

ANYONE CAN DO COLD CHAIN PACKAGING.

But there’s only one who can do climate-friendly, zero-waste, modular and payload-optimized pharmaceutical packaging that will save up to 1,000 kilograms of CO2* per 333-liter packaging box – and can do that in all temperature zones: eutecma!














Get ready for retecma!








It’s the one and only AI-supported reverse logistics system that fights to keep all resources in the lifecycle as long as possible.


4 FOREWORD GREEN VISION
6 Building a Healthier and More Environmentally Sustainable Future
Environment, Health, Safety and Sustainability (EHS&S) refers to the practices to protect the health and safety of employees and the public as well as the environment. Strong EHS&S management requires the implementation of systems and processes to assess and control the risks of environmental impacts and health and safety hazards. Besides assuring compliance with applicable legislation, EHS&S management systems drive continuous improvement and learning. EFPIA member companies strive to invent, produce, and distribute new medicines and vaccines in a safe and environmentally responsible manner. Furthermore, we are actively providing a safe and healthy workplace while reducing the environmental impact in our operations and those of our supply partners around the world. A risk management approach is employed to create transformational health innovations, while protecting our employees and employing practical aspects of environmental sustainability.
8 Pharmaceuticals in the Environment: A Threat to the Health of Ecosystems, Animals, and Humans
Pharmaceutical pollution can be found on all continents. Pharmaceuticals can enter the environment throughout their life cycle and cause serious damage to ecosystems and contribute to antimicrobial resistance (AMR). There are many actors across the pharmaceutical value chain, who with the support of improved regulation, must all take action to effectively reduce this environmental and health threat. Jean-Yves Stenuick, Safer Pharma Programme Manager at Health Care Without Harm (HCWH) Europe discusses the work, with the healthcare sector to minimise pharmaceutical pollution and its contribution to AMR through policy advocacy and upstream initiatives.
11 International Responses to the Emerging Threat of Antimicrobial Resistance
The problem of antimicrobial resistance is not confined to a few countries but rather is a challenge that affects the entire world. The extent and pattern in which antimicrobials are utilised can, however, have a significant impact on the degree of urgency or severity of the condition. The problem has gradually but steadily extended to every single country in the globe, posing a threat to the safety of human, animal, and plant health as well as the security of food production. The consistent rise in the consumption of antibiotics in both the human and animal sectors raises serious concerns about a future in which not a single antibiotic will be available for the treatment of even the most prevalent bacterial diseases. Hina Malik, Wasimuddin and Randhir Singh outline the international responses to the emerging threat of antimicrobial resistance.
14 The Green Discussion
In 2020, we decided that the theme for our year would be regenerating the veterinary world. I had been running The Webinar Vet for a decade and begun the online revolution for vets, which had taken millions of miles off the roads over the last decade. However, we wanted to do more to show leadership in the veterinary industry. As a digital educational business, it is arguably simpler to be sustainable than a large pharma company. However, we calculated our carbon footprint; offset double what we produced during the pandemic year; started a veterinary sustainability podcast and planted a wildflower meadow at the Liverpool Science Park along with encouraging our landlord to embrace renewable energy providers.
18 New Paper-based Security Seals Revolutionise Pharma Packaging
Since the 60s, the use of plastic has increased twentyfold. The EU is therefore focusing on a sustainable economy and is pulling the emergency brake on plastic packaging in particular. The Single-use Plastics Directive is only one measure to achieve this goal. The first step was the ban on plastic bags - products such as cotton swabs and drinking straws are also already adopting alternative packaging materials. In the future, there will be penalties for packaging that is not designed to be recyclable within the European Union. According to Securikett CEO, Werner Horn, "A switch to our new paper-based VOID seals and VOID tapes will therefore pay off for all businesses by eliminating the risk of non-compliance with material requirements of new regulations. The pulp material used by Securikett can be recycled together with folding cartons and complies with the new EU general circular economy package."
20 Meeting the World’s Evolving needs for a More Sustainable Future: How to Realise a Circular Approach to Drug-delivery-solutions
The world is looking for companies across all industries to take leadership positions that not only advance their traditional business metrics, but more importantly improve their impact on the environment and people’s lives around the world. When it comes to sustainability, there is not a single easy solution to solve all issues. Instead, taking a holistic view across various aspects of our business has led Aptar along the path to meeting its sustainability goals. Companies are increasingly adopting the principles of the circular economy where waste and pollution are eliminated or reduced as they are designed out of products throughout their lifecycle.
26 Inside Sustainability at Airnov: A Look at How Healthcare Packaging Solutions Expert Airnov Continues to Pioneer Sustainable Products and Develop Sustainable Working Practices Across its Business
Sustainability is a core priority for almost all packaging producers, including those which support the healthcare sector. As the world transitions towards a more circular way of operating, both in a personal and business sense, the ability to make sustainable enhancements down to the very last detail matters more and more. At Airnov, a pioneer of innovative solutions for the healthcare and medical packaging sectors, sustainability and circularity underpin all of its business activities. Here, we will explore some of the company’s latest products and initiatives that are helping to enhance its environmental credentials and those of its customers.
30 The Pharmaceutical Supply Chain in the Age of Sustainability
The drive for sustainability has impacted every business in recent years and continues to place new demands on all aspects of the pharmaceuticals supply chain. The team at Recipe Design, a London based consultancy who have worked in the industry for over 12 years, see positive developments coming both from legislative pressures and the shifts in values of the customers and end users its clients aim to serve.
32 A Greener Future for the Inhalation Industry: A Critical Year for our Climate

This article explores the legislative and economic drivers of why the industry needs to adapt, and crucially why now is the time to act. It is also important to ground ourselves and remember why we develop medicines; to better the health of the public and save lives. Adam Kay at Pharmaserve North West discusses the challenge to produce sustainable products cannot compromise the safety, efficacy and patient adherence to inhaled medicines.
COLLABORATION
36 Understanding the Role of Energy-Efficiency in Achieving Pharmaceutical Sustainability Targets
The pharmaceutical industry is growing. A 2020 report valued the worldwide life science manufacturing market at $405bn USD, with an expected compound annual growth rate of 11.34% over the following 8 years. As the quantity and diversity of pharmaceutical product continues to rise, so too does the burden this place on our environment. While the pressure to operate sustainably is beginning to be applied by a variety of interested parties, just 42% of the pharmaceutical industry has established a clear carbon reduction. Keith Beattie and Jamie Young of EECO2, explains that to deliver a more sustainable future, the life science industry needs to align the ambition for a cleaner, healthier future with objectives and targets that will deliver this aspiration.
42 A Journey of Generations
Today more than ever, businesses are uniquely qualified to address the impacts of climate change and social inequality. PCI has formally established the Global ESG Program as the foundation for our sustainable business practices. We believe in creating the changes we want to see in the world. PCI’s ESG commitment has gained recognition from global sustainability agencies, having been awarded Bronze Medal status by leading environmental, social, and ethical performance evaluation platform EcoVadis, and Gold Medal status at its Philadelphia Headquarters and Biotech Centre of Excellence.
46 How to Save 1,000 kilos of CO2 per 333-litre Refrigerated Packaging – Thanks to the First-ever Circular Economy Pharmaceutical Packaging
The year of 2019 was a turning-point. It was the year when fears of palpable global climate change gripped society, including the worlds of business and industry. In 2020 the COVID-19 pandemic led to a second dramatic realisation. To increase the resilience of global supply chains there is a radical need for greater sustainability. Sven Rölle of Eutecma GmbH describes the enormous potential which climate-friendly, no-waste packaging can offer the temperaturecontrolled transportation of pharmaceuticals.
SHOWCASE
50 Medicines that Don’t Cost the Earth: New Awards Showcase Innovations
“We know that climate change is bad for our health. What is less well known is that healthcare is bad for the environment,” said Nazneen Rahman while chairing a panel discussion showcasing innovations in sustainable packaging. The session was held on 14 September in Geneva at Connect in Pharma, a new annual event connecting innovators in pharma and biopharma to the world’s leading suppliers and manufacturers. Rahman, a physician, scientist, and AstraZeneca Non-Executive Director, said she had this epiphany two years ago and was compelled to devote the next phase of her working life to making healthcare more sustainable.
YOUR SECURE SUPPLIER FOR TAMPER EVIDENT SEALING WITH SUSTAINABLE PRODUCTS
The PAPER-based security seal with VOID technology is now available!



• Works on almost all cardboard surfaces
• Underlying text and printed codes remain legible

• Compliant with FMD safety requirement

• Serialisation of each individual product

• 'One package - one material' - this way you improve the recycling process and make a positive contribution to the recycling management

• Paper seals are produced from renewable raw materials

Our seals are easy to understand multi functional security locks against tam pering and counterfeiting. They are used and tested on pharmaceutical packaging worldwide.

The entire world has heightened its focus on environmental sustainability. Driven to address the global threat of climate change and safeguard our planet, organisations across a variety of sectors are seeking new ways to operate more sustainably and reduce environmental impact. The pharmaceutical industry is no exception. For drug developers and manufacturers, embracing greener practices and processes has become a key strategic priority.
The pharmaceutical industry often involves significant processing for a relatively small amount of active ingredients. Industry-wide, efforts are focused on reducing the waste generated by processing and making sure waste product is used rather than disposed of. As biotechnology and pharmaceutical organisations turn to outsourced service providers, they are increasingly focused on identifying partners that take environmental sustainability seriously.
The development and production of drugs and devices inherently consume large quantities of natural, human, and economic resources. As such, it represents a risk to the environment and the sustainability of the pharmaceutical industry.
However, with these challenges comes opportunity –many key players in the pharmaceutical industry, have committed to environmental stewardship across their production lifecycle, from discovery right through to disposal.
By minimising the environmental impact of operations, the pharmaceutical industry is becoming a driver of positive change towards a sustainable future.
We are always looking for new ways to enhance environmental sustainability and better manage waste throughout the lifecycle of products. Key issues from on-site improvements like energy-efficient lighting and bulk nitrogen systems, to more significant investments like combined heat and power plants that drive progress toward energy self-sufficiency. The industry needs to commit to addressing a full range of pharmaceutical waste, treating waste waters at our on-site biological treatment plant, handling hybrid waste through anaerobic digestion, and finding ways to recover and reuse solvent waste.
Transparency is key to success on the journey towards a sustainable future, not only to ensure the industry sticks to its sustainability targets but also to help in gaining and maintaining stakeholder confidence.
Overall, the pharmaceutical industry has made great steps towards this by identifying realistic sustainability targets however, we need more pharmaceutical companies to be accountable and join the sustainability movement now.
It is extremely important that large, profitable organisations ‘give back’ to local communities and contribute to the economic, social, environmental, and cultural sustainability of the societies in which we live and work. Sustainability is not just about the environment or improving patient access to medicines, it is also about empowerment, using resources to support local causes and activating communities to take charge of their health and wellbeing.
We have some clarity over the desired end point of zero carbon within the next 30 years (or sooner for some pharma companies), but our industry is uncertain how we will achieve this objective. As pharma engineers, we will all play a role in delivering this endpoint through the continuous improvement of existing facilities and by designing, equipping, and building new facilities that will be operating in 2050. The zero-carbon scenario requires us to make radical changes while simultaneously improving supply chain quality and resilience.
As we undertake this “decarbonization” process of designing for a future with a “100% green grid,” the lack of clear timetables for the various breakpoints challenges all pharmaceutical engineers. Even when we achieve a green grid, there will be no “magic bullet” for zero carbon because pharma companies will remain significant users of heat and other energy-intensive products. Process intensification, continuous processing, and modular containment technologies promise to deliver future facilities that will consume less energy but remain complex. Vendors supplying equipment for these facilities need clear guidance (user requirement specifications) on the specific needs of the facility to achieve optimized equipment designs – and providing this specification requires a strategic vision for the site.
The pharmaceutical industry is making strides towards greater environmental sustainability, and the right partner can empower you to minimise your environmental footprint today.
Together, we can reduce our impact and make a meaningful change.
In this journal entitled “Pharma Nature Positive”, we have drawn on the term “Nature Net Positive” – Give more back to nature than what we take away from it.
The goal of this journal is to create a dedicated platform where all stakeholders of the pharmaceutical and healthcare industry can discuss, and share technologies and innovations which will enable the industry to attain the challenge of reaching zero carbon, and any engineer with the bandwidth to develop the breadth of technical know-how needed to clarify, evaluate, and implement energy-efficient strategies will be in a good position to add value in the years ahead.
Mark A. Barker Managing Director, Senglobal Ltd


Environment, Health, Safety and Sustainability Building a Healthier and More Environmentally Sustainable Future
Environment, Health, Safety and Sustainability (EHS&S) refers to the practices to protect the health and safety of employees and the public as well as the environment. Strong EHS&S management requires the implementation of systems and processes to assess and control the risks of environmental impacts and health and safety hazards. Besides assuring compliance with applicable legislation, EHS&S management systems drive continuous improvement and learning.
Equally important, the rapidly growing rate of resource consumption throughout the world is unsustainable. The pharmaceutical industry recognises that reversing the use of natural resources, the degradation of ecosystems and the disruption of the environmental systems that support human life, are critical for the benefit of current and future generations. Therefore, we believe that an increased focus on environmental sustainability is key for the future health of our planet.
EFPIA member companies strive to invent, produce, and distribute new medicines and vaccines in a safe and environmentally responsible manner. Furthermore, we are actively providing a safe and healthy workplace while reducing the environmental impact in our operations and those of our supply partners around the world. A risk management approach is employed to create transformational health innovations, while protecting our employees and employing practical aspects of environmental sustainability.
Building a Healthier and More Environmentally Sustainable Future

The pharmaceutical industry is committed to building a healthier and more environmentally sustainable future. We do this by driving an agile, innovative, evidence-based sustainability strategy to enable the pharmaceutical industry to embrace evolutions in science, technology & society and to integrate sustainability across our entire value chain to deliver quality-based, healthy, and green outcomes while positively impacting on the lives of patients.
EFPIA welcomes and embraces the Commission’s focus on the Green Agenda and a more sustainable Europe and looks forward to engaging constructively on the roll-out of their policy priorities.
Our members are dedicated to making a positive impact on the lives of patients whilst operating in a sustainable manner. As we have a responsibility toward the health of the population, we are moving forward in making a beneficial impact by actively addressing climate change and the transition to a circular economy with changes throughout the value chain, as we continue to innovate.
What are we doing?
Environmental sustainability is a key value driver to accelerate delivery, improve efficiency and sustain transformational health innovation. Our industry encourages appropriate use of a risk-based approach to environmental challenges and undertakes initiatives to promote greater environmental responsibility.

The European pharmaceutical industry is committed to continue playing an active role in addressing concerns around risks associated with Pharmaceuticals in the Environment (PiE). Minimising the impact of medicines on the environment while safeguarding access to effective treatments for patients is a critical issue across all sectors of healthcare.
At EFPIA, we believe that a collaborative approach allows us to increase our mutual knowledge and understanding on how to proactively address any potential risks imposed by the presence of PiE. To this end, EFPIA, AESGP and Medicines for Europe have developed the Eco-Pharmaco-Stewardship (EPS) framework that applies the widely accepted principles of product stewardship and is implemented across the industry and with broader stakeholders in the healthcare and environmental sector.

The pharmaceutical sector is one of the most regulated in Europe and the world. Accordingly, the pre-approval of manufacturing plants, clinical trials and marketing authorisations should be given consideration when implementing and interpreting some elements of EU Chemicals legislation. The long development timelines and highly regulated nature of this industry are fundamental aspects of the ability to react to changes in legislation (e.g. restriction of chemicals). Chemical processes comprise a significant portion of a medicine’s environmental footprint, and responsible use of chemicals is a key environmental stewardship priority for the sector.
Our member companies lead projects and initiatives to minimise the impact of their manufacturing process, including reducing the generation of hazardous waste, and using greener solvents. Due to the global operation of our companies and increasing number of countries with
emerging chemical legislation, it is crucial that collaborations take place between industry and regulators globally. EFPIA supports the need to develop a sustainable chemicals strategy and to promote research & development for the transformation of the chemical industry and the creation of green and sustainable manufacturing.
Direct or indirect human activities have altered the composition of the global atmosphere and increased carbon dioxide emissions, driving up temperatures. This has led to what we often observe as more extreme weather conditions and is referred to as climate change.
The pharmaceutical industry contributes to a healthy environment while demonstrating leadership in mitigating climate change. Our activities support the ambition the European Commission expressed through their European Climate policies. EFPIA member companies are committed to:
• Establishing climate change policies and strategies based on materiality and impact for individual companies and addressing their entire value chains.
• Pursuing science based CO2e reduction targets.
• Contributing to reduced energy consumption and increased energy efficiency and seeking opportunities to use energy from renewable sources throughout the value chain.
• Annually and publicly disclosing CO2 performance calculated according to recognized methodologies such as e.g., the World Resources Institute Greenhouse Gas Protocol.
The Pharmaceutical Industry is supportive of a circular approach to its operations and products and is aligned with the European Commission's Circular Economy Action Plan.
We will achieve the goals of the Circular Economy Action Plan concurrent with our aspiration to safeguard the future supply of pharmaceuticals for patients and improve human health.
The pharmaceutical industry’s approach to circularity builds on our long experience in environmental sustainability, while recognising the constraints (e.g., speed of transition), from operating in a highly regulated industry. Circularity and regulation of pharmaceuticals should be carefully balanced. The innovation to enable circularity will drive new opportunities for growth, promote greater resource efficiency, create a more competitive economy and reduce pollutants.
Implementation of a circular economy is fundamental to help limit the global temperature increase to less than or equal to 1.5C, and we welcome the opportunity to be part of the solution by working collaboratively with the EU in shaping the legislative framework and within our organizations to mitigate our impacts.

EFPIA
The European Federation of Pharmaceutical Industries and Associations (EFPIA) represents the biopharmaceutical industry operating in Europe. Through its direct membership of 37 national associations, 38 leading pharmaceutical companies and a growing number of small and mediumsized enterprises (SMEs), EFPIA’s mission is to create a collaborative environment that enables our members to innovate, discover, develop and deliver new therapies and vaccines for people across Europe, as well as contribute to the European economy.
Pharmaceuticals in the Environment: A Threat to the Health of Ecosystems, Animals, and Humans

Pharmaceutical pollution can be found on all continents. Pharmaceuticals can enter the environment throughout their life cycle and cause serious damage to ecosystems and contribute to antimicrobial resistance (AMR). There are many actors across the pharmaceutical value chain, who with the support of improved regulation, must all take action to effectively reduce this environmental and health threat.
Pharmaceuticals have a Global Presence
More than 700 pharmaceutical agents or their metabolites have been detected in the environment1 across the world. These pharmaceutical residues are found in both soil and water systems, including surface and groundwater used for drinking water. In the environment, drugs can have harmful effects on animal and plant life threatening whole ecosystems. There are also questions about how humans can be affected by continuous, long-term exposure to low concentrations in drinking water.
In 2015, the potential adverse effects associated with exposure to environmentally persistent pharmaceutical pollutants2 (EPPPs) on human health and the environment were recognised within the Strategic Approach to International Chemicals Management (SAICM) global policy framework.

In 2020, UN Environment’s Assessment Report on Issues of Concern recommended to expand the scope of EPPPs to include pharmaceuticals in the environment.
Therapeutic classes of particular concern in the environment are anti-inflammatory drugs and sedatives consumed in large quantities, cytostatics that are cytotoxic by design, synthetic hormones that can act as endocrine disruptors, and antibiotics. Negative effects include renal failure in vulture populations, detrimental impact on the genetic material of aquatic organisms, reproductive failure in fish, and inhibition of the growth of certain aquatic species.
The Threat of Antimicrobial Resistance
The biggest concern of pharmaceuticals in the environment, however, is the contribution to the development and spread of antimicrobial resistance (AMR), a serious health and development threat that makes infections increasingly difficult or impossible to treat. It is estimated that AMR causes the direct deaths of 1.27 million people3 worldwide every year. Without effective action, AMR’s death toll could spiral up to 10 million people annually by 2050.4
Increasing AMR linked to the discharge of drugs and particular chemicals into the environment has been described by UN Environment as one of the most worrying health threats today.5 The main pollutant sources that exacerbate AMR6 include poor sanitation, sewage and waste effluent; effluent
and waste from pharmaceutical production, healthcare facilities, and animal production; as well as antimicrobial use and manure in crop production.
In 2022, the One Health Leaders Group on AMR, which brings together world leaders and experts to accelerate political action on AMR, called for a reduction in antimicrobial discharges from food systems,7 manufacturing facilities, and human health systems into the environment. Their recommendations include strengthening governance and oversight, improving surveillance and data availability, improving discharge management, and promoting research and development.

Main Pathways into the Environment
Pharmaceuticals can reach the environment throughout their life cycle, during manufacturing, excretion, and disposal. Wastewater treatment plants, which are primarily designed to eliminate biodegradable substances, have varying capacity to eliminate pharmaceutical substances in wastewater and even a low removal efficiency for most drugs. In addition, they do not capture diffuse sources of pharmaceutical solution such as surface run-off from agriculture.
Excretion after consumption is considered the most common entry pathway for pharmaceuticals into the environment with 30%–90% of orally administered drugs8 being excreted as active substance in the urine and faeces of animals and humans. Waste from unused medicine is another important problem, representing 10% of wastewater pollution9 with patients flushing medication down the toilets or sink.
Manufacturing pollution, however, has received growing attention in recent years with extremely high pharmaceutical concentrations found in water streams close to pharmaceutical plants, which is of serious concern for local ecosystems and the development of resistance. In Hyderabad, India, the concentration of the antibiotic Ciprofloxacin in the effluent from a wastewater treatment plant serving bulk drug manufacturing plants exceeded levels toxic to some bacteria by over 1000-fold.10
It is particularly concerning for antibiotics as the pharmaceutical industry has offshored the vast majority of its
production to countries with cheap labour and weak oversight and regulation. China currently produces 80%–90% of antibiotic active pharmaceutical ingredients (APIs)11 while India leads the production of ‘finished dose’ antibiotic products. Huge manufacturing pollution scandals have been reported in these countries.
Highest Concentrations of Active Pharmaceutical Ingredients
A large research project led by the University of York,12 United Kingdom, recently monitored over 1,000 sampling sites in 100+ countries for pharmaceutical pollution. It observed highest cumulative API concentrations in Sub-Saharan Africa, South Asia, and South America, particularly in areas associated with poor wastewater and waste management infrastructure and pharmaceutical manufacturing.
The most frequently detected APIs (found in more than half the sites monitored), were Carbamazepine, used to treat epilepsy and nerve pain, Metformin used to treat type-2 diabetes, and Caffeine used to treat tiredness and improve the effect of some pain relievers (in addition to lifestyle use). Overall, concentrations of at least one API were above levels considered safe for aquatic organisms or of concern in terms of selection for AMR at a quarter of all monitored sites.
The five most detected antibiotics in the study were Trimethoprim, Sulfamethoxazole, Ciprofloxacin, Metronidazole, and Clarithromycin. At one sampling site, close to a pharmaceutical manufacturing plant in Barisal, Bangladesh, the concentration of Metronidazole was more than 300 times higher than the safe target. However, even low concentrations are of concern as they can drive resistance and increase the likelihood that resistance genes transfer to human pathogens.
The EU Regulatory Framework
The European Union is the second market in the world13 in terms of pharmaceutical sales (25% for human consumption and 31% for veterinary consumption), behind the United States. However, there are no specific rules in the EU regulating the emissions from pharmaceutical production into the environment; active pharmaceutical ingredients (APIs) are not covered by the REACH regulation, which addresses the production and use of chemical substances.
Environmental Risk Assessments (ERAs), which aim to valuate and limit potential adverse effects of medicines on the environment, were introduced in 2005 for veterinary pharmaceuticals and in 2006 for human pharmaceuticals. However, ERA guidelines were not applied retroactively meaning that medicines that entered the EU market through the centralised procedure prior to these dates often lack an adequate ERA and for many there are is no data on potential environmental impact.
For human pharmaceuticals, environmental risks are not even criteria in the benefit-risk assessment in the marketing authorisation process. Environmental aspects are therefore not taken into account when the European Medicines Agency (EMA) makes a marketing recommendation for new human drugs. As a result, pharmaceutical companies tend not to prioritise ERAs during drug development14 and Member States tend not to develop appropriate resources for the evaluation of ERAs.15
There are also concerns that ERAs do not consider manufacturing risks, the risks of AMR development, nor the risks that degradation products, metabolites, and combination effects can pose – despite growing evidence that mixtures of pharmaceuticals can have a greater joint toxicity.16 ERA data is not fully publicly available, making environmental information on APIs difficult to research and leading to weak oversight.
The European Commission is currently revising the EU general legislation on human medicines17 with a proposal for a regulation expected later in 2022. This revision provides a unique opportunity to strengthen the EU regulatory framework to better mitigate the risks that pharmaceuticals in the environment can pose to ecosystems and human health through their contribution to the development and spread of AMR.
Need for a Multi-stakeholder Approach
There is no silver bullet to address pharmaceuticals in the environment. However, a number of stakeholders involved across the pharmaceutical value chain can take a series of measures, which together can significantly reduce the risks. These include the pharmaceutical industry, healthcare professionals and procurers, patients, veterinarians and food producers, wastewater treatment plant operators, drinking water utilities, and civil society.
Intergovernmental organisations, national government, and regulatory agencies and authorities also have a key role to play in building a strong regulatory framework with a life-cycle approach that includes a mix of source-directed, use-orientated, and end-of-pipe measures18 to protect the environment and safeguard public health. End-of-pipe measures alone are insufficient, as upgrading wastewater treatment plant is costly and energy-intensive.
The Role of the Healthcare Sector
Health Care Without Harm (HCWH) Europe19 works closely with the healthcare sector to minimise pharmaceutical pollution and its contribution to AMR through policy advocacy and upstream initiatives. Whilst healthcare facilities are only responsible for approximately 20% of APIs20 found in municipal sewage systems, they emit particularly high concentrations of pharmaceutically active compounds and administer specialised pharmaceuticals that are not commonly taken at home.
The healthcare sector is a key actor in the chain approach to reduce pharmaceutical pollution. It can enhance responsible pharmaceutical use, and strengthen green procurement, as well as raise awareness on the adverse effects of pharmaceuticals entering the environment and influence the purchasing, use and disposal of medicines. It can promote take-back schemes for household drugs and establish best management practices for collection and disposal schemes at healthcare facilities.
Medical doctors, in particular, as drug prescribers can take simple practices to reduce unnecessary emissions and waste21 and educate patients about pharmaceutical pollution to improve purchasing and disposal behaviour. By leveraging their trusted voice, they can also engage with healthcare leaders to change procurement and prescription practices and advocate environmental criteria when buying medicines and developing formularies.
REFERENCE
https://www.umweltbundesamt.de/en/databasepharmaceuticals-in-the-environment-0
http://www.saicm.org/Portals/12/documents/meetings/ ICCM4/doc/K1606013_e.pdf
https://www.thelancet.com/journals/lancet/article/ PIIS0140-6736(21)02724-0/fulltext
https://amr-review.org/sites/default/files/160525_Final paper_with cover.pdf
https://www.unep.org/pt-br/ node/20131#:~:text=Nairobi%2C%205%20December%20 2017%20%E2%80%93%20Growing,solutions%20in%20the%20 environmental%20space.
https://wedocs.unep.org/bitstream/ handle/20.500.11822/38373/antimicrobial_R.pdf
https://www.amrleaders.org/resources/m/item/ reducing-antimicrobial-discharges-from-food-systemsmanufacturing-facilities-and-human-health-systems-intothe-environment
https://ec.europa.eu/health/system/files/2016-11/study_ environment_0.pdf
https://saicmknowledge.org/sites/default/files/ publications/position_arzneimittel_englisch.pdf
https://www.researchgate.net/publication/6134174_ Effluent_From_Drug_Manufactures_Contains_Extremely_ High_Levels_of_Pharmaceuticals
https://changingmarket.wpengine.com/wp-content/ uploads/2016/12/BAD-MEDICINE-Report-FINAL.pdf
https://www.globalpharms.org/
https://op.europa.eu/en/publication-detail/-/ publication/5371e7bd-25db-11e9-8d04-01aa75ed71a1/ language-en
https://pubmed.ncbi.nlm.nih.gov/24447908/
https://ec.europa.eu/health/system/files/2016-11/study_ environment_0.pdf
https://www.oecd.org/environment/resources/ pharmaceutical-residues-in-freshwater-policy-highlights.pdf
https://ec.europa.eu/info/law/better-regulation/ have-your-say/initiatives/12963-Evaluation-and-revisionof-the-general-pharmaceutical-legislation_en
https://www.oecd.org/environment/resources/ pharmaceutical-residues-in-freshwater-policy-highlights. pdf
https://noharm-europe.org/

http://www.pills-project.eu/PILLS_summary_english.pdf
https://www.youtube.com/watch?v=9PTpylVotd8
Jean-Yves Stenuick
(HCWH)
International Responses to the Emerging Threat of Antimicrobial Resistance

The problem of antimicrobial resistance is not confined to a few countries but rather is a challenge that affects the entire world. The extent and pattern in which antimicrobials are utilised can, however, have a significant impact on the degree of urgency or severity of the condition. The problem has gradually but steadily extended to every single country in the globe, posing a threat to the safety of human, animal, and plant health as well as the security of food production. The consistent rise in the consumption of antibiotics in both the human and animal sectors raises serious concerns about a future in which not a single antibiotic will be available for the treatment of even the most prevalent bacterial diseases.
The drivers of AMR are present in human health, animal production, environment, and plant production sectors. Therefore, the problem requires joint and global efforts to address the challenge. In this pursuit, the first and foremost step is to measure the extent of the problem in different sectors. There is a coordinated effort underway to address this potential calamity including a wide range of international organisations. In response to the impending socioeconomic concerns of AMR, the World Health Organization (WHO) in its 68th World Health Assembly (WHA) adopted the Global Action Plan (GAP) on antimicrobial resistance, which was jointly developed by WHO, the Food and Agriculture Organization (FAO) and the World Organization of Animal Health (OIE) (1). This action plan emphasizes the importance of a sustainable "one health" strategy for cooperation between multiple worldwide sectors and actors, including human and veterinary medicine, agriculture, the environment, finance, and informed consumers.
The global action plan on antibiotic resistance specifies the following five strategic goals:1
(1) To improve AMR awareness understanding among the general population
(2) To utilise effective surveillance for generating evidence
(3) To minimise the disease and infection events
(4) To regulate the usage of antimicrobial agents in the human and animal sector

(5) To invest in research and development to counter antimicrobial resistance
For as long as practicable, the action plan aims to maintain access to effective medicines for treating and preventing infectious diseases, with the availability and accessibility of safe and quality-assured drugs which are used responsibly by all those who require their usage.
All 194 WHO Member States are urged by the World Health Assembly decision to align their National Action Plan
on Antimicrobial Resistance (NAP) with GAP-AMR. The United Nations General Assembly's High-Level Meeting on AMR further reinforced the commitment of global leaders to address AMR.2 Many countries have operationalised NAP-AMR; however, a few underdeveloped nations are in the midst of developing effective and fully functionalised NAP.3
These international bodies have operationalised surveillance and monitoring of AMR and Antimicrobial usage (AMU) at regional and integrating information at global. WHO started Global Antimicrobial Surveillance System (GLASS) in 2016 to collect official data on AMR and AMU.4 As of May 2021, 109 countries are enrolled in this surveillance system and sharing data, however, there are limited countries contributing data on AMU.5 Antibiotic usage in the major driver for AMR, therefore information on its extent of usage can provide valuable insight on its influence on the problem.
In another surveillance program started in 2005 by WHO as WHO-Global Salmonella Surveillance system which was later renamed as Global Foodborne Infections Network (GFN).6 Surveillance was committed to enhancing the capacities of countries to detect respond and prevent foodborne and other enteric infections from farm to table. The network work with countries to build national capacities for integrated surveillance and promote collaboration among various sectors. The network has also developed manuals and protocols for detection of various pathogens, Antibiotic sensitivity testing (AST) protocol and molecular detection methods for implementation of surveillance system in and harmonised manner.7
WHO also established Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) to support and build national capacities on integrated surveillance of AMR & AMU.8 In its 6th review meeting it was agreed to develop and standardise protocols with One Health approach. ESBL E. coli Tricycle Surveillance, a model of integrated surveillance of AMR
with harmonises protocol with single key indicator was started to assess the frequency of ESBL producing E. coli in human, food chain and the environment.9
Food and Agriculture Organization (FAO) which looks after agriculture and food sector is also active in surveillance of AMR and has developed FAO Assessment Tool for Laboratories and AMR Surveillance System (FAO-ATLASS).10 For this they have developed platform, International FAO Antimicrobial Resistance Monitoring data (InFARM) for collection, analysis and reporting of AMR data from food and agriculture sector at national level, including AMU data in plants and crops.11
World Organization of animal health (OIE) has mandate to improve animal health, welfare and veterinary public health. The organisation has set standards related to AMR & AMU which are available in Terrestrial Animal Health code and Aquatic Health code.12 The standards and protocols are aimed to harmonise national, AMR surveillance and monitoring program. On antibiotic usage OIE collect data from member countries on usage of OIE listed antimicrobials of veterinary importance and is regularly publishing report. OIE in its ongoing efforts is working on an AMU database project for
the countries to have tailor made tool of their need, setup tool/software to help countries in annual collection of data on AMU.
Accepting the importance of AMR across different sectors and to retain the effectiveness of antimicrobials in treating diseases, promote health of the people and food safety, the three international organisations in its resolution in 68th WHA stressed upon the collaborative and multisectoral ‘One Health’ approach to address the problem. As a consequence of this, in May 2018 the three organisation signed a Memorandum of Understanding (MoU) as a Tripartite agreement for joint cooperation to address the issue of AMR.13 The Tripartite also involved United Nation Environmental Program (UNEP) to integrate environment as well in their efforts to combat AMR. They have developed Tripartite workplan to be implemented in 10 pilot countries where impact of AMR is likely to be greatest. The workplan will help in implementing multisectoral National Action Plan (NAP) on AMR across the human, animal, plant, food and environment. The Tripartite agreement has also advocated for a common platform Tripartite Integrated Surveillance System (TISSA) where harmonised surveillance data from different sectors will come through their respective

organisation and available at one point for analysis of trends and policies decisions.14
Whereas in 2019, due to the pandemic, the aggressive efforts employed to combat AMR were redirected to protect the world from the immediate threat. The lack of a solid public health system, which may contribute to the emergence of antibiotic resistance throughout healthcare institutions, nations, and the globe, was painfully obvious. In the year 2019, estimates for the number of deaths associated to bacterial AMR ranged between 3.62 million and 4.95 million.15
International leadership united with more comprehensive measures after fully comprehending the implications of AMR. The Global Leaders Group on Antimicrobial Resistance was created in January 2021 for the purpose of collaborating for long-term political action on AMR.16 In collaboration with UNEP, a Tripartite Strategic Framework on AMR for the years 2022–2026 was prepared. The "Call to Action" for the UN General Assembly High-Level Dialogue 2021, which was endorsed by 35 non-state entities and signed by 113 Member States, included AMR as a key component.17
REFERENCES
1. Global Action Plan on Antimicrobial Resistance. Microbe Mag. 10, 354-355 (2015).
2. www.un.org/pga/71/event-latest/high-level-meeting-onantimicrobial-resistance/, visited on August 22, 2022.
3. https://amrcountryprogress.org/#/map-view, visited on August 22, 2022.
4. World Health Organization. Global antimicrobial resistance surveillance system (GLASS): technical meeting on the early implementation phase. (2016).
5. https://www.who.int/publications/i/item/9789240027336, visited on August 22, 2022.

6. Arthur, R. R., LeDuc, J. W., Hughes J. M. Global Surveillance for Emerging Infectious Diseases. Tropical Infectious Diseases: Principles, Pathogens and Practice. 9. 105. (2011)
7. https://www.cdc.gov/ncezid/dfwed/pdfs/gfn.pdf, visited on August 22, 2022.
8. World Health Organization. Integrated surveillance of antimicrobial resistance in foodborne bacteria: application of a one health approach: guidance from the WHO Advisory Group on Integrated Surveillanec of Antimicrobial Resistance (AGISAR). (2017).
9. WHO. Global Tricycle Surveillance – ESBL E.Coli - Integrated Global Surveillance on ESBL-Producing E. Coli Using a “One Health” Approach: Implementation and Opportunities. (2021).
10. FAO. FAO Assessment Tool for Laboratories and AMR Surveillance Systems (ATLASS). Available online at: http:// www.fao.org/antimicrobial-resistance/resources/tools/ fao-atlass/en/ , visited on August 22, 2022.
11. https://www.fao.org/antimicrobial-resistance/resources/ database/infarm/en/, visited on August 22, 2022.
12. https://www.woah.org/en/what-we-do/standards/ codes-and-manuals/, visited on August 22, 2022.
13. FAO, OIE, WHO. MoU Regarding cooperation to combat health risks at the animal-human-ecosystems interface in the context of the “One Health” approach and including AMR. (2018)
14. WHO, FAO, WOAH, UNEP. The Tripartite Workplan on
antimicrobial resistance. J Lang Relatsh. (2019)
15. Murray, C. J., Ikuta, K. S., Sharara, F., Swetschinski, L., Robles Aguilar, G., Gray, A., Han, C., Bisignano, C., Rao, P., Wool, E., Johnson, S. C., Browne, A. J., Chipeta, M. G., Fell, F., Hackett, S., Haines-Woodhouse, G., Kashef Hamadani, B. H., Kumaran, E. A. P., McManigal, B., Naghavi, M. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet, 399(10325), 629–655, (2022).
16. https://www.amrleaders.org/#tab=tab_1, visited on August 22, 2022.
17. WHO. WHO Strategic Priorities on Antimicrobial Resistance. (2022).
Hina Malik
The author holds a graduation degree in Veterinary Science and a master’s degree in Veterinary Public Health. She has been working as a veterinarian in the Uttarakhand Animal Husbandry Department for the past seven years. Currently she is pursuing a doctorate in veterinary public health and epidemiology at the Centre for One Health, Guru Angad Dev University of Veterinary and Animal Sciences in Ludhiana, India. Her study focuses on zoonotic diseases with emerging antibiotic resistance in one health framework.

Email: hinamalik.vet@gmail.com
Wasimuddin
Senior scientist at Centre for Cellular and Molecular Biology, Hyderabad, India. The focus area of research of the author is the One Health framework focusing mainly on human and environmental health in terms of shifts in microbiome and the emergence of antimicrobial resistance. The author has many research and review articles to his credit on host-pathogen interaction and microbiome dysbiosis in many international journals. Currently, he is working on the surveillance of antimicrobial resistance in human and the environment in India.
Email: wasim.bt@gmail.com
Randhir Singh

Professor at the Centre for One Health, Guru Angad Dev University of Veterinary and Animal Sciences in Ludhiana, India. The focus area of research of the auther include zoonoses, food safety and antimicrobial resistance in One Health framework. The author has many research and review papers and popular articles to his credit on zoonoses, food safety and antimicrobial resistance emergence published in various national and international journals.
Email: sainirandhir72@gmail.com
The Green Discussion Forum
In 2020, we decided that the theme for our year would be regenerating the veterinary world. I had been running The Webinar Vet for a decade and begun the online revolution for vets, which had taken millions of miles off the roads over the last decade. However, we wanted to do more to show leadership in the veterinary industry. As a digital educational business, it is arguably simpler to be sustainable than a large pharma company. However, we calculated our carbon footprint; offset double what we produced during the pandemic year; started a veterinary sustainability podcast and planted a wildflower meadow at the Liverpool Science Park along with encouraging our landlord to embrace renewable energy providers.
Part of beginning a sustainability journey is that the further you get down the road, the more you realise more can still be done. Many larger companies have not yet started, because they don’t know how to start. Small steps are enough at the start to build the company owner’s confidence.
Part of this year’s journey was to try to facilitate bringing the veterinary industry together to see how we can collaboratively move faster into a more sustainable future. I was at COP26 and I realised how government, industry and individuals need to work closely together to achieve the environmental targets that have been set for this decisive decade. Carbon seems to be the main focus that everyone concentrates on but it is not very holistic. When I decided to organise the forum, I wanted to look at resource use and biodiversity as well as climate change and carbon.
It's very hard to get a lot of big companies in the room initially. Companies want to know who else is turning up and, inevitably, there is some distrust in being too open in sharing where they are in their sustainability journey. From the start of the process, I made it clear that this would be a safe place to discuss topics and that Chatham House rules would apply. Sustainability and regeneration are such big topics and companies are learning as they go. Every company that is taking these topics seriously have strengths and weaknesses in its approaches and I believe we will learn quicker if we work together. Collegiality and collaboration are more important than competing against each other in the environmental arena.
This was the background that led me, as a vet in an independent business, to start bringing companies in the veterinary space together to collaborate in this essential area of veterinary regeneration and sustainability. This journey will not be finished next year. It is a long journey, but one with a clear goal of reducing carbon production by 50%; creating a

more circular, less wasteful economy and having 30% of the land of high biodiverse value by 2030 (30 by 30).

The Webinar Vet opened for business 12 years ago and is well-trusted in the industry. It is set apart from the pharmaceutical, feed and corporate veterinary practice groups and can, hopefully, act as a facilitator. It’s possible that an association can also take this role. At the inaugural forum, we were incredibly fortunate to have our regulator, the Royal College of Veterinary Surgeons, present as well as the British Veterinary Association, The British Small Animal Veterinary Association, The British Cattle Veterinary Association and VetSustain. This, obviously, enhanced the credibility of the forum.
The Webinar Vet has never held a physical conference. Indeed, during the pandemic, we helped over 50 associations and companies take their events online due to our decade-long experience of running online events. However, we decided that a green discussion forum had to take place at a physical venue and so we decided to hold it at Brockholes Nature Reserve just outside Preston. Holding it at a nature reserve would help to ground us and show clearly how protecting the environment was essential. The centre at Brockholes is an architectural masterpiece designed by the esteemed architect, Adam Khan. It floats on the lake and rises and falls as the lake depth varies due to floods and drought. As a result, its design has protected it from being inundated on several occasions There is much to learn from the design of the visitor centre which can help with the design of buildings on floodplains. It is a truly stunning creation using wood extensively to give it a natural, warm feeling.
We were keen to bring the representatives of the companies together to facilitate networking and discussion and the content of the forum was mainly created by the delegates. Although, we had a keynote speaker for every session of the two-day
forum which also included an afternoon working in the reserve to rake cuttings off one of the wildflower meadows or removing invasive Crassula helmsii or New Zealand pygmyweed from the main lake. This session was characterised by the number of breaks that delegates took to chew the cud with fellow delegates and carefully remove the myriads of small toads and frogs that we found on the meadow. Everybody clearly enjoyed working on the land!
After much planning and hard work, we managed to get 40 individuals in the room, representing 24 companies and associations. Inaugural meetings are always tricky. Some companies are more willing to take a risk on a new event in the calendar whilst others wait to see if it fails or not. All industries should be attempting these collaborations. It is better to attempt such an event that fails rather than not to try. I must admit that I was worried and a bit stressed before the event and decided that I would mainly walk to the event as well as using public transport so that I could immerse myself in the beautiful natural landscape and calm myself before the event. This was a very good move before the forum began.
Over the course of two days, we spent time listening to each other and the keynote speakers as well as discussing the important questions in small groups. Our keynote speakers were dialled in virtually to keep our carbon footprint as low as possible. We recorded everybody’s journey to the congress and offset the carbon emitted to make it a carbon-neutral event. It was also not for profit with several thousand pounds being donated to the Lancashire Wildlife Trust which own Brockholes.
Climate Change and Greenhouse Gases
Our first main keynote speaker was Juliet Davenport, the founder of Good Energy. Juliet spoke about the ongoing energy crisis which was partly due to the slow uptake of renewable energy. She also spoke about the journey she undertook to start Good Energy at the beginning of the millennium when no one else was really interested in the space. Insulation must also be a part of our journey towards net zero as this would massively reduce our energy needs. This must become a key part of every individual’s and company’s environmental commitment.
One of our delegates had done magnificent work moving their company’s car fleet away from diesel and petrol to hybrids and electric cars. Again, because of government tax reforms, this is not only good for the environment but also for employees’ pockets. She explained the benefit in kind advantages of these schemes. I am a committed environmentalist but if we can encourage a change of behaviour using money as the carrot this will guarantee greater uptake from people who do not understand the environmental issues.
One of our delegates was a chief sustainability officer at a leading insurance company. Scope 3 is a challenge for a lot of bigger companies. What are their suppliers doing on their sustainability journey? This will affect the emissions that another company is indirectly responsible for. If they did not need their materials, then they wouldn’t be produced and lower concentrations or fewer greenhouse gases would be emitted. Many companies have not truly grasped the importance of scope 3 to calculate their own carbon footprint but also to encourage their suppliers to begin the journey to net zero. The general consensus was that Scope 3 can be ignored until the
company’s own emissions are quantified and can be lowered and offset. The next stage of the journey to deal with scope 3 is more complicated but many important skills will have been learnt dealing with scope 1 and 2 emissions that will make dealing with scope 3 easier. The veterinary industry’s journey to a net zero future will be a series of stepping stones and we need to move from one stone to the next to reach our net zero destination.
The delegates then broke into small groups and discussed some circulated questions:
• What is your company doing to reduce its carbon usage in the next decade? What is the target?
• Is it time for the industry to follow the World Veterinary Association’s lead and declare a climate emergency or do we need to work closely together to create a set of standards?
• What are your thoughts about offsetting against carbon?
• How can we produce our own energy as businesses?
• The Amazon rainforest is now emitting carbon. How does that make you feel?
• How can we as an industry lobby government to move faster?
• Are practice groups discussing Scope 3 with you?
The discussion was so important to allow people to get to know each other as sustainability leads in their business but also to listen deeply and learn from each other. To facilitate the listening the symbol for listening in Chinese was displayed. This shows that listening involves the ears but also the eyes, heart and indeed the whole being. Delegates were encouraged not to interrupt other delegates who were speaking. A stone was used which every person speaking had to hold and only relinquish when they had finished speaking. This system was not abused by dominant personalities refusing to give the stone away!
The rest of the first day was spent networking and working on conservation projects on the nature reserve. At the end of the day, we returned to our hotel which was only 1 mile away to get spruced up. Many of the delegates walked through the reserve to get back to the hotel. We returned to the nature reserve in the evening to enjoy some fine food which had been locally sourced and was of good provenance along with some fine Italian organic wines from Vintage Roots which everyone enjoyed. Tom Burditt was our guest of honour and the CEO of the Lancashire Wildlife Trust. I’d asked him to speak about natural capital for about 10–15 minutes but he spoke for 55 minutes and had everybody spellbound. So many people spoke favourably about his passion for the topic. If we obsess too much about carbon, we forget about the inherent value of habitats already there which are biodiverse and have no carbon value but natural capital.
Many of the delegates walked to the reserve on the second day to see lovely views of deer and waterbirds by the River Ribble and on Brockholes Lake.
Our second keynote speaker was Professor David Goulson, author of The Silent Earth which bemoans the massive reduction of insect life which also affects their predators like spotted flycatchers whose populations have crashed in the UK. There are many causes for this precipitous loss, however, the
advent of very potent insecticides since the banning of DDT has certainly been a factor and the profession now worried that some of those insecticides used to kill fleas and other problematic insects may also be getting washed into streams and affecting aquatic life.
The day continued with talks on large and small animal parasiticide usage. A veterinary parasitologist described his work on a group of farms where reduced parasiticide usage and increased lungworm vaccine usage had led to healthier, more productive cattle. Justine Shotton, BVA president, spoke about the need to reduce preventive parasiticide usage in dogs and cats to prevent contamination of the environment with medicines. This is a problem across all medicine types including antibiotics and also across the various medical professions.
Sue Paterson from the Royal College of Veterinary Surgeons moved away from this topic to explain that the practice standards scheme run by the college now included an award for sustainability to encourage more practices to think positively about environmental concerns.
Some of the questions discussed in the small groups were as follows:
Biodiversity
• How much do you think the veterinary industry is part of the solution or part of the problem?
• How can we encourage practices to make their spaces more biodiverse?
• Should vets be the only one to prescribe anthelmintics?
• How can we make sure that products are safe in the environment?
• Are accreditations like Investors in the Environment and Bcorp worthwhile?

We then broke for questions followed by lunch.
The final afternoon we discussed resource use in the profession. Jen Gale was our keynote speaker discussing resource use and the circular economy. She is a veterinary surgeon and famous as the author of “Sustainable-ish Living Guide.” She spent a year not buying anything new and also is famous for emptying her bin for the first time this year in August. The circular economy was discussed. There is so much work needing to be done in this area to improve individual, corporate and government responsibility. During this session, other veterinary businesses discussed some of the work they were doing with biodegradable packaging and targets to reduce the rubbish going to landfill. Having a well-thoughtthrough sustainable plan was also deemed to be essential.
The forum finished with a discussion amongst the small groups around resource use.
Resource Use
• What are your targets for recycling and using recycled material in packaging?
• Any new innovations we can talk about?
• Could the industry come together to encourage recycling in practices?
• Which areas should the profession be concentrating on?
• Does the industry need to develop take-back schemes for packaging?
What Should We Be Copying from Other Industries?
The forum was very well received, and we are intending to run this at nature reserves until 2030 to act as encouragers for the various veterinary companies attending to work towards targets around carbon, biodiversity and resource use. This is the decisive decade and it is so important that we work together as industries to tackle the ongoing environmental challenges.
Anthony Chadwick

Anthony Chadwick BVSc CertVD MRCVS qualified from Liverpool University in 1990 and received his certificate in Veterinary Dermatology in 1995 from the Royal College of Veterinary Surgeons. Anthony was involved in first opinion practice and dermatology referrals until 2016. In 2010 Anthony set up The Webinar Vet, the first online training platform for veterinarians and nurses, in an attempt to make veterinary education more accessible and affordable across the world. Since that time tens of thousands of veterinarians and nurses have accessed the platform from all over the world. The Webinar Vet’s first virtual conference took place in 2013. During the pandemic, The Webinar Vet helped to take over 40 veterinary meetings and conferences online including WVAC2020 and WCVD9. In 2021, Anthony took the business carbon negative, helping to stand by The Webinar Vet’s principles of being as sustainable as possible and delivering exceptional quality training, internationally via remote means. The Webinar Vet is an Investor in the Environment Green Accredited business.
Web: www.thewebinarvet.com
IPI
Peer Reviewed, IPI looks into the best practice in outsourcing management for the Pharmaceutical and BioPharmaceutical industry.



www.international-pharma.com


INSIGHT / KNOWLEDGE / FORESIGHT SUPER PUBLICATIONS FOR SUPER PHARMACEUTICALS


JCS
Peer Reviewed, JCS provides you with the best practice guidelines for conducting global Clinical Trials. JCS is the specialist journal providing you with relevant articles which will help you to navigate emerging markets. www.journalforclinicalstudies.com


PHARMA’S DNA
Listen to industry experts on the latest in drug discovery, development, research, industry regulations and much more at Pharma,s DNA, the podcast channel by Senglobal Ltd., available on Sound Cloud, Spotify, iTunes and YouTube.
IAHJ
Peer Reviewed, IAHJ looks into the entire outsourcing management of the Veterinary Drug, Veterinary Devices & Animal Food Development Industry. www.international-animalhealth.com


IBI
Peer reviewed, IBI provides the biopharmaceutical industry with practical advice on managing bioprocessing and technology, upstream and downstream processing, manufacturing, regulations, formulation, scale-up/technology transfer, drug delivery, analytical testing and more. www.international-biopharma.com
New Paper-based Security Seals Revolutionise Pharma Packaging
EU-wide Amendment of the Packaging Law & Plastic Reduction
Since the 60s, the use of plastic has increased twentyfold. The EU is therefore focusing on a sustainable economy and is pulling the emergency brake on plastic packaging in particular. The Single-use Plastics Directive is only one measure to achieve this goal. The first step was the ban on plastic bags – products such as cotton swabs and drinking straws are also already adopting alternative packaging materials. In the future, there will be penalties for packaging that is not designed to be recyclable within the European Union. Further bans on plastics for certain applications are not ruled out. According to Securikett CEO, Werner Horn, "A switch to our new paper-based VOID seals and VOID tapes will therefore pay off for all businesses by eliminating the risk of non-compliance with material requirements of new regulations. The pulp material used by Securikett can be recycled together with folding cartons and complies with the new EU general circular economy package."
The trend toward use of recyclable and renewable materials is not just a European issue: recycling-friendly packaging based on renewable resources is in demand worldwide!
‘One Package – One Material‘
If the packaging and label are made of the same material, the cost, efficiency, and effectiveness of the recycling process is significantly enhanced. Thus, paper security seals optimise the recyclability of all cardboard or paper packaging.

"By using the latest paper-based security seals, pharma companies can not only package their products in a tamper-proof way that complies with all international standards, but also make a sustainable contribution to environmental protection and avoid plastic," says the CEO of Securikett, Werner Horn, who is committed to producing high performance security products in an effective, efficient and sustainable manner. Securikett’s PaperVOIDs therefore combine all benefits from FMD-compliant closure seals with sustainability and environment protection.
Sustainable Packaging and Product Digitalisation in One Step

Securikett was the first in the market to launch paper-based tamper evident security labels with an integrated QR code. These labels provide distinct proof of opening, the so-called VOID effect, and give the manufacturer the ability to communicate further information about the product directly to the customer via a simple scan of the label. In this way, product digitalisation is simple and sustainable. The unique codes are created
A Superlabel Made from Renewable Raw Material that Can Do it All
The new PaperVOID labels can be fully customised to the customer's corporate design. When it comes to product properties such as sustainability, origin, authenticity, supply chain integrity, ethics, etc., digital Securikett technology enables this information to be linked directly to each individual sales unit of product. In this way, the properties can be communicated to the customer in a trustworthy, transparent and personal way and increase the integrity and perception of the product.
Innovation Comes from Austria
Securikett, an Austrian company specialising in digital and physical security systems for a broad spectrum of product sectors, is attracting attention with its invention of the new paper security seal. The company has been a key supplier to major industrial companies around the globe for years and is a global leader in the development and supply of transparent and opaque security closure seals.
"Our VOID labels turn all types of packaging into safes," says Werner Horn, the company's CEO, explaining how they work. "We are pleased that we can now offer this special paper-based
From left to right: 1. label applied on box, 2. peeling off the
VOID technology solution. Paper is one of the most renewable raw materials and recycling of paper is well established. Pulp fibers can be recycled up to 8 times."
The security closure seals are characterised by the fact that an irreversible effect remains on the packaging when it is peeled off. Opening and tampering of the packaging is thus immediately and clearly obvious. The company produces these new paper VOID seals in various designs, sizes and colors.

3.
on
After opening or peeling off the
such as “VOID” or “OPEN” become visible due to a color change.

Paper-based Security Seals


Securikett's research department has been working for a long time on the goal of being able to produce paper-based security seals with equivalent performance and security as the plasticbased security seals that have been more commonly produced up to now. The goal included development of both opaque and transparent security seals, in which the special security VOID effect is displayed when the seal is peeled off.


Vanessa Mitterer, Msc. Head of the Research Department, says: "Thanks to technical innovations, we have succeeded in producing a seal from the renewable and recyclable raw material paper that is superior to the plastic seal in many aspects. PaperVOID deforms when peeled off and is particularly resistant to manipulation with water and liquids."
business
one-stop SMART
integrating manipulation evidence, security and IoT services. The company was founded 2001 and engages 90 employees. Applications by Securikett are used on luxury goods, spirits, medicines, spare parts and shipping boxes. Based in Austria, Securikett currently distributes products to 45 countries around the world.
Meeting the World’s Evolving needs for a More Sustainable Future: How to Realise a Circular Approach to Drug-delivery-solutions
The world is looking for companies across all industries to take leadership positions that not only advance their traditional business metrics, but more importantly improve their impact on the environment and people’s lives around the world. When it comes to sustainability, there is not a single easy solution to solve all issues. Instead, taking a holistic view across various aspects of our business has led Aptar along the path to meeting its sustainability goals. Companies are increasingly adopting the principles of the circular economy where waste and pollution are eliminated or reduced as they are designed out of products throughout their lifecycle.
And it’s not just manufacturers who are supporting these potential solutions. Consumers are also demanding higher recyclability of products and are willing to pay more for them. In a recent Aptar Pharma survey conducted in 2022 with German, French and American participants, 77% of in total 840 respondents indicated that it was important or very important that the products they buy can be recycled. Further, 60% of respondents consider the recyclability of products when making purchasing decisions and 70% said they were willing to pay more for a product that they could recycle. Participants also voiced that they were willing to adapt their consumption habits to contribute to a reduced environmental impact, with six of ten respondents willing to use a refill/reload system to reduce environmental impact. These survey results confirm that consumer demand for increased sustainability, recycling and circular economy initiatives will be reflected in their buying decisions moving forward.
At Aptar, we’ve strategically assessed our businesses, which encompass pharmaceuticals, beauty, personal care, home care, food, and beverages, and have worked to embed sustainability throughout our business and within our product offerings. As the pharmaceutical industry is subject to very strict regulations, the introduction of such sustainability initiatives within our Pharma business must achieve not only the sustainability objectives but also maintain adherence to all regulatory, GMP (Good Manufacturing Practice) and quality requirements. We have categorised our sustainability programs into the three main action areas: Care, Collaboration and Circularity.

Care
As a company we show care to our employees, communities, and environment by continuously improving our impact and reducing our footprint. Our focus on care – for people and for our shared environment – as a key element of how we shape our future and achieve our objectives. Last year, Aptar named a Global Top 10 Female-Friendly Company
by Forbes for its progress on leading the way to support women inside and outside of their workforce. Notably, at Aptar in 2021, women accounted for 20 percent of leaders at the Vice President level and above. We have also grown our Employee Resource Groups (ERGs) to include the ALIGN Women’s Network, BOLD (Black/African American or African Descent) and ARC (LGBTQ+ community and their allies). We support these people focused initiatives, as Aptar firmly believes in the diversity and importance of every employee and the potential benefit they bring to Aptar, our customers and the world.
A second aspect of our care action area is reducing our footprint on the environment. We established a science-based target for the reduction of our Scope 1 and Scope 2 emissions by 28% by 2030 from a 2019 baseline. By the end of 2021, Aptar proudly achieved 74% of the goal, well in advance of the original target date. Aptar remained on track to achieve goals to source more renewable energy. In 2021, renewables accounted for 96% of electricity purchased, with the goal of 100% targeted by 2030. Also of note, 63% of Aptar’s facilities had achieved Landfill
Free site certification at year-end of 2021 through our dedication to our internal waste reduction certification programs. We have additional sustainability targets related to both people and the planet and we remain committed to building a better future. (see table 1).

Our care action area demonstrates that through care for both people and the environment, Aptar can achieve broader objectives benefitting the company, customers and the planet.
Collaborate
We collaborate with customers, suppliers, industry coalitions and nonprofits to progress towards goals and better outcomes for people and the planet. These collaborations are important as they allow Aptar to gain and share knowledge and develop innovations that deliver economic, performance and environmental value.

Table 1: Aptar defined corporate targets that aim at bringing forward our sustainability efforts. The group is on track and even surpassed some of the goals at year-end 2021.
Examples of Aptar’s collaborations across segments and industries include:
• Partnering with PureCycle Technologies as its preferred technical partner to test and transform their Ultra-Pure Recycled plastic for food, beverage and cosmetics applications
• Active participation with the Ellen MacArthur Foundation (Cowe’s, UK) as a signatory of the “New Plastics Economy Global Commitment”, within a recyclability working group through the CE100 Network, and piloting their “Circulytics” circular economy assessment tool
• Expansion of domestic North American production of Activ-Film™ technology to ensure COVID-19 test kit integrity and accuracy through the support of a $19 million U.S. Government contract
• Dedicated supplier engagement on sustainability objectives. By the end of 2021 Aptar had engaged approximately 40% of its supply base to manage their emission reduction efforts through a collaboration with EcoVadis
As system thinkers and change-makers, Aptar is committed to working alongside, and often leading, others by identifying solutions, processes and products that enable us all to move forward together.
Circularity
We are helping the industry advance system-scale changes that will benefit people today and generations to come by addressing climate change and waste crisis through Circularity.
The traditional take-make-dispose linear production cycle is rapidly being replaced with the concept of the circular economy or circularity. This circularity approach is based on developing designs and processes that emphasize resource reduction, extended use, reuse, recycling or composting of products or services and regenerate natural systems. These objectives must not compromise performance, safety or health requirements, which are especially important to Aptar Pharma’s products as many save or improve human lives.
Aptar Eco-Design Tool Advances
Aptar partnered with an internationally respected consulting firm to develop an Eco-design tool that incorporates Lifecycle Assessment (LCA) functionalities that assess how products could impact the environment. The recently enhanced Eco-Design approach and LCA tools help our product design professionals better assess inputs and outputs and potential environmental impacts of the product system through the entire lifecycle of the product. Key performance indicators assessed by the tool is the CO2 footprint, recyclability and circularity. The new tool is now applied to the development of every new Aptar Pharma product in order to enhance their circularity and reduce the CO2 footprint of Aptar products globally.

Recyclable Metal Free Nasal Spray Pump Solution Using Eco-Design Tool
Aptar Pharma is in the advanced stages of developing a recyclable, metal free, nasal spray pump composed only of plastic components with no metal parts. This latest nasal spray pump was designed using our Eco-Design tool to maximise
recyclability from the earliest design stages. The full plastic nasal spray device will offer a very high degree of recyclability while delivering the superior level of reliability and precision expected of Aptar Pharma’s advanced nasal spray technologies including support for preservative free formulations. This metal free, nasal spray pump was primarily designed to provide a recyclable solution for nasal saline formulations and comparable nasal spray products (see table 2).
4Rs – Reduce, Reuse, Replace, Recycle
A concept that is critical to sustainability and engaging in the circular economy includes the four R’s. They are objectives to strategically Reduce, Reuse, Replace and Recycle at every opportunity throughout our business. Aptar Pharma has made great strides in many of these areas as part of our comprehensive sustainability objectives globally.

1. Reduce
As a drug delivery technology manufacturer, Aptar Pharma has been highly focused on ways to reduce the use of materials in device production and to minimise drug formulation waste. One effective way Aptar Pharma contributes to reduced material consumption is by designing drug delivery technologies that by function and inputs conserve materials when compared to other existing drug delivery technology options.
Aptar Pharma Ophthalmic Squeeze Dispenser (OSD) Compared to BFS Vials
Aptar’s unique multi-dose ophthalmic eye drop dispensing device has been designed for the ocular delivery of preservative-
free formulations. The design includes a purely mechanical tip-seal that eliminates the need for preservatives or additives such as silver ions to prevent contamination of the formulation. When compared to a traditional single-use blow-fill-seal (BFS) vials the OSD technology presents a more sustainable option for preservative-free eye drops. We recently performed an analysis that compares Aptar Pharma’s OSD against BFS vials for the same treatment course. For simplicity this comparison focuses only on the raw material extraction and production phase differences (see graphic 1).
With single-use BFS vials, residual medication must be discarded from opened vials after every use. With Aptar Pharma’s OSD, all of the medication can be used to the final drop. Based on a very conservative assumption a once daily treatment consists of 2 drops per eye with a 30–40 µl per drop, and 160 µl of product administered. These Blow-Fill-Seal (BFS) vials are filled with 300 µl of formulation in order to deliver the required dose, resulting in 140 µl of waste formulation. This discarded formulation represents approximately 46% of the total medication filled in each BFS vial.1 A single OSD eye drop dispenser with a 10 ml fill can deliver approximately 60 days of this dosing regimen. It would require a pack of 60 BFS vials (18ml total formulation fill) to match the equivalent treatment duration of 60 days. The BFS single use vial treatment course would use nearly 10x the primary packaging material as compared to a single Aptar Pharma OSD 2 in order to deliver the 10ml of formulation to the patient/customer. With Aptar Pharma’s Ophthalmic Squeeze Dropper (OSD), one multidose device can deliver enough formulation for the entire
Graphic 1: Aptar Pharma's multi-dose eye dropper for preservative-free formulations, OSD, has been compared to single-use BFS vials for primary packaging material use. Based on the standard OSD formulation volume of 10 ml, OSD offers an 83% reduction of primary packaging material as compared to BFS vials. OSD generates an 87% reduction in Global Warming Potential (GWP) based on the comparison with single-use BFS vials.

1) All calculations have been made with Aptar's EcoDesign LCA tool. No third party review was conducted. Secondary packaging / cartonage has not been considered in this approach.
treatment with virtually no residual formulation to discard. Additional sustainability advantages for OSD over BFS vial eye drop delivery (based on materials 10 ml formulation volume requirement) includes:3
• Reduction of packaging materials reducing global warming potential (GWP) by 87%
2) 1000 pieces stands for 1000 packaging units used for 10ml eye drops. For BFS that would be appr. 33,333 single use vials. feasible and would clash with current regulatory requirements. Aptar Pharma continues to pursue the development of solutions enabling compliant reuse or refill drug delivery systems, made possible through innovative rethinking of our approach to such drug delivery systems. In fact, significant preliminary progress on reuse has been achieved with our Consumer Health Care lines, that can have a significant impact on the market overall. This is a complex challenge for the industry, but Aptar’s efforts are positioning it to be a leader in the pursuit of solutions.
• Use of 100,000 OSD devices instead of the equivalent 3.3 million single use BFS vials, saves 8,090 kg of CO2 equivalents
• OSD generates lower carbon emissions during transportation than BFS. If you consider the volume and mass of both wasted formulation solution and bulky plastic waste differences, the OSD option would take considerably less space on a shipping pallet and consume less energy to transport, thereby markedly reducing the CO2 footprint as it is transported to the consumer.
• 67% more medication per product unit provides up to 3x longer treatment duration
• Overall, Aptar Pharma’s OSD greatly reduces plastic material use and minimises the waste formulation discarded as compared to single use BFS vial systems.
2. Reuse
Although reuse is an important part of the 4 R’s it is more challenging than most to institute in the highly regulated pharmaceutical industry. The primary function of pharmaceutical drug delivery devices is to reliably deliver pure and efficacious drug product to the patient in a form that meets current regulatory and safety requirements. Reuse of such devices is difficult in the medical space as the units are typically self-contained and sealed against external contaminants. As a practical safety measure reuse of such devices is generally not
3. Replace
Aptar Pharma continues to seek ways to replace harmful or polluting technologies or materials with those having that have a less negative impact on the environment and support increased sustainability.
Sustainable Pressurised Metered Dose Inhalers (pMDIs)
With decarbonisation efforts in high gear in the respiratory or pulmonary drug delivery category, Aptar Pharma is fully engaged in helping our clients in the transition from the current widely used hydrofluoroalkane (HFA) propellants, such as HFA 227 and HFA 134a and other Hydrofluorocarbons (HFCs). They are now restricted as part of the Kigali Amendment (2016) to the Montreal Protocol, which seeks to phase down HFA/HFC use by 85% by 2047. Both patient safety and functional drug delivery aspects must be proven in studies before converting existing products to a new propellant. HFO1234ze and HFA 152a are two potential low global warming potential (GWP) propellants under assessment at Aptar Pharma. Ongoing studies are underway, involving multiple Aptar Teams, to ensure both mechanical and chemical compatibility between Aptar’s metering valve technologies (and respective materials) and the propellants and respective formulations (including any necessary
excipients). HFA 152a has undergone exhaustive full inhalation propellant toxicology studies showing promising results and no adverse findings to date. The University of Manchester has also demonstrated that replacing HFA 134a with HFA 152a would reduce climate change and global warming impacts of inhalers in the UK by 90–92%.4
HFO1234ze has the potential to provide an even greater GWP reduction of 99% with more research and data being developed with customers and partners. However, less toxicology and safety data is available within the public domain to date, while additional toxicology data may be shared with interested clients under confidential disclosure. Aptar Pharma chose to work on replacement options seeking beneficial alternatives to current pMDI propellant gases. When customers eventually transition to these new alternative propellants, Aptar Pharma will be there to support them with data and services. Aptar Pharma supports customers with device selection, formulation development, analytical services and regulatory support. To help further de-risk and accelerate our clients’ low GWP pMDI propellant replacement programs, Aptar Pharma also provides mixing and filling services at its Le Vaudreuil facility. The facility recently installed and validated an ATEX rated filling suite, where our technical experts work hand in hand with customers utilising pMDI propellants in our pilot plant, in order to efficiently develop and optimise device-ready formulations. Aptar Pharma also offers proprietary and novel Respitab™ technology that eliminates the need for the mixing process, saving cost and reducing risk. Our SmartTrack™ tool can be applied to make clinical endpoint studies redundant, saving time and money. Most critically, Aptar Pharma offers regulatory support including clinically relevant in-vitro/in-silico methods to eliminate the need for clinical endpoint studies. Aptar understands the complex requirements of converting to more environmentally friendly propellants and is fully equipped to support customers making these important advancements for a more sustainable future.
Chemical Phase-Out
Another example of replacement includes our Chemical Phase-Out Initiative. Aptar is committed to the Chemical Phase-Out of a number of potentially hazardous substances that
are commonly used in consumer packaging by 2025. This includes the elimination of these chemicals in the packaging production process or replacement with an alternative substance. We have used the chemical phase-out initiative as an opportunity to also improve the recyclability of our packaging and in anticipation of future regulations. We continuously monitor the regulatory environment and work towards alignment with our practices, particularly in the pharmaceutical sector where regulations are among the strictest.
Phasing chemicals out typically requires that each impacted packaging item must undergo extensive product-process performance and robustness testing to ensure both performance and safety.

4. Recycle
Recyclability is critical to our device designs as we move forward. One measure of our success is through the classifications we receive for our technologies from institutions such as the cyclosHTP. cyclos-HTP is a reputable German organisation that assesses product packaging recyclability and responsibility for European countries and provides classifications for individual devices or packaging systems. Aptar Pharma is constantly looking for opportunities to improve the recyclability of their products through both design and innovation. This drive to improve Aptar’s devices through innovative design for recyclability has led to favourable ratings from cyclos-HTP for a number of our systems. As a member organisation of RecyClass, an organisation dedicated to the enhancing the recyclability of plastics, we are continually evaluating our products and optimising them for higher levels of recyclability. These recyclability improvements include designing mono-material systems that enable simple and complete recycling of devices without compromising functionality or safety. We also shift to using medical grade source materials for pharmaceutical applications that support recycling capabilities. Higher levels of recyclability can also be achieved by eliminating harder to recycle device component materials such as metals so that the entire device can be recycled without significant intervention or material separation steps. Aptar has looked at a variety of ways to enhance the recyclability of its products and invested in making these opportunities a reality across a number of its product lines.
Proventu – Mono-material Recyclability
The strategic development of mono-material systems is one way to increase a product’s recyclability. Proventu is the company’s first mono-material tube system designed to meet pharma product standards. Every part is composed entirely of medical grade polypropylene (PP). This eliminates the need for a separate elastomer valve that would reduce the recyclability. The tethered cap allows for one-handed closing and enables the entire device to be recycled with ease. Aptar Pharma strives to design and implement mono-material and highly recyclable systems as part of its recyclability programs.
Airless+ – High Recyclability Rated
Aptar Pharma’s Airless+ range of highly recyclable products for dermal drug delivery benefitted from using our Eco-Design tools. Tighter regulations regarding patient protection outlined in US Pharmacopeia (USP) <661> for “Plastic packaging systems and their materials of construction” were addressed in the design stage by using medical-grade resins in the manufacture of the device. Airless+ dermal delivery systems deliver precise dosing and a high evacuation rate that results in leaving a minimal amount of residual semi-solid formulation in the device when fully utilised. With only moulded components and no metal parts, the systems in the majority of the applications can seamlessly go into existing recycling streams with no additional preparation. Airless+ systems meet cyclos-HTP’s (Aachen, Germany) certification requirements and are rated "Class AAA" with an "excellent recyclability" rate (for raw, natural packaging without décor and label).
Bag-on-Valve (BOV) – Rated for Good Recyclability
Aptar Pharma’s Bag-on-Valve (BOV) continuous dispensing systems incorporate the circular economy and patient care considerations in one device line. The BOV system completely separates the product from the propellant providing a clean and advanced nasal or dermal delivery system. The compressed air or nitrogen propellant reduces greenhouse gas emissions compared to other commonly used propellants. The BOV system may contain recyclable aluminium, have removable actuators and offer high-evacuation rates, all contributing to higher recyclability. Designed for recyclability, our BOV systems achieved the cyclos-HTP qualification for “good recyclability” (Class A) of the raw packaging assembly. This certification was specifically assigned for Aptar Pharma’s BOV 30 ml, Pacifica Actuator (71% recyclable) and the BOV 400 ml, Nasal EP Actuator including cap and standard aluminium can. The Aptar Pharma BOV system provides industry leading nasal or dermal drug delivery with strong recyclability characteristics sign.
Supporting Mass Balance
Aptar Pharma’s sustainability objectives have continued to gain traction across the company. Another initiative we have successfully implement is the use of plastic resins following the mass-balance approach. Mass-balance is a system that is designed to trace the flow of materials through the complex value chain. It essentially tracks and securely documents the amount and sustainability characteristics of circular and/or bio-based content. Plastic components using resins following the mass balance approach will allow for gradually increasing the share of bio-based or circular feedstocks used in your production process. Having this knowledge gives you control over your material composition and allows you to make claims, based on the verifiable records you maintain (see graphic 2).
At Aptar Pharma, our Villingen and Mezzovico sites have deployed mass-balance approaches and achieved the ISCC PLUS certification from the International Sustainability & Carbon Certification organisation. The ISCC is composed of over 200 international stakeholders and provides the leading sustainability certifications across a range of industries. As a result, we have been able to incorporate renewable feedstock into our production sites, with the program continuing to expand to other sites.
For example, our Airless+ systems are manufactured in a facility that earned both ISO 14001 and ISO 50001 certifications and is certified for renewable feedstock use. With International Sustainability Carbon Certification (ISCC) PLUS qualifications, the mass-balance approach offers accountable renewable material flow across the value chain.
A successful mass-balance implementation could also help to lower the CO2 footprint of the product in a verifiable way. Aptar Pharma will continue to expand the application of the mass-balance approach across products and sites to help achieve its sustainability objectives and lower the CO2 footprint of its drug delivery products.
Dedication to Sustainability
Aptar Pharma has demonstrated that a lot can be accomplished in a short period of time when it comes to sustainability and the circular economy. But we are not even close to being done. We have more long-term objectives for future phases of our sustainability plans centered around our main action areas of Care, Collaboration and Circularity. Aptar Pharma stays ahead of the curve, keeping its eye on rapidly changing demands of consumers, customers and regulators. Doing our part for sustainability, recyclability and participating in the circular economy, Aptar Pharma will continue towards the future we all strive for on a healthier planet.
REFERENCES
1. Marguerite B. McDonald, MD: „Eye care moving toward multidose preservative free bottles for medications, tears.” Occular Surgery News, May,10, 2019. https://www.healio.com/ news/ophthalmology/20190502/eye-care-moving-towardmultidose-preservativefree-bottles-for-medications-tears
2. Calculation based on 10ml packed in 37.2g, hence 18ml packed in 66.6g (60 vials BFS) versus 10ml packed in 6.2g (one OSD)
3. All calculations have been made with Aptar’s EcoDesign LCA tool. No third-party review was conducted. Secondary packaging / cartonage has not been considered in this approach.
4. Jeswani H., Azapagic A., Life Cycle Environmental Impacts of Inhalers”. J Clean Prod, November 2019, Vol 237, Article 117733.
Stefan Ritsche
Christophe Marie
Inside Sustainability at Airnov A Look at How Healthcare Packaging Solutions Expert Airnov Continues to Pioneer Sustainable Products and Develop Sustainable Working Practices Across its Business

Sustainability is a core priority for almost all packaging producers, including those which support the healthcare sector.
As the world transitions towards a more circular way of operating, both in a personal and business sense, the ability to make sustainable enhancements down to the very last detail matters more and more.
At Airnov, a pioneer of innovative solutions for the healthcare and medical packaging sectors, sustainability and circularity underpin all of its business activities. Here, we will explore some of the company’s latest products and initiatives that are helping to enhance its environmental credentials and those of its customers.
Part 1 – Developing Sustainable Products

The most recent sustainable-oriented product line released by Airnov is HAT-B
A highly innovative vial, the product line has been developed and produced for applications in the pharmaceutical, diagnostic and nutraceutical markets, is available in two sizes and is compatible with existing filling lines. Furthermore, they contain an adjustable desiccant quantity and incorporate versatile sorbent materials to fit stability requirements – these include silica gel and molecular sieve.
HAT-B carries numerous sustainable features designed to limit carbon footprint, the most obvious and impactful being the fact it uses an optimised/reduced amount of plastic.
DRICARDTM, meanwhile, is a flat moisture absorber comprised of calcium chloride desiccant laminated between film layers. As with desiccant packets, DRICARD is designed to maintain a dry package environment to keep nutraceuticals, pharmaceuticals and diagnostics safe from the damaging effects of moisture.
Airnov has recently developed a new, more sustainable version that provides twice the capacity in the same size card, uses less plastic and is printed with high-contrast blue ink on a white background, meaning it requires 80% less ink to produce than the current orange DRICARD.
As with HAT-B, performance has not been compromised as a result of sustainability gains. On the contrary, it has actually been improved.
For example, its double thickness provides twice the moisture absorption capacity as its predecessor, all the while maintaining the same overall size and surface.
Furthermore, the rigid structure of the card allows high speed automatic insertion by card dispensers or pick-andplace systems, while its flat profile is designed for use in applications that require desiccation in small and narrow packaging spaces. The blue-on-white printing is high-contrast and high-visibility where it is essential to minimise confusion with the end-product.
The new DRICARD is available in a variety of standard sizes and can be tailored to custom sizes if required. It is also US FDAcompliant for use in nutraceutical and pharma applications.
Also, recently off the Airnov innovation production line is its new Light 27mm desiccant stopper, which was showcased in May of this year to industry stakeholders at the Pharmapack conference in Paris, France.
Fitted with a tamper evident security and easy to open features, this solution uses fewer raw materials and is made with a new sustainable polymer – two key features which are helping customers to reduce their carbon footprints.
New variations of Airnov’s laser marked canisters have also been launched.

Specifically, the company has released 2g and 3g sizes to round-out the product line. These canisters, which require fewer raw materials to make, use laser technology to create visible marks on the canister body without the use of inks, varnishes, adhesives, and other extraneous materials. This makes for a more sustainable product with less risk of contamination.
Part 2 – Deploying Sustainable Initiatives
In addition to manufacturing products with circularity and sustainability in mind, Airnov is also continuously assessing and adapting its own working processes.
ECOVADIS
Airnov’s stated aim is to devise and enact processes that add value to products and improve customer and employee experiences while lessening the company’s impact on the environment.
Central to this is minimising raw material and energy consumption in its production operations, as well as engagement in continuous assessment and improvement of its working methods and products to ensure that they are safe, sustainable and acceptable from the perspective of employees, customers, the public and all other stakeholders.
To achieve these goals, every facility at Airnov has implemented numerous initiatives, the company very much operating with the motto that even the smallest actions can have a huge impact.
In terms of ECOVADIS progress, Airnov France achieved ECOVADIS’ SILVER in August 2022. The status is in recognition of its implementation of ESG activities over the preceding 12 months, the aim now being to further improve its rating and use the French business as a template for other Airnov sites to follow. For example, the company will begin its ECOVADIS certification journey at its facility in Belen, New Mexico.
To further help achieve this end, the organisation recently implemented a Sustainability Committee, its major objective being to monitor the progress of ESG projects.
Secondary Packaging

One key initiative taking place at Airnov’s plant in Belen, and which will feed into its ECOVADIS certification process, is converting secondary packaging from plastic to cardboard. Traditional plastic pails are more difficult to recycle than carton boxes, chiefly because they are made from non-renewable polymer resins and difficult to compact after use. Cardboard, on the other hand, is a widely recyclable material that also naturally decomposes a lot faster.
By switching to carton-based secondary packaging in New Mexico, Airnov will be able to pull hundreds of tons of plastic out of its operational processes every year. In addition, cardboard alternatives are sourced from local forest, lighter in weight and more space efficient, helping to optimise transportation and storage. Indeed, the initiative aims to reduce transportation emissions by using double-stackable cartons. These new cartons, are designed to be more durable than standard cardboard, allowing pallets to be double stacked with 50% more material in the same footprint as a single pallet.
By adopting these storage and transport containers, Airnov is also helping customers to reduce freight costs while also cutting down on the number of trucks needed to transport goods, saving money, reducing waste of plastic pails and carbon emissions.
Crucially, once again performance is not sacrificed in the name of sustainability. The carton packaging adopted by Airnov performs equally to plastic pails by utilising high-barrier aluminum bags to provide the same shelf-life of three years.

Measuring Our Impact
None of these initiatives would be worthwhile without being able to measure the exact impact they are having.
This is why Airnov is also investing time and resources in building out its carbon footprint evaluation capabilities. In July 2022, the company partnered with a consulting firm to evaluate the total amount of greenhouse gases (GHG) generated by Airnov’s activities during a chosen year of reference.
The project is structured around three streams – mobilisation of Airnov employees and data collection, carbon footprint
measurement and carbon reduction roadmap definition. It is currently going through its first stage, framed around Airnov’s French site in Romorantin, the plan being to extend to other sites in the future.
In time, this work will enable Airnov to generate a superior understanding of how its operations generate carbon impact, and thus will help define its reduction targets and improvement roadmap moving forwards.

Airnov Healthcare Packaging
Airnov Healthcare Packaging designs and manufactures controlled atmosphere packaging that protects healthcare products from humidity and oxygen to maintain drug stability and extend shelf life, for pharmaceutical, nutraceutical & diagnostic applications. Products include industry-leading desiccant canisters and packets; tubes and desiccant stoppers; IDC ® Integrated Desiccant Closure; ADP® Advanced Desiccant Polymer directly embedded into plastic packaging with no dusting, keeping desiccant permanently integrated with packaging during shelf life and avoiding accidental swallowing; HAT® (Handy Active Tubes) vials for test strips and nutraceuticals; EQIUS™ Equilibrium RH Stabilizers that maintain a certain humidity level in pharmaceutical packaging; Oxygen scavengers; Oxynov™ barrier bottles effectively block oxygen ingress and provide an excellent barrier against moisture; Stablus™ shelf-life predictive simulation service; Compatilus™ program that ensures full compatibility with various automatic desiccant inserters for drop-in desiccants.

Airnov provides critical industries with high-quality, controlled atmosphere packaging, so that critical healthcare industries can protect their products from moisture and oxygen.


Our solutions benefit from our innovative research and development to offer the best protection to your drugs, pharmaceuticals or any type of sensitive products. All this while integrating our efforts to reduce the impact on the environment.
OUR SOLUTIONS

The Pharmaceutical Supply Chain in the Age of Sustainability
The drive for sustainability has impacted every business in recent years and continues to place new demands on all aspects of the pharmaceuticals supply chain. The team at Recipe Design, a London based consultancy who have worked in the industry for over 12 years, see positive developments coming both from legislative pressures and the shifts in values of the customers and end users its clients aim to serve.
The COO on Meeting the New Expectations of Businesses as a Strategic Supplier
As a strategic design agency with decades of experience working with healthcare and pharmaceutical companies, we have always been set up to be compliant with the rigorous processes and policies that our clients demand from us as a supplier. In recent years, our client’s business objectives have shifted markedly towards ever more challenging sustainability goals, resulting in significant pressure on every part of the supply chain to contribute to positive change.


The launch in 2015 of the United Nations Sustainable Development Goals (‘a universal call to action to end poverty, protect the planet and improve the lives and prospects of everyone, everywhere’) confirmed a new level of political will and we saw clients increasingly link their business plans to UN goals and KPIs – not only aligning them to legislative shifts but demonstrating a commitment to their own people that many found helped enhance engagement around ‘change for good’. Net zero objectives had become essential statements for any brand wanting to demonstrate market leadership – even if the detail in the plan was at times a little hazy.
Seven years on, new levels of intent have translated into a whole host of policies and measures that we as a small business have to get to grips with. As a relative minnow providing R&D support services (and not directly manufacturing components, drugs or devices) we pride ourselves on our ability to remain agile and adapt to the changing needs of the businesses we serve (which span healthcare, pharma and the consumer wellness space).
As a key supplier to a number of companies in Pharma, we align to the foundational commitments of the PSCI (Pharmaceutical Supply Chain Initiative) focusing on human rights, environmental sustainability and doing business responsibly. As a small business it hasn’t been easy, but in recent years we have introduced and reinforced policies that
ensure we meet all required standards in ethics, labour, health & safety, environment, and management systems.
Today, we are expected to report on Science Based Target measures (to the SBTi) including greenhouse gas emissions and with a much greater level of accountability than just a few years ago when as a small studio it may have felt unnecessarily onerous or even a barrier to engagement. We will now publicly report on our environmental performance to the Carbon Disclosure Programme (CDP) annually, playing our part in the move toward industry wide transparency that will drive further improvement.
One of the upsides of the recent pandemic for Recipe was being forced to operate as a fully decentralised team, using online collaboration tools (such as Miro and Zoom) in place of the open plan studio culture that hitherto was considered an essential part of the creative agency model. Our experience surprised all of us and within weeks we decided to radically downsize our London offices and invest in remote working for the long term. With necessity being the mother of invention, it also became the ideal time to quickly develop remote research tools which have now become a more efficient, less costly and less environmentally impactful choice over ethnographic studies conducted in situ around the world.
In 2021, Recipe Design was rated within the top 6% of its industry by EcoVadis – the world’s most trusted business sustainability index – and was awarded a silver medal in recognition of achievements across key measures that define Corporate Social Responsibility (CSR). We are committed to demonstrating continuous improvement year on year and in doing so, holding our own suppliers to equally high standards in contributing to healthy society, planet, and business.
After a tumultuous time of pandemic, war, economic turmoil and a climate emergency acting as a crisis multiplier, realising those UN Sustainable Development Goals feels very challenging indeed – but never more important.
The CSO on Understanding the New Expectations of End-users and HCPs


Whilst legislation is driving businesses to take action on responsible resource consumption, device and packaging reuse, disassembly and repurposing; consumers desires and expectations far outstrip the legislative push.
Our Meaning Centred Design™ approach has taken us all over the world (both physically and digitally through remote research). Speaking to the patients and Healthcare Professionals interacting daily with the diagnostic equipment, prescription medications, devices and paraphernalia surrounding conditions from respiratory and dermatological health to diabetic care, migraine management and oncological therapies. While the key meanings and values of importance vary greatly across condition, continent and experience, almost all patients are united in the desire to reduce their material impact on the world.
It's hardly surprising that as consumers develop a more complete understanding of their ethical impact on the world, their concerns transition from daily food and beverage ingredients and packaging to the items of their health management. But the balance of ethical considerations in the pharmaceuticals category is one the most complex and nuanced.
Patients’ relationships with the objects and items of their health management can be one that leaves them empowered and freed or ever more aware of their condition. As waste and detritus accumulates with each interaction, an empowered experience can quickly become overwhelming as singleuse devices and non-recyclable materials create a parallel between the personal burden of disease management and our ethical footprint.
As patients look for the reassurance of ‘hygiene’, ‘performance’ and ‘clinical advancement’ they often initially seek the strong, sturdy and single-use, only to be halted as they consider the disconnect with their consumer behaviours focused on minimal material use, reduction and reuse. Here context is vital with very different values enacted in an emergency event versus long-term condition management. Similarly, the contextual requirement for ‘ease’ will sometimes drive patients towards design for practicality rather than the best practices that align with their core values.
The Covid-19 pandemic demonstrated how quickly consumer resonant meanings and values can shift. Almost overnight the desire for reusables and natural cleaning products were replaced with plastic-wrapped single-use devices, bleach, item quarantine and sterilisation. Thankfully as our understanding and management of the pandemic has developed consumers have quickly returned to their core values and the importance of ethical considerations in healthcare management. This has only been heightened by the visuals of discarded single-use masks, aprons and gloves and the realisation of the scale of impact of single-use materials and the need for them to be reserved for the essential needs of protection and preservation.
Just like consumer categories, patients are aware that the ethical claims made in healthcare may not always be what they seem. As patients become more informed, they are
increasingly looking for transparency, integrity and detail, but most importantly, without overwhelm. Unlike other consumer categories, in healthcare ethics must come without compromise or burden, allowing patients to focus on their wellness whilst reassured they are living by their values; whether avoiding component ingredients and processes or looking to prepare devices and packaging for reuse or responsible disposal.
Our extensive work with patients and Healthcare professionals through in-depth interviews, ethnographic research, usability studies and longer-term handling studies allows us to see and unpick the changing relationship between pharmaceutical devices and the complex issues within ethics and sustainability. Intersecting these cultural shifts with the possibilities of verified ethical credentials and material, process and technical advancement allows us to define strategic visions for the future of sustainable healthcare.
With influences from the top down and bottom up increasingly vaster in their strength and complexity, it’s impossible for pharma brands not to start embracing this new era. The future of their business depends upon it and working in partnership with trusted suppliers, the sustainability challenge can in fact be their greatest opportunity.
Simon Browning
With over 23 years experience as an award winning designer, strategist and business leader, Simon has a proven track record in delivering innovation programmes for brands worldwide. Simon’s approach puts people at the heart of the design planning process and is equally passionate about harnessing new insights and perspectives that can unlock business growth.
Email: simon@recipe-design.com
Sarah Sanders
Being trained in industrial design at Northumbria University gives Sarah’s semiotic and trends research a unique perspective. She has an eye for what the rest of us miss, observing the changing patterns of culture with laser-sharp focus.
Email: sarah@recipe-design.com
Recipe Design
Recipe Design is a London based strategic design agency who have delivered world class solutions to clients spanning the Healthcare and Pharmaceutical sectors including Roche, Sanofi and Mundipharma. Using a proprietary process called Meaning Centred Design™ the team at Recipe deliver product, packaging and brand pipelinesfrom concept definition to commercialisation -by aligning human needs and desires with emerging science and technology.
A Greener Future for the Inhalation Industry: A Critical Year for our Climate


The urgent threat of climate change is the age-defining issue of our generation, and we are reaching a defining moment for our industry. The inhalation industry is committed to reducing their carbon footprint and establishing processes which are more sustainable. A global commitment to establishing a greener future must happen now; the industry’s proactive approach will address the major environmental concerns of pMDI manufacture – the carbon footprint of the propellant. The adverse effects of greenhouse gases on our climate are well documented. This is not a new revelation, rather, this industry has gone through a similar issue before! The issue being adapting the way we formulate MDIs to develop more sustainable products, whilst maintaining the safety and functionality.
This article explores the legislative and economic drivers of why the industry needs to adapt, and crucially why now is the time to act. It is also important to ground ourselves and remember why we develop medicines; to better the health of the public and save lives. The effective delivery of medicines still remains the priority. The challenge to produce sustainable products cannot compromise the safety, efficacy and patient adherence of inhaled medicines.
The 1987 Montreal Agreement was the catalyst for the first-wave development of sustainable pMDIs. The successful phase out of CFCs with F-gases had a positive impact on ozone depletion and to some extent the carbon footprint.1 Through significant investment by the industry, less ozone-depleting alternatives were developed (HFA-134a, HFA-227 & DPIs). Manufacturers of pMDIs understand that sustainable products cannot be developed on sentiment alone – it is the commitment to invest that ultimately allows the change to alternative propellant systems!
Two major pharmaceutical companies, Astra Zeneca and Chiesi Group, have recently committed to investing in alternative propellant pMDIs. Both companies have committed to launch a ‘greener’ inhaler by 2025. 2,3 Other major companies have yet to publicise their commitment. Given the timelines for pharmaceutical product development and regulatory approval of new medicines, 2025 may seem challenging. The importance of acting now is evident for both environmental and economic issues. It is clear the environmental crisis cannot wait; if and when the current propellant supply is phased out, commercial production of current inhalers becomes unrealistic.
As a producer of reliever medication, Pharmaserve North West has committed to future-proofing our facilities, products and processes. The importance of safeguarding the supply of
critical medicines has been highlighted through the COVID-19 crisis. It is a collective responsibility to ensure global delivery of life-saving pMDIs. The issues and challenges posed by the investment into two prospective greener propellants have been addressed as a matter of urgency. Working closely with industry leaders to ensure our approach to the ‘greener’ inhaler does not impact the health of the public.
Proposed Solutions

HFO-1234ze and HFA-152a have been highlighted as potential replacements of HFA-134a and HFA-227ea. Isobutane was also considered and is currently used in topical aerosols; the inhalation safety concerns make this a less favourable option.1 Both propellants address the environmental concerns of pMDIs, however each has limitations and challenges which must be overcome before commercial production.
It has been well documented that HFA-152a is actively being developed for use in pMDIs. The properties are similar to those of HFA-134a – but critically for suspension formulations,
HFA-134a HFA-152a HFO-1234ze
Liquid Density (g/mL) 1.21 2.7 1.29 ODP 0 0 0 GWP 1300 138 < 1
Atmospheric Lifetime 13 years 1.4 years 18 days Flammability n/a LFL 3.8% n/a
the liquid density is significantly lower. Despite this, early formulation studies have shown that the performance is surprisingly good.4x Although the data is promising, it is critical that further studies are performed to understand the propellant performance for more inhaled APIs and also solution formulations. The performance of HFO-1234ze may look more promising, given the closeness of its properties to HFA-134a. The transferability, due to these similarities, may expedite the development process.
Both propellants have been shown to have good compatibility with currently used materials in MDI componentry. Particular focus has been on the behaviour observed when the propellant contacts elastomer and polymer materials used in the valve constructions. In light of the change, the concern of compatibility is actively being addressed by componentry suppliers, with commitment to understanding and developing new ‘sustainable’ componentry.
The critical path activity of the development of a new propellant is the generation of long-term safety data. HFA-152a is expected to finalise the DMF in 2022; the FDA granted approval to proceed with clinical trials which began in February 2020.5 The generation of the toxicological data package is also backed by Chiesi to help achieve their target of a commercial product launch in 2025.3,6
The current uses for HFO-1234ze are focused on commercial and industrial products, although the manufacturer does have a pharmaceutical division and patents for use as a medical propellant. The initial safety data shows that it has a low acute toxicity. Any timelines for extensive toxicological assessment have yet to be published and will no doubt be the rate-limiting step.
The appeal of HFO-1234ze is clear – due to its lower flammability the propellant can be directly transferred to existing equipment and facilities. Whereas, HFA-152a is considered flammable and significant investment is required to mitigate the flammability risks and ensure safe handling. Although the technology is established for topical sprays (historically this technology has been used to safely fill cans with isobutane), the timeline to scale up production is somewhat hindered by the safety requirements. Novel manufacturing methods using ‘tablet dispersion drug loading’ are also being considered, which form stable suspensions when the propellant is added – the separation of the propellant filling stages, reduced batch sizes and mixing requirement mitigates some safety concerns.7
It is also important to consider the DPI approach. Whilst the product, technology and processes are established today, there are considerations to be made to ensure the
global access to inhaled medications to all demographics. The breathing ability of paediatric and geriatric patients makes DPIs less feasible, particularly in reliever medicines.
The economic impact of a wholesale switch was studied, showing the prescribing costs of the switch would increase around 10% on average.8 It is also important to note this is not feasible for all patients – the pMDI must remain and hence the environmental challenge remains.
Legislative & Environmental Drivers
With the rising concern in the urgency of the environmental crisis, the Kigali amendment to the Montreal protocol was signed in 2016 and presented the industry with a new challenge – tackling greenhouse gas emissions. The amendment aims to phase out global consumption of HFA by around 85 % by 2047 to address the environmental concerns.9,10

There is currently no legal or regulatory requirement to change propellant outside of the Montreal agreement in the US. More and more pressure is being applied by environmental lobbyists to phase out these high global warming potential propellants. The corporate responsibility to protect the environment is evident – however the ‘choice’ of the solution has commercial motivation. It is clear that all solutions are positive; for the environment and the enhancement to patient. Multiple therapeutic solutions allow additional factors to be considered when prescribing medicines: patient ability, preference, costs and adherence.
Economic Drivers
Besides from the moral obligations as residents of this planet, there are also commercial incentives to act promptly. The economic driving factors of propellant price increases and supply chain issue serve as a warning sign for currently marketed products.
Figure 3 (Koura) is based on probable phased withdrawal of ‘F’ gas quota exemption by the EU commission over six years. The increase in cost is mainly due to the need, under the revised regulation, to purchase quotas. This also applies to HFA-227ea, which may lead to it becoming unavailable for medical use. This is a median case; there are more aggressive scenarios. This applies to both 134a and 227ea. In addition, regulation of industrial uses of 227ea may lead to it becoming unavailable for medical use.
Medical Propellant Price Change (%/base year 2018)
By performing early-stage feasibility studies, a commercial strategy can be defined. The translation of existing medicines can follow several routes (DPI, alternative propellant, nasal spray). It is critical for both the patient and business to have assurance that their current product portfolios are safeguarded against the industrial change. The initial investment to assess the feasibility of product translations
to sustainable alternatives is relatively small, with relatively big dividends. The focus on being the ‘first-to-market’ for each medicine is becoming more evident. The market share of current HFA-134a pMDIs cannot be maintained going forward. Capitalisation on this industrial change ensures businesses are not cannibalised by the lack of agility on this issue.
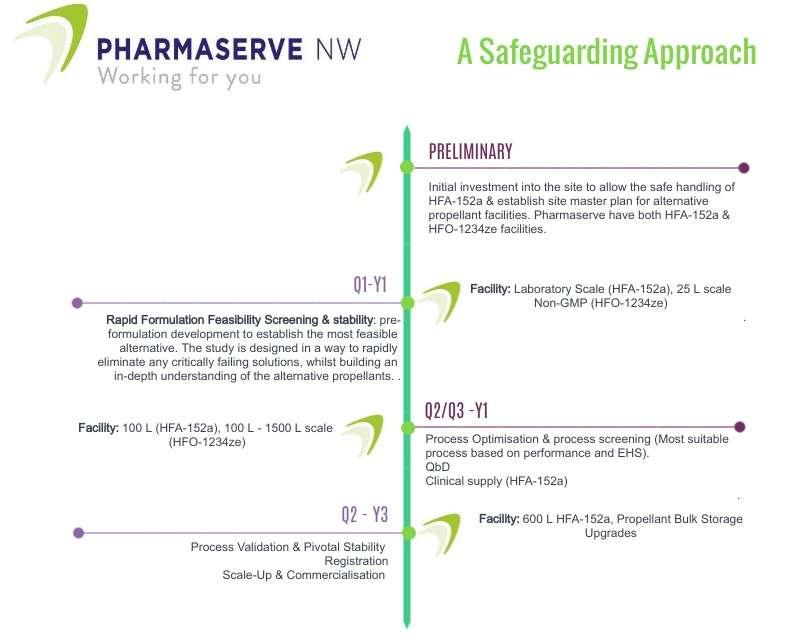
Safeguarding Approach
Pharmaserve have outlined a safeguarding approach to streamline the development and supply of sustainable pMDIs. Through working closely with industry leaders and suppliers, a suggested approach and timeline has been outlined, primarily to ensure the maintenance and supply of safe sustainable products, whilst maintaining a commercially viable operation within the inhalation industry.
REFERENCES
1. Medical and Chemical Technical Options Committee, 2018 report. Available from: https://ozone.unep.org/sites/default/ files/2019-04/MCTOC-Assessment-Report-2018.pdf
2. AstraZeneca. Investing in a sustainable future for patients with respiratory disease. Available from: https://www.astrazeneca. com/media-centre/articles/2020/investing-in-a-sustainablefuture-for-patients-with-respiratory-disease.html.

3. Farmaceutici C. Chiesi outlines €350 million investment and announces first carbon minimal pressurised metered dose inhaler (pMDI) for Asthma and COPD 04/ 12/2019. Available from: https://www.chiesi.com/en/chiesi-outlines-350million-investment-and-announces-first-carbon-minimalpressurised-metered-dose-inhaler-pmdi-for-asthma-andcopd/.
4. Noakes T, Corr S. The future of propellants for pMDIs; Drug Delivery to the Lungs 27; pp. 61-64, 2016.
5. Koura. ‘Green’ medical propellant receives FDA approval to proceed to clinical trials. Available from: https://www.prnewswire. com/news-releases/green-medical-propellant-receives-fdaapproval-to-proceed-to-clinical-trials-300998598.html.
6. Corr S. Development of HFA-152a as an environmentally sustainable propellant for pressurized metered dose inhalers; Inhalation 14; pp. 12-17, Oct 202.
7. Taylor G, Warren S, Tran C inventors; Cardiff Scintigraphics Ltd assignee. Pressurized metered dose inhalers and method of manufacture. United States Patent 9981092B2. 2018. May 29.
8. Pritchard JN. The Climate is Changing for Metered-Dose Inhalers and Action is Needed. Drug Des Devel Ther. 2020 Jul 29;14:30433055. doi: 10.2147/DDDT.S262141. PMID: 32801643; PMCID: PMC7410333.
9. United Nations Environment Program. The Kigali Amendment to the Montreal Protocol: HFC Phase-Down. OzonAction Fact Sheet OZFS/16/11_1. Paris, France: United Nations Environmental Program; 2016.
10. European Commission. Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006 Text with EEA Relevance. Available from: Https:// eur-lex.europa.eu/legal-content/EN/TXT/ F/?Uri=CELEX:\\ 32006R0842&qid=1594982827385&from=EN.
Adam Kay
A chemical engineering graduate from the University of Leeds, Adam has spent four+ years focusing on inhalation product development. His experience includes the end-to-end development of inhalation products, from formulation to commercialisation, leading EMA product submissions – as well as process development, optimisation and equipment commissioning. In 2020, Adam took the role of Business Development Manager to bring his technical expertise in product & process development to support the existing BD team.

Understanding the Role of Energy Efficiency in Achieving Pharmaceutical Sustainability Targets
The pharmaceutical industry is growing. A 2020 report1 valued the worldwide life science manufacturing market at $405bn USD, with an expected compound annual growth rate of 11.34% over the following 8 years. As the quantity and diversity of pharmaceutical product continues to rise, so too does the burden this places on our environment. Efforts to limit this burden can already be measured, for instance, global pharmaceutical manufacturer AstraZeneca has reported a 60% decrease in carbon emissions since 2015,2 but still requires noteworthy progress if it is to achieve a 100% reduction in emissions by the 2025 target.3 Evidence gathered by lab sustainability experts, My Green Lab, indicates that only 4% of life science organisations are set to deliver a future aligned to the 2030 emissions goals4 that are outlined in Article 2 of The Paris Agreement of COP21.5
Planning
While the pressure to operate sustainably is beginning to be applied by a variety of interested parties, including investors, stakeholders, employees and even customers, just 42% of the pharmaceutical industry has established a clear carbon reduction objective6 and of this 42%, just three companies currently have targets that will limit planetary warming to 1.5°C by 2030.
The challenge is clear, in order to deliver a more sustainable future, the life science industry needs to align the ambition for a cleaner, healthier future with objectives and targets that will deliver this aspiration.
Ultimately, each business needs to determine its own pathway to net zero and beyond, but to do this, external guidance can provide not only a realistic and attainable plan for decarbonisation but also give an insight into how this will affect business operations. For a recent project, the client in question had recently published ambitious sustainability targets, aimed at greatly reducing the carbon and environmental impact of business operations. While the drive to achieve a net zero future was clear, the way in which the organisation could do this was still to be decided. In order to determine this, a full breakdown of challenges and opportunities was created, considering all aspects of the value chain, which are defined by the ET Index Research7 across three scopes:
• Scope 1 – “All direct emissions”. Essentially, emissions from sources that the organisation directly controls, such as the burning of gas on site and release of refrigerant gases or solvents.
• Scope 2 – “Indirect emissions generated from the purchase of electricity” – the emissions cost of procuring electricity from off-site sources.

• Scope 3 – “All other indirect emissions, both upstream and downstream, such as distribution of goods, transportation of purchased goods, transportation of waste, disposal of waste, employee commuting, business travel or investments. Scope 3 emissions are usually the largest percentage of a company’s total GHG emissions”.
The first undertaking of the project was to review the existing organisational approach and gather information from 10 sample sites that were specifically chosen based on their representative nature and wider applicability to the client’s other sites. These sites were also selected due to their carbon intensity, representing 76% of the organisation’s Scope 1 and 2 emissions. Following this, a gap analysis was performed to identify potential risks and opportunities in the emissions reduction plan, alongside a pricing sensitivity analysis (see figure 1) to provide a clear insight into the possible OpEx paths of the corporation versus a business-as-usual (BAU) projection.
Roadmaps were then finalised and agreed upon, which provided the client with a clear timeline of implementation. This also detailed the management systems that would best deliver the net zero ambition, with a full assessment of capital and operational costs to support the roadmaps.
Ultimately, the client was able to understand the next steps on the road to a zero-carbon future, with a clear list of prioritised recommendations that covered all three scopes. In scopes 1 & 2, the decarbonisation plan represented a 100% reduction in emissions, totalling an annual decrease of over 400,000 tonnes CO2e.

Identifying
Setting realistic goals that are in line with an organisation’s carbon reduction challenge is the first step towards

decarbonisation and is central to the entire net-zero ambition. But beyond this, consideration for how each individual site can practically reduce energy consumption is needed. Many sites face unique challenges that require tailored solutions, with each of these sites benefitting from a specific roadmap that outlines a pathway to total carbon reduction.

One reality for pharmaceutical sites is that there is not always an awareness of the best decarbonisation opportunities. These opportunities can be separated into three areas: utilities, water, and heating, ventilation & air conditioning (HVAC). HVAC typically comprises the greatest opportunity for improved energy efficiency, as the greatest energy-saving projects8 and potential for decreased consumption are often found in this area (see figure 2).
Securing buy-in from all stakeholder departments is also a pivotal challenge in delivering successful energy reductions on-site, with alignment between quality, engineering and production departments playing a key role in successful project identification. Site teams are often experts in their own facilities,
so combining their knowledge of the facility with current engineering best practice and experience in improving energy efficiency in GMP areas typically uncovers previously unknown opportunities for optimisation. In order to secure buy-in from all stakeholders, identified projects need to account for energy and carbon saving in line with organisational sustainability objectives and consider the financial impact of implementation, with additional thought towards how the proposed measures could impact GMP compliance. Project payback can be calculated in support of this endeavour by calculating the total investment cost minus the annual cost saving identified via energy/resource reduction, with the typical payback in HVAC system projects amounting to approximately four years or less. With this information now outlined, a detailed roadmap that acts as a step-by-step guide now provides a clear plan for the site team to follow in order to achieve significant carbon and cost reduction. Of course, with the continual rise in energy prices, particularly in Europe,9 even opportunities that previously had long-term payback will become increasingly appealing to many pharmaceutical organisations who are attempting to manage this continual rise in operational expenditure.

>62% HVAC Energy Cost
>50% HVAC Energy Consumption >60% HVAC Carbon Emissions
Figure 3 – A detailed energy consumption breakdown of a typical non-sterile manufacturing site.

With water being a crucial resource in pharmaceutical manufacturing,10 the topic of conserving and enhancing the efficiency of water-based systems is rising on the agenda for countless global life science businesses, as was the case at a pharmaceutical site in South Africa. The local facility team were facing significant challenges with water due to low winter rainfall. This situation was exacerbated by the rapidly approaching summer season that would limit water accessibility even further in the region. To combat this, a water reduction strategy was devised that considered the urgent need for identifying and implementing water conserving solutions. Taking into account the local site team knowledge, alongside EECO2 experience in engineering best practice, a total of 24 varying opportunities were explored and presented to the client team. These included water metering, enhanced reporting procedures, water recycling, rainwater harvesting and more efficient water-cooling systems that totalled in identifying water savings of 47% of site water consumption, assisting the site to not only overcome the immediate threat of water shortage but also improve the long-term sustainability of the site and safeguard against similar challenges occurring in the future.
But assessing energy and water consumption is not the only way for life science companies to understand the environmental ramifications of site operations. Pharmaceutical production has far-reaching consequences for the environment in which this production takes place, with a 2014 review11 finding that over 600 pharmaceutical substances have been detected across a variety of environments worldwide. Of course, for a pharmaceutical industry that is coming to terms with its global operations, this impact on nature represents a significant challenge.
On-site, there are a number of different approaches that can be taken to negate these consequences. Principal to this is proper waste management, which focuses on not only limiting the amount of waste produced but also managing the way in which this waste is stored. GSK have recorded significant success in this aspect, noting a 78% decrease in
waste to landfill since 2010.2 Biodiversity is another core aspect of nature-focused sustainability, most notably, organisations such as the aforementioned GSK have admirably included biodiversity targets within their own sustainability goals12 in an effort to go beyond the typical net zero objective and deliver a net-positive impact on nature by 2030.
Understanding
Once relevant opportunities have been identified, some opportunities requiring limited investment and low technical risk can progress directly to implementation. Normally these will be projected to achieve paybacks of less than 2 years and be relatively straightforward to implement. However, more complex and higher investment projects may need further study to refine the solution and mitigate uncertainty or risk. is often required to understand their feasibility. Whilst the assessment process will have considered this to a reasonable degree, this is often the final step prior to implementation and realising the carbon and energy savings that were first noted when uncovering the opportunity.
Putting this into practice in a recent project, a life science site in France was able to outline a pathway to total carbon reduction. For context, the site had already switched to a sustainable renewable electricity source, so emissions typically associated with scopes 1 & 2 were significantly reduced. However, in order to achieve total site decarbonisation in line with the organisation’s goals for carbon reduction, the site needed to explore options to nullify the burning of natural gas. To do this, the feasibility of a electric heat pump opportunity was explored. Heat pumps utilise a refrigeration cycle to boost heat output to 3 or more times the electrical input. However, they have certain limitations which must be considered carefully in the application as this can have a huge impact on viability and long-term operational costs. The feasibility study included a , this involved a site survey, close collaboration with the site team and a full report of potential options to fully enable the final client decision. By installing a heat pump with some other operational changes, it was clarified determined that a 100% reduction could be achieved in gas usage, effectively
decarbonising the site and bringing the facility in line with the organisation’s goals for reducing carbon intensity.
Monitoring
A present challenge in the pharmaceutical industry is not only monitoring the success of energy efficiency improvements but also understanding how these results fit into the net zero ambition. In order for fully sustainable operations to take place, all sites must in some capacity be aware of their greatest energy consumers and be able to monitor these consistently.
Transparency in energy consumption, particularly in leased and rented commercial operations can be difficult. An accurate breakdown of equipment energy consumption is hard to access in scenarios where energy metering information is not made readily available. In overcoming this issue in a recent project, a client was able to ascertain a full breakdown of the largest energy consumers across three separate sites. The client was driven by a 2040 net zero objective and as such, required a non-invasive metering solution at multiple plasma service facilities. The data from the non-invasive metering solution was presented in a digital energy dashboard format that allowed the client to monitor energy consumption from a range of equipment sources across the portfolio of facilities. This was paired with a behaviour change programme, with the eventual outlook to monitor the success of this behaviour change programme via the energy dashboard solution. As a result, the client was able to understand the key areas to target in order to improve sustainability at the facilitieson the three sample sites, these ranged from utilising and lighting controls, optimising HVAC setpoints and informingeducating the site teams on efficient usage of freezers and utilities. All of the actions identified required little or no investment and are expected to deliver 10-20% energy reduction for each facility. In is foreseeable that with some investment in changing to more efficient equipment a further 10–20% reduction could be achieved.
Whilst monitoring energy performance is clearly good practice, it’s the insight and resultant behavioural changes of staff as it relates to energy use that should be the key outcome. Influencing staff to become more aware and take appropriate action is a very underused strategy and is going to become essential if Pharma is going to realise its carbon zero ambitions. New technology will be part of the solution, but one should consider how people can help get the most out of that technology.
Innovating
To go above and beyond current decarbonisation objectives, the life science industry needs to consider methods to reduce energy consumption in all areas, including cleanrooms. To do this innovative solutions are required to maintain GMP compliance as well as deliver a more energy-efficient space.
The challenge in improving cleanroom energy efficiency is significant. Cleanrooms have been recorded to consume up to 67% of total facility energy,13 with much of this derived from the HVAC system that provides airflow into the controlled environment. On a global scale, there are now well over 20,000 cleanroom facilities in operation, accounting for more than $1bn USD annual spend on energy.14 Such a massive
consumption of energy poses a serious barrier for pharma organisations attempting to achieve their sustainability objectives.
Cleanrooms are however unavoidable in pharmaceutical production, the requirement to maintain and control critical parameters such as temperature, humidity, pressure and cleanliness is fundamental to producing compliant and safe products. The airflow into the space helps to dictate the rate at which cleanroom air is changed (ACR). Within each classification of cleanroom, there are strict limits specifying at what rate air must be changed for the space to remain compliant, this can make reducing energy consumption difficult.
While airflow must remain sufficient to provide correct temperature and humidity, dilute the airborne concentration of particles below the limits for the cleanroom classification and maintain a differential pressure cascade between different cleanroom spaces to restrict the movement of airborne particles,15 there is potential to lessen cleanroom airflow below the levels commonly found in many pharmaceutical facilities. Indeed, the cleanrooms of today typically operate under a static airflow regime, meaning the airflow to the room is unresponsive to the contamination challenge at any given time, providing a constant but sometimes unnecessarily high ACRsupply air flow rate, resulting in high air change rates.
Theoretically, a more dynamic approach to air change rates (ACR) is one solution to cleanroom energy consumption. By utilising particle counters, it is possible to monitor the contamination in the environment in real-time and then only provide sufficient airflow to combat this contamination challenge. Of course, the ACR remains within the setpoint of the classification limit, so as to still remain compliant but also provide a dynamic solution that adapts to the demand of the space at any given time. At times of low particle generation rates, such as unoccupancy, a dynamic cleanroom control system will lessen airflow to provide a low ACR and therefore significantly lessen energy consumption. In the event of increasing particle generation rates, the system increases airflow to the necessary level to abate this new contamination challenge well before it can reach levels that would challenge product or room compliance limits.
Such technology has recently been installed at the Cambridge Pharma Limited facility in the Cambridge Research Park, UK. The Intelligent Cleanroom Control System (iCCS®) operates as a commissioned but unvalidated not qualified control system that works alongside a validated qualified environmental monitoring system to provide a fully compliant solution. From a compliance performance aspect, the facility is operating between 20–30% of the class limit for an ISO Class 7 cleanroom, which is well within the tolerable margins of a compliant production facility. In terms of energy performance, early data is demonstrating a minimum reduction of 50% fan energy consumption when compared with a theoretical static system operating at 15 air changes per hour – which is very much at the lower level of air changes found in many grade CISO 7 cleanrooms.
Technology like ICCS® is highlighting the need to bring innovation and engineering best practice into the realm of
sustainability, without which, hard-to-tackle emissions such as those associated with energy-intensive cleanrooms, would remain undisturbed, proving to be a thorn in the side of pharmaceutical organisations attempting to lessen the energy intensity of their sterile aseptic and non-sterile manufacturing spaces.
Conclusion
The challexnge for the pharmaceutical sector is great, as an industry that is dependent on energy consuming processes such as HVAC, there will always be a requirement for energy use. Energy efficiency exists as not just a solution to the challenge ahead of pharma, but as an opportunity to build a net positive future for the planet. Events such as the rising cost of energy across the globe9 are demonstrating the importance of energy conservation and the need to improve energy efficiency serves as a pathway to sustainable operations, both monetarily and environmentally.
REFERENCES
1. (2020) Pharmaceutical Manufacturing Market Size, Share & Trends Analysis Report By Molecule Type, By Drug Development Type, By Formulation, By Routes of Administration, By Sales Channel, By Age Group, And Segment Forecasts, 2021 – 2028. Market Analysis Report, pp. 1. Retrieved from < https://www.grandviewresearch. com/industry-analysis/pharmaceutical-manufacturingmarket >
2. Okereke, M. (2021) How Pharmaceutical Industries Can Address the Growing Problem Of Climate Change. The Journal of Climate Change and Health, pp 2. Retrieved from < https://www.sciencedirect.com/science/article/ pii/S2667278221000468?via%3Dihub >
3. (2021) Ambition Zero Carbon. AstraZeneca, pp. 1. Retrieved from < https://www.astrazeneca.com/sustainability/ environmental-protection/ambition-zero-carbon.html#! >
4. (2021) New Study Finds That Just 4% of Biotech & Pharma Companies Currently on Track to Meet Paris 2030 Climate Goals. My Green Lab, pp. 1. Retrieved from < https://www. mygreenlab.org/blog-beaker/my-green-lab-measurescarbon-impact-of-biotech-and-pharma >
5. United Nations / Framework Convention on Climate Change (2015) Adoption of the Paris Agreement, 21st Conference of the Parties, Paris: United Nations. United Nations, pp. 3. Retrieved from < https://unfccc.int/sites/ default/files/english_paris_agreement.pdf >
6. Connelly, J. et al. (2021) The Carbon Impact of Biotech & Pharma A Roadmap To 1.5°C. My Green Lab in collaboration with Urgentem, pp. 8. Retrieved from < https://www.mygreenlab.org/carbon_impact_of_ biotech_and_pharma.html >
7. Harris, J. (2015) Special Report The Emerging Importance of Carbon Emissions-Intensities and Scope 3 (Supply Chain) Emissions in Equity Returns. ET Index Research, pp 2. Retrieved from < https://papers.ssrn.com/sol3/papers. cfm?abstract_id=2666753 >
8. Regnier, C. and Mathew, P. and Robinson, A. et al. (2022) System Retrofits in Efficiency Programs: Track Record and Outlook. Lawrence Berkley National Laboratory, pp. 15. Retrieved from < https://escholarship.org/uc/ item/2g6574qn >
9. Sgaravatt, G. and Tagliapietra, S. and Zachmann,
G. (2022) National Policies to Shield Consumers From Rising Energy Prices. Bruegel Datasets, pp. 1. Retrieved from < https://www.mononews.gr/wp-content/ uploads/2022/03/220320134424_1.-Bruegel-EnergyCrisis-National-policies-Upd-8.2.2022-1.pdf >
10. Strade, E. et al. (2020) Water efficiency and safe re-use of different grades of water – Topical issues for the pharmaceutical industry. Water Resources and Industry, pp. 2. Retrieved from < https://reader.elsevier.com/ reader/sd/pii/S2212371720300676?token=DBC1387474E F221876555462A14D2CCBF74FCC20DA8FEE310EE36D46 1ED193828609E8FB7C8A9E849483EDE940225339&orig inRegion=eu-west-1&originCreation=20220908145341 >
11. Küster, A. and Adler, N. (2014) Pharmaceuticals in the environment: scientific evidence of risks and its regulation. Philosophical Transactions of The Royal Society, pp. 1-2. Retrieved from < https://royalsocietypublishing.org/ doi/10.1098/rstb.2013.0587 >
12. (2021) Our Biodiversity Targets. GSK, pp. 1. Retrieved from < https://www.gsk.com/en-gb/responsibility/ environmental-sustainability/biodiversity/#:~:text=To%20 address%20biodiversity%20in%20our,and%20 deforestation%2Dfree%20by%202030. >
13. Tschudi, W. & Xu, T. (2001) Cleanroom Energy Benchmarking Results. American Society of Heating, Refrigeration, and Air Conditioning Engineers conference, pp. 4. Retrieved from < https://www.osti.gov/servlets/ purl/840976 >
14. The McIlvaine Company (2011) $10.3 Billion Cleanroom Market in 2011. The McIlvaine Company, pp. 1. Retrieved from < http://home.mcilvainecompany.com/index. php?option=com_content&view=article&id=158 >
15. Appleby, C. et al (2017) Applying QRM to Air Change Reduction. The International Society for Pharmaceutical Engineering, pp. 1. Retrieved from < https://ispe. org/pharmaceutical-engineering/july-august-2017/ applying-qrm-improve-sustainability-pharma >
Keith Beattie
Keith has over 22 years of experience in the pharmaceutical industry in lead roles, most recently focusing on empowering various life science organisations to achieve their sustainability objectives. As a leading practitioner in his field, Keith is a member of ISPE’s global HVAC Sustainability Steering Group and has also contributed to code of practice guidelines and standards including ISO14644.

Jamie Young
Over recent years, Jamie has devoted his time to understanding and promoting methods of achieving decarbonisation in the pharmaceutical industry. In doing this, Jamie has written articles published in Cleanroom Technology magazine and Clean Air & Containment Review amongst others, aimed at exploring opportunities for decarbonising cleanrooms and enhancing HVAC energy performance.


COLLABORATION
A Journey of Generations
Key Learnings in Implementing Sustainable Practices at a Global CDMO, Contributing to a Better Future that Works for All
Our Global Environmental Social Governance (ESG) Program
Today more than ever, businesses are uniquely qualified to address the impacts of climate change and social inequality. PCI has formally established the Global ESG Program as the foundation for our sustainable business practices. We believe in creating the changes we want to see in the world.
PCI’s corporate ESG commitment has gained recognition from global sustainability agencies, having been awarded Bronze Medal status by leading sustainability performance ratings organisation EcoVadis. PCI's Philadelphia Headquarters and Biotech Center of Excellence have been awarded Gold Medal status by EcoVadis.

Measuring Progress: Selecting the Right Partners

We embarked on our ESG journey in 2020, appointing Proof as an agile partner for data management, able to provide the level of support and expertise to launch our program in the most efficient way possible. Proof demonstrated that they were well-versed in sustainability frameworks and reporting guidelines, extremely knowledgeable of the ESG landscape and receptive to feedback on products and services. They quickly became an extension of our ESG team, which is vital to the success of our program. Proof has helped us transition beyond ESG to articulate PCI's Impact on global communities.
By mining disjointed data across PCI's global business sites through direct system integrations and translating it into actionable metrics, analytics, and insights, Proof provides our key decisionmakers, investors, and stakeholders with an informed vantage point that allows better understanding of our businesses and empowers them to actively live and breathe our values.
Following data submission, we needed to identify a partner to assess and benchmark our results. We selected the EcoVadis Enterprise Platform for our sustainable procurement program, and leveraged the existing EcoVadis network to access suppliers scorecards to begin understanding the work that suppliers have done along their own sustainability journeys.
To maintain a steady cadence of data reporting and analysis, we internally measure our performance across the nine ESG impact categories prioritised by PCI every six months allowing us to compare and track progress. Externally, we have committed to assessing our global sustainability performance annually via EcoVadis with the aim to continuously improve our
score year on year. In addition, we will be publishing our first Sustainability Report to communicate our goals and achieve better transparency with our clients and vendors.
Building Sustainable Facilities

Following the ESG program launch and with the right teams in place, we began leveraging best practices across sites for waste management, energy and water consumption. For example, our Philadelphia site now purchases electricity from 100% renewable sources, with the rest of PCI sites taking steps towards procuring 100% renewable energy by 2030.
As result of the these actions and our key learnings, we are now able to weave ESG into the fabric of PCI’s growth by implementing ESG efforts into both new and existing sites. For example, we recently announced the opening of our New England Clinical Center of Excellence in Bridgewater, Massachusetts, which was constructed to reflect ESG and sustainability efforts. The site’s features include a storm water runoff system, an underground oil and sedimentation separation tank, electric vehicle chargers and the exclusive use of LED lighting.
Overall, our goal is to integrate ESG practices into PCI’s DNA, making it a priority element when implementing new practices, procedures, the building of new or the expanding of existing facilities.
Exploring Uncharted Territory:
A Green House Gas Case Study (GHG)
For a company that has never reported on GHG emissions, accurate and comprehensive reporting can be a daunting task. PCI utilised our partnership with Proof to set the boundaries of its emissions reporting. The GHG emissions dimensions that were most material to our business and stakeholders were identified, also considering site data availability. We were able to determine data sources for each of the selected emissions categories, such as electricity bills and energy meters. PCI deployed Proof’s GHG Emissions Reporting Tool to automatically calculate estimated GHG emissions based on data inputs from our business sites in the following categories:

1. Measure Understanding the current emissions performance and tracking improvement over time.

• On-site fuel consumption via diesel generators, refrigerants and natural gas power generated onsite.
• Vehicle fuel consumption from cars owned or rented by PCI.
• Electricity purchased (from utility provider). PCI sites reported data based on utility bills. Proof applied grid emissions factors based on each site’s location to calculate GHG emissions.
• Business travel. Based on the availability of data and a materiality assessment, business travel was selected as an important scope 3 emissions category to track. We elected to report data through our Concur travel and expense corporate software system, which feeds travel data directly into the Proof platform.
2. Reduce
Meaningful interventions to radically reduce carbon emissions. With an understanding of our baseline emissions across business sites, we quickly shifted our focus to emissions reduction strategies:
• Identifying performance benchmarks based on industry databases and competitors’ public data, to understand how PCI compares and to identify other companies’ best practices for emissions management.
• Putting in place ambitious yet realistic targets for the future.
• Tracking the potential environmental and financial impact of organisational changes on emissions (e.g. emissions reduction from transitioning to renewable energy).
• Engaging stakeholders in emissions reduction activities.
• Identifying verified emissions reduction partners at the cutting-edge of carbon offset and removal.

• When one site’s normalised GHG emissions were higher than
expected, for example, the PCI team looked at the underlying data to find a root cause (e.g. electricity consumption) and planned actions to reduce emissions from this area. This includes transitioning to more renewable energy purchases, improving equipment efficiency to require less fuel, and installing smart light meters in warehouses.
3. Scale
Expanding the boundaries of organisational impact. Based on the results of this assessment, Proof is working with PCI to expand our impact reporting beyond GHG emissions to include other types of impact performance datasets, such as:
• Environmental impacts including clean energy, waste and water management, climate risk, and biodiversity.
• Social impacts including employee diversity, workplace equity, employee engagement, health and safety, and training.
• Governance impacts such as data privacy and security, legal and regulatory compliance, board governance and diversity, and transparency and disclosure.
The PCI ESG Journey Continues
Through a commitment to impact measurement that is rooted in transparency and continuous improvement, we are living out our commitment to fostering environmentally sustainable performance, honoring a diverse and inclusive culture, and creating positive impact on employees, supply chain partners, customers, investors, patients, and the communities in which we live and work.
Our ESG journey is constantly evolving as we improve efficiencies in data capture with automation and implementation with global systems, and execute our site action plans across the PCI network. To date, we have documented existing initiatives site by site, established company baselines against the nine impact categories, and created a multi-year ESG Program Roadmap with targets to drive progress, global alignment, and enhanced governance.
Gigi Bat-Erdene
Gigi Bat-Erdene, Global ESG Program Manager, originally from Mongolia. Gigi is passionate about empowering individuals and businesses to play a bigger role in creating a more inclusive future for all. She joined PCI shortly after completing her undergraduate program at Columbia University as a Sustainable Development major, and is now leading the buildout of PCI’s ESG program, driving enhancements across impact and reporting strategy, including activities related to climate change, human rights, DEI and sustainable procurement.

PCI
At PCI, ensuring life-changing medicines reach those who need it most is our highest priority. As a truly integrated global CDMO, we are manufacturing, packaging and supply chain experts, harnessing our experience and expertise to deliver you a seamless solution, with the ultimate aim of improving the lives of patients.


How to Save 1,000 kilos of CO2 per 333-litre Refrigerated Packaging –Thanks to the First-ever Circular Economy Pharmaceutical Packaging

2019 was a turning-point. It was the year when fears of palpable global climate change gripped society as a whole, including the worlds of business and industry. In 2020 the COVID-19 pandemic led to a second dramatic realisation. In order to increase the resilience of global supply chains there is a radical need for greater sustainability. eutecma's objective now is to tap into the enormous potential which climate-friendly, no-waste packaging can offer the temperature-controlled transportation of pharmaceuticals. At the end of a three-year process of evaluation and development, we have created, for example, the 333-litre PROTECT cooling box, estimated to be reusable 15 times and can save up to 1,000 kilos of CO2. This is possible thanks to the first-ever circular economy system in the pharmaceutical packaging sector. The so-called retecma Loop, which is based on artificial intelligence (AI), ensures that not a single gram of expanded polystyrene (EPS) material is lost.
eutecma's drive towards increased sustainability in the temperature-controlled transportation of pharmaceuticals began in 2019. Our strategic concept could be compared with that of a bifocal lens. Just as the lens is designed for both close-up and distance vision, eutecma's development projects have followed two directions. On the one hand, the challenge was to make existing products more sustainable quickly; on the other hand, the medium-term goal was to redesign the pharmaceutical supply chain packaging radically.
Bifocal – Close up: Rapid Successes with Sustainable Products
This has resulted in ecological product alternatives which became available as early as 2020. For example, the foil composites of our ICECATCH© passive energy storage units consist of 50% sugar cane waste material. The latter bears the quality seal "I'm green", developed by Braskem, the Brazilian producer of polyethylene based on sugar cane waste. In the case of the PROTECT packaging systems, the customer has a choice between packaging boxes made from conventional Virgin EPS/styrofoam and others made from recycled styrofoam (Styrofoam Ccycled). This secondary material consists of pyrolysis oil, which is produced by the world's largest chemical company BASF from plastic waste. Both innovative products improve our customers' CO2 balance significantly. Last but not the least, we have launched an information campaign with the aim of informing customers how PROTECT packaging and ICECATCH© passive energy storage units can be used not just once but multiple times – without jeopardising the temperature integrity or the quality of the transportation.
Bifocal – the Distant View: Rethinking Pharmaceutical Supply Chain Packaging

While more rapidly sustainable product alternatives were

being shipped from our company headquarters in the Port of Mannheim, we were at the same time working on a "big bang". This was because, we recognised that to achieve a drastic reduction in CO2 levels and also in waste material, packaging in the pharmaceutical supply chain needs to be completely reconfigured. The fact that there were numerous requests for a "100% green and temperature-regulated packaging" indicated that customers would welcome such a development. All too often, supposedly sustainable products had turned out to the mere "greenwashing" in the marketplace. We interpreted these requests not only as confirmation that we are on the right track, but also as a challenge to fully rework the issue of pharmaceutical packaging.
Step 1: The Choice of Material
We began with the question: Must temperatureregulated high-tech packaging really have to consist of EPS? Can we not assume that wool, straw and hemp are much more sustainable materials? So, we launched a two-year evaluation process, to be conducted without any preconceived views. This presented no problems to us, since eutecma is not a producer of EPS, but simply a provider, which means that we don't have to bear the burden of production and machineries. Accordingly, it would have been possible for us to switch to an alternative material immediately without incurring losses. We investigated the following materials: paper, cardboard, wool, hemp, cotton, used textiles and various plastics.
A specific phenomenon appeared repeatedly. Although materials showed many good properties during testing, ultimately the problem was that they failed to meet all the evaluation criteria. Wool, for example, is an excellent material for insulated packaging, but as PROTECT packaging had to be
usable worldwide, and Australia forbids the import of wool, we had to rule out wool for our purposes.
The use of old textiles was also ruled out, in this case because all too frequently they are heavily contaminated by pesticides. A box made of straw can be pressed into any shape, but often has inferior insulation properties due to the development of moisture. In addition, it should be sealed with foil to prevent dust harming the pharmaceutical product.
In the end, two evaluation criteria proved critical: universal formability and complete recyclability. The eutecma system comprising ICECATCH© passive energy storage units and PROTECT boxes attains a high degree of reproducibility, since the former are secured in insertion slots throughout the transportation process and consequently supply energy to the products continuously and reliably. To achieve this result with other materials, these would need to be combined with a second material. For example, paper packaging requires a thin layer of plastic to prevent the formation of thermal bridges.

However, this type of hybrid packaging is almost impossible to recycle completely. This is because separation of the different components precedes the recycling process – and this calls for a huge amount of energy, which in turn can be saved if a mono-material is used. Therefore, the smaller the number of components contained in the packaging, the simpler the recycling process.
Step 2: The "Mental Paradigm Shift"
What would happen if from now on the used EPS packaging was no longer a waste material that had to be disposed of quickly? What would happen if from now on used EPS packaging was valuable raw material? Would it then not be logical to make every possible effort to ensure that the material EPS had as much "life" in it as possible? That was the initial thinking behind a strategy which is now resulting in the introduction of retecma: the first-ever AI-supported closed-loop material processing system in the pharmaceutical packaging sector. Of key importance here are the three success factors: digitalisation, regionalisation, and high-quality recycling.
Success Factor: Digitalisation
If EPS is a valuable material which should be reused as long as possible, then the packaging must not be regarded as a WHOLE; instead, it should be broken down into its component parts. Accordingly, each element in a PROTECT Box and an ICECATCH© passive energy storage unit will soon be clearly identifiable by way of an RFID chip. This RFID chip indicates not only the type of element (for example: L-shaped stacking frame) and the target weight but also the number of times the
element has already been used. In addition, each PROTECT Box is assigned a QR code which can be read out at the final destination by means of a eutecma app.

The eutecma App Offers the Following Options
1. Reusing the packaging >
Click to get the handling instructions depending on the duration of transport and temperature profiles
2. Sending packaging to the Refreshment Centre >
Click the address of the nearest Refreshment Centre
3. Sending packaging to Recycling >
Click the address of the nearest recycling company
Option 2 is a new and unique feature: the components of all PROTECT Boxes and ICECATCH© passive energy storage units are overhauled in eutecma's Refreshment Centres and made ready for their next application. The AI-supported system performs two fully automated tests on the individual components and either one or two sanitising procedures:
1. Weight test: If the target weight is not reached, material damage is assumed, and the component is withdrawn.
2. Photo-optical test: If the camera detects damage, the component is withdrawn.
3. Ultraviolet light is used to sanitise each component.
4. Steam cleaning (only if required): If the camera detects severe contamination, the component is steam-cleaned.
All the Refreshment Centres operate with Artificial Intelligence, which means their knowledge increases from one day to the next. Thus, the system can distinguish between superficial scratches and defects which threaten the integrity of the product. In addition, since the Refreshment Centres can

be networked with one another across different continents, this acquisition of know-how takes on a global dimension. The aim of the refreshment process is to ensure that the elements are reusable as far as possible.

Success factor: Regionalisation
To keep the CO2 footprint as small as possible and to restrict unnecessary journeys by empty delivery vehicles over long distances to a minimum, retecma will as soon as possible be available on those continents where the main flows of goods are destined, namely Europe (Germany), Asia, South America, North America as well as Middle East. Up until now standard practice has been either to dispose of packaging at the destination or to return it empty to the point of origin. With the retecma Loop system the procedure is different: in the future all PROTECT Boxes and ICECATCH© passive energy storage units will remain in the destination region. If the recipients have no use for them,

they will be collected in the destination region and can then be transported either to the Refreshment Centre or to authorised recycling partners.
Success Factor: Top-quality Recycling
All too often, however, discarded EPS components end up on a rubbish tip. This will not happen with eutecma's Reverse Logistic System, because part of the retecma pledge is that unusable EPS components are to undergo a high-quality recycling process. To this end we are working with certified regionally based partners who are carefully selected and audited. These regional partners ensure that by means of mechanical or chemical recycling it is possible to produce new valuable, high-quality EPS raw material from EPS which had previously been discarded in a manner detrimental to the environment. This new EPS raw material can be used, for example, in the production of new PROTECT boxes.
Step 3: Saving Up to 1,000 Kilos of CO2 with a 333-litre Cooling Box
According to the trend study "Pharma Management Radar" undertaken by Camelot Management Consultants, the interviewees consider product packaging to be an important means of achieving ecological improvements. Nevertheless, the survey, carried out in 2020, indicated that these efforts are thwarted by the lack of alternatives. However, all that has now changed with retecma Loop, the first-ever circular economy for pharmaceutical packaging. Instead of a piecemeal solution to augment their supply chain, retecma enables pharmaceutical companies with an interest in sustainability to leverage the enormous CO2 potential. Our calculations show that with a predicted quota of 15 reuses/usage cycles the PROTECT Europallet box with a capacity of 333 litres saves up to 1,000 kilos of CO2. Similar forecasts have been made for all other boxes in the PROTECT range.
Thus, each reused PROTECT Box and each reused ICECATCH© passive energy storage unit saves energy and resources, CO2 and waste. In addition, thanks to the AI-based Refreshment Centres, retecma will soon know how many lifecycles the individual components pass through and therefore will also be able to utilise the last kilometre that is still possible with the EPS packaging. And even after their final usage, no EPSs are lost in the retecma system. Thanks to sophisticated recycling processes, valuable new material is produced, from which high-quality EPS products can in turn be obtained.

And what's more: eutecma will in future be doing even more to help protect the planet. We are measuring our direct and indirect emissions to determine the complete CO2 footprint which our company and our products are leaving on the planet. In those areas in which we can achieve direct effects, for example by converting to green energy, we will do so, while in those areas in which CO2 emissions are unavoidable, for example during the initial production of PROTECT packaging and ICECATCH © passive energy storage units, we will
compensate for this by specifically promoting climate protection projects.
Conclusion
The time for greater sustainability in the pharmaceutical supply chain is not just ripe – it is over-ripe. For quite some time now, companies have been coming to realise that improving one's brand image is not the be-all and end-all. Ecological sustainability can also be used to make the global supply chains more robust. And this is a powerful asset in times of unexpected pandemics, sudden declarations of war and skyrocketing inflation. That's why eutecma is now acting as a pioneer and investing substantial amounts in the introduction of retecma, the first-ever closedloop material recycling system for pharmaceutical packaging. We are firmly convinced that only an intentional scarcity of goods as a result of strict multiple usage, supported by digitalisation, regionalisation and high-quality recycling, will make the pharmaceutical supply chain climate-friendly and waste-free. And thus, also counteract risks which threaten the pharmaceutical industry worldwide. According to the Camelot study, this includes growing cost pressure and supplier stability –factors mentioned by 86% and 52% of interviewees respectively.
Sven Rolle
Sven Rölle has been on the staff of eutecma from its earliest days. Having held various posts in the packaging industry, including SCA Packaging, he has played a key role in numerous milestones in the history of the eutecma company. The economics graduate is Head of Sales and is in close contact with many of the global players who do business with eutecma. With his deep understanding of intelligent solutions, characterised by "out-of-the-box" thinking, he and his team are making significant strides in the development of new groundbreaking cooling systems and supporting services.

Medicines that Don’t Cost the Earth: New Awards Showcase Innovations
“We know that climate change is bad for our health. What is less well known is that healthcare is bad for the environment,” said Nazneen Rahman while chairing a panel discussion showcasing innovations in sustainable packaging. The session was held on 14 September in Geneva at Connect in Pharma, a new annual event connecting innovators in pharma and biopharma to the world’s leading suppliers and manufacturers.
Rahman, a physician, scientist, and AstraZeneca Non-Executive Director, said she had this epiphany two years ago and was compelled to devote the next phase of her working life to making healthcare more sustainable.

Rahman went on to found sustainable healthcare pioneer YewMaker. Through YewMaker, Rahman started the Sustainable Medicines Partnership, a not-for-profit organisation that describes itself as a “public-private, multi-stakeholder global action collaborative”. To date, the partnership counts 42 organisations within its membership, including AstraZeneca, Walgreens, Pfizer and Deloitte.
She was in Geneva at Connect in Pharma to not only chair the sustainable packaging panel presentation, but to announce the winners of the Sustainable Medicines Packaging Awards, an initiative launched by YewMaker, with the award ceremony hosted at the first edition of Connect in Pharma.
Rahman says Connect in Pharma was a natural fit to launch the Sustainable Medicines Packaging Awards: “Collaboration and innovation are essential to delivering the changes needed to make sustainability core to how pharmaceutical packaging is designed and used. The audience of our awards marry perfectly with the audience of this new conference, which is all about bringing together innovators involved in the pharmaceutical supply chain. The partnership helped us bring a sharper focus on sustainability innovations in the sector.”
The awards focussed on innovations in two categories: the first category recognised sustainable packaging design, and the second looked to reward companies that had developed processes that brought greater circularity into the pharmaceutical supply chain.

WINNERS ANNOUNCED Design Award
The Design Award showcases innovations in sustainable packaging design. For medicine and medical device packaging to help reduce waste and increase sustainability, a pharma company might change to alternative materials that can be recycled or change the size or shape of packaging so that less
natural resources are used. Design innovations may involve materials, constituents, size or shape of medicine or medical device packaging to reduce waste and increase sustainability.
GOLD – Phill Box – Parcel Health
Phill Box is a recyclable and compostable alternative to plastic pill bottles. It has a 30% smaller carbon footprint, is water-resistant and light-proof.

SILVER – Protecting the Product and the Environment –Essentra Packaging
Essentra is producing a new carton-board package that replaces the need for plastic equivalents. Working with a pharmaceutical company that specialises in allergy diagnostics, they created a cartonboard option that eliminated 1.67 tons of plastic every year in the transportation vials for diagnosis to doctors.
Joint BRONZE – Dual Chamber Blister – Pantec AG/Pharmapan
The new Dual Chamber Blister device includes two high barrier chambers, the first filled with a mixing agent and the second with the drug formulation. With a simple finger compression, the ingredients are safely mixed and applied or injected. The design saves materials due to its lean structure compared to the classical vial/syringe solution, and its lower weight reduces shipping and energy costs.

Joint BRONZE – Qube Pro – Jones Healthcare Group
The Qube Pro multi-medication system is an automated sealing system for machine filling trays that uses eco-friendly, foil-free backing. A smaller overall size means lower shipping costs and less waste.
HIGHLY COMMENDED – EcoSecur Type 2 Glass Vials –Stoelzle Glass Group
Stoelze Pharma has developed a new, resource-efficient process for the inner surface treatment of type 2 glass vials. The new innovative process enables reliable and precise dosing tailored to the size of the glass, with fewer chemicals used and no toxic substances. Environmental impact is achieved through precise and optimal dosage of ammonium sulphate.
CIRCULARITY AWARD
The Circularity Award recognises packaging services, processes, partnerships or products that reduce single-use packaging, bringing circularity into the pharma packaging supply chain. Some packaging materials have great functional benefits, and they can’t be changed or recycled easily. This means there is a large volume of high-quality single-use packaging generated by the industry. Yet many see value in this ‘waste’. The Circularity Award aims to recognise innovations in the packaging services and processes, which may, for example, find ways of reusing packaging. If a producer can’t change the design, innovators in this category might be able to change something else to keep packaging out of landfills.
Joint GOLD – Sharpsmart Reusable Containers –Sharpsmart Ltd
Sharpsmart reusable container systems offer an environmentally responsible pharmaceutical waste management system, estimated to have saved over 45 tonnes of single use plastic and eliminated the manufacture and subsequent incineration of 46,938 disposable containers over the last 12 months.
Joint GOLD – Circularity Solutions for Thermoformed Plastics Along the Pharma Value Chain – Plastic Ingenuity
Plastic Ingenuity offer a range of services that aim to reduce waste, including a sustainable packaging assessment, a life cycle analysis and a recyclability consulting service and a vertically integrated take-back programme. One project allowed them to grind and recycle autoinjector handling trays, keeping them out of drug filling sites and saving an estimated $2.5 million.
BRONZE – Circularity-as-a-Service for Medicine Plastics –Automedi
Automedi combines recycling with a decentralised and distributed 3D printing fleet to recycle harvested medicine plastics into products at the touch of a button. By commandeering
machines across a country, the service can make millions of items from available waste in minutes.
HIGHLY COMMENDED – eZCooler – Zuellig Pharma
The eZCooler solution is a thermal insulation system that can be customised to provide temperatures down to -40 degrees Celsius. It is able to maintain a required temperature for up to four days without the need for an external energy source, and the container can be reused up to 200 times over five years, offering an eco-friendly way to ensure the integrity of temperature-sensitive products on the road.
HIGHLY COMMENDED – Smart Reusable Packaging –Monoceros and Emball’Iso
These companies have partnered to create a smart reusable medicinal packaging system. The “smart” element allows the package to be monitored live to track location and temperature conditions. The package can then be recalled and reused to deliver a new batch of medicines.
Pharmaceuticals are special, but the tide is turning
Millions of single-use pharmaceutical packaging items are discarded every year. The manufacturing process of medicines can consume high amounts of energy and contribute to carbon dioxide emissions. “If the global health sector were a country, it would be the fifth largest emitter on the planet,” said Rahman at the Connect in Pharma panel in Geneva.


Pharmaceuticals have been cushioned from complying with environmental regulations faced by other industries due to their “special nature”. Regulations governing the percentage of recycled content needed in a cereal box, for example, have not applied to medical packaging, at least not yet. Regulators have given the industry a grace period, in recognition that the main function of medical packaging is to maintain the efficacy and safety of medicines in different temperatures and humidity. Yet the pharmaceutical industry is facing increasing pressure from governments, investors, employees and the public to deliver solutions. “Everyone is demanding change,” said Rahman. YewMaker’s goal is to drive science-based, scalable, sustainable solutions to these challenges.
The Time is Now YewMaker Chief Scientific Officer Shazia Mahamdallie explains why the time was right to launch these awards: “We have to start now if we are to achieve national and global climate change goals. We launched these awards to showcase the
innovations that are here today, and to inspire and motivate innovators to find tomorrow’s sustainable packaging solutions.”
Mahamdallie points to Paul Anastas and John Warner’s ground-breaking book, Green Chemistry: Theory and Practice, published in 1998. The principles outlined within their book motivated academic and industrial scientists past and present. Working and scientific groups were established to bring Green Chemistry Principles into the manufacturing processes of medicines.
Since then, the industry has compiled over two decades of high-quality science research and development in the industry that have a shared goal in environmental sustainability. “By the nature of their business, pharma is an innovative industry. And we are beginning to see this inventive spirit being brought to packaging, logistics and distribution, to overcome challenges and hurdles,” says Mahamdallie.
“Today, I think all industries are making moves towards prioritising sustainability, pharma included, as it’s becoming imperative to business and important for the consumer,” she added. Change is coming, and YewMaker created these awards to highlight and champion the innovation emerging in the industry.
“At YewMaker’s core are scientists who have a shared belief in reducing waste and improving access to medicines. We believe healthcare can be good for us, good for our planet and good for business,” said Rahman.
Shortlisted Innovators Bring New Perspectives

Sustainability featured very highly in the discussion throughout the two days of the inaugural Connect in Pharma in Geneva last month, demonstrating the increasing spotlight on the industry to address its impact on the environment. The sustainability panel that was part of the programme featured innovators whose projects had been shortlisted for the new award scheme. Panelists discussed some of their biggest challenges in pushing through new forms of packaging and finding new ways of making business practices more sustainable.
Théophile Griveau-Billion, CEO of smart reusable packaging start-up Monoceros, said one of the biggest challenges has been getting people to change their habits. But he found success when he could demonstrate that making items reusable and keeping them in the circular economy means “being
sustainable is also cheaper.” He recommended finding people who want to change – wherever they are in the company, while also demonstrating how products that remain in circulation can save the company money.
Laura Lopez, Marketing Manager at Essentra Packaging, noted that any change in packaging has to go through an approval process, and companies want to ensure that changes don’t affect the speed of the production line. Longer production time can cut into profits and require more energy, which can be counterproductive for sustainability. Measuring sustainability was also a challenge, she noted, without industry-wide agreements on standards and the lack of a common language in place.

Melinda Su-En Lee, Co-Founder and CEO of Parcel Health, agreed that innovators face a public and industry too often stuck in its ways. As a pharmacist, she was horrified to be part of an industry that generated so much waste in packaging. As a child in Malaysia, she recalls seeing the environmental devastation caused by the plastic waste shipped there from the US and Europe. She was determined to create a recyclable, compostable packaging that also improves the functionality of the packaging.
Lee demonstrated her plastic-free pill dispensing design and is now looking to get US-based pharmacies on board to use the design as an alternative to the orange plastic pill containers ubiquitous in the US. “I would love to see more recyclable and compostable packages be used, and in the future hopefully the plastic pill bottles or even the over-the counter bottles will be sitting in a science museum as a relic of the past, and sustainable options like these will be the mainstay product,” she said.
Several panel members agreed that the complexity of the pharmaceutical supply chain compounds the challenge. Packaging passes through many hands from the initial design to manufacturing to distribution and disposal. There was agreement that solutions that are truly scalable and sustainable need the whole supply chain to be linked by common goals. “We need all the stakeholders at the table, and then we can measure progress,” said Kiley Djupstrom, Commercial Development Leader at Plastic Ingenuity.
And the Winner is… At the end of the session, Rahman announced the winners.
Djupstrom accepted a joint gold award in the circularity category in recognition of the company’s work to develop solutions with its thermoformed plastics that can be reused along the pharma value chain.
Lee accepted the gold award for the design category. On winning the award, Lee said: “Being able to share our product, our vision and our story with a larger audience is amazing.” Griveau-Billion from Monoceros noted that taking part in the event and panel discussion led to positive leads. “We had interesting people coming to speak to us afterwards. A pharma company we have been trying to reach out to for months and never came back to us came up to me afterwards and said: ‘Oh, we would like to try that!’”
For the team at YewMaker and the organisers of Connect in Pharma, that means success. “We wanted to tell the stories and highlight the creativity behind how medicines can be packaged sustainably,” said Mahamdallie. “Just as packaging is one of the unsung heroes in the story of global access to medicines, so are those working to make the pharmaceutical industry more sustainable. “
“The engagement of industry and the calibre of entries exceeded our hopes and gives us great optimism for the future of sustainable pharmaceutical packaging,” said Rahman.
Renan Joel, Divisional Director at Connect in Pharma, said: “Having an environmental focus was crucial at Connect in Pharma, and we were thrilled to be able to host these awards at our inaugural edition of the event. Connect in Pharma is designed to focus on innovation and technology in the pharma and biopharma community, and sustainable medicine packaging design is an important area of innovation in the sector. We are delighted to be coupling innovation with sustainability while putting the future of the planet in the spotlight. We have plans to grow this focus for the second edition of the show with more content and features dedicated to this important area for pharma.”
The next edition of Connect in Pharma will take place on 14 & 15 June 2023, and it will again be held at Palexpo in Geneva.
“Our exact plans for the content structure are still being planned, but we expect to dedicate more conference sessions and more focus on sustainability in year two. The issues of sustainability and circularity are only going to become more important, and we will reflect that in our event format,” said Joel.
“Sustainability doesn’t start or end at packaging, and it’s becoming clear that these are issues that need to be addressed throughout the supply chain. We aim to provide a forum for the key parts of this supply chain to meet and have a meaningful discussion on the way forward.”
More information on the awards and Connect in Pharma can be found at www.connectinpharma.com/sustainablemedicines-packaging-awards-shortlist/
More information on YewMaker and the Sustainable Medicines Partnership can be found at www.yewmaker.com
Connect in Pharma
Connect in Pharma brings together suppliers, associations, clusters and world-leading companies in pharmaceutical and biotechnological production.
Connect in Pharma Exhibition is the one-stop-platform for the development, industrialisation and contract manufacturing of drug delivery systems and pack & filling technology. From the concept phase to the finished product, every aspect of Connect in Pharma is designed to inspire collaboration and innovation between packaging suppliers, drug delivery systems, CMO/CDMO and filling & assembling technology.

CPHI Frankfurt is back, this Autumn!
Don’t miss your chance to meet the entire pharma supply chain in the heart of Europe. Register now and join Connect to Frankfurt, online on 28th September, to get a head start on making connections. Then join us in Frankfurt on 1-3 November for 3 days of in-person networking and learning to help you grow in your knowledge and expertise, face-to-face!
Special Offer: Register now and receive a 20% discount on your Attendee EARLY ACCESS pass!

PARTICIPANTS
Page 26–29
Airnov Healthcare Packaging
Page 20–25 AptarGroup, Inc.
Page 50–53 Connect in Pharma
Page 36–41 EECO2
Page 46–49 Eutecma GmbH
Page 11–13 Guru Angad Dev Veterinary and Animal Sciences University
Page 8–10 Healthcare Without Harm
Page 42–45 PCI Pharma Services
Page 32–35 Pharmaserve North West Ltd
Page 30–31 Recipe Design
Page 18–19 Securikett Ulrich & Horn
Page 14–16 The Webinar vet
Join thousands of pharma professionals and industry experts from across the entire supply chain to grow your knowledge and expand your understanding of your Environmental Social Governance. PNP is the place for you to make meaningful connections, enhance your knowledge and expertise, and to grow your business potential. Companies who practice sustainable applications can reap the benefits, from reduced resource and production costs to enhanced recruitment ability, increased sales, and brand popularity.
Subscribe today at Email: info@senglobalcoms.com www.pharmanaturepositive.com
Join us at the heart of realisation & discussion where innovation and collaboration combine to drive business forward.

We create product, packaging and brand pipelines from concept definition to commercialisation.
Recipe is a strategic design consultancy, dedicated to helping clients achieve growth through product and service innovation across the pharmaceutical, healthcare and home sectors.
The team at Recipe has spent over a decade pioneering the use of Meaning Centred Design™ to deliver award-winning work for market leading brands including Circassia, Mundipharma, Sanofi and Roche.
Recipe Design was awarded a silver medal in recognition of achievements across key measures that define Corporate Social Responsibility by EcoVadis – the world’s most trusted business sustainability index - and rated within the top 6% of its industry.
To find out more about Recipe’s Meaning Centred DesignTM approach, please visit our website, or contact:

Working daily to improve the health of our patients and our planet


As a leader in drug delivery, Aptar Pharma works daily to deliver innovative technology platforms that have a positive impact on patients and the planet.
In line with our public sustainability targets for Beauty + Home and Food + Beverage products, Aptar Pharma continues to invest in renewable feedstock resins, further develop mono-material-based products and prepare for the introduction of new low GWP propellants in the pMDI market.

Furthermore, our focus on sustainable design has enabled us to make important product breakthroughs with both our Airless+ dermal drug delivery range and our Bag-on-Valve (BOV) continuous dispensing technology, both achieving cyclos-HTP recyclability certifications with AAA and A classifications, respectively.


It is Aptar’s deep commitment to create solutions that respect the environment, conserve natural resources, improve life on earth, and safeguard patient welfare. Because for us, helping to keep the planet and its people healthy shouldn’t be a compromise.

A journey of a thousand miles begins with a single step. To find out how Aptar can help you on your sustainability journey, to learn more about our initiatives and to read our 2020 Corporate Sustainability Report, visit www.aptar.com/sustainability



Delivering solutions, shaping the future.




