
6 minute read
New Water Purification Technology Helps Turn Seawater Into Drinking Water Without Tons of Chemicals
Cutting acid and base treatments from conventional desalination plants could save billions of dollars globally, making seawater a more affordable option for drinking water.
Water desalination plants could replace expensive chemicals with new carbon cloth electrodes that remove boron from seawater, an important step of turning seawater into safe drinking water. A study describing the new technology has been published in Nature Water by engineers at the University of Michigan and Rice University.

technology that’s fairly scalable and can remove boron in an energy-efficient way compared to some of the conventional technologies.”
Boron is a natural component of seawater that becomes a toxic contaminant in drinking water when it sneaks through conventional filters for removing salts. Seawater’s boron levels are around twice as high as the World Health Organization’s most lenient limits for safe drinking water, and five to 12 times higher than the tolerance of many agricultural plants.
“Most reverse osmosis membranes don’t remove very much boron, so desalination plants typically have to do some post treatment to get rid of the boron, which can be expensive,” said Jovan Kamcev, U-M assistant professor of chemical engineering and macromolecular science and engineering and a co-corresponding author of the study. “We developed a new p Jovan Kamcev, an assistant professor of chemical engineering and macromolecular science and engineering at U-M, places a filter membrane between two electrodes, which measure how well the membrane conducts electricity. This helps his team predict how well it can purify water.
In seawater, boron exists as electrically neutral boric acid, so it passes through reverse osmosis membranes that typically remove salt by repelling electrically charged atoms and molecules called ions. To get around this problem, desalination plants normally add a base to their treated water, which causes boric acid to become negatively charged. Another stage of reverse osmosis removes the newly charged boron, and the base is neutralized afterward by adding acid. Those extra treatment steps can be costly.
“Our device reduces the chemical and energy demands of seawater desalination, significantly enhancing environmental sustainability and cutting costs by up to 15 percent, or around 20 cents per cubic meter of treated water,” said Weiyi Pan, a postdoctoral researcher at Rice University and a study co-first author.
Given that global desalination capacity totaled 95 million cubic meters per day in 2019, the new membranes could save around $6.9 billion annually. Large desalination plants – such as San Diego’s Claude “Bud” Lewis Carlsbad Desalination Plant – could save millions of dollars in a year.


This diagram shows how boron is removed by the researchers’ electrodes. First a majority of the salt ions are removed with reverse osmosis. Then the water flows into a cell containing a membrane with positive (pink) and negative (orange) layers. Similarly charged electrodes face the membrane layers, and when a current is applied, water molecules at the interface of the membranes split into hydrogen and hydroxide ions. The hydroxide ions stick to boron, causing it to stick the electrode.

Kamcev, Kamcev Research Lab, University of Michigan, and Weiyi Pan, Elimelech Research Lab, Rice University
Those kinds of savings could help make seawater a more accessible source of drinking water and alleviate the growing water crisis. Freshwater supplies are expected to meet 40% of demand by 2030, according to a 2023 report from the Global Commission on the Economics of Water.
The new electrodes remove boron by trapping it inside pores studded with oxygen-containing structures. These structures specifically bind with boron while letting other ions in seawater pass through, maximizing the amount of boron they can capture.
But the boron-catching structures still need the boron to have a negative charge. Instead of adding a base, the charge is created by splitting water between two electrodes, creating positive hydrogen ions and negative hydroxide ions. The hydroxide attaches to boron, giving it a negative charge that makes it stick to the capture sites inside the pores in the positive electrode. Capturing boron with the electrodes also enables treatment plants to avoid spending more energy on another stage of reverse osmosis. Afterward, the hydrogen and hydroxide ions recombine to yield neutral, boron-free water.
“Our study presents a versatile platform that leverages pH changes that could transform other contaminants, such as arsenic, into easily removable forms,” said Menachem Elimelech, the Nancy and Clint Carlson Professor of Civil and Environmental Engineering and Chemical and Biomolecular Engineering at Rice University, and a cocorresponding author of the study.
“Additionally, the functional groups on the electrode can be adjusted to specifically bind with different contaminants, facilitating energy-efficient water treatment,” Elimelech said.
The research is funded by the National Alliance for Water Innovation, the U.S. Department of Energy, the U.S. National Science Foundation, and the U.S.-Israel Binational Science Foundation. The electrodes were studied at the Michigan Center for Materials Characterization.
READ: https://news.umich.edu/new-water-purification-technology-helps-turn-seawater-into-drinkingwater-without-tons-of-chemicals/
STUDY: A highly selective and energy-efficient approach to boron removal overcomes the Achilles heel of seawater desalination (DOI: 10.1038/s44221024-00362-y) p (From left) SNUs Department of Mechanical Engineering researcher Seongmin Jeong (co-first author), Korea Institute of Science and Technology researcher Jaeho Shin (co-first author), and SNU's Professor Seung Hwan Ko (corresponding author).
Seoul National University College Of Engineering
Filter-Free Microbubble Air Purification System
Professor Seung Hwan Ko’s Research Team at Seoul National University Develops Filter-Free Microbubble Air Purification System. It solves both fine dust and CO2 problems using water-based purification inspired by the human respiratory and circulatory systems.
Seoul National University College of Engineering announced that a research team led by Professor Seung Hwan Ko from the Department of Mechanical Engineering has developed an eco-friendly air purification system using microbubble filters instead of conventional solid filters.
Enclosed indoor environments cause air pollution due to reduced oxygen and carbon dioxide accumulation, as well as fine dust and volatile organic compounds. In this case, ventilation carries the risk of introducing external contaminants, requiring more advanced purification methods.
The filters used in conventional air purification systems are unsuitable for enclosed rooms because they deteriorate due to the accumulation of fine dust and struggle to remove molecular substances, such as volatile organic compounds. Furthermore, the waste generated from filters that require regular cleaning and replacement has contributed to environmental pollution, highlighting the increasing demand for a new, eco-friendly air purification technology.

In response, the research team has developed a comprehensive air purification system inspired by the gas exchange principles of the human respiratory and circulatory systems. This innovative system not only removes indoor fine dust but also expels accumulated carbon dioxide and volatile organic compounds outdoors while supplying fresh oxygen to address oxygen deficiencies.
The human circulatory and respiratory systems prevent the entry of external pollutants while supplying oxygen to cells via the bloodstream and expelling unnecessary carbon dioxide. This process involves natural gas exchange in the alveoli and capillaries, effectively blocking the infiltration of fine dust. Simultaneously, waste materials are excreted through the p Figure 2. Actual configuration of an air filtration system simulating the human circulatory system/respiratory tract. (a) Schematic of a circulating air purification system consisting of water, a gas exchange unit, and a circulation pump. (b) Structure of the gas exchange unit and elastic micro-pore filter using microbubbles. (c) Actual appearance of the MEF and an optical‐microscope image showing the micro‐holes array within the MEF. (d) Photograph taken with an ultra-high-speed camera during actual microbubble generation.


Seoul National University College of Engineering
4. Animal test to identify and resolve the problem of indoor oxygen deprivation/carbon dioxide buildup due to breathing (top). Measured activity of rats with and without a circulating air purification system (bottom).
Seoul National University College of Engineering
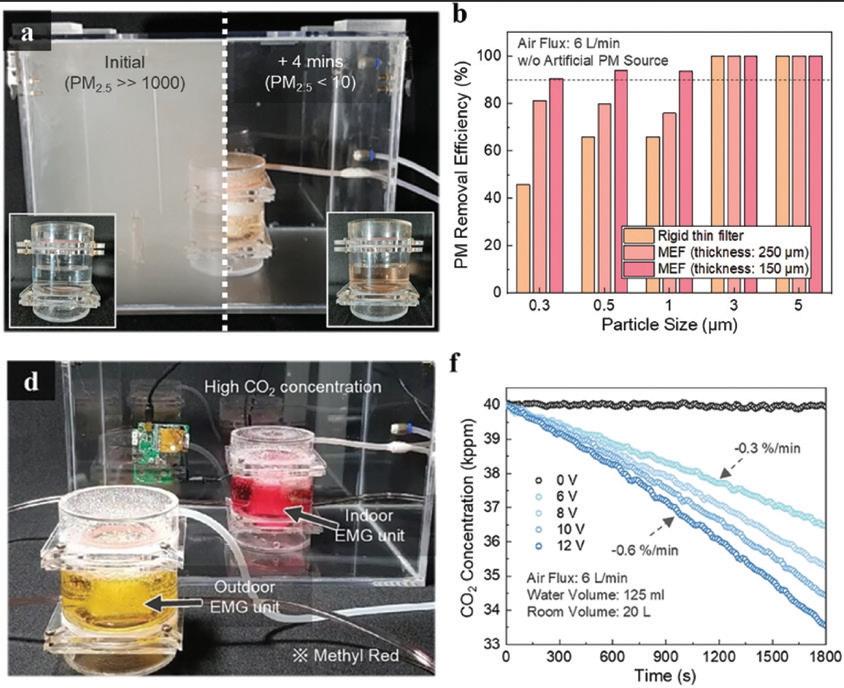
Figure 3. Evaluation of particulate matter and CO2 removal performance of human-simulated circulating air purification systems. Seoul National University College of Engineering kidneys, ensuring the body’s efficient purification and detoxification mechanisms.
(a) Photograph of the particulate matter (PM) removal process in an enclosed space.
(b) Filter efficiency measurement results for different PM sizes.
(d) Photo of CO2 removal process in an enclosed space.
(f) Measurement of CO2 removal performance as a function of water circulation rate.

Inspired by this principle, the research team developed a water circulation system that mimics blood circulation. Through this innovation, they demonstrated the ability to maintain normal carbon dioxide levels in indoor air. Additionally, they proved that an elastic filter, developed using laser technology, can generate smaller and more uniform microbubbles compared to traditional bubble production methods.
The microbubble-based gas exchange system features a simple principle and structure, enabling easy scalability by increasing the size or number of devices. The research team experimentally demonstrated its applicability across a range of settings, from compact tabletop and vehicle-mounted units to larger spaces such as offices and conference rooms.
Professor Seung Hwan Ko said, “This environmentally friendly technology, which replaces traditional filters with a simple water-based mechanism, purifies both particulate and molecular pollutants without generating filter waste, providing a sustainable alternative to existing filtration systems.”
The results of the research, which was supported by the Ministry of Science and ICT and the National Research Foundation of Korea, were published on October 10 2024 in Advanced Materials, an internationally renowned journal in the field of materials.
READ: https://en.snu.ac.kr/research/ highlights?md=v&bbsidx=151077










