8 minute read
Identification of Three Phenotypes of Cardiogenic Shock
Dr Ishikawa proposes TH in combination with LV mechanical unloading as a treatment for STEMI patients. TH, during the 30-minute period of LV unloading prior to reperfusion, as recommended by the STEMI-Door-toUnload pilot clinical trial, could enhance the impact of both treatments.17 A meta-analysis of preclinical studies from around the world over the past 40 years established that mechanical unloading reduces infarct size.18 Mechanical LV unloading decreases pressure–volume area, reducing myocardial oxygen consumption (MVO2) for mechanical work, whereas MVO 2 for non-mechanical work remains unchanged.19 TH improves myocardial energy efficiency by decreasing heart rate and MVO2 for nonmechanical work.20,21 LV unloading stabilises haemodynamics and prevents lung congestion through increased blood flow, mitigating TH complications of increased arrhythmia and impaired diastolic function.22,23 Combination therapy may also limit reperfusion injury by LV unloadingmediated reduction in wall stress and TH-mediated decrease in myocardial temperature. The appropriate TH method of cooling, administration timeline and potential side-effects of combined therapy, such as thrombogenesis, should be determined in future clinical studies.
1. Hale SL, Kloner RA. Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am J Physiol 1997;273:H220–7. https://doi.org/10.1152/ajpheart. 1997.273.1.H220; PMID: 9249493. 2. Tissier R, Ghaleh B, Cohen MV, et al. Myocardial protection with mild hypothermia. Cardiovasc
Advertisement
Res 2012;94:217–25. https://doi.org/10.1093/cvr/cvr315; PMID: 22131353. 3. Simkhovich BZ, Hale SL, Kloner RA. Metabolic mechanism by which mild regional hypothermia preserves ischemic tissue. J Cardiovasc Pharmacol Ther 2004;9:83–90. https://doi.org/10.1177/ 107424840400900203; PMID: 15309244. 4. Hale SL, Kloner RA. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol 1997;92:351–7. https://doi.org/10.1007/
BF00788947; PMID: 9486356. 5. Hale SL, Kloner RA. Myocardial temperature reduction attenuates necrosis after prolonged ischemia in rabbits. Cardiovasc Res 1998;40:502–7. https:///doi.org/10.1016/s00086363(98)00191-6; PMID: 10070490. 6. Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res 2003;59;715–22. https://doi. org/10.1016/s0008-6363(03)00456-5; PMID: 14499873. 7. Hale SL, Herring MJ, Kloner RA. Delayed treatment with hypothermia protects against the no-reflow phenomenon despite failure to reduce infarct size. J Am Heart Assoc 2013;2:e004234. https://doi.org/10.1161/JAHA.112.004234; PMID: 23525431. 8. Dai W, Hale SL, Kloner RA. Delayed therapeutic hypothermia protects against the myocardial no-reflow phenomenon independently of myocardial infarct size in a rat ischemia/reperfusion model. Int J Cardiol 2017;236:400–4. https://doi.org/10.1016/j.ijcard.2017.01.079; PMID: 28108128. 9. Herring MJ, Dai W, Hale SL, Kloner RA. Rapid induction of hypothermia by the ThermoSuit system profoundly reduces infarct size and anatomic zone of no reflow following ischemiareperfusion in rabbit and rat hearts. J Cardiovasc Pharmacol Ther 2015;20:193–202. https://doi. org/10.1177/1074248414535664; PMID: 24906542. 10. Dai W, Herring MJ, Hale SL, Kloner RA. Rapid surface cooling by ThermoSuit system dramatically reduces scar size, prevents post-infarction adverse left ventricular remodeling, and improves cardiac function in rats. J Am Heart Assoc 2015;4:e002265. https://doi.org/10.1161/
JAHA.115.002265; PMID: 26116692. 11. Shi J, Dai W, Kloner RA. Therapeutic hypothermia reduces the inflammatory response following ischemia/reperfusion injury in rat hearts. Ther Hypothermia Temp Manag 2017;7:162–70. https://doi.org/10.1089/ther.2016.0042; PMID: 28338422. 12. Shi J, Dai W, Carreno J, et al. Therapeutic hypothermia improves long-term survival and blunts inflammation in rats during resuscitation of hemorrhagic shock. Ther Hypothermia Temp Manag
2020;10:237–43. https://doi.org/10.1089/ther.2020.0024; PMID: 32833603. 13. Dallan LAP, Giannetti NS, Rochitte CE, et al. Cooling as an adjunctive therapy to percutaneous intervention in acute myocardial infarction: COOL=MI InCor Trial. Ther Hypothermia Temp Manag 2020. https://doi.org/10.1089/ther.2020.0018; PMID: 32552523; epub ahead of press. 14. Keeble TR, Karamasis GV, Noc M, et al. Effect of intravascular cooling on microvascular obstruction (MVO) in conscious patients with ST-elevation myocardial infarction undergoing primary PCI: results from the COOL AMI EU pilot study. Cardiovasc Revasc Med 2019;20:799–804. https://doi.org/10.1016/j.carrev.2018.09.017; PMID: 30414797. 15. Wang YS, Zhang J, Li YF, et al. A pilot clinical study of adjunctive therapy with selective intracoronary hypothermia in patients with ST-segment elevation myocardial infarction.
Catheter Cardiovasc Interv 2018;92:e433–40. https://doi.org/10.1002/ccd.27864;
PMID: 30265431. 16. Erlinge D, Götberg M, Noc M, et al. Therapeutic hypothermia for the treatment of acute myocardial infarction–combined analysis of the RAPID MI-ICE and the CHILL-MI Trials. Ther
Hypothermia Tem Manag 2015;5:77–84. https://doi.org/10.1089/ther.2015.0009;
PMID: 25985169. 17. Swain L, Reyelt L, Bhave S, et al. Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J Am Coll Cardiol 2020;76:684–99. https://doi.org/10.1016/j. jacc.2020.06.031; PMID: 32762903. 18. Miyashita S, Kariya T, Yamada KP, et al. Left ventricular assist devices for acute myocardial infarct size reduction: meta-analysis. J Cardiovasc Transl Res 2020. https://doi.org/10.1007/ s12265-020-10068-7; PMID: 32860130; epub ahead of press. 19. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support.
J Am Coll Cardiol 2015;66:2663–74. https://doi.org/10.1016/j.jacc.2015.10.017; PMID: 26670067. 20. Mikane T, Araki J, Suzuki S, et al. O2 cost of contractility but not of mechanical energy increases with temperature in canine left ventricle. Am J Physiol 1999;277:H65–73. https:// www.doi.org/10.1152/ajpheart.1999.277.1.H65; PMID: 10409183. 21. Bergman R, Braber A, Adriaanse MA, et al. Haemodynamic consequences of mild therapeutic hypothermia after cardiac arrest. Eur J Anaesthesiol 2010;27:383–7. https://doi.org/10.1097/
EJA.0b013e3283333a7d; PMID: 19858724. 22. Zhang W, et al. Therapeutic hypothermia increases the risk of cardiac arrhythmia for perinatal hypoxic ischaemic encephalopathy: a meta-analysis. PLoS One 2017;12:e0173006. https://doi. org/10.1371/journal.pone.0173006; PMID: 28273115. 23. Post H, Schmitto JD, Steendijk P, et al. Cardiac function during mild hypothermia in pigs: increased inotropy at the expense of diastolic dysfunction. Acta Physiol (Oxf) 2010;199:43–52. https://doi.org/10.1111/j.1748-1716.2010.02083.x; PMID: 20102340.
Presented by Elric Zweck, MD
German Center for Diabetes Research, Heinrich-Heine-Universität, Düsseldorf, Germany
Dr Elric Zweck is a doctoral researcher in the Westenfeld laboratory at the German Center for Diabetes Research.
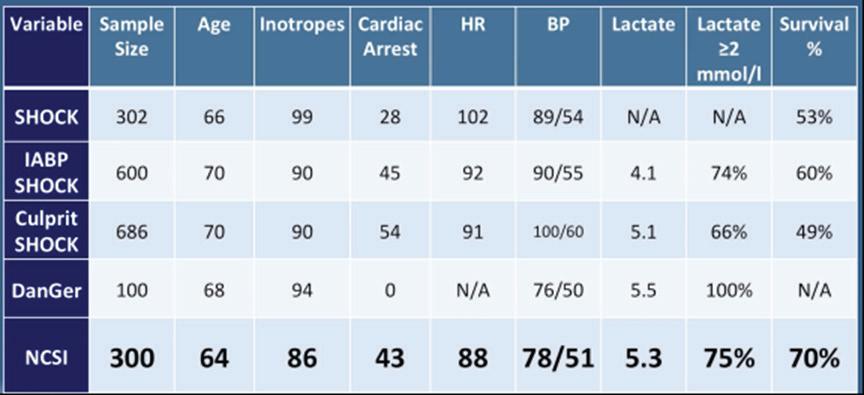
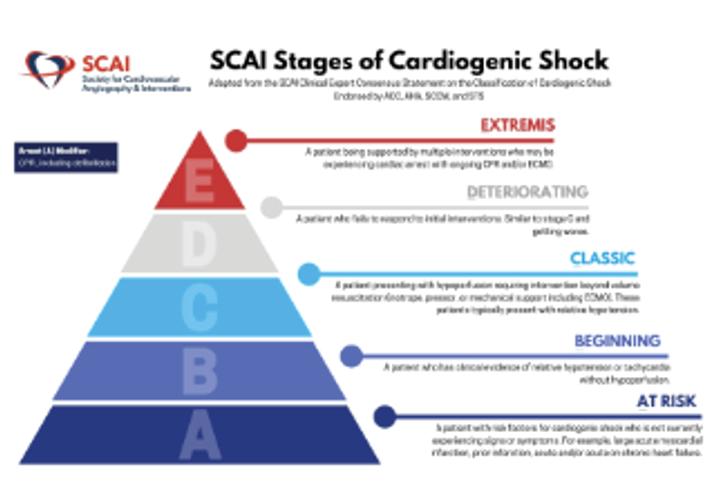
Dr Zweck began his talk by defining cardiogenic shock (CS) as a heterogenous clinical syndrome with unacceptably high mortality. Despite numerous innovations, in-hospital mortality for CS remains at 30–60%. Dr Zweck proposed that recent clinical trials in CS have been unable to identify superior new treatment strategies due to the heterogeneity of included patients. Current CS classifications, such as the 2019 classification released by the Society for Cardiovascular Angiography and Interventions (SCAI), are primarily derived through expert consensus, instead of being data driven.1 Dr Zweck’s research hypothesis is that a data-driven machine learning analysis could identify distinct phenotypes of CS with clinical applicability. Semi-supervised analysis using consensus K means clustering on clinically relevant mortality-driving variables identified by machine learning was applied to 1,835 CS patients from three international cohorts: the Danish Retroshock Registry (DRR), a two-centre registry of patients in CS after MI; the Cardiogenic Shock Working Group–Myocardial Infarction Registry (CSWG-MI), a US multicentre cohort of patients in CS after MI; and the Cardiogenic Shock Working Group–Heart Failure Registry (CSWG-HF), a
Figure 1: Consensus K Clustering Producing 3 Phenotypes (I, II, III) Derived from the Cardiogenic Shock Working Group–Myocardial Infarction Registry (CSWG-MI) and Validated by the Danish Retroshock Registry (DRR) Figure 2: In-hospital Mortality Differentiated by Machine Learning Phenotypes (I, II, III), MI, Heart Failure (HF), Combination of MI and HF and Society for Cardiovascular Angiography and Interventions (SCAI) Stage
CSWG-MI Derivation CSWG-MI
DRR Validation DRR
US multicentre group of CS patients with acute-on-chronic heart failure. Clusters were derived from the CSWG-MI and cross-validated in the DRR and CSWG-HF.
Clustering techniques with many variables may provide granularity, but often lack generalisation to their applied disease states. Dr Zweck’s research team limited cluster variables to those driving patient mortality to generate a clinically actionable phenotype. Fitting a random forest model to the CSWG-MI cohort identified six variables driving mortality to cluster based on availability, variable orthogonality and predictive importance: glomerular filtration rate (GFR), alanine aminotransferase (ALT), lactate, HCO
3, platelets and white blood cell (WBC) count.
Independent consensus clustering of GFR-CKDEPI, ALT, lactase, HCO3, platelets and WBCs to the CSWG-MI and DRR data identified three distinctly different CS clinical profiles (Figure 1). A three-cluster model provided stable or well-defined boundaries in visual representations of the data, as opposed to a two- or four-cluster model for both data cohorts.
Each cluster differs clinically in congestive profiles, haemodynamics, metabolic variables, cardiac function and in-hospital mortality. The key features of each cluster lead to their identification as non-congested CS/ phenotype I, cardiorenal CS/phenotype II and cardiometabolic CS/ phenotype III. Each phenotype demonstrated distinct in-hospital mortality profiles and applicability to CS patients with heart failure. Application of all three CS phenotypes within the SCAI shock classification scheme provides further stratification for mortality (Figure 2). SCAI stage and mortality associated with MI, HF or a combination of both primarily increased with phenotype classification number; non-congested/ phenotype I CS patients often exhibit a classic SCAI stage with the least in-hospital mortality, whereas cardiometabolic/phenotype III CS patients exhibit an extreme SCAI stage with the greatest in-hospital mortality.
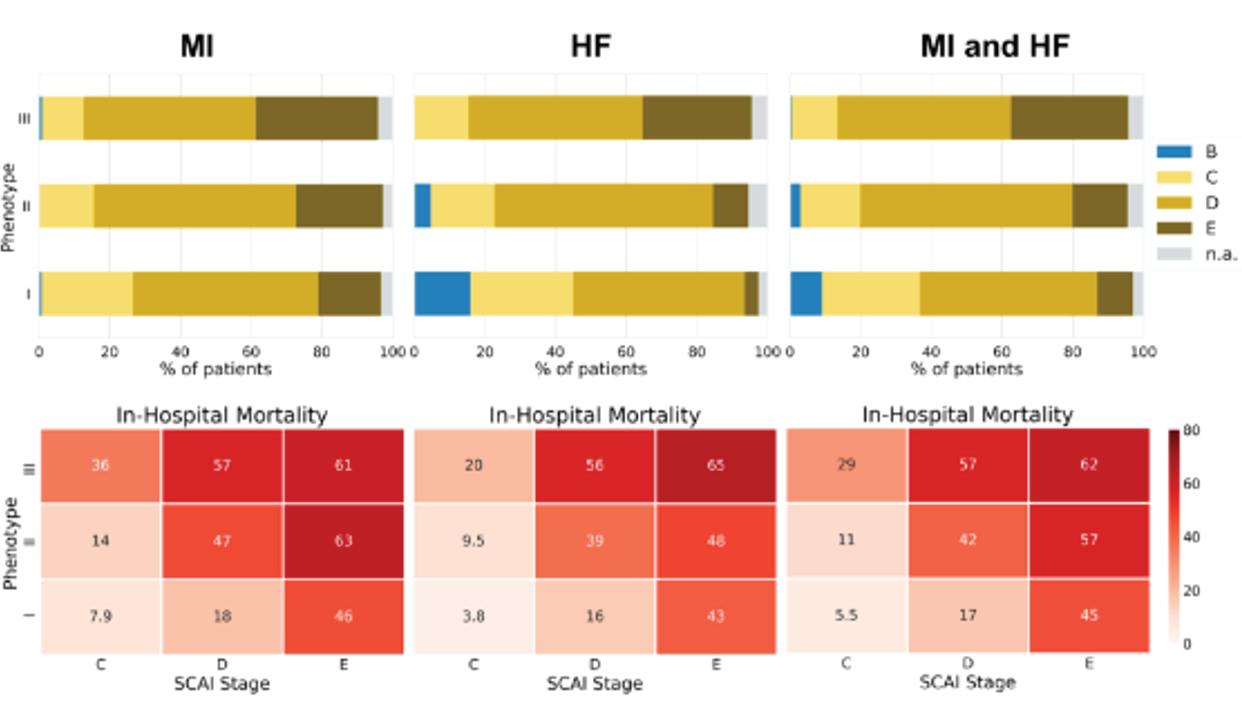
Dr Zweck concluded that an unbiased machine learning approach identified three distinct clinically applicable phenotypes of cardiogenic shock.2 These phenotypes exhibit different metabolic and haemodynamic profiles, and show a reproducible association with mortality. Each phenotype was classified by their defining features of non-congested shock, cardiorenal shock and cardiometabolic shock, and are compatible with, and enhance, SCAI stages. Future studies may further characterise these phenotypes and apply them in the conception of more targeted clinical trials, instead of a one-size-fits-all solution.
1. Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29. https://doi.org/10.1002/ccd.28329; PMID: 31104355. 2. Thayer K, Zweck E, Helgestad O, et al. Derivation and validation of three novel phenotypes of cardiogenic shock. J Heart Lung Transplant 2020;39(Suppl):S55. https://doi.org/10.1016/j. healun.2020.01.1241.