New and Emerging Therapies for Multiple Myeloma: Case Studies for Nurses



Slides available for download at: https://www.imf ons.myeloma.org

Please help us start on time.

Please do not save seats. Please silence cell phones.
Thank you for coming!
This activity is supported by independent educational grants from AbbVie, Inc.; Bristol Myers Squibb Company; Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC; Karyopharm Therapeutics; Pfizer Inc.; Sanofi; and Takeda Pharmaceuticals U.S.A., Inc.













Meeting space has been assigned to provide a symposia supported by the International Myeloma Foundation (IMF) during the Oncology Nursing Society’s (ONS) 48th Annual Congress, April 26 –April 30, 2023 in San Antonio, TX. The Oncology Nursing Society’s assignment of meeting space does not imply product endorsement










ONS Disclaimer
Accreditation/Certification




Postgraduate Institute for Medicine (PIM) is accredited as a provider of nursing continuing professional development education by the American Nurses Credentialing Center’s Commission on Accreditation.


A maximum of 1.5 contact hours may be earned for successful completion of this activity.

Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires faculty, planners, and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest (COI) are thoroughly vetted and mitigated according to PIM policy.

The existence or absence of COI for everyone in a position to control educational content will be disclosed to participants prior to the start of each activity.






Please access full disclosures here: https://imf ons.myeloma.org/disclosures/



Patient names, demographics, and identifying characteristics have been masked to be HIPAA compliant.




Off label use of drugs may be discussed.








Slides available for download at: https://www.imf ons.myeloma.org


HIPAA = Health Insurance Portability and Accountability Act.
Faculty Introductions
Chair

Beth Faiman, PhD, RN, MSN, APRN-BC, AOCN, BMTCN, FAAN



Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH
Faculty
Amy Pierre, RN, MSN, ANP-BC

Memorial Sloan Kettering Cancer Center, Montvale, NJ
Tiffany Richards, PhD, ANP-BC

MD Anderson Cancer Center, Houston, TX
Charise Gleason, MSN, NP-BC, AOCNP
Winship Cancer Institute of Emory University, Atlanta, Georgia
6
Learning Objectives
As a result of this program, you will be able to:




Discuss new and emerging treatment regimens for patients with newly diagnosed or relapsed multiple myeloma, including appropriate patient management and patient education
Identify healthcare disparities faced by patients with multiple myeloma who are part of underrepresented groups and strategies to overcome these
Describe strategies to support the patient’s input in therapeutic decisions through shared decision-making

7
Q1. Which of the following is TRUE about multiple myeloma in Black vs White patients?
1. Black patients tend to be diagnosed with myeloma at an older age
2. Black patients tend to have higher-risk disease
3. Black and White patients have a similar incidence of myeloma
4. Black patients may have superior outcomes when treated with standard of care

5. I don’t know

8
Q2. Which of the following is a recommended approach to supporting shared decision making for patients with multiple myeloma?
1. Encourage the patient to make a decision before leaving the office where they have the opportunity to get their concerns addressed by the medical team
2. Provide resources and/or tools to help the patient assess the type of treatment they want to receive

3. Emphasize the treatment option with the highest overall response rate
4. Explain the value of saving the most effective treatment for relapse
5. I don’t know




9
Q3. Which of the following is TRUE when caring for myeloma patients?
1. Bone strengtheners should only be given to treat patients with myeloma who experience hypercalcemia of malignancy
2. For patients with myeloma and chronic kidney disease, it is safer to take ibuprofen than Tylenol
3. Prophylactic anticoagulation is recommended for patients receiving myeloma regimens that contain IMiDs (immunomodulatory drugs) or carfilzomib

4. Nurses should obtain a blood type and cross match before initiating treatment with either a proteasome inhibitor or SINE inhibitor.
5. I don’t know

10
Q4. Your patient is admitted to the hospital for their first dose of a bispecific antibody therapy. Which of these acute side effects should you be more worried about?
1. Hypertension

2. Dehydration
3. Tumor lysis syndrome (TLS)
4. Cytokine release syndrome (CRS)
5. I don’t know
11


























Diagnosed Multiple Myeloma,
Newly
Including Treatment Disparities
compliant; not actual patient name.
CASE 1: Margaret* *HIPAA
Amy Pierre, RN, MSN, ANP BC
HIPAA = Health Insurance Portability and Accountability Act. International Myeloma Foundation 800 452 CURE (2873) http://myeloma.org
Beth Faiman, PhD, RN, MSN, APRN BC, AOCN, BMTCN, FAAN
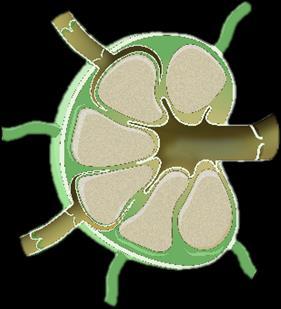
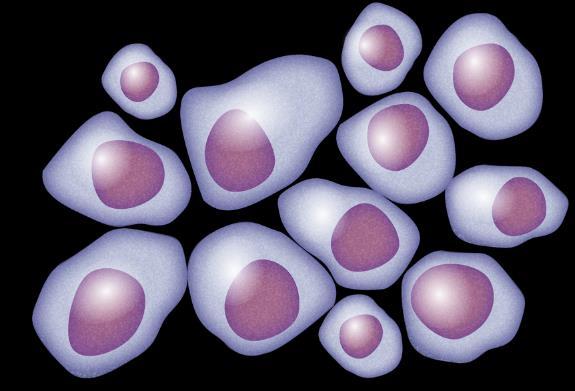
Myeloma Is a Cancer of Plasma Cells
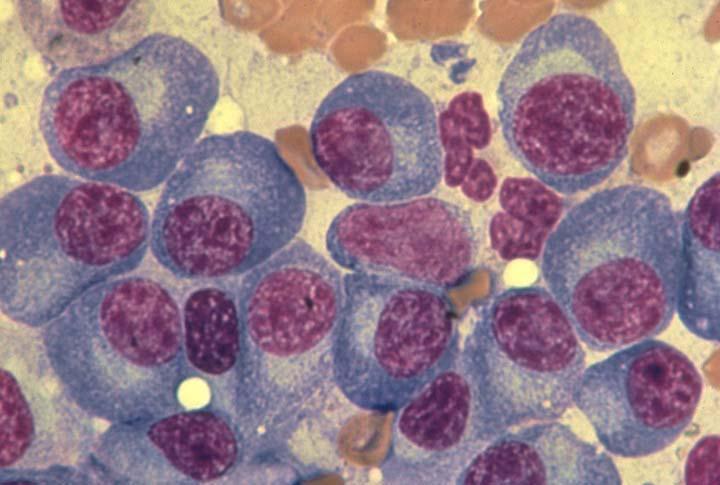

Bone Marrow of a Patient With MM

13
MM = multiple myeloma. Kyle RA, et al. Mayo Clin Proc. 2003;78:21-33.
Image courtesy of: American Society of Hematology
Plasma Cells Are Differentiated B Cells That Produce Antibodies

















B-cell Malignancies

Pre-B acute lymphoblastic leukemia (ALL)

Chronic lymphocytic leukemia (CLL) with unmutated IGHV
Burkitt lymphoma (BL)
Follicular lymphoma (FL)

Diffuse large B-cell lymphoma (DLBCL)
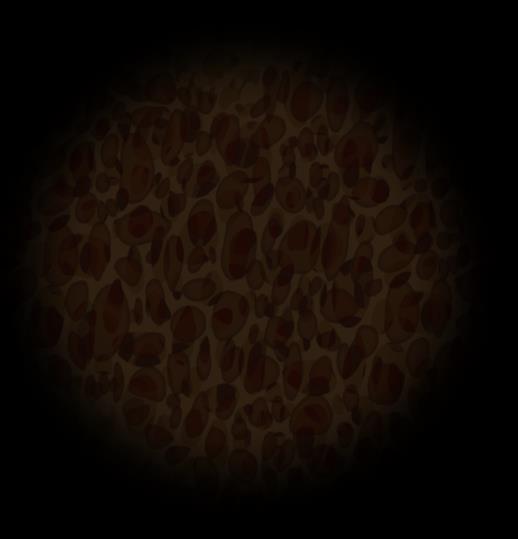
Activated B-cell diffuse large B-cell lymphoma (ABC-DLBCL)

Mantle cell lymphoma (MCL)






Marginal zone lymphoma (MZL)

Chronic lymphocytic leukemia (CLL with mutated IGHV)
Multiple myeloma (MM)
Waldenström macroglobulinemia (WM)

































FDC = follicular dendritic cell; GC = germinal center; IGHV = immunoglobulin heavy chain variable. Pal Singh S, et al. Mol Cancer. 2018;17(1):57. Pasqualucci L. Immunol Rev. 2019;288(1):240-261. Hematopoietic stem cell (in bone marrow) Immature B cell (in bone marrow) Transitional B cell Mature B cell T cell Plasmablast Plasma cell Memory B cell 6 11 1 3 2 9 Lymph node 5
B-cell malignancies have characteristics similar to the stages of B-cell development.
10 8 4 1 2 3 4 5 6 7 8 9 10 11 Bone 7
14
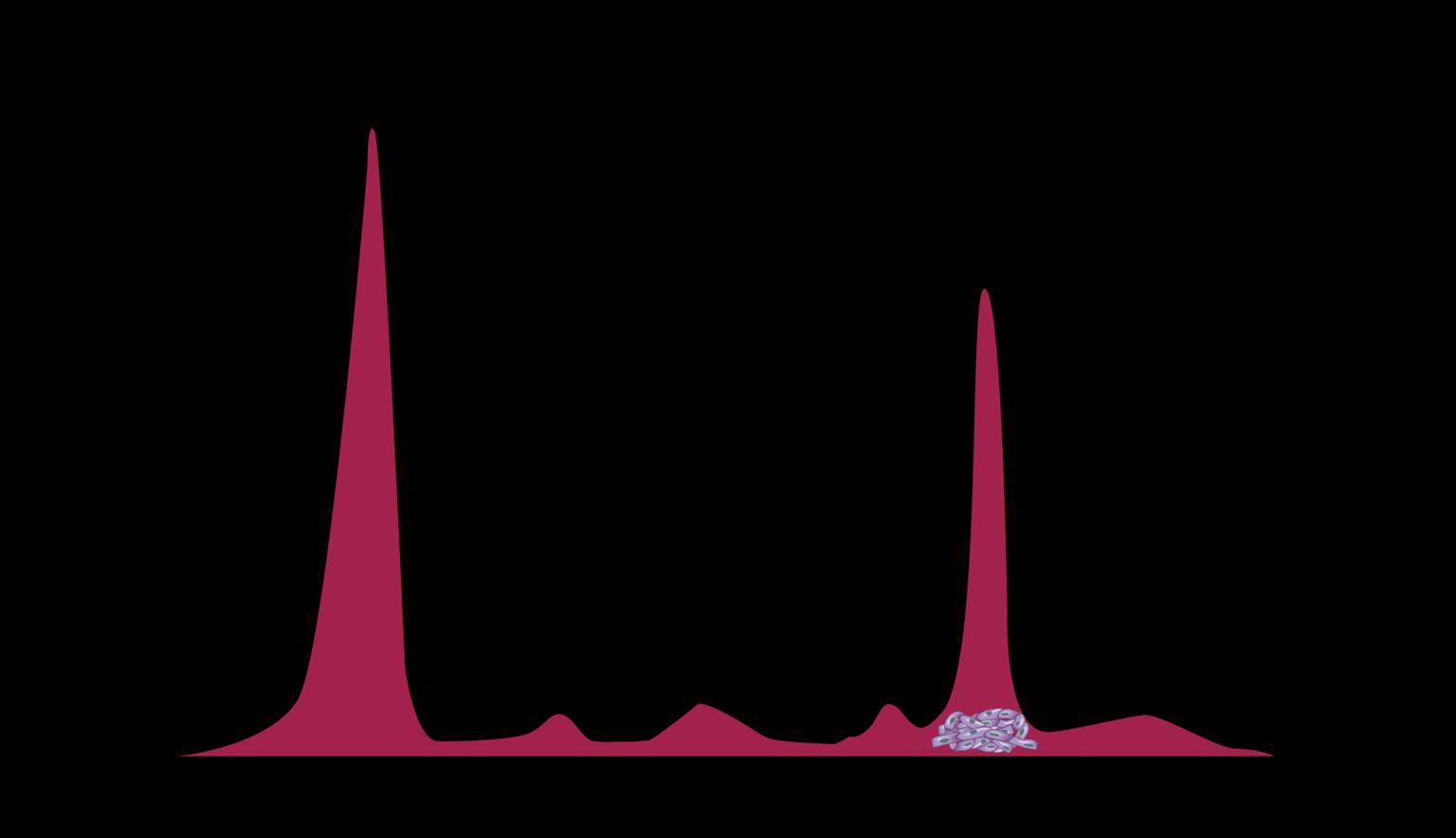
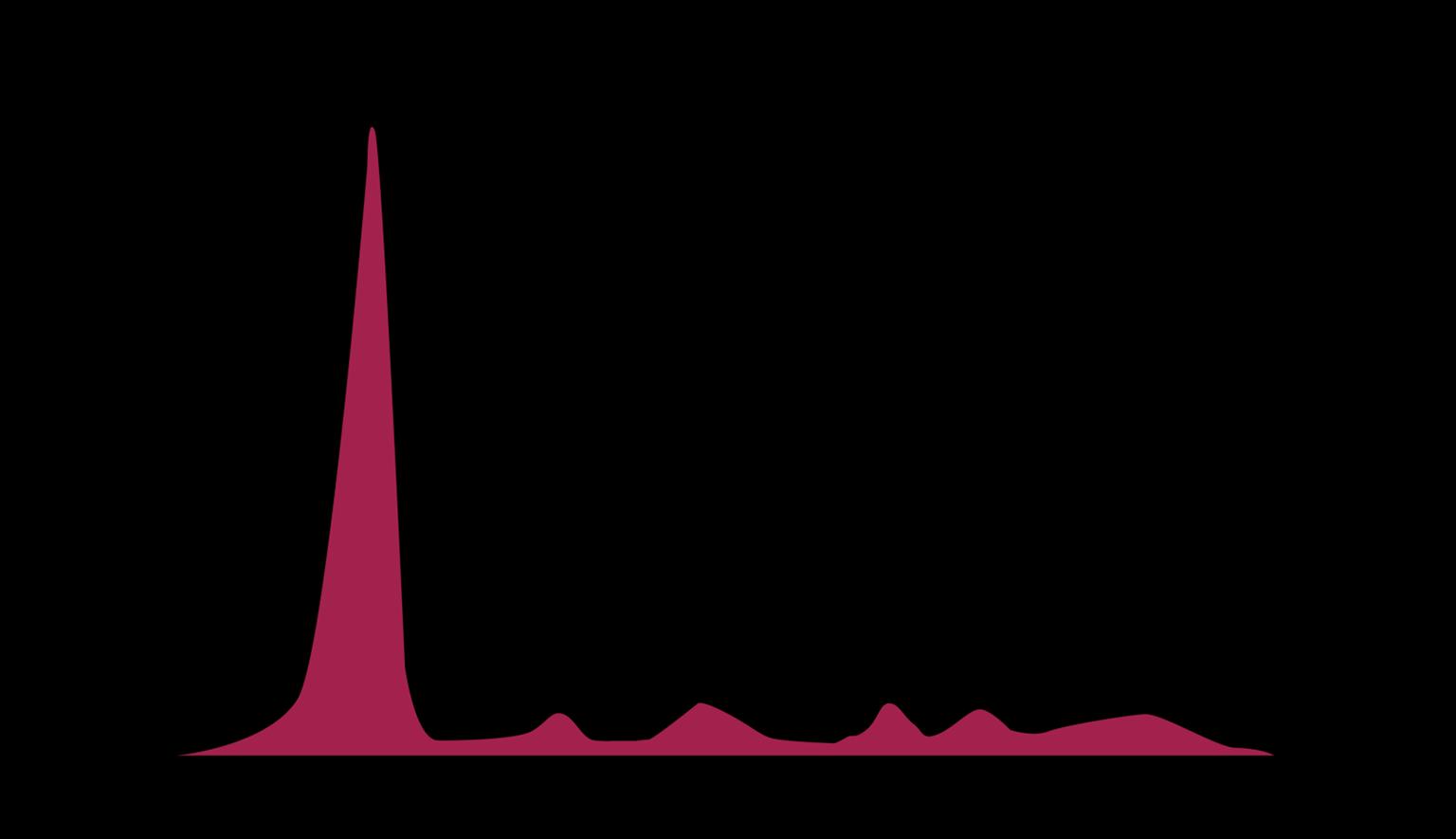
Myeloma Cells Can Produce Myeloma Protein Continually: Detectable







in Plasma and Urine

Note: Some patients have nonsecretory disease that does not produce detectable myeloma protein.
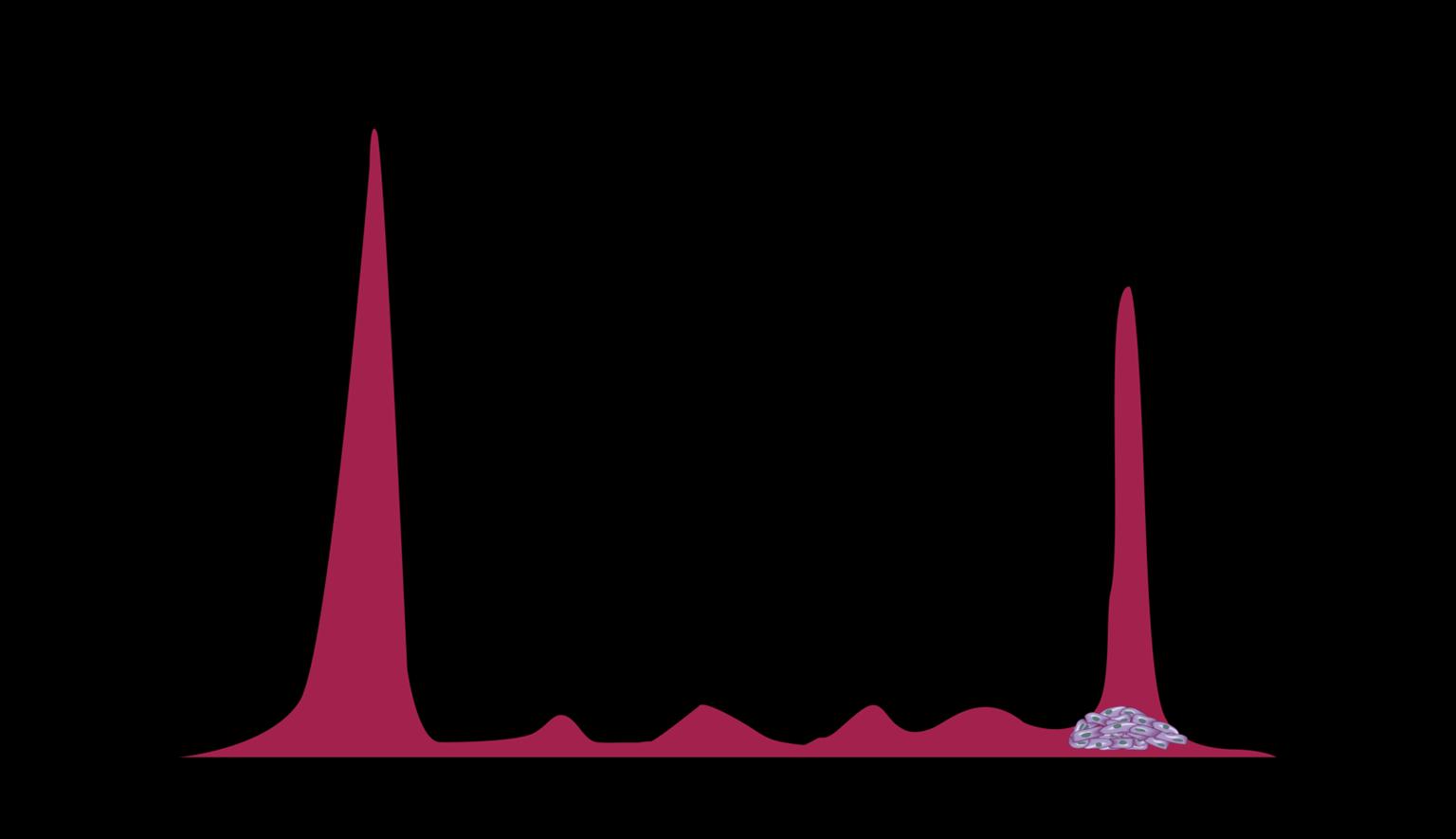

15
Understanding Your Test Results, International Myeloma Foundation 2018.
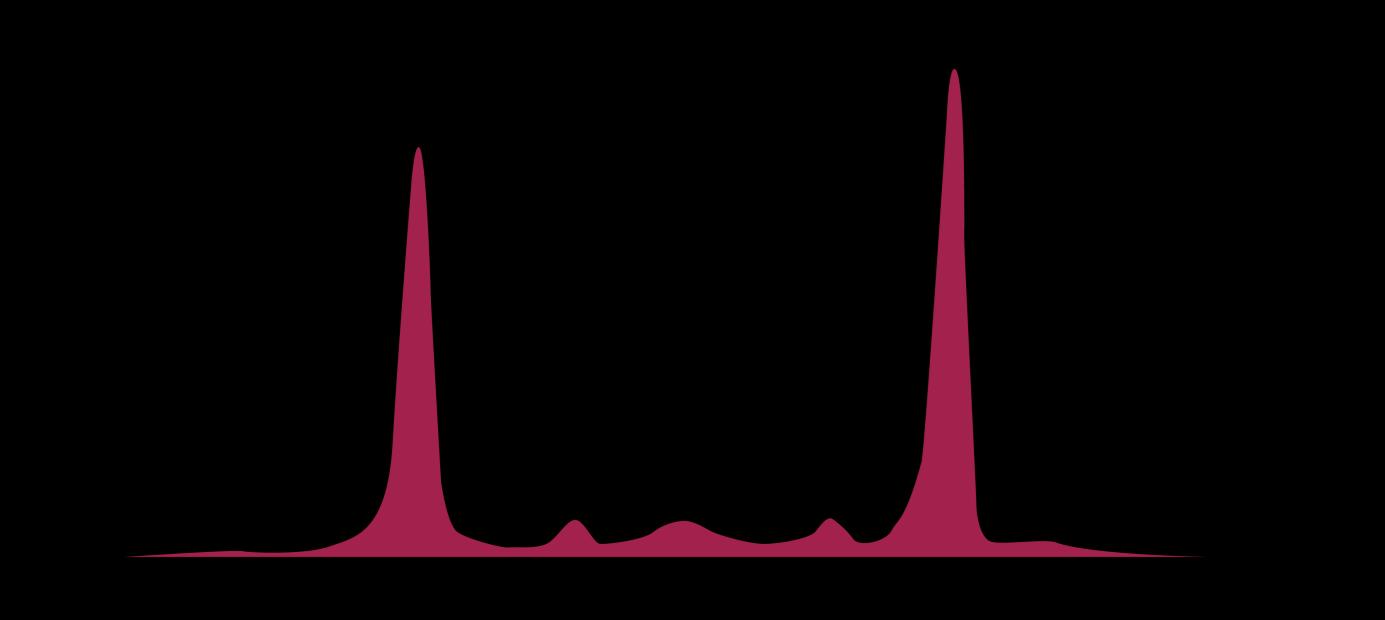
marrow Normal Myeloma Albumin alpha-1 alpha-2 beta gamma Myeloma
Bone
Characteristics Put Some at Higher Risk for MM
Risk Factors
MGUS or other plasma disorder
Obesity
Environmental or Occupational Exposures (eg, pesticides)

Male Age
MM = multiple myeloma; MGUS = monoclonal gammopathy of undetermined significance.
Family History of MM
Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment (PDQ®)–Patient Version - NCI. Published December 9, 2022. Accessed April 1, 2023. https://www.cancer.gov/types/myeloma/patient/myeloma-treatment-pdq. Tariman JD. Multiple myeloma. In: Yarbro CH, Frogge MH, Goodman M, eds. Cancer Nursing: Principles and Practice. Jones and Bartlett Publishers; 2005:1460-1489. Sergentanis TN, et al. Clin Lymphoma Myeloma Leuk. 2015;15(10):563-577.
Black Race
16
Multiple Myeloma Continuum: Premalignant Conditions
ACTIVE MM

CRAB Criteria
Calcium elevation
SMM
Low risk
Risk of progression: ≈ 1% per year
MGUS
Spike on SPEP/UPEP
Abnormal free light test
Bone marrow < 10% PCs
Renal dysfunction
Anemia
Bone lesions
High-risk: likely to progress to active MM within 2 years

M-spike ≥ 2 g/dL
Serum Free Light Chain Assay
(kappa/lambda ratio ≥ 20)
SLiM / MDEs
Bone marrow ≥ 60% PC
Serum Free Light Chain Assay
(kappa/lambda ratio ≥ 100)

Bone marrow ≥ 20% PCs
CLINICAL TRIAL
MGRSa TREAT PREMALIGNANT CONDITIONS MONITOR
MRI ≥ 1 focal lesion ≥ 5mm
aMonoclonal gammopathy of renal significance (MGRS) does not meet criteria for myeloma but has kidney manifestation; kidney biopsy is the gold standard for diagnosis. CRAB = calcium elevation, renal dysfunction, anemia, bone lesions; M-spike = monoclonal spike; MDE = myeloma-defining event; MGRS = monoclonal gammopathy of renal significance; MGUS = monoclonal gammopathy of undetermined significance; MM = multiple myeloma; MRI = magnetic resonance imaging; PC = plasma clone; SLiM = sixty, light chain, MRI; SMM = smoldering multiple myeloma; SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis.
Rajkumar SV, et al. Lancet Oncol. 2014;15(12):e538-48. Bridoux F, et al. Kidney Int. 2015;87(4):698-711. Terpos E, et al. Lancet Oncol. 2021;22(3):e119-e130. Hillengass J, et al. Lancet Oncol. 2019;20(6):e302-e312. Ludwig H, et al. Lancet. 2023;58:101910.
17
CASE 1
Margaret*
PATIENT NOTES:
• 62-year-old woman

• Seen by rheumatologist for chronic pain; rheumatologist identified
M-protein




• Referred to hematologist; diagnosed with SMM
• Friend urged her to get a second opinion from academic center
• Academic center recommended ECOG trial (Rd ± Dara)
BLOOD WORK: Serum free light chain assay
kappa/lambda ratio: 25
24-HOUR URINE: 2.3 g/dL M-protein
BONE MARROW: 30% plasma cells
Dara = daratumumab; ECOG = Eastern Cooperative Oncology Group; HIPAA = Health Insurance Portability and Accountability Act; M-protein = monoclonal protein; Rd = lenalidomide dexamethasone; SMM = smoldering multiple myeloma. 18
*HIPAA-compliant, not actual patient name, stock photo.
Clinical Trials: Early Access to Promising Treatments
Preclinical

ANIMAL STUDIES
PHASE 1
FIRST INTRODUCTION OF AN INVESTIGATIONAL DRUG INTO HUMANS
• Determine metabolism and PK/PD actions, MTD, and DLT
• Identify AEs
• Gain early evidence of effectiveness, studied in many conditions; typically, 20 to 80 patients; everyone gets agent
PHASE 2
EVALUATION OF EFFECTIVENESS IN A CERTAIN TUMOR TYPE
• Determine short-term AEs and risks; closely monitored
• Includes up to 100 patients, typically
PHASE 3
GATHER ADDITIONAL EFFECTIVENESS AND SAFETY INFORMATION COMPARED TO STANDARD OF CARE

• Placebo may be involved if no standard of care exists; 100s to several thousand patients
• Often multiple institutions; single or double blind
PHASE 4
APPROVED AGENTS IN NEW POPULATIONS OR NEW DOSE FORMS
AE = adverse event; DLT = dose-limiting toxicity; MTD = maximum tolerated dose; PD = pharmacodynamics; PK = pharmacokinetics. Faiman B, et al. Adv Pract Oncol. 2016;7:17-29.
19
Importance of Participation by Diverse Populations in Clinical Trials
[P]eople from racial and ethnic minorities and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products.
–

FDA
US Cancer Centers of Excellence Strategies for Increased Inclusion of Racial and Ethnic Minorities in Clinical Trials
Leadership and commitment
Community engagement practices
Investigator hiring, training, and mentoring practices
Patient engagement practices

20
FDA = Food and Drug Administration. Regnante JM, et al. J Oncol Pract. 2019;15(4):e289-e299. FDA website. Clinical Trial Diversity. Accessed March 31, 2022. https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity.
Clinical Trial Myths: Importance of Dispelling Inaccuracies
MYTH: If I participate in a clinical trial, I might get a placebo, not active treatment
MYTH: If I participate in a clinical trial, I can't change my mind
v
• Phase 1 and 2, everyone gets active treatment
• Phase 3 standard of care vs. new regimen: often standard regimen with/without additional agent in MM trials
• Patients can withdraw their consent for clinical trial participation at any time
MYTH: Patients (whatever demographic/ distance from clinic/etc) never participate in clinical trials so I won't mention it
• Mention the option and give the patient the opportunity; implicit and explicit biases can limit participation
• Some groups may need more information about clinical trials to feel comfortable with participation

MYTH: Clinical trials are dangerous because they have new medicines and practices
• Some risk is involved with every treatment, but medicines are used in clinical trials with people only after they have gone through testing to indicate that the drug is likely to be safe and effective for human use
MYTH: Clinical trials are expensive and not covered by insurance
• Research costs are typically covered by the sponsoring company
• Standard patient care costs are typically covered by insurance
• Check with clinical trial team/insurers; costs such as transportation, hotel, etc. may not be reimbursed and are paid by patient
= multiple myeloma. PhRMA website. Accessed April 6, 2023. https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/A-C/CLINICAL-TRIALS-MYTH-FACT-PRINT.pdf?hsCtaTracking=f6689b95-1626-40d98c87-c6b8d31600a4%7C35221aa8-d487-4db3-9416-b9c3c35e3bac.
MM
21
iStopMM Clinical Study: New Insights About MGUS
• 5.0% of people ≥50 years had MGUS
– IgA MGUS subtype does not increase with age, like other MGUS subtypes
• 75,422 individuals screened in Iceland
• Represents 51% of all Icelanders aged ≥50

• Analysis of study data has provided new insights

– 9.4% of patients had multiple paraproteins which was more common than previously observed
– No association between MGUS and chronic kidney disease or autoimmune disease
• Multivariate model to predict the risk of ≥10% BMPC based on laboratory tests. Available at https://istopmm.com/riskmodel/
• When using mass spectrometry (MS) as the benchmark, most individuals with transient M-protein spikes on SPEP/IFE are false negatives
• Covid vaccines are safe for people with MGUS: No evidence for MGUS progression after SARS-CoV-2 vaccination, regardless of the number of doses or type of vaccine
WATCH FOR New iStopMM results as analyses continue
BMPC = bone marrow plasma clone; IFE = immunofixation electrophoresis; IgA = Immunoglobulin A; iStopMM = Iceland Screens Treats or Prevents Multiple Myeloma; MGUS = monoclonal gammopathy of undetermined significance; MS = mass spectrometry; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SPEP = serum protein electrophoresis. Love TJ, et al. ASH 2022. Abstr #103. Eythorsson E, et al. ASH 2022. Abstr #107. Palmason R, et al. ASH 2022. Abstr #967. Palmason R, et al. ASH 2022. Abstr #105. Rögnvaldsson S, et al. ASH 2022. Abstr #4504. Sverrisdottir IR, et al. ASH 2022. Abstr #4507. Long TE, et al. ASH 2022. Abstr #4541. iStopMM website. Accessed March 28, 2023. https://istopmm.com/riskmodel/.
22
Treating High Risk SMM Patients in Clinical Trials
GEM-CESAR: Phase 2 study
• Patients with high-risk SMM (N=90)
• KRd × 6 cycles → ASCT → KRd consolidation → R maintenance up to 2 years
Results
• 94% alive and progression-free at a median follow-up of 65.8 months
• 68% MRD-negative (10-5) after KRd consolidation
• 23% maintained MRD-negative (10-5) status 4 years after ASCT (2 years after the end of maintenance treatment)
ASCENT: Phase 2 study
• Patients with high-risk SMM (n=87)
• Dara-KRd × 12 cycles → Dara-R maintenance for 12 cycles
Results



• ORR = 97%
• 84% of patients became MRD-negative

• 3-year PFS rate of 90%
• Regimen was well tolerated
WATCH FOR Updates in SMM treatment
ASCT = autologous stem cell transplant; Dara = daratumumab; KRd = carfilzomib, lenalidomide, dexamethasone; ORR = overall response rate; MRD = minimal residual disease; ORR = overall response rate; PFS = progression-free survival; R = lenalidomide; SMM = smoldering multiple myeloma.
Mateos MV, et al. ASH 2022. Abstr #118. Kumar SK, et al. ASH 2022. Abstr #757.
How Patients With Myeloma Commonly Present
ROUTINE PHYSICAL


• Patient with few/ no symptoms
• Abnormal bloodwork or test result
VISIT FOR SPECIFIC COMPLAINT
• Bone pain, fatigue, or injury
• Abnormal test result (eg, x-ray, blood test)
NON-EMERGENCY; More time for shared decision-making

EMERGENCY ROOM
• Severe pain—often spinal fractures
• Kidney failure
MEDICAL EMERGENCY; Need immediate treatment!
24
Brigle K, et al. J Adv Pract Oncol. 2022;13(suppl 4):7-14. Brigle K, et al. Clin J Oncol Nurs. 2017;21(5 suppl):60-76. Faiman B, et al. J Adv Pract Oncol. 2016;2016:7(suppl 1):17-29. Kurtin S, et al. J Adv Pract Oncol. 2016;7(suppl 1):59-70.
Multiple Myeloma Continuum: Active MM
ACTIVE MM

CRAB Criteria
Calcium elevation
SMM
Low risk
Risk of progression: ≈ 1% per year
MGUS
Spike on SPEP/UPEP
Abnormal free light test
Bone marrow < 10% PCs
Renal dysfunction
Anemia
Bone lesions
High-risk: likely to progress to active MM within 2 years

M-spike ≥ 2 g/dL
Serum Free Light Chain Assay
(kappa/lambda ratio ≥ 20)
SLiM / MDEs
Bone marrow ≥ 60% PC
Serum Free Light Chain Assay
(kappa/lambda ratio ≥ 100)

Bone marrow ≥ 20% PCs
CLINICAL TRIAL
MGRSa TREAT PREMALIGNANT CONDITIONS MONITOR
MRI ≥ 1 focal lesion ≥ 5mm
aMonoclonal gammopathy of renal significance (MGRS) does not meet criteria for myeloma but has kidney manifestation; kidney biopsy is the gold standard for diagnosis. CRAB = calcium elevation, renal dysfunction, anemia, bone lesions; M-spike = monoclonal spike; MDE = myeloma-defining event; MGRS = monoclonal gammopathy of renal significance; MGUS = monoclonal gammopathy of undetermined significance; MM = multiple myeloma; MRI = magnetic resonance imaging; PC = plasma clone; SLiM = sixty, light chain, MRI; SMM = smoldering multiple myeloma; SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis.
Rajkumar SV, et al. Lancet Oncol. 2014;15(12):e538-48. Bridoux F, et al. Kidney Int. 2015;87(4):698-711. Terpos E, et al. Lancet Oncol. 2021;22(3):e119-e130. Hillengass J, et al. Lancet Oncol. 2019;20(6):e302-e312. Ludwig H, et al. Lancet. 2023;58:101910.
25
Diagnostic Workup for Multiple Myeloma

LAB TESTS
• CBC + differential + platelet count + chemistry (including albumin and B2M) + LDH
• Peripheral blood smear
• Creatinine clearance
• Serum free light chain (FLC) assay



• Serum protein electrophoresis (SPEP)
• Urine protein electrophoresis (UPEP)-24 hr




BONE MARROW BIOPSY
• FISH
• Immunohistochemistry (IHC) and/or
multiparameter flow cytometry





• Clonal plasma cell percentage
FISH detects abnormalities in MM cells by using fluorescent probes







B2M = beta-2 microglobulin; CBC = complete blood count; FISH = fluorescence in situ hybridization; FLC = free light chain; IHC = Immunohistochemistry; LDH = lactate dehydrogenase; MM = multiple myeloma. SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis.

26
del(17)p
gain(1q)
t(4;14)
Brigle K, et al. J Adv Pract Oncol. 2022;13(suppl 4):7-14. Ghobrial IM, et al. Blood. 2014;124:3380-3388. Rajkumar SV, et al. Lancet Oncol. 2014;15:e538-3548. Faiman B. Clin Lymphoma Myeloma Leuk. 2014;14:436-440.
Imaging for Multiple Myeloma
SEVERAL OPTIONS FOR BONE IMAGING
Whole Body
Low-Dose CT (WBLDCT)


Best for early screening for bone disease
PET/CT
Response assessment: active residual disease

MRI
Whole body (WB) or spine + pelvis
Gold standard to assess bone
Image: Gavriatopoulou M, et al. Blood Cancer J. 2020;10:93
marrow involvement
CT = computed tomography; DEXA = dual-energy x-ray absorptiometry; MM = multiple myeloma; MRI = magnetic resonance imaging; PET = positron emission tomography; WB = whole body; WBLDCT = whole-body low-dose computed tomography. Brigle K, et al. J Adv Pract Oncol. 2022;13(suppl 4):7-14. Hillengass J, et al. Lancet Oncol. 2019;20(6):e302-e312. Rome SI, et al. Clin J Oncol Nurs. 2017;21(5 suppl):47-59. Faiman B. Clin Lymphoma Myeloma Leuk. 2014;14:436-440. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-1556.
NOTE: Bone scan (DEXA) for bone density is not for MM
27
Risk With Multiple Myeloma
No abnormalities detected OR Abnormalities that are not defined as high-risk

• Trisomies
• t(11;14)
• t(6;14)
FISH = fluorescence in situ hybridization; MM = multiple myeloma; SMM = smoldering multiple myeloma.
aHigh risk if detected in SMM; intermediate risk in MM according to Rajkumar SV 2022. Rajkumar SV. Am J Hematol. 2022;97(8):1086-1107.
Identified by FISH
• t(4;14)a



• t(14;16)
• t(14;20)
• del(17p)
• gain(1q)a
Double hit: any 2 high-risk factors
Triple hit: any 3 high-risk factors
28
STANDARD RISK HIGH
RISK
-ISS Staging System for Multiple Myeloma
WATCH FOR Adoption of the proposed revision to R-ISS: R2-ISS

• ISS stage I (serum B2M level < 3.5 mg/L and serum albumin ≥ 3.5 g/dL)
• No high-risk CA [del(17p) and/or t(4;14) and/or t(14;16)]
• Serum LDH < ULN (varies by institution)
82% 55% II


• Not R-ISS stage I or III 62% 36% III
• ISS stage III (serum B2M level > 5.5 mg/L)
• High-risk CA [del(17p) and/or t(14;4) and/or t(14;16)] or high serum LDH 40% 24%
B2M = beta-2 microglobulin; CA = chromosomal abnormality; ISS = International Staging System; LDH = lactate dehydrogenase; MM = multiple myeloma; OS = overall survival; PFS = progression-free survival; R-ISS = Revised-ISS;
revision; ULN = upper limit of normal.
29
ISS
A,
R-ISS 5-YEAR OS 5-YEAR PFS
R2-ISS =
second
Palumbo
et al. J Clin Oncol. 2015;33:2863-2869. D’Agostino M, et al. J of Clin Oncol. 2022;40(29):3406-3418. STAGE
I
SURVIVAL
BETTER WORSE
Margaret*

PATIENT NOTES:

• Academic center: High-risk SMM based on labs
• ECOG trial suggested (Rd ± Dara) → Screening
*HIPAA-compliant, not actual patient name or stock photo.
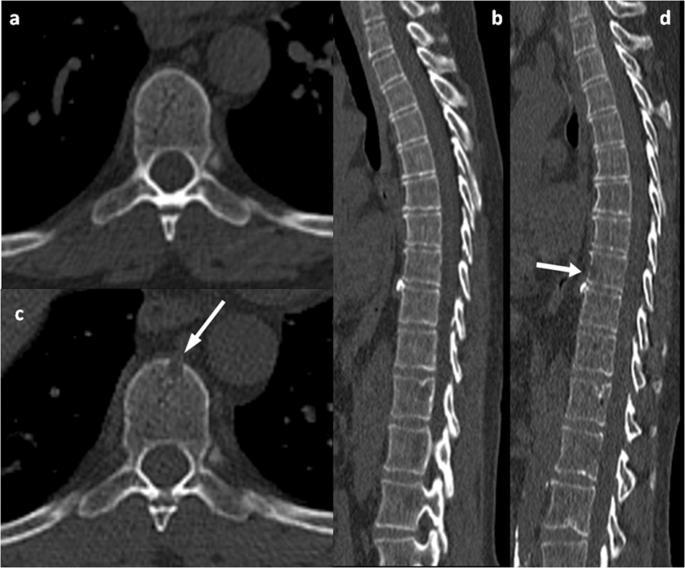
Whole Body PET-CT FDG-avid lesions T10-T12, lateral right ribs
Whole Spine MRI T10 to T12 lesions, intact spinal canal
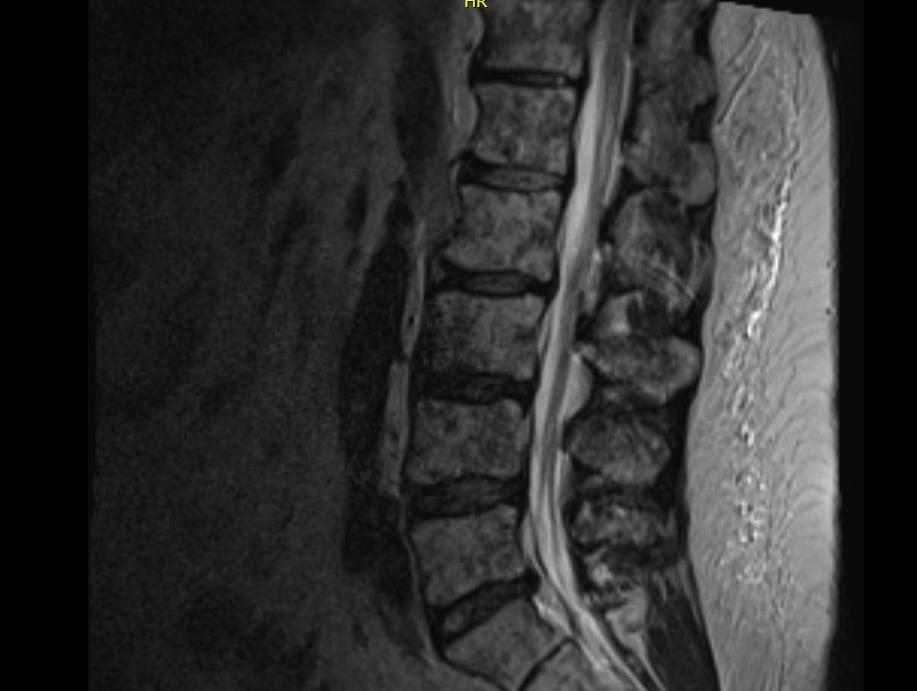
FISH Gain(1q)
Diagnosis Active MM, R-ISS II





not actual patient name, stock photo.

CASE 1
30 CT = computed tomography; Dara = daratumumab; ECOG = Eastern Cooperative Oncology Group; FDG = fluorodeoxyglucose; FISH = fluorescence in situ hybridization; HIPAA = Health Insurance Portability and Accountability Act; MM = multiple myeloma; MRI = magnetic resonance imaging; PET = positron emission tomography; R-ISS = Revised International Staging System; Rd = lenalidomide dexamethasone; SMM = smoldering multiple myeloma.
Median Age at Diagnosis of MM Varies by Race/Ethnicity









MM = multiple myeloma. These images are from http://www.shutterstock.com: 129923210, 80373427, 525699067, 603640064. Ailawadhi S, et al. Br J Haematol. 2012;158:91-98. National Cancer Institute. SEER Stat Fact Sheets: Myeloma. Surveillance, Epidemiology, and End Results Program website. Accessed February 11, 2021. http://seer.cancer.gov/statfacts/html/mulmy.html. THE MEDIAN AGE AT DIAGNOSIS FOR ALL PATIENTS IS 69 years 65 YEARS 66 YEARS 69 YEARS 71 YEARS 70 65 Hispanic Patients Black Patients Asian Patients White Patients 31 66 67 62 68 63 69 64 71 72
Health Disparities in Multiple Myeloma Among Black Patients
MM is a malignancy with one of the greatest disparities:
2- to 3-fold higher incidence of MM in Black vs White Americansa
Black patients with MM have more than double the mortality compared with White patients
Black patients are less likely to receive life-extending therapies such as ASCT, IMiDs, and PIs
~10% of Black patients have MGUS; MM is the most common blood cancer in Black patients
Black patients achieve better outcomes when they receive equal therapy

Black patients tend to have lower-risk disease due to biologic differences in MM
ASCT = autologous stem cell transplant; IMiD = immunomodulatory imide drug; MGUS = monoclonal gammopathy of undetermined significance; MM = multiple myeloma; PI = protease inhibitor. aData derived by calculating the ratio of the average age-adjusted incidence rates for Black and White patients from 2000 to 2013 for the 8 most common malignancies in Black patients, plus all cancer sites and MM. Incidence rates were obtained from National Cancer Institute. Fast stats. Surveillance, Epidemiology, and End Results Program website. Accessed March 3, 2022. https://seer.cancer.gov/.

Dong J, et al. Blood Cancer J. 2022;12(2):34. El-Khoury H, et al. ASH 2021. Abstr #152. Pierre A, Williams TH. Clin J Oncol Nurs. 2020;24(4):439-443. Greenberg AJ, et al. Blood Cancer J. 2015;4:e2713. Baker A, et al. Blood. 2013;12(16):3147-3152. Waxman AJ, et al. Blood. 2010;116(25):5501-5506. Hari PN, et al. Biol Blood Marrow Transplant. 2010;16:395-402. Saraf SL, et al. Bone Marrow Transplant. 2013;48:319-320. Rhotagi N, et al. Am J Clin Oncol. 2007;30(5):540-548. Ailawadhi S, et al. Br J Haematol. 2012;158:91-98. Doroshow D, et al. Ann Oncol. 2020;31:S1204. Hultcrantz M, et al. Blood Cancer Discov. 2020;1:234-243.

32
What Can Nurses Do to Combat Disparities in MM Care?

Be aware of higher rates and earlier age of onset of MGUS and MM in Black patients
Strive to become aware of potential conscious or unconscious biases
Ensure equal access to centers of excellence and treatments (eg, ASCT, IMiDs, PIs, clinical trials), and supportive care
Engage each patient; be aware of cultural differences
Encourage Black patients with MM to connect with International Foundation Outreach, eg, https://mpower.myeloma.org/

33
= autologous stem cell transplant; IMiD = immunomodulatory imide drug; MM = multiple myeloma; PI = protease inhibitor. Dong J, et al. Blood Cancer J. 2022;12(2):34. El-Khoury H, et al. ASH 2021. Abstr #152. Pierre A, Williams TH. Clin J Oncol Nurs. 2020;24(4):439-443. Greenberg AJ, et al. Blood Cancer J. 2015;4:e2713. Baker A, et al. Blood. 2013;12(16):3147-3152. Waxman AJ, et al. Blood. 2010;116(25):5501-5506. Hari PN, et al. Biol Blood Marrow Transplant. 2010;16:395-402. Saraf SL, et al. Bone Marrow Transplant. 2013;48:319-320. Rhotagi N, et al. Am J Clin Oncol. 2007;30(5):540-548. Ailawadhi S, et al. Br J Haematol. 2012;158:91-98. Doroshow D, et al. Ann Oncol. 2020;31:S1204. Hultcrantz M, et al. Blood Cancer Discov. 2020;1:234-243. International Myeloma Foundation website. Accessed March 4, 2022. http://www.myeloma.org.
ASCT
• Patient education is crucial but can be overwhelming




• The shock of diagnosis makes understanding and retaining information difficult


– Tell patients, but also give written or electronic information they can refer to




– Refer patients to reliable sources of information



– Engage care partners or extended family

IMF = International Myeloma Foundation.
35 Knowledge Is
IMF Website http://myeloma.org IMF TV Teleconferences Multiple Languages Free Download or Order From myeloma.org https://www.cancer.gov Leukemia & Lymphoma Society https://www.lls.org
Power: Steep Learning Curve for Newly Diagnosed Patients With Multiple Myeloma
https://www.cancer.org
Important Health Protection Education for Newly Diagnosed Patients With Multiple Myeloma

INFECTION PREVENTION
• Ensure handwashing, hygiene
• Growth factor (eg, filgrastim)
• IVIG for hypogammaglobulinemia
• Immunizations (NO live vaccines)
– COVID-19 vaccination + booster(s)
– Pneumococcal 20-valent conjugate vaccine
– Seasonal inactivated influenza vaccine (x2 or high-dose)
– Shingles vaccine: zoster vaccine recombinant, adjuvanted
• COVID-19 prevention
– Antibody levels
– Tixagevimab co-packaged with cilgavimab





• Avoid contact with sick people
KIDNEY HEALTH
Risks
• Active MM (M-protein, casts)
• High calcium Prevention
• Avoid certain medications (contrast dyes, NSAIDS)
• Hydration Treatment
• Address underlying myeloma causing kidney dysfunction
• Dose adjustments may be needed for reduced kidney function
BONE HEALTH
• Hypercalcemia from bone destruction can affect the kidneys
• ≈ 85% of patients with MM develop bone disease Monitor
• Report new or worsening bone pain
Medical testing or intervention
• Monitor serum calcium levels
• Imaging may be needed depending on type and location of pain (eg, MRI, PET-CT)
• Bone-modifying agents
CT = computed tomography; IVIG = intravenous immunoglobulin; M-protein = monoclonal protein; MM = multiple myeloma; MRI = magnetic resonance imaging; NSAID = nonsteroidal anti-inflammatory drug; PET = positron emission tomography. Brigle K, et al. J Adv Pract Oncol. 2022;13(suppl 4):7-14. Hillengass J, et al. Lancet Oncol. 2019;20(6):e302-e312. Faiman B, et al. Clin J Oncol Nurs. 2017;21(5 suppl):19-36. Faiman B, et al. Clin J Oncol Nurs. 2011;15(suppl):66-76. Miceli TS, et al. Clin J Oncol Nurs. 2011;15(4):9-23. Rome SI, et al. Clin J Oncol Nurs. 2017;21(5 suppl):47-59. Miceli TS, et al. Clin J Oncol Nurs. 2011;15(4 suppl):9-23. Dimopoulous M, et al. Leukemia. 2009;23(9):1545-1556. Brigle K, et al. Clin J Oncol Nurs. 2017;21(5 suppl):60-76. Faiman B, et al; IMF Nurse Leadership Board. Clin J Oncol Nurs. 2011;15(suppl):66-76. Miceli TS, et al. Clin J Oncol Nurs. 2011;15(4):9-23. Brigle K, et al. J Adv Pract Oncol [in press].
36
Bone Modifying Agents
Recommendation: Bone-strengthening agents should be administered for at least 12 months to all patients with newly diagnosed MM, with or without bone disease
Agent Notes
Zoledronic acid




• Preferred agent
• Also indicated for MM-related hypercalcemia
• PFS and OS benefit
Denosumab
• May also be used, particularly in patients with kidney impairment
• May prolong PFS in patients who are newly diagnosed with MM and are ASCT eligible


• Discontinuation can be challenging due to rebound effect
Pamidronic acid
• May be used if other agents are not available
37
ASCT = autologous stem cell transplant; MM = multiple myeloma; OS = overall survival; PFS = progression-free survival. Terpos E, et al. Lancet Oncol. 2021;22(3):e119-e130.
Expanding Treatment Options for Multiple Myeloma

CAR = chimeric antigen receptor; SC = subcutaneous; SINE = selective inhibitor of nuclear export. Tariman J. Nurs Clin North Am. 2017;52(1):65-81. DRUGS@FDA.gov. Alkylator Steroid Proteasome inhibitor (PI) Immunomodulatory imide drug (IMiD) Anthracycline 1986 High-Dose Dexamethasone 2003 Bortezomib 2019 Selinexor 2020 Isatuximab 1960 1970 1980 1990 2000 2010 2020 2020 Daratumumab SC 2021 2015 Daratumumab 2015 Ixazomib 2015 Elotuzumab 1958 Melphalan 1962 Prednisone 1983 Autologous Stem Cell Transplantation 2006 Lenalidomide 2006 Thalidomide 2007 Doxorubicin 2012 Carfilzomib 2018 Denosumab 2022 Ciltacabtagene Autoleucel 2021 Idecabtagene Vicleucel 2013 Pomalidomide 38 Monoclonal antibody (mAb) SINE CAR T-cell therapy 2022 Teclistamab Bi-specific antibody
Myeloma Primary Treatment: Quadruplet or Triplet Regimens
ASCT Followed by Maintenance
INDUCTION/ PRIMARY THERAPY

Transplant Eligible Common Regimens:
Transplant Ineligible
Common Regimens:
(for renal insufficiency)
(for renal insufficiency) RVd “light” (for frail)
ASCT Consolidation Maintenance Maintenance
ASCT = autologous stem cell transplant; Dara = daratumumab; IRd = ixazomib lenalidomide dexamethasone; KRd = carfilzomib lenalidomide dexamethasone; Rd = lenalidomide dexamethasone; RVd = lenalidomide bortezomib dexamethasone; NA = not applicable; VCd = bortezomib cyclophosphamide dexamethasone; VMP = bortezomib melphalan prednisone. Rajkumar SV. Am J Hematol. 2022;97(8):1086-1107. NCCN Guidelines® Multiple Myeloma. V3.2023. Accessed March 15, 2023.


39
±
Dara-RVd RVd KRd
VCd
RVd Dara-Rd KRd IRd
VCd
(oral regimen) Dara-VMP
SHARE Approach

Benefits to Health Care Professionals:
• Improved quality of care delivered

• Increased patient satisfaction

Benefits to Patients:
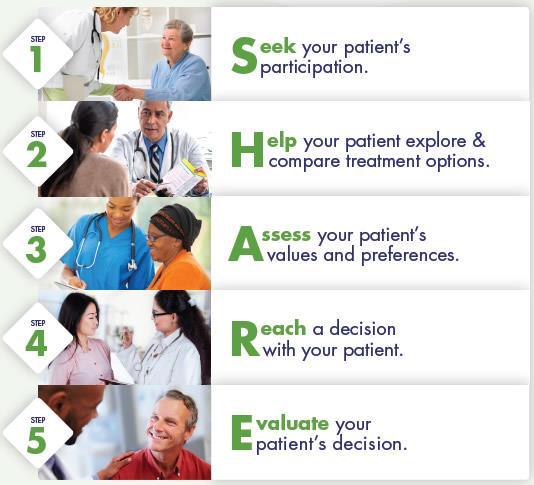
• Improved patient experience of care
• Improved patient adherence to treatment recommendations using the SHARE Approach builds a trusting and lasting relationship between health care professionals and patients
40
Agency for Healthcare Research and Quality website. Accessed April 5, 2023. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html. FREE professional education and
https://www.ahrq.gov/healthliteracy/professional-training/index.html
training
Daratumumab: Anti CD38 Monoclonal Antibody
• Monoclonal antibody targeting CD38
– Original IV dosing and SC formulation
• Multiple indications for MM
– See prescribing information for details
• Clinical pearls
– SC dose form for SC only; IV for IV only
– Antibody interference—type and cross BEFORE starting
– Premeds: corticosteroids, antipyretics, antihistamine, and montelukast
–
–
IRR with IV: ≈ 50% (mostly grade 1 and 2, in first or second infusion)
IRR with SC: ≈ 9%; systemic reactions 10%
– Post-med: oral corticosteroid for 2 days
– Herpes prophylaxis
– Remember appropriate prophylaxis for combination partner drugs
– Educate patients/care partners about expectations

SC injection
Dara-Rd, Dara-VMP (First-line non-transplant) Dara-VTd (First-line transplant eligible)
Dara-Vd, Dara-Pd, Dara-Kd (1-3 prior therapies)



Dara monotherapy (3 prior therapies or refractory to PI and IMiD)
Dara = daratumumab; Kd = carfilzomib dexamethasone; IMiD = immunomodulatory imide drug; IRR = infusion-related reaction; IV = intravenous; MM = multiple myeloma; Pd = pomalidomide dexamethasone; PI = proteasome inhibitor; Rd = lenalidomide dexamethasone; SC = subcutaneous; Vd = bortezomib dexamethasone; VMP = bortezomib melphalan prednisone; VTd = bortezomib thalidomide dexamethasone.
DARZALEX® (daratumumab) Prescribing Information. DARZALEX FASPRO™ (daratumumab and hyaluronidase-fihj) Prescribing Information. Gleason C, et al. J Adv Pract Oncol. 2016;7(suppl 1):53-57.
41
Daratumumab: Anti CD38 Monoclonal Antibody (Cont.)
IV Clinical Pearls
SLOW

then
on right or
cycle with Rd 3" 3"





4-week
• Schedule becomes less frequent

after 1st/2nd dose DAY 1 and 2 of CYCLE 1: 8 mg/kg SC or IV: SCHEDULES DEPEND on REGIMEN:
• If no injection/infusion reaction after 3 doses, consider discontinuing corticosteroid pre/post medications
42
IV = intravenous; SC = subcutaneous. DARZALEX® (daratumumab) Prescribing Information. DARZALEX FASPRO™ (daratumumab and hyaluronidase-fihj) Prescribing Information.
FIRST INFUSION
FASTER
CHECK
WEEKS 1-8 WEEKLY WEEKS 9-24
2 WEEKS WEEK 25
ALTERNATIVE: DIVIDED FIRST INFUSION 4 WEEKS
PRESCRIBING INFORMATION
EVERY
EVERY
SC Clinical Pearls
from navel
left side
15 mL into subcutaneous tissue over 3 to 5 minutes 1 2 3 DAY 1 mg/kg8 DAY 2 mg/kg8 ≈7 HOURS 3-4 HOURS
Pause or slow if patient experiences pain (9 doses) (5 doses) until progression INTO ABDOMEN
Example:
Inject
INJECTION
GRIFFIN: Dara Added to RVd Improves Response Rate and Depth of Response
GRIFFIN: Phase 3 clinical trial in newly diagnosed transplant-eligible patients with MM
• Dara-RVd (n=104) vs RVd (n=103)
• Followed by ASCT, consolidation, and maintenance for 24 months

Conclusions after 24 months of maintenance
• 99% ORR with Dara-RVd vs. 92% with RVd
• Dara doubled the percentage of MRD-negative patients
– 64.4% MRD-negative at 10-5 with Dara-RVd regimen vs 30.1% RVd (P < .0001)


sCR Rates with Dara-RVd and RVd
Dara added to RVd significantly improved ORR and depth of response, including sCR and MRD negativity
Continued use of Dara with RVd and R maintenance improved depth of response
43
Laubach JP, et al. ASH 2021. Abstr #79. End of Induction End of ASCT End of Consolidation After 1-Year Maintenance After 2-Year Maintenance RVd 7% 14% 32% 46% 47% Dara-RVd 12% 21% 42% 63% 66%
ASCT = autologous stem cell transplant; Dara = daratumumab; MM = multiple myeloma; MRD, minimal residual disease; ORR = overall response rate; R = lenalidomide; RVd = lenalidomide bortezomib dexamethasone; sCR = stringent complete response.
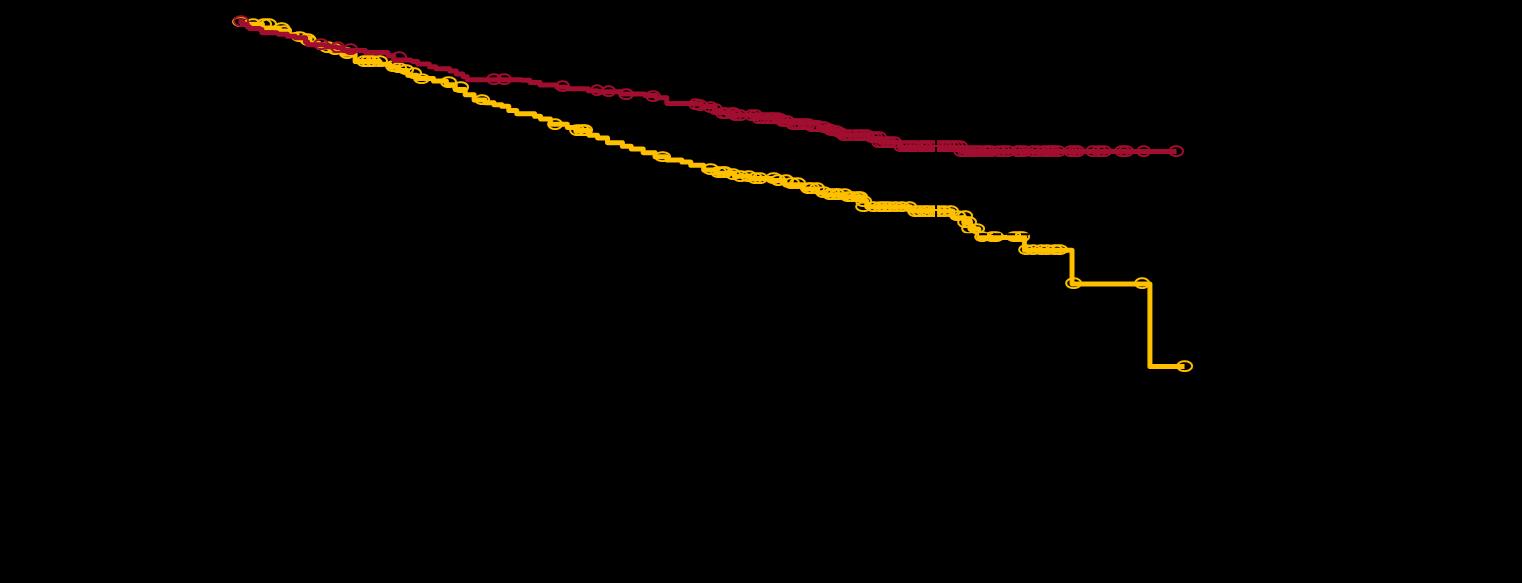
MAIA: Daratumumab Rd for Patients With MM Who Are Transplant Ineligible
MAIA Phase 3: PFS
Dara = daratumumab; HR = hazard ratio; MM = multiple myeloma; PFS = progression-free survival; Rd = lenalidomide dexamethasone; VMP = bortezomib melphalan dexamethasone. aKaplan-Meier estimate.






44
Facon T, et al. ASH 2018. Abstr #LBA-2. Facon T, et al Lancet Oncology. 2021;22(11):1582-1596.
At Risk, n Months Surviving without progression (%) Dara-Rd Rd At Risk, n Rd Dara-Rd




45
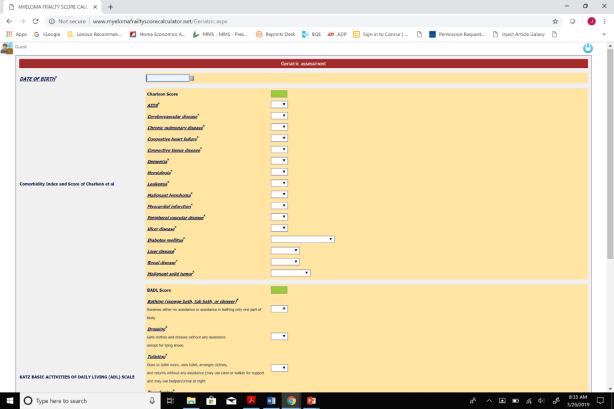
of Treatment Discontinuation Palumbo A, et al. Blood. 2015;125(13):2068-2074. International Myeloma Working Group. Myeloma Frailty Score Calculator. Accessed June 30, 2020. http://www.myelomafrailtyscorecalculator.net/ Score Patients (%) 3-Year Survival (%) Treatment Discontinuation (%) 0 39 84 17 1 31 76 22 ≥ 2 31 57 25 Online myeloma frailty score calculator at http://www.myelomafrailtyscorecalculator.net/ Fit = 0, intermediate = 1, frail ≥ 2 Calculator considers age, comorbidities, and ability to manage daily activities
Frailty Score Can Predict Survival and Rate
IFM 2017-03: Phase 3 Trial for Dexamethasone-Sparing
Treatment in Frail Patients With NDMM
Study Design
• Frail patients with NDMM
• Dara-R with only 2 months of steroids (n=199) or Rd (n=94)

Encouraging results, as Dara-R was associated with:
• Higher response: ORR 96% with Dara-R vs 85% with Rd
• Higher MRD negativity rates: 10% with Dara-R vs 3% with Rd
• Favorable safety profile and no increased risk of infection or pneumonia vs Rd
• Fewer discontinuations (32% with Dara-R vs 45% with Rd)

46
= complete response; Dara = daratumumab; MRD = minimal residual disease; NDMM = newly diagnosed multiple myeloma; ORR = overall response rate; PR = partial response; R =
Rd = lenalidomide dexamethasone; VGPR = very good partial response. Manier S, et al. ASH 2022. Abstr #569. 0 20 40 60 80 100 120 Dara-R Rd ORR VGPR 96% CR PR Patients (%)
CR
lenalidomide;
85% P=0.001 VGPR CR PR
Clinical Pearls for Steroids


• Patient education

– Steroids kill myeloma cells


– Don’t stop or change the dose without discussing with provider
• Consistent schedule (AM vs PM)
• Take with food
• Proactively manage side effects
– Glucose

–
Checklists
– Consider dose/timing adjustments with HCP
47
HCP = health care provider. King T, Faiman B. Clin J Oncol Nurs. 2107;21(2):240-249. Understanding Dexamethasone and Other Steroids. IMF Brochure.
Maintenance Therapy Nursing Implications

• Patients on therapy for a long time: AE management, adherence, treatment fatigue, no pregnancy with lenalidomide
• May encounter reimbursement challenges with PI (begin authorization early)
• Short-term vs long-term effects
– Many AEs subside after the first few months
– Health screening related to long-term use
• Patients living longer: survivorship care, coordination with PCP, emphasis on healthy behaviors
• Patient advocacy: understanding patient’s changing needs/desires; advocating with extended health care team
Lenalidomide maintenance: 10 or 15 mg on days 1 to 28 of a 28-day cycle
Ixazomib maintenance: 3 or 4 mg days 1, 8, and 15 in a 28-day cycle in TOURMALINE-MM3
Bortezomib maintenance: 1.3 mg/m2 every two weeks
Lenalidomide + bortezomib
Recommended for high-risk
48
AE = adverse event; PI = proteasome inhibitor; PCP = primary care physician. Bilotti E, et al. Clin J Oncol Nurs. 2011;15(4 suppl). Kurtin S. In: Tariman JD, et al, eds. Multiple Myeloma: A Textbook for Nurses. 2nd ed. 2015. Dimopoulous MA, et al. Lancet. 2019;393(10168):253-264. NCCN Guidelines®. Multiple Myeloma. V5.2022. Accessed March 15, 2022. Zhang S, et al. Blood Cancer J. 2020;10:33. Rajkumar SV. Am J Hematol. 2022;97(8):1086-1107.
Myeloma XI: Phase 3 Trial for Lenalidomide Maintenance
Study Design
• Transplant-eligible or ineligible patients with NDMM
• Patients randomized to lenalidomide maintenance until disease progression or observation
Results in Transplant-Eligible Patients
• Clear evidence that continuing R maintenance beyond 3-years is associated with improved PFS post-ASCT
• Benefit was consistent for standard and high-risk

– High-risk defined as the presence of del(17p), gain(1q), t(4,14), t(14;16) or t(14;20)
• Benefit of maintenance R after ASCT diminished over time, but more analysis and longer-term follow-up needed to define at what point

49
ASCT = autologous stem cell transplant; HR = hazard ratio.NDMM = newly diagnosed multiple myeloma; PFS = progression-free survival; R = lenalidomide. Pawlyn C, et al. ASH 2022. Abstr #570.
0 20 40 60 80 R maintenance Observation Median PFS VGPR 64 CR Months 32 HR 0.52 [95%CI 0.45, 0.61] P<0.001
Margaret*
• Shared decision-making:
– Treatment goals discussion
– Exploring treatment options:
• Clinical trial options

• Treatment risk vs benefit
• Side effects
–
–



Margaret’s priorities and preferences
Agreeing on a treatment plan
*HIPAA-compliant, not actual patient name, stock photo.
CASE 1 50 IMF Myeloma Treatment Discussion Tool. Accessed March 28, 2023. https://m-powercharlotte.myeloma.org/wp-content/uploads/Myeloma-Treatment-Discussion-Tool.pdf.
CASE 1 Margaret*
Remember:
✓ Shingles prevention


✓ DVT prophylaxis




TREATMENT: Dara-RVd Shared decision-making
✓ Monitor sugars

ASCT: Referral for consult with transplant center
MAINTENANCE: Planned: SWOG 1803 R-Dara (trial)

ASCT = autologous stem cell transplant; Dara = daratumumab; DVT = deep vein thrombosis; HIPAA = Health Insurance Portability and Accountability Act; R = lenalidomide; RVd = lenalidomide bortezomib dexamethasone; SWOG = National Cancer Institute–supported research group (formerly Southwest Oncology Group). 51
How Well Treatment Is Working: IMWG Myeloma Response and Relapse Criteria Assessment
CR
CR: myeloma protein undetectable in serum or urine (negative immunofixation); no more than 5% plasma cells in bone marrow; no new lytic lesions; plasmacytomas resolved
VGPR 90% reduction in myeloma protein
PR


At least 50% reduction in myeloma protein
Further categorization of CR: sCR, MRD-negative
For Nurses:

✓ Order labs regularly
✓ Encourage patients to know who is monitoring
✓ Monitor for relapse –

CRAB symptoms OR increase of 25% in M-protein from the lowest point
CR = complete response; CRAB = calcium elevation, renal dysfunction, anemia, bone lesions; IMWG = International Myeloma Worki response (only in relapsed); MRD = minimal residual disease; PD = progressive disease; PR = partial response; sCR= stringent response.
Palumbo A, et al; International Myeloma Working Group. J Clin Oncol. 2014;32:587-600. Durie BM, et al; International Myeloma Working Group. 2016;17(8):e328-e346.
MR SD PD
MRD-Negative Correlates With Better Patient Outcomes
ClonoSEQ MRD Results

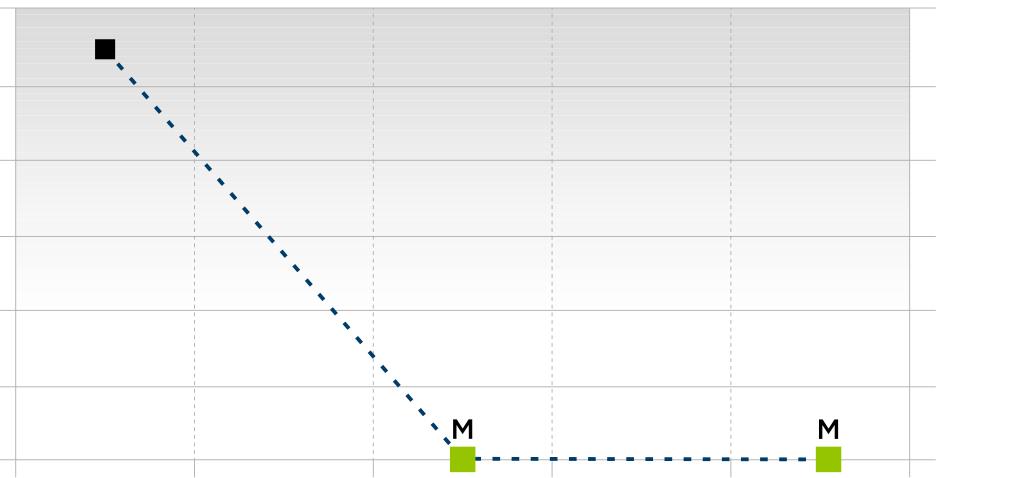


Role of the Nurse in MRD Testing:

The Why, When, How, and What
• Predict outcomes
WHY?
• Support treatment decisions?
• VGPR or better
WHEN?
• Often prior to transplant or cellular therapy
• Often retest at set intervals (eg, every 6 months)
HOW?
• ClonoSEQ—FDA cleared for diagnostic use
• Within a clinical trial protocol
WHAT DO THE TESTS MEAN FOR PATIENTS?
• Nice to know vs need-to-know information
53
ASCT = autologous stem cell transplant; CR = complete response; FDA = US Food and Drug Administration; MRD = minimal residual disease; VGPR = very good partial response. Munshi N, et al. Blood Adv. 2020;4(23):5988-5999. ClonoSEQ website. Accessed March 15, 20222. https://www.clonoseq.com/. ClonoSEQ® [technical summary]. Seattle, WA. Adaptive Biotechnologies; 2020. Accessed March 23, 2023. https://www.clonoseq.com/technical-summary/.
Total clonal cells/total nucleated cells 1 10-1 10-2 10-3 10-4 10-5 10-6 Collection date Dec 2019 June 2020 Dec 2020 June 2022 Dec 2022
CASE 1

Margaret*
TREATMENT: Dara-VRd





Remember:
✓ Shingles prevention


✓ DVT prophylaxis
✓ Monitor sugars
ASCT: Completed

MAINTENANCE: Clinical trial: SWOG 1803 R-Dara
Response CR after ASCT MRD testing planned every 6 months
ASCT = autologous stem cell transplant; CR = complete response; Dara = daratumumab; DVT = deep vein thrombosis; HIPAA = Health Insurance Portability and Accountability Act; R = lenalidomide; RVd = lenalidomide bortezomib dexamethasone; SWOG = National Cancer Institute–supported research group (formerly Southwest Oncology Group). 54
MM is a cancer of the plasma cells. Active MM, defined by CRAB criteria and/or myeloma-defining events (SLiM), requires treatment.







MGUS and SMM are premalignant conditions associated with multiple myeloma.
The workup for MM includes laboratory blood work, genetic testing (bone marrow biopsy), and imaging for bone involvement.
Disparities exist among patients with MM. Black patients tend to have lower risk disease and can achieve superior outcomes when treated with standard of care. Nurses are important to reducing disparities.
Maintenance treatment is recommended for all patients with MM after induction (usually a triplet) ± ASCT consolidation.
Nurses can support shared decision-making by using the SHARE model and encouraging patients to share their priorities and preferences with the health care team
ASCT = autologous stem cell transplant; CRAB = calcium elevation, renal dysfunction, anemia, bone lesions; MGUS = monoclonal gammopathy of undetermined significance; MGRS = monoclonal gammopathy of renal significance; MM = multiple myeloma; SLiM = ≥60% plasma cells, light-chain ratio ≥100, bone lesions on MRI; SMM = smoldering multiple myeloma. Kyle RA, et al. Mayo Clin Proc. 2003;78:21-33. Greenberg AJ, et al. Blood Cancer J. 2015;4:e271. Baker A, et al. Blood. 2013;12(16):3147-3152. Brigle K, et al. J Adv Pract Oncol. 2022;13(suppl 4):7-14. NCCN Guidelines®. Multiple Myeloma. V3.2023. Accessed March 28, 2023. O’Donnell EK, et al. Blood. 2019;134(suppl 1):3178. O’Donnell EK, et al. Br J Haematol. 2018;182(2):222-230. Gerber L. Nursing. 2018;48(4):55-58.

55
Summary
Relapsed Multiple Myeloma
























CASE 2: Henry*
*HIPAA compliant; not actual patient names.
Charise Gleason, MSN, NP BC, AOCNP
Beth Faiman, PhD, RN, MSN, APRN BC, AOCN, BMTCN, FAAN

HIPAA = Health Insurance Portability and Accountability Act.
International Myeloma Foundation 800 452 CURE (2873) http://myeloma.org
Patients With Multiple Myeloma Are Living Longer Than Ever



57
Rajkumar SV. Am J of Hematology. 2022;97(8):1086-1107. SEER Cancer Stat Facts: Myeloma. Accessed March 19, 2023. https://seer.cancer.gov/statfacts/html/mulmy.html. 0 10 20 30 40 50 60 70 80 90 1975 2000 2014 5year Survival Percentage Year ≈60% LIVE MORE THAN 5 YEARS after their diagnosis Many patients are living 10+ YEARS after their diagnosis!
Particularly those who are younger and/or have standard-risk disease
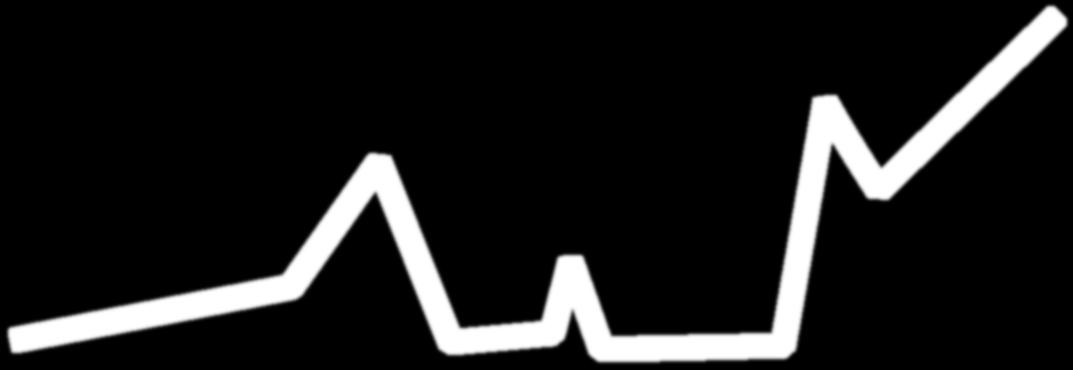
Clonal Evolution: The Relapsing Nature of Multiple Myeloma as Dominant Clones Change Over Time


58
M-protein = monoclonal protein; MGUS = monoclonal gammopathy of undetermined significance; MM = multiple myeloma; SMM = smoldering multiple myeloma. Adapted from Durie B. Keats JJ, et al. Blood. 2012;120(5):1067-1076. Clone 2.2 Dominant MM Clones Change Over Time Clone 2.1 Clone 1.2 Clone 1.1 Misc MProtein g/L 2 5 10 Time First-Line Therapy Second-Line Therapy ≥ Third-Line Therapy
Some Patients (≈17%) Do Not Relapse After Their First Treatment for MM



0 20 40 60 80 100 10 12 14 Percent Surviving Time from diagnosis (years) MM = multiple myeloma; Rx = prescription. Grieb B, et al. ASH 2018. Abstr #1912. 59
Mayo Clinic follow-up of 2125 patients with MM at ≥ 10 years ≥ 5-year remission off Rx ≥ 5-year remission on maintenance ≈ 17% of long-term MM survivors may represent patients “operationally cured”
20% Attrition Rate With Each Line of Therapy

Experts believe that “saving” a therapy for later is not in the patient’s best interest because of attrition at each line of therapy

60 ≈
Benda MA, et al. Cancers (Basel). 2023;15(3):962.
First Line Second Line Fourth Line Fifth Line Sixth Line 19% attrition rate 24% 27% Patients initiating line of therapy Ongoing therapy or still responding 19% Third Line 17%
Survivorship Care Plan: Recommended for Each Survivor and His/Her PCP

National Academy of Medicine recommendation: a survivorship care plan for each survivor

• Record of care
– Diagnosis, including diagnostic tests and results
– Treatments received, total dosage, responses, toxicities
– Other supportive services (psychosocial, etc.)
– Contact information for key providers
– Point of contact for continuing care
• Follow-up plan
–
–
–

Ongoing health maintenance therapy/testing
Recommended screenings
Late/long-term effects of treatments
– Recommendations/resources for healthy behaviors, support, etc.
61
PCP = primary care provider. Institute of Medicine. Cancer Survivorship Care Planning. Fact Sheet Nov 2005. Accessed March 17, 2023. https://apos-society.org/wp-content/uploads/2016/06/factsheetcareplanning.pdf. Salz T, et al. Cancer. 2014;120(5):722-730. Bilotti E, et al. Clin J Oncol Nurs. 2011;15(4 suppl). Kurtin S. In: Tariman JD, et al, eds. Multiple Myeloma: A Textbook for Nurses. 2nd ed. 2015.
How Patients With Myeloma Relapse
Myeloma protein



Psychologically, many patients find their first relapse harder than their initial diagnosis. Nurses are essential to supporting patients!

Asymptomatic Biochemical Relapse
• Sequentially rising myeloma protein or free light chain (>25% increase from low point)
• No other symptoms
• Decisions: if, when, how to treat
Symptomatic
• New, worsening bone pain
• Increasing fatigue, anemia
• Next step: relapse workup; many therapy choices
62
Noonan K, et al. J Adv Pract Oncol. 2022;13(suppl 4):15-21. Faiman B, et al. J Adv Pract Oncol. 2016:7(suppl 1):17-29. Kurtin S, et al. J Adv Pract Oncol. 2016;7(suppl 1):59-70. Gerber L. Nursing. 2018;48(4):55-58.






2
PATIENT
• 64-year-old man diagnosed with MM in 2015 – Standard risk – RVd → ASCT → R maintenance November 2020 • 69 years old, biochemical relapse – Light chains increasing >25% above the lowest point ASCT = autologous stem cell transplant; HIPAA = Health Insurance Portability and Accountability Act; MM = multiple myeloma; R = lenalidomide; RVd = lenalidomide bortezomib dexamethasone. 63 *HIPAA-compliant, not actual patient name, stock photo.
CASE
Henry*
NOTES
Relapse Workup

• CBC + differential + chemistry (metabolic panel)
Albumin


LAB TESTS
• Serum free light chain (FLC) assay
• Serum protein electrophoresis (SPEP)
• Urine protein electrophoresis (UPEP)
CONSIDER BONE MARROW BIOPSY
Cytogenetics and FISH
IMAGING
• PET/CT
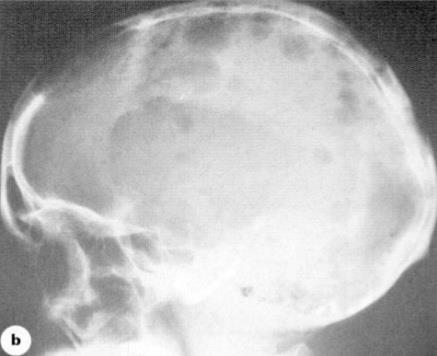
• WBLDCT
• MRI
Imaging type depends on individual’s symptoms and available testing options
CBC = complete blood count; CT = computed tomography; FISH = fluorescence in situ hybridization; FLC = free light chain; MRI = magnetic resonance imaging; PET = positron emission tomography; SPEP = serum protein electrophoresis; UPEP = urine protein electrophoresis; WBLDCT = whole-body low-dose computed tomography. Noonan K, et al. J Adv Pract Oncol. 2022;13(suppl 4):15-21. Rome SI, et al. Clin J Oncol Nurs. 2017;21(5 suppl):47-59. Hillengass J, et. Lancet Oncol. 2019;20(6):e302-e312. Ghobrial IM, et al. Blood. 2014;124:3380-3388. Rajkumar SV, et al. Lancet Oncol. 2014;15:e538-e548. Faiman B. Clin Lymphoma Myeloma Leuk. 2014;14:436-440.
gamma
64
alpha-1
alpha-2 beta
Practical Approach to the Treatment of Patients With Relapsed Myeloma
Disease-related factors

• Duration of response to initial therapy
• High-risk vs low-risk status
• Molecular relapse vs symptomatic relapse
• Other comorbid conditions, patient frailty
Treatment-related factors

• Previous/current therapy exposure and response (relapsed vs refractory)
• Toxicity/tolerability of the previous regimen
• Mode of administration (ie, PO or IV)
• Cost and convenience (out-of-pocket co-pays for IV vs PO)
• Patient preference
IV = intravenous; PO = by mouth. Noonan K, et al. J Adv Pract Oncol. 2022;13(suppl 4):15-21. Faiman B, et al. J Adv Pract Oncol. 2016;2016:7(suppl 1):17-29.
Many Treatment Options at Early Relapse

(1 3 Prior Therapies):
FDA-approved myeloma therapies Common Combinations
Bortezomib (SQ admin) VRd, Vd, VCd
Lenalidomide VRd, Rd
Carfilzomib KRd, Kd, Dara-Kd
Pomalidomidea Pd, Dara-Pd, EPd, PCd




Ixazomib IRd
Daratumumab Dara-Rd, Dara-Vd, Dara-Pd, Dara-VMp, Dara-Kd
Elotuzumab ERd, EPd
Isatuximaba Isa-Pd, Isa-Kd
Selinexor XVd
New agents or regimens in clinical trials are always an option

WATCH FOR
Evolving treatment paradigms: New data are constantly informing best practices
C = cyclophosphamide; d = dexamethasone; Dara = daratumumab; E = elotuzumab; Isa = isatuximab; I = ixazomib; K = carfilzomib; M = melphalan; p = prednisone; P = pomalidomide; R = lenalidomide; SQ = subcutaneous; V = bortezomib; X = selinexor.
a2 prior therapies.
Noonan K, et al. J Adv Pract Oncol. 2022;13(suppl 4):15-21. Steinbach M, et al. J Adv Pract Oncol. 2022;13(suppl 4):23-30. Moreau P, et al. Lancet Oncol. 2021;22(3):e105-e118. O’Donnell EK, et al. Br J Haematol. 2018;182(2):222-230. Devarakonda S, et al. Hematology Am Soc Hematol Educ Program. 2022;2022(1):560-568.
66
Carfilzomib Clinical Pearls
IV proteasome inhibitor

• Active in bortezomib refractory; common agent in regimens for MM, including trials
• Dosing
– Premedication with dexamethasone
– Hydration but not overhydration
– 1st dose @ 20 mg/m2 then escalate
–
Dose-dependent 10-min or 30-min infusion
• Full anticoagulation, especially for patients with high risk of VTE
• Herpesvirus prophylaxis
• Diuretic (furosemide or torsemide) or inhalers if needed
• Know cardiac and pulmonary status
– Optimize heart failure and blood pressure management
• Monitor
– Blood counts
– Response
– Signs of infection
• TIP: Avoid dyspnea over the weekend: start new patients’ first dose early in the week
• Patient education

Kd or Dara-Kd
≥ 1 prior linea 20/70 mg/m2
Once weekly 30-min infusion
Kd, Dara-Kd, K
≥ 1 prior linea 20/56 mg/m2
Twice weekly 30-min infusion
KRd or K
≥ 1 prior linea 20/27 mg/m2
Twice weekly 10-min infusion
a1 to 3 prior lines of therapy for DKd, KRd, or Kd.
Dara = daratumumb; IV = intravenous; K = carfilzomib; Kd = carfilzomib dexamethasone; KRd = carfilzomib lenalidomide dexamethasone; MM = multiple myeloma; Rd = lenalidomide dexamethasone; VTE = venous thromboembolism.
KYPROLIS® (carfilzomib) Prescribing Information. Stewart K, et al. N Engl J Med. 2015;372:142-152.
67
IMPEDE VTE Score Can Assess VTE Risk in Patients With Multiple Myeloma
IMPEDE VTE Score
…the IMPEDE VTE score outperformed the current IMWG guidelines and NCCN Guidelines® and could be considered the new risk stratification standard for VTE in MM



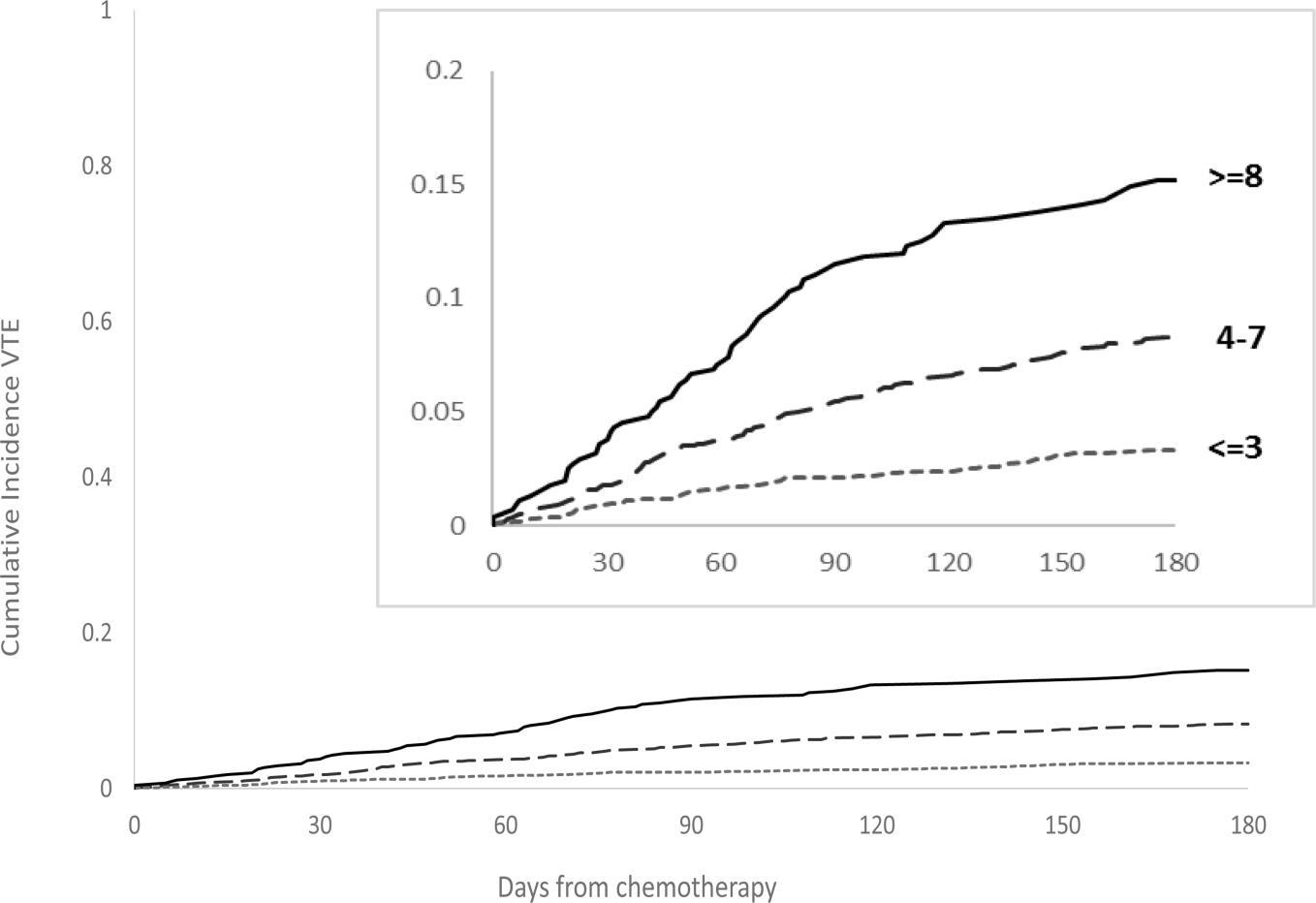

IMWG = International Myeloma Working Group; LWMH = low molecular weight heparin; MM = multiple myeloma; NCCN = National Comprehensive Cancer Network; VTE = venous thromboembolism. Sanfilippo KM, et al. Am J Hematol. 2019;94(11):1176-1184. Cumulative risk of VTE Days post-treatment 68
Predictor Acronym Score Immunomodulatory Drug I 4 Body Mass Index ≥ 25 kg/m2 M 1 Pelvic, Hip or Femur Fracture P 4 Erythropoiesis-stimulating Agent E 1 Doxorubicin D 3 Dexamethasone High-Dose Low-Dose 4 2 Ethnicity/Race = Asian/Pacific Islander E –3 History of Venous Thromboembolism before MM V 5 Tunneled Line Central Venous Catheter T 2 Existing Thromboprophylaxis: Therapeutic LMWH or Warfarin E –4 Existing Thromboprophylaxis: Prophylactic LMWH or Aspirin –3
Consider Full Anticoagulation for Patients on Carfilzomib
Regimens


Tip: rivaroxaban co-pay may be covered by an LLS grant or other cancer-related assistance IF indicated that it is necessary for myeloma treatment
Rivaroxaban may be a more effective antithrombotic agent for patients receiving carfilzomib and lenalidomide

= aspirin; KRd = carfilzomib lenalidomide dexamethasone; LLS = Leukemia & Lymphoma Society; RVd = lenalidomide bortezomib dexamethasone; VTE = venous thromboembolism. Piedra KM, et al. ASH 2019. Abstr #1835. 4.0% 16.5% 2.2% 1.0% 5.2% 2.2% 0% 4% 8% 12% 16% 20% RVd + ASA KRd + ASA KRd + rivaroxaban VTE Bleed
ASA
69
Pomalidomide Clinical Pearls
Oral immunomodulatory agent

• Active in R-refractory patients; common agent in regimens for MM, including trials
• Monitor
–
–
–
Blood counts—neutropenia most frequent grade 3/4 AE
Liver function
Response
• REMS program
• Proactive AE management
• Patient education

–
Oral adherence
– REMS process for refills
–
–
–
DVT prophylaxis
Common AEs: low blood counts, infection, GI
Refrain from smoking (reduces pomalidomide exposure)
– Protect renal health (renal excretion of pomalidomide)
• Hydration
• Avoid NSAIDS, IV contrast, other drugs with renal interactions
Dara-Pd (1-3 prior therapies)
EPd (≥ 2 prior therapies)
Pd (≥ 2 prior therapies)
AE = adverse event; Dara = daratumumab; DVT = deep vein thrombosis; GI = gastrointestinal; IV = intravenous; EPd = elotuzumab pomalidomide dexamethasone; MM = multiple myeloma; NSAID = nonsteroidal anti-inflammatory drug; Pd = pomalidomide dexamethasone; R = lenalidomide; REMS = Risk Evaluation and Mitigation Strategies. POMALYST® (pomalidomide) Prescribing Information. Faiman B, et al. J Adv Pract Oncol. 2016;7:45-52. EMPLICITI™ (elotuzumab) Prescribing Information. DARZALEX® (daratumumab) Prescribing Information.
70
Isatuximab: Anti CD38 Monoclonal Antibody
Safety
• IRR (38%): the most-common AR specific to isatuximab
• Isa-Pd common ARs: cytopenias, IRR, infections, dyspnea, GI ARs
Dosing
• Slower first and second infusions
• Weekly for 4 weeks then every 2 weeks
Clinical pearls
Isa-Kd (1-3 prior therapies)



Isa-Pd (≥ 2 prior therapies: IMiD and PI)
• IRR protection: premedicate with
– Dexamethasone: 40 mg oral or IV (or 20 mg for patients aged ≥ 75 years)
– Acetaminophen: 650 mg to 1000 mg
WEEKS 1-4
–
H2 antagonists
– Diphenhydramine: 25 mg to 50 mg oral or IV; IV preferred for at least the first 4 infusions

• Antibody interference—type and cross BEFORE starting
WEEKLY
Then ongoing EVERY 2 WEEKS (4 doses)
• Discontinue if IRR grade 3
• In combo with pomalidomide + dex: DVT prophylaxis
• Herpes prophylaxis
• No dose adjustments for isatuximab
AR = adverse reaction; dex = dexamethasone; DVT = deep vein thrombosis; GI = gastrointestinal; H = histamine; IMiD = immunomodulatory imide drug; Isa = isatuximab; IRR = infusion-related reaction; Isa-RVd = isatuximab lenalidomide bortezomib dexamethasone; IV = intravenous; Kd = carfilzomib dexamethasone; Pd = pomalidomide dexamethasone; PI = proteasome inhibitor. Wilmoth J, et al. Clin J Oncol Nurs. 2021;25(6):706-712. SARCLISA® (isatuximab) Prescribing Information. Goldschmidt H, et al. ASH 2021. Abstr #463.
71
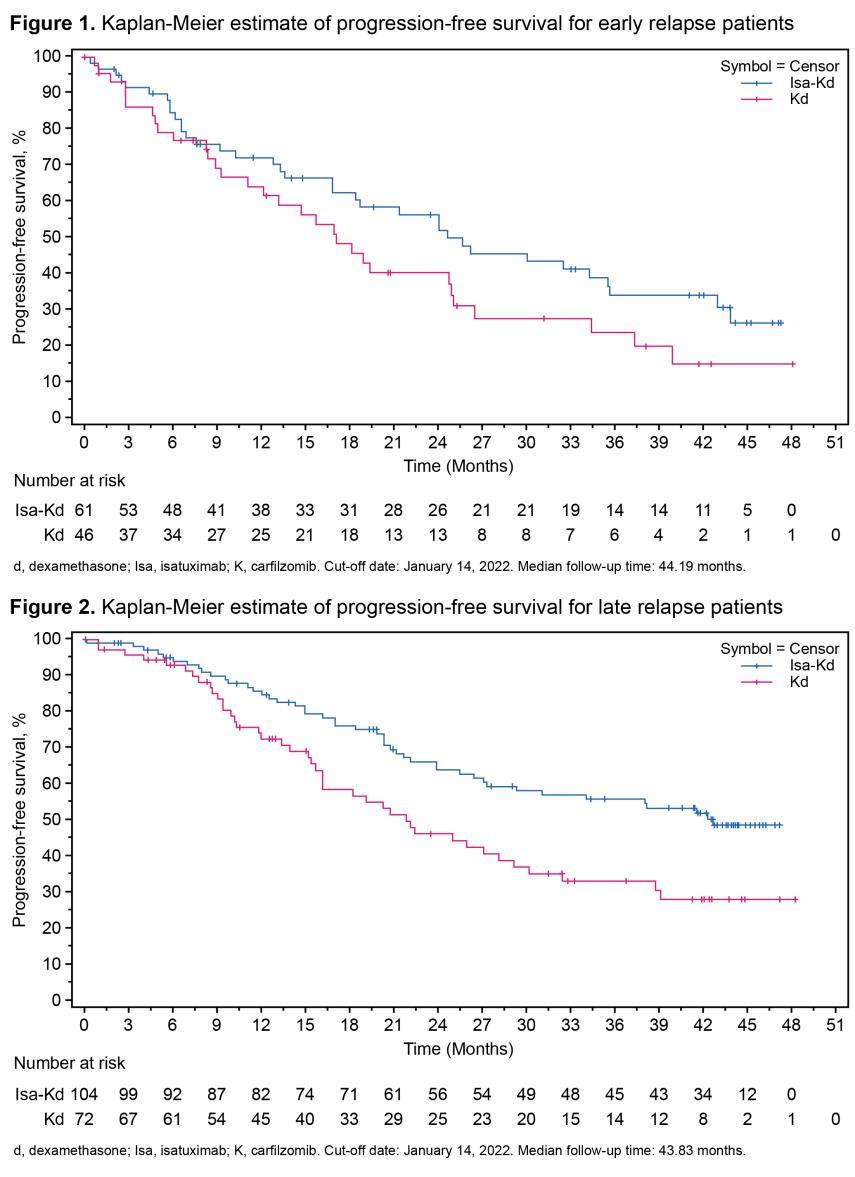
IKEMA Subgroup Analysis: Isa Added to Kd Improved PFS and Depth of Response in Patients With Early and Late Relapse
PFS in patients with early relapse
Study Design
• Patients with R/R MM (1-3 prior lines of therapy) randomized to receive Isa-Kd (n=179) or Kd (n=123)
• Patients defined as early relapse (n=107) if relapse <18 months from initiation of 1st line therapy or <12 months from initiation of 2nd or 3rd line therapy
Conclusions
• Isa added to Kd improved PFS and depth of response, with a manageable safety profile in both early and late relapse patients
• Results support Isa-Kd as a standard of care in patients with R/R MM regardless of early or late relapse
Isa = isatuximab; Kd = carfilzomib dexamethasone; MM = multiple myeloma; PFS = progression-free survival; R/R relapsed/refractory.
PFS in patients with late relapse

72
T, et al.
2022.
Facon
ASH
Abstr #753.
Isa-Kd Kd
Kd
Isa-Kd
ICARIA MM Clinical Trial: Isatuximab Pd Improved ORR and PFS vs
Pd alone
Isatuximab improved ORR: 60.4% Isa-Pd vs 35.3% Pd in patients with R/R MM
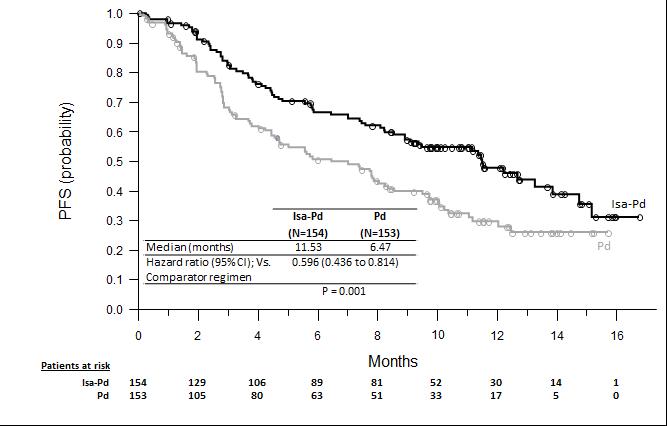

ICARIA-MM Phase 3 Clinical Trial







Significant increase in median PFS demonstrated with isatuximab + Pd vs Pd alone

•
73
Isa = isatuximab; MM = multiple myeloma; ORR = overall response rate; Pd = pomalidomide dexamethasone; PFS = progression-free survival; R/R relapsed/refractory. SARCLISA® (isatuximab) Prescribing Information.
In Pts With NDMM: Isa Added to RVd Improves Response Rate & Depth of Response; Isa KRd Induces Deep Responses in High Risk
GMMG-HD7: phase 3 clinical trial in transplanteligible patients with NDMM
• Isa-RVd (n=331) vs VRd (n=329)
• Followed by ASCT, maintenance (Isa-R or R)
GMMG-Concept Trial: phase 2 clinical trial in high-risk NDMM transplant-eligible patients
• n=153 patients with high-risk NDMM treated with Isa-KRd

MRD-Negative at the End of Induction
Odds Ratio: 1.83 (95% CI 1.34-2.51)
– High-risk defined as del(17p) or t(4;14) or t(14;16) or >3 copies 1q21 in combination with ISS stage 2 or 3 disease
Interim Analysis Results
• 67% MRD-negative (10-5) [3.2% MRD-positive; 24.7% timepoint not reached] in patients with high-risk NDMM treated with Isa-KRd
• Toxicity consistent with expectations
Isa-RVd (n=331) RVd (n=329)






Not assessable/missinga MRD status low: Isa-VRd, 10.6%; VRd, 15.2%
WATCH FOR
Quadruplets with Isa in the first line
ASCT = autologous stem cell transplant; Isa = isatuximab; KRd = carfilzomib lenalidomide dexamethasone; MM = multiple myeloma; MRD = minimal residual disease; NDMM = newly diagnosed multiple myeloma; R = lenalidomide; SAE = serious adverse event; RVd = lenalidomide bortezomib dexamethasone.
aDue either to loss to follow-up, missing bone marrow samples, or technical failures in measurement counted as nonresponders. Goldschmidt H, et al. ASH 2021. Abstr #463. Weisel KC, et al. ASH 2023. Abstr #759.
74
60 50 40 30 20 10 0
.001
P <
Patients (%) 50.1% 35.6%
Elotuzumab: Anti SLAMF 7 Monoclonal Antibody
• IV monoclonal antibody targeting SLAMF-7
• Prescribed with Rd or Pd
–
DVT prophylaxis (for R or P)
– Steroid side effects and schedule (AM vs PM)
• Clinical pearls
–
Prophylaxis for infusion reactions
– Infuse at a rate of 0.5 mL/min and escalate to 5 mL/min over time
–
–
Give weekly for 8 weeks, then twice monthly until progressive disease
Multiple dosing regimens—check prescribing information

• Monitoring
–
Blood counts (hold/adjust the dose if needed)
– Response assessment (monthly); interference
– Glucose (dexamethasone can affect)
– Kidney and liver function
EPd (≥ 2 prior therapies)
ERd (1-3 prior therapies)






WATCH FOR
Clinical trials with elotuzumab in combination with other MM drugs like iberdomide
DVT = deep vein thrombosis; EPd = elotuzumab pomalidomide dexamethasone; ERd = elotuzumab lenalidomide dexamethasone; IV = intravenous; MM = multiple myeloma;
75
P
SLAMF-7
EMPLICITI™ (elotuzumab) Prescribing Information. Gleason C, et al. J Adv Pract Oncol. 2016;7(suppl 1):53-57. Clinical trials.gov website. Accessed March 15, 2023. https://clinicaltrials.gov/.
R = lenalidomide;
= pomalidomide;
= signaling lymphocytic activation molecule 7 (also CD319, CS1).
Special Considerations With Antibody Therapy
• Potential interference with laboratory tests

– Co-migration of therapeutic antibodies with M-protein: overestimation of M-protein and reduced apparent CR rates
• Solutions
– Awareness
– Laboratory assays to minimize effects (eg, high-resolution mass spectrometry)
Daratumumab, elotuzumab, and isatuximab are all IgG antibodies
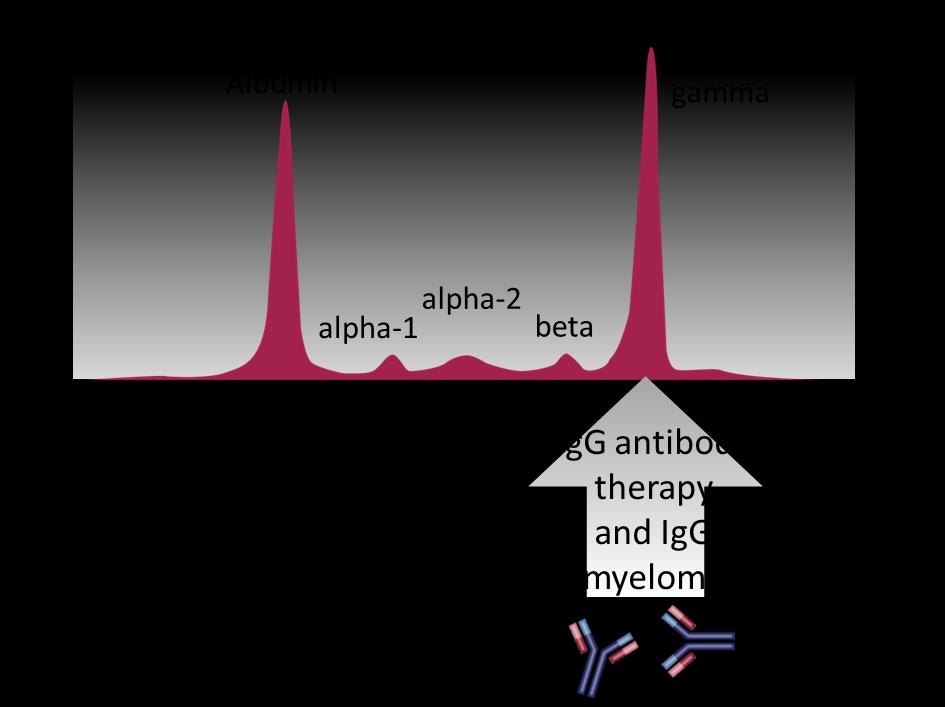
76
CR = complete response; Ig = immunoglobulin; M-protein = monoclonal protein. Mills JR, Murray DL. J Appl Lab Med. 2017;1(4):421-431.
Selinexor: Oral Selective Inhibitor of Nuclear Export (SINE)
• Oral nuclear export inhibitor: blocks tumor cells from exporting tumor suppressor proteins → selective apoptosis of tumor cells
• Clinical pearls
– Patient education

– Proactive AE management is crucial
• Patients must be given 2 antinauseants prophylactically for the management of nausea and anorexia (start ondansetron day 1; adding olanzapine and/or aprepitant)
XVd (≥ 1 prior therapy)
Xd
(≥ 4 prior therapies: refractory to 2 PIs, 2 IMiDs, anti-CD38 mAb)
• Thrombocytopenia and neutropenia (weekly blood counts in cycle 1)
• Hyponatremia (salty snacks, oral hydration)
• Diarrhea (oral hydration)
WATCH FOR New combination regimens with selinexor
AE = adverse event; IMiD = immunomodulatory imide drug; mAb = monoclonal antibody; MM = multiple myeloma; PI = proteasome inhibitor; SINE = selective inhibitor of nuclear export; Xd = selinexor dexamethasone; XVd = selinexor bortezomib dexamethasone.
XPOVIO™ (selinexor) Prescribing Information. Mikhael J, et al. Clin Lymphoma Myeloma Leuk. 2020;20(6):351-357.
77
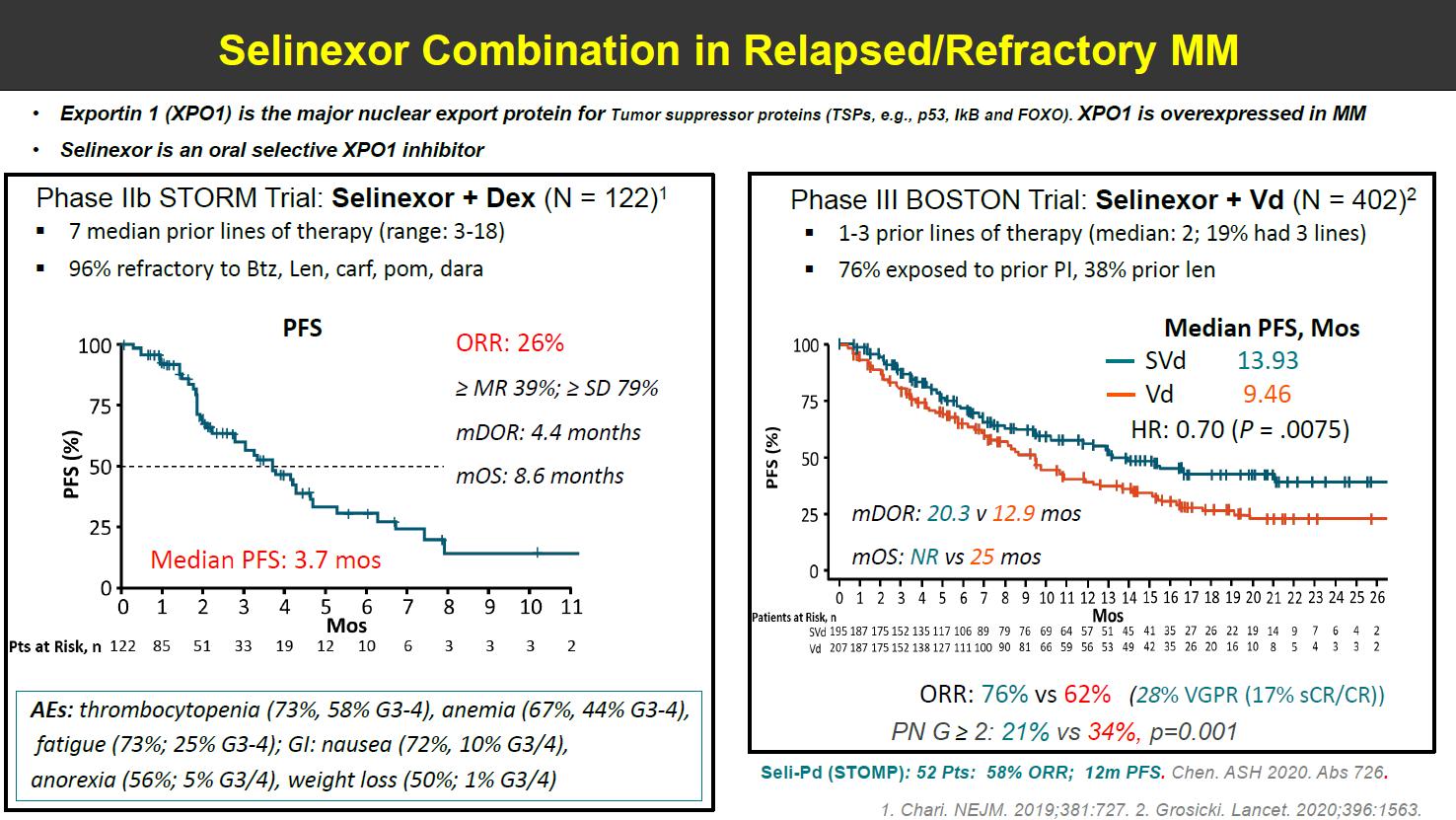
BOSTON Phase 3 Trial: XVd Combination Dosed Weekly
• Patients with MM with 1 to 3 lines of therapy
– 76% exposed to prior PI, 38% prior lenalidomide
• 402 patients: XVd (n=195) vs Vd (n=207)
Conclusions
• Once-weekly XVd significantly improved PFS and ORR compared to twice-weekly Vd – 76% ORR with XVd vs 62% with Vd
• Rates of PN were significantly reduced

– PN grade ≥ 2 was 21% with XVd vs 34% with Vd; P = .001
• Numerically fewer deaths on XVd vs Vd
Patients at Risk, n
months
months
HR = hazard ratio; mDOR = median duration of response; mOS = median overall survival; MM = multiple myeloma; NR = not reached; ORR = overall response rate;
PI = proteasome inhibitor; PN = peripheral neuropathy; PFS = progression-free survival; Vd = bortezomib dexamethasone; XVd = selinexor bortezomib dexamethasone. Dimopoulos MA, et al. ASCO 2020. Abstr #8501. Grosicki S, et al. Lancet. 2020;396(10262):1563-1573.
XVd Vd
Median PFS, Months Months
78
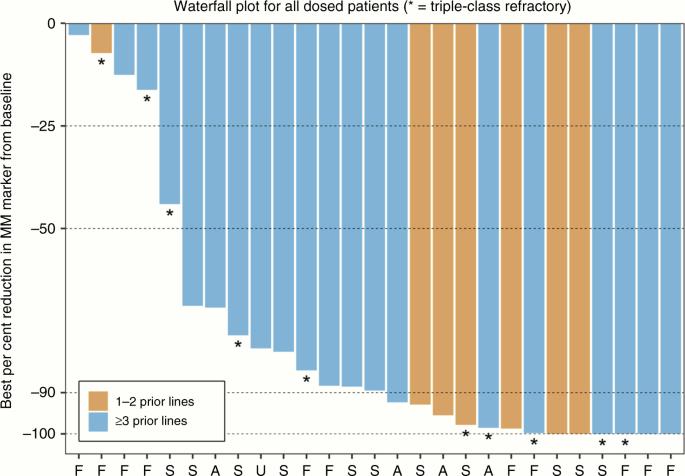
STOMP Trial: Once-Weekly Selinexor Carfilzomib Dexamethasone

STOMP Clinical Trial
• Patients (n=32) with R/R MM (median 4 prior therapies)
• ORR 78% (44% VGPR or better)
• Median PFS: 15 months
STOMP Subset Analysis in Triple-Class
Refractory


• Refractory to IMiDs, PIs, and anti-CD38 mAb
(n=12) – 66.7% of these with high-risk cytogenetics
del(17p), t(4;14), t(14;16), and/or gain 1q
• ORR: 66.7% (95% CI 34.9-90.1)
• mDOR: 12 months
mAb = monoclonal antibody; mDOR = median duration of response; MM = multiple myeloma; ORR = overall response rate; R/R = relapsed refractory; VGPR = very good partial response;
PFS = progression-free survival.
Gasparetto C, et al. Br J Cancer. 2022;126:718-725. Schiller GJ, et al. ASH 2022. Abstr #652.
79
Articles That Have Selinexor Use Guidance: Supportive Care and Monitoring



Published June 2020 in Clinical Lymphoma, Myeloma & Leukemia
Published July 2022 in
Lymphoma, Myeloma & Leukemia

80
Mikhael J, et al. Clin Lymphoma Myeloma Leuk. 2020;20(6):351-357. Nooka AK, et al. Clin Lymphoma Myeloma Leuk. 2022:22(7):e526-e531.
Selinexor: White Paper on Best Practices for Optimizing Treatment—Discussion With IMF Nurse Leadership Board


https://www.myeloma.org/ nlb-publications

Upfront discussions with patients should be individualized and include:
• Description of the unique MOA



• Oral route of administration and availability of all-oral regimens
• Toxicity profile (including potential GI AEs)
• Proactive strategies for managing AEs
• Requirement for potential prophylactic medications to prevent/mitigate/manage toxicities
• Set expectations for potential dosing changes based on tolerability
Patient support programs can help address patient-specific concerns or issues, such as insurance coverage, financial assistance, discussion of medications and/or AEs, and care coordination
81
AE = adverse event; GI = gastrointestinal; IMF = International Myeloma Foundation; MM = multiple myeloma; MOA = mechanism of action. IMF Nurse Leadership Board White Paper: Selinexor: Best Practices for Optimizing Treatment White Paper—Discussion With IMF Nurse Leadership Board. Accessed April 2, 2022. https://www.myeloma.org/nlb-publications.
Ixazomib: Oral Proteasome Inhibitor
• Oral proteasome inhibitor
– Indication: patients with MM who have received at least one prior therapy
– In combination with Rd
• Administration
– Oral capsule 1 × per week; do not crush, chew, or open the capsule
– Empty stomach: 1 hour before or 2 hours after food
• Clinical pearls
– Adherence, schedule, viral prophylaxis

– Rapid response (1.1 months); fast absorption (if vomit, do NOT repeat dose)
– Monitor blood counts: cyclic thrombocytopenia
–
Peripheral neuropathy, peripheral edema
– In combination with Rd, so DVT prophylaxis
Ixazomib + Rd (≥ 1 prior therapy)

All-oral regimen
WATCH FOR
• New regimens
• Clinical trials – IR in SMM – IPd in MM – Others
82
deep vein thrombosis; IR = ixazomib lenalidomide; IPd = ixazomib pomalidomide dexamethasone; MM = multiple myeloma; Rd = lenalidomide low-dose dexamethasone; SMM = smoldering MM. NINLARO® (ixazomib) Prescribing Information. Faiman B, et al. J Adv Pract Oncol. 2016;7:45-52. Clinical trials.gov.
DVT =
CASE 2



*HIPAA-compliant, not actual patient name, stock photo.
Henry*
PATIENT NOTES
• 69-year-old, biochemical relapse on R maintenance



November 2020
April 2021
• Increased anemia
• Shared decision-making
→ Isa-Kd
July 2021
• 90% reduction in light chains (VGPR)
83 Isa = isatuximab; Kd = carfilzomib dexamethasone; HIPAA = Health Insurance Portability and Accountability Act; MM = multiple myeloma; R = lenalidomide; VGPR very good partial response.
Henry*
PATIENT NOTES
• Now 71 years old


November 2022
• Symptomatic MM relapse (anemia, new bone lesions)
• FISH: t(11;14)
• Shared decision-making: Clinical trial
(CANOVA) venetoclax-d vs Pd



Provide tools and resources to enhance decisionmaking


84
CASE 2
*HIPAA-compliant, not actual patient name, stock photo. d = dexamethasone; FISH = fluorescence in situ hybridization; HIPAA = Health Insurance Portability and Accountability Act; MM = multiple myeloma; Pd = pomalidomide dexamethasone.
Venetoclax Clinical Pearls
• Venetoclax is a BCL-2 inhibitor currently FDA-approved for CLL and AML (off-label for MM)
• Deep responses in patients with MM, but increased infections, including serious infections, death

• Efficacy in patients with MM with t(11;14) or high expression of BCL2
• Plasma cell enrichment improves testing sensitivity
• AEs associated with venetoclax in other indications (eg, TLS) may not be like AEs in patients with MM
• Expanded access program provides free drug if insurance denies coverage to patients with MM
• Clinical trials are available
TO DO
Check that your lab does plasma cell enrichment or cytoplasmic immunoglobulinenhanced FISH, as recommended by the College of American Pathologists
WATCH FOR FDA approval in MM
CANOVA Phase 3 trial data
AE = adverse event; AML = acute myeloid leukemia; BCL-2 and BCL2 = B-cell lymphoma 2; CLL = chronic lymphocytic leukemia; FDA = US Food and Drug Administration; FISH = fluorescence in situ hybridization; MM = multiple myeloma; TLS = tumor lysis syndrome.

85
VENCLEXTA® (venetoclax) Prescribing Information. Kumar S, et al. ASCO 2020. Abstr #8509. Kumar SK, et al. Lancet Oncology. 2020;21(12):1630-1642.
D,
Basali
et al. Br J Haematol. 2020;189(6):1136-1140. Faiman B, Gleason C clinical experience. Read L. Enriching Our Understanding of Multiple Myeloma. Accessed March 29, 2023. https://thepathologist.com/diagnostics/enriching-our-understanding-of-multiple-myeloma.
BELLINI Phase 3 Clinical Trial: Vd ± Venetoclax
Design




• 291 patients with MM, 1 to 3 prior therapies
• Patients treated with Vd + Ven (n=194) or Vd (n=97)
Results for t(11;14) subset
(per investigator, with a median follow-up: 45.6 months)
Conclusions:
• Consistent with previous analysis, for Vd + Ven continues to reflect a favorable benefit-risk profile in patients with t(11;14) or high BCL-2 expression vs Vd – Higher
• Common AEs (all grades): diarrhea, nausea, constipation, fatigue

• Grade 3 AEs: hematologic events, pneumonia, diarrhea
• Increased infections and risk of death with venetoclax
*Reported per investigator with a median follow-up: 22.7 months.
Vd + Ven shows favorable results for patients with t(11;14) or high BCL-2 expression but is not appropriate for other patients with MM
AE = adverse event; BCL-2 = B-cell lymphoma; HR = hazard ratio; MM = multiple myeloma; MRD = minimal residual disease; NR = not reached; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; Vd = bortezomib dexamethasone; Ven = venetoclax.
Abstr
86
Vd + Ven Vd HR (95% CI) Median PFS t(11;14) 36.8 mo 9.3 mo 0.12 (0.03-0.44) OS NR NR 0.61 (0.16-2.32) ORR* 95% 47% Not reported MRD < 10-5* 25% 0% Not reported
PFS – Higher ORR – Deeper responses
Kumar S.
ASH
ASH 2021.
#653. Moreau P, et al.
2019. Abstr #1888. Kumar S, et al. ASCO 2020. Abstr #8509.
MM clones evolve over time and can become resistant to therapy. The relapse workup for MM includes laboratory blood work, genetic testing (FISH of bone marrow biopsy), and imaging for bone involvement and/or extramedullary disease.
There are many options for treating patients with relapsed MM; many considerations for treatment (eg, prior therapies, patient preference→ provide tools and resources to aid decision-making).
Anticoagulation is important for patients receiving certain myeloma therapies, including IMiD-, doxorubicin-, or carfilzomib-containing regimens.

Isatuximab is an anti-CD38 monoclonal antibody approved in combination with Kd or Pd; additional combination regimens are being explored in clinical trials.

Selinexor is a novel selective nuclear export (SINE) inhibitor approved for patients with ≥ 1 prior therapy, in combination with Vd; additional combination regimens are being explored in clinical trials.
Venetoclax is a BCL-2 inhibitor that is an appropriate treatment (off-label) for patients with MM containing t(11;14); deep responses, risk of infection.
= bortezomib dexamethasone.








87 Summary
BCL-2 = B-cell lymphoma 2; IMiD = immunomodulatory imide drug; FISH = fluorescence in situ hybridization; Kd = carfilzomib dexamethasone; MM = multiple myeloma; Pd = pomalidomide dexamethasone; SINE = selective nuclear export inhibitor; Vd
J, et al. Lancet Oncol. 2019;20(6):e302-e312. Ghobrial IM, et al. Blood. 2014;124:3380-3388. Rajkumar SV, et al. Lancet Oncol. 2014;15:e538-3548. Faiman B, et al. J Adv Pract Oncol. 2016;2016:7(suppl 1):17-29. NCCN Guidelines. Multiple Myeloma. V3.2023. Accessed March 19, 2021. SARCLISA® (isatuximab) Prescribing Information. XPOVIO™ (selinexor) Prescribing Information. Mikhael J, et al. Clin Lymphoma Myeloma Leuk
2020;20(6):351-357. VENCLEXTA®
Prescribing Information. Moreau P, et al. ASH 2019. Abstr #1888. Kumar S, et al. ASCO 2020. Abstr #8509.
Keats JJ, et al. Blood. 2012;120(5):1067-1076. Hillengass
.
(venetoclax)
Immunotherapies and Emerging Therapies



























CASE 4: Maria*
CASE 5: Paul*
*HIPAA compliant; not actual patient names.
Tiffany Richards, PhD, ANP BC
Beth Faiman, PhD, RN, MSN, APRN BC, AOCN, BMTCN, FAAN

HIPAA = Health Insurance Portability and Accountability Act. International Myeloma Foundation 800 452 CURE (2873) http://myeloma.org
BCMA Is a Target for Multiple Myeloma Therapy






BCMA (B-Cell Maturation Antigen)


• Member of TNF receptor superfamily







• BCMA is expressed on late memory B cells committed to PC differentiation and PCs
• BCMA plays a role in survival of long-lived PCs

• BCMA is expressed more abundantly on malignant PCs than on normal ones
89
BCMA = B-cell maturation antigen; MM = multiple myeloma; PC = plasma cell; TNF = tumor necrosis factor. Shah N, et al. Leukemia. 2020;34(4):985-1005. Yu B, et al. J Hematol Oncol. 2020;13:125.
BCMA MM cell
Mechanism of Action for CAR T and Bispecific Antibodies: -Cell Immune Activity Triggered Killing of Myeloma Cells
Car T cells
Viral vector



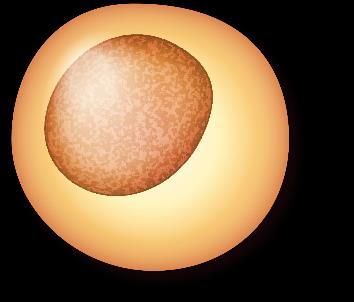
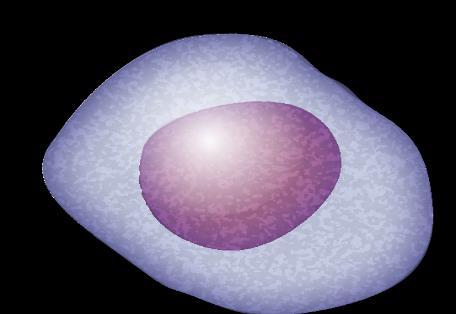
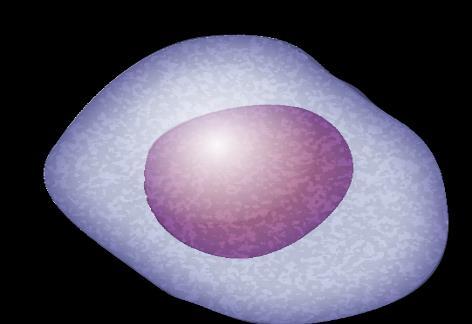



Bispecific Antibodies
Cytotoxic cytokines
T cell BCMA
BCMA is one target for bispecific antibodies
Cytotoxic cytokines

CD3

Bispecific antibody





MM cell death
MM cell death
BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; MM = multiple myeloma; scFV = single chain fragment variable. Shah N, et al. Leukemia. 2020;34(4):985-1005. Yu B, et al. J Hematol Oncol. 2020;13:125. 90
T cell MM
cell
Immune Activity Specific Side Effects
Cytokine Release Syndrome (CRS)




Immune effector cell–associated neurotoxicity syndrome (ICANS)
Neuro Toxicity

91
CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome.
CRS Severity Ranges From Mild to Life Threatening: Early Recognition and Treatment
RESPIRATORY
Hypoxia
Dyspnea
Capillary leak syndrome
Delirium
Somnolence




Dysphagia
Monitoring for CRS
• Vital signs (temperature, O2 saturation, etc.)
Hyperbilirubinemia













creatinine
Kidney insufficiency
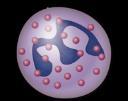
Anemia
HEMATOLOGIC Fever
Thrombocytopenia
Neutropenia
CONSTITUTIONAL
Fatigue, malaise
Headache
Sinus tachycardia
Hypotension
Arrhythmias
• Review of systems and physical exam
Nausea
Vomiting
Diarrhea
– Focus on cardiovascular, pulmonary, and neurologic systems

• Rule out infection
• Laboratory monitoring
–
Myalgia
Weakness
CRP
– Cytokines
–
–
Ferritin

LDH
ALP = alkaline phosphatase; CPK = creatine phosphokinase; CRP = C-reactive protein; CRS = cytokine release syndrome; LDH = lactate dehydrogenase; O2 = oxygen. Oluwole
CPK
92
OO,
ML. J Leukoc Biol
2016;100:1265-1272.
Science
JN.
et al.
DW, et al.
Davila
.
June CH, et al.
. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer
Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen,
J Immunother Cancer. 2018;6:56. Lee
Biol Blood Marrow Transplant. 2019;25:625-638.
MUSCULOSKELETAL GASTROINTESTINAL NEUROLOGIC CARDIOVASCULAR Serum
KIDNEY
Transaminitis ALP
LIVER
ASTCT CRS Consensus Grading
Hypotension None
Not requiring vasopressors
Requiring a vasopressor with or without vasopressin
Requiring multiple vasopressors (excluding vasopressin)


Hypoxia None
Requiring low-flow nasal cannulac or blow-by

Requiring high-flow nasal cannula,c facemask, nonrebreather mask, or Venturi mask
Requiring positive pressure (eg, CPAP, BiPAP, intubation and mechanical ventilation)
Organ toxicities associated with CRS may be graded according to CTCAE v5.0 but not influence CRS grading. aFever is defined as temperature ≥38°C not attributable to any other cause. In patients who have CRS and receive antipyretic or anti-cytokine therapy such as tocilizumab or steroids, fever is no longer required to grade subsequent CRS severity. In this case, CRS grading is driven by hypotension and/or hypoxia. bCRS grade is determined by the more severe event: hypotension or hypoxia not attributable to any other cause. For example, a patient with a temperature of 39.5°C, hypotension requiring one vasopressor, and hypoxia requiring low-flow nasal cannula is classified as grade 3 CRS. cLow-flow nasal cannula is defined as oxygen delivered at ≤6 L/minute. Low flow also includes blow-by oxygen delivery, sometimes used in pediatrics. High-flow nasal cannula is defined as oxygen delivered at >6 L/minute.
93
ASTCT = American Society for Transplant and Cellular Therapy; BiPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; CRS = cytokine release syndrome. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638. CRS Parameter Grade 1 Grade 2 Grade 3 Grade 4 Fevera Temperature ≥38°C Temperature ≥38°C Temperature ≥38°C Temperature ≥38°C With
And/orb
Managing CRS Is Institution Specific: Example Guideline

Grade Tocilizumab

1
2-3
4 (ICU/ critical care required)
Tocilizumab: Onset ≥ 72 h after infusion, treat symptomatically; onset < 72 h after infusion, consider tocilizumab
8 mg/kg IV over 1 h
Corticosteroids: Consider dexamethasone 10 mg IV every 24 h
Tocilizumab: 8 mg/kg IV over 1 h, repeat every 8 h as needed if not responsive to IV fluids or supplemental
Corticosteroids: Dexamethasone 10 mg IV every 12 h to 24 h
If no improvement in 24 h or rapid progression, repeat tocilizumab and escalate to dexamethasone 20 mg IV every 6 h to 12 h
If no improvement in 24 h or continued rapid progression, repeat tocilizumab and switch to methylprednisolone
2 mg/kg followed by 2 mg/kg divided 4 times/d
Tocilizumab: 8 mg/kg IV over 1 h, repeat every 8 h as needed if not responsive to IV fluids or supplemental
Corticosteroids: Dexamethasone 20 mg IV every 6 h
If no improvement in 24 h consider methylprednisolone (1 g to 2 g, repeat every 24 h if needed; taper as clinically indicated) or other anti–T-cell therapies
After two doses of tocilizumab, consider alternative anti-cytokine agents; do not exceed three doses of tocilizumab in 24 h, or four doses total
•
94
CRS = cytokine release syndrome; ICU = intensive care unit; IV = intravenous; O2 = oxygen. ABECMA™ (idecabtagene vicleucel) Prescribing Information. CARVYKTI™ (ciltacabtagene autoleucel) Prescribing Information.
O2
O2
Immune Activity Specific Side Effects
Cytokine Release Syndrome (CRS)




Immune effector cell–associated neurotoxicity syndrome (ICANS)

Neuro Toxicity

95
CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome.
Neurotoxicity: Rare but Potentially Serious AE
Hallucinations Altered wakefulness
Ataxia
Monitoring for immune effector cell–associated neurotoxicity syndrome (ICANS)
• ICE Screening Tool
Apraxia
• Review of systems and physical exam
– Focus on neurologic systems
• Rule out infection
Confusion
Neurotoxicity
Facial nerve palsy
• If ICANS suspected
– Neuroimaging (ideally MRI)
– Diagnostic lumbar puncture for opening pressure and infection tests
Headache
Encephalopathy
Seizures
Tremors
• Corticosteroids are typically indicated for ICANS ≥ grade 2
• Patient and care partner information

AE = adverse effect; CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome; ; ICE = Immune Effector Cell Encephalopathy; MRI = magnetic resonance imaging.
Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.
96
ICE Screening Tool for Neurological Assessment
Assessment Points


Naming
Ability to name 3 objects (eg, point to clock, pen, button)
Following commands
Ability to follow simple commands (eg, “Show me 2 fingers” or “Close your eyes and stick out your tongue”)
Writing
Ability to write a standard sentence (eg, “Our national bird is the bald eagle”)

Attention
Total Points
Ability to count backwards from 100 by 10
97
ICANS = immune effector cell–associated neurotoxicity syndrome; ICE = Immune Effector Cell Encephalopathy. Santomasso B, et al. Am Soc Clin Oncol Educ Book. 2019;39:433-444. Lee DW, et al. Biol Blood Marrow Transplant. 2018;15:47-62.
4
Orientation Orientation to year, month, city, hospital
3
1
1
1
10 Scoring 10 No impairment 7-9 Grade 1
3-6 Grade 2 ICANS 0-2 Grade 3 ICANS 0 due to patient unarousable and unable to perform ICE assessment
ICANS
Grade 4 ICANS
ASTCT ICANS Consensus Grading for Adults
Depressed level of consciousnessb Awakens spontaneously Awakens to voice Awakens only to tactile stimulus
Any clinical seizure focal or generalized that resolves rapidly or nonconvulsive seizures on EEG that resolve with intervention
Patient is unarousable or requires vigorous or repetitive tactile stimuli to arouse. Stupor or coma

Life-threatening prolonged seizure (>5 min); or repetitive clinical or electrical seizures without return to baseline in between

Elevated ICP/cerebral edema
Focal/local edema on neuroimagingd
Deep focal motor weakness such as hemiparesis or paraparesis
Diffuse cerebral edema on neuroimaging; decerebrate or decorticate posturing; or cranial nerve VI palsy; or papilledema; or Cushing’s triad
ASTCT ICANS Consensus Grading for Adults. ICANS grade is determined by the most severe event (ICE score, level of consciousness, seizure, motor findings, raised ICP/cerebral edema) not attributable to any other cause; for example, a patient with an ICE score of 3 who has a generalized seizure is classified as grade 3 ICANS.
aA patient with an ICE score of 0 may be classified as grade 3 ICANS if awake with global aphasia, but a patient with an ICE score of 0 may be classified as grade 4 ICANS if unarousable. bDepressed level of consciousness should be attributable to no other cause (eg, no sedating medication).cTremors and myoclonus associated with immune effector cell therapies may be graded according to CTCAE v5.0, but they do not influence ICANS grading. dIntracranial hemorrhage with or without associated edema is not considered a neurotoxicity feature and is excluded from ICANS grading. It may be graded according to CTCAE v5.0.
ASTCT = American Society for Transplant and Cellular Therapy; CTCAE = Common Terminology Criteria for Adverse Events; EEG = electroencephalogram; ICANS = immune effector cell–
associated neurotoxicity syndrome; ICE = Immune Effector Cell Encephalopathy; ICP = increased intracranial pressure; N/A = not applicable. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638.
Neurotoxicity Domain Grade 1 Grade 2 Grade 3 Grade 4 ICE screening tool scorea 7-9 3-6 0-2
(patient is unarousable; unable
ICE)
0
to perform
N/A N/A
Seizure
Motor findingsc N/A N/A N/A
N/A
N/A
98
CASE 4
Maria*
PATIENT NOTES
67-year-old woman diagnosed with IgA lambda MM, standard risk in 2009


• Rd then ASCT with CR—declined maintenance

• Bortezomib + dex—VGPR





TREATMENT:
• Elo + pom + dex—VGPR
• Carfilzomib + dex—CR
• Daratumumab + bortezomib + dex—VGPR
April 2022: Interested in CAR T-cell therapy
*HIPAA-compliant, not actual patient name, stock photo.
Provide tools and resources to enhance decisionmaking

99 ASCT = autologous stem cell transplant; CAR = chimeric antigen receptor; CR = complete response;
Health Insurance Portability
Accountability Act; IgA
A;
dex = dexamethasone; Elo = elotuzumab; HIPAA =
and
= immunoglobulin
MM = multiple myeloma; pom = pomalidomide; Rd = lenalidomide dexamethasone; VGPR = very good partial response.
CAR T-Cell Therapy: A New Treatment Approach
Patient education and REFERRAL

Refer patients to CAR T-cell therapy center early: Slots are limited
Patient education and screening
Confirm patient added to list for CAR T-cell slot
Bridging and lymphodepletion Apheresis
Infusion and inpatient monitoring
Post-discharge monitoring












Most centers require several screening steps, including financial consultation
Allocation of slots is institution specific— UNDERSTAND their process to help your patients navigate
CAR T-cell therapy takes a couple of months once a patient receives a CAR T-cell therapy slot!
• Extensive care partner support is typically required (especially for month post CAR T-cell infusion)
• Patients usually need to remain in proximity to the CAR T-cell therapy center for ~4 weeks after CAR T-cell infusion
• Patients should not drive for ~2 months after CAR T-cell infusion
At CAR T-Cell Therapy Center RETURN to care at home center
CAR = chimeric antigen receptor. Catamero D, et al. J Adv Pract Oncol. 2022;13(suppl 4):31-43. Teoh PJ, Chng WJ. Blood Cancer J. 2021;11(4):84. Shah UA, Mailankody S. BMJ. 2020;370:m3176. 100
Ethical Issues With CAR T Cell Therapy Slot Allocation

Published April 2023 in Transplantation and Cellular Therapy
Published April 2023 in Transplantation and Cellular Therapy

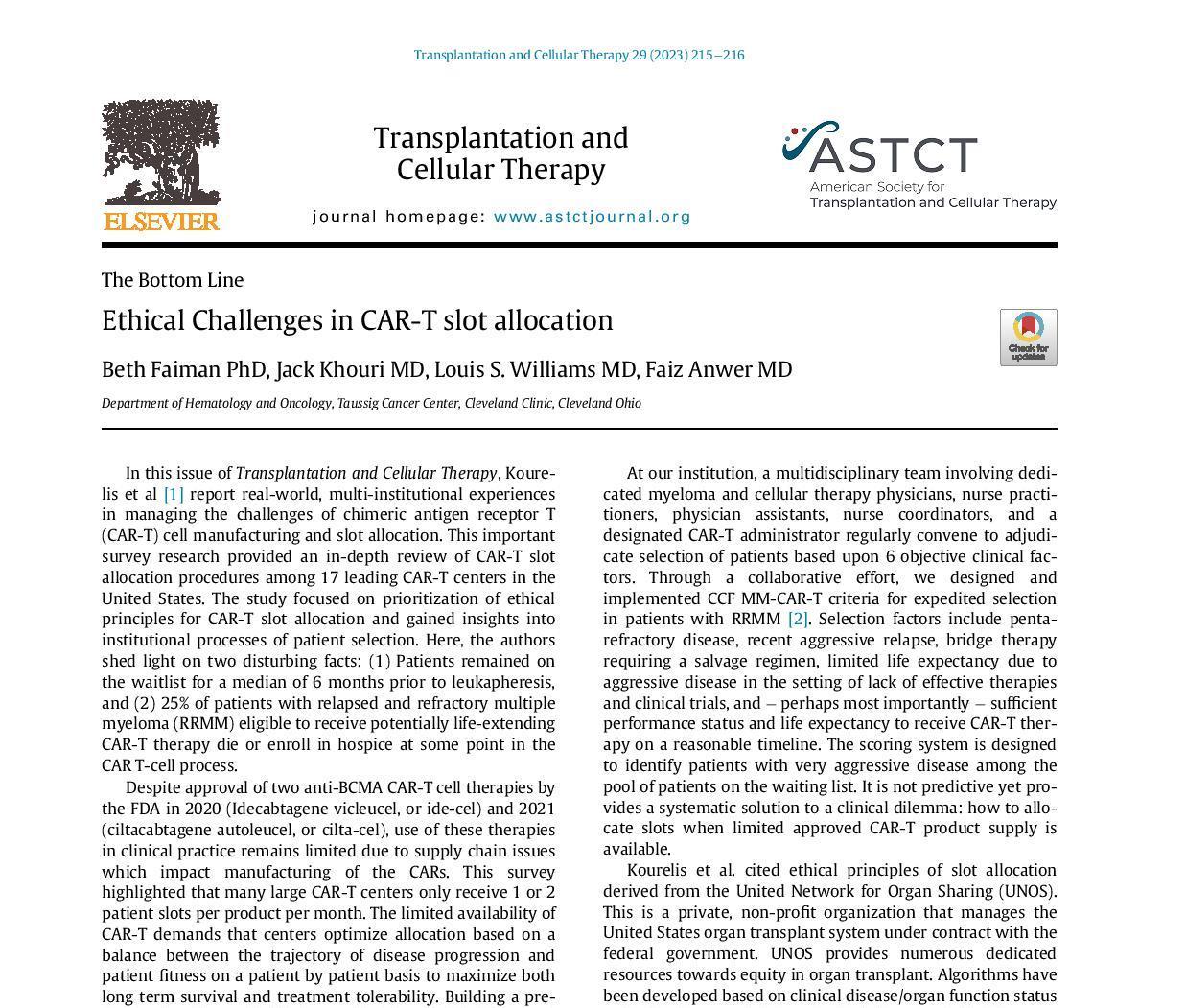



101
et al.
CAR = chimeric antigen receptor. Faiman B,
Transplant Cell Ther. 2023;29(4):215-216. Kourelis T, et al. Transplant Cell Ther. 2023;29(4):255-258.
Idecabtagene Vicleucel (Ide cel)
• CAR T-cell therapy targeting BCMA
• ORR = 72%; median DoR = 11 months
• Offered only in qualified centers (get on list)
• REMS program
• CRS
– Median time to onset: 1 day (range: 1-23)
– Median duration: 7 days (range 1-63)
–
CRS rates: 46% grade 1 30% grade 2
• Neurotoxicity
– Median time to onset: 2 days (range: 1-42)
– Median duration: 5 days (range 1-61)
–
NT rates: 17% grade 1 8% grade 2 4% grade 3 (No grade 4 or 5)

• Hemophagocytic lymphohistiocytosis (HLH); macrophage activation syndrome (MAS)
• Prolonged cytopenias




R/R MM after ≥ 4 lines of therapy (including a PI, an IMiD, and an anti-CD38 mAb) March 2021
WATCH FOR
• Real-world experience data
• Clinical trials in earlier lines of treatment
• Data on optimal therapy sequencing
BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; CRS = cytokine release syndrome; DoR = duration of response; HLH = hemophagocytic lymphohistiocytosis; ide-cel = idecabtagene vicleucel; IMiD = immunomodulatory imide drug; mAb = monoclonal antibody; MAS = macrophage activation syndrome; MM = multiple myeloma; NT = neurotoxicity; ORR = overall response rate; PI = proteasome inhibitor; REMS = risk evaluation and mitigation strategy; R/R = relapsed/refractory.
102
(idecabtagene vicleucel) Prescribing Information. Canonico D, et al. Poster presented at: 19th International Myeloma Society Annual Meeting 2022. ABECMA® website Accessed March 21.2023. https://www.abecmahcp.com/safety.
ABECMA®
Common Manifestations of CRS with Ide-cel (n=127) Pyrexia 98% Hypotension 41% Tachycardia 35% Chills 31% Hypoxia 20% Fatigue 12% Headache 10%
KarMMa 3: Ide-cel Improved PFS and OS vs Standard of Care in Patients With R/R MM (1 3 Prior Lines of Therapy)
KarMMa-3 Phase 3 Clinical Trial
• 386 patients with R/R MM (ide-cel n=254; standard regimen (5 options) n=132)
– 66% triple-class refractory
– 95% daratumumab refractory











Among those treated with ide-cel (n=225)
• 88% any-grade CRS (5% grade ≥3)
• 15% any-grade neurotoxicity (3% grade ≥3)
AE, adverse event; CRS = cytokine release syndrome; ide-cel = idecabtagene vicleucel; MM = multiple myeloma; OS = overall survival; ORR = overall response rate; PFS = progression-free survival; R/R = relpased/refractory; SoC = standard of care. Rodriguez-Otero P, et al. N Engl J Med. 2023;388:1002-1014.

Results 71 42 0 10 20 30 40 50 60 70 80 90 100 Ide-cel SoC ORR 103
13.3 4.4 0 2 4 6 8 10 12 14 16 Ide-cel SoC PFS Months HR = 0.49 95% CI 0.38-0.65 P < 0.001 93 42 0 10 20 30 40 50 60 70 80 90 100 Ide-cel SoC AEs Grade ≥3 Percent P < 0.001
Percent P < 0.001
KarMMa 2 Cohort 2a: Ide cel in Patients With MM With Early Relapse After Frontline ASCT
KarMMa Phase 2 Cohort 2a:
• Patients with high-risk MM (n=37)
– Progressive disease within 18 months of frontline ASCT
• Median follow-up 21.5 months (range 2-31)
• Lower AE rate in cohort 2a than in patients receiving ide-cel in later lines
Deep and durable responses in patients with high-risk MM

• ORR: 83.8%
• Median DoR: 15.7 months (range 7.62-19.81)
• Median PFS: 11.4 months (range 5.6-19.6)
• Median OS: Not reached







– 24-month OS rate: 84.7% (SE 6.31)
AE, adverse event; ASCT = autologous stem cell transplant; CR = complete response; CRS = cytokine release syndrome; DoR = duration of response; ide-cel = idecabtagene vicleucel; MM = multiple myeloma; OS = overall survival; ORR = overall response rate; PFS = progression-free survival; PR = partial response; sCR = stringent complete response; VGPR = very good partial response.
et al. ASH
Usmani S,
2022. Abstr #361.
104
0 20 40 60 80 100 Ide-cel ORR Patients (%) VGPR CR 83.8% sCR 45.9% ≥CR PR
Ciltacabtagene Autoleucel (Cilta cel)



• CAR T-cell therapy with 2 BCMA-targeting domains
• ORR = 97.9%; DoR = 21.8 months
• Offered only in qualified centers (get on list)
• REMS program
• CRS
– Median time to onset: 7 days (range: 1-12)
– Median duration: 4 days (range 1-40 days with 1 patient extending out to 97 days)
– CRS rates: 95% any grade

4% grade 3-4 1% grade
• ICANS
– Median time to onset: 8 days (range: 1-28)
– Median duration: 7.5 days (range: 2-927)
– Resolution in 77% (17/22); median time 6 days (range: 2-143)
– ICANS rates: 23% any grade
3% grade 3-4
2% grade 5
• Hemophagocytic lympohistiocytosis (HLH); macrophage activation syndrome (MAS)
• Prolonged cytopenias
R/R MM after ≥ 4 lines of therapy (including a PI, an IMiD, and an anti-CD38 mAb)
February 2022
WATCH FOR
• CARTITUDE-4 Phase 3 data
• Clinical trials in earlier lines of treatment
• Data on optimal therapy sequencing
BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; cilta-cel = ciltacabtagene autoleucel; CRS = cytokine release syndrome; DoR = duration of response; HLH = hemophagocytic lymphohistiocytosis; ICANS = immune effector cell–associated neurotoxicity syndrome; IMiD = immunomodulatory imide drug; mAb = monoclonal antibody; MAS = macrophage activation syndrome; MM = multiple myeloma; ORR = overall response rate; PI = proteasome inhibitor; REMS = risk evaluation and mitigation strategy; R/R = relapsed/refractory. CARVYKTI™ (ciltacabtagene autoleucel) Prescribing Information. CARVYKTI™ website. Accessed March 21, 2023. https://www.carvyktihcp.com/safety.
105
CARTITUDE 2 Cohort B: Cilta cel in Patients With MM With Early Relapse After Initial Therapy
CARTITUDE-2 Phase 2 Cohort B:

• Patients with high-risk MM (n=19)
– Relapse ≤12 months after ASCT or ≤12 months after start of initial treatment (no ASCT)
• Median follow-up 17.8 months (range 5.2-26.3)
Deep and durable responses in patients with high-risk MM treated with cilta-cel
• ORR: 100%
• MRD negativity at 10-5: 93% (14/15 evaluable)
• Median DoR: Not reached







• 12-month PFS: 90%
• AEs were consistent with expectations
= adverse event; ASCT = autologous stem cell transplant; cilta-cel = ciltacabtagene autoleucel; CR = complete response; DoR = duration of response; MM = multiple myeloma; MRD = minimal residual disease; OS = overall survival; ORR = overall response rate; PFS = progression-free survival; sCR = stringent complete response; VGPR = very good partial response. Van De Donk NWCJ, et al. ASH 2022. Abstr #3354.
AE
106
0 20 40 60 80 100 120 Cilta-cel ORR Patients (%) VGPR CR 100% 19/19 sCR 90% ≥CR
CARTITUDE 2 Cohort C: Cilta cel in Patients With R/R MM
Previously Treated With Noncellular Anti BCMA Therapy
CARTITUDE-2 Phase 2 Cohort C:
• Patients who progressed despite treatment with a PI, IMiD, anti-CD38 mAb, and noncellular anti-BCMA immunotherapy; median of 8 prior lines (range: 4-13; n=20)
– Antibody-drug conjugate (n=13)








– Bispecific monoclonal antibody (n=7)
Cilta-cel induced favorable responses in patients with R/R MM and prior exposure to anti-BCMA treatment
• Compared with non-responders, Cilta-cel responders had:
– Shorter duration of prior anti-BCMA therapy
– Longer duration since prior anti-BCMA therapy
• 7/10 evaluable patients were MRD negative

• Median DoR: 11.5 months (range 7.9-not estimable)
• Median PFS: 9.1 months (range 1.5-not estimable)
• AEs were consistent with expectations
AE, adverse event; BCMA = B-cell maturation antigen; cilta-cel = ciltacabtagene autoleucel; CR = complete response; DoR = duration of response; IMiD = immunomodulatory imide drug; mAb = monoclonal antibody; MM = multiple myeloma; PI = proteasome inhibitor; PR = partial response; ORR = overall response rate; PFS = progression-free survival; R/R = relapsed/refractory; sCR = stringent complete response; VGPR = very good partial response. Cohen AD, et al. Blood. 2023; 141(3):219-230.
107
0 20 40 60 80 Cilta-cel ORR Patients (%) PR VGPR 60% CR sCR
CASE 4

Maria* PATIENT NOTES
• June 2022: financial and medical consult at CAR T-cell center
• October 2022: symptomatic relapse
• DCEP (×1)
• November 2022: received slot for CAR T-cell therapy

• December 2022: CAR T-cell harvest
• Bortezomib-selinexor-dexamethasone bridging therapy



• January 2023: CAR T-cell therapy infusion
• Mild CRS (grade 1), cytopenias
• CR
*HIPAA-compliant, not actual patient name, stock photo.

CAR = chimeric antigen receptor; CR = complete response; CRS = cytokine release syndrome; DCEP = dexamethasone, cyclophosphamide, etoposide, cisplatin; HIPAA = Health Insurance Portability and Accountability Act.

108
Post CAR T Cell Therapy Patient Education

• Infection prevention
– Prophylaxis





– What to do if there are signs of infection

• Symptoms of neurotoxicity
• Patient handout to give to ER staff or other medical professionals
• Ongoing monitoring
• Transfusion possibility
109 CAR = chimeric antigen receptor; ER = emergency room. Catemero D, et al. Jadpro in press. ABECMA™ (idecabtagene vicleucel) Prescribing Information. Usmani S, et al. ASCO 2021. Abstr #8005. CARVYKTI™ (ciltacabtagene autoleucel) Prescribing Information. Shah UA, Mailankody S. BMJ. 2020;370:m3176. Catamero D, et al. J Adv Pract Oncol [in press].
CASE 5
*HIPAA-compliant, not actual patient name, stock photo.

• Male, standard-risk MM diagnosed 2016 at age 64
TREATMENT HISTORY
• Standard risk by cytogenetics
DIAGNOSED:
SMM IN 2010
MM IN 2016

• RVd then transplant + R maintenance (2 years)
• Carfilzomib/pomalidomide/dex





• Elotuzumab/lenalidomide/dex
• Daratumumab/pomalidomide/dex
January 2023 (71 years old):
• Biochemical relapse
• Patient considering bispecific antibody
Provide tools and resources to enhance decisionmaking

dex = dexamethasone; HIPAA = Health Insurance Portability and Accountability Act; MM = multiple myeloma; R = lenalidomide; RVd = lenalidomide bortezomib dexamethasone; SMM = smoldering multiple myeloma.

110
Paul*
Teclistimab: First Bispecific Antibody Approved for MM
• Bispecific antibody targeting BCMA
• Clinical pearls
– Step-up dosing
R/R MM after ≥ 4 lines of therapy (including a PI, an IMiD, and an anti-CD38 mAb)

October 2022
WATCH FOR
– CRS management and neurotoxicity management
– Individualize support: potential transfusion dependence
– Infection prophylaxis (acyclovir, Bactrim, IVIG if <400 mg/dL)
– Assessing care partner needs (formal or informal)
–
REMS program:
• Prescribers & institutions must be certified
• Nurses must be trained on AE monitoring requirements

• Driving restrictions during step-up dosing
– Inpatient vs outpatient reimbursement
• Clinical trials with combinations
• Real-world experience
AE = adverse event; BCMA = B-cell maturation antigen; CRS = cytokine release syndrome; IMiD = immunomodulatory imide drug; IVIG = intravenous immune globulin; mAb = monoclonal antibody; MM = multiple myeloma; PI = proteasome inhibitor; REMS = risk evaluation and mitigation strategy.
TECVAYLI™ (teclistamab-cqyv) prescribing information.
111
Dosing Schedule Day Dose Step-up dosing schedule Day 1 Step-up dose 1 0.06 mg/kg Day 4 Step-up dose 1 0.3 mg/kg Day 7 First treatment dose 1.5 mg/kg Weekly dosing schedule One week after first treatment dose and weekly thereafter Subsequent treatment doses 1.5 mg/kg once weekly
MajesTEC-1: Phase 1/2 Clinical Trial of Teclistamab
Study Design
• Patients with R/R MM with median of 5 prior lines of therapy



– 100% triple-class exposed; 77% triple-class refractory
– 69% penta-drug exposed; 29% penta-drug refractory
Durable responses that deepened over time in patients with R/R MM

• Median DoR: NE months (95% CI: 9.0, NE)
• Most frequent AEs (>20%): pyrexia, CRS, musculoskeletal pain, injection site reaction, fatigue, upper respiratory tract infection, headache, nausea, pneumonia
• Most frequent SAEs (>2%): pneumonia, urinary tract infection, hypertension, musculoskeletal pain, acute kidney injury, bone pain, pyrexia, upper respiratory tract infection, diarrhea, fatigue
AE = adverse event; BCMA = B-cell maturation antigen; CR = complete response; CRS = cytokine release syndrome; DoR = duration of response; MM = multiple myeloma; NE = not estimable; ORR = overall response rate; PR = partial response; R/R = relapsed/refractory; SAE = serious adverse event; sCR = stringent complete response; VGPR = very good partial response.
TECVAYLI™ (teclistamab-cqyv)
0 20 40 60 80 Teclistamab ORR
Patients (%) PR VGPR 61.8% CR or sCR
112
prescribing information. Moreau P, et al. ASH 2021.
E, et
2022.
Abstr #896. Searle
al. ASH
Abstr #160.




113
International Myeloma Foundation Website. Accessed March 15, 2023. https://www.myeloma.org/publications-videos/download-our-publications/tip-cards. NCCN Website. Accessed March 15, 2023. https://www.nccn.org/compendia-templates/compendia/drugs-and-biologics-compendia.
Resources for New Drugs
IMWG Infection Guidelines: Recommend Infection Prophylaxis and Vaccination

Examples of Salient Features and Key Recommendations:
• Infection remains the leading cause of death in patients with MM
• The periods of highest infectious risk are during the first 3 months after diagnosis and when treating R/R MM

• During periods of increased infectious risk, consider antibacterial prophylaxis with levofloxacin
• Acyclovir prophylaxis is used for patients who are seropositive for herpes simplex virus and varicella zoster virus, if tested. We also suggest use of acyclovir prophylaxis for patients receiving proteasome inhibitors or targeted monoclonal antibodies, specifically CD38-directed monoclonal antibodies

• We recommend immunizing patients with multiple myeloma with inactivated influenza vaccine (preferably with a two-dose series of high-dose influenza vaccine, regardless of age) on an annual basis, and an inactivated S pneumoniae vaccine (PCV13) followed by PPSV23 every 5 years
• We only recommend inactivated vaccines in patients with multiple myeloma
114
MM = multiple myeloma; R/R = relapsed/refractory. Raje NS, et al. Lancet Haematol. 2022;9:143-161. Published February 2022 in Lancet Hematology
Bispecific Antibodies in Development







Watch for different AEs associated with different targets



115
AE = adverse event; BCMA = B-cell maturation antigen; FcRH5 = Fc receptor-homolog 5; GPRC5D = G-protein coupled receptor family C group 5 member D; MM = multiple myeloma. Cho S-F, et al. Front Oncol. 12:1032775. Name Target on Myeloma Cells Elranatamab BCMA Linvoseltamab BCMA Alnuctamab BCMA (ABBV-383) TNB-383B BCMA Talquetamab GPRC5D RG6234 GPRC5D Cevostamab FcRH5 MM cell death CD3 Cytotoxic cytokines Bispecific antibody T cell MM cell
MonumenTAL 1: Phase 1/2 Study of Talquetamab, a GPRC5D Bispecific Antibody
• NEW mechanism: Bispecific antibody that targets G protein-coupled receptor family C group 5 member D (GPRC5D) that has limited expression in normal human tissue but is highly expressed on malignant plasma cells
• Patients with R/R MM all received talquetamab (N=143) at 0.4 OR 0.8 mg/kg; step-up dosing
–
–
Median 5 prior lines of therapy (range 2-13); 36% (51/143) BCMA therapy exposed
Triple-class: 100% exposed, 74% refractory. Penta-drug: 73% exposed, 29% refractory
Watch for FDA-approval of talquetamab

Novel agent with promising ORR of >70% in heavily pretreated patients with R/R MM
• ORR was similar across triple-class and penta-class refractory
• ORR in patients with prior BCMA therapy: 62.6%
• Most common AEs: CRS, dysgeusia, anemia, skin-related AEs, nail disorders, infection, and cytopenias (usually limited to first few cycles)
• Most CRS was grade 1 or 2 and occurred during step-up doses

• ICANS: ≈10% any grade; <2% grade 3; 0% grade 4+; no fatalities
• Infections: 57% any grade, 16.8% grade 3/4
• Available via compassionate use in some centers
*ORR was similar for 0.8 mg/kg dose.
AE = adverse event; BCMA = B-cell maturation antigen; CR = complete response; CRS = cytokine release syndrome; FDA = US Food and Drug Administration; ICANS = immune effector cell–associated neurotoxicity syndrome; GPRC5D = G-protein coupled receptor family C group 5 member D; MM = multiple myeloma; PR = partial response; R/R = relapsed/refractory; ORR = overall response rate; sCR = stringent complete response; VGPR = very good partial response. Chari AJ, et al. ASH 2022. Abstr #157.





116 0 20 40 60 80 0.4 mg/kg* ORR Patients (%) PR CR 74.1% VGPR
≥VGPR sCR
59.4%
BREAK THROUGH DESIGNATION
MagnetisMM 3 : Elranatamab in Patients With R/R MM
MagnetisMM-3 Phase 2
• Patients with R/R MM with no prior BCMA-targeted treatment (n=37)
– Refractory to ≥1 each of the following: PI, IMiD, and anti-CD38 mAb






• Premeds: acetaminophen, diphenhydramine, dexamethasone
• Step-up dosing in week 1, then 1x weekly; every 2 weeks beginning in cycle 7
Watch for
• FDA-approval of elranatamab


• MagnetisMM-3 updated data
• Clinical trials with combinations
Deep and durable responses in patients with R/R MM
• ORR: 61.0% (95% CI 51.8-69.6)
• Median DoR: NE (range 12-NE)
• Median PFS and OS: NE
• MRD negative (10-5): 90.9% of evaluable patients (n=22)
• Most common grade 3-4 AEs were hematological
• All CRS and ICANS were grade 1 or 2; no grade 5 neurotoxicity
AE, adverse event; BCMA = B-cell maturation antigen; CR = complete response; CRS = cytokine release syndrome; DoR = duration of response; iMiD = immunomodulatory drug; ICANS = immune effector cell–associated neurotoxicity syndrome; mAb = monoclonal antibody; MM = multiple myeloma; MRD = minimal residual disease; NE = not estimable; OS = overall survival; ORR = overall response rate; PI = proteasome inhibitor; PFS = progression-free survival; PR = partial response; R/R = relapsed/refractory; sCR = stringent complete response; VGPR = very good partial response. Bahlis N, et al. ASH 2022. Abstr #159.

117
0 20 40 60 80 Elranatamab ORR VGPR CR 61.0% sCR 27.6% PR
Patients (%) BREAK THROUGH DESIGNATION FAST TRACK
Paul*

• Male, standard-risk MM, 71-year-old, biochemical relapse
Considering
Treatment Options
• Teclistamab
• Elranatamab (clinical trial)




• Talquetamab (clinical trial)
Questions About clinical trials


*HIPAA-compliant, not actual patient name, stock photo.

118 HIPAA = Health Insurance Portability and Accountability Act; MM = multiple myeloma.
CASE 5
Resources to Find Clinical Trials and Avoid Bias
Clinicaltrials.gov
https://clinicaltrials.gov/

IMF Infoline
Just ASKTM Implicit Bias Training from Association of Community Cancer Centers Website. Accessed March 17, 2023. https://www.accccancer.org/home/attend/webinartemplate/2022/07/25/on-demand/justask-increasing-diversity-in-cancerclinical-research
US & Canada: 800-452 CURE (2873)
Worldwide: 1-818-487-7455
infoline@myeloma.org

119
CASE 5





Paul*

• Male, standard-risk MM, 71-year-old, biochemical relapse
Considering
Treatment Options
• Teclistamab
• Elranatamab (clinical trial)
• Talquetamab (clinical trial)
Monthly Monitoring

*HIPAA-compliant, not actual patient name, stock photo.
Informing treatment timing

120 HIPAA = Health Insurance Portability and Accountability Act; MM = multiple myeloma.
Therapies in Development for Treatment of Multiple Myeloma

CELMoD agents
• Iberdomide (cereblon E3 ligase)
• Mezigdomide (cereblon E3 ligase)

Monoclonal antibodies

• TAK-079 (CD38)
• Felzartamab (CD38)
Immunocytokine


• Modakafusp Alfa TAK-573
Antibody-drug conjugates


• Belantamab Mafadotin (BCMA)
• Lorvotuzumab Mertansine (CD56)
• Indatuximab Ravtansine (CD138)



CAR T-cell therapies
• P-BCMA-101 (BCMA)
• PHE885 (BCMA)
• ALLO-715 (BCMA)
CAR NK cells
















•
•
Checkpoint inhibitors
Nivolumab (PD-1)
Pembrolizumab (PD-1)
Bispecific antibodies/ T-cell engagers
• Elranatamab (BCMA)
• Alnuctamab (BCMA)
• Linvoseltamab (BCMA)


• ABBV-383 (BCMA)
• Talquetamab (GPRC5D)
• RG6234 (GPRC5D)
• Cevostamab (FcRH5)
BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; CELMoD = cereblon E3 ligase modulator; MHC = major histocompatibility complex; MM = multiple myeloma; NK = natural killer; PD-1 = programmed death protein 1; PD-L1 = programmed death ligand 1; TCR = T Cho S-F, et al. Front Oncol. 12:1032775. Shah UA, Mailankody S. BMJ. 2020;370:m3176. Yang . 2020;13:150.
. 2018;61:535-542. Lonial S, et al. ASH 2022. Abstr #1918. Richardson PG, et al. ASH2022. Abstr #568. Vogl DT, et al. ASH2022. Abstr #565. Gershman J. Pharmacy Times. Accessed March 29, 2023. https://www.pharmacytimes.com/view/a-look-at-multiple-myeloma-treatment-options-in-the-pipeline.
PD-1 PD-L1 MHC TCR T cell T cell T cell CAR NK cell CAR T cell Plasma cell 121 Immune
Bruins WSC, et al. Front Immunol. 2020;11:1155. Matyskiela ME, et al. J Med Chem
cells WATCH FOR
New drug approvals in MM
New data
Iberdomide + Dexamethasone in R/R MM With Prior BCMA Therapy Exposure
• Mechanism: Greater tumoricidal and immune-stimulatory effects compared with IMiDs; marked synergy with dex and other antimyeloma therapies in preclinical models
• Patients with R/R MM ≥3 lines of therapy (including IMiDs, a PI, an anti-CD38 mAb, and an anti-BCMA therapy) and progressive disease ≤60 days after last therapy* (n=38)
– CAR T-cell therapy 36.8%; antibody-drug conjugate 34.2%; T-cell engager 13.7%; other 10.5%
WATCH FOR
• Clinical trials with iberdomide
• Combinations with iberdomide


Iberdomide showed encouraging activity and safety in patients with triple-class-exposed R/R MM and prior anti-BCMA therapy
• Median DoR: 7.5 months (95% CI 3.2-not reached)
• Median PFS: 2.4 months (95% CI 3.2-6.3)
• Hematologic toxicities
• Low grade 3/4 nonhematologic toxicities
• No discontinuations due to treatment-emergent AEs

*Documented progressive disease if CAR T-cell therapy was the last therapy.
AE = adverse event; BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; CR = complete response; dex = dexamethasone; DoR = duration of response;
iMiD = immunomodulatory drug; mAb = monoclonal antibody; MM = multiple myeloma; ORR = overall response rate; PFS = progression-free survival; PI = proteasome inhibitor; PR = partial response; R/R = relapsed/refractory; VGPR = very good partial response.
122
Lonial S, et al. ASH 2022.
#1918. 0 20 40 Iberdomide ORR
Abstr
Patients (%) PR 36.8% CR VGPR
Mezigdomide (MEZI), a novel oral CELMoD® With Promising Activity
• Mechanism: enhanced tumoricidal and immune-stimulatory effects compared to IMiDs. MEZI induced maximal degradation of Ikaros and Aiolos, leading to increased apoptosis in myeloma cells
• Patients with R/R MM treated with MEZI + dexamethasone (n=101)
– ≥3 prior lines of therapy; progression ≤60 days of last myeloma therapy
– Refractoriness to IMiDs, a PI, a glucocorticoid, and an anti-CD38 mAb


WATCH FOR
Clinical trials with MEZI:
SUCCESSOR-1: MEZI with Vd in R/R MM
SUCCESSOR-2 MEZI with Kd in R/R MM
MEZI showed promising activity in heavily pretreated patients with MM
• Preliminary median DoR: 8.3 months (95% CI 5.4-not reached)
• Median PFS: 4.6 months (95% CI 3.2-6.3)
• ORR in patients with prior anti-BCMA therapy: 50% (n=30)
• Hematologic toxicities
• Low rate of grade 3/4 nonhematologic toxicities: gastrointestinal disorders (5.9%), fatigue (4.0%), and rash (1.0%)
• 5.9% discontinuation due to treatment-emergent AEs
BCMA = B-cell maturation antigen; CELMoD = Cereblon E3 ligase modulator; CR = complete response; DoR = duration of response; iMiD = immunomodulatory drug; Kd = carfilzomib dexamethasone; mAb = monoclonal antibody; MEZI = mezigdomide; MM = multiple myeloma; ORR = overall response rate; PI = proteasome inhibitor; PR = partial response; R/R = relapsed/refractory; sCR = stringent complete response; Vd = bortezomib dexamethasone; VGPR = very good partial response. Richardson PG, et al. ASH2022. Abstr #568.

0 20 40 60 Mezigdomide ORR
Patients (%) PR 39.6% CR
123
sCR VGPR
Modakafusp Alfa: First in Class Targeted Interferon Alpha
• First-in-class NEW mechanism: targeted delivery of IFNα to immune cells and myeloma cells
– 2 attenuated interferon (IFN)α2b molecules genetically fused to the Fc portion of an antiCD38 IgG4 mAb
• 100 pts with ≥3 prior lines of treatment: refractory/intolerant to ≥1 PI and ≥1 IMiD
WATCH FOR
Modakafusp alfa has a novel mechanism of action, a manageable safety profile, and encouraging anti-myeloma activity at 1.5 mg/kg Q4W, independent of peripheral blood immune cell CD38 expression
• Median DoR: Not reached (range 1.0-18.9 months)
• Hematologic toxicities
• No Grade 4 non-hematological toxicities

• IRR = 37% any grade (3% grade 3)
CR = complete response; DoR = duration of response; IgG4 = Immunoglobulin G4; iMiD = immunomodulatory drug; IFNα = interferon alpha; IRR = infusion-related reaction; mAb = monoclonal antibody; MM = multiple myeloma; ORR = overall response rate; PI = proteasome inhibitor; PR = partial response; Q4W = every 4 weeks; sCR = stringent complete response; VGPR = very good partial response.






124
DT, et al. ASH 2022. Abstr #565. 0 20 40 Modakafusp 1.5 mg/kg Q4W ORR (n=30)
Vogl
Patients (%) PR 43.3% CR VGPR
sCR
Clinical trials with modakafusp alpha
BCMA is a protein found on the surface of mature B cells and is one target for myeloma treatments.
CAR T-cell therapy (ide-cel & cilta-cel) engineers a patient’s own T cells; early referral to CAR T-cell therapy center for evaluation and preauthorization is important for access.
Bispecific antibodies act as a bridge between T cells and myeloma cells to use a patient’s immune system to target myeloma. Teclistamab is the first approved. Elranatamab and talquetamab approvals expected.
CRS is a common AE with both CAR T-cell therapy and bispecific antibodies that can be managed with tocilizumab and corticosteroids.
Neurotoxicity/ICANS is a rare but serious AE associated with CAR T-cell or bispecific antibodies therapy. Requires prompt intervention.

Many new drugs are in development with different targets, including CELMoD agents, monoclonal antibodies, bispecific monoclonal antibodies (T-cell engagers), and antibody-drug conjugates.

AE, adverse event; BCMA = B-cell maturation antigen; CAR = chimeric antigen receptor; CELMoD = cereblon E3 ligase modulator; cilta-cel = ciltacabtagene autoleucel; CRS = cytokine release syndrome; ICANS = immune effector cell–associated neurotoxicity syndrome; ide-cel = idecabtagene vicleucel; MM = multiple myeloma. Shah N, et al. Leukemia. 2020;34(4):985-1005. Yu B, et al. J Hematol Oncol. 2020;13:125. Oluwole OO, Davila ML. J Leukoc Biol. 2016;100:1265-1272. June CH, et al. Science. 2018;359:1361-1365. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Brudno JN, Kochenderfer JN. Blood Rev. 2019:34:45-55. Shimabukuro-Vornhagen, et al. J Immunother Cancer. 2018;6:56. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638. Brudno JN, Kochenderfer JN. Blood. 2016;127(26):3321-3330. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25:625-638. ABECMA® (idecabtagene vicleucel) Prescribing Information. CARVYKTI™ (ciltacabtagene autoleucel)
Prescribing Information. Catamero D, et al. J Adv Pract Oncol. 2022;13(suppl 4):31-43. Teoh PJ, Chng WJ. Blood Cancer J. 2021;11(4):84. Shah UA, Mailankody S. BMJ. 2020;370:m3176. Cho S-F, et al. Front Oncol. 12:1032775. Shah UA, Mailankody S. BMJ. 2020;370:m3176. Yang et al. J Hematol Oncol. 2020;13:150. Bruins WSC, et al. Front Immunol. 2020;11:1155. Matyskiela ME, et al. J Med Chem. 2018;61:535-542. Lonial S, et al. ASH 2022. Abstr #1918. Richardson PG, et al. ASH2022. Abstr #568. Vogl DT, et al. ASH2022. Abstr #565.

125
Summary
Thank You for Sharing in the Stories of Our Patients





126 *HIPAA-compliant, not actual patient name, stock photo. HIPAA = Health Insurance Portability and Accountability Act.
Paul*
Maria* Henry* Margaret*
We Hope You Had an Enjoyable and Educational Time
As a result of this program, you learned to:
Discuss new and emerging treatment regimens for patients with newly diagnosed or relapsed multiple myeloma, including appropriate patient management and patient education
Identify healthcare disparities faced by patients with multiple myeloma who are part of underrepresented groups and strategies to overcome these



Describe strategies to support the patient’s input in therapeutic decisions through shared decision-making

127
Q1. Which of the following is TRUE about multiple myeloma in Black vs White patients?
1. Black patients tend to be diagnosed with myeloma at an older age
2. Black patients tend to have higher-risk disease
3. Black and White patients have a similar incidence of myeloma
4. Black patients may have superior outcomes when treated with standard of care

5. I don’t know

128
Q1. Which of the following is TRUE about multiple myeloma in Black vs White patients?
1. Black patients tend to be diagnosed with myeloma at an older age
2. Black patients tend to have higher-risk disease
3. Black and White patients have a similar incidence of myeloma
4. Black patients may have superior outcomes when treated with standard of care

5. I don’t know

129
Q2. Which of the following is a recommended approach to supporting shared decision making for patients with multiple myeloma?
1. Encourage the patient to make a decision before leaving the office where they have the opportunity to get their concerns addressed by the medical team
2. Provide resources and/or tools to help the patient assess the type of treatment they want to receive

3. Emphasize the treatment option with the highest overall response rate
4. Explain the value of saving the most effective treatment for relapse
5. I don’t know




130
Q2. Which of the following is a recommended approach to supporting shared decision making for patients with multiple myeloma?
1. Encourage the patient to make a decision before leaving the office where they have the opportunity to get their concerns addressed by the medical team
2. Provide resources and/or tools to help the patient assess the type of treatment they want to receive

3. Emphasize the treatment option with the highest overall response rate
4. Explain the value of saving the most effective treatment for relapse
5. I don’t know




131
Q3. Which of the following is TRUE when caring for myeloma patients?
1. Bone strengtheners should only be given to treat patients with myeloma who experience hypercalcemia of malignancy
2. For patients with myeloma and chronic kidney disease, it is safer to take ibuprofen than Tylenol
3. Prophylactic anticoagulation is recommended for patients receiving myeloma regimens that contain IMiDs (immunomodulatory drugs) or carfilzomib

4. Nurses should obtain a blood type and cross match before initiating treatment with either a proteasome inhibitor or SINE inhibitor.
5. I don’t know

132
Q3. Which of the following is TRUE when caring for myeloma patients?
1. Bone strengtheners should only be given to treat patients with myeloma who experience hypercalcemia of malignancy
2. For patients with myeloma and chronic kidney disease, it is safer to take ibuprofen than Tylenol
3. Prophylactic anticoagulation is recommended for patients receiving myeloma regimens that contain IMiDs (immunomodulatory drugs) or carfilzomib

4. Nurses should obtain a blood type and cross match before initiating treatment with either a proteasome inhibitor or SINE inhibitor.
5. I don’t know

133
Q4. Your patient is admitted to the hospital for their first dose of a bispecific antibody therapy. Which of these acute side effects should you be more worried about?
1. Hypertension

2. Dehydration
3. Tumor lysis syndrome (TLS)
4. Cytokine release syndrome (CRS)
5. I don’t know
134
Q4. Your patient is admitted to the hospital for their first dose of a bispecific antibody therapy. Which of these acute side effects should you be more worried about?
1. Hypertension

2. Dehydration
3. Tumor lysis syndrome (TLS)
4. Cytokine release syndrome (CRS)
5. I don’t know
135
https://imf-ons.myeloma.org/












password: ons2023

136 MM = multiple myeloma.
Remember the Resources to Enhance Your Ability to Care for Your Patients With MM



137 Thank You for Your Attendance and Participation IMF = International Myeloma Foundation. On behalf of the International Myeloma Foundation with the generous support from AbbVie, Inc.; Bristol-Myers Squibb Company; Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC; Karyopharm Therapeutics; Pfizer Inc.; Sanofi; and Takeda Pharmaceuticals U.S.A., Inc., we thank you. Please Contact IMF for Further Information and Resources: 1-800-452-CURE (1-800-452-2873) http://myeloma.org Slides and Resources available at: http://imf-ons.myeloma.org Password: ons2023 TheIMF@myeloma.org Don’t forget to turn in your YELLOW eval for CNE credit





























































































































































































































































































































































































































































































