Helping patients quit the habit
David Lynch speaks to Public Health Specialist Dr Paul Kavanagh about new ‘stop smoking’ clinical guidelines
Biden’s reforms in the balance
It is shaping up to be a make-orbreak year for US President Joe Biden’s ambitious plans for healthcare. Bette Browne reports
PAGE
12-14
The shared dream of storytelling
Stories make us feel less alone, which is especially important at a time like this, writes Dr Lucia Gannon
PAGE 20
New HSE adult safeguarding policy in stasis
CATHERINE REILLY

A revised HSE adult safeguarding policy remains in draft form since 2019 with no imminent plans for roll-out, the Medical Independent (MI) can report.
The new policy is meant to extend to all of health and social care and replace a 2014 document that is only operational in social care.
Progressing implementation of the revised policy was first signalled in the HSE National Service Plan 2019. However, serious concerns about roles and responsibilities as described in the policy, under-investment in adult safeguarding, and the pandemic, have stymied implementation.
A HSE spokesperson told MI it is expected the 2014 policy will “remain extant for the foreseeable future”. Work will continue throughout 2022 “on designing a future operating model for safeguarding in line with the wider programme of health service reform”.

The Department of Health is also at “an advanced stage” of developing its own
national policy on adult safeguarding in health and social care, which will apply to all public, voluntary, and private health and social care settings and agencies under its remit. A public consultation is due in the first half of this year.
Development of the Department’s policy and underpinning legislation was approved in 2017. A Health (Adult Safeguarding) Bill is included in the spring legislative programme.
However, a number of organisations have emphasised the need for a whole of society legislative framework. The Law Reform Commission is currently developing a regulatory framework for adult safeguarding (across all sectors), which is due to be published this summer.
A Department spokesperson said it will consider any recommendations the Commission may make regarding legislation relevant to its functions and anticipates that other Departments will do likewise.
On whether it was intended to introduce any form of mandatory reporting of adult safeguarding concerns, the Department
Continued delays for ‘non-urgent’ breast cancer referrals – NCCP
PAUL MULHOLLAND
A meeting of the National Cancer Control Programme’s (NCCP) executive management committee in October heard the continued delays in patients being seen in symptomatic breast disease (SBD) clinics was concerning.
According to the minutes of the meeting, which were seen by the Medical Independent (MI) through Freedom of Information law, the clinic in St James’s Hospital in Dublin was particularly “challenged”, and management had been engaged with the NCCP for the previous six months to find solutions.
The meeting heard that potential solutions to delays in SBD clinics included outsourcing mammograms, additional clinics, and more resources allocated to radiology.
When asked about the current situation, a spokesperson for the NCCP told MI there are continued delays in seeing the ‘non-urgent’ patient cohort across all symptomatic breast disease units.
“This is due in part to the effects of Covid and the prioritisation of the patients in the urgent category as the cancer detection rate is higher.”
The NCCP has been engaging with the cancer centres, SBD clinics and rapid access clinics throughout 2020/2021 and has considered all potential solutions outlined in the October minutes, according to the spokesperson.
“The NCCP is continuing to engage with the cancer


said it would consider all relevant issues, including reporting structures and duties.
The spokesperson added that, under existing criminal law legislation, it is an offence to withhold information relating to the commission of certain offences against adults at risk.
“The HSE’s operational safeguarding policy includes a zero-tolerance approach requiring staff and services to report all abuse concerns. HIQA reviews all reports it receives, including from the public, when carrying out inspections of designated residential centres, and refers concerns as appropriate to the appropriate authorities,” they outlined.

Asked about investment to enhance safeguarding, the Department referred to measures under Budget 2022, including €600,000 “to employ additional social workers to provide safeguarding supports to older persons residing in nursing homes”. This will involve nine social work team leaders and nine social workers being assigned to new community support teams. See news feature, p4-6.
centres to address these Covid/cyberattack-related issues and the situation is being monitored closely,” they added.


In advance of World Cancer Day (4 February), the European Cancer Organisation called for urgent action on the fact that approximately one million cancer cases are undiagnosed across Europe as a result of the effects of Covid-19.
Through its ‘Time To Act’ data navigator, the Organisation makes key data available which maps out the impact of Covid-19 on cancer across European countries. For Ireland, the tool reveals significant “pandemic-induced burdens” across cancer control and care, with an estimated 58 per cent reduction in referrals to rapid access clinics at the lowest point and an overall 10-to-14 per cent shortfall in cancer diagnoses in 2020.
Meanwhile, at the September meeting of the NCCP executive manangement committee, there was a discussion on making breast cancer outpatient departments (OPD) ‘paperless’.
“The scoping exercise to determine how the NCIS [national cancer information system] can support a paperless environment in breast clinic OPDs is ongoing,” according to the minutes.
As a result of the cyberattack, it is now likely the full implementation of the NCIS will not occur until next year.

The dates for many hospital sites to ‘go-live’ with the system has been delayed by between six and 12 months, added the minutes.
Legal Category: S1A. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
XELJANZ® is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent.


XELJANZ® in combination with methotrexate (MTX) is indicated for the treatment of moderate to severe active rheumatoid arthritis (RA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying antirheumatic drugs.
XELJANZ® in combination with MTX is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior disease-modifying antirheumatic drug (DMARD) therapy. PP-XEL-IRL-0668
OUR PAPER IS NOW COMPOSTABLE, AS WELL AS RECYCLABLE NEWS 1-16 ● OPINION 17-22 ● MCQ s 22 ● CLINICAL 23-32 ● LIFE ● QUIZZES 34 ● FOOD & DRINK 35 ● MOTORING 36-37 ● RXDX 38 ● RECRUITMENT 39 10 FEBRUARY 2022 ● ISSUE 3 VOLUME 13 ● NEXT ISSUE 24 FEBRUARY 2022 €5.95
PAGE 10
Date of preparation: September 2021 INDIC A T E D FOR UC | RA | Ps A
Pictured (L-R) following the publication of a report into the outcomes of the Croí MySláinte digital cardiovascular disease prevention and recovery programme are Ms Irene Gibson, PhD candidate and Director of Programmes and Innovation, National Institute for Prevention and Cardiovascular Health, with Mr Neil Johnson, Chief Executive, Croí.
Keytruda is the first and only immunotherapy approved in the first-line treatment of M/uR HNSCC in Ireland1

KEYTRUDA (Pembrolizumab) is Now Publicly Available* as Monotherapy or in Combination with platinum and 5-fluorouracil (5-F U) chemotherapy for the first-line treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma in adults whose tumours express PD-L1 with a CPS ≥ 1.
The ESMO Clinical Practice Guidelines recommends Keytruda Monotherapy or in Combination with Platinum/5-FU for Metastatic or recurrent/persistent disease not amenable to curative RT or surgery in the ‘No platinum-based ChT during the last 6 months and PD-L1-positive tumour’ subset of recommendations.2
KEYTRUDA® (pembrolizumab) ABRIDGED PRODUCT INFORMATION

Refer to Summary of Product Characteristics before prescribing. PRESENTATION KEYTRUDA 25 mg/mL: One vial of 4 mL of concentrate contains 100 mg of pembrolizumab. INDICATIONS KEYTRUDA as monotherapy is indicated for the treatment of advanced (unresectable or metastatic) melanoma in adults. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with Stage III melanoma and lymph node involvement who have undergone complete resection. KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations. KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR or ALK positive mutations. KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA. KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD L1 with a combined positive score (CPS) ≥ 10. KEYTRUDA as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the firstline treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC) in adults whose tumours express PD-L1 with a CPS ≥ 1. KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic HNSCC in adults whose tumours express PD-L1 with a ≥ 50% TPS and progressing on or after platinum-containing chemotherapy. KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of advanced renal cell carcinoma (RCC) in adults. KEYTRUDA, in combination with lenvatinib, is indicated for the first line treatment of advanced renal cell carcinoma in adults. KEYTRUDA as monotherapy is indicated for the first line treatment of metastatic microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer in adults. KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic carcinoma of the oesophagus or HER-2 negative gastroesophageal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥ 10. KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumours express PD L1 with a CPS ≥ 10 and who have not received prior chemotherapy for metastatic disease. KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of advanced or recurrent endometrial carcinoma in adults who have disease progression on or following prior treatment with a platinum containing therapy in any setting and who are not candidates for curative surgery or radiation.
DOSAGE AND ADMINISTRATION See SmPC for full details. Therapy must be initiated and supervised by specialist physicians experienced in the treatment of cancer. The recommended dose of KEYTRUDA in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA as monotherapy in paediatric patients aged 3 years and older with cHL is 2 mg/kg bodyweight (up to a maximum of 200 mg), every 3 weeks administered as an intravenous infusion over 30 minutes. For use in combination, see the Summary of Product Characteristics (SmPC) for the concomitant therapies. KEYTRUDA must not be administered as an intravenous push or bolus injection. When administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should be administered first. Treat patients until disease progression or unacceptable toxicity (and up to maximum duration of therapy if specified for an indication). For the adjuvant treatment of melanoma, KEYTRUDA should be administered until disease recurrence, unacceptable toxicity, or for a duration of up to one year. KEYTRUDA, as monotherapy or as combination therapy, should be permanently discontinued (a) For Grade 4 toxicity except for: endocrinopathies that are controlled with replacement hormones; or haematological toxicity, only in patients with cHL in which KEYTRUDA should be withheld until adverse reactions recover to Grade 0-1; (b) If corticosteroid dosing cannot be reduced to ≤10 mg prednisone or equivalent per day within 12 weeks; (c) If a treatment-related toxicity does not resolve to Grade 0 1 within 12 weeks after last dose of KEYTRUDA; (d) If any event occurs a second time at Grade ≥ 3 severity. Patients must be given the Patient Alert Card and be informed about the risks of KEYTRUDA. Special populations Elderly: No dose adjustment necessary. Renal impairment: No dose adjustment needed for mild or moderate renal impairment. No studies in severe renal impairment. Hepatic impairment: No dose adjustment needed for mild hepatic impairment. No studies in moderate or severe hepatic impairment. Paediatric population: Safety and efficacy in children below 18 years of age not established except in paediatric patients with cHL. CONTRAINDICATIONS Hypersensitivity to the active substance or to any excipients. PRECAUTIONS AND WARNINGS Assessment of PD-L1 status When assessing the PD-L1 status of the tumour, it is important that a well-validated and robust methodology is chosen to minimise false negative or false positive determinations. Immune-related adverse reactions Immune-related adverse reactions, including severe and fatal cases, have occurred in patients receiving pembrolizumab. Most immune related adverse reactions occurring during treatment with pembrolizumab were reversible and managed with interruptions of pembrolizumab, administration of corticosteroids and/or supportive care. Immune related adverse reactions have also occurred after the last dose of pembrolizumab. Immune-related adverse reactions affecting more than one body system can occur simultaneously. Immune-related adverse reactions are immune-related pneumonitis, immune-related colitis, immune-related hepatitis, immune-related nephritis, immune-related endocrinopathies (including adrenal insufficiency, hypophysitis, type 1 diabetes mellitus, diabetic ketoacidosis, hypothyroidism, and hyperthyroidism), Immune-related skin adverse reactions (also including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)), Refer to SmPC for more information and management of immune-related adverse reactions. Complications of allogeneic Haematopoietic Stem Cell Transplant
For more information on Keytruda in HNSCC visit MSDConnect.ie or scan the QR code below with your phone
(HSCT): Cases of graft-versus-host-disease (GVHD) and hepatic veno-occlusive disease (VOD) have been observed in patients with classical Hodgkin lymphoma undergoing allogeneic HSCT after previous exposure to pembrolizumab. Infusion-related reactions: Grades 1, 2, 3 or 4 infusion reactions including hypersensitivity and anaphylaxis, could be seen with pembrolizumab treatment. Refer to SmPC for more information and management of infusion-related reactions. Overdose: There is no information on overdose with pembrolizumab. In case of overdose, monitor closely for signs or symptoms of adverse reactions and treat appropriately. INTERACTIONS No formal pharmacokinetic drug interaction studies have been conducted with pembrolizumab. No metabolic drug drug interactions are expected. The use of systemic corticosteroids or immunosuppressants before starting pembrolizumab should be avoided because of their potential interference with the pharmacodynamic activity and efficacy of pembrolizumab. Corticosteroids can be used as premedication, when pembrolizumab is used in combination with chemotherapy, as antiemetic prophylaxis and/or to alleviate chemotherapy-related adverse reactions. FERTILITY, PREGNANCY AND LACTATION Women of childbearing potential Women of childbearing potential should use effective contraception during treatment with pembrolizumab and for at least 4 months after the last dose of pembrolizumab. Pregnancy No data on use in pregnant women. Do not use during pregnancy unless the clinical condition of the woman requires treatment with pembrolizumab. Breast-feeding It is unknown whether pembrolizumab is secreted in human milk. A risk to newborns/ infants cannot be excluded. Fertility No clinical data available. SIDE EFFECTS Refer to SmPC for complete information on side effects. Pembrolizumab is most commonly associated with immune-related adverse reactions. Most of these reactions resolved with appropriate medical treatment or withdrawal of pembrolizumab. The most serious adverse reactions were immune-and infusion-related adverse reactions. When pembrolizumab is administered in combination with axitinib or lenvatinib, refer to the SmPC for axitinib or lenvatinib prior to initiation of treatment. For additional lenvatinib safety information related to advanced RCC see the SmPC for Kisplyx and for advanced EC see the SmPC for Lenvima. Monotherapy: Very Common: anaemia, hypothyroidism, decreased appetite, headache, dyspnea, cough, abdominal pain, nausea, vomiting, constipation, musculoskeletal pain, arthralgia, asthenia, oedema, pyrexia, diarrhoea, rash, pruritus, fatigue. Common: pneumonia, thrombocytopenia, neutropenia, lymphopenia, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, neuropathy peripheral, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, hyperthyroidism, thyroiditis, insomnia, dizziness, dysgeusia, pneumonitis, colitis, dry mouth, severe skin reactions, vitiligo, dry skin, alopecia, eczema, dermatitis acneiform, erythema, dermatitis, myositis, pain in extremity, arthritis, influenza like illness, chills, AST and ALT increases, hypercalcaemia, increase in blood alkaline phosphatase, blood bilirubin increased, blood creatinine increased, infusion related reaction. In combination with chemotherapy: Very Common: pneumonia, neutropenia, anaemia, thrombocytopenia, leukopenia, hypothyroidism, hypokalaemia, decreased appetite, insomnia, neuropathy peripheral, headache, dizziness, dyspnoea, cough, nausea, vomiting, diarrhoea, abdominal pain, constipation, alopecia, rash, pruritus, musculoskeletal pain, arthralgia, pyrexia, fatigue, asthenia, oedema, ALT increase, AST increased, blood creatinine increased. Common: febrile neutropenia, lymphopenia, infusion related reaction, hyperthyroidism, hyponatraemia, hypocalcaemia, lethargy, dysgeusia, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, pneumonitis, colitis, dry mouth, gastritis, hepatitis, severe skin reactions, erythema, dermatitis, dry skin, myositis, pain in extremity, arthritis, acute kidney injury, influenza-like illness, chills, hypercalcaemia, blood alkaline phosphatase increased, blood bilirubin increased. In combination with axitinib or lenvatinib: Very Common: urinary tract infection, anaemia, hypothyroidism, decreased appetite, headache, dysgeusia, hypertension, dyspnoea, cough, diarrhoea, abdominal pain, nausea, vomiting, constipation, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pain in extremity, fatigue, asthenia, oedema, pyrexia, lipase increased, alanine aminotransferase increased, aspartate aminotransferase increased, blood creatinine increased. Common: pneumonia, neutropenia, thrombocytopenia, lymphopenia, leukopenia, infusion-related reaction, adrenal insufficiency, hyperthyroidism, thyroiditis, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, dizziness, neuropathy peripheral, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), pneumonitis, colitis, pancreatitis, gastritis, dry mouth, hepatitis, severe skin reactions, dermatitis, dry skin, erythema, dermatitis acneiform, alopecia, arthritis, nephritis, influenza like illness, chills, amylase increased, blood bilirubin increased, blood alkaline phosphatase increased, hypercalcaemia. PACKAGE QUANTITIES KEYTRUDA 25 mg/mL: 4 mL of concentrate in a 10 mL Type I clear glass vial. Legal Category: POM. Marketing Authorisation numbers EU/1/15/1024/002 Marketing Authorisation holder Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands. Date of revision: November 2021. © Merck Sharp & Dohme B.V. 2021. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7 or from www. medicines.ie. II0104_105
Adverse
events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700) References 1. HSE Head and Neck Chemotherapy Regimens, Available at https://www.hse.ie/eng/services/list/5/cancer/profinfo/ chemoprotocols/headandneck/ 2. ESMO Clinical Guidelines, Available at https://www.annalsofoncology.org/action/showPdf?pii=S0923-7534%2820%2939949-X Accessed Dec21 3. Keytruda Summary of Product Characteristics, available at www.medicines.ie, accessed Dec 21 R/M = recurrent or metastatic, CPS = combined positive score, ESMO = European Society for Medical Oncology, ChT = chemotherapy, HNSCC = head and neck squamous cell carcinoma. Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7, Ireland. IE-KEY-00476 Date of Preparation: January 2022 *Reimbursed as of 20th December 2021 on the Oncology Drugs Management System
Now Publicly Available in Ireland*
5127_Keytruda_H&N_Reimbursement_250x346(5mm bleed)_Ad_v.indd 1 31/01/2022 21:01
Tobacco Free Ireland policy ‘should be reviewed’
DAVID LYNCH
The Government should review its Tobacco Free Ireland policy, according to the Chair of the guideline development group for new ‘stop smoking’ clinical guidance. Last month, the national clinical effectiveness committee released guidelines to help healthcare professionals assist adults to stop smoking.
Dr Paul Kavanagh, Specialist in Public Health Medicine and Chair of the guideline development group, told the Medical Independent “we are very proud of the huge progress we have made around controlling the amount of tobacco use in the population”. However, he added
Expenditure on strong opioid almost tripled
REILLY
State expenditure on the opioid tapentadol almost tripled –to over €3.75 million – and the number of items dispensed nearly quadrupled between 2014 and 2019, according to data released by the HSE to the Medical Independent Tapentadol, known by the brand name Pa–lexia, was added to the GMS reimbursement list in July 2011. Some 24,776 items were dispensed in 2014 at a cost of €1,141,457. In 2019, 91,620 items were dispensed at a cost of €3,754,472.
The drug is indicated for the management of severe chronic pain in adults that can be adequately managed only with opioid analgesics. It has the potential for abuse and addiction/dependence syndrome.
The National Centre for Pharmacoeconomics (NCPE) recommendation in 2011 was that tapentadol may be considered cost-effective, but should be reserved for patients who cannot tolerate existing strong oral opioids.
Oxycodone combination items had increased from 25,039 items dispensed in 2014, at a cost of €1,006,915, to 42,752 items in 2019 at a cost of €1,822,367.
The data from the Primary Care Eligibility and Reimbursement Service (PCERS) was presented in 2021 to a Medical Council working group devising strategies to reduce overprescribing of potentially addictive drugs.

The number of items dispensed for several opioids increased between 2014 and 2019, including morphine, oxycodone combinations, and codeine combinations. However, the costs of most of the drugs had been decreasing.
The number of gabapentinoid items dispensed had also increased.
In 2019, there were 613,598 pregabalin items dispensed at a cost of €19,930,257. The PCERS presentation noted that pregabalin was reference priced in April 2020, which will provide a further decrease in expenditure. However, the number of items dispensed “may remain static”.
In 2019 there were 131,301 gabapentin items dispensed at a cost of €2,795,368. It was reference priced in May 2020 and the number of items dispensed “may continue to increase”.
that there should be no “risk of complacency”.
The Tobacco Free Ireland policy was launched in October 2013 with a headline target to have a smoking prevalence rate of less than 5 per cent by 2025.

Dr Kavanagh told this newspaper the “reality” was that the goal was “looking increasingly unrealistic”. The Healthy Ireland Survey 2021 indicated a smoking prevalence rate of 18 per cent.
He praised the “very bold and ambitious goal that Gov-
ernment set for itself in 2013”. However, he added there should be an interim “review” of that policy and goal.
“Now, at the same time, I would say that shouldn’t be a reason for despondency or that we should back out of that goal,” stated Dr Kavanagh.
The 5 per cent goal should remain, but in light of the impact of the pandemic and the most recent Healthy Ireland Survey results, the timeline should be reviewed, he said. See news feature, p10.
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 3 News
1272_Advert-255x166_v2_MAT-IE-2100680(v1.0)_OL.indd 1 19/08/2021 18:01
CATHERINE
Between the lines of adult safeguarding in the HSE
In December, the HSE released part of an external review into the case of ‘Brandon’, a man with an intellectual disability who sexually assaulted and abused fellow residents at a HSE residential facility in Co Donegal.
It outlined prolonged sexual abuse of residents despite the knowledge of management and staff. One of the notable findings was that the HSE had “on occasions disregarded the advice and guidance” offered by the regional adult safeguarding and protection team (SPT) in terms of “how serious safeguarding concerns should be dealt with”.
The nine regional SPTs were set up under a 2014 HSE adult safeguarding policy in social care, but operate without any primary adult safeguarding legislation. The SPTs receive safeguarding concerns (‘preliminary screenings’) and safeguarding plans from HSE and HSE-funded disability and older persons services for specialist review and also directly manage cases in the community.
While the safeguarding teams did not exist for most of the period under review in the Brandon case (2003-2018), the SPT’s role after a whistle-blower raised concerns was “very illuminating in terms of the culture and attitude towards safeguarding concerns displayed by the management of this facility”, according to the report by the National Independent Review Panel, which was completed in 2020. There are ongoing calls for the HSE to release the full report to inform learning and promote transparency in adult safeguarding.
Speaking to the media in December, senior HSE management and Government Ministers were categorical that incidents such as the Brandon case were unacceptable. HSE CEO Mr Paul Reid said the Executive had introduced “dedicated safeguarding resources and procedures”.
SPT CHO 7
On 21 September 2021, the Principal Social Worker at the SPT in Dublin South, Kildare, and West Wicklow (DSKWW) emailed her line manager after a meeting that morning, where it was explained that the required resourcing for the team was not available.
The SPT in DSKWW, also known as Community Healthcare Organisation (CHO) 7, receives around 20 per cent of national safeguarding referrals, but its funding allocations have never reflected this workload.
Since 2016, records show successive principal social workers at the team appealed to management for the resourcing necessary to respond in a timely manner to safeguarding concerns – through communications, business cases, and risk assessments.
“The safeguarding team has not been adequately staffed since it started,” read a risk assessment dated September 2016, which identified vulnerabilities, such as increased risk of abuse of vulnerable adults
and exhausted and stressed staff.
An internal risk register from November 2021, also released under Freedom of Information (FoI) law, referred to “risk of harm” to adults at risk of abuse “due to inadequate number of safeguarding social workers per CHO population” to provide timely access to appropriately trained professionals to case manage, investigate allegations, and deliver training, etc.
In the intervening years, the problem had spiralled. In late 2020, 1,812 alleged abuse cases submitted by services had not been examined, with some dating to June 2019. The team also had 1,626 safeguarding plans, dating to 2016, that it had been unable to examine, as reported by the Medical Independent (MI) last year.
During the Covid-19 pandemic there were increasing community referrals and greater complexity of cases. The caseload included domestic violence, ongoing grooming and sexual exploitation of younger vulnerable adults, ‘cuckooing during cocooning’, and financial abuse. Two experienced staff were also involved in a safeguarding investigation in a facility in DSKWW.
However, despite the serious and sensitive nature of this work, and HSE plans to rollout a new policy across divisions, internal communications from DSKWW show its Chief Officer Ms Ann O’Shea described the national investment in adult safeguarding in 2021 as “minute”.
Delicate progress
By autumn 2021, amid ongoing resourcing challenges and considerable extra work and risks posed by the cyberattack, the SPT had made significant inroads on addressing the unreviewed cases. Some agency resourcing had been provided to assist with the backlog, but this arrangement was tenuous for several reasons, including the competitive employment market for social workers.
In September 2021, the backlog of preliminary screenings stood at around 600, dating to January 2020, and 1,636 safeguarding plans dating to 2016. However, on 21 September, the Principal Social Worker, Ms Celine O’Connor, also reported to management a “newer backlog” of 30 abuse concerns and 15 safeguarding plans dating to 8 September due to under staffing.
In an email to Dr Sinead Reynolds (PhD), DSKWW’s Head of Service for Quality, Safety and Service Improvement (QSSI), Ms O’Connor stated that she was “disappointed that no permanent resources can be found at this time” given the backlog dated to 2018 and “each time staffing reduces and agency resource is withdrawn we begin to grow the backlog again”.

While “grateful for the acknowledgement that we require more”, the lack of funding for permanent posts “has been very demoralising for the team”, according to records obtained under FoI law.
There was no waiting list for community cases, but due to the decreasing number of social workers, there were delays in providing intervention, reported Ms O’Connor, who gave an overview of staffing shortfalls and requirements, as well as the nature of recruitment and retention difficulties.
Community cases were remaining open for longer, there were gaps in getting interventions in place and people were being left at risk of abuse for longer. “It will also cause reputational damage to the HSE if [interventions in] abuse cases in community are being delayed and people are being harmed or die as a result,” outlined Ms O’Connor, who further noted the risks to running a service where agency staff outnumbered permanent staff. In her communications, Ms O’Connor also drew attention to the commitment of the social workers and administrative staff at the SPT.
Ms O’Connor and Dr Reynolds were in regular contact on SPT resourcing, the latter
becoming line manager for the team in spring 2021 as part of changes in the governance structure for SPTs nationally.
On 24 September, attaching a draft business case, Dr Reynolds responded that “much of what you discuss in the email below is well known and accepted in terms of the overall resourcing of CHO7”.
Dr Reynolds continued: “As discussed at our meeting we can submit information to national community operations every year as part of the estimates process and when the letter of determination is received by the HSE we are told what our budgets will be for each area for the following year. Once this happens we are legally obliged to make the best use of the resources that we have. There are many areas where resources are limited and we are challenged accordingly in terms of service provision. This is common to many public services.”
Dr Reynolds did not believe it was useful to “keep going over this in written correspondence as it takes time and distracts from the job at hand”. Her email indicated she was attempting to build a case for durable resourcing.

Ms O’Connor agreed that “having to repeatedly flag the gaps” was a waste of time. However, “when a serious incident does occur I won’t have it said that I haven’t actively tried to get resources for the team and the clients we assist.”
The Principal Social Worker, who acknowledged Dr Reynolds’ assistance, added: “It is disappointing that safeguarding people at risk of abuse is not seen as a priority area for the HSE to fund given its legal obligation to protect the health and welfare of the public. The team here will continue to use the resources we have in the most efficient manner and will continue to highlight when we can’t meet the need.”
In late January, a DSKWW spokesperson informed MI the SPT had completed the “historic backlog” of preliminary screenings. “Currently the majority of new preliminary screenings are being reviewed on the day that they are received. All current preliminary screenings are being reviewed within a week,” they outlined.
“There is an historic backlog of 1,784 safeguarding plans which go back as far as April 2016. These are plans put in place by services following preliminary screenings which have been viewed by the safeguarding team.”
Staffing levels “do impact the length of time that cases are open in the community” and priority is given to cases where the individual is at immediate risk of harm.
In 2021 additional funding was provided to the CHO SPT for two social worker team leader posts, a professionally qualified social worker post, and a grade VII (business manager) post (although internal correspondence showed the Principal Social Worker stated that these social worker posts would not nearly be enough for the workload requirements).
The CHO also provided “specified purpose funding for agency posts where this was achievable within funding limits available”.
DSKWW senior management had raised concern with senior management in Community Operations and the HSE National Safeguarding Office (NSO) in regard to SPT staffing.
Two “safeguarding risks” have recently been escalated from the QSSI risk register to the Chief Officer’s risk register, stated
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022
In the wake of the ‘Brandon’ review, HSE management provided assurances that adult safeguarding was being resourced but internal correspondences present a different picture. Catherine Reilly reports on ongoing resource and legislative deficits in the area
News Feature CATHERINE REILLY catherine@mindo.ie
the spokesperson.
According to the HSE, operational plans for 2022 were being finalised, and to date staffing in SPTs has not been adjusted to population or demand.
“The HSE is working at corporate and regional management levels to address the ongoing challenges operating the safeguarding policy in CHO 7…. No other CHO has reported a backlog, nor have any other backlogs been identified by the NSO.”
Meanwhile, the Department of Health referred to Budget 2022 measures including €600,000 to employ 18 additional social workers “to provide safeguarding supports” in nursing homes. These posts in new community support teams “will enhance” the nine SPTs.
Policy reform
A revised HSE adult safeguarding policy has remained in draft since 2019. There is widespread support for replacement of the 2014 policy, which is limited to social care and has been inconsistently interpreted and implemented. However, the proposed new policy
has raised a number of concerns.
Last May, Head of Quality and Patient Safety in HSE Community Operations, Mr JP Nolan, provided an “update” on policy implementation to National Director of Community Operations, Ms Yvonne O’Neill.
Based on current capacity, Mr Nolan did not foresee the revised policy becoming operational before 2022 and this would depend on the next national service plan “supporting a feasible operating model”. His email also referred to lack of investment in the SPTs in 2021 and an intention to introduce nursing safeguarding roles to the CHOs, a proposal that has perplexed some social workers due to existing deficits in safeguarding and the competencies involved.
According to his email, Mr Nolan had been engaging with the NSO, CHOs, the Department of Health and health sector unions, and found this area of practice to be “divisive”. In some respects, outside of the NSO and the SPTs, safeguarding was “poorly understood in comparison to other jurisdictions where I have worked”, added Mr Nolan, a former nurse with a broad range of clinical and advi-
sory experience in the nursing profession.
Defining pathways under the new policy would require “comprehensive engagement” with stakeholders. “I have already indicated to these parties that we will not choose between a specialist team and an ‘in-service’ model – we need both. That in turn at CHO level would probably look like the SPTs continuing their specialist services but also a safeguarding investment in either the five current care groups” – believed to be a reference to the main HSE care divisions –“or the future 96 CHNs” – a reference to the community healthcare networks planned under Sláintecare reforms.
In his note, Mr Nolan observed that the Department of Health was also developing an adult safeguarding policy, which could potentially require technical amendments to the HSE policy.
A part of the email was redacted and it resumed by stating there were some matters that in the interests of safety could not be conceded – “for example the position put to us
Continued
Lack of training for doctors in adult safeguarding
There is a lack of mandatory modules relating to adult safeguarding for doctors in training.
Higher specialist trainees in geriatric medicine are required to undertake a module titled ‘Respecting autonomy and safeguarding the rights of older people’.
It aims to help doctors “recognise the warning signs of elder abuse” and know how to report suspected cases.
However, this module is not a requirement for other trainees in the RCPI nor are there any specific adult safeguarding modules.
A RCPI spokesperson said adult safeguarding issues are often raised on trainee study days and within the College’s ethics courses.
The RCSI “offers” a module on adult safeguarding and the “rights of vulnerable patients” within its Human Factors and Patient Safety programme, which is mandatory for all trainees in surgery and emergency medicine.
“We have also included a mandatory module in active bystander intervention. There is also a section on safeguarding vulnerable adults on our trainee portal.”
The College of Psychiatrists said: “We do include the assessment of risk of abuse to ‘vulnerable’ adults in our training curriculum, supervisors teach and assess this with trainees and this subject may be examined in membership examinations.”
Feedback from individual psychiatrists indicated safeguarding was regarded as a social work function. However, lack of out-of-hours social work support was raised. An example was also provided of a safeguarding social worker believing a psychiatry MDT would manage a safeguarding concern as it was assumed this was preferred.
Another psychiatrist, Dr Patricia Walsh, emphasised to the Medical Independent (MI) that adult mental health services required “a 21st century infrastructure
of electronic patient records” to facilitate communication with agencies like Tusla, the gardaí, adult safeguarding, probation, etc, as well as promote safer prescribing practice and care. Dr Walsh said there needed to be clearer structures and processes across health and social care for management of adult safeguarding.
Adult safeguarding concerns in acute settings are usually referred to medical social workers, although some hospitals do not have a social worker or minimal staffing.
Ms Amanda Casey of the Irish Association of Social Workers, who is Principal Medical Social Worker at the Mater Hospital in Dublin, told MI referrals would often arise following presentations to the emergency department. Such referrals would usually come from nursing or medical staff.
An example would be a person admitted from a nursing home where there are signs of poor care or neglect. Financial abuse of the patient has also arisen during the application process for long-term care.
In Ms Casey’s experience, the medical social worker would take the lead role in doing the initial screen and safeguarding plan and link with other relevant services. There is a “reasonably well resourced” team at the Mater with the ability to assess and manage safeguarding cases. The medical social work team would often seek the advice of the relevant safeguarding and protection team (SPT) in community operations and refer the case onwards to them.
“We can make a referral to the SPT, but obviously one of the frustrating things is they don’t have an automatic right of entry to private nursing homes. Equally we can send concerns to HIQA, but they don’t investigate individual complaints,” noted Ms Casey.
There is a need for adult safeguarding training requirements for hospital-based
healthcare professionals, confirmed Ms Casey. “We are reliant on people having the wherewithal to notice it and do something about it,” she said. “That is contingent on staff having the time to notice these things and having the time to ask those questions, which is not always possible in that sort of an environment.”
Ms Casey said certain forms of abuse, such as coercive control, are “less seen and less obvious” particularly without training.
The core problem is the absence of adult safeguarding legislation, she added. “That legislation would need to involve consideration of things like mandatory reporting, of access to private nursing homes [etc]….”
Ms Casey said all health and social care professionals had a significant role in adult safeguarding. However, “it’s important that [the lead role] lies with a profession such as social work because of our training.”
“There is a mantra that ‘adult safeguarding is everyone’s responsibility’ and I always have a worry that by saying it is everyone’s responsibility, it becomes no-one’s responsibility…. There does need to be a lead profession who has a specific skillset and specific responsibility to manage these concerns, and coordinate what can often be incredibly complex situations… it makes sense that social work would continue to take a lead role in that.”
A HSE spokesperson said an adult safeguarding training programme on the HSeLanD (Health Service‘s e-Learning and development) platform was available to all health and social care workers including in voluntary hospitals.
MINDO NUMBERS
71
per cent of patients with a stroke were admitted to a stroke unit (below the target of 90 per cent), according to the Irish National Audit of Stroke National Report 2020
11
minutes was the median time to contact with a doctor after arrival at hospital (six minutes faster than in 2019), found the national stroke audit.
8.6
per cent of all ischaemic stroke patients had a thrombectomy in 2020. The current thrombectomy rate in Europe is 1.9 per cent.
511 admitted patients were waiting for beds on the first day of February, according to the Irish Nurses and Midwives Organisation Trolley Watch figures.
17,000 patients are currently on a waiting list to see a gastroenterologist –an increase of 7,000 since 2015, the IHCA recently highlighted.
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 5 Feature News
“There is a requirement that all personnel in HSE and HSE-funded services across both disability and older persons sectors undertake the online HSeLanD adult safeguarding training programme once every three years.” on p6 ▸
that only social workers can undertake safeguarding work. I have reiterated our acceptance, as per previous agreements, that social work are the lead profession for safeguarding, but it would be untenable for us to accept that no other profession has a role to play”.
All of the above was happening “against a narrative that views the HSE as the lead agency for the protection of adults at risk of abuse. While we should be always focused on what we can and should do, there are limits to our powers to act and roles for local government, justice, finance, and others that we can’t perform for them.”
Mr Nolan expressed hope that the policy and legislative landscape may evolve to “provide some clarity” on these issues.
Expertise
Adult safeguarding is regarded as complex and labour intensive, with casework involving many considerations including the rights of clients, right to self-determination and legal processes. In this context, many social workers view the proposed new policy as unworkable and not fit for purpose.
The policy introduces new roles of ‘safeguarding manager’ and ‘safeguarding coordinator’, but lacks clarity on who would assume such roles in clinical settings. It suggests a more distanced role for the SPTs.
A 2020 paper on adult safeguarding by the Irish Association of Social Workers (IASW) stated: “While the IASW agrees that it is essential that all health and social care professionals can identify risk and abuse, it is also vital that adults experiencing abuse and harm have direct access to the expertise of frontline safeguarding social work professionals.”
The IASW paper said safeguarding social workers had expert knowledge in the patterns and complex dynamics of abusive be-
haviours, including domestic violence, institutional abuse, coercive control, perpetrator grooming of professionals, and barriers to safety for those experiencing abuse and neglect. Safeguarding social workers were experts in co-working with An Garda Síochana, financial institutions and within the complexities of the Irish legal system.
On the proposed HSE policy, Ms Chris Cully, Assistant General Secretary at Forsa, told MI it was important the role of social worker was “not diluted and becomes anybody’s job”.
Ms Cully added: “We want to make sure the right professionals are involved at the right stages. Safeguarding is everybody’s responsibility, but there are certain responsibilities that should remain the domain of social workers from a statutory, advocacy point of view.”
Subject to negotiations, there could be more safeguarding case co-working between social workers in different settings, but also between social workers and other healthcare professionals, in the future. Ms Cully said the union needs to protect the terms and conditions of members and ensure there is not a blurring of lines between professions. She added: “I am not opposed to multidisciplinary team working as long as everyone understands their role within that MDT.”
MI understands some social workers in primary care are currently not taking on any safeguarding casework. The lack of adult safeguarding legislation and a health sector-wide policy, inconsistent implementation of the current policy, unclear referral thresholds in the safeguarding process, under-resourcing, and out of date job descriptions, are contributing factors. There is no Forsa instruction to primary care social workers that they should not take on safeguarding casework, the union confirmed.
The HSE’s spokesperson commented: “The current and only safeguarding policy in operation is the 2014 policy and it is expected
Inflation will ‘negate’ benefit of increased capital allocation – CSO
PAUL MULHOLLAND
The HSE Chief Strategy Officer (CSO) has said rising inflation will “negate some of the benefit” of increases in the allocation for capital healthcare projects. Mr Dean Sullivan also warned that expenditure on the National Children’s Hospital will “significantly impact funding for new projects in 2022 and 2023”.
Mr Sullivan provided an update on the development of the HSE’s new capital plan at a meeting of the Executive’s audit and risk committee on 12 November.
The core capital funding allocation for building and equipment in 2022 is €1.0265 billion.
Mr Sullivan also informed the committee that the plan will be informed by a number of other initiatives, including Project Ireland 2040: National Development Plan 20212030 and the Healthcare Capacity Review 2018, among others.
The committee was briefed on the challenges and risks currently being considered by HSE management in relation to capital funding and planning.
“The CSO highlighted that projects allocated funding in 2022 will significantly impact projected expenditure in 2023, 2024, and beyond, noting that capital funding levels post 2022 are uncertain,” according to minutes of the meeting.
“He also explained that despite the fact that Project Ireland 2040 indicates an 11.6 per cent increase in capital allocation for 2023, high levels of inflation will negate some of the benefit of this. Additionally, expenditure on the National Children’s Hospital will significantly impact funding for new projects in 2022 and 2023.”
The committee was also updated on the current situation in the construction sector, which has caused the HSE to have “difficulties” in procuring contractors to undertake works.
this will remain extant for the foreseeable future. Work will continue throughout 2022 on designing a future operating model for safeguarding in line with the wider programme of health service reform.”
On introducing the new role of safeguarding nurse, the spokesperson said: “While social work is, and will continue to be, the lead profession with respect to safeguarding, it is critical they are not the only discipline involved. Nurses are one of the largest professional groups in health and social care, and the majority of designated officers for safeguarding are nurses.”
“It is the intention of the HSE to introduce a small cohort of nurses working to support the implementation of the HSE safeguarding policy, specifically in the context of holistic nursing practice. Consultation with stakeholders is ongoing in this regard. “
DoH policy and legislation
As the HSE has been developing its revised policy and implementation plan (which is unlikely to be implemented in its current form) a parallel process has been ongoing at the Department of Health.
The Department is planning to publish its draft policy for consultation. In addition, a Health (Adult Safeguarding) Bill is included in the Government’s spring legislative programme, although not in the ‘priority legislation’ section (also listed is the Protection of Liberty Safeguards Bill).
However, organisations including HIQA and Safeguarding Ireland (a HSE-funded awareness-raising body) have emphasised the need for a whole-of-society legislative framework.
Ms Patricia Rickard-Clarke, Chair of Safeguarding Ireland, said that confining adult safeguarding legislation to health and social care did not go far enough. She noted that issues such as coercive control and financial
abuse involved a range of sectors of society. There was a need for a multidisciplinary “overarching authority which is independent of all service providers, who would have the statutory function to carry out investigations in certain cases”.
Safeguarding Ireland is working on a research paper, which it hopes to publish in March, looking at challenges and best practice considerations in relation to a legal framework for adult safeguarding. The Law Reform Commission is due to publish a regulatory framework for adult safeguarding in the summer.
The long-awaited launch of the decision support service, which is due to open in mid2022, will be an important advancement in regard to safeguarding, noted Ms Rickard-Clarke. Established under the Assisted Decision-Making (Capacity) Act 2015, the service will promote the rights and interests of adults who may require support to make decisions about their personal welfare, property, and affairs. Other legislative requirements include regulations under data protection law to fully address information sharing in the public interest, added Ms Rickard-Clarke.
In relation to the HSE, Ms Rickard-Clarke said its internal safeguarding capabilities would need to be enhanced, in tandem with the development of any independent investigative authority.
In the wake of scandals such as the Brandon case, MI asked about the scale of cultural change necessary in the HSE. She said: “It is a very large organisation and you find very good processes there on the one hand, and in other areas not so good. Remember, we are still working on a policy, we have no underlying legislation. So we need adult safeguarding legislation with very clear standards, very clear regulation, and very clear reporting avenues and we don’t have any of that. We need that framework to be put in place.”
Pandemic and capacity ‘slow pace’ of BowelScreen extension plans
CATHERINE REILLY
There is no implementation date as yet for extending the BowelScreen colorectal cancer screening programme to 59-year-olds, the HSE has stated.
“The pace of implementation has been affected by the Covid-19 pandemic and our need to match screening invites to our treatment capacity within the health service.”
In an interview with the Medical Independent last June, the programme’s interim Clinical Director Prof Pádraic MacMathuna said BowelScreen was planning to extend screening to 59-year-olds in 2022. He described colonoscopy capacity as the core issue that limited extension of the programme.
The National Cancer Strategy 2017-2026 stated that the HSE should ensure “appropriate” capacity to expand colorectal cancer screening to “all” 55-to-74-year-olds by the end of 2021.
First offered in October 2012, BowelScreen was established to provide screening initially to all eligible men and women aged 60-to-69 and ultimately to the full 55-to-74 age group. No expansion in the identified age range has
been implemented to date.
“Invitations are still affected by Covid-19, despite the lifting of restrictions in wider society. However, they are issued to ensure we can maximise the capacity available,” said the spokesperson.
“Some units have been impacted by the recent December and January wave of the Covid-19 virus. We anticipate that all units will return to normal operational capacity by the end of Q1, dependent on the situation with Covid-19 and continued easing of restrictions.”
There are no current plans to change the screening interval (FIT invite cycle). “The impact of Covid-19 means invitations in our current screening round (where we normally invite people for screening once every two years) are delayed by up to a year.”
This means if a person was due to be issued with a screening test kit in 2020, the programme aimed to issue their kit in 2021. If they were due to be invited to be screened in 2021, the programme aims to invite them in 2022.
“We are prioritising inviting people who have been waiting for screening longest, and new entrants to the screening programme.”
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 6
▸ Continued from p5 News
When a DPP-4 inhibitor is needed
Simplicity. Reinforced .
for a BROAD RANGE of adults with type 2 diabetes (T2D)
UNIQUE CONVENIENCE through always one dose, once daily 1 5mg once daily
Demonstrated
CV AND KIDNEY SAFETY PROFILE 2,3
PROVEN EFFICACY VS PLACEBO
for adults with T2D 1,4
References:
1. TRAJENTA® (linagliptin) Summary of Product Characteristics. SmPC available at: https://www.medicines.ie/
2. Rosenstock J, et al. JAMA. 2019;321:69–79
3. Rosenstock J, et al. Cardiovasc Diabetol. 2018;17:39
4. McGill JB, et al. Diabetes Care. 2013;36:237–44
Prescribing Information (Ireland) TRAJENTA® (Linagliptin)
Film-coated tablets containing 5 mg linagliptin. Indication: Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control as: monotherapy when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment; combination therapy in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control. Dose and Administration: 5 mg once daily. If added to metformin, the dose of metformin should be maintained and linagliptin administered concomitantly. When used in combination with a sulphonylurea or with insulin, a lower dose of the sulphonylurea or insulin, may be considered to reduce the risk of hypoglycaemia. Renal impairment: no dose adjustment required. Hepatic impairment: pharmacokinetic studies suggest that no dose adjustment is required for patients with hepatic impairment but clinical experience in such patients is lacking.
Elderly: no dose adjustment is necessary based on age. Paediatric population: the safety and ef cacy of linagliptin in children and adolescents has not yet been established. No data are available. The tablets can be taken with or without a meal at any time of the day. If a dose is missed, it should be taken as soon as possible but a double dose should not be taken on the same day. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and Precautions: Linagliptin should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis. Hypoglycaemia: Caution is advised when linagliptin is used in combination with a sulphonylurea and/or insulin; a dose reduction of the sulphonylurea or insulin may be considered. Acute pancreatitis: Acute pancreatitis has been observed in patients taking linagliptin. Patients should be informed of the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, Trajenta should be discontinued. If acute pancreatitis is con rmed, Trajenta should not be restarted. Caution
should be exercised in patients with a history of pancreatitis. Bullous pemphigoid: Bullous pemphigoid has been observed in patients taking Linagliptin. If bullous pemphigoid is suspected, Trajenta should be discontinued. Interactions: Linagliptin is a weak competitive and a weak to moderate mechanism-based inhibitor of CYP isozyme CYP3A4, but does not inhibit other CYP isozymes. It is not an inducer of CYP isozymes. Linagliptin is a P-glycoprotein substrate and inhibits P-glycoprotein mediated transport of digoxin with low potency. Based on these results and in vivo interaction studies, linagliptin is considered unlikely to cause interactions with other P-glycoprotein substrates. Effects of other medicinal products on linagliptin: The risk for clinically meaningful interactions by other medicinal products on linagliptin is low. Rifampicin: Multiple co-administration of 5 mg linagliptin with rifampicin, a potent inductor of P-glycoprotein and CYP3A4, decreased linagliptin steady state AUC and Cmax. Thus, full ef cacy of linagliptin in combination with strong P-glycoprotein inducers might not be achieved, particularly if administered long term. Coadministration with other potent inducers of P-glycoprotein and CYP3A4, such as carbamazepine, phenobarbital and phenytoin has not been studied. Effects of linagliptin on other medicinal products: In clinical studies linagliptin had no clinically relevant effect on the pharmacokinetics of metformin, glibenclamide, simvastatin, warfarin, digoxin or oral contraceptives (please refer to Summary of Product Characteristics for a full list of interactions and clinical data). Fertility, pregnancy and lactation: The use of linagliptin has not been studied in pregnant women. As a precautionary measure, avoid use during pregnancy. A risk to the breast-fed child cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from linagliptin therapy taking into account the bene t of breastfeeding for the child and the bene t of therapy for the woman.
No studies on the effect on human fertility have been conducted
for linagliptin. Undesirable effects: Adverse reactions reported in patients who received linagliptin 5 mg daily as monotherapy or as add-on therapies in clinical trials and from post-marketing experience. Frequencies are de ned as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) or very rare (<1/10,000). Adverse reactions with linagliptin 5 mg daily as monotherapy: Common: lipase increased. Uncommon: nasopharyngitis; hypersensitivity; cough; rash; amylase increased. Rare: pancreatitis; angioedema; urticaria; bullous pemphigoid. Adverse reaction with linagliptin in combination with metformin plus sulphonylurea: Very common: hypoglycaemia. Adverse reaction with linagliptin in combination with insulin: Uncommon: constipation. Prescribers should consult the Summary of Product Characteristics for further information on side effects. Pack sizes: 28 tablets. Legal category: POM. MA number: EU/1/11/707/003. Marketing Authorisation Holder: Boehringer Ingelheim International GmbH, D-55216 Ingelheim am Rhein, Germany. Prescribers should consult the Summary of Product Characteristics for full prescribing information. Additional information is available on request from Boehringer Ingelheim Ireland Ltd, The Crescent Building, Northwood, Santry, Dublin 9. Prepared in September 2021.
Adverse events should be reported. Reporting forms and information can be found at https:// www.hpra.ie/homepage/about-us/report-an-issue. Adverse events should also be reported to Boehringer-Ingelheim Drug Safety on 01 2913960, Fax: +44 1344 742661, or by e-mail: PV_local_UK_Ireland@boehringer-ingelheim.com
PC-IE-101415
professionals
Date of preparation: October 2021 This advertisement is intended for health care
practicing in Ireland only
HbA1c
IT gaps and governance review needed for future pandemic control – ISSPHM
DAVID LYNCH
A review of public health governance structures and addressing “longstanding” IT infrastructure deficits are among four key recommendations made by the Irish Society of Specialists in Public Health Medicine (ISSPHM) in its new position paper on pandemic control.
The document, titled For better pandemic control now and into the future , is the ISSPHM’s first ever position paper and is available on the Society’s website, which is now live (https://issphm.ie).
The ISSPHM was formally launched in December to represent the views of specialists in public health medicine in Ireland. Speaking at the online launch of the ISSPHM, Prof Breda Smyth, Interim Chair of the ISSPHM and Director of Public Health West, said the Society will “provide a strong cohesive voice for public health medicine”.
Last May, an historic agreement was signed between the HSE, Department of Health and IMO to establish 84 consultant in public health medicine posts, over three phases between June 2021 and December 2023.
The ISSPHM’s first position paper makes four recommendations to apply the “lessons learned from our collective experiences with Covid-19”.
Firstly, it recommends a review of “the governance structures for public health, incorporating all health protection functions from surveillance, to case and outbreak management, source identification, and contact tracing, as well as health improvement, health service improvement, and health intelligence”.
Secondly, the ISSPHM calls on the health authorities to “address longstanding and critical IT infrastructure gaps, including the lack of a case and outbreak management system, an integrated surveillance system, an immunisation reporting system”, as well as the development of a “fit for purpose” health information system capable of linking information across all service delivery sites.
Thirdly, the position paper recommends the further strengthening of “the capacities of regional departments of public health” beyond the current investment in health protection to enable the “delivery of the in -
Changes in HSE’s public health leadership
DAVID LYNCH
The HSE has confirmed to the Medical Independent (MI) a change in its public health leadership as a result of the retirement of Dr Kevin Kelleher last November.
Dr Kelleher held a number of significant roles within public health, including Assistant National Director and public health advisor to the Chief Clinical Officer 2020-21. The Executive told this newspaper that Dr Kelleher has been replaced in this role by Dr Mai Mannix, who is the Director of Public Health, HSE Midwest.
Dr Kelleher was also Acting Director of the Health Protection Surveillance Centre (HPSC) between 2016 and 2019.
Dr Kelleher’s retirement was discussed at meetings of the national flu planning steering group during the second half of last year, minutes of which MI has seen following a Freedom of Information request.
tegrated public health service required to support the full implantation of Sláintecare”.
The final of the four key recommendation in the position paper has a global scope. It argues Ireland should be promoted “as a global leader” for health systems recovery and resilience through the promotion of national and global policy that supports “proportionate investment in essential public health functions to promote health security and ensure economic and social prosperity”.
On the general experience of the last two years, the position paper notes that there was “no doubt that we were unprepared for Covid-19”, despite the known risk of a pandemic.
“Ireland is not alone in this, with more than 90 per cent of countries reporting disruptions to essential health services due to the pandemic,” according to the position paper.
“The truth is that Covid-19 has exposed the weaknesses in health systems globally, directly attributable in many cases to the chronic lack of investment in essential public health functions, including emergency planning and response, underpinned by a systems strengthening approach.”
The Society states that “given the likelihood of future and more devastating pandemics, we must learn from these lessons”.
It says the international experience has shown that “critical and longstanding weaknesses” in health systems “hindered the abilities of even well-resourced health systems to respond to this public health emergency”.
The weaknesses concerned information and surveillance systems, laboratory capacities, chronic under-investment and deprioritisation of essential public health functions including emergency preparedness and response, vaccine infrastructure and delivery systems, and an under-development of local response mechanisms and structures.
“As a result, lives and livelihoods were lost.”
The document notes that the Irish health system pre-pandemic was “experiencing significant challenges, many relating to chronic critical weaknesses within the health system”. This included a lack of capacity (including ICUs) within hospitals, “as well as limited capacities to support healthcare workers with infection prevention and control and occupational health services.” There were also “significant infrastructural deficits in IT, including the lack of an integrated health information system, the lack of suitable surveillance infrastructure, and the lack of a case and outbreak management system”.
While the document recognises the “significant and welcome investment in public health functions since the pandemic”, it states this “has come on the back of decades of under-investment, both regionally and nationally”.
“Despite all of this, there is no doubt that Ireland has performed well in many areas of the pandemic response so far,” the ISSPHM states.
The Society highlights such areas as the testing services, contact tracing system, “the tireless efforts of the medical and non-medical staff of regional departments of public health, to provide an un-contracted seven day a week service since the first wave of the pandemic in tandem with the contact management programme,” which resulted in the vast majority of cases being contact traced with the prevention of countless further cases.
In terms of risk communication, public information from the HSE was “rapidly developed, accessible, and available” and communication from the office of the Chief Medical Officer “was frequent, consistent, and clear”.
As well as his advisor role to the Chief Clinical Officer and position with the HPSC, Dr Kelleher was Assistant National Director of Health Protection, Public Health, Child Health 2005-2020.
“Dr Kelleher’s contribution to the HSE through these leadership roles has been wide-reaching and significant,” a HSE spokesperson told MI
In his role as advisor to the Chief Clinical Officer, “he provided public health expertise and leadership support to the HSE’s pandemic response.”
“He was a member of NPHET [national public health emergency team], the HSE’s national crisis management team, the public health senior management team, national flu planning steering committee, test and trace senior management team, and provided significant expertise, guidance, and support to our public health teams.”
Increase
in Department FoIs in 2021
DAVID LYNCH
The Department of Health saw an increase in Freedom of Information (FoI) requests last year, according to figures provided to this newspaper.
After a decline in requests in 2020 during the first year of the pandemic, the number increased in 2021.
In 2019 the Department received 582 requests, in 2020 it dropped to 480, while last year it increased by 200 to a total of 680.
A Department spokesperson told the Medical Independent (MI) “the vast majority of these are non-personal”.
“Since the introduction of the e-FoI system in late 2021, we have requested fees on two FoIs,” continued the spokesperson.
The HSE was not in a position to
provide figures for the number of FoI requests it received last year.
The 2021 data was being collated and validated and will be available on the Information Commissioner’s website in due course, an Executive spokesperson told MI

FoI requests to public bodies fell by 21 per cent during 2020, according to the Information Commissioner’s annual report for 2020. There were 31,591 requests made to public bodies in 2020, which was down 21 per cent on 2019.
Of these requests 40 per cent were for “Government and State bodies”, 27.7 per cent to the HSE, and 14.7 per cent to hospitals and mental health facilities. Half of all FoI requests were made by clients of public bodies, while 23 per cent were made by journalists.
8 THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 News
Genuair®-has it ‘clicked’ yet?

The ONLY prefilled inhaler with visual and audible feedback for confirmed dose delivery1-4
Genuair - a simple to use inhaler for patients with COPD4

Abbreviated Prescribing Information
Eklira® Genuair® 322 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and a green dosage button. Each delivered dose contains 375 µg aclidinium bromide equivalent to 322 µg of aclidinium. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).

Dosage: For inhalation use. Recommended dose is one inhalation of 322 micrograms aclidinium twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work differently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to aclidinium bromide or to any of the excipients. Warnings and Precautions: Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Use with caution in patients with symptomatic prostatic hyperplasia or bladder-neck obstruction or with narrow-angle glaucoma. Do not use in patients with rare hereditary problems of galactose intolerance, total lactose deficiency or glucose-galactose malabsorption.
Interactions: Do not administer with other anticholinergic-containing medicinal products. No other interactions expected. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Risk to newborns/infants cannot be excluded. Consider risk-benefit before using during lactation. Unlikely to affect fertility at the recommended dose. Side-effects: Common (1-10%): Sinusitis, nasopharyngitis, headache, cough, diarrhoea, nausea. Uncommon (0.1-1%): Dizziness, blurred vision, tachycardia, palpitations, dysphonia, dry mouth, stomatitis, rash, pruritus, urinary retention. Rare (0.01-0.1%): hypersensitivity. Not known: angioedema, anaphylactic reaction. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/12/778/002
Marketing Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: February 2020
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions to: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744.
Date of item: November 2020. IR-BRI-09-2020
Abbreviated Prescribing Information Brimica® Genuair® 340 micrograms/12 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and an orange dosage button. Each delivered dose contains 396 µg aclidinium bromide (equivalent to 340 µg of aclidinium) and 11.8 micrograms of formoterol fumarate dihydrate. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 340 µg/12 µg twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work differently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Warnings and Precautions: Do not use in asthma. Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Discontinue if increases in pulse rate, blood pressure or changes in ECG occur. Use with caution in patients with a history of or known prolongation of the QTc interval or treated with products affecting the QTc interval. Use with caution in patients with severe cardiovascular disorders, convulsive disorders, thyrotoxicosis and phaeochromocytoma. Hypokalaemia may occur, is usually transient and supplementation not needed. In patients with severe COPD, hypokalaemia may be potentiated by hypoxia and concomitant treatment. Use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Do not use in patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic and/or long-acting β2-adrenergic agonist containing medicinal products. Caution in use with methylxanthine derivatives, steroids, non-potassium-sparing diuretics, β-adrenergic blockers or medicinal products known to prolong the QTc interval. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Consider risk-benefit before using during lactation. Unlikely to affect fertility at the recommended dose. Side-effects: Common (1-10%): Nasopharyngitis, urinary tract infection, sinusitis tooth abscess, insomnia, anxiety, headache, dizziness, tremor, cough, diarrhoea, nausea, dry mouth, myalgia, muscle spasms, peripheral oedema, increased blood creatine phosphokinase. Uncommon (0.1- 1%): Hypokalaemia, hyperglycaemia, agitation, dysgeusia, blurred vision, tachycardia, electrocardiogram QTc prolonged, palpitations, angina pectoris, dysphonia, throat irritation, stomatitis, rash, pruritus, urinary retention, increased blood pressure. Rare (0.01-0.1%): Hypersensitivity, bronchospasm, including paradoxical. Not known: anaphylactic reaction, angioedema. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing

Authorisation Number: EU/1/14/963/001 Marketing Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: October 2019
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via
Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744. References: 1 MIMS Ireland November 2020 2. Eklira® Genuair® Summary of Product Characteristics, last updated November 2019 3 Brimica® Genuair® Summary of Product Characteristics, last updated August 2019 4 Magnussen, H et al. COPD. 2019 Apr;16(2):196-205
HPRA Pharmacovigilance,
LAMA + LABA
LAMA
Helping patients quit the habit
National stop smoking clinical guidelines have recently been launched. David Lynch speaks to the Chair of the group that developed the guidelines, Dr Paul Kavanagh, about the current evidence in reducing tobacco-related harm

Late last year, the Healthy Ireland Survey 2021 reported that 18 per cent of Irish adults currently smoke; 16 per cent smoke daily; and 2 per cent smoke occasionally.
It is estimated that 6,000 deaths per year in Ireland in recent years were attributed to smoking. In 2016, there were an estimated 55,000 hospital episodes (day case and inpatient) in publicly funded hospitals attributable to smoking and exposure to second-hand smoke.
Last month, the national clinical effectiveness committee released guidelines to help healthcare professionals assist adults to stop smoking.
The new guidelines were developed by a multidisciplinary guideline development group led by the HSE Tobacco Free Ireland Programme and chaired by Dr Paul Kavanagh, Specialist in Public Health Medicine.
“These new national clinical guidelines describe the evidence-based best practice for healthcare professionals providing people help to stop smoking.... we look forward to working with healthcare professionals and the public to implement these guidelines in day-to-day practice across the health services and working with them towards a tobacco-free Ireland," outlined Dr Kavanagh.
The drop in smoking levels in recent decades is widely regarded as a significant public health gain. Dr Kavanagh told the Medical Independent (MI) that "we're very proud of the huge progress we have made around controlling the amount of tobacco use in the population".
He pointed to statistics which showed that, two decades ago, smoking prevalence was as high as one-in-three in the population.
Dr Kavanagh added Ireland had witnessed the implementation of a "really ambitious, high impact programme of policy intervention", such as the bans on smoking in public and workplaces and the plain packaging reforms.
‘Hard won’
However, he said recent figures indicate that the reduction in smoking levels may have "stalled".
"I would say we do need to remain very alert, because those benefits have been very hard won and they are very precious from a public health perspective," he said.
Dr Kavanagh warned that there should be no "risk of complacency".
"While acknowledging the huge hard work and progress that has been made, I would urge caution that we need to stay on this issue. The progress that has been made could slip.”
To that end, Dr Kavanagh said the Government should review its Tobacco Free Ireland policy and then "pull together a very concrete and deliverable plan towards a tobacco-free Ireland".
Tobacco Free Ireland was launched in October 2013. The headline target was that Ireland would have a smoking prevalence rate of less than 5 per cent by 2025, effectively making the country a tobacco-free society.
Dr Kavanagh told MI the "reality" was that goal was "looking increasingly unrealistic".
He praised the "very bold and ambitious goal that Government set for itself in 2013", but added that there should be an interim "review" of that policy and goal.
"Now, at the same time I would say that shouldn't be a reason for despondency or that we should back out of that goal," added Dr Kavanagh, who said the 5 per cent goal should remain but the timeline should be reviewed.
“Our advice to the Department of Health and the Minister for Health would be that it is very timely given where we
are with Covid and given the findings of the most recent Healthy Ireland Survey to have an interim review of that goal. I'm not for a moment saying it is the wrong goal to have," he said.
“We need to match that goal and the ambition of that goal with a bold programme of endgame planning where we systematically identify the steps that we are going to take over a period of time to achieve that goal."
Pandemic
Dr Kavanagh's concern over a possible stalling in the reduction in tobacco prevalence in the population is based on the Healthy Ireland Survey figure of 18 per cent from last year,
which was 1 per cent more than the figure in 2019.
He noted that the "apparent" stalling "could be a blip in the statistics" from the recent survey. However, Dr Kavanagh added that the Central Statistics Office and international statistics showed that Covid-19 had impacted the smoking behaviour across the population "in a way that has probably been very mixed".
While some people have quit, others have relapsed due to the added stress and disruption to their lives in the pandemic.
He also noted that stop smoking services had been impacted, like all non-Covid services in the health service. Particularly in 2021, "there was a downturn of the people who accessed our phone support, certainly our face-toface support, were affected... as well." However, services are now available again.
With the fall in the number of people who smoke, there is the "concept of diminishing returns at the margins”, stated Dr Kavanagh.
"The effort of imagination that was required to reduce smoking prevalence across the population from what it was over 20 years ago when it was one-in-three people – to get it down to 18 per cent, I think we will require a multiple of that imagination and effort to bring smoking down to 10 per cent, never mind bring it down to the 2025 [target of] 5 per cent," Dr Kavanagh said.
Due to the general focus on Covid, there is a risk "that people might think that tobacco control is done and dusted. I think there is clearly an imperative that we continue to progress.”
On the new guidelines Dr Kavanagh said they "have a very wide application" across healthcare. But he agreed that primary care and GPs in particular “can play a really powerful role in working with people who smoke". Work has already commenced with the ICGP on the issue.
‘Tried and tested’ supports and guidance
Smoking is the "leading preventable cause of disease, disability and premature death in Ireland," according to Dr Paul Kavanagh, Specialist in Public Health Medicine.

"Yet there are three simple but powerful steps which every healthcare professional can take to maximise the chance that someone who smokes can successfully stop – asking about smoking; offering advice to stop; and providing practical support through arranging referral to a stop smoking advisor and stop smoking medicines."
The guidelines also advise that for people " who are currently interested in quitting, all healthcare professionals should recommend that behavioural support, either alone or in combination with pharmacological supports, increases the chances of successful quitting".
Healthcare professionals should recommend "varenicline (alone or in combination with nicotine replacement therapy (NRT)) as first-line treatment in the absence of a contraindication for those wishing to use pharmacological support". The guidelines further note that if varenicline is not suitable, combination NRT treatment should be recommended.
There are further recommendations on aspects dealing with pregnancy and mental health facilities.
E-cigarettes are mentioned in the guidelines, but they are not part of the recommendations. The guidelines note that they are "consumer products”.
“There is some regulation in place to protect
consumers of e-cigarettes, but not the same quality and safety system as would be in place for a licensed drug or medical device,” according to the document.
Dr Kavanagh told the Medical Independent that in other jurisdictions, such as the UK, medicine regulators are "increasingly interested in taking on a role in regulation e-cigarettes".
If the Health Products Regulatory Authority was to regulate e-cigarettes in the future, the considerations of the group may be different.
Currently, according to Dr Kavanagh, there is "a lot of ambiguity in the area" of e-cigarettes.
"It is evolving, it is very contested, and I think it's fair to acknowledge even across the tobacco control community in Ireland and internationally there are people who have different views on this... so this was one of the more challenging areas we had to address.”
He said that the evidence from studies on e-cigarettes was mixed. "We had to step back and look at the situation. Here we are with a tool kit that is already full of safe, effective, tried, and tested stop smoking supports... [but with e-cigarettes] we have all these questions and all these concerns.”
"From our perspective it was very hard to see how you would take a risk on something that wasn't proven, tried, and tested, when actually you have a whole set of interventions that are very tried and tested."
*A summary and the full guidelines can be found at www.gov.ie/en/publication/4828b-stop-smoking/
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 10
News Interview
Dr Paul Kavanagh
Lipocomb
GET LOWER
ESC TREATMENT TARGETS1
CV risk LDL-C target High** <1.8mmol/L
Very High* <1.4mmol/L
Lipocomb® (rosuvastatin (as zinc)/ezetimibe) Abbreviated Prescribing Information: Please refer to the Summary of Product Characteristics before prescribing. COMPOSITION* Lipocomb 10 mg/10 mg: hard capsule containing 10 mg rosuvastatin (as zinc) and 10 mg of ezetimibe. Lipocomb 20 mg/10 mg: hard capsule contains 20 mg rosuvastatin (as zinc) and 10mg of ezetimibe.
INDICATIONS*As adjunct to diet for treatment of primary hypercholesterolemia as substitution therapy in adult patients adequately controlled with the individual substances given concurrently at the same dose level as in the fixed dose combination, but as separate products. DOSAGE AND ADMINISTRATION* Recommended daily dose is one capsule of the given strength with or without food. Lipocomb is not suitable for initial therapy. Treatment initiation or dose adjustment if necessary, should only be done with the monocomponents and after setting the appropriate doses the switch to the fixed dose combination of the appropriate strength is possible. Lipocomb 10 mg/10 mg and 20 mg/10mg hard capsules are not suitable for the treatment of patients requiring 40 mg dose of rosuvastatin Children and adolescents: < 18 years should not be used. Elderly (age>70 years), moderate renal impairment (Clcr < 60 ml/min), race (Asian ancestry) and predisposing factors to myopathy: Start dose of 5 mg rosuvastatin is recommended. Mild to moderate renal impairment: no dose adjustment. Severe renal impairment (Clcr <30 ml/min): contraindicated. Hepatic impairment: Mild hepatic impairment (Child Pugh score 5 to 6): no dose adjustment. Moderate (Child Pugh score 7 to 9) or severe (Child Pugh score >9) liver dysfunction: not recommended. Active liver disease: contraindicated.
Genetic polymorphisms: a lower daily dose is recommended. Co-administration: contraindicated with ciclosporin, not recommended with combinations of ritonavir with atazanavir, lopinavir, and/or tipranavir (full list in section INTERACTIONS* below). CONTRAINDICATIONS* Hypersensitivity to the active substances (rosuvastatin, ezetimibe) or to any of the excipients, active liver disease including unexplained, persistent elevations of serum transaminases and any serum transaminase elevation exceeding 3 times the upper limit of normal (ULN), during pregnancy and breast-feeding and in women of childbearing potential not using appropriate contraceptive measures, severe renal impairment (creatinine clearance <30 ml/min), myopathy, concomitant ciclosporin. (see
WARNINGS*
INTERACTIONS* and PROPERTIES*). WARNINGS *Skeletal Muscle
Effects: Stop treatment if muscular symptoms with elevation of CK level > 5 xULN occur or if muscular symptoms are severe and cause daily discomfort (even if CK levels are ≤ 5 x ULN). Caution should be exercised when Lipocomb is used with fibric acid derivatives including gemfibrozil, ciclosporin, nicotinic acid, azole antifungals, protease inhibitors and macrolide antibiotics. Increased risk of myopathy with concomitant use of gemfibrozil. Co-administration with gemfibrozil is not recommended. Co-administration with systemic formulations of fusidic acid or within 7 days of stopping the treatment is not recommended. If the use is essential, Lipocomb

should be discontinued during fusidic acid treatment. Liver effects: Liver functions tests should be carried out 3 months following the initiation of rosuvastatin treatment. Stop treatment or reduce the dose if jaundice, hepatitis or marked elevation of hepatic enzymes (serum transaminases exceeding 3 times the upper limit of normal). Lipocomb use is not recommended in patients with moderate to severe hepatic impairment. Renal effects (proteinuria): Monitoring by dipstick testing recommended for patients treated with higher doses of rosuvastatin, in particular 40 mg. Race: Lipocomb may cause an increase in exposure in Asian subjects compared with Caucasians. Protease inhibitors: Concomitant use with certain protease inhibitors is not recommended unless adjustment. Interstitial lung disease: If suspected, treatment should be discontinued. Diabetes mellitus: Patients at risk (fasting glucose 5.6 to 6.9 mmol/L, BMI > 30 kg/m2, raised triglycerides, hypertension) should be monitored. Fibrates: If cholelithiasis is suspected in case of concomitant use with fenofibrate, treatment should be discontinued. Anticoagulants: If Lipocomb is added to warfarin, another coumarin anticoagulant, or fluindione, the INR should be appropriately monitored. Ciclosporin: Lipocomb is contraindicated in patients receiving concomitant ciclosporin. Paediatric population: not recommended in children < 18 years. Liver disease and alcohol: Use with caution in patients who consume excessive quantities of alcohol and/or have history of liver disease. INTERACTION(S)*Contraindicated: Ciclosporin. Not recommended combinations: protease inhibitors (e.g.atazanavir / ritonavir), transporter protein inhibitors (hepatic uptake transporter OATP1B1 / efflux transporter BCRP), gemfibrozil and other lipid-lowering products (fibrates and niacin(nicotinic acid)), fusidic acid. Precautions: Antacid, erythromycin, cytochrome P450 enzymes(inhibitor / inducer), vitamin K antagonists (e.g. warfarin or another coumarin anticoagulant), oral contraceptive/hormone replacement therapy (HRT), colestyramine, statins (atorvastatin, simvastatin, pravastatin, lovastatin, fluvastatin or rosuvastatin), other medicinal products(dapsone, dextromethorphan, digoxin, glipizide, tolbutamide, midazolam, cimetidine). PREGNANCY AND BREASTFEEDING*: Lipocomb is contraindicated in pregnancy and breastfeeding. FERTILITY* CONTRACEPTION* Women of childbearing potential should use appropriate contraceptive measures. DRIVE & USE MACHINES* Dizziness may occur. UNDESIRABLE EFFECTS* Common: Diabetes mellitus, headache, dizziness, constipation, nausea, abdominal pain, diarrhoea, flatulence, myalgia, asthenia, fatigue, ALT and/or AST increased. Uncommon: Decreased appetite, paraesthesia, hot flush, hypertension, cough, dyspepsia, gastrooesophageal reflux disease, dry mouth, gastritis, pruritus, rash, urticaria, arthralgia, muscle spasms, neck pain, back pain, muscular weakness, pain in extremity, chest pain, pain, oedema peripheral. Rare: Thrombocytopenia, hypersensitivity reactions including angioedema, pancreatitis, myopathy (including myositis), rhabdomyolysis, lupuslike syndrome, muscle rupture. Very rare: Polyneuropathy, memory loss, jaundice, hepatitis, arthralgia, haematuria, gynecomastia. Not Known: Hypersensitivity
(including anaphylaxis and angioedema), depression, peripheral neuropathy, sleep disturbances (including insomnia and nightmares), dyspnea, cholelithiasis, cholecystitis, Stevens Johnson syndrome, erythema multiforme, immune-mediated necrotising myopathy, tendon disorders, sometimes complicated by rupture. OVERDOSE*. PROPERTIES* Rosuvastatin is a selective and competitive inhibitor of HMG CoA reductase, the rate-limiting enzyme that converts 3-hydroxy3-methylglutaryl coenzyme A to mevalonate, a precursor for cholesterol. Ezetimibe is in a new class of lipid-lowering compounds that selectively inhibit the intestinal absorption of cholesterol and related plant sterols. PRESENTATION* Packs of 30hard capsules. Marketing Authorisation Holder: EGIS Pharmaceuticals PLC, Kereszturi ut 30-38, H-1106 Budapest, Hungary. Marketing Authorisation Number: PA1470/004/001002. Legal Classification for Supply: POM. Local Representative in Ireland: Servier Laboratories (Ireland) Ltd, Second Floor, 19 Lr. George’s Street, Dun Laoghaire, Co. Dublin A96 ER84, Ireland. Tel (01) 6638110, www.servier.ie.
*For complete information, please refer to the Summary of Product Characteristics available on www.medicines.ie
Date of last revision of the text: June 2021 (Date of last approved
SmPC: March 2019) Date of Preparation September 2021, 2122 C1 LCB Press Ad CBU.
* For complete information, please refer to the complete Summary of Product Characteristics for Lipocomb® at www.hpra.ie
1. Mach et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias, European Heart Journal (2019) 00, 1-78’ * Patients with: documented CVD, Diabetes Mellitus with organ damages, Severe CKD (chronic kidney disease), a calculated 10 year risk of fatal CVD ≥ 10%, Familial Hypercholesterolaemia with CVD/risk factor. **Patients with: Markedly elevated single risk factors (in particular cholesterol > 8mmol/L or BP ≥ 180/110 mmHg), Familial Hypercholesterolaemia, Diabetes Mellitus, Moderate CKD (chronic kidney disease), a calculated 10 year risk of fatal CVD ≥ 5% and <10%.
Rosuvastatin / Ezetimibe
Get your patient’s LDL-C down even lower to <1.8mmol/L1
Biden’s healthcare reforms in the balance
US President Joe Biden begins his second year in office fighting for the survival of his healthcare agenda in a deeply divided Congress and with time running out before congressional elections make the task even tougher.
The President’s Democratic party now holds a slim majority in both the House of Representatives and the Senate, but if Republicans win back control of both chambers in congressional elections in November, then Biden’s agenda will be stalled, or even doomed.

Democrats are also nervous about the trends of political history when it comes to midterm elections, which are held two years after the presidential election. Historically, the party that controls the White House frequently loses seats in Congress in the midterm election. It happened during the recent presidencies of Donald Trump, Barack Obama, and Bill Clinton. Therefore 2022 is shaping up to be a make-or-break year for Biden’s healthcare agenda.
Build Back Better
The healthcare changes the President wants Congress to enact are largely contained in his ‘Build Back Better’ Bill. Its provisions, which were passed in the House of Representatives late last year but remain stalled in the Senate, would herald sweeping advances in healthcare and social policies and propel the country closer than ever before to the goal of universal health coverage.
The plan is replete with measures that appeal to most Americans, according to polls. In one survey, 66 per cent of respondents including 61 per cent of independents and 39 per cent of Republicans said they supported the Build Back Better plan. However, at the end of 2021, another poll found support had dipped along with the President’s popularity, as the Covid-19 pandemic showed little sign of ending.
Politicised pandemic
Legal and political setbacks in his battle against Covid are seen as impacting the President’s wider healthcare agenda and weakening his political clout in pushing it through Congress. Indeed, some Democrats contend that for many Republicans this is part of their motivation for opposing the President’s push to get more Americans vaccinated.
Such politicisation of the pandemic was evident back in the summer of last year at a Conservative Political Action Conference in Texas, when supporters of President Biden’s predecessor, Donald Trump, cheered when a speaker said the Biden administration had failed to reach its target of getting 90 per cent of the country vaccinated.
Since then, however, Trump has urged the unvaccinated to take the jab, although his exhortations were often met with jeers from his supporters. Meanwhile, in this increasingly toxic atmosphere, the country’s death toll from the disease stood at over 850,000 people on the first anniversary of President Biden’s election.
This has left the President fighting a two-pronged battle – one against Covid and the other against opponents of his Build Back Better Bill. Its provisions would make health insurance more affordable for adults, expand healthcare access for children and improve benefits for older people and those with disabilities. It includes an expanded child tax credit that would give parents up to $300 (€260) per child per month.
The provisions, which would also boost investment in hous-
ing and implement measures to combat climate change, may not seem particularly radical. However, they would represent major advances, building on President Obama’s Affordable Care Act (ACA) a decade ago that insured millions.
The Biden plan would reduce premiums for more than nine million Americans who buy insurance through the Affordable Care Act by an average of $600 (€525) per person per year. For example, a family of four earning $80,000 (€70,000) per year would save nearly $3,000 (€2,270) per year on insurance premiums. Experts predict that more than three million people who would otherwise be uninsured will gain health coverage under the President’s plan.
framework will be the largest expansion of healthcare coverage since the Affordable Care Act.”

The administration said the plan would save most families more than half of their spending on childcare, deliver two years of free preschool for every three- and four-year-old, give more than 35 million families a major tax cut by extending the expanded Child Tax Credit, and expand access to homecare for older Americans and people with disabilities.
Costs
Yet for all its popularity among voters, a tug of war over its provisions has ensued in Congress, with opposition Republicans opposed to its $1.75 trillion price tag. Many are the same Republicans who enthusiastically backed tax cuts for the very wealthy in 2017 during Trump’s presidency, but are not happy this time because Biden wants to pay for his plan by increasing taxes on the largest corporations and the wealthiest Americans.
The administration stresses, however, that only those making over $400,000 (€350,000) would have to pay more taxes if the plan is passed.
President Biden had hoped to pass the Bill in the Senate before Christmas, but failed to do so when Democratic Senator Joe Manchin dramatically withdrew his support at the last minute and demanded changes to the plan. Voting was then postponed.
Progressive Democrats oppose any further changes to the Bill, citing Congressional Budget Office estimates that even if the Bill is passed, more than 27 million people would still remain uninsured including many undocumented immigrants.
“While the historic Affordable Care Act reduced the number of uninsured Americans by more than 20 million, extended critical consumer protections to more than 100 million people, and strengthened and improved the nation’s healthcare system, too many Americans continue to struggle to afford care,” according to a White House briefing.
“About 30 million people were uninsured in 2019 before President Biden took office, and coverage under the ACA (even with the premium subsidies) was too expensive for many families. In addition to investing in protecting Americans from future pandemics, the Build Back Better framework will expand access to affordable coverage. In all, the
The stalemate is causing President Biden problems on two fronts. It is highlighting his inability – so far at least – to get all the members of his party on board, thus weakening his authority in Congress and threatening passage of his agenda. Indeed, Senate Democrats are now beginning to realise they may have to accept a smaller list of accomplishments than what they hoped for at the start of last year when President Biden took office.
Voters are also increasingly questioning the President’s lack of progress. A University of Massachusetts Amherst/YouGov poll on 11 January, a week before the anniversary of his first
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 12
News Feature Continued on p14 ▸
This is shaping up to be a make-or-break year for US President Joe Biden’s ambitious plans for healthcare, with the Covid-19 pandemic still dominating the agenda. Bette Browne reports
In addition to investing in protecting Americans from future pandemics, the Build Back Better framework will expand access to affordable coverage
US President Joe Biden
For patients not adequately controlled on dual therapy with moderate to severe COPD
UNLEASH THE PROTECTION OF TRIXEO1,2
Significant protection against exacerbations*
TRIXEO Aerosphere is indicated as a maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid and a long-acting beta2-agonist or combination of a long-acting beta2-agonist and a long-acting muscarinic antagonist.1
*Significant reductions in the rate of moderate or severe exacerbations vs LAMA/LABA (24%, n=2137 vs n=2120, annual rates 1.08 vs 1.42, 95% CI 0.69–0.83; p<0.001) and ICS/LABA (13%, n=2137 vs n=2131, annual rates 1.08 vs 1.24, 95% CI 0.79–0.95; p=0.003).1,2 COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist. In the clinical trial programme for TRIXEO, LAMA/LABA refers to glycopyrronium/formoterol fumarate and ICS/LABA refers to budesonide/formoterol fumarate. ©AstraZeneca 2021. All rights reserved.
ABRIDGED PRESCRIBING INFORMATION
TRIXEO AEROSPHERE ® 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension (formoterol fumarate dihydrate/glycopyrronium/budesonide)
Consult Summary of Product Characteristics (SmPC) before prescribing
Indication: Trixeo Aerosphere is indicated as a maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid and a long acting beta2 agonist or combination of a long-acting beta2 agonist and a long acting muscarinic antagonist.
Presentation: Pressurised inhalation, suspension. Each single actuation (delivered dose, ex-actuator) contains 5 micrograms of formoterol fumarate dihydrate, glycopyrronium bromide 9 micrograms, equivalent to 7.2 micrograms of glycopyrronium and budesonide 160 micrograms.
Dosage and Administration: The recommended and maximum dose is two inhalations twice daily (two inhalations morning and evening). If a dose is missed, take as soon as possible and take the next dose at the usual time. A double dose should not be taken to make up for a forgotten dose. Special populations: Elderly: No dose adjustments required in elderly patients.
Renal impairment: Use at recommended dose in patients with mild to moderate renal impairment. Can also be used at the recommended dose in patients with severe renal impairment or end-stage renal disease requiring dialysis, only if expected benefit outweighs the potential risk. Hepatic impairment: Use at recommended dose in patients with mild to moderate hepatic impairment. Can also be used at the recommended dose in patients with severe hepatic impairment, only if expected benefit outweighs the potential risk. Paediatric Population: No relevant use in children and adolescents (<18 years of age). Method of administration: For inhalation use.
To ensure proper administration of the medicinal product, the patient should be shown how to use the inhaler correctly by a physician or other healthcare professional, who should also regularly check the adequacy of the patient’s inhalation technique. Patients who find it difficult to coordinate actuation with inhalation may use Trixeo Aerosphere with a spacer to ensure proper administration of the medicinal product..
Contraindications: Hypersensitivity to the active substances or to any of the excipients.
Warnings and Precautions: Not for acute use: Not indicated for treatment of acute episodes of bronchospasm, i.e. as a rescue therapy. Paradoxical bronchospasm: Administration of formoterol/glycopyrronium/budesonide may produce paradoxical bronchospasm with an immediate wheezing and shortness of breath after dosing and may be life threatening. Treatment should be discontinued immediately if paradoxical bronchospasm occurs.
Assess patient and institute alternative therapy if necessary. Deterioration of disease: Recommended that treatment should not be stopped abruptly. If patients find the treatment ineffective, continue treatment but seek medical attention. Increasing use of reliever bronchodilators indicates worsening of the underlying condition and warrants reassessment of the therapy. Sudden and progressive deterioration in the symptoms of COPD is potentially life threatening, patient should undergo urgent medical assessment.
Cardiovascular effects: Cardiovascular effects, such as cardiac arrhythmias, e.g. atrial fibrillation and tachycardia, may be seen after the administration of muscarinic receptor antagonists and sympathomimetics, including glycopyrronium and formoterol. Use with caution in patients with clinically significant uncontrolled and severe cardiovascular disease such as unstable ischemic heart disease, acute myocardial infarction, cardiomyopathy, cardiac arrhythmias and severe heart failure. Caution should also be exercised when treating patients with known or suspected prolongation of the QTc interval (QTc > 450 milliseconds for males or > 470 milliseconds for females), either congenital or induced by medicinal products. Systemic corticosteroid effects: May occur with any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur with
inhalation treatment than with oral corticosteroids. Systemic effects include Cushing’s syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, cataract and glaucoma. Potential effects on bone density should be considered particularly in patients on high doses for prolonged periods that have co existing risk factors for osteoporosis. Visual disturbances: May be reported with systemic and topical corticosteroid use. If patient presents symptoms such as blurred vision or other visual disturbances, consider ophthalmologist referral for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR). Transfer from oral therapy: Care is needed in patients transferring from oral steroids, since they may remain at risk of impaired adrenal function for a considerable time. Patients who have required high dose corticosteroid therapy or prolonged treatment at the highest recommended dose of inhaled corticosteroids, may also be at risk. These patients may exhibit signs and symptoms of adrenal insufficiency when exposed to severe stress. Additional systemic corticosteroid cover should be considered during periods of stress or elective surgery. Pneumonia in patients with COPD: An increase in the incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. Remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia include current smoking, older age, low body mass index (BMI) and severe COPD. Hypokalaemia: Potentially serious hypokalaemia may result from ß2-agonist therapy. This has potential to produce adverse cardiovascular effects. Caution is advised in severe COPD as this effect may be potentiated by hypoxia. Hypokalaemia may also be potentiated by concomitant treatment with other medicinal products which can induce hypokalaemia, such as xanthine derivatives, steroids and diuretics. Hyperglycaemia: Inhalation of high doses of ß2-adrenergic agonists may produce increases in plasma glucose. Blood glucose should be monitored during treatment following established guidelines in patients with diabetes.
Co-existing conditions: Use with caution in patients with thyrotoxicosis. Anticholinergic activity: Due to anticholinergic activity, use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Patients should be informed about the signs and symptoms of acute narrow-angle glaucoma and should be informed to stop using this medicinal product and to contact their doctor immediately should any of these signs or symptoms develop. Co-administration of this medicinal product with other anticholinergic containing medicinal products is not recommended. Renal impairment: Patients with severe renal impairment (creatinine clearance of <30 mL/min), including those with end-stage renal disease requiring dialysis, should only be treated with this medicinal product if the expected benefit outweighs the potential risk. Hepatic impairment: In patients with severe hepatic impairment, use only if the expected benefit outweighs the potential risk. These patients should be monitored for potential adverse reactions..
Drug Interactions: Co-treatment with strong CYP3A inhibitors, e.g. itraconazole, ketoconazole, HIV protease inhibitors and cobicistat-containing products are expected to increase the risk of systemic side effects. Should be avoided unless the benefit outweighs the increased risk, in which case patients should be monitored for systemic corticosteroid adverse reactions.
This is of limited clinical importance for short-term (1-2 weeks) treatment.
Since glycopyrronium is eliminated mainly by the renal route, drug interaction could potentially occur with medicinal products affecting renal excretion mechanisms. Other antimuscarinics and sympathomimetics: Coadministration with other anticholinergic and/or long-acting ß2-adrenergic agonist containing medicinal products is not recommended as it may potentiate known inhaled muscarinic antagonist or ß2-adrenergic agonist adverse reactions. Concomitant use of other beta-adrenergic medicinal products can have potentially additive effects. Caution required when prescribed concomitantly with formoterol. Medicinal product-induced hypokalaemia: Possible initial hypokalaemia may be potentiated by xanthine
derivatives, steroids and non potassium sparing diuretics. Hypokalaemia may increase the disposition towards arrhythmias in patients who are treated with digitalis glycosides. β -adrenergic blockers: ß-adrenergic blockers (including eye drops) can weaken or inhibit the effect of formoterol. Concurrent use of ß-adrenergic blockers should be avoided unless the expected benefit outweighs the potential risk. If required, cardio-selective ß-adrenergic blockers are preferred. Other pharmacodynamic interactions: Concomitant treatment with quinidine, disopyramide, procainamide, antihistamines, monoamine oxidase inhibitors, tricyclic antidepressants and phenothiazines can prolong QT interval and increase the risk of ventricular arrhythmias.
L-dopa, L-thyroxine, oxytocin and alcohol can impair cardiac tolerance towards beta2-sympathomimetics. Concomitant treatment with monoamine oxidase inhibitors, including medicinal products with similar properties such as furazolidone and procarbazine, may precipitate hypertensive reactions. Elevated risk of arrhythmias in patients receiving concomitant anaesthesia with halogenated hydrocarbons.
Pregnancy and Lactation: Administration to pregnant women/women who are breast-feeding should only be considered if the expected benefit to the mother justifies the potential risk to the foetus/child.
Ability to Drive and Use Machines: Dizziness is an uncommon side effect which should be taken into account.
Undesirable Events: Consult SmPC for a full list of side effects. Common (≥ 1/100 to < 1/10): Oral candidiasis, pneumonia, hyperglycaemia, anxiety, insomnia, headache, palpitations, dysphonia, cough, nausea, muscle spasms, urinary tract infection. Uncommon (≥ 1/1,000 to < 1/100): Hypersensitivity, depression, agitation, restlessness, nervousness, dizziness, tremor, angina pectoris, tachycardia, cardiac arrhythmias (atrial fibrillation, supraventricular tachycardia and extrasystoles), throat irritation, bronchospasm, dry mouth, bruising, urinary retention, chest pain. Very Rare (< 1/10,000): Signs or symptoms of systemic glucocorticosteroid effects, e.g. hypofunction of the adrenal gland, abnormal behaviour. Not known: Angioedema, vision blurred, cataract, glaucoma.
Product subject to prescription which may be renewed (B)
Legal Category:
Marketing Authorisation Number: EU/1/20/1498/002 120 actuations
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje, Sweden.
Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, Block B, Liffey Valley Office Campus, Dublin 22. Tel: +353 1 609 7100.
TRIXEO and AEROSPHERE are trademarks of the AstraZeneca group of companies.

Date of API preparation: 10/2021
Veeva ID: IE-3166
Adverse events should be reported directly to; HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
1. TRIXEO AEROSPHERE 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension. Summary of Product Characteristics. Available at www.medicines.ie
2. Rabe KF et al. Triple inhaled therapy at two glucocorticoid doses in modeate-to-very-severe COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/ NEJMoa1916046 COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046
Veeva ID: IE-3300 Preparation Date: November 2021
J13768 AZ Trixeo Advert IE-3300 250x346.indd 1 22/11/2021 10:11
Continued from p12 ▸
year in office, indicated that 55 per cent of adults felt he had “fallen short of expectations”.
Yet the hard reality for Democrats is that they have the narrowest of majorities in the Senate – 50-50 with the Vice President breaking ties in favour of Democrats – so the battle to improve access to healthcare and implement social reforms was always going to be tough for President Biden.
Sweeping changes usually only get passed through Congress when a President has big numbers in both chambers.
President Biden also faces another major challenge. The toxic political climate in America in 2022 makes change even tougher than for any previous President in modern times. For a start, he was the first President to be inaugurated after a coup attempt by his predecessor’s supporters who tried to prevent Congress from ratifying the election two weeks before he was due to assume office.
At a rally before the attack on Congress, Trump told his supporters that the election had been “stolen” from him and declared: “We fight like hell. And if you don't fight like hell, you're not going to have a country anymore.” A week after the riot, the House of Representatives impeached Trump for incitement of insurrection. The 6 January coup attempt was widely seen as one of the most dangerous threats ever faced by America’s democracy and it was in this political cauldron that Biden began his presidency just a year ago.
Covid-19 measures
At the same time, America, like the rest of the world, was also reeling from the Covid-19 pandemic. President Biden made the fight against the disease his top priority when he took office. “(This is) the worst pandemic in a century,” the President said in his first address to a joint session in April 2021. “The worst economic crisis since the Great Depression. The worst attack on our democracy since the Civil War.” But within months the battle against the disease had become politicised and Biden soon began to face political and legal challenges to his plans to fight the disease.
Still, he notched a number of impressive early wins. In his first days in office he signed a series of executive orders, including measures to ramp up vaccine manufacturing and distribution. At his urging, Congress poured tens of billions of dollars into state and local public health departments in response to the pandemic, paying for masks, contact tracers, and education campaigns to persuade people to get vaccinated.
He then pushed for immediate coronavirus legislation on a Covid relief package. This $1.9 trillion (€1.6 trillion) relief Bill, which was subsequently passed in Congress, provided for direct payments to support Americans hit by the effects of the pandemic, and also enhanced unemployment benefits and rental assistance.
It was the biggest piece of social and economic legislation since the 1960s and was widely seen as the major achievement for the President during his first 100 days. An NBC poll around that time showed 81 per cent of independents approved of how President Biden was handling the pandemic. But alarm bells were already ringing because the Bill was passed without a single Republican voting in favour.
Soon vaccine hesitancy, especially in Republican-controlled states, became a huge problem. Millions refused to get vaccinated or wear masks. Vaccine mandates were challenged by Republican governors in many states and legal challenges followed in the courts.
This year began with a major setback for President Biden’s plan to boost the number of people vaccinated when the Supreme Court ruled on 13 January against vaccine-or-test rules for businesses. This means that private businesses can now decide for themselves whether to impose a mandate. But without the legal cover, it is unlikely that many will do so, especially in Republican-led states that have made mandates illegal. A federal ruling would have overridden state policies allowing businesses to keep their mandates in place.
Republicans hailed the ruling. Louisiana Congressman Steve Scalise, in a tweet, congratulated the Supreme Court for blocking “Biden's authoritarian vaccine mandate for businesses. But let's be clear: This doesn’t go far enough. Healthcare workers should not be subjected to this tyrannical mandate either.”
Health experts in the US, however, argue that mandates work in getting more people vaccinated. They fear that without
one for some 80 million workers, more people will be at risk of getting hospitalised and dying.
“It is now highly unlikely that the US will hit the 85-to-90 per cent of Americans vaccinated to get to the other side of the pandemic,” tweeted Dr Celine Gounder, an infectious disease specialist and former member of the Biden administration's team.

President Biden has also indicated he is running out of patience with Americans who refuse to get vaccinated, even when offered inducements including cash giveaways and sweepstake prizes like cruises.
In remarks at the White House on 13 January, he again stressed that this was now a pandemic of the unvaccinated. “Right now, both vaccinated and unvaccinated people are testing positive, but what happens after that could not be more different,” he warned.
Mr Lawrence Gostin, a Public Health Law Professor at Georgetown University, said President Biden should mandate Covid vaccines for domestic travel.
According to the US publication, The Hill, Mr Gostin said: “He has wide authority over regulating the travel industry, and he’s already lawfully required masks in airports and on planes. He could do the same thing with vaccines, just like he requires it for international travellers.”
But airlines and other business groups oppose any vaccine and testing requirement for domestic air travel.
According to data from the Centres for Disease Control and Prevention (CDC), about 67 per cent of the eligible US population is fully vaccinated. The remaining group of unvaccinated
includes a term or condition that undermines efforts to stop the spread of Covid-19.
Arizona’s Republican Governor Doug Ducey will now have 60 days to redirect federal funds to eligible users or change the state programmes so they are in compliance.

If not, the federal government said it will move to recover the relief money. In a statement posted on Twitter, Governor Ducey countered by accusing the President of being “out of touch with the American people”.
He wrote: "When it comes to education, President Biden wants to continue focusing on masks. In Arizona, we’re going to focus on math and getting kids caught up after a year of learning loss."
Meanwhile, amid this increasingly toxic atmosphere, the country’s death toll from the disease has continued to soar in many states, not least Arizona where it is on track to be the leading cause of death in the state. The Arizona Department of Health Services reported an unofficial total of 24,229 Covid deaths as of 31 December 2021, in the state of seven million people. The surging numbers are a change from 2020, when the virus was the third-leading cause of death behind heart disease and cancer.
Unexpected medical charges
But the New Year also saw some positive healthcare news for President Biden when a ban on unexpected medical charges went into effect. The ‘No Surprises’ Act will apply to about 10 million such charges a year, according to federal estimates.
The new law protects patients when they receive emergency care or scheduled treatment from doctors and hospitals that are not in their insurance networks and that they did not choose. Consumers would be responsible only for their in-network cost-sharing in these situations.
Two-in-three adults say they worry about unexpected charges, according to the KFF. About one-in-five emergency room visits and up to one-in-six in-network hospitalisations include at least one surprise out-of-network bill. Patients can be hit with more than $1,200 (€1,000), on average, for anaesthesiologists' services and $2,600 (€2,272) for surgical assistants, according to a report by the Department of Health and Human Services.
The Biden administration is also in the process of implementing a plan to make 400 million N95 masks available to Americans for free in its latest effort to try to halt the deadly Covid surge.
holdouts is in large part fuelling the more than 1,700 deaths a day from Covid-19. As the Omicron variant has ripped through the country, healthcare professionals say hospitals and health systems have been overwhelmed in many places because of unvaccinated patients.
An analysis by the Kaiser Family Foundation (KFF) found there were 690,000 vaccine-preventable Covid-19 hospitalisations from June to November 2021 and the cost of treating these patients over that six-month period was $13.8 billion (€12 billion).
“That [cost] could have been prevented if people had chosen to get vaccinated. It's hard to project how the pandemic sort of plays out. But prevention measures do reduce longerterm costs. These are preventable deaths and hospitalisations,” said Ms Krutika Amin, a KFF Associate Director.
The administration is fighting back against some states. On 14 January, for example, it threatened to withdraw federal Covid-19 relief funding from Arizona unless the state stops directing the money to schools without mask mandates.
State programmes, funded with payments from the Coronavirus State and Local Fiscal Recovery Funds, impose conditions that discourage compliance with wearing masks in schools, contradicting guidance from the CDC on how to reduce Covid-19 transmission.
The funds are intended “to mitigate the fiscal effects stemming from the Covid-19 public health emergency, including by supporting efforts to stop the spread of the virus", the Treasury department stressed in a letter to Arizona state authorities. According to the administration, a recipient may not use the federal money for any programme or service that
But the President needs much more to show for his healthcare agenda. Unless he can sort out differences among his party’s senators to ensure passage of his Build Back Better Bill in the coming weeks and months his agenda may well be doomed after the November congressional elections.
Still, Biden can take some heart from a poll on 14 January from FiveThirtyEight that put his average approval rating at 42.2 per cent. But it remains to be seen if this will translate into boosting support for his Build Back Better Bill.
If his sweeping plan does fail to pass, some Democrats believe that all will not be lost. Senator Tim Kaine of Virginia has said that while he fears the $1.75 trillion Bill may be “dead”, core elements will probably still pass. “The most recent version of it is not going to happen,” Senator Kaine said on the CBS network on 16 January. He added that the Democrats will “find the core of the Bill – like education, reduced childcare costs, and healthcare support – and pass it”.
The President accepted as much at a news conference marking the anniversary of his first year in office. “It’s clear to me that we are going to have to probably break it up,” he said of his Build Back Better Bill.
But he emphasised the fight for those goals was not over. Instead, he said he would move on and try to achieve the objectives incrementally. “I think we can break the package up, get as much as we can now and come back and fight for the rest later.”
While some healthcare reform is better than none in this political climate, it seems Americans will again have to settle for incremental changes rather than the transformative advances proposed by the President.
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 14 News Feature
An analysis by the Kaiser Family Foundation found there were 690,000 vaccine-preventable Covid-19 hospitalisations from June to November
Dr Celine Gounder
Governor Doug Ducey
Once-weekly type 2 diabetes treatment with CV benefits† and superior efficacy1-9*
SUPERIOR GLYCAEMIC CONTROL1-9*
• Ozempic® -1.8% vs dulaglutide‡ -1.4%1,2,§
• Up to 79% achieved ADA target of HbA1c <7% (53 mmol/mol) vs other diabetes treatments1-10
SUPERIOR AND SUSTAINED WEIGHT LOSS1-9*
• More than double the weight loss vs dulaglutide (-6.5 kg vs -3.0 kg)1,2,§

• Weight loss sustained over 2 years1,3
PROVEN CV BENEFITS1,3†‡
• 26% CV risk reduction in patients with type 2 diabetes and high CV risk, compared to placebo in addition to standard treatment1,3,4,†
Ozempic® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events and the populations studied, see sections 4.4, 4.5 and 5.1. of the summary of product characteristics.1 CV=cardiovascular. SUSTAIN = Semaglutide Unabated Sustainability in treatment of Type 2 Diabetes. *Results apply to Ozempic® across SUSTAIN trials, which included placebo, sitagliptin, dulaglutide, canagliflozin, exenatide PR and glargine U100.1-9
†In SUSTAIN 6, Ozempic® reduced CV risk (CV death, nonfatal myocardial infarction [MI] or nonfatal stroke) versus placebo in patients with type 2 diabetes at high CV risk treated with standard of care.1,3 ‡When added to standard of care, which included oral antidiabetic treatments, insulin, antihypertensives, diuretics and lipid-lowering therapies. §SUSTAIN 7, Ozempic® 1.0 mg vs. dulaglutide 1.5 mg.


Abbreviated Prescribing Information
Ozempic®t semaglutide

Please refer to the Summary of Product Characteristics (SmPC) before prescribing. Ozempic® 0.25 mg solution for injection in pre-filled pen. Ozempic® 0.5 mg solution for injection in pre-filled pen. Ozempic® 1 mg solution for injection in pre-filled pen. One ml of solution contains 1.34 mg of semaglutide (human glucagonlike peptide-1 (GLP-1) analogue). Indication: Ozempic® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1 of the Ozempic® SmPC. Posology and administration: Administered once weekly at any time of the day, with or without meals. Injected subcutaneously in the abdomen, thigh or upper arm. Starting dose: 0.25 mg once weekly. After 4 weeks the dose should be increased to 0.5 mg once weekly. After at least 4 weeks with a dose of 0.5 mg once weekly, the dose can be increased to 1 mg once weekly to further improve glycaemic control. When Ozempic® is added to a sulfonylurea or insulin, a reduction in dose of sulfonylurea or insulin should be considered to reduce the risk of hypoglycaemia. Blood glucose self-monitoring is necessary to adjust the dose of sulfonylurea and insulin, particularly when Ozempic® is started and insulin is reduced. A stepwise approach to insulin reduction is recommended. Children: No data available. Elderly: No dose adjustment required, therapeutic experience in patients age ≥75 is limited. Renal impairment: No dose adjustment is required for patients with mild, moderate or severe renal impairment. Experience in patients with severe renal impairment is limited. Not recommended for use in patients with end-stage renal disease. Hepatic impairment: No dose adjustment is required for patients with hepatic impairment. Experience with severe hepatic impairment is limited. Caution should be exercised when treating these patients with semaglutide. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Special warnings and precautions for use: Should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis. Not a substitute for insulin. Diabetic ketoacidosis has been reported in insulin-dependent patients whom had rapid discontinuation or dose reduction of insulin. There is no experience in patients with congestive heart failure NYHA class IV and is therefore not recommended in these patients. Use of GLP-1 receptor agonists may be associated with gastrointestinal adverse reactions. This should be considered when treating patients with impaired renal function as nausea, vomiting, and diarrhoea may cause dehydration which could cause a deterioration of renal function. Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. Patients should be informed of the characteristic symptoms
of acute pancreatitis. If pancreatitis is suspected, semaglutide should be discontinued; if confirmed, semaglutide should not be restarted. Caution should be exercised in patients with a history of pancreatitis. Use of semaglutide in combination with a sulfonylurea or insulin may have an increased risk of hypoglycaemia, therefore consider reducing the dose of sulfonylurea or insulin when initiating treatment with Ozempic®. In patients with diabetic retinopathy treated with insulin and semaglutide, an increased risk of developing diabetic retinopathy complications has been observed. Caution should be exercised when using semaglutide in patients with diabetic retinopathy treated with insulin. These patients should be monitored closely and treated according to clinical guidelines. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy, but other mechanisms cannot be excluded. When semaglutide is used in combination with a sulfonylurea or insulin, patients should be advised to take precautions to avoid hypoglycaemia while driving and using machines. Fertility, pregnancy and lactation: Women of childbearing potential are recommended to use contraception when treated with semaglutide. Should not be used during pregnancy or breast-feeding. Discontinue at least 2 months before a planned pregnancy. Effect on fertility unknown. Undesirable effects: Very common (≥1/10); Hypoglycaemia when used with insulin or sulfonylurea, nausea, diarrhoea. Common:(≥1/100 to <1/10); Hypoglycaemia when used with other oral antidiabetic medications, decreased appetite, dizziness, diabetic retinopathy complications, vomiting, abdominal pain, abdominal distension, constipation, dyspepsia, gastritis, gastro-oesophageal reflux disease, eructation, flatulence, cholelithiasis, fatigue, increased lipase, increased amylase, weight decreased. Uncommon: (≥1/1,000 to <1/100); Dysgeusia, increased heart rate, acute pancreatitis, injection site reactions. Rare:(≥1/10,000 to <1/1,000) Anaphylactic reaction. The Summary of Product Characteristics should be consulted for a full list of side effects. MA Numbers: Ozempic® 0.25 mg pre-filled pen EU/1/17/1251/002. Ozempic® 0.5 mg pre-filled pen EU/1/17/1251/003. Ozempic® 1 mg prefilled pen EU/1/17/1251/005. Each pre-filled pen delivers 4 doses and includes 4 disposable NovoFine® Plus needles. Legal Category: POM. For complete prescribing information, please refer to the Summary of Product Characteristics which is available on www.medicines.ie or by email from infoireland@novonordisk.com or from the Clinical, Medical and Regulatory Department, Novo Nordisk Limited, 1st Floor, Block A, The Crescent Building, Northwood Business Park, Santry, Dublin 9 Ireland. Date last revised: March 2020
t This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare Professionals are asked to report any suspected adverse reactions to the Health Products Regulatory Authority. Information about adverse event reporting is available at www.hpra.ie. Adverse events should also be reported to Novo Nordisk Medical Department Tel; 1850 665 665.
References: 1. Ozempic® Summary of Product Characteristics www.medicines.ie 2. Pratley RE et al. Semaglutide versus dulaglutide once-weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018; 6: 275 - 286. 3. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844. 4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(suppl1):S1-S108. 5. Lingvay I, et al. Once weekly Semaglutide vs Canagliflozin in type 2 diabetes: results of the SUSTAIN 8 trial. https://doi.org/10.1016/S2213-8587(19)30311-0. 6. Ahmann AJ et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care 2018;41:258-266. 7. Aroda VR et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5: 355–66. 8. Sorli C et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 251–60. 9. Ahrén B et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017; 5: 341–54. 10. American Diabetes Association. Standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl1):S1-S159.
Novo Nordisk Limited, First Floor, Block A, The Crescent Building, Northwood Business Park Santry, Dublin 9, D09 X8W3, Ireland. Tel: 01 8629 700, Fax: 01 8629 725, Lo call: 1 850 665665 infoireland@novonordisk.com www.novonordisk.ie
Ozempic®, NovoFine® and the Apis bull logo are registered trademarks owned by Novo Nordisk A/S
Date of preparation: September 2020. IE20OZM00029

Patients with type 2 diabetes should expect more after metformin
The future of neurological research
Prof David Henshall, Ms Ciara Courtney, and Ms Bridget Doyle,
outline the latest developments from the FutureNeuro SFI
Research Centre
According to the Neurological Association of Ireland (NAI), there are over 800,000 people in Ireland living with a neurological condition. Brain diseases have a devastating impact on people living with neurological conditions, their families and carers. They are estimated to cost the economy €3 billion each year. The FutureNeuro Science Foundation Ireland (SFI) Research Centre was established in 2017 to address the significant unmet needs of people living with chronic and rare neurological disorders and to support the health professionals who care for them.
FutureNeuro is hosted by the RCSI University of Medicine and Health Sciences, with researchers based in six other Irish academic institutions: Trinity College Dublin (TCD); Dublin City University; University College Dublin (UCD); NUI Galway; University College Cork; and Waterford Institute of Technology. Operations began in October 2017 and since then, FutureNeuro has significantly increased in size. In total, there are 87 academic and clinical researchers and support staff based across these third level institutions and in the main hospitals associated with the Centre – Beaumont and St James’s Hospitals, Dublin, and Cork University Hospital.
FutureNeuro is a unique, multi-disciplinary and inter-institutional research centre focusing on epilepsy, motor neurone disease (MND), multiple sclerosis (MS), Parkinson’s disease, and a rare paediatric disease, and performing basic, applied and translational research in the area of diagnosis, therapeutics and e-health. Their work is focused in partnership with the health service, patient organisations and industry to make breakthrough discoveries on the causes of brain disease and the development of innovative technological solutions that help with diagnosis, treatment, and management.
FutureNeuro’s scientific strategy has focused on:
Developing stratification tools that facilitate diagnosis and clinical trials;
Discovery and validation of biomarkers for point-of-care diagnostics;
Application of genomic testing and pharmacogenomics to deliver precision medicine;
Bringing innovative and disease-modifying therapeutic leads into preclinical development;
Maximising adoption of e-health-enabled care and research including ‘big data’ and artificial intelligence (AI).
Some achievements during phase one of the centre include: (2017-present)
180 original publications including breakthroughs in diagnostics (discovery of novel class of circulating biomarkers and technology for their detection), therapeutics (new RNA metabolism pathways, potent disease-modifying therapies and their mechanisms of action) and e-health (applying innovative technological solutions
for self-monitoring of chronic disease).
Training of 19 PhDs, 17 postdoctoral fellows (of which five started their own laboratories).
Investigator pool growth from 11 to 23 and from 12 to 48 research projects.
Leadership of eight international consortia including TRICALS, EPIXCHANGE.
Industry
Working with the leading companies in neurology is critical to the Centre’s impact. During 2021, FutureNeuro worked collaboratively with multiple industry partners including Roche, UCB, Janssen, and Congenica. The projects address a range of topics including improved blood-based diagnostics, preclinical testing of new molecules to treat seizures, and ambulatory seizure monitoring.
Scientific highlights in 2021
Despite the impact of Covid on bench and clinical research, 2021 was another busy and successful year for FutureNeuro, with a number of important scientific breakthroughs. FutureNeuro is closely integrated into the national clinical neurology network, both adult and paediatric. This has facilitated access to key neurologists that have been critical to the success of the Centre and in turn has brought direct benefit to diagnostics and care. Both SFI-funded platform and industry research projects (eg, Congenica) led by Prof Gianpier Cavalleri and Prof Norman Delanty and through the work of Dr Katherine Benson have supported genomic clinical diagnosis in over 230 people, which has had a significant impact for these individuals and their families.
Elevated blood purine levels as a biomarker of seizures and epilepsy
Dr Tobias Engel and his team at RCSI, together with researchers in UK, Germany and clinical colleagues in Beaumont Hospital, identified a potential new biomarker that could lead to the diagnosis and detection of epilepsy via a novel, user-friendly point-ofcare device, which requires a drop of blood and gives results within minutes. The discovery was made by measuring blood purine concentrations using a point-of-care diagnostic technology based on the summated electrochemical detection of adenosine. Measurements of blood purine concentrations were carried out using finger prick samples from preclinical models and in video-electroencephalogram (EEG)-monitored adult patients with epilepsy. The paper is published in Epilepsia at: https://onlinelibrary.wiley.com/doi/full/10.1111/epi.16839
Identifying new mutations associated with early-onset dementia
Dr Matthew Campbell and his research team in TCD identified previously unreported mutations in a gene called colony stimulating factor-1 receptor (CSF-1R) in an ultra-rare form of Alzheimer’s disease.
This discovery may lead to better understanding of how Alzheimer’s and other dementias occur. Read the full study published in Embo Molecular Medicine at www.embopress.org/doi/full/10.15252/ emmm.202012889
FutureNeuro epilepsy clinical network
pivot to telecare during Covid-19
Before the onset of the Covid-19 pandemic, telemedicine was already an important part of the FutureNeuro Centre. Building on the HSE eHealth Ireland-funded epilepsy Lighthouse project, FutureNeuro researchers developed a pilot e-portal, allowing people to access and co-author their own epilepsy care records. The redeployment of staff to Covid19-care and the closure of outpatient services created an urgent need to provide safe remote patient care. With access to patient-co-authored records within the national epilepsy electronic patient record (EPR), Profs Colin Doherty (St James’s Hospital) and Norman Delanty (Beaumont Hospital) moved 1,600 patient clinics online using a mixture of telephone and virtual appointments. Research by the FutureNeuro e-health team into the facilitation of tele-clinics demonstrated no loss of care contact for patients with epilepsy, and the patient survey showed that telemedicine is seen as an effective and satisfactory method of delivering chronic outpatient care. This paper was published in Epilepsy Behaviour, and can be accessed here: www.epilepsybehavior.com/article/ S1525-5050(20)30855-6/fulltext
“The Irish national epilepsy electronic patient record (EPR) and the subsequent development of the electronic patient portal (e-portal) have facilitated changes in patients’ care pathways and improved patient-clinician contact and has engendered greater patient self-management (Power et al, 2020). These structures provide Irish policymakers with a template for expanding an EHR to other patient populations, and ultimately to establish a national EHR.”
In addition to this, in June last year, the Economic and Social Research Institute (ESRI) published the report Advancing a Digital Healthcare Future for Ireland citing the work of FutureNeuro, which can be accessed at: www.esri.ie/publications/developments-in-healthcare-information-systems-in-ireland-and-internationally
Prediction of caregiver quality-of-life in amyotrophic lateral sclerosis (ALS) using explainable machine learning
Dr Catherine Moone and PhD candidate
Anna Markella Antoniadi, both from UCD, and Prof Orla Hardiman (TCD), published a study in Scientific Reports on the identification of the predictors of a caregiver’s quality-of-life, in addition to the development of a model for clinical use, which would alert clinicians when a caregiver is at risk of experiencing low quality-of-life. The data was collected through the ALS registry and via interviews with 90 patient and caregiver pairs at three time-points.
The study is available here: www.nature. com/articles/s41598-021-91632-2
Building capacity to tackle
neurological disease
NeuroInsight
Tackling neurological disease remains FutureNeuro’s number one priority. Over a four-year period, the NeuroInsight Marie-Sklodowska Curie Fellowships programme will train 33 research fellows who conduct projects combining neurology and data. They will gain practical experience in hospitals and industry. The programme will facilitate high quality research, provide opportunities to interact with clinicians, patient groups and datasets, and establish a talented group of future research leaders. To find out more and how to apply, visit www.neuroinsight.eu
Empower
In October 2021, the Empower Data Governance Programme which was co-created by FutureNeuro was launched. As a health research centre, Empower will create a community of practice involving patients, their carers, clinicians, statutory bodies, industry and researchers to explore how and why patient data is used for healthcare improvement and research, and develop a culture that promotes the trustworthy use of data, to facilitate collaborative, transparent and citizen-centric opportunities for the safe and ethical use of health data.
Empower will harness the collective research capabilities across four participating SFI Centres – FutureNeuro, ADAPT, Insight, and Lero (programme lead). The programme will address many different types of data ecosystems, with health data being the prime focus of FutureNeuro. See more information at www.futureneurocentre.ie
Education and public engagement
Education and public engagement is a key focus for the entire FutureNeuro team. We have successfully partnered with NGOs, such as Epilepsy Ireland, to deliver a series of nationwide Epilepsy in English workshops addressing sensitive topics such as sudden unexpected death in epilepsy (SUDEP), temporal lobe epilepsy (TLE), gene therapy and managing epilepsy. We are prioritising public and patient involvement in our research. With the appointment of a part-time engagement expert, we are currently establishing a patient advisory council, representing both adult and paediatric patients with a lived experience of MS, MND, and epilepsy.
For more information on the work of FutureNeuro go to www.futureneurocentre.ie
AUTHORS: Prof David Henshall, FutureNeuro Centre Director and Professor of Molecular Physiology and Neuroscience at the RCSI University of Medicine and Health Sciences; Ms Ciara Courtney, Communications and Education and Public Engagement Lead, FutureNeuro; and Ms Bridget Doyle, Centre and Business Development Manager, FutureNeuro
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 16
News Feature
CAMHS scandal was disaster waiting to happen
Too often it takes a catastrophic failure for a situation to improve.
It is not going too far to give such a description to the lack of oversight, supervision, and accountability in child and adolescent mental health services (CAMHS) in South Kerry, which have recently come to light. The HSE recently published an independent review of the treatment of approximately 1,300 young people who attended South Kerry CAMHS between July 2016 and April 2021. While the authors of the report found no extreme harm was caused to the patients in these files, hundreds of children received “risky” treatment from one particular doctor and significant harm was caused to 46 of them.
The Chief Executive of the Mental Health Commission (MHC) Mr John Farrelly said: “This level of harm must be the catalyst for change.”
In a letter to the Minister of State for Mental Health Mary Butler, the MHC stated that while responsibility for improvement lies with the providers, independent oversight of the implementation of recommendations from the HSE report is necessary.
Significantly, the Commission pointed out its regulatory function only relates to approved inpatient mental health centres and does not extend to community mental health services.
The MHC told the Minister what has transpired in the Cork/Kerry community mental health services, regarding CAMHS, strongly supports the recommendation that it be given full regulatory powers over all areas of mental health services in Ireland.
The letter also informed the Minister that the Inspector of Mental
Health Services, Dr Susan Finnerty, will be conducting an independent review of the provision of CAMHS in accordance with her powers under the 2001 Mental Health Act. Dr Finnerty had scheduled to do this review prior to publication of the HSE report to assess whether there had been improvements in CAMHS provision since a similar review in 2017.
The MHC has formally advised the Minister that a number of actions should be undertaken as a matter of urgency. These consist of ensuring that the HSE “transparently and fully implement” all the recommendations emerging from the look-back review and that this process be supervised by a panel of independent experts who have relevant qualifications and experience.

The letter also said the national audit of all 72 CAMHS teams should have its terms of reference and scope agreed and overseen by independent experts. It further recommended that the HSE immediately review and update the ‘CAMHS Standard Operating Procedure 2015’ and the ‘CAMHS Operational Guideline 2019’, both of which are not underpinned by current best practice in the view of the MHC.
The MHC also refers to how there has been no National Director for Mental Health in the HSE since 2017. The Commission has advocated for the position to be filled since that time. It said the recent revelations have made such an appointment crucial.
The staffing deficiencies in CAMHS, which have been known about for a long time, are not a surprise. A disaster was waiting to happen. It can only be hoped that the spotlight shone on the service will help avoid similar occurrences now and in the future.
MINDO CARTOON
READER COMMENTS
REACTION TO OUR LEAD STORY, 'HRB CLINICAL TRIALS FUNDING DECISION COULD LEAD TO "INEQUITY" FOR THE WEST', 27 JANUARY
"We have enough health inequities in Ireland, without compounding it further #CareCantWait #ClincialTrials."
Dr Anne-Marie Baird, @BairdAM, 27 January
REACTION TO DR SARAH FITZGIBBON'S COLUMN, 'WHAT I MISS AND DON'T MISS ABOUT BEING A GP', 27 JANUARY
"Excellent – and very relatable.... Maith thú!" Dr Paul O'Dwyer, @ShirtnTie, 27 January
"Once again on the medical emotional button Sarah." laucullen, @laucullen1, 26 January
"Another honest and extremely well-written piece by @SarahFitzWiMIN on the realities of life as a GP.” MedisecIreland, @MedisecIreland, 26 January
"Lovely piece. Didn’t realise you were no longer practicing. I love my GP and no doubt it’s tough work. Congrats." Mairead D, @MDRN1, 26 January
REACTION TO OUR NEWS STORY, 'ONE-IN-FIVE TRAINEES REPORT "NO ACTION" FOLLOWING BULLYING COMPLAINTS – MEDICAL COUNCIL SURVEY', 27 JANUARY
"A helpful person recently explained to me that bullying wasn’t just a problem in hospitals, ‘it’s everywhere – teaching, banking, law….’ This doesn’t make it acceptable or inevitable. Bullying is unprofessional." Dr Suzanne Crowe, @itsthatgirlsuzi, 29 January
"We need to change this from within and from the top down! Not ok…." Dr Chiara Besani, @LastraChiara, 29 January
REACTION TO OUR NEWS STORY, 'WEEKLY "LOW-RISK" ALCOHOL GUIDELINES TO BE REVIEWED', 27 JANUARY
"We are very pleased to see progress on a key recommendation within our 2020 election manifesto." AlcoholActionIreland, @AlcoholIreland, 27 January
"It's about time. They are woefully out of step with the current science." Ireland Unlocked, @IrelandUnlocked, 27 January
Editor: Paul Mulholland paul@mindo.ie
News Editor: Catherine Reilly catherine@mindo.ie
Clinical Editor: Priscilla Lynch priscilla@mindo.ie
Journalists: David Lynch, david@mindo.ie, Pat Kelly, pat@greenx.ie
Sub-Editor: Emer Keogh emer@greenx.ie
Designer: Laura Kenny laura@greenx.ie
Advertisements: Graham Cooke graham@greenx.ie
Recruitment: Louis O’Hegarty louis@mindo.ie
Digital Marketing: Dermot Garland dermot@greenx.ie
Managing Director: Graham Cooke graham@greenx.ie

facebook.com/MedicalIndependent
twitter.com/med_indonews
If you have anything you would like to share, please email: info@mindo.ie
The Medical Independent is published by GreenCross Publishing Ltd., Top Floor, 111 Rathmines Road Lower, Dublin 6, Ireland Tel: 01 441 0024
Web: www.medicalindependent.ie. GreenCross Publishing (est. 2007) is owned by Graham Cooke (graham@greenx. ie). © Copyright GreenCross Publishing Ltd. 2022. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means –electronic, mechanical or photocopy recording or otherwise – whole or in part, in any form whatsoever for advertising or promotional purposes without prior written permission of the publishers. The Medical Independent endeavours to ensure accuracy of information given and of claims made in articles and advertisements. Nevertheless, no responsibility is accepted in respect of such information or claims. Any opinions expressed by contributors are entirely their own and do not purport to be the views of The Medical Independent
For subscriptions, contact: Daiva Maciunaite, daiva@greenx.ie Ireland €200 incl VAT. UK and International €275.00
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 17 Letters to: The Editor, The Medical Independent, Greencross Publishing Ltd, Top Floor, 111 Rathmines Road Lr, Dublin 6 or email paul@mindo.ie Editorial PAUL MULHOLLAND
Delivering value in Ireland across the entire lifecycle of new medicines.
The task of turning possibilities into reality for doctors and their patients is constant. More than 2,700 AbbVie employees support this goal at nine locations in Ireland including Cork, Dublin, Sligo and Westport. AbbVie has 135+ potential medicines under investigation. More than 75% are first in kind. An innovative medicine is created in four stages and AbbVie adds value in Ireland at each point in this journey.

Learn more at abbvie.ie
Supporting Discovery
• Creating treatment possibilities.
• Partnering with Science Foundation Ireland to fund research in university and hospital laboratories.
Public Access to New Medicines
• The ultimate value of innovation is to ensure those in medical need have new treatment options.
• Offers patients new hope and allows doctors advance the standard of care.
People. Passion. Possibilities.®
Clinical Trials and Data Generation
• 76 Irish clinical trial sites have tested the safety and efficacy of potential AbbVie medicines.
World-Leading Manufacturing
• Our industry works with IDA Ireland to ensure Ireland is an international medicines powerhouse.
• Irish plants are playing a key role in supporting AbbVie’s global supply of treatments.
Date of preparation: January 2022 | IE-ABBV-220003
Chaperones: A timely reminder
Ms Suzanne Creed , Clinical Risk Advisor at Medisec, provides advice concerning the use of chaperones for intimate medical examinations

and irrespective of the gender of the patient or clinician.
Selecting a chaperone
The role of the chaperone is to act as an impartial observer, providing reassurance to the patient and to raise any concerns about a doctor’s behaviour or actions. Ideally, the chaperone should be a trained health professional familiar with the examination being undertaken so that, if necessary, they can confirm the examination was appropriately conducted. A trained chaperone may also feel more comfortable raising questions or concerns about how the examination is conducted. If non-medical staff members, eg, secretary/administrator, are to undertake the chaperone role, you should ensure that they are fully trained, which includes training in maintaining patient confidentiality, familiarity with the procedure, where to position themselves during the examination, and how to raise concerns about a healthcare professional if misconduct occurs.
What to do if a chaperone is unavailable
There may be occasions when a chaperone may not be available, such as on home visits or in an out-of-hours setting. In these circumstances, you should consider whether the examination is urgent and necessary. If it is not urgent and the patient requests a chaperone, you could reschedule the appointment for a time when a chaperone is available.
If a patient declines a chaperone
Achaperone is an impartial observer, present during an intimate examination with a patient. They provide protection for both the patient and the clinician. This can prove important should a clinician face allegations of impropriety. In 2019, almost five per cent of all complaints made to the Medical Council included allegations about physical and intimate examinations. For any doctor an accusation of inappropriate behaviour towards a patient is devastating and the consequences can be far-reaching. Cases can take many months, and often years, to resolve, by which time the doctor concerned may have been through criminal, civil, and Irish Medical Council proceedings, as well as facing adverse publicity in the media. It is therefore important that clinicians are fully aware of their ethical responsibilities and protect themselves by complying with chaperone guidance.
Ethical obligations
The Medical Council’s Guide to Professional Conduct and Ethics for Registered Medical Practitioners provides clear guidance concerning the use of chaperones for intimate examinations. Paragraph 35 states:
35.1 Clinical assessments of patients often involve a physical examination as well as relevant history-taking. Before undertaking any physical examination, including an intimate examination, you should explain to patients why it is needed and what will be involved and get their consent.
35.2 You should respect patients’ dignity by giving them privacy to undress and
dress and keeping them covered as much as possible. You should not help the patient to remove clothing unless they have asked you to do so, or you have checked with them that they want your help.
35.3 Where an intimate examination is necessary, you must explain to the patient why it is needed and what it will entail. You must ask the patient if they would like a chaperone to be present – for example, a nurse or family member – and note in the patient’s record that a chaperone was offered. You should also record if a chaperone was present, had been refused, or was not available, but the patient was happy to proceed.
35.4 You must not carry out intimate examinations on anaesthetised patients unless the patient has given written consent to this in advance.
The use of a chaperone for intimate examinations is also endorsed by the HSE.
What is an intimate examination?
Examination of the breast, genitalia, and rectum are commonly recognised as intimate examinations. However, some patients may consider any examination in which the doctor needs to touch or be very close to them as intimate, for example, ophthalmic examination in a darkened room where physical proximity is necessary. Similarly, if a patient is vulnerable in any way, where prior misunderstandings exist or for cultural or religious reasons it may be appropriate for the doctor to offer a chaperone. It is therefore important that doctors use their clinical judgement to determine if an examination is intimate depending on the individual circumstances
The presence of a chaperone protects both the doctor and the patient. Even if a patient declines a chaperone, the doctor may feel it would be more prudent to have one for their own protection and/ or comfort. In such circumstances, the doctor should explain their position to the patient. If the patient still declines the offer of a chaperone, the doctor will need to determine whether they are happy to proceed with the examination or not. This is a personal decision that the
doctor will need to make, exercising their own clinical skills and judgement. If the doctor does proceed in the absence of a chaperone, they should carefully document that a chaperone was offered and declined. It is advisable that the doctor also makes a note detailing their rationale for undertaking the examination in the absence of a chaperone.
Record keeping
In all instances where a chaperone has been offered, it should be recorded in the patient’s clinical file whether it has been accepted or declined. Where a chaperone has been used, their identity, including name and job title, should also be recorded. If you use electronic records, you may find it useful to use a chaperone template to record the patient’s relevant decision.
Remain professional
Some clinicians may try to alleviate patients’ anxiety during sensitive examinations by using humour, making lighthearted comments, sharing personal stories, or minimisng the significance of the examination. While it’s natural to try to put patients at ease, the prevailing wisdom is that these types of comments can be misinterpreted by the patient and are best to be avoided.

In Medisec’s experience, the best way to minimise patient discomfort in these situations is to communicate clearly with the patient, be personable and compassionate and ensure that your personal behaviour remains professional throughout, so as to be beyond reproach.
A key focus in all clinical encounters should be an evaluation of whether to use a chaperone for some or all physical examinations. This should be done regardless of the gender of the patient or the clinician and irrespective of the long-term nature of the doctor-patient relationship. Chaperones can offer protection and comfort for doctors and patients, particularly during intimate examinations.
References on request
Practical tips to reduce your risk
1. Develop a chaperone policy for your practice/organisation. Consider displaying your policy on your website and/or in your waiting room via a poster.
2. If an intimate examination is required, you should explain the nature of the procedure/examination and obtain the patient’s consent to proceed. If you need to modify the examination while it is underway, inform the patient and reconfirm consent.
3. Allow the patient time to ask any questions about the procedure. Be aware of, and respect, any cultural or religious issues.
4. Offer a chaperone to all patients for intimate examinations (or examinations which could be construed as such) – irrespective of the gender of the patient
and the clinician.
5. Always respect patient privacy by providing an appropriate space to undress/re-dress. This applies to the clinician, your staff, and the chaperone.
6. Keep all conversations professional and avoid any personal or inappropriate comments.
7. The chaperone should stand in a position where they can see the patient and the process of the examination.
8. The chaperone should leave the room following the examination. They do not need to be present for any subsequent discussion of the patient’s condition or treatment.
9. Immediately after the consultation record the identity and job title of the chaperone, as well as any other relevant issues or concerns.
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 19 Medico-Legal Opinion
MS SUZANNE CREED
The shared dream of storytelling
Stories make us feel less alone, which is especially important at a time like this
DR LUCIA GANNON
 Read more by Dr Lucia Gannon at www.mindo.ie @LuciaGannon
Read more by Dr Lucia Gannon at www.mindo.ie @LuciaGannon
The thing about a story is that you dream it as you tell it, hoping that others might dream along with you, and in this way memory, imagination and language combine to make spirits in the head
Tim O’Brien – The Things They Carried
oe chose his visiting times carefully.
“Early afternoon was best,” he said. His father was usually alert at this time of day and his mother had completed her chores. They would be resting in their chaise recliners, either side of the charcoal cast iron stove, the forked flames visible through the toughened glass door panel. The perfect setting in which to tell a story.
“I don’t bring cakes or flowers,” he said, but I do like to bring a story.”
Joe and his parents lived separately on the far end of the Renvyle Peninsula, a wildly beautiful piece of land that juts out into the Atlantic. I had called him to ask about his father. It was the middle of the second lockdown. The summer visitors had gone home, all but the most essential shops and services were closed, and the weather was mostly wet and windy. Joe’s father was growing frail, he had poor mobility, his speech was almost gone, eating was a struggle, and he slept a lot.
“It passes an afternoon,” Joe said, “a break from Covid.”
I asked him if he would tell me his most recent story. I too, wanted to forget the pandemic, if only for a while.
Joe needed little persuasion. “It’s an interesting one,” he said, and he began.
“I was at the bog, stacking the last of the turf, a few weeks ago. It was getting dark and there was no one around. I am the only lunatic who goes there at this time of year. I was circling the stack of turf, throwing the last of the clods into a pile and tidying the place up when I noticed two figures in the distance. As I said, I have never seen another soul up there, so I stopped my mooching and stood waiting for them to get closer.”
As Joe spoke, I could see him alone on the bog, his hair in a low bun, sticking out from underneath his woolen cap; black gilet zipped to his chin; brown hiking boots under green khaki trousers. I could hear the wind and smell the turf, could see the light fade from the Connemara sky and the Twelve Bens turn from blue to grey in the late afternoon.
“The two boys approached,” Joe continued, “so I walked
out to meet them. I knew them both, a local archaeologist, and his companion. They were as surprised to see me as me them. Their story was that they were looking for an ancient well that they believed was somewhere in the general vicinity. I had been over most of that ground and I had never seen a well. Nevertheless, I decided to join them and the three of us set out across the windswept bog in the dwindling afternoon light. We were about to give up, when suddenly the wind calmed, the landscape changed from brown to stony grey, and there in the distance, like an oasis in the desert, was a patch of green fertile ground, out of which grew a cluster of trees, eerily still and silent except for the occasional tweet of a winter starling or thrush. And under the trees, a large grey stone slab, atop the ancient well. Three thousand years old, and God only knows when the last time was that any human set foot there. St Ceannanach’s Well. If we were believers, we would have been on our knees.”
Joe paused briefly before continuing. “My mother was very interested,” he said, “but my father’s eyes were closed, and I thought he was sleeping until I noticed him rousing himself. He asked me did I know who St Ceannanach was and I told him I did not. With all the energy he could muster, he told me that he was a missionary saint in the fifth century who fell afoul of a local king and got himself beheaded. But immediately his head fell to the ground, he bent down, picked it up, washed it in the waters of the well, then carried it to the neighboring village, lay down, and died.”
Joe explained that in his day, his father would have had much more to contribute, but he was grateful for this temporary connection, this glimpse of his father as he used to be. As I put down my phone, the words of Tim O’Brien came to mind and I thought about how stories do indeed help us dream along with each other and how when we emerge from this dreamlike state, we do not feel quite so alone.
The unhelpful concept of the ‘healthcare hero’
Militarised metaphors in medicine, which have been common in the pandemic, can be dangerous
DR MUIRIS HOUSTON
Read more by Dr Muiris Houston at www.mindo.ie
@muirishouston
Covid-19 has brought many changes to our lives. Some are beneficial, such as a much speeded-up approval of medicines and vaccines. Others are unhelpful, like the excessive isolation of older people.
One of the unhelpful concepts brought to healthcare since the pandemic hit in early 2020 is the metaphor of the ‘healthcare hero’. The militarised metaphor of healthcare heroes robs doctors and nurses of the ability to voice concerns for themselves and their patients. And militaristic metaphors in medicine can be dangerous to both doctors and patients. The danger of militarised language in medicine, even pre-Covid-19, is the promotion of aggression over receptivity, and the framing of doctors as soldiers, and disease, as well as patients, as the enemy.
Although it can be traced back centuries, the modern use of military terminology in medicine received a major boost when, in 1971, the then US President Richard Nixon publicly declared “war” on cancer and referred to it as a “relentless and insidious enemy”.
Yet how often do we read of the “collateral damage” of chemotherapy to describe the side effects of cancer treatments – a phrase defined as “the unintentional damage or incidental damage affecting facilities, equipment or per-
sonnel occurring as a result of military action directed against targeted enemy forces or facilities”?
Clearly military life cannot be distanced from the rest of the world, but do we really need terms like “killer virus” and “vaccine shots” when talking to patients about Covid-19? Covid-19 has produced a range of distinctly militarised gestures, and the consequence has been a social expectation for healthcare workers to unquestioningly serve their country while sacrificing their own health and, if necessary, their lives.
A more positive view of how Covid might change healthcare appeared in the Journal of the American Medical Association (JAMA). Written in the early stages of the pandemic by Dr Donald Berwick of the Institute of Healthcare Improvement, it framed a new post–Covid-19 normal not as predictions, but as a series of choices.
Of particular relevance when society was looking to identify healthcare heroes, he identifies health staff wellbeing as key: “Sadly, attention to healthcare worker safety has languished at far too low a level of priority for decades. Now it is evident how unwise that is, as millions of workers face personal risks that they would not encounter if protective equipment and preparatory procedures had been arranged in advance. Will the new normal address more adequately the physical safety and emotional support of the healthcare workforce in the future? Without a physically and psychologically safe and healthy workforce, excellent healthcare is not possible.”
Could we be better prepared for future large-scale health threats, Berwick asks? “The foundations of preparedness, most crucially a robust public health system, have been al-
lowed to erode or have never been laid in the first place,” he notes. And while the Covid-19 toll may be the largest paid so far for this failure, without taking public health and preparedness seriously, it won’t be the last. Whether it turns out to be another pathogen, massive trauma, cyberthreats to the electric grid, the question is: Will public health finally get its due?
Probably the most notable wake-up call of all is inequality. Headlines about the unequal toll of Covid-19 on the poor, the underrepresented minorities, the marginalised, the incarcerated, the indigenous peoples bear this out.
Berwick writes that the “most consequential question in the new normal for the future of global health is this: Will leaders and the public now at last commit to a firm, generous, and durable social and economic safety net? That would accomplish more for human health and wellbeing than any vaccine or miracle drug ever can.”

Here are the choices he outlines: Will science and fact gain the high ground in guiding resources and behaviours? Will solidarity endure? Will compassion and respect be restored for all of the people who make civilisation feasible, including a guarantee of decent livelihoods and security for everyone? Will the frenzied world of commerce take a breath and let technology help simplify work without so much harm to the planet and without so much stress on everyone?
And probably the ‘six-million-dollar question’ among the choices Berwick enumerates is: “Will society take a break from its obsessive focus on near-term gratification to prepare for threats ahead?”
Lots of questions, but ones that need to be asked if our post-Covid lives are to improve.
Opinion THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 20
But immediately his head fell to the ground, he bent down, picked it up, washed it in the waters of the well, then carried it to the neighboring village, lay down and died
FOR ALL THAT MATTERS



NEW WEBSITE LAUNCHING FEBRUARY 10
IN MEDICINE
Questioning the notion of ‘settled science’
It was scepticism, not unthinking belief, that prompted the emergence of modern medicine from the swamp of mumbo-jumbo
GEORGE WINTER

Bacteriologist Sir Almroth Wright (1861–1947) was Alexander Fleming’s boss and a great chum of George Bernard Shaw. Shaw made Wright the hero of his play The Doctor’s Dilemma (1906), where he appears as Sir Colenso Ridgeon. In Act 1 Ridgeon observes: “Opsonin is what you butter the disease germs with to make your white blood corpuscles eat them,” and I can imagine Shaw quizzing Wright on the truth of that line, with the latter modestly offering one of his better-known dictums: “Before making a decision on any subject, a man should always have numerous conversations with those who are expert in it.”
Confirming that his intellectual reach extended beyond his grasp of germs, Wright penned a letter to The Times, which was published on 28 March 1912 and in which he discarded all traces of diffidence to divulge that he was an “expert”. Wright’s letter explained his opposition to female suffrage by claiming that women’s participation in politics was made intolerable because of their physiology. Militant suffragettes, furthermore, were immoral, sexually embittered, and in his “expert” opinion “… there is mixed up with the women’s movement much mental disorder”.
A century later, it is unlikely that a contemporary purveyor of such misogynism would be invited onto the airwaves, yet a warm welcome awaits those who yield to the temptation of a daytime-telly sofa by permitting themselves to be described
MCQs
MULTIPLE CHOICE QUESTIONS
Question 1
Question 2
Question 3
Question 4
as an expert in something or other. But before lowering themselves onto a turquoise-hued Chesterfield they should remember that we live in an age where expert opinion is no longer accepted without demur. For example, in July 2005 the General Medical Council (GMC) struck off paediatrician Professor Sir Roy Meadow after finding him guilty of serious professional misconduct for giving “misleading” evidence in the Sally Clark case. Mrs Clark served three years in jail after being wrongly convicted of murdering her two sons, but she was exonerated after an appeal in 2003. During her trial, Sir Roy asserted that the probability of two natural unexplained cot deaths in the family was 73 million to one, a figure subsequently challenged by the Royal Statistical Society. The chairman of the GMC panel told Meadow: “You should not have strayed into areas that were not within your remit of expertise.” Like Wright, Meadow assumed that being an expert on something gives you range, whereas it only gives you focus.
The word “scientist” was first coined in 1834 by William Whewell (1794–1866) in an article for The Quarterly Review on Mary Fairfax Somerville’s book On the Connexion of the Physical Sciences. Up until then there had been “friends of science”, “cultivators of science” and “interpreters of nature” … but Whewell levered “scientist” into the language only relatively recently. Those who throw a protective cloak of “settled science” around what they consider sacrosanct areas of scientific knowledge when challenged by new evidence should remember physicist Dr Richard Feynman. The Nobel laureate who discovered the cause of the 1986 Space Shuttle disaster – and who JG Ballard praised for his ability “to tackle any problem in the most direct and simple way, ignoring the accumulated mass of conventional opinion” – defined science as “the belief in the ignorance of experts”.
But the internet can make us all experts – or so we think. The combination of universal access to data and self-absorption means that we mistake information for knowledge, we mistake knowledge for wisdom and there are few who can point out the difference.
Might objectivity be nothing more than an illusion? The notion of scientific objectivity was considered by molecular biologist Jacques Monod in his Chance and Necessity (1972). According to Monod, nature itself is objective because it lacks purpose. This prompted philosopher Dr Mary Midgley to pose this question in her Science as Salvation (1992): “… as he too was part of nature – was his own purpose in writing his books real or not? He doesn’t say.” More recently, the purpose of Dr Daniele Fanelli writing in PLoS ONE (2010; 5: e10271) was to ask whether academia’s publish or perish culture might “conflict with the objectivity and integrity of research, because it forces scientists to produce ‘publishable’, results at all costs”. Fanelli cites evidence to show that competition in academia can be seen as a threat to scientific integrity.
Whether it’s, say, the origins of heart disease, stomach ulcers or Covid-19, those who express scepticism about evidence adduced by so-called experts – whose opinions are often promoted without question by an apparently compliant mainstream media – are portrayed as “denialists” by those who shrink from disturbing the sleep of reason. But they forget that it was scepticism that prompted the emergence of modern medicine from the swamp of mumbo-jumbo.
As the late Prof Roy Porter observed: “Medicine is an enormous achievement, but what it will achieve practically for humanity, and what those who hold the power will allow it to do, remain open questions [my emphasis].”
Question 5
In hand, foot, and mouth disease
A. The cause is herpes simplex virus infection.
B. Teenagers are most likely to be affected.
C. The vesicular rash is confined to the hands, feet, and mouth.

D. There are little or no systemic symptoms.
E. Spontaneous resolution occurs within two-to-three days.
In older patients with community acquired pneumonia
A. Age alone is the strongest risk factor for fatality.
B. The majority of patients present with confusion.
C. Respiratory rate over 20 per minute should prompt hospital admission.
D. First-line antibiotic of choice is amoxycillin.
E. Follow-up is important.
Recognised auditory developmental milestones include

A. At one month, turns eye in direction of sound.
B. At six months, development of sound localisation.
C. By eight months, responds to name and simple commands.
D. By two years, incessant talk.
E. At four years, hears ticking of wristwatch three inches to right and left.
In the treatment of chronic gout, allopurinol
A. Acts by increasing urate excretion in the urine.
B. Possible side-effects include alopecia.
C. Should not be prescribed with NSAIDs.
D. Dose should be titrated up, in 100mg increments, from 100mg a day.
E. Use is contraindicated in patients with renal impairment.
Correct advice to give to patients with colostomies includes


A. Bleeding around the outside of the stoma when changing the appliance is normal.
B. Experiment with your diet.
C. Never use laxatives.
D. Consult the doctor if you experience the sensation of rectal fullness.
E. Parastomal hernias are better repaired while small.
E. FALSE. The majority are asymptomatic and only 10 per cent will require surgical correction if strangulated or severely affecting the patient’s quality-of-life.
D. FALSE. 70 per cent of patients will experience this a year after stoma surgery.
C. FALSE. Osmotic or bulkforming laxatives are preferable to stimulant types.
B. TRUE. Many people who have had ostomy are able to eat anything.
A. TRUE. From overzealous cleaning.
ANSWER 5
E. FALSE. Start at 50mg and titrate in smaller increments.
D. TRUE. Most adults require 300-500mg daily to maintain serum urate below 360μnol/l (6mg/dl).
C. FALSE. May be needed to treat flares due to urate crystal shedding into the synovial fluid when starting treatment.
B. TRUE. Also nausea and raised liver enzymes.
A. FALSE. Xanthine oxidase inhibitor that reduces urate production.
ANSWER 4
E. TRUE. Also hears whisper at same distance.
C. FALSE. Not until about 12 months.
B. TRUE. First horizontally, then below and above ear level.
A. FALSE. At one month will briefly quieten to sound. By three months dislikes harsh sounds and will turn eye in direction of sound.
ANSWER 3
E. TRUE. Eg, 16 per cent of male smokers will have an underlying lung cancer.
D. TRUE. 500mg to 1g three times a day.
C. FALSE. Over 30 per minute.
B. FALSE. With tiredness, breathlessness, pleuritic chest pain, cough.
A. FALSE. Comorbid disease, malnutrition, functional disability, cognitive impairment, are more important.
ANSWER 2
E. FALSE. Within about a week.
D. FALSE. The patient may be febrile and unwell.
C. FALSE. May also affect the nappy area.
B. FALSE. Young children are most affected.
A. FALSE. Is caused by Coxsackie virus.
ANSWER 1
D. TRUE. Remember hearing screening may be directly for deafness or for consequent speech failure.
THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 22 Opinion
Read more by George Winter at www.mindo.ie
THERESA LOWRY-LEHNEN, RGN, GPN, RNP, BSc, MSc, M. Ed, PhD, Clinical Nurse Specialist and Associate Lecturer, Institute of Technology Carlow
Peptic ulcer disease
Peptic ulcer disease is a significant source of morbidity and mortality worldwide, with a lifetime risk of development ranging from 5-to-10 per cent
Peptic ulcers are ulcerations exceeding 5mm in diameter that develop in the mucosal lining of the stomach or duodenum, resulting from an imbalance between factors promoting mucosal damage (such as gastric acid, pepsin, Helicobacter pylori (H. pylori) infection and non-steroidal anti-inflammatory drugs (NSAIDs)), and mechanisms promoting gastroduodenal defence, such as prostaglandins, mucus, bicarbonate, and mucosal blood flow. Ulcers smaller than this or without depth are classed as erosions. While peptic ulcers usually occur in the stomach and proximal duodenum, they may also occur in the lower oesophagus, distal duodenum, or jejunum.
Peptic ulcer disease (PUD) is a significant source of morbidity and mortality worldwide with a lifetime risk of development ranging from 5-to-10 per cent. Epidemiological studies, however, have shown a sharp decreasing trend in the past 20-to-30 years in the incidence rates of hospital admissions and mortality associated with the condition, due to improved hygiene, more effective treatments including the widespread use of PPIs, and judicious use of NSAIDs and aspirin.3
Duodenal ulcers are four times more common than gastric ulcers and are also more common in males than females.1,2 Gastric ulcers are most commonly located on the lesser curvature and duodenal ulcers are most common at the duodenal bulb. Duodenal ulcers occur most frequently in the first portion of the duodenum (over 95 per cent), with approximately 90 per cent located within 3cm of the pylorus and are usually less than or equal to 1cm in diameter.7 Peptic ulcers are round or oval in shape with a smooth base. Acute ulcers have regular borders, while chronic ulcers have elevated borders with inflammation.1,2
The mechanism of PUD results from an imbalance between gastric mucosal protective and destructive factors. In PUD there is usually a defect in the mucosa that extends to or beyond the muscularis mucosa. Once the protective superficial mucosal layer is damaged, the inner layers are susceptible to acidity and the ability of the mucosal cells to secrete bicarbonate is compromised. H. pylori can colonise the gastric mucosa and also impair the secretion of bicarbonate, promoting the development of acidity and gastric metaplasia.1,2
Aetiology
Common causes of PUD include H. pylori bacteria, NSAIDs and other medications. Rare causes include Zollinger-Ellison syndrome, malignancy, stress, viral infection, vascular insufficiency, radiation therapy, Crohn’s disease, and chemotherapy.1
H. pylori, a gram-negative bacillus found within the gastric epithelial cells, is responsible for 90 per cent of duodenal ulcers and 70-to-90 per cent of gastric ulcers. H. pylori causes an inflammatory response with neutrophils, lymphocytes, plasma cells, and
macrophages within the mucosal layer and causes epithelial cell degeneration and injury. H. pylori has a wide spectrum of virulence factors allowing it to adhere to and inflame the gastric mucosa. This results in hypochlorhydria or achlorhydria, leading to gastric ulceration.1 All patients found to have peptic ulcers should be tested for H. pylori 7
NSAID use is the second most common cause of PUD and approximately 25 per cent of regular NSAID users will develop PUD. Aspirin users are also twice as likely to develop peptic ulcers as the general population.7 NSAIDs damage the gastroduodenal mucosa through both systemic and local mechanisms, but the systemic inhibition of cyclooxygenase 1 (COX-1) derived prostaglandins is regarded as the main mechanism. The secretion of prostaglandin normally protects the gastric mucosa. NSAIDs block prostaglandin synthesis by inhibiting the COX-1 enzyme, resulting in decreased gastric mucus and bicarbonate production and a decrease in mucosal blood flow.1 Other medications including corticosteroids, bisphosphonates, potassium chloride, and fluorouracil have been implicated in the aetiology of PUD.3
About a fifth of PUD cases are not related to H. pylori, NSAIDs or aspirin, but the accuracy of this value has been challenged due to false negative H. pylori testing or under reported NSAID use.
Idiopathic PUD may be due to an im-
Gastric
Source: https://patient.gastro.org/peptic-ulcer-disease/
within 15-to-30 minutes following a meal in patients with a gastric ulcer, and two-tothree hours post prandial for patients with a duodenal ulcer. Nocturnal pain is common with duodenal ulcers.1,2 The most common complication of PUD is gastroduodenal bleeding. Perforation is a less frequent, but potentially life-threatening complication. Either of these may be the presenting symptom, particularly in patients taking NSAIDs.5
Investigations and diagnosis
A detailed history including medication use and lifestyle factors, physical examination, and invasive and non-invasive
antigen test and a full blood count. The urea breath test has high specificity; however, false-negative results can occur in the case of PPI use.6 Other investigations to consider may include fasting serum gastrin level and urine NSAID screen.4
CT scans can identify non-perforated peptic ulcers. However, many patients will need endoscopy, oesophagogastroduodenoscopy (OGD) for further evaluation. Barium endoscopy is an option for patients with contraindications to OGD.
Once the diagnosis of peptic ulcer disease has been made, it is important to establish the aetiology of the disease as this will help develop a treatment plan for the patient, not only acutely, but also a long-term plan to help prevent a recurrence.6
Differential diagnosis is important to rule out conditions which share similar signs or symptoms to PUD, including gastritis, gastro-oesophageal reflux disease (GORD), gastric cancer, pancreatitis, biliary colic, and cholecystitis. Life-threatening conditions that can also have similar presentations to PUD include myocardial infarction (MI), mesenteric ischaemia, and mesenteric vasculitis.1
Treatment and management
balance between factors that contribute to mucosal integrity and aggressive insults, including a hyper-secretory status.7
Symptoms
Approximately two-thirds of patients with PUD are asymptomatic. In symptomatic patients, the most common presenting symptom of peptic ulcer disease is epigastric pain, which may be associated with dyspepsia, bloating, abdominal fullness, nausea, or early satiety. Other signs include weight loss/gain, haematemesis and melaena. Signs and symptoms of PUD vary depending upon the age and location of the ulcer. Gastric and duodenal ulcers can be differentiated by the timing of their symptoms in relation to meals. Epigastric pain usually occurs
medical tests are important for an accurate diagnosis of PUD. A careful history should be obtained and noted for the presence of any complications. Any patient presenting with anaemia, melaena, haematemesis or weight loss should be further investigated for complications including bleeding, perforation, or cancer. A physical exam may reveal epigastric abdominal tenderness and signs of anaemia.1,3
Endoscopy is the gold standard for diagnosis of PUD. Although invasive, endoscopy allows for biopsy and includes a variety of methods for testing, such as histology, culture, or rapid urease test. Endoscopy can be used to detect H. pylori and can also rule out malignancy.7 Less invasive options include a H. pylori carbon-13 urea breath test or stool
The treatment plan for peptic ulcers is developed based on the degree of disease noted at diagnosis. Patients who present with complications, including perforation or bleeding, may require surgical intervention. However, the majority of patients are treated with anti-secretory agents to help reduce the amount of acid exposure to the ulcerated region, and in turn, provide symptomatic relief and promote healing. For patients who present with a history of heavy NSAID use, the first step is to advise them to avoid NSAIDs, as this is not only a possible aetiology, but also a cause of worsening symptoms. Smoking and alcohol cessation is also encouraged, as these may exacerbate symptoms.6
Treatment for H. pylori infection
Apart from exclusion of malignant disease,
Gastroenterology Clinical THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 23
Continued on p24 ▸
and duodenal ulcers can be differentiated by the timing of their symptoms in relation to meals. Epigastric pain usually occurs within 15-to-30 minutes following a meal in patients with a gastric ulcer, and two-to-three hours post prandial for patients with a duodenal ulcer
Continued from p23 ▸
detection of H. pylori infection with histology or rapid urease tests is essential to the subsequent treatment plan.3,4
First-line treatment for H. pylori-induced PUD is a triple regimen comprising two antibiotics and a proton pump inhibitor (PPI) (See Figure 1). PPIs should be avoided two weeks before testing for H. pylori, as they may increase the risk of a false negative result. Antimicrobials should also be avoided for four weeks before testing.5 Due to increasing antibiotic resistance in H. pylori, treatment has become more difficult, and the efficacy of triple therapy has fallen below 70 per cent in many countries.7
Post eradication testing must be carried out at least eight weeks following completion of therapy. In cases where the first-line regimen to eradicate H. pylori infection has failed, a different treatment regimen should be used as second-line.5 With failure of a second-line regimen the patient should be referred to gastroenterology for endoscopy with culture and antimicrobial susceptibility testing, to tailor therapy and increase the
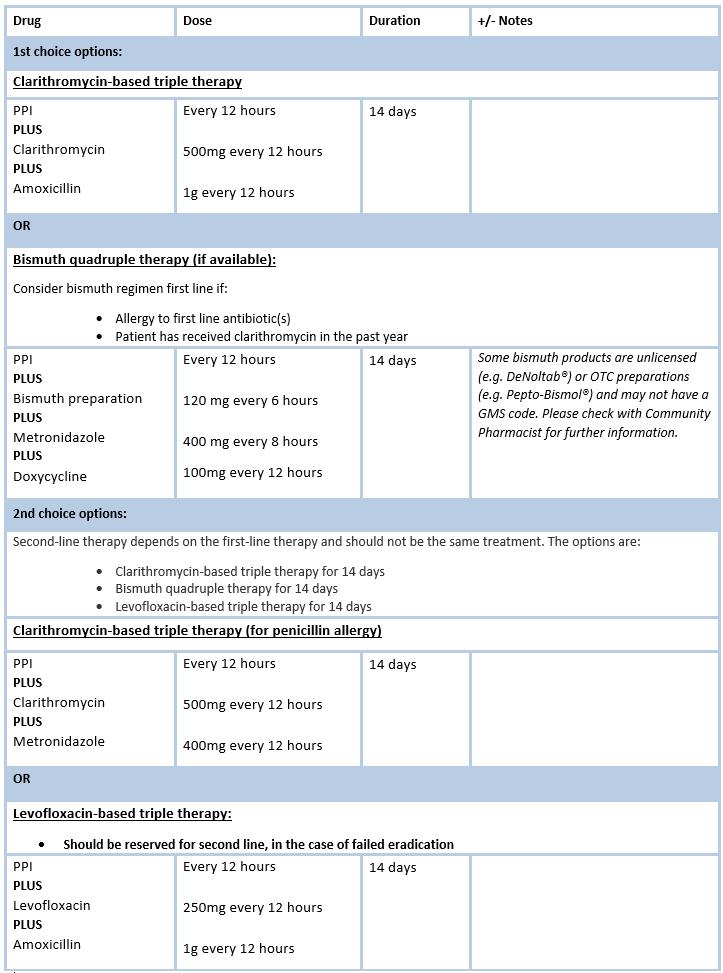
likelihood of eradication success.5
Anti-secretory drugs used for PUP include H2-receptor antagonists and PPIs. PPIs have largely replaced H2 receptor blockers due to their superior healing and efficacy. PPIs block acid production in the stomach, providing relief of symptoms and promote healing. Treatment may be incorporated with calcium supplements as long-term use of PPIs can increase the risk of bone fractures. The duration of PPI therapy varies for patients depending on the presenting symptoms, level of compliance, and the risk of recurrence. The majority of patients, however, do not require long-term anti-secretory therapy following H. pylori treatment, upon confirmation of eradication and if they remain asymptomatic.6
NSAID-induced PUD can be treated by stopping the use of NSAIDs or switching to a lower dose. Corticosteroids, bisphosphonates, and anticoagulants should also be discontinued if possible.1
Surgical treatment for PUD is indicated if the patient is non-compliant, unresponsive to medical treatment, or at high risk of complications. A refractory peptic ulcer is defined as one over 5mm in diameter that does
Latest treatment approaches to H. pylori eradication highlighted at ISG Winter Meeting
Pat Kelly
The Irish Society of Gastroenterology (ISG) 2021 Winter Meeting, held in early December, heard from Prof Javier Gisbert, Consultant Gastroenterologist at La Princesa University Hospital in Madrid, Spain, who spoke on the topic ‘European registry on H. pylori management: Most relevant results for clinical practice’.
Prof Gisbert provided an overview of eradication therapy and described how this European registry is a large, long-term registry of routine clinical practice by gastroenterologists in Europe, with 30 countries, more than 200 investigators and some 50,000 patients involved.
He gave the attendees a synopsis of a number of studies, one of which showed that prescription of tripletherapies in first-line treatment in Europe has markedly decreased from 50 per cent in 2013, to approximately 30 per cent in 2020.
The prescription of 14-day treatments has significantly increased, while sevenday treatment courses have reduced to practically zero, “which is in line with current guidelines”, said Prof Gisbert.
“All these changes in clinical practice have been associated with an increase in the overall efficacy of eradication therapy of approximately 10 per cent,” said Prof Gisbert.
However, overall, he said “management of H. pylori infection by European gastroenterologists is heterogeneous, suboptimal and discrepant with current recommendations”.
“Only quadruple therapies lasting for at least 10 days are able to achieve
more than 90 per cent eradication rates. European recommendations are being slowly and heterogeneously incorporated into routine clinical practice, which has been associated with a corresponding increase in effectiveness.”
Outlining other such studies in which he was involved, Prof Gisbert told the conference that optimised concomitant therapy shows significantly higher cure rates – higher than 90 per cent –compared to triple therapy. “The addition of metronidazole to the standard triple therapy increased eradication rates by 10 per cent, resulting in more mild adverse events, but without impairing compliance with therapy,” he said.
Another study looked at the addition of bismuth to standard triple therapy: “Due to the poor eradication rates of standard triple therapy, the addition of bismuth salts has been proposed for first-line eradication of H. pylori,” said Prof Gisbert.
He also outlined the evolution of antibiotic resistance in H. pylori over recent years and told the meeting that while resistance to clarithromycin remained stable, it has been higher than 15 per cent across these years. “This is the threshold that we generally consider as being indicative of high clarithromycin resistance.”
However, metronidazole resistance decreased over this time.
Another study reported by Prof Gisbert looked at the potential to improve overall H. pylori treatment. “Common mistakes include the use of standard triple therapy where it is ineffective, or to prescribe it as eradication therapy for only sevento-10 days,” said Prof Gisbert.
Source: www.hse.ie/eng/services/list/2/gp/antibiotic-prescribing/
not heal despite eight-to-12 weeks of PPI therapy. The common causes are persistent H. pylori infection, continued use of NSAIDs, or significant comorbidities that impair ulcer healing or other conditions like gastrinoma or gastric cancer. Surgical options include vagotomy or partial gastrectomy.1
Patient education is very important for those with PUD. It should include information about the primary causes of PUD, dietary advice, practices to avoid, such as NSAID use, and the risk of any interventions offered including the long-term use of PPIs if it is the therapy of choice for symptomatic management.6
Prognosis
Timely diagnosis and treatment of PUD and its sequelae are crucial to minimise associated morbidity and mortality, as is prevention of PUD among patients at high risk, including those infected with H. pylori and users of NSAIDs.2 Identifying the risk factors and mechanisms that lead to the development of PUD helps to understand the approach behind diagnostic and treatment strategies.7 Prognosis for individuals with PUD is very good if the underlying cause is successfully treated. For most people, treatment that targets the underlying cause is effective at eliminating PUD. Recurrence of peptic ulcers, however, is com-
mon, but recurrence may be prevented or reduced by maintaining good hygiene, avoiding alcohol, smoking and NSAIDs.
References
1. Malik T. (2021). Peptic ulcer disease. Available at: StatPearls Publications. www.statpearls.com/ ArticleLibrary/viewarticle/26913
2. Kavitt R, Lipowska A, Yeboa A, Grainek I. (2019). Diagnosis and treatment of peptic ulcer disease. Am J Med. Volume 132; Issue 4; pp447-456; April 2019
3. Lanas A, Chan, F. (2017). Lancet. Peptic ulcer disease. doi:10.1016/S0140-6736(16)32404-7
4. BMJ Best Practice (2020). Peptic ulcer disease. Available at: https://bestpractice.bmj.com/topics/ en-gb/3000205
5. HSE antibiotic prescribing. (2021). Helicobacter Pylori. Available at: www. hse.ie/eng/services/list/2/gp/antibioticprescribing/conditions-and-treatments/gastro/ helicobacter-pylori/
6. Woolf A, Haddad L, Ocasio G. (2021). Duodenal Ulcer (Nursing). In StatPearls Publishing. Available at: www.ncbi.nlm.nih.gov/books/NBK568678/
7. Narayanan M, Reddy K, Marsicano E. (2018). Peptic ulcer disease and Helicobacter Pylori infection. Mo Med. 2018 May-Jun; 115(3): 219–224. PMCID: PMC6140150
8. Image: https://patient.gastro.org/ peptic-ulcer-disease/
Clinical Gastroenterology THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 24
Figure 1: Treatment approach for H. pylori (HSE) 5
conditions-and-treatments/gastro/helicobacter-pylori/
Pioneering for patients to take control of the burden of IBD Working together with the IBD community to explore drivers of disease, address unmet needs and support optimal patient care. For more information contact: medicalaffairsuk@glpg.com We are Galapagos, a pioneering biotechnology company, focussed on making a difference to the lives of people with inflammatory diseases. GALAPAGOS and the GALAPAGOS logo are registered or pending trademarks of Galapagos NV. © 2021 Galapagos NV. All rights reserved. Date of preparation: November 2021 | IE-IBD-NA-202111-00001
DR SILE KELLY 1 , SpR in Public Health; and DR BREDA COSGROVE 1 , Specialist in Public Health Medicine. 1Department of Public Health Mid-West, Limerick
Food, (and not so) glorious food
Food is important in so many aspects of life and health. It can bring joy, love, comfort, cultural expression, and good health. But like many will know there is a dark side to food, in particular when it is inadequate, in excess, contaminated, or of poor quality. Food is powerful. Food and food choices can carry and increase the risk of diseases, result in malnutrition, contribute to climate destabilisation and even cause death. Trying to address these health issues can sometimes feel as helpless as facing an asteroid hurtling towards Earth. The impact of food-related disease can have a significant impact on an individual’s health, but also cause a chain-reaction that is felt across healthcare and society for many years to come. This article will review the public health consideration of food and nutrition, and the important interventions that physicians can integrate into their daily practice, benefitting patients and the wider population.
Food and disease
Communicable and non-communicable disease can arise from food and malnutrition. This is something that physicians encounter on a daily basis, through their management of people with obesity, cardiovascular disease, infectious colitis or gastroenteritis, and many more conditions.
Food and infectious disease
(i) H. pylori
The bacteria helicobacter pylori (H. pylori) can infect the stomach and cause a spectrum of symptoms and disease. It not only affects the infected individual, but also has wider healthcare implications. H. pylori is thought to be transmitted through contaminated food and water, as well as person-to-person spread. It can be carried asymptomatically, cause gastritis, ulcer formation, gastric adenocarcinoma and MALT lymphoma, as well as contribute to the burden of dyspepsia in the population. It has been estimated that approximately 40 per cent of the population will at some time in their lives experience dyspepsia, and 2 per cent will lose time from work because of it. Approximately 20-to-50 per cent of populations in Western countries are infected with H. pylori, and up to 80 per cent of populations in developing countries. Of these, 10 per cent will develop a duodenal or gastric ulcer, and up to 3 per cent may develop gastric cancer and MALT lymphoma. H. pylori is therefore an important health problem, and a significant contributor to the global burden of disease.
A ‘test and treat’ strategy has been shown to be effective in eradicating H. pylori. It also significantly reduces the need for endoscopy by approximately two-thirds of cases. This contributes to an overall reduction in healthcare costs. The annual national cost of elective day case upper gastrointestinal (GI) endoscopy performed in Ireland is an
estimated €29.9 million per year.
To maximise health and healthcare benefits, H. pylori should be eradicated. This in turn should be confirmed with follow-up investigations to ensure eradication has been successful. Eradication of H. pylori is the most effective way in preventing the recurrence of duodenal ulcer, and contributes to the reduction of the burden of dyspepsia and other negative outcomes of H. pylori infection (see Table 1). Population screening for H. pylori bacteria in the Irish context has previously been discussed. There are potential positives of screening, however, there are additional considerations such as antimicrobial stewardship, and demand on services. This is a particular consideration with increasing antimicrobial resistance as well as the pandemic having given rise to a backlog of healthcare delivery. An effective local implementation of a ‘test and treat’ strategy, with follow-up for eradication, has the potential to significantly decrease the burden of disease in our populations, decrease the use of long-term medications, and reduce the burden on endoscopy departments.
(ii)
Food poisoning
Another common communicable disease encountered in gastroenterology is ‘food poisoning’; gastroenteritis and infectious colitis. Verocytotoxigenic E. Coli (VTEC) is a particular issue in Ireland (see Figure 1), and a significant public health burden. VTEC can be spread from person-to-person, through contact with farm animals or through contaminated food and water. Ireland has the highest incidence of VTEC in Europe at a rate of 18.6/100,000. Once again, its impact is not only a risk to the infected individual, but additionally causes major challenges to close contacts and the healthcare setting.
There is a risk of developing haemolytic uraemic syndrome (HUS) in 5-to-12.5 per cent of people with VTEC, in particular in paediatrics and the elderly, which can be fatal in 3-to-5 per cent of people. Approximately 12 per cent can have long-term sequelae from HUS. VTEC can be suspected not only on clin-
ical grounds based on symptoms, but also on epidemiological grounds, such as contact with farm animals or using drinking water from an untreated private well. It has a low infectious dose, can be carried asymptomatically, and have prolonged shedding of the virus in stool.
In a healthcare setting, there are a number of vulnerable persons at higher risk of acquiring and spreading the infection. Healthcare workers play an important role in reducing that risk through good hand washing and food handling practices. A healthcare worker who contracts VTEC will also be required to submit two negative stool samples in order to return to work (ie, microbiological clearance). In adults, VTEC can take on average seven days due to viral shedding, and in children it could take several weeks following the resolution of diarrhoea. Antibiotics are ineffective in treating VTEC. Prevention is better than cure, and therefore handwashing is better than repeated stool samples.
Food and nutrition
There are also many non-communicable diseases associated with food and nutrition that have a wider public health impact. The healthcare system in Ireland faces the double burden of malnutrition that includes undernutrition as well as obesity.
(i) Undernutrition
Malnutrition is a significant health issue in Irish hospitals. One-in-three people admitted to hospital is malnourished. Undernutrition can increase a person’s risk of infection, pressure sores, falls, readmission, and death. There is also an associated increased healthcare cost and prolonged hospital stays in those who are malnourished. Overall it results in poorer outcomes for patients, with many of these potentially avoidable. Disease-related malnutrition annual costs are estimated to be €1.42 billion, representing more than 10 per cent of the total health and social care budget. Screening at-risk patients with a validated screening tool will result in early detection and management of those at risk. Widespread implementation of screening for malnutrition such as the Malnutrition Universal Screening Tool (MUST) at admission will result in more effective interventions, improve patient outcomes and reduce healthcare costs. Trainees can avail of nutrition study days, a core part of higher specialist training (HST), to support their education and deepen their understanding of their role in the prevention of malnutrition-related health outcomes.
(ii) Obesity
On the other end of the spectrum is obesity. Ireland has one of the highest levels of obesity in Europe with 60 per cent of adults and more than 20 per cent of children living with overweight and obesity. Obesity increases the risk of developing a number of GI health issues, including but not limited to Barrett’s oesophagus, gastroesophageal reflux disease (GORD), inflammatory bowel disease (IBD), colonic polyps, and some GI cancers such as oesophageal, gastric, colorectal, hepatocellular, and pancreatic cancer. The endoscopic investigation and management of these conditions is further complicated by obesity. Some studies have shown that people with obesity may require more aggressive bowel prep, and there is speculation that there may be increased difficul-
Clinical Gastroenterology THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 26
Continued on p28 ▸
OUTCOME NNT SOURCE Recurrence of duodenal ulcer at 12 months 2 Delaney, 2006 Eradication of functional dyspepsia 15 Delaney, 2006 Reduction of incidence of gastric cancer 124 Ford et al, 2020
Figure 1: VTEC cases in Ireland between 2015-2020 adapted from the HPSC website* (www.hpsc.ie/a-z/gastroenteric/vtec/ ). *2018 and 2019 are estimate figures only
Table 1: Estimated number needed to treat (NNT) for H. pylori eradication that results in a positive health outcome
The Evolution of Precision Science
The E volution of Precision Science
Contains a unique combination of two clinically researched synergetic strains

Contains a unique combination of two clinically researched synergetic strains.

Calcium: Contributes to the normal function of digestive enzymes.

Pantothenic Acid, Vitamin B6: Contribute to the reduction of tiredness and fatigue.
✝Calcium: Contributes to the normal function of digestive enzymes.
Pantothenic Acid, Vitamin B6: Contribute to the reduction of tiredness and fatigue.
New
+ TM Group
NEW ScientificallyStudied 1714 ScientificallyStudied
Continued from p26 ▸
ty achieving caecal intubation, potentially prolonging the procedure, increasing the risk to the patient, and the risk of an incomplete procedure. Gastroenterology clinics and endoscopy are seeing a rise in patients living with obesity. Screening and brief health interventions for obesity can be effective in supporting weight loss. In clinical practice, it is rare to make these opportunistic interventions on weight. Healthcare workers are among the most trusted in society as an information source, and screening opportunistically
and advising on weight loss can help support your patients to improve health. This can be further enhanced by supporting them through referral to an available community weight-loss programme. This may cost the health service initially, but one study in the UK showed that a community weight-loss programme is cost-effective from the third year and cost-saving from the ninth year onwards over an entire 20-year period, as it reduces the number of obesity-related health conditions. Ireland currently has some community weight-loss programmes on offer that may be available in your catchment area.
The only licensed treatment for the reduction in recurrence of episodes of overt hepatic encephalopathy ≥ 18 years1
(iii) Adequate nutrition
Adequate nutrition is one of the most effective (and least costly) ways to decrease the global burden of disease. Screening for malnutrition and early involvement of dietetics are important tools at a physician’s disposal. Moreover, to further support adequate nutrition, the HSE has a ‘Healthy Food for Life’ campaign with many readily available online resources. They have healthy eating guidelines for all people over the age of five years, including for older and pregnant or breastfeeding people. The 101 Square Meals recipe book is a freely available starting point for those people that are open to improving their nutrition.
Understanding and supporting nutrition is an important part of the national Gastroenterology and Hepatology Higher Specialist Training (HST) curriculum: “To understand energy homeostasis, under nutrition and be capable of determining nutritional status, applying that knowledge and appropriate skills to providing additional nutritional support….”
Despite the importance of nutrition as a leading cause of morbidity and mortality, nutrition being a recognised learning outcome in HST, and patients placing their trust in doctors as their information source on nutrition, many physicians are not confident in their nutrition-related counselling skills. This represents a gap in the needs of our patients and the delivery of care. To mitigate against the rising tide of nutrition-related illness, doctors should be skilled in meeting the needs of the people they serve and empower people to achieve better health outcomes. The Irish Society for Clinical Nutrition and Metabolism (IrSPEN) has a number of educational and professional resources, including the Life Long Learning (LLL) Programme for medical professionals.
Food and climate
IE Prescribing Information: Targaxan 550mg (rifaximin-α)
REFER TO FULL SUMMARY OF PRODUCT CHARACTERISTICS (SmPC) BEFORE PRESCRIBING
Presentation: Film-coated tablet containing rifaximin 550 mg. Uses: Targaxan is indicated for the reduction in recurrence of episodes of overt hepatic encephalopathy in patients ≥ 18 years of age. Dosage and administration:
Recommended dose: 550mg twice daily as long term treatment for the reduction in recurrence of overt episodes of overt hepatic encephalopathy. In the pivotal study, 91% of patients were using concomitant lactulose. TARGAXAN can be administered with a glass of water, with or without food. No dosage changes are necessary in the elderly or those with hepatic insufficiency. Use with caution in patients with renal impairment
The safety and efficacy in paediatric patients (aged less than 18 years) have not been established. Contraindications: Contraindicated in hypersensitivity to rifaximin, rifamycin-derivatives or to any of the excipients and in cases of intestinal obstruction. Warnings and precautions for use: The potential association of rifaximin treatment with Clostridium difficile associated diarrhoea and pseudomembranous colitis cannot be ruled out. The administration of rifaximin with other rifamycins is not recommended. Rifaximin may cause a reddish discolouration of the urine. Use with caution in patients with severe (Child-Pugh C) hepatic impairment and in patients with MELD (Model for End-Stage Liver Disease) score > 25. In hepatic impaired patients, rifaximin may decrease the exposure of concomitantly administered CYP3A4 substrates (e.g. warfarin, antiepileptics, antiarrhythmics, oral contraceptives). Both decreases and increases in international normalized ratio (in some cases
with bleeding events) have been reported in patients maintained on warfarin and prescribed rifaximin. If co-administration is necessary, the international normalized ratio should be carefully monitored with the addition or withdrawal of treatment with rifaximin. Adjustments in the dose of oral anticoagulants may be necessary to maintain the desired level of anticoagulation. Ciclosporin may increase the rifaximin Cmax. This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodiumfree’. Pregnancy and lactation: Rifaximin is not recommended during pregnancy. The benefits of rifaximin treatment should be assessed against the need to continue breastfeeding. Side effects: Common effects reported in clinical trials are dizziness, headache, depression, dyspnoea, upper abdominal pain, abdominal distension, diarrhoea, nausea, vomiting, ascites, rashes, pruritus, muscle spasms, arthralgia and peripheral oedema. Other effects that have been reported include: Clostridial infections, urinary tract infections, candidiasis, pneumonia cellulitis, upper respiratory tract infection and rhinitis. Blood disorders (e.g. anaemia, thrombocytopenia). Anaphylactic reactions, angioedemas, hypersensitivity. Anorexia, hyperkalaemia and dehydration. Confusion, sleep disorders, balance disorders, convulsions, hypoesthesia, memory impairment and attention disorders. Hypotension, hypertension and fainting. Hot flushes. Breathing difficulty, pleural effusion, COPD. Gastrointestinal disorders and skin reactions. Liver function test abnormalities. Dysuria, pollakiuria and proteinuria. Oedema. Pyrexia. INR abnormalities. Prescribers should consult the SmPC in relation to all adverse reactions.
IRELAND
Norgine B.V. Antonio Vivaldistraat 150, 1083 HP, Amsterdam, Netherlands Marketing Authorisation number: PA 1336/009/001 For further information contact: Norgine Pharmaceuticals Limited Norgine House Widewater Place, Moorhall Road, Harefield, Uxbridge, UB9 6NS, UK Telephone: +44 (0)1895 826 606 E-mail: Medinfo@norgine.com Ref: IE-HEP-XIF-2100010
Date of preparation: April 2021
Ireland
Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance. Website: www.hpra.ie. Adverse events should also be reported to Medical Information at Norgine Pharmaceuticals Ltd on: Tel. +44 (0)1895 826 606 Email. Medinfo@norgine.com
References:
1. National Institute for Health and Care Excellence. Rifaximin for preventing episodes of overt hepatic encephalopathy: appraisal guidance TA337 for rifaximin. Available from: http://www.nice.org.uk/guidance/ta337


2. TARGAXAN® 550 Summary of Product Characteristics. Available for Ireland from: www.medicines.ie


Product under licence from Alfasigma S.p.A. TARGAXAN is a registered trademark of the Alfasigma group of companies, licensed to the Norgine group of companies. NORGINE and the sail logo are registered trademarks of the Norgine group of companies.
Healthcare is a major contributor to climate destabilisation, and therefore paradoxically a contributor to the burden of disease in our patients. Food, including hospital food, has an important role in its intersectional and pivotal place in climate destabilisation. Globally, Ireland is considered a ‘major emitter’ of greenhouse gases which contribute to climate breakdown, and is considered one of the worst in the European Union. An estimated 71 per cent of emissions from healthcare are derived from the healthcare supply chain; the goods and services an organisation avails of. These are known as scope 3 emissions. They are as of a result of the activities of healthcare but are not owned or controlled by the institution itself, eg, pharmaceuticals, medical devices, personal protective equipment (PPE). Of these, 9 per cent are associated with the provision of food and agricultural products. It is estimated that up to 49 per cent of the food provided to patients in Irish hospitals is not eaten. Uneaten food is wasted food, is not contributing to nutrition, but directly contributing to increased costs and increased emissions. Early consultation with dietetics to tailor the food to the nutritional needs of the patient can reduce food waste, reduce the climate impact, and support your patients’ health.
Conclusion
Cost: €262.41 for 56 tablets Marketing Authorisation holder:


Legal category: Prescription only
IE-HEP-XIF-2100002


Date of preparation: May 2021
NASA and the European Space Agency (ESA) are currently involved in a mission that aims to nudge an asteroid off course and away from Earth. The asteroid causing concern is 11 million light years from Earth, and is not an immediate threat, but they are preparing now. Much like an asteroid, being conscious of the role food and nutrition plays in the health of our patients and implementing strategies to redirect their course toward better health outcomes is preventative. Food and nutrition play a powerful and complex role in health and healthcare in Ireland, and healthcare workers are an important driver in the direction of their patients’ health. Incorporating preventive practices on a daily basis for the future health of patients, wider society and the planet, can feel like nudging an asteroid that is impossibly far away. It takes awareness, extra time, uncertainty if it will work every time, and the results will take years to see. Unlike deflecting an asteroid, we have clear evidence that adequate nutrition is an effective and low cost way to reduce the global burden of disease. Taking the time in daily practice to screen for food-related infectious diseases, to screen for malnutrition on admission, to screen people living with obesity who may be ready and accepting of change, and to further our education in nutrition counselling may just nudge the trajectory of your patients’ health out of the path of disease.
References on request

Clinical Gastroenterology THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 28
Long-term prophylaxis in hepatic encephalopathy (HE)2
At home they are still at risk; …TARGAXAN® rifaximin-α reduces the risk of recurrence of overt hepatic encephalopathy.2
Latest modules
Chronic pain control - An overview
1.5 CPD Hours
Author: Eamonn Brady MPSI
Diabetic foot disease
2 CPD Hours
Authors: Theresa Lowry-Lehnen GPN, RNP, PhD, Clinical Nurse Specialist and Associate Lecturer at IT Carlow. Member of the Irish General Practice Nurses Educational Association (IGPNEA)
The spectrum of treatment for inflammatory bowel disease
2 CPD Hours
Authors: Dr Jayne Doherty, SpR in Gastroenterology, and Dr Garret Cullen, Consultant Gastroenterologist, Centre for Colorectal Disease, St Vincent’s University Hospital, Dublin
A B C doctorCPD.ie
www.medilearning.ie/doctorcpd Free CPD – now accessible on android, iPhone and tablet
Visit
Primary Care Dermatology Society of Ireland, Annual Meeting, 4-5 March 2022
Interactive two-day meeting supporting dermatology workload in general practice
Valerie Ryan speaks to Chair of the Primary Care Dermatology Society of Ireland, Dr Siobhán Twohig , about the Society’s upcoming Annual Meeting
The Annual Meeting of the Primary Care Dermatology Society of Ireland (PCDSI) on Friday
4 March and Saturday
5 March 2022 features a line-up of leading consultants and GPs with dermatologists participating from Ireland, England, Wales, and Spain.
Speakers are to present on a wide range of topics in dermatology, offering take-home messages and tips for general practitioners to optimise care for patients with dermatological conditions.

The new Chair of the Society, Dr Siobhán Twohig, a GP in Ennis and clinical skills tutor at the University of Limerick, spoke to the Medical Independent (MI) outlining the highlights of this year’s event, which will focus on conditions presented and managed in general practice. The meeting has been organised as a virtual, interactive two-day conference. The meeting is to facilitate breakout sessions for those attending to meet with the speakers and colleagues in small groups, as well as sponsors and exhibitors. Dr Johnny Loughnane, one of the founding members of the Society, has co-ordinated the programme.
The November 2021 meeting of the Society had focused on paediatric dermatology and the programme on Friday 4 March begins at 2pm with an update in paediatric dermatology from Dr Fiona Browne, a Consultant Dermatologist at Crumlin Children’s Hospital and Children’s University Hospital, Temple Street. She has a special interest in epidermolysis bullosa and leads the adult service at St James’s Hospital, Dublin.
The skin signs of internal disease are to be addressed by the next speaker Dr Brian Malcolm, a former GP and Associate Specialist from the UK, who has addressed Society meetings previously.
Dr Twohig said the talk will highlight the multidisciplinary work of the GP when conditions present with a rash. Dr Malcolm delivers skin clinics for the dermatology department of North Devon District Hospital, Barnstable.

Pruritis
Dr Colin Long, Consultant Dermatologist at Cardiff and Vale NHS Trust, is to discuss pruritis in the following presentations. He also works at the Royal Glamorgan Hospital and is interested in all aspects of dermatology including the management of inflammatory skin disorders: Eczema, psoriasis, urticaria, and acne as well as the surgical treatment of benign and malignant skin tumours.
Dr Alana Durack, Consultant Dermatologist at University Hospital Waterford, is to address an acute condition
seen more commonly in general practice than in the hospital dermatology services – bacterial skin infection – and will provide “six pearls for primary care” in relation to the topic.
Participants are to hear an update on “What's new in contact dermatitis” from Dr Johnny Bourke, Consultant Dermatologist at South Infirmary Victoria University Hospital, Cork, who has a special interest in the condition. Dr Twohig underlined this as an important area in general practice where GPs can provide vital patient support. She added the fine-tuning of history-taking techniques and management were key aspects of supporting patients.
Dr Chris Bower, Consultant Dermatologist, is Clinical Lead at the Royal Devon and Exeter NHS Trust Hospital. In one of his two talks to the Society this year, he is to speak on diagnosing and treating actinic keratosis in primary care. Dr Twohig said this accounted for a significant workload in general practice. It is a topic expected to be touched on again during Saturday's presentations on cryosurgery and benign skin lesions.
Acne
Dr Long will also provide an update to GPs on the “hugely” useful topic of dermatological emergencies. The next speaker, Prof Eugene Healy from the University of Southampton NHS Foundation Trust, will speak about acne and the latest NICE guidelines, outlining current first-line combination therapies as opposed to traditional treatments employed in general practice. Prof Healy practises general dermatology, and runs a specialist service for psoriasis and a specialist skin lymphoma service. The timing of referrals and how to optimise
patient education as key to care will also be discussed. The Friday programme ends with a talk by Prof Anne-Marie Tobin, Consultant Dermatologist in Tallaght University Hospital and Naas General Hospital and Clinical Associate Professor in Trinity College Dublin on rashes involving the flexures. Her clinical interests are in psoriasis, eczema, hidradenitis suppurativa, and acne.
The programme for Saturday 5 March opens at 9am with a talk entitled “How I manage urticaria” by Dr Malcolm. Dr Twohig said the topic is pertinent to general practice as the condition had both acute and chronic phases.
His presentation is to be followed by a talk on dermatology in skin of colour from London-based Consultant Dermatologist, Dr Daniel Creamer, who has a special interest in dermatological surgery, psoriasis, and photodermatology.
Dr Bower returns later in the morning to speak on leg ulcers followed after the break by Prof Tobin who is to address diagnosis and management of psoriasis in primary care, a chronic condition often seen as a first presentation in general practice.
A presentation at noon on “The child with fever and a rash – when to worry” is scheduled from Dr Browne.
Managing pattern hair loss in general practice is discussed by well-known Dublin Consultant Dermatologist, Dr Dmitri Wall. Dr Wall, St James's Hospital, Dublin, and Hair Restoration Blackrock, has a particular focus on the diagnosis and medical and surgical treatment of hair and scalp disease.
Book launch
On Saturday afternoon the latest Text-
book of Primary Care Dermatology is to be launched. Co-authored by GP Dr David Buckley and Dr Paola Pasquali, it is described as a textbook providing a comprehensive, practical guide to the identification of a range of common dermatological conditions encountered within primary care.
Dr David Buckley, a principal in general practice and GP trainer in Kerry, has developed a special interest and expertise in dermatology and skin surgery. A founding member of the PCDSI and the Primary Care Surgical Society, he has been a member of the European Academy of Dermatology and Venereology since 1993.
From Spain, co-author Dr Pasquali, Head of the Department of Dermatology, Pius Hospital de Valls, Tarragona, is a dermatologist with specialty in non-melanoma skin cancer treatment with special emphasis in cryosurgery, non-invasive imaging techniques and teledermatology. She is Past President of the International Society of Teledermatology, Chair of the American Academy of Dermatology International Member Committee and board member of the European Academy of Dermatology and Venereology.
The afternoon clinical session opens with Dr Pasquali, who is expected to offer “great take-home messages for GPs” on the use of cryotherapy. With so many GPs having access to cryotherapy, Dr Twohig said this engaging speaker is to highlight how to use cryotherapy correctly, the side-effects of cryosurgery and how best to avoid them to optimise patient care.
Dr Buckley is to speak on benign skin lesions, followed by the final presentation of the Annual Meeting by Dr Maeve McAleer, a Consultant Dermatologist in St James’s Hospital, and Children’s Health Ireland. She is to address the meeting on atopic eczema and how to best manage the condition, which is a significant part of a GP’s workload. Key to managing eczema in general practice was the education provided to parents of children with the condition, said Dr Twohig. She added that the Society has launched a new website with useful links for patients on conditions such as eczema.
Dr Twohig also reminded GPs of the available online app Dermabuddy, described as a secure resource to enable GPs to discuss dermatological issues with trusted experts in the field.
The Society has highlighted the varied online programme is specifically focused on general practice and is planning an in-person conference for 2023 to take place in Kilkenny.
Conference Preview THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 30





Skintelligence (where clever meets your skin) “ “ Oilatum Emollient* contains light liquid paraffin. Used to treat itchy, irritating dry skin conditions including eczema and dermatitis, particularly when they affect large areas of your body. Oilatum Junior Flare-up* contains light liquid paraffin, benzalkonium chloride and triclosan. Relieves itching, reduces redness associated with eczema flare-ups in children and helps protect against further drying. Avoid contact of the undiluted product with the eyes and the skin. If your condition does not improve, or if you experience any unwanted effects, stop using the product, consult your pharmacist or doctor. FOR EXTERNAL USE ONLY. *Fire Hazard: product burns more easily. Do not smoke or go near naked flames; clothing and bedding with this product dried on them can catch fire easily. Washing clothing and bedding may reduce product build-up but not totally remove it. A copy of the summary of product characteristics is available on request. PA 126/322/001 (Oilatum Emollient 63.4% Bath Additive) PA 126/323/001 (Oilatum Junior Flare Up bath Additive). Medicinal products not subject to medical prescription. PA Holder: Clonmel Healthcare Ltd., Waterford Road, Clonmel, Co. Tipperary. Also available, Oilatum Soap Bar, an emollient cleanser suitable for everyday cleansing for dry and sensitive skin. Always read the label. Date prepared: October 2021. 2021/ADV/OIL/103H Skilfully combining 50 years of scientific knowledge & expertise. Oilatum, a proven treatment for eczema and related dry skin conditions. skinSimplysmart Medically Proven Comprehensive range for adults and children Trusted for generations
Conference Agenda
Primary Care Dermatology Society of Ireland, Annual Meeting, 4-5 March 2022
AGENDA
Friday 4 March
13.55
14.00
14.40
15.20
16.00
16.30
17.10
17.50
18.30
19.00
19.40
20.20
Welcome – Dr Siobhán Twohig, Chairperson, PCDSI
What’s new in paediatric dermatology – Dr Fiona Browne, Dublin
Skin signs of internal disease – Dr Brian Malcolm, Barnstable, UK
Pruritus – Dr Colin Long, Cardiff, UK
Breakout
Bacterial skin infection: Six pearls for primary care – Dr Alana Durack, Waterford
What’s new in contact dermatitis – Dr Johnny Bourke, Cork
Diagnosing and treating actinic keratosis in primary care – Dr Chris Bower, Exeter, UK
Breakout
Dermatological emergencies – Dr Colin Long, Cardiff, UK
Acne: The latest NICE guidelines – Prof Eugene Healy, Southampton, UK
Rashes involving the flexures – Prof Anne-Marie Tobin, Dublin
21.00 Close
Saturday 5 March
9.00
9.40
10.20
11.00
11.30
12.10
12.50
13.30
13.50
14.00
14.40
15.20
How I manage urticaria – Dr Brian Malcolm, Barnstable, UK
Dermatology in skin of colour – Dr Daniel Creamer, London, UK

Leg ulcers – Dr Chris Bower, Exeter, UK
Breakout
Psoriasis: Diagnosis and management in primary care – Prof Anne-Marie Tobin, Dublin
The child with fever and a rash: When to worry – Dr Fiona Browne, Dublin
Managing pattern hair loss in general practice – Dr Dmitri Wall, Dublin
Breakout
Book Launch
Cryosurgery: Side-effects and how to avoid them – Dr Paola Pasquali, Spain
Benign skin lesions – Dr David Buckley, Tralee
Atopic eczema – Dr Maeve McAleer, Dublin
16.00 Close
32 THE MEDICAL INDEPENDENT |10 FEBRUARY 2022
Clinically proven to effectively treat acne lesions with high tolerability. Take care of your skin with PapiXTM



Take care of your skin with PapiXTM. A multi action defence against acne breakouts, oily, blemished, or acneic skin. With nicotinamide, salicylic acid and urea peroxide. An easy to use regime for acne prone skin.
RELIFETM. MY SKIN SAYS HOW I FEEL.
ACTIVE DEFENCE AGAINST ACNE


www.relife.ie
IE21038 IR-REL-141-2021
Distributed by: A.Menarini Pharmaceuticals Ireland Ltd, Castlecourt,Monkstown Farm, Monkstown, Glenageary, Co. Dublin.
OUR NEW ADDRESS
Post your answers to: Mindo Quizzes, The Medical Independent , Greencross Publishing Ltd,
1 Mortons Lane, Wicklow Town, A67RX38 Closing date for entries is 21 February 2022
SPORTS QUIZ WIN €50 10 February 2022
Q1 Who won his 21st Grand Slam singles title at the Australian Open last month?
Q2 Who became the new manager of Everton Football Club?
Q3 Which city will host the IAAF World Athletics Championships later this year?
Q4 The National Hurling League commenced last week. Which county are the defending champions?
Q5 Who will Katie Taylor fight, in what will be the first female boxing match to headline a bill at Madison Square Garden, when they face off in April?
Q6 Who will Ulster face in the last 16 round of the Heineken Champions Cup?
CROSSWORD COMPETITION
10 February 2022
The winner of the 13 January 2022 Sporting Quiz Competition is Dr Sorcha Dunne, Co Sligo
The winner of the 13 January 2022 Crossword is Dr Patricia McCarthy, Co Waterford
Q1 Who is the new PDC World Darts champion for 2022? A: Peter Wright
Q2 Name the Munster Rugby forwards coach and former England International who has just signed a two-year contract extension? A: Graham Rowntree
Q3 The Australian Open tennis championship serves off next week. Who is the defending ladies singles champion? A: Naomi Osaka
Q4 Name the South African who was named as head coach of the Ireland cricket team? A: Heinrich Malan
Q5 Who secured a unique double by winning the RTÉ Sports Personality and BBC Overseas Sports Personality of the Year? A: Rachael Blackmore
Q6 Which football team won the 2021/22 Scottish League Cup last month by beating Hibernian at Hampden Park? A: Celtic
ACROSS
1 Association created for mutual benefit (8)
5 Narrow point of land projecting into water (4)
9 Agreeable sound or tune (5)
10 Not with anybody (5)
11 Absurd (10)
14 University lecturer (6)
15 Occupation or profession (6)
17 Be a part of a whole (10)
20 External (5)
21 Exchange of tennis strokes (5)
22 Eg, use a straw (4)
23 Grow in number (8)
Down
DOWN
1 Military force (4)
2 Get beaten (4)
3 Practice of designing buildings (12)
4 Carry out (a crime) (6)
6 Put forward an idea (8)
7 Riches (8)
8 Maker (12)
12 Of great value (8)
13 Very attractive (of personality) (8)
16 Struggle to do something (6)
18 Earnest appeal (4)
19 Sort; variety (4)
(6)
18 - Earnest appeal (4)
19 - Sort; variety (4)
Life Mindo Quizzes THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 34 Solution to Crossword Competition
Answers to Sports Quiz Competition Solution to Sudoku A C C E P T I T O E H R R I D E R X P R O V O K E E I C O V A A N G E L I N S T I N C T G U I D H D E D S C A R E D F I E R C E O D D R S E M V B A C T E R I A E M A I L N A L O N E C O N T A I N N C O A C H C N E Y E F D E S I G N 7 5 2 8 4 3 6 1 9 6 9 8 5 7 1 4 2 3 3 1 4 2 9 6 8 5 7 5 4 1 3 2 8 9 7 6 2 3 9 6 5 7 1 4 8 8 7 6 4 1 9 2 3 5 4 8 5 7 6 2 3 9 1 1 2 3 9 8 5 7 6 4 9 6 7 1 3 4 5 8 2 3 9 5 8 6 9 1 9 8 2 4 9 8 2 1 7 6 1 2 7 5 9 6 6 7 8 SUDOKU SCRIBBLE BOX 13 JANUARY 2022 ANSWERS, SOLUTIONS, AND WINNERS WIN €50 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Across
(4)
10
11 - Absurd (10) 14 - University lecturer (6) 15 - Occupation or profession (6)
1 - Association created for mutual benefit (8) 5 - Narrow point of land projecting into water
9 - Agreeable sound or tune (5)
- Not with anybody (5)
17 - Be a part of a whole (10) 20 - External (5)
21 - Exchange of tennis strokes (5) 22 - Eg use a straw (4) 23 - Grow in number (8)
1 - Military force (4)
2 - Get beaten (4)
3 - Practice of designing buildings (12)
4 - Carry out (a crime) (6)
6 - Put forward an idea (8)
7 - Riches (8)
8 - Maker (12)
12 - Of great value (8)
13 - Very attractive (of personality) (8)
16 - Struggle to do something
The trials of the January diet
My BMI is fine – thanks for asking – but my usual relatively low-carb regime took a nosedive since early December, hence my need to drop a few kilos. I just prefer to be a shade less protuberant at the front, so I’m back to ignoring bread and rice and, of course, sugar, all of which crept in to my diet over the past few weeks. And pasta, of course. I discovered a source of great guanciale, the cured pig’s cheek that is absolutely essential for proper spaghetti carbonara, a dish that I perfected around the 10th of December, with predictably addictive consequences.
Not pancetta, then I hear (some of) you ask? No. It’s not the same. Okay, I use Parmesan instead of the classically stipulated Pecorino, because, well, Purist? Moi? Not at all....
Yes, I am missing the pasta, but not the spuds or the sweet things and certainly not the bread, although I do have an occasional hankering after the rillettes that Johann made from our leftover goose. Try eating them on something that isn’t crisp, like good toasted sourdough. But they will keep in the freezer, and when my shape returns to normal an occasional wild indulgence will be indicated.
January is always a pretty grim month, but because my low-carb regime involves plenty of cheese, charcuterie, salad, and good meat, I don’t feel deprived. I really do take off my cap to those who manage to embrace dry January and make it through to the end of the month. The weather is gloomy, there’s so much uncertainty out there and the world is perhaps a more dangerous place than it has been for quite a while. This is surely a very challenging and not terribly opportune time to make such changes. I prefer the drink-less-but-better approach, to be honest. As for Veganuary, why for heaven’s sake choose to eschew animal products at a time when fresh fruit and vegetables are at their scarcest? Or why at any time, given that claims of embracing a plant-based diet as a way of saving the planet are, at best, inaccurate and, at worst, deeply cynical ploys by huge vested interests in the processed food industry. But I’ve said all this before.
I see that researchers at the Francis Crick Institute in London have suggested that something they call “the Western diet” may be responsible for a sharp rise in autoimmune diseases, especially in parts of the world where they had been previously pretty much unknown.
They point out that genetics have not changed over the past few decades, but eating habits certainly have. There’s no suggestion that the Western diet causes such diseases, but the idea is that it may encourage their emergence in genetically susceptible people.
Interestingly, inflammatory bowel disease was virtually unknown in the Middle East and East Asia, but is now becoming common.
At the heart of the Crick Institute researcher suggestion is the question: What
has changed in the way people live over the past couple of decades? The adoption of the Western diet and – perhaps – the intensification of that diet is certainly a compelling candidate.

But it’s a vague term, even vaguer than the exceptionally hard-to-define and possibly non-existent so-called Mediterranean diet. However, I think we all know, almost instinctively, what it is, even if you just reflect on changes in Irish eating over the past half century.
A huge rise in fast food, the widespread use of refined seed oils, increased sugar consumption, the move to white bread, the emergence of highly processed foods, a lack of dietary fibre, so many poorly nourished microbiomes.... The list goes on and on. It’s perhaps significant that cases of autoimmune disease in the Western world started to climb about four decades ago. The rise elsewhere is much more recent.
Those of us who are – to use an awful phrase – serious about food, know what is meant by “proper food” and “real food”. It means eating things that have not been mucked about, that don’t require additives,
that are cooked rather than processed.
And, yes, I know there’s a food industry apologist who most annoyingly trots out the smart alec comment that cooking is food processing. Well, do you have hydrolysed vegetable protein in your cupboard?
High fructose corn syrup? Benzoates? Sorbates? No, I thought as much.
The American writer Michael Pollan has suggested, as a rule of thumb, that we eat nothing that our grandmothers (or
WINE OF THE MONTH
great-grandmothers in the case of younger readers) would not recognise. And I’m fine with that if I can create a kind of global, composite grandmother, including a Provençale and a Tuscan one.
Real wine is a thornier issue. We need to be aware of the fact that no matter how organic, biodynamic or “natural” a wine is, it still contains alcohol that behaves exactly the same way as the stuff in gin or, heaven forbid, a bottle of WKD.
But if you’re sensitive to sulphur, some low-intervention wines will be a godsend. On the other hand, the best way to get “real” wines is to trade up and, in the process, drink less in terms of volume while spending more.
A friend of mine who has taken to drinking only at the weekends has been blown away, in his words, by the difference when he spends a week’s budget on wines for just two nights. Okay, three nights, he grudgingly admits. It’s a good idea, but perhaps not for puritans who refuse to pay more than a tenner on a bottle of wine in any circumstances.

This month I want to suggest a wine that is well worth the extra spend, a white Burgundy that claims merely Bourgogne status on the label, but that tastes way superior to that. Domaine Latour-Giraud Bourgogne 2019, €35, whelehanswines.ie , is from a domaine in Meursault and this Bourgogne Blanc is made with the same care and attention as their top wines. Indeed, in a blind tasting it might well be confused for one and it’s streets ahead of many actual Meursaults from the big nègociants.
I’d be grateful if you could leave some for me.

Food & Drink Life THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 35 TOM DOORLEY Read more at www.mindo.ie @tomdoorley
January is always a pretty grim month, but because my low-carb regime involves plenty of cheese, charcuterie, salad, and good meat, I don’t feel deprived
THE MAZDA6 PUNCHES ABOVE ITS WEIGHT

Itook the Mazda6 on test after spending a week driving a car from a more prestigious marque, which was more expensive and in no way as luxurious or well-appointed as the Mazda6. This latest incarnation is just as handsome, well-appointed and fabulous to drive as its predecessors; something I’ve come to expect from Mazda’s premium saloon. When you drive a Mazda, you’re always reminded that the people who design and engineer these vehicles simply love cars. They’re not just fecking them together, they are clearly focused on creating something that both looks and drives beautifully.
The firm was founded in 1920 in Hiroshima, Japan, and to celebrate its centenary the marque has released some 100th anniversary edition models. My test car was one of this cohort, painted in snowflake white, powered by the interesting 2.5 litre Skyactive-G engine producing 194hp and 258nm of torque. My test car came with a lovely, smooth, automatic gearbox and a war chest of clever driving supports. Mazda is known for producing cars that handle very well, and this Mazda6 was no different. It drove like a much smaller and lighter vehicle, which was an absolute pleasure, especially on winding roads. The Skyactive
motor, which combines diesel and petrol engine principals to improve efficiency, achieves fuel consumption figures of 7.4l/ km, according to Mazda. I did most of my testing in the city, so my fuel figures were worse than that, but it wasn’t as balanced a test as would be required to challenge the official numbers.
The interior was wrapped in burgundy and stone Nappa leather and embossed with 100th anniversary logos. Inside this car was luxurious and comfortable space, however (and it’s a small ‘however’) the indicator stalks felt a bit cheap compared to the rest of the car. Pedantic, I know, but when everything else is so good, small issues can stand out. I’ve mentioned before that the Mazda infotainment system isn’t my favourite, but it does have Apple Car Play and Android Auto, so you can just plug in, avoid the Mazda interface and enjoy that magnificent Bose sound system.
My test car produced 167g/km of CO2 , putting it in the €420 tax band, which does seem a little steep compared to competitors. Prices for the Mazda6 start at €34,170, with the special 100th anniversary edition starting at €47,695. That is a lot of premium, executive saloon for less than €50,000 and as I mentioned at the start of this piece, some so called ‘premium’ cars wouldn’t be
able to hold a candle to the Mazda6, especially the entry models.

Mazda Europe are currently offering a three-year warranty for up to 100,000km, while you can also avail of other warranties, like the Mazda three-year paintwork warranty and 12-year warranty against corro-
sion. Mazda Europe’s service offer also covers you in the event you require breakdown assistance, or if the car is stolen or in a crash.
If you have €50,000 to spend on a new executive saloon, test drive this before you go to the Mercedes, BMW, Volvo or Audi showroom, for a different perspective.

THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 36 Life Motoring MORGAN FLANAGAN CREAGH Read more at www.mindo.ie @MorganFlanaganC
MAZDA6 100TH ANNIVERSARY EDITION
Engine: 2.5 Skyactiv-G

Power: 194ps/6000rpm
Torque: 258nm @ 4000rpm
0-100km/h: 8.1 seconds
Fuel economy (WLTP): 7.4l/km
Top speed: 204km/h
Spec: 100th Anniversary Edition
Colour: Snowflake white metallic
(not optional)
Transmission: Automatic
Drive: FWD
CO2 (WLTP): 167g/km
Price: €47,695 RRP
CX-5 starting price: €34,495

MAZDA6 STANDARD EQUIPMENT (GS-L)

LED Lights: Headlights, rear and fog, high beam control
17” Gunmetal alloy wheels Black cloth seats
Mazda radar cruise control
G-Vectoring Control Plus
Privacy glass
Multifunction leather steering wheel
Mazda Navigation System
Heated auto folding power door mirrors
8” Colour centre console display and multimedia commander with six speakers
Wireless Apple Car Play
Android Auto
USB ports (X2)
ISOFIX brackets
Hill hold assist
Automatic rain sensing wipers
Automatic dusk sensing lights
Electronic handbrake
i-stop/engine start stop button
Advanced Smart City Brake Support (front) with pedestrian recognition

Auto dimming inner mirror
Dual automatic air conditioning
Front and rear parking sensors
Blind spot monitoring with rear
cross traffic alert
Lane keep assist
Head up display
Traffic sign recognition
UPGRADE FEATURES (IN ADDITION) [PLATINUM LUX ADDS:]
Brown Nappa leather seats
Adaptive front lighting system
Steering wheel heater
Front seat heaters
Front seat ventilation
Power front seats with driver seat memory
Rear smart city brake support
Driver attention alert
19” Bright silver alloy wheels
BOSE Sound System with 12 speakers
Smart keyless entry
Sunroof
Rear view camera with 360 view
Wiper de-icer
7” TFT digital meter
Frameless auto dimming rear view mirror
Rear centre armrest w/x2 USBs
[100TH ANNIVERSARY EDITION ADDS:]
Burgundy & Stone Nappa leather
100th Anniversary embossed headrests
100th Anniversary floor mats
100th Anniversary key
100th Anniversary Exterior badging
100th Anniversary alloy centre caps
Snowflake Pearl paint
Motoring Life THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 37
GLUCORX AIDEX CONTINUOUS GLUCOSE MONITOR ON IRISH MARKET
clinical manifestations of psoriatic arthritis, such as physical function (as measured by the Health Assessment Questionnaire Disability Index [HAQ-DI]) and minimal disease activity (MDA) at week 24.
Highlights from the pivotal phase 3 programme:
In KEEPsAKE-1 and KEEPsAKE-2, 57.3 and 51.3 per cent of patients receiving risankizumab achieved the primary endpoint of ACR20 response at week 24, respectively, versus 33.5 and 26.5 per cent receiving placebo (p≤0.001).
Windzor Pharma has announced the launch of GlucoRX Aidex to the Irish market. Aidex is a continuous glucose monitor available for all patient types.

The sensors are small and secure and can be used on the arm or the abdomen. Each transmitter has a lifespan of three years and each sensor lasts up to 14
days. All data is submitted from the sensor to the GlucoRx Aidex app making it easy to track glucose levels during the day.
For more information, training or to order, contact orders@windzorpharma. com. Transmitters and sensors are available through UDW and Uniphar and PCO. Sensor GMS code is 85241.
ABBVIE EXPANDS IMMUNOLOGY PORTFOLIO IN THE EUROPEAN UNION WITH THE EUROPEAN COMMISSION APPROVAL OF SKYRIZI (RISANKIZUMAB) FOR TREATMENT OF ADULTS WITH ACTIVE PSORIATIC ARTHRITIS
AbbVie has announced that the European Commission (EC) has approved SKYRIZI (risankizumab, 150 mg, subcutaneous injection at week 0, week 4 and every 12 weeks thereafter) alone or in combination with methotrexate (MTX), for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). Marking the second indication for risankizumab, the marketing authorisation will be valid in all member states of the European Union, as well as Iceland, Liechtenstein, Norway and Northern Ireland.
Prof Brian Kirby, speaking about the European Commission approval of risankizumab for the treatment of adults with active psoriatic arthritis, said: “We know that almost 30 per cent of psoriasis patients will develop psoriatic arthritis. As a dermatologist having treatments available
that are effective both on skin and the joints provide reassurance when treating psoriasis patients. It is positive to see an additional treatment available for the treatment of PsA.”
“Discovering, developing, and delivering medicines that have a remarkable impact on patients’ lives is a complex endeavour that takes time, expertise, and unwavering innovation,” said Mr Andres Rodrigo, General Manager, AbbVie Ireland.
“This approval is a significant milestone for AbbVie in our pursuit to continually raise standards of care, helping alleviate the burden experienced by patients with psoriatic disease.”
Risankizumab received EC approval based on data from two phase 3 clinical studies, KEEPsAKE-1 and KEEPsAKE-2. In these studies, risankizumab met the primary endpoint of ACR20 response at week 24 versus placebo, and ranked secondary endpoints including, but not limited to, improvements in several
risankizumab in plaque psoriasis, with no new safety risks observed. Through week 24, serious adverse events occurred in 2.5 per cent and 4.0 per cent of patients treated with risankizumab in KEEPsAKE-1 and KEEPsAKE-2, respectively, compared with 3.7 per cent and 5.5 per cent on placebo. Rates of serious infections were 1.0 and 0.9 per cent in risankizumab-treated patients in KEEPsAKE-1 and KEEPsAKE-2, respectively, and 1.2 and 2.3 per cent in patients who received placebo.
risk of progressing to severe disease. This important supply agreement with the Irish Government enables access to sotrovimab for doctors managing cases of Covid-19 in medically vulnerable patients.”
GSK and Vir are committed to the ongoing evaluation of sotrovimab as the Covid-19 landscape continues to evolve at different rates across the globe
and new variants of concern and interest emerge.
In vitro data, published in bioRxiv, demonstrate that sotrovimab retains activity against all tested variants of concern and interest of the SARS-CoV-2 virus as defined by the World Health Organisation, including, but not limited to, Omicron (B.1.1.529), Delta (B.1.617.2), Delta Plus (AY.1 or AY.2) and Mu (B.1.621).
SALASO HEALTH TO CREATE 20 NEW JOBS FOLLOWING LATEST INVESTMENT
Risankizumab-treated patients showed significantly greater improvement from baseline in physical function as measured by HAQDI -0.31 and -0.22, compared to placebo -0.11 and -0.05 at week 24 (p≤0.001) in KEEPsAKE-1 and KEEPsAKE-2, respectively.
At week 24, 25.0 per cent and 25.6 per cent of risankizumab-treated patients achieved MDA, in KEEPsAKE-1 and KEEPsAKE-2 respectively, compared to 10.2 per cent and 11.4 per cent of those on placebo (p≤0.001).
The safety profile of risankizumab in psoriatic arthritis was consistent with the safety profile of
The rates of adverse events leading to discontinuation of the study drug were 0.8 per cent and 0.9 per cent of patients treated with risankizumab in KEEPsAKE-1 and KEEPsAKE-2, respectively, compared with 0.8 per cent and 2.3 per cent on placebo. In KEEPsAKE-1, there was one death in the risankizumab group not related to the study drug per investigator. There were no deaths reported in KEEPsAKE-2.
Risankizumab is part of a collaboration between Boehringer Ingelheim and AbbVie, with AbbVie leading development and commercialisation globally.
SUPPLY AGREEMENT BETWEEN GSK AND IRISH GOVERNMENT WILL ENABLE ACCESS TO COVID-19 TREATMENT XEVUDY (SOTROVIMAB)
GSK last month announced that agreement had been reached with the Irish Government for the supply of Covid-19 treatment Xevudy (sotrovimab), developed in partnership with Vir Biotechnology.
The contract is enabled by the 2021 Joint Procurement Agreement (JPA) between GSK, Vir, and the European Commission (EC) for the supply of up to 220,000 doses of sotrovimab to EU member states.
Following the December 2021 grant of marketing authorisation to Xevudy (sotrovimab) for the early treatment of Covid-19 by the EC, member states participating in the JPA, including Ireland, were able to proceed with ordering sotrovimab to support their pandemic response. Sotrovimab is now available in Ireland.
The grant of the marketing authorisation in the EU is based on data from the COMET-ICE phase III tri-
al, demonstrating that intravenous treatment with sotrovimab resulted in a 79 per cent reduction (adjusted relative risk reduction) (p<0.001) in all-cause hospitalisations for more than 24 hours or death due to any cause by day 29 compared to placebo, meeting the primary endpoint of the trial. In absolute numbers, 30 (6 per cent) of the 529 patients in the placebo arm progressed, compared to six (1 per cent) of the 528 patients receiving sotrovimab. Interim data were published in The New England Journal of Medicine on 27 October 2021 and final data were pre-published on 8 November 2021 on medRxiv.
Director of Medical Affairs for GSK Ireland, Dr Eavan Daly (PhD), said: “As we continue to navigate the pandemic using all the public health tools available to us, there remains a patient need for treatment options for those who test positive for Covid-19 and are at high
Salaso, the Kerry-based health technology company, is to create 20 new technology jobs in software development, data analytics, research and development and digital marketing and sales, having secured investment from the Davy EIIS Fund. The fund is managed by BES Management DAC, a joint venture between BDO and Davy.
CEO and founder of Salaso, Ms Aoife Ní Mhuirí, said the investment will allow Salaso to grow its expanding
international client base.
“This is a very exciting time for Salaso, as we grow our client base and continue to evolve and develop our cutting-edge digital therapeutics platform.
“Our team is highly skilled both in technology and delivering innovative evidence-based clinical solutions. This investment will enable Salaso to continue to expand the team and to grow internationally with a particular focus on the US market.”
LEADING EXPERT IN GUT INFLAMMATION AT APC MICROBIOME IRELAND SFI RESEARCH CENTRE AND UCC RECEIVES €5.6M THROUGH SFI PROFESSORSHIP AWARD
Some €5.6 million in funding has been awarded to Prof Subrata Ghosh, a global leader in research into Crohn’s disease and ulcerative colitis, to establish a world-leading research lab at University College Cork (UCC), through a prestigious Science Foundation Ireland (SFI) Research Professorship Programme award.
Prof Ghosh will lead the new AUGMENT project at UCC’s APC Microbiome Ireland SFI Research Centre, to investigate precision medicine in relation to gut inflammation and the microbiome. This will contribute to furthering research in IBD, Crohn’s disease and ulcerative colitis, which affect 40,000 people in Ireland and 10 million people globally every year.
The grant will resource a science research lab, and equipment, as well as 13 personnel, to investigate the nascent area of the microbiome in relation to gut inflammation and how it can be influenced by precision medicine to address critical health challenges.
Prof Ghosh is a Fellow of the Academy of Medical

Prof Subrata Ghosh
Sciences, UK and Fellow of the Canadian Academy of Health Sciences, the highest honours bestowed for leadership in life sciences and health research in those countries.
Attracted to UCC from the University of Birmingham, UK, due to the world-class research in microbes and food carried out at APC Microbiome Ireland and Teagasc, Prof Ghosh said many of the important clues as to what causes Crohn’s disease and ulcerative colitis are emerging from their research.
“Chronic inflammatory diseases and cancer are major causes of disability and death in Ireland and in the world. Current treatments are limited by their efficacy ceiling and adverse effects,” commented Prof Ghosh.
RXDX Product Focus THE MEDICAL INDEPENDENT |10 FEBRUARY 2022 38
GP ASSISTANT REQUIRED
GP assistant required in North Inishowen with a view to partnership. Short-term position also considered. Group practice/ fully computerised, full ancillary staff (APN, practice nurses, medical technicians and administration staff) in a purpose-built primary care centre.

For further information on this position contact Dr Seamus Kelly via email skelly@millbraesurgery.ie
Millbrae Surgery, Carndonagh, Co Donegal
Specialist Medical Accountant, RCSI Lecturer and author of Fit moneythe key to financial freedom
Now offering:
• Monthly Profit Growth Model
• Annual Accounts and Tax Returns
• Outsourced Bookkeeping and Payroll
• Support in Selling and Buying a Practice
Contact 01 801 3116 for help and support
GP REQUIRED
Vocationally trained GP required for Sutton Cross Surgery. 4-6 sessions. Flexible, friendly, coastal, Dublin teaching practice with a mix of GMS/private patients. We are fully computerised using Health One software and 15 minute appointments. Supported by excellent nursing and administrative staff. An attractive package is available for suitable candidates. Enquiries or CVs to manager@suttoncrosssurgery.ie 01 832 6438


GP required for a well-established practice in East Galway (6-8 sessions). 20 minute commute from Oranmore.


Fully computerised with Socrates and great practice support. Generous remuneration available to the right candidate.
Contact our Practice Manager Mary on 091 842144 or 087 203 5825 or email your CV to maryhoran.mh@gmail.com
We are currently seeking a vocationally trained (MICGP or equivalent) GP for 3-4 sessions (flexible) per week, with possible additional holiday cover in Falcarragh, Co Donegal, for immediate start. Our practice is fully computerised (Helix Practice Manager) with three GPs, two full-time practice nurses, practice manager, and administration staff. Falcarragh is a busy market town surrounded by beautiful scenery and mountains, near to white sandy beaches for surfing/watersports etc. Great work/life balance.
If interested, please send CV to Sheila O’Donnell Practice Manager at falcarraghmedicalMC21@gmail.com Tel 086 310 0732
walk away. There is a big concentration of new businesses in the area. Trinity College, Merrion Square, and Pearse Street are on the doorstep. Very favourable and flexible terms.
2 Suites available ● self-contained ● bathroom/waiting room/consulting rooms 440sq feet and 690sq feet both newly renovated ● high speed broadband, etc
Classifieds & Recruitment THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 39 Call: 086 803 0891 or Email: shirley.osullivan@matchmedics.com match medics are working with a number of vocationally trained European GPs ON THEIR JOURNEY TO IRELAND CONTACT SHIRLEY O’Sullivan TODAY TO KICK START THE PROCESS ARE YOU LOOKING TO HIRE A PERMANENT GP? Louth, Meath, Cavan, and Monaghan GP out-of-hours (NEDOC) and daytime surgery jobs Great rates available Contact us on 046 924 1533, Email: info@medsource.ie Visit our website: www.medsource.ie EXCELLENT GP JOB OPPORTUNITIES MEDICAL SUITES AVAILABLE IN DUBLIN 2 (close to Dawson Street Luas) Medical Suites available at 26 Clare Street, Dublin 2. Adjacent to the National Gallery. Suitable for a range of medical uses including GP practice - Private or GMSOut-of-hours service or walk-in clinic – physio clinic – beauty clinic, etc. Full medical planning permission. These suites would suit many uses due to the high footfall passing the door every day. The suites are 5 minutes walk from Grafton Street with several amenities close by. There is direct access from all areas of Dublin to the suites via the Dart and bus and the Luas runs to Dawson Street, 5 minutes
Please contact Mr Aidan Ringrose on +353 87 951 9786 or mornwill26@gmail.com for further information or to arrange viewing SHANE O’TOOLE GP REQUIRED GP REQUIRED
If you have anything you would like to share, please email: info@mindo.ie
A round-up of news and oddities from left field by Dr Doug
Witherspoon
Blazing saddles: The curious case of the exploding pants
January can normally be a difficult month, but as we emerge from Covid restrictions and head inexorably toward spring, there is much cause for optimism. Not so for the farmers of New Zealand in the 1930s, who were left scratching their heads – not to mention some other areas of the body – following an epidemic of exploding trousers among the profession. Amateur detectives and problem-solvers, read on
Between the two World Wars, there were advances in
COMBINING POWER AND CONFIDENCE
New Zealand's agricultural processes, particularly in dairy farming. This meant that more hectares were given over to cows rather than sheep. All seemed well until there emerged a pattern of farmers' trousers exploding or catching fire, sometimes with minimal consequences. But there were also sadly at least two deaths from the phenomenon, as well as a number of cases of serious burns.

One of the first cases to come to prominence was that of farmer Richard Buckley, as reported in the 21 April 1933
Suvezen is indicated for substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products, as adjunct to diet for treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolaemia1

for initial therapy.
Treatment initiation or dose adjustment, if necessary, should only be done with the monocomponents and after setting the appropriate doses the switch to the fixed dose combination of the appropriate strength is possible. Patient should use the strength corresponding to their previous treatment. The recommended dose is one Suvezen tablet daily. To be administered at any time of the day, with or without food. The tablet should be swallowed whole with a drink of water. If co-administered with bile acid sequestrant (BAS), administration of Suvezen should occur either ≥2 hours before or ≥4 hours after administration of a BAS. Special populations: Paediatric (<18 years): Safety and efficacy has not been established. Elderly (>70 years): Starting dose of 5 mg rosuvastatin is recommended. The combination is not suitable for initial therapy. Hepatic impairment: Mild: No dosage adjustment is required. Moderate/Severe: Treatment with Suvezen is not recommended. Renal impairment: Mild: No dose adjustment is necessary. Moderate (creatinine clearance <60 ml/ min): The recommended start dose is rosuvastatin 5mg. Race: The recommended start dose is rosuvastatin 5 mg for patients of Asian ancestry due to increased systemic exposure. The fixed dose combination is not suitable for initial therapy. Monocomponent preparations should be used to start the treatment or to modify the dose. Suvezen 40 mg/10 mg tablets are contraindicated in these patients. Genetic polymorphisms: In patients who are known to have specific types of genetic polymorphisms that can lead to increased rosuvastatin exposure, a lower daily dose of Suvezen is recommended. Dosage in patients with pre-disposing factors to myopathy: The recommended start dose is rosuvastatin 5mg in patients with pre-disposing factors to myopathy. Suvezen 40 mg/10 mg tablets are contraindicated in some of these patients. Concomitant therapy: The risk of myopathy (including rhabdomyolysis) is increased when Suvezen is administered concomitantly with certain medicinal products that may increase the plasma concentration of rosuvastatin (e.g. ciclosporin and certain protease inhibitors including combinations of ritonavir with atazanavir, lopinavir, and/or tipranavir).
Whenever possible, alternative medications should be considered, and, if necessary, consider temporarily discontinuing Suvezen therapy. In situations where co-administration of these medicinal products with Suvezen is unavoidable, the benefit and the risk of concurrent treatment and rosuvastatin dosing adjustments should be carefully considered. Contraindications: Hypersensitivity to the active substances or excipients.
Pregnancy, breast-feeding and in women of childbearing potential not using appropriate contraceptive measures. Active liver disease or any serum transaminase elevations which are unexplained, persistent or exceeding 3x the upper limit of normal (ULN). Severe renal impairment (creatinine clearance <30ml/min); myopathy or receiving concomitant ciclosporin. 40mg/10mg dose contraindicated in patients with predisposing factors for myopathy/rhabdomyolysis; such factors include: Moderate renal impairment (creatinine clearance <60ml/min), hypothyroidism, personal or family history of hereditary muscular disorders, previous history of muscular toxicity with another HMG-CoA reductase inhibitor or fibrate, alcohol abuse, situations where an increase in plasma levels of rosuvastatin may occur, Asian patients, concomitant use of fibrates.
Precautions and Warnings: Skeletal muscle effects: have been reported in rosuvastatin-treated patients with all doses and in particular with doses >20mg. As with other HMG-CoA reductase inhibitors, reporting rate for rhabdomyolysis is associated with use at doses >40mg. Post-marketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. If myopathy is suspected based on muscle symptoms or is confirmed by a creatine phosphokinase (CPK) level, Suvezen and any of these other agents that the patient is taking concomitantly should be immediately discontinued. All patients starting therapy with Suvezen should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness, particularly if associated with malaise or fever. Creatine kinase (CK) measurement: CK should not be measured following strenuous exercise or in the presence of a plausible alternative cause of CK increase. If CK levels are significantly elevated at baseline (>5x ULN) a confirmatory test should be carried out within 5 – 7 days. If the repeat test confirms a baseline CK >5x ULN, treatment should not be started. Patients with predisposing factors for myopathy/rhabdomyolysis: Caution should be exercised in these patients. Risk: benefit of treatment should be considered and clinical monitoring is recommended. CK levels should be measured in these patients. Therapy should be discontinued if CK levels are markedly elevated (>5x ULN) or if muscular symptoms are severe and cause daily discomfort. If symptoms resolve and CK levels return to normal, then consideration should be given to re-introducing treatment at the lowest dose. Immune-mediated necrotising myopathy (IMNM): Clinically characterised by proximal muscle weakness and elevated serum CK, has been reported very rarely during or after treatment with statins, including rosuvastatin, despite discontinuation of statin treatment. In clinical trials an increase in the incidence of myositis and myopathy has been seen in patients receiving other HMG-CoA reductase inhibitors together with fibric acid derivatives. Suvezen should not be used in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g. sepsis, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte disorders; or uncontrolled seizures). Liver effects: In controlled co-administration trials in patients receiving ezetimibe with statin, consecutive transaminase elevations ≥3x ULN have been observed. It is recommended that liver function tests be carried out prior to, and 3 months following, the initiation of treatment. Rosuvastatin should be discontinued or the dose reduced if the level of serum transaminases is >3x ULN. The reporting rate for serious events is higher at the 40mg dose. In patients with secondary hypercholesterolaemia caused by hypothyroidism or nephrotic syndrome, the underlying disease should be treated prior to initiating therapy with rosuvastatin. Liver disease and alcohol: As with other
HMG-CoA reductase inhibitors, rosuvastatin should be used with caution in patients who consume excessive quantities of alcohol and/or have a history of liver disease. Renal effects: Proteinuria has been observed in patients treated with higher doses of rosuvastatin and was transient or intermittent in most cases. Proteinuria has not been shown to be predictive of acute or progressive renal disease. An assessment of renal function should be considered during routine follow-up of patients treated with a dose of 40mg. Diabetes mellitus: Some evidence suggests that statins raise blood glucose and in some patients, at high risk of future diabetes, may produce a level of hyperglycaemia where formal diabetes care is appropriate. This risk, however, is outweighed by the reduction in vascular risk with statins and therefore should not be a reason for stopping statin treatment. Patients at risk (fasting glucose 5.6 – 6.9mmol/l, BMI >30kg/m2, raised triglycerides, hypertension) should be monitored both clinically and biochemically according to national guidelines. Interstitial lung disease: Exceptional cases have been reported with some statins, especially with long term therapy. Presenting features can include dyspnoea, non-productive cough and deterioration in general health (fatigue, weight loss and fever). If it is suspected, statin therapy should be discontinued. Protease inhibitors: Increased systemic exposure to rosuvastatin has been observed in subjects receiving rosuvastatin concomitantly with various protease inhibitors in combination with ritonavir. Consideration should be given both to the benefit of lipid lowering by use of Suvezen in HIV patients receiving protease inhibitors and the potential for increased rosuvastatin plasma concentrations when initiating and up titrating rosuvastatin doses in patients treated with protease inhibitors. The concomitant use with certain protease inhibitors is not recommended unless the dose of rosuvastatin is adjusted. Fibrates: The safety and efficacy of ezetimibe administered with fibrates have not been established. If cholelithiasis is suspected in a patient receiving Suvezen and fenofibrate, gallbladder investigations are indicated and therapy should be discontinued. Anticoagulants: If Suvezen is added to warfarin, another coumarin anticoagulant, or fluindione, the International Normalised Ratio (INR) should be appropriately monitored. Fusidic acid: Suvezen must not be co-administered with systemic formulations of fusidic acid or within 7 days of stopping fusidic acid treatment. In patients where the use of systemic fusidic acid is considered essential, statin treatment should be discontinued throughout the duration of fusidic acid treatment. There have been reports of rhabdomyolysis (including some fatalities) in patients receiving fusidic acid and statins in combination.
The patient should be advised to seek medical advice immediately if they experience any symptoms of muscle weakness, pain or tenderness. Statin therapy may be re-introduced seven days after the last dose of fusidic acid. In exceptional circumstances, where prolonged systemic fusidic acid is needed, e.g., for the treatment of severe infections, the need for co-administration of Suvezen and fusidic acid should only be considered on a case by case basis and under close medical supervision. Suvezen contains lactose monohydrate and sodium: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine. This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’. Interactions: Contraindicated combinations: Ciclosporin.
Not-recommended combinations: Fibrates and other lipid-lowering products, protease inhibitors, transporter protein inhibitors and fusidic acid. Other possible interactions: Cytochrome P450 enzymes, antacids, colestyramine, anticoagulants, Vitamin K antagonists, erythromycin, oral contraceptive/hormone replacement therapy. When co-administering rosuvastatin with other medicinal products known to increase exposure to rosuvastatin, doses should be adjusted (see SmPC for full details). The maximum daily dose should be adjusted so that the expected rosuvastatin exposure would not likely exceed that of a 40mg daily dose of rosuvastatin taken without interacting medicinal products. Fertility, Pregnancy and Breastfeeding: No clinical data are available on the use of ezetimibe during pregnancy. Potential risk from inhibition of HMG-CoA reductase outweighs the advantage of treatment during pregnancy. If a patient becomes pregnant during use of Suvezen, treatment should be discontinued immediately. Animal studies have shown excretion of medicinal product through breast milk. However, there are no data in humans. No clinical trial data on the effects of fertility in humans. Adverse Reactions: Adverse drug reactions previously reported with one of the individual components (ezetimibe or rosuvastatin) may be potential undesirable effects with Suvezen. Common (≥1/100 to <1/10): diabetes mellitus, headache, dizziness, constipation, nausea, abdominal pain, diarrhoea, flatulence, myalgia, ALT and/or AST increased, asthenia and fatigue. Uncommon (≥1/1,000 to <1/100): decreased appetite, paraesthesia, hot flush, hypertension, cough, dyspepsia, gastroesophageal reflux disease, nausea, dry mouth, gastritis, pruritus, rash, urticaria, arthralgia, muscle spasms, neck pain, back pain, muscular weakness, pain in extremity, ALT and/or AST increased, blood CPK increased, gamma-glutamyltransferase increased, liver function test abnormal, chest pain, pain, asthenia, oedema peripheral. Rare (≥1/10,000 to <1/1,000): thrombocytopenia, hypersensitivity reactions including angioedema, pancreatitis, increased hepatic transaminases, myopathy (including myositis), rhabdomyolysis, lupus-like syndrome and muscle rupture. Very rare (<1/10,000): polyneuropathy, memory loss, jaundice, hepatitis, arthralgia, haematuria, gynaecomastia. Not known: thrombocytopenia, hypersensitivity (including rash, urticaria, anaphylaxis and angioedema), depression, peripheral neuropathy, sleep disturbances (including insomnia and nightmares), dizziness, paraesthesia, cough, dyspnoea, diarrhoea, pancreatitis, constipation, hepatitis, cholelithiasis, cholecystitis, Stevens Johnson syndrome, erythema multiforme, immune-mediated necrotising myopathy, tendon disorders (sometimes complicated by rupture), myalgia, myopathy/rhabdomyolysis, oedema, asthenia. See SmPC for full details
issue of New Zealand's Evening Post : "Not long ago, in a country township, a man's trousers exploded with a loud report. Fortunately, the owner was not in them at the time. He was, however, in the same room and although dazed by the force of the explosion, he was able to seize the garment, which was hanging before the fire, and hurl it out onto the grass outside. There the trousers smouldered, with a series of minor detonations." With respect to today's crop of journalists, the reporting of yesteryear had a distinctly lyrical, almost poetic lilt to it.
Anyway, this was followed by cases such as that of another unfortunate farmer whose pants exploded while he was riding his horse. As these cases proliferated, albeit briefly, some sharp minds got to work on the problem.
The indirect cause, as you may have guessed, was the application of a certain chemical to grazing areas. As more land was given over to cows to graze, ragwort grew rampant. Cows normally avoid this poisonous weed, but its abundance was pushing down the amount of grass available to the cows for grazing. The problem became so severe that the farmers turned to the government of the time for help, and it was happy to oblige.
The government opted to use chemical herbicide sodium chlorate to kill the ragwort and distributed it widely to farmers to spray on their grazing land. The ragwort was indeed destroyed, but authorities had overlooked the fact that sodium chlorate is extremely caustic when dry. Whilst spraying the chemical onto their crops, the farmers were inadvertently turning themselves into walking timebombs. The worst affected were, of course, those who were wearing the trousers at the moment of combustion, but there are also reports of a man who lit a match in a dark room to check on his child, with predictable consequences.
As sodium chlorate had to be mixed with water for the spreading process, when the pants were subsequently dried-out, this left highly-volatile sodium chlorate crystals embedded in the fibres. This left the unfortunate farmers vulnerable to exploding trousers triggered by heat, a naked flame, or even just a strong impact.
Back to the Evening Post for an explanation in its historical context: "This strange behaviour of a quite respectable garment was due to the fact that its owner had been spraying sodium chlorate on ragwort, and incidentally had sprayed some on his trousers. Clothing and other burnable substances become highly inflammable when they absorb sodium chlorate. When clothes take the spray, the water evaporates rapidly, leaving the chlorate distributed through the fibres of the cloth. There is nothing visible to warn the owner that the affected portion of the dried-out clothing may catch fire (or even explode) by coming near a fire (there need not be actual contact with flame or spark), or by friction, or by the concussion of a sudden blow. Even sun-heat can cause ignition or explosion of clothes affected by sodium chlorate." The article goes on to helpfully point out that "it is an offence against the Explosives Act to mix chlorates with other substances with the intention of making an explosive".
If this has ignited your interest, see the 2004 paper written by scholar James Watson, titled: 'The significance of Mr Richard Buckley's exploding trousers: Reflections on an aspect of technological change in New Zealand dairy farming between the World Wars.'
The Dorsal View THE MEDICAL INDEPENDENT | 10 FEBRUARY 2022 40
20 mg /10 mg Rosuvastatin + Ezetimibe 10 mg /10 mg Rosuvastatin + Ezetimibe 40 mg /10 mg Rosuvastatin + Ezetimibe Single-pill combination of rosuvastatin and ezetimibe available in 3 doses* Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com Reference: 1. Suvezen Summary of Product Characteristics * LDL-C: Low-density lipoprotein Cholesterol ** Suvezen is available in 3 doses in Ireland. Suvezen 10mg/10mg, 20mg/10mg and 40mg/10mg: Each film-coated tablet contains 10mg; 20mg or 40mg of rosuvastatin (as rosuvastatin calcium) respectively, and 10mg ezetimibe. MAT-IE-2101262 (v1.0) – August 2021 Prescribing Information: Suvezen (rosuvastatin/ ezetimibe) film-coated tablets Please refer to the Summary of Product Characteristics (SmPC) for full prescribing details. Presentations: Suvezen 10mg/10mg, 20mg/10mg and 40mg/10mg: Each film-coated tablet contains 10mg; 20mg or 40mg of rosuvastatin (as rosuvastatin calcium) respectively,
Dosage
appropriate lipid-lowering
and 10mg ezetimibe. Indication: Suvezen is indicated for substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products, as adjunct to diet for treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolaemia.
and Administration: The patient should be on and continue, an
diet, during treatment with Suvezen. Suvezen is not suitable
on adverse reactions. Legal Category: POM. Marketing Authorisation Numbers: 10mg/10mg: PA0540/193/001; 20mg/10mg: PA0540/193/002; 40mg/10mg: PA0540/193/003. Marketing Authorisation Holder: Sanofi-Aventis Ireland Ltd. T/A SANOFI, Citywest Business Campus, Dublin 24, Ireland. Further information is available from: Sanofi, 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com Date of Preparation: December 2020.
LDL-C*
THE TREATMENT OF HYPERCHOLESTEROLAEMIA 11918_Suvezen_AD_10X4_AUG21_01.indd 1 30/08/2021 10:01
AGAINST
IN










































 Read more by Dr Lucia Gannon at www.mindo.ie @LuciaGannon
Read more by Dr Lucia Gannon at www.mindo.ie @LuciaGannon






















































