MATTERS OF LIFE & DEATH
Special feature: Pharmacists have fallen through the cracks of legal protection of conscience when it comes to euthanasia and assisted suicide


STICKY FINGERS
The family tradition of shoplifting from pharmacies is alive and well, writes
Fintan Moore
CLINICAL CONTENT
FEATURES ON ACNE, SMOKING CESSATION, AND EYE CARE


VOL 23 ISSUE 1 JANUARY 2022
FOR ALL THAT MATTERS IN MEDICINE

NEW WEBSITE COMING SOON
PAGE 2-12
PAGE 14-20
PAGE 22-23
PAGE 34-40
NEWS
National and international news in the world of pharmacy
PAGE 41-45
MATTERS OF LIFE AND DEATH
A special feature on the vulnerability of pharmacists in the assisted suicide process


PAGE 46-52
REGULATION
Some top-line statistics from the latest HPRA Annual Report

PAGE 53-54
SKIN DEEP
A look at the different types of acne and how to treat the troublesome problem

PAGE 55-56
EYE CARE

An overview of common dry eye complaints and Sjögren’s syndrome
KICKING THE HABIT
A clinical look at smoking cessation and the most effective ways to help patients to quit
PAGE 26-27
FINTAN MOORE
Editor
Pat Kelly, pat@greenx.ie
Creative Director
Laura Kenny, laura@greenx.ie
Administration Manager
Daiva Maciunaite, daiva@greenx.ie
Managing Director
Graham Cooke, graham@greenx.ie
PAGE 28-29
TERRY MAGUIRE
GreenCross Publishing was established in 2007. Publisher and Managing Director: Graham Cooke, graham@greenx.ie
© Copyright GreenCross Publishing Ltd 2022.
PAGE 30-31
DR DES CORRIGAN
The contents of Irish Pharmacist are protected by copyright. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means –electronic, mechanical or photocopy recording or otherwise – whole or in part, in any form whatsoever for advertising or promotional purposes without the prior written permission of the editor or publishers.

PRODUCT NEWS
A round-up of industry and product news

PAGE 32-33
ULTAN MOLLOY
Disclaimer
The views expressed in Irish Pharmacist are not necessarily those of the publishers, editor or editorial advisory board. While the publishers, editor and editorial advisory board have taken every care with regard to accuracy of editorial and advertisement contributions, they cannot be held responsible for any errors or omissions contained.
CONTENTS VOL 23 ISS 1 JANUARY 2022
FIESTA TIME
Morgan Flanagan Creagh reviews the Ford Fiesta mHEV ST-Line
FIP report gives snapshot of operation of online pharmacies across 79 countries
Increased co-operation between countries in developing regulation of online pharmacies is needed, according to the authors of a recent report, Online Pharmacy Operations and Distribution of Medicines , published by the International Pharmaceutical Federation (FIP).

The report, produced by FIP’s Community Pharmacy Section, presents the findings of a global survey of pharmacy organisations on a wide variety of aspects of online pharmacy, including the types of medicines these pharmacies supply in different countries, how the authenticity of online pharmacies can be verified, and the usage of eprescriptions and shared patient health records.
Of the 79 countries responding to the survey, 51 per cent said that no regulation of online pharmacies exists. Although online pharmacies offer convenience of increased access to medicines and services, a lack of regulation or lack of enforcement of regulation creates an avenue for illegal pharmacies and may impact the overall quality of medicines and services offered to consumers, the authors point out. They say that risks to patient safety include supply of substandard or falsified medicines, failure in supply-chain protocols (which can affect the quality of a medicine), and a lack of professional advice or medicines information. Furthermore, a quarter of the respondents reported cases of irresponsible or inappropriate self-medication by consumers who had purchased medicines through online pharmacies.
“Online pharmacies have now been around for over 20 years, but the Covid-19 pandemic has
rapidly shifted the e-commerce landscape, including in the pharmacy sector. It is likely that changed preferences for online services will remain and the importance of online pharmacies will continue to grow. It is important for health professionals to ensure consumers are informed and empowered to make good decisions related to the use of online pharmacies,” said Mr Lars-Åke Söderlund, immediate past-President of FIP’s Community Pharmacy Section and coeditor of the report.
The survey also reveals that community pharmacists in most countries do not have read-andwrite access to digital health
records and the authors say that this is a barrier to an effective contribution by community pharmacists to optimal patient care. “Evidence indicates that community pharmacists with access to digital health records provide more comprehensive reviews, better identification of medication-related issues and strengthened clinical recommendations. Pharmacists having access to digital health records is especially critical if a medicine is supplied via the Internet,” Mr Söderlund said.
“Consumers are increasingly wanting convenience, innovation and personalisation. This challenges pharmacists to pro -
vide a positive face-to-face experience for consumers utilising online channels. Nevertheless, digital transformation is critical and is an opportunity for pharmacies to further reinforce their place at the heart of their communities, with appropriate investment,” said FIP CEO Dr Catherine Duggan.
She added: “This report on online pharmacies is part of FIP’s programme of work on digital pharmacy practice. It aims to support developments necessary for our profession to continue providing quality healthcare — medicines and services, online or in a brick-and-mortar pharmacy — to meet evolving demands.”
NEWS 2 VOL 23 ISS 1 JANUARY 2022
ONLINE PHARMACY
Date published Jan 2022 ArthAd1 Rev1
IPU Secretary General to step down in April 2022
The Secretary General of the Irish Pharmacy Union, Mr Darragh O’Loughlin, has announced he will step down from his role in the organisation to engage in new business interests in April 2022.
IPU President Mr Dermot Twomey led tributes to Mr O’Loughlin following his announcement. “Throughout his tenure as Secretary General, Darragh O’Loughlin has led the Irish Pharmacy Union with distinction and this is most particularly the case over the past two years of the pandemic crisis, where he exercised all of his zeal and energy tirelessly and unstintingly on behalf of all of our members.
“The Executive Committee and membership of the Union will join with me in expressing our warmest possible appreciation to Darragh. He has been a trusted advocate and advisor across all of our strategies and policies, executing the wishes of the members, while advancing the status and essential role of the community pharmacy profession in the provision of world-class services in Ireland. The successes achieved, such as the roll-out of influenza vaccination and emergency contraception services, were ground-breaking, not only in an Irish but also in an international context and were introduced
ONCOLOGY DRUGS
with new and fair fees that recognised pharmacists’ professional input. In the lead-up to the 2020 budget, Darragh also led a successful fight against proposed cuts of up to €80 million to the fees earned in community pharmacy for administering Government schemes.
“More recently, Darragh fought to ensure that community pharmacists were in a position to play a pivotal role in the fight against Covid-19. Pharmacists are now regarded as the most trusted and accessible of the health professions. Darragh did not achieve these successes on his own, but through his leadership of the excellent staff of the IPU, from whom he earned and maintains great collegial respect.”
Mr O’Loughlin commented: “I have always been very proud to be a pharmacist and it has been an honour and a privilege to have led the IPU and served the profession of pharmacy during a period of great change; to have seen

such progress in the professional role of pharmacists, and to have overcome often considerable challenges for the profession.
“In my time as Secretary General, the role and the importance of community pharmacists and their teams in people’s lives have expanded greatly, especially during the Covid-19 pandemic, and it has been gratifying to see that reflected in the extraordinarily high esteem and affection which the public has for community pharmacy and our increased awareness and respect from Government and policy-makers.
“I have been privileged to have worked with some truly excellent people on IPU committees, and all of my management colleagues and staff, and to have served alongside five IPU Presidents, who all demonstrated vision, integrity and a determination to do their best for community pharmacy. In the same way that pharmacists are always there for their patients and communities, often in very adverse circumstances, I’m proud that the IPU has always been there for our members. I wish the IPU well and look forward to its continued success advocating for pharmacists and patients in the future, as I will also continue to do throughout my future career.”
Research reveals ‘huge step’ in tackling cancer resistance to treatment
New research has revealed the differences in mechanisms behind cancer’s resistance to the major class of anti-cancer drugs known as nucleoside analogues.
Nucleosides are molecules containing the genomic information that can be chemically modified into so-called nucleoside analogues to cause them to inhibit DNA formation in cancer cells, causing the cells to die. However, the presence of the enzyme SAMHD1 aids the cancer’s resistance to this treatment.
This new understanding, provided by the University of Kent’s School of Biosciences in the UK and the Institute of Medical Virology at Goethe-University, Germany, may lead to further breakthroughs in combatting resistance to anti-cancer therapies.
Researchers, supported by the Frankfurter Stiftung für krebskranke Kinder, investigated the drug candidate CNDAC, with hopes it would prevent SAMHD1’s involvement in the
cancer’s resistance to treatments.
The study confirmed that CNDAC does not inhibit SAMHD1 and so would not support nucleoside analogue treatments.
However, the research revealed differences in the resistance mechanisms between leukaemia cells immediately unresponsive to treatment and those that developed resistance over time.
In cells immediately unresponsive to treatment, resistance was found to be due to high levels of SAMHD1 inactivating the nucleoside analogues. In contrast, cells that had developed resistance were found to include the enzyme DCK, which is involved in the activation of nucleoside analogues.
Notably, developed resistance to nucleoside analogue treatments resulted in crossresistance to other closely-related nucleoside analogues activated by the same DCK enzyme. This profound revelation may help
inform further treatments for cancer in the future, said the authors.
Prof Martin Michaelis, University of Kent, said: “The differences between the mechanisms in resistances are a huge step in understanding why particular therapies have a lack of success in destroying the cancer cells and will help us to develop better cancer therapies.”
Prof Jindrich Cinatl, Goethe-University Frankfurt, added: “It is really encouraging that resistance to nucleoside analogues does not seem to be associated with cross-resistance to other therapies. This means that there is hope that there are additional therapy options for patients in whom first-line therapies have stopped working.”
The study, ‘Differences between intrinsic and acquired nucleoside analogue resistance in acute myeloid leukaemia cells’, is published in the Journal of Experimental & Clinical Cancer Research
NEWS 4 VOL 23 ISS 1 JANUARY 2022 IPU
Mr Darragh O’Loughlin
ABBREVIATED PRESCRIBING INFORMATION
Product Name: Brupro Cold & Flu 200 mg/30 mg lm-coated tablets. Composition: Each lm-coated tablet contains 200 mg ibuprofen and 30 mg pseudoephedrine hydrochloride. Description: Yellow, round, lm-coated tablets. Diameter: approx. 11 mm, height: approx. 5 mm. Indication(s): Adults and adolescents aged 12 years and older: For the symptomatic relief of nasal/sinus congestion with headache, fever and pain associated with the common cold and u. Dosage: 1 tablet every 6 hours if necessary. For more intense symptoms, 2 tablets every 6 hours if necessary, to a maximum total daily dose of 6 tablets (equivalent to 1200 mg ibuprofen and 180 mg pseudoephedrine hydrochloride). The maximum total daily dose of 6 tablets must not be exceeded. For short-term use. The patient should consult a doctor if symptoms worsen. The maximum duration of treatment is 4 days for adults and 3 days for adolescents aged 12 years and older. In situations where the symptoms predominantly consist of either pain/fever or nasal congestion, administration of single entity products is to be preferred. Undesirable e ects may be minimised by using the lowest e ective dose for the shortest duration necessary to control symptoms. The tablets should be swallowed whole without chewing with a large glass of water, preferably during meals. Contraindications: Hypersensitivity to ibuprofen, pseudoephedrine hydrochloride or to any of the excipients; Patients aged under 12 years; Pregnant women during the third trimester of pregnancy; Breast-feeding mothers; History of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy; Active, or history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding); Severe heart, liver or renal (glomerular ltration below 30ml/min) failure; Conditions involving an increased tendency to bleeding; Patients with known hypersensitivity or who have experienced asthma, urticaria, or allergic-type reactions after taking ibuprofen, aspirin or other NSAIDs; Severe cardiovascular disorders, coronary heart disease (heart disease, hypertension, angina pectoris), tachycardia; Hyperthyroidism; Diabetes; Pheochromocytoma; History of stroke or presence of risk factors for stroke; History of myocardial infarction; Closed-angle glaucoma; Urinary retention; History of seizures; SLE; Use of MAOIs. Warnings and Precautions for Use: Concomitant use with other NSAIDs including cyclo-oxygenase (COX)-2 selective inhibitors should be avoided. If symptoms persist beyond the recommended maximum duration of treatment with this medicinal product (4 days for adults and 3 days for adolescents), measures to be taken should be re-evaluated, in particular the possible usefulness of an antibiotic treatment. Acute rhinosinusitis, suspected to be of viral origin, is de ned by moderate intensity, bilateral rhinological symptoms dominated by nasal congestion with serious or puriform rhinorrhea, occurring in an epidemic context. The puriform appearance of rhinorrhea is common and does not systematically correspond to bacterial superinfection. Sinus pains, during the rst days of the illness, are associated with congestion of the sinus mucosa (acute congestive rhinosinusitis) and most often are resolved spontaneously. In the event of acute bacterial sinusitis, antibiotic therapy is justi ed. Special warnings related to pseudoephedrine hydrochloride: Discontinue in development of hypertension, tachycardia, palpitations, cardiac arrhythmias, nausea or any neurological signs such as onset or worsening of headache. Refer to the SPC for other warnings on use and discontinuation requirements. Precautions for use related to pseudoephedrine hydrochloride: Discontinue treatment several days before surgery if volatile halogenated anaesthetics are to be used due to risk of acute hypertension. Athletes: Possibility of positive results in doping tests. Interference with serological testing: Pseudoephedrine has the potential to reduce iobenguane i-131 uptake in neuroendocrine tumors, thus interfering with scintigraphy. Special warnings related to ibuprofen: Bronchospasm may be precipitated in patients su ering from, or with a history of bronchial asthma or allergic disease. Do not take in cases of asthma without prior consultation with a doctor, as an acute asthma attack can be precipitated, particularly when allergic to acetylsalicylic acid or an NSAID. Patients who have asthma associated with chronic rhinitis, chronic sinusitis and/or nasal polyposis have a higher risk of allergic reactions when taking acetylsalicylic acid and/or NSAIDs. Refer to the SPC for more information on medication overuse headache (MOH), blood clotting disorders, gastro-intestinal bleeding, ulceration or perforation

COLD & FLU?

(discontinue immediately in these cases), history of gastro-intestinal disease (ulcerative colitis, Crohn's disease), alcohol, risk of arterial thrombotic events (particularly at a high ibuprofen dose of 2400 mg/day), serious skin reactions. Discontinue at the rst appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity. Consider carefully in uncontrolled hypertension, congestive heart failure (NYHA II-III), established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease and high doses (2400 mg/day) should be avoided. Precautions for use related to ibuprofen: Monitor the elderly carefully, due to increased frequency of NSAID-related undesirable e ects, particularly gastro-intestinal bleeding and perforation, which can be fatal. Monitor in history of gastro-intestinal disease (such as peptic ulcer, hiatus hernia or gastrointestinal bleeding). Monitor urine output and renal function initially in heart failure, chronically impaired renal or hepatic function, patients taking diuretics, hypovolaemia as a result of major surgery. If visual disturbances occur during the course of treatment, a full ophthalmological examination should be carried out. Interactions: Refer to the SPC for detailed information on interactions. Combination of pseudoephedrine with: Non-selective MAOIs (iproniazid); Other indirectly-acting, orally or nasally administered sympathomimetics or vasoconstrictor agents, α-sympathomimetic drugs, phenylpropanolamine, phenylephrine, ephedrine, methylphenidate; Reversible inhibitors of monoamine oxidase A (RIMAs), linezolid, dopaminergic ergot alkaloids, vasoconstrictor ergot alkaloids: Volatile halogenated anaesthetics: Guanethidine, reserpine and methyldopa: Tricyclic antidepressants; Digitalis, chinidine or tricyclic antidepressants. Concomitant use of ibuprofen with: Other NSAIDs, including salicylates and COX-2 selective inhibitors; Digoxin; Corticosteroids; Anti-platelet agents; Acetylsalicylic acid; Anticoagulants: (e.g.: warfarin, ticlopidine, clopidogrel, tiro ban, epti batide, abciximab, iloprost); Phenytoin; Selective serotonin reuptake inhibitors (SSRIs); Lithium; Probenecid and sul npyrazone Diuretics, ACE inhibitors, beta-receptor blockers and angiotensin-II antagonists; Potassium sparing diuretics; Methotrexate; Ciclosporin; Tacrolimus; Zidovudine; Sulphonylureas; Quinolone antibiotics; Heparins; Gingko biloba. Pregnancy and Lactation: Contra-indicated during breastfeeding and the third trimester of pregnancy and should only be given if clearly necessary during the rst and second trimester. Ability to Drive and Use Machinery: Minor or moderate in uence on the ability to drive and use machines. Patients who experience dizziness, hallucinations, unusual headaches and visual or hearing disturbances should avoid driving or using machinery. Single administration or short-term use of this medicine does not usually warrant the adoption of any

Undesirable E ects: Common: Gastrointestinal discomfort, dyspepsia, abdominal pain, nausea, vomiting, atulence, diarrhoea, constipation, minor gastrointestinal blood loss in rare cases leading to anaemia. Refer to the SPC for other undesirable e ects. Marketing Authorisation Holder: Rowa Pharmaceuticals Ltd., Bantry, Co. Cork. Marketing Authorisation Number: PA0074/067/006. Further information and SPC are available from: Rowex Ltd., Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417 . E-mail: rowex@rowa-pharma.ie Legal Category: Not Subject to medical prescription. Date of Preparation: May 2021. Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing Rowex pv@rowa-pharma.ie If you need effective relief take it. If you don’t don’t.
holder: Rowa Pharmaceuticals Ltd., Newtown, Bantry, Co. Cork, Ireland. PA0074/067/006
of preparation: 6/21 CCF: 23764
NEW
special precautions.
MA
Date
Marketed by
INFLAMMATORY DISEASE
Researchers find new link between a disrupted body clock and inflammatory diseases
New research from the RCSI has demonstrated the significant role that an irregular body clock plays in driving inflammation in the body’s immune cells, with implications for the most serious and prevalent diseases in humans.
Published in Frontiers in Immunology, the research was led by the School of Pharmacy and Biomolecular Sciences at RCSI University of Medicine and Health Sciences.
The circadian body clock generates 24-hour rhythms that keep humans healthy and in time with the day/night cycle. This includes regulating the rhythm of the body’s own (innate) immune cells called macrophages. When these cell rhythms
HOSPITAL PHARMACY

are disrupted (due to things like erratic eating/sleeping patterns or shift work), the cells produce molecules that drive inflammation. This can lead to chronic inflammatory diseases such as heart disease, obesity, arthritis, diabetes and cancer, and can also impact our ability to fight infection.
In this study, the researchers looked at macrophages with and without a body clock under laboratory conditions. They were interested to understand if macrophages without a body clock might use or ‘metabolise’ fuel differently, and if that might be the reason these cells produce more inflammatory products.
The researchers found that macrophages without a body clock took up far more glucose and broke it down more quickly than normal cells. They also found that, in the mitochondria (the cells’ energy powerhouse), the pathways by which glucose was further broken down to produce energy were very different in macrophages without a clock. This led to the production of reactive oxygen species (ROS), which further fuelled inflammation.
Dr George Timmons, lead author on the study, said: “Our results add to the growing body of work showing why disruption of our body clock leads to inflamma-
tory and infectious disease, and one of the aspects is fuel usage at the level of key immune cells, such as macrophages.”
Dr Annie Curtis, Senior Lecturer at RCSI School of Pharmacy and Biomolecular Sciences and senior author on the paper, added: “This study also shows that anything which negatively impacts on our body clocks, such as insufficient sleep and not enough daylight, can impact on the ability of our immune system to work effectively.”
RCSI conducted the study in collaboration with researchers from Swansea University, Trinity College Dublin and the University of Bristol.
EAHP and ESCP collaborate to produce ‘Oath to Society’ for pharmacists
Ensuring the safe use of medication is one of the key activities of clinical and hospital pharmacists. However, many people don’t know that both professions carry out many more activities to ensure optimal treatment outcomes for patients, according to the European Association of Hospital Pharmacists (EAHP) and the European Society of Clinical Pharmacy (ESCP). The two bodies have collaboratively developed the ‘Oath to Society’, which acts as a contract for excellence in providing compassionate patient care, working as part of the healthcare team, advancing the pharmacy profession, and showcasing how clinical and hospital pharmacists work every day.
The Oath to Society is the promise that the members of EAHP and ESCP make to patients and the public they serve, the healthcare professionals they interact with and the health systems they work in, they said. The
Oath functions as a compass for pharmacists to adhere to the highest standards of ethics, integrity and professionalism, as they provide service to the community over the course of their careers, said the EAHP and ESCP. Touching on trust and respect, different aspects of the patient care pathway, the multidisciplinary care team, disease prevention and health promotion, education and
the future development of pharmacy practice, the Oath to Society is all-encompassing, they said.
Reflecting on their creation before the launch event, ESCP President Mr Derek Stewart commented: “The development of the Oath to Society for European clinical and hospital pharmacists was inspired by similar initiatives of pharmacists and our fellow healthcare profession-
als around the globe. The Oath to Society emphasises respect for patient privacy, the key contributions that our profession makes, and the need for clinical and hospital pharmacists to uphold the highest standards of ethics for both themselves and their colleagues.”
Commenting during the launch event, EAHP President Mr András Süle stated: “It is important to communicate closely with patients, their carers and our fellow healthcare professionals, such as physicians, nurses and pharmacy technicians, to offer the best possible treatment. As the healthcare system shifts towards efficiency while still ensuring a high degree of safety and quality, pharmacists — as valued members of the interprofessional healthcare team — can and should play an integral role, focusing on medication management, medication reconciliation, preventive care and patient education.”
NEWS 6 VOL 23 ISS 1 JANUARY 2022
For women with
Dryness and sensitive skin and membranes
Vaginal dryness, dry eyes and dry mouth are issues women typically experience around and after menopause. Omega 7 Pharma Nord is a formula developed to help maintain healthy and well hydrated mucosa at this stage of life.
• Scientifically documented
• Suited for vegetarians and vegans
• With vitamin A that supports normal skin, vision, and mucous membranes
Omega 7 Pharma Nord contains the SBA24 extract that is made from both the berries and seeds of sea buckthorn to ensure the widest spectrum of beneficial nutrients.
Sea buckthorn is one of nature’s richest sources of vitamin A, a nutrient that is best known for its ability to maintain normal skin, vision, and mucous membranes.
www.pharmanord.ie

IE_Omega7_Ad_IrishPharmacist_214x275_1221
A new qualitative study from researchers at the School of Nursing and Midwifery at Trinity College Dublin provides, for the first time, a depth of understanding of the everyday experience of the Covid-19 pandemic for older people in Ireland. As we move into the winter months and the possibility of further restrictive measures, the findings from the study provide important evidence that policymakers and Government should be mindful of regarding future possible public health restrictions, said the authors.
The report, launched recently in partnership with Safeguarding Ireland, found that older people made significant efforts to self-protect from Covid-19. However, this was at the expense of their physical and/ or mental health. While public health restrictions applied to the entire population, older
DIABETES
people were advised to follow guidance which limited their daily lives.
Unlike previous reports, participants gave a first-hand account of their experience and described their careful efforts to self-protect. The resilience of older people is a dominant feature of the report. The collection of data occurred between January and March 2021.
Key findings include:
Older people made substantial changes to their daily lives to comply with the Covid-19 shielding guidance.
Covid-19 had significant impacts on the health of older people in the community.
Older people reported a general stoic approach to living in the pandemic and they demonstrated resilience in multiple ways.
The use of technology assisted in managing social and practical activities, however, its
use, satisfaction and familiarity differed within the participants in the study.
Older people need more integrated support systems which maintain their personal, health and social needs.
Consideration needs to be given to pandemic-related information to avoid information fatigue, misinformation, and confusion.
Post-pandemic rehabilitation will be required to focus on restoring lost physical ability and address the consequences of social isolation and loneliness.
There is a need to ensure that ageist approaches do not underpin guidance. The rights of autonomy and self-determination need to be central considerations in future similar crises.
Prof Amanda Phelan, Professor in Ageing and Community Nursing and Principal Investigator of the study, said: “Older
people have made significant efforts to self-protect in the pandemic, however, there has been consequences for both their physical and mental health. The impact has been most profound in the older age groups due a disproportionate impact in mortality and morbidity rates. We need a short-term plan of engaging in rehabilitation through comprehensive geriatric assessments and care plans. While our study did not highlight safeguarding issues, it is also important to acknowledge that the conditions of the pandemic exacerbated elder abuse risk factors, and this may be occurring in Ireland under the radar of our data. Thus, a focus on awareness, prevention and early intervention are key considerations.”
To view the report, visit: https://www.tcd.ie/tcphi/assets/ pdf/older-people-shielding.pdf
New resource supports pharmacists in tackling public health threat of diabetes
The role that pharmacists can play in preventing the projected rise in diabetes to 700 million people by 2045 is highlighted in a new resource released by the International Pharmaceutical Federation (FIP). Diabetes prevention, screening and management: A handbook for pharmacists provides information on the wide range of pharmaceutical services that pharmacists can provide to reduce the global burden of this disease.
“It is imperative to ensure the healthcare workforce is prepared to care for people with diabetes and those at risk. In 2019, more than half of the 463 million adults with diabe -
tes were not aware they had it. While pharmacists are primarily trained to address health concerns through pharmacological means, they also have the necessary skills and knowledge to provide prevention and screening services,” said Mr Paul Sinclair, Chair, FIP Board of Pharmaceutical Practice.
“By leveraging their accessibility and the trust the public has in them, pharmacists can promote the importance of following a healthy lifestyle, including consuming a healthy diet and participating in regular physical activity — particularly important measures to prevent the development of type 2 diabetes, which accounts for
90-to-95 per cent of cases. This new FIP publication is part of the Federation’s new Practice Transformation Programme on Non-Communicable Diseases,” Mr Sinclair said.
In addition to prevention, screening and referral, the new FIP handbook covers medicines management, non-pharmacological management and advice, and prevention and management of complications such as diabetic neuropathy and retinopathy. It also addresses barriers to providing diabetes services.
“You need only spend a few minutes at your local pharmacy to appreciate the significant role the community
pharmacist plays in supporting community health. Pharmacists are active members of the healthcare team, providing trusted advice into a community to which, more often than not, they also belong. From a diabetes care perspective, it is a key role,” said Prof Andrew Boulton, President, International Diabetes Federation.
He added: “Advice, of course, should be based on the best available evidence. This publication from the International Pharmaceutical Federation provides pharmacists with everything they need to know to help guide community members to make healthy choices and adopt healthy habits.”
NEWS 8 VOL 23 ISS 1 JANUARY 2022
‘Ageist approaches’ must not underpin guidance for future crises — study
COVID-19
DON’T LET PAIN HOLD YOU BACK
For more detail and for any training requirements please contact your Perrigo Pharmacy Business Manager
*To verify contact verify@perrigo.com
ESSENTIAL INFORMATION
Solpa-Extra 500mg/65mg Soluble Tablets contain paracetamol and caffeine. For the treatment of mild to moderate pain. Adults and children over 16 years: 1-2 tablets dissolved in water every 4-6 hours. Max 8 tablets a day. Children 12-15 years: 1 tablet disolved in water every 4-6 hours. Max 4 tablets a day. Not suitable for children under 12 years. Contraindications: Hypersensitivity to the ingredients. Precautions: Particular caution needed under certain circumstances, such as renal or hepatic impairment, chronic alcoholism and malnutrition or dehydration. Precautions needed in asthmatic patients sensitive to acetylsalicylic acid, patients on a controlled sodium diet and with rare hereditary problems of fructose intolerance. Patients should be advised not to take other paracetamol containing products concurrently. Pregnancy and lactation: Not recommended during pregnancy and breastfeeding. Side effects: Rare: allergies. Very rare: thrombocytopenia, anaphylaxis, bronchospasm, hepatic dysfunction, cutaneous hypersentitivity reactions. Unknown: nervousness, dizziness. Further information is available in the SmPC. PA 1186/017/001. P. MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland.
Date of preparation: July 2020. Legal Class P – Pharmacy only.

IRE SOL1 2021 02
086-8189846086-4679113086-6011643086-1749626086-8189843086-1427717087-9533161
ALAN HARNETTRUTH GRANTCHARLIE RICKARDANNE MARIE O’NEILLBRENDAN O’KEEFFEPAUL FLYNNOLLIE HIGGINS Nth Leinster Midlands Greater DublinSouth LeinsterCork and Kerry Waterford/Tipperary/ Limerick/Clare West
A global call to action issued recently by the International Pharmaceutical Federation (FIP) sets out 18 urgent actions needed from academic institutions, professional organisations, policy-makers and key pharmaceutical stakeholders in order to help prevent the shortfall of 18 million health workers by 2030 predicted by the World Health Organisation, through advancing pharmacy and pharmaceutical sciences education.
“We can only meet the health and pharmaceutical needs of our societies if a flexible and adaptable pharmaceutical workforce is developed and deployed appropriately to apply its knowledge, skills, attitudes, behaviours and abilities to the maximum. As we draw closer to 2030, we are urging all stakeholders to take our recommended actions to advance pharmaceutical education now if we are to have any prospect of meeting the United Nations Sustainable Development Goals (SDGs),” said FIP CEO Dr Catherine Duggan.
Among these actions are to:
Increase capacity to provide a competent pharmaceutical workforce.
Support the development of early career and advanced specialist frameworks.
CANCER AND COVID
Ensure the quality of the pharmaceutical workforce by quality assuring pharmaceutical education and training.
Enhance interdisciplinary and interprofessional education and collaboration with key stakeholders.
Support the development of CPD programmes, such as for return to practice after career breaks and sector changes.
Generate pharmaceutical education intelligence to measure educational outcomes and inform investments.

The FIP said it has pledged to support the
achievement of the SDGs through implementing the FIP Development G oals across the entire pharmacy profession. The Federation has already been working on transforming the education sector through the FIP-UNESCO UNITWIN programme. At FIP-UNITWIN workshops in October 2021, academic institutions and key stakeholders worked together on SWOT analyses and identified priority FIP Development Goals for pharmaceutical education in each World Health Organisation region. The workshops resulted in regional roadmaps to advance pharmaceutical education, which have been consolidated in FIP’s global call to action.
Dr Duggan added: “FIP is committed to providing its networks with platforms, expertise, capacity, resources, guidance and global standards to advance pharmaceutical education, training and research. Regional roadmaps developed at our recent FIP-UNITWIN workshops will provide systematic and concerted frameworks to advance and transform pharmaceutical education globally, and FIP will establish regional centres for excellence through FIP-UNITWIN programme to implement these roadmaps.”
Progress on cancer survival at risk due to Covid disruption — Irish Cancer Society
Improved cancer survival figures revealed recently are being put at serious risk by pandemic-related disruption, according to the Irish Cancer Society.
Commenting on the National Cancer Registry Ireland (NCRI) annual report, the Society’s CEO Ms Averil Power said: “While it is heartening to hear that progress is being made for devastating cancers like breast, lung and prostate according to latest figures up to 2019, we are very worried that significantly less cancers were diagnosed last year.
“This will present a major challenge for years to come, and is unfortunately no surprise, as already struggling cancer services have been stretched to breaking point during the pandemic. Lengthy waiting lists and disruptions to vital diagnostic and screening services are now all too commonplace.
“Patients are telling us that they are terrified of having their treatment delayed given the current spike in Covid case numbers and are very distressed about the worrying consequences to their health from catching the virus, and the further risk of treatment delays that this would bring.
“We are particularly concerned about recent surgery cancellations, as the ‘non-urgent’ cases of today will only become more serious and difficult to treat the longer they are left, not to mention the mental anguish this causes for patients,” she continued.
“The Irish Cancer Society is collaborating with researchers from the Royal College of Surgeons in Ireland and others on a muchneeded project to get a clearer picture of the impact of the pandemic on cancer services. In the meantime, we need the Government to
step up and put a massive effort into clearing waiting lists and securing cancer services for patients nearly two years into this crisis.
“The NCRI report shows that as many as one-in-eight cancers that were predicted to be diagnosed in 2020 were not. Although there has been an encouraging trend of people seeking medical help so far [in 2021], waiting times for diagnostic tests remain too long and these must be addressed as a matter of urgency.
“It is frightening to think that there are people in our community with cancer who don’t know it yet. We would plead with anyone with a cancer concern or symptom to talk to their doctor or call our Freephone 1800 200 700 Support Line without delay, as we do not want potentially treatable cancers to go too far.”
NEWS 10 VOL 23 ISS 1 JANUARY 2022
Eighteen urgent actions needed in pharmaceutical education ‘to avoid health worker shortfall’
PHARMACY WORKFORCE
Genuair®-has it ‘clicked’ yet?








The ONLY pre lled inhaler with visual and audible feedback for confirmed dose delivery1-4

Genuair - a simple to use inhaler for patients with COPD4





Abbreviated Prescribing Information
Eklira® Genuair® 322 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and a green dosage button. Each delivered dose contains 375 µg aclidinium bromide equivalent to 322 µg of aclidinium. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 322 micrograms aclidinium twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents.

Contraindications: Hypersensitivity to aclidinium bromide or to any of the excipients. Warnings and Precautions: Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Use with caution in patients with symptomatic prostatic hyperplasia or bladder-neck obstruction or with narrow-angle glaucoma. Do not use in patients with rare hereditary problems of galactose intolerance, total lactose de ciency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic-containing medicinal products. No other interactions expected. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Risk to newborns/infants cannot be excluded. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Side-e ects:
Common (1-10%): Sinusitis, nasopharyngitis, headache, cough, diarrhoea, nausea. Uncommon (0.1-1%): Dizziness, blurred vision, tachycardia, palpitations, dysphonia, dry mouth, stomatitis, rash, pruritus, urinary retention. Rare (0.01-0.1%): hypersensitivity. Not known: angioedema, anaphylactic reaction. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing
Authorisation Number: EU/1/12/778/002 Marketing Authorisation holder: AstraZeneca AB, SE151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated:
February 2020







This medicinal product is subject to additional monitoring. This will allow quick identi cation of new safety information. Healthcare professionals are asked to report any suspected adverse reactions to: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744.
Date of item: November 2020. IR-BRI-10-2020









Abbreviated Prescribing Information Brimica® Genuair® 340 micrograms/12 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and an orange dosage button. Each delivered dose contains 396 µg aclidinium bromide (equivalent to 340 µg of aclidinium) and 11.8 micrograms of formoterol fumarate dihydrate. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 340 µg/12 µg twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Warnings and Precautions: Do not use in asthma. Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Discontinue if increases in pulse rate, blood pressure or changes in ECG occur. Use with caution in patients with a history of or known prolongation of the QTc interval or treated with products a ecting the QTc interval. Use with caution in patients with severe cardiovascular disorders, convulsive disorders, thyrotoxicosis and phaeochromocytoma. Hypokalaemia may occur, is usually transient and supplementation not needed. In patients with severe COPD, hypokalaemia may be potentiated by hypoxia and concomitant treatment. Use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Do not use in patients with rare hereditary problems of galactose intolerance, the Lapp lactase de ciency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic and/or long-acting β2-adrenergic agonist containing medicinal products. Caution in use with methylxanthine derivatives, steroids, non-potassium-sparing diuretics, β-adrenergic blockers or medicinal products known to prolong the QTc interval. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Sidee ects: Common (1-10%): Nasopharyngitis, urinary tract infection, sinusitis tooth abscess, insomnia, anxiety, headache, dizziness, tremor, cough, diarrhoea, nausea, dry mouth, myalgia, muscle spasms, peripheral oedema, increased blood creatine phosphokinase. Uncommon (0.1- 1%): Hypokalaemia, hyperglycaemia, agitation, dysgeusia, blurred vision, tachycardia, electrocardiogram QTc prolonged, palpitations, angina pectoris, dysphonia, throat irritation, stomatitis, rash, pruritus, urinary retention, increased blood pressure. Rare (0.01-0.1%): Hypersensitivity, bronchospasm, including paradoxical. Not known: anaphylactic reaction, angioedema. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/14/963/001 Marketing
Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: October 2019


This medicinal product is subject to additional monitoring. This will allow quick identi cation of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744.


References: 1 MIMS Ireland November 2020 2. Eklira® Genuair® Summary of Product Characteristics, last updated November 2019 3. Brimica® Genuair® Summary of Product Characteristics, last updated August 2019 4 Magnussen, H et al. COPD. 2019 Apr;16(2):196-205
LAMA + LABA LAMA
DRUG DISCOVERY
New computer modelling tool could boost drug discovery and improve treatment
Scientists from Queen’s University Belfast have developed a computer-aided data tool that could improve treatment for a range of illnesses. The computer modelling tool will predict novel sites of binding for potential drugs that are more selective, leading to more effective drug targeting, increasing therapeutic efficacy and reducing side-effects.
The data tool or protocol will uncover a novel class of compounds — allosteric drugs in G protein-coupled receptors (GPCRs).
GPCRs are the largest membrane protein family that transduce a signal inside cells from
DEMENTIA MEDICATIONS
A drug used to treat agitation in people with dementia is no more effective than a placebo, and might even increase mortality, according to a recent study published in The Lancet .
The research has shown that antidepressant mirtazapine offered no improvement in agitation for people with dementia and was possibly more likely to be associated with mortality than no intervention at all. The study was led by the University of Plymouth in the UK with Prof Iracema Leroi from the Global Brain Health Institute (GBHI), Trinity College Dublin, as coinvestigator on the study.
Agitation is a common symptom of dementia, characterised by inappropriate verbal, vocal or motor activity, and often involves physical and verbal aggression. Non-drug patient-centred care is the first intervention that should be offered but, when this doesn’t work, clinicians may
hormones, neurotransmitters, and other endogenous molecules. As a result of their broad influence on human physiology, GPCRs are drug targets in many therapeutic areas, such as inflammation, infertility, metabolic and neurological disorders, viral infections and cancer. Currently, over a third of drugs act via GPCRs. Despite the substantial therapeutic success, the discovery of GPCR drugs is challenging due to promiscuous binding and subsequent side-effects.
Recent studies point to the existence of other binding sites, called allosteric sites, that drugs can bind to and provide several
therapeutic benefits. However, the discovery of allosteric sites and drugs has been mostly serendipitous. Recent x-ray crystallography that determines the atomic and molecular structure, and cryo-electron microscopy that offers 3D models of several GPCRs, offer opportunities to develop computer-aided methodologies to search for allosteric sites.
The researchers developed a computer-aided protocol to map allosteric sites in GPCRs with a view to start rational search of allosteric drugs, presenting the opportunity for new solutions and therapies for a range of diseases.
School of Pharmacy at Queen’s University and senior author explained: “We have developed a novel, cost-effective and rapid pipeline for the discovery of GPCRs allosteric sites, which overcomes the limitations of current computational protocols, such as membrane distortion and non-specific binding.
“Our pipeline can identify allosteric sites in a short time, which makes it suitable for industry settings. As such, our pipeline is a feasible solution to initiate structure-based search of allosteric drugs for any membranebound drug targets that have an impact on cancer, inflammation, and CNS diseases.”
move to a drug-based alternative. Antipsychotics have been proven to increase death rates in those with dementia, along with other poor outcomes, and so mirtazapine has been routinely prescribed. This study was designed to add to the evidence base around its effectiveness.
Funded by the National Institute for Health Research (NIHR), the study recruited 204 people with probable or possible Alzheimer’s disease from 20 sites around the UK, allocating half to mirtazapine and half to placebo. The trial was double-blinded.
The results showed that there was no less agitation after 12 weeks in the mirtazapine group than in the control group. There were also more deaths in the mirtazapine group (seven) by week 16 than in the control group (only one), with analysis suggesting this was of marginal statistical significance.
The study is an example of the importance of clinical trials for dementia. Currently, less than 0.5 per cent of people with dementia participate in research in Ireland, despite the need for evidence to address the rapidly increasing impact of dementia on our aging society.
The study’s publication coincides with the launch of Dementia Trials Ireland (DTI), a five-year initiative funded by the Irish Health Research Board (HRB) and led by Prof Iracema Leroi and Prof Sean Kennelly, both faculty of GBHI at Trinity College. The aim of DTI is to significantly increase the opportunities for people with dementia to participate in clinical trials in Ireland.
Prof Leroi said: “It is only through conducting robust and high-quality clinical trials for Alzheimer’s disease and other forms of dementia that we can hope to combat this condition
which affects so many families in Ireland. It is every patient’s right to participate in research and we therefore have to ensure that they have the opportunity to do so.”
Lead researcher Prof Sube Banerjee, Executive Dean of the Faculty of Health and Professor in Dementia at the University of Plymouth, explained why the results of the study were so surprising, but important. “Dementia affects 46 million people worldwide — a figure set to double over the next 20 years. Poor life quality is driven by problems like agitation, and we need to find ways to help those affected. This study shows that a common way of managing symptoms is not helpful and could even be detrimental. It’s really important that these results are taken into account and mirtazapine is no longer used to treat agitation in people with dementia.”
NEWS 12 VOL 23 ISS 1 JANUARY 2022
Dr Irina Tikhonova from the
Common antidepressant should no longer be used to treat people with dementia, study says
























At Accord, our mandate is to deliver a ordable medicines that make a real di erence to patients’ lives. Date of preparation: February 2021 IE-01603 Associated member of: Oncology & Haematology Accord’s Commitment to Biosimilars
EUTHANASIA AND ASSISTED SUICIDE:
A VULNERABLE PROFESSION
INTRODUCTION
Euthanasia and assisted suicide are associated with the administration of lethal medications and/or lethal doses of medications. Pharmacists are pivotal to the euthanasia and assisted suicide process, as the process cannot occur without medications. Pharmacists in Great Britain have viewed their professional responsibility in euthanasia and assisted suicide to be more obligatory than a physician's, in having to provide the means for euthanasia/assisted suicide. The process is complex, involves a number of actors, involves a number of stages, engages morality, ethics, philosophy, law, medicine, pharmacy, religion, politics, human rights, etc. The outcome of the process is the intentional termination of human life. The three health professions whose roles in ‘assisted dying’ provision are most often described in the scientific literature are physicians, nurses, and pharmacists. If legislation to introduce euthanasia and assisted suicide is passed in Ireland, every pharmacist would need to make a personal decision on whether or not they would wish to be involved in providing or supporting a service resulting in the intentional ending of human life. This article is an Irish pharmacist’s reflection on some (limited) aspects of this complex grave topic.
BACKGROUND
The Dying with Dignity Bill 2020 was introduced in the Dáil by opposition deputies Gino Kenny, Mick Barry, Richard Boyd Barrett, Paul Murphy and Bríd Smith in 2020 and was voted to committee stage. The Justice Committee then carried out scrutiny of the Bill, including seeking public submissions on the topic. Over 1,400 public submissions, including a number from pharmacists (Box 1), were received by the deadline in January 2021. The Irish Human
Rights and Equality Commission (IHREC) has warned that the Bill did not ensure “adequate safeguards” to protect a person’s right to life. In July 2021, the Justice Committee Cathaoirleach James Lawless TD said the committee has determined that the Bill has “serious technical issues in several sections”, that it may have unintended policy consequences, and that the drafting of several sections of the Bill contains “serious flaws”. It was decided that the Bill should not progress to Committee Stage, but that a Special Oireachtas Committee should be established, at the earliest convenience, for detailed consideration. This committee is expected to commence in early 2022. If enacted, the so-called Dying with Dignity Bill 2020 would have introduced euthanasia and assisted suicide (ie, the intentional termination of human life/killing) to Ireland.
The Dying with Dignity Bill 2020 was a simple six-page document that made no reference to the pharmacist’s complex role in assisted suicide and euthanasia. The complexities involved in the roles of pharmacists were un-
Houses of the Oireachtas, Joint Committee on Justice, Report on Scrutiny of the Dying with Dignity Bill 2020 [PMB] July 2021 33/JC/11. Page 17 https://data.oireachtas.ie/ie/oireachtas/ committee/dail/33/joint_committee_on_ justice/reports/2021/2021-07-21_report-onscrutiny-of-the-dying-with-dignity-bill-2020pmb_en.pdf
In relation to Section 13 of the Bill, some of these submissions highlighted their concern that the right to conscientious objection does not stipulate that
recognised, unvalued, ignored, simplified or maybe not understood. The individual pharmacist was excluded from the “conscientious objection” section of the Bill. Her/his human and constitutional right to freedom of conscience was not acknowledged or protected. The consequences of this exclusion would have resulted in vulnerable individual pharmacists and a vulnerable profession.
VULNERABILITY
Vulnerability can be defined as a lack of autonomy and independence, bodily and psychological insecurity, marginalised or deviant status, or lack of acknowledgement within society. Vulnerable groups are exposed to discrimination, intolerant attitude, and subordination. Vulnerability is usually seen as an inherent quality of certain social groups (but not others). However, it has many dimensions and might be attributed to relatively ‘powerful’ groups. Doctors, pharmacists and nurses, ie, so-called powerful groups, are rarely characterised as vulnerable, but within certain
pharmacists can avail of this, even though they would also play a role in the process of assisted dying similar to other assisting healthcare professionals.
They also argue that the similar exclusion of pharmacists due to the narrow definition of assisting healthcare professionals in section 2 of the Bill means they are not being afforded sufficient legal protection if they do take part in the assisted dying of a patient, as stipulated in section 12 of the Bill.
FEATURE | Assisted dying & the pharmacist 14 VOL 23 ISS 1 JANUARY 2022
PHARMACISTS WORLDWIDE IN ALL AREAS OF THE PROFESSION HAVE FALLEN THROUGH THE CRACKS OF THE LEGAL PROTECTION OF CONSCIENCE WHEN IT COMES TO EUTHANASIA AND ASSISTED SUICIDE, WRITES DR BERNADETTE FLOOD (PHD) MPSI
Box 1: Joint Committee on Justice, Report on Scrutiny of the Dying with Dignity: Pharmacist Submissions
circumstances, they can be recognised as vulnerable. Euthanasia and assisted suicide may be depicted as no more than an individual patient's wish. This ignores the presence of vulnerable professionals in the process. Doctors, pharmacists and nurses are individually independent moral agents who must make his/ her independent and separate autonomous response, be required to justify it when asked, and take due responsibility for it.
As healthcare systems and societies are changing, the social positions of doctors, pharmacists, nurses and patients within them are changing too. In the past, clinical experts’ authority and patients’ autonomy have been in conflict. The current patient-centered/personcentered model of healthcare aims to establish egalitarian relationships between patients and professionals and other healthcare providers. However, the vulnerabilities of doctors, pharmacists and nurses would often appear to be invisible in the euthanasia and assisted suicide debates that include a focus on the personal autonomy of the patient. The relationship of the physician/pharmacist/nurse with the patient is a moral equation with rights and obligations on both sides and it must be balanced so that physicians/pharmacists/nurses and patients act beneficently toward each other, while respecting each other's autonomy. Beneficence and autonomy must be mutually reenforcing if the patient's good is to be served, if the physician/pharmacist/nurse's ability to serve that good is not to be compromised, and if their moral claim to autonomy and the integrity of the whole enterprise of medical ethics are to be respected.
Vulnerability is part of the human condition and is an all-pervasive phenomenon in healthcare. We as pharmacists can understand our humanity only by recognising our universal vulnerability and interdependence. ‘No man is an island.’ The Universal Declaration on Bioethics and Human Rights (UDBHR) text contains the first statement of bioethical principles
UDBHR, Article 8: Respect for human vulnerability and personal integrity In applying and advancing scientific knowledge, medical practice and associated technologies, human vulnerability should be taken into account.
Individuals and groups of special vulnerability should be protected and the personal integrity of such individuals respected.
accepted by governments (Box 2).
Experience in other countries shows that legalising assisted suicide, euthanasia or both can have a profound impact on pharmacy practice. The Royal Pharmaceutical Society in the policy statement ‘Assisted Dying’ advises that policy-makers need to recognise and be aware that the role of pharmacists goes far beyond supply of the required medication. Pharmacists in Ireland provide professional services in a variety of settings in response to local, national and international needs and priorities, with a focus on populations and/or individual patients, ie, palliative/hospice care, long-term care, intellectual disability care, hospitals, community, HIQA, HPRA, regulation, Department of Health, academia, research, education and the pharmaceutical industry. Pharmaceutical Public Health in Ireland includes services to populations, such as guidelines and treatment protocols, medicine use review and evaluation, national medicine policies and essential medicines lists, pharmacovigilance, needs assessment and pharmaco-epidemiology, etc. Individual pharmacists and the pharmacy profession will be challenged if euthanasia and assisted suicide are legalised in Ireland.
There are many euphemisms (‘dying with dignity’, ‘medical assistance in dying (MAiD)’,
‘physician-assisted dying/suicide’, etc) used to describe the euthanasia and assisted suicide and the lethal medication process. The following definitions are taken from the Houses of the Oireachtas, Joint Committee on Justice and Equality (2018) Report on the Right to Die with Dignity:
Euthanasia: Euthanasia is an intervention undertaken with the intention of ending a life to relieve suffering. In euthanasia, the doctor or other person involved ‘acts last’; it is their action that causes death.
Assisted suicide: The act of intentionally assisting a person to take their own life. Importantly, in assisted suicide, the person seeking to die ‘acts last’; they take the medicine/use whatever means selected that results in death. The assistance allows them to do so.
One concerning feature of the Dying with Dignity Bill 20203 is that it does not mention euthanasia or assisted suicide or lethal medications in any of the qualifying criteria or provisions. Instead, it uses confusing phrases such as ‘assistance in dying’, ‘the prescription of substances that can be orally ingested’, and ‘the substance or substances may be administered’. ‘Euthanasia’ and ‘assisted suicide’ are the terms used throughout the following reflective article.
There are many potential sources of vulnerability for individual pharmacists, and each constitutes a different, overlapping layer. Vulnerability is not static or fixed, with many sources of vulnerability overlapping in different situations arising from our human condition. Different elements can locate a person (ie, pharmacist) in a situation of personal and/or professional vulnerability. Vulnerability is dependent on the different positions we occupy in social space, or in the way we are supported (or not) by our social institutions, ie, legislation, professional organisations and regulation. “Vulnerability is experienced uniquely by each of us.” The vulnerabilities of pharmacists if euthanasia and assisted suicide are introduced to Ireland can be reflected on in relation to: (1)
Assisted dying & the pharmacist | FEATURE 15 VOL 23 ISS 1 JANUARY 2022
Box 2: UDBHR, Article 8: Respect for Human Vulnerability and Personal Integrity
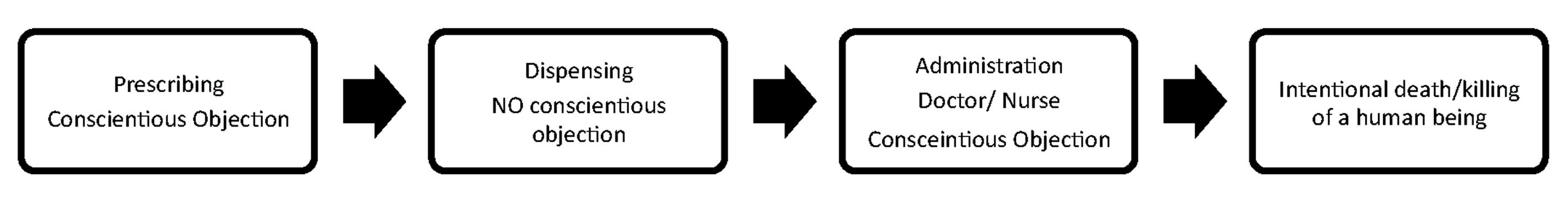
Figure 1: Lethal Medication Use Process and Conscientious Objection
personal vulnerability; (2) professional vulnerability; (3) societal vulnerability; and (4) lethal medication vulnerabilities.
1. PERSONAL VULNERABILITY
Pharmacists will be at the vortex of some of society’s most controversial moral dilemmas. Pharmacists are highly-trained professionals with moral, ethical and legal accountability. The role of pharmacists in the pharmaceutical care/medication use process at the end of life is often ignored, written out or overlooked. All forms of euthanasia and physician-assisted suicide require lethal medications dispensed by pharmacists. In many jurisdictions where euthanasia/assisted suicide are legalised/allowed, doctors and nurses are included in ‘conscientious objection’ legislative protections. In contrast, pharmacists worldwide have fallen through the cracks of the legal protection of conscience, and are often excluded from protection of their moral and human right to freedom of conscience and the derived right of ‘conscientious objection’ (Figure 1). Conscience is defined by the Cambridge Dictionary (2021) as “that part of you that judges how moral your own actions are and makes you feel guilty about bad things that you have done or things you feel responsible for”.
Pharmacists are vulnerable when their personal dignity as human beings is not acknowledged and the treatment they receive in regulation and legislation is not equal to that which doctors and nurses receive. All three professions are part of the lethal medication use process. Euthanasia and assisted suicide cannot occur without the provision of lethal medications/lethal doses of medication. Many pharmacists may have a moral and/or ethical objection (for a number of reasons) to participation in a process that results in the intentional ending of a human life.
2. PROFESSIONAL VULNERABILITY
Pharmacy as a profession is orientating itself towards patient interaction. Pharmacists are required to make difficult decisions in light of their personal judgement and they are also assuming responsibilities for patient outcomes. Pharmacy has been affected significantly by societal transformations such as mass manufacturing of pharmaceuticals, widespread availability of drug information, and inclusion
of pharmacists on healthcare teams. As such, its traditional roles, such as compounding, dispensing and advising, have been challenged. Pharmacy will be vulnerable in the future if the profession loses ground to other health professions in understanding identity challenges in pharmacy. These vulnerabilities will be magnified when pharmacists are unaware that their human rights (ie, freedom of conscience) are moral entitlements that every individual in the world possesses simply by virtue of the fact that he or she is a human being. In claiming our human rights, we are making a moral claim. Human rights belong to individual pharmacists — to ensure that every individual pharmacist can live a life of dignity and a life that is worthy of a human being.
Moral distress involves a threat to one’s moral integrity — that sense of wholeness and selfworth that comes from having clearly-defined values (ie, the value of human life) that are congruent with one’s perceptions and actions. Ethical dilemmas that may arise for pharmacists speak to the ethical justifications when considering alternative courses of action (clinical, legal and spiritual components). Moral distress begins after the fact, and involves the social and organisational issues at play, along with a consideration of personal feelings and explorations of accountability and responsibility. If pharmacists and pharmacy organisations are to remain true to stated core values, finding solutions to moral distress is an ethical imperative.
Moral distress is a growing problem in healthcare. It has been defined by Andrew Jameton in 1984 as the inability of a moral agent, ie, a pharmacist, to act according to his or her core values and perceived obligations due to internal and external constraints. Constraints such as risk of litigation, peer pressure, education by senior colleagues, practice norms, pressure by patients and pressure from regulatory bodies have been hypothesised as drivers for defensive practice by pharmacists. A broad definition of defensive practice is “deviation from sound medical practice that is induced primarily by a threat of liability”.
Irish pharmacists and pharmacy students are excluded from protection of their moral and human right to freedom of conscience and conscientious objection in the Health (Regulation of Termination of Pregnancy) Act 2018. Doctors and nurses and their students are included in the Conscientious Objection section
of the Act. This unequal treatment makes individual pharmacists and the profession of pharmacy vulnerable. Unequal treatment of pharmacists is also evident in the Conscientious Objection section of the Dying with Dignity Bill 2020. This is unequal, unjust and unfair and perpetuates the vulnerability of pharmacists and pharmacy. If doctors and nurses (and others) are included in the conscientious objection section, pharmacists should also be included.
The inclusion of one group in legislation excludes all groups not included. Politicians, legislators, regulators, society, or other professions may not recognise the value of pharmacists or understand the contributions of pharmacists to healthcare and public health in Ireland and their pivotal role in the lethal medication process. It is unclear to the vulnerable Irish pharmacist if legislative and regulatory activities affecting pharmacy practice (including new and proposed policy changes that impinge on the moral values of the individual pharmacist, such as the value of human life) are being monitored by any professional pharmacy organisation. Research into defensive practice in the pharmacy profession should inform future policy and legislation, which both protects vulnerable patients and empowers vulnerable pharmacists to provide high-quality care “without fear of litigation motivating unnecessarily conservative approaches to practice”.
Morality is that higher standard that helps each pharmacist to form her/his ethical standard of conduct. Moral philosophy drives ethics, because ethics is the systematic study of moral conduct and moral judgement. Codes of ethics should be based on moral foundation (not legal foundation or public opinion). Codes of ethics are fallible because people are fallible. Ethical codes cannot encompass what all individuals in a profession believe. Ethical codes that encompass true conscience clauses protect individuals from having to act against their own moral values and beliefs. Societies that deny the moral right to freedom of conscience, and thereby expect policy implementers such as individual pharmacists to lead morally-divided lives, are presented with a moral problem. Moral courage as defined by Miller has five major components: Presence and recognition of a moral situation; moral choice; behaviour; individuality; and fear (Figure 2). Pharmacists operate within a highly-regulated environment and are bound by strict legal frameworks
FEATURE | Assisted dying & the pharmacist 16 VOL 23 ISS 1 JANUARY 2022
medicine. The additive e ect of concomitantly administered products containing fructose (or sorbitol) and dietary intake of fructose (or sorbitol) should be considered.

Pregnancy
Side e ects: pruritus, rash, urticaria, angioedema, anaphylactic reaction, oral mucosal blistering,
pain, diarrhoea,
vomiting, dyspnoea, exacerbation of asthma.TR 2006/1/1. TR Holder: Kwizda Pharma GmbH, E ngergasse 21, A-1160 Vienna, Austria. IRE BRO 2021 21
and lactation: Not recommended.
abdominal
nausea,
and codes of professional conduct. This, combined with pharmacy having a strong ethical grounding, creates the potential for moral distress to occur in the profession. Understood as a psychological and emotional response to the experience of moral wrongdoing, there is evidence to suggest that — if unaddressed — it contributes to staff demoralisation, desensitisation and burnout and, ultimately, to lower standards of patient safety and quality of care.
Professional work is unique in society, because it exists in areas where there are no clear-cut 'right' answers — professionals work in complex environments, dealing with complex problems. They must act and make decisions based on judgment, wisdom, morality, ethics, and intuition. Professional selfidentity is a hallmark of professional status and an important part of what motivates and supports practice. Gregory and Austin found that in times of crisis, when it matters most, pharmacists may not identify as pharmacists and they may have weak or incomplete professional self-identity formation. Weak or incomplete professional self-identity formation in the pharmacy profession may adversely affect patient care and pharmacy practice. Negative defensive pharmacy practices may include increased referral to other healthcare practitioners, overly-conservative safety-netting strategies, or avoidance of treatment of certain conditions or supplying certain services, especially for high-risk patients.
Society has a social interest in pharmacists acting as gatekeepers to prevent the illicit use of prescription medications and to prevent harm to patients. Society desires pharmacists to be conscientious, but denies them legal protection of their human right to freedom of conscience, ie, wants them to be conscientious but not to have a conscience. This contradiction results in vulnerabilities for the pharmacist, as most professionals are not required to abandon their morals in the workplace. Pharmacy is vulnerable to becoming a robotic profession if pharmacists are dehumanised when required
to leave their morality (‘Thou shalt not kill’) and/or religion at home, when they go to work. Some US trainee physicians experiencing moral distress have reported developing detached and dehumanising attitudes towards patients as a coping mechanism, which may contribute to a loss of empathy.
Professional pharmacy organisations in many jurisdictions educate, support, and unify members. They also help influence and monitor pharmacy-related legislation, promote research in the field and standardisation, and strive to improve patient care. In the US, state pharmacy associations fight against legislation that can negatively affect pharmacies and proactively look for ways to expand pharmacy practice. It has been suggested that revising the regulation model governing pharmacy could offer pharmacists the flexibility needed to address the individuality of scenarios encountered in practice without fear of legislative implications. US trainee physicians have described how patient autonomy and a fear of lawsuits have contributed to moral distress.
3. SOCIETAL VULNERABILITY
International conventions attach equal importance to freedom of thought, of conscience and of religion. These freedoms are inextricably bound-up with freedom of expression, assembly and association, since without the latter, the former cannot be properly exercised. These freedoms are not always recognised under national constitutions and even if these rights are guaranteed under the constitution, it may be that the courts do not enforce them, or that groups in society actively impede their exercise or even persecute those who seek to have their right to exercise freedom of conscience recognised. When excluded in legislation from protection of their moral and human right to freedom of conscience, pharmacists are vulnerable. In a resolution adopted in 2014, the European Parliament has emphasised that “the right to freedom of thought, conscience and religion is a fundamental human right”.
Upholding the moral right to freedom of conscience is an essential principle of liberal democratic life that is reaffirmed in the courts to the greatest extent possible. However, such accommodation needs to be balanced against the rights of others and the duties of the state. It is reasonable to expect freedom of conscience/ conscientious objection claims to be facilitated by the pharmacy profession and society, given that pharmacist professionals are held to higher professional and private moral standards than most other professions. Indeed, conduct in a pharmacist’s private life can be cause for professional censure. “Make sure that your conduct at all times, both inside and outside your work environment, ensures public trust and confidence in the pharmacy profession.”
Opinions, preferences, etc, should not determine moral standards. Societal moral standards originate law. When people (ie, public opinion) abandon absolute moral standards, for example ‘Thou shalt not kill’, previously unacceptable behaviour (ie, intentional killing) is seen as acceptable. This results in the creation of new ‘rights’ that are turned into laws that potentially infringe on the conscience of those who adhere to the absolute moral standard. For a pharmacist to hold a sincere moral conviction is to say, ‘here I stand, and I can do no other’. Those who can best claim to be conscientious, Prof Kimberley Brownlee has argued, are those who are willing to be seen and who do not evade the risks of non-participation. From a societal public health point of view, accommodating freedom of conscience/conscientious objections offers a way to keep policy implementers such as pharmacists in their roles, while maintaining their moral integrity.
In a situation where euthanasia and assisted suicide (in its current form) is introduced in Ireland, pharmacists in all working environments (pharmaceutical industry, community pharmacy, hospital pharmacy, palliative care pharmacy, long-term care, care of people with disabilities, including intellectual disabilities, general practice pharmacists, HPRA, HIQA,
FEATURE | Assisted dying & the pharmacist 18 VOL 23 ISS 1 JANUARY 2022
Figure 2: Moral Courage (Miller)
pubic health, etc,) will be part of the lethal medication use process. In particular, Irish hospital pharmacists will be interested to note that the Second Annual Report on Medical Assistance in Dying in Canada 2020 details Canadian pharmacist reports that provide information about the type of pharmacy where euthanasia and assisted suicide medications were dispensed. (Both practitioners and pharmacists in Canada are required to report on euthanasia and assisted suicide: The practitioner when there is a written request received for euthanasia and/ or assisted suicide, and the pharmacist when euthanasia and assisted suicide medications drugs are dispensed.) Each jurisdiction in Canada has different guidelines regarding the dispensing of these types of medications. In Newfoundland and Labrador, Prince Edward Island, New Brunswick, Manitoba and Saskatchewan, euthanasia and assisted suicide medications are dispensed exclusively by hospital pharmacies. In Québec, the majority of drugs (98.9 per cent) are dispensed by hospital pharmacies, with the remainder (1.1 per cent) being dispensed by pharmacies located within long-term care residences.
The European Statements of Hospital Pharmacy reflect the importance of the hospital pharmacist as a key stakeholder within the hospital teams for providing optimal and safe patient care. The overarching goal of the hospital pharmacy service is to optimise patient outcomes through working collaboratively within multidisciplinary teams in order to achieve the responsible use of medicines across all settings. Pharmacists will be morally challenged and professionally vulnerable if they do not consider (for any number of reasons) the provision of lethal medications to be safe and optimal patient care.
4. LETHAL MEDICATION VULNERABILITIES
Many healthcare professionals (including pharmacists), politicians and members of the
general public may be under the impression that euthanasia and assisted suicide would be achieved by the administration of ‘a pill'. Promoters of assisted suicide and/or euthanasia often suggest that the use of medications during the assisted dying process results in a painfree, dignified death for all. This is not the case. Modern medicine cannot guarantee a painfree death (natural or intentional) for all.
The difficulties and complications of medication administration during the assisted suicide/euthanasia lethal medication process are rarely mentioned by those promoting the introduction of assisted suicide and/or euthanasia to intentionally end a human life. Patients and professionals are vulnerable in the process. It is important that the ‘correct’ medications are chosen. However, there are no drugs or devices that have been approved by the US FDA for assisted suicide or euthanasia. Failure to use the ‘correct’ medications in the assisted suicide and/or euthanasia process may lead to traumatic situations, such as an extended time to death or awakening of the patient, causing distress for the patient and the attending family and healthcare providers. One-third of cases identified in a study in 2013 involved the use of non-recommended drugs, mainly opioids or benzodiazepines. The authors suggested that: (a) Some physicians may overestimate the actual lethal effect of these drugs in persons at the very end of life; and (b) some physicians use drugs that are not associated with the recommended euthanasia procedure but with ‘palliative sedation’ as a strategy to reduce cognitive dissonance, that is, the mental discomfort experienced by a person with conflicting attitudes, as some clinicians find ‘palliative sedation’ emotionally less burdensome to perform than euthanasia.
There is currently an international scarcity of lethal medications suitable for oral administration during an assisted suicide. Self-administration of lethal medication for assisted suicide in Canada is permitted in
all jurisdictions except for Québec. The Second Annual Report on Medical Assistance in Dying in Canada 2020 indicated that there were fewer than seven reported cases of selfadministered lethal medications (assisted suicide) across Canada. All other intentional deaths were therefore euthanasia, where the doctor or the nurse are the final actor who administers the lethal medication. A combined total of 7,595 assisted suicide and euthanasia deaths were recorded in Canada in 2020. The total number of assisted suicide and euthanasia deaths reported in Canada since the enactment of legislation is 21,589. Annual growth in assisted suicide and euthanasia cases has been steadily increasing each year in Canada and in particular, rising 34.2 per cent (2020 over 2019), from 26.4 per cent (2019 over 2018).
Oral assisted suicide was used only seven times in Canada during 2020. In a number of jurisdictions, the usual drugs used during assisted suicide are 90-100 x 100mg secobarbital tabs, dissolved in liquid and swallowed quickly with an antiemetic pre-med, usually given to prevent vomiting. There is no international consensus on either the medications or the protocols for oral administration, nor a comprehensive understanding of the potential side-effects and complications associated with different regimens. The quality of evidence for ‘optimal’ assisted suicide medications is low. It is recognised that any suggested recommendations can only be informed by the global but generally anecdotal experience.
On occasions, when oral barbiturate drugs are available internationally and administered during assisted suicide, there are reports of patients being unable to self administer a complex cocktail of lethal medicines. Family members have reported having to scrape powder from 100 plus capsules with toothpicks to produce bitter powder to be mixed with sugar syrup. Medication for nausea and vomiting must be consumed before and during the process. In some countries, lethal cocktails have been ‘ex-
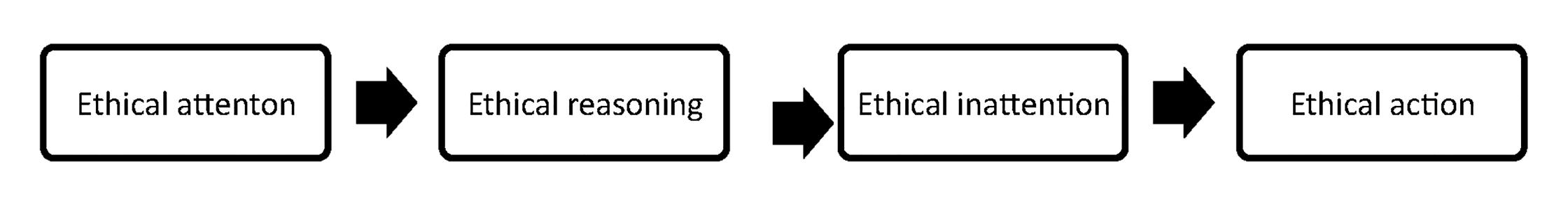
Assisted dying & the pharmacist | FEATURE 19 VOL 23 ISS 1 JANUARY 2022
Figure 3: Stages of Ethical Decision Making (Cooper, Bissell, Wingfield)
perimented’ with, due to difficulties sourcing licensed medicines for the purpose of assisted suicide and/or euthanasia, (Box 3).
A number of medicines used in assisted suicide and/or euthanasia were previously used in executions. (Pentobarbital became unavailable after drug-makers blocked its use in American death penalty executions.) Use of medicines during executions has been described as ‘inhumane’, with reports of people feeling ‘burning’ sensations throughout their bodies prior to death. A scoping review of the 163 studies that included technical summaries, institutional policies, practice surveys, practice guidelines and clinical studies that describe assisted suicide and/or euthanasia provision in adults, identified complications that may cause the patient, family and provider distress. These included prolonged duration of the dying process, difficulty in obtaining intravenous access, and difficulty in swallowing oral agents. In the Oregon Death with Dignity programme (1998-2016), there are reports of one patient having required up to 104 hours to die, at least six patients awoke after ingesting drugs, and for many patients, it is “unknown” whether complications occurred.
DISCUSSION
No medical association oversees assisted suicide or euthanasia, and no government committee helps fund research. In American states where the practice is legal, state governments provide guidance about which patients qualify, but say nothing about which drugs to prescribe. Research on this issue takes place on the margins of traditional science. It’s a part of medicine and pharmacy that is ‘practiced in the shadows’. Secrecy laws protect the identity of participating physicians, pharmacists, and drug suppliers, resulting in reduced transparency. Pharmacists providing medication for euthanasia/assisted suicide have been described as undertaking the role of an “accomplice”.
Pharmacists and the pharmacy profession must not be passive in the upcoming national debate concerning the introduction of euthanasia and assisted suicide to Ireland. Euthanasia and assisted suicide are a matter of life and death for vulnerable people and people at a vulnerable time in their lives. ‘Ethical passivity’ is a term used to describe pharmacists who were ethically
Docs In Northwest Tweak Aid-In-Dying Drugs To Prevent Prolonged Deaths. Aleccia J (2017). “The first Seconal alternative turned out to be too harsh, burning patients’ mouths and throats, causing some to scream in pain. The second drug mix, used 67 times, has led to deaths that stretched out hours in some patients — and up to 31 hours in one case…
"Patients and families are told to expect
sleep within 10 minutes and death within four hours. When it takes far longer, family members get worried, even distressed… The doctors say this can be addressed with larger doses of the three drugs they have been using — diazepam, often used to treat anxiety; digoxin, used to treat heart issues; and morphine, a narcotic pain reliever — plus another heart medication, propranolol, in a four-drug cocktail aimed at quickly inducing death… "
inattentive, who display limited forms of ethical reasoning, prioritise legalistic selfinterest, and are unable to act. Such ‘ethical passivity’ is argued to be inimical to healthcare practice and may be detrimental both to the welfare of vulnerable patients, and for pharmacy. Pharmacists have indicated that they were unable at times to do what they believed they should do and that when this happened, they simply did nothing or allowed supervening acts or the intervention of others to resolve an ethical situation. (Figure 2).
Moral distress can be distinguished from the concept of ethical dilemmas. Ethical dilemmas concern two or more courses of action which are in conflict (and will potentially have both positive and negative consequences) but where each action can be defended as viable and appropriate. In contrast, with moral distress, one action only is preferred by the pharmacist, but that pharmacist feels blocked from enacting that action by factors outside of the self.
There will be no going back for pharmacy or Irish society if assisted suicide and euthanasia are introduced to Ireland. The life or death of the individual will be at stake. The moral position and the freedom of conscience of the vulnerable pharmacist will be engaged. Vulnerable patients, people at a vulnerable time in their lives and vulnerable professionals will be at risk. Pharmacists without legal protection of their moral and human right to true freedom of conscience, and limited awareness among other professionals and the public of their complex role, will be one of the most vulnerable. Moral dilemmas will be ex-
perienced by pharmacists when their moral and/or professional autonomy is challenged by the behaviour/demands of patients and other health professionals. Vulnerable pharmacists will have to choose a morally justifiable action (‘Thou shalt not kill’), among several suboptimal action/inaction options.
Vulnerability is a shared condition in healthcare and this has an impact on the way of understanding vulnerability in regard to the relationships among patients, healthcare professionals, and institutions. Vulnerability can lead us pharmacists to better understand our shared condition and the effect this condition has on relationships and moral actions/ inactions. Reflecting on the vulnerability of the individual pharmacist and the pharmacy profession demands a response, generating responsibility and various wide-ranging implied obligations. These obligations are especially important from the perspective of pharmacists, healthcare professionals, institutions and the state. Vulnerability positions us in relation to each other as human beings and also suggests a relationship of responsibility between the state and the individual pharmacist. The success of a democracy depends on everyone contributing to its goals of prosperity, security, and non-tyranny.
It is crucial that pharmacists, student pharmacists, and pharmacy technicians get involved in advocacy efforts at all levels of government so as to positively affect the future of this vulnerable profession and to raise awareness of the complexities in the role of the pharmacist. ●
References on request
FEATURE | Assisted dying & the pharmacist 20 VOL 23 ISS 1 JANUARY 2022
Box 3: Tweaking Aid-In-Dying Drugs To Prevent Prolonged Deaths



Ireland’s No. 1 Provider of Ostomy Products & Services Contact our Ostomy Care team for further information A McKesson Company Telephone: 01 463 2300 Customer Care Over 20,000 calls handled annually from our customer care team. Complimentary Cutting Service Cutting up to 10,000 bags per week. Training Partnership Ostomy Excellence – we provide a complimentary education programme. Complimentary wipes and bags For Pharmacists to offer their patients. Expanding Range We continue to expand our range as Ireland’s leading stockist for Ostomy & Urology products.
HPRA PUBLISHES 2020 ANNUAL REPORT
THE STATISTICS SHOW THE DETENTION OF AN EXTRA 519,000 UNITS OF ILLEGAL OR COUNTERFEIT MEDICINES COMPARED TO 2019
The Health Products Regulatory Authority (HPRA) recently published its 2020 annual report, detailing its activities to regulate medicines, devices and other health products for the benefit of people and animals. The report outlines the HPRA’s programme of work in each of the health product areas it regulates, as well as highlights how the national regulator responded effectively and rapidly to significant developments, including Covid-19, said the Authority.
During 2020, the HPRA’s key activities included:
The authorisation of 251 (2019: 300) new human medicines for the Irish market through the national, mutual recognition and decentralised procedures. An additional 155 medicines were authorised through the centralised route — co-ordinated by the European Medicines Agency (EMA) — with the HPRA serving as rapporteur and co-rapporteur for 12 and six applications, respectively.
The authorisation of 57 new veterinary medicines for the Irish market through the national, mutual recognition and decentralised procedures contributing to the record figure of approximately 1,880 veterinary medicines now authorised for the Irish market. The HPRA also served as rapporteur or co-rapporteur under the centralised route for three veterinary medicines, while also acting as reference (lead) Member State for the assessment of three mutual recognition, seven decentralised and 14 repeat use procedures.
Approval for 73 clinical trials of human medicines, including seven Covid19-related trials.
The receipt of 7,752 adverse reaction reports in relation to human medicines, as well as 391 suspected adverse reactions and events reported in relation to veterinary medicines.
Lead member state for monitoring the safety of 70 nationally-authorised active substances, in addition to 56 centrally-authorised human medicines as part of its contribution to the work of the Pharmacovigilance Risk Assessment Committee at the EMA.
Undertaking 79 good manufacturing practice (GMP) inspections at manufacturing sites producing human and veterinary medicines and active substances across Ireland (2019: 110). The 2020 compliance programme also included inspections of five tissue establishments, four blood establishments and two organ procurement organisations/transplant centres.
The review of nine new applications to conduct clinical investigations of medical devices in Ireland, with six amendments to ongoing investigations.
The receipt and assessment of 1,668 medical device vigilance reports. There were 1,120 market surveillance cases undertaken during the year.
Evaluation of 300 surveillance cases as part of the work programme to monitor the safety and quality of cosmetic products.
GLOBAL IMPACT
Dr Lorraine Nolan, Chief Executive of the HPRA, emphasised the enormous global impact of Covid-19, which necessitated an almost immediate response from scientists and regulators alike to protect and benefit public health.
The detention of 1,610,295 dosage units of fake and other illegal medicines (2019: 1,018,678). Products detained included sedatives, erectile dysfunction medicines, analgesics and anabolic steroids.
The processing of 8,043 individual enforcement cases (2019: 6,167). Additionally, three prosecutions were initiated relating to the unauthorised supply of anabolic steroids.
“As the scale of the impact of the pandemic became clear, we also witnessed a direct and almost instant response from the global scientific community. This culminated in the authorisation of the first Covid-19 vaccines only one year after the identification a completely new virus. This was a remarkable achievement by all involved, facilitated by the speed and agility of the global medicines regulatory system.
FEATURE | Regulation 22 VOL 23 ISS 1 JANUARY 2022
As the scale of the impact of the pandemic became clear, we also witnessed a direct and almost instant response from the global scientific community
"The HPRA was an active participant in that response, both through our work at the EMA, and via our contribution to international efforts to co-ordinate and streamline regulatory processes and decision-making,” she said.

“At a national level throughout 2020, we provided expert regulatory support and input in respect of Covid-19 to colleagues across the broader health system, in addition to delivering engagement and communication with healthcare professionals and members of the public. Arising from this collaborative approach, and an increased focus on the key contribution of regulators, I believe there is now a greater understanding and recognition of the HPRA’s role as it relates to medicines, medical devices and other health products in protecting and enhancing public health. It is also worth noting that a similarly integrated partnership response significantly aided us in mitigating against
potential disruption to the supply of medicines resulting from Brexit.”
Dr Nolan continued: “I am very proud of what the HPRA has achieved in 2020, with colleagues demonstrating extraordinary commitment during this unprecedented period. While continuing to deliver on our core day-to-day activities, we quickly focused on ensuring an appropriate regulatory response to advance and progress the availability of medicines, devices and other health products. This response, adaptive and agile where necessary, has continued during 2021 and has seen the HPRA contribute significantly to the national public health effort during these most challenging of times,” Dr Nolan concluded.
In response to the Covid-19 pandemic, the HPRA states its key contributions included:
Active engagement in the assessment of Covid-19 vaccines and therapeutics at the EMA’s committee for human medicines (CHMP) and its Covid-19 EMA pan-
demic task force (COVID-EFT).
Participated in Covid-19 planning with the Department of Health, the HSE and the National Immunisation Office (NIO) through participation in NPHET.
Worked in partnership with the Department of Health and the HSE to minimise and address any risks posed to the ongoing supply of medicines to Irish patients.
Collaborated with the Department of Health and the Office of National Research Ethics Committee to support expedited regulatory review for Covid-19-related health research.
Actively contributing to and developing newly-established Covid-19 safety monitoring activities, at both national and European level.
Participating in the development of a procedure for remote assessment/distance inspection of manufacturers and distributors, in order to permit continued oversight during the Covid-19 pandemic. ●
Regulation | FEATURE 23 VOL 23 ISS 1 JANUARY 2022
MAKING SENSE OF OMICRON
As I write this, we are on the verge of entering potentially more lockdowns due to the spread of the Omicron variant. As the cute phrase goes, ‘it’s like deja vu all over again’ and I’m sure you have seen in the pharmacy and elsewhere the effects of ‘Covid fatigue’ among people around you. This will, of course, hamper compliance with any fresh restrictions imposed.
Naturally, it’s important to stay vigilant, especially for healthcare providers. But there are certain facts emerging regarding Omicron that are not as widely reported as they should be.

Just to be clear: I am not a ‘Covid denier’. Covid is real and for the first month or two after March 2019, we simply didn’t know what we were dealing with and how lethal it might be. Nor am I an ‘anti-vaxxer’. Vaccinations have been a revolution in public health. Nor do I believe Bill Gates is inserting nanobots into vaccines in order to control our spending habits/monitor our movements/etc.
However, we should also be wary when the term ‘anti-vaxxer’ is thrown around lightly — it is an ‘abracadabra’ term, often used to instantly discredit someone who may or may not have a valid evidence-based question or point to make. Terry Maguire put it better than I could in a recent column, where he bemoaned the lack of cool-headed scientific debate around the efficacy of the use of masks during Covid-19.

So now we have the Omicron variant. The ‘panic button’ has been pressed, despite many indications so far that it is much milder and less danger-
ous than the Delta variant. Still, as I write, the UK is considering another lockdown and Irish health authorities and politicians have decreed that pubs and restaurants must close at 8pm, which presumably is the time of the evening when Omicron comes out to play. The public must be sick of such seemingly nonsensical contradictions in logic — remember way back, when we were being told face masks were of little use? Some people still remember that.
Then there’s Dr Angelique Coetzee, a private practitioner and chair of South African Medical Association and one of the first to discover Omicron. Dr Coetzee is hoarse telling anybody who will listen that patients with Omicron have “very, very mild symptoms and none of them [doctors] so far have admitted patients to surgeries. We have been able to treat these patients conservatively at home”. This comes after a recent announcement that over 750,000 people in the UK have missed a cancer diagnosis since the start of the pandemic. The impact of missed diagnoses, delayed elective surgery and other procedures for our population here is yet to be determined, but in all likelihood the figures will be alarming.
Is it time to take a step back from the situation and consider the wider impact of more restrictions on our economy, mental health and wellbeing of our population, in the face of a far milder variant? As I write, only a handful of people worldwide have died from, or with, Omicron.
Am I missing something? Set me straight at the email address at the top of the page.
COMMENT | Editorial 24 VOL 23 ISS 1 JANUARY 2022 Send your comments to hello@irishpharmacist. ie or by post to Pat
,
Irish Pharmacist, GreenCross Publishing
Kelly
Editor,
Ltd, Top Floor, 111 Rathmines Road Lower, Dublin 6, D06K5F6.
Latest module
Asthma
This CPD module is focused on asthma in all its forms and the treatment opportunities. On completion of this module, it is expected that the reader will have an enhanced understanding of the causes, prevalence and distinctions between different severities of asthma, as well as evidence on the most effective treatment options.
Successful completion of this module will earn you 2 CPD credits Free CPD – now accessible on android, iPhone and tablet A B C Visit www.medilearning.ie/pharmacistcpd
Free independent CPD for Irish pharmacists by Irish pharmacists
FINTAN MOORE PUTS HIS REPORTER’S CAP ON AND EXAMINES WHAT ATTRIBUTES IT TAKES TO BECOME A SUCCESSFUL CAREER SHOPLIFTER
People often worry nowadays that with the increased use of mobile phones and other technology by children and teens, families will fail to pass on traditions from one generation to the next. Based on my experiences in work with one extended local family, I can confirm that some traditions remain alive and well, more’s the pity. Suffice it to say that the females of the clan that used to steal from me 15-to-20 years ago, before marrying-off and moving away, now have children of their own who were in to steal from me recently while back in the area for a funeral. The losses are an irritant, but the more galling thing is how nothing has evolved for these kids to have a better way to live. Let’s take a look now at the interview you will never hear.
In conversation with our reporter, two young shoplifters speak about the path they took to their chosen career. Amy Merrionvault-Delamere and Lucy Fotherington-Haughey.
Amy: “I was near the top of my year in Mount Amityville, and got 625 points in my Leaving Cert, but none of the options in the CAO really jumped out at me. Then I heard about shoplifting as a career, and it sounded really interesting.”
Reporter: “What in particular did you like about it?”
Amy: ‘It seemed like a great way to travel, and to experience different places, and shops of course. I’d done speech and drama, so I could act innocent and not attract suspicion. I’d captained the hock -
ey team, so I found the quick footwork useful too! (laughing)”
Lucy: “I went to Amexandrandia College, but I wasn’t as academic as Amy. I used to shoplift a little as a hobby, then somebody admired my work and I realised I had a gift for it.”
Reporter: “So that gave you the encouragement you needed?”
Lucy: “Ohmigod, yes — I just didn’t have the self-belief until then, but it’s amazing what the right motivation can do when you’re starting out.”
new Range Rovers that himself and mummy had ordered. Poor mummy started dropping me at the school gates instead of driving me into the car park. Daddy got his bonus a few months later when all the fuss was over, but the Range Rovers had registration plates for the second half of the year, so everyone knew they’d been delayed. Most girls were okay about it, but some were so mean.’
Reporter: “Thanks for sharing that, Amy — I can see how that must have hurt you.”
Lucy: “Ohmigod, that’s like, the year I was meant to go skiing with my family in Val D’Isere, and all the A-listers in my class were booked to go there at the same time. It was going to be so awesome, but there was no snow there that year and my brothers are such try-hard skiers, so we went to Austria or some other dump because the snow was better. I mean, I was wearing a €1,000 Helly Hansen ski-jacket that nobody could see because it was snowing! While everybody else was in Val D’Isere just partying because they couldn’t ski. It just wasn’t fair.”
Reporter: “And what do your families think of your choice of career?”

Despite the outward appearance of privilege, both of these young women have known setbacks in their lives when they felt stigmatised.
Amy: “There was one January when we were going back to school after Christmas hols, and daddy hadn’t got his annual bonus in December because his bank had laid off hundreds of people. So he didn’t have the money in his account to buy the
Amy: “They found it a little strange at the start — I’m the first in my family to branch out into shoplifting. But they can see that I’m happy and enjoying it, so they’re fine about it now.”
Lucy: “Well, my father is a personal injuries lawyer, so what I’m doing is a more honest way to make a living. He’s still coming to terms with that but we’re agreeing to disagree!”
COMMENT | Opinion 26 VOL 23 ISS 1 JANUARY 2022
The losses are an irritant, but the more galling thing is how nothing has evolved for these kids to have a better way to live
Reporter: “Thanks for being so open about your lives. I’m sure you’ll inspire others to follow in your footsteps. That’s strange… has either of you seen my wallet?”
STORM’S-A-COMING
It’s a perennial question, or maybe a hardy annual, but the problem of what to do when the weather turns bad has always been with us. As climate change looks set to bring more extremes of wind, rain and temperatures, it looks like there will be tough calls to make more often. The yellow, orange, red weather warnings that Met Éireann issues can be helpful, but it’s impossible for the forecast to cover all eventualities for all areas.

The recent Storm Barra had a patchwork quilt of colours across the country, but even within counties, people had different experiences on the ground.
As a general rule of thumb, it seems
reasonable for staff to travel to work if there’s a yellow warning, and unreasonable for an employer to expect them to travel through a red warning. Orange is the tricky one, because an orange at 8.30am when people are going to work could be a red a few hours later when they’re leaving. It’s also perfectly possible for an area with an orange warning to have localised episodes of ‘red’ weather. The prudent approach in an orange warning would seem to be that staff can travel if they feel safe, but if they don’t, they can take it as unpaid leave. This might not work everywhere, but seems fair to me.
The picture for employers is slightly simpler, or more difficult, depending on how you look at it! The buck stops with you, and if you don’t want to travel, nobody is pressuring you out the door. Personally, I’ll get to work come
hell or high water, and will work alone in the pharmacy if the staff can’t make it safely. The only time that came to pass was during ‘The Beast From The East’, when snows made roads nigh on impassable, although Brennan’s kept the bread arriving! My handiest way to work was by mountain-bike, but that’s a slightly niche approach. If I can’t afford to retire at a reasonable age and I’m still working when I’m 70, then I might need a plan B ●
Opinion | COMMENT 27 VOL 23 ISS 1 JANUARY 2022
INFORMATION
Fintan Moore graduated as a pharmacist in 1990 from TCD and currently runs a pharmacy in Clondalkin. His email address is: greenparkpharmacy@gmail.com.
CONTRIBUTOR
THE COST OF EVERYTHING; THE VALUE OF NOTHING
THERE IS A CLEAR VALUE FROM PHARMACIST INVOLVEMENT IN SUPPLYING EHC BUT COMMISSIONERS NEED TO START PAYING PROPERLY FOR THE SERVICE, WRITES TERRY MAGUIRE

Accusations of profiteering by Boots were in the newspapers in December.
That old chestnut, the retail price of Emergency Hormonal Contraception (EHC), is never far off the pages of the British liberal dailies. I was personally horrified that Boots would do such a thing; supply EHC so cheaply. I do cringe at the sales promotions of medicines generally, and Boots were really asking for trouble when they offered EHC as a Black Friday deal for £8.00. Bringing the price back to £15.99 after that American-themed retail-fest was going to hurt the bleeding hearts, especially when other caring UK pharmacies, such as the wonderfully-named Chemist4U, dish it out at £3.39.
I’m somewhat worried by all this, since I remain committed to the original retail price of £25, which justifiably contains a profit margin and a professional fee for my input. Of the 10 sales I do a month, it’s not my retirement fund, but it makes up for the poor margins on most of my frontof-shop. And I engage to earn my fee, but that engagement is now in question, which is interesting if not ironic.

Boots’s normal EHC price was attacked as a “sexist surcharge” by the British

28 COMMENT | Opinion VOL 23 ISS 1 JANUARY 2022
Pregnancy Advisory Service (BPAS), the UK’s largest abortion provider, and they told The Independent newspaper that emergency contraception should be free. I agree, and it is free within the NHS from GPs, A&E and sexual health clinics, but not commissioned in many pharmacies across the four UK nations. We had a successful pilot in my area some years ago and there are now plans to commission an EHC service as part of Pharmacy First across N Ireland.
Pharmacy First allows the public to visit the pharmacy first, and hopefully, when there, to get the advice, support and medicines they need on common and self-limiting conditions without having to go to the GP. This was one of those proposals that had been a good idea but never got commissioned, and then there was Covid.
When Covid-19 hit, GPs disappeared deep into their bunkers and their reception staff took up sniper positions should anyone have the temerity to approach the surgery door; the need for a Pharmacy First service has never been more acute or obvious.
Self-care has been a key UK government policy since the Blair-Brown years. Self-care is, as it states, care by patients themselves or their carers. Theory is that there will be huge efficiencies for the health service if patients take more care of themselves and the logic is that highly-accessible community pharmacy could take on a large chunk of this work, allowing GPs and A&Es to focus on more seriously ill patients. It hasn’t worked to date, for two reasons. The service has been poorly funded and badly designed, with its focus on supply of medicines as an outcome of the intervention and of course, there is the issue of trust.
For health commissioners, there perhaps remains concern that self-care services focused on community pharmacy might just see more OTC medicines ending up unused in home medicines cabinets while patients continue to visit the GP and A&E anyway. Ten years ago, our first Minor Ailments Scheme was abruptly modified due to abuse. Certain
multiple pharmacies, for example, imposed quotas on pharmacy managers to maximise profits from the scheme. This was irritating for most of us who kept to the rules and the spirit of the service. The Health Board should have directly challenged this excess. A strict letter to the responsible pharmacist would have provided cover against rapacious senior management, but the choice was to crash the scheme through lack of trust.
But now with the challenges of providing a health service in a post-Covid-19 world, Pharmacy First is back on the agenda and EHC will be one of the first commissioned services. It will be up to negotiators to determine the fees paid to contractors, but the scheme is likely to be for a defined age group, such as wom-
gle to get emergency contraception, that leads to unplanned pregnancy, and that leads to more women seeking an abortion, and that is not a good thing. No woman aspires to have an abortion. And of course there are wider issues, such as information and advice on safe sex and STDs, as well as watching out for sexual abuse.
So is the role of the pharmacist in the supply of EHC of any value? If so, what is this value and what can be justified as fair recompense? Seems the public are indifferent to our role as we retain our position in a twilight zone, being commercial retailers and professional practitioners.
Recently, a good friend was livid when his wife had her smile improved by an orthodontist for a price tag of £10k. My friend, no stranger to wheeling and dealing in the property business, met with the super-dentist to negotiate, and having offered a deal for cash, a promise to send more clients and a plea to the sanity of his invoice, the dentist calmly confirmed the £10k fee and expressed disappointment that his professionalism was being challenged. Okay, that’s perhaps a different ball-park, but the principle stands.

en between 16 years and 25 years. This would mean pharmacies supplying EHC; on a GP’s prescription, in the Pharmacy First Scheme, and OTC by counter sales.
The switch of EHC from POM to P was an unqualified success and the role of the pharmacist was central to ensuring the safe use of this medicine — so much so that, ironically, there are now calls for the product to become a General Sales List medicine, where it can be sold in any retail outlet. There will be dangers if the pharmacist is cut out of the supply of EHC and ironically, these are the very dangers highlighted by the bodies, like the consumer-vigilant BPSA, that initially opposed the OTC supply of EHC when MHRA first considered the switch because they deemed it too dangerous.
The safety profile of EHC and the public health gain from its use is down to its supply by pharmacists. If women strug-
Access to UK contraceptive services such as EHC was disrupted by the Covid-19 crisis and there have been funding issues affecting public health services generally that predate the pandemic. The pandemic shut or reduced clinics, while staff were transferred to work with Covid-19 patients or forced to self-isolate. There is a clear value from pharmacist involvement in the supply of EHC, so health commissioners need to start paying for an EHC service and the ongoing sniping by consumer groups about the costs of our products needs to stop. ●
CONTRIBUTOR INFORMATION

29 VOL 23 ISS 1 JANUARY 2022 Opinion | COMMENT
Terry Maguire owns two pharmacies in Belfast. He is an honorary senior lecturer at the School of Pharmacy, Queen’s University Belfast. His research interests include the contribution of community pharmacy to improving public health.
Ten years ago, our first Minor Ailments Scheme was abruptly modified due to abuse
DR DES CORRIGAN LOOKS AT SOME OF THE TAKE-HOME POINTS FROM FSAI ALERTS AND THE TRADITIONAL AND MODERN USE OF AYAHUASCA
he various alerts from the Food Safety Authority of Ireland (FSAI) make for interesting but disturbing reading, describing as they do, how some of our food is contaminated with salmonella, listeria, glass, plastic and metal particles. In the past year there have also been alerts calling for the removal from sale of supplements containing blueberries, ashwagandha (Withania), saw palmetto, green-lipped mussel and turmeric due to the presence of the unauthorised pesticide/fumigant ethylene oxide. Worryingly, the FSAI have had to issue seven separate alerts covering 40 different hemp or CBD products. Some were classified as 'unauthorised novel foods' that should not be on the EU market as their safety needs to be assessed, while many had levels of Delta-9-THC above those deemed safe by the European Food Safety Authority. The consumption of the latter products could cause problems for athletes, motorists and workers in companies that conduct routine or forcause drug testing because of the possibility of testing positive for cannabis.
The alert that intrigued me most was one relating to a series of products under the
brand name 'Herbs of the Gods'. This brought to mind the classical work by Schultes (who identified the 'Magic Mushrooms' and 'Morning Glories') and Hoffman (who discovered LSD), titled Plants of the Gods — Origins of Hallucinogenic Use. This book described a bewildering variety of hallucinogens, mainly of plant origin and largely from Central and
South America, including Mexico. When I read the list of plants in the FSAI alert, sure enough the first one mentioned was one that features prominently in a major hallucinogenic preparation from the Amazon called Ayahuasca. The plant in question is Banisteriopsis caapi. Since this botanical name is tortuous to write and pronounce, I will
just refer to it as 'caapi'. It is not the major component of the mixture of plants that go to make up Ayahuasca, that honour going to Psychotria viridis, source of the powerful hallucinogen Dimethyltryptamine (DMT). DMT is inactive when consumed orally, unless it is mixed with a monoamine oxidase inhibitor that blocks its metabolism and facilitates its transfer across the blood-brain barrier. This is where the caapi comes in, as it contains the β -carboline alkaloids harmaline and harmine, both powerful inhibitors of monoamine oxidase that increase serotonin levels by blocking deamination of the DMT. Remarkably, this sophisticated exploitation of drug metabolic processes was developed by what once might have been disparagingly referred to as 'primitive' Amazonian tribes as part of their religious practices. That ritual use of Ayahuasca has spread to the rest of the world, including Ireland, from the Amazon, through the proselytising activities of Brazilian groups such as the Church of Santo Daime. The hallucinations that kick-in after the vomiting characteristic of Ayahuasca (a purging process considered part of the ritual) are believed to facilitate communication between the Ayahuasca drinker and the supernatural world.
COMMENT | Opinion 30 VOL 23 ISS 1 JANUARY 2022
The alert that intrigued me most was one relating to a series of products under the brand name 'Herbs of the Gods'
It is no surprise that interest in the effects of DMT have spread from its original controlled ritual consumption to recreational use. Interestingly, a September 2021 article in the Irish Journal of Medical Science by Ivers et al lists DMT among the drugs reportedly used by Irish festival-goers both here and abroad. In this case, it is likely to have been DMT powder that they ingested by smoking, injecting or snorting, as all three routes will avoid metabolism, allowing the drug to cross the blood-brain barrier. DMT causes intense visual illusions, distortions of body image and speech disturbances. Euphoria or anxiety can occur, depending on the user's underlying mood ('set') or circumstances of use ('setting'). Psychotic-like symptoms such as thought disorders typical of schizophrenia generally disappear after a few days, but can persist in some users.
The second plant listed in the FSAI alert also contains the same monoamine oxidase inhibitory alkaloids as Caapi, ie, harmaline and harmine, but comes from the Middle East, hence its common name Syrian Rue or Peganum harmala. It has been used traditionally in the Middle East and China for thousands of years. Articles in the Journal of Ethnophar-
macology (2017) and the Archives of Pharmacological Research (2020) reviewed what is known about this plant. Traditionally used for cough, hypertension, diabetes and asthma, etc, there is evidence of effects in Alzheimer's, Parkinson's, cancer, diabetes and hypertension because of cholinesterase inhibition and the MAOI activity of the alkaloids. However, side-effects are listed as severe and include visual and auditory hallucinations, confusion, agitation and ataxia, along with bradycardia, nausea and vomiting. The plant is also believed to have abortifacient activity, so obviously it is contraindicated in pregnancy. This is hardly a plant that any regulator would wish to see on open sale as a food supplement.

Other plants in the FSAI alert are Blue Lotus Flower and its white counterpart. Also called Egyptian Water Lily, Blue Lotus contains, according to a 2017 paper in the Journal of Psychoactive Drugs, two alkaloids, apomorphine and nuciferine. Apomorphine is best known for its use in Parkinson’s disease because it improves motor function by stimulating dopamine receptors. Blue Lotus Flower has a traditional reputation as a sleep aid and anxiolytic, but it can cause euphoria and hallucinations at higher doses. A 2021 article in Military Medi-
cine describes five cases who presented to emergency departments with symptoms such as sedation and disturbances of perception, including one case of paranoia. White Lotus is botanically distinct from the blue variety. It is widely cultivated in Asia, Australia and North America for food use. Inedible parts of the seeds are used in TCM to enhance immunity and to protect the liver. It contains powerful antioxidants that might explain its effects on obesity and insulin resistance in obese mice. Like its blue counterpart, it too contains the alkaloid nuciferine that shares a receptor profile with the antipsychotic aripiprazole.
The final plant on the FSAI Alert is called Mexican Tarragon, which is not a 'Tarragon', but actually a species of marigold (Tagetes lucida). Traditionally used as an antidepressant and anxiolytic, animal studies have shown both positive and negative effects. Among the identified components are the analgesic β-Caryophyllene (also found in cloves) and according to one paper, methyl-chavicol or estragole, which presumably is responsible for the tarragon-like odour. Estragole is considered by the EMA as a genotoxic carcinogen, exposure to which should be as low as practically possible. Such exposure normally comes from dietary basil and tarragon or the herbal medicinal use of fennel and anise seeds, but use of Mexican tarragon could only add to that exposure and is therefore undesirable.
Overall, these plants are not suitable for use as food supplements and the FSAI has done us all a favour by having them removed from sale. ●
CONTRIBUTOR INFORMATION
Opinion | COMMENT 31 VOL 23 ISS 1 JANUARY 2022
Dr Des Corrigan, Best Contribution in Pharmacy Award (winner), GSK Medical Media Awards 2014, is a former Director of the School of Pharmacy at TCD and won the Lifetime Achievement Award at the 2009 Pharmacist Awards. He was chair of the Government’s National Advisory Committee on Drugs from 2000 to 2011. He currently chairs the Advisory Subcommittee on Herbal Medicines and is a member of the Advisory Committee on Human Medicines at the IMB. He is a National Expert on Committee 13B (Phytochemistry) at the European Pharmacopoeia in Strasbourg and he is an editorial board member of the Journal of Herbal Medicine and of FACT — Focus on Alternative and Complementary Therapy
WILL IT EVER END?
As I write this, we’re a couple of weeks out from Santa coming, and it’s a fairly hectic time of year. I wonder what shape we’ll be in come January, when this will be published?
We’re taking four days off, closed in both pharmacies. I think we all just need the break after two years of Covid-19, a busy November, and what’s shaping up to be a busier December, with work compacting into fewer days. We’ve the Covid-19 vaccination boosters on top of it now also, by choice, mind you. I’ve had it up to my back teeth with our restrictions, and am highly motivated to get as many as are willing to get vaccinated and boosted, just to help us move back toward the old normal. Vaccinating helps the finances somewhat too, of course, but comes at the cost of disruption, pharmacist and admin time, and resources.
“When is this going to finish?”; “I suppose we’ll be getting a vaccine every year for this now?”; and “Will the HSE send me on an up-to-date digital cert?” are among the many questions I don’t have answers for from many anxious customers and patients who are visiting us at present.
We don’t appear to have herd immunity developing. We have great vaccine uptake overall as a country, which is encouraging, but with:
A mutating virus;
Waning immunity post-infection and vaccination;
Increasing complacency in some cases post-vaccination, and in general with restriction fatigue;
Community transmission, potentially even from those who are vaccinated, with up to 60 per cent of people being asymptomatic, it’s hard
to see how we can drive Covid-19 out of our communities.
Management of it in public health through an annual vaccination, much like the flu vaccine, is a potential scenario I suspect could well play out.
What this means for us is, if we want to continue to offer vaccination as a community service through our pharmacies, we’ll need to resource it better and manage our inputs. Some local GPs have stopped offering the service, are referring people to us, and we’re a long way off having the whole community boosted, even in the recommended cohorts we’re presently prioritising.
tract due to an administrative change of beneficial ownership in the midst of things also. Sure, why not. Bring it on!
“You have them spoiled, you know?” said Sharon, who’s been with us as a locum, albeit now part of our family, since we opened in Ballindine over 14 years ago. I didn’t know, or consider it, to be honest. I do know, however, that familiarity can breed contempt both ways, and that minimising waiting times, driving efficiencies, having a friendly service, along with maintaining a high quality of care, can be a challenge. There’s a sweet spot there, isn’t there, between bending over backwards for customers, and treating them as an annoyance in one’s day. Managing their expectations is key.
Elf on the shelf is keeping a good eye on things on the home front. Our fiveyear-old is asking why her four-year-old sister is “saying lots of things that aren’t true” about the Elf coming alive at night, flying around and getting up to mischief, like putting lipstick on their dolls! Logic vs imagination and emotion at work. I’m starting to think it’s more nature than nurture when it comes to how and who we grow up into. They’re all great craic at the moment, mind you, and a fantastic antidote to stress on the work front.
We’re going through a PSI re-registration and application for a new GMS con-
That being said, many don’t have a ready reference point for an alternative pharmacy experience years on from initially joining us. We had one lady leave us last year, for example, and take herself and her husband’s scripts with her, as we were “not good enough”, having had three staff running around pulling items together for her prescription at one stage. The 10-minute wait was all too much for this retiree. Things to do, places to go and all that. I have given many a stick to beat us with though, driving efficiencies, that when they break down through a ‘bad day’, overload, training issues, a bottle-neck, or other, it can lead to customers getting very frustrated. I met this lady a few days later, let’s call her ‘L’, in the local supermarket and had forgotten about her telling me off for our poor service, etc, and said “Hi L, how are things... nice day out,” etc, and moved along. I remembered afterward mind you, but it felt good to normalise our ‘relationship’ somewhat with that little
COMMENT | Opinion 32 VOL 23 ISS 1 JANUARY 2022
LOCKDOWNS, SOCIAL RESTRICTIONS AND VACCINATIONS HAVE NOT SOLVED COVID AND CUSTOMERS ARE ASKING QUESTIONS THAT CAN’T BE ANSWERED, WRITES ULTAN MOLLOY
Some local GPs have stopped offering the service and are referring people to us
chat. She came back the following week with a bunch of flowers for me (the only one I’ve ever received from a woman, or a man, as it happens!) and asked could she come back to us with her prescriptions. Her new reference point was another local pharmacy, now closed, that dispensed several errors in her blister pack medicines, missed her prescription completely, and missed a page from her husband’s script from the blister pack. I was delighted to have her back, truth be told, but did give the condition that she would need to order ahead if there was anything urgent, and be patient at the counter when she came to us in future. It’s been all very easy with her since, thanks to her different reference point. Did you know that people in some countries in Asia, who may have to walk for a day to get to a doctor, are more satisfied with their health service than we are in Ireland? Reference point indeed. Unreasonable expectations are in the mix there somewhere also in many cases. I had another customer who left us after tripping on the mat at our front door.
We went to her at the time and asked was she okay, brought her to the doctor across the road, and subsequently dispensed some anti-inflammatories, checking in with her again at the time for her trouble. She took her script, and her family’s, elsewhere afterward, and I didn’t notice for some time. I rang her when I did to ask had something else happened, and she told me that the right thing for me to do would have been to ring her in the following days to see how she was. So there you go. I dropped the ball on that one. Hopefully she’s being fantastically cared for, with regular phone calls, in the pharmacy she’s moved to. Thirteen years of doing our best by her and her family, and off she goes. C’est la vie !
Anyway, so where was I. Reasonable expectations. One person’s ‘reasonable’ is often another person’s ‘unreasonable’ though, isn’t it. One has to benchmark, look in the mirror, consider our duty of care, and then back oneself and one’s team. I wasn’t great at the latter in the past, assuming that ‘the customer is always right’, and while I wouldn’t go as far
as Ryanair’s Michael O’Leary on The Late Late retorting, “the customer isn’t always right... they’re nearly always wrong”, they are certainly not always reasonable in their expectations of us community pharmacists. “It’s a job where you have to be able to plámás people” a colleague said recently, and she’s spot-on. You have to be able to flatter, to knock a bit of craic and cheer out of even the most miserable and demanding, and feed one’s soul on those customers who brighten our days just by coming through the door.

The ones who’ve complained about us to the PSI for having “medicines in the toilet” (untrue) after we let them use the staff facilities as they were under pressure, and another for cutting scored tablets to make the appropriate dose for their husband when his blood pressure tablets went short, are no doubt happily cared for elsewhere now. Many dead customers, I miss regularly when they come to mind. Some beautiful people. Many alive that have moved elsewhere, and I’m not sorry they’ve moved. I’ll leave it at that, lest I head down the Fr Ted Crilly Golden Cleric award route! It’s unfortunate that the empty cans can often take up a disproportionate amount of our good time and energy. I’m sure there are many I/we have dropped the ball on, and had taken for granted too.
Mea culpa
We’re lucky in community pharmacy though, in spite of our challenges, as I remind myself. We’ve been open through the pandemic. We’ve kept people in employment and cared for and supported our communities. Our team has pulled together and people have stepped up when they were needed. Here’s to more of that good stuff for all of us in the year ahead. ●

33 VOL 23 ISS 1 JANUARY 2022 Opinion | COMMENT Ultan Molloy is a business and professional performance coach, pharmacist, facilitator and development specialist. He works with other pharmacists, business owners and third parties to develop business strategies. Ultan can be contacted on 086 1693343. CONTRIBUTOR INFORMATION
DISTINGUISING AND TREATING ACNE

A CLINICAL OVERVIEW OF THE DIFFERENT SEVERITIES OF ACNE AND TREATMENT OPTIONS
Acne Vulgaris (referred to as 'acne' for short) is a disorder of skin glands that produce a natural oil (Sebum). The face, neck, shoulders, upper chest, and back are most frequently affected. It affects more than one-in-two young adults at some stage, usually beginning at puberty and clearing completely before the mid-twenties. In many adolescents, acne clears within two years. The reason acne is worse during puberty is due to an increase in sex hormones called androgens, especially testosterone (even women have small amounts of testosterone) and these excess hormones make oil glands overactive and enlarged, thus producing too much oil (sebum). Too much sebum causes the pores or hair follicles to become blocked with skin cells and a build-up of bacteria in these blockages. Testosterone levels increase during puberty, peaking in the late teens and then levelling-off, hence acne tends to reduce after puberty. Diet has little influence on acne. Despite what is commonly believed, sugary foods and fatty foods, such as chocolate and chips, have not been proven to contribute to acne.
SYMPTOMS
Definitions and terms used for symptoms of acne
Medically, clinicians use the term 'lesions' to describe the bumps on the
34 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Skin care
skin experienced during acne. Lesion is an overall term that can describe the different bumps caused by acne and include:
Papules: Raised bumps
Pustules: Raised bump often known as a pimple and filled with yellowish fluid known as pus; pus contains sebum (oil) that gets trapped in the pores mixed with dead skin cells and bacteria.
Comedones: raised bumps that can form blackheads and whiteheads.
Nodules: This is a bump under the skin which may not be visually obvious to someone else but can be painful.
Cysts: Look like a boil and can be filled with pus.
Comedonal acne is the name of acne which is characterised by inflamed skin blemishes, ie, papules which are like red bumps. These papules form due to hair follicles and pores becoming blocked with skin cells and oil, which leads to whiteheads and blackheads.
ROLE OF BACTERIA
Bacteria on the skin surface intensify the problem by producing chemicals that inflame and irritate the skin. The anaerobic bacterium Cutibacterium acnes (C.Acnes) plays an important role in maintenance of healthy skin, but acne creates an ideal environment for its excessive growth. An environment which includes inflammation, lesions with excess sebum and dead skin cells allows C.Acnes to multiply, thus exacerbating the problem and contributing to inflammation. Prior to 2016, Cutibacterium acnes was named Propionibacterium acnes (P.acnes) and was renamed C.Acnes to better reflect the biochemical and genomic make-up of this bacterium.
WHITEHEADS AND BLACKHEADS
Whiteheads are tiny little clogged pores just below the surface of the skin which are not inflamed. Blackheads are like whiteheads, except they are open to the air, which oxidises with the sebum and keratin in the skin and gives them a brown/black colour. Do not attempt to squeeze these, as this causes them to get irritated and inflamed
and cause a little red bump on the skin (a papule). Papules develop mainly on the Tzone (ie) forehead, nose, and chin. Healthy bacteria on the skin (ie, C.acnes) can form in these papules, causing them to fill with pus and develop a defined white or yellow head on the surface called a pustule.
MORE PRONOUNCED LUMPS
Cysts and nodules are large painful lumps underneath the skin which can take weeks to heal. The only difference between the two is that nodules are hard and don’t have an obvious white/yellow head, whereas cysts are fluid filled with a defined white or yellow head. Do not squeeze these, as it will cause scarring and possibly spread to surrounding pores.
isoniazid and ethionamide and the immunosuppressant ciclosporin, which is mainly used to prevent transplant rejection and inflammatory autoimmune conditions such as rheumatoid arthritis and psoriasis.
ACNE IS NOT LINKED TO ROSACEA
Rosacea is an inflammatory skin disease causing facial flushing, redness, papules, pustules and in some cases permanent dilation of small blood vessels (known as telangiectasia). In rosacea, the cheeks, chin, forehead, and nose are usually worse affected, with the rest of the face mainly unaffected. Rosacea can be temporary in some cases but is often recurrent or persistent. There are four subtypes of rosacea, which are: Erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea.
MEDICATION-INDUCED ACNE
Some medication can cause acne, so doctors and pharmacists must take this into account if patients prescribed these medications present with acne. They should only be reviewed and changed if acne is severe and only if the benefits of changing them outweigh the risks (ie, acne is causing severe psychological issues for the patient) as many of the medications implicated with acne are for serious long-term health conditions, so should not be changed without a suitable alternative, which may not always be available. Examples of medication that can cause acne-type symptoms include antiepileptics such as phenytoin, phenobarbital and carbamazepine, mood stabilisers such as lithium, anti-tuberculous medication such as rifampicin,
Rosacea is sometimes referred to as acne rosacea; however, this can cause confusion, as rosacea is unrelated to acne vulgaris . Rosacea is mostly diagnosed in people aged 40-to-59 years and is rare in under people under 30. Some of the treatment options like azelaic acid (Skinoren) and oral antibiotics (ie, minocycline, doxycycline) are similar for acne and rosacea. I discussed rosacea previously in Irish Pharmacist .
SELF-HELP
Most cases of mild acne will be helped by washing affected areas no more than twice daily; washing more often can irritate acne more.
Use a mild soap or cleanser and use luke-warm water, as very hot or cold water can worsen acne.
Avoid vigorous scrubbing while washing acne-affected skin. Avoid the use of abrasive soaps, cleansing granules, astringents, or exfoliating washes. A soft washcloth and fingers should be used instead.
Do not try to 'scrub off' blackheads. Scrubbing or picking acne will exacerbate acne.
Women should avoid overuse of
35 VOL 23 ISS 1 JANUARY 2022 Skin care | IN FOCUS
Bacteria on the skin surface intensify the problem by producing chemicals that inflame and irritate the skin
make-up and cosmetics and avoid greasy cosmetic products. Remove make-up completely at night.
If dry skin is a problem due to the medication used for acne, aim to use a fragrance-free, water-based emollient. Avoid ointments or oil-rich creams, as they clog pores.
Cream preparations tend to be less irritating to acne than gels.
Avoid food stuffs that patients feel worsen the problem, though there are no specific food groups that are thought to exacerbate acne. A healthy diet is the best recommendation.
While sunlight helps our skin produce vitamin D, it is a myth that sunlight improves acne.
TREATMENT OVER-THE-COUNTER PRODUCTS
Benzoyl peroxide
Benzoyl peroxide works by:
Preventing dead skin plugging hair follicles.
Unblocking skin pores and speedingup the production of new skin cells.
Killing bacteria on the skin that cause plugged follicles to become infected. This reduces antibiotic resistance when benzoyl peroxide is used with antibiotics.
Studies show that benzoyl peroxide may improve acne more rapidly than topical retinoids.
Availability of benzoyl peroxide
From 2012, pharmacy staff and consumers noticed many benzoyl peroxide products were discontinued by manufacturers, so brands like Panoxyl, Brevoxyl and Quinoderm, which contained benzoyl peroxide, disappeared off the shelves; though Quinoderm still offer a facewash only that does not contain benzoyl peroxide. There was little or no communication from manufacturers as to why they went of the market in Ireland and other countries. For example, Panoxyl, which was made by Stiefel (a subsidiary of GlaxoSmithKline), stopped production of Panoxyl due to
what it called a “global manufacturing supply issue” and was taken off the market along with other Stiefel acne products. Currently in Ireland, the only Panoxyl product available to order from wholesalers by pharmacies is Panoxyl 10% acne wash, which is unlicensed in Ireland (it’s an American product) so is only available to patients via a prescription here.
Acnecide gel contains 5% benzoyl peroxide and is the only licenced benzoyl peroxide product available over the counter in Ireland. It is advised to use Acnecide after washing with a mild cleanser and water, applying once or twice daily.
PREVENTING SIDE-EFFECTS OF BENZOYL PEROXIDE
Benzoyl peroxide should be used in moderation and not long-term. A sixweek course of treatment with benzoyl peroxide is required to clear acne. Drying and peeling of the skin can occur; drying and peeling can be prevented by reducing the frequency, ie, every second day. Those with sensitive skin can reduce application from twice daily to once daily before going to bed.
Benzoyl peroxide increases sensitivity to sunlight so when in strong sun, sunscreen is advised. Avoid contact with hair, clothes, towels, and bed linen, as benzoyl peroxide may bleach these. Wash hands after applying benzoyl peroxide products to prevent irritating the hands or inadvertently rubbing in the eyes. Consider referring to a GP if:
The acne is severe, angry-looking, inflamed, or widespread.
No improvement despite the correct use of benzoyl peroxide.
Any suspected case of medicationinduced acne.
Prescription medicines
In relation to the topical treatment options discussed below, a general consideration is that different formulations may be considered depending on skin type; for example, creams may be more beneficial for dry skin, and gels and topical solutions may be more beneficial for oily skin.
TREATMENT REGIMENS BY SEVERITY OF ACNE
1. MILD ACNE
Acne is classified as mild when it is limited to the face and lesions are not inflamed.
TREATMENT OPTIONS
Topical retinoids
Topical retinoids are the next option if there is no response from benzoyl peroxide. Topical retinoids work by reducing production of sebum while also preventing dead skin cells plugging hair follicles. Adapalene (Differin 0.1% Gel or Cream) is a topical retinoid available in Ireland. Retin A (Tretinoin) is now unlicensed in Ireland and isotretinoin (Isotrex) is not available in Ireland (only in the UK). Adapalene tends to be easier on the skin, so has fewer side-effects like redness and peeling than isotretinoin.
Topical retinoids can cause mild irritation and stinging of the skin. Apply topical retinoids sparingly and avoid excessive exposure to sunlight and UV. A pea-sized amount should be enough to cover the whole face. Starting topical retinoids too quickly/regularly increases risk of skin redness, soreness, and peeling. Some dermatologists’ advice is starting topical retinoids once weekly and increasing slowly up to a dose of once daily, thus allowing the skin to build up tolerance to the retinoid. It is best to apply topical retinoids at night, as sun causes degradation of retinoids, reducing the effect. Another reason acne treatments (not just retinoids) are recommended to apply at night is to prevent new spots developing overnight. Topical retinoids should be avoided during pregnancy, as they carry a risk of causing birth defects. A six-week course is usually required, but patients may need to continue it on an on-off basis.
2. MILD-TO-MODERATE ACNE
Mild-to-moderate acne primarily affects the face but may affect the neck, shoulders, upper chest and back. Topical therapy is first-line recommended and only consider oral medication if no improvement.
36 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Skin care
Clinically proven to effectively treat acne lesions with high tolerability. Take care of your skin with PapiXTM


Take care of your skin with PapiXTM. A multi action defence against acne breakouts, oily, blemished, or acneic skin. With nicotinamide, salicylic acid and urea peroxide. An easy to use regime for acne prone skin.
RELIFETM. MY SKIN SAYS HOW I FEEL.
ACTIVE DEFENCE AGAINST ACNE


www.relife.ie
IE21038 IR-REL-141-2021
Distributed by: A.Menarini Pharmaceuticals Ireland Ltd, Castlecourt,Monkstown Farm, Monkstown, Glenageary, Co. Dublin.
TREATMENT OPTIONS (ALL TOPICAL)
A. Benzoyl peroxide and topical retinoid combinations
Combination topical therapies should be considered if one product on its own does not work. Combined products are rarely needed for mild acne. Topical retinoids become less stable, hence less effective, when used in combination with benzoyl peroxide, so if using both they should be used at different times. However, the combination of topical benzoyl peroxide and Adapalene are stable when used together, so Epiduo gel is an all-in-one option containing benzoyl peroxide 2.5% and adapalene 0.1%.
B. Topical antibiotic options
Topical antibiotics reduce bacteria that infect plugged hair follicles. Topical antibiotics come as lotions or gels and are applied once or twice a day. A six-to-eightweek course is recommended. After this, treatment is usually stopped to reduce risk of antibiotic resistance. Side-effects such as minor irritation, redness, burning, and peeling are uncommon.
Topical antibiotic options for acne include clindamycin (Dalacin T), Zindaclin) and erythromycin (Zineryt). Topical erythromycin (Zineryt) may be reserved for patients with more sensitive skin who may experience more side-effects from clindamycin, benzoyl peroxide and/or topical retinoids. Monotherapy with topical antibiotics is generally not recommended because of risk of antibiotic resistance. Topical erythromycin is associated with more resistance than other antibiotics.
A topical benzoyl peroxide combined with a topical antibiotic is a popular option with clinicians in Ireland. An all-in-one combination option is Duac, which contains benzoyl peroxide 5% and clindamycin 1%. A topical retinoid combined with a topical antibiotic is another option and an example available is Treclin, which contains clindamycin 1% and tretinoin 0.025%. Topical retinoids are thought to improve antibiotic efficacy by improving penetration
and giving synergistic comedolytic and anti-inflammatory effects. All the combination topical treatments mentioned in this paragraph are applied once daily in the evening.
C. Azelaic acid
Azelaic acid (Skinoren) is a topical anti-inflammatory licensed for mild-tomoderate papular-pustular acne of the facial area. It works by dissolving dead skin and killing bacteria. It is applied twice daily or once daily for sensitive skin. It is considered less effective than benzoyl peroxide and topical retinoids. While azelaic acid can cause sideeffects like burning, itchiness, stinging, redness and dryness of the skin, these side-effects tend to be milder than benzoyl peroxide and topical retinoids. Tolerance to side-effects usually develops with ongoing use.
face and parts of the upper body) or acne that fails to respond to topical antibiotics.
TREATMENT OPTIONS
A. Oral antibiotics
Systemic treatments are the next option for moderate acne and are especially useful for harder-to-reach areas, such as acne on the back. Apart from the antimicrobial effect, antibiotics are thought to exert an anti-inflammatory effect on acne, thus contributing to their effectiveness.
Oral antibiotic options for acne include:
Doxycycline 100mg daily.
Lymecycline 408mg daily.
Minocycline 100mg (slow release) daily.
Erythromycin 500mg twice daily. It takes six weeks to notice significant improvement from oral antibiotics. More recent guidelines recommend treatment with oral antibiotics is discontinued after three months to reduce risk of antibiotic resistance (ie, to C.acnes), but can be repeated in future if recurrence occurs. Minocycline is less often recommended than other tetracyclines for acne due to increased risk of a lupus erythematous-like syndrome and autoimmune hepatitis, as well as a small risk of irreversible pigmentation.
Wash hands after each application to avoid irritating hands or inadvertently rubbing in the eyes. Avoid alcoholic cleansers, tinctures and astringents, abrasives and peeling agents when using azelaic acid to minimise irritation; this advice is also true for benzoyl peroxide and topical retinoids.
Azelaic acid can be considered as an alternative to benzoyl peroxide and topical retinoids when side-effects like skin irritation are an issue. Another advantage of azelaic acid is that it does not cause as much photosensitivity as benzoyl peroxide and topical retinoids, so there is no need to avoid sunlight.
3. MODERATE ACNE
Moderate acne is characterised by lesions affecting a greater area (ie, whole
Side-effects of tetracyclines include nausea, vomiting, and diarrhoea. Tetracyclines can make skin sensitive to sunlight. Avoid milk/indigestion remedies or iron/zinc supplements two hours before or after taking a tetracycline, as they reduce tetracycline absorption, and take on an empty stomach.
European acne guidance indicates increased microbial resistance to erythromycin, so it should only be used when tetracyclines are not tolerated or are contraindicated, ie, pregnancy, breast feeding. The most common side-effects of erythromycin are nausea and mild stomach pain.
To give improved patient outcomes and reduce resistance, adding an oral antibiotic to the topical treatment such as benzoyl peroxide or a topical retinoid is recommended. Oral antibiotics should not be used with topical antibi-
38 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Skin care
It takes six weeks to notice significant improvement from oral antibiotics
otics, as this increases risk of antibiotic resistance. None of the oral antibiotics are considered any more effective than the others and choice depends on the likes of possible resistance, side-effects and patient profile, ie, pregnant, intolerant to tetracyclines.
B. Combined oral contraceptives





Women with acne who require contraception or to control menstrual symptoms can take the oral contraceptive co-cyprindiol (Dianette), which contains a combination of the anti-androgen progesterone called cyproterone acetate 2mg (this is the acne-reducing component) and the oestrogen ethi-









nylestradiol 25mcg. Two-to-six months’ usage is needed before significant improvement in acne.











A combined oral contraceptive can be used in combination with topical treatments or oral antibiotics for female patients with more moderate-to-severe acne. If no contraception is needed, the doctor and patient will decide if hormonal treatment is a good option, which often comes down to the severity of the condition and success of previous treatments.
Progestogen-only contraceptives make acne worse, so should be avoided. From a contraceptive perspective, antibiotics that do not induce liver enzymes

such as doxycycline may be considered to use with oral contraceptives, as there is less risk of contraceptive failure.
SHOULD CO-CYPRINDIOL STILL BE THE FIRST-CHOICE ORAL CONTRACEPTIVE FOR ACNE?





































A Cochrane review from 2012 found that combined oral contraceptives are effective for inflammatory and non-inflammatory acne, but found little differences in the efficacy of the different types, including cyproterone acetate (Dianette). This was an interesting finding, considering cyproterone acetate has long been considered the oral contraceptive of choice for acne in females and is the only oral contraceptive licensed for acne.








Dianette is licensed for severe acne. However, it can increase risk of venous thromboembolism and a 2013 BMJ review questioned whether it should




39 VOL 23 ISS 1 JANUARY 2022 Skin care | IN FOCUS
www.citrinehealthcare.com | Tel: 01 445 7206 Protect the skin you’re in CLEARLY... ...different skin has different needs. 1 00% I R I S H OWNE D 1 %00 I R I S H ENWO D
Progestogen-only contraceptives make acne worse, so should be avoided
be first choice oral contraceptive compared to other combined oral contraceptives. This is due to the fact cyproterone has a 1.5-to-two times higher risk of venous thromboembolism compared to combined oral contraceptives containing levonorgestrel and has a similar risk of venous thromboembolism to oral contraceptives containing desogestrel, drospirenone and gestodene.
In many NHS Trusts in the UK, ethinyloestradiol 30 micrograms with norethisterone or levonorgestrel are recommended as first choice as a combined oral contraceptive for acne, which is move away from co-cyprindiol as first choice.
INTERACTION BETWEEN ANTIBIOTICS AND ORAL CONTRACEPTIVES
The theory that intestinal tract flora develop resistance to antibiotics after three weeks means women have been traditionally advised to take extra contraceptive precautions (ie, barrier contraceptive) for the first three weeks of antibiotic use. The theory is the reduction in intestinal tract flora due to antibiotics for the first three weeks reduces antibiotic absorption from the intestinal tract. However, this theory has been mainly dismissed in recent years, so no extra contraceptive method is generally advised with the contraceptive pill for the first three weeks of antibiotic use, as there is little evidence of contraceptive failure due to antibiotics. For example, an observational database study from BMJ (Evidence-Based Medicine) in August 2020 could not find increased risk of contraceptive failure with antibiotics. The exemption is the rifampicin antibiotics used for tuberculosis, which reduce contraceptive failure due to the liver enzyme-inducing effect (cytochrome P450) of rifampicin, which increases metabolism of the contraceptive, thus reducing effect.
4. SEVERE ACNE
Characterised by the condition being widespread on the face and upper body, especially if nodules and cysts occur

and scarring occurs. Can be considered if acne is causing severe psychological stress too. Referral to a dermatologist is advised for severe acne.
TREATMENT OPTIONS
Oral isotretinoin (Roaccutane) should be trialled on its own, especially if other treatment options such as systemic antibiotics have failed and especially if scarring is occurring and it is having a major psychological effect on the patient. Oral isotretinoin works like topical retinoids but has a more potent effect. Isotretinoin works in four ways:
Reduces bacteria, especially C.acnes .
Decreases size and secretions of sebaceous glands.
Stops comedones forming.
Reduces inflammation. Due to safety concerns and precautions, a dermatologist should initiate isotretinoin. Isotretinoin is used with caution with a history of depression, as it can exacerbate the condition. Despite initial concerns, there is no medical evidence that isotretinoin increases suicide risk.
Rarely, isotretinoin causes hepatic impairment, elevated serum lipid levels and pancreatitis, so it is necessary to do lipid and hepatic blood tests before starting isotretinoin. Isotretinoin is usually started at low dose (ie, 0.5mg/kg/day) and the blood test is repeated after one month at this dose before considering increasing to 1mg/kg/day after one month. These blood tests should be repeated after another one and two months at this full dose, and then every two months after that. Blood tests may be required more frequently if an abnormality is found (ie, lipid or hepatic abnormality).
A four-to-six-month course is a usual treatment range. Acne may get worse during the first seven days of treatment. This is normal and caused by the medication pushing out bacteria present in deeper layers of the skin. Isotretinoin carries elevated risk of causing serious birth defects. Women of childbearing age can only be prescribed isotretinoin following a negative pregnancy test. It is recommended this is repeated monthly
throughout isotretinoin treatment. Two forms of contraception are recommended while women use isotretinoin (oral contraceptive and carrier contraceptive). The progesterone-only contraceptive may be less effective while taking isotretinoin, so is not recommended and as mentioned earlier, progesteroneonly contraceptives can worsen acne.
If a further course of isotretinoin is required after the initial course (ie, after using for six months), then a minimum period of eight weeks is recommended before restarting isotretinoin. Combining isotretinoin with tetracyclines should be avoided due to the risk of intracranial pressure. Isotretinoin is recommended as monotherapy for acne.
COMMON SIDE-EFFECTS OF ISOTRETINOIN:
Inflammation, dryness and cracking of the skin and lips. This is relieved by applying moisturising cream and lip balm.
Dryness inside the nose leading to mild nosebleeds. Applying a thin layer of petroleum jelly to the inside the nose can prevent and ease.
Headaches.
Skin rash and peeling.
Inflammation of eyelids (blepharitis).
Inflammation and irritation of eyes.
Back, muscle and joint pain.
Blood in urine.
Bruising. ●
References: Available upon request
Disclaimer: Brands mentioned in this article are meant as examples only and not meant as preference to other brands.
40 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Skin care CONTRIBUTOR INFORMATION Written and researched by Eamonn Brady (MPSI), owner of Whelehans Pharmacies in Mullingar Tel 04493 34591 (Pearse St) or 04493 10266 (Clonmore). www. whelehans.inet. Eamonn specialises in the supply of medicines and training needs of nursing homes throughout Ireland. Email ebrady@whelehans.ie
THERE ARE MANY MINOR EYE CONDITIONS THAT ARE ASSOCIATED WITH THE CHANGING WINTER WEATHER, SUCH AS DRY EYE. HOWEVER, ONE CONDITION THAT CAN ALSO CAUSE EYE PROBLEMS IS SJÖGREN’S SYNDROME, AN UNDER-DIAGNOSED AUTOIMMUNE CONDITION THAT CAN HAVE A SERIOUS IMPACT ON QUALITY OF LIFE
People aged 60 years and older are particularly susceptible to eye problems and the pharmacist is in the best possible position to advise these patients. The Irish College of Ophthalmologists recommends lifestyle interventions to preserve eye health, including dietary advice. Foods high in antioxidants can help to prevent retinal damage, and certain eye conditions like cataracts and agerelated macular degeneration (AMD).
The harsh winter weather influences eye health due to factors such as increased heat indoors, as well as spending a lot of time outdoors in harsher weather conditions. Switching from cold weather to dry, heated indoor air can also have a detrimental effect
on eyes and can easily dry them out.
Diet is also an important factor and an antioxidant which hugely beneficial is lutein, which is found in many fruit and vegetables.1
Foods recommended for eye health that contain the antioxidant lutein include:
Broad leaf greens, such as kale and spinach.
Brightly-coloured fruit and vegetables, such as corn, carrots, orange sweet peppers and oranges.
Oily fish like salmon, tuna and mackerel.
Broccoli.
Eggs.
However, if a patient is suffering from dry eyes, dry mouth or dry skin and the cause has not been diagnosed, Sjögren’s syn-
drome is a potential reason.
Sjögren’s syndrome remains undiagnosed in many people. It is an autoimmune condition, meaning white blood cells attack other cells in the body. The cause is unknown. It was first discovered by Swedish ophthalmologist Henrik Sjögren in the 1930s and ‘Sjögren’ is pronounced ‘Show-gren’. In Sjögren’s syndrome, the body’s white blood cells attack other cells in the body.
Research has resulted in the identification of a number of factors (ie, immunological, genetic, hormonal, and inflammatory) that may be involved in causing the condition. One theory being postulated is that inflammation or abnormality of the body’s glands causes an autoimmune reaction.2
41 VOL 23 ISS 1 JANUARY 2022 Eye care | IN FOCUS
The main and most obvious symptoms of Sjögren’s syndrome are dry eyes and dry mouth, as well as enlargement of the parotid glands (salivary gland located in the cheeks just in front of the ears). Dry eyes and mouth occur in 95 per cent of cases.3
The hallmarks of Sjögren’s syndrome (ie, dry mouth and dry eyes) is known as sicca syndrome. Fatigue and joint and muscle pains are other debilitating features in many who have the condition.
For some people, the symptoms are no more than a nuisance. However, for others, they are more severe and have a profound effect on their quality of life if not treated adequately.
Whilst dry eyes and dry mouth are common features of Sjögren’s syndrome, most people who develop these symptoms do not have the disease; for example, dry eyes and dry mouth have been found to affect about 30 per cent of older people overall, and the majority of these cases are not due to Sjögren’s syndrome itself. Dry mouth and eyes can also be caused by many medicines, such as tricyclic antidepressants, antihistamines, decongestants, beta blockers (for blood pressure and heart conditions), codeine-type painkillers, diuretics, etc. Therefore, before a patient is diagnosed, it must be checked that they are
not taking one of these medicines that could be causing the dry eyes and mouth.
Dry eyes lead to itchy eyes, grittiness and soreness, and can lead to damage to the cornea if not controlled. Dry mouth may not be immediately obvious on presentation, and the person may not complain of dryness, but they may describe experiencing an unpleasant taste, insatiable thirst, difficulty eating dry food such as cream crackers, and soreness/discomfort.
In itself, dry mouth is not normally a serious problem, but if not addressed it can lead to:
Loss of taste.
Thrush (fungal infection) in the mouth.
Tooth decay and gum disease.
Swallowing problems and dysphagia (the feeling of something getting stuck in the throat on swallowing).
Sore or cracked tongue.
Difficulty talking.
According to the Mayo Clinic, other complications of Sjögren’s syndrome can include:4
MOST COMMON COMPLICATIONS INVOLVING EYES AND MOUTH:
Yeast infections. People with Sjogren's syndrome are much more likely to develop
oral thrush, a yeast infection in the mouth.
Dental cavities. Because saliva helps protect the teeth from the bacteria that cause cavities, people are more prone to developing cavities if their mouth is dry.
Vision problems. Dry eyes can lead to light sensitivity, blurred vision and corneal damage.
LESS COMMON COMPLICATIONS MAY INCLUDE:
Lymph node cancer. A small percentage of people with Sjogren's syndrome develop cancer of the lymph nodes (lymphoma).
Lungs, kidneys or liver. Inflammation can cause pneumonia, bronchitis or other problems in the lungs, lead to problems with kidney function, and cause hepatitis or cirrhosis in the liver.
Nerves. There may be numbness, tingling and burning in the hands and feet (peripheral neuropathy).
Typical effects of Sjogren's syndrome are illustrated in Figure 1.
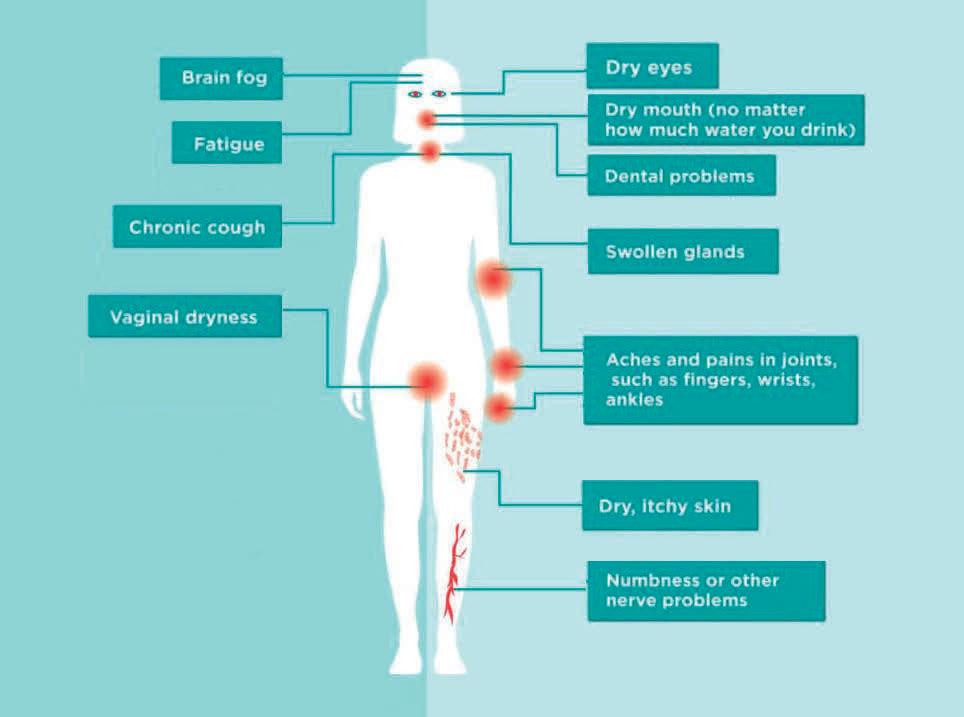
Other symptoms of Sjögren’s syndrome can include dryness of the airways (which can lead to dry cough and chest infections) and glandular swelling. Swelling of the parotid glands occurs in approximately one-third of cases and can be painful in some cases6 and swelling of other salivary glands located under the jaw or in the neck area can also be a problem. Muscle ache and aching joints can occur, and joint ache typically occurs in onethird of cases.6 Raynaud’s phenomenon — coldness in the extremities of the body, such as in the hands and fingers — occurs in about 20 per cent of cases of Sjögren’s syndrome.
As stated above, Sjögren’s syndrome can also cause peripheral neuropathy in about a quarter of cases. Peripheral neuropathy is damage to the nerve endings in the extremities such as fingers and toes and can cause numbness, tingling, itching, pins and needles, as well as other manifestations.7
Sjögren’s syndrome is referred to as ‘primary’ if it develops in isolation, and ‘secondary’ if it occurs in combination with other autoimmune or rheumatic conditions such as fibromyalgia, systemic sclerosis, rheumatoid arthritis, or systemic lupus erythematosus.
It is estimated that in 60 per cent of cases, Sjögren’s syndrome occurs with or is linked to other inflammatory autoimmune condi-
42 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Eye care
Figure 1: Typical Sjögren’s syndrome symptoms

















































































































HEAT CLEANSE Reusable Moist Heat Mask with HydroBead™ Technology Do your patients suffer with tired, itchy, dry eyes? The SCOPE Eye Care Regimen is designed to manage symptoms of Dry Eye Disease. Tea Tree Oil Lid Wipes and Cleansing Gel and Triple Action Spray-on, Leave-on Formula for improved Eyelid Hygiene Eye Spray for Mild-Moderate Dry Eye Disease Eye Drops and Ointment for 24 Hour Dry Eye Management* HYDRATE Visit scopeeyecare.com for more information *Daniel Nelson, J et al, TFOS DEWS II: New Dry Eye Report (The Ocular Surface, 2017) recommends a regime including lubricants, lid hygiene and heat applied to the eyes in the management of Dry Eye Disease and related eye conditions. Freephone: 1800 816 005 | IRL: + 353 1 525 3683 | email: info@scopeeyecare.com SCOPEIE165 PllAor d uctsare Preservat evi eerF ●
tions. Rheumatoid arthritis, for example, is a severe inflammatory condition that causes swelling, pain, and deformities in the joints and it tends to run in families.
Systemic lupus erythematosus (otherwise known as lupus) is nine times more common in women. Lupus can cause inflammation in all organs, leading to joint pain, muscle pain, fever, fatigue and damage to all major organs if not controlled. Lupus is more rare in Europe and more common in people of Afro-Caribbean decent.
Fibromyalgia is a chronic pain disorder characterised by extreme tiredness and fatigue, while scleroderma is an inflammatory condition that affects the skin, leading to hard skin and skin lesions, and can go on to damage other organs if not controlled.
Counselling patients with Sjögren’s syndrome is important to help them manage their own care, and there are a number of steps these patients can be advised to take to reduce the severity of their symptoms. These include:8
For joint pain:
Be more active, especially between flare-ups.
Rest when joints are swollen and painful.
Rest instead of trying to fight the fatigue during flare-ups.
For dry nose, throat and upper airways:
Regularly use saline nasal sprays.
A home humidifier may help.
For dry eyes:
Use lubricating eye ointments or gels at night.
Wraparound sunglasses can prevent moisture loss from the eyes.
Regularly use tear substitute eye drops, with single-dose preservative-free drops possibly showing more benefit.
Limit use of drops with preservatives, which can cause dryness and irritation.
Dry mouth and other dental/ oral issues:
Chewing sugar-free gum or sucking sugarfree hard sweets or lozenges stimulates saliva.
Regularly take small sips of water.
Avoid sugar-containing foods and drinks between meals.
Use an electric toothbrush.
Using products with artificial sweetener xylitol rather than sugar prevents tooth decay.
Get regular dental check-ups.
Use fluoride products specifically for dry mouth.
Brush and floss teeth regularly (after meals especially). If regular day-time brushing is not possible, rinse regularly with water.
Use artificial saliva in the form of mouth gels, mouthwashes and chewing gum.
To relieve dry eyes, artificial tears have proven effective and should be used regularly by the patient. They come in drop form and gel form (the gel form has a longer-lasting effect, so is especially suitable before going to bed). There are many brands available over the counter from pharmacies; there is no evidence that one brand is any more effective than the next, though preservativefree versions are recommended by some eye specialists to reduce the risk of a preservative causing irritation.
The tear film contains three layers — fatty oils, aqueous fluid, and mucus — and this combination normally keeps the surface of the eyes lubricated, smooth and clear. However, problems with any of these layers can cause dry eyes. The basic physiology of the tear ducts is illustrated in Figure 2.
There is no approved cure for Sjögren’s syndrome, so therefore the goal of any treatment methodology is to control symptoms using therapies, and to limit longterm damage. The patient must be referred to a rheumatologist for assessment and diagnosis, and an ophthalmologist is often involved in the treatment of the eye problems if they are severe enough. Smoking and drinking alcohol should be avoided and good oral hygiene should be maintained. Exercising as much as possible and
a healthy balanced diet are also important. Vaginal lubricants may be required to tackle certain symptoms and it should be noted that candidiasis (thrush) is more likely with Sjögren’s syndrome due to vaginal dryness.
DEWS II
Pharmacists should be aware of the TFOS DEWS II Definition and Classification system, which contains an evidence-based definition and a contemporary classification system for dry eye disease (DED). The algorithm provides detailed information on the management and therapy options for DED, including tear insufficiency, anti-inflammatory therapies, managament of the psychological aspects of DED, and a wide range of other important treatment considerations. Full details can be accessed at https://www.tfosdewsreport.org/report-management_and_therapy/147_36/en/.
OVER THE COUNTER
Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, can be prescribed over the counter to relieve muscle and joint pain. However, these should be used in moderation, as they can cause side-effects such as stomach irritation and ulcer, raised blood pressure and kidney problems. They should also be used in caution with other conditions such as asthma, heart disease and kidney disease. Mild corticosteroid cream such as hydrocortisone 1% cream — used sparingly and occasionally — may give some relief from the dry skin symptoms (although regular moisturisation is key to advised and prevent dry skin).
To relieve dry mouth, drinking plenty of fluids helps keep the mouth moist. Artificial saliva can also be prescribed over the counter and comes in the form of mouth gels, mouthwashes and chewing gum. Some brands available over the counter in pharmacies include BioXtra and Biotene. As well as moisturising, these therapies have enzymes that help stimulate the saliva glands. Another option for dry mouth is pilocarpine tablets and these are licensed for those who have some residual salivary function left.9 The dose is 5mg tablets to be taken four times daily, before each meal and at night.
MEDICATIONS
As stated above, Sjögren's syndrome may
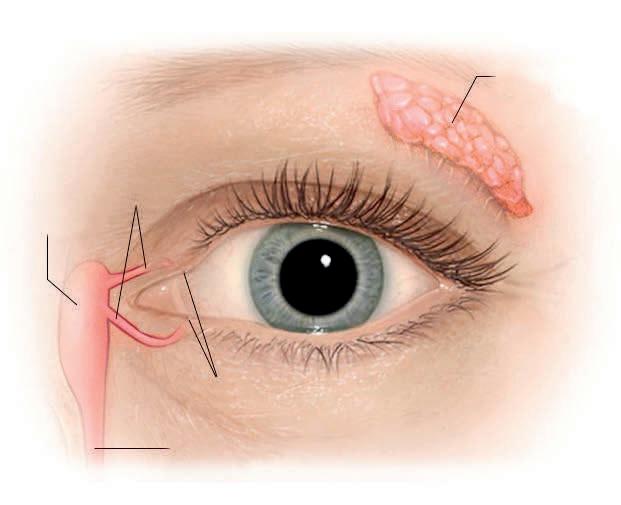
44 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Eye care
Figure 2: Tear glands and tear ducts. Image courtesy of Mayo Foundation for Medical Education and Research
Tear gland (Lacrimal gland)
Puncta Tear duct (Nasolacrimal duct)
Lacrimal sac Canaliculi
progress to involve organs such as the kidneys, lungs, skin and lymph glands and anti-inflammatory medication may be required in these situations. Options include:
Immunosuppressants: Careful monitoring with regular blood tests is required while taking immunosuppressants, as they can cause blood disorders and can affect the immune system. These drugs suppress the abnormal antibody production that causes Sjögren's syndrome, ie, they prevent the body ‘attacking itself’. Examples include methotrexate, azathioprine, penicillamine and hydroxychloroquine. They are reserved for more severe cases, as they can have side-effects and are only commenced by specialist rheumatologists. It is recommended that use of immunosuppressive agents is limited to cases where Sjögren's syndrome is affecting the major organs such as skin, lungs and kidneys.10
Steroids: These tablets are taken orally and reduce inflammation. They are usually limited to when symptoms are particularly troublesome, as they can cause side-effects if used long-term.
Methotrexate is often the first-choice DMARD for rheumatoid arthritis (RA), but less so in Sjögren’s syndrome. It can be taken on its own or in combination with another DMARD. The most common sideeffects associated with methotrexate are sickness, mouth ulcers, diarrhoea, hair loss or hair thinning, and rashes on the skin. Regular blood tests to monitor blood count and liver are required, as methotrexate can cause potentially serious liver and blood count problems. Very rarely, it can affect the lungs, so chest x-rays and possibly breathing tests are performed when starting methotrexate. This is to provide a comparison for if the patient develops shortness of breath or a persistent dry cough while taking methotrexate. Most people tolerate methotrexate well and more than 50 per cent of patients take it for at least five years.
Sulfasalazine has a slow onset of effect (one-to-three months).11 Patients may need to discontinue long-term treatment of sulfasalazine due to gastrointestinal complaints.
DMARDs help to ease symptoms and slow down the progression of inflammatory conditions. When antibodies attack the tissue in
the joints, they produce chemicals that can cause further damage to the bones, tendons, ligaments, and cartilage and in the case of Sjögren’s syndrome, they affect the eyes and salivary glands. DMARDs block the effects of these chemicals.
The earlier a DMARD is started, the more effective it will be but they must be started by a consultant rheumatologist. It is therefore important to seek treatment with a rheumatologist early if showing signs of a severe inflammatory aspect of Sjögren’s syndrome that is causing damage to other organs or if Sjögren’s syndrome is secondary to an accompanying autoimmune or rheumatic condition, such as rheumatoid arthritis or lupus.
The most common DMARDs include methotrexate, hydroxychloroquine, and sulfasalazine. These are now often referred to as conventional DMARDs since the advent of biological DMARDs in re -
REFERENCES
1. https://www.eyedoctors.ie/your-eye-health/ looking-after-your-eyes.asp.
2. Nikolov NP, Illei GG; Pathogenesis of Sjögren’s syndrome. Current Opinion on Rheumatology 2009 Sep; 21(5):465-70.
3. Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X. Primary Sjögren’s syndrome. British Medical Journal. 2012;344:e3821 doi: 10.1136.
4. Mayo Clinic: Sjögren’s syndrome. https://www.mayoclinic.org/diseasesconditions/sjogrens-syndrome/symptomscauses/syc-20353216.
5. Kassan SS. Managing dry eyes and dry mouth in Sjögren’s syndrome. American Journal of Managed Care. 2001;7 (supplement):S444-S450.
6. Chang HJ, Burke AE, Glass RM. Sjögren syndrome. JAMA, July 28, 2010, Volume 304, No 4.
7. Gemignani F, Marbini A, Pavesi G, et al. Peripheral neuropathy associated with primary Sjögren’s syndrome. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:983-986.
8. Arthritis Foundation. Sjögren’s’s syndrome. Self-Care, 2021.
cent years. Similar efficacy has been reported for methotrexate and sulfasalazine in studies of up to 12 months.12,13 The response of DMARDS is usually monitored every one-to-three months initially until symptoms improve.
PROGNOSIS
The outlook in terms of mortality for Sjögren’s is not unfavourable, even though quality of life is affected. The authors of a 2017 study wrote: “Overall, the prognosis of Sjögren’s syndrome is favorable. The life expectancy of pSS [primary Sjögren’s syndrome] patients is comparable with that of the general population… However, the patients’ quality of life is reduced by the diverse manifestations of the disease. Cardiovascular disease, infections, solid tumors, and lymphoma are the main causes of death. In patients with sSS, life expectancy is determined by the primary disease.”14 ●
9. Papas AS, Sherrer YS, Charney M, et al; Successful Treatment of Dry Mouth and Dry Eye Symptoms in Sjögren’s Syndrome. Patients with Oral Pilocarpine: A Randomised, PlaceboControlled, Dose-Adjustment Study. Journal of Clinical Rheumatology. 2004 Aug;10(4):169-177.
10. Ramos-Casals M, Tzioufas AG, Stone JH, Sisó A, Bosch X. Treatment of primary Sjögren’s syndrome: A systematic review. JAMA 2010; 304:452-60.
11. SPC for Salazopyrin®. Available at www.medicines.ie.
12. Lee D, Weinblatt M, Rheumatoid arthritis. The Lancet 2001; 358: 903-11.
13. Young A, Rheumatoid arthritis: Current approaches to drug treatment. Prescriber 19 Jan 2004; pages 48-55.
14. Ana-Luisa Stefanski, Dr med, Christian Tomiak, Dr med, Uwe Pleyer, Prof Dr med, Thomas Dietrich, Prof Dr med Dr med dent, Gerd Rüdiger Burmester, Prof Dr med, and Thomas Dörner, Prof Dr med. Diagnosis and Treatment of Sjögren’s Syndrome. Dtsch Arztebl Int. 2017 May; 114(20): 354–361.
45 VOL 23 ISS 1 JANUARY 2022 Eye care | IN FOCUS
KICKING THE HABIT
HELPING PATIENTS TO STOP SMOKING WILL BE ONE OF THE MOST COMMON TASKS FOR PHARMACISTS THIS JANUARY AND BEYOND
Ipreviously discussed smoking cessation in Irish Pharmacist and for the purpose of this article, I will concentrate on OTC medication to help give up rather than the negatives effects of smoking, which are well known to health professionals and the public alike. As a pharmacist, its rare to read through the causes of any condition that does not have smoking listed as one of the causes or exacerbating factors, so the benefits of giving up from a health perspective are obvious.
THREE STEPS TO GIVING UP
Deciding to give up smoking and really wanting to succeed are important steps in becoming a non-smoker. There are three steps to giving up smoking:
Preparing to stop;
Stopping; and
Staying stopped.
It can take up to three months to become a non-smoker, but it usually takes less time. The physical craving for a cigarette often disappears in less than a week, but the psychological craving can last longer.
STEP 1 - PREPARING TO STOP
It is important that people stop smoking because they want to. Think of the many benefits gained by stopping smoking. Giving up smoking is not easy, but the first three-to-four days will be the most difficult. If a person can, they should give up with a friend or family member who also wants to quit.
Here are a few tips to help give up:
Set a specific date to give up and cut down on the number of cigarettes smoked before that date.
46 VOL 23 ISS 1 JANUARY 2022
The support of family and friends will help give up.
Set a reward for the end of the first day, first week, first month.
Get rid of everything smoking-related, such as cigarettes, ashtrays and lighters, on the day before giving up.
STEP 2 - STOPPING
The initial goal is to get through the first day without smoking. If there is a need to put something in the mouth, chew sugarfree gum or something else that is healthy and non-fattening, such as fruit. If needing to do something with one’s hands, find something to fiddle with, such as a pencil, a coin, or a stress relief ball.
STEP 3 - STAYING STOPPED
Take it one day at a time. Thinking positively, remaining determined, and rewarding oneself can help. At the beginning, it may help to change normal routine to avoid situations that are normally associated with smoking. Avoiding alcohol for a while may also help. Most importantly, do not give up trying to quit, even if not successful the first time. Most people need several attempts at quitting long-term before they stop smoking completely.
HSE’S BRIEF INTERVENTION FOR SMOKING CESSATION (NATIONAL TRAINING PROGRAMME)
Pharmacological treatments should only be part of a treatment plan for someone giving up smoking. The HSE have a welldefined treatment advice platform for GPs, pharmacists and other clinicians involved in smoking cessation advice called Brief Intervention for Smoking Cessation (National Training Programme). Published in 2012, it advises clinicians in a well-defined manner how to aid the patient to give up smoking.
The Brief Intervention for Smoking Cessation training programme helps clinicians understand tobacco use and includes advice on how clinicians can:
Utilise brief interventions which involve opportunistic advice, discussion, negotiation, or encouragement.
Use the 'Five As' to help encourage and
support patients to give up, ie, Ask, Advise, Assess, Assist, Arrange.
Use motivational interviewing to help the patient through their smoking cessation journey. Motivational interviewing is a counselling technique that was introduced by American psychologists William Miller and Stephen Rollnick in 1983 and is used not just to aid smoking cessation, but in many other areas of mental health support.
Advise on pharmacological treatment. For this article, I concentrate on the overthe-counter treatment options to aid patients stop smoking. Due to word constraints, I will not discuss e-cigarettes or
zymes (primarily CYP1A2). It is believed the polycyclic aromatic hydrocarbons in tobacco smoke are responsible for the induction of cytochrome P450 rather than nicotine, meaning that nicotine replacement products do not have the same degree of drug interactions.
The HSE’s Brief Intervention for Smoking Cessation programme (Table 1) documents the main tobacco-drug interactions, with caffeine and fluvoxamine being considered the most clinically significant interactions.
Many of the tobacco-drug interactions involve drugs used for mental health disorders. It is well documented that people with mental health disorders have a higher propensity to smoke, so drug interactions can be more of an issue for these patients and need to be considered when patients are giving up smoking, as the concentration of the drug in the bloodstream may be increased as hepatic cytochrome P450 enzymes are not being induced by tobacco any longer. However, as evidenced in Table 1, many of the drugs which tend to have more significant interactions are used less frequently nowadays, ie, typical antipsychotics like haloperidol and chlorpromazine.
the two prescription-only smoking cessation drugs (bupropion and varenicline) in detail in this article, but I will return to this subject to discuss them in more detail in Irish Pharmacist in the future.
Pharmacological therapies (OTC or POM) should in no way be seen as standalone treatments to help patients quit. Psychological support and advice from clinicians should be central to the person’s road to becoming smoke-free.
DRUG INTERACTIONS WITH SMOKING
Interactions between tobacco smoke and drugs are often underestimated by patients and clinicians alike. There are pharmacokinetic reasons why tobacco smoke interacts with drugs; namely, tobacco smoke affects the absorption, distribution, metabolism (or elimination) of drugs, thus potentially giving an altered pharmacologic response. Tobacco smoke accelerates the metabolism of certain drugs by inducing hepatic cytochrome P450 en-
PHARMACOLOGICAL TREATMENT OPTIONS
To help give up, treatment options are available both over the counter in the pharmacy and on prescription.
Nicotine replacement therapy (NRT)
Nicotine replacement therapy (NRT) works by releasing nicotine steadily into the bloodstream at much lower levels than in a cigarette, without the tar, carbon monoxide and other poisonous chemicals found in tobacco smoke. This helps to control the cravings for a cigarette that occur when the body starts to miss the nicotine that smoking provides. The dose depends on the number of cigarettes smoked, intensity, and pattern of habit. NRT increases the rate of smoking cessation compared to placebo by 50-to-70 per cent.
NRT is the most common smoking cessation treatment. The National Institute of Health and Care Excellence in the UK (NICE, 2018) recommends a long-acting
47 VOL 23 ISS 1 JANUARY 2022 Smoking cessation | IN FOCUS
Use motivational interviewing to help the patient through their smoking cessation journey
Benzodiazepines Pharmacodynamic interaction: Decreased sedation and drowsiness.
May be caused by central nervous system stimulation by nicotine.
Beta-blockers Pharmacodynamic interaction: Less effective antihypertensive and rate control effects.
May be caused by nicotine-mediated sympathetic activation.
Caffeine Increased metabolism (induction of CYP1A2); clearance increased by 56%.
Caffeine levels may increase after cessation.
Chlorpromazine Decreased area under the curve (AUC) (36%) and serum concentrations (24%).
Smokers may experience less sedation and hypotension and require higher dosages than non-smokers.
Clozapine Increased metabolism (induction of CYP1A2); plasma concentrations decreased by 28%.
Flecainide Clearance increased by 61%; trough serum concentrations decreased by 25%.
Smokers may require higher dosages.
Fluvoxamine Increased metabolism (induction of CYP1A2); clearance increased by 25%; decreased plasma concentrations (47%).
Dosage modifications not routinely recommended but smokers may require higher dosages.
Haloperidol Clearance increased by 44%; serum concentrations decreased by 70%.
Heparin Mechanism unknown but increased clearance and decreased half-life observed.
Smokers may require higher dosages.
Insulin Insulin absorption may be decreased secondary to peripheral vasoconstriction; smoking may cause release of endogenous substances that antagonise the effects of insulin.
Smokers may require higher dosages.
Mexiletine Clearance (via oxidation and glucuronidation) increased by 25%; half-life decreased by 36%.
Olanzapine Increased metabolism (induction of CYP1A2); clearance increased by 40-98%.
Opioids (propoxyphene, pentazocine)
Dosage modifications not routinely recommended but smokers may require higher dosages.
Pharmacodynamic interaction: Decreased analgesic effect; higher dosages necessary in smokers.
Mechanism unknown.
Propranolol Clearance (via side chain oxidation and glucuronidation) increased by 77%.
Oral contraceptives Pharmacodynamic interaction: Increased risk of cardiovascular adverse effects (ie, stroke, myocardial infarction, thromboembolism) in women who smoke and use oral contraceptives.
Risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over age 35 years.
Tacrine Increased metabolism (induction of CYP1A2); half-life decreased by 50%; serum concentrations three-fold lower.
Smokers may require higher dosages.
Theophylline Increased metabolism (induction of CYP1A2); clearance increased by 58-100%; half-life decreased by 63%.
Theophylline levels should be monitored if smoking is initiated, discontinued, or changed.
Maintenance doses are considerably higher in smokers.
Table 1: Significant tobacco/drug interactions (Reference: Brief Intervention for Smoking Cessation (HSE) 2012)
product (ie, a patch) and a short-acting product, of which there are many varieties; these provide a dose of nicotine to help cravings. Most are absorbed sublingually (ie, vaping, gum, spray, or inhalator). The dose is usually titrated down over a 12-week period, however heavy smokers may need longer.
With a medical card, NRT products available include patches, gum, inhalers, oral sprays and lozenges once prescribed by a GP on the GMS scheme. Bear in mind, the pack sizes available on the GMS for the likes of nicotine gum are bigger dispensing packs rather than the OTC versions. Nicotine replacement products are not
available on the Drug Payment Scheme (DPS); despite having PCRS GMS codes.
If pregnant or breast-feeding, it is best for the health of mother and baby to stop completely and immediately, without the help of any smoking cessation treatment.
Side-effects of NRT can include:
Skin irritation from patches.
48 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Smoking cessation
Nicotine
of Quitting Smoking
*Provides significant improvement in quit rate vs patch alone. To verify contact: verify@perrigo.com Lindson N et al. 2019 Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Library. NiQuitin CLEAR 24 hrs transdermal patches are indicated for the relief of nicotine withdrawal symptoms including cravings as an aid to smoking cessation. Indicated in adults and adolescents aged 12 years and over. NiQuitin patches should be applied once a day, at the same time each day and preferably soon after waking and worn continuously for 24 hours. Apply a patch to non-hairy clean dry skin surface, a new skin site should be used every day. Therapy should usually begin with NiQuitin 21 mg/24 hrs and reduced according to the following dosing schedule: Step1: NiQuitin Clear 21 mg/24 hrs transdermal patches first 6 weeks. Step 2: NiQuitin Clear 14 mg/24 hrs transdermal patches next 2 weeks. Step 3: NiQuitin Clear 7 mg/24 hrs transdermal patches last 2 weeks. Light smokers (less than 10 cigarettes per day) are recommended to start at Step 2 (14 mg) for 6 weeks and decrease the dose to NiQuitin 7 mg/24 hrs for the final 2 weeks. In some instances (e.g. heavy smokers, those who have relapsed after NRT, or when one NRT product is not enough to control cravings), NiQuitin patches may be used in combination with a nicotine oral format (refer to the package leaflet for dosing guidance). Contraindications: Non-smokers, hypersensitivity, children under 12 years and occasional smokers. Precaution: Supervise use if hospitalised for MI, severe dysrhythmia or CVA, if haemodynamically unstable. Use with caution in patients with active oesophagitis, oral and pharyngeal inflammation, gastritis, peptic ulcers, GI disturbances, susceptible to angioedema, urticaria, renal/hepatic impairment, hyperthyroidism, diabetes, phaeochromocytoma, seizures & epilepsy. Discontinue if severe persistent skin rash. Pregnancy and lactation: Oral formats preferable to patches unless nauseous. Remove patches at bedtime. Side effects: Sleep disorders, abnormal dreams, insomnia, headache, dizziness, nausea, vomiting, application site reactions, nervousness, palpitations, dyspnoea, pharyngitis, cough, dyspepsia, upper abdominal pain, diarrhoea, constipation, dry mouth, sweating, localised pain, urticaria, hypersensitivity, tremor, nervousness, palpitations, tachycardia, contact & allergic dermatitis, photosensitivity, arthralgia, myalgia, asthenia, malaise, influenza-type illness, fatigue, chest or limb pain, pain, seizures and anaphylaxis. Legal classification: GSL: PA 1186/18/4, PA 1186/18/5 & PA 1186/18/6. MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. https://www.medicines.ie/medicines/niquitin-clear-7-mg-24-hours-transdermal-patch-33085/patient-info https://www.medicines.ie/medicines/niquitin- clear-14-mg-24-hours-transdermal-patch-33083/patient-info https://www.medicines.ie/medicines/niquitin-clear-21-mg-24-hours-transdermal-patch-33084/patient-info NiQuitin Mini 1.5mg/4mg Mint Lozenges are used for the treatment of tobacco dependence by relief of nicotine withdrawal symptoms and cravings. Indicated in adults and adolescents aged 12 years and over. NiQuitin Mini 1.5 mg are suitable for those who smoke who smoke 20 cigarettes or less a day. NiQuitin Mini 4 mg are suitable for smokers who smoke more than 20 cigarettes a day. Place a lozenge in the mouth whenever there is an urge to smoke, allow to dissolve completely. Do not chew or swallow whole. In heavy smokers, those who have relapsed after NRT, or when one NRT is not enough to control cravings, NiQuitin Minis may be used in combination with NiQuitin patches (refer to the package leaflet for dosing guidance). Abrupt cessation: Use a lozenge whenever there is an urge to smoke, maximum of 15 lozenges a day. Continue for up to 6 weeks, then gradually reduce lozenge use. Gradual cessation Use lozenges whenever there is an urge to smoke in order to reduce the number of cigarettes smoked for up to 6 weeks, followed by abrupt cessation. Adolescents (12-17 years): only with advice from a healthcare professional. Should not quit with a combination NRT regimen. Contraindications: hypersensitivity to nicotine or any of the excipients, children under the age of 12 years and non-smokers. Precaution: Supervised use in dependent smokers with a recent myocardial infarction, unstable or worsening angina pectoris including Prinzmetal’s angina, severe cardiac arrhythmias, uncontrolled hypertensions or recent cerebrovascular accident. Use with caution in those with; stable cardiovascular diseases, diabetes mellitus, susceptibility to angioedema & urticaria, renal/hepatic impairment, phaeochromocytoma &uncontrolled hyperthyroidism, GI disease & seizures. Side effects: Nausea, mouth/throat and tongue irritation, irritability, anxiety, sleep disorders, dizziness, headaches, cough, sore throat, vomiting, diarrhoea, GI and oral discomfort, flatulence, hiccups, heartburn, dyspepsia, dry mouth, constipation, ulcerative stomatitis, pharyngitis, nervousness, depression, palpitations, heart rate increased, dyspnoea, rash, angioedema, pruritus, erythema, hyperhidrosis, fatigue, malaise chest pain, anaphylactic reactions, hypersensitivity, tremor, dysgeusia, paresthesia mouth, seizures & epilepsy, dysphagia, eructation, salivary hypersecretion, influenza like illness. Legal classification: GSL: PA 1186/18/11 & PA 1186/18/12 MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. https://www.medicines.ie/medicines/niquitin-mini-1-5mg-mint-lozenges-33090/smpc https://www.medicines.ie/medicines/niquitin-mini-4mg-mint-lozenges-33091/smpc



“Since I quit smoking, I enjoy a healthier me”
Samantha
IRE NIQ 2021 40
Irritation of the nose, throat or eyes when using nasal spray.
Insomnia and vivid dreams.
Upset stomach.
Dizziness.
Headaches. Side-effects, if they occur, are generally mild. NRT is not considered as like swapping one addition for another. Smoking is very addictive mainly because it delivers nicotine to the brain very quickly. Nicotine levels in NRT are significantly lower than in tobacco and they deliver nicotine in a more measured way, making them less addictive than smoking.
Types include:
Transdermal patches (which stick to the skin). Available in formulations that release nicotine for either 16 hours or 24 hours.
Patches should be applied daily to clean, dry, hairless skin, ie, hip, upper arm, or chest. The 24-hour patch is advised for patients who need a cigarette as soon as they wake up, ie, Nicquitin CQ, Nicotinell. While the number of weeks advised per strength of patch varies by brand, the general advice is that patients who smoke more than 10 cigarettes a day should start with a 21mg patch for six-to-eight weeks, then taper down to a 14mg patch at weeks six-to-eight, and then a 7mg patch for week nine or 10 if they have no cravings.
Smokers who smoke less than 10 cigarettes a day are advised to start with the 14mg patch daily for four-to-six weeks than the 7mg patch for two-to-four weeks. The time periods between reducing strengths varies between brands (Nicquitin CQ, Nicotinell) and their classification between light smokers (less than 10 or 20 cigarettes daily) varies, so the best advice is to follow the guidance that comes with each specific brand.
Patients who do not need a cigarette first thing in the morning may be advised to use a 16-hour patch, ie, Nicorette. Based on the Nicorette Patch, patients who smoke more than 10 cigarettes a day should start with a 25mg patch daily for eight weeks, then taper down to 15mg daily for weeks nine and 10, and taper down to the 10mg patch daily for weeks 11 and 12 if no cravings. Those who smoke less than 10 cigarettes daily are ad-
vised to start the 15mg patch for eight weeks, and then the 10mg patch for four weeks. The advantages of patches are the patient can put the patch on in the morning and forget about it for the day. The disadvantage is that it is passive, meaning the patient cannot act when craving occurs; this can be particularly difficult for patients who miss 'smoking gestures' such as the hand-to-mouth action of having a cigarette; this is where the likes of nicotine inhalators, nicotine gum and e-cigarettes can have an advantage. Combination therapy may be advised for some patients, ie, wearing a patch and using an inhaler or gum for breakthrough cravings, though it should be limited, as if patients are having this level of craving, an alternative option like varenicline (Champix) should perhaps be considered.
rotating sites or brands may help. The 24hour patch can cause vivid dreams or sleep disturbance at night.
Chewing gum that is available with either 2mg or 4mg of nicotine
Brands include Nicorette, Nicotinell and Nicochew gum, which all come in 2mg and 4mg strengths. The 2mg gum is advised for smokers of less than 20 cigarettes daily, and the 4mg gum is advised for smokers of more than 20 cigarettes daily. The patient uses them every one-to-two hours as needed, which depends on nicotine cravings; usually, no more than 15 pieces a day is advised. After three months, gradually reduce using nicotine gum; this can be done by reducing the length of time each piece is chewed or reducing the number of times the patient chews gum daily. An option when close to getting off the nicotine gum is to cut the gum into smaller pieces. Another option to eventually get of it completely is to swap the nicotine gum for ordinary sugar-free chewing gum, so stopping nicotine gum completely.
Patches are not advised to be used while smoking, though if a patient does smoke while wearing a patch, the rewarding effect of smoking is reduced, as they already have nicotine in the system, so it should reduce the urge to continue smoking. Smoking while wearing a patch risks nicotine overdosing, which can cause dizziness. Patches are often started to aid smoking cessation for patients post-myocardial infarction (MI); this should be under a doctor’s supervision, so should ideally be prescribed by a doctor within four weeks post-MI rather than the patient buying over the counter, where medical supervision is less likely. Nicotine patches should be used with caution in patients with serious underlying arrhythmias and worsening angina, but in most cases the advantages of giving up smoking outweigh the risks. Nicotine patches are contraindicated during pregnancy and breastfeeding. Fifty per cent of patients have a skin reaction with nicotine patches but in most cases, this is mild and
The advantages of chewing gum are that the patient can use as required and can self-dose. It can be advantageous for patients who miss the 'gestures of smoking', as it involves the action of putting something in the mouth and chewing; however, nicotine inhalers are better in these patients. For maximum effect, avoid food and acidic drinks 15 minutes before and while using. Some patients experience jaw pain or fatigue from frequent chewing and the swallowing of saliva that occurs while chewing nicotine gum can cause nausea, heartburn, and hiccups.
Nicotine inhalers which look like a plastic cigarette and through which nicotine is inhaled
For example, Nicorette 15mg inhaler. The major advantage is that it mimics hand-tomouth action of smoking. Dose is one puff as required and the advised max daily dose is six cartridges.
Less is needed if using the inhaler as combination therapy, ie, if using while using a nicotine patch or a prescription smoking cessation therapy like varenicline. Combination therapy of a nicotine inhaler with another
50 VOL 23 ISS 1 JANUARY 2022 IN FOCUS | Smoking cessation
NRT is not considered as like swapping one addition for another
therapy like patches is generally not needed, but it may help some patients who still have some cravings, especially in the first weeks after stopping smoking. Combination therapy of inhaler and other smoking cessation therapy may be advised for patients who miss the hand-to-mouth action of cigarettes, but for whom nicotine inhalers are not sufficient on their own, ie, very heavy smokers.
A common misconception of nicotine inhalers is that the patient should inhale deeply; nicotine inhalers are orally absorbed, so there is no need to inhale deeply. Each cartridge lasts for 20-to-40 minutes (around 400 puffs), after that, replace the cartridge. Patients are advised to use the 'non-smoking hand' to hold the inhaler to help break the link from the habit of holding a cigarette. A disadvantage is that it is visible in the hand, so is less discreet. For maximum effect, avoid food and acidic drinks 15 minutes before and while using.
Caution is advised for asthmatics due to a very small risk of triggering asthma symptoms, however cigarettes are universally found to have more negative effects for asthmatics than nicotine inhalers. Side-effects are usually mild and can include cough and throat irritation, however these are side-effects often associated with stopping smoking, so these sideeffects may be more to do with this than actual side-effects of the nicotine inhaler.
Lozenges which are placed under the tongue
Brands include Nicquitin CQ, Nicotinell and Nicorette and come in 1mg, 2mg and 4mg lozenges, depending on brands. The 4mg strength is advised for smokers of 20 or more cigarettes daily and who smoke first cigarette within 30 minutes after waking, and the 2mg strength is advised for smokers of 20 or less cigarettes daily and who smoke the first cigarette more than 30 minutes after waking. Dose is one lozenge every one-to-two hours. Frequency of use varies per brand, but general advice is to use eight-to-12 lozenges per day (no more than 15) for first six weeks before tapering-off. Each lozenge should last 20-to-30 minutes. If cravings are still experienced after six weeks, the advice is to continue using the lozenges and to keep gradually
reducing the number of lozenges used and once down to a use of one-to-two lozenges a day, aim to stop using them completely.
The lozenge is placed (not chewed or swallowed) and allowed to dissolve between cheek and gum. Suck the lozenge until the taste becomes strong then rest between gums and cheek and suck again when the taste fades. Advantages are their ease of use and flexible dosing, and they are available over the counter. For maximum effect, avoid food and acidic drinks 15 minutes before and while using. As with nicotine chewing gum, the swallowing of saliva while using can cause nausea, heartburn, and hiccups.
Nicotine Mouth Spray (only brand is Nicorette Quickmist)
A nicotine mouth spray quickly releases nicotine into the body and is advertised by Nicorette to ease cravings within 30 seconds of use. Dose is one-to-two sprays when needed as cravings occur. Each spray delivers 1mg of nicotine. It can be used in combination with nicotine patches to quell breakthrough urges.
To use, point the spray nozzle towards the open mouth, holding as close to the mouth as possible, spraying into the side of the mouth, avoiding the lips. As it is absorbed orally, do not inhale while spraying and for maximum effect do not swallow for a few seconds after spraying. Maximum dose is four sprays an hour, or 64 sprays a day. Aim to eventually reduce usage down to two-tofour sprays a day, then stop and aim not to use longer than six months, though there is no proof longer use is harmful.
NRT NOT AVAILABLE IN IRELAND
Nicotine Nasal Spray (not launched in Ireland yet)
Nicorette launched a nicotine nasal spray in America (got FDA approval to sell over the counter in 2019) and in the UK. It has not been launched in Ireland, but it is likely to come on the market soon. Each spray delivers 1mg of nicotine, with a maximum of four sprays per hour and maximum of 64 sprays daily.
Micro Tablets
While Nicorette Microtabs are available in
the UK and other countries, they are not currently available in Ireland. They work by being placed under the tongue to dissolve and smokers of 20 or more cigarettes daily use one every one or two hours. They are very similar to Nicorette lozenges.
QUICK SUMMARY OF OTHER OPTIONS
E-cigarettes
Electronic cigarettes (e-cigarettes) are electronic devices that mimic cigarettes and release nicotine vapour. They allow inhalation of nicotine without the negative effects of tar and carbon monoxide. There are hundreds of different types of devices and juices available. As e-cigarettes are relatively new on the market, and research is ongoing on their benefits and negative effects, the HSE still doesn’t endorse e-cigarettes as an option to help give up cigarettes and recommends NRT (ie, nicotine patches) as the first option.
The World Health Organisation’s opinion is that e-cigarettes should not be recommended as a smoking cessation tool at a population level and warned they can reduce cessation by prolonging or increasing nicotine addiction. Advice from the likes of the Irish Heart Foundation and the Irish Cancer Society to an Oireachtas Committee in November 2021 was that ecigarettes need to face similar sale and advertising restrictions as tobacco products, and they are concerned at the number of teenagers and young people becoming addicted to nicotine through vaping products. Legislation will tighten on the sale and use of e-cigarettes in the coming years but while there are risks from them, they may ultimately share a more official role in aiding smoking cessation as their safety and efficacy continues to be analysed.
PRESCRIPTION MEDICATION
There are two main treatments available on prescription:
Varenicline (Champix)
Varenicline, whose brand name is Champix. It is available in tablet form. The dose is titrated, meaning the person smokes for the first eight-to-14 days while taking varenicline before quitting.
51 VOL 23 ISS 1 JANUARY 2022 Smoking cessation | IN FOCUS
The recommended course of Champix is generally 12 weeks. It is a partial agonist that prevents nicotine reaching receptors; it also releases dopamine to help with cravings, meaning it works in two ways — it reduces the enjoyment of a cigarette if a person does smoke while taking it, and it reduces the craving for a cigarette.
Varenicline may not suit everyone and has possible side-effect, including:
Nausea and vomiting.
Insomnia and vivid dreams.
Dry mouth.
Constipation and diarrhoea.
Headaches.
Drowsiness.
Dizziness.
It is not suitable for:
Children under 18.
During pregnancy or breastfeeding.
People with kidney problems.
People with epilepsy. Varenicline has no clinically significant medicine interactions.
Bupropion (Zyban)
Bupropion was originally developed as an antidepressant and the way in which it works in relation to helping stop smoking is not completely understood, but it is thought to work on the brain pathways involved in addiction and withdrawal. It is available in the form of tablets. A course usually lasts eight-to-12 weeks.
Side-effects can include:
Nausea and vomiting.
Insomnia.
Dry mouth.
Constipation and diarrhoea.
Dizziness.
Difficulty concentrating. It is not suitable for:
Children under 18.
During pregnancy or breastfeeding.
People with epilepsy.
People with bipolar disorder.
People with eating disorders.
Varenicline versus bupropion
Bupropion has more contraindications compared to varenicline. Contraindications for bupropion include increased risk of manic episodes in patients with bipolar disorder and a reduced seizure threshold for epilepsy patients. Analysis published in
1. NRT or varenicline or bupropion should be prescribed only for a smoker who commits to a target stop date.
2. The initial supply of the prescribed smoking cessation therapy should be sufficient to last only two weeks after the target stop date.
3. Normally, this will be two weeks of NRT or three-to-four weeks of varenicline or bupropion.
4. A second prescription should only be issued if the smoker demonstrates a continuing attempt to stop smoking.
5. If an attempt to stop smoking is unsuccessful, the health service (HSE if a patient is getting products via the GMS scheme) should not normally fund a second attempt within six months.
the Harm Reduction Journal in 2009 found varenicline to be more effective than bupropion and NRT for smoking cessation. Varenicline is way more commonly prescribed than bupropion nowadays.
GUIDANCE FOR PRESCRIBERS ON SMOKING CESSATION THERAPIES
It is difficult to get figures on the numbers of NRT and prescription-only smoking cessation drugs (bupropion and varenicline) prescribed in Ireland annually. The Primary Care Reimbursement Service (PCRS) Statistical Analysis of Claims and Payments figures are released annually and over the last 15 years, only twice has NRT been mentioned in the figures.
In 2014, NRT products were prescribed 137,192 times under the PCRS’s general medical card scheme (GMS) at a cost to the State of €4,931,977, making it 35th of the top 100 products by ingredient cost. In 2017, NRT products were prescribed 140,003 times under the PCRS’s general medical card scheme (GMS) at a cost to the State of €5,303,121, making it 18th of the top 100 products by ingredient cost that year. There is no recent data to indicate the breakdown of NRT by product on the GMS.
NRT became reimbursable on the GMS (ie, free to medical card patients) from 2001 and based on figures soon after they were allowed (2002), nicotine patch therapy accounted for 82.8 per cent of all NRT dispensed in 2002 on the GMS scheme and
based on dispensing trends seen in pharmacies, patches are still likely to account for over 80 per cent of NRT prescriptions on the GMS.
Prescribing advice taken from NICE in the UK (Table 2) and embraced by the HSE and aims to reduce wastage in the prescribing of NRT products. It advises that smaller amounts of NRT (two weeks) are prescribed initially, with more only be prescribed when it is seen that the patient has shown a continued commitment after the initial two-week period to continuing to give up.
SUPPORT FROM THE HSE
Contact the Quit Team for free support at Freephone: 1800 201 203, free text: QUIT to 50100, Email: support@quit.ie Twitter: @HSEQuitTeam. ●
References: Available upon request
Disclaimer: Brands mentioned in this article are meant as examples only and not meant as preference to other brands.
CONTRIBUTOR INFORMATION
Written and researched by Eamonn Brady (MPSI), owner of Whelehans Pharmacies in Mullingar Tel 04493 34591 (Pearse St) or 04493 10266 (Clonmore). www. whelehans.inet. Eamonn specialises in the supply of medicines and training needs of nursing homes throughout Ireland. Email ebrady@whelehans.ie

52 VOL 23 ISS 1 JANUARY 2022 IN FOCUS |
Smoking cessation
Table 2: NICE guidance for smoking cessation therapy. Reference: NICE (UK)
MORGAN FLANAGAN CREAGH REVIEWS THE FORD FIESTA MHEV ST-LINE
Unfortunately, while testing Ford’s new Fiesta mHEV, I had to get my mum to a certain South Dublin private hospital’s emergency room before it closed at 6pm. This plucky little Fiesta rose to the challenge of becoming a makeshift ambulance and proved, as the Fiesta has time and again, that it isn’t the size of the dog in the fight, it’s the size of the fight in the dog.
Having fallen in love with the Fiesta mHEV’s hardcore cousin, the full-fat 200hp Fiesta ST two years ago, I was sceptical about this ‘mild hybrid’ Fiesta, but boy, was I pleasantly surprised. My test car was a five-door ST-Line, with a 1.0-litre turbocharged engine, pushing 125hp through a rather sweet six-speed manual gearbox.
Utilising the new mild hybrid system, this Fiesta was fun to drive in a way that many supposed sporty cars would dream of. It was powerful, responsive, and accelerated smoothly throughout the gear range. It was a handsome little so-and-so, painted in metallic black with 17” alloy wheels and slightly tinted rear windows, for that sleek, black-on-black look. It also boasted some nice ST-Line body panels, such as the grille and spoiler.
Available in 125hp or 155hp, this turbocharged, mHEV Fiesta features a belt-driven integrated starter/generator (BISG) in place of the standard alternator, enabling recovery and storage of energy usually lost during braking and coasting, to charge a 48-volt lithium-ion air-cooled battery
pack. The BISG also acts as a motor, integrating with the engine and using the stored energy to provide torque assistance during normal driving and acceleration, as well as running the vehicle’s electrical ancillaries. Finally, this clever system also shuts down one of the engine’s cylinders when it detects it’s not needed, to enhance fuel efficiency and save the planet.
According to Mr Roelant de Waard, Vice-President, Marketing, Sales and Service at Ford of Europe: “Adding EcoBoost Hybrid technology to Fiesta’s best-in-class driving dynamics means customers can have even more power and still go further on a tank of fuel.”
The Fiesta is festooned with tech, like FordPass Connect, which allows the

53 VOL 23 ISS 1 JANUARY 2022 Motoring | LIFESTYLE
Ford’s versatile Fiesta can be all things to all people
driver to unlock their doors, start the car, locate the vehicle and check on the fuel level, alarm status, tyre pressures and oil life through the FordConnect app on their smartphone. My test car also had adaptive cruise control, which the driver can now use in stop-start traffic, as well as motorway driving, and a terrific Fiesta SYNC 3 infotainment system with Android Auto and Apple CarPlay. Also available are park assist, cross traffic alert, with active braking and a phenomenal B&O sound system with a subwoofer built into the wheel arch.
The four-door Fiesta is a perfect example of ‘less is more’. I packed up the boot with shopping on three occasions, as well as bags, boxes, and four adult humans, and despite what the crossover SUV propaganda would tell you, nothing went wrong, it was perfectly fine. So, do you really need a small SUV for your family of three when a good hatchback will do the job? I don’t think so.
The Fiesta is festooned with tech, like FordPass Connect, which allows the driver to unlock their doors, start the car, locate the vehicle... through the FordConnect app, on their smartphone
The entry-level Fiesta costs €18,949, but I don’t believe in promoting entry-level cars, as they’re usually lacking. My test car, the ST-Line, 1.0-litre, 125hp, EcoBoost-Hybrid starts at €23,947. However, once you add the optional extras, that price rises to €25,015. That’s not bad at all, in a world where people spend €45k on a Nissan Qashqai.
I can’t recommend this little bargain enough, especially if you’re looking for a family car that doesn’t feel cheap and won’t let you down in an emergency. ●
1.0T 125PS MHEV
EcoBoost Hybrid
Six-speed manual
Five-door
CO2 emissions: 116g/km (WLTP)
Colour: Agate Black metallic paint
Registration:
211 D 8915
Standard features include:
Rock Metallic Light 17”
5x2-spoke alloy wheels
ST-Line front fog lamps with cornering lights. Black headlining.
LED night signature to rear lights.
Keyless start with Ford power starter button.
Rear privacy glass.
Cruise control including ASLD (active speed limiter device).
Rear parking sensors.
Rain-sensing wipers and
FORD FIESTA MHEV ST-LINE
autodimming mirror. Sensico wrapped flat-bottom steering wheel with centre console with opening armrest, 2 x cupholders, red stitching 12v and USB. Ford SYNC 3 navigation with 8’’ touchscreen. Electric rear windows. DAB radio, Emergency Assistance, Apple CarPlay and Android Auto, 2 x USB sockets, Six speakers and FordPass Connect** (Embedded modem). Unique ST-Line upper grille and full body styling kit with ST-Line wing badges. Large body coloured rear spoiler. Sports tuned suspension.
Additional options on this model:
Driver assistance pack
– incl 4.2” TFT instrument cluster; (AEB) Autonomous emergency braking –incl pedestrians AEB, auto high beam, speed sign recognition, driver alert, intelligent adaptive cruise control (ACC), door edge protectors, BLIS (blind spot information system), advanced auto park includes front parking sensors and advanced auto park assistance, rear view camera = €1,068.
Price for Ford Fiesta
ST-Line 1.0 125PS
EcoBoost Hybrid from €23,947 = plus optional extras = €25,015. Entry price for Ford Fiesta from €18,949 (prices exclude delivery and related charges).

54 LIFESTYLE | Motoring VOL 23 ISS 1 JANUARY 2022
VITALITY AND IMMUNITY CLONMEL HEALTHCARE OFFERS IRELAND’S PHARMACIES A BROADER PORTFOLIO
AdditionofthePharmatonvitality/immunityrange strengthens Clonmel Healthcare’s offering as the go-to-partner for Irish pharmacies this winter.
Clonmel Healthcare is responsible for the marketing and sales of Sanofi’s consumer healthcare range of products in the Irish market. These include their allergy medicines Telfast, Nasacort and Opticrom, and the Dulcolax laxative range, Buscopan for IBS, and Maalox antacids. These form part of a wider agreement covering 20 countries.
Mr Jim Hanlon, Clonmel Healthcare’s General Manager, said: “The addition of such brands significantlystrengthensClonmel’spositionasa trusted partner to pharmacists, patients and consumers throughout the country.”
Clonmel Healthcare is now offering pharmacists, and their customers a broader range of consumer healthcare brands such as Pharmaton vitality/ immunity, Telfast, Nasacort, Opticrom, and the Dulcolax laxative range, as well as Buscopan for IBS, and Maalox antacids. These attractive brands are covered by an agreement whereby Clonmel’s parent group STADA is distributing and marketing Sanofi’s local portfolio of well-established consumer healthcare products across 20 countries.
The addition of such well known and strong brands like Pharmaton will enhance Clonmel’s existing portfolio of winter products, including the

company's recently launched Medithyme cough syrup and long-established Paralief (Paracetamol) and Easofen (Ibuprofen) ranges. With Clonmel Healthcare as a trusted partner, Irish pharmacists can be sure they are offering their customers an appealing and comprehensive offering for a range of symptoms.
“The addition of Sanofi’s portfolio to Clonmel Healthcare’s current offering will alter the company's ranked position in the market to No. 2 by volume, and will firmly place the company in the top five OTC companies in Ireland,” according to Mr Hanlon. He further stated: “Adding major brands like Buscopan and Dioralyte for rehydration further strengthens the company's position. We look forward to supporting pharmacy purchases with targeted and effective promotional campaigns for these brands."
Other brands in Clonmel Healthcare’s new offering include Antihistan Cream (Mepyramine Maleate) and Selsun anti-dandruff shampoo, which join Clonmel’s other products in the anti-fungal head care treatments, such as Nizoral Shampoo.
Clonmel Healthcare has a large portfolio of products covering three distinct business units: Prescription medicines, speciality medicines, and the OTC Consumer Division. The over-the-counter product range focuses on the three main areas: Women’s and babies' health, skincare, and pain management.
For further information, please contact Clodagh Lenehan, Ceede Media, Telephone: 087 958 6005 or email clenehan@ceedemedia.ie.
PAIN RELIEF CLONMEL HEALTHCARE LAUNCHES CO-TIPOL MAX SUPPOSITORIES
Clonmel Healthcare is pleased to announce the launch of Co-Tipol Max 1000mg/60mg Suppositories containing paracetamol and codeine phosphate hemihydrate.
Co-Tipol Max are indicated for moderate-to-severe pain and are GMS reimbursable.
Full prescribing information is available on request or alternatively, please go to www.clonmel-health.ie. Medicinal product subject to medical prescription. PA 126/332/003. PA Holder: Clonmel Healthcare Ltd., Waterford Road, Clonmel, Co Tipperary. Also available, Tipol (paracetamol) suppositories range as below:
Tipol 75mg Suppositories
Tipol 125mg Suppositories
Tipol 250mg Suppositories
Tipol 500mg Suppositories
Tipol Max 1000mg Suppositories
Medicinal product subject to medical prescription. For retail sale through pharmacy only.
PA 126/331/001-005. PA Holder: Clonmel Healthcare Ltd, Waterford Road, Clonmel, Co Tipperary. Please contact Clonmel Healthcare on 01-6204000 if you require any additional information.
Date prepared: October 2021. 2021/ADV/COT/092H.
The Hermitage Medical Clinic is using a new care management system which assists patients before and after robotic orthopaedic surgeries.
Due to an agreement between the Hermitage, Zimmer Biomet and Apple, the mymobility app can be used by any patients with a wearable device such as an iWatch or an iPhone.
Mymobility tracks the patient’s movements and prompts them when they need to do their postoperative exercises, while also providing guidance on whether they are doing them correctly.
It is used in conjunction with the ROSA Knee System, a technology designed to adapt to each surgeon’s workflow while maximising efficiency and
enhancing intraoperative feedback.
This the first robotic platform to be used in the Hermitage, where patients can now benefit from the “more personalised approach to recovery”, according to the hospital.
Mr David Cogley, a Consultant Orthopaedic Surgeon at the Hermitage who specialises in knee and hip surgery, said the system is helping patients in their recovery.
“An effective recovery journey is absolutely vital to the surgical process. What mymobility does is provide patients with the information they need to do the required exercises correctly.
“The medical team here at the Hermitage can
monitor patients’ progress remotely via mymobility. It helps doctors to identify immediately where problems exist and lets us know where extra support and guidance is needed. Using it helps to maximise the benefits of the robotic joint replacement, and it helps patients to stick to their post-op recovery plans and ultimately get back on their feet more quickly,” Mr Cogley said.
He added: “Being able to use mymobility in conjunction with ROSA will be of huge assistance in preparing patients for surgery in the coming years, and in aiding their recovery. It is a massive win for patients, and another example of how modern technology is delivering real results in orthopaedic surgery here at the Hermitage.”
PRODUCT NEWS 55 VOL 23 ISS 1 JANUARY 2022
CARE MANAGEMENT HERMITAGE MEDICAL CLINIC TO USE MYMOBILITY
HIGH-TECH MEDICINES
ACCORD HEALTHCARE MAINTAINING THEIR LEADING POSITION IN GENERIC HIGH-TECHS IN IRELAND
THINK HIGH-TECHS THINK ACCORD
The High-Tech medicines scheme was first introduced in Ireland in 1996. The scheme involves the supply and dispensing of High-Tech medicines through community pharmacies. Expenditure on this scheme has continually increased, with spend rising by 173 per cent between 2009 and 2019.
Consistency of supply is extremely important in the context of High-Tech medicines, as many of these medicines are prescribed for long-term illnesses. Accord Healthcare Ireland’s High-Tech portfolio consists of 16 medicines of which they are the leading generic supplier in 15 of these — 2022 will see many new High-Tech launches.
Demonstrating their commitment to these medicines, Accord conducted market research with Behaviour and Attitudes in 2021. The research demonstrated that 63 per
cent of pharmacists who use the High-Tech hub had a positive experience with it. On average, pharmacists placed orders for 33 scripts through the hub per month. Another positive highlighted in the research is that 59 per cent do not have concerns about other therapeutic areas joining the hub. Pharmacists also nominated Accord Healthcare Ireland as the supplier they would be most likely to recommend in this research. The reasons for this were reliability, product availability and price, to name a few.
Mr Padraic O’ Brien, Managing Director of Accord Healthcare Ireland, said: “We at Accord Healthcare Ireland have established ourselves as a leader in the generic HighTech market. We want Irish pharmacists to know that we are always here to support them and to ‘Think HighTechs, Think Accord’. We identified the opportunity in this market some time ago and our portfolio has increased by 45 per cent since 2017 alone. We have an extensive
range of generic high-tech medicines, demonstrating our commitment to ensuring more Irish patients have access to the medicines they need when they need them. The next 12 months will be an important time for the High-Tech space and Accord Healthcare Ireland will be playing its part as an industry leader, promising a very strong pipeline for the year ahead.”
As the generic High-Tech product is priced up to 60 per cent below the branded product, when pharmacists dispense items from the Accord Healthcare Ireland High-Tech portfolio, they are not only helping to provide a quality treatment for Irish patients, they are also helping the State to make considerable savings. In turn, these savings are used to provide access to new medicines, allowing more patients access to the treatments they require.
Think High-Techs, Think Accord.
A CONVERSATION ABOUT DEPRESSION
Former Waterford hurler Mr Maurice Shanahan and Dublin senior footballer Ms Nicole Owens are lending their support to Talking Depression, a new campaign to encourage open and honest conversations.
Launched by Janssen Sciences Ireland UC, the campaign comes as new research reveals that almost half of Irish adults (47 per cent) do not feel equipped to have a conversation with a family member or friend who they suspect may be experiencing a mental illness, despite almost two-thirds (62 per cent) having more empathy towards mental illnesses, such as depression, compared to before the pandemic.
A new resource, The Little Book of Big Conversations, has been created to support people living with all forms of depression, their family members and friends. The book is part of a larger global campaign created by Janssen in partnership with people living with depression and their loved ones and with the support of the Global Alliance of Mental Illness Advocacy Networks Europe and the European Federation of Associations of Families of People with Mental Illness.
The public survey of 1,000 people in Ireland, conducted by Empathy Research on behalf of Janssen, found that 78 per cent of adults would be likely to have a conversation with a family member or friend who they suspect may be experiencing mental illness. However, there was concern about upsetting the person (81 per cent), saying the ‘wrong thing’ (79 per cent), or not understanding
the other person’s experience (73 per cent).
In Ireland, it is estimated that approximately 150,000 people per year are living with severe depression (also known as major depressive disorder or MDD).

Recent patient research commissioned by Janssen captured the prevalence and unmet needs of people living with the most severe forms of depression.
This research, involving over 200 adults living with severe depression in Ireland, showed that over three-in-four adults reported the Covid-19 pandemic has had a negative impact on their depression, with half having had their access to support, therapy and treatment restricted. Three-in-five adults living with severe depression say it has a major impact on their quality of life as they experience challenging symptoms daily, including fatigue or loss of energy (74 per cent), poor sleep patterns (66 per cent), low mood and poor concentration (56 per cent) and
feelings of worthlessness (52 per cent).
Dr Thekiso B Thekiso, Senior Lecturer and Consultant Psychiatrist, Trinity College Dublin and Navan Mental Health Services, said: “Depression, and in particular severe depression also known as major depressive disorder, is more than just a bout of the blues. It is where you stop doing the things you normally enjoy doing and you can’t simply ‘snap out’ of it. Unfortunately, depression often goes undiagnosed and untreated in adults, and they may feel reluctant to seek help. The Covid-19 pandemic has without doubt made things much more challenging for people living with serious mental illness and now more than ever, people need support.”
Dr Thorsten Giesecke, General Manager, Commercial Business, Janssen Sciences Ireland UC, said: “Janssen has a strong heritage in helping to reduce the burden, disability and devastation caused by mental illness and is dedicated to broadening society’s understanding of and support for the different types of depression. Open and honest conversations about depression are an important step towards ensuring that people living with depression get the support they need, and we hope this campaign will help facilitate this.”
The Little Book of Big Conversations can be accessed at https://janssenwithme.ie/en-ie/littlebook-big-conversations
The Talking Depression video series can be accessed at https://bit.ly/3osX3dH
PRODUCT NEWS 56 VOL 23 ISS 1 JANUARY 2022
MENTAL HEALTH
ALMOST HALF OF IRISH ADULTS DO NOT FEEL EQUIPPED TO HAVE
AVAILABLE IN PRINT AND DIGITAL




The solution to all your sourcing pains For more information please contact: Free Phone 1800 440 440 I PharmaSource@uniphar.ie I www.uniphar.ie






















































































































































































































































































