17 minute read
Guttural Pouch Mycosis: An Incidental Finding? Gareth Fitch
CASE REPORT
GUTTURAL POUCH MYCOSIS: AN INCIDENTAL FINDING?
Gareth L. Fitch
leefitch@cityu.edu.hk
SIGNALMENT
A 15-year-old TB gelding presented for investigation of a respiratory noise and weight loss. The horse had been purchased 8 months previously and a pre-purchase examination was performed prior to purchase. The horse was then rested for one month prior to beginning exercise. The horse was reported to have a good appetite, normal faeces, regular dental maintenance and was regularly de-wormed.
EXAMINATION FINDINGS
On presentation, clinical parameters were all within normal limits. There was no nasal discharge or malodour of the breath. There was loss of topline musculature and body condition score was scored 4/9. Melanomas were present on the tail and around the perianal area. There were no palpable melanomas in the parotid area or visible elsewhere on the horse’s head or body.
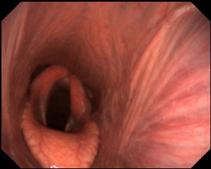
The horse was sedated with detomidine at 0.02 mg/kg IV and an endoscopic examination of the upper airway was performed. It was apparent that there was left-sided recurrent laryngeal neuropathy (RLN) grade 3.3 on the Havermeyer scale (Figure 1).
Pharyngeal tone and function appeared normal and there was a brisk swallowing reflex. There was no food material within the laryngeal aperture or trachea and no feed material present in the nasal passages suggestive of dysphagia. No discharge was seen exiting either guttural pouch ostium. As the horse had melanomas on the tail it was decided to examine the guttural pouches during the endoscopic examination to determine if there was any melanosis or melanomas visible in the guttural pouches or any enlargement of the retropharyngeal lymph nodes. There was a yellowish plaque, a few cm3, overlying the cranial nerve bundle at the point where it becomes visible within the caudal aspect of the left guttural pouch with some evidence of localised lymphoid swellings (Figure 2). Pigmentation was present over the surface of the left external carotid artery which was also present on the surrounding mucosa (Figure 3). No fungal-type plaque was present in the right guttural pouch, but pigmentation was also present.
Figure 1. Left-sided RLN. Pharyngeal tone and function appeared normal.
Figure 2. Infiltration of a mycotic plaque around the base of the cranial nerve bundle as it enters the guttural pouch in the medial compartment of the left guttural pouch. Localised swellings are present that likely represent reactive lymphoid tissue.


Figure 3. Pigmentation on the surface of the External carotid artery and surrounding mucosal surface indicative of melanosis.
A rectal examination was performed to determine if there was any extension of the melanomas deeper to the perineum, but this did not appear to be the case. An ultrasound examination of the abdomen including the spleen was performed and no abnormalities were detected.
TREATMENT
Following discussion with the owner it was decided to treat the fungal infection with a combined surgical and medical approach. A standing laser surgery to create a salpingopharyngeal fistula was planned two days following the examination so that the owner, who was also a veterinarian, could be present for the procedure.
The horse was positioned in standing stocks and sedated with detomidine hydrochloride IV and given 1g of phenylbutazone IV. Lidocaine hydrochloride 2% was sprayed over the walls of the nasopharynx and nasal passages. A salpingopharyngeal fistula was created into the left guttural pouch from the pharynx using a 980nm diode laser (Velas, Giga Laser, Wuhan) using a 400μm diameter fibre at 18W in continuous mode as described by Watkins and Parente 2018 (Figure 4).
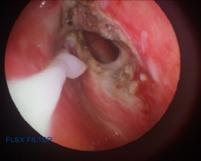
Figure 4. Salpingopharyngeal fistula created at the nasopharyngeal recess The laser was applied on a low power setting (5W) over the surface of the plaque, but due to its location over the cranial nerve bundle this was not a prolonged application to avoid any thermal neural damage. A portion of the left vocal cord was also removed whilst the horse was under sedation to address the respiratory noise that was occurring at exercise.
Topical application of an antifungal agent:
The endoscope was then passed through the fistula to access the fungal plaque and partial removal was achieved using endoscopic biopsy forceps via the instrument channel in combination with saline flushing. Following this, 2% miconazole gel (Decozol Oral Gel®, AFT Pharmaceuticals) was applied to the surface of the plaque using a 5ml Luer lock syringe and attached 14-gauge 1 inch needle inserted into plastic tubing which was passed via the injection port of the endoscope (Figure 5). The consistency of the gel allowed application via the tubing, as well as adherence and retention on the surface of the plaque. The fungal plaque was not submitted for culture as it was considered unlikely to alter the treatment decision due to the time taken to identify the fungal species and the limited choice of appropriate antifungal medications in New Zealand.
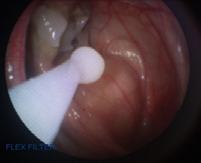
Figure 5. Application of miconazole gel to plaque using plastic tubing passed via the instrument channel of the endoscope.
Post-operative treatment and management:
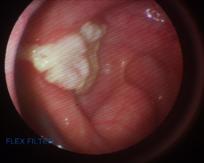
The horse was maintained on trimethoprim/sulphonamide antibiotic at 15mg/kg and phenylbutazone 1g, both given PO, BID for 5 days. Physical removal, saline flushing and treatment of the plaque was repeated daily for 7 days, then every other day for 7 days and then on 3 consecutive days prior to discharge. Eight days following surgery, there was a marked reduction in the size of the plaque, but the material was still adherent to the underlying mucosal tissue and could not be easily removed using the biopsy forceps and with flushing (Figure 6). At eighteen days post-operatively the plaque had regressed further and was easier to remove with the biopsy forceps and saline flush (Figure 7).
Twenty days following surgery and treatment only a thin layer of plaque remained (Figure 8), and the horse was discharged from the hospital with instructions to return within 2 weeks for a recheck endoscopy.
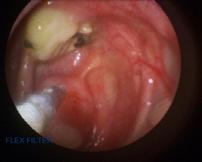
Figure 6. Marked reduction in the size of the plaque 8 days post-operatively – compare with Figure 2. Figure 7. The appearance of the plaque following 18 days post-operatively.
Figure 8. The appearance of the plaque 20 days post-operatively and prior to be discharged from the hospital. Lesion with miconazole gel applied (right).

Follow-up examination:
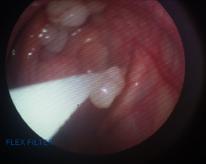
Eight days following discharge the horse returned for a follow-up endoscopic examination the guttural pouch was reexamined and there was just a small plaque remaining which was easily removed with an endoscopic biopsy forceps leaving a nodular area covered with inflamed mucosa (Figure 9). The horse was discharged with no arrangement for follow-up examinations unless there were signs of any problems. Eight months following discharge it was reported that his breathing was improved, but that he still made some respiratory noise at faster speeds. His body condition score was 5/9 with recovery of top line musculature. An occasional cough at the start of exercise was reported.


Figure 9. Appearance of the plaque at the final examination (left), and appearance after final removal of the plaque (right). Adjacent localised swellings interpreted as lymphoid tissue appear smaller.
Guttural pouch mycosis (GPM) is not considered a common condition in horses but in certain regions is sufficiently common, as is the case here, to warrant investigation where persistent nasal discharge is present. Mycosis can occur in other locations of the respiratory tract such as the sinuses (Hunter and Nation (2011). The infection is usually unilateral but can be bilateral (Lepage and Piccot-Crezollet (2005). In bilateral cases the infection has transferred from one pouch to the other via erosion and creation of a fistula between the two pouches (Dobesova et al. 2012). It generally affects adults, but the condition has been reported in a 6-month-old filly (Millar, 2006) and two foals (Chidlow and Slovis 2017). Nasal discharge is a common clinical sign, but not consistently present (Cook et al., 1968; Dobesova et al., 2012, Watkins and Parente, 2018). In a description of 28 cases that were referred for investigation of epistaxis or dysphagia, >60% presented with unilateral or bilateral nasal discharge with or without epistaxis (Dobesova et. al, 2012). It may be expected that the incidence of nasal discharge would be higher, but where epistaxis is present it may not be noticeable or have gone unnoticed. Of those 28 cases, five presented with nasal discharge as the only clinical sign and of the 13 cases that presented with dysphagia only 6 had nasal discharge. In this case there was no nasal discharge and also no signs of pharyngeal disfunction despite the plaque being relatively large and centered over the cranial nerve bundle that would include CNs IX, X, XI and XII. A diagnosis of mycosis was made based on appearance alone. It was regarded as highly likely to be of fungal origin and although no mycological examination was performed a decision was made to treat it as a local mycotic infection. Where mycological analysis is performed Aspergillus species are most commonly identified (Cook et al., 1968; Rawlinson and Jones, 1978; Gatineau and Ludwig, 2002; Ludwig, A., et al., 2005; Dobesova et al., 2012; Ludwig, A., et al., 2005). Of the cases described by Dobesova, mycological examination was performed in only 7 of the 28 cases and in 6 of the 7 cases Apergillus species were identified. Other species that that have been identified include Fusarium, Trichosporon, Acremonium Rhodotorula (Dobesova 2012) and Emericella nidulans (Cabanes et al., 2002; Kosuge, J., et al., 2000). Examination of a biopsy is more productive in identifying fungal infection than culture (Freeman and Hardy 2012, Dobesova 2012). The options for medical treatment of fungal infections are limited in New Zealand. In this case, there was concern that the location of the infection could result in dysphagia and so treatment was initiated as soon as possible.
This case presented for investigation of respiratory noise and was diagnosed with left recurrent laryngeal neuropathy (RLN). Laryngeal hemiplegia in cases of guttural pouch mycosis has been reported (Dobesova 2012; Lepage et al. (2021). In a description of neurologic dysfunction caused by guttural pouch disease (Borges and Wantanabe 2011) it was stated that vagal lesions at the guttural pouch site could affect all branches except the auricular and that dysphagia and inspiratory dyspnea (due to laryngeal paralysis or displacement of the soft palate) are the clinical signs that are observed with vagus lesions at the guttural pouch site. Of 28 cases presenting with GPM (Dobesova 2021), 10 also had RLN, two right-sided and 8 left-sided, and in all cases the hemiplegia corresponded to the side of the mycotic lesion. Interestingly, but perhaps not surprisingly, all the cases with RLN presented with dysphagia with or without epistaxis. The recurrent laryngeal nerve arises from the parent vagal nerve in the cranial thorax to return up the neck to the larynx without passing via the caudo-dorsal wall of the guttural pouch. Thus, if the plaque caused the RLN it would need to have markedly involved the left vagal nerve, that may be considered unlikely in the absence of other signs of vagal neuropathy, namely dysphagia. Although the RLN was left-sided in this case, as was the mycotic-like plaque, it still is very likely that it was an incidental finding and unrelated to the infection as there were no other signs of vagal neuropathy. A pre-purchase examination was performed prior to purchase, although endoscopy was not part of that procedure, no respiratory noise was identified at this time or immediately afterwards.
If left untreated, guttural pouch mycosis can result in a fatal haemorrhage where the infection is situated over the internal or external carotid arteries or branches thereof (Baptiste 2004; Delfs, K. C., et al. 2009; Archer 2012) and can cause irreversible damage to cranial nerves V, VII, IX, X, XI, or the cranial sympathetic trunk (Le Page et al., 2004). Dysphagia can result when there is localization over the nerve bundle of CN IX, X, XII as it passes through the guttural pouch. The complexity and fragility of the swallowing reflex was demonstrated in a case where the fungal infection was situated over the pharyngeal nerve only, causing only a unilateral pharyngeal dysfunction that was sufficient to cause dysphagia (Eichenttopf et al., 2016). Aggressive spread of the infection to involve adjacent structures can even cause pharyngeal fistula by eroding the dividing wall between both pouches (Dobesova 2012), middle ear infection, osteitis of underlying temporal bone, atlanto-occipital infection (Dixon and Rowlands, 1981; Ernst 2006), temporohyoid arthropathy (Walmsley, 1988), Horner syndrome and atrophy of the tongue (Ernst 2006; Cook et al., 1986; Survival rates where dysphagia is a presenting are poor and persistent dysphagia is a common reason for euthanasia (Lepage,. and PiccotCrezollet, 2005; Archer et al. 2012; Dobesova 2012).
Medical treatment alone generally gives unsatisfactory and doubtful results (Speirs et al., 1995). It has been suggested that horses can recover completely after surgical treatment without any medical therapy (Lepage et al., 2004; Leveille et al., 2000), but there are descriptions of cases where surgical treatment alone has not resulted in acceptable syndrome resolution and resulted in euthanasia or death (Ernst 2006; Eichentopf et al., 2016; Archer et al., 2012). Successful treatment of dysphagic horses with a combination of surgical and medical treatment is possible (Whitehead et al., 2018; pers. obs.), but the caveat is that treatment is often prolonged, intensive, and expensive and has a poor prognosis. Taking
this into account, treatment was initiated promptly in this case. Successful treatment of guttural pouch mycosis in 7 horses by creation of a salpingopharyngeal fistula using a diode laser has been described (Watkins and Parente, 2018). Cited advantages of this procedure were that it could minimise cost of treatment, be performed on an outpatient basis and provide better exposure of the infected area with few complications. That said, in this series of 7 horses, both medical and surgical treatment was carried out in some, and therefore it cannot be concluded that the surgical procedure alone would be successful. Resolution of the fungal plaques was reported to be between one to 6 months. In this case, a combination of surgical and medical treatment was chosen to resolve the infection as efficiently as possible and prior to development of any haemorrhage or cranial nerve dysfunction, especially dysphagia. In a description by Cousty et al., of 24 horses treated with ipsilateral ligation of the common carotid in combination with medical treatment for epistaxis secondary to guttural pouch mycosis, it was concluded that removal of the plaque and topical treatment of the underlying lesion appeared to speed up resolution of the mycotic lesions; in this case series the number of treatments ranged from 2-14. Although oral preparations of antifungals are available, topical application of an anti-fungal was chosen in this case as it was significantly cheaper compared to oral therapy. The preparation chosen is used for treatment of oral candidiasis in people, and its consistency allowed administration via tubing passed down the channel of the scope, although this required the use of a Luer lock syringe due to the pressure required. The gel adhered to the lesion, and with body heat it created a film that remained on the plaque. The entire 20day treatment only required a single 40g tube of Decozol Oral Gel®. Resolution of the fungal plaque was effectively complete at discharge after 20 days of treatment and the plaque was substantially reduced after only 8 days of treatment. Although spontaneous resolutions have been reported (Freeman and Hardy 2012); in cases where arterial occlusion has not been performed and where a salpingopharyngeal fistula has been created, application of an appropriate antifungal medication is relatively straight forward to perform and may hasten the resolution of the infection. It does require hospitalisation of the horse and the associated costs, but does enable confirmation that the infection has resolved. As previously stated, in this case there was concern that inflammation of the underlying nerves may result in dysphagia and therefore, the most rapid resolution was critical.
Fortunately, in this case the mycotic plaque was found prior to any signs of dysphagia had occurred but given the lack of any external clinical signs it would have been easy to miss, as a cause of the respiratory noise had been identified. In cases where persistent nasal discharge is present, endoscopy of the guttural pouches is warranted. A combination of surgical and medical treatment allowed rapid resolution of the plaque and no progression of clinical signs. Gracious thanks to the owner of the horse in this case Freja Hudspith BVSc, and the staff at Canterbury Equine Clinic, most particularly Danielle Guiver and Hayley Jackson for input to the success of this case, and to Maree Robertson for assistance with sourcing information for the manuscript.
References
Archer, R. M., et al. (2012). Guttural pouch mycosis in six horses in New Zealand. N Z Vet J 60(3): 203-209.
Baptiste, K. E. (2004). The mystery of guttural pouch mycosis: the paradox of advancing knowledge of a rare disease. Vet J 168(1): 1-2.
Borges, A. S., Marcos J. W. (2011), Guttural Pouch Diseases Causing Neurologic Dysfunction in the Horse. Vet Clin Nh Amer: Eq Pract 27(3): 545-572.
Cabanes, F. J., et al. (2002). [Guttural pouch mycosis by Emericella nidulans in a horse]. Rev Iberoam Micol 19(4): 208-211.
Chidlow, H. B., & Slovis, N. M. (2017). Guttural pouch mycosis in two foals. Eq Vet Edu 29(4), 213–218.
Cousty, M., et al. (2016). Ligation of the ipsilateral common carotid artery and topical treatment for the prevention of epistaxis from guttural pouch mycosis in horses. Vet Rec 178(2), 44-44.
Delfs, K. C., et al. (2009). Treatment of acute epistaxis secondary to guttural pouch mycosis with transarterial nitinol vascular occlusion plugs in three equids. J Am Vet Med Assoc 235(2): 189-193.
Dobesova, O., et al. (2012). Guttural pouch mycosis in horses: a retrospective study of 28 cases. Vet Rec 171(22): 561.
Eichentopf, A., et al. (2013). Dysphagia caused by focal guttural pouch mycosis: mononeuropathy of the pharyngeal ramus of the vagal nerve in a 20-year-old pony mare. Ir Vet J 66(1): 13.
Ernst, N. S., et al. (2006). Progression of mycosis of the auditory tube diverticulum (guttural pouch) after arterial occlusion in a horse with contralateral temporohyoid osteoarthropathy. J Am Vet Med Assoc 229(12): 1945-1948.
David E. Freeman, Joanne Hardy, (2012). Guttural Pouch, cg 46 in Equine Surgery, 4th ed. Eds: Jörg A. Auer, John A. Stick. W.B. Saunders: 623-642,
Hunter, B. and P. N. Nation (2011). Mycotic encephalitis, sinus osteomyelitis, and guttural pouch mycosis in a 3-year-old Arabian colt. Can Vet J 52(12): 1339-1341.
Kosuge, J., et al. (2000). [Detection of Emericella nidulans from bedding materials in horse breeding environment and its significance as a causative agent of guttural pouch mycosis in horses]. Nihon Ishinkin Gakkai Zasshi 41(4): 251-256.
Lepage, O. M., et al. (2021). The Effect of Topical Oxygen
Therapy in Horses Affected with Mycosis of the Guttural Pouch: An Experimental Pilot Study and a Case Series. Animals (Basel) 11(11): 3329.
Lepage, O. M. and C. Piccot-Crezollet (2005). Transarterial coil embolisation in 31 horses (1999-2002) with guttural pouch mycosis: a 2-year follow-up. Equine Vet J 37(5): 430-434.
Leveille, R., et al. (2000). Transarterial coil embolization of the internal and external carotid and maxillary arteries for prevention of hemorrhage from guttural pouch mycosis in horses. Vet Surg 29(5): 389-397. Ludwig, A., et al. (2005). Fungal isolation and identification in 21 cases of guttural pouch mycosis in horses (1998-2002). Vet J 169(3): 457-461.
Millar, H. (2006). Guttural pouch mycosis in a 6-month-old filly. Can Vet J 47(3): 259-261.
Watkins, A. R. and E. J. Parente (2018). Salpingopharyngeal fistula as a treatment for guttural pouch mycosis in seven horses. Equine Vet J 50(6): 781-786.
Whitehead, A. E., et al. (2018). Reversible dysphagia secondary to guttural pouch mycosis in a gelding treated medically with voriconazole and surgically with carotid occlusion and esophagostomy. Can Vet J 59(2): 165-170.
EXAMINATION OF HORSES | BLUE BOOK
The VPIS have just re written and released the 2021 5th Edition of Examination of Horses, also known as the Equine PPE Blue Book and it is available on the following web links: VPIS: https://www.vpis.org.nz/equine-ppe NZVA: https://www.nzva.org.nz/resource/equine/ PDF: HERE
WE’RE HERE TO HELP
CONTACT US TODAY (07) 888 8193

• 24/7 Emergency Referral
Hospital • Full Reproductive Service • Performance and Sports
Medicine • Sales and Yearling Consultancy and Radiology • Pre-purchase Examinations – including Hong Kong • Digital Radiography • Ultrasonography • Scintigraphy (Bone Scan) • Shockwave Therapy

Dave Keenan BVSc MANZCVS Andrea Ritmeester BVSc (Hons) MS DipACVS Leigh de Clifford BVSc CertAVP PGDipVPS MVSc Barbara Hunter DVM MS DAVCS-LA
ADVANCING VETERINARY SCIENCE IN THE HEART OF THE WAIKATO
Our dedicated team of highly experienced Equine Veterinarians provide the latest diagnostics, treatment and best care available for your horse.
362 Hinuera Road West Matamata (07) 888 8193 matamatavets.co.nz






