






Minimize your risks by prioritizing high quality, consistency and reliable GMP manufactured raw materials.
Our Benzalkonium Chloride is manufactured following cGMP guide ICH Q7 for APIs, the highest available quality standard in the industry.
Get peace of mind with full traceability, high product purity, full pharmacopoeia compliance (PhEur, USP/NF, JP, ChP), audit access, and 30+ years of cGMP manufacturing experience.
novonordiskpharmatech.com

MANAGING DIRECTOR
Mark A. Barker
BUSINESS DEVELOPMENT
Anthony Stewart anthony@senglobalcoms.com
Mark A. Barker mark@senglobalcoms.com
EDITORIAL MANAGER
Alice Phillips alice@senglobalcoms.com
DESIGNER
Jana Sukenikova www.fanahshapeless.com
EDITORIAL ASSISTANT
Carla Devine carla@senglobalcoms.com
ADMINISTRATOR Barbara Lasco
FRONT COVER istockphoto
PUBLISHED BY Senglobal Ltd. 46 Plover Way, London - SE16 7TT United Kingdom
Tel: +44 (0) 2045417569
Email: info@senglobalcoms.com www.pharmanaturepositive.com
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held responsible for any loss or damage incurred. This publication is protected by copyright.
Volume 4 Issue 1 2025 Senglobal Ltd.
04 FOREWORD GREEN INDUSTRY GROWTH
06 Accelerating Sustainability in Pharma – A Q&A with Silvia Forroova, Director, Partnerships and Sustainability, and founder of the Sustainability Collective at Informa Markets
CPHI promotes low-carbon practices, supports sustainable innovation, and provides platforms like the Sustainability Collective to drive industry alignment. Silvia Forroova of Informa Markets, discusses how CPHI is integrating sustainability into the pharmaceutical industry through strategic event design, cross-sector collaboration, and measurable impact.
GREEN VISION
10 Living and Breathing: The Inhalation Industry’s Green Transition
Bespak is leading the inhalation industry’s green transition through strategic investment in sustainable manufacturing low GWP pMDIs and circular economy practices. With a robust ESG strategy emissions reduction targets and strong community engagement Bespak is embedding sustainability across its operations balancing patient needs environmental responsibility and long-term industry leadership.
Brian Morrisey at Gaelic Laboratories explores how pharmaceutical manufacturing can embrace sustainability through pollution control, carbon reduction, circular economy practices and recyclable packaging. He highlights actionable strategies such as effluent monitoring, renewable energy use and reshoring supply chains, calling for a united industry approach to sustainability.
17 Staying Ahead of Green Propellant Regulations in pMDIs
As green regulations tighten, the pharmaceutical industry must transition to low-GWP propellants in pMDIs without compromising patient care. Strategic planning, deep product understanding and collaboration with expert partners will ensure performance, supply continuity and regulatory readiness. Craig Sommerville of Kindeva explains how innovation, not reactivity, is key to achieving both environmental goals and patient-centred outcomes.
20 The Next Frontier for Inhaled Therapies: Low Global Warming Propellants and the Future of pMDI Development
The pharmaceutical inhalation industry is transitioning to low global warming potential propellants like HFA152a and HFO1234ze. Joanne Mather of Proveris examines this shift demands innovation in formulation, testing, and regulation. Emerging tools like INVIDA enable faster, human-realistic performance analysis. Companies embracing these changes will improve sustainability, patient access, and lead future respiratory care advancements.
24 Study Designs and Digital Technologies for Sustainable Clinical Trials
The clinical trial industry contributes significantly to CO2 emissions, mainly from travel and site energy use. Bipin Patel of electronRx explains how sustainable solutions like patient-centric designs, decentralised trials, adaptive methodologies, ePROs, and digital technologies reduce environmental impact while improving data quality, recruitment, and retention. Overcoming adoption barriers is key to sustainable medical innovation.
26 Rethinking Growth Promoters Through the Lens of Green Chemistry
Alex Del Priore of Syngene International analyses how green chemistry is reshaping veterinary growth promoter manufacturing by replacing hazardous solvents, optimising synthesis routes, and reducing waste. Structured approaches like SELECT and DoE enable safer, scalable processes with lower environmental impact. This sustainable shift meets regulatory demands and stakeholder expectations, driving innovation and responsibility in animal health production.

GREEN LOGISTICS
28 Cold Chain in the Context of Global Warming
Global warming is challenging pharmaceutical cold chain logistics with unpredictable weather and rising temperatures. Niklas Adamsson of Enviortainer explains that to maintain efficiency and patient safety, the industry must adopt AI-driven planning, climateresilient packaging and cross-sector collaboration. Balancing sustainability with performance and building flexible infrastructure will be crucial to futureproof global medicine distribution systems.
30 The Impact of Data and Academic Partnerships on Cold Chain Innovation
Data-driven academic partnerships, advance sustainable pharmaceutical cold chain logistics. Cold Chain Technologies’ Niall Balfour explains by optimising reusable container use and minimising empty transport trips, they reduce carbon emissions and waste while ensuring product integrity, aligning operational efficiency with regulatory compliance and environmental responsibility.
BC Bespak Limited
Page 33 CPHI Frankfurt 2025
Page 34 DDL 2025
IBC Krautz Temax Group
IFC Novo Nordisk Pharmatech A/S
Page 23 Proveris Scientific Corporation
Page 3 Richter Biologics
Page 5 The Wool Packaging Company Limited



Richter BioLogics is a Germany-based GMP manufacturer specialized in products derived from bacteria and yeasts, with a proven 35-year track record.
Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide have already benefited from our commitment to good manufacturing practice and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody-like sca olds (VHH/Nanobody), cell-free expression and vaccines.
Your product – our competence and dedication. Contact us +49 40 55290-801 www.richterbiologics.eu LEARN MORE ABOUT OUR SERVICES AND CAPABILITIES


In 2025 the pharmaceutical industry is no longer debating the need for sustainability. The focus has shifted to how effectively it can be integrated across the entire value chain. This reflects a wider global movement that continues to gather pace. Expectations have evolved. Sustainability is no longer a long-term goal but a core operational priority.
Across the pharmaceutical landscape, organisations are responding with practical action. Sustainability goals are now influencing everything from manufacturing and packaging to clinical trials and cold chain logistics. The sector is moving away from fragmented initiatives toward integrated strategies that prioritise long-term environmental performance, regulatory compliance and patient outcomes.
This issue of Pharma Nature Positive brings together leading themes shaping the sustainability agenda in 2025. The drive to lower carbon emissions in pharmaceutical manufacturing is evident in the adoption of pollution control systems, renewable energy, and circular economy principles. Packaging is being redesigned with recyclability and material efficiency in mind. Supply chains are being re-evaluated for resilience and environmental impact, with many pharma sectors reshoring operations to reduce emissions and improve control.
One major area of transformation is the inhalation and respiratory medicines space. Global warming potential has become a defining consideration in product design, and the transition to low-GWP propellants is well underway. Industry players are not waiting for regulations to force change. They are proactively developing next-generation pressurised metered dose inhalers that meet both environmental and therapeutic goals. This includes investment in advanced testing tools and a deep rethinking of formulation and device compatibility.
Innovation is also enabling the cold chain sector to adapt to the realities of climate change. Rising temperatures and more frequent weather disruptions are challenging traditional distribution models. In response, logistics providers are turning to data and AI to optimise route planning and container efficiency. Reusable temperature-controlled containers are gaining ground, supported by improved design, predictive analytics, and academic partnerships that enhance operational performance while reducing waste.
Clinical trials, once considered an immovable part of the pharmaceutical carbon footprint, are also evolving. In 2025, decentralised and hybrid trials are becoming more common. Digital technologies such as electronic patient-reported outcomes and adaptive trial designs are helping reduce the environmental impact of site-based studies. These changes are improving access and efficiency while lowering emissions related to travel and site operations.
Veterinary medicine is also embracing sustainability. Growth promoters and other widely used active ingredients are being re-evaluated through the lens of green chemistry. This involves replacing hazardous reagents, streamlining synthetic routes, and
eliminating environmentally harmful steps in the manufacturing process. Structured methods such as SELECT and Design of Experiments are playing a key role in making these greener processes scalable and economically viable.
What connects all of these developments in 2025 is a shift in mindset. Sustainability is no longer treated as a side project. It is a core metric of success. Collaboration is essential, not just within organisations but across sectors. Events, partnerships and knowledge-sharing platforms are helping to align standards, accelerate innovation and support meaningful progress.
The regulatory landscape is also evolving. New standards for pharmaceutical packaging, emissions and materials use are pushing the industry to do better. Meeting these standards requires forward planning, clear measurement frameworks and transparency. Developers that take a proactive approach are finding that sustainability can be a source of competitive advantage as well as a compliance necessity.
This issue shows how far the pharmaceutical industry has come in building a more sustainable model. It also highlights how much work remains. The urgency is real. Climate pressures, rising energy costs, material scarcity and growing public expectations demand more than incremental change. They call for a complete rethinking of how medicines are developed, produced and delivered.
In 2025, pharmaceutical manufacturers are showing that environmental responsibility can go hand in hand with meeting patient needs. Continued progress will depend on practical solutions, collaboration and a clear focus on long-term goals.
Alice Phillips, Editorial Manager, Senglobal Ltd


The pharmaceutical industry is increasingly looking to meet the opportunities and needs of a broad agenda on sustainability, from the more efficient use of materials and reducing energy costs and carbon emissions to ensuring greater access to medicines and inclusivity of employment.
Sustainable transformation doesn’t come without its challenges and can be difficult to navigate at scale. However, momentum is building. With environmental impact now firmly on the strategic agenda, progress is being shaped by evolving regulation, investor expectations, and shifting societal demands.
Recognising this complexity, many companies are now moving from high-level commitments to more targeted action. As a result, sustainability is becoming an integral part of long-term strategic planning across the sector.
For events like CPHI, a series of global, annual pharmaceutical supply chain trade events, this shift brings a new sense of purpose. Long known as a global platform for pharma innovation and networking, CPHI is increasingly positioned as a space where sustainability challenges can be addressed directly and openly, and where actionable progress is enabled through collaboration.
To explore how trade events can serve as catalysts for sustainability in pharma, Pharma Nature Positive spoke to Silvia Forroova, Director, Partnerships & Sustainability at Informa Markets. In this conversation, she outlines how CPHI embeds sustainability into its event design, fosters collaboration across the sector, and creates space for new ideas to emerge.
Q: How does CPHI integrate sustainability into its event strategy?
Sustainability is embedded into every facet of the event. As part of Informa’s FasterForward programme, CPHI operates under a group-wide framework with these sustainability pillars:
• Faster to Zero (focusing on waste and emissions reduction)


These principles inform all aspects of how CPHI is planned and delivered, influencing decisions on procurement, venue partnerships, content programming, and year-round engagement. The aim is to reduce environmental impact while also equipping the pharmaceutical community with tools and platforms to support practical, achievable progress.
Q: Are there formal goals or KPIs used to track the progress of the sustainability initiatives?
Yes. Alongside Informa’s group-level targets, CPHI sets annual objectives tailored to each of our events. These include metrics for waste reduction, energy efficiency, travelrelated emissions, and sustainable procurement. Internally, performance is tracked using a structured framework known as the Fundamentals, which measures progress across 16 operational areas.

• Sustainability Inside (embedding sustainability inside every brand - whether it’s through content, events, intelligence, research, or training, this pillar helps customers accelerate their own sustainable development, creating growth opportunities and supporting progress in the specialist markets it serves)
• Impact Multiplier (broadening access to knowledge and networks)
Efforts are also underway to deepen the quality of data collected – for example, through more detailed tracking of materials use, venue energy sourcing, and stakeholder engagement. These metrics support both transparent reporting and ongoing refinement of sustainability practices across future events.
Q: What steps have been taken to ensure sustainability isn’t just a theme –but a tangible outcome?
Tangible progress is a core priority. One key initiative is the Better Stands Programme, a 10-point framework that encourages exhibitors to build modular, reusable stands rather than single-use installations. This programme has been adopted across multiple events and is part of the Net Zero Carbon Events Initiative to help give exhibitors and our value chain a consistent, industry-wide framework to encourage the adoption of reusable exhibition stands.
CPHI has also partnered with venues and suppliers on initiatives focused on waste reduction, including recyclable carpeting, digital signage, and lower-impact catering options. These decisions are shaped not only by environmental considerations but also by their potential to be scaled and applied consistently across future events.
Beyond operational practices, sustainability is treated as a strategic issue for the wider industry. CPHI convenes expert groups to explore shared challenges, curates content on critical topics like Scope 3 emissions and supply chain decarbonisation and supports practical knowledge-sharing across the pharma ecosystem.
Q: Can you share any examples of successful sustainability collaborations that have emerged through CPHI?
The launch of the Sustainability Collective at CPHI Milan in 2024 marked a significant step in advancing cross-sector collaboration on industry sustainability issues.1 The initiative brings together pharmaceutical sustainability professionals – including manufacturers, suppliers, associations, and consultants – to exchange insight, build connections, and identify shared priorities.
The Collective is built on three core principles: facilitating engagement, supporting knowledge-sharing, and fostering alignment across stakeholders. It serves as a neutral platform for open discussion, where ideas can be tested and turned into practical strategies without commercial pressure.
Since its inception, the Collective has formalised its governance with a chair and steering committee drawn from across the global pharma ecosystem. It has extended its presence to other CPHI portfolio events in various geographies.
Q: What role does CPHI play in surfacing sustainable innovation from within the pharma supply chain?
CPHI provides a range of opportunities for sustainabilityfocused innovation to gain visibility through exhibition areas, targeted programming, and structured networking.
One recent initiative is the Sustainability Supplier Zone, launching at this year’s event in Frankfurt. It brings together solution providers focused on emissions tracking, low-impact production, and supply chain transparency.2 The goal is to facilitate direct engagement between pharmaceutical stakeholders and the suppliers best positioned to support their sustainability objectives, while aligning with commercial requirements.
Alongside this, the Start-Up Market has become a key feature for early-stage companies developing sustainabilityrelated tools and technologies. The space has expanded significantly since launch and continues to highlight emerging approaches across the supply chain.
Q: Are there formal recognition programmes for sustainability-related initiatives?
Yes. The CPHI Pharma Awards, held on the first evening of the event, include a dedicated category for sustainability. Beyond that, environmental and social impact has become a judging criterion across all categories, encouraging entrants to incorporate sustainability considerations into every aspect of their submissions.
This shift reflects a conscious effort to move sustainability out of a siloed track and into the mainstream of industry recognition. Rather than treating it as a separate topic, the awards now promote a broader cultural change – one where sustainable practice is recognised as integral to excellence across all areas of pharmaceutical innovation.

Q: How does the event environment support visibility for breakthrough initiatives?
In addition to exhibition opportunities and formal recognition through the awards, CPHI highlights real-world sustainability efforts through its content programme. This includes innovation sessions, expert panels, fireside discussions, and technical workshops designed to surface practical examples and facilitate peer learning.
Topics range from low-carbon manufacturing and sustainable API production to the use of digital tools for managing Scope 3 emissions. For attendees seeking to benchmark or explore new approaches, the programme offers both strategic insight and operational detail.
Access to knowledge is also a priority. Guided by the FasterForward commitment to broadening participation, CPHI has developed initiatives such as the Start-Up Market and targeted content for under-represented voices. By removing financial and visibility barriers, the event creates space for smaller and early-stage companies to contribute meaningfully to the sustainability conversation.
Q: What can attendees expect from this year’s content across sustainability-related tracks?
This year’s programme includes three full days of sessions in the Sustainable Futures theatre, with additional sustainabilityfocused content integrated across six other theatres. Topics will include:
• Scope 3 emissions and decarbonising pharmaceutical supply chains
• Innovation in packaging materials and circular design
• ESG reporting standards and data governance
• Effective sustainability communication strategies
• AI-enabled tools for emissions tracking and resource efficiency
Other sessions will examine workforce skills, stakeholder engagement, and organisational change – all recognised as critical enablers of sustainable transformation.
A dedicated spotlight session, the Industry Power Hour, will be hosted on the Spotlight Stage (next to the Sustainability Centre, Hall 4), bringing together leading organisations such as the PSCI (Pharmaceutical Supply Chain Initiative), BioPhorum, and CiPPPA (Circularity in Primary Care Pharmaceutical Packaging) – to share insights and coordinated responses to sector-wide challenges.
Minimising Environmental Impact Through Event Design
Q: How does CPHI manage the environmental footprint of its own events?
Sustainability is embedded into operational planning and treated as a shared responsibility across venues, suppliers, and internal teams. This includes careful consideration of logistics, materials, signage, and sourcing.
CPHI works closely with host venues to reduce energy consumption and improve waste management. For example, catering providers are chosen based on their ability to deliver regionally sourced food and lower-impact options, such as vegetarian-led menus.
Carpet use has been cut significantly through a series of targeted measures: switching to recyclable materials, limiting use in non-essential areas, and ensuring proper post-event recycling. Wherever possible, CPHI replaces printed signage with digital alternatives to minimise waste and allow for live updates without reprinting.
Exhibitors also receive tailored guidance via the Better Stands Programme, with help available on site to answer questions and provide support.
Q: How do you track the outcomes of these operational efforts?
Performance is measured against the Fundamentals framework – a structured internal tool used to assess sustainability progress across multiple dimensions. This includes waste management, energy sourcing, signage decisions, travel patterns, and materials usage. We also compare ourselves to many other events and encourage the sharing of best practice between them. Outcomes are captured in post-event reporting and fed back to the wider team to inform future planning.
The goal is not just to demonstrate compliance or meet reporting expectations, but to identify opportunities for continuous improvement and to strengthen relationships with suppliers aligned on sustainability priorities.
CPHI’s Future Role in Industry Collaboration and Sustainability
Q: What is your vision for how CPHI’s role in sustainability will evolve over the next five years?
Large-scale events are increasingly expected to lead by example – not only in their operations, but in how they foster industry-wide collaboration. CPHI is well placed to do both.
The event has already shifted focus from awareness-raising to enabling practical change. This evolution will continue, with expanded sustainability programming, a broader international presence for the Sustainability Collective, and new tools to support alignment across the sector.
The goal is to make engagement more accessible, especially for companies in the early stages of developing a sustainability strategy or exploring where to begin.
Q: What trends do you anticipate shaping sustainability conversations at this year’s event and beyond?
A number of topics are expected to feature prominently. Scope 3 emissions remain a critical focus, particularly as companies seek more reliable access to supply chain data. Manufacturing decarbonisation continues to present technical and operational challenges, while sustainable packaging is
evolving beyond material substitution to include new models for reuse and circularity.
Other areas gaining traction include the application of AI and digital platforms for emissions tracking, increasing expectations around ESG reporting, and the role of internal communication in supporting organisational change. Many companies are also reassessing how they engage stakeholders, whether through supplier relationships, workforce initiatives, or investor dialogues.
In each of these areas, progress depends on stronger alignment across the sector. CPHI plays a role in supporting that alignment by facilitating focused discussions, enabling peer exchange, and keeping sustainability visible across its global platforms.
As sustainability ambitions mature across the pharmaceutical sector, the need for structured collaboration is becoming more pressing. Events like CPHI are increasingly recognised not just for their convening power, but for their ability to turn conversation into practical alignment. The next phase of progress depends on access to repeatable models, shared infrastructure, and sustained peer engagement — the platforms that support this will be central to accelerating sector-wide change.
1. https://www.cphi.com/en/about/about/sustainability-collective. html?utm_source=content&utm_medium=referral&utm_ campaign=hln25cpw-cl-barter-pnp&utm_ content=sustainability-interview
2. https://www.cphi.com/europe/en/exhibit/new-zones/ sustainability.html?utm_source=content&utm_ medium=referral&utm_campaign=hln25cpw-cl-barterpnp&utm_content=sustainability-interview

Silvia Foroova, Director Partnerships & Sustainability, and Founder of the Sustainability Collective at Informa Markets. With over 15 years of experience in global events, Silvia has consistently contributed to the success of multiple large-scale trade shows and conferences across international markets throughout her career. Silvia brings valuable expertise to her role with the CPHI portfolio while championing sustainable practices through the Sustainability Collective initiative. Her skills include strategic leadership, partnerships, sustainability strategy development, building impact networks, and she continues to drive innovation at the intersection of events strategy and sustainability.
Sustainability is multi-faceted. Beyond direct environmental impacts such as carbon emissions from manufacturing, a company's sustainable practices should also account for indirect impacts across the entire product lifecycle. For a Contract Development and Manufacturing Organisation (CDMO), a sustainability strategy should consider every stage of operations, spanning sourcing, development, and manufacturing, through to the emissions of the finished products and end-of-life disposal. Beyond environmental considerations, a company’s impact can also extend to the way the company and its activities affect its workforce and its local communities. Bespak ® – a specialist inhalation CDMO – is combining its expert focus on inhaled and nasal drug-device products with a dedication to true sustainability that is embedded in its DNA. Bespak’s mission is to enable the inhalation industry’s ‘green transition’, and with this aim, Bespak is working to go above and beyond, considering its operations and products holistically to help forge a greener future.
Sustainable Solutions for the Inhalation Industry
In an industry that centres on helping patients to breathe, the emissions of life-saving devices such as pressurised Metered Dose Inhalers (pMDIs) cannot be ignored. The industry is therefore in the midst of a transition to next-generation propellant gases in pMDIs that can reduce the carbon footprint of the devices. Currently used HFA propellants have a high Global Warming Potential (GWP) and are responsible for an estimated 3% of total carbon emissions from the UK’s National Health Service (NHS).1 As a result, pMDIs have been identified as a priority for decarbonisation efforts, leading to the development of two new low GWP propellants: HFA-152a and HFO-1234ze. These have GWPs 100 times and 1,000 times lower than currently used propellants, respectively. Enabling the industry to transition to these propellants is a central focus for Bespak. It has sparked adaptation and optimisation across Bespak’s processes, facilities and components, as the industry looks to CDMOs to help facilitate the shift.




growing demand for low carbon inhalers. The first commercialscale lines using HFO-1234ze are now fully operational, supplying the first regulatory-approved low carbon pMDI at commercial scale. In 2025, Bespak further expanded its operations with a clinical-scale manufacturing line capable of producing pMDIs using HFA-152a, in addition to commissioning a commercial-scale line, expected to commence commercial production in 2027.

In recognition of this responsibility, Bespak has been investing in its capabilities for the development and production of pMDIs using either of the next-generation propellants. Its product feasibility and development laboratories have been fully equipped to develop pMDIs with HFO-1234ze since 2022, and the CDMO has since invested in development and small-scale manufacturing capabilities for HFA-152a –providing necessary early-stage services for the industry. In 2024, Bespak committed to significant capacity expansions, installing a new high-speed pMDI filling line in anticipation of
Another key aspect of enabling the transition to low GWP propellants is the optimisation of pMDI components, such as valves, to ensure correct function with formulations containing the new propellants. Bespak has significant expertise in this area, producing almost half of the pMDI valves used globally. As a trusted market leader in pMDI components, Bespak is dedicated to adapting its valve technology for optimum performance with each of the next-generation propellants, so that pMDI developers can smoothly transition. This is something Bespak has been working on for several years, exploring the materials used in the valves for compatibility with HFA-152a and HFO-1234ze. Utilising advanced simulation technology and studies in the lab, the design optimisation involved an update to the elastomers in the valve to reduce the incidence of propellant leakage and moisture ingression with one of the new propellants. Bespak’s BK357 valve was the first to be approved by a regulatory agency, as a result of its selection for use in the first low carbon pMDI to achieve regulatory approval.

By providing the know-how, technology, infrastructure and manufacturing capacity needed, Bespak helps its customers to bring low carbon pMDIs to their patients and contributes to the decarbonisation of the pharmaceutical industry.
Where Carbon Counts:
Tackling Emissions Across Every Stage
In addition to the industry perspective, it is also important for companies to focus in on their individual footprints. For a CDMO that supplies billions of components, devices and drug-device combination products to patients around the world every year, the total environmental impact can be significant. This makes it necessary to identify hotspots or priority areas for reduction. Bespak mapped its emissions to gain a deeper understanding of its impacts, and found that for scope 1–2 emissions, 86% of its direct emissions in 2024 were from fugitive emissions. Scope 3 is the most significant output (Figure 2). Of these emissions, Use of Sold Products was the largest contributor to indirect emissions.
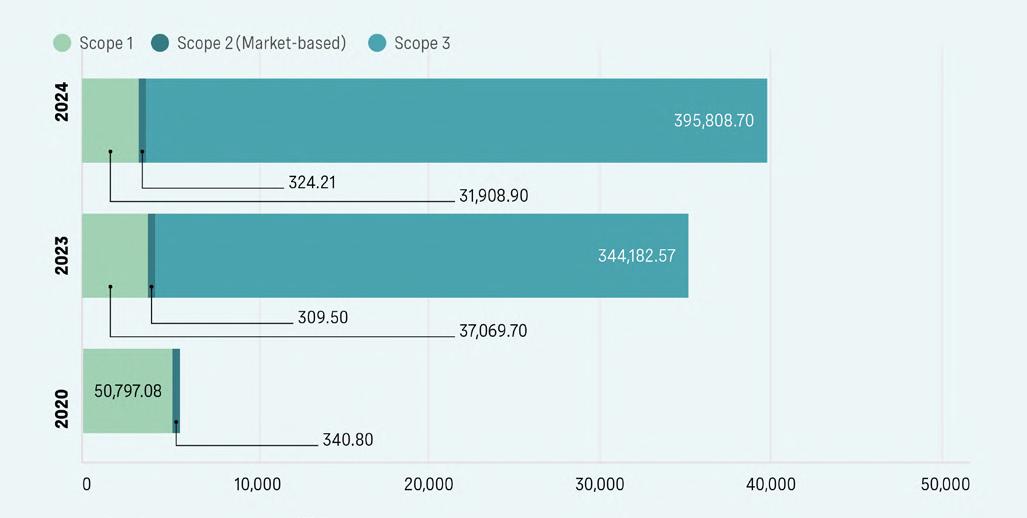
Emissions in this category increased by 28% from 2023 to 2024, primarily influenced by customers’ selection of propellants, with total Scope 3 emissions also increasing due to broader business growth. As a result, transitioning to production with low GWP propellants is Bespak’s biggest opportunity to minimise its total carbon footprint across all operations.
Embedding Sustainability Across the Value Chain
For a business-wide approach to sustainability, however, there are many other aspects of a CDMO’s operations that must be addressed. For example, Bespak is working to embed sustainability considerations throughout its product design philosophy, from product inception to end-of-life. Bespak has also completed its first independently verified Life Cycle Assessment (LCA) for its widely used BK357 valve platform, in line with ISO 14067:2018 standards. This project delivered:
• Improved understanding of the carbon impact of Bespak’s materials and manufacturing processes, helping to increase supply chain transparency.
• Benefits for Bespak’s industry partners and customers, who can use the verified LCA certification to drive transparency in their sustainability reporting and sustainable procurement frameworks, supporting their own transition to net zero carbon emissions.
• From the carbon hot-spot identification, a clear direction for future carbon reduction initiatives at Bespak for its BK357 valves.
Advancing a Circular Economy
Assessing resource use and working towards a more circular economy are also key aspects of sustainable manufacturing –
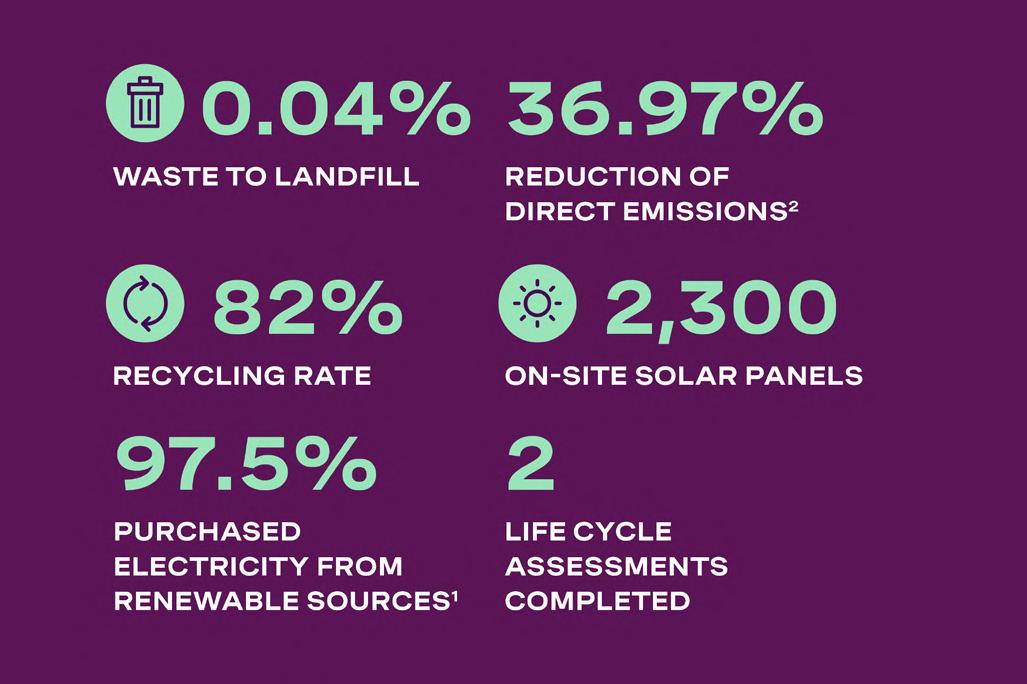
reducing consumption and waste wherever possible. To this end, Bespak has sought to engage customers and distribution partners in circular economy partnerships to avoid plastic waste. Its teams are also exploring a mechanical recycling process that will create product delivery trays with up to 80% recycled polymers. This transition will reduce CO2e emissions by ~29% compared to trays made with virgin polymers. Through Bespak’s Polymer Lab and Injection Moulding Academy, its polymer scientists are investigating sustainable material substitutions, such as incorporating International Sustainability and Carbon Certification (ISCC) certified materials across Bespak’s product portfolio. Quality management systems and operating standards used in manufacturing processes ensure minimal reject and waste material. As a result of these initiatives, 2024 data shows that only 0.04% of Bespak’s waste went to landfill, with an 82% recycling rate achieved.
In terms of energy use, Bespak installed LED lighting across office and manufacturing areas, and upgraded cleanroom HVAC filters, reducing electricity use by 52% for the upgraded HVAC systems. Renewable electricity has a role to play as well. At one of its sites, Bespak has 2,300 on-site solar panels, providing 7% of the site’s total energy consumption. In addition, Bespak’s sites procure 100% REGO-certified renewable electricity and offset 100% of their natural gas use.
With all these factors considered, Bespak has been able to achieve a 36.97% reduction of direct emissions in 2024 compared to its 2020 baseline.
Maximising Positive Local Impact
On a global scale, minimising emissions and resource consumption are some of the clearest priorities, but sustainability also requires positive impact on a smaller scale – thinking about the people, communities and ecosystems that are directly involved in a company’s operations. To this end, Bespak has worked to ensure that it has a positive impact on its local environment, from the communities at its UK sites – Holmes Chapel, Cheshire and King’s Lynn, Norfolk – to the natural ecosystems that surround them both. In fact, Bespak has a variety of initiatives underway to understand its impact and add benefit locally.
Organising Charitable Initiatives
To give back to its home communities, Bespak commits to partnering with two charities of the year (one in Holmes Chapel and one in King’s Lynn). To ensure that its charitable activities and wider sustainability efforts continue adding value to its local communities, Bespak worked with Samtaler, a women-owned small business, to conduct Local Community Needs Assessments at both sites in 2024. The assessment examined local trends such as ethnicity, income, employment, education and skills training, and more, to maximise Bespak’s ability to positively impact its local economies and communities. These initial assessments laid the groundwork for a mission-driven community engagement strategy going forward. This will continue the charity work that is already central to Bespak’s communities and also expand into partnerships with local enterprises, ultimately working to enhance the local economy, community health, public education and skills development.
Fostering a Positive Working Environment
Employees and workers in the value chain are another important area of consideration. A priority for Bespak is ensuring that the
working conditions and business practices of employees and workers in the value chain are in line with its core values. To help achieve this, Bespak expanded its supplier evaluation criteria to include a scorecard with questions related to business conduct, climate action, biodiversity and other sustainability topics. From a professional development perspective, Bespak provides apprenticeships and training in conjunction with local educational organisations through its Injection Moulding Academy in King’s Lynn and is taking this initiative even further. Its plans include expanding partnerships with local schools, colleges and universities to increase opportunities for apprenticeships, mentoring and project-based job training. By nurturing the local Science, Technology, Engineering and Mathematics (STEM) pipeline and providing students with hands-on work experiences in a product development and manufacturing environment, Bespak is helping to inspire and train the next generation of scientists and engineers within its local communities.
It is therefore particularly important to understand and preserve these local ecosystems, and each site will require a different approach. Bespak conducted on-site biodiversity assessments at both sites and will use these to develop Biodiversity Management Plans to help reduce the impact of its operations on local biodiversity and natural ecosystems.
Efforts also extend beyond Bespak’s two UK sites through a comprehensive Supply Chain Biodiversity Footprint (SCBF) assessment, which evaluates the impact of the supply chain on nature – from raw material sourcing and production processes to transportation and logistics. The assessment results identified raw materials and processes with the greatest biodiversity impact, enabling Bespak to develop phased action plans to protect and enhance biodiversity in high-risk areas of the supply chain.
A final aspect of Bespak’s work to understand and improve its local impact focuses on ecosystems. Whilst the King’s Lynn site is based on an industrial estate typical of a CDMO, Bespak’s Holmes Chapel headquarters is uniquely surrounded by green space. In a recent white paper published in the British Ecological Journal, Bespak discuss the importance of reflecting on reliance on biodiversity for raw materials and biochemical diversity.
Bringing together sustainable industry solutions and manufacturing practices with initiatives to protect and enhance local
communities provides a thorough approach to sustainability. However, these activities must align with business strategy in order to be maintainable long-term.
Bespak’s initiatives – from driving the transition to low carbon pMDIs to supporting charities, offering local apprenticeships, and identifying priority areas to reduce impact across the supply chain – stem from its overarching Environmental, Social and Governance (ESG) strategy. With this strategy established right at the start of Bespak’s journey as a standalone CDMO, it is possible for Bespak to balance ESG priorities with business growth, ensuring that ESG topics remain a central focus. Bespak formalised this commitment earlier this year with the release of its first ESG Update, setting new standards for transparency and accountability by clearly communicating progress, setting benchmarks and laying out actionable next steps.
The ESG Update is underpinned by fundamental strategy work to ensure business and sustainability priorities work in harmony. A key step was conducting a Double Materiality Assessment (DMA), engaging with internal and external stakeholders, including employees, suppliers, customers, local authorities, and management to identify key sustainability matters. For Bespak, the DMA has a dual purpose: helping to prepare for Corporate Sustainability Reporting Directive (CRSD) reporting, and informing the strategic prioritisation of sustainability matters. The DMA highlighted four key topics that are significant from an impact perspective, one from a financial perspective and 15 sub-topics that are deemed double material to Bespak. To further understand and assess sustainability engagement, Bespak conducted a comprehensive stakeholder mapping exercise (Figure 2). The goal was to identify areas of strength, uncover any gaps, and explore opportunities for deeper engagement. The material topics highlighted in the DMA and the insights gained from stakeholder mapping inform the companywide ESG strategy that will allow Bespak to decarbonise its operations, scale up circular economy efforts and improve the lives of workforce and community members.
Finally, to keep this work on track and drive progress, Bespak has established strong governance frameworks. The senior leadership team is highly involved, and one of the Board members has specific responsibility for ESG, helping to maintain a chain of accountability and keep sustainability goals central to both strategy and operations. The governance approach also includes ESG awareness and training sessions to foster engagement and a feeling of responsibility across the workforce.
With the DMA, stakeholder mapping, and governance frameworks in place, Bespak has officially announced its commitment to the Science Based Targets initiative (SBTi) Net-Zero Standard. Having implemented GHG emissions accounting software in 2024, Bespak’s next step will be to share its emissions reduction targets in line with the Science Based Targets initiative (SBTi). In 2026, the CDMO plans to conduct a climate risk assessment, and ultimately a Climate Transition Plan that will help it to meet its SBTi goals.
The vision and commitment guiding Bespak’s efforts has so far driven exciting achievements in its local communities and the wider inhalation industry. With an in-depth and long-term strategy firmly in place, the CDMO is set up for a more sustainable future. By leveraging its expertise, capabilities and network, Bespak is leading by example to drive positive change in the industry to answer real needs at all scales.
1. https://www.rpharms.com/recognition/all-our-campaigns/policya-z/sustainability-policy/policies
2. https://www.carbontrust.com/our-work-and-impact/ guides-reports-and-tools/what-are-scope-3-emissions-andwhy-do-they-matter

Bespak is the specialist inhalation CDMO committed to leading the transition to climate-friendly pMDIs. Bespak offers a fully integrated service to support customers from early-stage feasibility, analytical and formulation, product development and clinical supply, through to full-scale cGMP batch production, utilising HFA-152a and HFO-1234ze.
The environmental impact of the pharmaceutical industry is hard to quantify, but it has been suggested Pharma may be responsible for 300 million tons of plastic waste each year while its carbon footprint could make up 4.4% of total global emissions.1,2 There are big incentives for Pharma to become more sustainable; companies demonstrating progress in this area are more likely to attract investors, while complying with regulations and meeting regional targets. They are also more likely to help create a healthier world – not just through the medicines they deliver, but in the way they develop and manufacture these products.
Today’s pharma industry needs to go beyond basic regulations that require environmental risk assessments and minimise pollution. The EU's Circular Economy Action Plan is a key component of the European Green Deal that focuses on key value chains like electronics, batteries, packaging and textiles. Although Pharma might not fall precisely within those sectors, our industry could play an important part in transitioning towards a more sustainable, circular model, reducing waste and resource depletion.
Companies can adapt in a variety of ways to incorporate environmental responsibility into every process. Some examples, which will be discussed here, include the monitoring and management of effluent and air particulates, recycling materials from waste streams, sourcing of green energies, re-shoring programmes, and the use of recyclable materials wherever possible. Whether it’s reducing, re-using or recycling, any and all of these initiatives need to be embraced by Pharma.
Pollution Management
While the major source of pharmaceuticals in the environment is believed to result from patient use of medicine and subsequent excretion, other sources can include industrial emissions during the manufacture of pharmaceuticals.3 Active pharmaceutical ingredients (APIs) have been found in sewerage water, surface water, groundwater, soil, air and local ecosystems, in concentrations ranging from sub-ng/L to more than μg/L.4 Waste from pharmaceutical manufacturing sites can have harmful effects on the environment, and it has been linked to the development of antimicrobial resistance (AMR), which is recognised as one of the greatest public health challenges in the 21st century.


the AMR Industry Alliance published its Antibiotic Manufacturing Standard: ‘Minimising risk of developing antibiotic resistance and aquatic ecotoxicity in the environment resulting from the manufacturing of human antibiotics’.3 The Standard provides clear guidance to manufacturers in the global antibiotic supply chain to ensure that antibiotics are made responsibly, helping to minimise the risk of AMR in the environment.
Main sources of pharmaceuticals released into the environment include wastewater and air particulates discharged from manufacturing operations. Good practice methods for avoiding accidental release or leakages through these channels should be rigorously established. As a manufacturer of beta-lactams (penicillin), we adhere to the AMR Industry Alliance’s Manufacturing Standard.

High efficiency particulate air (HEPA) filters are an efficiency standard of air filters that – in Europe – must remove at least 99.95% of particles with a diameter of 0.3 μm, such as pollen, bacteria, viruses and submicron liquid aerosol. HEPA filters are commonly used at manufacturing sites for pharma products, including antibiotics, to avoid release of those products into the atmosphere. Where many companies fall down is the maintenance of these filters – a site’s GMP equipment checklist should have HEPA filters on a high-priority proactive maintenance plan. At Gaelic Laboratories, for example, HEPA filters are integrity-checked annually to ensure optimal performance, as well as compliance with relevant regulations and industry guidance.
This highlights the importance of effective control of API emissions from manufacturing but, in general, emissions of APIs are not regulated globally.3 Some companies go beyond basic regulatory compliance, by establishing environmental protection goals to evaluate and reduce potential risks. In 2022,
Effluent monitoring is the other key focus for avoiding accidental release of pharma products into the environment. It involves the collection, analysis and management of wastewater discharged from manufacturing facilities to ensure compliance with environmental regulations and minimise potential harm to ecosystems. This process typically includes assessing the composition of effluent, determining the presence of pharmaceuticals and other contaminants, and implementing
measures to reduce the impact of these pollutants. Our own experience suggests that this is best assured by performing regular routine testing in our own laboratories by an in-house expert lab team, complemented by additional independent testing at an external local council treatment platform. This dual testing approach ensures that – should any accidental leakages occur – they would be quickly detected and dealt with appropriately.
The climate footprint of healthcare is said to represent 4.4% of total global emissions and, of that, 71% of emissions derive from the healthcare supply chain, including the pharma industry.2 Given the carbon intensity and rapid growth of the biotech and pharma industries, it is critical and timely to examine ways in which companies can realistically reduce their carbon profiles.
Fortunately, many pharma companies are already making rapid progress in adopting the UN Race to Zero. A report published by My Green Lab last year suggested that 56% of companies (by revenue) had committed to the campaign, which was already a 10% increase over the previous year.2 These companies have pledged to cut total carbon emissions by 50% by 2030, and to reach net zero emissions by 2050 or sooner.
The question is why the other 44% of companies are delaying their commitment to these goals, when the steps that need to be taken often have no major cost penalties and are supported by other factors influencing Pharma. For example, recent geopolitical pressures, in the wake of the COVID pandemic, have led many companies to transform their supply chains. We have ourselves spent the past two years reshoring our supply chains, and we have been pleased to see that the effort has resulted not just in reduced carbon emissions and greenhouse gas pollution (as our supply chain now requires lesser transportation), but the business advantages of allowing us to be faster, more flexible and more competitive. In other words, we and many other companies that have reshored their supply chains, can confirm that taking this step has a sound business rationale, as well as an environmental one.
Another goal that these companies are embracing is a commitment to switching to at least 80% renewable power by 2030.2 This is easier for some companies than others, depending on the infrastructure available. For example, for companies located in countries like Ireland, where developing renewable energy is central to the national climate change strategy, this is a relatively easy commitment – we run on 100% green energy at Gaelic Laboratories, and it should be acknowledged that this has not been challenging for us to achieve. The Irish government aims to accomplish an 80% share of renewable electricity by 2030, with a focus on expanding both onshore and offshore wind and solar energy generation. We benefit directly from a government that is itself committed to energy security, cost competitiveness and sustainability. One more reason, perhaps, why Ireland’s pharma industry is thriving, and a lesson to other governments that are lagging behind.
Another important commitment is to achieve targets to reduce waste and to re-use materials in manufacturing.2 This aligns with the European Union’s Green Deal, which highlights the need for us all to work towards economic growth without increasing our
consumption of materials. The sources on which we rely for our energy, the efficient use of raw materials, and the approaches and actions required to protect our people and planet, can all be optimised with the advance of the circular economy. Achieving Zero Landfill in pharma manufacturing might seem to be a challenging prospect, but it is possible through the use of process changes, better material choices, waste recovery and re-use.
Across the pharma industry, value can be created from used materials, such as precious metal or solvents recovery, plastics to chemicals, or effluent treatment. A number of companies are emerging as solution providers in this area, offering advanced facilities and innovative processes to recover a wide range of materials from industrial streams. In addition, many of these treatment facilities create energy from the incineration of non-recyclable waste, and that energy can then be re-used at those same sites, feeding back into those green energy goals mentioned above.
Furthermore, these circular processes can save on material costs, reduce transportation costs, simplify supply chains, and remove the need for expensive disposal procedures. Taking solvents as an example, companies can typically expect to recover up to 95% of used solvents from an industrial stream, with overall significant cost savings.5
Zero Landfill is implemented in industry by aiming to eliminate or drastically reduce waste generated through operations, transitioning towards a circular economy in which resources are re-used and recycled instead of discarded. At Gaelic Laboratories, we have pledged to use resources responsibly, and that includes a commitment to Zero Landfill. This benefits the environment, while supporting our own green credentials, and those of our customers.
Recyclable packaging in the pharma industry is a growing area of focus, with companies shifting towards more sustainable materials like paper, cardboard and certain types of plastics. The goal is to reduce waste, lower carbon emissions, and align with growing consumer expectations for sustainability.
However, this remains one of the most challenging aspects of sustainability in pharma, as any changes in packaging material choices must still ensure patient safety and regulatory compliance. Recyclable cardboard outer packaging is a ‘no brainer’ but when it comes to packaging that is in contact with the drug product itself, any changes cannot compromise patient safety or increase risks of contamination. The direct contact between product and packaging can make this a significant challenge.
Nevertheless, there are ongoing efforts to tackle this issue. There is a large amount of investment going into the R&D of alternative materials beyond plastics for primary packaging of pharma products, within the industry, academia and research institutions globally.
At Gaelic Laboratories, we are most interested in alternative materials for blister packs. Blister packaging, which takes up about 30% of the global pharma packaging market, provides stable and reliable conditions, and it is safe, hygienic and convenient, with great adaptability and a long shelf life. However, blister packs

are a mix of plastic and aluminium foil, and separating these materials for recycling is exceptionally challenging. There is also a high risk of contamination, which is why most used blister packs end up being destroyed or incinerated.
One promising alternative is polypropylene blister packaging, which uses polypropylene as the primary material for the blister itself. It is favoured for its recyclability, transparency and suitability for pushing tablets through, offering a sustainable alternative to older materials like PVC, but there remain some challenges with packaging integrity and quality standards. Various initiatives and technological advancements are ongoing to make recyclable packaging – including polypropylene blister packaging – completely fit for purpose. We are watching those developments carefully, with plans to make that switch ourselves, as soon we can be confident that our blister packaging will be able to continue to meet the highest requirements.
Pharma makes incredible contributions to the health of our global population. It is an industry sector well known for its ambitious and mission-focused companies, nurturing a culture of innovation.
Pharma companies need to embrace those qualities and apply them not just to the health of the planet’s people, but also to the planet itself. Our sector is almost uniquely positioned to help lead the global fight against climate change. Through collective action, Pharma can effectively influence shared value chains, accelerate progress on carbon reductions, and serve as a model for other industry sectors.
Perhaps the most frustrating aspect of this argument is that, in so many areas, doing the ‘right thing’ doesn’t even cost more, or have negative business impacts. A circular economy, once established, should lead to cost, energy and resource savings. Sustainability in pharma manufacturing is not just achievable – it is the only sensible approach, from both a human and business perspective.
Pharma is already coming together, with a growing number of companies sharing a united vision for a more sustainable future. If your own company is not already on that path, it’s time to re-evaluate. Modern Pharma is using its innovation and expertise to create a fully healthy world.
1. Jacques, A. Pharma supply chains of the future will be sustainable, connected: LogiPharma 2022 key takeaways. Kinaxis, 20 April 2022; https://kinaxis.com
2. My Green Lab. The Carbon Impact of Biotech & Pharma: Crossing the Tipping Point of Industry Transformation.
3. AMR Industry Alliance. Manufacturing Standard: Minimizing risk of developing antibiotic resistance and aquatic ecotoxicity in the environment resulting from the manufacturing of human antibiotics, May 2022.
4. Desai, M., Njoku, A. & Nimo-Sefah, L. Comparing Environmental Policies to Reduce Pharmaceutical Pollution and Address Disparities. Int. J. Environ. Res. Public Health 19, 8292 (2022).
5. Barker, S. Pure and sustainable: high-end circular solvents. Spec Chem Mag 2005;May-June:66–67.

Brian Morrisey is General Manager of Gaelic Laboratories Limited, a manufacturer of beta-lactam (penicillin) products that is headquartered in Ireland. With a career spanning 30 years at leading companies such as Accord Healthcare, GSK, Sun Pharma and Abbotts, Brian has extensive experience in all areas of operations management in the medical device and pharmaceutical industry, including supply chain management, manufacturing, engineering, quality optimisation and environmental responsibility.
Email: brian.morrissey@gaeliclabs.com
Navigating the transition to green propellants, while maintaining performance and usability
The next few years will see substantial changes for pressurised metered-dose inhalers (pMDIs).
As a result of emerging regulations such as the European Union’s F-Gas legislation and the Kigali Amendment to the Montreal Protocol on Substances that Deplete the Ozone Layer, the pharmaceutical industry is working to transition to more sustainable inhalers with reduced environmental impact.
In this article, Craig Sommerville, Senior Vice President of the Metered-Dose Inhaler (MDI) business unit at Kindeva, will discuss how companies can respond to these changes without compromising performance or patient welfare, and how they can act strategically to ensure they are prepared for future opportunities and regulatory shifts.
As countries around the world look to mitigate the effects of climate change, there has been a renewed focus on the propellants used in inhalers.
In 1987, the Montreal Protocol was adopted, precipitating the phasing-out of ozone-depleting substances such as chlorofluorocarbons (CFCs) worldwide. At the time, collaboration and innovation enabled the industry to navigate a transition that has left a lasting legacy.
Nearly 30 years later, in 2016, the Kigali Amendment was signed, turning the world’s attention to the hydrofluorocarbons (HFCs) that effectively replaced them.
Companies are now looking to replace the current pMDI propellants – such as HFA-134a and HFA-227ea – with more environmentally friendly alternatives with a significantly reduced global warming potential (GWP). HFA-152a and HFO-1234ze have a GWP that is 90% and 99.9% lower than HFA-134a. Economics also plays a part in the shift. The price of HFA-227ea has risen considerably in recent years, and HFA-134a prices are expected to follow the same trend.
a


A 2024 study estimates that as many as 13.6 million patients in the five largest European Union countries required a pMDI in 2021, and any widespread change would involve a substantial time and cost investment.1 The pMDI enables the fast and effective delivery of medication directly to the lungs, and moving patients to other inhaler options may have knock-on effects that affect patient convenience and adherence to treatment.
Furthermore, it is important for companies to anticipate regulations that may arise in the future. Europe is presently taking the lead on HFA regulation, with the European Union’s F-Gas legislation aiming to phase out all such gases by 2050.2 The USA’s response is currently taking shape in the wake of the American Innovation and Manufacturing Act, with other territories such as India embarking on strategies to phase out HFCs over the next few decades.3,4 While the goals are clear, the precise path forward is still being shaped by industry experts and regulators.
With the regulatory focus currently trained on pMDIs, it may be tempting for companies to consider a switch away to other types of inhaler devices, such as dry powder inhalers (DPIs).
However, this is where companies should take a step back and take a more holistic, strategic view. While it is imperative to reduce environmental impact, the industry must also consider the impact of any change as a whole, and plan for longer-term sustainability and compliance.
As legislators continue to work toward more environmentally friendly practices, it is safe to expect that regulations affecting inhalers such as DPIs and soft mist inhalers (SMIs) are on the horizon. While propellants are the current area of focus, a recent study of the environmental impacts of inhalers notes that alternatives such as DPIs may have room to improve on factors such as recyclability and component materials.5
Experience of the previous major transition away from CFCs has demonstrated that it is vital to be proactive, and to investigate potential innovations and improvements in all potential propellants. The industry risks missing out on potentially transformative breakthroughs if it abandons lines of treatment.
By remaining open to multiple products, therapies and methods of delivery, strategic companies can maximise their ability to stay ahead of regulatory changes and breakthroughs. Partnering with the right contract development and manufacturing organisation (CDMO), with extensive experience in optimising different approaches, can therefore be an important step in preparing for the future.
Achieving the Best for the Planet, and the Patient The shift toward next-generation low-GWP propellants such as HFA-152a and HFO-1234ze is an important step. The challenge is to ensure that this can be achieved while maintaining – and even ultimately improving – the standards of efficacy, convenience and safety that patients expect.
It is therefore important to explore the ways in which changing the propellant used in pMDIs affects performance. Switching to next-generation propellants can result in changes to vapor pressure, density, surface tension, specific heat capacity and latent heat of vaporisation, all of which must be analysed and accommodated where necessary.
For example, HFA-152a and HFO-1234ze both have higher surface tension values than the currently used propellants, which can affect the size of the initial droplets formed in atomisation as well as the final size of the droplets in the lungs. Furthermore, the physical suspension stability of the low-GWP propellants could be impacted because of their lower density.
Analysing the effects of changing the propellant can be challenging due to the chaotic atomisation that takes place when the pMDI is actuated, and the propellant liquid depressurises rapidly. Nevertheless, it is crucial to develop a deeper understanding of how formulation and device are affected by the introduction of a new propellant – both inside and outside the device - so that future formulation and hardware adaptations can be made with confidence.
This will require companies to partner with experienced research partners and CDMOs, using a combination of compendial and novel methodologies. For example, initial explorations of spray performance at ultra-high-speed imaging facilities indicate that the vapor pressure of next-generation propellants is less sensitive to increased ethanol concentration than current propellants such as HFA-134a.6 As a result, manipulation of the ethanol cosolvent in the formulation may be one option for tuning the formulation and optimising performance.
Changes in inhaler hardware could also improve outcomes. For example, studies indicate that lengthening the pMDI orifice slightly can reduce the width of the spray, which makes it a potential mitigation route for modulating plume width and mixing.6
Insights such as these are crucial in helping companies to integrate next-generation propellants while maintaining performance and patient safety, as well as remaining compliant with upcoming regulations. However, it is important to note that some key aspects of regulation are yet to be determined. For example, it is not yet clear whether moving to pMDIs with next-generation propellants will require a New Drug Application (NDA). Furthermore, while the HFO-1234ze
next-generation propellant offers a substantially lower GWP than current propellants, it may yet fall under emerging European restrictions on per- and polyfluoroalkyl substances (PFAS).
As these regulations are shaped and refined, it is important that the industry shares its expertise to ensure that the best route forward is agreed. CDMOs with extensive experience in developing pMDIs at scale over many years are ideally placed to play a key role in this.
This transition will no doubt require careful planning and investment. That said, the priority must be to ensure that there is as little disruption to the patient as possible. This means working to adapt pMDIs so that there is no perceivable difference in product performance and taking all necessary steps to make this next generation of pMDIs available to patients with no impact on supply.
Any disruption to availability could result in patients moving to other options or interrupting their treatment. Therefore, companies that wish to respond to next-generation propellant regulations – and perhaps obtain a commercial advantage over competitors – should ensure that they work with partners who are sufficiently advanced in upgrading to next-generation propellants. Furthermore, partners should be able to demonstrate that they can handle, fill and manufacture pMDI products using these propellants at the required capacity to meet patient demand.
Forward-thinking CDMO partners have been investing heavily in recent years to ensure they can help this change take root. This involves scaling up both infrastructure and production lines and undertaking extensive re-designs to adapt to the unique storage needs of the new materials. Many are working with companies on more integrated drug-device development strategies, which enable them to consider formulation, device design and manufacturing in tandem. In this way, they can anticipate challenges and opportunities, avoid setbacks and make more informed choices.
Selecting proactive partners with a coherent and advanced strategy will minimise the impact on patients, and the companies themselves.
While the move away from current propellants represents a sizable and complex challenge, companies should also keep an eye on the future. The shift to next-generation propellants is almost certainly part of an ongoing trend toward reducing environmental impact in the industry as a whole. This trend will affect different products in different ways as regulations emerge and evolve.
As such, companies that wish to prepare for the long term must consider a number of crucial factors to make sure they are prepared for what comes next.
• Do not make decisions in haste: Switching to next-generation propellants may be complex in the short and medium term, and it may be tempting to react by switching to other inhaler options.

However, abandoning treatment options may cause companies to miss out on potential future innovations, and the cost and impact of switching at scale may lead to larger environmental impacts.
• Anticipate evolving regulations: Instead of simply reacting to the immediate challenges of current and imminent legislation, companies should develop an informed strategy that observes the direction of travel of the industry, and plans based on what is expected in the future.
This requires companies to be well-versed in current regulations, and ideally active in discussions about how regulations need to evolve in the coming years.
• Understand your product, and how it can be optimised: While the current focus of legislation is on propellants, it is probable that other components and practices will be discussed in future years.
By developing a highly detailed understanding of their products, companies can start to catalogue all the potential options that are open to them for product optimisation and can react with more confidence and insight as regulations emerge.
• Select informed and prepared partners: The right partner can provide invaluable guidance and expertise during a complex change. For example, an experienced CDMO with a background in multiple therapies, products and delivery methods can anticipate challenges, point out the best approaches, and potentially provide access to new and innovative techniques and methodologies.
It is also important to choose a partner who can provide the infrastructure and expertise necessary to switch to green propellants quickly, to maintain supply and minimise disruption.
• Keep the patient in mind: Ultimately, the aim is to provide treatments that are safe, effective, affordable and easy to use, while keeping environmental impact as low as possible.
While adapting to legislation, companies must strive to ensure that the patient is not affected. This means adapting formulation and product design to maintain efficacy, and maintaining supply so that treatments remain accessible to those who rely on them. With the phasedown of existing propellants likely to commence in 2027, it is likely that the move to next-generation propellants will dominate discussions in the industry in the near future.7
However, companies must resist the temptation to act reactively. By taking a wider view, the industry can put itself in a better position to adapt to future regulations. Furthermore, by continuing to explore how to optimise a variety of therapies and devices, we can maximise our chances of discovering innovative treatments which change lives down the line.
1. https://www.ipcrg.org/24040
2. https://climate.ec.europa.eu/eu-action/fluorinated-greenhousegases/f-gas-legislation_en
3. https://www.epa.gov/climate-hfcs-reduction/background-hfcsand-aim-act
4. https://india.mongabay.com/2025/01/the-journey-of-phasing-outozone-depleting-substances/
5. https://www.sciencedirect.com/science/article/abs/pii/ S0959652619325934?via%3Dihub
6. https://www.kindevadd.com/resources/low-gwp-pmdiperformance-webinar/
7. https://ecostandard.org/wp-content/uploads/2024/01/ ECOS_240125_F-Gas_Factsheet_FINAL.pdf

Craig Sommerville, Senior Vice President of Kindeva's Metered-Dose Inhaler (MDI) business unit, is responsible for shaping and executing Kindeva's MDI platform's global strategy, focusing on leading the industrialisation of the green propellant transition. With over 25 years of experience in increasingly senior roles across site leadership, supply chain strategy, operations, logistics, procurement and technical functions, Craig brings a proven track record of transforming challenges into opportunities.
The pharmaceutical inhalation industry stands at a pivotal crossroads. After decades of relative stability following the phase-out of CFCs in the 1990s, a second major transition is underway, this time driven by the urgent need to mitigate climate change. The move toward low-global-warming-potential (LGWP) propellants such as HFA152a and HFO-1234ze(E) represents not just an environmental imperative, but also an enormous opportunity to rethink how we develop, evaluate, and deliver pressurised metered-dose inhalers (pMDIs) to patients worldwide.
This article explores the significance of the shift to LGWP propellants, early performance data generated through the pioneering work of Proveris Laboratories and H&T Presspart, and the broader implications for product development, regulatory strategies, and patient access.
A Turning Point for pMDIs
pMDIs have long been a cornerstone of respiratory care, offering portable, fast-acting relief for asthma and chronic obstructive pulmonary disease (COPD) patients. However, their environmental impact, stemming primarily from their hydrofluoroalkane (HFA) propellants, notably HFA134a and HFA227, has come under increased scrutiny. These HFAs, although ozone-safe compared to CFCs, have high global warming potentials (GWP >1300), far exceeding carbon dioxide.
Enter the next generation: LGWP propellants, such as HFA152a and HFO1234ze, have GWPs below 150, making them eco-friendly alternatives for inhalers. While they address critical environmental concerns, their adoption demands careful evaluation of their physical properties and effects on inhaler performance to ensure effectiveness and safety.
These new propellants have distinct physicochemical profiles compared to legacy HFAs. As a result, they influence critical pMDI attributes, including aerosol generation, droplet size distribution, spray force, and patient deposition patterns, all of which directly impact drug delivery efficiency and therapeutic outcomes.
The Challenges of LGWP Propellants


In making the transition to the LWGP inhaler, it is important to consider material compatibility and the propellant’s safety profile, as well as to understand the regulatory uncertainty.
Swelling behaviour refers to how certain materials, particularly elastomers (rubber-like materials used in gaskets, seals, and valve components of inhalers), interact with propellants. When exposed to a propellant, elastomeric materials can absorb it, causing them to swell, soften, or change in mechanical properties. This can affect the integrity of seals, leading to potential leaks, or alter the performance of valve components, which are critical for consistent drug delivery in metered-dose inhalers (MDIs). Different propellants, such as HFA152a or HFO1234ze, have unique chemical properties (e.g., polarity and solubility) that may cause more or less swelling compared to traditional propellants like HFA134a. This necessitates rigorous testing to ensure that all components remain compatible and maintain performance over the inhaler’s shelf life.

Despite promising data, transitioning to LGWP propellants presents real technical hurdles, including increased volatility. HFA152a has a boiling point of -24.7°C, compared to -26.2°C for HFA134a, but its lower molecular weight (~66 g/mol vs. ~102 g/mol) affects vapor pressure and aerosolisation behaviour. This can influence Plume Geometry, droplet velocity, and drug particle size – factors critical for efficient lung deposition.1
Although HFA-152a is safe for inhalation at intended doses, its flammability at certain volumes – unlike the non-flammable HFA-134a – necessitates enhanced safety measures during manufacturing, such as explosion-proof equipment, inert gas storage systems, and compliance with hazardous material transport regulations. These measures, while feasible, increase operational costs. In contrast, HFO1234ze is a non-flammable LGWP propellant with a GWP of less than 1, offering significant advantages for manufacturing and logistics. Its non-flammability aligns with HFA134a’s safety profile, allowing manufacturers to leverage existing facilities without costly upgrades for spark-proof environments or specialised transport protocols. Additionally, in Europe, HFO1234ze faces potential classification as a per- and polyfluoroalkyl substance (PFAS)
under REACH regulations due to its environmental persistence, which could lead to regulatory restrictions and impact its adoption. While HFA152a avoids PFAS concerns, its flammability makes HFO1234ze an attractive alternative for manufacturers prioritising operational simplicity, provided regulatory hurdles are resolved.2
Understanding how LGWP behave in real-world inhalers is crucial for successful transition. Proveris Laboratories and H&T Presspart have been at the forefront of this effort, deploying innovative as well as traditional evaluation methods to study the performance of HFA152a and HFO1234ze formulations compared to standard HFA134a.
In recent studies, both Proveris and H&T Presspart labs evaluated:3
• Delivered Dose Uniformity (DDU) testing
• Nozzle Design Assessments (orifice diameter and jet length)
• Aerodynamic Particle Size Distribution (APSD) using Next Generation Impactor (NGI)
• Inhalation Deposition with a human-realistic breathing simulator
During the DDU study, the impact of three propellants – HFA134a, HFA152a, and HFO1234ze – on the DDU of an Ipratropium Bromide solution formulation was assessed. Figure 1 shows the DDU for all three formulations. The DDU is calculated as a percentage of the target value of 18 µg/dose. The results for all three propellants were consistent through-life and within allowable limits for each pMDI.
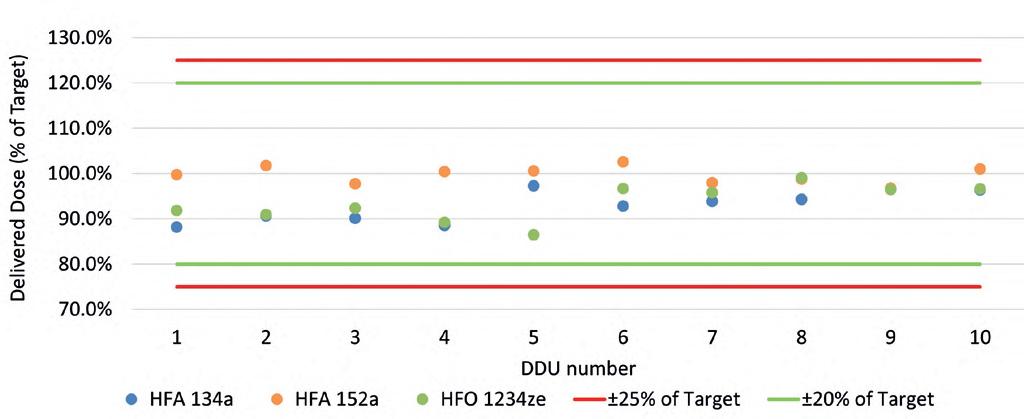
In an initial study done jointly between H&T Presspart and Proveris Laboratories, a comparison of the commercially available Atrovent was made to an inhaler of Ipratropium Bromide 20 µg/actuation with a 15 % w/w ethanol cosolvent and a citric acid excipient with HFA152a propellant.4 Three different Atrovactuator geometries were examined for inhaler deposition testing and APSD. Actuator geometries tested looked at varying the Orifice Diameter (0.25, 0.26 and 0.29mm OD) and Jet Length, (0.35, and 0.75mm), as shown in Table 1.
APSD testing revealed comparable aerodynamic profiles between the HFA134a and HFA152a propellants, with most deposition occurring in the induction port - a behaviour typical of pMDIs as shown in Figure 2. The effect of actuator geometry is illustrated in the levels of drug deposition measured in the NGI. Actuator 3 with a larger Orifice Diameter gave a higher Induction Port and a lower inter-stage deposition than A1 and A2 actuators. The Fine Particle Mass (FPM) of actuator A3 showed an agreement with Atrovent (p=0.857) while actuators A1 and A2 had higher FPM results than Atrovent.
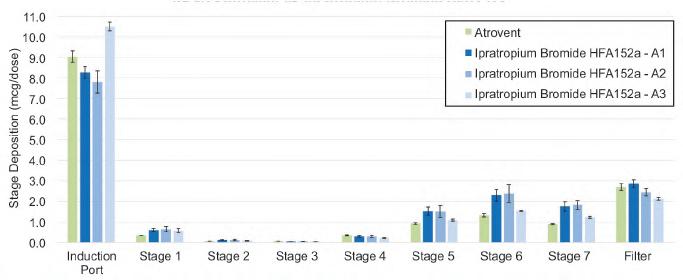
Regional deposition of the inhalers was performed using the In Vitro Inhaled Drug Analysis Platform (INVIDA® Platform), (Proveris Laboratories, USA). INVIDA is a groundbreaking, human-realistic inhalation deposition simulation testing service offered through Proveris Laboratories that mimics human-realistic testing of aerodynamic deposition, shown in Figure 3.
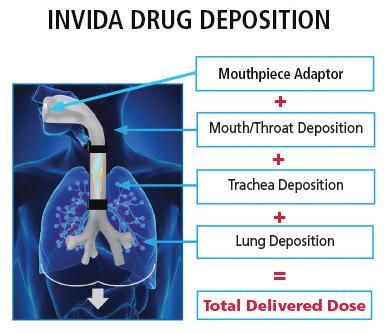
Drug deposition was measured across four anatomical regions using a realistic airway model: the mouthpiece adapter, mouth-throat, tracheobronchial region, and deep lung (captured by a downstream filter). INVIDA, offered as a service by Proveris Laboratories, pairs with the Proveris Human Breathing Simulator to replicate a human flow rate vs. time profile during inhalation. Three inhalers were tested per group – Atrovent and Ipratropium Bromide HFA-152a formulations – with six actuations per inhaler.
The inhalers were manually actuated into the INVIDA mouthpiece adaptor with one inhalation cycle and a peak inspiratory flow rate of 74.71 LPM, with an inhaled volume of 1.5 L. The regional deposition stages were extracted, and Ipratropium Bromide was quantified by HPLC. Deposition results for all 4 regions of the mouth-throat, trachea, and lungs indicated similar regional distribution between HFA134a and HFA152a. Results showed no statistically significant difference (p>0.05) for total drug delivered for any of the 3 HFA152a actuators tested.
Slight differences in deposition were observed in the mouth/ throat and lung regions (Figure 4).
Most notably, the INVIDA platform delivered performance insights comparable to traditional APSD testing, but in one-fifth of the time – demonstrating a powerful path toward faster and more efficient inhalation product development.
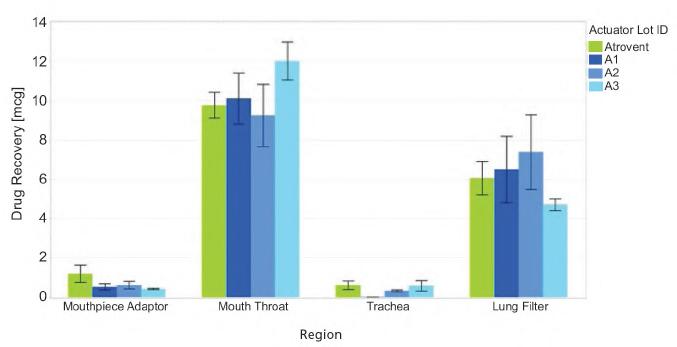
A key regulatory question for the pharmaceutical industry is whether transitioning an approved pMDI from HFA-134a to an LGWP propellant like HFA-152a requires submission of a new drug application (NDA), or whether the change can be managed through a Prior Approval Supplement (PAS).
Requiring full NDAs could lead to drug shortages, delay patient access to environmentally friendly inhalers, and significantly increase development costs. The PAS pathway, supported by robust analytical, performance, and clinical data, offers a scientifically sound and patient-centred approach to facilitate the transition to low global warming potential propellants like HFA152a.
A related question arises when developing a generic version of a previously approved product: can an LGWP propellant be used, and what regulatory pathway would that entail? According to the FDA’s draft guidance on quality considerations for metered dose and dry powder inhalers, and insights from industry experts, a PAS may be sufficient for reformulated pMDIs – provided the new formulation meets specific criteria:5
• Demonstrates equivalence in critical quality attributes (CQAs) such as delivered dose, particle size, Spray Pattern, and Plume Geometry.
• Establishes bioequivalence through pharmacokinetic bridging studies or in vitro – in vivo correlations (IVIVC).
• Confirms no new safety concerns arise from changes in formulation or device.
While regulators like the FDA have signalled support for science-driven approaches, definitive guidance on equivalence testing, clinical bridging requirements, and reformulation pathways continue to evolve. This creates ambiguity for sponsors planning their transition strategies.
The Future is LGWP, Human-Realistic, and rapid analysis. The pharmaceutical inhalation industry is poised for a once-in-ageneration shift. LGWP propellants like HFA152a and HFO1234ze are essential for meeting global climate goals, but their adoption demands innovation across formulation science, device engineering, analytical testing, and regulatory strategy. Early evidence, work combining DDU, APSD, and INVIDA testing, shows

that performance equivalence is achievable, albeit with a need for careful optimisation. Human-realistic testing platforms, faster workflows, and data-driven development models are not just desirable – they are now essential. Companies that embrace this change proactively will not only deliver better environmental outcomes but also emerge as leaders in respiratory care for the next decade and beyond.
The future of pMDIs is lighter, faster, greener – and above all, better for patients and the planet.
1. Buttini F, Glieca S, Sonvico F, Lewis DA. Metered dose inhalers in the transition to low GWP propellants: what we know and what is missing to make it happen. Expert Opin Drug Deliv. 2023 Jul-Dec;20(8):1131-1143. doi: 10.1080/17425247.2023.2264184. Epub 2023 Oct 16. PMID: 37767756.
2. HFO-1234ze(E): A Near-Zero GWP Propellant Supporting Sustainability Transition in Metered Dose Inhalers, Mark Boelens, Nilesh Wagh, Paul Giffen, Stefan Platz, Drug Delivery to the Lungs (DDL) – Edinburgh, UK – 11-13 Dec 2024
3. A Tale of Three Propellants: Understanding the In-Vitro Characteristics of a Solution pMDI Ameet Sule, Lynn Jordan, Lauren Liddle, Amala Xavier, John Howard, Sunita Sule, Ramesh Chand, Mohamed Eldam, & Ashaleni Tharmarajah Drug Delivery to the Lungs (DDL) – Edinburgh, UK – 11-13 Dec 2024
4. Investigation of In Vitro Techniques for Evaluating Ipratropium Bromide Formulations, Ameet Sule, Deborah Jones, Lynn Jordan, Ramesh Chand, Naveen Madamsetti, Sunita Sule, Lauren Liddle, John Howard, Amala Xavier, Steve McGovern Respiratory Drug Delivery (RDD) – Lisbon Portugal – 6-9 May 2025
5. U.S. Food and Drug Administration. (2018). Draft Guidance for Industry: Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products—Quality Considerations. Available at: www.fda.gov

Joanne Mather is a scientific marketing leader with many years of experience in the analytical science space. As senior director of Marketing at Proveris Scientific, she focuses on translating complex scientific and regulatory challenges into practical solutions that help companies in the OINDP space accelerate development and ensure product quality. With a strong background in analytical science and a customer-centric approach, she is dedicated to supporting the industry in bringing effective and reliable aerosolised drug products to market.

Scientific expertise and testing strategies for innovator and generic OINDPs
• Hand Actuation Parameter Design Studies
• Device and Formulation Screening
• Method Development
• Spray Characterization Studies
• Regional Deposition Studies (Human Realistic)
• In Vitro Testing
• IVBE Studies
• Development Stability Testing & Storage
• Method Validation & Transfer
• Device Reliability Studies
• Device Robustness

• Root Cause Analysis
• OOS/OOT Investigations
• Product Consultation when Design, Supply Chain or Manufacturing Changes Occur




The clinical trial industry is essential for ensuring the safety and efficacy of new medicines; however, it is also a significant contributor of greenhouse gas emissions. The Sustainable Healthcare Coalition estimates clinical trials may be responsible for up to 100 megatons of CO2 emissions annually, about the same emissions produced by a country the size of Belgium.1 The main sources of these emissions are thought to include trial-related travel by study participants and site staff, deliveries of trial equipment and energy usage by clinical study sites.2 Given the clear role of CO2 emissions in climate change, the clinical trial industry must adopt new solutions to improve the sustainability, while enhancing patient access and retention as well as the patient experience. Those solutions that are showing promise include patient-centric study designs, virtual clinical studies, adaptive study designs, electronic patient-reported outcomes (ePRO) and the use of digital technologies.
Patient-centric study designs consider a patient's needs and preferences with the aim of reducing the burden on study participants. Those patient-centric approaches that may impact trial sustainability include the use of shorter trial durations and reductions in the frequency and number of required clinic visits e.g., using remote monitoring technologies or telemedicine. An example of a patient-centric study design can be seen in the CHIEF-HF trial, which aimed to evaluate whether the SGLT2 inhibitor, canagliflozin significantly reduces symptom burden in patients with heart failure.3 This trial was designed to reduce the need for in-person visits with direct engagement of patients through a study website, electronic informed consent, direct home delivery of study medication, reporting of the primary endpoint by a mobile application, and use of a Fitbit to monitor activity.3 Patient-centric approaches can make trials more convenient for patients through fewer patient journeys, which in turn can lower fuel consumption and help reduce CO2 emissions. The involvement of patients in the design of clinical trials can also benefit patient recruitment and retention. It is estimated that more than 80% of clinical studies face problems with study recruitment resulting in delays and the need for additional study sites.4 Furthermore, almost a quarter of participants involved in cardiovascular clinical studies drop out before completion.5 Through improved recruitment and retention, patient-centric approaches can help reduce the number of participants required to meet the study outcomes and the resources needed to complete the study.
Decentralised



some of the trial activities take place at sites other than the clinical investigation site, such as the patient's home or a local clinic. DCTs often involve the use of digital tools and platforms to facilitate communication between research teams and study participants. A notable example includes the DeTAP study – a trial, involving the physiologic monitoring of patients with atrial fibrillation receiving oral anticoagulation therapy.6 The DeTAP study utilised telemedicine, eConsent, remote monitoring devices, and ePROs collected via a mobile application to fully decentralise research activities.6 DCTs can improve trial sustainability by reducing the distance or frequency participants travel to clinical study sites and CO2 emissions. The improved accessibility associated with DCTs can help speed up study recruitment, reducing timelines and the need for additional study sites. DCTs can also lead to the more efficient use of energy resources through reduced reliance on physical study sites and the generation and handling of study-related paperwork. However, the manufacture, distribution and use of digital tools and data storage platforms can also contribute to energy usage and CO2 emissions. Nonetheless, one study estimated that the full digitalisation of a traditional clinical study could lead to a more than 90% reduction in energy usage.7
Decentralised clinical trials (DCTs) describe trials where
Adaptive study designs allow for the modification of an ongoing clinical trial design based on evidence accumulated during the study. This is in contrast with traditional clinical studies, where the study design is fixed at the outset and does not change during the course of the study. These modifications may include refinement of sample sizes, targeting specific
patient subpopulations, treatment or dosage regimens, study endpoints and trial duration. Adaptive trial designs have been utilised in cardiovascular studies such as the ANTHEM-HFrEF, a study evaluating the impact of autonomic regulation therapy in heart failure patients, which employed a Bayesian approach for sample size selection.8 Adaptive study designs can help improve trial sustainability by reducing participant numbers and study timelines, potentially reducing the resources needed to complete the study. The optimisation of clinical trial designs can also help increase the likelihood that the study will meet its endpoints, saving the time and resources spent on failed studies.
ePROs are health outcomes directly reported by patients by means of electronic devices such as smartphones, tablets or web applications. ePROs are commonly used in clinical trials to provide information about a participant's symptoms, daily functioning, quality of life and response to therapy. Notably, more than a quarter of the clinical trials currently being conducted are thought to involve ePROs.9 The CHIEF-HF study is one such study, where the outcomes were assessed using an app version of the Kansas City Cardiomyopathy Questionnaire for quantification of symptom frequency and severity, and Fitbit monitoring of daily step counts.3 ePROs can enhance study data quality by enabling more frequent and real-time reporting of a study participant's health status. ePROs can contribute specifically to trial sustainability by reducing the number of appointments participants need to attend, thereby reducing required clinic time and trial-related travel and its environmental impacts. Additionally, the incorporation of ePROs into study designs can conserve resources by reducing study-related paperwork and the associated administrative burdens.
Digital technologies are increasingly being used to enhance data quality within the clinical trial setting by enabling the continuous and remote collection of health data from study participants during their everyday activities. These digital technologies range widely and may include software (e.g., smartphone apps), and hardware (e.g., wearables) solutions. The number of clinical trials incorporating digital technology was estimated at 11% in 2020 and is projected to reach 70% by 2025.10 One example is the LINK-HF study, which utilised a multisensor disposable patch to continuously monitor a range of physiological parameters and detect oncoming heart failure exacerbations.11 Another recent example, the eBRAVE study, utilised a smartphone app to screen for pulse wave irregularities and detect atrial fibrillation in individuals at risk of stroke.12 Digital technologies can contribute to trial sustainability by reducing the need for participants to travel to study sites to have measurements performed and conserving energy associated with study site operations. Additionally, digital tools can provide more accurate and real-world data as well as additional data points, helping to reduce the likelihood that the study will need to be extended or repeated.
New solutions are crucial for improving the sustainability of the clinical trial industry and reducing its contribution to climate change. The main contributing factor is CO2 emissions, resulting from trial-related travel and energy usage by
clinical study sites. Those solutions showing promise in this regard revolve around adjustments in study designs or the incorporation of digital technologies. These solutions can also improve recruitment and retention by enhancing the clinical trial experience for study participants. However, several barriers exist to their adoption, including concerns over data security and privacy, data quality, participant safety in the absence of physical visits, and accessibility for those with poor computer literacy. Addressing these concerns will not only help minimise the environmental impact of the industry but also help ensure the continued development of new medicines.
1. https://shcoalition.org/clinical-trials/, visited on 9 October 2024.
2. Sustainable Trials Study Group. Towards sustainable clinical trials. BMJ. 334(7595), 671–673 (2007).
3. Spertus, J.A., Birmingham, M.C., Nassif, M., et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patientcentered randomized trial. Nat. Med. 28(4), 809–813 (2022).
4. Desai, M. Recruitment and retention of participants in clinical studies: Critical issues and challenges. Perspect. Clin. Res. 11(2), 51 (2020).
5. Hutchison, E., Zhang, Y., Nampally, S., et al. Modeling Clinical Trial Attrition Using Machine Intelligence: A driver analytics case study using 1,325 trials representing one million patients. medRxiv. 2021.11.12.21266277 (2021).
6. Sarraju, A., Seninger, C., Parameswaran, V., et al. Pandemicproof recruitment and engagement in a fully decentralized trial in atrial fibrillation patients (DeTAP). NPJ Digit. Med. 5(1), 80 (2022).
7. Kohl, S.H., Schmidt-Lucke, C. Clinical trials to go green–A sustainable argument for decentralised digital clinical trials. PLOS Digit. Health. 2(10), e0000366 (2023).
8. Konstam, M.A., Udelson, J.E., Butler, J., et al. Impact of Autonomic Regulation Therapy in Patients with Heart Failure. Circ. Heart Fail. 12(11) (2019).
9. Vodicka, E., Kim, K., Devine, E.B., et al. Inclusion of patientreported outcome measures in registered clinical trials: Evidence from ClinicalTrials.gov (2007–2013). Contemp. Clin. Trials. 43, 1–9 (2015).
10. Chandrasekaran, R., Katthula, V., Moustakas, E. Patterns of Use and Key Predictors for the Use of Wearable Health Care Devices by US Adults: Insights from a National Survey. J. Med. Internet Res. 22(10), e22443 (2020).
11. Stehlik, J., Schmalfuss, C., Bozkurt, B., et al. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization. Circ. Heart Fail. 13(3) (2020).
12. Rizas, K.D., Freyer, L., Sappler, N., et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat. Med. 28(9), 1823–1830 (2022).

Bipin Patel Ph.D. is the CEO and Founder of electronRx, a deep-tech startup developing novel chronic disease and hospital patient management solutions. He is a key digital health thought-leader with over 20 years’ experience in medical engineering, drug development and commercialisation and holds a PhD in Medical Engineering from UCL, UK.
Email: enquiries@electronRx.com
As veterinary medicine continues to evolve, so too do the expectations around how treatments are developed and produced. In recent years, there has been a significant shift towards more sustainable and environmentally conscious pharmaceutical manufacturing. This is particularly relevant in animal health, where high-volume APIs like growth promoters are widely used – and where their method of production can carry a considerable environmental footprint.
The call for greener processes is not just a matter of compliance. It reflects growing industry-wide recognition that environmental responsibility must be integrated into the earliest stages of drug development. Regulators are introducing stricter guidelines around solvent usage, waste generation, and energy consumption. Meanwhile, customers, investors, and other stakeholders increasingly expect companies to align their manufacturing practices with broader sustainability goals.
Within this context, green chemistry has emerged as a critical tool — not simply as a cost-saving measure, but as a strategic imperative. And yet, implementing green chemistry principles in active pharmaceutical ingredient (API) synthesis remains a formidable challenge, particularly when working with legacy molecules and multi-step synthetic routes.
One of the most instructive areas for exploring these challenges – and potential solutions – is in the synthesis of growth promoters. These compounds, used to support feed efficiency and animal weight gain, are typically administered on a large scale. As such, even incremental improvements in their manufacturing process can result in substantial environmental benefits.
Rethinking Traditional Synthesis
Conventional synthesis of growth promoters often involves solvent-intensive processes, toxic reagents, and energy-heavy conditions. These routes may have been developed for speed or cost-effectiveness decades ago, but they frequently fall short by today’s environmental and safety standards.
In response, some development teams are now undertaking complete re-evaluations of synthetic routes, applying green chemistry principles from the ground up. One key focus has been the elimination of column chromatography in favour of crystallisation-based purification methods, which significantly reduce both silica waste and solvent consumption. In many cases, process steps are being telescoped – merged together without isolating intermediates – to minimise processing time and material usage. Hazardous solvents, including carcinogenic Class 2 options used in earlier processes, are being replaced with less toxic alternatives to


reduce health and environmental risks. Common choices include replacing metal lactate with lactic acid and substituting pyridinium salts with safer, more economical coupling agents. These changes not only support safety and sustainability but also improve compatibility across steps. Reagent selection is increasingly guided by cost-efficiency as well, ensuring that the switch to greener materials doesn't compromise commercial viability. Another area of emphasis is temperature control; by designing reactions to proceed at ambient conditions, developers can reduce energy usage and mitigate the risks associated with thermal hazards.
What’s increasingly clear is that sustainability-driven process design doesn’t happen by chance – it requires deliberate methodology. One effective framework gaining traction is the SELECT criteria approach, which evaluates potential process improvements across dimensions like Safety, Environment, Legal, Economics, Control, and Throughput.
Applied thoughtfully, SELECT allows chemists and engineers to balance complex trade-offs – for example, choosing a slightly lower-yielding reaction if it eliminates a hazardous solvent or significantly reduces waste.

Additionally, the use of Design of Experiments (DoE) and other systematic optimisation techniques is helping teams arrive at greener, more robust processes more efficiently. These tools not only fine-tune reaction conditions, but also help identify control strategies that ensure consistent product quality at scale.
Developing a green process at lab scale is one thing; implementing it reliably at manufacturing scale is another. A common pitfall is overlooking the nuances of scale-up – such as heat transfer, mixing, and reaction kinetics – that can affect yield or product purity.
Successful projects often involve close collaboration between process development and manufacturing teams, ensuring that what works in the flask can be reproduced consistently in

multi-kilogram or commercial batch settings. Early identification of critical process parameters (CPPs) and a clear understanding of critical quality attributes (CQAs) is key to bridging that scale-up gap.
One recent case involved the re-engineering of a multi-step growth promoter synthesis that originally relied on column chromatography and costly, hazardous reagents. Through a series of redesigns, including route modification, solvent swaps, and step consolidation, the process team was able to reduce waste, lower solvent usage by 20%, and improve overall yield by over 15% – all while maintaining the required purity and impurity profile for regulatory submission.
Critically, the process was developed to be chromatographyfree, enabling better reproducibility and significant environmental savings. The new route resulted in an 80% reduction in both the cumulated environmental factor (cE-factor) and process mass intensity (PMI) – two key metrics used to measure environmental impact in pharmaceutical manufacturing.
The example above is not unique, but it is illustrative. As the animal health sector becomes more global, more tightly regulated, and more attuned to environmental impact, the expectations around how veterinary drugs are developed will only rise.
Growth promoters – often produced in large volumes – are a prime target for sustainable innovation. But the same principles apply across a wide range of veterinary APIs, from anti-parasitics to anti-infectives. In each case, the question is the same: how can we make the process cleaner, safer, more efficient – and still meet the commercial and therapeutic goals?
For many companies, this will mean working more closely with development partners who bring a multidisciplinary perspective to the table: deep synthetic chemistry knowledge, process engineering expertise, and a commitment to sustainable practices. Whether in-house or through external
collaboration, it is this integration of science, strategy, and sustainability that will define the next generation of animal health manufacturing.
Green chemistry is not a passing trend in animal health – it is becoming central to how responsible, forward-looking companies develop products. As pressure mounts from regulators, investors, and end consumers, the ability to rethink established synthesis routes and adopt greener alternatives will increasingly define competitive advantage.
In this context, project success should be measured not just by yield or cost, but by how well the process aligns with broader goals of safety, sustainability, and scalability. Whether applied to new molecular entities or re-engineered legacy APIs, sustainable synthesis is a powerful lever for progress – one that aligns industry performance with environmental stewardship in a way that benefits everyone, from producers and veterinarians to animals and ecosystems.

Alex is the Senior Vice President, Manufacturing Services at Syngene International and has three decades of experience in developing, commercialising and life-cycle management of products in various life science industries. Holding positions in both the US and Europe at Syngene International, his experience includes senior roles with global P&L responsibility. As a member of the Executive Committee, Alex plays a techno-commercial role, providing technical expertise to the API plant at Mangalore while building a sustainable client base for the business in collaboration with the commercial and business development teams. In addition, Alex is also responsible for biologics operation at Syngene International.
Global distribution of life-saving pharmaceuticals is incredibly complex, with several different components from warehouse to final delivery. At each stage, providers must make sure strict temperature requirements are met across varying climates and infrastructures.
As a result of global warming, the increase of unpredictable weather patterns and increased temperatures is making distribution even more challenging. To combat this, manufacturers, logistics providers and distributors are having to work together to implement new strategies and routes whilst trying to keep costs down. However, someone vital is often being missed out of the conversation.
As witnessed during the pandemic, packaging providers play a crucial role in the delivery of lifesaving medicines. By building relationships with these providers now, logistics can adapt to ensure continued effectiveness of cold chain distribution as we prepare for the increase of extreme weather.
Cold Chain Needs to be Smarter, Not Just Stronger
Supply chain disruptions can easily cause issues with the delivery of supplies and treatments. Global warming will create unpredictable conditions, with flooding, landslides and storm damage. These extreme weather fluctuations will impact routes and mean that future packaging may need to handle freezing temperatures, extreme heat and humidity all in one journey.
Ensuring reliability and efficiency is vital. Availability of packaging solutions must be successfully managed, and the industry must position themselves to be able to predict and prepare for all disruptions. The extreme weather fluctuations will mean a one-size-fits-all approach will no longer be viable. Instead, data-driven risk analysis and route-specific adaptation will be key. Manufacturers will need to factor in seasonal and regional climate risks when planning distribution.
One solution to these evolving challenges is integrating AI into the cold chain. AI-driven insights can help optimise routes, reduce waste and lower costs. By analysing historical data and predicting climate patterns, the most efficient, reliable and unaffected delivery routes can be determined. This not only cuts costs but also supports the timely and reliable delivery of medicine and minimises environmental impact. AI will need real-time data on transportation conditions, such as weather patterns and temperature fluctuations, to determine the correct route and solution type needed for a successful delivery.


Although reducing the environmental impact of cold chain logistics is essential, it cannot come at the cost of efficiency and patient safety. AI also plays an important role here, through not only determining the best routes and solution choice, but by unlocking efficiencies to help meet sustainability requirements. There must be a focus on minimising waste through forever-use packaging, making sure it is returned and re-used wherever possible. Adopting lighter, space-efficient packaging can lower fuel consumption and reduce emissions, as well as optimise the amount of product shipped to reduce cost. However, to truly have an impact, sustainability requires collaboration across the entire supply chain.

As temperatures increase, so will the demand for enhanced cold chain infrastructure.
Packaging solutions with significant autonomy will be required to maintain temperature and safe delivery, even in the face of extreme conditions. Additionally, climate-related supply chain disruptions may call for alternative backup routes, meaning redundancy will need to be built into distribution systems.
More frequent extreme weather events, such as heatwaves, flooding, wildfires, and landslides, will significantly disrupt supply chains. These events can lead to road closures, disrupted shipping lanes, and airport shutdowns, making it difficult to maintain consistent transportation routes. To address this challenge, it is crucial to collaborate with partners who have a wide global network to mitigate the risk of delays. These partners must also demonstrate agility and proactivity in adjusting plans as needed to ensure that patients receive their vital medicines without disruption.


Handling Global Warming Requires a Delicate Balance
As learnt from any previous crisis, collaboration is key. Only by fostering collaboration between manufacturers, logistics partners and packaging providers can the pharmaceutical industry hope to balance sustainability, cost and reliability in the face of global warming. New technology and AI will be key drivers of this, along with the agility to react fast to any potential disruption. Companies that prepare now and find the right balance will be more efficient and gain a competitive edge in a market that demands both resilience and responsibility.

Niklas is the CEO Interim and COO at Envirotainer. Niklas brings more than 15 years of international leadership experience from various roles. Before joining Envirotainer, he held the position as Technical Director Northern Europe at Dassault Systemes. Furthermore, he has also worked within the Operations and Supply Chain domain at Accenture in Sweden.
Niall Balfour, CEO of Tower Cold Chain – a Cold Chain Technologies company – examines how data and academic partnerships are driving sustainability in the pharmaceutical cold chain to optimise logistics and reduce the environmental impact of transporting temperature-sensitive products.
Cold chain logistics are vital in the pharmaceutical and life sciences industries, where temperature-sensitive products such as vaccines, medications, and biological samples must be transported and stored under strict temperature controls. Ensuring the integrity of these products is essential, as their efficacy often relies on precise temperature regulation. However, managing the distribution of such sensitive goods presents significant challenges. With growing sustainability demands, the industry is increasingly relying on innovative solutions that reduce carbon emissions while upholding product integrity.
One such solution has been the collaboration between academic institutions and industry stakeholders to optimise cold chain logistics, with a growing focus on sustainability. A notable example is the partnership between Cardiff University’s Business School and Tower Cold Chain, aimed at utilising academic expertise to enhance operational efficiency while prioritising sustainable practices.
The Environmental Challenges of Cold Chain Logistics in the Pharmaceutical Industry
The pharmaceutical industry's cold chain logistics operations are highly energy-intensive, primarily due to the stringent temperature requirements needed to preserve the efficacy of drugs and biological materials. For example, vaccines typically need to be stored between 2–8°C (35–46°F), while gene therapies and biologics may require sub-zero or even cryogenic temperatures. Maintaining these conditions over long distances and periods can be technologically challenging and environmentally costly.
Traditional cold chain solutions often involve single-use packaging and active cooling systems, which require constant electricity and result in significant waste and carbon emissions. Additionally, the need for global logistics networks exacerbates the environmental impact. The pharmaceutical sector’s increased demand for reliable cold chain logistics, particularly during emergencies like the COVID-19 pandemic, has put further strain on the industry’s environmental footprint.


scenario poses a dual challenge: companies must meet regulatory standards for product preservation while minimising waste and emissions.
To address these challenges, reusable, passive containers have become a viable solution for maintaining temperature control without the need for continuous power or disposable cooling agents. These containers are engineered to preserve specific temperature ranges for extended durations, sometimes up to and beyond 120 hours, without relying on active refrigeration. This innovation allows pharmaceutical companies to lessen their dependence on single-use materials and energy-intensive cooling systems, providing a reusable approach that still ensures precise temperature management.
Equipped with advanced insulation and phase-change materials (PCMs), these containers absorb and release thermal energy, maintaining stable temperatures. With capabilities to sustain temperatures from -80°C to +20°C, they support the wide range of temperature-sensitive products in the pharmaceutical industry. These containers are also built for durability, often lasting up to 15 years, which reduces the environmental footprint tied to the production, shipping, and disposal of single-use alternatives.

The shift to reusable containers addresses key concerns in the industry. By decreasing the need for manufacturing and disposing of single-use containers, it helps reduce overall waste. Additionally, these containers contribute to a lower carbon footprint by minimising energy consumption. However, transitioning to this sustainable solution requires a comprehensive review of supply chain operations to ensure the approach is cost-effective and operationally efficient.
As the global demand for temperature-sensitive medicines rises, so does the need for a wider variety of solutions. This
While the sustainability advantages of reusable containers are clear, their implementation requires careful consideration of the total cost of ownership across the entire supply chain. The initial cost of reusable containers is often higher than that of singleuse options, but the long-term savings can be significant. These savings stem from reduced waste, fewer repair costs, lower shipping costs, and extended lifespans.

However, to realise these savings, companies must assess the broader logistics and distribution strategies they employ. The transition to reusable containers must be accompanied by a thorough analysis of how to streamline inventory management and shipping practices. Proper inventory management ensures that containers are available when and where needed, reducing delays and ensuring compliance with regulatory requirements.
Logistics networks must also be optimised to accommodate the new containers. For instance, organisations need to assess the most efficient routes and distribution methods to minimise transportation distances and reduce the environmental impact.
Offering an extensive, regional hub network and the use of a space-efficient conditioning system are also key factors in reducing carbon emissions. By offering shorter distances for servicing and redeployment, combined with lower energy requirements for container conditioning, logistics providers can help to decrease environmental impact while supporting more efficient operations.
The integration of reusable containers into any supply chain requires close coordination between all stakeholders to identify the best strategies for improving efficiency and reducing costs.
The collaboration between academic institutions and industry partners plays a crucial role in driving innovation and efficiency in cold chain logistics. Academic research provides valuable insights into operational optimisation, helping industry stakeholders make informed decisions about logistics strategies and container use. This collaboration is particularly important in
ensuring that sustainability goals are met without compromising operational requirements.
For example, Cardiff University’s Business School has worked with logistics providers to analyse demand patterns, shipping routes, and inventory management practices. By leveraging data analytics, the university’s research helps identify opportunities for optimising container usage across various global locations. This data-driven approach enables companies to streamline their supply chains, minimise inefficiencies, and reduce unnecessary carbon emissions.
One of the critical challenges identified in this research is the phenomenon of "empty leg" journeys, where containers travel to and from distribution centres without cargo. These journeys result in additional costs and unnecessary carbon emissions. By analysing data on shipping routes and container usage, Cardiff University’s research has helped cold chain providers reduce empty leg trips and increase the efficiency of transportation networks.
Through the partnership, research has also focused on resource allocation, ensuring that reusable containers are positioned in locations where they can be most effectively utilised. This approach minimises transportation distances and increases the frequency of fully loaded trips, further reducing environmental impact and costs.
As environmental regulations become increasingly stringent, pharmaceutical companies are under pressure to demonstrate their commitment to sustainability. The integration of sustainable practices into cold chain logistics not only helps companies

comply with these regulations but also supports their broader sustainability objectives. By prioritising the use of reusable containers and optimising logistics processes, companies can reduce their reliance on single-use packaging and cut down on waste.
This shift aligns with the broader industry trend toward adopting circular economy principles. By extending the lifespan of containers and reducing waste, pharmaceutical companies can significantly reduce their environmental impact. Furthermore, the adoption of sustainable practices often leads to long-term cost savings. Reduced packaging waste, improved logistics efficiency, and more effective resource allocation contribute to a more cost-effective and environmentally responsible supply chain.
The future of cold chain logistics in the pharmaceutical sector is moving toward greater sustainability. However, achieving this goal requires ongoing innovation and collaboration between industry players, academic researchers, and regulatory bodies. Initiatives like the partnership between Cardiff University and Tower Cold Chain serve as examples of how data analytics, operational efficiency, and sustainable technologies can work together to create a more environmentally responsible logistics landscape.
By integrating academic research with industry practice, these collaborations help to address the complexities of cold chain logistics, from regulatory compliance to operational optimisation. As pharmaceutical companies increasingly prioritise sustainability, they will need to embrace innovative solutions, such as reusable containers and data-driven optimisation strategies, to meet both environmental and operational goals.
This collaborative approach underscores the critical role of data analytics in transforming the pharmaceutical cold chain industry. By making informed decisions based on comprehensive data analysis, companies can reduce their environmental impact while ensuring that temperature-sensitive products are safely and efficiently transported. Moreover, the use of sustainable practices not only helps meet regulatory requirements but also
aligns with the growing demand for corporate responsibility in the face of climate change.
The future of pharmaceutical logistics will likely continue to evolve as sustainability becomes a central focus. As data analytics, technology, and academic research continue to shape the industry, pharmaceutical companies will be well-positioned to meet modern challenges while contributing to a more sustainable and resilient global supply chain.
The path toward sustainability in pharmaceutical cold chain logistics is an ongoing process, but collaborative efforts between industry stakeholders and academic institutions provide a promising blueprint for the future. By optimising logistics operations, adopting reusable containers, and leveraging data to drive efficiency, the pharmaceutical industry can significantly reduce its environmental impact. The integration of these practices into everyday operations will not only contribute to a more sustainable future but will also help pharmaceutical companies meet the evolving demands of the global healthcare landscape. As the industry continues to innovate and embrace new technologies, sustainability will undoubtedly become an increasingly integral part of the pharmaceutical cold chain.

Tower Cold Chain’s Chief Executive Officer, Niall Balfour, is a highly experienced and versatile director operating in the life science logistics and pharmaceutical supply markets. Joining Tower in 2015 as CEO, Niall brings 30+ years of industry knowledge to the company, with previous positions including Vice President, Commercial Operations, Biocair, Vice President Sales, Europe and Asia Pac, Marken, and Director, EU Sales & Marketing, Quest Diagnostics Clinical Trials. Niall is committed to driving Tower’s mission in being a dynamic and profitable company that creates value for all its stakeholders through innovation, regulatory compliance, and sustainability.
28-30 October 2025
Messe Frankfurt
Where the global pharma community gathers to shape the future.

62,000+ attendees
2,400 exhibitors


3 days of innovation, partnerships, and business growth

Experience
CPHI Frankfurt
Register now and save 10%






Bringing together an expert network to embed sustainability in the inhaled and nasal industry. Learn more in our first ESG update by scanning the QR code.