Embolic Pneumonia Associated with Udder Cleft Dermatitis in Dutch Dairy Cows
Preventing and Reducing the Impact of Neonatal Diarrhea in Calves


Establishment of Histomonas meleagridis Challenge Model in Turkeys: An Industry Perspective
Getting Your Product Approved Regulatory considerations for the EU and US Official


www.international-animalhealth.com PEER REVIEWED Volume 10 Issue 1
Supporting
Sponsored
-
Associations -
companies
Your partner for contract research
Royal GD is a partner within the animal health industry worldwide, performing in vivo, in vitro and field studies. We conduct safety and efficacy studies on veterinary biologicals and pharmaceuticals in compliance with the OECD principles of Good Laboratory Practice (GLP).

Our portfolio includes, but is not limited to:
• Safety and efficacy studies of veterinary biologicals and pharmaceuticals;
• Studies to obtain vaccine or challenge-strain candidates;


• Quality control tests on final products;

• Development of models to demonstrate the efficacy or safety of veterinary biologicals and pharmaceuticals;
• Surveys on the prevalence of (emerging) infectious diseases/agents.
Get in touch and plan a meet up with our account managers and research project team: support@gdanimalhealth.com
www.gdanimalhealth.com
AHEAD IN ANIMAL HEALTH
ROYAL GD IS AHEAD IN ANIMAL HEALTH WITH EXPERT AND INDEPENDENT CONTRACT RESEARCH
MANAGING DIRECTOR
Mark A. Barker
EDITORIAL MANAGER
Beatriz Romao beatriz@senglobalcoms.com
RESEARCH AND CIRCULATION
Virginia Toteva virginia@senglobalcoms.com
DESIGNER
Jana Sukenikova www.fanahshapeless.com
BUSINESS DEVELOPMENT
Jerome D’Souza info@senglobalcoms.com
ADMINISTRATOR
Jessica Chapman jessica@senglobalcoms.com
FRONT COVER
© istockphoto
PUBLISHED BY Senglobal Ltd.
Unit 5.02, E1 Studios, 7 Whitechapel Road, E1 1DU, United Kingdom
Tel: +44 (0) 2045417569
Email: info@senglobalcoms.com www.international-animalhealth.com
International Animal Health Journal – ISSN 2752-7697 is published quarterly by Senglobal Ltd.

04 FOREWORD REGULATORY & MARKETPLACE
06 Global Trends in the Animal Health Sector
The last few years was one of growth for the animal health sector. Global changes like population growth, public health, pet adoption, and sustainable societies have made good animal health more essential than ever before. The sector’s growth has been resilient over the past decade as industry fundamentals evolve. The value of the global animal health sector increased by 12% to $38.3 billion in 2021 with sales increasing across all geographies. Carel du Marchie Sarvaas at HealthforAnimals outlines the global trends in the aimal health sector.
09 Burnout and Compassion Fatigue: What Vet Professionals Need to Know
Being a veterinary professional is one of the most fulfilling occupations. However, it comes with a lot of hardship which needs to be managed otherwise we find ourselves in a downward spiral. Not only is burnout dangerous for the individual, but it is also detrimental for the veterinary business and the whole profession. Dr. Silvia Janská at The Webinar Vet. explains more about what vet professionals need to know when dealing with fatigue and compassion fatigue.
14 Getting your Product Approved –Regulatory Considerations for the EU and US
When developing a veterinary product for the global market, differences in product categorisation and applicable regulations can be difficult to navigate. The final article in the “From Molecule to Market” series, by Dr. Caroline Vanino, Dr. Heather Sedlacekand and Dr. Aaron Johnson at Argenta Clinical R&D, provides an overview of the regulation for animal health products in the European Union and the United States.
18 The Green Discussion Forum
The opinions and views expressed by the authors in this journal are not necessarily those of the Editor or the Publisher. Please note that although care is taken in the preparation of this publication, the Editor and the Publisher are not responsible for opinions, views, and inaccuracies in the articles. Great care is taken concerning artwork supplied, but the Publisher cannot be held responsible for any loss or damage incurred. This publication is protected by copyright.
Volume 10 Issue 1 Spring 2023
Senglobal Ltd.
In 2020, we decided that the theme for our year would be regenerating the veterinary world. Anthony Chadwick had been running The Webinar Vet for a decade and begun the online revolution for vets, which had taken millions of miles off the roads over the last decade. However, we wanted to do more to show leadership in the veterinary industry. As a digital educational business, it is arguably simpler to be sustainable than a large pharma company. However, we calculated our carbon footprint; offset double what we produced during the pandemic year; started a veterinary sustainability podcast and planted a wildflower meadow at the Liverpool Science Park along with encouraging our landlord to embrace renewable energy providers.
RESEARCH AND DEVELOPMENT
22 Embolic Pneumonia Associated with Udder Cleft Dermatitis in Dutch Dairy Cows
Udder cleft dermatitis (UCD; also bovine ulcerative mammary dermatitis or foul udder) is an inflammation of the udder skin and is most often located between the frontquarters and at the transition of the frontquarters and the abdominal wall. Cows with UCD may have
International Animal Health Journal 1 www.international-animalhealth.com
CONTENTS
increased risk of clinical mastitis, and associations between UCD and digital dermatitis have been suggested. The lesions can impair animal welfare, milk production, and milk quality and can lead to death and premature culling. Thus preventive measures are warranted, but in daily practice UCD is not always adequately detected by farmers and their cattle veterinarians. Christian Scherpenzeel and Klaas Peperkamp at Royal GD investigate the prevalence of cows with complicated UCD on postmortem examination, associated with embolic pneumonia as most likely cause of death.
24 Preventing and Reducing the Impact of Neonatal Diarrhea in Calves

Calf diarrhea, also known as calf scour, is one of the major causes of neonatal calf mortality, which has been estimated to cost farmers around 60 to 80 Euros per calf1, with up to three in four calves affected. Calf scour can result in a decrease in performance in the shortterm, and in the longer-term impact future production. It also has significant welfare impacts through the pain of the disease, combined with the risk of mortality, as well as increasing the use of antimicrobials. Matt Yarnall at Boehringer Ingelheim explains how to prevent and reduce the impact of neonatal diarrhea in calves.
28 Vaccination Against Infectious Bronchitis in Chickens: An Evolving Challenge
Infectious Bronchitis (IB) is a highly contagious respiratory disease of poultry, that causes widespread economic losses within the industry. Vaccination is key to the effective control of IB, but IBV has an inherently high mutation rate, and the continual emergence of new serotypes makes this challenging. For successful IB control, alternatives to homologous vaccines are necessary and protectotyping is one such concept that

has been suggested. Dr. Abdallah Makahleh at Kemin Biologics guides through the challenges in the vaccination against infectious bronchitis in chickens.
32 Establishment of Histomonas meleagridis Challenge Model in Turkeys: An Industry Perspective
Histomoniasis (blackhead disease, histomonosis, enterohepatitis) in turkeys, is caused by a protozoan parasite, Histomonas meleagridis. Currently, there are no commercial vaccines or prophylactic/therapeutic treatments available for blackhead disease. Due to the lack of vaccines, treatment and efficacious drugs, the turkey industry faces a huge economic impact due to mortality, morbidity and condemnations. In order to evaluate any vaccine or efficacious prophylactic/ therapeutic treatment, establishment of a robust challenge model is essential. Dr. Vijay Durairaj and Dr. Vander Veen at Huvepharma, Inc describe the establishment of a robust challenge model against recently isolated wild-type H. meleagridis isolates from USA.
AQUACULTURE
38
Tackling Cardiomyopathy Syndrome in Farmed Salmon
Cardiomyopathy Syndrome of farmed Atlantic salmon has become widespread since it was first recognised in Scotland and Norway in the 1980’s. The condition is responsible for significant losses particularly of larger, market size fish. In Norway it was the most important cause of mortality in 2020 and 2021. Mortalities may be chronically elevated and acute mortalities may occur, often associated with a stressful event such as handling for sea lice treatment. Bill Roy at Moredun Scientific explains how to tackle Cardiomyopathy Syndrome in Farmed Salmon.
Volume 10 Issue 1 2 International Animal Health Journal
CONTENTS



Animal health contract research Expert delivery of contract studies supporting product development and commercialisation. Efficacy studies • Broad portfolio of infectious disease models • New model and protocol development • VICH-GCP compliant studies • Studies in all species of livestock • Aquaculture studies Target animal safety testing • GLP compliant studies • All species of livestock • Aquaculture studies • All types of biological & pharmaceutical products www.moredun-scientific.com For further information please visit www.moredun-scientific.com or contact: Moredun Scientific, Pentlands Science Park, Bush Loan, Penicuik EH26 0PZ, Scotland, UK Tel: +44 (0)131 445 6206 Email: info@moredunscientific.com Follow us Facilities • GLP accredited animal accommodation • Conventional farm accommodation • Category 3 containment • Specific Pathogen Free • Gnotobiotic units • Aquaculture Testing Facilities (Seawater and Freshwater) • GLP accredited laboratory facilities
Welcome to the latest edition of the Journal. The animal pharmaceutical industry develops and sells antibiotic drugs and other products used for food and companion animals. However, the use of antibiotics in agriculture is under increasing scrutiny from policymakers and consumers. How animal pharma responds in terms of developing and marketing veterinary products has ramifications for agricultural production and meat prices. Trends in sales and development of veterinary antibiotics. Antibiotics sales for food-animal production in the United States and the European Union (EU) have shown declines, even as demand for food-animal products continues to increase. The number of veterinary drug and biological products brought through regulatory approval in the United States also declined and antibiotics for food-animal production account for a decreasing share of new drug approvals.
Perceived contemporary issues like food safety and food healthfulness, environment, sustainability, biotechnology, animal well-being, animals as food, and research funding. Food safety is the paramount contemporary issue, and environment and sustainability issues can be considered as a single issue.
Being a veterinary professional is one of the most fulfilling occupations. However, it comes with a lot of hardship which needs to be managed otherwise we find ourselves in a downward spiral. Not only is burnout dangerous for the individual, but it is also detrimental for the veterinary business and the whole profession. Dr. Silvia Janská at The Webinar Vet. explains more about what vet professionals need to know when dealing with fatigue and compassion fatigue.
In this journal, we will also explore more about some of the diseases in the animal health field. Calf diarrhea, also known as calf scour, is one of the major causes of neonatal calf mortality, which has been estimated to cost farmers around 60 to 80 Euros per calf, with up to three in four calves affected. Calf scour can result in a decrease in performance in the short-term, and in the longer-term impact future production. It also has significant welfare impacts through the pain of the disease, combined with the risk of mortality, as well as increasing the use of antimicrobials. Matt Yarnall at Boehringer Ingelheim explains how to prevent and reduce the impact of neonatal diarrhea in calves.
In this issue of the IAHJ, we bring you a portfolio of articles that defines our changing industry, and how to effectively harness these changes.
EDITORIAL ADVISORY BOARD
Amanda Burkardt, MSc, MBA – CEO of Nutripeutics Consulting
Germán W. Graff – Principal, Graff Global Ltd
The last few years was one of growth for the animal health sector. Global changes like population growth, public health, pet adoption, and sustainable societies have made good animal health more essential than ever before. The sector’s growth has been resilient over the past decade as industry fundamentals evolve. The value of the global animal health sector increased by 12% to $38.3 billion in 2021 with sales increasing across all geographies. Carel du Marchie Sarvaas at HealthforAnimals outlines the global trends in the Animal Health Sector.
I hope you all enjoy reading this edition, and my team and I look forward to bringing you more innovative articles in the net issue.
Kevin Woodword, Managing Director, KNW Animal Health Consulting
Fereshteh Barei – Health Economist & Strategy Advisor, Founder of BioNowin Santé Avenue Association
Carel du Marchie Sarvaas Executive Director Health For Animals
Kimberly H. Chappell – Senior Research Scientist & Companion Animal Product Development Elanco Animal Health
Dr. Sam Al-Murrani – Chief Executive Officer Babylon Bioconsulting & Managing Director at Bimini LLC
Sven Buckingham – Buckingham QA Consultancy Ltd.


Dan Peizer – Director Animal Health at Catalent Pharma Solutions
Dawn Howard – Chief Executive of the National Office of Animal Health (NOAH)
Jean Szkotnicki – President of the Canadian Animal Health Institute (CAHI)
Dr. Kevin Woodward – Managing Director KNW Animal Health Consulting
Norbert Mencke – VP Global Communications & Public Affairs Bayer Animal Health GmbH

Volume 10 Issue 1 4 International Animal Health Journal
FOREWORD










Project Management Pharmaceutical Sciences Novel Drug Delivery Technologies Pre-Clinical and Clinical Services Regulatory Affairs Data Management Commercial Manufacture All the product development and manufacturing expertise and capabilities you need, under one roof. www.argentaglobal.com Succeed Sooner. VICH GCP 1 1 + 1 2 M A Y , 2 0 2 3 M U N I C H , G E R M A N Y W O R K S H O P 2 0 t h K l i f o v e t
Global Trends in the Animal Health Sector
The last few years was one of growth for the animal health sector. Global changes like population growth, public health, pet adoption, and sustainable societies have made good animal health more essential than ever before.
The sector’s growth has been resilient over the past decade as industry fundamentals evolve. The value of the global animal health sector increased by 12% to $38.3 billion in 2021 with sales increasing across all geographies.


investing more in pet care. This is the case in large developed markets but also in emerging markets where growth in middle class populations is making pet ownership more common.
Foundational tools like vaccination, parasiticides and diagnostics are essential to protect pets and safeguard the surrounding household against the fleas, ticks, illnesses and other hazards that a animals can bring home. Emerging technologies in areas like immunotherapies and digital technologies are allowing for pets to share longer, heathier lives with their owners.
Looking ahead into 2023 and beyond, this article analyses major trends and expectations related to the animal health sector. Many of these trends will further accelerate in the coming years.
Growing Demand for Animal Protein
More people with more spending power are demanding more animal protein. Demand for all types of animal protein skyrocketing. The OECD and United Nations Food and Agriculture Organisation (FAO) estimate that livestock and fish production will increase 14% from 2020–2030. In richer countries, milk, egg, fish and white meat consumption is growing and in most of the emerging world there are strong consumption increases in all categories.

Sustainably meeting this demand will require better onfarm health to reduce livestock losses and improve efficiency. For some regions, this starts with greater uptake of vaccination, while in others better use of diagnostics and emerging digital tools can offer precision health.
Growing Disease Pressure
Recent years have seen a marked increase in the threat of deadly animal diseases. Illnesses like African Swine Fever continue to spread across Asia, while Avian Influenza is leading to the culling of hundreds of millions of birds across the globe. Incidence of animal disease creates significant economic and societal costs, which is spurring greater investment in and public support for animal health.
The likelihood of pathogen spread is also increasing with significantly more trade, travel and transport of humans and animals. More food is also traded, for example, poultry trade was up by 520% over the last 20 years.
There is also a significant relationship between animal disease and human disease with 13 zoonoses being the source of 2.4b cases of human illness and 2.2m deaths a year. Healthier animals lead to healthier humans and there are dramatic improvements in both to be made with a OneHealth mindset.

Growing Pet Populations and Growing Spend

Global pet populations are also rising, while owners are

Volume 10 Issue 1 6 International Animal Health Journal REGULATORY & MARKETPLACE
The Environment and sustainable Livestock Production
The livestock sector continues to grow worldwide to meet the demands of an ever-increasing global population. Supporting greater efficiency, productivity and sustainability is a core goal in all markets.
In emerging countries there is scaling-up, professionalization and significant investment in more efficient livestock products. Governments, the private sector, civil society and others are also investing, implementing practice changes and supporting research and innovation, to implement and incentivise more sustainable food production. This is the right thing to do.
Changing temperatures mean that vectors survive longer at higher latitudes – malaria, dengue and tick-borne encephalitis are now appearing in places they never did. Likewise, climate change is leading to more challenging production conditions with droughts and floods.
Urbanisation coupled with the increasing pressure on natural resources and competition for land, water, and feed means livestock must continually improve its sustainability. Livestock is an emitter of greenhouse gas emissions. The need for more sustainable production means the animal health sector has tremendous opportunities to provide solutions. These include more and better vaccination, better parasite management and other techniques that enable better overall animal health – less disease, less morbidity, less death and healthier more productive animals.
Evolution in the Animal Health Sector Company Offerings


There has been, and will continue to be, an evolution in the offerings of the animal health sector. Where traditional markets such as the United States and the European Union will remain essential, revenues in the emerging markets are increasing as food production and pet ownership increase.
The animal health sector has seen consolidation through mergers and acquisitions, but also through bolt-on acquisitions in areas such as vaccination, digital technology, diagnostics and so on. There are many successful vibrant smaller companies and funding for acquisitions is easily accessible.
One of the major changes which will continue is the application of integrated animal health solutions, including a much wider range of tools that integrate diagnostics, genetics, treatment, prevention, nutrition, prediction and so on. There is significant cross-over of techniques and approaches from other sectors such as the biotech human health and IT sectors.
The animal health sector understands that innovation is needed to meet the emerging challenges, which is why companies are increasing R&D spending across the sector. These investments will be crucial to meeting global objectives like the Sustainable Development Goals (SDGs) and strengthening defenses against potential future zoonotic pandemics.
Scientific and technical innovations are moving beyond the R&D phase with multiple areas of innovation including: new vaccine technologies, biotech based approaches, new enzymes, genomics, nutraceuticals – and also new offerings in herd management, data surveillance, epidemiology, etc. The increasing costs and requirements related to regulatory approvals favour larger companies with more resources.
Other technological solutions are mainstreaming. Think about the use of ‘big data’, satellite data, smart tags, wearable devices, remote monitoring, video-enabled engagement, predictive Approaches to disease outbreaks, telemedicine apps for veterinarians, e-prescription and so on. Many companies are investing in these areas.
Investments in Prevention
Disease prevention is the cornerstone of veterinary care. When illness is avoided, animals are spared from the devastating effects of disease while people are protected against zoonotic transfer. Animal health companies are supplying more disease prevention products than ever before, from vaccines to diagnostics to emerging digital tools, to help support these efforts.
As a result, since 2013, vaccines and parasiticides have grown from 56.7% to 62.6% of the product portfolio, while antimicrobials fell from 20.9% to 15.2% (a relative reduction

International Animal Health Journal 7 www.international-animalhealth.com REGULATORY & MARKETPLACE
of 28%). This is also a reflection of responsible antibiotic use strategies, built on a foundation of prevention, that have been applied in most markets.

The Evolution of Attitudes Toward Animals
People are increasingly more aware of what they eat, and they know more about food safety and balanced diets.
Consumers want more information about their food, and they want more choice. When it comes to animal protein more people care about how animals are raised and about their overall welfare.
Anti-farming, anti-meat, anti-technology campaigners challenge animal production. Commercial plant-based meat companies see this as an opportunity. Significant media attention for vegan and vegetarian lifestyles has not led to overall decreases in animal protein consumption. The cost-ofliving crisis has also seen a sharp decline in the consumption of plant-based meats and most analysts expect that this will remain a small niche market.
Pet ownership is increasing rapidly in countries like Brazil, India, China and elsewhere. Another trend is the increasing medicalisation of pets. Life expectancy is increasing, and pet owners are interested in ‘quality of life’ products to be able to treat conditions like skin problems, diabetes and arthritis. Obesity and related diseases are also increasing in pets. People are bringing their pets in for more regular checkups and adopting health management plans.

One of the most dramatic changes over the last few decades has been the further humanisation of pets. Pets have shifted from living outdoors to sharing the house, bedroom and – for some – the bed itself. People expect their pets not to have diseases, bugs, rashes, or smells. Pets are companions, whether we are very young or old. They improve our physical health and help many with loneliness or depression.
Conclusion
All of the underlying trends are positive for the animal health sector, but the spread of zoonotic disease presents significant challenges. The animal health sector is wellpositioned to help provide for the health needs of billions of animals. Success will mean more sustainable food systems, improved public health, continued companionship from our pets and more.

Carel du Marchie Sarvaas
Carel du Marchie Sarvaas is Executive Director of HealthforAnimals, the global animal health association. HealthforAnimals represents the top 10 global companies (Boehringer Ingelheim, Ceva, Elanco, IDEXX Merck/ MSD, Phibro, Vétoquinol, Virbac, Zenoaq, Zoetis) and 29 national associations. Carel joined HealthforAnimals after holding the position of Director at EuropaBio, the Biotechnology Association. Prior to EuropaBio, Carel worked at international consultancies and think tanks in Brussels and Washington DC. He is Dutch national, married, has four children, and holds degrees from the University of Leiden and the Johns Hopkins University.

Volume 10 Issue 1 8 International Animal Health Journal REGULATORY & MARKETPLACE
Sales per product category (%) (2013–2021)
Burnout And Compassion Fatigue: What Vet Professionals Need To Know
Having recently read a young girl’s letter entitled ‘My Dream Job’ provides a good illustration of some of the underlying reasons for compassion fatigue in the veterinary industry. Her dream job is full of recognition and love where she can treat sick animals that she can’t bear to see suffer; where she can alleviate their illness with her own two hands, and where her job is her calling. Fast forward and this girl will be met with reactions such as “You are a vet? What a beautiful profession, I also wanted to be a vet.” Yet behind the curtain she will be one of 74% of veterinarians who are stressed and burnout if she works in the UK (BVA, 2020), and one of 50.2% feeling burnout or one of 58.9% feeling compassion fatigue if she works in the US (Ouedraogo et al., 2021).
Being a veterinary professional is one of the most fulfilling occupations. However, it comes with a lot of hardship which needs to be managed otherwise we find ourselves in a downward spiral. Not only is burnout dangerous for the individual, but it is also detrimental for the veterinary business and the whole profession. A recent study from the Cornell Centre for Veterinary Business and Entrepreneurship has found that workplace burnout is costing the veterinary industry $2 billion annually in the US (Neill et al., 2022). While it’s not all about the money of course, putting this very human issue into financial terms may make it more tangible for some. This high burnout rate is followed by difficulties of retaining and recruiting veterinary professionals into clinical practice, with 43.7% of vets in the UK actively thinking about leaving their
employment (Hagen et al., 2020). The most frequent reason, at 41.2%, for wanting to leave is due to poor work-life integration.
What Does Work-life Integration Have to Do with Burnout?
Work-life balance or work-life integration is typically defined as a state of well-being that can be maintained through effective management of multiple work and non-work related responsibilities, and with minimal stress related outcomes (Figure 1). In the Cornell study mentioned above, they defined burnout according to the World Health Organisation (WHO) as a syndrome “resulting from chronic workplace stress that has not been successfully managed”. While reasons for any outcome are often multifactorial and there is no one best linear solution to a problem, we could sum-up that to aid retention of happy and fulfilled vets in clinical practice, we need to primarily improve their work-life integration, which largely means avoiding stress related outcomes that may lead to burnout in the long run.

Compassion Fatigue – A Type of Burnout
Burnout can be classified into three types:
1. Overload burnout, where the person works harder than ever to the point of risking their health, feels obligated to work at an unsustainable pace, and often results in physical and mental exhaustion
2. Under-challenge burnout, where the person loses passion and joy for their work, is not being stimulated by the job, and is often accompanied by feelings of boredom and lack of learning opportunities by doing too little

International Animal Health Journal 9 www.international-animalhealth.com REGULATORY & MARKETPLACE
Figure 1
3. Neglect burnout, where the person loses a sense of purpose, finds it hard to stay engaged and is unable to keep up with the demands because they feel helpless in the case of challenge
Compassion fatigue is a form of overload burnout, but the two terms are not interchangeable. While vets suffering from burnout become physically, emotionally, and mentally exhausted due to work-related stressors, vets with compassion fatigue continue to give themselves to the job despite their emotional exhaustion and the resulting inability to continue to empathise. Compassion fatigue usually follows on from burnout and secondary trauma. The physical symptom of both are similar, for example, disturbed sleeping patterns, changes in appetite, and feelings of sickness. Emotionally, during a burnout a person can feel helpless, demotivated, dissatisfied, and detached from work, while a person experiencing compassion fatigue will primarily lose the ability to empathise and the ability to separate personal and professional life.
Why Do Vets Get Compassion Fatigue?
As the little girl’s letter at the beginning of this article demonstrates, the veterinary profession attracts individuals who are highly compassionate and caring. Veterinarians find a sense of satisfaction in helping and caring for others. This is both a blessing and a curse. While caregiving is a positive and a desirable trait, repeated exposure to negative and traumatic events, such as euthanasia and disease, can lead to compassion fatigue. In fact, compassion fatigue is often known as “secondary traumatic stress”. As two psychology scholars put it, “exposure to patients to clients experiencing trauma or distress can negatively impact professional’s mental and physical health, safety and wellbeing…[and] can impact standards of patient care, relationships with colleagues, or lead to more serious mental health conditions such as posttraumatic stress disorder, anxiety or depression” (Cocker and Joss, 2016).
How to Prevent and Recover from Compassion Fatigue?
Time – a friend or a foe? Time, or the lack of it, is the enemy for those who are experiencing compassion fatigue. To compensate for the lack of time, vets would eat their lunch while writing up their notes, or skip lunch altogether to phone their clients, or skip taking a break to check on inpatients. They skip on the very thing that would help them the most to recharge and continue productively – a break, exercise, nonwork activities, or time with friends. Self-care practices are key to managing compassion fatigue. Investing in taking care of yourself will contribute to building resilience on all levels –behavioural, emotional, spiritual, physical, and cognitive. The following are commonly stated areas to focus on:
• Connect with your co-workers and colleagues to support each other and remind yourself that you are not alone
• Build resilience through adequate sleep, healthy nutrition, physical exercise, and active relaxation
• Take time off for self-reflection and to build selfawareness, to identify what is important to you, and to engage is some self-care activities
• Create your own ‘work principles’, guidelines of personal integrity that can help you stay within the parameters of your personal values, then live and work within these principles
Commonly, veterinary professionals start with ‘compassion satisfaction’ (as we can see in the young girl’s letter) and overtime and due to various personal and organisational pressures it turns into ‘compassion fatigue’. Compassion satisfaction is the pleasure and feeling of fulfilment a person

feels when helping others. The good news is, it can be developed intentionally and can then act as a preventative measure for compassion fatigue. In human nurse research, factors that influence a nurse’s level of compassion satisfaction include work hours per shift, team dynamics, physical exercise and healthy food facilities at workplace, quality of social support and professional counselling, and longer duration in current position (Jarrad and Hammad, 2020).
How Can the Workplace Minimise the Likelihood of Burnout and Compassion Fatigue?
Burnout, such as compassion fatigue, affects not only the individual in terms of job satisfaction and health but also the workplace. The workplace may suffer from decreased productivity, quality of work, client satisfaction and increased staff turnover, all of which result in increased financial burden for the business as stated earlier. How to prevent burnout at a workplace can be largely classified into the following three subheadings:
Leadership Behaviour
• Relationship-orientated leadership behaviour
• Effective leaders that promote a culture of caring
• Recognition and reward system
• Feedback and debriefing
• Effective management training to provide efficient support
Workplace Technicalities
• Appropriate staffing
• Professional development
• Flexible working opportunities
• Monitoring of workload and schedules
• Clarity of job role and responsibilities
Psychological Conditions
• Effective communication, teamwork, equality
• Psychological safety
• Eliminating mistrust and blame culture
• Promoting autonomy and fairness
• Promoting work-life integration and work boundaries
The above list is only a brief outline, it is not exhaustive, and different clinics will need to focus on different areas. However, a good starting point is to be proactive. It is noteworthy that, despite being common, compassion fatigue is only one type of burnout, therefore it is useful to be aware of some of the other reasons for burnout and direct attention to the appropriate mitigation and prevention strategies necessary in your workplace. There are numerous studies that highlight the effect of workload on the mental wellbeing of veterinarians. These studies state working hours and ethical dilemmas as the major stressors in a veterinary workplace; with others including financial strain and client demands (Pohl et al., 2022). Whatever the reasons, both, developing personal coping strategies and organisational support are important to help create a burnout-free culture and help cope with work-related stress.
While quite a bleak picture of the veterinary profession was painted here, they are not alone in suffering from burnout. According to the Microsoft’s 2021 Work Trend Index, over half of the world’s workforce is overworked. Depending on the source, health and patient care professionals are typically quoted within the top three industries with the highest burnout rates, accompanied by the hospitality and manufacturing industries. Whatever the profession, ignoring
Volume 10 Issue 1 10 International Animal Health Journal REGULATORY & MARKETPLACE
employee burnout is a risky business, and with the level of burnout in the veterinary profession, it’s better to be prepared than needing to put out fires. We can all relate to the age old saying: “prevention is better than cure”. Here too, prevention of burnout by having a proactive mindset and strong business acumen is a good first steppingstone.
REFERENCES
1. BVA (2020) Report of the Voice snapshot survey on Covid-19: 6 months on from lockdown. Available at: https://www.bva. co.uk/media/3780/voice-covid-survey-2020-results.pdf
(Accessed 9 January 2023).
2. Cocker, F. and Joss, N. (2016) Compassion Fatigue among Healthcare, Emergency and Community Service Workers: A Systematic Review. Int J Environ Res Public Health. 13(6): 618. doi:10.3390/ijerph13060618
3. Hagen, J. R., Weller, R., Mair T. S., Kinnison, T. (2020) Investigation of factors affecting recruitment and retention in the UK veterinary profession. Vet Rec. 187(9):354. doi:10.1136/vr.106044
4. Jarrad, R. A. and Hammad, S. (2020) Oncology nurses’ compassion fatigue, burnout and compassion satisfaction. Annals of General Psychiatry. 19(22). doi:doi.org/10.1186/ s12991-020-00272-9
5. Neill, C. L., Hansen, C. R., Salois, M. (2022) The Economic Cost
of Burnout in Veterinary Medicine. Front Vet Sci. 9(81404) doi:10.3389/fvets.2022.814104
6. Ouedraogo, F. B., Lefebvre, S. L., Hansen, C. R., Brorsen, B. W., (2021) Compassion satisfaction, burnout, and secondary traumatic stress among full-time veterinarians in the United States (20162018). J Am Vet Med Assoc. 258(11):1259-1270. doi:10.2460/ javma.258.11.1259.

7. Pohl, R., Botscharow, J., Bockelmann, I., Thielmann, B. (2022) Stress and strain among veterinarians: a scoping review. Ir Vet J. 75(15). doi:10.1186/s13620-022-00220-x

8. Sirgy, M., K. and Lee, DJ (2018) Work-Life Balance: an Integrative Review. Applied Research in Quality of Life. 13: 229-254. doi:https://doi.org/10.1007/s11482-017-9509-8
Dr. Silvia Janská
Dr. Silvia Janská BSc(Hons) MSc BVetMed
PgCertVBM MRCVS is a veterinarian and a business consultant, CEO of Flexee, Vice President of SPVS, and an advisory board chair for The Webinar Vet.

International Animal Health Journal 11 www.international-animalhealth.com REGULATORY & MARKETPLACE

Volume 10 Issue 1 12 International Animal Health Journal Control of fleas, tic k s , m i t e s , lungworms , roundworms , hookworm and For more information please contact your local Vetoquinol Representative. Felpreva® is a veterinary medicine and contains tigolaner, emodepside and praziquantel. Product information is available at https://www.ema.europa.eu/en/medicines/veterinary/EPAR/felpreva. Agency product number: EMEA/V/C/005464. Marketing authorisation holder: Vetoquinol SA. For more details please contact your local Vetoquinol representative: www.vetoquinol.com/en


International Animal Health Journal 13 www.international-animalhealth.com tapeworms Copyright © 2022, Vetoquinol SA. All rights reserved. Vetoquinol, 37 Rue de la Victoire, 75009, Paris, France THREE MONTHS’ parasite treatment & protection in a SINGLE SPOT-ON . Active for up to 13 weeks against fleas & ticks. NEW ACTIVE: Tigolaner breakthrough IN PARASITE PROTECTION FOR CATS A
Getting Your Product Approved – Regulatory Considerations for the EU and US

When developing a veterinary product for the global market, differences in product categorisation and applicable regulations can be difficult to navigate. The final article in the “From Molecule to Market” series provides an overview of the regulation for animal health products in the European Union (EU) and the United States (US).
Regulatory Agencies
European Union
In the EU there are different agencies responsible for regulation of veterinary medicinal products, immunologicals, biologicals, feed and feed additives.
The European Medicines Agency (EMA) is the agency of the European Union (EU) which regulates all types of Veterinary Medicinal Products (VMPs). Responsibility for scientific evaluation, supervision and safety monitoring is with the Committee for Medicinal Products for Veterinary Use (CVMP). The CVMP consists of representatives of the individual national EU and EEA (European Economic Area) authorities as well as additional experts and plays an important role during the initial assessment of marketing authorisation applications. After evaluation by CVMP the European Commission (EC) issues the marketing authorisation.
The national competent authorities of the EU Member States regulate the products authorised in the individual countries.
For feed and feed additives the European Food Safety Authority (EFSA) is responsible for the scientific assessment.
Biozides are regulated by the European Chemical Agency (ECHA).
United States
In the US, there are three agencies which regulate veterinary products: FDA, USDA, and EPA.
Veterinary drugs are regulated by the Food and Drug Administration (FDA) Center for Veterinary Medicine (CVM). The Federal Food, Drug, and Cosmetic Act gives FDA its authority to regulate animal drugs. From the Act, regulations are codified in title 21 of the Code of Federal Regulations (21CFR). Guidance documents (GFIs) provide CVM’s current thinking and interpretation on how to comply with the regulations.
Veterinary biologics are regulated by the United States Department of Agriculture Center for Veterinary Biologics (USDA-CVB). The Virus-Serum-Toxin Act gives USDA its authority to regulate animal biologics. From the Act, regulations are codified in title 9 of the CFR (9CFR). Guidance documents (VSMs) provide CVB’s current thinking and interpretation on how to comply with the regulations.
Certain topical veterinary products that kill pests without systemic action are regulated by the Environmental Protection Agency (EPA). This type of product is beyond the scope of this article.
Categorisation of Products European Union
Before the new Regulation (EU) 2019/6 came into effect, products had been classified either as ‘Veterinary Medicinal Products’ or ‘Immunological Veterinary Medicinal Products’. The new Regulation introduced the definition of biological products and biological substances for the first time. Consequently, a new product categorisation was implemented.
Products are therefore now classified as: ‘Veterinary Medicinal Products’ other than biological veterinary medicinal products and ‘Biological Veterinary Medicinal Products’. Biological products are further divided into: ‘Biological Veterinary Medicinal Products’ other than Immunologicals (i.e., Biologicals) and ‘Immunological Veterinary Medicinal Products’.
This product classification is based on the nature of the active substance (biological or chemical), the mode of action, and the intended claim. Because it is often difficult to differentiate between ‘immunological’ and ‘biological nonimmunological’ products, it is possible to discuss the correct categorization with EMA or the national competent authority upfront.
United States
CVM regulates animal drugs, which are defined as: “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of animal disease if the primary mechanism of action is not immunological or is undefined (unknown);” and “articles intended to solely affect the structure or function of the body of animals.”
Conversely, the USDA regulates veterinary biologics, which are defined as products: “intended for use to diagnose, cure, mitigate, treat, or prevent disease in animals and work primarily through an immune process” which includes “the direct stimulation, supplementation, enhancement, or modulation of the immune system or immune response.”

For products that do not clearly fall into either category, a Memorandum of Understanding (MOU 225-05-7000) between USDA and CVM establishes a committee with a liaison from each agency to coordinate issues concerning regulatory responsibilities.
Overview of the Regulatory Processes European Union

In the EU, different authorisation procedures are possible. One option is the Centralised Procedure (CP) via EMA, which leads to a single marketing authorisation (MA) valid throughout the EU and EEA. The CP is mandatory for some products e.g., products containing new active substances.
Another option is the Decentralised Procedure (DCP), where one Reference Member State (RMS) will be selected which carries out the scientific assessment. According to the countries in which the product is intended to be marketed, other Concerned Member States (CMS) will be selected by the applicant. At the end of a DCP, the applicant will receive an individual, national authorisation in each of the selected Member States (MS). A single national authorisation procedure
Volume 10 Issue 1 14 International Animal Health Journal REGULATORY & MARKETPLACE
with only one member state is also possible via the national procedure. Additional authorisations in other MS may be added later by a Mutual Recognition Procedure (MRP). The data requirements are the same for all procedures.
The time from submission to authorisation takes around one and a half years for all procedure types without extraordinary delays. The exact timelines for the centralized procedure are described in the table below.
United States - CVM
To start the regulatory process for a new animal drug product, an Investigational New Animal Drug (INAD) file needs to be opened with the CVM Office of New Animal Drug Evaluation (ONADE). All regulatory actions are conducted and submitted under the INAD, which allows the Sponsor to legally conduct clinical studies with an unapproved drug. The Sponsor should establish a series of meetings (pre-submission conferences) with CVM to agree on the pivotal requirements needed for approval of the drug product. Additional meetings may be
EU (EMA; centralised)
Submission Type Dossier in VNees structure
scheduled throughout the drug development process. Pivotal study protocols should be submitted for CVM concurrence before studies are conducted.
Each Technical Section is submitted independently for a phased review using the e-Submitter and the Electronic Submissions Gateway systems. Phased review allows each Technical Section to proceed through the review process as a stand-alone submission. This allows the Sponsor to respond to CVM concerns about one Technical Section while the other Technical Sections are still progressing. At the end of the review cycle, CVM issues a complete letter when all requirements are satisfied, or an incomplete letter with further questions. A response to an incomplete letter must be made to re-activate the Technical Section, which starts a new review cycle.
When all required Technical Section Complete Letters are received, an Administrative New Animal Drug Application (NADA) is submitted to CVM to complete the approval process and assign an NADA number. The CVM Office of Surveillance
US (FDA-CVM)
Technical Sections and Administrative NADA application
Submission components
Part 1 - Administrative (incl. SPC, Labelling, Product Leaflet, Critical Expert Reports and Certificates)
Part 2 – Quality (CMC)
Part 3 – Safety
Part 4 – Efficacy
210 days – clock stop at Day 120 for List of Questions, and at Day 180 for List of Outstanding Issues. CVMP opinion issued at day 210
European Commission Decision to grant or refuse a MA within 67 days of receipt of CVMP opinion.
Application Fee Actual fee set by EMA. From 77 900€ (Immunologicals) to 156 700€ in 2023.
5 Major Technical Sections: CMC, Target Animal Safety, Effectiveness, Environmental Impact, Human Food Safety (if applicable)
2 Minor Technical Sections: Labeling and All Other Information (AOI)
Administrative NADA submitted once all Technical Sections are completed
180 days for each major technical section review; 90 days for a shortened review; each Technical Section submitted when all data is available
Each section receives a ‘complete’ or ‘incomplete’ response

60 days for Administrative NADA
Set by Animal Drug User Fee Act (ADUFA); rates change annually; currently $659,364 USD for FY2023
Public Summary European Public Assessment Report (EPAR) Freedom of Information (FOI) Summary
Raw data requirements
Protocol Review
Agency Interactions
Only required upon request.
Not an EMA process.
Scientific Advice at EMA. Fee ranges from 15 400€ to 46 900€ depending on type of questions. Possibility for follow-up request (fee required). Possibility for pre-submission and ITF (Innovation Task Force) meetings.
EMA vs. FDA-CVM Comparison table
Required for all pivotal studies; original raw data copies
Not required, but gaining protocol concurrence is strongly encouraged before conducting pivotal studies
No fees for pre-submission conferences (meetings); – no fee; can have numerous meetings throughout course of development
International Animal Health Journal 15 www.international-animalhealth.com REGULATORY & MARKETPLACE
Registration nomenclature Marketing Authorisation Approval Review times
and Compliance (OS&C) monitors drug products after approval and marketing.
United States – USDA
A development plan is submitted to USDA to begin the regulatory process for a new animal biologic product. The development plan allows for optional pre-submission meetings to discuss the product and assess what data and/ or testing will be required for the Product License Application.
The application for a US Veterinary Biological Product License (APHIS Form 2003) is an evolving document which is continuously updated with new information as it becomes available during the application process. Information present in the Product License application includes: the Outline of Production and Special Outlines as applicable, Master Seed and Cell Reports, Summary Information Formats, supporting data, as well as protocols for studies of host animal immunogenicity /efficacy /safety and other required animal testing. It is strongly encouraged that applicants have CVB review protocols prior to starting a study.

The firm is responsible for conducting and documenting studies, and assuring they were performed per protocol. The USDA reviewer is responsible for assuring the submitted material meets the regulatory requirements to obtain a product license. The applicant must have an approved Veterinary Biologics Establishment license in addition to the Veterinary Biologics Product License. For a new applicant, product license and establishment license review occur concurrently.
Limited Market – EU Regulation (EU) 2019/6 introduced the legal basis for granting marketing authorisations for limited market products and defines the conditions. To benefit from reduced data requirements for safety and efficacy, the applicant must provide evidence that the product falls under the definition.
According to Article 4(29) ‘limited market’ means:
• Veterinary medicinal products for the treatment or prevention of diseases that occur infrequently or in limited geographic areas; - or -

• Veterinary medicinal products for animal species other than cattle, sheep for meat production, pigs, chickens, dogs, and cats.
To evaluate the eligibility, applicants need to address two questions as outlined in Article 23.
Is the Product Intended for a Limited Market as Defined in Article 4 (29) and Art. 23 (1) b of the Regulation?
Products intended for limited market targets diseases that occur infrequently or in limited geographical areas. The decision whether a product falls into this category is based on the estimated potential size of the market applying the following formula:
Estimated potential size of the market % =
total annual number of animals potentially treated x 100 EU (EEA) target species population
EEA = European Economic Area
There are many factors which influence the potential market size (e.g., number of potential animals, treatment course, etc.). This variability highlights the need for case-by-
case decisions and the difficulty for an applicant to predict the results.
Does the Benefit of the Availability on the Market Outweigh the Risk that Certain Documentation has not Been Provided in a Dossier (Art. 23 (1) a)?
The benefit is proposed to be accepted if the product is intended to treat a serious or life-threatening disease/ condition or addresses an ‘unmet medical need’. Serious or life-threatening illness is defined to be associated with morbidity that has substantial impact or is associated with mortality, is zoonotic (except antimicrobials and parasiticides) or has the potential to cause significant economic impact for individual producers.
The term ‘unmet medical need’ means a condition for which no satisfactory method of diagnosis, prevention or treatment in the Union exists or if such methods exist, for which the product will be of major therapeutic advantage. It has to be shown that either no available therapy exists for the same intended use, or the new product is expected to be safer, more effective, or clinically superior.
The MA for a limited market under Article 23 is valid for five years and may either be, based on a re-examination, prolonged for further periods of five years, or upgraded to an unlimited full MA when providing the missing information on safety and efficacy.
As a consequence, a faster market access can be achieved. For any product, it will be a case by case decision.
Conditional Approval - US
Conditional approval allows a Sponsor to legally market and sell their product prior to establishing substantial evidence of effectiveness. The Effectiveness Technical Section for a conditionally approved product is called “Reasonable Expectation of Effectiveness” and can often be demonstrated through pilot work in the target species. All other major technical sections must be completed to the same standard as for full approval. The indication for conditional approval should be the same as that pursued for full approval. A conditional approval is for one year and can be renewed annually, up to five years, while the Sponsor continues to work towards a full approval. Extra-label use of conditionally approved products is not permitted.
There are two pathways for a conditional approval: MUMS and Expanded Conditional Approval.
MUMS (Minor Use or Minor Species)
A MUMS determination requires that either the incidence of the desired condition represents a minor use in a major
Volume 10 Issue 1 16 International Animal Health Journal REGULATORY & MARKETPLACE
species, (i.e., less than 50,000 horses, 80,000 dogs, 150,000 cats, 310,000 cattle, 1.45M pigs, 14M turkeys, 72M chickens), or represents a minor species. A Sponsor can submit their request for conditional approval eligibility when submitting for their minor use determination.


Expanded Conditional Approval
Expanded conditional approval incentivises drug development for certain major uses in major species where demonstration of effectiveness would be particularly challenging. Eligibility criteria for expanded conditional approval requires that the new animal drug is intended to: Treat a serious or life-threatening disease or condition – OR - address an unmet animal or human health need; – AND –A demonstration of effectiveness would require a complex or particularly difficult study or studies.
Additional detail for these requirements is outlined in CVM Guidance for Industry #261. A formal request for eligibility must be submitted to CVM under an INAD early in development.
Conclusion
There are many similarities but also differences when registering new animal health products in the EU and US. While general data requirements to demonstrate safety, effectiveness, and quality are similar, the regulatory processes can vary significantly. The most notable differences include the level of interaction with the regulatory agency during the development process, differences in EU authorisation procedures (e.g., centralized versus decentralized), and CVM’s phased submission process. However, opportunities exist to leverage similarities across agencies to develop a streamlined development approach, which can help save a sponsor time and money. We highly recommend that you consult with Klifovet/Argenta early in the drug development process to achieve the most effective plan to product approval.
Dr. Caroline Vanino is a veterinarian with over 10 years of experience in the animal health industry. She is the Regulatory Affairs Group Leader for the pharmaceutical products at Argenta/Klifovet with responsibilities that include clinical, technical, and regulatory consulting for animal health sponsors.
Dr. Heather Sedlacek is a veterinarian with over 26 years of clinical and animal health industry experience in a variety of roles. She is the Regulatory Affairs Manager at Argenta Clinical R&D with responsibilities that include pre-clinical, clinical, and regulatory consulting for animal health sponsors.
Dr. Aaron Johnson is a veterinarian with over 18 years of clinical and animal health industry experience. He is the Senior Regulatory Manager at Argenta Clinical R&D and provides pre-clinical, clinical, and regulatory consulting for animal health sponsors. He is the Immediate Past President of the Indiana Veterinary Medical Association.
Marlene Burisch is a pharmacist with an experience of 8 years in the animal health industry. She is a Regulatory Affairs Manager at Klifovet GmbH (part of the Argenta Group) with responsibilities that include regulatory consulting for animal health sponsors.
International Animal Health Journal 17 www.international-animalhealth.com
Dr. Caroline Vanino
Dr. Heather Sedlacek
Dr. Aaron Johnson
Marlene Burisch
REGULATORY & MARKETPLACE
The Green Discussion Forum



In 2020, we decided that the theme for our year would be regenerating the veterinary world. I had been running The Webinar Vet for a decade and begun the online revolution for vets, which had taken millions of miles off the roads over the last decade. However, we wanted to do more to show leadership in the veterinary industry. As a digital educational business, it is arguably simpler to be sustainable than a large pharma company. However, we calculated our carbon footprint; offset double what we produced during the pandemic year; started a veterinary sustainability podcast and planted a wildflower meadow at the Liverpool Science Park along with encouraging our landlord to embrace renewable energy providers.
Part of beginning a sustainability journey is that the further you get down the road, the more you realise more can still be done. Many larger companies have not yet started, because they don’t know how to start. Small steps are enough at the start to build the company owner’s confidence.
Part of this year’s journey was to try to facilitate bringing the veterinary industry together to see how we can collaboratively move faster into a more sustainable future. I was at COP26 and I realised how government, industry and individuals need to work closely together to achieve the environmental targets that have been set for this decisive decade. Carbon seems to be the main focus that everyone concentrates on but it is not very holistic. When I decided to organise the forum, I wanted to look at resource use and biodiversity as well as climate change and carbon.
It's very hard to get a lot of big companies in the room initially. Companies want to know who else is turning up and, inevitably, there is some distrust in being too open in sharing where they are in their sustainability journey. From the start of the process, I made it clear that this would be a safe place to discuss topics and that Chatham House rules would apply. Sustainability and regeneration are such big topics and companies are learning as they go. Every company that is taking these topics seriously have strengths and weaknesses in its approaches and I believe we will learn quicker if we work together. Collegiality and collaboration are more important than competing against each other in the environmental arena.
This was the background that led me, as a vet in an independent business, to start bringing companies in the veterinary space together to collaborate in this essential area of veterinary regeneration and sustainability. This journey will not be finished next year. It is a long journey, but one with a clear goal of reducing carbon production by 50%; creating a more circular, less wasteful economy and having 30% of the land of high biodiverse value by 2030 (30 by 30).
The Webinar Vet opened for business 12 years ago and is well-trusted in the industry. It is set apart from the pharmaceutical, feed and corporate veterinary practice groups and can, hopefully, act as a facilitator. It’s possible that an association can also take this role. At the inaugural
forum, we were incredibly fortunate to have our regulator, the Royal College of Veterinary Surgeons, present as well as the British Veterinary Association, The British Small Animal Veterinary Association, The British Cattle Veterinary Association and VetSustain. This, obviously, enhanced the credibility of the forum.
The Webinar Vet has never held a physical conference. Indeed, during the pandemic, we helped over 50 associations and companies take their events online due to our decadelong experience of running online events. However, we decided that a green discussion forum had to take place at a physical venue and so we decided to hold it at Brockholes Nature Reserve just outside Preston. Holding it at a nature reserve would help to ground us and show clearly how protecting the environment was essential. The centre at Brockholes is an architectural masterpiece designed by the esteemed architect, Adam Khan. It floats on the lake and rises and falls as the lake depth varies due to floods and drought. As a result, its design has protected it from being inundated on several occasions There is much to learn from the design of the visitor centre which can help with the design of buildings on floodplains. It is a truly stunning creation using wood extensively to give it a natural, warm feeling.
We were keen to bring the representatives of the companies together to facilitate networking and discussion and the content of the forum was mainly created by the delegates. Although, we had a keynote speaker for every session of the two-day forum which also included an afternoon working in the reserve to rake cuttings off one of the wildflower meadows or removing invasive Crassula helmsii or New Zealand pygmyweed from the main lake. This session was characterised by the number of breaks that delegates took to chew the cud with fellow delegates and carefully remove the myriads of small toads and frogs that we found on the meadow. Everybody clearly enjoyed working on the land!
After much planning and hard work, we managed to get 40 individuals in the room, representing 24 companies and associations. Inaugural meetings are always tricky. Some companies are more willing to take a risk on a new event in the calendar whilst others wait to see if it fails or not. All industries should be attempting these collaborations. It is better to attempt such an event that fails rather than not to try. I must admit that I was worried and a bit stressed before the event and decided that I would mainly walk to the event as well as using public transport so that I could immerse myself in the beautiful natural landscape and calm myself before the event. This was a very good move before the forum began.
Over the course of two days, we spent time listening to each other and the keynote speakers as well as discussing the important questions in small groups. Our keynote speakers were dialled in virtually to keep our carbon footprint as low as possible. We recorded everybody’s journey to the congress and offset the carbon emitted to make it a carbonneutral event. It was also not for profit with several thousand pounds being donated to the Lancashire Wildlife Trust which own Brockholes.
Volume 10 Issue 1 18 International Animal Health Journal REGULATORY & MARKETPLACE
Climate Change and Greenhouse Gases
Our first main keynote speaker was Juliet Davenport, the founder of Good Energy. Juliet spoke about the ongoing energy crisis which was partly due to the slow uptake of renewable energy. She also spoke about the journey she undertook to start Good Energy at the beginning of the millennium when no one else was really interested in the space. Insulation must also be a part of our journey towards net zero as this would massively reduce our energy needs. This must become a key part of every individual’s and company’s environmental commitment.
One of our delegates had done magnificent work moving their company’s car fleet away from diesel and petrol to hybrids and electric cars. Again, because of government tax reforms, this is not only good for the environment but also for employees’ pockets. She explained the benefit in kind advantages of these schemes. I am a committed environmentalist but if we can encourage a change of behaviour using money as the carrot this will guarantee greater uptake from people who do not understand the environmental issues.
One of our delegates was a chief sustainability officer at a leading insurance company. Scope 3 is a challenge for a lot of bigger companies. What are their suppliers doing on their sustainability journey? This will affect the emissions that another company is indirectly responsible for. If they did not need their materials, then they wouldn’t be produced and lower concentrations or fewer greenhouse gases would be emitted. Many companies have not truly grasped the importance of scope 3 to calculate their own carbon footprint but also to encourage their suppliers to begin the journey to net zero. The general consensus was that Scope 3 can be ignored until the company’s own emissions are quantified and can be lowered and offset. The next stage of the journey to deal with scope 3 is more complicated but many important skills will have been learnt dealing with scope 1 and 2 emissions that will make dealing with scope 3 easier. The veterinary industry’s journey to a net zero future will be a series of stepping stones and we need to move from one stone to the next to reach our net zero destination.
The delegates then broke into small groups and discussed some circulated questions:
• What is your company doing to reduce its carbon usage in the next decade? What is the target?

• Is it time for the industry to follow the World Veterinary Association’s lead and declare a climate emergency or do we need to work closely together to create a set of standards?
• What are your thoughts about offsetting against carbon?
• How can we produce our own energy as businesses?
• The Amazon rainforest is now emitting carbon. How does that make you feel?

• How can we as an industry lobby government to move faster?
• Are practice groups discussing Scope 3 with you?
The discussion was so important to allow people to get to know each other as sustainability leads in their business but also to listen deeply and learn from each other. To facilitate the listening the symbol for listening in Chinese was displayed. This shows that listening involves the ears but also the eyes, heart and indeed the whole being. Delegates were encouraged not to interrupt other delegates who were speaking. A stone was used which every person speaking had to hold and only relinquish when they had finished speaking. This system was not abused by dominant personalities refusing to give the stone away!
The rest of the first day was spent networking and working on conservation projects on the nature reserve. At the end of the day, we returned to our hotel which was only 1 mile away to get spruced up. Many of the delegates walked through the reserve to get back to the hotel. We returned to the nature reserve in the evening to enjoy some fine food which had been locally sourced and was of good provenance along with some fine Italian organic wines from Vintage Roots which everyone enjoyed. Tom Burditt was our guest of honour and the CEO of the Lancashire Wildlife Trust. I’d asked him to speak about natural capital for about 10–15 minutes but he spoke for 55 minutes and had everybody spellbound.
International Animal Health Journal 19 www.international-animalhealth.com REGULATORY & MARKETPLACE
So many people spoke favourably about his passion for the topic. If we obsess too much about carbon, we forget about the inherent value of habitats already there which are biodiverse and have no carbon value but natural capital.
Many of the delegates walked to the reserve on the second day to see lovely views of deer and waterbirds by the River Ribble and on Brockholes Lake.
Our second keynote speaker was Professor David Goulson, author of The Silent Earth which bemoans the massive reduction of insect life which also affects their predators like spotted flycatchers whose populations have crashed in the UK. There are many causes for this precipitous loss, however, the advent of very potent insecticides since the banning of DDT has certainly been a factor and the profession now worried that some of those insecticides used to kill fleas and other problematic insects may also be getting washed into streams and affecting aquatic life.
The day continued with talks on large and small animal parasiticide usage. A veterinary parasitologist described his work on a group of farms where reduced parasiticide usage and increased lungworm vaccine usage had led to healthier, more productive cattle. Justine Shotton, BVA president, spoke about the need to reduce preventive parasiticide usage in dogs and cats to prevent contamination of the environment with medicines. This is a problem across all medicine types including antibiotics and also across the various medical professions.
Sue Paterson from the Royal College of Veterinary Surgeons moved away from this topic to explain that the practice standards scheme run by the college now included an award for sustainability to encourage more practices to think positively about environmental concerns.
Some of the questions discussed in the small groups were as follows:
Biodiversity
• How much do you think the veterinary industry is part of the solution or part of the problem?
• How can we encourage practices to make their spaces more biodiverse?
• Should vets be the only one to prescribe anthelmintics?
• How can we make sure that products are safe in the environment?
• Are accreditations like Investors in the Environment and Bcorp worthwhile?
We then broke for questions followed by lunch.
The final afternoon we discussed resource use in the profession. Jen Gale was our keynote speaker discussing resource use and the circular economy. She is a veterinary surgeon and famous as the author of “Sustainable-ish Living Guide.” She spent a year not buying anything new and also is famous for emptying her bin for the first time this year in August. The circular economy was discussed. There is so much work needing to be done in this area to improve individual, corporate and government responsibility. During this session, other veterinary businesses discussed some of the work they were doing with biodegradable packaging and targets to reduce the rubbish going to landfill. Having a well-thoughtthrough sustainable plan was also deemed to be essential.
The forum finished with a discussion amongst the small groups around resource use.
Resource Use
• What are your targets for recycling and using recycled material in packaging?

• Any new innovations we can talk about?
• Could the industry come together to encourage recycling in practices?

• Which areas should the profession be concentrating on?
• Does the industry need to develop take-back schemes for packaging?
What Should We Be Copying from Other Industries?
The forum was very well received, and we are intending to run this at nature reserves until 2030 to act as encouragers for the various veterinary companies attending to work towards targets around carbon, biodiversity and resource use. This is the decisive decade and it is so important that we work together as industries to tackle the ongoing environmental challenges.
Anthony Chadwick
Anthony Chadwick BVSc CertVD MRCVS qualified from Liverpool University in 1990 and received his certificate in Veterinary Dermatology in 1995 from the Royal College of Veterinary Surgeons. Anthony was involved in first opinion practice and dermatology referrals until 2016. In 2010 Anthony set up The Webinar Vet, the first online training platform for veterinarians and nurses, in an attempt to make veterinary education more accessible and affordable across the world. Since that time tens of thousands of veterinarians and nurses have accessed the platform from all over the world. The Webinar Vet’s first virtual conference took place in 2013. During the pandemic, The Webinar Vet helped to take over 40 veterinary meetings and conferences online including WVAC2020 and WCVD9. In 2021, Anthony took the business carbon negative, helping to stand by The Webinar Vet’s principles of being as sustainable as possible and delivering exceptional quality training, internationally via remote means. The Webinar Vet is an Investor in the Environment Green Accredited business. Web: www.thewebinarvet.com

Volume 10 Issue 1 20 International Animal Health Journal REGULATORY & MARKETPLACE
Lyophilization and Sterile Manufacturing

THE PCI WAY
Specialist expertise and experience in driving development and connecting commercialization of sterile and lyophilized life-changing therapies, delivering peace of mind for our customers.



•
•
OUR END-TO-END BIOLOGIC SOLUTIONS INCLUDE:

www.pci.com talkfuture@pci.com YOUR BRIDGE BETWEEN LIFE-CHANGING THERAPIES AND PATIENTS
• Sterile Formulation & Lyophilization Cycle Development
• Lyophilization and Sterile Fill-Finish Manufacturing (including Complex Formulations)
• Analytical Support
Clinical & Commercial Labeling & Packaging
Refrigerated/Frozen Storage & Distribution
Embolic Pneumonia Associated with Udder Cleft Dermatitis in Dutch Dairy Cows
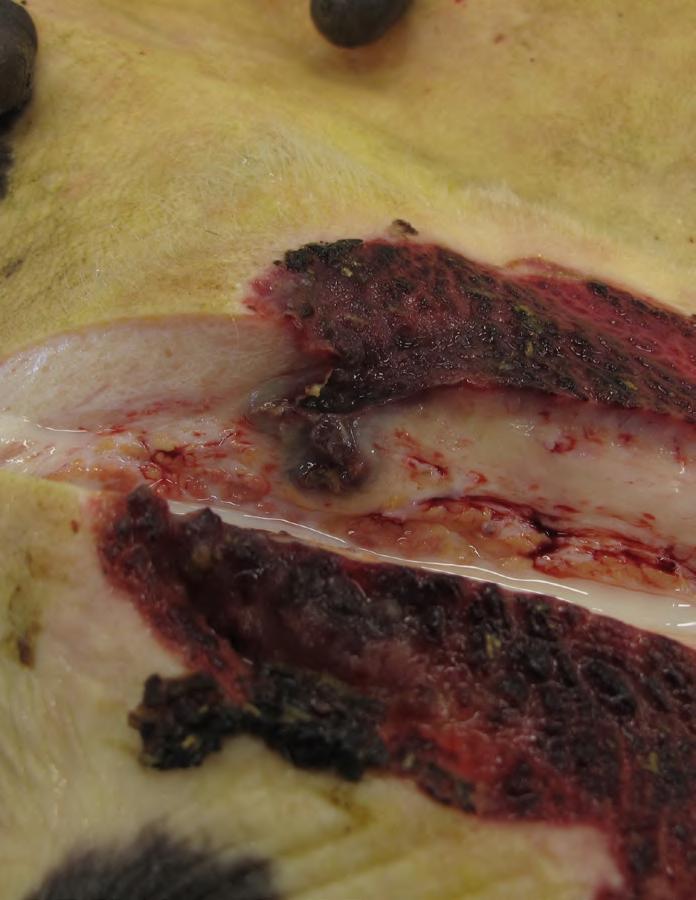
Udder cleft dermatitis (UCD; also bovine ulcerative mammary dermatitis or foul udder) is an inflammation of the udder skin and is most often located between the frontquarters and at the transition of the frontquarters and the abdominal wall. Cows with UCD may have increased risk of clinical mastitis, and associations between UCD and digital dermatitis have been suggested. The lesions can impair animal welfare, milk production, and milk quality and can lead to death and premature culling. Thus preventive measures are warranted, but in daily practice UCD is not always adequately detected by farmers and their cattle veterinarians.
Objective
The objective of this study was to investigate the prevalence of cows with complicated UCD on postmortem examination, associated with embolic pneumonia as most likely cause of death.
Material and Methods
Royal GD’s registered veterinary pathologists diagnosed 39 dairy cows from different Dutch dairy herds with mild to severe UCD from 1 January 2019–31 December 2019 based on postmortem examination in the necropsy room.

Histological examination and microscopy, as well as additional bacteriology testing was carried out in the GD laboratory. The pathology department is part of the GD laboratory, which is ISO 17025 accredited for a large number of laboratory tests and based on these accredited procedures the postmortem examinations were performed. Photographs were taken from the relevant lesions.
Results
From 20 of the 39 dairy cows (51%) with UCD on postmortem examination in 2019 it is most likely that a complicated UCD
was the fatal cause of death, due to the fact that no other causal factor for death was identified by the veterinary pathologists. Based on histology from the udder skin, a necrotic inflammation of the subcutaneous abdominal (milk) vein was observed with many bacteria involved, besides an ulcerative dermatitis of the skin. A comparable inflammation of the blood vessels in the lungs, with extension to the surrounding tissue, was observed, which is typical for embolic pneumonia. Bacteriological culture of the lung tissue revealed seven times Helcococcus spp., 12 times Trueperella pyogenes, and in two cases a mixed infection of these bacteria was isolated. In some cases E. coli, streptococci, and/or anaerobic bacteria were cultured from the diseased lung tissue. All the isolated bacteria can be considered as opportunistic bacteria.

Interestingly, in all of these 20 cases, the clinical signs during life of these cows (that were reported by the veterinarian on the accompanying submission form) were fever, respiratory distress, milk drop and death, despite antimicrobial treatment. In only one case the history taken by the veterinarian reported UCD.
From 19 of the 39 dairy cows (49%) with UCD on postmortem external examination in 2019 passed the necropsy room with uncomplicated UCD. In these cows ulcerative dermatitis was described, although there was no related embolic pneumonia observed, but other most likely causes that lead to death of these cows.
Conclusions
• 51% of the postmortem examination of cows resulted in the diagnosis of embolic pneumonia associated with UCD;
• In these cases, UCD lesions, which were apparently not important enough for the farmers and the veterinarians

Volume 10 Issue 1 22 International Animal Health Journal RESEARCH AND DEVELOPMENT
Multifocal embolic Necropurulent inflammation of the lung tissue (1a) in the context of a metastatic pneumonia as a result of Udder Cleft Dermatitis on the udder skin (1b) and on the cut surface of the lesion with vasculitis of the milk vein. (1c).
to report (or not even observed), are thought to have been the initiating source of infection;

• It is crucial that both farmers and veterinarians are made more aware of the possible severe consequences of udder cleft lesions and that they adopt strategies to allow early detection of UCD;

• More research is needed to find out which interventions can be initiated effectively in order to detect as early as possible.
Christian Scherpenzeel


Christian Scherpenzeel studied veterinary medicine at Ghent University and the Faculty of Veterinary Medicine, Utrecht University. After graduation in Utrecht, he started working as dairy veterinarian in a large dairy practice with specific interests in udder health and veterinary communication. After several years in dairy practice he went back to the veterinary school in Utrecht, where he was employed as lecturer/researcher at the Department of Farm Animal Health, Faculty of Veterinary Medicine, Utrecht University. Since 2011 he works as a dairy specialist at GD in Deventer, the Netherlands. In this job, his challenge is to integrate state-of-the-art scientific udder health expertise with the implementation of practical expertise, working closely together with dairy farmers and associated veterinarians. His research activities focus on udder health, antimicrobial resistance and social aspects of modern herd health management. Dr. Scherpenzeel published a number of peer-reviewed publications in the Journal of Dairy Science. He received his Ph.D. in Veterinary Medicine from Utrecht University and his thesis was focused on the effects of selective dry cow treatment in dairy cows for udder health, antimicrobial use, economics and social influences. Together with his team, carrying out the ‘Animal Health Approach’, the combination of scientific and practical expertise on modern dairy health management triggers a lot of farm visits each year and resulted in major experience in dairy consultancy and veterinary communication skills.
Klaas Peperkamp
In 1986 Klaas Peperkamp qualified as a veterinary surgeon at Utrecht University. After a few years in mixed practice he was employed as a veterinary laboratory diagnostician at the Animal Health Service (Royal GD) and specialized in pathology in collaboration with the Department of Veterinary Pathology, Utrecht University. In 1996 he was registered as a specialist by The Royal Netherlands Veterinary Association. Since that time he was engaged in large outbreaks of farm animal diseases like Classical Swine Fever, Blue Tongue virus disease, Q-fever and Schmallenberg virus disease. In the meantime his job comprised postmortem examination, histological and cytological examination of farm and companion animals and horses. He was also involved in the monitoring programme of animal diseases of Royal GD, which commenced in 2001. Klaas is (co-)author of various scientific articles with reference to his broad interest in veterinary pathology.
International Animal Health Journal 23 www.international-animalhealth.com RESEARCH AND DEVELOPMENT
Preventing and Reducing the Impact of Neonatal Diarrhea in Calves
Calf diarrhea, also known as calf scour, is one of the major causes of neonatal calf mortality, which has been estimated to cost farmers around 60 to 80 Euros per calf,1 with up to three in four calves affected.2 Calf scour can result in a decrease in performance in the shortterm, and in the longer-term impact future production. It also has significant welfare impacts through the pain of the disease, combined with the risk of mortality, as well as increasing the use of antimicrobials.
Causes of Calf Diarrhea

Neonatal diarrhea is most commonly caused by infectious agents or nutritional disturbances. The main infectious agents are Enterotoxigenic E. coli (F5 adhesin) or ETEC, bovine rotavirus, bovine coronavirus, cryptosporidiosis, Salmonella, coccidiosis and other minor pathogens. It is important to note that the outbreaks often include multiple pathogens. For practitioners, it is worth considering the impact of determining the pathogen(s) involved in the outbreak in terms of changes to treatment and prevention protocols, as well as public health-related issues (e.g. zoonosis potential, identification of a reportable/notifiable disease).
Treatment of Calf Diarrhea
One of the key components of a treatment plan for calf scour is rehydration, as mortalities caused by infectious pathogens are a result of the lack of fluid replacement. The estimation of the volume of fluid required in a calf is based on clinical signs, which estimate the proportion of fluid lost by the calf, for example a loss of 5% bodyweight for a 40 kg calf is 2 liters. Replacement of fluids can be delivered orally or by intravenous fluid therapy, depending on the severity of the clinical signs. Additionally, the use of non-steroidal anti-inflammatories (NSAIDs) is important for reducing the intestinal damage, caused by the response of the immune system to the presence of an antigen. As part of
the treatment, a single injection of meloxicam (Metacam®) was reported to improve all relevant clinical symptoms of diarrhea including fever, allowing a faster recovery. It was also shown to increase feed consumption, either milk or solid feed, and decrease discomfort that is associated with sickness behavior.3 Another important aspect is the isolation of sick calves, either as individuals or as a group. Biosecurity measures like the disinfection of tools, equipment and boots will also aid in reducing the transmission on the farm.
Ensuring the correct treatment protocol is in place is important for improving the success of treatment and reducing the degree of short and long-term production effects and overall mortality and morbidity.


Prevention is Key
The main enteric pathogens are found in adult bovine feces, which act as a reservoir of infection. Therefore, one of the key strategies to prevent the spread of calf diarrhea is hygiene through the reduction of exposure of calves to enteric pathogens in either the calving area or calf rearing area. Calves need to be removed from the source of infection and other calves that are shedding the pathogen because of current infection.
Another crucial measure for the prevention of calf scour on farm is excellent colostrum management to ensure the transfer of passive immunity from the dam to the calf. Colostrum, the first milk from the dam, is hugely important for neonatal calves, as they do not receive any maternal antibodies in utero from the dam and are entirely dependent on the innate immune system and passive transfer from maternal antibodies. Colostral immunoglobulins are pivotal for bridging the gap between specific local pathogenic antigens and the innate immune activities of the calf. A failure to receive sufficient colostral antibodies has been reported to increase the chance of mortality and diarrhea by 2.16 and 1.51 times respectively.4

Volume 10 Issue 1 24 International Animal Health Journal RESEARCH AND DEVELOPMENT
Figure 1. An overview of the components of bovine colostrum.
The general rules of thumb for optimum colostrum management are as follows:
• Volume: recommendations for volume of colostrum fed are 10–15% bodyweight in the first feed to ensure that the critical amount of immunoglobulin is consumed.
• Quality: the quality of the colostrum ensures that there is an adequate concentration of immunoglobulin in the colostrum fed.
• Timing: the recommendation is that calves receive colostrum within the first 2 to 6 hours of their life as there is an increase in intestinal wall permeability at birth to facilitate the absorption of immunoglobulin which decreases over time and has disappeared by 24 to 36 hours of life.
• Cleanliness: colostrum quality is impacted by cleanliness, as the immunoglobulin in the colostrum will bind any pathogens that are present in the colostrum, thus making them unavailable for absorption into the bloodstream.
One method of boosting colostrum antibodies to fecal pathogens is through vaccination of the dam during the dry period to promote the production of antibodies against rotavirus, E. coli and coronavirus. Vaccination of the dam is required as the vaccines have limited use in calves during the risk period (< 3 weeks of age) because of their immature immune system and the interference of maternally derived antibodies (MDA).5
Successful vaccination and excellent colostrum management are inseparably connected: The vaccination of the dam relies on delivery of around 10% of birthweight by volume of clean, high-quality colostrum within 2 to 6 hours of birth, as it will otherwise not have any impact.
Holistic Approach:
Vaccination and Lean Calf Management
Boehringer Ingelheim has just launched the first vaccine licensed for prevention of calf scour. It prevents diarrhea caused by rotavirus and E. coli F5 (K99) adhesin and reduces the incidence and severity of diarrhea caused by coronavirus. Viral shedding in calves infected by rotavirus and coronavirus was also reduced. This level of protection is unique among calf scour vaccines. Launched following extensive and recent registration studies, it is the only single-shot annual vaccine in the market that harnesses an oil-free adjuvant to boost the immune response to the three pathogens.
The level of protection was demonstrated through a series of laboratory studies with 40 cows vaccinated, and 20 cows as controls. Calves were challenged 12 hours after birth in the E. coli study and seven days after birth in the coronavirus and rotavirus studies to investigate onset of immunity, and after 14 days in the coronavirus study to assess duration. Calves were fed colostrum and transition milk from vaccinated or control dams in a supervised manner for seven days in all studies after birth, followed by experimental challenge.
Comprehensive monitoring of both the dams and calves was undertaken, including blood samples collected from calves on the day of birth, on the day of challenge and at the end of the study to assess the degree of passive immunisation received. Fecal samples were taken daily for seven days from the day of challenge in the viral challenge studies, to determine the amount of virus excreted.
The studies demonstrated unrivalled protection against calf scour in calves from vaccinated dams, achieving the unique claim of prevention against calf scour caused by bovine rotavirus and E. coli F5. A visual representation of the laboratory efficacy studies performed can be seen in figure 2.
The colors of each calf represent the outcome of the challenge with regards to calf scour. Normal, healthy feces and calves are represented by the grey calves. Yellow calves represent animals with signs of mild diarrhea. Orange calves represent the ones with moderate diarrhea and red calves represent cases with severe clinical signs. The black calves represent the animals that died as a result of diarrhea.
The graphic visually demonstrates the impact of protection by vaccination: Calves from vaccinated dams showed no scour following challenge for either the rotavirus or E.coli study. Whereas the control group showed six severe cases of diarrhea and three calves dying from it. For the seven day coronavirus challenge, there was also no scour in the calves from vaccinated dams, with only two calves showing mild symptoms of diarrhea in the 14 day challenge.

Further tests have shown significant increases in antibody levels in dams’ serum, colostrum, and calves’ serum, as well as reduced viral shedding.

To realise the full potential of the benefits of colostrum and the vaccine antibodies it contains, quality and quantity of colostrum and timing are crucial. This is where the lean process management approach comes in and has proven to be extremely valuable.
The history of lean management goes back to Japan in the 1980s and 1990s when a big automotive company outperformed its competitors by focusing on efficiency increase. The key to this success was “less of everything” and the lean management describes exactly that.
Boehringer Ingelheim provides a holistic lean approach to cattle farming through its training and support services. This is aimed at veterinarians to allow them to further improve calf management and ensure optimal gut and immune health, inspiring excellence in calf rearing.
Gerald Behrens, Global Head of Ruminants at Boehringer Ingelheim, says: “We are excited to have launched the first
International Animal Health Journal 25 www.international-animalhealth.com RESEARCH AND DEVELOPMENT
Figure 2. Visualisation of the outcome of the laboratory studies
vaccine in the market that prevents calf scour. Together with our lean management trainings for vets, it supports excellence in calf management, particularly around the vital feeding of colostrum to calves.”
As farming has changed towards increasing productivity, so has the role of the farmers shifted from craftspeople, working one on one with their animals and people, to managers and leaders. The use of lean management helps them optimise processes and hence achieve greater efficiency, increased consistency, reduced costs, and bigger production.
“Boehringer Ingelheim will provide guidance to veterinarians for the use of lean management tools to maximise the impact of our vaccine on farm and help farmers produce healthier and more productive calves”, Behrens concludes.


REFERENCES
1. ADAS UK Ltd. (2012) Economic impact of health and welfare issues in beef cattle and sheep in England (p. 35–36)
2. Johnson KF, Chancellor N, Burn CC, Wathes DC (2017) Vet Rec Open. 4(1):e000226.
3. Todd CG et al. (2010) Journal of Animal Science 88(6): 20192028
4. Raboisson D, Trillat P, Cahuzac C (2016) PLOS ONE 11(3): e0150452

5. Chase CC, Hurley DJ, Reber AJ (2008) Vet Clin North Am Food Anim Pract. 24(1): 87-104
Matt Yarnall
Matt Yarnall is the Senior Global Technical Manager for Ruminant vaccines at Boehringer Ingelheim. He holds a veterinary degree from The Royal (Dick) School of Veterinary Studies in Edinburgh, a Master’s degree in International Animal Health (University of Edinburgh) and a Master’s degree in Business Administration (Henley Business School). His interests are in Bovine Virusdiarrhoe (BVD), Bovine Respiratory Disease (BRD) and calf scour, and he enjoys engaging with vets, farmers, and industry leaders on all aspects of disease prevention.
Volume 10 Issue 1 26 International Animal Health Journal RESEARCH AND DEVELOPMENT
MEET NORDSON EFD
Where reliability meets advanced animal health packaging

Industry-Leading Drug Packaging & Delivery
Nordson EFD Dial-A-Dose™ and Posi-Dose® disposable dosing syringes allow users to tailor multiple, accurate doses based on treatment requirements.

• Molded from FDA-approved resins
• Wide range of nozzles for varied applications
• Oral, topical, intramammary, and rectal uses
• Self-venting feature prevents air entrapment
• Reliable, accurate, repeatable dosing
• Global customer support

International Animal Health Journal 27 www.international-animalhealth.com
EFD dosing syringes feature a unique ‘lead-in’ aid that facilitates high-speed filling.
nordsonefd.com/ReliableAH WATCH VIDEO
Vaccination Against Infectious Bronchitis in Chickens: An Evolving Challenge
Infectious Bronchitis (IB) is a highly contagious respiratory disease of poultry, that causes widespread economic losses within the industry. Vaccination is key to the effective control of IB, but IBV has an inherently high mutation rate, and the continual emergence of new serotypes makes this challenging. For successful IB control, alternatives to homologous vaccines are necessary and protectotyping is one such concept that has been suggested.
Infectious bronchitis is caused by the Infectious Bronchitis Virus (IBV), a Gammacoronoavirus belonging to the Coronaviridae family, and it was first reported in 1931.1 IBV affects the respiratory, urinary and reproductive systems and clinical signs depend on the tissue tropism of the infecting virus. Coughing, sneezing, tracheal rales and ocular discharge are commonly seen, and the disease tends to be more severe in young birds. In addition, mortality rates are higher in those strains with a renal tropism (nephropathogenic strains), or where secondary infection occurs.
Structure of the Infectious Bronchitis Virus
IBV is an enveloped, single stranded RNA virus. Three virusspecific proteins (the spike, membrane and nucleocapsid glycoproteins) are encoded by the IB viral genome, and it is the spike protein that is particularly important when it comes to both infectivity of the virus and vaccine efficacy.
The spike proteins are comprised of two glycopolypeptides, termed S1 and S2, and they project from the surface of the virus, enabling attachment to the cell surface and entry into host cells. The S1 glycoprotein determines serotype and enables binding of the virus to the host cell receptor. It contains a number of epitopes (or antigenic determinants) to which antibodies attach, and indeed it is known that most haemagglutination inhibition (HI) and serum neutralisation antibodies are directed against this glycoprotein.2
Serotype and the S1 Glycoprotein: An Evolving Threat
While biosecurity measures are important, control of IB is focused on vaccination. However, despite widespread use of vaccines dating back to the 1950s, the disease continues
to circulate widely, with a devastating impact on the poultry industry.
The lack of progress in disease control, is largely due to the inherently high mutation rate of IBV, which results in continuous genetic and antigenic changes of the circulating virus, and the emergence of new strains or serotypes.3 The majority of these genetic mutations occur in the S1 glycoprotein, and new serotypes can result from very few changes in the amino acid sequence. In the face of this constant change, successful control of IBV by vaccination is challenging.
Homologous Vaccines and Infectious Bronchitis Control
Having a homologous, serotype-specific vaccine to protect against different serotypes as they arise is the ideal scenario. With an S1 glycoprotein amino acid sequence homologous to the circulating serotype, such vaccines may give full protection. However, given the remarkable ability of IBV to mutate and the continual emergence of new serotypes, it would be impossible to develop such a vaccine against all new strains. Even if it was possible, many of these variant serotypes disappear from circulation and may no longer be of significance by the time a vaccine was ready for use.
Heterologous Vaccines and Cross Protection
No single vaccine can protect against all emerging serotypes; however it has been shown that a heterologous vaccine virus may provide cross protection. In other words, the vaccine confers protective immunity against an IB serotype that shares cross-reacting antigens with the vaccine virus. This is possible, because despite the frequent mutations in the S1 protein that result in new serotypes, the majority of the viral genome, including some of the S1 epitopes, remains unchanged. In addition, the S2 protein, which genetically is much more stable than S1, also has epitopes that may be involved in the production of protective antibodies.
The Protectotyping Concept
Due to the constantly changing picture in the field, an alternative to the use of homologous vaccines is necessary if IB control is to be successfully achieved. Protectotyping is one such concept that has been suggested.4

Volume 10 Issue 1 28 International Animal Health Journal RESEARCH AND DEVELOPMENT
Treatment group Vaccine regime Challenge (day 28) Challenge dose Number of birds Vaccination (day zero) Booster (day 14) T01 None None None 25 T02 None None IBV QX 4LOG10 EID50/bird 25 T03 IB-Var 2 IB-H120 25 T04 IB-Var 2 + IB-H120 None 25 T05 IB-Var 2 + IB-H120 IB-H120 25 Table 1
Protectotyping involves combining vaccine virus strains that are antigenically different into a vaccination programme. Typically, a classical strain is combined with a variant strain, producing a synergistic effect and an increased level of protection than if the two strains were used separately. Studies have shown that this approach, using two or more live attenuated vaccines, can successfully confer protection against heterologous serotypes.5
An element of trial-and-error may be used in the field to determine the optimum vaccine or combination of vaccines. However, identifying serotypes that are prevalent in the region and using these antigenically dominant strains in a vaccination programme will maximise the chances of success, as there is likely to be significant cross-protection between emerging serotypes.
A Protectotyping Case Study: Evaluation of IB-H120 and IB-Var 2 Infectious Bronchitis Vaccines

A study6 was carried out on 150 specific pathogen free (SPF) chickens to evaluate two live attenuated vaccines, MEVAC IB H-120 and MEVAC IB-Var 2 (Figure 1). Birds were allocated to one of five treatment groups (Table 1), and vaccines were administered by eye drop. Apart from control group T01, all birds were challenged on day 28 by administration of IBV strain QX by eyedrop.


A number of parameters were evaluated to assess vaccination efficacy:
• Serological Response to Vaccination: ELISA Testing
Mean IBV titers increased significantly in all vaccinated groups, with the highest titers in treatment groups T04 and T05 (Figure 2).
• Serological Response to Vaccination: Haemagglutination Inhibition (HI) Testing
Haemagglutination is a reaction that causes clumping of red blood cells in the presence of some viruses, including the IBV. The reaction is inhibited in the presence of HI antibodies. At days 14 and 28, all birds apart from those in the control groups had HI antibodies against the challenge virus, IBV QX.
• Viral Shedding: qPCR on Tracheal Swabs
There was a clear reduction in viral shedding for all treatment groups, showing that the vaccines were able to inhibit the replication of the IBV QX challenge virus in the respiratory tract. Treatment group T04 showing the strongest reduction of all groups (Figure 3).

• Ciliostasis Scoring
Tracheal cilia form part of the mucociliary apparatus, a defence mechanism to help protect the body against respiratory pathogens such as IBV. Loss of cilia negatively impacts the body’s natural defences against IBV and when assessing vaccinated birds, the lower the score, the better the level of protection provided by the vaccination regime.
Mean total ciliostasis scores were lowest in treatment group T05, vaccinated with a combination of IB-Var2 and IB-H120 at day zero and IB-H120 at day 14 and in addition 64 percent of birds in this group showed normal ciliary activity over both days on which scoring was carried out.
• Tracheal histopathology
Infection of the nasal and tracheal mucosa by IBV causes loss of ciliated epithelium3 and impaired mucociliary clearance.
Tracheal lesions were assessed according to set histological parameters, including loss of cilia, epithelial degeneration, epithelial atrophy, the presence of exudate and congestion of capillaries. The parameters were then
International Animal Health Journal 29 www.international-animalhealth.com RESEARCH AND DEVELOPMENT
Figure 1
Figure 2
Figure 3
scored from zero to three, based on severity and location, with a score of zero where no lesions were present, up to a maximum of three for severe or diffuse lesions. For vaccinated birds, the lowest scores were observed in the T05 group (Figure 4).
provide broad coverage through cross-protection. Greatest success is achieved where a classical strain (such as IBH120) is used in combination with a variant strain (such as IB-Var 2). This approach is likely to play a key role in the control of infectious bronchitis both now and in the future.
REFERENCES
1. Schalk, A.F. & Hawn, M.C. (1931) An apparently new respiratory disease of baby chicks. Journal of the American Veterinary Medical Association, 78, 41-422

2. Butcher, G.D., Shapiro, D. & Miles, R.D. University of Florida. Infectious Bronchitis Virus: Classical and Variant Strains (2018)
3. Cook, J. K., et al. (2012) The long view: 40 years of infectious bronchitis research. Avian Pathol. 41:239–250
4. Lohr, J.E. (1988) Differentiation of IBV strains. In E.F. Kaleta & U. Heffels-Redmann (Eds), Proceedings of the First International Symposium on Infectious Bronchitis, Rauischholzhausen , Germany (19-207)
5. Cook, J.K.A., et al. (1999) Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology, 28, 477-478
6. Protective efficacy of different vaccination regimes against challenge with IBV QX, Kemin Study report (2022)
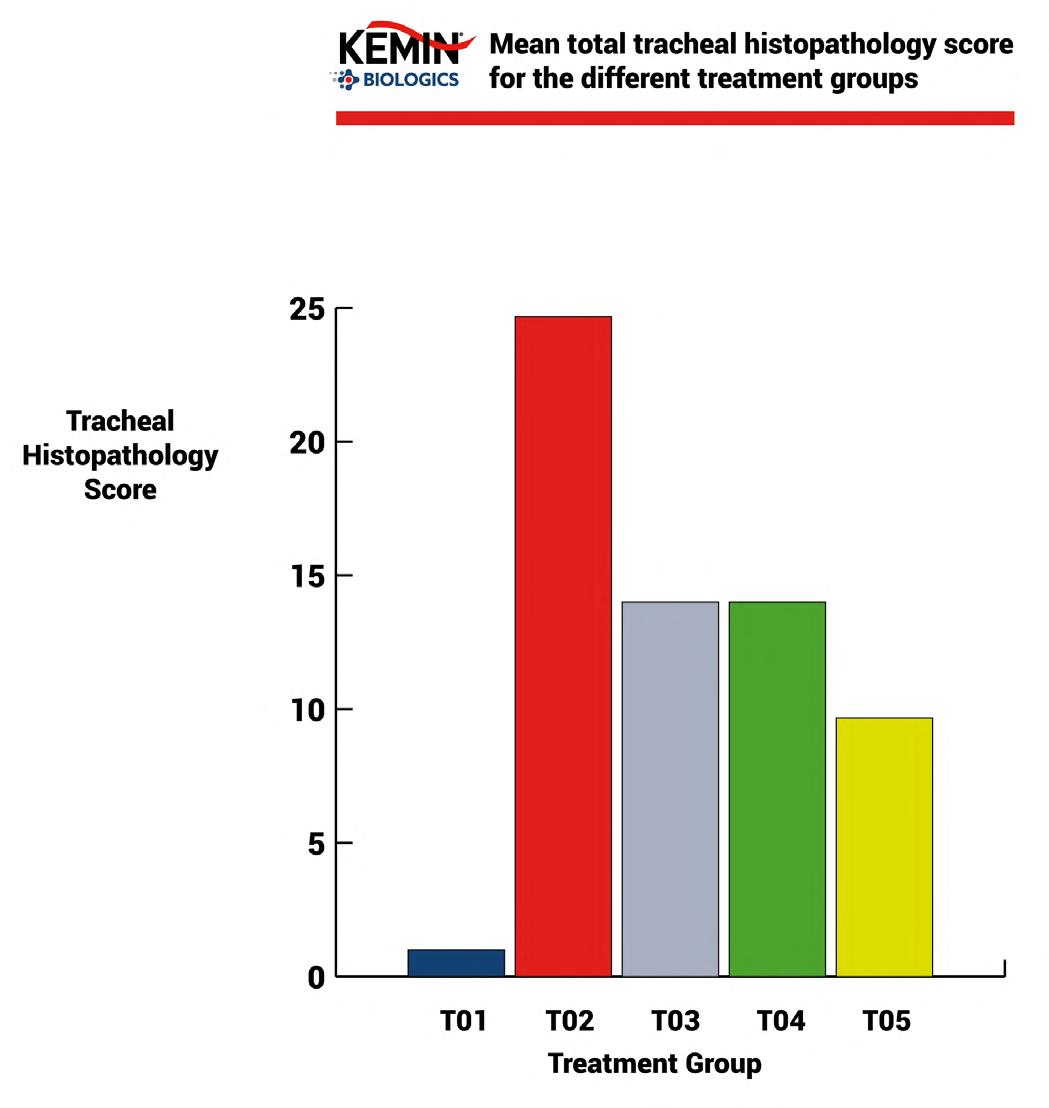
This study demonstrates the benefits of protectotyping. Even though IB QX was not included in the vaccine virus portfolio, a combination of variant (IB Var2) and classical (MEVAC H120) vaccine strains, provided good protection against challenge.
In Conclusion
No one combination of vaccines will provide complete protection against all IB serotypes. However, after identifying the serotypes that are prevalent in a region, protectotyping, in which antigenically different live attenuated virus strains are included in a vaccination programme, can be used to
Dr. Abdallah Makahleh, the Global Technical Support Manager for Kemin Biologics, has demonstrated expertise in the field of animal diseases, particularly in poultry. He began his journey with Elanco Animal Health in 2012, serving as a Technical Consultant for the Middle East region. In 2015, Abdallah joined Kemin, where he provided technical support for the company's products in the Middle East, North Africa and Pakistan. Currently, he works with Kemin Biologics to providing technical support globally.


Email: abdullah.makahleh@kemin.com

Volume 10 Issue 1 30 International Animal Health Journal RESEARCH AND DEVELOPMENT
Dr. Abdallah Makahleh
Figure 4
Media and Communications
IPI

Peer Reviewed, IPI looks into the best practice in outsourcing management for the Pharmaceutical and BioPharmaceutical industry.

www.international-pharma.com
JCS
Peer Reviewed, JCS provides you with the best practice guidelines for conducting global Clinical Trials. JCS is the specialist journal providing you with relevant articles which will help you to navigate emerging markets.

www.journalforclinicalstudies.com
IAHJ
Peer Reviewed, IAHJ looks into the entire outsourcing management of the Veterinary Drug, Veterinary Devices & Animal Food Development Industry.

www.international-animalhealth.com
IBI
Peer reviewed, IBI provides the biopharmaceutical industry with practical advice on managing bioprocessing and technology, upstream and downstream processing, manufacturing, regulations, formulation, scale-up/technology transfer, drug delivery, analytical testing and more.

www.international-biopharma.com

PNP
Pharma Nature Positive, is a platform for all stakeholders in this industry to influence decision making by regulators, governments, investors and other service providers to achieve Nature Net Positive Results. This journal will enable pharma the ability to choose the right services to attain this goal.

www.pharmanaturepositive.com
PHARMA’S DNA
Listen to industry experts on the latest in drug discovery, development, research, industry regulations and much more at Pharma,s DNA, the podcast channel by Senglobal Ltd., available on Sound Cloud, Spotify, iTunes and YouTube.
senglobalcoms.com
Establishment of Histomonas meleagridis Challenge Model in Turkeys: An Industry Perspective


Histomoniasis (blackhead disease, histomonosis, enterohepatitis) in turkeys, is caused by a protozoan parasite, Histomonas meleagridis. Currently, there are no commercial vaccines or prophylactic/therapeutic treatments available for blackhead disease. Due to the lack of vaccines, treatment and efficacious drugs, the turkey industry faces a huge economic impact due to mortality, morbidity and condemnations. In order to evaluate any vaccine or efficacious prophylactic/ therapeutic treatment, establishment of a robust challenge model is essential. This manuscript describes the establishment of a robust challenge model against recently isolated wild-type H. meleagridis isolates from USA.
Keywords
Histomonas meleagridis, challenge model, histomoniasis, histomonosis, blackhead disease, turkeys.
Histomonas meleagridis is the causative organism of histomoniasis (blackhead disease, histomonosis, enterohepatitis). H. meleagridis affects all gallinaceous birds including turkeys and chickens. Turkeys are highly susceptible to H. meleagridis compared to other gallinaceous birds. H. meleagridis can be directly introduced into the flock or may be carried by vectors such as Heterakis gallinarum, earthworms and insects. Fomites, sharing equipment and movement between farms may facilitate disease transmission. Once H. meleagridis enters the flock it is transmitted between the birds by cloacal route and fecal-oral transmission. Histomoniasis causes general clinical signs such as dullness, depression, ruffled feathers and drooping of wings. H. meleagridis affects ceca, induces typhlitis and cecal core formation (Figure 1) and then reaches liver by systemic circulation. It causes liver degeneration and necrotic foci (Figure 1). In later stages of the
disease, sulfur-yellow feces results in yellow staining around the vent (Figure 2). The clinical signs are usually seen one to two weeks after infection and increased mortalities may be noticed from the second week onwards. Histomoniasis can cause severe mortality in turkeys up to 80–100%. However, some field outbreaks of histomoniasis have been documented with low mortalities and morbidity. In some cases, the turkeys may not show any clinical signs but have higher condemnations in the processing plants due to visceral lesions. Thus, the severity of histomoniasis outbreaks varies between flocks. Many factors, such as the virulence of H. meleagridis isolate, management method, age of the bird, season, immune status, concurrent infections and gut health, play a role in histomoniasis outbreaks.

Recently, increased incidences of histomoniasis have been documented in many countries after the withdrawal/ ban of previously licensed prophylactic/therapeutic products. Currently, neither commercial vaccines nor prophylactic/ therapeutic treatments are available in USA for histomoniasis. In order to find a solution for histomoniasis, a robust challenge model needs to be established to screen the vaccine candidates or test the efficacy of prophylactic/therapeutic products. Thus, choosing an ideal challenge isolate and administering by an


Volume 10 Issue 1 32 International Animal Health Journal
Figure 1. Field outbreak of histomoniasis in turkeys: Characteristic lesions in liver and ceca.
A. Necrotic foci in the liver. B. Necrotic cecal mucosa. C. Cecal core.
RESEARCH AND DEVELOPMENT
Figure 2. Sulfur-yellow dropping in turkeys challenged with Histomonas meleagridis
appropriate challenge route plays a critical role in establishing the challenge model. The aim of our studies was to develop a robust challenge model for histomoniasis with recently isolated wild-type H. meleagridis isolates from USA.
Materials and Methods
Histomonas meleagridis challenge isolates
Three H. meleagridis field isolates (HMA, HMB and HMK) were evaluated. HMA was isolated from a histomoniasis outbreak in turkeys (2020) in Midwest, USA. HMB was isolated from a histomoniasis outbreak in turkeys (2020) in Southeastern, USA. HMK was isolated from a broiler breeder flock (2021) with histomoniasis incidences in Southeastern, USA. All isolates were confirmed by PCR and sequencing. Cecal samples were collected and transported in plug-sealed cap flasks with modified Dwyer’s media. The flasks were shipped in an insulated container under warm temperature. The culture was incubated at 41°C for a period of 2–3 days and observed under the inverted microscope. After visual observation of histomonads, the culture was further sub cultured and stored in LN2 containers with 10% DMSO. Few days before challenge, the culture was retrieved back from LN2 containers and incubated at 41°C. After subculturing, the culture was examined under the inverted microscope and histomonads were enumerated.

Evaluation of Gross Lesions in Liver and Ceca
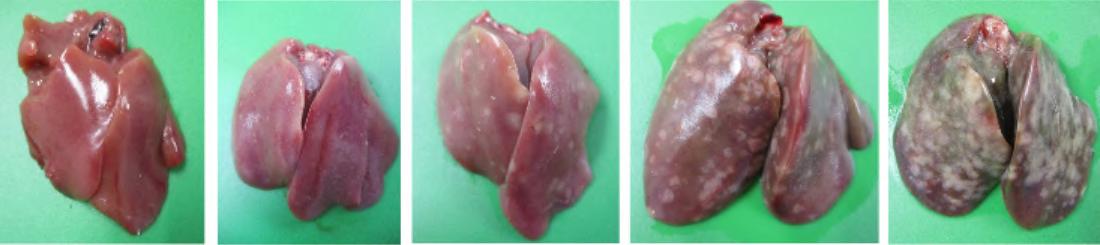

All birds enrolled in the studies were euthanised on the last day of the study, necropsied and evaluated for gross lesions in liver and ceca. Any bird that died during the study period was necropsied and evaluated to identify the cause of death and gross lesions of liver and ceca were evaluated. Gross lesions in the ceca (Figure 3) and liver (Figure 4) were scored as per the criteria (Table 1).
Study 1: Intra-cloacal Challenge Model
Fifty-two poults were randomly assigned into four groups (13 birds/group) and housed in separate isolators. All birds were fed with ad libitum feed and water throughout the study. Group 1 served as a negative control. At 16 days-of-age, groups 2–4 (n=13 birds/group) were challenged by intra-cloacal route with H. meleagridis isolates (HMA, HMB, HMK) at the dose of 1x105, 2x105, 1x105 histomonads/0.5 ml, respectively. A blunt-ended oral gavage needle was attached to the 1-ml syringe and used for intra-cloacal inoculation (Figure 5). Five days post-challenge, (DPC) 6 birds from each group were necropsied and all the remaining birds were necropsied eight DPC.
Study 2: Cloacal Drop Challenge Model
Nineteen poults were randomly assigned into two groups and housed in separate isolators. All birds were fed with ad

International Animal Health Journal 33 www.international-animalhealth.com
Figure 3. Cecal gross lesion scoring guide for histomoniasis
Score 0 Score 0 Score 1 Score 1 Score 2 Score 2 Score 3 Score 3 Score 4 Score 4
Figure 4. Liver gross lesion scoring guide for histomoniasis
RESEARCH AND DEVELOPMENT
0 No visible lesions
1 Mild – Very few, pale and mild necrotic foci on the liver.
2
Moderate – Presence of necrotic foci (white or pale-yellow) in less than 25% of liver surface.
No visible lesions
Mild – Healthy mucosal membrane without any thickening of the cecal wall. Few petechiae on the mucosa may be rarely seen. Presence of normal cecal contents.
Moderate – Distinctly thickened cecal wall. Presence of many petechiae on the mucosa/serosa. Few cecal ulcers may be seen on the mucosa. Abnormal cecal contents and may contain few grains or caseous clots. Absence of cecal core.
3
Severe – Presence of necrotic foci (white or pale-yellow) covering 25–75% of liver surface.
Severe – Distinctly thickened cecal wall. Presence of many petechiae on the mucosa/serosa. Presence of many cecal ulcers on the mucosa. Abnormal cecal contents with solid cecal core.
4
Very severe – Presence of coalescing necrotic foci (white or pale-yellow) covering >75% of the liver surface.
Very severe – Severely thickened, fibrinonecrotic cecal wall. Presence of hemorrhage and necrosis of the serosa. Presence of many cecal ulcers, hemorrhage, and necrosis of the mucosa. Presence of solid cecal core adhered to the necrotic mucosa or covered by blood.
libitum feed and water throughout the study. Group 1 (n=9) served as a negative control. At 27 days of age, group 2 (n=10) was challenged with wild-type H. meleagridis isolate (HMA) at the dose of 1x103 histomonads/0.5 ml dose by cloacal drop method (Figure 6). Before cloacal drop inoculation, the protuberance on the dorsal lip of the cloaca was wiped with a clean paper towel to stimulate the cloacal drinking reflex. The challenge material (0.5 ml/bird) was administered at the tip of the cloaca using a 1 ml syringe. Fifty percent of the birds were necropsied at 9 DPC and the remaining birds were necropsied between 12–14 DPC.

Study 3: Cloacal Spray Challenge Model
Thirty-five poults were randomly assigned into three groups and housed in separate isolators. All birds were fed with ad libitum feed and water throughout the study. Group 1 (n=11) served as negative control. At 27 days of age, groups 2 and 3 (n=12/group) were challenged with wild-type H. meleagridis isolate (HMA) at the dose of 1x105 and 3.2x105 histomonads /0.5 ml dose by cloacal spray method. A 3.0 ml syringe was attached to a spray device and sprayed at the cloaca (0.5 ml/bird) after wiping the cloaca with a clean paper towel. The study was completed 14 DPC.
Results
Study 1: Intra-cloacal Challenge Model
Five days post-challenge: Histomoniasis associated gross lesions were noticed only in the challenged birds (Table 2).
The HMA isolate induced mild (2/6) to severe lesions (2/6) in ceca and induced mild lesions in liver (1/6). The HMB isolate induced mild (2/6) to severe lesions (4/6) in ceca and mild lesions in liver (3/6). The HMK induced mild lesions only in the ceca (3/6).
Eight days post-challenge: Histomoniasis associated gross lesions were noticed only in the challenged birds (Table 3). The HMA isolate induced mild (1/7), moderate (1/7) and severe lesions (3/7) in ceca and mild (1/7) to severe lesions (4/7) in the liver. The HMB isolate induced severe (3/7) to very severe lesions (3/7) in ceca and moderate (1/7) to severe lesions (5/7) in liver. The HMK isolate induced mild lesions only in the ceca (7/7) and did not induce any lesions in the liver.
Study 2: Cloacal Drop Challenge Model
Nine days post-challenge: Histomoniasis associated gross lesions were noticed only in the challenged birds (Table 4). The HMA isolate induced moderate (2/5) to severe lesions (3/5) in ceca and mild (1/5), moderate (1/5) to very severe lesions (3/5) in the liver.
Twelve-fourteen days post-challenge: Histomoniasis associated gross lesions were noticed only in the challenged
Volume 10 Issue 1 34 International Animal Health Journal RESEARCH AND DEVELOPMENT
Score Liver Ceca
Group Liver score Cecal score 1 (6 birds) 0 0 0 0 0 0 0 0 0 0 0 0 2 (6 birds) 0 1 3 3 0 0 0 0 0 0 1 1 3 (6 birds) 1 0 3 3 1 1 3 1 0 0 1 3 4 (6 birds) 0 0 1 1 0 0 0 0 0 0 1 0 Group Liver score Cecal score 1 (7 birds) 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 (7 birds) 0 3 0 3 1 3 3 3 3 0 0 1 3 2 3 (7 birds) 3 0 3 0 3 3 4 3 2 3 4 4 3 3 4 (7 birds) 0 0 1 1 0 0 1 1 0 0 1 1 0 1
Table 1: Scoring guide for histomoniasis associated gross lesions in the liver and ceca. Table 2: Necropsy on 5 days post-challenge (Study 1)
Table 3: Necropsy on 8 days post-challenge (Study 1)
birds (Table 5). The HMA isolate induced moderate (2/5), severe (2/5) and very severe lesions (1/5) in ceca and severe (3/5) to very severe lesions (2/5) in the liver. Two mortalities were documented in the challenged birds, one on 12 DPC, and the other on 14 DPC.
Group 2 birds challenged with HMA at the dose of 1x105 histomonads/0.5 ml induced mild (3/12), severe (3/12) and very severe lesions (1/12) in the ceca, whereas severe (3/12) and very severe lesions (1/12) were noticed in the liver. Group 2 had two mortalities, one on 11 DPC and the other on 13 DPC. Group 3 birds challenged with HMA at the dose of 3.2x105 histomonads/0.5 ml induced mild (3/12), moderate (2/12), severe (2/12) and very severe lesions (3/12) in the ceca, whereas severe (2/12) and very severe (5/12) lesions were induced in the liver. Group 3 had six mortalities, one mortality occurred on 10 DPC, two mortalities occurred on 11 DPC and three mortalities occurred on 13 DPC.
Discussion
Several studies have been conducted in the past by various research labs for establishing histomoniasis challenge model. Methods, such as exposing turkeys to contaminated litter, feeding infected tissues, oral gavage, parenteral administration and intra-cloacal administration, have been tried to establish the challenge model. Histomoniasis challenge models have been tried using H. meleagridis, Heterakis gallinarum or earthworms. In our studies, we evaluated two established methods for H. meleagridis challenge (intra-cloacal and cloacal drop) and tried a new method (cloacal spray) with recently isolated wild-type H. meleagridis isolates.


Study
Fourteen days post-challenge: Histomoniasis associated gross lesions were noticed only in the challenged birds (Table 6).

International Animal Health Journal 35 www.international-animalhealth.com RESEARCH AND DEVELOPMENT
Group Liver score Cecal score 1 (11 birds) 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 (12 birds) 4B 0 3B 1 3C 0 3C 0 0 0 0 1 0 0 0 0 3 0 4 1 0 3 0 3 3 (12
4A 0 3A 1 4B 0 3B 1 4B 0 2B 0 4C 0 2C 0 3C 4 4C 4 3C 0 4C 1 Group Liver score Cecal score 1 (5 birds) 0 0 0 0 0 0 0 0 0 0 2 (5 birds) 4 3 4 2 4 2 2 3 1 3 Group Liver score Cecal score 1 (4 birds) 0 0 0 0 0 0 0 0 2 (5 birds) 3A 3A 3B 4B 4 2 3 3 4 2
post-challenge
birds)
Table 4: Necropsy on 9 days
(Study 2)
Table 5: Necropsy between 12 and 14 days post-challenge (Study 2)
Table 6: Necropsy at 14 days post-challenge (Study 3)
ADead on 12 DPC, BDead on 14 DPC. ADead on 10 DPC, BDead on 11 DPC, CDead on 13 DPC.
Figure 5. Intra-cloacal route of inoculation in turkeys
3: Cloacal Spray Challenge Model
Figure 6. Cloacal drop challenge method in turkeys
In study 1, wild-type field isolates HMA and HMB challenged by intra-cloacal routes resulted in severe lesions in ceca (Figure 7) and liver (Figure 8), while the HMK isolate induced only mild lesions in the ceca but not in the liver. At 5 DPC, HMA and HMB induced pronounced lesions only in the ceca. At 8 DPC, HMA and HMB induced
pronounced lesions in the liver and ceca. At 5 and 8 DPC, HMK isolate induced only mild lesions in the ceca, but not in the liver. No mortalities were documented by any of these isolates until 8 DPC. Among the three wild-type H. meleagridis isolates evaluated, two of these isolates (HMA and HMB) were virulent and induced severe lesions





Volume 10 Issue 1 36 International Animal Health Journal
Figure 7. Pathological manifestation of Histomonas meleagridis in ceca. A. Hyperemic, inflamed and thickened cecal wall. Feathers around the vent are sulfur-yellow in color. B. Hyperemic and thickened cecal wall engorged with cecal core. C. Cross section of the cecal core. D. Cecal core attached to the mucosa by fibrin strands. E. Cecal core with numerous coalescing petechiae on the cecal mucosa. F. Necrotized ceca and the core.
RESEARCH AND DEVELOPMENT
Figure 8. Pathological manifestation of Histomonas meleagridis in the liver. A. Numerous red centered necrotic foci circumscribed by tan colored periphery resulting in discoloration of the liver. B. Numerous coalescing white and speckled necrotic foci resulting in discoloration of the liver.
RESEARCH AND DEVELOPMENT
in the ceca and liver, while the isolate HMK resulted in mild lesions only in the ceca.
In study 2, wild-type field isolate HMA challenged by cloacal-drop route resulted in severe lesions in ceca (Figure 7) and liver (Figure 8). Pronounced lesions were noticed in liver and ceca at 9 DPC and mortalities were noticed starting from 12 DPC.
In study 3, wild-type field isolate HMA challenged by cloacal spray route induced severe lesions in ceca (Figure 7) and liver. Pronounced lesions were seen in both cloacal spray groups and more severe lesions were seen in the higher dose challenge group. Mortalities were seen in the lower dose challenge group starting 11 DPC, while in higher dose challenge group mortalities started from 10 DPC. A dose response infectivity was observed in this study.
In summary, choosing the challenge isolate for histomoniasis challenge model plays a crucial role. Among the three isolates evaluated, only two isolates were capable of inducing severe lesions in liver and ceca. Cloacal contact of H. meleagridis is very important in establishing the infection. The cloacal contact of the challenge isolate could be achieved either by intra-cloacal route, cloacal drop or cloacal spray. The gross lesions in the ceca were noticed as early as 5 DPC and mortalities were noticed after 10 DPC. In addition, a dose related response has been observed in the severity of the gross lesions. If a vaccine or a pharmaceutical product needs to be evaluated for its efficacy against gross lesions of ceca and liver, the necropsy can be performed at 9–10 DPC. If a vaccine or a pharmaceutical product needs to be evaluated for its efficacy against reduction in the gross lesions of ceca and liver as well as reduction in the mortalities, then the necropsy can be performed around 14–15 DPC or later. The two wild-type field isolates described in this study can be considered as ideal candidates for establishing an H. meleagridis challenge model by cloacal contact (intra-cloacal, cloacal drop, and cloacal spray).
Acknowledgment
We would like to thank Dr. Steven Clark, Dr. Nicholas Brown, and Dr. Brandon Doss for the field support. We appreciate the support of Laura Trygstad, Nerissa Ahern, April Cruse, Jess Houchen, Jake Campbell, Juan Gonzalez, Deborrah Higuchi and Emily Barber for their valuable contributions.
REFERENCES
1. Armstrong PL, McDougald LR. The infection of turkey poults with Histomonas meleagridis by contact with infected birds or contaminated cages. Avian Diseases. 55:48-50; 2011.
2. Bilic I, Jaskulska B, Souillard R, Liebhart D, Hess M. Multi-locus typing of Histomonas meleagridis isolates demonstrates the existence of two different genotypes. PLOS ONE. 9:e92438; 2014.
3. Clark S, Kimminau E. Critical review: Future control of blackhead disease (histomoniasis) in Poultry. Avian Diseases. 61:281-288; 2017.
4. Durairaj V, Drozd M, Lin G, De Keyser K, Vander Veen R. Pathological investigation of a histomoniasis outbreak in turkeys. EC Veterinary Science. 7.1:78-82; 2022.
5. Durairaj V, Higuchi D, Clark S, Vander Veen R, Campi T. Isolation of Histomonas meleagridis and Pentatrichomonas hominis in an enterohepatitis outbreak in turkeys. Proceedings at the western poultry disease conference. Vancouver, BC, Canada. 62-63; 2022.
6. Durairaj V, Higuchi D, Vander Veen R. Lessons learned from pathogenesis studies of Histomonas meleagridis in Turkeys. Proceedings at the north central avian disease conference. Virtual platform. 45; 2021.
7. Durairaj V, Nezworski J, Drozd M, Clark S, Vander Veen R. Case report: Clinical investigation of a turkey barn with a recurrent history of histomoniasis. Abstract at the AAAP symposium & scientific program. Philadelphia, PA. 77; 2022.
8. Hauck R, Armstrong PL, McDougald LR. Histomonas meleagridis (Protozoa: Trichomonadidae): analysis of growth requirements in vitro. Journal of Parasitology. 96:1–7; 2010.
9. Hauck R, Hafez HM. Experimental infections with the protozoan parasite Histomonas meleagridis: a review. Parasitology Research. 112:19-34; 2013.
10. Hess M, McDougald LR. Histomoniasis. In: Swayne D, Boulianne M, Logue C, McDougald L, Nair V, Suarez D, deWit S, Grimes T, Johnson D, Kromm M, et al., editors. Diseases of poultry. 14th ed. Ames (IA): Wiley-Blackwell. p. 1223–1230; 2020.
11. Hu J, Fuller L, McDougald LR. Infection of turkeys with Histomonas meleagridis by the cloacal drop method. Avian Diseases. 48:746–750; 2004.
12. Liebhart D, Hess M. Oral infection of turkeys with in vitrocultured Histomonas meleagridis results in high mortality. Avian Pathology. 38:223-227; 2009.
13. McDougald LR, Hu J. Blackhead disease (Histomonas meleagridis) aggravated in broiler chickens by concurrent infection with cecal coccidiosis (Eimeria tenella). Avian Diseases. 45:307-312; 2001.
14. Tyzzer, E.E. and Collier, J. Induced and natural transmission of blackhead in the absence of Heterakis. Journal of Infectious Diseases. 37: 265–276; 1925.

Dr. Vijay Durairaj

Dr. Vijay Durairaj is a board-certified poultry veterinarian with more than 15 years of experience in working with poultry health, diagnostics and vaccines. He is currently working as senior scientist at Huvepharma Inc, USA. Previously, he worked in Boehringer Ingelheim Animal Health, USA for five years and held various positions in R&D. He received his BVSc degree from Veterinary College and Research Institute- Namakkal, Tamil Nadu Veterinary and Animal Sciences (TANUVAS) University, India. He received his MS degree from the Center of Excellence in Poultry Science, University of Arkansas, USA. He earned his PhD degree from Poultry Diagnostic and Research Center, The University of Georgia, USA. Vijay has vast experience in working with viral, bacterial, parasitic and metabolic diseases of chickens and turkeys. Vijay’s research interests involve identifying potential vaccine candidates, develop novel vaccine strategies and therapeutics to prevent poultry diseases.
Corresponding Author – Vijay.Durairaj@huvepharma.us
Dr. Vander Veen
Dr. Vander Veen has more than 15 years of experience in the animal health industry. He is currently the Research and Development Director at Huvepharma, Inc. Previously, he has worked for Boehringer Ingelheim Animal Health, Zoetis, and other animal health companies and has held various positions in R&D, manufacturing, and diagnostics. He received his BS degree in Microbiology from South Dakota State University and his PhD in Immunobiology from Iowa State University. Ryan has vast experience in livestock vaccine development and licensure in multiple species.

International Animal Health Journal 37 www.international-animalhealth.com
Tackling Cardiomyopathy Syndrome in Farmed Salmon


Cardiomyopathy Syndrome of farmed Atlantic salmon has become widespread since it was first recognised in Scotland and Norway in the 1980’s. The condition is responsible for significant losses particularly of larger, market size fish. In Norway it was the most important cause of mortality in 2020 and 2021.1 Mortalities may be chronically elevated and acute mortalities may occur, often associated with a stressful event such as handling for sea lice treatment. Affected fish typically represent a large financial investment for the producer and the loss of high quality fish so close to harvest can be demoralising for farm staff.
Wild Atlantic salmon hatch from eggs laid in freshwater streams and rivers. Fry develop rapidly in freshwater to become smolts which are physiologically able to survive in saltwater. During the seawater phase they feed and grow, typically undergoing extensive marine migrations before returning to their home river to spawn. Sea trout, likewise, spawn and hatch in freshwater and migrate to sea but tend to remain in coastal areas close to their freshwater origin.
Affected fish may show no external signs other than sudden mortality. Clinical signs, where present, are typically those associated with heart failure and include exophthalmia, ventral skin haemorrhages and raised scales due to oedema. Internally, affected fish may show ascites, dark liver and enlarged hearts often with blood or blood clots in the pericardium.2 Histologically, characteristic changes including inflammatory cell infiltration, cell degeneration and cell necrosis are apparent in the heart muscle.
The primary cause of the condition is understood to be infection with Piscine Myocarditis Virus (PMCV), a doublestranded RNA virus with genetic similarities to members of the family Totiviridae. To date, however, PMCV has not been isolated in pure culture. This limitation continues to hinder efforts to develop effective control measures.
In order to provide a platform to study the condition and evaluate methods for control, Moredun Scientific has recently validated an experimental model of Cardiomyopathy Syndrome in Atlantic salmon post-smolts. The challenge model uses an injectable suspension prepared from heart tissue from clinically affected salmon. Source material was homogenised in tissue culture medium, and purified by filtration and centrifugation. Homogenates were screened by PCR to confirm the presence of PMCV and to exclude interfering viruses causing similar pathologies. Viability of the PMCV preparation was confirmed by demonstration of cytopathic effect using a susceptible fish cell line.
In the validation study, groups of 40 fish were challenged by intra-peritoneal injection with the tissue homogenate preparation at three dose levels. The control group was injected with tissue culture medium only. All experimental fish injected with the homogenate preparation were to be PMCV positive at 4 weeks and 10 weeks post challenge. All control fish were PMCV negative. Experimentally challenged fish showed no gross signs of cardiomyopathy syndrome
and the pathological effect was determined by scoring of histopathological changes in the heart (Figure 1) using a scoring scheme developed to assess the degree of cardiac myopathy associated with disease outbreaks on farms.3 The experimental challenge resulted in a substantial increase in heart scores in fish challenged using the tissue homogenate preparation.
The challenge model offers new scope to develop practical methods for the control of Cardiomyopathy Syndrome on commercial farms.
The PMCV challenge is one of several experimental models available from Moredun Scientific. Please contact us for further information (info@moredun-scientific.com) or visit our website www.moredun-scientific.com .
REFERENCES
1. Sommerset I., Walde C.S., Bang Jensen B., Wiik-Nielsen J., Bornø G., Oliveira V.H.S, Haukaas A. and Brun E. Norwegian Fish Health Report 2021, Norwegian Veterinary Institute Report, series #2a/2022, published by the Norwegian Veterinary Institute in 2022

2. Garseth, A.H., Fritsvold, C., Svendsen, J.C., Bang Jensen, B. and Mikalsen, A.B. (2018). Cardiomyopathy syndrome in Atlantic salmon Salmo salar L.: A review of the current state of knowledge. J. Fish Diseases 41, 11-26.
3. Fritsvold, C., Kongtorp, R.T., Taksdal, T., Orpetveit, I., Heu,. M. and Poppe, T.T. (2009). Experimental transmission of cardiomyopathy syndrome (CMS) in Atlantic salmon. Dis Aquat Org 87, 225-234.
Bill Roy
Bill Roy is the Head of Aquaculture at Moredun Scientific, responsible for the development and delivery of commercial projects on farmed fish health and welfare. He has a PhD in aquaculture from the Institute of Aquaculture, University of Stirling, and more than 30 years of experience in aquaculture R&D, leading studies to identify, evaluate and support registration of fish vaccines, veterinary medicines and feed additives.
Email: wroy@moredun-scientific.com
Volume 10 Issue 1 38 International Animal Health Journal AQUACULTURE
Figure 1. Cardiomyopathy Syndrome histology

I hope this journal guides you progressively, through the maze of activities and changes taking place in the animal health industry.

IAHJ is also now active on social media. Follow us on: www.twitter.com/AHMJournal www.facebook.com/Animal-Health-Media www.plus.google.com/+Animalhealthmediajournal www.animalhealthmedia.tumblr.com/

Volume 10 Issue 1 40 International Animal Health Journal AD INDEX BC Alltech Page 5 Argenta Limited Page 39 Connect in Pharma
Page
Page 27 Nordson
PCI
Senglobal
Subscribe today at www.international-animalhealth.com or email info@senglobalcoms.com
IBC Controlant
3 Moredun Scientific
Page 21
Pharma Services IFC Royal GD Page 31
Ltd. Page 12 & 13 Vetoquinol Global
Real-time supply chain visibility is driving the industry forward
Cold Chain as a Service® Integrated technology and services for your end-to-end supply chain and vaccine distribution.

Real-time temperature monitoring Product location traceability
Mission-critical analytics & insights 24/7 monitoring and response services
Validated for all lanes—air, road, sea. Pay as you go.
Controlant is pioneering the development of next-generation visibility solutions for digitally connected global supply chains that keep products safe. Our Cold Chain as a Service® Digital Visibility Platform solution consists of reusable Internet of Things (IoT) data loggers that send mission-critical quality data and insights in real-time to a proprietary, cloud-enabled software platform, and costreducing operational services..
Businesses are leveraging the improved visibility to collaborate with stakeholders, support their corporate sustainability objectives, and achieve supply chain improvements leading to a substantial return on investment.
For more information, visit controlant.com contact@controlant.com
Follow us on Twitter @controlant

Volume 10 Issue 1 42 International Animal Health Journal Benefits of Allzyme® enzymes Profit Performance Planet Optimizes feed cost savings Improves nutrient absorption and digestibility Decreases excess nutrient excretion into the environment Alltech’s range of feed enzymes was developed to maximize feed efficiency and nutrient absorption — lowering costs and improving bird performance while reducing the environmental impact. Contact your local representative or scan the QR code below to find out more about our portfolio of enzyme solutions. you could optimize animal performance and lower your feed costs? What if...


































































































