














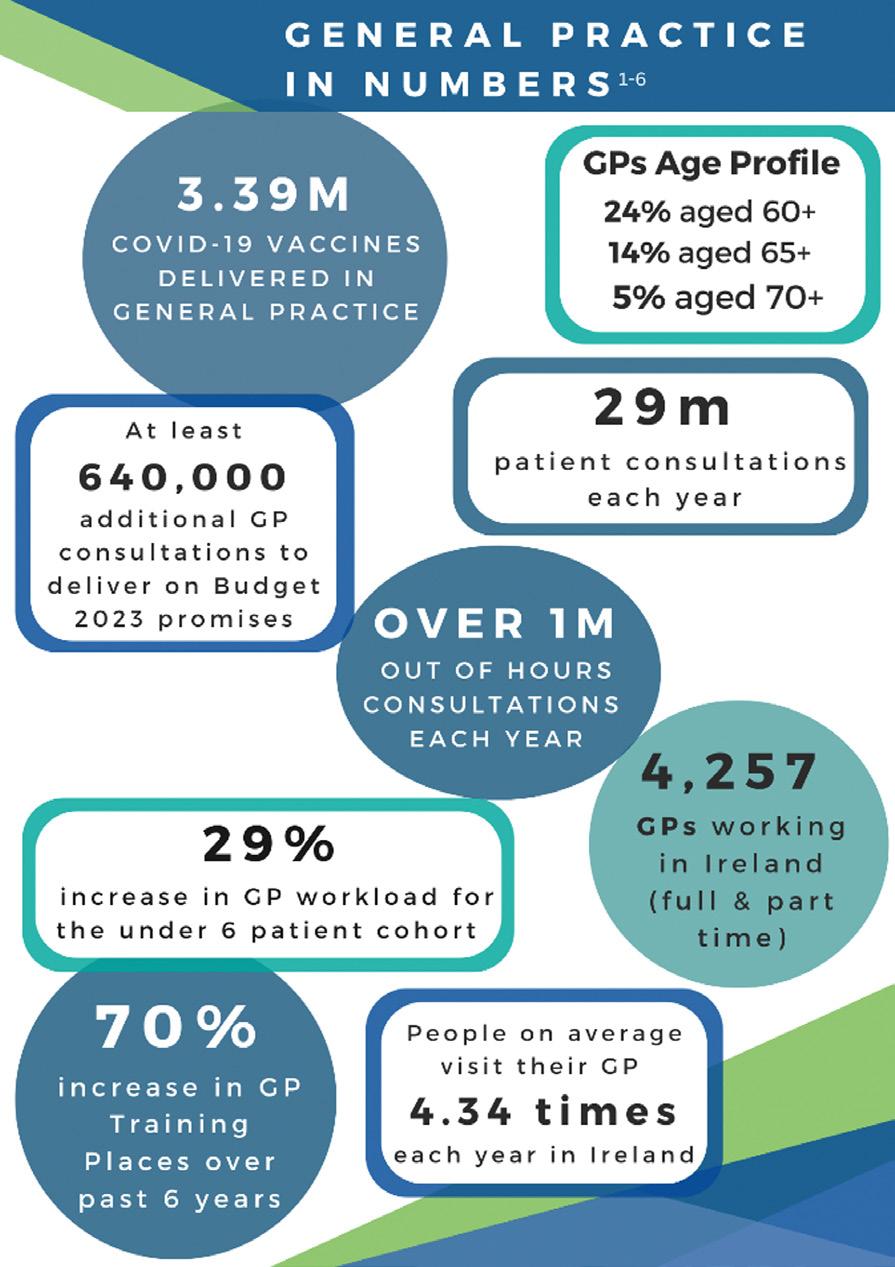
Welcome to the final edition of Nursing in General Practice for 2022. Once again, this month’s edition reflects the diverse nature of the general practice nurse’s (GPN) role. Whether you are new to general practice nursing or an experienced GPN, the articles featured in this issue provide both new knowledge and an opportunity to update.

Firstly, there is a double feature on immunisation, with one article focusing on influenza and Covid-19 vaccines and a second article on monkeypox vaccine by Dr Tom Barrett from the National Immunisation Office, who is well known to GPNs for his frequent support. Immunisation is such a rapidly changing area and accounts for a large volume of the GPN’s workload. We welcome Dr Barrett’s contribution to this edition.
Iron deficiency in cardiology is the focus of a new NurseCPD module. Dr Patrick O’Callaghan, Consultant Cardiologist, and Dr Robert Evans, SpR in Cardiology, are the authors of this module. The module provides an overview of the latest approaches and evidence in treating iron deficiency in patients with cardiovascular issues. This module is available on NurseCPD at: www.medilearning.ie/nursecpd/iron-deficiency-in-cardiology-_-2022.
Priscilla Lynch outlines the major highlights from the Irish Osteoporosis Society's Annual Medical Conference in October. She discusses the impact, prevention, and management of bone

disease, 'health passports' for children, the importance of pelvic floor training, and other issues of focus from the event.
November is a busy month for health awareness days, with two of the chronic disease management conditions being featured – diabetes and COPD. This year sees World Diabetes Day being held on 14 November. Every year, the World Diabetes Day campaign focuses on a dedicated theme that runs for one or more years. The theme for World Diabetes Day 2021-23 is ‘Access to diabetes care.’ Access to diabetes care in Ireland has improved dramatically over the past 20 years. As hospital diabetes teams are slowly being integrated into primary care, this will make diabetes care even more accessible for patients, as care will be delivered locally. Priscilla Lynch
provides a comprehensive overview of the new NICE Guidelines for type 1 diabetes management.
Also taking place, is World COPD Day. The 2022 theme for World COPD Day is ‘Your lungs for life’ and it takes place on 16 November. This year's theme aims to highlight the importance of lifelong lung health. From development to adulthood, keeping lungs healthy is an integral part of future health and wellbeing. This campaign focuses on the contributing factors to COPD from birth to adulthood and what we can do to promote lifelong lung health, as well as protect vulnerable populations. The article in this issue provides an overview on diagnosis and management of stable COPD.
We would like to thank all the contributors to Nursing in General Practice during 2022 for their expertise in producing relevant, up-to-date, evidence-based articles, and we look forward to working with you again in 2023, while welcoming new contributors.
Wishing all our readers a peaceful Christmas and a happy, healthy, and prosperous New Year. l
NiGP is now a fully independent publication and is no longer the official journal of the IGPNEA. If you are interested in writing an article for NiGP, please email denise@greenx.ie.
The latest Irish healthcare news
INTERVIEW
Oncology Nurse Specialist, Geraldine Mullan, talks to NiGP after winning ‘Local Hero’ award 14
CPD: IRON DEFICIENCY IN CARDIOLOGY
Cardiology experts, Dr Robert M Evans and Dr Patrick O’Callaghan from University Hospital Waterford provide an evidencedbased update on iron deficiency in cardiology patients
Ruth Morrow delivers an extensive overview of the diagnosis and management of stable COPD
Highlights from the
EDITOR
Denise Doherty denise@greenx.ie
CONSULTING EDITOR Ruth Morrow
SUB-EDITOR Emer Keogh emer@greenx.ie
CREATIVE DIRECTOR Laura Kenny laura@greenx.ie
ADVERTISEMENTS Graham Cooke graham@greenx.ie
WINTER VACCINES
Irish Osteoporosis Society’s annual Medical Conference 32
Vaccination updates for Covid-19 and flu from Dr Apama Keegan as the winter season approaches
MONKEYPOX Updates and recommendations for managing the current monkeypox outbreak by Dr Tom Barrett, Senior Medical Officer of the National Immunisation Office
ADMINISTRATION
Daiva Maciunaite daiva@greenx.ie
Please email editorial enquiries to Denise Doherty denise@greenx.ie
Nursing in General Practice is produced by GreenCross Publishing Ltd (est. 2007).
© Copyright GreenCross Publishing Ltd. 2022
Please email publishing enquiries to Publisher and Director, Graham Cooke graham@greenx.ie
TYPE 1 DIABETES Priscilla Lynch provides a comprehensive summary of the new NICE guidelines 48
PRODUCT NEWS

The latest news in healthcare and pharmaceutical products
The contents of Nursing in General Practice are protected by copyright. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means – electronic, mechanical or photocopy recording or otherwise –whole or in part, in any form whatsoever for advertising or promotional purposes without the prior written permission of the editor or publishers.
The views expressed in Nursing in General Practice are not necessarily those of the publishers, editor or editorial advisory board. While the publishers, editor, and editorial advisory board have taken every care with regard to accuracy of editorial and advertisement contributions, they cannot be held responsible for any errors or omissions contained.
Stroke is the second leading cause of death in middle to higher income countries and the leading cause of acquired adult neurological disability in Ireland. The HSE National Clinical Programme for Stroke marked World Stroke Day on 29 October by launching the new National Stroke Strategy 2022-2027. The programme has prioritised an ambitious, but realistic strategy, to improve resourcing of stroke services. It also seeks commitment from Government for a structured implementation, review, and a follow-on stroke strategy to commence in 2026.
The overall aim of the strategy is to achieve full realisation of the Stroke Action Plan for Europe by 2031. It also recognises the need for an all-age and complexitytiered approach to neuro-rehabilitation. The new strategy has identified four primary pillars of focus, primarily in view of the predicted increases in strokes in Ireland. They are:
1. Stroke prevention;
2. Acute care and cure;
3. Rehabilitation and restoration to living;
4. Education and research.
Stroke prevention highlights that strokes are preventable and that principal risk factors, such as hypertension and atrial fibrillation (AF), are also increasing. Key objectives for stroke prevention in the strategy include:
Develop a pathway for the case-finding, diagnosis, and treatment of high blood pressure in over 45-year-olds;
Develop a pathway for the prevention and case detection of AF;
Ensure all hospitals receiving acute stroke patients have a specialist-led, rapid access stroke service or access to such a service within their hospital network;
Ensure that all patients recovering from a stroke have access to a specialist secondary prevention stroke service and diagnostics.
Acute care objectives include:
Acute stroke services must have adequate staffing and diagnostic resources to provide 24/7 acute stroke care and treatment;
All hospitals receiving acute stroke patients must have an acute stroke unit;
Appropriate staffing of specialist stroke units with a number of trained physicians, nurses, healthcare assistants, health and social care professionals;
All patients must have 24/7 access to emergency acute stroke assessment and treatment by a stroke specialist.
Rehabilitation and restoration to living objectives include:
Early supported discharge teams to be fully commissioned across 21 high activity sites over a three-year period to cover 92 per cent of the stroke inpatient population; Stroke key worker resource to be appointed in each Community Health Organisation (CHO), so that discharged stroke patients and their families have access to the specific support and advice; All stroke patients to have a stroke passport (to contain information about their stroke, healthcare provider contacts, their condition, stroke medication, etc).
Education and Research objectives include: Funding for a sustained public awareness campaign on stroke; Creation of professorships in neurovascular and stroke medicine in six medical schools to improve Irish research and teaching in stroke;

Creation of three stroke research fellowships to enhance research and career opportunities and help retain our medical graduates in stroke medicine.
Dr Colm Henry, Chief Clinical Officer, HSE, said: “I welcome the launch of the new National Stroke Strategy, which has been developed to provide safe, effective stroke care with improved outcomes for patients. The strategy will bring stroke care in Ireland in line with other allied national strategies and the Stroke Action Plan for Europe 20182030 of the European Stroke Organisation. I am confident it will pay significant dividend for patients, healthcare, and society as a whole for years to come.”
The full document is available at: www. hse.ie/eng/services/publications/clinicalstrategy-and-programmes/national-strokestrategy-2022-2027.pdf.
Aglobal study, co-led by University of Galway, into causes of stroke has found that high and moderate alcohol consumption is associated with increased odds of stroke. The INTERSTROKE research looked at the alcohol consumption of almost 26,000 people worldwide, of which one-quarter were current drinkers and two-thirds abstained completely. The study included 12,913 cases of acute stroke from 27 countries with a diverse range of ethnic backgrounds. It also included 12,935 control participants. Countries where over 95 per cent of
the populations report never drinking alcohol, such as Pakistan, Kuwait, Iran, and Saudi Arabia were excluded.
Low alcohol intake was defined as one-seven drinks/week; Moderate alcohol intake was defined as seven-14 drinks/week for females and seven-21 drinks/week for males;
High intake was defined as >14 drinks/week for females and >21 drinks/week for males;
Heavy episodic (binge) drinking was defined as >5 drinks in one day, at least once a month.
Results: One-in-four people studied were current drinkers, one-in-six were
former drinkers, and the rest were never drinkers. In the study, those classified as current drinkers were linked with a 14 per cent increase in risk of all stroke and a 50 per cent increased risk of intracerebral haemorrhage. No increased risk of ischaemic stroke was noted in this group. Heavy episodic or binge drinking was linked with a 39 per cent increase in all stroke, a 29 per cent increase in ischaemic stroke, and a 76 per cent increase in intracerebral haemorrhage. High alcohol intake was associated with a 57 per cent increase in stroke. The study also found that there was no link between low level drinking and stroke.
Senior Lecturer at University College Cork’s (UCC) Department of General Practice and GP, Dr Tony Foley, has highlighted that there is no specific dementia training given to healthcare professionals in clinical practice, despite the prevalence of the disease. Around 25 per cent of all over 65-year-olds in hospital in Ireland have dementia or cognitive impairment and there are currently 64,000 people living with the disorder across the country. This figure is expected to reach 142,000 by 2050. In view of these figures, Dr Foley has called for an urgent, structured, pro-active model
of care for dementia patients, rather than a reactive approach, and advocates that dementia care become part of a National Structured Chronic Disease Management Programme in Ireland.

Dr Foley said: “The figures
for dementia are very high, not just here in Ireland, but across the globe. Dementia is now the second leading cause of death in the UK and Australia. It is one of the greatest health and social care challenges of our time. There
is an urgent need to act now to transform health, social, and palliative care services to meet the projected growth in palliative care needs.”
The GP and academic was speaking at a UCC Interprofessional Learning Workshop event, Understanding Dementia Together: A new collaborative multi-disciplinary approach, last month. The workshop is in its fourth year at the university, initially starting with occupational therapy and physiotherapy students, and expanding to include students from 11 different healthcare disciplines this year, ranging from nursing, to medicine, dentistry, pharmacy, and others.
The HSE National Forensic Mental Health Service (NFMHS) was officially opened on 4 November by the Minister for Health, Stephen Donnelly TD; Minister of State for Mental Health and Older People, Mary Butler TD; and Minister of State for Law Reform, James Browne TD. The relocation of the Central Mental Hospital (CMH) in Dundrum to the new, stateof-the-art, purpose-built facility in Portrane, Co Dublin, brings much anticipated and necessary reform to Ireland’s mental health services. This is the country’s largest capital project, costing in the region of €200 million.
Speaking at the opening, Stephen Mulvany, CEO, HSE said: “I am delighted to be here to mark this historic day. While the Dundrum site has a capacity of 96 patients, the National Forensic Mental Health Service increases that capacity to 110 beds initially, with a further expansion to 130 beds to occur in 2023. The opening of the Intensive Rehabilitation Care Units (ICRU) is also due to progress in 2023, treating 30 patients who require specific interventions, and will inform the strategic roll-out of a number of other facilities nationally.”
The new NFMHS will have the capacity to care for 170 patients on campus when fullyoperational. The facility also offers community and prison in-reach services, Forensic
Child and Adolescent Mental Health Service (FCAMHS), and an Intensive Care Rehabilitation Unit (ICRU) that will operate on site next year. NFMHS will also an additional five clusters of forensic mental health care, including a pre-discharge unit, female unit, mental health intellectual disability unit (MHID-F), high secure unit, and a medium secure unit.
The care and services that will now be more readily available to the most vulnerable and in-need populations, such as those engaged with prison, legal, and addiction services, was paid particular acknowledgement by several of the ministers present on the day. Minister Browne anticipates that the facility will enable more shared-care between services, and help many people towards the pathway to recovery and a subsequently improved qualityof-life. Minister for Justice, Helen McEntee TD, who was also present, echoed these sentiments and went on to
highlight that the new services would go beyond helping service users towards the path to recovery, but would also help to keep them there. She said: “We know that many of those who end up engaging with our criminal justice system have higher rates of mental health and addiction challenges than the rest of the population, and if we are to create safer communities and reduce crime, we have to ensure we have properly resourced, appropriately located systems of care in place for the most vulnerable people in society." Also present was Minister of State for Public Health, Wellbeing and National Drugs Strategy, Frank Feighan TD. He too made specific reference to vulnerable populations and the collaborative, multidisciplinary approach to their care that the new NFMHS would facilitate. He said: “People with severe mental health difficulties can also experience addiction issues, referred to as dual diagnosis."
The design concept for the
new facility is to support the underlying aim of providing therapeutic care and security with dignity, delivering a unique world class hospital that embodies the best principles of high secure mental healthcare design. 130 single patient bedrooms are laid out in small wards around shared indoor and outdoor spaces, in which collective activities and therapies take place. A ‘village centre’ provides shared recreational facilities, including a horticultural area, a gym, a woodwork workshop, and a music room, while a series of courtyards and secure perimeter gardens allow patients direct access to nature from each ward. The village centre also houses mental health therapeutic services, a GP and a dentist.
The new facility will also: Ensure that patients are living in accommodation appropriate to their needs, risks and modern healthcare standards; Improve the quality-of-life of the patients and carers using the services;

Increase the number of high and medium secure beds in accordance with international comparisons and national need;
Deliver specialist secure care for MHID-F and CAMH-F patients;
Reduce the operating costs of the service pro rata;
Modernise and improve clinical practice;
Reduce costs to the HSE for placement of patients in the UK, prisons and other HSE services.
Patrick Bergin, Head of Service at NFMHS, described the opening as a welcome day for patients and “a key milestone in the delivery of a modern forensic service to our patients”. He went on to say: “We now have the opportunity to be a centre of excellence and evolve our delivery of treatment and care… and this new facility provides us with opportunities to be a world leader in this specialist field.”
The state-of-the-art centre doesn’t just promote a holistic and individualised approach to mental health, but also takes measures to improve planetary health as well. Eight hectares of new native woodland will be established as part of the project and up to 20,000 trees will be planted; 2000 of which will be certified Irish native trees. The new development is also responsible for improving and diversifying adjacent
wetland habitats, including opening up and reprofiling drainage ditches, and creating new ponds and wader habitats within the wetlands. Landscaping within the grounds of the new hospital includes substantial pollinator friendly planting that will provide foraging for a variety of nectar feeding invertebrates including butterflies, moths and hoverflies, and a large wildflower meadow.
Minister Butler thanked
those involved in the project and commended the new services and the positive impacts they will have. She said: “I welcome the opening of today’s new complex, which will enable the HSE to support the enhanced delivery of person-centred care, underpinned by human rights for persons using the service and their families. This is one of the most modern forensic mental health facilities in Europe."
The Irish College of General Practitioners (ICGP) has proposed 10 potential solutions to the growing shortage of GPs in Ireland, and the subsequent issues this is causing that we outlined in the previous issue of NiGP. The solutions are part of the ICGP’s comprehensive Discussion Paper Shaping the Future of General Practice that was launched at their Autumn conference last month. The 10 proposed solutions outlined in the document are:
1. Expand GP-led multidisciplinary teams;
2. At least double the number of GP practice nurses;
3. Resource the career expectations of future GPs;
4. Provide suitable premises for GP-led multidisciplinary teams;
5. Fast-track suitably-qualified GPs to take on GMS lists;
6. Increase remote consulting;
7. Introduce a career pipeline for
rural general practice;
8. Develop the role of practice manager;
9. Increase exposure to general practice in medical schools;
10. Invest in GP data-informatics to drive policy and practice.
Prof Tom O’Dowd, former ICGP President and GP in Tallaght, chaired the ICGP group which produced the paper. He said: “We propose solutions in this paper, but it is the key stakeholders working collectively that will help produce the solutions. This is an urgent problem that cannot be solved overnight, and while we welcome the Minister’s decision to establish a strategic review of general practice, we urge the Minister to act immediately and to begin the process of finding innovative solutions to this crisis. This review and establishment of a working group is needed as a matter of urgency if the extension of free GP care is to proceed.”





































The Irish College of General Practitioners (ICGP) has launched its Quick Reference Guide on Menopause, which is now available for its members to access.
The guide is designed to assist GPs and practice nurses in the management of menopause and its myriad symptoms through evidence-based interventions.
Last month, the Minister for Health, Stephen Donnelly TD, launched a government awareness campaign for menopause as a direct response to the demand from Irish women for greater knowledge and understanding of the major life event. They want better access to accurate information and supports, so that they can proactively manage their experience and maintain a high quality-of-life during it. The campaign is intended to meet their demands by increasing awareness of and encouraging open conversation about menopause, its symptoms, and associated stigma.
Welcoming the launch of the Quick Reference Guide on Menopause, Minister Donnelly said: "When launching the Women's Health Action Plan in March 2022, I committed to changing the approach to menopause care in Ireland,
and increasing the supports available to women before, during and after menopause. Women told us they needed more when it came to support and treatment for this significant life event. We have been responding to this ask, for example, through the development of specialised menopause clinics nationally, and today, the ICGP has responded by publishing this reference guide, which will assist GPs across the country in treating people experiencing menopause."
As well as the new guide for ICGP members, the College has also published a series of online videos about the menopause to help educate and guide the general public, as well as increase awareness. These online resources have been created by the ICGP’s Director of Women’s Health, Dr Nóirín O’Herlihy, and the ICGP’s GP Clinical Lead in Women’s Health, Dr Ciara McCarthy. The five short videos are designed to provide some general information about menopause, what it is, how it is diagnosed, and what to expect from its treatment.
Speaking about the new ICGP guide, Dr McCarthy said: "Menopause is a major life event affecting all women in a variety of ways, with up to 80 per cent
of women experiencing physical and/ or emotional symptoms during that time. Most of these symptoms can be managed in general practice and this guide comprises evidence-based information on the management of perimenopause and menopause in general practice by GPs and practice nurses. It provides advice on diagnosis, lifestyle interventions, prescribing HRT safely, alternative options to HRT, and specific advice for women with a history of breast cancer. An overview of the management of premature ovarian failure is included." Dr O'Herlihy, added: "This reference guide is a significant milestone in the care of women with perimenopause and menopause in Ireland and part of the widening of knowledge and understanding. It is funded by the Women's Health Taskforce and the National Women and Infants Programme, and will provide GPs with updated and comprehensive knowledge on menopause treatment for their patients."
The Quick Reference Guide on Menopause is available for ICGP members at www.icgp.ie/MenopauseQRG.
The series of public videos about the menopause is available at www.icgpnews. ie/menopause-patient-information/.
Nurses and midwives employed by the public service have accepted the proposals in the review of Building Momentum by 97 per cent. Included in the review, as outlined in the previous issue of Nursing in General Practice, are a 3 per cent increase on
annualised salary to be back paid in February 2023; a further 2 per cent increase on annualised salary from 1 March 2023; and a 1.5 per cent increase (or €750, whichever is the greater) from 1 October next year. This is in addition to the 1 per cent (or €500 increase if greater) due to members from 1 October
2022, as previously agreed. Nurse manager grades were notified this week that the circular to apply the 3.28 per cent increase from February 1 2022 has been issued.
The INMO has written to the HSE to confirm that these payments will be made urgently.
As the winter arrives, bringing with it the associated demands and strains on the health services, the HSE has outlined actions that will be taken to support the sector and manage the expected increase in the number of patients requiring care over the coming months. 2022 has already seen a 5.36 per cent increase in overall attendances to the emergency department (ED); a 0.74 per cent increase in hospital admissions; and a 12.92 per cent increase in ED attendances by those aged over 75 years, when compared to 2019. Funding of just over €169 million has been assigned to implement the measures outlined in the Winter Plan, which will include the recruitment of 608 posts across a range of services and the vaccination programme for influenza and Covid-19.
Speaking about the plan, Chief Clinical Officer of the HSE, Dr Colm Henry, outlined how our third winter with Covid-19, alongside other seasonal viruses, carries much uncertainty, and has the potential to add substantial pressure on our already strained services. He said: “Our response has been to create additional much-needed capacity and to diversify access to healthcare and reduce reliance on hospitals. While these measures are all necessary and helpful, we can all act an individual level to reduce the impact of Covid-19 and influenza by getting our winter vaccines as soon as possible.”
Major actions outlined in the plan include: Delivering additional capacity in acute and community services. This will include the ongoing delivery of additional acute and community beds and increasing staffing capacity in line with the Safe Staffing and Skill-Mix Framework. The HSE also plans to extend the opening hours of a number of local injury units during the winter period.
Improving pathways of care for patients: Alternative patient pathways will be implemented during the winter period to help reduce the number of presentations and admissions to hospital and improve patient flow and discharge. This includes enhanced community care (ECC) supports.
Roll-out of the vaccination programme: The influenza and Covid-19 vaccination programmes continue to be rolled out. Targeted communications will be used to increase awareness and uptake for winter vaccine programmes.
Implementation of the Pandemic Preparedness Plan: The Public Health Plan will be implemented as appropriate, which includes a surge and emergency response plan, in the event of a significant surge in Covid-19 infections.
Mary Day, HSE National Director for Acute Operations acknowledged the progress and investment and also the persistent challenges faced in healthcare. She said: “No matter what the time of year, HSE services are seeing and treating more patients in hospitals and in the community than ever before. In our acute hospitals this year, over 1.6 million people will have an inpatient or day case procedure or use maternity services. We have also provided 14.2 million home support hours this year to date to enable people to remain at home and to support their families to care for them.”
Yvonne O'Neill, HSE National Director for Community Operations added: “The fact that we are continuously growing the amount of healthcare provided to the population is good news, but the growing demand also results in delays and waiting lists, and the measures being announced today will go some way to easing that in what may be a very busy period.”
Some of these additional measures in the plan include:
A further €4.5 million for aids and appliances to enable patients be discharged home or to a community facility as quickly as possible.
Expansion of patient flow and discharge teams in hospitals and community to minimise delays in discharge or transfer to other hospitals or step-down facilities.
Additional funding for complex care packages, which will enable hospitals to discharge patients with complex need by giving them the supports they need to be cared for at home.
Extra funding to allow treatment of more patients in the private hospital system.
Funding for additional access to diagnostics for GPs to enable them to refer patients directly for x-rays or scans, rather than it being necessary for them to be sent to EDs in order to access such diagnostic services. Over 188,516 scans have been completed as part of the GP access to diagnostics initiative so far this year.
Additional funding to the GP out-of-hours service, including expansion to provide full coverage in rural areas in Community Healthcare Organisation (CHO) West.
Investment to support expansion of Community Intervention Teams (CIT) across the country with a particular focus on the Mid-West and North-West regions.
Funding to enhance palliative care services during the winter, which will deliver 1,340 nights of night nursing to 380 patients and families.
Funding has been provided for an integrated action team funds, which each Hospital Group and CHO can request access for specific initiatives during the winter period.
Reinforcement of the key role of Covid-19 and flu vaccines in reducing the level of severe respiratory illness
The full plan is available at : www.hse. ie/eng/services/news/media/pressrel/ winter-plan-2022-23.pdf.
The Institute of Public Health (IPH) has launched a free digital learning course to help healthcare professionals support older people towards achieving and maintaining the recommended 150+ minutes of physical activity per week. The new Getting Active for Better Ageing course is part of the IPH’s ongoing Public Health Matters programme, which continues to create and provide digital resources to help promote and improve public health. The online modules and courses are suitable for the public, as well as healthcare professionals and policy makers. Both the platform and app are regularly updated to include new podcast episodes, blogs, videos, and other content and resources relevant to many aspects of public health.
This latest course was developed following research findings published by the IPH in 2021 that identified the need for
tailored education, training, and resources to support healthcare professionals to promote physical activity in routine practice. Getting Active for Better Ageing is available on the IPH’s Public Health Matters app or desktop platform. The resource is aimed at general practice nurses (GPNs), GPs, physiotherapists and all healthcare professionals that interact with older adults. It is also suitable for carers and members of the public with an interest in
promoting and supporting activity in older age. The new resource sets out key facts, guidelines, and tools on how to support behaviour change in older adults, as well as other essential information.

IPH Director of Ageing Research and Development Prof Roger O’Sullivan said: “The evidence is clear, physical activity plays an important role in our health at all stages of life, including in older age, which is sometimes overlooked. This new free learning resource is available on our Public Health Matters platform and was developed as a practical programme to assist healthcare professionals to support older persons to be more physically active.”
You can access the Public Health Matters desktop platform at: https://learning.publichealth.ie/ or you can download the Public Health Matters app for free on the Google Play (Android) and iTunes store (Apple).
GP practice nurse required in friendly practice in Rathfarnham
12 hours per week over 3 sessions
l To work alongside other full-time practice nurse
l Previous experience desirable but not essential
l Training provided l Good location l Modern bright purpose-built premises l Free parking
Duties to include, but not exclusive to: Phlebotomy, ear syringing, childhood vaccines and other immunisations, smear taking, chronic disease management, ECG, 24 hour ABPM, removal of sutures/ wound dressings, diabetes, assist with minor procedures, telephone triage, Covid/flu vaccinations.
Please send CV to practice manager Grace: gablesmedc@gmail.com
Practice nurse required in Ballinteer, Dublin 16 (just off M50) from 01/02/2023.
Busy 1 (full-time male) GP +2 (part-time female) GP practice.
At least 5 (3 hour) sessions, some flexibility with day mornings/afternoons.
Experience in phlebotomy, childhood immunisations and cervical smear taking is necessary.
Experience in antenatal care, BP monitoring, chronic disease management an advantage. Very nice family orientated type practice.
Please contact 086 8132303 (text) or email drmaevemoloney@gmail.com for further information.
If you would like to place a recruitment advert in the next edition, please contact Louis@mindo.ie


Helps rebalance the gut microbiota1 4 to support immune development and long-term health5-8
IMPORTANT NOTICE: Breastfeeding is best. Neocate Syneo is a food for special medical purposes for the dietary management of Cow’s Milk Allergy, Multiple Food Protein Allergies and other conditions where an amino acid based formula is recommended. It should only be used under medical supervision, after full consideration of the feeding options available including breastfeeding. Suitable for use as the sole source of nutrition for infants under one year of age. Refer to label for details. 1. Burks AW et al. Pediatr Allergy Immunol. 2015 Mar 31; 26(4):316–322. 2. Candy DCA, et al. Pediatr Res. 2018 Dec 6; 83(3):677-686. 3. Fox A.T, et al. Clin. Transl. Allergy. 2019 Jan15; 9.5 4. Chatchatee P, et al. J Allergy Clin Immunol. 2021 Jul 2; S0091-6749(21)01053-8. 5. Martin R et al. Benef Microbes. 2010. 1(4):367–82. 6. Wopereis H et al. Pediatr Allergy Immunol. 2014. 25:428–38. 7. West CE et al. J Allergy Clin Immunol. 135(1):3–13. 8. Walker WA et al. Pediatr Res. 2015. 77(1):220–228. 9. Sorensen K, et al. Nutrients. 2021 Mar 14; 13(3):935. 10. Sorensen K, et al. Nutrients. 2021 Jun 27; 13(7):2205. ± Exploratory outcomes from randomised control trials, Neocate Syneo vs Neocate LCP; † Systematic review of 4 randomised controlled trials, Neocate Syneo vs Neocate LCP. ¥ UK Observational study of real world evidence in THIN GP database, Neocate Syneo vs Alfamino, Feb 2021 (Alfamino is not currently GMS listed in ROI); ^ Clinical journey: asymptomatic and not requiring hypoallergenic formula prescription for at least 3 months. § Product can be provided to patients upon the request of a healthcare professional. They are intended for the purpose of professional evaluation only. Nutricia Ireland, Block 1 Deansgrange Business Park, Deansgrange, Co. Dublin. Date of publication: 02/2022

Just over two years ago, Geraldine Mullan’s six-yearold daughter Amelia explained that her little heart had four corners; one for her, one for her older brother, one for her mammy, and one for her daddy. Only months later, Geraldine’s own heart would be irreparably broken, and three of its four corners would be left empty.
On 20 August 2020, she was the sole survivor of a car accident that claimed the lives of her beloved husband, John, and their children, Tomás (14) and Amelia (6). Since then, the devastated Oncology Nurse Specialist has worked tirelessly to keep their memory alive and to spread a little hope through her local community.
Speaking to Nursing in General Practice (NiGP), Geraldine describes the horrific event that took those she loved most and its aftermath. She and her family were returning home to Moville in Co Donegal after what she now describes as “a very precious family day” at the cinema complex in Derry that night, busy making plans for going back to school and enjoying each other’s company. The weather was particularly aggressive that evening, with violent waves and winds lashing the Inishowen Peninsula. Geraldine tells NiGP how she vividly remembers

THE VIBRANT SPACE HAS BECOME A HUB FOR LOCAL FAMILIES, VISITORS, AND MANY OF TOMÁS AND AMELIA’S FRIENDS
every single second and detail of the horrendous few minutes that changed her life forever. She doesn’t elaborate and doesn’t need to, as words simply don’t exist to describe the horror.
While words fail, Geraldine’s strength, determination, and generosity since the tragedy have not, despite the infinite grief she is left with. Her mission since burying her family is to keep their memory alive and to give back to the local community that she says has been a rock to her throughout the trauma. Originally from Galway, Geraldine now considers herself a local in Inishowen and describes the overwhelming support she received, and continues to receive from those around her. Geraldine was nominated by that very community for the national Goss.ie Local Hero Award 2022, and has been hailed as an inspiration in the area for her work since the tragedy.
Instead of watching John’s much loved garden centre close after his death, his wife has transformed it into the Mullan HOPE Centre in his and their children’s memory. The vibrant space has become a hub for local families, visitors, and many of Tomás and Amelia’s friends. Geraldine has dedicated herself to creating a place that oozes joy, hope and love, where families can enjoy precious time together and make memories like those that now help her get out of bed each morning. “I won’t see my own children grow up, but I will see their friends having fun and getting older,” Geraldine smiles sadly as she describes the sense of comfort she gets from being with those who were close to her family. “My memories are what keep me going. I love to see their friends having fun and smiling. I know they are still with me and I can hear John telling me to keep going. He was a wonderful, supportive husband and I was so blessed to have had the time we shared with our beautiful children. Sometimes I live day-by-day,
others it's hour-by-hour, and on the very bad days, it’s a minute at a time. They [John, Tomás and Amelia] give me strength. They say grief is the price we pay for love. There was a lot of love.”

The pictures that hang in every corner of the Mullan HOPE Centre reflect a family that indeed radiated love and hope, and that is what Geraldine is determined to keep alive in their memory. She is busy organising the Christmas markets that will be hosted at the centre next month, after welcoming hundreds of dressed-up children and their families for a hugely successful ‘Spooktacular Farmers Market’ over Halloween. During the summer, Geraldine spread her message of hope to thousands when she created the Field of Hope, a two-and-a-halfacre field of fabulous sunflowers that she nurtured herself, again, with some extra help from those around
her. Seeds from a sunflower grown by little Amelia were among those sown in the field. She and Tomás had been growing the flowers to enter a competition with the help of John, the garden centre expert. “Sunflowers were Amelia’s favourite,” Geraldine explains with a smile as she remembers. The project literally evolved to scream hope, when a maze was carved out within the plantation in the shape of the letters H-O-P-E. At the end of August last year, to mark the second anniversary of the accident, Geraldine opened the field to the public. Over 15,000 people from all over the country visited the sea of gold that stretched towards the sky in memory of her family, in the name of hope, and in solidarity with others that had experienced loss.
The Mullan HOPE Centre and Geraldine’s impact on the local area led to her nomination for ‘Local Hero’ at this year’s Goss.ie Women of the Year Awards. Geraldine, who also recently won Community Champion of the Year at the Derry Journal People of the Year Awards, and RSVP ’s Unsung Hero Award, was named the overall winner at a ceremony hosted by Gráinne Seoige at the Royal Marine Hotel in Dun Laoghaire last month. “I’m humbled,” Geraldine says and bows her head. “I am very grateful, but I just want to share some hope. It’s all I have and sharing it and the memory of John, Tomás, and Amelia is what keeps me going. I just hope that our story can bring a little hope to those that need it most.”
Geraldine continues to work as an Oncology Nurse Specialist in Letterkenny University Hospital and describes it as the place she can just be Geraldine, and not the woman who lost everything she loved most in the world. Her job is also a huge support structure and she intends to keep practising, and to continue to host events for her community for as long as she can in memory of her beloved family. l
SOMETIMES I LIVE DAY-BY-DAY, OTHERS IT'S HOUR-BY-HOUR, AND ON THE VERY BAD DAYS, IT’S A MINUTE AT A TIME

Iron deficiency and anaemia are two significant comorbidities that are present in a large proportion of cardiology patients.1,2 Iron deficiency is a well-recognised cardiovascular risk factor in heart failure,3,4 with the European Society of Cardiology (ESC) recommending parenteral iron replacement as part of standard care for chronic heart failure patients that are found to be iron deficient (Class IIa recommendation, Level of Evidence: A).5
There is a growing hypothesis that iron deficiency may have a significant impact on cardiovascular health at all levels and conditions, and that replenishment may also be of benefit to the general cardiovascular disease population.
Iron deficiency can be classed as either absolute iron deficiency (AID), or functional iron deficiency (FID). AID is defined as serum ferritin <100μg/L, with FID defined as a combination of serum ferritin <100-300μg/L in combination with a transferrin saturation (TSAT) <20 per cent.
This article will discuss the significance of iron deficiency in cardiology patients, the available evidence relating to iron deficiency in these patients, and the recommendations from current guidelines. It will also discuss anaemia due to its close association with iron deficiency.
The causes of iron deficiency and anaemia are often multifactorial. Nutritional deficiency, chronic inflammation, blood loss, and unexplained presentations are common causes within the general population. 2 The causes of iron deficiency and anaemia in the cardiology patient cohort may be due to their cardiac condition,
pass through or around a prosthetic valve; including transcatheter aortic valve implantations (TAVI). This can also occur in the setting of a severely stenotic native aortic valve and may be exacerbated when combined with small bowel angiodysplasia, which will result in slow, often difficult to localise, gastrointestinal (GI) bleeding, known as Heyde’s syndrome. Heyde’s syndrome is due to von Willebrand
their comorbidities, or their medication. Cardiac conditions themselves can lead to, or exacerbate, both iron deficiency and anaemia. Chronic heart failure causes chronic inflammation, as well as hypervolaemia, both of which can affect gut absorption of dietary iron, exacerbating anaemia. Patients with valvular heart disease can often be anaemic and/or iron deficient too.6 Microangiopathic haemolytic anaemia (MAHA) is a non-immune haemolysis often caused by intravascular blood cell fragmentation as the red cells
factor destruction as it passes through the valve, with subsequent abnormal clotting and spontaneous GI bleeding, in comparison to the intravascular haemolysis of MAHA.
Infective endocarditis (IE) persists as a significant, critical illness in the cardiology population with valvular dysfunction, which may lead to anaemia, as outlined above. Subacute IE results in profound chronic inflammation and patients often present with anaemia due to their diseased valve and their chronic inflammatory state.7 Many cardiology
INFECTIVE ENDOCARDITIS (IE) PERSISTS AS A SIGNIFICANT, CRITICAL ILLNESS IN THE CARDIOLOGY POPULATION WITH VALVULAR DYSFUNCTION, WHICH MAY LEAD TO ANAEMIA
patients have concurrent chronic renal disease, which can result in reduced erythropoietin production and anaemia at a bone marrow level.
With regards to medication in the cardiology population, both antiplatelet and anticoagulation agents are associated with increased bleeding and subsequent anaemia risks, especially GI bleeding. These are used ubiquitously in the cardiology population, for primary and secondary prevention of coronary artery disease (CAD) and arrhythmias. Stress ulceration of the GI tract, in the setting of acute coronary syndrome, can often contribute to this, which has been recognised since the 1940s. 8 Previous studies also suggest other drug-induced causes, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) that reduce haemoglobin levels via reninangiotensin system inhibition. 9 Finally, in relation to patients with IE, prolonged parenteral antimicrobial therapy can contribute to anaemia, with some classes of antibiotics causing haemolytic anaemia or myelosuppression.
Most of the available evidence regarding the role of iron deficiency and anaemia in cardiology is in association with heart failure. Both iron deficiency and anaemia are independently associated with poor outcomes in heart failure.6
In patients with iron deficiency without anaemia, there is reduced uptake of iron by non-erythroid tissues and bone marrow. Within these nonerythroid tissues, iron has multiple key roles because of its ability to bind oxygen. As a result, it is required in metabolic cellular reactions, such as oxidative phosphorylation and production of reactive oxygen species (ROS).10 Iron acts as an electron transporter in sites of oxygen utilization, including the mitochondria, that are abundant in high-energy areas, such as skeletal muscle or cardiac myocytes. Mitochondrial function relies
on iron homeostasis.11
Mitochondrial failure due to iron deficiency leads to cellular anaerobic metabolism, resulting in less effective cellular energy production. In mouse models, disruption of this pathway, forcing anaerobic metabolism, resulted in a severe form of cardiomyopathy and resultant death within 14 days.12 Anaemia results in reduced oxygen delivery to tissues, leading to increased workload, causing left ventricular hypertrophy and remodelling; worsening the oxygen demand present prior to this. Groenveld et al (2008) concluded that anaemia doubled the relative risk of death in a meta-analysis of 150,000 patients across 33 studies. 13
Iron replacement as a treatment option for iron deficiency, with or without anaemia, has been used for many years in both primary and secondary care. Oral iron replacement is often the initial treatment of choice, however, there is evidence that it is relatively unsuccessful at replenishing iron stores. IRONOUT-HF was a large double blinded, randomised, placebo-controlled trial of 225 heart failure with reduced ejection fraction (HFrEF) patients, with AID or FID, treated with oral iron replacement for 16 weeks. Peak oxygen update (VO 2 Max) did not change during cardiopulmonary exercise testing, nor did the six-minute walk test (6MWT) results, or NT-proBNP levels. 14 Oral iron replacement can also cause significant adverse GI effects, including constipation, nausea, and dark stool. 40 per cent of patients in IRONOUT-HF experienced such issues.
Parenteral/Intravenous (IV) iron replacement is available in several formulations. Compliance, tolerance and absorption are all superior in the IV route compared to the oral route.
Iron formulations include, but are not limited to, ferric carboxymaltose (FCM) (Ferinject), ferric sucrose (Venofer), ferric dextran (Cosmofer) and ferric derisomaltose (Monover). Previous clinical trials regarding iron replacement in heart failure have primarily used FCM.
Parenteral iron replenishment is generally given in secondary care in Ireland due to administration and monitoring requirements during infusions. IV iron usually requires multiple doses as well as the potential for maintenance treatment, therefore, proper organisational and infrastructural support is required for treating large numbers of patients. This generally includes administrative and specialist nursing support, which is more readily available in acute hospital settings, but it is anticipated that this could be transitioned to more community-based care in the future.
IV FCM, as the most evidencebased formulation, is generally administered up to 1g as an infusion over 15-30minutes. Infusion reactions are common and in the region of 10 per cent. These include infusion site irritation, extravasation, rash, dizziness, and a range of hypersensitivity/ allergy symptoms that can reach lifethreatening anaphylaxis. More severe reactions were previously more common with early-generation IV iron, but have become less common as modern variations are generally better tolerated. Patients with an allergy to a single formulation are often able to tolerate different formulations, but caution should be advised with subsequent administrations of same.
Blood transfusion is frequently used in an acute setting to treat severe anaemia. Packed red blood cells, although a reliable source of iron, are a scarce commodity and have risks of transfusion-associated side-effects or reactions. Research advocates for restrictive use of transfusion in
hospitalisations after acute myocardial infarction (MI), 15 and transfusion is not recommended routinely in the management of chronic cardiovascular conditions in isolation, unless indicated for other reasons.
Chronic renal disease, with resultant relative erythropoietin deficiency, can contribute to anaemia in heart failure. Erythropoietin stimulating agents (ESAs) have been successfully used in renal disease, where there is a benefit in avoiding blood transfusions. However, RED-HF16 failed to show significant clinical benefit of ESAs for patients with systolic heart failure and haemoglobin of 9-12 g/dL. In fact, increased risks of thromboembolic events and strokes resulted in worse clinical outcomes in this cohort.
FAIR-HF (Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency) 17 was the initial trial investigating iron replacement in chronic heart failure patients, with or without anaemia. The trial included 459 patients. Dosage was dependent on participant weight and haemoglobin level at screening, with weekly administration until repletion, with four weekly maintenance infusions. Whilst there was no significant effect on all-cause mortality, there was improvements in New York Heart Association (NYHA) class and outcomes scores.
CONFIRM-HF18 was designed similarly to FAIR-HF. 6MWT (10 endpoint) was significantly improved (33m +/- 11m) after IV iron. Patients were treated for longer (one year vs six months) and received higher dosages than the FAIR-HF population. Secondary endpoints also showed improvement including NYHA levels and patient global assessment scores.
Primarily, both trials confirmed iron replacement was associated with improved functional capacity and quality-of-life. However, neither trial identified a mortality benefit. CONFIRM-HF did show a reduction in heart failure hospitalisation, but this was on post-hoc analysis alone and the trial was not designed or powered for this hypothesis. Metaanalyses of available studies identified that FCM infusion significantly reduces hospitalisation, heart failure hospitalisations and overall mortality (RR 0.59; p = 0.009). 19
Three trials are currently underway which are powered for the primary endpoint of heart failure hospitalisation or cardiovascular death in patients with HFrEF and iron deficiency. IRON-MAN is the first trial to assess with intravenous ferric derisomaltose. FAIR-HF2 is the subsequent trial from FAIR-HF, again using ferric carboxymaltose. Meanwhile, HEART-FID is designed to assess the effects of ferric carboxymaltose on all cause death, heart failure hospitalisation, and 6MWT improvement.
At the time of writing, a randomised control trial (RCT) assessing the role of parenteral iron replenishment in heart failure with preserved ejection fraction (HFpEF) has not been published, however, FAIRHFpEF should answer that question. Our knowledge in HFpEF relies on systematic reviews and metaanalyses of HFpEF/iron deficiency that demonstrate negative impacts on NYHA, 6MWT, and quality-of-life measures. From this, we infer that iron deficiency has an important role in HFpEF, as in HFrEF. It is hypothesised that these patients may benefit from IV iron, however, clear evidence in the form of an RCT will confirm this.
Although most previous research on the impact of iron deficiency in the cardiology population has been in heart failure, iron deficiency, with
or without anaemia, has been linked to several other cardiac pathologies. These include CAD, arrhythmia, and major adverse cardiac events (MACE).
Iron deficiency is present at high rates among patients with atrial fibrillation (AF), as observed in several small studies. Larger studies are ongoing to confirm these observations. IRON-AF is currently assessing the impact of iron infusion in patients with AF. This initial short trial (12 week followup) will assess peak oxygen uptake, similar to initial HF trials. Secondary outcomes being examined include transthoracic echocardiography (TTE) parameters, AF burden via quality-oflife (QoL), Holter, and death.
It has been highlighted in several landmark trials that anaemia is associated with worse cardiovascular outcomes. When patients were followed for four years, a 1g/ dL decrease in haemoglobin was associated with an increased relative risk of cardiovascular death (RR=1.28) and MACE events (RR=1.23), 20 whilst a 14,000 patient-community cohort trial, with a mean of six years follow-up, identified that anaemic patients were more likely to have a MACE event than non-anaemic patients (HR=1.41). 21
Patients undergoing percutaneous coronary intervention (PCI), for either known CAD or MI, have been shown to have worse 30-day MACE and one year survival rate if anaemic, with the adverse outcomes directly related to the severity of anaemia. 22
The ESC and American Heart Association (AHA) provide no guidance on iron replacement in CAD due to a paucity of evidence in regard to its benefit. In 1981, Sullivan et al 23 hypothesised that iron was a chronic cardiotoxic, and despite its role in ROS, may promote atherosclerosis and CAD. These claims have since
FIGURE 1: Pathology of iron deficiency in heart failure4 https://ars.els-cdn.com/content/image/1-s2.0-S0735109717420134-fx1_lrg.jpg
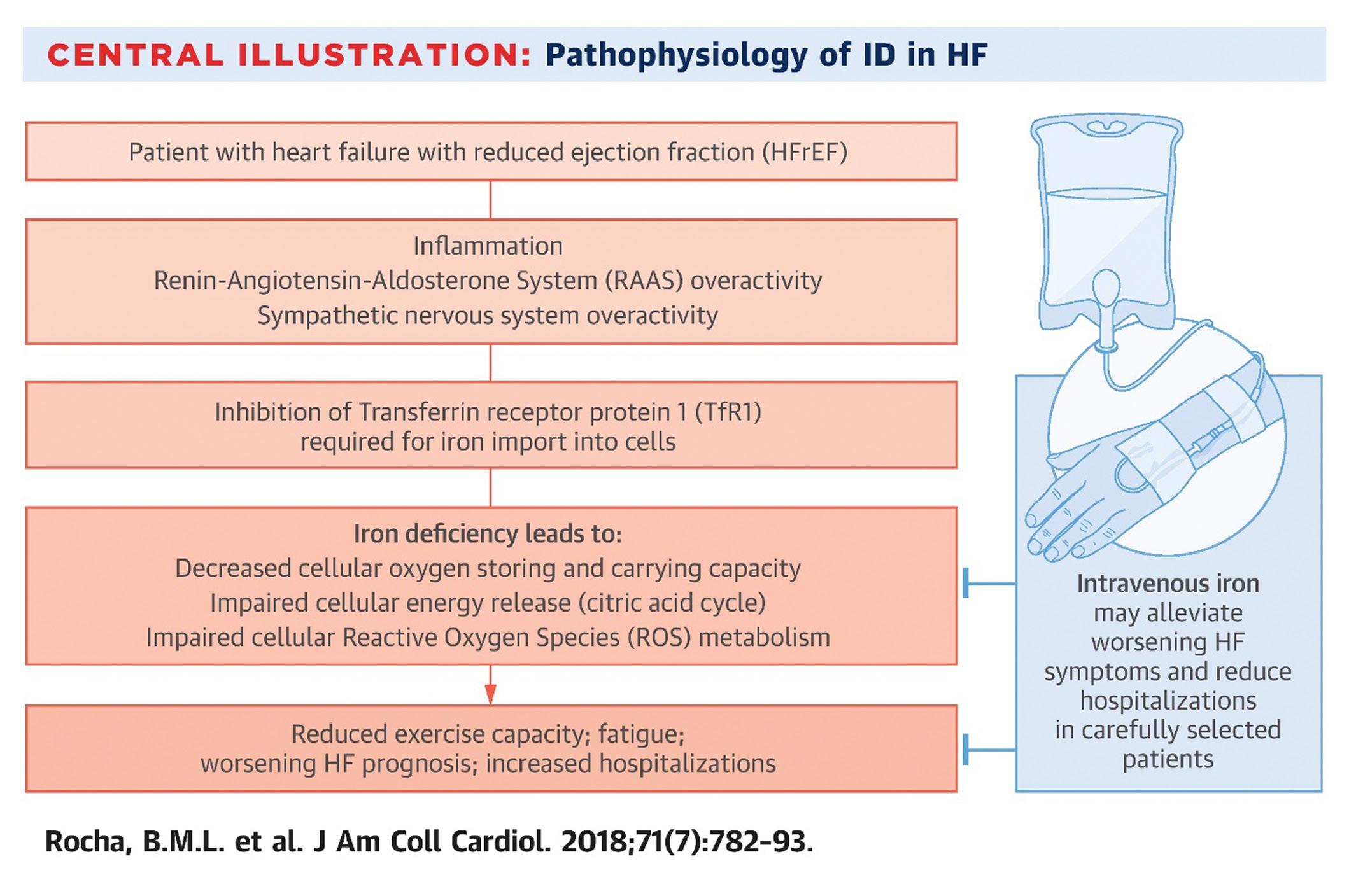
been disproven by multiple interval studies. 24 Unfortunately, the time taken to disproving that iron overload was not toxic has resulted in a slow movement towards research into iron deficiency and its role in overall cardiovascular health.
Subsequently, the Ludwigshafen Cardiac cohort trial 25 suggested that those with the lowest levels of iron stores are at highest risk of CAD compared to iron replete sex-matched patients. In contrast to this, a 2015 meta-analyses of 17 studies, with 9,236 patients with CAD, published conflicting evidence. 26 These patients were assigned into three groups for various iron store markers. There were no significant associations between
differing groups for serum ferritin, total iron binding capacity (TIBC), and iron. Higher TSAT did have a lower combined risk ratio of CAD/MI (RR =0.82).
Schrage et al, 27 studied 12,164 individuals from three European population-based cohorts, reviewing associations between AID/FID and incidental CAD, cardiovascular mortality, and all-cause mortality. FID was significantly associated with increased incidence of all three outcome measures, with 64 per cent of the cohort meeting FID criteria. AID however was only statistically significantly associated with incidental CAD, with all-cause mortality being statistically significant in those with severe AID (Ferritin <30). Given FID
includes TSAT, these findings match up to the 2015 meta-analyses, but highlight the difficulties in defining iron deficiency. The hope is for an RCT to investigate the effect of treating iron deficiency in the general cardiology or non-cardiology population.
Iron deficiency and anaemia are common conditions and have significant impacts on cardiology patients. It is often overlooked and undertreated. Current trials, soon to publish their findings, will give us stronger heart failure outcome evidence, which may lead to stronger evidence-based recommendations from the ESC and other organisations.
In the meantime, there is sufficient QoL and functional improvement evidence to support that iron replenishment should be a routine part of standard care, along with the use of left ventricular sparing medications. Iron infusion on a large patient scale is currently restricted, mostly to acute hospital care, requiring our chronic heart failure patients to travel for this potentially outpatient
service. The transition to enable iron infusions in community care settings should be prioritised as we transition the care of chronic conditions to the community, but resources must be put in place to support this. Although the evidence in heart failure is stronger than in other cardiology conditions, there is a suggestion that functional iron deficiency and low TSATs has a significant cardiovascular
morbidity and mortality impact on all cardiology patients. Future research into the role of iron deficiency in the general cardiac population will guide healthcare professionals as to whether there is a benefit in advocating for iron replenishment in these patients. Stronger evidence than that currently available is required before any changes to guidelines can be made. l
Q1. Iron deficiency affects more than two billion people worldwide.
True or false?
Q2. Iron deficiency in cardiology patients can occur without anaemia/reduced haemoglobin.
True or false?
Q3. Serum iron is a reliable indicator of iron stores.
True or false?
Q4. Transferrin saturation (TSAT) measures absolute iron deficiency.
True or false?
Q5. Oral iron supplementation is as effective as parenteral iron supplementation in patients with heart failure.
True or false?
Q6. Iron supplementation improves exercise capacity in both anaemic and nonanaemic heart failure patients.
True or false?
Q7. Iron supplementation has been shown to prospectively improve mortality in HFrEF and HFpEF.
True or false?
Q8. IV iron generally requires multiple treatments over time rather than a single once-off infusion.
True or false?
Q9. Iron is cardiotoxic and promotes atherosclerosis and earlier coronary artery disease.
True or false?
Q10. ESC guidelines state that iron deficiency should be assessed and replaced for all patients with coronary artery disease.
True or false?
Check your answers against the latest module on nursecpd.ie Successful completion of this module will earn you 2 CPD credits


Authors: Dr Robert M Evans, SpR in Cardiology, and Dr Patrick O’Callaghan, Consultant Cardiologist, University Hospital Waterford
1. Rymer JA, Rao SV. Anemia and coronary artery disease: Pathophysiology, prognosis, and treatment. Coron Artery Dis. 2018;29(2):161-7. doi: 10.1097/ MCA.0000000000000598
2. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-9. doi: 10.1182/ blood-2018-05-815944
3. Von Haehling S, Gremmler U, Krumm M, Mibach F, Schön N, Taggeselle J, et al. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: The PrEP Registry. Clin Res Cardiol. 2017;106(6):436-43. doi: 10.1007/s00392-016-1073-y
4. Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: Therapeutic approach. J Am Coll Cardiol. 2018;71(7):782-93. doi: 10.1016/j. jacc.2017.12.027
5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the taskforce for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24(1):4-131. doi: 10.1093/eurheartj/ehab368
6. Kvaslerud AB, Hussain AI, Auensen A, Ueland T, Michelsen AE, Pettersen KI, et al. Prevalence and prognostic implication of iron deficiency and anaemia in patients with severe aortic stenosis. Open Heart 2018;5(2):e000901. doi: 10.1136/ openhrt-2018-000901
7. Parsons WB, Jr Cooper T, Scheifley CH. Anemia in bacterial endocarditis. JAMA. 1953;153(1):14-6. doi: 10.1001/ jama.1953.02940180016005
8. Branwood AW. A case of perforated duodenal ulcer and cardiac infarction. Br Heart J. 1947;9(4):263-6. doi: 10.1136/hrt.9.4.263
9. Leshem-Rubinow E, Steinvil A, Zeltser D, Berliner S, Rogowski O, Raz R, et al. Association of angiotensin-converting enzyme inhibitor therapy initiation with a reduction in hemoglobin levels in patients without renal failure. Mayo Clin Proc. 2012;87(12):118995. doi: 10.1016/j.mayocp.2012.07.020
10. Ratcliffe PJ, O'Rourke JF, Maxwell PH, Pugh CW. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J Exp Biol. 1998;201(Pt 8):1153-62. doi: 10.1242/jeb.201.8.1153
11. Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 2012;111(8):1091-106
12. Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 2015;13(3):533-45. doi: 10.1016/j. freeradbiomed.2018.08.010
13. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients a systematic review and metaanalysis. J Am Coll Cardiol. 2008;52(10):81827. doi: 10.1016/j.jacc.2008.04.061
14. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF Randomised Clinical Trial. JAMA. 2017;317(19):1958-66. doi: 10.1001/ jama.2017.5427
15. Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand-Zaleski I, et al. Effect of a restrictive vs liberal blood transfusion strategy on major cardiovascular events among patients with acute myocardial infarction and anemia: The REALITY Randomised Clinical Trial. JAMA. 2021;325(6):552-60. doi: 10.1001/ jama.2021.0135
16. Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. New Engl J Med. 2013;368(13):1210-9. doi: 10.1056/NEJMoa1214865
17. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New Engl J Med. 2009;361(25):2436-48. doi: 10.1056/ NEJMoa0908355
18. Ponikowski P, van Veldhuisen DJ, CominColet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in
patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36(11):65768. doi: 10.1093/eurheartj/ehu385
19. Anker SD, Kirwan B-A, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in irondeficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018;20(1):125-33. doi: 10.1002/ejhf.823
20. Muzzarelli S, Pfisterer M. Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J. 2006;152(5):991-6. doi: 10.1016/j. ahj.2006.06.014
21. Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, et al. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40(1):2733. doi: 10.1016/s0735-1097(02)01938-1
22. Lee PC, Kini AS, Ahsan C, Fisher E, Sharma SK. Anemia is an independent predictor of mortality after percutaneous coronary intervention. J Am Coll Cardiol 2004;44(3):541-6. doi: 10.1016/j. jacc.2004.04.047
23. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):12934. doi: 10.1016/s0140-6736(81)92463-6
24. Ma J, Stampfer MJ. Body Iron Stores and Coronary Heart Disease. Clinical Chemistry. 2002;48(4):601-3. doi: 10.1056/ NEJM199404213301604
25. Grammer TB, Kleber ME, Silbernagel G, Pilz S, Scharnagl H, Tomaschitz A, et al. Hemoglobin, iron metabolism and angiographic coronary artery disease (The Ludwigshafen Risk and Cardiovascular Health Study). Atherosclerosis. 2014;236(2):292-300. doi: 10.1016/j.atherosclerosis.2014.07.002
26. Das De S, Krishna S, Jethwa A. Iron status and its association with coronary heart disease: Systematic review and meta-analysis of prospective studies. Atherosclerosis. 2015;238(2):296-303. doi: 10.1016/j.atherosclerosis
27. Schrage B, Rübsamen N, Ojeda FM, Thorand B, Peters A, Koenig W, et al. Association of iron deficiency with incident cardiovascular diseases and mortality in the general population. ESC Heart Fail 2021;8(6):4584-92. doi: 10.1002/ehf2.13589
AUTHOR: Ruth Morrow, Registered Advanced Nurse Practitioner (Primary Care); Respiratory Nurse Specialist (WhatsApp Messaging Service, Asthma Society of Ireland; and Nurse Educator and Consultant
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease that is characterised by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.

The chronic airflow limitation that is characteristic of COPD is caused by a mixture of small airways disease (eg, obstructive bronchiolitis) and parenchymal destruction (emphysema), the relative contributions of which vary from person to person (GOLD, 2022).
In 2019, the National Institute for Health Research in the UK carried out a systematic review, which explored the prevalence of COPD from 19902019 in 65 countries. The study found that the global prevalence of COPD was 10.3 per cent in 30-to-79 year-olds; equivalent to 391.9 million people.
The study also found that the key risk factors for developing COPD include being male, current smoker, having a BMI of less than 18.5, exposure to biomass fuels, and occupational exposure to dust or smoke (Adeloye et al, 2022). Other contributing factors
FIGURE 1: The revised ABCD assessment tool (GOLD)
include genetic factors, lung growth and development, socioeconomic status, asthma and airway hyper-reactivity, chronic bronchitis, and infections.
The paradigm of COPD as a predominantly male disease is changing as smoking rates in both males and females are now similar, and the number of deaths due to COPD among women has surpassed that of men. Women appear more susceptible to the effects of cigarette smoke, developing COPD earlier and with lower cigarette exposure than men. Women also commonly exhibit a COPD phenotype with airway dominant disease in comparison with emphysema and they also vary in response to treatment.
The diagnosis of COPD involves a detailed history, spirometry, investigations, assessment of symptoms.
The patient history should include: Medical and surgical history; Smoking history including pack year history; Occupational history.
Post-bronchodilator FEV1/FVC ratio of 70 per cent or 0.7 indicates COPD. Table 1 illustrates the severity of obstruction using FEV1
Blood screen to include FBC and TFTs; Chest x-ray; ECG.
The measurement of fractional inhaled nitrous oxide (FENO) is a relatively new concept and is now
IN PATIENTS WITH FEV1/FVC <70%
GOLD 1 Mild FEV1 > 80% predicted
GOLD 2 Moderate 50% < FEV1 < 80% predicted
GOLD 3 Severe 30% < FEV1 < 50% predicted
GOLD 4 Very severe FEV1 < 30% predicted
TABLE 1: GOLD (2022) classification based on FEV1
recommended as one of the tools to assess if inhaled corticosteroid therapy (ICS) is indicated for these patients. Approximately 40 per cent of patients with COPD have raised eosinophils. Patients with high eosinophil levels have an increased risk of exacerbations of COPD if they are not on ICS therapy.
The characteristic symptoms of COPD are chronic and progressive dyspnoea, cough, and sputum production that can be variable from day-to-day. Dyspnoea is usually progressive, persistent, and characteristically worse with exercise. Patients may have an intermittent cough, which may be unproductive, but many patients will commonly cough up white/clear non-purulent sputum. Symptoms and their impact on qualityof-life can be assessed using the COPD assessment tool (CAT) test and the Medical Research Council (MRC) dyspnoea scale ( Table 2). The CAT test is a validated eight-item measure of health status impairment in COPD (www.catestonline.org).
0 No breathlessness except with strenuous exercise
1 Shortness of breath when hurrying on the level or walking up a slight hill

2 Walks slower than people of the same a ge on the level because of breathlessness or has to stop for breath when walking at own pace on the level
3 Stops for breath after walking about 100 metres or after a few minutes on the level
4 Too breathless to leave the house or breathless when dressing or undressing
TABLE 2: Medical Research Council dyspnoea scale
The ABCD assessment tool is a useful tool for assessing severity of COPD. The assessment of COPD has been refined to include assessment of symptoms and risk of future exacerbations ( Figure 1).
Pharmacological therapies are used to reduce COPD symptoms, reduce the severity and frequency of exacerbations, and improve exercise tolerance and health status.
Bronchodilator therapy remains the mainstay of the management of stable COPD and has been shown to reduce hyperinflation. The main groups of COPD medications include:
Beta-agonists – these relax smooth
A
SABA OR SAMA
B
LABA or LAMA
SABA: Salbutamol, terbutaline
SAMA: Ipratropium bromide
LABA: Salmeterol, formoterol, indacaterol, olodaterol
LAMA: Tiotropium, umeclidinium, aclidinium, glycopyrronium
MDI with spacer, Easi-breathe, Diskus, Turbohaler
MDI with spacer, Turbohaler, Breezhaler, Respimat
Respimat, Handihaler, Genuair, Elipta, Breezhaler
C
ICS and LABA or LAMA
ICS/LABA: Budesonide/formoterol, vilanterol/fluticasone
ICS/LAMA: Aclidinium/formoterol
LAMA: Tiotropium, umeclidinium, aclidinium, glycopyrronium
Turbohaler, Spiromax, Easyhaler, Elipta
Genuair
Respimat, Handihaler, Genuair, Elipta, Breezhaler
D
LABA with ICS and LAMA
ICS/LABA: Budesonide/formoterol, vilanterol/fluticasone
ICS/LAMA: Aclidinium/formoterol
LAMA: Tiotropium, umeclidinium, aclidinium, glycopyrronium
TABLE 3: Pharmacological treatment options based on COPD classification (GOLD, 2022)
muscle by stimulating the beta2 adrenergic receptors. Beta-agonists can be classified into short-acting (SABA) and long-acting (LABA); eg, salbutamol (SABA), salmeterol (LABA), indacaterol (LABA), vilanterol (LABA), formoterol (LABA), olodaterol (LABA).
Antimuscarinic drugs block the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors. These can also be classified into short-acting (SAMA) and longacting (LAMA); eg, ipratropium (SAMA), tiotropium (LAMA), umeclidinium (LAMA), aclidinium bromide (LAMA), glycopyrronium (LAMA).
Combining bronchodilators may increase the degree of bronchodilation, whilst lowering the risk of side-effects compared to increasing the dose of a single bronchodilator agent.
Methylxanthines – this group of drugs remain controversial as to their
mechanism of action. There is evidence of bronchodilation in stable COPD. Theophylline is the most commonly used methylxanthine. However, there are significant drug interactions with its use and clearance of the drug declines with age.
ICS should not be used as a single agent in the management of COPD. In patients with moderate to severe COPD, the use of ICS combined with a LABA is more effective than using either agent alone in improving lung function, health status, and reducing exacerbations. Their use in patients with high eosinophil levels have been shown to be beneficial (GOLD, 2022).
To-date, there is no conclusive clinical trial evidence that any existing medications for COPD modify the long-term decline in lung function (GOLD, 2022).
Pharmacological algorithms are
Turbohaler, Spiromax, Easyhaler, Elipta
Genuair
Respimat, Handihaler, Genuair, Elipta, Breezhaler
given for the initiation, escalation, or de-escalation of treatment according to the individual assessment of symptoms and exacerbation risk. In previous publications of GOLD reports, recommendations were only given for treatment initiation. Table 3 illustrates the pharmacological treatment options according to the patient’s COPD classification.
The assessment of inspiratory effort is important to ensure that the patient has sufficient inspiratory flow to optimise deposition of medication to the lungs. Inspiratory effort can be assessed using the In Check Dial metre. Inhaler devices have different inspiratory flow requirements, with dry powder devices requiring a greater effort that soft mist or metre dose inhalers. To improve technique,
≥2 moderate exacerbations or ≥1 leading to hospitalisation
0 or 1 moderate exacerbations (not leading to hospital admission)
Group C
LAMA
Group D
LAMA or LAMA + LABA* or ICS + LABA**
* Consider if highly symptomatic (eg, CAT >20)
** Consider if eos ≥300
Group A A bronchodilator
Group B
A long-acting bronchodilator (LABA or LAMA)
mMRC 0-1 CAT <10
mMRC ≥2 CAT ≥10
FIGURE 2: Initial pharmacological treatment with the ABCD assessment tool (GOLD, 2022)
it is recommended that patients are educated and trained with the appropriate devices. The choice of device should be tailored to the individual, depending on the patient’s ability to use it, their manual dexterity, cognitive function, and taking their preference into account.
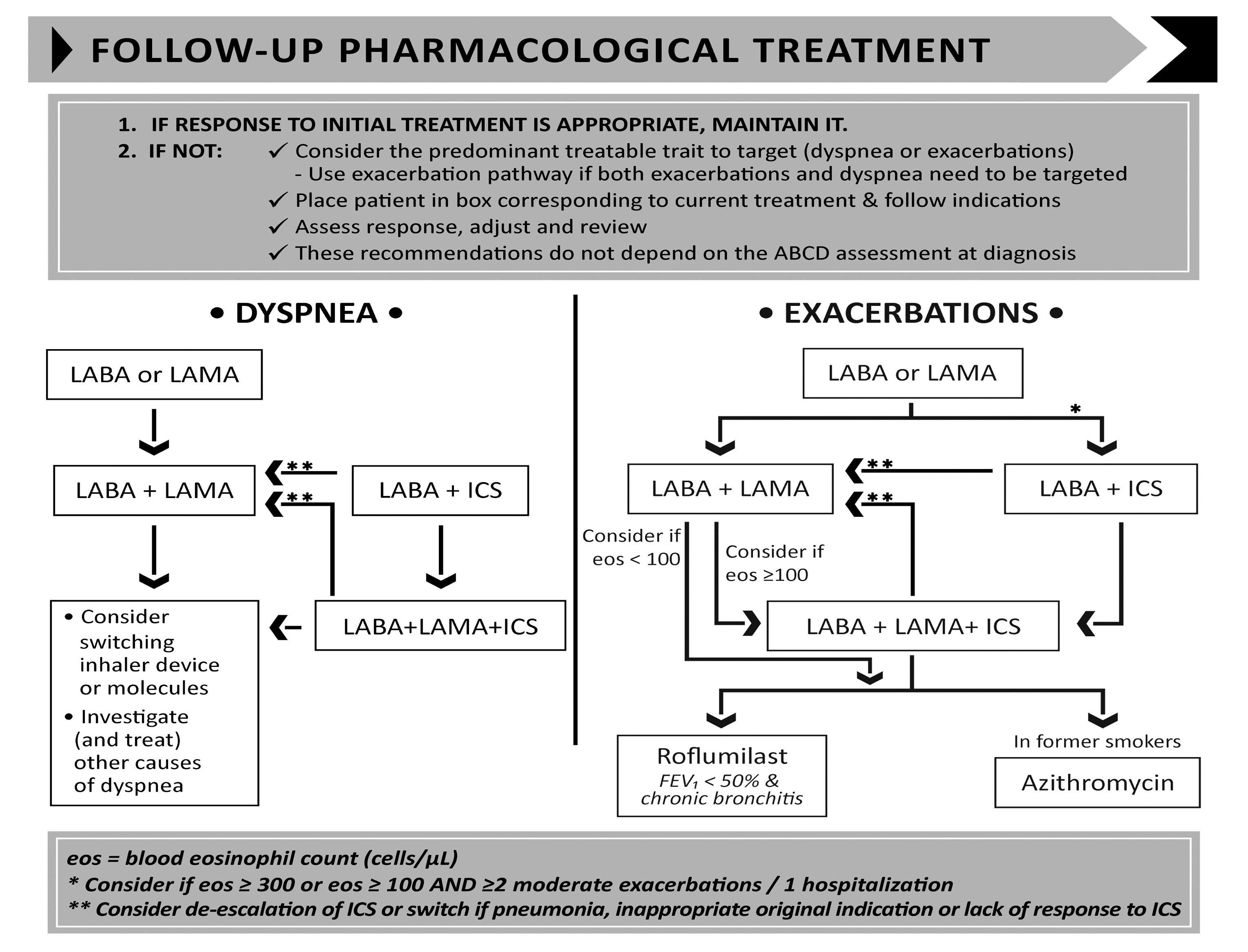
Ongoing monitoring and follow-up GOLD (2022) recommends that regular review of COPD should focus on dyspnoea and exacerbations. Whichever is the most problematic for the patient should be the focus of the review. For patients with persistent breathlessness or exercise limitation on LABA/ICS treatment, LAMA can be added to escalate to triple therapy. Alternatively, switching from LABA/ICS to LABA/LAMA should be considered if the original indication for ICS was inappropriate (eg, an ICS was used to treat symptoms in the absence of a history of exacerbations), or there has been a lack of response to ICS treatment, or if ICS side-effects
AT ALL STAGES, DYSPNOEA DUE TO OTHER CAUSES (NOT COPD) SHOULD BE INVESTIGATED AND TREATED APPROPRIATELY
warrant discontinuation (GOLD, 2021). At all stages, dyspnoea due to other causes (not COPD) should be investigated and treated appropriately. Inhaler technique and adherence should be considered as causes of inadequate treatment response. Where exacerbations are the predominant trait, prophylactic macrolide antibiotics should be considered following cardiovascular assessment. Assessment of eosinophils can be useful when the patient is experiencing exacerbations. If the
eosinophils are above 0.3 or if the patient has experienced two or more exacerbations with one hospitalisation, initiating or escalating ICS can be beneficial (GOLD, 2021). Roflumilast, another treatment option, is a selective inhibitor of phosphodiesterase-4 (PDE-4) that has unique antiinflammatory activity and is used to treat and prevent exacerbations.
Smoking cessation is of paramount importance in the management of COPD regardless of disease severity. Support given by health professionals significantly increases quit rates over self-initiated strategies. Even a brief (three-minute) period of counselling to urge a smoker to quit results in smoking quit rates of 5-to-10 per cent. Smoking cessation should be encouraged at all severities of the condition. Nicotine replacement therapy (nicotine gum, nasal spray, transdermal patch, sublingual tablet, or lozenge) as well as treatment with varenicline reliably increases longterm smoking abstinence rates and are significantly more effective than placebo (GOLD, 2022).
Pulmonary rehabilitation has been proven to show significant benefits in reducing dyspnoea, fatigue and exacerbations, and improving qualityof-life in people with COPD. Although an effective pulmonary rehabilitation programme is six weeks, the longer the programme continues, the more effective the results. If exercise training is maintained at home, the patient’s health status remains above prerehabilitation levels (McCarthy et al, 2015).
Patients should be encouraged to be as physically active as possible. The aim is to achieve 30 minutes of aerobic


FIGURE 3: Follow-up treatment (GOLD, 2022)
activity daily, eg, walking, cycling, and swimming. Exercises such as chest and shoulder exercises, shoulder raises, step ups, and sit-to-stand exercises should be encouraged as this will assist in maintaining upper body strength with the ultimate aim of prevention of muscle wasting.
Breathing exercises, such as pursedlip breathing and the active cycle of breathing, can help manage dyspnoea
and assist in expectorating sputum. These techniques are available on www.copd.ie .
Energy conservation is based on the 4Ps:
Make a list of what you have to do;
Place the task in order of importance, into what you need to do, want to do, and should do; Get rid of any unnecessary tasks; Decide if someone else can do some tasks for you; Change between light and heavy tasks.
Work at a slow steady pace;
Allow plenty of time for rest and relaxation, at least five-to-10 minutes every hour;
Use breathlessness as a guide;
Space difficult and heavy tasks evenly throughout the week.
Change the activities to keep the energy you use low to do the job;
Sit rather than stand when possible, use a perching or high stool
when possible;
Organise yourself, your home, and your working environment;
Put items you frequently use in an easy to reach place;
Consider using a bag or basket to carry things;
Plan tasks around when help is available and when your energy levels are high.
Push or pull objects, rather than lifting;
Use your legs, not your back; Use your strongest, largest joints; Keep your arm movements at a low rate;
Avoid bending, reaching or twisting.
Patients require ongoing education and support to assist them to live and maintain optimal lifestyles. Education about the disease process, inhaler technique, adherence to medication, immunisations, pulmonary rehabilitation, smoking cessation, and long-term oxygen therapy are required. All patients should have a COPD communication card, which outlines their treatment, including oxygen therapy, and gives guidance on how to recognise worsening COPD. (Available at www.COPD.ie)
Long-term oxygen therapy is indicated for stable patients who have:
PaO 2 at or below 7.3kPa (55mmHg) or SaO 2 at or below 88 per cent, with or without hypercapnia confirmed twice over a three-week period; or PaO 2 between 7.3kPa (55mmHg) and 8.0kPa (60mmHg), or SaO 2 of 88 per cent, if there is evidence of pulmonary hypertension, peripheral oedema suggesting congestive cardiac failure, or polycythaemia (haematocrit >55 per cent).
Once placed on long-term oxygen therapy (LTOT) the patient should be
re-evaluated after 60-to-90 days with repeat arterial blood gas (ABG) or oxygen saturation while inspiring the same level of oxygen or room air to determine if oxygen is therapeutic and still indicated, respectively (GOLD, 2022).
Many patients with COPD have coexisting illness such as diabetes, cardiovascular disease, osteoporosis, and depression to mention but a few. In general, the presence of comorbidities should not affect COPD treatment and co-morbidities should be treated according to standards and guidelines. Lung cancer is common in patients with COPD due to smoking history. About 50 per cent of patients who experience exacerbations of their COPD and require hospital admission have undiagnosed cardiovascular disease (Steer et al, 2022). Addressing this reduces subsequent admissions to hospital. It is recommended that these patients should have an
echocardiograph, BNP and cardiac MRI. Gastroesophageal reflux is common and is associated with an increased risk of exacerbations and therefore should be managed optimally. Osteoporosis is common due to recurrent use of oral steroids, lack of weight-bearing exercise, smoking, and being over- or underweight. GOLD recommends that treatments for co-morbidities should be kept as simple as possible to avoid polypharmacy (GOLD, 2022).
This article has focused on the diagnosis, management, and prevention of COPD. The definition, classification, pharmacological, nonpharmacological, the importance of inhaler technique, and co-morbidities have been addressed. There have been significant changes to the assessment tool which has simplified the classification of COPD, which will enable practitioners to individualise the patient’s management and treatment, and ultimately improve patient outcomes and quality-of-life. l
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of COPD. 2022. Available at: www.goldcopd.org/
2. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I; 2022, NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir Med . 2022 May;10(5):447-458. doi: 10.1016/
S2213-2600(21)00511-7

3. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015 Feb 23;(2):CD003793. doi: 10.1002/14651858.CD003793.pub3
4. Steer J, Kibbler DP, Bourke SC, Ripley DP. 2022. Undiagnosed and undertreated heart disease in hospitalised patients with exacerbation of chronic obstructive pulmonary disease (COPD).
European Respiratory Society Congress 2022 oral presentation

































Please refer to the Summary of Product Characteristics (SmPC) before prescribing Sondelbay® (teriparatide) 20 micrograms/80 microlitres solution for injection in pre- lled pen Presentation: One pre- lled pen of 2.4 ml contains 600 μg of teriparatide. Each dose contains 20 μg of teriparatide in 80 μL. Indications: Indicated in adults. Treatment of osteoporosis in postmenopausal women and in men at increased risk of fracture. In postmenopausal women, signi cant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures have been demonstrated; Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage and Administration: Recommended dose of Sondelbay is 20 μg administered once daily. Maximum total duration of treatment should be 24 months. The course should not be repeated over a patient’s lifetime. Patients should receive supplemental calcium and vitamin D supplements if dietary intake is inadequate. Following cessation of Sondelbay therapy, patients may be continued on other osteoporosis therapies. Elderly: Dosage adjustment is not required. Renal impairment: Must not be used in patients with severe renal impairment but should be used with caution in patients with moderate renal impairment. No special caution is required for patients with mild renal impairment. Hepatic impairment: Should be used with caution as no data are available. Paediatric population and young adults with open epiphyses: Should not be used in paediatric patients (<18 years), or young adults (>18 to 29 years) with open epiphyses due to lack of safety and e cacy data. Method of administration: Sondelbay should be administered once daily by subcutaneous injection in the thigh or abdomen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or to any of the excipients; Pregnancy and breast-feeding; Pre-existing hypercalcaemia; Severe renal impairment; Metabolic bone diseases other than primary osteoporosis or glucorticoidinduced osteoporosis; Unexplained elevations of alkaline phosphatase; Prior external beam or implant radiation therapy to the skeleton; Patients with skeletal malignancies or bone metastases. Warnings and Precautions: Traceability: The name and batch number of the administered product should be clearly recorded. Serum and urine calcium: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection.
Routine calcium monitoring during therapy is not required. Urolithiasis: Sondelbay should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. Orthostatic hypotension: In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. Renal impairment: Caution should be exercised in patients with moderate renal impairment. Younger adult population (>18 to 29 years): Experience is limited (including premenopausal women). Treatment should only be initiated if the bene t clearly outweighs risks in this population. Excipient: Contains less than 1 mmol sodium (23 mg) per dosage unit, that is to say essentially “sodium-free”. E ects on ability to drive and use machines: No or negligible in uence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Fertility, Pregnancy & Lactation: Women of childbearing potential: E ective methods of contraception should be used during use of teriparatide. If pregnancy occurs, Sondelbay should be discontinued. Pregnancy and breast-feeding: Contraindicated for use. It is not known whether teriparatide is excreted in human milk. Fertility: The e ect on human foetal development has not been studied. The potential risk for humans is unknown. Adverse Events include: Adverse events which could be considered serious: Common: Syncope, dyspnoea, hiatus hernia. Uncommon: tachycardia, nephrolithiasis. Rare: anaphylaxis, renal failure/impairment. Other Very Common adverse events: Pain in limb. Other Common adverse events: Anaemia, hypercholesterolaemia, depression, dizziness, headache, sciatica, vertigo, palpitations, hypotension, nausea, vomiting, gastro-oesophageal re ux disease, sweating increased, muscle cramps, fatigue, chest pain, asthenia, mild and transient injection site events. See SmPC for details of other adverse events. Shelf Life: Unopened vial – 2 years. Pack size: available in pack sizes of 1 pre- lled pen or 3 pre- lled pens. Each pre- lled pen contains 28 doses of 20 micrograms (per 80 microliters). Marketing Authorisation Numbers: EU/1/22/1628/001-002. Marketing Authorisation Holder: World Trade Centre, Moll de Barcelona s/n, Edi ci Est, 6ª Planta, 08039, Barcelona, Spain. Legal Category: POM. Full prescribing information including the SmPC is available on request from Accord Healthcare Ireland Ltd, Euro House, Little Island, Co. Cork, Tel: 021-4619040 or www.accord-healthcare.ie/products. Adverse reactions can be reported to Medical Information at Accord Healthcare Ltd. via email: medinfo@accord-healthcare.com or Tel: +44(0)1271385257. Date of Generation of API: June 2022. IE-01851.
that danced in the summer of love, that brought three children into the world, that worked hard until retirement, that will stand up to osteoporosis and dance some more.
SONDELBAY® is indicated in adults for treatment of osteoporosis in postmenopausal women and in men at increased risk of fracture.1
Ireland should introduce a system of ‘health passports’ for children to support them in keeping physically active in an effort to stave off chronic disease and ill health later in life, according to Prof Niall Moyna, Professor of Clinical Exercise Physiology, Dublin City University.
During a very well-received presentation at the IOS 2022 Annual Medical Conference, Prof Moyna highlighted the importance of physical activity and a healthy, active lifestyle in maintaining good health and preventing the development of chronic conditions. “There is compelling evidence for the benefits of physical activity in relation to health.”
Speaking during the questions and answers session, he said that physical activity levels in Irish children are inadequate and below the recommended levels in the majority, and their fitness levels are not monitored or supported (outside of competitive sports and PE).
However, Prof Moyna suggested that a ‘health passport’ system, where children would be assessed in school on their fitness over regular intervals, in a fun, 'child-centric way,' using validated scores, would provide an ideal opportunity to intervene early on when needed and would educate children on the importance of physical activity for good health. This would encourage children to remain fit throughout their life and provide important data to help inform policy at a national level.
“The whole idea would be to identify kids early on who are at risk and, in a very sensitive way, to bring that to the attention of the public health nurse and
let them intervene rather than wait until it’s too late,” he said.
He pointed out that one-third of people over the age of 18 years have a chronic condition, which rises to one-in-two aged over 50 years, “which is quite alarming.” These conditions, including chronic obstructive pulmonary disease, heart disease, and neurological conditions, accounted for 76 per cent of all deaths in Ireland in 2021, and lead to years of ill health, increased risk of hospitalisation, and premature death.
Prof Moyna quoted a number of studies showing that people with various levels of physical activity have much better health, and are less likely/slower to develop chronic health conditions.
He quoted supporting data, adding that modern medicine tends to focus far more on medication or surgery rather than lifestyle adjustments, despite the overwhelming evidence on the benefits, which he said needs to be addressed.
Prof Moyna discussed the findings of a recent major meta-analysis of studies involving over 115,000 people, which showed the positive health benefits of physical activity in reducing various negative health risks and outcomes.
“If you can increase your function capacity just by one MET [metabolic equivalent of task], you will get a 13 per cent reduction in all-cause mortality, a 15 per cent reduction in cardiovascular events, a 7 per cent decrease in waist circumference, a 5 per cent decrease in systolic blood pressure, and improvements in triglycerides and resting blood pressure…. So every one

MET increase we can have between four and 10 METs is hugely important in relation to longevity and risk of death.”
Prof Moyna also looked at international physical activity guidelines, which advocate a mixture of daily moderate activity and more vigorous activity a couple of times a week. He said that daily walking, even for just 30 minutes, dramatically reduces the risk of developing chronic diseases, as well as aiding bone mineral density.
Speaking to NiGP after the conference, Prof O’Brien praised Prof Moyna’s presentation and suggestions on improving child fitness. “I agree with Prof Moyna where he states that prevention of diseases should begin in childhood.
“His idea of having health passports for children would make both logical and financial sense, [and] is something that the Government should look very seriously at investing in.
“Such an initiative would significantly decrease the long-term financial burden on our already creaking health system. It would also ensure that our younger generations would live healthier lifestyles than previous generations, and they would carry this through to their adulthood and old age.
“Physical activity should be compulsory in all schools from pre-school right through to when the student finishes school, by which stage physical activity has become a way of life.
“Personally, I cannot see why our Government would not initiate this type of programme. I am unsure that if we want a healthier Ireland, spending millions on bicycle lanes is the answer.
The Irish Osteoporosis Society (IOS) Annual Medical Conference for Health Professionals was held virtually on Saturday, 22 October. The well-attended conference featured a varied range of presentations from expert speakers on topical issues relating to osteoporosis.
President of the Society, Prof Moira O’Brien, told NiGP she was delighted attendees could get updates in so many areas of bone health on the day.
“Osteoporosis is the commonest bone disease worldwide, but it is preventable, and it is treatable [for the majority],” stated Prof O’Brien.
“Dr Kevin McCarroll spoke about the importance of normal vitamin D levels and parathyroid hormone on bone and the
different issues that can arise.
“Prof Bernard Walsh discussed the horrendous secondary impact Covid-19 has had on people’s bone health, including the increased risk of falls.
“I believe many people were shocked when they heard from [physiotherapist] Aoife Ni Eochaidh that 75 per cent of people with incontinence issues can reverse the problem [with pelvic floor training] and the other 25 per cent can improve the issue.
“Mr Derek Bennett spoke about the importance of not only fixing bone fractures, but increased diagnosis of osteoporosis by orthopaedic surgeons.
“The presentation on denosumab by Dr Rosie Lannon clarified that patients on this treatment should not be put on 'drug
holidays' from it, as unfortunately some patients have been told to stop/put on hold their osteoporosis medications for dental work to be done, which is placing these patients at risk of fractures.”
Prof O’Brien also praised the presentation given by Prof Niall Moyna on exercise and chronic diseases.
“I graduated medical school in 1956 and I have been involved in sports medicine since the 1960s, and at 89 I am still working as an osteoporosis consultant.
“In my opinion Prof Niall Moyna’s idea of health passports, for children, is by far the most ingenious yet simplest one that I have heard presented to date.”
More information and resources on osteoporosis are available on the IOS website, www.irishosteoporosis.ie.
Three-quarters of women with incontinence issues who have pelvic floor muscle training see improvement or resolution of their symptoms, the IOS 2022 Annual Medical Conference heard.
Pelvic floor training also leads to significant improvement in faecal incontinence, constipation, pelvic organ prolapse, and sexual dysfunction and erectile dysfunction in men, Ms Aoife Ni Eochaidh, Chartered Physiotherapist, Clinical Specialist Physiotherapist, Women’s and Men’s Health and Continence, Bon Secour Consultant’s Clinic, Galway, reported during her presentation on women’s health.
She noted that bowel obstruction and constipation are a key cause of hospitalisation in older adults, with an over-dependence on laxatives and surgery
despite conservative methods giving good results. “Training the pelvic floor will help the bowel fill, store, and empty.”
Ms Ni Eochaidh looked at issues caused by hormonal changes in menopause, which impact smooth muscle and affect the pelvic floor, “leading to atrophy, loss of bulk… and huge problems during menopause such as itchy vaginal skin… bacterial vaginitis, thrush, UTIs [urinary tract infections], etc.”
She stressed the importance of regular exercise, pelvic floor training, and hormone replacement therapy and probiotics, and other suitable supplements, where indicated, in helping address these issues.
She also noted the risk of osteoporosis in this cohort and the impact of osteoporosis on the pelvic floor, ie, incontinence issues, which can raise the risk of falls.
“The physical mechanical changes with osteoporosis affect the pelvic floor…
the woman needs to be doing weightbearing exercise, but you need a good pelvic floor so that it can be enjoyable. Start first with the pelvic floor in any training programme. Don’t forget the fluids and the fibre and then the weight bearing [exercise].”
Ms Ni Eochaidh said her clinic can also provide ‘blended’ pelvic floor training programmes, through online video sessions, which can be more convenient and cost-effective for some patients.
Concluding, she stressed that “no amount of urine leakage is normal” and “pelvic floor muscle training is for life, it is not a quick fix. I think it should be called ‘patience floor training’ because you need patience. It can take 12 months, but in general six months… it is essential for good bone health and in menopause, as well as during and after pregnancy.”

For the first time, influenza (flu) vaccination rates in Ireland for people aged 65 years and older reached the World Health Organisation (WHO) target of 75 per cent during the 2021/2022 season. This is a significant milestone for Ireland and is thanks to the concerted effort of all who plan and deliver the flu vaccine programme. Re-vaccination is recommended every flu season because circulating strains of the influenza virus change and immunity declines over time after vaccination.
Influenza is a very common acute viral respiratory illness which affects all age groups. The virus is seen all year round, but peaks every winter. The degree of influenza infection is unpredictable, however, and each year in Ireland influenza is responsible for between 200 and 500 deaths.
Although there was no flu circulating in Ireland during 2020/2021 due to public health restrictions in place because of the Covid-19 pandemic, flu activity returned in Ireland and across Europe during the 2021/2022 season as restrictions eased.
As we head into winter, the 2022/2023 flu season presents a significant challenge to the delivery of healthcare services both in primary care and in hospitals, as flu and Covid-19 are expected to co-circulate.
The WHO’s latest surveillance report states: “Countries are recommended to prepare for the cocirculation of influenza and SARS-CoV-2 viruses. They are encouraged to enhance integrated surveillance to monitor influenza and SARS-CoV-2 at the same time, and step-up their influenza vaccination campaign to prevent severe disease and hospitalisations associated with influenza. Clinicians should consider influenza in differential diagnosis, especially for high-risk groups for influenza, and test and treat according to national guidance.”
Each year, influenza statistics reinforce that flu is more severe in older adults, those with long-term heart or respiratory illness, in young children, and those who are pregnant. Eightto-nine out of every 10 flu-related deaths occur in older adults, therefore it is important that older adults are protected early in every flu season.
The best way to prevent Influenza is by vaccination. Each year, the WHO recommends the vaccine compositions based on the four virus strains most likely to be circulating in the coming season.
The 2022/2023 HSE seasonal vaccination programme is offering three vaccines:
Quadrivalent live attenuated influenza vaccine (LAIV) nasal application for children aged twoto-17 years. Brand available:
• Fluenz Tetra nasal spray suspension influenza vaccine (live attenuated, nasal) manufactured by AstraZeneca AB.
Inactivated quadrivalent influenza vaccine (QIV) available for all other eligible populations, including those aged two-to-17 years with contraindications

2,185 (399 A(H3)), 8 A(H1) pdm09 and 1,771 A not subtyped
11 and 1 with influenza type not reported
TABLE 1: Total of 2,197 influenza cases in Ireland for 2021/2022 (between week 40 2021 to week 20 2022)
Medium 32 (interquartile range 21-64 years)
to LAIV (QIV is licensed for those six months of age and older). Two brands of injectable vaccine will be distributed and are interchangeable where two doses are required:
• Quadrivalent influenza vaccine (split virion, inactivated) manufactured by Sanofi Pasteur. • Influvac Tetra marketed by Mylan.
The National Immunisation Advisory Committee (NIAC) recommends annual influenza vaccination for the following groups: Aged 65 years and older; Aged two-to-17 years; Healthcare workers; Pregnant women; Those living in a nursing home or other long-term care facility; and Those in regular contact with pigs, poultry or waterfowl.
People with the following conditions can also get a free flu vaccine: Chronic heart disease, including acute coronary syndrome; Chronic liver disease; Chronic kidney failure; Chronic respiratory disease, including chronic obstructive pulmonary disease (COPD), cystic fibrosis, moderate or severe asthma, or bronchopulmonary dysplasia; Chronic neurological disease, including multiple sclerosis, and hereditary and degenerative disorders of the central nervous system; Diabetes; Down syndrome; Haemoglobinopathies; A body mass index (BMI) over 40; Immunosuppression due to disease or treatment (including asplenia or hyposplenism, and all cancer patients); Children with a moderate-to-severe neurodevelopmental disorder, such as cerebral palsy; Children on long-term aspirin therapy; and
Any condition that can compromise respiratory function, like spinal cord injury, seizure disorder or other neuromuscular disorders, especially people also attending special schools or day centres.
Free flu vaccines can be offered to carers or household contacts of people who have: A health condition listed above; or Down syndrome.
People eligible for the HSE flu vaccine should not be charged an administration fee or be charged for the HSE flu vaccine.
pharmacist for vaccination has been shown to increase vaccine uptake.
The nasal spray flu vaccine is recommended for all children aged two-to-17 years. The nasal flu vaccine protects children from flu-related morbidity and mortality.
Children are among the most susceptible to influenza infection and are twice as likely as adults to catch the virus. Approximately 10 per cent of children aged under 15 years attend their GP with influenzalike illness in an average influenza season. Influenza in children can cause serious complications such as pneumonia, bronchitis, otitis media, croup, and bronchiolitis.
Significant improvements have been made in recent years to improve vaccination uptake, notably in older adults, with a 75.4 per cent uptake recorded among people aged 65 years and older for the 2021/2022 season. However, vaccination rates for other at-risk groups, particularly children, remain low and have yet to reach the WHO target of 75 per cent. Therefore, it is important to encourage uptake of the influenza vaccine this flu season.
Those most at risk from flu are dependent on their healthcare providers — nurses, doctors, and pharmacists — for information on influenza vaccination. A recommendation by a trusted healthcare professional for their patients who are at an increased risk of influenza-related complications to attend their GP or
Children attending crèches, day-care centres and schools are important transmitters of influenza in the community. This means that vaccinating children decreases the number of people with influenza, the number of hospital admissions, transmission of influenza to older adults and persons in at-risk groups, and transmission to healthcare workers in families with children.
Children also transmit the flu virus for a longer period than adults; they can transmit the virus for 10 or more days, compared to six days in adults, therefore increasing the spread of the disease, increasing the pressure on the healthcare system, and contributing significantly to influenza outbreaks.
A meta-analysis of LAIV in 2012 suggested an efficacy against confirmed disease of 83 per cent (95 per cent, CI 69-91) in younger children.
Vaccinating children not only protects them against severe illness, but may also assist in reducing influenza transmission to other vulnerable populations, such as those aged 65 years and older and those with long-term health conditions.
CHILDREN ARE AMONG THE MOST SUSCEPTIBLE TO INFLUENZA INFECTION AND ARE TWICE AS LIKELY AS ADULTS TO CATCH THE VIRUS
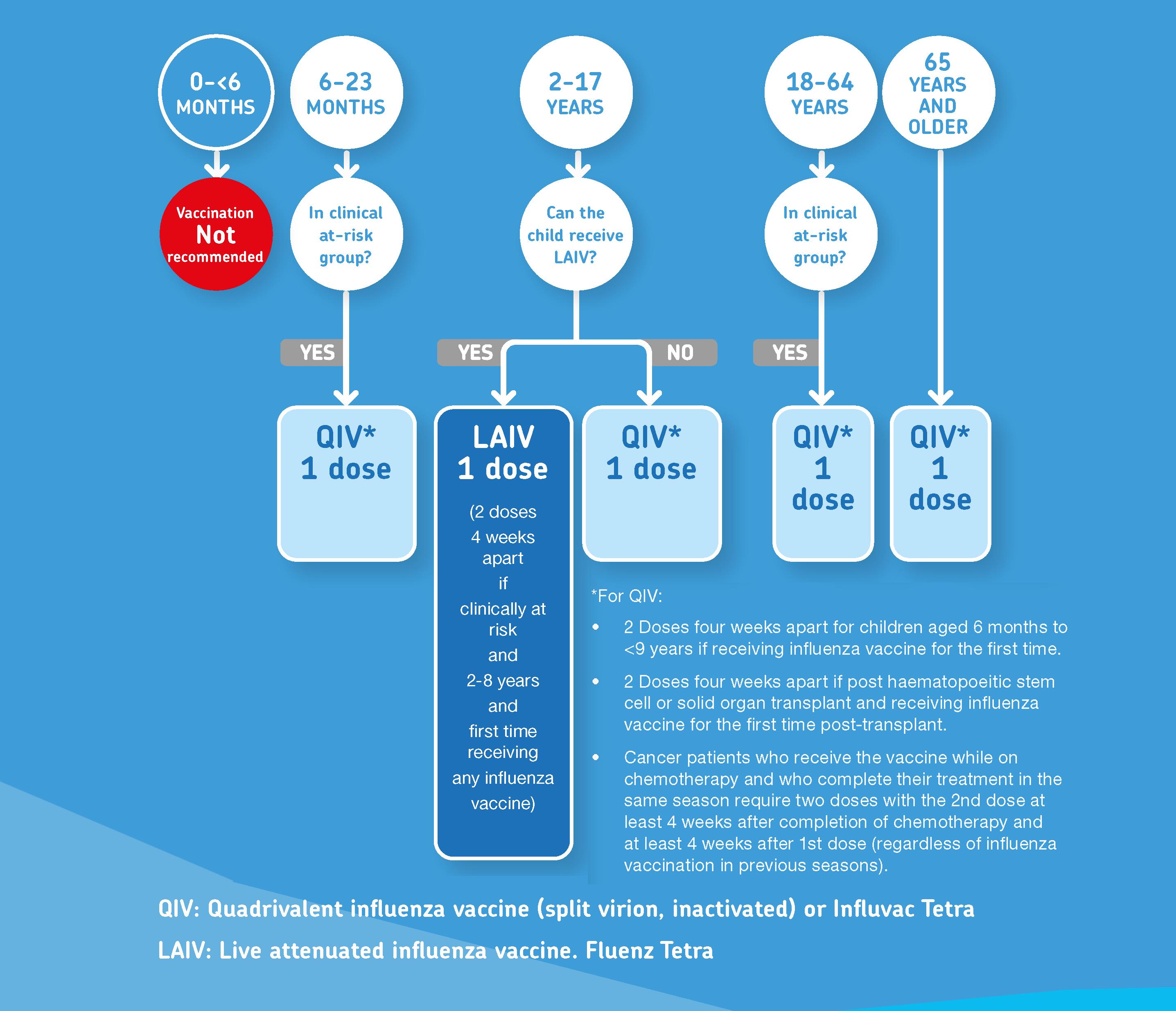
Healthcare staff are up to 10 times more likely to get influenza compared to the general population. It is estimated that one-in-five healthcare workers are infected with flu every year and many
continue to work despite being ill, which increases the risk of spread of influenza to their colleagues and patients.
Because the influenza virus is transmissible before symptoms develop, many healthcare workers transmit the disease to their highrisk patients. During hospitalisation,
patients in general are five-to-35 times more likely to acquire influenza if exposed to infected patients or healthcare workers. At-risk patients rely on healthcare workers to be vaccinated for their own protection, as some cannot generate an adequate immune response from
vaccination themselves.
Flu vaccination of healthcare workers results in an up to 40 per cent reduction in influenza-related patient deaths. Reduced rates of influenza-like illness, hospitalisation, and deaths from influenza in older adults, and a reduction in healthcare worker sick leave, have also been observed in institutions with high levels of healthcare worker immunisation in Europe.
This flu season, HSE healthcare workers can avail of their free flu vaccine from peer vaccinators in their workplace or from participating GPs
and pharmacies. It is recommended that all staff in general practice get their flu vaccine this season too.
While rates of vaccine uptake among healthcare workers in Ireland have improved in recent years, the latest uptake rates of healthcare workers in public hospitals saw a reduction from 71.0 per cent in 2020/2021, to 64.0 per cent for the 2021/2022 influenza season.
Achieving higher healthcare worker vaccination uptake is more important now than ever in preventing the spread of influenza and the double threat of flu and Covid-19 on
healthcare services. Leadership by senior medical and nursing staff has been shown to be a key factor in better uptake rates.
NIAC has advised that all influenza vaccines and other vaccines (except PCV13 for children aged 12-to-23 months) may be administered at the same time or at any interval.
The influenza vaccine and Covid-19
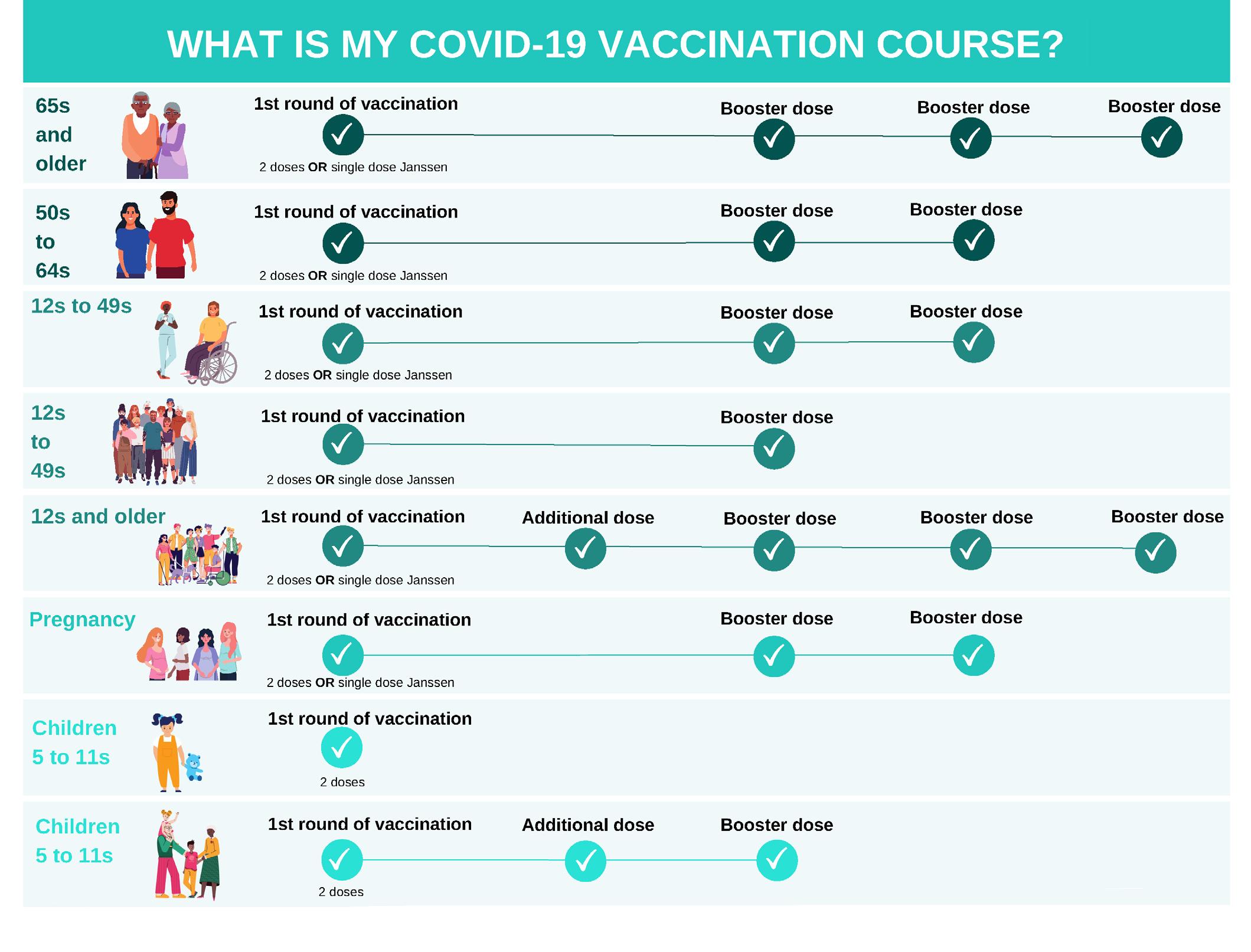
vaccines can be administered at the same time to eligible groups. If they are getting a Covid-19 booster, there should be an interval of at least four months since the last Covid-19 vaccine or Covid-19 infection (post completed primary Covid-19 vaccination course).
As it is not known if Covid-19 vaccine reactogenicity is increased with co-administration, the flu vaccine and Covid-19 vaccines should be preferably given in different limbs. It is recommended that when people get vaccinated, they are informed that there may be a slight increase in short-term mild adverse events after co-administration of the flu vaccine and a Covid-19 vaccine. These can include pain at the site of injection, fatigue, headache, and myalgia.
1. A third mRNA Covid-19 booster vaccine is recommended for: Those aged 65 years and older (fifth Covid-19 vaccine dose); and Those aged 12-to-64 years with immunocompromise associated with a suboptimal response to vaccines at the time of their primary or booster vaccination (sixth Covid-19 vaccine dose).
When practicable, these booster doses of Covid-19 vaccine and influenza vaccine can be given at one visit.
2. A second mRNA Covid-19 booster vaccine is now recommended for: Those aged 50-to-64 years (fourth Covid-19 vaccine dose); Those aged 12-to-49 years who have underlying medical conditions associated with a higher risk of severe Covid-19, ie, diabetes (fourth
a booster vaccine in the current pregnancy (fourth Covid-19 vaccine). This timing is to enhance protection to the mother and the infant.
5. A first mRNA Covid-19 booster vaccine is now recommended for those aged five-to-11 years with immunocompromise associated with a suboptimal response to vaccines at the time of their primary or additional vaccination (third Covid-19 vaccine).
It is important to follow the recommended Covid-19 vaccine journey for each patient (See Figure 2).
The pneumococcal polysaccharide vaccine, PPV23 (Pneumovax) can also be administered to eligible groups at the same time or at any interval as the influenza and Covid-19 vaccines.
As influenza and Covid-19 are caused by two different viruses, it is important for eligible groups to get both vaccines.
This winter, the HSE is encouraging those eligible — especially anyone aged 50 or older who is yet to get their Covid-19 booster, those aged 65 years and older, healthcare workers, children, people with certain longterm conditions, and anyone pregnant — to top-up their immunity by getting their Covid-19 booster and flu vaccine to make sure they are protected in the months ahead.
Using available mRNA Covid-19 vaccines, NIAC advises that the following groups are recommended for a Covid-19 booster:
Covid-19 vaccine dose); and Those aged 12-to-49 years with underlying medical conditions who are residents of long-term care facilities (fourth Covid-19 vaccine does).
3. A second mRNA Covid-19 booster vaccine is recommended for: Healthcare workers, and when practicable this booster dose can be given at the same time as the influenza vaccine (fourth Covid-19 vaccine dose).
4. To enhance maternal protection and provide optimal benefit to the infant, an additional mRNA Covid-19 booster vaccine is recommended in pregnancy at 16 weeks’ gestation or later for those who have not received
Pneumococcal disease is a serious, but vaccine-preventable disease. It is caused by the common bacteria, Streptococcus pneumonia. Like influenza, pneumococcal bacteria can spread from person-to-person by coughing, sneezing, or close contact. People can carry the pneumococcal infection in their nose and throat without being sick and can easily spread the bacteria to others. This means that pneumococcal disease can cause complications that range from mild to very severe.
When pneumococcal bacteria spread from the nose and throat to the ears or sinuses, it typically causes mild infections, such as ear and sinus infections. However, when the bacteria spreads to other parts of the body, it can cause life-threatening illnesses like pneumonia, septicaemia, and meningitis. When pneumococcal infection leads to these more serious complications, it is known as invasive pneumococcal disease (IPD).
Anyone can get pneumococcal disease, and you can get it any time of the year. However, most cases tend
WHEN PNEUMOCOCCAL BACTERIA SPREAD FROM THE NOSE AND THROAT TO THE EARS OR SINUSES, IT TYPICALLY CAUSES MILD INFECTIONS
to occur in the winter months. Older adults, the very young, and those with a weakened immune system are most at risk of severe illness and IPD.
All those aged 65 and older are at risk of pneumococcal disease and invasive infection. Other risk factors include having diabetes; a weakened immune system due to disease or treatment; Down syndrome; or certain chronic conditions.
Over the years, the pneumococcal bacterium has become resistant to many medications. This makes treating pneumococcal disease much more difficult. But it also means that prevention of pneumococcal disease by vaccination is more important
than ever. The pneumococcal polysaccharide vaccine, PPV23, offers the best protection against pneumococcal infection and IPD.
There is no change in the recommendations for pneumococcal polysaccharide vaccine, PPV23, ie, for those aged 65 years and older and those in specific at-risk groups.
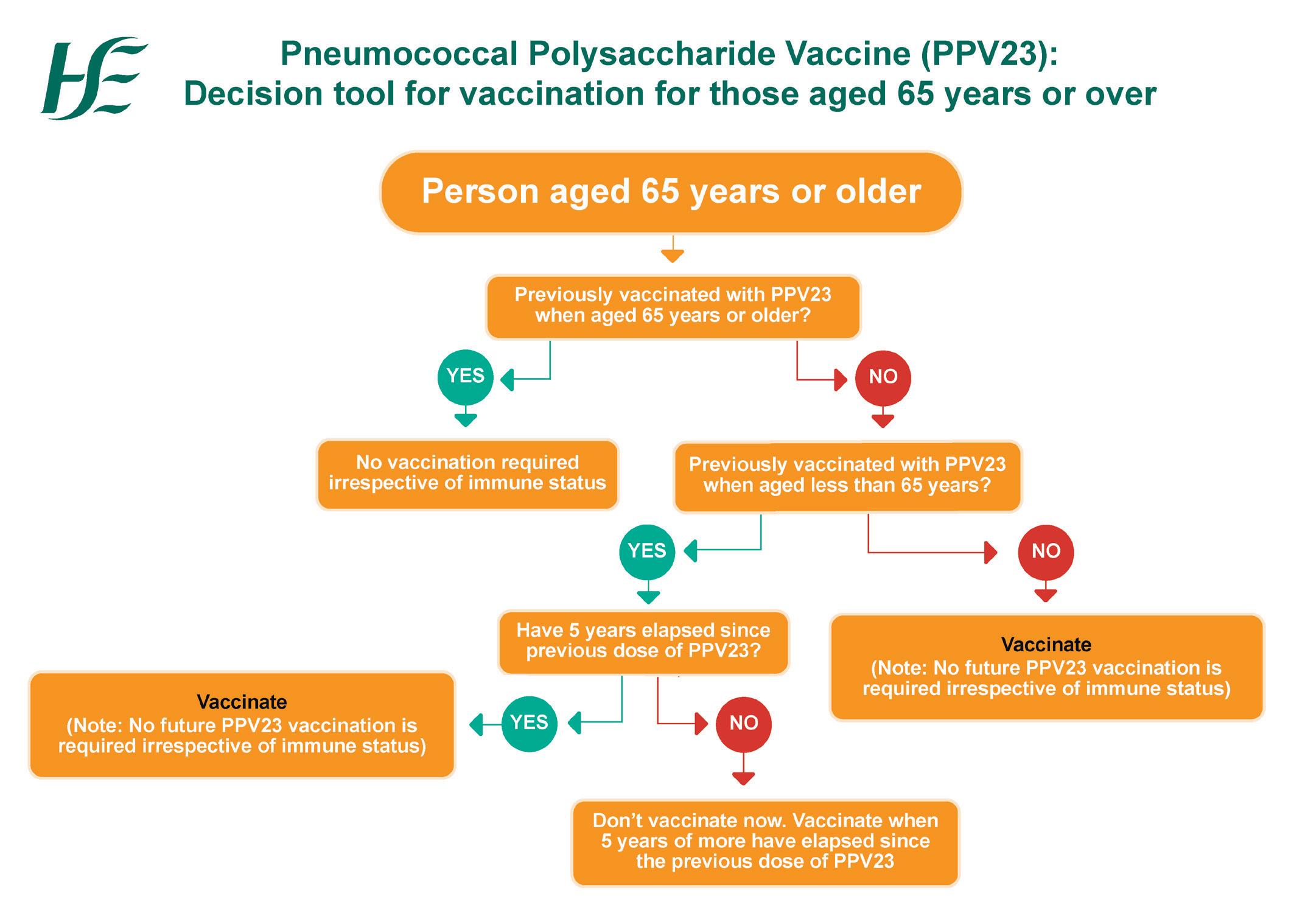
It is important to remember that patients aged 65 years and older who have never previously received PPV23 require a once-only dose of PPV23.
Administering PPV23 to those we care for who are most at-risk is the best way to protect them from pneumococcal disease.
A new HSeLanD training module
called Pneumococcal Polysaccharide Vaccine (PPV23), and algorithms (see Figures 3 and 4), have been developed on the PPV23 programme.
Public information materials about influenza vaccines, including HSE patient leaflets and posters, are available on www.hse.ie/flu. Information for healthcare workers is also available on www.immunisation.ie.
HSeLanD training programmes are for: Influenza vaccination – LAIV (two modules) and QIV (one module); Covid-19 vaccination and PPV23 are available at www.hseland.ie. l
FIGURE 4: Algorithm for PPV23 for people aged two-to-64 years old
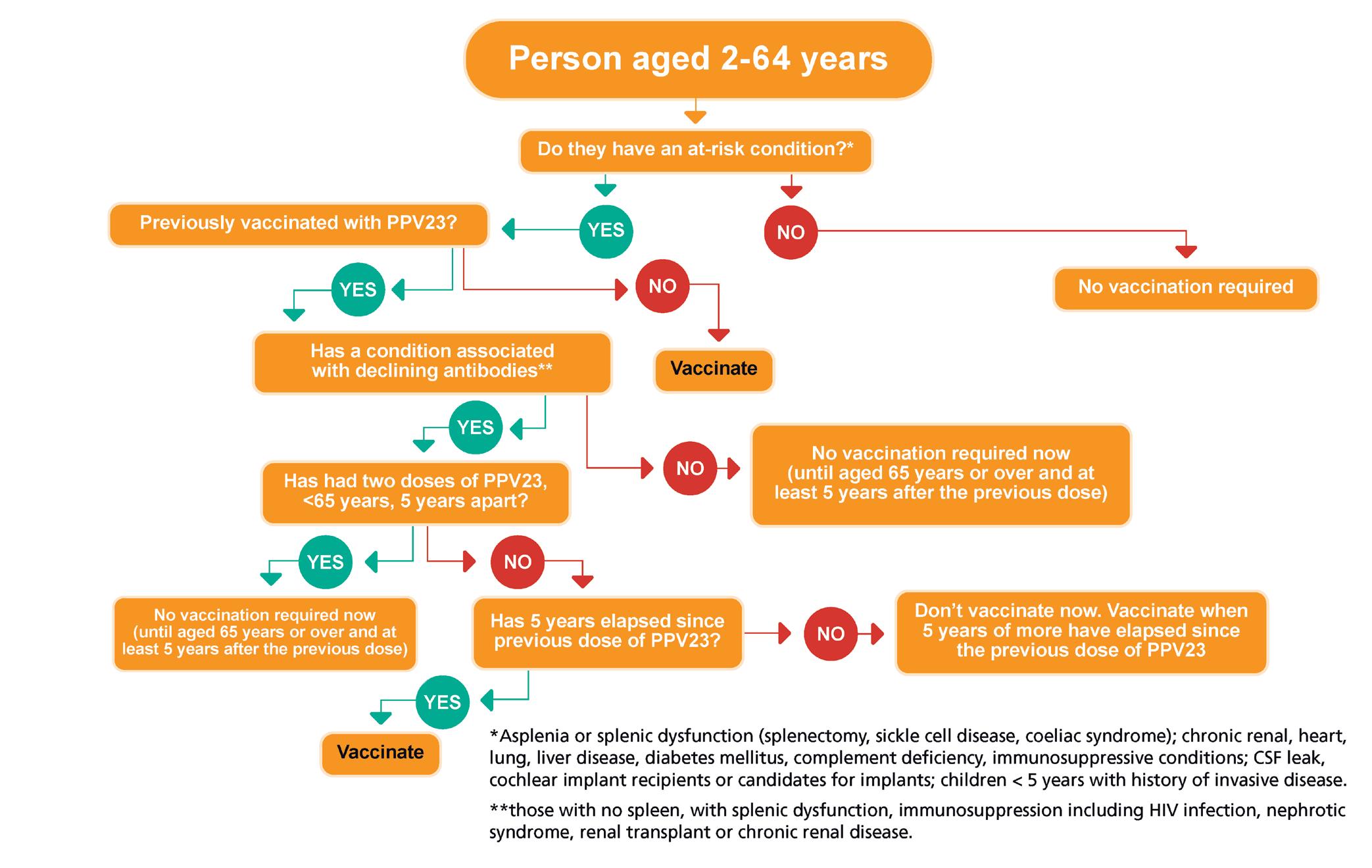
1. Antonova EN, Rycroft CE, Ambrose CS, Heikkinen T, Principi N. Burden of paediatric influenza in Western Europe: A systematic review. BMC Public Health. 2012 Nov 12;12:968
2. NACI. Canadian immunisation guide chapter on influenza and statement on seasonal influenza vaccine for 2022/2023. Avail able at: www.canada.ca/en/ public-health/services/publica tions/vaccines-immunization/ canadian-immunization-guidestatement-seasonal-influenza-vac cine-2022-2023.html
3. Finnish Institute for Health and Welfare. Vaccination programme for children and adolescents [online]. Available at: https://thl. fi/en/web/vaccination/nationalvaccination-programme/vaccina
tionprogramme-for-childrenand-adolescents
4. Health Protection Surveillance Centre (HPSC). Annual epide miological report, influenza and other seasonal respiratory viruses in ireland, 2018/2019. (December 2019). Available at: www.hpsc. ie/a-z/respiratory/influenza/season alinfluenza/surveillance/influenza surveillancereports/previousinflue nzaseasonssurveillancereports/20 182019season/Influenza%2020182019%20Season_Summary.pdf
5. HSE Seasonal Influenza Vaccination Programme 2022/2023. Available at: www.hse.ie/eng/health/i mmunisation/hcpinfo/fluinfo/
6. Caspard H, Mallory R M, Yu J, Ambrose CS. Live-attenuated influenza vaccine effectiveness in
children from 2009 to 2015-2016: A systematic review and metaanalysis. Open Forum Infect Dis 2017 Summer; 4(3): ofx111
7. National Immunisation Advi sory Committee. Immunisation Guidelines Chapter 11 – Influenza (online). (2022). Available at: www. hse.ie/eng/health/immunisation/ hcpinfo/guidelines/chapter11.pdf.
8. National Immunisation Advi sory Committee. Immunisation Guidelines Chapter 5a – Covid-19 (online). (2022). Available at: www. hse.ie/eng/health/immunisation/ hcpinfo/guidelines/covid19.pdf
9. Quadrivalent Live Attenuated Influenza (LAIV), nasal, Fluenz Tetra (AstraZeneca Pharmaceuticals (Ireland) DAC) SmPC. Available at: www.ema.europa.eu/en/ medicines/human/EPAR/fluenz-
tetra#product-information-section
10. National Health Service. The NHS National Flu Immunisation Programme 2022/2023. Available at: www.gov.uk/government/publi cations/national-flu-immunisationprogramme-plan
11. Pebody RG, Green HK, Andrews N, Zhao H, Boddington N, et al. Uptake and impact of vaccinating school age children against influenza during a season with circulation of drifted influenza A and B strains, England, 2014/15. Surveillance and outbreak report. www.eurosurveillance.org/images/ dynamic/EE/V20N39/art21256.pdf
12. World Health Organisation. Influenza update N°427. (2022). Available at: www.who.int/ publications/m/item/influenzaupdate-n-427
Monkeypox was first recognised in 1958, when two outbreaks of a pox-like disease occurred in macaque monkeys in a research facility in Copenhagen. The first human case was recorded in 1970 in the Democratic Republic of the Congo (DRC), who was initially diagnosed with smallpox, as the two diseases can resemble one another. Both viruses are orthopoxviruses. Since then, the infection has been reported in a number of central and Western African countries.
Monkeypox is endemic to tropical rainforest regions of Central and West Africa. Most cases are reported from the DRC and Nigeria.
Over the last few years, cases and the geographical extent of human monkeypox infection have been increasing. Extensive outbreaks of human monkeypox infection in Nigeria and DRC are not uncommon. Prior to 2017, Nigeria had registered no human monkeypox infection for almost 40 years. However, since 2017, an upsurge in human monkeypox disease was identified and is currently ongoing. Before 2022, cases had been reported in seven countries,
and were rarely reported outside Africa. Those cases were generally related to international travel. Travellers infected in Nigeria have been identified in Israel in 2018, in the UK in 2018, 2019, 2021, and 2022, in Singapore in 2019, and in the US in 2021.
range of African mammals, including primates, can become infected with the monkeypox virus.
On 7 May, 2022, monkeypox was identified in the UK in a person with recent travel to Nigeria. Since then, hundreds of cases have been reported in non-endemic countries without a history of travel to Africa.
On 23 July 2022, WHO declared the global monkeypox outbreak a public health emergency of international concern, with the vast majority of cases in the European region. The cases in Ireland are part of an ongoing multi-country outbreak of monkeypox in Europe, the Americas and many other regions worldwide.

In 2003, monkeypox was recorded in the US when an outbreak occurred in humans and pet prairie dogs following importation of rodents from Africa. The human infections followed contact with an infected pet and all patients recovered.
Monkeypox virus has been isolated from a number of animal hosts in sub-Saharan Africa, including small mammals such as rodents. A wide
There are two clades (strains) of monkeypox, the Central African and West African strains. A recent systematic review reported a case fatality rate of 10.6 per cent for the Central African strain and 3.6 per cent for the West African strain in a Nigerian population. However, most of this data is from countries where access to healthcare might be variable. The West African lineage is generally associated with milder
THERE ARE TWO CLADES (STRAINS) OF MONKEYPOX, THE CENTRAL AFRICAN AND WEST AFRICAN STRAINS
disease and is responsible for the 2022 outbreak.
As of 2 November 2022, there were 77,573 cases of monkeypox reported from 109 countries and regions to the European Centre for Disease Control (ECDC) and the WHO Regional Office for Europe. Nine-hundred and fiftythree of these cases were in seven countries that historically report monkeypox. Thirty deaths from monkey pox have been reported, four in Europe. No case linked to occupational exposure has been reported to date.
In Ireland, as of 2 November 2022, there were 208 cases reported, 206 were male and two were female, with a median age of 36 years. Eighteen
people have been hospitalised. Eleven cases were admitted for clinical care, two were admitted for isolation purposes, and the reason for admission for the remaining five remains unknown. Currently, data shows that gay, bisexual and other men who have sex with men (gbMSM) make up the majority of cases in this current monkeypox outbreak. However, anyone who has been in close personal contact with someone that has monkeypox is at risk. Sexual orientation is known for 186 people affected, 185 of whom self-identified as gbMSM. The epidemiological picture to date in Ireland is similar to that seen in other countries, whereby cases are
primarily in gbMSM populations.
In Africa, the majority of human monkeypox infection cases have traditionally been infected through direct contact with African wild mammals and consumption of bushmeat. In this current outbreak, human-to-human transmission of monkeypox virus is evident, through direct contact with lesion exudate, crust material, lesion scabs, body fluids or contaminated materials like bedding, clothing, and surfaces. The virus enters the body through broken skin, the respiratory tract or mucous membranes (eyes, nose, mouth, anus or vagina). It usually takes close physical contact with a symptomatic individual for transmission to occur. The incubation period is generally six-to-13 days, but can range between five-to-21 days.
Initial symptoms include sudden onset of fever (38.5-40.5 o C), severe headache, profound weakness, back pain, myalgia, and generalised lymph node enlargement involving posterior auricular, cervical and axillary nodes. In more severe disease, the inguinal nodes may also be enlarged.
The rash appears within one-to-10 days of development of fever, usually within one-to-three days, beginning on the face and then spreading to other parts of the body. The lesions are initially maculopapular, pruritic and about 2-5mm in diameter. They then become vesicular, then pustular and painful, before ulcerating, crusting and scabbing. The lesions seen in monkeypox are similar to those of varicella, however, unlike varicella, the lesions in monkeypox infection evolve at the same time and
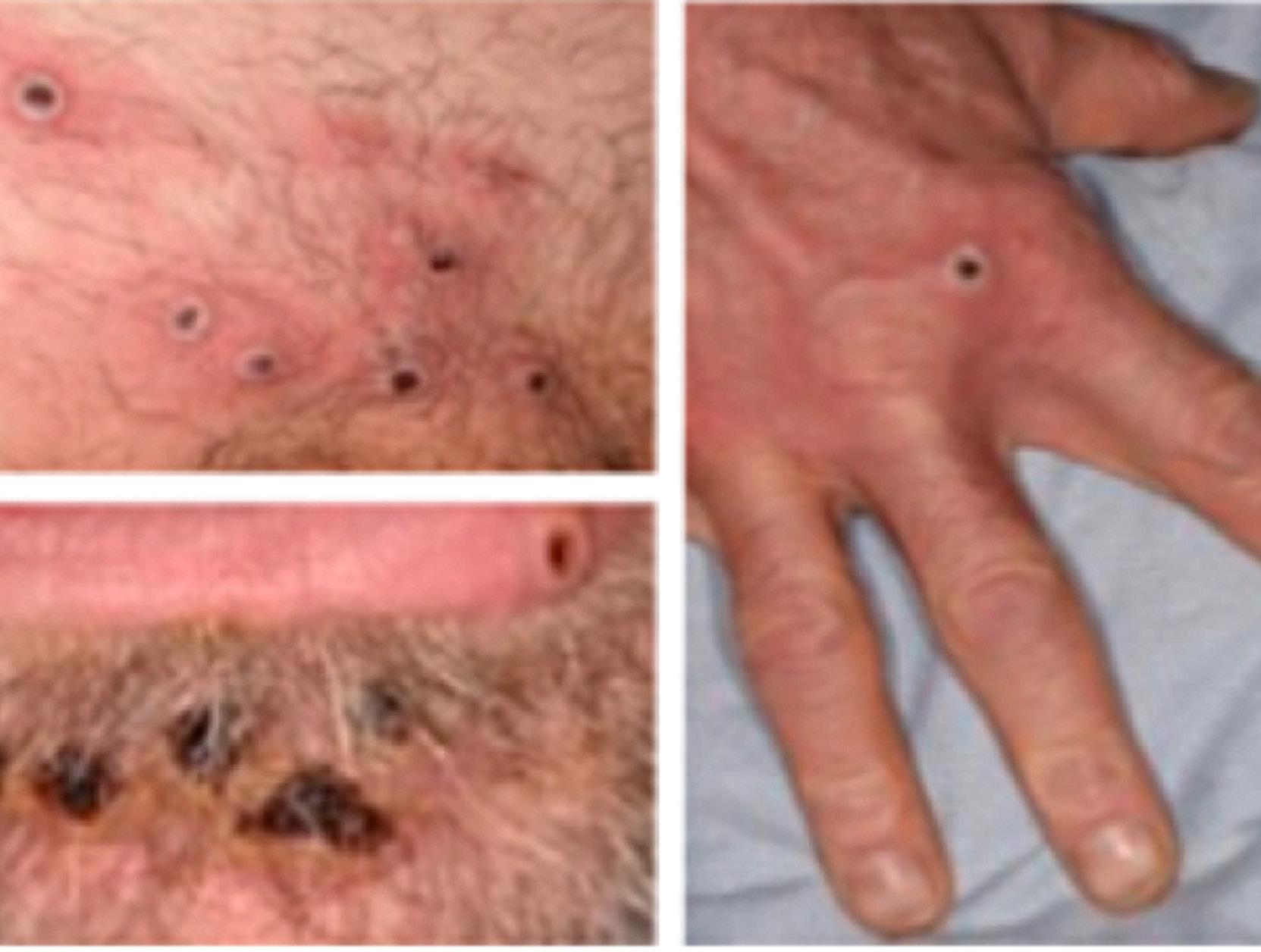
THE RASH APPEARS WITHIN ONE-TO-10 DAYS OF DEVELOPMENT OF FEVER, USUALLY WITHIN ONE-TO-THREE DAYSFIGURE 1: Monkeypox skin lesions
do become pustular.
The rash was typically only seen on the face, palms of the hands, soles of the feet, and occasionally in the mouth. However, during the upsurge of human monkeypox infection cases seen in Nigeria since 2017, more than two-thirds of cases developed genital infections, indicating the likelihood that monkeypox virus could be transmitted through close skin-to-skin contact during sexual intercourse. In the current outbreak, genital lesions are also being seen. The entire cycle, from initial presentation until lesion desloughing and skin healing, takes between two and four weeks, depending on the severity of the disease. This evolution is shown in Figure 1
Monkeypox infection is usually a self-limiting illness and symptoms generally last for two-to-four weeks. The disease is more severe in young children, pregnant women, older persons, and those who are severely immunocompromised, especially if this is related to HIV. Secondary bacterial skin infection is not uncommon in monkeypox infection and requires early and aggressive intervention.
The HSE has established a Monkeypox Crisis Management Team (CMT) to co-ordinate the multi-agency, multidisciplinary response to the outbreak in Ireland. The HSE also continues to actively monitor the evolving international situation. To assist in Ireland’s response, monkeypox has been made a notifiable disease. Any patient who presents with symptoms of monkeypox, or any close contacts of confirmed monkeypox cases, should be referred to the local Department of Public Health for follow-up.
There is no specific treatment for
human monkeypox infection. The monkeypox vaccine distributed in the EU contains Smallpox Modified Vaccinia Ankara – Bavarian Nordic (MVA-BN). Imvanex, a thirdgeneration smallpox vaccine, is authorised by the European Medicines Agency (EMA) for the prevention of smallpox, monkeypox, and disease caused by vaccinia virus in adults. It is marketed as Jynneos in the US, where it is authorised by the Food and Drug Administration (FDA) for the prevention of monkeypox. During the current monkeypox outbreak, both Jynneos and Imvanex are being used across the EU in line with EMA recommendations. The vaccine contains a non-replicating form of vaccinia virus that does not cause disease in humans, as it cannot replicate in human cells.
People who are considered part of
a high-risk group can now book a pre-exposure vaccine through this HSE website: www2.hse.ie/ conditions/monkeypox/vaccine/#. They will receive the vaccine in a sexual health clinic or at a vaccination centre. Two doses of the vaccine are given intradermally or subcutaneously in the forearm, 28 days apart, as per the National Immunisation Advisory Committee immunisation guidelines. The vaccine is effective 14 days after the second dose. Vaccines may develop adverse reactions, similar to the prodromal symptoms of monkeypox infection, during the first 48 hours after vaccination. There may be erythema, induration or itching at the intradermal vaccine site, or mild injection site skin discoloration lasting six or more months. Any reported adverse events should be notified to the Health Products Regulatory Authority (HPRA). l

National Immunisation Advisory Committee immunisation guidelines, Monkeypox/smallpox (variola) chapter. Available at: www.hse. ie/eng/health/immunisation/ hcpinfo/guidelines/ch13a.pdf
Monkeypox in Ireland - latest
update (HPSC). Available at: www.hpsc.ie/a-z/ zoonotic/monkeypox/ title-22106-en.html
CDC 2022 Outbreak Cases and Data. Available at: www. cdc.gov/poxvirus/monkeypox/ response/2022/index.html.
Information for the public is available at: www2.hse.ie/ conditions/monkeypox/

Prescribing Information: Toujeo (insulin glargine 300 units/ml)
®
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Presentation: Toujeo SoloStar and DoubleStar pre-filled pens. Each ml contains 300 units of insulin glargine. SoloStar pen contains 1.5ml (450 units) of solution for injection. DoubleStar pen contains 3ml (900 units) of solution for injection. Indication: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. Dosage and Administration: Toujeo is administered subcutaneously, by injection into the abdominal wall, the deltoid or the thigh, once daily, at any time of the day, preferably at the same time every day. Injection sites must be rotated within a given injection area from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis. The dose regimen (dose and timing) should be adjusted according to individual response. Do not administer intravenously. In type 1 diabetes mellitus, Toujeo must be combined with short-/rapid-acting insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, recommended daily starting dose is 0.2 units/kg followed by individual dose adjustments. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch between insulin glargine 100 units/ ml and Toujeo: Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not directly interchangeable. When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit to unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels. When switching from Toujeo to insulin glargine 100 units/ml, the dose should be reduced (approximately by 20%). Switching from other basal insulins to Toujeo: A change of dose and/or timing of the basal insulin and concomitant anti hyperglycaemic treatment may be required. Dose adjustments may also be required if the patient’s weight or lifestyle changes, the timing of insulin dose is changed or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia. Toujeo must not be mixed or diluted with any other insulin or other medicinal products. Close metabolic monitoring is recommended during a switch and in the initial weeks thereafter. SoloStar 1-80 units per single injection in steps of 1 unit and DoubleStar 2-160 units in steps of 2 units. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number then the dose must be increased or decreased by 1 unit. Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Special Populations: Insulin requirements may be diminished in the elderly or patients with renal or hepatic impairment. Paediatric: When switching basal insulin to Toujeo, dose reduction of basal and bolus insulin needs to be considered on an individual basis, in order to minimise the risk of hypoglycaemia. The safety and efficacy of Toujeo in children and adolescents below 6 years of age have not been established. Contraindications: Hypersensitivity to insulin glargine or any excipients. Precautions and Warnings: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Toujeo is not the insulin of choice for treatment of diabetic ketoacidosis. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing lipodystrophy and cutaneous amyloidosis. There is a potential risk of delayed insulin absorption and worsened glycaemic control following insulin injections at sites with these reactions. A sudden change in the injection site to an
References: 1. Toujeo® Summary of Product Characteristics
Date of preparation: September 2020 | MAT-IE-2001062 (v1.0)
unaffected area has been reported to result in hypoglycaemia. Blood glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar pre-filled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There is no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Legal Category: POM. Marketing Authorisation Number: SoloStar 3 Pen pack: EU/1/00/133/034, DoubleStar EU/1/00/133/038. Marketing Authorisation Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2020.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
AUTHOR: Priscilla LynchType 1 diabetes is a chronic autoimmune disease that arises following the destruction of insulinproducing beta cells in the pancreas. As a result, people with type 1 diabetes require insulin therapy to adequately regulate blood glucose levels. In the short-term, people with type 1 diabetes may face significant challenges to daily living, such as hyperglycaemia, hypoglycaemia, and ketoacidosis, while long-term complications can occur in the form of both microvascular complications, such as diabetic retinopathy and neuropathy, and macrovascular complications, such as stroke and coronary artery disease.
The additional strain placed on healthcare resources when diabetes patients are hospitalised illustrates that diabetes-related complications impose not only a significant burden on patients and the healthcare system, but can also have a substantial societal impact due to productivity losses (such as days off work because of illness).
This year the UK’s National Institute for Health and Care Excellence (NICE) has updated a number of its diabetesrelated clinical guidelines, including its one on type 1 diabetes care for adults (Type 1 diabetes in adults:
Diagnosis and management (NG17)).
This guideline covers care and treatment for adults (aged 18 and over) with type 1 diabetes and includes advice on diagnosis, education and support, blood glucose management, cardiovascular risk, and identifying and managing long-term complications. It is presented here in an abridged format, highlighting the key elements.
In August 2022, NICE also amended its recommendations on blood pressure targets in people with diabetes to make them consistent with its recommendations on blood pressure control in its guidelines on chronic kidney disease and hypertension.
1.1.1 Make an initial diagnosis of type 1 diabetes on clinical grounds in adults presenting with hyperglycaemia. Bear in mind that people with type 1 diabetes typically (but not always) have one or more of:
Ketosis; Rapid weight loss; Age of onset under 50 years; Body mass index (BMI) below 25kg/m2; Personal and/or family history of autoimmune disease. [2015, amended 2022]
1.1.2 Do not use age or BMI alone to exclude or diagnose type 1 diabetes in adults. [2022]
1.1.3 Take into consideration the possibility of other diabetes subtypes and revisit the diagnosis at subsequent clinical reviews. Carry out further investigations if there is uncertainty (see recommendations 1.1.7 and 1.1.8). [2022]
1.1.4 Measure diabetes-specific autoantibodies in adults with an initial diagnosis of type 1 diabetes, taking into account that:
The false negative rate of diabetesspecific autoantibody tests is lowest at the time of diagnosis.
The false negative rate can be reduced by carrying out quantitative tests for two different diabetesspecific autoantibodies (with at least one being positive). [2022]
1.1.5 Do not routinely measure serum C peptide to confirm type 1 diabetes in adults. [2022]
1.1.6 In people with a negative diabetes-specific autoantibody result, and if diabetes classification remains uncertain, consider measuring nonfasting serum C peptide (with a paired blood glucose). [2022]

1.1.7 At subsequent clinical reviews, consider using serum C peptide to revisit the diabetes classification if there is doubt that type 1 diabetes is the correct diagnosis. [2022]
1.1.8 Take into account that the discriminative value of serum C peptide to diagnose type 1 diabetes increases the longer the test is done after initial diagnosis of diabetes. [2022]
1.1.9 For people aged 60 and over presenting with weight loss and new-onset diabetes, follow recommendations on assessing for pancreatic cancer in the section on pancreatic cancer in the NICE guideline on suspected cancer: Recognition and referral. [2022]
1.1.10 At diagnosis (or, if necessary, after managing critically decompensated metabolism), the diabetes professional team should work with adults with type 1 diabetes to develop a plan for their early care. This will generally require: Medical assessment to:
• Ensure the diagnosis is accurate (see recommendations 1.1.1 to 1.1.5);
• Ensure appropriate acute care is given when needed;
• Review medicines and detect potentially associated disease;
• Detect adverse vascular risk factors.
Environmental assessment to understand:
• The social, home, work, and recreational circumstances of the person and their carers;
• Their lifestyle (including diet and physical activity);
• Other relevant factors, such as substance use.
Cultural and educational assessment to:
• Find out what they know about diabetes;
• Help with tailoring advice, and with planning treatments, and diabetes education programmes. Assessment of their emotional wellbeing to decide how to pace diabetes education. [2004]
1.1.11 Use the results of the initial diabetes assessment to agree a future care plan. This assessment should include: Acute medical history; Social, cultural, and educational history, and lifestyle review; Complications history and symptoms; Diabetes history (recent and long-term); Other medical history; Family history of diabetes and cardiovascular disease; Medication history; Vascular risk factors; Smoking; General examination; Weight and BMI; Foot, eye, and vision examination; Urine albumin:creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR); Psychological wellbeing; Attitudes to medicine and self-care; Immediate family and social relationships, and availability of informal support. [2004, amended 2021]
1.1.12 Include the following in an individualised and culturally appropriate diabetes plan: When and where they will have their diabetes education, including their dietary advice (see the sections on education and information and dietary management).
Initial treatment, including guidance on insulin injection and insulin regimens (see the sections on insulin therapy and insulin delivery in the full guideline).
Self-monitoring and targets (see the section on blood glucose management). Symptoms, and the risk of
hypoglycaemia and how it is treated. Management of special situations, such as driving.
Communicating with the diabetes professional team (how often and how to contact them).
Management of cardiovascular risk factors (see the section on control of cardiovascular risk).
Implications for pregnancy and family planning advice (see NICE’s guideline on diabetes in pregnancy).
How often they will have follow-up appointments, and what these will cover (including review of HbA1c levels, experience of hypoglycaemia, and annual reviews). [2004, amended 2015]
1.1.13 After the initial plan is agreed, implement it without inappropriate delay. Based on discussion with the adult with type 1 diabetes, modify the plan as needed over the following weeks. [2004]
1.3.1 Offer all adults with type 1 diabetes a structured education programme of proven benefit, for example, the DAFNE (dose adjustment for normal eating) programme. [2015] Full details of this section are available at: www.nice.org.uk/guidance/ng17/.
1.4.1 Offer carbohydrate counting training to adults with type 1 diabetes as part of structured education programmes for self-management (see the section on education and information). [2015]
1.4.2 Consider carbohydrate counting courses for adults with type 1 diabetes who are waiting for a more detailed structured education programme or who are unable to take part in a standalone structured education programme. [2015]
1.4.3 Do not advise adults with type 1 diabetes to follow a low glycaemic index diet for blood glucose control. [2015]
1.4.4 Offer dietary advice to adults with type 1 diabetes about issues other than blood glucose control (such as managing weight and cardiovascular risk), as needed. [2015]
consultations. Follow the principles on communication in NICE’s guideline on patient experience in adult NHS services. [2015]
1.6.5 If HbA1c monitoring is invalid because of disturbed erythrocyte turnover or abnormal haemoglobin type, estimate trends in blood glucose control using one of the following:
Fructosamine estimation.
1.6.9 Diabetes services should document the proportion of adults with type 1 diabetes who reach an HbA1c level of 53mmol/mol (7 per cent) or lower. [2015]
1.6.22 Advise adults with type 1 diabetes to aim for: A fasting plasma glucose level of 5-to-7mmol/litre on waking; and A plasma glucose level of 4-to7mmol/litre before meals at other times of the day. [2015]
CONSIDER CARBOHYDRATE COUNTING COURSES FOR ADULTS WITH TYPE 1 DIABETES WHO ARE WAITING FOR A MORE DETAILED STRUCTURED EDUCATION PROGRAMME
1.6.1 Measure HbA1c levels every three to six months in adults with type 1 diabetes. [2015]
1.6.2 Consider measuring HbA1c levels more often in adults with type 1 diabetes if their blood glucose control is suspected to be changing rapidly; for example, if their HbA1c level has risen unexpectedly above a previously sustained target. [2015]
1.6.3 Measure HbA1c using methods calibrated according to International Federation of Clinical Chemistry (IFCC) standardisation. [2015]
1.6.4 Tell adults with type 1 diabetes their HbA1c results after each measurement and have their most recent result available at
Quality-controlled blood glucose profiles.
Total glycated haemoglobin estimation (if abnormal haemoglobins). [2015]
1.6.6 Support adults with type 1 diabetes to aim for a target HbA1c level of 48mmol/mol (6.5 per cent) or lower, to minimise the risk of longterm vascular complications. [2015]
1.6.7 Agree an individualised HbA1c target with each adult with type 1 diabetes. Take into account factors, such as their daily activities, aspirations, likelihood of complications, comorbidities, occupation, and history of hypoglycaemia. [2015]
1.6.8 Ensure that aiming for an HbA1c target is not accompanied by problematic hypoglycaemia in adults with type 1 diabetes. [2015]
1.6.23 Advise adults with type 1 diabetes who choose to measure after meals to aim for a plasma glucose level of 5-to-9mmol/litre at least 90 minutes after eating. (This timing may be different in pregnancy – for guidance on plasma glucose targets in pregnancy, see NICE’s guideline on diabetes in pregnancy.) [2015]
1.6.24 Agree bedtime target plasma glucose levels with each adult with type 1 diabetes. Take into account the timing of their last meal of the day and the related insulin dose, and ensure the target is consistent with the recommended fasting level on waking (see recommendation 1.6.22). [2015]
1.10.10 Explain to adults with type 1 diabetes that a fast acting form of glucose is needed for managing hypoglycaemic symptoms or signs in people who can swallow. [2004, amended 2015]
1.10.11 Adults with type 1 diabetes who have a decreased level of consciousness because of hypoglycaemia and so cannot safely take oral treatment should be: Given intramuscular glucagon by a family member or friend who has
been shown how to use it (intravenous glucose may be used by healthcare professionals skilled in getting intravenous access).
Checked for response at 10 minutes, and then given intravenous glucose if their level of consciousness is not improving significantly.
Then given oral carbohydrate when it is safe to administer it, and put under continued observation by someone who has been warned about the risk of relapse. [2004, amended 2015]
1.10.12 Explain to adults with type 1 diabetes that:
It is very common to experience some hypoglycaemic episodes with any insulin regimen.
They should use a regimen that avoids or reduces the frequency of hypoglycaemic episodes, while maintaining the most optimal blood glucose control possible. [2004]
1.10.13 Make hypoglycaemia advice available to all adults with type 1 diabetes, to help them find the best possible balance with any insulin regimen. (See the sections on insulin therapy and insulin delivery.) [2004]
1.10.14 If hypoglycaemia becomes unusually problematic or increases in frequency, review the following possible causes:
Previous physical activity.
Lack of appropriate knowledge and skills for self-management. [2004]
1.10.15 Manage nocturnal hypoglycaemia (symptomatic or detected on monitoring) by:
Reviewing knowledge and selfmanagement skills.
Reviewing current insulin regimen, evening eating habits, and previous physical activity.
Choosing an insulin type and regimen that is less likely to cause low glucose levels at night. [2004, amended 2015]
1.10.16 If early cognitive decline occurs in adults on long-term insulin therapy, then in addition to normal investigations consider possible brain damage from overt or covert hypoglycaemia, and the need to manage this. [2004]
Ketone self-monitoring to prevent diabetic ketoacidosis.
1.11.1 Consider ketone monitoring (blood or urine) as part of ‘sick day rules’ for adults with type 1 diabetes, to help with self-management of hyperglycaemia. [2015]
density lipoprotein [HDL] and lowdensity lipoprotein [LDL] cholesterol, and triglycerides); Age; Family history of cardiovascular disease; Abdominal adiposity. [2004, amended 2015 and 2021]
1.13.3 For guidance on tools for assessing risk of cardiovascular disease in adults with type 1 diabetes, see the recommendations on full formal risk-assessment in NICE’s guideline on lipid modification. [2015]
1.13.4 For guidance on the primary prevention of cardiovascular disease in adults with type 1 diabetes, see the section on primary prevention for people with type 1 diabetes in NICE’s guideline on lipid modification. [2015]
Inappropriate insulin regimens (incorrect dose distributions and insulin types).
Meal and activity patterns, including alcohol.
Injection technique and skills, including insulin resuspension if necessary.
Injection site problems.
Possible organic causes, including gastroparesis.
Changes in insulin sensitivity (including drugs affecting the renin–angiotensin system and renal failure).
Mental health problems.
1.13.1 Do not offer aspirin for the primary prevention of cardiovascular disease in adults with type 1 diabetes. [2015]
1.13.2 Assess cardiovascular risk factors annually, including: eGFR and urine ACR; Smoking; Blood glucose control; Blood pressure; Full lipid profile (including high-
1.13.5 Give adults with type 1 diabetes who smoke advice on stopping smoking and stop smoking services, including NICE guidance recommended therapies (see the NICE topic page on smoking and tobacco). Reinforce these messages annually for people who currently do not plan to stop smoking, and at all clinical contacts if there is a prospect of the person stopping. [2004]
1.13.6 Advise adults who do not smoke never to start smoking. [2004, amended 2021]
1.13.7 Provide intensive management for adults who have had myocardial infarction or stroke, according to relevant non diabetes guidelines. For angina or other ischaemic heart disease, beta-blockers should be considered (for insulin use in these circumstances, see the section on caring for adults with type 1
diabetes in hospital). For guidance on secondary prevention of myocardial infarction, see NICE’s guideline on acute coronary syndromes. [2004, amended 2015]
1.13.8 In adults with type 1 diabetes aim for blood pressure targets as follows:
For adults with a urine ACR less than 70mg/mmol, aim for a clinic systolic blood pressure less than 140mmHg (target range 120-to139mmHg) and a clinic diastolic blood pressure less than 90mmHg.
1.13.10 Start a trial of a reninangiotensin system blocking drug as first-line therapy for hypertension in adults with type 1 diabetes. [2004, amended 2015]
are indicated; Low-dose thiazides may be combined with beta blockers; When prescribing calcium channel antagonists, only use long-acting preparations;
For adults with an ACR of 70mg/ mmol or more, aim for a clinic systolic blood pressure less than 130mmHg (target range 120-to129mmHg) and a clinic diastolic blood pressure less than 80mmHg.
1.13.11 Provide information to adults with type 1 diabetes on how lifestyle changes can improve their blood pressure control and associated outcomes, and offer help to achieve their aims in this area. [2004]
Ask adults directly about potential side-effects of erectile dysfunction, lethargy and orthostatic hypotension with different drug classes. [2004, amended 2015]
In adults aged 80 or more, whatever the ACR, aim for a clinic systolic blood pressure less than 150mmHg (target range 140-to149mmHg) and a clinic diastolic blood pressure less than 90mmHg.

Use clinical judgement for adults with frailty, target organ damage (damage to organs because of diabetes, for example, to nerves or eyes) or multimorbidity. See the recommendations on pharmacotherapy in NICE’s guideline on chronic kidney disease, and NICE’s guidelines on hypertension in adults and multimorbidity. [2004, amended 2022]
1.13.9 Discuss the following with adults with type 1 diabetes who have hypertension to help them make an informed choice:
Reasons for the choice of intervention level;
The substantial potential gains from small improvements in blood pressure control;
Any possible negative consequences of therapy. [2004, amended 2015]
1.13.12 Do not allow concerns over potential side-effects to inhibit advising and offering the necessary use of any class of drugs, unless side-effects become symptomatic or otherwise clinically significant. In particular: Do not avoid selective betablockers for adults on insulin if these
1.13.13 This recommendation has been removed as the previous link to NICE’s guideline on chronic kidney disease no longer provides relevant information.
Full Type 1 diabetes in adults: Diagnosis and management (NG17) guideline is available at: www.nice.org.uk/guidance/ng17/. l
There are a number of national guidelines for diabetes-related care published by the HSE’s National Clinical Programme for Diabetes, while the ICGP also published integrated care guidelines for the management of type 2 diabetes in general practice in 2016, which were developed in conjunction with the National Clinical Programme for Diabetes Working Group.
In relation to type 1 diabetes specifically, in 2018 the adult type
1 diabetes mellitus guideline was published ( Adult type 1 diabetes mellitus: National Clinical Guideline No. 17 ), which can be accessed here: www.hse.ie/eng/about/who/ cspd/ncps/diabetes/resources/ adult-type-1-diabetes-mellitus.pdf The document was put together by a Guideline Development Group, supported by the HSE National Clinical Programme for Diabetes, and developed through contextualisation of NICE’s 2015 Type 1 diabetes in adults: Diagnosis and management (NG17) guideline document.
Accord Healthcare Ireland have announced the launch of Sondelbay to the Irish market. Sondelbay is a biosimilar, subcutaneous injection pen that is self-administered once daily to optimise bone health. It is indicated for the treatment of osteoporosis in postmenopausal women and for men at an increased risk of fracture. In postmenopausal women, the product has displayed a notable reduction in the incidence of vertebral and non-vertebral fractures, however, no significant reduction in hip fractures has yet been demonstrated. It is also
recommended in the treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in
women and men.
Tracy Kivlehan, Head of Hospitals and Speciality Brands at Accord Healthcare said: “We are proud to launch Sondelbay in Ireland as an addition to our emerging Bone Health franchise. We know the problems patients with osteoporosis face, such as weak hands, poor grip, and diminished dexterity. That’s why we are delighted to bring our product to the market. We have taken patient and healthcare professional concerns into consideration when creating our device and this has influenced the design.”
SMA PRO Follow-on Milk has received a Gold Award at the ninth annual National Parenting Product Awards (NPPAs) at a ceremony hosted by Ireland’s specialist parenting publisher and content agency, Zahra. The NPPAs are Ireland’s first and only independently-verified consumer awards for products and services designed for young families. SMA PRO Follow-on Milk’s win in the highly competitive ‘Best Follow-on Formula’ category is in recognition of the brand's robust commitment to research and development in achieving the highest standards in baby nutritional products.

Both Nursing in General Practice and SMA Nutrition acknowledge the superiority of breastmilk over formula and advocate that breastfeeding continue for as long as possible. In

the event that breastfeeding is not possible, optimal infant nutrition is vital, and the company has been pioneering research for over 100 years, including over 70 years of breast milk research. SMA PRO Follow-on Milk is tailored for babies from six months as part of a varied weaning diet. It is enriched with iron and also contains 2'Fucosyllactose (2’FL), the most abundant
oligosaccharide found in breast milk and the brands latest breakthrough in baby nutrition.
Catherine O’Connor, Senior Brand Manager at Nestlé Nutrition Ireland said: We are delighted that SMA PRO Follow-on Milk has received gold in the ‘Best Follow-on Formula’ category at this year’s National Product Awards. This win in a highly competitive category and in a year of recordhigh entries to the awards overall is testament to our commitment to pioneering research and excellence in baby nutrition. This award is particularly special for us as a team, given that it has been voted for by parents, which shows that SMA PRO Follow-on Milk is not only highlyregarded within the industry, but is also a trusted product among our most important critics – Irish parents and their babies.”







Colours are important, they can signal what’s going on.



it is easy to see if you are below, within or above your range.
you on, straight
the
are trademarks of Google LLC.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG. Inc, and any use of such marks by LifeScan IP Holdings, LLC is under license.
Other trademarks and trade names are those of their respective owners.
© 2021-2022 LifeScan IP Holdings, LLC. GL-VRF-2100078 (22-004)

Very common side effects include decreased appetite, nasal congestion/rhinorrhoea, malaise.1
The blue spray is for illustration purposes only. Fluenz Tetra is colourless to pale yellow, clear to opalescent. Small white particles may be present.1
1,2
Prophylaxis of influenza in children and adolescents from 24 months to less than 18 years of age1
For full prescribing information including contraindications for use, please consult the SmPC1 and the HSE Immunisation Guidelines3 prior to administration.
FLUENZ® TETRA nasal spray suspension Influenza vaccine (live attenuated, nasal)
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: Prophylaxis of influenza in children and adolescents from 24 months to less than 18 years of age. The use of Fluenz Tetra should be based on official recommendations.

Presentation: Nasal spray, suspension.
Reassortant influenza virus* (live attenuated) of the following four strains**: A/Victoria/2570/2019 (H1N1)pdm09 - like strain (A/Victoria/1/2020, MEDI 340505) 107.0±0.5 FFU***
A/Darwin/9/2021 (H3N2) - like strain (A/Norway/16606/2021, MEDI 355293) 107.0±0.5 FFU***
B/Austria/1359417/2021 - like strain (B/Austria/1359417/2021, MEDI 355292) 107.0±0.5 FFU***
B/Phuket/3073/2013 - like strain (B/Phuket/3073/2013, MEDI 306444) 107.0±0.5 FFU*** per 0.2 ml dose
* propagated in fertilised hens’ eggs from healthy chicken flocks.
** produced in VERO cells by reverse genetic technology. This product contains genetically modified organisms (GMOs).
*** fluorescent focus units.
Dosage and administration: 0.2ml (administered as 0.1ml per nostril). Children not previously vaccinated against seasonal influenza should be given a second dose after an interval of at least 4 weeks. Should not be used in individuals below 24 months of age because of safety concerns regarding increased rates of hospitalisation and wheezing in this population. Method of administration: Nasal administration only. Do not inject Fluenz Tetra. Fluenz Tetra is administered as a divided dose in both nostrils. After administering half of the dose in one nostril, administer the other half of the dose in the other nostril immediately or shortly thereafter. The patient can breathe normally while the vaccine is being administered – there is no need to actively inhale or sniff.
Contraindications: Hypersensitivity to the active substances, any of the excipients (e.g. gelatin), or gentamicin (a possible trace residue). Severe allergic reaction (e.g. anaphylaxis) to eggs or to egg proteins (e.g. ovalbumin). Children and adolescents who are clinically immunodeficient due to conditions or immunosuppressive therapy such as: acute and chronic leukaemias; lymphoma; symptomatic HIV infection; cellular immune deficiencies; and high-dose corticosteroids. Not contraindicated for use in individuals with asymptomatic HIV infection; or individuals who are receiving topical/inhaled corticosteroids or low-dose systemic corticosteroids or those receiving corticosteroids as replacement therapy, e.g. for adrenal insufficiency. Contraindicated in children and adolescents younger than 18 years of age receiving salicylate therapy because of the association of Reye’s syndrome with salicylates and wild-type influenza infection.
Warnings and precautions: Clearly record name and batch number of administered product to improve traceability. Medical treatment and supervision should always be readily available in case of an anaphylactic event following administration. Fluenz Tetra should not be administered to children and adolescents with severe asthma or active wheezing because these individuals have not been adequately studied in clinical studies. Vaccine recipients should be informed that Fluenz Tetra is an attenuated live virus vaccine and has
References
the potential for transmission to immunocompromised contacts. Vaccine recipients should attempt to avoid, close association with severely immunocompromised individuals (e.g. bone marrow transplant recipients requiring isolation) for 1-2 weeks following vaccination. Where contact is unavoidable, the potential risk of transmission of the influenza vaccine virus should be weighed against the risk of acquiring and transmitting wild-type influenza virus. No data exists regarding the safety in children with unrepaired craniofacial malformations.
Drug Interactions: Do not administer Fluenz Tetra to children and adolescents receiving salicylate therapy. Salicylates must not be used for 4 weeks following vaccination unless medically indicated. Co-administration of trivalent Fluenz with the live attenuated vaccines: No clinically meaningful changes in immune responses to measles, mumps, varicella, orally-administered poliovirus or Fluenz have been observed. Immune response to rubella vaccine was significantly altered. This might not be of clinical relevance with the two dose immunisation schedule of the rubella vaccine. This observation with trivalent Fluenz is relevant to the use of Fluenz Tetra as Fluenz Tetra (influenza vaccine-live attenuated, nasal) is identical to Fluenz with the only difference being the addition of a fourth strain (a second B strain) to Fluenz Tetra. Co-administration of Fluenz Tetra with inactivated vaccines has not been studied. Concurrent use with antiviral agents active against influenza A and/or B viruses has not been evaluated. Based upon the potential for influenza antiviral agents to reduce the effectiveness of Fluenz Tetra, it is recommended not to administer the vaccine until 48 hours after the cessation of influenza antiviral therapy. Administration of influenza antiviral agents within two weeks of vaccination may affect the response of the vaccine. If influenza antiviral agents and Fluenz Tetra are administered concomitantly, revaccination should be considered based on clinical judgment.
Fertility, Pregnancy and Lactation: Not recommended during pregnancy. Should not be used during breast-feeding. No data on the effects of Fluenz Tetra on male and female fertility.
Undesirable events: Consult SmPC for a full list of side-effects. Very common: decreased appetite, nasal congestion/rhinorrhoea, malaise. Common: headache, myalgia, pyrexia. Uncommon: hypersensitivity reactions (including facial oedema, urticaria and very rare anaphylactic reactions), epistaxis, rash. Very rare reports of Guillain-Barré syndrome and exacerbation of symptoms of Leigh syndrome (mitochondrial encephalomyopathy) have also been observed in the post-marketing setting.
Legal Category: Product subject to prescription which may not be renewed (A).
Marketing Authorisation Number: EU/1/13/887/004.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden.
Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15, Ireland. Tel: +353 1 609 7100.
FLUENZ is a trademark of the AstraZeneca group of companies.
Date of API Preparation: 07/2022
Veeva ID: IE-4109
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
1. Fluenz Tetra SmPC. https://www.medicines.ie/medicines/fluenz-tetra-nasal-spray-suspension-34994/spc
2. Principi N et al. Arch Dis Child. 2004;89:1002–1007.
3. HSE - Influenza. Chapter 11. National Immunisation Advisory Committee guidelines. © AstraZeneca 2022. All rights reserved. Veeva ID: IE-4168 | Date of Preparation: September 2022