II.2.b.ii. Mapping the stakeholders
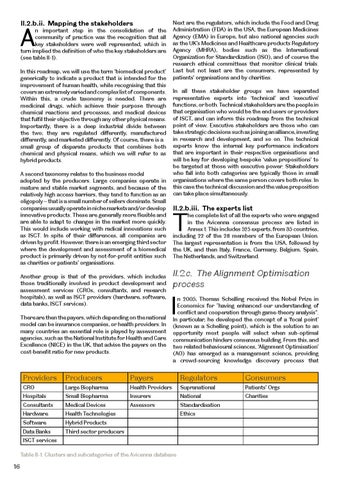
Next are the regulators, which include the Food and Drug Administration (FDA) in the USA, the European Medicines Agency (EMA) in Europe, but also national agencies such as the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), bodies such as the International Organization for Standardization (ISO), and of course the research ethical committees that monitor clinical trials. Last but not least are the consumers, represented by patients’ organisations and by charities.
A
n important step in the consolidation of the community of practice was the recognition that all key stakeholders were well represented, which in turn implied the definition of who the key stakeholders are (see table II-1). In this roadmap, we will use the term ‘biomedical product’ generically to indicate a product that is intended for the improvement of human health, while recognising that this covers an extremely varied and complex list of components. Within this, a crude taxonomy is needed. There are medicinal drugs, which achieve their purpose through chemical reactions and processes, and medical devices that fulfil their objective through any other physical means. Importantly, there is a deep industrial divide between the two; they are regulated differently, manufactured differently, and marketed differently. Of course, there is a small group of disparate products that combines both chemical and physical means, which we will refer to as hybrid products.
In all these stakeholder groups we have separated representative experts into ‘technical’ and ‘executive’ functions, or both. Technical stakeholders are the people in that organisation who would be the end users or providers of ISCT, and can inform this roadmap from the technical point of view. Executive stakeholders are those who can take strategic decisions such as joining an alliance, investing in research and development, and so on. The technical experts know the internal key performance indicators that are important in their respective organisations and will be key for developing bespoke ‘value propositions’ to be targeted at those with executive power. Stakeholders who fall into both categories are typically those in small organisations where the same person covers both roles. In this case the technical discussion and the value proposition can take place simultaneously.
A second taxonomy relates to the business model adopted by the producers. Large companies operate in mature and stable market segments, and because of the relatively high access barriers, they tend to function as an oligopoly – that is a small number of sellers dominate. Small companies usually operate in niche markets and/or develop innovative products. These are generally more flexible and are able to adapt to changes in the market more quickly. This would include working with radical innovations such as ISCT. In spite of their differences, all companies are driven by profit. However, there is an emerging third sector where the development and assessment of a biomedical product is primarily driven by not-for-profit entities such as charities or patients’ organisations. Another group is that of the providers, which includes those traditionally involved in product development and assessment services (CROs, consultants, and research hospitals), as well as ISCT providers (hardware, software, data banks, ISCT services). There are then the payers, which depending on the national model can be insurance companies, or health providers. In many countries an essential role is played by assessment agencies, such as the National Institute for Health and Care Excellence (NICE) in the UK, that advise the payers on the cost-benefit ratio for new products.
II.2.b.iii. The experts list
T
he complete list of all the experts who were engaged in the Avicenna consensus process are listed in Annex 1. This includes 525 experts, from 35 countries, including 22 of the 28 members of the European Union. The largest representation is from the USA, followed by the UK, and then Italy, France, Germany, Belgium, Spain, The Netherlands, and Switzerland.
II.2.c. The Alignment Optimisation process
I
n 2005, Thomas Schelling received the Nobel Prize in Economics for “having enhanced our understanding of conflict and cooperation through game-theory analysis”. In particular, he developed the concept of a ‘focal point’ (known as a Schelling point), which is the solution to an opportunity most people will select when sub-optimal communication hinders consensus building. From this, and two related behavioural sciences, ‘Alignment Optimisation’ (AO) has emerged as a management science, providing a crowd-sourcing knowledge discovery process that
Providers
Producers
Payers
Regulators
Consumers
CRO
Large Biopharma
Health Providers
Supranational
Patients’ Orgs
Hospitals
Small Biopharma
Insurers
National
Charities
Consultants
Medical Devices
Assessors
Standardisation
Hardware
Health Technologies
Software
Hybrid Products
Data Banks
Third sector producers
ISCT services Table II-1. Clusters and subcategories of the Avicenna database
16
Ethics