads, resulting in reduced wound healing, without any clinically apparent presentation. Introduction of this new terminology seems to help address this diagnostic gap.
Clinical Implications
In this study, CSS did not correlate with high bacterial loads. In fact, CSS were largely missing, and all 5 IWGDF criteria were infrequent. This suggests that clinical assessment alone is not a suitable method of detecting high bacterial loads, as there is frequently an absence of signs and symptoms even when there are many bacteria present. The term ‘CIBL’ illustrates this important pathogenic state. There is no connection between CIBL and any bacterial threshold, it merely describes the state in which a persistently elevated bacterial load results in pathology, including delayed healing. There is also no link to infection status of the wound.
CIBL may exist in a wound at any point beyond contamination, regardless of clinical markers of infection; it can therefore be addressed with bacteria-based wound care, which may include debridement and cleansing.1,11 This concept acknowledges the role of the biofilm in wound chronicity and is also suited to holistic wound infection prevention and management.
In clinical practice, CIBL can be utilized as an indicator for intervention prior to the onset of infection. Current clinical guidelines do not respond to asymptomatic but potentially pathogenic bacterial loads, despite acknowledging the value of their early and proactive management. This may be due to the inability to clinically detect them. Fl-imaging overcomes this diagnostic limitation, as it can visualize high bacterial loads across the entire wound and periwound.
Previous evidence has demonstrated high positive predictive values (PPV) of FL-imaging, and sensitivity greater than that of the CSS of infection. This study is consistent with previous findings, and has also demonstrated that while the sensitivity of CSS is not proportional to bacterial load, there is a proportional increase in sensitivity of FL-imaging.1 Furthermore, DFUs often present with macerated tissue. This allows futher bacterial colonization,
What is the Role of Point-Of-Care Fluorescence Imaging for Bacterial Load Identification in Diabetic Foot Ulcers? Wound Masterclass - Vol 2 - March 2023 95
What is the Role of Point-Of-Care Fluorescence Imaging for Bacterial Load Identification in Diabetic Foot Ulcers?
particularly of organisms like Pseudomonas aeruginosa and Staphylococcus aureus
FL-imaging provides critically important information on the distribution of CIBL. Imaging in this study demonstrated that 84.2% of DFUs contain elevated bacterial loads in the periwound region and in callus tissue. Clinically, this might indicate that aggressive wound hygiene strategies like sharp debridement may be warranted when CIBL is identified in the DFU periwound, given the depth to which bacteria can extend. In future, the periwound should be recognized as a region frequently colonized with subsurface bacterial loads.1
Strengths and Limitations
One of the main strengths of the study was its generalizability to the overall DFU population. Minimal participant exclusion criteria were enforced, leaving a heterogenous sample of patients. Recruitment was also wide, from numerous clinical sites, and the clinicians were from a range of wound specialties. The diagnostic accuracy of the FL-imaging was ensured by completing quantitative culture analysis to confirm bacterial loads.1
The major limitation of the study was the lack of experience of the clinicians with the technology, which may have influenced sensitivity data. There are also other systemic contributors which influence wound healing which were not considered here.
Conclusion
Current practice does not include a strategy for the treatment of high bacterial loads that are asymptomatic. This presents a clinical problem, as high bacterial presence at the wound site has been associated with ongoing wound chronicity and increased infection risk.
The lack of sufficient and reliable means of identifying the presence of high bacterial loads in asymptomatic patients has resulted in a gap in understanding of the associated clinical implications. FL-imaging provides a solution to this; by facilitating appropriate identification of bacterial burden, there is a potential to aid early intervention and monitor treatment effectiveness, during and after local wound treatments. There is also a possible means of more effectual antimicrobial stewardship, to limit antibiotic and antimicrobial dressing
prescriptions and to improve wound healing outcomes.
The prevalence and distribution of bacterial burden in DFUs has historically been underestimated, and its pathogenicity is unclear. There has been an absence of a clinical definition for such a finding in asymptomatic patients. It is anticipated that the definition of CIBL will spark a paradigm shift in DFU wound assessment and management, and it is hoped that a clearer clinical definition of bacterial burden in patients, where typical symptoms of infection are absent, will encourage earlier intervention along the bacterial infection continuum, thereby preventing sequelae of infection and supporting improved DFU outcomes.
References
1. Armstrong DG, Edmonds ME, Serena TE. Point-ofcare fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers. Int Wound J. 2023;20(2):554-66.
2. Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280.
3. Jia L, Parker CN, Parker TJ, Kinnear EM, Derhy PH, Alvarado AM, et al. Incidence and risk factors for developing infection in patients presenting with uninfected diabetic foot ulcers. PLoS One. 2017;12(5):e0177916.
4. Xu L, McLennan SV, Lo L, Natfaji A, Bolton T, Liu Y, et al. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care. 2007;30(2):378-80.
5. Rippon MG, Westgate S, Rogers AA. Implications of endotoxins in wound healing: a narrative review. J Wound Care. 2022;31(5):380-92.
6. Phillipson M, Kubes P. The Healing Power of Neutrophils. Trends Immunol. 2019;40(7):635-
47.
7. Lantis JC, 2nd, Marston WA, Farber A, Kirsner RS, Zhang Y, Lee TD, et al. The influence of patient and wound variables on healing of venous leg ulcers in a randomized controlled trial of growth-arrested allogeneic keratinocytes and fibroblasts. J Vasc Surg. 2013;58(2):433-9.
8. Goldberg SR, Diegelmann RF. What Makes Wounds Chronic. Surg Clin North Am. 2020;100(4):681-93.
9. Johani K, Malone M, Jensen S, Gosbell I, Dickson H, Hu H, et al. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. International Wound Journal. 2017;14(6):1160-9.
10. Le L, Baer M, Briggs P, Bullock N, Cole W, DiMarco D, et al. Diagnostic Accuracy of Pointof-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv Wound Care (New Rochelle). 2021;10(3):123-36.
11. Wounds International. International Consensus Update 2016 International Wound Infection Institute (IWII) Wound Infection in Clinical Practice: Principles of best practice. 2016 [cited June 22, 2022]. Available from: https://www.woundsinternational.com/resources/details/woundinfection-inclinical-practice-principles-of-bestpractice
12. Rennie MY, Dunham D, Lindvere-Teene L, Raizman R, Hill R, Linden R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics (Basel). 2019;9(1).
13. Raizman R, Little W, Smith AC. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics (Basel). 2021;11(2).
14. Jones LM, Dunham D, Rennie MY, Kirman J, Lopez AJ, Keim KC, et al. In vitro detection of porphyrin-producing wound bacteria with real-time fluorescence imaging. Future Microbiol. 2020;15:319-32.
15. Serena TE, Bowler PG, Schultz GS, D’Souza A, Rennie MY. Are Semi-Quantitative Clinical Cultures Inadequate? Comparison to Quantitative Analysis of 1053 Bacterial Isolates from 350 Wounds. Diagnostics (Basel). 2021;11(7).
16. Andersen CA, McLeod K, Steffan R. Diagnosis and treatment of the invasive extension of bacteria (cellulitis) from chronic wounds utilising point-of-care fluorescence imaging. International Wound Journal.
2022;19(5):996-1008.
96 Wound Masterclass - Vol 2 - March 2023
foam


H&R H 3 Redc T: + 44
When it comes to consistency in quality, care and savings look no further than Kliniderm foam silicone. A trusted choice whatever your supply route – savings of up to 47%*.
patients
hurting
*Drug Tariff prices correct from January 2022, based on 10 x 10cm dressing size.
silicone Heal your
without
your budgets www.kliniderm.co.uk
GUIDES
Introduction
This Masterclass Guide provides a concise overview of critical limb ischaemia.
Critical Limb Ischaemia
Keywords
■ Peripheral arterial disease (PAD)
■ Atherosclerosis
■ Critical limb ischaemia
What is Critical Limb Ischaemia?
■ Critical limb ischaemia occurs in 5-10% of patients with Peripheral Arterial Disease (PAD), a chronic condition, usually caused by atherosclerosis (Figure 1), that results in lower extremity arterial obstruction and poor circulation. Early stages of PAD are often asymptomatic. As the disease progresses, patients may develop intermittent claudication or chronic limb-threatening ischemia (CLTI)1,2
■ It is characterized by resting lower extremity pain, ulceration, gangrene, increased risk of cardiovascular events, amputation, and death1,2
■ Prevalence is high among elderly patients with PAD affecting between 1520% of individuals over the age of 70 years3
■ As lipids accumulate within vessel walls there is subsequent hardening causing a significant reduction in blood flow to target tissues4
■ The corresponding reduction in oxygenation at peripheral tissue can lead to claudication or resting pain5,6
■ CLTI carries a mortality rate of 20% within 6 months of diagnosis and 50% at 5 years5,6
Diagnosis
■ The diagnosis of CLTI involves clinical findings (such as ischemic rest pain) and objective findings including ankle-brachial pressures
■ (<50 mmHg with rest pain or <70 mmHg with foot ulcer or gangrene), toe systolic pressure (<30 mmHg with rest pain or <50 mmHg with foot ulcer or gangrene), and transcutaneous oxygen pressure (<30 mmHg with foot ulcer or gangrene) (Figure 2)7
Impact
■ The prevalence of CLTI is projected to grow in coming years as the trend in risk factors such as age, diabetes, smoking, and the unknown effects of vaping product use increases
■ The poor clinical prognosis associated with CLTI leads to significant reduction in quality of life (QOL) due to ischemic resting pain, non-healing ulcers, and recurrent infection8
■ Chronic limb ischemia not only affects the patient, but also impacts caregivers who must handle the patient’s lack of mobility, pain control needs, wound care, and other issues
■ Economically, the annual cost of new Medicare patients with CLTI alone is $12 billion9
■ This healthcare cost is largely attributed to high inpatient expenditure, need for complex wound care and interventions, prolonged hospitalizations, and high readmission rates
■ Vascular disease
■ Ankle brachial pressure index (ABPI)
■ Diabetes
Presence of chronic ischemic rest pain plus Ankle pressure <50 mmHg or Toe pressure <30 mmHg
Presence of foot ulcers or gangrene plus Ankle pressure <70 mmHg
Toe systolic pressure <50 mmHg or TcPO2 <30 mmHg
98 Wound Masterclass - Vol 2 - March 2023
Masterclass
Figure 2:
© Copyright. Wound Masterclass. 2023
Figure 1: Atherosclerosis.
Critical Limb Ischaemia
Management and Treatment of Critical Limb Ischaemia
■ Due to the complex poly-vasculopathy associated with PAD, a holistic approach to management is needed7
■ Management with early-stage PAD or advanced stage CLTI should focus on pain management, promotion of wound healing, reduction of cardiovascular risk factors, and optimization of glycemic control
■ Pain management is fundamental to improve quality of life
■ Common modalities for treating pain include peripheral revascularization; however, for those unable to undergo revascularization acetaminophen and nonsteroidal antiinflammatory drugs are often first line
■ Scheduled dosing of analgesics is superior to an as-needed regimen for sustained relief
■ Some pain associated with PAD may be neuropathic, prompting a careful differential in evaluating pain as the use of neuropathic pain agents may provide the best control
■ Cardiovascular risk factors should be managed with a multifaceted approach
■ Smoking cessation should begin early and routinely addressed as it has been shown to reduce mortality and improve amputation-free survival10
■ Uncontrolled dyslipidemia contributes to the development and progression of PAD and can be treated with statin therapy to reduce cardiovascular events11
■ Hypertension should be controlled according to guidelines
■ Hyperglycemia is a risk factor of PAD and attention to glycemic control is required. Glycemic control involves both medication management and subsequent monitoring of HbA1c, which has been shown to be a predictor of major amputation12,13
■ Antiplatelet therapy with aspirin or clopidogrel is indicated for secondary prevention in patients with PAD
GUIDES
■ Use of intermittent pneumatic compression (IPC) devices have become an alternative treatment for patients unable to undergo angioplasty, open reconstruction, or without reconstruction options
■ IPC devices utilize sequential inflation and deflation compression pressure targeted to the affected limb
■ Mechanistic evaluation of IPC devices has revealed increased distal perfusion through increased popliteal, gastrocnemial, collateral arterial, and skin blood flow in patients with CLTI14
■ Evaluation with Doppler ultrasound identifies similar increases in both cutaneous and arterial perfusion following IPC use15-18
■ It has been postulated that IPC devices enhance blood flow through increasing the arteriovenous pressure gradient14
■ As blood is forced proximally out of the dependent foot and calf there is a reduction in the venous pressure19
■ Reduced venous pressure increases the arteriovenous pressure gradient augmenting arterial inflow20
■ The integrity of the arteriovenous pressure gradient is an important component of local vasoregulation via the venoarteriolar reflex (VAR) and has been shown to be impaired in patients with PAD. The VAR is an adaptive mechanism that creates venous distention, precapillary vasoconstriction, and subsequent decrease in capillary uptake in the sitting position21,22
■ As blood is moved proximally out of the dependent leg there is a decrease in venous pressure that allows precapillary sphincters to dilate again
■ Use of IPC devices has been found to temporarily suspend the VAR by increasing blood flow and temporarily abolishing postural vasoconstriction20
■ To summarize, proposed mechanisms for the effects of IPC include increased arteriovenous pressure gradient from release of nitric oxide casued by wall shear stress of the rapid cuff inflations, stimulation of endothelial vasodilation, suspension of the VAR, and stimulation of collateral artery arteriogenesis14,20,23
■ IPC devices are available as foot only, calf only, foot and calf, or foot, calf, and thigh, utilizing high pressure compression in the range of 80 - 140 mmHg with variable cycling each minute; they are typically prescribed to be used for 3 - 6 hours if CLTI has resulted in wounds, or rest pain is present24
■ A recent systematic review of available IPC devices found improvements in limb salvage, major adverse cardiovascular events, pain relief, quality of life, wound healing, and hemodynamics, varying by protocol and device used24
Wound Masterclass - Vol 2 - March 2023 99
Masterclass
Holistic approach
© Copyright. Wound Masterclass. 2023
Intermittent Pneumatic Compression and Venoarteriolar Reflex
GUIDES
Stimulation of the lymphatics and production of Nitric Oxide (NO) hypothesis
■ Underrecognized in the context of PAD and the use of arterial stimulation by IPC are the effects seen within the lymphatic system. Similar to the arterial endothelium, the lymphatics produce Nitric Oxide (NO) during contraction as a result of shear flow25
■ The lymphatic bulb-valve regions appear to contain the largest density of eNOS, producing approximately a 30 - 50% higher NO concentration compared to the tubular regions25
■ Under environments of increased fluid shear across the lymphatic endothelium there is increased production of NO which causes suppression of lymphatic contraction and pumping (Figure 3)26-29
■ However, the role of NO within the lymphatic system during periods of high lymphatic flow can be thought of as a 'brake' that decreases contractility while simultaneously increasing the diastolic relaxation period, thereby allowing adequate lymphatic refilling and stroke volume30,31
■ This paradoxical effect of NO on lymphatic contractility does not account for increased fluid movement unless the importance of diastolic relaxation is appreciated. IPC use in patients with PAD seems to result in improved fluid management and redistribution from the tissue beds
■ It is hypothesized that stimulation of the lymphatics and production of NO via increased shear force results in increased diastolic relaxation and subsequent filling within the lymphatics and therefore allows for a delayed, albeit increased stroke volume of lymph fluid increasing the propensity of immune cell recognition and activation and increased immune surveillance
■ Beyond increased immune surveillance, NO is also known to exert innate broad-spectrum antimicrobial activity through multiple mechanisms described in the literature without any observation of antimicrobial resistance32-35
■ This is an important area of continued study as systemic production of NO via IPC use or supplementation with L-arginine as a precursor to NO production may be of benefit from not just an arterial point of view, but lymphatic as well
Critical Limb Ischaemia
Case study
■ To further illustrate this notion, a case-example is given of a patient presenting to the wound clinic after smoking cessation 3 years prior for progressive wounds. Additional pertinent information includes recent angiogram and stent placement, tibial run off, and an ABI 0.65.The patient was evaluated and brought to the operative suite for wound debridement using ultrasonic debridement (Misonix, Farmingdale NY, United States) with a polyhexamethylene biguanide solution. Initial consultation and post-operative debridement is shown in Figure 4 and Figure 5, respectively
■ Following debridement, the patient was prescribed moist-to-moist hypochlorous acid (Vashe®, Urgo Medical North America, Fort Worth, TX), changes 3 times daily while in the hospital in addition to a robust micronutrient regimen including: 1 mg folate daily, 1 mg vitamin B12 daily, 10,000 IU vitamin D daily, 1 tablet MPFF twice daily, and 2,000 IU vitamin C Daily. She was prescribed IPC (ArtAssist® AA-1000, ACI Medical, San Marcos, California, United States) use dosing for a total of 6 hours daily with her leg in a gravity dependent position. Initially, the patient could not tolerate compression on the affected right side due to pain and was only applied to her unaffected contralateral limb. After approximately 3 weeks, granulation tissue on the affected right leg improved with decreased pain, allowing bilateral IPC compression use
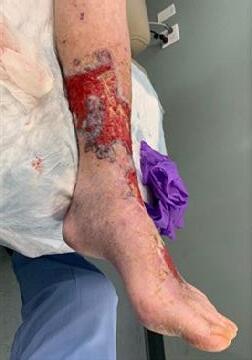

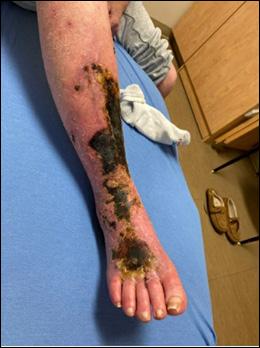

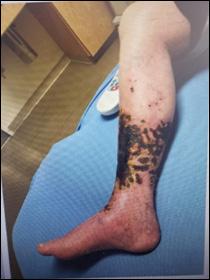
■ The goal of using the IPC device on the unaffected limb was to establish a systemic increase in NO production for possible benefit in the wound dependent limb until IPC could be tolerated. Direct manual-lymph drainage (MLD) was also utilized like dermal stimulation with fuzzy-whale 8mm longitudinal compression (Edemawear®, Quart Medical, Kitchener, Ontario, Canada). Edemawear® works through dermal microdeformation, similar to the effects seen in negative pressure wound therapy (NPWT) that has been well documented to support wound healing36,37
■ Microdeformation in the totality of the wound bed results in a pulling shear force stimulating glycocalyx (GCX) restoration and biofluid movement. This concept was validated in a recent murine model study, but also highlighted the stimulation to endothelial GCX regeneration is vitally linked to the lymphatic regeneration capabilities which resonates with the improve fluid mobility seen in the patient.38 IPC use in patients with CLTI also stimulates the lymphatics, which would teleologically be appropriate given the decreased rates of cellulitis, improved angiogenesis, and dermal regeneration seen, all of which relate to lymphatic system functionality
■ This regimen provides a well-tolerated form of compression for patients with ABIs of 0.4 and above. As the 8mm compression is not constrictive, it is well tolerated by patients with pain. Functionally, there are benefits from the effects of stimulating dermal lymphatic functionality, decreasing interstitial edema in cases of extreme CLTI, and therefore increasing microvascular perfusion at the 5-micron level with resolution of underlying interstitial edema
Masterclass
Figure 4: Initial consultation right lower extremity CLTI. Figure 5: Post-operative debridement right lower extremity CLTI.
Image available from article: Weinbaum, S., Cancel, L.M., Fu, B.M. et al. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc Eng Tech 12, 37–71 (2021). © Copyright. Wound Masterclass. 2023
Figure 3: Shear-induced Nitric Oxide Production via Heparan Sulfate and Glypican-1 Mechanotransduction and PECAM1- Phosphorylation leading to eNOS activation and NO synthesis.
Critical Limb Ischaemia
Discussion
■ There is a significant proportion of patients with PAD or CLTI who are not candidates for conventional revascularization procedures. In these patients there is clear benefit from the use of IPC devices, including decreased rates of amputation, improved wound healing, increased QOL, and reduced pain17,24,39






■ Although causality study designs are lacking, numerous proposed mechanisms for the microvascular changes associated with PAD/ CLTI have been published
■ The potential effects IPC devices may have on these microvascular changes have unfortunately not been established to date. The improved oscillatory micro shear and subsequent re-establishment of GCX intracellular signal transduction with continued IPC use may play a role in the regeneration of healthy vascular endothelium
■ Pre- and post-treatment biopsy evaluation following IPC use may allow for the objective and anatomical understanding of the microvascular effects seen with IPC use in PAD/CLTI


■ The importance of continued investigation into the systemic effects of endothelial cell dysfunction remains prudent to advancing arterial, venous, and lymphatic disease

Masterclass
Wound Masterclass - Vol 2 - March 2023 101
GUIDES
1. Elkady R, Tawfick W, Hynes N, Kavanagh EP, Jordan F, Sultan S. Intermittent pneumatic compression for critical limb ischaemia. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No.: CD013072. DOI: 10.1002/14651858.CD013072. 2. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Vasc Surg. 2007 Jan; 45 SupplS5-67. 3. Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler Thromb Vasc Biol. 2020 Aug;40(8):1808-1817. doi: 10.1161/ATVBAHA.120.314595. Epub 2020 Jun 25. PMID: 32580632; PMCID: PMC7377955. 4. Peach G, Griffin M, Jones KG, Thompson MM, Hinchliffe RJ. Diagnosis and management of peripheral arterial disease. British Medical Journal 2012;345:e5208. 5. Adam DJ, Beard JD, Cleveland T, et al; BASIL trial participants. Bypassversus angioplasty in severe ischaemia of the leg (BASIL): multicentre,randomised controlled trial. Lancet. 2005;366(9501):1925–1934. 6. Stoyioglou A, Jaff MR. Medical treatment of peripheral arterial disease: a comprehensive review. Vasc Interv Radiol. 2004;15(11):1197–1207. 7. Uccioli L, Meloni M, Izzo V, Giurato L, Merolla S, Gandini R. Critical limb ischemia: current challenges and future prospects. Vascular Health and Risk Management. 2018;14:63-74 8. Monaro S, West S, Gullick J. An integrative review of health-related quality of life in patients with critical limb ischaemia. Clin Nurs. 2017 Oct;26(19-20):2826-2844. doi: 10.1111/jocn.13623. Epub 2017 Mar 20. PMID: 27808440. 9. Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE, etal. De-terminantsoflong-termoutcomesandcostsinthemanagementofcrit-icallimbischemia:apopulation-basedcohortstudy.JAmHeartAssoc2018;7(16):e009724. 10. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. Vasc Surg. 2014;60(6):1565–1571. 11. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans affairs high-density lipoprotein cholesterol intervention trial study group. N Engl Med. 1999;341(6):410–418. 12. Giurato L, Vainieri E, Meloni M, et al. Limb salvage in patients with diabetes is not a temporary solution but a life-changing procedure. Diabetes Care. 2015;38(10):e156–e157. 13. Uccioli L, Gandini R, Giurato L, et al. Long-term outcomes of diabetic patients with critical limb ischemia followed in a tertiary referral diabetic foot clinic. Diabetes Care. 2010;33(5):977–982. 14. Labropoulos N, Leon LR Jr, Bhatti A, Melton S, Kang SS, Mansour AM, Borge M. Hemodynamic effects of intermittent pneumatic compression in patients with critical limb ischemia. Vasc Surg. 2005 Oct;42(4):710-6. doi: 10.1016/j.jvs.2005.05.051. PMID: 16242559. 15. Eze A.R., Comerota A.J., Cisek P.L., Holland B.S., Kerr R.P., Veeramasuneni R., Comerota Jr, A.J. Intermittent calf and foot compression increases lower extremity blood flow.Am Surg. 1996; 172 (discussion 135): 130-134 16. Kavros S.J., Delis K.T., Turner N.S., Voll A.E., Liedl D.A., Gloviczki P., Rooke T.W.Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up.J Vasc Surg. 2008; 47: 543-549 17. van Bemmelen P.S., Mattos M.A., Faught W.E., Mansour M.A., Barkmeier L.D., Hodgson K.J., et al.Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression.J Vasc Surg. 1994; 19: 1052-1058 18. Abu-Own A., Cheatle T., Scurr J.H., Coleridge Smith P.D.Effects of intermittent pneumatic compression of the foot on the microcirculatory function in arterial disease.Eur J Vasc Surg. 1993; 7: 488-49 19. Labropoulos N., Cunningham J., Kang S.S., Mansour M.A., Baker W.H. Optimising the performance of intermittent pneumatic compression devices.Eur Vasc Endovasc Surg. 2000; 19: 593-597 20. Husmann M, Willenberg T, Keo HH, Spring S, Kalodiki E, Delis KT. Integrity of venoarteriolar reflex determines level of microvascular skin flow enhancement with intermittent pneumatic compression. Vasc Surg. 2008 Dec;48(6):1509-13. doi: 10.1016/j.jvs.2008.07.016. Epub 2008 Oct 1. PMID: 18829220. 21. Delis K.T., GiannouKas A.D., Intermittent pneumatic compression (IPC) in the treatment of peripheral arterial occlusive disease (PAOD).Eur Vasc Endovasc Surg. 2007; 33: 309-310 22. Henriksen O. Orthostatic changes of blood flow in subcutaneous tissue in patients with arterial insufficiency of the legs.Scand J Clin Lab Invest. 1974; 34: 103-109 23. Delis, KT. The case for intermittent pneumatic compression of the lower extremity as a novel treatment in arterial claudication. Perspect Vasc Surg Endovasc Ther 2005; 17: 29–42. 24. Moran PS, Teljeur C, Harrington P, Ryan M. A systematic review of intermittent pneumatic compression for critical limb ischaemia. Vasc Med. 2015 Feb;20(1):41-50. doi: 10.1177/1358863X14552096. Epub 2014 Sep 30. PMID: 25270409. 25. Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am Physiol Heart Circ Physiol. 2011;301(5):H1897-H1906. doi:10.1152/ajpheart.00260.2011 26. Ferguson MK, DeFilippi VJ. Nitric oxide and endothelium-dependent relaxation in tracheobronchial lymph vessels. Microvasc Res47: 308–317, 1994 27. Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. Physiol540: 1023–1037, 2002 28. Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol575: 821–832, 2006 29. Leak LV, Cadet JL, Griffin CP, Richardson K. Nitric oxide production by lymphatic endothelial cells in vitro. Biochem Biophys Res Commun217: 96–105, 1995 30. Granger HJ. Role of the interstitial matrix and lymphatic pump in regulation of transcapillary fluid balance. Microvasc Res18: 209–216, 1979 31. Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am Physiol Heart Circ Physiol264: H1283–H1291, 1993 32. Hetrick, E. M.; Shin, J. H.; Stasko, N. A.; Johnson, C. B.; Wespe, D. A.; Holmuhamedov, E.; Schoenfisch, M. H. BactericidalEfficacy of Nitric Oxide-Releasing Silica Nanoparticles. ACS Nano2008, 2, 235−246. 33. Fang, F. C. Mechanisms of Nitric Oxide-Related AntimicrobialActivity. J. Clin. Invest. 1997, 99, 2818−2825. 34. Yang, L.; Feura, E. S.; Ahonen, M. J. R.; Schoenfisch, M. H.Nitric Oxide−Releasing Macromolecular Scaffolds for AntibacterialApplications. Adv. Healthcare Mater. 2018, 7, 1800155. 35. Privett, B. J.; Broadnax, A. D.; Bauman, S. J.; Riccio, D. A.;Schoenfisch, M. H. Examination of Bacterial Resistance to ExogenousNitric Oxide. Nitric Oxide 2012, 26, 169−173. 36. Wiegand C, White R. Microdeformation in wound healing. Wound Repair Regen. 2013 Nov-Dec;21(6):793-9. doi: 10.1111/wrr.12111. Epub 2013 Oct 17. PMID: 24134318. 37. Ehmann S, Walker KJ, Bailey CM, DesJardins JD. Experimental Simulation Study to Assess Pressure Distribution of Different Compression Applications Applied Over an Innovative Primary Wound Dressing. Wounds. 2020 Dec;32(12):353-363. Epub 2020 Aug 17. PMID: 33370244. 38. Nelson TS, Nepiyushchikh Z, Hooks JST, et al. Lymphatic remodelling in response to lymphatic injury in the hind limbs of sheep. Nat Biomed Eng. 2020;4(6):649-661. doi:10.1038/s41551-019-0493-1 39. Sultan, S., Tawfick, W., Zaki, M., Mohamed, M., Elsherif, M., Sharkawy, M., Kavanagh, E., Hynes, N. Sequential Pneumatic Compression Biomechanical Home Therapy Device in the Management of Critical Lower Limb Ischemia for No-Option Patients. Vascular Disease Management. 2016;13(7):E147-E15513. 147-155. 40. Ait-Aissa, K., Nguyen, Q.M., Gabani, M. et al. MicroRNAs and obesity-induced endothelial dysfunction: key paradigms in molecular therapy. Cardiovasc Diabetol19, 136 (2020). https://doi.org/10.1186/s12933-020-01107-3 41. Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Techniques in Vascular and Interventional Radiology 2016;19(2):91-5. 42. Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. The burden of critical limb ischemia: a review of recent literature. Vasc Health Risk Manag. 2019;15:187-208. Published 2019 Jul 1. doi:10.2147/VHRM.S209241 43. Gangqi Wang, Sarantos Kostidis, Gesa L. Tiemeier, Wendy M.P.J. Sol, Margreet R. de Vries, Martin Giera, Peter Carmeliet, Bernard M. van den Berg, Ton J. Rabelink. 2020. Shear Stress Regulation of Endothelial Glycocalyx Structure Is Determined by Glucobiosynthesis. AHA Volume 40, Issue 2, February 2020;, Pages 350-364 https://doi.org/10.1161/ATVBAHA.119.313399 44. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). 45. McPhee JT, Nguyen LL, Ho KJ, Ozaki CK, Conte MS, Belkin M. Risk prediction of 30-day readmission after infrainguinal bypass for critical limb ischemia. Vasc Surg. 2013;57(6):1481-8 46. Menzel D, Haller H, Wilhelm M, Robenek H. L-Arginine and B vitamins improve endothelial function in subjects with mild to moderate blood pressure elevation. Eur Nutr. 2018;57(2):557-68. 47. Mitra, R., Qiao, J., Madhavan, S. et al. The comparative effects of high fat diet or disturbed blood flow on glycocalyx integrity and vascular inflammation. transl med commun3, 10 (2018). https://doi.org/10.1186/s41231-018-0029-9 48. Mitra R, O’Neil GL, Harding IC, Cheng MJ, Mensah SA, Ebong EE. Glycocalyx in Atherosclerosis-Relevant Endothelium Function and as a Therapeutic Target. Curr Atheroscler Rep. 2017;19(12):63. Published 2017 Nov 10. doi:10.1007/s11883-017-0691-9 49. Möckl L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front Cell Dev Biol. 2020;8:253. Published 2020 Apr 15. doi:10.3389/fcell.2020.00253 50. Moretto, P, Karousou, E, Viola, M, Caon, I, D’Angelo, ML, De Luca, G, Passi, A, Vigetti, D. Regulation of hyaluronan synthesis in vascular diseases and diabetes. Diabetes Res. 2015;2015:167283. doi: 10.1155/2015/167283 51. Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. Vasc Surg 2011;54(4):1021-31 52. Schmitz B, Niehues H, Lenders M, Thorwesten L, Klose A, Krüger M, Brand E, Brand SM. Effects of high-intensity interval training on microvascular glycocalyx and associated microRNAs. Am Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1538-H1551. doi: 10.1152/ajpheart.00751.2018. Epub 2019 Apr 12. PMID: 30978133. 53. Tarbell, J. M., and M. Y. Pahakis. 2006. “Mechanotransduction and the Glycocalyx.” Journal of Internal Medicine 259 (4): 339–50. https://doi.org/10.1111/j.1365-2796.2006.01620.x. 54. van den Berg, BM, Wang, G, Boels, MGS, Avramut, MC, Jansen, E, Sol, W, Lebrin, F, Jan van Zonneveld, A, de Koning, EJP, Vink, H, et al. Glomerular function and structural integrity depend on hyaluronan synthesis by glomerular endothelium. Am Soc Nephrol. 2019;30:1886–1897. doi: 10.1681/ASN.2019020192 55. Wilkes R, Zhao Y, Cunningham K, Kieswetter K, Haridas B. 3D strain measurement in soft tissue: demonstration of a novel inverse finite element model algorithm on MicroCT images of a tissue phantom exposed to negative pressure wound therapy. Mech Behav Biomed Mater. 2009 Jul;2(3):272-87. doi: 10.1016/j.jmbbm.2008.10.006. Epub 2008 Nov 5. PMID: 19627832. 56. Weinbaum, S., Cancel, L.M., Fu, B.M. et al. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc Eng Tech 12, 37–71 (2021). https://doi.org/10.1007/s13239-020-00485-9 Masterclass Guide: Critical Limb Ischaemia. Wound Masterclass. Volume 2. No 4. March 2023. How to Cite this Article Authors Dr Tristan Pennella Department of Family Medicine, Mayo Clinic Health System Mankato MN, United States Dr Charles Andersen Chief of Vascular/ Endovascular Surgery, United States Army Milton WA, United States Dr Thom Rooke Cardiologist, Professor of Medicine, the Mayo Clinic College of Medicine and Science Rochester MN, United States Dr Tony Comerota Medical Director Eastern Region at Inova Heart and Vascular Institute, Inova Alexandria Hospital Alexandria VA, United States Dr Paul Van Bemmelen Vascular Surgeon Philadelphia PA, United States Dr M. Mark Melin M Health Fairview, Medical Director Wound Healing Institute Minneapolis MN, United States Dr Eno Ebong Associate Professor at Northeastern University Boston MA, United States Dr Leonhard Möckl Max Plank Institute Erlangen, Germany © Copyright. Wound Masterclass. 2023 Visit the Wound Masterclass website
References
EWMA 2023
THE 33RD CONFERENCE OF THE EUROPEAN WOUND MANAGEMENT ASSOCIATION
WOUND CARE – FROM ART TO SCIENCE

DALL’ARTE ALLA SCIENZA: L’EVOLUZIONE DELLA CURA DELLE FERITE
OTHER COLLABORATORS:
MILAN, ITALY 3
5 MAY 2023
–
How
Global Wound Care Leaders
Including:
Prof Sebastian Probst | EWMA President; Professor of Tissue Viability and Wound Care at the School of Health Sciences, Geneva, Switzerland
Prof Amit Gefen | Professor of Biomedical Engineering, Tel Aviv University, Israel
Dr M. Mark Melin | Medical Director of the M Health Wound Healing Institute; Minnesota, USA
Dr Mitch Sanders | CSO and EVP Alira Health. CEO of WoundForce Inc. and Firefly Innovations LLC, Boston, USA
Dr Windy Cole | Director of Wound Care Research, Kent State University of Podiatric Medicine, Ohio
Ms Terry Swanson | Vice Chair, International Wound Infection Institute, Victoria, Australia
Ms Trish Idensohn | Wound Nurse Specialist, Consultant and Educator
Dr Honda Hsu | Plastic Surgeon and Associate Professor, Tzu Chi General Hospital
Mr Frank Aviles | Wound Care Clinical Coordinator, Natchitoches Regional Medical Center
Dr Aliza Lee | Clinical Research Investigator, Department of Veterans Affairs
woundmasterclass.com/Events woundmasterclass.com/Register
Improving Patient Outcomes: Evidence-Based Technology
Advances in Dressings
Wound Bed Prep and Debridement
Surgical Site Infection
Venous Leg Ulcer (VLU)
Diabetic Foot Ulcer (DFU)
Pressure Injuries
Lymphedema
Obstetric Wounds
Biofilm
Oxygen Therapy
Skin Substitutes
Three Dimensional Printing
Advanced Modalities

31st
Global Innovation in Wound Care Summit | May
2023
to Improve Patient Outcomes: Evidence-Based Technology

201-AD004 Rev.03 08/22 URGO Medical North America, LLC. 3801 Hulen Street, Suite 251, Fort Worth, TX 76107 To order, call 1-855-888-8273 or visit www.urgomedical.us © 2022 Urgo Medical North America, LLC. All rights reserved. Vashe, Urgo, and the Urgo logo are registered trademarks of Urgo Medical. *as a preservative within the solution References: 1. Sampson CM, Sampson MN. Hypochlorous acid: A safe and efficacious new wound therapy. Poster presented at: World Union of Wound Healing Societies; 2008; Toronto, Ontario, Canada. 2. Vashe Wound Solution data developed from USP 51 Antimicrobial testing. 3. Robson MC. Treating chronic wounds with hypochlorous acid disrupts biofilm. Today’s Wound Clinic. 2014;8(9). 4. Nagoba BS, Suryawanshi NM, Wadher B, et al. Acidic environment and would healing: A review. Wounds. 2015;27(1):5-11. Using Purehypochlorous acid (pHA) is recommended by science and consensus guidelines, including the NPIAP, IWII, and WHD/WRR. Vashe is the solution. Never Compromise. Pure Hypochlorous Acid pHA Vashe never compromises the safety of the patient or key cells required for healing.1 Vashe is backed by quality evidence for efficacy in the removal of planktonic & biofilm microbes.2-3 Vashe contains Pure hypochorlous acid (pHA)* , supporting an ideal trajectory for healing.4
Safety Efficacy
Ideal pH
MasterSeries 60 Minutes Interactive



Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro



Introduction

Wounds progress from hemostasis to inflammatory, proliferative, and remodelling phases, but wounds are generally considered hard-to-heal if they are stalled in the inflammation phase and have not entered the proliferation phase after 4 weeks of standard of care therapy.
Venous Leg Ulcers (VLUs) account for up to 80% of the 2.5 million leg ulcer cases per year, with an increased incidence in an ever-increasing elderly population; aside from the associated reduced quality of life, the costs involved with this are substantial, involving a high frequency of hospitalizations, wound clinic visits, home healthcare, decreased work capacity, and job loss or time lost from work; in the United States, these costs amount to nearly $15 billion annually.
MasterSeries: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro includes a panel of global experts including Dr Abigail Chaffin, Associate Professor of Surgery and Medical Director of the MedCentris Wound Healing Institute at Tulane University; editorial board



member Dr M. Mark Melin, Medical Director of the M Health Wound Healing Institute; Dr Lee Ruotsi, Medical Director at the Saratoga Hospital Centre for Wound Healing and Hyperbaric Medicine; Dr Peter Gloviczki, Editor-in-chief of the Journal of Vascular Surgery and Professor of Surgery (Emeritus) at the Mayo Clinic Rochester, Minnesota, and Dr Monika Gloviczki, scientist and artist at Vascular Science and Art.
Associate Professor of Surgery and Medical Director, MedCentris Wound Healing Institute at Tulane University New Orleans LA, United States








Reconstructive Considerations

In the 4 weeks of standard of care, there should be a 50% reduction in the wound size. If not, it may be necessary to signal for a change or additional therapies or advanced extracellular matrices (ECM). This is important, because generally wound area reduction after 4 weeks is predictive of complete wound healing by 24 weeks. Local and national guidelines should be followed, with incorporation of evidence-based modalities that lead to the highest quality outcomes with the most appropriate resource utilization in an integrated approach.
The goals are to alleviate pain, rapidly heal the ulcer

106 Wound Masterclass - Vol 2 - March 2023
Global expert Dr Abigail Chaffin
Moderator Miss Negin Shamsian Consultant Plastic and Reconstructive Surgeon (Locum) London, United Kingdom Supported by woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro Concise. Interactive. Accredited. Supported by MasterSeries 60 Minutes Interactive Held: January 24th 2023: 1pm EST | 6pm GMT woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register MasterSeries 60 Minutes Interactive Better wound care. Better content. Better clinical articles. Get accredited. January 24th: 1pm EST | 6pm GMT Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro Supported by Moderator Miss Negin Shamsian Global expert Dr Abigail Chaffin Global expert Co-chairperson Dr M. Mark Melin Mineapolis MN, United States Global expert Co-chairperson Dr
Global expert Dr
Global expert Dr
© Copyright. Wound Masterclass. 2023
Monika Gloviczki
Peter Gloviczki
Lee Ruotsi
and to prevent long term sequelae.
General treatment algorithms for VLUs include commencing with standard compression, leg elevation, and then a thorough wound bed preparation with a sharp excisional debridement, a high consideration for bacterial control with appropriate wound cleansers (it is possible to use a pure hypochlorous acid preserved wound cleanser); early use of ECMs when appropriate for coverage of vital structures, dermal regeneration and accelerating primary healing of small VLUs (preferred option would be to use an ovine forestomach matrix graft). Split Thickness Skin Grafts (STSG) should be considered for definitive coverage of larger VLUs.
Pure Hypochlorous Acid (pHA) Preserved Cleanser
This is a pure Hypochlorous Acid (pHA) preserved cleanser (Vashe®, Urgo Medical North America, Fort Worth, TX), manufactured at 300 ppm (parts per million); it has a pH range of 3.5 - 5.5, which is conducive to healing.2 It is not cytotoxic,3 unlike standard sodium hypochlorite solutions, which are considered toxic even at low concentrations. This product is consumed by organic matter in the wound and dissipates in seconds, so it is safe to use with ECMs in the same setting.4 For wounds that have microbial adherent aggregates, 5-8 minutes of wound contact with the solution should be considered,5 in combination with sharp debridement. This mechanical disruption of germs and associated necrotic debris is a rapid process, and does not require long exposure like other some other modalities may.
Some evidence and very recent consensus documents and guidelines suggest discontinuation of cytotoxic cleansing agents such as Dakin’s or solutions that contain cytotoxic agents such as chlorhexidine gluconate. In 2022 consensus guidelines were published by the International Wound Infection Institute,6 which included discussions from global experts on the use of hypochlorous acid versus sodium hypochlorite. A key section of this guideline discusses the issue of margin of safety; this is the therapeutic index.7 Hypochlorous acid as a
preservative has a higher therapeutic index compared to the hypochlorite present in Dakin’s, and also some cleansers that combine variable proportions of elements of hypochlorous acid (harmless) and hypochlorite (toxic). The pH of 3.5 to 5.5 of pHA is well below the pKa of HOCl (7.5), with the added attribute that the pHA preserved cleanser has a greater efficacy with a lower toxicity to the tissue.
The hypochlorous acid molecule, a naturally evolving substance inside the human tissue, forms part of the cellular barrier immunity. Humans are designed to live with hypochlorous acid in their tissue, which explains the lack of cytotoxicity of the pHA based cleanser.
A study in an academic centre looking at sulfamylon or mafenide and bacterial adherent aggregates reports that the mafenide solution had a flat curve; very little effects occurred on the bacterial adherent aggregates, as compared to the pHA based cleanser solution. Antibiotic Stewardship is a key topic to incorporate into clinical practice and It is vital not to overuse antibiotics to prevent the ever evolving challenge of drug resistance. Mafenide was also unable to remove Candida colonies, while the pHA preserved cleanser did so easily; which also illustrates that while the pHA preserved cleanser is able to kill planktonic germs in seconds in a situation where microbial aggregates adherent to tissue is involved, it may be appropriate to soak the wound for 5 - 8 minutes.
Ovine (Sheep) Forestomach Matrix
An effective advanced ECM for use in VLUs is ovine forestomach matrix (OFM) (EndoformTM Antimicrobial; EndoformTM Natural; Myriad MatrixTM, Aroa Biosurgery, Auckland, New Zealand). After years of research, the forestomach of sheep in New Zealand was identified as an ideal starting tissue to construct advanced ECM technologies for soft tissue repair. This source tissue from juvenile sheep (less than 12 months of age) is highly abundant in New Zealand, and the animals are disease free. The forestomach, even from these young animals, is very large, meaning large sheets of ECM can be easily manufactured. The
MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro Wound Masterclass - Vol 2 - March 2023 107 © Copyright. Wound Masterclass. 2023
“Humans are designed to live with hypochlorous acid in their tissue, which explains the lack of cytotoxicity of the pure hypochlorous acid (pHA) based cleanser.”
forestomach is a highly vascular tissue, designed for digestion that is integral for rapid nutrient absorption. This also means that residual vascular channels in the graft help it to revascularize more quickly, and then this tissue undergoes more rapid remodelling, where soft tissues are continuously renewed in the body; it has a high concentration of secondary macromolecules, which are known to be important for healing.12
A study of ovine forestomach matrix usage in a military veteran hospital in 201713 shows that in nine months, cellular or tissue-based graft unit usage decreased by 60%; expenditures on these grafts decreased by 66%, with a higher and faster rate of healing.
Chronic wounds tend to be deficient in a quality ECM; this is comprised of a complex process of cells, blood vessels, and macromolecules.10 The ECM is important for structural and biochemical support. Chronic wounds often have deficient ECM, senescent fibroblasts and increased matrix metalloproteinases (MMPs). Enhancing and optimising the ECM with a replacement ECM to heal these ulcers more effectively should be considered. To that end, it is important to consider what features make an ideal replacement? An ideal advanced ECM affords a rapid 100% or near 100% wound closure, long term tissue durability, cost effectiveness, and an ease of storage and transport. The outcomes that are measured by multiple studies include the percentage of closed wounds, the time to closure, wound recurrence or infections, need for amputation or hospitalization, and a time to return to baseline Activities of Daily Living (ADLs), pain reduction, odour and exudate reduction, and also other adverse effects.
Patients that have failed healing of large venous ulcers with standard of care in conjunction with a granulated wound base, with no bone or tendon exposure and a high quality of skin coverage is desired, a STSG may be considered.
Utilizing Synergistic Effects of Both Modalities

An ideal operative algorithm for STSGs could be considered as the following: a combination of performing a thorough intra-operative ultrasonic debridement with pure hypochlorous acid solutions; sharp debridement with a curette; and ovine forestomach matrix coverage. The graft can then be stapled or sutured in place with a thrombin sealant. A standard dressing protocol can be used which can include: a non-adherent silver silicone dressing with Negative Pressure Wound Therapy (NPWT); appropriate 4 or 5 layer compression wrap dressings, and then immobilization at the ankle or knee as indicated. The most complex patients are often admitted, put on bed rest, and some require a stay for optimal take of the graft; and compression and post-operative patient education.
The Typical Venous Leg Ulcer Checklist
It is important to run a typical VLU checklist like an airplane pilot’s pre-flight checks to maximize outcomes and achieve consistency in terms of treatment. To begin, a vital check is to make sure that there is adequate blood flow; in current demographics, about 20-25% of patients have some component of either diabetes or a metabolic syndrome which can compromise both microvascular as well as macrovascular arterial flow. Investigation with venous competency ultrasound is critical in identifying both saphenous and non-saphenous reflux and perforator status. The EVRA study and the ESCAR study suggest that axial and non-axial venous reflux should be treated early, managing the venous hypertension as a basis for wound healing. To attempt healing without managing the venous
MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro 108 Wound Masterclass - Vol 2 - March 2023
“Decellularized extracellular matrix–based biomaterials are of great clinical utility in soft tissue repair applications due to their regenerative properties. ”
Global expert Dr M. Mark Melin
Medical Director of the M Health Wound Healing Institute
Mineapolis MN, United States
© Copyright. Wound Masterclass. 2023
hypertension is not found to be effective practice.
A nutrition evaluation typically falls way down on the checklist; it is established that the micronutrient components to maximize nitric oxide production which improves lymphatic function, micro arterial vasodilation, venous tone and immune function are all incredibly beneficial to these patients who often times may be micronutrient deficient. It is vital to focus on the B vitamins (B12, B6 folate), and high dose vitamin C to help with decreasing reactive oxygen species and antioxidants; a caveat on vitamin C is that if the patient has a history of renal stones, it may be necessary to be careful and discuss with the primary care physician. Vitamin D can also have an impact, not just on bone health, but on microvascular perfusion. Arginine certainly contributes to nitric oxide production and micronized purified flavonoid fractions (MPFFs). A new way to look at albumin is not necessarily as a marker of overall nutritional health; it is actually a component of the endothelial glycocalyx, it helps as a permeability layer, so when the albumin is low increased levels of microvascular hyperpermeability may be seen, which can influence subcutaneous edema, potentially compromising microvascular arterial perfusion, as well as immune function.
Certainly, looking at the medication list is important, because medications like amlodipine or other calcium channel blockers can contribute directly to lower extremity edema; typically amlodipine needs to be stopped to maximize edema control. Oncology patients undertaking chemotherapy, or who are on hydroxyurea, are certainly at a high risk of inhibited long term healing.
Good hyperglycaemic control is important; again, hyperglycaemia can shed the very fragile layer of the endothelial glycocalyx, which can result in more edema.
It is prudent to consider biopsy; concerning atypical vs typical ulcerations, if an atypical is missed such as a malignancy, or pyoderma gangrenosum, and it is treated as a typical venous leg ulcer, there will not
be a good outcome.
Evidence shows that the wound pH should be controlled at a mildly acidic, low pH range of between 3 to 6. It is established through peer reviewed, validated data that decreasing wound pH can help with decreasing pathogen proliferation, improving angiogenesis, and improving accelerated epithelialization, when with other types of adjunctive modalities.
Another important consideration is compression, which is critical; it is important to get certified lymphedema therapists involved and begin utilizing inelastic Velcro; this is easy to get on and off over the top of dressings, and it is important not to forget about both the foot component and the calf component.
Cost effective wound bed modulation, as shown with ovine forestomach, should be opted for. Also, smoking has to be ceased, including vaping.
For bacteria proliferation management, a pHA preserved cleanser is recommended; it has a stable shelf life of approximately 18 months, a pH range over its shelf life at 3.5 to 5.5, and it is noncytotoxic for wound and peri-wound environments; it decreases inhibitory wound bed pathogens; it can be used in conjunction with NPWT after surgical debridement to maximize lowering of the wound pH, and avoid recurrence of formation of adherent germ aggregations; it is applicable to all wound sites. It has been used in trauma, pyoderma wounds, and diabetic foot ulcerations. In terms of the extracellular matrix, ovine forestomach matrix should be used in the operating room, and Endoform™ Antimicrobial or Endoform™ Natural in the clinic. Myriad Morcells™ is another option that is available; it is a structured retention of architecture from the forestomach of young sheep, typically less than 12 months old. The stomach of sheep has to heal very quickly to maintain the health of the animal, so the ovine forestomach matrix has a diverse array of collagens including 1, 2, 3, and 5; as well as an abundance of glycoproteins and proteoglycans. All of these are preserved through a
MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro Wound Masterclass - Vol 2 - March 2023 109 © Copyright. Wound Masterclass. 2023
“It is prudent to consider biopsy; concerning atypical vs typical ulcerations, if an atypical is missed such as a malignancy, or pyoderma gangrenosum, and it is treated as a typical venous leg ulcer, there will not be a good outcome.”
very specific process and retention of vascular channels, and it is cost effective.
It is an important consideration to try to focus on decreasing interstitial edema, and maximizing lymphatic functionality, also resulting in immune function enhancement, as by decreasing subcutaneous edema we can maximize microvascular arterial perfusion at the 5-micron level. To consider peri-wound lymphatic stasis, it is occurring in almost every wound; the epiboly we see in wounds is actually built-up lymphatic stasis, and when lymphatics are static, the immune system does not function well. By maximizing peri-wound lymphatic stasis resolution, overall wound healing can be improved.11,14,15
To conclude, the steps should include the checklist for VLUs with special attention to avoiding missing the identification of Peripheral Arterial Disease (PAD). It has been observed a growing demographic with diabetes and metabolic syndrome in the population. Another focus point is avoiding undertreating lymphedema; recognising malnutrition and treating it appropriately, not just the protein component but the micronutrients component. In terms of investigations venous ultrasounds must be done early. Vigilance with medications contributing to lower extremity edema and modify them. Help patients to wean themselves off tobacco.
and skin changes such as hemosiderosis (extravasated red blood cells in the breakdown of haemoglobin). Chronic inflammation and fibrosis of skin can often be mistaken for cellulitis. The morphology of VLUs tends to be irregularly shaped, full thickness ulcerations with moderate to large exudate, with typically no exposed underlying bone, tendon, or other structures.
General treatment guidelines include vascular workup; ensuring an adequate arterial workup, including ankle brachial indices and toe brachial indices to support that gold standard of multi-layer compression. It is important to remember that both clinically applied compression and the post-closure garments that the patients wear at home have to be tailored to the patient’s life status. Utilizing arteriograms, Computed Tomography Angiography (CTA) and Magnetic Resonance Arteriography (MRA).
Lymphedema is a common presentation to outpatient wound centers commonly presenting with open wounds with a multitude of challenges. Most outpatient wound centres are poorly equipped to manage lymphedema, and many lymphedema providers and clinics are unwilling to accept patients with open wounds. Therefore, this brings about a dilemma that these lymphoedematous wounds need to be closed before the lymphedema can actually be managed appropriately, with the skill and technique that can really only be provided by lymphedema therapists.
Arterial Insufficiency Ulceration
The Differential Diagnosis of Lower Extremity Ulcerations

VLUs begin distally with the feet and ankles, moving proximally as venous hypertension develops. Over time varicosities develop, and this is worse after prolonged standing or activity and the venous hypertension symptoms such as aching, discomfort
In this condition the clinical features tend to be punched out, with well-defined edges almost as though the wound was created with a drill-bit. They tend to occur over distal bony prominences; in appearance, the wound beds tend to have an unhealthy and pale granulation tissue. They tend to be fairly dry, with minimal exudate, black to brown eschars, and the surrounding skin tends to be ruborous and hairless. In terms of management of arterial insufficiency ulceration, it is vital to make the diagnosis and then refer to an appropriate vascular surgeon. It is important that there is an adequate arterial
MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro 110 Wound Masterclass - Vol 2 - March 2023
“The critical catalyst for Buerger’s disease is cigarette smoking; in a patient who is not a cigarette smoker, a diagnosis of this disease cannot be made.”
Global expert Dr Lee Ruotsi
Medical Director at the Saratoga Hospital Center for Wound Healing and Hyperbaric Medicine
Saratoga NY, United States
© Copyright. Wound Masterclass. 2023
supply. Priorities should be completing the VLU checklist; thinking out of the box and ensuring that you make that diagnosis of arterial insufficiency in a timely fashion. Don’t always rely on your fingers; remember to use a hand-held doppler, MRA, and CTA.
Debridement should be performed cautiously, especially in the ischemic limb; and on the advice from the National Pressure Injury advisory panel: it is important not to debride dry stable heal eschar; leave it alone allowing it to demarcate.
Thromboangiitis Obliterans
Also known as Buerger’s disease, this is an arterial clotting in the hands and feet, with the resultant ulceration and gangrene. It is a vaso-occlusive, inflammatory vasculopathy, where inflammatory thrombi occlude vessels but spare the vessel walls. The critical catalyst for Buerger’s disease is cigarette smoking; in a patient who is not a cigarette smoker, a diagnosis of this disease cannot be made.
Vasculitis
Vasculitis can occur in any organ system, however interestingly the skin is the most common organ system affected. The bilateral lower legs are the most common location. The typical description is a painful palpable purpura. Biopsy is done for diagnosis; doing a biopsy on the most immature of the areas will lead to the highest return in diagnosis. Leukocytoclastic of inflammatory vasculitis is the most common form of cutaneous vasculitis.
Scleroderma
Scleroderma, most commonly the C.R.E.S.T. syndrome, and also sometimes known as progressive system sclerosis, is an autoimmune disorder of unknown etiology ulcers, usually over the digits, pre-tibial area, and bony prominences. One of the most troubling features of this disease process if the subcutaneous calcification, known as calcinosis cutis. There are yellow areas around the periphery of these wounds, so sometimes those areas are able to be distracted or extracted, almost like dental extractions,
but getting these wounds to epithelialize is very difficult.
These are painful ulcers of varying depth and size, with purulent wound beds and a blue-black wound edge. PG is most commonly associated with underlying autoimmune and occasionally malignant disease. The most common association is with inflammatory bowel disease such as ulcerative colitis or Crohn’s disease. System corticosteroids and other disease modifying agents such as cyclosporin are often used in treatment. A very important concept with pyoderma gangrenosum is that of pathergy where, if the ulcers are sharply debrided, they grow large.
This is an unusual form of squamous cell, or occasionally basal cell carcinoma. Marjolin’s ulcers were originally described by French surgeon JeanNicholas Marjolin in the late 1700’s, as a squamous cell cancer arising in an area of prior skin trauma. In the United States, these are colloquially called scar carcinomas or burn carcinomas, and are now known to be roughly 80% squamous cell and 20% basal cell. A 73-year-old lady presented to the clinic with a large VLU in an area where she had prior venous leg ulceration. It appeared as a fairly typical looking venous ulcer. She was quite mobility impaired, so home nursing care was arranged and she was brought back in 4 weeks for a follow-up. When she returned she had an odd looking area in the superior aspect of the wound; this was biopsied and it turned out to be a poorly differentiated squamous cell carcinoma. This patient’s prior area of skin damage ended up being her recurrent VLU; biopsy early and biopsy often; there are very few wounds that can be negatively affected with a 5mm punch biopsy.
Pyoderma Gangrenosum
Marjolin’s Ulcer
MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro Wound Masterclass - Vol 2 - March 2023 111 © Copyright. Wound Masterclass. 2023
“Biopsy early and biopsy often; there are very few wounds that can be negatively affected with a 5mm punch biopsy.”
Diabetic Foot Ulcer
5 year mortality of neuropathic Diabetic Foot Ulcer (DFU) is almost 50%. Vascular assessment is of critical importance. Remember the tri-neuropathy; we always think of the sensory neuropathy, but remember that we have the motor neuropathy that leads to the bony deformities, and then the autonomic neuropathy that leads to the dry foot, leading to fissuring and cracking. Infection and osteomyelitis are common.
Treat reflux early; vascular specialists should be consulted as soon as possible because we want to treat the underlying incompetence (whether it is superficial or perforated) early.32
Treat iliofemoral venous obstruction; if the patient has associated deep venous obstruction, we certainly need to do this. This can be done very effectively with relatively low risk but good clinical success.
Interventions for Treatment of Chronic Venous Insufficiency
Chronic venous disease is a huge spectrum of medical conditions. This is one of the most prevalent medical conditions in the world. With the increasing age of the population, there is likely to be a higher prevalence of venous ulcerations, and the associated economic cost of this is well understood. Although it affects elderly patients more frequently, there is a significant economic burden because of loss of working days in people who are still working, and it is not infrequent to see these patients having to retire early, with a reduced quality of life.
1,26
There are guidelines on how to treat patients. These guidelines need upgrades frequently, not only because there is a large number of new venous ulcer treatments that are not interventional, but because there have been tremendous changes in vascular reconstruction because of the endovascular revolution.

27,28,29,30
Strategy for VLU
In establishing the diagnosis of venous ulcers, duplex scanning is extremely important to establish the status of venous incompetence, and excluding the underlying arterial disease is important, as is performing the VLU checklist, looking at diabetes vasculitis and other rare ulcerations. Compression treatment is of course a usual part of immediate treatment, and treatment of infection is extremely important. Cleanse and debride the ulcer and apply dressing. Maintain a moist surface, absorb and excess fluid, and protect the surrounding skin.
For persistent/ recurrent ulcers, treat residual reflux or obstruction. If stenting is not possible, the Palma procedure or surgical bypass should be performed. For nonhealing wound ulcers, duplex scanning should be repeated to look for new or recurrent reflux; the effectiveness here of skin grafting and bilayer cellular therapy is well evidenced. To summarize, the best strategy to treat venous ulcers is to confirm the right diagnosis, optimize the local care, treat the infection, initiate compression therapy, and treat the underlying reflux and obstruction early to prevent/ treat, if there is ulcer recurrence.
Scientific Societies' Guidelines
The last guidelines of the American Venous Forum and Society for Vascular Surgery suggest nutrition assessment in any patient with VLU and evidence of malnutrition; nutritional supplementation should be provided if malnutrition is identified. For long standing or large venous ulcers, the 2 societies recommended adjunctive therapy with pentoxifylline or Micronized Purified Flavonoid Fraction (MPFF).
The guidelines of the Wound Healing Society attributed recommendations of Level I for pentoxifylline and MPFF, in conjunction with compression therapy to improve healing of venous ulcers.
Just recently the ESVS published Clinical Guidelines on the management of Chronic Venous Disease and included adjuvant pharmacotherapy to the algorithm of treatment of patients with active venous ulcers. In the recommended pharmacologic treatments, based on the level A evidence, the guidelines listed MPFF, hydroxyethylrutosides, pentoxifylline or sulodexide, combined with compression and local wound care. All the guidelines were using the same definition of scientific evidence, levels A, B, and C.

MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in
From Micro to Macro 112 Wound Masterclass - Vol 2 - March 2023
Chronic Venous Disease;
Global expert Dr Monika Gloviczki Scientist and Artist at Vascular Science and Art Scottsdale AZ, United States
Global expert Dr Peter Gloviczki
Editor-in-Chief of the Journal of Vascular Surgery Publications.Professor of Surgery (Emeritus), Mayo Clinic, Rochester, Minnesota Scottsdale AZ, United States
© Copyright. Wound Masterclass. 2023
Scientific Evidence for Nutritional and Pharmacologic Treatment in VLU
Nutrition
Published January 2023, a systematic review on nutrition stated that VLU patients tend to have a deficit of nutrients such as vit A, D, or zinc and an excess of lipids and carbohydrates. A clinical trial with an oral nutritional supplement found that high calorie, high protein, enriched oral supplementation had a positive effect on ulcer healing. Another systematic review concluded that omega-3 fatty acids, Mg, zinc, vit A, C, D and resveratrol with probiotics improved VLU healing.

Pharmacologic compounds
As for pharmacologic treatment, the scientific evidence available for MPFF is a meta-analysis including 5 RCTs. This meta-analysis demonstrated a significantly better healing rate in the MPFF group and shorter median time to healing: 16 weeks for MPFF vs 21 in the control group.
The cost-effectiveness analysis showed that MPFF adjuvant therapy improved the cost-effectiveness ratio by 45%. For Pentoxifylline the Cochrane systematic review concluded that it is an effective adjunct to compression bandaging for treating VLUs and may be effective in the absence of compression. The whole group of flavonoids was also an object of the Cochrane review for VLUs treatment. It included MPFF and hydroxyethylrutosides and was inconclusive in despite of significant ulcer healing improvement documented, as some trials were considered to be poorly reported. The sulodexide’s Cochrane review concluded that it may increase the healing of VLUs, however the evidence was judged to be of low quality.
In conclusion, nutritional supplements are indicated for patients with vitamin/ mineral deficiencies. For venous leg ulcers, in addition to standard wound care and compression therapy, an adjunctive treatment with MPFF, pentoxifylline, hydroxyethylrutosides and sulodexide was established in several RCTs, metaanalysis and Cochrane’s systematic reviews.
See all Wound Masterclass MasterSeries on demand: bigmarker.com/wound-masterclass
References
1. Gloviczki ML, Kalsi H, Gloviczki P, Gibson M, Cha S, Heit JA. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for estimating the prevalence of venous ulcer. J Vasc Surg Venous Lymphat Disord. 2014 Oct;2(4):362-7. doi: 10.1016/j.jvsv.2014.03.002. Epub 2014 May 10. PMID: 26993538.
2. Nagoba BS et al. Acidic environment and wound healing: a review, Wounds 2015;27(1):5-11
3. Hidalgo E et al. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness. Chem Biol Interact. 2002 Mar 20;139(3):265-82.
4. C. Winterbourn et al. Modeling the Reactions of Superoxide and Myeloperoxidase in the Neutrophil Phagosome. Implications for Microbial Killing. J Biol Chem Volume 281. No. 52. Dec. 29, 2006. 39860 - 39869;
5. Robson MC. Treating chronic wounds with hypochlorous acid disrupts biofilm. Wound Prevention and Management 2020;66 (5):9-10
6. Swanson, T., Ousey, K., Haesler, E., Bjarnsholt, T., Carville, K., Idensohn, P., Kalan, L., Keast, D. H., Larsen, D., Percival, S., Schultz, G., Sussman, G., Waters, N., & Weir, D. (2022). IWII Wound Infection in Clinical Practice Consensus Document: 2022 Update. Journal of wound care, 31(S12), S10-S21. https://doi.org/10.12968/jowc.2022.31.Sup12.S10
7. Eriksson E, Liu PY, Schultz GS, Martins-Green MM, Tanaka R, Weir D, Gould LJ, Armstrong DG, Gibbons GW, Wolcott R, Olutoye OO, Kirsner RS, Gurtner GC. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022 Mar;30(2):156-171.
doi: 10.1111/wrr.12994. Epub 2022 Feb 7. Erratum in: Wound Repair Regen. 2022 Jul;30(4):536. PMID: 35130362; PMCID: PMC9305950.
8. Wound Repair and Regeneration – the international journal of tissue repair and regeneration. Available from: https://onlinelibrary.wiley.com/journal/1524475x
9. Melphine M. Harriott, Nayan Bhindi, Salam Kassis, Blair Summitt, Galen Perdikis, Blair A. Wormer, Timothy M. Rankin, Christodoulos Kaoutzanis, Mario Samaha, Charles Stratton, Jonathan E. Schmitz. Comparative Antimicrobial Activity of Commercial Wound-Care Solutions on Bacterial and Fungal Biofilms, Ann Plast Surg. 83(4): 404-410, 2019.
10. Optical Microscopy and the Extracellular Matrix Structure: A review, Cells 2021, 10(7),1760; https://doi.org/10.3390/cells10071760
11. Sandi G. Dempsey, Christopher H. Miller, Ryan C. Hill, Kirk C. Hansen, and Barnaby C. H. May, Journal of Proteome Research 2019 18 (4), 1657-1668, DOI: 10.1021/acs.jproteome.8b00908
12. Topps J, Kay et al (1968). Digestion of concentrate and of hay diets in the stomach and intestines of ruminants. Br J Nutr 22, 261-280, 2. Engelhardt W and Hales J (1977). Partition of capillary blood flow in rumen, reticulum, and omasum of sheep. American Journal of Physiology- Endocrinology and Metabolism 232(1 ): E53. 3. Baldwin, R. L; McLeod, K. R; Klotz, J. L.; Heitmann, R. N.Rumen development,
Day DJ, May BCH. A novel chemotactic factor derived from the extracellular matrix protein decorin recruits mesenchymal stromal cells in vitro and in vivo. PLoS One. 2020 Jul 13;15(7):e0235784. doi: 10.1371/journal.pone.0235784. Erratum in: PLoS One. 2020 Sep 3;15(9):e0238964. PMID: 32658899; PMCID: PMC7357784.
15. Suami H, Scaglioni MF. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin Plast Surg. 2018 Feb;32(1):5-11. doi: 10.1055/s-0038-1635118. Epub 2018 Apr 9. PMID: 29636647; PMCID: PMC5891651.
16. Kubik S, Manestar M. Topographic relationship of the ventromedial lymphatic bundle and the superficial inguinal nodes to the subcutaneous veins. Clin Anat. 1995;8(1):25-8. doi: 10.1002/ca.980080104. PMID: 7535176.
17. Kirk C. Hansen, Angelo D’Alessandro, Cristina C. Clement, Laura Santambrogio, Lymph formation, composition and circulation: a proteomics perspective, International Immunology, Volume 27, Issue 5, May 2015, Pages 219–227, https://doi. org/10.1093/intimm/dxv012
18. Zwart SR, Laurie SS, Chen JJ, Macias BR, Lee SMC, Stenger M, Grantham B, Carey K, Young M, Smith SM. Association of Genetics and B Vitamin Status With the Magnitude of Optic Disc Edema During 30-Day Strict Head-Down Tilt Bed Rest. JAMA Ophthalmol. 2019 Oct 1;137(10):11951200. doi: 10.1001/jamaophthalmol.2019.3124. PMID: 31415055; PMCID: PMC6696878.
19. Broekhuizen LN, Mooij HL, Kastelein JJ, Stroes ES, Vink H, Nieuwdorp M. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr Opin Lipidol. 2009 Feb;20(1):57-62. doi: 10.1097/ MOL.0b013e328321b587. PMID: 19106708.
20. Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann Vasc Surg. 2018 Jan;46:380-393. doi: 10.1016/j.avsg.2017.06.131. Epub 2017 Jul 6. PMID: 28688874.
21. Weinbaum, S., Cancel, L.M., Fu, B.M. et al. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc Eng Tech 12, 37–71 (2021). https://doi.org/10.1007/s13239-020-00485-9
H, Takemura G, Suzuki K, Takada C, Tomita H, Zaikokuji R, Hotta Y, Miyazaki N, Yano H, Muraki I, Kuroda A, Fukuda H, Kawasaki Y, Okamoto H, Kawaguchi T, Watanabe T, Doi T, Yoshida T, Ushikoshi H, Yoshida S, Ogura S. BrainSpecific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible
22. Ando Y,
MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro Wound Masterclass - Vol 2 - March 2023 113
intestinal growth and hepatic metabolism in the pre- and post weaning ruminant. J. Dairy Sc. 2004, 87 (Suppl. E), E55-E65
13. Ferreras, D. T., S. Craig and R. Malcomb (2017). “Use of an Ovine Collagen Dressing with Intact Extracellular Matrix to Improve Wound Closure Times and Reduce Expenditure in a US Military Veteran Hospital Outpatient Wound Center”. Surg Technol Int 30:61-69
14. Dempsey SG, Miller CH, Schueler J, Veale RWF,
Okada
© Copyright. Wound Masterclass. 2023
Contribution for Blood Brain Barrier. Sci Rep. 2018 Nov 30;8(1):17523. doi: 10.1038/ s41598-018-35976-2. PMID: 30504908; PMCID: PMC6269538.
23. Potje SR, Paula TD, Paulo M, Bendhack LM. The Role of Glycocalyx and Caveolae in Vascular Homeostasis and Diseases. Front Physiol. 2021 Jan 13;11:620840. doi: 10.3389/fphys.2020.620840. PMID: 33519523; PMCID: PMC7838704.
24. Suzuki, Akio et al. Form Follows Function: The Endothelial Glycocalyx. Translational Research, Volume 247, 158 – 167. DOI: https://doi.org/10.1016/j. trsl.2022.03.014
25. Mitra R, Nersesyan A, Pentland K, Melin MM, Levy RM, Ebong EE. Diosmin and its glycocalyx restorative and anti-inflammatory effects on injured blood vessels. FASEB J. 2022 Dec;36(12):e22630. doi: 10.1096/fj.202200053RR. PMID: 36315163.
26. Kolluri R, Lugli M, Villalba L, Varcoe R, Maleti O, Gallardo F, Black S, Forgues F, Lichtenberg M, Hinahara J, Ramakrishnan S, Beckman JA. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc Med. 2022 Feb;27(1):63-72. doi: 10.1177/1358863X211028298. Epub 2021 Aug 16. Erratum in: Vasc Med. 2021 Sep 22;:1358863X211048020. PMID: 34392750; PMCID: PMC8808361.
27. O’Donnell TF Jr, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, Lurie F, Henke PK, Gloviczki ML, Eklöf BG, Stoughton J, Raju S, Shortell CK, Raffetto JD, Partsch H, Pounds LC, Cummings ME, Gillespie DL, McLafferty RB, Murad MH, Wakefield TW, Gloviczki P; Society for Vascular Surgery; American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. J Vasc Surg. 2014 Aug;60(2 Suppl):3S-59S. doi: 10.1016/j.jvs.2014.04.049. Epub 2014 Jun 25. PMID: 24974070.
28. Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, Lohr JM, McLafferty RB, Meissner MH, Murad MH, Padberg FT, Pappas PJ, Passman MA, Raffetto JD, Vasquez MA, Wakefield TW; Society for Vascular Surgery; American Venous Forum. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011 May;53(5 Suppl):2S-48S. doi: 10.1016/j.jvs.2011.01.079. PMID: 21536172.
29. Gloviczki P, Lawrence PF, Wasan SM, Meissner MH, Almeida J, Brown KR, Bush RL, Di Iorio M, Fish J, Fukaya E, Gloviczki ML, Hingorani A, Jayaraj A, Kolluri R, Murad MH, Obi AT, Ozsvath KJ, Singh MJ, Vayuvegula S, Welch HJ. The 2022 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities.
Part I. Duplex Scanning and Treatment of Superficial Truncal Reflux: Endorsed by the Society for Vascular Medicine and the International Union of Phlebology. J Vasc Surg Venous Lymphat Disord. 2022 Oct 12:S2213-333X(22)00417-6. doi: 10.1016/j. jvsv.2022.09.004. Epub ahead of print. PMID: 36326210.
30. Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why Venous Leg Ulcers
Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J Clin Med. 2020 Dec 24;10(1):29. doi: 10.3390/jcm10010029. PMID: 33374372; PMCID: PMC7795034.
31. Goldschmidt E, Schafer K, Lurie F. A systematic review on the treatment of nonhealing venous ulcers following successful elimination of superficial venous reflux. J Vasc Surg Venous Lymphat Disord. 2021 Jul;9(4):1071-1076.e1. doi: 10.1016/j. jvsv.2020.12.085. Epub 2021 Feb 26. PMID: 33647527.
32. Gohel MS, Heatly F, Liu X et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med 2018; 378:2105-114 DOI: 10.1056/NEJMoa1801214
33. Gloviczki P, Bergan JJ, Rhodes JM, Canton LG, Harmsen S, Ilstrup DM. Mid-term results of endoscopic perforator vein interruption for chronic venous insufficiency: lessons learned from the North American subfascial endoscopic perforator surgery registry. The North American Study Group. J Vasc Surg. 1999 Mar;29(3):489-502. doi: 10.1016/s0741-5214(99)70278-8. PMID: 10069914.
34. Köksoy C, Bahçecioğlu İB, Çetinkaya ÖA, Akkoca M. Iliocaval outflow obstruction in patients with venous ulcers in a small comparison study between patients with primary varicose veins and chronic deep vein disease. J Vasc Surg Venous Lymphat Disord. 2021 May;9(3):703-711. doi: 10.1016/j.jvsv.2020.08.019. Epub 2020 Aug 19.
PMID: 32827736.
35. Lawrence PF, Hager ES, Harlander-Locke MP, Pace N, Jayaraj A, Yohann A, Kalbaugh C, Marston W, Kabnick L, Saqib N, Pouliot S, Piccolo C, Kiguchi M, Peralta S, Motaganahalli R. Treatment of superficial and perforator reflux and deep venous stenosis improves healing of chronic venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020 Jul;8(4):601-609. doi: 10.1016/j.jvsv.2019.09.016. Epub 2020 Feb 21.
PMID: 32089497.
36. Marston W, Ta ng J, Kirsner RS, Ennis W. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair Regen. 2016 Jan- Feb;24(1):136-44. doi:

10.1111/wrr.12394. PMID: 26663616.
37. De Maeseneer MG, Kakkos SK, Aherne T, Baekgaard N, Black S, Blomgren
L, Giannoukas A, Gohel M, de Graaf R, Hamel-Desnos C, Jawien A, JaworuckaKaczorowska A, Lattimer CR, Mosti G, Noppeney T, van Rijn MJ, Stansby G, Esvs Guidelines Committee, Kolh P, Bastos Goncalves F, Chakfé N, Coscas R, de Borst
GJ, Dias NV, Hinchliffe RJ, Koncar IB, Lindholt JS, Trimarchi S, Tulamo R, Twine CP, Vermassen F, Wanhainen A, Document Reviewers, Björck M, Labropoulos N, Lurie F, Mansilha A, Nyamekye IK, Ramirez Ortega M, Ulloa JH, Urbanek T, van Rij AM, Vuylsteke ME. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2022
Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur J Vasc Endovasc Surg. 2022 Feb;63(2):184-267. doi: 10.1016/j. ejvs.2021.12.024. Epub 2022 Jan 11. Erratum in: Eur J Vasc Endovasc Surg. 2022 AugSep;64(2-3):284-285. PMID: 35027279.
38. García-Rodríguez MT, Rodríguez-Parrado M, Seijo-Bestilleiro R, González-Martín
C. Influence of Nutrition Status and Compression Therapy on Venous Ulcer Healing: A Systematic Review. Adv Skin Wound Care. 2023 Jan 1;36(1):45-53. doi: 10.1097/01.
ASW.0000902492.97059.f2. PMID: 36537775.
39. Melo PG, Mota JF, Nunes CAB, Malaquias SG, Coelho ASG, Soriano JV, Bachion MM. Effects of Oral Nutritional Supplementation on Patients with Venous Ulcers: A Clinical Trial. J Clin Med. 2022 Sep 26;11(19):5683. doi: 10.3390/jcm11195683. PMID: 36233551; PMCID: PMC9570985.
40. de Souza T, Monteiro JDC, Curioni CC, Cople-Rodrigues C, Citelli M. Nutrients with Antioxidant Properties and Their Effects on Lower-Limb Ulcers: A Systematic Review. Int J Low Extrem Wounds. 2022 Jan 24:15347346221074861. doi: 10.1177/15347346221074861. Epub ahead of print. PMID: 35072533.
41. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a metaanalysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005 Aug;30(2):198-208. doi: 10.1016/j.ejvs.2005.04.017. PMID: 15936227.
42. Simka, M., Majewski, E. The Social and Economic Burden of Venous Leg Ulcers. Am J Clin Dermatol 4, 573–581 (2003). https://doi.org/10.2165/00128071-20030408000007
43. Jull AB, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst
44.

Rev. 2012 Dec 12;12(12):CD001733. doi: 10.1002/14651858. CD001733.pub3. PMID: 23235582; PMCID: PMC7061323.
Wu B, Lu J,
pub2.
PMCID: PMC9308373. 45. Scallon C, Bell-Syer SE, Aziz Z. Flavonoids for treating venous leg ulcers. Cochrane Database Syst Rev. 2013 May 31;(5):CD006477. doi: 10.1002/14651858.CD006477. pub2. PMID: 23728661. MasterSeries 60 Minute Interactive: Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro 114 Wound Masterclass - Vol 2 - March 2023 woundmasterclass.com Introducing Wound Masterclass Video woundmasterclass.com/Video Image licenced from Adobe Stock. Credit: BillionPhotos.com Sponsored by Urgo Medical and Aroa Biosurgery. All production resources provided by Urgo Medical and Aroa Biosurgery. © Copyright. Wound Masterclass. 2023
Yang M, Xu T. Sulodexide for treating venous leg ulcers. Cochrane Database Syst Rev. 2016 Jun 2;2016(6):CD010694. doi: 10.1002/14651858.CD010694.
PMID: 27251175;
RELIEVE PAIN STIMULATE HEALING
Chronic wounds can also be extremely painful –between 50% and 60% of patients with a chronic wound experience persistent pain.[1,2] One major issue is that pain can make gold standard treatments, such as compression, unbearable.[3]
Electrical stimulation has been used globally in specialist clinics for more than a decade to relieve pain and accelerate healing in chronic wounds. With nine meta analyses and over 35 randomised controlled trials published, electrical stimulation is one of the most evidence-based technologies in wound management.
Electrical stimulation therapy is now available in a simple to use, single use, wearable device, enabling its more widespread use across different care settings. Accel-Heal Solo electrical stimulation wound therapy is a one-off, interventional, 12-day treatment which is applied alongside existing care to relieve pain [4] and accelerate healing [4,5]. Accel-Heal Solo improves the quality of life for the patient [5] and helps facilitate the use of compression therapy where wound pain has been an issue.[6]
To find out more about Accel-Heal Solo, please contact: customerservices@accelheal.com

References. 1. Price P, et al. Managing painful chronic wounds: the Wound Pain Management Model. Int Wound J. 2007;4(1). 2. Hellström A, et al. Leg ulcers in older people: a national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatr. 2016;16(1):25. 3. Atkin L, et al. Accel-Heal Made Easy. Wounds UK. 2019. https://www.wounds-uk.com/resources/details/made-easy-accel-heal-electrical-stimulation 4. Guest J, et al. Cost-effectiveness of an electroceutical device in treating non-healing venous leg ulcers: results of an RCT. J Wound Care. 2018;27(4):230-243. 5. Turner N, L. Ovens. Clinical outcome results and quality of life improvements using electroceutical treatment* - Patient perspectives. Presented at: EWMA 2018. 6. Ovens L. Presented at: Wounds UK. ; 2014.
There have been significant advances in chronic wound management treatments over the last 30 years, however, hard-to-heal wounds are an increasing problem.
MasterSeries 60 Minutes Interactive

Topical Oxygen Therapy: All Your Questions Answered
Introduction
This MasterSeries is on Topical Oxygen Therapy. We have a global panel of experts who have been using this form of cyclical topical oxygen for many years in their clinical practice. We will be delving into the clinical indications for the usage, the types of patients that generally benefit, how to administer the therapy, as well as substantial evidence for the usage. We get a personal view with tips and tricks as well as other pearls of clinical wisdom. You will have the opportunity to watch the whole interactive, immersive event at this link:

woundmasterclass.com/Video


Our global experts joining us for this MasterSeries are Dr Matthew G. Garoufalis, Past President of the American Podiatric Medical Association; Frank Aviles of the Natchitoches Regional Medical Center; Dr Anil Hingorani, vascular surgeon; Kara Couch, director of wound care services at George Washington University Hospital, and Dr Paul Haser, vascular surgeon.
The Role of Oxygen in Wound Healing

Oxygen therapy has been very helpful in treating a variety of wounds in recent times. It has been used in Sickle Cell Disease (SCD), radiation burns, and pyoderma gangrenosum, that has been very difficult to treat. With more and more success stories, there has been a huge change at the molecular level, changing the way that cells function and the way they communicate to each other and therefore allowing healing to finally occur in these hard-to-heal wounds.
The scope for the wounds that can be amenable to oxygen therapy is quite wide, and yet there still remains a large percentage of the population of clinicians, nurses and hyperbaric technicians that are reluctant to incorporate oxygen therapy into their clinical practice, as part of an advanced modality to be used alongside standard of care.
How is Oxygen Delivered to the Wound?
For topical oxygen therapy, presently there is continuous diffusion, which has been around for a while with a variety of devices; and then there is cyclical pressurised topical oxygen, which is a newer

116 Wound Masterclass - Vol 2 - March 2023
Moderator Miss Negin Shamsian Consultant Plastic and Reconstructive Surgeon (Locum) London, United Kingdom
Supported by
© Copyright. Wound Masterclass. 2023
Global expert Dr Matthew G. Garoufalis Past President, American Podiatric Medical Association Chicago IL, United States
technology having been around for about ten years now; this method actually drives oxygen into the wound under pressure. This is how I first became involved with topical oxygen; I had used many devices in the past, and with an understanding of hyperbarics, but not always having access to that, which in my hands at least did not always get good or consistent results. So when cyclical pressure topical oxygen became available, I was interested in it from a scientific point of view because I understood the theories behind it from my experience in lymphedema and venous stasis treatment. Even though there was no science at that time in 2015, for this reason I started to use it in my practice.
Now, however, continuous diffusion and cyclical pressure devices do have some very good science and studies. Cyclical pressure now has level 1A Randomized Controlled Trials (RCT’s) and excellent studies which have all been reviewed and accepted into medical literature. We are in a much better position with this modality than we were many years ago.
Evidence Base
At time of writing, there has been a plethora of articles that have been peer reviewed and published. I will review two studies on cyclical pressure topical oxygen that gives us some remarkable data.
The first is an RCT that was able to demonstrate that using this modality we were able to heal wounds sixtimes faster in twelve weeks, with a recurrence rate six-times lower at one year, compared with the study arm that did not get topical oxygen.1
The second is a real world evidence study that was done in a Veteran’s Affairs (VA) population, which as most of us will understand is a population with many co-morbidities. This study was conducted in two different sites with two hundred patients. All the patients in the study were undergoing regular treatments for their wounds using skin substitutes, grafts, different dressings, etc. They were all receiving top line modalities to heal their wounds,
the only difference being that one arm received cyclical pressurised topical oxygen therapy, the other arm did not. This study was able to demonstrate an 88% reduction in hospitalizations after one year, and a 71% reduction in amputations after one year, in the group that got oxygen therapy.2
This evidence tells us that this is a modality that can make a difference in patient’s lives, and also make a difference in costs.
We now have data that is definitive, peer-reviewed and has undergone meta-analysis by several different groups that were all extremely positive.
Limb Assessment and Patient Selection
The patient need to be assessed for adequate blood flow, whether the wound has been debrided adequately, and that they are relatively free of infection. A four-week model of good wound care and good standard of care medicine like collagen or foam is a good start. The patient’s wound needs to be measured in size and needs to progress by 50% by the end of the four-week model. If by the end of the four weeks there wasn’t a decrease in size by 50%, different modalities must be used, such as skin substitutes. Topical oxygen, being an adjunctive therapy, can be used at the four-week level, however those who have used it and seen its efficacy are more encouraged to use it. Patients that have undergone topical oxygen initially had a huge difference in their recovery times and in the way the wound closed because the hypoxic level of that chronic wound was unaffected.
Wound assessment is key, since you have to pick the right wound. Many of these patients have to go to revascularization before treatment is started to make sure that there is enough blood supply to heal the wound.
Coverage
Right now, the coverage in the U.S. is only in the VA setting. In New York it is covered by Medicaid,
MasterSeries 60 Minute Interactive: Topical Oxygen Therapy Wound Masterclass - Vol 2 - March 2023 117 © Copyright. Wound Masterclass. 2023
“We now have data that is definitive, peer-reviewed and has gone under meta-analysis by several different groups that were all extremely positive.”
however that will soon be changing as Medicaid in other states will begin paying for it, hopefully, in the coming new year. We also hope that the Centers for Medicare and Medicaid Services (CMS) will allow it to be covered by CMS, at which point we think that not only will it have CMS and Medicaid coverage, but the private insurers will then fall in line, too. Right now, the most restrictive part of the coverage is in New York Medicaid where the wound needs to be present for at least thirty days, and then it is reassessed every 30 days, which is appropriate, but other than that there are no other restraints on when and how this technology is used.
Technical Challenges
This modality can certainly be used with compression hosiery because oxygen can penetrate. It is very challenging to put on and take off compression hosiery, so if the patient doesn’t have to do that, things are much easier. This technology can also be used with compression wraps, such as a multilayer compressive system; it can penetrate all these materials. It has been used with total contact casts many times, and the technology has been developed with these things in mind, such as to accommodate a total contact cast.
Conclusion
I was the biggest sceptic in the world about topical oxygen. After many years of practice and now having used it on hundreds of patients, I’ve now decided to become the CMO of that company, in order to educate others on what this can do for their patients and practice.
Incorporation of Oxygen Into Wound Healing Therapy
In the beginning, topical oxygen got a bad reputation because, initially, there were very poor studies. The evidence now, however, for its use in many conditions, is significant. For practitioners, the way they practice will definitely change as soon as the results are noticed.

We know the importance of oxygen in that it is vital to sustain life, but after having an injury the vasculature in the body will limit the oxygen to the area. Having a hypoxic wound is needed and that’s acute hypoxia, because that’s what starts some of the processes, but when you have inflammation that is going to increase the oxygen demand. So, if you have prolonged hypoxia, that wound is not going to move to the proliferative phase. The oxygen has to get to the actual site. With inflammation, it is well known that oxygen helps with taking care of bacteria, and angiogenesis, but if we have this hypoxic wound, we are not going to progress.
Also, nutrition and cell health is a very important factor that needs to be considered. If the metabolic cell does not have adequate nutrients and oxygen getting to it, it will be very inactive.
MasterSeries 60 Minute Interactive: Topical Oxygen Therapy 118 Wound Masterclass - Vol 2 - March 2023
“Oxygen helps with taking care of bacteria, and angiogenesis, but if we have this hypoxic wound, we are not going to progress.
Global expert
Mr Frank Aviles
Natchitoches Regional Medical Center
© Copyright. Wound Masterclass. 2023
Natchitoches LA, United States
Application
When we were first introduced to topical oxygen, we were very sceptical. We therefore used this modality on patients with whose wounds we had tried everything, given optimal care, and nothing had worked. We were amazed at how quickly some of these patients turned around.
The application of the technology is quite broad, and this is definitely one of the advantages. When it comes to patients with significant infection, this modality helps. The oxygen is toxic to bacteria; we have evidence from studies that infection is better with these patients receiving topical oxygen therapy in terms of wound cultures.
This is an adjunctive technique to what you are already doing; you’re already assessing the arteries, veins, local wound care, infection, etc. This modality adds to what you’re already doing, and augments the healing. It doesn’t change what you’re doing. You can leave it over almost any type of dressing, even those that are all the way up to the knee.
they are in the intensive care unit, etc. Any level of amputation is terrible for the patient, of course, but for the upper extremities the prosthetic technology has not really caught up, and these patients have a much more difficult time than those with lower extremity amputations.
Another group of patients would be those who suffer from Raynaud’s syndrome, a very painful condition that can result in wounds. This modality would help them, particularly in the winter. These are patients that we sometimes bring into the hospital for IV treatment for five days, but this would be a much preferable treatment, especially in an outpatient setting at home.
There are a wide variety of suitable patients. I think that the key in using topical oxygen is the need for a committed patient, so that if they are using it in the outpatient setting, they are doing so consistently and you can ensure that the therapy is being done daily, or as prescribed. You have to choose the patient appropriately to ensure the best outcome.



An excellent advantage in using this modality is the rapid pain reduction. This has been the most striking thing I have noticed about this therapy in addition to the obvious healing benefits. The patients have really been so thrilled about this; I really cannot emphasise enough how badly pain impacts quality of life, and if we can get patients off pain medication, this is obviously a benefit.
What Patients Do You Use This Modality On?
We use this modality on lower extremity wounds, and I have also used it on upper extremity wounds to help with digital ischemia for patients who I cannot bring to the hyperbaric chamber because
What Patients Do You Use This Modality On?
One of the great secondary effects of topical oxygen therapy, aside from wound healing, is that it shows a marked reduction in pain. We have had a really
MasterSeries 60 Minute Interactive: Topical Oxygen Therapy Wound Masterclass - Vol 2 - March 2023 119
“An excellent advantage in using this modality is the rapid pain reduction. This has been the most striking thing I have noticed about this therapy in addition to the obvious healing benefits.”
Global expert
Ms Kara Couch
Director, Wound Care Services at The George Washington University Hospital Arlington VA, United States
Global expert
Dr Paul Haser Vascular Surgeon New York City NY, United States
Global expert
Dr Anil Hingorani Vascular Surgeon
New York City NY, United States
© Copyright. Wound Masterclass. 2023
good compliance with the patients doing this; firstly, it is very easy to use, it’s really quite comfortable for most patients, partly because with this modality the patients often find that their wound hurts a lot less.
We consider topical oxygen therapy in diabetic patients, or those who otherwise have nonrevascularizable ischemic issues. Right now, we are limited in terms of insurance reimbursement in the U.S. but I do think at some point down the road we may decide to start this therapy immediately as a very useful adjunct to what we are already doing.

See all Wound Masterclass MasterSeries on demand: bigmarker.com/wound-masterclass
References
1. Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes; Advances in Wound Care; 2021
2. A Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers; The TWO2 Study; Diabetes Care 2020;43:616-624 | https://doi.org/10.2337/dc19-0476.
MasterSeries 60 Minute Interactive: Topical Oxygen Therapy 120 Wound Masterclass - Vol 2 - March 2023 Sponsored By AOTI. All production resources provided by AOTI. © Copyright. Wound Masterclass. 2023
Evidence Based Therapy:

Demonstrated in both Randomized Controlled Trial and Real World Evidence to offer superior healing for Diabetic Foot Ulcers (DFUs).
A Unique Delivery System:
Targets multiple aspects of wound healing with OXYGEN, CYCLICAL COMPRESSION and HUMIDIFICATION

A Game Changer for Patients and Clinicians: Drives compliance with a self-administered, at-home therapy and overcomes traditional healthcare barriers. TWO2 can be used with gas permeable dressings, CCD, UNNA Boot and TCC.
6X 6X
88% 71% DELIVERING EXCEPTIONAL OUTCOMES
MORE LIKELY TO HEAL DFUs in 12 weeks
LOWER RECURRENCE rate at 12 months
REDUCTION in Hospitalizations at 12 months
REDUCTION in Amputations at 12 months
REAL WORLD EVIDENCE STUDY RANDOMIZED CONTROLLED TRIAL
A seamless addition to your care plan.
Visit www.AOTInc.net

for
more.
research articles, patient and physician testimonials – and
O2
REFERENCES: - A Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers; Robert G. Frykberg, Peter J. Franks, et al. The TWO2 Study; Diabetes Care 2020;43:616-624 | https://doi.org/10.2337/dc19-0476. - Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes; Advances in Wound Care; 2021
PROVEN SUSTAINED HEALING
MasterSeries 60 Minutes Interactive




Surgical Site Infection (SSI): All Your Questions Answered
This is a really important topic and for this MasterSeries on SSI we have an esteemed global panel, including:
Introduction
Surgical site infection (SSI) presents a real challenge for healthcare systems across the world, but they also have a substantial impact on patients. Statistics have shown that about 60% of these surgical site infections are actually completely preventable; despite that, the rates are still high. Each patient undergoing a surgical procedure has a 5% chance of developing an SSI, which is a considerable number.
Relating to SSI, NHS costs are between £10,000 and £100,000 per patient, and it is also associated with extended hospital stays, delays in going back to work and returning to normal life for the patient.

On another note, SSI is caused by bacteria/ microorganisms that get into the body through the incisions that we make in surgery, and treating them involves use of antibiotics. This has contributed a lot to the spread of antibiotic resistance, particularly in low and middle income countries; about 11% of patients in these countries are infected, and in Africa as a whole, one in five women who have a cesarean section end up having an SSI.
Dr Jonathan Johnson, the founder and surgical director of the comprehensive wound care service in Washington DC; Dr Mark Melin, the Medical Director of the M Health Wound Healing Institute in Fairview, Minnesota, United States; Dr Hüseyin Kemal Raşa, the European president of the Surgical Infections Society; Dr Windy Cole, Director of Wound Care Research, Kent State University of Podiatric Medicine; and Dr Michael Magro consultant obstetrician and gynaecologist in London.
Global expert Dr Jonathan Johnson Surgical Director, Comprehensive Wound Care Services Washington DC, United States


Initial Management of the Surgical Infection
Being a surgeon and working on wounds for multiple years, I can say one of the most important aspects we look at as far as SSI’s are concerned is making sure that:
• We adequately close the wound
• We make sure we have a post-op review of the wound

122 Wound Masterclass - Vol 2 - March 2023
Moderator Miss Negin Shamsian Consultant Plastic and Reconstructive Surgeon (Locum) London, United Kingdom
© Copyright. Wound Masterclass. 2023 woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register Join global wound care experts to answer all your questions about Surgical Site Infection (SSI) Concise. Interactive. Accredited. Supported by MasterSeries 60 Minutes Interactive December 13th 2022: 1pm EST | 6pm GMT
Supported by
If the patient needs antibiotics, start them on antibiotics and if you do see any signs and symptoms of SSI; erythema in duration, active purulent drainage, etc., you have to treat it very aggressively.
A post-op surgical review and post-op surgical followup is key; with an outpatient patient we want to make sure they come back within at least three to five days for a review.
What Do You Do Pre-Operatively to Minimize the Risk of SSI?
Firstly, you must have great communication with your patient, so they understand that this is a team-based approach; pre-operative instructions are a vital part of controlling and minimizing SSI’s. Another important thing to make sure they are taking their pre-operative antibiotics, and they make sure that they are adequately cleaning the area prior to surgery and not scrubbing the skin. After that, everything should work in an active process; firstly, prep the patient in the operating room and explain all education issues prior to surgery; secondly, in the operating room or at the bedside before starting the procedure, we need to have a timeout and make sure that everyone involved understands what their role is, like prep of the area, explaining to the patient what is going on and what the procedure is, making a clean line incision, and making sure throughout the process to extensively close everything. Another important thing is to make sure there is adequate post-op dressing on the scar.
Before making the incisions, its important to note who is at a higher risk for developing SSI, and patients with diabetes, problems with coagulopathy, ischemic problems, or comorbidities are at higher risk. In addition, patients who have had surgeries near the site of the current surgery or the same surgical incision are at a higher risk, as sometimes patients have adverse effects in the same area. Patients with autoimmune issues and nutritional defects need to be adequately prepped prior to performing the procedure.
What is your Go-To Surgical Prep?
Prepping the wound depends on whether the patient has acute or increased colonization or critical colonization, as it is required to remove the biofilm as much as possible for a clean wound bed, prior to grafting or any type of surgical debridement. If there is increased colonization at the site, then it must be removed, depending on what is available and the patient’s body and how it reacts. It’s important to remove the biofilm as much as possible; we use a device which looks at the fluorescence of the wound, at the increased amount of bio-burden and the increase in the amount of bacteria at the wound site prior to debriding, but then also after debriding to make sure we removed as much of it as possible; it’s a specific protocol that depends specifically on the patient.
How Do You Identify SSI Early in Your Patients, In Person and Remotely?
Telehealth has newly emerged within the last couple of years due to the pandemic; it’s a great tool to use for surveillance but also to monitor our patients. The number one thing to look for or to tell the patient or patient’s caregiver, is to look for:
• Redness or erythema around the wound site
• Induration/ thickness or hardness around the wound site
• Active purulent drainage or decolourization coming from the wound site
• Warmth or pain at the wound site
If the surgical site has sutures or staples that are intact, this could contribute to the wound being infected, causing pain or erythema; its best to seek medical attention as quickly as possible.
MasterSeries 60 Minute Interactive: Surgical Site Infection (SSI) Wound Masterclass - Vol 2 - March 2023 123 © Copyright. Wound Masterclass. 2023
“You must have great communication with your patient, so they understand that this is a team-based approach; pre-operative instructions are a vital part of controlling and minimizing SSI’s.”
What Group of Patients May Not Present Typical Symptoms?
Patients with autoimmune issues, diabetes, and extensive comorbidities may not show active signs and symptoms of infections acutely, compared to patients that do not have these comorbidities. It’s very important to make sure patients with comorbidities, specifically in patients that are diabetic, that you’re regularly checking on the wound, discussing any issues with changes of the skin, and any of the previously mentioned signs or symptoms. One of the other major things that we need to focus on is patients that have darker skin tones, and this goes back to a lot of research and looking at the bacterial burden and fluorescence at the wound site in patients that have darker skin. If we look at what we call the Fitzpatrick scale, we have FP1 all through all the way through to FP6; typically patients that are FP1 are fair skinned and they typically burn and tan, and then we have patients that are FP6; typically darker skinned and they don’t burn or tan, but the signs and symptoms of erythema in duration, pain and changes in the skin warmth and temperature may not be apparent in these patients. Typically these are your patients that are FP4, FP5, and FP6.
We did a study utilizing bacterial fluorescent imaging because with this device we can see the increased amount of that bacterial burden and fluorescence in the patients that may not have what we call active clinical signs and symptoms through simple observation.
So, this group of patients that may not present the typical signs and symptoms are patients with comorbidities and patients with darker skin tones.
How Do You Do An Initial Management Of Somebody With An SSI?
One thing we do first of all is to observe the wound; take a look at the wound, determine the amount of erythema, determine the amount of induration; determine the amount of active purulent or abnormal colour of exudate at the wound site. Observe, talk
to your patient; is there increased pain? Is there increased temperature at the wound site?
We want to look at whether there are foreign bodies at the wound site; does the patient have staples, does the patient have sutures; are there implants at the site?
We want to make sure we can decrease the infection as quickly as possible and to make sure that the patient improves quickly. Another issue we should look at is whether the patient is post-op, and if they have active drainage; for example, if they have active drainage from a total hip or a total knee arthroplasty, then we should put a dressing over the top of the site to see if we can decrease the amount of infection actively and topically before you start the antibiotics. Sometimes in long-term care if the patient does have active infection and drainage from the wound site we will use a silver alginate dressing or a product that has a silver component.
Are There Any Adjuncts To Clinical Skills That You Use As An Initial Point Of Investigation?
We typically focus on utilizing bacterial fluorescence technology, but there is a role for culturing the wound and obviously biopsying of the wound, if the wound is not progressing after you’ve initiated the standard of care management; we do utilize these if the wound continues to decline after we’ve used POA antibiotics, or after we’ve used topical antibiotics, but typically that is what we call a ‘game time decision’, or a ‘wound time decision’ (if I may put this into the lexicon) because it just depends on how the patient reacts postoperatively; if you do need to utilize the culture or the biopsy it is an option available to us, but if not, what we should focus on is standard of care.
MasterSeries 60 Minute Interactive:
124 Wound Masterclass - Vol 2 - March 2023 © Copyright. Wound Masterclass. 2023
“Patients that may not present the typical signs and symptoms are patients with comorbidities and patients with darker skin tones.”
Surgical Site Infection (SSI)
“The idea of wound hygiene has been taking hold in the wound care space, so we should consider wound hygiene post-operatively, too. Currently, most hospitals are not doing a good job with treating post-operative sites to prevent biofilm formation.”
• Practicing aseptic techniques
• Screening our patients; making certain that we understand our patients, their comorbidities, and their underlying conditions
The Role of Biofilm
Biofilm is a colony of polymicrobials; multiple organisms, bacteria, fungus, yeast, etc., that form on the wound surface. Planktonic bacteria are free floating bacteria that will implant on the surface of a wound or a surgical incision and then they start to connect with one another. They do something we call quorum sensing; they start to share information. They may share DNA or RNA; they could share antibiotic resistance, and they start to mutate, and begin to secrete a kind of extra polymeric substance. This substance forms a micro-dome that protects the biofilm. Interestingly, the organisms within a biofilm are not affected by oral and IV antibiotics and some topical antibiotics or antimicrobials have difficulty penetrating through the biofilm construct because of this extra polymeric substance, so these wounds initially are not necessarily infected, but they are contaminated with surface bacteria. The number of complexities of the microbes that are involved in this biofilm on the surface increases the patient’s risk of developing a wound infection. We also know that the presence of biofilm will cause inflammation, and cause wounds to be chronic, so if we have biofilms that are in the area of a surgical incision, and we allow them to develop, if we don’t treat them appropriately we can get an SSI.
We can also get inflammation that continues, and these wounds won’t heal; this becomes a particularly problematic situation for a lot of our patients postoperatively, so we all must practice strategies to prevent infection and SSI. Some of the strategies that are most commonly known in practice are these six:
• Hygiene; washing our hands frequently
• Environmental hygiene; cleaning our spaces
• Surveillance; closely monitoring our patients, especially post-operatively

• Practicing antibiotic stewardship
Associated SSI must not be neglected; this is the main cause of most hospital acquired infections, and the development of SSI is typically due to the microbial contamination of the surgical site. This is either from endogenous or exogenous sources, although most commonly it is the endogenous with superficial site infection, but they can go into deeper tissues. This causes severe patient outcomes with the infection affecting underlying organisms, organs, and then leading to more surgical procedures that are necessary and long term, amputations.
The idea of wound hygiene has been taking hold in the wound care space, so we should consider wound hygiene post-operatively, too. Currently, most hospitals are not doing a good job with treating postoperative sites to prevent biofilm formation.
Choosing the appropriate dressing is vital; we potentially manage, control, and remove the exudate from the incision site. There are newer bandages that have entered the market which also have antimicrobial properties that can assist in wound hygiene, hopefully prevent biofilm bacteria from reforming, and help to control any kind of SSI.
Antimicrobial stewardship is very important as the world is facing a crisis because we have a rising rate of bacterial resistance, due to our overuse of antibiotic agents. The bacterial resistance is directly related to overuse; most patients when they go to their primary care doctors will be prescribed antibiotics, and the statistics say that 90% of antibiotics prescriptions are unnecessary. Prior treatment with commonly used antibiotics actually increases the risk of infection in
MasterSeries 60 Minute Interactive: Surgical Site Infection (SSI) Wound Masterclass - Vol 2 - March 2023 125
Global expert Dr Windy Cole
© Copyright. Wound Masterclass. 2023
Director of Wound Care Research, Kent State University of Podiatric Medicine Streetsboro OH, United States
patients, which also increases the risk of morbidity, hospital stays, and an overall increase in health care costs and mortality. So, we must practice better antimicrobial stewardship which helps support better clinical care, outcomes and lowers healthcare costs.
The first step we must follow is to avoid antibiotics when they’re not indicated, which means wound infection should be diagnosed clinically, and there must be signs and symptoms of infection. Just because there is a wound, and they’re mostly colonized, this does not indicate the need to prescribe antibiotics, although antimicrobials, surface topical agents, dressings, might be useful in some of these patients, we’re going to prescribe the appropriate regimen to narrow a spectrum for the likely bacteria present. Culture results are important to treat the most common or frequent pathogens, as it is unnecessary to treat low virulence bacteria, and antibiotics should be used for the correct duration, just long enough to achieve symptom resolution. Patients should be treated with topical therapy and usually for about one to two weeks maximum for soft tissue infection, and six weeks for bone infection. Choosing the agent that shows the least risk and best outcomes to avoid adverse reactions to patients is important.
There are many antimicrobial agents out there, for example; silver products, hypochlorous acid solutions, surfactant agents that are antimicrobial, and many new innovative dressings on the market.
temperature management is critical. Telehealth management has its place because we can fit patients in if they’re concerned about their incisions, but it can also complicate making a diagnosis. I would encourage everybody that if there’s ever a question which makes you suspect SSI in a Telehealth interview, bring the patient in for a physical examination and bring that patient in soon.
For signs and symptoms, we’ve all been taught in our medical training to listen to the patient, ask the patient the symptoms; do they have fevers, chills? Are there cognitive changes? Sometimes getting a history from a family member can be very important, and when we’re looking at the wound we’re looking for redness, increasing pain, increasing drainage, or change in the drainage.
In accurately identifying SSI, we have to be aware of the atypicals. In the immunosupressed patient, the elderly, or those with cognitive disorders, SSI may present in a relatively atypical fashion; having a high degree of suspicion is so important as clinicians.
Nutrition
Top Tips for Identifying Surgical Infection

Early identification of suspected SSI is critical; this is going to be based upon pre-operative risk factor assessment, interoperative factors, such as length of procedure, complications, transfusions, and of course perioperative and interoperative body
To emphasize prevention, one of the least talked about components with pre-operative management of patients is nutrition. I think albumin has gotten a little bit of a bad reputation in terms of being a marker for nutrition, and it may actually not be as good a marker for nutrition, as it’s a more important marker for degree of vascular hyperpermeability. The albumin is a significant component of something called the endothelial glycocalyx. It helps with permeability, much like Gore-Tex within a rain jacket. As a constituent of the endothelial glcocalyx, when albumin is low there’s increased miscrovascular hyperpermeabilty which results in increased tissue edema, which can compromise microvascular arterial perfusion at the five micron level, where all oxygen and nutrient delivery truly occurs. I think we need to start thinking about albumin not as a true marker of nutrition but as a marker of endothelial cell health, and helping ultimately with oxygen nutrient delivery, indirectly, to the incisions that
MasterSeries 60 Minute Interactive: Surgical Site Infection (SSI) 126 Wound Masterclass - Vol 2 - March 2023
“To emphasize prevention, one of the least talked about components with preoperative management of patients is nutrition.”
Global expert Dr M. Mark Melin
Medical Director of the M Health Wound Healing Institute
Mineapolis MN, United States
© Copyright. Wound Masterclass. 2023
by 45% when comparing a
we’re making in our patients and expect to heal. Also, consider control of perioperative hyperglycemia; almost counter-intuitively, preoperative carbohydrate loading in patients can actually help with postoperative hyperglycemia management. This has been recognised in multiple surgical guidelines, and I would counsel you to seek out those guidelines and talk to your nutritionist within the hospital about preoperative carbohydrate loading, which is now a very validated method for assisting in glycemic control perioperatively. The other important thing to focus on is micronutrients; within our wound clinic, we routinely use micronutrients, in addition to standard of care with protein, and the micronutrients we are using are all focusing on endothelial cell function. Vitamin D has an important role of emphasising microvascular functionality. In Minnesota we have a lot of vitamin D deficiency, and it’s not unusual for us to be using 3,000 - 5,000 international units of vitamin D on a daily basis. Vitamin C at 1000mg can help decrease inflammation, which will certainly help improve microvascular perfusion; vitamin B12, B6 and methylfolate can all be part of a methylenetetrahydrofolate reductase pathway that’s connected with homocysteine ultimately resulting in recoupling endothelial nitric oxide synthase to maximise nitric oxide production which results in vasodilation, lymphangion contractility, as well as immune functionality. Vitamin A, for patients who have recently been on steroids, helps with collagen production. Micronized Purified Flavonoids Fractions (MPFF) such as diosmin and hesperidin have also been shown to be a significant component in improving microvascular health; they are inflammation quenching, decreasing ICAM-1 and VCAM-1; they improve venous function and lymphangion functionality.
Conclusion
As professionals we really have to choose counsel wisely and appropriately, and choose the correct time and pre-operative intervention such as compression, edema reduction, and nutrition maximization to result in decreased and minimized post-operative complication risk.
Strategies for Reducing Post-Cesarean Section Infections

The National Institute for Clinical Excellence (NICE) produced guidelines in 2021 using randomized controlled trials (RCT) data which show that after cesarean section, if we used a dressed called Leukomed® Sorbact® we could reduce SSI’s and as a consequence of that, also save money. Leukomed® Sorbact® is a bacteria binding, sterile wound dressing. It can be applied for up to seven days, and not only does it act as a barrier for infection, it ‘sucks’ the bacteria away from the wound and traps it in the dressing so that the bacteria load at the surgical site is less. It is also waterproof, meaning that patients can shower with it on.
We have had a very good success story at the Barking, Havering and Redbridge University Hospitals in reducing SSI after cesarean section (CS), using Leukomed® Sorbact® dressings.
For us, the dressings were roughly £10 per person, and our previous dressings were much cheaper at £1 per person, so we wanted to see if we used a much more expensive wound dressing whether it would make a difference for us over time, and whether it would be a worthwhile investment.
Our SSI rate reduced by 45% when comparing a sixmonth period in using Leukomed® Sorbact®, to the same period previously using the old dressings. For the women that were readmitted, we reduced the days spent in hospital by 61%. Antibiotic prescriptions for women with post-cesarean SSI were reduced by 36%.
We had great improvements and are really pleased with these results, and this is something we will be
MasterSeries 60 Minute Interactive: Surgical Site Infection (SSI) Wound Masterclass - Vol 2 - March 2023 127
“Our SSI rate reduced
six-month period in using Leukomed® Sorbact®, to the same period previously using the old dressings.”
Global expert
Dr Michael Magro
© Copyright. Wound Masterclass. 2023
Consultant Obstetrician and Gynaecologist London, United Kingdom
continuing long term.
In terms of cost savings, when we looked at the number of SSIs that we had reduced in a six-month period, and considering the average costs involved with SSIs, we were able to save nearly £140,000, even with the additional cost of the wound dressings. It is important to note that this excludes the savings from reduced antibiotic prescription, so the real figure will be even higher.
Conclusion
There is a lot of scepticism when companies approach you with their product. I had the same attitude, which is why we didn’t implement this just based on the NICE guidelines, and we kept this audit data. We were very clear that if SSI rates didn’t improve in the sixmonth period, and save us money, we would go back to the old dressing.

I would urge clinicians to try this, even if it is just in a similar six-month period, and see if your SSI rates improve. It is a very simple thing to do, and at worst you will only spend a little more money.
Guidelines
There are many SSI prevention guidelines including those from the American College of Surgeons (ACS) - 2016, the World Health Organization (WHO) - 2016, modified in 2018 because of the hyperbaric oxygen recommendation, and the Centers for Disease Control and Prevention (CDC) - 2017.
I accept that these guidelines can be tiresome, but I have no doubt that with them we can prevent half of all SSI’s. There are strong consensuses across the guidelines, as well as some conflicts.
To summarise the strongest agreements between guidelines, we have five recommendations:
• Parenteral antimicrobial prophylaxis; this is the most significant recommendation in all these three guidelines
• Alcohol-based skin preparation
• Perioperative glycemic control
• Temperature regulation to normothermia
• Maintenance of normal tissue oxygenation
Implementation of Guidelines
When we compare SSI rates in developed countries to low and middle income countries, there is a huge difference; in low and middle income countries SSI is the most surveyed and most frequent type of infection, and incidence rates range from 1.2 and 23.6 per 100 surgical procedures, with a pooled incidence of 11.8%. If we consider the clinical outcomes of SSI, it leads to significant morbidity and mortality, and extended hospital stays. Patients who develop SSI have a 2 to 11 fold higher risk of death; 75% of deaths in patients with SSI are directly attributable to the SSI.
The remaining recommendations did not achieve unanimous agreement across these three guidelines. There are however strong considerations, such as smoking cessation, screening and decolonization; colorectal surgery bundles, perioperative surgical attire; operating room traffic control, operating room disinfection, and use of the WHO perioperative checklist. These have become cornerstones of SSI prevention.
To summarise some of the conflicts and controversies when it comes to SSI prevention, they include:
• Preoperative chlorhexidine gluconate bathing
• Local antibiotics
• Surgical wound irrigation
• Antiseptic drapes
• Antiseptic-impregnated sutures
• Advanced dressings
• Wound protectors
• Prophylactic negative pressure wound therapy
MasterSeries 60 Minute Interactive: Surgical Site Infection (SSI) 128 Wound Masterclass - Vol 2 - March 2023
“We were able to save nearly £140,000, even with the additional cost of the wound dressings.”
Global expert Dr Hüseyin Kemal Raşa Surgical Infections Society, European President Kocaeli, Turkey
© Copyright. Wound Masterclass. 2023
I believe most of these controversies will be cleared up over the next few years, and we will have a clearer consensus.
I am very proud to be part of the team that, in 2018, published the WHO implementation manual, designed to bring this dogmatic knowledge in the guidelines to the clinician and to the bedside.
We have an Inter-Process Communication (IPC) multimodal improvement strategy which involves five categories:
Build it: Training and education
What type of training should be used to ensure that the intervention will be implemented in line with evidence-based policies and how frequently?
Does the facility have trainers, training aids, and the necessary equipment?
Teach it: System change
What infrastructures, equipment, supplies and other resources (including human) are required to implement the intervention?
Does the physical environment influence health worker behaviour? How can ergonomics and human factor approaches facilitate adoption of the intervention?
Are certain types of health workers needed to implement the intervention?
Check it: Monitoring and feedback
How can you identify the gaps in IPC practices or other indicators in your setting to allow you to prioritize your intervention?
How can you be sure that the intervention is being implemented correctly and safely, including at the bedside? For example, are there methods in place to observe or track practices?
How and when will feedback be given to the target audience and managers? How can patients also be informed?
Sell it: Reminders and communications
How are you promoting an intervention to ensure that there are cues to action at the point of care and messages are reinforced to health workers and patients?
Do you have capacity/ funding to develop promotional messages and materials?
Live it: Culture change
Is there demonstrable support for the intervention at every level of the health system? For example, do senior managers provide funding for equipment and other resources? Are they willing to be the champions and role models for IPC improvement?
Are teams involved in co-developing or adapting the intervention? Are they empowered, and do they feel ownership and the need for accountability?
Take Home Messages
The prioritisation of feasible but high-impact pilots, such as SSI in intensive care units, will be a good place to start.
Slowly scaling-up, in a stepwise manner, and moving from paper to electronic forms is a good trick.
The mainstay of these strategies is multidisciplinary collaboration, and mentorship, such as yearly surveillance seminars, site support visits to assess case findings, and denominator data.
The integration of HAI and AMR surveillance efforts including a multidisciplinary working group and a master trained in surveillance that can provide leadership is also an important concept.
We definitely need careful consideration of definitions
MasterSeries 60 Minute Interactive: Surgical Site Infection (SSI) Wound Masterclass - Vol 2 - March 2023 129 © Copyright. Wound Masterclass. 2023
“I believe most of these controversies will be cleared up over the next few years, and we will have a clearer consensus.”
Surgical Site Infection (SSI)
before use; this is critical. We need to discuss factors such as the use of validated standards, consistency and feasibility of data collection. We must use the same terminology and definitions.
We also need clear procedures for data management and promotion of data for action.
In almost all success stories we have incorporated an evaluation strategy to monitor performance and provide feedback to frontline staff. Monitoring and feedback can heighten the sense of urgency, promote accountability, and show clinicians how they are performing.
References
57-62, February 2009. | DOI: 10.1097/MOL.0b013e328321b587
3. Aldecoa, C., Llau, J.V., Nuvials, X. et al. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann. Intensive Care 10, 85 (2020). https://doi.org/10.1186/s13613-020-00697-1
4. Yuwen, P., Chen, W., Lv, H. et al. Albumin and surgical site infection risk in orthopaedics: a meta-analysis. BMC Surg 17, 7 (2017). https://doi.org/10.1186/ s12893-016-0186-6
5. D. G. Armstrong, J. R. Hanft, V. R. Driver, A. P. S. Smith, J. L. Lazaro-Martinez, A. M. Reyzelman, G. J. Furst, D. J. Vayser, H. L. Cervantes, R. J. Snyder, M. F. Moore, P. E. May, J. L. Nelson, G. E. Baggs, A. C. Voss, on behalf of the Diabetic Foot Nutrition Study Group
6. Chronic Wound Care Management and Research 2015:2 129-136. International Journal of Cell Biology, Volume 2015, Article ID 701738
7. Summers S, Yakkanti R, Ocksrider J, Haziza S, Mannino A, Roche M, Hernandez
VH. Effects of Venous Insufficiency in Patients Undergoing Primary Total Knee Arthroplasty: An Analysis of 1.2 Million Patients. J Knee Surg. 2021 Aug 31. doi:
10.1055/s-0041-1733901. Epub ahead of print. PMID: 34464986.
8. Carmichael J, Dennis D, Jennings J, Stevens-Lapsley J, Bade M. Feasibility and initial efficacy of a multimodal swelling intervention after total knee arthroplasty: A prospective pilot study with historical controls. Knee. 2022 Mar;35:25-33. doi:
10.1016/j.knee.2022.01.008. Epub 2022 Feb 17. PMID: 35183923.
9. Summers S, Yakkanti R, Haziza S, Vakharia R, Roche MW, Hernandez VH. Nationwide analysis on the impact of peripheral vascular disease following primary total knee arthroplasty: A matched-control analysis. Knee. 2021 Aug;31:158-163. doi: 10.1016/j.knee.2021.06.004. Epub 2021 Jun 29. PMID: 34214955.
10. Singh TP, Velu RB, Quigley F, Golledge J. Association of chronic venous disease with major adverse cardiovascular events. J Vasc Surg Venous Lymphat Disord. 2022 May;10(3):683-688. doi: 10.1016/j.jvsv.2021.08.021. Epub 2021 Sep 8. PMID: 34506962.
11. Patel M, Lee SI, Akyea RK, Grindlay D, Francis N, Levell NJ, Smart P, Kai J, Thomas KS. A systematic review showing the lack of diagnostic criteria and tools developed for lower-limb cellulitis. Br J Dermatol. 2019 Dec;181(6):1156-1165. doi: 10.1111/ bjd.17857. Epub 2019 Jun 28. PMID: 30844076; PMCID: PMC6916392.
12. MacLeod BG, Klarich CS, Wessman LL, Gaddis KJ, Konstantinov NK, Wubben A, Melin MM. Fluorescent Imaging as a Component of Diagnosing Pyoderma Gangrenosum: A Case Report. Adv Skin Wound Care. 2022 Jun 1;35(6):1-6. doi: 10.1097/01.ASW.0000820248.26138.bc. Epub 2022 Mar 29. PMID: 35426849.
13. Ehmann S, Walker KJ, Bailey CM, DesJardins JD. Experimental Simulation Study to Assess Pressure Distribution of Different Compression Applications Applied Over an Innovative Primary Wound Dressing. Wounds. 2020 Dec;32(12):353-363. Epub 2020 Aug 17. PMID: 33370244.
14. Anatone AJ, Shah RP, Jennings EL, Geller JA, Cooper HJ. A risk-stratification algorithm to reduce superficial surgical site complications in primary hip and knee arthroplasty. Arthroplast Today. 2018 Oct 15;4(4):493-498. doi: 10.1016/j. artd.2018.09.004. PMID: 30560182; PMCID: PMC6287286.
15. Kwon J, Staley C, McCullough M, Goss S, Arosemena M, Abai B, Salvatore D, Reiter D, DiMuzio P. A randomized clinical trial evaluating negative pressure therapy to decrease vascular groin incision complications. J Vasc Surg. 2018 Dec;68(6):17441752. doi: 10.1016/j.jvs.2018.05.224. Epub 2018 Aug 17. PMID: 30126781.
16. Report on the Burden of endemic health care- associated infection worldwide. Geneva: World Health Organization; 2011 (http://www.who.int/infectionprevention/publications/burden_hcai/en/)
17. Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta- analysis. Lancet. 2011; 377:228–41
18. Bagheri Nejad S, Allegranzi B, Syed SB, Ellis B, Pittet
D. Health care-associated infection in Africa: a systematic review. Bull World Health Organ. 2011; 89:757–65.
19. Monahan M, Jowett S, Pinkney T, Brocklehurst P, Morton DG, Abdali Z, Roberts TE. Surgical site infection and costs in low- and middle-income countries: A systematic review of the economic burden. PLoS One. 2020 Jun 4;15(6):e0232960. doi: 10.1371/ journal.pone.0232960. PMID: 32497086; PMCID: PMC7272045.
20. Liu Y, Xiao W, Wang S, Chan CWH. Evaluating the direct economic burden of health care-associated infections among patients with colorectal cancer surgery in China. Am J Infect Control. 2018 Jan;46(1):34-38. doi: 10.1016/j.ajic.2017.08.003. Epub
2017 Sep 28. PMID: 28967510.
21. Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59-74.
22. Global Guidelines for the Prevention of Surgical Site Infection. WHO; 2018.
23. Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784-791. doi:10.1001/jamasurg.2017.0904
24. Fields AC, Pradarelli JC, Itani KMF. JAMA. 2020 Mar 17;323(11):1087-1088.

25. Schmitt C, Lacerda RA, Turrini RNT, Padoveze MC. Improving compliance with surgical antibiotic prophylaxis guidelines: A multicenter evaluation. Am J Infect Control. 2017 Oct 1;45(10):1111-1115. doi: 10.1016/j.ajic.2017.05.004. Epub 2017 Jun
16. PMID: 28629754.
26. Allen J, David M, Veerman JL. Systematic review of the cost-effectiveness of preoperative antibiotic prophylaxis in reducing surgical-site infection. BJS Open. 2018 Apr 14;2(3):81-98. doi: 10.1002/bjs5.45. PMID: 29951632; PMCID: PMC5989978.
27. Ho YH, Wang YC, Loh EW, Tam KW. Antiseptic efficacies of waterless hand rub, chlorhexidine scrub, and povidone-iodine scrub in surgical settings: a meta-analysis of randomized controlled trials. J Hosp Infect. 2019 Apr;101(4):370-379. doi:
10.1016/j.jhin.2018.11.012. Epub 2018 Nov 28. PMID: 30500384.
28. Nthumba PM, Stepita-Poenaru E, Poenaru D, Bird P, Allegranzi B, Pittet D, Harbarth S. Cluster-randomized, crossover trial of the efficacy of plain soap and water versus alcohol-based rub for surgical hand preparation in a rural hospital in Kenya. Br J Surg. 2010 Nov;97(11):1621-8. doi: 10.1002/bjs.7213. PMID: 20878941.
29. Mehtar S, Wanyoro A, Ogunsola F, Ameh EA, Nthumba P, Kilpatrick C, Revathi G, Antoniadou A, Giamarelou H, Apisarnthanarak A, Ramatowski JW, Rosenthal VD, Storr J, Osman TS, Solomkin JS. Implementation of surgical site infection surveillance in low- and middle-income countries: A position statement for the International Society for Infectious Diseases. Int J Infect Dis. 2020 Nov;100:123-131. doi: 10.1016/j. ijid.2020.07.021. Epub 2020 Jul 24. PMID: 32712427; PMCID: PMC7378004.
30. Ariyo P, Zayed B, Riese V, Anton B, Latif A, Kilpatrick C, Allegranzi B, Berenholtz S. Implementation strategies to reduce surgical site infections: A systematic review. Infect Control Hosp Epidemiol. 2019 Mar;40(3):287-300. doi: 10.1017/ice.2018.355. Epub 2019 Feb 21. PMID: 30786946. 31. Rasa K, Kilpatrick C. Implementation of World Health Organization Guidelines in the Prevention of Surgical Site Infection in Low- and Middle-Income Countries:What We Know and Do Not Know. Surg Infect (Larchmt). 2020 Sep;21(7):592-598. doi: 10.1089/sur.2020.163. Epub 2020 Jun 1. PMID: 32478641.
MasterSeries 60 Minute
130 Wound Masterclass - Vol 2 - March 2023
Interactive:
1. Aslam MS, Ahmad MS, Riaz H, Raza SA, Hussain S, Qureshi OS, et al. Role of Flavonoids as Wound Healing Agent. Phytochemicals - Source of Antioxidants and Role in Disease Prevention [Internet]. 2018 Nov 7; Available from: http://dx.doi. org/10.5772/intechopen.79179
2. Broekhuizen, Lysette Na; Mooij, Hans La; Kastelein, John JPa; Stroes, Erik SGa; Vink, Hansa,c; Nieuwdorp, Maxa,b. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Current Opinion in Lipidology 20(1):p
Sponsored By Essity. All production resources provided by Essity. © Copyright. Wound Masterclass. 2023 See all Wound Masterclass MasterSeries on demand: bigmarker.com/wound-masterclass
Leukoplast®
Leukomed® Sorbact®

SSIs are the third most commonly reported type of healthcare-acquired infection and the most costly¹. They place a significant impact on patient welfare² as well as presenting a heavy financial burden for the NHS³.
New NICE Medical Technologies Guidance
New NICE medical technologies guidance recommends the use of Leukomed® Sorbact® for prevention of surgical site infection (SSI) in wounds with low to moderate exudate after caesarean section and vascular surgery.

To view please go to https://www.nice.org.uk/guidance/mtg55
The guidance states that Leukomed Sorbact:
• reduces SSI in caesarean section and vascular surgery
• may reduce antibiotic use
• may reduce readmissions from wound complications
For more information, please contact support.leukomedsorbact@leukoplast.com
Leukoplast® and Leukomed® are registered trademarks of BSN medical Gmbh. Sorbact® is a registered trademark of ABIGO Medical AB. © NICE 2021 Leukomed Sorbact for preventing surgical site infection. Available from https://www.nice.org.uk/guidance/mtg55/ All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this publication.
SSI prevention: now in your hands
1. Wounds UK (2020) Best Practice Statement: Post-operative wound care – reducing the risk of surgical site infection. Wounds UK, LondonCcc 2.
L,
E,
S, Seckam A (2020) Reducing SSI rates for women
by
J Community Nurs 34(3): 50–3
financial
elimination
SSI
Infect 2014; 86(1):24–33.
5813/421
Taylor
Mills
George
birthing
caesarean section.
3. Jenks PJ, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection (SSI) and predicted
consequences of
of
from an English hospital. J Hosp
https://doi.org/10.1016/j.jhin.2013.09.012


For more information contact info@omni-stat.com visit www.omni-stat.com 1. In Vitro and In Vivo Data on file at Omni-stat Medical Inc. 2. Millner R, Lockhart AS, Marr R.Chitosan arrests bleeding in major hepatic injuries with clotting dysfunction: an in vivo experimental study in a model of hepatic injury in the presence of moderate systemic heparinisation.Ann R Coll Surg Engl 2010; 92(7):559-561 3. Snyder RJ, Sigal BD.The importance of hemostasis in chronic wound care: an open-label controlled clinical study of OMNI-STAT (chitosan) versus standard of care in post-debridement treatment of patients with chronic wounds with or without concomitant use of anticoagulants.Wound Care Hyperb Oxygen 2013; 4(2):9-16 MTO.21.110 STOP THE BLEEDING START THE HEALING Rapid bleeding control for surgical and non surgical wounds A fast acting, safe and easy to use temporary topical external hemostat Controls minor, moderate and severe bleeding1 Stops bleeding in as little as 1 minute1 Effective even in the presence of common anticoagulants and clotting dysfunction2 Does not damage healthy tissue3 R Powered by Celox™ Technology




















































































 Mr Bernard Ross
Mr Bernard Ross
 CEO of Sky Medical Technology
CEO of Sky Medical Technology























 Dr Peter Gloviczki
Dr Peter Gloviczki


















































































































 Dr Marcus F. Yarbrough Alpha Wound Care Solutions and Wellness
Dr Marcus F. Yarbrough Alpha Wound Care Solutions and Wellness










































































































































