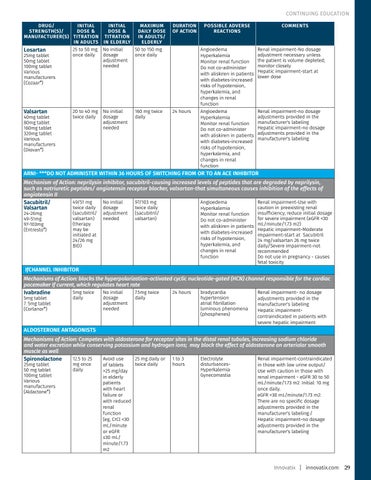
CONTINUING EDUCATION DRUG/ INITIAL STRENGTH(S)/ DOSE & MANUFACTURER(S) TITRATION IN ADULTS 25 to 50 mg Losartan once daily 25mg tablet 50mg tablet 100mg tablet Various manufacturers (Cozaar®)
INITIAL MAXIMUM DOSE & DAILY DOSE TITRATION IN ADULTS/ IN ELDERLY ELDERLY No initial 50 to 150 mg dosage once daily adjustment needed
DURATION OF ACTION
Valsartan
No initial dosage adjustment needed
24 hours
40mg tablet 80mg tablet 160mg tablet 320mg tablet Various manufacturers (Diovan®)
20 to 40 mg twice daily
160 mg twice daily
POSSIBLE ADVERSE REACTIONS Angioedema Hyperkalemia Monitor renal function Do not co-administer with aliskiren in patients with diabetes-increased risks of hypotension, hyperkalemia, and changes in renal function Angioedema Hyperkalemia Monitor renal function Do not co-administer with aliskiren in patients with diabetes-increased risks of hypotension, hyperkalemia, and changes in renal function
COMMENTS
Renal impairment-No dosage adjustment necessary unless the patient is volume depleted; monitor closely Hepatic impairment-start at lower dose
Renal impairment-no dosage adjustments provided in the manufacturer's labeling Hepatic impairment-no dosage adjustments provided in the manufacturer's labeling
ARNI- ***DO NOT ADMINISTER WITHIN 36 HOURS OF SWITCHING FROM OR TO AN ACE INHIBITOR Mechanism of Action: neprilysin inhibitor, sacubitril-causing increased levels of peptides that are degraded by neprilysin, such as natriuretic peptides/ angiotensin receptor blocker, valsartan-that simultaneous causes inhibition of the effects of angiotensin II 49/51 mg No initial 97/103 mg Angioedema Renal impairment-Use with Sacubitril/ twice daily dosage twice daily caution in preexisting renal Valsartan Hyperkalemia 24-26mg 49-51mg 97-103mg (Entresto®)
(sacubitril/ valsartan) (therapy may be initiated at 24/26 mg BID)
adjustment needed
(sacubitril/ valsartan)
Monitor renal function Do not co-administer with aliskiren in patients with diabetes-increased risks of hypotension, hyperkalemia, and changes in renal function
insufficiency; reduce initial dosage for severe impairment (eGFR <30 mL/minute/1.73 m2) Hepatic impairment-Moderate impairment-start at Sacubitril 24 mg/valsartan 26 mg twice daily/Severe impairment-not recommended Do not use in pregnancy - causes fetal toxicity
IƒCHANNEL INHIBITOR Mechanisms of Action: blocks the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel responsible for the cardiac pacemaker If current, which regulates heart rate 5mg twice No initial 7.5mg twice 24 hours bradycardia Renal impairment- no dosage Ivabradine 5mg tablet 7. 5mg tablet (Corlanor®)
daily
dosage adjustment needed
daily
hypertension atrial fibrillation luminous phenomena (phosphenes)
adjustments provided in the manufacturer's labeling Hepatic impairmentcontraindicated in patients with severe hepatic impairment
ALDOSTERONE ANTAGONISTS Mechanisms of Action: Competes with aldosterone for receptor sites in the distal renal tubules, increasing sodium chloride and water excretion while conserving potassium and hydrogen ions; may block the effect of aldosterone on arteriolar smooth muscle as well 12.5 to 25 Avoid use 25 mg daily or 1 to 3 Electrolyte Renal impairment-contraindicated Spironolactone 25mg tablet 50 mg tablet 100mg tablet Various manufacturers (Aldactone®)
mg once daily
twice daily of tablets >25 mg/day in elderly patients with heart failure or with reduced renal function (eg, CrCl <30 mL/minute or eGFR ≤30 mL/ minute/1.73 m2
hours
disturbancesHyperkalemia Gynecomastia
in those with low urine output/ Use with caution in those with renal impairment - eGFR 30 to 50 mL/minute/1.73 m2: Initial: 10 mg once daily. eGFR <30 mL/minute/1.73 m2: There are no specific dosage adjustments provided in the manufacturer's labeling./ Hepatic impairment-no dosage adjustments provided in the manufacturer's labeling
Innovatix | innovatix.com 29