14 minute read
PLANT FEATURE: MIGHTY PLANT MITOCHONDRIA
MIGHTY PLANT MITOCHONDRIA
BY CAROLINE WOOD
Advertisement
In biology textbooks, mitochondria are typically portrayed as little more than the ‘energy powerhouses’ of cells, but researchers are uncovering an increasing number of processes that involve these ancient organelles. Caroline Wood takes a look at recent highlights from the SEB journals that portray the diverse roles mitochondria can assume in plant cells.
SIGNALLING STRESS
As climate change causes our weather patterns to become increasingly extreme, plant scientists are under pressure to develop crops that can better tolerate environmental stresses. So far, these efforts have typically focused on anatomical adaptations and physiological adjustments, rather than the molecular functioning of plant mitochondria. But as Oliver Berkowitz (La Trobe University, Melbourne, Australia) explains, this presents a missed opportunity. ‘Any plant response that requires energy or a shift in metabolic activity will also need to adjust the function of the mitochondria,’ he says. ‘Consequently, during stress responses, mitochondria play a key role by acting as sensors that link nuclear transcription with cellular energetics.’
In particular, mitochondria influence gene expression by communicating their functional status to the nucleus, a process known as retrograde signalling. Current evidence suggests that retrograde signalling is initiated by low-molecular-weight molecules, such as reactive oxygen species (ROS) and calcium ions. However, the extent to which this process contributes to stress responses remained unclear. To investigate this, Oliver led a study within Professor James Whelan’s laboratory group that focused on plant responses to submergence stress during flooding.1 Working on the model plant Arabidopsis thaliana, he found that impaired mitochondrial retrograde signalling led to significantly reduced flooding tolerance. Specifically, mutants with impaired ANAC017, a master regulator of gene transcriptional responses, and cyclin-dependent kinase E1 (CDKE1), a regulator of energy signalling, showed greater tissue damage during submergence and slower recovery.
To probe the mechanistic basis of these phenotypes, the team performed transcriptomic analysis of these mutant lines to identify gene networks that failed to respond during submergence. The results were compared with those from genetically diverse wild-type Arabidopsis accessions that showed naturally greater sensitivity to submergence. This
identified the transcription factors WRKY40 and WRKY45, previously identified as regulators of mitochondrial retrograde signalling.
Follow-up experiments with corresponding mutants and overexpression lines confirmed that these have a functional role in submergence tolerance. ‘Interestingly, the expression of these two WRKYs was increased already before the onset of stress under normal growth conditions in tolerant Arabidopsis accessions, suggesting that this altered constitutive expression is beneficial for stress tolerance,’ says first author Xiangxiang Meng. Furthermore, WRKY40 and WRKY45 have also been implicated in other stress responses, including abscisic acid (ABA)-dependent responses and leaf senescence, suggesting that mitochondrial retrograde signalling may play a role across a broad range of plant stresses.
Although his laboratory is already starting to investigate the impact of mitochondrial signalling for stress responses in rice, Oliver warns that using this work to develop improved crop cultivars is going to be challenging, and will take many years. ‘Mitochondria are deeply integrated with other processes, especially those of the chloroplasts,’ he says. ‘Impacting one will impact the function of the other with possible unforeseen or even detrimental effects.’
A TIME TO DIE
Besides stress signalling, new evidence suggests that mitochondria may also have an important role in the regulatory cascades that orchestrate programmed cell death (PCD) in plants. Animal mitochondria are already known to act as important signalling hubs during PCD, with various proteins in the mitochondrial membrane participating in apoptotic signalling cascades. But so far, no homologues of these signalling proteins have been found in plant mitochondria. ‘This meant that, traditionally, it was thought that mitochondria had a more limited role during PCD in plants,’ says Keiko Yoshioka (University of Toronto, Canada). ‘Specifically, their main contribution appeared to be in providing energy and generating ROS during senescence, a highly regulated process that occurs at the end of the plant life cycle or under unfavourable conditions.’ However, recent work led by Keiko’s group is starting to overturn this view of mitochondria only being ‘supporting actors’ during PCD.2
Keiko’s suspicions were first triggered by the ttm1 Arabidopsis mutant, which exhibits delayed natural and dark-induced senescence. The affected gene encodes triphosphate tunnel metalloenzyme 1 (TTM1), a tail-anchored protein in the outer mitochondrial membrane with three phosphorylation sites. Functional complementation assays in ttm1 mutants revealed that phosphorylation of a specific residue, serine 437, was critical for the protein’s role in senescence. ‘Interestingly, although plant senescence is regulated by the hormones ABA, ethylene and jasmonic acid, we found that TTM1 is specifically connected to ABA-mediated senescence, and that exogenous ABA or prolonged dark treatment leads to serine 437 phosphorylation,’ says co-author Wolfgang Moeder.
Using in vitro kinase and pull-down assays to identify upstream signalling components, the group demonstrated that serine 437 interacts with, and is phosphorylated by, the MAP kinase proteins MPK3 and MPK4, which had not previously been connected to senescence. Although the mechanistic link between serine 437 phosphorylation and PCD is not yet clear, Wolfgang suggests that the conformational change induced by phosphorylation may activate TTM1’s enzymatic activity or affect its interactions with other proteins.
But the surprises weren’t over. Confocal microscopic analysis of yellow fluorescent protein-tagged TTM1 variants revealed that phosphorylation at the other sites, serine 10 and 490, caused the protein to be removed from the mitochondrial membrane and degraded by the proteasome. ‘The fact that phosphorylation of these residues triggers TTM1 protein turnover suggests that TTM1 is required for a specific and short window during senescence to positively regulate cell death execution,’ says Wolfgang.
Having established this novel link between mitochondria and PCD, Keiko’s laboratory is now investigating the potential applications for agricultural crops. ‘We are studying the role of TTM1 in crops such as tomato and soybean, where a small delay in senescence can have a significant impact on the shelf life,’ she says.
LINKING CELLULAR ENERGY TO CELL CYCLE AND SEED DEVELOPMENT
Besides stress and senescence responses, mitochondria may also play important roles in
Left Mitochondrial DNA is the small circular chromosome found inside mitochondria. These organelles found in cells have often been called the powerhouse of the cell. 3D illustration
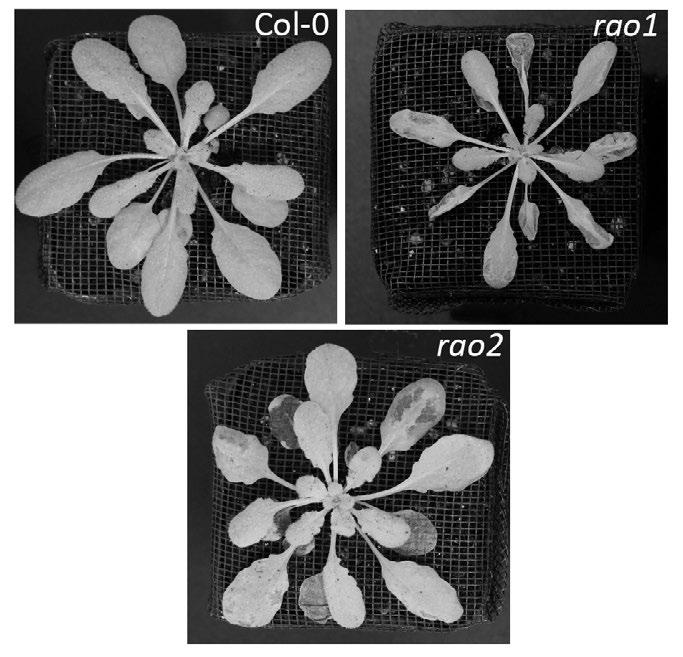
Top Phenotypes of the wild type (Col-0) and two mitochondrial signalling mutants (rao1/cdke1 and rao2/ anac017) after 2 days of submergence and 1 day recovery Photo credit Dr Xiangxiang Meng
developmental processes, as recently demonstrated by the opaque18 maize mutant. This mutant shows impaired embryo and endosperm development yet is economically important because the seeds contain nearly twice as much of the essential amino acid lysine as wild-type cultivars. Understanding the mechanistic basis of the seed opacity phenotype has been a challenge occupying plant researcher Guifeng Wang (Henan Agricultural University, China) for many years.
Using map-based cloning, Guifeng’s team validated the causal gene for the opaque18 phenotype to be ZmRIBA1, an enzyme that catalyses the first step in riboflavin biosynthesis.3 ‘It was initially unclear how riboflavin may be linked to the phenotype but we suspected that it could involve two riboflavin derivatives, flavin mononucleotide and flavin adenine dinucleotide (FAD),’ Guifeng says. ‘Because these are important electron carriers, we speculated that the assembly and activity of mitochondrial respiratory complexes could be impaired in opaque18 seeds.’
In support of their hypothesis, opaque18 mitochondria were structurally disrupted in the developing endosperm cells. Subsequent experiments revealed that loss of function of OPAQUE18 specifically disrupts the assembly of respiratory complexes I and II, shifting metabolic flux from the mitochondrial citric acid (tricarboxylic acid) cycle to glycolysis and thereby limiting cellular energy production.
Using flow cytometry of cells in developing opaque18 endosperm, they found that this lack of cellular energy ultimately led to cell-cycle arrest. ‘The strong deprivation of energy leads to cellcycle arrest of endosperm cells, probably by either restricting the activity of cyclin-dependent kinases or by triggering specific cell-cycle checkpoints,’ says Guifeng.
But although opaque18 mutants displayed cellcycle arrest, they exhibited increased expression of cell-cycle genes, suggesting that additional machinery may be implicated in this regulatory control. The group found that this gene upregulation correlated with increased methylation of the DNA packaging protein histone H3, which is required for cell-cycle progression. Guifeng suggests that this results from impaired activity of a key histone H3 demethylase enzyme, lysine-specific demethylase 1, given that this requires FAD as an essential cofactor.

‘Ultimately, this gene induction cannot reverse cell-cycle arrest, but this indicates the presence of an epigenetic control of cell cycle’ Guifeng says. ‘Thus, we propose that this histone methylationmediated regulation may be a back-up mechanism of cell-cycle progression under unfavourable conditions, including insufficient energy.’
AN UNLIKELY MODEL FOR HUMAN DISEASE
Beyond developing improved crops, research on plant mitochondria could also have applications for human health. Mutations in genes encoding mitochondrial proteins can cause devastating diseases, including cardiomyopathy, Leigh syndrome and neurodegenerative disorders. Many of the causative mutations affect complex I, an enzyme of the mitochondrial electron transfer chain; however, the molecular mechanisms driving the clinical symptoms remain poorly understood. ‘Most complex I-linked diseases in humans correspond to mutations that do not map to the known complex I structural genes, suggesting that genes encoding for yet-to-be-discovered assembly and biogenesis factors remain to be uncovered,’ says Patrice Hamel (The Ohio State University, USA).
A frustration for researchers into human mitochondrial diseases is that many popular nonhuman experimental models, including bacteria and the yeast Saccharomyces cerevisiae, either have a simplified complex I or lack it altogether. Fortunately, the plant kingdom has offered an unlikely alternative model: the unicellular photosynthetic alga Chlamydomonas reinhardtii. ‘Chlamydomonas is ideally suited for the study of mitochondrial function because mutants deficient for mitochondrial respiration are viable as long as they rely on photosynthesis for energy conversion,’ explains Patrice. Furthermore, Chlamydomonas with impaired mitochondrial function are unable to carry out acetate-dependent growth in the dark (which requires mitochondrial respiration), providing a ready-made phenotypic screen. And because complex I subunits are highly conserved among eukaryotes, genetic discoveries in Chlamydomonas are likely to be applicable to humans also.
It was this rationale that drove Patrice and his team to screen 54,000 insertional Chlamydomonas mutants to uncover genes that had not been previously associated with mitochondrial complex I.4,5 This yielded 13 complex I-deficient mutants, with two of the affected loci, AMC9 and AMC5, found to contain structural genes, verifying the screen as yielding bona fide complex I mutants. These mutants were later employed as the scaffold upon which to explore the effect of patient-derived mutations on complex I function, highlighting the utility of Chlamydomonas in understanding the molecular basis of human diseases.
Some of the other mutants revealed genes encoding novel mitochondrial complex I biogenesis factors. For example, the low-complexity protein AMC1 is required for expression of the mitochondrial gene nad4, which encodes a membrane subunit of complex I.6 The mechanism of AMC1 remains unknown for now, but Patrice expects that a similar protein must also operate in human mitochondria. His current plan is to dissect the molecular mechanism by which impaired AMC1 affects mitochondrial gene expression and complex I assembly.
‘Ultimately, we hope that we can apply these results in a chemical genetics approach to find molecules that can be developed as therapeutics,’ he says. ‘For instance, high-throughput screening of bioactive molecules restoring complex I assembly or activity to an algal mutant strain could lead to treatment options for patients with complex I-linked genetic diseases.’
A MITOCHONDRIAL ‘SOCIAL NETWORK’
It’s not just the biochemical and molecular properties of mitochondria that are of interest to researchers. In plants, individual mitochondria show highly dynamic behaviour, moving across the cell, coming together and occasionally fusing – something which captivated Iain Johnston (University of Bergen, Norway) when he first saw it captured on camera. ‘It was a natural phenomenon begging to be understood and for me raised a clear question: why does the plant cell invest so much energy in driving this strikingly dynamic mitochondrial motion?’ he says.
Iain suspected the answer might be linked to the diverse plant mitochondrial genome. In animal cells, the mitochondrial genome is present in hundreds or thousands of highly similar mitochondrial DNA (mtDNA) molecules. In plants, however, mtDNA molecules are highly variable, and individual mitochondria may contain an incomplete set of mtDNA genes, or even none at all. Iain hypothesised that plant mitochondria might compensate for this variability by sharing contents through transient fusion and fission events that allow intra-mitochondrial exchange of DNA, membranes and proteins. ‘This sharing, like trade on a social network, allows each mitochondrion to be effectively interconnected even without simultaneous physical linkage, and to maintain a full set of mtDNA gene products, despite only ever carrying a subset of the mtDNA genome,’ says Iain. In addition, mtDNA recombination, which is rarely seen in animal cells, but much more frequent in plants, enables genetic information to be mixed when different mitochondria meet, helping to prevent mutational damage from accumulating.
However, combining single-cell live imaging of mitochondrial dynamics with modelling and
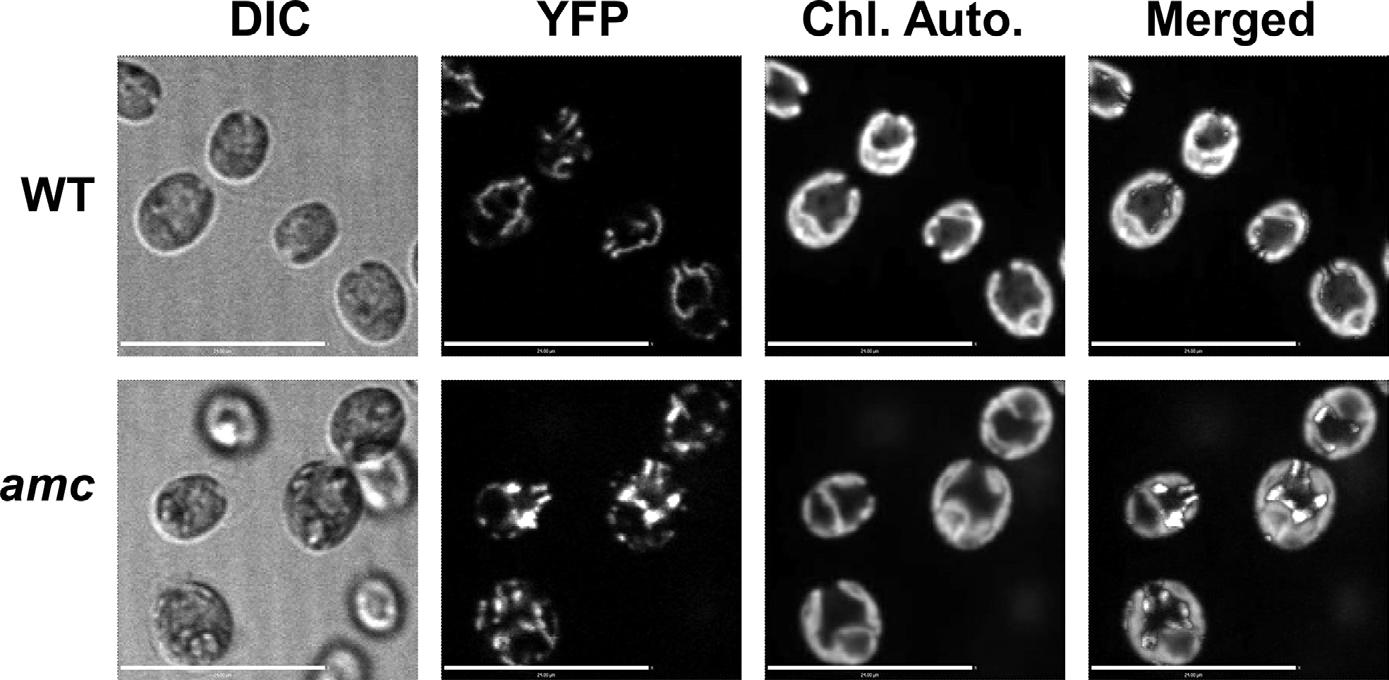
Left Images of wild-type (WT) and complex I-deficient (amc) Chlamydomonas strains expressing mitochondriatargeted yellowfluorescent protein (YFP) were obtained by confocal fluorescence microscopy. Cells are from mid-exponential phase cultures in mixotrophic conditions, where the alga rely on both photosynthesis and respiration. Scale bar: 24 µm. DIC: differential interference contrast. Photo credit Andrew Castonguay
Bottom Left Lightbox image of mature kernels in wild-type maize (left) and opaque18 mutants (right) showing the opaque phenotype in the mutant line Photo credit Guifeng Wang network analysis revealed an inevitable tradeoff between regular social encounters and the benefits that come from mitochondria being evenly distributed throughout the cell. ‘Spacing mitochondria throughout the cell ensures an even energy supply, avoids local build-up of damaging chemical “exhaust” and means that no other cell components will ever be particularly far from a mitochondrion,’ Iain explains. ‘This creates a tension between maintaining mitochondrial spacing and facilitating co-localisation. We believe plant cells resolve this by having dynamic mitochondria with high potential for information exchange.’
To confirm whether balancing these two priorities is a general principle underlying mitochondrial dynamics, Iain’s team investigated the effect of reducing the payoff of social interactions. ‘We wondered if we challenged the ability of mitochondria to recombine mtDNA in each single interaction event, would they allow more social encounters to take place to make up the difference?’ To explore this, the team created a new Arabidopsis mutant by combining the msh1 mutant (impaired in mtDNA recombination) with mitochondrially targeted GFP, and characterised the mitochondrial dynamics with a combination of single-cell time-lapse microscopy, computational tracking and network analysis.7 The results showed that mitochondrial social networks were indeed much denser, supporting more encounters between individuals to compensate for the reduced recombination taking place during each one.
‘Our results suggest that the balance of priorities between even spread and transient proximity may be a fundamental principle underlying mitochondrial organisation. We’re now investigating whether these social network principles also apply to interactions with other organelles, such as mitochondria–chloroplast encounters, and also to other organisms with heterogeneous mtDNA, including fungi, corals and sponges,’ says Iain.8
Reference:
1. Meng X, Li L, Narsai R, et al. Mitochondrial signalling is critical for acclimation and adaptation to flooding in Arabidopsis thaliana. Plant J 2020; 103: 227–247. 2. Karia P, Yoshioka K, Moeder W. Multiple phosphorylation events of the mitochondrial membrane protein TTM1 regulate cell death during senescence. Plant J 2021; 108: 766–780. 3. Tian Q, Wang G, Ma X, et al. Riboflavin integrates cellular energetics and cell cycle to regulate maize seed development.
Plant Biotechnol J 2022; 20: 1487–1501. 4. Subrahmanian N, Castonguay AD, Fatnes TA et al.
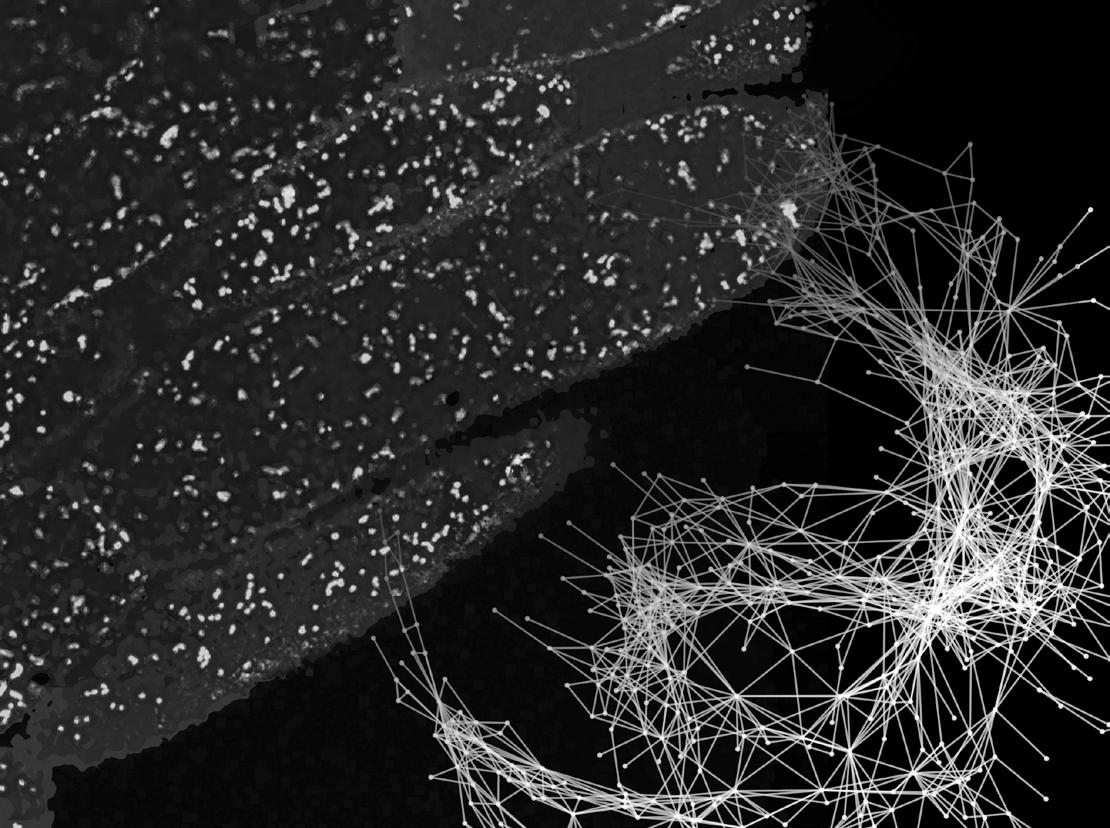
Chlamydomonas reinhardtii as a plant model system to study mitochondrial complex I dysfunction. Plant Direct 2020; 4: e00200. 5. Barbieri MR, Larosa V, Nouet C, et al. A forward genetic screen identifies mutants deficient for mitochondrial complex I assembly in Chlamydomonas reinhardtii. Genetics 2011; 188: 349–358. 6. Subrahmanian N, Castonguay AD, Remacle C, et al. Assembly of mitochondrial complex i requires the low-complexity protein AMC1 in Chlamydomonas reinhardtii. Genetics 2020; 214: 895–911. 7. Chustecki JM, Etherington RD, Gibbs DJ, et al. Altered collective mitochondrial dynamics in the Arabidopsis msh1 mutant compromising organelle DNA maintenance. J Exp Bot 2022; 73: 5428–5439. 8. Edwards DM, Røyrvik EC, Chustecki JM, et al. Avoiding organelle mutational meltdown across eukaryotes with or without a germline bottleneck. PLoS Biol 2021; 19: e3001153. Left ‘Social networks’ of plant mitochondria. In the background, a snapshot of several Arabidopsis hypocotyl cells (cell walls in purple) from a video taken with a laser microscope, with mitochondria shown in orange. In the foreground is a constructed social network, where each point is a mitochondrion and each line between two points means that a physical encounter has occurred between those two mitochondria as they move within the cell. Photo credit Iain Johnston and Joanna Chustecki

JOURNALS - JXB .................... 34
YSAS WINNERS ..................... 36
SPOTLIGHT ON CHIMWEMWE TEMBO ...... 44
SPOTLIGHT ON LISANDRA MARI ........ 46
SPOTLIGHT