
28 minute read
CPD: Melanoma
Continuing Professional Development CPD CPD
60 Second Summary
Malignant melanoma is the fifth most common invasive cancer nationally, with rising incidence. The main risk factor for its development is ultraviolet radiation exposure, especially intense and intermittent exposures and sun burn in fair-skinned individuals. Most cases of melanoma affect middle-aged individuals, though a significant proportion affect young adults. Males over 50 tend to present with thicker tumours, often on the trunk, and have a worse prognosis than females. Most melanomas occur de novo, with approximately 30% arising within a pre-existing mole.
Early detection and prompt excision of melanoma remains the most important element of management, with rapidaccess pigmented lesions set up nationally for this purpose. Early stages of melanoma have excellent prognosis. Historically, the prognosis for advanced and metastatic melanoma was very poor, though dramatic improvements in the landscape of melanoma treatment have been made in the last decade with the advent of a variety of systemic therapies for advanced and metastatic disease. These include targeted therapies such as BRAF and MEK inhibitors for BRAF-mutated melanoma, and immunotherapy agents in the form of immune checkpoint inhibitors and programmed cell-death protein 1 (PD-1) inhibitors.
Primary prevention strategies and public health messaging emphasise the importance of adequately protecting skin from the sun and selfsurveillance for early detection of suspicious lesions.
AUTHOR:
Dr Emma Porter. Dermatology Registrar, University Hospital Limerick
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice? 2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area. 3. PLAN - If I have identified a knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs? 5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the 4 previous steps, log and record your findings. Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Malignant Melanoma of the Skin
Introduction
Malignant melanoma is the fifth most common invasive cancer in Ireland, with approximately 20 cases per 100,000 per year - one of the highest incidence rates globally - and rising incidence.1 Ultraviolet (UV) radiation exposure is the main risk factor for development of melanoma, particularly intense and intermittent exposures in fair-skinned individuals.2,3 Sun bed exposure is widely recognised to increased risk of melanoma, particularly when used in younger age groups.4 Further risk factors include multiple melanocytic naevi, dysplastic naevus syndrome, a family history of melanoma, and immunosuppression. Familial genetic mutations with high penetrance are rare, with CDKN2A most frequently reported in these cases. In genome-wide association studies, single nucleotide polymorphisms in multiple genes related to nevogenesis and pigmentation have been associated with melanoma, including melanocortin 1 receptor, the gene which underlies red hair and freckles.5
Median age at diagnosis was 64 years for melanoma between 2011 and 2015 in Ireland. Melanoma patients, particularly females, tend to have a younger age profile than those with non-melanoma skin cancers, with almost 31% of all female patients and 20% of males diagnosed before age 50. Paediatric melanoma is rare.6 Males over 50 tend to present with thicker tumours, often on the trunk, and have worse prognosis. Epidemiological studies suggest that women with melanoma have a better prognosis, regardless of whether pre or postmenopausal and when adjustments for tumour characteristics are taken into account.7,8
The majority of melanomas occur de novo, with 30% arising within a pre-existing naevus. There is emerging evidence suggesting differing characteristics of these melanomas, with naevusassociated melanoma associated with lower depth of invasion and similarities in body sites.9 Histopathological subtypes of melanoma include superficial spreading, the most common, representing a radial growth phase, and nodular (vertical growth phase). Other types include acral (hands and feet), spitzoid and desmoplastic (spindle cells). In-situ melanoma is considered pre-cancerous, non-invasive and is limited to the epidermis, without evidence of dermal invasion on histology. Lentigo maligna is a subset of melanoma in situ with histological evidence of photodamage, typically seen on sun-exposed areas such as the face in elderly individuals.
Diagnosis
When diagnosed and treated early, melanoma has an excellent prognosis. Stage 1A carries a 5-year survival rate of 99%. Diagnostic and therapeutic advances contribute to further improvement in survival in all stages, though advanced melanoma still carries a poor prognosis and one fifth of patients are at stage III or IV at the time of diagnosis.1 The National Cancer Control Programme (NCCP) has developed the National Melanoma GP Referral Guidelines (Figure 1) for assessment and referral of suspicious lesions via a standardised pathway to a dermatologist or plastic surgeon, the National Pigmented Lesion GP Referral form.10,11 There are 14 rapid access centres nationally to where referrals can be sent. Standardised referral forms support inclusion of relevant clinical data for pigmented lesions including the ‘ABCDE’ system of lesion abnormalities (see Figure 1) and environmental and genetic risk factors.

Melanoma is usually clinically suspected before histopathological confirmation. The use of dermoscopy by dermatologists is used as standard in analysis of pigmented lesions and can facilitate differentiation from benign lesions, and prompt excision where atypical features are present.12 In special cases and where available, confocal reflectance microscopy is a further diagnostic tool in improving clinical diagnosis. The COVID-19 pandemic introduced unprecedented challenges to healthcare systems worldwide, and limited access to non-emergency services resulted in lower levels of referral, and later diagnosis of melanoma and skin cancer in general. 13,14 Many centres report more advanced tumours at diagnosis, though national figures are not currently available.
Management Primary excision
Early detection of melanoma and prompt, complete excision influences prognosis and survival and is the first line of management. A 2mm surgical margin around the lesions and a cuff of subcutaneous fat is taken on primary excision. Some centres offer sameday excision where resources allow. After histopathological confirmation, cases are discussed at dedicated multidisciplinary meetings. The diagnosis of melanoma is explained in detail to patients in a clinic setting, and information provided, usually with support from a dedicated skin cancer clinical nurse specialist. Following primary excision, histopathological findings are used to determine staging – based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging system, shown in Figure 2.15 Breslow thickness, or the depth in millimetres of tumour invasion into the dermis, is the most important pathological feature, followed by the presence of ulceration. Tumours with a Breslow thickness of <0.8mm and without ulceration are pT1a, carrying an excellent prognosis (99% 5-year survival). In contrast, for example, a 50-year-old male with a melanoma with Breslow thickness >4mm with ulceration on an extremity, and no metastatic spread at diagnosis, has a 5-year survival rate of 70%.16
Wide local excision
Wide local excision is performed for all melanomas, with the aim to reduce the risk of local recurrence. Clinical margins as recommended by NICE Guidelines are 0.5cm for Stage 0 or in situ melanoma, 1cm for Stage I melanoma and 2cm for Stage II.17 In some cases, topical imiquimod can be considered for melanoma in situ, for example if complete surgical excision would cause unacceptable morbidity or disfigurement.
Sentinel lymph node biopsy
Sentinel lymph node biopsy (SLNB) involves sampling of the first node(s) in the draining nodal basin of the melanoma, e.g. the axillary nodes for a melanoma on the upper limb. This is a prognostic or staging procedure rather than therapeutic, is typically carried out by plastic surgeons at the time of wide local excision, and requires general anaesthetic. This is offered to patients with tumours ≥0.8mm Breslow thickness, estimated to carry a 5% risk of sentinel lymph node positivity. Preceding 2017, the standard of care was complete lymph node dissection for those with a positive SLNB, however the landmark studies MSLT-I and MSLT-II showed that there is no melanoma-specific survival benefit for those that have SLNB, nor for those with a positive SLNB who undergo immediate lymph node dissection versus ultrasound surveillance.18,19 However, identification of occult nodal metastasis with SLNB can allow consideration of systemic adjuvant therapy with an aim to reduce the risk of relapse and improve survival.
Radiological imaging
NICE Guidelines 2015 and European consensus guidelines from 2019 for the management of melanoma outline recommendations for baseline staging imaging for melanoma patients – including stage IIC melanoma and above.12 A summary of these recommendations is illustrated in Table 1 (on page 34). These are general guidelines, and an individualised plan is made for each patient following multidisciplinary review.
Figure 1. National Melanoma GP Referral Guidelines
Systemic therapy in advanced/ metastatic disease
Metastatic disease has historically been associated with poor survival.20 Ten years ago, median overall survival of those with advanced-stage melanoma was 6-9 months.21 With the advent of targeted therapy and immunotherapy for the treatment of metastatic melanoma in the last decade, outcomes are dramatically improving.22 In 2010, targeted treatment for BRAFmutated melanoma was groundbreaking.23 This has evolved to treatment with a combination of BRAF and MEK inhibition, to reduce resistance seen in BRAF inhibitor monotherapy.24 However, less than 25% of melanomas in Ireland are BRAF-mutated, lower than other countries where this figure is closer to 40%.25 Melanoma is an immunogenic tumour, and a BRAF mutation is not required for immunotherapy. Agents include immune checkpoint inhibitors including anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), such as ipilimumab, and programmed cell-death protein 1 (PD-1) inhibitors such as pembrolizumab and nivolumab.26 Toxicity with treatment, however, is a significant factor considered by oncologists when exploring options with patients with advanced melanoma, and not all patients will be suitable or have a preference for treatment. Several
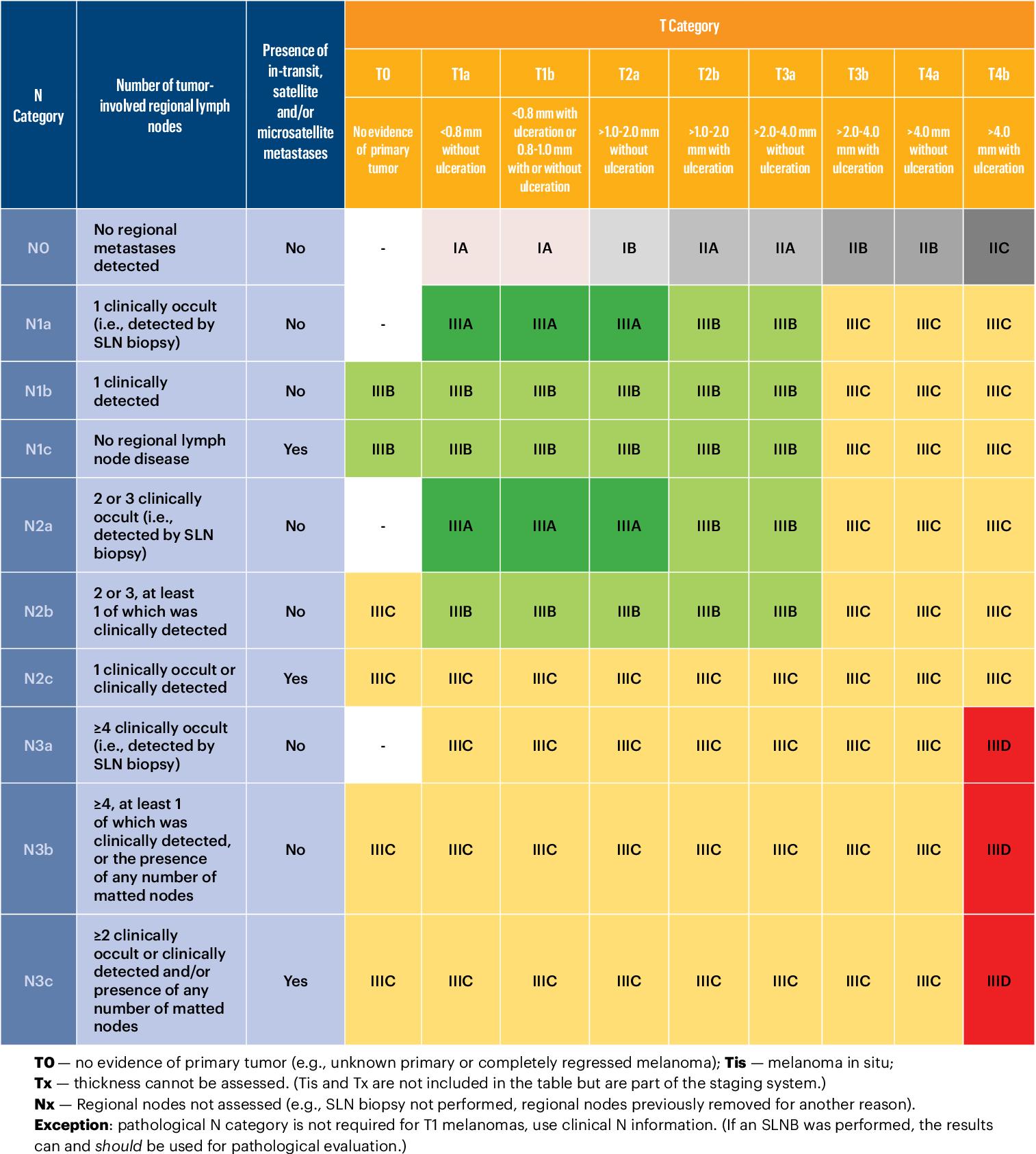
Figure 2: AJCC 8th Figure 2: AJCC 8th edition melanoma staging system. Source: Keung E, Balch C, Gershenwald J, et al. Key changes in the AJCC eighth edition melanoma staging system. Source: Keung E, Balch C, Gershenwald J, et al. Key edition melanoma staging system. Melanoma Lett.changes in the AJCC eighth edition melanoma staging system. Melanoma Lett. 2018;36(1):1–9. 2018;36(1):1–9
Wide local excision
Wide local excision is performed for all melanomas, with the aim to reduce the risk of local recurrence. Clinical margins as recommended by NICE Guidelines are 0.5cm for Stage 0 or in situ melanoma, 1cm for Stage I melanoma and 2cm for Stage II.17 In some cases, topical imiquimod can be considered for melanoma in situ, for example if complete surgical excision would cause unacceptable morbidity or disfigurement.
Guideline Stage
NICE Guidelines 2015
European consensus guidelines 2019 Stage IIC no SLNB and
Stage III
Stage IIC Staging at baseline Surveillance Schedule of surveillance
CT TAP +/- Brain
CT or MRI brain if age <24 years
US
CT TAP / PET-CT
MRI brain CT TAP
+/- Brain
LN US Every 6 months for the first 3 years
Every 3 – 6 months for the first 1-3 years
CT TAP / PET-CT
MRI Brain Every 6 months for the first 1-3 years
Stage III & higher US
CT TAP / PET-CT
CT TAP / PET-CT
MRI Brain 3 – 6 months for the first 1-3 years
of the immunotherapy agents are approved for reimbursement by the HSE, including pembrolizumab, ipilimumab, nivolumab, along with multiple agents for BRAF mutation-positive unresectable or metastatic melanoma.27
Adjuvant treatment of stage III melanoma
Adjuvant therapy in melanoma aims to reduce the risk of relapse and improve overall survival in patients with moderate to high risk resected melanoma. Funding was made available for nivolumab in February 2021 as monotherapy for the adjuvant treatment of adults with stage III melanoma and lymph node involvement who have undergone complete resection, and pembrolizumab in May 2021. It is anticipated that licensing and funding for further agents will continue to expand in the near future.
Clinical follow up
Patients with melanoma are followed up in a dermatology clinic with full skin examination and assessment of draining lymph node basins on a regular basis, frequency and duration depending on clinical stage. The first five years are most important, as this is the period in which 90% of metastases occur.12 Early stage melanoma, such as melanoma in situ and Stage IA melanoma, may only require a small number of clinic reviews over the space of the first year before discharge, as risk of recurrence is much lower.13 Regular clinical review facilitates identification of recurrent disease, and monitors for development of a second primary melanoma (which can occur in up to 10% of patients) or a non-melanoma skin cancer. It also provides the opportunity for further patient education regarding photoprotection and selfsurveillance, and psychological support. Patients with later stage melanoma may require surveillance imaging (see Table 1).
Vitamin D
Following a diagnosis of melanoma, the change in behaviour in reducing sun exposure is likely to result in lower levels of vitamin D synthesis over time, with suboptimal levels common in the general population at a baseline.28 There is some evidence that vitamin D plays a role in melanoma survival; higher serum vitamin D is associated with thinner primary melanoma and better outcome, although a causal mechanism has not been established.29 Serum 25-hydroxyvitamin D3 levels are typically measured for melanoma patients to allow advice on supplementation, with an ideal range considered to be 60-85 nmol/L.
Primary Prevention
Public health campaigns for prevention of melanoma, and skin cancer in general, focus on safe sun practices of avoiding sun burn, seeking shade, covering up, using sunscreens and avoiding the use of sun beds. The SunSmart campaign, with the ‘5 S’s’ of Slip, Slap, Slop, Slide and Seek shade, has been most widely adopted, introduced first in Australia over 30 years ago where it has been shown to have a positive impact on sun-related behaviours.30 This is the primary messaging used in Ireland, promoted by Healthy Ireland, and emphasised each year typically beginning in May with media campaigns. These sun protection practices are recommended especially between 11am and 3pm from April to September even on a cloudy day in Ireland, and when the UV Index is 3 or greater. Children and outdoor workers should have
management guidelines. Abbreviations: CT TAP – Computed tomography of thorax, abdomen and pelvis. MRI – Magnetic resonance imaging. PET-CT – Positron emitted tomography-computed tomography. US – Ultrasound. Systemic therapy in advanced/metastatic disease particular care taken in these measures. Furthermore, healthcare professionals can play an important role in further promoting these primary prevention practices for all patients we encounter.
References available on request
21 With the advent of targeted therapy and immunotherapy for the treatment of metastatic melanoma in the last decade, outcomes are dramatically improving.22 In 2010, targeted treatment for BRAF-mutated melanoma was groundbreaking. 23 This has evolved to treatment with a combination of BRAF and MEK inhibition, to reduce resistance seen in BRAF inhibitor monotherapy. 24 However, less than 25% of melanomas in Ireland are BRAF-mutated, lower than other countries where this figure is closer to 40%.25 Melanoma is an immunogenic tumour, and a BRAF mutation is not required for immunotherapy. Agents include
Table 1. Summary of recommendations for baseline and surveillance imaging from most recent melanoma management guidelines. Abbreviations: CT TAP – Computed tomography of thorax, abdomen and pelvis. MRI – Magnetic resonance imaging. PET-CT – Positron emitted tomography-computed tomography. US – Ultrasound
CPD Questions
1. Which of the following is the strongest exogenous risk factor for melanoma development?
a. Smoking
b. Chronic low intensity UV exposure
c. Intense intermittent UV exposure
d. Multiple melanocytic naevi
2. True or False: Survival rates for melanoma are better in males than females.
3. For melanoma with a positive sentinel lymph node biopsy, which of the following is the first management step?
a. Complete lymph node dissection
b. Ultrasound surveillance
c. Commencement of adjuvant immunotherapy
d. Discussion at multidisciplinary meeting
4. True or false: Systemic treatment options for a patient with metastatic non-
BRAF-mutated melanoma include immunotherapy such as pembrolizumab and nivolumab, and targeted therapies such as BRAF and
MEK inhibitors
5. What is the lowest stage of melanoma for which baseline imaging is recommended by NICE
Guidelines 2015?
a. Stage IB
b. Stage IIA
c. Stage IIC
d. Stage IIIA

Tip of the iceberg: the hidden health consequences of polycystic ovary syndrome
Written by Lauren Madden Doyle1,2, Michael W. O’Reilly1,2 1. Department of Endocrinology, Beaumont Hospital 2. Endocrinology Research Group, Department of Medicine, Royal College of Surgeons in Ireland (RCSI)
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting women of reproductive age, with an estimated prevalence of 6-13% depending on diagnostic criteria applied.1,2 The Rotterdam diagnostic criteria were introduced in 2003 and largely replaced the 1990 National Institutes of Health (NIH) criteria. As per Rotterdam, PCOS can be diagnosed on identification of two of the following three criteria: (i) oligo- or amenorrhoea, (ii) clinical and/or biochemical evidence of androgen excess or (iii) sonographic evidence of polycystic ovaries. This was an expansion on the previous NIH criteria, which did not include ultrasound as a diagnostic factor for PCOS.
Typically, patients report a constellation of symptoms which include features of androgen excess such as hirsutism, acne or alopecia, as well as irregular or absent periods, subfertility and in many cases difficulty losing weight. Traditionally, the health impact of PCOS has focused on reproductive dysfunction, with much emphasis on anovulatory infertility and oligomenorrhoea. In recent years, focus has shifted to the overarching associated Lauren Madden Doyle Michael W. O’Reilly




metabolic derangements and other long term health complications.2,6 This transition in our understanding of PCOS as a chronic metabolic condition with lifelong health implications is important, and increasing awareness amongst healthcare professionals and the general public remains a challenge. Up to 50% of women with underlying PCOS remain undiagnosed, and the first international clinical practice consensus guideline was only published in 2018.27
The role of androgen excess and insulin resistance
Before considering the excess metabolic morbidity and health complications of PCOS, a brief discussion of the underlying pathogenesis is warranted. Androgen excess is a cardinal pathological feature of PCOS, with both ovarian and adrenal phenotypes of androgen excess reported. The pathogenesis of androgen excess in PCOS reflects a complex interplay between insulin resistance and androgen metabolism, with hyperinsulinaemia driving androgen generation at the level of the ovary, adrenal and even adipose tissue.22 Weak adrenal androgenic precursors including androstenedione (A4) and dehydroepiandrosterone (DHEA) may be activated in peripheral tissues into more potent androgens such as testosterone (T) and dihydrotestoterone (DHT), while T may also be produced and secreted directly by ovarian luteal cells.
Clinically, patients will present with classic symptoms of androgen excess, namely hirsutism, acne & frontotemporal alopecia. Generally these develop over a more protracted timeframe, and features of overt virilisation such as clitoromegaly or deepening of the voice, as observed in virilising ovarian or adrenal neoplasms, are notably absent. Cosmetic implications of androgen excess have traditionally been the focus of management in PCOS, however recent studies have linked biochemical hyperandrogenism with a direct impact on cardiovascular & metabolic health. Elevated serum T alongside low sex hormone binding globulin (SHBG) levels are closely linked with metabolic perturbations in PCOS9,10,. These include insulin resistance, impaired glucose tolerance (IGT), type 2 diabetes mellitus (T2DM), dyslipidaemia and non-alcoholic fatty liver disease (NAFLD). Rather than purely associative, we see emerging evidence that androgen excess is directly complicit in mediating some of the metabolic health complications in women with PCOS.
Insulin resistance, a strong risk factor for development of overt hyperglycaemia, has been linked with PCOS since early models of pathogenesis. Whilst not included in diagnostic criteria, up to 75% of women with PCOS demonstrate features of insulin resistance.3,4 This observation is independent of BMI, and in vivo data suggest an intrinsic defect of insulin signalling in skeletal muscle in PCOS. The direction of causality linking androgen excess and insulin resistance in PCOS is unclear, and the associations between these metabolic perturbations remains a key focus of research in this condition.
The concept of PCOS as a disorder of insulin resistance is not a new one, with metformin employed as a therapeutic agent for menstrual cycle regulation and to enhance rates of spontaneous pregnancy in those with PCOS for decades. Initially insulin resistance results in reduced peripheral glucose uptake at key sites such as muscle, with compensatory hyperinsulinaemia maintaining euglycaemia for many years. Ultimately, however, beta cell failure can lead to overt hyperglycaemia, a process that appears to be expedited by the presence of obesity and androgen excess.2,4,5
PCOS & metabolic disease: type 2 diabetes and beyond
Two large meta-analyses have demonstrated the association between PCOS and both IGT and T2DM7.8 These showed a three-fold increase in risk of IGT in women with a diagnosis of PCOS, with the highest risk demonstrated in patients of Asian ethnicity. Similarly, the risk of T2DM was also shown to be four-fold increased compared to the general population. The implications of insulin resistance in PCOS extend far beyond an impact on glycaemic control. Similar to IGT and T2DM, a large UK-based population study derived from primary care records has demonstrated an increased risk of NAFLD in women with PCOS. This study of 63,000 women compared with 121,000 controls found that a diagnosis of PCOS conferred a 2.0-2.4 fold increase in the risk of NAFLD. Significantly, this also linked circulating T levels with an increased risk of NAFLD, which highlights a potential independent role for hyperandrogenism as a potential pathogenic factor in the aetiology of PCOS-related fatty liver disease.2,9,10
Traditionally, women during their reproductive years have been perceived as having low overall cardiovascular risk, particularly in comparison to their male counterparts. A diagnosis of PCOS however, appears to change this risk profile. Overall, while the absolute risk of major adverse cardiovascular events (MACEs) remains low, data has shown there is an increased risk of cardiovascular morbidity in the PCOS cohort. A recent retrospective cohort study of 176,000 women with PCOS in the UK CPRD dataset demonstrated a 26% increase in nonfatal myocardial infarction (MI), revascularisation & angina, when compared to age & weight adjusted controls.1, 11,12 Further analysis highlighted risk of progression was highest in those with increased BMI, T2DM & from regions of socioeconomic deprivation. While morbidity is increased, overall cardiovascular mortality and risk of cerebrovascular events is unchanged, likely due to the young age profile. Mechanistically, there are a number of potential drivers of this increased risk of cardiovascular events. At a biochemical level, insulin resistance appears to cause vasoconstriction due to reduced nitric oxide production, and leads to increased lipid trafficking and lipolysis, with patients at a subsequent increased risk of hypertension, dyslipidaemia and likely atherosclerosis. Patients with PCOS demonstrate significant derangements in lipid profiles, with reduced HDL cholesterol levels & increased triglycerides, while LDL-C levels remain unchanged.12
Anovulatory infertility and reproductive morbidity in PCOS
It is long established that women with PCOS experience anovulatory cycles which manifest as oligo- or amenorrhoea. The pathological basis of anovulation appears also to be multifactorial; increased follicle recruitment may be driven by an interplay between luteninising hormone (LH) secretion and insulin, while androgen excess may lead to subsequent arrest of follicular development and consequent failure of ovulation. Strategies to help with ovulation in PCOS primarily include weight loss through a combination of dietary and lifestyle modifications, and metformin. Both are employed in an effort to reduce peripheral insulin resistance and address hyperinsulinaemia, with a secondary knock-on effect on ovulation. Several studies have demonstrated the efficacy of metformin in restoring ovulation through its mechanism as an insulin sensitising agent.23,24 Additional ovulation induction agents utilised to overcome anovulatory infertility include clomiphene and letrozole. Clomiphene is a selective oestrogen receptor modulator (SERM), which enhances follicular development via hypothalamic stimulation and FSH/LH production. Letrozole, an aromatase inhibitor, also enhances endogenous FSH levels and is used to induce ovulation in those desiring fertility. Reproductive outcomes may be better for letrozole compared to clomiphene,25 and the former is recommended as first line therapy for ovulation induction in the 2018 clinical practice guideline; however larger scale randomised controlled trials are required to confirm this.27 Antenatally, patients with PCOS have an increased risk of pregnancy related complications, largely due to its associations with metabolic risk factors. One retrospective study in Japan of 1,000,000 patients on a national database highlighted an increased risk of first trimester miscarriage in the context of a prior diagnosis of PCOS. This appeared to be reduced when metformin was used as an agent for ovulation induction.17 Another metaanalysis also identified PCOS as an independent risk factor for multiple antenatal and neonatal complications including gestational diabetes mellitus (GDM), pregnancy induced hypertension (PIH) & pre-eclampsia (PET), preterm delivery, caesarean delivery, neonatal hypoglycaemia and neonatal death.18
Mental Health & Quality of Life Indices in PCOS
Multiple studies have demonstrated an increased risk of depression and anxiety in patients with a diagnosis of PCOS.2,16,19,20 This is likely multifactorial, with cosmetic manifestations such as hirsutism, increased BMI & poor body image playing a role, in addition to concerns regarding subfertility. However, even when controlling for these factors, PCOS has emerged as an independent risk factor for depression & anxiety. The role of hyperandrogenism & insulin resistance on cortical pathways may be a potential driver of this phenomenon.19,20 It is not surprising given its multiple


implications, that PCOS is also associated with reduced health related quality of life scores.
Emerging health issues in PCOS
The long-term health impacts of PCOS extend beyond the increased cardiometabolic risk profile. Women with PCOS have a significantly increased risk of endometrial cancer compared to the background female population. This is due to the unopposed proliferative effect of oestrogen on the endometrial lining caused by anovulatory cycles, with ectopic oestrogen production by increased adipose tissue also contributing in those with increased BMI.2,6,14 Theoretically, this increased oestrogen exposure may also predispose to breast cancer, however meta-analyses have thus far failed to demonstrate a concrete link between PCOS & breast cancer.14
PCOS also increases risk of obstructive sleep apnoea (OSA), independent of BMI. A recent observational cohort study of over 76,000 women with PCOS in the U.K. showed an increased risk of OSA across all ranges of BMI, with the highest risk observed in those with elevated BMI and documented anovulation or hirsutism. Insulin resistance, androgen excess and low luteal phase progesterone levels have all been postulated as potential mechanistic factors in the pathogenesis of OSA in PCOS.15 There is increasing evidence of neurological sequelae of androgen exposure in PCOS. Idiopathic intracranial hypertension (IIH) shares similar demographic features to PCOS, largely affecting women of reproductive age with co-existent obesity. A recent study highlighted the presence of a convincing androgen excess phenotype in women with IIH, potentially implicating hyperandrogenism in its pathogenesis.26 Whether this represents a severe variant of PCOS or a distinct clinical androgen excess phenotype remains unclear at this point. However its importance is clear not only a potential therapeutic target for IIH, but also in the identification of androgen excess as a driver of other co-morbidities in the PCOS population.
Conclusion
PCOS is a complex heterogeneous condition, with a spectrum of severity and multiple different phenotypes, and our understanding of the condition is continuing to expand. Our current perspective has evolved dramatically since the turn of the century, and it is now clearly defined as a complex chronic condition with far-reaching metabolic, reproductive and other multi-system complications. Current research in PCOS is targeted at identifying those at highest risk of metabolic manifestations, and further elucidating the relationship between insulin resistance & hyperandrogenism. The diversity of clinical phenotypes of women with PCOS remains a diagnostic & therapeutic challenge. It has become increasingly clear it is a condition with expansive long term health consequences largely attributed to its metabolic risk. As a condition which affects an estimated 5% of the population, given its metabolic burden, it undoubtedly has far-reaching public health consequences. PCOS has often been dismissed as a purely reproductive health problem, but the bulk of emerging evidence reframes it as a chronic systemic metabolic disorder. This shift in understanding is crucial. Future horizons for PCOS are centred on establishing which patient cohort shoulders the burden of this metabolic risk, and identifying a targeted treatment for its pathogenesis.
References
1. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25 2. Allen LA, Shrikrishnapalasuriyar N, Rees DA. Long-term health outcomes in young women with polycystic ovary syndrome: A narrative review. Clin Endocrinol (Oxf). 2021 Oct 7. 3. Rosenfield RL, Ehrmann DA.The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016 Oct;37(5):467520. 4. Moghetti, P., Tosi, F. Insulin resistance and PCOS: chicken or egg?. J Endocrinol Invest 44, 233–244 (2021) 5. Andrea Dunaif, Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis, Endocrine Reviews, Volume 18, Issue 6, 1 December 1997, Pages 774–800, 6. Cooney, L., Dokras, A. Beyond fertility: polycystic ovary syndrome and long-term health, Fertility and Sterility Vol. 110, No. 5, October 2018 0015-0282 7. Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update 2018;4:455–67. 8. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and metaanalysis. Hum Reprod Update 2010;16:347–63. 9. Kumarendran, B., O'Reilly, M. W., Manolopoulos, K. N., Toulis, K. A., Gokhale, K. M., Sitch, A. J., Wijeyaratne, C. N., Coomarasamy, A., Arlt, W., & Nirantharakumar, K. (2018). Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS medicine, 15(3), e1002542. https://doi.org/10.1371/journal.pmed.1002542 10. Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. doi:10.1016/j. molmet.2020.01.001 11. Berni TR, Morgan CL, Rees DA. Women with polycystic ovary syndrome have an increased risk of major cardiovascular events: a population study. J Clin Endocrinol Metab. 2021;106:3369 12. Wekker V, van Dammen L, Koning A, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26(6):942-960. doi:10.1093/humupd/ dmaa029 13. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012 Nov-Dec;18(6):618-37. doi: 10.1093/humupd/dms030. Epub 2012 Jul 4. PMID: 22767467 14. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014 Sep-Oct;20(5):748-58. 15. Kumarendran B, Sumilo D, O'Reilly MW, et al. Increased risk of obstructive sleep apnoea in women with polycystic ovary syndrome: a population-based cohort study. Eur J Endocrinol. 2019;180(4):265-272 16. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman's longterm health using data linkage. J Clin Endocrinol Metab. 2015 Mar;100(3):911-9. doi: 10.1210/jc.2014-3886. Epub 2014 Dec 22. Erratum in: J Clin Endocrinol Metab. 2015 Jun;100(6):2502 17. Pan ML, Chen LR, Chen KH. The Risk of Subsequent Miscarriage in Pregnant Women with Prior Polycystic Ovarian Syndrome: A Nationwide Population-Based Study. Int J Environ Res Public Health. 2021 Aug 4;18(16):8253. 18. Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and metaanalysis. Medicine (Baltimore). 2016 De ;95(51):e4863 19. Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017 May 1;32(5):1075-1091 20. Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, Epperson N, Teede H. Androgen Excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018 May;109(5):888-899 21. Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020 Aug 1;105(8):e2695–709. 22. O'Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017 Sep 1;102(9):3327-3339 23. Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebocontrolled trial. J Clin Endocrinol Metab. 2002 Feb;87(2):569-74 24. Sharpe A, Morley LC, Tang T, Norman RJ, Balen AH. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2019, Issue 12 25. Franik S, Eltrop SM, Kremer JAM, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2018, Issue 5 26. O'Reilly MW, Westgate CS, Hornby C, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight. 2019;4(6):e125348. Published 2019 Mar 21. 27. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network.Fertil Steril. 2018.










