
5 minute read
The Benefits of Meltdown Technology for Implantable Nonwovens
Dr. Jonny Hunter, Research & Development Manager, Fibre Extrusion Technology Limited,
ibre Extrusion Technology Limited (FET), based in Leeds, UK has a long history of R&D and innovation, driven by its mission to help customers find solutions to specific technical problems. In this example, we examine the role of meltblown systems (Figure 1) developed for the medical market.
What Is Meltblown Technology?
Meltblown systems use a one-step process in which high-velocity air blows a molten thermoplastic resin from an extruder die on to a conveyor to form a fine fibrous and self-bonding web. The fibres in the meltblown web are laid together by a combination of entanglement and cohesive sticking. The ability to form a web directly from a molten polymer without controlled stretching gives meltblown technology a distinct cost advantage over other systems (Figure 2).
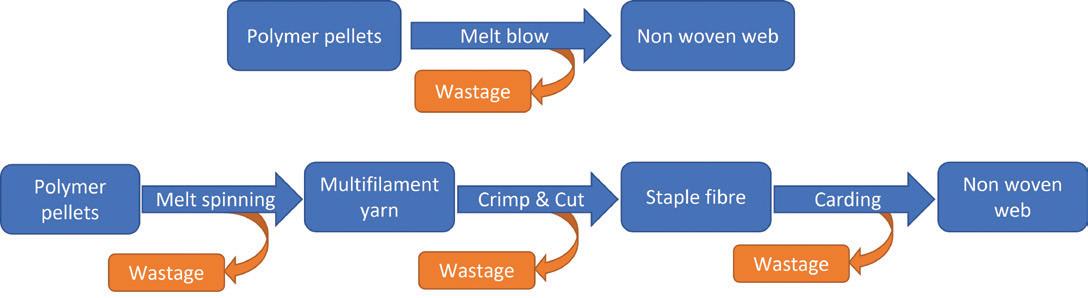
Meltblown to Implantable Nonwoven
As a result of innovative development work by FET, nonwoven structures can now be readily made from “difficult to process” polymers. The new processing technology is a unique adaptation of the meltblowing process and makes possible the conversion of a wide range of polymers into nonwoven webs, using a single processing stage. For many of our clients, the benefits of single-stage manufacturing and the flexibility provided solve many processing problems and give them a substantial competitive edge. This can be coupled with the fact that FET already has a strong capability in processing medical grade polymers used as sutures meaning that the same materials can be used to make a novel range of absorbable nonwoven products. This can significantly de-risk new product development as the base polymers have already been shown to be biocompatible.
New processes have been developed in particular for polymers that have properties which would in normal circumstances make them unsuitable for meltblowing, such as high melt viscosity (IV ≈ 3 dL/g) or susceptibility to degradation. These versatile systems enable the extrusion of meltblown medical nonwovens with a broad span of structures and mechanical properties allowing usage in products like:
• Hernia repair patches
• Staple reinforcement buttresses
• Periodontal & gingival repair
• Adhesion barriers
• Skin substitutes (burns dressing and cosmetic surgery)
• Cell culture support structure (Figure 3)
• Some example resorbable polymers processed include PHA, PGA, PLGA and PDO.
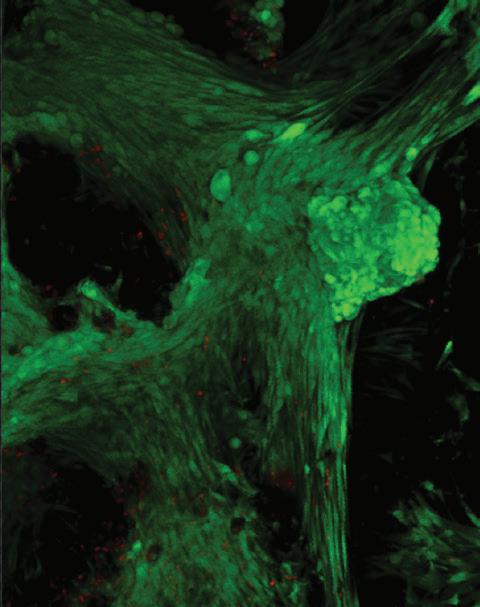
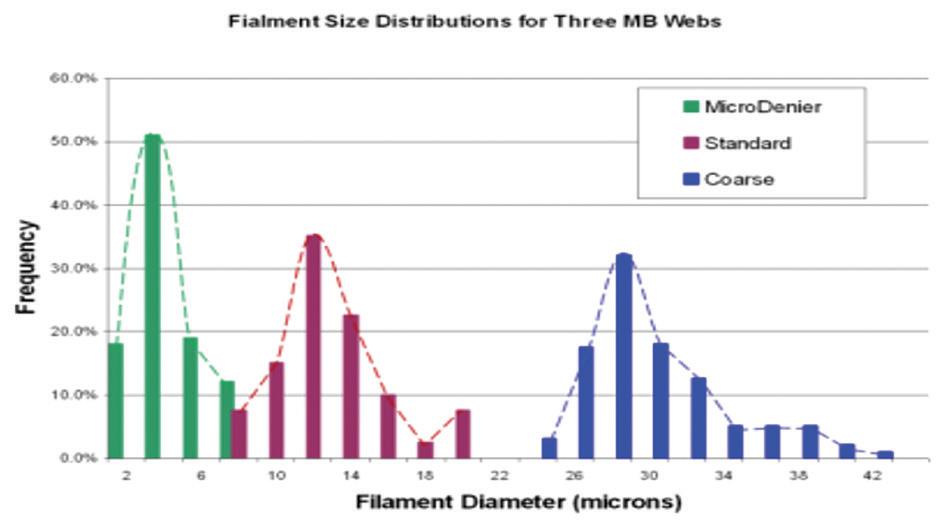
For the reader’s general interest, FET has also been able to produce meltblown nonwovens from other challenging polymers used in other industries. These polymers include PEEK, PET and TPU and are used as filters, clothing and sanitary products. Figure 4 shows a range of filament sizes which can be readily achieve through the use of melt blowing technologies.
Case Study
In this example, a leading medical device manufacturer (MDM) was interested in applying the benefits of FET’s meltblowing technology to produce new materials and products using their existing range of bioresorbable polymers (Figure 5). This case study provides an example of the Research Team at FET working closely with the MDM Project Team to provide a rapid and low risk route to complete the product development and achieve manufacturing capability. The high yield of product from polymer was key to the MDM as the customer’s resin was worth over £6,000 per kg!

Stage 1: Proof of Capability
The first step was for FET to use the in-house meltblowing pilot line to run a Proof of Capability trial. This threeday trial successfully demonstrated that the FET system could use one of their resins to produce a nonwoven web that matched their target structure (Figure 6).
Stage 2: Initial Structure & Polymer Evaluation
A detailed confidentiality agreement was put in place and the MDM selected a range of polymers for further processing trials. These were attended by MDM staff who worked with FET to produce a range of samples with different structures and compositions. Each resin had different processing properties and the equipment configuration and processing conditions needed to be adapted for each one.
Samples were provided for evaluation by the MDM along with a detailed report of the processing properties of each polymer formula-tion, allowing the MDM to select the best polymer for further work.
Stage 3: Lead Option Optimization
The third stage was carried out on the lead option polymer, comprising a series of short trials to produce samples with different structures and properties. This also provided the opportunity for FET to refine the equipment design and processing conditions for the selected polymer and discover how to gain tight control over the filament and web formation processes.
Modifications were made to the design of the spinneret and air blades, which improved the structure and consistency of the web. This iterative process led to the specification of the preferred polymer formulation and web structure, generating the scale up data that was required to design and specify the production unit.
Step 4: Build and Development
Whilst the production line was being built, the progress of the MDM’s project was accelerated by FET producing batches of the specified nonwoven for the customer to continue trials and testing of their prototype product. The nonwovens were made to an agreed SOP covering all aspects of the machine preparation, sample production and data recording.
Step 5: Installation
On completion of the equipment manufacture, FET invited the MDM to attend a factory acceptance test (FAT). This included a detailed review of the equipment and a demonstration of it operating within tight conditions as per determined from the earlier stages of the project. Following this, the equipment was shipped to the MDM where it was installed and a site acceptance test (SAT) was conducted to ensure the equipment was still performing to a high standard after international shipping.
Stage 6: Verification and Training
In the final stage for FET, the R&D staff helped the MDM complete a rigorous verification of the processing systems on the new production line and provided detailed training for the new operators.
Stage 7: Process Qualification and Manufacturing
Recently, the MDM has confirmed they have full qualified the production process and are building the stock to launch the new product. In fact, the initial feedback from focus groups has been so positive that the MDM is looking to repeat the cycle with an alternative polymer to expand their product development pipeline. This will allow them to produce a range of implantable medical nonwovens devices which will degrade over a specific time frame. The product will also be available in a range of sizes, shapes and thicknesses.

Conclusion
This is a brief overview of a new product development process supported by FET in a highly regulated industry. The whole project has taken a number of years to come to completion but shows that novel machine development can have huge impacts, enabling novel products to be made. This ethos of continued machine and process development at FET is a corner stone of the business and puts customers’ needs at the heart of what FET does. www.fetuk.com
Dr. Jonny Hunter is Research & Development Manager at Fibre Extrusion Technology Limited, based in Leeds, UK. Hunter brings a wealth of academic credentials to the department, including a Master’s in Medicinal and Biological Chemistry and a PhD in Sustainable Chemistry. His strong academic background is complemented by over 10 years’ R&D experience in industry, including FMCG and in particular medical devices, which encompasses wound care, the medical device manufacturing process and regulatory environment. Email: jonathan. hunter@fetuk.com, Tel: + 44 (0) +44 113 253 7676 or Mobile: + 44 (0) 7563243181.










