
Journal for Clinical Studies PEER REVIEWED Volume 15 Issue 1
and Conduct of Trials in Alzheimer’s Disease Following the Impending Approvals of Disease-Modifying Agents CRM Study Coordinators Making a Difference in Clinical Trial Conduct in Malaysia Beyond Modernisation Unlocking the Value of Regulatory Information Management Rethinking Statistical Significance in a Rare Disease Context www.journalforclinicalstudies.com
Design
Harnessing decades of global drug product development and commercialization, you can rely on us for global, integrated CDMO services. speedsolutions™ combine expertise and services to simplify the supply chain, spanning the cycle from development to launch.



Introducing speedsolutions
Your bridge between life-changing therapies and patients speedtostudy™ speedtopatient™ speedtoapproval™ speedtolaunch™ PH II PH III COMMERCIALIZATION PH I Let’s talk future™ talkfuture@pci.com | pci.com Accelerating your product through development to commercialization and beyond Development & Manufacturing | Clinical Trial Services | Commercial Packaging
™
Journal for Clinical Studies
MANAGING DIRECTOR
Mark A. Barker
BUSINESS DEVELOPMENT info@senglobalcoms.com
EDITORIAL MANAGER
Beatriz Romao beatriz@senglobalcoms.com
DESIGNER
Jana Sukenikova www.fanahshapeless.com
RESEARCH & CIRCULATION MANAGER
Jessica Chapman jessica@senglobalcoms.com
ADMINISTRATOR
Barbara Lasco barbara@senglobalcoms.com
FRONT COVER istockphoto
PUBLISHED BY Senglobal Ltd.
Unite 5.02, E1 Studios, 7 Whitechapel Road, E1 1DU, United Kingdom
Tel: +44 (0) 2045417569
Email: info@senglobalcoms.com www.journalforclinicalstudies.com
Journal for Clinical Studies – ISSN 1758-5678 is published quarterly by Senglobal Ltd.
4 FOREWORD
WATCH PAGES
6
The Role of Confirmatory Trials After Accelerated Approval
Initially developed for the HIV/AIDS crisis in 1992, the US Food and Drug Administration (FDA) established the accelerated approval program to expedite the approval of drugs for serious or life-threatening conditions. The FDA grants these drugs accelerated approval based on a surrogate endpoint, which helps to speed up the drug development process. Since inauguration of the program, the FDA has typically given accelerated approval for anti-cancer therapies. Jennifer Nguyen at Clarivate explains the role of confirmatory trials after accelerated approval.
8 Rethinking Statistical Significance in a Rare Disease Context
Biopharma Excellence recently hosted a panel discussion featuring experts in biotech and the evolving regulatory environment to discuss the challenge of achieving statistical significance with novel and highly targeted therapies. In a recent panel debate moderated by Christian K Schneider at Biopharma Excellence, reviews emerging approaches to clinical data collection where patient populations are small.
REGULATORY
10
Data Protection and Clinical Trial
The highly regulated nature of the pharmaceutical industry means that organisations within this sector are no strangers to the requirement to abide by the various strict rules and regulations throughout the lifecycle of drug discovery, testing and production. However, when thinking about privacy and data protection regulations, clinical trials are certainly where it is most pertinent. Due to the volume of sensitive personal data that needs to be processed to demonstrate the safety and efficacy of a drug, this creates the requirement for significant data protection considerations. Rob Masson at The DPO Centre, sets out four of the most important data protection factors that life sciences organisations must take into account when sponsoring a clinical trial that involves EU or UK residents.
14 The Year Ahead for Life Sciences Regulatory Operations
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held responsible for any loss or damage incurred. This publication is protected by copyright.
Volume 15 Issue 1 Spring 2023
Senglobal Ltd.
Regulatory has always been the “spider in the web” within a pharmaceutical company – collecting all of the relevant information for submission of the registration: Clinical, CMC, administrative data, etc – then compiling a valid eCTD by the submission deadline. In future, all of this will be data driven, requiring a different approach to managing everything and ensuring its quality. Amplexor asked some of its closest partners for their views on the most important developments in Life Sciences regulatory operations in 2022, and for their predictions for 2023. Renato Rjavec of Amplexor Life Sciences shares their input.
16 Beyond Modernisation– Unlocking the Value of Regulatory Information Management
Biopharmaceutical companies are now at an important inflexion point. Most have completed their modernisation of the Regulatory function globally, bringing improvements to efficiency, according to newlypublished Gens & Associates research, the 2022 World Class RIM Survey. Steve Gens reveals how respondents are planning to harness regulatory information management (RIM) as an enterprise asset.
Journal for Clinical Studies 1 www.journalforclinicalstudies.com
Contents
18 Next-level Clinical Trial Technology Can Avoid Dropouts and Accelerate Drug Development
Despite the recent shift to hybrid and decentralised clinical trials (DCT), low enrolment, poor retention, and outdated processes are still holding research back. But it’s not the technology that’s the problem – it is the way we are using it. From ride hailing to mobile banking, technology is light years ahead of what it was just a few years ago. Yet many solutions in the clinical sphere have not kept pace with the level of choice, control, and user experience people have come to expect. It all adds up to a clear disparity between the experience in clinical trials and the daily experience of the average consumer. Sam Whitaker at Mural Health highlights how next-level clinical trial technology can avoid dropouts and accelerate drug development.
20 Digital Clinical Trials: Time to Throw Out the Old Rule Book

Traditional industries are regularly disrupted by new technologies, and the life sciences industry is no exception. Electronic data capture (EDC) arrived on the scene decades ago. It has now become the de facto tool for data management and collection, and over time it has bought with it several important benefits. Where previously it would take 8 to 16 weeks for data recorded during a patient’s visit to appear in the data management workstream, EDC has reduced that lag time from weeks to days, even hours in some cases. Richard Young at Veeva Systems discusses the ways that digital clinical trials need to improve. MARKET REPORT
22 Developing a Pre-IND Packing Strategy to Expedite Clinical Trial Timelines
As biotechnology and pharmaceutical companies seek innovative solutions to expedite their timelines, efficient management of a clinical trial starts with strategic planning. Submission of an Investigational New Drug (IND) application is a major milestone in new drug development. It marks the transition from bench research to clinical studies in human participants. In this article, PCI Pharma Services shares insights into the IND application process, the benefits of applying a Pre-IND strategy utilising a Canadian-based CDMO, and how this approach can deliver reduced First Patient-In (FPI) timelines by 6–8 weeks.
24 CRM Study Coordinators – Making a Difference in Clinical Trial Conduct in Malaysia
The clinical trial industry is developing rapidly in Malaysia. Since 2012 there have been more than 2000 clinical trials across different Therapeutic Areas, hence promoting Malaysia as a preferred country for sponsored clinical trials. The commitment to deliver clinical trials with Speed, Reliability and Quality is the essence to this success. This achievement is critically delivered by the invisible hands of Study Coordinators (SC). Intan Munirah bt Mohd Murad, Nor Hafiza bt Johari, Venoo Kuppusamy and Joanne Yeoh at Clinical Research Malaysia clarify how SCs are the invisible hands in clinical trials who contributes to the success of each trial and provides supports to the investigators.
28 Who Cares About Diversity in Clinical Trials?
The imperative to obtain a diverse body of clinical trial participants has been stressed in public statements for decades, including formal promotion by the U.S. National Institutes of Health (NIH), regulatory authorities such as the U.S. Food and Drug Administration (FDA), and recently, with the Consolidated Appropriations Act of 2023, even the
U.S. Federal Government1-4. Despite the duration and high profile of this issue, however, parties executing clinical trials, including sponsors, clinical research organisations (CROs), and academic centers, have remained insufficiently effective in addressing it. Denise Calaprice, Ryan Whitty, Christina Moon and Neva Hidajat at WCG Avoca, outline the importance of diversity in clinical trials.
TECHNOLOGY
32 Clinical Research: More than Words
Art and science are often positioned as opposites, with scientific illustration at the intersection. However, visuals contribute greatly to scientific communication, allowing complex ideas or theories to be presented clearly and in an accessible way. Whether it’s a flyer in the doctor’s office, a poster at a conference or a research paper Fabricio Pamplona at Mind the Graph explains that the communication of clinical studies research requires more than just words.
THERAPEUTICS
37 Design and Conduct of Trials in Alzheimer’s Disease
Following the Impending Approvals of Disease-Modifying Agents
Fortunately, after nearly two decades of negative studies, three amyloid-lowering drugs have recently received accelerated approval for Alzheimer's Disease (AD) by the FDA, and one of these drugs is likely to receive full approval by July of this year. Submissions have also been made to regulatory authorities in Asia and Europe, and it is possible there will be global approval by the end of 2023, or early 2024. Smith M, Smith R & Drosopoulou N. at Worldwide Clinical Trials outline how the design and conduct of trials in alzheimer’s disease following the impending approvals of disease-modifying agents.
2 Journal for Clinical Studies Volume 15 Issue 1 Contents
RESEARCH & DEVELOPMENT
Thermal Blankets
Development
(+15°C +25°C) and (+2°C +30°C) Worldwide
Qualifications & Validations

- Multilayer thermal blanket for PMC -ULD – Euro and Block pallets

- Temax-4000 blanket with integrated 4x multiple reflection technique
- Stress tested in summer (+46°C) and winter ( -15°C) profiles
- Tarmac tested on solar power and greenhouse effects
Ecological notes
- Recyclable + Low ecological footprint
- NON-laminated composition = easy to dismantle
- Re-manufacturing of recycled compounds

and manufacturing
available at freight forwarder s KRAUTZ - TEMAX Group Development and manufacturing Belgium - Europe
+32-11.26.24.20
www.krautz.org Email info@krautz.org
Americas LTD Hanover park – Illinois – USA
certified
for the temperature protection of pharmaceuticals and healthcare products in airfreight
Phone
Website
Temax
ISO-9001:2015
Development of disease-modifying treatments for Alzheimer's disease (AD) has been challenging, with no drugs approved to date. Alzheimer's disease affects more than 20 million people worldwide. More people will be impacted as the cohort of adults aged 65 and older rises and if disease modifying therapies are not fully approved. Clinical trials for Alzheimer's disease are scientifically and operationally challenging. Sponsors need innovative solutions to drive new therapies to market.
Fortunately, after nearly two decades of negative studies, three amyloid-lowering drugs have recently received accelerated approval for Alzheimer's Disease (AD) by the FDA, and one of these drugs is likely to receive full approval by July of this year. Submissions have also been made to regulatory authorities in Asia and Europe, and it is possible there will be global approval by the end of 2023, or early 2024. Smith M, Smith R & Drosopoulou N. at Worldwide Clinical Trials outline how the design and conduct of trials in alzheimer’s disease following the impending approvals of disease-modifying agents.
The highly regulated nature of the pharmaceutical industry means that organisations within this sector are no strangers to the requirement to abide by the various strict rules and regulations throughout the lifecycle of drug discovery, testing and production. However, when thinking about privacy and data protection regulations, clinical trials are certainly where it is most pertinent. Due to the volume of sensitive personal data that needs to be processed to demonstrate the safety and efficacy of a drug, this creates the requirement for significant data protection considerations. Rob Masson at The DPO Centre, sets out four of the most important data protection factors that life sciences organisations must take into account when sponsoring a clinical trial that involves EU or UK residents.
As biotechnology and pharmaceutical companies seek innovative solutions to expedite their timelines, efficient management of a clinical trial starts with strategic planning. Submission of an Investigational New Drug (IND) application is a major milestone in new drug development. It marks the transition from bench research to clinical studies in human participants. In this article, PCI Pharma Services shares insights into the IND application process, the
JCS – Editorial Advisory Board
• Ashok K. Ghone, PhD, VP, Global Services MakroCare, USA
• Bakhyt Sarymsakova – Head of Department of International Cooperation, National Research Center of MCH, Astana, Kazakhstan
• Catherine Lund, Vice Chairman, OnQ Consulting
• Cellia K. Habita, President & CEO, Arianne Corporation
• Chris Tait, Life Science Account Manager, CHUBB Insurance Company of Europe
• Deborah A. Komlos, Principal Content Writer, Clarivate
• Elizabeth Moench, President and CEO of Bioclinica – Patient Recruitment & Retention
• Francis Crawley, Executive Director of the Good Clinical Practice Alliance – Europe (GCPA) and a World Health Organization (WHO) Expert in ethics
• Georg Mathis, Founder and Managing Director, Appletree AG
benefits of applying a Pre-IND strategy utilising a Canadian-based CDMO, and how this approach can deliver reduced First Patient-In (FPI) timelines by 6-8 weeks.
In this journal, we will also explore more about the need to obtain a diverse body of clinical trial participants has been stressed in public statements for decades, including formal promotion by the U.S. National Institutes of Health (NIH), regulatory authorities such as the U.S. Food and Drug Administration (FDA), and recently, with the Consolidated Appropriations Act of 2023, even the U.S. Federal Government. Despite the duration and high profile of this issue, however, parties executing clinical trials, including sponsors, clinical research organisations (CROs), and academic centers, have remained insufficiently effective in addressing it. Denise Calaprice, Ryan Whitty, Christina Moon and Neva Hidajat at WCG Avoca, outline the importance of diversity in clinical trials.
I would like to thank all our authors and contributors for making this issue an exciting one. We are working relentlessly to bring you the most exciting and relevant topics through our journals.
Beatriz Romao, Editorial Manager Journal for Clinical Studies

• Hermann Schulz, MD, Founder, PresseKontext
• Jeffrey W. Sherman, Chief Medical Officer and Senior Vice President, IDM Pharma.
• Jim James DeSantihas, Chief Executive Officer, PharmaVigilant
• Mark Goldberg, Chief Operating Officer, PAREXEL International Corporation
• Maha Al-Farhan, Chair of the GCC Chapter of the ACRP
• Rick Turner, Senior Scientific Director, Quintiles Cardiac Safety Services & Affiliate Clinical Associate Professor, University of Florida College of Pharmacy
• Robert Reekie, Snr. Executive Vice President Operations, Europe, AsiaPacific at PharmaNet Development Group
• Stanley Tam, General Manager, Eurofins MEDINET (Singapore, Shanghai)
• Stefan Astrom, Founder and CEO of Astrom Research International HB
• Steve Heath, Head of EMEA – Medidata Solutions, Inc
4 Journal for Clinical Studies Volume 15 Issue 1 Foreword
Ramus Corporate Group
is a union between Ramus Medical, Medical Diagnostic Laboratory Ramus and Medical Centre Ramus. All the companies are situated in the Ramus building in Sofia, Bulgaria. They are certified in compliance with the requirements of ISO 9001:2015.
Ramus Medical is full service CRO, working CTs in a variety of therapeutic areas and medical device.
• Medical writing for drugs and devices
• Scientific review of documentation
• Clinical trial management
• Monitoring
• Data management
• Regulatory advising and services during clinical trial

Medical Diagnostic Laboratory Ramus (SMDL-Ramus)
• 30 clinical laboratories in Bulgaria and North Macedonia
• 325 affiliates for sampling in Bulgaria and North Macedonia
• More than 20 years’ experience in the CT field as central and safety laboratory;
• Largest PCR laboratory in Bulgaria

• Laboratory System integrates cluster generation, sequencing, and data analysis
• Total laboratory automation with Abbott GLP-System
• Bioanalytical laboratory – ISO/IEC 17025:2017 accredited
Medical Centre Ramus with Phase I Unit
• PK/PD studies
• Medical devices investigations
• Phase I–IV
• Non-interventional studies
Others:
• Readability user testing
• Bridging report
• Carriage and storage of dangerous goods in compliance with ADR principles
Medical Diagnostic Laboratory Ramus Ltd 26 Kapitan Dimitar Spisarevski Street, 1592 Sofia, Bulgaria
Tel/Fax: +359 2 944 82 06 www.ramuslab.com email: info@ramuslab.com
Ramus Medical Ltd


26 Kapitan Dimitar Spisarevski Street, 1592 Sofia, Bulgaria
Tel./Fax: +359 2 841 23 69 www.ramusmedical.com email: office@ramusmedical.com
Mihaylov Marketing Director

Corporate Profile
Journal for Clinical Studies 5
S ct a e f r e r , o f c ast, to V i e C r o e Tut
Dimitar
!
www.journalforclinicalstudies.com
The Role of Confirmatory Trials After Accelerated Approval
Initially developed for the HIV/AIDS crisis in 1992, the US Food and Drug Administration (FDA) established the accelerated approval program to expedite the approval of drugs for serious or life-threatening conditions. The FDA grants these drugs accelerated approval based on a surrogate endpoint, which helps to speed up the drug development process. Since inauguration of the program, the FDA has typically given accelerated approval for anti-cancer therapies.
After receiving accelerated approval, drug companies are still required to conduct confirmatory trials to verify clinical benefit. If a confirmatory trial fails to do this, products may be withdrawn by the companies or by the FDA after a public hearing. In addition, the FDA notes that confirmatory trials must be completed with “due diligence.” They should be conducted by the time an accelerated approval application is submitted and if the product will be assessed based on the surrogate endpoint or intermediate clinical endpoint.1,2
Project Confirm is an initiative by the FDA Oncology Center of Excellence (OCE), designed to provide information and transparency on the accelerated approval process.3 It includes lists of accelerated approvals for drugs that have requirements for ongoing confirmatory trials, products that have been withdrawn, and agents that were verified through post-marketing trials and granted traditional approval. More information on accelerated approval and confirmatory trial requirements can also be found in the FDA guidance for industry, Expedited Programs for Serious Conditions –Drugs and Biologics.2
When Confirmatory Trials are Needed for Approval
In September 2022, the FDA’s Oncologic Drugs Advisory Committee (ODAC) met to discuss the efficacy and safety of a few products under or seeking accelerated approval, including 1) Pozenveo (poziotinib), from Spectrum Pharmaceuticals, Inc, for the treatment of patients with previously treated, locally advanced, or metastatic non–small cell lung cancer (NSCLC) harboring HER2 exon 20 insertion mutations; and 2) Pepaxto (melphalan flufenamide), from Oncopeptides AB, for use in combination with dexamethasone to treat adult patients with relapsed or refractory multiple myeloma who have received ≥4 prior lines of therapy and whose disease is refractory to ≥1 proteasome inhibitor, 1 immunomodulatory agent, and 1 cluster of differentiation 38–directed monoclonal antibody. The committee found concerns related to the confirmatory trials for these products.

Pozenveo was submitted for accelerated approval with data from phase 2 study, ZENITH20. The primary endpoint for the trial was the overall response rate, which the FDA found to be low compared to other available therapies for NSCLC. A high rate of adverse events and poor tolerability were also associated with the
proposed dose of Pozenveo. Due to these concerns, a confirmatory trial would be important for verifying clinical benefit. However, the sponsor planned to use a different dosing regimen for the phase 3 confirmatory trial, PINNACLE, than the dose used for ZENITH20. Another major issue was that the confirmatory trial started several months after the sponsor submitted the marketing application. Because results were not anticipated until at least 2026, patients could be exposed to toxicity risks for a prolonged amount of time, the FDA noted.
At the same ODAC meeting, the FDA shared concerns related to the phase 3 confirmatory trial, OCEAN, for Pepaxto. The agency initially granted Pepaxto accelerated approval in February 2021 based on the results from phase 2 study, HORIZON. After identifying issues with OCEAN, the FDA issued a safety alert for Pepaxto that cited an increased risk of death.4 This led to the sponsor’s request in October 2021 to withdraw its new drug application (NDA) for Pepaxto from the market. However, the sponsor rescinded the withdrawal request and presented additional post hoc analyses at the ODAC meeting. From its review of the new data, the FDA concluded that OCEAN failed to meet its primary endpoint, progression-free survival, and showed higher death rates after treatment with Pepaxto. In addition, the agency noted that subgroup analyses could not be used to confirm clinical benefit. Overall, OCEAN suggested that the benefitrisk profile for Pepaxto was unfavorable, and another clinical study would be needed to identify a safer dose of the drug.

The ODAC agreed with the FDA’s assessments for both confirmatory trials and did not support the approvals for Pozenveo or Pepaxto. A complete response letter was subsequently issued to Spectrum in November 2022, indicating that Pozenveo could not be approved at the time.5 The FDA also requested for the withdrawal of Pepaxto in December 2022.6
Addressing Concerns with Confirmatory Trials
FDA staff members from the Office of Oncologic Diseases at the Center for Drug Evaluation and Research (CDER) and the OCE, including the OCE’s director, Richard Pazdur, MD, commented on recent concerns related to accelerated approval and confirmatory trials in the New England Journal of Medicine 7 There are issues related to the lengthy process of completing confirmatory trials or removing drugs from the market when confirmatory trials fail to show clinical benefit. The authors noted that “a comprehensive strategy is needed” to “focus on the timely generation of evidence” because delayed withdrawals may put patients at risk.
The article includes a few alternative strategies to consider for accelerated approval in the future. They call for conducting confirmatory trials earlier and including randomised confirmatory trials instead of only single-arm studies. The authors suggested that “off-ramp” approaches for accelerated approval such as implementing time limits for confirmatory trials and alternative
6 Journal for Clinical Studies Volume 15 Issue 1
Pages
Watch
processes for withdrawals are important. However, the “onramp” procedures that focus on establishing clinical efficacy (e.g., conducting randomised clinical trials) are equally significant. Overall, these strategies may improve accelerated approval and help expedite access to therapeutics that are safe and effective, the authors concluded.
REFERENCES
1. Accelerated Approval. Food and Drug Administration webpage. https:// www.fda.gov/patients/fast-track-breakthrough-therapy-acceleratedapproval-priority-review/accelerated-approval
2. Guidance for Industry: Expedited Programs for Serious Conditions— Drugs and Biologics. Food and Drug Administration webpage. https:// www.fda.gov/regulatory-information/search-fda-guidance-documents/ expedited-programs-serious-conditions-drugs-and-biologics
3. Project Confirm. Food and Drug Administration webpage. https://www. fda.gov/about-fda/oncology-center-excellence/project-confirm
4. FDA alerts patients and health care professionals about clinical trial results showing an increased risk of death associated with Pepaxto (melphalan flufenamide). Food and Drug Administration webpage. https://www.fda.gov/drugs/drug-safety-and-availability/fda-alertspatients-and-health-care-professionals-about-clinical-trial-resultsshowing-increased
5. Spectrum Pharmaceuticals Receives Complete Response Letter from U.S. Food and Drug Administration for Poziotinib; Reaffirms Focus on
the Commercialization of ROLVEDON™ (eflapegrastim-xnst) injection. Spectrum. https://investor.sppirx.com/news-releases/news-releasedetails/spectrum-pharmaceuticals-receives-complete-response-letter-us
6. Oncopeptides provides update on Pepaxto US marketing authorization. Oncopeptides. https://www.oncopeptides.com/en/media/press-releases/ oncopeptides-provides-update-on-pepaxto-us-marketing-authorization
7. Fashoyin-Aje LA, Mehta GU, Beaver JA, Pazdur R. The On- and Off-Ramps of Oncology Accelerated Approval. N Engl J Med. 2022; 387(16):1439-1442. https://pubmed.ncbi.nlm.nih.gov/36129992/
Jennifer Nguyen, PhD, is a Senior Content Editor for the Cortellis suite of life science intelligence solutions at Clarivate. She previously worked as a medical writer, which involved writing and editing scientific journal articles and materials for science conferences. Her current role includes reporting on FDA advisory committee meetings, drug approvals, and workshops.
Email: jennifer.nguyen@clarivate.com

Journal for Clinical Studies 7 www.journalforclinicalstudies.com
Watch Pages
Jennifer Nguyen
Rethinking Statistical Significance in a Rare Disease Context
Highlights of a recent panel debate moderated by Christian K Schneider, head of Biopharma Excellence, reviewing emerging approaches to clinical data collection where patient populations are small.
Biopharma Excellence recently hosted a panel discussion featuring experts in biotech and the evolving regulatory environment to discuss the challenge of achieving statistical significance with novel and highly targeted therapies.
Daniel O’Connor of the UK’s MHRA noted that the challenge of achieving statistical significance when target populations are tiny is nothing new, but conceded that randomised trials aren’t always viable – especially with very rare conditions. He urged drug developers to seek scientific advice before finding workarounds.
When Trial Data is Lacking
The panel considered the potential for supplementary data sets – such as pharmacodynamic read-outs, histological evidence and historical controls – in enabling regulators to reach robust decisions.
Nick Sireau, of AKU Society, talked about his early experiences of trying to get new drugs approved for Alkaptonuria (AKU); his two sons both suffer from the ultra-rare disease. His organisation relies

heavily on grant funding and donations, with access to funds often difficult. It doesn’t help that the authorities’ requirements for clinical evidence have been quite restrictive up to now.
Fifteen years ago, 40 patients were recruited to an AKU drug trial that lasted three years and focused on a single endpoint (hip rotation), Nick explained. “The trouble is, that AKU affects patients very differently,” he noted. “So to just look at 40 patients with a single endpoint proved futile.” Although patients were reporting that they could walk further, that their pain had reduced, that they were feeling better since joining the trial, the study itself failed.
This led AKU Society to form a consortium of linked organisations across Europe, and a composite end point – a proposition which by then was well received in the EU. “That changed everything,” he said.
Regulatory Evolution
Daniel argued that the industry is now witnessing an evolution in regulatory thinking, resulting in a much more proportionate approach with regard to looking at the disease condition based on how much is already known: “not just thinking about either a randomised study or a single-arm study.”
Oncology’s use of basket studies has helped shine a light on what’s possible, he added. “But there’s also a lot of work now looking at shared molecular entities in rare diseases – something
8 Journal for Clinical Studies Volume 15 Issue 1 Watch Pages
the International Rare Diseases Research Consortium (IRDiRC) is moving on quite quickly now.” The toolbox to get to the data now needed is visibly expanding, he said.


Likely next steps will need to come from a collaborative discussion and feasibility conversation – with patients; with researchers; with the regulators – about what's viable with a clinical development program. “We need to think about timelines, too,” Daniel added. “Three years is a long time to run a study.”
Reimbursement: Aligning Endpoints
The discussion moved on to the topic of reimbursement, which in turn requires clarity about endpoints. Developers and regulators may be thinking about patient risk/benefit, while payers are more likely to be weighing up the cost/benefit.
The industry as a whole must think more broadly in terms of endpoints that really matter, the panel concluded – particularly when it comes to very rare diseases for which there have previously been no real treatment options; and include the patient voice as early as possible.
Nick said he sensed that while regulators now seemed to be accommodating the patient perspective more structurally, the big pharma companies tend to be less proactive about patient engagement. “Most drug companies will start working on something and then get in touch with us right at the last minute when things have started to go wrong,” he said. “There doesn't seem to be any systematic way that pharma companies engage with patient groups, particularly in the rare disease space.”
Involving patients at an earlier stage, finding out what’s most important to them and using this to direct research, is a growing area of focus among those thinking about all of this more strategically. For instance, factors such of quality of life can be hugely significant to patients.
Sought-after Stimuli
The panellists ended by offering their own personal wish-lists for change. Rachelle Jacques of Akari Therapeutics, felt that multistakeholder approaches to overcoming practical barriers at a macro level would be important to stimulate progress – rather than one company/ patient advocacy organisation/regulatory body at a time
– if the 95% of today’s remaining unmet needs are to be addressed within an acceptable timescale.
Nick said he hoped for a funding model geared to ultra-rare diseases, to fund studies that are otherwise are just not commercially viable. He pointed to the Chan Zuckerberg Initiative in the US as potential inspiration to other regions.
Christian K Schneider
Christian K Schneider, head of Biopharma Excellence and Chief Medical Officer for biopharma at PharmaLex, is a former regulator at the British MHRA and the Danish Medicines Agency.
Email: christian.schneider@pharmalex.com
Nick Sireau
Nick Sireau is CEO and Chair of Trustees for the AKU Society which is dedicated to improving the lives of Alkaptonuria (AKU) patients.


Rachelle Jacques
Rachelle Jacques is President and CEO of Akari Therapeutics, a late-stage biotechnology company developing advanced therapies for auto-inflammatory and orphan diseases.

Daniel O’Connor
Daniel O’Connor is Deputy Director with responsibility for the Innovation Accelerator and Regulatory Science at UK healthcare agency, the MHRA.
Journal for Clinical Studies 9 www.journalforclinicalstudies.com Watch Pages
Data Protection and Clinical Trial
The highly regulated nature of the pharmaceutical industry means that organisations within this sector are no strangers to the requirement to abide by the various strict rules and regulations throughout the lifecycle of drug discovery, testing and production. However, when thinking about privacy and data protection regulations, clinical trials are certainly where it is most pertinent. Due to the volume of sensitive personal data that needs to be processed to demonstrate the safety and efficacy of a drug, this creates the requirement for significant data protection considerations. For clinical trials involving individuals located within the EU or UK, the General Data Protection Regulation, widely considered to be the ‘gold standard’ for safeguarding individuals’ personal data, will be at the centre of such considerations. And, due to the GDPR’s applicability being determined by the location of the individuals whose personal data is being processed and not the location of the entities doing the processing, trial sponsors located anywhere in the world are subject to the requirements set out within it.
In this article we set out four of the most important data protection factors that life sciences organisations must take into account when sponsoring a clinical trial that involves EU or UK residents:
• Basic data protection requirements
• Data transfers
• Local jurisdictional requirements
• Appointing a DPO and DPR
It is worth noting at the outset that following Brexit, the UK has enacted the GDPR into its own domestic legislation – the UK GDPR. At present, the EU and UK GDPRs are in practice the same, so unless stated otherwise, both will be referred to as the GDPR.
Basic Requirements
Data Controller
In all but very rare cases, trial Sponsors are deemed to be the ‘Data Controller’ under the GDPR for the personal data collected as part of a clinical trial. A Data Controller is the entity that determines the ‘means and purposes for processing’ personal data. Given the Sponsor is generally the organisation that writes the protocol (the purpose) as well as contracts with the various organisations that will run the trial and collect the data (the means), and even though the Sponsor may only have access to coded (pseudonymised) personal data, they are still considered the Controller under the GDPR. This means that as the Controller, Sponsors must comply with the more onerous accountability responsibilities required by the GDPR. This includes being responsible for identifying the appropriate lawful basis for the processing, implementing appropriate agreements to legitimise cross boarder data transfers, informing individuals about the intended processing, dealing with individuals’ rights requests

and ensuring the organisations you appoint as your Data Processors comply with their own obligations and apply ‘appropriate technical and organisations measures’ to protect the data being processed.
Lawful Basis
The first principle of the GDPR states that personal data must be processed lawfully. Therefore a lawful basis must be identified for each processing activity. Article 6 of the GDPR sets out the six lawful bases that organisations can choose from. In the context of a clinical trial, most sponsors can rely on ‘Legal Obligation’ to process data for reporting and safety reasons. However, for the main research purpose of a study, the three most common lawful bases to rely on are:
• Public task
• Consent
• Legitimate interest
The one that is the most appropriate will depend upon the context. Where a trial is commissioned by a public/government body, Public Task may be appropriate. If Public Task does not apply, sponsors will have to choose between Consent and Legitimate Interest and, depending on where data subjects are located, it could be different. In the UK for example, the preferred lawful basis is Legitimate Interest. However, in Germany the regulator requires Consent. It is therefore essential to investigate the specific guidance and rules within each different jurisdiction where trial participants are located, remembering that even Member States within the EU may differ on this point.
It is also worth noting that in many instances, personal data used in one clinical trial may also be used subsequently to benefit future research. Where this is the case, you will not need to identify an additional lawful basis for this additional research provided that the purpose of the additional processing is compatible with the purpose for which the data was originally collected. Recital 50 of the UK and EU GDPRs indicate that “Further processing for... scientific or historical research purposes... should be considered to be compatible.”
Similar to a lawful basis, where health data is being processed, as is the case for most clinical trials, an additional condition for processing must be identified. This is because under the GDPR, information relating to things such as an individual’s health; genetics; and sex life are considered ‘special category data’ and thus are afforded extra protections under Article 9 GDPR.
Data Protection Impact Assessments
Another requirement that falls at the feet of sponsors is the need to conduct a Data Protection Impact Assessment (DPIA) to assess the risks of the personal data processing involved in a clinical trial and ensure that mitigations for identified risks are in place. This is an essential step in demonstrating a trial’s compliance with data protection laws, which is a key part of complying with the GDPR’s Accountability principle.
10 Journal for Clinical Studies Volume 15 Issue 1 Regulatory
International Data Transfers
As mentioned above, a sponsor may be subject to the GDPR even if it is not itself located within the EU or UK, but your trial participants that reside within these jurisdictions. Similarly, other vendors involved in the trial may also be located outside of the EU/ UK but still subject to the GDPR, such as your Contract (or Clinical) Research Organisation (CRO). Therefore, the personal data of EU/ UK individuals may be transferred to countries outside of this area (known as third countries). Where this is the case, the GDPR requires that an appropriate transfer mechanism be put in place to safeguard the personal data being transferred and ensure that it is protected in an ‘essentially equivalent’ manner to if it had remained within the EU/UK.
Currently, 14 countries (including Argentina, Canada, Israel, Japan and New Zealand etc. – but not including the US), have been deemed ‘adequate’ by the European Commission (and the ICO for the UK), meaning that the laws in these countries have been deemed to provide a level of protection of personal data that is ‘essentially equivalent’ to that provided by the GDPR. This means that data can flow freely between these countries without the need for further safeguards. The EU and UK have also granted each other Adequacy.
Given most countries have not been granted this status, organisations making personal data transfers to these countries (most notably, the US) must ensure that additional safeguards are in place. In most cases, in the EU context these safeguards come in the form of EU-specific Standard Contractual Clauses (EU SCCs) that need to be included within an agreement between the sharing organisations. The EU SCCs guarantee rights and protections for data subjects when their data is being transferred to a third country. If your organisation is exporting data from the UK, to a non-adequate country, you cannot rely on SCCs and should instead rely on either the UK’s Addendum to the EU SCCs or the UK-specific International Data Transfer Agreement (IDTA). In addition, organisations relying on EU SCCs (or the UK equivalent) to transfer personal data, must now complete a Transfer Impact Assessment prior to the sharing commencing. A TIA is used as a mechanism to assess the laws of the importing entity’s country and consider whether the risks posed by the non-adequate jurisdiction can be adequately mitigated.
Whilst the above is currently virtually the only way to transfer personal data to the US, there is potential for a new international agreement that could see transfers become easier. The US and the EU are at present in political talks to create a new Trans-Atlantic Privacy Framework, which would allow for the seamless flow of data between the EU and the US. In October 2022, President Biden signed an Executive Order to help with putting this agreement in motion. The UK, since it has left the EU, would need to create its own agreement with the US, but may well follow the EU’s lead in the near future.
Jurisdictional Requirements
If conducting a trial involving multiple investigator sites located in different countries, it is important to be aware that there will likely be varying data protection requirements (beyond which lawful basis to select) affecting each location, even if all locations are within EU Member States.
The Clinical Trials Regulation (CTR) that came into force in the EU in early 2022 did go some way to harmonising the rules in this area, and the new Clinical Trials Information System (CTIS) that is set to become mandatory for trial applications in the EU from January 2023 will go further to achieve this and making it easier to run trials
across multiple Member States. The CTR allows Sponsors to submit one application, via the CTIS portal, for approval to run the trial in multiple Member States. To gain approval, life sciences organisations will need to provide a statement on their compliance with the GDPR and any other local jurisdictional requirements. The Sponsor then declares this on the CTIS submission.
However, despite these recent changes, sponsors still need to be aware that jurisdiction-specific requirements are very much still relevant in some countries. For example, any trial involving French residents must submit an MR-001 declaration form, to demonstrate that the Sponsor has developed a robust data protection framework, has completed a DPIA, and that they have appointed an EU Data Protection Representative (DPR). The CNIL, the French Data Protection Authority, has also released specific guidelines on personal data processing in clinical trials, including a requirement that only anonymised or pseudonymised data can leave the EU, with the anonymisation/pseudonymisation occurring before export.
As a non-EU country, the UK is not party to the CTR or CTIS. The clinical trial regulator for the UK is the Medicines and Healthcare Products Regulatory Authority (MHRA). For the data protection requirements related to trials conducted within the UK, sponsors must look to the UK GDPR, the MHRA’s requirements and any additional rules and guidance from the Information Commissioner’s Office (ICO).
Data Protection Officers (DPO) and EU/UK Data Protection Representatives (DPR)
Life sciences organisation conducting trials in the EU or UK must appoint a DPO. A DPO’s role is to inform, advise and monitor compliance with data protection legislation. This involves assisting with the carrying out of DPIAs; undertaking Data Processor (vendor/ supplier/partner) due diligence and ensuring the required agreements are in place; creating policies and procedures to maintain compliance; responding to individuals’ rights requests, such as data subject access requests (DSARs) and requests for deletion, and maintaining other required documentation.
In addition to a DPO, an EU and/or UK Data Protection Representative (DPR) is required when a Sponsor does not have a presence with the EU/UK but processes the personal data of individuals located within these areas. The Representative must be established in one of the countries where the processing is taking place and act as a point of contact for the regulatory authorities and for the data subjects. If your trial is being conducted in multiple EU Member States, you will only need one DPR, ideally located in the country where the largest proportion of data subjects are located. But, if your trial takes places in the UK and EU, you will need two separate Representatives – as per Article 27 of UK and EU GDPR.
Contracts and Agreements
A final consideration to note is that as data controllers, clinical trial sponsors will need to ensure that they have implemented appropriate contracts and agreements with each third party involved in the processing of the trial’s personal data. These will include:
• Data processing agreements – Under Article 28 GDPR, this type of agreement is required between a Controller and Processor. These agreements set out the Processor’s obligations including the mandatory reporting of breaches to the Controller; retention of data upon termination of the contract; and any audit rights the Controller has over the Processor. These types of agreements would normally be implemented with your CRO and laboratories
Journal for Clinical Studies 11 www.journalforclinicalstudies.com Regulatory
• Joint controller agreements – If Sponsors intend to share the role of Controller with another entity (i.e. jointly determining the means and purpose of processing the trial data), a Data Sharing Agreement setting out the responsibilities of each party in relation to the personal data being processed must be in place. These types of agreements may be implemented with your investigator sites
Conclusion
Conducting a clinical trial within the EU and/or the UK brings with it a number of data protection considerations that must be accounted for. Appointing an experienced DPO will help to ease this burden by guiding the sponsor through the relevant obligations to ensure compliance. It is vital for trial sponsors to ensure that they fully understand their data protection obligations in the EU and UK because, ultimately, this could lead to trial delays or failure to gain approval.

Rob Masson
As founder and CEO of The DPO Centre, Rob is actively driving innovation, transformation and thought leadership in data protection and privacy. For over 30 years, Rob has been involved in delivering solutions to some of the world's largest and most respected organisations. Supported by the DPO Centre's large team of privacy professionals, Rob advises on evolving data protection legislation and how, when implemented well, compliance builds trust, confidence, loyalty and engagement. The DPO Centre assists a broad range of bioscience, genomics, therapeutics, healthcare and pharma companies globally to comply with EU data protection laws such as the GDPR.

12 Journal for Clinical Studies Volume 15 Issue 1 Regulatory
to the Japanese

Journal for Clinical Studies 13 www.journalforclinicalstudies.com
More information at cphi.com/japan CPHI Japan is the ideal business platform for international pharma professionals to join in order to grow business in the rapidly changing Japanese pharmaceutical market. 20,000+ Attendees 90+ Countries in attendance 420+ Exhibitors
Your gateway
market 19-21 April 2023 Tokyo, Japan
The Year Ahead for Life Sciences Regulatory Operations

Amplexor asked some of its closest partners for their views on the most important developments in Life Sciences regulatory operations in 2022, and for their predictions for 2023. Renato Rjavec of Amplexor Life Sciences shares their input.
Of the most prominent issues facing Regulatory functions, Steve Gens sensed renewed urgency around improving global ways of working, particularly around reducing the time for market approvals.
Gens & Associates, which has been tracking RIM-related improvement activity closely, has observed increased application of modern technology and digitisation focused on improved ways of working and process enhancement (change control, label management, regulatory intelligence) at an end-to-end level.
There is general agreement now that the shift away from inconsistent manual, document-oriented processes towards agreed use of standards-based data will be pivotal to transforming key processes. Remco Munnik noted that, up to now, Regulatory has always been the “spider in the web” within a pharmaceutical company – collecting all of the relevant information for submission of the registration: Clinical, CMC, administrative data, etc – then compiling a valid eCTD by the submission deadline. In future, all of
this will be data driven, he said – requiring a different approach to managing everything and ensuring its quality.
Kelly Hnat observed that the current advance toward structured data and new submission paradigms would have benefits for Regulatory, and across the enterprise, as the value of clear, accurate and complete data representations of registered medicinal products is tapped.
Resourcing to Meet Condensed Timeframes
The potential for transformed processes may be considerable, but the industry faces ongoing challenges in getting to where it would ideally like to be. Tris Nockles highlighted the challenges for organisations in assembling the right resources to meet increasingly condensed timeframes for submissions.
“Finding people with the right skills to work in Regulatory, which has been problematic historically, seems to have become a more difficult and lengthy pursuit in recent years,” she said, noting that it can take 6–12 months to onboard mid-level and senior staff these days. “On top of that, technology is under-delivering in many cases,” she added. “It’s not uncommon for companies to augment their RIM capabilities with more friendly technologies to capture and feed data in, or look to flexible resourcing to enter data on behalf of their Regulatory staff.”
14 Journal for Clinical Studies Volume 15 Issue 1 Regulatory
With the twin challenge of speed of delivery in a highly competitive job market, Tris said, companies will need to consider new and innovative ways of filling the staffing void, whether through flexible resourcing (outsourcing/offshoring) and technology and automation to enable routine operational work, or adjusted talent strategies that value technology-savvy newcomers in the workforce.
Evolving Organisational Structures
Other considerations as companies extend their ambitions are the need for more formal organisational change, as the growing flow of standardised data paves the way for new process fluidity across functional boundaries.
As Steve Gens put it, “Companies that are modernising RIM now are focused on evolving the regulatory operation organisation – driven by the data sciences and advanced technology including robotic process automation, structured content generation, and connectivity to other critical functions such as Quality, Manufacturing, Safety, and Clinical.

“We are in the early stages of the ‘data connectivity’ period, he said. This in turn requires a clear focus and excellence around crossfunctional data governance, he explained. It is where the promise of cross-functional business analytics, real-time information visualisation, and advanced technology will play out. “What we’re witnessing is the birth of the data science era,” he summarised.
Becoming Data-driven
With EMA’s direction toward agile development now, pharmaceutical companies too must become agile - in terms of adapting their own processes and systems, Remco added. “The biggest challenge ahead is for companies to become more data-driven organisations, and to adapt complex processes and transform the content from large volumes of legacy documents into more dynamic, reusable data,” he said.
Ultimately the challenge ahead is a business process one, though. Kelly urged change in the way Regulatory product teams work with technology to facilitate this.
To get to where they want to be, companies now need to develop a clear digitisation strategy in conjunction with other functions such as R&D and Manufacturing, Steve said. That’s in addition to improved information exchange at a local affiliate or “last mile” level, where further innovation is required. These two factors, if addressed appropriately, promise to greatly enhance both the efficiency and effectiveness of the Regulatory organisation.
Above all, companies need to reset their strategy now, adapting as they go, so that they can deliver more data-driven ways of working without the scale of the opportunity, and the associated challenge, becoming too daunting.
Business Processes Trump Technology
In 2023, companies need to become better at central, regional, and local affiliate collaboration and information management, Steve said. “Lost opportunities persist when it comes to improving how real-time regulatory information is available globally, to support day-to-day operational tasks across different markets,” he warned.


On top of that there is substantial untapped scope to strategically improve the ‘time to patient’ for new products – especially in the smaller markets around the globe. Improved regulatory intelligence sharing; global dossier management excellence; and the central
organisation working more seamlessly with the local affiliate will be critical success factors going forward.
Kelly highlighted that the key to success will ultimately always be manifested through business process and organisational culture: “Top-performing organisations excel at the process and organisational work, along with strong implementation execution. These strengths can overcome the weaknesses with almost any software platform... but the opposite is never true.” Clearly, organisations should be careful not to rely too heavily on technology and tools to solve their problems.
Steve Gens
Steve Gens, Managing Partner, Gens & Associates, a boutique Life Science benchmarking and advisory firm specialising in operational performance improvement, benchmarking, World Class RIMSM, and organisational transition for the regulatory domain – including closer integration with clinical, commercial, safety, quality, and health authorities.
Kelly Hnat
Kelly Hnat, founder and Principal of K2 Consulting (k2rim.com), a Regulatory Information Management and IDMP expert and active participant in the EMA SPOR Task Force.

Tris Nockles
Tris Nockles, Labeling Networks Lead at Navitas Life Sciences, a technology-backed global clinical research organisation.

Renato Rjavec
Renato Rjavec is Director of Product Management at Amplexor Life Sciences. Amplexor helps organisations that are developing pharmaceutical drugs, medical devices, and biotechnology to launch products and break into new markets quickly using innovative end-to-end regulatory and quality management solutions. Its solutions and services expedite the management of highlystructured data and the creation and delivery of consistent, compliant global content. Amplexor’s services include technology consultancy, implementation, and management services.
Web: www.amplexorlifesciences.com Email: renato.rjavec@amplexor.com
Journal for Clinical Studies 15 www.journalforclinicalstudies.com
Remco Munnik
Regulatory
Remco Munnik, Associate Director, Iperion –a Deloitte business, a subject matter expert in RIM, eCTD, xEVMPD and ISO IDMP.
Beyond modernisation– Unlocking the Value of Regulatory Information Management
Biopharmaceutical companies are now at an important inflexion point. Most have completed their modernisation of the Regulatory function globally, bringing improvements to efficiency, according to newly-published Gens & Associates research, the 2022 World Class RIM Survey. Founder Steve Gens reveals how respondents are planning to harness regulatory information management (RIM) as an enterprise asset.
It’s now 10 years since my company began tracking the ambitions of life science organisations around the world in relation to improving the management of regulatory information, through our World-Class RIM survey series. Our newly-published 2022 research confirms that, as we predicted, a majority of companies have now largely completed the foundational phase of their Regulatory function modernisation in which RIM becomes an important enterprise asset.
Those leading the way have also begun to deepen the strategic partnership with other functions such as clinical, quality, and manufacturing – to improve regulatory pathways/expedite new product approvals in all markets; to ensure compliance for existing products; and to harness regulatory information management (RIM) as an enterprise asset. They also believe that further digitisation is required to fully unlock regulatory potential and contribution.
Next-level Process Optimisation
As others catch up, and as the leading transformers look to the next level for process optimisation and more, a whole new chapter is going to unfold. But what is the opportunity that lies ahead, and what specific plans are emerging?
When we compare our very latest survey with previous ones, we see a number of clear trends. RIM-related investment is becoming more strategic and ‘outward looking’. As a critical mass of biopharmaceutical companies complete the groundwork of a process and system modernisation program that started in earnest in around 2013, they are shifting their focus toward organisational priorities, advanced technology, and cross-functional information sharing.
Although a strategic priority, most organisations have yet to fully realise global RIM adoption, with just 32% claiming this in 2022 and another 54% still working towards this goal. Even with an increase in end-to-end process work and affiliate access to most global systems, there is a gap in the ability to deliver full-scale process transformation.
For this to be possible, innovation is now needed at an affiliate level:
• in the way that local regulatory intelligence is shared globally and integrated to the global dossier process, to reduce time to submission and health authority approval;
• in resource allocation, via improved submission planning and forecasting, to better inform local teams to upcoming life cycle management submission (which typically accounts for 80% of portfolio work); and
• in improved control of the deviation process for label management, to ensure proper label management.
Since the majority of local affiliates are infrequent users of RIM systems and processes, innovation promises to simplify complex regulatory activities requiring global coordination.
Data Quality Underpins Real-time Insights
As working with real-time data becomes the default, and as more teams look to share and work from the same agreed data sets, the responsibility for the quality and integrity of the underlying data becomes everyone’s responsibility – and on an ongoing basis.
On the one hand, there will need to be a rebalancing of roles to emphasise data custodianship/governance and data science. But, equally, a data quality/data-first mentality needs to be fostered across and between teams, with senior leaders advocating for a culture of quality. This is to ensure that there are no weak links, and that pivotal data can be continuously relied upon as a source of product truth.
In all aspects of associated transformation, innovation must begin with the organisation supporting pragmatic experimentation; teams must also appreciate their responsibilities beyond their immediate function.
3D Technology Model Brings Advanced Capabilities
Technology alone cannot deliver the depth and scale of change and improvement now needed. Yet, without optimal application of transactional and advanced technology, transformation potential will be limited. Intelligent tools to manage, combine, interrogate, and share data cross-functionally will become ever more critical, for instance.
The vision is extending beyond basic systems and singleuse automation tools to encompass a more ambitious and complementary application of smart technology, spanning three dimensions:
• transactional (e.g. robotic process automation, natural language generation etc.);
• strategic (e.g. data lakes, AI etc.); and
• foundational digitisation capabilities (e.g. master data management).
Combining these three strands offers the potential for a complete set of advanced capabilities to manage and achieve more with regulatory information – as long as there is a clear and coordinated strategy and plan.
Key to delivering this broader and more ambitious plan will be the involvement of regulatory, R&D and enterprise organisation layers. Up to now, the biopharmaceutical industry and many technology providers have focused their attention for information/process digitalisation primarily on the transaction level to achieve quick wins, potentially sacrificing the long-term view and investment portfolio. The challenge and opportunity now is to fulfil all of the above through a single, coordinated roadmap.
16 Journal for Clinical Studies Volume 15 Issue 1 Regulatory
In our 2022 research, looking at the more advanced end of the scale, we see 39% of companies now working toward data hubs; 32% on collaborative submission platforms; 24% on AI/machine learning; 18% on knowledge management; 15% on natural language processing, and 8% on natural language generation. The vast majority are large multinationals and mid-tier companies who are actively investigating and conducting proof of concepts with these different types of technologies.
It is no coincidence that most companies (~75%) have settled on a single-platform strategy for their RIM transactional systems to underpin all of their diverse ambitions, rather than persisting with what could be 5–7 different best-of-breed applications. We see this in automated document quality checks and document creation giving way to intelligent search of past health authority responses.

Investments are shifting too toward intelligent label management; to automated extraction of product metadata into RIM systems (so companies can find it more easily and do more with it); and to smarter resource planning (through the analysis of pipelines, submissions planning, etc.)
Vision to Realise Value
For most companies there is still a way to go with all of this. While the top performers have largely realised significant speed, quality, real-time information access, reduced complexity, and process integration benefits, most organisations are still at the beginning of that process of extrapolating the fuller benefits of their evolving RIMbased digital process transformations. However, with a clear vision, many biopharmaceutical companies are set to realise the value from their investment in the shape of increased cross-functional insight and capability, and organisational agility.
No one can be sure what the future will hold, but we can expect a lot of refinement over the next five years to maximise regulatory value. Much of this will be complemented by a continued focus on and investment in data quality and related roles and responsibilities. Role-wise, leading companies are ahead with the planned appointment of strong data skills, as well as regulatory intelligence strategy and analyst roles.
Top-performing companies share some well-defined organisational and culture attributes. They tend to have a clear and well understood regulatory strategy and vision; a right-first-time data quality mindset, linked to rewards systems; a willingness to try new processes and technologies; and a strong willingness and ability to work across functional boundaries. The research is clear – armed with a shared vision, a focus on data quality and the flexibility to work in different ways, companies will be in a great position to maximise the value of their RIM investment.
Steve Gens is the managing partner of Gens & Associates, a global life sciences advisory and benchmarking firm specialising in strategic planning, RIM programme development, industry benchmarking, and organisational performance.
Email: sgens@gens-associates.com

Journal for Clinical Studies 17 www.journalforclinicalstudies.com Regulatory
Steve Gens
Next-level Clinical Trial Technology Can Avoid Dropouts and Accelerate Drug Development

Despite the recent shift to hybrid and decentralised clinical trials (DCT), low enrolment, poor retention, and outdated processes are still holding research back. But it’s not the technology that’s the problem – it is the way we are using it.

From ride hailing to mobile banking, technology is light years ahead of what it was just a few years ago. Yet many solutions in the clinical sphere have not kept pace with the level of choice, control, and user experience people have come to expect. It all adds up to a clear disparity between the experience in clinical trials and the daily experience of the average consumer.
Recruitment, Retention, Inclusion
Translating discoveries into products has remained a stubborn challenge, with clinical trials facing several well-documented bottlenecks.
Chief among them is recruitment, a process that can take up to 30% of development timelines, and commonly delays study start by between one and six months.1 That comes as no surprise when we consider that 11% of clinical research sites fail to enroll a single participant, and 37% under-enroll.1
Even when a study has enough participants to proceed, up to 40% go on to drop out before the completion date,2 draining study power and putting the entire project at risk.
There is also a very real need to widen inclusion and increase representation in clinical research. Black/African Americans, who make up more than 13% of the US population, account for just 7% of clinical trial participants, for example.3
Compounding all these well-established challenges is that the advent of precision medicine has shrunk the pool of eligible participants, sometimes to those with a single genetic mutation.
Technology-enabled Research
DCTs and hybrid trials, which allow sponsors to cast a wider geographical net and reduce burden on participants, have emerged as a solution to many research challenges.
Key to unlocking the benefits of DCTs is making it as easy as possible for people to sign up to, and stick with, a clinical trial. After all, improving recruitment is worthless if sponsors are unable to ensure trial cohorts are more representative at the outset, or if swathes of data are lost to subsequent dropouts.
We can take our cue here from the consumer sector, which would never dream of asking users to jump through the hoops some clinical trial technology platforms put up.
Currently, for example, trial participants have to interact with a multitude of solutions or platforms, each with a different log-in system and interfaces. It is burdensome and far from convenient.
Many payment and reimbursement systems charge fees, provide a limited choice of payment methods, and block participants from raising their own payment requests. In a world where people are used to instant, easy to use mobile banking, such restrictions create barriers to trial registration and continuation.

Even within the DCT model, people will usually need to visit a site at some point. Yet many sponsors ask their participants to pay the fare and claim the money back, without considering whether the cost could be prohibitive. In addition, they may place the onus for organising travel on their already busy research sites.
It is also worth considering that DCTs have the potential to erode the participant/ site relationship. If effective communication is not enabled, patients may feel isolated, and sites can miss drop-out warning signals.

User Experience
We therefore need to make it as easy as possible for people to take part in clinical trials. That means providing solutions that give
18 Journal for Clinical Studies Volume 15 Issue 1 Research & Development
people the same level of control and user experience they have come to expect in their everyday lives.
Combining services into a single platform, for example, provides ease of use and removes the “clunkiness” that can cause people to disengage.
Payment systems should offer the same level of security, control, and oversight that people expect from their bank. They should be able to choose how they get paid, whether that’s by PayPal, bank transfer, or even check, raise their own payment requests, and easily track incoming funds. Crucially, they should never incur ATM fees, monthly inactivity fees, or any other extraneous charges.
When people face difficulties in getting to sites, it results in site productivity-draining lateness, or, even worse, no shows and missing data. One solution is enabling centrally charged travel within the patient-facing platform. At Mural, for example, we have partnered with HIPPA-compliant Uber Health. It means that rather than relying on sites to make travel arrangements, participants simply hail a ride to their appointment without incurring any out of pocket costs.


Underpinning all of this is communication. Solutions that provide two-way messaging, in a format people are used to using on their smart phones every day, allow participants to raise queries and concerns. They allow sites to offer reassurance, as well as send appointment reminders and payment notifications.
Integrated systems that focus on meeting patient need can take this one step further. Regular satisfaction surveys, for example, allow sites to spot and respond to the falling engagment that can result in dropouts. They also provide sponsors with the insights they need for the continual improvement of patient-centered processes.
Next Generation trial Technology
Wrap around, consumer-quality patient support solutions are the next logical step in clinical trial evolution.
Combining advanced payment products, a centrally charged travel concierge service, two-way site/patient communication channels, and participant insight collection tools makes it easy for people to take part in research. It allows sponsors to fully embrace the potential of DCTs – and set clinical advancement free.
REFERENCES
1. Chaudhari, N., Ravi, R., at el. (2020). Recruitment and retention of the participants in clinical trials: challenges and solutions. Perspectives in clinical research, 11(2), 64.
2. 5 reasons clinical trial drop-out rates are on the rise. (2021). Available at: https://www.pmlive.com/pmhub/clinical_research/couch_integrated_ marketing/white_papers_and_resources/5_reasons_clinical_trial_ drop-out_rates_are_on_the_rise Last accessed: 30 November 2021.

3. Clinical trial diversity and representation. (n.d.). Available at: https:// www.amgen.com/science/clinical-trials/clinical-trial-diversity Last accessed: 30 November 2022.
Jason Dong
Jason co-founded Mural Health with the goal of using technology to elevate patient experience in clinical trials. When Jason was an investor in pharma technology businesses at Advent International, he had an off-the-cuff conversation with co-founder Sam Whitaker about what it would look like to modernise the patient experience. That conversation snowballed, and over the course of the next year, Mural Health was founded. Jason is excited to bring his experience and insights in building a world class organisation at Mural Health. Jason grew up in New Zealand and came to the US to attend Harvard. Previously, he was a management consultant at McKinsey & Co.
Sam Whitaker
Mural Health represents a natural evolution to Sam’s past work; the Mural Link product leverages a next-generation participant payment technology to drive deeper benefits to the participants and more meaningful value to both sites and sponsors executing the study. Prior to co-founding Mural Health, Sam founded Greenphire in 2008, where he was responsible for inventing the first payment technologies that were vertically integrated into the clinical trials industry. Sam is from suburban Philadelphia (Delco!) and is a graduate of the University of Pennsylvania.
Journal for Clinical Studies 19 www.journalforclinicalstudies.com Research & Development
Digital Clinical Trials: Time to Throw Out the Old Rule Book

Unless we create a new rule book with user experience at the forefront, operational excellence will remain a distant dream.
Traditional industries are regularly disrupted by new technologies, and the life sciences industry is no exception. Electronic data capture (EDC) arrived on the scene decades ago. It has now become the de facto tool for data management and collection, and over time it has bought with it several important benefits. Where previously it would take 8 to 16 weeks for data recorded during a patient’s visit to appear in the data management workstream, EDC has reduced that lag time from weeks to days, even hours in some cases.
However, these changes did bring trade-offs with them. Traditional EDC, for example, transferred the responsibility of data entry from sponsors to sites. This fragmented the data model whilst ignoring the underlying challenges around data collection. The pandemic saw another crucial change, with the industry introducing new processes and technology that allowed organisations to oversee and execute trials remotely. Patients now had new methods of participating in studies, however, it meant site users were left juggling trial data originating from multiple sources.
These trade-offs that prioritise the needs of one set of users (e.g.sites, patients, regulators, etc.) over equally important stakeholder groups can have a detrimental effect. Ultimately, there needs to be a realignment between operational efficiency and scientific rigour. Rather than having to choose between trial execution and scientific excellence, we instead need a fresh approach to these challenges. If we’re ever to deliver on the potential of digital clinical trials, we need to place the user experience at the centre of our efforts.
Refocusing on Speed and Simplicity
At a recent Veeva event for more than 500 European R&D and quality leaders, the discussion topics were surprisingly consistent. The industry is increasingly frustrated with the silo-driven approach suppressing operational efficiency. Obstacles include fragmented data, inefficient processes, and the heavy demands of manual tasks, not to mention the difficulty of breaking long-established ways of working.
It’s easy to see how disjointed technology and processes could hinder progress in areas that should be advancing faster. In data management, for example, the complexity of today’s clinical trials means it is far more challenging to be efficient. Trials that were linear at the start of my career are now four-dimensional. We’ve morphed into an era of platform, bucket, and umbrella trials that require amendments after almost every visit. Tanya du Plessis, chief data and solutions officer at CRO Bioforum, summarizes the challenge: “We’ve gone from linear to almost circular [in data management]. It’s hard to determine where one step starts and the other ends, and that’s not even accounting for the volume or veracity of data.”
It’s fairly common now to see studies with 15 or more data sources or types. Yet, as an industry, we are not at the point where our systems and processes can easily manage non-conventional data
at scale. The outcome is patchwork solutions that require complex data integrations. Instead of more automation, we rely heavily on manual solutions (such as emails and spreadsheets) to track data queries and cleanliness. This makes the data manager’s job harder and does nothing to boost productivity.
Even more troubling, a siloed approach to complex trials raises the risk of poor decision-making. Companies have introduced technology to collect data from sources that did not exist (nor were accepted) by regulators until a few years ago. Millions of new data points have had a huge impact, slowing data cleaning and reviews. It has become critical to gain central oversight of these tasks that are now undertaken in a decentralized manner.
How can we create a more connected approach? First, we need to focus more on the total experience in clinical trials across stakeholders. I believe today’s clinical trials are like a complex Rubik’s Cube, with each side representing an important user group. Each time you try to solve a problem for one set of users, you need to specifically review the experience of other users to determine the overall impact. Just as you can’t solve a Rubik’s Cube with one move, the challenges we face in clinical trials are too big to address with one phase of digital transformation. Instead, we need to take incremental steps and focus our innovation efforts on eliminating daily timeconsuming tasks.
For instance, data managers need better tools to aggregate, clean, and provide data with less manual effort. We can automate activities like data cleaning, medical coding, safety signals, and predictive analyses, which are all too often still on paper or in spreadsheets. Technology can also eliminate manual processes to simplify the data manager’s job, like end-of-study data or serious advance-event reconciliation.
Having a holistic view of clean and concurrent data is important. Not only does our database need to scale to match today’s complex clinical trials, but we also need the capability to harmonize data from multiple sources without custom coding. The data model we require needs to be simultaneously flexible to adjust to future trial requirements and robust enough to handle today’s complexity.
Simplifying the User Experience
There are more tools available than ever before and greater opportunities to run studies more efficiently. However, by introducing an avalanche of new systems, we have significantly increased the burden on users with seemingly few efficiency gains.
If we are to make a real difference in productivity, the priority should be to work with sites to understand their frustrations and burdens. On the patient side, simplifying the number of tools patients use could improve participation and choice. Then, we can move on to offering them a consumer-grade experience that meets specific
20 Journal for Clinical Studies Volume 15 Issue 1 Research & Development
needs, like easy methods of reminder management or dispensing and travel support.
The life sciences industry must recapture the essence of its goal, which is ensuring that patients can participate in the right clinical studies – to do this, they need to be enrolled as quickly and as seamlessly as possible. To deliver medicines and therapies to the broader patient population in a timely manner, we must take responsibility for speeding this process up. It’s time to embrace different ways of working, and through a mix of pragmatism, optimism, and enthusiasm, we can draft a new rule book that brings truly effective change.
Research & Development

Richard Young is Vice President, Vault CDMS, at Veeva Systems. With over 25 years of experience in life sciences, Richard is known for his executive vision and proven operational experience in data management, eClinical solutions, and advanced clinical strategies. His role at Veeva is helping to transform the way we approach clinical data, unifying every contributor and consumer in a single platform.

Journal for Clinical Studies 21 www.journalforclinicalstudies.com
Richard Young
Developing a Pre-IND Packing Strategy to Expedite Clinical Trial Timelines
As biotechnology and pharmaceutical companies seek innovative solutions to expedite their timelines, efficient management of a clinical trial starts with strategic planning. Submission of an Investigational New Drug (IND) application is a major milestone in new drug development. It marks the transition from bench research to clinical studies in human participants. While awaiting the FDA Safe to Proceed letter for their IND, companies are able to take advantage of a Pre-IND labelling and packaging strategy for their clinical study by working with a CDMO operating out of Canada, such as PCI Pharma Services. In this article, we share insights into the IND application process, the benefits of applying a Pre-IND strategy utilising a Canadian-based CDMO, and how this approach can deliver reduced First Patient-In (FPI) timelines by 6–8 weeks.
Understanding the IND application process

Successful IND submissions require careful planning and compliance with FDA regulatory requirements and although not required, a Pre-IND meeting with the FDA is valuable. It is the company’s first opportunity to ask specific questions of an FDA review panel pertaining to their specific product, reducing the risk of a clinical hold and delaying time of product delivery to clinic. Pre-IND meetings provide early FDA feedback on regulatory and scientific issues relating to clinical trial design, non-clinical research, and product related CMC questions. The specific feedback will assist in future drug development plans and preparation for submitting a complete IND application.
For companies entering the Pre-IND phase for the first time, seeking support from an experienced CDMO with a specialist regulatory consulting team can prove to be beneficial. Not only can the CDMO team represent the sponsor company at FDA meetings, but they can also provide valuable insight and assist in preparing the meeting package to ensure it contains appropriate information, such as clear focused questions for the review panel to answer, product overview information, design of preclinical and/or initial IND studies, and appropriate clinical / manufacturing and quality control data.
In terms of the process, following IND submission the FDA will review the application within 30 days for safety and assess whether the human subjects are incurring an unreasonable risk. At the end of the review cycle, the agency will either issue a Safe to Proceed letter or inform the sponsor that the study is on clinical hold. If placed on hold, the FDA will provide an explanation within 30 days and the sponsor will need to address the noted deficiencies. Submitted responses will then be reviewed and the FDA will determine whether the hold can be lifted. If the sponsor does not hear from the FDA within 30 calendar days after the initial submission, the IND goes into effect or becomes active on day 31. At that point, the study may proceed as submitted once it has been approved by an Institutional
Review Board (IRB) whose purpose is to ensure the protection of the rights and welfare of the clinical trial participants.
During the 30 day review period, it is important to note that the FDA does not allow packaging or labelling of clinical trial material at any US facility. Even if a sponsor has filed an IND and received an IND number, they still must wait until day 31, when the IND application is in effect before they can package their clinical material within the US. In some circumstances, sponsors can import drug product into the US under “quarantine.” However, shipping under quarantine does not give the sponsor approval to proceed with clinical packaging and the IND number must be available for import documentation.
Recognising the Benefits of Pre-IND Labelling and Packaging in Canada

Many companies are unaware that a strategy exists allowing upstream labelling and /packaging of clinical material prior to obtaining their IND Safe to Proceed notification from the FDA. The 30 day review period does not necessarily have to be a period of waiting.
While sponsors are awaiting authorisation from the FDA to conduct a human clinical trial in the US, Pre-IND labelling and packaging is possible in a GMP facility operating in Canada. The Canadian site can label and package the Investigational Medicinal Product (IMP) and have finished goods ready to ship to US investigator sites immediately upon receiving the Safe to Proceed notification. This strategy expedites upstream processing and can accelerate timelines for companies with critical FPI milestones.
If a company has not yet filed an IND application and does not have an IND number, it is still possible to send the clinical drug product into Canada for labelling and packaging. Canadian import laws do not require an IND approval nor IND number in the product import documentation.
Importing, Labelling, and Packaging Clinical Trial Material in Canada
The process for importing, labelling, and packaging is relatively straightforward, and an experienced clinical trial service partner can help guide sponsors on the process requirements as well as support the completion of the necessary Health Canada documents.
Once the drug product is manufactured, an Annex A document is filed with Health Canada to notify of the drug product import. According to the Conditions for Provision of Packaging/Labelling Services for Drugs under Foreign Ownership (GUIDE-0067), Part 1 of Annex A must be filed with Health Canada 10 days prior to the arrival of the drug product and accompanying shipment into Canada.
The sponsor then arranges for the physical transportation of the clinical material from the manufacturer to the clinical trial service
22 Journal for Clinical Studies Volume 15 Issue 1 PCI Pre-IND Application Note
facility in Canada for labelling and packaging under GMP conditions. The Canadian-based clinical trial service partner will then hold the finished goods until exportation into the US can occur.
On day 31 following the submission of the IND application, the sponsor can consider the IND approval granted by the FDA. The Canadian clinical trial service partner will complete Part 2 of the Annex A document and file with Health Canada 10 days prior to exportation of the clinical finished goods into the US.
If the FDA does not allow the IND to proceed, the product cannot enter the US. However, any risks associated with labelling and packaging prior to receiving the Safe to Proceed notification are minimal because sponsors are simply reporting the data that they have already collected on the molecule. If the sponsor has solid safety data and promising outcomes from their test data, they will be well advised as to whether their IND will be allowed to proceed.
Understanding Regulatory Requirements for Importing Material into Canada
Canadian regulatory requirements vary based on the status of the IND approval and plans to distribute the product within Canada. The following are required:
• A bill of lading
• Packing slip
• Canadian customs invoices
• An active IND number (if available)
• An end-use letter
• Part 1 of Annex A filed with Health Canada 10 days prior to the arrival of the drug product and must accompany the shipment into Canada (If an approved IND is not in place)
If any of the material is intended to be used in Canada or distributed to Canadian clinical investigator sites, then a No Objection Letter (NOL) is required, which is the Canadian equivalent of the FDA IND Study May Proceed letter. Health Canada provides approval for products used in human studies via a Clinical Trial Application (CTA) and provides a formal response to the CTA via an NOL.
Supporting your Pre-IND strategy with PCI Pharma Services Our clients come to us seeking solutions for their often complex and unique products and for our renowned reputation in offering unmatched flexibility, a client-centric experience and consultative approach, delivering a seamless clinical service including pharmaceutical development, clinical manufacturing, labelling, packaging, storage and distribution.
As the only Canadian clinical trial services company with the experience and expertise to deliver integrated end-to-end clinical solutions spanning packaging, labelling, kit design, storage (including cold chain), global distribution, returns and destruction, we can ensure that your life changing clinical medicines reach patients when needed upon receiving the Safe to Proceed designation.
Expediting First Patient-In timelines our speedsolutionsTM combine value-added services and expertise, delivering an integrated approach for every clinical project, aimed at accelerating drug products through the earliest stages of development towards commercialisation.
Complementing our global services across development and manufacturing and clinical trial services, our expert Pre-IND support services to accelerate your project phase include:
• Bespoke Quality and Regulatory Services from support writing IND applications to representation and consulting with regulatory authorities such as the FDA and Health Canada

• Pre-IND clinical solutions facilitating the packaging and labelling of clinical prior to IND approval
• Industry leading expertise in Clinical Supply Project Management with PCI Clinical SMARTTM
• Faster First-In-Human manufacturing technologies including Xcelodose® microdosing and sterile robotic technologies
Clients can rely on our Pre-IND speedsolutionsTM to accelerate timelines to meet First Patient-In dosing milestones, providing financial benefits. With access to a global network of manufacturing and packaging facilities with scalable sterile and non-sterile solutions, sponsors can de-risk their supply chain by eliminating the need to transfer to alternative suppliers, regardless of where they are in their drug product lifecycle journey.
As a fully integrated global CDMO, PCI truly spans the drug product lifecycle, connecting development and commercialisation, de-risking supply chains and delivering true speed to market on behalf of our clients. We are a strategic partner of choice and an integral part of the supply chain as the bridge between life-changing therapies and patients.
Sharlett Burgess

Sharlett Burgess is the Quality Director for the PCI Pharma Services sites in Canada. She has responsibility for the clinical site in Burlington, Ontario as well as the Commercial site in Mississauga, Ontario. After completing her studies in chemistry and microbiology she began her career as an analytical chemist working with Eli Lilly for 5 years. She went on to work with AstraZeneca for 15 years in various quality roles in their commercial and clinical business. Sharlett has over 25 years of pharmaceutical experience and has successfully led the Clinical and Commercial sites over the past 9 years at PCI (formerly Bellwyck Pharma Services). She strives for excellence in Quality and Compliance, ensuring that the sites have robust quality management systems that meet client and regulatory requirements.
Christopher Hamlin
Christopher Hamlin MSc is Senior Manager of Regulatory Affairs – CMC at PCI Pharma Services, where he leads a Regulatory Affairs team supporting PCI’s manufacturing sites across the United States and in Europe. Prior to joining PCI, he was an employee at Division of AIDS at the National Institute of Allergy and Infectious Disease with the U.S. National Institute of Health (DAIDS/NIAID/NIH) where he contributed to the development and translation of HIV vaccine candidates into clinical trials. He was with the Walter Reed Army Institute of Research (WRAIR) and participated in the U.S. Military’s HIV Research Program in the Laboratory of Antigen and Adjuvant Research where his protein characterization work contributed to multiple scientific publications. He holds a Master of Science degree in Biotechnology from the Catholic University of America in Washington, DC and Bachelor of Science in Biochemistry Marietta College in Marietta, OH.
Journal for Clinical Studies 23 www.journalforclinicalstudies.com Application Note PCI Pre-IND
CRM Study Coordinators –Making a Difference in Clinical Trial Conduct in Malaysia

CRM Initiative – Boosting Excellent Study Coordinator Service
The clinical trial industry is developing rapidly in Malaysia. Since 2012 there have been more than 2000 clinical trials across different Therapeutic Areas, hence promoting Malaysia as a preferred country for sponsored clinical trials. The commitment to deliver clinical trials with Speed, Reliability and Quality is the essence to this success. This achievement is critically delivered by the invisible hands of Study Coordinators (SC).
Although the Principal Investigators (PI) are responsible for the clinical trial conduct, very often the success of these studies relies on the study team which consists of SCs. SCs are specialised research professionals working with the PI to support, facilitate, and coordinate daily clinical trial activities and play a critical role in the conduct of the study. It is essential that SCs and PIs work hand in hand to ensure study conduct is in accordance with the protocol and principles of Good Clinical Practice (GCP).
CRM SCs support investigators at site to quicken the start-up process (from submission of Ethics Committee approval to site initiation) by assisting to collect and compile relevant documents within 14 days of request. The SCs are also trained to adhere to study timeline including PD reporting, SAE reporting, data entry etc. to ensure the study processes are carried out in a timely manner.
Growth of CRM Study Coordinators
A study published by Papke A. et. al shows that adding a coordinator to a research team significantly improves subject recruitment numbers, enhances subject retention, and increases general study efficiency.1
In 2012, CRM had 22 SCs placed throughout Malaysia at various sites conducting industry-sponsored research; this number has grown to more than 160 SCs in 2022, placed mainly at Ministry of Health (MOH) hospitals, few MOH clinics and private hospitals. CRM has received increasing interest to support investigators and sites in Ministry of Higher Education (MOHE) hospitals and private hospitals over the years. The growth of CRM SCs over the span of 7 years is as illustrated in Graph 1.
In ensuring high quality SC service is provided to stakeholders, CRM conducts regular trainings and provides opportunities to further develop the SCs, continually evaluating the quality of service through Performance Management System (PMS) and treat customer’s feedback as gold nuggets to be more effective in answering their needs.
a) Training for CRM SCs
The pharma industry does recognize that the performance quality of sites varies dramatically. Jim Kremidas, executive director of Association of Clinical Research Professionals (ACRP) believes the root cause of this variance is the lack of consistency in how staff, including principal investigators (PIs) and SCs at sites are screened, hired, trained, and validated for their competency.2
With ISO 9001:2015, CRM has strengthened and standardized our services with proper Standard Operating Procedure (SOP) ensuring the right candidate is hired, trained and the delivery of the studies is similar across the country. Having an efficient SOP in place minimizes errors, clears the way forward by avoiding uncertainties, and serves as a vital tool to transfer knowledge and skill.3
It is recommended GCP certified personnel to refresh their GCP every three years to stay updated with current regulations, standards, and guidelines.4 CRM mandates that all SCs must be equipped with the adequate knowledge and GCP certification to perform their duties. Internal Post GCP Assessment Test (PGAT) is also incorporated as part of quality metrics to ensure SCs are tested on GCP knowledge every three years post GCP certification.
CRM has developed structural training programs to support the onboarding process for SCs especially those with no prior experience in clinical research. Training for SCs continues even after the probation period. This includes the yearly Training to Improve Performance of Study Coordinators (TIPS) introduced in 2019. The training modules are designed to meet expectation of the industry with support from Sponsors/CROs to deliver the trainings to SCs. There are also regional Continuous Medical Education (CME) conducted monthly for SCs to share their knowledge and best practices.

The continuous training also includes our in-house newsletter called “Q-Bites”. It contains practical knowledge focussing on GCP, local Ethics Committee guidelines, regulatory guidelines in conducting high quality study which can be applied to SC’s tumultuous daily life at work.
b) Adequate supervision by Line Manager
To ensure we maintain our service quality, the line managers conduct supervisory visits on quarterly basis to provide guidance
24 Journal for Clinical Studies Volume 15 Issue 1
Market Report
94 113 128 130 132 150 168 0 20 40 60 80 100 120 140 160 180 2016 2017 2018 2019 2020 2021 2022 (YTD) GROWTH OF CRM SCS OVER THE LAST 7 YEARS # CRM SCs Graph 1: Growth of CRM SCs over the last 7 years Graph 1: Growth of CRM SCs over the last 7 years
and support to the SCs throughout the year. The work performance of the SCs is appraised by the respective line managers at least every 6 months through the structural mid-year performance and yearend performance review system.
c) Opportunities for career development
CRM creates opportunities for the SCs to grow their careers in a transparent, competence-based systems as depicted in Diagram 2. These career tracks allow CRM to increase job satisfaction of the SCs as each employee can grow their individual passion and career.
Customer Satisfaction Survey on SC Services
In 2021, CRM conducted Customer Satisfaction Survey (CSS) through a third-party company, Vase.ai. The online survey was conducted between Dec 2021 and Jan 2022. The nationwide survey covered four services provided by CRM including the Study Coordinator service. 234 respondents from stakeholders such as Investigators, Sponsors and CRO have taken part in the survey and the detailed breakdown is as shown in Figure 1.
211 out of 234 respondents have experience in engaging with CRM SCs for their clinical trials. The respondents rated CRM SCs performance at site on three areas:
1. Knowledge and compliance of study protocol and GCP.
2. SC’s competency in performing delegated tasks and helpfulness in subject recruitment activities.
3. Speed/responsiveness to perform delegated tasks and maintenance of Investigator Site File (ISF) within the timelines.

SC understands study requirements per protocol and complying

SC understands GCP and complying
study tasks based on requirements and what have been delegated
speed/responsiveness on delegated tasks within specific timelines
updates and maintains the Investigator Site File (ISF) in a timely manner
Journal for Clinical Studies 25 www.journalforclinicalstudies.com Market Report Development of our Talent Pool – grow from within SC Level 1 Associate Regional Manager (ARM) Senior SC SC Level 2 Clinical Operations Manager (COM) Senior COM Head of Clinical Operations Clinical operation Executive Recruitment Specialist Project Manager Diagram 2: Career Advancement Ladder for SCs within Clinical Operations Department N = 234. Base = All Respondents 3 Figure 1: Respondents for Customer Satisfaction Survey 2021 4% 12% 15% 70% 0% 10% 20% 30% 40% 50% 60% 70% 80% Others, please specify Sponsor CRO Investigator % of Respondents N = 211. Base = Respondents who selected Yes in "Do you have experience in engaging /working with CRM SC(s) in your clinical trials?" Figure 2: Ratings on CRM SC services 1%4% 1% 2% 1% 1% 11% 9% 15% 14% 10% 8% 13% 33% 35% 32% 34% 32% 30% 35% 55% 47% 50% 46% 55% 60% 51% 1% 4% 2% 4% 1% 1% 1% 0% 20% 40% 60% 80% 100%
SC
SC’s
SC
Overall, how do you find CRM’s Study Coordinator (SC) performance at site?
SC's
helpfulness in subject recruitment activities
performs
Very poor Poor Fair Good Very good N/A
Diagram 2: Career Advancement Ladder for SCs within Clinical Operations Department
Figure 1: Respondents for Customer Satisfaction Survey 2021
Figure 2: Respondents Ratings
Figure 2 shows the detailed ratings provided by respondents.
Through the initiatives conducted by CRM, the customers gave 89% satisfactory rate (rated ‘Good and above’) towards CRM SC services. Feedback was received that the top three areas for improvement are related to workload management, retention of SCs and training and development. CRM values the feedback from respondents and has taken action to mitigate the issues. One of the new strategies introduced in 2022 is the hiring of additional SCs that are mobile and support site with high activities. New trainings including effective communication was carried out to improve the communication skills of the SCs. In addition to this, CRM credits the outstanding performance of the company to its people. The people are the greatest asset to the organisation and CRM has implemented new schemes in 2022 to improve the employee’s status and benefit in the company.
Conclusion
The SCs are the invisible hands in clinical trials who contributes to the success of each trial and provides supports to the Investigators. Finding qualified candidates for SC position can be quite challenging5. However, with proper skills and knowledge equipped
Intan Munirah bt Mohd Murad
Intan Munirah is the Senior Clinical Operations Manager in Clinical Research Malaysia (CRM), actively supporting process improvement initiatives in the company. She obtained her Bachelor of Biomedical Science from National University Malaysia and has been in clinical research field for ten years.


Email: intan@clinicalresearch.my
Nor Hafiza bt Johari
Nor Hafiza Johari graduated from Universiti Putra Malaysia with Bachelor of Science (Hons) Biology. She started her career as a full-time study coordinator, managing trials from few therapeutic areas. Currently she is the Clinical Operations Manager working with Clinical Research Malaysia (CRM). For the past 10 years, she is engaging with different sponsors, CROs, investigators, and trial sites to assist on the trial conduct and later demonstrated her capabilities through people management.
Email: norhafiza@clinicalresearch.my
to each SC by CRM, they can carry out the clinical trials not only at a high quality, but also maintain high integrity throughout the trial conduct.

REFERENCES
1. Papke A. The ACRP National Job Analysis of the Clinical Research Coordinator. The Monitor. 1996 Winter;45–53. at 46.
2. The Changing Landscape for Clinical Trial Sites, 01Sep2019, Cindy H. Dubin https://www.appliedclinicaltrialsonline.com/view/changinglandscape-clinical-trial-sites
3. Amare G. Reviewing the values of a standard operating procedure. Ethiop J Health Sci. 2012;22(3):205-208. (https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC3511899/)
4. National Institutes of Health, n.d. Good Clinical Practice Training. Retrieved 05Nov2022 from: https://grants.nih.gov/policy/clinical-trials/ good-clinical-training.htm
5. Barriers to Patient Enrolment in Therapeutic Clinical Trials for Cancer; A Landscape Report (https://www.fightcancer.org/sites/default/files/ National%20Documents/Clinical-Trials-Landscape-Report.pdf)
Venoo Kuppusamy

Venoo Kuppusamy graduated from Moscow Medical Academy with a Medical Degree and went on to obtain a second degree from Universiti Sains Malaysia in Bachelor of Arts (Malay Literature). He currently works in Clinical Research Malaysia as an Associate Regional Manager. He has 10 years of experience as a Study Coordinator assisting in clinical trials mainly Industry Sponsored Research and 2 years of experience managing Study Coordinators and clinical trial sites.
Email: venoo@clinicalresearch.my
Joanne Yeoh

Joanne Yeoh graduated from University of Melbourne, Australia with a Bachelor of Biomedical Science. She has vast experience in clinical research which she has done for 15 years and was supporting clinical operations across Malaysia, Singapore, Taiwan, and Hong Kong in various CROs prior to joining Clinical Research Malaysia (CRM). Joanne is currently the Head of Clinical Operations in CRM.
Email: joanne.yeoh@clinicalresearch.my
26 Journal for Clinical Studies Volume 15 Issue 1 Market Report



Journal for Clinical Studies 27 www.journalforclinicalstudies.com Asia’s premier pharmaceutical event 19-21 June 2023 | Shanghai New International Expo Center To register please visit www.cphi.com/china Contact salesoperations@informa.com China’s pharmaceutical market is forecast to expand from CNY1.07trn (USD155.1bn) in 2020 to CNY1.3trn (USD203.8bn) by 2025. 50,000+ Pharmaceutical professionals 3,000+ Exhibiting companies 80+ Conferences & activities
Who Cares About Diversity in Clinical Trials?
The imperative to obtain a diverse body of clinical trial participants has been stressed in public statements for decades, including formal promotion by the U.S. National Institutes of Health (NIH), regulatory authorities such as the U.S. Food and Drug Administration (FDA), and recently, with the Consolidated Appropriations Act of 2023, even the U.S. Federal Government.1–4 Despite the duration and high profile of this issue, however, parties executing clinical trials, including sponsors, clinical research organisations (CROs), and academic centers, have remained insufficiently effective in addressing it. According to the 2020 U.S. Census, an estimated 33% of people in the U.S. identify as members of racial or ethnic groups that are underrepresented in medicine.5 However, between 1997 and 2014, only 4 to 5% of participants in trials of drugs submitted for approval by the FDA were from these groups.6 In 2017, nonHispanic Whites of European ancestry still comprised more than 90% of participants in clinical trials7 and even in 2020, the year during which the first Covid-19 vaccines were developed, and issues of racial diversity were prominent, 75% of clinical trial participants remained White.8 Although demographic variables other than race, such as gender, socioeconomic status, stage of disease, and residential location, are known also have critical impacts on medical outcomes and have also been mentioned in FDA Guidance, enrollment of diverse clinical research participants in these respects has received even less attention.
This state of affairs has persisted despite assertion by the vast majority of research professionals that DEI (Diversity, Equity, and Inclusion) is important. In the 2020 WCG Avoca Industry Survey, in which clinical research professionals were asked to provide confidential opinions about the importance of diversity among clinical trial participants, 81% of 215 respondents felt that at least one aspect of diversity (e.g., race, gender, socioeconomic) was important or critically important, including 40% who endorsed diversity as “critically important,” the extreme of the scale.9–10 This result was replicated in 2021 (75% and 37% of 101 respondents, respectively). 11 Similarly, in a survey conducted at a 2020 meeting of the Clinical & Translational Science Awards (CTSA) consortium leaders (funded by the NIH National Center for Advancing Translational Sciences), 94% of 231 respondents said they believed DEI in clinical and translational science to be “important,” and 86% were “committed” to making changes in CTSA processes to improve DEI.1 Clearly, there is a disconnect between what the vast majority of professionals voice to be important and the results their enterprises have actually achieved.
The present study seeks to gain insight into this disconnect by performing a detailed analysis of data regarding the DEI-related values and motivations of individuals executing clinical research projects. Herein we revisit the data from the 2021 WCG Avoca industry survey, asking specifically who cares (and does not care) about diversity in clinical research participation and why, rather than simply how many and how much. Our working hypotheses are1 that there may be systematic motivational differences across clinical research professionals that result in DEI-related decisions not being made in a manner that reflects the values of the wider group, and

(2) that beliefs about why diversity is important (i.e., ethical vs scientific vs regulatory) may impact how critical to research quality diversity is felt to be. Although the authors fully recognise the challenges associated with enrolling diverse participants (and have discussed these at length elsewhere9–11), we believe that the values and motivations of acting parties – and not just forces beyond their control – play a role when it comes to making difficult decisions about practices that impact the DEI results achieved.
Methods and Respondents
The 2021 WCG Avoca Industry Survey was designed to gain deeper comprehension of respondents’ values, views, and experiences regarding diversity in clinical research execution and clinical research participants.11 An open invitation to participate was extended to attendees at the 2021 WCG Avoca Quality & Innovation Summit, sent to contacts in the company’s database, posted on its website and on LinkedIn. Data were collected during the four-month period from September to December 2021.
The 101 respondents included clinical research personnel from 64 sponsor companies, 35 providers, and two academic research organisations. Approximately 38% of respondents held positions within clinical development/operations, 34% within quality assurance, and the remainder within a wide spectrum of management and functional roles. Thirty-seven percent of respondents represented companies that sponsored or conducted more than 50 clinical trials during 2021, and 20% represented companies that sponsored/conducted five or fewer. Most respondents worked for companies headquartered in the U.S., but approximately one-third represented companies headquartered in Northern/Western Europe (19%), Japan (5%), or other parts of the world (9%). Most (78%) of the respondents resided in the U.S.
Data analysed for the present study derived from two key questions in the 2021 survey. Survey respondents were asked to rate the importance of different types of diversity (i.e., racial/ethnic, gender, socioeconomic, etc.) to clinical research quality on a scale of 1 (no importance) to 5 (critically important). They were then asked to indicate whether their importance ratings were driven mostly by scientific, regulatory, ethical, or marketing and sales considerations. The impacts of “demographic” variables including respondent age, time in company, time in role (irrespective of company), functional area, and location were then investigated using multiple regression and ANOVA techniques.
RESULTS
Respondent Traits vs. Importance of Diversity
Figures 1 and 2 and Table 1 depict the average “importance ratings” (on a scale of 1 to 5) across respondents for each of five different types of diversity, as well as the statistical relationships between the quantitative respondent characteristics and these ratings. Of these variables, time in role demonstrated the most robust correlation with importance ratings across all of the diversity categories and was the only respondent characteristic to bear a statistical relationship to diversity importance ratings if corrections for multiple tests were applied. Specifically, the more time participants spent in their roles, the less important they generally rated every type of diversity. On
28 Journal for Clinical Studies Volume 15 Issue 1 Market Report
average, respondents who had worked less than 10 years in their current roles rated the importance of the various diversity categories between 3.3 and 4.3; for participants who had worked more than 10 years in their roles, in contrast, average ratings ranged between 2.1 and 3.0. This relationship could not be explained by age and/ or tenure within respondents’ companies (Table 1). Separately, we examined the possible impacts of respondent functional area and location; neither of these either bore independent statistically significant effects on importance ratings or covaried with the continuous variables in a way that would confound the results.
To determine whether the effect of Time in Role could be accounted for by time in any specific role, we looked separately at respondents in each of the two most-represented functional areas in the survey: clinical development and quality assurance. The statistically significant negative relationships between time in role and importance ratings for each aspect of diversity persisted for each of these functional areas taken separately, at least at the trend level (except in the case of race, within the group of respondents from Clinical Development). Again, the relationships could not be explained by confounding effects of age and/or tenure within respondents’ companies.

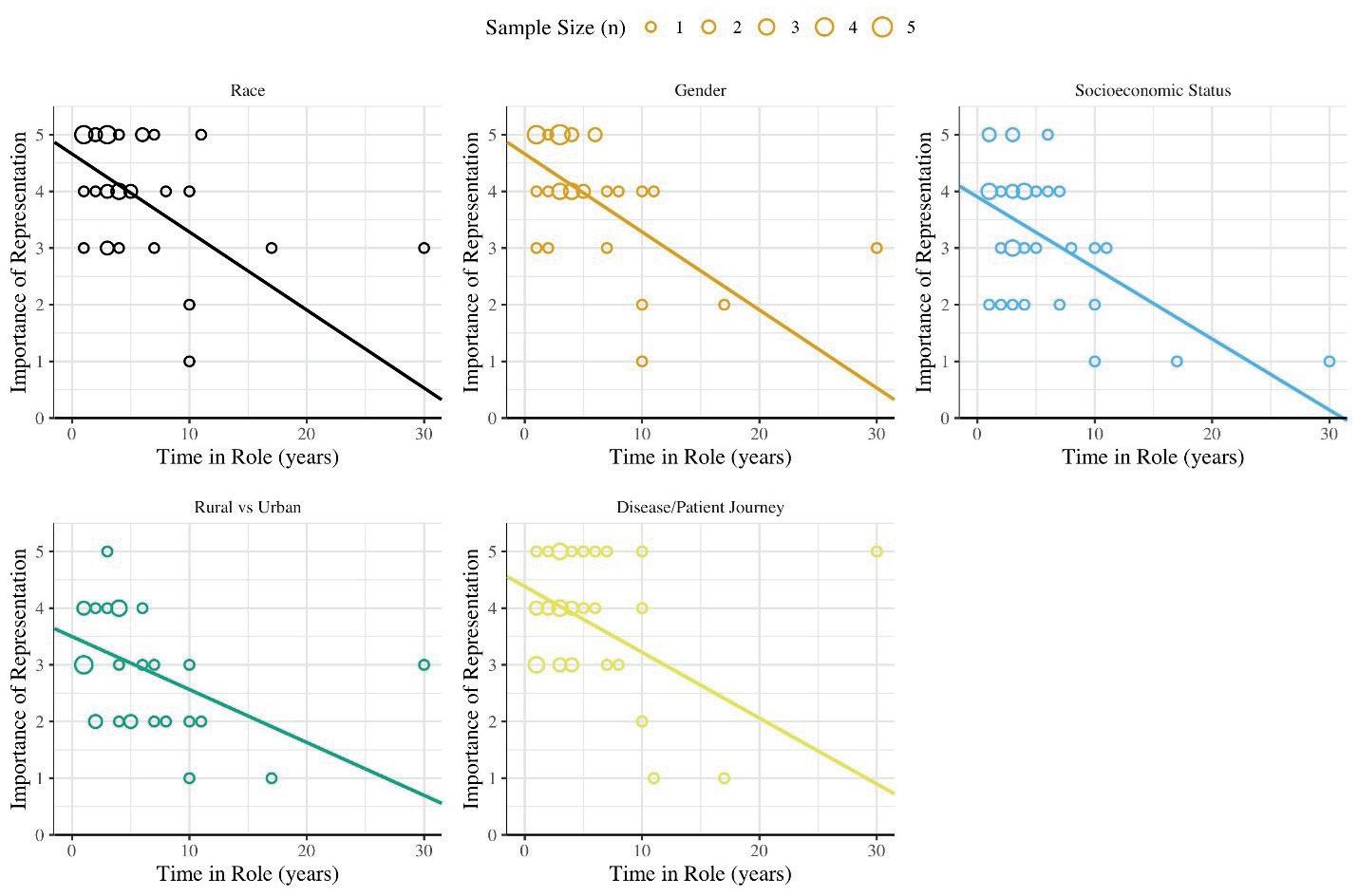
Journal for Clinical Studies 29 www.journalforclinicalstudies.com Market Report
Figure 1. Importance Ratings for Diversity in Clinical Research Participation vs. Time in Role
Figure 2. Importance Ratings for Diversity in Clinical Research Participation vs. Time in Company
Why Diversity is Important vs. How Important It Is
We also examined relationships between the reasons participants felt that diversity was important and how important they felt it to be. Our hypothesis was that feelings about diversity’s importance might be stronger if the propelling values were, e.g., scientific or ethical as opposed to regulatory or marketing. In general, this was found to be true. Across all aspects of diversity, respondents whose ratings were driven by scientific or ethical considerations felt more strongly about the importance of diversity than did those who felt diversity to be important primarily for regulatory or marketing reasons (Table 2).
Discussion
The goal of the present analysis was to better understand what drives differences across research personnel in how critical diversity among clinical research participants is believed to be. Understanding these differences and drivers may provide insight into motivational factors that underlie the disconnect between the stated importance of diversity (by groups of research professionals) and the DEI-related results actually achieved, and may thus inform efforts to improve performance in this respect. Of the variables we explored, time in role was, by a margin, the strongest predictor of the perceived importance of every aspect of diversity explored, with longer times in role associated with lower importance ratings for diversity. These effects were independent of the effects of age, functional area, location, and time in company, the last of which also bore independent negative relationships to diversity importance ratings in areas other than race/ethnicity and gender.
Responses to open-ended survey questions requesting the rationale for provided responses did not provide an explicit explanation for the observed relationships; however, they did shed light on important themes that may be at work. Most respondents described cultural difficulties and structural restrictions that posed challenges to incorporating DEI in clinical research, including “upper management stagnation” and hesitance to depart from “old ways.” One respondent in clinical development implied a lack of open-mindedness at the senior levels at which decisions were made: “Top decision-making people have to fund, invest, trade... and for that, they first have to be open-minded….” Disincentives for doing things in new ways were also described, including the “old school sponsor mentality that it must always be done a certain way because that way has proven successful in the past. There is a place for risk management built in, yet the folks executing the trial have hoops to jump through…such that it becomes a disincentive to be a leader in the unknown…to be an innovative trial leader.” One respondent mentioned the increased costs associated with DEI
initiatives: “When it increases costs due to the need for more clinical sites to achieve diversity and/or it prolongs the time to enroll the target numbers in all subgroups, then sponsors and shareholders are going to need to be sold on this”; and another called for “education based on solid evidence that variability and flexibility [in clinical trial execution, that would support diversity] will be accepted by regulators.”
It would stand to reason that those who have worked longer in their roles, particularly if they have also worked for a long time in their companies, are more likely to be entrenched in “old ways” of doing things; more likely to directly hold budget, timeline, and regulatory responsibilities for their departments; more likely to either be or report to “upper management” and shareholders; and more likely to have had long experience with the challenges of incorporating new practices. It would further stand to reason that direct, long-standing experience with the difficulties and risks of implementing new practices could negatively influence how critical such changes were felt to be. Consequentially from the perspective of implementing DEI-oriented programs, individuals who have spent more time in their roles are more likely than junior staff to make decisions regarding clinical trial execution, and thus their beliefs about what is (and is not) critical carry more weight. Skepticism from such individuals about the importance of diversity is unfortunate because it is exactly these more senior individuals on whom successful implementation of DEI-related programs would rely. When asked to describe the key enablers of variability and flexibility in clinical trial execution in support of DEI, one respondent insightfully responded: “experienced clinical scientists, supported by leadership, to drive clinical operations to execute the plans.”
Another useful finding from this analysis was the relationship between how important participants believe participant diversity to be, and why they believe it to be important. Not surprisingly, respondents who saw the pursuit of diversity as a scientific or ethical imperative were more passionate about its importance than were those who valued diversity for other reasons. Every aspect of diversity studied here is arguably important from a scientific perspective, as well as from an ethical perspective. As reflected by one respondent: “Appropriate representation of ethnic groups and sex are critical to the scientific validity of research findings, and their absence can lead to false conclusions. Representation of patients across the spectrum of disease is crucial to ensure deep understanding of new drug therapies. While, initially, the push for diversity may be driven by the ethics of inclusion, ultimately, it
30 Journal for Clinical Studies Volume 15 Issue 1 Market Report Race Gender Socioeconomic status (SES) Rural/Urban Stage in Patient Journey Time in Company 0.56 0.56 0.03 0.005 0.03 Time in Role o.0006 0.0004 0.00002 0.0001 0.0005 Age 0.26 0.31 0.26 0.65 0.03
Racial/Ethnic Gender Economic Rural/Urban Patient Journey Primary Reason Diversity is Believed to be Important Mostly Scientific 4.1 (50) 4.0 (68) 3.4 (17) 3.4 (20) 4.0 (71) Mostly Ethical 3.9 (33) 3.9 (18) 3.5 (63) 3.1 (47) 3.9 (13) Mostly Regulatory 3.9 (11) 3.6 (9) 3 (3) 2.3 (3) 3.5 (6) Mostly Marketing 3.0 (1) 0 (0) 2.2 (12) 2.5 (25) 3.2 (5)
Mean (N) Importance Ratings Type of Diversity
Table 1. Statistical Significance Levels (p value) for Impacts of Respondent Characteristics on Importance Ratings
Table 2. Importance Ratings for Diversity vs. Primary Reasons Diversity was Considered to be Important
benefits drug developers by...ensuring the medicines being tested work as expected in different ethnicities and at different points along the disease pathway.” Nevertheless, significant fractions of the respondents saw the pursuit of diversity, at least of some types, not to be driven by these values.
Understanding the patterns unveiled in this study’s analyses can inform policies and programs designed to effectively encourage personnel to make diversity-oriented decisions. If people motivated by scientific and ethical considerations are more likely than others to make decisions that prioritise diversity, then perhaps education surrounding the scientific and ethical rationales for diversity, and selection of leaders who understand and embrace these rationale will lead to more diversity-oriented decisions. Further, as senior staff will always play key roles in determining how clinical research is executed and will make such decisions based on their own beliefs and experiences, it is important to augment the findings of this study, which included only a minority of respondents in this category, by expanding participation of such individuals in the dialogue to more fully understand their perspectives. Another limitation of this study is that participants were not asked to report personal demographic data including their own genders, races, and ethnicities. Diversity of research staff is known to be highly associated with diversity of trial participants,11 and conceivably, respondents who had spent less time in their roles and at their companies may themselves have been more diverse. Additional studies and analyses such as the ones performed herein promise to more fully elucidate the reasons for continued failures to achieve DEI goals in clinical research, and thus will serve to support the design of DEI-promoting policies and programs.
REFERENCES

1. Boulware, LE, Corbie, G, et al. 2022. Combating Structural Inequities — Diversity, Equity, and Inclusion in Clinical and Translational Research.
N Engl J Med 386;3.
2. US FDA: Draft Guidance for Industry, 2022. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry (fda. gov)
3. US FDA. 2020. Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry | FDA
4. Sutter, S. 2022. Clinical Trial Diversity Action Plans Required under US Funding Bill. Pink Sheet, 21 Dec 2022.
5. Association of American Medical Colleges. Diversity and inclusion: underrepresented in medicine definition. 2021.
6. Knepper TC, McLeod HL. When will clinical trials finally reflect diversity? Nature 2018;557:157-9.
7. Ma, MA, Gutierrez, DE et al. 2021. Minority Representation in Clinical Trials in the United States: Trends Over the Past 25 Years. Mayo Clinic Proceedings 96:1.
8. FDA Data Snapshots: Features - PharmaNewsIntelligence
9. Calaprice, D. 2021. How CRO-Sponsor Partnerships Spurred Innovation in 2020. Life Science Leader. June 1. 2021.
10. Calaprice, D. 2021. How do we finish what Covid started? WCG Avoca 2020 Industry Research on Innovation and Diversity.
11. Calaprice, D. 2022. Diversity in Clinical Research Execution and Participation. Life Science Leader, June 1, 2022.
12. Tufts Center for Study of Drug Development. 2021. Diversity of Site Staff Highly Associated with Diversity of Patients Enrolled in Trials. As summarized in Applied Clinical Trials, Nov 2, 2021.
Denise Calaprice
Denise Calaprice, PhD, is a Senior Consultant and industry research lead at WCG Avoca. After receiving her bachelor’s degree in biology from Harvard University and her PhD from Princeton, Dr. Denise Calaprice entered the world of clinical research as an NIH fellow at Columbia University’s College of Physicians and Surgeons in 1994. Over the subsequent decades, she acquired extensive experience in directing clinical research programs and creating innovative development and operational strategies in a variety of settings, including academia, federal government, nonprofit, CRO, big pharma, and biotech/med tech start-ups.
Ryan Whitty
Ryan Whitty is a Research Assistant with University of California, Berkeley from the Computer Science department.

Christina Moon
Christina Moon is a Clinical Research Associate with the Brain Inflammation Collaborative.


Neva Hidajat
Neva Hidajat is a Clinical Research Intern with the Brain Inflammation Collaborative.

Journal for Clinical Studies 31 www.journalforclinicalstudies.com
Market Report
Technology
Clinical Research: More Than Words
Art and science are often positioned as opposites, with scientific illustration at the intersection. However, visuals contribute greatly to scientific communication, allowing complex ideas or theories to be presented clearly and in an accessible way. Whether it’s a flyer in the doctor’s office, a poster at a conference or a research paper – the communication of clinical studies research requires more than just words.
For many years, researchers have used both text and images to communicate their findings. Perhaps the earliest documentation of scientific drawing comes from fifteen thousand years ago, in the form of a cave drawing of a mammoth with a smudge over its heart. It has been hypothesized that this was to help guide early hunters to where the mammoth’s heart was located for when hunting.

Greek physician Herophilus later pioneered illustration as a technical tool, as he dissected and drew his subjects to gain a better understanding of human anatomy. In the 16th Century, Leonardo Da Vinci illustrated the human heart, to show that it had four chambers and to communicate this observation on how the aortic valve opens and closes, so blood can only flow in one direction. Fast forward to today and visuals have become fundamental to the communication of clinical research, both peer-to-peer, in education and with the wider public. So why is that, and what is best practice when doing so?
As humans, we are visual by nature. The human brain processes images 60,000 times faster than text, and 90 per cent of information transmitted to the brain is visual. Visuals provide a way of breaking
down medical phenomena in a way that allows us to enhance data processing and organisational effectiveness.
Communicating With Researchers
In clinical research papers, illustrations and infographics can simplify highly complex systems to make concepts easier to grasp. One option, which is growing in popularity, is visual abstracts. Often a single image, these help readers gain an overview of a research article in just a few minutes and usually contains the overall idea of experimental design, hypothesis and an indication of results.
Visual abstracts enable fast screening of the information, while also stimulating further exploration of the research. According to a famous study published in The Economist “Graphic Details: the importance of diagrams to science”, having an infographic as a visual element in a paper increases citations by 120 per cent.

There are three main styles of visual abstract: diagram style (often used when molecular structures are involved), infographic style (preferred when you have images with high visual impact that need to be incorporated with text), and comic type (useful for communicating in magazines). The Cell Press Graphical Abstract Guidelines include examples of different styles of visual abstracts and gives insight into how to improve them.
Conference Posters
During conferences or other scientific gatherings, researchers are frequently granted the opportunity to share their clinical knowledge in the form of a presentation or a poster. Poster presentations enable direct interaction between the clinical researcher and the conference participant, acting as a springboard for discussion, and they are a good tool for networking. The best posters at research symposiums

32 Journal for Clinical Studies Volume 15 Issue 1

Journal for Clinical Studies 33 www.journalforclinicalstudies.com Technology
are often awarded prizes or other accolades, based both on their content and visual appeal.
Poster requirements may differ from conference to conference, so ensure you check the layout before you begin. However, there are poster templates available online that can act as a strong starting point.
Presentations
Often, giving an interesting an informative presentation is easier said than done, but visual content can help this process significantly. Using images might seem like the go-to option, but good shots that explain medical procedures and scientific detail are hard to come by. When illustrating harsh procedures like surgeries, choosing images that are graphic can even compromise the audience’s recollection. Several psychological studies have shown that trauma and shock can negatively impact memory, so seeing real images with lots of blood and sectioned bodies could confuse the audience. In these instances, illustrations or infographics can provide a compelling alternative.
Communicating With Patients
Medical technology is a complex phenomenon, every device and piece of technology has its own features, benefits and technical information that relevant audiences must process. For example, if manufacturers are releasing a new tongue depressor or insulin pump, effective marketing to patients is a must.

Any information about medical technology is naturally complex to an untrained audience, so manufacturers can use images to help patients understand quickly and easily. For example, visual instructions of how to operate a glucose monitor patch is far easier to understand than text-based instructions.
Medical technology manufacturers can use images and visuals in multiple ways to inform consumers and persuade them that their product is the most effective solution on the market. Infographics are powerful digital tools that provide patients, healthcare professionals and other readers with the knowledge they need to understand a specific technology’s features, wider industry trends and different conditions and procedures.
34 Journal for Clinical Studies Volume 15 Issue 1 Technology

Journal for Clinical Studies 35 www.journalforclinicalstudies.com Technology
Consider this example. A manufacturer begins marketing a newly approved line of syringes designed for intravenous therapy purposes and is reaching out to local healthcare facilities and patients. When supplying a batch, the manufacturer could attach an infographic to the standard product guide, featuring the various features and injection techniques. By presenting this information as images, manufacturers can better engage with their audience and enhance understanding.
Creating Imagery for Use in Clinical Science

It can be a challenge to create an accurate, visually appealing graphic, especially when the subject matter is highly complex. One important early consideration is the color palette. Research has indicated that colors evoke varying emotions. For example, blue evokes trust, and red suggests urgency – as indicated by traffic lights and warning signs. Therefore, red encourages readers to stop and take in the message being presented to them. The most prominent colors can therefore be chosen based on the theme of the research, using color theory to help influence the audience’s reaction.
When planning the aesthetics of a design, color combinations with contrast work well. This can be opposing hues on the color wheel, or two contrasting shades of the same hue. Something to avoid is using too many distinct colors, which can clutter the image; try to strike a balance between realism and visual aesthetics.
Importantly, color has an impact on readability. A light and clean colored background can improve the visibility of the text, particularly if it is black. In addition, an untextured, plain background can help draw attention to the written information. Remember to make the most of white space; while it can be tempting to fill the space with as much information as possible, this can make it more difficult to read.
In addition, it is important to keep the text used to the minimum needed to communicate your key points. It’s also recommended to pick a clear and consistent font – nothing that distracts from
the information. Some popular options in science include Arial, Baskerville, Helvetica, Georgia, Garamond, Caslon and Times New Roman. Headings should be written in sans serif font and made big enough so the reader can make them out. Serif fonts are best for body text as they are easier to read when printed, and bold and italics are helpful design choices to draw attention to key points.
One recommendation is a to create trial print before making any final decisions. By printing your design in the format it will be presented in, you can see how it will look when you submit your paper or present your poster. Check it carefully for errors, unusual symbols and fonts, and ensure that the placement of the figures is correct. Having someone else to proofread it can help. They can spot mistakes you may have missed, as well as check it against the journalor conference-specific requirements.
In scientific communication, art and science go hand in hand. By learning the basics of color theory and graphic design, clinical researchers and educators can better illustrate their points, and make a lasting impact on their audiences.
Fabricio Pamplona
Fabricio Pamplona is the founder of Mind the Graph – a tool used by over 400K users in 60 countries. He has a Ph.D. and solid scientific background in Psychopharmacology and experience as a Guest Researcher at the Max Planck Institute of Psychiatry (Germany) and Researcher in D'Or Institute for Research and Education (IDOR, Brazil). Fabricio holds over 2500 citations in Google Scholar. He has 10 years of experience in small innovative businesses, with relevant experience in product design and innovation management.

36 Journal for Clinical Studies Volume 15 Issue 1 Technology
Design and Conduct of Trials in Alzheimer’s Disease Following the Impending Approvals of Disease-Modifying Agents
Fortunately, after nearly two decades of negative studies, two amyloid-lowering drugs have recently received accelerated approval for Alzheimer's Disease (AD) by the FDA, and one of these drugs is likely to receive full approval by July of this year. Submissions have also been made to regulatory authorities in Asia and Europe, and it is possible there will be global approval by the end of 2023, or early 2024. Notably, these drugs go beyond symptom management and appear to directly affect the disease pathology. Given the long dearth of new approvals and the shift from symptomatic treatment to disease modification, the clinical trial landscape will need to undergo substantial change when one or more of these drugs receives full approval. To aid sponsors and other stakeholders to navigate this new potential environment with success, several concepts and considerations are reviewed here.
Recruitment & Retention
While the regulatory authorities deliberate on the disease modifying therapies (DMT) in consideration for approval, research sites continue to try to recruit patients to ongoing AD clinical trials. Recruitment into study protocols in the more severe stages of the disease is less likely to be affected by these approvals, although the expected large influx of enquiries for treatment with one of the DMTs is bound to result in a resource issue at many trial sites, in terms of staffing. The real challenge for research sites will be the continued recruitment into DMT protocols in the Early AD (eAD) space. In the US, patients and families might prefer to wait for full approval by the FDA, rather than commit themselves to a long-duration, placebo-controlled trial with no guarantee of success, or an unknown safety profile. The Centers for Medicare and Medicaid Services (CMS) have intimated that if a monoclonal antibody directed against amyloid for the treatment of Alzheimer’s disease subsequently receives traditional FDA approval, CMS will provide broader coverage of reimbursement. Theoretically, at least in the US, this would mean that those patients with appropriate health insurance coverage would have their costs for treatment partially or fully reimbursed, although this of course needs to be confirmed. It is less clear, at this moment in time, whether the national or private health systems of countries outside the US will allow the reimbursement of the DMT upon approval from the applicable health/drug authorities.
Assuming that at least one DMT receives full approval, and CMS (or the corresponding national health system in other countries) agrees on a reimbursement package, typical eAD patients and caregivers will infer that approval confirms efficacy and a reasonable safety profile, while providers understand that all investigational compounds come with uncertainty on both fronts. Sponsors will need to give significant thought to educational programs and materials to equip site personnel to understand differences between the proposed investigational compound and approved treatments in the same space. This knowledge
is critical for two reasons: 1) to speak to potential reasons to consider an investigational compound over an approved treatment, and 2) to ensure that site staff form educated opinions on the relative merits of treatment options for their patients.
Cost-benefit analysis of pursuing approved treatments will play significantly different roles in patient/caregiver decision-making outside the US. The US Centers for Medicare and Medicaid Services (CMS) and private insurers may come to different conclusions regarding the predicted cost of care for patients at different stages of disease, and therefore the appropriateness to reimburse for newly approved treatments. By contrast, patients in most other countries where approved treatments are available may have relatively fewer financial factors influencing decision-making when pursuing treatment via approved drug or a clinical trial. As a result, recruitment rates might be more significantly adversely impacted in the US in the short term and less so in the long term, as compared to other countries.
Sponsors should ask sites to have candid and practical conversations with patients about their realistic potential to receive approved treatments, particularly in cases where patients might be at risk of soon declining beyond the boundary of eligibility for clinical trials.

Retention of patients in clinical trials may also become more problematic. Some patients may enter a trial such that all diagnostic investigations are conducted free of charge, and then withdraw consent when the opportunity arises to receive an approved treatment. The setting of expectations with patients is paramount to predictable realisation of screened to enrolled subjects, and therefore to recruitment.
Study Design
Any approval of a DMT in Early AD, reimbursed either via health insurance or state, may also have an impact on study design. Once a treatment becomes a standard of care, it is imperative to consider both placebo-controlled studies and those with an active control, using an approved DMT. Several important issues must be considered, including:
• Different rates of decline: Early AD trials inevitably include patients with either mild cognitive impairment (MCI) due to AD or patients with Mild AD. There is a need to examine the differential rates of decline of these two populations of patients to determine whether the intuitive notion that MCI due to AD patients may take longer to show a deterioration is indeed correct. Current psychometric tests used for efficacy measures may not be adequate to detect decline in this very early group of patients, and alternatives are needed to enable the reduction of treatment time currently used in such trials. Eighteen months on placebo may not be a palatable option for many patients, but a shorter period may be more acceptable and therefore more likely to encourage participation.

Journal for Clinical Studies 37 www.journalforclinicalstudies.com
Therapeutics
• Delayed administration of trial medication: In DMT-controlled studies, there is a need to ensure that the short-term commonly reported side effects have run their course before adding a trial medication. This may require that patients spend at least 3–6 months on an approved DMT before initiating treatment with the new study drug being tested, incurring obvious cost and clinical implications.
• DMT control costs: Assuming a DMT-controlled, 18-month study, there is a need to establish whether payment for the DMT will be covered by insurance or state funding. Given the likely cost of these drugs, it is highly unlikely that any small biotech and pharma companies will have the resources to pay for initiation and 18 months treatment of the approved drug.
• Lengthy period requiring availability of DMT control: Clearly if a trial is of minimally 18 months’ duration, the approved DMT must be made available for the full study period. In other words, the terms of reimbursement of the drug cannot be limited, or potentially based on treatment success.

• Practical challenges to global reimbursement: Although approved, reimbursement of DMTs will remain a significant
challenge, primarily in the US but potentially in other countries later. Consequently, studies beginning in 2023 and 2024 may suffer from restrictions on which countries can be included due to differing approval timelines, outcomes, and levels of reimbursement.
• Global differences in clinical meaningfulness: Recent work discussing clinical meaningfulness in randomized controlled trials lays the foundation for critical next steps in demonstrating benefit to patients and therefore adequate impact for regulator consideration.1 While globally applicable, this discussion is somewhat US-centric. If international regulatory authorities utilize different criteria, then the ability to use approved DMT drugs may be compromised. More specifically, consideration should be given to the potential for labels to restrict treatment to patients with milder disease. As DMT drug developers have come to understand the likely mechanisms of most study drugs – namely, to slow or stop decline – the focus has been plainly put on identifying mechanisms of action with effect at earlier stages of disease progression. Were evidence to demonstrate, in regulators’ eyes, that a given DMT is not suitable for more severely impaired patients, trial recruitment might be accordingly impacted. Further, if long-term treatment objectives are focused on patients with
38 Journal for Clinical Studies Volume 15 Issue 1
Therapeutics
MCI or pre-symptomatic AD, sponsors may be faced with the significant challenge that approved treatments compete directly for the same subpopulation sought for clinical trials.
• Likelihood of segregation of trial- and treatment-focused sites in the US and associated knock-on effects: Perhaps uniquely to the United States, many clinical trial sites do not serve as dedicated treatment centers. As a result, patients might receive an approved DMT from one physician and practice while seeking concomitant treatment in a clinical trial conducted at another provider’s facility. Potential impacts may include: 1) reduced data quality/ completeness, 2) diminished safety monitoring, 3) inconsistent dosing or treatment cessation practices in the event of approvedDMT-related AEs, and more.
Equity, Diversity & Inclusion
Finally, reimbursement and cost of care paradigms in the US will likely have different implications for people of different races, ethnicities, cultures, and socioeconomic statuses. Well documented differences in diagnoses and care rates among under-served people of different races and ethnicities2 are microcosms of the potential factors at play in over- and underrepresentation of subpopulations of patients globally. As each trial and context will differ, sponsors should conduct a rigorous review of study design, mechanism of action, and AD subpopulation to be treated as factors impacting the potential demographic composition of the eventually enrolled population of subjects. For example, any ethnic, racial, and socioeconomic differences might impact the likelihood of reimbursement by managed care companies in the US. The representation of American people of colour might be unduly impacted in clinical trials due to mistrust and stigma associated with medical care,3,4 as patients from these backgrounds might more readily choose approved DMTs over a treatment offered via clinical trial.
In the context of potentially poorly representative clinical trial populations, it is important to consider not only the implications for study acceptance by regulators – in light of recommendations (and likely requirements) to achieve reasonably diverse study populations5 – but also to possibly impact signal. Racial and ethnic differences in AD biomarker studies have been widely observed in recent years,6,7 significantly calling into question whether AD treatments act similarly across these populations. If clinical trial population diversity is abjectly affected by the availability of approved DMTs, stakeholders must be prepared to mitigate these imbalances during study design, site selection, and recruitment stages to help ensure adequate study of investigational compounds across all populations intended to be treated.
Summary
Given the strong possibility of regulatory full approval being granted to at least one DMT later this year, serious thought needs to be given to the likely impact on both ongoing RCTs in AD and future clinical studies. Both recruitment and retention may be more challenging, and clinical trialists may need to adapt trial design and conduct to reflect the new standard of care that will arise.
REFERENCES
1. Petersen RC; Aisen PS; Andrews JS; et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimer's Dement. 2023;17. https://doi.org/10.1002/alz.12959.
2. Alzheimer's Association. 2018 Alzheimer's disease facts and figures. Alzheimer’s Dement. 2018;14(3):367-429.
3. Chin AL, Negash S & Hamilton R. Diversity and Disparity in Dementia: The Impact of Ethnoracial Differences in Alzheimer Disease. Alzheimer Disease & Associated Disorders 25(3):187-195, July–September 2011. DOI: 10.1097/
WAD.0b013e318211c6c9
4. Chiong W, Tsou AY, Simmons Z, Bonnie RJ & Russell JA, on behalf of the Ethics, Law, and Humanities Committee (a joint committee of the American Academy of Neurology, American Neurological Association, and Child Neurology Society). Ethical Considerations in Dementia Diagnosis and Care, AAN Position Statement. Neurology Jul 2021, 97 (2) 80-89; DOI: 10.1212/ WNL.0000000000012079.
5. Dark HE & Walker KA. New IDEAS about amyloid, race and dementia disparities. Nat Rev Neurol 19, 5–6 (2023). https://doi.org/10.1038/s41582022-00748-0.

6. See FDA, Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry. https://www.fda.gov/regulatory-information/searchfda-guidance-documents/diversity-plans-improve-enrollment-participantsunderrepresented-racial-and-ethnic-populations. 87 Fed. Reg. 22211-01 (Apr. 14, 2022).
7. Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, Fagan AM, Grant E, Gordon BA, Holtzman DM & Xiong C. Assessment of Racial Disparities in Biomarkers for Alzheimer Disease. JAMA Neurol. 2019 Mar 1;76(3):264-273. DOI: 10.1001/jamaneurol.2018.4249. PMID: 30615028; PMCID: PMC6439726.
Michael T. Smith

Michael T Smith, MSc is a Project Manager in Neuroscience at Worldwide Clinical Trials and speech language pathologist specializing in neurodegenerative disease. His focuses include the delivery of highquality clinical data in large AD clinical trials, the optimization of trial outcome measurement, and the continued refinement of trial methodology to recruit appropriate and diverse study populations. His contributions to the literature include many conference presentations and several peer-reviewed articles, particularly in behavioral assessment methods.
Email: michael.th.smith@worldwide.com
Robert Smith
Robert Smith, PhD is an Executive Director in the Scientific Solutions Group at Worldwide Clinical Trials. His PhD is in Neurology, and he spent 15 years as a Principal Investigator at a Memory Clinic in the UK, working on over 100 clinical trials in Alzheimer’s Disease. Since 2004, he has worked in two CRO’s, predominantly, in large Phase III, global programmes of Monoclonal Antibodies in Early AD.
Email: robert.smith@worldwide.com
Natalia E. Drosopoulou
Natalia E. Drosopoulou, PhD is a Senior Vice President of Project Management in Neuroscience at Worldwide Clinical Trials. She received her PhD in Biochemistry, specialised in Developmental Neurobiology from King’s College of London. With over 20 years in the clinical research industry, Dr Drosopoulou’s experience spans from small intricate Phase I studies to large global Phase III programmes with a special emphasis in AD trials.
Email: natalia.drosopoulou@worldwide.com

Journal for Clinical Studies 39 www.journalforclinicalstudies.com
Therapeutics
Page 13
Page 27
CPHI Japan
CPHI China
IBC Connect in Pharma
IFC + 22 & 23
Page 5
Page 3
PCI Pharma Services
Ramus Medical Ltd.
KRAUTZ-TEMAX Europe
Trilogy Writing & Consulting GmbH Subscribe today at Email: info@senglobalcoms.com www.journalforclinicalstudies.com
BC
Journal for Clinical Studies
I hope this journal guides you progressively, through the maze of activities and changes taking place in the pharmaceutical industry


JCS is also now active on social media. Follow us on:
www.twitter.com/jforcsjournal www.facebook.com/Journal-for-Clinical-Studies www.plus.google.com/+Jforcsjournal www.journalforclinicalstudies.com
40 Journal for Clinical Studies Volume 15 Issue 1 Ad Index






Trilogy experience will change the way you think about medical writing. Our writers are masters of their trade. Experts in engaging reviewers through crystallized messages, able to guide and manage complex teams, Trilogy offers precision writing backed by years of dedication to the craft. Which means one less thing for you to worry about.
The
MEET THE MASTERS OF MEDICAL WRITING Visit us at TrilogyWriting.com writers@TrilogyWriting.com www.TrilogyWriting.com/TriloTalk Listen to our podcast Apple Podcasts, Google Podcasts, and Spotify
Dr. Julia Forjanic Klapproth, Senior Partner Master Skills — Owning the process, leading with expertise, lighting up a room





















































































