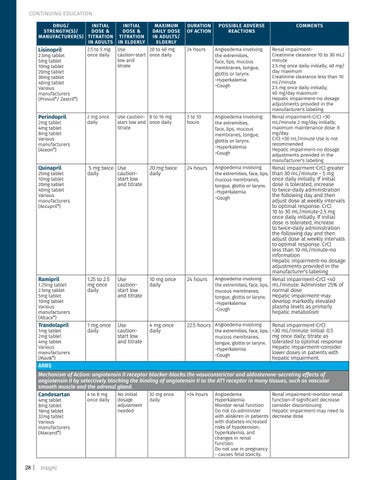
CONTINUING EDUCATION DRUG/ INITIAL STRENGTH(S)/ DOSE & MANUFACTURER(S) TITRATION IN ADULTS 2.5 to 5 mg Lisinopril once daily 2.5mg tablet 5mg tablet 10mg tablet 20mg tablet 30mg tablet 40mg tablet Various manufacturers (Prinivil®/ Zestril®)
INITIAL MAXIMUM DOSE & DAILY DOSE TITRATION IN ADULTS/ IN ELDERLY ELDERLY Use 20 to 40 mg caution-start once daily low and titrate
Perindopril
2 mg once daily
Use caution- 8 to 16 mg start low and once daily titrate
Quinapril
5 mg twice Use daily cautionstart low and titrate
Ramipril
1.25 to 2.5 mg once daily
Trandolapril
1 mg once daily
2mg tablet 4mg tablet 8mg tablet Various manufacturers (Aceon®)
25mg tablet 10mg tablet 20mg tablet 40mg tablet Various manufacturers (Accupril®)
1.25mg tablet 2.5mg tablet 5mg tablet 10mg tablet Various manufacturers (Altace®) 1mg tablet 2mg tablet 4mg tablet Various manufacturers (Mavik®)
DURATION OF ACTION
POSSIBLE ADVERSE REACTIONS
24 hours
Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx. -Hyperkalemia -Cough
3 to 10 hours
Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx. -Hyperkalemia -Cough
20 mg twice daily
24 hours
Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx. -Hyperkalemia -Cough
Use cautionstart low and titrate
10 mg once daily
24 hours
Use cautionstart low and titrate
4 mg once daily
22.5 hours Angioedema involving
COMMENTS
Renal impairmentCreatinine clearance 10 to 30 mL/ minute 2.5 mg once daily initially; 40 mg/ day maximum Creatinine clearance less than 10 mL/minute 2.5 mg once daily initially; 40 mg/day maximum Hepatic impairment-no dosage adjustments provided in the manufacturer's labeling Renal impairment-CrCl >30 mL/minute 2 mg/day initially; maximum maintenance dose: 8 mg/day. CrCl <30 mL/minute Use is not recommended Hepatic impairment-no dosage adjustments provided in the manufacturer's labeling
Renal impairment-CrCl greater than 30 mL/minute - 5 mg once daily initially. If initial dose is tolerated, increase to twice-daily administration the following day and then adjust dose at weekly intervals to optimal response. CrCl 10 to 30 mL/minute-2.5 mg once daily initially. If initial dose is tolerated, increase to twice-daily administration the following day and then adjust dose at weekly intervals to optimal response. CrCl less than 10 mL/minute-no information Hepatic impairment-no dosage adjustments provided in the manufacturer's labeling Angioedema involving Renal impairment-CrCl <40 the extremities, face, lips, mL/minute: Administer 25% of normal dose mucous membranes, tongue, glottis or larynx. Hepatic impairment-may develop markedly elevated -Hyperkalemia plasma levels as primarly -Cough hepatic metabolism the extremities, face, lips, mucous membranes, tongue, glottis or larynx. -Hyperkalemia -Cough
Renal impairment-CrCl <30 mL/minute: Initial: 0.5 mg once daily; titrate as tolerated to optimal response Hepatic impairment-consider lower doses in patients with hepatic impairment.
ARBS Mechanism of Action: angiotensin II receptor blocker-blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. 4 to 8 mg No initial 32 mg once >24 hours Angioedema Renal impairment-monitor renal Candesartan 4mg tablet 8mg tablet 16mg tablet 32mg tablet Various manufacturers (Atacand®)
28 |
Insight
once daily
dosage adjustment needed
daily
Hyperkalemia Monitor renal function Do not co-administer with aliskiren in patients with diabetes-increased risks of hypotension, hyperkalemia, and changes in renal function. Do not use in pregnancy - causes fetal toxicity.
function-if significant decrease consider discontinuing Hepatic impairment-may need to decrease dose