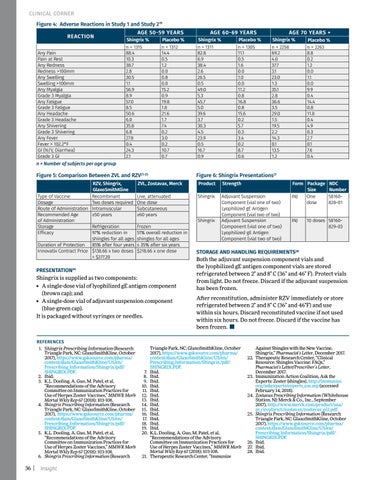
CLINICAL CORNER Figure 4: Adverse Reactions in Study 1 and Study 219 REACTION Any Pain Pain at Rest Any Redness Redness >100mm Any Swelling Swelling >100mm Any Myalgia Grade 3 Myalgia Any Fatigue Grade 3 Fatigue Any Headache Grade 3 Headache Any Shivering Grade 3 Shivering Any Fever Fever > 102.2°F GI (N/V, Diarrhea) Grade 3 GI
AGE 50-59 YEARS Shingrix % n = 1315 88.4 10.3 38.7 2.8 30.5 1.1 56.9 8.9 57.0 8.5 50.6 6.0 35.8 6.8 27.8 0.4 24.3 2.1
Placebo % n = 1312 14.4 0.5 1.2 0.0 0.8 0.0 15.2 0.9 19.8 1.8 21.6 1.7 7.4 0.2 3.0 0.2 10.7 0.7
AGE 60-69 YEARS Shingrix % n = 1311 82.8 6.9 38.4 2.6 26.5 0.5 49.0 5.3 45.7 5.0 39.6 3.7 30.3 4.5 23.9 0.5 16.7 0.9
Placebo % n = 1305 11.1 0.5 1.6 0.0 1.0 0.0 11.2 0.8 16.8 0.8 15.6 0.2 5.7 0.3 3.4 0.2 8.7 0.6
AGE 70 YEARS + Shingrix % n = 2258 69.2 4.0 37.7 3.1 23.0 1.3 35.1 2.8 36.6 3.5 29.0 1.5 19.5 2.2 14.3 0.1 13.5 1.2
Placebo % n = 2263 8.8 0.2 1.2 0.0 1.1 0.0 9.9 0.4 14.4 0.8 11.8 0.4 4.9 0.3 2.7 0.1 7.6 0.4
n = Number of subjects per age group
Figure 5: Comparison Between ZVL and RZV21-25 RZV, Shingrix, GlaxoSmithKline Type of Vaccine Recombinant Dosage Two doses required Route of Administration Intramuscular Recommended Age ≥50 years of Administration Storage Refrigeration Efficacy 97% reduction in shingles for all ages Duration of Protection 85% after four years Innovatix Contract Price $138.66 x two doses = $277.20
ZVL, Zostavax, Merck
Product
Strength
Live, attenuated One dose Subcutaneous ≥60 years
Shingrix
Adjuvant Suspension Component (vial one of two) Lyophilized gE Antigen Component (vial two of two) Adjuvant Suspension Component (vial one of two) Lyophilized gE Antigen Component (vial two of two)
Frozen 51% overall reduction in shingles for all ages ≤ 35% after six years $218.66 x one dose
PRESENTATION26 Shingrix is supplied as two components: ■■ A single-dose vial of lyophilized gE antigen component (brown cap); and ■■ A single-dose vial of adjuvant suspension component (blue-green cap). It is packaged without syringes or needles.
REFERENCES 1. Shingrix Prescribing Information (Research Triangle Park, NC: GlaxoSmithKline, October 2017), https://www.gsksource.com/pharma/ content/dam/GlaxoSmithKline/US/en/ Prescribing_Information/Shingrix/pdf/ SHINGRIX.PDF. 2. Ibid. 3. K.L. Dooling, A. Guo, M. Patel, et al, “Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines,” MMWR Morb Mortal Wkly Rep 67 (2018): 103-108. 4. Shingrix Prescribing Information (Research Triangle Park, NC: GlaxoSmithKline, October 2017), https://www.gsksource.com/pharma/ content/dam/GlaxoSmithKline/US/en/ Prescribing_Information/Shingrix/pdf/ SHINGRIX.PDF. 5. K.L. Dooling, A. Guo, M. Patel, et al, “Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines,” MMWR Morb Mortal Wkly Rep 67 (2018): 103-108. 6. Shingrix Prescribing Information (Research
36 |
Insight
Figure 6: Shingrix Presentations27
Shingrix
Form Package Size INJ One dose INJ
NDC Number 58160828-01
10 doses 58160829-03
STORAGE AND HANDLING REQUIREMENTS28 Both the adjuvant suspension component vials and the lyophilized gE antigen component vials are stored refrigerated between 2° and 8° C (36° and 46° F). Protect vials from light. Do not freeze. Discard if the adjuvant suspension has been frozen.
After reconstitution, administer RZV immediately or store refrigerated between 2° and 8° C (36° and 46°F) and use within six hours. Discard reconstituted vaccine if not used within six hours. Do not freeze. Discard if the vaccine has been frozen.
Triangle Park, NC: GlaxoSmithKline, October 2017), https://www.gsksource.com/pharma/ content/dam/GlaxoSmithKline/US/en/ Prescribing_Information/Shingrix/pdf/ SHINGRIX.PDF. 7. Ibid. 8. Ibid. 9. Ibid. 10. Ibid. 11. Ibid. 12. Ibid. 13. Ibid. 14. Ibid. 15. Ibid. 16. Ibid. 17. Ibid. 18. Ibid. 19. Ibid. 20. K.L. Dooling, A. Guo, M. Patel, et al, “Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines,” MMWR Morb Mortal Wkly Rep 67 (2018): 103-108. 21. Therapeutic Research Center, “Immunize
Against Shingles with the New Vaccine, Shingrix,” Pharmacist’s Letter, December 2017. 22. Therapeutic Research Center, “Clinical Resource: Shingles Vaccine: FAQs,” Pharmacist’s Letter/Prescriber’s Letter, December 2017. 23. Immunization Action Coalition, Ask the Experts: Zoster (shingles), http://immunize. org/askexperts/experts_zos.asp (accessed February 14, 2018). 24. Zostavax Prescribing Information (Whitehouse Station, NJ: Merck & Co., Inc., September 2017), http://www.merck.com/product/usa/ pi_circulars/z/zostavax/zostavax_pi2.pdf. 25. Shingrix Prescribing Information (Research Triangle Park, NC: GlaxoSmithKline, October 2017), https://www.gsksource.com/pharma/ content/dam/GlaxoSmithKline/US/en/ Prescribing_Information/Shingrix/pdf/ SHINGRIX.PDF. 26. Ibid. 27. Ibid. 28. Ibid.