DRIVING
FUNCTIONAL
AN EXPANDING VIEW OF ATAXIA
NEW

When future generations look back to take stock of our era, there will be many foundational moments to consider for the front page headline. But I’m convinced that “the age of understanding and treating brain disease” will be on their shortlist of candidates. Brain science, whether it’s foundational research or clinical therapies, is at a pivotal moment. There is a palpable sense when surveying the breadth of brain disorders and diseases that we are beginning to have the tools and the understanding to win real ground. And the progress happening every day in treating formerly intractable diseases bolsters the overall mood of optimism. This publication is intended to do just that, to share the O’Donnell Brain Institute’s sense of optimism at this specific moment and some of our reasons for it.
If you’re taking the time to read this publication, it’s likely because you’re in the small group of people poised to make a real difference in the current explosion of brain research and clinical treatment. We know your time is incredibly valuable, both to you and to the world that depends on the work you’re doing now, so we’ve endeavored to make this piece as informative and powerful as it is brief.
My tenure here at the Peter O’Donnell Jr. Brain Institute at UT Southwestern has been the profound honor of my career. I’m regularly humbled by the dedication and insight of my colleagues. That dedication and insight is what this publication is about. We have chosen to highlight some of the recent scientific and human stories that most exemplify the spirit of the age of discovery we’re currently spearheading.
We’re not the only brain institute that combines foundational scientific research with clinical care. But the degree to which we’ve structured our program around that collaboration sets the O’Donnell Brain Institute apart in our field. I’ve witnessed a collaboration between a laboratory scientist and a neurosurgeon which lead to the placement of a rapid tissue freezer in the operating room – so that they could better study unique human brain samples – enabling unprecedented insight into the structural basis of abnormal protein accumulation in neurodegeneration. I’ve seen the growth of our roster of incredibly talented clinicians and laboratory and clinical scientists as they learn that this is the place where unique opportunities and partnerships are changing the future of brain science and care.
The stories that follow are the stories that are changing how the world understands and treats the brain. Our vision is that together, our work will bring about an end to countless brain diseases sooner than anyone a short time ago would have imagined possible.
Thank you,
William T. Dauer, M.D. Director of the Peter O’Donnell Jr. Brain Institute
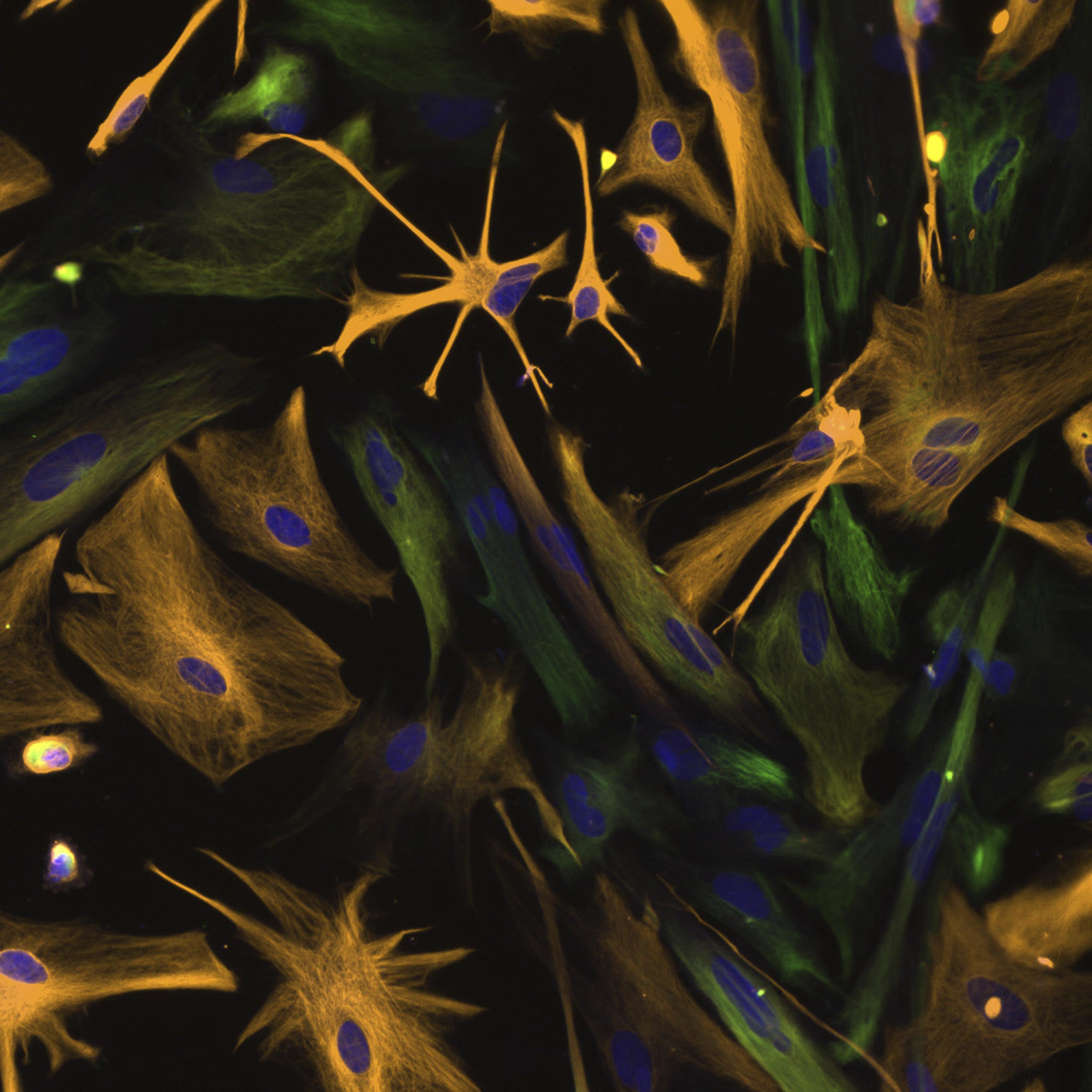
With its broad array of scientific talent, UT Southwestern’s Center for Alzheimer’s and Neurodegenerative Diseases is bringing exciting new advances to the study of Alzheimer’s disease and related disorders.
UT Southwestern’s Center for Alzheimer’s and Neurodegenerative Diseases (CAND) was launched in 2014 with a unique mission to develop mechanism-based approaches to diagnose and treat Alzheimer’s disease and related disorders.
Part of the Peter O’Donnell Jr. Brain Institute (OBI) at UT Southwestern Medical Center, CAND exemplifies OBI’s ideal of uniting scientists with diverse interests around the common goal of curing brain disorders. The center’s founding director is Marc Diamond, M.D., a Professor in the Department of Neurology, whose vision has been to recruit a diverse and collaborative group of scientists focused on fulfilling that primary mission.
“An important aspect of the center is how it integrates basic science with clinical knowledge,” Dr. Diamond says. “CAND scientists work closely with clinicianinvestigators at UT Southwestern and beyond to study human samples and to bring new approaches to the clinic for diagnosis and treatment.”
“We are beginning to understand how a single tau monomer changes shape to form a pathological monomer and then a pathological aggregate.”LUKASZ JOACHIMIAK, PH.D. Associate Professor of the Center for Alzheimer’s and Neurodegenerative Diseases
The study of neurodegenerative diseases has evolved from describing manifestations of abnormal brain function and neuropathology to understanding molecular mechanisms. Today, it is well known that many seemingly disparate neurodegenerative disorders share a common etiology based in abnormal protein folding.
“The vast majority of these diseases currently have no effective treatments, although the last few years have seen amazing breakthroughs with new FDAapproved drugs,” Dr. Diamond says. “It is for this reason that we continue to foster new collaborative research across various disciplines – we are trying to add substantially to the limited treatments currently available.”
The center’s active group is made up of eight principal investigators and five affiliated faculty members who have farreaching and diverse interests, ranging from machine learning, structural biology, and biochemistry to advanced imaging and interventions using highintensity focused ultrasound.
Now approaching nearly a decade of operation, the CAND continues to be a destination for talented faculty with complementary skills. Barbara Stopschinski, M.D., joined the faculty in July 2023 as an instructor and fellow in behavioral neurology. Her lab studies the molecular and cellular mechanisms driving tau uptake and propagation, and the mechanisms connecting neuroinflammation and neurodegeneration in tauopathies.
In keeping with the OBI mission, her research is directly tied to her clinical practice, where she treats patients with cognitive impairment and dementia.
“Our approach is unique in that we truly operate as a multidisciplinary team,” Dr. Diamond says. “In traditional academic environments, collaborations are expected to evolve organically, but here we cultivate a much more intentional environment by organizing weekly meetings, faculty events, and even dinners to discuss our research.”
As discoveries in the field continue to accelerate, Dr. Diamond and his team recognize that understanding the interplay of non-neuronal systems in relation to the changes that occur within the brain is essential. Consequently, they’ve started to focus on the immune system and its components –disentangling the shared dysfunctions that occur across the spectrum of neurodegenerative disorders.
“A substantial portion of the center’s prior work has been devoted to structural biology and biochemistry, with the aim of utilizing that knowledge to target proteins that assume a pathological conformation,” Dr. Diamond says.
Building upon this foundation, an expanding direction for future research is to investigate the impact of the immune system and its contribution to these misfolding processes. The researchers hypothesize that immunemediated mechanisms may play a key role in the pathogenesis of many neurodegenerative diseases.
“A plethora of immune factors have been associated with increased risk of neurodegeneration in recent genomewide association studies,” Dr. Diamond explains. “Teasing out which signals are helpful and which are detrimental when devising strategies for immune modulation against neurodegeneration is one of our next major challenges.”
Strategically, the Department of Immunology is situated only one floor away from CAND scientists – a deliberate decision to promote collaboration.
“All major neurodegenerative diseases are relentlessly progressive, and virtually all are linked to the accumulation of protein amyloids.”MARC DIAMOND, M.D. Director of the Center for Alzheimer’s and Neurodegenerative Diseases
In addition to leading the CAND, Dr. Diamond has made original discoveries linking common disorders such as Alzheimer’s disease to rare infectious prion disorders. He holds multiple patents and is very focused on how to translate basic discoveries into effective therapy.
The Diamond Lab studies neurodegeneration from the standpoint of prion biology and protein aggregation. His ideas and the tools that have been developed in his lab have helped transform the scientific community’s understanding of how neurogenerative diseases progress.
“All major neurodegenerative diseases are relentlessly progressive, and virtually all are linked to the accumulation of protein amyloids,” Dr. Diamond
says. “My lab focuses primarily on the tau protein, whose aggregation underlies neurodegeneration.”
Like the prion protein, tau adopts many distinct pathological conformations that replicate within cells and underlie different tauopathies, Dr. Diamond explains. In recent years, he and his team have uncovered how abnormal assemblies of the tau protein move between cells and serve as templates for their own replication.
“Our findings explain the relentless progression through brain networks and the phenotypic diversity of the myriad disorders termed ‘tauopathies,’ which include Alzheimer’s disease, the frontotemporal dementias, and chronic traumatic encephalopathy,” he says.
In collaboration with Lukasz Joachimiak, Ph.D., an Associate Professor at CAND and a member of the OBI, Dr. Diamond is using cell models to study fundamental events in the pathogenesis of tauopathies.
“We are using detailed knowledge of tau’s structure to engineer proteins that target its pathological conformations and then test predictions of efficacy in mouse models,” Dr. Joachimiak says. “This involves studying how different conformations of tau originate in Alzheimer’s disease versus other disorders.”
The co-investigators anticipate that a clearer understanding of tau conformations will have important implications for the development of better diagnostic and therapeutic strategies.
In 2018, Dr. Diamond and Dr. Joachimiak published two seminal papers proposing a new idea –that the tau protein exists in two separable states, shifting from an “inert” form to one that is “seed-competent.”
“We discovered that tau populates two distinct conformational ensembles,” Dr. Diamond says. “The inert form is present in control brains, whereas the seed-competent form is present in disease states.”
The researchers traced this conformational change to a key component of the tau protein, proposing that a type of “hairpin” structure hides an amyloidogenic sequence in the inert monomer, whereas in the seed-competent monomer the tau “unfolds” locally to expose the amyloidogenic sequence so the protein can subsequently aggregate.
Most recently, the duo followed up these findings with a report describing how the tau monomer converting from the “inert” form to “seed-competent” form represents the first detectable step in the initiation of tauopathy. They are now using computational approaches coupled with experimental molecular genetics to decipher how tau folds to form pathological aggregates and what cellular factors might trigger this process.
“We are beginning to understand how a single tau monomer changes shape to form a pathological monomer and then a pathological aggregate,” Dr. Joachimiak explains. “A better knowledge of this process may contribute to the design of interventions that target diseasespecific tau conformations.”

A next direction for the research is the development of tauopathy-specific therapies, Dr. Diamond says, adding, “We plan to leverage our insights to develop novel diagnostic and therapeutic interventions for Alzheimer’s disease and possibly other tauopathies.”
Dr. Diamond acknowledges that while more studies are needed, clinical translation of their findings is imminent, largely due to support received from the OBI.
“Future investigations will be needed to fully elucidate the mechanistic origins of tauopathy – nevertheless, we’re thrilled about the progress made so far,” Dr. Diamond says. “With the backing of the O’Donnell Brain Institute, we’re confident our translational efforts will be a success.”
According to Dr. Diamond, OBI investigators experience unique scientific direction and growth through participation in recruitment, mentorship, and planning committees. In addition, he explains that partnerships within the Institute foster scholarly excellence and enhance connections among faculty working on brain science-related research.
“It is a great honor to work and collaborate with so many brilliant minds,” he says. “We are all excited about the generational impact the Institute is making in training future cohorts of researchers and clinicians.”
Marc Diamond, M.D., is a Professor in the Department of Neurology and Director of the Center for Alzheimer’s and Neurodegenerative Diseases at UT Southwestern Medical Center. He holds the Effie Marie Cain Distinguished University Chair in Alzheimer’s Research. His research focuses on neurodegenerative diseases linked to protein aggregation.
Lukasz Joachimiak, Ph.D., is an Associate Professor in the Center for Alzheimer’s and Neurodegenerative Diseases at UT Southwestern Medical Center, where he is a Marie Cain Endowed Scholar in Medical Research. His research focuses on the role of chaperones and protein misfolding in neurodegenerative diseases.

Humans rely on their ability to draw upon memories to adaptively guide interactions with the world. Cognitive behavior, including memory formation and retrieval, requires cooperative but distributed neuronal firing. A prime example is self-organization of neurons within the hippocampus, the area of the brain responsible for coordinating memory.
“Episodic memories, or conscious memories of unique events, represent a key aspect of how our brains organize and respond to the world,” says Bradley Lega, M.D., an Associate Professor in the Department of Neurological Surgery at UT Southwestern Medical Center and a member of the O’Donnell Brain Institute. “By improving our understanding of how the brain supports complex cognitive behavior, we want to find ways to slow or even prevent memory loss caused by Alzheimer’s disease and other types of dementia.”
As a neurosurgeon physician-scientist, Dr. Lega studies how electrophysiological insights, gathered in human patients, can be used to fix the ability to form memories in patients with brain disease.
Episodic memory requires our brain to understand time, which scientists call “temporal context.” Understanding temporal context requires the hippocampus. In patients undergoing neurosurgical procedures, recordings of individual neurons in the hippocampus are sometimes possible using special equipment. By taking these recordings while patients perform memory tests, Dr. Lega has identified unique groups of memory-sensitive neurons.
In a recent study published in NeuroImage, Dr. Lega and colleagues described hippocampal neurons that code for temporal context information.
“We discovered that these memory-sensitive neurons exhibit important properties,” Dr. Lega says. “The most significant finding was learning that these neurons fire with different timing when a memory is first being formed, compared to when it is being retrieved.”
Dr. Lega explains that this slight difference in timing, termed “phase offset,” has not been reported in humans before. These results explain how the brain can “re-experience” an event but also keep track of whether the memory is something new or something previously encoded.
Strikingly, this neuronal activity could have relevance to schizophrenia because hippocampal dysfunction contributes to a schizophrenics’ inability to decipher between memories and hallucinations or delusions.
“Hallucinations and delusions in people with a psychotic illness appear to be actual memories, processed through neural memory systems like ‘normal’ memories, even though they are corrupted,” Dr. Lega says. “It’s important to understand how to use this ‘phase offset’ mechanism to modify these corrupted memories.”
Building upon these findings, Dr. Lega and Brad Pfeiffer, Ph.D., an Assistant Professor in the Department of Neuroscience and a member of the O’Donnell Brain Institute, are beginning to establish links between “neuronal assemblies” — co-firing groups of neurons that represent a fundamental neurophysiological unit — and human episodic memory.
“These assemblies are important because we think this is a fundamental unit of how the brain works, especially for creating new memories,” Dr. Lega explains.
Previous research from animal models of hippocampal-dependent memory indicates that organized firing occurs on time scales of approximately 25 milliseconds. This time window is set by key brain waves involved in memory called gamma oscillations.
By firing so close together in time, the brain waves can organize the timing of when assembly neurons fire and promote connections between them, Dr. Lega notes. While these assemblies have been well established in lab models, they had not previously been seen in humans, and it was unknown if this same basic mechanism applied to human brain activity.
New research published in Nature Communications by Drs. Lega and Pfeiffer and their colleagues supports the existence of neuronal assemblies and has uncovered novel mechanistic insights into their role in humans.
To establish the existence of the neuronal assemblies, the researchers worked with patients at UT Southwestern’s Epilepsy Monitoring Unit, specifically those who stay at the facility for several days before undergoing surgery for intractable seizures.
“Electrodes implanted in their brains not only help surgeons precisely identify the seizure foci but also provide valuable information in a research context,” Dr. Lega says.
In the study, the researchers recorded activity from electrodes placed in each patient’s medial temporal lobe (MTL). Analysis of these recordings revealed the human brains exhibited the same phenomenon as in rodents, with groups of neurons firing together on time scales of about every 25 milliseconds, but rather than being nearest neighbors, these neurons were often scattered across the patients’ MTLs.
“We noticed a key difference compared to the findings in rodents,” Dr. Pfeiffer says. “Rather than these assemblies being permanently fixed, they were typically flexible, with neural members joining and dropping out over 20 to 30 minutes. The more flexible a patient’s assemblies behaved, the better the individual performed on the memory task.”
According to Dr. Pfeiffer, because this flexibility appears to be key to successful episodic memory, it could be a useful biomarker for interventions that aim to help people with memory loss.

“These findings establish principles for identifying possible targets for neuromodulation using these approaches,” Dr. Pfeiffer says. “The importance of assembly identification may extend beyond memory processing, including strategies for treatment of psychiatric illness in humans.”
On the clinical side, Dr. Lega specializes in the evaluation and surgical treatment of brain tumors and epilepsy. As the surgical leader of UT Southwestern’s Comprehensive
Epilepsy Program, one of the top programs of its kind in the world, he is recognized as a national expert in using stereoelectroencephalography, also termed stereo EEG, to locate the origin of epileptic seizures in the brain.
“The multidisciplinary team at UT Southwestern is at the forefront of solving the mysteries surrounding epilepsy. On a daily basis, we’re making the kind of scientific and clinical breakthroughs that can change our patients’ lives,” Dr. Lega says.
The program offers patients with epilepsy a comprehensive and personalized approach to care, from a comprehensive EEG evaluation to involvement in the latest clinical trials and brain-mapping methods for pinpointing and controlling seizures. In addition, it houses one of the largest epilepsy teams in the country, specializing in care for patients in North Texas and beyond.

Drawing upon knowledge from hundreds of epilepsy and tumor cases, Dr. Lega tailors his mapping and treatment strategies to the unique circumstances of each patient. This includes using new, less-invasive approaches along with traditional surgical approaches.
“It’s a very exciting time to be working in this field,” Dr. Lega says. “New tools, such as stereo EEG and NeuroPace, are giving options to patients who may have previously been told surgical treatments weren’t available to them.”
Dr. Lega adds that laser interstitial therapy, or LITT, is an emerging minimally invasive option that is suited for some patients with epilepsy and brain tumors. Numerous reports have been published describing treatment of lesions ranging from tumors to epileptogenic foci, but the indications for LITT continue to evolve.
“Laser therapy requires an expert team working together, and the close collaborative environment at UT Southwestern is really necessary for these complex cases,” Dr. Lega says. “Brain tumors are often linked to seizures, and our integrated team provides the best approach for their treatment.”
He notes that magnetoencephalography (MEG) is also being used with increased frequency. UT Southwestern’s MEG team brings together experts from neurology and radiology who use a collaborative approach to identify seizure “hot spots.” MEG information is critical for improving rates of seizure freedom by helping neurologists understand seizure networks before
brain mapping. The team is also developing new methods to identify brain structure changes that are linked with seizure onset, such as cortical dysplasia.
Another important innovation includes a dedicated program to counsel epilepsy patients before and after surgery. The program’s goal is to alleviate the patient’s stress of going through surgery and help patients and families cope in general. The program, pioneered by Chadrick Lane, M.D., an Assistant Professor of Psychiatry and the inaugural Clinical Neuroscience Scholar within the O’Donnell Brain Institute at UT Southwestern Medical Center, is unique across the state of Texas.
“With the tools we have at UT Southwestern, the expertise of our team, and our experience as one of the busiest centers in the country, I am proud to say that the care we provide patients with these conditions is truly top-notch,” Dr. Lega says.
Dr. Lega and his colleagues have been widely recognized for their contributions to understanding how the brain creates memories. One day, he hopes to restore memory function to patients whose brain has been affected by tumors or epilepsy.
“For now, this work has begun to provide us with a deeper understanding of how best to preserve vital functions for patients who need surgery,” he says.
“New tools, such as stereo EEG and NeuroPace, are giving options to patients who may have previously been told surgical treatments aren’t available to them.”BRADLEY LEGA, M.D. Associate Professor in the Department of Neurological Surgery
Bradley Lega, M.D., is an Associate Professor in the Department of Neurological Surgery at UT Southwestern Medical Center. He has secondary appointments in Neurology and Psychiatry. His research focuses on preserving memory function and restoring memory to patients with brain injuries or brain tumors.
Brad Pfeiffer, Ph.D., is an Assistant Professor in the Department of Neuroscience and is the Southwestern Medical Foundation Scholar in Biomedical Research at UT Southwestern Medical Center. His research focuses on how experience is encoded at the neuronal level, how neuronal representations of experience are consolidated into long-term memory, and how neuronal representations of past experience are recalled and used to guide or inform behaviors.

A veteran clinician-researcher seeks to advance knowledge of schizophrenia and related disorders.
Schizophrenia and related psychotic disorders have a lifetime prevalence of approximately 3.5% and account for a major health care burden in the United States. The disorders are also associated with reduced life expectancy – about 15 years shorter than the general population and a 5% to 10% risk of death by suicide.
While antipsychotic medications are effective for the management of psychotic symptoms (e.g., hallucinations, delusions), these drugs essentially all have the same function and targets: blocking dopamine and serotonin receptors. Recent developments in genetics, neuroimaging, and preclinical research have begun to untangle the mechanisms underlying schizophrenia and related psychotic disorders.
“The medications used to treat schizophrenia were discovered serendipitously and not based on objective brain characteristics matching the underlying mechanisms of disease,” says Elena Ivleva, M.D., Ph.D., an Associate Professor in the UT Southwestern Department of Psychiatry’s Division of Translational Neuroscience Research in Schizophrenia and a member of the O’Donnell Brain Institute.
Dr. Ivleva is a dedicated physician-scientist in the field of neurobiological psychosis research. She focuses on understanding the neurobiological mechanisms of schizophrenia and developing human in vivo biomarkers for psychotic disorders, primarily using cognition and multimodal brain imaging approaches.

According to Dr. Ivleva, schizophrenia syndrome is a clinically and neurobiologically complex and heterogeneous illness, as reflected in the large number of independent risk genes for the condition and extensive variability in clinical presentation, outcomes, and neurobiological features.
Importantly, this notion suggests schizophrenia syndrome may in fact represent a combination of unique diseases with overlapping clinical presentations.
“In recent years, several studies have investigated this heterogeneity using a range of approaches,” Dr. Ivleva says. “MRI studies in schizophrenia have typically utilized case-control designs to identify group-level brain alterations in patient cohorts.”
The findings of these studies have led to the identification of regional gray matter alterations, such as decreased volume, cortical thickness, or surface area in many regions of the brain –but most prominent in frontal and
temporal lobes – as well as widely distributed white matter alterations. While these group-level findings are of interest, Dr. Ivleva explains, the severity and specific features of neuroanatomical alterations vary widely among individual patients.
“It still remains unclear whether neuroanatomic variability is driven by varying degrees of illness severity or by qualitatively distinct schizophrenia subtypes,” she says.
Dr. Ivleva is working within the fivesite Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) team, led by Carol A. Tamminga, M.D., Chair of the UT Southwestern Department of Psychiatry and Director of the Psychosis Research Program. The team is looking at multiple biomarkers of brain function to delineate illness groups and predict psychosis responses. The B-SNIP consortium is studying the genetic, neurophysiological, imaging, and cognitive markers of severe mental illnesses. UT Southwestern is the consortium’s lead site; others are in Boston, Chicago, Hartford, and Athens.
To better grasp the complexity of the neuroanatomic milieu surrounding psychosis, Dr. Ivleva is employing the B-SNIP biomarker strategies, harnessing the power of MRI along with the clinical biomarkers to identify neurobiologically distinct subtypes within schizophrenia and related disorders. These strategies have the potential to move diagnostic and therapeutic practice away from a reliance on symptom and behavioral features, Dr. Ivleva notes.
“Whether quantitative imaging biomarkers can identify discrete subgroups of patients, thereby fostering personalized medicine approaches for patients, remains unclear,” she says.
Dr. Ivleva adds that neuroimaging is wellpositioned for biomarker development in schizophrenic psychosis, explaining that it could capture phenotypic variations in molecular and cellular disease targets, or in the brain circuits themselves.
“These mechanistically based biomarkers collected in B-SNIP may represent a direct measure of the pathophysiological underpinnings of the
disease process,” Dr. Ivleva says. “We are using biomarkers to explore novel treatment targets, aid in prediction of response, determine optimal treatment regimens, and provide a rationale for personalized medicine approaches.”
Historically, many different clustering algorithms were used to classify patients with schizophrenic psychosis into subgroups based on similarities in symptoms or imaging features. However, no consensus was reached as to the definition of a “cluster.”
A novel density peak-based clustering (DPC) algorithm has recently been proposed by a group of researchers, which, when compared with conventional clustering algorithms, may be able to better detect nonspherical clusters and to automatically find the correct number of clusters, without initialization and multiple iterations.
In a recent study, Dr. Ivleva and colleagues examined cortical (surface area, cortical thickness) and subcortical (volume) morphology in a cohort of acutely ill patients with never-treated first-episode schizophrenia and clinically stable chronic patients with midcourse schizophrenia. The researchers matched each of these cohorts with a healthy control group.
In the study, gray matter was selected as the subgrouping feature because it consists of neuronal cell bodies, neuropil, glial cells, and synapses and is well documented to be altered in patients with schizophrenia. In addition, the novel DPC algorithm was used to classify individuals with schizophrenia into subgroups with distinct neuroanatomical features.
“The primary aim of the study was to identify the number and features of discrete subgroups in a sample of firstepisode schizophrenia patients,” Dr. Ivleva says.
After analyzing the data, Dr. Ivleva and her colleagues successfully identified three subgroups of patients, defined by distinct patterns of regional cortical and subcortical morphometric features. Interestingly, a similar threesubgroup pattern was identified in the independent dataset of patients from the multisite B-SNIP consortium.
“Similarities of classification patterns across our two patient cohorts indicate the three-group typology is relatively stable over the course of illness,” Dr. Ivleva explains. “These findings provide new clues into distinct subgroups of patients with psychosis based on structural brain features.”
Based on these results, Dr. Ivleva believes that anatomic magnetic resonance subgrouping – along with other cognition, EEG, and eye-tracking biomarkers – may be able to be leveraged to separate neurobiologically distinct subgroups of individuals with psychosis. This represents an important step toward differentiating subtypes of psychotic disorders for understanding and treating the disorder.
“Clinical and behavioral features have been shown over many years to have limited utility in stratifying schizophrenia patients for the purpose of clinical trials and personalized treatment,” Dr. Ivleva says. “Our results represent an important step toward the stratification of patients on the basis of a potential biomarker, as opposed to behavioral features.”
In addition to her research efforts, Dr. Ivleva serves as the Director of the Early Psychosis Clinical and Research Program at UT Southwestern.
“Psychosis is a mental condition in which a person misinterprets information or loses touch with reality – it can impact thoughts, feelings, and behaviors, as well as what people believe, hear, or see,” Dr. Ivleva says. “Early psychosis refers to an initial phase of the illness – within the first two to three years – when a person first exhibits psychotic symptoms.”
Dr. Ivleva notes that prompt, comprehensive treatment is the best way to help manage the symptoms of early psychosis and set a person on the best possible long-term life trajectory. Within UT Southwestern Medical Center’s HOPE program, which stands for “Healing Over Psychosis Early,” she and her team provide innovative and comprehensive intervention for early psychosis in a compassionate, collaborative environment.
“We provide interventions including medication, individual psychotherapy, group psychotherapy, family support and education, employment and education services, and individualized case management,” Dr. Ivleva explains. “Our aim is to alleviate symptoms and restore functioning, with an ultimate goal of having patients live productive, fulfilling lives.”
While the mechanisms of psychotic disorders remain poorly understood, multiple factors are implicated, including certain drugs, psychological trauma, brain injuries, and genetic factors.
According to Dr. Ivleva, society often portrays individuals with psychosis negatively. However, with treatment, many people are able to achieve functional recovery and live personally meaningful lives. She says it is important to view psychosis as an obstacle to overcome rather than as a preset course with poor outcomes.
“Over the past two decades, we have seen an increased focus on early intervention in psychotic disorders in research and clinical practice.”ELENA IVLEVA, M.D., PH.D. Associate Professor in the Department of Psychiatry’s Division of Translational Neuroscience Research in Schizophrenia
In addition to exploring schizophrenia subtypes, Dr. Ivleva is also pioneering clinical trials for patients with early psychosis. In collaboration with Dr. Tamminga, she is conducting studies exploring brain-based biomarkers, including MRI, EEG, ocular motor function, and cognitive assessments. They are also exploring genetic and immunological features, as well as developing novel treatment protocols.
“Over the past two decades, we have seen an increased focus on early intervention in psychotic disorders in research and clinical practice,” Dr. Ivleva says. “Early symptom levels appear to be strongly predictive of later illness trajectories.”
Other researchers have proposed the idea of an “early therapeutic window,” she explains, where patients in the
early phase of the psychotic illness –due to high levels of brain plasticity – might derive the highest benefit from interventions. As a result, many studies are now investigating the effect of early interventions aiming to reduce the duration of untreated psychosis as well as multidisciplinary, prolonged early interventions combining psychopharmacological, cognitive, and psychosocial interventions.
While specialized early interventions have been found to be more successful in reducing symptom burden and improving outcomes in individuals with psychosis, Dr. Ivleva acknowledges that we still do not possess the definitive key on therapies that can provide longterm symptom reduction and functional recovery. Thus, the development of new therapeutic interventions is needed.
The heart of psychosis research at UT Southwestern is to improve diagnosis of these illnesses and, ultimately, to find new targets for therapies.
Despite theories and accumulating data, even the most seasoned clinicians and researchers do not fully understand the mechanisms of therapeutic action underlying most of the current treatment options.
While it takes many years to accumulate the necessary data to confidently bring new treatment approaches to the clinic, Dr. Ivleva hopes to provide renewed optimism for clinicians, inspired by novel clinical and basic science research efforts.
Elena Ivleva, M.D., Ph.D., is an Associate Professor in the Department of Psychiatry’s Division of Translational Neuroscience Research in Schizophrenia at UT Southwestern Medical Center. She also serves as Director of the department’s Early Psychosis Clinical and Research Program. Her research focuses on understanding neurobiological mechanisms of schizophrenia and related disorders and developing human in vivo biomarkers for psychotic disorders.
Carol Tamminga, M.D., is Chair of UT Southwestern’s Department of Psychiatry and Chief of its Translational Neuroscience Research Program in Psychosis. She holds the Stanton Sharp Distinguished Chair in Psychiatry. Her research focuses on understanding the mechanisms underlying psychosis and designing brain biomarkers to support informative research in psychosis and its memory dysfunction.


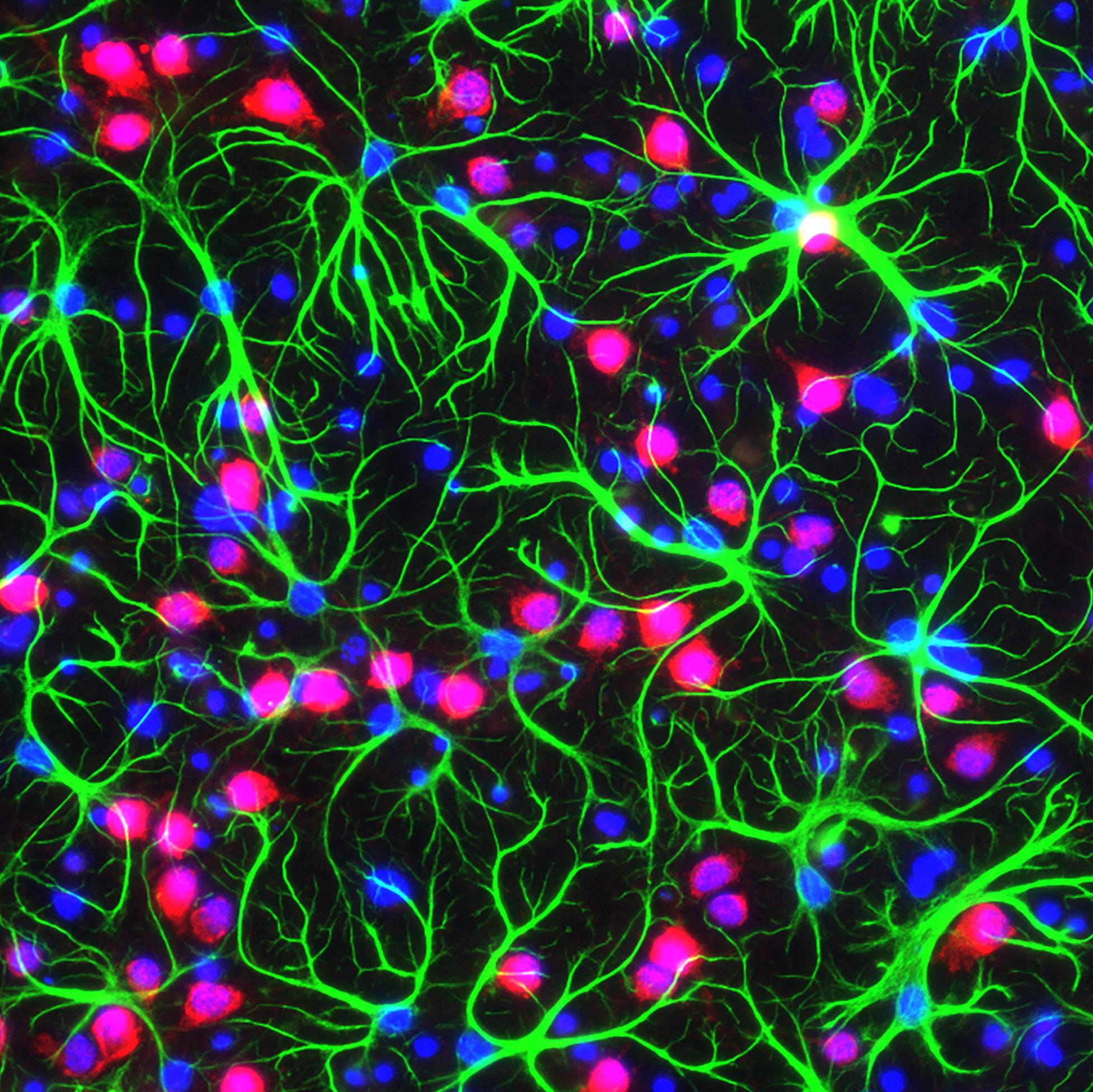
A specialist in functional neurological disorders calls for greater attention to these common neuropsychiatric conditions.

Despite accounting for the secondmost common reason someone visits a neurology clinic, behind only headaches, functional neurological disorders (FNDs) have been largely neglected by the medical community until recently. Previously known as a “conversion disorder” prior to the fifth edition of the DSM, an FND is a brainbased disorder that impacts emotion and cognition, as well as motor and sensory processes.
Two of the most frequently encountered clinical presentations of FNDs are functional seizures (also called dissociative or psychogenic nonepileptic seizures) and functional movement disorders (FMDs). Other
subtypes of FNDs include functional cognitive disorder, functional speech disorder, and functional dizziness, now known as persistent postural-perceptual dizziness (PPPD).
“Neuropsychiatry, a field of medicine committed to understanding brain-behavior relationships, is well positioned to advance our understanding of contributing risk factors and the neural mechanisms underlying FNDs,” says Chadrick Lane, M.D., Assistant Professor of Psychiatry and the inaugural Clinical Neuroscience Scholar within the Peter O’Donnell Jr. Brain Institute at UT Southwestern Medical Center. “Due to various reasons, including a need for
more training in FNDs, clinicians often feel ill-equipped to debrief patients about their diagnosis. This can be remedied with multidisciplinary clinical programs and curricular development in medical education.”
Dr. Lane’s clinical and research efforts are focused on neuropsychiatry. He co-directs the Department of Psychiatry’s FND Multidisciplinary Clinic, is part of the UT Southwestern Epilepsy Team, and is an active member of both the American Neuropsychiatric Association and Functional Neurological Disorder Society.

For effective treatment, neurologists, psychiatrists, psychotherapists, and rehabilitative therapists must all collaborate closely in the care of patients, Dr. Lane explains, adding that currently there is a dearth of FND-focused specialty care across the Unites States.
“FNDs are one of the most common reasons for new outpatient neurological consultations,” Dr. Lane says. “Studies suggest an incidence of 10 to 15 per 100,000, translating to an estimated prevalence of 250,000 to 300,000 people in the United States alone.”
Dr. Lane notes that neuroimaging studies have illustrated differences in those living with an FND compared to those without an FND. Networks in the brain responsible for emotional regulation, allocation of attention, and the perception of volitional control over movements may be working less effectively in those with an FND.
“FNDs have a high association with a history of prior trauma, as well as medical comorbidities, such as mild traumatic brain injury, fibromyalgia, chronic fatigue syndrome, and chronic pain syndrome,” Dr. Lane explains.
“FNDs have a high association with a history of prior trauma, as well as medical comorbidities, such as mild traumatic brain injury, fibromyalgia, chronic fatigue syndrome, and chronic pain syndrome.”
CHADRICK LANE, M.D. Assistant Professor of Psychiatry
In years past, an FND was regarded as a diagnosis of exclusion. A patient seeking care would receive a neurological workup, and when nothing returned consistent with a structural neurological condition, the patient would receive a diagnosis of FND. In the past decade, important progress has been made in making FND a diagnosis of inclusion, based on positive exam and history findings as well incongruity with other neurological disorders.
The gold standard in the diagnosis of functional seizures is the use of video EEG (vEEG). This procedure records brain wave activity while the patient is observed in the hospital. If a typical seizure is captured on video, presents with functional characteristics, and epileptic activity is present on the EEG, a reliable diagnosis can be made. Common signs of functional seizures are fluctuating asynchronous limb or side-to-side head movements, long duration of events, ictal eye closure and crying, pelvic thrusting, peri-ictal responsiveness, and postictal memory recall.
“vEEG results require experienced interpretation by epileptologists,” Dr. Lane says. “Many patients lack access to specialized monitoring units or have an ‘indeterminate’ diagnosis due to equivocal or uneventful monitoring.”
The International League Against Epilepsy (ILAE) published methods of diagnosing functional seizures based
on levels of certainty. While vEEG is the highest level of certainty, this system allows for patients to receive a workup when they may not have access to an epilepsy monitoring unit for vEEG evaluation.
While research on biomarkers to differentiate epileptic from functional seizures is ongoing, at present, clinical history, individual risk factors, and vEEG are the standard of care. Dr. Lane explains that neuropsychiatry is an essential part of the treatment picture given the high rates of psychiatric comorbidity.
For an FMD, establishing a proper diagnosis rests on key elements of clinical history and characteristic signs on examination (e.g., specific movement patterns). In addition, clinical tests such as imaging and labs can play an important role.

Importantly, prompt diagnosis allows for more rapid implementation of appropriate evidence-based interventions. The mainstay of treatment, Dr. Lane notes, is psychotherapy and rehabilitative therapies, including physical, occupational, and speech therapies. Medications can have a role in the management of co-occurring conditions, including depression, trauma-related disorders, and anxiety.
“Multidisciplinary treatment teams are fundamental to the successful care of patients with an FND,” Dr. Lane says.
“As is often noted in the literature, treatment begins with diagnosis. Assuring patients that their symptoms are real and brain-based, providing a helpful metaphor such as FND being a ‘software’ rather than a ‘hardware’ problem, and delivering the diagnosis with compassion, empathy, and confidence are all critical to setting a patient on the right treatment trajectory.”

The Peter O’Donnell Jr. Brain Institute Functional Neurological Disorder Clinic is made up of neurologists, psychotherapists, psychiatrists, rehabilitative therapists, and speechlanguage pathologists. It is funded in part by an award from the OBI’s Clinical Neuroscience Scholars Program and is one of only a handful of centers in the nation to take a comprehensive approach to managing FNDs, with staff working together to address the physical, psychological, and social aspects of these disorders.
Upon initial presentation, patients are evaluated by both a psychiatrist and psychotherapist, who then formulate a treatment plan. Subsequently, most patients are referred to the clinic’s eight-week, evidence-based group therapy program, designed specifically for the treatment of FNDs.
“Only a few centers can provide this particular type of therapeutic intervention,” Dr. Lane says. “We bring together a collaborative team of neurologists, psychiatrists, psychotherapists, and rehabilitative therapists, each actively participating in the patient’s care.”
While it’s too early to draw conclusions, Dr. Lane says he and his colleagues are excited to analyze the clinic’s outcomes in the coming months and years. If research based on similar programs at other institutions is any indication, the OBI Functional Neurological Disorder Clinic will prove beneficial to the region.
The fundamental pathophysiology of all FNDs remains incomplete. Current understanding includes overactivity of the limbic system, symptom modeling as part of a predictive coding framework, and dysfunction of self-agency networks. Studies have demonstrated brain anatomical differences (gray matter volume and cortical thickness) and connectivity differences using fMRI between patients with an FND compared to those without.
“These early studies, though in their infancy, note alterations in brain function that may provide clues to the neural underpinnings of FNDs –however, longitudinal studies are still needed to address the question of causality,” Dr. Lane says.
As to optimal treatment strategies, commonalities have begun to emerge, including the benefit of therapy, with evidence increasingly supporting tailored multidisciplinary approaches. Neuromodulation, particularly transcranial magnetic stimulation, may hold promise.
“Our understanding of FNDs and their treatments has advanced, but we have far to go. Importantly, there is hope, and people can experience improved functionality, quality of life, and – possibly – symptom control,” Dr. Lane says.
He adds that further research is needed to determine the optimal dose and duration of various approaches and to assess the value of combination strategies, stressing that therapeutic success hinges on a diagnostic approach that validates the patient’s symptoms and disability, allowing for full understanding and acceptance by the patient.
“FNDs are real conditions that arise from extraordinarily complex biological, psychological, and social interactions. Recovery is possible,” he concludes.
Chadrick Lane, M.D., is an Assistant Professor of Psychiatry and the inaugural Clinical Neuroscience Scholar within the Peter O’Donnell Jr. Brain Institute at UT Southwestern Medical Center. His clinical and research focus is on neuropsychiatry, a subspecialty of medicine that explores brain-behavior relationships to improve the diagnosis and treatment of people living with complex brain disorders.

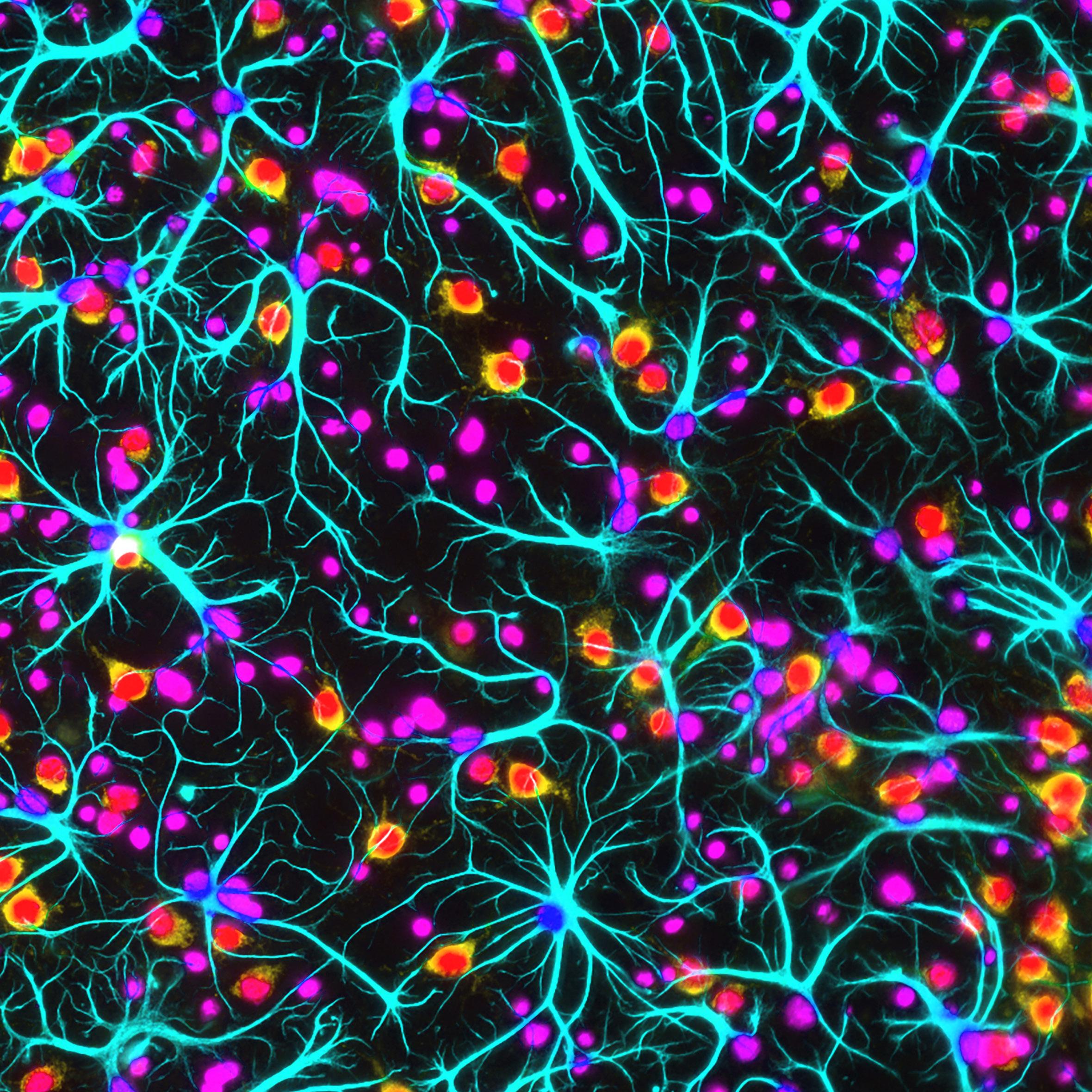
A renowned clinical research program seeks new insights into ataxia and related disorders.

UT Southwestern’s Multidisciplinary Ataxia Clinic in the O’Donnell Brain Institute is a hub for research and care of patients with ataxia. This group of poorly understood, often disabling neurological disorders affects up to 150,000 people in the U.S., resulting in uncoordinated limb and trunk movements and falls, frequently leading to wheelchair confinement.
Led by Vikram Shakkottai, M.D., Ph.D., Associate Professor and Vice Chair for Basic Research in the Department of Neurology, as well as a member of the O’Donnell Brain Institute, the Multidisciplinary Ataxia Clinic is equipped to make important and needed strides in the diagnosis, study, and treatment of this difficult disease.
“We are building a truly comprehensive program with notable expansions across our clinical and research components,” Dr. Shakkottai says. “Our providers really think deeply about a patient referred with ataxia, or labeled as having it, and revise a diagnosis if necessary. This label is, many times, incorrect.”
While the etiologies of ataxia are diverse, recent advances have led to the discovery of novel genetic causes for ataxia and a more comprehensive understanding of the biological pathways critical for normal cerebellar function.
Importantly, when these molecular pathways become dysfunctional, patients develop ataxic symptoms, Dr. Shakkottai explains.
“The underlying causes of ataxia are incredibly diverse, ranging from infectious, immune-mediated, to genetic, and knowledge about the etiology of these disorders, even among neurologists, is poor,” Dr. Shakkottai says.
He adds that differentiating inherited causes from potential immune causes is vital to both avoid unnecessary treatment and to initiate appropriate treatment early. Historically, treatments for ataxia
were thought to be ineffective, leaving many patients with ataxia untreated. In February 2023, the U.S. FDA approved the first drug specifically for the treatment of Friedreich’s ataxia. This therapy, called omaveloxolone (Skyclarys), works by counteracting the effects of Friedreich’s ataxia in nerve and muscle cells.
“Friedreich’s ataxia is a debilitating neuromuscular disorder that, over time, robs patients of their mobility and independence,” Dr. Shakkottai explains. “The recent approval of omaveloxolone represents the first disease-specific drug available for patients with this debilitating disease.”
“Overall, identification of a diseaseenriched, early terminated transcript at FXN represents a significant step forward in understanding molecular pathology of the disease.”MAREK NAPIERALA, PH.D. Associate Professor of Neurology
On the clinical side, patients in UT Southwestern’s Multidisciplinary Ataxia Clinic who are diagnosed with ataxia or a related disorder are provided sufficient information, in the right setting, to ensure they understand what the future holds for them and what approaches – both pharmacological and nonpharmacological – can resolve the etiology or symptoms of the disease.
Along with neurologists, the clinic also involves specialists in physical therapy, speech pathology, and occupational therapy to give patients broad-based care for the various aspects of their condition, which can include impairments of balance, speech, and hand dexterity. The clinic’s physical therapists can provide important input at the same time as clinical visits, a feature that is vital for patients with ataxia, who often travel long distances to receive expert treatment.
“Patients come from all over the country to receive care at the clinic,” Dr. Shakkottai says. “It’s incredibly humbling to know that we are one of the few ataxia programs – nationally or internationally – providing state-of-the-art clinical care.”
In addition, Dr. Shakkottai notes the availability of comprehensive genetic testing through the clinic. Because ataxia can be misdiagnosed as other neurodegenerative disorders, genetic testing is often useful for identifying the underlying cause.
“Through genetic testing, families have been able to identify the cause of their ataxia. It has also been incredibly helpful in identifying treatable causes of the disorder, in predicting future disability, and in family planning,” he adds.
Currently, about 20% of genes in families with a dominant or recessive pedigree remain undiscovered, Dr. Shakkottai
explains, anticipating that genetic testing of patients in the clinic will provide information for novel gene discoveries.
The Multidisciplinary Ataxia Clinic is also becoming a global hub for research. Dr. Shakkottai is currently leading a multiinstitutional, longitudinal, observational study of participants with inherited ataxia that he previously initiated at the University of Michigan.
Dr. Shakkottai also conducts clinical and laboratory research on a mission to determine whether alterations in neuronal physiology contribute to motor dysfunction and to translate preclinical mouse model research to human clinical trials for cerebellar ataxia.
Recent work in his laboratory has identified shared dysfunction in the cerebellum in mouse models of ataxia, with identification of several ion channels that act in concert, converging on large-conductance calciumactivated potassium channels.
“Basic research into mechanisms of disease and development of therapy is an important endeavor for UT Southwestern’s ataxia program,” he says.
To further research progress, UT Southwestern recently recruited Marek Napierala, Ph.D., an Associate Professor of Neurology, whose research interests include understanding the molecular mechanisms of Friedreich’s ataxia.
Additionally, Elan Louis, M.D., M.S., Chair of Neurology at UT Southwestern, is renowned for his study of cerebellar anatomical changes in tremor. With six faculty members within the department studying various aspects of cerebellar impairment, UTSW has one of the largest groups of cerebellar researchers in the country.

New research published in Human Molecular Genetics by Dr. Napierala and colleagues has uncovered new mechanistic insights into the pathogenesis of Friedreich’s ataxia.
“Friedreich’s ataxia is an autosomal recessive disease primarily caused by biallelic expansion of the guanineadenine-adenine (GAA) trinucleotides located in intron1 of the FXN gene,” Dr. Napierala explains. “In most patients, frataxin – the protein encoded by the FXN gene – does not carry any mutations; instead, the severe decrease of its levels leads to this progressive multisystem disorder.”
Dr. Napierala notes that a block in transcription progression caused by expanded GAA repeats has been recurrently proposed as the mechanism of FXN mRNA deficiency in Friedreich’s ataxia. While there is a
strong correlation between mature FXN mRNA expression and the length of the expanded GAA repeats, however, the direct mechanism of the transcriptional impairment remains unknown.
In the study, the researchers used multiple transcriptomic approaches to determine the molecular mechanism of transcription inhibition caused by long GAAs, uncovering that transcription of FXN in patient cells is prematurely terminated upstream of the expanded repeats, leading to the formation of a novel, truncated, and stable RNA.
“Mechanistically, this FXN early terminated transcript (FXN-ett) undergoes alternative, nonproductive splicing and does not contribute to the synthesis of functional frataxin,” Dr. Napierala says. “In our study, we showed that transcription progression is indeed affected at the FXN locus, resulting in
the GAA-length dependent formation of an aberrant, early terminated, and alternatively spliced transcript.
“Overall, identification of a diseaseenriched, early terminated transcript at FXN represents a significant step forward in understanding molecular pathology of the disease,” he explains.
Importantly, the FXN transcriptional defect, FXN-ett, could be a very attractive target for therapeutic intervention.
While currently there is insufficient evidence for a direct pathogenic role of FXN-ett, formation of this stable transcript may interfere with transcription of the full-length canonical FXN pre-mRNA, thereby reducing overall transcriptional output of the FXN locus in Friedreich’s ataxia cells, Dr. Napierala explains.
“Targeting GAAs with antisense oligonucleotides or excision of the repeats eliminates the transcription impediment, diminishing expression of the aberrant FXN-ett while increasing levels of FXN mRNA and frataxin,” Dr. Napierala says. “Nonproductive transcription may represent a common phenomenon and attractive therapeutic target in diseases caused by repeatmediated transcription aberrations.”
In light of these findings, Dr. Napierala believes a strategy of blocking nonproductive splicing of the FXN-ett RNA may lead to a natural upregulation of FXN transcription and increase of frataxin production. He and his team are conducting further experiments exploring this potential treatment approach.
The Napierala lab, led by Dr. Napierala and Jill S. Napierala, Ph.D., an Assistant Professor of Neurology at UT Southwestern, is focused on finding a cure for Friedreich’s ataxia by elucidating molecular mechanisms causing the disease, developing novel cellular and animal models of disease, and identifying disease biomarkers, in addition to testing novel therapeutic approaches.
“The creation of our lab was a collaborative effort between the Department of Neurology and the O’Donnell Brain Institute,” Dr. Napierala says. “At the core of the mission is to foster a spirit of collaboration between our worldrenowned researchers and clinicians.”
The Napierala lab takes a multidisciplinary approach to study the molecular mechanisms underlying Friedreich’s ataxia. While developing
therapeutic strategies aimed at reactivating transcription of the FXN gene is a key focus for the lab, its researchers are also pioneering other projects.
“We’re also characterizing the molecular phenotypes of Friedreich’s ataxia neuronal and cardiac cells derived from induced pluripotent stem cells (iPSCs) and generating novel cellular and mouse models of disease,” he adds.

In collaboration with the Friedreich’s Ataxia Research Alliance, as well as David Lynch, M.D., Ph.D., of the Children’s Hospital of Philadelphia, the Napierala lab recently established the Friedreich’s Ataxia Cell Line Repository, which houses numerous Friedreich’s ataxia and control cell lines that are available to the research community.
The repository is the world’s largest bank of primary Friedreich’s ataxia fibroblasts and iPSCs that currently holds more than 100 lines derived from different Friedreich’s ataxia patients.
“The bank includes more than 80 disease and control fibroblast lines and 20 disease and control iPSC lines,” Dr. Napierala says. “Included in the iPSC lines are three sets of patient and isogenic, CRISPR-Cas9edited paired lines.”
Dr. Napierala is also highly involved in the Friedreich’s ataxia patient community. The Napierala lab hosts yearly meetings with patients and their caregivers and participates in rideATAXIA events. He is also an active member of the Scientific Advisory Board of the Friedreich’s Ataxia Research Alliance.
Collectively, ongoing discoveries in the Department of Neurology and the O’Donnell Brain Institute may assist in the design of therapeutic strategies for ataxia and related disorders.
At OBI, researchers and clinicians work closely together, by design. Combining basic and translational research with advanced clinical care produces scientific breakthroughs that can move from the labs to patients in the clinic faster than ever.
“Within ataxia, the field is advancing rapidly – especially around novel approaches to care and curative treatments,” Dr. Shakkottai says. “From a research standpoint, there are many studies being done in this area involving our department and others across the country.”
“As discoveries continue to accelerate, we recognize that understanding the exact mechanisms of disease is essential,” Dr. Napierala says. “It is for this reason that our continued focus is on studying the molecular mechanisms of disease – uncovering the common genetic aberrations that occur across seemingly disconnected diseases.”
“Within ataxia, the field is advancing rapidly – especially around novel approaches to care and curative treatments.”VIKRAM SHAKKOTTAI, M.D., PH.D.
Associate Professor and Vice Chair for Basic Research in the Department of Neurology
Vikram Shakkottai, M.D., Ph.D., is an Associate Professor and Vice Chair for Basic Research in the Department of Neurology at UT Southwestern Medical Center. He is also the Dedman Family Distinguished Chair in Neurological Disease. His research focuses on whether alterations in neuronal physiology contribute to motor dysfunction and on translating preclinical murine model research to human clinical trials for cerebellar ataxia.
Marek Napierala, Ph.D., is an Associate Professor of Neurology at UT Southwestern Medical Center. His research interests include understanding the molecular mechanisms of Friedreich’s ataxia with the long-term goal of developing novel therapeutic strategies for this disease.
Jill S. Napierala, Ph.D., is an Assistant Professor of Neurology at UT Southwestern Medical Center. Her research interests include specifying gene and protein expression patterns unique to Friedreich’s ataxia in order to identify novel therapeutic targets and disease biomarkers.
Signaling pathway thought to be driving oncogenesis gets called into question.

The epidermal growth factor receptor (EGFR) is a key oncogene that is frequently amplified in glioblastoma, and evidence has suggested that EGFR is an important oncogenic driver in this highly invasive brain tumor. However, multiple clinical trials of EGFR targeting have failed as an effective treatment.
Surprising findings, published in Nature Cell Biology by Amyn Habib, M.D., a Professor of Neurology and member of both the Harold C. Simmons Comprehensive Cancer Center and Peter O’Donnell Jr. Brain Institute at UT Southwestern, suggest a tumor suppressive role of EGFR in a subset of EGFR-amplified glioblastomas.
“Glioblastoma’s invasive property is perhaps its most formidable barrier to treatment,” Dr. Habib says. “The prevailing paradigm was that EGFR activation was uniformly oncogenic in human cancer. In contrast, we discovered a tumor suppressive function of EGFR signaling that actually suppresses glioblastoma invasion.”
Surprising new findings suggest a tumor suppressive role of EGFR in a subset of EGFR-amplified glioblastomas.

The study initially focused on analyzing data from The Cancer Genome Atlas (TCGA), a landmark cancer genomics database of over 20,000 primary cancer and matched normal samples spanning 33 cancer types. The researchers looked at the combined effect of all seven EGFR ligands, uncovering that EGFR ligands are in fact tumor suppressive in EGFRamplified glioblastoma.
Consistent with this unexpected finding, experimental data showed that ligand-activated EGFR signaling suppresses tumor invasion. Dr. Habib and his colleagues believe this
suppression of invasion mediates the tumor-suppressive effect of liganddependent EGFR signaling in EGFRamplified glioblastomas.
“Previous studies have reported that EGFR stimulates invasion in glioblastoma-established cell lines that have lost EGFR amplification,” Dr. Habib says. “Our data show that the level of EGFR expression determines whether the outcome of liganddependent EGFR signaling is either increased or decreased invasion.”
The TCGA analysis confirmed these findings, revealing that in non-EGFR
amplified glioblastomas, EGFR ligands are oncogenic while, in a striking reversal, EGFR-amplified glioblastomas’ EGFR ligands are tumor suppressive. A key reason is likely the differential upregulation of BIN3, a cytoskeletal protein, in EGFRamplified tumors, Dr. Habib explains.
“In patients with EGFR-amplified glioblastomas, with high EGFR ligands, EGFR inhibition could actually be detrimental, since EGFR ligands and high phosphorylation of the EGFR confer a better prognosis.”
AMYN HABIB,M.D. Professor of Neurology
Dr. Habib and his team were the first to connect ligand-dependent EGFR signaling and suppression of invasion by upregulation of BIN3.
Using a series of siRNA and mass spectrometry experiments to identify BIN3-interacting proteins, the researchers discovered the guanine nucleotide exchange factor DOCK7, which regulates the small G proteins RAC1 and CDC42, and prioritized further investigation of the protein owing to its known role in HGF-induced invasion of glioblastoma cells.
Mechanistically, ligand-induced EGFR activation leads to upregulation of BIN3 and tyrosine phosphorylation of DOCK7, leading to a physical association of BIN3 and DOCK7. DOCK family members have a RhoGEF domain and function as guanine nucleotide exchange factors for the RhoGTPase family of signaling proteins.
Together, the experiments indicated that ligand-induced EGFR activation leads to decreased invasiveness by a BIN3-mediated inhibition of a DOCK7RhoGTPase pathway.
“These findings show that a patient stratification based on the level of EGFR and EGFR ligands may be helpful to predict subsets of patients likely to benefit from targeting the EGFR,” Dr. Habib says. “In patients with EGFRamplified glioblastomas, with high EGFR ligands, EGFR inhibition could actually be detrimental, since EGFR ligands and high phosphorylation of the EGFR confer a better prognosis.”
On the other hand, in EGFR-amplified tumors with a low level of ligands, a brain-penetrant drug such as tofacitinib, a selective JAK1/JAK3 inhibitor that upregulates BIN3 through upregulation of EGFR ligands and suppresses invasion, could be very useful, he adds.

From preclinical studies through regulatory approval, the average timeline for discovering, developing and bringing to market a new drug extends more than a decade and can cost hundreds of millions of dollars. With thousands of drugs now FDA-approved for use in humans, new developments have opened opportunities for potentially expanding indications of marketed drugs to other diseases.
“Tofacitinib is a drug already used to treat rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis,” Dr. Habib says. “Tofacitinib could be a unique and effective treatment for glioblastoma that specifically targets invasion and upregulates BIN3 levels.”
The researchers confirmed the effect of tofacitinib in vivo in several orthotopic PDX models. In these experiments, they engineered tumor cells to express the EGFR ligand TGFα, so that tumor cells secreted this ligand in an autocrine manner.
Dr. Habib further explains that this type of treatment could be used to suppress invasion prior to surgical resection and shrink the main tumor mass. Since invasion is thought to be a key determinant of glioblastoma recurrence, tofacitinib could also be used later in the course of the disease to prevent or treat recurrence.
More generally, for EGFR non-amplified tumors with a high level of ligands, or possibly for EGFR-amplified tumors with a low level of ligands, EGFR inhibition combined with blunting of the accompanying adaptive response could be a useful therapeutic strategy, Dr. Habib says.
In collaboration with Pfizer, Dr. Habib and Michael Youssef, M.D. – an Assistant Professor of Neurology and Hematology-Oncology and member of both the Simmons Cancer Center and the O’Donnell Brain Institute at UT Southwestern – are leading a new clinical trial examining the effects of tofacitinib in patients with recurrent glioblastoma. The study (NCT05326464) is currently accruing patients at Simmons Cancer Center.
The co-investigators anticipate the trial has the potential to advance the standard of care and improve the quality of life of patients with this debilitating malignancy. Moreover, if the results are positive, it could prompt the FDA to approve the drug for this indication.
“We have already validated the feasibility of tofacitinib in preclinical models of amplified EGFR glioblastoma tumors,” Dr. Youssef says. “This trial provides us with the opportunity to confirm these observations with evidence from a Phase 3 study.”
In the trial, eligible patients with recurrent glioblastoma are being treated with 10 milligrams of tofacitinib twice daily until evidence of progression, intolerance of treatment, withdrawal of consent, or death.
The primary outcome is progressionfree survival as defined by RANO criteria. Secondary outcomes include overall survival, safety, and tolerability, and tumor response by RANO criteria up to two years after study treatment.
“We expect this trial to also provide further insight into the mechanisms underlying recurrent disease, which has not been well characterized,” Dr. Youssef adds.
As the trial continues to accrue, the researchers eagerly await the forthcoming results, which they expect by mid to late 2024.
“We expect this trial to also provide further insight into the mechanisms underlying recurrent disease, which have not been well characterized.”MICHAEL YOUSSEF, M.D. Assistant Professor of Neurology and Hematology-Oncology

A next direction for the research could be to determine whether distinct ligands differentially affect EGFR signaling downstream from EGFR, Dr. Habib says.
“Further studies may also test how distinct ligands differentially affect downstream signaling, and the ability to block EGFR signaling at multiple nodes both upstream and downstream of this receptor tyrosine kinase,” Dr. Habib notes. “These findings could inform future clinical
approaches in patients with EGFRamplified glioblastoma.”
More generally, additional research is needed to understand how the current findings affect our treatment regimens using EGFR inhibitors, Dr. Habib explains.
Dr. Habib acknowledges that in theory, adapting the current precision-medicine workflow by stratifying patients by their levels of EGFR ligands represents an opportunity to improve outcomes but could present practical challenges.
“If we were to stratify patients, we’d have to determine how and when a patient’s level of EGFR ligands would be assessed,” he adds.

CONCLUDING REMARKS
While a clinical paradigm that incorporates EGFR amplification status may benefit some patients, curative treatment regimens will probably continue to rely on combination therapy.
According to Dr. Habib, glioblastoma has historically proved intractable to treatment, likely due to incomplete understanding of the complex signaling networks required for tumor cells to proliferate, invade, and develop resistance to treatment.
Future studies, both in the clinical and basic science realms, will be needed to fully elucidate whether outcomes in patients with EGFR-amplified glioblastoma can be improved by targeting oncogenic functions of EGFR while sparing its potential tumorsuppressive roles.
“The O’Donnell Brain Institute is truly a unique environment for neuroscience and clinical care,” Dr. Habib says. “Abundant grant and funding opportunities, such as the Investigator Program and the Sprouts Grant Program, encourage creative, highquality research that continues to make a difference in our patients’ lives.”
This study was funded by grants from the Department of Veterans Affairs (VA) (2I01BX002559-08) and the National Institutes of Health (1R01CA244212-01A1 and 1R01NS119225-01A1). The VA has filed a patent on the use of tofacitinib in glioblastoma, listing Dr. Habib as the inventor. The clinical trial will be conducted at the Simmons Cancer Center.
Amyn Habib, M.D., is a Professor of Neurology at UT Southwestern Medical Center and a Staff Physician at the VA North Texas Health Care System/Dallas Veterans Affairs Medical Center. His research interests include investigation of growth factor signaling pathways in glioblastoma and other cancers.
Michael Youssef, M.D., is an Assistant Professor of Neurology and HematologyOncology at UT Southwestern Medical Center. His research interests include glioblastoma, primary and rare brain tumors, and neurologic complications of systemic cancer therapy.
Interventional psychiatry answers unmet needs in treatment-resistant depression.

With the rising number of survivors into old age, depression among the elderly is growing, but the new crisis is among the young, whose rate of suicide increased 62% from 2007 to 2021.
The O’Donnell Brain Institute (OBI) has amassed a large multidisciplinary team of researchers and clinicians to improve existing treatments and discover new treatment options. On the clinical front, a comprehensive initial evaluation spares patients from navigating a maze of specialists and treatment options on their own. Instead, they are readily channeled to the most appropriate provider and most promising treatment or clinical trials.
Housed together, OBI’s fully integrated, broad spectrum of mental health researchers and practitioners go beyond developing and testing therapies within their siloes to understanding how they fit in relation to one another.
Among the leaders of this interventional psychiatry enterprise are Carol Tamminga, M.D., Chair of Psychiatry; Madhukar H. Trivedi, M.D., Professor of Psychiatry and Founding Director of the Center for Depression Research and Clinical Care; Nader Pouratian, M.D., Ph.D., Chair of the Department of Neurological Surgery; Kala Bailey, M.D., Vice Chair for Clinical Affairs for the Department of Psychiatry; and Frederick Hitti, M.D., Ph.D., an Assistant Professor of Neurological Surgery and Psychiatry and specialist in novel treatments for depression.
Despite the growing number of available treatments for depression, even when a diagnosis is made, treatment selection has long been largely trial and error.
The challenge of providing truly individualized care is at the heart of the OBI’s mission. Together, our psychiatrists, neuropsychologists, neurosurgeons, bioinformaticians, and basic researchers are creating algorithms to enable an earlier match between an individual’s depression and the specific therapy with the greatest chance of alleviating his or her suffering.
Our team is also developing novel pharmacological, neuromodulation, and behavioral therapy techniques to address the challenge of treatment-resistant depression and suicide.
“We want to understand the different dimensions of depression, which is a very heterogenous disease – how they are represented in the brain and which circuits are involved in driving the symptoms,” Dr. Pouratian says. “Then we can tailor our therapy based on those particular symptoms and those circuits.”
Psychotherapy and trials of medication continue to be the first-line treatment for depression. For the approximately 30% of patients who are resistant or refractory to these therapies, however, the options have never been more promising.
Historically, electroconvulsive therapy (ECT) continues to be a first-line option for some severely ill patients or for those who fail other interventions. However, Dr. Bailey says that for some patients with depression, the group recommends transcranial magnetic stimulation (TMS), in
which the stimulus is applied from outside the brain to the left dorsolateral prefrontal cortex. The OBI offers both repetitive TMS (rTMS) and intermittent theta-burst TMS (iTMS) therapies.
“ECT has been the only real choice [for patients with severe treatment-resistant depression], yet only about 1% of these patients receive this therapy. Now, there are new treatments such as TMS that, in some cases, is a more accessible and desirable step before ECT is considered,” Dr. Bailey says. “TMS works for depression about 40% of the time, though there are some exciting data on how accelerated protocols or neural navigation may improve TMS efficacy.”
Dr. Tamminga, whose research focuses mostly on psychosis, is particularly interested in TMS because of the overlap between psychosis and depression.
“Early indications show that TMS holds promise in alleviating depression symptoms in some patients with psychoses just as they do in patients with solely a depression diagnosis,” she says.
Other therapies offered for treatmentresistant depression include low-dose ketamine, which is proving particularly useful in patients who have attempted suicide. Practitioners are also using ketamine as an ongoing maintenance treatment for depression.
“About 50-60% of medication-refractory patients are finding significant benefit,” Dr. Bailey notes.
OBI practitioners are now using deep brain stimulation (DBS) for a growing number of treatment-refractory patients and committing significant resources to DBS research, which holds promise for widespread applications and compellingly rapid symptom relief.
“We want to understand the different dimensions of depression… which circuits are involved in driving the symptoms.”
NADER POURATIAN,M.D.,
PH.D.Chair and Professor of the Department of Neurological Surgery
This promise led OBI neurosurgeons, psychiatrists, and neurologists to convene with NIH and industry representatives to brainstorm how to move forward with new DBS studies. What followed was the OBI team initiating two randomized control trials of DBS for depression.
The first trial seeks to evaluate whether advanced imaging can be used to enhance the effectiveness of subcallosal cingulate DBS for depression. The researchers are using MRI diffusion tractography in 12 patients and looking at connections within the white matter in the brain. This is part of the quest to precisely target the brain anatomy and circuits related to depression for each patient.
“We are using magnetic resonance tractography to find the precise spot for DBS to help with treatment-resistant
depression. We think that the ‘best spot’ can be found based on how the different parts of the brain connect with one another,” Dr. Pouratian says.
Dr. Pouratian is leading another DBS trial, collaborating with Baylor University, to evaluate the optimal location, proper timing, and amplitude of stimulation. Six patients will be enrolled at UT Southwestern in this first study to look at both imaging and physiology at the same time, learning if next-generation precision DBS with steering capability can be safely used to engage targeted networks and make adjustments that reduce depression.
In this work, the concept is to use the symptomatic network – rather than structural brain regions – to define the
target location. The researchers will examine how brain waves change as symptoms of depression improve and how stimulation can push those brain waves toward a “less depressed” state.
“With movement disorders, we can see the tremor, we can feel the rigidity, and we can adjust the stimulation settings to fix those things. But with affective disorders, the symptoms don’t change quickly,” Dr. Hitti says.
Dr. Pouratian explains the team’s approach for narrowing in on the correct targets.
“We are not only implanting stimulators but additional electrodes across the brain, especially the prefrontal regions that are thought to be critical for mood regulation” Dr. Pouratian says. “The team uses brain signals from these recordings to judge how effective stimulation is going to
be and how to make a stimulation strategy even more effective.”
The ultimate goal is to find signatures of these activities and use them to precisely match brain pathology to the location and type of stimulation, thereby accurately targeting specific presentations such as anhedonia.
“There are people for whom the reward circuit is really the major culprit, and others for whom the control and cognition circuit or the amygdala fear-and-stress circuit are affected. We have to learn to define the problem for these various groups,” Dr. Hitti says.
Intracranial stimulation has other applications beyond depression and movement disorders. Dr. Tamminga and Dr. Pouratian are leading a project, in
“But, if the research supports it, we are looking toward using DBS and magnetic seizure therapy (MST) in the future for a broad spectrum of these disorders.”CAROL TAMMINGA, M.D. Chair of the Department of Psychiatry and Chief of the Division of Translational Research in Psychosis
collaboration with neurological surgeon Bradley Lega, M.D., exploring DBS for patients with psychosis.
“Currently, our patients with psychoses may receive TMS or ECT, and we are also using these modalities to great advantage for autism, bipolar disorder, and other serious mental illnesses,” Dr. Tamminga says. “But, if the research supports it, we are looking toward using DBS and magnetic seizure therapy (MST) in the future for a broad spectrum of these disorders.”
Tackling the biological piece of the depression puzzle, researchers led by Dr. Trivedi are electronically combing patients’ physiological and clinical histories and using MRI diffusion tractography to identify biomarkers of depression.


“We are asking what all the factors we can gather about individual patients can tell us about how all these treatments work in the brain, to help establish brain and blood tests that can be used to individualize treatment targets,” Dr. Trivedi says.
Toward this end, he launched a large Framingham-type longitudinal study, the Texas Resilience Against Depression (T-RAD) study, to create a description of the pathology for each individual – from cells, to molecules, to circuits, to behavior. Biological samples are accessed through the nationally acclaimed University of Texas biobank.
Dr. Trivedi’s team has also published extensively on inflammation biomarkers, proteomic markers, and metabolic markers and is now looking at RNA sequencing to observe its effect on the initiation and continuation of depression.
Dr. Hitti, whose work straddles the neurosurgery and psychiatry realms, is currently performing basic research on rodents to look at depression-related brain circuits. Using viral-delivery techniques, he is turning brain areas off and on with a drug.
“We are applying stress to the rodents to look at emotional regulation processes,” Dr. Hitti explains. “For example, if I turn a brain area off, will that make their social withdrawal go away? The goal is to determine what part of the brain does what in different individuals and situations.”
Other modalities are also being used and researched for depression resolution. OBI clinicians are part of a multicenter study called RECOVER that is investigating the effect of vagus nerve stimulation (VNS) in Medicare patients with treatment-resistant depression. In related work, they are using responsive neurostimulation (RNS) for patients with obsessive-compulsive disorder and addiction, wherein a signal will stimulate the subthalamic nucleus of the brain when it begins to trigger unwanted thought patterns.
Supported by a National Institutes of Health grant, Dr. Trivedi is researching cutting-edge treatments for teenagers who have attempted suicide.
“There may also be patient populations who could be good candidates for research on psychedelics such as psilocybin,” he says. “These drugs can, in essence, push a reset button that breaks a pathological neural circuit they are stuck in, whether it is a fear-based circuit involving the amygdala or the reward circuit affecting motivation and pleasure, for example.”
Statistically, ECT is still the most triedand-true therapy for treatment-resistant depression, but given the potential cognitive side effects, OBI researchers are investigating MST as an alternative.
“We have a grant to learn more about MST, and we are hoping to find that it has the same antidepressant effects as ECT but with a lower risk of cognitive side effects,” Dr. Tamminga says.
The OBI team is also testing new drugs, including KarXT, a muscarinic agonist and antagonist combination that targets the M1 and M2 receptors in the brain.
“It will likely be brought out for psychosis treatment, but it may be a cognitive enhancer for patients with depression as well,” Dr. Tamminga says.
“We are asking what all the factors we can gather about individual patients can tell us about how all these treatments work in the brain, to help establish brain and blood tests that can be used to individualize treatment targets.”
MADHUKAR TRIVEDI, M.D. Professor of the Department of Psychiatry
The depth of depression research at the OBI is matched by its breadth, which includes outward-facing work that our clinicians, across the board, embrace.
Through funding from the Texas Child Mental Health Care Consortium (TCMHCC), for example, OBI psychiatrists partner with practitioners in primary care and pediatric settings in the DallasFort Worth area and are involved in a statewide research network for youth with depression and at risk for suicide. They also lead a statewide network providing prevention and mental health awareness programs in high schools.
“A pediatrician or school counselor can call a psychiatrist for advice if their students are experiencing mental health problems,” Dr. Tamminga says. “To enable us to help young people in the future, we have created registries of children who have been involved in trauma and children who have had childhood depression, and we use those registries in our studies.”
Many OBI interventional psychiatry and psychology leaders are involved in the pediatric and adult educational programs at UT Southwestern Medical Center and
Children’s Medical Center Dallas, as well as Parkland Memorial Hospital and the Dallas Veterans Affairs Medical Center.
Importantly, the state and city, along with UT Southwestern and Children’s Medical Center, are taking another giant leap forward in care for patients with severe mental illnesses by building a new psychiatric hospital, the Texas Behavioral Health Center at UT Southwestern, scheduled to open in 2025.
“We are very excited about this hospital that will have 200 adult beds and 96 children’s beds,” Dr. Tamminga says. “It will be designed for small groups to be treated with others who have similar illnesses. We have not previously had a psychiatric hospital available within 90 miles – this will be a chance for Dallas as a community to be served and will also enable new and impactful research on serious mental illness.”
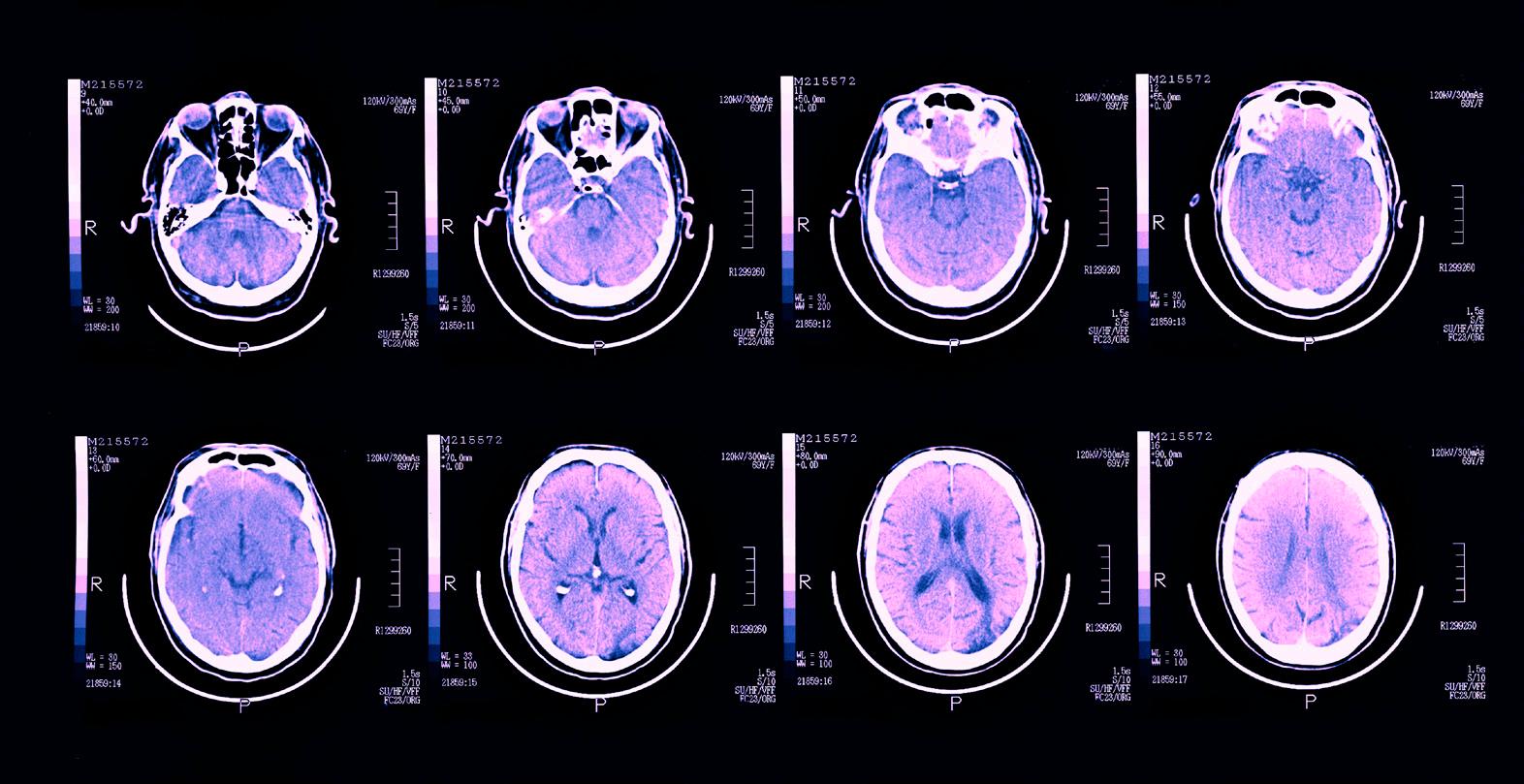

Kala Bailey, M.D., is Vice Chair for Clinical Affairs for the Department of Psychiatry. She is also a member of the Peter O’Donnell Jr. Brain Institute Clinical Leadership Committee. Her clinical expertise is in interventional psychiatry, utilizing neurostimulation and other innovative methods to treat medication-refractory mental disorders.
Frederick Hitti, M.D., Ph.D., is an Assistant Professor in the Department of Neurological Surgery and the Department of Psychiatry. He specializes in neuromodulation to treat epilepsy and movement disorders. He also works on the development of novel therapies for psychiatric disorders, such as treatment-resistant depression.
Bradley Lega, M.D., is an Associate Professor in the Department of Neurological Surgery. He has secondary appointments in Neurology and Psychiatry. His research focuses on preserving memory function and restoring memory to patients with brain injuries or brain tumors.
Nader Pouratian, M.D., Ph.D., is Chair and Professor of the Department of Neurological Surgery. He holds the Lois C.A. and Darwin E. Smith Distinguished Chair in Neurological Surgery. His research focuses on developing brain mapping techniques to improve the precision and targeting of neurosurgical procedures.
Carol Tamminga, M.D., holds the Stanton Sharp Distinguished Chair in Psychiatry. She is Chair of the Department of Psychiatry and Chief of the Division of Translational Research in Psychosis. Her research focuses on the mechanisms underlying schizophrenia, especially its most prominent symptoms, psychosis, and memory dysfunction.
Madhukar Trivedi, M.D., is a Professor in the Department of Psychiatry, Chief of the Division of Mood Disorders, and the founding Director of the Center for Depression Research and Clinical Care, where he holds the Betty Jo Hay Distinguished Chair in Mental Health and the Julie K. Hersh Chair for Depression Research and Clinical Care. He specializes in treating depression.


Progress in joint replacement procedures has led to ever-shorter surgeries and broadened options, yet innovations in treating spine pathology – the leading cause of pain in adults the world over – has remained in the risk-averse shadows. Too often, these patients travel on diagnostic or treatment odysseys before receiving appropriate personalized treatment.
Spine practitioners at the O’Donnell Brain Institute (OBI) Spine Center are working to properly align treatment to the needs of the individual patient. As part of a truly transformative perioperative program, they are leading the way in integrating novel techniques and devices to bring the best interventions out of the shadows.

“The fact that we have one of the most integrated spine care programs in the country allows us to evaluate and treat patients holistically, without a subspecialty bias,” says Nader Pouratian, M.D., Ph.D., Chair and Professor of the Department of Neurological Surgery. “If core strengthbuilding and lifestyle changes help, great! But if an intervention – whether an injection or surgery – is in the patient’s best interest, I believe there is no place where the risks of adverse events are better mitigated.”

Established in 2016, the OBI Spine Center, which encompasses four specialties –neurosurgery, physical medicine and rehabilitation, orthopaedic spine surgery, and anesthesiology – is one of few medical centers in the country to apply Enhanced Recovery After Surgery (ERAS) to spine surgery. Leading the OBI’s advances in perioperative optimization is Salah Aoun, M.D., Director of ERAS, who has authored more than 80 publications on the topic in the past two years.
“ERAS is the quintessential crystallization of our Spine Center’s efforts to improve surgical patient management,” he says. “What defines success for the patient is largely whether they are prepared beforehand and monitored afterward. Many spine services will involve perioperative difficulties, so our patients see pain management doctors in advance to optimize their narcotic regimen before and after surgery. Then, every patient undergoing a complex surgical reconstruction sees a psychiatry practitioner to make sure they understand the goals and timeline for healing.”
Patients with first-tier procedures such as anterior cervical fusion disc replacement, small discectomies, and small laminectomies in the lower back require only an overnight stay and rarely have complications. ERAS makes the largest impact on the middle tier of procedures, such as short segment fusions and short 360s. Patients with these procedures generally stay in the hospital for two days and have a higher risk of complications and narcotic dependence.
The OBI also integrates ERAS to improve outcomes in its most complex surgeries, such as scoliosis reconstruction.
Through ERAS, surgical candidates
can meet patients who have had their procedure, hear their testimonials, and watch videos that feature their practitioners’ work. Addressing the balancing act of pain management, while minimizing narcotic intake, starts with a six-week prehab, so patients know what’s expected of them after surgery, boosting their motivation to walk early and commit to rehabilitation.
One of the Spine Center team’s strategies for managing pain in patients with spine deformities is administering epidural analgesia and long-lasting incisional blocks after surgery. Compared with a typical pain regimen, this can make a huge difference in patients avoiding narcotic dependency.
“It’s been remarkable – at three months and six months, they just aren’t renewing their narcotics scripts,” Dr. Aoun says.
The Spine Center team was the first to adapt a joint replacement epidural anesthetic called Exparel to complex spine surgery patients. Exparel consists of microsomal lidocaine or Marcaine enclosed in tiny fatty molecules that release over time. Its use significantly reduces pain for four days so that patients are able to walk, which reduces wound infections, blood clots, constipation, and urine retention.
“These patients require less pain medication both during and after surgery,” Dr. Aoun says. “We are tracking all that data through our patientcontrolled anesthesia (PCA) software. It makes a huge difference in reducing narcotic dependence.”
Spine Center researchers are continuing to gather data to publish results on the anesthetic’s effectiveness and are
establishing a general use protocol on Exparel for both neurosurgery and orthopaedic surgery.
The team is also using epidurals in a novel way with patients undergoing highest-tier procedures such as scoliosis corrections, resection of tumors requiring anterior and posterior access, and multilevel fusion. This enables patients to walk in the first few postoperative days without oral narcotics.
Dr. Aoun says the operative time for big cases has dropped from as long as 12-15 hours to 4-6 hours, thanks to the just-intime effect built into the ERAS program.
“We are making sure that every time we look at a case, we know how many hours it should take, and we have a process for making sure all the tools, equipment, and
For patients with complex spine deformities, the center is pioneering new enhancements in surgical techniques and aides.
staff are at the ready,” Dr. Aoun says. “On our scoliosis team, we have the same three people who do the instrumentation. From techs to nurses to surgeons, there is a lot of pride that goes into reaching our goals.”
For patients undergoing highest-tier surgeries, the OBI has also made a groundbreaking leap by establishing two dedicated ICUs within the institute, making it one of a handful of spine centers in the nation to provide this level of care. The team is conducting research through these ICUs, including several traumatic brain injury trials to optimize decompression and medication management.
For patients with complex spine deformities, the center is pioneering new enhancements in surgical techniques and
aides. One of these utilizes life-size 3D prints of the spine that enable Dr. Aoun and his team to more precisely plan and execute procedures.
“Instead of wasting an hour and a half figuring out where we’re going to put the screws to line up the vertebra, this model acts as a reference I hold in my hands and can move 360 degrees,” Dr. Aoun says.
The Spine Center is one of the first to offer the new Intracept radiofrequency ablation procedure for vertebrogenic low-back pain and currently performs seven to eight of these procedures per month.
Robotic techniques still play a narrow, but valuable, role in Spine Center surgeries, primarily helping with precise placement of hardware in areas of high risk for displacement, such as the L5 S1, where the bone angle and hardness make manual placement precarious.

The Spine Center is at the forefront of trials for implant materials that are revolutionizing spine surgery.
One is a two-year trial on a Medtronic implant technique, where an artificial vertebra or other bone replacement is micro-etched to resemble bone on a microscopic level. When fusion takes place, instead of overproducing inflammatory agents to help colonize cells, this material sidesteps the inflammatory phase in healing.
“The body thinks the hardware is just a bone graft,” Dr. Aoun says. “Now, the same technology is used in screws as well.”
This technique may also be a solution for smokers, older patients, and those with poor bone integrity, whose bodies don’t produce the inflammation necessary to engender fusion healing. Dr. Aoun is scanning cervical spine patients at three months to compare progress against those implanted with unetched titanium or plastic materials.
In another innovation, the Spine Center is in phase 2 trials on implanting custom rods. Patient images are sent to the rod manufacturer beforehand, where they calculate the degrees that the patient is off alignment, prepare a plan that accounts for the curve and line-up of the screws, and then manufacture rods to fit the specific patient. During the procedure, the surgeons can make changes if needed and later send postsurgical X-rays to compare actual and expected outcomes for continuous improvement and self-monitoring.
More pliable discs are also finding their way into the Spine Center surgical suite. The spine team is providing feedback on new cervical discs that mimic the natural disc. Instead of sliding into flexion and extension, they enable the joints to move 360 degrees and in unison.
“We are seeing a significant improvement in neck pain, which should reduce the risk of surgery in the future. In a younger person with a herniated disc, this implant winds the clock back to before the herniation,” Dr. Aoun says.
The OBI is currently participating in two stem cell trials. In one phase 1 safety study, stem cells are injected into the spinal cord of patients with paraplegia, and thus far the study has proceeded without safety concerns. In a second trial, the team is working with colleagues at Parkland Memorial Hospital testing anti-inflammatory interleukin molecules within 48 hours of acute spinal cord injury to see if nerve damage is mitigated or even reversed.
Also in conjunction with Parkland, the Spine Center is in several ICUrelated trials. One is looking at when to decompress a patient with traumatic brain injury (TBI) and when to give them medication or transfusions.
“When someone comes in with a TBI, we have found that transfusing these patients at higher blood volume increases their chances of living longer, more functional lives,” Dr. Aoun says. “This led to our publishing new transfusion guidelines on TBI and spine fusion.”
“Most spine patients don’t need surgery, so if we can get that patient better without it, that is our initial goal.”
KAVITA TRIVEDI, D.O.Medical
Director ofthe Multidisciplinary Spine Center
In an enterprise that serves more than 2,300 patients per month, Kavita Trivedi, D.O., Medical Director of the Spine Center, heads operations and helps practitioners keep up with the new evidence-based research in the field.
“I feel very passionate about providing good spine care to patients. A lot of people have come to us having undergone multiple spine surgeries without an improvement in their initial symptoms or perhaps having gone without any meaningful treatment before finally coming to us,” Dr. Trivedi says. “Many of these patients may benefit from different types of physical therapy, injections, nerve blocks, radiofrequency ablations, or minimally invasive surgeries.”
Of utmost importance to the center’s mission to provide integrative, holistic
spine care is ensuring communication among nonsurgical and surgical providers alike.
“We all have the same values and priorities of conservative care, when possible,” Dr. Trivedi says. “Most spine patients don’t need surgery, so if we can get that patient better without it, that is our initial goal.”
An advantage of being under one roof is the ease of having weekly meetings to discuss patients and lend expertise on cases.
“Physicians from all four disciplines may bring in cases where, for example, the patient may either have small nerve disease or something pressing on the nerve, and we share opinions. Residents, trainees, and all spine providers are all welcome to present challenging cases,” Dr. Trivedi says.
Through a multidisciplinary quarterly journal club, different members of the spine team present findings to the group from some of the “hottest” new journal articles for potential incorporation into the center’s practice.


The communication priority Dr. Trivedi promotes is pervasive throughout the center.
“If you don’t educate patients about what’s causing their symptoms, they’re not going to be compliant with your recommendations,” Dr. Trivedi notes.
She adds that this starts with a holistic initial evaluation, looking at root causes of spine problems and their connections with other problems such as shoulder, hip, or knee pathologies.
“Depending on what they have, the first line of treatment may be something like physical therapy, but there are different types of treatment for spine issues,” she says. “The thing I always ask my patients is, ‘What are you not able to do in your
normal life that you want to do?’ It may be ‘I can’t play soccer with my kid,’ or ‘I can’t work,’ or ‘I can’t go to a movie with my girlfriend.’ That gives us a goal. I also ask them what approach they want to take, because if they don’t believe in what they’re doing, they’re not going to do it, and then they’re not going to get better.”
The team offers several community lectures per year, presented by one of the providers, where patients, families, and others can learn about spine and musculoskeletal health. Dr. Aoun leads an online course for patients with a spine deformity such as scoliosis, explaining how the surgery is going to be done and what to expect before and after.
“It’s a good time to be a patient at the OBI – the gadgets are great, and patient care has undergone a sea change. I think we have an exciting 20 years ahead, building on these innovations,” Dr. Aoun says.
“It’s a good time to be a patient at the OBI – the gadgets are great, and patient care has undergone a sea change. I think we have an exciting 20 years ahead, building on these innovations.”
SALAH AOUN, M.D. Assistant Professor in the Department of Neurological Surgery and Director of the ERAS Program
Nader Pouratian, M.D., Ph.D., is Chair and Professor of the Department of Neurological Surgery at UT Southwestern.
Salah Aoun, M.D., is an Assistant Professor in the Department of Neurological Surgery and Director of the ERAS Program at UT Southwestern.
Kavita Trivedi, D.O., is an Associate Professor in the Department of Physical Medicine and Rehabilitation (PM&R) and Medical Director of the Multidisciplinary Spine Center at UT Southwestern.

UT SOUTHWESTERN’S CHIEF OF STROKE REHABILITATION DISCUSSES PROGRESS MADE IN CLINICAL CARE, RESEARCH, AND EDUCATION
The field of stroke rehabilitation has made rapid advances in recent decades, propelled by innovations in early, coordinated, and multidisciplinary care. Still, the greatest era of innovation lies ahead with the advent of precision rehabilitation, biomarkers, and related technologies.
Nneka Ifejika, M.D., M.P.H., a Professor of Physical Medicine and Rehabilitation and Neurology at UT Southwestern Medical Center, is a leading rehabilitation expert with a clinical and research focus in acute stroke rehabilitation, including transitions of care, outcomes, and health disparities.

Dr. Ifejika has played an active leadership role in conducting and disseminating practice-changing research internationally and is the only female Physical Medicine and Rehabilitation (PM&R) physician who is a Fellow of the American Heart Association’s Stroke Council. In addition, she has been recognized by the National Institutes of Health for teaching and mentorship of underrepresented medical students, residents, and fellows.
“The field of stroke rehabilitation is advancing at a rapid pace – especially around novel approaches to stroke care and treatment,” Dr. Ifejika says. “While most individuals with stroke survive, they do typically live with chronic impairments in body function and activity limitations that impact their quality of life.”
While there is debate regarding the precise timing and intensity of early rehabilitation after an acute stroke, experts generally accept rehabilitation’s beneficial effect on functional disability. Consequently, initiation of stroke recovery efforts from the time of symptom onset can help guide care and improve outcomes.
“Our goal is to bridge the gap between the hospitalization for acute stroke and the initiation of the ensuing rehabilitation process,” Dr. Ifejika says.
She notes that the stroke rehabilitation program at UT Southwestern –which incorporates evidence-based rehabilitation paradigms through all levels of stroke care – is highly
innovative compared to other programs nationally.
Dr. Ifejika believes that a successful stroke rehabilitation program requires a multidisciplinary, team-based approach, one that involves neurologists, nurses, physical therapists, occupational therapists, speech therapists, social workers, and other professionals trained specifically in stroke rehabilitation and recovery.
“UT Southwestern has a highly collaborative environment, and in our program, we implement stroke recovery efforts from the time of symptom onset,” she says.
The typical stroke patient follows a complex path from the onset of symptoms to the years following the event. Along this journey, transitions
take place between the respective spheres of care.
“Transitions of care for stroke patients are complex,” Dr. Ifejika explains.
“Major transitions of care include from the place of stroke onset to acute care hospital and from there to other medical care facilities and then home.”
To simplify these transitions, Dr. Ifejika and her team at UT Southwestern developed and tested a new transition of care model that facilitates process flow from acute stroke hospitalization to post-acute rehabilitation.
Implementation of the team’s multistep care model resulted in multiple improved outcomes, including a reduced length of time from acute stroke admission to inpatient rehabilitation care admission, an increase in referrals to the inpatient rehabilitation facility, and a decrease in referrals to skilled nursing facilities.
“The identification of biomarkers, combined with data on disease, patient characteristics, and functional assessment, will accelerate the application of precision medicine in rehabilitation therapy.”
NNEKA IFEJIKA, M.D., M.P.H. Professor of Physical Medicine and Rehabilitation and Neurology
Dr. Ifejika and her colleagues recently published these findings in Archives of Physical Medicine and Rehabilitation.
“Our model includes weekday interdisciplinary huddle rounds, which create a pathway to ensure stroke patients receive comprehensive rehabilitation care,” Dr. Ifejika explains. “We’ve also incorporated a virtual rounding tool, allowing clinicians to evaluate plan-of-care facilitation using the electronic medical record (EMR).”
Based on their findings, the researchers plan to further validate the model, with the long-term goal of implementing it in stroke centers across the country.
Precision rehabilitation involves applying the optimal type and dose of treatment at the ideal time to maximize return of function for individual patients. Currently, this strategy is of considerable interest to providers and has the potential to substantially reduce long-term disability.
“While precision rehabilitation is conceptually appealing, building a robust research base for the approach has remained challenging,” Dr. Ifejika notes. “The identification of biomarkers, combined with data on disease, patient characteristics, and functional assessment, will accelerate the application of precision medicine in rehabilitation therapy.”
She adds that there are many potential use cases of blood-based biomarkers in stroke care, including earlier diagnosis, aiding in triage, risk stratification for acute management, prognostication of functional recovery, and others.
In an ongoing pilot study, Dr. Ifejika and her team are identifying biomarker signals at specific time intervals during the acute stroke hospitalization period. With their findings, they hope to define a “golden window” phase during which use of a multimodal treatment intervention can boost stroke recovery.
“We’re assessing for relevant biomarker signals within the early acute stroke interval,” Dr. Ifejika explains. “In the angiography suite, we draw blood samples before the clot is even removed from the brain – and following this intervention, we recheck to see if the biomarker levels have changed.”
As data analysis continues, the researchers eagerly await forthcoming results, which they expect could inform the development of larger-scale biomarker studies.
Dr. Ifejika is also working to address racial disparities in stroke care and outcomes.
“Racial differences in the treatment of post-stroke sequelae may contribute to known disparities in post-stroke function,” she says. “Improving our understanding of racial differences in functional outcomes may help home in on the underlying causes.”
According to Dr. Ifejika, significant racial disparities exist in the treatment of common post-stroke sequelae within large hospitals across the United States.
In a recent study, Dr. Ifejika and her team analyzed EMR data from 65 large health care organizations to identify a cohort of non-Hispanic white, Black, and Hispanic hospitalized acute stroke
Data from 65 large health care organizations … found that compared to non-Hispanic white patients, Black patients were significantly less likely to receive treatment for every condition at nearly every timepoint measured.

patients. The researchers’ goal was to quantify the magnitude and timing of racial differences in the treatment of common post-stroke complications. After analyzing the data, they found that compared to non-Hispanic white patients, Black patients were significantly less likely to receive treatment for every condition at nearly every timepoint measured (14-, 90-, and 365-days post-stroke).
Overall, the team found the differences were greatest at 14 days, indicating that acute hospitalization and rehabilitation are crucial to identify and treat complications to reduce disparities in post-stroke function. These findings were presented at the 2023 International Stroke Conference in Dallas, where Dr. Ifejika’s group received the Stroke Rehabilitation Award and the Paul Dudley White International Scholar Award for the highest-scored abstract in the U.S.
While physiatry residency training provides a variety of multidisciplinary clinical experiences, research might not be pursued by residents due to several factors, including limited research exposure and uncertainty of how to begin a project.
“Limited resident participation in clinical research negatively affects the growth of physiatry as a field and medicine as a whole,” Dr. Ifejika says. “Resident research trainees are uniquely positioned to become future leaders of multidisciplinary and multispecialty collaborative teams.”
To combat the shortfall, leaders within the Department of PM&R and the Peter O’Donnell Jr. Brain Institute have

taken steps to create resident research programs. The research team within the Department of PM&R at UT Southwestern consists of residents, each of whom is assigned a primary mentor, a sponsor, secondary mentors, and collaborators.
“As a resident, you get to develop your own research question and design the project with guidance from the primary mentor,” notes Audrie A. Chavez, M.D., M.P.H., a former resident in the Department of PM&R, who recently completed a brain injury medicine fellowship at Harvard University. “Residents in the team synergistically lead their own projects and collaborate on their co-residents’ projects.”
Dr. Chavez adds that this dynamic is important because team members learn to work cohesively to implement the project and collect data despite variations in individual workload.
“We call it a ‘research family,’” Dr. Ifejika says. “By instilling a sense of community in the work environment, accountability rises amongst our group members, and research productivity increases as a result.”
Dr. Ifejika is excited about the future of stroke rehabilitation and hopes that an improved understanding of transitions of care, outcomes, and health disparities will continue to aid efforts in recovery and reduction of long-term disability.
“We hope our work will lead to more investigation, as the unmet medical need for stroke patients remains high,” she says.
Nneka Ifejika, M.D., M.P.H., is a Professor of Physical Medicine and Rehabilitation and Neurology at UT Southwestern Medical Center. She serves as the Section Chief of Stroke Rehabilitation and is the only African American woman physician in the United States with this distinction. Her research focuses on acute stroke rehabilitation, including transitions of care, outcomes, and health disparities.
Audrie A. Chavez, M.D., M.P.H., is a former resident in the Department of Physical Medicine and Rehabilitation at UT Southwestern Medical Center.