

2024 Boca Raton Patient and Family Seminar
March 15 & 16, 2024













Friday Agenda
• 1:00 – 1:10 PM
Welcome and Agenda Review
• 1:10 – 1:25 PM Hot Topics in Myeloma
• 1:25 – 1:45 PM
Shared Decision Making
• 1:45 – 2:00 PM Myeloma.org: Resource Review
• 2:00 – 2:15 PM Q&A w/ Panel
• 2:15 – 2:45 PM BREAK
• 2:45 – 3:00 PM
• 3:00 – 3:40 PM
• 3:40 – 4:10 PM
• 4:10 – 4:50 PM
• 4:50 – 5:00 PM
• 5:00 – 7:00 PM
Advanced Care Planning
Myeloma 101 & Understanding Your Labs
Financial Considerations in Myeloma
Clinical Trials & Patient/Care Partner Testimonial w/ Q&A
Day 1 Recap, Day 2 Announcements & Evaluations
Welcome Reception & Networking


The IMF Support Group Team is Here For You!
Shared Experiences Help to Better Understand the Myeloma Journey
• Support Groups Empower Patients & Care Partners with information, insight, & hope
• The IMF provides educational support to a network of over 150 myeloma specific groups

Support.myeloma.org



We are happy to help connect you with an existing support group or help form a new one! We assist with virtual, in-person, and hybrid options for meetings.
Reach out to us at
SGTeam@myeloma.org

Local
Support Groups: You Are Not Alone!
Miami Multiple Myeloma Support Group
Meets virtually on the 4th Wednesday of each month at 6:30PM
Melbourne Multiple Myeloma Support Group
Meets in-person on the 4th Monday of each month at 10:30AM
Palm Beach County Multiple Myeloma Support Group
Meets in a hybrid format on the 1st nonholiday Monday of each month at 6:30PM

Maitland Multiple Myeloma Support Group
Meets in-person on the 2nd Monday of each month at 6:30pm


Fort Myers Multiple Myeloma Support Group
Meets in-person on the 3rd Tuesday of each month at 6pm
Hollywood Multiple Myeloma Support Group
Meets virtually on the 1st Tuesday of each month at 6PM
Jacksonville Multiple Myeloma Support Group
Meets in a hybrid format on the 2nd Wednesday of each month at 6PM
Local Support Groups: You Are Not Alone!
Brooksville / Nature Coast Multiple Myeloma Support Group
Meets virtually on the 3rd Wednesday of each month at 6PM
Tampa CentralMultiple Reasons Support Group
Meets virtually on the 2nd Thursday of each month at 11AM


North Tampa Multiple Myeloma Support Group
Meets in-person on the 3rd Saturday of each month at 10:30AM

Naples Multiple Myeloma Support Group
Meets in-person on the 3rd Thursday of each month at 6pm
Tampa Bay/St Petersburg Multiple Myeloma Educational Group
Meets virtually on the 1st Saturday of each month at 10:30AM
Local Support Groups: You Are Not Alone!
Palm Coast Multiple Myeloma Support Group
Meets in-person on the 2nd Thursday of each month at 3:30PM
Ocala Multiple Myeloma Support Group
Meets in-person on the 2nd Saturday of each month at 11AM

Sarasota Multiple Myeloma Network & Education Group
Meets in-person on the 4th Friday of each month at 11AM

The Villages Multiple Myeloma Support Group
Meets in-person on the 1st Tuesday of each month at 1PM

Panama City Multiple Myeloma Support Group
Meets in-person on the 2nd Saturday of each month at 10AM
Tallahassee Multiple Myeloma Support Group
Meets in-person on the 4th Monday of each month at 5:30PM
IMF – Special Interest Virtual Groups

Special interest groups are designed as a supplemental support for specific populations of patients, in addition to their local Support Groups
Las Voces de Mieloma
Designed for Spanish speaking patients only
Living Solo & Strong with Myeloma
Designed for patients without a care partner

Coming Soon!
Care Partners Only
Designed to address the needs of care partners only

Smolder Bolder
Created for people living with Smoldering Multiple Myeloma
MM Families

High Risk Multiple Myeloma
Designed to address the needs of the high-risk MM population
MGUS 4 Us
Created for people living with MGUS
For patients/care partners with young children

EVALUATION
Please be sure to complete your program evaluation today.
Questions 1 – 5 can be completed before the program begins.
Questions 7 & 8 can be worked on after each presentation.
If you are attending Friday program only, we ask that you turn the survey in at the end of the day.
If you are coming back for the Saturday sessions, please hold onto your survey, bring it back tomorrow and turn it in at the end of the program. We greatly appreciate your time and feedback!


Hot Topics in Myeloma
Joseph Mikhael, MD, MEd, FRCPC, FACPChief Medical Officer, International Myeloma Foundation
Professor, Translational Genomics Research Institute
City of Hope Cancer Center


Shared Decision
Making:
Be An Active Member Of Your Health Care Team

Teresa Miceli, RN BSN OCN
International Myeloma Foundation - InfoLine Advisor, NLB Member, Support Group Leader (MMSS, Smolder Bolder)
Mayo Clinic – Myeloma Nurse Navigator
National Cancer Institute - Myeloma Patient Advocate
Goals
Review Share Decision Making (SDM)
Identify influencing factors to Treatment Decision Making
Discuss strategies to enhance patient empowerment & promote Shared Decision Making
Individual Beliefs & Preference s
Transplant
Eligible Patients
Individual Care Partner
Family
Initial Therap y
Transplant
Ineligible Patients
A Person With `
Treating Myeloma
Transplant (ASCT) Maintenance
Everyone
Social Network & Obligations
Treatment of Relapsed disease
Consolidation / Maintenance
Continued therapy
Myeloma Symptom s & Treatment Options
Supportive Care
Employme nt & Finances
Terpos E, Mikhael J, Hajek R, Chari A, Zweegman S, Lee HC, Mateos MV, Larocca A, Ramasamy K, Kaiser M, Cook G, Weisel KC, Costello CL, Elliott J, Palumbo A, Usmani SZ. Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety
“The aim of shared decision making is to ensure that:
- Patients understand their options and the pros and cons of those options.
- Patient's goals and treatment preferences are used to guide

Steps in Shared Decision-Making

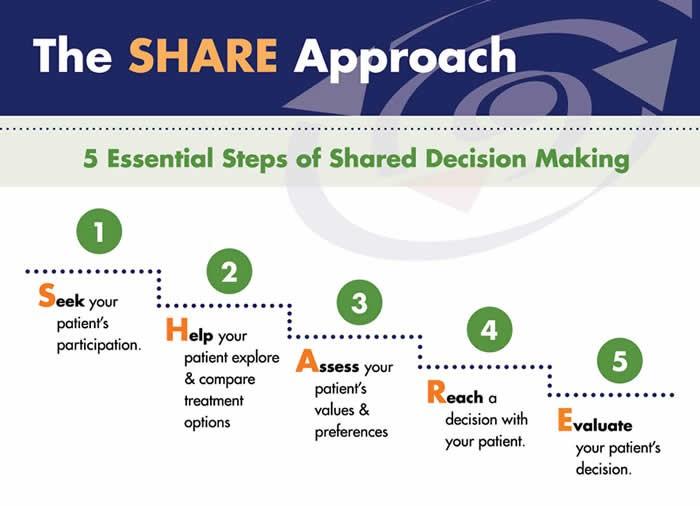
Recognizing and acknowledging that a decision is needed:
The HCP informs the patient that a decision is to be made and that the patient's opinion is important (Choice talk).
Knowing and understanding the best available evidence-based options:
The HCP explains the options and their pros and cons. The patient expresses their preferences, and the HCP supports the patient in deliberation (Option talk).
The HCP and patient discuss the patient's wish to take part in the decision making and incorporate the patient's values and preferences into the decision (Decision talk).
The
https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.htm
Stiggelbout
Advantages to Partaking in SDM
Patients, regardless of age, want to be a part of treatment
Requires staying informed
Reduces uncertainty and alleviates concerns
Decisions reflect personal and family values
Promotes patient and care partner engagement and sense of empowerment
Positive impact on QOL
Lower demand on health care resources

Terpos, et al.
“The 'efficacy' of treatment means different things to different patients, and treatment decision-making in the context of personalized medicine must be guided by an individual's composite definition of what constitutes the best treatment choice.” Terpos, et al.
2021
https://www.ahrq.gov/cahps/quality-improvement/improvement-guide/6-strategies-for-improving/communication/strategy6i-shared-decisi onmaking.html#6i1
Influencing Factors to Treatment Decision-Making


Disease-derived
Time: Stage, risk stratification, Urgent intervention needed vs time to consider options
Treatment: Availability/access, effectiveness, toxicity, current research
Patient-derived
Provider-derived Time limitations
Support for patient involvement
Provider bias and preference
Understanding complex treatment options
Physical and emotional wellness
Comfort in speaking up “Doctor knows best”
Choon-Quinones, Mimi, Hose D, Kaló Z, Zelei T, Harousseau JL, Durie B, Keown P, Barnett M, Jakab I. Patient and Caregiver Experience Decision Factors in Treatment Decision Making: Results of a Systematic Literature Review of Multiple Myeloma Decision Aids. Value Health. 2023 Jan;26(1):39-49. doi: 10.1016/j.jval.2022.04.003. Epub 2022 May
PMID: 35613958.
Financial, Cultural and Religious factors
Care partner & social network, transportation
https://www.ahrq.gov/sites/de fault/files/wysiwyg/cahps/qua lity-improvement/improveme nt-guide/6-strategies-for-impr oving/communication/cahps-s trategy-section-6-i.pdf
https://www.valueinhealthjournal.com/action/showFullTableHTML?isHtml=true&tableId=tbl4&pii=S1098-3015%2822%290019
Patient Empowerment

Stay informed, understand options
Use reliable and current sources of information
Use caution considering stories of personal experiences
Consider your priorities
Discuss with your care partner
Consider your goals/values/preferences
Be a part of the conversation, create a dialog
Ask questions & Express your goals/values/preferences
Ask for time to consider options, if needed
Arrive at a treatment decision together
Arrange follow up to review and adjust, if needed
HCP
Clinical Experience
Research Results
TREATMENT DECISION


Your Preference

Decision Aids available at Myeloma.org



























Know the Members of Your Care Team

Understand their different roles
Myeloma specialist and General Heme/Onc
Primary care: for health screening, general check ups, vaccinations
Sub-specialists: specialty needs
Keep a contact list of your providers

Primary Care Provider (PCP)

Family/Support Network

Subspecialists

You & Your Care Partner

Allied Health Staff

General Hem/Onc


Myeloma Specialist
Prepare
Prepare For Medical Visits
Medications: Bring a current list of prescribed and over-thecounter
Questions: Prioritize questions & concerns including financial issues
Paperwork needing medical signature (ex FMLA, prior authorizations)
Inform
Updates: Medical or life changes since your last visit
Symptoms: How have they changed (improved, worsened, stable)? Keep a symptom diary. Bring it along
Communicate effectively so your health care team can help
Follow Up
“Next Steps”: Future appointments, medication changes, treatment plan. Ask for the information in writing or on your patient portal Include a care partner, especially for pivotal appointments
Prepare For Medical Visits
Check with your healthcare team –
Is telemedicine an option?
What is the process and what technology is needed?
Are labs needed in advance? Do you need an order?
Preparation is similar for “in-person” appointment PLUS:
Location: quiet, well-lit location with strong Wi-Fi is best
Yourself: Do you need to show a body part - wear accessible clothing
Vital signs (blood pressure, temp, heart rate, weight) selfserve blood pressure cuff is available at many pharmacies and for purchase
Include a care partner, especially for pivotal appointments
You & Your Care Partner
Myeloma causes the highest burden of symptoms, most commonly effecting people of older age with other medical issues. Care partner support is valuable in SDM
Care partners assist in many ways
Attending medical appointments, being present to learn and discuss possible treatment options and alert the medical team of side effects to treatment
Some treatment options available only if care partner support exists
Care partners can be one person or a rotation of many people
Building a partnership is based in good communication
Finding the balance:

- helping the patient with needed activities while maintaining a sense of independence
- allowing the care partner to have time for good self-care




Resource List

Bylund CL, Eggly S, LeBlanc TW, Kurtin S, Gandee M, Medhekar R, Fu A, Khurana M, Delaney K, Divita A, McNamara M, Baile WF. Survey of patients and physicians on shared decision-making in treatment selection in relapsed/refractory multiple myeloma. Transl Behav Med. 2023 Apr 15;13(4):255-267. doi: 10.1093/tbm/ibac099. PMID: 36688466.
Chari A, Romanus D, DasMahapatra P, Hoole M, Lowe M, Curran C, Campbell S, Bell JA. Patient-Reported Factors in Treatment Satisfaction in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Oncologist. 2019 Nov;24(11):1479-1487. doi: 10.1634/theoncologist.2018-0724. Epub 2019 Aug 1. PMID: 31371520; PMCID: PMC6853123.
Choon-Quinones, Mimi, Hose D, Kaló Z, Zelei T, Harousseau JL, Durie B, Keown P, Barnett M, Jakab I. Patient and Caregiver Experience Decision Factors in Treatment Decision Making: Results of a Systematic Literature Review of Multiple Myeloma Decision Aids. Value Health. 2023 Jan;26(1):3949. doi: 10.1016/j.jval.2022.04.003. Epub 2022 May 22. PMID: 35613958.
Rifkin RM, Bell JA, DasMahapatra P, Hoole M, Lowe M, Curran C, Campbell S, Hou P, Romanus D. Treatment Satisfaction and Burden of Illness in Patients with Newly Diagnosed Multiple Myeloma. Pharmacoecon Open. 2020 Sep;4(3):473-483. doi: 10.1007/s41669-019-00184-9. PMID: 31605300; PMCID: PMC7426337.
3718 Cytokine Release Syndrome: The Patient, Caregiver and Healthcare Professional Experience. Janelle Soong, Giuseppe De Carlo, Naziah Lasi-Tejani, Sumanjit K. Sethi, Natacha Bolaños, Martine Elias, Yelak Biru, Solène Clavreul, G. Scott Chandler, Klaus Finzler, Yann Nouet, Antonio Giuseppe Del Santo. Blood (2023) 142 (Supplement 1): 3718
Terpos E, Mikhael J, Hajek R, Chari A, Zweegman S, Lee HC, Mateos MV, Larocca A, Ramasamy K, Kaiser M, Cook G, Weisel KC, Costello CL, Elliott J, Palumbo A, Usmani SZ. Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J. 2021 Feb 18;11(2):40. doi: 10.1038/s41408-021-00432-4. PMID: 33602913; PMCID: PMC7891472.
https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
https://www.ahrq.gov/cahps/quality-improvement/improvement-guide/6-strategies-for-improving/communication/strategy6i-shared-decisionmaking.ht ml#6i1
EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Myeloma.org
RobinTuohy
Vice President, Support Groups
International Myeloma Foundation


EVALUATION
Please be sure to complete your program evaluation today.
Questions 7 & 8 can be worked on after each presentation.
If you are attending Friday program only, we ask that you turn the survey in at the end of the day.
If you are coming back for the Saturday sessions, please hold onto your survey, bring it back tomorrow and turn it in at the end of the program.
We greatly appreciate your time and feedback!


BREAK













Myeloma 101 & Understanding Your Labs
Joseph Mikhael, MD, MEd, FRCPC, FACP
Chief Medical Officer, International Myeloma Foundation
Teresa Miceli, RN, BSN, OCN
International Myeloma Foundation Nurse Leadership Board Member


Q&A with Teresa and Dr. Joe: Understanding Myeloma Basics
Joseph Mikhael, MD, MEd, FRCPC, FACP
Professor, Applied Cancer Research and Drug Discovery, Translational Genomics
Research Institute (TGen), City of Hope Cancer Center
Chief Medical Officer, International Myeloma Foundation
Consultant Hematologist and Director, Myeloma Research, Phase 1 Program, HonorHealth Research Institute
Adjunct Professor, College of Health Solutions, Arizona State University


Teresa S. Miceli RN BSN OCN
Mayo Clinic, Rochester, MN
• Mayo Associate
• Assistant Professor of Nursing
• Myeloma Research RN Navigator
International Myeloma Foundation
• InfoLine Advisor
• Nurse Leadership Board
• Support Group Leader
NCI Myeloma Steering Committee





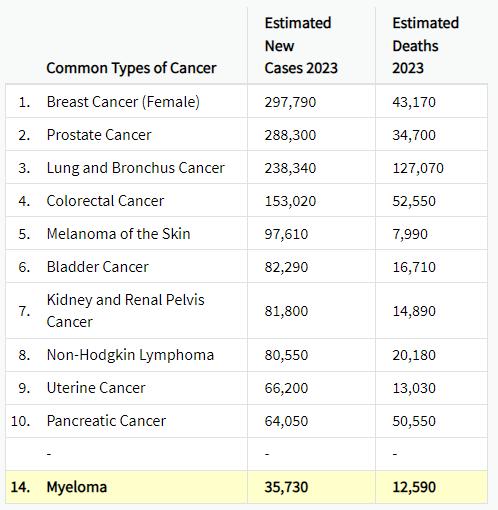
Myel0ma Statistics
Estimated New Cases in 2023 35,730 %
Median Age At Diagnosis 69 years

How common is Myeloma?





Bone Marrow Cells – Good & Bad


Platelets

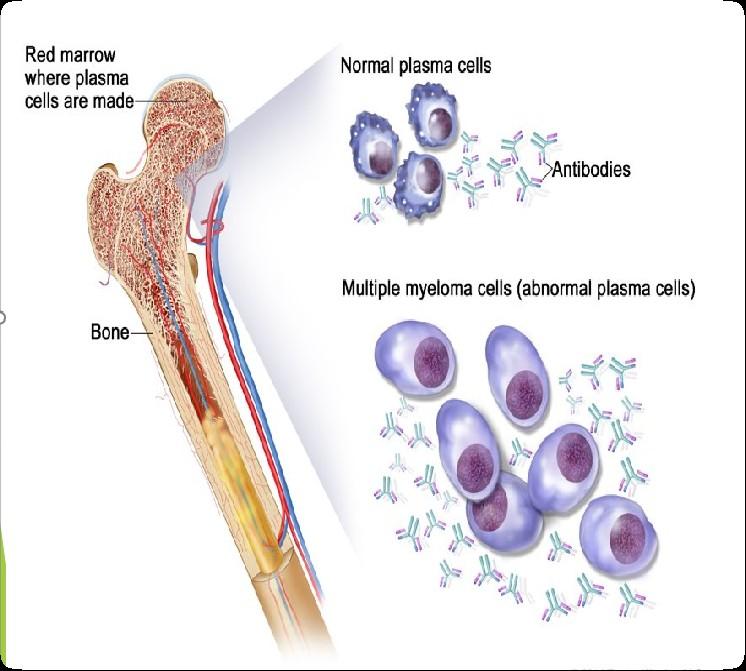


 Hematopoietic stem cell
Myeloid progenitor cell
Hematopoietic stem cell
Myeloid progenitor cell
(Mono)clonal Plasma Cells

Clonal
Heavy Chain: G, A, M, D, E

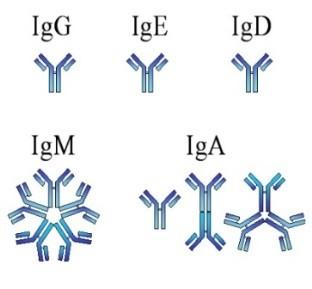

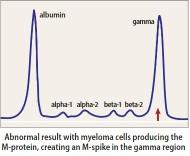
Heavy Chain = M-Spike
65% IgG – most common
20% IgA – associated with AL Amyloid
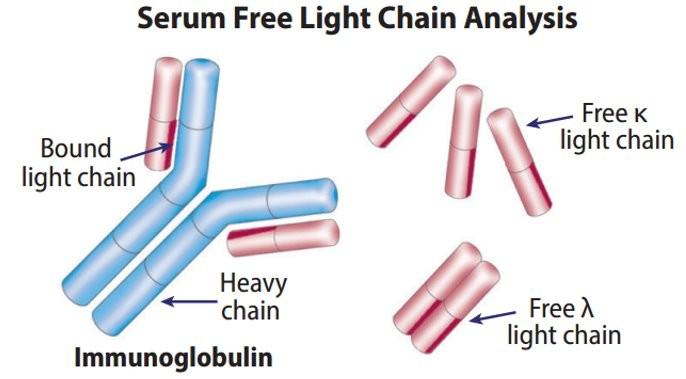
5% to 10% light chain-only (kappa, lambda)
Uncommon: IgD, IgE, IgM
OS, Palsson R., et al. Defining new reference intervals for serum free light chains in individuals with chronic kidney

Normal Ranges vary between labs.
Note the unit of measure (mg/dL vs mg/L): Results adjusted for renal function
eGFR = estimated glomerular filtration rate; M-spike = monoclonal spike; Ig = Immunoglobulin

of the iStopMM study. Blood Cancer J. 2022 Sep 14;12(9):133. doi: 10.1038/s41408-022-00732-3


Spectrum of Monoclonal Protein Disorders
Condition
Clonal plasma cells in bone marrow
MGUS1-4 (Monoclonal Gammopathy of Undetermined Significance)
SMM1-5,8 (Smoldering Multiple Myeloma)
• AL-Amyloid
• POEMS
• Light or Heavy Chain Deposition Disease
• MGRS = Renal
• MGNS = Neuro
Active Multiple Myeloma6-8
Presence of Myeloma
Defining Events None None Yes
Likelihood of progression ~1% per year ~10% per year Not Applicable
Treatment No; observation
1. Kyle RA, et al. N Engl J Med. 2007;356:2582-90.
2. IMWG. Br J Haematol. 2003;121:749-57.
3. Jagannath S, et al. Clin Lymphoma Myeloma Leuk. 2010;10(1):28-43.
Yes for high risk*; No for others Yes
* In clinical trial
5. Mateos M-V, et al. Blood. 2009;114:Abstract 614.
6. Durie BG, Salmon SE. Cancer. 1975;36:842-854.
7. Durie BG, et al. Leukemia. 2006;20(9):1467-1473.



4. Kyle RA, et al. Curr Hematol Malig Rep. 2010;5(2):62-69.
8. Rajkumar SV, et al. Lancet Oncology 2014; 15:e538-
Clonal Bone Marrow
BMPC ≥ 60% S Li
C



Testing For Myeloma: Blood & Urine
Test Name
CBC + differential
Complete metabolic panel
Beta-2 Microglobulin (B2M)
Lactate Dehydrogenase (LDH)
Serum Immunofixation and Protein
electrophoresis (SPEP+IFE)
Immunoglobulins (G, A, M, D, E)
Free light chain assay with kappa/lambda ratio
Urine immunofixation & protein
electrophoresis (UPEP+IFE)
What it means
Hemoglobin, WBC, Platelets
Creatinine, Calcium, Albumin, Liver function
Part of staging and risk stratification

Measures the level of normal and clonal protein
Identifies the type of clonal protein

Measures the level of normal and clonal protein
Identifies the type of clonal protein

This


CBC= Complete Blood Count; WBC = White Blood Cell


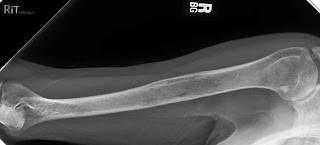
Testing For Myeloma: Imaging
Imaging:
–Skeletal survey: Series of X-rays; less sensitive than other techniques
–Whole body low dose (CTWB-LD CT )
–Positron Emission Tomography (PET/CT)
–Magnetic Resonance Imaging (MRI)

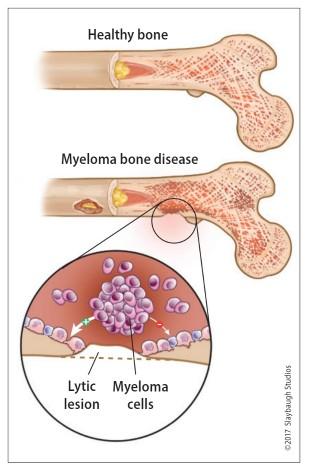
Healthy bone versus myeloma bone disease




Testing For Myeloma: Bone Marrow
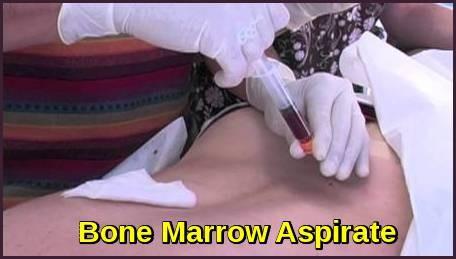

Bone marrow biopsy & aspirate
Bone marrow plasma cells (%)
Congo Red staining if concern for AL-Amyloid

Bone marrow genetics
Cytogenetics
Fluorescence in situ hybridization (FISH)
Next generation sequencing (NGS)
High Risk FISH Results*
Deletions Translocations Gain 1p17p(p53del)
*15-20% of people with NDMM
t(4;14) t(14;16) t(14;20)
1q+



Staging and Risk Stratification

International Staging System (ISS)
Stage Result
1 β2M < 3.5 mg/L; serum albumin ≥ 3.5 g/dL
2 β2M < 3.5 mg/L; serum albumin < 3.5 g/dL; or β2M 3.5 to 5.5 mg/L, irrespective of serum albumin
3

Revised International Staging System (RISS), adding LDH & FISH Stage Result 1





Myeloma Treatment Schema
Transplan
t Eligible Patients
Transplan t
Ineligible Patients
Initial Therap y
Transplant (ASCT) Maintenan
ce Treatme nt of Relapse d disease
Consolidation / Maintenance
Continued therapy
Everyone
Supportive Care
HCP Clinical Experience


TREATMENT DECISION
Your Preference
Research Results
Philippe Moreau. ASH 2015.




Drug Class Overview

IMiD
immunomodulatory drug
Pomalyst (pomalidomide) P or Pom
Revlimid (lenalidomide) R, Rev, Len
Thalomid (thalidomide) T or Thal
Oral (PO)
Proteasome inhibitor Velcade (bortezomib) V or Vel or B SC/SQ or IV IV
Kyprolis (carfilzomib) C or K or Car
Ninlaro (ixazomib) N or I Oral
Chemotherapy
Steroids
Monoclonal Antibodies
Cytoxan (cyclophosphamide) C, CTX Oral IV
Alkeran or Evomela (melphalan) M or Mel
Decadron (dexamethasone) Dex or D or d Oral IV
Prednisone P or Pred
Darzalex (daratumumab)
Sarclisa (isatuximab)
Empliciti (elotuzumab)
Dara Isa Elo
IV or SQ IV IV
XPO1 Inhibitors Xpovio (selinexor) X or Sel Oral


Drug Class Overview

Peptide Drug Conjugate* Pepaxto (Melphalan Flufenamide) Melflufen IV
BCMA Targeted Antibody Drug Conjugate (ADC)* Blenrep (belantamab mafodotinblmf) Bela, Belamaf, or B IV CAR T Cell therapy
Bispecific Antibodies
Pipeline
Abecma (idecabtagene vicleucel) Ide-cel IV
Carvykti (ciltacabtagene vicleucel) Cilta-cel
Tecvayli (teclistimab)
Talvey (Talquetamab)
Elrexfio (Elranatamab)
Tec Talq Elra SC/SQ
Cevostamab, Iberdomide, Mezigdomide, Venetoclax Linvoseltamab, LCAR-B38M …………………………………….. MORE TO COME!
* These agents are currently off the market but available through special programs


Measuring Disease Response: IMWG Response Criteria

















R e s p o n s e
Flow MRD negative*
sCR
Molecular CR CR
VGPR PR MR SD PD
Negative by next generation flow (NGF) (minimum sensitivity 1 in 10-5 nucleated cells or higher)*
mCR AND normal Free Light Chain ratio, Bone Marrow negative by flow, 2 measures
CR AND negative PCR
Complete Response: Negative immunofixation (IFE); no more than 5% plasma cells in BM; 2 measures
Very Good Partial Response: 90% reduction in myeloma protein
Partial Response: at least 50% reduction in myeloma protein
Minimal Response
Stable Disease: Not meeting above criteria
Progressive Disease: At least 25% increase in identified myeloma protein from lowest level
MRD = Minimal Residual Disease
sCR = Stringent Complete Response; BM = Bone Marrow


Kumar, S., Paiva, B., Anderson, K. C., Durie, B., Landgren, O., Moreau, P., ... & Dimopoulos, M. (2016). International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The lancet oncology, 17(8), e328-e346.
When Do I Need A New Treatment?
Biochemical or Symptomatic Progression/Relapse
Not every relapse requires immediate therapy
Each case is different
Symptomatic or extramedullary disease
Asymptomatic high-risk disease or rapid doubling time or extensive marrow involvement
Initiate Treatment
Asymptomatic biochemical relapse on 2 consecutive assessments
Consider Treatment
Patient-/Disease-Specific Monitor Carefully
Consider Observation
Monitor Carefully



Targets on the Myeloma Cell Surface and Therapeutic Antibodies

Bi-Specific Antibodies
Talquetamab
CAR-T






Antibody Drug
Elotuzumab
Bi-Specific Antibodies
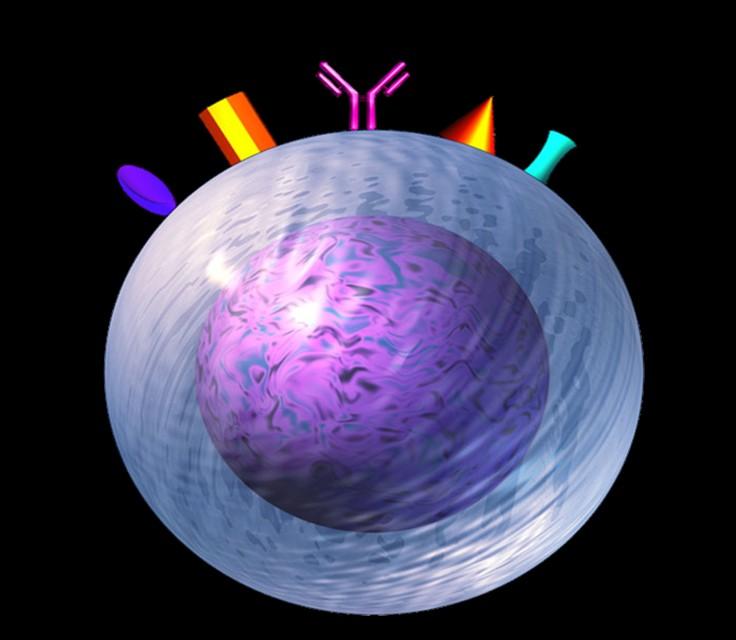


Bi-Specific Antibodies CAR-T
Antibody Drug
Daratumumab and Darzalex Faspro
Isatuximab
TAK-079
MOR202


Immune Therapies
Ide-cel CAR-T
Cilta-cel CAR-T
Teclistamab
Other CAR-Ts
Other Bi-Specific Antibodies

 BCMA
CD38
GPRC
BCMA
CD38
GPRC
Antibody Drug Conjugates
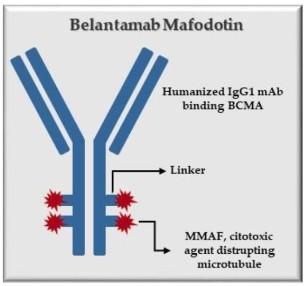

How it works:
An antibody directed at a target (BCMA) combined with a cytotoxic agent (chemotherapy)

ADC = Antibody-Drug Conjugate
BCMA = B-Cell Maturation Antigen
ADCP/ADCC = Antibody-Dependent Cellular
Cytotoxicity & Phagocytosis


Image
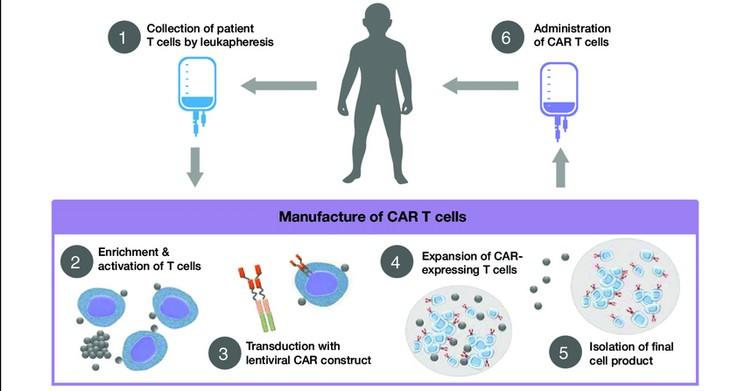
The Process of CAR T Cell Therapy





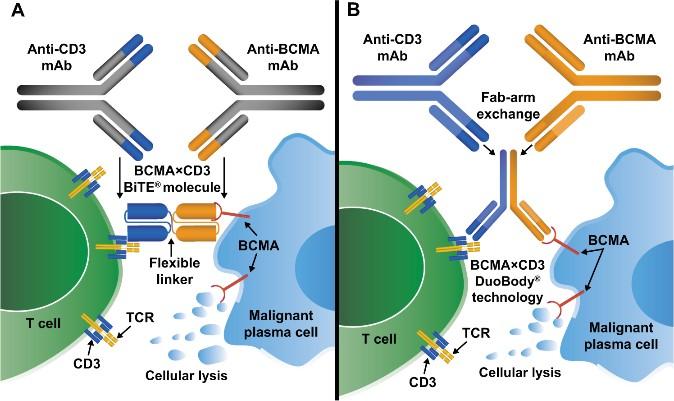
Bispecific Antibodies: Mechanism of Action
• Incorporates 2 antibody fragments to target and bind both tumor cells and T cells
• Brings target-expressing MM cells and T cells into close proximity, enabling T cells to induce tumor-cell death

Antibody-Drug
Targets of Bispecific Molecule Vary
“Off the Shelf” Advantage
• No manufacturing process, unlike CAR T-cell therapy (but like ADC/belantamab therapy)
• Thus, no delay between decision to treat and administration of drug


The Evolution of Myeloma Therapy
VD
Rev/Dex
CyBorD VTD VRD KRD D-VMP DRD
ASCT Tandem ASCT (?)
Nothing Thalidomide?
Bortezomib
Ixazomib
Lenalidomide Combinations
Bortezomib
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Panobinostat
Daratumumab Ixazomib
Elotuzumab Isatuximab
Belantamab mafodotin
Melphalan flufenamide
Idecabtagene autoleucel
Ciltacabtagene autoleucel
Teclistamab, Talquetamab
Elranatamab
D-VRD Isa-VRD D-KRD Isa-VRD
“More” induction?
Daratumumab?
Carfilzomib?
Lenalidomide + PI
CAR T Cell Therapy
Bispecific/Trispecific Antibodies
Cell Modifying Agents
Venetoclax
PD/PDL-1 Inhibition?
Small Molecules


What about Disease Control and Cure in Myeloma?

Biochemical or Symptomatic Progression/Relapse
Control is the immediate priority with active disease
Cure remains the overall goal
Defining “Cure” has many considerations:
Minimal Residual Disease Negative (MRD-)
Time Off Therapy
Functional Cure
Active Disease
Requiring Treatment
Stable or Unmeasurable Disease, Receiving Treatment
Unmeasurable Disease, Receiving No Treatment
https://seer.cancer.gov/statfacts/html/mulmy.html;



















EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Advanced Care Planning

 Wendy Thomas, RN, MSN, CHPN
University of Kansas Cancer Center
Wendy Thomas, RN, MSN, CHPN
University of Kansas Cancer Center
Advance Care Planning


 Wendy Thomas, RN, MSN, CHPN
Wendy Thomas, RN, MSN, CHPN

CHPN
• Outpatient Palliative Care Nurse Navigator
• Kansas City Area Myeloma
Support Group Leader
About me:
• Nurse 27 years
• 14 years in blood and marrow transplant
• 8 years in palliative care
• 10 years as a myeloma support group leader
• Worked for the University of Kansas Health System for 17 years
• Based at the Bloch Cancer Care Pavilion, Westwood Kansas


Advance Care Planning
What is Advance Care Planning?
• Discussing and preparing for future medical care decisions
• Important at any stage of life
• Crucial for anyone with a serious illness



Do you have an Advance Directive?

• Who would you want to speak for you if you were unable to speak for yourself?
• Do your family/loved ones know what your wishes would be in a healthcare emergency?
• Do you know what your wishes would be?






What are your wishes?
Code Status
• What is a Code Status?
– Cardiopulmonary Resuscitation or CPR
• Why do they keep asking?
– Code status expires at discharge
– Out of hospital DNR
– Living will
• How aggressive do you want care to be?
– ICU

– Mechanical Ventilation
– Medically administered nutrition


Deciding about CPR
CPR
• No pulse, not breathing
• One of the few treatments that patients to NOT have performed
• A physician order is REQUIRED to NOT perform CPR
• Older people and people with cancer may have survival & quality of life after CPR
• You can CHOOSE to allow a NATURAL prefer


Level of Medical Interventions?
Pulse present &/or still breathing
• Full treatment
ICU and intubation with mechanical ventilation
• Midlevel treatment
Antibiotics, fluids, medication to support blood pressure
• Best supportive care
Treat with dignity and respect, comfort-focused medical treatment


Out of hospital DNR
• Patients should complete with physician
• Requires physician signature
• Original form stays with patient
• Copy should be provided to all of your healthcare providers/health systems
• Some states have transportable DNR laws



What to do with Forms & Documents?

• Make certain your family/loved ones know the location
• Give a copy to your healthcare providers/health systems
• Easy to find in case of emergency
• These documents DO NOT belong in your safe deposit box


Other Practical issues
Planning ahead
• Eases the burden for family/loved ones
• Protects your assets
• Allows you to manage your personal effects
New Complications
• The electronic era brings new challenges
• Cellphones, computers, online accounts, social media and photos
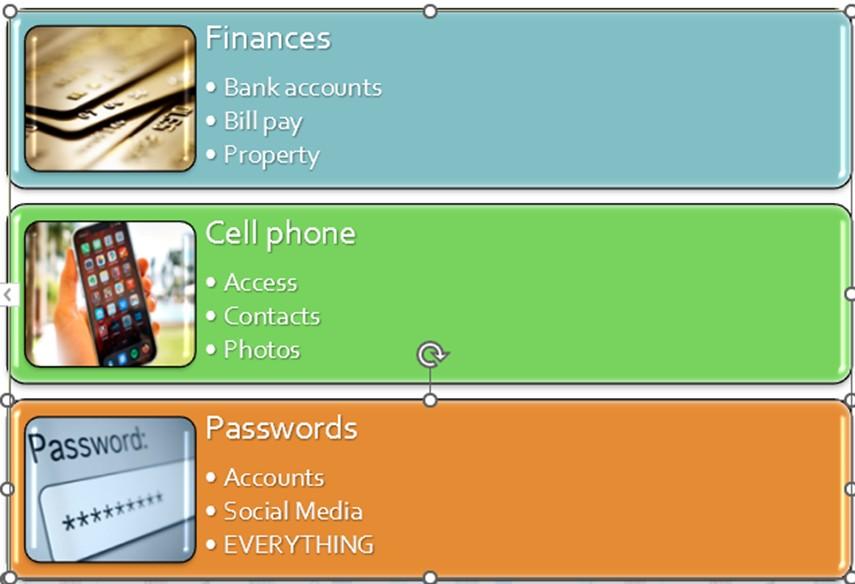



The Most Important Part
Talking with your loved ones about your healthcare wishes





EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Financial Considerations in Myeloma
Amanda Goodstadt, Esq.
Triage Cancer




Financial Considerations in Myeloma
Amanda Goodstadt, Esq. Senior Staff Attorney, Triage CancerThis presentation provides general information on the topics presented. The authors and presenters are not engaged in rendering any legal, medical, or professional services by its presentation or distribution. Although this content was reviewed by a professional, it should not be used as a substitute for professional services.
No part of this presentation may be reproduced, distributed, or transmitted in any form or by any means, without the prior written permission of the author, except properly attributed, noncommercial uses permitted by copyright law. For permission requests, contact the authors at info@triagecancer.org

Triage Cancer is a national, nonprofit organization that provides free education on the legal and practical issues that may impact individuals diagnosed with cancer and their caregivers.
About Triage Cancer


Triage Cancer’s Free Resources

• TriageCancer.org
• Educational Events

• Triage Cancer Conference: 5/17 & 5/18
• Live & Recorded Webinars
• CancerFinances.org
• Quick Guides & Checklists
• Animated Videos

• State Resources & Chart of State Laws
• Legal & Financial Navigation Program



Contributors to Financial Toxicity
• Health Insurance Status
Financial Health Factors That Tip the Scale
• Adequate coverage
• Effective navigation of policies
• Consumer Protections
• Medical Bills
• Employment Changes
• To work or not to work - accommodations
• Disability Insurance
• Life Changes
• Marriage/divorce, moving, graduating from school, etc.



Not


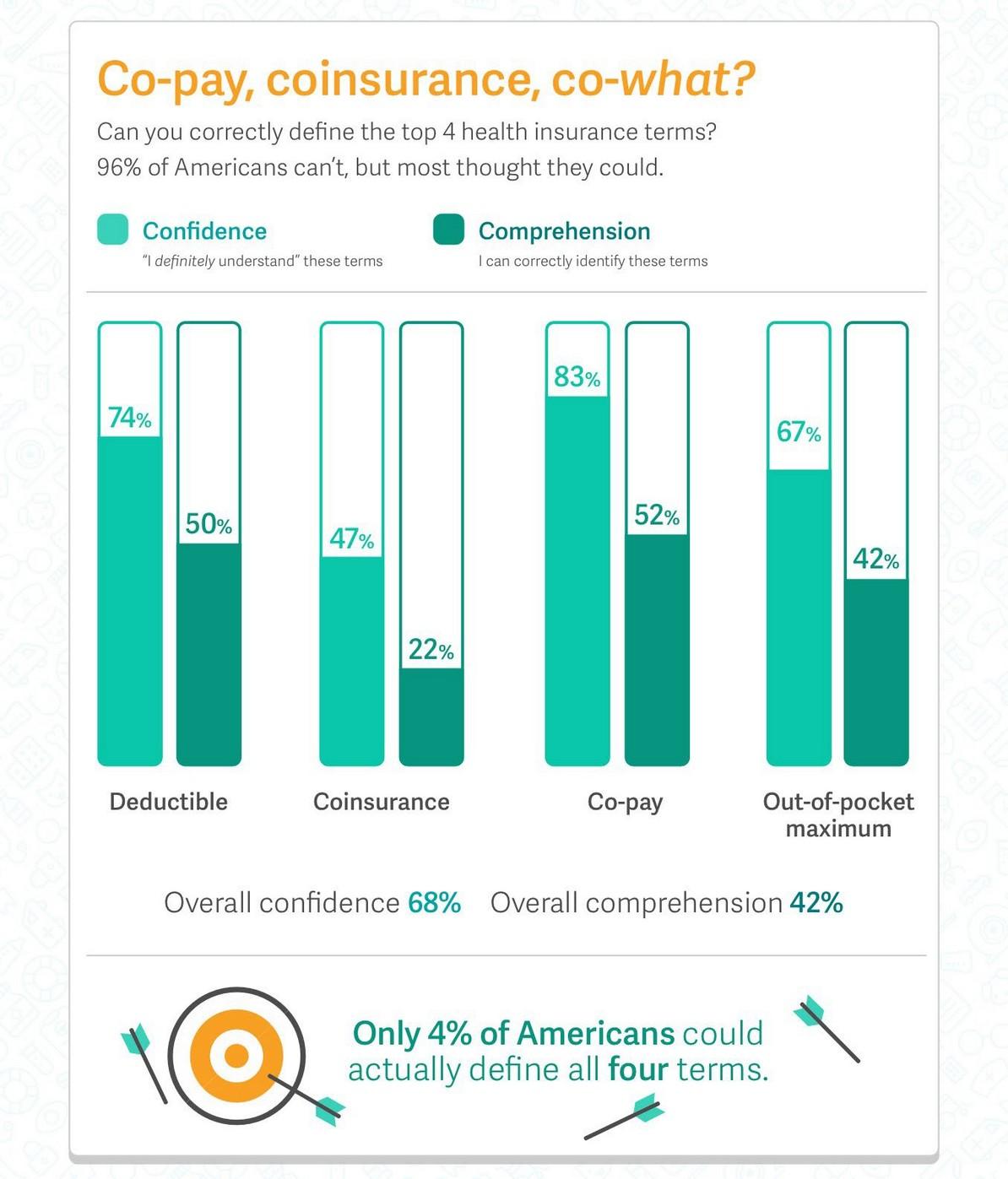
How much time do Americans spend reviewing their health insurance options?
< 30 minutes
< 20 minutes







“As fast as I can!”
> 2 hours per day!

Knowing How to Use Your Health Insurance


Health Insurance Terms: Costs
Cost to Have Health Insurance
• Premium – each month (fixed $ amount)
Costs When You Use Your Health Insurance
• Deductible – each year (fixed $ amount)
• Co-Payment – each time you get care (fixed $ amount)
• Co-Insurance or Cost-Share – each time you get care (%)
• Out-of-Pocket Maximum (fixed $ amount) =
deductible + co-payments + co-insurance

Meet Dan
Dan’s Plan: Deductible = $2,000 Co-insurance = 80/20 plan
OOP Max = $8,000
If Dan has a $102,000 hospital bill, what does he pay?
1. His deductible of $2,000 $102,000-$2,000 = $100,000 left
2. His co-insurance amount of 20% 20% of $100,000 = $20,000

But OOP max is $8,000. So, he would only pay the $2,000 deductible + $6,000 of the $20,000 co-insurance amount, for a total of $8,000.

There may be a separate out-of-pocket maximum for out-of-network services
Individual vs. Family Plans
• e.g., Individual $5,000 and Family $10,000
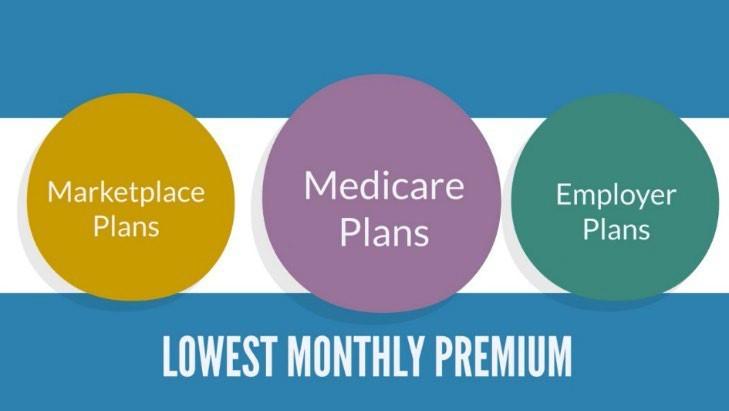
Marketplace Plans
•Out-of-pocket max = deductible + co-payments + co-insurance (medical care & drugs)
Some Employer Plans
• Doesn’t include deductibles
• Out-of-pocket max = co-payments + co-insurance
• Doesn’t include deductibles or co-payments
• Out-of-pocket max = co-insurance
• Doesn’t include prescription drugs
• Separate out-of-pocket max for prescription drugs = co-payments + co-insurance




Medicare
As of September 2023: covers more than 66 million people (~20% of U.S. population)
• Eligibility
• U.S. Citizen or legal resident
• Legal residents = live in U.S. for at least 5 years in a row, including the 5 years just before applying
• And be:
• 65+ years old; or
• Receiving SSDI 2+ years; or
• Have ESRD or ALS

Medicare Options: Pick a Lane
Lane 1: Original Medicare


Do you want an Rx plan (Part D)?

Do you want a Medigap plan?

Lane 2: Medicare Advantage (Part C)


Do you also need a Rx plan (Part D)?

Has OOP Max

Medicare Part D 2024 After the ACA
Beneficiary pays $545 deductible out of pocket
After total out-of-pocket drug costs* = $8,000**
Beneficiary pays 25%
Beneficiary pays nothing

Medicare pays 75%
Medicare pays 100%
*Includes certain payments made on behalf of the beneficiary (e.g., drug company discounts)
**Actual OOP if taking only brand-name drugs ~$3,300

Inflation Reduction Act of 2022
• 2024: Eliminates 5% catastrophic coverage payments (total oop drug costs capped at $8000)
• 2025: Caps out-of-pocket drug costs at $2,000
• Applies to both Part D plans and Part C plans with drug coverage
• Currently, no cap for Part D & Part C out-of-pocket max does not include drugs!
• If plan has a drug deductible, that will count towards the cap
• Cap could increase over time
• 2025: Part D plans will allow out-of-pocket costs to be spread out through the year, rather than a lump sum payment (e.g., in January)


Medicaid
• Federal program
• Provides free or low-cost health insurance coverage
• As of 9/23: 88.4 million people on Medicaid & CHIP (~1 in 4 Americans)
• Federal – state funding partnership
• State administration
• State waivers to customize program


Medicaid as of 1/1/14
Eligibility: low income + low resources +
Adults with household income under 138% of FPL
“Aged, Blind, Disabled”

Minor kids or people with minor kids

Pregnant women for 6-12 months after baby’s birth

Breast & Cervical Cancer Treatment

State Medicaid Expansion 2024
Updated: 2/9/2024 (info changes frequently, please check for updates)
1 MI expansion began 4/1/14
2 PA expansion began 1/1/15
3 NH expansion began 1/1/16
4 IA & AR implemented expansion through premium assistance & wrap around Medicaid
5 AK expansion began 9/1/15
6 MT expansion began 1/1/16; reauthorized with work requirement from 1/1/20-12/31/24 – waiting for fed approval
7 LA Governor signed EO for expansion; began 7/1/16
8 ME expansion began 2/1/19; coverage retroactive to 7/2/18
9 VA expansion began 1/1/19
10 UT full expansion began 1/1/20
11 ID expansion began 1/1/20
12 NE expansion begins 10/1/20
13 OK voters approved a ballot measure 6/20 to expand 7/1/21
14 MO voters approved a ballot measure 8/4/20 to expand 7/1/21, court reinforced expansion requirement
15 SD voters approved a ballot measure 11/22 to expand 7/23
16 NC expansion began 12/1/23
TriageCancer.org/Medicaid-Expansion

State Health Insurance Marketplaces
• “Exchanges” = insurance shopping mall
• Benefits:
• Cap on OOP max: $9,450 individual / $18,900 family (2024)
• Financial help
• Premium tax credits
• Cost-sharing subsidies (aka “reduction”)
Ins. Company

Government: Medicare, Medicaid, Military, State & Local Programs, etc.

Employer

Marketplace Plan Options
Standardized cost-share:

Catastrophic coverage (under 30)

Additional Financial Help Now Available
Cost-Sharing Subsidies
Note: Eligibility for 2024 premium tax credits based on 2023 FPL

Will continue through 2025 under IRA
400% + (2023) $ help reduce monthly premiums to 8.5% of household income

When to Enroll?
• Employer plans: varies (often in the Fall)
• Medicaid: accepted year round
• Medicare: Oct. 15 – Dec. 7*
• Marketplace: Nov. 1 – Jan. 15*
• Enroll by Dec. 15 for coverage that starts Jan. 1

• Some states may have longer open enrollment periods (e.g., CA and NJ until Jan. 31)
*Plans are for a calendar year


Total Annual Cost

Do the Math!
Note: for in-network providers only
Total possible costs for year = 12 months of premiums + OOP max
#1: $200x12 = $2,400
+ OOP = $8,000
Total = $10,400
#2: $275x12 = $3,300
+ OOP = $6,000
Total = $9,300
#3: $400x12 = $4,800
+ OOP = $2,000
Total = $6,800

Key Considerations
• Cost
• Premiums, co-payments, deductibles, co-insurance, out-ofpocket maximums
• Network of providers and facilities
• Check if your providers and facilities (hospitals, labs, imaging centers, etc.) are covered
• Prescription drug coverage
• Which drugs are covered (i.e., formulary)?
• Is there a separate out-of-pocket maximum for drugs?




Communications Post-treatment
• From your insurance company:
We have received a claim
We are processing your claim
Explanation of Benefits



Managing Medical Bills
•
From your provider:
• The bill
• Doesn’t always happen in this order!
• Wait for the EOB before paying any bills
• Keep track and communicate with providers
• Ask questions
• Do you qualify for hospital charity care?
• Apply for help from Dollar For:
https://dollarfor.org/Triage Cancer
• Appeal denials



Reviewing Medical Bills
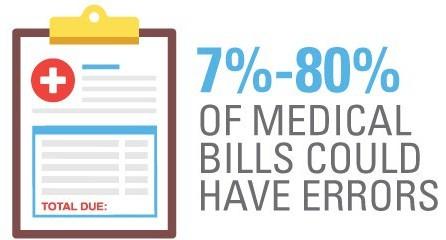
• Review bills for accuracy
• Don’t be afraid to ask your provider to clarify codes!
• Medical procedure billing codes: CPT (Current Procedural Terminology) codes, www.nlm.nih.gov/research/umls/sourcereleasedocs/current/CPT/sourcerepresentation.html
• Medical diagnosis codes: International Classification of Diseases (ICD) codes, www.who.int/standards/classifications/classification-of-diseases
• Review EOBs
• Tips on reading EOBs: https://nhhealthcost.nh.gov/guide/question/how-do-i-read-explanation-benefits-eob
• Consider professional bill reviewer/medical claims org
• Alliance of Claims Assistance Professionals (ACAP) www.claims.org

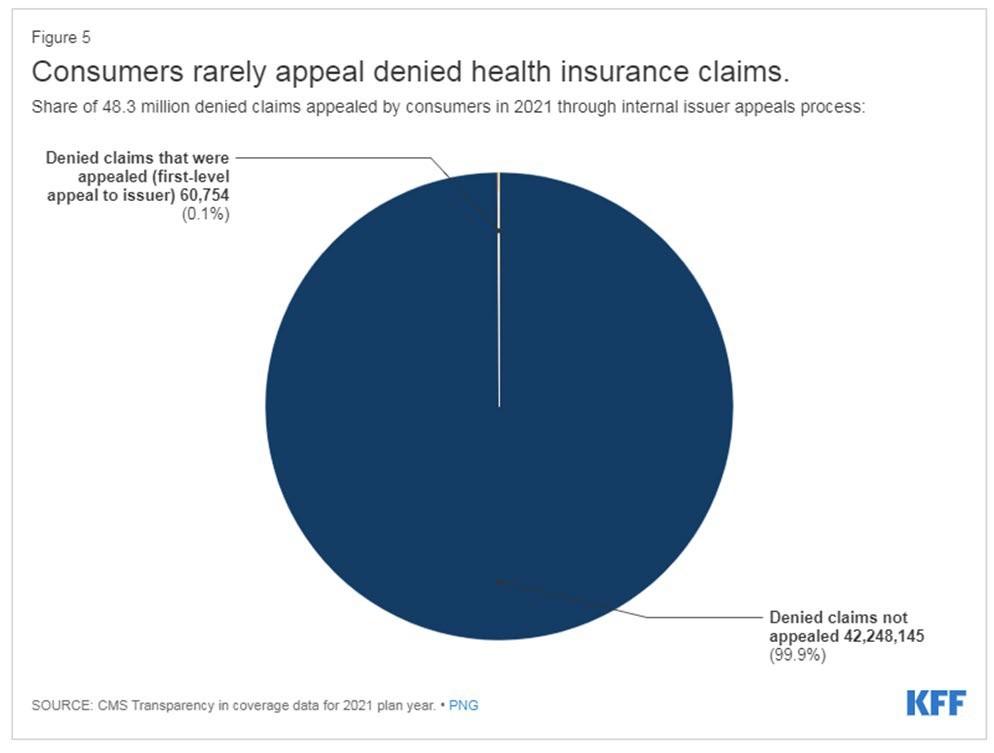
Consumer Protections: Appeals
• Denials of coverage (aka “adverse benefit determination” (ABD))
• Internal appeals
• External appeals (individual and employer plans)
• AKA: Independent or External Medical Review
• Conducted by an independent medical review organization (IRMO) or independent review entity (IRE)
• State Health Insurance Agency: Triagecancer.org/StateResources
• Cost: $0 if HHS process. Up to $25 if issuer contracts with IRO or uses state process


Hurdle: Knowledge



Think Creatively About Financial Assistance





Triage Cancer Conferences
Educational events for:
• Individuals diagnosed with cancer
• Caregivers
• Health care professionals
•Advocates & others Topics:
• Being an Advocate
• Health Insurance
• Finances
• Being Prepared
• Employment
Online: May 17 & 18 October 25 & 26

TriageCancer.org/Conferences
*Free CEs/Contact Hours for nurses, social workers, & patient advocates
*Free PDCs for HR professionals

Triage Cancer Webinar Series
Upcoming Topics:
• February 27 ~ Managing Medical Bills & Getting Financial Help
• March 26 ~ Improving Access to Fertility Preservation
• April 30 ~ Estate Planning
Full Schedule & Registration: TriageCancer.org/Webinars
Recordings of Past Webinars: TriageCancer.org/Past-Webinars
*Free Contact Hour/CE for nurses, social workers, & patient advocates
*Free PDCs for HR professionals


Free, one-on-one help for:
• Individuals diagnosed with cancer
• Caregivers
• Health care professionals
Health Insurance, Employment, Disability Insurance, Finances, Estate Planning, & More
Our Navigation services:
• Explain options
• Provide accurate information
• Empower you to take next steps

Start Online:
TriageCancer.org/GetHelp
For Spanish:
TriageCancer.org/ConsigueAyuda




EVALUATION
Please take a moment to reflect and respond to the program evaluation.
Questions 7 & 8 can be worked on after each presentation.
We greatly appreciate your time and feedback!


Clinical Trials
Joseph Mikhael MD, MEd, FRCPC Chief Medical Officer, International MyelomaFoundation
Professor, Translational Genomics Research Institute, City of Hope Cancer Center


Objectives
• Provide the rationale for clinical trials
• Outline the phases of clinical trials
• Discuss the risks and benefits of clinical trials
• Listen to patients who have been on a clinical trial
Clinical Trials - Overview
Remember some of the important principles of clinical trials:
• The drive of research has brought us to where we are
• No one is expected to be a “guinea pig” with no potential benefit to them
• Research is under very tight supervision and standards
• Open, clear communication between the physician and the patient is fundamental
Clinical Trials – Why Me??
• Every patient is unique and must be viewed that way
• Benefits of trials are numerous and include:
• Early access to “new” therapy
• Delay use of standard therapy
• Contribution to myeloma world – present and future
• Financial access to certain agents
• Must be balanced with potential risks
• “toxicity” of side effects
• Possibility of lack of efficacy
Why Are Cancer Clinical Trials Important?
• Clinical trials translate results of basic scientific research into better ways to prevent, diagnose, or treat cancer
• The more people that take part, the faster we can:
• Answer critical research questions
• Find better treatments and ways to prevent cancer


Overview of New Drug Development

Identify a target for therapy in the laboratory
Confirm the anticancer activity in laboratory and animal studies
Clinical trials (human studies) to determine safety, dosing and effectiveness
The whole process costs millions of dollars and years of effort!
Even Before Phase I
• Most agents are tested in lab models
• Various “myeloma cell lines” = in vitro
• Next step is animal model
• We are more like mice than you think!!
• Earliest study in phase I is called “First in Human”
• Often uses extremely low dose of drug to ensure safety

Clinical Trials - Phases
Phase I Phase II Phase III
Tests safety Tests how well treatment works Compares new treatment to standard treatment
Phase 1 Clinical Trials
• All patients receive the experimental therapy
• Phase 1 trials find the optimal dose of a new drug or drug combination
• Patients get higher doses as the study continues
• Determine side effects of new drugs or combinations
• Explore how the drug is metabolized by the body
• Important for all stages of myeloma
Phase 2 Clinical Trials
• Determine if a new drug or combination is effective against the cancer
• May be added to a phase 1 study once the ideal dose is found
• Patients usually receive the experimental therapy
• In some cases, the study may include two “arms” comparing either two different doses or a different treatment (another combination of drugs)
Phase 3 Clinical Trials
• Highest form of clinical evidence. Typically a large number of patients are required…usually required for full FDA approval
• Patients receive either an experimental therapy (one or more drugs) or the current standard treatment
• The patient is randomly assigned to a treatment—a process called randomization
• Neither the physician or the patient can determine which treatment is given
• May be placebo controlled, if no standard treatments are available
• Very closely monitored for effectiveness and side effects
Clinical trial study design or protocol
•
Each cancer clinical trial has a written detailed study design called a protocol that includes:
• Why the clinical trial is needed
• Purpose of the clinical trial
• What drug or drug(s) are being tested, with a treatment and follow-up schedule
• Safety measures throughout the clinical trial program
• How outcomes will be measured
• Who is eligible for the clinical trial
• How the clinical trial will be organized, one site or multiple sites
• If the clinical trial is a multi-site trial, all participating physicians must follow the same protocol
Benefits of Participation
Possible benefits:
• Patients will receive, at a minimum, the best standard treatment
• If the new treatment or intervention is proven to work, patients may be among the first to benefit
• Patients have a chance to help others and improve cancer care
Risks of Participation
Possible risks:
• New treatments or interventions under study are not always better than, or even as good as, standard care
• Even if a new treatment has benefits, it may not work for every patient
• Health insurance and managed care providers do not always cover clinical trials
Why Do So Few Cancer Patients
Participate in Trials?
Patients may:
• Be unaware of clinical trials
• Lack access to trials
• Fear, distrust, or be suspicious of research
• Have practical or personal obstacles
• Face insurance or cost problems
• Be unwilling to go against their physicians’ wishes
• Not have physicians who offer them trials
• Have a disconnect with their healthcare team
Diversity in Clinical Trials
• There has been a lack of diverse representation in clinical trials in myeloma. In the U.S., approximately 20% of all myeloma patients are of African descent, but only 5%–8% of patients in myeloma clinical trials are of African descent.
• This is significant for the following reasons:
All patients of all races and ethnicities should be able to benefit from clinical trials.
Diverse patient representation in clinical trials is required to ensure that the outcomes are applicable to all patients.
• Reasons for underrepresentation in clinical trials are complex and include systemic racism, accessibility of clinical trials, sensitivity to diversity by medical professionals, misconduct in medicine in the past, the lack of trust in the system, and more.
Why Do So Few Cancer Patients
Participate in Clinical Trials?
Doctors might:
• Lack awareness of appropriate clinical trials
• Be unwilling to “lose control” of a person’s care
• Believe that standard therapy is best
• Be concerned that clinical trials add administrative burdens
Commonly Asked Questions
How does the study work? How often will I need to see my doctor or visit the cancer center?
Will I need to undergo additional tests?
What is currently known about the new drug or combination?


What benefits can I expect?
What side effects should I expect? Who should I notify if I have side effects?


Can I take my vitamins or other medications?
Can I get the treatment with my local doctor?
Will my insurance pay for my participation in the clinical trial?




• Discuss whether or not you are eligible for a clinical trial with your physician
• Work with your physician to determine the best trial for you
• Meet with the clinical research nurse or trials coordinator to discuss the trial
• Carefully review the provided “Informed Consent”
• Describes the study and any potential safety concerns related to the experimental medication


Wrap Up
- Day 1 Recap
- Welcome Reception and Networking: Royal Palms Ballroom
5pm
- Day 2 Announcements
- Saturday breakfast at 7 – 8 am: Veranda Ballroom
- Program begins at 8 am
- Hotel check-out is 11:00 AM
- Evaluations


EVALUATION
Please be sure to complete your program evaluation today.
If you are attending Friday program only, we ask that you turn the survey in at the end of the day.
If you are coming back for the Saturday sessions, please hold onto your survey, bring it back tomorrow and turn it in at the end of the program.
We greatly appreciate your time and feedback!













