
16 minute read
EMERGENCE
Compiled by Ken Norberg, IFN Editorial & Production Manager
International Filtration News Explores Trending Innovation
IFN highlights significant research from universities and institutions around the world. If you are a part of a project you would like to highlight, email csmith@inda.org. Please write “IFN Emerging Research Submission” in your subject line in order to apply. Please send a completed press release and/or summary of the research as you would want it to be printed, a link to the university online story (if applicable), and all high resolution photographs/charts/graphs, short researcher bio(s). All selections could be edited for length.
University Of Minnesota
Study Shows Air Filtration Systems Significantly Reduce PRRSV Outbreaks
By Jim Eadie
Agroundbreaking study from the University of Minnesota Department of Veterinary Population Medicine, sponsored by AAF International, a global leader in air filtration solutions, has revealed that air filtration systems can dramatically reduce the occurrence of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in U.S. breeding herds.
A comprehensive longitudinal study analyzed 16 years of data from 413 sow farms participating in the University’s Morrison Swine Health Monitoring Project (MSHMP), spanning the period from 2009 to 2024. The research represents more than 1.5 million sows and, for the first time, compares negative versus positive pressure filtration, while also making a solid financial business case for using air filtration to help reduce the occurrence of PRRSV in breeding herds.
Key Findings
The study found that farms with commercial air filtration systems experienced significantly lower risk of PRRSV outbreak compared to unfiltered operations:
• Negative pressure filtered farms: 51% lower risk of PRRSV outbreak.
• Positive pressure filtered farms: 58% lower risk of PRRSV outbreak.
“PRRSV continues to pose significant challenges to the U.S. swine industry,” said Dr. Cesar Corzo, DVM, MS, PhD,
University of Minnesota. “While air filtration methods combined with biosecurity measures have demonstrated effectiveness in preventing PRRSV introductions, this study is the first to comprehensively address the impact of different ventilation pressure types while controlling for regional pig density which is a main risk factor for disease occurrence.”
Study Methodology
The research, led by Dr. Xiaomei Yue, postdoctoral associate with the Morrison Swine Health Monitoring Project, analyzed breeding herd health status data from 413 sow farms, accounting for 1.5 million sows, including 238 unfiltered operations, 128 farms with negative pressure filtration, and 47 with positive pressure filtration systems. Researchers calculated total PRRSV occurrences and weeks at risk for each farm based on air filtration status, while accounting for herd size, the number of farms within a 35-kilometer radius, and the number of pigs in the county.
Using Generalized Additive Models (GAM), the study provided robust statistical evidence while controlling for herd size and regional pig density and comparing filtered versus unfiltered farms within nearby geographic areas.
The findings offer valuable data for swine producers considering air filtration investments. “A single PRRSV occurrence can devastate a farm financially, so this research gives producers evidence-based guidance for implementing air filtration strategies as part of their biosecurity measures to protect their herds from airborne viruses like PRRSV,” comments Dr. Yue.
“For the first time ever, sow farmers can now make a concrete business case for investing in air filtration technology,” said Carlos Lora, Global Director of the Animal Science Division at American Air Filter International. “The numbers don’t lie. In this study, filtration did an excellent job at preventing the risk of PRRSV outbreaks, which is just as much about biosecurity as it is about protecting their businesses.”
READ: https://www.swineweb.com/animalhealth/ new-independent-university-of-minnesota-studyshows-air-filtration-systems-significantly-reduceprrsv-outbreaks-in-breeding-herds/
Penn State
Imperfect Underground Processes Help Filter Wastewater in Florida Keys
Researchers at Penn State find that microbial and other processes do not completely clear wastewater shallowly injected into groundwater of potentially harmful contaminants
By Adam Smeltz
For seaside communities reducing their pollution, nitrogen is a prime target. Often found in agricultural runoff and human waste, nitrogen and the nitrogencontaining nitrate molecule can enter coastal waters as a critical nutrient for algae. Its abundance leads to a surplus of algal blooms, upsetting delicate balances of plant and marine life.
Many South Florida communities dispose of treated wastewater – which contains nitrate and more – by shallowly injecting it into the ground below the groundwater table. Microbes living in the groundwater within the porous limestone bedrock convert and consume wastewater-derived nitrate to nitrogen gas or ammonium. But underground microbes are an imperfect – if still helpful – antidote for wastewater nitrogen in the Florida Keys, a finding that researchers at Penn State said may help other coastal areas with their cleanup strategies. The scientists reported their findings and potential applications in The Depositional Record, a journal in sedimentology.

A Penn State team previously evaluated phosphate – a nutrient involved in many biological processes and applied in industry products, like fertilizer – and its removal from shallowly injected wastewater near a treatment facility in Marathon, Florida.
The treatment facility releases effluent 60 to 90 feet below ground into the porous limestone bedrock near the coastline. Drawing samples from an array of groundwater wells positioned between the effluent injection well and the coastlines of the Florida Bay and Boot Key
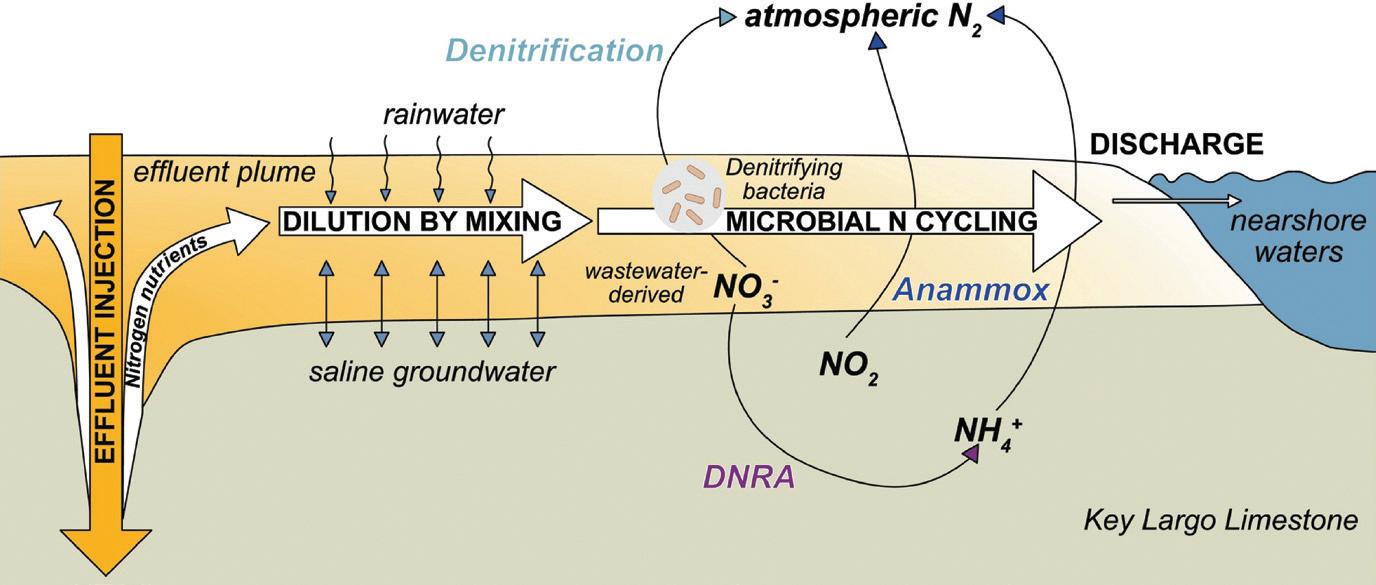
Harbor, which both lead into the Atlantic Ocean, between 2021 and 2023, the researchers consistently found appreciable nitrogen and phosphorus had migrated toward the shore. This indicates that while the underground microbes converted and consumed some of the nitrate and phosphate, they did not successfully capture all of the nutrients.
“Both nitrate and phosphate are greatly reduced between injection and the time the effluent reaches nearshore waters,” said lead author Miquela Ingalls, an assistant professor of geosciences in Penn State’s College of Earth and Mineral Sciences. “Yet the contaminant levels shifted widely over time. How much nitrate and phosphate had already been removed from the water, or still remained, varied by orders of magnitude.”
The variability is likely tied to seasonal differences in wastewater volume and to phosphate’s interactions with porous carbonate bedrock, Ingalls said, explaining additional research would be needed to confirm.
Funded by the Environmental Protection Agency, both phosphate and nitrogen studies centered on contaminant sampling in the Florida Keys National Marine Sanctuary.
The team also wanted to establish whether shallow injections there serve as a “functional equivalent” of the direct discharge of untreated sewage. They found the injections aren’t equal to direct discharges to the ocean, explaining that biogeochemical cycles occurring within the route the waste material takes back to the surface water significantly filter its content compared to a direct discharge.
However, the nitrogen findings signal that effluent may need more travel time from the injection point to coastal waters to better filter the contaminants and avoid adverse ecosystem effects.
One fix may be to modify the effluent’s chemical makeup for greater salinity and density. That approach could keep the discharge from buoying as quickly to the surface, giving it more filtration time, Ingalls explained.
Further research will delve more into the process called adsorption, when phosphate binds to the carbonate bedrock, made of ancient coral reefs. In a follow-up project, researchers are exploring how long the phosphate stays attached and how easily it can dissolve back into the water.
READ: https://www.psu.edu/news/earth-andmineral-sciences/story/imperfect-undergroundprocesses-help-filter-wastewater-florida
THE FULL RESEARCH: https://onlinelibrary.wiley. com/doi/10.1002/dep2.70018
NATIONAL INSTITUTE OF ENVIRONMENTAL HEALTH SCIENCES (NIEHS)
Creating Thermo-Responsive Water Filters
Rollie Mills, Ph.D., developed a novel filter for water contaminants that responds to temperature changes.
By Michelle Zhao
Responsive membranes – materials that change properties in response to different conditions – can sustainably and economically filter pollutants from water, according to Rollie Mills, Ph.D., formerly with the University of Kentucky Superfund Research Center. Mills described his research, which aims to treat water contaminated with chemicals like PFAS.
PFAS are a large group of stable compounds that can leach into water and persist for many years. These chemicals have been linked to many health issues. Existing methods to remove PFAS from water, such as activated carbon or nanofiltration, have drawbacks that limit widespread usefulness.
Developing a Responsive Filter
Mills sought to develop a flexible filter that was not only efficient and affordable but also easily and sustainably reusable. He created a specialized membrane coated with a thermo-responsive polymer that could attract or repel PFAS

Growing up in Kryoneri, Greece, which depended on a nearby waterfall for fresh water, Mills learned the importance of clean water from an early age. Rollie Mills depending on the temperature.
“At temperatures above 35 degrees Celsius, about the temperature of a hot summer’s day, the membrane can attract and capture PFAS from the water,” explained Mills. “Then, at lower temperatures, the material will repel PFAS and allow us to clean and reuse the filter.”
Mills also tested flexible membrane filters that could be used to remove other contaminants in different environments. He incorporated the thermo-responsive polymer into a membrane that could degrade polychlorinated biphenyls (PCBs) and found that the new filter could capture and destroy PCBs more efficiently than the membrane alone. Additionally, Mills found that light could be used to stimulate and heat membranes, introducing the possibility of using sunlight to treat contaminated water.
He said that, in addition to PFAS and PCBs, membrane filters could also be used to remove a range of pollutants, including trichloroethylene and volatile organic compounds.
More Applications of Membranes
In addition to creating membranes for filtering water contaminants, Mills developed an aerosol filter that can neutralize COVID-19 viral particles. The filter, which can be placed in N95 masks, contains enzymes that deactivate disease-causing proteins on the virus within 30 seconds. The membranes are also long-lasting and are capable of degrading viral particles for weeks after their initial use. Michelle Zhao is a science writer for MDB, Inc., a contractor for the NIEHS Division of Extramural Research and Training.
READ: https://factor.niehs.nih.gov/2025/5/ science-highlights/water-filters
ARGONNE/UNIVERSITY OF CHICAGO
Argonne Researchers Develop New Membrane Technology to Extract Lithium From Water
Securing the future supply of a vital resource to modern technology – Argonne and the University of Chicago researchers have developed an advanced membrane technology that extracts lithium from water.
Lithium, the lightest metal on the periodic table, makes it ideal for electric vehicles, cellphones, laptops and military technologies where every ounce counts. As demand for lithium skyrockets, concerns about supply and reliability are growing.
To help meet surging demand and possible supply chain problems, scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have developed an innovative membrane technology that efficiently extracts lithium from water. Several team members also hold joint appointments with the Pritzker School of Molecular Engineering (PME) at the University of Chicago.
“The new membrane we have developed offers a potential low-cost and abundant alternative for lithium extraction here at home,” said Seth Darling, chief science and technology officer for Argonne’s Advanced Energy Technologies directorate.
Right now, most of the world’s lithium comes from hard-rock mining and salt lakes in just a few countries. Yet most of the Earth’s lithium is actually dissolved in seawater and underground saltwater reserves. Extracting it from these unconventional sources has been prohibitively expensive, energy-hungry and inefficient. Traditional methods struggle to separate lithium from other, more abundant elements like sodium and magnesium.
In salt water, lithium and other elements exist as cations. These are atoms that have lost one or more electrons, giving them a positive electric charge. The key to efficient lithium extraction lies in filtering out the other cations based on both size and degree of charge.
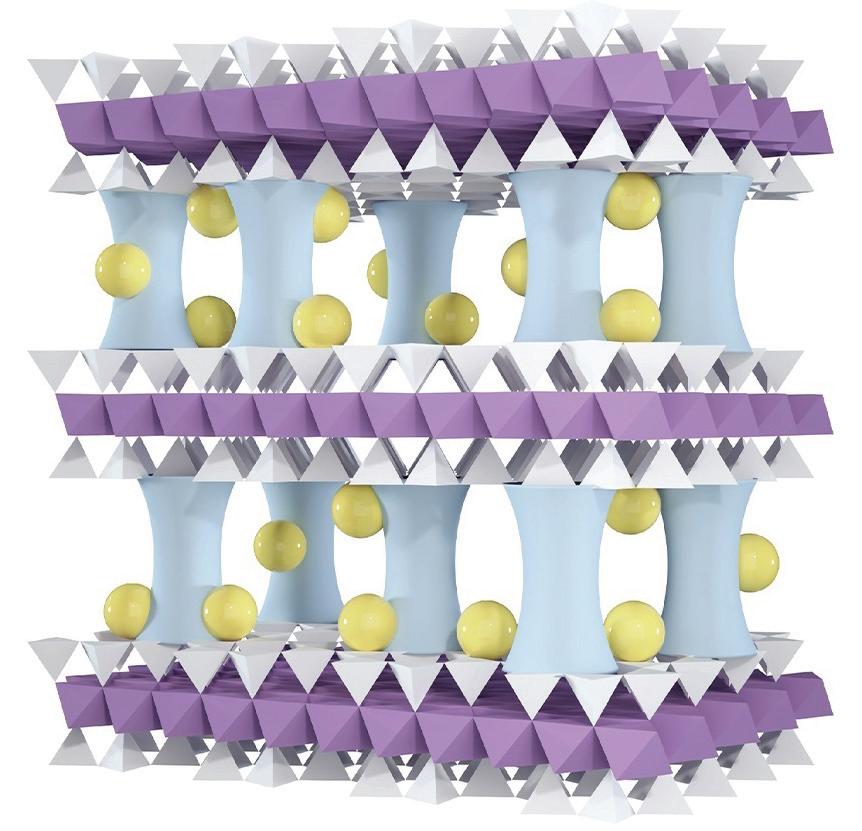
The new membrane offers a promising low-cost solution. It’s made from vermiculite, a naturally abundant clay that costs only about $350 per ton. The team developed a process to peel apart the clay into ultrathin layers – just a billionth of a meter thick – and then restack them to form a kind of filter. These layers are so thin they’re considered 2D.

Untreated, the clay layers fall apart in water within half an hour due to their strong affinity to it. To solve this problem, researchers inserted microscopic aluminum oxide pillars between the layers, giving the structure the look of a high-rise parking lot under construction – with many solid pillars holding each “floor” in place. This architecture prevents collapse while neutralizing the membrane’s nega- tive surface charge, a crucial step for subsequent modifications.
H-shaped cell for studying membrane transport behavior: one half has a salt water mixture (blue liquid), the other shows result after membrane separation (clear liquid).
Next, sodium cations were introduced into the membrane, where they settled around the aluminum oxide pillars. This changed the membrane’s surface charge from neutral to positive. In water, both magnesium and lithium ions carry a positive charge, but magnesium ions carry a higher charge (+2) compared with lithium’s (+1). The membrane’s positively charged surface repels the higher charged magnesium ions more forcefully than it does the

Sorptive materials for customized filtration solutions
Applications
- Cabin air filtration in aircraft, agricultural and landfill vehicles
- Odour removal in households, such as in kitchen hoods, refrigerators, and vacuum cleaners
- Surgical smoke purification
- Purification of contaminated breathable and process air
- Filtration of hazardous substances in air conditioning systems
- Indoor air purifiers
- Solvent recovery systems
Get in contact:
Foam filter
- Variable dimensions, shapes, and adsorbent content
- High adsorbent capacity at very low pressure drop
Pleatable filter media
- Very well suited for limited construction space
- Combined solutions with particle filters or different covers
Customized coating of sorptive composite materials
- Variable layer structure and widths
- Carrier, cover material and roll size according to customer specifications
- Provision of the required materials possible: and granulated etc. lithium ions. This difference allows the membrane to capture lithium ions more easily while keeping magnesium ions out.
To further refine performance, the team added even more sodium ions. This decreased the membrane’s pore size. The result is that the membrane allows the smaller ions like sodium and potassium to pass through while catching the larger lithium ions.
“Filtering by both ion size and charge, our membrane can pull lithium out of water with much greater efficiency,” said first author Yining Liu, a Ph.D. candidate at UChicago and a member of the AMEWS team. “Such a membrane could reduce our dependence on foreign suppliers and open the door to new lithium reserves in places we never considered.”
The researchers believe this breakthrough could have broader applications, from recovering other key materials like nickel, cobalt and rare earth elements, to removing harmful contaminants from water supplies.
This research was funded by AMEWS, an Energy Frontier Research Center funded by the DOE Office of Basic Energy Sciences.
The findings first appeared in the journal Advanced Materials. In addition to Darling and Liu, Argonne authors include Yuqin Wang, Bratin Sengupta, Omar Kazi, Alex B. F. Martinson and Jeffrey W. Elam. Liu, Wang, Kazi, Elam and Darling also hold joint appointments with PME.
READ: https://www.anl.gov/article/argonneresearchers-develop-new-membrane-technologyto-extract-lithium-from-water
RICE UNIVERSITY
A Smarter Membrane for Cleaner Water
New process modeling framework aims to take guesswork out of catalytic membrane design.
By Alexandra Becker
As climate change and population growth place mounting pressure on global water resources, communities around the world are seeking sustainable ways to reclaim water from nontraditional sources such as stormwater, agricultural runoff and municipal wastewater. A team of researchers led by Menachem Elimelech and his former postdoctoral researcher Yanghua Duan at Rice University has taken a major step toward solving one of water purification’s biggest puzzles: how to best design catalytic membranes that simultaneously filter and transform contaminants in a single step.

Menachem Elimelech, the Nancy and Clint Carlson Professor of Civil and Environmental Engineering at Rice. Gustavo Raskosky/Rice University
“Our work addresses a long-standing limitation in the field,” said Elimelech, the Nancy and Clint Carlson Professor of Civil and Environmental Engineering. “Until now, most progress in reactive nanofiltration membranes has been empirical. We’ve lacked a solid framework to understand and optimize how these membranes actually work.”
Reactive nanofiltration membranes offer a powerful promise: the ability to remove salts, heavy metals and small stubborn organic pollutants all at once. But behind that promise has been a challenge – performance that’s hard to predict and scale due to the complex interplay between chemical reactions and mass transport.
To tackle that complexity, Elimelech and Duan developed the first mechanistic model that simulates how oxidants and pollutants move through and react inside catalytic membranes under realistic operating conditions.
“We hypothesized that membrane performance is fundamentally governed by the interplay between reaction kinetics and solute transport,” said Duan, who is now an assistant professor of civil and environmental engineering at Colorado State University. “By capturing these interactions in a model, we can move beyond trial-and-error design.”
Their framework, recently published in Nature Water, simulates how variables like catalyst placement, membrane thickness, pore size and water flow affect the removal of contaminants.
“We discovered that the same membrane can behave completely differently depending on where the catalysts are located,” Duan said. “At low water flux, surface-loaded catalysts dominate, but at higher flux, the action shifts inside the membrane. That has big implications for how we design systems for different water treatment needs.”
One major discovery was identifying the ideal range for catalyst loading. Too little catalyst limits the reaction rate, while too much creates a transport bottleneck.
“We showed that more isn’t always better,” Elimelech said. “There’s an optimal catalyst distribution, and we now know how to find it.”
Elimelech and Duan also introduced new performance metrics that go beyond conventional removal rates – tools that can help engineers better compare and refine membrane systems across the board.
“We’re shifting the field from reactive experimentation to predictive design,” Duan said. “That opens the door to membranes that are not only more effective but also more scalable, energy-efficient and adaptable to different water qualities.”
Importantly, the study lays out design principles for tailoring membranes to specific goals such as minimizing salt contamination, reducing energy use or maximizing contaminant selectivity. The researchers also evaluated how different oxidants, such as hydrogen peroxide and persulfate, behave inside the membrane, showing that the charge of the oxidant strongly influences its accessibility and reactivity.
“Water is too essential to be left to guesswork,” Elimelech added. “Our goal is to empower the global water community with the tools to design smarter, cleaner and more sustainable solutions.”
The research was supported by the Rice Center for Membrane Excellence, the
National Institutes of Health and in part by the Yale University Superfund Research Program, which is supported by a grant from the National Institute of Environmental Health Sciences.
READ: https://news.rice.edu/news/2025/smartermembrane-cleaner-water
DREXEL UNIVERSITY
Drexel to Lead Multinational Effort to Produce MXene Materials for Water Desalination and Medical Diagnostics
Drexel University is embarking on a three-year, $5-million multinational collaboration to produce MXene nanomaterials. The project, which is a collaboration with Kalifa University in the United Arab Emirates, the University of Padua in Italy and the Kyiv, Ukraine-based MXene manufacturing company Carbon-Ukraine, seeks to use the promising nanomaterial, first discovered at Drexel, to provide clean drinking water for arid areas of the world and improve cell labeling and tracking technology for biomedical analysis.
Drexel’s collaboration, MX-Innovation, is part of a broad initiative, funded by Kalifa’s Research & Innovation Center for Graphene and 2D Materials (RIC2D), to translate two-dimensional materials into commercial innovations in areas that include water treatment, energy, health care, and technology infrastructure, among others.
“We are thrilled to begin work on this exciting project,” said Yury Gogotsi, PhD, Distinguished University and Charles T. and Ruth M. Bach professor of Materials Science and Engineering in Drexel’s College of Engineering, and director of the A.J. Drexel Nanomaterials Institute, who is heading the project.
Gogotsi and his collaborators in Drexel’s College of Engineering have been studying MXenes, a family of two-dimensional nanomaterials they discovered in 2011, and testing them in a variety of applications, from telecommunications to energy storage to electromagnetic shielding. This two-dimensional nanomaterial has proven to be exceptionally versatile and easy to integrate into existing technologies because it can be produced in dozens of different chemical configurations, which allows researchers to optimize for each application.

While the material boasts more than 70 patents and has been licensed by the Japanese company Murata Manufacturing Company for development in electronicsrelated applications, its broad commercialization has been slowed by the lack of a commercial-scale production of materials designed for specific applications – an impediment that the MX-Innovation team aims to remove by 2028.
An effort like this to boost the availability of MXenes could enable their widespread use for industrial applications, as well as academic research, according to Gogotsi.
Making More Drinking Water
MXenes have already demonstrated an exceptional acuity for liquid filtration and ion separation. The nanomaterials’ layered structure and adjustable chemical composition allow them to be customized for straining a wide variety of ions or chemicals out of a solution. MXInnovation will harness this capability as it designs a pilot-level device that can turn salt water into drinking water using a physical and electrochemical filtration process.
The UAE already produces 42% of its drinking water through desalination, primarily via an energy-intensive process involving evaporation and condensation of water. But producing more potable water is quickly becoming a priority for large swaths of the world, with some estimates suggesting more than 844 million people do not have access to clean drinking water, and many have to travel long distances to find it.
Drexel researchers have conducted preliminary research in using MXenes for desalination, which has already shown promising results. Focusing on this goal as part of the initiative could speed progress toward the development of a hybrid capacitive deionization (HCDI) technology. It could also reveal other desalination methods that may benefit from the use of MXenes.
“Our preliminary results show that the MXene electrodes with bi-stacked architecture exceed the salt removal performance demonstrated by nanostructured carbon electrodes,” said Yuan Zhang, PhD, a Humboldt Fellow and postdoctoral researcher in the Nanomaterial Institute, who will be helping to lead the group’s desalination research.
Getting a Clearer Look at Cells
The second goal of the MX-Innovation team is to develop MXenes as a cell labeling technology that could improve early detection of cancers, outcomes for transplant patients and possibilities for tissue regeneration.
MXenes will be developed and tested for use in a cell analysis technique, called
Cytometry by Time of Flight (CyTOF), that uses metal materials as tags or labels on the surface and interior of cells to observe and quantify their behaviors and study the interactions of proteins, carbohydrates or lipids within a cell.
This is another area where the multifaceted MXenes have the potential to expand current capabilities. Over the years, Drexel researchers, in collaboration with Lucia Delogu, PhD, from Khalifa University and Laura Fusco, PhD, a Marie Curie Fellow and former postdoctoral researcher in the Drexel Nanomaterials Institute, from the University of Padua; have refined the process of making the nanomaterials to the point that they can uniquely tailor a flake of MXene to latch onto nearly any type of cell –and even the organelles inside cells.
Because MXenes are nontoxic and readily detectable by mass spectrometry technology, they are prime for use have already demonstrated an exceptional acuity for liquid filtration and ion separation. The nanomaterials’ layered structure and adjustable chemical composition allow them to be customized for straining a wide variety of ions or chemicals out of a solution. in this type of biomedical analysis. And the MX-Innovation team has taken the first steps toward a pilot technology that could be improved as part of this effort.
The Goal in Sight
By the end of the project, the team aims to scale up its MXene production and lay the groundwork for a commercial manufacturing facility for the products created by this collaboration.
Carbon-Ukraine will focus on developing a process for low-cost synthesis of MAX Phase – the precursor ingredient for MXenes – which will enable their production at an industrial scale. While this process has been demonstrated and tested to ensure the properties of the MXene are not affected when they are produced in large quantities, it has not yet been implemented in a dedicated manufacturing facility.
READ: https://drexel.edu/news/archive/2025/July/ MXene-desalination-medical-diagnostics-KalifaPadua-Carbon-Ukraine









