6 minute read
Health Canada's recent approvals in 2025
by Brigitte Leonard, Ph.D.
Health Canada has approved four new innovative medications since the last issue of the E3 Advocacy in April 2025 (Table 1).
A New Option For Canadians Diagnosed With Pompe Disease
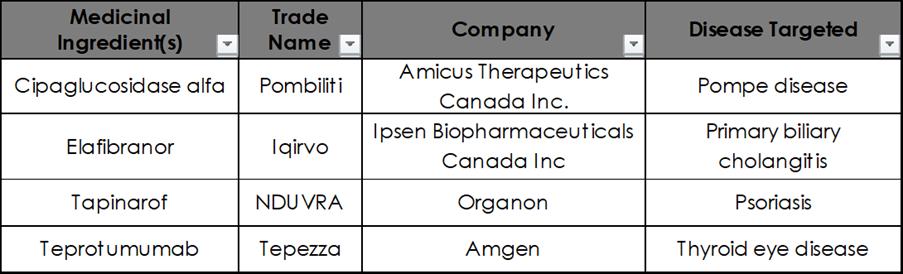
Pompe disease is a rare genetic disorder which damages muscle and nerve cells throughout the body. Pompe disease is caused by mutations in the gene (GAA) responsible for producing an enzyme (acid alpha-glucosidase) which breaks down glycogen into glucose within the cell. The excess carbs that we eat are stored in our bodies as glycogen in the liver and muscle cells. Glycogen will be broken down into glucose to provide energy on demand. People with Pompe disease cannot access this energy reserve, and the accumulation of glycogen leads to progressive muscle weakness, particularly in the heart, skeletal muscle, liver and nervous system. Without effective treatment, Pompe disease can lead to life-threatening complications.
Health Canada approved Amicus Therapeutics' innovative treatment, Pombiliti (Cipaglucosidase alfa), in April 2025 Pombiliti is an enzyme replacement therapy that can be used in combination withmiglustattotreatPompedisease
The approval of Pombiliti has been based on a phase III clinical trial called PROPEL. Pombiliti plus miglustat provide potentially clinically meaningful improvements in motor and respiratory function compared with the current standard therapy (alglucosidase alfa plus placebo) with a comparable safety profile.
NewHopeForCanadiansWithPrimaryBiliaryCholangitis(PBC)
Primary Biliary Cholangitis (PBC) is a rare, chronic, progressive autoimmune disease of the liver affecting mainly women PBC is caused by antibodies attacking the bile duct cells, progressively destroying the small bile ducts in the liver and impeding bile flow. The bile accumulates in the liver, causing damage, fibrosis and cirrhosis. Cirrhosis increases the risk of complications, including liverfailureandapotential needforatransplant.

Symptoms are severe pruritus, weakness, fatigue, dry eyes and mouth, abdominal discomfort, osteoporosis, high cholesterol, and jaundice. Current treatment efficacy is limited to addressing symptoms and slowing down the progression. UDCA, a tertiary hydrophilic bile acid, is the first-line drug approved for patients with PBC; however, 40% of patients do not respond adequately and experience significant adverse effects. Liver transplant is an extreme procedure with several limitations.[1] Obeticholic acid, a selective farnesoid X receptor agonist, is approved as a second-line therapy. However, less than 50% of patients achieve a biochemical response. It can aggravate pruritus.[1] These limitations of currently available treatment options highlight the pressing need for an effective and better-tolerated therapy for patients suffering from PBC.
Health Canada approved Ipsen Pharmaceutical's innovative treatment, Iqirvo (elafibranor), in April 2025. Iqirvo is an oral treatment targeting two forms of a molecule called PAPR. Iqirvo allows for reducing bile acid production and improving bile flow, which helps alleviate liver damage. The approval of Iqirvo has been based on a phase III clinical trial called ELATIVE. Iqirvo provides a significant improvement in biochemical response for PBC patients with an inadequate response or intolerance to UDCA. Iqirvo is a crucial option for helping to reduce disease activity and ease symptoms. However, we're still awaiting data to see whether these translate into long-term health benefits through real-world evidence analysis and the Phase III trial called ELFIDENCE.

Introduction Of A New Treatment Option For Canadians With Psoriasis
Psoriasis is a common, long-term, chronic skin disease that causes a rash with itchy, scaly patches, most commonly on the knees, elbows, trunk and scalp. In psoriasis, the life cycle of skin cells is significantly accelerated, leading to a buildup of dead cells on the epidermis surface. It can be painful, interfere with sleep and make it hard to concentrate. The condition tends to fluctuate in cycles, with periods of flaring for a few weeks or months, followed by periods of subsiding for a while. Common triggers in people with a genetic predisposition to psoriasis include infections, cuts or burns, and certain medications. Treatments are available to help you manage symptoms. You can also try lifestyle habits and coping strategies to help you manage psoriasis more effectively.
However, treatment failure rates for psoriasis can be relatively high, ranging from 48% to 75%, depending on the treatment type and individual patient factors, underscoring the need for additional options.
Health Canada approved Organon's innovative cream, NDUVRA (tapinarof), in April 2025. The approval of NDUVRA is based on the results from two phase III pivotal studies called PSOARING 1 and PSOARING2.
NDUVRA was highly efficacious in reducing pruritus across multiple patient-reported outcome measures, with rapid, statistically significant, and clinically meaningful improvements. The high proportion of patients achieving the treatment target of an itch-free state at week 12 (50%) is a noteworthy clinical outcome for a non-steroidal topical cream in the treatment of mild to severe plaque psoriasis.
And What About Thyroid Eye Disease?
Thyroid Eye Disease (TED), also known as Graves' eye disease, is an autoimmune disorder that affects the tissues and muscles around the eyes, often causing swelling, bulging eyes, and other vision problems It's not a direct disease of the thyroid itself, but somewhat related to an abnormal immune response
The body produces autoantibodies (typically against TSH receptors the primary receptor that controls thyroid activity but other components can be involved). These autoantibodies and related immune cells attack tissues within the eye socket (orbit).
The conventional treatment for TED focuses on reducing inflammation and relieving symptoms while preserving vision. They varied from eye drops and lubricants to antiinflammatory medication, surgery, and radiotherapy. While current treatments can reduce disease activity and improve symptoms, a significant unmet need remains for more effective, less invasive, and personalized therapies, particularly in the areas of chronic disease management, disease prevention, and enhancing health-related quality of life.

Health Canada's recent approvals in 2025 cont'd
Health Canada approved Amgen's innovative therapy, Tepezza (teprotumumab), in April 2025. Tepezza is indicated in adults for the treatment of moderate to severe active TED. It is an antibody that targets the insulin-like growth factor-1 receptor (IGF-1R) on orbital tissues, thereby disrupting inflammation and tissue swelling in TED.
The approval of Tepezza has been based on two phase III clinical trials called OPTIC and OPTIC-J. Teprotumumab significantly improved eye bulging compared to placebo in longstanding, low-inflammation TED, demonstrating efficacy regardless of disease duration or activity. The safety profile was comparable to that previously reported.
Brigitte Léonard, Ph.D.
Brigitte has had the privilege of working in Pharma for over 20 years, contributing to bringing life-changing treatments to patients with the highest ethical standards. Now, she wants to share her knowledge and utilize her scientific, strategic, and communication skills to help the patients’ community.
She obtained her Ph.D. in Biomedical Sciences from Université de Montréal in 2003. Her doctoral research was conducted at Maisonneuve-Rosemont Hospital Research Center. She developed a quantitative diagnostic assay in non-Hodgkin's lymphoma and evaluated the relevance of this marker in the patient's outcome.












