




As a top state for biomedical engineering graduates, Michigan is pioneering the next generation of medicine with advancements in everything from vaccine manufacturing to genomic research. Discover the world-class life sciences talent that make it all possible and seize your opportunity at MICHIGANBUSINESS.ORG

Michigan’s life sciences ecosystem thrives because of the partnerships between our world-renowned research institutions and universities, flourishing startups, and established medical device and pharmaceutical industry giants.
It is with great enthusiasm that I welcome you to this edition of BioMatters™. The issue highlights some of the exceptional individuals and organizations that make Michigan a leader in the life sciences industry, not only nationally, but globally.
I am filled with immense pride in the remarkable growth and innovation that continues to define Michigan’s life sciences community. Our state has long been recognized for its industrial ingenuity and educational excellence. However, what is truly exciting today is how we continue to be a powerhouse in the life sciences industry, albeit one that flies somewhat below the radar screen, from groundbreaking biosciences research to transformative medical technologies to biopharmaceutical production. Michigan is carving a unique path forward in bioscience innovation and manufacturing.
What makes this community so special is the collaboration amongst its stakeholders. Michigan’s life sciences ecosystem thrives because of the partnerships between our world-renowned research institutions and universities, flourishing startups, and established medical device and pharmaceutical industry giants. Across the state, we are witnessing the real power of collective effort in solving some of the world’s most pressing life challenges with groundbreaking work in therapeutics, medical products, diagnostics, agricultural biotech, and more.
At its core, Michigan’s bio-industry is home to a vibrant network of talent, from brilliant scientists and engineers to visionary entrepreneurs and compassionate bioscience professionals. Together, they are advancing scientific knowledge and creating tangible impacts on the lives of millions of people.
At the Michigan Biosciences Industry Association (MichBio), our mission is to foster collaboration, support innovation, and advocate for policies that advance scientific discovery and economic growth. This year, we’ve seen remarkable progress in public-private partnerships, research discovery and commercialization, and manufacturing prowess.
Because of such efforts, I am incredibly optimistic about the future of Michigan’s life sciences cluster. Together, we are laying the foundation for a healthier, more vibrant tomorrow. As we look ahead, let us continue to build on our successes, embrace the opportunities that lie ahead, and focus on improving the quality of life for all Michiganders and the world beyond.
Thanks to Michigan’s life sciences community for the dedication, passion, and commitment to making the state a beacon of excellence in life sciences. The best is yet to come.
Sincerely,

STEPHEN RAPUNDALO, PHD President and CEO, MichBio
Every day, from discovery to delivery, Avantor is an essential partner to the scientific community — pioneers, scientists, innovators and educators — relentlessly focusing on breakthroughs that help solve the world’s most complex challenges.
Our proven expertise and trusted portfolio of products and services, combined with a global reach and ability to provide customized materials of the highest quality for highly regulated applications move science forward.
As a global leader in life sciences, we fulfill our mission: to set science in motion to create a better world


MichBio is the biosciences trade association for the state of Michigan. Our goal is to drive the growth of the state’s bio-industry through advocacy, education, connections, and supportive resources.







Stephen Rapundalo, PhD President and CEO
Emily Brockman Director, Member Relations
Carrie Hranko Manager, Administrative Services
Jamie Krajny Editor Director, Marketing and Industry Engagement
CHAIR
Ken Massey, PhD
Wayne State University
Senior Director, Venture Development Technology Commercialization
VICE CHAIR
Kevin McLeod C2Dx
Founder, President and CEO
Christy Bigelow
Emergent Biosolutions
Vice President and General Manager, Manufacturing Operations
Chuck Bird
Neogen Corporation
Senior Director of Government Relations and Industry Affairs
Rhonda DeLuca
Terumo Cardiovascular Group Sr. Vice President Global Human Resources
Rose Gillesby, DVM Zoetis
Vice President, Veterinary Medicine Research and Development
Christine Haakenson, PHD Bio-industry Executive
Charles Hasemann, PhD
Michigan State University
Assistant Vice President for Innovation and Economic Development
Ken Massey, PhD
Wayne State University
Senior Director, Venture Development Technology Commercialization
MichBio
3600 Green Court, Suite 780 Ann Arbor, MI 48105-1175
734-527-9150
info@michbio.org michbio.org
Designer: Daina Fuson Designs
Printer: Progressive Printing
© Copyright Michigan Biosciences Industry Association, DBA MichBio
PRESIDENT AND CEO
Stephen Rapundalo, PhD
MichBio
President and CEO
SECRETARY
Vacant
TREASURER Vacant
Kevin McLeod
C2Dx
Founder, President and CEO
Dave Morin
Care Technology Advisors, President & Chief Operating Officer
Kate Remus
University of Michigan
Senior Associate Director &
Business Development Group Lead
Stephen Rapundalo, PhD MichBio
President and CEO
John J.H. Schwarz, MD
Former U.S. Representative
Uma Sharma, PhD MMS Holdings Chief Executive Officer
Ashlea Souffrou
SxanPro CEO and Founder

The MichBio Preferred Purchasing Program leverages the collective purchasing power of our member companies to obtain steep discounts from our vetted and endorsed vendor portfolio.
Members have saved upwards of 900 times their annual membership dues.






















BY DAN ROSS, PRESIDENT AND CEO, TRANSPHARM PRECLINICAL SOLUTIONS
On January 22, 2007, Pfizer closed its Ann Arbor, Michigan facility, bringing significant change to the region’s life sciences community Just two days later, TransPharm Preclinical Solutions was established, transforming a challenging moment into an opportunity for growth and innovation in preclinical research.
TransPharm Preclinical Solutions specializes in in vivo efficacy testing within the infectious disease therapeutic area. The company collaborates with clients from across the globe, testing experimental compounds targeting diseases caused by bacteria, fungi, viruses, and parasites. By utilizing murine models, the company provides essential efficacy data to support therapeutic advancements. While in vitro assays are part of its offerings, TransPharm’s core expertise lies in developing novel animal models and refining existing ones. These capabilities ensure that clients receive high-quality, reliable data to further their research.
Beyond precision research, the company offers shortterm leasing of lab and vivarium spaces. TransPharm’s facility includes clean room bioBubbles and three separate vivarium areas to safely isolate studies. The rodent surgical suite, equipped with two anesthesia stations, further enhances its capabilities. For clients aiming to generate preliminary data for investors, the option to lease space for a limited duration provides an accessible and cost-effective solution. Additionally, TransPharm’s long-established Institutional Animal Care and Use Committee (IACUC)


assists clients with creating Animal Use Protocols. With AAALAC accreditation and PHS assurance, the company is well-positioned to support clients, including those working with grant funding.
Recognition has followed TransPharm’s dedication to excellence. The company was named one of Michigan’s “Top 50 Companies to Watch” in 2011 and highlighted on ABC World News Tonight. Locally, it has garnered numerous awards for its contributions to both the life sciences industry and its surrounding community.
TransPharm’s impact extends well beyond its scientific pursuits. The company is deeply committed to supporting education and inspiring the next generation of scientists. Through longstanding participation in Junior Achievement, TransPharm has engaged 5th and 6th-grade students in discussions about the critical role animals play in research.
One of its hallmark initiatives, the “Girls in Science Weekend,” was created in partnership with YMCA Camp Storer. This program provides 5th and 6th-grade girls interested in science with a weekend-long experience filled with hands-on learning and engagement. TransPharm sponsors tuition for all participants and plays a key role in formalizing the program, which has earned recognition from the YMCA.
TransPharm also supports high school students through the Irish Hills STEM Scholarship. Each year, one senior


from each of four local high schools receives $1,000 to apply toward college expenses at a Michigan-based institution. To complement financial support, the company collaborates with schools to provide unique learning opportunities. These include lab tours and handson activities, such as those offered to Adrian High School’s Project Lead The Way class. The Lenawee Intermediate School District also organizes annual visits to TransPharm, allowing 25-35 science-focused students to explore laboratory settings and engage in interactive Q&A sessions.
TransPharm’s commitment to fostering careers in STEM continues through its internship programs for college students. The program immerses students in the full research process, from reading protocols and executing experiments to collecting, graphing, and reporting data. Recently, TransPharm began a partnership with Albion College to pioneer an internship model that grants college credit to participants. This initiative reflects TransPharm’s commitment to strengthening the Michigan life sciences talent pool by equipping students with practical experience.
Through its focus on innovation, collaboration, and community outreach, TransPharm Preclinical Solutions has built a reputation not only as a scientific leader but also as a company that values its role in shaping the future of life sciences.
By combining technical expertise with comprehensive educational support, TransPharm continues to make a lasting impact on the scientific community and the next generation of innovators in Michigan and beyond.





BY CADENSOFT, LLC
In today’s rapidly evolving healthcare landscape, efficient and accurate data management is crucial for delivering high-quality patient care. Zest Pediatrics a client company faced a significant challenge in migrating patient data from their existing Pediatric EMR Atlas system to a new instance in a usable, distinct field specific format rather than a cumbersome single document format.
Recognizing the complexity and importance of this undertaking, they partnered with Cadensoft to ensure a seamless and secure migration. This article explores the challenges faced, the solutions implemented, and the outcomes achieved, highlighting Cadensoft’s expertise in providing dependable data migration services.
Zest Pediatrics needed to transition critical patient records from an outdated Pediatric EMR Atlas instance to a new system without disrupting operations or compromising data integrity.
THE PROJECT INVOLVED TRANSFERRING LARGE VOLUMES OF SENSITIVE DATA, INCLUDING:
• Patient Demographics: Personal and familial medical history and contact information.
• Patient Visits: Patient notes, vitals, growth parameters, labs, and OT forms in PDF format.
• Macros: A comprehensive list of dot phrases/ templates.
• Contacts: Related contact information, including phone, fax, and departmental details.
Key challenges included ensuring data accuracy, maintaining continuity in patient care, and completing the migration with minimal downtime.
Cadensoft applied its expertise in healthcare data migration to deliver a comprehensive solution, addressing the project’s unique requirements.
THE SCOPE OF WORK INCLUDED:
DATA TRANSFORMATION
• Accurate mapping of data fields from the original Pediatric EMR Atlas instance to the new system.
DATA LOADING
• Importing transformed data into the new Pediatric EMR Atlas instance.
• Verifying the integrity and accuracy of loaded data.
DATA VALIDATION AND VERIFICATION
• Conducting thorough validation checks post-migration.
• Resolving discrepancies to ensure 100% data accuracy.
DELIVERABLES
• A complete and secure transfer of all data categories.
• A detailed final report documenting the data migration process, validation results, and resolutions.

The project began with a detailed discovery phase to understand Zest Pediatrics’ specific needs and data structure. Cadensoft’s team developed a tailored migration plan, focusing on precision and security. Regular updates ensured transparency, while collaborative and timely problem-solving addressed unexpected challenges swiftly.
THE SUCCESSFUL COMPLETION OF THE PROJECT DELIVERED MEASURABLE BENEFITS, INCLUDING:
• Accurate and Complete Data Migration: All patient records were seamlessly transferred to the new EMR system, ensuring uninterrupted access to critical information.
• Improved Operational Efficiency: The new system, coupled with clean and accurate data, streamlined workflows and enhanced patient care delivery.
• Comprehensive Reporting: The final migration report provided Zest Pediatrics with clear documentation of the process, instilling confidence in the integrity of their data.
Zest Pediatrics’ EMR migration highlights how Cadensoft’s expertise in healthcare data solutions can effectively address complex challenges. Through precise data transformation, secure migration, and thorough validation, Cadensoft ensured seamless access to accurate and structured patient records. This approach not only supported uninterrupted operations but also enhanced patient care delivery, showcasing Cadensoft’s commitment to delivering impactful and reliable technology solutions for their clients.
Cadensoft specializes in delivering custom technology solutions for healthcare organizations, combining technical expertise with deep industry knowledge to solve complex challenges. From EMR migrations to full-scale platform development, Cadensoft partners with clients to achieve measurable outcomes that drive success.
“ “
At Zest Pediatrics we faced the daunting task of migrating a large volume of sensitive patient data from a prior EMR instance into a new Pediatric EMR system. We weren’t simply moving files—we needed the data to be functional, structured, and accurately mapped into discrete, usable fields rather than static PDF attachments. This level of detail was critical to supporting our clinical workflows and ensuring continuity of care for our patients.
Cadensoft approached the project with precision and thoughtfulness. They conducted a thorough discovery process to understand the structure and content of our existing patient data, then developed a tailored migration plan that prioritized accuracy, data integrity, and security. Rather than settling for a generic document dump, they painstakingly transformed and validated our patient records— ensuring that vitals, visit notes, growth charts, macros, and contact information were all imported into the correct fields within the new system. Their technical capabilities were matched by their excellent communication and responsive support throughout the entire process.
The result was a smooth transition that preserved the quality and functionality of our patient records. We were able to continue operations without interruption, and our clinical team gained immediate access to patient charts.
Cadensoft’s work has had a lasting impact on our ability to deliver highquality pediatric care, and we’re incredibly grateful for their partnership on this critical project.


ECOVIA RENEWABLES
BY KOUSAY SAID, CEO, ECOVIA RENEWABLES INC.
Ecovia Renewables Inc. is a spin-off from the University of Michigan. It was founded with a commitment to addressing the dual challenges of reducing greenhouse gas emissions and eliminating microplastic pollution. The founders, however, were determined not to pursue a technology that failed to overcome the primary obstacles of biopolymer commercialization.
THESE OBSTACLES INCLUDE:
1. Higher costs compared to synthetic alternatives
2. Performance limitations
3. Inconsistent regulations
4. Research and development challenges
5. Supply chain and feedstock availability
Through an extensive high-throughput screening approach, that encompassed biology, process, and customer expectations, and after receiving encouraging research findings, the founders chose to focus on the development and scale-up of gamma polyglutamic acid (γ-PGA).

Gamma polyglutamic acid, or γ-PGA, was accidentally produced nearly a thousand years ago as a byproduct of soybean fermentation in the Japanese food natto. It wasn’t until 1942 that researchers successfully extracted γ-PGA from the fermentation broth of Bacillus subtilis. Today, γ-PGA is a remarkable anionic biopolymer that has gained significant attention across various industries due to its exceptional properties and versatile applications. It is a naturally occurring polymer known for its water retention, biodegradability, and non-toxic nature. These qualities position γ-PGA as a promising ingredient for sustainable innovations in skincare, agriculture, medicine, food technology, and hygiene.
Ecovia’s core mission is to commercialize γ-PGA. At the heart of its approach is the development of novel microbial strains and processes that overcome cost and performance barriers. While γ-PGA has traditionally been used as a humectant and drug delivery scaffold, among other industrial uses, Ecovia has identified new applications that were previously considered unfeasible due to the higher cost of the material. Notably, Ecovia has developed multiple grades of superabsorbent polymers that are biobased, low-cost, biodegradable, and high-performing.
Ecovia markets its linear form of γ-PGA under the brand name AzuraBase and the cross-linked gel form under the name AzuraGel. These products offer a range of benefits and applications.

One of the most notable properties of γ-PGA is its exceptional water-retention capacity. Research has shown that γ-PGA can carry more water than hyaluronic acid, making it an increasingly popular ingredient in skincare formulations. Its moisture-binding ability is a significant factor in its rising demand.
γ-PGA’s water-absorbent properties extend to agriculture. When introduced to soil, γ-PGA enhances water retention, creating drought-resistant growing conditions. It enables the soil to gradually release moisture, promoting seedling growth even under osmotic stress. Additionally, γ-PGA’s hydrophilic carboxyl groups improve soil bulkiness, porosity, and overall health, particularly in sandy soils. These qualities make it a valuable tool for sustainable farming, especially in regions prone to water scarcity.
Due to its biodegradable, non-toxic, and non-immunogenic nature, γ-PGA is ideal for medical applications. Research has explored its potential for drug delivery systems. Additionally, γ-PGA produced by certain Bacillus strains has demonstrated antimicrobial properties, with applications against various pathogens. Some forms of γ-PGA also exhibit angiotensin-converting enzyme (ACE) inhibitory activities, which could benefit cardiovascular health.

Rheology modifiers, or thickeners, alter the formulations’ viscosity and flow behavior across various industries, including cosmetics, paints, coatings, food, pharmaceuticals, and agriculture. Most rheology modifiers are synthetically derived. Ecovia has partnered with industry experts to introduce a biobased, biodegradable rheology modifier that meets technical requirements while offering a lower carbon footprint and eliminating microplastics.
γ-PGA can also replace sodium polyacrylate in cold packs, making them biodegradable. Similarly, γ-PGA can serve as a moisture absorber in packaging for raw meats, poultry, fish, and produce, reducing food spoilage during transport.
Hygiene product manufacturers are under increasing pressure to create more sustainable and biodegradable products. Ecovia has developed AzuraGel, a superabsorbent polymer that matches the performance characteristics of synthetic products. Ecovia plans to launch this product commercially in Q4 2025.
Gamma polyglutamic acid, from its early identification in fermented foods to its discovery in bacterial capsules, has evolved into a multifunctional biopolymer with exceptional properties. Its extraordinary waterretention capacity, biodegradability, and nontoxic nature have made it a valuable ingredient across skincare, agriculture, medicine, food technology, and hygiene. As Ecovia continues to develop its potential, γ-PGA offers a promising example of how naturally occurring compounds can provide sustainable solutions to modern industry challenges.

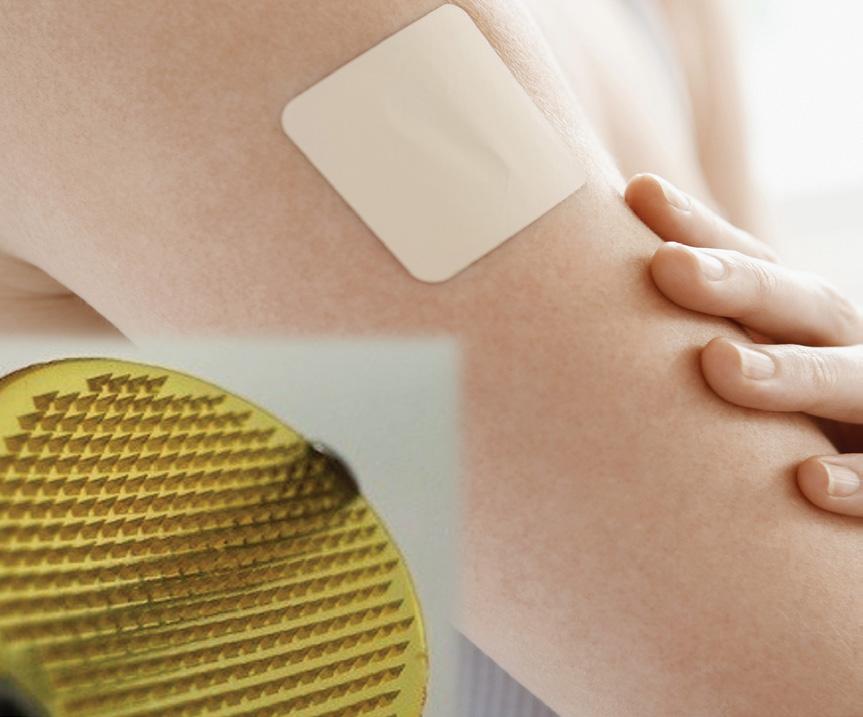
TSRL, INC.
BY ELKE LIPKA, PHD, MBA, CEO, TSRL, INC.
The transition from early-stage research to clinical drug development is one of the most challenging phases in the pharmaceutical industry, with many promising drug candidates failing due to insufficient preclinical data, strategic guidance, and funding.
At TSRL, Inc., we’ve built a strong reputation as a preclinical accelerator, helping biotech startups and academic researchers bridge this gap as an earlystage funding partner and product development advisory team.
Now, we’re expanding our role with comprehensive Contract Research Organization (CRO) services, providing the expertise, capabilities, and support to advance drug candidates toward clinical trials efficiently. Our mission is to deliver highquality preclinical testing that supports regulatory submissions, de-risks pipelines, and accelerates drug candidates toward clinical success.
Our Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited research facility in Ann Arbor, MI, features a vivarium and advanced instrumentation like HPLC, LC-MS/MS, and spectrophotometry, enabling precise, high-quality data delivery. All studies follow TSRL SOPs, client-approved protocols, and rigorous quality control for accurate, reproducible results.
BIOANALYSIS: PRECISION DRUG QUANTIFICATION
Accurate quantification of drug exposure is critical for determining efficacy, safety, and dosing strategies. TSRL’s bioanalytical services include:
• HPLC and LC-MS/MS quantification of drugs and metabolites
• Method development and validation for bioanalytical assays
• Non-GLP preclinical and clinical sample analysis
Our advanced analytical capabilities are customizable to support everything from early-stage research to International Council for Harmonisation (ICH) M10 compliance, providing high-quality data for regulatory filings and clinical trial preparations.
FORMULATION DEVELOPMENT: OPTIMIZING DRUG DELIVERY
One of the biggest hurdles in drug development is ensuring effective drug delivery. TSRL’s formulation development services focus on enhancing bioavailability, stability, and manufacturability through:
• Solubility screening and optimization
• Formulation screening for oral, injectable, and transdermal delivery
• Preclinical formulation stability testing
We develop tailored drug formulations that improve performance and meet target profiles.
PK/ADME & TOXICOLOGY: UNDERSTANDING DRUG BEHAVIOR
• In vitro ADME (absorption, distribution, metabolism, and excretion) profiling
• Blood/plasma stability and protein binding assays
• Microsomal, hepatocyte, and intestinal metabolism studies
• In vivo PK studies in rodents
• Mechanistic absorption studies
• Exploratory toxicology
• Biodistribution and exposure assessments
Early-stage pharmacokinetic and toxicology insights support candidate selection, optimize dosing strategies, and reduce risk before clinical development.


As TSRL expands its CRO offerings, we continue to provide high-level strategic guidance to ensure drug candidates meet regulatory and clinical development milestones.
OUR CONSULTING EXPERTISE INCLUDES:
Regulatory Strategy Development – Ensuring preclinical studies align with FDA/EMA guidelines
Clinical Planning – Designing data-driven clinical trial strategies
Intellectual Property Strategy – Strengthening patents and licensing assets
SBIR Grant Strategy – Strengthening the small business funding pipeline
TSRL combines scientific rigor with strategic planning to optimize preclinical programs for regulatory approval and market success.
Alongside our services, TSRL is advancing next-generation drug delivery technologies to improve therapeutic efficacy and patient outcomes.
Our MAP formulations provide a self-administered drug delivery system that avoids the inconvenience of traditional injections. This hydrogel-based microarray:
• Provides a drug-releasing reservoir upon skin application
• Delivers therapeutics directly into systemic circulation
• Reduces the need for clinic visits and improves patient compliance
MAP technology represents a delivery alternative for suboptimal drug products, improving bioavailability, consistency of dosing, and ultimately patient compliance.

PRODRUG PLATFORM: ENHANCING ORAL DRUG ABSORPTION
TSRL’s patent-protected prodrug technology is designed to increase membrane permeability and improve oral bioavailability of challenging compounds. This platform:
• Optimizes drug absorption in the GI tract
• Reduces variability in drug exposure
• Provides a strategy for targeted delivery
• Minimizes side effects while enhancing therapeutic efficacy
This approach can potentially to improve the pharmacological profile of drug candidates, making them more clinically viable and commercially attractive.
TSRL is positioned as a full-service preclinical partner that goes beyond traditional contract research. Unlike many CROs that only offer testing and data generation, we provide end-to-end drug development support, helping clients:
• Secure non-dilutive funding for early-stage development
• Generate high-quality preclinical data to support IND applications
• Optimize drug formulations and delivery methods
• Develop regulatory strategies to streamline approval processes

Whether you are a biotech startup, pharmaceutical company, or academic research team, TSRL’s expanded CRO capabilities provide the scientific expertise, technical resources, and strategic insight needed to accelerate drug candidates from discovery to clinical trials. TSRLINC.COM


BY ABLE MEDICAL DEVICES IN COLLABORATION WITH NORTHERN MICHIGAN UNIVERSITY
The Upper Peninsula community of Marquette, idyllically located on the shore of Lake Superior, is home to three medical device manufacturers. Its relatively small population of about 21,000 limits the pool of prospective employees, so the companies have devised innovative approaches to training and retaining a qualified workforce to fuel their rapid growth. One example is their collaboration on a Work Scholars pilot program with Northern Michigan University’s Engineering Technology Department.
From their freshman year forward, NMU students receive paid, work-based learning experiences under the guidance of professional mentors. The goal is to prepare them for a successful career in a well-paying, high-demand sector that will allow them to remain in this desirable area of Michigan after graduation. The manufacturers benefit by providing curriculum insight that ensures students acquire the necessary skills and knowledge to meet evolving industry demands. They also maintain impactful connections with these potential employees throughout their education, which will hopefully aid in recruitment and retention.
Able Medical Devices is one of the companies that has focused on reviving a collaboration with NMU that was originally established by Pioneer Surgical Technology in the 1990s, based on the vision of founder Matthew Songer.
“Matthew had a vision of creating a high-skilled labor force that required people around here get up to speed on CNC equipment,” said Adam Paltzer, vice president of Business Development at Able, who worked at Pioneer and has more than 25 years’ experience in medical device manufacturing. “There was no other place to draw talent from, so we had to develop something ourselves. Our management worked closely with NMU’s CNC program and, based on that success, Able wanted to reignite that relationship and align with the university in a similar way. This partnership requires a two-way investment by both sides–not just financially, but in time, energy and passion–to have the impact it does on creating jobs, acquiring talent locally and keeping people in the area.”
NMU has a full CNC lab, a full manual machine shop lab, a print lab and 3D modeling. Able, a portfolio organization of Michigan-based asset manager Longyear, made a financial contribution so NMU could purchase two Swiss turn machines to add to its arsenal of training tools. Robert

Kinney, an Able employee with expertise in their operation, spends his afternoons as an adjunct instructor in NMU’s Engineering Technology Department, mentoring students on the machines’ use in manufacturing.
“Having an industry presence in the classroom is key to this whole system,” said NMU Engineering Technology Professor Cale Polkinghorne. “It’s a huge advantage for students to know that they’re learning from a professional in the field who has day-to-day technical expertise in CNC machining. It also helps me because it keeps me connected to the industry and confirms that we’re doing things correctly in terms of educating their future workforce. Having that direct link to industry adds legitimacy to the curriculum and the work our students are doing in the labs.”
“It used to be that students completed one semester and were hired; there was no incentive to stay and get a degree,” Kinney added. “At Able, we promote the degree and make sure education comes first. We’ll provide work-based experience around their school schedules and support them on their educational journey, but a job only becomes available to them after they fulfill their obligation to Northern. We also continue to promote education after they’re hired full time, allowing them to enroll in four credit hours per semester to advance to the next degree level.”
Dave Nyberg, NMU executive director of Business Engagement and Economic Development, said the university is fortunate to collaborate with the medical device manufacturing cluster in Marquette on the Work Scholars pilot program. It began in fall 2024 and offers a student experience that is more expansive and impactful than a typical internship.

“Research shows that if students have an opportunity to try out a work environment and build good relationships within it, they are more likely to stay with that employer and in the region,” Nyberg said.
“This aligns with Northern’s mission and core values, as well as the Growing Michigan Together Council’s emphasis on retaining talent and increasing the state’s population. The higher education work group to the council called out work-based learning and post-graduation incentives from employers as critical opportunities to help students stay in Michigan.”
Able Medical Devices is a global company offering an array of services and capabilities for the orthopedic and medical device industry, from design and development to comprehensive finished goods manufacturing.

47,815* Bioscience Jobs 3,319* Bioscience Establishments 9 th
$110,204* Average Bioscience Salary
MICHIGAN BIO-INDUSTRY QUICK FACTS * 5 th /9 th 6 th /10 th 16 th
BioPharma State by number of establishments / employment
Medical Device State by number of establishments / employment
Agri-Biosciences by number of establishments
Bioscience State by employment
DISTRIBUTION OF RESEARCH FUNDS
$ MILLIONS,
BIOSCIENCE-RELATED VENTURE CAPITAL INVESTMENTS
$ MILLIONS, 2019-2023
PHARMACEUTICAL & BIOTECHNOLOGY DIGITAL

Anchored by several large hubs, this representative sample of Michigan life sciences companies shows the breadth of the industry footprint.

OAKLAND/MACOMB/



Intero Biosystems, a spin-out from the University of Michigan, is revolutionizing preclinical testing with its cutting-edge stem cell-derived mini-organs. The traditional drug development pipeline heavily relies on testing drug candidates in simple cell or animal models before advancing to clinical trials. However, this approach has a staggering failure rate—90% of new drugs fail in human clinical trials. This not only wastes years of research and billions of dollars but also delays the availability of effective treatments for patients in need. The industry faces an urgent need for more accurate, human-relevant models that can reliably predict drug toxicity and efficacy before clinical trials begin.
To address this critical challenge, Intero Biosystems has developed GastroScreen, the first stem cell-derived Human Intestinal Organoid that replicates the complexity
and functionality of the human intestine. GastroScreen contains all major intestinal cell types, mimicking the organ’s natural structure and behavior. It features functional smooth muscle, perfusable blood vessels, and neurons that respond to stimuli—creating a true “mini gut” in a dish. This innovative platform can be used in preclinical assays, disease modeling, and functional tests for intestinal processes such as peristalsis, the rhythmic squeezing of the intestine.
By offering a predictive, human-relevant platform, GastroScreen has the potential to transform drug development—failing ineffective compounds early, reducing risks, and paving the way for safer, more effective treatments for patients worldwide.
Michigan continues to be a leader in advancing biosciences research and commercialization, thanks to its rich pipeline of intellectual property. A whole new crop of startups are germinating in the state, which is good news for the regional cluster of established companies that are focused on innovation in therapeutics, medical devices, healthcare technologies, clinical diagnostics and agri-/ industrial biotechnology.



Magsorbeo Biomedical is redefining orthopedic fixation with all-natural, absorbable metal implant technology and a passionate mission to eliminate the risks of lifelong complications caused by devices left in the body.
Over 2 million bone fixation surgeries occur in the U.S. annually, with over 80% of patients being forced to keep their implants, which cause pain and complications, risking infection and repeat hospital visits. Implant removal surgery is costly and includes a traumatic patient journey, yet these patients report significant improvements in pain and function.
Combining titanium-like strength with optimized absorption, Curasorb® implants are designed to heal fractures, restore natural function, and completely and safely disappear. With this fit-for-purpose approach offering superior outcomes, lower costs, and streamlined patient recovery, Curasorb is poised to become a new standard of care in trauma, cosmetic, and reconstructive ortho surgeries.
Curasorb® is the first absorbable metal platform with properly controlled and adjustable degradation without utilizing a coating, to ensure proper implant performance over a wide variety of applications. The proprietary alloy technology overcomes the challenges of first-generation absorbable alloys, which have uncontrolled degradation leading to bone loss around the implant, resulting in high clinical failure rates.
Magsorbeo product development is focused on ortho extremities and maxillofacial fixation, with 90% of surgeons showing interest in adopting Curasorb implants, following strong preclinical performance outcomes. As a fast follower in extremities fixation, Magsorbeo anticipates First in Human clinical studies in 2026, 510(k) clearance in 2027, followed by De Novo clearance of the maxillofacial fixation system.
MAGSORBEO.COM
BY DAVID ESPOSITO, PRESIDENT AND CHIEF EXECUTIVE OFFICER OF ONL THERAPEUTICS
ONL Therapeutics (ONL) is a clinical-stage biopharmaceutical company committed to developing first-in-class therapeutics to protect and improve the vision of patients with retinal disease. By advancing a breakthrough technology designed to protect key retinal cells from Fas-mediated cell death, ONL is pioneering a new approach to preserving vision.
Headquartered in Ann Arbor, MI, ONL is developing a platform of products for use in a wide range of blinding diseases, including geographic atrophy, glaucoma, and retinal detachment. As the first and only ophthalmology company targeting the Fas-mediated cell death pathway—a fundamental cause of retinal damage—ONL is addressing a critical unmet need impacting millions of patients worldwide.
The foundation of ONL’s breakthrough technology originated in the research lab of David Zacks, MD, PhD, a world-renowned retina specialist at the University of Michigan Kellogg Eye Center. Conversations with patients suffering from irreversible retinal diseases motivated Dr. Zacks to pursue new treatment options, especially when confronted with telling them, “There is nothing more I can do for you.”
Founded in 2011, ONL filed patents in major markets worldwide to protect its innovative neuroprotective platform that preserves retinal cells. Activation of the Fas receptor in the retina simultaneously triggers cell

death and harmful inflammation. ONL’s lead therapeutic candidate, ONL1204, employs a unique and differentiated mechanism of action that blocks the Fas receptor, inhibiting both cell death and inflammation and potentially improving patient vision.
ONL has conducted human clinical trials across multiple disease indications with data demonstrating signals of biological activity and target tissue engagement.
THE COMPANY’S COMPLETED TRIALS INCLUDE:
• Phase 1 and 2 trials in rhegmatogenous retinal detachment (macula-off)
• Phase 1b trial in geographic atrophy (GA) associated with age-related macular degeneration (AMD)
• Phase 1b trial in open-angle glaucoma
ONL is now preparing for its Galaxy Phase 2 trial in dry AMD/GA while advancing its glaucoma program.
Advancing biotech innovations from discovery to clinical application requires significant capital, enduring perseverance, and a bold risk-taking spirit. Michigan’s angel investment community exemplified this courage and vision by providing ONL with early seed funding when it was just beginning as a university spinout, helping advance its promising technology. Many of these Michigan angels continued investing in ONL through multiple financing rounds over the past decade.
“At ONL, we’re pioneering a new approach to vision-threatening eye diseases that affect millions of people with limited treatment option.”
David Zacks, MD, PhD | Co-Founder and Chief Scientific Officer
The level of scientific innovation in the Midwest rivals major global markets. Combined with a vibrant community to support, encourage, and scale innovation, the region has emerged as a burgeoning biotechnology center ready to lead the nation’s life science advancements while maintaining an exceptional quality of life.”

The company recently completed a $65 million Series D financing, attracting an elite roster of investors and strategic partners to support its Galaxy Phase 2 study in dry AMD/GA over the next several years.
ONL and fellow life science innovators across the Midwest present a compelling investment opportunity, providing potentially outsized investment returns and value creation distinguished from traditional biotech centers like Boston and San Francisco.
The biotech journey from discovery through clinical development and ultimately to commercialization demands strategic and visionary leadership—a principle central to ONL’s success. At the helm, seasoned biotech CEO David Esposito leverages leadership experience from several Michigan-based life science companies and his time as a combat veteran.
The senior leadership team combines world-class talent with Midwest roots whose credentials would open doors globally, yet they’ve deliberately chosen to build companies in their own backyard. Their commitment inspires rising talent seeking to build thriving careers and fulfilling lives in America’s heartland.
“This regional commitment extends to creating opportunities for diverse leadership. While industry discussions continue to highlight the importance of women in life sciences, ONL Therapeutics, in typical Midwest fashion, quietly demonstrates the principle in practice. The company’s diverse team—with women comprising 75% and serving in key leadership roles— delivers performance that validates this purposeful approach while proving that exceptional life science innovation and outstanding leaders can thrive beyond coastal hubs.


ONL is poised to fulfill its vision of helping patients see the future through first-in-class therapeutics to protect and improve the vision of millions with retinal diseases around the globe. The company also serves as a blueprint for Midwest biotech excellence—demonstrating how visionary leadership can drive medical breakthroughs and economic vitality while cultivating regional talent beyond traditional innovation centers.


BY JOHN HALL, PRESIDENT, ADVANCED MEDICAL DEVICE INNOVATIONS

Advanced Medical Device Innovations (AMDI) assists its clients with cutting edge medical device development, additive manufacturing (AM), and engineering solutions. AMDI is shaping the future of manufacturing technologies, mentoring students, and advancing the medical device and engineering sectors. John Hall, a 30+ year medical device innovator with an extensive portfolio of patents and product introductions leads the AMDI team.
Through collaborations with companies like EnvisionTEC, Markforged, and 3DXTech, AMDI remains at the forefront of AM technology, promoting research, innovation and training. AMDI’s lab is equipped with state-of-the-art industrial grade 3D printing technology, including the Envision One cDLM XL, Metal X, and Gearbox HT2. These machines support a wide range of materials, from photopolymers to metals like stainless steel and copper to high temperature and strength thermoplastics, enabling AMDI to produce high-quality prototypes for medical devices and other industries. Over 35 students and professionals have been trained in AM technology at AMDI.
In 2024, AMDI initiated West Michigan Additive Manufacturing Quarterly Meetings to bring together industry professionals and enthusiasts. These events, supported by Grand Valley State University (GVSU) and Automation Alley, provide insights into AM technology and networking opportunities. Past events hosted at GVSU, 3DXTech, and Accel Digital Solutions highlighted developments in AM education, hightemperature polymer melt extrusion and multi-jet fusion applications and flame-retardant material.
AMDI plays a vital role mentoring some of GVSU’s engineering co-op students. In 2024, AMDI supported former co-op student Cara Franke, whose research on “Influence of Process Parameters on the Microstructure and Material Properties of Material Extruded (MEX) Three-Dimensional Steel Parts” was presented at the GVSU Reach Higher Showcase. Co Nguyen, another past co-op student, presented her paper on “Impact of Lattice Structure on Compressive Performance and Strength to Weight Ratio in Continuous Stereolithography” at the North American Manufacturing Research Conference (NAMRC), demonstrating AMDI’s real-world training impact. Graduate intern Dasani Gorrelle engaged in research and development for lattice structures intended for fabrication with additive manufacturing.
AMDI collaborates closely with GVSU and industry partners. AMDI worked with Dr. Brad Burke’s 2Innovate company and Dr. Abishek Balsamy-Kamaraj to help prototype fall prevention and gait assist technology. GVSU student support has included 3D printing heat exchangers for a graduate project and 3D printing steel multi-tool components for a student team’s entry into American Makes Additive in Steel Competition, showcasing AMDI’s lab capabilities and involvement in student-driven research and projects.

Rigorous data driven product testing is conducted in AMDI’s lab with calibrated instruments and includes standardized (e.g. ASTM, ISO) and custom methods to verify feasibility, performance, and reliability. Method development frequently entails creating 3D models for test fixtures in SolidWorks® that can be fabricated same day with internal AM equipment.
AMDI plans to continue its growth in 2025 by expanding its client base and increasing its team. The company aims to hire new co-op students, a graduate assistant, and summer interns. AMDI also plans to enhance its collaboration with GVSU and evaluate adding new AM equipment to its toolbox.
AMDI’s dedication to fostering innovation, collaboration, and student mentorship positions the company as a leader in the additive manufacturing and medical device sectors. By continuing to push the boundaries of AM technology and partnering with educational institutions and industry leaders, AMDI is shaping the future of engineering solutions.

BY MARQUICIA PIERCE HENDERSON, PHD, MBA, FOUNDER AND PRINCIPAL CONSULTANT, RUBY LEAF MEDIA
For the past decade, Ruby Leaf Media has served as a storytelling engine for tech founders—especially those coming out of academia. Many began as scholars, postdocs, or graduate students driven by a desire to turn their lab work into real-world innovation. Ruby Leaf helps them translate their ideas into a business narrative that resonates with funders, stakeholders, and strategic partners.
From our roots in building community among trainees at Vanderbilt University to our current partnerships with innovation programs across the Midwest, we’ve stayed focused on the same mission: to help life science entrepreneurs articulate their value in a way that moves their technology—and their story—forward.

Out of this mission came the Strategic Partnership Playbook, a Michigan-centered tool designed to help earlystage life science founders identify and prioritize the right connections to grow their business.
WE BUILT THE PLAYBOOK AROUND THREE PILLARS:
• Formulate your story: Capture your tech’s potential in a clear, testable narrative.

• Validate with data: Pressure-test assumptions through customer discovery, funding alignment research, and ecosystem mapping.
• Communicate with purpose: Develop the right materials—one-pagers, pitch decks, intro emails, or even grant proposals—to approach strategic partners with confidence.
The framework equips life science companies with a comprehensive overview of potential funding and strategic partnerships opportunities. Through it, entrepreneurs surface two to three key “next-step” connections— regulatory consultants, research and co-development partners, or contract manufacturers—based on the gaps in their commercialization journey.
It’s a way to stop building in silos and start building in partnership—with clarity and momentum.


In 2025, Ruby Leaf is extending this same ethos of connection and clarity to early-career talent with our firstever Life Science Communication Fellowship.
This paid opportunity is designed for students and scholars who want hands-on experience supporting Michigan’s life science innovation ecosystem.
FELLOWS WILL WORK ON SCOPED PROJECTS ACROSS THREE FOCUS AREAS:
• Science Communication & Storytelling
• Ecosystem Market Research & Mapping
• Entrepreneurship & Innovation Support
The fellowship is grounded in the same structure as the Playbook. Fellows will learn how to formulate effective narratives, validate them through research and data collection, and communicate in formats that drive action— whether that’s a grant brief, a partner pitch, or a press release.
Whether we’re helping a founder tell their story for the first time or guiding a fellow through their first market research deep-dive, Ruby Leaf Media remains committed to one thing: helping people build out loud.
We believe that strategic storytelling is how ecosystems grow—through clear articulation, shared knowledge, and intentional connection.
If you’re a scholar looking to level up your science communication skills—or a founder ready to connect with your next strategic partner—reach out to us or keep an eye out for the upcoming fellowship announcement.











From groundbreaking discoveries to transformative solutions, together we can achieve extraordinary breakthroughs that redefine industries. Scan the QR codes to learn more about some of the pioneering research being done at MSU or visit our website below to learn how your organization can join forces with our award winning faculty innovators and tap into a wealth of expertise and resources to accelerate your R&D efforts.
www.msuinnovationcenter.com/empower
The IP & Legal Affairs Committee serves as a vital resource for thought leaders in intellectual property and legal services across Michigan’s biosciences industry. Committed to safeguarding innovation and navigating complex regulatory landscapes, the committee provides a platform to address key legal issues impacting life sciences organizations.
Whether it’s protecting groundbreaking ideas or shaping policies, the group advances the business of biotech through collaborative expertise.

ASHLEY SLOAT, PH.D.
President and Director of Patent Strategy, Aurora Patents
Ashley leads Aurora Patents in building strategic patent portfolios for earlystage life sciences, medical device, and software companies. Aiming for strong and enforceable patent protection, her team provides patent drafting, prior art analysis, and lifecycle guidance to align IP strategies with clients’ goals.


MELVIN J. MUSKOVITZ
Senior Counsel, Dykema
Melvin specializes in labor and employment law, advising employers on policies, agreements, and compliance. With decades of experience, he offers practical solutions for matters spanning pre-hire to post-termination, helping clients resolve disputes and avoid litigation.
THOMAS T. MOGA
Member, Dykema
Thomas, a seasoned patent attorney, brings over 30 years of expertise in domestic and international IP portfolio management and enforcement. Focused on biological sciences, he also oversees IP enforcement and anticounterfeiting efforts in Asia.
Seek Additional details: The IP & Legal Affairs Committee welcomes collaboration from the biosciences community to foster innovation through sound legal strategies.

Sr. Technology Manager, Michigan State University
Anupam leverages his life sciences expertise to guide IP protection, license negotiation, and startup creation for MSU’s technology transfer team. His portfolio includes diverse applications in health and AI/ML tools.


KRISTEN PURSLEY
President, Dobrusin IP Law
Kristen advises on global IP portfolios, specializing in patents, trademarks, and copyrights. Her clients span the automotive, medical device, and consumer product industries, where she excels in drafting, agreements, and dispute resolution.
MICHELLE LARKIN
Director of Medical Device
Licensing, University of Michigan
Michelle combines expertise in medical device development and university technology transfer to advance IP protection and licensing efforts, bridging academia with innovation.
HAVE A QUESTION FOR THE COMMITTEE? MICHBIO MEMBERS HAVE ACCESS TO OUR COMMITTEE AS AN EXCLUSIVE MEMBER BENEFIT. CONTACT US AT ASKIPLEGAL@MICHBIO.ORG .
BY STATE REP. JULIE ROGERS (D-KALAMAZOO) AND STATE REP. GREG VANWOERKOM (R-NORTON SHORES)
As legislators, one of our primary responsibilities is to ensure Michigan remains competitive in the rapidly evolving global economy. Central to achieving this goal is fostering an environment where research and development (R&D), innovation and commercialization, like in the life sciences, are not only encouraged but thrive.
In a significant move to bolster economic growth, job creation, and technological advancement, a tax credit capped at $100 million was enacted that aims to incentivize businesses to conduct R&D activities within the state, aligning Michigan with over 30 other states that offer similar programs. The bipartisan package of bills included HB 4368 and 5099-5102 was signed into law by Governor Whitmer and took full effect on April 1.
For large companies (i.e., > 250 employees), the credit allows claims of 3% of qualifying R&D expenses up to a base amount, and 10% of expenses exceeding the base amount, capped at $2 million per year.
Importantly, the new credit strategically recognized that small, early-stage and mid-sized businesses (i.e., those with < 250 employees) are the backbone of Michigan’s high-tech industries and was structured in a way to level the playing field. It did so by not only reserving $25 million of the total credit for small businesses but allows them to claim 15% of qualifying R&D expenses exceeding the base amount up to a cap of $250,000 per year.
In addition, an extra 5% credit is available for businesses outsourcing their R&D to Michigan-based research
universities, with a cap at $200,000 per year. Further, the credit is refundable, meaning that if the credit exceeds the business’ tax liability, the difference will be refunded, a particularly advantageous benefit to early-stage, pre-revenue companies.
By design, the Michigan R&D Tax Credit is one of the most generous and competitive nationally. And coupled with the introduction of the Michigan Innovation Fund, with $60 million allocated to support startups and early-stage businesses, provides additional resources for life science companies in their formative years.
In short, the R&D tax credit is a win for Michigan’s life science companies. By offering this credit, the state hopes to attract and retain top-tier life science companies, foster collaboration between businesses and academic institutions, and ultimately accelerate the commercialization of new products and technologies.


The goal is to make Michigan a more attractive location for both life science startups and established companies. Moreover, the credit is meant to have a ripple effect, encouraging the growth of suppliers, contractors, and services that support the life sciences industry.
Collectively, the R&D tax credit is meant to be a multiplier, fostering and growing an ecosystem of talent and industry that attracts even more investment. In turn, this helps strengthen and diversity the state’s economy, create more jobs and enhance Michigan’s reputation as a hub for bioscience innovation.
Make no mistake...the R&D tax credit is not just a financial incentive – it is a strategic investment in Michigan’s future. We’ve seen how other states with robust R&D incentives have transformed into bioscience powerhouses. Michigan can do the same, and in our own way: by harnessing our unmatched work ethic, deep research assets and manufacturing capabilities, along with an expanded investment in STEM and trades training.
To the researchers, entrepreneurs, investors and manufacturers out there: Michigan is open for bioscience innovation. Let’s the build a bold future for life sciences together.

BY KHANH COURTNEY, PH.D., TECHNICAL MANAGER, BIOLOGICS , ELEMENT MATERIALS TECHNOLOGY
The biologics sector continues to experience remarkable growth, significantly outpacing traditional smallmolecule therapeutics. As monoclonal antibodies mature in the market, next-generation modalities including antibody-drug conjugates, multispecific antibodies, gene editing and cell and gene therapies are transforming treatment paradigms across multiple disease areas. For manufacturers in this competitive landscape, effectively navigating technical complexities will directly impact development timelines and their ability to secure a first-tomarket advantage.
The structural complexity of biologics presents researchers with unique challenges throughout the development process. While small molecules have well-defined structures, biologics exhibit exponentially greater structural variability with complex folding patterns and post-translational modifications. Industry feedback suggests that analytical characterization remains a primary development bottleneck, with substantial portions of development timelines consumed by safety testing and analytical method development. Post-translational modifications, protein aggregation, degradation pathways, and host cell protein contamination require increasingly sophisticated orthogonal testing strategies as products advance toward clinical manufacturing.
For manufacturers of biological therapeutics, safety testing represents a critical path constraint. The structural complexity and microheterogeneity inherent to biological products directly impact immunogenicity profiles and clinical outcomes—frequently in ways that preclinical models fail to predict. This translational gap means
adverse events may only emerge during late-stage clinical trials or post-marketing surveillance, potentially derailing development programs after significant investments.
Current biomarker initiatives offer promising approaches to predictive safety assessment, but implementation remains challenging. The identification and validation of reliable biomarkers—particularly for novel modalities such as gene therapies—requires sophisticated analytical capabilities and specialized expertise. Manufacturers developing multispecific antibodies face additional challenges, as these molecules can exhibit unexpected binding characteristics and complex pharmacokinetic profiles requiring specialized assay development.
Regulatory requirements for biologics continue to evolve in response to emerging safety considerations and novel modalities. Minor manufacturing or formulation modifications can trigger extensive comparability studies, bioequivalence studies, and regional regulatory divergence necessitates customized submission strategies across major markets.
Novel therapeutic approaches such as gene-edited cell therapies and personalized interventions further challenge conventional regulatory paradigms. These products often involve patient-specific manufacturing processes that defy traditional consistency requirements, requiring innovative approaches to quality control and safety assessment that satisfy regulatory expectations without impeding innovation.


Recent analytical technology implementations are delivering meaningful productivity improvements across the biologics development, and even routine QC testing, landscape. Multi-attribute monitoring (MAM) using highresolution mass spectrometry has significantly reduced characterization and Critical Quality Attributes (CQA) testing timelines in post-approval change assessments, while simultaneously detecting critical modifications that conventional methods may miss entirely.
Next-generation sequencing (NGS) for cell line characterization has transformed viable contaminant testing, demonstrating superior sensitivity for adventitious agent detection compared to traditional methods, such as mycoplasma, bacterial, fungal, and adventitious viral contaminations. Leading biologics developers have successfully implemented integrated NGS characterization to reduce cell line development timelines while enhancing safety profiles.
Raw materials and ancillary reagents used in Biologics manufacturing are frequently custom made for a specific product, or non-compendial, because there is not yet standardization. Setting up methodologies to test such raw materials can be costly, and time consuming for manufacturers. The development and standardization of common raw materials, ancillary reagents, and their CQA testing methodologies will drive productivity across the industry and eliminate unnecessary redundancy.
Industry experience has identified key strategies that consistently compress biologics development timelines without compromising quality or compliance:
• Parallel workflow optimization: Integration of analytical and process development teams reduces method development timelines compared to traditional siloed approaches
• Strategic regulatory engagement: Pre-IND meetings focusing on control strategy rationales reduce postsubmission information requests and accelerate review timelines
• Risk-based analytical approach: Implementing tiered testing based on critical quality attribute and safety risk assessments improves submission success rates while optimizing resource allocation
• Enhanced technology transfer: Standardized knowledge management systems, reproducibility between labs, and phase-appropriate co-validations reduce site-to-site method transfer times
• Predictive stability modeling: Utilizing accelerated condition data to establish correlations with long-term stability outcomes reduces overall stability timelines
The biologics revolution continues to accelerate, with increasingly sophisticated molecules targeting previously undruggable pathways.
Several emerging trends will reshape biologics development and quality testing:
• Continuous manufacturing adoption will require real-time monitoring capabilities and advanced analytical methods
• AI-enabled predictive analytics will increasingly identify potential safety and efficacy concerns earlier in development
• Regulatory convergence initiatives like FDA/EMA parallel scientific advice will streamline global strategies
• Multi-specific biologics platforms will require increasingly sophisticated characterization methodologies and minimize unnecessary redundancy
• Advanced delivery technologies including lipid nanoparticles will introduce additional requirements
Organizations seeking to navigate these development challenges effectively may benefit from partnerships with specialized testing providers like Element Materials Technology, whose comprehensive biologics testing services provide on-demand access to critical testing capabilities— from sterility testing and virology to extractables/leachables studies and advanced biopharmaceutical analysis.
Such partnerships enable developers to confidently advance biologics with fast, reliable results, ensure regulatory compliance across global markets, and maintain the flexibility to scale testing resources in alignment with evolving program requirements.

Dr. Khanh Courtney specializes in solving complex analytical challenges for biopharmaceuticals through expert method development and validation. With a Ph.D. in biochemistry and professional background in CMC, she creates robust characterization studies and documentation for IND/BLA applications, bridging R&D innovation with CMC compliance to enable smoother regulatory pathways.






JUNE 16-19 IN BOSTON, MA
The BIO International Convention is the premier gathering for the biotechnology industry, bringing together over 20,000 global leaders reshaping the future of healthcare, research, and innovation. MichBio is proud to support Michigan’s thriving biotech community alongside the Michigan Economic Corporation and some of the state’s most forward-thinking companies.
DISCOVER GROUNDBREAKING ADVANCEMENTS FROM MICHIGAN-BASED COMPANIES, INCLUDING:
• AAPHARMASYN
• AKADEUM LIFE SCIENCES
• ANCHORBIO SOLUTIONS
• AMBIENT BIOSCIENCES
• CORIUM INTERNATIONAL
• ELEMENT MATERIALS TECHNOLOGY
• INTERO BIOSYSTEMS
• MMS
• PHARMOPTIMA
• TRANSPHARM PRECLINICAL SOLUTIONS
• TSRL
• UMICH INNOVATION PARTNERSHIPS
• WAYNE STATE TECH COMMERCIALIZATION
• …AND MANY MORE!
MichBio
3520 Green Court Suite 175, Ann Arbor, MI 48105
WE INVITE YOU TO BECOME AN ACTIVE MEMBER OF MICHIGAN’S THRIVING BIOSCIENCES COMMUNITY. JOIN MICHBIO. Proudly Serving Michigan’s Bio-Industry Sectors
