CLINICAL CORNER CONTRAINDICATIONS Do not administer RZV to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine or after a previous dose of Shingrix.10 WARNINGS AND PRECAUTIONS
■■ A patient’s vaccination history should be reviewed for any vaccine-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of RZV.
■■ Immunosuppressive therapies may reduce the effectiveness of RZV.11
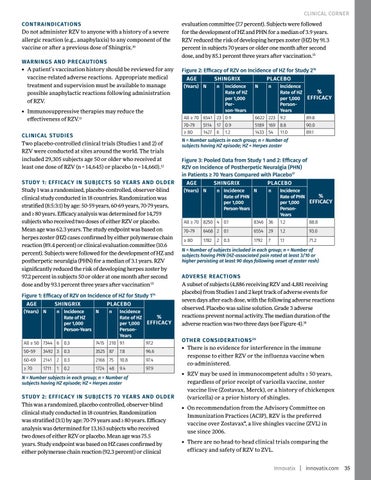
CLINICAL STUDIES Two placebo-controlled clinical trials (Studies 1 and 2) of RZV were conducted at sites around the world. The trials included 29,305 subjects age 50 or older who received at least one dose of RZV (n = 14,645) or placebo (n = 14,660).12 STUDY 1: EFFICACY IN SUBJECTS 50 YEARS AND OLDER Study 1 was a randomized, placebo-controlled, observer-blind clinical study conducted in 18 countries. Randomization was stratified (8:5:3:1) by age: 50-59 years, 60-69 years, 70-79 years, and ≥ 80 years. Efficacy analysis was determined for 14,759 subjects who received two doses of either RZV or placebo. Mean age was 62.3 years. The study endpoint was based on herpes zoster (HZ) cases confirmed by either polymerase chain reaction (89.4 percent) or clinical evaluation committee (10.6 percent). Subjects were followed for the development of HZ and postherpetic neuralgia (PHN) for a median of 3.1 years. RZV significantly reduced the risk of developing herpes zoster by 97.2 percent in subjects 50 or older at one month after second dose and by 93.1 percent three years after vaccination 13 Figure 1: Efficacy of RZV on Incidence of HZ for Study 114 AGE
SHINGRIX
(Years) N
PLACEBO
n Incidence N Rate of HZ per 1,000 Person-Years
n
Incidence % Rate of HZ per 1,000 EFFICACY PersonYears 210 9.1 97.2
All ≥ 50 7344 6 0.3
7415
50-59
3492 3
0.3
3525 87
7.8
96.6
60-69
2141 2
0.3
2166 75
10.8
97.4
≥ 70
1711
0.2
1724 48
9.4
97.9
1
N = Number subjects in each group; n = Number of subjects having HZ episode; HZ = Herpes zoster
STUDY 2: EFFICACY IN SUBJECTS 70 YEARS AND OLDER This was a randomized, placebo-controlled, observer-blind clinical study conducted in 18 countries. Randomization was stratified (3:1) by age: 70-79 years and ≥ 80 years. Efficacy analysis was determined for 13,163 subjects who received two doses of either RZV or placebo. Mean age was 75.5 years. Study endpoint was based on HZ cases confirmed by either polymerase chain reaction (92.3 percent) or clinical
evaluation committee (7.7 percent). Subjects were followed for the development of HZ and PHN for a median of 3.9 years. RZV reduced the risk of developing herpes zoster (HZ) by 91.3 percent in subjects 70 years or older one month after second dose, and by 85.1 percent three years after vaccination.15 Figure 2: Efficacy of RZV on Incidence of HZ for Study 216 AGE
SHINGRIX
(Years) N
n
Incidence Rate of HZ per 1,000 Person-Years All ≥ 70 6541 23 0.9 70-79 5114 17 0.9 ≥ 80 1427 6 1.2
PLACEBO N
n
Incidence Rate of HZ per 1,000 PersonYears 6622 223 9.2 5189 169 8.8 1433 54 11.0
% EFFICACY 89.8 90.0 89.1
N = Number subjects in each group; n = Number of subjects having HZ episode; HZ = Herpes zoster
Figure 3: Pooled Data from Study 1 and 2: Efficacy of RZV on Incidence of Postherpetic Neuralgia (PHN) in Patients ≥ 70 Years Compared with Placebo17 AGE
SHINGRIX
(Years) N
PLACEBO
n Incidence N Rate of PHN per 1,000 Person-Years
All ≥ 70 8250 4 0.1
8346 36
Incidence % Rate of PHN EFFICACY per 1,000 PersonYears 1.2 88.8
70-79
6468 2 0.1
6554
29
1.2
93.0
≥ 80
1782
1792
7
1.1
71.2
2 0.3
n
N = Number of subjects included in each group; n = Number of subjects having PHN (HZ-associated pain rated at least 3/10 or higher persisting at least 90 days following onset of zoster rash)
ADVERSE REACTIONS A subset of subjects (4,886 receiving RZV and 4,881 receiving placebo) from Studies 1 and 2 kept track of adverse events for seven days after each dose, with the following adverse reactions observed. Placebo was saline solution. Grade 3 adverse reactions prevent normal activity. The median duration of the adverse reaction was two-three days (see Figure 4).18 OTHER CONSIDERATIONS 20 ■■ There is no evidence for interference in the immune response to either RZV or the influenza vaccine when co-administered.
■■ RZV may be used in immunocompetent adults ≥ 50 years,
regardless of prior receipt of varicella vaccine, zoster vaccine live (Zostavax, Merck), or a history of chickenpox (varicella) or a prior history of shingles.
■■ On recommendation from the Advisory Committee on
Immunization Practices (ACIP), RZV is the preferred vaccine over Zostavax®, a live shingles vaccine (ZVL) in use since 2006.
■■ There are no head-to-head clinical trials comparing the efficacy and safety of RZV to ZVL.
Innovatix | innovatix.com 35