Spotlight | By Herman Cheung and Laurence MacPhie, Bereskin & Parr LLP
Patent rights and the coming of age
of cell-based immunotherapies
I
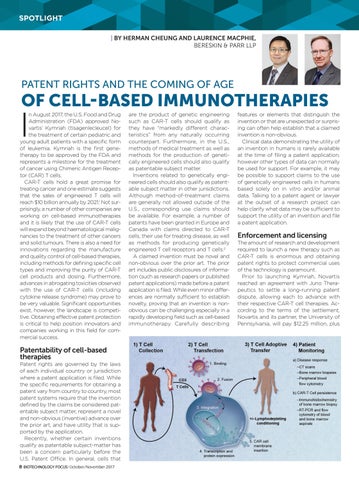
n August 2017, the U.S. Food and Drug Administration (FDA) approved Novartis’ Kymriah (tisagenlecleucel) for the treatment of certain pediatric and young adult patients with a specific form of leukemia. Kymriah is the first genetherapy to be approved by the FDA and represents a milestone for the treatment of cancer using Chimeric Antigen Receptor (CAR) T cells. CAR-T cells hold a great promise for treating cancer and one estimate suggests that the sales of engineered T cells will reach $10 billion annually by 2021.1 Not surprisingly, a number of other companies are working on cell-based immunotherapies and it is likely that the use of CAR-T cells will expand beyond haematological malignancies to the treatment of other cancers and solid tumours. There is also a need for innovations regarding the manufacture and quality control of cell-based therapies, including methods for defining specific cell types and improving the purity of CAR-T cell products and dosing. Furthermore, advances in abrogating toxicities observed with the use of CAR-T cells (including cytokine release syndrome) may prove to be very valuable. Significant opportunities exist, however, the landscape is competitive. Obtaining effective patent protection is critical to help position innovators and companies working in this field for commercial success.
Patentability of cell-based therapies Patent rights are governed by the laws of each individual country or jurisdiction where a patent application is filed. While the specific requirements for obtaining a patent vary from country to country, most patent systems require that the invention defined by the claims be considered patentable subject matter, represent a novel and non-obvious (inventive) advance over the prior art, and have utility that is supported by the application. Recently, whether certain inventions qualify as patentable subject-matter has been a concern particularly before the U.S. Patent Office. In general, cells that 8 BIOTECHNOLOGY FOCUS October/November 2017
are the product of genetic engineering such as CAR-T cells should qualify as they have “markedly different characteristics” from any naturally occurring counterpart. Furthermore, in the U.S., methods of medical treatment as well as methods for the production of genetically engineered cells should also qualify as patentable subject matter. Inventions related to genetically engineered cells should also qualify as patentable subject matter in other jurisdictions. Although method-of-treatment claims are generally not allowed outside of the U.S., corresponding use claims should be available. For example, a number of patents have been granted in Europe and Canada with claims directed to CAR-T cells, their use for treating disease, as well as methods for producing genetically engineered T cell receptors and T cells.2 A claimed invention must be novel and non-obvious over the prior art. The prior art includes public disclosures of information (such as research papers or published patent applications) made before a patent application is filed. While even minor differences are normally sufficient to establish novelty, proving that an invention is nonobvious can be challenging especially in a rapidly developing field such as cell-based immunotherapy. Carefully describing
features or elements that distinguish the invention or that are unexpected or surprising can often help establish that a claimed invention is non-obvious. Clinical data demonstrating the utility of an invention in humans is rarely available at the time of filing a patent application, however other types of data can normally be used for support. For example, it may be possible to support claims to the use of genetically engineered cells in humans based solely on in vitro and/or animal data. Talking to a patent agent or lawyer at the outset of a research project can help clarify what data may be sufficient to support the utility of an invention and file a patent application.
Enforcement and licensing The amount of research and development required to launch a new therapy such as CAR-T cells is enormous and obtaining patent rights to protect commercial uses of the technology is paramount. Prior to launching Kymriah, Novartis reached an agreement with Juno Therapeutics to settle a long-running patent dispute, allowing each to advance with their respective CAR-T cell therapies. According to the terms of the settlement, Novartis and its partner, the University of Pennsylvania, will pay $12.25 million, plus