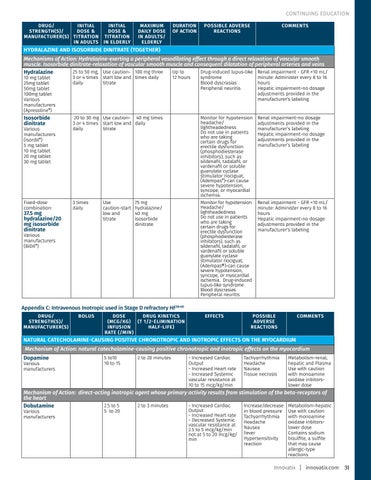
CONTINUING EDUCATION DRUG/ INITIAL STRENGTH(S)/ DOSE & MANUFACTURER(S) TITRATION IN ADULTS
INITIAL DOSE & TITRATION IN ELDERLY
MAXIMUM DAILY DOSE IN ADULTS/ ELDERLY
DURATION OF ACTION
POSSIBLE ADVERSE REACTIONS
COMMENTS
HYDRALAZINE AND ISOSORBIDE DINITRATE (TOGETHER) Mechanisms of Action: Hydralazine-exerting a peripheral vasodilating effect through a direct relaxation of vascular smooth muscle. Isosorbide dinitrate-relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins 25 to 50 mg, Use caution- 100 mg three Up to Drug-induced lupus-like Renal impairment - GFR <10 mL/ Hydralazine 10 mg tablet 25mg tablet 50mg tablet 100mg tablet Various manufacturers (Apresoline®)
3 or 4 times start low and times daily daily titrate
Isosorbide dinitrate
20 to 30 mg Use caution- 40 mg times 3 or 4 times start low and daily daily titrate
Fixed-dose combination:
3 times daily
Various manufacturers (Isordil®) 5 mg tablet 10 mg tablet 20 mg tablet 30 mg tablet
37.5 mg hydralazine/20 mg isosorbide dinitrate
Use caution-start low and titrate
12 hours
75 mg hydralazine/ 40 mg isosorbide dinitrate
Various manufacturers (BiDil®)
syndrome Blood dyscrasias Peripheral neuritis
minute: Administer every 8 to 16 hours Hepatic impairment-no dosage adjustments provided in the manufacturer's labeling
Monitor for hypotension headache/ lightheadedness Do not use in patients who are taking certain drugs for erectile dysfunction (phosphodiesterase inhibitors), such as sildenafil, tadalafil, or vardenafil or soluble guanylate cyclase stimulator riociguat, (Adempas®)-can cause severe hypotension, syncope, or myocardial ischemia. Monitor for hypotension Headache/ lightheadedness Do not use in patients who are taking certain drugs for erectile dysfunction (phosphodiesterase inhibitors), such as sildenafil, tadalafil, or vardenafil or soluble guanylate cyclase stimulator riociguat, (Adempas®)-can cause severe hypotension, syncope, or myocardial ischemia. Drug-induced lupus-like syndrome Blood dyscrasias Peripheral neuritis
Renal impairment-no dosage adjustments provided in the manufacturer’s labeling Hepatic impairment-no dosage adjustments provided in the manufacturer’s labeling
Renal impairment - GFR <10 mL/ minute: Administer every 8 to 16 hours Hepatic impairment-no dosage adjustments provided in the manufacturer's labeling
Appendix C: Intravenous Inotropic used in Stage D refractory HF58-60 DRUG/ STRENGTH(S)/ MANUFACTURER(S)
BOLUS
DOSE DRUG KINETICS (MCG/KG) (T 1/2-ELIMINATION INFUSION HALF-LIFE) RATE (/MIN)
EFFECTS
POSSIBLE ADVERSE REACTIONS
COMMENTS
NATURAL CATECHOLAMINE-CAUSING POSITIVE CHRONOTROPIC AND INOTROPIC EFFECTS ON THE MYOCARDIUM Mechanism of Action: natural catecholamine-causing positive chronotropic and inotropic effects on the myocardium Dopamine
Various manufacturers
5 to10 10 to 15
2 to 20 minutes
- Increased Cardiac Output - Increased Heart rate - Increased Systemic vascular resistance at 10 to 15 mcg/kg/min
Tachyarrhythmia Headache Nausea Tissue necrosis
Metabolism-renal, hepatic and Plasma Use with caution with monoamine oxidase inbitorslower dose
Mechanism of Action: direct-acting inotropic agent whose primary activity results fram stimulation of the beta-receptors of the heart 2.5 to 5 2 to 3 minutes - Increased Cardiac Increase/decrease Metabolism-hepatic Dobutamine Various manufacturers
5 to 20
Output - Increased Heart rate - Decreased Systemic vascular resistance at 2.5 to 5 mcg/kg/min not at 5 to 20 mcg/kg/ min
in blood pressure Tachyarrhythmia Headache Nausea Fever Hypersensitivity reaction
Use with caution with monoamine oxidase inbitorslower dose Contains sodium bisulfite, a sulfite that may cause allergic-type reactions
Innovatix | innovatix.com 31